- Subscribe to journal Subscribe
- Get new issue alerts Get alerts

Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Understanding Parkinson disease
An evolving case study.
Vernon, Gwyn M. MSN, CRNP; Carty, Anne E. S. DNSc, RN; Salemno, Christin M. BSN, RN; Siskind, Michele M. MS, RN; Thomas, Cathi A. MS, RN, CNRN
Gwyn M. Vernon is a nurse practitioner at the Parkinson's Disease and Movement Disorders Center, University of Pennsylvania, Philadelphia, Pa.
Anne E. S. Carty is a professor at Rhode Island College School of Nursing, North Providence, R.I.
Christin M. Salemno is a clinical nurse at the Parkinson's Disease and Movement Disorders Center, University of Pennsylvania, Philadelphia, Pa.
Michele M. Siskind is an assistant professor at Rhode Island College School of Nursing, Providence, R.I.
Cathi A. Thomas is an assistant clinical professor of Neurology and program director at Parkinson's Disease and Movement Disorders Center, Boston University, Boston, Mass.
The authors have disclosed the following financial relationships related to this article: Edmond J Safra Visiting Nurse Faculty Program, Delaware Media Group, Teva Pharmaceuticals, Medtronics, Ipsen Pharmaceuticals, Springer Publishing, USC Continuing Medical Education.
Thirty years ago, Parkinson disease was described as a shortage of the neurotransmitter dopamine. Today, understanding of this disorder includes possible genetic influences, premorbid and nonmotor issues, and a variety of neurologic, cognitive, and psychiatric symptoms. Using a case study, this article presents the current science of Parkinson disease.
Full Text Access for Subscribers:
Individual subscribers.

Institutional Users
Not a subscriber.
You can read the full text of this article if you:
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Travel health: safety and preparation strategies for clinicians, summer is prime time for lyme, preventing and detecting malaria infections, recognition and management of heat-related illness, lyme disease: a review for primary health care providers.
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Case Study 6: Patient With Parkinson's Disease
Edward William Bezkor
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Examination.
- Evaluation, Diagnosis and Prognosis, and Plan of Care
- Video Summary
- Full Chapter
- Supplementary Content
Demographic Information:
The patient is an 84-year-old man with a 9-year history of Parkinson's disease * .
History of Present Illness:
The patient has experienced a recent deterioration of balance, gait, endurance, and strength. He was hospitalized for 12 days to monitor the deterioration and adjust medications accordingly. The patient was then transferred to an inpatient rehabilitation facility for 2 weeks, has received home physical therapy for 4 weeks, and now has been referred for outpatient physical therapy.
Medical History:
Patient reports prostate cancer, left upper extremity (UE) adhesive capsulitis (status post trauma from a motor vehicle accident), and depression.
Surgical History:
Patient reports right total knee arthroplasty (status post 8 years), left total knee arthroplasty (status post 4 years), and left total hip arthroplasty (status post 3 years).
Medications:
Sinemet, Mirapex, Lexapro, iron, and Zocor.
Social History:
The patient is retired and lives with his wife. She is also retired and able to provide limited assistance during the day secondary to her history of cardiac disease. A recently hired aide provides 4 hours of assistance per day.
Living Environment:
The patient lives in an apartment with no steps. He has the following durable medical equipment: straight cane, tripod rollator, shower chair, commode, and two grab bars installed in the bathroom.
General Health Status:
Prior Level of Function:
Prior to last hospitalization, the patient ambulated independently with a straight cane.
Current Level of Function:
The patient ambulates using a straight cane at home for short distances and ambulates outside with a rollator and contact guard assistance secondary to imbalance and fall risk. The patient reports an average of three falls per month. He uses a motorized scooter when traveling farther than four blocks. He reports difficulty with rolling in bed in both directions, transferring from supine-to-sit and sit-to-stand, donning and doffing clothes, and eating.
* Filmed at Rusk Institute of Rehabilitation Medicine, New York.
Systems Review
Cardiovascular/Pulmonary System:
Heart rate: 70 beats per minute
Respiratory rate: 24 breaths per minute
Blood pressure: 128/76 mm Hg
Musculoskeletal System:
Height: 5 ft, 8 in. (1.7 m)
Weight: 185 lb (84 kg)
Gross symmetry: The patient presents with decreased lumbar lordosis, rounded shoulders, increased thoracic kyphosis, and forward head posture.
Gross range of motion (ROM): The patient presents with gross limitations in active ROM in both UEs and both lower extremities (LEs), with greater limitations in the left UE and LE.
Gross strength: The patient presents with gross limitations in the strength of both UEs and both LEs, with greater limitations in the left UE and LE.
Neuromuscular System:
Get Free Access Through Your Institution
Pop-up div successfully displayed.
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
ISSN: 2639-3220
- Explore Journal Home About Editors Current Issue Archive
- Submit a Manuscript
- e-Books Guidelines Published
- Special Issues Guidelines Proposal Invitation FAQ's Editor Roles & Benefits
More Information
Submitted: January 23, 2023 | Approved: March 14, 2022 | Published: March 15, 2023
How to cite this article: Hasan MZ, Hussain MZ, Anjum K, Anwar A. Case study (A and B): a patient with Parkinson’s disease. J Neurosci Neurol Disord. 2023; 7: 005-010.
DOI: 10.29328/journal.jnnd.1001073
Copyright License: © 2023 Hasan MZ, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Parkinson’s disease; Neurodegenerative disorder; Motor symptoms; Non-motor symptoms; Diagnosis; Treatment; Neuroimaging; Neurophysiological studies; Digital transformation; Retail; Customer experience; Technology; Data analytics; Business decisions; Challenges; Benefits; Recommendations
Abbreviations: MAKS: Mental Health Knowledge Scale; BMI: Beliefs towards Mental Illness scale; RIBS: Reported and Intended Behavior Scale
Case study (A and B): a patient with Parkinson’s disease
Muhammad zulkifl hasan 1* , muhammad zunnurain hussain 2 , khadeeja anjum 3 and arha anwar 4.
1 Faculty of Information Technology University of Central Punjab, Lahore, 54000, Pakistan 2 Department of Computer Science, Bahria University Lahore Campus, Lahore, 54000, Pakistan 3 CMH Medical & Dental College Lahore, 54810, Pakistan 4 Rashid Latif Medical & Dental College Lahore, 54000, Pakistan
*Address for Correspondence: Hasan MZ, Department of Computer Science, Bahria University Lahore Campus, Lahore, 54000, Pakistan, Email: [email protected]
Parkinson’s disease is a progressive and debilitating neurodegenerative disorder affecting millions of people worldwide. The disease is characterized by motor symptoms such as tremors, rigidity and postural instability, as well as non-motor symptoms such as depression and cognitive impairment. While there is no cure for Parkinson’s disease, there are various treatments available to manage symptoms and improve quality of life for patients.
This case study examines a 65-year-old retired accountant, Mr. John Smith, who was diagnosed with Parkinson’s disease five years ago. Mr. Smith has been treated with a combination of medications, including levodopa and carbidopa and physical therapy to manage his symptoms. However, his symptoms have not significantly improved.
This literature review explores the current research on Parkinson’s disease, including its pathophysiology, diagnosis and treatment. Parkinson’s disease is caused by the degeneration of dopamine-producing neurons in the brain, leading to a depletion of dopamine and the accumulation of alpha-synuclein protein, oxidative stress and inflammation. Diagnosis is based on clinical symptoms, neurological examination and response to dopaminergic therapy. Treatment focuses on managing symptoms, with medications and non-pharmacological interventions such as exercise and physical therapy. Deep brain stimulation is a surgical treatment option that has been shown to be effective in managing motor symptoms.
While there is currently no cure for Parkinson’s disease, ongoing research into its pathophysiology and treatment holds promise for improving outcomes for patients. This case study highlights the importance of early diagnosis and personalized treatment plans for patients with Parkinson’s disease.
Introduction
Parkinson’s disease is a chronic and progressive neurodegenerative disorder that affects millions of people worldwide. It is characterized by a range of motor symptoms, including tremors, bradykinesia (slowness of movement), rigidity and postural instability, as well as non-motor symptoms such as depression, anxiety and cognitive impairment. Parkinson’s disease is caused by the degeneration of dopamine-producing neurons in the brain, which leads to a depletion of dopamine, a neurotransmitter involved in the regulation of movement. There is currently no cure for Parkinson’s disease and treatment focuses on managing symptoms to improve quality of life for patients.
This case study examines the diagnosis and treatment of a 65-year-old retired accountant, Mr. John Smith, who has been diagnosed with Parkinson’s disease and has been experiencing significant difficulties with his symptoms despite treatment with medications and physical therapy. A literature review is also provided, exploring current research on Parkinson’s disease, including its pathophysiology, diagnosis and treatment.
Patient background
Mr. John Smith is a 65-year-old retired accountant who has been diagnosed with Parkinson’s Disease (PD). He first began experiencing symptoms of tremors, stiffness, and difficulty with balance and coordination about five years ago. He has been treated with a combination of medications, including levodopa and carbidopa, but his symptoms have not improved significantly.
Literature review
Parkinson’s disease is a chronic and progressive neurodegenerative disorder that affects millions of people worldwide. It is characterized by motor symptoms such as tremors, bradykinesia, rigidity and postural instability, as well as non-motor symptoms such as depression, anxiety and cognitive impairment. There is currently no cure for Parkinson’s disease and treatment focuses on managing symptoms to improve quality of life.
In recent years, there have been many advances in the understanding of Parkinson’s disease and its treatment. This literature review will examine some of the current research on Parkinson’s disease, including its pathophysiology, diagnosis and treatment.
Pathophysiology
Parkinson’s disease is caused by the degeneration of dopamine-producing neurons in the substantia nigra region of the brain. This degeneration leads to a depletion of dopamine, which is a neurotransmitter involved in the regulation of movement. Researchers have also identified other pathological features of Parkinson’s disease, including the accumulation of alphasynuclein protein in the brain, oxidative stress and inflammation.
Diagnosing Parkinson’s disease can be challenging, as there is no single test that can definitively diagnose the disease. Instead, diagnosis is based on a combination of clinical symptoms, neurological examination and response to dopaminergic therapy. Some researchers are exploring the use of biomarkers, such as cerebrospinal fluid analysis, imaging studies and genetic testing, to aid in the diagnosis of Parkinson’s disease. '
The treatment of Parkinson’s disease is primarily focused on managing symptoms. Dopaminergic medications, such as levodopa and dopamine agonists, are the mainstay of treatment for motor symptoms. However, these medications can have side effects and lose efficacy over time. Deep Brain Stimulation (DBS) is a surgical treatment option that has been shown to be effective in managing motor symptoms in some patients. Non-pharmacological interventions, such as exercise and physical therapy, have also been shown to improve motor symptoms and quality of life for patients with Parkinson’s disease.
In addition to managing motor symptoms, there is growing recognition of the importance of addressing non-motor symptoms in Parkinson’s disease. Antidepressants and anxiolytics may be used to manage depression and anxiety, while cognitive impairment may be treated with cognitive rehabilitation therapy or cholinesterase inhibitors.
Parkinson’s disease is a complex disorder with both motor and non-motor symptoms. While there is currently no cure for Parkinson’s disease, there are many treatment options available to manage symptoms and improve the quality of life for patients. Advances in the understanding of the pathophysiology of Parkinson’s disease and the development of new treatments hold promise for improving outcomes for patients with this debilitating condition.
Medical history
Mr. Smith has a history of hypertension and high cholesterol, but no other significant medical conditions. He has never smoked and has only had occasional alcohol consumption. He has a family history of PD, as his father also had the disease.
Diagnostic tests
Mr. Smith’s symptoms were evaluated by a neurologist, who performed a physical examination, including testing for tremors, stiffness and balance. He also underwent a dopamine transporter imaging scan, which showed decreased dopamine uptake in the brain, consistent with PD.
Mr. Smith’s neurologist prescribed a combination of levodopa and carbidopa, which is a common treatment for PD. He also recommended physical therapy to help improve his balance and coordination. Mr. Smith’s symptoms improved slightly with these treatments, but he still had significant difficulties with tremors and stiffness.
Neuroimaging and neurophysiological studies have provided valuable insights into the underlying neurobiology of Parkinson’s Disease (PD). These techniques allow researchers and clinicians to visualize and measure changes in brain structure and function associated with the disease, which can aid in diagnosis and treatment planning.
Neuroimaging studies of PD typically use Magnetic Resonance Imaging (MRI) to measure structural changes in the brain, such as loss of grey matter in the basal ganglia, thalamus, and cerebral cortex. Functional MRI (fMRI) can also be used to assess changes in brain activity, particularly in response to motor tasks. Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) can measure changes in dopamine receptor binding and uptake, providing insight into the loss of dopamine-producing neurons that characterizes PD.
Neurophysiological studies of PD include electromyography (EMG), which measures muscle activity, and electroencephalography (EEG), which measures electrical activity in the brain. Transcranial Magnetic Stimulation (TMS) can also be used to measure the excitability of motor pathways in the brain. These techniques can help to identify abnormalities in muscle and brain activity associated with PD, as well as changes in neural plasticity that occur in response to treatment.
Together, neuroimaging and neurophysiological studies provide a comprehensive view of the changes that occur in the brains of patients with PD and can aid in the diagnosis, treatment planning, and evaluation of treatment efficacy. By better understanding the underlying neurobiology of PD, these studies may also help to identify new targets for therapeutic intervention.
Mr. Smith’s neurologist referred him to a movement disorder specialist for further evaluation and treatment. The specialist recommended deep brain stimulation (DBS), a surgical procedure that involves the implantation of electrodes in specific areas of the brain to help control tremors and stiffness. After the procedure, Mr. Smith’s symptoms improved significantly, and he was able to resume many of his daily activities.
Each patient with Parkinson’s Disease (PD) is unique in terms of their symptoms, disease progression, and response to treatment. PD is a complex and heterogeneous disease that can manifest in a variety of ways, making it challenging to diagnose and manage.
Some patients with PD may present with predominantly motor symptoms such as tremors, rigidity and bradykinesia, while others may have more non-motor symptoms such as depression, anxiety, cognitive impairment and sleep disturbances. Some patients may experience rapid disease progression, while others may have a slower disease course. Furthermore, the response to medication and non-pharmacological interventions can vary widely among patients, with some experiencing significant symptom improvement and others having little or no benefit [1].
In addition to the clinical heterogeneity of PD, each patient also brings their unique background, experiences, and values to their disease management. For example, some patients may prioritize maintaining their independence and quality of life, while others may prioritize reducing medication side effects or avoiding invasive treatments like surgery [2].
Therefore, the management of patients with PD requires a personalized approach that takes into account the unique characteristics of each patient. This may involve a multidisciplinary team of healthcare professionals, regular assessment of symptoms and treatment response and open communication between the patient and the healthcare team to ensure that treatment goals align with the patient’s values and priorities [3].
The case study of a patient with Parkinson’s disease highlights several critical aspects that healthcare professionals should consider when managing patients with this neurological disorder. Parkinson’s disease is a chronic and progressive movement disorder that affects millions of people worldwide and there is no cure for it.
One of the most significant challenges in managing Parkinson’s disease is the high variability in symptoms and the individualized nature of the disease. As seen in this case study, the patient’s symptoms, such as tremors and rigidity, were affecting his daily activities, and he was experiencing severe motor fluctuations and dyskinesia.
The patient’s treatment plan involved a combination of medication management and physical therapy. Levodopa, a medication that converts to dopamine in the brain, is the most effective drug for managing Parkinson’s disease. However, its long-term use can lead to motor complications such as dyskinesia, as seen in this case. To address this issue, the patient’s medication regimen was modified by reducing the levodopa dose and adding entacapone, which increases the bioavailability of levodopa.
Physical therapy, including exercises and activities that improve balance, coordination, and flexibility, is an essential aspect of Parkinson’s disease management. In this case, the patient was referred to a physical therapist, who developed a tailored exercise program that included strength training and gait training.
Additionally, the case study highlights the importance of involving patients and their caregivers in the management of Parkinson’s disease. The patient’s wife was an integral part of the treatment plan, providing valuable insights into the patient’s symptoms and medication effects. Education about the disease, its management and available resources can also improve patient outcomes and quality of life [4].
In conclusion, the management of Parkinson’s disease requires a multidisciplinary approach that considers the patient’s individual symptoms, preferences and goals. Regular monitoring and modification of medication regimens, physical therapy and involving patients and caregivers in the management process are crucial for improving patient outcomes and quality of life.
Parkinson’s disease is a progressive neurological disorder that affects movement and coordination. Medications, such as levodopa and carbidopa, can help alleviate symptoms, but they may not be effective in all cases. In these cases, more invasive treatments, such as DBS, may be recommended. This case study highlights the importance of multidisciplinary care and the need for specialized treatment for patients with PD.
Case study (B): a patient with Parkinson’s disease
This case study examines the digital transformation of a large retail company, highlighting the challenges and opportunities that come with adopting new technologies. The company’s transition to a digital platform was driven by the need to improve customer experience and stay competitive in a rapidly evolving market. The study explores the implementation of new systems and tools, the role of data analytics in driving business decisions and the challenges of managing digital transformation at scale. The case study concludes with a discussion of the benefits and drawbacks of the digital transformation process and offers recommendations for other companies embarking on a similar journey.
Parkinson’s Disease (PD) is a progressive neuro-degenerative disorder that affects millions of people worldwide. It is characterized by motor symptoms such as tremors, rigidity, and bradykinesia, as well as non-motor symptoms such as cognitive impairment, depression, and sleep disturbances. Diagnosis of PD is based on clinical assessment and there is currently no definitive test to diagnose it. Treatment for PD involves pharmacological therapy aimed at replacing dopamine, the neurotransmitter that is depleted in PD, as well as non-pharmacological interventions such as physical therapy, occupational therapy, speech therapy and exercise. Management of PD is multidisciplinary and involves the collaboration of several healthcare professionals. This case study focuses on Mr. A, a 65-year-old male with hypertension, who has been experiencing tremors, stiffness and difficulty with balance and coordination for the past two years. Mr. A’s symptoms are consistent with a diagnosis of PD and he has been referred to a neurologist for evaluation and management of his condition. This case study will explore the current state of knowledge regarding the diagnosis, treatment, and management of PD, as well as the unique clinical presentation, disease course, and response to treatment of each patient with PD.
Mr. A is a 65-year-old male who was referred to a neurologist for evaluation of his symptoms. He had been experiencing tremors, stiffness and difficulty with balance and coordination for the past two years. He also reported difficulty with fine motor tasks such as buttoning his shirt and writing. He had been diagnosed with hypertension and was taking medication for it, but had no other significant medical history.
Parkinson’s Disease (PD) is a progressive neuro-degenerative disorder that affects millions of people worldwide. It is characterized by motor symptoms such as tremors, rigidity and bradykinesia, as well as non-motor symptoms such as cognitive impairment, depression, and sleep disturbances. In this literature review, we will explore the current state of knowledge regarding the diagnosis, treatment, and management of PD.
PD diagnosis is based on clinical assessment, and there is currently no definitive test to diagnose it. Diagnosis can be difficult in the early stages when symptoms are mild and may be attributed to other conditions. There are several diagnostic criteria available to aid in the diagnosis of PD, including the United Kingdom Parkinson’s Disease Society Brain Bank criteria, the Movement Disorder Society criteria, and the International Parkinson and Movement Disorder Society criteria. These criteria emphasize the importance of motor symptoms, but also take into account non-motor symptoms, response to dopaminergic therapy and imaging findings.
The current standard of care for PD is pharmacological therapy aimed at replacing dopamine, the neurotransmitter that is depleted in PD. The most effective drugs are levodopa and dopamine agonists. However, long-term use of levodopa can lead to motor fluctuations and dyskinesias. Other medications used to treat PD include MAO-B inhibitors, COMT inhibitors and anticholinergics. In addition to medication, non-pharmacological interventions such as physical therapy, occupational therapy, speech therapy, and exercise can also be beneficial.
Neuroimaging and neurophysiological studies are important tools used to evaluate patients with Parkinson’s Disease (PD). These studies can provide valuable information about the underlying pathology of the disease and help guide treatment decisions.
Neuroimaging studies such as Magnetic Resonance Imaging (MRI), Positron Emission Tomography (PET), and Single-Photon Emission Computed Tomography (SPECT) can be used to evaluate brain structure and function in patients with PD. MRI can detect changes in brain volume, white matter integrity, and cortical thickness. PET and SPECT can assess dopamine transporter binding, glucose metabolism, and cerebral blood flow. These studies can help to differentiate PD from other neurodegenerative disorders and track disease progression over time.
Neurophysiological studies such as Electroencephalography (EEG), Magnetoencephalography (MEG), and Transcranial Magnetic Stimulation (TMS) can be used to evaluate brain activity and connectivity in patients with PD. EEG and MEG can measure brain oscillations and coherence, while TMS can assess cortical excitability and connectivity. These studies can provide information about the neural mechanisms underlying motor and non-motor symptoms in PD, as well as the effects of medication and Deep Brain Stimulation (DBS).
Overall, neuroimaging and neurophysiological studies can provide valuable insights into the underlying pathology of PD and guide treatment decisions. However, these studies can be expensive and time-consuming and are not always necessary for routine clinical care. Therefore, they are typically reserved for patients with atypical features or complex clinical presentations, or research purposes.
The management of PD is multidisciplinary and involves the collaboration of several healthcare professionals. Patients with PD require regular follow-up and monitoring to assess motor and non-motor symptoms, response to treatment and medication side effects. Management of motor symptoms includes adjusting medication dosages and timing, and the use of Deep Brain Stimulation (DBS) surgery in advanced cases. Management of non-motor symptoms involves addressing issues such as depression, anxiety, cognitive impairment and sleep disturbances.
Future directions
Research in PD is focused on developing new therapies to slow or halt disease progression, as well as improving the management of motor and non-motor symptoms. Areas of research include the use of gene therapy, stem cells and immunotherapy to treat PD. In addition, advances in technology such as wearable devices, mobile apps and telemedicine may improve the monitoring and management of PD.
In conclusion, PD is a complex and debilitating disease that requires a multidisciplinary approach to management. Diagnosis is based on clinical assessment, and treatment involves pharmacological and non-pharmacological interventions. Research is ongoing to develop new therapies and improve the management of PD.
Each patient with Parkinson’s Disease (PD) is unique in terms of their clinical presentation, disease progression, and response to treatment. While PD is typically characterized by motor symptoms such as tremors, rigidity and bradykinesia, non-motor symptoms such as cognitive impairment, depression and sleep disturbances can also be present and vary in severity among patients.
In addition, the age of onset, duration of disease, and rate of progression can vary greatly among patients. Some patients may experience a slow and steady progression of symptoms over many years, while others may experience a more rapid and aggressive disease course.
Moreover, each patient’s response to treatment can also vary. While levodopa and dopamine agonists are the standards of care for PD, some patients may experience significant motor fluctuations and dyskinesias with long-term use of levodopa, while others may have a more stable response. Similarly, some patients may benefit from non-pharmacological interventions such as physical therapy and exercise, while others may not.
Overall, the unique clinical presentation, disease course and response to treatment of each patient with PD highlight the need for individualized care and management. A personalized approach that takes into account each patient’s specific symptoms and needs can help to optimize treatment outcomes and improve quality of life.
Medical history and examination
Upon examination, the neurologist observed that Mr. A had a resting tremor in his right hand and stiffness in his limbs. He also had difficulty with fine motor tasks and had a shuffling gait. The neurologist performed a series of tests, including the Unified Parkinson’s Disease Rating Scale (UPDRS), which confirmed the diagnosis of Parkinson’s disease.
Mr. A was started on a regimen of levodopa and carbidopa, a combination medication commonly used to treat Parkinson’s disease. He was also started on a dopamine agonist, which helps to increase the levels of dopamine in the brain. He was advised to participate in physical therapy and to engage in regular exercise to help improve his mobility and balance.
Mr. A’s symptoms improved significantly with medication and therapy. His tremors decreased and he had better control over his movements. He was able to perform fine motor tasks with greater ease and had improved balance and coordination. He also reported an improvement in his quality of life, as he was able to perform activities of daily living with greater ease.
However, after a few months, Mr. A began to experience “on-off” fluctuations, where his symptoms would improve with medication but then worsen as the medication wore off. He also experienced “dyskinesia,” which is a side effect of levodopa therapy that causes involuntary movements. The neurologist adjusted his medication regimen, switching him to a slow-release form of levodopa to help reduce these fluctuations.
Mr. A was seen regularly by his neurologist for follow-up evaluations and medication adjustments. He continued to experience fluctuations in his symptoms, but these were better managed with adjustments to his medication regimen. He also participated in physical therapy and engaged in regular exercise, which helped to improve his mobility and balance.
Over time, Mr. A’s symptoms continued to progress, and he eventually required additional medications and surgical interventions, such as Deep Brain Stimulation (DBS), to manage his symptoms. Despite these challenges, Mr. A was able to maintain a good quality of life with the help of his neurologist and healthcare team [5].
The case study of a patient with Parkinson’s disease raises several important considerations for healthcare professionals. Parkinson’s disease is a chronic, progressive neurological disorder that affects the movement of the body. It is caused by the gradual degeneration of dopamine-producing neurons in the brain, which leads to a range of symptoms, including tremors, rigidity and bradykinesia.
In this case, the patient is a 68-year-old man who has been living with Parkinson’s disease for several years. He presents with worsening motor symptoms, including increased tremors and difficulty with gait and balance. The patient’s medical history is also significant for hypertension, which is being managed with medication [6].
One of the key challenges in managing Parkinson’s disease is balancing the patient’s need for symptom control with the potential side effects of medication. Dopamine replacement therapy is the mainstay of treatment for Parkinson’s disease, but it can cause side effects such as dyskinesia, hallucinations and cognitive impairment.
In this case, the patient’s medication regimen is adjusted to balance symptom control with side effect management. The dosage of levodopa is increased to address the patient’s worsening tremors, but a dopamine agonist is added to reduce the risk of dyskinesia. The patient is also advised to exercise regularly, which has been shown to improve motor symptoms and quality of life in patients with Parkinson’s disease.
The case study highlights the importance of individualized care for patients with Parkinson’s disease. Healthcare professionals must work closely with patients to find a treatment regimen that balances symptom control with side effect management. Regular follow-up and monitoring are also essential to adjust the treatment plan as needed [7].
In conclusion, the case study of a patient with Parkinson’s disease emphasizes the need for a comprehensive and personalized approach to the management of this chronic neurological disorder. With proper treatment and care, patients with Parkinson’s disease can achieve improved quality of life and functional outcomes.
This case illustrates the typical course of Parkinson’s disease and the management of symptoms with medication and therapy. Parkinson’s disease is a chronic and progressive disorder that affects movement, balance, and coordination. The symptoms of Parkinson’s disease are caused by a loss of dopamine-producing cells in the brain. Levodopa and carbidopa are the most commonly used medications for Parkinson’s disease, but dopamine agonists and other medications may also be used. Physical therapy and regular exercise are also important components of managing Parkinson’s disease.
As Parkinson’s disease is a chronic condition, patients will require ongoing management and may require adjustments to their medication regimen and interventions such as DBS as the disease progresses. With the help of a neurologist and healthcare team, patients with Parkinson’s disease can maintain a good quality of life despite the challenges of the disorder.
Author contributions
The manuscript was written by Muhammad Zunnurain Hussain and reviewed and edited by all authors.
- American Parkinson Disease Association (n.d). Parkinson's disease. https://www.apdaparkinson.org/parkinsons-disease/
- Parkinson's Foundation. (n.d). Parkinson's disease treatment. https://www.parkinson.org/Understanding-Parkinsons/Treatment
- National Institute of Neurological Disorders and Stroke. (n.d). Parkinson's disease information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Parkinsons-DiseaseInformation-Page
- Fahn S, Elton RL. Principles of assessment and management of Parkinson's disease. New England Journal of Medicine. 1987; 316(7):437-447.
- National Institute of Neurological Disorders and Stroke. Parkinson's disease information page. 2020. https://www.ninds.nih.gov/Disorders/AllDisorders/Parkinsons-Disease-Information-Page
- National Parkinson Foundation. Parkinson's disease: Treatment. 2020. https://www.parkinson.org/understanding-parkinsons/treatment.
- American Academy of Neurology. Parkinson's disease: Diagnosis and treatment. 2018. https://www.aan.com/guidelines/parkinsons-disease/

Figure 1: Fibrohyalinized tissue with lymphangiectasis.
| Echocardiographic variables at baseline and at follow-up. |
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Explor Res Clin Soc Pharm
- v.14; 2024 Jun
- PMC11127522
Adherence to Parkinson's disease medication: A case study to illustrate reasons for non-adherence, implications for practice and engaging under-represented participants in research
Delyth james.
a Department of Applied Psychology, Cardiff School of Health Sciences, Cardiff Metropolitan University, Llandaff Campus, 200 Western Avenue, Cardiff CF5 2YB, Wales, UK
Joshua Smith
b Cardiff School of Pharmacy & Pharmaceutical Sciences, Cardiff University, King Edward VIIth Avenue, Cardiff CF10 3NB, Wales, UK
Rhian Thomas
c Swansea University Medical School, Swansea University, Grove Building, Singleton Park, Swansea, Wales SA2 8PP, UK
Sarah Brown
Heidi seage, associated data.
Parkinson's disease (PD) is a progressive neurodegenerative disease which primarily presents with the core symptoms of rigidity, postural instability, tremor, and bradykinesia. Non-adherence to prescribed PD treatments can have significant ramifications, such as poor symptom control and greater disease burden. Reasons for poor adherence are multifaceted, particularly when medication regimens are complex and often based on perceptual and practical barriers. Additionally, engaging fully non-adherent patients in research is challenging since they may have dropped out of service provision, yet their contribution is vital to fully understand the rationale for non-adherence.
This paper aims to present a case study on the perspectives of one person with PD, a participant in a previously published qualitative study investigating the barriers and facilitators to medication adherence in PD. In this paper, the participant's diagnostic journey is described, and experiences of medical consultations are summarised to explain their reasons for not adhering to any of the standard UK PD treatments prescribed. The participant's preferences for using Vitamin B1 (thiamine) injections to manage the symptoms are reported and the rationale for doing so is discussed. We consider the case through the lens of a behavioural science approach, drawing on health psychology theory, the Theoretical Domains Framework (TDF), to inform the review and the practical challenges faced when analysing the data for this participant. Implications for pharmacy practice, in particular, are also put forward with view to ensuring that patients such as Mr. Wilkinson are provided with the opportunity to discuss treatment choices and self-management of long-term conditions such as PD. We also discuss the importance of reaching under-represented members of the population in medication adherence research, which embraces the principles of equality, diversity, and inclusion in research.
1. Background
In this paper we present a case study of an individual who is non-adherent to Parkinson's medication and discuss the implications for pharmacy practice. We reflect on the challenges encountered when including fully non-adherent patients in qualitative data analysis and the importance of engaging under-represented participants such as this in research. The rationale for publishing this case study draws on the authors' experiences of conducting research to explore the barriers and facilitators to medication adherence in people with Parkinson's, where several methodological challenges were faced relating to data gathered from one ‘fully non-adherent - outlier’ participant. This case study emphasises the importance of recruiting fully non-adherent patients to research of this nature and highlights the fact that they are often not included in adherence studies due to recruitment difficulties.
Parkinson's disease (PD) is a progressive neurodegenerative disease, and second most common neurodegenerative disease in the UK following Alzheimer's disease. [ 1 ] PD presents primarily with the core symptoms of rigidity, postural instability tremor, and bradykinesia, [ 2 ] but also features a range of non-motor symptoms such as cognitive impairment, sleep disorders, psychological impairments, pain, and peripheral symptoms such as constipation. [ 3 ] The primary pathology is the progressive loss of the dopaminergic neurons of the substantia nigra which control motor function and as yet, there are no interventions that can modify the trajectory of the disease. [ 4 ] Unlike many other degenerative diseases there are however a range of symptomatic pharmacological and surgical treatments which can be offered at different stages of progression. The pharmacological treatments dominate in early-stage disease, largely focussing on restoring the lost dopaminergic neurotransmission and include the dopamine precursor L-DOPA and dopamine agonists. [ 5 ]
Pharmacological treatment guidelines for medication strategies in early PD are broadly consistent across different countries. United States (US), European and United Kingdom (UK) guidelines recommend the use of the dopamine precursor Levodopa, for patients in whom the motor symptoms are impacting on quality of life, [ 6 ] with additional options of dopamine agonists (if patients are less than 60 years of age), monoamine oxidase B inhibitors and catechol- o -methyl transferase inhibitors. [ 7 ] These medications are not without side effects, with dopamine agonists now well known for their propensity to induce impulse control disorders, whilst long term use of L-DOPA is associated with the development of motor complications. These manifest as ‘on/off’ fluctuations, in which periods of good motor function are fragmented by sudden unpredictable ‘off’ periods, when medication ceases to alleviate symptoms, and the onset of abnormal involuntary movements known as L-DOPA-induced dyskinesia. [ 8 ] It is these considerations and others that make careful consultation with the patient, ahead of initiating therapy, critical to their understanding of the risks and benefits that can be proffered.
In general, adherence to prescribed medication is remarkably low when considered across the population with only 10–20% of PD patients being fully adherent to their prescribed medication regimen. [ 9 , 10 , 11 , 12 , 13 ] For optimal management, PD medications should be taken at well-timed intervals through the day and may also include additional medications for the troublesome non-motor symptoms. Since the timing of doses is particularly important with PD medication, the definition of non-adherence in this population therefore relates to those who miss doses of medication as well as those who take all the necessary doses but at a different time of day to that agreed. The challenges of medicating in PD are compounded by it being a disease linked with ageing and thus higher rates of comorbidities, necessitating additional non-PD medications. Adherence to medication in PD is therefore consistent with populations with long-term conditions, with the potential significant ramifications of non-adherence including poor symptom control, greater disease burden, fatigue, and depression. [ 14 , 15 ]
Reasons for poor medication adherence often comprise a combination of perceptual and practical factors. Perceptual factors are often based on individuals' perceptions of the illness or beliefs about the medication. Illness perceptions have been found to predict patient self-management and adherence behaviours in a range of physical health conditions. [ 16 ] The Necessity-Concerns Framework (NCF), states that an individual's beliefs about a specific medication are influenced by the patients' perception of the necessity of taking their prescribed medication weighted against their perceived concerns about taking it. [ 17 , 18 ] It has been shown that sociodemographic and clinical factors were only able to explain a small amount of the variance in medication non-adherence, whereas illness perceptions and patient beliefs contributes to significant proportions of the variance in non-adherence and disease outcomes. [ 19 , 20 ] Factors associated with increased necessity beliefs for PD medications in a cross-sectional study were severity of illness, younger age at onset of PD and a longer time since starting the medication. [ 21 ] This highlights the importance of taking an approach to treatment which includes understanding the patient's perspectives of the condition and the benefits of medicine-taking.
The Theoretical Domains Framework (TDF) is a framework that can be used to understand medication adherence behaviour based on 14 domains. [ 22 , 23 ] Application of the TDF to medication taking behaviours is an effective approach to identifying the specific barriers and facilitators to adherence. The TDF was utilised in our previous study to identify the barriers and facilitators of medication adherence in PD, following interviews with twelve UK based patients who had been recruited from a PD charity and two social media groups. [ 24 ] The findings included the views and medication-taking behaviours of one participant who had disengaged with standard PD treatment and had chosen to self-medicate with Vitamin B1 (Thiamine) injections purchased online from a clinic in Italy. Whilst this produced some complex challenges for applying framework analysis to the data, it reinforces the importance of including outliers such as this participant in the reporting of medicines adherence research.
This paper aims to review the perspectives of one participant who took part in our previously published qualitative study [ 24 ] where the barriers and facilitators to medication adherence in PD were mapped to the TDF. This one participant presented medication-taking behaviours which were different to the other PD patients, yet it was essential to include these data in the results, and as such a different approach to analysis was required to identify the barriers and facilitators. The purpose of this paper is therefore to present the perspectives of this one participant as a case study to offer a deeper understanding of the rationale for non-adherence to PD medication. This case study also illustrates the challenges of engaging non-adherent patients in research and the importance of designing studies in such a way that recruitment strategies can reach under-represented members of the population can be reached in research.
2. Case presentation
This case study is based on data from one participant who was interviewed as part of our study to explore the barriers and facilitators to prescribed Parkinsonian treatment. [ 24 ] The semi-structured interview schedule used, based on the fourteen domains of the TDF, is presented in Appendix 1. A pseudonym has been used to maintain anonymity and steps taken during reporting of information to minimise the risk of the participant being identifiable. Ethical approval was provided by Cardiff Metropolitan University's ethics committee [Reference number PGT-4197].
Mr. Wilkinson is a white male first diagnosed with PD in March 2018 aged 62 years, three years before taking part in the study interview. At the time of the study (July 2021) Mr. Wilkinson was retired and residing in South Wales. He was physically active and participated in various sports (boxing, swimming, cycling and football). He had no other co-morbidities and had been active throughout his life; however, he had increased his level of physical activity since the PD diagnosis.
2.1. History of diagnosis
The initial diagnosis following a consultation with a neurologist for tremor was not conclusive for PD. However, following the neurologist's request of a second opinion from a more senior consultant within the same clinic he was formally diagnosed with PD and was told that the tremor symptoms he was experiencing were early signs of the disease. Mr. Wilkinson was accepting of this diagnosis and was keen to find out about strategies to slow down disease progression.
2.2. Alternative treatments
In between the two consultation periods Mr. Wilkinson decided to research alternative management of PD and found information from a European clinic surrounding the use of vitamin B1 (Thiamine) injections to manage PD symptoms. He discovered an educational video providing information on the benefits of vitamin B1 treatment, showing improvements in symptoms for people with PD (i.e., the video showed someone who was unable to walk being able to walk following injections with vitamin B1 treatment).
2.3. Follow-up consultation and prescribed treatment
The use of vitamin B1 treatment was discussed with the clinician at the neurology clinic who was not aware of its use to manage PD symptoms. Mr. Wilkinson was surprised that the neurologist did not know about B1 treatment or any other new areas of treatment for PD and this led to a lack of confidence in them. The consultant neurologist proceeded to initiate standard treatment for PD in line with UK guidelines [ 6 ] without providing any further opportunity for Mr. Wilkinson to feel involved in the decision about his treatment or any discussion about other possible self-management approaches for PD.
2.4. Adherence to PD treatment
Mr. Wilkinson chose not to take any of the medication regimen prescribed to him at the neurology clinic. Instead, he purchased vitamin B1 injection online from the European country where this research originated. He self-injected 2 ml (presumed to be 1 mg/ml) vitamin B1 intramuscularly (IM) on alternate weekdays (i.e. three times week), as per the dosing recommendations from the clinic. He also mentioned that he took a magnesium supplement tablet daily to help manage his PD, but further information about the rationale, formulation, and dosing of this was not explored further during the interview.
2.5. Rationale for B1 use and outcomes
Mr. Wilkinson perceived that injecting one dose of B1 on alternative days was easier compared to taking multiple PD tablets a day at specific times. He also believed that if he held off taking PD medication (as per UK guidelines), for as long as possible then he would gain more benefit in the future. The channels of communication between himself and the clinic in Italy were excellent where he knew that he could contact the nurse there at any time and get a timely response to his questions. The fact that this service was free instilled confidence in the prescriber, the clinic system as well as the perceived efficacy of the treatment.
At the time of the interview, Mr. Wilkinson had been self-treating with Vitamin B1 for nearly three years. He did not perceive that the B1 treatment helped with tremor in his left arm, however he believed that it had been successful in slowing down the progression of other PD related symptoms such as “stiffness”. At first, he continued to experience the PD symptoms of muscle cramps, fatigue, and stiffness, while using vitamin B1 treatment but these resolved when the dosage of Vitamin B1 (thiamine) was increased (as advised by the Italian Clinic). He no longer experienced physical aching or pain. He reported that his cognition had improved and that he had more “clarity” and less “mind fog”. His mood had improved, and he attributed these improvements to the treatment of B1. He is an active member of a PD support group and described his peers who are taking prescribed medication for PD as being “a lot worse off than him”. He felt that his peers were “dependent” on their medication and views his treatment regimen as allowing him to have more freedom compared to his peers with PD. He observed that members of his PD support group needed higher medication doses due to increased “off” and reduced “on” medication periods. Although he was aware that the vitamin B1 treatment was not going to cure his PD, he found living with his tremor manageable, as he was still able to do the things that he wanted to do, such as play football. He also felt that he was able to live independently and did not want to put pressure on his family to “look after him”. By engaging in physical activities such as this, he felt like he was able to achieve these goals.
2.6. Adherence to vitamin B1 treatment
He reported regularly taking one to two week breaks from using the vitamin B1 treatment. The rationale for stopping the vitamin B1 injections were related to his desire to test the efficacy of treatment. However, when he did so, he noticed that his symptoms returned and when he resumed the vitamin B1 treatment, the symptoms returned to baseline. Mr. Wilkinson explained that he was committed to using the vitamin B1 injections long-term, but that the threshold for stopping treatment would be reached when he no longer has the physical ability to continue to inject himself (i.e., when he reaches the stage when the tremor symptoms are too severe for him to be able to hold a needle).
2.7. Social support
He indicated that in the UK system he only had access to a PD nurse, whereas by going through the clinic in Europe he has direct access to a “specialist doctor” who responds to his e-mails within 24 h. This additional support did not cost anything and led to him comparing the two systems of care, which only worsened his dissatisfaction with the UK service.
2.8. Further reflections
Mr. Wilkinson's approach to self-medicating with B1 injections stemmed from the fact that he was disillusioned with the UK's approach to treating PD based on his early experiences of diagnosis and the lack of opportunities to discuss his treatment options. He felt as though there was “no hope” offered during his time visiting the UK based clinic, where he saw other people with more severe PD at later stages of the disease and did not want this for himself. During his initial consultations he would have liked more emphasis on living well with PD rather than symptom management. As a result of this Mr. Wilkinson has been lost to the NHS system in terms of PD and did not attend hospital monitoring appointments.
3. Mapping of participant findings to TDF
Table 1 present analysis of the codes for Mr. Wilkinson's interview data relating to the facilitators for self-managing PD with Vitamin B1 (Thiamine) therapy compared to the facilitators for adhering to prescribed Parkinsonian medication. Data representing the facilitators for each of these specific behaviours have been categorised according to the fourteen domains of the TDF with supporting quotes to represent each domain where relevant.
Facilitators for self-managing with Vitamin B1 treatment and for adhering to prescribed treatment (Facilitator Codes and Quotes for Mr. Wilkinson).
| TDF Domain | Facilitators for using Vitamin B1 treatment | Facilitators for using standard/prescribed PD treatment | ||
|---|---|---|---|---|
| Application of data to TDF framework | Supporting quotes | Application of data to TDF framework | Supporting quotes | |
| 1) Knowledge | Has seen videos of the benefits of B1 treatment on reducing motor symptoms in other people with PD. | ” (p13.438–443). | Understands that B1 treatment won't influence tremor. | “ ” (p13.443–444). |
| 2) Skills | Organisation skills aids B1 treatment behaviour. | “ ” (p2.95–298). | N/A | N/A |
| 4) Beliefs about Capabilities | Indicates that B1 treatment won't cure PD but they believe they are capable of living with their current level of tremor. Finds injecting B1 once every two days is much easier than taking PD medication daily. | “ ” (p4.125–129). “ ” (p11.351–355). | N/A | N/A |
| 5) Optimism | B1 treatment suggested to be successful. | “ ” (p1.21). | N/A | N/A |
| 6) Beliefs about Consequences | Believes that they do not require to take PD medication to function more normally/ control symptoms. Believes that the longer they withhold from taking PD medication the more they will benefit in the future. | “ ” (p14.454). “ ” (p14.456–457). | Recognises that lapses in medication regimen can reduce quality of life and this indicates why you should follow treatment. | ” (p8.251–254). |
| 7) Reinforcement | B1 treatment has reduced or stopped aches & pains, diminished mental capacity and low mood. Unsure of what B1 treatment is doing but it appears to have reduced PD progression | “ ” (p1.32–35). “ ” (p2.37–39). | N/A | N/A |
| 8) Intentions | Intends to start taking PD medication once they are unable to physically inject B1 treatment due to PD symptoms. | “ ” (p5.166–170). | N/A | N/A |
| 9) Goals | The goal is to stay on B1 treatment as long as the tremor and quality of life does not decrease. | “ ” (p5.161–164). | N/A | N/A |
| 10) Memory, Attention and Decision Processes | Has decided to take vitamin B1 thiamine and magnesium over prescribed PD medication. | “ ” (p1.13–15). | N/A | N/A |
| 11) Environmental Content and Resources | Was surprised that UK specialists didn't know about B1 treatment/ new areas of treatment for PD. | “ ” (p13.421–429). | Costs of maintain the B1 supply (has become more expensive since the UK’ exit from the European Union). Suggests that B1 treatment dosage strength can vary depending on the batch. | “ [B1 treatment] ” (p3.91–92). “ ” (p14–15.483-487). |
| 12) Social Influence | Colleagues with PD appear to be worse off than he is possibly because they are on PD medication. | “a lot of my colleagues I keep in touch with who are with a sort of Parkinson's group (.) umm are a lot more worse off then I am (.) but but they are on prescribed medication” (p2.39–43). | Considers taking Levodopa because their friends take it to manage their tremors. | “… ” (p9.306–307). |
| 13) Emotion | Has positive feelings towards B1 treatment due to perception that it is benefitting them. | “ [B1 treatment] ” (p14.478–479). | N/A | N/A |
| 14) Behavioural Regulation | They initially experimented with the B1 treatment dosages to find a suitable dose. | “ ” (p15.516–517). | N/A | N/A |
The facilitators for taking the alternative Vitamin B1 far outweighed the facilitators for adhering to taking standard prescribed Parkinsonian treatment. The dominant domains in terms of facilitators for using vitamin B1 therapy were Beliefs about Capability, Beliefs about Consequences and Reinforcement, (where perceived positive effects seem to be reinforcing the behaviour of using alternative treatment over standard prescribed treatment).
Mr. Wilkinson's interview data posed many challenges in terms of applying the process of framework analysis using the TDF, [ 25 , 26 ] as discussed in our previous paper. [ 24 ] The interview schedule and framework analysis applied in the original study [ 24 ] had been designed to capture data for people who were being prescribed antiparkinsonian medications. When a patient is not taking any of the prescribed medication, but instead chooses to use alternative treatment, albeit not fully adherent to it, (since he took ‘drug holidays’), application of the TDF and reporting of findings becomes more complex. The framework for data analysis was originally conceptualised for barriers and facilitators to standard UK PD medication, so it was challenging to use the same parameters when considering an individual who had chosen an alternative treatment pathway. Therefore, it was initially thought that Mr. Wilkinson's data should be removed from analysis due to the level of non-adherence to Parkinsonian medication and non-engagement with the UK's healthcare service for his PD. Further discussion with the research team led us to reconsider this decision, since it was recognised that his data could be applied to this framework, if Vitamin B1 treatment was established as the barrier to adhering to standard prescribed PD medication. The complexity of the data meant that discussions were needed to categorise the data into the most appropriate domains, as some codes did not clearly fit into TDF domains and others could be placed into more than one. Although this added additional complexity to the analysis, we considered the facilitators to his vitamin B1 treatment behaviour as a barrier to adherence to standard therapy and vice versa. The TDF-informed interview schedule enabled Mr. Wilkinson to share relevant information linking to the related behaviours, which in turn aided mapping to the TDF. To further illustrate this complexity, we have extracted the coding framework with supporting quotes for the facilitators and barriers to Parkinsonian medication for this participant (Appendix 2).
4. Discussion
This case study focuses on an individual's experience of self-managing PD symptoms using vitamin B1 which highlighted a number of interesting and important issues. Mr. Wilkinson was a participant in a previously published qualitative study which investigated the barriers and enablers to adherence to antiparkinsonian medications. [ 24 ] The inclusion of Mr. Wilkinson's interview data in the earlier full study and the presentation of his details in this case study has provided many interesting areas for discussion. This case study also offers healthcare professionals a unique insight into the management of PD as it captures the experiences of an underrepresented voice in pharmacy research – the non-adherent patient.
This case study used the TDF framework to identify factors that facilitated Mr. Wilkinson's alternative treatment choices as well as behaviours that created barriers to adherence to standard PD medication. Beliefs about Capabilities of administering vitamin B1 and Beliefs about Consequences of using this treatment were dominant factors for this individual as well as the effect of positive Reinforcement on symptom management gained from the effects of vitamin B1 therapy. These are illustrated by the following TDF descriptions and corresponding extracts of quotes as presented in Table 1 and summarised in Box 1 below.
Illustrative Quotes and Descriptions of Dominant TDF Domains for Mr. Wilkinson.
| Mr. Wilkinson finds injecting vitamin B1 once every two days is much easier than taking PD medication daily: |
| Mr. Wilkinson believes that the longer they withhold from taking PD medication the more they will benefit in the future: |
| Mr. Wilkinson believes that vitamin B1 treatment has reduced or stopped aches and pains, diminished mental capacity and low mood: Mr. Wilkinson is unsure of what B1 treatment is doing but it appears to have reduced PD progression: “ ”. |
Alt-text: Box 1
Although Beliefs about Consequences arose as a dominant facilitator across the whole group researched in our previous study, on the whole Beliefs about Capability and Reinforcement did not feature as strong enablers to PD medication adherence. [ 24 ] This is somewhat surprising since taking a complex medication regimen, such as that prescribed for PD management needs a high degree of capability for good adherence. Similarly, it would be expected that the reinforcement gained from the control (or absence) of PD symptoms after taking prescribed medication would also act as an enabler to adherence, but this was not apparent across the data for the other eleven participants interviewed. Mr. Wilkinson's beliefs about his capability to manage a complex medication regime were likely to be influential in his decision to research and source an alternative PD treatment. It seems unlikely that he would have researched his preferred treatment if he had low capability beliefs and this behaviour suggests that he had high levels of health literacy which may contrast with the wider group of participants, although this was not captured as part of this study.
Further quantitative research is needed to establish the prevalence of these barriers and enablers in a large sample of PD patients. We are in the process of developing a structured questionnaire to establish the prevalence of these barriers and facilitators to medication adherence in a large sample of PD patients. To date, medication non-adherence studies in PD have focused on patients who choose to take the treatment offered by the prescriber but might not be fully adherent to the mediation regimen, [ 27 ] rather than choosing not to take any of the medication. Further analysis of this one outlier (or deviant) participant offers great insight for clinical practice and added value for qualitative researchers by allowing different perspectives which are often missed, to be fully explored in a case study. [ 28 ] This research also demonstrates the need for recruitment strategies that support the inclusion of patients such as Mr. Wilkinson. Had we conducted this study in a clinical setting, this individual and those like him who do not take any of their prescribed treatment may not be in the system and as such would not have been recruited. Although non-adherent patients will still require monitoring and will be utilising the PD services to some extent. There is a need to continue to support patients who chose not to follow standard treatment to engage in research of this nature. Developing recruitment strategies that go beyond the clinical setting is one way of achieving this. For example, utilising social media, support groups and other innovative methods of recruitment offers the opportunity to engage those participants who would not necessarily take part in adherence research.
Several of the TDF domains were facilitators in Mr. Wilkinson's decision to manage PD symptoms via vitamin B1 injections. Knowledge of PD treatment and beliefs about the consequences of taking antiparkinsonian medicine underpinned Mr. Wilkinson's desire to source alternative treatment. Horne & Weinman's [ 17 ] Necessity-Concerns Framework can be used to understand how Mr. Wilkinson's medication beliefs influence adherence. He perceived that the benefit to postponing treatment with antiparkinsonian medications (i.e., that this would improve the medication's effectiveness long term) outweighed the risks of not taking the medication. He was also concerned that once he began taking antiparkinsonian medications, he would become dependent on the medication for symptom control. These beliefs about the consequences of taking antiparkinsonian medication contrast with those expressed by adherent PD participants who considered the medication to be essential for symptom control. [ 24 ]
Social comparison also played an important role in Mr. Wilkinson's treatment decisions; he is a member of PD support group and is in contact with people with different levels of disease progression. He measured the efficacy of B1 treatment by making comparisons between his own health status to others within this group; such downward social comparison (comparing oneself to those who have more pronounced symptoms or disability) reinforced Mr. Wilkinsons treatment decisions and led him to view the perceived side effects of taking antiparkinsonian medication as outweighing current benefits. It is interesting to note that whilst Mr. Wilkinson was fully non-adherent to prescribed PD medication, he occasionally took breaks from injecting the vitamin B1 treatment to gauge whether it was still working. This behaviour of taking a ‘medication holiday’ is well documented in the literature [ 29 ] and is captured by the Intentional Non-Adherence Scale (INAS) which measures the behaviour of ‘testing treatment’ where patients take less doses of medication than prescribed to see if it is still needed. [ 30 , 9 ]
Although there is some emerging evidence that vitamin B1 deficiency may influence the risk of developing PD [ 31 , 32 , 33 ] there is an absence of robust clinical evidence that symptoms of PD can be improved with B1 supplementation. [ 32 , 33 , 34 ] A series of small, case study reports by one Italian research clinic, implicate vitamin B1 as beneficial for a range of movement disorders and for post-stroke and multiple sclerosis related fatigue, with a similar small open label study in PD which was then extended into a larger series of patients. [ 35 ] A very recent correlative study (which was published later) also suggested that there was a relationship between vitamin B1 intake and lower levels of PD symptoms. [ 36 ] Although minimal clinical evidence exists, this sits on a broader base of preclinical literature that is more convincing. Mr. Wilkinson developed his understanding of the therapeutic benefits of B1 through online resources and health forums. Prior to being diagnosed with PD, he attended an online appointment with an Italian centre for PD treatment, which provided one-to-one advice and training on how to administer vitamin B1 injections. The internet is a common way to gain information about symptoms or health conditions, individuals report that they are more likely to look online for information about “new” symptoms than contact their healthcare providers. Health information is associated with a greater knowledge of treatment options [ 37 ] yet it can be challenging to navigate the complexity of healthcare information available to patients and to ascertain the validity of information that is presented about treatment options. However, more recently, the well-trusted Science of Parkinson's Blog ( www.scienceofparkinsons.com ) has been introduced for PD, which includes an entry on the use of Vitamin B1 (thiamine) (Be one with Vitamin B1 – The Science of Parkinson's ( scienceofparkinsons.com ). Furthermore, Parkinson's UK and the Michael J Fox foundation both have webpages about the use of vitamin B1 on their websites.
In terms of limitations, it is important to note that the qualitative study from which this case study derives, did not intend to explore the reasons for taking alternative treatments to the standard UK prescribing guidelines. In this sense, Mr. Wilkinson was adherent to vitamin B1 injections (bar a few medication holidays to check efficacy), but not adherent to the recommended treatment guidelines in the UK. An investigation of adherence to PD services in other parts of the world may have yielded different interpretations of the findings of framework analysis. Had this been the aim, we would have redesigned the interview schedule to explore the rationale and behaviours relating to the use of alternative and concomitant treatments (in this case vitamin B1 injections and magnesium supplements respectively) in more depth.
The authors of this paper do not endorse the use of vitamin B1 or magnesium supplements for the treatment of PD since these are not part of the UK prescribing guidelines. However, the general public is able to access a range of materials beyond the scientific literature and will form their own opinions on the evidence available, which may be at odds with the medical reality. For example, a lay person may interpret this pre-clinical data to be more clinically relevant than it is, and it is our responsibility as clinical practitioners to help patients put this information into context. As previously discussed, all the major Parkinson's organisations have summaries of the evidence for vitamin B1, but much of the material is also provided through blogs and books. The Italian clinic itself provides powerful videos extoling the benefits of vitamin B1 treatment, however, there is clearly a potential conflict of interest if patients are only receiving information from this source. Linking this back to the theoretical perspective of the Necessity-Concerns Framework, the availability of these resources creates a compelling reason for using alternative treatments by describing their benefits in this way. As healthcare practitioners, we need to be equipped to engage in conversations about the pros and cons of conventional treatments over newer ones, which may not yet have the evidence-base.
This case study raises a number of implications for pharmacy practice, in particular, the need to ensure that patients such as Mr. Wilkinson are provided with the opportunity to discuss treatment choices and self-management options for long-term conditions such as PD. Mr. Wilkinson is highly motivated to control his condition and perceives to be self-managing PD effectively, albeit with therapy that is outside the recommended UK guidelines. This highlights the need for healthcare professionals to explore, discuss and acknowledge the patient's beliefs, goals and preferences about their condition and its treatment. Mr. Wilkinson has indicated that he may return to the ‘standard’ medication in the future. The risk of not acknowledging the patient's views and their agency to take an alternative approach means that the opportunity for continued conversation/shared decision-making both at treatment initiation and throughout the course of the disease could be lost. To that end, there is a need to keep the lines of communication open to allow that discussion to evolve over time and avoid patients dropping out of the system, particularly in the early stages where the patient tries to evaluate initial treatment decisions. Beliefs and illness perceptions may change over time, particularly as patients evaluate their treatment choices, illustrated by Mr. Wilkinson who takes ‘drug holidays’ and compares his progress to peers.
As healthcare providers we can learn from this case study by being aware of the breadth of unverified treatments being discussed, particularly online (including those in private UK clinics and overseas) so that we are able to support patients to make informed decisions about their self-management options, referring them to trusted, balanced sources of information. For this to happen, healthcare professionals need to keep themselves updated on new/alternative treatments, to enable an informed discussion about all available treatment options, whether within UK guidelines or not. In this case study, the prescriber's lack of knowledge of vitamin B1 as a potential treatment option was a pivotal point, after which Mr. Wilkinson lost confidence in the consultant as a credible source of information and potentially altered his perception of the support available within the system.
This case study has also highlighted the potential consequences when there is a perceived lack of support or dissatisfaction with the opportunities to discuss different treatment options within the clinical consultation which are essential for shared decision-making. When patients feel involved in the decision-making for their treatment, by engaging in discussions about treatment choices, they are more likely to follow the treatment plan. [ 38 ] Considering the patients' personal preferences, values and needs is important when discussing treatment options since these factors have a significant impact on perception of illness control, subsequent self-management behaviours and adherence to medication. [ 39 , 40 , 41 ]
To summarise, this case study offers healthcare professionals a unique insight into the management of PD as it captures the experiences of an underrepresented voice in pharmacy research – the non-adherent patient. The paper highlights the need within PD services to consider opportunities for how to support individuals who engage in treatments that are not offered within UK healthcare settings.
5. Conclusion
In conclusion, this paper presents interesting findings to Illustrate one case study's reasons for not taking standard PD medication and how the lack of a shared decision-making approach during initial consultations led to alternative PD treatment being sought. The importance of engaging under-represented participants in adherence research is also demonstrated along with the methodological and analytical challenges for dealing with ‘outlier’ cases such as this. Researchers who routinely analyse qualitative data through a behavioural science lens, may lead to a narrow view, since as highlighted in this paper, in many ways Mr. Wilkinson was adherent, just not adherent to the treatment offered in the UK. Finally, important implications for pharmacy practice are raised, in particular with regards to the need to recognise the influence that the patient's perspective has on self-management of symptoms and medication-taking behaviour and how these are addressed within the consultation. As part of clinical practice there is a need to consider opportunities for how cases such as Mr. Wilkinson could be supported through PD services, even if they are also receiving treatment elsewhere.
This research received no specific grant from any finding agency in the public, commercial, or not for profit sectors. The work was completed as part of a MSc in Health Psychology at Cardiff Metropolitan University which was self-funded by JS.
CRediT authorship contribution statement
Delyth James: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Joshua Smith: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Emma Lane: Writing – review & editing, Writing – original draft. Rhian Thomas: Writing – review & editing. Sarah Brown: Writing – review & editing. Heidi Seage: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This paper and the research behind would not have been possible without the agreement and consent of the participant involved.
Appendix A Supplementary data to this article can be found online at https://doi.org/10.1016/j.rcsop.2024.100450 .
Appendix A. Supplementary data
Supplementary material
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Case Reports
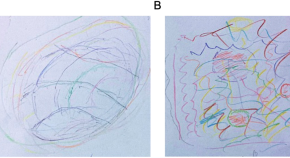
Narrative medicine pinpoints loss of autonomy and stigma in Parkinson’s disease
- Barend W. Florijn
- Raoul Kloppenborg
- Bastiaan R. Bloem
A severe neurodegenerative disease with Lewy bodies and a mutation in the glucocerebrosidase gene
- Jussi O. T. Sipilä
- Laura Kytövuori
- Kari Majamaa
Pathophysiological evaluation of the LRRK2 G2385R risk variant for Parkinson’s disease
- Toshiki Tezuka
- Daisuke Taniguchi
- Nobutaka Hattori
Neuropathology of Parkinson’s disease after focused ultrasound thalamotomy
- Shunsuke Koga
- Mariam Ishaque
- Dennis W. Dickson
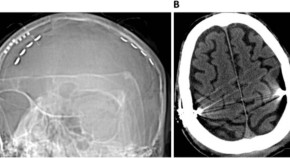
Extradural Motor Cortex Stimulation might improve episodic and working memory in patients with Parkinson’s disease
- Carla Piano
- Marco Ciavarro
- Antonio Daniele
A curious case of DBS radiofrequency programmer interference
- Sanjeet S. Grewal
- Karim ReFaey
- Robert E. Wharen Jr
Cognitive behavioral therapy for insomnia in Parkinson’s disease: a case series
- Meghan Humbert
- James Findley
- Lana M. Chahine
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Parkinson's Disease: Challenges, Progress, and Promise
Introduction.
Following Alzheimer’s disease, Parkinson's disease (PD) is the second-most common neurodegenerative disorder in the United States. Most people diagnosed with PD are age 60 years or older, however, an estimated 5 to 10 percent of people with PD are diagnosed before the age of 50. Approximately 500,000 Americans are diagnosed with PD, but given that many individuals go undiagnosed or are misdiagnosed the actual number is likely much higher. Some experts estimate that as many as 1 million Americans have PD. Of course, given the progressive nature of the disabilities associated with PD, the disease affects thousands more wives, husbands, children, and other caregivers.
In the United States alone, the cost of treating PD is estimated to be $14 billion annually. Indirect costs, such as those associated with the loss of productivity, are conservatively estimated to total $6.3 billion each year. As the U.S. population ages, these figures are expected to rise rapidly. The number of people diagnosed with PD in the United States is expected to double by 2040.
The National Institute of Neurological Disorders and Stroke ( NINDS ), part of the National Institutes of Health ( NIH ), has a long history of supporting PD research. For decades, NINDS-funded researchers working nationwide have developed treatment options that have greatly improved motor symptoms for people with PD. For example, dopamine replacement therapy with Sinemet, a mainstay therapy in the treatment of PD, has helped alleviate motor symptoms particularly in the early stages of disease. Deep brain stimulation (DBS) can reduce tremor, rigidity, stiffness, and improve movement. However, much work remains to be done. Despite their many successes, these therapies have limitations. There is no currently available therapy that slows the progression of the underlying disease or adequately relieves the wide range of symptoms in people with more advanced PD.
The NINDS brings scientists, health care providers, individuals with PD, caregivers, advocacy groups, and other stakeholders together to assess the state of PD research, define key challenges, and set priorities for advancing PD research. Most recently, the NINDS held the “Parkinson's Disease 2014: Advancing Research, Improving Lives” conference, which resulted in a series of prioritized recommendations that will inform ongoing and future efforts in PD research. This booklet highlights the recent progress made in PD research and maps out the challenges and priorities for the road ahead.
About Parkinson's
PD’s effects on the central nervous system are both chronic (meaning they persist) and progressive (meaning the symptoms grow worse over time). By the time a diagnosis is made, PD has typically already progressed to a point where people have difficulty controlling the movement of their bodies due to tremors (involuntary shaking), bradykinesia (slowness of movement and reflexes), stiffness in their limbs or the trunk of their body, and impaired balance. As these symptoms progress, walking, talking, swallowing, and completing other simple tasks can become challenging.
In addition to these motor-related symptoms, non-motor symptoms such as cognitive impairment, mood and behavioral problems, sleep disorders, and constipation can significantly impair quality of life and require careful symptom-based treatment. Some non-motor symptoms such as hyposmia (reduced ability to detect odors), REM sleep-behavior disorder (acting out vivid dreams), and constipation typically precede the motor symptoms by several years. Other non-motor symptoms such as cognitive impairment commonly appear after the onset of motor symptoms.
Many people with PD eventually develop dementia, but the time from the onset of movement symptoms to the onset of dementia symptoms varies greatly from person to person. Dementia is a leading reason for people with PD to transition from independent living at home to long-term care facilities.
PD disease processes begin well before people start exhibiting motor symptoms. This is the preclinical phase of the disease. During this phase people may experience a range of nonspecific, non-motor symptoms such as hyposmia, depression, anxiety, and sleep disorders. People may also experience disturbances of the autonomic nervous system that manifest as problems with digestion, respiration, salivation as well as excessive sweating, bladder dysfunction, or sexual dysfunction. This phase may last for several years. The onset of motor symptoms marks the clinical phase of PD. People may have a variety of symptoms including resting tremor, bradykinesia, rigidity (resistance to passive movement of the limbs), and balance problems. The progression of these symptoms is typically gradual, often involving only one side of the body at first. This includes things like a reduction of arm swing on one side when walking, soft speech, or intermittent tremor.
More research is needed to better understand, characterize, and identify features of the preclinical phase of PD. A high priority is placed on finding biological identifiers, or biomarkers, of these early phases so that people at high risk for progressing to the clinical phase of PD can be identified. In the future, therapeutics or other interventions may be available to prevent or slow the onset of the clinical phase of the disease among those at high risk for PD.
Currently available PD medications do offer valuable symptomatic relief, but as PD progresses, their use is often associated with significant and sometimes intolerable side effects. For example, levodopa, one of the most effective treatments for PD can normalize motor function for years but later cause involuntary muscle movements known as dyskinesia and dystonia (sustained muscle contractions). In addition, people in the mid to late stages of PD often experience a wearing-off of the beneficial effects of PD drugs and a re-emergence of motor and non-motor symptoms before their next scheduled dose. In more advanced PD, drug-resistant motor symptoms (e.g, postural instability, freezing of gait, loss of balance, frequent falls), behavioral changes (impulse control disorders, hallucinations, and psychosis), and often dementia are leading causes of impairment.
In addition to new therapeutic options, better diagnostic tools are needed to identify PD earlier in the course of the disease. By the time a person exhibits classic motor symptoms and is diagnosed with PD, substantial and widespread loss of brain cells and functions of the brain and autonomic nervous system have already occurred. Earlier diagnosis may provide a therapeutic window to slow or prevent the progression of PD prior to the onset of motor impairments.
Understanding the Pathology
The nervous system is made up of individual units called nerve cells or neurons. Neurons serve as a "communication network" within the brain and throughout a person’s body. Parkinson’s disease develops when neurons in the brain and elsewhere in the nervous system fail to function normally or die. The hallmark symptoms of PD — bradykinesia, tremor, postural instability, and rigidity — result primarily from the death of neurons in the substantia nigra, a region in the midbrain critical for motor control.
In order to communicate, neurons use chemical messengers called neurotransmitters. Neurotransmitters send information between neurons by crossing the space between them, called the synapse. Normally, neurons in the substantia nigra produce a neurotransmitter known as dopamine. Dopamine is critical for movement and it helps transmit messages within the brain to make sure muscles produce smooth, purposeful movement. Loss of dopamine results in abnormal nerve firing patterns that impair movement. By the time Parkinson’s is diagnosed, most people have lost an estimated 60 to 80 percent of their dopamine-producing cells in the substantia nigra.
While loss of dopamine accounts for the characteristic features of the disease, recent studies have revealed that a number of other brain systems are also damaged. These include the brain structures that regulate the chemical pathways that depend on norepinephrine, serotonin, and acetylcholine. The changes in these neurotransmitters and circuits may account for many of the non-motor features of PD.
A factor believed to play a fundamental role in the development of PD involves abnormalities of a protein called alpha-synuclein. In the normal brain, alpha-synuclein is located in nerve cells in specialized structures called presynaptic terminals. These terminals release neurotransmitters which carry signals between neurons. This signaling system is vital for normal brain function.
While normal alpha-synuclein functions are related to the storage and release of neurotransmitters, evidence suggests the buildup of excessive and abnormal alpha-synuclein plays a key role in the development of PD. There are rare examples of families in which certain genetic mutations in alpha-synuclein have been shown to cause the alpha-synuclein protein to misfold into an abnormal configuration. Most individuals with PD do not have a mutation in alpha-synuclein, but even when there is no mutation present, nearly every case of PD is associated with a buildup of abnormal and misfolded alpha-synuclein. As the misfolded protein accumulates, it clumps together into aggregates, or collections, that join together to form tiny protein threads called fibrils. Fibrils are the building blocks for Lewy bodies, abnormal structures that form inside nerve cells in the substantia nigra and elsewhere in the brain. Lewy bodies are a pathological hallmark of PD. Research suggests that the harmful buildup of alpha-synuclein may affect normal function and trigger nerve cell death.
Lewy bodies were discovered more than 100 years ago, and there are still unanswered questions about their role in disease. They are found in the brain of almost every patient affected by PD, but whether the Lewy bodies themselves contribute to the death of neurons is still unclear. Alternatively, the accumulation of protein in Lewy bodies may be part of an unsuccessful attempt to protect the cell from the toxicity of aggregates of alpha-synuclein.
A key objective for researchers moving forward is to better understand the normal and abnormal functions of alpha-synuclein and its relationship to genetic mutations that impact PD.
Genetic Studies
In the past decade, NINDS-funded researchers have discovered much about the genetic factors that contribute to PD. In most instances the cause of PD is unknown, however, a small proportion of cases can be attributed to genetic factors. An estimated 15 to 25 percent of people with Parkinson’s disease have a family history of the disorder. It is relatively rare for PD to be caused by a single mutation of one of several specific genes. This only accounts for about 30 percent of cases in which there is a family history of PD and only 3 to 5 percent of sporadic cases — instances with no known family history.
Researchers increasingly believe that most, if not all, cases of PD probably involve both a genetic and environmental component. Early-onset Parkinson's disease is relatively rare and is more likely to be influenced by genetic factors than the forms of the disease that develop later in life.
Multiple NIH projects helped build an infrastructure for PD genetics research. The Human Genome Project and the International HapMap Project laid the groundwork for this research, producing tools to help researchers find genetic contributions to common diseases. Using these tools, researchers supported the Parkinson’s Disease Genome Wide Association Study (PD-GWAS). Funded by both the NINDS and the National Institute on Aging ( NIA ), this effort aims to detect genetic risk factors for PD from groups around the world. Included in PD-GWAS are data from nearly 14,000 people with PD and more than 95,000 people without PD. By comparing these two groups, researchers can identify patterns in certain regions, or loci, of the human genome where genes that cause or increase the risk of PD are likely to reside. Much like a zip code, genetic loci describe the general neighborhood of a gene.
Based on an analysis of PD-GWAS data and other sources, NIH-funded scientists have identified 28 loci believed to be independently associated with PD risk and many more loci have been tentatively linked to the disorder.
Next generation genetic technologies have led to a number of new discoveries and allowed scientists learn more about what genetic factors contribute to the risk of developing PD. The first successes were a result of high-content genotyping, a method of identifying common variants in the human genome. Currently, there is a great deal of excitement regarding next generation sequencing — methods of genetic sequencing that allow for rapid sequencing of DNA base pairs in particular loci of the genome. These methods have significantly cut the time and costs required to identify genes involved with PD and will continue to facilitate the identification of PD-related genes in the future.
Another breakthrough in genetic sequencing is NeuroX, the first DNA chip able to identify genetic variants in a person’s genome to determine any risk for developing a number of late-onset neurodegenerative diseases, including PD. A joint venture between the NINDS and investigators at the NIA , the NeuroX chip was developed as a result of a 2011 NINDS workshop. The workshop led to an analysis of data from worldwide PD-GWAS investigations. Those studies helped correlate genetic variants and common traits among people with PD, which made the NeuroX chip possible.
Despite these innovations, significantly more research is needed to identify PD-related genes and the cellular processes they support in order to understand how these functions contribute to the onset and progression of PD. Common genetic variations alone cannot fully explain how genetics contributes to the risk of developing PD. Instead, researchers hypothesize there must be additional genetic contributions from variants that are not common enough to be detected by PD-GWAS investigations.
Known Genetic Mutations
Inherited PD has been found to be associated with mutations in a number of genes including SNCA , LRRK2 , PARK2 , PARK7 , and PINK1 . Many more genes may yet be identified. Genome-wide association studies have shown that common variants in these genes also play a role in changing the risk for sporadic cases.
Mutations in other types of genes, including GBA , the gene in which a mutation causes Gaucher’s disease, do not cause PD, but appear to modify the risk of developing the condition in some families. There may also be variations in other genes that have not been identified that contribute to the risk of the disease.
- Gene for alpha-synuclein ( SNCA )
In 1997, scientists identified the first genetic mutation ( SNCA ) associated with PD among three unrelated families with several members affected with PD. The SNCA gene provides instructions for making the protein alpha-synuclein, which is normally found in the brain as well as other tissues in the body. Finding this mutation led to the discovery that alpha-synuclein aggregates were the primary component of the Lewy body. This is an example of how a disease-causing rare mutation can shed light on the entire disease process.
PD related to SNCA gene mutations is autosomal dominant, meaning that just one mutated copy of the gene in each cell is sufficient for a person to be affected. People with this mutation usually have a parent with the disease.
Though more than a dozen mutations in the SNCA gene have been linked to PD, these mutations are considered a relatively rare cause of the disease. In some cases, SNCA gene mutations are believed to cause the alpha-synuclein protein to misfold. Other SNCA mutations create extra copies of the gene, leading to excessive production of the alpha-synuclein protein. Even when no mutation is present, buildup of abnormal synuclein is a hallmark of PD. The NINDS is funding multiple studies aimed at determining how misfolded and excessive levels of alpha-synuclein might contribute to developing PD.
- Gene for leucine-rich repeat kinase 2 ( LRRK2 )
Mutations of the LRRK2 gene are the most common genetic cause of autosomal dominant PD. These mutations play a role in about 10 percent of inherited forms of PD and about 4 percent of people who have no family history of the disease. Studies show that one particular LRRK2 mutation, G2019S, accounts for up to 20 percent of PD in specific groups, such as the Ashkenazi Jewish population.
Researchers are still studying exactly how LRRK2 gene mutations lead to PD, but it appears these mutations influence both the manufacturing and disposal of unwanted proteins in multiple ways. PD associated with LRRK2 mutations involves both early- and late-onset forms of the disease. The LRRK2 gene is a kinase enzyme, a type of protein that tags molecules within cells with chemicals called phosphate groups. This process of tagging, called phosphorylation, regulates protein enzymes by turning them “on” or “off” and it is fundamental to basic nerve cell function and health.
NINDS-supported investigators at the Udall Center at Johns Hopkins University (JHU) have found that LRRK2 mutations increase the rate at which the gene’s protein tags ribosomal proteins, a key component of the protein-making machinery inside cells. This can cause the machinery to manufacture too many proteins, leading to cell death.
LRRK2 gene mutations also are believed to inhibit a waste disposal method called autophagy, the process by which cells breakdown nutrients, recycle cellular components, and get rid of unusable waste. Autophagy is a critical means for quality control by enabling the cell to eliminate damaged organelles and abnormal proteins.
LRRK2 gene mutations inhibit a type of autophagy called chaperone-mediated autophagy. During this type of autophagy a “chaperone” protein escorts a damaged protein to the lysosome, spherical vesicles within cells that contain acid that help breakdown unwanted molecules. As a result, the LRRK2 gene mutations may lead to the buildup of alpha-synuclein into toxic aggregates within the cells. Researchers are exploring whether certain compounds might be capable of overriding LRRK2 gene mutation effects by rebooting the chaperone-mediated disposal system.
- Gene for parkin ( PARK2 )/ Gene for PTEN induced putative kinase 1, or PINK1 ( PARK6 )
PARK2 mutations are the most common genetic mutations associated with early-onset PD, which first appear at age 50 or younger. PARK6 gene mutations also are associated with early-onset PD, but they are far more rare. Both types of mutations are associated with autosomal recessive PD, meaning that two mutated copies of the gene are present in each cell and that anyone affected may have unaffected parents who each carried a single copy of the mutated gene.
Findings from a NINDS-funded study suggest that people with PARK2 mutations tend to have slower disease progression compared with those who do not carry PARK2 mutations.
The genes PARK2 , PARK6 , PINK1 , along with the protein parkin, are all involved at different points along a pathway that controls the integrity of mitochondria, the powerhouses inside cells that produce energy by regulating quality control processes. Brain cells are especially energetic and dependent upon mitochondrial energy supply. Specifically, parkin and PINK1 regulate mitochondrial autophagy — a process known as mitophagy. These processes are critical for maintaining a healthy pool of mitochondria by providing a means to eliminate those that no longer function properly.
Much work remains to be done to understand the association of PARK2 and PARK6 mutations and mitochondrial dysfunction, as well as to investigate if and how mitochondrial dysfunction leads to PD. Evidence suggests that parkin and PINK1 function together. When PINK1 (which is located on mitochondria) senses mitochondrial damage, it recruits parkin to get the process of mitophagy underway.
NINDS researchers are exploring ways to stimulate the PINK1 /parkin pathway to encourage mitophagy. Scientists hope this will help them develop treatments for people with mitochondrial diseases, including certain forms of PD. Additionally, NINDS researchers are screening chemicals to identify agents that may be able to stimulate the expression of PINK1 , and looking for other genes that may affect the functions of PINK1 and parkin.
Evidence suggests that parkin is a factor in several additional pathways leading to PD, including sporadic forms of the disease associated with alpha-synuclein toxicity.
- Gene for DJ-1 ( PARK7 )
The PARK7 gene encodes for the protein DJ-1. Several mutations in the gene for DJ-1 are associated with some rare, early-onset forms of PD. The function of the DJ-1 gene remains a mystery. However, one theory is it can help protect cells from oxidative stress. Oxidative stress occurs when unstable molecules called free radicals accumulate to levels that can damage or kill cells. Some studies suggest that the DJ-1 gene strengthens the cells’ ability to protect against metal toxicity and that this protective function is lost in some DJ-1 mutations. Animal studies suggest DJ-1 plays a role in motor function and helps protect cells against oxidative stress.
- Gene for beta-glucocerebrosidase ( GBA )
Mutations in the gene encoding the lysosomal enzyme beta-glucocerebrosidase ( GBA ) are associated with a lysosomal storage disorder, Gaucher’s disease. People with Gaucher’s disease are also more likely to have parkinsonism, a group of nervous disorders with symptoms similar to Parkinson's disease. This has spurred investigators to look for a possible link between the two diseases. NIH-funded researchers have conducted studies of individuals with both disorders to assess their brain changes, family histories, and to screen tissues and DNA samples, which have helped confirm this link.
An NIH-led, multicenter study involving more than 10,000 people with and without PD showed that people with PD were more than 5 times more likely to carry a GBA mutation than those without the disease. Mutation carriers also were more likely to be diagnosed with PD earlier in their lives and to have a family history of the disease. Scientists have observed that depletion of beta-glucocerebrosidase results in alpha-synuclein accumulation and neurodegeneration.
Further research is needed to understand the association between GBA gene mutations and PD. The NINDS supports many lines of research investigating the role of GBA gene mutations. Projects are aimed at estimating the risk of PD associated with being a GBA carrier and identifying the phenotypic traits.
Studying the genes responsible for inherited cases of PD can help shed light on both inherited and sporadic cases of PD. The same genes and proteins that are altered in inherited cases of PD may play a role in sporadic cases of the disease. In some cases genetic mutations may not directly cause PD but may increase the susceptibility of developing the disease, especially when environmental toxins or other factors are present.
Cellular and Molecular Pathways to PD
What happens in a person’s brain that causes him or her to develop PD? To answer this question scientists are working to understand the cellular and molecular pathways that lead to PD.
Mitochondrial Dysfunction
Research suggests that damage to mitochondria plays a major role in the development of PD. Mitochondria are unique parts of the cell that have their own DNA entirely separate from the genes found in the nucleus of every cell.
Mitochondrial dysfunction is a leading source of free radicals — molecules that damage membranes, proteins, DNA, and other parts of the cell. Oxidative stress is the main cause of damage by free radicals. Oxidative stress-related changes, including free radical damage to DNA, proteins, mitochondria, and fats has been detected in the brains of individuals with PD. A number of the genes found to cause PD disturb the process by which damaged mitochondria are disposed of in the neuron (mitophagy).
To learn more about how the process of mitophagy relates to PD, scientists have turned to RNA interference (RNAi), a natural process occurring in cells that helps regulate genes. Scientists are able to use RNAi as a tool to turn off genes of interest to investigate their function in cultured cells or animal models of PD. A technique known as high-throughput RNAi technology enabled NIH scientists to turn off nearly 22,000 genes one at a time. This process helped scientists identify dozens of genes that may regulate the clearance of damaged mitochondria. Researchers continue to study how these genes regulate the removal of damaged mitochondria from cells and the genes identified in this study may represent new therapeutic targets for PD.
One mechanism that helps regulate the health of mitochondria is autophagy, which allows for the breakdown and recycling of cellular components. Scientists have long observed that disruptions in the autophagy processes are associated with cell death in the substantia nigra and the accumulation of proteins in the brains of people with PD as well as other neurodegenerative diseases.
Ubiquitin-proteasome System
Another area of PD research focuses on the ubiquitin-proteasome system (UPS), which helps cells stay healthy by getting rid of abnormal proteins. A chemical called ubiquitin acts as a “tag” that marks certain proteins in the cell for degradation by proteasomes, structures inside cells that launch chemical reactions that break peptide bonds. Researchers believe that if this disposal symptom fails to work correctly, toxins and other substances may accumulate to harmful levels, leading to cell death. Impairment of the UPS is believed to play a key role in several neurodegenerative disorders, including Alzheimer's, Parkinson's, and Huntington's diseases.
The contribution of UPS to the development of PD appears to be multifactorial, meaning UPS influences the interactions of several genes. NINDS-funded researchers have found that UPS is critical for the degradation of misfolded alpha-synuclein in cells. Conversely, evidence suggests that abnormal or misfolded alpha-synuclein may also inhibit the proper functioning of UPS. A feedback loop may exist whereby abnormal alpha-synuclein inhibits the functions of UPS, causing more abnormal alpha-synuclein to accumulate and additional suppression of UPS activity. NINDS-funded researchers have also identified proteins that accumulate in the absence of parkin that contribute to the loss of dopaminergic neurons.
Several NINDS-funded investigators are exploring ways of enhancing UPS function as a potential therapeutic strategy.
Cell-to-cell Transmission of Abnormally-folded Proteins
Researchers have learned more about how PD-related damage spreads to various parts of the brain and nervous system. A characteristic pattern has emerged by which Lewy bodies are distributed in various regions of the brain. The earliest brain changes appear to involve Lewy bodies in the brain stem region (medulla oblongata and pontine tegmentum, as well as the olfactory bulb).
Braak staging is a six-tier classification method used to identify the degree of postmortem pathology resulting from PD. According to this classification, people in Braak stages 1 and 2 are generally thought to be presymptomatic. As the disease advances to Braak stages 3 and 4, Lewy bodies spread to the substantia nigra, areas of the midbrain, the basal forebrain, and the neocortex.
More recent evidence suggests that even before such brain changes have occurred, alpha-synuclein aggregates and Lewy bodies can be found in the nervous system of the gastrointestinal tract and in the salivary glands, a finding that supports the theory that PD many originate not in the brain but in the autonomic nervous system. Non-motor symptoms such as constipation may in fact be a sign of the disease affecting nerves outside the brain before the disease moves into the brain where it later affects regions that control movement.
Researchers at the Udall Center at the Perelman School of Medicine of the University of Pennsylvania injected mice with a synthetic form of abnormal alpha-synuclein and found that misfolded alpha-synuclein appeared to spread throughout the brain. The researchers hypothesize that the injected abnormal alpha-synuclein may act like a seed that triggers the mouse’s own alpha-synuclein to misfold, leading to a cell-to-cell transmission of PD-like brain changes, especially in regions of the brain important for motor function. The mice also exhibited PD-like motor symptoms.
Understanding more about how abnormal proteins spread through the nervous system may provide a potential window for a therapeutic strategy that interrupts the process of protein transmission and slows or halts disease progression. For example, NINDS-funded investigators are looking at immune therapy and antibodies or immunization against alpha-synuclein, to block PD transmission in the brains of mice.
Environmental Influences Environmental circumstances are thought to impact the development of PD. Exposure to certain toxins may have a direct link to the development of PD. This was the case among people exposed to MPTP, a by-product accidentally produced in the manufacture of a synthetic opioid with effects similar to morphine. During the 1980s, street drugs contaminated with this substance caused a syndrome similar to PD. MPTP is also structurally similar to some pesticides. The brain converts MPTP into MPP+, which is toxic to substantia nigra neurons. MPP+ exposure produces severe, permanent parkinsonism and has been used to create animal models of PD.
In other cases, exposure to the metal manganese among those with working in the mining, welding, and steel industries has been associated with an increased risk of developing parkinsonism. Some evidence suggests that exposure to certain herbicides such as paraquat and maneb increase the risk of PD. Scientists believe that there are other yet-to-be identified environmental factors that play a role in PD among people who are already genetically susceptible to developing the disease.
The National Institute of Environmental Health Sciences ( NIEHS ) is the lead institute at the NIH investigating the association between PD and environmental influences such as pesticides and solvents as well as other factors like traumatic brain injury. For example, NIEHS is funding a project at the University of Washington aimed at developing and validating biomarkers to identify early-stage neurological disease processes associated with toxic agents such as chemicals, metals, and pesticides. Animal models are being developed to study the impact of pesticides on farmworkers and metals on professional welders.
The NIEHS also funds the Parkinson’s, Genes & Environment study. The study is designed to determine the role genes as well dietary, lifestyle, and environmental factors play on the risk for developing PD and their potential to cause the illness. The more than 500,000 study participants were originally recruited in 1995 as part of the National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study. Researchers will continue to follow participants over time to address some of the most interesting theories about the causes of PD. Already they have found, for example, that people who consume low levels of healthy dietary fats, such as those from fish, or high levels of saturated fats are more vulnerable to developing PD after being exposed to neurotoxins such as pesticides. The findings need to be confirmed, however, they suggest the possibility that diets rich in healthy fats and low in saturated fats may reduce the risk of PD.
The development of PD is a complex interplay between environmental, genetic, and lifestyle factors. Scientists are increasingly aware that in any given individual, there may be multiple factors that cause the disease.
In some cases, environmental factors may also have a protective effect. Population-based studies have suggested, for example, that people with high levels of vitamin D in their blood have a much lower risk of developing PD compared with people with very low concentrations of vitamin D. Further research is need to determine if vitamin D deficiency puts people at higher risk for PD, but such findings suggest the possibility that vitamin D supplements may have a beneficial effect. However, there may be genetic factors that cause people with low vitamin D levels to have higher rates of PD in which case vitamin D supplements would not be helpful.
To answer to this question, researchers at the Udall Center at the University of Miami are examining the pharmacogenetics of vitamin D. The investigators are studying a large dataset to confirm the finding that low levels of vitamin D is a risk factor for PD. At the same time, they are trying to identify any potential genetic modifiers of vitamin D’s effect on PD risk.
Certain drugs and chemicals available as a supplement or in a person’s diet also have been shown to have a neuroprotective effect for PD and other disorders. For example, regular use of caffeine (coffee, tea) was found to reduce the loss of dopamine-producing neurons. Studies hope to define the optimal caffeine dose in treating movement disorders like PD while gaining a better understanding of the mechanisms involving caffeine’s benefit. Uric acid, because of its antioxidative effect, may lower the risk for multiple neurodegenerative disorders, in particular, PD. A preliminary clinical trial funded by the Michael J. Fox Foundation examined the effectiveness of the drug inosine to safely raise uric acid levels and possibly slow the progression of Parkinson’s disease.
Neuroinflammation Neuroinflammation is a protective biological response designed to eliminate damaged cells and other harmful agents in nervous system tissue. Mounting evidence suggests that neuroinflammation plays a role in PD. Several lines of research funded by the NINDS are investigating this connection.
Compared to people without PD, those with PD tend to have higher levels of pro-inflammatory substances known as cytokines in their cerebrospinal fluid. Immune cells in the brain called microglia also are more likely to be activated in the brains of individuals with PD. Epidemiological studies suggest that rates of PD among people who frequently use non-steroidal anti-inflammatory drugs (NSAIDS) are lower than in those who do not use NSAIDS.
Evidence from animal studies also suggests that elevated levels of the protein alpha-synuclein may trigger microglia to become activated in the brains of people with PD.
Currently, scientists are investigating whether inflammation itself is a cause of brain cell death or if it is a response to an already occurring process that contributes to the development of a disease. If researchers can interrupt the neuroinflammatory processes, they may be able to develop neuroprotective treatments for people with PD that prevent or slow the progression of the disease by halting, or at least reducing, the loss of neurons.
Models for Studying PD Much of the research advancing our understanding and treatment of PD would not be possible without research models — yeast, fruit flies, worms, fish, rodents, and non-human primates — that have specific characteristics that mimic PD biology in humans. Scientists depend on these models to investigate questions about what goes wrong in PD, how cellular processes fit into the context of neuronal circuits, and how potential new treatments affect these disease processes.
The NINDS supports ongoing studies at the Udall Centers and elsewhere to refine existing research models and develop new ones. Better models are needed to more accurately mimic human disease in animals and to study PD’s mechanisms and potential treatments. Currently, none of the models express all the key pathologic features of PD or reflect the complement of clinical motor and non-motor features of the disease in humans.
In addition to creating new animal models, NINDS-funded researchers also look for ways of combining different types of models (i.e., genetic and toxin-induced) to better understand the interplay between genetic and environmental factors that contribute to the development of PD.
Genetic Models The identification of genetic mutations among some families with hereditary forms of PD led to the development of animal models (rodent, non-human primate, worm, and fly) engineered to have mutations or deletions of PD genes. Each model has its strengths and shortcomings in helping researchers study the disease.
For example, mice with SNCA mutations develop an adult-onset degenerative disease characterized by movement dysfunction and aggregation of alpha-synuclein, but these mice have no loss of dopaminergic neurons. Other mice have been engineered to express LRRK2 mutations, but show little evidence of PD symptoms. Fruit flies and worms engineered to overexpress LRRK2 exhibit reductions in motor abilities and loss of dopamine neurons, but they do not adequately reflect the disease as it occurs in humans.
Scientists have developed numerous models aimed at interrupting key cellular functions known to play a role in PD. For example, the MitoPark mouse model disrupts the functioning of the mitochondria, leading to some PD-like motor symptoms that respond to levodopa treatment.
Toxin-induced Models For decades, the most widely used models for studying PD involved those in which toxins were used to induce PD-like motor symptoms. Such models were used to evaluate potential therapies. The first toxin-induced models relied on MPTP or the neurotoxin 6-hydroxydopamine to kill dopamine-producing neurons in the substantia nigra, causing PD-like motor symptoms. Later, researchers developed another type of model that examined how toxins interfered with the activities of mitochondria. Toxins for this purpose included the pesticide rotenone and the herbicides paraquat and maneb. Rats exposed to such toxins develop large inclusions in substantia nigra neurons that resemble Lewy bodies and contain alpha-synuclein and ubiquitin. The animals also developed bradykinesia, rigidity, and gait problems. Such toxin models are helpful for studying the consequences of dopamine depletion. However, they are limited in their ability to model the all the factors that cause PD in humans.
Induced Pluripotent Stem Cells Genetic engineering is another mechanism for modeling some of the processes that go wrong in PD. Recently scientists developed a breakthrough modeling mechanism using induced pluripotent stem cells (iPSCs), which are cells that can become any type of cell in the body. Researchers take samples of skin, blood, hair follicles, or other types of tissue from a person with PD and then manipulate those cells to become iPSCs. These cells are then programmed to become dopaminergic neurons, making it possible for scientists to study the molecular and cellular mechanisms that lead to PD as well as potential treatments. NIH-funded researchers have also coaxed iPSCs to become tissue from other parts of the body such as the gastrointestinal tract and the heart, allowing them to study the mechanisms of PD in other regions of the body.
NINDS-funded researchers at the Udall Center at Johns Hopkins University have used iPSCs from people with PD as well as presymptomatic people who carry PARK6 or LRRK2 genetic mutations to develop brain cells to study specific aspects of mitochondrial functioning. They also are testing potential ways of intervening to reverse mitochondrial dysfunction.
The ability to create neurons or other cell types from an individual with PD presents the possibility of providing a personalized treatment approach. iPSC-derived neurons may prove useful for testing the effectiveness of a drug before giving it to people with PD.
The NINDS created and supports an open-access repository of iPSCs from people who have genetic mutations associated with PD. Specimens in the repository are collected and characterized by a team of collaborating researchers at seven major institutions participating in the Parkinson’s iPSC Consortium. The iPSCs are available through the NINDS Repository for researchers to study the causes of PD, as well as to screen potential drug therapies.
Improving Diagnosis
There is no single definitive test for diagnosing PD in a living person and there is no way to track disease progression on a biological level. Aside from finding a cure, the holy grail of PD research is the discovery of biomarkers — detectable and measurable changes in the body that can be used to predict, diagnose, and monitor disease activity and progression. Biomarkers can be identified through a number of different methods, including imaging scans (e.g., MRI, CT), biological samples (e.g., cerebrospinal fluid, plasma), and genetic studies. The risk for heart disease, for example, can be detected by measuring cholesterol or blood pressure. People at risk for PD currently lack a similar means for risk detection.
The ideal PD biomarker would be one that can be easily tested, varies with disease severity, and is abnormal during the preclinical phase of the illness before a person has any symptoms. Reliable biomarkers would allow physicians to screen and identify people at increased risk of developing PD and more accurately monitor disease progression among people who have been diagnosed with the disease.
Biomarkers would also greatly accelerate clinical research efforts by shortening the timeframe needed to show that a drug has successfully engaged a disease-specific target in the brain or nervous system. Such measures may be available long before meaningful clinical changes are evident after a person has tried a particular therapy or intervention. Biomarkers may also be useful for determining optimal drug dosage.
Progress toward the development of biomarkers is occurring on several fronts.
The U.S. Food and Drug Administration (FDA) has approved the use of brain imaging technology to detect dopamine transporters (DaT), an indicator of dopamine neurons, to help evaluate adults with suspected parkinsonism. The DaTscan uses an iodine-based radioactive chemical along with single-photon emission computed tomography (SPECT, imaging involving blood flow to tissue) to determine whether there has been a loss of dopamine-producing neurons in a person’s brain. However, DaTscan cannot diagnose PD, nor can it accurately distinguish PD from other disorders that involve a loss of dopamine neurons.
NINDS scientists are trying to develop additional ways of imaging the brain and measuring neurochemicals in order to look for early signs of PD. In one study, researchers are comparing brain images from people with PD with images from people who might have early symptoms of PD, as well as people without signs of PD. The objective is to provide a picture of how PD affects the brain over time.
Given the critical contribution that alpha-synuclein is believed to play in the development of PD, a high-priority goal is to develop a positron emission tomography (PET) imaging agent that can show alpha-synuclein accumulation in the brain. Currently, alpha-synuclein levels and localization in the brain can only be confirmed by an autopsy. The ability to detect the protein with an imaging technology in a living person would enable physicians to track the severity of alpha-synuclein accumulation over time, as well as to provide a means to gauge the success or failure of therapies aimed at reducing alpha-synuclein levels. Such a tool would be a game changer for accelerating drug development.
PET imaging produces a three-dimensional image of functional processes in the body. The technique requires the injection of a radiotracer agent to target the alpha-synuclein protein so that it can be visualized. Several NINDS-funded researchers and a consortium of researchers assembled by the Michael J. Fox Foundation are working to develop such an alpha-synuclein radiotracer.
NINDS researchers are conducting a longitudinal study of a large population of people — half of whom have multiple risk factors for PD while the other half have no obvious risk factors — as a way of identifying and validating biomarkers for predicting the development of PD. Many of the biomarkers being tested measure functioning of the autonomic nervous system because, as research suggests, non-motor symptoms associated with the autonomic nervous system often precede motor symptoms.
Advancing Treatments
A personalized medicine approach that treats an individual with PD in a timely manner with the optimal treatment requires understanding the enormously complex and diverse set of factors that contribute to PD. The disease processes that lead to PD involve numerous potential variables and pathways operating at cellular and molecular levels. Most of these processes unfold over the course of many years and begin well before individuals start having symptoms. People with PD may also differ significantly in terms of the symptoms they experience, the severity of those symptoms, disease progression, and their response to treatment and risk of complications.
Improving our understanding of what causes the complexity and diversity of PD is a major challenge for researchers. Tools are needed to group people with similar types of PD so that individuals who are most likely to benefit from clinical trials can be studied and their responses to treatment can be compared in a meaningful way.
Neuroprotection and Disease Modification
A current NINDS study is focused on a potentially neuroprotective treatment that modulates calcium levels for newly-diagnosed individuals with PD. Cells in the body, including dopamine neurons in the brain, maintain optimal levels of calcium by pumping it in and out of their membranes through pore-like openings called channels. When calcium levels are too low, cells do not function properly. If they are too high, cells die. Scientists have long observed that imbalances in calcium may play a role in the development of PD.
Recent research also suggests that modifying the effects of calcium with calcium channel blockers — some of which are already on the market for treating high blood pressure — may potentially slow the progression of PD. Some population studies report that people who take calcium channel-blocking medications have decreased risk of PD. Using a mouse model of PD, researchers at the Northwestern Udall Center have shown that the calcium channel blocker isradipine can protect dopamine neurons from a variety of toxins. A preliminary study of isradipine in people with PD demonstrated relative safety. Researchers hope to confirm results in a larger, ongoing multicenter trial that is currently recruiting early-stage PD patients. Other NINDS-funded researchers continue to screen additional calcium channel blocking agents in order to develop potential neuroprotective treatments for people with PD.
In people with sporadic forms of PD, evidence suggests that parkin, normally neuroprotective, becomes inactive, pointing to a possible link between parkin and sporadic PD. NINDS-funded researchers have discovered ways of modifying the parkin protein to boost its neuroprotective activity. Since PD is caused by the death of dopamine-producing neurons, a trial of embryonic cell replacement was attempted but did not demonstrate benefit. As researchers learn more about induced pluripotent stem cells they may be able to create healthy dopamine cells that can be transplanted into the brain as a form of therapy.
Animal models and clinical studies suggest that the body’s immune system may contribute to the pathology of Parkinson's disease. NINDS-supported researchers are looking at whether a drug called sargramostim, which is a synthetic version of a substance that helps bone marrow manufacture new white blood cells to fight infection, can be used to restore immune system functions.
Gene Therapy
Glial cell derived neurotrophic factor (GDNF) is a protein that may help protect and strengthen brain cells that produce dopamine. Researchers are testing the ability of these cells to deliver GDNF to key areas of the brain with the help of a viral vector known as adeno-associated virus (AAV). Using a brain infusion technique, researchers deliver AAVs that have been programmed to produce GDNF into a person’s brain. The therapeutic approach is being tested among people with advanced PD.
Deep Brain Stimulation
The U.S. Food and Drug Administration first approved deep brain stimulation (DBS) for the treatment of PD-related tremor in 1997. The NINDS supported pioneering research contributing to the development of DBS, which has become widely used and is one of the most effective options for treating PD once levodopa treatment becomes problematic. Much of the research that led to the development of DBS was performed by NINDS-funded scientist Dr. Mahlon DeLong and his colleagues, who have been instrumental in defining the complex circuits in the brain that malfunction in PD. Ongoing NINDS funded research is currently building upon this scientific foundation to understand the therapeutic mechanisms and long-term effects of circuit-based treatment of PD by DBS.
DBS involves the implantation of electrodes into deep parts of the brain, typically the subthalamic nucleus or the globus pallidus. A pulse generator is also implanted under the individual’s collarbone to send finely controlled electrical signals to the DBS electrodes through wires placed under the skin. When turned on externally, the pulse generator and electrodes stimulate the brain to block signals that cause many of the motor symptoms of PD. How DBS helps control the symptoms of PD is not well understood.
In a study conducted by the NINDS and the Department of Veterans Affairs, bilateral DBS was found to more successfully control PD motor function symptoms and improve quality of life than even the most effective medications. DBS provides symptom relief for many people with PD, but it does not work for everyone. PD symptoms persist in some people despite DBS treatment. Researchers continue to look for ways of improving DBS so that it benefits a greater number of people.
For example, NINDS-supported researchers are attempting to deliver a more highly targeted stimulation of specific regions of the brain—the globus pallidus interna (GPi) and the subthalamic nucleus (STN) — to see if it makes a difference in terms of the duration of motor improvements. Other researchers are studying the effects of combining STN DBS with stimulation of the pedunculopontine nucleus (PPN, located in the brain stem) to improve gait control in people who continue to have difficulty walking and talking following STN DBS alone.
NINDS-funded researchers are also investigating different forms of brain stimulation that may be less invasive than DBS. Transcranial direct current stimulation (tDCS) involves attaching electrodes to the skin, or just beneath it, to deliver low doses of electrical current to the brain. Researchers, with support and funding from the NINDS , have also developed ParkinStim, a device that people with PD wear while sleeping. People with PD often feel worst in the morning because the medication they took the night before has worn off. Stimulation during the night may help these individuals wake up feeling better. While tDCS may not replace DBS, it may allow people to delay starting DBS therapy. It may also help individuals with PD decrease the amount or frequency of their medication.
Other NINDS-funded investigators aim to improve DBS success by understanding how DBS works. For example, NINDS-funded researchers developed a device known as WINCS (wireless instantaneous neurotransmitter concentration sensor system) that measures the release of chemicals or neurotransmitters in the brain. The WINCS device is being used in conjunction with functional MRI (fMRI) to look at brain activity and neurotransmitter release during DBS. Such information may be used to design closed-loop controllers capable of monitoring neurochemical activity so that DBS stimulation can be adjusted accordingly.
Taken together, these advances in understanding, tools, and techniques may begin to point to entirely new ways of modulating the brain’s circuits that will benefit people with treatment-resistant PD. For example, researchers at the Udall Center at Emory University are using animal model systems to understand the effects of DBS and other neurosurgical interventions on brain network elements downstream from the basal ganglia, the part of the brain responsible for voluntary motor function. These studies will not only allow researchers to better understand how DBS works but also to improve treatment and care for people with PD.
Drug-Induced, Treatment-resistant, Non-motor symptoms A major objective of PD research is to develop treatments for symptoms that do not respond to currently available medications or DBS. Therapies are still lacking for motor symptoms such as freezing of gait and non-motor symptoms such as cognitive impairment, dementia, sleep disorders, and symptoms involving the autonomic nervous system. The NINDS supports many studies that address these features.
- Levodopa-induced dyskinesias. Early on, Parkinson’s disease can generally be effectively managed for many years with dopaminergic treatments using a drug known as levodopa. However, the majority of people using this drug eventually develop levodopa-induced dyskinesias (e.g., tics, tremors). Based on the results from animal studies, one hypothesis is that levodopa may be associated with neurovascular changes that alter the ability of the drug to pass through the blood-brain barrier.
The Udall Center at the Feinstein Institute for Medical Research is leading investigations into strategies for preventing drug-induced symptoms, which are such an important quality of life issue for many people with PD. Using advanced PET imaging, Feinstein researchers are examining blood flow dynamics among people with and without levodopa-induced dyskinesias. Using an animal model, the researchers hope to determine whether changes in blood flow are associated with structural changes in the tiny blood vessels surrounding the brain or with the permeability of the blood-brain barrier.
- Dementia. NINDS-supported researchers are conducting several clinical trials aimed at gaining a better understanding PD-related dementia, which affects a substantial portion of people with PD and for which there are virtually no treatments. Among the many lines of research addressing PD-related dementia, one longitudinal study is following people with PD and healthy volunteers over time. Participants take thinking and memory tests as researchers measure their brain activity using imaging studies, among other tests. Researchers also analyze participant’s brain tissue after they die. Investigators hope that these studies will provide information on the pathology occurring in regions of the brain that are affected in people who have PD-related dementia.
Several Udall Centers, including the Pacific Northwest Udall Center (PANUC) and the Penn Udall Center also have projects devoted to PD-related dementia and cognitive impairment. In a study of more than 600 people with PD, PANUC researchers found that at baseline nearly 60 percent had mild cognitive impairment and 22 percent had dementia. Men were more likely to have cognitive impairment than women.
- Disruption of sleep . Excessive daytime sleepiness and an inability to sleep throughout the night are some of the most common and most disabling non-motor symptoms of PD. Mechanisms leading to impaired sleep are not well understood and treatment options are limited. NINDS-supported researchers are examining markers of the circadian system — which controls the body's "biological clock" — sleepiness, and sleep quality in people with PD and healthy controls. They are also looking at the effects of bright light exposure to see if it has an effect on circadian rhythms and sleepiness.
- Freezing of gait. This condition is a common and disabling symptom of PD, often leading to significant declines in quality of life. Walking requires shifting from one leg to the other. A person suffering from freezing of gait experiences a sense of falling every time he or she lifts a foot up off the floor. Every step forward resembles a controlled fall. Research has shown that auditory stimuli (sounds of a metronome) or visual cues (a flash of light or lines on the floor indicating stride length) can reduce episodes of freezing, but how these cues work is a mystery. NINDS-supported researchers are trying to determine the best way to treat freezing of gait. For example, researchers at the Udall Center for Excellence at the University of Michigan are using innovative Positron emission tomography (PET) imaging techniques to examine the mechanisms involved with gait, postural control, and attentional function.
- Neurogenic orthostatic hypotension. The autonomic nervous system controls blood pressure. People with diseases that disrupt the autonomic nervous system, such as PD, are therefore at risk of sudden drops in blood pressure that can lead to fainting. Research funded by the NINDS led the FDA to approve the use of Northera capsules (droxidopa) for the treatment of neurogenic orthostatic hypotension in 2014.
Living Well with Parkinson's
While medication and DBS surgery are the most effective treatments for PD, individuals often choose to delay these treatments because of their adverse side effects. Until a therapy is developed that can halt the progression of PD, there is a significant need for strategies that provide symptom relief without causing negative side effects.
Diet, Exercise, and Stress Reduction
Findings from several studies suggest that exercise has the potential to provide relief from certain PD symptoms. Anecdotally, people with Parkinson’s disease who exercise typically do better. However, many questions remain. Among them is whether exercise provides a conditioning effect by strengthening muscles and improving flexibility or whether it has a direct effect on the brain.
In an NINDS-funded trial comparing the benefits of tai chi, resistance training, and stretching, tai chi was found to reduce balance impairments in people with mild-to-moderate PD. People in the tai chi group also experienced significantly fewer falls and greater improvements in their functional capacity.
The NINDS funds many studies aimed at determining how exercise benefits PD and identifying exercise regimens that improve PD symptoms. An important question is whether exercise provides people with newly-diagnosed PD a means for delaying treatment with drug therapy or DBS. NINDS-supported researchers are comparing the effects of moderate and vigorous exercise regimens with no exercise (control group) in a clinical trial to see if it can help slow the progression of symptoms.
Another study is using neuroimaging techniques to compare the neurophysiologic effects of tango dancing, treadmill training, and stretching (control group) on brain function and connectivity. The results may help explain how exercise influences function in PD and help identify which brain regions are involved. The hope is that these findings will lead to better treatments for gait difficulties by identifying specific exercise interventions and targets for DBS.
Technologies that Improve Quality of Life
New technologies may provide measurable quality of life improvements among people with PD. For example, wearable “smart home” devices may present a far more accurate and nuanced picture of an individual’s symptom status compared to a typical physical exam performed in a physician’s office. NINDS has funded a technology laboratory at the University of Rochester to develop and test technologies for PD research and the care of patients. Scientists there have worked with Apple to develop smartphone apps to assess PD symptoms. NINDS researchers are testing the feasibility of using a portable computer module, called a quantitative motor assessment tool (QMAT), to collect information about a person’s disease impairment — all without requiring a trip to a medical center.
The NINDS also supports the development of adaptive technologies that enable people with neurological disorders to independently perform daily activities. The NINDS funding led to the development of the Liftware spoon, a chargeable electronic spoon that uses a microchip and sensors to detect the direction and force of a tremor before motoring the spoon in the opposite direction to cancel out the movement and make it easier to eat. Studies show that the spoon reduces the disruption of tremor by 70 percent.
Research using brain tissue, donated after death, is critical to advancing the understanding of Parkinson’s disease and other neurodegenerative diseases. However, this precious resource is in short supply. New approaches to brain banking are necessary and better communication is needed with all stakeholders, including people with neurodegenerative diseases and their families. The NINDS supports several projects aimed securing resources for research.
- The NIH NeuroBioBank ( https://neurobiobank.nih.gov ) is a network of brain and tissue repositories throughout the United States that coordinates the collection, evaluation, processing, storage, and distribution of nervous system tissue and associated clinical data. The project, funded by the NINDS, the National Institute of Mental Health, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development brings together researchers, NIH program staff, information technology experts, disease advocacy groups, and individuals seeking information about opportunities to donate. Repositories in the network are dedicated to collecting specimens in a standardized and transparent way so they can be made available for use by the broader research community. The repositories are linked through a common informatics platform, providing researchers with easy access to a centralized resource housing thousands of biospecimens from donors with a variety of diseases of the nervous system.
- The National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders at the Banner Sun Health Research Institute in Sun City, Arizona, conducts ongoing clinical assessments of healthy elderly individuals and people with PD and related disorders who are willing to donate their brain and other biospecimens for research purposes. Participants are autopsied when they die and biospecimens are stored and available to the broader research community.
- The NINDS Human Genetics DNA and Cell Line Repository at the Coriell Institute ( https://catalog.coriell.org/1/NINDS ) provides researchers with resources for studying genetic causes of nervous system disorders. The bank includes a variety of samples including iPSCs from participants with Parkinson’s disease as well as other forms of parkinsonism. Also included in the collection are samples from participant’s family members and normal healthy controls.
PD research has progressed enormously in recent years. Scientists are rapidly working to unlock the mysteries of Parkinson’s, and treatments that restore lost function, halt disease progression, and prevent the condition are now realistic goals. Many of these advances are the result of discoveries from NINDS-funded basic, translational, and clinical investigators across the United States as well as NINDS-supported research at the Udall Parkinson’s Disease Research Centers of Excellence. Studies funded by the NIH have identified several genetic mutations that make individuals susceptible to Parkinson’s disease and breakthroughs in genetic research make finding new genetic factors easier and more efficient. A number of promising new therapies have been developed and are currently being tested in animals as well as people. As scientists work to learn more about the underlying biology of the disease and the complex interplay between genetic and environmental influences, new biomarkers will be discovered, therapies for relieving PD symptoms will continue to improve, and ultimately the disease may be halted, reversed, or even prevented from occurring at all.
Sidebar: Morris K. Udall Centers of Excellence for Parkinson's Disease Research
The Morris K. Udall Parkinson’s Disease Research Act of 1997 authorized the NIH to greatly accelerate and expand PD research efforts by launching the NINDS Udall Centers of Excellence, a network of research centers that provide a collaborative, interdisciplinary framework for PD research. Udall Center investigators, along with many other researchers funded by the NIH, have made substantial progress in understanding PD, including identifying disease-associated genes; investigating the neurobiological mechanisms that contribute to PD, developing and improving PD research models, and discovering and testing potential therapeutic targets for developing novel treatment strategies.
The Udall Centers continue to conduct critical basic, translational, and clinical research on PD including: 1) identifying and characterizing candidate and disease-associated genes, 2) examining neurobiological mechanisms underlying the disease, and 3) developing and testing potential therapies. As part of the program, Udall Center investigators work with local communities of patients and caregivers to identify the challenges of living with PD and to translate scientific discoveries into patient care. The Centers also train the next generation of physicians and scientists who will advance our knowledge of and treatments for PD. See the full list of Udall Centers .
Sidebar: NINDS Steps Up Pursuit of PD Biomarkers
In 2012, the NINDS dramatically accelerated efforts to identify biomarkers by establishing the Parkinson’s Disease Biomarkers Program (PDBP). This unprecedented program unites a range of stakeholders from basic and clinical researchers to healthcare professionals, the NINDS staff, information technology experts, and people living with PD and their families.
PDBP supports research and builds resources aimed at accelerating the discovery of biomarkers to ultimately slow the progression of PD. For example, the program has established a repository of biological specimens and a Data Management Resource (DMR) system maintained by the NIH Center for Information Technology. The DMR allows researchers to access clinical, imaging, genetic, and biologic data, while a complementary PDBP-supported project develops statistical tools to analyze vast quantities of data so that patterns can be identified across these diverse sources of information.
PDBP supports several new and existing clinical studies that collect and analyze biospecimens such as blood, urine, and cerebrospinal fluid from people with all stages of PD as well as those without the disease. Several lines of research are looking at various proteins in these biospecimens to explore their value as markers of PD and its progression. Biospecimens are analyzed along with detailed clinical information on signs and symptoms such as gait, balance, sleep problems, memory deficits, and hyposmia. Imaging techniques are used at different stages of disease to analyze brain function in areas associated with movement and cognition.
Once a potential biomarker is identified, the next step is to validate it to make sure that it consistently and reliably provides meaningful information about PD. The PDBP studies complement work being done through the Michael J. Fox Foundation’s biomarker project and the Parkinson’s Progression Markers Initiative (PPMI), which seeks to validate biomarkers. The NINDS also works with the Michael J. Fox Foundation on BioFIND, a two-year observational clinical study in which investigators collect blood and cerebrospinal fluid from people with and without PD. The samples can be used in multiple research projects designed to discover and verify biomarkers of PD.
Sidebar: Advances in Circuitry Research
The brain contains numerous connections among neurons known as neural circuits.
Research on such connections and networks within the brain have advanced rapidly in the past few years. A wide spectrum of tools and techniques can now map connections between neural circuits. Using animal models, scientists have shown how circuits in the brain can be turned on and off. For example, researchers can see correlations between the firing patterns of neurons in a zebrafish’s brain and precise behavioral responses such as seeking and capturing food.
Potential opportunities to influence the brain’s circuitry are starting to emerge. Optogenetics is an experimental technique that involves the delivery of light-sensitive proteins to specific populations of brain cells. Once in place, these light-sensitive proteins can be inhibited or stimulated by exposure to light delivered via fiber optics. Optogenetics has never been used in people, however the success of the approach in animal models demonstrates a proof of principal: A neural network can be precisely targeted.
Thanks in part to the BRAIN Initiative, research on neural circuitry is gaining momentum. The “Brain Research through Advancing Innovative Neurotechnologies” Initiative is accelerating the development and application of new technologies that enable researchers to produce dynamic pictures of the brain that show how individual brain cells and complex neural circuits interact at the speed of thought.
BRAIN is expected to yield tools and technologies that will deepen our understanding of how the nervous system functions in health and disease. These advances are likely to shed light on many neurological diseases, including PD.
"Parkinson's Disease: Challenges, Progress, and Promise", NINDS . September 30, 2015.
NIH Publication No. 15-5595
Prepared by: Office of Communications and Public Liaison National Institute of Neurological Disorders and Stroke National Institutes of Health Bethesda, MD 20892
NINDS health-related material is provided for information purposes only and does not necessarily represent endorsement by or an official position of the National Institute of Neurological Disorders and Stroke or any other Federal agency. Advice on the treatment or care of an individual patient should be obtained through consultation with a physician who has examined that patient or is familiar with that patient's medical history.
All NINDS-prepared information is in the public domain and may be freely copied. Credit to the NINDS or the NIH is appreciated.
- Corpus ID: 264553687
Case Study Case study (A and B): a patient with Parkinson’s disease
- M. Z. Hasan , M. Z. Hussain , +1 author Arha Anwar
3 References
National institute of neurological disorders and stroke, [parkinson's disease]., related papers.
Showing 1 through 3 of 0 Related Papers
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Chief Complaint:
“My husband is slowing down and his hands won’t stop shaking. “
History of Present Illness:
Mr. R.D. is a 73 year old white male who was referred to the Neurology clinic by his PCP. Prior to retiring in 2009, Mr. R.D. was a math professor. In his free time, he enjoyed playing the piano and cooking. After retirement, he and his wife were active in senior groups and also volunteered at the local high school tutoring students in math. Over the past 9-12 months, his wife noticed that he was less interested in attending senior functions and was less active in general. She thought he was just tired and encouraged him to take naps, which initially helped. Over the last 6 months, he continued to be fatigued (with little activity) and had stopped cooking. Mr. R.D. has also stopped enjoying his food. Recently, he has begun complaining that food does not taste as good as in the past. His wife commented that she thought they were just “getting older”, however when she realized it was taking much longer to solve math problems—something he previously completed very quickly—she “knew something was wrong”. Upon questioning, Mrs. R.D. indicated that he had occasional urinary hesitancy and constipation. She also stated that his hands are “shaking constantly”.
Past Medical History:
- Tonsillectomy/adenoidectomy at age 5
- R ulnar fx. And R femur fx. From MVA at age 18
- Hyperlipidemia
- Hypertension
- Diabetes Type II (controlled with diet and exercise)
Pertinent Family history:
- Mother passed away from MI
- Father was killed in an MVA
- Both parents had hypertension
Pertinent Social History:
- Math professor for 30 years
- Avid reader
- Piano player
- Enjoyed cooking
- Previously very active in his senior group
- Enjoyed an active lifestyle of walking, bike riding, and swimming
- No known drug allergies
Medications:
- Lipitor 10mg daily
- Lisinopril 20 mg daily
- Atenolol 50mg daily
Focused physical exam:
- A&Ox4, cooperative
- Flat affect, minimal facial expressions
- Hands resting on thighs, tremors noted bilaterally
- Reflexes delayed when checked in knees
- When standing, patient noted to be bent forward, arms stiffly at sides, jerking movements
- When asked to walk to the door, noted to take short, shuffling steps, leading with his head
- His wife stood at the door and stopped him
- Neurodegeneration
- Neurodegenerative Diseases
- Biological Science
- Neuroscience
- Parkinson's Disease
Case Study on Patient with Parkinson's Disease

- central council for research in yoga and naturopathy, New Delhi

- CENTRAL COUNCIL FOR RESEARCH IN YOGA AND NATUROPATHY
- This person is not on ResearchGate, or hasn't claimed this research yet.
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Balaram Pradhan

- OXID MED CELL LONGEV

- Rajagopal Selladurai Abiramasundari

- J. Jankovic

- VSM Gudlavalleti

- PARKINSONISM RELAT D

- Per Svenningsson

- Joelle M. Abi-Rached
- W.R.G. Gibb
- ARCH NEUROL-CHICAGO

- Ming X Tang

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Treatments for progressing Parkinson's disease: a clinical case scenario study
Affiliation.
- 1 Hôpital de la Citadelle, Liège, Belgium. [email protected]
- PMID: 19902812
Objective: A 'case scenario' study on clinical decisions in progressing Parkinson's disease (PD) was developed to complement scientific evidence with the collective judgment of a panel of experts.
Methods: The opinions of 9 experts in movement disorders on the appropriateness of 9 common pharmacological treatments for 33 hypothetical patient profiles were compared to those of 14 general neurologists. Before rating the case scenarios, all participants received a document integrating European and US guidelines for the treatment of patients with advanced PD. Case scenarios showing disagreement or with inconsistencies in appropriateness ratings were discussed at a feedback meeting. A tool for interactive discussion on the clinical case scenarios included was developed based on the outcome of the study.
Results: Current guidelines are often insufficient to adequately guide the management of patients with progressing PD. The case scenario study did not reveal major differences in opinions between experts in movement disorders and general neurologists about the appropriateness of certain drug choices for specific case scenarios. However in about 1 out of 5 treatment decisions where experts stated appropriateness or inappropriateness, the general neurologists panel had no or dispersed opinions.
Conclusions: This study reveals more uncertainty about treatment of advanced PD in general neurologists compared with experts in movement disorders and underlines the need for additional support for guiding treatment decisions in clinical practice.
PubMed Disclaimer
Similar articles
- [Neuroprotection in Parkinson's disease: analysis though group of experts' methodology]. Linazasoro G, Sesar A, Valldeoriola F, Compta Y, Herrero MT, Martínez Castrillo JC, López Lozano JJ, Bergaretxe A, Vela L, Fernández JM, Castro A, Kulisevski J, Lezcano E, Vaamonde J, López Del Val J, Chacón J, Vivancos F, Luquin R, Aguilar M, Burguera JA, Salvador C, Menéndez Guisasola L, Catalán MJ, Mir P, Campos V, Grandas F, Mínguez A, Balaguer E, Yáñez R, Leiva C, García Ruiz P, Cubo E. Linazasoro G, et al. Neurologia. 2009 Mar;24(2):113-24. Neurologia. 2009. PMID: 19322690 Spanish.
- Physical therapy in Parkinson's disease: evolution and future challenges. Keus SH, Munneke M, Nijkrake MJ, Kwakkel G, Bloem BR. Keus SH, et al. Mov Disord. 2009 Jan 15;24(1):1-14. doi: 10.1002/mds.22141. Mov Disord. 2009. PMID: 18946880 Review.
- Nonphysicians' and physicians' knowledge and care preferences for Parkinson's disease. Swarztrauber K, Graf E. Swarztrauber K, et al. Mov Disord. 2007 Apr 15;22(5):704-7. doi: 10.1002/mds.20945. Mov Disord. 2007. PMID: 17377921
- [Comment on guidelines for treatment of Parkinson's disease]. Kondo T. Kondo T. Rinsho Shinkeigaku. 2004 Nov;44(11):830-2. Rinsho Shinkeigaku. 2004. PMID: 15651305 Review. Japanese.
- The consistency of panelists' appropriateness ratings: do experts produce clinically logical scores for rectal cancer treatment? Hodgson DC, Brierley JD, Cernat G, Bondy S, Slaughter PM, Pinfold SP, Paszat LF. Hodgson DC, et al. Health Policy. 2005 Jan;71(1):57-65. doi: 10.1016/j.healthpol.2004.05.004. Health Policy. 2005. PMID: 15563993
Publication types
- Search in MeSH
LinkOut - more resources
- Genetic Alliance
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Open access
- Published: 18 June 2024
Exploring the lived experiences of individuals with Parkinson’s disease and their relatives: insights into care provision experiences, disease management support, self-management strategies, and future needs in Germany (qualitative study)
- Theresia Krieger 1 ,
- Leonie Jozwiak 1 ,
- Georg Ebersbach 2 ,
- Thorsten Suess 2 ,
- Björn Falkenburger 3 ,
- Tim Feige 3 ,
- Carsten Eggers 4 ,
- Tobias Warnecke 5 ,
- Winfried Scholl 6 ,
- Christian Schmidt-Heisch 7 ,
- Ann-Kristin Folkerts 1 ,
- Elke Kalbe 1 &
- Ümran Sema Seven 1
BMC Neurology volume 24 , Article number: 208 ( 2024 ) Cite this article
19 Accesses
Metrics details
Parkinson’s disease (PD) significantly impacts the health-related quality of life of affected individuals and their relatives. In order to support the affected individuals and their families in coping with PD, it is essential to offer comprehensive information about their experiences. A comprehensive understanding of their lived experiences with the disease, the healthcare system, applied self-management strategies and their needs is considered crucial for developing a PD support program. Therefore, we aimed to explore the lived experiences and support needs of individuals with PD and their relatives in Germany.
This non-interventional, qualitative study conducted an explorative status quo and needs assessment. It generated knowledge through semi-structured focus groups and interviews with individuals with PD at various disease stages and their relatives. The interviews were digitally recorded, transcribed verbatim, and analysed using content analysis.
Fifty-two individuals with PD and 29 relatives participated in eight focus groups and 13 paired and 13 individual interviews. Four themes with corresponding subthemes emerged: (1) experiences, revealing individuals’ experiences around their diagnosis and with disease-specific care provision; (2) management support offers, clarifying who provides support and the type of support offered; (3) self-management, including comprehensibility, meaningfulness and manageability; and (4) future needs, differentiating between deficits and needs. Most participants expressed a sense of abandonment when obtaining self-management strategies and mastering their lives with PD, often referred to as ‘life 2.0’. They identified the lack of structured and adequate provision of information, system orientation and social awareness.
Conclusions
In Germany, there is an urgent need for a comprehensive PD care program that addresses the needs of individuals with PD and their relatives from the start of their care trajectory. It could assist individuals in gaining a comprehensive understanding of the disease, obtaining self-management strategies, building a support network, and becoming experts in self-managing their disease. Moreover, it may positively influence their care trajectory and reduce burdens, such as overburdening, fear of progression, and health anxiety.
Trial Registration
German Clinical Studies Register ( https://www.drks.de/DRKS00030090 , No. DRKS00030090, Date of registration: 15.12.2022).
Peer Review reports
Parkinson’s disease and its disease-related burden on affected individuals and their relatives
Parkinson’s disease (PD) is a progressive neurodegenerative disorder with a rapidly increasing prevalence that affects the lives of those affected at various levels [ 1 ]. In Germany, around 400,000 individuals are affected by PD, and the impact of PD will increase further due to the ageing population in Western societies [ 2 ].
PD’s complex nature is associated with considerable limitations in the health-related quality of life of people with PD (PwPD) and their relatives [ 3 ]. The diversity of motor symptoms (e.g. bradykinesia, rigidity, tremor and postural instability) and non-motor symptoms (e.g. cognitive dysfunction and depression) constitutes a severe burden not only for PwPD but also for their relatives, typically the spouse or a child of the person with PD [ 4 , 5 ]. Clinicians have tended to frequently focus on the key motor symptoms and neglect other symptoms (e.g. depression) or needs (e.g. psychosocial issues) [ 6 , 7 ]. However, non-motor symptoms strongly determine the quality of life of PwPD [ 8 ]. Moreover, ‘reducing’ PD to a merely biomedical issue is problematic since it potentially compromises the well-being of both PwPD and their relatives [ 9 ].
PD is also considered to impose a significant burden on the relatives of PwPD, including fear of disease progression [ 10 ]. Spouses or other family members can suddenly find themselves in the position of becoming an ‘informal’ caregiver, generally without financial compensation for their effort [ 11 , 12 ]. Due to insufficient disease-specific social support or inadequate care provision, informal caregivers may experience constraints on their relationship and everyday life, concerns about the future and lack of employment of the PwPD, which may adversely affect their emotional and physical well-being (e.g. anxiety or depression) [ 10 , 11 , 13 ].
Care and support needs
Few qualitative studies have explored the lived experiences and support needs of PwPD and their relative caregivers, especially in early-stage PD [ 14 ]. Furthermore, there is a paucity of research examining the potential for implementing an advanced care approach for this group [ 15 ]. PD requires the care of a multidisciplinary team of healthcare professionals and individualised medication [ 16 ]. Intensive interdisciplinary cooperation between healthcare professionals and a single point of contact or a helpline providing individual support is considered highly beneficial for PwPD and their families [ 17 ]. The strong involvement of PwPD in therapy decisions correlates significantly with their satisfaction regarding the consultation and distress relief [ 18 ]. Above all, a self-management approach plays a significant role in empowering PwPD and their caregivers to overcome problems, make decisions, activate resources, build physician-patient relationships and implement measures [ 19 ].
Care and support approaches in Germany
Germany currently lacks a patient-centred, nationally coordinated and well-established holistic approach to care that focuses on both supporting PwPD and their families [ 20 ]. A lack of effective communication between healthcare professionals and people with Parkinson’s disease (PwPD) and their relatives is a significant issue. This communication gap can result in unintended changes to treatment, which may lead to suboptimal therapeutic outcomes and hospital admissions [ 21 ].
In certain German regions PD networks exist. Their objective is to enhance the quality of care for PwPD, reduce the incidence of unnecessary hospital admissions and associated costs, and facilitate the sharing of data and resources among different healthcare providers operating across various sectors [ 22 ]. However, most PD networks rely heavily on local initiatives, where consultation is primarily provided by resident neurologists and, in many cases, in specialized PD clinics [ 20 ]. Moreover, recently integrated concepts have been introduced, which have been demonstrated to enhance the quality of life and satisfaction of PwPD with regard to healthcare services [ 20 , 21 ]. Meanwhile, a status quo analysis of current care practice was conducted that explored extensive routine data for Saxony in Germany, indicating a need for innovative care concepts [ 23 ].
In order to fill the gap in holistic care, a nationwide systematic PD support programme should be implemented by providing needs-based training with a focus on self-management. It should be integrated into a ‘Parkinson’s school’ for PwPD and their families [ 24 ]. To establish this school at a the federal level and ensure coverage by statutory health insurance, a number of mandatory steps must be undertaken, including development, practical adaptation, and evaluation [ 25 ]. Given the dearth of studies investigating the lived experiences of German PwPD and their relatives with the current support system and their desires regarding a disease-related school, the initial step was to conduct a systematic status quo and needs assessment within the ‘WissensPARK’ (‘KnowledgePARK’) project. This step was funded by the German Parkinson Association (Deutsche Parkinson Vereinigung; 08/2022–08/2023). WissensPARK´s aim was to explore the lived experiences of PwPD and their relatives with the diagnosis and subsequent consultation and care, as well as their ‘preferences and needs’ regarding the content and didactics of a potential self-management support program.
This article specifically reports on the first part of the larger WissensPARK study, which comprehensively assesses the current status quo and identifies the support needs of the target population. The findings regarding the desired content and didactics for the support program (school) will be published elsewhere.
We aimed to explore the lived experiences of German PwPD and their relatives with PD, PD-related care provision, perceived disease management support, and self-management strategies, as well as their future support needs.
WissensPARK was a non-interventional, explorative, mixed-method qualitative study [ 26 ]. Qualitative research tends to be small in order to support the depth of case-oriented analysis and to find information-rich material [ 26 ]. This approach provides a multifaceted and comprehensive understanding of the research topic [ 26 , 27 ]. New knowledge was generated through semi-structured individual (II), paired (PI), and focus group (FGI) interviews. A flexible data collection approach was necessary to permit all potentially interested individuals to participate by acknowledging their PD severities and availabilities in a given time slot.
Setting and sampling
This study used purposeful sampling [ 28 ]. Its data were collected nationwide, and recruitment efforts were facilitated through collaborations with clinics, institutes and stakeholders from the German Parkinson Association and affiliated self-help groups, who assisted in identifying and reaching out to suitable candidates (see the Acknowledgements).
In qualitative research, the concept of sample adequacy concerns the suitability of the composition and size of the sample [ 29 ]. In selecting participants, a number of parameters were taken into account, including the scope of the study, the nature of the topic (in terms of complexity and accessibility), and the quality of the data [ 26 ]. We did not aim for saturation, but our data collection strategy was focused on gaining comprehensive insights into the diverse needs of PwPD at different stages of PD, characterised by the Hoehn and Yahr stage classification [ 30 ]. In order to understand the specific needs of newly diagnosed PwPD and their relatives, we defined one special group (‘de novo’), which included PwPD who had received their diagnosis within the last two years. Our recruitment strategy was flexible to the circumstances at the respective locations and cooperation partners (e.g. consent or availability of PwPD and their relatives). Further collaboration partners, such as experts with their own experiences group ‘Parkinson Paten’ (PwPD that act as PD mentors), were also included in this study.
Participants
The study participants were PwPD and their relatives; however, they were not required to participate in pairs. The inclusion criteria were PwPD at all Hoehn and Yahr stages, including those with new diagnoses [ 30 ], of both sexes who were aged ≥ 18 years (no upper age limit) and cognitively able to participate (assessed by the cooperating partners and researchers). We also included relatives of PwPD at all Hoehn and Yahr stages [ 30 ], regardless of whether or not they were informal caregivers. All participants had to be native German speakers or have an excellent command of it and unrestricted or sufficiently corrected vision and hearing abilities. The PwPD’s Hoehn and Yahr stage [ 30 ] was assessed by the cooperating partners or within the medical documentation of the treating neurologist before data collection.
Data collection
The participants were asked to complete a sociodemographic information form that asked for data such as education, employment status, and living situation.
It was initially planned to collect qualitative data face-to-face in various healthcare settings specialising in PD, such as university hospitals or rehabilitation clinics. However, due to geographic distance and the PD-associated burden, three FGIs had to be conducted online via the ‘Zoom’ online meeting platform. Some participants interviewed face-to-face were inpatients at cooperating clinics, while others travelled to the respective data collection locations. Moreover, participation in an FGI was not appropriate for all participants due to their physical circumstances or availability, so they were offered a PI or II.
Between January and April 2023, data were collected by three researchers with backgrounds in psychology, nursing and public health (ÜSS, LJ and TK). Data collection was guided by the same semi-structured interview guidelines. Our instrument was based on a previously co-developed and piloted guideline used by one of the cooperating partners [ 31 ]. Our research team adapted it to our specific research needs and piloted it before data collection (Annex 1).
Interviews with PwPD and relatives were conducted separately. Whenever possible, both groups were subdivided into four groups based on the Hoehn and Yahr stage (de novo, H&Y 1, H&Y 2–3 and H&Y 4–5) [ 30 ], ensuring that PwPD were grouped with those at the same stage during data collection, and relatives were grouped with those whose affected relatives were currently at the same stage.
Two team members conducted the FGIs, while a single team member conducted the PIs or IIs. All interviews were audio recorded, and notes were taken. During face-to-face FGIs, important issues were visualised on flipcharts parallel to the data collection and used for clarification or periodisation. The photo-documented flipcharts assisted in the analysis process.
Data analysis
This study followed the Consolidated Criteria for Reporting on Qualitative Studies (COREQ) guidelines [ 32 ]. The qualitative content analysis was conducted between April and July 2023 [ 33 ]. First, all audio files were transcribed verbatim by an external transcription bureau, considering the standards of social research [ 34 ]. Anonymisation was achieved by assigning each participant a pseudonym in the form of an ID number, which provided information only about the study site and the Hoehn and Yahr stage [ 30 ] of the PwPD or, respectively, the participating relative. Second, transcripts were analysed using MAXQDA (version 22), with three researchers (TK, LJ and ÜSS) participating in the coding process. Two researchers independently coded each transcript using a deductive-inductive content analysis [ 33 ]. The initial coding tree was created deductively in alignment with the interview guidelines. Third, based on emerging themes in the interview material, additional themes and subthemes were incorporated inductively, grouped and condensed. Illustrative quotes were identified and highlighted. In order to gain a deeper understanding of the meaning, perceived differences among the coders were discussed. The discussions between the coders and the entire research team continued until a consensus was achieved and a final coding tree was agreed upon. The quotes integrated into this article were translated from German to English by a fluent English-speaking research team member (TK).
Sociodemographic description
Eighty-one individuals participated in WissensPARK (Table 1 ). The participants included inpatients at a PD clinic, PwPD living at home who travelled to the study sites to participate, members of a Young Parkinson’s group (‘JuPa-Group’), and a group of experts with their own lived experiences (‘Parkinson Paten’ [Parkinson mentors]) and their relatives. There were no dropouts; all participants completed the interviews.
Interview data
New knowledge was generated from 34 qualitative data collection sessions: 8 FGIs, 13 PIs and 13 IIs. Of these, 31 were conducted face-to-face in collaboration with cooperating clinics at various locations across Germany (see the Acknowledgements); three FGIs were conducted online with participants distributed throughout Germany.
Altogether, 2836 min of audio material were recorded, with interview durations ranging from 32 to 119 min (mean = 83 min). The findings presented in this article are based on the first part of the interview guideline (the status-quo assessment, Annex 1), which constitutes approximately one-third of the audio material; the other parts focus on the support needs, emphasising information transmission (e.g. content or conditions), and will be published elsewhere.
Four main themes emerged from the interviews with the PwPD and their relatives: (1) experiences, (2) PD-management support offers, (3) self-management strategies and (4) future necessities. The outcomes will be discussed sequentially, first for the PwPDs and then for the relatives.
Table 2 summarises the four main themes and their corresponding subthemes from the perspectives of the PwPD and their relatives. The quotes presented below belong to two groups: PwPD or the relatives of PwPD (labelled as ‘R’).
Theme 1 - experiences
The lived experiences of pwpd around their diagnosis.
The lived experiences of PwPD when being informed of their diagnosis ranged from ‘very good’ to ‘very unsatisfactory’. Among those who were unsatisfied, a clear pattern emerged of disappointment with how their physician delivered their diagnosis. The PwPD complained about receiving only a very brief explanation from their physician. One participant reported receiving only a phone call with no additional information other than the diagnosis, while another reported that they were first informed of their diagnosis through a casual remark made by a nurse.
For many participants, the process of identifying PD had been lengthy and debilitating, with a long period between the initial perception of symptoms and the final diagnosis. Both PwPD and their relatives reported fatiguing, exhausting feelings of uncertainty.
The described symptoms at the time of diagnosis were very diverse, with their intensity increasing as the disease progressed. They ranged from tremors and micrographia to limb pain, excessive movements, insomnia, disturbing dreams, depressive periods, swallowing difficulties, loss of smell, gait instability, stumbling or freezing of gait.
However, reaching the point of obtaining a diagnosis was perceived as challenging. Many PwPD felt that their initial symptoms (e.g. loss of smell or taste and feelings of numbness in the arms or legs) were not attributed to PD. In the search for a cause, they had to undergo a strenuous journey involving several screening procedures with medical professionals. The health of a few PwPD was even damaged due to incorrect diagnosis and, consequently, incorrect medications. For PwPD whose diagnosis was reached more quickly, the process was often driven by their intuition or premonition or a premonition from family members or friends.
Regarding their initial emotional reactions, the answers of PwPD were very heterogeneous, ranging from shock to sadness, anger, or fear. Some were overwhelmed with the information, while others immediately took the initiative. Some denied the diagnosis at first, while others felt a certain vindication about finally having a diagnosis that explained their symptoms. Some PwPD withdrew from social life. Initially, the emerging questions mainly focussed on the nature of PD, future prospects and treatment options, and independence and work life, all corresponding to a constant fear of disease progression.
Relatives’ experiences around the diagnosis
The diagnosis was typically delivered by a neurologist. Relatives often described a similarly lengthy and debilitating process that they had to endure between the initial symptoms and the final diagnosis, which was perceived as challenging. More frequently than in the PwPD, relatives stated that they had a clear premonition about the nature of the symptoms observed in their affected relative. Even in cases where participants could not specifically connect the symptoms to PD, they simply ‘noticed that something was wrong ’ (R 2, H&Y 1). This realisation often occurred long before any physicians were involved.
Once a final diagnosis was reached, relatives often noted that they were not adequately informed, and they felt alone in dealing with the shock and fear. Some complained about not being believed about the severity of the changes they had noticed in their affected relative.
When asked about their initial emotional reactions, the relatives’ answers were just as diverse as those of the PwPD. Many described feelings of relief about finally having diagnostic certainty. Other reactions ranged from shock, helplessness, distress and grief to fear or denial.
Questions arising after receiving the diagnosis focused on general information about PD, causes and possible symptoms, treatment options, disease progression, independence, employment, available resources and entitlements. At this time, many thought about how to cope emotionally with the expected changes in their relationship and daily lives.
PwPDs’ experiences with care provision
Disease-specific medical care was primarily provided by neurologists, with general practitioners occasionally involved. Finding a ‘ suitable and competent neurologist ’ was described as challenging (PwPD 41, H&Y stage 2–3). PwPD expressed frustration about feeling lost in the ‘maze’ or ‘jungle’ of different medical professionals (e.g. general physician, neurologist or physiotherapist). In contrast, those who had a stayover in a PD-specific rehabilitation clinic generally felt well-supported. Satisfaction with therapies such as physiotherapy or occupational therapy was generally good, although some PwPD felt the therapeutic effect was limited.
Medication treatment was almost always offered as the first therapeutic step. However, many PwPD expressed that the information about side effects was scarce, and especially those who experienced impulse control disorders due to their medication saw a great need for better clarification from physicians. Since medication is perceived as an ‘ abiding theme ’ (PwPD 38, de novo), a more holistic approach is universally desired by the PwPD. Deep brain stimulation was a particularly sensitive topic. Many participants expressed fear of such an intrusive operation, while others saw it as a kind of ‘last resort’ and refused it because it would mean that all measures had been taken, and there was nothing else they could hope for to ease their symptoms.
Significant differences became evident regarding how well or quickly participants gained orientation with the possible care and therapy options. This process seems to be strongly influenced by the dedication and willingness of the respective neurologist to invest time in informing the PwPD. Since everyone in Germany has health insurance, some PwPD highlighted that they had hoped for more comprehensive support, especially regarding support offers (e.g. system orientation or finding a suitable neurologist).
Relatives’ experiences with care provision
Family members of PwPD reported that despite the early manifestation of symptoms in their affected relatives, the diagnosis was often delayed. Criticism was directed at physicians for sometimes recognising PD belatedly. Typically, the diagnosis was communicated by a neurologist. Family members often perceived insufficient information and found themselves in shock after the diagnosis. Physician-patient conversations were frequently time-constrained, offering little time for individual concerns to be addressed or in-depth explanations by physicians. Neurologists specialising in PD were perceived as particularly supportive, but the search for them posed an initial challenge for those affected.
Relatives found it particularly burdensome that current information about available services and their availability is difficult to access. The information they and their affected family members encountered was perceived as extensive and confusing, contributing to a sense of contradiction. Medication intake was a central concern, yet knowledge gaps exist regarding its mechanisms and side effects. Navigating the healthcare system and understanding support and care structures, including assistive devices, were considered challenging and deficient. A holistic needs assessment for PwPD and their families is lacking, as is professional guidance throughout the disease process. Relatives often felt abandoned, which, together with assuming caregiving responsibilities, experiencing pressure, and the absence of specific, established resources, led to psychological distress. They clearly expressed the need for psychological support.
Theme 2 - PD management support offers
Subtheme 1 and 2, the views of pwpd regarding supporters and the type of support.
PwPD recognised different support groups within the healthcare system: outpatient professionals, especially their general practitioner and neurologist, and specialised clinics. Moreover, individualised support activities or training were occasionally provided by physiotherapists, occupational therapists, psychotherapists, neurologists, speech therapists, nutrition counsellors or spiritual advisors. Moreover, they perceived that the national and regional PD self-help associations offered support (e.g. regional self-help groups). In these cases, the coordinator of the self-help group acted as a contact person for further issues (e.g. complex questions), which was highly appreciated by the PwPD. Unfortunately, these different groups seldom interact with each other.
Most PwPD did not receive any training or utilise any support services besides self-help groups. Around 25% of the PwPD participated in some form of individual therapeutic training (e.g. offered by the physiotherapist or speech therapist) or attended PD information sessions (e.g. offered by PD specialised care centres). Younger PwPD found it especially challenging to find programs, groups, or other support offerings that were suited to their needs and appropriate for those who are younger or at earlier PD stages. Only some PwPD attended topic-specific information sessions (e.g. focusing on new treatments or nutrition) offered by clinics or organised self-help groups.
Relatives’ views regarding supporters and the type of support
Many relatives knew of existing support services, but almost all stated they had not received any training or assistance besides self-help groups. Those whose partners had visited specialised PD clinics described them as very helpful. Neurologists and other physicians only sporadically helped connect their affected relatives to any groups or services.
Some relatives visited informational assemblies or other informative events and considered them very valuable. Moreover, the relatives considered therapies (e.g. physiotherapy, occupational therapy or speech therapy) as fundamental to their management strategies, not only regarding the effects of the specific PD-related symptoms but also from a psychosocial perspective.
Theme 3 - self-management strategies
The views of pwpds on achieving comprehensibility.
When asking for a self-assessment of the comprehensibility of their disease, the level of information provided to PwPD varied widely. The process of obtaining information differed based on the duration and severity of PD and the willingness of PwPD to engage with the available material. PwPD with higher Hoehn and Yahr stages reported less need for information. Those diagnosed relatively recently estimated their knowledge as ‘bad’ to ‘mediocre’ or ‘satisfactory’, although almost all stated that there was room for improvement. Only two participants stated they felt no need for further information. A few mentioned that they intentionally kept their knowledge low due to a fear of becoming overburdened.
When the PwPD were asked about the sources and channels of information they used, their answers were again very diverse. Almost all had regularly used the internet. Other important channels were, along with their physicians, self-help groups and PD associations, family and friends, print media, television and special events hosted by hospitals or PD clinics. Occasionally, nursing services or outpatient physiotherapists provided information.
Many of the PwPD revealed how burdensome the condition was for their relationships. They frequently talked about the sorrow they feel when seeing their family members suffer because of their PD diagnosis. Some mentioned that they were secretly hoping to die before depending too heavily on their partner’s care. The distribution of roles and the dynamics within their relationships were extremely diverse; the PwPD were the dominant force for managing their condition in some cases, while their partner took over this role in others. Some PwPD frequently perceived a lack of understanding from their partner and wished for them to be better informed about the symptoms of PD. For one PwPD, their partner terminated their relationship right after their diagnosis. However, most PwPD expressed gratitude for their relative’s support.
Relatives’ views on achieving comprehensibility
Throughout, all relatives had a general understanding of the basics of PD. However, most stated that significant room for improvement existed regarding their knowledge. Again, those with partners in the later stages expressed less need for information.
The intensity with which the relatives had immersed themselves in the topic differed greatly. While some had been the driving force in understanding all circumstances, others only knew what their affected partner had told them. When asked about topics for which they still felt the need for more information, their answers centred on the same subjects as in the PwPD: general information about PD, including its symptoms, causes and progression; possible treatments, therapies and medication; the future need for caregiving, support options and entitlements for healthcare and financial support.
Channels used for gathering information were primarily the internet: ‘Your only friend is Google’ ( R 22 of PwPD, H&Y stage 2–3), followed by the responsible medical professionals. The relatives further consulted their family and friends, print media, television, and, in some cases, informative events hosted by clinics, private PD support groups or official PD associations. ‘I did most of the research myself. There is not much coming from the physicians’ (R 19 of PwPD, de novo).
The views of PwPD on achieving meaningfulness
After initially strong emotional reactions, most PwPD reported they had found ways to cope relatively well with PD and had accepted it. The PwPD described appreciable individual differences during this process. Many in advanced PD stages explained that working on their mindset and finding a positive attitude towards their PD was necessary.
A few attempted to only concern themselves with their illness as little as necessary and continue their familiar routines, as far as possible in a sense that the ‘PD should not dominate’ (PwPD 52, H&Y stage 1).
Relatives’ view on achieving meaningfulness
In some cases, the relatives accepted the new situation and took a more carefree stance towards the condition. In others, the relatives tended to ignore the disease and symptoms of the PwPD to protect themselves.
Overall, relatives attempted to offer encouragement and support to their affected relatives through active involvement in managing necessary treatments and therapy, a positive mindset, and open communication. Open communication about PD and its symptoms and progression was particularly challenging for many relationships.
The views of PwPD on achieving manageability
Most of the PwPD found ways to actively shape their ‘life 2.0’ (PwPD 17, H&Y stage 3), including new hobbies, new social networks and adjusting daily routines. Some actively disclosed their condition to get along with others.
All PwPD stated that it was helpful for them to ‘not to look too far into the future’ (PwPD 4, H&Y stage 2–3) and only concern themselves with their current or upcoming PD stage at most. When asked how they manage their condition in daily life, the younger PwPD usually responded with information about their work life and children. Family support eases the burden of managing PD. However, those who still work found it challenging to open up about their diagnosis; nonetheless, when they did, it was often perceived as relieving. PwPD actively sought support in their daily lives through medical aids or household assistance.
Relatives’ views on achieving manageability
The relatives described diverse challenges they had faced since their affected relatives had been diagnosed and adjusted to their new lives. Many initially felt overwhelmed by the situation. Witnessing the changes in their affected relatives’ behaviour, character, and mental and cognitive well-being caused them great distress.
Many relatives also struggled with resistance and defensive attitudes toward medical treatment, physical activity or other forms of therapy on behalf of their affected relatives. Time management was a further issue, especially when balancing work and caregiving. Young families with children faced particularly difficult situations in terms of explaining the disease to their children. Most who still worked had adjusted their work conditions to accommodate their partner’s needs. However, many stated that in adjusting to the new situation, they inevitably had to prioritise their partner’s needs over their own and tended to place themselves in the background.
Some relatives talked about their search for support networks (i.e. family caregiver support groups and self-help groups) and had both positive and negative experiences. Those who found suitable groups appreciated the sense of community, the support, and the openness in discussing PD-related topics. However, some were less successful in their search for a suitable support network. Locally available self-help groups were often not perceived as appropriate for their individual needs, and in some cases, being confronted with PwPD at higher Hoehn and Yahr stages caused severe fear of progression for those whose partner had only recently received the diagnosis.
Most relatives considered physical activity and exercise programs crucial and integrated them into their daily lives with PD. The relatives also demonstrated a strong willingness to accompany their affected partners to these activities and, in some cases, participated themselves.
It became apparent that orientation within the healthcare system and support structures outside the healthcare system depended strongly on the self-initiative of the relative or their affected partner, respectively.
Theme 4 - future needs
The perceived needs of pwpd.
Most of the PwPD desired a holistic and comprehensive healthcare concept and early offers of structured capacity-building activities (e.g. patient school). In such training, the PwPD wished to gain a condensed overview of PD as a whole, with a high demand for ‘valid information about nutrition ’ (PwPD 21, H&Y stage 4) and information on medication and possible side effects; the same was true for comorbid conditions, such as depression. They especially desired more orientation about existing support structures.
Many wished to be more connected to other PwPD (e.g. in self-help groups), ideally with those of a similar age and PD stage. Several participants expressed a desire for psychological support. Finally, since family members play an important role, their support is also considered ‘ obligatory ’ (PwPD 52, H&Y 1).
Relatives’ perceived needs
Most importantly, relatives’ desires were consistent with those of the PwPD in requesting a holistic care concept for PD. They also expressed the need for networking: Self-help groups should also focus on the partners of those affected by PD.
The deficits perceived by PwPD
The greatest deficits perceived by PwPD were the availability of structured information, orientation and lack of time on the part of physicians. The PwPD felt challenged by the lack of navigation between different non-collaborating healthcare professionals and institutions. Many felt lost in the maze of possible therapies and interventions, entitled benefits and different medical professionals who only sporadically collaborated. In addition, insufficient information about the side effects of medications was expressed as very problematic. The PwPD reported deficits in the PD-specific capabilities of the medical professionals. Some described harmful experiences while staying in the hospital for something non-PD related and being treated with medication that was not previously aligned with their PD medication or not suitable for PwPD in the first place.
While the internet was an important information channel, most of the PwPD perceived the amount and quality of (sometimes discrepant) information as overwhelming and unsettling. Their information needs depended on how involved or dedicated their neurologist was and ‘what bridges they build or don’t build’ (PwPD 24, H&Y stage 2–3).
Finally, the societal perception of PD was described as ‘very difficult’ (PwPD 29, H&Y stage 2–3). Some PwPD had experienced staring or comments from others in public that were caused by a lack of understanding of PD symptoms. This distorted public image was described as burdensome and placed PwPD at risk of social isolation.

Relatives’ perceived deficits
The relatives perceived substantial deficits in information about available support services and their accessibility. Time constraints on the part of the responsible physicians deprived them of opportunities to ask questions and discuss available treatment options in more depth. They considered treatment management a critical issue, yet knowledge gaps persisted in how medications worked and their potential side effects. They perceived navigating the healthcare system and understanding support and supply structures as significant challenges that could have been simplified.
With their caregiving responsibilities, the relatives felt tremendous pressure, and the absence of concrete, established support offers for family members caused them distress. Societal perceptions and attitudes toward PD were perceived as further complicating caring for their affected partner.
Further illustrative quotes are provided in Table 3 .
Our study aimed to comprehensively explore the lived experiences of German PwPDs and their relatives with PD, PD-related care provision, and how they manage PD and their needs. To our knowledge, this study is the first detailed and multicentre exploration of the lived experiences of PwPD and their relatives, including those with the PD diagnosis process and care provision, and examination of their associated needs.
The experiences of PwPD and their relatives
The diagnosis, universally characterised by PwPD and their relatives as a ‘life-interrupting event’ (PwPD 2, H&Y stage 1), elicited sentiments akin to ‘standing in the rain without an umbrella’ (PwPD 8, H&Y stage 2–3) or being under shock and facing the fear of progression. At this critical moment, the lack of support system guidance, coupled with the deficiency or unstructured provision of valid or comprehensible information (e.g. about the side effects of medical treatment), was experienced as increasing their emotional burden, manifesting as feelings of anxiety, overburdening, repression or helplessness.
Most PwPD experienced medical support from outpatient neurologists, general practitioners or specialised clinics; unfortunately, the existence of regional self-help groups was noted only in some cases. Several PwPD across all disease stages complained that they had not been offered individual training, and only some felt that information sessions (e.g. within clinical settings) could help to ‘quench their thirst for knowledge’ (R 7 of PwPD, H&Y stage 4–5). Especially in the early PD stages, most PwPD felt abandoned and had difficulty mastering their new circumstances. However, over time, they obtained self-management strategies, leading to an enhanced understanding of PD, giving it meaning (e.g. acceptance) and facilitating the navigation of their ‘life 2.0’. However, their learning about the disease and constructing of these strategies were mainly self-directed.
Notably, our study underscored various deficiencies within the existing PD care support system. Pronounced issues included the lack of structured and adequate information provision, challenges in support system orientation and constraints related to the time and capabilities of healthcare professionals. Since many participants experienced discriminating or intimidating situations, the social perception of PD was perceived as ‘immature’ (PwPD 47, H&Y stage 2–3). Consequently, PwPD and their relatives require a holistic and comprehensive PD care concept. A structured training program that targets developing self-management strategies is needed to address these challenges efficiently. Furthermore, investing in network building, peer and relative support, and offering psychological support, especially during the ‘so-called PD honeymoon period’ (PwPD 1, H&Y stage 1), were considered obligatory.
Our findings showed that the participants’ experiences were heterogeneous. Most harmful experiences were associated with how the diagnosis was delivered and the lack of a comprehensive support system for PD care. While various studies have described the general physical and psychological challenges of living with PD [ 5 , 7 , 14 ], more extensive efforts are needed to explore the context-specific challenges and support needs of PwPD and their relatives, aiming to mitigate PD symptoms and address the requirements of PwPD and their relatives. Promoting the future well-being of those affected aligns with previous studies [ 17 , 35 ].
In our study, many of the PwPD experienced the communication about their diagnosis and the conditions under which it was delivered as inappropriate and intimidating. They attributed this to a lack of empathy, insufficient time for questions, limited informational material, and the minimal involvement of relatives. This observation seems similar to a Dutch study, where PwPD and their relatives desired better information and emotional support from healthcare professionals and greater active involvement in clinical decision-making [ 17 ]. In order to ease this situation, we recommend that healthcare professionals invest further in refining their communication skills and developing therapeutic relationships. Following protocols that are abbreviated as SPIKES, BREAKS or ABCDE may help increase communication quality when delivering a diagnosis perceived as ‘bad news’ [ 36 , 37 , 38 ].
PD management support offers
The PwPD and their relatives described the actual PD care support they received as unstructured, with their trajectory being influenced by the physicians’ deliberation and system orientation. While there are promising self-help and care structures in Germany, they are unfortunately locally organised and not universally accessible to all PwPD [ 20 ]. Ethical considerations underscore the need for advocacy and compassion for PwPD and their families [ 11 ]. Regrettably, only some PwPD are immediately connected to support structures through the initiative of their neurologists; some were informed, and others were not.
Accessibility to different types of support systems, comprehensive care across health sectors, and acknowledgement of PwPD and their relatives in managing their health are vital for managing PD [ 39 ]. Many PwPD rely on locally organised self-help groups. However, the existence and operational level of these groups decreased during the COVID-19 pandemic, and some groups no longer exist (e.g. due to the lack of group leaders or meeting rooms). Since these groups have demonstrated the potential to enhance PD adjustment, reduce psychiatric symptomatology, and increase coping skills and life satisfaction [ 40 , 41 ], we recommend considering organised self-help groups as an integral component of the PD support system.
In the German healthcare system, PwPD typically have appointments with their neurologist every three months. However, many participants expressed the need for comprehensive information and system orientation along their trajectory. While a medicolegal barrier exists in Germany, distributing a list of PD-experienced therapists (e.g. speech therapists, physiotherapists or psychologists) could support the orientation needs of PwPD [ 42 ]. Moreover, the joint investment of self-help groups in designing understandable, tailored information material (e.g. by applying quality check support instruments such as the User-friendly Patient Information Material Checklist [UPIM-check]) or applying a patient-centred needs questionnaire might be advantageous for both affected families and health professionals [ 17 , 43 , 44 ]. Engaging with relatives as early as possible is considered helpful, particularly in preparing them for their future roles [ 12 ]. Specifically, regarding the management of multiple PD drugs, physicians could explicitly invite relatives to join their consultations with the PwPD to ensure compliance.
- Self-management
Our findings indicate that PwPD and their relatives differ in how they cope with PD, but they share many of their needs and desires, which is consistent with previous studies [ 35 , 45 ]. Wieringa et al. highlighted that maintaining a coherent sense of self, feeling in control and holding a positive mind set were imperative for managing PD [ 35 ]. While obtaining self-management strategies is considered vital for managing chronic progressive diseases such as PD, our participants expressed regret that no self-management programs were available. We agree with the recent research by Tuijt et al. on PD self-management, which identified medication management, physical exercise, self-monitoring methods, psychological strategies, maintaining independence, encouraging social engagement, and providing knowledge and information to both PwPD and their relatives as crucial [ 45 ].
In Germany, patient-centred care is considered important but has not yet been reached [ 20 ]. Providing appropriate self-management strategies will empower PwPD to share decision-making and lead to a patient-centred approach [ 17 ]. We encourage offering systematic and tailored self-management as soon as possible after diagnosis to help reduce misunderstandings, health anxiety, and fear of progression and possibly empower the entire family system. Moreover, further research is needed to identify the self-management needs of PwPD and their relatives, considering factors such as demographics and the Hoehn and Yahr stages [ 45 ].
Future needs
Our findings underscore the lack of a holistic and comprehensive PD care concept in Germany, particularly one emphasising self-management and care coordination. Similar deficiencies have been observed in other European countries. Navarta-Sanchez et al. identified four unmet needs: (i) personalised care for changing needs, (ii) accessibility of different types of support systems, (iii) comprehensive care across health sectors and (iv) acknowledgement of PwPD and their relatives in managing their health [ 39 ]. Vlaanderen et al. also noted that self-management, interdisciplinary collaboration between healthcare professionals, time to discuss the future and a single point of access to healthcare professionals are insufficiently addressed in the Netherlands [ 46 ]. The authors of both studies argued the need for a holistic, patient-centred, and collaborative care approach [ 39 , 46 ]. The Dutch integrated care model seems promising when aiming to improve the quality of life of PwPD [ 47 ]. Such initiatives to create integrated PD care networks exist in some regions of Germany and have shown significant improvements in the quality of life of PwPD compared to the standard neurological practice [ 48 ].
Our data show that the current quality of care for PwPD predominantly depends on strong self-initiative and, in some cases, on coincidence. Since increased satisfaction with PD care may increase treatment compliance and outcomes, consideration should be given to involving PwPD in their care. Therefore, a holistic, comprehensive and universally accessible PD care concept is urgently needed in Germany, as our findings suggest. While we can learn from such programmes in Sweden and the Netherlands, a contextual adaption will be required [ 20 , 47 , 49 , 50 ]. During the programme´s developing and piloting phase, the action research approach will be applied [ 51 ]. Elements such as structured and adequate information provision (e.g. PD and its treatment), system orientation, network building, peer support and improvements in social perception should be addressed. Furthermore, a central ‘point of contact’ [ 46 ] (e.g. a PD nurse) to support newly diagnosed patients and their relatives would be beneficial [ 52 ]. This service must be available to all and actively highlighted by neurologists or other responsible physicians. Valuable insights can be gained from new integrated care models such as PRIME-Parkinson (Proactive and Integrated Management and Empowerment in Parkinson’s Disease) [ 53 ].
With 81 participants, this is considered a substantial qualitative study [ 26 ]. By generating qualitative mixed methods data through FGIs, PIs and IIs, we gained a comprehensive understanding of the lived experiences and needs of PwPD and their relatives in Germany. Conducting FGIs was perceived as valuable by both the researchers and the participants. The flexible and intensive interaction with other participants during FGIs facilitated the identification of interlinkages (e.g. how missing information leads to underestimation of medication side effects) and a nuanced understanding of specific needs at the different PD stages (e.g. distinguishing the needs of newly diagnosed PwPD and those ay later Hoehn and Yahr stages), age specific needs or geographic differences. Using IIs, PIs, and FGIs allowed us to address participants’ wishes (e.g. including those at later Hoehn and Yahr stages or who did not feel comfortable joining a group discussion), which could be relevant beyond the German context.
The semi-structured interview guidelines and the researchers’ methodological experiences facilitated data collection, providing flexibility in exploring emerging insights. Validity was enhanced by the researchers directly addressing any misunderstandings or uncertainties. While the participant groups were not entirely equivalent among the Hoehn and Yahr stages, we gained a representative qualitative sample. Moreover, the support of the self-help group enabled us to highlight the needs of younger PwPD. We consider our sample size sufficient since saturation was reached.
Limitations
Our data are specific to the German context, so their transferability to other settings and healthcare systems might be limited. However, the fact that data were collected in various regions across Germany, each with its distinct characteristics (e.g. infrastructure, urban/rural profiles and sociocultural influences from East to West), lends a degree of generalisability to our results, potentially making them applicable outside of Germany. In addition, only two study participants had migrant backgrounds, possibly because a good command of German was required. Future research should explore the cultural-specific needs of PwPD.
The dearth of suitable participants in some settings forced us to conduct PIs or IIs, even when applying strategies to reduce recall bias in interview studies [ 54 ]. It became evident that PwPD with cognitive impairments (H&Y 4–5 stages) found it challenging to participate in our study, with less than 10% of the participating PwPD coming from this group. A bias in willingness to participate also became apparent, especially among newly diagnosed PwPD.
We attempted to reduce the limitations related to subjectivity in the data coding and analysis by having two coders independently code all materials and then discuss them until a consensus was reached.
We gained a thorough understanding of the current situation of German PwPD and their relatives (e.g. their diagnosis experiences and management support offers), self-management strategies, and further needs (e.g. comprehensive training). Our findings provide clinicians with unique insights into how PwPD and their families perceive current support, and offer practice-based suggestions for improvement. The study may raise awareness among health professionals that PwPD and their families need comprehensive care that goes beyond medical or pharmaceutical treatment. The results of the study do serve as a foundation for developing and implementing a needs-driven, comprehensive PD care support program in Germany. Such a program should address the needs of PwPD and their relatives from the initial stages of their PD trajectory. Offering such a program should help comprehensively address understanding PD, obtaining self-management strategies, building a support network and becoming an expert on one’s disease. It should address the needs of PwPD and their families from the earliest stages of their PD journey. This approach is of high clinical relevance as it has the potential to improve the quality of care and reduce the burden of illness, health anxiety and fear of progression in PwPD.
Data availability
Due to the sensitive nature of the questions asked in this study, the respondents were assured that the raw data would be kept confidential and not be shared.
Tolosa E, Garrido A, Scholz SW, et al. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021;20(5):385–97. https://www.sciencedirect.com/science/article/pii/S1474442221000302 .
Article CAS PubMed PubMed Central Google Scholar
Heinzel S, Berg D, Binder S, et al. Do we need to rethink the epidemiology and Healthcare utilization of Parkinson’s Disease in Germany? Front Neurol. 2018;9:500. https://doi.org/10.3389/fneur.2018.00500 . [published Online First: 29 June 2018].
Article PubMed PubMed Central Google Scholar
Zhao N, Yang Y, Zhang L, et al. Quality of life in Parkinson’s disease: a systematic review and meta-analysis of comparative studies. CNS Neurosci Ther. 2021;27(3):270–79. https://doi.org/10.1111/cns.13549 . [published Online First: 28 December 2020].
Article PubMed Google Scholar
Rutten S, van den Heuvel OA, de Kruif AJTCM, et al. The subjective experience of living with Parkinson’s Disease: a Meta-ethnography of qualitative literature. J Parkinsons Dis. 2021;11(1):139–51.
Khoo TK, Yarnall AJ, Duncan GW, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology. 2013;80(3):276–81.
Todorova A, Jenner P, Ray Chaudhuri K. Non-motor Parkinson’s: integral to motor Parkinson’s, yet often neglected. Pract Neurol. 2014;14(5):310–22. https://doi.org/10.1136/practneurol-2013-000741 . [published Online First: 3 April 2014].
Nègre-Pagès L, Grandjean H, Lapeyre-Mestre M, et al. Anxious and depressive symptoms in Parkinson’s disease: the French cross-sectionnal DoPaMiP study. Mov Disord. 2010;25(2):157–66.
Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399–406. https://doi.org/10.1002/mds.23462 . [published Online First: 24 January 2011].
Haahr A, Groos H, Sørensen D. Striving for normality’ when coping with Parkinson’s disease in everyday life: a metasynthesis. Int J Nurs Stud. 2021;118:103923. https://doi.org/10.1016/j.ijnurstu.2021.103923 . [published Online First: 4 March 2021].
Geerlings AD, Kapelle WM, Sederel CJ, et al. Caregiver burden in Parkinson’s disease: a mixed-methods study. BMC Med. 2023;21(1):247. https://doi.org/10.1186/s12916-023-02933-4 . [published Online First: 10 July 2023].
Bhimani R. Understanding the Burden on caregivers of people with Parkinson’s: a scoping review of the literature. Rehabil Res Pract. 2014;2014:718527. https://doi.org/10.1155/2014/718527 . [published Online First: 14 September 2014].
Mosley PE, Moodie R, Dissanayaka N. Caregiver Burden in Parkinson Disease: a critical review of recent literature. J Geriatr Psychiatry Neurol. 2017;30(5):235–52. https://doi.org/10.1177/0891988717720302 . [published Online First: 26 July 2017].
Lau K-M, Au A. Correlates of Informal Caregiver Distress in Parkinson’s Disease: a Meta-analysis. Clin Gerontologist. 2011;34(2):117–31.
Article Google Scholar
Morel T, Cleanthous S, Andrejack J, et al. Patient experience in early-stage Parkinson’s Disease: using a mixed methods analysis to identify which concepts are Cardinal for Clinical Trial Outcome Assessment. Neurol Ther. 2022;11(3):1319–40. https://doi.org/10.1007/s40120-022-00375-3 . [published Online First: 1 July 2022].
Churm D, Dickinson C, Robinson L, et al. Understanding how people with Parkinson’s disease and their relatives approach advance care planning. Eur Geriatr Med. 2022;13(1):109–17. https://doi.org/10.1007/s41999-021-00548-7 . [published Online First: 16 August 2021].
Lidstone SC, Bayley M, Lang AE. The evidence for multidisciplinary care in Parkinson’s disease. Expert Rev Neurother. 2020;20(6):539–49. https://doi.org/10.1080/14737175.2020.1771184 . [published Online First: 1 June 2020].
Article CAS PubMed Google Scholar
van der Eijk M, Faber MJ, Al Shamma S, et al. Moving towards patient-centered healthcare for patients with Parkinson’s disease. Parkinsonism Relat Disord. 2011;17(5):360–64. https://doi.org/10.1016/j.parkreldis.2011.02.012 . [published Online First: 10 March 2011].
Grosset KA, Grosset DG. Patient-perceived involvement and satisfaction in Parkinson’s disease: effect on therapy decisions and quality of life. Mov Disord. 2005;20(5):616–19.
Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7.
van Munster M, Tönges L, Loewenbrück KF, et al. Building a Parkinson-Network-experiences from Germany. J Clin Med. 2020;9(9):2743. https://doi.org/10.3390/jcm9092743 . [published Online First: 25 August 2020].
Eggers C, Wellach I, Groppa S, et al. Versorgung Von Parkinson-Patienten in Deutschland: Status quo und Perspektiven Im Spiegel Des Digitalen Wandels: care of patients with Parkinson’s disease in Germany: status quo and perspectives as reflected in the digital transition. Nervenarzt. 2021;92(6):602–10. https://doi.org/10.1007/s00115-020-01027-3 . [published Online First: 16 November 2020].
Eggers C, Wolz M, Warnecke T, et al. Parkinson-Netzwerke in Deutschland: Zukunft Oder Utopie? Parkinson Networks in Germany: future or Utopia? Fortschr Neurol Psychiatr. 2020;88(9):586–89. https://doi.org/10.1055/a-1113-7751 . [published Online First: 6 April 2020].
Timpel P, Tesch F, Müller G, et al. Versorgungssituation Von Parkinson-Patienten in Sachsen Eine sekundärdatenbasierte Analyse Der Inanspruchnahme Im Beobachtungszeitraum 2011 Bis 2019: treatment practice of patients with Parkinson’s disease in Saxony A secondary data-based analysis of utilization in the observation period 2011–2019. Nervenarzt. 2022;93(12):1206–18. https://doi.org/10.1007/s00115-022-01273-7 . [published Online First: 14 March 2022].
Kessler D, Liddy C. Self-management support programs for persons with Parkinson’s disease: an integrative review. Patient Educ Couns. 2017;100(10):1787–95. https://doi.org/10.1016/j.pec.2017.04.011 . [published Online First: 17 April 2017].
Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. https://doi.org/10.1136/bmj.a1655 . [published Online First: 29 September 2008].
Morse JM, Cheek J. Making room for qualitatively-driven mixed-method research. Qual Health Res. 2014;24(1):3–5.
Greene JC. Mixed methods in social inquiry. 1st ed. San Francisco, CA: Jossey-Bass; 2007.
Google Scholar
Robinson OC. Sampling in interview-based qualitative research: a theoretical and practical guide. Qualitative Res Psychol. 2014;11(1):25–41.
Vasileiou K, Barnett J, Thorpe S, et al. Characterising and justifying sample size sufficiency in interview-based studies: systematic analysis of qualitative health research over a 15-year period. BMC Med Res Methodol. 2018;18(1):148. https://doi.org/10.1186/s12874-018-0594-7 . [published Online First: 21 November 2018].
Hoehn MM, Yahr MD, Yahr MD. Neurology , 1967:427–42.
Tennigkeit J, Feige T, Haak M, et al. Structured Care and Self-Management Education for Persons with Parkinson’s Disease: Why the First Does Not Go without the Second-Systematic Review, Experiences and Implementation Concepts from Sweden and Germany. J Clin Med. 2020;9(9). https://doi.org/10.3390/jcm9092787 . [published Online First: 28 August 2020].
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57. https://doi.org/10.1093/intqhc/mzm042 . [published Online First: 14 September 2007].
Kuckartz U, Rädicker S. Qualitative inhaltsanalyse. Methoden, Praxis, Computerunterstützung: Grundlagentexte Methoden: Kapitel 6.1. Qualitative content analysis. Methods, practice, computer support: comment. Wiesbaden: Springer VS.
Dresing TPT. Praxisbuch Interview, Transkription & Analyse: Anleitungen Und Regelsysteme für qualitativ Forschende. Practice Book Interview, transcription & analysis: instructions and Rule systems for qualitative researchers. 8th ed. Eigen; 2018.
Wieringa G, Dale M, Eccles FJR. Adjusting to living with Parkinson’s disease; a meta-ethnography of qualitative research. Disabil Rehabil. 2022;44(23):6949–68. https://doi.org/10.1080/09638288.2021.1981467 . [published Online First: 30 September 2021].
Baile WF, Buckman R, Lenzi R, et al. SPIKES-A six-step protocol for delivering bad news: application to the patient with cancer. Oncologist. 2000;5(4):302–11.
Narayanan V, Bista B, Koshy C. BREAKS’ protocol for breaking Bad News. Indian J Palliat Care. 2010;16(2):61–5.
Rabow MW, McPhee SJ. Beyond breaking bad news: how to help patients who suffer. West J Med. 1999;171(4):260–63.
CAS PubMed PubMed Central Google Scholar
Navarta-Sánchez MV, Palmar-Santos A, Pedraz-Marcos A, et al. Perspectives of people with Parkinson’s disease and family carers about disease management in community settings: a cross-country qualitative study. J Clin Nurs. 2023;32(15–16):5201–18. https://doi.org/10.1111/jocn.16636 . [published Online First: 2 February 2023].
Charlton GS, Barrow CJ. Coping and self-help group membership in Parkinson’s disease: an exploratory qualitative study. Health Soc Care Community. 2002;10(6):472–78.
Markó-Kucsera M, Kullmann L, Paulik E. Measuring quality of life in individuals with Parkinson’s disease attending a self-help club: cross-sectional study in Hungary. Int J Rehabil Res. 2018;41(1):81–3.
Warnecke T, Lummer C, Rey JW, et al. Parkinson-Krankheit. Innere Medizin. 2023;64(2):131–38. https://doi.org/10.1007/s00108-022-01444-3 . [published Online First: 8 December 2022].
Krieger T, Salm S, Mollenhauer J et al. User-friendly Patient Information Material Checklist - UPIM-Check 2020.
van der Eijk M, Faber MJ, Ummels I, et al. Patient-centeredness in PD care: development and validation of a patient experience questionnaire. Parkinsonism Relat Disord. 2012;18(9):1011–16. https://doi.org/10.1016/j.parkreldis.2012.05.017 . [published Online First: 15 June 2012].
Tuijt R, Tan A, Armstrong M, et al. Self-management components as experienced by people with Parkinson’s Disease and their carers: a systematic review and synthesis of the qualitative literature. Parkinsons Dis. 2020;2020:8857385. https://doi.org/10.1155/2020/8857385 . [published Online First: 15 December 2020].
Vlaanderen FP, Rompen L, Munneke M, et al. The Voice of the Parkinson customer. J Parkinsons Dis. 2019;9(1):197–201.
Bloem BR, Henderson EJ, Dorsey ER, et al. Integrated and patient-centred management of Parkinson’s disease: a network model for reshaping chronic neurological care. Lancet Neurol. 2020;19(7):623–34. https://doi.org/10.1016/S1474-4422(20)30064-8 . [published Online First: 25 May 2020].
Eggers C, Dano R, Schill J, et al. Patient-centered integrated healthcare improves quality of life in Parkinson’s disease patients: a randomized controlled trial. J Neurol. 2018;265(4):764–73. https://doi.org/10.1007/s00415-018-8761-7 . [published Online First: 1 February 2018].
Macht M, Gerlich C, Ellgring H, et al. Patient education in Parkinson’s disease: formative evaluation of a standardized programme in seven European countries. Patient Educ Couns. 2007;65(2):245–52. https://doi.org/10.1016/j.pec.2006.08.005 . [published Online First: 11 September 2006].
Lang C, Timpel P, Müller G, et al. Exploration Potenzieller Barrieren für die Akzeptanz eines interdisziplinären sektorenübergreifenden versorgungsnetzwerkes für Patient*innen mit Morbus Parkinson: exploration of potential barriers for the acceptance of an interdisciplinary cross-sectoral care network for patients with Parkinson’s disease. Präv Gesundheitsf. 2023;18(2):253–60.
Harorani M, Jadidi A, Zand S, et al. Spiritual care in hospitalized patients in Iran: an Action Research Study. J Relig Health. 2022;61(5):3822–39. https://doi.org/10.1007/s10943-021-01302-w . [published Online First: 16 June 2021].
Mai T. Stand und Entwicklung Der Rolle als Parkinson Nurse in Deutschland – Eine Online-Befragung: Status and development of the role as Parkinson nurse in Germany – an online survey. Pflege. 2018;31(4):181–89. https://doi.org/10.1024/1012-5302/a000617 . [published Online First: 26 June 2018].
Tenison E, Smink A, Redwood S, et al. Proactive and Integrated Management and Empowerment in Parkinson’s Disease: Designing a New Model of Care. Parkinsons Dis. 2020;2020:8673087. https://doi.org/10.1155/2020/8673087 . [published Online First: 30 March 2020].
Bergelson I, Tracy C, Takacs E. Best practices for reducing Bias in the interview process. Curr Urol Rep. 2022;23(11):319–25. https://doi.org/10.1007/s11934-022-01116-7 . [published Online First: 12 October 2022].
Download references
Acknowledgements
We are grateful to all the individuals with Parkinson’s disease and their relatives for sharing their valuable experiences. We thank the German Parkinson Association (Deutsche Parkinson Vereinigung; No. 4201-9514-0002) for funding this project. We also thank our cooperation partners for supporting the study: the Movement Disorder Clinic, Kliniken Beelitz; the Department of Neurology, University of Technology Dresden; the Department of Neurology, University Hospital Munster; the Department of Neurology, Knappschaftskrankenhaus Bottrop; the Department of Neurology, University Hospital Cologne; the organised self-help groups of the Young Parkinson Association (JuPa) in the federal states Rhineland-Palatinate and Hesse; and the support network Parkinson Pate.
The WissensPARK project was funded by the German Parkinson Association (Deutsche Parkinson Vereinigung [dPV] e.V.; No. 4201-9514-0002). We acknowledge support for the Article Processing Charge from the DFG (German Research Foundation, 491454339).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Medical Psychology | Neuropsychology and Gender Studies, Centre for Neuropsychological Diagnostics and Intervention (CeNDI), Faculty of Medicine, University Hospital Cologne, University of Cologne, Cologne, Germany
Theresia Krieger, Leonie Jozwiak, Ann-Kristin Folkerts, Elke Kalbe & Ümran Sema Seven
Movement Disorder Clinic, Kliniken Beelitz, Beelitz-Heilstätten, Germany
Georg Ebersbach & Thorsten Suess
Department of Neurology, University of Technology Dresden, Dresden, Germany
Björn Falkenburger & Tim Feige
Knappschaftskrankenhaus Bottrop, Department of Neurology, Bottrop, Germany
Carsten Eggers
Department of Neurology, University Hospital Munster, Munster, Germany
Tobias Warnecke
Young Parkinson Association, Schneckenheim, Germany
Winfried Scholl
Parkinson Pate Organization, Hamburg, Germany
Christian Schmidt-Heisch
You can also search for this author in PubMed Google Scholar
Contributions
TK: conceptualisation, investigation, methodology, formal analysis, validation, visualisation, writing—original draft, and project management. LJ: investigation, formal analysis, resources, and writing—original draft and reviewing. EG: project administration, resources, and writing—review and editing. TS: project administration, resources, and writing—review and editing. BF: project administration, resources, and writing—review and editing. TF: project administration, resources, and writing—review and editing. CE: project administration, resources, and writing—review and editing. WT: project administration, resources, and writing—review and editing. WS: project administration, resources, and writing—review and editing.CSH: project administration, resources, and writing—review and editing. AKF: supervision, validation, resources, and writing—reviewing and editing. EK: supervision, validation, resources, and writing—reviewing and editing. ÜSS: conceptualisation, investigation, methodology, formal analysis, validation, visualisation, and writing—reviewing and editing.
Corresponding author
Correspondence to Theresia Krieger .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of the Faculty of Medical at the University of Cologne (No. 22-1346) and adhered to the principles of the Declaration of Helsinki and relevant national and European data protection regulations. Before data collection, the researchers provided written and oral information to each participant, explaining the study’s procedures and objectives. Written informed consent was then obtained from each participant. Each participant was offered €30 compensation to acknowledge their participation.
Consent for publication
Not applicable.
Competing interests
The authors TK, LJ, GE, TS, NF, TF, CE, TW, WS, CSH and ÜSS declare they have no competing interests.EK has received grants from the German Ministry of Education and Research, the German General Joint Committee, the German Parkinson Society, and STADAPHARM GmbH (Berlin, Germany) as well as honoraria from AbbVie GmbH (Ludwigshafen, Germany) and memodio GmbH (Potsdam, Germany) and license fees from Prolog GmbH (Landau, Germany). EK is one of the authors of the cognitive intervention series “NEUROvitalis” but receives no corresponding honoraria.AKF has received grants from the German Parkinson Society, the German Alzheimer’s Society, the German Parkinson Foundation, STADAPHARM GmbH and the German General Joint Committee, as well as honoraria from Springer Medizin Verlag GmbH (Heidelberg, Germany), Springer-Verlag GmbH (Berlin, Germany), ProLog Wissen GmbH (Cologne, Germany), Seminar- und Fortbildungszentrum (Rheine, Germany), LOGOMANIA, Fendt & Sax GbR (Munich, Germany), LOGUAN (Ulm, Germany), dbs e.V. (Moers, Germany), STADAPHARM GmbH (Bad Vilbel, Germany), NEUROPSY (St. Konrad, Austria), Multiple Sclerosis Society Vienna (Vienna, Austria), and Gossweiler Foundation (Bern, Switzerland). AKF is one of the authors of the cognitive intervention series ‘NEUROvitalis’ but receives no corresponding honoraria.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Krieger, T., Jozwiak, L., Ebersbach, G. et al. Exploring the lived experiences of individuals with Parkinson’s disease and their relatives: insights into care provision experiences, disease management support, self-management strategies, and future needs in Germany (qualitative study). BMC Neurol 24 , 208 (2024). https://doi.org/10.1186/s12883-024-03696-y
Download citation
Received : 09 February 2024
Accepted : 29 May 2024
Published : 18 June 2024
DOI : https://doi.org/10.1186/s12883-024-03696-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Parkinson’s disease
- Qualitative research
- Lived experience
- Experiences of illness
- Informational needs
- Management support needs
BMC Neurology
ISSN: 1471-2377
- General enquiries: [email protected]
Young-Onset Parkinson's

Young-onset Parkinson’s disease (YOPD) occurs in people younger than 50 years of age. Most people with idiopathic, or typical, PD develop symptoms at 50 years of age or older.
YOPD affects about four percent of the one million people with PD in the U.S. Symptoms are similar to late onset PD but it is important to understand the challenges YOPD individuals often face at a financial, family and employment level.
In rare instances, Parkinson's-like symptoms can appear in children and teenagers. This form of the disorder is called juvenile Parkinsonism and is often associated with specific, high-PD risk genetic mutations.
Young-onset PD is diagnosed similarly to late-onset PD with symptoms including:
- The tremors in people with YOPD can sometimes appear faster and somewhat different from the classic parkinsonian resting tremor seen in those with late-onset PD.
- Rigidity of the limbs and trunk
- Bradykinesia (slowness of movement)
- Postural instability or impaired balance and coordination
People with YOPD may experience the same non-motor symptoms as others with PD, including:
- Sleep disturbances
- Changes in memory and thinking
- Constipation or urinary problems
How is young-onset PD different?
People diagnosed with YOPD have a more frequent family history of Parkinson’s disease and a longer survival. People living with young-onset PD may experience:
- Slower progression of PD symptoms over time, staying functional and cognitively intact for longer duration
- Less frequent cognitive problems such as dementia
- More side effects from dopaminergic medications, such as more frequent dyskinesias (involuntary body movements)
- Earlier and more frequent dystonias (cramping and abnormal postures) such as arching of the foot
Why is distinguishing young-onset Parkinson's important?
Socially, people who are affected by PD at a younger age experience the disease differently — they may be at a different stage of their career and often have less time to engage in their own care. They may also have children or are planning to have children and have questions regarding passing on PD genes. Medically, doctors tailor treatment when it is a younger person with PD. The younger you are, the more likely the disease is genetic. Your care team may offer genetic testing or counseling. Younger brains also have higher neuroplasticity (the brain’s ability to grow and change response to therapy) potential which allows the brain to handle and respond to disease and therapy differently.
Causes and Theories
For most people with PD, the disease is caused by a combination of genetics and environmental exposures. However, genetics plays a larger role in YOPD. Scientists have discovered genes that can cause or increase the risk of developing Parkinson's at a younger age.
People who have both early-onset PD and a strong family history of the disease are more likely to carry genes linked to PD, such as SNCA, PARK2, PINK1 and LRRK2 . In fact, a recent study found that 65 percent of people with PD onset under 20 years old and 32 percent of people with onset between 20 and 30 had a genetic mutation believed to increase PD risk.
However, some people with these genes may not develop Parkinson’s at all. Genetic tests are not generally available, but the Parkinson's Foundation genetics initiative, PD GENEration: Mapping the Future of Parkinson's Disease , is the first national Parkinson's study to offer free genetic testing plus counseling for Parkinson's-related genes through medical professionals. This flagship study will ultimately provide genetic information that will lead to improving care, expanding research and accelerating enrollment in clinical trials.
Theoretically, genes may play a larger role in young-onset PD, while environmental factors may play more of a role in sporadic PD. But to date researchers have found this hard to prove, as we are still improving our understanding of the biological mechanisms of the disease.
Navigating YOPD After Diagnosis
While a diagnosis can be disruptive and unexpected, it doesn’t have to stop you from reaching your goals. When you are ready, think about your current family and career responsibilities, and set up a new long-term plan. Take one step at a time as you move toward achieving your priorities.
Build a Care Team
Find a movement disorder specialist or a neurologist with expertise in movement disorders. This doctor will help find the medication that is right for you and help you determine which other specialists can help you further your goals. The Parkinson’s Foundation Helpline at 1-800-4PD-INFO (1-800-473-4636) can recommend PD specialists in your area.
Talk to Family
Parkinson’s affects everyone in the family. You may be concerned that telling your children you have Parkinson’s will cause undue worry. Fostering an open, honest and age-appropriate conversation early can help children understand PD-related changes and feel secure.
Tell Your Employer When You are Ready
Many people with YOPD are still working. You decide when, with whom and how to share your diagnosis. Learn more about Managing YOPD in the workplace .
Discover Community
Whether finding a support group, educational program or getting involved with a non-profit, there are many ways to connect with others living with PD. Consider getting involved with the Parkinson’s Foundation.
Genetic Testing
People with YOPD who have children, or who are considering pregnancy, may wonder whether they carry a PD-related gene. The Parkinson’s Foundation PD GENEration national testing initiative offers Parkinson’s-related genetic testing and counseling at no cost to people with Parkinson’s.
Begin Planning
While it may seem challenging, planning for the future can alleviate stress and foster security. Familiarize yourself with your disability and long-term care insurance policies. Take legal, financial and insurance planning into consideration.
Therapy and Treatment
When it comes to medical treatment, people with YOPD have a significantly greater risk of developing the following:
- Dyskinesias (involuntary movements) and dystonias (cramping and abnormal postures), sometimes as a side effect of carbidopa/levodopa (the drug prescribed most often to treat Parkinson’s)
- Motor fluctuations when taking levodopa
Each person’s treatment is unique and can require fine adjustments of multiple medications. Deep brain stimulation remains a surgical option for people with young-onset PD.
Page reviewed by Dr. Jun Yu, Movement Disorders Fellow at the University of Florida, a Parkinson’s Foundation Center of Excellence.
Related Materials
Episode 10: young-onset parkinson’s 101, episode 124: characteristics of young-onset parkinson’s disease, episode 125: young-onset parkinson’s disease: lifestyle, family, and counseling, related blog posts.

10 Helpful Young-Onset Parkinson's Resources

Managing Young Onset Parkinson’s Disease in the Workplace

Navigating the Challenges of Young-Onset Parkinson's
Parkinson's connection, personal information.
Factors associated with self-rated health in people with late-stage parkinson’s and cognitive impairment
- Open access
- Published: 18 June 2024
Cite this article
You have full access to this open access article

- Jennifer S. Pigott ORCID: orcid.org/0000-0002-0951-7941 1 ,
- Megan Armstrong ORCID: orcid.org/0000-0001-6773-9393 2 , 3 ,
- Nathan Davies ORCID: orcid.org/0000-0001-7757-5353 2 ,
- Daniel Davis ORCID: orcid.org/0000-0002-1560-1955 4 ,
- Bastiaan R. Bloem ORCID: orcid.org/0000-0002-6371-3337 5 ,
- Stefan Lorenzl ORCID: orcid.org/0000-0002-1165-0821 6 , 7 , 8 ,
- Wassilios G. Meissner ORCID: orcid.org/0000-0003-2172-7527 9 , 10 ,
- Per Odin ORCID: orcid.org/0000-0002-0756-7478 11 , 12 ,
- Joaquim J. Ferreira ORCID: orcid.org/0000-0003-3950-5113 10 ,
- Richard Dodel ORCID: orcid.org/0000-0003-2044-6299 13 &
- Anette Schrag ORCID: orcid.org/0000-0002-9872-6680 1
25 Accesses
4 Altmetric
Explore all metrics
To investigate the contributors to self-rated health in people with late-stage Parkinson’s disease (PD) and cognitive impairment.
A secondary analysis of baseline data from the international Care of Late-Stage Parkinsonism (CLaSP) cohort study was conducted. Participants with PD and either dementia or mild cognitive impairment or MMSE < 24/30 in the absence of major depression were included if they had completed the EQ-5D-3L assessment (n = 277). Factors associated with self-rated health (EQ-5D-3L Index and Visual Analogue Scale) were investigated through multivariable linear regression.
More severe PD (motor and non-motor) was associated with worse self-rated health. The EQ-5D-3L dimensions of Mobility, Self-Care and Usual Activities were almost universally affected; the latter two particularly severely. Being unable to perform usual activities or having moderate to extreme anxiety or depression were significantly associated with EQ-5D-3L Visual Analogue Scale, suggesting these are particularly valued. Worse motor impairment and function and the non-motor symptom domains of mood, perception, sexual function, and miscellaneous (e.g., pain) were associated with worse self-rated health, whereas greater burden of gastrointestinal symptoms was associated with better self-rated health in multivariate analysis. Better self-rated health was associated with recent PD nurse consultation, and higher doses of dopaminergic medication.
Improvement of activities of daily living, mood and anxiety should be prioritised in clinical practice, with consideration of perception and sexual function in this population. Recent nurse consultations and higher antiparkinsonian doses are associated with better self-rated health, suggesting there is no room for a therapeutic nihilism in this population of people within a complex phase of PD.
Similar content being viewed by others

Quality of life in a German cohort of Parkinson’s patients assessed with three different measures
Asymmetric responsiveness of disability and health-related quality of life to improvement versus decline in parkinson’s disease, health-related quality of life in parkinson’s disease: a cross-sectional study focusing on non-motor symptoms.
Avoid common mistakes on your manuscript.
Plain English Summary
Parkinson’s is a complex and progressive health condition that causes a wide range of symptoms and significantly impacts daily life. ‘Cognitive’ problems include memory and thinking problems. These are common in Parkinson’s, particularly in later stages. However, research about the experience of people with Parkinson’s who have these symptoms is limited.
We sought to understand how people with Parkinson’s who have cognitive problems perceive their health, and what factors influence this.
We found that performing usual activities, low mood and anxiety are particularly important to people with this condition. A range of Parkinson’s-related problems, including low mood, were associated with how people perceived their health, as has been the case for other groups of people with Parkinson’s in past research. More severe movement problems, limitations in ability to perform activities, perception symptoms such as hallucinations, and sexual problems were important, and may be more specific to this group of people. We also found that people who had seen a Parkinson’s nurse within the last 3 months and people taking more Parkinson’s medication reported better health.
We suggest that the factors identified as important should be addressed as a priority by healthcare professionals. Our findings also show the importance of the Parkinson’s nurse role for people with Parkinson’s and cognitive problems, and in ensuring medication is reviewed.
Parkinson’s disease (PD) is a complex neurodegenerative condition with increasing prevalence [ 1 ] and progressive course, conferring a heterogeneous range of impairments. Advancing PD is characterised by increasing dependence on caregivers for activities of daily living (ADLs), owing to treatment-resistant motor or non-motor symptoms, including cognitive decline [ 2 ]. Cognitive symptoms are common in PD, increasing with PD duration and more severe PD [ 3 , 4 ] and dementia is often considered a hallmark of advanced disease [ 5 ]. Cognitive impairment in PD is associated with increased dependence, higher caregiver burden and higher economic cost [ 4 , 6 , 7 , 8 , 9 , 10 , 11 ]. Despite the high prevalence of cognitive impairment in advanced stages of PD, there is however relatively little information on patients’ health status from their point of view. Although many studies have evaluated factors associated with worse self-rated health in PD overall, often using PD-specific measures, most have been conducted in early to mid-stage disease (e.g. [ 12 , 13 , 14 , 15 ]), while people with dementia were often excluded [ 14 , 16 , 17 ] or not represented [ 13 ]. Several reviews have identified non-motor symptoms, in particular depression, and functional impairment or dependence as being key predictors of self-rated health in PD overall [ 18 , 19 , 20 , 21 ], and the impact of PD is more pronounced in advanced disease [ 20 ]. Impairment in cognitive function, particularly attention and executive function, has also been associated with poorer self-rated health even in early disease stages [ 22 , 23 ]. Literature on this specific population is however sparse. A recent study from China investigated determinants of self-rated health according to cognitive status in PD, although in relatively early disease with relatively young participants (dementia group mean age 62.8 ± 6.93 years) [ 24 ]. Motor function was the strongest predictor of self-rated health for those with normal cognition, whereas depression was the strongest predictor for those with mild cognitive impairment or dementia. Bodily discomfort, cognition and mobility domains of the PDQ-39, a disease specific measure of self-rated health, were most affected for those with cognitive impairment.
To our knowledge, no studies have investigated determinants of self-rated health specifically for those with cognitive impairment in late-stage PD. Through greater understanding of factors that influence self-rated health, including healthcare and social care factors, interventions and services could be targeted to try to improve it.
Our aim was to investigate the factors associated with self-rated health of people with PD and cognitive impairment.
Care of late-stage parkinsonism (CLaSP) study
This is a multi-centre, prospective cohort study of people with late-stage parkinsonism and caregivers (either individual patients or patient-caregiver dyads) over 18 months, conducted in six European countries: Germany, Portugal, Sweden, UK, France and the Netherlands. Late-stage parkinsonism was operationally defined by disease duration of seven years or more; and Hoehn and Yahr stage IV or V, or Schwab and England stage 50% or less in the “On”-state. Details of the study have been published previously [ 25 ]. Participant identification and recruitment was adapted to healthcare arrangements in each country to target this hard-to-reach group. Patients are seen to withdraw from specialized medical care once they reach advanced stages of PD [ 2 ], so a variety of recruitment methods were employed, with extensive efforts made to recruit patients beyond specialist settings to minimize selection bias. Primary and secondary care providers, community settings such as nursing homes, and patient organizations (self-help groups and advocates) were contacted. The study sites included neurology, care of the elderly, and palliative care settings. Data was collected by trained researchers, through face-to-face interviews with participants and their caregiver, with breaks and repeated visits provided as required to facilitate completion. Ethical approval was granted locally for each site, and participants provided consent.
Participants
The CLaSP study included people with late-stage parkinsonism, as defined above, and excluded secondary parkinsonism or dementia with clear onset before motor symptoms. For the present analysis, baseline data for the subgroup of patients with Parkinson’s disease and cognitive impairment. Atypical and vascular Parkinsonism were excluded. Cognitive impairment was operationally defined as a pre-established diagnosis of dementia or Mild Cognitive Impairment, or a Mini Mental State Examination (MMSE) score < 24/30 in absence of major depression, as suggested by a score of 4 on the Unified Parkinson’s Disease Rating Scale (UPDRS) Part-I Question 3 (“Sustained depression with vegetative symptoms and suicidal thoughts or intent”). Participants who did not meet the disease duration of at least 7 years inclusion for the main CLaSP study could still be included in our analysis since the focus was cognitive impairment which can be marked even after relatively short disease duration [ 4 ] but is often considered an indicator of advanced Parkinson’s [ 26 ]. Analyses of variables only relevant to participants living in their own homes (professional and informal care provision) were applied to that subgroup.
Assessments
A range of assessments were conducted during the CLaSP study [ 25 ]; those included in this secondary analysis are shown in Table 1 . For the primary outcome of this analysis, self-rated health was assessed using the EuroQoL (EQ-5D-3L), since this was applied to all participants according to study protocol and has been validated for those with dementia in PD [ 27 , 28 ]. It also allows comparison with other studies including non-PD populations. It is a two-part instrument: five dimensions are each assessed with one question with three possible response levels as shown in Table 1 , plus participants are asked to indicate their overall perceived health status on a visual analogue scale (EQ VAS). The dimensions provide a description of self-rated health. An index is calculated to synthesise these dimension responses based on published population-specific value sets. For consistency and comparability we used the UK value sets [ 29 , 30 ] for all participants as recommended by the EuroQoL group for international studies. Studies suggest that across Western European countries values are broadly similar [ 31 , 32 ]. The range of possible scores for this calculated index is -0.594 to 1, with lower score indicating worse health status.
Statistical analysis was performed in Stata 17 [ 33 ]. Distributions were assessed visually. Descriptive statistics are presented as mean and standard deviation, and median and interquartile range (latter only for ordinal data), and numbers and percentages for categorical data, with numbers having completed each assessment presented. Correlation between the EQ-5D-3L Index and EQ VAS was assessed through Spearman rank correlation analysis. Relative contributions of the five dimensions of the EQ-5D-3L descriptive system to the EQ VAS were investigated by linear regression analysis with EQ VAS as the outcome. Missing data for the outcome variable were examined through comparison of those with and those without the questionnaire data and through univariate logistic regression with completion of the EQ-5D-3L questionnaire as the outcome measure.
Univariate analysis was conducted through simple linear regression analysis with each self-rated health outcome measure: EQ-5D Index and EQ VAS. For multivariable analysis, first we assessed four aspects of self-rated health determinants: (1) demographic factors, (2) clinical factors, (3) social care factors, and (4) healthcare factors, to identify factors of importance. Subsequently, we fitted the final model using the important variables: UPDRS Parts I-III to represent disease severity due to its clinical importance, and variables with p < 0.1 in the initial aspect-specific multivariable models. For multivariable analyses, the UPDRS parts, age and Zarit carer burden were standardised as z-scores (by mean and standard deviation), and NMSS domain scores converted to percentage of total possible scores to facilitate meaningful coefficients and comparisons. The Schwab and England Scale was not included in multivariable analyses due to overlap with other measures. Variance inflation factors were calculated to check for multicollinearity.
Analyses were conducted as complete case analysis, and a sensitivity analysis was conducted with the same multivariable models run with missing data imputed. Both complete case and imputed datasets have theoretical advantages and disadvantages where data may be missing not-at-random, so the sensitivity analyses quantify these differences in estimated coefficients [ 34 ]. Multiple Imputation by Chained Equations was used to impute missing data [ 35 ]. This technique was chosen for its flexibility: it can be applied to variables with different characteristics and distributions.
Of 342 participants with PD and cognitive impairment, 277 had been assessed with the EQ-5D-3L questionnaire. Participants were from Germany (n = 78), Portugal (n = 68), Sweden (n = 58), UK (n = 31), France (n = 24) and the Netherlands (n = 18). Of 277 participants, 185 had a pre-established diagnosis of dementia, one had a pre-established diagnosis of Mild Cognitive Impairment (MCI), and 91 scored less than 24/30 on the MMSE in the absence of major depression but without a formal diagnosis of dementia or MCI. Six participants had a disease duration of less than 7 years. 273 had completed all questions of the EQ-5D-3L descriptive system and so have a EQ-5D Index (4 missing); 249 had completed the EQ VAS (28 missing), and both were complete for n = 247. The participants predominantly had severe motor PD as indicated by Hoehn and Yahr staging [ 36 ]: n = 28 stages II-III, n = 142 stage IV and n = 107 stage V. The demographic, clinical and resource utilisation findings for the sample (n = 277) are displayed in Table 2 , with further detailed breakdowns in Online Resource 4.
Comparison of completers and non-completers
Factors associated with non-completion of the outcome measure (EQ-5D-3L) were worse cognition (MMSE), more severe PD (UPDRS Parts I-III), greater functional dependence (Schwab & England), female gender, nursing home residents, and site differences (higher proportions missing for Nijmegen, Bordeaux and London). Participation of a caregiver, age, marital status, disease duration, non-motor symptoms (NMSS total), and dopaminergic or dementia medication use were not associated with non-completion of the outcome measure.
Health status outcomes
Figure 1 displays the self-rated health results for the sample, described by response levels in the five dimensions, the Index (calculated from the dimension scores) and the EQ VAS. Almost all participants (96%) experienced some or severe problems with mobility. Severe problems with self-care and with usual activities were reported by 58% and 64% respectively, and some problems by 39% and 34% respectively. The mean Index was 0.12 (sd 0.33, range -0.59 to 0.82) and median EQ VAS score was 50 (IQR 30–55). Spearman correlation between EQ-5D Index and Visual Analogue Scale showed a moderate positive correlation between the scales, (n = 247, Spearman’s rho 0.3805, P < 0.0001) as illustrated in Fig. 2 .

EQ-5D-3L Outcomes: Bar charts of Dimension Response Levels and Histograms of EQ-5D Index and EQ VAS

Scatterplot of EQ-5D Index and EQ VAS
Analysis of the relative contribution of the five dimensions measured by the EQ-5D-3L descriptive system to the overall self-rated health, measured by the EQ VAS is shown in Fig. 3 (model detailed in Online Resource 1). It demonstrated that being unable to perform usual activities (p = 0.035) and being moderately (p = 0.012) or extremely (p = 0.034) anxious or depressed were significant predictors of the overall self-rated health recorded by EQ VAS.

Coefficient plot for regression model showing the relationship between responses on the EQ-5D-3L Descriptive System and the EQ VAS
Predictors of self-rated health
Univariate analyses are provided in Online Resource 2 and all multivariable analyses are provided in Online Resource 3. On multivariable analysis, no statistically significant associations were identified for demographic or care factors for either outcome measure. For the EQ VAS, non-significant trends toward female gender and greater caregiver burden being associated with worse self-rated health were identified (β = 5.11, p = 0.070 and β = -3.12, p = 0.089 respectively).
Numerous clinical factors were associated with the EQ-5D Index, but few for the EQ VAS. Higher UPDRS Parts-II and -III (ADL and Motor Examination) were significantly associated with worse self-rated health (Index: β = -0.12, p < 0.001 and β = -0.09, p = 0.001 respectively). Mood and perception symptoms were significantly associated with worse self-rated health (β = -0.004, p < 0.001 and β = -0.002, p = 0.04 respectively), whereas gastrointestinal symptoms and higher UPDRS Part-IV (Complications of Therapy) were associated with better self-rated health, for the Index (β = 0.002, p = 0.02 and β = 0.04, p = 0.04 respectively). Only miscellaneous symptoms (which include pain) were associated with worse self-rated health measured by the EQ VAS (β = -0.13, p = 0.05). With regard to healthcare utilisation, having had a PD Nurse consultation in the preceding 3 months was significantly associated with better self-rated health for both outcomes (Index: β = 0.19, p = 0.01; EQ VAS: β = 9.73, p = 0.02).
Important variables from these aspect-specific models were incorporated into a combined model, with UPDRS Parts I-III to control for PD severity, for each outcome, shown in Table 3 . Different variables were significant for the different EQ-5D-3L outcomes: UPDRS Part-II (ADL) and -III (motor examination) and NMSS domains mood, perception, and sexual function were all significantly associated with worse self-rated health for the EQ-5D Index; and NMSS gastrointestinal domain and having had a PD nurse consultation in the preceding three months were associated with better self-rated health status for the EQ-5D Index. For the EQ VAS, UPDRS Part-III (motor examination) and NMSS miscellaneous symptom domain were significantly associated with worse self-rated health, whereas higher doses of dopaminergic medication (LEDD) were associated with better self-rated health. The amount of variance accounted for by the combined models (calculated by R 2 ) for the EQ-5D Index was 46%, and for the EQ VAS was 24%.
Sensitivity analysis
All models were also run with missing data imputed, detailed in Online Resource 3, with some minor discrepancies identified. The combined models for the EQ-5D Index were similar, but the NMSS perception/hallucinations domain and PD Nurse consultations no longer reached significance with imputed data. The combined models for the EQ VAS were also similar, but the association between UPDRS Part-I and worse self-rated health reached significance with missing data imputed.
Summary of findings
In patients with cognitive impairment in late-stage PD the health dimensions of Self-Care and Usual Activities as well as Mobility were severely affected, and overall health as rated on the EQ VAS was particularly associated with being unable to perform usual activities and having moderate or extreme anxiety or depression. The two outcome components of the EQ-5D-3L instrument, the Index and EQ VAS score, were associated with slightly different clinical factors. For the Index, both more severe motor and non-motor features (UPDRS Parts-II and -III, NMSS mood, perception and sexual function domains) were associated with worse self-rated health. GI symptoms and having had a recent PD nurse consultation were associated with better self-rated health, although with imputation of missing data the association of self-rated health with PD nurse consultation and with perception symptoms was no longer statistically significant. For the EQ VAS, motor impairment (UPDRS Part-III) and the NMSS miscellaneous symptoms domain, which includes pain, were associated with worse self-rated health. Higher dose of dopaminergic medication was associated with better self-rated health on the EQ VAS, indicating that those who are treated more aggressively experience better health. With missing data imputed, the UPDRS Part-I (Mentation, Behaviour and Mood) was also significantly associated with worse self-rated health on the EQ VAS.
Context of previous research
Cognitive impairment in PD has been associated with reduced self-rated health in previous studies (e.g. [ 9 , 24 , 37 ]). The descriptive report of self-rated health provided by the EQ-5D-3L in this study allows comparison to other samples, as in Table 4 . All dimensions of health showed greater impairment compared to the general European population and to a sample of people with dementia (varied pathologies but predominantly Alzheimer’s disease). Problems with mobility were almost universal, comparable with another PD sample (without dementia), and pain/discomfort scores were also similar [ 13 ]. On the other hand, problems with self-care and usual activities were considerably more prominent than in the previous earlier PD sample. There was also an association between not being able to perform usual activities and subjective valuation of overall health status on the EQ VAS, highlighting the importance of independence in daily tasks. In a previous study in PD [ 24 ], motor impairment was also an important determinant to self-rated health in patients with PD-Mild Cognitive Impairment but this was not the case in those with PD-dementia. However, motor severity was markedly less in these groups in that study than in our sample. This likely reflects the late-stage of our sample, where motor impairments still play an important role in self-rated health in those with severe cognitive impairment.
Dopaminergic medication, which was associated with better self-rated health, is known to improve motor and some non-motor functions and self-rated health in PD [ 38 ], but it becomes more difficult to dose adequately in late-stage PD [ 2 ]. Doses typically must be balanced against the risk of adverse effects, including a worsening of neuropsychiatric and cognitive symptoms, often leading to a very cautious approach. Earlier work formed in comparably advanced PD populations revealed that many individuals are relatively undertreated [ 39 ]. Our findings are in line with growing recognition that there is still room for therapeutic improvement, even in this complex population with late-stage PD.
Whilst the marked association of depression with poorer self-rated health is consistent with previous reports in both PD [ 18 , 19 , 20 ] and in those with cognitive impairment in PD [ 24 ], depression/anxiety dimension scores were notably higher than the previous PD sample without dementia. The NMSS mood domain was associated with self-rated health on the EQ-5D Index, and with missing data imputed, ‘Mentation, Behaviour and Mood’ (UPDRS Part-I) was associated with the EQ VAS. This is important as cognitive impairment or dementia can be a barrier to assessment and treatment of depression, although treatment options are available [ 40 ]. It highlights that even in the presence of cognitive impairment, depression should be addressed as a treatable symptom.
We did not find significant associations of age and gender with self-rated health on multivariable analyses, although univariate analysis suggested a weak association between male gender and better self-rated health. Disease duration was not significantly associated with self-rated health, unlike in previous studies [ 8 , 24 ], but all patients were at the late stage of the disease, reflected in the long duration of disease (median 15, IQR 10–21 years).
Perceptual and sexual function symptoms, which we found to be associated with worse self-rated health, were not significantly associated with self-rated health in PD in most previous studies, and where they have, it had been in broader concepts of ‘autonomic symptoms’ [ 41 ] and ‘psychiatric symptoms’ [ 42 ]. They were not included in the previous studies of self-rated health in PD with cognitive impairment where the importance of these symptoms may be more distinctive. Sexual dysfunction in PD is complex [ 43 , 44 ]. Although studies are sparse, there seems to be an association between sexual symptoms and depression, anxiety and cognition in PD [ 45 , 46 ]. A qualitative study in caregivers found that relationship satisfaction and intimacy decreased with evolution of cognitive impairment [ 47 ]. Sexual symptoms are under-recognised, under-researched, and undertreated in PD [ 48 ], as well as dementia [ 49 ], so this finding highlights a need for consideration of sexual symptoms in this population. Psychosis and other neuropsychiatric symptoms are known to be associated with cognitive impairment [ 50 ], and have a particular negative impact on caregivers [ 51 ].
The association between gastrointestinal symptoms and better self-rated health is surprising. It diverges from previous studies, albeit in different populations [ 41 , 52 ], although it is interesting to note that this direction of relationship only occurred in our multivariable analysis. In contrast, univariate analysis showed an association between gastrointestinal symptoms and poorer self-rated health, and specifically for constipation, indicating that it may be primarily a function of overall disease severity. This suggests that it is the relative proportion of non-motor symptoms that impact self-rated health, and perhaps gastrointestinal symptoms are relatively well managed amongst these.
Health and social care factors have rarely been reported in past studies, but the importance of the PD nurse seen in our sample was also the case for those with late-stage PD without dementia where these factors were included [ 37 ]. However, when modelled after multiple imputations, this relationship was not statistically significant, weakening the findings. Since these are both from cross-sectional studies, causation cannot be inferred. Indeed, although there is widespread conviction that PD nurses offer crucial benefits to people living with PD, regardless of disease stage, there is to date only very modest evidence to support this assumption.
Strengths, limitations and future research
The CLaSP study successfully collected data on a previously understudied population and included a large sample with cognitive impairment, often excluded from research, offering improved generalisability of results to the population of those with late-stage PD and cognitive impairment. Furthermore, our sample had larger numbers with more severe cognitive impairment than previous studies of cognitive impairment in PD (dementia group n = 79 [ 24 ] and n = 25 [ 8 ]). The use of the EQ-5D-3L instrument facilitates evaluation of important patient-reported outcomes and has been validated in late-stage PD and found to be useful for those with dementia, although is not PD-specific.
Several limitations must be considered. First, this was an observational study so we cannot infer causal relationships between factors. There are likely to be a range of unmeasured factors that contribute further to self-rated health. Of note ethnicity and socioeconomic status data was not collected so could not be incorporated into the models but may play a role in self-rated health, though previous findings reported in the literature are mixed, and these factors are likely less important with increased age [ 53 , 54 , 55 ]. Personal factors such as self-efficacy [ 56 ], sense of coherence [ 57 ] and culture, and from a healthcare perspective, the quality rather than frequency of healthcare contacts may influence self-rated health. The overlap in concepts between the EQ-5D-3L and the clinical measures poses potential risk to the investigation of structural relationships between them, which is a recognised problem, for example, depressive symptoms on the NMSS and the Anxiety & Depression dimension of the EQ-5D-3L. However, PD is a complex condition with a broad spectrum of symptoms, which is why it impacts health so significantly. To exclude all clinical measures that overlap with EQ-5D dimensions from analysis would no longer represent PD. However, our use of initial aspect-specific models helps to disentangle this issue, for example, evaluating the impact of demographic factors independently of clinical symptoms. Moreover, the EQ VAS provides a self-rating of health that is not directly driven by ratings in these dimensions so does not explicitly measure overlapping constructs.
We also recognise limitations of the outcome measure itself: Although well validated, including in related populations [ 28 , 55 ], the EQ-5D-3L has been seen to have ceiling effects for those with better health, and may overestimate ill health in those with multimorbidity and chronic disease [ 58 ]. Furthermore, whilst the use of UK-derived value sets in the calculation of the EQ-5D Index provides consistency, it may not be representative of the values in non-UK countries. Missing data could have introduced information bias, since those with greater severity of PD and cognitive impairment were more likely to have missing outcome data. Some differences were seen on sensitivity analysis, when missing data was imputed, though most findings were consistent with the main analysis.
Future research would benefit from collecting additional data that may influence perception of health, such as ethnicity and self-efficacy; and more information regarding healthcare use, such as quality of consultations and ease of access to services. Intervention studies are warranted, addressing the factors we have identified, to further demonstrate causation, and importantly, to hopefully help improve self-rated health for this late-stage PD population.
Conclusions
The association between a range of motor and non-motor symptoms and self-rated health affirms the importance for a holistic clinical approach to the management of late-stage patients with cognitive impairment in PD. The relationship between PD nurse consultations and better self-rated health supports the importance of this role but discrepancies on sensitivity analysis warrant further investigation. Investigation of EQ-5D-3L dimensions highlights the widespread and powerful impact on self-care and usual activities, and the importance of usual activities and depression/anxiety to overall self-rated health in people with PD and cognitive impairment. Self-care, usual activities, mood and anxiety should therefore be priorities in clinical practice. A high proportion of variance in self-rated health remains unaccounted for so future research should investigate the role of personal factors and quality of healthcare.
Data availability
Data available upon request.
Ray Dorsey, E., Elbaz, A., Nichols, E., Abd-Allah, F., Abdelalim, A., Adsuar, J. C., Ansha, M. G., Brayne, C., Choi, J. Y. J., Collado-Mateo, D., Dahodwala, N., Do, H. P., Edessa, D., Endres, M., Fereshtehnejad, S. M., Foreman, K. J., Gankpe, F. G., Gupta, R., Hankey, G. J., … Murray, C. J. L. (2018). Global, regional, and national burden of parkinson’s disease, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurology, 17 , 939–953. PMID: 30287051.
Article Google Scholar
Coelho, M., & Ferreira, J. J. (2012). Late-stage parkinson disease. Nature Reviews. Neurology, 8 , 435–442.
Article CAS PubMed Google Scholar
Hely, M. A., Reid, W. G. J., Adena, M. A., Halliday, G. M., & Morris, J. G. L. (2008). The Sydney multicenter study of parkinson’s disease: The inevitability of dementia at 20 years. Movement Disorders, 23 , 837–844. PMID: 18307261.
Article PubMed Google Scholar
Aarsland, D., Batzu, L., Halliday, G. M., Geurtsen, G. J., Ballard, C., Chaudhuri, K. R., & Weintraub, D. (2021). Parkinson disease-associated cognitive impairment. Nature Reviews Disease Primers, 7 (1), 47.
Kempster, P. A., Williams, D. R., Selikhova, M., Holton, J., Revesz, T., & Lees, A. J. (2007). Patterns of levodopa response in parkinson’s disease: A clinico-pathological study. Brain, 130 , 2123–2128. PMID: 17586867.
Vossius, C., Larsen, J. P., Janvin, C., & Aarsland, D. (2011). The economic impact of cognitive impairment in parkinson’s disease. Movement Disorders, 26 , 1541–1544. PMID: 21538519.
Weir, S., Samnaliev, M., Kuo, T.-C., Tierney, T. S., Autiero, S. W., Taylor, R. S., & Schrag, A. (2018). Short- and long-term cost and utilization of health care resources in parkinson’s disease in the UK. Movement Disorders, 33 (6), 974–981.
Leroi, I., McDonald, K., Pantula, H., & Harbishettar, V. (2012). Cognitive impairment in parkinson disease: Impact on quality of life, disability, and caregiver burden. Journal of Geriatric Psychiatry and Neurology, 25 , 208–214. PMID: 23172765.
Lawson, R. A., Yarnall, A. J., Duncan, G. W., Khoo, T. K., Breen, D. P., Barker, R. A., Collerton, D., Taylor, J. P., & Burn, D. J. (2014). Severity of mild cognitive impairment in early parkinson’s disease contributes to poorer quality of life. Parkinsonism & Related Disorders, 20 , 1071–1075. PMID: 25074728.
Lawson, R. A., Yarnall, A. J., Johnston, F., Duncan, G. W., Khoo, T. K., Collerton, D., Taylor, J. P., & Burn, D. J. (2017). Cognitive impairment in parkinson’s disease: Impact on quality of life of carers. International Journal of Geriatric Psychiatry, 32 , 1362–1370. PMID: 27925292.
Rosqvist, K., Schrag, A., Odin, P., & Consortium, C. (2022). Caregiver burden and quality of life in late stage parkinson’s disease. Brain Sciences, 12 (1), 111. PMID: 35053854.
Article PubMed PubMed Central Google Scholar
Skorvanek, M., Martinez-martin, P., Kovacs, N., Zezula, I., Rodriguez-violante, M., Corvol, J., Taba, P., Seppi, K., Levin, O., Schrag, A., Aviles-olmos, I., Alvarez-sanchez, M., Arakaki, T., Aschermann, Z., Benchetrit, E., Benoit, C., Bergareche-yarza, A., Cervantes-arriaga, A., Chade, A., … Stebbins, G. T. (2018). Relationship between the MDS-UPDRS and quality of life: A large multicenter study of 3206 patients. Parkinsonism & Related Disorders, 52 , 83–89.
Balzer-Geldsetzer, M., Klotsche, J., Reetz, K., Spottke, A., Storch, A., Baudrexel, S., Mollenhauer, B., Berg, D., Liepelt, I., Kassubek, J., Kalbe, E., Wittchen, H. U., Dodel, R., & Riedel, O. (2018). Quality of life in a German cohort of parkinson’s patients assessed with three different measures. Journal of Neurology, 265 , 2713–2722. PMID: 30209651.
Kudlicka, A., Clare, L., & Hindle, J. V. (2014). Quality of life, health status and caregiver burden in parkinson’s disease: Relationship to executive functioning. International Journal of Geriatric Psychiatry, 29 , 68–76. PMID: 23625583.
Schrag, A., Jahanshahi, M., & Quinn, N. P. (2001). What contributes to depression in parkinson’s disease? Psychological Medicine, 31 , 65–73. PMID: 11200961.
Tu, X., Hwang, W., Ma, H., Chang, L., & Hsu, S. (2017). Determinants of generic and specific health- related quality of life in patients with parkinson’s disease. PLoS ONE, 12 (6), e0178896.
Sanchez-Luengos, I., Lucas-Jlmenez, O., Ojeda, N., Pena, J., Gomez-Esteban, J. C., Gomez-Beldarraln, M. A., Vazquez-Picon, R., Foncea-Betl, N., & Ibarretxe-Bilbao, N. (2022). Predictors of HrQoL in PD – impact of overlap in clinical and QoL measures. Quality of Life Research, 31 (11), 3241–3252.
Den Oudsten, B. L., Van Heck, G. L., & De Vries, J. (2007). Quality of life and related concepts in parkinson’s disease: A systematic review. Movement Disorders, 22 , 1528–1537. PMID: 17523198.
Soh, S.-E., McGinley, J. L., Watts, J. J., Iansek, R., Murphy, A. T., Menz, H. B., Huxham, F., & Morris, M. E. (2013). Determinants of health-related quality of life in people with parkinson’s disease: A path analysis. Quality of Life Research, 22 , 1543–1553.
Schrag, A. (2006). Quality of life and depression in parkinson’s disease. Journal of the Neurological Sciences, 248 , 151–157.
Schrag, A., & Quinn, N. (2020). What contributes to quality of life in parkinson’s disease: A re-evaluation. Journal of Neurology, Neurosurgery & Psychiatry, 91 (6), 563–565. PMID: 32139651.
Lawson, R. A., Yarnall, A. J., Duncan, G. W., Breen, D. P., Khoo, T. K., Williams-Gray, C. H., Barker, R. A., Collerton, D., Taylor, J. P., & Burn, D. J. (2016). Cognitive decline and quality of life in incident parkinson’s disease: The role of attention. Park. Relat. Disord., 27 , 47–53. PMID: 27094482.
Klietz, M., Schnur, T., Drexel, S., Lange, F., Tulke, A., Rippena, L., Paracka, L., Dressler, D., Höglinger, G. U., Wegner, F., & Portillo, M. C. (2020). Association of motor and cognitive symptoms with health-related quality of life and caregiver burden in a german cohort of advanced parkinson’s disease patients. Parkinson’s Disease, 2020 , 5184084.
CAS PubMed PubMed Central Google Scholar
Fan, Y., Liang, X., Han, L., Shen, Y., Shen, B., Chen, C., Sun, Y., Wang, J., & Tang, Y. (2020). Determinants of quality of life according to cognitive status in parkinson’s disease. Frontiers in Aging Neuroscience, 12 , 269.
Balzer-Geldsetzer, M., Ferreira, J., Odin, P., Bloem, B. R., Meissner, W. G., Lorenzl, S., Wittenberg, M., Dodel, R., & Schrag, A. (2018). Study protocol: Care of late-stage parkinsonism (CLaSP): A longitudinal cohort study. BMC Neurology, 18 , 1–8.
Gonzalez, M. C., Dalen, I., Maple-Grødem, J., Tysnes, O. B., & Alves, G. (2022). Parkinson’s disease clinical milestones and mortality. NPJ Parkinson’s Disease, 8 (1), 58.
Rabin, R., & de Charro, F. (2001). EQ-5D: A measure of health status from the EuroQoL group. Annals of Medicine, 33 , 337–343. PMID: 11491192.
Ramadhan, M., Schrag, A., CLaSP Consortium. (2022). The validity of health-related quality of life instruments in patients with late-stage parkinson’s disease. Journal of geriatric psychiatry and neurology, 36 (3), 089198872211199.
Google Scholar
Ramos-Goni, J. M., & Rivero-Arias, O. (2011). eq5d: A command to calculate index values for the EQ-5D quality-of-life instrument. The Stata Journal, 11 , 120–125. https://www.stata-journal.com/article.html?article=st0220 .
Szende, A., Oppe, M., & Devlin, N. (2007). EQ-5D value sets: Inventory, comparative review and user guide (2007th ed.). Springer.
Book Google Scholar
Olsen, J. A., Lamu, A. N., & Cairns, J. (2018). In search of a common currency: A comparison of seven EQ-5D-5L value sets. Health economics, 27 , 39–49. PMID: 29063633.
EuroQol Group. (1990). EuroQol — a new facility for the measurement of health-related quality of life. Health policy (Amsterdam, Netherlands), 16 (3), 199–208. PMID: 10109801.
StataCorp. (2021). Stata Statistical Software: Release 17.
Jakobsen, J. C., Gluud, C., Wetterslev, J., & Winkel, P. (2017). When and how should multiple imputation be used for handling missing data in randomised clinical trials – a practical guide with flowcharts. BMC Medical Research Methodology, 17 , 1–10. PMID: 29207961.
Azur, M. J., Stuart, E. A., Frangakis, C., & Leaf, P. J. (2011). Multiple imputation by chained equations: What is it and how does it work? International Journal of Methods in Psychiatric Research, 20 , 40–49. PMID: 18543368.
Hoehn, M. M., & Yahr, M. D. (1967). Parkinsonism: onset, progression, and mortality. Neurology, 17 (5), 427–442.
Rosqvist, K., Odin, P., Lorenzl, S., Meissner, W. G., Bloem, B. R., Ferreira, J. J., Dodel, R., & Schrag, A. (2021). CLaSP consortium, factors associated with health-related quality of life in late-stage parkinson’s disease. Movement Disorders Clinical Practice, 8 , 563–570.
Martinez-Martin, P., Rodriguez-Blazquez, C., Forjaz, M. J., & Kurtis, M. M. (2015). Impact of pharmacotherapy on quality of life in patients with parkinson’s disease. CNS Drugs, 29 , 397–413.
Weerkamp, N. J., Zuidema, S. U., Tissingh, G., Poels, P. J. E., Munneke, M., Koopmans, R. T. C. M., & Bloem, B. R. (2012). Motor profile and drug treatment of nursing home residents with parkinson’s disease. Journal of the American Geriatrics Society, 60 , 2277–2282. PMID: 23231550.
Goodarzi, Z., & Ismail, Z. (2017). A practical approach to detection and treatment of depression in parkinson disease and dementia. Neurology Clinical Practice, 7 (2), 128–140.
Gallagher, D. A., Lees, A. J., & Schrag, A. (2010). What are the most important nonmotor symptoms in patients with parkinson’s disease and are we missing them? Movement Disorders, 25 , 2493–2500. PMID: 20922807.
Antonini, A., Barone, P., Marconi, R., Morgante, L., Zappulla, S., Pontieri, F. E., Ramat, S., Ceravolo, M. G., Meco, G., Cicarelli, G., Pederzoli, M., Manfredi, M., Ceravolo, R., Mucchiut, M., Volpe, G., Abbruzzese, G., Bottacchi, E., Bartolomei, L., Ciacci, G., … Colosimo, C. (2012). The progression of non-motor symptoms in Parkinson’s disease and their contribution to motor disability and quality of life. Journal of Neurology, 259 , 2621–2631. PMID: 22711157.
Santa Rosa Malcher, C. M., da Roberto Silva Gonçalves Oliveira, K., FernandesCaldato, M. C., Lopes dos Santos Lobato, B., da Silva Pedroso, J., & de Tubino Scanavino, M. (2021). Sexual disorders and quality of life in parkinson’s disease. Sexual medicine, 9 (1), 100280.
Bronner, G. (2011). Sexual problems in parkinson’s disease: The multidimensional nature of the problem and of the intervention. Journal of the Neurological Sciences, 310 , 139–143.
Kotková, P., & Weiss, P. (2013). Psychiatric factors related to sexual functioning in patients with parkinson’s disease. Clinical Neurology and Neurosurgery, 115 , 419–424. PMID: 22831909.
Kummer, A., Cardoso, F., & Teixeira, A. L. (2009). Loss of libido in parkinson’s disease. The Journal of Sexual Medicine, 6 , 1024–1031. PMID: 19040621.
Vatter, S., McDonald, K. R., Stanmore, E., Clare, L., McCormick, S. A., & Leroi, I. (2018). A qualitative study of female caregiving spouses’ experiences of intimate relationships as cognition declines in parkinson’s disease. Age and Ageing, 47 , 604–610. PMID: 29617933.
Bhattacharyya, K. B., & Rosa-Grilo, M. (2017). Sexual dysfunctions in parkinson’s disease: An underrated problem in a much discussed disorder. International Review of Neurobiology, 134 , 859–876.
D’cruz, M., Andrade, C., & Rao, T. S. S. (2020). The expression of intimacy and sexuality in persons with dementia. Journal of Psychosexual Health, 2 , 215–223.
Leroi, I., Pantula, H., Mcdonald, K., Harbishettar, V., Royal, S., Foundation, N. H. S., & Salford, M. (2012). Neuropsychiatric symptoms in parkinson’s disease with mild cognitive impairment and dementia. Parkinson’s Disease, 2012 , 1–10.
Martinez-Martin, P., Rodriguez-Blazquez, C., Forjaz, M. J., Frades-Payo, B., Agüera-Ortiz, L., Weintraub, D., Riesco, A., Kurtis, M. M., & Chaudhuri, K. R. (2015). Neuropsychiatric symptoms and caregiver’s burden in parkinson’s disease. Parkinsonism & Related Disorders, 21 , 629–634. PMID: 25892660.
Lubomski, M., Davis, R. L., & Sue, C. M. (2021). Health-related quality of life for parkinson’s disease patients and their caregivers. Journal of Movement Disorders, 14 , 42–52.
Shaw, J. W., Johnson, J. A., Chen, S., Levin, J. R., & Coons, S. J. (2007). Racial/ethnic differences in preferences for the EQ-5D health states: results from the U.S. valuation study. Journal of Clinical Epidemiology, 60 (5), 479–490. PMID: 17419959.
Schneider, P., Love-Koh, J., McNamara, S., Doran, T., & Gutacker, N. (2022). Socioeconomic inequalities in HRQoL in England: An age-sex stratified analysis. Health and Quality of Life Outcomes, 20 , 1–9. PMID: 35918765.
Buchholz, I., Marten, O., & Janssen, M. F. (2022). Feasibility and validity of the EQ-5D-3L in the elderly Europeans: A secondary data analysis using SHARE(d) data. Quality of Life Research, 31 , 3267–3282. PMID: 35624409.
Rosqvist, K., Hagell, P., Odin, P., Ekström, H., Iwarsson, S., & Nilsson, M. H. (2017). Factors associated with life satisfaction in parkinson’s disease. Acta Neurologica Scandinavica, 136 , 64–71. PMID: 27726132.
Gison, A., Rizza, F., Bonassi, S., Dall’Armi, V., Lisi, S., & Giaquinto, S. (2014). The sense-of-coherence predicts health-related quality of life and emotional distress but not disability in Parkinson’s disease. BMC Neurology, 14 , 1–6. PMID: 25304029.
Thompson, A. J., & Turner, A. J. (2020). A comparison of the EQ-5D-3L and EQ-5D-5L. PharmacoEconomics, 38 , 575–591.
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., & Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in parkinson’s disease. Movement Disorders, 25 , 2649–2685.
Fahn, S., Elton, R., Members of the UPDRS Development Committee. (1987). Unified parkinson’s disease rating scale. In S. Fahn, C. Marsden, M. Goldstein, & D. Calne (Eds.), Recent Developments in Parkinson’s Disease. (Vol. 2). Florhan Park, NJ: Macmillan Healthcare Information.
Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12 (3), 189–198.
Chaudhuri, K. R., Martinez-Martin, P., Brown, R. G., Sethi, K., Stocchi, F., Odin, P., Ondo, W., Abe, K., MacPhee, G., MacMahon, D., Barone, P., Rabey, M., Forbes, A., Breen, K., Tluk, S., Naidu, Y., Olanow, W., Williams, A. J., Thomas, S., … Schapira, A. H. V. (2007). The metric properties of a novel non-motor symptoms scale for parkinson s disease. Movement Disorders, 22 (13), 1901–1911.
Von Campenhausen, S., Winter, Y., Rodriguese Silva, A., Sampaio, C., Ruzicka, E., Barone, P., Poewe, W., Guekht, A., Mateus, C., Pfeiffer, K.-P., Berger, K., Skoupa, J., Bötzel, K., Geiger-Gritsch, S., Siebert, U., Balzer-Geldsetzer, M., Oertel, W. H., Dodel, R., & Reese, J. P. (2011). Costs of illness and care in parkinson’s disease: An evaluation in six countries. European Neuropsychopharmacology, 21 (2), 180–191.
Schwab, R., & England, A. (1969). Projection technique for evaluating surgery in Parkinson’s disease. In F. H. Billingham & M. C. Donaldson (Eds.), Third Symposium on Parkinson’s Disease (pp. 152–157). Edinburgh: Churchill Livingstone.
Zarit, S. H., Reever, K. E., & Bach-Peterson, J. (1980). Relatives of the impaired elderly: Correlates of feelings of burden. The Gerontologist, 20 , 649–655.
Janssen, M. F., Pickard, A. S., & Shaw, J. W. (2021). General population normative data for the EQ-5D-3L in the five largest European economies. The European Journal of Health Economics, 22 , 1467–1475. PMID: 34117986.
Nelis, S. M., Wu, Y. T., Matthews, F. E., Martyr, A., Quinn, C., Rippon, I., Rusted, J., Thom, J. M., Kopelman, M. D., Hindle, J. V., Jones, R. W., & Clare, L. (2019). The impact of co-morbidity on the quality of life of people with dementia: Findings from the IDEAL study. Age and Ageing, 48 , 361–367. PMID: 30403771.
Canadian Agency for Drugs and Technologies in Health. (2018). Clinical Review Report – Levodopa/Carbidopa. https://www.ncbi.nlm.nih.gov/books/NBK539562/pdf/Bookshelf_NBK539562.pdf .
Fernandez, H. H., Standaert, D. G., Hauser, R. A., Lang, A. E., Fung, V. S. C., Klostermann, F., Lew, M. F., Odin, P., Steiger, M., Yakupov, E. Z., Chouinard, S., Suchowersky, O., Dubow, J., Hall, C. M., Chatamra, K., Robieson, W. Z., Benesh, J. A., & Espay, A. J. (2015). Levodopa-carbidopa intestinal gel in advanced parkinson’s disease: Final 12-month, open-label results. Movement Disorders, 30 , 500–509. PMID: 25545465.
Download references
Acknowledgements
We thank the participants of the CLaSP study as well as all those who recruited and collected data at all of the study sites.
This report is independent research funded by the Rosetrees Trust and the Royal College of Physicians [RCP-2020\11]. The CLaSP study was funded by the European Commission (Joint Programme – Neurodegenerative Disease Research “European research projects for the evaluation of health care policies, strategies and interventions for Neurodegenerative Diseases”) through national funding bodies in all six countries [Economic and Social Research Council ES/L009250/1; BMBF, Marburg, Germany 01ED1403A, Munich, Germany 01ED1403B, Bordeaux, France: ANR-13-JPHC-0001–07, Lisbon, Portugal: HC/0002/ 2012, Lund, Sweden: HC-559–002, Nijmegen, Holland, 733051003].
Author information
Authors and affiliations.
Clinical Neurosciences, Queen Square Institute of Neurology, University College London, Royal Free Hospital, Rowland Hill Street, London, NW3 2PF, UK
Jennifer S. Pigott & Anette Schrag
Centre for Ageing Population Studies, Research Department of Primary Care and Population Health, University College London, London, UK
Megan Armstrong & Nathan Davies
Centre For Psychiatry and Mental Health, Queen Mary University of London, London, UK
Megan Armstrong
MRC Unit for Lifelong Health and Ageing, University College London, London, UK
Daniel Davis
Donders Institute for Brain, Cognition and Behavior, Department of Neurology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands
Bastiaan R. Bloem
Institute for Palliative Care, Paracelsus Medical University, Salzburg, Austria
Stefan Lorenzl
Department of Palliative Medicine, University Hospital, LMU Munich, Munich, Germany
Department of Neurology and Palliative Care, University Hospital Agatharied, Hausham, Germany
Service de Neurologie des Maladies Neurodégénératives, IMNc, IMN, UMR 5293, CHU de Bordeaux, Univ. de Bordeaux, CNRS, 33000, Bordeaux, France
Wassilios G. Meissner
Dept. Medicine, University of Otago, Christchurch, and New Zealand Brain Research Institute, Christchurch, New Zealand
Wassilios G. Meissner & Joaquim J. Ferreira
Division of Neurology, Department of Clinical Sciences Lund, Lund University, Skåne University Hospital, Lund, Sweden
Faculdade de Medicina, Instituto de Medicina Molecular João Lobo Antunes, Universidade de Lisboa, Lisboa, Portugal
Department of Geriatric Medicine, University Duisburg Essen, Essen, Germany
Richard Dodel
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization and execution of the CLaSP study: Anette Schrag Bastiaan R. Bloem, Stefan Lorenzl, Wassilios G. Meissner, Per Odin, Joaquim J. Ferreira, Richard Dodel. Design of this analysis: Jennifer S Pigott, Anette Schrag, Daniel Davis; execution of analysis: Jennifer S Pigott; supervision & review of analysis: Anette Schrag, Daniel Davis, Nathan Davies, Megan Armstrong. Writing: The first draft of the manuscript was written by Jennifer S Pigott; subsequently reviewed by all authors.
Corresponding author
Correspondence to Anette Schrag .
Ethics declarations
Competing interests.
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The study was approved by the ethical committees of all participating study sites (London, Camden, and Islington NRES Committees 14/LO/0612; Bordeaux, South West, and Overseas Protection Committee III [South West and Overseas Protection Committee], 2014-A01501–46; Lisbon, Centro Hospitalar Lisboa Norte, DIRCLN-19SET2014– 275; Lund, EPN regional ethics name Lund, JPND NC 559–002; Marburg, Ethics Commission at the State Medical Association Hesse, MC 309/2014; Munich, ethics committee at the LMU Munchen, 193–14; Nijmegen, Radboud University Medical Center, Group Staff Quality and Safety Human Research Committee, Arnhem-Nijmegen region, DJ/CMO300). To obtain consent, detailed oral and written information were given to the patients and their informant to ensure that the patient fully understood the potential risks and benefits of the study. If patients were unable to provide consent, consent was obtained with the legal representative, in accordance with national law.
Consent to Participate
Informed consent was obtained for all individual participants included in the study: from the participant themselves if they had capacity, or from a legal guardian, in accordance with local ethical and legal regulations, if participants lacked capacity.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 168 KB)
Supplementary file2 (pdf 248 kb), supplementary file3 (pdf 365 kb), supplementary file4 (pdf 168 kb), supplementary file5 (pdf 204 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Pigott, J.S., Armstrong, M., Davies, N. et al. Factors associated with self-rated health in people with late-stage parkinson’s and cognitive impairment. Qual Life Res (2024). https://doi.org/10.1007/s11136-024-03703-2
Download citation
Accepted : 28 May 2024
Published : 18 June 2024
DOI : https://doi.org/10.1007/s11136-024-03703-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cognitive impairment
- Parkinson’s disease
- Parkinson’s disease dementia
- Self-rated health.
- Find a journal
- Publish with us
- Track your research
- Find A Therapist
- A Case Study on the Impact of Digital Therapy in Parkinson’s Disease Management
admin June 19th, 2024
– Written By
Rakshitha S
MASLP (Master’s in Audiology and Speech Language Pathology)
Consultant Speech Swallow pathologist, Digital practitioner -SLP
Introduction
Living with Parkinson’s Disease can present many challenges, particularly in terms of speech and communication. Today, I want to share an inspiring story about Gadha, a 63-year-old woman who has shown remarkable progress through digital therapy. Gadha and her caretakers opted for digital therapy due to their remote location and the unavailability of her caretakers for frequent travel. This approach ensures consistent and accessible care despite the logistical challenges they face. This blog will highlight her journey, the activities she engaged in, and how digital therapy has empowered her to improve her speech and communication skills.
Understanding Parkinson’s Disease
Parkinson’s Disease is a progressive neurological disorder that affects movement and can impact speech and cognitive functions. Symptoms often include tremors, stiffness, difficulty with balance and coordination, and speech difficulties such as:
- Reduced range of tongue movement
- Slow rate of speech with interrupted fluency
- Reduced vocal loudness and a monotonous voice quality
- Challenges with word retrieval and answering in sequence
- Mild executive function deficits (planning and organization)
Gadha’s Initial Challenges
When Gadha first started her therapy, she faced several significant challenges:
- Difficulty biting hard food
- Slow and interrupted speech
- Reduced vital capacity and mean length of utterance
- Difficulty retrieving words and answering in sequence
- Reduced vocal loudness and a breathy, monotonous voice quality
- Mild executive function deficits
- Generally intact attention and memory skills with some variability
Despite these hurdles, Gadha was determined to improve her speech and communication abilities.
The Power of Digital Therapy
Digital therapy leverages technology to provide therapeutic interventions that can be accessed remotely. This approach offers flexibility, accessibility, and personalized care, making it a powerful tool for individuals like Gadha.
1. Oromotor and Brain Gym Exercises:
Enhanced range of tongue movement and oral motor skills.
Improved cognitive abilities, including attention and memory.
2. Reading and Explaining Proverbs:
Boosted word retrieval abilities and fluency.
Engaged cognitive skills by understanding and explaining proverbs.
3. Describing Pictures:
Improved mean length of utterance and vital capacity.
Encouraged the use of descriptive language and longer sentences.
4. LSVT Technique for Breath Support and Sound Quality:
Increased vocal loudness and improved voice quality.
5. Calendar Tasks:
Helped comprehend and express days of the week, enhancing cognitive organization.
6. Identifying Birds and Animals:
Strengthened categorization skills and vocabulary.
7. Performing Complex Commands:
Improved ability to follow multi-step instructions, aiding executive function.
8. Identifying Emotions through Digital Activities:
Enhanced emotional recognition and expression.
9. Singing Along with Film Songs:
Improved intonation and rhythmic speech patterns.
10. Comprehending and Expressing Various Categories:
Practiced naming professionals, zodiac signs, and more.
11. Sequencing Skills through Reciting Cooking Recipes:
Strengthened sequencing abilities and verbal organization.
12. Number Sequence Tasks and Cognitive Skill Exercises:
Enhanced cognitive processing and number recognition.
13. Sorting and Identifying Spices, Discussing Recipes:
Combined sensory stimulation with cognitive and language skills.
14. Expressing Names of Kannada Serials and Their Timings:
Practiced memory and time-related vocabulary.
15. Naming Things Related to Different Rooms:
Enhanced environmental vocabulary and contextual usage.
16. Answering WH Questions and Riddles:
Improved comprehension and critical thinking.
17. Identifying Addresses and Recalling Phone Numbers:
Strengthened memory and practical language use.
18. Repeating Kannada Proverbs:
Practiced cultural language and fluency.
19. Identifying Hindu Mythology Characters and Their Roles:
Enhanced cultural literacy and narrative skills.
20. Reading Numbers for Digit Recognition:
Improved number recognition and reading skills.
21. Discussing Morals from Stories and Songs:
Engaged critical thinking and moral reasoning.
22. Telling Time from a Displayed Clock:
Practiced time-telling and temporal concepts.
23. Identifying and Expressing the Use of Objects:
Improved functional language and vocabulary.
24. Recognizing Logos:
Enhanced visual recognition and associative language skills.
Gadha’s Progress
Through consistent practice and dedication, Gadha has shown significant improvements:
- Increased range of tongue movement
- Enhanced ability to bite and chew food
- Faster, more fluent speech
- Improved word retrieval and sequencing abilities
- Increased vocal loudness and a more varied voice quality
- Better planning and organizational skills
Gadha’s journey demonstrates the effectiveness of digital therapy. Her ability to engage in these therapeutic activities from the comfort of her home has played a crucial role in her progress.
Gadha’s story is a testament to the power of digital therapy in managing Parkinson’s Disease. With the right tools and support, individuals can make meaningful progress in their speech and communication skills. If you or a loved one are facing similar challenges, consider exploring digital therapy options. Remember, with perseverance and the right guidance, improvement is always within reach. Thank you for reading! If you found this story inspiring, please share it with others who might benefit from it. Together, we can spread awareness and support those on their journey with Parkinson’s Disease.
XceptionalLEARNING platform offer customized solutions designed to meet the specific requirements of both therapists and clients. Whether you’re looking for advanced features to enhance your practice or specialized tools for educational therapy, our platforms provide the flexibility and support you need. Contact us today to explore our comprehensive range of services and discover how we can support you in reaching your therapy goals. Our dedicated team is here to provide personalized assistance and ensure you have access to the best resources available. Reach out now and take the first step towards achieving excellence in therapy.
Recent Post
- Comprehensive Guide to Pediatric Occupational Therapy: Enhancing Children’s Development and Well-being
- Transforming Communication: A Case Study on Speech Therapy for an 8-Year-Old Boy with Autism Spectrum Disorder (ASD)
- Empowering Special Education Through Technology
- The Impact of Early Intervention: Why It’s Essential
Study aims to understand genetics of Parkinson’s disease in Black people
Erin Foster , an associate professor of occupational therapy, and Scott Norris, MD , an associate professor of neurology, have established a site at Washington University School of Medicine in St. Louis for the Black and African American Connections to Parkinson’s Disease (BLAAC PD) study, an international study aimed at understanding the gene changes that may lead to Parkinson’s disease in people with African ancestry. Parkinson’s is a neurodegenerative disease characterized by slow and unsteady movement. Foster and Norris are collecting clinical and behavioral data from people with Parkinson’s and healthy people who identify as Black or African American in the St. Louis area.
By joining the study, Washington University also joins the Parkinson’s Genetics Program, a global initiative geared at promoting a more comprehensive view of Parkinson’s disease by collecting genetic data from 150,000 people representing diverse populations around the world. Parkinson’s is a debilitating disease that affects people of all backgrounds, but it has historically been understudied in many populations, including Black, Latino, Asian, Native American, LGBTQ+, those in lower socioeconomic groups and people living in underserved geographies (rural and urban).
Comments and respectful dialogue are encouraged, but content will be moderated. Please, no personal attacks, obscenity or profanity, selling of commercial products, or endorsements of political candidates or positions. We reserve the right to remove any inappropriate comments. We also cannot address individual medical concerns or provide medical advice in this forum.
You Might Also Like
Latest from the Record
Announcements.
Parking shares latest update
Staff leadership program applications due May 31
Peace Park planting May 18
Bose named Fulbright Scholar
Oppenheimer named Religion & Politics executive editor
Lucey receives sleep science award
Stan H. Braude, professor of practice in Arts & Sciences, 62
Liz Colletta, longtime accounting employee, 55
Eduardo Slatopolsky, professor emeritus of medicine, 89
Research Wire
Sampling eDNA for global biodiversity census
Biologists take closer look at stress response in cells
The View From Here
Washington people.
Sadie Williams Clayton
Caitlyn Collins
Kim Thuy Seelinger
Who Knew WashU?
Who Knew WashU? 1.27.21
Who Knew WashU? 1.13.21
Who Knew WashU? 12.9.20
F.M. ALEXANDER STUDIES
- Marigold Smith
- Sep 21, 2018
Parkinson's Disease: A Case Study
Here is case study of a course of lessons with a Parkinson's Disease patient, from Marigold Smith, an Alexander Technique teacher from Studio Evolving at Healesville, Victoria . She published this in 2009 and when I noticed that this was no longer on her web-site I asked Marigold for permission to publish it here.
There are numerous. anecdotal accounts of the effectiveness of the Alexander Technique in dealing with the symptoms of Parkinson's Disease. And there are also a very few small scientific research papers with promising results, but as drug companies can't make any money out of non-drug interventions, there is so far no funding for large double-blind clinical trials.I have put some links to some links to Youtube videos and scientific papers at the bottom of this case-study.
David Moore

Parkinson's Disease - A Case Study
Introducing Valma Scott
Val was diagnosed with Parkinson’s disease over 10 years ago. She is 76 years of age and married to child hood sweetheart, Jim for 60 years. They have 6 children including 2 sets of twins and now 17 grandchildren.
I have been working with Val since November last Year. I feel privileged and honored getting to know Val and her remarkable partner Jim. They wish to share their journey with the Alexander Technique and hope their experience will inspire other people with Parkinson’s disease to explore the Technique for themselves.
Jim found the English Alexander Technique and Parkinson’s disease study on the Internet, and with Val decided to investigate the Technique. Val’s well-being and quality of life was their main concern They live in Alexandra in country Victoria and I was the nearest Alexander Technique teacher, a good 100 kilometers away in Healesville.
A Typical Day
Val is confined to a wheel chair unable to stand, dependent on Jims support. She is hoisted by crane from bed to chair. Val had both knees replaced over 30 years ago and bears horrific scars and no kneecaps. Riding a bike and walking until a few years ago, her knees finally were so painful she ground to a stop. Further surgery is impossible because of the nervous disability bought about with the Parkinson symptoms. In one sense Val’s problem with her knees is unrelated to Parkinson’s disease, but in another it is entirely relevant to the issues she faces. When people have additional problems to their Parkinson’s symptoms these are still part of the ‘whole person’s’ experience of daily life.
A usual day in Val and Jim’s life revolves around the rituals of the hoist. With Jim lifting or hoisting Val from bed to bath or to toilet or wheel chair, the tasks can take a whole day. Each day depends on her symptoms of nervousness, shakes, depression, motivation and co-operation. Television doesn’t interest her, reading she finds difficult, the radio is O.K. particularly music. She has an amazingly date retentive memory of family events, every family members birthday and names. On a ‘bad day’ her short-term memory is affected, causing panic, anxiety, dependency, clinging and insecurity. From one day to the next Val’s experience varies greatly.
Initial Presentation
When I first met Val she presented with these problems:
•Bladder - incontinency
•Bowel problems - constipated
•Collapsed head and neck
•Difficulty Speaking
•Difficulty swallowing
•Fear of falling
•Poor circulation in feet (cold, discolored, poor feeling)
•Cannot stand - legs fixed in one direction
Introduction
The following is a summary of of Valma's progress derived from records kept after each visit . My first Alexander lesson with Val was on November 20th 2003. Lessons have been maintained on a weekly basis with some short breaks of two to three weeks during holidays etc. The work involves mainly table work which has extended to more free work on the floor and on a chair over a period of months. The notes below are presented as a weekly report or monthly summary depending on Val's progress.
Summary of Progress
November - December 2003
Started with table work with books under her head and bolsters under legs. After the first lesson we saw some immediate changes - less shakes, speaking more freely but could only open legs a little. Val said she felt relaxed and sleepy and slept well that night. At the end of November she looked more upright in the chair and calmer Started giving directions to help her short shallow breathing. More movement in left leg.
December - January 2004
Positive changes include less neck restriction / speaking more freely - lucid humor, teasing and joking - calmer and less shaking. Val said she felt relaxed and sleepy. Jim was going to provide a firm board on Val's bed to try semi-supine once a day.
Third Visit
Val more upright, smiling and welcoming with a kiss. Laid flatter on the table - easier to help neck / head / jaw. Can now find room to put my hands around her neck. Jim has noticed change in bowel and bladder control. When I put my hands on Val her breathing became easier and the shakes go. Tried a pillow between her legs to help spacing between legs - to change pattern.
Fourth Visit
Jim thinks Val's skin, feet and legs feel different - feet have better colour, skin more elastic texture. Val seems wider and longer on the table -moving feet and toes herself, but lack co-ordination and control - better on one side.
Fifth Visit
Val loves being touched down legs from hip to each toe. Discussed Home Maintenance with Jim. Her head position at home in the wheelchair and in the car was important - explained the effect of 'collapse' on breathing and speech. Jim will try to change pillow height and support in her bed.
Sixth Visit
Val able to put feet flat on the table at hip width apart with a pillow spacing. Val asks "when will she be walking".
Three week break. Val continued with her home self maintenance program of semi-supine, pillow changes and exercises with pillow between legs. Upon her return for the seventh visit her breathing had improved and was able to move her legs independently. Mobility improved being able to put hands on her knees and rotate her knees together and away by herself. Val used to love riding her bicycle so we played with her pedaling her feet into my hands.
February 2004
Val's back is stronger and her neck tone improved. She sits straighter with little or no support. Bladder control better and only misses if she sleeps in.
Val is very bright and talkative. Jim reported after a family visit at the weekend all had noticed changes in her. Muscle tone improved - now soft and less squidgy. Skin feels beautiful and has good colour Feet not blue and are more sensitive. Losing weight - less fluid retention.
Doesn't need Ventalin anymore.
Val bright and lively. Sat her upright on the table with legs over side - first supported, then free. (Which showed her back was strong, her balance good and she was not afraid of falling forward). Introduced the yoga "bridge" - lifting the pelvis up supported on the feet - to help tone and strengthen as well as to develop hip mobility and muscle tone to the feet. Moved her bent legs side to side. Sat on wooden chair in the sunny studio with her feet on the floor and looking out at my dogs. Was able to roll a ball under her feet. Was able to bend forward and put her hands on the back of a chair - very stable and strong. When her family visited at Easter they were all thrilled with her wellness and brightness. She was full of family history and stories.
Val tells me she stood up when she got out of bed in the night. Jim was not happy about her midnight exploits. By mid-may she was sitting unsupported on a chair moving freely from hips with feet on the floor (even though her ankles are weak and collapsing inward). With Jim's and my support Val came to standing.
Surprise ! Val came in her walking shoes and I took this to mean a strengthening in her resolve and determination to try walking. A physio visit assessment suggested exercises to strengthen her legs if Val was motivated. Val is not keen on exercises!. She is happy to play at them with me but can't maintain interest at home.
Jim put Val on the floor with the hoist. She lay back with her head supported then experimented with rolling over onto her sides. It was easier rolling to the left than the right and Val really enjoyed the sense of freedom and mobility.
August 2004
Val was rolling over more easily in both directions and pulling herself half up to the crawling position, showing her strength, mobility and independence. She came up from lying to bend forward and untie her shoe laces (Big changes). When sitting she can now push foot into a shoe with a person or something solid for support and then tie up the laces. She has a strong motivation for independence and self sufficiency.
After almost one year Val can now
•Sit unsupported
•Tie her own shoes
•Crawl (pulling herself up into crawling)
•Swallow without using a straw
•Talk easily, and is socializing, interacting with her family
•Balance herself when sitting upright (can hold her own great grandchild)
•Stand up (weight bearing) with support
•(is) Continent / regular
•Motivated (to be involved) with increased self esteem and
•Has reduced or discontinued some medications (particularly
analgesics and benzodiazepines)
My work with Val has made an invaluable contribution to my experience as an Alexander Teacher . Val and I will continue to work together . The experience for Val is equally if not more important as she has said herself that her day to day life experience and well being has improved.
Some links:
How I used Alexander Technique to temporarily mitigate the effects of Parkinson's Disease
Parkinson's Balance and the Alexander Technique
How Alexander Technique can help Parkinson's Disease
Alexander Technique - Mayo Clinic
Lighten Up: Specific Postural Instructions Affect Axial Rigidity and Step Initiation in Patients with Parkinson's Disease
Randomized Controlled Trial of the Alexander Technique for Idiopathic Parkinson's Disease
Retention of Skills learned in Alexander Technique lessons
#ParkinsonsDisease #AlexanderTechnique
Recent Posts
Protecting and Strengthening our Knees
How much flexibility do we need?
A Report on our workshop for stroke survivors
- Patient Care & Health Information
- Diseases & Conditions
- Parkinson's disease
Parkinson's disease is a progressive disorder that affects the nervous system and the parts of the body controlled by the nerves. Symptoms start slowly. The first symptom may be a barely noticeable tremor in just one hand. Tremors are common, but the disorder also may cause stiffness or slowing of movement.
In the early stages of Parkinson's disease, your face may show little or no expression. Your arms may not swing when you walk. Your speech may become soft or slurred. Parkinson's disease symptoms worsen as your condition progresses over time.
Although Parkinson's disease can't be cured, medicines might significantly improve your symptoms. Occasionally, a health care professional may suggest surgery to regulate certain regions of your brain and improve your symptoms.
Products & Services
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Parkinson's disease symptoms can be different for everyone. Early symptoms may be mild and go unnoticed. Symptoms often begin on one side of the body and usually remain worse on that side, even after symptoms begin to affect the limbs on both sides.
Parkinson's symptoms may include:
- Tremor. Rhythmic shaking, called tremor, usually begins in a limb, often your hand or fingers. You may rub your thumb and forefinger back and forth. This is known as a pill-rolling tremor. Your hand may tremble when it's at rest. The shaking may decrease when you are performing tasks.
- Slowed movement, known as bradykinesia. Over time, Parkinson's disease may slow your movement, making simple tasks difficult and time-consuming. Your steps may become shorter when you walk. It may be difficult to get out of a chair. You may drag or shuffle your feet as you try to walk.
- Rigid muscles. Muscle stiffness may occur in any part of your body. The stiff muscles can be painful and limit your range of motion.
- Impaired posture and balance. Your posture may become stooped. Or you may fall or have balance problems as a result of Parkinson's disease.
- Loss of automatic movements. You may have a decreased ability to perform unconscious movements, including blinking, smiling or swinging your arms when you walk.
- Speech changes. You may speak softly or quickly, slur, or hesitate before talking. Your speech may be more of a monotone rather than have the usual speech patterns.
- Writing changes. It may become hard to write, and your writing may appear small.
When to see a doctor
See a health care professional if you have any of the symptoms associated with Parkinson's disease — not only to diagnose your condition but also to rule out other causes for your symptoms.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
In Parkinson's disease, certain nerve cells called neurons in the brain gradually break down or die. Many of the symptoms of Parkinson's are due to a loss of neurons that produce a chemical messenger in your brain called dopamine. When dopamine levels decrease, it causes irregular brain activity, leading to problems with movement and other symptoms of Parkinson's disease.
The cause of Parkinson's disease is unknown, but several factors appear to play a role, including:
Genes. Researchers have identified specific genetic changes that can cause Parkinson's disease. But these are uncommon except in rare cases with many family members affected by Parkinson's disease.
However, certain gene variations appear to increase the risk of Parkinson's disease but with a relatively small risk of Parkinson's disease for each of these genetic markers.
- Environmental triggers. Exposure to certain toxins or environmental factors may increase the risk of later Parkinson's disease, but the risk is small.
Researchers also have noted that many changes occur in the brains of people with Parkinson's disease, although it's not clear why these changes occur. These changes include:
- The presence of Lewy bodies. Clumps of specific substances within brain cells are microscopic markers of Parkinson's disease. These are called Lewy bodies, and researchers believe these Lewy bodies hold an important clue to the cause of Parkinson's disease.
- Alpha-synuclein found within Lewy bodies. Although many substances are found within Lewy bodies, scientists believe that an important one is the natural and widespread protein called alpha-synuclein, also called a-synuclein. It's found in all Lewy bodies in a clumped form that cells can't break down. This is currently an important focus among Parkinson's disease researchers. Researchers have found the clumped alpha-synuclein protein in the spinal fluid of people who later develop Parkinson's disease.
Risk factors
Risk factors for Parkinson's disease include:
- Age. Young adults rarely experience Parkinson's disease. It ordinarily begins in middle or late life, and the risk increases with age. People usually develop the disease around age 60 or older. If a young person does have Parkinson's disease, genetic counseling might be helpful in making family planning decisions. Work, social situations and medicine side effects are also different from those of an older person with Parkinson's disease and require special considerations.
- Heredity. Having a close relative with Parkinson's disease increases the chances that you'll develop the disease. However, your risks are still small unless you have many relatives in your family with Parkinson's disease.
- Sex. Men are more likely to develop Parkinson's disease than are women.
- Exposure to toxins. Ongoing exposure to herbicides and pesticides may slightly increase your risk of Parkinson's disease.
Complications
Parkinson's disease is often accompanied by these additional problems, which may be treatable:
- Thinking difficulties. You may experience cognitive problems, such as dementia, and thinking difficulties. These usually occur in the later stages of Parkinson's disease. Such cognitive problems aren't usually helped by medicines.
Depression and emotional changes. You may experience depression, sometimes in the very early stages. Receiving treatment for depression can make it easier to handle the other challenges of Parkinson's disease.
You also may experience other emotional changes, such as fear, anxiety or loss of motivation. Your health care team may give you medicine to treat these symptoms.
- Swallowing problems. You may develop difficulties with swallowing as your condition progresses. Saliva may accumulate in your mouth due to slowed swallowing, leading to drooling.
- Chewing and eating problems. Late-stage Parkinson's disease affects the muscles in the mouth, making chewing difficult. This can lead to choking and poor nutrition.
Sleep problems and sleep disorders. People with Parkinson's disease often have sleep problems, including waking up frequently throughout the night, waking up early or falling asleep during the day.
People also may experience rapid eye movement sleep behavior disorder, which involves acting out dreams. Medicines may improve your sleep.
- Bladder problems. Parkinson's disease may cause bladder problems, including being unable to control urine or having difficulty in urinating.
- Constipation. Many people with Parkinson's disease develop constipation, mainly due to a slower digestive tract.
You may also experience:
- Blood pressure changes. You may feel dizzy or lightheaded when you stand due to a sudden drop in blood pressure (orthostatic hypotension).
- Smell dysfunction. You may experience problems with your sense of smell. You may have trouble identifying certain odors or the difference between odors.
- Fatigue. Many people with Parkinson's disease lose energy and experience fatigue, especially later in the day. The cause isn't always known.
- Pain. Some people with Parkinson's disease experience pain, either in specific areas of their bodies or throughout their bodies.
- Sexual dysfunction. Some people with Parkinson's disease notice a decrease in sexual desire or performance.
Because the cause of Parkinson's is unknown, there are no proven ways to prevent the disease.
Some research has shown that regular aerobic exercise might reduce the risk of Parkinson's disease.
Some other research has shown that people who consume caffeine — which is found in coffee, tea and cola — get Parkinson's disease less often than those who don't drink it. Green tea also is related to a reduced risk of developing Parkinson's disease. However, it is still not known whether caffeine protects against getting Parkinson's or is related in some other way. Currently there is not enough evidence to suggest that drinking caffeinated beverages protects against Parkinson's.
Parkinson's disease care at Mayo Clinic
- Loscalzo J, et al., eds. Parkinson's disease. In: Harrison's Principles of Internal Medicine. 21st ed. McGraw Hill; 2022. https://accessmedicine.mhmedical.com. Accessed April 4, 2022.
- Parkinson's disease: Hope through research. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Parkinsons-Disease-Hope-Through-Research. Accessed April 4, 2022.
- Ferri FF. Parkinson disease. In: Ferri's Clinical Advisor 2022. Elsevier; 2022. https://www.clinicalkey.com. Accessed April 4, 2022.
- Chou KL. Diagnosis and differential diagnosis of Parkinson disease. https://www.uptodate.com/contents/search. Accessed April 4, 2022.
- Hornykiewicz O. The discovery of dopamine deficiency in the parkinsonian brain. Journal of Neural Transmission Supplementum. 2006; doi:10.1007/978-3-211-45295-0_3.
- Spindler MA, et al. Initial pharmacologic treatment of Parkinson disease. https://www.uptodate.com/contents/search. Accessed April 4, 2022.
- Relaxation techniques for health. National Center for Complementary and Integrative Health. https://nccih.nih.gov/health/stress/relaxation.htm. Accessed April 4, 2022.
- Taghizadeh M, et al. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson's disease: A randomized, double-blind, placebo-controlled trial. Neurochemistry International. 2017; doi:10.1016/j.neuint.2017.03.014.
- Parkinson's disease: Fitness counts. Parkinson's Foundation. http://www.parkinson.org/pd-library/books/fitness-counts. Accessed April 4, 2022.Green tea. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed April 4, 2022.
- Green tea. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed April 4, 2022.
- Tarsy D. Nonpharmacologic management of Parkinson disease. https://www.uptodate.com/contents/search. Accessed April 4, 2022.
- Caffeine. Natural medicines. https://naturalmedicines.therapeuticresearch.com. Accessed April 4, 2022.
- Jankovic J. Etiology and pathogenesis of Parkinson disease.
- Thomas A. Allscripts EPSi. Mayo Clinic. April 22, 2022.
- Post B, et al. Young onset Parkinson's disease: A modern and tailored approach. Journal of Parkinson's Disease. 2020; doi:10.3233/JPD-202135.
- Bower JH (expert opinion). Mayo Clinic. May 16, 2023.
- Robbins JA, et al. Swallowing and speech production in Parkinson's disease. Annals of Neurology.1986; doi:10.1002/ana.410190310.
- Hauser RA, et al. Orally inhaled levodopa (CVT-301) for early morning OFF periods in Parkinson's disease. Parkinsonism and Related Disorders. 2019; doi:10.1016/j.parkreldis.2019.03.026.
- Dashtipour K, et al. Speech disorders in Parkinson's disease: Pathophysiology, medical management and surgical approaches. Neurodegenerative Disease Management. 2018; doi:10.2217/nmt-2018-0021.
- Mishima T, et al. Personalized medicine in Parkinson's disease: New options for advanced treatments. Journal of Personalized Medicine. 2021; doi:10.3390/jpm11070650.
- Jenner P, et al. Istradefylline — A first generation adenosine A2A antagonist for the treatment of Parkinson's disease. Expert Review of Neurotherapeutics. 2021; doi:10.1080/14737175.2021.1880896.
- Isaacson SH, et al. Blinded SAPS-PD assessment after 10 weeks of pimavanserin treatment for Parkinson's disease psychosis. Journal of Parkinson's Disease. 2020; doi:10.3233/JPD-202047.
- Al-Shorafat DM, et al. B-blocker-induced tremor. Movement Disorders Clinical Practice. 2021; doi:10.1002/mdc3.13176.
- Haahr A, et al. 'Striving for normality' when coping with Parkinson's disease in everyday life. A metasynthesis. International Journal of Nursing Studies. 2021; doi:10.1016/j.ijnurstu.2021.103923.
- Mehanna R, et al. Age cutoff for early-onset Parkinson's disease: Recommendations from the International Parkinson and Movement Disorder Society task force on early-onset Parkinson's disease. Movement Disorders Clinical Practice. 2022; doi:10.1002/mdc3.13523.
- Siderowf A, et al. Assessment of heterogeneity among participants in the Parkinson's Progression Markers Initiative cohort using alpha-synuclein seed amplification: A cross-sectional study. The Lancet Neurology. 2023; doi:10.1016/S1474-4422(23)00109-6.
- Berg D, et al. Alpha-synuclein seed amplification and its uses in Parkinson's disease. The Lancet Neurology. 2023; doi:10.1016/S1474-4422(23)00124-2.
Associated Procedures
- Acupuncture
- Deep brain stimulation
- Massage therapy
- Parkinson's test
News from Mayo Clinic
- Empowering Lives: Navigating Parkinson's disease with hope June 11, 2024, 04:30 p.m. CDT
- Recognizing symptoms of Parkinson's disease April 13, 2024, 11:00 a.m. CDT
- Science Saturday: Removal of both ovaries in younger women associated with increased risk of Parkinson's Jan. 14, 2023, 12:00 p.m. CDT
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Phoenix/Scottsdale, Arizona, and Mayo Clinic in Jacksonville, Florida, have been ranked among the best Neurology & Neurosurgery hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be part of the cutting-edge research and care that's changing medicine.
Queensland researchers target gut health to slow or stop progression of Parkinson's disease
Australian researchers are working on developing drugs that target bugs in the guts of Parkinson's disease patients in a radical new treatment approach they hope will slow or even stop the progression of the debilitating illness.
Queensland University of Technology neuroscientist Richard Gordon said the research followed emerging evidence suggesting the gut was as important as the brain in the development of Parkinson's.
Associate Professor Gordon, based at the Translational Research Institute in Brisbane, said studies showed differences in the complex gut ecosystems of Parkinson's disease patients compared with healthy people.
He said people with Parkinson's disease were known to experience persistent inflammation and activation of the immune system, believed to be closely linked to an imbalance of microbes in their guts.
"The inflammation, over a prolonged period, has been shown to damage the vulnerable dopamine-producing neurons that are gradually lost in people with Parkinson's," Associate Professor Gordon said.
Two years ago, Ross Martin began to notice a tremor in his hand.
The 66-year-old Queenslander is not the first in his family to be diagnosed with Parkinson's — his uncle also had the disease.
"Being on a computer, you go to play a game or something [and] your left hand is hitting all the wrong keys," he said.
"It's the little things at the moment, but the longer-term things and knowing where I'm heading and what life I'm heading towards is something I don't want to think about too much."
He said the the study gave him hope.
"We are pro-science, and it's obvious the good work is being done," Mr Martin said.
Rise in cases linked to 'chemical exposure'
Parkinson's disease is a progressive movement disorder, characterised by degeneration of dopamine-producing neurons in the brain.
The decrease in dopamine levels results in impaired mobility – including tremors, stiffness of the arms and legs, slow movement, and poor balance.
Other symptoms can include an impaired sense of smell, disturbed sleep, anxiety and depression, fatigue, gut problems, and speech changes.
Drug treatments, such as levodopa, which increases the amount of dopamine in the brain, help alleviate some patients' symptoms rather than slow the progression of the illness.
In what he described as "a radical new way of thinking" about Parkinson's disease, Associate Professor Gordon's team has been awarded $4 million over four years by the US Department of Defense to work on new therapeutics targeting the gut microbiome.
He said military personnel were considered at increased risk of developing neurological conditions, such as Parkinson's disease, because of chemical exposures during their service.
"There is this rapid increase in the prevalence of Parkinson's globally," Associate Professor Gordon said.
"We believe it's linked to … chemical exposures."
The gut is a new target
The Queensland research will involve both human and animal studies to identify new classes of therapeutics to treat Parkinson's disease, first described more than 200 years ago by London doctor, James Parkinson.
Scientists will study blood, urine, and faecal samples from at least 70 Parkinson's patients and compare them to those of similarly aged healthy volunteers.
"One of the ways we study the gut microbiome is by sequencing the bacteria that's present in people's guts," Associate Professor Gordon said.
They hope to be able to identify so-called "healthy bugs" that may disappear in people with Parkinson's.
"Then we're going to use that knowledge to develop drugs, or improve the drugs that we have, to target the microbes rather than just target the brain, which we've done in the past," Associate Professor Gordon said.
'Bugs as drugs'
In what he termed a "bugs as drugs" approach, he said the team would also engineer bacteria and test their potential to slow or stop Parkinson's progression by altering the gut ecosystem.
"These studies would be done in animals initially," he said.
"Once we know that it's safe and it's effective the next phase of this work will take that towards clinical trials."
The research team includes scientists from QUT's School of Biomedical Sciences and neurologists from the Royal Brisbane and Women's and Princess Alexandra hospitals.
They will partner with researchers at the University of Georgia in the US.
An estimated 200,000 Australians have Parkinson's disease.
- X (formerly Twitter)
Related Stories
Deep brain electrodes, adjusted by wi-fi, are bringing 'miraculous' results to parkinson's patients.
Perfect Match helped them find each other 31 years ago. Now they're facing their greatest challenge
Parkinson's can show up in the way you move '15 to 20 years' before a diagnosis — could motion capture tech spot it?
- Academic Research
- Brain and Nervous System
- Doctors and Medical Professionals
- Medical Research
- Parkinson s Disease

- Wearable technology, smartphones may aid Parkinson’s research
Devices can be used to track symptoms, assess changes, study shows

by Patricia Inácio, PhD | June 18, 2024
Share this article:
Remote assessments using smartphones and smartwatches could be used to detect and monitor changes in the early stages of Parkinson’s disease , according to findings from the WATCH-PD study.
Over the course of one-year of follow-up, an Apple Watch paired with an iPhone was able to detect declines in gait and increase in tremors in early-stage patients.
“Digital measures hold the promise to provide objective, sensitive, real-world measures of disease progression in Parkinson’s disease,” Jamie Adams, MD, an associate professor of neurology at the University of Rochester Medical Center, and the study’s lead researcher, said in a university press release . “This study shows that data generated by smartwatches and smartphones can remotely monitor and detect changes in multiple domains of the disease. These digital assessments could help evaluate the efficacy of future therapies.”
The study, “ Using a smartwatch and smartphone to assess early Parkinson’s disease in the WATCH-PD study over 12 months ,” was published in njp Parkinson’s Disease.
Wearable technologies are offering a new way to monitor motor symptoms in Parkinson’s disease patients. While symptoms used to only be assessed in the clinic, wearable technologies allow for continuous, at-home monitoring of things like changes in gait and tremor. Smartwatches may even help identify people at risk for Parkinson’s years before their symptoms become evident. And finger tapping and voice recording can help detect early signs of speech impairment , a little known feature of the disease.
Data from the observational WATCH-PD study show that remote assessments of motor and nonmotor features using smartphones and smartwatches could be used in clinical trials to track the severity of Parkinson’s disease.
The study enrolled 82 early-stage, untreated patients (mean age, 63.3) and 50 aged-matched (mean age, 60.2 years) people without the disease who served as controls at more than a dozen sites across the U.S.
Companies partner on wearable apomorphine injection system
Monitoring parkinson’s symptoms remotely, digitally.
Here, researchers at the University of Rochester Medical Center, in New York, along with members of the Parkinson’s collaborative group Critical Path for Parkinson’s Consortium 3DT Initiative report the feasibility of digitally monitoring Parkinson’s manifestations over a year. Smartphone assessments were conducted at each clinic visit and at home every two weeks, with results showing patients saw significant declines in measures of gait, as assessed by arm swing, along with the number of daily steps. An increase in tremor and modest changes in speech were also reported.
For example, smartwatch assessments of arm swing in the clinic fell from a mean of 25.9 degrees to 19.9 degrees. At the beginning of the study, Parkinson’s patients walked significantly less than controls — a mean of 238 versus 362 steps an hour.
The proportion of patients having tremor while awake at home significantly increased from 19.3% at the study’s start to 25.6%, supporting the use of smartwatches as a “promising measure for assessing the efficacy of therapies aimed at reducing tremor,” the researchers wrote.
Digital speech composite scores, which are designed to capture changes in speech and language over time, assessed at home changed significantly after 12 months in patients, while no change was seen in healthy controls. The smartphone also detected speech changes even when clinicians didn’t notice any. The findings support using at-home speech monitoring to differentiate Parkinson’s patients from healthy controls, said the researchers.
WATCH-PD will continue to follow patients for 18 more months, an extension supported by the Michael J. Fox Foundation .
“On behalf of Critical Path Institute, we are delighted to see the astounding progress of this unique project,” said Diane Stephenson, PhD, executive director of Critical Path for Parkinson’s consortium and a co-author of the study. “The early and often feedback from regulators have shaped this study in ways that now can link the clinical meaningfulness of symptoms measured by digital health technologies to the voice of people with lived experience. By partnering with patients, regulators, industry, and academic experts this project is serving as a precedent for other disease areas to follow. ”
About the Author

Recent Posts
- Vitamin B1 for Parkinson’s disease wasn’t the miracle we’d hoped for
- Scientists discover how PINK1 pathway activates to protect cells
- A New Way to Reduce Stress: Probiotics
- Levodopa boosts brain connections to ease Parkinson’s resting tremor
Recommended reading
Genetic variants tied to mortality, motor progression in Parkinson’s

People with Parkinson’s tremor asked to take part in survey

Parkinson’s tremors have made handwriting impossible for my dad
Subscribe to our newsletter.
Get regular updates to your inbox.

IMAGES
VIDEO
COMMENTS
This mixed-methods case study explored the experiences of patients with PD in a palliative phase and their family caregivers, over a 12-month period. ... Palliative care in People with Parkinson's disease. Guidelines for professional healthcare workers on the assessment and management of palliative care needs in Parkinson's disease and ...
Parkinson's disease is a chronic and progressive. neurodegenerative disorder that affects millions of. people worldwide. It is characterized by a range of motor. symptoms, including tremors ...
Abstract. Thirty years ago, Parkinson disease was described as a shortage of the neurotransmitter dopamine. Today, understanding of this disorder includes possible genetic influences, premorbid and nonmotor issues, and a variety of neurologic, cognitive, and psychiatric symptoms. Using a case study, this article presents the current science of ...
The patient is an 84-year-old man with a 9-year history of Parkinson's disease *. History of Present Illness: The patient has experienced a recent deterioration of balance, gait, endurance, and strength. He was hospitalized for 12 days to monitor the deterioration and adjust medications accordingly.
Parkinson's Disease usually develops after the age of 40 years, with mean age of onset at 60 years. It is one of the most common neurodegenerative diseases among the aged, with approximately 60,000 new diagnoses each year in the United States. Between 5 and 15 percent of cases manifest before the age of 40, and are considered "young onset ...
This case study examines the diagnosis and treatment of a 65-year-old retired accountant, Mr. John Smith, who has been diagnosed with Parkinson's disease and has been experiencing significant difficulties with his symptoms despite treatment with medications and physical therapy. A literature review is also provided, exploring current research ...
"No One Can Tell Me How Parkinson's Disease Will Unfold": A Mixed Methods Case Study on Palliative Care for People with Parkinson's Disease and Their Family Caregivers J Parkinsons Dis. 2022;12(1) :207-219. ... Methods: A mixed methods case study design. Health care professionals included patients for whom the answer on the question "Would you ...
To optimize care for persons with PD, we envisage a personalized care management model that is tailored to each individual's needs and preferences, with the following core elements: (1) care coordination, (2) patient navigation, (3) information provision, (4) early detection of signs and symptoms through proactive monitoring, and (5) process ...
This paper aims to present a case study on the perspectives of one person with PD, a participant in a previously published qualitative study investigating the barriers and facilitators to medication adherence in PD. ... Colangeli M. High-dose thiamine as initial treatment for Parkinson's disease. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013 ...
Narrative medicine pinpoints loss of autonomy and stigma in Parkinson's disease. Barend W. Florijn. Raoul Kloppenborg. Bastiaan R. Bloem. Case Report Open Access 01 Nov 2023.
Introduction Following Alzheimer's disease, Parkinson's disease (PD) is the second-most common neurodegenerative disorder in the United States. Most people diagnosed with PD are age 60 years or older, however, an estimated 5 to 10 percent of people with PD are diagnosed before the age of 50. Approximately 500,000 Americans are diagnosed with PD, but given that many individuals go undiagnosed ...
A study titled "Mindfulness or meditation therapy for Parkinson's disease: A systematic review and meta‐analysis of randomized controlled trials" by Hongfu Lin, Ka Wai Tam, Yi-Chun Kuan explored the relation between Parkinson's disease (PD) and mindfulness. Parkinson's disease is the second most common neurodegenerative disorder worldwide.
This case study examines a 65-year-old retired accountant, Mr. John Smith, who was diagnosed with Parkinson's disease fi ve years ago. Mr. Smith has been treated with a combination of medications, including levodopa and carbidopa and physical therapy to manage his symptoms. However, his symptoms have not signifi cantly improved.
History. Chief Complaint: "My husband is slowing down and his hands won't stop shaking. History of Present Illness: Mr. R.D. is a 73 year old white male who was referred to the Neurology clinic by his PCP. Prior to retiring in 2009, Mr. R.D. was a math professor. In his free time, he enjoyed playing the piano and cooking.
Thirty years ago, Parkinson disease was described as a shortage of the neurotransmitter dopamine. Today, understanding of this disorder includes possible genetic influences, premorbid and nonmotor issues, and a variety of neurologic, cognitive, and psychiatric symptoms. Using a case study, this article presents the current science of Parkinson ...
4454. Case Study on Patient with Parkinson's Disease. Author: Dr. Rakesh Gupta 1 Consultant Physician (Y&N) 1 ,Siddappa Naragatti 2 Yoga Therapist 2. Central Council for Research in Yoga ...
Abstract. Objective: A 'case scenario' study on clinical decisions in progressing Parkinson's disease (PD) was developed to complement scientific evidence with the collective judgment of a panel of experts. Methods: The opinions of 9 experts in movement disorders on the appropriateness of 9 common pharmacological treatments for 33 hypothetical ...
Parkinson's disease and its disease-related burden on affected individuals and their relatives. Parkinson's disease (PD) is a progressive neurodegenerative disorder with a rapidly increasing prevalence that affects the lives of those affected at various levels [].In Germany, around 400,000 individuals are affected by PD, and the impact of PD will increase further due to the ageing ...
Study with Quizlet and memorize flashcards containing terms like Meet the Client: Leo White, a 64-year-old male, was diagnosed with Parkinson's disease 4 years ago. He has been married to his second wife, Nancy, for 10 years. Leo has 2 grown children who live out of state, and Nancy has one grown daughter who lives close by. Until recently, Leo and Nancy had always been active in the community.
Hallucinations, which are common in Parkinson's, can also occur, according to case studies. A 67-year-old man would see faceless, hooded or cloaked figures or animals while awake during the night.
Parkinson's affects about one million people in the U.S. and 10 million worldwide. The main finding in brains of people with PD is loss of dopaminergic neurons (these regulate movement and play a key role in Parkinson's progression) in an area of the brain known as the substantia nigra. Genetics. Genetics cause about 10 to 15% of all Parkinson's.
Young-onset Parkinson's disease (YOPD) occurs in people younger than 50 years of age. Most people with idiopathic, or typical, PD develop symptoms at 50 years of age or older. ... In fact, a recent study found that 65 percent of people with PD onset under 20 years old and 32 percent of people with onset between 20 and 30 had a genetic ...
Purpose To investigate the contributors to self-rated health in people with late-stage Parkinson's disease (PD) and cognitive impairment. Methods A secondary analysis of baseline data from the international Care of Late-Stage Parkinsonism (CLaSP) cohort study was conducted. Participants with PD and either dementia or mild cognitive impairment or MMSE < 24/30 in the absence of major ...
Understanding Parkinson's Disease. Parkinson's Disease is a progressive neurological disorder that affects movement and can impact speech and cognitive functions. Symptoms often include tremors, stiffness, difficulty with balance and coordination, and speech difficulties such as: Reduced range of tongue movement
Erin Foster, an associate professor of occupational therapy, and Scott Norris, MD, an associate professor of neurology, have established a site at Washington University School of Medicine in St. Louis for the Black and African American Connections to Parkinson's Disease (BLAAC PD) study, an international study aimed at understanding the gene changes that may lead to Parkinson's disease in ...
David Moore. Parkinson's Disease - A Case Study. Introducing Valma Scott. Val was diagnosed with Parkinson's disease over 10 years ago. She is 76 years of age and married to child hood sweetheart, Jim for 60 years. They have 6 children including 2 sets of twins and now 17 grandchildren.
Over 10 years' follow-up, 16 of the people predicted for Parkinson's went on to develop the disorder, researchers said. The new study was published Tuesday in the journal Nature Communications .
People usually develop the disease around age 60 or older. If a young person does have Parkinson's disease, genetic counseling might be helpful in making family planning decisions. Work, social situations and medicine side effects are also different from those of an older person with Parkinson's disease and require special considerations. Heredity.
The Queensland research will involve both human and animal studies to identify new classes of therapeutics to treat Parkinson's disease, first described more than 200 years ago by London doctor ...
The study, "Using a smartwatch and smartphone to assess early Parkinson's disease in the WATCH-PD study over 12 months," was published in njp Parkinson's Disease. Wearable technologies are offering a new way to monitor motor symptoms in Parkinson's disease patients.