To read this content please select one of the options below:
Please note you do not have access to teaching notes, developing a robust case study protocol.
Management Research Review
ISSN : 2040-8269
Article publication date: 4 July 2023
Issue publication date: 11 January 2024
Case study research has been applied across numerous fields and provides an established methodology for exploring and understanding various research contexts. This paper aims to aid in developing methodological rigor by investigating the approaches of establishing validity and reliability.

Design/methodology/approach
Based on a systematic review of relevant literature, this paper catalogs the use of validity and reliability measures within academic publications between 2008 and 2018. The review analyzes case study research across 15 peer-reviewed journals (total of 1,372 articles) and highlights the application of validity and reliability measures.
The evidence of the systematic literature review suggests that validity measures appear well established and widely reported within case study–based research articles. However, measures and test procedures related to research reliability appear underrepresented within analyzed articles.
Originality/value
As shown by the presented results, there is a need for more significant reporting of the procedures used related to research reliability. Toward this, the features of a robust case study protocol are defined and discussed.
- Case study research
- Systematic literature review
Burnard, K.J. (2024), "Developing a robust case study protocol", Management Research Review , Vol. 47 No. 2, pp. 204-225. https://doi.org/10.1108/MRR-11-2021-0821
Emerald Publishing Limited
Copyright © 2023, Emerald Publishing Limited
Related articles
All feedback is valuable.
Please share your general feedback
Report an issue or find answers to frequently asked questions
Contact Customer Support
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Cardiovasc Echogr
- v.28(3); Jul-Sep 2018

How to Write a Research Protocol: Tips and Tricks
Matteo cameli.
Department of Cardiovascular Diseases, University of Siena, Siena, Italy
Giuseppina Novo
1 Biomedical Department of Internal Medicine and Medical Specialties, Cardiology Unit, University of Palermo, Palermo, Italy
Maurizio Tusa
2 Division of Cardiology, IRCCS Policlinico San Donato, Italy
Giulia Elena Mandoli
Giovanni corrado.
3 Department of Cardiology, Valduce Hospital, Como, Italy
Frank Benedetto
4 Division of Cardiology, Bianchi-Melacrino-Morelli Hospital, Reggio Calabria, Italy
Francesco Antonini-Canterin
5 High Specialization Rehabilitation Hospital, ORAS, Motta di Livenza, Treviso, Italy
Rodolfo Citro
6 Heart Department, University Hospital “San Giovanni di Dio e Ruggi D’Aragona”, Salerno, Italy
The first drafting of the protocol for a new research project should start from a solid idea with one or more of these goals:
- Overcoming the limits of the current knowledge in a determinate field with the aim of bridging a “knowledge gap”
- Bringing something new in a scarcely explored field
- Validating or nullifying previous results obtained in limited records by studies on a wider population.
A research proposal born with the intent to convince the others that your project is worthy and you are able to manage it with a complete and specific work plan. With a strong idea in mind, it is time to write a document where all the aspects of the future research project must be explained in a precise, understandable manner. This will successively help the researcher to present it and process and elaborate the obtained results.[ 1 ] The protocol manuscript should also underline both the pros and the potentialities of the idea to put it under a new light.[ 2 ]
Our paper will give the authors suggestions and advices regarding how to organize a research protocol, step by step [ Table 1 ].
Main sections and subsections in a complete research protocol
| Main investigator |
| Name |
| Address |
| Phone/fax |
| Number of involved centers (for multi-centric studies) |
| Indicate the reference center |
| Title of the study |
| Protocol ID (acronym) |
| Keywords (up to 7 specific keywords) |
| Rationale of the study (describe current scientific evidence in support of the research with a possible sub-section for the references) |
| Study design |
| Monocentric/multicentric |
| Perspective/retrospective |
| Controlled/uncontrolled |
| Open-label/single-blinded or double-blinded |
| Randomized/nonrandomized |
| parallel branches/ overlapped branches |
| Experimental/observational |
| Others |
| Primary objective |
| Endpoints (main primary and secondary endpoints to be listed) |
| Expected results |
| Analyzed criteria |
| Main variables/endpoints of the primary analysis |
| Main variables/endpoints of the secondary analysis |
| Safety variables |
| Quality of life (if applicable) |
| Health economy (if applicable) |
| Visits and examinations |
| Therapeutic plan and goals |
| Visits/controls schedule (also with graphics) |
| Comparison to treatment products (if applicable) |
| Dose and dosage for the whole time period |
| Formulation and power of the studied drugs |
| Method of administration of the studied drugs |
| Informed consent |
| Study population |
| Short description of the main inclusion and exclusion criteria |
| Sample size |
| Estimate of the duration of the study |
| Best supposed perspective |
| Safety advisory |
| Classification needed |
| Requested funds |
| Additional features |
| On the main concept of the study |
A research protocol must start from the definition of the coordinator of the whole study: all the details of the main investigator must be reported in the first paragraph. This will allow each participant to know who ask for in case of doubts or criticalities during the research. If the study will be multicentric, in the first section must be written also the number of the involved centers, each one possibly matched with the corresponding reference investigator.
Second section: Specific features of the research study
After completing the administrative details, the next step is to provide and extend title of the study: This is made for identifying the field of research and the aim of the study itself in a sort of brief summary of the research; the title must be followed by a unique acronym, like an ID of the protocol. If the protocol has been already exposed and approved by the Ethical Committee, it is appropriate to include also protocol number.
A list of 3–7 keywords must be listed to simplify the collocation of the protocol in its field of research, including, for example, disease, research tools, and analyzed parameters (e.g. three-dimensional echocardiography, right ventricle, end-stage heart failure, and prognosis).
The protocol must continue stating the research background that is the rational cause on the base on which the study is pursued. This section is written to answer some of these questions: what is the project about? What is already available in this field in the current knowledge? Why we need to overcome that data? and How will the community will from the present study?
As for an original research manuscript, the introduction to the project must include a brief review of the literature (with corresponding references). It is also fundamental to support the premises of the study, to underline the importance of the project in that particular time period and above all, of the materials and methods that will be employed. The rationale should accurately put in evidence the current lack in that field of scientific knowledge, following a precise, logical thread with concrete solutions regarding how to overcome the gaps and to conclude with the hypothesis of the project. A distinct paragraph can be dedicated to references, paying attention to select only the previous papers that can help the reader to focus the attention on the topic and to not excessively extend the list. In the references paragraph, the main studies regarding the object of the research but also state-of-art reviews updating the most recent discoveries in the field should be inserted.
The section should successively expose the study design: monocentric or multicentric, retrospective or prospective, controlled or uncontrolled, open-label or blinded, randomized or nonrandomized, and observational or experimental. It should also be explained why that particular design has been chosen.
At this point, the author must include the primary objective of the research, that is, the main goal of the study. This is a crucial part of the proposal and more than 4–5 aims should be avoided to do not reduce the accuracy of the project. Using verbs as “to demonstrate,” “to assess,” “to verify,” “to improve,” “to reduce,” and “to compare” help to give relevance to this section. Add also a description of the general characteristics of the population that will be enrolled in the study (if different subgroups are planned, the criteria on the base of which they will be divided should be specified); primary and secondary end-points, including all the variables that represent the measure of the objective (e.g., all-cause death, cardiovascular death, hospitalization, and side effects of a drug) follow in this section.
All the single parameters and variables that will be assessed during the study must be accurately and precisely listed along with the tools, the methods, the process schedule timing, and the technical details by which they will be acquired; Here, the author should explain how the Investigators who work in the other involved centers have to sent their results and acquired data to the Core Laboratory (e.g. by filled databases or by sending images).
A special attention must then be paid to clarify the planning of each examination the study patients will undergo: basal evaluation, potential follow-up schedule, treatment strategy plan, comparison between new and already-in-use drugs, dose and dosage of the treatment in case of a pharmacological study. This part can be enhanced by flowcharts or algorithms that allow a more immediate comprehension and interpretation of the study strategy.
This section may result more complete if one more subsection, illustrating the expected results, is included. Considering the idea at the base on the project, the endpoints and the pre-arranged objectives, the author can explain how its research project will
- Contribute to optimize the scientific knowledge in that specific field
- Give real successive implications in clinical practice
- Pave the way for future scientific research in the same or similar area of interest, etc.
The study population must be specified in detail, starting from inclusion criteria (including age and gender if it is planned to be restricted) and exclusion criteria: the more precise are the lists, the more accurate the enrollment of the subjects will be to avoid selection biases. This will also help to raise the success rate of the project and to reduce the risks of statistical error during the successive analysis of the data. The sample size should be planned and justified on the base of a statistic calculation considering the incidence and prevalence of the disease, frequency of use of a drug, etc., and possibly also indicating if the study considers a minimal or maximal number of subjects for each enrollment center (in case of multicentric studies).
This section of the protocol should end with some indications regarding timing and duration of the study: Starting and end of enrollment date, starting and end of inclusion date, potential frequency of control examinations, and timing of the analysis of the acquired data. If already settled, it can be useful to indicate also the type of statistical analysis that the investigators will apply to the data.
It is always necessary to prepare an informed consent to be proposed to the patient where premises, methods, and aims of the research together with advantages (e.g., some visits or diagnostic examinations for free) and possible risks derived from the participation to the study.
In this short section, various pieces of information regarding safety of the study must be added (a classification is fundamental in case of studies that expect the use of invasive procedures or drugs use). Usually, for nonobservational studies, an insurance coverage must be considered.
If the investigators have requested or plan to request funding or financial support, all the obtained resources must be listed to avoid conflicts of interest.
C ONCLUSION
Writing a complete and detailed document is a paramount step before starting a research projects. The protocol, as described in this paper, should be simply and correctly written but must clarify all the aspects of the protocol. The document could be divided into three different sessions to give all the parts the appropriate attention.
R EFERENCES
- Search Menu
- Sign in through your institution
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- Reasons to Submit
- About Journal of Surgical Protocols and Research Methodologies
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, contents of a research study protocol, conflict of interest statement, how to write a research study protocol.
- Article contents
- Figures & tables
- Supplementary Data
Julien Al Shakarchi, How to write a research study protocol, Journal of Surgical Protocols and Research Methodologies , Volume 2022, Issue 1, January 2022, snab008, https://doi.org/10.1093/jsprm/snab008
- Permissions Icon Permissions
A study protocol is an important document that specifies the research plan for a clinical study. Many funders such as the NHS Health Research Authority encourage researchers to publish their study protocols to create a record of the methodology and reduce duplication of research effort. In this paper, we will describe how to write a research study protocol.
A study protocol is an essential part of a research project. It describes the study in detail to allow all members of the team to know and adhere to the steps of the methodology. Most funders, such as the NHS Health Research Authority in the United Kingdom, encourage researchers to publish their study protocols to create a record of the methodology, help with publication of the study and reduce duplication of research effort. In this paper, we will explain how to write a research protocol by describing what should be included.
Introduction
The introduction is vital in setting the need for the planned research and the context of the current evidence. It should be supported by a background to the topic with appropriate references to the literature. A thorough review of the available evidence is expected to document the need for the planned research. This should be followed by a brief description of the study and the target population. A clear explanation for the rationale of the project is also expected to describe the research question and justify the need of the study.
Methods and analysis
A suitable study design and methodology should be chosen to reflect the aims of the research. This section should explain the study design: single centre or multicentre, retrospective or prospective, controlled or uncontrolled, randomised or not, and observational or experimental. Efforts should be made to explain why that particular design has been chosen. The studied population should be clearly defined with inclusion and exclusion criteria. These criteria will define the characteristics of the population the study is proposing to investigate and therefore outline the applicability to the reader. The size of the sample should be calculated with a power calculation if possible.
The protocol should describe the screening process about how, when and where patients will be recruited in the process. In the setting of a multicentre study, each participating unit should adhere to the same recruiting model or the differences should be described in the protocol. Informed consent must be obtained prior to any individual participating in the study. The protocol should fully describe the process of gaining informed consent that should include a patient information sheet and assessment of his or her capacity.
The intervention should be described in sufficient detail to allow an external individual or group to replicate the study. The differences in any changes of routine care should be explained. The primary and secondary outcomes should be clearly defined and an explanation of their clinical relevance is recommended. Data collection methods should be described in detail as well as where the data will be kept secured. Analysis of the data should be explained with clear statistical methods. There should also be plans on how any reported adverse events and other unintended effects of trial interventions or trial conduct will be reported, collected and managed.
Ethics and dissemination
A clear explanation of the risk and benefits to the participants should be included as well as addressing any specific ethical considerations. The protocol should clearly state the approvals the research has gained and the minimum expected would be ethical and local research approvals. For multicentre studies, the protocol should also include a statement of how the protocol is in line with requirements to gain approval to conduct the study at each proposed sites.
It is essential to comment on how personal information about potential and enrolled participants will be collected, shared and maintained in order to protect confidentiality. This part of the protocol should also state who owns the data arising from the study and for how long the data will be stored. It should explain that on completion of the study, the data will be analysed and a final study report will be written. We would advise to explain if there are any plans to notify the participants of the outcome of the study, either by provision of the publication or via another form of communication.
The authorship of any publication should have transparent and fair criteria, which should be described in this section of the protocol. By doing so, it will resolve any issues arising at the publication stage.
Funding statement
It is important to explain who are the sponsors and funders of the study. It should clarify the involvement and potential influence of any party. The sponsor is defined as the institution or organisation assuming overall responsibility for the study. Identification of the study sponsor provides transparency and accountability. The protocol should explicitly outline the roles and responsibilities of any funder(s) in study design, data analysis and interpretation, manuscript writing and dissemination of results. Any competing interests of the investigators should also be stated in this section.
A study protocol is an important document that specifies the research plan for a clinical study. It should be written in detail and researchers should aim to publish their study protocols as it is encouraged by many funders. The spirit 2013 statement provides a useful checklist on what should be included in a research protocol [ 1 ]. In this paper, we have explained a straightforward approach to writing a research study protocol.
None declared.
Chan A-W , Tetzlaff JM , Gøtzsche PC , Altman DG , Mann H , Berlin J , et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials . BMJ 2013 ; 346 : e7586 .
Google Scholar
- conflict of interest
- national health service (uk)
| Month: | Total Views: |
|---|---|
| January 2022 | 201 |
| February 2022 | 123 |
| March 2022 | 271 |
| April 2022 | 354 |
| May 2022 | 429 |
| June 2022 | 380 |
| July 2022 | 405 |
| August 2022 | 560 |
| September 2022 | 539 |
| October 2022 | 690 |
| November 2022 | 671 |
| December 2022 | 609 |
| January 2023 | 872 |
| February 2023 | 1,165 |
| March 2023 | 1,277 |
| April 2023 | 1,183 |
| May 2023 | 1,292 |
| June 2023 | 1,117 |
| July 2023 | 1,040 |
| August 2023 | 936 |
| September 2023 | 941 |
| October 2023 | 1,028 |
| November 2023 | 993 |
| December 2023 | 910 |
| January 2024 | 1,251 |
| February 2024 | 1,051 |
| March 2024 | 1,411 |
| April 2024 | 991 |
| May 2024 | 895 |
| June 2024 | 677 |
Email alerts
Citing articles via.
- Advertising and Corporate Services
- Journals Career Network
- JSPRM Twitter
Affiliations
- Online ISSN 2752-616X
- Copyright © 2024 Oxford University Press and JSCR Publishing Ltd
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- No results found
THE CASE STUDY PROTOCOL
Research methodology.
The case study protocol defines the procedures and general rules to be followed using the protocol which is different from a survey questionnaire” (Yin, 2009, p. 79). The case study protocol and a survey questionnaire are both directed at a single data point, whether it’s a single case or a single respondent (Yin, 2009). A case study protocol is always needed when
performing a multiple-case study (Yin, 2009). The protocol is a major way of increasing the
reliability of case study research and is intended to guide the researcher in carrying out data
collection from a single case (Yin, 2009). A case study protocol should have at least the following sections (Yin, 2009, p. 81):
1. Overview of the case study project (project objectives and auspices, case study issues, and relevant readings about the topic being investigated).
2. Field procedures (presentation of credentials, access to the case study “sites”, language pertaining to the protection of human subjects, sources of data, and procedural reminders).
3. Field procedures (the specific questions that the case study must keep in mind in collecting data, “table shells” for specific arrays of data, and the potential sources of information for answering each question …).
4. Investigator guide for the case study report (outline, format of the data, use and presentation of other documentation, and bibliographical information).
The importance of the protocol helps the researcher to remain focused on the topic and problem areas. This intuitive knowledge of the context and perspective will guide the researcher in the search for supporting information. By writing an overview of the case study, the researcher allows potential knowledge seeker to capitalize on the products of the case study and understand beforehand, the intent and depth of the case study research. There are also potential guidelines for field procedure. A researcher’s “field procedure of the protocol need to emphasize the major tasks in collecting data, including gaining access to key organizations or interviewees” (Yin, 2009, p. 85):
1. Having sufficient resources while in the field – including a personal computer, writing instruments, paper, paper clips, and a pre-established, quiet place to write notes privately.
2. Developing a procedure for calling for assistance and guidance, if needed, from other case study investigators or colleagues.
3. Making a clear schedule of the data collection activates that are expected to be completed within specified periods of time.
4. Providing for unanticipated events, including changes in the availability of interviewees as well as changes in the mood and motivation of the case study investigator.
“The heart of the protocol is a set of substantive questions reflecting your actual line of inquiry” (Yin, 2009, p. 86). Each question should be “posed to you, the investigator, not to an
interviewee” and linked to a source of evidence (Yin, 2009, p. 86). Each question of this protocol should reflect a specific type/level potentially categorized by Yin’s five levels of questions below (Yin, 2009, p. 86):
1. Level 1: question asked of specific interviewees.
2. Level 2: questions asked of the individual case (these are the questions in the case study protocol to be answered by the investigator during a single case, even when the single case is part of a larger, multiple-case study).
3. Level 3: questions asked of the pattern of findings across multiple cases.
4. Level 4: questions asked of the entire study – for example, calling the information beyond the case study evidence and including other literature or published data that may have been reviewed.
5. Level 5: normative questions about policy recommendations and conclusions, going beyond the narrow scope of study.
“The questions should cater to the unit of analysis of the case study, which may be at a different level from the unit of data collection of the case study” (Yin, 2009, p. 88). “The common
confusion begins because the data collection sources may be individual people (e.g., interviews with individuals), whereas the unit of analysis of your case study may be a collective (e.g., the organization to which the individual belongs) - a frequent design when the case is about the organization, community, or social group” (Yin, 2009, p. 88). Table 6 below illustrates design verses data collection using different units of analysis:
Individual behavior Individual attitudes Individual perceptions
Individual employee records Interview with individual’s supervisor; other employees
How organization works
Why organization works Personnel policiesOrganization outcomes
Abo ut a n indi vidua l Abo ut a n or ga ni za tion De sig n
From an individual From an organization
Data Collection Source
Table 6: Design verses Data Collected
Table 6 above, Design verses Data Collection, helps the researcher to identify exactly what data is desired and ensures parallel information is collected from different sites as during a multiple case study (Yin, 2009, p. 89). The researcher should include an outline in the protocol to guide
in the collection, presentation, and formatting of data (Yin, 2009). This rigor allows other researchers to follow the case (Yin, 2009). The researcher may choose a pilot case to discover unforeseen issues or challenges (Yin, 2009). The protocol helps align the researcher’s data collection efforts.
The case study protocol defines the procedures and general rules to be followed using the protocol (Yin, 2009). Yin (2009) reminds the researcher that the protocol is a major way of increasing the reliability of case study research and is intended to guide the researcher in carrying out data collection from a single case. The case study protocol should contain at minimum the following sections (Yin, 2009): (1) Overview of the case study project; (2) Field procedures (credentials); (3) Field procedures (questions); and (4) a form of investigator guide for the case study report. The importance of the protocol helps the researcher to remain focused on the topic and problem areas. Design verses Data Collection helps the researcher to identify exactly what data is desired and ensures parallel information is collected from different (Yin, 2009). The case study protocol is used in the collection of case study evidence as described in the next section.
- PROJECT MANAGEMENT SYSTEMS (PMS)
- PMS: PMBOK PERSPECTIVE
- CYBERNETICS: PRELUDE TO THE VSM
- VIABLE SYSTEM MODEL (VSM)
- ORIGINS OF THE VSM
- SYSTEM FOUR
- CASE STUDY RESEARCH
- COMPONENTS OF RESEARCH DESIGN
- CASE STUDY DESIGNS
- THE CASE STUDY PROTOCOL (You are here)
- COLLECTING CASE STUDY EVIDENCE
- FRAMEWORK DEVELOPMENT
- DATA COLLECTION STRATEGIES
- ROLE OF THE RESEARCHER
- PROJECT Q: A CASE STUDY
Related documents
Study protocol
Publishing study protocols in BMC Public Health is part of our commitment to improving research standards by promoting transparency, reducing publication bias, and enhancing the reproducibility of study design and analysis. We consider study protocols for proposed or ongoing prospective clinical research that provide a detailed account of the hypothesis, rationale and methodology of the study, and the associated ethical requirements. By publishing your protocol with us, it becomes a fully citable open-access article.
We evaluate study protocol submissions on a case-by-case basis and consider only those for proposed or ongoing studies that have not completed participant recruitment at the time of submission. We encourage authors to submit their study protocols well in advance of participant recruitment completion and confirm the study status within the cover letter.
Study protocols for pilot or feasibility studies are not considered, and authors are encouraged to submit the pilot results as a research article and the study protocol for the definitive study. Additionally, we may not consider study protocols where authors have other articles published or under consideration relating to the same protocol. Please note that study protocols for systematic reviews are not considered by the BMC Series journals.
If a proposed study protocol has obtained formal ethical approval and undergone independent peer-review from a major funding body, it will usually be considered for publication without further peer-review. Please ensure the Declarations section in your manuscript outlines the funding information, including whether it was peer reviewed and information about ethical approval. In some cases, the Editor may request to see the peer-review reports from the funder and/or may send the protocol for additional peer review.
Please note that study protocols without major external funding or ethics approval may not be considered for publication.
Protocols of randomized controlled trials should follow the SPIRIT guidelines and must have a trial registration number included in the last line of the abstract, as described in our editorial policies. Please provide a completed SPIRIT checklist as a supplementary file when submitting your protocol.
When conducting peer-review of study protocols, the intention is not to change the study design. Instead, we ask our reviewers to evaluate and report on the study's adequacy in testing the hypothesis, whether there is sufficient detail for replication or comparison, the appropriateness of the planned statistical analysis, and the acceptability of the writing.
The final decision on whether to consider a study protocol for publication will rest with the Editor, and appeals will not be considered.
To ensure a smooth submission process, please take note of the following before submitting:
- Confirm the status of your study within the cover letter.
- Ensure that the Declarations section is complete and contains ethical approval and funding information.
- For protocols describing clinical trials, provide a completed copy of the SPIRIT checklist as a supplementary file.
Professionally produced Visual Abstracts
BMC Public Health will consider visual abstracts. As an author submitting to the journal, you may wish to make use of services provided at Springer Nature for high quality and affordable visual abstracts where you are entitled to a 20% discount. Click here to find out more about the service, and your discount will be automatically be applied when using this link.
What we are looking for
The journal considers protocols for ongoing or proposed large-scale, prospective studies related to public health and public health management activities and submissions should provide a detailed account of the hypothesis, rationale and methodology of the study. We are particularly interested in protocols for public health interventions aimed at improving health outcomes of populations.
Protocols for clinical research will not be considered and should be submitted to the relevant BMC Series medical journal.
Preparing your manuscript
The information below details the section headings that you should include in your manuscript and what information should be within each section.
Please note that your manuscript must include a 'Declarations' section including all of the subheadings (please see below for more information).
The title page should:
- "A versus B in the treatment of C: a randomized controlled trial", "X is a risk factor for Y: a case control study", "What is the impact of factor X on subject Y: A systematic review"
- or for non-clinical or non-research studies: a description of what the article reports
- if a collaboration group should be listed as an author, please list the Group name as an author. If you would like the names of the individual members of the Group to be searchable through their individual PubMed records, please include this information in the “Acknowledgements” section in accordance with the instructions below
- Large Language Models (LLMs), such as ChatGPT , do not currently satisfy our authorship criteria . Notably an attribution of authorship carries with it accountability for the work, which cannot be effectively applied to LLMs. Use of an LLM should be properly documented in the Methods section (and if a Methods section is not available, in a suitable alternative part) of the manuscript
- indicate the corresponding author
The Abstract should not exceed 350 words. Please minimize the use of abbreviations and do not cite references in the abstract. The abstract must include the following separate sections:
- Background: the context and purpose of the study
- Methods: how the study will be performed
- Discussion: a brief summary and potential implications
- Trial registration: If your article reports the results of a health care intervention on human participants, it must be registered in an appropriate registry and the registration number and date of registration should be in stated in this section. If it was not registered prospectively (before enrollment of the first participant), you should include the words 'retrospectively registered'. See our editorial policies for more information on trial registration
Three to ten keywords representing the main content of the article.
The Background section should explain the background to the study, its aims, a summary of the existing literature and why this study is necessary or its contribution to the field.
Methods/Design
The methods section should include:
- the aim, design and setting of the study
- the characteristics of participants or description of materials
- a clear description of all processes, interventions and comparisons. Generic drug names should generally be used. When proprietary brands are used in research, include the brand names in parentheses
- the type of statistical analysis used, including a power calculation if appropriate.
This should include a discussion of any practical or operational issues involved in performing the study and any issues not covered in other sections.
List of abbreviations
If abbreviations are used in the text they should be defined in the text at first use, and a list of abbreviations should be provided.
Declarations
All manuscripts must contain the following sections under the heading 'Declarations':
Ethics approval and consent to participate
Consent for publication, availability of data and materials, competing interests, authors' contributions, acknowledgements.
- Authors' information (optional)
Please see below for details on the information to be included in these sections.
If any of the sections are not relevant to your manuscript, please include the heading and write 'Not applicable' for that section.
Manuscripts reporting studies involving human participants, human data or human tissue must:
- include a statement on ethics approval and consent (even where the need for approval was waived)
- include the name of the ethics committee that approved the study and the committee’s reference number if appropriate
Studies involving animals must include a statement on ethics approval and for experimental studies involving client-owned animals, authors must also include a statement on informed consent from the client or owner.
See our editorial policies for more information.
If your manuscript does not report on or involve the use of any animal or human data or tissue, please state “Not applicable” in this section.
If your manuscript contains any individual person’s data in any form (including any individual details, images or videos), consent for publication must be obtained from that person, or in the case of children, their parent or legal guardian. All presentations of case reports must have consent for publication.
You can use your institutional consent form or our consent form if you prefer. You should not send the form to us on submission, but we may request to see a copy at any stage (including after publication).
See our editorial policies for more information on consent for publication.
If your manuscript does not contain data from any individual person, please state “Not applicable” in this section.
All manuscripts must include an ‘Availability of data and materials’ statement. Data availability statements should include information on where data supporting the results reported in the article can be found including, where applicable, hyperlinks to publicly archived datasets analysed or generated during the study. By data we mean the minimal dataset that would be necessary to interpret, replicate and build upon the findings reported in the article. We recognise it is not always possible to share research data publicly, for instance when individual privacy could be compromised, and in such instances data availability should still be stated in the manuscript along with any conditions for access.
Authors are also encouraged to preserve search strings on searchRxiv https://searchrxiv.org/ , an archive to support researchers to report, store and share their searches consistently and to enable them to review and re-use existing searches. searchRxiv enables researchers to obtain a digital object identifier (DOI) for their search, allowing it to be cited.
Data availability statements can take one of the following forms (or a combination of more than one if required for multiple datasets):
- The datasets generated and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]
- The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
- All data generated or analysed during this study are included in this published article [and its supplementary information files].
- The datasets generated and/or analysed during the current study are not publicly available due [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
- Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
- The data that support the findings of this study are available from [third party name] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [third party name].
- Not applicable. If your manuscript does not contain any data, please state 'Not applicable' in this section.
More examples of template data availability statements, which include examples of openly available and restricted access datasets, are available here .
BioMed Central strongly encourages the citation of any publicly available data on which the conclusions of the paper rely in the manuscript. Data citations should include a persistent identifier (such as a DOI) and should ideally be included in the reference list. Citations of datasets, when they appear in the reference list, should include the minimum information recommended by DataCite and follow journal style. Dataset identifiers including DOIs should be expressed as full URLs. For example:
Hao Z, AghaKouchak A, Nakhjiri N, Farahmand A. Global integrated drought monitoring and prediction system (GIDMaPS) data sets. figshare. 2014. http://dx.doi.org/10.6084/m9.figshare.853801
With the corresponding text in the Availability of data and materials statement:
The datasets generated during and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]. [Reference number]
If you wish to co-submit a data note describing your data to be published in BMC Research Notes , you can do so by visiting our submission portal . Data notes support open data and help authors to comply with funder policies on data sharing. Co-published data notes will be linked to the research article the data support ( example ).
All financial and non-financial competing interests must be declared in this section.
See our editorial policies for a full explanation of competing interests. If you are unsure whether you or any of your co-authors have a competing interest please contact the editorial office.
Please use the authors initials to refer to each authors' competing interests in this section.
If you do not have any competing interests, please state "The authors declare that they have no competing interests" in this section.
All sources of funding for the research reported should be declared. If the funder has a specific role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript, this should be declared.
The individual contributions of authors to the manuscript should be specified in this section. Guidance and criteria for authorship can be found in our editorial policies .
Please use initials to refer to each author's contribution in this section, for example: "FC analyzed and interpreted the patient data regarding the hematological disease and the transplant. RH performed the histological examination of the kidney, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript."
Please acknowledge anyone who contributed towards the article who does not meet the criteria for authorship including anyone who provided professional writing services or materials.
Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements section.
See our editorial policies for a full explanation of acknowledgements and authorship criteria.
If you do not have anyone to acknowledge, please write "Not applicable" in this section.
Group authorship (for manuscripts involving a collaboration group): if you would like the names of the individual members of a collaboration Group to be searchable through their individual PubMed records, please ensure that the title of the collaboration Group is included on the title page and in the submission system and also include collaborating author names as the last paragraph of the “Acknowledgements” section. Please add authors in the format First Name, Middle initial(s) (optional), Last Name. You can add institution or country information for each author if you wish, but this should be consistent across all authors.
Please note that individual names may not be present in the PubMed record at the time a published article is initially included in PubMed as it takes PubMed additional time to code this information.
Authors' information
This section is optional.
You may choose to use this section to include any relevant information about the author(s) that may aid the reader's interpretation of the article, and understand the standpoint of the author(s). This may include details about the authors' qualifications, current positions they hold at institutions or societies, or any other relevant background information. Please refer to authors using their initials. Note this section should not be used to describe any competing interests.
Footnotes can be used to give additional information, which may include the citation of a reference included in the reference list. They should not consist solely of a reference citation, and they should never include the bibliographic details of a reference. They should also not contain any figures or tables.
Footnotes to the text are numbered consecutively; those to tables should be indicated by superscript lower-case letters (or asterisks for significance values and other statistical data). Footnotes to the title or the authors of the article are not given reference symbols.
Always use footnotes instead of endnotes.
Examples of the Vancouver reference style are shown below.
See our editorial policies for author guidance on good citation practice
Web links and URLs: All web links and URLs, including links to the authors' own websites, should be given a reference number and included in the reference list rather than within the text of the manuscript. They should be provided in full, including both the title of the site and the URL, as well as the date the site was accessed, in the following format: The Mouse Tumor Biology Database. http://tumor.informatics.jax.org/mtbwi/index.do . Accessed 20 May 2013. If an author or group of authors can clearly be associated with a web link, such as for weblogs, then they should be included in the reference.
Example reference style:
Article within a journal
Smith JJ. The world of science. Am J Sci. 1999;36:234-5.
Article within a journal (no page numbers)
Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjønneland A, et al. Meat consumption and mortality - results from the European Prospective Investigation into Cancer and Nutrition. BMC Medicine. 2013;11:63.
Article within a journal by DOI
Slifka MK, Whitton JL. Clinical implications of dysregulated cytokine production. Dig J Mol Med. 2000; doi:10.1007/s801090000086.
Article within a journal supplement
Frumin AM, Nussbaum J, Esposito M. Functional asplenia: demonstration of splenic activity by bone marrow scan. Blood 1979;59 Suppl 1:26-32.
Book chapter, or an article within a book
Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. In: Bourne GH, Danielli JF, Jeon KW, editors. International review of cytology. London: Academic; 1980. p. 251-306.
OnlineFirst chapter in a series (without a volume designation but with a DOI)
Saito Y, Hyuga H. Rate equation approaches to amplification of enantiomeric excess and chiral symmetry breaking. Top Curr Chem. 2007. doi:10.1007/128_2006_108.
Complete book, authored
Blenkinsopp A, Paxton P. Symptoms in the pharmacy: a guide to the management of common illness. 3rd ed. Oxford: Blackwell Science; 1998.
Online document
Doe J. Title of subordinate document. In: The dictionary of substances and their effects. Royal Society of Chemistry. 1999. http://www.rsc.org/dose/title of subordinate document. Accessed 15 Jan 1999.
Online database
Healthwise Knowledgebase. US Pharmacopeia, Rockville. 1998. http://www.healthwise.org. Accessed 21 Sept 1998.
Supplementary material/private homepage
Doe J. Title of supplementary material. 2000. http://www.privatehomepage.com. Accessed 22 Feb 2000.
University site
Doe, J: Title of preprint. http://www.uni-heidelberg.de/mydata.html (1999). Accessed 25 Dec 1999.
Doe, J: Trivial HTTP, RFC2169. ftp://ftp.isi.edu/in-notes/rfc2169.txt (1999). Accessed 12 Nov 1999.
Organization site
ISSN International Centre: The ISSN register. http://www.issn.org (2006). Accessed 20 Feb 2007.
Dataset with persistent identifier
Zheng L-Y, Guo X-S, He B, Sun L-J, Peng Y, Dong S-S, et al. Genome data from sweet and grain sorghum (Sorghum bicolor). GigaScience Database. 2011. http://dx.doi.org/10.5524/100012 .
Figures, tables and additional files
See General formatting guidelines for information on how to format figures, tables and additional files.
Submit manuscript
Important information
Editorial board
For authors
For editorial board members
For reviewers
- Manuscript editing services
Annual Journal Metrics
2022 Citation Impact 4.5 - 2-year Impact Factor 4.7 - 5-year Impact Factor 1.661 - SNIP (Source Normalized Impact per Paper) 1.307 - SJR (SCImago Journal Rank)
2023 Speed 32 days submission to first editorial decision for all manuscripts (Median) 173 days submission to accept (Median)
2023 Usage 24,332,405 downloads 24,308 Altmetric mentions
- More about our metrics
Peer-review Terminology
The following summary describes the peer review process for this journal:
Identity transparency: Single anonymized
Reviewer interacts with: Editor
Review information published: Review reports. Reviewer Identities reviewer opt in. Author/reviewer communication
More information is available here
- Follow us on Twitter
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
- Corpus ID: 39692556
Designing a Case Study Protocol for Application in IS Research
- Hilangwa Maimbo , G. Pervan
- Published in Pacific Asia Conference on… 2005
- Computer Science
Figures and Tables from this paper

87 Citations
Guidelines for conducting and reporting case study research in software engineering, designing a case study template for theory building, integrating process inquiry and the case method in the study of is failure, an interpretive approach for data collection and analysis, exploratory case study research: outsourced project failure, using interpretive qualitative case studies for exploratory research in doctoral studies: a case of information systems research in small and medium enterprises, use of a quality monitoring methodology and platform in industrial software development – a feasibility study, it project portfolio assessment criteria: development and validation of a reference model, product derivation in practice, case studies research in the bioeconomy: a systematic literature review, 19 references, case study research: a multi‐faceted research approach for is.
- Highly Influential
Using case study research to build theories of IT implementation
Using a positivist case study methodology to build and test theories in information systems : illustrations from four exemplary studies, case study research: design and methods, a paradigmatic and methodological examination of information systems research from 1991 to 2001, research methods for business : a skill building approach (5th edition), combining is research methods: towards a pluralist methodology, on the use of construct reliability in mis research: a meta-analysis, is/it investment and organisational performance in the financial services sector: a credit union case study, developing an historical tradition in mis research, related papers.
Showing 1 through 3 of 0 Related Papers

Alternative Quality Management Systems for Highway Construction (2015)
Chapter: appendix c: case study protocol.
Below is the uncorrected machine-read text of this chapter, intended to provide our own search engines and external engines with highly rich, chapter-representative searchable text of each book. Because it is UNCORRECTED material, please consider the following text as a useful but insufficient proxy for the authoritative book pages.
APPENDIX C: CASE STUDY PROTOCOL C.1 Overview of Case Study Background Information Delivering highway projects using alternative project delivery methods demands a shift in the traditional agency quality assurance (QA) and quality control (QC) programs to accommodate the faster pace of design and construction as well as the redistribution of responsibilities among project stakeholders. Thus, the objectives of this research are to: ï§ Identify and understand alternative quality management systems ï§ Develop guidelines for their use in highway construction projects Alternative project delivery in highway construction often requires the application of alternative quality management systems that emphasize contractor quality control and quality assurance. These new systems allow owners to have confidence through a verification of contractor quality system processes. They also permit state transportation agencies (STAs) to satisfy due diligence requirements for federal- aid highway projects. For example, the International Organization for Standardization (ISO) 9001 quality management system regulates quality management at all levels from material suppliers through the contractors to owners. It requires a formal project performance evaluation after completion and uses that information to publish contractor performance ratings, which can then be used for future contractor prequalification. The U. S. Army Corps of Engineersâ quality management system relies on detailed guide specifications and rigorous on-site testing by contractors. The Corps has used alternative project delivery on a routine basis for over thirty years on a wide variety of heavy civil projects that include roads and bridges, and as a result, furnishes an excellent analog from which to draw lessons learned and best practices that apply to highway design and construction. Research is needed to provide guidance on the use of alternative quality management systems for highway construction projects using alternative delivery methods. This research needs to address the major quality issues associated with these methods including accelerated project timelines and the change of the designer-of-recordâs (DOR) contractual relationships with the owner to permit the required level of integration with the construction contractor. Issues of quality are further complicated by the addition of private funding of public projects in public-private partnerships (PPP). On these projects, an argument can be made that since the concessionaire is at risk for project performance the public agency has few if any reasons to involve itself in the quality management process. From current and past projects, there exists a limited but rapidly expanding body of experience associated with alternate methods of assuring quality. The purpose of this research is to bring together this relatively new body of experience and summarize it in one easily accessible reference treating the subject of QA in alternative projects. The case studies for this research will be used to learn how existing projects have managed quality in light of non-traditional delivery methods. After analyzing and comparing the various case studies, the information gathered will be condensed into working theories and used to modify what the authors of this research are calling the Integrated Quality Management Model (IQ2M) shown in Figure C1. The model was developed to be generic to all forms of project delivery and furnish a foundation for assigning quality management responsibilities between the owner, the designer, and the constructor. As such, it acts as a framework to structure the analysis of other 167
alternative project delivery quality systems that are common in highway construction and will be used in that manner in this research. Figure C1 â Integrated Quality Management Model (IQ2M) (adapted from Synthesis 376 (Gransberg and Molenaar 2008)) Relevant Definitions Across the highway construction and engineering industry, terms relating to quality often have multiple meanings that in some cases overlap with one another and in others supersede each other. To prevent confusion among several vital terms important to this study, the following definitions have been provided. These definitions are in accordance with the most recent issuance of the TRB Circular Glossary of Highway Quality Assurance Terms E-C137 and the NCHRP Synthesis 376. ⢠Quality: (1) The degree of excellence of a product or service. (2) The degree to which a product or service satisfies the needs of a specific customer. (3) The degree to which a product or service conforms to a given requirement. ⢠Quality Assurance (QA): All those planned and systematic actions necessary to provide confidence that a product or facility will perform satisfactorily in service. [QA addresses the overall problem of obtaining the quality of a service, product, or facility in the most efficient, economical, and satisfactory manner possible. Within this broad context, QA involves continued evaluation of the activities of planning, design, development of plans and specifications, advertising and awarding of contracts, construction, and maintenance, and the interactions of these activities.] TRB E-C074. 168
⢠Quality Control (QC): Also called process control. Those QA actions and considerations necessary to assess and adjust production and construction processes, so as to control the level of quality being produced in the end product. TRB E-C074. ⢠Quality Management (QM): The overarching system of policies and procedures that govern the performance of QA and QC activities. The totality of the effort to ensure quality in design and/or construction. ⢠Design-Bid-Build (DBB): A project delivery method where the design is completed either by in- house professional engineering staff or a design consultant before the construction contract is advertised. Also called the âtraditional method.â ⢠Design-Build (DB): A project delivery method where both the design and the construction of the project are simultaneously awarded to a single entity. ⢠Construction Manager-General Contractor (CMGC): A project delivery method where the contractor is selected during the design process and makes input to the design via constructability, cost engineering, and value analysis reviews. Once the design is complete, the same entity builds the projects as the general contractor. CMGC assumes that the contractor will self-perform a significant amount of the construction work. ⢠Construction Manager-at-Risk (CMR): A project delivery method similar to CMGC, but where the CM does not self-perform any of the construction work. ⢠Public Private Partnership (P3): A project delivery method where the agency contracts with a concessionaire organization to design, build, finance and operate an infrastructure facility for a defined extended period of time. ⢠Design deliverable: A product produced by the design-builderâs design team that is submitted for review to the agency (i.e. design packages, construction documents, etc.). ⢠Construction deliverable: A product produced by the design-builderâs construction team that is submitted for review to the agency (shop drawings, product submittals, etc.). Statement of Purpose The primary research objectives and research questions for this project are as follows: Objectives ⢠Document and categorize current practices and applications of Quality Management Systems (both traditional and alternative) in highway construction for all project delivery methods ⢠Explore how highway construction projects of all project delivery methods are effectively applying alternate quality management systems. (developing and implementing quality management systems) ⢠Identify benefits and limitations of the approaches 169
⢠Explore how to implement and apply quality management system for all methods of project delivery ⢠Produce a guidebook that will match appropriate quality management systems to selected alternative delivery methods o Describes the quality systems in the I2QM model o Discusses the barriers to each system o Gives guidance for individual roles in development and adoption of alt. quality management models in their agency ⢠Produce a research report that addresses the implications of adopting the guidelines and the barriers to implementation Research Questions 1. What is the fundamental definition of quality and what is the underlying purpose of a âquality program?â 2. How are projects using alternative delivery methods currently applying quality management systems? 3. What are the advantages and disadvantages to the contractor and the owner of alternative quality management systems relating to various project delivery alternatives? 4. What changes must be made to the baseline quality management system to adapt to evolving project delivery methods? Relevant Readings The protocol is based largely on the following documents and research reports. ï§ NCHRP Project 10-83 Proposal ï§ Coding Structure for NCHRP Project 10-83 ï§ TRB Circular E-C137 Glossary of Highway Quality Assurance Terms ï§ NCHRP Synthesis 376 ï§ NCHRP Synthesis 40-02 170
C.2 Field Procedures Project Researchers The following is a list of the project investigators and their contact information. ï§ Keith R. Molenaar, Ph.D. â Principal Investigator K. Stanton Lewis Chair and Associate Professor Construction Engineering and Management Program Department of Civil, Environmental, and Architectural Engineering University of Colorado at Boulder Campus Box 428, ECOT 643 Boulder, Colorado 80309-0428 Telephone: 303-735-4276 Facsimile: 303-492-7317 E-mail: [email protected] ï§ Douglas D. Gransberg, Ph.D., P.E. â Co-Principal Investigator Donald and Sharon Greenwood Chair and Professor of Construction Engineering Construction Engineering and Management Program Department of Civil, Construction, & Environmental Engineering Iowa State University 456 Town Engineering Ames, IA 50011 Telephone: 515-294-4148 E-mail: [email protected] ï§ David N. Sillars, Ph.D. â Co-Principal Investigator R.C Wilson Chair and Associate Professor Construction Engineering and Management Program Civil and Construction Engineering Oregon State University 220 Owen Hall Corvallis, Oregon 97331 Telephone: 541 737-8058 Fax: 541 737-3300 Email: [email protected] ï§ Elizabeth R. Kraft Graduate Student Construction Engineering and Management Program Department of Civil, Environmental, and Architectural Engineering University of Colorado at Boulder Campus Box 428 Boulder, Colorado 80309-0428 171
Telephone: Facsimile: Email: [email protected] ï§ Nickie West Graduate Student Construction Engineering and Management Program Department of Civil, Construction, & Environmental Engineering Iowa State University 456 Town Engineering Ames, IA 50011 Telephone: Facsimile: E-mail: [email protected] ï§ Landon S. Harman Graduate Student Construction Engineering and Management Program Civil and Construction Engineering Oregon State University 220 Owen Hall Corvallis, Oregon 97331 Telephone: Email: [email protected] Case Study delegation Note: The information in this section will not be available until after potential case studies have been identified and selected for study. When this information is available, this section will list who will be contacting each case study, what the scope of their questions/role will be, and who will be following up to garner additional information or thank participants for their time and effort. Case Study Identification and Schedule Case study project selection criteria In the original research proposal, it was stated that âa concerted effort will be made to select case study projects from transportation agencies that have mature experience with at least two different project delivery methods.â A total of 6 â 12 case studies will be performed with at least two of the case studies coming from a non-STA (USACE or the FTA). Additionally 2 pilot studies will be conducted and reviewed prior to the remaining studies to validate the case study protocol and data coding and to ensure that the research objectives will be met by the data collected. Potential case studies will be identified in Task 1 of the research as a part of the initial survey that aims to identify alternative quality management systems currently in use. The results of this survey will be used to populate an initial list of potential case studies. Case study project selection protocol will involve three tiers of project information that must be present to move a potential case study project into the 172
final list of candidates. The case study candidates will be forwarded to the NCHRP Panel for approval. The tiers are as follows: 1. Project Factor Information: This tier seeks to create a uniform set of data points for every case study project to ensure that trends or disconnects found during analysis can be uniformly mapped across the entire case study population (Yin 2008). Examples of this information are project location, size, major type of construction, initial and final budget amounts, initial and final delivery periods, delivery method, and other factors as required. 2. Project Quality Factor Information: This tier seeks to explicitly define the precise details of the system used to manage quality across the case study projectâs life cycle. Examples of this information are quality plans, quality organization composition, use of consultants for independent technical review or independent assurance/oversight, quality audits, division of quality management responsibility between the various stakeholder in the project and other factors as required. 3. Project Performance Information: This tier seeks to measure, if possible, the success of the quality management system employed in each project. It will use to the extent possible the stipulated performance metrics for scope, schedule, cost, quality and risk that were created for each case study project and will attempt to back-calculate performance metrics for common areas in all the projects to furnish a means of comparison and to identify and quantify the magnitude of the trends and disconnects in the case study project population. In addition to the above, a concerted effort will be made to select case study projects from transportation agencies that have mature experience with at least two different project delivery methods. The FHWA Report to Congress on Design-Build Effectiveness (2006) identified more than 30 state STAs that had been authorized to delivery design-build projects under SEP-14 authority. However, of that sample 12 had not completed a single design-build project, another eight had only completed one design-build project, and only six had finished more than five design-build projects. Thus, at that point in time, for this project delivery method the quality management systems of only six STAs could have been impacted by multiple project experiences. Since that time additional experience has been gained and NCHRP Synthesis 376 (2008) reported that four STAs had completed five to 10 design-build projects and nine had completed greater than 10. Thus, depending on the final criterion for design-build experience, as many as 13 STAs will have potential case study design-build projects that had quality management systems that were tailored for DB project delivery. That is not the case in construction management at risk. NCHRP Synthesis 40-02 (2010) reported that only Florida and Utah have completed more than a single construction management at risk project. Thus, case study projects with construction management at risk tailored quality management systems will have to come from those two STAs or from another mode of transportation such as transit. To summarize, the primary criterion for case study project selection will be the requirement to have come from an agency that has sufficient experience with a given project delivery method that the potential exists that the project was designed and built using a quality management system that was modified from the baseline design-bid-build system based on actual experience, using the USACE cyclical quality management system where quality management experience is fed back into the quality planning process to continuously improve the performance of the system itself. 173
This list of potential case studies created from the previously mentioned protocol will be supplemented by the Industry Advisory Board and the investigatorsâ industry contacts. The following three pieces of information will have been collected for each case study as a part of the survey in Task 1: (1) name and location of the project; (2) description of the project delivery, procurement, and contracting method in use; and (3) description of the quality management system. The goal for selecting the case studies will be to generate a cross section of cases that allow for analysis of the advantages and disadvantages of the quality management systems across the various project delivery method characteristics. To ensure that this goal is met, the following criteria will be placed on the case study selection will include: ⢠Quality management systems for design and construction; ⢠Quality assurance by all parties and independent auditors; ⢠Project quality assurance including independent verification and independent acceptance; ⢠Project delivery methods including design-build, construction manager-at-risk, and PPP; ⢠Procurement methods including best value, A+B, and qualifications-based selection; and ⢠Payment methods including incentives, lump sum, and guaranteed maximum price. Case study informant selection Once a case study project has been selected, several members of the team directly associated with creating and implementing the quality management plan will need to be interviewed. While many people are responsible for ensuring quality on a project during its lifecycle from conception through construction, we will seek to speak with â at a minimum â enough project team members to fully satisfy the research objectives and goals. This may include speaking with representatives from the ownerâs, designerâs, and contractorâs project team to develop a full picture of the quality management systems utilized on a project and their relation to each other. Potential interviewees include the following: ï§ Agency project manager, contracting manager, quality manager, etc. ï§ Project design manager, construction manager, design quality manager, construction quality manager, etc. ï§ Designer quality manager, project manager ï§ Contractor preconstruction manager, quality manager, project manager ï§ Third party quality assurance/quality control inspectors 174
Case Study Basic Data and Research Delegation Tables # Case Study Name Location Organization Contact (Information) 1 2 3 4 5 6 7 8 # Case Study Name Contacted? Lead Investigato r Interview Type Intervie w Date Follow-up Date 1 2 3 4 5 6 7 8 175
# Case Study Name Materials/Documents Received 1 2 3 4 5 6 7 8 C.3 Requested Documents The following is a list of documents that will be requested. However, successful completion of the research study does not require that each of the documents is collected. A subset of these documents will likely be collected depending upon the unique attributes of the project being studied. ⢠Project RFP/RFQ ⢠Project RFP/RFQ Response ⢠Project Quality Management Plan ⢠Project Design Quality Plan ⢠Project Construction Quality Plan ⢠Agency/Company Quality Plan ⢠Project Quality Organizational Chart ⢠Copy of the contract with the engineer/contractor/consultant ⢠Organizational document that outlines its approach to quality assurance on project Case Study Questions This section seeks to formalize the interview questions asked of each case study to allow for easier comparison and analysis between case studies later on. Each interview will be unique and largely guided by the case study participants. To draw out the pertinent information, specific questions may be needed while some of the questions listed here may not be relevant. In modifying the protocol questions, generality should still be maintained so that the results can still be readily compared and categorized according to the coding structure. 176
Questionnaire The purpose of the questionnaire is to identify how state highway agencies (SHA) have implemented alternative QA programs and from that baseline, identify commonly used practices for dissemination and use by SHAs that intend to implement alternative procurement on future projects or alternative QA methods in their current program. The questionnaire consists of closed ended questions which will allow the researchers to perform a quantitative analysis. The data gathered by the questionnaire will be used to validate the case study findings. Ideally this questionnaire will be completed by the respondent prior to the interview. If it is not completed prior to the interview then it will be requested that the respondent complete it during the interview. The questionnaire is included in APPENDIX B. Background and Overall Quality Questions Background ï§ Background information to keep track of who we spoke with on each project ï§ Relevant prior experience used to potentially weight their opinions in later comparison 1. Name, occupation, employer 2. What are you current duties, especially related to QM, QA, QC? 3. Have you held any positions prior to your current position related to QM, QA, or QC? If so, please briefly list that information. 4. How long have you held your current position? 5. Have you worked on projects of different project delivery methods? If so specifically what project delivery methods do you have experience with? 6. How many years of experience do you with projects using baseline/DBB quality systems? 7. Name and location of the project for which you are answering project-specific questions Overall Understanding of Quality ï§ Examines the overall understanding of quality and the informants attitudes towards quality ï§ Establishes a baseline of traditional/DBB quality for comparison 8. How do you define project quality? 9. What is your understanding of the differences between quality management, quality assurance, and quality control? 10. In your experience, how has quality management been approached on a traditional/DBB project? ï§ Project by project basis? ï§ During the RFP process? ï§ Dictated by owner agency? If so, how is it dictated?? Specifications, performance⦠11. What are the critical elements/milestones of a quality management plan on a traditional/DBB project? 12. How are the quality roles and responsibilities divided on a traditional/DBB project? 13. In your experience, what quality systems/procedures have been successful on traditional/DBB projects? 177
14. What are some characteristics of a successful quality management plan, regardless of the project delivery method, contracting method, or procurement method? Organizational Questions ï§ Provides the needed categories and statistics (from the coding structure) to later sort and group the enterprise level QM information we receive. As not everyone selected for an interview will have answered the survey, these questions are not redundant Please answer the following questions related to quality management at different phases of a project for the organization you work for as a whole. Please be as thorough as possible when discussing what quality management systems may exist and be utilized within your organization. 15. Of the projects your organization designs/builds/owns what percent of the projects are managed using the following delivery methods: ï§ Design-bid-build (DBB): ______ ï§ Design-build (DB): _______ ï§ Construction Manager/General Contractor (CM/GC): _______ ï§ Public-Private-Partnership (PPP): _______ ï§ Other (please list): _______ 16. Of the projects your organization designs/builds/owns what percent of the projects are procured using the following procurement methods: ï§ Cost: ______ ï§ Best-value: _______ ï§ Qualifications: _______ ï§ Design: _________ 17. Of the projects your organization designs/builds/owns what percent of the projects utilize the following contract payment methods: ï§ Payment type: ________ ï§ Incentive type: ________ 18. If you work for a STA, what due diligence requirements must your organization meet in your state for quality assurance purposes on public projects? ï§ Please list applicable laws, reporting requirements, or processes required in your state ï§ Can you utilize new or alternative quality management practices that produce equal or superior quality projects to meet these requirements? If not, what legal barriers prevent you from doing so? ï§ If so, what steps are required to do this? Has this been done before? Please describe if it has. 19. Does your organization perform any in-house design work? ï§ On what percent of your projects do you perform in-house design? ï§ Do you have formal quality assurance systems in place for this process? ï§ If so, please describe this process. 20. Does your organization self-perform any construction work? ï§ On what percent of your projects do you self-perform some amount of construction? ï§ Do you have formal quality assurance systems in place for this process? ï§ If so, what are they? 178
Design Phase QM ï§ Examines design QM and is broken up into components according to the coding structure to allow for easier comparison between case studies later on ï§ How is design quality management managed on all project delivery types whether design is kept in-house or out of house 21. Does your organization have any design quality assurance systems in place? ï§ If so, please describe the systems, policies, procedures, techniques, or standards used to ensure the quality of the designs you produce or receive. Can you provide us with an electronic or hard copy of these systems, policies, procedures, techniques, or standards? ï§ Have you found these systems to be effective at producing quality designs? If yes, what makes them superior to other systems you are familiar with or function well? If no, what changes would you suggest to increase their efficacy? ï§ Are there any additional procedures that would further improve the quality of designs you produce or receive? If so, please describe them. 22. Does your organization have any design quality control systems in place? ï§ If so, please describe the systems, policies, procedures, techniques, or standards used for quality control of the designs you produce or receive. Can you provide us with an electronic or hard copy of these systems, policies, procedures, techniques, or standards? 23. Does your organization utilize any form of peer review or 3rd party/independent design quality assurance? ï§ If so, please describe how this is conducted and how the results are utilized to improve the quality of the original design. ï§ If this process is formalized, can you provide us with any documents detailing it? Construction Phase QM ï§ Examines construction QM from a STAâs point of view and is broken up into components according to the coding structure to allow for easier comparison between case studies later on ï§ The primary focus of many QM plans and where the bulk of the information gathered at an enterprise level may be found 24. How does your organization perform construction quality assurance? Who has primary responsibility? ï§ Does the process change based on the project type, contract style, or delivery method? If so, how is the process tailored? Who decides which method will be used? ï§ Has your organization experimented with new quality assurance methods? Are you actively implementing any now? If so, what are they and how have you evaluated their efficacy? ï§ Has your organization modified traditional quality assurance processes to make them applicable to projects with alternative delivery methods? If so, how have you modified them? 25. Who is responsible for construction quality control on projects your organization is a part of? ï§ What is the process used for quality control? If this process changes due to project type, contract style, or delivery method, how does it change? ï§ Do you still utilize traditional quality control methods such as control charts? ï§ Has your organization modified traditional quality control processes to make them applicable to projects with alternative delivery methods? If so, how have you modified them? 179
ï§ Has your organization experimented with new quality control methods? Are you actively implementing any now? If so, what are they and how have you evaluated their efficacy? 26. What role does independent assurance (IA) play in your projects? ï§ Which parties â owner, designer, or builder â utilize IA to ensure a quality product? 27. How is owner verification testing used in your traditional projects? ï§ What role does it have in your projects? ï§ What statistical tests are used to verify the contractorâs results? ï§ Who conducts the testing the owner or a third party? ï§ Is it used on your non-traditional projects as well? If so, what function does it serve on those projects? 28. How is owner acceptance testing utilized on this project? ï§ Is this seen as sufficient justification of quality construction to ensure final payment? How are the results used? ï§ Who conducts the testing, the owner or a third party? ï§ If it is used on non-traditional projects, what function does it serve? 29. Does your organization make use of or offer any form of post-construction quality assurance? Please describe this if you can. ï§ Is this part of a warranty or of an operate-and-maintain contract? If not, what is it? ï§ If you are engaged in contracts with warranties or operate-and-maintain clauses, how does this impact your quality management model before and during construction? Project Specific Questions Please answer the following questions for the specific project you were contacted about. While not every question may apply to your project, please be as thorough as possible. If you have additional information regarding the quality management process on you project that you would like to provide, please feel free to add that below or contact us directly. Procurement and delivery method ï§ Used to categorize the case studies for later comparison, these identifying questions will help focus future analysis ï§ Seeks to explore the relationship (if one exists) between the delivery method selected and the QM techniques used 30. What delivery method is being/was used for this project (DBB, DB, CM/GC, PPP, etc.)? 31. What procurement method (cost, best-value, qualifications based, design based, A + B, multiparameter bidding, etc.) was used to select the designer/builder/concessionaire on this project? 32. Was a prequalification process used? If so, please describe that process. ï§ If a prequalification process was used, were interested parties required to submit a quality management plan (QMP)? Were they required to identify a dedicated quality manager? If you still have the QMPs from the initial solicitations, can you provide us with copies? 33. What requirements related to Quality were included in the RFP? 180
ï§ Submittal of a QMP prior to award, qualifications of the quality staff on the project, submittal of a QMP after award, etc. 34. Were either the procurement or delivery methods selected partially or in whole because of their effect on quality management? If so, why were the particular methods selected? 35. What contract payment approach was selected for the project, payment or incentive? ï§ What effect did this have/is this having on quality management on the project? Quality Management Plan ï§ Determine roles and responsibilities for creating the overall quality management plan ï§ Determine the purpose/requirements of the quality management plan ï§ Determine how the Quality Management plan was developed and approved 36. How was the Quality Management Plan (QMP) created? ï§ Was it a stock plan modified for the project? Was it created from scratch? 37. Who was responsible for creating the Quality Management Plan? 38. How was the overall quality management hierarchy created for this project? ï§ What is the overall quality management hierarchy? Can you provide us with a copy of the org chart? 39. What were the Agencyâs quality objectives/requirements for this project and how were these communicated? 40. Describe the QMP approval process. 41. How did the QMP differ from a traditional DBB project QMP? ï§ Why did this projectâs QMP need to be different from a traditional DBB? 42. How was the QMP development process different from a traditional DBB project? ï§ Why was this projects QMP development process different from a traditional DBB QMP development? 43. How was this QMP and its development process successful? ï§ What about the QMP and its development process could be improved for the next project? 44. What other management plans were required for this project and what were the quality roles and responsibilities associated? ï§ Design, construction, environmental, traffic, etc⦠45. Describe how the quality management on this project is different from a traditional DBB project ï§ What procedures/processes/systems were different? ï§ What project factors required the design quality management plan to be different from the traditional (project delivery, project complexity, funding), Design phase QM ï§ Details the specific design QM techniques used on this project as broken down by the coding structure and gathers any additional information related to this process 181
ï§ Seeks to understand the relationship between the techniques used on the project and those recommended at the agency-wide level ï§ Seeks to understand how the design QM was developed and approved and requests the needed documents 46. How was the design Quality Management Plan (QMP) created and by whom? ï§ What requirements was it based on? 47. What was your organizationâs role/responsibilities in developing and implementing the design QMP on this project? 48. What was the project hierarchy (org chart) for this phase of the project? Who was responsible for design QA/QC on this project? ï§ Can we get a copy of that chart? 49. What was the approval process for the Design QM? 50. How were the Agencyâs design quality requirements communicated to the Designer? ï§ By RFP, design guidelines, performance specifications, etc. 51. Describe how the design quality management on this project is different from a traditional DBB project ï§ What procedures/processes/systems were different? ï§ What project factors required the design quality management plan to be different from the traditional (project delivery, project complexity, funding), 52. What was the basic premise of the design QMP for this project? ï§ Over the shoulder reviews, spot checks, design checks at certain milestones, etc. 53. Was quality assurance incorporated in the design phase of this project? If so, how was it implemented on this project? ï§ Who was responsible for QA, the owner or designer? ï§ Was a formal plan drafted detailing how design QA would be considered? If so, can you provide us with a copy of that report? 54. Were design quality control processes utilized on this project? If so, how? ï§ Are there documents outlining these processes? If so, can you provide us with a copy? 55. Was a dedicated design quality manager assigned by the owner, designer, CM, or concessionaire? What were their responsibilities? What authority did they have to make changes to the design or QA processes? 56. Was a peer review or independent assurance component included in design phase quality management? ï§ If so, what was its role and how did it impact the final design? 57. What problems were discovered and corrected through the design phase quality management process? ï§ What problems were not discovered until the construction phase of the project? Could these have been avoided with a more robust design quality management process? If so, what changes would need to be made to your process to catch these problems in the future? 182
58. Was the design phase QMP used on this project a standardized system used on most or all of your organizationâs projects? If not, was it tailored to meet the needs of this project from an existing process or created from scratch for this project? ï§ How much time/what resources did creating the design QMP for this project require? ï§ If an existing design QMP was modified for this project, can you provide copies of both for comparison? 59. How effective was the design QMP on this project? ï§ How could it have been improved? Construction Phase QM ï§ Details the specific construction QM techniques used on this project as broken down by the coding structure and gathers any additional information related to this process ï§ Seeks to understand the relationship between the techniques used on the project and those recommended at the agency-wide level ï§ Seeks to understand how the design QM was developed and approved and requests the needed documents 60. How was the Construction Quality Management Plan (QMP) created and by whom? 61. How much of the quality systems were developed during the RFP/contracting phase? ï§ Was a quality plan required at RFP submittal? after award? ï§ Was the quality plan developed collaboratively? 62. What was the approval process for the construction QMP? 63. How were the Agencyâs design quality requirements communicated to those crafting the construction QMP? ï§ By RFP, design guidelines, performance specifications, etc. 64. How were this projectâs QM, QA, and QC systems different from a project using traditional DBB? ï§ Roles/responsibilities, liabilities, etc. ï§ What project factors required the design quality management plan to be different from the traditional (project delivery, project complexity, funding), 65. Was there a dedicated quality manager for this project? Who employed the manager, the owner, builder, designer, concessionaire, etc.? ï§ What responsibility and authority to make changes did this manager have? 66. How was construction quality assurance put into place on this project? 67. What role did construction quality control play on this project? If a problem was discovered in the QC process, were changes made quickly enough to prevent future problems? ï§ What facilitated or hindered this rapid communication? ï§ How was QC implemented on this project? 68. Was owner verification testing used on this project to check the contractorâs QC process? ï§ Who performed the testing, the owner or a 3rd party? ï§ Were significant discrepancies discovered on this project? If so, did the QMP provide an effective way to deal with these? How? 69. Was owner acceptance testing included on this project before final payment? 183
ï§ Who performed this testing? ï§ Were significant issues discovered? ï§ How were they handled? Were all parties satisfied with the outcome? 70. Was independent assurance included as part of the construction QMP on this project? ï§ If so, what role did it play in quality management on the project? 71. What other features/systems were parts of the QMP during the construction phase of this project? ï§ What additional features could have prevented quality issues from arising on this project? 72. How was QM in the construction phase affected by the contract delivery method? ï§ By the procurement method? 73. Can you provide a copy of the construction QMP for this project? ï§ Did you modify an existing QMP for the construction phase of this project or develop one from scratch? If you modified an existing plan, can you provide copies of both for comparison? 74. What challenges did the QMP on this project face and how were they overcome? 75. Overall, were you satisfied with the quality management of the construction phase of this project? ï§ Which particular aspects of the QMP were you especially pleased with? ï§ If not, what would you change regarding quality management for your next project? 76. Based on your experience, would any of the quality techniques/systems used on this project be beneficial if applied to a traditional/DBB project? Post-Construction QM ï§ Explores whether a formal policy for QM relating to the post-construction period exists and if such a policy exists, seeks to understand its relationship to the QMP implemented during design and construction of the project 77. Did/does this project include any warranty or operate-and-maintain provisions in the contract? If no, skip the rest of this section. If yes, please answer the following questions. ï§ How did the inclusion of these provisions affect the QMP during construction? ï§ When compared with other projects NOT having these provisions, how did these provisions affect the overall quality of the project? Additional Questions ï§ This section is designed to allow for further refinement of the interview (in the pilot study phase) ï§ Offers the participant the opportunity to add any additional information he/she believes is relevant to our study that we may have overlooked or not asked about for lack of project specific knowledge 78. As a case study participant, how easy to understand did you find the interview questions? Were they straight forward? Did you struggle to understand the intent of some questions? In addition to any answers to these questions, please provide a rating from 1 to 10 (10=easy to understand, 1=so hard to understand it was difficult to finish). 184
ï§ Which questions did you find especially difficult to understand or answer? 79. Did the interview seem repetitive to you? If so, what sections/questions seemed to contain overlapping material? 80. Was the interview burdensome to complete? Were you able to answer each question to the extent of your knowledge without fatigue? 81. Given our project objectives, what additional information can you provide that would help us to better understand alternative quality management? What other QM processes, procedures, or ideas are you aware of (for any phase of a project) that you have not shared with us thus far or have been unable to utilize first-hand? 82. Given our project objectives, are there additional questions that you feel we should be asking? If so, what questions would you suggest? 83. Do you know of any other co-workers or industry peers with a position related to quality management that would be interested in speaking with us? If so, can you provide their contact information? 84. Are you aware of any projects that have utilized or are utilizing excellent quality management procedures on a project with a non-traditional delivery method (DB, CM/GC, PPP, etc.)? If so, can you provide us with the name, location, and any other pertinent information for the project? C.4 Data Analysis ⢠The complete data analysis plan is described in the project Work Plan. The main points of the analysis include: ⢠Advantages and disadvantages to each system from the agencyâs and the designerâs/constructorâs point of view; ⢠Identification of trends and common finding between the lit review, survey and case studies; ⢠Triangulate the common findings from these three sources of data to arrive at valid conclusions; ⢠Case studies will be summarized individually in the lens of the IQ2M model; ⢠Using literature review and survey information, compare key attributes of the baseline approach to key attributes in the IQ2M models. (design quality is a gap, but rigorous comparison of construction quality control, construction quality assurance and independent audit procedures will be made); ⢠Individual findings will analyzed across the cases using pattern matching techniques; and ⢠Comparison to baseline quality management system approaches. 185
C.5 Case Study Contact Flowchart #1 Phone â¢Call contacts for identified case studies and secure their agreement to act as a champion for the case study process ⢠Set up a date and time for the case study interview during this contact #2 Letter ⢠Send letter #1 from the case study protocol to the contact confirming their agreement to act as a champion â¢This letter should include a brief outline of our project goals and documents we will request #3 Letter ⢠Send letter #2 from the protocol and the questionnaire to participant and ask them to fill it out before the interview if possible â¢This letter should also include a list of the documents we will be requesting, ask them to collect them ahead of time if possible #4 In-Person â¢Conduct the case study interview at the agreed time and date â¢Before leaving the interview, be sure that at a minimum: the questionnaire has been filled out and the needed documents have been collected #5 Letter ⢠Send letter #3 from the protocol thanking the participant for their time and assistance and offer to share the results of the research with them when it is finished 186
C.6 Sample Letters Letter #1 MEMORANDUM DATE TO: Survey Participant FROM: Keith Molenaar Principal Investigator SUBJECT: NCHRP 10-83 Case Study Thank you for agreeing to participate in the NCHRP 10-83 Research Project case study concerning alternative quality management procedures for highway construction projects utilizing non-traditional contracts. We have enclosed some brief background information about the research project, its objectives, goals, and methods. We are currently scheduled to (meet with/call) you on (insert day/month) at (insert time) to conduct our interview. If for some reason this no longer works for you, please contact me as soon as possible to reschedule. Before our interview, we will send you a questionnaire related to the topics we would like to cover in the interview. Please review the questionnaire prior to the interview to become acquainted with the nature of the questions that we will be discussing. Iâve attached a brief outline of our research interests along with a list of documents related to the project that we would like to collect. If you have any questions, please feel free to contact me by telephone at 303-735-4276 or by email at [email protected]. Regards, Keith Molenaar Requested Documents Below is a list of documents we would like to collect regarding the project this case study is focusing on. While your project may not have all of the listed documents, we need to obtain a copy of any of the documents included on your project as well as any additional documents not listed that you believe are relevant to our research objectives as outlined below. While we would prefer (hard/electronic) copies, 187
either will suffice. Additionally, are there any online resources we can examine (FTP sites, project websites, etc.) before the interview to familiarize ourselves with the project? ⢠Copy of the contract with the engineer/ contractor/ consultant ⢠Organizational document that outlines its approach to quality assurance on project ⢠Project RFP/RFQ ⢠Project Quality Organizational Chart ⢠Project RFP/RFQ response ⢠Project Construction quality plan ⢠Project Quality Management Plan ⢠Agency/Company quality plan ⢠Project design quality Plan ⢠Project Background The NCHRP 10-83 research project has four primary research objectives. They are to: ⢠Document and categorize current practices and applications of Quality Management Systems (both traditional and alternative) in highway construction for all project delivery methods ⢠Explore how highway construction projects of all project delivery methods are effectively applying alternate quality management systems. (developing and implementing quality management systems) ⢠Identify benefits and limitations of the approaches ⢠Explore how to implement and apply quality management system for all methods of project delivery In the course of meeting those objectives, we will seek to answer these four questions: 1. What is the fundamental definition of quality and what is the underlying purpose of a âquality program?â 2. How are projects using alternative delivery methods currently applying quality management systems? 3. What are the advantages and disadvantages to the contractor and the owner of alternative quality management systems relating to various project delivery alternatives? 4. What changes must be made to the baseline quality management system to adapt to evolving project delivery methods? To answer these questions, we are performing several in-depth project case studies, covering both traditional and alternative delivery methods. The case studies will be used to learn how existing projects have managed quality in light of non-traditional delivery methods. After analyzing and comparing the various case studies, the information gathered will be condensed into working theories, used to modify what we are calling the Integrated Quality Management Model (IQ2M), and ultimately used to prepare a final research report for the NCHRP and a set of guidelines for when implementing certain AQM systems may be useful in light of other project characteristics. 188
Letter #2 MEMORANDUM April 17, 2015 TO: Survey Participant FROM: Keith Molenaar Principal Investigator SUBJECT: NCHRP 10-83 Case Study Questionnaire Thank you again for your assistance with this research effort investigating alternative quality management procedures for highway construction projects utilizing non-traditional contracts. Enclosed is a questionnaire that touches on many of the topics we would like to cover in our in-depth interview. The questionnaire will be used as a baseline to generate easily comparable data across all of the different case studies we will be performing. It will be a starting point for our discussion and we would greatly appreciate it if you would take the time to look it over and fill it out to the best of your ability before our scheduled interview on (insert day/month) at (insert time). Also enclosed is a list of the documentation we are seeking for this case-study. While we would prefer (hard/electronic) copies, either will suffice. If this information is available on a website for FTP site, please let us know so that we may familiarize ourselves with the information ahead of time. If you have any questions, please feel free to contact me by telephone at 303-735-4276 or by email at [email protected]. Regards, Keith Molenaar 189
Letter #3 MEMORANDUM April 17, 2015 TO: Survey Participant FROM: Keith Molenaar Principal Investigator SUBJECT: NCHRP 10-83 Case Study Follow-Up Thank you for your participation in the NCHRP 10-83 interview process. We recognize that this process is time consuming and very much appreciate your assistance in helping us better understand alternative quality systems in this industry. Your insight and experience with this project will be invaluable as we compare it with projects from across the country and try to develop a better understanding of which systems work well and which do not. The research report and guidelines will not be finished until the summer of 2012, but we would be happy to share the research results with you then if you would like. Again, thank you for your time! If you have any questions, please feel free to contact me by telephone at 303-735-4276 or by email at [email protected]. Regards, Keith Molenaar 190
C.7 Questionnaire INTRODUCTION/BACKGROUND: The purpose of this questionnaire is to identify how state highway agencies (SHA) have implemented alternative QA programs and from that baseline, identify commonly used practices for dissemination and use by SHAs that intend to implement alternative procurement on future projects or alternative QA methods in their current program. DEFINITIONS: The research will use TRB Circular E-C074, Glossary of Highway Quality Assurance Terms to standardize its terminology. The following are terms that must be carefully understood to properly complete this survey. Quality: (1) The degree of excellence of a product or service. (2) The degree to which a product or service satisfies the needs of a specific customer. (3) The degree to which a product or service conforms with a given requirement. Quality Assurance (QA): All those planned and systematic actions necessary to provide confidence that a product or facility will perform satisfactorily in service. [QA addresses the overall problem of obtaining the quality of a service, product, or facility in the most efficient, economical, and satisfactory manner possible. Within this broad context, QA involves continued evaluation of the activities of planning, design, development of plans and specifications, advertising and awarding of contracts, construction, and maintenance, and the interactions of these activities.] TRB E-C074. Quality Control (QC): Also called process control. Those QA actions and considerations necessary to assess and adjust production and construction processes, so as to control the level of quality being produced in the end product. TRB E-C074. Quality Management (QM): The overarching system of policies and procedures that govern the performance of QA and QC activities. The totality of the effort to ensure quality in design and/or construction. Design-Bid-Build (DBB): A project delivery method where the design is completed either by in-house professional engineering staff or a design consultant before the construction contract is advertised. Also called the âtraditional method.â Design-Build (DB): A project delivery method where both the design and the construction of the project are simultaneously awarded to a single entity. Construction Manager-General Contractor (CMGC): A project delivery method where the contractor is selected during the design process and makes input to the design via constructability, cost engineering, and value analysis reviews. Once the design is complete, the same entity builds the projects as the general contractor. CMGC assumes that the contractor will self-perform a significant amount of the construction work. Construction Manager-at-Risk (CMR): A project delivery method similar to CMGC, but where the CM does not self-perform any of the construction work. Public Private Partnership (P3): A project delivery method where the agency contracts with a concessionaire organization to design, build, finance and operate an infrastructure facility for a defined extended period of time. 191
Design deliverable: A product produced by the design-builderâs design team that is submitted for review to the agency (i.e. design packages, construction documents, etc.). Construction deliverable: A product produced by the design-builderâs construction team that is submitted for review to the agency (shop drawings, product submittals, etc.). Requested Documents Below is a list of documents we would like to collect regarding the project this case study is focusing on. While your project may not have all of the listed documents, we need to obtain a copy of any of the documents included on your project as well as any additional documents not listed that you believe are relevant to our research objectives as outlined below. While we would prefer electronic copies if available, but either will suffice. ⢠Copy of the contract with the engineer/ contractor/ consultant ⢠Organizational document that outlines its approach to quality assurance on project ⢠Project RFP/RFQ ⢠Project Quality Organizational Chart ⢠Project RFP/RFQ response ⢠Project Construction quality plan ⢠Project Quality Management Plan ⢠Agency/Company quality plan ⢠Project design quality Plan ⢠Additionally, are there any online resources we can examine (FTP sites, project websites, etc.) before the interview to familiarize ourselves with the project? General Information -STA: 7. US state in which the respondent is employed: 8. Name of Agency: 9. Names and groups/sections of interviewees: 10. Please check the appropriate boxes for your agencyâs project delivery program (PDM). Project Delivery Method Legislative/Legal Authority Number of years of experience with PDM DBB âNA; âPilot projects only; âGeneral authorization âNA; â1-5; â5-10;â > 10 CMGC âNA; âPilot projects only; âGeneral authorization âNA; â1-5; â5-10;â > 10 DB âNA; âPilot projects only; âGeneral authorization âNA; â1-5; â5-10;â > 10 P3 âNA; âPilot projects only; âGeneral authorization âNA; â1-5; â5-10;â > 10 Other âNA; âPilot projects only; âGeneral authorization âNA; â1-5; â5-10;â > 10 11. Are your Quality Management systems different between Project Delivery Methods? âYes âNo 192
12. What is the approximate proportion of in-house design versus outsourced design services? In-house design services - Click here to enter text.% Outsourced design services - Click here to enter text. % Administrative prequalification: A set of procedures and accompanying forms/documentation that must be followed by a designer or construction contractor to qualify to submit bids on construction projects using traditional project delivery. Performance based prequalification: A set of procedures and back-up documents that must be followed by a designer or construction contractor to qualify to submit a bid on a construction project based on quality, past performance, safety, specialized technical capability, project-specific work experience, key personnel, and other factors. 3. Does your agency use a prequalification program for design firms? âYes âNo If the answer to the previous question is YES, please answer for your agencyâs program to prequalify design firms. Designer prequalification program factors Prequalification Type Administrative Performance Based Prequalification required for all projects â â Prequalification required for selected projects only â â Prequalification standards are the same for all projects â â Prequalification standards are different by project class â â 4. Does your agency use a prequalification program for construction contractors? âYes âNo If the answer to the previous question is YES, please answer for your agencyâs construction contractor prequalification program. Construction prequalification program factors Prequalification Type Administrative Performance Based Prequalification required for all projects â â Prequalification required for selected projects only â â Prequalification standards are the same for all projects â â Prequalification standards are different by project class â â Case Study Project Information and Data 1. Project Name and location: 2. Project scope of work: 193
3. Original Total Awarded Value of project: $ Final Total Value of project: $ 4. Date preliminary design contract awarded: Date project advertised: 5. Date final design contract awarded: Date construction contract awarded: [Note: same if DB] 6. Original Project Delivery Period (including design) Final Project Delivery Period (including design) Explanatory notes: 7. Project delivery method used on this project: Design-Bid- Build CM-at- Risk Design- Build P3 Please explain what effect this choice had on the overall quality of the project: 8. Which of the following were reasons why your agency selected the delivery method used for this project? Check all that apply. Reduce/compress/accelerate project delivery period Establish project budget at an early stage of design development Get early construction contractor involvement Encourage innovation Facilitate Value Engineering Encourage price competition (bidding process) Compete different design solutions through the proposal process Redistribute risk Complex project requirements Flexibility needs during construction phase Reduce life cycle costs Provide mechanism for follow-on operations and/or maintenance 194
Innovative financing Other: Explain 9. Which of the above was the single most significant reason for the delivery method decision on this project? 10. Please explain the process that led you to the choice of the project delivery system for this project . Case Study Project Quality Management Policy/Procedures Information: The following questions will break up the quality management process into the following four phases: ⦠Procurement phase: Actions taken regarding the quality management process that are reflected in the agencyâs contractor prequalification requirements and/or solicitation documentation such as in the Invitation for Bids (IFB), Request for Qualifications (RFQ) and the Request for Proposals (RFP). ⦠Design Phase (in-house): Actions taken after approval to start design work regarding ensuring the quality of the design deliverables as well as that the final design complies with contractual requirements. OR ⦠Design Phase (out-source): Actions taken after design contract award regarding ensuring the quality of the design deliverables as well as that the final design complies with contractual requirements. ⦠Construction Phase: Actions taken after contract award regarding the quality of the final constructed product to ensure that it complies with both the completed design and other contractual requirements. The research team understands that the term âApprovalâ has a variety of slightly different meanings from state to state. It is used here to indicate the process by which the agency indicates that it is satisfied with the quality of the design or construction deliverable and is willing to make payment for satisfactory completion of that task if asked. Procurement phase: 1. What procurement method was used to select the designer, builder, or concessionaire? (low bid, best-value, qualifications based selection, etc.) 195
2. Please answer for the case study project. If your process was conducted in more than one way, please answer for the most prevalent set of procedures. Do your project advertising/solicitation documents (i.e. IFB, RFQ, RFP, etc.) contain the following? Required proposal/ bid package submittal? If YES: Is it evaluated to make the award decision? If NO: Is it a required submittal after contract award? Yes No Yes No Yes No Qualifications of the Design Quality Manager â â â â â â Qualifications of the Construction Quality Manager â â â â â â Qualifications of other Quality Management Personnel (design reviewers, construction inspectors, technicians, etc.) â â â â â â Design quality management plan â â â â â â Design quality assurance plan â â â â â â Design quality control plan â â â â â â Construction quality management plan â â â â â â Construction quality assurance plan â â â â â â Construction quality control plan â â â â â â Quality management roles and responsibilities â â â â â â Design criteria checklists â â â â â â Construction testing matrix â â â â â â Quality-based incentive/disincentive features â â â â â â Warranties â â â â â â Optional warranties â â â â â â 3. Was your procurement method selected in part because of its effect on quality management? If so, what effect did the method you chose have on overall project quality? Design Phase: 4. Did this project have a formal design quality assurance program for any design performed in- house? âYes âNo 5. Did this project have a formal design quality assurance program for design performed by design consultants? âYes âNo 6. Did this project combine in-house design services with projects delivered by alternative methods (CMGC, DB, P3)? âYes âNo 196
For this project who performed the following design quality management tasks? (Check all that apply) Does not apply Agency design staff Agency project manage- ment staff Project design consult- ant Project con- struction staff in CMGC, DB, P3 Indepen- dent quality consultan t Other Please specify below Technical review of design deliverables â â â â â â â Checking of design calculations â â â â â â â Checking of quantities â â â â â â â Acceptance of design deliverables â â â â â â â Review of specifications â â â â â â â Approval of final construction plans & other design documents â â â â â â â Approval of progress payments for design progress â â â â â â â Approval of post-award design QM/QA/QC plans â â â â â â â Construction Phase: For this project who performed the following construction quality management tasks? (Check all that apply) Does not apply Agency design staff Agency project manage- ment staff Project design consult- ant Project con- struction staff in CMGC, DB, P3 Indepen- dent quality consultan t Other Please specify below Technical review of construction shop drawings â â â â â â â Technical review of construction material submittals â â â â â â â Checking of pay quantities â â â â â â â Routine construction inspection â â â â â â â Quality control testing â â â â â â â Verification testing â â â â â â â Acceptance testing â â â â â â â Approval of progress payments for construction progress â â â â â â â Approval of construction post-award QM/QA/QC plans â â â â â â â Report of nonconforming work or punchlist. â â â â â â â Quality Management Planning: Please answer the following questions about the project based on your experience. 12. Are the design QM plans used on this project different from the QM plans used on traditional design projects? 197
âNo â Yes If yes, what is the major difference? Click here to enter text. 13. Are the construction QM plans used on this project different from the QM plans used on traditional DBB construction projects? âNo â Yes If yes, what is the major difference? Click here to enter text. 14. Did the agency mandate the use of standard agency specifications? âNo â Yes 15. Did the agency mandate the use of standard agency design details? âNo â Yes 16. Did the agency mandate the use of standard agency construction means and/or methods? âNo â Yes If no, what was required in their place? 17. Did the agency mandate a specific set of qualifications for the quality management staff of design consultants and construction contractors on this project? âNo â Yes If yes, what are those qualifications? Click here to enter text. 18. Did your agency utilize contractor quality assurance test results for acceptance on this project? âYes. âNo. Quality Management Procedures: 2. Do you think that the agency held the CMGC/design-builder/P3 concessionaireâs design/construction quality management staff to a higher standard of care than it sets for its internal staff? âYes âNo 198
Comments? Click here to enter text. 3. Does your organization have a document that outlines its approach to quality assurance on project? âYes âNo If yes, was it used on this project? If no, what was used in its place? 4. Does your agency use an approach to quality management that is substantially different than that used by other agencies and might be considered an âalternative QM systemâ? (i.e. statistical analysis of material test reports that eliminates the need for agency acceptance testing) âYes âNo Describe: 5. Which of the below best describes your agencyâs approach to QA on this project? Interviewer: select appropriate delivery method DBB CMGC DB P3 âDesign consultant primarily responsible for QA/Agency audits consultant program âContractor primarily responsible for QA/Agency audits contractor program âAgency retains traditional QA roles âAgency retains an independent party to perform QA roles âAgency uses two or more of the above depending on the project âNone of the above âDesign consultant primarily responsible for QA/Agency audits consultant program âContractor primarily responsible for QA/Agency audits designers and CMGCâs program âAgency retains traditional QA roles âAgency retains an independent party to perform QA roles âAgency uses two or more of the above depending on the project âNone of the above âDesign-builder primarily responsible for QA/Agency audits design-builderâs program âAgency retains traditional QA roles âAgency retains an independent party to perform QA roles âAgency uses two or more of the above depending on the project âNone of the above âConcessionaire primarily responsible for QA/Agency audits concessionaireâs program âAgency retains traditional QA roles âAgency retains an independent party to perform QA roles âAgency uses two or more of the above depending on the project âNone of the above If âNone of the aboveâ was selected, please describe the approach that was used instead: 6. What was the biggest quality challenge in the procurement phase? 199
7. What was the biggest quality challenge in the design phase? 8. What was the biggest quality challenge in the construction phase? 9. Please rate the following factors for their impact on the quality of this project: Factor Very High Impact High Impact Some Impact Slight Impact No Impact Qualifications of agency design staff â â â â â Qualifications of agency project management staff â â â â â Qualifications of agency construction staff â â â â â Qualifications of the design consultantâs staff â â â â â Design consultantâs past project experience â â â â â Qualifications of the construction contractorâs staff â â â â â Construction contractorâs past project experience â â â â â Submittal of Quality management plans prior to work start â â â â â Level of agency involvement in the QM process â â â â â Use of agency specifications and/or design details â â â â â Level of detail expressed in the procurement documents (IFB/RFQ/RFP) â â â â â Use of manuals, standards and specifications developed for DBB type projects â â â â â Allowing flexibility in choice of design standards and construction specifications â â â â â Use of performance criteria/specifications â â â â â Detailed design criteria â â â â â Warranty provisions â â â â â Incentive/disincentive provisions â â â â â Follow-on maintenance provisions â â â â â Innovative financing (PPP/concession) â â â â â 200
C.8 Case Study Selection Matrix Case Study Selection Matrix Delivery Method Procurement Method # Case Study Name State Est. Value (millions) Pilot Design- Bid-Build Design- Build CM/GC PPP No Prequalifications Designer Prequalification Contractor Prequalification 1 Hastings River Bridge MN $ â â â â â â â â 2 Willamette River Bridge OR $ â â â â â â â â 3 $ â â â â â â â â 4 $ â â â â â â â â 5 $ â â â â â â â â 6 $ â â â â â â â â 7 $ â â â â â â â â 8 $ â â â â â â â â
TRB’s National Cooperative Highway Research Program (NCHRP) Web-Only Document 212: Alternative Quality Management Systems for Highway Construction documents the research process, data collection and analysis used to develop NCHRP Report 808: Guidebook on Alternative Quality Management Systems for Highway Construction .
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
Case Study Protocol for the Analysis of Sustainable Business Models
- Conference paper
- First Online: 22 June 2021
- Cite this conference paper

- Joaquin Sanchez-Planelles 3 &
- Marival Segarra-Oña 3
Part of the book series: Springer Proceedings in Business and Economics ((SPBE))
1631 Accesses
1 Citations
This paper is encompassed in the process of the development of a theory about sustainability. Our aim is to present a case study protocol for performing multiple-case studies about corporate sustainability. It has been designed according to the methodology about case study research. It includes a combination of frameworks that will help researchers to draw and understand how sustainability is integrated through the value chain of the company, and it also has a set of questions that will help to know the environmental practices that the target company deploys from a strategic point of view to the most common operations. Finally, once all the information has been retrieved, it will be possible to identify the way that value generated by sustainability practices flows through the company’s activities and the way customers perceive it.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

Practice Review of Business Models for Sustainability

Sustainable business model: A review and framework development

Business Models for Sustainability
https://www.consum.es/ .
Bengtsson, M. (2016). How to plan and perform a qualitative study using content analysis. NursingPlus Open, 2 (2016), 8–14.
Article Google Scholar
Bocken, N., Short, S., Rana, P., & Evans, S. (2013). A value mapping tool for sustainable business modelling. Corporate Governance, 13 (5), 482–497.
Bower, J. L. (1986). Managing the resource allocation process: A study of corporate planning and investment . Harvard Business School Press.
Google Scholar
Carlile, P., & Christensen, C. (2005). The cycles of theory building in management research.
Chairul, F. (2019). Business development of coffee farmers group using triple layered business model canvas. GATR Journals, Global Academy of Training and Research (GATR) Enterprise . https://EconPapers.repec.org/RePEc:gtr:gatrjs:jber182 .
Cheng, C. C. J., Yang, C.-L., & Sheu, C. (2014). The link between eco-innovation and business performance: A Taiwanese industry context.
Christensen, C., & Johnson, M. (2009). What are business models, and how are they built? Harvard Business School Module Note 610–019, August 2009. (Revised May 2016).
Downe-Wambolt, B. (1992). Content analysis: Method, applications and issues. Health Care for Women International, 13, 313–321.
Eccles, R. G., Ioannou, I., & Serafeim, G. (2014). The impact of corporate sustainability on organizational processes and performance. Management Science, 60 (11), 2835–2857.
Esty, D. C., & Winston, A. S. (2006). Green to gold: How smart companies use environmental strategy to innovate, create value, and build competitive advantage . Yale University Press.
França, C. L., Bromana, G., Karl-Henrik, R., Basile, B., & Trygg, L. (2016). An approach to business model innovation and design for strategic sustainable development. Journal of Cleaner Production, 140 (Part 1), 155–166.
Freeman, R. E. (1984). Strategic management: A stakeholder approach . Pitman.
Guinee, J. B. (2002). Handbook on life cycle assessment operational guide to the ISO standards. International Journal of Life Cycle Assessment, 7, 311.
Hillebrand, B., Kok, R., & Biemans, W. G. (2001). Theory-testing using case studies: A comment on Johnston, Leach, and Liu. Industrial Marketing Management, 30, 651–657.
Ioannou, I., & Serafeim, G. (2019). Corporate sustainability: A strategy? (January 1, 2019). Harvard Business School Accounting & Management Unit Working Paper No. 19–065.
Johnston, W. J., Leach, M. P., & Liu, A. H. (1999). Theory testing using case studies in business-to-business research. Industrial Marketing Management, 28, 201–213.
Joyce, A., & Paquin, R. (2016). The triple layered business model canvas: A tool to design more sustainable business models. Journal of Cleaner Production, 135 (2016), 1474–1486.
Krippendorff, K. (2004). Content analysis: An introduction to its methodology . Sage.
Myers, M. D. (2013). Qualitative research in business & management (2nd ed.). Sage.
Nidumolu, R., Prahalad, C. K., & Rangaswami, M. R. (2009). Why sustainability is now the key driver of innovation. In Harvard Business Review September Issue .
Osterwalder, A., & Pigneur, Y. (2010). Business model generation: A handbook for visionaries, game changers, and challengers . Wiley.
Patton, M. Q. (1990). Qualitative evaluation and research methods . Sage.
Porter, M. E. (1985). Competitive advantage. Creating and sustaining superior performance (p. 557). Free Press.
Porter, M. E., & Kramer, M. R. (2011, January–February). Creating shared value. Harvard Business Review, 89 (1–2), 62–77.
Rodríguez-Vilá, O., & Bharadwaj, S. (2017, September–October). Competing on social purpose. Harvard Business Review .
Sanchez-Planelles, J., & Segarra-Oña, M. (2019a). Conference paper: Modelos de negocio sostenibles y su implementación en el mercado. II Congreso Iberoamericano AJICEDE. Valencia, 28–29th November.
Sanchez-Planelles, J., & Segarra-Oña, M. (2019b). Reshaping Business Models with an Environmental Perspective. Corporate Social Responsibility in the Manufacturing and Services Sectors, EcoProduction. Springer Nature 2019.
Sanchez-Planelles, J., & Segarra-Oña, M. Conference paper: Modelos de negocio sostenibles y su implementación en el mercado. II Congreso Iberoamericano AJICEDE. Valencia, 28–29th November.
Sanchez-Planelles, J., Segarra-Oña, M., & Peiro-Signes, A. (2021). Building a Theoretical Framework for Corporate Sustainability. Sustainability , 13 (1), 273.
Schaltegger, S., Hansen, E., & Lüdeke-Freund, F. (2016). Business models for sustainability: Origins, present research, and future avenues. Organization & Environment., 29, 3–10.
Secretaría Técnica del Laboratorio de Ecoinnovación. (2016). Informe deTendencias #1: Modelos de Negocio Ecoinnovadores. Available from https://www.laboratorioecoinnovacion.com/informes_de_tendencias .
Segarra Oña, M., Merello Giménez, P., Segura Maroto, M., Peiró Signes, A., & Maroto Álvarez, M. (2012). Proactividad medioambiental en la empresa: Clasificación empírica y determinación de aspectos clave. Tec Empresarial, 6 (1), 35–48.
Segarra-Oña, M., Peiró-Signes, A., Mondéjar-Jiménez, J., & Sáez-Martínez, F. J. (2016). Friendly environmental policies implementation within the company: An ESG ratings analysis and its applicability to companies environmental performance enhancement. Global NEST Journal, 18 (4), 885–893.
Steckler, A., McLeroy, K. R., Bird, S. T., & McCormick, L. (1992). Toward integrating qualitative and quantitative methods: An introduction. Health Education Quarterly, 19 (1), 1–8.
Walsham, G. (2006). Doing interpretive research. European Journal of Information Systems, 15, 320–330.
Wohlin, C. (2014). Guidelines for snowballing in systematic literature studies and a replication in software engineering. In EASE ‘14.
Yin, R. (1993). K Applications of case study research . Sage.
Yin, R. K. (2013). Validity and generalization in future case study evaluations. Evaluation, 19 (3), 321–332. https://doi.org/10.1177/1356389013497081 .
Yin, R. K. (2003). Case study research: Design and methods . Sage.
Download references
Acknowledgements
We acknowledge Mr. Elías Amor Montiel from the ESG Department of Consum for his help and collaboration in the interview and providing us with data.
Author information
Authors and affiliations.
Universitat Politècnica de València, Valencia, Spain
Joaquin Sanchez-Planelles & Marival Segarra-Oña
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Joaquin Sanchez-Planelles .
Editor information
Editors and affiliations.
University of West Attica, Athens, Greece
Vicky Katsoni
University of South Africa, Pretoria, South Africa
Ciná van Zyl
Questions about Holistic Sustainability
Checking the company’s sustainable policies against these questions will allow to identify how board members deal with the subject matter and know what kind of strategies is executing the company.
Question 1: What motivated the company to become sustainable? What is the company’s purpose?
Source of data:
CEO (Chief Executive Officer)
CSO (Chief Strategy Officer)
CSO (Chief Sustainability Officer)
Sample strategies :
Mission, vision and values statements from website.
Media press.
Question 2: How is the market dealing with sustainability? Is this company a leader, follower or laggard on the implementation of sustainable practices?
Product manager
Product manager assistant
Sales manager
Products portfolio
List the direct competitors of the company.
Identify which attributes about sustainability their products have.
Identify which attributes about sustainability those companies have.
Gather data about when sustainable practices were announced by direct competitors in order to determine which one is the leader, follower and laggard.
Question 3: Analysis of the influence of sustainability in the decision-making process. Are environmental criteria taken into account during the decision-making process? List the environmental criteria used for the decision-making process. Is the company’s statement about sustainability aligned with the decision-making process?
Identify the decision-making process established by the board members of the company.
Estimate how much environmental concerns are taken into consideration during the decision-making process.
Create a framework or diagram of the decision-making process.
Question 4: What is the process for detecting market needs focused on sustainable attributes?
CMO (Chief Marketing Officer)
Sales Manager
Identify if there is a department focused on detecting market trends.
Gather data about providers that offer services related to markets analysis, consumer studies, etc.
Question 5: Analysis of the relationship with stakeholders and the influence of sustainability in the relationship between company and stakeholders.
Determine what kind of relationships has the company with:
Capital market
Networks and associations
Policymakers
Business partners
Local stakeholders
Civil society and NGO’s
Question 6: Identify if the company’s board members establish environmental goals for the short, medium and long term.
UN Sustainable Development Goals
Materiality matrix
Questions about Sustainable Business Models:
Checking the company’s business model against these questions will allow to identify how the company creates superior value to customers improving the society and reducing the environmental impact.
Question 7: Identify which activities generate value through sustainability and determine flows of value through activities.
Draw the Porter’s value chain and complete each activity
Draw the value flows between activities from the value chain.
Question 8: Classify the sustainable business model developed by the company: circular economy, sustainable production, servitisation and sustainable consumption.
Draw the flows of inputs and outputs that take part in the business.
Create an organisational chart of the company that shows the different business lines and possible sustainable business models within the company (e.g. circular economy procedures to revalorise waste).
Whom does the business model supervise?
What customer segment does the business model target? Is it targeting external or internal customers?
Question 9: Are eco-friendly products and/or services addressed to a specific market niche or are they launched to broad customer segments?
Reports about the market sector.
News or press notes published in mass media.
Question 10: How does the company informs or communicates the sustainable practices to customers, users and other groups of interest?
Chief of Staff
Identify the channels used to deliver information: videos, seminars, conferences, online courses, short sessions, etc.
Question 11: Does the company consider the degree of sustainability of its providers or partners? If does, what are those criteria?
Purchasing manager
Identify the most key partners and providers of the company.
Examine what criteria o requirements need to be matched in order to work with the company. For instance: ISO 14001, EMAS, green certificates, eco certificates, etc.
Questions about Sustainable Operations.
Checking the company’s operations against these questions will allow to identify how business processes from the operational level might reduce the environmental impact.
Question 12: List the products and/or services which incorporate eco-innovative attributes.
Draw a chart with the products and services managed by business line.
Retrieve information about eco-innovative technologies and practices developed for the last three years.
Check the eco-innovative practices that have been integrated in the company’s products or services.
Question 13: What decision-making process or criteria is considered by the company to invest resources in the development and release of eco-innovative products / services?
R&D manager
Retrieve information about eco-innovative technologies and practices developed for the last three years and identify characteristics which are similar between each other.
Identification of the customers’ needs that try to solve the eco-innovative products / services.
Question 14: Identify the business areas that create value through sustainable activities.
Complete the business model canvas (Osterwalder & Pigneur, 2010 ).
Complete the triple-layered business model canvas (Joyce & Paquin, 2016 ).
Question 15: Are the channels to deliver products and services to your customers sustainable?
CLO (Chief Logistics Officer)
Identify the channels used to deliver products and services to customers: vehicle fleet, logistics, shops, offices, stores, etc.
Identify which eco-innovations or sustainable practices have been implemented in each channel. For instance, energy efficiency processes in cooling systems, eco-innovative trucks, etc.
Question 16: Have the company implemented any measure to reduce the environmental impact of its assets? For instance, energy efficiency measures in offices and production plants, emissions-reduction devices, etc.
Production Manager
Chief of Maintenance
Identify the strategic assets of the company. For instance, production centre, factory, stores, shops, offices, vehicle fleets, fields, etc.
Examine what environmental improvements have been implemented recently in the facilities and assets. For instance, acquisitions of eco-innovative production systems, installation of solar panels, implementation of sustainable architecture principles in the company’s buildings, etc.
Questions about Sustainable Methodologies.
These questions will show if methodologies designed for implementing sustainable practices among companies are widely used by managers and which of them are the most commonly applied.
Question 17: Identify if the company has any green certificate (e.g. ISO 14,001, EMAS, BREAM, Ecologic label, etc.) or if it is working to achieve one.
Information retrieved from website.
Question 18: Examine if the company works with any international standard to report its sustainable practices.
Some of the most common international standards for measuring the implementation of sustainable practices are:
GRI (Global Reporting Initiative)
Rainforest Alliance
Question 19: Did the company use any framework or methodology to implement sustainable practices?
Some of the most common sustainable methodologies and frameworks are:
Triple-Layered Canvas
Framework for Strategic Sustainable Development
Shareholder-value framework
Value Mapping Tool
Questions about the Evolution of the Corporate Sustainability.
This question will classify the company in the stage of the corporate sustainability evolution and will enlighten the potential practices that might be deployed in order to move forward to a sustainable business model or a new business platform.
Question 20: According to the different stages of the corporate sustainability evolution, in which stage does the company fit?
Sustainability memories of the company.
Classify the company according to the following corporate sustainability stages:
Sustainable Value Chain
Eco-innovative practices
Sustainable Business Model
New business platforms
Rights and permissions
Reprints and permissions

Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper.
Sanchez-Planelles, J., Segarra-Oña, M. (2021). Case Study Protocol for the Analysis of Sustainable Business Models. In: Katsoni, V., van Zyl, C. (eds) Culture and Tourism in a Smart, Globalized, and Sustainable World. Springer Proceedings in Business and Economics. Springer, Cham. https://doi.org/10.1007/978-3-030-72469-6_10
Download citation
DOI : https://doi.org/10.1007/978-3-030-72469-6_10
Published : 22 June 2021
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-72468-9
Online ISBN : 978-3-030-72469-6
eBook Packages : Business and Management Business and Management (R0)
Share this paper
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- [email protected]
- Connecting and sharing with us

- Entrepreneurship
- Growth of firm
- Sales Management
- Retail Management
- Import – Export
- International Business
- Project Management
- Production Management
- Quality Management
- Logistics Management
- Supply Chain Management
- Human Resource Management
- Organizational Culture
- Information System Management
- Corporate Finance
- Stock Market
- Office Management
- Theory of the Firm
- Management Science
- Microeconomics
- Research Process
- Experimental Research
- Research Philosophy
- Management Research
- Writing a thesis
- Writing a paper
- Literature Review
- Action Research
- Qualitative Content Analysis
- Observation
- Phenomenology
- Statistics and Econometrics
- Questionnaire Survey
- Quantitative Content Analysis
- Meta Analysis
The Case Study Protocol
A case study protocol has only one thing in common with a survey questionnaire: Both are directed at a single data point—either a single case (even if the case is part of a larger, multiple-case study) or a single respondent.
Beyond this similarity are major differences. The protocol is more than a questionnaire or instrument. First, the protocol contains the instrument but also contains the procedures and general rales to be followed in using the protocol. Second, the protocol is directed at an entirely different party than that of a survey questionnaire, explained below. Third, having a case study protocol is desirable under all circumstances, but it is essential if you are doing a multiple-case study.
The protocol is a major way of increasing the reliability of case study research and is intended to guide the investigator in carrying out the data collection from a single case (again, even if the single case is one of several in a multiple-case study). Figure 3.2 gives a table of contents from an illustrative protocol, which was used in a study of innovative law enforcement practices supported by federal funds. The practices had been defined earlier through a careful screening process (see later discussion in this chapter for more detail on “screening case study nominations”). Furthermore, because data were to be collected from 18 such cases as part of a multiple-case study, the information about any given case could not be collected in great depth, and thus the number of the case study questions was minimal.

As a general matter, a case study protocol should have the following sections:
- an overview of the case study project (project objectives and auspices, case study issues, and relevant readings about the topic being investigated),
- field procedures (presentation of credentials, access to the case study “sites,” language pertaining to the protection of human subjects, sources of data, and procedural reminders),
- case study questions (the specific questions that the case study investigator must keep in mind in collecting data, “table shells” for specific arrays of data, and the potential sources of information for answering each question—see Figure 3.3 for an example), and
- a guide for the case study report (outline, format for the data, use and presentation of other documentation, and bibliographical information).
A quick glance at these topics will indicate why the protocol is so important. First, it keeps you targeted on the topic of the case study. Second, preparing the protocol forces you to anticipate several problems, including the way that the case study reports are to be completed. This means, for instance, that you will have to identify the audience for your case study report even before you have conducted your case study. Such forethought will help to avoid mismatches in the long run.

The table of contents of the illustrative protocol in Figure 3.2 reveals another important feature of the case study report: In this instance, the desired report starts by calling for a description of the innovative practice being studied (see item Cl in Figure 3.2)—and only later covers the agency context and history pertaining to the practice (see item C4). This choice reflects the fact that most investigators write too extensively on history and background conditions. While these are important, the description of the subject of the study—the innovative practice—needs more attention.
Each section of the protocol is discussed next.
1. Overview of the Case Study Project
The overview should cover the background information about the project, the substantive issues being investigated, and the relevant readings about the issues.
As for background information, every project has its own context and perspective. Some projects, for instance, are funded by government agencies having a general mission and clientele that need to be remembered in conducting the research. Other projects have broader theoretical concerns or are offshoots, of earlier research studies. Whatever the situation, this type of background information, in summary form, belongs in the overview section.
A procedural element of this background section is a statement about the project which you can present to anyone who may want to know about the project, its purpose, and the people involved in conducting and sponsoring the project. This statement can even be accompanied by a letter of introduction, to be sent to all major interviewees and organizations that may be the subject of study. (See Figure 3.4 for an illustrative letter.) The bulk of the overview, however, should be devoted to the substantive issues being investigated. This may include the rationale for selecting the case(s), the propositions or hypotheses being examined, and the broader theoretical or policy relevance of the inquiry. For all of these topics, relevant readings should be cited, and the essential reading materials should be made available to everyone on the case study team.
A good overview will communicate to the informed reader (that is, someone familiar with the general topic of inquiry) the case study’s purpose and setting. Some of the materials (such as a summary describing the project) may be needed for other purposes anyway, so that writing the overview should be seen as a doubly worthwhile activity. In the same vein, a well-conceived overview even may later form the basis for the background and introduction to the final case study report.
2. Field Procedures
Chapter 1 has previously defined case studies as studies of events within their real-life context. This has important implications for defining and designing the case study, which have been discussed in Chapters 1 and 2.
For data collection, however, this characteristic of case studies also raises an important issue, for which properly designed field procedures are essential. You will be collecting data from people and institutions in their everyday situations, not within the controlled confines of a laboratory, the sanctity of a library, or the structured limitations of a survey questionnaire. In a case study, you must therefore learn to integrate real-world events with the needs of the data collection plan. In this sense, you do not have the control over the data collection environment as others might have in using the other research methods discussed in Chapter 1.
Note that in a laboratory experiment, human “subjects” are solicited to enter into the laboratory—an environment controlled nearly entirely by the research investigator. The subject, within ethical and physical constraints, must follow the investigator’s instructions, which carefully prescribe the desired behavior. Similarly, the human “respondent” to a survey questionnaire cannot deviate from the agenda set by the questions. Therefore, the respondent’s behavior also is constrained by the ground rules of the investigator. Naturally, the subject or respondent who does not wish to follow the prescribed behaviors may freely drop out of the experiment or survey. Finally, in the historical archive, pertinent documents may not always be available, but the investigator can inspect what exists at his or her own pace and at a time convenient to her or his schedule. In all three situations, the research investigator closely controls the formal data collection activity.

Doing case studies involves an entirely different situation. For interviewing key persons, you must cater to the interviewee’s schedule and availability, not your own. The nature of the interview is much more open-ended, and an interviewee may not necessarily cooperate fully in sticking to your line of questions. Similarly, in making observations of real-life activities, you are intruding into the world of the subject being studied rather than the reverse; under these conditions, you are the one who may have to make special arrangements, to be able to act as an observer (or even as a participant- observer). As a result, your behavior—and not that of the subject or respondent—is the one likely to be constrained.
This contrasting process of doing data collection leads to the need to have explicit and well-planned field procedures encompassing guidelines for “coping” behaviors. Imagine, for instance, sending a youngster to camp; because you do not know what to expect, the best preparation is to have the resources to be prepared. Case study field procedures should be the same way.
With the preceding orientation in mind, the field procedures of the protocol need to emphasize the major tasks in collecting data, including
- gaining access to key organizations or interviewees;
- having sufficient resources while in the field—including a personal computer, writing instruments, paper, paper clips, and a preestablished, quiet place to write notes privately;
- developing a procedure for calling for assistance and guidance, if needed, from other case study investigators or colleagues;
- making a clear schedule of the data collection activities that are expected to be completed within specified periods of time; and
- providing for unanticipated events, including changes in the availability of interviewees as well as changes in the mood and motivation of the case study investigator.
These are the types of topics that can be included in the field procedures section of the protocol. Depending upon the type of study being done, the specific procedures will vary.
The more operational these procedures are, the better. To take but one minor issue as an example, case study data collection frequently results in the accumulation of numerous documents at the field site. The burden of carrying such bulky documents can be reduced by two procedures. First, the case study team may have had the foresight to bring large, prelabeled envelopes, to mail the documents back to the office rather than carry them. Second, field time may have been set aside for perusing the documents and then going to a local copier facility and copying only the few relevant pages of each document—and then returning the original documents to the informants at the field site. These and other operational details can enhance the overall quality and efficiency of case study data collection.
A final part of this portion of the protocol should carefully describe the procedures for protecting human subjects. First, the protocol should repeat the rationale for the IRB-approved field procedures. Then, the protocol should include the “scripted” words or instructions for the team to use in obtaining informed consent or otherwise informing case study interviewees and other participants of the risks and conditions associated with the research.
3. Case Study Questions
The heart of the protocol is a set of substantive questions reflecting your actual line of inquiry. Some people may consider this part of the protocol to be the case study “instrument.” However, two characteristics distinguish case study questions from those in a survey instrument. (Refer back to Figure 3.3 for an illustrative question from a study of a school program; the complete protocol included dozens of such questions.)
General orientation of questions. First, the questions are posed to you, the investigator, not to an interviewee. In this sense, the protocol is directed at an entirely different party than a survey instrument. The protocol’s questions, in essence, are your reminders regarding the information that needs to be collected, and why. In some instances, the specific questions also may serve as prompts in asking questions during a case study interview. However, the main purpose of the protocol’s questions is to keep the investigator on track as data collection proceeds.
Each question should be accompanied by a list of likely sources of evidence. Such sources may include the names of individual interviewees, documents, or observations. This crosswalk between the questions of interest and the likely sources of evidence is extremely helpful in collecting case study data. Before arriving on the case study scene, for instance, a case study investigator can quickly review the major questions that the data collection should cover.
(Again, these questions form the structure of the inquiry and are not intended as the literal questions to be asked of any given interviewee.)
Levels of questions. Second, the questions in the case study protocol should distinguish clearly among different types or levels of questions. The potentially relevant questions can, remarkably, occur at any of five levels:
Level 1: questions asked of specific interviewees;
Level 2: questions asked of the individual case (these are the questions in the case study protocol to be answered by the investigator during a single case, even when the single case is part of a larger, multiple-case study);
Level 3: questions asked of the pattern of findings across multiple cases;
Level 4: questions asked of an entire study—for example, calling on information beyond the case study evidence and including other literature or published data that may have been reviewed; and
Level 5: normative questions about policy recommendations and conclusions, going beyond the narrow scope of the study.
Of these five levels, you should concentrate heavily on Level 2 for the case study protocol.
The difference between Level 1 and Level 2 questions is highly significant. The two types of questions are most commonly confused because investigators think that their questions of inquiry (Level 2) are synonymous with the specific questions they will ask in the field (Level 1). To disentangle these two levels in your own mind, think again about a detective, especially a wily one. The detective has in mind what the course of events in a crime might have been (Level 2), but the actual questions posed to any witness or suspect (Level 1) do not necessarily betray the detective’s thinking. The verbal line of inquiry is different from the mental line of inquiry, and this is the difference between Level 1 and Level 2 questions. For the case study protocol, explicitly articulating the Level 2 questions is therefore of much greater importance than any attempt to identify the Level 1 questions.
In the field, keeping in mind the Level 2 questions while simultaneously articulating Level 1 questions in conversing with an interviewee is not easy. In a like manner, you can lose sight of your Level 2 questions when examining a detailed document that will become part of the case study evidence (the common revelation occurs when you ask yourself, “Why am I reading this document?”). To overcome these problems, successful participation in the earlier seminar training helps. Remember that being a “senior” investigator means maintaining a working knowledge of the entire case study inquiry. The (Level 2) questions in the case study protocol embody this inquiry.
The other levels also should be understood clearly. A cross-case question, for instance (Level 3), may be whether the larger school districts among your cases are more responsive than smaller school districts or whether complex bureaucratic structures make the larger districts more cumbersome and less responsive. However, this Level 3 question should not be part of the protocol for collecting data from the single case, because the single case only can address the responsiveness of a single school district. The Level 3 question cannot be addressed until the data from all the single cases (in a multiple-case study) are examined. Thus, only the multiple-case analysis can cover Level 3 questions. Similarly, the questions at Levels 4 and 5 also go well beyond any individual case study, and you should note this limitation if you include such questions in the case study protocol. Remember: The protocol is for the data collection from a single case (even when part of a multiple-case study) and is not intended to serve the entire project.
Undesired confusion between unit of data collection and unit of analysis. Related to the distinction between Level 1 and Level 2 questions, a more subtle and serious problem can arise in articulating the questions in the case study protocol. The questions should cater to the unit of analysis of the case study, which may be at a different level from the unit of data collection of the case study. Confusion will occur if, under these circumstances, the data collection process leads to an (undesirable) distortion of the unit of analysis.
The common confusion begins because the data collection sources may be individual people (e.g., interviews with individuals), whereas the unit of analysis of your case study may be a collective (e.g., the organization to which the individual belongs)—a frequent design when the case study is about an organization, community, or social group. Even though your data collection may have to rely heavily on information from individual interviewees, your conclusions cannot be based entirely on interviews as a source of information (you would then have collected information about individuals’ reports about the organization, not necessarily about organizational events as they actually had occurred). In this example, the protocol questions therefore need to be about the organization, not the individual.
However, the reverse situation also can be true. Your case study may be about an individual, but the sources of information can include archival records (e.g., personnel files or student records) from an organization. In this situation, you also would want to avoid basing your conclusions about the individual from the organizational sources of information only. In this example, the protocol questions therefore need to be about the individual, not the organization.
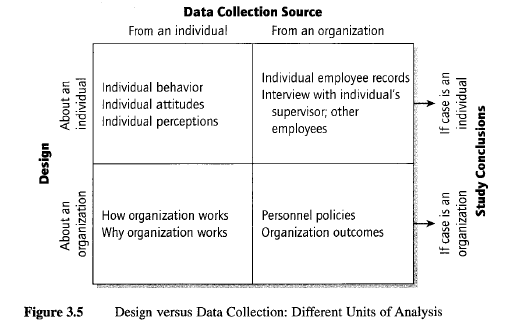
Figure 3.5 illustrates these two situations, where the unit of analysis for the case study is different from the unit of data collection.
Other data collection devices. The protocol questions also can include empty “table shells” (for more detail, see Miles & Huberman, 1994). These are the outlines of a table, defining precisely the “rows” and “columns” of a data array—but in the absence of having the actual data. In this sense, the table shell indicates the data to be collected, and your job is to collect the data called forth by the table. Such table shells help in several ways. First, the table shells force you to identify exactly what data are being sought. Second, they ensure that parallel information will be collected at different sites, where a multiple- case design is being used. Finally, the table shells aid in understanding what will be done with the data once they have been collected.
4. Guide for the Case Study Report
This element is generally missing in most case study plans. Investigators neglect to think about the outline, format, or audience for the case study report until after the data have been collected. Yet, some planning at this preparatory stage—admittedly out of sequence in the typical conduct of most research— means that a tentative outline can (and should) appear in the case study protocol. (Such planning accounts for the arrow between “prepare” and “share” in the figure at the outset of this chapter.)
Again, one reason for the traditional, linear sequence is related to practices with other research methods. One does not worry about the report from an experiment until after the experiment has been completed, because the format of the report and its likely audience already have been dictated by the conventional formats of academic journals. Most reports of experiments follow a similar outline: the posing of the research questions and hypotheses; a description of the research design, apparatus, and data collection procedures; the presentation of the data collected; the analysis of the data; and a discussion of findings and conclusions.
Unfortunately, case study reports do not have such a uniformly acceptable outline. Nor, in many instances, do case study reports end up in journals (Feagin et al., 1991, pp. 269-273). For this reason, each investigator must be concerned, throughout the conduct of a case study, with the design of the final case study report. The problem is not easy to deal with.
In addition, the protocol also can indicate the extent of documentation for the case study report. Properly done, the data collection is likely to lead to large amounts of documentary evidence, in the form of published reports, publications, memoranda, and other documents collected about the case. What is to be done with this documentation, for later presentation? In most studies, the documents are filed away and seldom retrieved. Yet, this documentation is an important part of the “database” for a case study (see Chapter 4) and should not be ignored until after the case study has been completed. One possibility is to have the case study report include an annotated bibliography in which each of the available documents is itemized. The annotations would help a reader (or the investigator, at some later date) to know which documents might be relevant for further inquiry.
In summary, to the extent possible, the basic outline of the case study report should be part of the protocol. This will facilitate the collection of relevant data, in the appropriate format, and will reduce the possibility that a return visit to the case study site will be necessary. At the same time, the existence of such an outline should not imply rigid adherence to a predesigned protocol. In fact, case study plans can change as a result of the initial data collection, and you are encouraged to consider these flexibilities—if used properly and without bias—to be an advantage of the case study method.
EXERCISE 3.4 Developing a Case Study Protocol
Select some phenomenon in need of explanation from the everyday life of your university or school (past or present). Illustrative topics might be, for example, why the university or school changed some policy or how it makes decisions about its curriculum requirements. For these illustrative topics (or some topics of your own choosing), design a case study protocol to collect the information needed to make an adequate explanation. What would be your main research questions or propositions? What specific sources of data would you seek (e.g., persons to be interviewed, documents to be sought, and field observations to be made)? Would your protocol be sufficient in guiding you through the entire process of doing your case study?
Source: Yin K Robert (2008), Case Study Research Designs and Methods , SAGE Publications, Inc; 4th edition.
13 Aug 2021
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Username or email address *
Password *
Log in Remember me
Lost your password?

Before you submit
In this section:
- NEW! Featured Author Support
- How to choose a journal
- Submitting to a Topic Collection
- See our calls for papers for Topic Collections
- See our published Topic Collections
- Become a Guest Editor
- Reporting guidelines
- Patient and public partnership
- Study protocols
- Scientific misconduct
- Trial registration
- Authorship and contributorship
- Research Ethics
- Patient consent and confidentiality
- Competing interests
- From research to publication
- Language editing services
- Writing for online visibility
Why publish study protocols?
- Keep researchers and funding bodies up-to-date in their fields
- Give exposure to research activity that otherwise may not get publicised
- Enable more collaboration in the research community
- Prevent unnecessary duplication of work
- Increase transparency by making more information available than required by trial registries
- Give others the opportunity to see and understand deviations that occur during the study
BMJ requires authors of clinical trials submitted as research articles to upload a protocol for their study as a supplementary file. This protocol will be published alongside other materials if the article is accepted.
If your protocol is for a randomised trial
We encourage authors to adhere to the SPIRIT recommendations .
The SPIRIT (Standard Protocol Items for Randomized Trials) statement is an evidence-based tool developed through systematic review of a wide range of resources and consensus. It closely mirrors the CONSORT statement and reflects important ethical considerations.
If your protocol is for a systematic review or meta-analysis

Macro Comparisons without the Pitfalls: A Protocol for Comparative Research
- https://kellogg.nd.edu/opportunities/nd-faculty/working-papers
Usage metrics

Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Deep learning for flash drought detection: a case study in northeastern brazil.

Share and Cite
Barbosa, H.A.; Buriti, C.O.; Kumar, T.V.L. Deep Learning for Flash Drought Detection: A Case Study in Northeastern Brazil. Atmosphere 2024 , 15 , 761. https://doi.org/10.3390/atmos15070761
Barbosa HA, Buriti CO, Kumar TVL. Deep Learning for Flash Drought Detection: A Case Study in Northeastern Brazil. Atmosphere . 2024; 15(7):761. https://doi.org/10.3390/atmos15070761
Barbosa, Humberto A., Catarina O. Buriti, and T. V. Lakshmi Kumar. 2024. "Deep Learning for Flash Drought Detection: A Case Study in Northeastern Brazil" Atmosphere 15, no. 7: 761. https://doi.org/10.3390/atmos15070761
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Assessing biomarkers of remission in female patients with anorexia nervosa (REMANO): a protocol for a prospective cohort study with a nested case-control study using clinical, neurocognitive, biological, genetic, epigenetic and neuroimaging markers in a French specialised inpatient unit
Affiliations.
- 1 Institute of Psychiatry and Neuroscience of Paris (IPNP), INSERM U1266, Team "Vulnerability to Psychiatric and Addictive Disorders", Université Paris Cité, Paris, France [email protected].
- 2 Clinique des Maladies Mentales et de l'Encéphale, Hôpital Sainte-Anne, GHU Paris Psychiatrie et Neurosciences, Paris, France.
- 3 Institute of Psychiatry and Neuroscience of Paris (IPNP), INSERM U1266, Team "Vulnerability to Psychiatric and Addictive Disorders", Université Paris Cité, Paris, France.
- 4 Institute of Psychiatry and Neuroscience of Paris (IPNP), INSERM U1266, IMA-Brain, Université Paris Cité, Paris, France.
- 5 Service de Neuroradiologie, Hôpital Sainte-Anne, GHU Paris psychiatrie et neurosciences, Paris, France.
- 6 CNRS, UMR 9193 - SCALab - Sciences Cognitives et Sciences Affectives, University of Lille, Villeneuve d'Ascq, France.
- PMID: 38925688
- PMCID: PMC11208877
- DOI: 10.1136/bmjopen-2023-077260
Background: Anorexia nervosa (AN) is a severe psychiatric disorder associated with frequent relapses and variability in treatment responses. Previous literature suggested that such variability is influenced by premorbid vulnerabilities such as abnormalities of the reward system. Several factors may indicate these vulnerabilities, such as neurocognitive markers (tendency to favour delayed reward, poor cognitive flexibility, abnormal decision process), genetic and epigenetic markers, biological and hormonal markers, and physiological markers.The present study will aim to identify markers that can predict body mass index (BMI) stability 6 months after discharge. The secondary aim of this study will be focused on characterising the biological, genetic, epigenetic and neurocognitive markers of remission in AN.
Methods and analysis: One hundred and twenty-five (n=125) female adult inpatients diagnosed with AN will be recruited and evaluated at three different times: at the beginning of hospitalisation, when discharged and 6 months later. Depending on the BMI at the third visit, patients will be split into two groups: stable remission (BMI≥18.5 kg/m²) or unstable remission (BMI<18.5 kg/m²). One hundred (n=100) volunteers will be included as healthy controls.Each visit will consist in self-reported inventories (measuring depression, anxiety, suicidal thoughts and feelings, eating disorders symptoms, exercise addiction and the presence of comorbidities), neurocognitive tasks (Delay Discounting Task, Trail-Making Test, Brixton Test and Slip-of-action Task), the collection of blood samples, the repeated collection of blood samples around a standard meal and MRI scans at rest and while resolving a delay discounting task.Analyses will mainly consist in comparing patients stabilised 6 months later and patients who relapsed during these 6 months.
Ethics and dissemination: Investigators will ask all participants to give written informed consent prior to participation, and all data will be recorded anonymously. The study will be conducted according to ethics recommendations from the Helsinki declaration (World Medical Association, 2013). It was registered on clinicaltrials.gov on 25 August 2020 as 'Remission Factors in Anorexia Nervosa (REMANO)', with the identifier NCT04560517 (for more details, see https://clinicaltrials.gov/ct2/show/record/NCT04560517). The present article is based on the latest protocol version from 29 November 2019. The sponsor, Institut National de la Santé Et de la Recherche Médicale (INSERM, https://www.inserm.fr/), is an academic institution responsible for the monitoring of the study, with an audit planned on a yearly basis.The results will be published after final analysis in the form of scientific articles in peer-reviewed journals and may be presented at national and international conferences.
Trial registration number: clinicaltrials.gov NCT04560517 .
Keywords: Adult psychiatry; Eating disorders; Magnetic resonance imaging.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
PubMed Disclaimer
Conflict of interest statement
Competing interests: None declared.
Similar articles
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
- Inpatient versus outpatient care, partial hospitalisation and waiting list for people with eating disorders. Hay PJ, Touyz S, Claudino AM, Lujic S, Smith CA, Madden S. Hay PJ, et al. Cochrane Database Syst Rev. 2019 Jan 21;1(1):CD010827. doi: 10.1002/14651858.CD010827.pub2. Cochrane Database Syst Rev. 2019. PMID: 30663033 Free PMC article.
- [The psychiatric comorbidity of anorexia nervosa: A comparative study in a population of French and Greek anorexic patients]. Kountza M, Garyfallos G, Ploumpidis D, Varsou E, Gkiouzepas I. Kountza M, et al. Encephale. 2018 Nov;44(5):429-434. doi: 10.1016/j.encep.2017.07.005. Encephale. 2018. PMID: 29102367 French.
- Individual psychological therapy in the outpatient treatment of adults with anorexia nervosa. Hay PJ, Claudino AM, Touyz S, Abd Elbaky G. Hay PJ, et al. Cochrane Database Syst Rev. 2015 Jul 27;2015(7):CD003909. doi: 10.1002/14651858.CD003909.pub2. Cochrane Database Syst Rev. 2015. PMID: 26212713 Free PMC article. Review.
- [Anorexia nervosa in the light of neurocognitive functioning: New theoretical and therapeutic perspectives]. Martinez G, Cook-Darzens S, Chaste P, Mouren MC, Doyen C. Martinez G, et al. Encephale. 2014 Apr;40(2):160-7. doi: 10.1016/j.encep.2012.06.004. Epub 2013 Mar 28. Encephale. 2014. PMID: 23541918 Review. French.
- Trends Endocrinol Metab. 2019 Dec;30(12):915-928 - PubMed
- Lancet Psychiatry. 2016 Aug;3(8):706-707 - PubMed
- J Int Neuropsychol Soc. 2009 Sep;15(5):695-703 - PubMed
- J Neuroendocrinol. 2017 Oct;29(10): - PubMed
- Nutrients. 2021 Feb 23;13(2): - PubMed
Publication types
- Search in MeSH
Associated data
- Search in ClinicalTrials.gov
Related information
Linkout - more resources, full text sources.
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Open access
- Published: 27 June 2024
Chemotherapy-related cardiotoxicity and its symptoms in patients with breast cancer: a scoping review
- Hyunjoo Kim 1 , 2 ,
- Bomi Hong 3 ,
- Sanghee Kim 4 ,
- Seok-Min Kang 5 &
- Jeongok Park ORCID: orcid.org/0000-0003-4978-817X 4
Systematic Reviews volume 13 , Article number: 167 ( 2024 ) Cite this article
22 Accesses
Metrics details
Chemotherapy-related cardiotoxicity is a significant concern because it is a major cause of morbidity. This study aimed to provide in-depth information on the symptoms of chemotherapy-related cardiotoxicity (CRCT) by exploring literature that concurrently reports the types and symptoms of CRCT in patients with breast cancer.
A scoping review was performed according to an a priori protocol using the Joanna Briggs Institute’s guidelines. The participants were patients with breast cancer. The concept was the literature of specifically reported symptoms directly matched with CRCT and the literature, in English, from 2010, and the context was open. The search strategy included four keywords: “breast cancer,” “chemotherapy,” “cardiotoxicity,” and “symptoms.” All types of research designs were included; however, studies involving patients with other cancer types, animal subjects, and symptoms not directly related to CRCT were excluded. Data were extracted and presented including tables and figures.
A total of 29 articles were included in the study, consisting of 23 case reports, 4 retrospective studies, and 2 prospective studies. There were no restrictions on the participants’ sex; however, all of them were women, except for one case report. The most used chemotherapy regimens were trastuzumab, capecitabine, and doxorubicin or epirubicin. The primary CRCT identified were myocardial dysfunction and heart failure, followed by coronary artery disease, pulmonary hypertension, and other conditions. Major tests used to diagnose CRCT include echocardiography, electrocardiography, serum cardiac enzymes, coronary angiography, computed tomography, and magnetic resonance imaging. In all case reports, CRCT was diagnosed through an incidental checkup according to the patient’s symptom presentation; however, only 10 of these studies showed a baseline checkup before chemotherapy. The five most common CRCT symptoms were dyspnea, chest pain, peripheral edema, fatigue, and palpitations, which were assessed by patient-reported symptom presentation rather than using a symptom assessment tool. Dyspnea with trastuzumab treatment and chest pain with capecitabine treatment were particularly characteristic. The time for first symptom onset after chemotherapy ranged from 1 hour to 300 days, with anthracycline-based regimens requiring 3–55 days, trastuzumab requiring 60–300 days, and capecitabine requiring 1–7 days.
Conclusions
This scoping review allowed data mapping according to the study design and chemotherapy regimens. Cardiac assessments for CRCT diagnosis were performed according to the patient’s symptoms. There were approximately five types of typical CRCT symptoms, and the timing of symptom occurrence varied. Therefore, developing and applying a CRCT-specific and user-friendly symptom assessment tool are expected to help healthcare providers and patients manage CRCT symptoms effectively.
Peer Review reports
Breast cancer is currently the most common cancer worldwide. Its incidence and mortality rates in East Asia in 2020 accounted for 24% and 20% of the global rates, respectively, and these rates are expected to continue increasing until 2040 [ 1 ]. In the USA, since the mid-2000s, the incidence rate of breast cancer has been increasing by 0.5% annually, while the mortality rate has been decreasing by 1% per year from 2011 to 2020 [ 2 ]. Despite the improved long-term survival rate in patients with breast cancer due to the development of chemotherapy, the literature has highlighted that cardiotoxicity, a cardiac problem caused by chemotherapy, could be a significant cause of death among these patients [ 3 ]. Chemotherapy-related cardiotoxicity (CRCT) can interfere with cancer treatment and progress to congestive heart failure during or after chemotherapy [ 4 ], potentially lowering the survival rate and quality of life of patients with cancer [ 5 ].
The term cardiotoxicity was first used in the 1970s to describe cardiac complications resulting from chemotherapy regimens, such as anthracyclines and 5-fluorouracil. The early definition of cardiotoxicity centered around heart failure, but the current definition is broad and still imprecise [ 6 ]. The 2022 guidelines on cardio-oncology from the European Society of Cardiology (ESC) define cardiotoxicity as including cardiac dysfunction, myocarditis, vascular toxicity, arterial hypertension, and cardiac arrhythmias. Some of these definitions reflect the symptoms. For example, cardiac dysfunction, which accounts for 48% of cardiotoxicity in patients with cancer, is divided into asymptomatic and symptomatic cardiac dysfunction. Asymptomatic cardiac dysfunction is defined based on left ventricular ejection fraction (LVEF), myocardial global longitudinal strain, and cardiac biomarkers. Symptomatic cardiac dysfunction indicates heart failure and presents with ankle swelling, breathlessness, and fatigue [ 7 ]. The ESC guidelines for heart failure present more than 20 types of symptoms [ 8 ]; however, to the best of our knowledge, few studies have been conducted to determine which heart failure symptoms and their characteristics are associated with CRCT in patients with breast cancer. Similarly, there is a lack of information related to vascular toxicity such as myocardial infarction [ 7 ].
Professional societies in cardiology and oncology have proposed guidelines for the prevention and management of cardiotoxicity in patients with cancer. According to the American Society of Clinical Oncology and the ESC, it is recommended to identify high-risk patients, comprehensively evaluate clinical signs and symptoms associated with CRCT, and conduct cardiac evaluations before, during, and after chemotherapy [ 7 , 9 , 10 ]. In addition, guidelines for patients with cancer, including those for breast cancer survivorship care, emphasize that patients should be aware of the potential risk of CRCT and report symptoms, such as fatigue or shortness of breath to their healthcare providers [ 7 , 11 , 12 ]. Although these guidelines encompass cardiac monitoring as well as symptom observation, many studies have focused solely on objective diagnostic tests, such as echocardiography, cardiac magnetic resonance, and cardiac biomarkers [ 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 ], which means that there is little interest in CRCT symptoms in patients under breast cancer care.
This lack of interest in CRCT symptoms may be related to the absence of a specific symptom assessment tool for CRCT. Symptom monitoring of CRCT in patients with breast cancer was conducted through patient interviews and reported using the appropriate terminology [ 23 ]. In terms of interviews, patients with cancer experienced the burden of expressing symptoms between cardiovascular problems and cancer treatment. Qualitative research on patients with cancer indicates that these patients experience a daily battle to distinguish the symptoms they experience during chemotherapy [ 24 ]. To reduce the burden of identifying CRCT symptoms, it is crucial to educate patients with breast cancer undergoing chemotherapy about these symptoms. To report cardiotoxicity, healthcare providers in oncology can use a dictionary of terms called the Common Terminology Criteria for Adverse Events (CTCAE) for reporting adverse events in patients with cancer [ 25 ]. Patients can also use Patient-Reported Outcome (PRO), which allows unfiltered reporting of symptoms directly to the clinical database [ 26 ]. PRO consists of 78 symptomatic adverse events out of approximately 1,000 types of CTCAE [ 27 ]. Basch et al. suggested that PRO could enable healthcare providers to identify patient symptoms before they worsen, thereby improving the overall survival rate of patients with metastatic cancer [ 28 ]. This finding implies that symptoms can provide valuable clues for enhancing the timeliness and accuracy of clinical assessments of CRCT [ 29 ]. Therefore, it is necessary to explore the scope of research focusing on CRCT symptoms for prevention and early detection of CRCT in patients with breast cancer. The detailed research questions are as follows:
What are the general characteristics of the studies related to CRCT in patients with breast cancer?
What diagnostic tools and monitoring practices are used to detect CRCT?
What are the characteristics and progression of symptoms associated with CRCT?
A scoping review is a research method for synthesizing evidence that involves mapping the scope of evidence on a particular topic [ 30 ]. It aims to clarify key concepts and definitions, identify key characteristics of factors related to a concept, and highlight gaps or areas for further research [ 30 ]. This study used a scoping review methodology based on the Joanna Briggs Institute (JBI) framework. The JBI methodology, refined from the framework initially developed by Arksey and O’Malley [ 31 ], involves developing a research question, establishing detailed inclusion and exclusion criteria, and selecting and analyzing literature accordingly [ 32 ]. In contrast to systematic reviews, scoping reviews can encompass a variety of study designs and are particularly suitable when the topic has not been extensively studied [ 33 ]; hence, the decision was made to conduct a scoping review.
Development of a scoping review protocol
To conduct this review, an a priori scoping review protocol was developed to enhance transparency and increase the usefulness and reliability of the results. The protocol included the title, objective, review questions, introduction, eligibility criteria, participants, concept, context, types of evidence source, methods, search strategy, source of evidence selection, data extraction, data analysis and presentation, and deviation from the protocol [ 34 ] (Supplementary File 1).
Eligibility criteria
A participant-concept-context (PCC) framework was constructed based on the following research criteria. The participants were patients with breast cancer. The concept was that studies that specifically reported symptoms directly matched to CRCT in patients with breast cancer and the literature, published in English since 2010, in line with the year the CRCT guidelines were announced by the Cardio-Oncology Society. The context was open. We included all types of research designs. The exclusion criteria were studies that included patients with other types of cancer, involved animal subjects, and reported symptoms not directly related to CRCT.
Search strategy
The keywords consisted of “breast cancer,” “chemotherapy,” “cardiotoxicity,” and “symptoms.” The keywords for “cardiotoxicity” were constructed according to the clinical cardiotoxicity report and ESC guidelines [ 7 , 35 ]. The keywords for “symptoms” included 40 specific symptoms of arrhythmia, heart failure, and cardiac problems [ 36 , 37 ] (Supplementary Table 1). We used PubMed, Embase, and CINAHL.
Source of evidence selection
Duplicate studies were removed using EndNote 21. The titles and abstracts were then reviewed according to the inclusion criteria, the primary literature was selected, and the final literature was selected through a full-text review. Any disagreements were resolved through discussions between the investigators.
Data extraction
The data from the literature included the general characteristics of the study, as well as information on the patients, chemotherapy, cardiotoxicity, and symptoms. The general characteristics of the study included author, publication year, country of origin, study design; patient information including sample size, sex, age, cancer type, and cancer stage; chemotherapy information including chemotherapy regimen; cardiotoxicity information including type of cardiotoxicity, diagnostic tests, and times of assessment; and symptom information including type of symptom, characteristics of symptom worsening or improvement, onset time, progression time, and time to symptom improvement. Information on whether to receive chemotherapy after the diagnosis of cardiotoxicity was explored.
Data analysis and presentation
The contents of the included studies were divided into three categories: (1) general characteristics, which encompassed study designs, patients, and medications; (2) type of CRCT and cardiac assessment for CRCT; and (3) characteristics and progression of the symptoms associated with CRCT. CRCT symptom-related data are presented in tables and figures.
In total, 487 studies were identified through database searches, and 116 duplicates were subsequently removed. After reviewing the titles and abstracts, we excluded 197 studies in which participants had cancers other than breast cancer, no symptoms, or symptom-related expressions. Of the remaining 174 studies, 146 were excluded after full-text review. Among the excluded studies, 79 were mainly clinical trials that the symptoms were not directly related to CRCT, 62 did not report specific symptoms, four were in the wrong population, and one was unavailable for full-text review. An additional study was included after a review of references, bringing the final count to 29 studies included in the analysis (Fig. 1 ).

Preferred reporting items for systematic reviews flowchart
General characteristics of studies including designs, sex and age, chemotherapy regimen, and CRCT criteria
Table 1 presents the general characteristics of the studies included in this review. The majority of these studies were published in the USA ( n =14), with Japan ( n =3), and Romania ( n =2) following. The study designs primarily consisted of case reports ( n =23), retrospective studies ( n =4), and prospective studies ( n =2).
All case reports involved female patients, except for one involving a male patient. Five quantitative studies did not specify or limit the sex of the participants, and one retrospective study included only female patients. In terms of cancer stage, the majority of studies involved patients with advanced breast cancer ( n =13), while a smaller number involved patients with early-stage breast cancer ( n =4). Twelve studies did not specify the cancer stage. Approximately 20 types of chemotherapy regimens are currently in use. Trastuzumab, which is a human epidermal growth factor receptor 2 (HER2) blocker, was mentioned in the majority of studies ( n =8), followed by capecitabine (an antimetabolite) ( n =7), and doxorubicin or epirubicin (anthracycline-based chemotherapy) ( n =6). Current chemotherapy and previous treatment methods were described together, with the exception of eight studies. Six quantitative studies defined the CRCT criteria, five of which were based on decreased LVEF and one of which was based on significant cardiac symptoms and/or electrocardiogram changes. Twenty-three case reports described the cardiovascular diagnosis as CRCT.
Diagnostic tools and monitoring practice for CRCT
Table 2 displays the types of CRCT, diagnostic tools, and times of cardiac assessment according to chemotherapy regimens. The most prevalent CRCT were myocardial dysfunction and heart failure, identified in 12 case studies, respectively. This was followed by coronary artery disease, represented in 8 case studies, pulmonary hypertension in 2 case studies, and a single case study of periaortitis. The most used test for diagnosing CRCT was echocardiography ( n =22), followed by EKG ( n =20), various types of cardiac enzymes ( n =16), coronary angiography (CAG, n =12), computed tomography ( n =6), and magnetic resonance imaging (MRI, n =4). Regarding the CRCT symptom assessment tools, the CTCAE was used in two studies, the New York Heart Association classification for heart failure in two studies, the dyspnea assessment scale in one study, and symptoms of cardiac origin, which consisted of chest pain, dyspnea, and palpitations in one study.
Regarding the times of cardiac evaluation, two studies performed regular cardiac checkups including before, during, and after chemotherapy. There were 10 case studies and six quantitative studies describing cardiac function testing before chemotherapy, of which seven studies performed regular cardiac screening tests and two studies mentioned cardiac screening even after the completion of chemotherapy. The frequency of regular checkups varied from every 3 months to every two to four cycles. In all case reports ( n =23), CRCT were diagnosed through incidental checkups based on patients’ symptom presentation, and in most cases, several tests were performed subsequentially for CRCT diagnosis. In one case study, cardiac evaluation was conducted 3 days after the patient’s initial symptom presentation, when the symptoms became more severe.
Characteristics and progression of symptoms associated with CRCT
Table 3 shows the descriptive scope of the CRCT-related symptoms according to the chemotherapy regimens used in the included studies. The mapping factors included initial symptoms, symptom onset or severity, symptom progression, medical management, and CRCT results. One of the most frequent symptoms associated with CRCT was dyspnea, which was discussed in 19 studies and described as difficulty in breathing, shortness of breath, or New York Heart Association (NYHA) class II or III. When dyspnea appeared as the initial symptom of CRCT, the symptom progression was worsening in eight case studies and persistent in two cases. Chest pain was described in 12 studies as a symptom characterized by a squeezing, tingling, burning, tightened, or atypical feeling that was relieved by rest and exacerbated by exertion. Other symptoms included peripheral edema ( n =6), fatigue ( n =5), and palpitation ( n =2). The symptoms were assessed by patient-reported symptom presentation rather than using a symptom assessment tool.
The symptoms could be categorized based on the type of chemotherapy regimens used. In the case studies involving anthracycline-based regimen and HER2 blockers, dyspnea was the most frequently observed symptom ( n =7), followed by peripheral edema ( n =2), and chest pain or discomfort ( n =2). In case studies where antimetabolites were used, specifically capecitabine, chest pain was a common and prominent symptom. This chest pain typically manifested between 1 and 7 days after drug administration and persisted until treatment. Notably, four out of seven patients reported this symptom on the first day of chemotherapy, according to the case reports. The time for first symptom onset after chemotherapy ranged from 1 hour to 300 days, with anthracycline-based regimens requiring 3–55 days, trastuzumab requiring 60–300 days, and capecitabine requiring 1–7 days. Figure 2 shows the progression of symptoms in case studies, detailing the time of symptom onset, the date of symptom reporting, and the date of treatment completion following the use of chemotherapy. The studies that did not specify any of the dates of symptom onset, reporting, and completion of treatment were excluded from the figure.

Figure 3 shows symptoms according to the main types of chemotherapy regimens reported in case studies. Dyspnea with trastuzumab and chest pain with capecitabine are particularly characteristic. A retrospective study included in this scoping review reported that chest pain was the most common symptom associated with capecitabine, followed by dyspnea and palpitation [ 40 ]. Furthermore, peripheral edema was primarily observed with anthracycline, alkylating, and HER2 blockers, while fatigue was noted with various anticancer drugs, irrespective of the type of chemotherapy regimen.

Ongoing chemotherapy was discontinued after CRCT was detected in 20 case studies. When patients presented symptoms indicative of CRCT, the majority were promptly hospitalized for further evaluation, medication, or interventional treatment. The majority of studies indicated the initiation of cardiac medication ( n =21), with three case studies involving coronary intervention and two involving treatment with wearable devices. Most management procedures were conducted in a general ward or an intensive care unit.
In most case studies, symptoms improved following cardiac treatment, with either complete or partial recovery of LVEF observed in 19 instances. However, a few studies reported a poor prognosis, including two instances of death. LVEF recovered in most patients within 6 months when treated with an anthracycline-based regimen and HER2 blockers (Fig. 2 ). A retrospective study reported that the rates of complete or partial recovery of CRCT following treatment with doxorubicin-based chemotherapy and trastuzumab were 42.9% and 86.1%, respectively [ 39 ]. Another retrospective study noted that the recovery time of CRCT when treated with HER2 blockers increased in correlation with the severity of the NYHA class, ranging from 8 to 80 weeks [ 38 ]. In the case of the antimetabolite capecitabine, all patients recovered within a day to a week, except one patient who did not recover.
This scoping review was conducted to explore the scope of studies focusing on CRCT symptoms, including the general characteristics of the studies, diagnostic tools, monitoring practices related to detecting CRCT, and the characteristics and progression of symptoms associated with CRCT. The primary findings of this review were as follows: (1) common symptoms related to CRCT and differences in symptoms according to the chemotherapy regimens used were identified; (2) the symptoms reported by the patient served as clues to suspect a specific type of CRCT; and (3) regular monitoring practices for CRCT prevention and detection were insufficient.
First, the current study identified common symptoms such as dyspnea, chest pain, peripheral edema, fatigue, and palpitation associated with CRCT, as well as variations in symptoms depending on the chemotherapy regimen used in patients with breast cancer. Among these symptoms, dyspnea, edema, and chest pain were frequently observed in patients receiving anthracycline-based and/or HER2 blocker drugs. These symptoms, which are associated with heart failure, appeared later compared to those observed with capecitabine, as depicted in Fig. 2 . This may be due to the known impact of anthracycline-based and/or HER2 blocker regimens on cardiomyocytes and other cells, leading to myocardial damage [ 42 ]. Therefore, the symptoms are related to heart failure, potentially resulting from the impairment of ventricular filling or ejection in patients undergoing treatment with these regimens [ 43 ].
In a similar vein, Attin et al. (2022) documented the occurrence of symptoms such as lower extremity edema, chest pain, difficulty breathing, and fatigue before the diagnosis of CRCT in women undergoing breast cancer treatment. They conducted a retrospective and longitudinal investigation of the symptoms, signs, and cardiac tests of 15 patients who experienced CRCT, using their electronic medical records. In their study, cardiotoxicity was defined by an echocardiogram or MRI showing a decrease in LVEF of 5 to 10%, with a specialist’s confirmation note. They compared the number of symptom occurrences during the first half of the year with those during the second half of the year prior to the diagnosis of cardiotoxicity. Specifically, the frequency of lower-extremity edema significantly increased from three occurrences in the first half of the year to 17 occurrences in the second half of the year. The frequency of symptoms for dyspnea and chest pain also increased from 10 and 8 times, respectively, to 16 and 14 times in the second half of the year. While there was limited information on the doses or timing of chemotherapy, 87% of the patients received the same chemotherapy regimens, namely anthracyclines and/or HER2 blockers [ 44 ]. This suggests that the increase in symptom occurrence over time may be related to the accumulation of anthracycline and the duration of anti-HER2 therapy [ 45 ].
Salyer et al. (2019) conducted a study on the prevalent symptoms of heart failure and their clustering. They identified three symptom clusters: sickness behavior, gastrointestinal disturbance, and discomfort of illness. Notably, dyspnea, edema, and pain were grouped into the discomfort of illness cluster, which aligns with the symptoms we observed in patients treated with anthracyclines and/or HER2 blockers [ 46 ]. Therefore, it is crucial for patients undergoing treatment with anthracyclines and/or HER2 blockers to be vigilant for symptoms such as dyspnea, edema, or chest pain, as these are indicative of heart failure.
Chest pain caused by vasospasm was a predominant symptom in patients taking antimetabolite regimens such as oral capecitabine, and it manifested as the following types of cardiotoxicities: vasospasm-related arrhythmia, myocardial disease, and ischemia [ 47 ]. Vasospasm can be triggered by endothelial dysfunction, hypersensitive vascular smooth muscle, reactive oxidative stress, or chemotherapy regimens [ 48 , 49 ]. According to previous studies, in patients using antimetabolite drugs such as 5-fluorouracil or capecitabine, chest pain was usually reported to occur from several hours to 72 hours after the first administration [ 47 , 50 , 51 , 52 , 53 ]. To detect chemotherapy-related coronary vasospasm in the early stage, it is recommended to carefully monitor typical or atypical symptoms of chest pain and EKG monitoring during drug infusion [ 54 ]. Muco et al. (2022) reported severe outcomes resulting from delayed management of vasospastic angina symptoms. The patient’s cardiac evaluation was performed 3 days after the onset of symptoms, and unfortunately, she did not recover from brain damage caused by coronary vasospastic sequelae. The authors stressed the importance of medical teams recognizing the symptoms of CRCT through vigilant monitoring and patient education [ 55 ].
As seen in the symptoms of CRCT caused by heart failure and vasospasm, careful observation of symptoms and conducting appropriate tests are crucial to prevent cardiotoxicity and minimize damage. These characteristics of CRCT and the associated symptoms related to chemotherapy regimens can provide crucial educational content for healthcare providers and patients preparing for chemotherapy. In addition, CRCT and symptom progression according to chemotherapy regimens could be used to formulate research questions for future systematic reviews.
Second, the preventive management of CRCT necessitates adherence to recommended guidelines. The 2022 ESC guidelines on cardio-oncology have updated the classification of CRCT and the monitoring protocols based on the chemotherapy regimens used [ 7 ]. The CRCT identified in the current study aligns with the drug toxicity outlined in the 2022 ESC guidelines. These guidelines advocate for regular cardiac monitoring before, during, and after chemotherapy to prevent and manage CRCT induced by anthracycline and HER2 blockers [ 7 , 12 ]. In this scoping review, two of 23 records described cardiac monitoring before, during, and after chemotherapy. An Australian multicenter study revealed that 59% of patients were referred to a cardiologist before CRCT occurred, but only 15% of patients diagnosed with CRCT had consulted a cardiologist before chemotherapy [ 41 ]. Given the declining mortality rates among cancer patients, managing CRCT requires a collaborative approach between oncology and cardiology to minimize mortality and morbidity in patients with breast cancer undergoing chemotherapy [ 7 ]. Therefore, it remains crucial to emphasize adherence to cardiac monitoring guidelines and foster cooperation between oncology and cardiology.
Additionally, symptom assessment is important for the early detection of patients with CRCT. The studies included in the current scoping review assessed whether patients’ symptoms could detect CRCT using interviews with patients, the New York Heart Association classification, a dyspnea assessment scale, and CTCAE tools. The United States National Cancer Institute recommends that healthcare providers use CTCAE and patients with cancer use PRO to report adverse events, including symptoms. CTCAE is a broad and comprehensive terminology that encompasses adverse events related to cancer treatment, has been used since the 1980s [ 25 ], and is not specialized in cardiotoxicity. Additionally, a discrepancy between CTCAE and PRO discovered that healthcare providers often underestimate both the incidence and duration of symptoms compared to the patients [ 56 , 57 , 58 ]. Specifically, healthcare providers tend to report symptom severity as lower than that reported by patients. For instance, there are notable discrepancies between healthcare providers and patients when reporting severe or very severe symptoms of fatigue, dyspnea, and limb edema in patients with early-stage breast cancer undergoing chemotherapy. The reported rates were 8% and 22% for fatigue, 0% and 4% for dyspnea, and 0% and 5% for limb edema, from healthcare providers and patients, respectively. Therefore, it is necessary to develop a user-friendly questionnaire to assess the various symptoms of CRCT.
Finally, we found that once CRCT was confirmed, cardiac treatment was promptly initiated and chemotherapy was frequently halted until CRCT resolution. A Delphi study on the use of anthracycline and trastuzumab proposed altering the treatment schedule or discontinuing treatment until there was an improvement in LVEF [ 59 ]. However, the professional societies did not provide definitive recommendations regarding continuing or ceasing ongoing chemotherapy. Instead, they suggested that the decision to continue or discontinue ongoing chemotherapy should be made based on the patient’s potential risks and benefits [ 60 ]. For example, Polk et al. (2016) reported that out of 22 patients with CRCT resulting from capecitabine, six continued medications with or without cardiac treatment; some of these patients experienced the same symptoms, while others did not exhibit significant symptoms [ 40 ]. Further research is required to explore the continuation or discontinuation of chemotherapy when CRCT is confirmed.
This study has some limitations. First, although we did not restrict the patients’ sex when reviewing the literature, most patients, except for one, were female. This may be related to the lower incidence of breast cancer in men. Second, although this scoping review mapped CRCT symptoms according to chemotherapy regimens, including anthracycline-based drugs, HER2 blockers, and antimetabolites, it did not cover cardiotoxicity related to other types of chemotherapy regimens. Thus, exploring the symptoms by focusing on expanded chemotherapy regimens and cardiovascular toxic diseases will assist in overcoming this limitation. Third, of the 29 studies, 23 were case reports with some grey literature, which may be justified by the nature of scoping reviews that allow for inclusion irrespective of the data source [ 61 ] and the study type. Experimental or observational clinical studies use objective criteria, such as diagnostic tests to generate primary evidence. However, case reports have led to new medical discoveries regarding the prevention and treatment of diseases [ 62 ]. Given the nature of case reports, specific symptoms that could provide clues for evaluating CRCT in patients with breast cancer are most often found in these reports. We incorporated grey literature to gather more comprehensive information on CRCT-related symptoms. However, to mitigate the potential issue of unverified quality in grey literature, we initially organized 16 studies from peer-reviewed literature and subsequently incorporated the grey literature into our findings. This approach helped to clarify the results of the peer-reviewed literature, particularly the types of chemotherapy regimens [ 63 ]. Finally, regarding the literature selection criteria, we examined articles written in English and published since 2010, the year the cardio-oncology guidelines were announced, thereby excluding articles published before 2010.
This scoping review allowed data mapping according to the study design and chemotherapy regimens. The key messages included a type of CRCT, cardiac assessment, and in-depth information regarding the CRCT symptoms. There were approximately five typical CRCT symptoms, including dyspnea, chest pain, peripheral edema, fatigue, and palpitations, and the timing of symptom occurrence varied. The symptoms were assessed by patient-reported symptom presentation rather than using a symptom assessment tool. Therefore, developing and applying a CRCT-specific and user-friendly symptom assessment tool are expected to help healthcare providers and patients manage CRCT symptoms effectively.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, Soerjomataram I. Current and future burden of breast cancer: global statistics for 2020 and 2040. The Breast. 2022;66:15–23.
Article PubMed PubMed Central Google Scholar
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Article PubMed Google Scholar
Agha A, Wang X, Wang M, Lehrer EJ, Horn SR, Rosenberg JC, Trifiletti DM, Diaz R, Louie AV, Zaorsky NG. Long-term risk of death from heart disease among breast cancer patients. Front Cardiovasc Med. 2022;9: 784409.
Oikawa M, Ishida T, Takeishi Y. Cancer therapeutics-related cardiovascular dysfunction: Basic mechanisms and clinical manifestation. J Cardiol. 2023;81(3):253–9.
Piepoli MF, Adamo M, Barison A, Bestetti RB, Biegus J, Böhm M, Butler J, Carapetis J, Ceconi C, Chioncel O, et al. Preventing heart failure: a position paper of the Heart Failure Association in collaboration with the European Association of Preventive Cardiology. Eur J Heart Fail. 2022;24(1):143–68.
Chung R, Ghosh AK, Banerjee A: Cardiotoxicity: precision medicine with imprecise definitions. In., vol. 5: Archives of Disease in childhood; 2018: e000774.
Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). European Heart Journal - Cardiovascular Imaging. 2022;23(10):e333–465.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O et al: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022, 24(1):4-131.
Armenian SH, Lacchetti C, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract. 2017;13(4):270–5.
Lanza O, Ferrera A, Reale S, Solfanelli G, Petrungaro M, Tini Melato G, Volpe M, Battistoni A: New insights on the toxicity on heart and vessels of breast cancer therapies. Med Sci (Basel) 2022, 10(2).
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(1):43–73.
Lee GA, Aktaa S, Baker E, Gale CP, Yaseen IF, Gulati G, Asteggiano R, Szmit S, Cohen-Solal A, Abdin A, et al. European Society of Cardiology quality indicators for the prevention and management of cancer therapy-related cardiovascular toxicity in cancer treatment. Eur Heart J Qual Care Clin Outcomes. 2022;9(1):1–7.
Alexandraki A, Papageorgiou E, Zacharia M, Keramida K, Papakonstantinou A, Cipolla CM, Tsekoura D, Naka K, Mazzocco K, Mauri D et al: New insights in the era of clinical biomarkers as potential predictors of systemic therapy-induced cardiotoxicity in women with breast cancer: a systematic review. Cancers (Basel) 2023, 15(13).
Di Lisi D, Manno G, Madaudo C, Filorizzo C, Intravaia RCM, Galassi AR, Incorvaia L, Russo A, Novo G: Chemotherapy-related cardiac dysfunction: the usefulness of myocardial work indices. Int J Cardiovasc Imaging 2023.
Kar J, Cohen MV, McQuiston SA, Malozzi CM. Can global longitudinal strain (GLS) with magnetic resonance prognosticate early cancer therapy-related cardiac dysfunction (CTRCD) in breast cancer patients, a prospective study? Magn Reson Imaging. 2023;97:68–81.
Article CAS PubMed Google Scholar
Lim A, Jang H, Jeon M, Fadol AP, Kim S. Cancer treatment-related cardiac dysfunction in breast cancer survivors: a retrospective descriptive study using electronic health records from a Korean tertiary hospital. Eur J Oncol Nurs. 2022;59: 102163.
Liu W, Li W, Li H, Li Z, Zhao P, Guo Z, Liu C, Sun L, Wang Z. Two-dimensional speckle tracking echocardiography help identify breast cancer therapeutics-related cardiac dysfunction. BMC Cardiovasc Disord. 2022;22(1):548.
Article CAS PubMed PubMed Central Google Scholar
Mauro C, Capone V, Cocchia R, Cademartiri F, Riccardi F, Arcopinto M, Alshahid M, Anwar K, Carafa M, Carbone A et al: Cardiovascular side effects of anthracyclines and HER2 inhibitors among patients with breast cancer: a multidisciplinary stepwise approach for prevention, early detection, and treatment. J Clin Med 2023, 12(6).
Okushi Y, Saijo Y, Yamada H, Toba H, Zheng R, Seno H, Takahashi T, Ise T, Yamaguchi K, Yagi S et al: Effectiveness of surveillance by echocardiography for cancer therapeutics-related cardiac dysfunction of patients with breast cancer. J Cardiol 2023.
Ositelu K, Trevino A, Tong A, Chen MH, Akhter N: Challenges in cardiovascular imaging in women with breast cancer. Curr Cardiol Rep 2023.
Terui Y, Sugimura K, Ota H, Tada H, Nochioka K, Sato H, Katsuta Y, Fujiwara J, Harada-Shoji N, Sato-Tadano A, et al. Usefulness of cardiac magnetic resonance for early detection of cancer therapeutics-related cardiac dysfunction in breast cancer patients. Int J Cardiol. 2023;371:472–9.
Thavendiranathan P, Shalmon T, Fan CS, Houbois C, Amir E, Thevakumaran Y, Somerset E, Malowany JM, Urzua-Fresno C, Yip P, et al. Comprehensive cardiovascular magnetic resonance tissue characterization and cardiotoxicity in women with breast cancer. JAMA Cardiol. 2023;8(6):524–34.
Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25(32):5121–7.
White J, Byles J, Williams T, Untaru R, Ngo DTM, Sverdlov AL. Early access to a cardio-oncology clinic in an Australian context: a qualitative exploration of patient experiences. Cardiooncology. 2022;8(1):14.
PubMed PubMed Central Google Scholar
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P: CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003, 13(3):176-181.
Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, Mendoza TR, Hay J, Atkinson TM, Abernethy AP et al: Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014, 106(9).
Kluetz PG, Chingos DT, Basch EM, Mitchell SA. Patient-reported outcomes in cancer clinical trials: measuring symptomatic adverse events with the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Educ Book. 2016;36:67–73.
Article Google Scholar
Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–8.
Liu L, Suo T, Shen Y, Geng C, Song Z, Liu F, Wang J, Xie Y, Zhang Y, Tang T, et al. Clinicians versus patients subjective adverse events assessment: based on patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Qual Life Res. 2020;29(11):3009–15.
Munn Z, Pollock D, Khalil H, Alexander L, Mclnerney P, Godfrey CM, Peters M, Tricco AC. What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI Evidence Synthesis. 2022;20(4):950–2.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005;8(1):19–32.
Peters M, Godfrey C, McInerney P, Munn Z, Tricco A, Khalil H: Chapter 11: scoping reviews (2020 version). In: JBI Manual for Evidence Synthesis. edn. Edited by Aromataris E MZ: JBI; 2020.
Munn Z, Peters MD, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC medical research methodology. 2018;18:1–7.
Peters MDJ, Godfrey C, McInerney P, Khalil H, Larsen P, Marnie C, Pollock D, Tricco AC, Munn Z. Best practice guidance and reporting items for the development of scoping review protocols. JBI Evid Synth. 2022;20(4):953–68.
Bohdan M, Kowalczys A, Mickiewicz A, Gruchala M, Lewicka E: Cancer therapy-related cardiovascular complications in clinical practice: current perspectives. J Clin Med 2021, 10(8).
Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932–63.
Bozkurt B, Coats A, Tsutsui H: Universal definition and classification of heart failure. J Card Fail 2021.
Aldiab A. Cardiotoxicity with adjuvant trastuzumab use in breast cancer: a single institution»s experience. J Saudi Heart Assoc. 2010;22(3):133–6.
Russell SD, Blackwell KL, Lawrence J, Pippen JE Jr, Roe MT, Wood F, Paton V, Holmgren E, Mahaffey KW. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant Breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28(21):3416–21.
Polk A, Shahmarvand N, Vistisen K, Vaage-Nilsen M, Larsen FO, Schou M, Nielsen DL: Incidence and risk factors for capecitabine-induced symptomatic cardiotoxicity: a retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ Open 2016, 6(10).
Clark RA, Marin TS, McCarthy AL, Bradley J, Grover S, Peters R, Karapetis CS, Atherton JJ, Koczwara B. Cardiotoxicity after cancer treatment: a process map of the patient treatment journey. Cardiooncology. 2019;5:14.
Anjos M, Fontes-Oliveira M, Costa VM, Santos M, Ferreira R. An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity. Life Sci. 2021;280: 119760.
Malik A, Brito D, Vaqar S, Chhabra L: Congestive heart failure. In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC.; 2023.
Attin M, Reifenstein K, Mehta S, Arcoleo K, Lin CD, Storozynsky E. Reported signs, symptoms, and diagnostic tests before cardiotoxicity among women with breast cancer: a pilot study. J Cardiovasc Nurs. 2022;37(2):104–11.
Huang P, Dai S, Ye Z, Liu Y, Chen Z, Zheng Y, Shao X, Lei L, Wang X. Long-term tolerance and cardiac function in breast cancer patients receiving trastuzumab therapy. Oncotarget. 2017;8(2):2069–75.
Salyer J, Flattery M, Lyon DE. Heart failure symptom clusters and quality of life. Heart Lung. 2019;48(5):366–72.
Padegimas A, Carver JR. How to diagnose and manage patients with fluoropyrimidine-induced chest pain: a single center approach. JACC CardioOncol. 2020;2(4):650–4.
Sheth MA, Widmer RJ, Dandapantula HK. Pathobiology and evolving therapies of coronary artery vasospasm. Proc (Bayl Univ Med Cent). 2021;34(3):352–60.
PubMed Google Scholar
Hokimoto S, Kaikita K, Yasuda S, Tsujita K, Ishihara M, Matoba T, Matsuzawa Y, Mitsutake Y, Mitani Y, Murohara T, et al. JCS/CVIT/JCC 2023 guideline focused update on diagnosis and treatment of vasospastic angina (coronary spastic angina) and coronary microvascular dysfunction. J Cardiol. 2023;82(4):293–341.
Kanduri J, More LA, Godishala A, Asnani A. Fluoropyrimidine-associated cardiotoxicity. Cardiol Clin. 2019;37(4):399–405.
Garbis K, Rafiee MJ, Luu J. 5-fluorouracil-induced coronary vasospasm: a cardiovascular magnetic resonance imaging case report. Glob Cardiol Sci Pract. 2023;2023(3): e202316.
Dyhl-Polk A, Vaage-Nilsen M, Schou M, Vistisen KK, Lund CM, Kümler T, Appel JM, Nielsen DL. Incidence and risk markers of 5-fluorouracil and capecitabine cardiotoxicity in patients with colorectal cancer. Acta Oncol. 2020;59(4):475–83.
Becker K, Erckenbrecht JF, Häussinger D, Fueling T. Cardiotoxicity of the antiprolif erative compound fluorouracil. Drugs. 1999;57(4):475–84.
Lestuzzi C, Vaccher E, Talamini R, Lleshi A, Meneguzzo N, Viel E, Scalone S, Tartuferi L, Buonadonna A, Ejiofor L, Schmoll HJ. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: an underestimated risk. Ann Oncol. 2014;25(5):1059–64.
Muco E, Patail H, Shaik A, McMahon S. Capecitabine-associated coronary vasospasm and cardiac arrest. Cureus. 2022;14(8): e28184.
Montemurro F, Mittica G, Cagnazzo C, Longo V, Berchialla P, Solinas G, Culotta P, Martinello R, Foresto M, Gallizioli S, et al. Self-evaluation of adjuvant chemotherapy-related adverse effects by patients with breast cancer. JAMA Oncol. 2016;2(4):445–52.
Atkinson TM, Ryan SJ, Bennett AV, Stover AM, Saracino RM, Rogak LJ, Jewell ST, Matsoukas K, Li Y, Basch E. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669–76.
Galizia D, Milani A, Geuna E, Martinello R, Cagnazzo C, Foresto M, Longo V, Berchialla P, Solinas G, Calori A, et al. Self-evaluation of duration of adjuvant chemotherapy side effects in breast cancer patients: a prospective study. Cancer Med. 2018;7(9):4339–44.
Gavila J, Seguí M, Calvo L, López T, Alonso JJ, Farto M. Sánchez-de la Rosa R: Evaluation and management of chemotherapy-induced cardiotoxicity in breast cancer: a Delphi study. Clin Transl Oncol. 2017;19(1):91–104.
Leong DP, Lenihan DJ. Clinical practice guidelines in cardio-oncology. Heart Fail Clin. 2022;18(3):489–501.
Munn Z, Pollock D, Khalil H, Alexander L, McLnerney P, Godfrey CM, Peters M, Tricco AC. What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI Evid Synth. 2022;20(4):950–2.
Li YR, Jia Z, Zhu H. Understanding the value of case reports and studies in the context of clinical research, research design and evidence-based practice. J Case Reports and Studies. 2013;1(2):1–4.
Conn VS, Valentine JC, Cooper HM, Rantz MJ. Grey literature in meta-analyses. Nurs Res. 2003;52(4):256–61.
Download references
Acknowledgements
The authors thank Nawon Kim, a librarian at the Yonsei University Medical Library, for building search terms and guiding the database searches.
This research is supported by the Brain Korea 21 FOUR Project founded by the National Research Foundation (NRF) of Korea, Yonsei University College of Nursing.
Author information
Authors and affiliations.
Graduate School, College of Nursing, Yonsei University, 50 Yonsei-ro, Seoul, South Korea
Hyunjoo Kim
Severance Cardiovascular Hospital, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
College of Nursing and Brain Korea 21 FOUR Project, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
College of Nursing and Mo-Im Kim Nursing Research Institute, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
Sanghee Kim & Jeongok Park
Department of Internal Medicine, Division of Cardiology, Heart Failure Center, Severance Hospital, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
Seok-Min Kang
You can also search for this author in PubMed Google Scholar
Contributions
HK, BH, SK, and JP contributed to the study conception and design. The literature search and record screening were performed by HK and BH under the supervision of JP. Material preparation, data collection, and analysis were performed by HK, BH, and JP. The first draft of the manuscript was written by HK and JP commented on each updated version of the manuscript. The tables and figures were prepared by BH under the instruction of JP. SK helped to interpret the data and provided critical feedback on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jeongok Park .
Ethics declarations
Ethics approval and consent to participate.
This study was exempted from ethical approval by the Yonsei University Institute Review Board, and consent to participate was not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kim, H., Hong, B., Kim, S. et al. Chemotherapy-related cardiotoxicity and its symptoms in patients with breast cancer: a scoping review. Syst Rev 13 , 167 (2024). https://doi.org/10.1186/s13643-024-02588-z
Download citation
Received : 25 December 2023
Accepted : 14 June 2024
Published : 27 June 2024
DOI : https://doi.org/10.1186/s13643-024-02588-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Chemotherapy
- Cardiotoxicity
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Case study protocol is a formal document capturing the entire set of procedures involved in the collection of empirical material . It extends direction to researchers for gathering evidences, empirical material analysis, and case study reporting . This section includes a step-by-step guide that is used for the execution of the actual study.
The reliability of a case study is best established by developing what he calls a 'case study protocol' (ibid., pp. 101-104). A case study protocol should have the following constituent elements: (a) an overview of the entire study including its objectives, (b) a detailed description of field procedures including the techniques of data ...
Case study protocol Field phase a. Contact b. Interact Reporting phase a. Case study reporting Foundation Phase This is the first and foremost step in conducting the case study. This phase is based on some considerations that research stu-dents should carefully look into. If there is ambiguity in under-
Based on a systematic review of relevant literature, this paper catalogs the use of validity and reliability measures within academic publications between 2008 and 2018. The review analyzes case study research across 15 peer-reviewed journals (total of 1,372 articles) and highlights the application of validity and reliability measures.
Open in a separate window. First section: Description of the core center, contacts of the investigator/s, quantification of the involved centers. A research protocol must start from the definition of the coordinator of the whole study: all the details of the main investigator must be reported in the first paragraph.
A Case Study Protocol (CSP) is a set of guidelines that can be used to structure and govern a. case research project (Yin 1994). It therefore outlines the procedures and rules governing the. conduct of researcher(s) before, during and after a case research project. In addition, a case.
Designing and Conducting Case Studies. This guide examines case studies, a form of qualitative descriptive research that is used to look at individuals, a small group of participants, or a group as a whole. Researchers collect data about participants using participant and direct observations, interviews, protocols, tests, examinations of ...
A study protocol is an essential part of a research project. It describes the study in detail to allow all members of the team to know and adhere to the steps of the methodology. Most funders, such as the NHS Health Research Authority in the United Kingdom, encourage researchers to publish their study protocols to create a record of the ...
The link between the purpose of the study and the data to be collected is explained in a case study protocol based on the templates suggested by Yin (2009) and Brereton et al. (2008), which is ...
The case study protocol defines the procedures and general rules to be followed using the protocol (Yin, 2009). Yin (2009) reminds the researcher that the protocol is a major way of increasing the reliability of case study research and is intended to guide the researcher in carrying out data collection from a single case. The case study ...
Protocol Templates Download Version; Descriptive Study Template: This template should only be used for for studies limited to (1) the use of existing data or specimens, (2) where the only study procedure is a retrospective chart review or use of existing biological samples and (3) where the analysis plan is limited to purely descriptive summary statistics.
We evaluate study protocol submissions on a case-by-case basis and consider only those for proposed or ongoing studies that have not completed participant recruitment at the time of submission. We encourage authors to submit their study protocols well in advance of participant recruitment completion and confirm the study status within the cover ...
Case Study Protocol Background Information The following information is summarized from the Interim Report: Alternative contracting methods (ACM), such as Design-build (DB), Construction Management/General Contractor (CMGC), and Public-Private Partnerships (P3), have been proven to accelerate the construction, reconstruction, and rehabilitation ...
A set of guidelines that may be used by researchers in the development of Case Study Protocols are presented, which are an integral part of the case research design and contains the procedures for conducting the research, the research instrument itself, and the guidelines for data analysis. A review of the literature has shown that there is a growing call for the use of the case research ...
Case Study Protocol NCPI Project 5.1 Introduction Project 5.1 of the National Center for Postsecondary Improvement is primarily responsible for researching the dynamics and effects of the assessment policies and practices of regional accrediting associations and state governments. More specifically, the project focuses on the
Case Study Interview Protocol 29 6.3 Case Study Questions The following outline shows the general format and flow of the D-B case study process. Where appropriate, the outline highlights how this layout differed between D-B and CM-GC projects. Again, to mitigate response bias from agency representatives, the questions and topics were inten ...
APPENDIX C: CASE STUDY PROTOCOL C.1 Overview of Case Study Background Information Delivering highway projects using alternative project delivery methods demands a shift in the traditional agency quality assurance (QA) and quality control (QC) programs to accommodate the faster pace of design and construction as well as the redistribution of ...
Pradeep Teregowda) : Abstract. In order to undertake a series of case studies aimed at investigating systematic literature reviews. we have developed a case study protocol template. This paper introduces the template and discusses our experiences of using the template and the resulting case study protocol. Study Monitoring Plan .
This case study protocol has been designed according to the guidelines stated by Yin (), and it has been deployed a literature review of other case study protocols throughout the selection of the next keywords and data strings: protocol case research, case study research and sustainability in Web of Science or Scopus.In addition, a deep comprehension of the integration of sustainability among ...
Case Study Protocol - Characterizing SPL Scoping and Requirements. 1. Background. Qualitative research, such as, case study, involves an interpretive, naturalistic approach to the world, investigating things in their natural settings, attempting to make sense of, or interpret, phenomena in terms of the meanings people bring to them (Denzin ...
The protocol is a major way of increasing the reliability of case study research and is intended to guide the investigator in carrying out the data collection from a single case (again, even if the single case is one of several in a multiple-case study). Figure 3.2 gives a table of contents from an illustrative protocol, which was used in a ...
Increase transparency by making more information available than required by trial registries. Give others the opportunity to see and understand deviations that occur during the study. BMJ requires authors of clinical trials submitted as research articles to upload a protocol for their study as a supplementary file.
Later on, Tellis (1997) developed four stage of the methodology based on a modification of the methodology devised by Yin (1984): design the case study protocol (determine the required skills and develop and review the protocol), conduct the case study (prepare for data collection, distribute questionnaire, and conduct interviews), analyze case ...
Abstract Comparative analysis is, with statistical and case study approaches, one of the three main tools for studying macrophenomena in the social sciences. This paper begins by delimiting its essential characteristics in contrast to the other two approaches, noting that it owes much of its strength to cases studies even though it focuses, like statistical methods, on explaining how phenomena ...
A description of the protocol, medications, laboratory monitoring, human milk analysis including macronutrients, oligosaccharides, and hormones is presented. Discussion: This is the fourth case to date known in the literature of a transgender woman with successful lactation induction, and the third case to remain on antiandrogen therapy during ...
A case is known of a chimpanzee with Down syndrome that survived to 23 months of age thanks to the ... area of the lumen of the lateral and ASCs in the ampullary zone and in the nonampullary zone was also measured using a protocol designed for this study that consists of placing a transverse plane to the corresponding canal and measuring the ...
Flash droughts (FDs) pose significant challenges for accurate detection due to their short duration. Conventional drought monitoring methods have difficultly capturing this rapidly intensifying phenomenon accurately. Machine learning models are increasingly useful for detecting droughts after training the models with data. Northeastern Brazil (NEB) has been a hot spot for FD events with ...
Assessing biomarkers of remission in female patients with anorexia nervosa (REMANO): a protocol for a prospective cohort study with a nested case-control study using clinical, neurocognitive, biological, genetic, epigenetic and neuroimaging markers in a French specialised inpatient unit
Chemotherapy-related cardiotoxicity is a significant concern because it is a major cause of morbidity. This study aimed to provide in-depth information on the symptoms of chemotherapy-related cardiotoxicity (CRCT) by exploring literature that concurrently reports the types and symptoms of CRCT in patients with breast cancer. A scoping review was performed according to an a priori protocol ...
For the second time in two years, the public learned of where the Supreme Court was headed in a major abortion case before the justices had formally handed down their ruling, spoiling the court ...