Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 08 April 2021
- Antoni Torres ORCID: orcid.org/0000-0002-8643-2167 1 , 2 , 3 , 4 ,
- Catia Cilloniz ORCID: orcid.org/0000-0002-4646-9838 1 , 2 , 3 , 4 ,
- Michael S. Niederman ORCID: orcid.org/0000-0003-0293-386X 5 ,
- Rosario Menéndez 6 ,
- James D. Chalmers 7 ,
- Richard G. Wunderink ORCID: orcid.org/0000-0002-8527-4195 8 &
- Tom van der Poll 9
Nature Reviews Disease Primers volume 7 , Article number: 25 ( 2021 ) Cite this article
211k Accesses
231 Citations
696 Altmetric
Metrics details
- Respiratory tract diseases
Pneumonia is a common acute respiratory infection that affects the alveoli and distal airways; it is a major health problem and associated with high morbidity and short-term and long-term mortality in all age groups worldwide. Pneumonia is broadly divided into community-acquired pneumonia or hospital-acquired pneumonia. A large variety of microorganisms can cause pneumonia, including bacteria, respiratory viruses and fungi, and there are great geographical variations in their prevalence. Pneumonia occurs more commonly in susceptible individuals, including children of <5 years of age and older adults with prior chronic conditions. Development of the disease largely depends on the host immune response, with pathogen characteristics having a less prominent role. Individuals with pneumonia often present with respiratory and systemic symptoms, and diagnosis is based on both clinical presentation and radiological findings. It is crucial to identify the causative pathogens, as delayed and inadequate antimicrobial therapy can lead to poor outcomes. New antibiotic and non-antibiotic therapies, in addition to rapid and accurate diagnostic tests that can detect pathogens and antibiotic resistance will improve the management of pneumonia.

Similar content being viewed by others
Etiological and epidemiological features of acute respiratory infections in China
Respiratory viral infections in pragmatically selected adults in intensive care units
Usual interstitial pneumonia: a clinically significant pattern, but not the final word
Introduction.
Pneumonia is a common acute respiratory infection that affects the alveoli and distal bronchial tree of the lungs. The disease is broadly divided into community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP, which includes ventilation-associated pneumonia (VAP)) (Box 1 ). Aspiration pneumonia represents 5–15% of all cases of CAP; however, its prevalence amongst patients with HAP is not known 1 . The lack of robust diagnostic criteria for aspiration pneumonia may explain why the true burden of this type of pneumonia remains unknown 1 .
The causative microorganisms for CAP and HAP differ substantially. The most common causal microorganisms in CAP are Streptococcus pneumoniae , respiratory viruses, Haemophilus influenzae and other bacteria such as Mycoplasma pneumoniae and Legionella pneumophila . Conversely, the most frequent microorganisms in HAP are Staphylococcus aureus (including both methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA)), Enterobacterales, non-fermenting gram-negative bacilli (for example, Pseudomonas aeruginosa ), and Acinetobacter spp. 2 , 3 . In health-care-associated pneumonia (HCAP), owing to patient risk factors, the microbial aetiology is more similar to that in HAP than to that in CAP. However, difficulties in standardizing risk factors for this population, coupled with the heterogeneity of post-hospital health care worldwide, suggest that the concept of HCAP has little usefulness, and indeed, HCAP was not included in recent guidelines for CAP and HAP 3 , 4 , 5 .
Differences in microbiology between CAP and HAP depend on whether pneumonia was acquired in the community or health care environment and on host risk factors, including abnormal gastric and oropharyngeal colonization. In addition, the aetiopathogenesis of CAP is different from that of HAP. In general, mild CAP is treated on an outpatient basis, moderately severe CAP in hospital wards, and severe CAP in intensive care units (ICUs) with or without mechanical ventilation 6 . The need for mechanical ventilation is used as a sub-classification of interest for prognosis and stratification in randomized clinical trials.
Both CAP 7 and HAP 4 can occur in either immunosuppressed or immunocompetent patients. To date, most research data have been based on studies of immunocompetent patients and, therefore, we rely on such sources in this Primer. However, CAP, HAP and VAP in immunosuppressed patients have attracted the attention of researchers, and more investigation is to come.
In this Primer, we cover and summarize the most important and recent updates related to epidemiology, pathophysiology, diagnostic screening, prevention, management, quality of life, and research perspectives. Additionally, owing to the profound impact of the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2), we summarize the main features of SARS-CoV-2 pneumonia (Box 2 ).
Box 1 Classifications of pneumonia
Community-acquired pneumonia (CAP)
Pneumonia acquired outside the hospital in individuals who have not been hospitalized during the month prior to symptom onset.
Hospital-acquired pneumonia (HAP)
Pneumonia acquired after at least 2 days of hospitalization and when no suspicion of disease incubation before hospital admission is present.
Ventilator-associated pneumonia (VAP)
HAP occurring >48 h after endotracheal intubation.
Aspiration pneumonia
Pneumonia occurring as a result of inhalation of contents from the stomach or mouth into the lungs. It is best considered as part of the continuum between CAP and HAP, and not as a distinct entity.
Health-care-associated pneumonia (HCAP)
Pneumonia acquired in non-hospital care institutions.
Box 2 COVID-19 features
Frequent symptoms
Shortness of breath
Less-common symptoms
Hyposmia (decreased sense of smell) and hypogeusia (decreased sense of taste)
Sore throat
Rhinorrhoea (runny nose)
Muscle pain
Diarrhoea and vomiting
Main complications
Acute respiratory distress syndrome (ARDS)
Sepsis and septic shock
Multiple organ failure
Secondary infection
Epidemiology
Global incidence.
Data from the 2019 Global Burden of Diseases (GBD) study 8 showed that lower respiratory tract infections (LRTIs) including pneumonia and bronchiolitis affected 489 million people globally. Children of <5 years of age and adults of >70 years of age are the populations most affected by pneumonia, according to the 2019 GBD study 8 . In 2019, there were 489 million incident cases of LRTI, and 11 million prevalent cases of LRTI. In the 2016 GBD study, the global incidence of LRTI was 155.4 episodes per 1,000 adults of >70 years of age and 107.7 episodes per 1,000 children of <5 years of age 9 . Finally, aspiration pneumonia contributes 5–15% of all cases of CAP and is associated with worse outcomes, especially in older patients with multiple comorbidities 10 , 11 . There is a lack of data about the incidence of aspiration pneumonia in patients with HAP 1 , 12 .
In the USA, the Etiology of Pneumonia in the Community (EPIC) study 13 found that the annual incidence of CAP was 2.4 cases per 1,000 adults, with the highest rates amongst adults of 65–79 years of age (6.3 cases per 1,000 individuals) and those of ≥80 years of age (16.4 cases per 1,000 people). In Europe, the annual incidence of CAP has been estimated at 1.07–1.2 cases per 1,000 people, increasing to 14 cases per 1,000 people amongst those of ≥65 years of age and with a preponderance in men 14 . Differences in epidemiology between the USA and Europe might be explained by the higher proportion of the adult population who received the pneumococcal vaccine in the USA (63.6% of adults of ≥65 years of age, compared with pneumococcal vaccination rates of 20% to 30% in most European countries 15 , 16 ); in addition, in 2015 in the USA, ~69% of adults of ≥65 years of age had received an influenza vaccine within the previous 12 months. Another possible contributing factor is the decreased rate of smoking in the USA: between 2005 and 2016, the percentage of smokers who quit increased from 51% to 59% 17 . Finally, marked differences between US and European health systems can influence epidemiological data.
The South American Andes region had the highest incidence of adults of >70 years of age with LRTIs (406.5 episodes per 1,000 people), while South Asia had the greatest number of LRTI episodes amongst adults of >70 years of age. Incidence per global region was 171.1 per 1,000 people in Central Europe, eastern Europe and central Asia; 234.4 per 1,000 people in Latin America and the Caribbean; 130.8 per 1,000 people in Southeast Asia, eastern Asia and Oceania; 246.6 per 1,000 people in North Africa and the Middle East; and 229.3 per 1,000 people in sub-Saharan Africa 9 .
According to the 2016 GBD study 9 , Oceania had the highest incidence of LRTI in children (171.5 per 1,000 children of <15 years of age), while South Asia had the greatest number of LRTI episodes amongst children of <5 years of age. Incidence per global region was: 107.1 per 1,000 children in Central Europe, eastern Europe, and central Asia; 94.9 per 1,000 children in Latin America and the Caribbean; 120.4 per 1,000 children in Southeast Asia, eastern Asia and Oceania; 133.2 per 1,000 children in North Africa and the Middle East; and 100.6 per 1,000 children in sub-Saharan Africa.
The epidemiology of pneumonia is constantly changing, owing to the development of molecular diagnostic tests, novel antimicrobial therapies and implementation of preventive measures. Since the beginning of the 21st century, pneumonia has been the most common cause of pandemic infections that have effects on its own epidemiology. In the 2009 influenza pandemic, the influenza virus A H1N1 infected ~200 million people and caused almost 250,000 deaths, with infectivity higher in children than in adults 18 . By contrast, in the current SARS-CoV-2 pandemic, 106 million people had been infected and >2 million had died worldwide by 9 February 2021. However, unlike the influenza virus A H1N1, SARS-CoV-2 affects adults more often than children 19 .
The annual incidence of HAP in adults ranges from 5 to 10 cases per 1,000 hospital admissions globally, whereas VAP affects 10–25% of all patients on mechanical ventilation 3 . HAP is the second most frequent hospital infection after urinary tract infection, and VAP is the most common cause of nosocomial infection and death in the ICU 3 , 4 . The incidence of HAP is highest amongst immunocompromised, post-surgical and older patients 20 . In the USA, the incidence of VAP is estimated to range from 2 to 6 cases per 1,000 ventilator-days 21 , and the incidence of non-ventilator-associated HAP is estimated to be 3.63 cases per 1,000 patient-days 22 . A 2018 systematic review and meta-analysis of studies of VAP in adults from 22 Asian countries found an overall incidence of 15.1 cases per 1,000 ventilator-days 23 . In 2015, data from the prospective French multicentre OUTCOMEREA database (1996–2012) indicated that the risk of VAP was ~1.5% per ventilator-day, decreasing to <0.5% per day after 14 days of mechanical ventilation 24 .
The 2019 GBD study 8 showed that LRTI was responsible for >2.49 million deaths, with mortality highest amongst patients of >70 years of age (1.23 million deaths). These data indicate that mortality due to LRTI is higher than mortality due to tuberculosis (1.18 million deaths) and HIV (864,000 deaths), making it the leading cause of infectious disease mortality worldwide. Indeed, data from a systematic review and meta-analysis on the global and regional burden of hospital admissions for pneumonia estimated that 1.1 million pneumonia-related hospital deaths occurred in 2015 amongst older adults 25 .
In 2016, the highest LRTI mortality rates amongst children of <5 years of age were in the Central African Republic (460 deaths per 100,000 children), Chad (425 deaths per 100,000) and Somalia (417 deaths per 100,000) 9 . Interestingly, data from the 2017 GBD study 26 showed that mortality due to LRTI decreased by 36.4% between 2007 and 2017 for children of <5 years of age, whereas it increased by an estimated 33.6% in adults of ≥70 years of age. LRTI-related deaths amongst children have substantially reduced as a result of the implementation of vaccines (against S. pneumoniae and H. influenzae ), antibiotic therapy, the continuous improvements in education, nutrition, water, sanitation and hygiene, and female empowerment. Nevertheless, in many areas the progress is slow; Nigeria, India, Pakistan, Ethiopia and the Democratic Republic of Congo are the five countries with the highest child mortality 27 .
Conversely, the increased mortality in adults of >70 years of age might be associated with the increasing longevity of the frail older population, chronic diseases, comorbidities 28 , multiple medication use and functional disability, especially in high-income countries. In low-income countries, the high mortality is associated with the effect of air pollution; smoke and alcohol consumption are the main risk factors for pneumonia in this age group.
Globally, amongst children and adults, mortality in those with CAP is related to the treatment setting: <1% in outpatient care, ~4–18% in hospital wards and up to 50% in the ICU 29 , 30 , 31 . However, in adults, age and comorbidities influence mortality. A study that investigated the effects of age and comorbidities on CAP mortality found a mortality of 5% in patients of <65 years of age, 8% amongst patients of 65–79 years and 14% amongst patients of ≥80 years of age 32 , and these rates increased to 20%, 42% and 43%, respectively, in patients with more than one comorbidity. On the basis of studies on long-term mortality across 1–10 years 33 , 34 , 35 , approximately one in three adults will die within one year of being hospitalized with CAP 36 . The estimated in-hospital mortality in patients with chronic obstructive pulmonary disorder (COPD) and CAP has been reported to be 6% during hospitalization and 12%, 24% and 33% within 30 days, 6 months and 1 year from discharge, respectively 37 . Interestingly, 30-day mortality amongst those with pneumococcal pneumonia remained fairly stable in a 20-year study 33 , and this was further confirmed in a review on the burden of pneumococcal CAP in Europe 38 .
Globally, HAP and VAP are considered the leading causes of death due to hospital-acquired infection 39 , 40 , 41 . The estimated global mortality due to HAP is 20–30%, whereas global mortality due to VAP is 20–50% 20 , 42 . Mortality due to VAP in the USA was ~13% 4 . By contrast, a prospective study in central Europe 43 indicated that 30-day mortality due to VAP was 30%. In a large French cohort of patients admitted to the ICU for >48 h, both non-ventilator-associated HAP and VAP were associated with an 82% and a 38% increase in the risk of 30-day mortality, respectively 44 . However, analysis of data from trials on antibiotic therapy for bacterial HAP and VAP to characterize all-cause mortality showed that mortality differed notably within and across studies; all-cause mortality at day 28 was 27.8% in bacterial HAP, 18% in bacterial VAP and 14.5% in non-ventilation-associated bacterial HAP 45 .
In a systematic review and meta-analysis 10 , aspiration pneumonia was significantly associated with increased in-hospital mortality (relative risk 3.62) and 30-day mortality (relative risk 3.57) in patients with CAP treated outside of the ICU. One of the largest studies in aspiration pneumonia demonstrated that mortality in patients with aspiration pneumonia (29%) was more than twice that in patients with CAP (12%) 11 .
Risk factors and differences in epidemiology
Children of <5 years of age 46 and older adults 13 , particularly those of of ≥65 years of age and with comorbidities 14 , 47 , have an increased risk of CAP (Table 1 ). In children, prematurity, malnutrition, household air pollution, ambient particulate matter or suboptimal breastfeeding are the main CAP-related risk factors 48 . In adults, respiratory disease (for example, COPD), diabetes mellitus, cardiovascular disease and chronic liver disease are the most frequent comorbidities that increase the risk of CAP 14 . Of note, men have a higher risk of CAP than women, which may be explained by differences in anatomy, and behavioural, socioeconomic and lifestyle factors 49 .
A US study on the incidence, outcomes and disease burden in >18,000 hospitalized patients with COPD 37 found that, during the 2-year study, 3,419 patients had pneumonia; the annual incidence for CAP was 93.6 cases per 1,000 in the COPD population. In patients without COPD, the incidence was 5.09 cases per 1,000. In the USA, 506,953 adults with COPD are estimated to be hospitalized every year due to pneumonia 37 .
Immunocompromised patients have a higher risk of CAP than the general population 7 , 14 . A secondary analysis of an international, multicentre study from 54 countries worldwide found that almost one in five patients hospitalized with CAP were not immunocompetent 7 . Amongst patients with CAP, 18% had one or more risk factors for immunodeficiency, with chronic steroid use (45%), haematological cancer (25%) and chemotherapy (22%) being the most frequent.
Several studies have also demonstrated an association between lifestyle factors and the risk of CAP, including smoking, high alcohol consumption, being underweight (owing to under-nutrition or underlying conditions that compromise the immune response), living conditions, such as a large household or regular contact with children, and others 14 . Smoking is associated with colonization by pathogenic bacteria and an increased risk of lung infection, especially by S. pneumoniae 50 . Consumption of 24 g, 60 g and 120 g of pure alcohol daily (one standard alcoholic beverage equals 10 ml or 8 g of pure alcohol, and it is the approximate amount of alcohol that the average adult can process in an hour) resulted in relative risks for CAP of 1.12, 1.33 and 1.76, respectively, compared with no consumption 51 . In addition, exposure to air pollution may increase the risk of pneumonia in the short and long term; a study in 345 hospitalized patients with CAP and 494 controls (patients who were admitted in the same period but for non-pneumonia reasons) demonstrated that long-term exposure (1–2 years) to high levels of air pollutants (particulate matter 2.5 μm and nitrogen dioxide) was associated with increased hospitalization in those of ≥65 years of age 52 .
Factors that increase the risk of HAP can be categorized into patient-related and treatment-related groups (Table 1 ). Oropharyngeal colonization is the main mechanism underlying HAP. However, much attention has been shifted to oropharyngeal colonization in critically ill patients (present at ICU admission or occurring during ICU stay) 53 . A study from Japan investigating oral colonization in residents in long-term care facilities found that 38% of these individuals were colonized with antibiotic-resistant pathogens, mainly Acinetobacter spp., Enterobacterales and Pseudomonas spp. The presence of these pathogens represents a potential risk for pneumonia 54 . Indeed, current international guidelines have suggested that previous colonization by antibiotic-resistant pathogens be considered when identifying patients with an increased risk of HAP due to such pathogens 3 , 4 .
Colonization and biofilm formation were present within 12 h of intubation and remained for >96 h in most patients 55 . Underpinning an important association between intubation and VAP pathogenesis, this study also showed that colonization in patients undergoing mechanical ventilation occurred in the oropharynx and stomach first, followed by the lower respiratory tract and, thereafter, the endotracheal tube 55 . Intubation and mechanical ventilation can increase the risk of developing VAP by 6–21-fold, with the highest risk within the first 5 days of intubation 53 . Endotracheal tubes enable the direct entry of bacteria into the lower respiratory tract, interfere with normal host defence mechanisms and serve as a reservoir for pathogenic microorganisms.
Multiple risk factors are related to aspiration pneumonia, each one increasing the chance of gastric contents reaching the lungs. The most frequent of these factors are impaired swallowing, decreased consciousness and an impaired cough reflex 1 (Table 1 ).
Microbial aetiology
Knowledge of pathogens associated with pneumonia is crucial to provide more targeted empiric antibiotic therapy, prevent the emergence of antimicrobial resistance through selection pressure and reduce health-care-associated costs.
The microbial aetiology of CAP differs by its severity at clinical presentation and by season 2 , 56 , 57 , 58 . However, the microbial aetiology of CAP is not detected in ~50% of patients; possible reasons include the failure to obtain a respiratory sample adequate for culture or before the initiation of antibiotic therapy and the inconsistent availability of newly improved molecular tests 59 . S. pneumoniae remains the most frequent pathogen in CAP, although a study in North America found that its incidence has decreased owing to the introduction of polysaccharide vaccines 60 and a reduced smoking rate 61 , 62 . No such decrease has been observed in Europe 2 , 63 , 64 , 65 (Fig. 1 ).
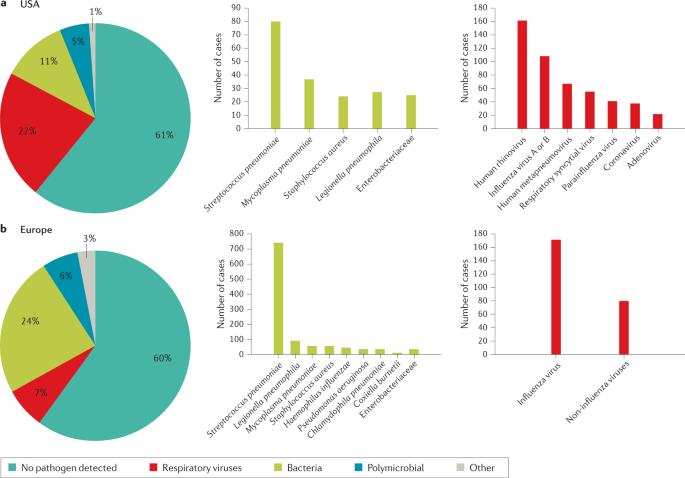
a | Aetiology of community-acquired pneumonia (CAP) in the adult population in the USA from 2010 to 2012 (from 2,488 cases) 9 . b | Aetiology of CAP in the adult population in Europe from 2003 to 2014 (from 3,854 cases) 6 . Possible reasons that may explain the challenge in identifying the aetiology of pneumonia include difficulty in obtaining samples from the lower respiratory tract, the effect of antibiotic use prior to sample collection and low sensitivity of some diagnostic tests.
In a small proportion of patients, CAP is caused by MRSA and antibiotic-resistant gram-negative bacteria (for example, P. aeruginosa and Klebsiella pneumoniae ) 2 , 66 . As antibiotic resistance complicates clinical management, clinicians need to recognize risk factors for these pathogens and initiate adequate empirical therapy in response (Box 3 ). The main risk factors for multidrug-resistant (MDR) pathogens in CAP include immunosuppression, previous antibiotic use, prior hospitalization, use of gastric acid-suppressing agents, tube feeding and non-ambulatory status 67 . Various scoring systems can help to determine the risk of infection by antibiotic-resistant pathogens.
The P. aeruginosa , extended-spectrum β-lactamase (ESBL)-positive Enterobacterales and MRSA (PES) score 68 is based on several risk factors, including age 40–65 years and male sex (one point each), age >65 years, previous antibiotic use, chronic respiratory disorder and impaired consciousness (two points each), and chronic renal failure (three points). The PES score has been validated in general wards, ICUs and a very old population (age ≥80 years). One study 69 demostrated that there is an 80% probability of detecting a PES pathogen with the PES score, demonstrating good accuracy of the score. In another study 70 , the accuracy of the PES score in patients of ≥80 years of age with CAP was ~64%, highlighting differences in clinical characteristics of this population who are more susceptible to infections, recurrent pneumonia and sepsis.
The drug resistance in pneumonia (DRIP) score 71 is based on both major and minor risk factors. Major risk factors (two points each) include previous antibiotic use, residence in a long-term care facility, tube feeding and prior infection by a drug-resistant pathogen (within the past year). Minor risk factors (one point each) include hospitalization within the previous 60 days, chronic pulmonary disease, poor functional status, gastric acid suppression, wound care and MRSA colonization (within the past year).
The use of new diagnostic molecular techniques has led to an increased interest in the role of respiratory viruses as potential aetiological agents in CAP. Recent studies have reported that respiratory viruses account for 7–36% of CAP cases with a defined microbial aetiology 13 , 72 , 73 . A recent study from China reported that in patients with viral CAP, influenza virus, non-influenza virus and mixed viral infections were the cause of CAP in 63%, 27% and 10% of patients, respectively (Fig. 2 ). The outcomes were similar between patients with CAP due to influenza virus and those with CAP due to non-influenza viruses, although in patients with CAP due to non-influenza viruses the incidence of complications was higher 74 . In another study, 3% of all patients with a diagnosis of CAP admitted to the emergency department had pure viral sepsis 75 . Viral sepsis was present in 19% of those admitted to ICU, and sepsis was present in 61% of all patients with viral CAP.
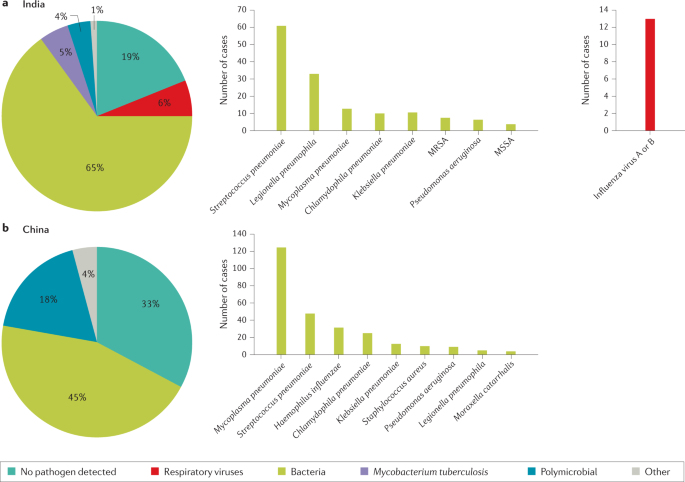
a | Aetiology of community-acquired pneumonia (CAP) in the adult population in India from 2013 to 2015 (from 225 cases) 54 . b | Aetiology of CAP in the adult population in China from 2004 to 2005 (from 593 cases) 55 . Possible reasons that may explain the challenge in identifying the aetiology of pneumonia include difficulty in obtaining samples from the lower respiratory tract, the effect of antibiotic use prior to sample collection and low sensitivity of some diagnostic tests. MRSA, methicillin-resistant Staphylococcus aureus ; MSSA, methicillin-susceptible Staphylococcus aureus .
Respiratory viruses are detected in more than half of children with CAP 76 . Respiratory viruses were the most frequent cause of pneumonia (66%) in children with an aetiological diagnosis in the USA, with respiratory syncytial virus, rhinovirus and metapneumovirus being the most common ones 76 . Bacterial pathogens were the cause of CAP in 8% of patients, with S. pneumoniae and S. aureus being the most common bacteria. Bacteria–virus co-infections were detected in 7% of patients.
Box 3 Pathogen-specific risk factors
Streptococcus pneumoniae : Dementia, seizure disorders, congestive heart failure, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), HIV infection, overcrowded living conditions and smoking
Legionella pneumophila : Smoking, COPD, compromised immune system, travel to outbreak areas, residence in a health-care facility and proximity to cooling towers or whirlpool spas
MRSA : Previous MRSA infection or colonization, residence in a nursing home or long-term care facility and prior hospitalization within the previous 90 days
Pseudomonas aeruginosa : Pulmonary comorbidity
Enterobacterales: Residence in a nursing home
MRSA, methicillin-resistant Staphylococcus aureus
Data on microbial aetiology of HAP have mostly been obtained from patients with VAP. However, studies in patients with HAP or VAP with known microbial aetiology have shown that both HAP and VAP have similar microbial aetiology, with P. aeruginosa and S. aureus being the most frequent pathogens. Other pathogens such as Acinetobacter spp. and Stenotrophomonas spp. are more frequently reported in VAP 4 , 77 .
Antibiotic resistance is the main concern with HAP and VAP. Assessing risk factors for MDR organisms (resistant to at least one agent in three or more groups of antibiotics), extensively drug-resistant organisms (XDR; resistant to one or more agents in all but one or two antibiotic groups) and pandrug-resistant organisms (resistant to almost all groups of approved antibiotics) is central to managing patients with these pathogens 78 . In general, we can classify the risk into three categories: (1) local epidemiology (for example, ICU with high rates of MDR pathogens); (2) patient risk factors (including structural pulmonary diseases (for example, bronchiectasis), antibiotic use during the 90 days prior to HAP or VAP onset, hospitalization (2–5 days) during the 90 days prior to HAP or VAP onset, septic shock at VAP onset, acute respiratory distress syndrome (ARDS) preceding VAP, at least 5 days of hospitalization prior to VAP onset, and acute renal replacement therapy prior to VAP onset) 42 ; and (3) previous colonization or infection with MDR pathogens 42 . Anaerobes and gram-negative bacilli (for example, E. coli , K. pneumoniae and P. aeruginosa ) are the most frequent microorganisms found in aspiration pneumonia 1 .
Mechanisms/pathophysiology
From colonization to infection.
The mechanisms that drive LRTIs have become increasingly known. Most instances of bacterial pneumonia are caused by microorganisms that translocate from the nasopharynx to the lower respiratory tract 79 , 80 . Bacteria enter the nasopharynx after shedding from a colonized individual. Pathogens can spread between individuals via direct or indirect contact, droplets and aerosols 81 . Transmission success depends on many variables, including environmental conditions, gathering of people and host factors, such as the distribution of pattern recognition receptors in the epithelial cells of the airways 81 . Pathogen adherence to the upper airway epithelium is a crucial first step in colonization and subsequent infection. Once in the nasopharynx, bacteria escape from mucus and attach to the epithelium using multiple strategies to evade host clearance, including expression of host-mimicking or antigenically varying molecules 82 (that is, molecules that imitate the structure of host molecules or can vary their antigens to avoid recognition by host immune cells). Microorganisms gain entry to the lower airways through inhalation or, less frequently, by pleural seeding from blood. Selection of colonizing mutants that can evade immune clearance is considered to precede infection 79 . Infection occurs when host defences are impaired and/or there has been exposure to a highly virulent microorganism or a large inoculum. Several factors can facilitate the transition from colonization to infection, including preceding viral infection and chronic lung diseases. Other mechanisms involved in the increased susceptibility to infection include loss of barrier integrity and impaired host defences due to complex interactions amongst anatomical structures, microorganisms (and their virulence factors) and the host immune system 79 , 80 , 83 .
Of note, it has become clear that healthy lungs are not sterile; instead, they harbour a unique microbiota that includes ~100 different taxa 84 . The main genera in healthy lower airways are Prevotella , Streptococcus , Veillonella , Fusobacterium and Haemophilus 84 . The pathogenesis of pneumonia has been suggested to include a change in the lung microbiota, from a physiological, homeostatic state to dysbiosis, in association with a low microbial diversity and high microbial burden, and with corresponding immune responses 84 , 85 To further support this concept, longitudinal lung microbiota studies are required to document transitions from homeostatic to dysbiotic states during the development and resolution of pneumonia. An additional area of research lies in analysing the virome and mycobiome in airways and their influence on host defence against pneumonia. The mechanisms by which lung microbiota affect immunity in the airways have been partially elucidated. Bacteria present in the upper airways that potently stimulate nucleotide-binding oligomerization domain-containing (NOD)-like receptors ( Staphylococcus aureus and Staphylococcus epidermidis ) increase resistance to pneumonia through NOD2 and induction of release of granulocyte–macrophage colony-stimulating factor 86 .
Mechanisms of infection
A general mechanism of infection of the lower airways is difficult to define. The many different microorganisms that can cause pneumonia do not seem to express specific features. Even in specific populations (for example, young children, hospitalized patients, older individuals), a spectrum of pathogens, rather than a specific microorganism, can cause pneumonia. This finding has led to the assumptions that the development of pneumonia largely depends on the host response to the microbe in the airways, with pathogen characteristics playing a less prominent role 83 . Nonetheless, virulence factors expressed by microorganisms do contribute to the ability of specific pathogens to cause pneumonia 79 , 80 . For example, pneumolysin, a virulence factor expressed by S. pneumoniae , is a member of the cholesterol-dependent cytolysin family that can form large pores in (and thereby injure) eukaryotic cells with cholesterol-containing membranes 87 . S. aureus expresses several virulence factors, such as α-haemolysin (also known as α-toxin), a pore-forming toxin that causes cell death via activation of the inflammasome 88 . α-Haemolysin binds to the disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) and results in disruption of the barrier function of the respiratory epithelium 88 . Finally, toxins secreted by the type III secretion system are a key element in P. aeruginosa virulence in the lung. Genes encoding type III-secreted toxins are induced in P. aeruginosa upon contact with host cells, eliciting a plethora of effects, including cytotoxicity 89 .
Once an LRTI has occurred, the maintenance of lung homeostasis whilst in the presence of microbes depends on an adequate balance between two seemingly opposing processes, immune resistance and tissue resilience, that are largely mediated by the same cell types. Whilst immune resistance seeks to eliminate invading microbes, tissue resilience strives to prevent or resolve tissue damage caused by the immune response, the pathogen or both 83 . The organized actions of immune resistance and tissue resilience determine whether and how an LRTI progresses or resolves. Inadequate or unfitting immune responses can result in adverse outcomes, such as ARDS, defined as the acute onset of non-cardiogenic pulmonary oedema, hypoxaemia and the need for mechanical ventilation 90 , 91 . Unbalanced immune responses during pneumonia can also result in extrapulmonary complications, some of which can occur up to years after the respiratory illness (see below).
Immune resistance
Anatomical barriers present the first line of defence against pneumonia. Mucociliary clearance, mediated by mucous and liquid layers and cilia on the surface of respiratory epithelial cells, is considered the primary innate defence mechanism 92 . The respiratory epithelium produces a robust barrier composed of secretory products, surface glycocalyces and membranes, and intercellular junctional proteins linked to the actin cytoskeleton 92 . Cell-associated and secreted mucins form a polymeric glycoconjugate layer that can bind and transport pathogens from the airways 92 . The branching bronchial tree provides an additional defence mechanism by preventing particles of >3 µm in diameter from entering the lower airways 92 . If microbes do reach the lower respiratory tract, the host defence becomes shaped by an interplay between resident and recruited immune cells and mechanisms (Fig. 3 ).
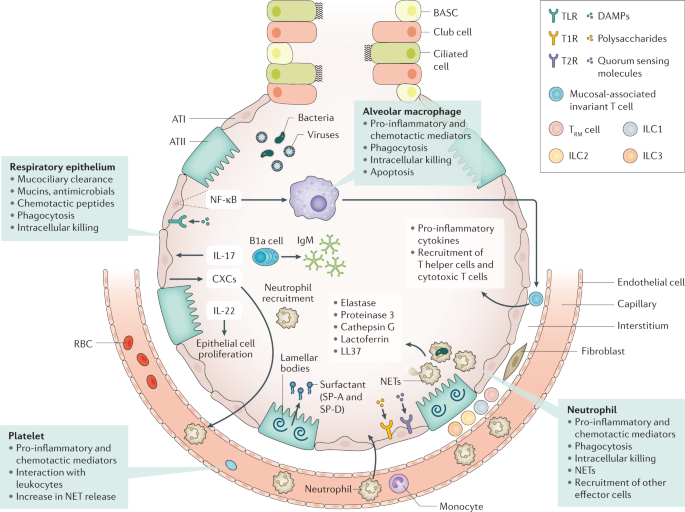
Immune resistance aims to eradicate microorganisms that invade the airways. Respiratory epithelial cells are covered by cell-associated and secreted mucins that form a layer of polymeric glycoconjugates that remove pathogens from the airways. The epithelium can also remove pathogens through phagocytosis and intracellular killing. The quiescent alveolar space contains many alveolar macrophages that, upon activation, can phagocytose and kill pathogens, which is improved by apoptosis. Innate lymphoid cells (ILCs) are tissue-resident cells populating the pulmonary mucosa. Together with natural killer cells, ILCs boost host defence during airway infection. Neutrophils migrate to the airways attracted by chemotactic proteins released by respiratory epithelial cells and alveolar macrophages; these chemotactic proteins also promote the recruitment of other leukocyte subsets. The lung contains a marginated pool of neutrophils tethered to the vasculature, enabling rapid neutrophil recruitment into tissue upon infection. Adequate pulmonary immunity entails neutrophil-mediated killing of invading microbes by several effector mechanisms, including the release of neutrophil extracellular traps (NETs). Platelets can form complexes with leukocytes, facilitating NET formation and the release of microbicidal agents. Resident memory T (T RM ) cells are generated after exposure to pathogens and reside in the quiescent lung. ATI, alveolar type I cell; ATII, alveolar type II cell; BASC, bronchioalveolar stem cell; CXCs, CXC chemokines; DAMPs, damage-associated molecular patterns; NF-κB, nuclear factor-κB; RBC, red blood cell; SP, surfactant protein; T1R, G-protein-coupled sweet taste receptor; T2R, G-protein-coupled bitter taste receptor; TLR, Toll-like receptor.
Innate immunity
Various innate immune cells reside in quiescent airways to provide the next line of defence against pathogens. Lung epithelial cells can be triggered through a variety of receptors that recognize not only pathogens but host-derived molecules as well, including damage-associated molecular patterns (released upon cell injury) and cytokines. Many pattern recognition receptors (for example, toll-like receptors) then induce nuclear factor ĸB, which is a major driver of protective immunity in the epithelium 93 , 94 . In the alveoli, surfactant proteins SP-A and SP-D produced by type II epithelial cells can directly inhibit microbes 95 . Recently, G-protein-coupled bitter taste receptors (T2R) and sweet taste receptors (T1R) were identified in respiratory epithelial cells 96 ; bacterial quorum-sensing molecules can trigger bitter taste receptors, whilst sugars can activate sweet receptors, and these interactions may then modify host defence mechanisms 97 . IL-17 and IL-22 mediate protection during pneumonia largely through epithelial cell activation 98 . IL-17 stimulates the epithelium to secrete antimicrobial proteins and CXC chemokines that trigger neutrophil recruitment. The protective properties of IL-22 are linked to its function in stimulating epithelial cell proliferation, which is indispensable for repair following injury 99 .
Alveolar macrophages (AMs), which reside on lower airway surfaces, have essential roles in both immune resistance and tissue resilience 100 . During homeostasis, they limit the effect of potentially noxious environmental stimuli through anti-inflammatory effects. The crucial role of AMs in immune resistance during pneumonia is illustrated by studies showing impairment of the host defence when AM function is disrupted 94 . Microbes can activate AMs via several pattern recognition receptors and nuclear factor ĸB, leading to the production of pro-inflammatory cytokines that orchestrate subsequent, innate immune responses necessary for resistance. In addition, stimulated by AM apoptosis, activated AMs can phagocytose and kill pathogens 101 . By contrast, AM death via non-apoptotic pathways, such as necroptosis, impairs antibacterial defence during pneumonia 102 . The complex role of necroptosis in the host response to bacterial infection is illustrated by reports linking necroptosis to exaggerated inflammation and impaired bacterial clearance during S. aureus pneumonia 103 , whereas it has a protective, anti-inflammatory effect associated with improved bacterial clearance during systemic S. aureus infection 104 . Local conditions may instruct AMs in providing the most suitable response.
Innate lymphoid cells (ILCs) serve as counterparts to T cells by regulating immune responses via the production of effector cytokines and by influencing functions of other innate and adaptive immune cells 105 . These cells are especially abundant on the mucosal surfaces of the lung. There are three major groups of ILCs, namely, ILC1, ILC2 and ILC3. ILC classification reflects these cells’ capacity to secrete types 1, 2 and 17 cytokines, respectively. Beneficial roles for ILC1s and ILC2s have been reported in viral pneumonia models 106 , 107 ; lung ILC3s have a protective role in pneumonia by secreting IL-17 and IL-22 (refs 108 , 109 ). Mucosal-associated invariant T cells are other innate-like T lymphocytes that are abundant in the lung mucosa 110 . These cells probably have a role in protective immunity during airway infection through a variety of mechanisms, including production of pro-inflammatory cytokines, macrophage activation and recruitment of effector helper and cytotoxic T cells 111 .
When resident cells are unable to eradicate invading pathogens, mechanisms are activated to attract additional effector cells to the site of infection. Neutrophils are the first and most profusely recruited cells in response to infection 112 . Primed neutrophils have a strongly increased capacity to phagocytose microbes and initiate a respiratory burst response 112 . In addition, neutrophil products, such as elastase, proteinase 3 (also known as myeloblastin), cathepsin G, lactoferrin and LL-37, exert potent antimicrobial activities 113 . Neutrophil extracellular traps, comprising decondensed chromatin fibres that carry histones and antimicrobial peptides, are also released to kill pathogens 113 . The crucial role of neutrophils in pulmonary immune resistance is illustrated by the increased susceptibility found in patients with neutropenia or neutrophil deficiencies and mouse pneumonia models, in which neutrophil depletion has been shown to exacerbate infection with several pathogens 112 . In addition to AMs, newly recruited inflammatory monocytes–macrophages are involved in immune resistance during pneumonia 114 . In mice, induction of K. pneumoniae -associated pneumonia has been found to lead to the recruitment of inflammatory monocytes to the lungs where they mediate the influx of protective ILCs producing IL-17 through the release of tumour necrosis factor 109 . Innate-like B1 B cells mainly reside in the pleural space. In response to infection, B1a B cells migrate to the lung parenchyma to produce polyreactive immunoglobulin M and contribute to protective immunity 115 . Platelets also provide immune resistance during pneumonia through various mechanisms, including platelet–bacteria interactions and complex formation with leukocytes. Other mechanisms include facilitating neutrophil extracellular trap formation and stimulating the release of microbicidal agents that can directly lyse bacteria 116 . Thrombocytopenia is associated with impaired antibacterial defence during murine pneumonia 117 , 118 .
Finally, several distant organs can affect immune resistance in the respiratory tract. For example, depletion of gut microbiota by broad-spectrum antibiotics has been shown to impair host defence during viral and bacterial pneumonia in mice 119 , 120 . This protective gut–lung axis has been hypothesized to be mediated, at least in part, by gut-derived microbial products that can improve host defence mechanisms in other tissue 121 . The existence of a liver–lung axis has been suggested in many studies; pneumonia elicits a robust acute-phase protein response in the liver, probably mediated by cytokines released into circulation, and distinct acute-phase proteins can improve antibacterial defence through several mechanisms, for example, by enhancing opsonophagocytosis (phagocytosis mediated by opsonins) and respiratory burst activity by immune cells and by limiting iron availability to bacteria.
Adaptive immunity
Previous encounters with respiratory pathogens shape memory defence mechanisms against pneumonia. Evidence highlights roles of innate immune cells (for example, epithelial cells and AMs) that had been modified by a prior infection to trigger epigenetic alterations in a so-called process of ‘trained immunity’ 122 . Trained immunity has received attention within the context of pneumonia in humans. The Bacille Calmette–Guérin vaccination induces trained immunity. When administered to older patients after hospital discharge, the vaccination increased time to first infection, and most of the protection was observed against respiratory tract infections of probable viral origin 123 . Humoral response to microbes improves host defence by producing pathogen-specific antibodies, as illustrated by the efficacy of vaccines in diminishing the risk of pneumonia.
The airways contain pools of memory cells that are assembled in tertiary lymphoid organs in the upper airways and in bronchus-associated lymphoid tissue in the lower airways. Together, these cells protect against infection through local and systemic antibody production 124 . The majority of CD4 + T cells and CD8 + T cells in the quiescent lung have a memory phenotype (hence they are named resident memory T (T RM ) cells) and are generated in response to exposure to respiratory pathogens 125 . In experimental mouse models, the lung is enriched with T RM cells specific for multiple viral and bacterial pathogens following a respiratory infection, and these cells contribute to future protective immunity. For example, lobar pneumococcal pneumonia in mice leads to the accumulation of CD4 + T RM cells in the infected lobe, but not in other areas of the lung. This T RM cell-populated lobe expresses better defence against reinfection by S. pneumoniae than other lobes 126 .
Tissue resilience
Tissue resilience is essential in controlling excessive inflammation whilst sustaining effective protection against microbes (Fig. 4 ). AMs contribute to tissue resilience by producing anti-inflammatory cytokines, such as IL-10 and IL-1 receptor antagonist, and through the phagocytosis of apoptotic leukocytes. This process is named efferocytosis and protects tissue in two manners: by preventing the release of pro-inflammatory and toxic contents from dying cells and by concurrently prompting the release of anti-inflammatory and repair factors, including transforming growth factor β1, prostaglandin E 2 , and pro-resolving lipid mediators 100 . Pro-resolving lipid mediators (resolvins, protectins and maresins) can mediate a large variety of immune responses in pneumonia, both increasing bacterial killing and promoting tissue repair 127 . Such mediators have been shown to have important protective roles in mouse models of bacterial pneumonia 128 , 129 .
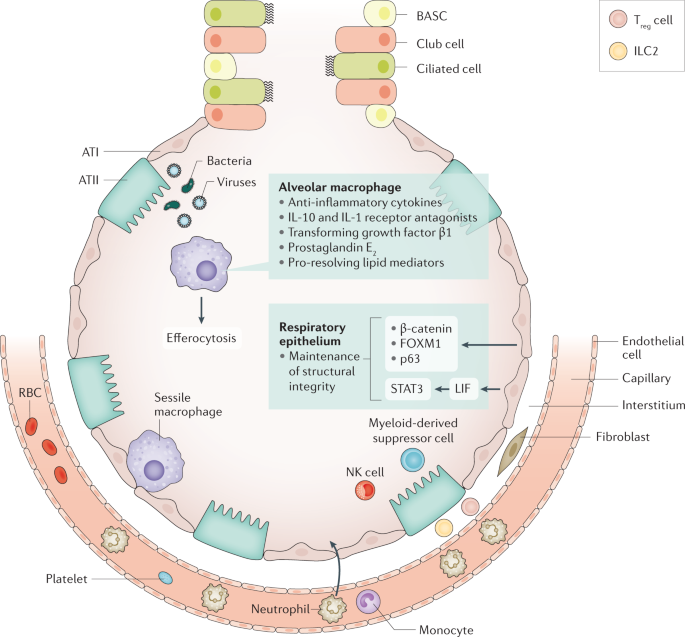
Tissue resilience controls excessive inflammation whilst safeguarding protection against pathogens. The respiratory epithelium is an important player in tissue resilience. Maintenance of the structural integrity of the epithelial barrier is a crucial factor here. Alveolar macrophages also have an important role, via release of anti-inflammatory mediators and efferocytosis (phagocytosis of apoptotic leukocytes). Sessile macrophages adhere to the epithelium, where they probably contribute to tissue resilience. Cell types recruited to the site of infection during pneumonia that are involved in tissue resilience include myeloid-derived suppressor cells, regulatory T (T reg ) cells, type 2 ILC2s and natural killer (NK) cells. ATI, alveolar type I cell; ATII, alveolar type II cell; BASC, bronchioalveolar stem cell; FOXM1, forkhead box protein M1; ILC, innate lymphoid cell; LIF, leukaemia inhibitory factor; RBC, red blood cell; STAT3, signal transducer and activator of transcription 3.
The structural integrity of the epithelial barrier in the respiratory tract is crucial to tissue resilience. Contributors to epithelial resilience include β-catenin (also known as catenin β1) 130 , forkhead box protein M1 (FOXM1) 131 , tumour protein 63 (p63) 132 and signal transducer and activator of transcription 3 (STAT3) 133 , 134 . Interestingly, a deficiency of STAT3 in airway epithelial cells results in exaggerated lung injury during experimental pneumonia 133 , 134 . Epithelial cell-derived leukaemia inhibitory factor (LIF) has been implicated as an important inducer of STAT3 in the respiratory epithelium, and inhibition of LIF has been shown to increase lung injury in pneumonia 135 . Several immune cells recruited to the site of infection during pneumonia are known to contribute to tissue resilience, including myeloid-derived suppressor cells 136 , regulatory T cells 137 , ILC2s 138 and natural killer cells 139 , 140 .
Lung pathology
With respect to the histopathology of bacterial pneumonia, four stages have classically been described: congestion, red hepatization, grey hepatization and resolution (Fig. 5 ). The term hepatization refers to an increased firmness of inflamed lung tissue that renders the tissue consistency similar to that attributed to hepatic tissue. In the early stages of bacterial pneumonia, lung tissue shows mild intra-alveolar oedema and congestion of the capillaries within the alveolar septa 141 . This stage is followed by inflammatory exudation with an accumulation in the alveolar spaces of neutrophils, red blood cells and fibrin, and a subsequent, gradual disintegration of red blood cells and neutrophils. The exudates are eventually transformed into intra-alveolar fibromyxoid moulds, consisting of macrophages and fibroblasts, and gradual resolution follows thereafter.
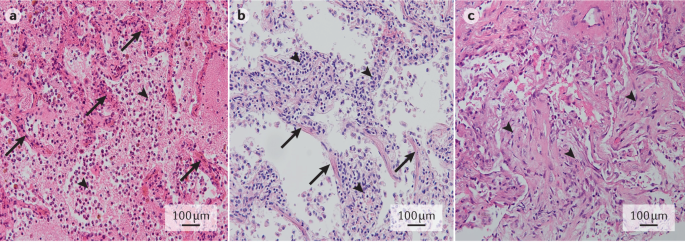
a | Early stage bacterial pneumonia, with congestion of septal capillaries (arrows) and intra-alveolar presence of oedema, neutrophils and a meshwork of fibrin strands (arrowheads). b | Early stage viral pneumonia, with interstitial lymphocytic infiltrates (arrowheads) and diffuse alveolar damage, as evidenced by the presence of hyaline membranes (arrows). c | Organizing pneumonia, with intra-alveolar fibroblast plugs (arrowheads) and few remnant fibrin deposits. Haematoxylin and eosin staining; original magnification ×20. Images in parts a – c courtesy of J.J.T.H. Roelofs, Amsterdam UMC, Netherlands.
Viral pneumonia is typically associated with interstitial inflammation and diffuse alveolar damage 142 . Interstitial inflammation involves the alveolar walls, which widen and usually contain an inflammatory infiltrate of lymphocytes, macrophages and plasma cells in some cases. Alveolar damage is characterized by pink hyaline membranes lining the alveolar septa that follow a pattern of organization and resolution similar to that of intra-alveolar inflammation in bacterial pneumonia.
In addition to these features, specific microorganisms may cause different histopathological changes such as granulomas, multinucleated giant cells or specific viral inclusions.
Extrapulmonary complications
Extrapulmonary complications are extremely common in patients with pneumonia, including those without sepsis. Such complications entail both acute and long-term adverse sequelae. Patients who have been hospitalized for pneumonia have higher rates of all-cause hospitalization and an increased mortality risk for 10 years after discharge 35 compared with matched patients hospitalized for other pneumonia-unrelated conditions.
Sepsis, defined as a life-threating organ dysfunction caused by a dysregulated host response to an infection 143 , is most often caused by pneumonia (up to half of all patients with sepsis) 144 . Conversely, of patients who are hospitalized with CAP 145 or HAP 146 , 36% and 48% have been reported to develop sepsis, respectively. Both pro-inflammatory and anti-inflammatory reactions characterize host response to sepsis, which varies strongly between individuals. Pro-inflammatory responses include the release of cytokines, activation of the complement and coagulation system (which could result in disseminated intravascular coagulation), and disruption of the normal barrier and anticoagulant function of the vascular endothelium. Anti-inflammatory responses can result in immune suppression, in part due to apoptotic loss of lymphoid cells 147 , 148 .
Cardiovascular disease
Pneumonia particularly affects the cardiovascular system, and its effects include depression of left ventricular function, myocarditis, arrhythmias, ischaemia and infarction 149 . Patients hospitalized for pneumonia have an increased short-term and long-term risk (up to ten years) of cardiovascular disease 150 . A meta-analysis of the incidence of cardiac events within 30 days of pneumonia diagnosis found new or worsening heart failure in 14% of all patients, new or worsening arrhythmias in 5% and acute coronary syndromes in 5% 151 . Approximately 90% of cardiac complications occur within 7 days of a pneumonia diagnosis, and more than half occur within the first 24 h 149 . In a multicentre study, one third of patients hospitalized for CAP experienced intrahospital cardiovascular events, mainly involving the heart, and such occurrence was associated with a fivefold increase in 30-day mortality. Independent risk factors for cardiovascular events were severity of pneumonia and pre-existing heart failure 152 . Additionally, hospitalization for pneumonia is associated with an increased risk of new-onset heart failure in the intermediate and long term, with a hazard ratio of 2 after 5 years 34 . In patients with pneumonia who require ICU treatment within 24 h of hospital admission, approximately half have diagnostic criteria for myocardial infarction 153 ; cardiac complications are the direct or main cause of death in 27% of patients hospitalized for pneumonia 154 . Notably, whilst the increased risk for myocardial infarction associated with pneumonia is proportional to disease severity, it is not restricted to patients with pneumonia-induced sepsis 155 . Even mild respiratory infection is associated with an increased risk of myocardial infarction for several months after the onset of infection 155 .
The mechanisms underlying an increased risk of cardiovascular disease after pneumonia are probably multifactorial. Hypoxaemia due to impaired gas exchange and ventilation–perfusion mismatching, as well as endothelial dysfunction causing vasoconstriction, may increase vulnerability to ischaemic events 149 . Systemic inflammation during pneumonia can increase inflammatory activity within coronary atherosclerotic plaques, rendering them prone to rupture 149 . The systemic host response during pneumonia also entails endothelial dysfunction and procoagulant changes, which can promote thrombus formation at the site of a ruptured coronary plaque 149 . Indeed, as reflected by elevated markers of coagulation activation in the circulation, the majority of patients admitted to hospital for pneumonia have a procoagulant phenotype 156 , 157 .
Patients with pneumonia and acute coronary syndromes show higher platelet-aggregating activity than patients with acute coronary syndromes without pneumonia 149 . Notably, the connection between pneumonia and cardiovascular disease is probably bidirectional. For example, pre-existing heart failure is a risk factor for pneumonia, perhaps partially related to impaired immune responses 149 . Preclinical investigations suggest that lung congestion can facilitate the growth of common respiratory pathogens in the airways 149 . With regard to long-term risk, investigations in mice predisposed to developing atherosclerosis 158 and post mortem examinations in humans 159 have suggested that infection can elicit pro-inflammatory responses in atherosclerotic lesions and result in increased vulnerability for coronary and cerebrovascular events. For example, acute lung inflammation induced by intratracheal administration of lipopolysaccharide in mice prone to atherosclerosis resulted in destabilization of atherosclerotic plaques; neutrophil depletion prevented this destabilization, suggesting a role for neutrophils in plaque weakness elicited by lung injury 160 . In addition, systemic inflammation and coagulation are sustained in many patients with pneumonia and have been associated with an increased risk of cardiovascular death 161 , 162 . Left ventricular dysfunction during pneumonia may be secondary to depressant activity of pro-inflammatory cytokines in circulation and/or altered vascular reactivity 149 .
Other complications
Additional extrapulmonary complications of pneumonia include a decline in cognition and functional status 163 , 164 . Pneumonia is associated with a 57% increase in the risk of dementia 164 . Encephalopathy associated with acute infectious disease has been studied in the context of sepsis 165 , 166 . Mechanisms involved include impaired circulation in the brain secondary to hypotension, a disturbed vasoreactivity, endothelial dysfunction and microvascular thrombosis, which can result in ischaemic and haemorrhagic lesions. The blood–brain barrier can be disturbed through increased activity of pro-inflammatory cytokines and reactive oxygen species produced at least in part by astrocytes. Activation of microglia can further contribute to neuronal damage in the brain 166 .
Approximately one fifth of patients hospitalized with pneumonia are readmitted to the hospital within 30 days; pneumonia, cardiovascular disease and (chronic obstructive) pulmonary disease are the most common diagnoses 167 . An increased susceptibility for infection after pneumonia may be related to a relatively immunocompromised state, as has been described in patients with sepsis 147 . Knowledge of immunological defects contributing to recurrent pneumonia (usually defined as a new episode of pneumonia within several months of the previous one, separated by at least a 1-month asymptomatic interval and/or radiographic clearing of the acute infiltrate) 168 is limited. A small study involving 39 patients suggested that immunoglobulin deficiency and an inability to react to polysaccharide antigens are associated with an increased incidence of recurrent pneumonia 169 . Further, a study in mice found a reduced capacity of AMs to phagocytose E. coli and S. aureus following recovery from primary pneumonia, a reduction mediated by signal-regulatory protein-α (also known as tyrosine–protein phosphatase non-receptor type substrate 1) and associated with an impaired host defence after secondary infection of the lower airways 170 .
Diagnosis, screening and prevention
The most common symptoms of pneumonia are cough, breathlessness, chest pain, sputum production and fatigue 171 , 172 . Symptoms are not a part of the initial severity assessment of patients, as the initial symptom burden does not influence outcome. Exceptions include delirium, which is associated with an increased risk of mortality 173 , and pleuritic chest pain, which is associated with an increased risk of para-pneumonic effusion and complicated (infected) para-pneumonic effusion 174 , 175 . Usually mild disease refers to patients with CAP who do not require hospitalization, moderate disease to those cared for in conventional hospital wards, and severe disease to those admitted to the ICU.
It is not possible to differentiate bacterial and viral pneumonia based on symptoms in adults or children, as patients report similar symptoms regardless of microbial aetiology 176 . A recent study found that artificial intelligence was also unable to differentiate microbial aetiology based on symptoms, clinical features and radiology 177 .
CAP is usually clinically suspected in the presence of acute (≤7 days) symptoms of LRTI, such as cough, expectoration, fever and dyspnoea, as well as the presence of new infiltrates on chest radiographs (CXRs) 178 . In older patients, symptoms are typically less evident, and fever can be absent in as many as 30% of patients 179 . Symptoms may also be less evident in patients treated with steroids, NSAIDs and antibiotics 6 . Other pulmonary diseases — most frequently pulmonary embolism and lung cancer — may present with fever and pulmonary infiltrates that can mimic CAP. Interstitial and systemic diseases can also mimic CAP. When diagnosing CAP, it is extremely important to review prior chest CXRs if available, as an additional means to help rule out the disease.
Although HAP is also suspected clinically, symptoms may be hidden by either other medications or the cause of admission. No studies exist about symptom duration in HAP before diagnosis; however, it is usually suspected when patients present with pyrexia (fever) and/or tachypnoea (rapid breathing). HAP diagnosis is believed to be usually delayed, which could explain the higher mortality observed in this population than in patients with VAP.
VAP is suspected when there are at least two of the following symptoms: fever or hypothermia, leukocytosis or leukopenia, and evidence of purulent secretions in an endotracheal tube or tracheostomy 4 . For VAP diagnosis, clinicians often rely on clinical parameters; radiological and laboratory parameters help initiate antimicrobial treatment. Scores have been proposed to facilitate diagnosis. For example, the clinical pulmonary infection score (CPIS) 180 is the most common one, and it is based on points assigned to various signs and symptoms of pneumonia. A CPIS score of >6 suggests VAP, although score sensitivity and specificity are not perfect. In fact, the FDA does not accept this score to diagnose VAP in randomized controlled trials studying antibiotics. In patients with VAP, fever and pulmonary infiltrates can present as atelectasis (collapse of parts of the lung), alveolar haemorrhage and pulmonary thromboembolism, amongst other conditions. In a landmark study using immediate post mortem lung histopathology and microbiology as a gold standard, the presence of two clinical criteria plus the presence of infiltrates on CXRs had a 70% sensitivity and 75% specificity in the diagnosis of VAP 181 .
Radiographic confirmation is essential for the diagnosis of pneumonia. CXRs provide important information about the site, extent and associated features of pneumonia (for example, the lobes involved and the presence of pleural effusion and cavitation) 5 (Fig. 6 ). CXRs have a sensitivity and specificity of 43.5% and 93%, respectively, for detecting pulmonary opacities 182 . In CAP, sensitivity and specificity of 66% and 77%, respectively, have been reported 183 using CT scans as the gold standard. The presence of either pleural fluid or multilobar pneumonia serve as indicators of severity 5 . In CAP, the development of pulmonary infiltrates that were not previously present on a simple posterior–anterior (PA) CXR is essential for CAP diagnosis. The standard CXR for CAP consists of a PA and lateral images; the use of lateral projection images increases diagnostic performance of PA images. In HAP, radiographic evidence of infiltrates is usually determined by CXR examination alone. In VAP, new infiltrates are usually detected by anterior–posterior projection in the supine position; however, in this situation, CXRs are insufficiently sensitive and specific.
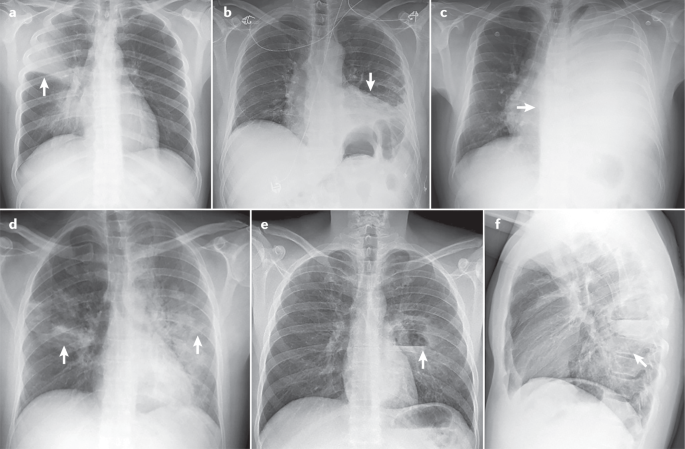
Pneumonia in upper right lobe (arrow) (part a ); pleural effusion on the left side (arrow) (part b ); massive pleural effusion in the left lung (arrow) (part c ); bilateral pneumonia (arrows) (part d ); lateral image showing left parahilar cavitation with air–fluid level in the lower left lobe (arrow) (part e ); front-to-back image in the same individual as in part e .
In studies in patients hospitalized with CAP, CT identified up to 35% of patients with CAP who had not initially been caught by CXRs 184 . In many patients with COVID-19, CT scans detect pulmonary infiltrates not observed on simple CXRs 185 . In patients with CAP, CT scans serve as a practical complement to CXRs in several cases: when radiographic findings are non-specific, when pulmonary complications such as empyema (pus in the pleural space) or cavitation are present, when there is suspicion of an underlying lesion such as lung carcinoma, and when recurrent pneumonia or non-resolving pneumonia is present 186 . Although this supporting role of CT scans is assumed to apply to patients with HAP as well, supporting evidence is lacking.
Ultrasonography
Lung ultrasonography is a non-invasive imaging method that is now frequently used in many emergency departments and ICUs. Advantages over CT include the absence of radiation exposure, ready use at the bedside and reasonable diagnostic sensitivity and specificity 187 . However, the technique has a steep learning curve, especially in mechanically ventilated patients. In a systematic review, lung ultrasonography was shown to have a sensitivity of 88% and a specificity of 89%, with a ~90% probability of diagnosing pneumonia 188 . Echographic diagnosis is more complex in patients with VAP, and only a few observational studies have been conducted to date 188 . The best of these studies have shown that such diagnosis had better accuracy than the CPIS score alone; the addition of direct Gram stain examination in quantitative cultures of endotracheal aspirates further improved accuracy 189 , 190 . On the basis of on these results, the ventilator-associated pneumonia lung ultrasound score (VPLUS) was developed, and has a sensitivity of 71% and a specificity of 69% for VAP diagnosis 190 .
Microbiology and laboratory tests
Recommendations for microbiological diagnosis in CAP vary according to disease severity (Table 2 ). Of note, microbiological diagnosis in CAP cannot be obtained in up to 50% of patients 5 . In patients with CAP who do not need hospital admission, obtaining samples such as sputum and pharyngeal swabs is optional or not recommended in recent guidelines 5 . In patients requiring hospitalization, obtaining good-quality sputum and blood samples, as well as pharyngeal swabs (for PCR), is recommended. Sputum is the most common respiratory sample in patients with CAP, and samples should be collected before antibiotic treatment. The sensitivity of Gram staining for a sputum sample is ~80% in patients with pneumococcal pneumonia and 78% in patients with pneumonia caused by Staphylococcus spp., and the specificity is 93–96% 191 , 192 . Most health care institutions perform viral PCR on pharyngeal swabs during the influenza season. In the COVID-19 pandemic, it is recommended that all patients admitted with CAP receive a PCR test for the detection of SARS-COV-2.
In patients requiring ICU admission, in addition to all tests mentioned above, bronchoscopic samples, such as bronchoalveolar lavage (BAL) in intubated patients, are not difficult to obtain and provide information on the lower respiratory tract microbiota. Urinary antigen detection tests for S. pneumoniae and L. pneumophila have good sensitivity and specificity, are not extremely expensive and are recommended in all hospitalized patients.
In patients with HAP or VAP, international guidelines 4 recommend obtaining distal respiratory samples for semiquantitative or quantitative cultures (Table 3 ). In patients with HAP, bronchoscopy is not easy to perform, and sputum samples are not often collected. In patients with VAP, distal respiratory samples are preferred. BAL (performed with or without concomitant bronchoscopy) is the sample that provides most information, as, in addition to cultures, cellularity analysis and PCR can be performed on the fluid. A recent meta-analysis showed that Gram staining of BAL performs well in detecting S. aureus 193 . Respiratory samples from patients with HAP or VAP have to be collected before the initiation of a new antibiotic treatment to avoid false-negative cultures. International guidelines 4 do not recommend using procalcitonin (PCT) for the initial diagnosis of HAP or VAP, as several studies have shown that it lacks diagnostic value 194 .
Since the 2000s, owing to multiple outbreaks, epidemics and pandemics caused by respiratory viruses in particular, several molecular tests have been developed, which have contributed to widened availability of molecular testing for the aetiological diagnosis of CAP. Molecular tests have several advantages, including detecting low levels of microbial genetic material, remaining unaffected by prior antibiotic therapy, and providing results within a clinically relevant time frame 195 . Molecular tests based on multiplex PCR have been developed to simultaneously detect and quantify multiple respiratory pathogens, as well as some genes related to antimicrobial resistance. Several commercial multiplex platforms are currently available for comprehensive molecular testing for respiratory pathogens that cause pneumonia (respiratory viruses, bacteria and fungi) and for the main resistance genes of the most common bacteria causing pneumonia 195 , 196 , 197 , 198 .
The WHO currently recommends COVID-19 diagnosis by molecular tests that detect SARS-CoV-2 RNA. SARS-CoV-2 viral sequences can be detected by real-time reverse transcriptase (RT-PCR) in nasopharyngeal swab samples 199 . The disadvantage of this method is that it requires specialized equipment and trained personnel. Additionally, two types of rapid tests are available for COVID-19 diagnosis. The direct SARS-CoV-2 antigen test detects viral components present during infection in samples such as nasopharyngeal secretions, and, therefore, can indicate whether an individual is currently carrying the virus. The indirect antibody test detects antibodies that can be found in serum as part of the immune response against the SARS-CoV-2; thus, it can yield false-negative results if performed before the antibody response has developed and cannot distinguish between past and current infections. These two tests are relatively simple to perform and interpret, requiring limited test operator training 199 .
Some biomarkers may be helpful in identifying which patients are likely to have bacterial pneumonia, in deciding whether antibiotic therapy should be administered, in determining prognosis and in facilitating decisions related to the site of care. However, biomarkers should only be used as an adjunctive tool when managing CAP, as no biomarker has proven full utility in predicting clinical outcomes in patients.
The most widely used biomarkers are acute phase reactants such as C-reactive protein (CRP) and PCT 200 . However, their serum kinetics differ: CRP levels increase after the first 3 days of infection (peak time from infection is 36–50 h), whereas PCT levels rise rapidly (peak time from infection is 12–24 h) in response to microbial toxins or host responses. These properties are useful in differentiating CAP from other non-infectious causes. CRP levels increase in response to any inflammation, and can be modified by the presence of corticosteroids and previous antibiotic therapy, whereas PCT is more specific in bacterial pneumonia. Viral infection-related cytokines attenuate induction of CRP and PCT; however, some elevation in their levels can occur when pneumonia is caused by atypical pathogens (for example, Mycoplasma spp., Chlamydia spp. and Legionella spp.) 201 .
Both CRP and PCT can assist in the clinical diagnosis of pneumonia, but CRP and PCT cannot be used in isolation as a basis for treatment decisions. A second test after 24–48 h is mandatory to monitor for any increases. Clinicians should also consider the pattern in the days preceding symptom onset in patients with CAP and whether a patient is taking medication that could have modified these values. For patients with radiographic CAP, PCT levels can be used with clinical assessment to identify those individuals from whom antibiotic therapy can be safely withheld. This assessment can be combined with a PCR test to identify viral infection, especially as new data show that viruses can frequently be a cause of CAP 13 , 75 . However, caution should be used when a mixed viral–bacterial infection is considered. The new American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) CAP 5 guidelines do not recommend using PCT to determine the need for initial antibacterial therapy. The current recommendation is that empirical antibiotic therapy should be initiated in adults with clinically suspected and radiographically confirmed CAP, regardless of initial PCT level.
In studies in patients with HAP or VAP, in whom biomarkers had been monitored serially since before infection, steady increases or persistent elevations in CRP levels were shown to be associated with a high risk of VAP 202 . However, no such pattern was shown for PCT values (crude values or kinetics), with poor diagnostic accuracy for VAP 203 . Thus, a recent international consensus concluded that a combination of clinical assessment including PCT levels in well-defined antibiotic stewardship algorithms could improve diagnosis of bacterial infections and support antibiotic effectiveness 204 .
Prevention of CAP
Many factors increase the risk of CAP and can generally be divided into host factors (for example, age, and the presence of COPD and other chronic pulmonary diseases, diabetes mellitus and chronic heart failure), unhealthy habits (for example, smoking and excessive alcohol consumption) and medications (for example, immunosuppressive drugs, sedating medications such as opioids, and proton pump inhibitors within the first 3 months of administration 205 ). Prevention of CAP is crucial, especially in individuals with these risk factors. Available preventive measures include smoking and alcohol use cessation, improvements in dental hygiene, physical exercise, avoiding contact with children with respiratory infections, and pneumococcal and influenza vaccinations 14 . Implementing these measures in primary and specialized care could help reduce the burden of CAP. Presently, pneumococcal and influenza vaccination are the cornerstones of CAP prevention.
The 23-valent pneumococcal polysaccharide vaccine (PPV23) and the 13-valent pneumococcal conjugate vaccine (PCV13) are currently used in adults. Owing to the demonstrated effectiveness of PPV23 in preventing invasive pneumococcal disease (IPD) in people of ≥65 years of age, the use of the vaccine in this population is recommended in many countries 206 . However, PPV23 effectiveness in preventing non-IPD or CAP due to any cause is much less clear. The effectiveness of PPV23 has been reported to range from 25% to 63% in pneumococcal pneumonia 207 , 208 ; the effectiveness of PCV13 in preventing the first episode of CAP, non-bacteraemic and non-invasive CAP, and IPD due to serotypes contained in the vaccine amongst adults of ≥ 65 years of age has been reported to be 45.6%, 45% and 75%, respectively 209 . Efficacy persisted through the mean follow-up period of 4 years 209 . A post-hoc analysis based on data from the CAPITA trial showed that the effectiveness of PCV13 ranged from 43% to 50.0% for pneumococcal CAP, 36% to 49% for non-bacteraemic and non-invasive pneumococcal CAP, and 67% to 75% for pneumococcal IPD 210 . Of note, the most important measure in reducing pneumococcal CAP burden (bacteraemic and non-bacteraemic) in adults is conjugate vaccine programmes in children. Vaccination with pneumococcal conjugate vaccine in children substantially reduces disease in adults owing to the interruption of transmission and herd protection 211 , 212 .
Influenza vaccination can reduce the risk of complications of influenza, such as pneumonia, and is associated with a decrease in severity, hospitalization, ICU admission and mortality associated with influenza 213 , 214 . All age groups can be affected by influenza virus infection; however, older individuals, young children, pregnant women and those with underlying medical conditions have the highest risk of severe complications. In 2019, a study 75 found that viral sepsis was present in 19% of patients with CAP admitted to ICU and in 61% of patients with viral CAP; influenza virus was the main aetiology. More recently, a study 215 found influenza virus in 23% of patients with LRTI; 57% of these patients had radiographically confirmed CAP. The authors reported 35% vaccine effectiveness against influenza virus LRTI and 51% against influenza-associated CAP. These data demonstrate the importance of an annual influenza vaccination, especially in at-risk groups.
Prevention of HAP
HAP is the leading cause of death from hospital-acquired infection; however, only limited effort has been made in developing prevention strategies. HAP occurs owing to pharyngeal colonization with pathogenic organisms and, in the case of VAP, subsequent aspiration. Thus, oral care and precautions against aspiration may attenuate some of the risk. Although oral and/or digestive decontamination with antibiotics may also be effective, this approach could increase the risk of selecting resistant organisms. Other preventive measures, including isolation practices, remain theoretical or experimental. Indeed, most potential prevention strategies for HAP remain unproven 216 .
The individual measures included in prevention bundles can be divided into non-pharmacological and pharmacological categories. To date, most of our knowledge in HAP prevention is extrapolated from prevention strategies for VAP. An important concept in these strategies is that no single measure is deemed adequate to ensure prevention, with prevention bundles advocated instead. A prospective, interventional, multicentre study in Spain, the Pneumonia Zero project 217 , which included 181 ICUs and built on the experience from a previous study 218 , suggested VAP prevention via a bundle of mandatory and highly recommended measures. The mandatory measures were education and training of medical staff in airway management, hand hygiene with alcohol solutions, oral hygiene with an antiseptic (chlorhexidine), semirecumbent positioning and promotion of procedures and protocols that safely avoid or reduce duration of mechanical ventilation. The highly recommended measures were aspiration of subglottic secretions (removal of secretions that accumulate above the endotracheal tube cuff, in patients who were expected to be mechanically ventilated for >72 h), selective digestive decontamination (SDD)), and selective oropharyngeal decontamination (SOD) (prophylactic strategies to prevent or minimize infections in critically ill patients, based on the application of non-absorbable antibiotics in the oropharynx and gastrointestinal tract (SDD) or oropharynx (SOD) of patients). When implemented, these measures enabled a decrease in adjusted frequency of VAP from 9.83 to 4.34 per 1,000 ventilator-days over 21 months; similarly, the percentage of patients with VAP significantly decreased from 2.4% to 1.9%. In the ICUs with prolonged participation in the study (19–21 months), the incidence of VAP significantly decreased further to just 1.2%. Finally, significant decreases were observed in VAP recurrence rates (from 10.9% to 7.7%).
Non-pharmacological measures
Good hand hygiene using alcohol solution before airway management is firmly established as a fundamental component of clinical practice. Its inclusion in the VAP care bundle represents an opportunity to audit compliance with, and optimize the quality of, hand hygiene practices 217 , 219 .
Remaining in the supine position 220 , the use of gastric tubes and the presence of contents in the stomach contribute to the reflux of gastric contents, aspiration and VAP. Semirecumbent positioning at 30–60° may help to avoid these problems, as found in a 2016 meta-analysis 221 . The lateral Trendelenburg body position (the patient is positioned inclined with head down and feet elevated) has shown no substantial benefit, with research even showing an increase in the number of adverse events 222 . However, based on the results of a post-hoc analysis of the Gravity VAP trial, patients without pulmonary infiltrates at intubation and with no contraindications for the approach may benefit from this position for a short period 222 . The prone position is used to improve hypoxaemia in patients with severe ARDS 223 . This measure is frequently used in COVID-19-associated ARDS 224 , 225 . This approach might decrease the incidence of VAP, as it facilitates the drainage of secretions compared with a semirecumbent position 226 . Further confirmation is needed to assess the beneficial effect in reducing VAP in patients with COVID-19.
Endotracheal tubes also have an important role in the pathogenesis of VAP, and removing contaminated oropharyngeal secretions can reduce the risk of VAP. In a meta-analysis from 2016, evidence supported the use of endotracheal tubes with subglottic secretion drainage to decrease the rate of VAP 227 . Maintaining cuff pressure at >25 cmH 2 O may further prevent the leakage of bacterial pathogens into the lower respiratory tract 217 , and continuous cuff pressure regulation could be superior to intermittent control for preventing VAP 228 . Finally, the tube cuff shape and material may have a role in the aspiration of secretions; a randomized, multicentre trial showed that cuffs made of polyurethane or of a conical shape were not superior to conventional cylindrical polyvinyl chloride cuffs in preventing tracheal colonization and VAP 229 .
Pharmacological measures
Oral washing with chlorhexidine seems to be effective in preventing VAP; however, a recent meta-analysis 230 showed a trend for increased mortality in patients who received chlorhexidine. Consequently, recent international guidelines 3 did not recommend its use. It is plausible that this increased mortality could be due to direct lung toxicity from aspirated chlorhexidine.
Furthermore, the use of either SOD or SDD remains controversial, with most studies to date being performed in settings with low prevalence rates of MDR or XDR microorganisms. These studies have shown a decrease in both the incidence of VAP and overall mortality 231 . However, in a recent cluster randomized clinical trial performed in units with high rates of MDR or XDR pathogens, SOD and SDD were not effective in decreasing bacteraemia caused by those microorganisms 232 . SDD and SOD are not applied in many centres in the USA and in Europe, primarily for fear of inducing microbial resistance. Owing to the unclear balance between a potential reduction in pneumonia rate and a potential increase in mortality, the 2017 international guidelines 3 decided not to issue a recommendation on the use of chlorhexidine for SOD in patients requiring mechanical ventilation until more safety data becomes available. However, the guidelines did suggest the use of SOD — but not SDD — in settings with low rates of antibiotic-resistant bacteria and low antibiotic consumption. Although establishing a cut-off value for low and high resistance settings is a dilemma, the committee felt that a 5% threshold was reasonable.
Prevention of recurrent pneumonia
Recurrent pneumonia affects ~9% of patients hospitalized with CAP 233 , 234 . The main factors related to recurrent pneumonia are age ≥65 years, lack of pneumococcal vaccination, previous episode of pneumonia, COPD and corticoid therapy. S. pneumoniae is the most frequently identified pathogen in patients with recurrent pneumonia 233 , 234 . The main preventive measures for recurrent pneumonia are vaccination and adequate control of prior comorbidities, especially in an older population who have an increased risk of infection.
Antibiotics are the mainstay of therapy for pneumonia; however, the agents used depend on a variety of host and pathogen factors. Ideally, therapy should be pathogen-directed, even though a pathogen is often not identified. Nevertheless, as therapy must be started promptly, empirical therapy directed at the most likely aetiological pathogens is required. Because empirical therapy may be more broad-spectrum than definitive therapy, it is often necessary to narrow and target antibiotics once diagnostic testing results become available, usually after 48–72 h. Such a strategy is referred to as a ‘de-escalation’ of therapy 235 . Rapid comprehensive multiplex molecular methods have been cleared by the FDA and provide results within 2–4.5 h, prior to obtaining final diagnostic testing data. These methods include antibiotic resistance markers and facilitate identification of specific viruses and bacteria, thereby aiding in therapeutic choices and the escalation, de-escalation or cessation of antibiotics.
Considerations for therapeutic choices
Relevant host factors for choosing the type of empirical therapy are severity of illness, the presence of specific medical comorbidities and certain historical data. In detail, these include: chronic lung, heart or liver disease; diabetes mellitus; asplenia; alcohol use disorder; malignancy; malnutrition; recent hospitalization, antibiotic use or colonization by drug-resistant bacteria; the presence of risk factors for aspiration of gastric contents into the lungs (such as impaired swallowing, vomiting, altered consciousness and impaired cough reflex); and recent contact with a health care environment (for example, patients requiring haemodialysis) 236 . It is also important to know epidemiological data regarding individual patients. Seasonal viruses such as influenza viruses are worth examining during the autumn and winter. Contact with someone known to have an illness transmitted by an airborne route (for example, tuberculosis) is also relevant. Similarly, residence in an area with endemic mycoses is a risk for certain fungal pneumonias. Finally, an ICU with a high rate of drug-resistant pathogens poses a risk factor for VAP caused by such organisms 3 .
The site of pneumonia acquisition is also an important consideration, namely, in the community, hospital or ICU, or whilst on mechanical ventilation. Since the late 1990s, guidelines have been developed for patients with pneumonia in each of these settings; however, recent data suggest that patient risk factors, and not the site of infection, should be the main determinant for empirical antibiotic choice. Recently, a unified algorithm based on these risk factors has been proposed for all patients with pneumonia 236 .
In addition to choosing an antibiotic that is likely to target the aetiological pathogens, it is equally important to determine the right dose and route of administration, to ensure that the drug penetrates into the site of infection. In general, oral therapy is used in patients with less severe illness, whilst intravenous therapy is administered in patients with more serious illness. Aerosolized therapy can be used to boost drug delivery to infected lung tissue, especially if the chosen drugs penetrate into the lung poorly. When treating a critically ill patient with pneumonia and a MDR pathogen, it may be necessary to use high doses to ensure reaching bactericidal drug concentrations at the site of infection. Continuous or prolonged infusion may be needed in the case of β-lactam antibiotics to maximize the time during which the drug concentration exceeds the minimum inhibitory concentration (MIC) of the target organism. Other drugs, such as aminoglycosides, kill bacteria in a concentration-dependent fashion and are best administered at high dosages given once daily 237 . In young patients with pneumonia and sepsis, drug clearance by the kidney may be accelerated (augmented renal clearance), and dosing will need to be increased appropriately 238 . In those with renal impairment, dosing or the frequency of administration may need to be reduced and can be optimized by therapeutic drug monitoring, if available.
CAP therapy
Guidelines for CAP recommend empirical therapy based on the severity of illness and presence of risk factors for specific complex pathogens 5 , 53 , 239 (Table 4 ). In the past, patients with risk factors that included contact with a health care environment (haemodialysis, recent hospitalization, residence in a nursing home) were considered to have HCAP and were treated differently from patients with CAP. The new guidelines have eliminated HCAP as a category and recommended that these patients be treated as having CAP. Without forgoing consideration of the local frequency of penicillin and macrolide resistance, every patient with CAP should be treated for pneumococcus in most parts of the world. In addition, atypical pathogens may have a role, often as co-infecting agents; studies showed improved patient outcomes when macrolides or quinolones were added to β-lactam therapy in patients with CAP, particularly those with more severe illness 240 , suggesting a need to treat atypical pathogens in many patients with CAP. Patients with more severe illness may need empirical therapy for MRSA and/or P. aeruginosa , especially if colonization had occurred previously following influenza (in the case of MRSA) or after prior use of broad-spectrum antibiotics (for both pathogens) 241 .
Although in many patients CAP may have a viral aetiology, either as a single pathogen or as part of a mixed infection, antiviral therapy is not routinely recommended. However, for documented influenza-associated pneumonia, current guidelines recommend the use of an anti-influenza agent such as oseltamivir, regardless of illness duration 5 . Nonetheless, the benefit of these agents is greatest within the first 48 h of infection onset. Thus, in patients with a high suspicion of influenza, therapy should be started, whilst results from diagnostic testing are pending. Additionally, even with documented influenza, antibiotics should be used empirically to account for possible bacterial superinfection 5 .
Outpatients
For outpatients without comorbidities or risk factors for MDR pathogen infection, current guidelines recommend monotherapy with respiratory fluroquinolone or combination with amoxicillin–clavulanate or a cephalosporin and macrolide or doxycycline 5 . Regardless of the prevalence of resistance, good experience with macrolide monotherapy has been reported, suggesting that in vitro resistance is not always clinically relevant unless it is high-level resistance (resulting from a ribosomal mechanism) and not lower-level resistance (caused by efflux pumps) 242 . For example, in a Canadian study, patients with CAP who received macrolide therapy (usually as monotherapy) had lower mortality and hospitalization rates than those receiving alternative therapies 243 . For outpatients with comorbid illnesses, current guidelines recommend therapy with a β-lactam and macrolide combination or monotherapy with a respiratory fluoroquinolone, even though recent concerns about fluoroquinolone toxicity have limited their use 5 .
Hospitalized patients
In patients with CAP in hospital wards, therapy should be a β-lactam–macrolide combination or a quinolone (levofloxacin or moxifloxacin) alone (Table 4 ). In areas with a high prevalence of endemic tuberculosis, caution should be exercised with the use of a quinolone, as it can mask the presence of tuberculosis and select for drug-resistant tuberculosis. β-Lactams include ceftriaxone, ceftaroline and ampicillin–sulbactam, whilst macrolides should comprise azithromycin or clarithromycin; some recent data have shown more frequent cardiac complications with the use of erythromycin 244 . Many studies have shown that the addition of a macrolide to the β-lactam, particularly in those with moderately severe illness or with Legionella spp. infection, is associated with a lower mortality rate than β-lactam monotherapy 245 .
All ICU-admitted patients should receive a combination therapy of a β-lactam and either a macrolide or a quinolone. Admission to ICU should be guided by the presence of one of two major criteria (need for mechanical ventilation or septic shock requiring vasopressors) or three of nine minor criteria, as per the 2007 ATS/IDSA guidelines 239 . In this population, a macrolide is generally preferred, although some studies have shown that a quinolone may prove more effective if Legionella spp. infection is highly suspected or documented 246 . If the patient has risk factors for P. aeruginosa or MRSA infection, then treatment for such pathogens should be added.
HAP therapy
Patients can develop HAP in or outside the ICU and can be managed with or without mechanical ventilation, although as many as 30% of patients with HAP who are not initially ventilated will require mechanical ventilation 247 . In patients with a predicted mortality risk of <15% based on the presence or absence of septic shock, monotherapy is associated with lower mortality than combination therapy. In patients with a predicted mortality risk of >25%, combination therapy is associated with reduced mortality; the type of therapy has no effect on mortality in those with a predicted mortality risk of 15–25% 248 . MDR pathogen infection should be considered in patients with a history of prior antibiotic therapy or prolonged hospitalization in the previous 3 months, as well as patients hospitalized in an ICU with a >25% rate of MDR pathogen infections. Although empirical therapy can be guided by patient features, each ICU has its own unique bacteriology; thus, therapy should be guided by knowledge of the local antibiogram 3 , 249 .
Patients with a low mortality risk (estimated from published data in relation to the presence of sepsis and shock) and no MDR pathogen risk factors should receive monotherapy (Table 5 ). In patients with a mortality risk of >15% and/or risk factors for MDR pathogens but who are not in septic shock, monotherapy can be adequate (provided that the chosen antibiotic can target >90% of the gram-negative pathogens in the ICU). Although there is controversy in many hospitals about the need for combination therapy, two agents are often necessary to provide a >90% likelihood of appropriate therapy, especially in the high-risk population and in those with septic shock. The combination regimen should target P. aeruginosa and ESBL-producing Enterobacterales. In all patients with HAP, anti-MRSA therapy should be considered and, if necessary, administered with either vancomycin or linezolid. Depending on local epidemiology, some patients will be at risk of infection with Acinetobacter baumanii , carbapenem-producing Enterobacterales or Stenotrophomonas maltophilia , each one requiring a unique therapy approach. For VAP due to MDR pathogens, such as Acinetobacter baumanii , adjunctive inhaled antibiotics (amikacin or colistin) have been added to systemic therapy, with no proven mortality benefit; efficacy may vary with the type of aerosol delivery system used 250 .
The duration of HAP therapy is between 7 and 14 days, although most patients are successfully treated within only 7 days 251 . Although not all experts agree, the European guidelines list the following groups as exceptions to short duration therapy: patients with MDR pathogen infection, such as P. aeruginosa and Acinetobacte r spp.; those who received inappropriate therapy initially; those who are severely immunocompromised; and those receiving second-line antibiotic agents 217 , 252 , 253 . Current guidelines do not strongly endorse biomarkers such as PCT to guide therapy duration for HAP and VAP, although some randomized trial data do show efficacy for this approach 254 .
Therapy in immunocompromised patients
Immunocompromised patients can develop pneumonia due to the common community and nosocomial pathogens present in the setting as well as other pathogens related to a specific type of immune dysfunction and/or resistant bacteria, viruses, fungi and parasites. Common conditions that impair the immune system include malignancy, HIV infection with a CD4 + T cell count of <200 cells per mm 3 , and solid organ or stem cell transplantation. Therapies that cause immune suppression include prednisone, biological disease modifiers, and chemotherapeutic agents such as azathioprine, methotrexate and cyclophosphamide.
Although empirical therapy is often used, the range of possible pathogens in this population is so broad that aggressive diagnostic testing is necessary, including sampling of deep lower respiratory tract secretions with bronchoscopy in most patients 255 . In patients with HIV infection and a low CD4 + T cell count or with recent corticosteroid tapering, therapy should target common pathogens and Pneumocystis jirovecii 256 . Patients with severe neutropenia, steroid-induced immune suppression and those receiving biologic response modifiers (such as tumour necrosis factor inhibitors) can be infected with fungi such as Aspergillus spp. or Mucorales. Diagnostic testing in those with malignancy or drug-induced immune suppression should also consider other opportunistic pathogens, including cytomegalovirus, Varicella zoster virus, Nocardia spp., parasites such as Strongyloides stercoralis and Toxoplasma gondii , and Mycobacterium tuberculosis (for example, owing to a re-emergence of latent infection).
Aspiration pneumonia therapy
Patients with witnessed macro-aspiration of gastric or oral contents into the lung can develop chemical or bacterial pneumonitis, or simply have bland aspiration. If bacterial pneumonia occurs, patients should receive antibiotics aimed at common community or nosocomial pathogens that were likely to be colonizing the oral and gastric tract at the time of aspiration. In community aspiration, therapy is the same as in CAP unless the patient has poor dentition, which can make infection by anaerobic pathogens possible owing to favourable growth conditions for such microbes in the patient’s mouth. When patients with poor dentition have a lung infiltrate after a witnessed or clinically suspected aspiration event, therapy should be a β-lactam such as ampicillin–sulbactam or amoxicillin–clavulanate, or a quinolone, such as levofloxacin or moxifloxacin. Any of these drugs could also be used if dentition is normal; alternatively, ceftriaxone would be effective 1 . For those with nosocomial aspiration, therapy should be based on the presence of risk factors for MDR pathogens and aimed at common, local and drug-resistant organisms, similar to therapy in other forms of nosocomial pneumonia. There is no need to add specific anti-anaerobic coverage, as these organisms are uncommon in patients who aspirate whilst in hospital or chronic care facilities 257 .
Adjunctive therapy
In addition to antibiotics, patients with severe illness might benefit from adjunctive corticosteroid therapy. In general, this therapy should be restricted to those with severe CAP and a high inflammatory response 258 . In one trial, methylprednisolone was more effective than placebo, leading to less treatment failure (especially late failure) in a population with both severe CAP and elevated CRP levels in the serum 259 . However, before using corticosteroids, it is necessary to rule out influenza, as it may worsen with this line of therapy 260 . By contrast, studies in patients with COVID-19 and hypoxaemic respiratory failure have shown a benefit of corticosteroid therapy with dexamethasone 261 . Similarly, IgM-enriched immunoglobulin may be useful in patients with severe CAP, and high CRP levels and low IgM levels in the serum. In a randomized, double-blind, placebo-controlled trial, IgM-enriched immunoglobulin led to a reduction in mortality and an increase in ventilator-free days in this population, when compared with placebo 262 .
Another adjunctive and supportive therapy includes management of hypoxaemia with respiratory failure, which may necessitate mechanical ventilator support. However, some studies show that patients with CAP can be managed with either non-invasive ventilation or high-flow oxygen. Either modality can reduce the need for mechanical ventilation and, therefore, avoid some of the complications associated with endotracheal intubation and ventilation 263 .
Follow-up of patients after pneumonia
In some patients with CAP, pneumonia can be the start of an inexorable downhill course. In one study, the long-term mortality of patients of >65 years of age hospitalized with CAP far exceeded the in-hospital mortality (33.6% and 11%, respectively) 264 . In some studies, this long-term effect has been attributed to cardiac events that were initiated by acute lung infection 155 .
Pneumonia recurrence can occur in all forms of pneumonia. Recurrence should be classified on the basis of the site of infection. If re-infection occurs at the same site as the original infection, consideration should be given to local factors such as endobronchial obstruction (due to a tumour or foreign body), focal bronchiectasis, insufficient duration of therapy, or infection with a drug-resistant or inadequately treated pathogen. Recurrence elsewhere could be due to immune impairment (due to comorbid illness or certain medications), a non-infectious pulmonary process or recurrent aspiration.
Routine follow-up chest radiography after CAP is not generally recommended. However, if it is prescribed (to monitor resolution of a pleural effusion or infiltrate suggestive of a possible lung mass), it should be delayed for 4–6 weeks if the patient is responding well to therapy 5 . During follow-up, patients should be monitored for undiagnosed or ineffectively managed comorbid illness and encouraged to avoid cigarette smoking. Patients should also have up-to-date pneumococcal and influenza vaccinations. The 30-day readmission rate for patients with CAP has been found to vary from 16.8% to 20.1% 167 . Pneumonia itself was the cause of readmission in only 17.9–29.4% of patients; however, other common causes were exacerbations of congestive heart failure or COPD 167 . Patients with health-care-associated risk factors have a higher probability of readmission than patients with uncomplicated CAP 265 .
Quality of life
The effect of pneumonia is heavily influenced by both the origin of the disease (within the community or in health care environments) and its severity 266 . Most data regarding the effect on quality of life have been obtained in patients with CAP 171 . Antibiotic treatment starts to improve pneumonia symptoms rapidly; acute symptoms typically improve within 3–5 days in patients with mild CAP (outpatients) and 5–10 days in hospitalized patients with more severe CAP not requiring ICU admission; however, return to baseline levels of symptoms and function seems to take substantially longer 172 , 267 , 268 , 269 . In mild-to-moderate CAP, in most patients symptoms such as cough and breathlessness resolve within 14 days, although up to 6 months are required for full recovery 267 . Thus, the greatest burden seems to be a loss of function in the long term. Delayed recovery is associated with the number of comorbid conditions. In most cases, the presence of ongoing health impairment is largely related to a decompensation of underlying diseases rather than the ongoing acute symptoms of CAP 267 . A modelling study showed that in hospitalized patients with CAP, these acute symptoms reduced in intensity by ~50% within the first 3–5 days, and resolved in nearly all patients by day 28 (ref. 268 ). There does not seem to be a meaningful difference in symptom intensity or time to symptom resolution between viral and bacterial pneumonia 270 .
A French study in patients with pneumococcal pneumonia followed for 12 months after hospital discharge used the EQ-5D-3L questionnaire to evaluate health status 271 . Patients experienced a progressive improvement in quality of life after discharge, plateauing at six months. Importantly, quality of life either did not improve or deteriorated after discharge in 34% of patients; recovery was worse in old patients than in young patients. In a US study in patients with CAP, on average, patients were able to return to normal productivity in 3 weeks and missed 2 weeks of work 272 . Recovery was slowest in patients with comorbidities such as COPD, leading to recovery times of 2 months on average. Even after recovery, symptom scores in patients with CAP are worse than those in the general population, partially because CAP has a long-term effect on health. Another partial reason for these lower scores is the development of CAP in patients with high-risk comorbidities, which make these patients more symptomatic than the general population 273 . Lastly, long-term mortality is increased in patients with CAP compared with the general population 35 . LRTIs without radiographic infiltrates (non-CAP LRTIs) are associated with a similar impairment in quality of life to CAP 274 .
Studies comparing quality of life between patients with CAP and the general population have shown consistently worse quality of life up to 12 months after CAP. With a few exceptions, most of these studies used generic quality of life and productivity tools. A systematic review identified five CAP-specific, patient-reported outcome measures, of which the CAP symptom questionnaire (CAP-sym) was the most widely used 275 . This review concluded that most CAP-specific tools have thus far been evaluated in highly specific populations and may not be fully representative, and it recommends continuing to use generic tools until better tools are available.
Improved diagnostics
The key to a switch to pathogen-specific therapy is an accurate aetiological diagnosis, and the availability of rapid molecular diagnostic tests makes clinical trials and subsequent clinical use of these targeted therapies feasible. Most progress in diagnostics can be observed in two areas: rapid identification of pathogens in positive blood cultures and detection of respiratory viral pathogens. However, bacteraemia is uncommon in pneumonia and, therefore, the effect of these molecular assays on management is limited. By contrast, PCR diagnosis of respiratory viral infections has now become the standard of care. The greatest issue with these assays obtained from nasopharyngeal specimens is whether results reflect upper respiratory tract infections only or accurately detect the cause of pneumonia. In addition, negative nasopharyngeal samples have occurred in patients with positive concurrent bronchoalveolar samples for influenza and SARS-CoV-2 (ref. 276 ).
Several multiplex PCR platforms are available for clinical use for bacterial pneumonia, with approval based on comparison with standard diagnostic tools, specifically culture 277 , 278 . However, as culture itself is not a gold standard, the true operating characteristics of the tests remain unknown. One alternative is metagenomics sequencing to determine all microbiota present; clinically relevant platforms are available 279 , 280 . Generally, these molecular assays are more sensitive than culture, especially for fastidious microorganisms; nevertheless, none of the current multiplex assays detect all of the relevant pathogens and, therefore, cannot replace cultures. In addition, a limited ability to provide information on antibiotic susceptibility is a major weakness. Despite such limitations, substantial impact on antibiotic prescription is possible. Most evaluations to date comprise observational studies and analyses of the theoretical benefit if antibiotic decisions based on molecular assays were applied prospectively. Perhaps the best demonstration of such potential is to limit the use of vancomycin or linezolid for suspected MRSA pneumonia 197 . Multiple sensitive and specific gene targets for S. aureus identification are available, whilst the absence of the mecA gene detection essentially excludes methicillin resistance in that isolate; thus, a negative assay eliminates the need for MRSA coverage. However, the greatest hurdle for molecular assays is clinicians’ willingness to base antimicrobial treatment on results obtained from these novel diagnostic platforms; even a BAL assay with a 98% negative predictive value did not result in a decrease in empirical treatment of VAP 281 . Implementation trials are required to demonstrate the true benefit of more accurate diagnostics.
Improved diagnostic testing may enable a host of unanswered epidemiological matters surrounding pneumonia to be addressed. A leading question in the field of pneumonia is its cause in immunocompromised patients; only expert opinion guides treatment recommendations 256 . The COVID-19 pandemic also illustrates the probable high frequency of additional viral agents that may cause CAP of seemingly unknown aetiology 13 . The role of fungal superinfection of viral pneumonia also remains controversial owing to diagnostic uncertainty 282 .
Antibiotic therapy
For most of the ~75-year history of antibiotic treatment of pneumonia, the backbone of therapy has been a β-lactam 283 . The emergence of bacterial resistance to β-lactams has been tackled with two strategies: newer generations or types of β-lactams (penicillins, cephalosporins and carbapenems) 284 , 285 , 286 , 287 , 288 and combinations with β-lactamase inhibitors (BLIs). Ceftolozane is the newest β-lactam on the market; it has improved activity against P. aeruginosa compared with other cephalosporins 284 . Each BLI has slightly different activity against the variety of resistance mechanisms in Enterobacterales, including carbapenem-resistant and ESBL-producing Enterobacterales, which may affect local efficacy owing to geographical differences in resistance patterns 289 .
Each new drug had been intended to replace the prior generation, gain a large proportion of market share and, therefore, justify the large development costs for the pharmaceutical industry. However, the majority of infections, especially community-acquired 13 , remain susceptible to cheap generic antibiotics even today, and the probability of a new blockbuster drug that would garner a large market share is progressively in decline 290 . This and multiple other factors, including increased costs for registration trials, a regulatory environment and challenges in clinical trial design, have led many pharmaceutical firms to abandon antibiotic development, as it offers a poor return on investment 291 .
Nevertheless, the paradigm for antibiotic development has shifted and, since the 2000s, niche antibiotics, particularly for gram-negative pathogens, have progressively emerged, developed by small biotech companies. These niche antibiotics specifically address gaps in standard antibiotic treatment coverage, yet leverage high prices to compensate for a small market share. The future success of these niche antibiotics could be increased by the emergence of rapid diagnostic tests that can detect specific pathogens or specific resistance markers immediately.
New antibiotics
The first generation of niche antibiotics were new β-lactams or BLIs developed for individual MDR or XDR pathogens 292 . The greatest unmet need for pneumonia due to gram-negative pathogens is for treatment of carbapenem-resistant Acinetobacter spp.; the only agent in development specifically for Acinetobacter spp. is a combination of two BLIs 293 . Both BLIs also have intrinsic β-lactam activity but are being studied in combination with a carbapenem for serious Acinetobacter spp. infections, including pneumonia.
Agents specific for Pseudomonas spp. are also in development. Murepavadin is the first of a new class of antibiotics that inhibit the outer membrane assembly of P. aeruginosa ; other drugs targeting outer membrane assembly are in development, including phage-derived endolysins 294 . Small molecule inhibitors of the type-III secretion apparatus in P. aeruginosa , a crucial component of its pathogenesis, are also in development.
One exception to the niche drug approach is cefiderocol, an extremely broad-spectrum agent with activity against almost all MDR pathogens. Cefiderocol links ceftazidime and cefepime together, maintaining the β-lactam bactericidal mechanism whilst enhancing bacterial uptake 295 . Bacteria take up cefiderocol through iron channels, and this mechanism is extremely appealing, as many MDR gram-negative pathogens, including P. aeruginosa , Acinetobacter spp. and Stenotrophomonas spp., avidly take up iron, and a major component of the acute-phase host response is to sequester iron from pathogens. Cefiderocol was non-inferior to high-dose extended-infusion meropenem for HAP due to gram-negative pathogens 296 , but it was associated with a higher mortality than the best available therapy for pneumonia and bacteraemia, specifically due to carbapenem-resistant Acinetobacter spp. 292 .
Lefamulin is the first truly new antibiotic class since the oxazolidinone linezolid. The mechanism of action of lefamulin is via protein synthesis inhibition, and lefamulin is approved for the treatment of CAP based on equivalence to moxifloxacin 297 , 298 . This drug can be used as a single agent to target MRSA and other CAP pathogens resistant to macrolide, β-lactam and fluoroquinolone antibiotics, and possibly in cases of treatment failure and/or in patients with multiple drug allergies. Unfortunately, lefamulin does not have substantial activity against ESBL-producing gram-negative pathogens, which is an unmet need in CAP.
Non-antibiotic therapy
Monoclonal or polyclonal antibodies to specific MDR pathogens, including S. aureus and P. aeruginosa , are the ultimate narrow-spectrum agents, being both extremely safe and having the great advantage of not disturbing the commensal microbiota 299 , 300 . Antibodies against the P. aeruginosa type-III secretion apparatus, alginate and other unique targets have entered clinical trials. Several anti- S. aureus antibodies have also been developed 301 . The challenge for specific antibodies is whether they should be used for prevention or as adjuncts to antibiotic therapy. The lack of sensitive risk factors or predictive markers for pneumonia caused by a specific pathogen make prophylactic trials difficult and potential clinical use expensive; thus, development for preventive indications has been abandoned for several agents, and attention has shifted to adjunctive use, despite this being associated with loss of the microbiota-sparing effect with this strategy.
Case reports have been published on bacteriophage therapy as an alternative to antibiotics in patients with extremely difficult-to-treat pneumonia 302 . However, major logistic issues must be overcome before phage therapy becomes a legitimate option 303 : the individual patient’s isolate must be tested for susceptibility against a battery of bacteria-specific phages; a cocktail of at least three phages is usually needed, owing to the emergence of resistance to any single phage; and the availability of phages and susceptibility testing facilities remain extremely limited. Furthermore, the optimal delivery method, namely aerosolization, instillation or venous infusion, remains unclear. No large-scale clinical trials have been completed.
Lastly, the COVID-19 pandemic has generated a large number of studies of adjuvant treatments focusing on host response to SARS-CoV-2. It remains unclear whether any adjuvant treatments other than corticosteroids that may provide benefit in SARS-CoV-2 infection can be used for influenza or other serious viral pneumonias. However, the COVID-19 pandemic has clearly increased interest in both host-directed therapy and newer antivirals.
Mandell, L. A. & Niederman, M. S. Aspiration pneumonia. N. Engl. J. Med. 380 , 651–663 (2019). A review article about aspiration pneumonia, including new insights about microbial aetiology and antibiotic treatment .
CAS PubMed Google Scholar
Cillóniz, C. et al. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax 66 , 340–346 (2011).
PubMed Google Scholar
Torres, A. et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur. Respir. J. 50 , 1700582 (2017). In these international European and Latin American guidelines, a panel of experts present recommendations about diagnosis, risk factor for antibiotic resistance and type and duration of treatment for HAP and VAP. PICO questions and GRADE methodology were used .
Kalil, A. C. et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 63 , e61–e111 (2016). These guidelines provide risk factors for suspected MDR or XDR microorganisms and recommendations for empirical treatments, use of biomarkers and duration of antibiotic administration .
PubMed PubMed Central Google Scholar
Metlay, J. P. et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 200 , e45–e67 (2019). These guidelines include new recommendations for microbiological diagnostic tests, in particular for empirical treatments in outside and in-hospital patients .
Prina, E., Ranzani, O. T. & Torres, A. Community-acquired pneumonia. Lancet 386 , 1097–1108 (2015).
Di Pasquale, M. F. et al. Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin. Infect. Dis. 68 , 1482–1493 (2019).
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1204–1222 (2020).
Google Scholar
GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 18 , 1191–1210 (2018).
Komiya, K. et al. Prognostic implications of aspiration pneumonia in patients with community acquired pneumonia: a systematic review with meta-analysis. Sci. Rep. 6 , 38097 (2016).
CAS PubMed PubMed Central Google Scholar
Lindenauer, P. K. et al. Variation in the diagnosis of aspiration pneumonia and association with hospital pneumonia outcomes. Ann. Am. Thorac. Soc. 15 , 562–569 (2018).
Neill, S. & Dean, N. Aspiration pneumonia and pneumonitis: a spectrum of infectious/noninfectious diseases affecting the lung. Curr. Opin. Infect. Dis. 32 , 152–157 (2019).
Jain, S. et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N. Engl. J. Med. 373 , 415–427 (2015).
Torres, A., Peetermans, W. E., Viegi, G. & Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 68 , 1057–1065 (2013).
Norris, T., Vahratian, A. & Cohen, R. A. Vaccination coverage among adults aged 65 and over: United States, 2015. NCHS Data Brief No. 281 (CDC, 2017).
Fedson, D. S. et al. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert. Rev. Vaccines 10 , 1143–1167 (2011).
Jamal, A. et al. Current cigarette smoking among adults – United States, 2016. MMWR 67 , 53–59 (2018).
Louie, J. K. et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 302 , 1896–1902 (2009).
Wu, C. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 180 , 934–943 (2020).
Barbier, F., Andremont, A., Wolff, M. & Bouadma, L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr. Opin. Pulm. Med. 19 , 216–228 (2013).
Rosenthal, V. D. et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am. J. Infect. Control. 40 , 396–407 (2012).
Giuliano, K. K., Baker, D. & Quinn, B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am. J. Infect. Control. 46 , 322–327 (2018).
Bonell, A. et al. A systematic review and meta-analysis of ventilator-associated pneumonia in adults in Asia: an analysis of national income level on incidence and etiology. Clin. Infect. Dis. 68 , 511–518 (2019).
Bouadma, L. et al. Ventilator-associated events: prevalence, outcome, and relationship with ventilator-associated pneumonia. Crit. Care Med. 43 , 1798–1806 (2015).
Shi, T. et al. Global and regional burden of hospital admissions for pneumonia in older adults: a systematic review and meta-analysis. J. Infect. Dis. 222 , S570–S576 (2020).
GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 , 1736–1788 (2018).
JustActions. The Missing Piece. Why Continued Neglect of Pneumonia Threatens the Achivement of Health Goals (JustActions, 2018).
Nunes, B. P., Flores, T. R., Mielke, G. I., Thumé, E. & Facchini, L. A. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch. Gerontol. Geriatr. 67 , 130–138 (2016).
Arnold, F. W. et al. Mortality differences among hospitalized patients with community-acquired pneumonia in three world regions: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir. Med. 107 , 1101–1111 (2013).
Heo, J. Y. & Song, J. Y. Disease burden and etiologic distribution of community-acquired pneumonia in adults: evolving epidemiology in the era of pneumococcal conjugate vaccines. Infect. Chemother. 50 , 287–300 (2018).
Cillóniz, C. et al. Community-acquired pneumonia in outpatients: aetiology and outcomes. Eur. Respir. J. 40 , 931–938 (2012).
Luna, C. M. et al. The impact of age and comorbidities on the mortality of patients of different age groups admitted with community-acquired pneumonia. Ann. Am. Thorac. Soc. 13 , 1519–1526 (2016).
Cillóniz, C. et al. Twenty-year trend in mortality among hospitalized patients with pneumococcal community-acquired pneumonia. PLoS ONE 13 , e0200504 (2018).
Corrales-Medina, V. F. et al. Intermediate and long-term risk of new-onset heart failure after hospitalization for pneumonia in elderly adults. Am. Heart J. 170 , 306–312 (2015).
Eurich, D. T., Marrie, T. J., Minhas-Sandhu, J. K. & Majumdar, S. R. Ten-year mortality after community-acquired pneumonia. A prospective cohort. Am. J. Respir. Crit. Care Med. 192 , 597–604 (2015).
Ramirez, J. A. et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology and mortality. Clin. Infect. Dis. 65 , 1806–1812 (2017).
Bordon, J. et al. Hospitalization due to community-acquired pneumonia in patients with chronic obstructive pulmonary disease: incidence, epidemiology and outcomes. Clin. Microbiol. Infect. 26 , 220–226 (2020).
Torres, A. et al. Burden of pneumococcal community-acquired pneumonia in adults across Europe: a literature review. Respir. Med. 137 , 6–13 (2018).
Magill, S. S. et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 370 , 1198–1208 (2014).
Micek, S. T., Chew, B., Hampton, N. & Kollef, M. H. A case-control study assessing the impact of nonventilated hospital-acquired pneumonia on patient outcomes. Chest 150 , 1008–1014 (2016).
Melsen, W. G. et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 13 , 665–671 (2013).
Bassetti, M. et al. Risk stratification and treatment of ICU-acquired pneumonia caused by multidrug-resistant/extensively drug-resistant/pandrug-resistant bacteria. Curr. Opin. Crit. Care 24 , 385–393 (2018).
Herkel, T. et al. Epidemiology of hospital-acquired pneumonia: results of a Central European multicenter, prospective, observational study compared with data from the European region. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 160 , 448–455 (2016).
Ibn Saied, W. et al. A comparison of the mortality risk associated with ventilator-acquired bacterial pneumonia and nonventilator ICU-acquired bacterial pneumonia. Crit. Care Med. 47 , 345–352 (2019).
Talbot, G. H. et al. Evidence-based study design for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. J. Infect. Dis. 219 , 1536–1544 (2019).
McAllister, D. A. et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob. Health 7 , e47–e57 (2019).
Weir, D. L., Majumdar, S. R., McAlister, F. A., Marrie, T. J. & Eurich, D. T. The impact of multimorbidity on short-term events in patients with community-acquired pneumonia: prospective cohort study. Clin. Microbiol. Infect. 21 , 264.e7–264.e13 (2015).
CAS Google Scholar
Bradley, J. S. et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 53 , e25–e76 (2011).
Barbagelata, E. et al. Gender differences in community-acquired pneumonia. Minerva Med. 111 , 153–165 (2020).
Mutepe, N. D. et al. Effects of cigarette smoke condensate on pneumococcal biofilm formation and pneumolysin. Eur. Respir. J. 41 , 392–395 (2013).
Samokhvalov, A. V., Irving, H. M. & Rehm, J. Alcohol consumption as a risk factor for pneumonia: a systematic review and meta-analysis. Epidemiol. Infect. 138 , 1789–1795 (2010).
Neupane, B. et al. Long-term exposure to ambient air pollution and risk of hospitalization with community-acquired pneumonia in older adults. Am. J. Respir. Crit. Care Med. 181 , 47–53 (2010).
American Thoracic Society & Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171 , 388–416 (2005).
Le, M. N.-T. et al. Oral colonisation by antimicrobial-resistant Gram-negative bacteria among long-term care facility residents: prevalence, risk factors, and molecular epidemiology. Antimicrob. Resist. Infect. Control. 9 , 45 (2020).
Feldman, C. et al. The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur. Respir. J. 13 , 546–551 (1999).
Cilloniz, C. et al. Seasonality of pathogens causing community-acquired pneumonia. Respirology 22 , 778–785 (2017).
Para, R. A., Fomda, B. A., Jan, R. A., Shah, S. & Koul, P. A. Microbial etiology in hospitalized North Indian adults with community-acquired pneumonia. Lung India 35 , 108–115 (2018).
Tao, L.-L. et al. Etiology and antimicrobial resistance of community-acquired pneumonia in adult patients in China. Chin. Med. J. 125 , 2967–2972 (2012).
Shoar, S. & Musher, D. M. Etiology of community-acquired pneumonia in adults: a systematic review. Pneumonia 12 , 11 (2020).
Moberley, S., Holden, J., Tatham, D. P. & Andrews, R. M. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. 2013 , CD000422 (2013).
PubMed Central Google Scholar
Centers for Disease Control and Prevention (CDC). Current cigarette smoking among adults - United States, 2011. MMWR 61 , 889–894 (2012).
Luca, D. L. et al. Impact of pneumococcal vaccination on pneumonia hospitalizations and related costs in Ontario: a population-based ecological study. Clin. Infect. Dis. 66 , 541–547 (2017).
Johansson, N., Kalin, M., Tiveljung-Lindell, A., Giske, C. G. & Hedlund, J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin. Infect. Dis. 50 , 202–209 (2010).
Rozenbaum, M. H. et al. The role of Streptococcus pneumoniae in community-acquired pneumonia among adults in Europe: a meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 32 , 305–316 (2013).
Huijts, S. M. et al. Diagnostic accuracy of a serotype-specific antigen test in community-acquired pneumonia. Eur. Respir. J. 42 , 1283–1290 (2013).
Aliberti, S. et al. Multidrug-resistant pathogens in hospitalised patients coming from the community with pneumonia: a European perspective. Thorax 68 , 997–999 (2013).
Shindo, Y. et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 188 , 985–995 (2013).
Prina, E. et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann. Am. Thorac. Soc. 12 , 153–160 (2015).
Ceccato, A. et al. Validation of a prediction score for drug-resistant microorganisms in community-acquired pneumonia. Ann. Am. Thorac. Soc. 18 , 257–265 (2021).
Cilloniz, C. et al. Difficult to treat microorganisms in patients over 80 years with community-acquired pneumonia: the prevalence of PES pathogens. Eur. Respir. J. 56 , 2000773 (2020).
Webb, B. J. et al. Derivation and multicenter validation of the drug resistance in pneumonia clinical prediction score. Antimicrob. Agents Chemother. 60 , 2652–2663 (2016).
Karhu, J., Ala-Kokko, T. I., Vuorinen, T., Ohtonen, P. & Syrjälä, H. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin. Infect. Dis. 59 , 62–70 (2014).
Wu, X. et al. Incidence of respiratory viral infections detected by PCR and real-time PCR in adult patients with community-acquired pneumonia: a meta-analysis. Respiration 89 , 343–352 (2015).
Zhou, F. et al. Disease severity and clinical outcomes of community acquired pneumonia caused by non-influenza respiratory viruses in adults: a multicenter prospective registry study from CAP-China Network. Eur. Respir. J. 54 , 1802406 (2019).
Cillóniz, C. et al. Pure viral sepsis secondary to community-acquired pneumonia in adults: risk and prognostic factors. J. Infect. Dis. 220 , 1166–1171 (2019).
Jain, S. et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 372 , 835–845 (2015). This study is a prospective multicentre investigation of the CAP microbial aetiology in hospitalized patients. Very importantly, PCR tests for the detection of viral pathogens , Legionella spp. and Mycoplasma pneumoniae were systematically used in the diagnostic work-up. With this approach, viruses represented the first cause of CAP .
Weber, D. J. et al. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect. Control. Hosp. Epidemiol. 28 , 825–831 (2007).
Magiorakos, A.-P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18 , 268–281 (2012).
Parker, D., Ahn, D., Cohen, T. & Prince, A. Innate immune signaling activated by MDR bacteria in the airway. Physiol. Rev. 96 , 19–53 (2016).
Grousd, J. A., Rich, H. E. & Alcorn, J. F. Host-pathogen interactions in gram-positive bacterial pneumonia. Clin. Microbiol. Rev. 32 , e00107-18 (2019).
Kutter, J. S., Spronken, M. I., Fraaij, P. L., Fouchier, R. A. & Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 28 , 142–151 (2018).
Siegel, S. J. & Weiser, J. N. Mechanisms of bacterial colonization of the respiratory tract. Annu. Rev. Microbiol. 69 , 425–444 (2015).
Quinton, L. J., Walkey, A. J. & Mizgerd, J. P. Integrative physiology of pneumonia. Physiol. Rev. 98 , 1417–1464 (2018).
Dickson, R. P., Erb-Downward, J. R. & Huffnagle, G. B. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir. Med. 2 , 238–246 (2014). A review–opinion article about new insights into the aetiopathogenesis of pneumonia based on changes in the microbiota .
Pettigrew, M. M., Tanner, W. & Harris, A. D. The lung microbiome and pneumonia. J. Infect. Dis. https://doi.org/10.1093/infdis/jiaa702 (2020).
Article Google Scholar
Brown, R. L., Sequeira, R. P. & Clarke, T. B. The microbiota protects against respiratory infection via GM-CSF signaling. Nat. Commun. 8 , 1512 (2017).
Nishimoto, A. T., Rosch, J. W. & Tuomanen, E. I. Pneumolysin: pathogenesis and therapeutic target. Front. Microbiol. 11 , 1543 (2020).
von Hoven, G., Qin, Q., Neukirch, C., Husmann, M. & Hellmann, N. Staphylococcus aureus α-toxin: small pore, large consequences. Biol. Chem. 400 , 1261–1276 (2019).
Hauser, A. R. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 7 , 654–665 (2009).
Ferguson, N. D. et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 38 , 1573–1582 (2012).
Matthay, M. A. et al. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 5 , 18 (2019).
Whitsett, J. A. & Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 16 , 27–35 (2015).
Cheng, D. et al. Airway epithelium controls lung inflammation and injury through the NF-κB pathway. J. Immunol. 178 , 6504–6513 (2007).
Quinton, L. J. et al. Functions and regulation of NF-κB RelA during pneumococcal pneumonia. J. Immunol. 178 , 1896–1903 (2007).
Han, S. & Mallampalli, R. K. The role of surfactant in lung disease and host defense against pulmonary infections. Ann. Am. Thorac. Soc. 12 , 765–774 (2015).
Carey, R. M. & Lee, R. J. Taste receptors in upper airway innate immunity. Nutrients 11 , 2017 (2019).
CAS PubMed Central Google Scholar
Lee, R. J. & Cohen, N. A. The emerging role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Am. J. Rhinol. Allergy 27 , 283–286 (2013).
McAleer, J. P. & Kolls, J. K. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol. Rev. 260 , 129–144 (2014).
Aujla, S. J. et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14 , 275–281 (2008).
Allard, B., Panariti, A. & Martin, J. G. Alveolar macrophages in the resolution of inflammation, tissue repair, and tolerance to infection. Front. Immunol. 9 , 1777 (2018).
Preston, J. A. et al. Alveolar macrophage apoptosis-associated bacterial killing helps prevent murine pneumonia. Am. J. Respir. Crit. Care Med. 200 , 84–97 (2019).
González-Juarbe, N. et al. Pore-forming toxins induce macrophage necroptosis during acute bacterial pneumonia. PLoS Pathog. 11 , e1005337 (2015).
Kitur, K. et al. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 11 , e1004820 (2015).
Kitur, K. et al. Necroptosis promotes Staphylococcus aureus clearance by inhibiting excessive inflammatory signaling. Cell Rep. 16 , 2219–2230 (2016).
Panda, S. K. & Colonna, M. Innate lymphoid cells in mucosal immunity. Front. Immunol. 10 , 861 (2019).
Kaiko, G. E., Phipps, S., Angkasekwinai, P., Dong, C. & Foster, P. S. NK cell deficiency predisposes to viral-induced Th2-type allergic inflammation via epithelial-derived IL-25. J. Immunol. 185 , 4681–4690 (2010).
Jayaraman, A. et al. IL-15 complexes induce NK- and T-cell responses independent of type I IFN signaling during rhinovirus infection. Mucosal Immunol. 7 , 1151–1164 (2014).
Van Maele, L. et al. Activation of type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection. J. Infect. Dis. 210 , 493–503 (2014).
Xiong, H. et al. Innate lymphocyte/ly6c(hi) monocyte crosstalk promotes Klebsiella pneumoniae clearance. Cell 165 , 679–689 (2016).
Hinks, T. S. C. et al. Steroid-induced deficiency of mucosal-associated invariant T cells in the chronic obstructive pulmonary disease lung. Implications for nontypeable Haemophilus influenzae infection. Am. J. Respir. Crit. Care Med. 194 , 1208–1218 (2016).
Meierovics, A. I. & Cowley, S. C. MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection. J. Exp. Med. 213 , 2793–2809 (2016).
Liu, J. et al. Advanced role of neutrophils in common respiratory diseases. J. Immunol. Res. 2017 , 6710278 (2017).
Castanheira, F. V. S. & Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 133 , 2178–2185 (2019).
Xiong, H. et al. Distinct contributions of neutrophils and CCR2+ monocytes to pulmonary clearance of different Klebsiella pneumoniae strains. Infect. Immun. 83 , 3418–3427 (2015).
Winter, C. et al. Important role for CC chemokine ligand 2-dependent lung mononuclear phagocyte recruitment to inhibit sepsis in mice infected with Streptococcus pneumoniae. J. Immunol. 182 , 4931–4937 (2009).
Weber, G. F. et al. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J. Exp. Med. 211 , 1243–1256 (2014).
de Stoppelaar, S. F. et al. Thrombocytopenia impairs host defense in gram-negative pneumonia-derived sepsis in mice. Blood 124 , 3781–3790 (2014).
van den Boogaard, F. E. et al. Thrombocytopenia impairs host defense during murine Streptococcus pneumoniae pneumonia. Crit. Care Med. 43 , e75–e83 (2015).
Ichinohe, T. et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl Acad. Sci. USA 108 , 5354–5359 (2011).
Schuijt, T. J. et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65 , 575–583 (2016).
Haak, B. W. & Wiersinga, W. J. The role of the gut microbiota in sepsis. Lancet Gastroenterol. Hepatol. 2 , 135–143 (2017).
Netea, M. G., Schlitzer, A., Placek, K., Joosten, L. A. B. & Schultze, J. L. Innate and adaptive immune memory: an evolutionary continuum in the host’s response to pathogens. Cell Host Microbe 25 , 13–26 (2019).
Giamarellos-Bourboulis, E. J. et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell 183 , 315–323.e9 (2020).
Hwang, J. Y., Randall, T. D. & Silva-Sanchez, A. Inducible bronchus-associated lymphoid tissue: taming inflammation in the lung. Front. Immunol. 7 , 258 (2016).
Snyder, M. E. & Farber, D. L. Human lung tissue resident memory T cells in health and disease. Curr. Opin. Immunol. 59 , 101–108 (2019).
Smith, N. M. et al. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol. 11 , 220–235 (2018).
Serhan, C. N. & Levy, B. D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128 , 2657–2669 (2018).
Flitter, B. A. et al. Pseudomonas aeruginosa sabotages the generation of host proresolving lipid mediators. Proc. Natl Acad. Sci. USA 114 , 136–141 (2017).
Sham, H. P. et al. 15-epi-lipoxin A4, resolvin D2, and resolvin D3 induce NF-κB regulators in bacterial pneumonia. J. Immunol. 200 , 2757–2766 (2018).
Zemans, R. L. et al. Neutrophil transmigration triggers repair of the lung epithelium via β-catenin signaling. Proc. Natl Acad. Sci. USA 108 , 15990–15995 (2011).
Liu, Y. et al. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J. Exp. Med. 208 , 1473–1484 (2011).
Kumar, P. A. et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147 , 525–538 (2011).
Matsuzaki, Y. et al. Stat3 is required for cytoprotection of the respiratory epithelium during adenoviral infection. J. Immunol. 177 , 527–537 (2006).
Quinton, L. J. et al. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am. J. Respir. Cell Mol. Biol. 38 , 699–706 (2008).
Quinton, L. J. et al. Leukemia inhibitory factor signaling is required for lung protection during pneumonia. J. Immunol. 188 , 6300–6308 (2012).
Poe, S. L. et al. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol. 6 , 189–199 (2013).
D’Alessio, F. R. et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Invest. 119 , 2898–2913 (2009).
Monticelli, L. A. et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12 , 1045–1054 (2011).
Laidlaw, B. J. et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41 , 633–645 (2014).
Xu, X. et al. Conventional NK cells can produce IL-22 and promote host defense in Klebsiella pneumoniae pneumonia. J. Immunol. 192 , 1778–1786 (2014).
Kradin, R. L. & Digumarthy, S. The pathology of pulmonary bacterial infection. Semin. Diagn. Pathol. 34 , 498–509 (2017).
Pritt, B. S. & Aubry, M. C. Histopathology of viral infections of the lung. Semin. Diagn. Pathol. 34 , 510–517 (2017).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315 , 801–810 (2016).
Angus, D. C. & van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 369 , 840–851 (2013).
Dremsizov, T. et al. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest 129 , 968–978 (2006).
Giuliano, K. K. & Baker, D. Sepsis in the context of nonventilator hospital-acquired pneumonia. Am. J. Crit. Care 29 , 9–14 (2020).
Hotchkiss, R. S. et al. Sepsis and septic shock. Nat. Rev. Dis. Prim. 2 , 16045 (2016).
van der Poll, T., van de Veerdonk, F. L., Scicluna, B. P. & Netea, M. G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 17 , 407–420 (2017).
Corrales-Medina, V. F., Musher, D. M., Shachkina, S. & Chirinos, J. A. Acute pneumonia and the cardiovascular system. Lancet 381 , 496–505 (2013).
Corrales-Medina, V. F. et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 313 , 264–274 (2015). The short-term and long-term risk of cardiovascular diseases after CAP hospitalization is shown in this capital study .
Corrales-Medina, V. F. et al. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med. 8 , e1001048 (2011).
Violi, F. et al. Cardiovascular complications and short-term mortality risk in community-acquired pneumonia. Clin. Infect. Dis. 64 , 1486–1493 (2017).
Ramirez, J. et al. Acute myocardial infarction in hospitalized patients with community-acquired pneumonia. Clin. Infect. Dis. 47 , 182–187 (2008).
Mortensen, E. M. et al. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch. Intern. Med. 162 , 1059–1064 (2002).
Musher, D. M., Abers, M. S. & Corrales-Medina, V. F. Acute infection and myocardial infarction. N. Engl. J. Med. 380 , 171–176 (2019). A review article showing the evidence of acute respiratory viral infection and the increased risk of myocardial infarction .
Milbrandt, E. B. et al. Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol. Med. 15 , 438–445 (2009).
van Vught, L. A. et al. Comparative analysis of the host response to community-acquired and hospital-acquired pneumonia in critically Ill patients. Am. J. Respir. Crit. Care Med. 194 , 1366–1374 (2016).
Naghavi, M. et al. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation 107 , 762–768 (2003).
Madjid, M., Vela, D., Khalili-Tabrizi, H., Casscells, S. W. & Litovsky, S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex. Heart Inst. J. 34 , 11–18 (2007).
Jaw, J. E. et al. Lung exposure to lipopolysaccharide causes atherosclerotic plaque destabilisation. Eur. Respir. J. 48 , 205–215 (2016).
Yende, S. et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am. J. Respir. Crit. Care Med. 177 , 1242–1247 (2008).
Yende, S. et al. Elevated hemostasis markers after pneumonia increases one-year risk of all-cause and cardiovascular deaths. PLoS ONE 6 , e22847 (2011).
Iwashyna, T. J., Ely, E. W., Smith, D. M. & Langa, K. M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304 , 1787–1794 (2010).
Shah, F. A. et al. Bidirectional relationship between cognitive function and pneumonia. Am. J. Respir. Crit. Care Med. 188 , 586–592 (2013).
Girard, T. D., Dittus, R. S. & Ely, E. W. Critical illness brain injury. Annu. Rev. Med. 67 , 497–513 (2016).
Chung, H.-Y., Wickel, J., Brunkhorst, F. M. & Geis, C. Sepsis-associated encephalopathy: from delirium to dementia? J. Clin. Med. 9 , 703 (2020).
Prescott, H. C., Sjoding, M. W. & Iwashyna, T. J. Diagnoses of early and late readmissions after hospitalization for pneumonia. A systematic review. Ann. Am. Thorac. Soc. 11 , 1091–1100 (2014).
Dang, T. T., Majumdar, S. R., Marrie, T. J. & Eurich, D. T. Recurrent pneumonia: a review with focus on clinical epidemiology and modifiable risk factors in elderly patients. Drugs Aging 32 , 13–19 (2015).
Ekdahl, K., Braconier, J. H. & Svanborg, C. Immunoglobulin deficiencies and impaired immune response to polysaccharide antigens in adult patients with recurrent community-acquired pneumonia. Scand. J. Infect. Dis. 29 , 401–407 (1997).
Roquilly, A. et al. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat. Immunol. 21 , 636–648 (2020).
Lamping, D. L. et al. The community-acquired pneumonia symptom questionnaire: a new, patient-based outcome measure to evaluate symptoms in patients with community-acquired pneumonia. Chest 122 , 920–929 (2002).
Metlay, J. P. et al. Measuring symptomatic and functional recovery in patients with community-acquired pneumonia. J. Gen. Intern. Med. 12 , 423–430 (1997).
Chalmers, J. D. et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax 65 , 878–883 (2010).
Chalmers, J. D. et al. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community-acquired pneumonia. Thorax 64 , 556–558 (2009).
Falguera, M. et al. Predictive factors, microbiology and outcome of patients with parapneumonic effusion. Eur. Respir. J. 38 , 1173–1179 (2011).
Bhuiyan, M. U. et al. Combination of clinical symptoms and blood biomarkers can improve discrimination between bacterial or viral community-acquired pneumonia in children. BMC Pulmonary Med. 19 , 71 (2019).
Lhommet, C. et al. Predicting the microbial cause of community-acquired pneumonia: can physicians or a data-driven method differentiate viral from bacterial pneumonia at patient presentation? BMC Pulmonary Med. 20 , 62 (2020).
Torres, A., & Cillóniz, C. Clinical Management of Bacterial Pneumonia (Springer, 2015).
Cilloniz, C., Ceccato, A., San Jose, A. & Torres, A. Clinical management of community acquired pneumonia in the elderly patient. Expert Rev. Respir. Med. 10 , 1211–1220 (2016).
Schurink, C. A. M. et al. Clinical pulmonary infection score for ventilator-associated pneumonia: accuracy and inter-observer variability. Intensive Care Med. 30 , 217–224 (2004).
Fàbregas, N. et al. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post-mortem lung biopsies. Thorax 54 , 867–873 (1999). The most complete immediate post-mortem study of VAP to validate clinical diagnosis .
Self, W. H., Courtney, D. M., McNaughton, C. D., Wunderink, R. G. & Kline, J. A. High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am. J. Emerg. Med. 31 , 401–405 (2013).
Laursen, C. B. et al. Diagnostic performance of chest X-ray for the diagnosis of community acquired pneumonia in acute admitted patients with respiratory symptoms. Scand. J. Trauma. Resusc. Emerg. Med. 21 , A21 (2013).
Claessens, Y.-E. et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 192 , 974–982 (2015).
Ding, X., Xu, J., Zhou, J., Long, Q. & Chest, C. T. findings of COVID-19 pneumonia by duration of symptoms. Eur. J. Radiol. 127 , 109009 (2020).
Franquet, T. Imaging of community-acquired pneumonia. J. Thorac. Imaging 33 , 282–294 (2018).
D’Amato, M. et al. Assessment of thoracic ultrasound in complementary diagnosis and in follow up of community-acquired pneumonia (CAP). BMC Med. Imaging 17 , 52 (2017).
Long, L., Zhao, H.-T., Zhang, Z.-Y., Wang, G.-Y. & Zhao, H.-L. Lung ultrasound for the diagnosis of pneumonia in adults: a meta-analysis. Medicine 96 , e5713 (2017).
Mongodi, S. et al. Lung ultrasound for early diagnosis of ventilator-associated pneumonia. Chest 149 , 969–980 (2016).
Bouhemad, B., Dransart-Rayé, O., Mojoli, F. & Mongodi, S. Lung ultrasound for diagnosis and monitoring of ventilator-associated pneumonia. Ann. Transl. Med. 6 , 418 (2018).
Musher, D. M., Montoya, R. & Wanahita, A. Diagnostic value of microscopic examination of Gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin. Infect. Dis. 39 , 165–169 (2004).
Fukuyama, H., Yamashiro, S., Kinjo, K., Tamaki, H. & Kishaba, T. Validation of sputum Gram stain for treatment of community-acquired pneumonia and healthcare-associated pneumonia: a prospective observational study. BMC Infect. Dis. 14 , 534 (2014).
Ranzani, O. T. et al. Diagnostic accuracy of Gram staining when predicting staphylococcal hospital-acquired pneumonia and ventilator-associated pneumonia: a systematic review and meta-analysis. Clin. Microbiol. Infect. 26 , 1456–1463 (2020).
Torres, A., Artigas, A. & Ferrer, R. Biomarkers in the ICU: less is more? No. Intensive Care Med. 47 , 97–100 (2021).
Torres, A., Lee, N., Cilloniz, C., Vila, J. & Van der Eerden, M. Laboratory diagnosis of pneumonia in the molecular age. Eur. Respir. J. 48 , 1764–1778 (2016). In-depth revision of available molecular diagnostic techniques for bacterial and viral pneumonia .
Schulte, B. et al. Detection of pneumonia associated pathogens using a prototype multiplexed pneumonia test in hospitalized patients with severe pneumonia. PLoS ONE 9 , e110566 (2014).
Paonessa, J. R. et al. Rapid detection of methicillin-resistant Staphylococcus aureus in BAL: a pilot randomized controlled trial. Chest 155 , 999–1007 (2019).
Gastli, N. et al. Multicentric evaluation of BioFire FilmArray Pneumonia Panel for rapid bacteriological documentation of pneumonia. Clin. Microbiol. Infect. https://doi.org/10.1016/j.cmi.2020.11.014 (2020).
Article PubMed Google Scholar
Centers for Disease Control and Prevention. Overview of Testing for SARS-CoV-2 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html (CDC, 2020).
Karakioulaki, M. & Stolz, D. The case of procalcitonin for lower respiratory tract infections. BRN Rev. 5 , 277–293 (2019).
Krüger, S. et al. Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with CAP: results from the German Competence Network CAPNETZ. Respir. Res. 10 , 65 (2009).
Ramirez, P. et al. Sequential measurements of procalcitonin levels in diagnosing ventilator-associated pneumonia. Eur. Respir. J. 31 , 356–362 (2008).
Luyt, C.-E. et al. Usefulness of procalcitonin for the diagnosis of ventilator-associated pneumonia. Intensive Care Med. 34 , 1434–1440 (2008).
Schuetz, P. et al. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin. Chem. Lab. Med. 57 , 1308–1318 (2019).
Liapikou, A., Cilloniz, C. & Torres, A. Drugs that increase the risk of community-acquired pneumonia: a narrative review. Expert Opin. Drug Saf. 17 , 991–1003 (2018).
Niederman, M. S. et al. Efficacy and effectiveness of a 23-valent polysaccharide vaccine against invasive and non-invasive pneumococcal disease and related outcomes: a review of available evidence. Expert Rev Vaccines https://doi.org/10.1080/14760584.2021.1880328 (2021).
Maruyama, T. et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ 340 , c1004 (2010).
Falkenhorst, G. et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta-analysis. PLoS ONE 12 , e0169368 (2017).
Bonten, M. J. M. et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 372 , 1114–1125 (2015).
Patterson, S. et al. A post hoc assessment of duration of protection in CAPiTA (Community Acquired Pneumonia immunization Trial in Adults). Trials Vaccinol. 5 , 92–96 (2016).
Millar, E. V. et al. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin. Infect. Dis. 47 , 989–996 (2008).
Hammitt, L. L. et al. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J. Infect. Dis. 193 , 1487–1494 (2006).
Chung, J. R. et al. Effects of influenza vaccination in the United States during the 2018-2019 influenza season. Clin. Infect. Dis. 71 , e368–e376 (2020).
Restivo, V. et al. Influenza vaccine effectiveness among high-risk groups: a systematic literature review and meta-analysis of case-control and cohort studies. Hum. Vaccin. Immunother. 14 , 724–735 (2018).
Chow, E. J. et al. Vaccine effectiveness against influenza-associated lower respiratory tract infections in hospitalized adults, Louisville, Kentucky, 2010-2013. Open. Forum Infect. Dis. 7 , ofaa262 (2020).
Lyons, P. G. & Kollef, M. H. Prevention of hospital-acquired pneumonia. Curr. Opin. Crit. Care 24 , 370–378 (2018).
Álvarez-Lerma, F. et al. Prevention of ventilator-associated pneumonia: the multimodal approach of the Spanish ICU “Pneumonia Zero” Program. Crit. Care Med. 46 , 181–188 (2018).
Palomar, M. et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit. Care Med. 41 , 2364–2372 (2013).
Ma, S. et al. A meta analysis of the effect of enhanced hand hygiene on the morbidity of ventilator-associated pneumonia. Zhonghua Wei Zhong Bing. Ji Jiu Yi Xue 26 , 304–308 (2014).
Drakulovic, M. B. et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet 354 , 1851–1858 (1999).
Wang, L. et al. Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. Cochrane Database Syst. Rev. 2016 , CD009946 (2016).
Li Bassi, G. et al. Randomized, multicenter trial of lateral Trendelenburg versus semirecumbent body position for the prevention of ventilator-associated pneumonia. Intensive Care Med. 43 , 1572–1584 (2017).
Guérin, C. et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 368 , 2159–2168 (2013).
Douglas, I. S. et al. Safety and outcomes of prolonged usual care prone position mechanical ventilation to treat acute coronavirus disease 2019 hypoxemic respiratory failure. Crit. Care Med. 49 , 490–502 (2021).
Shelhamer, M. C. et al. Prone positioning in moderate to severe acute respiratory distress syndrome due to COVID-19: a cohort study and analysis of physiology. J. Intensive Care Med. 36 , 241–252 (2021).
Sud, S., Sud, M., Friedrich, J. O. & Adhikari, N. K. J. Effect of mechanical ventilation in the prone position on clinical outcomes in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. CMAJ 178 , 1153–1161 (2008).
Mao, Z. et al. Subglottic secretion suction for preventing ventilator-associated pneumonia: an updated meta-analysis and trial sequential analysis. Crit. Care 20 , 353 (2016).
Marjanovic, N. et al. Multicentre randomised controlled trial to investigate the usefulness of continuous pneumatic regulation of tracheal cuff pressure for reducing ventilator-associated pneumonia in mechanically ventilated severe trauma patients: the AGATE study protocol. BMJ Open 7 , e017003 (2017).
Philippart, F. et al. Randomized intubation with polyurethane or conical cuffs to prevent pneumonia in ventilated patients. Am. J. Respir. Crit. Care Med. 191 , 637–645 (2015).
Klompas, M., Speck, K., Howell, M. D., Greene, L. R. & Berenholtz, S. M. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta-analysis. JAMA Intern. Med. 174 , 751–761 (2014).
de Smet, A. M. G. A. et al. Decontamination of the digestive tract and oropharynx in ICU patients. N. Engl. J. Med. 360 , 20–31 (2009).
Wittekamp, B. H. et al. Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: a randomized clinical trial. JAMA 320 , 2087–2098 (2018).
Dang, T. T., Eurich, D. T., Weir, D. L., Marrie, T. J. & Majumdar, S. R. Rates and risk factors for recurrent pneumonia in patients hospitalized with community-acquired pneumonia: population-based prospective cohort study with 5 years of follow-up. Clin. Infect. Dis. 59 , 74–80 (2014).
Garcia-Vidal, C. et al. Aetiology of, and risk factors for, recurrent community-acquired pneumonia. Clin. Microbiol. Infect. 15 , 1033–1038 (2009).
Liu, P. et al. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established Antimicrobial Stewardship Program. BMC Infect. Dis. 16 , 751 (2016).
Maruyama, T. et al. A therapeutic strategy for all pneumonia patients: a 3-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin. Infect. Dis. 68 , 1080–1088 (2018).
Abdul-Aziz, M. H., Lipman, J. & Roberts, J. A. Antibiotic dosing for multidrug-resistant pathogen pneumonia. Curr. Opin. Infect. Dis. 30 , 231–239 (2017).
Tsai, D., Lipman, J. & Roberts, J. A. Pharmacokinetic/pharmacodynamic considerations for the optimization of antimicrobial delivery in the critically ill. Curr. Opin. Crit. Care 21 , 412–420 (2015).
Mandell, L. A. et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44 (Suppl 2), S27–S72 (2007).
Sligl, W. I. et al. Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis. Crit. Care Med. 42 , 420–432 (2014).
Torres, A. et al. Challenges in severe community-acquired pneumonia: a point-of-view review. Intensive Care Med. 45 , 159–171 (2019).
Niederman, M. S. Macrolide-resistant pneumococcus in community-acquired pneumonia. Is there still a role for macrolide therapy? Am. J. Respir. Crit. Care Med. 191 , 1216–1217 (2015).
Asadi, L. et al. Guideline adherence and macrolides reduced mortality in outpatients with pneumonia. Respir. Med. 106 , 451–458 (2012).
Postma, D. F. et al. Cardiac events after macrolides or fluoroquinolones in patients hospitalized for community-acquired pneumonia: post-hoc analysis of a cluster-randomized trial. BMC Infect. Dis. 19 , 17 (2019).
Garin, N. et al. β-Lactam monotherapy vs β-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern. Med. 174 , 1894–1901 (2014).
Gershengorn, H. B., Keene, A., Dzierba, A. L. & Wunsch, H. The association of antibiotic treatment regimen and hospital mortality in patients hospitalized with Legionella pneumonia. Clin. Infect. Dis. 60 , e66–e79 (2015).
Niederman, M. S. Antibiotic treatment of hospital-acquired pneumonia: is it different from ventilator-associated pneumonia? Curr. Opin. Crit. Care 24 , 353–360 (2018).
Kumar, A., Safdar, N., Kethireddy, S. & Chateau, D. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit. Care Med. 38 , 1651–1664 (2010).
Martin-Loeches, I. et al. Potentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: the interaction of ecology, shock and risk factors. Intensive Care Med. 39 , 672–681 (2013).
Niederman, M. S. et al. Inhaled amikacin adjunctive to intravenous standard-of-care antibiotics in mechanically ventilated patients with Gram-negative pneumonia (INHALE): a double-blind, randomised, placebo-controlled, phase 3, superiority trial. Lancet Infect. Dis. 20 , 330–340 (2020).
Chastre, J. et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 290 , 2588–2598 (2003). A seminal article comparing 8 or 15 days of antibiotic treatment in VAP .
Garnacho-Montero, J. et al. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med. 41 , 2057–2075 (2015).
Timsit, J.-F., Pilmis, B. & Zahar, J.-R. How should we treat hospital-acquired and ventilator-associated pneumonia caused by extended-spectrum β-lactamase-producing enterobacteriaceae? Semin. Respir. Crit. Care Med. 38 , 287–300 (2017).
de Jong, E. et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect. Dis. 16 , 819–827 (2016).
Sousa, D. et al. Community-acquired pneumonia in immunocompromised older patients: incidence, causative organisms and outcome. Clin. Microbiol. Infect. 19 , 187–192 (2013).
Ramirez, J. A. et al. Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest 158 , 1896–1911 (2020).
El-Solh, A. A. et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am. J. Respir. Crit. Care Med. 167 , 1650–1654 (2003).
Siemieniuk, R. A. C. et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann. Intern. Med. 163 , 519–528 (2015).
Torres, A. et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 313 , 677–686 (2015).
Rodrigo, C., Leonardi-Bee, J., Nguyen-Van-Tam, J. & Lim, W. S. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst. Rev. 3 , CD010406 (2016).
Recovery Collaborative Group. et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 384 , 693–704 (2021).
Welte, T. et al. Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: a randomized, placebo-controlled, double-blind, multicenter, phase II trial (CIGMA study). Intensive Care Med. 44 , 438–448 (2018).
Frat, J.-P. et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 372 , 2185–2196 (2015).
Kaplan, V. et al. Pneumonia: still the old man’s friend? Arch. Intern. Med. 163 , 317–323 (2003).
Shorr, A. F. et al. Readmission following hospitalization for pneumonia: the impact of pneumonia type and its implication for hospitals. Clin. Infect. Dis. 57 , 362–367 (2013).
Chalmers, J. D. et al. Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study. Clin. Infect. Dis. 53 , 107–113 (2011).
El Moussaoui, R. et al. Long-term symptom recovery and health-related quality of life in patients with mild-to-moderate-severe community-acquired pneumonia. Chest 130 , 1165–1172 (2006).
Wootton, D. G. et al. A longitudinal modelling study estimates acute symptoms of community acquired pneumonia recover to baseline by 10 days. Eur. Respir. J. 49 , 1602170 (2017).
Marrie, T. J., Lau, C. Y., Wheeler, S. L., Wong, C. J. & Feagan, B. G. Predictors of symptom resolution in patients with community-acquired pneumonia. Clin. Infect. Dis. 31 , 1362–1367 (2000).
Almirall, J. et al. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur. Respir. J. 15 , 757–763 (2000).
Andrade, L. F. et al. Health related quality of life in patients with community-acquired pneumococcal pneumonia in France. Health Qual. Life Outcomes 16 , 28 (2018).
Wyrwich, K. W., Yu, H., Sato, R. & Powers, J. H. Observational longitudinal study of symptom burden and time for recovery from community-acquired pneumonia reported by older adults surveyed nationwide using the CAP Burden of Illness Questionnaire. Patient Relat. Outcome Meas. 6 , 215–223 (2015).
Carratala, J. et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann. Intern. Med. 142 , 165–172 (2005).
Mangen, M.-J. J., Huijts, S. M., Bonten, M. J. M. & de Wit, G. A. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect. Dis. 17 , 208 (2017).
Lloyd, M., Callander, E., Karahalios, A., Desmond, L. & Karunajeewa, H. Patient-reported outcome measures in community-acquired pneumonia: a systematic review of application and content validity. BMJ Open. Respir. Res. 6 , e000398 (2019).
Gao, C. A. et al. Comparing nasopharyngeal and BAL SARS-CoV-2 assays in respiratory failure. Am. J. Respir. Crit. Care Med. 203 , 127–129 (2021).
Peiffer-Smadja, N. et al. Performance and impact of a multiplex PCR in ICU patients with ventilator-associated pneumonia or ventilated hospital-acquired pneumonia. Crit. Care 24 , 366 (2020).
Murphy, C. N. et al. Multicenter evaluation of the biofire filmarray pneumonia/pneumonia plus panel for detection and quantification of agents of lower respiratory tract infection. J. Clin. Microbiol. 58 , e00128-20 (2020).
Pendleton, K. M. et al. Rapid pathogen identification in bacterial pneumonia using real-time metagenomics. Am. J. Respir. Crit. Care Med. 196 , 1610–1612 (2017).
Chiu, C. Y. & Miller, S. A. Clinical metagenomics. Nat. Rev. Genet. 20 , 341–355 (2019).
Hellyer, T. P. et al. Biomarker-guided antibiotic stewardship in suspected ventilator-associated pneumonia (VAPrapid2): a randomised controlled trial and process evaluation. Lancet Respir. Med. 8 , 182–191 (2020).
Blot, S. I. et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 186 , 56–64 (2012).
Bassetti, M., Welte, T. & Wunderink, R. G. Treatment of Gram-negative pneumonia in the critical care setting: is the beta-lactam antibiotic backbone broken beyond repair? Crit. Care 20 , 19 (2016).
Kollef, M. H. et al. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 19 , 1299–1311 (2019). A randomized clinical trial comparing ceftolozane–tazobactam with meropenem in ventilated HAP and VAP. A post-hoc analysis in ventilated HAP demonstrated superiority of ceftolozane–tazobactam .
Kollef, M. H. et al. A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit. Care 16 , R218 (2012).
File, T. M. et al. FOCUS 1: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J. Antimicrob. Chemother. 66 , iii19–iii32 (2011).
Biedenbach, D. J., Kazmierczak, K., Bouchillon, S. K., Sahm, D. F. & Bradford, P. A. In vitro activity of aztreonam-avibactam against a global collection of Gram-negative pathogens from 2012 and 2013. Antimicrob. Agents Chemother. 59 , 4239–4248 (2015).
Awad, S. S. et al. A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia. Clin. Infect. Dis. 59 , 51–61 (2014).
David, S. et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat. Microbiol. 4 , 1919–1929 (2019).
Watkins, R. R. & File, T. M. Lefamulin: a novel semisynthetic pleuromutilin antibiotic for community-acquired bacterial pneumonia. Clin. Infect. Dis. 71 , 2757–2762 (2020).
Spellberg, B., Bartlett, J., Wunderink, R. & Gilbert, D. N. Novel approaches are needed to develop tomorrow’s antibacterial therapies. Am. J. Respir. Crit. Care Med. 191 , 135–140 (2015).
Matteo Bassetti, R. E. et al. Efficacy and safety of cefiderocol for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): results of a phase 3 randomised, open-label, parallel-assigned, pathogen-focused study. Lancet 21 , 226–240 (2021).
Barnes, M. D. et al. Targeting multidrug-resistant Acinetobacter spp.: sulbactam and the diazabicyclooctenone β-lactamase inhibitor ETX2514 as a novel therapeutic agent. mBio 10 , e00159-19 (2019).
Lehman, K. M. & Grabowicz, M. Countering gram-negative antibiotic resistance: recent progress in disrupting the outer membrane with novel therapeutics. Antibiotics (Basel) 8 , 163 (2019).
Wu, J. Y., Srinivas, P. & Pogue, J. M. Cefiderocol: a novel agent for the management of multidrug-resistant gram-negative organisms. Infect. Dis. Ther. 9 , 17–40 (2020).
Wunderink, R. G. et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a phase 3, randomised, double-blind, non-inferiority study. Lancet Infect. Dis. 21 , 213–225 (2020).
File, T. M. et al. Efficacy and safety of IV-to-oral lefamulin, a pleuromutilin antibiotic, for treatment of community-acquired bacterial pneumonia: the phase 3 LEAP 1 trial. Clin. Infect. Dis. 69 , 1856–1867 (2019).
Alexander, E. et al. Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia: the LEAP 2 randomized clinical trial. JAMA 322 , 1661–1671 (2019).
Que, Y.-A. et al. Assessment of panobacumab as adjunctive immunotherapy for the treatment of nosocomial Pseudomonas aeruginosa pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 33 , 1861–1867 (2014).
François, B. et al. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized, double-blind, placebo-controlled trial. Crit. Care Med. 40 , 2320–2326 (2012).
François, B. et al. Safety and tolerability of a single administration of AR-301, a human monoclonal antibody, in ICU patients with severe pneumonia caused by Staphylococcus aureus: first-in-human trial. Intensive Care Med. 44 , 1787–1796 (2018).
Maddocks, S. et al. Bacteriophage therapy of ventilator-associated pneumonia and empyema caused by Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 200 , 1179–1181 (2019).
Wunderink, R. G. Turning the phage on treatment of antimicrobial-resistant pneumonia. Am. J. Respir. Crit. Care Med. 200 , 1081–1082 (2019).
Sicot, N. et al. Methicillin resistance is not a predictor of severity in community-acquired Staphylococcus aureus necrotizing pneumonia – results of a prospective observational study. Clin. Microbiol. Infect. 19 , E142–E148 (2013).
Download references
Acknowledgements
A.T. is the recipient of ICREA award from Generalitat de Catalunya. C.C. is the recipient of the SEPAR fellowship 2018, a grant 2019 from the Fondo de Investigación Sanitaria (PI19/00207), and the SEPAR fellowship “Programa Mentor”. We thank J.J.T.H. Roelofs (Department of Pathology, Amsterdam UMC, Amsterdam, Netherlands) for his invaluable assistance with the section on lung pathology and in providing representative histopathology slides.
Author information
Authors and affiliations.
Department of Pneumology, Hospital Clinic of Barcelona, Barcelona, Spain
Antoni Torres & Catia Cilloniz
August Pi i Sunyer Biomedical Research Institute (IDIBAPS), Barcelona, Spain
University of Barcelona, Barcelona, Spain
Biomedical Research Networking Centers in Respiratory Diseases (CIBERES and CIBERESUCICOVID study), Barcelona, Spain
Division of Pulmonary and Critical Care, New York Presbyterian/Weill Cornell Medical Center, New York City, NY, USA
Michael S. Niederman
Department of Pneumology, Hospital Universitario y Politécnico La Fe, Valencia, Spain
Rosario Menéndez
Scottish Centre for Respiratory Research, University of Dundee, Ninewells Hospital and Medical School, Dundee, UK
James D. Chalmers
Division of Pulmonary and Critical Care, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Richard G. Wunderink
Center of Experimental and Molecular Medicine, Division of Infectious Diseases, Amsterdam University Medical Centers, Location Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands
Tom van der Poll
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (C.C. and A.T.); Epidemiology (C.C. and R.M.); Mechanisms/pathophysiology (T.v.d.P.); Diagnosis, screening and prevention (C.C. and A.T.); Management (M.S.N.); Quality of life (J.D.C.); Outlook (R.G.W); Overview of Primer (A.T. and C.C.).
Corresponding authors
Correspondence to Antoni Torres or Catia Cilloniz .
Ethics declarations
Competing interests.
A.T. has been a paid consultant to Pfizer, Jansen, and MSD, and a speaker for Pfizer and MSD. M.S.N. has received research grants from Shionogi, Bayer and Merck. He has been a paid consultant to Bayer, Merck, Paratek, Abbvie, Nabriva, and Thermo-Fisher. J.D.C. has received research funding from Astrazeneca, Boehringer-Ingelheim, Gilead Sciences, Glaxosmithkline, Insmed and Novartis; he has received consultancy fees from Chiesi, Grifols and Zambon. R.G.W. is a consultant to Merck, Shionogi, Polyphor, Microbiotix, bioMerieux, Curetis, KBP Biosciences, Idorsia and Accelerate. All other authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks Y. Arabi, C. Ginocchio, K. Klugman, M. Metersky, N. Suttorp and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Torres, A., Cilloniz, C., Niederman, M.S. et al. Pneumonia. Nat Rev Dis Primers 7 , 25 (2021). https://doi.org/10.1038/s41572-021-00259-0
Download citation
Accepted : 26 February 2021
Published : 08 April 2021
DOI : https://doi.org/10.1038/s41572-021-00259-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Risk factors and predicting nomogram for the clinical deterioration of non-severe community-acquired pneumonia.
- Cheng-bin Xu
- Shan-shan Su
BMC Pulmonary Medicine (2024)
Validating the accuracy of deep learning for the diagnosis of pneumonia on chest x-ray against a robust multimodal reference diagnosis: a post hoc analysis of two prospective studies
- Jeremy Hofmeister
- Nicolas Garin
- Virginie Prendki
European Radiology Experimental (2024)
Hospitalization, case fatality, comorbidities, and isolated pathogens of adult inpatients with pneumonia from 2013 to 2022: a real-world study in Guangzhou, China
- Zhufeng Wang
- Jinping Zheng
BMC Infectious Diseases (2024)
A methodical exploration of imaging modalities from dataset to detection through machine learning paradigms in prominent lung disease diagnosis: a review
- Sunil Kumar
- Harish Kumar
- Manoj Diwakar
BMC Medical Imaging (2024)
Prediction of new-onset atrial fibrillation with the C2HEST score in patients admitted with community-acquired pneumonia
- Daniele Pastori
- Danilo Menichelli
- Roberto Cangemi
Infection (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Community-acquired pneumonia: Trends in and research on drug resistance and advances in new antibiotics
Affiliations.
- 1 Laboratory of Pathobiology, Ministry of Education, Department of Pathophysiology, College of Basic Medical Sciences, Jilin University, Changchun, Jilin, China.
- 2 Department of Health Policy and Management, International University of Health and Welfare, Tokyo, Japan.
- 3 Department of Breast Surgery, China-Japan Union Hospital, Jilin University, Changchun, Jilin, China.
- 4 Department of Respiratory Medicine, China-Japan Union Hospital, Jilin University, Changchun, Jilin, China.
- 5 Department of Radiation Oncology, The Second Affiliated Hospital, Jilin University, Changchun, Jilin, China.
- PMID: 34483225
- DOI: 10.5582/bst.2021.01342
Community-acquired pneumonia (CAP) refers to infectious inflammation of the lung parenchyma developing outside of a hospital. CAP has quite a high mortality and morbidity rate worldwide, and especially among elderly patients. The increasing burden of CAP is due to antibiotic resistance, the growth of the elderly population, and underlying comorbidities. Streptococcus pneumoniae remains the most common bacterial pathogen causing CAP, but multi-drug resistance bacteria and potential pathogens have increased the difficulty and challenges of managing CAP. Although preventive measures, diagnostic techniques, and treatment strategies are constantly advancing and improving, the susceptibility of multi-drug resistant pathogens, such as including Methicillin-Resistant Staphylococcus aureus (MRSA), Klebsiella pneumoniae, and Pseudomonas aeruginosa, has not improved significantly in recent decades, thus highlighting the importance and necessity of developing new antibiotics for the treatment of CAP. New antimicrobials have been approved over the past few years that will expand treatment options for CAP, and especially for patients with potential comorbidities. This situation also offers the chance to reduce the abuse of antibiotics, their toxicities, and their adverse reactions and to provide effective personalized antibiotic treatment.
Keywords: Community-acquired pneumonia (CAP); antimicrobial resistance; epidemiology; treatment guidelines.
PubMed Disclaimer
Similar articles
- Risk factor-based analysis of community-acquired pneumonia, healthcare-associated pneumonia and hospital-acquired pneumonia: Microbiological distribution, antibiotic resistance, and clinical outcomes. Hyun H, Song JY, Yoon JG, Seong H, Noh JY, Cheong HJ, Kim WJ. Hyun H, et al. PLoS One. 2022 Jun 29;17(6):e0270261. doi: 10.1371/journal.pone.0270261. eCollection 2022. PLoS One. 2022. PMID: 35767562 Free PMC article.
- Advances in antibiotic therapy for community-acquired pneumonia. Viasus D, Garcia-Vidal C, Carratalà J. Viasus D, et al. Curr Opin Pulm Med. 2013 May;19(3):209-15. doi: 10.1097/MCP.0b013e32835f1c0b. Curr Opin Pulm Med. 2013. PMID: 23422416 Review.
- Methicillin-resistant Staphylococcus aureus in community-acquired and health care-associated pneumonia: incidence, diagnosis, and treatment options. Dean N. Dean N. Hosp Pract (1995). 2010 Feb;38(1):7-15. doi: 10.3810/hp.2010.02.274. Hosp Pract (1995). 2010. PMID: 20469619 Review.
- Community-acquired pneumonia with risk for drug-resistant pathogens. Fleming V, Buck B, Nix N, Kumar P, Southwood R. Fleming V, et al. South Med J. 2013 Mar;106(3):209-16. doi: 10.1097/SMJ.0b013e318287fe71. South Med J. 2013. PMID: 23462490
- Identification of Risk Factors for Multidrug-Resistant Organisms in Community-Acquired Bacterial Pneumonia at a Community Hospital. Covington EW, Rufe A. Covington EW, et al. J Pharm Pract. 2023 Apr;36(2):303-308. doi: 10.1177/08971900211039700. Epub 2021 Aug 18. J Pharm Pract. 2023. PMID: 34406082
- Pneumonia-Plus: a deep learning model for the classification of bacterial, fungal, and viral pneumonia based on CT tomography. Wang F, Li X, Wen R, Luo H, Liu D, Qi S, Jing Y, Wang P, Deng G, Huang C, Du T, Wang L, Liang H, Wang J, Liu C. Wang F, et al. Eur Radiol. 2023 Dec;33(12):8869-8878. doi: 10.1007/s00330-023-09833-4. Epub 2023 Jun 30. Eur Radiol. 2023. PMID: 37389609
- Rational medication management mode and its implementation effect for the elderly with multimorbidity: A prospective cohort study in China. Tang Q, Wan L, Lu J, Wu W, Wu H, Liu Z, Zhao S, Li C, Chen G, Lu J. Tang Q, et al. Front Public Health. 2022 Sep 6;10:992959. doi: 10.3389/fpubh.2022.992959. eCollection 2022. Front Public Health. 2022. PMID: 36148363 Free PMC article.
- Health Status and Individual Care Needs of Disabled Elderly at Home in Different Types of Care. Tang Q, Yuan M, Wu W, Wu H, Wang C, Chen G, Li C, Lu J. Tang Q, et al. Int J Environ Res Public Health. 2022 Sep 9;19(18):11371. doi: 10.3390/ijerph191811371. Int J Environ Res Public Health. 2022. PMID: 36141656 Free PMC article.
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- J-STAGE, Japan Science and Technology Information Aggregator, Electronic
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplements
- Virtual Roundtables
- Journal of Antimicrobial Chemotherapy
- JAC-Antimicrobial Resistance
- Author Guidelines
- Submission Site
- Open Access
- About the Journal of Antimicrobial Chemotherapy
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Contact BSAC
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
New trends in the epidemiology of community-acquired pneumonia (cap), recent changes in the microbial aetiology of cap, risk factors and clinical presentation, cap versus healthcare-associated pneumonia: a blurring distinction and term in disuse, novel therapeutics for cap, transparency declarations.
- < Previous
Community-acquired pneumonia: a US perspective on the guideline gap
- Article contents
- Figures & tables
- Supplementary Data
Maricar Malinis, Lilian Abbo, Jose A Vazquez, Luis Ostrosky-Zeichner, Community-acquired pneumonia: a US perspective on the guideline gap, Journal of Antimicrobial Chemotherapy , Volume 79, Issue 5, May 2024, Pages 959–961, https://doi.org/10.1093/jac/dkae050
- Permissions Icon Permissions
Community-acquired pneumonia continues to be one of the most common causes of morbidity and mortality due to infectious disease. The aetiologies, clinical presentations, diagnostic modalities and therapeutic options are changing and outpacing the creation of management guidelines. This educational article summarizes a roundtable activity sponsored by an unrestricted educational grant by Paratek that included US experts discussing these changes and identifying gaps in the current guidelines.
CAP continues to be one of the most common causes of morbidity and mortality due to infectious disease in all patients. Despite the COVID-19 pandemic, it continues to be a neglected, but common infection. Interestingly, there is a lack of urgency and disease awareness when one discusses CAP, perhaps because of its commonly used term ‘walking pneumonia’, not realizing that the mortality associated with CAP remains between 10% and 20%. 1 According to the American Thoracic Society (ATS)/IDSA guidelines from 2019, CAP continues to be a growing health problem globally, with an annual global burden of approximately 350 million cases in 2016. 2 In the USA, there are over 1 million hospitalizations due to CAP per year, not including those associated with SARS-CoV-2. 3
There are several challenges that remain when discussing the evolving epidemiology of CAP in the USA. The most important of these is being the increase in the number of pathogens that are now routinely diagnosed in patients with CAP. This is especially true for the viral causes of pneumonia such as SARS-CoV-2, respiratory syncytial virus and human metapneumovirus. In addition, certain fungal infections such as Histoplasma capsulatum and Coccidioides immitis are also increasing in frequency as causes of non-bacterial CAP in endemic areas. Finally, it is important to point out that in the past 12–24 months there has been an increase in Mycobacterium tuberculosis , especially in the southwest part of the USA. This is possibly due to the large migration of individuals. 4
Overall, bacterial and viral organisms constitute the most common causes of CAP. Pathogens such as Streptococcus pneumoniae and Haemophilus influenzae continue to be the most common bacterial pathogens recovered from sputum and occasionally, the bloodstream. However, more recently we are seeing an increase in CAP due to Staphylococcus aureus and Klebsiella pneumoniae . In addition, the intracellular pathogens or ‘atypical pneumonias’ frequently constitute up to 30% of causative organisms in large clinical trials. Among these, Mycoplasma pneumoniae and Chlamydophila pneumoniae continue to be the most common. 1 , 4 , 5 Among the viral pathogens, influenza virus, rhinovirus and SARS-CoV-2 are the most common organisms detected in the USA. 4
It is also important to point out that despite all of the advances that have been made in diagnostics over the past decade, only 30%–40% of patients who are admitted with CAP have a microbiological diagnosis. This leads to empirical treatment of CAP in the majority of situations.
Established risk factors for CAP include older age, chronic comorbidities, viral infection, impaired airway protection (i.e. increased risk for microaspiration), smoking and environmental exposure (water, animal, toxin exposures). 4 , 6 Over the years, novel risk factors for CAP have emerged, and these mainly involve the use of immunosuppressive therapies in the treatment of chronic diseases. For example, anti-TNF antibodies, ibrutinib and B-cell depleting agents increase the risk for infections due to non-tuberculous mycobacteria, Aspergillus spp. and respiratory viruses, respectively. 7–9 Globalization and immigration play a considerable role in the transmission of respiratory infections. In the last 3 years, we have witnessed a novel virus (SARS-CoV-2) emerging, and certain host factors predispose to severe disease, such as older age, high BMI, socioeconomic background, race, and sex.
The clinical presentation of CAP can range from mild disease characterized by fever, cough and dyspnoea to severe disease characterized by sepsis and respiratory failure. Symptom severity highly depends on pathogen virulence, the burden of exposure, and the host’s immune response. Signs and symptoms of pneumonia may be subtle among patients with advanced age and/or impaired immune systems. Notably, chest radiographs may be poorly sensitive and not able to detect evidence of pneumonia in individuals with impaired immunity. Hence, other radiographical modalities, such as CT scan of the chest, may have improved sensitivity in demonstrating abnormal lung findings. It is essential to highlight that with COVID-19, atypical presentations such as anosmia, dysgeusia, dermatological findings (maculopapular rash, vesicular eruptions, reddish nodules), conjunctivitis, gastrointestinal symptoms and thromboembolic complications have been reported.
Per the definition in the 2005 ATS/IDSA guideline, healthcare-associated pneumonia (HCAP) is a pneumonia in non-hospitalized patients with significant healthcare system experience that predisposes an individual to increased risk for MDR organism (MDRO) infection. 10 These risk factors include prior hospitalization for at least 2 days in the preceding 90 days, residence in a nursing home or extended-care facility, interventions such as home infusion therapy, chronic dialysis, home wound care and exposure to family members with MDRO infection. The landmark study of Kollef et al. 11 that defined HCAP involved a large US inpatient database demonstrating higher rates of MDRO (mainly MRSA, Pseudomonas and Enterobacter ) amongst patients meeting the HCAP criteria of the study and was associated with increased mortality. However, since then, several studies have evaluated the validity of that report. The study by Chalmers et al. 12 found increased mortality among patients meeting the criteria for HCAP but associated it with the patient’s comorbidities rather than the presence of an MDRO. A meta-analysis and systematic review, 13 which included 24 prospective and retrospective studies, found that guideline-defined HCAP did not accurately identify resistant pathogens, nor did it determine mortality risk with the presence of an MDRO. The accompanying editorial commented that HCAP criteria may not accurately identify risk for MDROs due to the diversity of pathogens across the healthcare system and the different antibiotic policies from country to country. 14 Finally, a Veterans Affairs (VA)-based study also reported that guideline-concordant therapy in non-severe HCAP patients did not improve overall survival. 15
The 2019 guideline did comment on possibly ‘moving away’ from the HCAP definition and its management as it has caused the overuse of broad-spectrum antibiotics. 2 Therefore the term HCAP is broadly now in disuse and the preferred approach is individualized risk for MDROs based on local epidemiology. An effort to perform an appropriate collection of respiratory cultures (and/or blood cultures) at the time of diagnosis of pneumonia (i.e. within 48–72 h) will improve the yield of cultures. Newer studies involving multiplex PCR can expedite diagnosis. Appropriate modification of antibiotics based on microbiological data can help reduce prolonged broad-spectrum antibiotic exposure.
Adjuvant therapy has been generally relegated to supportive therapy, but a recent breakthrough study found that adding hydrocortisone to antibiotics in the setting of severe CAP was associated with decreased risk of mortality by Day 28, therefore this intervention is likely to become standard of care. 16
Omadacycline is a tetracycline antibiotic and was approved in 2018 for treatment of bacterial CAP (CABP). It overcomes the resistance by tetracycline efflux and ribosomal protection mechanisms and has activity against Legionella pneumophila , M. pneumoniae and C. pneumoniae . Thus, it can be used as a single agent to treat CABP as an alternative to the empirical combination of a β-lactam and a macrolide. The efficacy and safety of omadacycline were tested on a Phase III randomized control trial (OPTIC) comparing omadacycline in 388 patients with moxifloxacin in 386 patients with CABP followed by oral omadacycline or moxifloxacin. The early clinical response was 81.1% in the omadacycline group compared with 82.7% in the comparator group. In the post-treatment evaluation, clinical response rate was 87.6% in the omadacycline arm compared with 85.1% in the moxifloxacin arm. The rate of adverse events leading to treatment discontinuation was 5.5% with omadacycline compared with 7% with moxifloxacin. 17 , 18
Lefamulin is a novel pleuromutilin antibiotic and was approved in August 2019 by the US FDA for use in CABP. Lefamulin inhibits protein synthesis by inhibition of the 50S bacterial ribosome. It has activity against S. pneumoniae , MRSA, VRE, MDR Neisseria gonorrhoeae , C. pneumoniae , L. pneumophila , M. pneumonia e and H. influenzae . Lefamulin exhibits time-dependent killing, with higher concentrations in epithelial lining fluid than in plasma. Lefamulin was non-inferior to moxifloxacin in 551 adults with CABP in a Phase III (LEAP-1) clinical trial. Early clinical response was 87.3% versus 90.2%. The rate of drug discontinuation was 2.9% in the lefamulin arm and 4.4% in the moxifloxacin arm. In the second Phase III clinical trial (LEAP-2), oral lefamulin was compared with moxifloxacin in 738 patients with CABP Lefamulin was non-inferior to moxifloxacin for CABP (90.8% versus 90.8%), clinical response (87.5% versus 89.1%) and clinically evaluable population (89.7% versus 93.6%). 17 , 18
Cefiderocol is the first in a class of siderophore cephalosporins with activity against ESBL- and carbapenemase-producing Gram-negative bacteria (carbapenem-resistant Enterobacterales, carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumannii ), MDR Stenotrophomonas maltophilia and Burkholderia cepacia . Potential indications include complicated urinary tract infection, healthcare-associated pneumonia/ventilator-associated pneumonia, bloodstream infection and sepsis caused by MDR Gram-negative isolates. It is a potent broad-spectrum agent that is not routinely recommended for first-line CAP treatment unless there is clinical failure to first-line therapy, in addition to risk factors for MDROs. 17 , 18
Solithromycin is a novel, fourth-generation macrolide, known as a fluoroketolide, which inhibits protein synthesis by binding to the bacterial ribosome. 19 It has activity against macrolide-resistant S. pneumoniae , H. influenzae and atypical pathogens, with potential indication for use in CABP. Oral solithromycin was non-inferior to oral moxifloxacin in 860 adults with CABP in a Phase III trial (SOLITAIRE-ORAL). Early clinical response was 78.2% versus 77.9%. Elevation of ALT was observed in 5.4% of the solithromycin group compared with 3.3% in the moxifloxacin group, and AST was elevated in 2.5% of the solithromycin group compared with 1.9% in the comparator group. Solithromycin (IV to oral) was non-inferior to moxifloxacin (IV to oral) in 863 adults with CABP in a Phase III clinical trial (SOLITAIRE-IV). The early clinical response in ITT was 79.3% in the solithromycin arm compared with 79.7% in the moxifloxacin arm, with a higher rate of adverse drug reactions in the solithromycin arm. This agent exhibits activity against macrolide-resistant strains of S. pneumoniae ; however, it is not FDA approved for this use.
The video is playable in the HTML version at https://doi.org/10.1093/jac/dkae050 .
This Virtual Roundtable was supported by an unrestricted educational grant from Paratek Pharmaceuticals, Inc.
Relevant to this manuscript: M.M. reports nothing to declare; L.A. reports consulting for ViiV, Shionogi, Ferring, La Jolla Pharmaceuticals, bioMérieux, Pfizer, and being of the board of director for the Infectious Diseases Society of America; J.A.V. reports consulting for Cidara, Melinta, AbbVie, Insmed and Vedanta, and speaking for AbbVie and Melinta; L.O.-Z. reports consulting for GSK, Melinta, Pfizer, Gilead and Viracor.
Alibert S , Dela Cruz CS , Amati F et al. Community acquired pneumonia . Lancet 2021 ; 398 : 906 – 19 . https://doi.org/10.1016/S0140-6736(21)00630-9
Google Scholar
Metlay JP , Waterer GW , Long AC et al. Diagnosis and treatment of adults with community-acquired pneumonia . Am J Respir Crit Care Med 2019 ; 200 : e45 – 67 . https://doi.org/10.1164/rccm.201908-1581ST
Divino V , Schranz J , Early M et al. The annual economic burden among patients hospitalized for community-acquired pneumonia (CAP): a retrospective US cohort study . Curr Med Res Opin 2020 ; 36 : 151 – 60 . https://doi.org/10.1080/03007995.2019.1675149
Ramirez JA , Wiemken TL , Peyrani P et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality . Clin Infect Dis 2017 ; 65 : 1806 – 12 . https://doi.org/10.1093/cid/cix647
Cilloniz C , Torres A , Niederman M et al. Community acquired pneumonia related to intracellular pathogens . Intensive Care Med 2016 ; 42 : 1374 – 86 . https://doi.org/10.1007/s00134-016-4394-4
Torres A , Peetermans WE , Viegi G et al. Risk factors for community-acquired pneumonia in adults in Europe: a literature review . Thorax 2013 ; 68 : 1057 – 65 . https://doi.org/10.1136/thoraxjnl-2013-204282
Winthrop KL , Chang E , Yamashita S et al. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-α therapy . Emerg Infect Dis 2009 ; 15 : 1556 – 61 . https://doi.org/10.3201/eid1510.090310
Bercusson A , Colley T , Shah A et al. Ibrutinib blocks Btk-dependent NF-ĸB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis . Blood 2018 ; 132 : 1985 – 8 . https://doi.org/10.1182/blood-2017-12-823393
Dib RW , Ariza-Heredia E , Spallone A et al. Respiratory viral infections in recipients of cellular therapies: a review of incidence, outcomes, treatment, and prevention . Open Forum Infect Dis 2023 ; 10 : ofad166 . https://doi.org/10.1093/ofid/ofad166
American Thoracic Society ; IDSA . Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia . Am J Respir Crit Care Med 2005 ; 171 : 388 – 416 . https://doi.org/10.1164/rccm.200405-644ST
Kollef MH , Shorr A , Tabak YP et al. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia . Chest 2005 ; 128 : 3854 – 62 . https://doi.org/10.1378/chest.128.6.3854
Chalmers JD , Taylor JK , Singanayagam A et al. Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study . Clin Infect Dis 2011 ; 15 : 107 – 13 . https://doi.org/10.1093/cid/cir274
Chalmers JD , Rother C , Salih W et al. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis . Clin Infect Dis 2014 ; 58 : 330 – 9 . https://doi.org/10.1093/cid/cit734
Restrepo MI , Aliberti S . Healthcare-associated pneumonia: where do we go next? Clin Infect Dis 2014 ; 58 : 340 – 1 . https://doi.org/10.1093/cid/cit738
Attridge RT , Frei CR , Restrepo MI et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia . Eur Respir J 2011 ; 38 : 878 – 87 . https://doi.org/10.1183/09031936.00141110
Dequin PF , Meziani F , Quenot JP et al. Hydrocortisone in severe community-acquired pneumonia . N Engl J Med 2023 ; 388 : 1931 – 41 . https://doi.org/10.1056/NEJMoa2215145
Nair GB , Niederman MS . Updates on community acquired pneumonia management in the ICU . Pharmacol Ther 2021 ; 217 : 107663 . https://doi.org/10.1016/j.pharmthera.2020.107663
Al-Tawfiq JA , Momattin H , Al-Ali AY et al. Antibiotics in the pipeline: a literature review (2017–2020) . Infection 2022 ; 50 : 553 – 64 . https://doi.org/10.1007/s15010-021-01709-3
File TM , Rewerska B , Vucinic-Mihalovic V et al. SOLITAIRE-IV: a randomized, double-blind, multicenter study comparing the efficacy and safety of intravenous-to-oral solithromycin to intravenous-to-oral moxifloxacin for treatment of community-acquired bacterial pneumonia . Clin Infect Dis 2016 ; 63 : 1007 – 16 . https://doi.org/10.1093/cid/ciw490
- community acquired pneumonia
- communicable diseases
- knowledge acquisition
| Month: | Total Views: |
|---|---|
| May 2024 | 4,722 |
| June 2024 | 286 |
Email alerts
Citing articles via.
- About Journal of Antimicrobial Chemotherapy
- Recommend to your Library
Affiliations
- Online ISSN 1460-2091
- Print ISSN 0305-7453
- Copyright © 2024 British Society for Antimicrobial Chemotherapy
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Conversations with Leaders
- Advanced search

Advanced Search
Community-acquired pneumonia: Strategies for triage and treatment
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected]
- Figures & Data
- Info & Metrics
Community-acquired pneumonia significantly contributes to patient morbidity and healthcare costs. As our understanding of this common infection grows, collaborative efforts among researchers and clinical societies provide new literature and updated guidelines informing its management. This review discusses diagnostic methods, empiric treatment, and infection prevention strategies for patients with suspected community-acquired pneumonia.
Systematically stratifying patients with suspected community-acquired pneumonia based on mortality risk can aid in designating the safest level of care for each patient.
Empiric treatment should be informed by the local antibiogram (ie, local patterns of antibiotic resistance) with multidrug-resistant organism coverage added based on individual patient and institutional risk factors.
Prompt de-escalation to targeted antimicrobial therapy, guided by diagnostic testing, can reduce antibiotic resistance and antibiotic-related adverse drug reactions.
Appropriate clinical and radiographic follow-up after antibiotic course completion to assess for treatment failure is a subject of ongoing debate.
While physicians have treated pneumonia for centuries, each stage of the clinical decision-making process still poses challenges, from determining the most appropriate setting of care for a patient with suspected pneumonia to planning follow-up after antibiotic completion. Over the years, physicians have witnessed the advent of new medical and respiratory therapies as well as the development of antibiotic resistance in the management of this common infection.
Inpatients with pneumonia fall into 2 categories: those with community-acquired pneumonia (CAP) who are admitted, and those who develop either hospital-acquired or ventilator-associated pneumonia while already hospitalized. Each patient population faces unique organism exposures, and thus, recommended diagnostic tests, empiric treatment regimens, and goals for infection prevention vary.
This article reviews guidelines by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) 1 and interprets recent studies to address questions that arise specifically in the inpatient management of CAP.
- COMMON AND COSTLY
CAP is a significant health concern, with one study reporting 915,500 episodes in adults at least 65 years of age in the United States every year, and medical costs associated with CAP exceeding $10 billion in 2011. 2 , 3
The National Center for Health Statistics reported 1.7 million visits to emergency departments in the United States in 2017 in which pneumonia was the primary discharge diagnosis, and listed pneumonia as the cause of death for 49,157 people in 2017. 4
- RISK-STRATIFICATION OF COMMUNITY-ACQUIRED PNEUMONIA
The IDSA/ATS 2019 guidelines 1 emphasize the importance of first determining what level of patient care is needed: Is outpatient treatment appropriate, or does the patient need to be admitted to the hospital, or even to the intensive care unit? Appropriate triage can prevent stresses on the patient and the healthcare system associated with under- or overestimating illness severity. Patients at high risk of death whose acuity is not fully appreciated face inadequate support, while those admitted despite low risk of death may be unnecessarily subjected to the risks of the hospital setting, such as infections from healthcare-associated multidrug-resistant organisms.
Risk calculators are routinely used to help physicians triage their patients in everyday practice, although they have not been specifically validated to predict the need for admission.
CURB-65 is a simple calculator based on 5 risk factors first identified in 1987 ( Table 1 ). 1 Patients receive 1 point each for c onfusion, high blood u rea nitrogen, high r espiratory rate, low b lood pressure, and age 65 or older; the higher the total score, the higher the 30-day mortality risk. According to the IDSA/ATS, patients with scores of 0 or 1 can be managed as outpatients, those with scores of 2 should be admitted to the hospital, and those with scores of 3, 4, or 5 need care in the intensive care unit.
- View inline
The CURB-65 calculator
An abbreviated version of this calculator, CRB-65, allows risk-stratification of outpatients without laboratory work. 1
The Pneumonia Severity Index incorporates 20 risk factors to place patients into 5 classes correlated with mortality risk ( Table 2 ). 5 The authors suggest outpatient management for those in classes I or II and inpatient management for those in risk classes IV and V. Patients in class III may be safely treated in an outpatient setting with adequate support or in an inpatient observation unit.
Pneumonia Severity Index calculator and associated risk classes
While CURB-65 may be better in busy clinical settings, as it is a shorter risk stratification scale for CAP, the Pneumonia Severity Index is preferred by the IDSA/ATS 2019 guidelines as it has been more extensively studied and validated. 1
The IDSA/ATS guidelines list a separate set of major and minor criteria to define “severe pneumonia” to determine which patients with suspected CAP merit intensive care. 1 At least 1 of the major criteria or at least 3 of the minor criteria are required for the diagnosis of severe pneumonia ( Table 3 ).
Severe pneumonia: Infectious Diseases Society of America and American Thoracic Society criteria
The Pneumonia Patient Outcomes Research Team study , a multicenter, prospective controlled study of both ambulatory and hospitalized patients with CAP, also devised a list of risk factors associated with death within 30 days. 6 These risk factors include altered mental status, uremia, leukopenia, and hypoxemia. Chronic liver failure was a risk factor highlighted in this study but was not included in the IDSA/ATS criteria.
Yet none of these scoring systems can fully capture all medical or psychosocial comorbidities that may prevent successful recovery in the outpatient setting. A retrospective chart review of more than 1,800 patients found that 45% of patients who had “low-risk” CAP by the Pneumonia Severity Index were nevertheless admitted. 7 Patients with cognitive impairment, coronary artery disease, diabetes mellitus, pulmonary disease, multilobular radiographic opacities, home oxygen therapy, corticosteroid use, or use of antibiotics prior to presentation had increased odds of hospitalization.
Clinical judgment should be applied to the results of any of these calculators to appropriately triage patients with pneumonia.
- DIAGNOSIS OF COMMUNITY-ACQUIRED PNEUMONIA
After triaging a patient with suspected CAP to the safest level of care, several radiographic and laboratory methods can be used to verify the diagnosis and identify the organism most likely responsible for the ongoing infection. Chest radiographs with demonstrable infiltrates are required to diagnose CAP and to distinguish it from upper respiratory tract infection. 1
Different organisms can be associated with characteristic infiltrate patterns, which often manifest within 12 hours of symptom onset:
Focal nonsegmental or lobar pneumonia ( Figure 1 ). Typical bacterial pneumonias caused by organisms such as Streptococcus pneumoniae tend to manifest with an airspace opacity in 1 segment or lobe, though antibiotic use can alter their pathophysiology to create a patchy, multilobular opacity pattern.
- Download figure
- Open in new tab
- Download powerpoint
Focal lobar pneumonia.
Multifocal bronchopneumonia or lobar pneumonia. Bronchopneumonias, similarly characterized by a patchy pattern, are most commonly caused by Staphylococcus aureus , Haemophilus influenzae , and fungi. 8
Focal or diffuse “interstitial” pneumonia ( Figure 2 ). Atypical bacterial organisms including Legionella pneumophila , Mycoplasma pneumoniae , and Chlamydophila pneumoniae frequently involve the lung bases in a diffuse, bilateral, reticulonodular pattern, but can start as isolated lobar opacities on chest radiography. 9 Viral organisms are associated with diffuse, bilateral lung involvement as well.
Diffuse interstitial pneumonia.
Early radiographic identification of pulmonary complications, such as pleural effusions or cavitating lesions, can provide more clues to the causative organism and allow for timely intervention. 9

How accurate is chest radiography?
The utility of chest radiographs in diagnosing CAP is ultimately subject to interobserver variability, with some studies citing 65% ac curacy in diagnosing viral pneumonia, 67% in diagnosing bacterial pneumonia, and no statistical reliability for differentiating bacterial from nonbacterial pneumonias. 10 A Swedish retrospective chart review of 103 outpatients with suspected CAP noted that just 88% of patients with high clinical concern for CAP demonstrated radiographic evidence of infection. 11
Microbiology
A thorough social history should be gathered for every patient with suspected CAP to screen for potential occupational, travel, or endemic exposures. This will guide microbiologic testing and empiric antibiotic treatment. 1 For example, patients presenting during flu season or with known exposures to poultry in areas of prior influenza outbreaks should be screened for influenza A and B with a nasopharyngeal swab.
Isolating a specific organism in outpatients with CAP may not be necessary but is recommended to guide de-escalation of empiric antibiotic regimens. 1 Pretreatment Gram stain and culture in patients able to adequately expectorate a good-quality specimen or endotracheal aspirate in intubated patients should be collected. Patients fulfilling criteria for severe pneumonia as defined by the IDSA/ATS guidelines merit blood and sputum cultures as well as urinary antigen tests for L pneumophila and S pneumoniae ( Table 4 ). 1
Indications for blood culture testing in suspected community-acquired pneumonia
Overall, active surveillance of more than 2,200 patients with CAP requiring hospitalization noted that 38% of blood and sputum cultures, nasopharyngeal and oropharyngeal swabs, and urinary antigens yielded a causative organism. 12 Viral organisms accounted for 25% of these cases and bacterial organisms accounted for 14%; 5% of patients with viral pneumonias were coinfected with either another respiratory virus or a bacterial organism.
Procalcitonin testing
Procalcitonin testing can help differentiate viral from bacterial pathogens in patients admitted for CAP, preventing the use of unnecessary antibiotics and allowing prompt de-escalation of empiric therapy more effectively than clinical judgment alone. 13 While any infectious pneumonia can precipitate elevations of this serum biomarker, typical bacteria tend to result in higher procalcitonin levels than atypical bacteria or viruses. 14 Cytokines, associated with bacterial infections, enhance procalcitonin release, while interferons, associated with viral infections, inhibit procalcitonin release. This biomarker is not perfect, however, and will not be elevated in up to 23% of typical bacterial infections. 14
For this reason, procalcitonin should not replace clinical judgment in guiding the decision to initiate antimicrobial therapy for patients with suspected CAP but can be used in conjunction with clinical judgment to de-escalate therapy. In patients whose clinical histories suggest alternative causes of respiratory distress or improvement with concomitantly administered therapies such as diuresis, a negative procalcitonin can help guide cessation of antibiotics. On the other hand, in patients with polymerase chain reaction-proven influenza, an elevated procalcitonin can suggest continuation of antibiotics to treat bacterial superinfection.
- MANAGEMENT OF COMMUNITY-ACQUIRED PNEUMONIA
Antibiotic therapy
The selection of antibiotics before a causative pathogen is identified should be informed by the patient’s risk factors and degree of illness ( Table 5 , Table 6 ). 1
Common organisms in community-acquired pneumonia
Initial antibiotic therapy for community-acquired pneumonia
Patients on a medical floor should be started on either a respiratory fluoroquinolone or a combination of a beta-lactam plus a macrolide; intensive care patients should receive a beta-lactam plus either a macrolide or a respiratory fluoroquinolone. Doxycycline can be used as an alternative to the macrolide or respiratory fluoroquinolone to cover atypical organisms such as Chlamydia pneumoniae, Legionella pneumophila, and Mycoplasma pneumoniae in patients with prolonged QTc. In penicillin-allergic patients, aztreonam should be used in combination with an aminoglycoside and a respiratory fluoroquinolone.
Patients who may have been exposed to influenza or who have a history of injection drug use or structural lung disease or who have a lung abscess, cavitary infiltrates, or endobronchial obstruction also merit coverage against community-acquired methicillin-resistant S aureus (MRSA) with vancomycin or linezolid. Those with confirmed or suspected influenza A presenting within 48 hours of symptom onset or with severe illness should be treated with oseltamivir. 1
If an organism is identified by culture, polymerase chain reaction, or serology, the empiric antibiotic regimen should be tailored to this organism. MRSA nares screening can be reliably used to guide empiric and targeted antimicrobial regimens; patients started on vancomycin or linezolid based on the above-stated risk factors can be safely de-escalated on the basis of a negative nasal swab. 15 The pneumococcal urinary antigen has a similarly reliable negative predictive value and can also be used to de-escalate empiric antimicrobial therapy. 16
Should microbiologic evaluation fail to identify a causative organism, the patient’s individual risk factors as listed above must be considered in de-escalating therapy to a final regimen with coverage for MRSA, Pseudomonas aeruginosa , or atypical pathogens as indicated. Pseudomonal pneumonia has been associated with higher risk of mortality and relapse than pneumonia caused by other pathogens.
Corticosteroids as adjunctive therapy
The use of adjunctive corticosteroids for CAP management has been widely contested. The IDSA/ATS guidelines recommend against corticosteroid use for adjunctive treatment of CAP except in patients with refractory septic shock. 1
Later management
Patients who are hemodynamically stable, can ingest medications safely, and have a normal gastrointestinal tract can be discharged on oral therapy without waiting to observe the clinical response. Antibiotics should be given for at least 5 days, though longer durations may be needed in immunocompromised patients or in those with pulmonary or extra-pulmonary complications. 1
An infectious disease consultation may be beneficial if long-term intravenous antibiotic therapy is anticipated or if the patient progressively deteriorates on guideline-based antimicrobial therapy.
Pulmonary consultation may be needed for bronchoscopy to obtain deep respiratory samples, especially if the patient is clinically worsening and the causative pathogen remains unidentified. We acknowledge that the yield of bronchoscopy and bronchoalveolar lavage samples is reduced with longer durations of antibiotic therapy, yet believe that in the context of clinical worsening in spite of antibiotics, bronchoalveolar lavage may help successfully identify multidrug-resistant or atypical pathogens which may not be covered by the ongoing antibiotic regimen. Pulmonology consultation is also indicated for patients with complications of pneumonia such as empyema that require procedural intervention.
- TAKE-HOME POINTS
CAP continues to contribute to patient morbidity and mortality as well as healthcare costs.
Professional societies have released collaborative guidelines to streamline practice patterns and provide evidence-based protocols for the diagnosis, treatment, and prevention of this common infection.
Further research is needed to delineate appropriate strategies to de-escalate antibiotics in the absence of a causative organism, define the dose and duration of adjunctive steroid use, and clarify patient follow-up after discharge from the hospital.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.
- Metlay JP ,
- Waterer GW ,
- Long AC , et al
- Jackson ML ,
- Neuzil KM ,
- Thompson WW , et al
- Pfuntner A ,
- Centers for Disease Control and Prevention (CDC)
- Yealy DM , et al
- Mortensen EM ,
- Singer DE , et al
- Labarere J ,
- Scott Obrosky D , et al
- Domingo ML ,
- Moberg AB ,
- Fransson SG ,
- Wunderink RG , et al ; CDC EPIC Study Team
- Schuetz P ,
- Christ-Crain M ,
- Thomann R , et al ; ProHOSP Study Group
- Grijalva CG , et al
- Parente DM ,
- Mylonakis E ,
- Timbrook TT
- McCauley LM ,
- Sorensen JS ,
- Jephson AR ,
In this issue

- Table of Contents
- Table of Contents (PDF)
- Index by author
- Complete Issue (PDF)
Thank you for your interest in spreading the word on Cleveland Clinic Journal of Medicine.
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Linkedin Share Button
Jump to section
Related articles.
- Google Scholar
Cited By...
- An unending ode to pneumonia
More in this TOC Section
- Diabetes technology: A primer for clinicians
- Preexposure prophylaxis for preventing HIV infection: Routine practice in primary care
- Gastroparesis for the nongastroenterologist
Similar Articles
- Infectious Diseases
- Pulmonology
- Hospital Medicine
- Emergency Medicine
- Critical Care
- Open access
- Published: 29 May 2024
Highly diverse sputum microbiota correlates with the disease severity in patients with community-acquired pneumonia: a longitudinal cohort study
- Jing Yang 1 , 2 , 3 na1 ,
- Jinman Li 4 na1 ,
- Linfeng Zhang 1 , 2 na1 ,
- Zijie Shen 1 , 2 ,
- Yan Xiao 4 , 5 , 6 ,
- Guoliang Zhang 7 ,
- Mingwei Chen 8 ,
- Fuhui Chen 9 ,
- Ling Liu 10 ,
- Ying Wang 4 ,
- Lan Chen 4 , 5 ,
- Xinming Wang 4 , 5 ,
- Li Zhang 1 ,
- Lu Wang 1 ,
- Zhang Wang 11 ,
- Jianwei Wang 4 , 5 na2 ,
- Mingkun Li 1 , 2 na2 &
- Lili Ren 4 , 5 , 6 na2
Respiratory Research volume 25 , Article number: 223 ( 2024 ) Cite this article
274 Accesses
Metrics details
Community-acquired pneumonia (CAP) is a common and serious condition that can be caused by a variety of pathogens. However, much remains unknown about how these pathogens interact with the lower respiratory commensals, and whether any correlation exists between the dysbiosis of the lower respiratory microbiota and disease severity and prognosis.
We conducted a retrospective cohort study to investigate the composition and dynamics of sputum microbiota in patients diagnosed with CAP. In total, 917 sputum specimens were collected consecutively from 350 CAP inpatients enrolled in six hospitals following admission. The V3-V4 region of the 16 S rRNA gene was then sequenced.
The sputum microbiota in 71% of the samples were predominately composed of respiratory commensals. Conversely, 15% of the samples demonstrated dominance by five opportunistic pathogens. Additionally, 5% of the samples exhibited sterility, resembling the composition of negative controls. Compared to non-severe CAP patients, severe cases exhibited a more disrupted sputum microbiota, characterized by the highly dominant presence of potential pathogens, greater deviation from a healthy state, more significant alterations during hospitalization, and sparser bacterial interactions. The sputum microbiota on admission demonstrated a moderate prediction of disease severity (AUC = 0.74). Furthermore, different pathogenic infections were associated with specific microbiota alterations. Acinetobacter and Pseudomonas were more abundant in influenza A infections, with Acinetobacter was also enriched in Klebsiella pneumoniae infections.
Collectively, our study demonstrated that pneumonia may not consistently correlate with severe dysbiosis of the respiratory microbiota. Instead, the degree of microbiota dysbiosis was correlated with disease severity in CAP patients.
Introduction
Community-acquired pneumonia (CAP) is an acute respiratory infection acquired outside the hospital, affecting alveoli and distal airways, with variable symptoms including cough, fever, dyspnea, and expectoration [ 1 ]. The incidence of lower respiratory tract infection (LRI), which includes CAP, was 5,837 cases and 6,832 cases per 100,000 population among females and males, respectively [ 2 ]. It resulted in high morbidity and mortality rates in all age groups, especially in children and the elderly [ 2 ]. LRI remained the fourth leading cause of global years of life lost in 2019 before the COVID-19 pandemic [ 3 ].
Recent culture-independent studies revealed that the respiratory tract was not sterile in healthy individuals [ 4 ], and the lower respiratory tract microbiota contributed to the ecological and immunological homeostasis of the lung, influencing lung health and susceptibility to infections [ 5 ]. Although pathogen invasion is considered the cause of CAP, the causative agents are detected in fewer than 50% of CAP patients [ 4 ]. Studies have identified significant differences in the respiratory microbiota between CAP patients and healthy individuals, with the former being less diverse and enriched with pathogenic microbes such as Pseudomonas , Staphylococcus , and Klebsiella [ 6 , 7 , 8 , 9 ]. Additionally, the respiratory microbiota may influence pneumonia susceptibility via impeding colonization and immunological modulation [ 10 , 11 ]. However, previous studies primarily focused on high-risk populations, such as human immunodeficiency virus (HIV) patients, lung transplant recipients, and children, and often with a small size of patients [ 6 , 7 , 8 ]. The association between respiratory microbiota and CAP in immunocompetent adults remains unclear. The interpretation is further complicated by diverse pathogens, the use of antibiotics, intubation, and corticosteroid therapies in CAP patients. Therefore, a comprehensive microbiota study in the general population, especially those untreated, is needed.
The respiratory microbiota is heterogeneous due to various host and environmental factors, including genetic background, mode of birth, feeding type, and inhaled pollutants [ 12 , 13 , 14 ]. Thus, clarifying the role of a specific variable in shaping the respiratory microbiota is challenging in cross-sectional studies. In contrast, longitudinal studies can pinpoint particular microbiota changes associated with a specific condition by controlling other covariates. Although longitudinal studies have been conducted on lung transplantation, chronic obstructive pulmonary disease (COPD), cystic fibrosis, COVID-19, and ventilator-associated pneumonia [ 15 , 16 , 17 , 18 , 19 ], studies on the lower respiratory microbiota in CAP patients are limited. Studying microbiota changes during the disease process will provide insights into the role of the respiratory microbiota in disease development.
In this study, we collected time-series sputum samples from CAP patients starting from the first day after admission, prior to therapy administration. We identified a correlation between the composition and dynamics of the sputum microbiota and disease severity, revealing distinct microbiota compositions in patients with different pathogens. This suggests that the dysbiosis of the sputum microbiota could potentially serve as a valuable diagnostic and prognostic marker for pneumonia. Furthermore, it presents a possible target for intervention in the management of the condition.
Overview of the samples and sequencing data
Longitudinal sputum samples (1,065) were collected from 367 inpatients diagnosed with CAP in six hospitals across representative geographical locations in China. Following quality filtering, 917 samples from 350 patients and 25 negative controls (NCs) were used for subsequent analysis (Fig. 1 A-C). The composition of sputum microbiota of CAP patients was notably different from that of NCs (PERMANOVA, R 2 = 0.2, p = 0.001, Fig. S1 A), and the five most abundant taxa in NCs ( Sphingomonas , Blastomonas , Methylobacterium , Bosea , and Propionibacterium , Fig. S1 B) comprised 53.1% of all NCs sequence, while comprising 2.7% of all sequence in CAP samples, indicating minimal background contamination.
The median days from symptoms onset to admission were six (IQR 3–7). Forty-one (12.0%) patients had chronic pulmonary diseases, including COPD, asthma, and bronchiectasis. Before admission, 66 (19.5%) patients took antibiotics within five days, and 15 (5.5%) patients used immunosuppressants. Fifty-five (16.3%) patients were diagnosed with severe cases and seven of them died. Notably, five clinical severity indicators, including the use of invasive mechanical ventilation, CURB65 scores, pneumonia severity index (PSI) scores, duration of oxygen supplementation, and length of hospital stay, were all significantly higher in severe cases than in non-severe cases (Fisher’s exact test or Wilcoxon signed-rank test, p < 0.05). More demographic and clinical information was provided in Table 1 and Table S1 . Meanwhile, 876 sputum microbiotas in Chinese healthy individuals (with no acute or chronic respiratory diseases) from three previous studies were used as the healthy controls (HCs) in the study (Table S2 and S3 ) [ 20 , 21 , 22 ].
Study design and sputum microbiota composition. ( A ) Geographic distribution of the samples. n: sample size. ( B ) Sampling strategy. d: days after admission. ( C ) Summary of the collected samples. ( D ) Abundance of bacteria in CAP patients and negative controls. The top 15 bacteria with the highest average relative abundance in CAP patients are shown. Bacteria that are more enriched in CAP patients than in all three HCs are labeled in red, while those enriched in HCs are labeled in blue
Sputum microbiota composition in CAP patients
Six commensal microbes that are frequently observed in the respiratory tract, including Streptococcus , Veillonella , Neisseria , Prevotella , Rothia , and Haemophilus , showed the highest relative abundance and accounted for 51.2% of CAP microbial reads, 38.0% of HC microbial reads, and 1.4% in NCs (Fig. 1 D). The sputum microbiota diversity and composition in CAP patients were significantly different from HCs (PERMANOVA, mean R 2 = 0.13, p < 0.001; Fig. S1 A). Possible pathogens, including Pseudomonas , Enterobacteriaceae , Sphingomonas , and Stenotrophomonas , were significantly enriched in CAP samples compared to all three HC populations (Fig. S1 C).
The major component of sputum microbiotas in CAP patients showed significant heterogeneity among different individuals (Fig. 1 D). Employing clustering algorithms on the microbiota data revealed the presence of nine distinct clusters (CSs) (Fig. 2 A, Fig. S2 A). The robustness of these clusters was confirmed by bootstrap analysis (Fig. S2 B, mean Rand index = 0.85, see Supplementary methods). These CSs could be further classified into three microbiota types: CS2, CS3, and CS4 (CS2-4), which were found in 71.1% of CAP patients, exhibited higher alpha diversity than other clusters, except for CS6 (Fig. 2 B). CS2-4 were dominated by commensal bacteria (Fig. 2 A) and showed higher similarity to healthy controls (Fig. 2 C). In contrast, CS1, CS5, and CS7-9 (CS1,5,7,8,9) were dominated by possible pathogens. They had lower alpha diversity, higher dominant bacteria abundance, and were more distinct from HCs compared to CS2-4 (Fig. 2 B-C, Fig. S2 C). Microbiotas in CS6 exhibited the highest similarity to NCs and were more prevalent in specific individuals than randomly distributed (Fig. 2 D, Fig. S2 D-F), suggesting that the sputum samples of CS6 were either relatively sterile or challenging to collect. Additionally, CS6 did not appear to be detected more frequently in the later period of hospitalization, suggesting no association with post-admission treatment (Fig. S2 G). Notably, the severity rate (incidence of severe condition) in CS2-4 patients was 12.9%, similar to CS6 (4.7%, Fisher’s exact test, p = 0.213), but significantly lower than CS1,5,7,8,9 (36.4%, Fisher’s exact test, p < 0.001).
The composition of sputum microbiota clusters in CAP patients. ( A ) The compositions of bacteria in different clusters. Bacteria with average relative abundances greater than 5% in at least one cluster are shown. The sample numbers in each cluster and NCs were labeled above the figure. ( B ) Shannon index of each cluster and HCs. ( C ) JSD distance between different CAP clusters and healthy individuals. The microbiota composition of the three HC groups was averaged and used as the HC to calculate the distance. ( D ) JSD distance between different CAP clusters and NCs. In B and C, statistical significance was determined by comparing each cluster with all the HCs, and the color of the plot in B, C, and D denotes the proportion of severe cases in each CAP cluster. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
Association between sputum microbiota and disease severity
We then investigated the association between clinical and demographic features and the sputum microbiota. To minimize the impact of antibiotic use and other medical interventions after admission, only 238 samples collected on the first day after admission were used for the subsequent analyses. We found that disease severity (diagnosed by the clinician, see Methods), as well as five clinical indicators that correlated with disease severity, including CURB65 scores, PSI scores, duration of oxygen supplementation, length of hospital stay, and clinical outcome, were all significantly correlated with the sputum microbiota, after controlling for the possible confounders (Table 1 , confounders: variables 1–10). In individual geographic sites, the correlation with disease severity remained statistically significant in Wuhan, with the largest sample size ( n = 142, PERMANOVA, R 2 = 0.021, p < 0.05), suggesting that the correlation was not influenced by the differences in patient enrollment across various geographic locations. Notably, the use of antibiotics and immunosuppressive drugs before admission, as well as days from onset, showed no significant impact on the microbiota composition (Table 1 ). This might be attributed to the limited sample size in this study. Nevertheless, variables 1–10 in Table 1 were all included as covariates whenever applicable in subsequent multivariate analyses.
First, we found that the alpha diversity of sputum microbiotas in non-severe patients was less deviated from the three healthy cohorts compared to severe patients (Fig. 3 A). Moreover, the microbiotas in 53.8% of severe patients were dominated by bacteria with abundances greater than 50%, whereas the fraction was 22.0% and 0% in non-severe patients and healthy individuals, respectively (Fig. 3 B). Additionally, dominant bacteria with abundances greater than 50% comprised more possible pathogens, especially in severe patients (Fig. 3 C).
The composition of sputum microbiota differed considerably between severe and non-severe patients (multivariate PERMANOVA R 2 = 0.02, p < 0.05; Fig. 3 D), which were both distinct from healthy individuals (PERMANOVA, p < 0.001; Fig. S3 A). Microbiotas in severe patients were more disrupted relative to healthy individuals than those in non-severe patients (Fig. 3 E). LEfSe analysis revealed increased abundance of possible pathogens, including Enterobacteriaceae , Acinetobacter , and Enterococcus in severe cases, while commensal bacteria, including Haemophilus , Neisseria , and Prevotella were more abundant in non-severe cases ( p < 0.05, Fig. 3 F). MaAsLin2 analysis identified enrichment of Enterobacteriaceae in severe cases, and its abundance was also positively correlated with the duration of oxygen supplementation, while adjusting for covariates (Fig. S3 B). Additionally, a classifier utilizing the L1 regularized logistic regression model could distinguish the severe cases from non-severe cases using microbiota with moderate accuracy (AUC = 0.74; Fig. 3 G). Key features selected for identifying severe cases included high abundances of Enterobacteriaceae and Corynebacterium , along with a low abundance of Neisseria . Furthermore, the analysis of patients from individual cities confirmed the enrichment of Enterobacteriaceae in severe patients (in Wuhan), suggesting that the identified signature was not an artifact due to variations in the patients enrolled from different cities.
The functional potential of the sputum microbiota was predicted using PICRUSt analysis [ 23 ]. Five of the top 10 pathways enriched in the severe cases (MaAsLin2 analysis) were related to menaquinol biosynthesis (Fig. S3 C and D), with all five pathways contributed by Enterobacteriaceae . Menaquinones are involved in the post-translational modifications of proteins needed for blood coagulation [ 24 ], and their dysfunction has been proposed as a risk factor for the severity of CAP [ 25 , 26 ]. Meanwhile, four pathways involving the fermentation of butanoate, primarily contributed by Porphyromonas and Fusobacteria , were enriched in non-severe cases (Fig. S3 E). Butanoate has been shown to enhance T cell proliferation and activation while suppressing inflammatory reactions [ 27 , 28 ].
Difference in the sputum microbiota between CAP patients with varying degrees of severity and healthy individuals. ( A ) Shannon index of the microbiota of CAP patients on admission and healthy individuals. ( B ) Distribution of the abundance of the predominant bacterium in CAP patients and HCs. The y-axis indicates the proportion of patients with a dominating bacterium whose abundance is greater than that indicated on the x-axis. The number of patients with a dominating bacterium whose abundance is greater than 0, 25%, 50%, 75%, and 100% is shown below the x-axis. The p-value was calculated by log-rank test. ( C ) Proportion of dominant bacteria in CAP patients and HCs. Bacterial genera and families containing at least one known (opportunistic) pathogen, are highlighted with red boxes. The numbers in brackets on the x-axis indicate the number of samples. A list of pathogenic bacteria is provided in Table S4 . Samples with dominating bacterium abundance higher than 50% and lower than 50% were shown separately. ( D ) PCoA plot of samples from severe and non-severe patients based on the JSD distance. R 2 was calculated by PERMONAVA analysis. ( E ) JSD distance to healthy individuals of severe and non-severe CAP patients. The microbiota composition of the three HC groups was averaged and used as the HC to calculate the distance. ( F ) Bacteria correlated with the disease severity identified by LEfSe (LDA score > 4, p < 0.05). ( G ) ROC curve for the disease severity classifier based on the L1 regularized logistic regression model. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
Dynamics of the sputum microbiota and its association with the disease severity
We further investigated how microbiota dynamics varied between non-severe cases and severe cases. First, the alpha diversity of microbiota in non-severe cases was significantly higher than that in severe cases at the first three time points (Fig. 4 A), with no significant difference observed between different time points within the same group. Second, severe cases showed a larger longitudinal change in the microbiota composition (Fig. 4 B), becoming more deviated from the initial state during hospitalization (Fig. 4 C). Third, neither the severe nor the non-severe patients’ sputum microbiota altered toward a healthy state during hospitalization (Fig. S4 A).
Then, we explored the CS transition pattern between Day 1 and Day 5, which encompassed the largest number of sample pairs (33 severe cases and 172 non-severe cases). First, cluster switching occurred more frequently in severe cases (66.7% vs. 43.6%, Fisher’s exact test, p < 0.05, Fig. 4 D). Furthermore, transmissions between different CSs were likely non-random, as all three CS8 samples on Day 1 switched to CS5 on Day 5 in severe cases, whereas other CSs were rarely transmitted to CS5 (100% vs. 10.5%, Fisher’s exact test, p < 0.01, Fig. 4 D). Specifically, all those three CS8 samples were dominated by Enterobacteriaceae (abundance > 62.8%) on Day 1, with abundance decreasing to less than 28.1% on Day 5, while Acinetobacter increased from less than 1.2% to more than 54% (NJ17037, NJ17043, and NJ17054 in Fig. 4 E). Besides, all six severe patients with CS5 microbiota on Day 5 received invasive mechanical ventilation during hospitalization, suggesting that the expansion of Acinetobacter might be associated with secondary infection following the use of invasive mechanical ventilation. However, not all intubated patients transmitted to CS5 (6 out of 11; Fig. 4 F) despite that the probability is much higher than that in non-intubated patients (54.6% vs. 2.1%, Fisher’s exact test, p < 0.01).
To explore the association between the dynamics of microbial interaction and disease severity in CAP, correlation networks were constructed for samples collected at different time points and in different groups. We found that the interactions between bacteria were remarkably sparser (with a small number of edges and degrees in the network) in severe patients than in non-severe patients at all time points (Fig. 4 G, Fig. S4 B). Meanwhile, we noted that the network contained more potential pathogens, such as Enterobacteriaceae , in severe patients compared to non-severe patients and HCs (Fig. S4 B and C), suggesting a possible dysbiotic state of the sputum microbiota in severe patients. Furthermore, the number of network connections in the severe group decreased markedly but remained unchanged in the non-severe group, indicating that the sputum microbiota in severe patients may become more disordered during hospitalization.
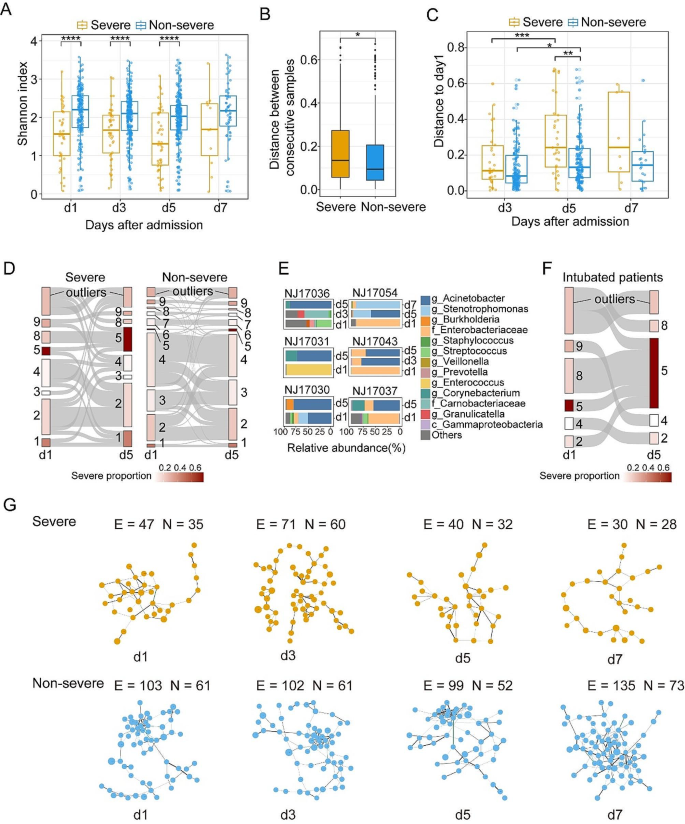
Dynamics of the sputum microbiota and its association with disease severity. ( A ) Shannon index of sputum microbiota in severe cases and non-severe cases at different time points after admission. ( B ) Differences in JSD distance between two consecutive samples from severe and non-severe cases. ( C ) JSD distance between samples on admission and samples collected at different sampling time points. ( D ) The transitional Sankey diagram of different microbiota clusters from day1 to day5 in severe and non-severe CAP cases. Outliers are samples that could not be assigned to any of the nine clusters. ( E ) The microbiota composition of six severe patients whose microbiota belonged to CS5 on day5. ( F ) The transitional Sankey diagram of microbiota clusters in 11 patients underwent invasive mechanical ventilation from day1 to day5. ( G ) Giant component of concurrent networks constructed by SpiecEasi in severe and non-severe CAP cases at different time points. Each node denotes a bacterial microbe and the size of nodes represents the mean abundance of microbes. Black lines represent positive correlations between microbes while green lines represent negative correlations. The thickness of the lines denotes the magnitude of the correlation. The number of edges (E) and nodes (N) are shown in the Figure. The same networks with microbial labels of nodes were shown in Fig. S4 B. * p.adj < 0.05, ** p.adj < 0.01, *** p.adj < 0.001, **** p.adj < 0.0001
Sputum microbiotas varied between patients infected by different pathogens
Possible pathogens were identified in 548 samples from 256 patients by the FTD® Respiratory Pathogens 33 assay (Fig. 5 A). Notably, there was good consistency between the result of 16 S rRNA gene sequencing and the FTD assay (Fig. S5 A). To avoid secondary infection, only 216 patients with a positive FTD result within the first three days after admission were used in subsequent analyses (11 patients positive for Pneumocystis jirovecii were excluded due to the small sample size). Ninety patients were suspected to be infected by at least one bacterial pathogen, 88 patients were suspected to be infected by viruses, and Thirty-eight patients were coinfected by both bacterial and viral pathogens (mix). We observed a significant difference in the microbiota composition between bacterial and viral infections, as well as between viral and mixed infections, and microbiotas under the three conditions were all different from that in HCs (PERMANOVA, p < 0.05, Fig. S5 B), with the bacterial infection samples showed greater deviations (Fig. 5 B). Different bacteria were enriched in three distinct types of infections, whereas some commensal bacteria, such as Fusobacterium , were significantly depleted in all three types (Fig. S5 C).
We then classified the infections into subgroups based on the pathogen detected, considering only those infecting more than fifteen patients (Rhinovirus, Mycoplasma pneumoniae , Klebsiella pneumoniae , and Influenza A) after excluding coinfection samples. Out of 18 patients detected with Mycoplasma pneumoniae , only three exhibited a predominance of Mycoplasma in their sputum microbiota (CS7, median Mycoplasma abundance = 42.4%), while the remaining samples were dominated by respiratory commensals (14 from CS2-4, one dominated by Lautropia , median Mycoplasma abundance = 2.6%). Similarly, only one of the 18 Klebsiella pneumoniae -positive patients was assigned to Enterobacteriaceae -dominant CS5, indicating that the pathogen was not obligatory as the predominant bacterium. The microbiota composition (excluding the pathogen itself) in all four infections differed from that in HCs (PERMANOVA, p < 0.05, Fig. S5 D). Although no significant difference in alpha diversity was observed between patients infected with different pathogens (Fig. S5 E), the microbiota alterations relative to the HCs in Mycoplasma pneumoniae infections was less significant than in other infections (Fig. 5 C). Specifically, we noted that rhinovirus infections were enriched with Enterococcus and Stenotrophomonas , influenza A infections with Acinetobacter and Pseudomonas , Mycoplasma pneumoniae with Rothia and Carnobacteriaceae , while Acinetobacter was enriched in Klebsiella pneumoniae infections (Fig. 5 D). The microbiota composition differed between Mycoplasma pneumoniae infections and the Klebsiella pneumoniae infections, as well as between Mycoplasma pneumoniae infections and rhinovirus infections (PERMANOVA, R 2 = 0.0.81 and 0.078, p < 0.05, Fig. S5 D).
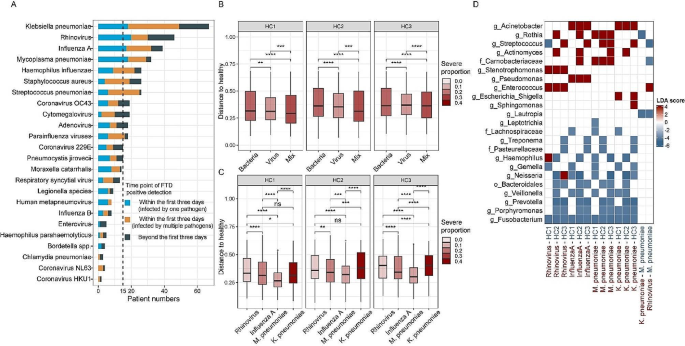
Microbiota features in patients infected with different pathogens. ( A ) Number of patients infected by different pathogens. ( B ) JSD distance to HCs for patients infected by bacteria, viruses, and mixed infection. ( C ) JSD distance to HCs for samples infected by Rhinovirus, Influenza A, Mycoplasma pneumoniae , and Klebsiella pneumoniae . ( D ) Bacteria that associated with different types of infections identified by LEfSe (|LDA| score > 4, p < 0.05). The LDA score denotes the extent of enrichment of the bacterium in the infection type that is labeled in red on the x-axis. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
Recent studies proposed that respiratory microbiota dysbiosis, especially low community diversity, was implicated in pneumonia development [ 9 , 19 ]. However, due to the small sample size and the underrepresentation of immunocompetent patients in previous studies, characteristics of the lower respiratory microbiota in CAP patients remain largely unknown. In this study, we revealed key features of sputum microbiota in 350 CAP patients through an examination of 917 longitudinal sputum samples.
The sputum microbiota in CAP patients is highly diverse. In contrast to previous studies that identified a limited number of microbiota community types in healthy populations and patients with pneumonia or other pulmonary diseases [ 7 , 29 , 30 ], we identified a more heterogeneous microbiota composition in CAP patients in this study, with nine distinct microbiota clusters being identified, which may be attributed to a larger sample size, better sample representation, and diverse pathogen types. The commensal bacteria that are typically found in the respiratory tract of healthy populations make up the majority of the sputum microbiota in most patients, suggesting potential resistance or resilience of the respiratory microbiota against acute infection. Meanwhile, a sizeable proportion of samples (14.0%) had microbiota with unusually high abundances of possible pathogens, including Enterobacteriaceae , Pseudomonas, Acinetobacter, Mycoplasma , and Stenotrophomonas , all previously proposed as pneumonia-causing pathogens [ 19 , 31 , 32 , 33 , 34 ], suggesting abnormal pathogen growth. In addition, 10.3% of samples had a microbiota predominated by non-typical pathogenic bacteria, such as Corynebacterium , Rothia , and Haemophilus , highlighting the complexity of the CAP microbiota (Fig. S2 I). A special group of patients with a relatively sterile microbial community was also identified, a phenomenon previously observed in the bronchoalveolar lavage fluid of healthy individuals and COPD patients [ 5 , 29 ]. However, the presence of such a low microbial load in CAP patients is unexpected, given that CAP is typically associated with the proliferation of invasive or colonized bacteria, triggering an inflammatory response [ 5 , 35 ]. The severity rate of those patients was similar to that of commensals-dominated patients (CS2-4), and lower than that of the patients dominated by possible pathogens (CS1,5,7,8,9). We hypothesize that a stronger immune response or lack of sufficient resources might have suppressed the growth of both commensal and pathogenic microbes in these patients.
Second, the degree of sputum microbiota dysbiosis correlated with disease severity in CAP patients. In line with previous findings, severe CAP patients exhibited lower alpha diversity compared to healthy controls upon admission [ 6 , 8 , 9 ]. However, we noticed that the sputum microbiota in non-severe cases had alpha diversity less deviated from healthy controls, despite that their microbiota was still more likely to be predominated by a specific bacterium. Meanwhile, their microbiota compositions were more similar to those of healthy controls compared to severe cases. The most significant enriched bacterium in the sputum of severe cases is Enterobacteriaceae , commonly found in the gastrointestinal tract [ 36 ]. This increase may be due to the growth of colonizing bacteria or the translocation of gut bacteria to the respiratory tract, triggering systemic inflammation [ 37 ]. Furthermore, we observed a high transition rate from an Enterobacteriaceae- dominant microbiota to an Acinetobacter -dominant microbiota post-mechanical ventilation, suggesting increased vulnerability to ventilation-induced secondary infection in Enterobacteriaceae- dominant cases. Thus, a high level of Enterobacteriaceae in the sputum seems to predict a poor prognosis in CAP patients.
Third, the sputum microbiota in severe cases was more vulnerable and susceptible to significant changes during hospitalization, evidenced by higher compositional alteration, more frequent cluster switching, and more significant changes in the microbial network. This pattern resembles observations in other respiratory diseases like COPD and COVID-19 [ 16 , 38 ], potentially influenced by both medical intervention and disease progression [ 12 , 19 ]. However, distinguishing the specific impact of each factor is challenging. Moreover, the duration of altered microbiota and its relationship with the persistence of respiratory symptoms remain unknown, warranting a longer follow-up study for clarification.
Fourth, the alteration of sputum microbiota was associated with the infected pathogen. Rhinovirus infections exhibited enrichment of Enterococcus and Stenotrophomonas , aligning with previous studies reporting coinfection of Rhinovirus with Stenotrophomonas maltophilia or Enterococcus faecium [ 39 , 40 ]. Meanwhile, influenza A infections showed enrichment of Acinetobacter and Pseudomonas , indicating a possible increased susceptibility to Acinetobacter baumannii and Pseudomonas aeruginosa after infection influenza A [ 41 , 42 , 43 ]. The underlying mechanism may involve viral infections damaging respiratory airways and concurrently impairing both innate and acquired immune responses. This creates a favorable environment for bacterial growth, adherence, and invasion into healthy sites of the respiratory tract [ 44 ]. Besides, Klebsiella pneumoniae infections, which were associated with a higher incidence of severe illness, showed more deviation from HCs (more dysbiotic) compared to Mycoplasma pneumoniae infections, which had a lower risk of severe illness. However, it is unclear to what extent the accompanying microbiota change, in addition to the pathogen’s direct influence, affects disease progression, as most cases with Klebsiella pneumoniae -positivity or Mycoplasma pneumoniae -positivity still possessed sputum microbiotas dominated by respiratory commensal. Such analysis is constrained by a small sample size and a diverse background microbiota, which could be overcome by conducting intervention experiments in animal models.
Our study has several limitations. First, pneumonia is a lung infection caused by various pathogens, hence the samples from the lungs (e.g., biopsy, bronchoalveolar lavage fluid) are particularly valuable. However, obtaining such samples involves invasive procedures, and longitudinal sampling is challenging. While sputum is commonly used as a proxy for lung samples [ 45 ], it inevitably contains upper respiratory tract microbes. The accuracy of sputum microbiota in reflecting lung microbiota is still debated [ 46 , 47 ]. Second, the healthy microbiota data were obtained from three previous studies on the Chinese population, potentially differing from the population investigated in this study. We compared the CAP microbiota to different healthy datasets and reported only consistent results, making our conclusions more robust. Third, the use of antibiotics may influence sputum microbiota during hospitalization, but controlling this confounding factor is challenging as patients were not treated following the same protocol. Therefore, our analyses primarily focused on the samples taken upon admission when limited medical intervention had been applied. Fourth, the utilization of 16 S rRNA gene sequencing restricted our study to primarily assessing the relationship between the abundance of genus-level microorganisms and the disease, while the functional attributes of the sputum microbiota were merely predicted by the bioinformatic method. Further investigations employing metagenomic and metatranscriptomic technologies are warranted to elucidate the more precise role exerted by airway microorganisms in respiratory infectious diseases.
In summary, our study demonstrated diverse sputum microbiota compositions in CAP patients, with many, especially in non-severe patients, resembling those in healthy individuals. Severe CAP cases were more likely to have microbiota dominated by potentially pathogenic bacteria and underwent greater changes during hospitalization. Further studies, especially prospective and intervention studies, are needed to decipher the causality between the respiratory microbiota change and disease severity.
Patients and sample collection
Spontaneous sputum samples were collected on days 1, 3, 5, 7, and 9 after admission from 367 CAP inpatients from six hospitals (Tongji Hospital, The Second Affiliated Hospital of Harbin Medical University, The First Affiliated Hospital of Xi’an Jiaotong University, The Third People’s Hospital of Shenzhen, ZhongDa Hospital, Fujian Provincial Hospital) located in different cities representing distinct geographical locations in mainland China between 2014 and 2017 (Fig. 1 A). Sputum quality was assessed by the presence of polymorphonuclear neutrophils (PMNs) and squamous epithelial cells (SECs) per low-power (microscopic) field (LPF) [×10 objective]. Only qualified samples (> 25 PMNs and < 10 s per LPF) were included in the study [ 48 ]. The sputum samples were immediately placed into a viral transport medium and stored at -80℃ until transported to the lab for processing (normally within a year).
Patients in this study were diagnosed with CAP through guidelines for the diagnosis and treatment of community-acquired pneumonia [ 49 ], meeting inclusion criteria included clinical manifestations of acute infection, respiratory symptoms, inflammatory changes revealed by chest X-rays or computed tomography, and no history of healthcare system exposure. In addition, the study primarily included patients who developed symptoms within 7 days. Patients who had been ill for more than 7 days and experienced a sudden worsening of symptoms during treatment, suggestive of a possible secondary infection, were also included. Cases of pneumonia caused by non-infectious factors were excluded. The severity of the patients was determined following the Guideline of the American Thoracic Society and Infectious Diseases Society of America [ 50 ]. Specifically, CAP patients must meet one primary criterion or three secondary criteria to be classified as clinically severe CAP cases. Primary criteria included 1). requirement for invasive mechanical ventilation; 2). presence of septic shock necessitating vasopressor therapy. Secondary criteria were 1). respiratory rate ≥ 30 breaths/minute; 2). PaO2/FiO2 ratio ≤ 250; 3). Multilobar infiltrates; 4). altered mental status or disorientation; 5). Renal dysfunction (blood urea nitrogen level ≥ 20 mg/dL); 6). Leukopenia (white blood cell count < 4 × 10^9/L); 7). Thrombocytopenia (platelet count < 100 × 10^9/L); 8). Hypothermia (core body temperature < 36.0 °C); 9). Hypotension requiring aggressive fluid resuscitation. Patients diagnosed with severe pneumonia at any time during hospitalization are recorded as severe cases.
Statistical analysis
The alpha diversity was calculated by the estimate_richness function in R package phyloseq(v.1.38.0) [ 51 ]. Beta diversity represented by Jensen-Shannon Divergence (JSD) distance was calculated by Phyloseq R package (v4.0.3) [ 51 ]. Permutational multivariate analysis of variance (PERMANOVA) was used to compare the microbiota composition between different groups [ 52 ], p-value was calculated based on 999 permutations. All the possible confounders (variables 1–10 in Table 1 ) were used for multivariate PERMANOVA. Wilcoxon signed-rank test was used to compare continuous variables in different groups. Fisher’s exact test was used to test the correlation between categorical variables. P-values were adjusted for multiple testing using the Benjamini-Hochberg method.
Additional methods applied in the study were described in the supplementary methods.
Data availability
Raw sequencing data have been deposited in the GSA in the National Genomics Data Center (HRA002709). All statistical analyses were implemented in RStudio and the scripts and data could be accessed at https://github.com/zhanglinfeng164/CAP_sputum_microbiota.
Abbreviations
chronic obstructive pulmonary disease
body-mass index
permutational multivariate analysis of variance
community-acquired pneumonia
negative controls
healthy controls
Jensen–Shannon divergence
principal coordinate analysis
linear discriminant analysis effect size
linear discriminant analysis
receiver operator characteristic
sparse inverse covariance estimation for ecological association inference
Shoar S, Musher DM. Etiology of community-acquired pneumonia in adults: a systematic review. Pneumonia. 2020;12(1):11.
Article PubMed PubMed Central Google Scholar
Age-sex differences. In the global burden of lower respiratory infections and risk factors, 1990–2019: results from the global burden of Disease Study 2019. Lancet Infect Dis. 2022;22(11):1626–47.
Article Google Scholar
Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England). 2020;396(10258):1204-22.
Wu BG, Segal LN. The Lung Microbiome and its role in Pneumonia. Clin Chest Med. 2018;39(4):677–89.
Segal LN, Clemente JC, Tsay J-CJ, Koralov SB, Keller BC, Wu BG, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol. 2016;1(5):16031.
Article CAS PubMed PubMed Central Google Scholar
Man WH, van Houten MA, Mérelle ME, Vlieger AM, Chu MLJN, Jansen NJG, et al. Bacterial and viral respiratory tract microbiota and host characteristics in children with lower respiratory tract infections: a matched case-control study. Lancet Respiratory Med. 2019;7(5):417–26.
Shenoy MK, Iwai S, Lin DL, Worodria W, Ayakaka I, Byanyima P, et al. Immune Response and Mortality Risk relate to distinct lung microbiomes in patients with HIV and Pneumonia. Am J Respir Crit Care Med. 2017;195(1):104–14.
Shankar J, Nguyen M-H, Crespo M, Kwak E, Lucas S, McHugh K et al. Looking beyond respiratory cultures: Microbiome-Cytokine signatures of bacterial pneumonia and Tracheobronchitis in Lung Transplant recipients. Am J Transplantation. 2016;16(6):1766-78.
de Steenhuijsen Piters WAA, Huijskens EGW, Wyllie AL, Biesbroek G, van den Bergh MR, Veenhoven RH, et al. Dysbiosis of upper respiratory tract microbiota in elderly pneumonia patients. ISME J. 2016;10(1):97–108.
Article PubMed Google Scholar
Thibeault C, Suttorp N, Opitz B. The microbiota in pneumonia: from protection to predisposition. Sci Transl Med. 2021;13(576).
Dickson RP, Erb-Downward JR, Huffnagle GB. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir Med. 2014;2(3):238–46.
Man WH, de Steenhuijsen Piters WAA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15(5):259–70.
Adar SD, Huffnagle GB, Curtis JL. The respiratory microbiome: an underappreciated player in the human response to inhaled pollutants? Ann Epidemiol. 2016;26(5):355–9.
Carney SM, Clemente JC, Cox MJ, Dickson RP, Huang YJ, Kitsios GD, et al. Methods in lung Microbiome Research. Am J Respir Cell Mol Biol. 2020;62(3):283–99.
Das S, Bernasconi E, Koutsokera A, Wurlod D-A, Tripathi V, Bonilla-Rosso G, et al. A prevalent and culturable microbiota links ecological balance to clinical stability of the human lung after transplantation. Nat Commun. 2021;12(1):2126.
Mayhew D, Devos N, Lambert C, Brown JR, Clarke SC, Kim VL, et al. Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax. 2018;73(5):422–30.
Frayman KB, Armstrong DS, Carzino R, Ferkol TW, Grimwood K, Storch GA, et al. The lower airway microbiota in early cystic fibrosis lung disease: a longitudinal analysis. Thorax. 2017;72(12):1104–12.
Sulaiman I, Chung M, Angel L, Tsay JJ, Wu BG, Yeung ST, et al. Microbial signatures in the lower airways of mechanically ventilated COVID-19 patients associated with poor clinical outcome. Nat Microbiol. 2021;6(10):1245–58.
Zakharkina T, Martin-Loeches I, Matamoros S, Povoa P, Torres A, Kastelijn JB, et al. The dynamics of the pulmonary microbiome during mechanical ventilation in the intensive care unit and the association with occurrence of pneumonia. Thorax. 2017;72(9):803–10.
Du S, Shang L, Zou X, Deng X, Sun A, Mu S, et al. Azithromycin exposure induces transient Microbial composition shifts and decreases the Airway Microbiota Resilience from Outdoor PM(2.5) stress in healthy adults: a Randomized, Double-Blind, placebo-controlled trial. Microbiol Spectr. 2023;11(3):e0206622.
Cai X, Luo Y, Zhang Y, Lin Y, Wu B, Cao Z, et al. Airway microecology in rifampicin-resistant and rifampicin-sensitive pulmonary tuberculosis patients. BMC Microbiol. 2022;22(1):286.
Lin L, Yi X, Liu H, Meng R, Li S, Liu X, et al. The airway microbiome mediates the interaction between environmental exposure and respiratory health in humans. Nat Med. 2023;29(7):1750–9.
Article CAS PubMed Google Scholar
Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38(6):685–8.
Lippi G, Franchini M. Vitamin K in neonates: facts and myths. Blood Transfus = Trasfusione del sangue. 2011;9(1):4–9.
PubMed Google Scholar
Arslan S, Ugurlu S, Bulut G, Akkurt I. The association between plasma D-dimer levels and community-acquired pneumonia. Clin (Sao Paulo). 2010;65(6):593–7.
Agapakis DI, Tsantilas D, Psarris P, Massa EV, Kotsaftis P, Tziomalos K, et al. Coagulation and inflammation biomarkers may help predict the severity of community-acquired pneumonia. Respirology. 2010;15(5):796–803.
Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858–76.
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50.
Ren L, Zhang R, Rao J, Xiao Y, Zhang Z, Yang B et al. Transcriptionally active lung Microbiome and its Association with bacterial biomass and host inflammatory status. mSystems. 2018;3(5).
Mac Aogáin M, Narayana JK, Tiew PY, Ali N, Yong VFL, Jaggi TK, et al. Integrative microbiomics in bronchiectasis exacerbations. Nat Med. 2021;27(4):688–99.
Dickson RP, Schultz MJ, van der Poll T, Schouten LR, Falkowski NR, Luth JE, et al. Lung microbiota predict clinical outcomes in critically ill patients. Am J Respir Crit Care Med. 2020;201(5):555–63.
Garnacho-Montero J, Timsit JF. Managing Acinetobacter baumannii infections. Curr Opin Infect Dis. 2019;32(1):69–76.
Kishaba T. Community-Acquired Pneumonia caused by Mycoplasma pneumoniae: how physical and radiological examination contribute to successful diagnosis. Front Med (Lausanne). 2016;3:28.
Kanderi T, Shrimanker I, Mansoora Q, Shah K, Yumen A, Komanduri S. Stenotrophomonas maltophilia: an Emerging Pathogen of the respiratory tract. Am J Case Rep. 2020;21:e921466.
Pahal PRV, Rajasurya V, Sharma S. Typical Bacterial Pneumonia. Treasure Island (FL): StatPearls Publishing; 2024.
Martinson JNV, Pinkham NV, Peters GW, Cho H, Heng J, Rauch M, et al. Rethinking gut microbiome residency and the Enterobacteriaceae in healthy human adults. ISME J. 2019;13(9):2306–18.
Dickson RP, Singer BH, Newstead MW, Falkowski NR, Erb-Downward JR, Standiford TJ, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. 2016;1(10):16113.
Ren L, Wang Y, Zhong J, Li X, Xiao Y, Li J, et al. Dynamics of the Upper Respiratory Tract Microbiota and its Association with Mortality in COVID-19. Am J Respir Crit Care Med. 2021;204(12):1379–90.
Hung HM, Yang SL, Chen CJ, Chiu CH, Kuo CY, Huang KA, et al. Molecular epidemiology and clinical features of rhinovirus infections among hospitalized patients in a medical center in Taiwan. J Microbiol Immunol Infect. 2019;52(2):233–41.
Jacobs SE, Soave R, Shore TB, Satlin MJ, Schuetz AN, Magro C, et al. Human rhinovirus infections of the lower respiratory tract in hematopoietic stem cell transplant recipients. Transpl Infect Disease: Official J Transplantation Soc. 2013;15(5):474–86.
Article CAS Google Scholar
Zhou Y, Du J, Wu JQ, Zhu QR, Xie MZ, Chen LY, et al. Impact of influenza virus infection on lung microbiome in adults with severe pneumonia. Ann Clin Microbiol Antimicrob. 2023;22(1):43.
Jie F, Wu X, Zhang F, Li J, Liu Z, He Y, et al. Influenza virus infection increases host susceptibility to secondary infection with Pseudomonas aeruginosa, and this is attributed to neutrophil dysfunction through reduced myeloperoxidase activity. Microbiol Spectr. 2023;11(1):e0365522.
Liu WJ, Zou R, Hu Y, Zhao M, Quan C, Tan S, et al. Clinical, immunological and bacteriological characteristics of H7N9 patients nosocomially co-infected by Acinetobacter Baumannii: a case control study. BMC Infect Dis. 2018;18(1):664.
Manna S, Baindara P, Mandal SM. Molecular pathogenesis of secondary bacterial infection associated to viral infections including SARS-CoV-2. J Infect Public Health. 2020;13(10):1397–404.
Rogers GB, van der Gast CJ, Cuthbertson L, Thomson SK, Bruce KD, Martin ML, et al. Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax. 2013;68(8):731.
Feng Z-H, Li Q, Liu S-R, Du X-N, Wang C, Nie X-H et al. Comparison of composition and diversity of bacterial Microbiome in Human Upper and Lower Respiratory Tract. Chin Med J. 2017;130(9).
An SQ, Warris A, Turner S. Microbiome characteristics of induced sputum compared to bronchial fluid and upper airway samples. Pediatr Pulmonol. 2018;53(7):921–8.
Gal-Oz A, Kassis I, Shprecher H, Beck R, Bentur L. Correlation between Rapid Strip Test and the quality of Sputum. Chest. 2004;126(5):1667–71.
He LX. Guidelines for the diagnosis and treatment of community-acquired pneumonia: learning and practicing. Chin J Tuberculosis Respiratory Dis. 2006;29(10):649–50.
Google Scholar
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72.
McMurdie PJ, Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8(4):e61217.
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin P, O’Hara RB et al. Vegan: Community Ecology Package. R Package Version. 2.0–10. CRAN. 2013.
Download references
Acknowledgements
We thank all the participants who donated their specimens to this study.
This study was supported by funding from the National Key R&D Program of China (grant no. 2022YFA1304300 to L.R. and M.L.), Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (grant no. 2019PT310029 to L.R.), the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (grant no. 2021-I2M-1-038 to J.W.), the Fundamental Research Funds for the Central Universities (grant no. 3332021092 to J.W.), the National Natural Science Foundation of China (grant no. 32100098 to L.Z.), the Beijing Municipal Natural Science Foundation of China (grant no. Z190017 to L.R.), the Beijing Nova Program (grant no. Z191100006619102 to J.W., Z211100002121135 to L.Z.), and Fondation Merieux (grant no. N/A to J.W.). The funders had no role in the design of this study and did not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Author information
Jing Yang, Jinman Li and Linfeng Zhang contributed equally to this study.
Jianwei Wang, Mingkun Li and Lili Ren contributed equally as senior authors to this work.
Authors and Affiliations
Beijing Institute of Genomics, Chinese Academy of Sciences, China National Center for Bioinformation, Beijing, 100101, China
Jing Yang, Linfeng Zhang, Zijie Shen, Li Zhang, Lu Wang & Mingkun Li
University of Chinese Academy of Sciences, Beijing, 100049, China
Jing Yang, Linfeng Zhang, Zijie Shen & Mingkun Li
Changping Laboratory, Beijing, 102206, China
NHC Key Laboratory of Systems Biology of Pathogens and Christophe Mérieux Laboratory, National Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100730, China
Jinman Li, Yan Xiao, Ying Wang, Lan Chen, Xinming Wang, Jianwei Wang & Lili Ren
Key Laboratory of Respiratory Disease Pathogenomics, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100730, China
Yan Xiao, Lan Chen, Xinming Wang, Jianwei Wang & Lili Ren
State Key Laboratory of Respiratory Health and Multimorbidity, National Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100730, China
Yan Xiao & Lili Ren
Shenzhen Third People’s Hospital, Shenzhen, 518112, China
Guoliang Zhang
The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710061, China
Mingwei Chen
The Second Affiliated Hospital of Harbin Medical University, Harbin, 150001, China
Jiangsu Provincial Key Laboratory of Critical Care Medicine, Department of Critical Care Medicine, School of Medicine, Zhongda Hospital, Southeast University, Nanjing, 210009, China
Institute of Ecological Sciences, South China Normal University, Guangzhou, 510631, China
You can also search for this author in PubMed Google Scholar
Contributions
L.R., M.L., and J.W. designed the study. J.Y., J.L. and L.F.Z. conducted the experiments, performed the statistical analysis, and drafted the initial draft of the manuscript. Y.X., Y.W., L.C., and X.W. contributed to the process of the specimens. G.Z., M.C., F.C., L.L. participated in the recruitment of subjects and contributed to clinical data acquisition. Z.S., L.Z., Z.W. and L.W. contributed to computational analysis. M.L., L.R., and J.W. revised the manuscript. J.Y., J.L., L.F.Z., L.R., M.L., and J.W. have assessed and verified the underlying data reported in the manuscript. All authors contributed to this article and approved the submitted versions.
Corresponding authors
Correspondence to Jianwei Wang , Mingkun Li or Lili Ren .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Institutional Review Board of Ethics of hospitals where sampling was conducted and the Institute of Pathogen Biology, Chinese Academy of Medical Sciences (record number: IPB-2018-3), and written informed consent was obtained from each subject before inclusion. The private information of all patients was confidential.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Yang, J., Li, J., Zhang, L. et al. Highly diverse sputum microbiota correlates with the disease severity in patients with community-acquired pneumonia: a longitudinal cohort study. Respir Res 25 , 223 (2024). https://doi.org/10.1186/s12931-024-02821-2
Download citation
Received : 17 January 2024
Accepted : 24 April 2024
Published : 29 May 2024
DOI : https://doi.org/10.1186/s12931-024-02821-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Community-acquired pneumonia
- Sputum microbiota
- Longitudinal study
- Disease severity
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Community-Acquired Pneumonia
Key concepts.
- • Community-acquired pneumonia (CAP) is the most common cause of admission of adults in the USA.
- • Diagnosis of community-acquired pneumonia is relatively easy in previously healthy patients but may be challenging in those with underlying cardiopulmonary disease or in the elderly.
- • The highest yield for diagnostic testing for CAP etiology is in the critically ill and those with risk factors for drug-resistant pathogens.
- • The majority of hospitalized CAP patients can be treated with either a respiratory fluoroquinolone or cephalosporin/macrolide combination.
- • Alternative antibiotic treatment should be based on presence of multiple risk factors for drug-resistant pathogens (i.e. healthcare-associated pneumonia), specific risks (e.g. travel or zoonotic risks), or unique syndromes (e.g. toxin-mediated community-acquired MRSA syndrome).
- • Decisions regarding initial placement in an intensive care unit (ICU) of tenuous CAP patients should be based on the number of minor physiologic factors and laboratory abnormalities associated with risk of subsequent deterioration.
- • Number of deaths by pneumonia is decreasing in the world linked to child vaccination of pneumococcus and large-scale use of antibiotics in India/China.
Introduction
Captain of the men of death … Sir William Osler MD
Community-acquired pneumonia (CAP) is one of the most underappreciated medical illnesses in the USA. The combination of pneumonia and influenza is the ninth leading cause of death overall and the most common cause of infectious death in the USA, causing an estimated 50 000 deaths in 2010. 1 This number is likely an underestimate because many deaths caused directly by CAP are coded as sepsis, for which pneumonia is the most common source, 2 or attributed to an underlying condition (such as cancer and Alzheimer's disease), for which pneumonia is the terminal event. For example, the proximate cause of death in >40% of patients with dementia is pneumonia. 3 Lower respiratory tract infection remains the leading cause of infectious death in the world as well, exceeding deaths from tuberculosis, human immunodeficiency virus (HIV) and malaria combined. 4
CAP is the most common reason for hospital admission of adults in the USA. 5 It is a common cause of severe complications, including septic shock, 2 acute respiratory distress syndrome (ARDS) and acute renal failure. Even in survivors, hospital admission for CAP has been associated with increased subsequent mortality and with accelerated cognitive decline.
CAP is also costly, with the estimated annual cost of CAP in the USA being $10.8 billion. 6 , 7 Indirect costs are also substantial: CAP is a major cause of work days and days of school lost to illness.
The mortality rate from CAP has changed very little until the last decade. Two factors likely have contributed to this recent decrease: widespread use of conjugate pneumococcal vaccines in children, with a beneficial effect of herd immunity on adults, 8 and more extensive management of adults according to guideline-recommended antibiotic therapy. 9
Diagnosis of classic CAP is not difficult in patients without underlying cardiopulmonary disease. A triad of (1) evidence of infection (fever or chills, leukocytosis) with (2) signs or symptoms localizing to the respiratory system (cough, increased sputum production, shortness of breath, chest pain, abnormal pulmonary exam with crackles, signs of consolidation, or finding of a pleural effusion), accompanied by (3) a new or changed radiographic infiltrate, usually accurately defines a patient with CAP. In patients with lung cancer, pulmonary fibrosis or other chronic infiltrative lung diseases, and congestive heart failure (CHF), 10 the diagnosis of CAP can be very difficult. Atypical presentations also complicate diagnosis. Confusion may be the only presenting symptom in the elderly, leading to delay in diagnosis. 11
The differential diagnosis of CAP ( Table 28-1 ) results from either noninfectious inflammatory disorders that also cause radiographic infiltrates or concurrent non-lower respiratory tract infection with other causes of infiltrate. Viral upper respiratory tract infection (URI) in association with worse CHF is probably the most common pneumonia mimic, given the frequency of both disorders.
Differential Diagnosis of Community-Acquired Pneumonia
| Abnormal CXR | Normal CXR |
|---|---|
| Congestive heart failure | Acute exacerbation of COPD |
| Aspiration pneumonitis | Influenza |
| Pulmonary infarction | Acute bronchitis |
| Acute exacerbation of pulmonary fibrosis | Pertussis |
| Acute exacerbation of bronchiectasis | Asthma |
| Acute eosinophilic pneumonia | |
| Hypersensitivity pneumonitis | |
| Pulmonary vasculitis | |
| Cocaine-induced (‘crack lung’) | |
CXR, chest radiograph.
Radiographic infiltrates may also be subtle: an individual radiologist may miss infiltrates in up to 15% of cases and two radiologists reading the same chest radiograph disagree in 10% of cases. 12 Computed tomography (CT) detects alveolar infiltrates in a not inconsequential number of patients with manifestations of CAP but normal chest radiographs. 13 The inflammatory reaction caused by antibiotic-induced bacterial lysis and aggressive fluid resuscitation will often unmask these otherwise radiographically occult infiltrates on a subsequent chest radiograph.
The most difficult radiographic challenge is detection of acute pneumonia in the setting of chronic lung disease, such as pulmonary fibrosis, pneumoconioses, bronchiectasis, cystic fibrosis and even emphysema or CHF. Comparison to chest radiographs at a time of clinical stability is very important for these cases. If not available, other clinical manifestations, including use of biomarkers, 10 may be required to avoid excessive antibiotic therapy.
The pattern of radiographic infiltrates is occasionally helpful in the differential diagnosis of etiology. Cavitary CAP ( Figure 28-1 ) has a limited differential diagnosis ( Table 28-2 ), although it varies somewhat by geographic location. Conversely, even though viral or atypical bacterial pneumonia more commonly cause diffuse interstitial infiltrates, this pattern is not distinctive enough to guide antibiotic therapy.

Cavitary pneumonia in an otherwise healthy young adult caused by methicillin-resistant Staphylococcus aureus (MRSA).
Differential Diagnosis of Cavitary/Necrotizing CAP in Non-immunocompromised Patients
| , including MRSA spp. |
Radiographic pattern is also associated with prognosis. Initial presence of bilateral infiltrates is consistently associated with greater mortality and need for ICU care. 9 A rapid increase in radiographic infiltrates, whether due to uncontrolled infection or development of ARDS, in the initial 24–48 hours, is also associated with antibiotic failure and need for ICU care. 14 Conversely, presence of a pleural effusion has been associated with better prognosis.
Pathophysiology
Pneumonia results from the proliferation of microbial pathogens, most commonly bacteria, but occasionally by viruses, fungi, parasites and other infectious agents, in the alveoli and the host's response to those pathogens. The latter is critically important since recent data have demonstrated the presence of a normal bacterial microbiome in the alveoli.
Bacterial Invasion
Infection of the lower respiratory tract can occur at each level, with a varying proportion of viral and bacterial etiologies at each level, and can be confused with pneumonia. Respiratory bronchiolitis due to respiratory syncytial virus (RSV) in children is a classic example of confusion between CAP and more proximal level infection, with the increased secretions and airway narrowing due to RSV airway infection leading to a radiographic infiltrate from atelectasis. Conversely, infection can progress through the entire respiratory tract, such as influenza URI followed by cough and wheezing from tracheobronchitis, culminating in hypoxemia and infiltrates from influenza pneumonia.
Classically, pneumonia is thought to result from introduction of pathogens into the lower respiratory tract through four pathways. Aspiration from the oropharynx is likely the most common for bacterial pneumonia. Small-volume aspiration occurs frequently during sleep (especially in the elderly) and in patients with decreased levels of consciousness. Viruses and tuberculosis are inhaled as contaminated droplets. Rarely, pneumonia occurs via hematogenous spread (e.g., from tricuspid endocarditis) or by contiguous extension from an infected pleural or mediastinal space.
Recognition that a normal flora exists at the alveolar level of the lung, rather than the distal lung being sterile, raises an alternative mechanism for development of bacterial pneumonia. The normal lung microbiome is similar to that of the normal oropharynx, predominantly streptococci (including the pneumococcus) but also including Haemophilus , Mycoplasma and other CAP pathogens, but at significantly lower concentrations. 15 , 16 CAP may therefore result from a perturbation in the normal balance, for example a viral URI, resulting in disruption of the balance and outgrowth of a specific species. This hypothesis is very consistent with the frequent association between antecedent or concomitant viral infection and bacterial CAP.
Host Defenses
For pneumonia to occur, lung host defenses must be overcome. The normal lung host defenses are formidable, given that the lung represents the greatest amount of surface area in contact with the external environment and is therefore routinely exposed to infectious micro-organisms. As a result, the lungs and entire respiratory tract have effective and redundant host defense mechanisms in order to respond to this infectious challenge.
Mechanical factors are critically important for inhaled pathogens; the hairs and turbinates of the nares and the branching architecture of the tracheobronchial tree trap microbes on the airway lining, where mucociliary clearance and local antibacterial factors either clear or kill potential pathogens. The gag reflex and cough play major roles in protection from aspiration challenges.
By adhering to mucosal cells of the oropharynx, normal flora prevent attachment of pathogenic bacteria and thereby decrease risk of aspirating these more virulent bacteria. Disruption of the normal microbiome of both oropharynx and lung by antibiotics, viruses, or other factors not only leads to increased risk of pneumonia but also predisposes to more antibiotic-resistant pathogens.
When these mechanical barriers are overcome or when the micro-organisms are small enough to be directly inhaled to the alveolar level, resident alveolar macrophages are extremely efficient at clearing and killing pathogens. Macrophages are assisted by the alveolar epithelial cells, which produce proteins (e.g., surfactant proteins A and D) with opsonic properties or direct antibacterial or antiviral activity. Once engulfed by the macrophage, the pathogens – even if they are not killed – are eliminated via either the mucociliary elevator or the lymphatics.
Only when the capacity of the alveolar macrophages to ingest or kill the micro-organisms is exceeded does clinical pneumonia become manifest. In that situation, the alveolar macrophages initiate the inflammatory response to bolster lower respiratory tract defenses.
Localizing infection to the alveolar space is an important but underappreciated component of host immunity. Factors preventing bacteremia and defending the vascular space are poorly understood. Even the presence of bacterial DNA in peripheral blood appears to correlate with mortality and organ dysfunction. 17 Clearly, preformed antibody is important, since the most incontrovertible evidence of pneumococcal vaccine efficacy is prevention of invasive disease, including bacteremia. 18 Ability to opsonize bacteria is also important since deficiencies in mannose-binding lectin and complement are also associated with increased bacteremia and invasive pneumococcal disease. 19 , 20 Splenic clearance of opsonized bacteria is also important for the pneumococcus and other encapsulated bacteria.
Clinical Manifestations
The host inflammatory response, rather than simply proliferation of micro-organisms, triggers the clinical syndrome of pneumonia. Release of inflammatory mediators, such as interleukin (IL)-1 and tumor necrosis factor (TNF), results in fever. Chemokines, such as IL-8 and granulocyte colony-stimulating factor, stimulate bone marrow release of neutrophils and homing to the lung, producing both peripheral leukocytosis and increased purulent secretions. Erythrocytes crossing the alveolar–capillary membrane in the stage of red hepatization result in hemoptysis. Inflammatory mediators released by macrophages and the newly recruited neutrophils cause an alveolar capillary leak equivalent to that demonstrated for ARDS, although initially localized in pneumonia. Radiographic infiltrates and rales detectable on auscultation are a direct result of the alveolar–capillary leak syndrome. Hypoxemia results from alveolar filling but may be exacerbated by paralysis of the hypoxemic vasoconstriction that would normally occur with fluid-filled alveoli by some bacterial pathogens. Increased respiratory drive as part of the systemic inflammatory response syndrome leads to respiratory alkalosis. Decreased compliance due to capillary leak, hypoxemia, increased respiratory drive, increased secretions and occasionally infection-related bronchospasm all lead to dyspnea and, if severe enough, respiratory failure.
Immunocompromise
People with no recognizable defect in any component of host defense can develop CAP. However, the more severe the manifestations and the less virulent the pathogen, the more likely some component of the host defense is deficient. Genetic deficiencies in every component of host defense have been described and the list of primary immunodeficiency syndromes increases yearly. 21 Extrinsic factors such as cigarette smoke, alcohol intoxication and particulate matter inhalation can contribute. However, the most important risks are age and co-morbid illnesses, such as diabetes mellitus, CHF, emphysema, cirrhosis and liver failure. 22 Even in cases of overt immunocompromise, such as neutropenia from chemotherapy, leukemia and HIV disease, the usual CAP pathogens are still important although the differential of etiologies becomes much larger.
The series of pathologic changes seen in classic lobar bacterial pneumonia is described in Table 28-3 . The gray hepatization phase corresponds with successful containment of the infection and improvement in gas exchange, with restoration of the normal hypoxic vasoconstrictor response. This classic pattern does not apply to pneumonia of all etiologies, especially viral or Pneumocystis pneumonia . If microaspiration is the underlying mechanism, a bronchopneumonia pattern is seen and the corresponding phases may not occur.
Pathologic Phases of Classic Lobar Pneumonia
Epidemiology
CAP occurs in every ecological niche in the world from the Arctic regions to deserts to jungle, although the most frequent pathogens may vary. Table 28-4 lists geographic and zoonotic considerations for etiology. A general seasonal pattern occurs, with higher rates in the winter/rainy season, tracking most closely with respiratory viruses such as influenza and RSV.
Zoonoses and Geographic Considerations
| Travel | Pathogen(s) | Exposure | Pathogen(s) |
|---|---|---|---|
| Ohio/Mississippi/St Lawrence river valleys | Bird or bat dung | ||
| Southwestern USA | spp. Hantavirus | Pet birds | |
| Upper Midwest USA woods | Rabbits | ||
| South East Asia | Avian influenza spp. | Exposure to sheep, goats, parturient cats | |
| Hotel or cruise ship stay in last 2 weeks | spp. | Sick dogs | |
CAP occurs in all ages but incidence and mortality are greatest in the extremes of age. 23 In infants, lack of humoral immunity to common pathogens such as influenza, RSV and Streptococcus pneumoniae is the major factor. In the elderly, a senescent host immune system and high frequency of co-morbid illnesses play the greatest role. Females are slightly more likely to develop CAP while males are more likely to die from CAP.
In the USA, 80% of CAP patients are treated as outpatients. Of hospitalized patients, 15–20% require ICU monitoring or interventions.
The major etiologies of CAP are listed in Table 28-5 . By far, the most common bacterial etiology is Strep. pneumoniae . The actual proportion caused by viruses is difficult to determine since the majority of detections are from the upper respiratory tract, and it is unclear whether the virus present in the oropharynx is causing the pneumonia, predisposed to a superinfection bacterial pneumonia, or is simply an innocent bystander. This dilemma is most obvious for human rhinovirus detection in adults.
Common Etiologies of CAP *
| spp. spp. spp. spp. sp. |
SARS, severe acute respiratory syndrome; MERS, Middle East respiratory syndrome.
Less common etiologies are usually associated with specific geographic areas or exposure to specific zoonoses (see Table 28-4 ). Occasionally, more chronic pulmonary infections can masquerade as acute CAP ( Table 28-6 ) and should be considered in endemic areas and if the time course is more indolent. 24
Chronic Pulmonary Infections that May Present as Acute Pneumonia
| ) |
Healthcare-Associated Pneumonia (HCAP)
Concern has been raised about community-onset pneumonia caused by pathogens usually associated with hospital-acquired pneumonia or even ventilator-associated pneumonia, including methicillin-resistant Staphylococcus aureus (MRSA) and multidrug resistant (MDR) gram-negative pathogens. 9 , 25 , 26 , 27 Several community-onset pneumonia syndromes at risk for more drug-resistant pathogens can be defined ( Table 28-7 ). In the USA, transfer of hospitalized patients to long-term ventilator-weaning facilities or acute rehabilitation institutes, rather than completing their recovery in an acute care hospital, does not decrease their risk of the typical hospital-acquired pathogens. These patients have previously been lumped together with those in lower-risk settings such as nursing homes and chronic dialysis units. An episode of aspiration weeks prior to presentation without intervening medical attention is the classic predisposition for anaerobic pneumonia, often complicated by empyema as well. Otherwise, anaerobes play a minor role in usual CAP.
Community-Onset Pneumonia Syndromes in Special Populations
| Syndrome | Examples |
|---|---|
| Hospital-acquired | Recent discharge, long-term weaning facilities, rehabilitation institutes |
| Healthcare-associated | Nursing homes, chronic hemodialysis |
| Immunocompromised | Chemotherapy, HIV disease, transplant, acute leukemia/lymphoma |
| Aspiration | Severe alcoholism, seizure disorder, stroke |
HCAP was proposed as a discrete entity with the goal of identifying those patients who were more likely to receive initially inappropriate antibiotic therapy, and have an associated higher mortality risk. 27 , 28 While early observational studies of culture-positive cases suggest improved outcome from broad-spectrum antibiotic therapy in persons with HCAP risk factors, 27 , 28 prospective studies using the same definition find lower rates of antibiotic-resistant pathogens and many culture-negative cases. 26 , 29 , 30 Even more concerning were reports of adverse outcomes among persons with HCAP risk factors treated with broad-spectrum antibiotic therapy. 26 , 31
Rather than using the original definition derived from healthcare-associated bacteremia, a prospective multicenter study identified six independent risk factors ( Table 28-8 ) for pneumonia caused by pathogens resistant to the usual inpatient antibiotic regimens recommended by Infectious Diseases Society of America (IDSA)/ American Thoracic Society (ATS) guidelines. 26 While the risk factors were similar to the original, the incidence of drug-resistant pathogens was not significantly increased until three or more risk factors are present. A separate analysis specifically for MRSA found that presence of one MRSA-specific risk factor (prior MRSA infection/colonization, chronic hemodialysis, or heart failure) and another pneumonia-specific risk factor may warrant MRSA coverage (but not dual anti-pseudomonal antibiotics). Importantly, this new definition would result in significantly fewer patients receiving broad-spectrum antibiotics than the original HCAP definition. 9
Criteria for Healthcare-Associated Pneumonia (HCAP)
| Original Criteria | Pneumonia-Specific Criteria |
|---|---|
| Hospitalization for ≥2 days in previous 90 days | Hospitalization for ≥2 days in previous 90 days |
| Nursing home or extended care facility residents | Antibiotics in previous 90 days |
| Chronic home infusion therapy | Non-ambulatory status |
| Chronic dialysis within 30 days | Tube feedings |
| Home wound care | Immunocompromise |
| Family member with MDR pathogen | Gastric acid suppressive agents |
| Immunosuppressive disease/therapy | |
Community-Acquired MRSA CAP
The MRSA identified in patients with HCAP risk factors is likely a hospital-acquired strain. However, in the USA a specific USA300 strain of MRSA causes CAP in previously healthy patients, specifically without HCAP or other risk factors for MDR pathogens. 32 , 33 Many of the characteristic presenting features of this MRSA strain ( Table 28-9 ), as well as the methicillin-sensitive variant, are a result of exotoxin production. 32 The Panton–Valentine Leukocidin (PVL) gene is an efficient marker of toxigenic strains but is not the main exotoxin involved in the increased lethality. 33 The USA300 strain is increasingly being found in hospital-acquired MRSA infections, blurring some of the epidemiologic distinctions.
Clinical Features Suggesting Community-Acquired MRSA Pneumonia
| Cavitary infiltrate or necrosis | Neutropenia |
| Rapidly increasing pleural effusion | Erythematous skin rash |
| Gross hemoptysis (not just blood-streaked) | Skin pustules |
| Concurrent influenza | Young, previously healthy |
| Severe CAP in summer months | |
Determination of Etiology
While the diagnosis of CAP is relatively straightforward, determination of etiology is very difficult. 9 Even with aggressive use of currently available diagnostic tests, the etiology remains unknown in >50% of cases.
A complete history of travel, pets and hobbies is critical for suspicion of the less common pathogens (see Table 28-4 ), as well as CAP mimics (see Table 28-1 ). Unfortunately, diagnosis of many of these pathogens requires acute and convalescent serology or tests sent to a reference laboratory, making most treatment empirical.
In general, the greater the likelihood of unusual bacterial pathogens, the greater the yield of diagnostic tests. Patients with severe CAP requiring ICU admission 34 and/or HCAP risk factors 26 started on broad-spectrum antibiotics have the clearest indication for extensive diagnostic testing, including attempts at obtaining sputum culture. The yield of testing is higher in the critically ill CAP patient, possibly because endotracheal intubation allows direct sampling of the lower respiratory tract. Other indications and the corresponding appropriate tests are listed in Table 28-10 . 9
TABLE 28-10
Indications for More Aggressive Diagnostic Testing in Cap 9
| ICU admission | Cirrhosis/severe chronic liver disease |
| HCAP risk factors | Severe chronic obstructive lung disease |
| Failure of outpatient antibiotic therapy | Asplenia (anatomic and functional) |
| Cavitary infiltrates on presentation | Recent travel (within 2 weeks) |
| Leukopenia | Positive or pneumococcal urinary antigen test |
| Active alcohol abuse | Pleural effusion |
Biomarkers have been used in an attempt to differentiate viral from bacterial pneumonia. The best validated is procalcitonin (PCT). 10 , 35 This pro-hormone is elevated in uncontrolled bacterial infections and actively suppressed by the interferon response induced in many viral pneumonias. However, PCT may be low in atypical pathogen CAP as well and is clearly elevated in severe viral CAP, such as seen in the 2009–2010 influenza A pandemic, with or without evidence of superimposed bacterial pneumonia. C-reactive protein (CRP) is more nonspecific than PCT in CAP but may be a better predictor of treatment failure. 14
Almost every antibiotic approved by the US Food and Drug Administration in the past four decades has an indication for CAP. In general, keys to appropriate therapy are adequate coverage of Strep. pneumoniae and the atypical bacterial pathogens ( Mycoplasma , Chlamydophila , Legionella ). The recommended regimens from the IDSA/ATS guideline are listed in Table 28-11 . 9 European guidelines differ in that β-lactam antibiotics (typically amoxicillin) remain the recommended agent for mild–moderate CAP. 36 , 37 A recent study from the Netherlands suggests that a strategy of empirical treatment for moderately severe CAP with β-lactam monotherapy is noninferior to either β-lactam–macrolide combination therapy or fluoroquinolone monotherapy. 38 The primary factors to discriminate among the antibiotic options, therefore, should be local resistance patterns in community organisms, recent antibiotic use, which increases the risk of class resistance, 39 and cost.
TABLE 28-11
IDSA/ATS Recommended Empirical Antibiotic Therapy 9
| Disposition | Recommended Class | Typical Examples |
|---|---|---|
| Outpatient | Macrolide | Azithromycin 500 mg po once, then 250 mg q day |
| Doxycycline | Clarithromycin 500 mg po BD | |
| Recent oral antibiotics | Change antibiotic class Consider: Fluoroquinolone Amoxicillin ± clavulanate | |
| Non-ICU inpatient | Respiratory fluoroquinolone | Moxifloxacin 400 mg q day or |
| Levofloxacin 750 mg po q day | ||
| Ceftriaxone 1–2 g q day | ||
| β-lactam and macrolide | Ampicillin–sulbactam 2 g iv q8h plus | |
| Azithromycin 500 mg q day | ||
| ICU patient | Ceftriaxone plus Azithromycin Respiratory fluoroquinolone | |
Since outpatient treatment failure is rare and the guideline-compliant therapy covers 90% of etiologies in hospitalized patients, deviation from these guidelines should have appropriate justification. Presence of risk factors for MDR (see Table 28-8 ) or zoonotic/geographic-specific pathogens (see Table 28-4 ) may justify alternative empirical coverage but should be accompanied by aggressive attempts at diagnosis, in order to appropriately de-escalate broader-spectrum antibiotic therapy. 26 , 40 Quality improvement projects consistently show that as compliance with IDSA/ATS guideline antibiotics increases, mortality rates and length of stay decrease. 41 , 42 Conversely, continuing broad-spectrum antibiotics for CAP patients without documented MDR pathogens is associated with excess mortality. 26 , 31
Macrolides appear to have beneficial effects in excess of their coverage of atypical pathogens, especially in the more severely ill patient. 43 , 44 These benefits may be due to immunomodulatory effects on the host, less cell lysis-induced cytokine release, or inhibition of bacterial virulence factors, such as biofilms, quorum sensing and toxin production.
CA-MRSA would require specific coverage since the regimens in Table 28-11 have inadequate MRSA coverage. For patients with HCAP-MRSA risk factors, linezolid has a 15% better clinical response rate than vancomycin. 45 Because manifestations of the USA300 strain of CA-MRSA CAP are disproportionately exotoxin-mediated (see Table 28-7 ), 32 treatment with antibiotics that suppress toxin production, such as linezolid or clindamycin (added to vancomycin), are preferred and have been associated with lower mortality. 33 Ceftaroline, the only antibiotic approved for CAP recently, has MRSA activity as well.
One of the most critical elements of treatment is early initiation of appropriate antibiotic therapy after the diagnosis of CAP has been made. The first dose should be given in the emergency department (ED) to allow closer monitoring of the initial response and to assure that the initial dose is given promptly. 9 Timing of the first dose is even more important when the patient presents with septic shock; the goal should be initial antibiotic within the first hour. 46
For uncomplicated bacterial CAP, the usual duration of treatment should be 5–7 days. Certain pathogens, such as Legionella , may require up to 2 weeks of therapy. Conversion to an equivalent oral agent is appropriate whenever the patient is clinically improving and able to tolerate food.
Influenza Treatment
Treatment of influenza pneumonia has not been prospectively studied specifically. Experience during the 2009–10 pandemic and retrospective analysis 47 suggest that antivirals should be used if a patient has a radiographic infiltrate, no matter the duration of symptoms. The potential for oseltamivir-resistant strains should be monitored from CDC and local health department information as each influenza season progresses. The major issue is whether antibiotics are always needed for influenza CAP, with no clear data or consensus. For a full description of the use of antiviral therapy, see Chapter 154.
Other Management
Disposition.
The major determinant of the cost of CAP care is the physician's decision to hospitalize. Of CAP patients who present to the ED, 40–60% are admitted, 22 , 48 , 49 with considerable variability in admission for patients with similar clinical characteristics. Use of scoring systems, such as the Pneumonia Severity Index (PSI) 22 and the CURB-65 Score 50 that were developed specifically to guide admission decisions, result in fewer admissions of low acuity patients with no increase in adverse outcomes. 48 PSI is a complex score, requiring formal scoring or electronic decision support whereas CURB-65 (confusion, uremia, respiratory rate, blood pressure, age >65 years) is both easy to remember and calculate, although not as well validated as PSI. Both scores are valid for analysis of groups of CAP admissions, but admission of low score patients is legitimate, for both objective reasons (e.g. low arterial saturations) and subjective (e.g. unreliable home support, concern regarding compliance).
Decisions regarding initial ICU placement of tenuous CAP patients probably have the greatest potential impact on mortality. Patients transferred to the ICU within 48 hours of initial admission to a general medical service have higher mortality than those with an obvious need for ICU care (mechanical ventilation or hypotension requiring vasopressors) at the time of admission. 49 , 51 , 52 The fraction of hospitalized pneumonia patients admitted to the ICU also varies widely (5–20%) depending on hospital and health system characteristics. 49 , 53 , 54 , 55
The IDSA/ATS guidelines suggest that presence of > 3 of a group of nine minor criteria ( Table 28-12 ) warrant consideration for ICU admission. 9 Other scores to predict clinical deterioration with similar parameters have also been developed and validated. 53 , 54 , 55 For each, the probability of need for invasive ventilatory or vasopressor therapy increases with increasing number of criteria or points, with a threshold score around three to consider ICU admission. All these ICU admission scores are overly sensitive, resulting in substantially more ICU admissions if followed rigidly. 9 , 49 The most appropriate use of these scores may be to focus attention on patients with high scores while still in the ED. A quality-improvement study demonstrated that increased attention in the ED to patients with > 3 IDSA/ATS minor criteria resulted in decreased mortality (23.4% to 5.7%) and fewer floors to ICU transfers (32.0% to 14.8%) without significantly increasing direct ICU admissions. 49
TABLE 28-12
Minor Criteria for Consideration of ICU Admission for Severe CAP
| IDSA/ATS Criteria | Other Criteria |
|---|---|
| Confusion | Lactic acidosis |
| Uremia (BUN >20 mg/dL) | pH <7.30–7.35 |
| Tachypnea (RR >30/min) | Low albumin |
| Bilateral radiographic infiltrates | Hyponatremia (<130 mEq/L) |
| Severe hypoxemia (P/F <250) | Leukocytosis >20 x10 /L |
| Thrombocytopenia | Hypoglycemia |
| Hypotension requiring aggressive fluid resuscitation | |
| Hypothermia | |
| Leukopenia | |
BUN, blood urea nitrogen; RR, respiratory rate; P/F, P aO 2 / F iO 2 ratio.
Pleural Effusion
A new pleural effusion in a patient admitted with CAP should always prompt concern for empyema or complicated parapneumonic effusion (generally pleural fluid pH <7.2). Early diagnosis by thoracentesis, placement of a chest tube and use of tissue plasminogen activator combined with DNAase can prevent the need for surgical intervention in the majority of cases. 56 Management of pleural effusions in patients with CHF and intermittent pleural effusions is less straightforward but thoracentesis in all unclear situations is warranted.
Adjunctive Treatment
Some patients benefit from aerosolized β-agonist bronchodilators for wheezing or other bronchial hygiene maneuvers for difficult expectoration. Patients with viral lower respiratory tract infections occasionally require anticholinergic aerosols to control nonproductive cough.
Use of systemic corticosteroids in CAP patients who have no other indication, e.g. asthma or COPD exacerbation associated with pneumonia, remains controversial. In moderate disease, a potential benefit of shortening hospitalization is counterbalanced by an increased risk of superinfection. 57 In severe viral pneumonia, either SARS or the 2009–2010 influenza pandemic, 58 steroid use was associated with worse outcomes.
Exacerbation of Co-Morbid Illnesses
As mentioned, CAP can exacerbate underlying chronic illnesses such as asthma and COPD, diabetes mellitus and CHF. Up to 15–20% of patients admitted with pneumococcal CAP can have a new cardiovascular diagnosis during the acute hospitalization, including acute myocardial infarction, atrial fibrillation and other arrhythmias, or CHF. 59 Destabilization of co-morbid illness is more likely to cause hospital readmission than complications of CAP or its treatment.
The main CAP preventive measures are vaccination and smoking cessation. 9 Even among patients without obstructive lung disease, smokers are at increased risk of pneumococcal CAP.
Influenza Vaccination
Two forms of influenza vaccine are available – intramuscular inactivated influenza vaccine and intranasal live-attenuated cold-adapted influenza vaccine. The latter is contraindicated in immunocompromised patients. Specific vaccine components are reassessed yearly based on the main circulating strains in the opposite hemisphere. In the event of an influenza outbreak, unprotected patients at risk from complications should be vaccinated immediately and given chemoprophylaxis with oseltamivir for 2 weeks, at which time vaccine-induced antibody levels should be sufficiently protective.
Pneumococcal Vaccine
A pneumococcal polysaccharide vaccine (PPV23) and a protein conjugate vaccine (PCV13) are both available in the USA. The vaccine efficacy of PPV23 has been questionable, particularly in the elderly and other at-risk populations. Administration of the protein conjugate vaccine to children has led to an overall decrease in the prevalence of antimicrobial-resistant pneumococci and in the incidence of invasive pneumococcal disease among both children and adults. 18 , 60 However, vaccination may result in replacement of vaccine serotypes with nonvaccine serotypes, as was seen with serotypes 19A and 35B after introduction of the original 7-valent conjugate vaccine. 61 The 13-valent conjugate vaccine is now also recommended for the elderly and for younger immunocompromised patients (see also Chapter 177).
Key References
- Americam Thoracic Society, Infectious Diseases of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005; 171 (4):388–416. [ PubMed ] [ Google Scholar ]
- Attridge R.T., Frei C.R., Restrepo M.I. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011; 38 :878–887. [ PubMed ] [ Google Scholar ]
- Charles P.G., Wolfe R., Whitby M. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008; 47 :375–384. [ PubMed ] [ Google Scholar ]
- Charlson E.S., Bittinger K., Haas A.R. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011; 184 :957–963. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dean N.C., Bateman K.A., Donnelly S.M. Improved clinical outcomes with utilization of a community-acquired pneumonia guideline. Chest. 2006; 130 :794–799. [ PubMed ] [ Google Scholar ]
- Fine M.J., Auble T.E., Yealy D.M. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997; 336 :243–250. [ PubMed ] [ Google Scholar ]
- Griffin M.R., Zhu Y., Moore M.R. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013; 369 :155–163. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lim H.F., Phua J., Mukhopadhyay A. IDSA/ATS minor criteria aided pre-ICU resuscitation in severe community-acquired pneumonia. Eur Respir J. 2014; 43 :852–862. [ PubMed ] [ Google Scholar ]
- Maisel A., Neath S.X., Landsberg J. Use of procalcitonin for the diagnosis of pneumonia in patients presenting with a chief complaint of dyspnoea: results from the BACH (Biomarkers in Acute Heart Failure) trial. Eur J Heart Fail. 2012; 14 :278–286. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mandell L.A., Wunderink R.G., Anzueto A. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007; 44 (Suppl. 2):S27–S72. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Musher D.M., Rueda A.M., Kaka A.S. The association between pneumococcal pneumonia and acute cardiac events. Clin Infect Dis. 2007; 45 :158–165. [ PubMed ] [ Google Scholar ]
- Rahman N.M., Maskell N.A., West A. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med. 2011; 365 :518–526. [ PubMed ] [ Google Scholar ]
- Rello J., Lisboa T., Lujan M. Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest. 2009; 136 :832–840. [ PubMed ] [ Google Scholar ]
- Shindo Y., Ito R., Kobayashi D. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013; 188 :985–995. [ PubMed ] [ Google Scholar ]
- Journal club
- Subscriptions
- Advanced search

Advanced Search
Hot topics and current controversies in community-acquired pneumonia
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected]
- Info & Metrics
Community-acquired pneumonia (CAP) is one of the most common infectious diseases, as well as a major cause of death both in developed and developing countries, and it remains a challenge for physicians around the world. Several guidelines have been published to guide clinicians in how to diagnose and take care of patients with CAP. However, there are still many areas of debate and uncertainty where research is needed to advance patient care and improve clinical outcomes. In this review we highlight current hot topics in CAP and present updated evidence around these areas of controversy.
Community-acquired pneumonia is the most frequent cause of infectious death worldwide; however, there are several areas of controversy that should be addressed to improve patient care. This review presents the available data on these topics. http://bit.ly/2ShnH7A
- Introduction
Community-acquired pneumonia (CAP) is the most frequent cause of death in developing countries [ 1 ]. CAP kills more people than all other infectious diseases around the globe [ 2 ], and is responsible for more than 3 million deaths a year. Despite the mortality burden CAP has been recently recognised as a neglected disease [ 3 ]. CAP also has an important economic cost to healthcare systems, with more than USD 10 billion a year spent to treat CAP patients in the USA alone [ 4 , 5 ]. More prevalent in patients younger than 5 years old and older than 65 years old, CAP is a more severe and more frequently fatal disease in older adults [ 6 ].
Many guidelines have been published to help clinicians diagnose and take care of CAP patients. The American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines are the most frequently cited and most widely adopted worldwide [ 7 ]. However, the most recent version of these guidelines was published more than 10 years ago, although a new version is expected to be published later this year. During the past decade new evidence has been published in the CAP field: new treatments are now available, extensive data has been published regarding risk factors for drug-resistant pathogens and there has been substantial focus on short- and long-term complications arising in patients with CAP [ 8 – 11 ]. In this review we will highlight current hot topics in pneumonia and discuss the state of the current evidence regarding these areas of controversy.
- What is the role of serum biomarkers in diagnosis, prognosis and antibiotic stewardship strategies in CAP patients?
Biomarkers are molecules that represent normal biological pathways, pathogenic processes or pharmacological response to therapeutic interventions. These molecules have been used to diagnose diseases or assess effects of a certain treatments [ 12 ]. Among biomarkers that have been assessed in the setting of CAP, C-reactive protein (CRP) and procalcitonin (PCT) are the most extensively studied. Both have been used in numerous clinical scenarios with varying results, but it is generally accepted that these biomarkers have some utility in the diagnosis and prognosis of CAP and may also be useful to guide antibiotic stewardship strategies, in particular limiting the duration of antibiotic therapy [ 13 ]. Other serum biomarkers, such as pro-adrenomedullin, interleukin (IL)-6 and fibroblast growth factor (FGF)21, have recently emerged as promising molecules but there is insufficient evidence at present to have a clear consensus on their clinical utility in CAP [ 12 , 14 ].
Biomarkers may be helpful in the diagnosis of CAP, especially in patients who present with atypical signs and symptoms or comorbid conditions that could make the diagnosis challenging. There are several studies that have demonstrated benefits of CRP and PCT in CAP patients [ 12 , 15 ]. CRP has been shown to have an area under the curve (AUC) between 0.76 and 0.84 for CAP diagnosis, with better accuracy when it is combined with classical pneumonia clinical findings (AUC: 0.92). CRP has a positive likelihood ratio (LR + ) of five when CRP concentration is above 200 mg·L −1 and a negative likelihood ratio (LR − ) <0.2 when is below 75 mg·L −1 [ 15 , 16 ]. However, CRP might be increased by other clinical situations and currently there is no consensus about which cut-off value should be used for CAP diagnosis. In a recent systematic review including a total of 2194 patients, values of CRP ≤20 mg·L −1 had a LR + of 2.1 and a LR − of 0.33, values ≤50 mg·L −1 had a LR+ of 3.43 and a LR − of 0.34 and values >100 mg·L −1 had LR+ 5.01 and LR − of 0.54 for CAP diagnosis. This information suggests that CRP is not sensitive or specific enough to diagnose CAP [ 17 ].
With these limitations in mind, interest has grown around PCT and other biomarkers. A recent study showed that a PCT >0.1 ng·mL −1 could help identify patients with CAP in the emergency department with a sensitivity of 78% and a specificity of 80% [ 18 ]; however, other studies have shown different outcomes. L e B el et al. [ 19 ] showed that PCT >0.25 μg·L −1 only reached a sensitivity of 50% and a specificity of 64.7%. PCT is elevated in patients with bacterial pneumonia and not in patients with viral CAP in the absence of bacterial coinfection [ 20 , 21 ]. This ability to discriminate between viral and bacterial infection is also true in patients with severe pneumonia [ 22 ]. However, some data published by the CAPNETZ network showed that PCT may not be elevated in CAP when the pathogen is Mycoplasma pneumoniae or Legionella pneumophila , which is an important limitation [ 23 ]. At present, no biomarker is accurate enough to be used to determine whether CAP is present or not, or to determine if empiric antibiotic therapy can be withheld because of a presumptive viral pathogen.
CRP and PCT might be useful to determine the prognosis of CAP patients. Higher levels of CRP or PCT reflect a greater inflammatory response that could be related to more severe infection and therefore worse outcomes [ 12 ]. Many studies have been conducted to study the relationship between certain biomarkers and both severity and mortality in CAP [ 24 ]. Consistent with uncontrolled inflammation being a bad prognostic sign, failure to reduce CRP levels by at least 50% after 3 days is independently associated with higher mortality [ 25 ]. Patients with higher 30-day mortality risk have elevated concentrations of CRP, PCT, IL-6 and IL-8. Importantly, IL-6 and CRP are independently associated with mortality [ 26 ]. When CRP is added to CURB65 (confusion, urea >7 mmol·L −1 , respiratory rate ≥30 breaths·min −1 , blood pressure <90 mmHg (systolic) or ≤60 mmHg (diastolic), age ≥65 years), the AUC for the 30-day mortality prediction improves from 0.82 to 0.85 [ 27 ]. Additionally, PCT had an AUC of 0.65 to predict treatment failure in patients with CAP [ 28 ] and elevated serum PCT was associated with increased 1-year mortality (HR 1.8) [ 18 ]. While these are all interesting observations, at present there are no apparent cut-off values for CRP or PCT that enable them to be routinely used to aid clinical assessment of individual patient prognosis.
New putative biomarkers are frequently reported but have so far failed to become widely available. For example, FGF21 was recently found to be effective to discriminate patients with moderate-to-severe pneumonia, predict longer length of hospital stay and 30-day mortality when compared with PCT and CRP [ 29 ]. Mid-regional pro-adrenomedullin is another recently described biomarker with an AUC of 0.74 for CAP diagnosis and higher levels predicting greater complications [ 30 ]. Further research is needed to determine if these and other new biomarkers have real utility in the general clinical setting.
Both CRP and PCT may be useful for antibiotic stewardship strategies [ 12 ], because they can be monitored to evaluate effectiveness of antibiotic treatment and may reduce antibiotic duration, especially when this exceeds the normal duration of 5–7 days [ 13 ]. In this regard, CRP could be used to identify patients ready for hospital discharge [ 31 ]. In a large prospective controlled randomised trial with 1359 patients using a PCT-based algorithm to guide antibiotic duration led to lower antibiotic exposure in patients with CAP. The authors suggested that PCT >0.25 μg·L −1 should be used to start antibiotics and recommended to cease antibiotics when, after 3, 5 or 7 treatment days, control PCT was below 0.25 μg·L −1 . They also recommended that when values are very high, withholding antibiotics should occur when patient had a decrease of the peak value by 80–90% [ 32 ].
As there are no data to suggest empiric antibiotic therapy can safely be withheld in patients with CAP, the main role for PCT is in reducing the duration of antibiotic therapy. As all trials that have shown PCT to be useful had a control arm with a duration of well over 7 days, the utility of PCT is likely to be much higher in centres that have problems convincing clinicians to use shorter, conventional durations of therapy.
- Is a macrolide compulsory in severe CAP?
Severe CAP (sCAP) is known to be associated with higher morbidity, mortality and worse clinical outcomes [ 33 , 34 ]. Several severity scores have been proposed to identify patients with sCAP [ 35 , 36 ]. The Pneumonia Severity Index (PSI) and the British Thoracic Society simplified prediction model (CURB-65) are two of the most frequently used scores. However, these scores do not perform well at predicting which patients will require intensive care unit (ICU) admission, because they tend to overestimate disease severity in patients with advanced age or chronic organ failure. Another strategy to identify patients with sCAP are the severity criteria proposed by the ATS/IDSA guidelines, which have a low positive predictive value biased by the major criteria [ 37 – 43 ]. However, the 2007 IDSA/ATS guidelines recommended using the modified ATS/IDSA criteria specifying that prospective validation of these criteria is still needed [ 7 ].
The question of whether macrolides should be used routinely in sCAP has been around since 1994 [ 44 ]. In 2004, B addour et al. [ 45 ] identified that, in patients with severe pneumococcal pneumonia (defined by a Pitt score>4), the use of macrolide in a combination treatment was associated with lower 14-day mortality, independent even of in vitro activity of the prescribed antibiotics. In a study of patients with severe sepsis due to pneumonia the use of a macrolide was associated with a decrease in 30-day (HR: 0.3) and 90-day mortality [ 46 ]. In a study of intubated patients with sCAP, M artin- L oeches et al. [ 47 ] found that the use of combination therapy (β-lactam/macrolide) was associated with lower ICU mortality. This lower mortality with combination therapy (β-lactam/macrolide) was also observed in a more recent study by O kumura et al. [ 48 ] in which the OR for 30-day mortality was 0.28 compared with monotherapy with a β-lactam. In this study 75.3% had severe pneumonia based on PSI [ 48 , 49 ]. In contrast, A drie et al. [ 50 ] reported an observational cohort study in patients with sCAP admitted to the ICU in which they observed that initial adequate antibiotic therapy, according to current guidelines, was associated with better survival than dual therapy (β-lactam/macrolide versus β-lactam/quinolone). In another study of patients with CAP admitted to the ICU, the authors found that early antibiotic administration and use of combination therapy (macrolide/β-lactam and quinolone/β-lactam) resulted in a lower mortality rate. However, due to the sample size, no difference was observed between combination therapy with a macrolide versus quinolone schemes [ 51 ].
There are only two randomised controlled trials trying to address the issue of the value of additional macrolide therapy. G arin et al. [ 52 ] performed a randomised noninferiority trial including patients with sCAP, defined by 2007 IDSA/ATS severity criteria or PSI category V. After 7 days of treatment, they were not able to show that monotherapy with a β-lactam was not inferior to combination therapy (macrolide/β-lactam). P ostma et al. [ 53 ] conducted a “pragmatic” randomised controlled trial and found no advantage of the addition of a macrolide. However, this trial had major problems with 25% of patients having no radiological confirmation of pneumonia and 40% of patients in the “monotherapy” arm being given empiric combination therapy that included a macrolide. Furthermore, the macrolide in the “combination therapy” arm was overwhelming erythromycin, whereas in the “monotherapy” arm, when a macrolide was given it was either azithromycin or clarithromycin. These problems make it impossible to interpret the findings of P ostma et al. [ 53 ].
Finally, a systematic review that evaluated mortality as an endpoint when comparing macrolide therapy with other regimens in critically ill patients with sCAP, which did not include the two studies previously mentioned, found that macrolide use was associated with a significant 18% (3% absolute) reduction in mortality when compared with non-macrolide schemes [ 54 ]. It is important to highlight that using a macrolide in combination with a β-lactam may have beneficial outcomes not only due to its coverage of atypical pathogens, but because macrolides may also have immunomodulatory effects; such as disruption of biofilm formation, inhibition of quorum sensing, inhibition of bacterial protein synthesis, reduction of bacterial toxin formation ( e.g. pneumolysin and streptolysin), reduced adherence and bacterial motility [ 55 ]. In addition, macrolides also reduce neutrophil chemotaxis, adhesion and accumulation of inflammatory cells, and enhance macrophage phagocytosis and reduce secretion of proinflammatory cytokines [ 56 ]. Macrolides also have some specific effects on the production of pneumolysin, a pore-forming toxin produced by Streptococcus pneumoniae, that is well known to be capable of activating the inflammasome and inducing necroptosis in alveolar macrophages [ 57 – 60 ], which are important mechanisms to induce sCAP. Finally, macrolides can improve mucociliary clearance and inhibit inducible nitric oxide synthase [ 56 ].
With current available data, macrolides should be considered a standard of care in patients with sCAP. In patients admitted with nonsevere CAP a macrolide should probably also be included in the antibiotic regimen; however, the data are less strong. Recent retrospective data suggest that to gain the benefit of the macrolide it may need to be given prior to other antibiotics, but this remains to be confirmed [ 61 ].
- Are macrolides still appropriate as monotherapy in outpatient CAP?
The use of macrolides in outpatients diagnosed with CAP is convenient, due to the simple administration regimen and to their generally sufficient coverage for most frequently isolated pathogens ( S. pneumoniae , Staphylococcus aureus , Haemophilus influenzae and intracellular pathogens) [ 62 ]. However, there is a growing concern about using macrolides in CAP patients due to their cardiovascular effects [ 63 ] and burgeoning resistance [ 64 ].
Macrolide resistance has been reported with increasing frequency worldwide, ranging from 4 to 100% [ 65 ]. Several global surveillance studies such as the Alexander Project and the PROTEKT study were developed to monitor prevalence and distribution of antimicrobial resistance among respiratory pathogens [ 65 ]. The Alexander Project indicated that between 1996 and 1997 the global rate of pneumococcal macrolide resistance was 16.5–21.9%, but it had increased to 24.6% by 2000 in France, Spain and the USA [ 66 , 67 ]. Data from the PROTEKT study also showed a high incidence of pneumococcal resistance to macrolides (31%) in the USA; however, in 2002 a small reduction was documented (27.9%) after introduction of the 7-valent pneumococcal vaccine [ 68 , 69 ]. However, these antibiotic resistance rates relate to macrolides in general and not to pathogens exclusively causing CAP.
In 2008, Y e et al. [ 65 ] conducted an analysis to compare treatment failure among patients with CAP treated with levofloxacin or macrolides (azithromycin, clarithromycin or erythromycin) in an outpatient setting. Out of 7526 patients included in the analysis, 60.6% were treated with macrolides. They found that treatment failure with macrolides was 22.7%. S kalsky et al. [ 70 ] performed a systematic review and meta-analysis of randomised controlled trials comparing macrolides versus quinolones for outpatients with CAP treatment. They did not find strong evidence to support use of macrolide or quinolone monotherapy to treat outpatients diagnosed with CAP. However, they found higher treatment success with quinolones, possibly related to the rising macrolide resistance in S. pneumoniae [ 70 ]. Cardiovascular events (arrhythmias and cardiovascular death) are frequent in patients treated with macrolides [ 71 ]. However, in a systematic review and meta-analysis carried out by W ong et al. [ 63 ] most of the information came from observational studies and not from randomise controlled trials, and the authors found no association for long-term risk ranging from >30 days to >3 years.
With the presented information it is important to emphasise the importance of having the local susceptibility pattern of S. pneumoniae resistance to define whether a macrolide can be used in outpatients diagnosed with CAP. It is also important to highlight that current evidence shows that communities with resistance levels above 20% should not use macrolides as first-line treatment. Finally, it should always be in clinicians' minds that macrolides may induce adverse cardiovascular events, especially in patients with abnormal QT segment or previous arrhythmias, thus, it is mandatory to evaluate the risk/benefit of using macrolides in patients at higher risk of cardiovascular events.
- What are the most useful coadjutant treatments for severe CAP? Should corticosteroids be used?
It is well known that patients with sCAP have an excessive local and systemic inflammatory response that induces tissue destruction, systemic complications and worse clinical outcomes [ 26 , 72 ]. Therefore, researchers have hypothesised that anti-inflammatory and pulmonary protective adjuvants might be good strategies to improve clinical outcomes in CAP patients; however, the available data are controversial [ 73 – 75 ].
Corticosteroid administration is one of the alternatives proposed as coadjutant treatment for CAP [ 76 ]. There are now as many published meta-analyses of corticosteroids in CAP as there are primary studies, something that should always trigger alarm bells [ 76 – 81 ]. The general, but not universal, consensus of these meta-analyses, which do not include the studies mentioned earlier, has been that glucocorticoids reduce mortality in sCAP, but not in nonsevere CAP. It is, however, critically important that clinicians understand how poor the evidence base is for glucocorticoids in CAP and how flawed the meta-analyses are due to their failure to properly critique the studies included. Equally, the potential risks of moderate doses of corticosteroids have been significantly understated [ 76 – 81 ].
The major driver of a mortality advantage in all the meta-analyses is the study by N afae et al. [ 82 ]. This study was a single-centre, single-blinded trial in adults with CAP. 60 patients were randomised to corticosteroids and 20 to placebo. The authors reported a mortality benefit in the steroid group (6.7% versus 31.6%, p<0.05). However, although the manuscript states that randomisation was stratified by severity, no details of the stratification were provided and severity details are generally lacking. More importantly, although the authors report no significant differences in baseline characteristics between the groups, reanalysis of the table provided (assuming a normal distribution given they provide t-scores) shows a very significant difference in the degree of renal impairment at randomisation in the placebo group compared with the corticosteroid group: mean± sd creatinine 1.5±0.8 mg·dL −1 versus 1.14±0.5, p=0.02; mean± sd urea 41.8±19.5 versus 31.4±14.2 mg·dL −1 , p=0.01. It is hardly surprising that a group with normal renal function at enrolment did better than a group with significant renal impairment.
There are also significant problems with bias at baseline in a second study by S abry et al . [ 83 ]. 80 patients were randomised on a 1:1 basis in this multicentre, double-blind, placebo controlled trial in adults with sCAP based on ATS/IDSA criteria [ 7 ]. First, mortality was measured at day eight, not hospital survival, where there was a statistically nonsignificant trend towards lower mortality in the steroid group (38 versus 34, p=0.3). Secondly, while the authors report no significant differences at baseline, their table shows 34 out of 40 patients in the placebo group required mechanical ventilation at baseline (85%), compared with only 26 out of 40 patients in the steroid group (65%). The authors report the p-value as 0.144; however, by Chi-squared it is 0.04 and Fisher's exact test it is 0.07. With 20% more patients requiring mechanical ventilation at study entry, any trend towards improved mortality must be highly suspect.
With respect to other potential adverse effects of steroids, there are two significant concerns. First, there is a reasonable amount of observational data suggesting that steroid use in the setting of influenza may be associated with significantly greater mortality [ 84 ]. Secondly, there is evidence that even a short duration of steroid therapy is associated with complications in the following 90 days, including higher rates of sepsis, pulmonary emboli and fractures [ 85 ]. While not specific to pneumonia, these data underline the point that steroids are not benign drugs, but to demonstrate the adverse impact you need larger studies with longer periods of follow-up [ 86 ].
In summary, it is possible that corticosteroid therapy might be of benefit in a very small subset of patients with sCAP, but the evidence at present is distinctly underwhelming and the risks have been understated and understudied. Extracting tables from manuscripts and compiling the results without critically examining the underlying studies is fraught with problems, especially when the total number of patients enrolled in all the studies is actually quite small. We would strongly recommend that clinicians wait for the results of the several studies that are currently underway to properly identify if there is a subgroup of patients where there is a clear benefit of corticosteroids before considering adding them to routine care.
- Should CAP patients have secondary prevention to avoid systemic complications?
Systemic complications during and after CAP are very frequent [ 87 ], especially in patients with several comorbid conditions and sCAP [ 88 ]. Major cardiovascular events (MACE) are by far the most frequent cardiovascular events associated with CAP [ 8 ]. In several epidemiological studies it has been documented that up to 30% of patients admitted due to CAP may develop MACE [ 89 – 95 ]. Cardiovascular complications include new or worsening arrhythmias, heart failure, myocardial infarction and stroke [ 96 ]. Importantly, patients who develop MACE have an increased mortality when compared with patients with CAP alone. A higher risk of MACE has been identified during acute hospitalisation due to CAP and, importantly, a 10-year increase in risk after CAP was recently identified [ 97 ]. Several underlying mechanisms for MACE have been described; however, it is not clear why some patients develop MACE and others do not. We have recently published that S. pneumoniae, the most frequently identified bacteria in CAP patients, is capable of reaching the heart and inducing cell death with subsequent scar formation during acute pneumonia [ 9 , 11 , 98 , 99 ].
Pathophysiology of MACE in CAP patients has been explained as secondary to inflammatory molecules, hypoxia and oxidative stress; recent studies have also demonstrated dissemination of the causative pathogen to extrapulmonary tissues, in this case the myocardium. For instance, S. pneumoniae has been associated with extrapulmonary tissue spreading and myocardial invasion, dependent on adhesins, choline binding protein A and phosphorylcholine [ 100 ]. Pneumolysin, a pore forming toxin and the most important pneumococcal virulence factor, is not only able to induce necroptosis in alveolar macrophages and cardiomyocytes, but has also been shown to have a direct pro-arrhythmic effect [ 101 ]. A lhamdi et al. [ 101 ] found an important association between cardiac injury and pneumolysin presence in a murine model, in which not only could the toxin induce cardiomyocyte death, but also at non-lysing concentrations it could alter a cell's contractile function.
Risk factors for developing MACE during or after CAP have been recently identified [ 102 ]. C orrales- M edina et al. [ 94 ] compared prediction of cardiovascular events in patients hospitalised due to CAP using a scoring system for stratification of 30-day risk of cardiac complications (age, medical conditions, pulse rate, blood pressure, laboratory and radiographic findings) with PSI score; revealing suboptimal calibration of the latter in this matter. Still, there is no consensus about how to determine risks for developing MACE and how to identify patients at higher risk of developing these fatal complications.
There is a high cardiovascular risk in CAP patients [ 103 , 104 ], thus, finding a way to reduce MACE in these patients must be a priority for the scientific community. Statins are widely used as part of anti-ischaemic treatment in patients who have higher cardiovascular risk, not only for lowering serum cholesterol as they also stabilise already formed atherosclerotic plaques. Moreover, they have anti-inflammatory pleiotropic effects reducing cytokine release, endothelial permeability and overexpressed inducible nitric oxide [ 105 , 106 ]. Therefore, these medications may be strategies to prevent MACE in CAP patients, however, currently there is no data to recommend their routine use.
- Is HCAP a dead concept?
The term healthcare-associated pneumonia (HCAP) was introduced for first time in the 2007 ATS/IDSA guidelines to differentiate a group of patients that, although they were not admitted to the hospital, developed pneumonia due to multidrug-resistant pathogens previously thought to be exclusive to “hospital-acquired pneumonia” [ 107 ]. In addition, HCAP patients had greater morbidity and mortality than regular CAP patients [ 108 , 109 ].
HCAP represents a heterogeneous group of patients that have a close relationship with healthcare systems and thus, may have different microbiology, severity and clinical outcomes. HCAP patients are those living in healthcare facilities such as nursing homes, those in contact with dialysis centres, those having chronic intravenous fluid therapy or wound care at home, and those with hospitalisation within the past 3 months. Since its introduction HCAP has been extensively studied in multiple settings and the conclusion is that it has poor validity outside of a few centres in the USA [ 110 – 112 ].
In the original studies, the comparison between CAP and HCAP showed a higher prevalence of aetiologies that require treatment with broad spectrum antibiotics, such as methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa and extended-spectrum β-lactamase-producing Enterobacteriaceae , in HCAP patients [ 108 , 110 , 113 ]. K ollef et al. [ 114 ] published the original manuscript describing HCAP in which they reported higher in-hospital mortality rates and longer length of hospital stay compared with regular CAP patients. They proposed that severity, prognosis and microbiological characteristics of HCAP resemble hospital-acquired pneumonia. A major limitation of these studies is that the cohorts only included culture-positive pneumonia patients, reported in a multi-institutional administrative database in the USA. This is a big limitation because it is well documented that only around 37% of CAP patients have culture-positive pneumonia, which is an important selection bias [ 4 ]. Prevalence of multidrug-resistant pathogens in the USA is another fact to keep in mind since healthcare systems are very different around the world and these data may not be generalisable for other countries. Nursing homes in the USA are centres with a wide range of patients, including patients with a lot of comorbid conditions and requiring several in-house procedures (such as i.v. fluid administration and i.v. antibiotics, among others). By contrast, nursing homes globally only take care of senior citizens that usually do not require healthcare interventions.
Most studies carried out after the study by K ollef et al. [ 114 ] have failed to confirm the prevalence of multidrug-resistant pathogens reported in the original manuscript [ 115 ]. M etersky et al. [ 116 ] used a cohort of 61 651 patients with HCAP criteria in the United States Veterans Health Administration dataset and documented that 1.9% were diagnosed with Pseudomonas pneumonia and 1% with MRSA pneumonia, which is far from the prevalence described by K ollef et al. [ 114 ]. Moreover, excess mortality described in HCAP does not necessarily have to be associated with pneumonia per se , because a patient's age and comorbid conditions are important predictors of worse outcomes. Since HCAP patients are usually over 60 years old with several comorbid conditions, this is an important bias for the HCAP term and its clinical characteristics. To support this, S hindo et al. [ 115 ] observed in a prospective study that age and comorbid conditions might play a stronger role in patients infected with multidrug-resistant pathogens than the HCAP category. Similar conclusions have been reached in more recent studies [ 117 , 118 ].
To further characterise this important clinical problem, we developed the Global Initiative for MRSA pneumonia (GLIMP study) [ 119 ]. In this study, we enrolled more than 3700 patients in more than 120 hospitals across six continents; showing that MRSA pneumonia is very uncommon, with a global prevalence of around 5%. We did not find an association between previously described HCAP risk factors with the development of MRSA pneumonia or with CAP due to P. aeruginosa [ 120 ]. In contrast, we found that sCAP, previous MRSA colonisation and recurrent skin infections were risk factors for MRSA pneumonia [ 10 , 119 ]. Moreover, we found that very severe COPD, previous documented bronchiectasis, chronic use of tracheostomy and requiring mechanical ventilation and/or vasopressors were risk factors for P. aeruginosa infection in CAP patients [ 120 ]. We also reported a very different epidemiology of MRSA and P. aeruginosa infection across continents, and even among countries within the same continent. As we and other authors have pointed out in recent publications regarding HCAP utility, there are two findings consistent with infections by MRSA or P. aeruginosa : detection of the pathogen prior the actual hospitalisation and sCAP, since these findings bring more implications for the patient in case the aetiology is not covered properly with empiric treatment [ 121 – 124 ].
Evidence suggests that HCAP is not a concept that will remain in clinical practice or research, since it is not as useful as it seemed when first introduced. Instead of being useful, this concept might be very confusing for clinicians taking care of patients with CAP. We strongly believe that is better to identify individual risk factors for each possible aetiological pathogen in CAP patients [ 10 , 119 , 120 , 122 – 124 ], rather than attempting to categorise patients in a very heterogeneous group such as HCAP and provide the same treatment for all of them. One size does not fit all our patients.
CAP has accompanied humanity since the beginning of civilisation and still represents a public health issue all around the world. The questions discussed in this review only represent a small part of all the areas of uncertainty that physicians face in their clinical practice. CAP is usually misconceived in real life as a simple disease, but as Steve Jobs once said: “simple can be harder than complex”.
Procalcitonin and C-reactive protein are widely available biomarkers useful for diagnosis, prognosis and stewardship strategies in community-acquired pneumonia (CAP) patients. New biomarkers are promising to improve patient care; however, more data are needed.
Macrolide usage in combination therapy with a β-lactam should be the standard of care in patients with severe CAP.
Knowledge of local susceptibility patterns in Streptococcus pneumoniae is mandatory to define whether macrolides can be used in outpatients with CAP.
Corticosteroids should not be routinely used in CAP, especially when influenza is the aetiological pathogen.
Major adverse cardiovascular events are an important cause of death and morbidity in CAP patients; studies are needed to determine how to prevent them.
The term healthcare-associated pneumonia (HCAP) is not a useful concept for clinical practice or for research and should be abandoned.
Conflict of interest: D. Severiche-Bueno has nothing to disclose.
Conflict of interest: D. Parra-Tanoux has nothing to disclose.
Conflict of interest: L.F. Reyes has nothing to disclose.
Conflict of interest: G.W. Waterer has nothing to disclose.
- Copyright ©ERS 2019
Breathe articles are open access and distributed under the terms of the Creative Commons Attribution Non-Commercial Licence 4.0.
- ↵ GBD 2015 LRI Collaborators . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015 . Lancet Infect Dis 2017 ; 17 : 1133 – 1161 . OpenUrl CrossRef PubMed
- ↵ GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015 . Lancet 2016 ; 388 : 1459 – 1544 . OpenUrl CrossRef PubMed
- Aliberti S ,
- Dela Cruz CS ,
- Sotgiu G , et al.
- Wunderink RG , et al.
- Wunderink RG ,
- Cilloniz C ,
- Polverino E , et al.
- Mandell LA ,
- Anzueto A , et al.
- Restrepo MI ,
- Hinojosa CA , et al.
- Chalmers JD ,
- Aliberti S , et al.
- Gilley RP ,
- Gonzalez-Juarbe N ,
- Shenoy AT , et al.
- Shaddock EJ
- Restrepo MI
- Harbarth S ,
- Stolz D , et al.
- Ruiz-González A ,
- Utrillo L ,
- Bielsa S , et al.
- Truong QA ,
- Gaggin HK , et al.
- Hausfater P ,
- Chenevier-Gobeaux C , et al.
- Christ-Crain M ,
- Pfister R ,
- Kochanek M ,
- Leygeber T , et al.
- Marre R , et al.
- Simonetti A ,
- Garcia-Vidal C , et al.
- Schuetz P ,
- Zimmerli W , et al.
- D'Angelo G ,
- Kellum JA , et al.
- Menéndez R ,
- Martínez R ,
- Reyes S , et al.
- Morgenthaler NG ,
- Ebrahimi F ,
- Wolffenbuttel C ,
- Blum CA , et al.
- Zhao H , et al.
- Coelho LM ,
- Salluh JI ,
- Soares M , et al.
- Wolbers M , et al.
- Martin-Loeches I , et al.
- Cavallazzi R ,
- Wiemken T ,
- Arnold FW , et al.
- Schembri S ,
- Chalmers JD
- Meduri GU ,
- Mortensen EM , et al.
- de Roux A ,
- Bauer T , et al.
- Marrie TJ ,
- Obrosky DS , et al.
- Macfarlane JT
- van der Eerden MM ,
- Laing R , et al.
- Aronsky D ,
- Gaillat J ,
- Sedallian A
- Baddour LM ,
- Klugman KP , et al.
- Mortensen EM ,
- Waterer GW , et al.
- Martin-Loeches I ,
- Rodriguez A , et al.
- Okumura J ,
- Takahashi K , et al.
- Schwebel C ,
- Garrouste-Orgeas M , et al.
- Gattarello S ,
- Lagunes L ,
- Vidaur L , et al.
- Carballo S , et al.
- Postma DF ,
- van Werkhoven CH ,
- van Elden LJ , et al.
- Eurich DT , et al.
- Mantero M ,
- Emmet O'Brien M ,
- Martin-Loeches I
- Brissac T ,
- Shenoy AT ,
- Patterson LA , et al.
- Durandt C ,
- Mitchell TJ , et al.
- Reboul CF ,
- Whisstock JC ,
- Dunstone MA
- Metersky ML ,
- Musher DM ,
- Anand S , et al.
- Jacobs MR ,
- Bajaksouzian S ,
- Zilles A , et al.
- Sikirica V ,
- Schein JR , et al.
- Klugman KP ,
- Felmingham D ,
- Jacobs MR , et al.
- Whitney CG ,
- Farley MM ,
- Hadler J , et al.
- Skalsky K ,
- Lador A , et al.
- Pugh MJ , et al.
- Tuomanen EI ,
- Wunderink R , et al.
- Cruz CS D ,
- Christiani DC , et al.
- Pastores SM , et al.
- Confalonieri M ,
- Potena A , et al.
- Wang Y , et al.
- Avni T , et al.
- Siemieniuk RA ,
- Alonso-Coello P , et al.
- Chen Y , et al.
- Haranaga S , et al.
- Amany F , et al.
- Sabry NAO EE
- Rodrigo C ,
- Leonardi-Bee J ,
- Nguyen-Van-Tam J , et al.
- Waljee AK ,
- Rogers MA ,
- Lin P , et al.
- Feldman C ,
- Linde-Zwirble W ,
- Mayr F , et al.
- Corrales-Medina VF
- Cangemi R ,
- Falcone M , et al.
- Corrales-Medina V , et al.
- Corrales-Medina VF ,
- Alvarez KN ,
- Weissfeld LA , et al.
- Taljaard M ,
- Yende S , et al.
- Fine MJ , et al.
- Shachkina S , et al.
- Soto-Gomez N ,
- Anzueto A ,
- Bradley KM ,
- Gao G , et al.
- Alhamdi Y ,
- Abrams ST , et al.
- Griffin AT ,
- Wiemken TL ,
- Calvieri C ,
- Mandal P , et al.
- Albert MA ,
- Danielson E ,
- Rifai N , et al.
- ↵ American Thoracic Society , Infectious Diseases Society of America . Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia . Am J Respir Crit Care Med 2005 ; 171 : 388 – 416 . OpenUrl CrossRef PubMed
- Montravers P ,
- Rodrigo-Troyano A ,
- Shindo Y , et al.
- Ottosen J ,
- Niederman MS ,
- Chastre J , et al.
- Dalhoff K ,
- Carratalà J ,
- Mykietiuk A ,
- Fernández-Sabé N , et al.
- Kollef MH ,
- Tabak YP , et al.
- Kobayashi D , et al.
- Mortensen EM
- Tomczyk S ,
- Bramley AM , et al.
- Jones MM , et al.
- Faverio P , et al.
- Reyes LF , et al.
- Radovanovic D ,
- Jankovic M , et al.
- Gramegna A ,
- Di Pasquale M , et al.
- Carugati M ,

- Table of Contents
- Index by author
Thank you for your interest in spreading the word on European Respiratory Society .
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Respiratory clinical practice
- Respiratory infections and tuberculosis
- Tweet Widget
- Facebook Like
- Google Plus One
More in this TOC Section
- TB screening in the European migrant population: current practices
- Imaging in the diagnosis and management of fibrosing ILDs
- Respiratory problems associated with liver disease in children
Related Articles
- Qazi Corner
- Biosimilars Spotlight
- DME & nAMD On-Demand Presentation

- Multimedia Series
- Conferences
- Advisory Board
The Changing Community-Acquired Pneumonia Landscape with Thomas File, MD, MSc, MACP
File joins Lungcast to review the ATS-IDSA 2019 guideline updates for community-acquired pneumonia, and advances to diagnostics and care.
Episode Higlights
0:16 Intro 2:29 Updated community-acquired pneumonia guidelines 5:41 Diagnostic tools for CAP following COVID-19 9:35 Procalcitonin levels as a biomarker 12:30 The CAP treatment algorithm 18:36 Best practices for antibiotic stewardship 20:40 New CAP therapies on the horizon 22:36 ACIP recommendations for vaccination 24:30 Remaining areas of CAP uncertainty 26:30 Final thoughts 27:25 Outro
Among the most commonly encountered and morbid conditions globally, community-acquired pneumonia (CAP) is associated with nearly 5 million annual outpatient and emergency room visits in the US annually. It is the second most common cause of hospitalization and is associated with a notable risk of recurring disease; approximately 1 in 10 patients hospitalized with CAP are re-hospitalized with a new episode in the same year.
The means to treat the highly common and burden condition, though, are well-established and even gradually improving. In fact, updates to guidelines originally published by the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) in 2019 reflect progress being made in CAP diagnostics and care. 1
In the June 2024 episode of Lungcast , Thomas M. File, Jr., MD, MSc, MACP, distinguished physician in the infectious disease division at Summa Health, and professor emeritus of internal medicine and master teacher of the infectious disease section at Northeast Ohio Medical University, joins to provide a comprehensive update on the modern management and research into CAP.
Among the topics File reviewed with host Albert Rizzo, MD, chief medical officer of the American Lung Association, were the ATS-IDSA guidelines around the standard treatment algorithm for CAP, the evolution of diagnostic tools following the COVID-19 pandemic and introduction of mRNA technology, as well as the role of potential biomarkers including procalcitonin.
File and Rizzo additionally discussed pneumonia therapies in development, improved focus on antibiotic stewardship in CAP following derailment due to the pandemic, and File’s 2023 review of gaps in CAP research, including the role of cardiovascular events. 2
Lungcast is a monthly respiratory news podcast series hosted by Albert Rizzo, MD, chief medical officer of the ALA, and produced by HCPLive.
Subscribe to Lungcast on Spotify here , or listen to the episode below.

Pulmonology Month in Review: May 2024

Diabetes Dialogue: GLAM and CRISTAL, Updates in Pregnancy

FDA Extends Dupilumab COPD Decision to September 2024

Don't Miss a Beat: Semaglutide and the Future of Kidney Disease, with Brendon Neuen, MBBS, PhD

Traditional Assessment Tools May Underestimate Cardiovascular Risk in AATD

NOTUS Trial Confirms Dupixent Benefit in COPD with Type 2 Inflammation
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

- Help & FAQ
Community-Acquired Pneumonia in Childhood
Research output : Chapter in Book/Report/Conference proceeding › Chapter
The diagnosis of pneumonia in children is challenging due to overlapping clinical features of viral and bacterial etiologies of pneumonia, in addition to other lower respiratory tract pathologies. Although limited, chest radiography remains the reference standard for diagnosis. In all pediatric age groups excluding neonates, viral pathogens are the most common etiology of pneumonia, with S. pneumoniae as the most common typical bacterial pathogen. Diagnostic testing should be focused to those at higher risk for pathogen detection or in situations where diagnostic testing results will change management. Treatment for bacterial pneumonia should begin with narrow-spectrum beta-lactam antibiotics, but broadening to third-generation cephalosporins may be appropriate in certain situations.
| Original language | English (US) |
|---|---|
| Title of host publication | Encyclopedia of Respiratory Medicine, Second Edition |
| Publisher | |
| Pages | 119-131 |
| Number of pages | 13 |
| Volume | 6 |
| ISBN (Electronic) | 9780081027240 |
| ISBN (Print) | 9780081027233 |
| DOIs | |
| State | Published - Jan 1 2021 |
- Bacterial pneumonia
- Chest radiography
- Community-acquired pneumonia
- Lower respiratory tract infection
- Lung ultrasound
- Pneumonia biomarkers
- Viral pneumonia
ASJC Scopus subject areas
- General Medicine
Access to Document
- 10.1016/B978-0-08-102723-3.00013-5
Other files and links
- Link to publication in Scopus
- Link to the citations in Scopus
Fingerprint
- Diagnosis Medicine and Dentistry 100%
- Community-Acquired Pneumonia Medicine and Dentistry 100%
- Childhood Medicine and Dentistry 100%
- communities INIS 100%
- pneumonia INIS 100%
- Pneumonia Medicine and Dentistry 75%
- Pathogen Medicine and Dentistry 75%
- Etiology Medicine and Dentistry 50%
T1 - Community-Acquired Pneumonia in Childhood
AU - Popovsky, Erica Y.
AU - Florin, Todd A.
N1 - Publisher Copyright: © 2022 Elsevier Ltd. All rights reserved
PY - 2021/1/1
Y1 - 2021/1/1
N2 - The diagnosis of pneumonia in children is challenging due to overlapping clinical features of viral and bacterial etiologies of pneumonia, in addition to other lower respiratory tract pathologies. Although limited, chest radiography remains the reference standard for diagnosis. In all pediatric age groups excluding neonates, viral pathogens are the most common etiology of pneumonia, with S. pneumoniae as the most common typical bacterial pathogen. Diagnostic testing should be focused to those at higher risk for pathogen detection or in situations where diagnostic testing results will change management. Treatment for bacterial pneumonia should begin with narrow-spectrum beta-lactam antibiotics, but broadening to third-generation cephalosporins may be appropriate in certain situations.
AB - The diagnosis of pneumonia in children is challenging due to overlapping clinical features of viral and bacterial etiologies of pneumonia, in addition to other lower respiratory tract pathologies. Although limited, chest radiography remains the reference standard for diagnosis. In all pediatric age groups excluding neonates, viral pathogens are the most common etiology of pneumonia, with S. pneumoniae as the most common typical bacterial pathogen. Diagnostic testing should be focused to those at higher risk for pathogen detection or in situations where diagnostic testing results will change management. Treatment for bacterial pneumonia should begin with narrow-spectrum beta-lactam antibiotics, but broadening to third-generation cephalosporins may be appropriate in certain situations.
KW - Bacterial pneumonia
KW - Chest radiography
KW - Community-acquired pneumonia
KW - Lower respiratory tract infection
KW - Lung ultrasound
KW - Pediatrics
KW - Pneumonia biomarkers
KW - Viral pneumonia
UR - http://www.scopus.com/inward/record.url?scp=85143097694&partnerID=8YFLogxK
UR - http://www.scopus.com/inward/citedby.url?scp=85143097694&partnerID=8YFLogxK
U2 - 10.1016/B978-0-08-102723-3.00013-5
DO - 10.1016/B978-0-08-102723-3.00013-5
M3 - Chapter
AN - SCOPUS:85143097694
SN - 9780081027233
BT - Encyclopedia of Respiratory Medicine, Second Edition
PB - Elsevier

IMAGES
VIDEO
COMMENTS
This review focuses on advances in the research and care of community-acquired pneumonia in the past two decades. We summarize the evidence around our understanding of pathogenesis and diagnosis, discuss key contentious management issues including the role of procalcitonin and the use or non-use of corticosteroids, and explore the relationships ...
Although community-acquired pneumonia has traditionally been viewed as an acute disease of the lungs, the current understanding is that it is a multisystem disease that can result in acute and ...
Background: This document provides evidence-based clinical practice guidelines on the management of adult patients with community-acquired pneumonia. Methods: A multidisciplinary panel conducted pragmatic systematic reviews of the relevant research and applied Grading of Recommendations, Assessment, Development, and Evaluation methodology for clinical recommendations.
Community-acquired pneumonia is a leading cause of hospitalization and mortality and incurs significant healthcare costs. As disease presentation varies from a mild illness that can be managed as an outpatient to a severe illness requiring treatment in the intensive care unit, diagnosing early and determining the appropriate level of care is important for improving outcomes.[1][2][3][4][5]
Pneumonia is a respiratory infection of the distal airways; it can be acquired in the community or in the hospital, and it can be caused by several types of bacteria, viruses, fungi and other ...
Community-acquired pneumonia is one of the most common infections seen in emergency department patients. There is a wide spectrum of disease severity and viral pathogens are common. After a careful history and physical examination, chest radiographs may be the only diagnostic test required. The first step in management is risk stratification ...
Community-acquired pneumonia is not usually considered a high-priority problem by the public, although it is responsible for substantial mortality, with a third of patients dying within 1 year after being discharged from hospital for pneumoniae. Although up to 18% of patients with community-acquired pneumonia who were hospitalised (admitted to hospital and treated there) have at least one risk ...
Community-Acquired Pneumonia. Long recognized as a major cause of death, pneumonia has been studied intensively since the late 1800s, the results of which led to many formative insights in modern ...
Abstract. Community-acquired pneumonia is not usually considered a high-priority problem by the public, although it is responsible for substantial mortality, with a third of patients dying within 1 year after being discharged from hospital for pneumoniae. Although up to 18% of patients with community-acquired pneumonia who were hospitalised ...
Pneumonia is a leading infectious cause of hospitalization and death among adults in the United States, 1,2 with medical costs exceeding $10 billion in 2011. 3 Routine administration of the ...
Introduction Despite improvements in medical science and public health, mortality of community-acquired pneumonia (CAP) has barely changed throughout the last 15 years. The current SARS-CoV-2 pandemic has once again highlighted the central importance of acute respiratory infections to human health. The "network of excellence on Community Acquired Pneumonia" (CAPNETZ) hosts the most ...
TOPICS. AI in Medicine; Climate Crisis and Health; ... Although both scores are valid for the analysis of groups of admissions for quality improvement or research in community-acquired pneumonia ...
Severe community-acquired pneumonia (SCAP) is usually defined as CAP admitted to an intensive care unit (ICU). The mortality associated with SCAP is still very high, particularly in patients needing mechanical ventilation (30%) [ 1 ]. Indeed, these patients represent an important target population for future research.
Community-acquired pneumonia (CAP) refers to infectious inflammation of the lung parenchyma developing outside of a hospital. CAP has quite a high mortality and morbidity rate worldwide, and especially among elderly patients. The increasing burden of CAP is due to antibiotic resistance, the growth o …
Despite decades of advances in clinical management protocols and new antibiotics, pneumonia continues to be a leading cause of morbidity and mortality worldwide. The 2019 Global Burden of Disease Study indicated that lower respiratory infections, including pneumonia, were the fourth leading cause of disability-adjusted life-years across all ages.1 People at the extremes of age, specifically ...
Abstract. Community-acquired pneumonia continues to be one of the most common causes of morbidity and mortality due to infectious disease. The aetiologies, clinical presentations, diagnostic modalities and therapeutic options are changing and outpacing the creation of management guidelines.
Clinical presentation of community-acquired pneu-monia varies widely, ranging from mild pneumonia characterised by fever and cough, to severe pneumonia with sepsis and respiratory failure, and depends on the interaction between the patient's immune system, patient's characteristics, and pathogen's virulence.
INTRODUCTION. —. Community-acquired pneumonia (CAP) is a leading cause of morbidity and mortality worldwide. The clinical presentation of CAP varies, ranging from mild pneumonia characterized by fever and productive cough to severe pneumonia characterized by respiratory distress and sepsis.
Community-acquired pneumonia significantly contributes to patient morbidity and healthcare costs. As our understanding of this common infection grows, collaborative efforts among researchers and clinical societies provide new literature and updated guidelines informing its management. This review discusses diagnostic methods, empiric treatment, and infection prevention strategies for patients ...
Community-acquired pneumonia is a leading cause of mortality and hospital admissions. The aetiology remains unknown in 30-65% of the cases. Molecular tests are available for multiple pathogen detection and are under research to improve the causal diagnosis. Methods. We carried out a prospective study to describe the clinical characteristics ...
Community-acquired pneumonia (CAP) is an acute respiratory infection acquired outside the hospital, affecting alveoli and distal airways, with variable symptoms including cough, fever, dyspnea, and expectoration [].The incidence of lower respiratory tract infection (LRI), which includes CAP, was 5,837 cases and 6,832 cases per 100,000 population among females and males, respectively [].
Design. A psychometric study within an international, prospective, randomized, double-blind study. The CAP-symptom questionnaire (CAP-Sym) is a new, 18-item, patient-reported outcome measure that evaluates the bothersomeness of CAP-related symptoms during the past 24 h using a 6-point Likert scale. We used "gold standard" psychometric ...
Topic Collections; CHEST® COVID-19 Articles; Special Sections; ... Biomedical Research in Endstage and Obstructive Lung Disease, Member of the German Center for Lung Research, Hannover, Germany ... Community-acquired pneumonia (CAP) is a leading cause of morbidity and mortality worldwide. Limited evidence is available on the most effective ...
Community-acquired pneumonia (CAP) is one of the most underappreciated medical illnesses in the USA. The combination of pneumonia and influenza is the ninth leading cause of death overall and the most common cause of infectious death in the USA, causing an estimated 50 000 deaths in 2010. 1 This number is likely an underestimate because many ...
Introduction. Community-acquired pneumonia (CAP) is the most frequent cause of death in developing countries [].CAP kills more people than all other infectious diseases around the globe [], and is responsible for more than 3 million deaths a year.Despite the mortality burden CAP has been recently recognised as a neglected disease [].CAP also has an important economic cost to healthcare systems ...
Dive into the research topics of 'Advances in the causes and management of community acquired pneumonia in adults'. Together they form a unique fingerprint. ... abstract = "Community acquired pneumonia remains a common cause of morbidity and mortality. Usually, the causal organism is not identified and treatment remains empiric. ...
Pneumonia is a frequent complication of solid organ transplantation that adversely impacts both graft and recipient survival. There is a paucity of data on community-acquired pneumonia (CAP) in transplant recipients, particularly the long term outcomes. We conducted a study to compare the clinical characteristics and outcomes of pneumonia in solid organ transplant (SOT) recipients to those in ...
Among the most commonly encountered and morbid conditions globally, community-acquired pneumonia (CAP) is associated with nearly 5 million annual outpatient and emergency room visits in the US annually. It is the second most common cause of hospitalization and is associated with a notable risk of recurring disease; approximately 1 in 10 patients hospitalized with CAP are re-hospitalized with a ...
1. Introduction. Community-acquired pneumonia (CAP) is a dominant cause of hospitalization, death, and economic burden worldwide [].The prevalence of CAP is high among all age groups, especially for the elderly and patients with comorbidities [1 - 3].Aspiration pneumonia (AP) is an infectious process caused by the inhalation of oropharyngeal secretions that are colonized by pathogenic ...
The diagnosis of pneumonia in children is challenging due to overlapping clinical features of viral and bacterial etiologies of pneumonia, in addition to other lower respiratory tract pathologies. Although limited, chest radiography remains the reference standard for diagnosis. In all pediatric age groups excluding neonates, viral pathogens are ...