- Campus Directory
- Current Students
- Faculty & Staff


Acute Renal Failure Case Study
Our kidneys are incredible organs that get rid of toxins, retain substances needed by our bodies, and maintain the right balance of electrolytes, minerals, and water. Find out what happens to this 27-year-old when toxins accumulate in her kidneys leading to acute renal failure.
Module 11: Acute Tubular Necrosis
A 27 year old female was seen by medical personnel at an after...
Renal Failure - Page 1

The patient was admitted to the hospital by the patient's...
Renal Failure - Page 2

Case Summary
Summary of the Case
Renal Failure - Summary

Answers to Case Questions
Renal Failure - Answers

Professionals
Health Professionals Introduced in Case
Renal Failure - Professionals
Additional Links
Opptional links to explore further
Renal Failure - Links

- Previous Article
Presentation
Clinical pearls, case study: man with type 2 diabetes and stage 1 kidney disease on atkins-like diet.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Deborah Thomas-Dobersen , Lynn Casey; Case Study: Man With Type 2 Diabetes and Stage 1 Kidney Disease on Atkins-Like Diet. Clin Diabetes 1 January 2005; 23 (1): 46–48. https://doi.org/10.2337/diaclin.23.1.46
Download citation file:
- Ris (Zotero)
- Reference Manager
C.S. is a 45-year-old Hispanic man with a 10-year history of type 2 diabetes. He has a glycated hemoglobin of 7.0% and a blood pressure of 130/80 mmHg, treated with an angiotensin-converting enzyme inhibitor for the past 2 years. He has stable background retinopathy and is a nonsmoker. His BMI has been 30 (height 5′10″, weight 210 lb) for the past year. However,lately, he has put himself on the latest high-protein diet (i.e., the Atkins diet).
His weight has dropped by 10 lb, his fasting serum triglyceride level has fallen from 185 to 130 mg/dl, and his blood pressure has decreased to 120/78 mmHg. His LDL cholesterol has remained stable at 102 mg/dl on a statin. His serum creatinine is 0.9 mg/dl, and his 24-hour urine shows a significant increase in microalbumuria from 100 mg/24 hours last year to the current 200 mg/24 hours. He has stage 1 chronic kidney disease indicating kidney damage,with a normal glomerular filtration rate (GFR) of 98 ml/min/1.73 m 2 .
Would the weight reduction, blood pressure, and lipid-lowering accomplished by this high-protein, low-carbohydrate diet be an acceptable choice for a patient who is at significant risk of cardiovascular disease?
What are the recommendations of the American Heart Association (AHA), the National Kidney Foundation (NKF), the National Academy of Sciences, and the American Diabetes Association (ADA) regarding this type of diet for diabetes and/or weight loss?
What has research revealed about appropriate levels of macronutrients for patients such as C.S.?
It is likely that microalbuminuria is the start of a continuum progressing to macroalbuminuria and proteinuria. Microalbuminuria predicts renal disease in diabetes (both type 1 and type 2) and relates to premature mortality. Microalbuminuria is also a marker for pronounced diabetic vascular disease(endothelial dysfunction and chronic low-grade inflammation). Abnormal albuminuria is a major risk factor for cardiovascular complications,predicting increased cardiovascular morbidity and mortality. 1
Twenty to thirty percent of patients with type 2 diabetes develop evidence of nephropathy. Some patients already have microalbuminuria or overt nephropathy upon diagnosis. Without intervention, 20-40% of those with microalbuminuria progress to overt nephropathy. For those on the continuum from overt nephropathy to end-stage renal disease (ESRD), the greater risk of death from coronary artery disease (CAD) may intervene. 2
The average adult protein intake in the United States is 15-20% of total calories and has remained consistent from 1909 to the present. 3 Most Americans eat 50% more protein than they need. The Recommended Dietary Allowance (RDA) is 0.8 g of good quality protein per kilogram body weight per day for men and women. The high-protein Atkins and Zone diets recommend 125 g/day (36% kcal from protein) and 127 gm/day (34% kcal from protein),respectively. 4 The initial phases of the South Beach diet are similar, but no specific nutrient intake can be found in the diet's literature. In C.S., the Atkins diet would contribute 1.3 g protein/kg body weight and 36% of total daily calories from protein. Thus, high-protein diets promote a significantly abnormally high protein intake.
There is some evidence that a sustained high-protein diet can adversely affect renal function, especially in people with diabetes with or without mild renal insufficiency. In patients without renal insufficiency, a high-protein diet may act by acutely increasing the GFR and causing intraglomerular hypertension, which may cause progressive loss of renal function. In the Nurses Health Study, 1,624 female nurses between 30 and 55 years of age were followed for a period of > 11 years. The highest quartile of total protein intake, an average of 93 g/day, was significantly associated with a decline in GFR in women with mild renal insufficiency, thus worsening renal disease. 5 Previous studies had shown mixed results of high-protein diets on renal function but had limitations such as small patient numbers, limited follow-up, and a narrow range of protein intake.
Looking at this relationship from another angle, a meta-analysis recently showed that protein restriction retards the rate of decline in GFR, thus lessening kidney damage. The resulting decrease in kidney damage was small and not impressive. However, when studies looking at people with diabetes were combined, a total of 102 patients given a mean protein restriction of 0.7 g/kg/day versus a control group given 1 g/kg/day (a narrow range), showed a more impressive improvement in renal function independent of the original renal function over 22 months. 6 A crosssectional study of > 2,600 people with type 1 diabetes found that a protein intake > 20% of calories was associated with an increased urinary albumin excretion rate. Researchers concluded that people with diabetes should not exceed a protein intake of 20% of calories. 7 Any study in type 1 diabetes is applicable to type 2 diabetes as it relates to nephropathy. Therefore, there is evidence to recommend avoidance of high protein intakes in patients at risk for renal disease, i.e. all patients with type 1 or type 2 diabetes.
Nutrient analysis of high-protein diets is a concern. With some high-protein diets, such as Atkins, come carbohydrate restrictions. Yet high-carbohydrate foods, such as fruits, vegetables, and low-fat dairy products, provide potassium, magnesium, and calcium, which modestly reduce blood pressure. 8 Normal blood pressure is critically important in preventing CAD and microalbuminuria. With high-protein diets and carbohydrate restrictions come decreased-fiber diets. High-fiber diets have many beneficial effects,including weight loss and lower cardiovascular and cancer risks. With high-protein diets come higher intakes of saturated fats, which are potentially atherogenic. 9 In addition, experimental evidence indicates that a high-protein diet and the resultant increase in saturated fat intake may accelerate the progression of renal disease. Increased LDL cholesterol can stimulate mesangial hypertrophy and stimulate cytokine formation, which may ultimately cause tissue injury. In both type 1 and type 2 diabetes, hypercholesterolemia is a predictor of deteriorating kidney function. 10
The RDA for carbohydrate is set at 130 g carbohydrate/day for adults and children based on the average minimum amount of glucose utilized by the brain to ensure optimal brain function. 11 That pretty much omits Atkins (28-33 g/day) and the early phases of the South Beach diet. Recent AHA guidelines discourage high-protein diets for weight loss,citing potential increased risk for coronary heart disease and renal disease. 12 The most recent ADA technical review on nutrition states that high-protein diets are not recommended until further research establishes their safety. 3 Concerns include renal function and cardiovascular disease. The NKF states in its Kidney Disease Outcomes Quality Initiative guidelines for chronic kidney disease that there is no benefit from a protein intake higher than the RDA of 0.8 g/kg body weight and that this is a reasonable level to recommend for patients with chronic kidney disease in stages 1-3. 13 Thus, many respected nonprofit health care organizations discourage the use of high-protein, low-carbohydrate diets.
Literature reviews of research on the effect of high-protein,low-carbohydrate diets on obesity and lipid levels are not convincing. A review of the literature describing adult outpatient recipients of low-carbohydrate, high-protein diets compared a wide variety of study designs,carbohydrate levels, durations, and calorie levels. Only five studies evaluated low-carbohydrate, high-protein diets for > 90 days, and these were nonrandomized, uncontrolled studies. The three variables that most predicted weight loss were calorie level, duration of calorie restriction, and number of very obese participants in the study. Reduced carbohydrate content was not significantly associated with weight loss. 14
Another review concluded that populations at risk for renal disease, such as patients with diabetes, should avoid high-protein diets. The authors also caution that evidence suggested that protein intakes in excess of two to three times the RDA may have harmful effects on calcium homeostasis and possibly bone mass, 15 a problem for a population already predisposed to osteoporosis. In addition, a comparison of high-protein, low-carbohydrate diets versus a low-fat diet for weight loss shows them equally effective after 1 year in duration. 16 A recent small, randomized, clinical trial comparing a low-carbohydrate (< 30 g) to a conventional low-fat diet in severely obese patients, including individuals with diabetes, showed no significant difference in weight loss after 1 year, although weight loss was minimal (11 vs. 7 lb). Of interest was that the weight loss on the low-carbohydrate diet did not appear to be sustainable and that blood urea nitrogen levels increased more in the low-carbohydrate group. 17
Reduced energy intake is an important therapeutic objective for the patient in the case described above. Reduced energy intake would reduce his blood pressure and serum lipids as well as improve his glycemic control. Weight loss was effective in lowering his blood pressure and serum triglycerides, as one would expect. However, the macronutrient content of his diet may have exacerbated the microalbuminuria. Therefore, a patient such as C.S. would be illadvised to stay on the high-protein diet because of the potential risk to his kidney function as shown by his elevated microalbuminuria.
With guidance from a registered dietitian, C.S. started a 1,500-kcal,low-fat diet with a walking program of 2 miles/day, 6 days/week. He was very tired of the restrictive nature of the high-protein diet and welcomed a change. His urine microalbumin level fell to < 50 mg/24 hours.
Two important studies show strategies that work to yield long-term weight loss. In order to determine what strategies work for long-term weight loss,the National Weight Control Registry elicited and studied information from> 800 people who have been successful in this endeavor. Only half had lost weight through weight loss programs. The remainder had lost weight without medical intervention. Keys to success were an average calorie intake of ∼1,400 kcal/day, a low-fat diet (24% of kcal), and a high energy expenditure through exercise (2,800 kcal/week). 18 The Diabetes Prevention Program also documented that a low-fat diet, increased physical activity, and educational sessions with frequent follow-up allowed participants to lose 7% of their body weight and maintain a 5% weight loss for 3 years. 19
High protein intakes cause higher workloads for kidneys, whose function is to handle amino acid fragments during protein degradation and excrete nitrogen as urea.
There is no research documenting that a high-protein diet maintains weight reduction any better than a low-fat diet, which is safer and offers long-term results.
Safety and efficacy of high-protein, low-carbohydrate diets are a concern for patients with diabetes, regardless of documented kidney disease.
Additional Information
Concerns about the low-carbohydrate diet craze of 11 leading nonprofit consumer, nutrition, and public health organizations are discussed in a format appropriate for both health professionals and patients at the Partnership for Essential Nutrition website: www.essentialnutrition.org .
Deborah Thomas-Dobersen, RD, MS, CDE, is a diabetes educator at the Center for Diabetes and Endocrinology in Arvada, Colo. Lynn Casey, RD, CSR, is a renal dietitian at Renal Care Group, Inc., in Denver, Colo.
Email alerts
- Online ISSN 1945-4953
- Print ISSN 0891-8929
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Case report
- Open access
- Published: 04 August 2009
A 60-year-old man with chronic renal failure and a costal mass: a case report and review of the literature
- Germán Campuzano-Zuluaga 1 ,
- William Velasco-Pérez 1 &
- Juan Ignacio Marín-Zuluaga 1
Journal of Medical Case Reports volume 3 , Article number: 7285 ( 2009 ) Cite this article
42k Accesses
7 Citations
3 Altmetric
Metrics details
Introduction
Brown tumors are a rare focal manifestation of osteitis fibrosa cystica, which results from hyperparathyroidism. Chronic kidney failure may lead to secondary or tertiary hyperparathyroidism and thus to osteitis fibrosa cystica and brown tumors.
Case presentation
A 60-year-old man with a history of diabetes mellitus and chronic kidney failure presented with a 15-day history of dyspnea, cough, malaise and fever. Initially, there was little correlation between his history and his physical examination. Various pulmonary, cardiac and infectious etiologies were ruled out. A chest X-ray showed a costal mass that was further verified by tomography and gammagraphy. The mass was suspected of being neoplastic. After a failed biopsy, the mass was removed surgically and on histopathology was compatible with a giant-cell tumor versus a brown tumor caused by hyperparathyroidism. Laboratory tests showed elevated calcium, phosphate and parathyroid hormone concentrations. The patient was diagnosed with a brown tumor secondary to refractory hyperparathyroidism.
Tending towards a diagnosis because it is more frequent or it implies more risk for the patient may delay the consideration of other diagnostic options that, although rare, fit well into the clinical context. The patient presented here was suspected to have an osseous neoplasia that would have had major implications for the patient. However, reassessment of the case led to the diagnosis of a brown tumor. Brown tumors should be an important diagnostic consideration in patients with chronic kidney failure who have secondary or tertiary hyperparathyroidism and an osseous mass.
The first case in the literature reporting a brown tumor was published in 1953 and described a fronto-ethmoidal brown tumor [ 1 ]. However, previous reports of patients with localized forms of osteitis fibrosa cystica (OFC) suggest that the clinical entity was described earlier, at a time when there were few treatment options for chronic kidney failure (CKF) and consequently chronic hyperparathyroidism was more prevalent. Brown tumors are rare osseous lesions that represent a focal manifestation of OFC resulting from hyperparathyroid states. Patients suffering from CKF may develop secondary or tertiary hyperparathyroidism due to altered phosphorus and calcium metabolism. Persistent hyperparathyroidism leads to altered osseous metabolism with bone resorption and tissue changes collectively known as OFC. Our case report describes a patient with poorly controlled CKF who presented with a non-specific clinical picture and no clear diagnosis. Incidentally a costal mass was found and the diagnostic workup that followed led to an unexpected diagnosis.
A 60-year-old man was transferred from the hemodialysis unit to the emergency room because of a 15-day history of malaise, subjective fever, shortness of breath, dry cough, abdominal pain and diarrhea. He also complained of mild anterior thoracic pain not associated with other symptoms and which was not irradiated. He had a 20-year history of type 2 diabetes mellitus (DM) that required insulin, with micro- and macro-vascular complications such as diabetic retinopathy and CKF. He was on hemodialysis and had a history of multiple failed dialysis accesses. He also suffered from arterial hypertension, upper and lower extremity peripheral arterial disease, carotid artery disease, a first degree atrioventricular heart block and had smoked one packet of cigarettes per day for the last 20 years. He was being treated with sevelamer, erythropoietin, folic acid, lovastatin, gemfibrozil, NPH insulin, amlodipine and acetylsalicylic acid, but was not receiving calcium or a vitamin D supplement.
A physical examination revealed the patient to be in a fair condition, with no apparent distress, hydrated, alert and well oriented. He had a heart rate of 92 beats per minute, respiratory rate of 14 breaths per minute, blood oxygen saturation of 97%, arterial blood pressure of 130/70 mmHg and no fever. He had bilateral blindness and mild epistaxis through the left nostril. The thorax was tender to palpation in some costochondral unions, but pain was poorly localized. The vesicular murmur had reduced intensity and no pathologic sounds were auscultated. Peripheral pulses were weak in both the upper and the lower limbs. He had a translumbar hemodialysis catheter. The remaining physical examination was unremarkable.
The patient had stable vital signs and had no signs of systemic inflammatory response. However, because of the patient's previous history of DM, CKF and the presence of leukocytosis, neutrophilia and elevated C-reactive protein upon admission (Table 1 ), we initially ruled out a gastrointestinal or lung infection, or any cardiac cause for the patient's symptoms. The electrocardiogram showed no signs of ischemia, and the chest X-ray showed cardiomegaly, a small left pleural effusion, a circular opacity in the right inferior thoracic region and no signs of consolidation. These findings were initially interpreted as a pulmonary infection, probably a lung abscess, an abscedated nodule or pulmonary tuberculosis. A contrast tomography scan of the chest was ordered for further characterization. Though it showed no parenchymal compromise, a 4 × 1.3 cm lesion was observed on the right dorsal region of the eighth rib. The lesion showed thinning of cortical bone in some areas, preserved cortex and lacked periosteal reaction (Figure 1 ). The radiology staff considered a bone metastasis as a first diagnostic option, and a thoraco-abdomino-pelvic tomography scan was done in search for more lesions and a probable primary tumor. Additional hypodense lesions were observed, including one on the left lamina of L4, acetabulum, and head and neck of the right femur. There was no lymph-node or internal organ compromise. A Tc 99 m Medronate osseous gammagraphy reported a hypermetabolic focus compatible with a neoplastic lesion, concordant in size and location with the costal mass reported in the previous imaging studies. It also revealed generalized osseous compromise compatible with renal osteodystrophy and did not confirm the other lesions described on tomography. A tomography-guided biopsy specimen (Figure 1 ) was obtained, but histopathological analysis reported normal tissue components.

Tomographic image during guided biopsy procedure . Note the heterogeneous 4 × 1.3 cm mass (arrow), with preserved cortical bone and no periosteal reaction or other inflammatory signs. No cysts were identified.
Not being able to reach a clear diagnosis, a careful reassessment of the patient's clinical record led to considering the alternative diagnosis of renal osteodystrophy. This was supported by a history of poorly controlled CKF, elevated calcium (11.2 mg/dl) and phosphorus (5.3 mg/dl) concentrations, a phosphocalcic product of 59.36 mg 2 /dl 2 , and a bone gammagraphy that showed changes compatible with OFC. However, the possibility of neoplasia was still being considered so the mass was removed surgically. Histopathological studies reported an osseous tissue with spindles of fusiform cells in a storiform disposition with abundant multinucleated giant cells, some macrophages and some mononuclear cells. Scarce mitotic activity was observed, and there were no signs of malignancy (Figure 2 ). The pathologist concluded that the findings were compatible with a giant-cell tumor or a brown tumor, both histologically very similar [ 2 ]. Parathyroid hormone (PTH) concentration was 1377 pg/ml. These findings were compatible with refractory hyperparathyroidism, and a diagnosis of a brown tumor of hyperparathyroidism associated with CKF was reached.
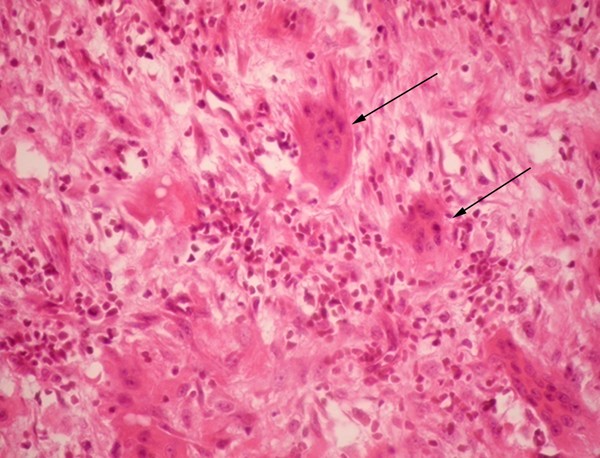
Microscopic pathology of surgical specimen . Presence of various multinucleated giant cells (arrows) and spindle arranged cells. Hemosiderin deposits were not observed in the sample. Hematoxylin-eosin stain at 40 × magnification.
The patient continued ambulatory medical treatment with vitamin D, calcium and sevelamer. Two months after discharge, the parathyroid level was 1900 pg/ml and a Tc 99 m Sestamibi scan revealed hyperfunctioning glands despite aggressive pharmacological treatment. Serum calcium and phosphorus levels were within normal limits, 9.4 mg/dl and 3.4 mg/dl, respectively. At the time of writing, the patient was awaiting parathyroidectomy as definite treatment for tertiary hyperparathyroidism associated with severe renal osteodystrophy.
Brown tumors are unusual bone lesions that represent a localized manifestation of OFC induced by hyperparathyroidism, independent of its cause. Increased PTH levels and locally produced tumour necrosis factor α and interleukin 1 (IL-1) by marrow monocytes induce the proliferation and differentiation of pluripotent bone-marrow cells into osteoblasts. These cells produce granulocyte macrophage colony stimulating factor, IL-6, IL-11 and stem-cell factor that induce the migration and differentiation of monocytes into osteoclasts, increasing the number of the latter in the bone tissue. Enhanced activity of osteoclasts and osteoblasts leads to bone resorption and a reduction of bone mineral concentration with an increased proliferation of fibrous tissue and extracellular matrix [ 3 ]. Brown tumors develop in 3% to 4% of patients with primary hyperparathyroidism and in 1.5% to 1.7% of patients with secondary causes of hyperparathyroidism [ 4 ]. However, around half of patients with CKF may develop OFC due to secondary hyperparathyroidism making brown tumors more frequent in these patients. Brown tumors have been reported in patients with primary hyperparathyroidism due to adenomas [ 5 ] and carcinomas [ 6 ] of the parathyroid gland; vitamin D deficiency due to lack of sunlight exposure [ 7 ] or due to intestinal malabsorption syndromes [ 8 ]; and secondary [ 9 ] or tertiary hyperthyroidism [ 10 ] in patients suffering CKF. Hyperphosphatemia with hypocalcemia caused by tubular damage and impaired vitamin D metabolism explains hyperparathyroidism in these patients.
Brown tumors are either mono- or polyostotic benign masses, painless and usually found incidentally. However, they may cause tissue damage to adjacent structures and compressive manifestations such as pain, neuropathies [ 11 ] and myelopathy [ 12 ]. The majority of cases report the maxilla and mandible as the main sites of occurrence [ 9 ]. Other common sites are the clavicles, scapula, pelvis and ribs; however, these lesions may appear in any osseous structure [ 7 ], including chondral tissue [ 13 ]. They are associated with an increased risk of fractures if localized in weight-bearing areas [ 14 ].
Brown tumors arise from foci of OFC and represent a reparative bone process rather than true neoplastic lesions, as there is no hyperplasia or clonal cell proliferation. Typical histopathology describes spindle cells or fibroblasts in areas of osseous lysis, multinucleated giant cells (probably osteoclasts), increased vascularization and accumulation of hemosiderin-laden macrophages, with micro-hemorrhages which confer a brownish appearance to the affected tissue. Cysts and areas of necrosis may be found [ 2 , 5 ]. Brown tumors are histologically similar to giant-cell tumors, giant-cell regenerative granulomas, cherubism and aneurismatic osseous cysts [ 2 , 4 ].
On X-ray imaging, brown tumors appear as lytic lesions with thinned cortical bone that may be fractured. Concurrent changes that suggest OFC such as osteopenia, a "salt-and-pepper" bone appearance, subperiosteal bone resorption and disappearance of the lamina dura around the roots of the teeth, may help differentiate it from other entities [ 4 ]. Tomographic imaging shows an osseous mass, with no cortical disruption, no periosteal reaction or inflammatory signs, a heterogeneous center and areas that suggest cysts [ 14 ]. Magnetic resonance imaging (MRI) shows variable intensities on T2-weighted images and intense enhancement on T1-weighted contrast MRI. MRI may be better for determining the presence of cysts or fluid filled levels; a finding that is very suggestive of a brown tumor [ 14 ]. Osseous gammagraphy is not indicated for the diagnosis of brown tumors; however, isolated hypermetabolic lesions or simultaneous hypercaptation of bone lesions and parathyroid adenomas, when done with Tc 99 m Sestamibi, have been described [ 15 ].
Although differential diagnoses for an isolated bone lesion are extensive, when confronted with a patient with CKF, an osseous mass and laboratory data that show increased levels of calcium, phosphate, phosphocalcic product as well as alkaline phosphatase, it is imperative to determine PTH levels to rule out hyperparathyroidism. Histopathological analysis of the osseous lesion is needed to confirm the diagnosis of a brown tumor. In the case presented here, parathyroid levels were not assessed earlier because another diagnosis, osseous neoplasia, was suspected which posed major prognostic value and risk for the patient. A parathyroid hormone measurement six months earlier reported 570 pg/ml; thus, it is probable that the pathological process evolved during this brief time.
Treatment of brown tumors relies on a definitive control of the underlying hyperparathyroid state. In a patient with CKF, this is achieved through the administration of phosphorus chelators, and calcium and vitamin D supplementation. In patients presenting with tertiary hyperparathyroidism, parathyroidectomy may be required. Osseous lesions usually cease to grow, then shrink and eventually ossify without further consequences for the patient. Surgery is required under certain circumstances, such as: 1) compressive neurologic symptoms over peripheral nerves, cauda equina or spinal medulla; 2) a significant anatomical deformity; 3) risk of a pathologic fracture; 4) when the symptoms or pain do not resolve despite adequate medical treatment and control of the hyperparathyroid state; and 5) when the biopsy does not yield a clear diagnosis, as with the present case [ 9 , 11 , 12 ].
The case presented here illustrates how brown tumors, though rare, should be considered in patients with CKF and an osseous mass. The initial clinical presentation of this patient, a history of DM with a non-compensated CKF and the laboratory studies suggested an infectious process. Retrospectively, these initial complaints and findings could be explained by the patient's renal condition with volume overload, severe anemia, hydro-electrolyte disturbances, as well as altered calcium and phosphate metabolism. Early diagnosis and proper management of CKF enable an optimal control of bone-mineral metabolism, thus decreasing the incidence of OFC and making brown tumors rare lesions. Nevertheless, when confronted with a patient with CKF and an osseous mass, a brown tumor caused by hyperparathyroidism should always be considered in the differential diagnosis.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
chronic kidney failure
diabetes mellitus
interleukin 1
interleukin 6
interleukin 11
magnetic resonance imaging
osteitis fibrosa cystica
parathyroid hormone.
Guarnaccia E: [Brown fronto-ethmoidal tumor; contribution to the knowledge of cranial localizations of the fibrocystic osteopathies]. Otorinolaringol Ital. 1953, 21: 175-189.
CAS PubMed Google Scholar
Mafee MF, Yang G, Tseng A, Keiler L, Andrus K: Fibro-osseous and giant cell lesions, including brown tumor of the mandible, maxilla, and other craniofacial bones. Neuroimaging Clin N Am. 2003, 13: 525-540. 10.1016/S1052-5149(03)00040-6.
Article PubMed Google Scholar
Hruska K: New concepts in renal osteodystrophy. Nephrol Dial Transplant. 1998, 13: 2755-2760. 10.1093/ndt/13.11.2755.
Article CAS PubMed Google Scholar
Takeshita T, Tanaka H, Harasawa A, Kaminaga T, Imamura T, Furui S: Brown tumor of the sphenoid sinus in a patient with secondary hyperparathyroidism: CT and MR imaging findings. Radiat Med. 2004, 22: 265-268.
PubMed Google Scholar
Fernandez-Sanroman J, Anton-Badiola IM, Costas-Lopez A: Brown tumor of the mandible as first manifestation of primary hyperparathyroidism: diagnosis and treatment. Med Oral Patol Oral Cir Bucal. 2005, 10: 169-172.
Pahlavan PS, Severin MC: Parathyroid carcinoma: A rare case with mandibular brown tumor. Wien Klin Wochenschr. 2006, 118: 175-179. 10.1007/s00508-006-0566-5.
Erturk E, Keskin M, Ersoy C, Kaleli T, Imamoglu S, Filiz G: Metacarpal brown tumor in secondary hyperparathyroidism due to vitamin-D deficiency A case report. J Bone Joint Surg Am. 2005, 87: 1363-1366. 10.2106/JBJS.D.02250.
Ehrlich GW, Genant HK, Kolb FO: Secondary hyperparathyroidism and brown tumors in a patient with gluten enteropathy. AJR Am J Roentgenol. 1983, 141: 381-383.
Jeren-Strujic B, Rozman B, Lambasa S, Jeren T, Markovic M, Raos V: Secondary hyperparathyroidism and brown tumor in dialyzed patients. Ren Fail. 2001, 23: 279-286. 10.1081/JDI-100103500.
Pinto LP, Cherubinim K, Salum FG, Yurgel LS, de Figueiredo MA: Highly aggressive brown tumor in the jaw associated with tertiary hyperparathyroidism. Pediatr Dent. 2006, 28: 543-546.
Tarrass F, Ayad A, Benjelloun M, Anabi A, Ramdani B, Benghanem MG, Zaid D: Cauda equina compression revealing brown tumor of the spine in a long-term hemodialysis patient. Joint Bone Spine. 2006, 73: 748-750. 10.1016/j.jbspin.2006.01.011.
Kaya RA, Cavusoglu H, Tanik C, Kahyaoglu O, Dilbaz S, Tuncer C, Aydin Y: Spinal cord compression caused by a brown tumor at the cervicothoracic junction. Spine J. 2007, 7: 728-732. 10.1016/j.spinee.2006.07.013.
Perrin J, Zaunbauer W, Haertel M: Brown tumor of the thyroid cartilage: CT findings. Skeletal Radiol. 2003, 32: 530-532. 10.1007/s00256-003-0664-7.
Takeshita T, Takeshita K, Abe S, Takami H, Imamura T, Furui S: Brown tumor with fluid-fluid levels in a patient with primary hyperparathyroidism: radiological findings. Radiat Med. 2006, 24: 631-634. 10.1007/s11604-006-0068-4.
Yapar AF, Aydin M, Reyhan M, Bal N, Yapar Z, Yologlu NA: Simultaneous visualization of a mandibular brown tumor with a large parathyroid adenoma on Tc-99 m MIBI imaging. Clin Nucl Med. 2005, 30: 433-435. 10.1097/01.rlu.0000162970.49398.4c.
Download references
Acknowledgements
We thank the following persons: the patient and his family for the information provided and their approval for the publication of this case; the medical staff at the Hospital Pablo Tobón Uribe, especially the Internal Medicine, Radiology, Surgery and Pathology Departments, and the Nephrology and Dialysis Unit; Dr. Victoria Eugenia Murillo for histopathological analysis, case discussion and photomicrography; Dr. John M. Lopera, Dr. Jorge H. Donado and Ana Isabel Toro for manuscript revision and editing.
Author information
Authors and affiliations.
Department of Internal Medicine, Hospital Pablo Tobón Uribe, Calle 78B No. 69-240, Medellín, Colombia
Germán Campuzano-Zuluaga, William Velasco-Pérez & Juan Ignacio Marín-Zuluaga
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Germán Campuzano-Zuluaga .
Additional information
Competing interests.
The authors declare that they have no competing interests regarding this case report.
Authors' contributions
GCZ summarized and interpreted the patient's medical record and was part of the medical staff, did the literature review and wrote the manuscript. WV and JIMZ helped to interpret the patient's medical record, were part of the medical staff and helped to write and review the manuscript. JIMZ was the principal attending physician and responsible for most medical decisions and interpretations expressed in the article. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/3.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Campuzano-Zuluaga, G., Velasco-Pérez, W. & Marín-Zuluaga, J.I. A 60-year-old man with chronic renal failure and a costal mass: a case report and review of the literature. J Med Case Reports 3 , 7285 (2009). https://doi.org/10.4076/1752-1947-3-7285
Download citation
Received : 23 December 2008
Accepted : 24 December 2008
Published : 04 August 2009
DOI : https://doi.org/10.4076/1752-1947-3-7285
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hyperparathyroidism
- Parathyroid Adenoma
- Renal Osteodystrophy
- Brown Tumor
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
Day 1: A 62-year old, recently widowed male Hispanic patient, named Mr. Kevin Ulyses Blanco (K. U. B.) was brought in to the emergency department (ED) by his daughter for progressively worsening shortness of breath, fatigue, a lingering non-productive cough, and generalized edema. One month prior, he noticed dyspnea upon exertion, loss of appetite, nausea, vomiting and malaise, which he attributed to the flu. In the emergency department, he appeared anxious and pale, and had a dry yellow tint to the skin. He denied any chest pain, and he could not recall the last time he urinated. He has history of benign prostatic hyperplasia, diabetes mellitus type 2, hypertension, dyslipidemia, and renal insufficiency for the past two years. His ED assessment findings included: 1+ pedal edema, basilar crackles in the lungs bilaterally, and a scant amount of urine according to a bladder scan. His lab results indicated a glomerular filtration rate (GFR) of 12. Based on his subjective and objective symptoms, he was admitted with a diagnosis of progression of chronic kidney disease (CKD) to end-stage renal disease (ESRD). The plan of care was focused on managing his symptoms and consulting with his nephrologist regarding need for hemodialysis.
Day 3: Mr. K.U.B had an AV graft placed in his forearm to receive dialysis and a dual-lumen hemodialysis catheter for temporary use. His symptoms were worsening despite medical interventions and hemodialysis was needed urgently. The plan was to continue his medications to manage anemia, HTN, diabetes, and renal disease. The nurse identified psychosocial stressors of financial concern and having to live alone with his worsening health condition. With his daughter living far away, he was worried he wouldn’t have support. He stated that he was worried about the financial burden of hemodialysis and struggled with facing the reality of his diagnosis and what his quality of life would be like in the next few years of his life. A recommendation was made for a social worker and psychiatric consult.
Day 8: By the end of day 8, most of his acute symptoms had been relieved and he was stable enough to be discharged. He had been in contact with case management for his follow up appointment had been made with his primary physician and discharge teaching was given.
- What modifiable factors could Mr. K.U.B. have addressed to slow the progression of his renal disease?
- What collaborative interventions could be used to enhance his care and ensure continuity of care after discharge?
- What affect did uncontrolled hypertension and poor medication compliance have on his disease process?
Nursing Case Studies by and for Student Nurses Copyright © by jaimehannans is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
Sorry. You need a frames capable broswer to view this page.
Global case studies for chronic kidney disease/end-stage kidney disease care
Affiliations.
- 1 Kidney Research Center, Department of Nephrology, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taoyuan, Taiwan.
- 2 Centre for Transplantation and Renal Research, Westmead Institute for Medical Research, University of Sydney, Sydney, New South Wales, Australia.
- 3 Institute of Biomedical Ethics and the History of Medicine, University of Zurich, Zurich, Switzerland.
- 4 Renal Division, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA.
- 5 Division of Nephrology, The University of Tokyo School of Medicine, Hongo, Japan.
- 6 State Key Laboratory of Organ Failure Research, National Clinical Research Center for Kidney Disease, Division of Nephrology, Nanfang Hospital, Southern Medical University, Guangzhou, China.
- 7 Servicio de Nefrologia, Hospital Civil de Guadalajara Fray Antonio Alcalde, University of Guadalajara Health Sciences Center, Hospital 278, Guadalajara, Jalisco, Mexico.
- 8 Almughtaribeen University, Khartoum, Sudan.
- 9 Department of Nephrology, Dalal Jamm Hospital, Cheikh Anta Diop University Teaching Hospital, Dakar, Senegal.
- 10 Dialysis Unit, CASMU-IAMPP, Montevideo, Uruguay.
- 11 Division of Nephrology, Department of Internal Medicine, Rajavithi Hospital, Bangkok, Thailand.
- 12 Department of Medicine, Chulalongkorn Hospital, Bangkok, Thailand.
- 13 Division of Nephrology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
- 14 Bhumirajanagarindra Kidney Institute, Bangkok, Thailand.
- 15 SEHA Dialysis Services, Abu Dhabi, United Arab Emirates.
- 16 Department of Nephrology and Clinical Research Centre, Hospital Serdang, Jalan Puchong, Kajang, Selangor, Malaysia.
- 17 Department of Nephrology, Barts Health NHS Trust, London, UK.
- 18 Centre for Nephrology, University College London, London, UK.
- 19 Malawi Ministry of Health, Queen Elizabeth Central Hospital, Blantyre, Malawi.
- 20 Parklands Kidney Centre, Nairobi, Kenya.
- 21 Department of Medicine, The Aga Khan University Hospital, Nairobi, Kenya.
- 22 Paediatric Intensive and Critical Unit, Red Cross War Memorial Children's Hospital, University of Cape Town, Cape Town, South Africa.
- 23 Division of Nephrology, College of Medicine, Seoul National University, Seoul, Korea.
- 24 School of Medicine and Dentistry, College of Health Sciences, University of Ghana, Legon, Accra, Ghana.
- 25 Department of Medicine, University of Calgary, Calgary, Alberta, Canada.
- 26 Pan American Health Organization/World Health Organization's Coordinating Centre in Prevention and Control of Chronic Kidney Disease, University of Calgary, Calgary, Alberta, Canada.
- 27 International Society of Nephrology, Brussels, Belgium.
- PMID: 32149007
- PMCID: PMC7031689
- DOI: 10.1016/j.kisu.2019.11.010
The prevalence of chronic kidney disease and its risk factors is increasing worldwide, and the rapid rise in global need for end-stage kidney disease care is a major challenge for health systems, particularly in low- and middle-income countries. Countries are responding to the challenge of end-stage kidney disease in different ways, with variable provision of the components of a kidney care strategy, including effective prevention, detection, conservative care, kidney transplantation, and an appropriate mix of dialysis modalities. This collection of case studies is from 15 countries from around the world and offers valuable learning examples from a variety of contexts. The variability in approaches may be explained by country differences in burden of disease, available human or financial resources, income status, and cost structures. In addition, cultural considerations, political context, and competing interests from other stakeholders must be considered. Although the approaches taken have often varied substantially, a common theme is the potential benefits of multistakeholder engagement aimed at improving the availability and scope of integrated kidney care.
Keywords: chronic kidney disease; dialysis; end-stage kidney disease; transplantation.
© 2020 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Publication types
Chronic Kidney Disease (CKD) Case Study (45 min)
Watch More! Unlock the full videos with a FREE trial
Included In This Lesson
Study tools.
Access More! View the full outline and transcript with a FREE trial
Mr. Stinson is a 52-year-old male with a history of HTN, DM Type II, CKD, and CHF. He presented to the Emergency Department (ED) complaining of severe itching, nausea, and vomiting. He appeared pale and is lethargic. He reported shortness of breath and the nurse notes crackles in his lungs. He has now been admitted to your unit.
What additional nursing assessments should be performed?
- Full set of vital signs
- Auscultate heart and lung sounds, as well as peripheral pulses
- Assess skin turgor and edema
- Assess the patient’s dialysis access site for functionality or bleeding
What diagnostic or lab tests would you expect the provider to order?
- Complete metabolic panel (electrolytes, renal function, etc.
- Complete blood count
- Possibly an ABG to assess for acidosis
- Possibly a BNP to assess volume overload and its effect on the heart
Upon further questioning, the patient reports he normally gets dialysis Monday, Wednesday, Friday, but that he skipped dialysis yesterday because he was “not feeling well”. He has +2 pitting edema in his legs. Vital signs are as follows:
HR 102 RR 24
BP 153/97 SpO 2 90%
The patient’s labs result and show the following:
BUN 62 mg/dL Na 134 mg/dL
Cr 3.9 mg/dL Ca 7.8 mg/dL
GFR 13 mL/min/m 2 Phos 5.0 mg/dL
K 6.3 mEq/L Mg 1.6 mg/dL
Gluc 224 mg/dL H/H 8.2 / 30%
pH 7.32 pCO 2 32 HCO 3 – 16
BNP 247 pg/mL
Interpret these lab results and explain their meaning.
- The BUN/Cr and GFR indicate the patient is definitely in kidney failure as his glomerulus is not filtering the blood like it should and the waste products are building up
- His electrolyte abnormalities (hyperkalemia, hyponatremia, hypocalcemia, hyperphosphatemia, and hypomagnesemia) are all indicative of kidney disease and acidosis. The kidneys would normally retain sodium and excrete potassium. In kidney failure, they do the opposite and potassium levels can get very high.
- He is in metabolic acidosis, likely because his kidneys are not able to retain the bicarb buffer like they normally would – this also contributes to the hyperkalemia. As the body tries to balance the H+ ions, it kicks K+ out into the bloodstream.
- His BNP is also elevated, indicating volume overload – this is probably caused both by the kidney failure and not getting dialysis and by the heart failure
- He is anemic – chronic anemia is common in chronic kidney disease patients due to the lack of erythropoietin.
What is going on with Mr. Stinson physiologically?
- Because of his CKD, Mr. Stinson requires dialysis to perform the normal functions of the kidneys, since his aren’t working. He likely felt sick because his potassium was elevated and because of the azotemia (toxins building up in his blood).
- He missed dialysis and therefore he is now even more volume overloaded and azotemic
- This will cause a risk to his heart and lungs because of the overload and the hyperkalemia
The nephrologist is consulted and determines that the patient needs hemodialysis. As soon as possible. The charge nurse of the dialysis unit is working to create a bed for him and will call back as soon as one is available, hopefully within the hour.
What do you, the nurse, need to consider and assess for Mr. Stinson PRIOR to sending him to dialysis?
- ALWAYS hold antihypertensives before HD (obtain provider order)
- Hold any medications that may be dialyzed off as they will not have their therapeutic benefit (confirm with pharmacist and obtain provider order)
- May require potassium-lowering medications before dialysis if the wait is going to be too long – hyperkalemia can be deadly
- Determine if any medications should be held prior to HD
- Assess full set of vital signs
- Obtain a weight, preferably on a standing scale
- Assess heart and lung sounds, as well as skin/edema
Mr. Stinson goes to hemodialysis, where they are able to pull of 3 L of fluid. He tolerates the procedure well and returns to his room.
What would you need to assess for Mr. Stinson AFTER he returns from Dialysis?
- Obtain a weight, preferable on a standing scale, to compare to the pre-HD weight. This helps determine how much fluid was pulled off (1 kg = 1 L)
- Obtain a full set of vital sign
- Re-draw a renal function panel as ordered to ensure electrolytes are not in a dangerous range (requires provider order)
What are some important patient education topics for Mr. Stinson before discharge?
- Importance of hemodialysis – he likely didn’t feel well because he NEEDED dialysis.
- Reasons to “skip” dialysis typically involve severe infections and fevers, in which case he should go the following day whenever possible or notify his nephrology team
- Should also reinforce teaching regarding nutrition – foods to avoid (high in potassium) and when to take medications with or without food (especially Phos-Lo and Calcium supplements)
View the FULL Outline
When you start a FREE trial you gain access to the full outline as well as:
- SIMCLEX (NCLEX Simulator)
- 6,500+ Practice NCLEX Questions
- 2,000+ HD Videos
- 300+ Nursing Cheatsheets
“Would suggest to all nursing students . . . Guaranteed to ease the stress!”
Nursing Case Studies

This nursing case study course is designed to help nursing students build critical thinking. Each case study was written by experienced nurses with first hand knowledge of the “real-world” disease process. To help you increase your nursing clinical judgement (critical thinking), each unfolding nursing case study includes answers laid out by Blooms Taxonomy to help you see that you are progressing to clinical analysis.We encourage you to read the case study and really through the “critical thinking checks” as this is where the real learning occurs. If you get tripped up by a specific question, no worries, just dig into an associated lesson on the topic and reinforce your understanding. In the end, that is what nursing case studies are all about – growing in your clinical judgement.
Nursing Case Studies Introduction
Cardiac nursing case studies.
- 6 Questions
- 7 Questions
- 5 Questions
- 4 Questions
GI/GU Nursing Case Studies
- 2 Questions
- 8 Questions
Obstetrics Nursing Case Studies
Respiratory nursing case studies.
- 10 Questions
Pediatrics Nursing Case Studies
- 3 Questions
- 12 Questions
Neuro Nursing Case Studies
Mental health nursing case studies.
- 9 Questions
Metabolic/Endocrine Nursing Case Studies
Other nursing case studies.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Risk Factors
- Providing Care
- Living with Diabetes
- Clinical Guidance
- DSMES for Health Care Providers
- Prevent Type 2 Diabetes: Talking to Your Patients About Lifestyle Change
- Employers and Insurers
- Community-based Organizations (CBOs)
- Toolkits for Diabetes Educators and Community Health Workers
- National Diabetes Statistics Report
- Reports and Publications
- Current Research Projects
- National Diabetes Prevention Program
- State, Local, and National Partner Diabetes Programs for Public Health
- Diabetes Self-Management Education and Support (DSMES) Toolkit
Kidney Failure and Diabetes
- End-stage kidney disease (ESKD) is kidney failure that requires dialysis or a transplant for the person to survive.
- It can lead to disability and early death.
- ESKD is expensive to treat, and cases are on the rise.
What did this study examine?
The study looked at trends in ESKD cases during 2000–2019. It also looked at the main causes of ESKD and the populations that were most affected.
Study results
During 2000–2019, the number of reported cases of kidney failure increased in the United States. The number of new cases increased by about 42% (from 92,660 to 131,422 cases). The number of existing cases more than doubled (from 358,247 to 783,594 cases).
Diabetes and high blood pressure were the main causes of ESKD. Diabetes was the main cause for most cases of ESKD, and cases caused by high blood pressure increased the most.
Compared to White people, Black, Hispanic, and American Indian or Alaska Native people had higher chances of having diabetes. These groups of people also had a higher chance of developing ESKD. Asian, Native Hawaiian, other Pacific Islander, and Hispanic people had the largest increases in ESKD cases. American Indian and Alaska Native people, and adults 45 and younger, had the smallest increase in ESKD cases.
Why is this study important?
Since the main causes of ESKD are diabetes and high blood pressure, managing these conditions helps to prevent or delay ESKD. Looking at trends over time can help show if health programs are successful in preventing ESKD.
For example, some diabetes programs for American Indian people show a savings of over $500 million in avoided ESKD cases. The success of these programs could explain why American Indian and Alaska Native people had the smallest increase in ESKD cases.
Kidney disease testing and management could be key to reducing the number of people living with ESKD. Programs that address other health conditions that can lead to ESKD may also help reduce ESKD cases.
Diabetes is a chronic disease that affects how your body turns food into energy. About 1 in 10 Americans has diabetes.
For Everyone
Health care providers, public health.
- Open access
- Published: 10 January 2022
Chronic kidney disease and its health-related factors: a case-control study
- Mousa Ghelichi-Ghojogh 1 ,
- Mohammad Fararouei 2 ,
- Mozhgan Seif 3 &
- Maryam Pakfetrat 4
BMC Nephrology volume 23 , Article number: 24 ( 2022 ) Cite this article
19k Accesses
10 Citations
8 Altmetric
Metrics details
Chronic kidney disease (CKD) is a non-communicable disease that includes a range of different physiological disorders that are associated with abnormal renal function and progressive decline in glomerular filtration rate (GFR). This study aimed to investigate the associations of several behavioral and health-related factors with CKD in Iranian patients.
A hospital-based case-control study was conducted on 700 participants (350 cases and 350 controls). Logistic regression was applied to measure the association between the selected factors and CKD.
The mean age of cases and controls were 59.6 ± 12.4 and 58.9 ± 12.2 respectively ( p = 0.827). The results of multiple logistic regression suggested that many factors including low birth weight (OR yes/no = 4.07, 95%CI: 1.76–9.37, P = 0.001), history of diabetes (OR yes/no = 3.57, 95%CI: 2.36–5.40, P = 0.001), history of kidney diseases (OR yes/no = 3.35, 95%CI: 2.21–5.00, P = 0.001) and history of chemotherapy (OR yes/no = 2.18, 95%CI: 1.12–4.23, P = 0.02) are associated with the risk of CKD.
Conclusions
The present study covered a large number of potential risk/ preventive factors altogether. The results highlighted the importance of collaborative monitoring of kidney function among patients with the above conditions.
Peer Review reports
Chronic kidney disease (CKD) is a non-communicable disease that includes a range of different physiological disorders that are associated with an abnormal renal function and progressive decline in glomerular filtration rate (GFR) [ 1 , 2 , 3 ]. Chronic kidney disease includes five stages of kidney damage, from mild kidney dysfunction to complete failure [ 4 ]. Generally, a person with stage 3 or 4 of CKD is considered as having moderate to severe kidney damage. Stage 3 is broken up into two levels of kidney damage: 3A) a level of GFR between 45 to 59 ml/min/1.73 m 2 , and 3B) a level of GFR between 30 and 44 ml/min/1.73 m 2 . In addition, GFR for stage 4 is 15–29 ml/min/1.73 m 2 [ 4 , 5 ]. It is reported that both the prevalence and burden of CKD are increasing worldwide, especially in developing countries [ 6 ]. The worldwide prevalence of CKD (all stages) is estimated to be between 8 to 16%, a figure that may indicate millions of deaths annually [ 7 ]. According to a meta-analysis, the prevalence of stage 3 to 5 CKD in South Africa, Senegal, and Congo is about 7.6%. In China, Taiwan, and Mongolia the rate of CKD is about 10.06% and in Japan, South Korea, and Oceania the rate is about 11.73%. In Europe the prevalence of CKD is about 11.86% [ 8 ], and finally, about 14.44% in the United States and Canada. The prevalence of CKD is estimated to be about 11.68% among the Iranian adult population and about 2.9% of Iranian women and 1.3% of Iranian men are expected to develop CKD annually [ 9 ]. Patients with stages 3 or 4 CKD are at much higher risk of progressing to either end-stage renal disease (ESRD) or death even prior to the development of ESRD [ 10 , 11 ].
In general, a large number of risk factors including age, sex, family history of kidney disease, primary kidney disease, urinary tract infections, cardiovascular disease, diabetes mellitus, and nephrotoxins (non-steroidal anti-inflammatory drugs, antibiotics) are known as predisposing and initiating factors of CKD [ 12 , 13 , 14 ]. However, the existing studies are suffering from a small sample size of individuals with kidney disease, particularly those with ESRD [ 15 ].
Despite the fact that the prevalence of CKD in the world, including Iran, is increasing, the factors associated with CKD are explored very little. The present case-control study aimed to investigate the association of several behavioral and health-related factors with CKD in the Iranian population.
Materials and methods
In this study, participants were selected among individuals who were registered or were visiting Faghihi and Motahari hospitals (two largest referral centers in the South of Iran located in Shiraz (the capital of Fars province). Cases and controls were frequency-matched by sex and age. The GFR values were calculated using the CKD-EPI formula [ 16 , 17 ].
Data collection
An interview-administered questionnaire and the participant’s medical records were used to obtain the required data. The questionnaire and interview procedure were designed, evaluated, and revised by three experts via conducting a pilot study including 50 cases and 50 controls. The reliability of the questionnaire was measured using the test-retest method (Cronbach’s alpha was 0.75). The interview was conducted by a trained public health nurse at the time of visiting the clinics.
Avoiding concurrent conditions that their association may interpreted as reverse causation; the questionnaire was designed to define factors preceding at least a year before experiencing CKD first symptoms. Accordingly participants reported their social and demographic characteristics (age, sex, marital status, educational level, place of residency), history of chronic diseases (diabetes, cardiovascular diseases, hypertension, kidney diseases, family history of kidney diseases, autoimmune diseases and thyroid diseases [ 18 ]). Also history of other conditions namely (smoking, urinary tract infection (UTI), surgery due to illness or accident, low birth weight, burns, kidney pain (flank pain), chemotherapy, taking drugs for weight loss or obesity, taking non-steroidal anti-inflammatory drugs, and taking antibiotic) before their current condition was started. Many researchers reported recalling birth weight to be reliable for research purposes [ 19 ]. Moreover, we asked the participants to report their birth weight as a categorical variable (< 2500 g or low, 2500- < 3500 g or normal, and > 3500 g or overweight). Medical records of the participants were used to confirm/complete the reported data. In the case of contradiction between the self-reported and recorded data, we used the recorded information for our study.
Verbal informed consent was obtained from patients because the majority of the participants were illiterate. The study protocol was reviewed and approved by the ethical committee of Shiraz University of Medical Sciences (approval number: 1399.865).
Sample size
The sample size was calculated to detect an association between the history of using antibiotics (one of our main study variables) and CKD as small as OR = 1.5 [ 20 ]. With an alpha value of 0.05 (2-sided) and a power of 80%, the required sample size was estimated as large as n = 312 participants for each group.
Selection of cases
The selected clinics deliver medical care to patients from the southern part of the country. In this study, patients with CKD who were registered with the above centers from June to December 2020 were studied. A case was a patient with a GFR < 60 (ml/min/1.73 m 2 ) at least twice in 3 months. According to the latest version of the International Classification of Diseases (2010), Codes N18.3 and N18.4 are assigned to patients who have (GFR = 30–59 (ml/min/1.73 m 2 ) and GFR = 15–29 (ml/min/1.73 m 2 ) respectively [ 21 ]. In total, 350 patients who were diagnosed with CKD by a nephrologist during the study period.
Selection of the controls
We used hospital controls to avoid recall-bias. The control participants were selected from patients who were admitted to the general surgery (due to hernia, appendicitis, intestinal obstruction, hemorrhoids, and varicose veins), and orthopedic wards from June to December 2020. Using the level of creatinine in the participants’ serum samples, GFR was calculated and the individuals with normal GFR (ml/min/1.73 m 2 ) GFR > 60) and those who reported no history of CKD were included ( n = 350).
Inclusion criteria
Patients were included if they were ≥ 20 years old and had a definitive diagnosis of CKD by a nephrologist.
Exclusion criteria
Participants were excluded if they were critically ill, had acute kidney injury, those undergone renal transplantation, and those with cognitive impairment.
Statistical analysis
The Chi-square test was used to measure the unadjusted associations between categorical variables and CKD. Multiple logistic regression was applied to measure the adjusted associations for the study variables and CKD. The backward variable selection strategy was used to include variables in the regression model. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. All p -values were two-sided and the results were considered statistically significant at p < 0.05. All analyses were conducted using Stata version 14.0 (Stata Corporation, College Station, TX, USA).
In total, 350 cases and 350 age and sex-matched controls were included in the analysis. The mean age of cases and controls were 59.6 ± 12.4 and 58.9 ± 12.2 respectively ( p = 0.83). Overall, 208 patients (59.4%) and 200 controls (57.1%) were male ( p = 0.54). Also, 149 patients (42.6%) and 133 controls (38.0%) were illiterate or had elementary education ( p = 0.001). Most cases (96.9%) and controls (95.7%) were married ( p = 0.42). The mean GFR for CKD and control groups were 38.6 ± 11.4 and 78.3 ± 10.2 (ml/min/1.73 m2) respectively.
Result of univariate analysis
Table 1 illustrates the unadjusted associations of demographic and health-related variables with CKD. Accordingly, significant (unadjusted) associations were found between the risk of CKD and several study variables including education, history of chronic diseases (diabetes, cardiovascular, hypertension, kidney diseases, autoimmune diseases, and hypothyroidism), family history of kidney diseases, smoking, UTI, surgery due to illness or accident, low birth weight, burns, kidney pain, chemotherapy, taking non-steroidal anti-inflammatory drugs, and taking antibiotics) ( P < 0.05 for all).
Results of multivariable analysis
Table 2 illustrates the adjusted associations between the study variables and the risk of CKD. Most noticeably, low birth weight (OR yes/no = 4.07, 95%CI: 1.76–9.37, P = 0.001), history of surgery (OR yes/no = 1.74, 95%CI: 1.18–2.54, P = 0.004), family history of kidney diseases (OR yes/no = 1.97, 95%CI: 1.20–3.23, P = 0.007), and history of chemotherapy (OR yes/no = 2.18, 95%CI: 1.12–4.23, P = 0.02) were significantly associated with a higher risk of CKD. On the other hand, education (OR college/illiterate or primary = 0.54, 95%CI: 0.31–0.92, P = 0.025) was found to be inversely associated with CKD.
The results of the present study suggested that several variables including, education, history of diabetes, history of hypertension, history of kidney diseases or a family history of kidney diseases, history of surgery due to illness or accident, low birth weight, history of chemotherapy, history of taking non-steroidal anti-inflammatory drugs, and history of taking antibiotics may affect the risk of CKD.
In our study, the level of education was inversely associated with the risk of CKD. This finding is in accordance with the results of a study conducted by K Lambert et.al, who suggested that illiteracy or elementary education may raise the risk of CKD [ 22 ]. The fact that education level is associated with health literacy, may partly explain our results that lower education and inadequate health literacy in individuals with CKD is associated with worse health outcomes including poorer control of biochemical parameters, higher risk of cardiovascular diseases (CVDs); a higher rate of hospitalization, and a higher rate of infections [ 23 ].
In the current study, the history of diabetes was associated with a higher risk of CKD. This finding is consistent with the results of other studies on the same subject [ 20 , 21 , 24 , 25 , 26 , 27 ]. It is not surprising that people with diabetes have an increased risk of CKD as diabetes is an important detrimental factor for kidney functioning as approximately, 40% of patients with diabetes develop CKD [ 27 ].
The other variable that was associated with an increased risk of CKD was a history of hypertension. Our result is consistent with the results of several other studies [ 20 , 24 , 25 , 28 ]. It is reported that hypertension is both a cause and effect of CKD and accelerates the progression of the CKD to ESRD [ 29 ].
After controlling for other variables, a significant association was observed between family history of kidney diseases and risk of CKD. Published studies suggested the same pattern [ 24 ]. Inherited kidney diseases (IKDs) are considered as the foremost reasons for the initiation of CKD and are accounted for about 10–15% of kidney replacement therapies (KRT) in adults [ 30 ].
The importance of the history of surgery due to illness or accident in this study is rarely investigated by other researchers who reported the effect of surgery in patients with acute kidney injury (AKI), and major abdominal and cardiac surgeries [ 31 , 32 ] on the risk of CKD. Also, AKI is associated with an increased risk of CKD with progression in various clinical settings [ 33 , 34 , 35 ]. In a study by Mizota et.al, although most AKI cases recovered completely within 7 days after major abdominal surgery, they were at higher risk of 1-year mortality and chronic kidney disease compared to those without AKI [ 31 ].
The present study also showed that low birth weight is a significant risk factor for CKD. This finding is consistent with the results of some other studies. However, the results of very few studies on the association between birth weight and risk of CKD are controversial as some suggested a significant association [ 19 , 36 , 37 ] whereas others suggested otherwise [ 36 ]. This may be explained by the relatively smaller size and volume of kidneys in LBW infants compared to infants that are normally grown [ 38 ]. This can lead to long-term complications in adolescence and adulthood including hypertension, decreased glomerular filtration, albuminuria, and cardiovascular diseases. Eventually, these long-term complications can also cause CKD [ 39 ].
Another important result of the current study is the association between chemotherapy for treating cancers and the risk of CKD. According to a study on chemotherapy for testicular cancer by Inai et al., 1 year after chemotherapy 23% of the patients showed CKD [ 40 ]. Another study suggested that the prevalence of stage 3 CKD among patients with cancer was 12, and < 1% of patients had stage 4 CKD [ 41 , 42 ]. Other studies have shown an even higher prevalence of CKD among cancer patients. For instance, only 38.6% of patients with breast cancer, 38.9% of patients with lung cancer, 38.3% of patients with prostate cancer, 27.5% of patients with gynecologic cancer, and 27.2% of patients with colorectal cancer had a GFR ≥90 (ml/min/1.73 m 2 ) at the time of therapy initiation [ 43 , 44 ]. The overall prevalence of CKD ranges from 12 to 25% across many cancer patients [ 45 , 46 , 47 ]. These results clearly demonstrate that, when patients with cancer develop acute or chronic kidney disease, outcomes are inferior, and the promise of curative therapeutic regimens is lessened.
In our study, the history of taking nephrotoxic agents (antibiotics or NSAIDs drugs) was associated with a higher risk of CKD. Our result is following the results reported by other studies [ 48 , 49 ]. Common agents that are associated with AKI include NSAIDs are different drugs including antibiotics, iodinated contrast media, and chemotherapeutic drugs [ 50 ].
Strengths and limitations of our study
Our study used a reasonably large sample size. In addition, a considerably large number of study variables was included in the study. With a very high participation rate, trained nurses conducted the interviews with the case and control participants in the same setting. However, histories of exposures are prone to recall error (bias), a common issue in the case-control studies. It is to be mentioned that the method of selecting controls (hospital controls) should have reduced the risk of recall bias when reporting the required information. In addition, we used the participants’ medical records to complete/ confirm the reported data. Although the design of the present study was not able to confirm a causal association between the associated variables and CKD, the potential importance and modifiable nature of the associated factors makes the results potentially valuable and easily applicable in the prevention of CKD.
Given that, chemotherapy is an important risk factor for CKD, we suggest the imperative for collaborative care between oncologists and nephrologists in the early diagnosis and treatment of kidney diseases in patients with cancer. Training clinicians and patients are important to reduce the risk of nephrotoxicity. Electronic medical records can simultaneously be used to monitor prescription practices, responsiveness to alerts and prompts, the incidence of CKD, and detecting barriers to the effective implementation of preventive measures [ 51 ]. Routine follow-up and management of diabetic patients is also important for the prevention of CKD. We suggest a tight collaboration between endocrinologists and nephrologists to take care of diabetic patients with kidney problems. In addition, surgeons in major operations should refer patients, especially patients with AKI, to a nephrologist for proper care related to their kidney function. Treatment of hypertension is among the most important interventions to slow down the progression of CKD [ 12 ]. Moreover, all patients with newly diagnosed hypertension should be screened for CKD. We suggest all patients with diabetes have their GFR and urine albumin-to-creatinine ratio (UACR) checked annually. Finally, the aging population and obesity cause the absolute numbers of people with diabetes and kidney diseases to raise significantly. This will require a more integrated approach between dialectologists/nephrologists and the primary care teams (55).
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to their being the intellectual property of Shiraz University of Medical Sciences but are available from the corresponding author on reasonable request.
Abbreviations
- Chronic kidney disease
End-stage renal disease
Glomerular filtration rate
Renal replacement treatment
Urinary tract infection
Odds ratios
Confidence intervals
Hypertension
Acute kidney injury
Ghelichi Ghojogh M, Salarilak S, Taghezadeh Afshari A, Khalkhali HR, Mohammadi-Fallah MR, Mkhdoomi K. The effect of body mass index on patient and graft survival rate in kidney transplanted patients in Iran. Nephrourol Monthly. 2017;9(4):e14386.
Zeba Z, Fatema K, Sumit AF, Zinnat R, Ali L. Early screening of chronic kidney disease patients among the asymptomatic adult population in Bangladesh. J Prev Epidemiol. 2020;5(1):e10–e.
Article Google Scholar
Mahajan C, Tiwari V, Divyaveer SS, Patil MR, Banerjee A, Bagur V, et al. Spectrum of renal biopsies; a three-year data from a tertiary care Centre of eastern India. J Nephropharmacol. 2020;9(2):e20–e.
Article CAS Google Scholar
Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic Hyperuricemia: a randomized trial. Am J Kidney Dis. 2018;72(6):798–810.
Article CAS PubMed Google Scholar
Foster MC, Hwang S-J, Larson MG, Lichtman JH, Parikh NI, Vasan RS, et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham heart study. Am J Kidney Dis. 2008;52(1):39–48.
Article PubMed PubMed Central Google Scholar
Rachmi CN, Agho KE, Li M, Baur LA. Stunting, underweight and overweight in children aged 2.0–4.9 years in Indonesia: prevalence trends and associated risk factors. PLoS One. 2016;11(5):e0154756.
Asghari G, Momenan M, Yuzbashian E, Mirmiran P, Azizi F. Dietary pattern and incidence of chronic kidney disease among adults: a population-based study. Nutr Metab. 2018;15(1):1–11.
Ruggles DR, Freyman RL, Oxenham AJ. Influence of musical training on understanding voiced and whispered speech in noise. PLoS One. 2014;9(1):e86980.
Moazzeni SS, Arani RH, Hasheminia M, Tohidi M, Azizi F, Hadaegh F. High incidence of chronic kidney disease among Iranian diabetic adults: using CKD-EPI and MDRD equations for estimated glomerular filtration rate. Korean Diabetes J. 2021;45(5):684-97.
Salam SN, Eastell R, Khwaja A. Fragility fractures and osteoporosis in CKD: pathophysiology and diagnostic methods. Am J Kidney Dis. 2014;63(6):1049–59.
Zahmatkesh M, Tamadon MR. World kidney day 2018; chronic kidney disease in women. J Nephropathol. 2017;7(1):4–6.
Noble R, Taal MW. Epidemiology and causes of chronic kidney disease. Medicine. 2019;47(9):562–6.
Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80(4):1029–35.
Sepahi MA, Niknafs B. Multifaceted role of apolipoprotein L1 risk variants and nephropathy. J Nephropathol. 2020;9(1):1-3.
Cohen JB, Tewksbury CM, Landa ST, Williams NN, Dumon KR. National postoperative bariatric surgery outcomes in patients with chronic kidney disease and end-stage kidney disease. Obes Surg. 2019;29(3):975–82.
Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. Chronic kidney disease: common, harmful and treatable–world kidney day 2007. Am J Nephrol. 2007;27(1):108–12.
Article PubMed Google Scholar
Argulian E, Sherrid MV, Messerli FH. Misconceptions and facts about hypertrophic cardiomyopathy. Am J Med. 2016;129(2):148–52.
Rhee CM. The interaction between thyroid and kidney disease: an overview of the evidence. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):407.
Article CAS PubMed PubMed Central Google Scholar
Al Salmi I, Hoy WE, Kondalsamy-Chennakes S, Wang Z, Healy H, Shaw JE. Birth weight and stages of CKD: a case-control study in an Australian population. Am J Kidney Dis. 2008;52(6):1070–8.
Yacoub R, Habib H, Lahdo A, Al Ali R, Varjabedian L, Atalla G, et al. Association between smoking and chronic kidney disease: a case control study. BMC Public Health. 2010;10(1):1–6.
Saucier NA, Sinha MK, Liang KV, Krambeck AE, Weaver AL, Bergstralh EJ, et al. Risk factors for CKD in persons with kidney stones: a case-control study in Olmsted County, Minnesota. Am J Kidney Dis. 2010;55(1):61–8.
Lambert K, Mullan J, Mansfield K, Lonergan M. A cross-sectional comparison of health literacy deficits among patients with chronic kidney disease. J Health Commun. 2015;20(sup2):16–23.
Fraser SD, Roderick PJ, Casey M, Taal MW, Yuen HM, Nutbeam D. Prevalence and associations of limited health literacy in chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2013;28(1):129–37.
Ji MY, Park YS, Yi SE. A case-control study to identify the risk factors of school accidents. Korean J Epidemiol. 2005;27(2):80–94.
Google Scholar
Khajehdehi P, Malekmakan L, Pakfetrat M, Roozbeh J, Sayadi M. Prevalence of chronic kidney disease and its contributing risk factors in southern Iran a cross-sectional adult population-based study; 2014.
Li H, Lu W, Wang A, Jiang H, Lyu J. Changing epidemiology of chronic kidney disease as a result of type 2 diabetes mellitus from 1990 to 2017: estimates from global burden of disease 2017. J Diabetes Investig. 2021;12(3):346.
Xu Y, Surapaneni A, Alkas J, Evans M, Shin J-I, Selvin E, et al. Glycemic control and the risk of acute kidney injury in patients with type 2 diabetes and chronic kidney disease: parallel population-based cohort studies in US and Swedish routine care. Diabetes Care. 2020;43(12):2975–82.
Sepanlou SG, Barahimi H, Najafi I, Kamangar F, Poustchi H, Shakeri R, et al. Prevalence and determinants of chronic kidney disease in northeast of Iran: results of the Golestan cohort study. PLoS One. 2017;12(5):e0176540.
Pugh D, Gallacher PJ, Dhaun N. Management of hypertension in chronic kidney disease. Drugs. 2019;79(4):365–79.
Torra R, Furlano M, Ortiz A, Ars E. Genetic kidney diseases as an underecognized cause of chronic kidney disease: the key role of international registry reports. Clin Kidney J. 2021;14(8):1879-85.
Mizota T, Dong L, Takeda C, Shiraki A, Matsukawa S, Shimizu S, et al. Transient acute kidney injury after major abdominal surgery increases chronic kidney disease risk and 1-year mortality. J Crit Care. 2019;50:17–22.
Madsen NL, Goldstein SL, Frøslev T, Christiansen CF, Olsen M. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int. 2017;92(3):751–6.
Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168(6):609–16.
Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171(3):226–33.
James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123(4):409–16.
Esmeijer K, de Vries AP, Mook-Kanamori DO, de Fijter JW, Rosendaal FR, Rabelink TJ, et al. Low birth weight and kidney function in middle-aged men and women: the Netherlands epidemiology of obesity study. Am J Kidney Dis. 2019;74(6):751–60.
White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54(2):248–61.
Harer MW, Charlton JR, Tipple TE, Reidy KJ. Preterm birth and neonatal acute kidney injury: implications on adolescent and adult outcomes. J Perinatol. 2020;40(9):1286–95.
Al Salmi I, Hannawi S. Birth weight and susceptibility to chronic kidney disease. Saudi J Kidney Dis Transplant. 2020;31(4):717.
Inai H, Kawai K, Ikeda A, Ando S, Kimura T, Oikawa T, et al. Risk factors for chronic kidney disease after chemotherapy for testicular cancer. Int J Urol. 2013;20(7):716–22.
Launay-Vacher V, Oudard S, Janus N, Gligorov J, Pourrat X, Rixe O, et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110(6):1376–84.
Launay-Vacher V. Epidemiology of chronic kidney disease in cancer patients: lessons from the IRMA study group. Semin Nephrol. 2010;30(6):548–56.
Launay-Vacher V, Janus N, Deray G. Renal insufficiency and cancer treatments. ESMO Open. 2016;1(4):e000091.
Janus N, Launay-Vacher V, Byloos E, Machiels JP, Duck L, Kerger J, et al. Cancer and renal insufficiency results of the BIRMA study. Br J Cancer. 2010;103(12):1815–21.
Kitchlu A, McArthur E, Amir E, Booth CM, Sutradhar R, Majeed H, et al. Acute kidney injury in patients receiving systemic treatment for Cancer: a population-based cohort study. J Natl Cancer Inst. 2019;111(7):727–36.
Kidney Disease Improving Global Outcomes (KDIGO). KDIGO clinical practice guideline for acute kidney injury. kdigo.org/wpcontent/uploads/2016/10/KDIGO-2012-AKI-Guide line-Engli sh.pdf . Accessed 23 Mar 2020.
Königsbrügge O, Lötsch F, Zielinski C, Pabinger I, Ay C. Chronic kidney disease in patients with cancer and its association with occurrence of venous thromboembolism and mortality. Thromb Res. 2014;134(1):44–9.
Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 2016;90(1):212–21.
Menon S, Kirkendall ES, Nguyen H, Goldstein SL. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr. 2014;165(3):522–7.e2.
Perazella MA, Izzedine H. New drug toxicities in the onco-nephrology world. Kidney Int. 2015;87(5):909–17.
Luyckx VA, Tuttle KR, Garcia-Garcia G, Gharbi MB, Heerspink HJL, Johnson DW, et al. Reducing major risk factors for chronic kidney disease. Kidney Int Suppl. 2017;7(2):71–87.
Download references
Acknowledgments
This paper is part of a thesis conducted by Mousa Ghelichi-Ghojogh, Ph.D. student of epidemiology, and a research project conducted at the Shiraz University of Medical sciences (99-01-04-22719). We would like to thank Dr. Bahram Shahryari and all nephrologists of Shiraz University of medical sciences, interviewers, and CKD patients in Shiraz for their voluntary participation in the study and for providing data for the study.
Shiraz University of Medical Sciences financially supported this study. (Grant number: 99–01–04-22719).
Author information
Authors and affiliations.
Candidate in Epidemiology, Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran
Mousa Ghelichi-Ghojogh
HIV/AIDS research center, School of Health, Shiraz University of Medical Sciences, P.O.Box: 71645-111, Shiraz, Iran
Mohammad Fararouei
Department of Epidemiology, School of Health, Shiraz University of Medical Sciences, Shiraz, Iran
Mozhgan Seif
Nephrologist, Shiraz Nephro-Urology Research Center, Department of Internal Medicine, Emergency Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Maryam Pakfetrat
You can also search for this author in PubMed Google Scholar
Contributions
MGG: Conceptualization, Methodology, Statistical analysis, Investigation, and writing the draft of the manuscript. MP: were involved in methodology, writing the draft of the manuscript, and clinical consultation. MS: was involved in the methodology and statistical analysis. MF: was involved in conceptualization, methodology, supervision, writing, and reviewing the manuscript. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Mohammad Fararouei .
Ethics declarations
Ethics approval and consent to participate.
The study protocol was reviewed and approved by the ethical committee of Shiraz University of Medical Sciences (approval number: 1399.865). All methods were performed in accordance with the relevant guidelines and regulations of the Declaration of Helsinki. The participants were assured that their information is used for research purposes only. Because of the illiteracy of a considerable number of the patients, verbal informed consent was obtained from the participants. Using verbal informed consent was also granted by the ethical committee of Shiraz University of Medical Sciences.
Consent for publication
Not applicable.
Competing interests
None of the authors declare disclosures of direct relevance to the submitted work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ghelichi-Ghojogh, M., Fararouei, M., Seif, M. et al. Chronic kidney disease and its health-related factors: a case-control study. BMC Nephrol 23 , 24 (2022). https://doi.org/10.1186/s12882-021-02655-w
Download citation
Received : 14 August 2021
Accepted : 24 December 2021
Published : 10 January 2022
DOI : https://doi.org/10.1186/s12882-021-02655-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Related factors
- Case-control
BMC Nephrology
ISSN: 1471-2369
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Diabetes & Primary Care
- Vol:26 | No:02
Diabetes Distilled: Predicting risk of kidney failure and mortality – a new tool
20 May 2024
Share this article + Add to reading list – Remove from reading list ↓ Download pdf
Clinical prediction models can be used to estimate whether a condition is present (diagnostic models) or will occur in the future (prognostic models) (Kengne et al, 2024). The NICE (2021) NG203 guideline recommends the use of the Kidney Failure Risk Equation (KFRE) in people with chronic kidney disease (CKD; defined as persisting eGFR <60 mL/min/1.73 m 2 or ACR ≥3 mg/mmol for 90 days), which uses age, sex, eGFR and ACR to predict the 1-year and 5-year likelihood of developing end-stage renal disease (ESRD). People with a 5-year risk of 5% or greater are recommended to be referred for specialist assessment, along with other groups, in the NG203 guideline.
The KFRE calculator is interactive and allows users to explore the impact on risk progression of tight blood pressure control or addition of an ACE inhibitor/ARB, SGLT2 inhibitor or, if type 2 diabetes is also present, addition of finerenone. Most clinicians find the tool useful, although it is not fully integrated into all electronic record systems.
The KDpredict tool
In the present study, Liu and colleagues developed the KDpredict tool (available at https://kdpredict.com ) using artificial intelligence and a “super learner” algorithm to identify the best algorithm and model for predicting progression to ESRD and mortality. Compared with KFRE, the KDpredict algorithm used data from an older age group (median 77–80 years compared with 69–70 years) with lower eGFRs (<45 vs <59 mL/min/1.73 m 2 ) at the point of their initial diagnosis with CKD of at least stage 3b. KDpredict was then evaluated for use in cohorts in Denmark and Scotland, where it was compared with KFRE for predictive accuracy. Although both calculators use the same four core risk factors (age, sex, eGFR and ACR), KDpredict can also include diabetes and cardiovascular disease as predictors ( Figure 1 ).
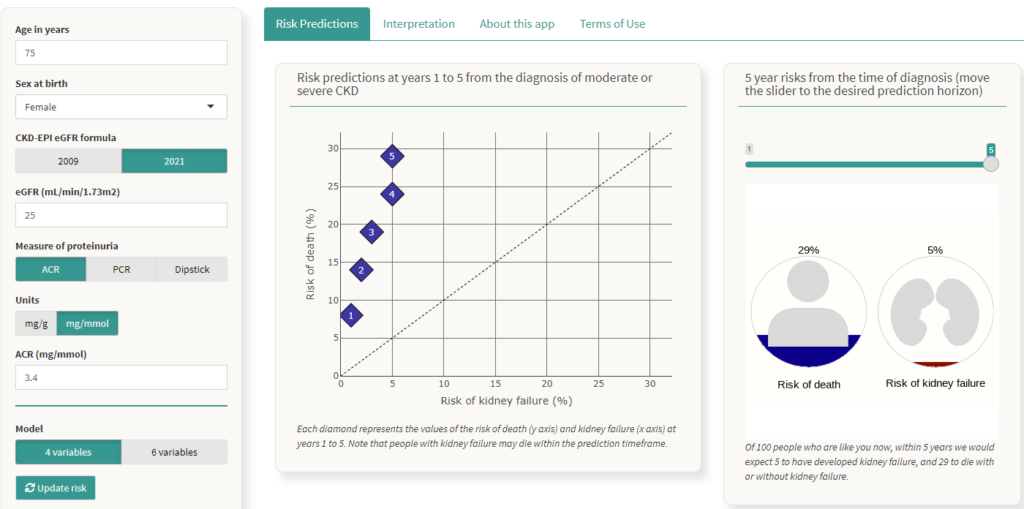
In the Scottish study, conducted in nearly 8000 people with stage G3b–G4 CKD and a median age of 77–80 years, KDpredict consistently outperformed KFRE in predicting kidney failure risk at 1–5 years, as well as estimating all-cause mortality. CKD risk predictions differed significantly between the two tools, with KFRE providing higher risk estimates as it does not take into account the competing risk of mortality. Faced with values above and below the specialist referral threshold of 5% over 5 years, clinicians would need reassurance that the value predicted by KDpredict should be used, thus avoiding uncertainty about the most appropriate management. It is suggested the mortality risk data would be used alongside the ESRD risk to aid person-centred decision making and discussions regarding the competing risks, so that an informed choice can be made about whether specialist referral is appropriate. The authors conclude that this tool could be incorporated into electronic medical record systems and could be adapted to different health systems.
Practice implications
CKD affects up to 10% of adults in general, and up to 30% of those with type 2 diabetes. Large numbers of people with CKD, with and without diabetes, remain undiagnosed. Furthermore, diagnosis is often inaccurately coded and many people remain unaware of their diagnosis despite our best efforts to share this at point of measurement. People with CKD are at high risk of cardiovascular disease and this is the main cause of death, so managing this risk and minimising progression to ESRD remain the priorities. Usually these aims require similar management: smoking cessation; good blood pressure control, including use of ACE inhibitors/ARBs; lipid control with statins and newer therapies; glycaemic control with drugs that reduce risk of cardiovascular events and CKD progression, such as SGLT2 inhibitors and GLP-1 receptor agonists; and addition of finerenone if appropriate.
It is not clear whether this tool’s ability to predict mortality risk will alter management significantly, and this will need to be sensitively handled if we choose to share it to aid decision making. The KDpredict tool is designed to be used once – at the time of diagnosis of at least CKD3b – whereas KFRE can be used repeatedly.
As clinicians, it is useful for us to understand the benefits and limitations of the prediction tools we currently use, and how AI can be harnessed to develop better tools to help us optimise care delivery. This paper, and its accompanying editorial and Fast Facts article explaining risk prediction models (Kengne et al, 2024; Gerd and Ravani, 2024), will help us better understand this in relation to CKD. However, right now, we still need to optimise our screening for CKD, code accurately, and ensure we help people reduce their risks of cardiovascular disease and CKD progression as much as possible using lifestyle changes and the drugs available.
NICE (2021) continues to recommend use of the KFRE and a 5% 5-year threshold as one criterion for consideration of referral, so we should continue to follow this recommendation, even if we use KDpredict to inform our discussions with older people with CKD.
Gerds TA, Ravani P (2024) Predicting the risks of kidney failure and death in adults with moderate to severe chronic kidney disease. BMJ 385 : q721
Kengne AP, George C, Ameh OI (2024) Predicting the outcomes of chronic kidney disease in older adults. BMJ 385 : q749
Liu P, Sawhney S, Heide-Jørgensen U et al (2024) Predicting the risks of kidney failure and death in adults with moderate to severe chronic kidney disease: Multinational, longitudinal, population based, cohort study. BMJ 385 : e078063
NICE (2021) Chronic kidney disease: assessment and management [NG203]. Available at: www.nice.org.uk/guidance/ng203
Editorial: Updated guidance on prescribing incretin-based therapy, cardiovascular risk reduction and the wider uptake of CGM
Updated guidance from the pcds and abcd: managing the national glp-1 ra shortage, at a glance factsheet: tirzepatide for management of type 2 diabetes, how to diagnose and treat hypertension in adults with type 2 diabetes, interactive case study: non-diabetic hyperglycaemia – prediabetes, impact of freestyle libre 2 on diabetes distress and glycaemic control in people on twice-daily pre-mixed insulin, diabetes distilled: fib-4 – a diagnostic and prognostic marker for liver and cardiovascular events and mortality.

Jane Diggle highlights advice on preventing eye damage when initiating new incretin-based therapies.

Advice on selecting alternative glucose-lowering therapies when GLP-1 RAs used in the management of type 2 diabetes in adults are unavailable.

Indications, benefits and side effects of tirzepatide, plus tips for prescribing.

Diagnosing and treating hypertension in accordance with updated NICE guidelines.
Sign up to all DiabetesontheNet journals
- CPD Learning
- Journal of Diabetes Nursing
- Diabetes Care for Children & Young People
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 07 May 2024
Influence of acute kidney injury and its recovery subtypes on patient-centered outcomes after lung transplantation
- Jin Ha Park 1 ,
- Jae‑Kwang Shim 1 ,
- Mingee Choi 2 ,
- Hyun-Soo Zhang 3 ,
- Na Hyung Jun 4 ,
- Seokyeong Choi 1 &
- Young-Lan Kwak 1
Scientific Reports volume 14 , Article number: 10480 ( 2024 ) Cite this article
208 Accesses
Metrics details
Acute kidney injury
- Outcomes research
- Quality of life
- Respiratory tract diseases
- Risk factors
This study aimed to investigate the association between acute kidney injury (AKI) recovery subtypes and days alive out of hospital within the first 3 months (DAOH-90) in patients undergoing lung transplantation. Patients who underwent lung transplantation from January 2012 to December 2021 were retrospectively analyzed and stratified into three groups: no-AKI, early recovery AKI (within 7 days), and non-early recovery AKI group. AKI occurred in 86 (35%) of patients, of which 40 (16%) achieved early recovery, and the remaining 46 (19%) did not. The median DAOH-90 was 21 days shorter in the AKI than in the no-AKI ( P = 0.002), and 29 days shorter in the non-early recovery AKI group than in the no-AKI group ( P < 0.001). Non-early recovery AKI and preoperative tracheostomy status were independently associated with shorter DAOH-90. The prevalence of CKD (76%), and 1-year mortality (48%) were highest in the non-early recovery AKI group. Postoperative AKI was associated with an adverse patient-centered quality measure for perioperative care, and shorter DAOH-90. The non-early recovery AKI group exhibited the worst prognosis in terms of DAOH-90, CKD progression, and 1-year mortality, highlighting the important role of AKI and early-recovery AKI on both the quality of life and clinical outcomes after lung transplantation.
Similar content being viewed by others

Acute heart failure

Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup
Introduction.
Lung transplantation is a life-saving therapy for patients who suffer from end-stage lung disease. While pulmonary function dramatically improves after lung transplantation, the quality of life is often limited by exercise intolerance, sedentary behavior, and skeletal muscle weakness 1 , 2 , 3 . If complications ensue in the early postoperative period, however, it leads to a prolonged use of mechanical ventilation and intensive care unit (ICU) stay, resulting in delayed rehabilitation, reduced daily exercise activity, and prolonged hospital stay or rehospitalization up to 3–6 weeks or longer 1 , 3 . In this regard, days alive out of hospital (DAOH) after lung transplantation is an appropriate patient-centered quality measure of perioperative outcome since it not only combines overall hospitalization, including death, hospital stay and rehospitalization but also has a marked impact on quality improvement 4 .
Regarding the hindrances to postoperative recovery, acute kidney injury (AKI) frequently occurs after lung transplantation, with incidence rates ranging from 40% to as high as 75%, and is strongly associated with increased mortality 5 , 6 , 7 , 8 . Furthermore, the influence of AKI on patient outcomes differs depending on the AKI subtype in terms of its recovery pattern after diagnosis, as well as the severity of AKI in various post-surgical patients including those underwent lung transplantation 6 , 9 , 10 , 11 , 12 , 13 . Notably, previous studies have focused on long-term mortality or renal function; however, only a few studies have considered the impact of the various AKI subtypes on the quality of patient recovery, such as DAOH in patients who underwent lung transplantation.
This retrospective study aimed to evaluate the association of AKI and AKI recovery types after diagnosis, with DAOH within the first 3 months (DAOH-90), and 1-year mortality, and thereby, understand the role of renal recovery status on patient-centered as well as clinical outcomes after lung transplantation.
Materials and methods
This study was approved by the Institutional Review Board (IRB) of Severance Hospital, Yonsei University College of Medicine (IRB number: 4-2022-1144). The requirement for informed consent was waived owing to the retrospective nature of the study by the Institutional Review Board of Severance Hospital, Yonsei University College of Medicine. This study was conducted in accordance with the principles of the Declaration of Helsinki. We retrospectively reviewed the electronic medical records of 262 patients who underwent lung transplantation between January 2012 and December 2021 at the Severance Hospital, Seoul, Korea. Exclusion criteria were age < 19 years, preoperative renal replacement therapy, re-transplantation during the study period, combined organ transplantation, and simultaneous cardiac surgery. One patient was also excluded because he was lost to follow up. A total of 245 patients were included in this study (Fig. 1 ).
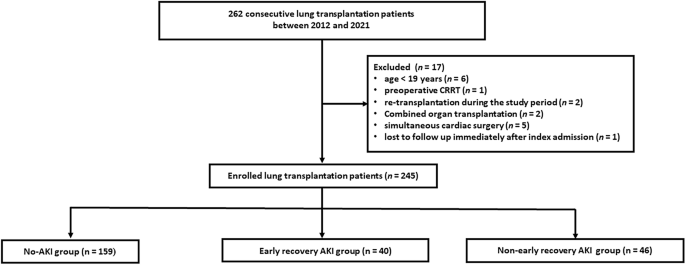
Flowchart of the study population. AKI acute kidney injury, CRRT continuous renal replacement therapy.
Patient data were retrospectively retrieved up to February 28, 2023. Preoperative data included patients’ demographics, including age, sex, body mass index, medical history, smoking status, preoperative functional status and laboratory data. Intraoperative data included operative and anesthesia time, fluid balance, transfusion, single or double lung transplantation, and intraoperative extracorporeal membrane oxygenation (ECMO) weaning. Immediate postoperative data included the presence of grade 3 primary graft dysfunction (PGD) within 72 h of lung transplantation, mechanical ventilation day, duration of ECMO support after lung transplantation, and length of hospital and ICU stay. Postoperative day (POD) 7 data included failure to achieve mechanical ventilation weaning, failure to achieve ECMO weaning, tracheostomy rate among patients who did not undergo tracheostomy before lung transplantation, reoperation, and incidence of postoperative complications, such as bronchopleural fistula, pneumonia, and atrial fibrillation, 7 days after surgery. The incidence of AKI and AKI recovery type were assessed. Mid and long-term postoperative data included the incidence of chronic kidney disease (CKD), DAOH-90, and 1-year mortality.
AKI and CKD were determined by changes in serum creatinine levels according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria 14 , and AKI was defined as an increase in serum creatinine concentration to 0.3 mg/dL within 2 days after lung transplantation or a 50% increase within the first 7 postoperative days. Baseline creatinine value was defined as the most recently documented serum creatinine level before lung transplantation. Because serum creatinine level was measured daily during the hospitalization, as well as on every outpatient follow-up visit, serum creatinine levels were retrieved from the electronic medical records up to 1 year after lung transplantation. Urine output criteria were not used because the data were not available. CKD was defined as abnormalities in kidney structure and function of > 3 months. Functional criteria for CKD are glomerular filtrate rate < 60 mL/min/1.73 m 2 .
Early recovery AKI was defined as an absence of AKI within 7 days of the onset of AKI 11 , 15 . Non-early recovery AKI was defined as all AKI cases not meeting the definition of early recovery AKI 16 , 17 . Patients with relapsing AKI occurring more than 7 days after early recovery were classified as the early-recovery AKI group. Among patients receiving renal replacement therapy, those who failed to discontinue renal replacement therapy within 7 days after onset of AKI were classified into non-early recovery AKI group, while those who discontinued were classified into the early recovery AKI group.
PGD was graded based on diffuse pulmonary oedema on a chest radiograph and a PaO 2 /FiO 2 ratio, according to the criteria of the International Society of Heart and Lung Transplant (ISHLT) Working Group 18 .
DAOH was defined as previously described by Myles et al. 19 , 20 DAOH-90 was calculated from the total postoperative duration of the index and subsequent hospital stays during POD 90 after surgery. For instance, DAOH-90 was calculated as 90 − (index length of stay [LOS] + subsequent LOS within postoperative 90 days + the length until the day of death before POD 90). If a patient died during the index hospitalization, the DAOH was 0 (zero).
The primary outcome of the study was DAOH-90 after lung transplantation. Specifically, the relationship between AKI recovery types and DAOH-90 was the research question of interest. Therefore, patients were stratified into three groups: patients without AKI (no-AKI), patients with early recovery AKI, and patients with non-early recovery AKI groups. The secondary outcomes were the incidence of CKD and 1-year mortality rate. In addition, patients’ characteristics associated with DAOH-90 were investigated.
Statistical analysis
Sample sizes of each AKI recovery group, Q-Q plots of normality, and normality tests such as the Kolmogorov–Smirnov test were used to for a nonparametric presentation of the descriptive statistics. Hence, continuous variables were presented as median (interquartile range) or mean (± standard deviation), and categorical variables as N (%). The Kruskal–Wallis test (one-way ANOVA on ranks) was used to compare the group ranks of continuous variables, while the chi-square test, or Fisher’s exact test when needed, was used to compare the group proportions of categorical variables in the three AKI recovery groups. Kaplan–Meier survival curves were constructed to compare 1-year mortality between the AKI recovery groups.
For variables that showed statistically significantly different mean values or proportions between the AKI recovery groups, post-hoc pairwise comparisons were conducted. Specifically, Tukey’s adjustment for continuous variables and Fisher’s exact test with false discovery rate adjustment for categorical variables were performed to verify which pairs among the three AKI groups showed such a difference. For a similar purpose, a random intercept linear mixed model was utilized to determine the pairs of AKI recovery groups with different mean serum creatinine levels over time during the 1-year follow-up period.
Among the independent variables considered for potential association with DAOH-90 in lung transplantation patients, an exposure variable of interest was postoperative AKI. Other independent variables initially considered were patient age, sex, smoking, preoperative tracheostomy, intraoperative ECMO weaning, grade 3 PGD within postoperative 72 h, and postoperative atrial fibrillation within 7 days, based on statistical tests of association with DAOH-90, a priori clinical knowledge, and evidence from previous studies on the prognosis of lung transplantation patients 5 , 6 , 9 , 21 . A correlation analysis among these independent variables revealed a high correlation between sex and smoking and between intraoperative ECMO weaning and grade 3 PGD within postoperative 72 h, which were 0.72 and 0.69, respectively. To prevent multi-collinearity and based on clinical grounds, smoking status and grade 3 PGD within postoperative 72 h were removed from further multivariable analyses.
Quantile regression analysis 22 was used to investigate the potential risk factors of shorter DAOH-90 among lung transplantation patients, as the bimodal shape of DAOH-90 posed challenges for assuming a parametric distribution. Thus, the τ-th conditional quantile of DAOH-90, given a matrix of independent variables, was modelled as a linear function of the independent variables, for which the regression coefficients, standard errors, and P -values were estimated using the quantreg package in R 23 . Since the quantile τ ranges from 0 to 1, tests of difference in the estimated regression coefficients by different values of τ were conducted for τ values of 0.25, 0.5, and 0.75, from which non-significant differences were confirmed. Thus, τ = 0.5 was chosen, and the median of DAOH-90 was regressed upon the independent variables considered.
A Sankey flow diagram was also drawn using the networkD3 package in R 24 . The diagram visualizes patient prognosis trajectories based on AKI status and AKI recovery groups, resulting in different numbers or proportions of CKD and mortality in the study population throughout the 1-year follow-up 25 .
The patient characteristics associated with AKI status and AKI recovery groups (the main exposures of interest) were examined in an exploratory manner. The statistical methods are described in detail in the Supplementary Methods.
All tests were two-sided, and a P < 0.05 was considered statistically significant. R 4.1.2 (The R Foundation for Statistical Computing, Vienna, Austria) and SAS 9.4 (Cary, NC, USA) were used for the analyses.
Of the 245 patients, AKI occurred in 86 patients (35%). Among them, 40 patients recovered early after AKI (early recovery AKI group), and the remaining 46 patients were classified as the non-early recovery AKI group. Patients in the non-early recovery AKI group were more frequently female, more often received tracheostomy before lung transplantation, and were less likely to be smokers than patients in the no-AKI group (Table 1 ).
All patients received cardiopulmonary support during surgery, with the majority undergoing ECMO.The success rate of intraoperative ECMO weaning was significantly lower in the non-early AKI group than in the no-AKI group. The number of patients who received colloid (all patients received 6% hydroxyethylstarch 130/0.4 in a balanced solution, Volulyte ® ; Fresenius Kabi, Bad Homburg, Germany) intraoperatively was higher in the non-early recovery AKI group than in the no-AKI group. Grade 3 PGD within 72 h occurred more frequently in the non-early recovery AKI group than in the no-AKI and early recovery AKI groups. The incidence of failure to achieve weaning from mechanical ventilation and reoperation within POD 7 was higher in the non-early recovery AKI group than in the no-AKI and early recovery AKI groups. The incidence of atrial fibrillation was significantly lower in the no-AKI group than in the early and non-early recovery AKI groups. The length of ICU stay was longer in the non-early recovery AKI group compared to the no-AKI group. The length of hospital stay was longer in the non-early recovery AKI group than in the no-AKI and early recovery AKI groups (Table 2 ).
Serum creatinine levels were higher in the non-early recovery AKI group than in the no-AKI group throughout the 1-year follow-up period (except at POD 0 and 3 months after surgery) and in the early recovery AKI group at POD 5 and 7, and 1 year after surgery. Serum creatinine levels were higher in the early recovery AKI group than in the no-AKI group during POD 0–3, whereas there were no intergroup differences thereafter (Fig. 2 ).
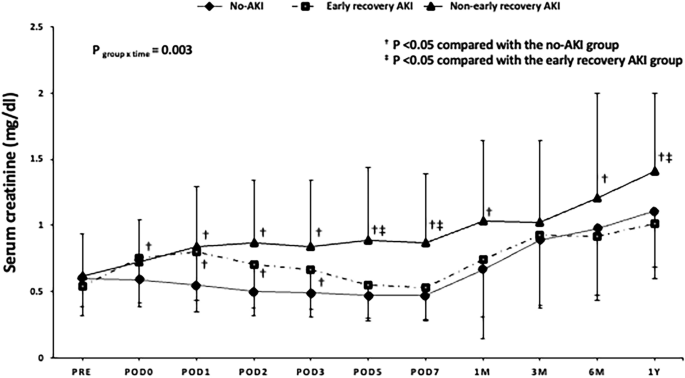
Changes in serum creatinine levels in patients who underwent lung transplantation by AKI recovery group. AKI acute kidney injury, POD postoperative day.
Thirty (75%), eight (20%) and two (5%) patients in the early recovery AKI group and 16 (35%), 12 (26%) and 18 (39%) patients in the non-early recovery AKI group developed stage 1, stage 2, and stage 3 AKI, respectively ( P < 0.001). Among the 20 patients with AKI stage 3, 14 (70%) required renal replacement therapy within 7 days after the onset of AKI. A post-hoc analysis of the difference in the incidence of early recovery according to the AKI stage revealed a difference only between patients with stage 1 and 3 AKI ( P < 0.001). Differences in the DAOH-90 were found only between patients with stage 1 and 3 AKI ( P = 0.01) with DAOH-90 of 27, 26, and 0 days in stage 1, stage 2 and stage 3 AKI, respectively. The prevalence of CKD was higher in the non-early recovery AKI group (76%) than in the early recovery AKI group (49%) or the no-AKI group (39%) (both P < 0.001). The median DAOH-90 was shorter in the non-early recovery AKI group than in the no-AKI group ( P < 0.001). The number of patients with DAOH-90 value of 0 was significantly higher in the early and non-early recovery AKI groups (35% and 52%, respectively) than in the no-AKI group (19%, P < 0.001). The 1-year mortality rate was significantly higher in the non-early recovery AKI group than in the no-AKI group (Table 3 ). Kaplan–Meier survival curves confirmed a significantly worse survival at 1 year after lung transplantation in the non-early recovery AKI group than in the no-AKI group ( P < 0.001, Fig. 3 ).
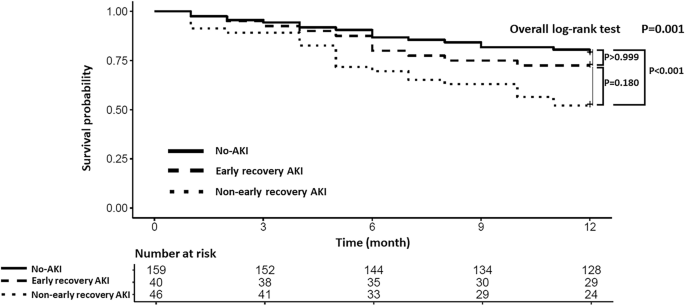
Kaplan–Meier survival curves for 1-year overall survival after lung transplantation. AKI acute kidney injury.
Unlike its recovery status, AKI stages were not associated with CKD progression ( P = 0.417) or 1-year mortality rate ( P = 0.214) (Fig. 4 ).
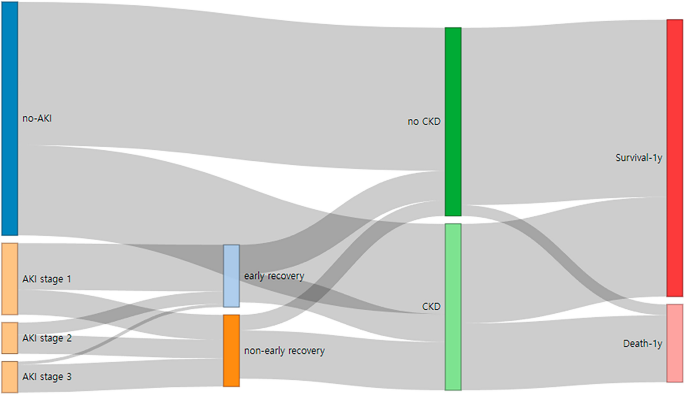
Sankey flow diagram showing prognosis trajectories by AKI status and AKI recovery groups throughout the 1-year of follow-up after lung transplantation. AKI acute kidney injury, CKD chronic kidney disease.
Table 4 shows the results of multivariable quantile regression of DAOH-90, by either AKI occurrence or AKI recovery groups. In each model, the median DAOH-90 calculated using regression coefficient was 21 days shorter in patients with AKI than in patients with no-AKI ( P = 0.002), and 29 days shorter in the non-early recovery AKI group than in the no-AKI group ( P < 0.001). Patients who underwent tracheostomy before lung transplantation also showed a shorter median DAOH-90.
Patient characteristics associated with AKI occurrence were the female sex, preoperative lower neutrophil counts, preoperative mechanical ventilation status, failure to achieve intraoperative ECMO weaning and intraoperative use of colloid (Table S1). Patient characteristics associated with non-early recovery AKI were preoperative tracheostomy status and intraoperative use of colloid (Table S2).
In this single-center retrospective study, the occurrence of AKI was associated with adverse patient-centered mid-term outcome in the AKI group (shorter DAOH-90 by 21 days than in the no-AKI group), which was even worse in the non-early recovery AKI group (shorter DAOH-90 by 29 days than in the no-AKI group). Shorter DAOH-90 was independently associated with non-early recovery AKI and preoperative tracheostomy status. Moreover, the non-early recovery AKI group exhibited the worst prognosis in terms of CKD progression and 1-year mortality rate. In contrast, there was no significant difference in DAOH-90 between the no-AKI and early recovery AKI groups.
Lung transplantation is often associated with poorer health outcomes than other solid organ transplantation. Among various perioperative complications, AKI is one of the most common postoperative complications 5 , 8 closely related to short-and long-term prognosis 26 . Prolonged hypoxemia, hemodynamic instability, blood transfusion, inflammatory response, and ischemia–reperfusion injury induced by ECMO or cardiopulmonary bypass have been reported to contribute to AKI development after lung transplantation 5 , 6 . Consistent with previous reports, in this study, AKI occurred in 35% of the patients, which was significantly associated with the female sex, preoperative mechanical ventilation, and failure to achieve weaning from ECMO intraoperatively. Additionally, colloid use increased the risk of AKI as well as non-early recovery AKI; however, there was no difference in the amount of colloid per body weight administered between the groups. Although this result should be generalized with caution due to the small size of the study population, previous meta-analyses, and narrative reviews have reported that a high volume of intraoperative hydroxyethyl starch increases the risk of AKI after lung transplantation 5 , 6 . Regarding long-term renal function after lung transplantation, attention needs to be paid to the higher overall incidence of CKD (47%), indicating that kidney injury is not a one-time event but is a continuous process in this subset of patients.
Concerning the primary endpoint of this study, a remarkable decrease in DAOH-90 was observed in non-early recovery AKI group compared with no-AKI group, while early recovery AKI group have similar DAOH-90 to those of the no-AKI group. DAOH, a composite patient-centered outcome, emerged as the most desirable outcome for patients—being free from complications and readmissions, and returning to normal life promptly 19 . Compared with mid- and long-term mortality endpoint in patients undergoing major surgery, DAOH accounts for multiple outcome parameters reflecting the days spent healthily after surgery, and its use has been augmented. In such context, DAOH after lung transplantation could be a valuable metric to assess postoperative quality of life and recovery, while it has received limited attention. In previous studies, a prolonged hospitalization after lung transplantation not only increased the risk of early complications, such as C. difficile infection, but also showed a strong association with late survival 27 , and shorter DAOH was also related to higher 1-year mortality after surgery 28 , all of which indicated a long-term prognostic value of DAOH.
In the present study, the occurrence of AKI, especially non-early recovery AKI, significantly shortened DAOH-90. AKI per se is reported to increases the length of hospital stay, and also through the contribution to the development of PGD and prolonged mechanical ventilation after lung transplantation 9 , 29 . Of interest, the incidence of grade 3 PGD within 72 h and failure to achieve weaning from mechanical ventilation within POD 7 were higher only in the non-early recovery AKI group and not in the early recovery AKI group compared with the no-AKI group. By extension, a significantly shorter DAOH-90 was observed only in the non-early recovery AKI group and not in the early recovery AKI group. Moreover, multivariable quantile regression revealed non-early recovery AKI as an independent risk factor of shorter DAOH-90, even when adjusting for other major confounders. Our results align with previous literatures showing that AKI recovery subgroups differently affect long-term outcomes 11 , 13 , 16 . These results indicate the necessity of risk stratification and close monitoring of patients susceptible to prolonged manifestation of AKI in the management of lung transplantation patients to achieve clinical goals of being able to return to life outside the hospital and recovery of quality of life. Besides AKI, patients who received tracheostomy before lung transplantation were independently experienced shorter DAOH-90, many of whom often required mechanical ventilation preoperatively, which was an independent predictor of prolonged length of hospital stay after lung transplantation in the previous study 27 .
Regarding the long-term influence of AKI, the 1-year mortality rate and Kaplan–Meier 1-year survival curve showed significant differences only between the no-AKI and non-early recovery AKI groups in this study and were worse in the non-early recovery AKI group. Notably, CKD progression was most prominent in the non-early AKI group (even when compared with the early recovery AKI group), which subsequently led to increased 1-year mortality (Fig. 4 ). These results further highlighted the importance of early recovery AKI in improving the postoperative quality of life in patients undergoing lung transplantation.
In terms of AKI stages, out of 86 patients with AKI, 46 (53%) had stage 1 AKI, of which 30 (65%) achieved early recovery, and 20 (23%) had stage 3 AKI, of which only 2 (10%) achieved early recovery. The median DAOH-90 in patients with stage 3 AKI was 0 in contrast to 27 days in patients with stage 1 AKI ( P = 0.01). Unlike the differences in recovery type and DAOH-90 according to the AKI stages, there were no differences in the incidence of CKD ( P = 0.417). Thus, it can be considered that not only the stages of AKI but also the recovery status should be emphasized in the longitudinal assessment of renal dysfunction. These results were further supported by the lack of difference in the serially assessed serum creatinine levels up to 1-year between the no-AKI and early recovery AKI groups after POD 5.
This study had some limitations. First, there were inherent limitations related to being a single-centered, retrospective design. Second, like in many other studies, urine output criteria were not used for AKI diagnosis because these data were unavailable 6 , 9 . This may have led to the underestimation of the incidence of AKI 30 . Third, DAOH-30 has been proposed as a more suitable index in elective surgeries, considering the confounding influence of postoperative mortality 19 , 28 . However, DAOH-30 in our study population was 0, and to account for the high-risk of lung transplantation, DAOH-90 was a more suitable index of patient-centered outcome in this subset of patients.
In conclusion, the current study first demonstrated that AKI significantly worsened patient recovery in terms of both patient-centered (DAOH-90) and clinical outcome (CKD progression and 1-year mortality) measures. Moreover, the non-early recovery AKI group exhibited the worst prognosis in terms of DAOH-90, CKD progression, and 1-year mortality, highlighting the important role of AKI and its early-recovery on both the quality of life and clinical outcomes in patients receiving lung transplantation.
Data availability
The data analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
Chronic kidney disease
Days alive out of hospital
Days alive out of hospital within the first 3 months
Extracorporeal membrane oxygenation
Intensive care unit
Length of stay
Primary graft dysfunction
Postoperative day
Abidi, Y. et al. Lung transplant rehabilitation: A review. Life (Basel) 13 , 506 (2023).
ADS PubMed Google Scholar
Hoffman, M., Chaves, G., Ribeiro-Samora, G. A., Britto, R. R. & Parreira, V. F. Effects of pulmonary rehabilitation in lung transplant candidates: A systematic review. BMJ Open 7 , e013445 (2017).
Article PubMed PubMed Central Google Scholar
Hume, E. et al. Exercise training for lung transplant candidates and recipients: A systematic review. Eur Respir Rev 29 , 200053 (2020).
M’Pembele, R. et al. Life impact of VA-ECMO due to primary graft dysfunction in patients after orthotopic heart transplantation. ESC Heart Fail. 9 , 695–703 (2022).
Article PubMed Google Scholar
Jing, L. et al. Acute kidney injury after lung transplantation: a narrative review. Ann. Transl. Med. 9 , 717 (2021).
Article CAS PubMed PubMed Central Google Scholar
Doricic, J. et al. Kidney injury after lung transplantation: Long-term mortality predicted by post-operative day-7 serum creatinine and few clinical factors. PLoS One 17 , e0265002 (2022).
Chan, E. G. et al. Postoperative acute kidney injury and long-term outcomes after lung transplantation. Ann. Thorac. Surg. 116 , 1056–1062 (2023).
Lertjitbanjong, P. et al. Acute kidney injury after lung transplantation: A systematic review and meta-analysis. J. Clin. Med. 8 , 1713 (2019).
Scaravilli, V. et al. Longitudinal assessment of renal function after lung transplantation for cystic fibrosis: Transition from post-operative acute kidney injury to acute kidney disease and chronic kidney failure. J. Nephrol. 35 , 1885–1893 (2022).
Andrew, B. Y. et al. Identification of trajectory-based acute kidney injury phenotypes among cardiac surgery patients. Ann. Thorac. Surg. 114 , 2235–2243 (2022).
Kellum, J. A., Sileanu, F. E., Bihorac, A., Hoste, E. A. & Chawla, L. S. Recovery after acute kidney injury. Am. J. Respir. Crit. Care Med. 195 , 784–791 (2017).
Bhatraju, P. K. et al. Acute kidney injury subphenotypes based on creatinine trajectory identifies patients at increased risk of death. Crit. Care 20 , 372 (2016).
Bhatraju, P. K. et al. Integrated analysis of blood and urine biomarkers to identify acute kidney injury subphenotypes and associations with long-term outcomes. Am. J. Kidney Dis. 82 , 311-321e311 (2023).
Article CAS PubMed Google Scholar
Lameire, N. H. et al. Harmonizing acute and chronic kidney disease definition and classification: Report of a kidney disease—improving global outcomes (KDIGO) consensus conference. Kidney Int. 100 , 516–526 (2021).
Forni, L. G. et al. Renal recovery after acute kidney injury. Intensive Care Med. 43 , 855–866 (2017).
Bhatraju, P. K. et al. Association between early recovery of kidney function after acute kidney injury and long-term clinical outcomes. JAMA Netw. Open 3 , e202682–e202682 (2020).
Bhatraju, P. K. et al. Acute kidney injury subphenotypes based on creatinine trajectory identifies patients at increased risk of death. Crit. Care 20 , 1–10 (2016).
Article Google Scholar
Snell, G. I. et al. Report of the ISHLT Working group on primary lung graft dysfunction, part I: Definition and grading—a 2016 consensus group statement of the international society for heart and lung transplantation. J. Heart Lung Transplant. 36 , 1097–1103 (2017).
Myles, P. S. et al. Validation of days at home as an outcome measure after surgery: A prospective cohort study in Australia. BMJ Open 7 , e015828 (2017).
Huang, L., Frandsen, M. N., Kehlet, H. & Petersen, R. H. Days alive and out of hospital after enhanced recovery video-assisted thoracoscopic surgery lobectomy. Eur. J. Cardiothorac. Surg. https://doi.org/10.1093/ejcts/ezac148 (2022).
Van Slambrouck, J. et al. A focused review on primary graft dysfunction after clinical lung transplantation: A multilevel syndrome. Cells 11 , 745 (2022).
Koenker, R. Quantile Regression (Cambridge University Press, 2005).
Book Google Scholar
Koenker, R. Quantreg: Quantile regression. R package version 5.97 (2023). http://CRAN.R-project.org/package=quantreg (2023).
Allaire, J. et al. Package ‘networkD3’. D3 JavaScript network graphs from R (2017).
Otto, E. et al. Overview of Sankey flow diagrams: Focusing on symptom trajectories in older adults with advanced cancer. J. Geriatr. Oncol. 13 , 742–746 (2022).
Boyer, N., Eldridge, J., Prowle, J. R. & Forni, L. G. Postoperative acute kidney injury. Clin. J. Am. Soc. Nephrol. 17 , 1535–1545 (2022).
Banga, A. et al. Hospital length of stay after lung transplantation: Independent predictors and association with early and late survival. J Heart Lung Transplant. 36 , 289–296 (2017).
Spurling, L. J., Moonesinghe, S. R. & Oliver, C. M. Validation of the days alive and out of hospital outcome measure after emergency laparotomy: A retrospective cohort study. Br. J. Anaesth. 128 , 449–456 (2022).
Tagawa, M. et al. Acute kidney injury as an independent predictor of infection and malignancy: The NARA-AKI cohort study. J. Nephrol. 32 , 967–975 (2019).
Quan, S. et al. Prognostic implications of adding urine output to serum creatinine measurements for staging of acute kidney injury after major surgery: A cohort study. Nephrol. Dial. Transplant. 31 , 2049–2056 (2016).
Download references
Author information
Authors and affiliations.
Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, 50 Yonsei-ro, Seodaemun-gu, Seoul, 03722, Republic of Korea
Jin Ha Park, Jae‑Kwang Shim, Seokyeong Choi & Young-Lan Kwak
Department of Preventive Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea
Mingee Choi
Biostatistics Collaboration Unit, Department of Biomedical Systems Informatics, Yonsei University College of Medicine, Seoul, Republic of Korea
Hyun-Soo Zhang
Departments of Anesthesiology and Pain Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Republic of Korea
Na Hyung Jun
You can also search for this author in PubMed Google Scholar
Contributions
J.H.P., J.K.S. and Y.L.K. designed the study, collected and analyzed data, participated in the performance of the research, writing, review and editing. M.C. and H.S.Z. performed the statistical analysis. N.H.J. and S.C analyzed the data, and participated in the performance of the research. J.H.P. and Y.L.K. participated in supervision. All authors reviewed the manuscript.
Corresponding author
Correspondence to Young-Lan Kwak .
Ethics declarations
Competing interests.
The authors declare no competing interests.

Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Park, J.H., Shim, J., Choi, M. et al. Influence of acute kidney injury and its recovery subtypes on patient-centered outcomes after lung transplantation. Sci Rep 14 , 10480 (2024). https://doi.org/10.1038/s41598-024-61352-4
Download citation
Received : 18 February 2024
Accepted : 05 May 2024
Published : 07 May 2024
DOI : https://doi.org/10.1038/s41598-024-61352-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 13 May 2024
Lipidomic studies revealing serological markers associated with the occurrence of retinopathy in type 2 diabetes
- Mingqian He 1 na1 ,
- Guixue Hou 2 na1 ,
- Mengmeng Liu 1 na1 ,
- Zhaoyi Peng 1 ,
- Hui Guo 1 ,
- Yue Wang 1 ,
- Jing Sui 3 ,
- Hui Liu 4 ,
- Xiaoming Yin 5 ,
- Meng Zhang 1 ,
- Ziyi Chen 1 ,
- Patrick C.N. Rensen 1 , 6 ,
- Liang Lin 2 , 8 ,
- Yanan Wang 1 , 7 &
- Bingyin Shi 1
Journal of Translational Medicine volume 22 , Article number: 448 ( 2024 ) Cite this article
289 Accesses
1 Altmetric
Metrics details
The duration of type 2 diabetes mellitus (T2DM) and blood glucose levels have a significant impact on the development of T2DM complications. However, currently known risk factors are not good predictors of the onset or progression of diabetic retinopathy (DR). Therefore, we aimed to investigate the differences in the serum lipid composition in patients with T2DM, without and with DR, and search for potential serological indicators associated with the development of DR.
A total of 622 patients with T2DM hospitalized in the Department of Endocrinology of the First Affiliated Hospital of Xi’an JiaoTong University were selected as the discovery set. One-to-one case–control matching was performed according to the traditional risk factors for DR (i.e., age, duration of diabetes, HbA1c level, and hypertension). All cases with comorbid chronic kidney disease were excluded to eliminate confounding factors. A total of 42 pairs were successfully matched. T2DM patients with DR (DR group) were the case group, and T2DM patients without DR (NDR group) served as control subjects. Ultra-performance liquid chromatography–mass spectrometry (LC–MS/MS) was used for untargeted lipidomics analysis on serum, and a partial least squares discriminant analysis (PLS-DA) model was established to screen differential lipid molecules based on variable importance in the projection (VIP) > 1. An additional 531 T2DM patients were selected as the validation set. Next, 1:1 propensity score matching (PSM) was performed for the traditional risk factors for DR, and a combined 95 pairings in the NDR and DR groups were successfully matched. The screened differential lipid molecules were validated by multiple reaction monitoring (MRM) quantification based on mass spectrometry.
The discovery set showed no differences in traditional risk factors associated with the development of DR (i.e., age, disease duration, HbA1c, blood pressure, and glomerular filtration rate). In the DR group compared with the NDR group, the levels of three ceramides (Cer) and seven sphingomyelins (SM) were significantly lower, and one phosphatidylcholine (PC), two lysophosphatidylcholines (LPC), and two SMs were significantly higher. Furthermore, evaluation of these 15 differential lipid molecules in the validation sample set showed that three Cer and SM(d18:1/24:1) molecules were substantially lower in the DR group. After excluding other confounding factors (e.g., sex, BMI, lipid-lowering drug therapy, and lipid levels), multifactorial logistic regression analysis revealed that a lower abundance of two ceramides, i.e., Cer(d18:0/22:0) and Cer(d18:0/24:0), was an independent risk factor for the occurrence of DR in T2DM patients.
Disturbances in lipid metabolism are closely associated with the occurrence of DR in patients with T2DM, especially in ceramides. Our study revealed for the first time that Cer(d18:0/22:0) and Cer(d18:0/24:0) might be potential serological markers for the diagnosis of DR occurrence in T2DM patients, providing new ideas for the early diagnosis of DR.
Introduction
Type 2 diabetes mellitus (T2DM) is a common chronic disease in many countries, and its prevalence is growing as people’s lifestyles are changing [ 1 ]. Diabetes causes various complications, classified as either macrovascular complications (such as cardiovascular disease and stroke) or microvascular complications (such as kidney disease) [ 2 ]. Diabetic retinopathy (DR), a specific microvascular complication of diabetes, is the most common cause of vision loss in people of working age [ 3 , 4 ]. Poor glycemic control, hypertension, and diabetes duration are major risk factors for DR [ 5 ]. Although intensive risk factor control reduces the risk of DR progression and vision loss, many diabetic patients continue to develop DR with strict glycemic and blood pressure control [ 6 ]. Despite increasing research supporting the efficacy of routine DR screening to prevent DR and early treatment to reduce the risk of vision loss, there are no specific biomarkers for diagnosing the onset and early progression of DR. Additionally, new and more effective strategies are awaited to prevent and treat the progression of DR.
Accumulating evidence suggests that disruption in lipid metabolism is an early event in the pathogenesis of diabetes complications. Previous studies found that levels of multiple lipid species, including glycerophospholipids, sphingolipids and glycerolipids, are critical risk factors for T2DM and its complications [ 7 , 8 ]. Lysophosphatidylcholine (LPC) is a main glycerophospholipid known for its essential role in lipid and glucose metabolism, and LPC has been intensively studied in the development of metabolic diseases including T2DM [ 9 ]. Sphingolipids, including ceramides (Cer), sphingomyelins (SM) and gangliosides, have a variety of intra- and extracellular effects on glucose homeostasis and metabolic disease [ 10 ] Numerous studies suggest Cer, a crucial lipid intermediate in sphingolipid metabolism, is a major contributing factor for insulin resistance, and inhibition or depletion of enzymes driving de novo ceramide synthesis can prevent the development of diabetes in mice [ 7 , 11 , 12 ]. In contrast, a decrease in very long chain Cer is correlated with the development of macroalbuminuria in diabetes [ 13 ]. Accelerated sphingolipid catabolism’ leading to an increase in glucosylceramide or glycosphingolipids might contribute to the neuronal pathologies of DR [ 14 ]. In addition, SM produced by the transfer of a phosphocholine moiety from phosphatidylcholine to the ceramide backbone has been linked to insulin resistance [ 15 , 16 ] and is also an independent marker of cardiovascular disease [ 17 ]. Thus, dysregulated lipid metabolism is a major contributor to the pathogenesis of T2DM and its complications, and specific lipid species that are responsible for the occurrence of DR are rather obscure.
Lipidomics offers solid platforms for identifying novel lipid mediates in biochemical processes of lipid metabolism, thus providing new opportunities for disease prediction and detection [ 18 , 19 ]. Lipidome analysis is performed by liquid chromatography and electrospray ionization-tandem mass spectrometry (LC–MS/MS) for molecular lipid identification and quantification and multiple reaction monitoring (MRM) for targeted quantification of those lipid species. Lipid-based biomarkers offer unique options for precision medicine by providing sensitive diagnostic tools for disease prediction and monitoring [ 20 ]. Using a quantitative metabolomics approach, Emil et al. compared the aqueous humor and serum concentrations of metabolites in senior adults with an without diabetes who underwent cataract surgery [ 21 ]. However, the field of lipidomics studies of DR is still in its early stages, with few studies published and little replication of results [ 22 ].
In this study, we aimed to find reliable serum lipid-based biomarkers for the presence of DR in patients with T2DM by using two cohorts. To this end, serum samples of the discovery cohort was subjected to untargeted lipidomics analysis to search for differentially abundant lipids between individuals without and with DR. In the validation cohort, the observed differential lipid molecules were validated using mass spectrometry MRM targeting techniques. We hypothesized that DR has a distinctive serum lipid signature and that particular lipid species can act as biomarkers for T2DM patients with DR.
Research design and methods
Participants.
A total of 622 participants with T2DM hospitalized in the Endocrinology Department of the First Affiliated Hospital of Xi’an JiaoTong University were screened as the discovery set. Participants with chronic kidney disease [estimated glomerular filtration rate (eGFR) < 90 (mL/min/1.73 m 2 )] were excluded from the selection. We conducted pair matching according to the traditional risk factors for DR (including age, duration of diabetes, HbA1c level, and hypertension). For the discovery cohort, we selected 42 T2DM patients with DR (DR group). The control participants were 42 T2DM patients without DR (NDR group), and they were matched to patients in the DR group by age (in 5-year bands), diabetes duration (in 5-year bands), HbA1c levels (in 0.5% bands), and hypertension status.
Lipid markers of DR identified from the discovery cohort were quantified in a separate sample cohort (validation cohort). We first screened 531 T2DM patients. Individuals with chronic kidney disease [eGFR < 90 (mL/min/1.73 m 2 )] were excluded from the selection. Then, we conducted 1:1 propensity score matching (PSM) (matching tolerance = 0.02) by age, diabetes duration, HbA1c level, hypertension status, sex, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), and eGFR. For the validation cohort, 95 T2DM patients with DR (DR group) and 95 T2DM patients without DR (NDR group) were included.
Sample collection
Fasting blood samples and clinical data were collected from the individuals. All blood samples were collected at the First Affiliated Hospital of Xi’an JiaoTong University physical examination center. Blood samples were centrifuged for 20 min at 1500 rpm and 4 °C. Then, serum was collected and stored at -80 °C until analysis. HbA1c was measured using an automatic HbA1c analyzer (TOSOH BIOSCIENCE, INC.; HLC-723G8). Total cholesterol (CHOL), triglyceride (TG), high density lipoprotein-cholesterol (HDL-c), low density lipoprotein-cholesterol (LDL-c), uric acid (UA), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), total bilirubin (TBIL), direct bilirubin (DBIL), total protein (TP), albumin (ALB), glucose (GLU), blood urea nitrogen (BUN), creatinine (CRE) were measured using standard reagents on an automatic biochemistry analyzer (HITACHI, Inc.; LAbOSPECT, 008AS). Blood pressure was measured in triplicate using an Omron HBP-9020 digital automatic blood pressure machine (Kyoto, Japan).
Lipid extraction
The serum samples were thawed slowly at 4 °C, 100 µL of the sample was placed in a 96-well plate, 300 µL of isopropanol (prechilled at -20 °C) spiked with internal standards (SPLASH® LIPIDOMIX® Mass Spec Standard, Avanti, USA) was added, and the samples were vortexed and mixed for 1 min and then centrifuged at 4 °C for 20 min at 4000 rcf after resting overnight at -20 °C as previously reported [ 23 ]. The supernatant was injected for LC–MS/MS analysis, and 10 µL of each supernatant was mixed into quality control (QC) samples to assess the reproducibility and stability of the LC–MS analysis process.
LC–MS/MS analysis
Lipids were separated and detected by an UPLC (CSH C18 column, 1.7 μm 2.1*100 mm, Waters, USA) equipped with a Q Exactive Plus high-resolution mass spectrometer (Thermo Fisher Scientific, USA) as previously reported [ 24 ]. The following gradient was used for elution: 0–2 min, 40-43% mobile phase B (10 mM ammonia formate, 0.1% formic acid, 90% isopropyl alcohol, and 10% acetonitrile); 2–2.1 min, 43-50% liquid B; 2.1–7 min, 50-54% solution B; 7–7.1 min, 54-70% liquid B; 7.1–13 min, 70-99% liquid B with a flow rate of 0.35 mL/min. Mobile phase A was an aqueous solution containing 10 mM ammonia formate, 0.1% formic acid and 60% acetonitrile in water.
All samples were analyzed in data-dependent acquisition (DDA) mode with the following positive/negative ionization settings: spray voltage, 3.8/–3.2 kV; aux gas heater temperature, 350 °C; and capillary temperature, 320 °C. The full scan mass range was 200–2000 m/z with 70,000 mass resolution at m/z 200 and AGC set to 3e6 with a maximum ion injection time of 100 ms. The top three precursors were selected for subsequent MS fragmentation with a maximum ion injection time of 50 ms and resolution of 17,500 at m/z 200, and the AGC was 1e5. The stepped normalized collision energy was set to 15, 30, and 45 eV.
Data preprocessing and quality control
The raw data obtained from the LC–MS/MS detection were imported into LipidSearch v.4.1 (Thermo Fisher Scientific, USA) for lipid identification and quantification. The following parameters were used for lipid identification and peak extraction: the type of identification was Product, the mass deviation of the parent and daughter ions was 5 ppm, and the response threshold was set to 5.0% of the relative response deviation of the daughter ions; the quantitative parameters were set to calculate the peak areas of all identified lipids, and the peak extraction mass deviation was set to 5 ppm. For ESI + data, [M + H]+, [M + NH4]+, and [M + Na] + were selected as adducts, while for ESI- data, [M-H]-, [M-2 H]-, and [M-HCOO]- were selected as adducts. The peak alignment was performed for all identified lipids, and those not marked as “rejected” were considered for inclusion in the subsequent analysis.
For data preprocessing, raw data exported from LipidSearch were further analyzed by meta X [ 25 ]. The data preprocessing included (1) Removing lipid molecules with more than 50% missing information in QC samples and more than 80% missing information in experimental samples (i.e., LipidIon in the table); (2) Filling the missing values using the k-nearest neighbor (KNN) algorithm; (3) Correcting the batch effect using quality control-based robust LOESS signal correction (QC-RLSC); (4) Using probabilistic quotient normalization (PQN) to normalize the data to obtain the relative peak areas; and (5) Removing the lipid molecules with a coefficient of variation (CV) greater than 30% of the relative peak areas from all QC samples.
Data quality was assessed by the reproducibility of QC sample assays. The assessment included chromatogram overlap of QC samples, principal component analysis (PCA), number of extracted peaks, and differences in peak response intensity.
Data processing
A combination of multivariate statistical analysis and univariate analysis was used to screen for lipids of which the abundance differed between groups. The multivariate statistical analysis methods used were principal component analysis (PCA) and partial least squares method-discriminant analysis (PLS-DA). PCA is an unsupervised pattern recognition method, and PLS-DA is a supervised pattern recognition method. The univariate analyses were fold change (FC) and Student’s t test. The FC was obtained by fold change analysis, and the p value pairs of the t test were corrected for the false discovery rate (FDR) to obtain a q-value. The differential lipid molecule screening conditions were as follows: (1) variable importance in the projection (VIP) ≥ 1 for the first two principal components of the PLS-DA model; (2) fold change ≥ 1.2 or ≤ 0.83; and (3) p value < 0.05.
Targeted lipid quantification by MRM in validation samples
The identified differential lipids were further quantified by multiple reaction monitoring (MRM). For lipid extraction, the procedure was consistent with the untargeted experiment as described. The MRM transition list is shown in Table S1 . For MRM quantification, all validation samples were analyzed on a QTRAP 5500 mass spectrometer with a CSH C18 column (1.7 μm 2.1*100 mm, Waters, USA) for separation. All lipids were subjected to targeted quantification in ESI + mode with a specific transition setting.
Statistical analysis
The clinical data of samples are presented as the mean ± standard deviation (SD) for normally distributed variables or the median (interquartile range) for abnormal distribution. Comparisons between the case group and the control group were made using a two-tailed t test or Mann-Whitney U test for continuous data and the X 2 test for categorical data. The calculation of the area under the curve (AUC) in receiver operating characteristic (ROC) curve analysis was used to evaluate the discriminatory ability of the markers. Logistic regression models were applied to assess the relationship between lipid molecules and the presence of DR. The odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for the molecules with 1-SD changes. The known risk factors for DR, such as CHOL, TGs, LDL-c, and HDL-c, were added to multivariate logistic regression to calculate the adjusted odds ratios. Ordinal logistic regression models were used to assess the relationships between lipid molecules and DR stages [NDR, nonproliferative DR (NPDR) and proliferative DR (PDR)].
Characteristics of the discovery cohort
Table 1 shows the clinical characteristics of individuals selected for the discovery cohort. There were no significant differences in age and sex between the DR and NDR groups. In fact, these groups were comparable for most metabolic characteristics, such as BMI, diabetes duration, and HbA1c, and there were no significant between-group differences for hypertension status, antihypertensive agent use, hypoglycemic therapy status or NSAID use. The blood pressure and glucose of the participants were treated and controlled. Compared with control subjects with T2DM, T2DM patients with DR had higher levels of LDL-c levels, AST, TBIL, and BUN (Table 1 and Table S2 ).
Untargeted lipidome-derived biomarkers for diabetic retinopathy: results from the discovery cohort
A total of 1721 lipids were detected. The number of lipids with an RSD (CV) less than or equal to 30% in the QC samples was 1421. The ratio of the number of lipids with CV less than or equal to 30% to the number of all detected lipids in QC samples was 81%.
Fifteen candidate lipids were identified from the discovery cohort. Compared with those of the NDR group, the levels of three Cer and seven SM were significantly lower in the DR group. In contrast, two SM, two LPC and one PC were significantly higher in the DR group (Fig. 1 A and B). More specifically, compared with T2DM patients without DR, T2DM patients with DR showed lower levels of Cer(d18:0/24:0), Cer(d18:0/22:0), Cer(d42:3), SM(d22:0/16:0), SM(d18:1/24:1), SM(d42:0), SM(d40:0), SM(d39:0), SM(d38:0), and SM(d36:0), and higher levels of SM(d20:1/16:1), SM(d34:1), LPC(18:2), LPC(16:0) and PC(34:2). The heat map shows the distribution of these lipids between individuals of the NDR and DR groups (Fig. 1 C). The results of ROC analysis and the odds ratios of the lipid markers in the basic logistic regression models are shown in Table 2 . The AUC values for the 15 lipids ranged from 0.72 to 0.94. All lipids retained significant ORs after adjusted for CHOL, TG, LDL-c, and HDL-c (adjusted ORs are shown in Table 2 ). Furthermore, we used ordinal logistic regression, which estimated the odds of being in one higher category of the DR stage (from NDR to PDR) for lipid species, to test the associations between lipid species and DR stage (Table S3 ; n = 42 in the NDR group, n = 37 in the NPDR group, n = 5 in the PDR group), and we analyzed the data while excluding participants with diabetic macular edema (DME) ( n = 4 in the DR group), as before, all lipids retained significant ORs (Table S4 ).
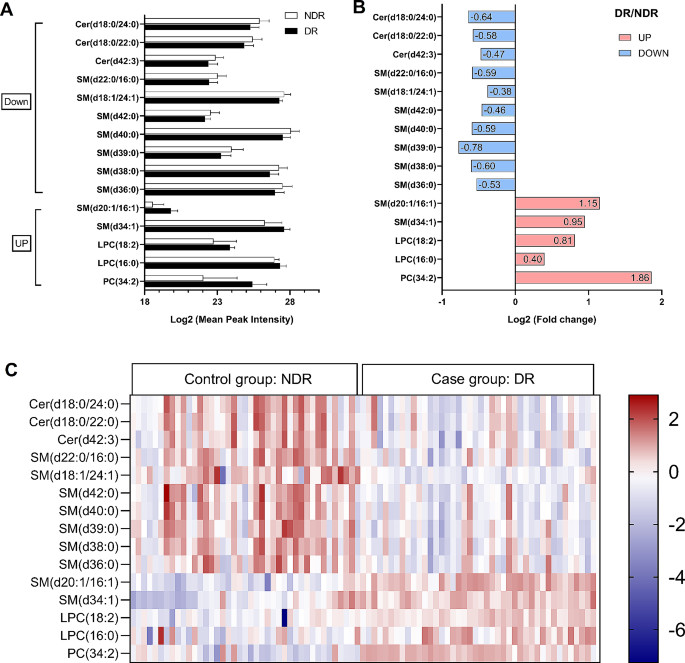
Lipidome-derived markers identified from the discovery cohort. Lipidomic analysis identified fifteen candidate lipids of which serum levels were different between 42 T2DM patients with DR (DR group) and 42 T2DM patients without DR (NDR group) from the discovery cohort. ( A ) Mean peak intensity of lipids was analyzed after Log2 transformation of the data. ( B ) Fold change in DR/NDR was analyzed after Log2 transformation of the data. ( C ) Heatmap showing the distribution of lipid markers. Each row in the figure represents a different lipid, and each column represents a sample. Different colors indicate different intensities, and Log2 conversion was used for the data
Characteristics of the validation cohort and targeted lipidomics analysis
The 15 differential lipids found from the discovery cohort were validated in another set of samples. The clinical characteristics of individuals selected for the validation cohort are shown in Table 3 . Most metabolic and clinical features were comparable (Table S5 ), and there was no significant difference in LDL-c between the DR and NDR groups.
In the validation cohort, when compared with subjects in the NDR group, T2DM patients with DR showed lower levels of Cer(d18:0/24:0), Cer(d18:0/22:0), Cer(d42:3) and SM(d18:1/24:1) by univariate logistic regression, which was consistent with the results of the discovery cohort. However, the levels of SM(d20:1/16:1), LPC(18:2) and LPC(16:0) were lower in T2DM patients with DR from the validation cohort, opposite to the result obtained in the discovery cohort (Fig. 2 A and B). The AUC values for these lipids were higher than 0.61. The other 8 lipids did not significantly differ between the DR and NDR groups in the validation cohort (Table 4 ). Of note, compared with those in T2DM patients, the peak area (after Log2 transformation) of Cer(d18:0/24:0) (20.48 ± 0.82 vs. 20.12 ± 0.99, p = 0.006, Fig. 2 C) and Cer(d18:0/22:0) (19.91 ± 0.75 vs. 19.64 ± 0.92, p = 0.028, Fig. 2 C) remained significantly lower in T2DM patients with DR, and the levels of these two lipids retained significant ORs when adjusted for known risk factors (i.e., CHOL, TG, LDL-c and HDL-c). In the ordinal regression, these two lipids maintained significant ORs (Table S7 , n = 95 in the NDR group, n = 87 in the NPDR group; n = 8 in the PDR group), and were also significant while excluding patients with DME (Table S6 , n = 2 in the DR group). These findings imply that levels of Cer(d18:0/24:0) and Cer(d18:0/22:0) were independent markers for T2DM patients with DR in both the discovery cohort (Table 2 ) and validation cohort (Table 4 ).
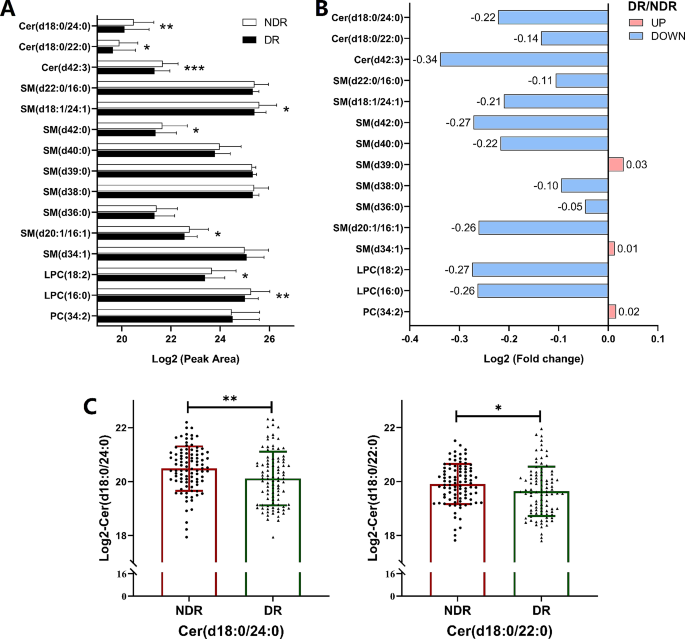
The results of targeted lipidomics analysis in the validation cohort. For the validation cohort, the cases were 95 T2DM patients with DR (DR group), and the control subjects were 95 T2DM patients who had no DR (NDR group). ( A ) Peak area of lipids was analyzed after Log2 transformation of the data. ( B ) Fold change in DR/NDR was analyzed after Log2 transformation of the data. ( C ) The log2 conversion was used for the intensities of Cer(d18:0/24:0) and Cer(d18:0/22:0). All data are presented as the mean ± standard deviation (SD). Each symbol represents an individual participant. * p < 0.05, ** p < 0.01, pairwise comparisons of change scores between the groups were evaluated by t test
DR is the most common microvascular complication of diabetes and the main factor contributing to visual impairment in working-age individuals [ 3 ]. T2DM patients often develop DR despite of proper control of systemic risk factors, indicating the involvement of other pathogenic factors for DR development. To find new and more effective strategies for preventing and treating DR, it is necessary for us to identify novel biomarkers for DR screening or detection. Lipidomics will aid in understanding the mechanism of DR at various stages of the disease, early diagnosis, and the identification of new therapeutic targets. In this study, by using two clinical cohorts, we found that the serum lipidomic profiles in T2DM patients with DR showed significant differences from those in T2DM patients without DR. The differential lipid species in the DR group were linked to disturbances in sphingolipid metabolism. Compared with those in the NDR group, the levels of Cer(d18:0/24:0) and Cer(d18:0/22:0) were significantly lower in the DR group after adjusting for covariates, i.e. known risk factors in both the discovery and validation cohorts. These findings suggest that these two lipid species may be potential serological markers for the diagnosis of DR in patients with T2DM.
In this study, we found two ceramide molecules that were significantly lower in T2DM patients with DR, indicating that they may have disturbed ceramide metabolism compared to T2DM patients without DR. Ceramide is sphingolipid [ 11 ] and can be found in VLDL, LDL, and HDL. Consistent with our findings, Fort et al. found a significantly lower abundance of Cer in central retinal tissue obtained postmortem from T2DM patients with DR compared to those without DR [ 26 ]. Similarly, ceramide levels were shown to be lower and glucosylceramide levels higher in the retinas of diabetic rodents [ 27 ]. This indicates that diabetes reduces the retinal ceramide content and may suggest that dysregulated sphingolipid metabolism may cause retinal resistance to insulin action [ 27 ]. These findings imply that ceramide is diverted from the overall pools of retinal sphingolipids toward the glycosylated forms due to hyperglycemia. In contrast, Levitsky et al. found that diabetes-induced increases in mitochondrial ceramide led to impaired mitochondrial function in the retinal pigment epithelial (RPE) cells of the retina [ 28 ], and disruption of the blood-retinal barrier might be caused by diabetes-induced overexpression of acid sphingomyelinase. Additionally, inflammation is a common underlying factor in DR, and inflammation generates Cer from SM in the serum membrane. This induces death receptor ligand formation and leads to apoptosis of RPE and photoreceptor cells [ 29 ]. In addition to diabetes, circulating Cer was shown to strongly correlate with future adverse cardiovascular events. It has recently been discovered that in individuals with atherosclerotic CVD, serum levels of specific Cer species can predict the future risk of cardiovascular death. In the Corogene study, higher concentrations of Cer(d18:1/16:0), Cer(d18:1/18:0), and Cer(d18:1/24:1) and lower concentrations of Cer(d18:1/24:0) were associated with a higher risk of fatal myocardial infarction [ 30 ]. Our study found that Cer(d18:0/24:0) and Cer(d18:0/22:0) were significantly lower in T2DM patients with DR compared to those without DR, which suggests that different numbers of carbons and double bonds in ceramides might play differential roles in DR and CVD. The distinct ceramides and ceramide metabolites involved in metabolic regulation play unanticipated roles [ 31 ]. Watt et al. discovered that circulating ceramides present in LDL particles were sufficient to induce insulin resistance in vitro and in vivo [ 32 ]. However, how these two identified ceramides influence lipid metabolism in T2DM remains unclear and needs further exploration. Thus, disturbed Cer metabolism may contribute to dysfunction in DR, and therapeutic strategies to restore normal Cer metabolism might be an effective approach for treatment of DR.
In the discovery cohort, LPC(18:2) and LPC(16:0) were significantly higher in T2DM patients with DR. However, these two lipids were significantly lower in DR in the validation cohort. The previous findings point to a change in sphingolipid composition between control and T2DM [ 33 ]. LPC is an inflammatory phospholipid and an important atherogenic substance in LDL that contributes to diabetic complications [ 34 ]. Lipoprotein-associated phospholipase A2 (Lp-PLA2) plays a crucial role in diabetes-related retinal vasopermeability, a response mediated by LPC, and inhibiting Lp-PLA2 reduces diabetes-induced retinal vasopermeability [ 35 ]. LPC O-16:0, LPC P-16:0, LPC O-18:0, and LPC 18:1 were all found to be inversely related to incident T2DM [ 36 ]. The differences between the discovery and validation cohorts may be related to the populations studied, medications used, and stages of diabetic retinopathy [ 37 ].
There are some limitations of this study. First, only a Chinese ethnic group was selected, and future validation of our findings in other races or ethnic groups is warranted. Second, instead of chronic risk factors associated with the development of DR, some of the identified lipid markers might only represent temporary metabolic perturbations in this cross-sectional study. Third, the exact mechanism of DR development in patients with T2DM through which ceramide functions has not been explained. Therefore, more extensive preclinical and clinical studies are needed to clarify the mechanisms behind the potential effects of specific lipids.
Overall, the deregulation of sphingolipid metabolism in the diabetic retina appears to be a significant and seldom-studied element of DR pathophysiology. The precise mechanism underlying this disease is still unknown and requires further investigation. We showed the potential value of lipidomics research in understanding the pathophysiology of DR, and the results suggest that lipidomics profiling may be capable of identifying early-stage DR diagnostic indicators in high-risk Chinese populations. In addition, the findings from this study may help in the elucidation of new therapeutic targets for DR prevention and treatment.
Data availability
All relevant data and materials have been included in the article and its supplementary data files. Further inquiries can be directed to the corresponding authors.
Abbreviations
- Type 2 diabetes mellitus
Diabetic retinopathy
Liquid chromatography–mass spectrometry
Partial least squares discriminant analysis
Variable importance in the projection
Propensity score matching
Sphingomyelins
Phosphatidylcholine
Lysophosphatidylcholines
Multiple reaction monitoring
Systolic blood pressure
Diastolic blood pressure
Total cholesterol
Triglyceride
High density lipoprotein-cholesterol
Low density lipoprotein-cholesterol
Aspartate aminotransferase
Alanine aminotransferase
Alkaline phosphatase
Gamma-glutamyl transpeptidase
Total bilirubin
Direct bilirubin
Total protein
Blood urea nitrogen
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14.
Article CAS PubMed Google Scholar
Wong TY, Cheung CM, Larsen M, Sharma S, Simo R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012.
Article PubMed Google Scholar
American Diabetes A. 10. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S105-S18.
Jampol LM, Glassman AR, Sun J. Evaluation and care of patients with Diabetic Retinopathy. N Engl J Med. 2020;382:1629–37.
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Article PubMed PubMed Central Google Scholar
Beulens JW, Patel A, Vingerling JR, Cruickshank JK, Hughes AD, Stanton A, et al. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52:2027–36.
Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Reviews Endocrinol. 2017;13:79–91.
Article CAS Google Scholar
Zeng W, Beyene HB, Kuokkanen M, Miao G, Magliano DJ, Umans JG, et al. Lipidomic profiling in the strong heart study identified American indians at risk of chronic kidney disease. Kidney Int. 2022;102:1154–66.
Article CAS PubMed PubMed Central Google Scholar
Knuplez E, Marsche G. An updated review of Pro- and Anti-inflammatory properties of plasma lysophosphatidylcholines in the Vascular System. Int J Mol Sci. 2020;21.
Tonks KT, Coster ACF, Christopher MJ, Chaudhuri R, Xu AM, Gagnon-Bartsch J, et al. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity. 2016;24:908–16.
Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018;19:175–91.
Chaurasia B, Tippetts TS, Mayoral Monibas R, Liu J, Li Y, Wang L, et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Volume 365. New York, NY: Science; 2019. pp. 386–92.
Google Scholar
Klein RL, Hammad SM, Baker NL, Hunt KJ, Al Gadban MM, Cleary PA, et al. Decreased plasma levels of select very long chain ceramide species are associated with the development of nephropathy in type 1 diabetes. Metab Clin Exp. 2014;63:1287–95.
Fox TE, Han X, Kelly S, Merrill AH 2nd, Martin RE, Anderson RE, et al. Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes. 2006;55:3573–80.
Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends Endocrin Met. 2012;23:391–8.
Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286:27855–62.
Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:2614–8.
Hyotylainen T, Oresic M. Analytical Lipidomics in metabolic and clinical research. Trends Endocrin Met. 2015;26:671–3.
Xuan Q, Ouyang Y, Wang Y, Wu L, Li H, Luo Y, et al. Multiplatform Metabolomics reveals novel serum metabolite biomarkers in Diabetic retinopathy subjects. Adv Sci (Weinh). 2020;7:2001714.
Hyotylainen T, Oresic M. Optimizing the lipidomics workflow for clinical studies-practical considerations. Anal Bioanal Chem. 2015;407:4973–93.
Grochowski ET, Pietrowska K, Godlewski A, Gosk W, Buczynska A, Wojnar M et al. Simultaneous comparison of aqueous humor and serum metabolic profiles of Diabetic and nondiabetic patients undergoing cataract Surgery—A targeted and quantitative Metabolomics Study. Int J Mol Sci. 2023;24.
Liew G, Lei Z, Tan G, Joachim N, Ho IV, Wong TY, et al. Metabolomics of Diabetic Retinopathy. Curr Diab Rep. 2017;17:102.
Sarafian MH, Gaudin M, Lewis MR, Martin FP, Holmes E, Nicholson JK, et al. Objective set of criteria for optimization of sample preparation procedures for ultra-high throughput untargeted blood plasma lipid profiling by ultra performance liquid chromatography-mass spectrometry. Anal Chem. 2014;86:5766–74.
Li J, Li L, Liu R, Zhu L, Zhou B, Xiao Y, et al. Integrative lipidomic features identify plasma lipid signatures in chronic urticaria. Front Immunol. 2022;13:933312.
Wen B, Mei Z, Zeng C, Liu S. metaX: a flexible and comprehensive software for processing metabolomics data. BMC Bioinformatics. 2017;18:183.
Fort PE, Rajendiran TM, Soni T, Byun J, Shan Y, Looker HC et al. Diminished retinal complex lipid synthesis and impaired fatty acid β-oxidation associated with human diabetic retinopathy. Jci Insight. 2021;6.
Fox TE, Han XL, Kelly S, Merrill AH, Martin RE, Anderson RE, et al. Diabetes alters sphingolipid metabolism in the retina - A potential mechanism of cell death in diabetic retinopathy. Diabetes. 2006;55:3573–80.
Levitsky Y, Hammer SS, Fisher KP, Huang C, Gentles TL, Pegouske DJ et al. Mitochondrial Ceramide effects on the retinal pigment epithelium in diabetes. Int J Mol Sci. 2020;21.
Mondal K, Mandal N. Role of bioactive sphingolipids in inflammation and Eye diseases. Adv Exp Med Biol. 2019;1161:149–67.
Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37:1967–76.
Summers SA, Chaurasia B, Holland WL. Metabolic messengers: ceramides. Nat Metabolism. 2019;1:1051–8.
Article Google Scholar
Zhang QJ, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, et al. Ceramide mediates vascular dysfunction in Diet-Induced obesity by PP2A-Mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 2012;61:1848–59.
Wilmott LA, Grambergs RC, Allegood JC, Lyons TJ, Mandal N. Analysis of sphingolipid composition in human vitreous from control and diabetic individuals. J Diabetes Complications. 2019;33:195–201.
Shi AH, Yoshinari M, Wakisaka M, Iwase M, Fujishima M. Lysophosphatidylcholine molecular species in low density lipoprotein of type 2 diabetes. Horm Metab Res. 1999;31:283–6.
Canning P, Kenny BA, Prise V, Glenn J, Sarker MH, Hudson N, et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) as a therapeutic target to prevent retinal vasopermeability during diabetes. Proc Natl Acad Sci U S A. 2016;113:7213–8.
Zhong J, Cheung CYY, Su X, Lee CH, Ru Y, Fong CHY, et al. Specific triacylglycerol, diacylglycerol, and lyso-phosphatidylcholine species for the prediction of type 2 diabetes: a ∼ 16-year prospective study in Chinese. Cardiovasc Diabetol. 2022;21:234.
Krischer JP, Liu X, Lernmark A, Hagopian WA, Rewers MJ, She JX, et al. The influence of type 1 diabetes genetic susceptibility regions, Age, Sex, and Family History on the Progression from multiple autoantibodies to type 1 diabetes: a TEDDY Study Report. Diabetes. 2017;66:3122–9.
Download references
Acknowledgements
Y.N.W. is supported by the China “Thousand Talents Plan” (Young Talents), Shaanxi province “Thousand Talents Plan” (Young Talents) and Foundation of Xi’an Jiaotong University (Plan A).
This study was supported by grants from The Natural Science Foundation Program of Shaanxi (2024JC-YBQN-0828) and National Key R&D Program of China (No. 2018YFC1311501).
Author information
Mingqian He, Guixue Hou and Mengmeng Liu contributed equally to this work.
Authors and Affiliations
Department of Endocrinology, the First Affiliated Hospital of Xi’an JiaoTong University, No.277, West Yanta Road, Xi’an, Shaanxi, 710061, P.R. China
Mingqian He, Mengmeng Liu, Zhaoyi Peng, Hui Guo, Yue Wang, Meng Zhang, Ziyi Chen, Patrick C.N. Rensen, Yanan Wang & Bingyin Shi
BGI-SHENZHEN, No. 21 Hongan 3rd Street, Yantian District, Shenzhen, Guangdong, 518083, P.R. China
Guixue Hou & Liang Lin
Department of Endocrinology and International Medical Center, the First Affiliated Hospital of Xi’an JiaoTong University, No.277, West Yanta Road, Xi’an, Shaanxi, 710061, P.R. China
Biobank, The First Affiliated Hospital of Xi’an JiaoTong University, Xi’an, Shaanxi, 710061, China
Chengdu HuiXin Life Technology, Chengdu, Sichuan, 610091, P.R. China
Xiaoming Yin
Department of Medicine, Division of Endocrinology, Leiden University Medical Center, P.O. Box 9600, Leiden, 2300 RA, The Netherlands
Patrick C.N. Rensen
Med-X institute, Center for Immunological and Metabolic Diseases, the First Affiliated Hospital of Xi’an JiaoTong University, Xi’an JiaoTong university, Xi’an, Shaanxi, 710061, P.R. China
Building NO.7, BGI Park, No. 21 Hongan 3rd Street, Yantian District, Shenzhen, Guangdong, 518083, P.R. China
You can also search for this author in PubMed Google Scholar
Contributions
B.S., Y.W., and L.L. conceived this review and critically revised the manuscript. M.H., G.H., and M.L. drafted the manuscript. Z.P., H.G., Y.W., and J.S. drew the figures and collected the related references. H.L., X.Y., M.Z., Z.C. and P.C.N. supervised and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Liang Lin , Yanan Wang or Bingyin Shi .
Ethics declarations
Ethics approval and consent to participate.
This study was conducted with approval from the Institutional Review Board at the First Affiliated Hospital of Xi’an Jiaotong University, Shaanxi, China (approval number: XJTU1AF2018LSK-055). Written informed consent was obtained from all participants.
Consent for publication
All authors critically reviewed and approved the final manuscript.
Competing interests
All authors declared no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
He, M., Hou, G., Liu, M. et al. Lipidomic studies revealing serological markers associated with the occurrence of retinopathy in type 2 diabetes. J Transl Med 22 , 448 (2024). https://doi.org/10.1186/s12967-024-05274-9
Download citation
Received : 21 August 2023
Accepted : 04 May 2024
Published : 13 May 2024
DOI : https://doi.org/10.1186/s12967-024-05274-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Retinopathy
- Serological markers
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Nephrol

Chronic kidney disease and its health-related factors: a case-control study
Mousa ghelichi-ghojogh.
1 Candidate in Epidemiology, Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran
Mohammad Fararouei
2 HIV/AIDS research center, School of Health, Shiraz University of Medical Sciences, P.O.Box: 71645-111, Shiraz, Iran
Mozhgan Seif
3 Department of Epidemiology, School of Health, Shiraz University of Medical Sciences, Shiraz, Iran
Maryam Pakfetrat
4 Nephrologist, Shiraz Nephro-Urology Research Center, Department of Internal Medicine, Emergency Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Associated Data
The datasets generated and/or analyzed during the current study are not publicly available due to their being the intellectual property of Shiraz University of Medical Sciences but are available from the corresponding author on reasonable request.
Chronic kidney disease (CKD) is a non-communicable disease that includes a range of different physiological disorders that are associated with abnormal renal function and progressive decline in glomerular filtration rate (GFR). This study aimed to investigate the associations of several behavioral and health-related factors with CKD in Iranian patients.
A hospital-based case-control study was conducted on 700 participants (350 cases and 350 controls). Logistic regression was applied to measure the association between the selected factors and CKD.
The mean age of cases and controls were 59.6 ± 12.4 and 58.9 ± 12.2 respectively ( p = 0.827). The results of multiple logistic regression suggested that many factors including low birth weight (OR yes/no = 4.07, 95%CI: 1.76–9.37, P = 0.001), history of diabetes (OR yes/no = 3.57, 95%CI: 2.36–5.40, P = 0.001), history of kidney diseases (OR yes/no = 3.35, 95%CI: 2.21–5.00, P = 0.001) and history of chemotherapy (OR yes/no = 2.18, 95%CI: 1.12–4.23, P = 0.02) are associated with the risk of CKD.
Conclusions
The present study covered a large number of potential risk/ preventive factors altogether. The results highlighted the importance of collaborative monitoring of kidney function among patients with the above conditions.
Chronic kidney disease (CKD) is a non-communicable disease that includes a range of different physiological disorders that are associated with an abnormal renal function and progressive decline in glomerular filtration rate (GFR) [ 1 – 3 ]. Chronic kidney disease includes five stages of kidney damage, from mild kidney dysfunction to complete failure [ 4 ]. Generally, a person with stage 3 or 4 of CKD is considered as having moderate to severe kidney damage. Stage 3 is broken up into two levels of kidney damage: 3A) a level of GFR between 45 to 59 ml/min/1.73 m 2 , and 3B) a level of GFR between 30 and 44 ml/min/1.73 m 2 . In addition, GFR for stage 4 is 15–29 ml/min/1.73 m 2 [ 4 , 5 ]. It is reported that both the prevalence and burden of CKD are increasing worldwide, especially in developing countries [ 6 ]. The worldwide prevalence of CKD (all stages) is estimated to be between 8 to 16%, a figure that may indicate millions of deaths annually [ 7 ]. According to a meta-analysis, the prevalence of stage 3 to 5 CKD in South Africa, Senegal, and Congo is about 7.6%. In China, Taiwan, and Mongolia the rate of CKD is about 10.06% and in Japan, South Korea, and Oceania the rate is about 11.73%. In Europe the prevalence of CKD is about 11.86% [ 8 ], and finally, about 14.44% in the United States and Canada. The prevalence of CKD is estimated to be about 11.68% among the Iranian adult population and about 2.9% of Iranian women and 1.3% of Iranian men are expected to develop CKD annually [ 9 ]. Patients with stages 3 or 4 CKD are at much higher risk of progressing to either end-stage renal disease (ESRD) or death even prior to the development of ESRD [ 10 , 11 ].
In general, a large number of risk factors including age, sex, family history of kidney disease, primary kidney disease, urinary tract infections, cardiovascular disease, diabetes mellitus, and nephrotoxins (non-steroidal anti-inflammatory drugs, antibiotics) are known as predisposing and initiating factors of CKD [ 12 – 14 ]. However, the existing studies are suffering from a small sample size of individuals with kidney disease, particularly those with ESRD [ 15 ].
Despite the fact that the prevalence of CKD in the world, including Iran, is increasing, the factors associated with CKD are explored very little. The present case-control study aimed to investigate the association of several behavioral and health-related factors with CKD in the Iranian population.
Materials and methods
In this study, participants were selected among individuals who were registered or were visiting Faghihi and Motahari hospitals (two largest referral centers in the South of Iran located in Shiraz (the capital of Fars province). Cases and controls were frequency-matched by sex and age. The GFR values were calculated using the CKD-EPI formula [ 16 , 17 ].
Data collection
An interview-administered questionnaire and the participant’s medical records were used to obtain the required data. The questionnaire and interview procedure were designed, evaluated, and revised by three experts via conducting a pilot study including 50 cases and 50 controls. The reliability of the questionnaire was measured using the test-retest method (Cronbach’s alpha was 0.75). The interview was conducted by a trained public health nurse at the time of visiting the clinics.
Avoiding concurrent conditions that their association may interpreted as reverse causation; the questionnaire was designed to define factors preceding at least a year before experiencing CKD first symptoms. Accordingly participants reported their social and demographic characteristics (age, sex, marital status, educational level, place of residency), history of chronic diseases (diabetes, cardiovascular diseases, hypertension, kidney diseases, family history of kidney diseases, autoimmune diseases and thyroid diseases [ 18 ]). Also history of other conditions namely (smoking, urinary tract infection (UTI), surgery due to illness or accident, low birth weight, burns, kidney pain (flank pain), chemotherapy, taking drugs for weight loss or obesity, taking non-steroidal anti-inflammatory drugs, and taking antibiotic) before their current condition was started. Many researchers reported recalling birth weight to be reliable for research purposes [ 19 ]. Moreover, we asked the participants to report their birth weight as a categorical variable (< 2500 g or low, 2500- < 3500 g or normal, and > 3500 g or overweight). Medical records of the participants were used to confirm/complete the reported data. In the case of contradiction between the self-reported and recorded data, we used the recorded information for our study.
Verbal informed consent was obtained from patients because the majority of the participants were illiterate. The study protocol was reviewed and approved by the ethical committee of Shiraz University of Medical Sciences (approval number: 1399.865).
Sample size
The sample size was calculated to detect an association between the history of using antibiotics (one of our main study variables) and CKD as small as OR = 1.5 [ 20 ]. With an alpha value of 0.05 (2-sided) and a power of 80%, the required sample size was estimated as large as n = 312 participants for each group.
Selection of cases
The selected clinics deliver medical care to patients from the southern part of the country. In this study, patients with CKD who were registered with the above centers from June to December 2020 were studied. A case was a patient with a GFR < 60 (ml/min/1.73 m 2 ) at least twice in 3 months. According to the latest version of the International Classification of Diseases (2010), Codes N18.3 and N18.4 are assigned to patients who have (GFR = 30–59 (ml/min/1.73 m 2 ) and GFR = 15–29 (ml/min/1.73 m 2 ) respectively [ 21 ]. In total, 350 patients who were diagnosed with CKD by a nephrologist during the study period.
Selection of the controls
We used hospital controls to avoid recall-bias. The control participants were selected from patients who were admitted to the general surgery (due to hernia, appendicitis, intestinal obstruction, hemorrhoids, and varicose veins), and orthopedic wards from June to December 2020. Using the level of creatinine in the participants’ serum samples, GFR was calculated and the individuals with normal GFR (ml/min/1.73 m 2 ) GFR > 60) and those who reported no history of CKD were included ( n = 350).
Inclusion criteria
Patients were included if they were ≥ 20 years old and had a definitive diagnosis of CKD by a nephrologist.
Exclusion criteria
Participants were excluded if they were critically ill, had acute kidney injury, those undergone renal transplantation, and those with cognitive impairment.
Statistical analysis
The Chi-square test was used to measure the unadjusted associations between categorical variables and CKD. Multiple logistic regression was applied to measure the adjusted associations for the study variables and CKD. The backward variable selection strategy was used to include variables in the regression model. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. All p -values were two-sided and the results were considered statistically significant at p < 0.05. All analyses were conducted using Stata version 14.0 (Stata Corporation, College Station, TX, USA).
In total, 350 cases and 350 age and sex-matched controls were included in the analysis. The mean age of cases and controls were 59.6 ± 12.4 and 58.9 ± 12.2 respectively ( p = 0.83). Overall, 208 patients (59.4%) and 200 controls (57.1%) were male ( p = 0.54). Also, 149 patients (42.6%) and 133 controls (38.0%) were illiterate or had elementary education ( p = 0.001). Most cases (96.9%) and controls (95.7%) were married ( p = 0.42). The mean GFR for CKD and control groups were 38.6 ± 11.4 and 78.3 ± 10.2 (ml/min/1.73 m2) respectively.
Result of univariate analysis
Table 1 illustrates the unadjusted associations of demographic and health-related variables with CKD. Accordingly, significant (unadjusted) associations were found between the risk of CKD and several study variables including education, history of chronic diseases (diabetes, cardiovascular, hypertension, kidney diseases, autoimmune diseases, and hypothyroidism), family history of kidney diseases, smoking, UTI, surgery due to illness or accident, low birth weight, burns, kidney pain, chemotherapy, taking non-steroidal anti-inflammatory drugs, and taking antibiotics) ( P < 0.05 for all).
Unadjusted associations between demographic and health related variables with the risk of CKD
*Chi-square test; ** HTN Hypertension; *** UTI Urinary tract infection
Results of multivariable analysis
Table 2 illustrates the adjusted associations between the study variables and the risk of CKD. Most noticeably, low birth weight (OR yes/no = 4.07, 95%CI: 1.76–9.37, P = 0.001), history of surgery (OR yes/no = 1.74, 95%CI: 1.18–2.54, P = 0.004), family history of kidney diseases (OR yes/no = 1.97, 95%CI: 1.20–3.23, P = 0.007), and history of chemotherapy (OR yes/no = 2.18, 95%CI: 1.12–4.23, P = 0.02) were significantly associated with a higher risk of CKD. On the other hand, education (OR college/illiterate or primary = 0.54, 95%CI: 0.31–0.92, P = 0.025) was found to be inversely associated with CKD.
Adjusted association between the study variables and risk of CKD
a The full model included education, history of chronic diseases (diabetes, cardiovascular, hypertension, kidney diseases, autoimmune diseases and hypothyroidism), family history of kidney diseases, smoking, UTI, surgery due to illness or accident, low birth weight, burns, kidney pain, chemotherapy, taking non-steroidal anti-inflammatory drugs, and taking antibiotics); b OR odds ratio; c CI confidence interval
The results of the present study suggested that several variables including, education, history of diabetes, history of hypertension, history of kidney diseases or a family history of kidney diseases, history of surgery due to illness or accident, low birth weight, history of chemotherapy, history of taking non-steroidal anti-inflammatory drugs, and history of taking antibiotics may affect the risk of CKD.
In our study, the level of education was inversely associated with the risk of CKD. This finding is in accordance with the results of a study conducted by K Lambert et.al, who suggested that illiteracy or elementary education may raise the risk of CKD [ 22 ]. The fact that education level is associated with health literacy, may partly explain our results that lower education and inadequate health literacy in individuals with CKD is associated with worse health outcomes including poorer control of biochemical parameters, higher risk of cardiovascular diseases (CVDs); a higher rate of hospitalization, and a higher rate of infections [ 23 ].
In the current study, the history of diabetes was associated with a higher risk of CKD. This finding is consistent with the results of other studies on the same subject [ 20 , 21 , 24 – 27 ]. It is not surprising that people with diabetes have an increased risk of CKD as diabetes is an important detrimental factor for kidney functioning as approximately, 40% of patients with diabetes develop CKD [ 27 ].
The other variable that was associated with an increased risk of CKD was a history of hypertension. Our result is consistent with the results of several other studies [ 20 , 24 , 25 , 28 ]. It is reported that hypertension is both a cause and effect of CKD and accelerates the progression of the CKD to ESRD [ 29 ].
After controlling for other variables, a significant association was observed between family history of kidney diseases and risk of CKD. Published studies suggested the same pattern [ 24 ]. Inherited kidney diseases (IKDs) are considered as the foremost reasons for the initiation of CKD and are accounted for about 10–15% of kidney replacement therapies (KRT) in adults [ 30 ].
The importance of the history of surgery due to illness or accident in this study is rarely investigated by other researchers who reported the effect of surgery in patients with acute kidney injury (AKI), and major abdominal and cardiac surgeries [ 31 , 32 ] on the risk of CKD. Also, AKI is associated with an increased risk of CKD with progression in various clinical settings [ 33 – 35 ]. In a study by Mizota et.al, although most AKI cases recovered completely within 7 days after major abdominal surgery, they were at higher risk of 1-year mortality and chronic kidney disease compared to those without AKI [ 31 ].
The present study also showed that low birth weight is a significant risk factor for CKD. This finding is consistent with the results of some other studies. However, the results of very few studies on the association between birth weight and risk of CKD are controversial as some suggested a significant association [ 19 , 36 , 37 ] whereas others suggested otherwise [ 36 ]. This may be explained by the relatively smaller size and volume of kidneys in LBW infants compared to infants that are normally grown [ 38 ]. This can lead to long-term complications in adolescence and adulthood including hypertension, decreased glomerular filtration, albuminuria, and cardiovascular diseases. Eventually, these long-term complications can also cause CKD [ 39 ].
Another important result of the current study is the association between chemotherapy for treating cancers and the risk of CKD. According to a study on chemotherapy for testicular cancer by Inai et al., 1 year after chemotherapy 23% of the patients showed CKD [ 40 ]. Another study suggested that the prevalence of stage 3 CKD among patients with cancer was 12, and < 1% of patients had stage 4 CKD [ 41 , 42 ]. Other studies have shown an even higher prevalence of CKD among cancer patients. For instance, only 38.6% of patients with breast cancer, 38.9% of patients with lung cancer, 38.3% of patients with prostate cancer, 27.5% of patients with gynecologic cancer, and 27.2% of patients with colorectal cancer had a GFR ≥90 (ml/min/1.73 m 2 ) at the time of therapy initiation [ 43 , 44 ]. The overall prevalence of CKD ranges from 12 to 25% across many cancer patients [ 45 – 47 ]. These results clearly demonstrate that, when patients with cancer develop acute or chronic kidney disease, outcomes are inferior, and the promise of curative therapeutic regimens is lessened.
In our study, the history of taking nephrotoxic agents (antibiotics or NSAIDs drugs) was associated with a higher risk of CKD. Our result is following the results reported by other studies [ 48 , 49 ]. Common agents that are associated with AKI include NSAIDs are different drugs including antibiotics, iodinated contrast media, and chemotherapeutic drugs [ 50 ].
Strengths and limitations of our study
Our study used a reasonably large sample size. In addition, a considerably large number of study variables was included in the study. With a very high participation rate, trained nurses conducted the interviews with the case and control participants in the same setting. However, histories of exposures are prone to recall error (bias), a common issue in the case-control studies. It is to be mentioned that the method of selecting controls (hospital controls) should have reduced the risk of recall bias when reporting the required information. In addition, we used the participants’ medical records to complete/ confirm the reported data. Although the design of the present study was not able to confirm a causal association between the associated variables and CKD, the potential importance and modifiable nature of the associated factors makes the results potentially valuable and easily applicable in the prevention of CKD.
Given that, chemotherapy is an important risk factor for CKD, we suggest the imperative for collaborative care between oncologists and nephrologists in the early diagnosis and treatment of kidney diseases in patients with cancer. Training clinicians and patients are important to reduce the risk of nephrotoxicity. Electronic medical records can simultaneously be used to monitor prescription practices, responsiveness to alerts and prompts, the incidence of CKD, and detecting barriers to the effective implementation of preventive measures [ 51 ]. Routine follow-up and management of diabetic patients is also important for the prevention of CKD. We suggest a tight collaboration between endocrinologists and nephrologists to take care of diabetic patients with kidney problems. In addition, surgeons in major operations should refer patients, especially patients with AKI, to a nephrologist for proper care related to their kidney function. Treatment of hypertension is among the most important interventions to slow down the progression of CKD [ 12 ]. Moreover, all patients with newly diagnosed hypertension should be screened for CKD. We suggest all patients with diabetes have their GFR and urine albumin-to-creatinine ratio (UACR) checked annually. Finally, the aging population and obesity cause the absolute numbers of people with diabetes and kidney diseases to raise significantly. This will require a more integrated approach between dialectologists/nephrologists and the primary care teams (55).
Acknowledgments
This paper is part of a thesis conducted by Mousa Ghelichi-Ghojogh, Ph.D. student of epidemiology, and a research project conducted at the Shiraz University of Medical sciences (99-01-04-22719). We would like to thank Dr. Bahram Shahryari and all nephrologists of Shiraz University of medical sciences, interviewers, and CKD patients in Shiraz for their voluntary participation in the study and for providing data for the study.
Abbreviations
Authors’ contributions.
MGG: Conceptualization, Methodology, Statistical analysis, Investigation, and writing the draft of the manuscript. MP: were involved in methodology, writing the draft of the manuscript, and clinical consultation. MS: was involved in the methodology and statistical analysis. MF: was involved in conceptualization, methodology, supervision, writing, and reviewing the manuscript. The authors read and approved the final manuscript.
Shiraz University of Medical Sciences financially supported this study. (Grant number: 99–01–04-22719).
Availability of data and materials
Declarations.
The study protocol was reviewed and approved by the ethical committee of Shiraz University of Medical Sciences (approval number: 1399.865). All methods were performed in accordance with the relevant guidelines and regulations of the Declaration of Helsinki. The participants were assured that their information is used for research purposes only. Because of the illiteracy of a considerable number of the patients, verbal informed consent was obtained from the participants. Using verbal informed consent was also granted by the ethical committee of Shiraz University of Medical Sciences.
Not applicable.
None of the authors declare disclosures of direct relevance to the submitted work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Leptospirosis: What Every Dog Owner Should Know
F RIDAY, May 17, 2024 (HealthDay News) -- Mice, rats and other wildlife can pick up bacteria called leptospira from water or soil and excrete it in urine.
If your dog contacts any soil or water contaminated by the germ (especially if they have an open wound) they can easily develop a potentially deadly disease called leptospirosis.
"Every dog that has access to the outdoors is at risk of getting leptospirosis," stressed Dr. Emmanuelle Butty , a veterinarian and assistant clinical professor of veterinary medicine at Tufts University in Boston.
"It's heartbreaking when we see these cases," Butty added, because there's been an effective canine vaccine against leptospirosis for the past two decades.
Humans can also become infected, although cats are thought to be resistant to leptospirosis.
In dogs, the symptoms of leptospirosis include lethargy, vomiting and loss of appetite. Signs of thirst may appear, and your dog's eyes could turn yellow with jaundice.
Symptoms do vary, however, so be sure to get your dog checked out by a vet if you have any concerns.
In most cases, antibiotics clear up a case of canine leptospirosis, Butty said.
However, the disease can progress to harm the kidneys, triggering kidney failure that may require dialysis.
"If we buy time [with dialysis]," Butty said, "we have a chance that the body will recover."
In a study she conducted, 16 of 22 dogs whose leptospirosis progressed to kidney failure survived.
"All of them would have died without dialysis because their kidneys were completely shut down," she said, "but almost 75% of them were able to get out of the hospital. Even if things look really bad, there is a decent chance we will be able to save this animal."
These treatments are costly, however, so Butty suggested pet insurance to cover such emergencies. "It can definitely save a life," she said.
Recovering from a severe case of leptospirosis still leaves dogs with long-term kidney issues.
"It's sad when dogs have chronic kidney disease at one or two years old," Butty said. "Their lifespan is going to be reduced significantly."
Of course the best way to help your pooch avoid leptospirosis is through vaccination.
"We have a good way to prevent the disease and to prevent the most severe cases of the disease, and that is the vaccine," Butty said. It's a two-shot regimen spaced a month apart, and can be given to dogs as young as 12 weeks of age.
Yearly boosters are recommended after that, Butty said, and sticking to a schedule is crucial.
"If the booster is not on time, they are not considered vaccinated anymore and have to be restarted with the first two doses," Butty explained. "Owners absolutely have to be on top of this and get an appointment with the vet before the due date."
The vaccine is very safe: Dogs might get mildly under the weather for a couple days after their shots, but most bounce back quickly and serious reactions are rare.
More information
The American Veterinary Medical Association has more on leptospirosis .
SOURCE: Tufts University, news release, May 15, 2024

Predictors of Adverse Drug Reaction-Related Hospitalisations Among People with Dementia: A Retrospective Case-Control Study
- Original Research Article
- Open access
- Published: 13 May 2024
Cite this article
You have full access to this open access article

- Anum Saqib Zaidi ORCID: orcid.org/0000-0003-1293-2289 1 ,
- Gregory M. Peterson ORCID: orcid.org/0000-0002-6764-3882 1 , 2 ,
- Colin M. Curtain ORCID: orcid.org/0000-0001-5029-7541 1 &
- Mohammed S. Salahudeen ORCID: orcid.org/0000-0001-9131-7465 1
233 Accesses
2 Altmetric
Explore all metrics
Introduction
Adverse drug reactions (ADRs) are common among people with dementia; however, little is known about the magnitude and predictors associated with ADR-related hospitalisation among these individuals. This study aimed to determine the magnitude, types, drugs implicated and predictors of ADRs associated with hospitalisation among people with dementia.
This retrospective case-control study analysed medical records of individuals aged ≥ 65 years with dementia admitted to major public hospitals in Tasmania, Australia, from July 2010 to July 2021. Adverse drug reactions and implicated drugs were identified using administrative data and cross-checked with hospital medical records, with consensus reached among the research team.
Of the 7928 people admitted to hospital at least once within the study period, 1876 (23.7%) experienced at least one ADR-related hospitalisation. Of these, 300 case patients with 311 ADRs and 300 control patients were randomly selected. The most common types of ADRs were renal (acute kidney injury; AKI) (36.0%), followed by neuropsychiatric (17.6%), cardiovascular (16.0%) and haematological (13.1%). Diuretics, renin-angiotensin system (RAS) inhibitors and anti-thrombotics constituted the main implicated drug classes. The ADR-related hospitalisation was associated with: chronic kidney disease (CKD) (OR 8.00, 95% CI 2.63–24.28, p < 0.001), Australian-born (OR 1.62, 95% CI 1.08–2.43, p = 0.019), hypertension (OR 1.48, 95% CI 1.01–2.17, p = 0.044) and the number of medicines (OR 1.06, 95% CI 1.00–1.12, p = 0.022). Potentially inappropriate medication use and anticholinergic burden did not predict ADR-related hospitalisation.
Conclusions
These predictors could help identify the individuals at the highest risk and enable targeted interventions to be designed.
Similar content being viewed by others

Prevalence and Nature of Medication Errors and Medication-Related Harm Following Discharge from Hospital to Community Settings: A Systematic Review
Interpreting global trends in type 2 diabetes complications and mortality

Polypharmacy in elderly people
Avoid common mistakes on your manuscript.
1 Introduction
An adverse drug reaction (ADR) is defined by the World Health Organization (WHO) as “any response to a drug which is noxious and unintended, and which occurs at doses normally used in man for prophylaxis, diagnosis or therapy of disease, or for the modification of physiological function” [ 1 ]. Adverse drug reactions represent a significant clinical challenge, as they can lead to important adverse outcomes, including higher rates of morbidity and mortality, extended hospitalisation, and increased health care expenditures [ 2 ]. An Australian study found that ADRs were responsible for 18.9% of unplanned admissions to medical wards among elderly patients [ 3 ]. A systematic review reported that ADR-related hospitalisations had a median prevalence rate of 6.0% of all admissions [ 4 ].
The ageing population results in an exponential increase in the incidence of neurodegenerative disorders, such as dementia [ 5 ]. The global number of individuals living with dementia is expected to rise significantly from 57 million in 2019 to 153 million by 2050 [ 6 ]. The Australian Institute of Health and Welfare (AIHW) estimated that in 2022, there were around 401,300 individuals living with dementia in Australia, where dementia also ranks as the second leading cause of death [ 7 ]. Similarly, in 2023 it was estimated that there were more than 10,300 people living with all forms of dementia in Tasmania [ 8 ]. Impaired cognition, possible reduction in adherence, age, polypharmacy and increased sensitivity to drugs with anticholinergic properties make people with dementia more prone to ADRs [ 9 ]. Evidence suggests that ADRs are common among people with dementia [ 10 ], but the predictors of ADR-related hospitalisation in these individuals have not been captured. Such information is crucial to address any modifiable predictors causing hospitalisation. Developing strategies to reduce ADR-related hospitalisation is dependent on determining the nature of ADRs, drugs most frequently implicated and the clinical impact of ADRs. Multiple studies have identified several predictors of ADRs causing hospitalisation among general population [ 4 , 11 ]; however, medical practitioners may lack awareness of factors predicting ADRs leading to hospitalisation among people with dementia. Hence, this research aimed to determine the magnitude, types, drugs implicated and potential predictors of ADRs associated with hospitalisation among people with dementia.
2.1 Study Design and Setting
This study used a retrospective case-control design, from the population of Tasmania, an Australian state with a population of 558,000 [ 12 ]. The data for the study were retrospectively collected from the four major public hospitals. This study was reported following the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist for case-control studies (Supplementary Table ST1 of the Electronic Supplementary Material [ESM]).
2.2 Dataset
For this study, the Admitted Patient Care National Minimum Dataset (APC-NMDS) [ 13 ] and Digital Medical Records (DMR) were utilised to obtain patient information. The APC-NMDS is a collection of coded clinical and administrative data elements from Australian public hospitals, that includes summary information about each patient’s sociodemographic characteristics, principal diagnosis, and any additional diagnoses associated with their admission, based on the International Statistical Classification of Diseases and Related Health Problems, tenth edition, Australian Modification (ICD-10-AM) [ 13 ]. The DMR contains scanned copies of all documents related to a patient’s episode of care in a public hospital. It contains a detailed record of sociodemographic characteristics, primary and secondary diagnoses, comorbidities, medication history, and laboratory data.
2.3 Study Population and Admissions
The study population comprised people aged ≥ 65 years who had a medical admission to one of the four major Tasmanian public hospitals between July 2010 and July 2021, with a primary or secondary diagnosis of dementia, based on ICD-10-AM. The study sample consisted of two groups (i) cases: a randomly selected subset of 300 patients from the APC-NMDS who had at least one ADR-related admission during the study period, and (ii) controls: another randomly selected group of 300 patients from the APC-NMDS with no admission codes related to ADRs, i.e., the control group included patients with dementia not admitted with an ADR (patients admitted for other medical reasons) during the study period.
We used a two-step process in identifying the ADRs and implicated drugs, through the external cause codes and diagnostic codes in the APC-NMDS, followed by cross-checking and verifying the ADRs and implicated drugs using the information provided in the DMR. An ICD-10-AM external cause code between Y40 and Y59 was recorded in the APC-NMDS as a diagnosis associated with the hospitalisation. These codes align with the WHO’s ADR definition and are commonly used for ADR identification [ 14 , 15 ]. The diagnostic codes (Du et al. codes) mainly refer to drug-induced symptoms or conditions without explicitly specifying the external cause of the ADR [ 16 ]. Code categories A1, A2, C and D were included, where A1: The ICD-10 code description includes the phrase ‘induced by medication/drug’, A2: The ICD-10 code description includes the phrase ‘induced by medication or other causes’, C: Adverse drug event deemed to be very likely although the ICD-10 code description does not refer to a drug, and D: Adverse drug event deemed to be likely although the ICD-10 code description does not refer to a drug. Patients coded with C and D code categories were said to have ADR-related hospitalisations when the association between the ADR and drug was stated in the DMR. The admission was not classified as ADR-related if the ADR occurred during the hospital stay. Each patient’s first ADR admission during the study period was defined as their index admission. The primary researcher (ASZ) independently reviewed all cases of suspected ADRs and cases without suspected ADRs for the presence of ADRs. If any uncertainty existed when identifying an ADR and associated drug (for the reasons outlined in Supplementary Figure SF1 of the ESM), a meeting of the research team was held to reach a consensus.
2.4 Sample Size Calculation for Identifying the Predictors of ADR-related Hospitalisations
The sample size calculation was based on the guidelines for multiple logistic regression by Peduzzi et al [ 17 ]. A rate of 0.235 (23.5%) was assumed for positive cases; our previous study investigating the trends in the incidence of ADR-related hospitalisations among people with dementia showed that 23.5% of hospitalised people experienced at least one ADR-related hospitalisation over a 10-year period [ 18 ]. An estimated 7 independent variables were included in the analysis. The minimum sample size was approximately 300 people. We doubled this number to 600 patients to ensure ample power, and any dropouts (missing or unclear information) were replaced.
2.5 Sampling Strategy
A random sampling strategy was used for recruiting the cases and controls. The patients were assigned a study ID number, and for each case, one control was randomly selected, using a web-based random number generator programme [ 19 ]. Controls were not matched with cases based on clinical characteristics such as age and sex. This decision was made for two primary reasons: first, matching variables would hinder the assessment of these variables’ impact on the outcome; second, matching has the potential to introduce bias in case-control study designs [ 20 ].
2.6 Definitions and Measures
2.6.1 potentially inappropriate medication.
Potentially inappropriate medications (PIMs) are drugs that carry a high risk of causing harm to patients and should be avoided in an individual due to the health risks outweighing the clinical benefits [ 21 ]. These medications may be inappropriate due to factors such as a lack of efficacy, the potential for ADRs or interactions, or the availability of safer alternatives. Given that the patients were elderly and had dementia, the use of PIMs was identified using the STOPPFrail criteria [ 22 ].
2.6.2 Drug-Drug Interactions
The Lexicomp ® (Wolters Kluwer Health Inc. Riverwoods, IL, USA) database system was used to identify potential drug-drug interactions (DDIs), which are classified into five types (A, B, C, D, X) based on their level of clinical significance. Only the most severe DDIs (X) on admission were considered for this study.
2.6.3 Anticholinergic Burden
Anticholinergic drug burden is defined as the cumulative effect of drugs with anticholinergic activity that a patient is taking [ 23 ]. The Anticholinergic Cognitive Burden (ACB) Scale was utilised to ascertain the anticholinergic burden on admission in each patient [ 24 ].
2.6.4 Charlson Co-Morbidity Index
The Charlson Co-Morbidity Index (CCI) comprises 19 medical conditions that are weighted with scores ranging from 1–6, resulting in a total score range of 0–37 [ 25 ]. A pre-validated age-adjusted Microsoft Excel spreadsheet was utilised to compute the score for each patient [ 25 ].
2.6.5 Postcode
The residential postcode of the patient was used to determine the following.
a. Rurality
The Australian Bureau of Statistics Accessibility/Remoteness Index of Australia plus (ARIA+ 2016) was employed to determine the geographic location [ 26 ]. The ARIA+ classifies geographic regions into five groups: major cities, inner regional, outer regional, remote and very remote Australia. However, in Tasmania, no regions are classified as major cities based on ARIA+ 2016 [ 26 ]. Consequently, postcodes were reassigned to inner regional, outer regional, and remote/very remote Australia. The University of Sydney ARIA Lookup tool was used to generate ARIA+ classifications [ 27 ], and areas with multiple classifications were identified using the ARIA+ 2016 demonstration map [ 28 ].
b. Socio-economic status
We utilised the Socio-economic Indexes for Areas-Index of Relative Socio-economic Advantage and Disadvantage (SEIFA-IRSAD) based on the 2016 census data from the Australian Bureau of Statistics to ascertain the socio-economic status of each patient [ 29 ]. The SEIFA-IRSAD offers a concise summary of various census variables that pertain to the relative socio-economic disadvantage and/or advantage of geographical regions in Australia. These indices are assigned deciles (1 to 10) based on the patient’s residential postcode, with decile 1 representing the most socio-economically disadvantaged region and decile 10 representing the most socio-economically advantaged. We grouped these deciles into three categories: 1–3 (low socio-economic class), 4–6 (middle socio-economic class), and 7–10 (high socio-economic class) in order to achieve an approximately equal distribution of the study sample across the three groups.
2.7 Statistical Analysis
Statistical Package for Social Sciences (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, version 28.0. Armonk, NY, USA: IBM Corp.) and Microsoft Office Excel 2019 were used for data analysis. Variables were checked for normality of distribution via visual inspection of histograms. Normally distributed continuous variables were reported as mean ± standard deviation (SD), and non-normally distributed variables as median (interquartile range [IQR]). Frequency (percentage) was used to report proportions and categorical variables. A chi-square test/Student’s t -test/ANOVA/Mann-Whitney U test/Kruskal Wallis test was applied, as appropriate, to make comparisons between groups. Multiple logistic regression analysis was used to determine the predictors of ADR-related hospitalisation. A p -value < 0.05 was considered statistically significant. The collinearity was determined through collinearity diagnostics, and variables with a high variance inflation factor (VIF) (≥ 5) were excluded from the model.
Of the 7928 people admitted to hospital at least once within the study period with a primary or secondary diagnosis of dementia, 1876 (23.7%) experienced at least one ADR-related hospitalisation. Of these 1876 people, 300 patients were randomly selected as cases, while 300 patients were randomly selected as controls from the rest of the study population. The numbers of pre-existing chronic conditions and regular medicines upon presentation, and the ACB score were significantly greater for the ADR-related admission group, as detailed in Table 1 . The proportion of Australian-born individuals was significantly greater among people with ADR-related admissions, and the estimated glomerular filtration rate (eGFR) at presentation was significantly lower among people with ADR-related admissions. Nevertheless, the assessment of eGFR at the time of presentation was determined to be an unreliable measure for further analysis because the lower eGFR values observed were probably influenced by the presence of renal ADRs among individuals who had experienced ADR-related hospital admissions. Common comorbid conditions, including hypertension, diabetes, chronic kidney disease (CKD), ischaemic heart disease and cerebrovascular disease, were more prevalent in ADR-related admissions.
A total of 311 ADRs was identified among 300 patients with ADR-related hospitalisations. Table 2 and Fig. 1 represents the most common ADRs and the implicated drugs/drug class(es). The most common type of ADRs were renal (acute kidney injury; AKI) (36.0%), followed by neuropsychiatric (17.6%), cardiovascular (16.0%) and haematological (13.1%). A detailed description of the types of ADR causing or contributing to hospitalisations among people with dementia is given in Supplementary Table ST2 of the ESM. Of the total 2487 drugs being taken by the 300 patients with ADR-related hospitalisations, 399 drugs were implicated in the 311 ADRs. Diuretics, renin-angiotensin system (RAS) inhibitors and anti-thrombotics comprised the main drug classes implicated in ADR-related hospitalisations (Supplementary Table ST3 of the ESM).
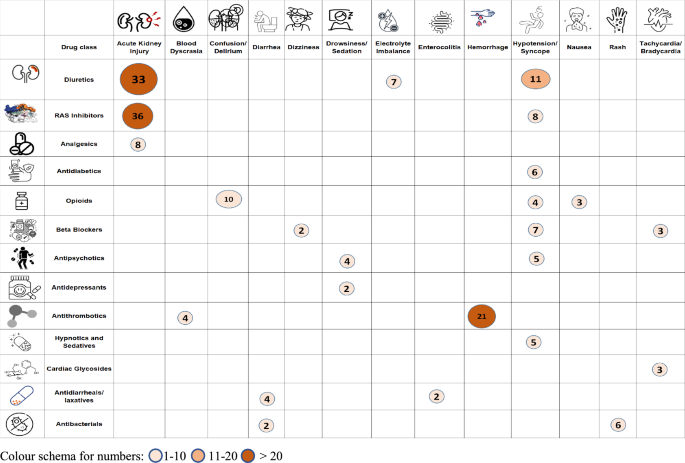
Most common ADRs causing or contributing to hospitalisations and the drug classes most frequently implicated. ADRs adverse drug reactions, RAS renin-angiotensin system
The median number of PIMs, DDIs and anticholinergic burden score among cases and controls was 1 (IQR for cases = 1–2; IQR for controls = 0–2, respectively), 0 and 1 (IQR of 1–3 for cases and 0–2 for controls), respectively. The detailed prevalence of PIMs, DDIs and anticholinergic burden among cases and controls is given in Supplementary Tables ST4-ST7 of the ESM.
Logistic regression was used to identify the predictors of ADR-related hospitalisation (Table 3 ). Four predictors were identified to be independently associated with ADR-related hospitalisation: CKD (odds ratio [OR] 8.00, 95% confidence interval [CI] 2.63–24.28, p < 0.001), Australian-born (OR 1.62, 95% CI 1.08–2.43, p = 0.019), hypertension (OR 1.48, 95% CI 1.01–2.17, p = 0.044) and the number of medicines (OR 1.06, 95% CI 1.00–1.12, p = 0.022).
4 Discussion
Our study provides valuable insights into the burden of ADR-related admissions on the health system and the patients in its care. Nearly one-quarter of those admitted to hospital at least once within the study period with a primary or secondary diagnosis of dementia, experienced at least one ADR-related hospitalisation. Older people with dementia often require multiple drugs to treat cognitive difficulties, manage behavioural and neuropsychiatric symptoms, and treat various coexisting conditions [ 30 ]. Consequently, polypharmacy is common among people with dementia [ 31 ]. In addition to the physiological changes associated with aging that alter the activity and metabolism of drugs, individuals with dementia exhibit increased sensitivity to the cognitive effects of medications [ 32 ]. Additionally, these individuals are frequently excluded from clinical trials and observational studies, resulting in a significant scarcity of high-quality data. Therefore, the lack of substantial evidence impedes the capacity to establish precise guidelines for appropriate prescribing practices [ 33 ]. All of these factors collectively contribute to a substantial increase in the risk of ADRs among individuals with dementia.
Identifying the nature of ADRs, implicated drugs and predictors of ADR-related hospitalisations among people with dementia is crucial for developing strategies to reduce harm in this cohort. Our study showed that the most common ADR was AKI associated with RAS inhibitors and diuretics. Similar findings have been reported from studies conducted among the older population [ 34 , 35 , 36 ]. Most people with dementia are elderly with chronic medical conditions such as CKD, diabetes mellitus, heart failure and cancer, who regularly take medications and are at increased risk of AKI [ 37 ]. Additionally, AKI can be exacerbated by dehydration, which is a common issue among individuals with dementia, who may lose their ability to recognise thirst [ 38 ]. To address these concerns, it is essential for patients with dementia to receive regular renal function tests and primary care reviews. During these reviews, health care professionals should engage with caregivers to discuss the risk of AKI resulting from the patients’ diminished capacity for self-care, particularly in regulating fluid intake when unwell [ 37 ].
Neuropsychiatric ADRs, including confusion and delirium, were the second most common type of ADRs among people with dementia. While long-term treatment with some CNS-acting medications may be necessary for individuals with dementia, there is abundant evidence indicating that benzodiazepines, opioids, and antipsychotics may be excessively or inappropriately used in this population [ 39 ]. Opioid-induced neurotoxicity is a complex syndrome that can manifest a range of symptoms, from mild confusion and drowsiness to more severe symptoms, such as hallucinations, delirium, and seizures [ 40 ]. Older adults, especially those with pre-existing cognitive impairment, are at a greater risk of developing confusion and delirium as a result of opioid use [ 41 ].
Cardiovascular ADRs are frequently reported in the literature [ 11 , 42 ]. This is due to a combination of drug and patient-related factors, including the impact of heart disease on drug pharmacokinetics, which can lead to reduced volume distribution and clearance in patients with congestive heart failure [ 44 , 45 ]. Individuals with dementia may experience amplified age-related changes, further increasing the risk of cardiovascular ADRs.
Haematological complaints were the fourth most common ADRs identified. Around 20% of elderly individuals with dementia are estimated to experience atrial fibrillation (AF) [ 43 ]. People with dementia are prescribed anticoagulants or aspirin to prevent stroke in patients with AF [ 44 ]. However, individuals with advanced dementia and AF receive limited clinical benefit in terms of life prolongation from anticoagulation therapy, accompanied by an increased risk of bleeding [ 43 ]. It is crucial to regularly review the need for anti-thrombotics and deprescribe according to treatment goals.
Understanding the factors that predispose people with dementia to ADRs is crucial in developing effective preventive measures. Furthermore, enhancing prescriber education by emphasising the identification of predictors for ADRs and the significance of conducting risk-benefit assessments prior to prescribing any medication is imperative for enhancing the safety of pharmacological treatments for individuals with dementia. Our study identified the presence of CKD, being Australian-born, having hypertension and the number of medications as the predictors of ADR-related hospitalisations among people with dementia.
Patients with pre-existing renal disease exhibited a higher likelihood of hospitalisation due to ADRs than patients with normal renal function (Supplementary Table ST8 of the ESM), as observed in previous studies [ 11 , 45 ]. Acute kidney injury (the most common type of ADR) was experienced by three-quarters of the individuals with ADR-associated hospitalisations who had CKD as a comorbidity (Supplementary table ST9 of the ESM). Additionally, 49% of people with comorbid CKD were prescribed at least one of the most commonly implicated drug classes (RAS inhibitors and diuretics).
In 2021 it was estimated that 15% of the Tasmanian population were individuals who were born overseas [ 46 ]. One of the predictors of ADR-related hospitalisation was being born in Australia. The most common type of ADR (AKI) and use of commonly implicated drug classes in ADRs (diuretics, RAS inhibitors and anti-thrombotics) were predominant among Australian-born individuals (Supplementary table ST10 of the ESM). The causative reasoning behind this predictor is unclear.
Hypertension is as common in people with dementia as in other populations and is as commonly treated with antihypertensive drugs [ 47 ]. Hypertension can lead to vascular changes and compromised blood flow in the brain, which can make individuals with dementia more vulnerable to the adverse effects of medications. The impaired blood supply to the brain may exacerbate the negative impact of ADRs, potentially leading to severe symptoms and hospitalisation [ 47 ]. Moreover, approximately 70% of patients with hypertension require two or more drugs to achieve their target blood pressure [ 48 ]. The use of multiple antihypertensives in elderly patients has been identified as a frequent cause of hospital admission [ 49 ].
Patients who experienced an ADR were found to be taking significantly more medications than patients without ADRs. This is consistent with findings in other studies [ 50 , 51 ]. According to estimates, individuals who take two drugs face a 13% chance of encountering an ADR. However, this risk significantly escalates to 58% and 82% when an individual takes five or seven or more drugs per day, respectively [ 52 ]. People with dementia are often subject to “prescribing cascade” [ 53 ]. There might be a greater risk of error due to more complicated regimens and also a greater chance of drug-drug and drug-disease interactions among this group of people [ 54 ]. To address these concerns and minimise the risks associated with inappropriate polypharmacy in older adults living with dementia, it is crucial to integrate medication reviews and deprescribing plans into routine care [ 55 ].
Our study showed that PIM use did not predict ADR-related hospitalisation. There is controversy regarding whether the use of PIM leads to hospital admission. Adverse drug reaction-related hospitalisation among older people can be explained by idiosyncratic response or the predisposition of these patients to develop adverse drug events, whether or not drugs are classed as PIMs [ 56 ]. Similarly, we did not demonstrate DDIs to be a predictor of ADR-related hospitalisations. In contrast to other studies conducted among the general population [ 57 , 58 ], our study only considered the most severe type of DDIs. The disparity in our findings compared to other studies can be attributed to various factors, including differences in the study population, methods employed for detecting DDIs and the type of DDIs included in the study. Our study showed that anticholinergic burden was not associated with ADR-related hospitalisation. A systematic review conducted among older population also showed that the use of anticholinergic medicines was not associated with hospitalisation [ 59 ]. Anticholinergic medications can cause a wide range of side effects, including cognitive impairment, dry mouth, constipation, blurred vision, and urinary retention. However, not all of these will lead to hospital admissions.
Our study has some limitations. We relied on administrative hospital coding and hospital datasets for the identification of ADR-related hospitalisations, which might result in the underestimation of ADRs. The ADR-related admissions are detected at a much lower rate using administrative datasets. It has been estimated that only 18%–35% of ADR-related admissions captured prospectively can be identified via administrative data sources, although these rates can be affected by the ICD codes used with this methodology [ 41 , 60 ]. Additionally, some ADRs such as falls/fractures might not have been documented in the hospital dataset because they are not always seen as ADR related. Second, information related to some variables (such as frailty status) that might be predictors of ADR-related hospitalisation, was missing for most patients and was not incorporated in the final analysis. Third, the assessment of ADRs was not done through any formal causality assessment tool. However, the concomitant use of an administrative dataset and hospital DMR, followed by consensus between the research team, allowed for a robust evaluation of ADRs and the implicated drugs. Finally, the ACB scale utilised in this study might not capture the full extent of the anticholinergic burden because anticholinergic load is cumulative, and over-the-counter (OTC) drugs, which may not have been included in the drug list, could potentially contribute to the overall burden.
Our study is the first to identify independent predictors of ADR-related hospitalisation among people with dementia over an extensive period of time. Tasmania is an ideal location for tracking hospital admissions over time. Its relative isolation makes it reasonable to expect that almost all eligible patients’ hospital admissions would occur within the Tasmanian health system and be captured in our dataset.
5 Conclusions
The most common type of ADR was renal (AKI), followed by neuropsychiatric, cardiovascular and haematologic. Diuretics, RAS inhibitors and anti-thrombotics constituted the drug classes most potentially implicated in causing ADR-related hospitalisation in patients with dementia. Four predictors associated with ADR-related hospitalisation were CKD, being Australian-born, hypertension and the number of medicines. These factors could help identify the individuals at the highest risk and enable targeted interventions to be designed.
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–9.
Article CAS PubMed Google Scholar
Wu TY, Jen MH, Bottle A, Molokhia M, Aylin P, Bell D, et al. Ten-year trends in hospital admissions for adverse drug reactions in England 1999–2009. J R Soc Med. 2010;103(6):239–50.
Article PubMed PubMed Central Google Scholar
Parameswaran Nair N, Chalmers L, Bereznicki BJ, Curtain C, Peterson GM, Connolly M, et al. Adverse drug reaction-related hospitalizations in elderly Australians: a prospective cross-sectional study in two Tasmanian hospitals. Drug Saf. 2017;40(7):597–606.
Article PubMed Google Scholar
Angamo MT, Chalmers L, Curtain CM, Bereznicki LR. Adverse drug reaction related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Saf. 2016;39(9):847–57.
Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M. World Alzheimer Report 2015-The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. 2015.
Alzheimer's Research UK. Worldwide dementia cases to triple by 2050 to over 150 million people. 2022 [cited 2023 17 March]; Available from: https://www.alzheimersresearchuk.org/worldwide-dementia-cases-to-triple-by-2050-to-over-150-million/ . Accessed 17 Mar 2023.
Australian Institute of Health and Welfare. Dementia in Australia. 2023 [cited 2023 17 March]; Available from: https://www.aihw.gov.au/reports/dementia/dementia-in-aus/contents/summary . Accessed 17 Mar 2023.
Dementia Australia. Dementia in Australia; 2023. Available from: https://www.dementia.org.au/sites/default/files/2023-03/Prevalence-Data-2023-Updates.pdf . Accessed 9 Dec 2023.
Laroche ML, Perault-Pochat MC, Ingrand I, Merle L, Kreft-Jais C, Castot-Villepelet A, et al. Adverse drug reactions in patients with Alzheimer’s disease and related dementia in France: a national multicentre cross-sectional study. Pharmacoepidemiol Drug Saf. 2013;22(9):952–60.
Zaidi AS, Peterson GM, Bereznicki LR, Curtain CM, Salahudeen M. Outcomes of medication misadventure among people with cognitive impairment or dementia: a systematic review and meta-analysis. Ann Pharmacother. 2021;55(4):530–42.
Angamo MT, Curtain CM, Chalmers L, Yilma D, Bereznicki L. Predictors of adverse drug reaction-related hospitalisation in Southwest Ethiopia: a prospective cross-sectional study. PLoS ONE. 2017;12(10): e0186631.
Australian Bureau of Statistics. Snapshot of Tasmania. 2022 [cited 2023 11 March]; Available from: https://www.abs.gov.au/articles/snapshot-tas-2021 . Accessed 11 Mar 2023.
Australian Institute of Health and Welfare. Admitted patient care NMDS 2022–23. 2023 [cited 2023 11 March]; Available from: https://meteor.aihw.gov.au/content/742173 . Accessed 11 Mar 2023.
Veeren JC, Weiss M. Trends in emergency hospital admissions in England due to adverse drug reactions: 2008–2015. J Pharm Health Serv Res. 2017;8(1):5–11.
Article Google Scholar
Walter SR, Day RO, Gallego B, Westbrook JI. The impact of serious adverse drug reactions: a population-based study of a decade of hospital admissions in New South Wales, Australia. Br J Clin Pharmacol. 2017;83(2):416–26.
Du W, Pearson SA, Buckley NA, Day C, Banks E. Diagnosis-based and external cause-based criteria to identify adverse drug reactions in hospital ICD-coded data: application to an Australia population-based study. Public Health Res Pract. 2017;27(2):e2721716.
Long JS, Long JS. Regression models for categorical and limited dependent variables. Berlin: Sage; 1997.
Google Scholar
Zaidi AS, Peterson GM, Bereznicki LRE, Curtain CM, Salahudeen MS. Ten-year trends in adverse drug reaction-related hospitalizations among people with dementia. Ther Adv Drug Saf. 2022;13:20420986221080796.
CalculatorSoup®. Random Number Generator. 2023 [cited 2023 3 April]; Available from: https://www.calculatorsoup.com/calculators/statistics/random-number-generator.php . Accessed 3 Apr 2023.
Rose S, Laan MJ. Why match? Investigating matched case-control study designs with causal effect estimation. Int J Biostat. 2009;5(1):1.
Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update Arch Intern Med. 1997;157(14):1531–6.
Lavan AH, Gallagher P, Parsons C, O’Mahony D. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): consensus validation. Age Ageing. 2017;46(4):600–7.
PubMed Google Scholar
Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15(1):31.
Campbell NL, Maidment I, Fox C, Khan B, Boustani M. The 2012 Update to the Anticholinergic Cognitive Burden Scale. J Am Geriatr Soc. 2013;61(S1):S142–3.
Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;20(4):94.
Glover JD, Tennant SK. Remote areas statistical geography in Australia: notes on the Accessibility/Remoteness Index for Australia (ARIA+ version): Public Health Information Development Unit, the University of Adelaide; 2003.
The University of Sydney. Aria lookup tool. 2023 [cited 2023 9 March]; Available from: https://www.pocog.org.au/aria/default.aspx . Accessed 9 Mar 2023.
The University of Adelaide. ARIA+ 2016 Demonstration Map. [cited 2023 11 March]; Available from: https://services.spatial.adelaide.edu.au/giscaportal/apps/webappviewer/index.html?id=417801ba9b844792af44ea4f766a3e30 . Accessed 11 Mar 2023.
Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2016. 2018 [cited 2023 8 March]; Available from: https://www.abs.gov.au/AUSSTATS/[email protected]/DetailsPage/2033.0.55.0012016?OpenDocument . Accessed 8 Mar 2023.
Eshetie TC, Nguyen TA, Gillam MH, Kalisch Ellett LM. A narrative review of problems with medicines use in people with dementia. Expert Opin Drug Saf. 2018;17(8):825–36.
Parsons C. Polypharmacy and inappropriate medication use in patients with dementia: an underresearched problem. Ther Adv Drug Saf. 2017;8(1):31–46.
Bishara D, Harwood D. Safe prescribing of physical health medication in patients with dementia. Int J Geriatr Psychiatry. 2014;29(12):1230–41.
Rollin-Sillaire A, Breuilh L, Salleron J, Bombois S, Cassagnaud P, Deramecourt V, et al. Reasons that prevent the inclusion of Alzheimer’s disease patients in clinical trials. Br J Clin Pharmacol. 2013;75(4):1089–97.
Pedrós C, Formiga F, Corbella X, Arnau JM. Adverse drug reactions leading to urgent hospital admission in an elderly population: prevalence and main features. Eur J Clin Pharmacol. 2016;72:219–26.
Schmiedl S, Rottenkolber M, Szymanski J, Drewelow B, Siegmund W, Hippius M, et al. Preventable ADRs leading to hospitalization—results of a long-term prospective safety study with 6,427 ADR cases focusing on elderly patients. Expert Opin Drug Saf. 2018;17(2):125–37.
Jennings EL, Murphy KD, Gallagher P, O’Mahony D. In-hospital adverse drug reactions in older adults; prevalence, presentation and associated drugs—a systematic review and meta-analysis. Age Ageing. 2020;49(6):948–58.
UK Renal Registry. Guidance for mental health professionals on the management of acute kidney injury; 2016. Available from: https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Guidance-for-mental-health-patients-2018.pdf . Accessed 12 May 2023.
Beales A. An innovative approach to hydration for a patient with dementia. Nurs Older People. 2017;29(4):26–9.
Meeks TW, Culberson JW, Horton MS. Medications in long-term care: when less is more. Clin Geriatr Med. 2011;27(2):171–91.
Matzo M, Dawson KA. Opioid-induced neurotoxicity. Am J Nurs. 2013;113(10):51–6.
Parameswaran Nair N, Chalmers L, Peterson GM, Bereznicki BJ, Curtain CM, Bereznicki LR. Prospective identification versus administrative coding of adverse drug reaction-related hospitalizations in the elderly: a comparative analysis. Pharmacoepidemiol Drug Saf. 2018;27(11):1281–5.
Conforti A, Costantini D, Zanetti F, Moretti U, Grezzana M, Leone R. Adverse drug reactions in older patients: an Italian observational prospective hospital study. Drug Healthc Patient Saf. 2012;4:75–80.
Ouellet GM, O’Leary JR, Leggett CG, Skinner J, Tinetti ME, Cohen AB. Benefits and harms of oral anticoagulants for atrial fibrillation in nursing home residents with advanced dementia. J Am Geriatr Soc. 2023;71(2):561–8.
Subic A, Cermakova P, Religa D, Han S, von Euler M, Kåreholt I, et al. Treatment of atrial fibrillation in patients with dementia: A cohort study from the Swedish Dementia Registry. J Alzheimers Dis. 2018;61(3):1119–28.
Article CAS PubMed PubMed Central Google Scholar
Caamaño F, Pedone C, Zuccalà G, Carbonin P. Socio-demographic factors related to the prevalence of adverse drug reaction at hospital admission in an elderly population. Arch Gerontol Geriatr. 2005;40(1):45–52.
tasmania Ph. Primary health tasmania, health needs assessment 2022–23 to 2024–2; 2022. Available from: https://www.primaryhealthtas.com.au/wp-content/uploads/2023/05/Health-in-Tasmania-Comprehensive-Needs-Assessment-2022-25.pdf . Accessed 17 Mar 2024.
Welsh TJ, Gladman JR, Gordon AL. The treatment of hypertension in people with dementia: a systematic review of observational studies. BMC Geriatr. 2014;14(1):19.
Guerrero-Garcia C, Rubio-Guerra AF. Combination therapy in the treatment of hypertension. Drugs Context. 2018;7: 212531.
Parameswaran Nair N, Chalmers L, Connolly M, Bereznicki BJ, Peterson GM, Curtain C, et al. Prediction of hospitalization due to adverse drug reactions in elderly community-dwelling patients (the PADR-EC score). PLoS ONE. 2016;11(10): e0165757.
Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS ONE. 2009;4(2): e4439.
Osanlou R, Walker L, Hughes DA, Burnside G, Pirmohamed M. Adverse drug reactions, multimorbidity and polypharmacy: a prospective analysis of 1 month of medical admissions. BMJ Open. 2022;12(7): e055551.
Zazzara MB, Palmer K, Vetrano DL, Carfi A, Onder G. Adverse drug reactions in older adults: a narrative review of the literature. Eur Geriatr Med. 2021;12(3):463–73.
Trenaman SC, Bowles SK, Kirkland S, Andrew MK. An examination of three prescribing cascades in a cohort of older adults with dementia. BMC Geriatr. 2021;21(1):297.
Pfister B, Jonsson J, Gustafsson M. Drug-related problems and medication reviews among old people with dementia. Bmc Pharmacol Toxico. 2017;18(1):52.
Sawan MJ, Moga DC, Ma MJ, Ng JC, Johnell K, Gnjidic D. The value of deprescribing in older adults with dementia: a narrative review. Expert Rev Clin Pharmacol. 2021;14(11):1367–82.
Varallo FR, Capucho HC, Planeta CdS, Mastroianni PdC. Safety assessment of potentially inappropriate medications (PIM) use in older people and the factors associated with hospital admission. J Pharm Pharm Sci. 2011;14(2):283–90.
Dechanont S, Maphanta S, Butthum B, Kongkaew C. Hospital admissions/visits associated with drug–drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2014;23(5):489–97.
Ayalew MB, Tegegn HG, Abdela OA. Drug related hospital admissions; a systematic review of the recent literatures. Bull Emerg Trauma. 2019;7(4):339.
Cardwell K, Hughes CM, Ryan C. The association between anticholinergic medication burden and health related outcomes in the ‘oldest old’: a systematic review of the literature. Drugs Aging. 2015;32:835–48.
Hohl CM, Kuramoto L, Yu E, Rogula B, Stausberg J, Sobolev B. Evaluating adverse drug event reporting in administrative data from emergency departments: a validation study. BMC Health Serv Res. 2013;13(1):473.
Download references
Acknowledgements
ASZ gratefully acknowledges the material and financial support of the University of Tasmania in the form of a Tasmania Graduate Research Scholarship
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and affiliations.
School of Pharmacy and Pharmacology, University of Tasmania, Hobart, 7001, Australia
Anum Saqib Zaidi, Gregory M. Peterson, Colin M. Curtain & Mohammed S. Salahudeen
Faculty of Health, University of Canberra, Canberra, Australia
Gregory M. Peterson
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Anum Saqib Zaidi .
Ethics declarations
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval
The study was conducted with approval from the Tasmanian Health and Medical Human Research Ethics Committee (reference number H0018582).
Consent to Participate
Not applicable.
Consent for Publication
Availability of data and material.
The data utilised in this analysis were sourced from a third party, namely the Tasmanian Department of Health and Human Services. It is important to note that these data are not publicly accessible in Australia, and the research team has not been granted permission to make them publicly available. To access the data, necessary ethics approvals must be obtained. Upon securing these approvals, the research team will be able to request access to the data in writing by contacting: The Secretary, Department of Health, GPO Box 125, Hobart, Tasmania, 7001, Australia.
Code Availability
The code of this study is available from the corresponding author upon reasonable request.
Author Contributions
Anum Saqib Zaidi: Conceptualisation; Methodology; Formal analysis; Writing – original draft. Gregory M. Peterson: Conceptualisation; Methodology; Investigation; Supervision; Visualization; Writing—review & editing. Colin M. Curtain: Conceptualisation; Methodology; Investigation; Supervision; Writing—review & editing. Mohammed S. Salahudeen: Conceptualisation; Methodology; Investigation; Supervision; Writing—review & editing. All authors read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 224 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Zaidi, A.S., Peterson, G.M., Curtain, C.M. et al. Predictors of Adverse Drug Reaction-Related Hospitalisations Among People with Dementia: A Retrospective Case-Control Study. Drug Saf (2024). https://doi.org/10.1007/s40264-024-01435-3
Download citation
Accepted : 16 April 2024
Published : 13 May 2024
DOI : https://doi.org/10.1007/s40264-024-01435-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Presentation of Case. Dr. Eugene P. Rhee: A 74-year-old man was evaluated in the nephrology clinic of this hospital because of chronic kidney disease. The patient had been in his usual state of ...
Abstract. The prevalence of chronic kidney disease and its risk factors is increasing worldwide, and the rapid rise in global need for end-stage kidney disease care is a major challenge for health systems, particularly in low- and middle-income countries. Countries are responding to the challenge of end-stage kidney disease in different ways ...
Acute Renal Failure Case Study. Our kidneys are incredible organs that get rid of toxins, retain substances needed by our bodies, and maintain the right balance of electrolytes, minerals, and water. Find out what happens to this 27-year-old when toxins accumulate in her kidneys leading to acute renal failure.
1. Background. Prevalence of chronic kidney disease (CKD) is increasing significantly and it has poor outcomes if not diagnosed and treated early in its course [].CKD is a public health issue that affects 9 to 12% of the population in the USA [2, 3].When management is early and adequate, the rate of progression to kidney failure can be slowed, comorbidities prevented, and the morbidity and ...
A 42-year-old Caucasian man was referred because of proteinuria and decreased renal function. He had developed hypertension several years earlier and was being treated with hydrochlorothiazide and telmisartan. The heart rate was 64/min, while the blood pressure was 146/90 mm Hg. The personal and family histories were negative.
Here, in part 2, we address disease complications and treatment for kidney failure. As in part 1, the case study of Anna Lowry, a 49-year-old woman with CKD, will be used for illustration, offering nurses specific guidance in helping patients to better understand and manage their CKD. (This case is a composite based on the authors' experience.)
CKD, chronic kidney disease; ESKD, end-stage kidney disease. C-W Yang et al.: Global case studies ISN public affairs Kidney International Supplements (2020) 10, e24-e48 e25
Chronic kidney disease (CKD) is a disorder in which both the kidneys may lose their capacity to function and is a long-standing and progressive, irreversible condition. ... A case study of an 82-year-old female Mrs. SD who is nondiabetic, hypertensive patient with stage 5 CRF. The patient's chief complaints were lack of appetite, vomiting ...
successful early detection of CKD, including screening and case-finding (Table 1). Table 1 - List of Pre-Requisites for Successful Early Detection of CKD 1. Document the Burden of Kidney Failure 2. Leadership from Kidney Society 3. Partnership with Government 4. National Health Insurance / Medicare 5. Method of CKD Identification: Screening or Case
The NKF states in its Kidney Disease Outcomes Quality Initiative guidelines for chronic kidney disease that there is no benefit from a protein intake higher than the RDA of 0.8 g/kg body weight and that this is a reasonable level to recommend for patients with chronic kidney disease in stages 1-3. 13 Thus, many respected nonprofit health care ...
Introduction Brown tumors are a rare focal manifestation of osteitis fibrosa cystica, which results from hyperparathyroidism. Chronic kidney failure may lead to secondary or tertiary hyperparathyroidism and thus to osteitis fibrosa cystica and brown tumors. Case presentation A 60-year-old man with a history of diabetes mellitus and chronic kidney failure presented with a 15-day history of ...
While the epidemiology and risk factors for renal disease are not well understood in Guatemala, available evidence including from the Global Burden of Disease Study suggests that, as in other LMICs, the burden of CKD is significant and rising. 1 2 WHO estimates that renal failure mortality in Guatemala in 2007 was 24.7 per 100 000 people, one ...
Day 1: A 62-year old, recently widowed male Hispanic patient, named Mr. Kevin Ulyses Blanco (K. U. B.) was brought in to the emergency department (ED) by his daughter for progressively worsening shortness of breath, fatigue, a lingering non-productive cough, and generalized edema. One month prior, he noticed dyspnea upon exertion, loss of ...
M.D. Resources. Self eval. Case #1. The patient is a 41 year-old male who has a longstanding history of hypertension and diabetes and presents with a complaint of pruritis, lethargy, lower extremity edema, nausea and emesis. He denies any other medical illnesses. On physical exam the patient is a well-developed, well-nourished male in moderate ...
The prevalence of chronic kidney disease and its risk factors is increasing worldwide, and the rapid rise in global need for end-stage kidney disease care is a major challenge for health systems, particularly in low- and middle-income countries. ... Global case studies for chronic kidney disease/end-stage kidney disease care Kidney Int Suppl ...
Outline. Mr. Stinson is a 52-year-old male with a history of HTN, DM Type II, CKD, and CHF. He presented to the Emergency Department (ED) complaining of severe itching, nausea, and vomiting. He appeared pale and is lethargic. He reported shortness of breath and the nurse notes crackles in his lungs.
Study results. During 2000-2019, the number of reported cases of kidney failure increased in the United States. The number of new cases increased by about 42% (from 92,660 to 131,422 cases). The number of existing cases more than doubled (from 358,247 to 783,594 cases). Diabetes and high blood pressure were the main causes of ESKD.
Background Chronic kidney disease (CKD) is a non-communicable disease that includes a range of different physiological disorders that are associated with abnormal renal function and progressive decline in glomerular filtration rate (GFR). This study aimed to investigate the associations of several behavioral and health-related factors with CKD in Iranian patients. Methods A hospital-based case ...
KDpredict, a new kidney failure prediction tool which also estimates mortality risk over 1-5 years, demonstrated improved predictive accuracy for end-stage renal disease development compared with the current Kidney Failure Risk Equation in this study published in the BMJ.KDpredict was developed using innovative machine learning algorithms to predict the competing endpoints in a large ...
26 At the end of follow-up in this study, one patient was known to have died, and 25% of patients progressed to kidney failure. Only 1 patient was known to have had a renal response, which was lower than that of LCDD and HCDD in our center (34% and 65%, respectively).
This study aimed to investigate the association between acute kidney injury (AKI) recovery subtypes and days alive out of hospital within the first 3 months (DAOH-90) in patients undergoing lung ...
A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump ... Drummond N, Singer A, et al. Integrating risk-based care for patients with chronic kidney disease in the community: study protocol for a cluster randomized trial. Can J Kidney Health Dis. 2019; 6:2054358119841611. doi ...
Global case studies for chronic kidney disease/end-stage kidney disease care Chih-Wei Yang1, David C.H. Harris2, Valerie A. Luyckx3,4, Masaomi Nangaku5, Fan Fan Hou6, Guillermo Garcia Garcia7, Hasan Abu-Aisha8, Abdou Niang9, Laura Sola10, Sakarn Bunnag11, Somchai Eiam-Ong12, Kriang Tungsanga13,14, Marie Richards15, Nick Richards15, Bak Leong Goh16, ...
Chronic kidney disease (CKD) is a multifactorial disease whose prevalence is increasing worldwide. CKD affects 700 million to 1 billion people worldwide, with a prevalence of 9.1% to 13.4%. In Iran, the reported prevalence of CKD is 15.14%, even higher than the global prevalence. Some studies introduced heavy metals as possible risk factors of CKD. We conducted the first study in Iran to ...
One-to-one case-control matching was performed according to the traditional risk factors for DR (i.e., age, duration of diabetes, HbA1c level, and hypertension). All cases with comorbid chronic kidney disease were excluded to eliminate confounding factors. A total of 42 pairs were successfully matched.
Chronic kidney disease (CKD) is a non-communicable disease that includes a range of different physiological disorders that are associated with abnormal renal function and progressive decline in glomerular filtration rate (GFR). This study aimed to investigate the associations of several behavioral and health-related factors with CKD in Iranian ...
In a study she conducted, 16 of 22 dogs whose leptospirosis progressed to kidney failure survived. ... Recovering from a severe case of leptospirosis still leaves dogs with long-term kidney issues ...
This retrospective case-control study analysed medical records of individuals aged ≥ 65 years with dementia admitted to major public hospitals in Tasmania, Australia, from July 2010 to July 2021. ... including hypertension, diabetes, chronic kidney disease (CKD), ischaemic heart disease and cerebrovascular disease, were more prevalent in ADR ...