An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

National Institute of Environmental Health Sciences
Your environment. your health., what is ethics in research & why is it important, by david b. resnik, j.d., ph.d..
December 23, 2020
The ideas and opinions expressed in this essay are the author’s own and do not necessarily represent those of the NIH, NIEHS, or US government.

When most people think of ethics (or morals), they think of rules for distinguishing between right and wrong, such as the Golden Rule ("Do unto others as you would have them do unto you"), a code of professional conduct like the Hippocratic Oath ("First of all, do no harm"), a religious creed like the Ten Commandments ("Thou Shalt not kill..."), or a wise aphorisms like the sayings of Confucius. This is the most common way of defining "ethics": norms for conduct that distinguish between acceptable and unacceptable behavior.
Most people learn ethical norms at home, at school, in church, or in other social settings. Although most people acquire their sense of right and wrong during childhood, moral development occurs throughout life and human beings pass through different stages of growth as they mature. Ethical norms are so ubiquitous that one might be tempted to regard them as simple commonsense. On the other hand, if morality were nothing more than commonsense, then why are there so many ethical disputes and issues in our society?
Alternatives to Animal Testing
Alternative test methods are methods that replace, reduce, or refine animal use in research and testing
Learn more about Environmental science Basics
One plausible explanation of these disagreements is that all people recognize some common ethical norms but interpret, apply, and balance them in different ways in light of their own values and life experiences. For example, two people could agree that murder is wrong but disagree about the morality of abortion because they have different understandings of what it means to be a human being.
Most societies also have legal rules that govern behavior, but ethical norms tend to be broader and more informal than laws. Although most societies use laws to enforce widely accepted moral standards and ethical and legal rules use similar concepts, ethics and law are not the same. An action may be legal but unethical or illegal but ethical. We can also use ethical concepts and principles to criticize, evaluate, propose, or interpret laws. Indeed, in the last century, many social reformers have urged citizens to disobey laws they regarded as immoral or unjust laws. Peaceful civil disobedience is an ethical way of protesting laws or expressing political viewpoints.
Another way of defining 'ethics' focuses on the disciplines that study standards of conduct, such as philosophy, theology, law, psychology, or sociology. For example, a "medical ethicist" is someone who studies ethical standards in medicine. One may also define ethics as a method, procedure, or perspective for deciding how to act and for analyzing complex problems and issues. For instance, in considering a complex issue like global warming , one may take an economic, ecological, political, or ethical perspective on the problem. While an economist might examine the cost and benefits of various policies related to global warming, an environmental ethicist could examine the ethical values and principles at stake.
See ethics in practice at NIEHS
Read latest updates in our monthly Global Environmental Health Newsletter

Many different disciplines, institutions , and professions have standards for behavior that suit their particular aims and goals. These standards also help members of the discipline to coordinate their actions or activities and to establish the public's trust of the discipline. For instance, ethical standards govern conduct in medicine, law, engineering, and business. Ethical norms also serve the aims or goals of research and apply to people who conduct scientific research or other scholarly or creative activities. There is even a specialized discipline, research ethics, which studies these norms. See Glossary of Commonly Used Terms in Research Ethics and Research Ethics Timeline .
There are several reasons why it is important to adhere to ethical norms in research. First, norms promote the aims of research , such as knowledge, truth, and avoidance of error. For example, prohibitions against fabricating , falsifying, or misrepresenting research data promote the truth and minimize error.
Join an NIEHS Study
See how we put research Ethics to practice.
Visit Joinastudy.niehs.nih.gov to see the various studies NIEHS perform.

Second, since research often involves a great deal of cooperation and coordination among many different people in different disciplines and institutions, ethical standards promote the values that are essential to collaborative work , such as trust, accountability, mutual respect, and fairness. For example, many ethical norms in research, such as guidelines for authorship , copyright and patenting policies , data sharing policies, and confidentiality rules in peer review, are designed to protect intellectual property interests while encouraging collaboration. Most researchers want to receive credit for their contributions and do not want to have their ideas stolen or disclosed prematurely.
Third, many of the ethical norms help to ensure that researchers can be held accountable to the public . For instance, federal policies on research misconduct, conflicts of interest, the human subjects protections, and animal care and use are necessary in order to make sure that researchers who are funded by public money can be held accountable to the public.
Fourth, ethical norms in research also help to build public support for research. People are more likely to fund a research project if they can trust the quality and integrity of research.
Finally, many of the norms of research promote a variety of other important moral and social values , such as social responsibility, human rights, animal welfare, compliance with the law, and public health and safety. Ethical lapses in research can significantly harm human and animal subjects, students, and the public. For example, a researcher who fabricates data in a clinical trial may harm or even kill patients, and a researcher who fails to abide by regulations and guidelines relating to radiation or biological safety may jeopardize his health and safety or the health and safety of staff and students.
Codes and Policies for Research Ethics
Given the importance of ethics for the conduct of research, it should come as no surprise that many different professional associations, government agencies, and universities have adopted specific codes, rules, and policies relating to research ethics. Many government agencies have ethics rules for funded researchers.
- National Institutes of Health (NIH)
- National Science Foundation (NSF)
- Food and Drug Administration (FDA)
- Environmental Protection Agency (EPA)
- US Department of Agriculture (USDA)
- Singapore Statement on Research Integrity
- American Chemical Society, The Chemist Professional’s Code of Conduct
- Code of Ethics (American Society for Clinical Laboratory Science)
- American Psychological Association, Ethical Principles of Psychologists and Code of Conduct
- Statement on Professional Ethics (American Association of University Professors)
- Nuremberg Code
- World Medical Association's Declaration of Helsinki
Ethical Principles
The following is a rough and general summary of some ethical principles that various codes address*:

Strive for honesty in all scientific communications. Honestly report data, results, methods and procedures, and publication status. Do not fabricate, falsify, or misrepresent data. Do not deceive colleagues, research sponsors, or the public.

Objectivity
Strive to avoid bias in experimental design, data analysis, data interpretation, peer review, personnel decisions, grant writing, expert testimony, and other aspects of research where objectivity is expected or required. Avoid or minimize bias or self-deception. Disclose personal or financial interests that may affect research.

Keep your promises and agreements; act with sincerity; strive for consistency of thought and action.

Carefulness
Avoid careless errors and negligence; carefully and critically examine your own work and the work of your peers. Keep good records of research activities, such as data collection, research design, and correspondence with agencies or journals.

Share data, results, ideas, tools, resources. Be open to criticism and new ideas.
Transparency
Disclose methods, materials, assumptions, analyses, and other information needed to evaluate your research.

Accountability
Take responsibility for your part in research and be prepared to give an account (i.e. an explanation or justification) of what you did on a research project and why.

Intellectual Property
Honor patents, copyrights, and other forms of intellectual property. Do not use unpublished data, methods, or results without permission. Give proper acknowledgement or credit for all contributions to research. Never plagiarize.

Confidentiality
Protect confidential communications, such as papers or grants submitted for publication, personnel records, trade or military secrets, and patient records.

Responsible Publication
Publish in order to advance research and scholarship, not to advance just your own career. Avoid wasteful and duplicative publication.

Responsible Mentoring
Help to educate, mentor, and advise students. Promote their welfare and allow them to make their own decisions.

Respect for Colleagues
Respect your colleagues and treat them fairly.

Social Responsibility
Strive to promote social good and prevent or mitigate social harms through research, public education, and advocacy.

Non-Discrimination
Avoid discrimination against colleagues or students on the basis of sex, race, ethnicity, or other factors not related to scientific competence and integrity.

Maintain and improve your own professional competence and expertise through lifelong education and learning; take steps to promote competence in science as a whole.

Know and obey relevant laws and institutional and governmental policies.

Animal Care
Show proper respect and care for animals when using them in research. Do not conduct unnecessary or poorly designed animal experiments.

Human Subjects protection
When conducting research on human subjects, minimize harms and risks and maximize benefits; respect human dignity, privacy, and autonomy; take special precautions with vulnerable populations; and strive to distribute the benefits and burdens of research fairly.
* Adapted from Shamoo A and Resnik D. 2015. Responsible Conduct of Research, 3rd ed. (New York: Oxford University Press).
Ethical Decision Making in Research
Although codes, policies, and principles are very important and useful, like any set of rules, they do not cover every situation, they often conflict, and they require interpretation. It is therefore important for researchers to learn how to interpret, assess, and apply various research rules and how to make decisions and act ethically in various situations. The vast majority of decisions involve the straightforward application of ethical rules. For example, consider the following case:
The research protocol for a study of a drug on hypertension requires the administration of the drug at different doses to 50 laboratory mice, with chemical and behavioral tests to determine toxic effects. Tom has almost finished the experiment for Dr. Q. He has only 5 mice left to test. However, he really wants to finish his work in time to go to Florida on spring break with his friends, who are leaving tonight. He has injected the drug in all 50 mice but has not completed all of the tests. He therefore decides to extrapolate from the 45 completed results to produce the 5 additional results.
Many different research ethics policies would hold that Tom has acted unethically by fabricating data. If this study were sponsored by a federal agency, such as the NIH, his actions would constitute a form of research misconduct , which the government defines as "fabrication, falsification, or plagiarism" (or FFP). Actions that nearly all researchers classify as unethical are viewed as misconduct. It is important to remember, however, that misconduct occurs only when researchers intend to deceive : honest errors related to sloppiness, poor record keeping, miscalculations, bias, self-deception, and even negligence do not constitute misconduct. Also, reasonable disagreements about research methods, procedures, and interpretations do not constitute research misconduct. Consider the following case:
Dr. T has just discovered a mathematical error in his paper that has been accepted for publication in a journal. The error does not affect the overall results of his research, but it is potentially misleading. The journal has just gone to press, so it is too late to catch the error before it appears in print. In order to avoid embarrassment, Dr. T decides to ignore the error.
Dr. T's error is not misconduct nor is his decision to take no action to correct the error. Most researchers, as well as many different policies and codes would say that Dr. T should tell the journal (and any coauthors) about the error and consider publishing a correction or errata. Failing to publish a correction would be unethical because it would violate norms relating to honesty and objectivity in research.
There are many other activities that the government does not define as "misconduct" but which are still regarded by most researchers as unethical. These are sometimes referred to as " other deviations " from acceptable research practices and include:
- Publishing the same paper in two different journals without telling the editors
- Submitting the same paper to different journals without telling the editors
- Not informing a collaborator of your intent to file a patent in order to make sure that you are the sole inventor
- Including a colleague as an author on a paper in return for a favor even though the colleague did not make a serious contribution to the paper
- Discussing with your colleagues confidential data from a paper that you are reviewing for a journal
- Using data, ideas, or methods you learn about while reviewing a grant or a papers without permission
- Trimming outliers from a data set without discussing your reasons in paper
- Using an inappropriate statistical technique in order to enhance the significance of your research
- Bypassing the peer review process and announcing your results through a press conference without giving peers adequate information to review your work
- Conducting a review of the literature that fails to acknowledge the contributions of other people in the field or relevant prior work
- Stretching the truth on a grant application in order to convince reviewers that your project will make a significant contribution to the field
- Stretching the truth on a job application or curriculum vita
- Giving the same research project to two graduate students in order to see who can do it the fastest
- Overworking, neglecting, or exploiting graduate or post-doctoral students
- Failing to keep good research records
- Failing to maintain research data for a reasonable period of time
- Making derogatory comments and personal attacks in your review of author's submission
- Promising a student a better grade for sexual favors
- Using a racist epithet in the laboratory
- Making significant deviations from the research protocol approved by your institution's Animal Care and Use Committee or Institutional Review Board for Human Subjects Research without telling the committee or the board
- Not reporting an adverse event in a human research experiment
- Wasting animals in research
- Exposing students and staff to biological risks in violation of your institution's biosafety rules
- Sabotaging someone's work
- Stealing supplies, books, or data
- Rigging an experiment so you know how it will turn out
- Making unauthorized copies of data, papers, or computer programs
- Owning over $10,000 in stock in a company that sponsors your research and not disclosing this financial interest
- Deliberately overestimating the clinical significance of a new drug in order to obtain economic benefits
These actions would be regarded as unethical by most scientists and some might even be illegal in some cases. Most of these would also violate different professional ethics codes or institutional policies. However, they do not fall into the narrow category of actions that the government classifies as research misconduct. Indeed, there has been considerable debate about the definition of "research misconduct" and many researchers and policy makers are not satisfied with the government's narrow definition that focuses on FFP. However, given the huge list of potential offenses that might fall into the category "other serious deviations," and the practical problems with defining and policing these other deviations, it is understandable why government officials have chosen to limit their focus.
Finally, situations frequently arise in research in which different people disagree about the proper course of action and there is no broad consensus about what should be done. In these situations, there may be good arguments on both sides of the issue and different ethical principles may conflict. These situations create difficult decisions for research known as ethical or moral dilemmas . Consider the following case:
Dr. Wexford is the principal investigator of a large, epidemiological study on the health of 10,000 agricultural workers. She has an impressive dataset that includes information on demographics, environmental exposures, diet, genetics, and various disease outcomes such as cancer, Parkinson’s disease (PD), and ALS. She has just published a paper on the relationship between pesticide exposure and PD in a prestigious journal. She is planning to publish many other papers from her dataset. She receives a request from another research team that wants access to her complete dataset. They are interested in examining the relationship between pesticide exposures and skin cancer. Dr. Wexford was planning to conduct a study on this topic.
Dr. Wexford faces a difficult choice. On the one hand, the ethical norm of openness obliges her to share data with the other research team. Her funding agency may also have rules that obligate her to share data. On the other hand, if she shares data with the other team, they may publish results that she was planning to publish, thus depriving her (and her team) of recognition and priority. It seems that there are good arguments on both sides of this issue and Dr. Wexford needs to take some time to think about what she should do. One possible option is to share data, provided that the investigators sign a data use agreement. The agreement could define allowable uses of the data, publication plans, authorship, etc. Another option would be to offer to collaborate with the researchers.
The following are some step that researchers, such as Dr. Wexford, can take to deal with ethical dilemmas in research:
What is the problem or issue?
It is always important to get a clear statement of the problem. In this case, the issue is whether to share information with the other research team.
What is the relevant information?
Many bad decisions are made as a result of poor information. To know what to do, Dr. Wexford needs to have more information concerning such matters as university or funding agency or journal policies that may apply to this situation, the team's intellectual property interests, the possibility of negotiating some kind of agreement with the other team, whether the other team also has some information it is willing to share, the impact of the potential publications, etc.
What are the different options?
People may fail to see different options due to a limited imagination, bias, ignorance, or fear. In this case, there may be other choices besides 'share' or 'don't share,' such as 'negotiate an agreement' or 'offer to collaborate with the researchers.'
How do ethical codes or policies as well as legal rules apply to these different options?
The university or funding agency may have policies on data management that apply to this case. Broader ethical rules, such as openness and respect for credit and intellectual property, may also apply to this case. Laws relating to intellectual property may be relevant.
Are there any people who can offer ethical advice?
It may be useful to seek advice from a colleague, a senior researcher, your department chair, an ethics or compliance officer, or anyone else you can trust. In the case, Dr. Wexford might want to talk to her supervisor and research team before making a decision.
After considering these questions, a person facing an ethical dilemma may decide to ask more questions, gather more information, explore different options, or consider other ethical rules. However, at some point he or she will have to make a decision and then take action. Ideally, a person who makes a decision in an ethical dilemma should be able to justify his or her decision to himself or herself, as well as colleagues, administrators, and other people who might be affected by the decision. He or she should be able to articulate reasons for his or her conduct and should consider the following questions in order to explain how he or she arrived at his or her decision:
- Which choice will probably have the best overall consequences for science and society?
- Which choice could stand up to further publicity and scrutiny?
- Which choice could you not live with?
- Think of the wisest person you know. What would he or she do in this situation?
- Which choice would be the most just, fair, or responsible?
After considering all of these questions, one still might find it difficult to decide what to do. If this is the case, then it may be appropriate to consider others ways of making the decision, such as going with a gut feeling or intuition, seeking guidance through prayer or meditation, or even flipping a coin. Endorsing these methods in this context need not imply that ethical decisions are irrational, however. The main point is that human reasoning plays a pivotal role in ethical decision-making but there are limits to its ability to solve all ethical dilemmas in a finite amount of time.
Promoting Ethical Conduct in Science

Do U.S. research institutions meet or exceed federal mandates for instruction in responsible conduct of research? A national survey

Read about U.S. research instutuins follow federal manadates for ethics in research
Learn more about NIEHS Research
Most academic institutions in the US require undergraduate, graduate, or postgraduate students to have some education in the responsible conduct of research (RCR) . The NIH and NSF have both mandated training in research ethics for students and trainees. Many academic institutions outside of the US have also developed educational curricula in research ethics
Those of you who are taking or have taken courses in research ethics may be wondering why you are required to have education in research ethics. You may believe that you are highly ethical and know the difference between right and wrong. You would never fabricate or falsify data or plagiarize. Indeed, you also may believe that most of your colleagues are highly ethical and that there is no ethics problem in research..
If you feel this way, relax. No one is accusing you of acting unethically. Indeed, the evidence produced so far shows that misconduct is a very rare occurrence in research, although there is considerable variation among various estimates. The rate of misconduct has been estimated to be as low as 0.01% of researchers per year (based on confirmed cases of misconduct in federally funded research) to as high as 1% of researchers per year (based on self-reports of misconduct on anonymous surveys). See Shamoo and Resnik (2015), cited above.
Clearly, it would be useful to have more data on this topic, but so far there is no evidence that science has become ethically corrupt, despite some highly publicized scandals. Even if misconduct is only a rare occurrence, it can still have a tremendous impact on science and society because it can compromise the integrity of research, erode the public’s trust in science, and waste time and resources. Will education in research ethics help reduce the rate of misconduct in science? It is too early to tell. The answer to this question depends, in part, on how one understands the causes of misconduct. There are two main theories about why researchers commit misconduct. According to the "bad apple" theory, most scientists are highly ethical. Only researchers who are morally corrupt, economically desperate, or psychologically disturbed commit misconduct. Moreover, only a fool would commit misconduct because science's peer review system and self-correcting mechanisms will eventually catch those who try to cheat the system. In any case, a course in research ethics will have little impact on "bad apples," one might argue.
According to the "stressful" or "imperfect" environment theory, misconduct occurs because various institutional pressures, incentives, and constraints encourage people to commit misconduct, such as pressures to publish or obtain grants or contracts, career ambitions, the pursuit of profit or fame, poor supervision of students and trainees, and poor oversight of researchers (see Shamoo and Resnik 2015). Moreover, defenders of the stressful environment theory point out that science's peer review system is far from perfect and that it is relatively easy to cheat the system. Erroneous or fraudulent research often enters the public record without being detected for years. Misconduct probably results from environmental and individual causes, i.e. when people who are morally weak, ignorant, or insensitive are placed in stressful or imperfect environments. In any case, a course in research ethics can be useful in helping to prevent deviations from norms even if it does not prevent misconduct. Education in research ethics is can help people get a better understanding of ethical standards, policies, and issues and improve ethical judgment and decision making. Many of the deviations that occur in research may occur because researchers simply do not know or have never thought seriously about some of the ethical norms of research. For example, some unethical authorship practices probably reflect traditions and practices that have not been questioned seriously until recently. If the director of a lab is named as an author on every paper that comes from his lab, even if he does not make a significant contribution, what could be wrong with that? That's just the way it's done, one might argue. Another example where there may be some ignorance or mistaken traditions is conflicts of interest in research. A researcher may think that a "normal" or "traditional" financial relationship, such as accepting stock or a consulting fee from a drug company that sponsors her research, raises no serious ethical issues. Or perhaps a university administrator sees no ethical problem in taking a large gift with strings attached from a pharmaceutical company. Maybe a physician thinks that it is perfectly appropriate to receive a $300 finder’s fee for referring patients into a clinical trial.
If "deviations" from ethical conduct occur in research as a result of ignorance or a failure to reflect critically on problematic traditions, then a course in research ethics may help reduce the rate of serious deviations by improving the researcher's understanding of ethics and by sensitizing him or her to the issues.
Finally, education in research ethics should be able to help researchers grapple with the ethical dilemmas they are likely to encounter by introducing them to important concepts, tools, principles, and methods that can be useful in resolving these dilemmas. Scientists must deal with a number of different controversial topics, such as human embryonic stem cell research, cloning, genetic engineering, and research involving animal or human subjects, which require ethical reflection and deliberation.
The Importance of Ethics in Research Essay
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
Introduction
Factors that increase vulnerability of subjects to research abuse, the solution to reduce vulnerability of subjects to research abuse.
In science and medical research, ethics is essential in enhancing the safety and well-being of the subjects or participants. Different studies globally expose vulnerable populations or subjects to abuse, affecting their overall health. In the same case, researchers are employing diverse strategies to enhance ethics and reduce subjects’ vulnerabilities to negative implications of studies and abuse. For these reasons, it is essential to examine the factors that enhance subjects’ vulnerability to abuse and maltreatment during scientific studies. These reasons are economic and financial problems, impractical hope, improper patient advocacy, as well as non-compliance to research ethics. Contrarily, encouraging compliance with ethical principles during research would reduce subjects’ susceptibility to abuse and negative research implications.
Economic and financial issues are among the factors that increase subjects’ vulnerability to abuse during research. Kelly (2013) indicates that “people struggling to put food on the table and a roof over their heads” are vulnerable to abuse during clinical trials and pharmaceutical studies. Washington (2008) also notes that “jobless white men turned their noses at the disgusting work and partly pay” when referring to a perilous clinical experiment conducted in 1935 by the University of Pennsylvania. The sentiment indicates that the university conducted unethical and dangerous medical research on subjects with economic and financial problems (Washington, 2008). The phrase “when you see an opportunity to feed your starving family, […], or get treatment for a terminal disease?” also indicates how financial issues make subjects partake in unsafe clinical trials (Kelly, 2013). Thus, these studies increase the health risks, burden, disparity, and complications among subjects from developing and low-income countries as well as communities.
Unrealistic hope is another factor that increases the risks of abuse among subjects of research activities. According to Washington (2008), patients desperate for healing from diseases, surviving, living longer, and stressed about their health conditions are vulnerable to abuse during medical studies. The phrase “was described to him as his last chance at a meaningful life” shows that James Quinn was a victim of research abuse because of hoped to live a productive life after artificial heart implantation (Washington, 2008). The quote, “Do you have a choice about participating when you see an opportunity to (…) get treatment for a terminal disease,” indicates that subjects are always hopeful of improving their health after clinical studies (Kelly, 2008). This makes patients accept risky treatments or clinical trials that are mentally or physically abusive to their health, hoping to enhance their lifespan.
Improper patient advocacy and education are also major factors that increase the dangers of abusing subjects during clinical research. Washington (2008) questions how medical researchers and providers empower patients about the risks and benefits of clinical trials before treatments. Washington (2008) asks, “But are such warnings offered in a fair and intelligible manner?” The quote proves that researchers obtain patients’ consent for treatments when they are mentally, emotionally, and cognitively incapable of making informed decisions about these interventions. Kelly (2013) also agrees that healthcare researchers fail to provide accurate and quality patient information before clinical trials. The sentence “one of the most commonly cited ethical qualms with clinical trials tends to be misinformation” indicates that patients’ advocacy teams misinform subjects before clinical trials (Kelly, 2013). The wrong information affects the ability of patients to make informed decisions about participating or not partaking in medical studies.
Finally, non-compliance to research ethics and regulations among researchers also makes subjects vulnerable to abuse during studies. Washington (2008) indicates that “the informed consent process consists of much more than obtaining a patient signature on a piece of paper.” The quote implies that medical researchers are violating the informed consent ethics of research that requires patients’ participation only after knowing all the risks and benefits of a treatment. In the statement, “one of the most commonly cited ethical qualms with clinical trials tends to be misinformation,” Kelly (2013) supports Washington (2008) about the voluntary and involuntary deception of subjects to participate in their studies. Generally, violation of ethics makes vulnerable subjects partake in medical or scientific studies that harm their health and those around them.
To reduce the susceptibility of patients or subjects to abusive medical and scientific studies, adherence or compliance to research ethics is essential. For instance, Kelly (2013) suggests that continuous investigation by ethics boards on researchers violating ethical requirements would enhance compliance with research regulations, integrity, and morals. The statement “informed consent is an ongoing process of patient notification and education” also implies that continuous patient advocacy, edification, and communication before treatments and clinical trials is essential in reducing non-compliance to research ethics (Washington, 2008). Moreover, when seeing patient permission for research inclusion, Washington (2008) proposes that researchers should make patients aware of treatment risks through the phrase “the scientist must […] knows all the known risks and must inform the subject proxy.” This will make subjects decide to participate in research after knowing the benefits and negative implications on them and the people around them.
Conclusively, the lack of compliance with ethical research principles among scientists and the economic issues of patients make them susceptible to abuse during studies. Unrealistic hope, desperation, and inappropriate patient advocacy and education make subjects vulnerable to research abuse. Based on these, developing, implementing, and complying with research ethics is a feasible approach to reducing the vulnerability of research subjects to abuse. Therefore, patient abuse is a systemic issue in medical and scientific studies. This means that this problem requires universal or systemic solutions to achieve the desired outcome of protecting patients’ interests and well-being during and after research.
Kelly, S. (2013). Testing drugs on the developing world . The Atlantic. Web.
Washington, H. (2008). Medical apartheid: The dark history of medical experimentation on Black Americans from colonial times to the present. Psychiatric Services , 58 (10), 1380-1381.
- Employment Non-Compliance in Australia in 2008-12
- Donnie Darko by Richard Kelly Review
- Feldman and Kelly's Views on Disagreement
- Practice-Informed Research: Case Analysis
- Ethical Issues Associated with Online Research
- Ethical Principles in Research
- Discussion: Ethics and Fashion
- Abortion Rights: The Ethical Issues
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, April 1). The Importance of Ethics in Research. https://ivypanda.com/essays/the-importance-of-ethics-in-research/
"The Importance of Ethics in Research." IvyPanda , 1 Apr. 2024, ivypanda.com/essays/the-importance-of-ethics-in-research/.
IvyPanda . (2024) 'The Importance of Ethics in Research'. 1 April.
IvyPanda . 2024. "The Importance of Ethics in Research." April 1, 2024. https://ivypanda.com/essays/the-importance-of-ethics-in-research/.
1. IvyPanda . "The Importance of Ethics in Research." April 1, 2024. https://ivypanda.com/essays/the-importance-of-ethics-in-research/.
Bibliography
IvyPanda . "The Importance of Ethics in Research." April 1, 2024. https://ivypanda.com/essays/the-importance-of-ethics-in-research/.
- Essay Guides
- Other Essays
- How to Write an Ethics Paper: Guide & Ethical Essay Examples
- Speech Topics
- Basics of Essay Writing
- Essay Topics
- Main Academic Essays
- Research Paper Topics
- Basics of Research Paper Writing
- Miscellaneous
- Chicago/ Turabian
- Data & Statistics
- Methodology
- Admission Writing Tips
- Admission Advice
- Other Guides
- Student Life
- Studying Tips
- Understanding Plagiarism
- Academic Writing Tips
- Basics of Dissertation & Thesis Writing
- Research Paper Guides
- Formatting Guides
- Basics of Research Process
- Admission Guides
- Dissertation & Thesis Guides
How to Write an Ethics Paper: Guide & Ethical Essay Examples

Table of contents
Use our free Readability checker
An ethics essay is a type of academic writing that explores ethical issues and dilemmas. Students should evaluates them in terms of moral principles and values. The purpose of an ethics essay is to examine the moral implications of a particular issue, and provide a reasoned argument in support of an ethical perspective.
Writing an essay about ethics is a tough task for most students. The process involves creating an outline to guide your arguments about a topic and planning your ideas to convince the reader of your feelings about a difficult issue. If you still need assistance putting together your thoughts in composing a good paper, you have come to the right place. We have provided a series of steps and tips to show how you can achieve success in writing. This guide will tell you how to write an ethics paper using ethical essay examples to understand every step it takes to be proficient. In case you don’t have time for writing, get in touch with our professional essay writers for hire . Our experts work hard to supply students with excellent essays.
What Is an Ethics Essay?
An ethics essay uses moral theories to build arguments on an issue. You describe a controversial problem and examine it to determine how it affects individuals or society. Ethics papers analyze arguments on both sides of a possible dilemma, focusing on right and wrong. The analysis gained can be used to solve real-life cases. Before embarking on writing an ethical essay, keep in mind that most individuals follow moral principles. From a social context perspective, these rules define how a human behaves or acts towards another. Therefore, your theme essay on ethics needs to demonstrate how a person feels about these moral principles. More specifically, your task is to show how significant that issue is and discuss if you value or discredit it.
Purpose of an Essay on Ethics
The primary purpose of an ethics essay is to initiate an argument on a moral issue using reasoning and critical evidence. Instead of providing general information about a problem, you present solid arguments about how you view the moral concern and how it affects you or society. When writing an ethical paper, you demonstrate philosophical competence, using appropriate moral perspectives and principles.
Things to Write an Essay About Ethics On
Before you start to write ethics essays, consider a topic you can easily address. In most cases, an ethical issues essay analyzes right and wrong. This includes discussing ethics and morals and how they contribute to the right behaviors. You can also talk about work ethic, code of conduct, and how employees promote or disregard the need for change. However, you can explore other areas by asking yourself what ethics mean to you. Think about how a recent game you watched with friends started a controversial argument. Or maybe a newspaper that highlighted a story you felt was misunderstood or blown out of proportion. This way, you can come up with an excellent topic that resonates with your personal ethics and beliefs.
Ethics Paper Outline
Sometimes, you will be asked to submit an outline before writing an ethics paper. Creating an outline for an ethics paper is an essential step in creating a good essay. You can use it to arrange your points and supporting evidence before writing. It also helps organize your thoughts, enabling you to fill any gaps in your ideas. The outline for an essay should contain short and numbered sentences to cover the format and outline. Each section is structured to enable you to plan your work and include all sources in writing an ethics paper. An ethics essay outline is as follows:
- Background information
- Thesis statement
- Restate thesis statement
- Summarize key points
- Final thoughts on the topic
Using this outline will improve clarity and focus throughout your writing process.
Ethical Essay Structure
Ethics essays are similar to other essays based on their format, outline, and structure. An ethical essay should have a well-defined introduction, body, and conclusion section as its structure. When planning your ideas, make sure that the introduction and conclusion are around 20 percent of the paper, leaving the rest to the body. We will take a detailed look at what each part entails and give examples that are going to help you understand them better. Refer to our essay structure examples to find a fitting way of organizing your writing.
Ethics Paper Introduction
An ethics essay introduction gives a synopsis of your main argument. One step on how to write an introduction for an ethics paper is telling about the topic and describing its background information. This paragraph should be brief and straight to the point. It informs readers what your position is on that issue. Start with an essay hook to generate interest from your audience. It can be a question you will address or a misunderstanding that leads up to your main argument. You can also add more perspectives to be discussed; this will inform readers on what to expect in the paper.
Ethics Essay Introduction Example
You can find many ethics essay introduction examples on the internet. In this guide, we have written an excellent extract to demonstrate how it should be structured. As you read, examine how it begins with a hook and then provides background information on an issue.
In this example, the first sentence of the introduction makes a claim or uses a question to hook the reader.
Ethics Essay Thesis Statement
An ethics paper must contain a thesis statement in the first paragraph. Learning how to write a thesis statement for an ethics paper is necessary as readers often look at it to gauge whether the essay is worth their time.
When you deviate away from the thesis, your whole paper loses meaning. In ethics essays, your thesis statement is a roadmap in writing, stressing your position on the problem and giving reasons for taking that stance. It should focus on a specific element of the issue being discussed. When writing a thesis statement, ensure that you can easily make arguments for or against its stance.
Ethical Paper Thesis Example
Look at this example of an ethics paper thesis statement and examine how well it has been written to state a position and provide reasons for doing so:
The above thesis statement example is clear and concise, indicating that this paper will highlight the effects of dishonesty in society. Moreover, it focuses on aspects of personal and professional relationships.
Ethics Essay Body
The body section is the heart of an ethics paper as it presents the author's main points. In an ethical essay, each body paragraph has several elements that should explain your main idea. These include:
- A topic sentence that is precise and reiterates your stance on the issue.
- Evidence supporting it.
- Examples that illustrate your argument.
- A thorough analysis showing how the evidence and examples relate to that issue.
- A transition sentence that connects one paragraph to another with the help of essay transitions .
When you write an ethics essay, adding relevant examples strengthens your main point and makes it easy for others to understand and comprehend your argument.
Body Paragraph for Ethics Paper Example
A good body paragraph must have a well-defined topic sentence that makes a claim and includes evidence and examples to support it. Look at part of an example of ethics essay body paragraph below and see how its idea has been developed:
Ethics Essay Conclusion
A concluding paragraph shares the summary and overview of the author's main arguments. Many students need clarification on what should be included in the essay conclusion and how best to get a reader's attention. When writing an ethics paper conclusion, consider the following:
- Restate the thesis statement to emphasize your position.
- Summarize its main points and evidence.
- Final thoughts on the issue and any other considerations.
You can also reflect on the topic or acknowledge any possible challenges or questions that have not been answered. A closing statement should present a call to action on the problem based on your position.
Sample Ethics Paper Conclusion
The conclusion paragraph restates the thesis statement and summarizes the arguments presented in that paper. The sample conclusion for an ethical essay example below demonstrates how you should write a concluding statement.
In the above extract, the writer gives final thoughts on the topic, urging readers to adopt honest behavior.
How to Write an Ethics Paper?
As you learn how to write an ethics essay, it is not advised to immediately choose a topic and begin writing. When you follow this method, you will get stuck or fail to present concrete ideas. A good writer understands the importance of planning. As a fact, you should organize your work and ensure it captures key elements that shed more light on your arguments. Hence, following the essay structure and creating an outline to guide your writing process is the best approach. In the following segment, we have highlighted step-by-step techniques on how to write a good ethics paper.
1. Pick a Topic
Before writing ethical papers, brainstorm to find ideal topics that can be easily debated. For starters, make a list, then select a title that presents a moral issue that may be explained and addressed from opposing sides. Make sure you choose one that interests you. Here are a few ideas to help you search for topics:
- Review current trends affecting people.
- Think about your personal experiences.
- Study different moral theories and principles.
- Examine classical moral dilemmas.
Once you find a suitable topic and are ready, start to write your ethics essay, conduct preliminary research, and ascertain that there are enough sources to support it.
2. Conduct In-Depth Research
Once you choose a topic for your essay, the next step is gathering sufficient information about it. Conducting in-depth research entails looking through scholarly journals to find credible material. Ensure you note down all sources you found helpful to assist you on how to write your ethics paper. Use the following steps to help you conduct your research:
- Clearly state and define a problem you want to discuss.
- This will guide your research process.
- Develop keywords that match the topic.
- Begin searching from a wide perspective. This will allow you to collect more information, then narrow it down by using the identified words above.
3. Develop an Ethics Essay Outline
An outline will ease up your writing process when developing an ethic essay. As you develop a paper on ethics, jot down factual ideas that will build your paragraphs for each section. Include the following steps in your process:
- Review the topic and information gathered to write a thesis statement.
- Identify the main arguments you want to discuss and include their evidence.
- Group them into sections, each presenting a new idea that supports the thesis.
- Write an outline.
- Review and refine it.
Examples can also be included to support your main arguments. The structure should be sequential, coherent, and with a good flow from beginning to end. When you follow all steps, you can create an engaging and organized outline that will help you write a good essay.
4. Write an Ethics Essay
Once you have selected a topic, conducted research, and outlined your main points, you can begin writing an essay . Ensure you adhere to the ethics paper format you have chosen. Start an ethics paper with an overview of your topic to capture the readers' attention. Build upon your paper by avoiding ambiguous arguments and using the outline to help you write your essay on ethics. Finish the introduction paragraph with a thesis statement that explains your main position. Expand on your thesis statement in all essay paragraphs. Each paragraph should start with a topic sentence and provide evidence plus an example to solidify your argument, strengthen the main point, and let readers see the reasoning behind your stance. Finally, conclude the essay by restating your thesis statement and summarizing all key ideas. Your conclusion should engage the reader, posing questions or urging them to reflect on the issue and how it will impact them.
5. Proofread Your Ethics Essay
Proofreading your essay is the last step as you countercheck any grammatical or structural errors in your essay. When writing your ethic paper, typical mistakes you could encounter include the following:
- Spelling errors: e.g., there, they’re, their.
- Homophone words: such as new vs. knew.
- Inconsistencies: like mixing British and American words, e.g., color vs. color.
- Formatting issues: e.g., double spacing, different font types.
While proofreading your ethical issue essay, read it aloud to detect lexical errors or ambiguous phrases that distort its meaning. Verify your information and ensure it is relevant and up-to-date. You can ask your fellow student to read the essay and give feedback on its structure and quality.
Ethics Essay Examples
Writing an essay is challenging without the right steps. There are so many ethics paper examples on the internet, however, we have provided a list of free ethics essay examples below that are well-structured and have a solid argument to help you write your paper. Click on them and see how each writing step has been integrated. Ethics essay example 1
Ethics essay example 2
Ethics essay example 3
Ethics essay example 4
College ethics essay example 5
Ethics Essay Writing Tips
When writing papers on ethics, here are several tips to help you complete an excellent essay:
- Choose a narrow topic and avoid broad subjects, as it is easy to cover the topic in detail.
- Ensure you have background information. A good understanding of a topic can make it easy to apply all necessary moral theories and principles in writing your paper.
- State your position clearly. It is important to be sure about your stance as it will allow you to draft your arguments accordingly.
- When writing ethics essays, be mindful of your audience. Provide arguments that they can understand.
- Integrate solid examples into your essay. Morality can be hard to understand; therefore, using them will help a reader grasp these concepts.
Bottom Line on Writing an Ethics Paper
Creating this essay is a common exercise in academics that allows students to build critical skills. When you begin writing, state your stance on an issue and provide arguments to support your position. This guide gives information on how to write an ethics essay as well as examples of ethics papers. Remember to follow these points in your writing:
- Create an outline highlighting your main points.
- Write an effective introduction and provide background information on an issue.
- Include a thesis statement.
- Develop concrete arguments and their counterarguments, and use examples.
- Sum up all your key points in your conclusion and restate your thesis statement.
Contact our academic writing platform and have your challenge solved. Here, you can order essays and papers on any topic and enjoy top quality.

Daniel Howard is an Essay Writing guru. He helps students create essays that will strike a chord with the readers.
You may also like

Imagine living in a world where people only lie, and honesty is becoming a scarce commodity. Indeed, modern society is facing this reality as truth and deception can no longer be separated. Technology has facilitated a quick transmission of voluminous information, whereas it's hard separating facts from opinions.
The moral implications of dishonesty are far-reaching as they undermine trust, integrity, and other foundations of society, damaging personal and professional relationships.
Honesty is an essential component of professional integrity. In many fields, trust and credibility are crucial for professionals to build relationships and success. For example, a doctor who is dishonest about a potential side effect of a medication is not only acting unethically but also putting the health and well-being of their patients at risk. Similarly, a dishonest businessman could achieve short-term benefits but will lose their client’s trust.
In conclusion, the implications of dishonesty and the importance of honesty in our lives cannot be overstated. Honesty builds solid relationships, effective communication, and better decision-making. This essay has explored how dishonesty impacts people and that we should value honesty. We hope this essay will help readers assess their behavior and work towards being more honest in their lives.
When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open.
- PLOS Biology
- PLOS Climate
- PLOS Complex Systems
- PLOS Computational Biology
- PLOS Digital Health
- PLOS Genetics
- PLOS Global Public Health
- PLOS Medicine
- PLOS Mental Health
- PLOS Neglected Tropical Diseases
- PLOS Pathogens
- PLOS Sustainability and Transformation
- PLOS Collections
Understanding Scientific and Research Ethics

How to pass journal ethics checks to ensure a smooth submission and publication process
Reputable journals screen for ethics at submission—and inability to pass ethics checks is one of the most common reasons for rejection. Unfortunately, once a study has begun, it’s often too late to secure the requisite ethical reviews and clearances. Learn how to prepare for publication success by ensuring your study meets all ethical requirements before work begins.
The underlying principles of scientific and research ethics
Scientific and research ethics exist to safeguard human rights, ensure that we treat animals respectfully and humanely, and protect the natural environment.
The specific details may vary widely depending on the type of research you’re conducting, but there are clear themes running through all research and reporting ethical requirements:
Documented 3rd party oversight
- Consent and anonymity
- Full transparency
If you fulfill each of these broad requirements, your manuscript should sail through any journal’s ethics check.

If your research is 100% theoretical, you might be able to skip this one. But if you work with living organisms in any capacity—whether you’re administering a survey, collecting data from medical records, culturing cells, working with zebrafish, or counting plant species in a ring—oversight and approval by an ethics committee is a prerequisite for publication. This oversight can take many different forms:
For human studies and studies using human tissue or cells, obtain approval from your institutional review board (IRB). Register clinical trials with the World Health Organization (WHO) or International Committee of Medical Journal Editors (ICMJE). For animal research consult with your institutional animal care and use committee (IACUC). Note that there may be special requirements for non-human primates, cephalopods, and other specific species, as well as for wild animals. For field studies , anthropology and paleontology , the type of permission required will depend on many factors, like the location of the study, whether the site is publicly or privately owned, possible impacts on endangered or protected species, and local permit requirements.
TIP: You’re not exempt until your committee tells you so
Even if you think your study probably doesn’t require approval, submit it to the review board anyway. Many journals won’t consider retrospective approvals. Obtaining formal approval or an exemption up front is worth it to ensure your research is eligible for publication in the future.
TIP: Keep your committee records close
Clearly label your IRB/IACUC paperwork, permit numbers, and any participant permission forms (including blank copies), and keep them in a safe place. You will need them when you submit to a journal. Providing these details proactively as part of your initial submission can minimize delays and get your manuscript through journal checks and into the hands of reviewers sooner.
Consent & anonymity
Obtaining consent from human subjects.
You may not conduct research on human beings unless the subjects understand what you are doing and agree to be a part of your study. If you work with human subjects, you must obtain informed written consent from the participants or their legal guardians.
There are many circumstances where extra care may be required in order to obtain consent. The more vulnerable the population you are working with the stricter these guidelines will be. For example, your IRB may have special requirements for working with minors, the elderly, or developmentally delayed participants. Remember that these rules may vary from country to country. Providing a link to the relevant legal reference in your area can help speed the screening and approval process.
TIP: What if you are working with a population where reading and writing aren’t common?
Alternatives to written consent (such as verbal consent or a thumbprint) are acceptable in some cases, but consent still has to be clearly documented. To ensure eligibility for publication, be sure to:
- Get IRB approval for obtaining verbal rather than written consent
- Be prepared to explain why written consent could not be obtained
- Keep a copy of the script you used to obtain this consent, and record when consent was obtained for your own records
Consent and reporting for human tissue and cell lines
Consent from the participant or their next-of-kin is also required for the use of human tissue and cell lines. This includes discarded tissue, for example the by-products of surgery.
When working with cell lines transparency and good record keeping are essential. Here are some basic guidelines to bear in mind:
- When working with established cell lines , cite the published article where the cell line was first described.
- If you’re using repository or commercial cell lines , explain exactly which ones, and provide the catalog or repository number.
- If you received a cell line from a colleague , rather than directly from a repository or company, be sure to mention it. Explain who gifted the cells and when.
- For a new cell line obtained from a colleague there may not be a published article to cite yet, but the work to generate the cell line must meet the usual requirements of consent—even if it was carried out by another research group. You’ll need to provide a copy of your colleagues’ IRB approval and details about the consent procedures in order to publish the work.
Finally, you’re obliged to keep your human subjects anonymous and to protect any identifying information in photos and raw data. Remove all names, birth dates, detailed addresses, or job information from files you plan to share. Blur faces and tattoos in any images. Details such as geography (city/country), gender, age, or profession may be shared at a generalized level and in aggregate. Read more about standards for de-identifying datasets in The BMJ .
TIP: Anonymity can be important in field work too
Be careful about revealing geographic data in fieldwork. You don’t want to tip poachers off to the location of the endangered elephant population you studied, or expose petroglyphs to vandalism.
Full Transparency
No matter the discipline, transparent reporting of methods, results, data, software and code is essential to ethical research practice. Transparency is also key to the future reproducibility of your work.
When you submit your study to a journal, you’ll be asked to provide a variety of statements certifying that you’ve obtained the appropriate permissions and clearances, and explaining how you conducted the work. You may also be asked to provide supporting documentation, including field records and raw data. Provide as much detail as you can at this stage. Clear and complete disclosure statements will minimize back-and-forth with the journal, helping your submission to clear ethics checks and move on to the assessment stage sooner.
TIP: Save that data
As you work, be sure to clearly label and organize your data files in a way that will make sense to you later. As close as you are to the work as you conduct your study, remember that two years could easily pass between capturing your data and publishing an article reporting the results. You don’t want to be stuck piecing together confusing records in order to create figures and data files for repositories.
Read our full guide to preparing data for submission .
Keep in mind that scientific and research ethics are always evolving. As laws change and as we learn more about influence, implicit bias and animal sentience, the scientific community continues to strive to elevate our research practice.
A checklist to ensure you’re ethics-check ready
Before you begin your research
Obtain approval from your IRB, IACUC or other approving body
Obtain written informed consent from human participants, guardians or next-of-kin
Obtain permits or permission from property owners, or confirm that permits are not required
Label and save all of records
As you work
Adhere strictly to the protocols approved by your committee
Clearly label your data, and store it in a way that will make sense to your future self
As you write, submit and deposit your results
Be ready to cite specific approval organizations, permit numbers, cell lines, and other details in your ethics statement and in the methods section of your manuscript
Anonymize all participant data (including human and in some cases animal or geographic data)
If a figure does include identifying information (e.g. a participant’s face) obtain special consent
The contents of the Peer Review Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
The contents of the Writing Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
There’s a lot to consider when deciding where to submit your work. Learn how to choose a journal that will help your study reach its audience, while reflecting your values as a researcher…
Public Health Notes
Your partner for better health, research ethics: definition, principles and advantages.
October 13, 2020 Kusum Wagle Epidemiology 0

Table of Contents
What is Research Ethics?
- Ethics are the set of rules that govern our expectations of our own and others’ behavior.
- Research ethics are the set of ethical guidelines that guides us on how scientific research should be conducted and disseminated.
- Research ethics govern the standards of conduct for scientific researchers It is the guideline for responsibly conducting the research.
- Research that implicates human subjects or contributors rears distinctive and multifaceted ethical, legitimate, communal and administrative concerns.
- Research ethics is unambiguously concerned in the examination of ethical issues that are upraised when individuals are involved as participants in the study.
- Research ethics committee/Institutional Review Board (IRB) reviews whether the research is ethical enough or not to protect the rights, dignity and welfare of the respondents.
Objectives of Research Ethics:
- The first and comprehensive objective – to guard/protect human participants, their dignity, rights and welfare .
- The second objective – to make sure that research is directed in a manner that assists welfares of persons, groups and/or civilization as a whole.
- The third objective – to inspect particular research events and schemes for their ethical reliability, considering issues such as the controlling risk, protection of privacy and the progression of informed consent.
Principles of Research Ethics:
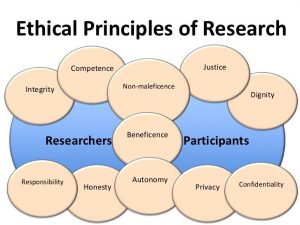
The general principles of research ethics are:
|
| Being honest with the beneficiaries and respondents. Being honest about the findings and methodology of the research. Being honest with other direct and indirect stakeholders. |
| Ensuring honesty and sincerity. Fulfilling agreements and promises. Do not create false expectations or make false promises. | |
|
| Avoiding bias in experimental design, data analysis, data interpretation, peer review, and other aspects of research. |
| It includes: | |
| Maximize the benefits of the participants. Ethical obligation to maximize possible benefits and to minimize possible harms to the respondents. | |
| Do no harm. Minimize harm/s or risks to the human. Ensure privacy, autonomy and dignity. | |
| Responsibly publishing to promote and uptake research or knowledge. No duplicate publication. | |
| It means keeping the participant anonymous. It involves not revealing the name, caste or any other information about the participants that may reveal his/her identity.
| |
| Protecting confidential information, personnel records. It includes information such as: | |
| Avoid discrimination on the basis of age, sex, race, ethnicity or other factors that are violation of human rights and are not related to the study. | |
| Be open to sharing results, data and other resources. Also accept encouraging comments and constructive feedback. | |
| Be careful about the possible error and biases. Give credit to the intellectual property of others. Always paraphrase while referring to others article, writing. Never plagiarize. | |
| The obligation to distribute benefits and burdens fairly, to treat equals equally, and to give reasons for differential treatment based on widely accepted criteria for just ways to distribute benefits and burdens. |
Broad Categorization of Principles of Research Ethics:
Broadly categorizing, there are mainly five principles of research ethics:
1. MINIMIZING THE RISK OF HARM
It is necessary to minimize any sort of harm to the participants. There are a number of forms of harm that participants can be exposed to. They are:
- Bodily harm to contributors.
- Psychological agony and embarrassment.
- Social drawback.
- Violation of participant’s confidentiality and privacy.
In order to minimize the risk of harm, the researcher/data collector should:
- Obtain informed consent from participants.
- Protecting anonymity and confidentiality of participants.
- Avoiding misleading practices when planning research.
- Providing participants with the right to withdraw .
2. OBTAINING INFORMED CONSENT
One of the fundamentals of research ethics is the notion of informed consent .
Informed consent means that a person knowingly, voluntarily and intelligently gives consent to participate in a research.
Informed consent means that the participants should be well-informed about the:
- Introduction and objective of the research
- Purpose of the discussion
- Anticipated advantages, benefits/harm from the research (if any)
- Use of research
- Their role in research
- Methods which will be used to protect anonymity and confidentiality of the participant
- Freedom to not answer any question/withdraw from the research
- Who to contact if the participant need additional information about the research
3. PROTECTING ANONYMITY AND CONFIDENTIALITY
Protecting the anonymity and confidentiality of research participants is an additionally applied constituent of research ethics.
Protecting anonymity: It means keeping the participant anonymous. It involves not revealing the name, caste or any other information about the participants that may reveal his/her identity.
Maintaining confidentiality: It refers to ensuring that the information given by the participant are confidential and not shared with anyone, except the research team. It is also about keeping the information secretly from other people.
4. AVOIDING MISLEADING PRACTICES
- The researcher should avoid all the deceptive and misleading practices that might misinform the respondent.
- It includes avoiding all the activities like communicating wrong messages, giving false assurance, giving false information etc.
5. PROVIDING THE RIGHT TO WITHDRAW
- Participants have to have the right to withdraw at any point of the research.
- When any respondent decides on to withdraw from the research, they should not be stressed or forced in any manner to try to discontinue them from withdrawing.
Apart from the above-mentioned ethics, other ethical aspects things that must be considered while doing research are:
Protection of vulnerable groups of people:
- Vulnerability is one distinctive feature of people incapable to protect their moralities and wellbeing. Vulnerable groups comprise captive populations (detainees, established, students, etc.), mentally ill persons, and aged people, children, critically ill or dying, poor, with learning incapacities, sedated or insensible.
- Their participation in research can be endorsed to their incapability to give an informed consent and to the need for their further safety and sensitivity from the research/researcher as they are in a greater risk of being betrayed, exposed or forced to participate.
Skills of the researcher:
- Researchers should have the basic skills and familiarity for the specific study to be carried out and be conscious of the bounds of personal competence in research.
- Any lack of knowledge in the area under research must be clearly specified.
- Inexperienced researchers should work under qualified supervision that has to be revised by an ethics commission.
Advantages of Research Ethics:
- Research ethics promote the aims of research.
- It increases trust among the researcher and the respondent.
- It is important to adhere to ethical principles in order to protect the dignity, rights and welfare of research participants.
- Researchers can be held accountable and answerable for their actions.
- Ethics promote social and moral values.
- Promote s the ambitions of research, such as understanding, veracity, and dodging of error.
- Ethical standards uphold the values that are vital to cooperative work , such as belief, answerability, mutual respect, and impartiality.
- Ethical norms in research also aid to construct public upkeep for research. People are more likely to trust a research project if they can trust the worth and reliability of research.
Limitations of Research Ethics:
For subjects:
- Possibilities to physical integrity, containing those linked with experimental drugs and dealings and with other involvements that will be used in the study (e.g. measures used to observe research participants, such as blood sampling, X-rays or lumbar punctures).
- Psychological risks: for example, a questionnaire may perhaps signify a risk if it fears traumatic events or happenings that are especially traumatic.
- Social, legal and economic risks : for example, if personal information collected during a study is unintentionally released, participants might face a threat of judgment and stigmatization.
- Certain tribal or inhabitant groups may possibly suffer from discrimination or stigmatization, burdens because of research, typically if associates of those groups are recognized as having a greater-than-usual risk of devouring a specific disease.
- The research may perhaps have an influence on the prevailing health system: for example, human and financial capitals dedicated to research may distract attention from other demanding health care necessities in the community.
How can we ensure ethics at different steps of research?
The following process helps to ensure ethics at different steps of research:
- Collect the facts and talk over intellectual belongings openly
- Outline the ethical matters
- Detect the affected parties (stakeholders)
- Ascertain the forfeits
- Recognize the responsibilities (principles, rights, justice)
- Contemplate your personality and truthfulness
- Deliberate innovatively about possible actions
- Respect privacy and confidentiality
- Resolve on the appropriate ethical action and be willing to deal with divergent point of view.
References and For More Information:
http://dissertation.laerd.com/principles-of-research-ethics.php
https://researchethics.ca/what-is-research-ethics/
https://www.who.int/ethics/Ethics_basic_concepts_ENG.pdf
https://www.niehs.nih.gov/research/resources/bioethics/whatis/index.cfm
https://research.ku.edu/sites/research.ku.edu/files/docs/EESE_EthicalDecisionmakingFramework.pdf
https://www.who.int/ethics/research/en/
https://www.ufrgs.br/bioetica/cioms2008.pdf
https://www.who.int/ethics/research/en/#:~:text=WHO%20Research%20Ethics%20Review%20Committee,financially%20or%20technically%20by%20WHO .
https://www.who.int/reproductivehealth/topics/ethics/review_bodies_guide_serg/en/
https://www.who.int/ethics/indigenous_peoples/en/index13.html
https://www.who.int/bulletin/archives/80(2)114.pdf
https://www.who.int/about/ethics
https://www.slideshare.net/uqudent/introduction-to-research-ethics
https://libguides.library.cityu.edu.hk/researchmethods/ethics#:~:text=Methods%20by%20Subject-,What%20is%20Research%20Ethics%3F,ensure%20a%20high%20ethical%20standard .
https://www.apa.org/monitor/jan03/principles
https://www.hsj.gr/medicine/what-are-the-major-ethical-issues-in-conducting-research-is-there-a-conflict-between-the-research-ethics-and-the-nature-of-nursing.php?aid=3485
https://www.skillsyouneed.com/learn/research-ethics.html
- advantages of research ethics
- difference between confidentiality and anonymity in research
- minimizing the risk of harm in research
- obtaining informed consent in research
- principles of research ethics
- PROTECTING ANONYMITY AND CONFIDENTIALITY
- what are the advantages of research ethics
- what are the limitations of research ethics
- what are the principles of research ethics
- what is obtaining informed consent in research
- what is research ethics
- what is right to withdraw in research
- what is ROTECTING ANONYMITY AND CONFIDENTIALITY in research
Copyright © 2024 | WordPress Theme by MH Themes
- Original article
- Open access
- Published: 13 July 2021
Assisting you to advance with ethics in research: an introduction to ethical governance and application procedures
- Shivadas Sivasubramaniam 1 ,
- Dita Henek Dlabolová 2 ,
- Veronika Kralikova 3 &
- Zeenath Reza Khan 3
International Journal for Educational Integrity volume 17 , Article number: 14 ( 2021 ) Cite this article
18k Accesses
12 Citations
4 Altmetric
Metrics details
Ethics and ethical behaviour are the fundamental pillars of a civilised society. The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law. In fact, ethics gets precedence with anything that would include, affect, transform, or influence upon individuals, communities or any living creatures. Many institutions within Europe have set up their own committees to focus on or approve activities that have ethical impact. In contrast, lesser-developed countries (worldwide) are trying to set up these committees to govern their academia and research. As the first European consortium established to assist academic integrity, European Network for Academic Integrity (ENAI), we felt the importance of guiding those institutions and communities that are trying to conduct research with ethical principles. We have established an ethical advisory working group within ENAI with the aim to promote ethics within curriculum, research and institutional policies. We are constantly researching available data on this subject and committed to help the academia to convey and conduct ethical behaviour. Upon preliminary review and discussion, the group found a disparity in understanding, practice and teaching approaches to ethical applications of research projects among peers. Therefore, this short paper preliminarily aims to critically review the available information on ethics, the history behind establishing ethical principles and its international guidelines to govern research.
The paper is based on the workshop conducted in the 5th International conference Plagiarism across Europe and Beyond, in Mykolas Romeris University, Lithuania in 2019. During the workshop, we have detailed a) basic needs of an ethical committee within an institution; b) a typical ethical approval process (with examples from three different universities); and c) the ways to obtain informed consent with some examples. These are summarised in this paper with some example comparisons of ethical approval processes from different universities. We believe this paper will provide guidelines on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Introduction
Ethics and ethical behaviour (often linked to “responsible practice”) are the fundamental pillars of a civilised society. Ethical behaviour with integrity is important to maintain academic and research activities. It affects everything we do, and gets precedence with anything that would include/affect, transform, or impact upon individuals, communities or any living creatures. In other words, ethics would help us improve our living standards (LaFollette, 2007 ). The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law, but is also gaining recognition in all disciplines engaged in research. Therefore, institutions are expected to develop ethical guidelines in research to maintain quality, initiate/own integrity and above all be transparent to be successful by limiting any allegation of misconduct (Flite and Harman, 2013 ). This is especially true for higher education organisations that promote research and scholarly activities. Many European institutions have developed their own regulations for ethics by incorporating international codes (Getz, 1990 ). The lesser developed countries are trying to set up these committees to govern their academia and research. World Health Organization has stated that adhering to “ ethical principles … [is central and important]... in order to protect the dignity, rights and welfare of research participants ” (WHO, 2021 ). Ethical guidelines taught to students can help develop ethical researchers and members of society who uphold values of ethical principles in practice.
As the first European-wide consortium established to assist academic integrity (European Network for Academic Integrity – ENAI), we felt the importance of guiding those institutions and communities that are trying to teach, research, and include ethical principles by providing overarching understanding of ethical guidelines that may influence policy. Therefore, we set up an advisory working group within ENAI in 2018 to support matters related to ethics, ethical committees and assisting on ethics related teaching activities.
Upon preliminary review and discussion, the group found a disparity in understanding, practice and teaching approaches to ethical applications among peers. This became the premise for this research paper. We first carried out a literature survey to review and summarise existing ethical governance (with historical perspectives) and procedures that are already in place to guide researchers in different discipline areas. By doing so, we attempted to consolidate, document and provide important steps in a typical ethical application process with example procedures from different universities. Finally, we attempted to provide insights and findings from practical workshops carried out at the 5th International Conference Plagiarism across Europe and Beyond, in Mykolas Romeris University, Lithuania in 2019, focussing on:
• highlighting the basic needs of an ethical committee within an institution,
• discussing and sharing examples of a typical ethical approval process,
• providing guidelines on the ways to teach research ethics with some examples.
We believe this paper provides guidelines on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Background literature survey
Responsible research practice (RRP) is scrutinised by the aspects of ethical principles and professional standards (WHO’s Code of Conduct for responsible Research, 2017). The Singapore statement on research integrity (The Singapore Statement on Research integrity, 2010) has provided an internationally acceptable guidance for RRP. The statement is based on maintaining honesty, accountability, professional courtesy in all aspects of research and maintaining fairness during collaborations. In other words, it does not simply focus on the procedural part of the research, instead covers wider aspects of “integrity” beyond the operational aspects (Israel and Drenth, 2016 ).
Institutions should focus on providing ethical guidance based on principles and values reflecting upon all aspects/stages of research (from the funding application/project development stage upto or beyond project closing stage). Figure 1 summarizes the different aspects/stages of a typical research and highlights the needs of RRP in compliance with ethical governance at each stage with examples (the figure is based on Resnik, 2020 ; Žukauskas et al., 2018 ; Anderson, 2011 ; Fouka and Mantzorou, 2011 ).
Summary of the enabling ethical governance at different stages of research. Note that it is imperative for researchers to proactively consider the ethical implications before, during and after the actual research process. The summary shows that RRP should be in line with ethical considerations even long before the ethical approval stage
Individual responsibilities to enhance RRP
As explained in Fig. 1 , a successfully governed research should consider ethics at the planning stages prior to research. Many international guidance are compatible in enforcing/recommending 14 different “responsibilities” that were first highlighted in the Singapore Statement (2010) for researchers to follow and achieve competency in RRP. In order to understand the purpose and the expectation of these ethical guidelines, we have carried out an initial literature survey on expected individual responsibilities. These are summarised in Table 1 .
By following these directives, researchers can carry out accountable research by maximising ethical self-governance whilst minimising misconducts. In our own experiences of working with many researchers, their focus usually revolves around ethical “clearance” rather than behaviour. In other words, they perceive this as a paper exercise rather than trying to “own” ethical behaviour in everything they do. Although the ethical principles and responsibilities are explicitly highlighted in the majority of international guidelines [such as UK’s Research Governance Policy (NICE, 2018 ), Australian Government’s National Statement on Ethical Conduct in Human Research (Difn website a - National Statement on Ethical Conduct in Human Research (NSECHR), 2018 ), the Singapore Statement (2010) etc.]; and the importance of holistic approach has been argued in ethical decision making, many researchers and/or institutions only focus on ethics linked to the procedural aspects.
Studies in the past have also highlighted inconsistencies in institutional guidelines pointing to the fact that these inconsistencies may hinder the predicted research progress (Desmond & Dierickx 2021 ; Alba et al., 2020 ; Dellaportas et al., 2014 ; Speight 2016 ). It may also be possible that these were and still are linked to the institutional perceptions/expectations or the pre-empting contextual conditions that are imposed by individual countries. In fact, it is interesting to note many research organisations and HE institutions establish their own policies based on these directives.
Research governance - origins, expectations and practices
Ethical governance in clinical medicine helps us by providing a structure for analysis and decision-making. By providing workable definitions of benefits and risks as well as the guidance for evaluating/balancing benefits over risks, it supports the researchers to protect the participants and the general population.
According to the definition given by National Institute of Clinical care Excellence, UK (NICE 2018 ), “ research governance can be defined as the broad range of regulations, principles and standards of good practice that ensure high quality research ”. As stated above, our literature-based research survey showed that most of the ethical definitions are basically evolved from the medical field and other disciplines have utilised these principles to develop their own ethical guidance. Interestingly, historical data show that the medical research has been “self-governed” or in other words implicated by the moral behaviour of individual researchers (Fox 2017 ; Shaw et al., 2005 ; Getz, 1990 ). For example, early human vaccination trials conducted in 1700s used the immediate family members as test subjects (Fox, 2017 ). Here the moral justification might have been the fact that the subjects who would have been at risk were either the scientists themselves or their immediate families but those who would reap the benefits from the vaccination were the general public/wider communities. However, according to the current ethical principles, this assumption is entirely not acceptable.
Historically, ambiguous decision-making and resultant incidences of research misconduct have led to the need for ethical research governance in as early as the 1940’s. For instance, the importance of an international governance was realised only after the World War II, when people were astonished to note the unethical research practices carried out by Nazi scientists. As a result of this, in 1947 the Nuremberg code was published. The code mainly focussed on the following:
Informed consent and further insisted the research involving humans should be based on prior animal work,
The anticipated benefits should outweigh the risk,
Research should be carried out only by qualified scientists must conduct research,
Avoiding physical and mental suffering and.
Avoiding human research that would result in which death or disability.
(Weindling, 2001 ).
Unfortunately, it was reported that many researchers in the USA and elsewhere considered the Nuremberg code as a document condemning the Nazi atrocities, rather than a code for ethical governance and therefore ignored these directives (Ghooi, 2011 ). It was only in 1964 that the World Medical Association published the Helsinki Declaration, which set the stage for ethical governance and the implementation of the Institutional Review Board (IRB) process (Shamoo and Irving, 1993 ). This declaration was based on Nuremberg code. In addition, the declaration also paved the way for enforcing research being conducted in accordance with these guidelines.
Incidentally, the focus on research/ethical governance gained its momentum in 1974. As a result of this, a report on ethical principles and guidelines for the protection of human subjects of research was published in 1979 (The Belmont Report, 1979 ). This report paved the way to the current forms of ethical governance in biomedical and behavioural research by providing guidance.
Since 1994, the WHO itself has been providing several guidance to health care policy-makers, researchers and other stakeholders detailing the key concepts in medical ethics. These are specific to applying ethical principles in global public health.
Likewise, World Organization for Animal Health (WOAH), and International Convention for the Protection of Animals (ICPA) provide guidance on animal welfare in research. Due to this continuous guidance, together with accepted practices, there are internationally established ethical guidelines to carry out medical research. Our literature survey further identified freely available guidance from independent organisations such as COPE (Committee of Publication Ethics) and ALLEA (All European Academics) which provide support for maintaining research ethics in other fields such as education, sociology, psychology etc. In reality, ethical governance is practiced differently in different countries. In the UK, there is a clinical excellence research governance, which oversees all NHS related medical research (Mulholland and Bell, 2005 ). Although, the governance in other disciplines is not entirely centralised, many research funding councils and organisations [such as UKRI (UK-Research and Innovation; BBSC (Biotechnology and Biological Sciences Research Council; MRC (Medical Research Council); EPSRC (Economic and Social Research Council)] provide ethical governance and expect institutional adherence and monitoring. They expect local institutional (i.e. university/institutional) research governance for day-to-day monitoring of the research conducted within the organisation and report back to these funding bodies, monthly or annually (Department of Health, 2005). Likewise, there are nationally coordinated/regulated ethics governing bodies such as the US Office for Human Research Protections (US-OHRP), National Institute of Health (NIH) and the Canadian Institutes for Health Research (CIHR) in the USA and Canada respectively (Mulholland and Bell, 2005 ). The OHRP in the USA formally reviews all research activities involving human subjects. On the other hand, in Canada, CIHR works with the Natural Sciences and Engineering Research Council (NSERC), and the Social Sciences and Humanities Research Council (SSHRC). They together have produced a Tri-Council Policy Statement (TCPS) (Stephenson et al., 2020 ) as ethical governance. All Canadian institutions are expected to adhere to this policy for conducting research. As for Australia, the research is governed by the Australian code for the responsible conduct of research (2008). It identifies the responsibilities of institutions and researchers in all areas of research. The code has been jointly developed by the National Health and Medical Research Council (NHMRC), the Australian Research Council (ARC) and Universities Australia (UA). This information is summarized in Table 2 .
Basic structure of an institutional ethical advisory committee (EAC)
The WHO published an article defining the basic concepts of an ethical advisory committee in 2009 (WHO, 2009 - see above). According to this, many countries have established research governance and monitor the ethical practice in research via national and/or regional review committees. The main aims of research ethics committees include reviewing the study proposals, trying to understand the justifications for human/animal use, weighing the merits and demerits of the usage (linking to risks vs. potential benefits) and ensuring the local, ethical guidelines are followed Difn website b - Enago academy Importance of Ethics Committees in Scholarly Research, 2020 ; Guide for Research Ethics - Council of Europe, 2014 ). Once the research has started, the committee needs to carry out periodic surveillance to ensure the institutional ethical norms are followed during and beyond the study. They may also be involved in setting up and/or reviewing the institutional policies.
For these aspects, IRB (or institutional ethical advisory committee - IEAC) is essential for local governance to enhance best practices. The advantage of an IRB/EEAC is that they understand the institutional conditions and can closely monitor the ongoing research, including any changes in research directions. On the other hand, the IRB may be overly supportive to accept applications, influenced by the local agenda for achieving research excellence, disregarding ethical issues (Kotecha et al., 2011 ; Kayser-Jones, 2003 ) or, they may be influenced by the financial interests in attracting external funding. In this respect, regional and national ethics committees are advantageous to ensure ethical practice. Due to their impartiality, they would provide greater consistency and legitimacy to the research (WHO, 2009 ). However, the ethical approval process of regional and national ethics committees would be time consuming, as they do not have the local knowledge.
As for membership in the IRBs, most of the guidelines [WHO, NICE, Council of Europe, (2012), European Commission - Facilitating Research Excellence in FP7 ( 2013 ) and OHRP] insist on having a variety of representations including experts in different fields of research, and non-experts with the understanding of local, national/international conflicts of interest. The former would be able to understand/clarify the procedural elements of the research in different fields; whilst the latter would help to make neutral and impartial decisions. These non-experts are usually not affiliated to the institution and consist of individuals representing the broader community (particularly those related to social, legal or cultural considerations). IRBs consisting of these varieties of representation would not only be in a position to understand the study procedures and their potential direct or indirect consequences for participants, but also be able to identify any community, cultural or religious implications of the study.

Understanding the subtle differences between ethics and morals
Interestingly, many ethical guidelines are based on society’s moral “beliefs” in such a way that the words “ethics”‘and “morals” are reciprocally used to define each other. However, there are several subtle differences between them and we have attempted to compare and contrast them herein. In the past, many authors have interchangeably used the words “morals”‘and “ethics”‘(Warwick, 2003 ; Kant, 2018 ; Hazard, GC (Jr)., 1994 , Larry, 1982 ). However, ethics is linked to rules governed by an external source such as codes of conduct in workplaces (Kuyare et al., 2014 ). In contrast, morals refer to an individual’s own principles regarding right and wrong. Quinn ( 2011 ) defines morality as “ rules of conduct describing what people ought and ought not to do in various situations … ” while ethics is “... the philosophical study of morality, a rational examination into people’s moral beliefs and behaviours ”. For instance, in a case of parents demanding that schools overturn a ban on use of corporal punishment of children by schools and teachers (Children’s Rights Alliance for England, 2005 ), the parents believed that teachers should assume the role of parent in schools and use corporal or physical punishment for children who misbehaved. This stemmed from their beliefs and what they felt were motivated by “beliefs of individuals or groups”. For example, recent media highlights about some parents opposing LGBT (Lesbian, Gay, Bisexual, and Transgender) education to their children (BBC News, 2019 ). One parent argued, “Teaching young children about LGBT at a very early stage is ‘morally’ wrong”. She argued “let them learn by themselves as they grow”. This behaviour is linked to and governed by the morals of an ethnic community. Thus, morals are linked to the “beliefs of individuals or group”. However, when it comes to the LGBT rights these are based on ethical principles of that society and governed by law of the land. However, the rights of children to be protected from “inhuman and degrading” treatment is based on the ethical principles of the society and governed by law of the land. Individuals, especially those who are working in medical or judicial professions have to follow an ethical code laid down by their profession, regardless of their own feelings, time or preferences. For instance, a lawyer is expected to follow the professional ethics and represent a defendant, despite the fact that his morals indicate the defendant is guilty.
In fact, we as a group could not find many scholarly articles clearly comparing or contrasting ethics with morals. However, a table presented by Surbhi ( 2015 ) (Difn website c ) tries to differentiate these two terms (see Table 3 ).
Although Table 3 gives some insight on the differences between these two terms, in practice many use these terms as loosely as possible mainly because of their ambiguity. As a group focussed on the application of these principles, we would recommend to use the term “ethics” and avoid “morals” in research and academia.
Based on the literature survey carried out, we were able to identify the following gaps:
there is some disparity in existing literature on the importance of ethical guidelines in research
there is a lack of consensus on what code of conduct should be followed, where it should be derived from and how it should be implemented
The mission of ENAI’s ethical advisory working group
The Ethical Advisory Working Group of ENAI was established in 2018 to promote ethical code of conduct/practice amongst higher educational organisations within Europe and beyond (European Network for Academic Integrity, 2018 ). We aim to provide unbiased advice and consultancy on embedding ethical principles within all types of academic, research and public engagement activities. Our main objective is to promote ethical principles and share good practice in this field. This advisory group aims to standardise ethical norms and to offer strategic support to activities including (but not exclusive to):
● rendering advice and assistance to develop institutional ethical committees and their regulations in member institutions,
● sharing good practice in research and academic ethics,
● acting as a critical guide to institutional review processes, assisting them to maintain/achieve ethical standards,
● collaborating with similar bodies in establishing collegiate partnerships to enhance awareness and practice in this field,
● providing support within and outside ENAI to develop materials to enhance teaching activities in this field,
● organising training for students and early-career researchers about ethical behaviours in form of lectures, seminars, debates and webinars,
● enhancing research and dissemination of the findings in matters and topics related to ethics.
The following sections focus on our suggestions based on collective experiences, review of literature provided in earlier sections and workshop feedback collected:
a) basic needs of an ethical committee within an institution;
b) a typical ethical approval process (with examples from three different universities); and
c) the ways to obtain informed consent with some examples. This would give advice on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Setting up an institutional ethical committee (ECs)
Institutional Ethical Committees (ECs) are essential to govern every aspect of the activities undertaken by that institute. With regards to higher educational organisations, this is vital to establish ethical behaviour for students and staff to impart research, education and scholarly activities (or everything) they do. These committees should be knowledgeable about international laws relating to different fields of studies (such as science, medicine, business, finance, law, and social sciences). The advantages and disadvantages of institutional, subject specific or common (statutory) ECs are summarised in Fig. 2 . Some institutions have developed individual ECs linked to specific fields (or subject areas) whilst others have one institutional committee that overlooks the entire ethical behaviour and approval process. There is no clear preference between the two as both have their own advantages and disadvantages (see Fig. 2 ). Subject specific ECs are attractive to medical, law and business provisions, as it is perceived the members within respective committees would be able to understand the subject and therefore comprehend the need of the proposed research/activity (Kadam, 2012 ; Schnyder et al., 2018 ). However, others argue, due to this “ specificity ”, the committee would fail to forecast the wider implications of that application. On the other hand, university-wide ECs would look into the wider implications. Yet they find it difficult to understand the purpose and the specific applications of that research. Not everyone understands dynamics of all types of research methodologies, data collection, etc., and therefore there might be a chance of a proposal being rejected merely because the EC could not understand the research applications (Getz, 1990 ).
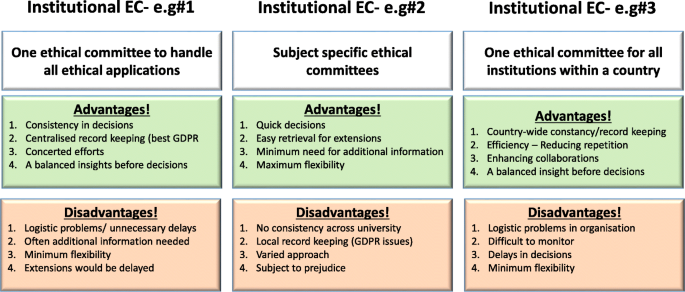
Summary of advantages and disadvantages of three different forms of ethical committees
[N/B for Fig. 2 : Examples of different types of ethical application procedures and forms used were discussed with the workshop attendees to enhance their understanding of the differences. GDPR = General Data Protection Regulation].
Although we recommend a designated EC with relevant professional, academic and ethical expertise to deal with particular types of applications, the membership (of any EC) should include some non-experts who would represent the wider community (see above). Having some non-experts in EC would not only help the researchers to consider explaining their research in layperson’s terms (by thinking outside the box) but also would ensure efficiency without compromising participants/animal safety. They may even help to address the common ethical issues outside research culture. Some UK universities usually offer this membership to a clergy, councillor or a parliamentarian who does not have any links to the institutions. Most importantly, it is vital for any EC members to undertake further training in addition to previous experience in the relevant field of research ethics.
Another issue that raises concerns is multi-centre research, involving several institutions, where institutionalised ethical approvals are needed from each partner. In some cases, such as clinical research within the UK, a common statutory EC called National Health Services (NHS) Research Ethics Committee (NREC) is in place to cover research ethics involving all partner institutions (NHS, 2018 ). The process of obtaining approval from this type of EC takes time, therefore advanced planning is needed.
Ethics approval forms and process
During the workshop, we discussed some anonymised application forms obtained from open-access sources for qualitative and quantitative research as examples. Considering research ethics, for the purpose of understanding, we arbitrarily divided this in two categories; research based on (a) quantitative and (b) qualitative methodologies. As their name suggests their research approach is extremely different from each other. The discussion elicited how ECs devise different types of ethical application form/questions. As for qualitative research, these are often conducted as “face-to-face” interviews, which would have implications on volunteer anonymity.
Furthermore, discussions posited when the interviews are replaced by on-line surveys, they have to be administered through registered university staff to maintain confidentiality. This becomes difficult when the research is a multi-centre study. These types of issues are also common in medical research regarding participants’ anonymity, confidentially, and above all their right to withdraw consent to be involved in research.
Storing and protecting data collected in the process of the study is also a point of consideration when applying for approval.
Finally, the ethical processes of invasive (involving human/animals) and non-invasive research (questionnaire based) may slightly differ from one another. Following research areas are considered as investigations that need ethical approval:
research that involves human participants (see below)
use of the ‘products’ of human participants (see below)
work that potentially impacts on humans (see below)
research that involves animals
In addition, it is important to provide a disclaimer even if an ethical approval is deemed unnecessary. Following word cloud (Fig. 3 ) shows the important variables that need to be considered at the brainstorming stage before an ethical application. It is worth noting the importance of proactive planning predicting the “unexpected” during different phases of a research project (such as planning, execution, publication, and future directions). Some applications (such as working with vulnerable individuals or children) will require safety protection clearance (such as DBS - Disclosure and Barring Service, commonly obtained from the local police). Please see section on Research involving Humans - Informed consents for further discussions.
Examples of important variables that need to be considered for an ethical approval
It is also imperative to report or re-apply for ethical approval for any minor or major post-approval changes to original proposals made. In case of methodological changes, evidence of risk assessments for changes and/or COSHH (Control of Substances Hazardous to Health Regulations) should also be given. Likewise, any new collaborative partners or removal of researchers should also be notified to the IEAC.
Other findings include:
in case of complete changes in the project, the research must be stopped and new approval should be seeked,
in case of noticing any adverse effects to project participants (human or non-human), these should also be notified to the committee for appropriate clearance to continue the work, and
the completion of the project must also be notified with the indication whether the researchers may restart the project at a later stage.
Research involving humans - informed consents
While discussing research involving humans and based on literature review, findings highlight the human subjects/volunteers must willingly participate in research after being adequately informed about the project. Therefore, research involving humans and animals takes precedence in obtaining ethical clearance and its strict adherence, one of which is providing a participant information sheet/leaflet. This sheet should contain a full explanation about the research that is being carried out and be given out in lay-person’s terms in writing (Manti and Licari 2018 ; Hardicre 2014 ). Measures should also be in place to explain and clarify any doubts from the participants. In addition, there should be a clear statement on how the participants’ anonymity is protected. We provide below some example questions below to help the researchers to write this participant information sheet:
What is the purpose of the study?
Why have they been chosen?
What will happen if they take part?
What do they have to do?
What happens when the research stops?
What if something goes wrong?
What will happen to the results of the research study?
Will taking part be kept confidential?
How to handle “vulnerable” participants?
How to mitigate risks to participants?
Many institutional ethics committees expect the researchers to produce a FAQ (frequently asked questions) in addition to the information about research. Most importantly, the researchers also need to provide an informed consent form, which should be signed by each human participant. The five elements identified that are needed to be considered for an informed consent statement are summarized in Fig. 4 below (slightly modified from the Federal Policy for the Protection of Human Subjects ( 2018 ) - Diffn website c ).
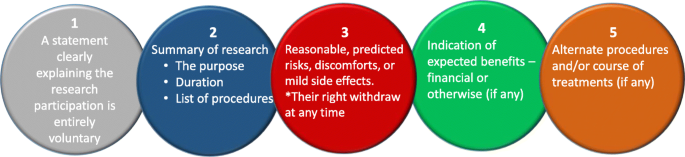
Five basic elements to consider for an informed consent [figure adapted from Diffn website c ]
The informed consent form should always contain a clause for the participant to withdraw their consent at any time. Should this happen all the data from that participant should be eliminated from the study without affecting their anonymity.
Typical research ethics approval process
In this section, we provide an example flow chart explaining how researchers may choose the appropriate application and process, as highlighted in Fig. 5 . However, it is imperative to note here that these are examples only and some institutions may have one unified application with separate sections to demarcate qualitative and quantitative research criteria.
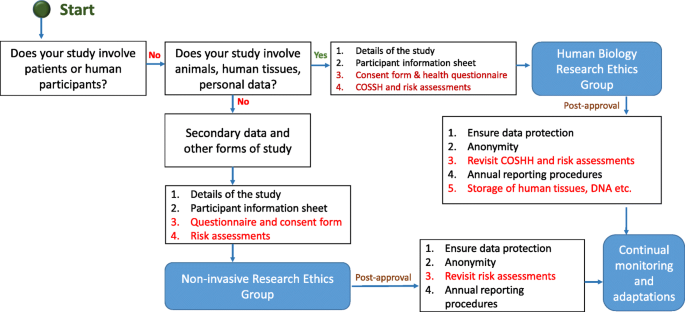
Typical ethical approval processes for quantitative and qualitative research. [N/B for Fig. 5 - This simplified flow chart shows that fundamental process for invasive and non-invasive EC application is same, the routes and the requirements for additional information are slightly different]
Once the ethical application is submitted, the EC should ensure a clear approval procedure with distinctly defined timeline. An example flow chart showing the procedure for an ethical approval was obtained from University of Leicester as open-access. This is presented in Fig. 6 . Further examples of the ethical approval process and governance were discussed in the workshop.
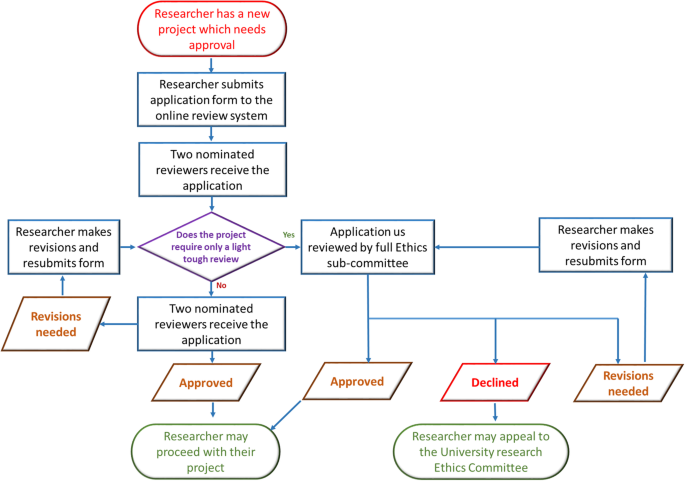
An example ethical approval procedures conducted within University of Leicester (Figure obtained from the University of Leicester research pages - Difn website d - open access)
Strategies for ethics educations for students
Student education on the importance of ethics and ethical behaviour in research and scholarly activities is extremely essential. Literature posits in the area of medical research that many universities are incorporating ethics in post-graduate degrees but when it comes to undergraduate degrees, there is less appetite to deliver modules or even lectures focussing on research ethics (Seymour et al., 2004 ; Willison and O’Regan, 2007 ). This may be due to the fact that undergraduate degree structure does not really focus on research (DePasse et al., 2016 ). However, as Orr ( 2018 ) suggested, institutions should focus more on educating all students about ethics/ethical behaviour and their importance in research, than enforcing punitive measures for unethical behaviour. Therefore, as an advisory committee, and based on our preliminary literature survey and workshop results, we strongly recommend incorporating ethical education within undergraduate curriculum. Looking at those institutions which focus on ethical education for both under-and postgraduate courses, their approaches are either (a) a lecture-based delivery, (b) case study based approach or (c) a combined delivery starting with a lecture on basic principles of ethics followed by generating a debate based discussion using interesting case studies. The combined method seems much more effective than the other two as per our findings as explained next.
As many academics who have been involved in teaching ethics and/or research ethics agree, the underlying principles of ethics is often perceived as a boring subject. Therefore, lecture-based delivery may not be suitable. On the other hand, a debate based approach, though attractive and instantly generates student interest, cannot be effective without students understanding the underlying basic principles. In addition, when selecting case studies, it would be advisable to choose cases addressing all different types of ethical dilemmas. As an advisory group within ENAI, we are in the process of collating supporting materials to help to develop institutional policies, creating advisory documents to help in obtaining ethical approvals, and teaching materials to enhance debate-based lesson plans that can be used by the member and other institutions.
Concluding remarks
In summary, our literature survey and workshop findings highlight that researchers should accept that ethics underpins everything we do, especially in research. Although ethical approval is tedious, it is an imperative process in which proactive thinking is essential to identify ethical issues that might affect the project. Our findings further lead us to state that the ethical approval process differs from institution to institution and we strongly recommend the researchers to follow the institutional guidelines and their underlying ethical principles. The ENAI workshop in Vilnius highlighted the importance of ethical governance by establishing ECs, discussed different types of ECs and procedures with some examples and highlighted the importance of student education to impart ethical culture within research communities, an area that needs further study as future scope.
Declarations
The manuscript was entirely written by the corresponding author with contributions from co-authors who have also taken part in the delivery of the workshop. Authors confirm that the data supporting the findings of this study are available within the article. We can also confirm that there are no potential competing interests with other organisations.
Availability of data and materials
Authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
ALL European academics
Australian research council
Biotechnology and biological sciences research council
Canadian institutes for health research
Committee of publication ethics
Ethical committee
European network of academic integrity
Economic and social research council
International convention for the protection of animals
institutional ethical advisory committee
Institutional review board
Immaculata university of Pennsylvania
Lesbian, gay, bisexual, and transgender
Medical research council)
National health services
National health services nih national institute of health (NIH)
National institute of clinical care excellence
National health and medical research council
Natural sciences and engineering research council
National research ethics committee
National statement on ethical conduct in human research
Responsible research practice
Social sciences and humanities research council
Tri-council policy statement
World Organization for animal health
Universities Australia
UK-research and innovation
US office for human research protections
Alba S, Lenglet A, Verdonck K, Roth J, Patil R, Mendoza W, Juvekar S, Rumisha SF (2020) Bridging research integrity and global health epidemiology (BRIDGE) guidelines: explanation and elaboration. BMJ Glob Health 5(10):e003237. https://doi.org/10.1136/bmjgh-2020-003237
Article Google Scholar
Anderson MS (2011) Research misconduct and misbehaviour. In: Bertram Gallant T (ed) Creating the ethical academy: a systems approach to understanding misconduct and empowering change in higher education. Routledge, pp 83–96
BBC News. (2019). Birmingham school LGBT LESSONS PROTEST investigated. March 8, 2019. Retrieved February 14, 2021, available online. URL: https://www.bbc.com/news/uk-england-birmingham-47498446
Children’s Rights Alliance for England. (2005). R (Williamson and others) v Secretary of State for Education and Employment. Session 2004–05. [2005] UKHL 15. Available Online. URL: http://www.crae.org.uk/media/33624/R-Williamson-and-others-v-Secretary-of-State-for-Education-and-Employment.pdf
Council of Europe. (2014). Texts of the Council of Europe on bioethical matters. Available Online. https://www.coe.int/t/dg3/healthbioethic/Texts_and_documents/INF_2014_5_vol_II_textes_%20CoE_%20bio%C3%A9thique_E%20(2).pdf
Dellaportas S, Kanapathippillai S, Khan, A and Leung, P. (2014). Ethics education in the Australian accounting curriculum: a longitudinal study examining barriers and enablers. 362–382. Available Online. URL: https://doi.org/10.1080/09639284.2014.930694 , 23, 4, 362, 382
DePasse JM, Palumbo MA, Eberson CP, Daniels AH (2016) Academic characteristics of orthopaedic surgery residency applicants from 2007 to 2014. JBJS 98(9):788–795. https://doi.org/10.2106/JBJS.15.00222
Desmond H, Dierickx K (2021) Research integrity codes of conduct in Europe: understanding the divergences. https://doi.org/10.1111/bioe.12851
Difn website a - National Statement on Ethical Conduct in Human Research (NSECHR). (2018). Available Online. URL: https://www.nhmrc.gov.au/about-us/publications/australian-code-responsible-conduct-research-2018
Difn website b - Enago academy Importance of Ethics Committees in Scholarly Research (2020, October 26). Available online. URL: https://www.enago.com/academy/importance-of-ethics-committees-in-scholarly-research/
Difn website c - Ethics vs Morals - Difference and Comparison. Retrieved July 14, 2020. Available online. URL: https://www.diffen.com/difference/Ethics_vs_Morals
Difn website d - University of Leicester. (2015). Staff ethics approval flowchart. May 1, 2015. Retrieved July 14, 2020. Available Online. URL: https://www2.le.ac.uk/institution/ethics/images/ethics-approval-flowchart/view
European Commission - Facilitating Research Excellence in FP7 (2013) https://ec.europa.eu/research/participants/data/ref/fp7/89888/ethics-for-researchers_en.pdf
European Network for Academic Integrity. (2018). Ethical advisory group. Retrieved February 14, 2021. Available online. URL: http://www.academicintegrity.eu/wp/wg-ethical/
Federal Policy for the Protection of Human Subjects. (2018). Retrieved February 14, 2021. Available Online. URL: https://www.federalregister.gov/documents/2017/01/19/2017-01058/federal-policy-for-the-protection-of-human-subjects#p-855
Flite, CA and Harman, LB. (2013). Code of ethics: principles for ethical leadership Perspect Health Inf Mana; 10(winter): 1d. PMID: 23346028
Fouka G, Mantzorou M (2011) What are the major ethical issues in conducting research? Is there a conflict between the research ethics and the nature of nursing. Health Sci J 5(1) Available Online. URL: https://www.hsj.gr/medicine/what-are-the-major-ethical-issues-in-conducting-research-is-there-a-conflict-between-the-research-ethics-and-the-nature-of-nursing.php?aid=3485
Fox G (2017) History and ethical principles. The University of Miami and the Collaborative Institutional Training Initiative (CITI) Program URL https://silo.tips/download/chapter-1-history-and-ethical-principles # (Available Online)
Getz KA (1990) International codes of conduct: An analysis of ethical reasoning. J Bus Ethics 9(7):567–577
Ghooi RB (2011) The nuremberg code–a critique. Perspect Clin Res 2(2):72–76. https://doi.org/10.4103/2229-3485.80371
Hardicre, J. (2014) Valid informed consent in research: an introduction Br J Nurs 23(11). https://doi.org/10.12968/bjon.2014.23.11.564 , 567
Hazard, GC (Jr). (1994). Law, morals, and ethics. Yale law school legal scholarship repository. Faculty Scholarship Series. Yale University. Available Online. URL: https://digitalcommons.law.yale.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=3322&context=fss_papers
Israel, M., & Drenth, P. (2016). Research integrity: perspectives from Australia and Netherlands. In T. Bretag (Ed.), Handbook of academic integrity (pp. 789–808). Springer, Singapore. https://doi.org/10.1007/978-981-287-098-8_64
Kadam R (2012) Proactive role for ethics committees. Indian J Med Ethics 9(3):216. https://doi.org/10.20529/IJME.2012.072
Kant I (2018) The metaphysics of morals. Cambridge University Press, UK https://doi.org/10.1017/9781316091388
Kayser-Jones J (2003) Continuing to conduct research in nursing homes despite controversial findings: reflections by a research scientist. Qual Health Res 13(1):114–128. https://doi.org/10.1177/1049732302239414
Kotecha JA, Manca D, Lambert-Lanning A, Keshavjee K, Drummond N, Godwin M, Greiver M, Putnam W, Lussier M-T, Birtwhistle R (2011) Ethics and privacy issues of a practice-based surveillance system: need for a national-level institutional research ethics board and consent standards. Can Fam physician 57(10):1165–1173. https://europepmc.org/article/pmc/pmc3192088
Kuyare, MS., Taur, SR., Thatte, U. (2014). Establishing institutional ethics committees: challenges and solutions–a review of the literature. Indian J Med Ethics. https://doi.org/10.20529/IJME.2014.047
LaFollette, H. (2007). Ethics in practice (3rd edition). Blackwell
Larry RC (1982) The teaching of ethics and moral values in teaching. J High Educ 53(3):296–306. https://doi.org/10.1080/00221546.1982.11780455
Manti S, Licari A (2018) How to obtain informed consent for research. Breathe (Sheff) 14(2):145–152. https://doi.org/10.1183/20734735.001918
Mulholland MW, Bell J (2005) Research Governance and Research Funding in the USA: What the academic surgeon needs to know. J R Soc Med 98(11):496–502. https://doi.org/10.1258/jrsm.98.11.496
National Institute of Health (NIH) Ethics in Clinical Research. n.d. Available Online. URL: https://clinicalcenter.nih.gov/recruit/ethics.html
NHS (2018) Flagged Research Ethics Committees. Retrieved February 14, 2021. Available online. URL: https://www.hra.nhs.uk/about-us/committees-and-services/res-and-recs/flagged-research-ethics-committees/
NICE (2018) Research governance policy. Retrieved February 14, 2021. Available online. URL: https://www.nice.org.uk/Media/Default/About/what-we-do/science-policy-and-research/research-governance-policy.pdf
Orr, J. (2018). Developing a campus academic integrity education seminar. J Acad Ethics 16(3), 195–209. https://doi.org/10.1007/s10805-018-9304-7
Quinn, M. (2011). Introduction to Ethics. Ethics for an Information Age. 4th Ed. Ch 2. 53–108. Pearson. UK
Resnik. (2020). What is ethics in Research & why is it Important? Available Online. URL: https://www.niehs.nih.gov/research/resources/bioethics/whatis/index.cfm
Schnyder S, Starring H, Fury M, Mora A, Leonardi C, Dasa V (2018) The formation of a medical student research committee and its impact on involvement in departmental research. Med Educ Online 23(1):1. https://doi.org/10.1080/10872981.2018.1424449
Seymour E, Hunter AB, Laursen SL, DeAntoni T (2004) Establishing the benefits of research experiences for undergraduates in the sciences: first findings from a three-year study. Sci Educ 88(4):493–534. https://doi.org/10.1002/sce.10131
Shamoo AE, Irving DN (1993) Accountability in research using persons with mental illness. Account Res 3(1):1–17. https://doi.org/10.1080/08989629308573826
Shaw, S., Boynton, PM., and Greenhalgh, T. (2005). Research governance: where did it come from, what does it mean? Research governance framework for health and social care, 2nd ed. London: Department of Health. https://doi.org/10.1258/jrsm.98.11.496 , 98, 11, 496, 502
Book Google Scholar
Speight, JG. (2016) Ethics in the university |DOI: https://doi.org/10.1002/9781119346449 scrivener publishing LLC
Stephenson GK, Jones GA, Fick E, Begin-Caouette O, Taiyeb A, Metcalfe A (2020) What’s the protocol? Canadian university research ethics boards and variations in implementing tri-Council policy. Can J Higher Educ 50(1)1): 68–81
Surbhi, S. (2015). Difference between morals and ethics [weblog]. March 25, 2015. Retrieved February 14, 2021. Available Online. URL: http://keydifferences.com/difference-between-morals-and-ethics.html
The Belmont Report (1979). Ethical Principles and Guidelines for the Protection of Human Subjects of Research. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. Retrieved February 14, 2021. Available online. URL: https://www.hhs.gov/ohrp/sites/default/files/the-belmont-report-508c_FINAL.pdf
The Singapore Statement on Research Integrity. (2020). Nicholas Steneck and Tony Mayer, Co-chairs, 2nd World Conference on Research Integrity; Melissa Anderson, Chair, Organizing Committee, 3rd World Conference on Research Integrity. Retrieved February 14, 2021. Available online. URL: https://wcrif.org/documents/327-singapore-statement-a4size/file
Warwick K (2003) Cyborg morals, cyborg values, cyborg ethics. Ethics Inf Technol 5(3):131–137. https://doi.org/10.1023/B:ETIN.0000006870.65865.cf
Weindling P (2001) The origins of informed consent: the international scientific commission on medical war crimes, and the Nuremberg code. Bull Hist Med 75(1):37–71. https://doi.org/10.1353/bhm.2001.0049
WHO. (2009). Research ethics committees Basic concepts for capacity-building. Retrieved February 14, 2021. Available online. URL: https://www.who.int/ethics/Ethics_basic_concepts_ENG.pdf
WHO. (2021). Chronological list of publications. Retrieved February 14, 2021. Available online. URL: https://www.who.int/ethics/publications/year/en/
Willison, J. and O’Regan, K. (2007). Commonly known, commonly not known, totally unknown: a framework for students becoming researchers. High Educ Res Dev 26(4). 393–409. https://doi.org/10.1080/07294360701658609
Žukauskas P, Vveinhardt J, and Andriukaitienė R. (2018). Research Ethics In book: Management Culture and Corporate Social Responsibility Eds Jolita Vveinhardt IntechOpenEditors DOI: https://doi.org/10.5772/intechopen.70629 , 2018
Download references
Acknowledgements
Authors wish to thank the organising committee of the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania for accepting this paper to be presented in the conference.
Not applicable as this is an independent study, which is not funded by any internal or external bodies.
Author information
Authors and affiliations.
School of Human Sciences, University of Derby, DE22 1, Derby, GB, UK
Shivadas Sivasubramaniam
Department of Informatics, Mendel University in Brno, Zemědělská, 1665, Brno, Czechia
Dita Henek Dlabolová
Centre for Academic Integrity in the UAE, Faculty of Engineering & Information Sciences, University of Wollongong in Dubai, Dubai, UAE
Veronika Kralikova & Zeenath Reza Khan
You can also search for this author in PubMed Google Scholar
Contributions
The manuscript was entirely written by the corresponding author with contributions from co-authors who have equally contributed to presentation of this paper in the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania. Authors have equally contributed for the information collection, which were then summarised as narrative explanations by the Corresponding author and Dr. Zeenath Reza Khan. Then checked and verified by Dr. Dlabolova and Ms. Králíková. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Shivadas Sivasubramaniam .
Ethics declarations
Competing interests.
We can also confirm that there are no potential competing interest with other organisations.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sivasubramaniam, S., Dlabolová, D.H., Kralikova, V. et al. Assisting you to advance with ethics in research: an introduction to ethical governance and application procedures. Int J Educ Integr 17 , 14 (2021). https://doi.org/10.1007/s40979-021-00078-6
Download citation
Received : 17 July 2020
Accepted : 25 April 2021
Published : 13 July 2021
DOI : https://doi.org/10.1007/s40979-021-00078-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Higher education
- Ethical codes
- Ethics committee
- Post-secondary education
- Institutional policies
- Research ethics
International Journal for Educational Integrity
ISSN: 1833-2595
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
6.3 Principles of Research Ethics
There are general ethical principles that guide and underpin the proper conduct of research. The term “ethical principles” refers to those general rules that operate as a foundational rationale for the numerous specific ethical guidelines and assessments of human behaviour. 7 The National Statement on ‘ethical conduct in human research’ states that ethical behaviour entails acting with integrity, motivated by a deep respect and concern for others. 8 Before research can be conducted, it is essential for researchers to develop and submit to a relevant human research ethics committee a research proposal that meets the National Statement’s requirements and is ethically acceptable. There are five key ethical research principles – respect for autonomy, beneficence, non-maleficence, and justice 8, 9 (Figure 6.1). These principles are universal, which means they apply everywhere in the world, without national, cultural, legal, or economic boundaries. Therefore, everyone involved in human research studies should understand and follow these principles.
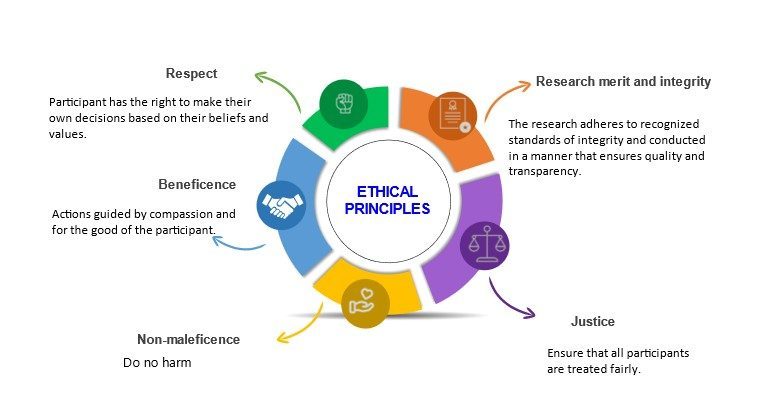
Research merit and integrity
Research merit and integrity relate to the quality or value of a study in contributing to the knowledge base of a particular field or discipline. 8,9 This is determined by the originality and significance of the research question, the soundness and appropriateness of the research methodology, the rigor and reliability of the data analysis, the clarity and coherence of the research findings, and the potential impact of the research on advancing knowledge or solving practical problems. Research must be developed with methods that are appropriate to the study’s objectives, based on a thorough analysis of the relevant literature, and conducted using facilities and resources that are appropriate for the study’s needs. In essence, research must adhere to recognised standards of integrity and be designed, reviewed and conducted in a manner that ensures quality and transparency. 8 Examples of unacceptable practices include plagiarism through appropriation or use of the ideas or intellectual property of others; falsification by creating false data or other aspects of research (including documentation and consent of participants); distortion through improper manipulation and/or selection of data, images and/or consent.
Respect for persons
Respect for humans is an acknowledgement of their inherent worth, and it refers to the moral imperative to regard the autonomy of others. 8,10 Respect entails taking into account the well-being, beliefs, perspectives, practises, and cultural heritage of persons participating in research, individually and collectively. It involves respecting the participants’ privacy, confidentiality, and cultural sensitivity. 8 Respect also entails giving adequate consideration to people to make their judgements throughout the study process. In cases where the participants have limited capacity to make autonomous decisions, respect for them entails protecting them against harm. 8 This means that all participants in research must participate voluntarily without coercion or undue influence, and their rights, dignity and autonomy should be respected and adequately protected.
Beneficence
The ethical principle that requires actions that promote the well-being and interests of others is known as beneficence. 10 It is the fundamental premise underlying all medical health care and research. 8 Beneficence requires the researcher to weigh the prospective benefits and hazards and make certain that projects have the potential for net benefit over harm. 8,10 Researchers are responsible for: (a) structuring the study to minimise the risks of injury or discomfort to participants; (b) explaining the possible benefits and dangers of the research to participants; and (c) the welfare of the participants in the research setting 8. Thus, the study participants must always be prioritised over the research methodology, even if this means invalidating data. 8
Non-maleficence
This is the ethical principle that requires actions that avoid or minimize harm to others. According to the principle of non-maleficence, participating in a study shouldn’t do any harm to the research subject. This principle is closely related to beneficence; however, it may be difficult to keep track of any damage to study participants. 11 Different types of harm could occur, including physical, mental, social, or financial harm. While the physical injury may be quickly recognised and then avoided or reduced, less evident issues such as emotional, social, and economic factors might hurt the subject without the researcher being aware. 11 It is essential to note that all research involve cost to participants even if just their time, and each research study has the potential to hurt participants, hence it is important to ensure that the merit of research outweighs the costs and risks. There are five categories into which studies may be categorised based on the possible amount of injury or discomfort that they may expose the participants to. 11
- No anticipated effects
- Temporary discomfort
- Unusual levels of temporary discomfort
- Risk of permanent damage
- Certainty of permanent damage
The concept of fairness and the application of moral principles to ensure equitable treatment. According to this research tenet, the researcher must treat participants fairly and always prioritise the needs of the research subjects over the study’s aim. 8,11 Research participants must be fairly chosen, and that exclusion and inclusion criteria must be accurately stated in the research’s findings. 8 In addition, there is no unjust hardship associated with participating in research on certain groups, and the participant recruitment method is fair. Furthermore, the rewards for research involvement are fairly distributed; research participants are not exploited, and research rewards are accessible to everybody equally. 8
An Introduction to Research Methods for Undergraduate Health Profession Students Copyright © 2023 by Faith Alele and Bunmi Malau-Aduli is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Afr J Emerg Med
- v.10(Suppl 2); 2020
Principles of research ethics: A research primer for low- and middle-income countries
Cindy c. bitter.
a Saint Louis University School of Medicine, Division of Emergency Medicine, St. Louis MO, USA
Annet Alenyo Ngabirano
b Aga Khan University, Kampala, Uganda
e Makerere University College of Health Sciences, Kampala, Uganda
Erin L. Simon
c Cleveland Clinic Akron General, Department of Emergency Medicine, Akron, OH, USA
f Northeast Ohio Medical University, Rootstown, OH, USA
David McD. Taylor
d University of Melbourne, Department of Medicine, Parkville, Victoria, Australia
g Austin Health, Heidelburg, Victoria, Australia
Ethical oversight in the form of review boards and research ethics committees provide protection for research subjects as well as guidance for safe conduct of studies. As the number of collaborative emergency care research studies carried out in low- and middle-income countries increases, it is crucial to have a shared understanding of how ethics should inform choice of study topic, study design, methods of obtaining consent, data management, and access to treatment after closure of the study. This paper describes the basic principles of Western research ethics – respect for persons, beneficence, and justice - and how the principles may be contextualized in different settings, by researchers of various backgrounds with different funding streams. Examples of lapses in ethical practice of research are used to highlight best practices.
African relevance
- • Low and middle income countries (LMICs) have a high burden of acute illness, but insufficient research on emergency care
- • Ethical barriers and lack of oversight impede emergency care research in LMICs
- • Ethical clinical research in LMICs builds capacity and contributes to the evidence base for emergency care
The International Federation for Emergency Medicine global health research primer
This paper forms part 7 of a series of how to papers, commissioned by the International Federation for Emergency Medicine. This primer discusses ways to ensure that research is carried out ethically. It discusses principles for researchers from the global north involved in emergency care research projects in LMICs and provides guidance for LMIC researchers who may not have experience for navigating ethical issues in research. We have also included additional tips and pitfalls that are relevant to emergency medicine researchers.
Despite a disproportionally high burden of acute illnesses in low-and middle-income countries (LMICs), there is insufficient emergency care research. In a survey carried out through the African Federation for Emergency Medicine (AFEM), 67.3% of respondents agreed with this statement [ 1 ]. Research that targets local health challenges, such as context-specific health service delivery and drug development for diseases prevalent in LMIC, has the potential to improve population health in host countries [ [2] , [3] , [4] ].
Ethical barriers and oversight have been cited as an important barrier to research in LMIC [ 5 ]. Emanuel et al. provided a framework for conducting ethical research that stressed collaborative partnerships, social value, scientific validity, fair selection of study populations, favourable risk-benefit ratio, independent review, informed consent, and respect for recruited participants and study communities [ 6 ]. In their review of the framework, Tsoka-Gwegweni and colleagues concluded that most recurring ethical issues relating to research in LMICs were informed consent, scientific validity, fair participant selection and on-going respect for participants [ 7 ]. Research priorities are often determined outside the host country, raising concerns of outsourcing risk and exploitation of uninformed populations [ 8 ].
The value of building ethical clinical research in LMICs goes beyond the specific information collected in a study. Conducting research has substantial positive impact in a community by raising local scholarly standards and encouraging independent thinking and creativity as many LMICs work towards building evidence-based emergency care systems. Each project builds confidence and skills which allows local researchers to participate fully in the scientific process which impacts the care of their local community. This may lead to intellectual and financial resources for the community which in turn encourages local empowerment and self-sufficiency for additional research.
Bioethical principles
Review of research protocols by experts not involved with the study is one way of ensuring protection for human research subjects. In the United States of America (US), such review committees are called Institutional Review Boards (IRBs). In other countries, these committees may be called Human Research Ethics Committees (HREC) or have other titles. Several articles discuss the evolution of HRECs in sub Saharan Africa [ 9 , 10 ].
Most Western scholars agree on the main principles of autonomy, beneficence, and justice for clinical ethics, with some adjustments in how these principles are applied in the research setting. However, these principles are not universally acknowledged. Some authors argue that these principles are less relevant in populations where community rather than individual values are stressed. Others argue that local development of research ethics is crucial to ensuring buy-in and avoiding bioethical imperialism [ 11 , 12 ]. Acknowledging the past abuses that took place under the guise of research, Western ethical thought now insists on voluntary consent, with special protections for traditionally vulnerable populations such as children, women, and ethnic minorities. Emergency care research, however, introduces a unique type of vulnerability in unconscious or other otherwise mentally altered patients who do not meet criteria for giving informed consent. In some Western countries like the US and the United Kingdom (UK), such patients may still be enrolled in studies through a different process called an exemption from informed consent or waiver of consent [ [13] , [14] , [15] ]. The same principle may be applied in LMICs, however, a detailed assessment of the research components necessary to guide HRECs in order to reach decisions that protect this vulnerable group from harm or unnecessary risk.
International standards
In 1964, the World Medical Association adopted the Declaration of Helsinki. This document and subsequent revisions lay out the principles of minimizing risks, informed consent, privacy, special protections for vulnerable groups, access to beneficial treatment after trial, and dissemination [ 16 ]. The first Declaration was adopted largely in response to the unethical experiments carried out by Nazi researchers during the Holocaust, in which prisoners were exposed to infectious disease, extremes of temperature, and experimental drugs without their consent, often resulting in death [ 17 ].
The Declaration is a guideline of research ethics but is not legally binding. Individual countries are responsible for implementing legislation that reflects the principles. In the United States, discovery of ongoing research abuses such as the Tuskegee syphilis study in the 1970's led to adoption of the Belmont Report [ 18 ]. The Belmont Report requires that researchers uphold three basic principles: respect for persons, beneficence, and justice. Researchers in the US must have the protocol approved by an IRB prior to enrolling subjects. IRBs are charged with operationalizing the principles outlined in the Belmont Report and subsequent regulations specified in the Common Rule [ 19 ]. Certain research may be exempted from IRB review and these typically include surveys, interviews, and research using existing specimens, records or data. The concept of “minimal risk to participants” plays a key role in allowing this exemption and should be considered when conducting research in LMIC's. “Minimal risk” is defined as that “ordinarily encountered in daily life or the performance of routine physical and psychological testing.”
These previous regulations, however, were written without consideration for Emergency Care research. Therefore in 1996, the US' Food and Drug Administration recommended procedures for enrolling patients in emergency situations into research studies through exemption of informed consent [ 20 ]. Similar procedures were adopted by the United Kingdom in 2006 [ 21 ]. This concept of “waiver of consent” in emergency care research should be considered in LMICs where contextualized processes are critical for development of sustainable emergency care systems.
Guidelines intended for global adoption often lack details required for context-specific implementation and can be slow to respond to emerging realities. Barugahare uses an analogy to jurisprudence to discuss how local HRECs can guide implementation of global ethical principles within their own contexts [ 22 ]. With that in mind, we will go through the guiding principles and discuss pitfalls in implementation.
Respect for persons
Respect for persons requires that research subjects freely participate in research after informed consent. Subjects should be counselled on the known risks of the drug or treatment being examined, the expected course of illness without intervention, and any compensation or benefit they may receive from participating. This must occur in the subjects' native language. If documents require translation from the researchers' language to the local language, the documents should be translated back to the original language and reviewed to ensure consistency with the originals. Only after the subject has shown comprehension and has had any questions answered can they be considered to have given informed consent. There is some disagreement about the need for a signature on a consent form, but researchers must treat consent as an ongoing process rather than a piece of paper. Subjects must also understand they are free to withdraw from the study at any time.
Research in the global context highlights challenges to the notion of consent. In the global north, the concept of respect for persons is often supplanted by the principle of autonomy. But many cultures outside the global north stress the importance of the family unit and communitarian values over individual decisions [ 23 ]. Community leaders and heads-of-household should be involved in the process, often before approaching individual subjects [ 24 ]. This is especially critical in emergency situations where “waiver of consent” protocols require involvement of family or communities of unconscious or otherwise incapacitated patients. Consent processes may also be challenging when approaching female subjects, who may require approval of a male head of household in addition to their own consent [ [25] , [26] , [27] ]. While 18 years is considered the age of adulthood in many Western countries, other cultures may consider younger persons to have reached maturity [ 28 , 29 ]. In one qualitative study of 15–19 year old participants in HIV research in Kenya, 50% thought parental consent should be sought, 25% felt this was not necessary, and 25% had mixed feelings [ 30 ].
Lack of education, limited health literacy, poor access to medical care, and the sense of urgency that accompanies disease outbreaks also call into question the notion of voluntary, informed consent. The Pfizer meningitis trial in Nigeria reported that 100% of patients approached were enrolled in the protocol, suggesting patients were desperate to access care [ 31 ]. All participants in a clinical trial for malaria believed procedures followed solely to meet research objectives were a required part of their individual curative treatment [ 32 ]. Some participants in an Ebola vaccine trial enrolled in hopes of accessing free healthcare for other conditions [ 33 ].
Beneficence
Beneficence requires that researchers obtain scientifically valid data with useful applications, while minimizing risks within the study protocol and protecting subjects during the trial.
Although several guidelines recommend research in LMIC focus on health conditions that contribute substantially to the local burden of disease, research objectives are set by funders and do not always meet this guideline. Research on cancer therapeutics focuses on lung and breast cancer, even when trials are performed in LMIC which have a large burden of cervical, liver and gastric cancers [ 34 ]. While some risk is unavoidable when testing new drugs, research subjects should expect benefits to accrue for their population.
Use of rigorous methodology with adequate power and appropriate control groups is essential to ethical research. Underpowered research, poorly designed studies, and falsified data will not yield valid results and are inherently unethical. The use of placebo controls has been an area of contention since early studies of maternal-fetal transmission of HIV [ 35 ]. While placebo-controlled trials are optimal from a methodological standpoint, patients should be treated with their standard local regimen if effective treatment is available. Cheah et al. provide a compelling rationale for a placebo arm in their malaria trial, as use of the placebo is equivalent to current clinical practice, thus the subjects do not incur additional risk [ 36 ]. Researchers should ensure that interventions proven successful will be sustainable in their study population [ 37 ].
Unproven interventions may be utilized during emergency and disaster situations. The World Health Organization convened a panel to help guide use of novel therapies during epidemics [ 38 ]. They stress the importance rigorous data collection to assess effectiveness and safety of such interventions, and transparency and fairness in decisions regarding access to investigational treatments.
Minimizing risk means building safety into the protocol and monitoring for adverse events throughout the trial. Adverse events may require unblinding, a report to the HREC, or cessation of the trial. Dissemination of all results, regardless of the success of the trial, is crucial to ensure future study participants are not exposed to risks of unsuccessful interventions and funding can be distributed to projects more likely to result in improved health [ 39 ].
Protection of privacy is another important principle of beneficence. Data should only be accessible to the research team. Surveys and other paper documents must be kept in a locked cabinet or drawer in a secure room. Files should be password protected. Study ID numbers should be substituted for individual identifiers. Master lists that could be used to link data back to participants should be destroyed after data is cleaned. Data must be de-identified before sharing data with anyone outside the original study team. Data-sharing is important for verification of results and avoidance of duplicate efforts, but regulations and guidelines have not kept pace with emerging technologies [ 40 , 41 ]. Privacy laws vary widely between countries, with the European Union having some of the strictest protections. Although it would seem that outside researchers would adhere to the standards they must uphold to in their own settings, this is not always the case. Medical students from the US are more likely to violate patients' privacy online during international electives than during rotations at home [ 42 ].
Justice requires that the costs and benefits of research are distributed fairly within the population. Even this statement is open to interpretation, as there are no global norms of fairness [ 43 ]. Justice is a key consideration in the research framework proposed by Pratt and Loff [ 44 ].
The global distribution of harms and benefits in research is particularly problematic in the setting of clinical trials. The cost of research is significantly lower in LMIC but there is concern that pharmaceutical companies are also benefitting from lax regulatory oversight to conduct high-risk research that would not be tolerated in the global north [ 45 ]. Millions of persons participate in clinical research each year, but the number of adverse events is difficult to determine and likely underreported [ 46 , 47 ]. Deaths in clinical trials are not distributed evenly across nations, with higher rates of morbidity and mortality in LMIC. While deaths in the global north tend to be rare and highly publicized, India had 2644 deaths during clinical trials in the years 2005–12 [ 48 ]. Laws regarding compensation for injuries sustained during research are markedly different between nations, adding another layer of disparities [ 49 ].
Compensation for participation in research is another potential pitfall. Western research ethics considers whether compensation for participation is enough to exert undue influence or coercion. Paradoxically, using this standard in LMIC research would decrease the value of compensation as smaller amounts would likely constitute a larger proportion of subjects' income [ 50 ]. Participation “gifts” of nominal value, such as soap or sugar, may carry hidden meanings in the local context and should be discussed with community leaders [ 51 ]. One proposed solution would be to consider participation as work, to be compensated according to local custom [ 52 ].
A final area of justice concerns authorship of manuscripts. There is an inherent imbalance of power when outsiders approach a community to do research. Researchers should strive to build capacity in local communities and ensure all members of the study team receive appropriate credit. The International Committee of Medical Journal Editors requires the following criteria be met to be listed as an author: contributions to study design, data acquisition, or data analysis, drafting or substantial revisions to the manuscript, final approval of submission, and agreement to be accountable for integrity of the work [ 53 ]. All persons who meet these criteria should be listed. Guest authorship has been reported as common and usually involves including superiors or subject experts that did not contribute substantially to the manuscript [ 54 ]. Some authors fear that the criteria to draft/substantially revise and approve the final document discriminates against researchers from LMIC whose native language is not commonly used for international publications [ 55 ]. Determination of authorship and order of authors may cause great angst, particularly if not done early in the process.
Emerging challenges
Data security.
Collection of genetic material, even blood samples, may jeopardize anonymity. Consensus is lacking on how best to use “big data” to improve public health while protecting individual privacy concerns.
Tips on this topic
- • Research in LMICs, contextualized to their local health systems, is necessary to ensure patients receive the most appropriate care.
- • Researchers should ensure sound methodology and data management practices in their studies.
- • Community engagement is one way of ensuring patients' values are incorporated into setting research priorities, the consent process, risk assessment, and dissemination of results.
- • HRECs have a responsibility to protect human subjects but also serve to protect researchers from allegations of improper behaviour. Ethical approval for a protocol is required before researchers approach participants and is required for publication by most journals.
Pitfalls to avoid
- • Failure to consider local norms in obtaining informed consent
- • Failure to execute the study in exact accordance with the approved study protocol and other documentation may undermine the ethical integrity of the project and violate the ethical principles of research. Any changes to approved study protocols must be reviewed and approved by the local HREC prior to adoption.
- • Inadequate procedures for confidentiality and data security
Annotated bibliography
- • Biruk [ 51 ] uses the example of soap as a gift for participation in surveys to discuss ethical compensation for participants and duties of researchers.
- • Das et al. [ 32 ] discuss participants' limited understanding of the overlap of clinical care and research in malaria studies.
- • Emanuel et al. [ 6 ] established benchmarks for ethical research in the global setting.
- • The WHO advisory panel report [ 38 ] and Ezeome and Simon [ 31 ] discuss ways of minimizing ethical pitfalls when performing research during epidemics – these articles are applicable to the discussion of experimental treatments for the ongoing COVID-19 pandemic.
- • Groves et al. [ 30 ] used qualitative methods to explore adolescents' views on research requirements for parental involvement in studies of HIV in youth.
- • Ochieng et al. [ 10 ] describe the development of research ethics within Uganda, starting with early regulations on protection of human subjects, through revisions to applicable statues, and training of local experts.
- • Tangwa [ 8 ] critiques the lack of inclusion of African researchers in the efforts surrounding HIV vaccine trials and the response to the Ebola outbreak of 2013–16.
- • World Medical Association Declaration of Helsinki [ 16 ] describes the principles that should guide research. Most recent version available at https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ .
Academic Life in Emergency Medicine: Society for Academic Emergency Medicine Research Learning Series provides helpful guidance for junior researchers in many stages of starting a research project. In particular, episode 3 deals with IRB pitfalls and episode 8 discusses waivers from informed consent.
University of Oxford Global Health podcast has an interview with Dr. Phaik Yeong Cheah on the ethics of research in vulnerable populations (Oct 5, 2015).
Authors' contribution
Authors contributed as follow to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content: CB contributed 50%; AAN contributed 30%; and ES and DMT contributed 10% each. All authors approved the version to be published and agreed to be accountable for all aspects of the work.
Declaration of competing interest
The authors declared no conflicts of interest.
Frequently asked questions
Why do research ethics matter.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe.
Frequently asked questions: Methodology
Attrition refers to participants leaving a study. It always happens to some extent—for example, in randomized controlled trials for medical research.
Differential attrition occurs when attrition or dropout rates differ systematically between the intervention and the control group . As a result, the characteristics of the participants who drop out differ from the characteristics of those who stay in the study. Because of this, study results may be biased .
Action research is conducted in order to solve a particular issue immediately, while case studies are often conducted over a longer period of time and focus more on observing and analyzing a particular ongoing phenomenon.
Action research is focused on solving a problem or informing individual and community-based knowledge in a way that impacts teaching, learning, and other related processes. It is less focused on contributing theoretical input, instead producing actionable input.
Action research is particularly popular with educators as a form of systematic inquiry because it prioritizes reflection and bridges the gap between theory and practice. Educators are able to simultaneously investigate an issue as they solve it, and the method is very iterative and flexible.
A cycle of inquiry is another name for action research . It is usually visualized in a spiral shape following a series of steps, such as “planning → acting → observing → reflecting.”
To make quantitative observations , you need to use instruments that are capable of measuring the quantity you want to observe. For example, you might use a ruler to measure the length of an object or a thermometer to measure its temperature.
Criterion validity and construct validity are both types of measurement validity . In other words, they both show you how accurately a method measures something.
While construct validity is the degree to which a test or other measurement method measures what it claims to measure, criterion validity is the degree to which a test can predictively (in the future) or concurrently (in the present) measure something.
Construct validity is often considered the overarching type of measurement validity . You need to have face validity , content validity , and criterion validity in order to achieve construct validity.
Convergent validity and discriminant validity are both subtypes of construct validity . Together, they help you evaluate whether a test measures the concept it was designed to measure.
- Convergent validity indicates whether a test that is designed to measure a particular construct correlates with other tests that assess the same or similar construct.
- Discriminant validity indicates whether two tests that should not be highly related to each other are indeed not related. This type of validity is also called divergent validity .
You need to assess both in order to demonstrate construct validity. Neither one alone is sufficient for establishing construct validity.
- Discriminant validity indicates whether two tests that should not be highly related to each other are indeed not related
Content validity shows you how accurately a test or other measurement method taps into the various aspects of the specific construct you are researching.
In other words, it helps you answer the question: “does the test measure all aspects of the construct I want to measure?” If it does, then the test has high content validity.
The higher the content validity, the more accurate the measurement of the construct.
If the test fails to include parts of the construct, or irrelevant parts are included, the validity of the instrument is threatened, which brings your results into question.
Face validity and content validity are similar in that they both evaluate how suitable the content of a test is. The difference is that face validity is subjective, and assesses content at surface level.
When a test has strong face validity, anyone would agree that the test’s questions appear to measure what they are intended to measure.
For example, looking at a 4th grade math test consisting of problems in which students have to add and multiply, most people would agree that it has strong face validity (i.e., it looks like a math test).
On the other hand, content validity evaluates how well a test represents all the aspects of a topic. Assessing content validity is more systematic and relies on expert evaluation. of each question, analyzing whether each one covers the aspects that the test was designed to cover.
A 4th grade math test would have high content validity if it covered all the skills taught in that grade. Experts(in this case, math teachers), would have to evaluate the content validity by comparing the test to the learning objectives.
Snowball sampling is a non-probability sampling method . Unlike probability sampling (which involves some form of random selection ), the initial individuals selected to be studied are the ones who recruit new participants.
Because not every member of the target population has an equal chance of being recruited into the sample, selection in snowball sampling is non-random.
Snowball sampling is a non-probability sampling method , where there is not an equal chance for every member of the population to be included in the sample .
This means that you cannot use inferential statistics and make generalizations —often the goal of quantitative research . As such, a snowball sample is not representative of the target population and is usually a better fit for qualitative research .
Snowball sampling relies on the use of referrals. Here, the researcher recruits one or more initial participants, who then recruit the next ones.
Participants share similar characteristics and/or know each other. Because of this, not every member of the population has an equal chance of being included in the sample, giving rise to sampling bias .
Snowball sampling is best used in the following cases:
- If there is no sampling frame available (e.g., people with a rare disease)
- If the population of interest is hard to access or locate (e.g., people experiencing homelessness)
- If the research focuses on a sensitive topic (e.g., extramarital affairs)
The reproducibility and replicability of a study can be ensured by writing a transparent, detailed method section and using clear, unambiguous language.
Reproducibility and replicability are related terms.
- Reproducing research entails reanalyzing the existing data in the same manner.
- Replicating (or repeating ) the research entails reconducting the entire analysis, including the collection of new data .
- A successful reproduction shows that the data analyses were conducted in a fair and honest manner.
- A successful replication shows that the reliability of the results is high.
Stratified sampling and quota sampling both involve dividing the population into subgroups and selecting units from each subgroup. The purpose in both cases is to select a representative sample and/or to allow comparisons between subgroups.
The main difference is that in stratified sampling, you draw a random sample from each subgroup ( probability sampling ). In quota sampling you select a predetermined number or proportion of units, in a non-random manner ( non-probability sampling ).
Purposive and convenience sampling are both sampling methods that are typically used in qualitative data collection.
A convenience sample is drawn from a source that is conveniently accessible to the researcher. Convenience sampling does not distinguish characteristics among the participants. On the other hand, purposive sampling focuses on selecting participants possessing characteristics associated with the research study.
The findings of studies based on either convenience or purposive sampling can only be generalized to the (sub)population from which the sample is drawn, and not to the entire population.
Random sampling or probability sampling is based on random selection. This means that each unit has an equal chance (i.e., equal probability) of being included in the sample.
On the other hand, convenience sampling involves stopping people at random, which means that not everyone has an equal chance of being selected depending on the place, time, or day you are collecting your data.
Convenience sampling and quota sampling are both non-probability sampling methods. They both use non-random criteria like availability, geographical proximity, or expert knowledge to recruit study participants.
However, in convenience sampling, you continue to sample units or cases until you reach the required sample size.
In quota sampling, you first need to divide your population of interest into subgroups (strata) and estimate their proportions (quota) in the population. Then you can start your data collection, using convenience sampling to recruit participants, until the proportions in each subgroup coincide with the estimated proportions in the population.
A sampling frame is a list of every member in the entire population . It is important that the sampling frame is as complete as possible, so that your sample accurately reflects your population.
Stratified and cluster sampling may look similar, but bear in mind that groups created in cluster sampling are heterogeneous , so the individual characteristics in the cluster vary. In contrast, groups created in stratified sampling are homogeneous , as units share characteristics.
Relatedly, in cluster sampling you randomly select entire groups and include all units of each group in your sample. However, in stratified sampling, you select some units of all groups and include them in your sample. In this way, both methods can ensure that your sample is representative of the target population .
A systematic review is secondary research because it uses existing research. You don’t collect new data yourself.
The key difference between observational studies and experimental designs is that a well-done observational study does not influence the responses of participants, while experiments do have some sort of treatment condition applied to at least some participants by random assignment .
An observational study is a great choice for you if your research question is based purely on observations. If there are ethical, logistical, or practical concerns that prevent you from conducting a traditional experiment , an observational study may be a good choice. In an observational study, there is no interference or manipulation of the research subjects, as well as no control or treatment groups .
It’s often best to ask a variety of people to review your measurements. You can ask experts, such as other researchers, or laypeople, such as potential participants, to judge the face validity of tests.
While experts have a deep understanding of research methods , the people you’re studying can provide you with valuable insights you may have missed otherwise.
Face validity is important because it’s a simple first step to measuring the overall validity of a test or technique. It’s a relatively intuitive, quick, and easy way to start checking whether a new measure seems useful at first glance.
Good face validity means that anyone who reviews your measure says that it seems to be measuring what it’s supposed to. With poor face validity, someone reviewing your measure may be left confused about what you’re measuring and why you’re using this method.
Face validity is about whether a test appears to measure what it’s supposed to measure. This type of validity is concerned with whether a measure seems relevant and appropriate for what it’s assessing only on the surface.
Statistical analyses are often applied to test validity with data from your measures. You test convergent validity and discriminant validity with correlations to see if results from your test are positively or negatively related to those of other established tests.
You can also use regression analyses to assess whether your measure is actually predictive of outcomes that you expect it to predict theoretically. A regression analysis that supports your expectations strengthens your claim of construct validity .
When designing or evaluating a measure, construct validity helps you ensure you’re actually measuring the construct you’re interested in. If you don’t have construct validity, you may inadvertently measure unrelated or distinct constructs and lose precision in your research.
Construct validity is often considered the overarching type of measurement validity , because it covers all of the other types. You need to have face validity , content validity , and criterion validity to achieve construct validity.
Construct validity is about how well a test measures the concept it was designed to evaluate. It’s one of four types of measurement validity , which includes construct validity, face validity , and criterion validity.
There are two subtypes of construct validity.
- Convergent validity : The extent to which your measure corresponds to measures of related constructs
- Discriminant validity : The extent to which your measure is unrelated or negatively related to measures of distinct constructs
Naturalistic observation is a valuable tool because of its flexibility, external validity , and suitability for topics that can’t be studied in a lab setting.
The downsides of naturalistic observation include its lack of scientific control , ethical considerations , and potential for bias from observers and subjects.
Naturalistic observation is a qualitative research method where you record the behaviors of your research subjects in real world settings. You avoid interfering or influencing anything in a naturalistic observation.
You can think of naturalistic observation as “people watching” with a purpose.
A dependent variable is what changes as a result of the independent variable manipulation in experiments . It’s what you’re interested in measuring, and it “depends” on your independent variable.
In statistics, dependent variables are also called:
- Response variables (they respond to a change in another variable)
- Outcome variables (they represent the outcome you want to measure)
- Left-hand-side variables (they appear on the left-hand side of a regression equation)
An independent variable is the variable you manipulate, control, or vary in an experimental study to explore its effects. It’s called “independent” because it’s not influenced by any other variables in the study.
Independent variables are also called:
- Explanatory variables (they explain an event or outcome)
- Predictor variables (they can be used to predict the value of a dependent variable)
- Right-hand-side variables (they appear on the right-hand side of a regression equation).
As a rule of thumb, questions related to thoughts, beliefs, and feelings work well in focus groups. Take your time formulating strong questions, paying special attention to phrasing. Be careful to avoid leading questions , which can bias your responses.
Overall, your focus group questions should be:
- Open-ended and flexible
- Impossible to answer with “yes” or “no” (questions that start with “why” or “how” are often best)
- Unambiguous, getting straight to the point while still stimulating discussion
- Unbiased and neutral
A structured interview is a data collection method that relies on asking questions in a set order to collect data on a topic. They are often quantitative in nature. Structured interviews are best used when:
- You already have a very clear understanding of your topic. Perhaps significant research has already been conducted, or you have done some prior research yourself, but you already possess a baseline for designing strong structured questions.
- You are constrained in terms of time or resources and need to analyze your data quickly and efficiently.
- Your research question depends on strong parity between participants, with environmental conditions held constant.
More flexible interview options include semi-structured interviews , unstructured interviews , and focus groups .
Social desirability bias is the tendency for interview participants to give responses that will be viewed favorably by the interviewer or other participants. It occurs in all types of interviews and surveys , but is most common in semi-structured interviews , unstructured interviews , and focus groups .
Social desirability bias can be mitigated by ensuring participants feel at ease and comfortable sharing their views. Make sure to pay attention to your own body language and any physical or verbal cues, such as nodding or widening your eyes.
This type of bias can also occur in observations if the participants know they’re being observed. They might alter their behavior accordingly.
The interviewer effect is a type of bias that emerges when a characteristic of an interviewer (race, age, gender identity, etc.) influences the responses given by the interviewee.
There is a risk of an interviewer effect in all types of interviews , but it can be mitigated by writing really high-quality interview questions.
A semi-structured interview is a blend of structured and unstructured types of interviews. Semi-structured interviews are best used when:
- You have prior interview experience. Spontaneous questions are deceptively challenging, and it’s easy to accidentally ask a leading question or make a participant uncomfortable.
- Your research question is exploratory in nature. Participant answers can guide future research questions and help you develop a more robust knowledge base for future research.
An unstructured interview is the most flexible type of interview, but it is not always the best fit for your research topic.
Unstructured interviews are best used when:
- You are an experienced interviewer and have a very strong background in your research topic, since it is challenging to ask spontaneous, colloquial questions.
- Your research question is exploratory in nature. While you may have developed hypotheses, you are open to discovering new or shifting viewpoints through the interview process.
- You are seeking descriptive data, and are ready to ask questions that will deepen and contextualize your initial thoughts and hypotheses.
- Your research depends on forming connections with your participants and making them feel comfortable revealing deeper emotions, lived experiences, or thoughts.
The four most common types of interviews are:
- Structured interviews : The questions are predetermined in both topic and order.
- Semi-structured interviews : A few questions are predetermined, but other questions aren’t planned.
- Unstructured interviews : None of the questions are predetermined.
- Focus group interviews : The questions are presented to a group instead of one individual.
Deductive reasoning is commonly used in scientific research, and it’s especially associated with quantitative research .
In research, you might have come across something called the hypothetico-deductive method . It’s the scientific method of testing hypotheses to check whether your predictions are substantiated by real-world data.
Deductive reasoning is a logical approach where you progress from general ideas to specific conclusions. It’s often contrasted with inductive reasoning , where you start with specific observations and form general conclusions.
Deductive reasoning is also called deductive logic.
There are many different types of inductive reasoning that people use formally or informally.
Here are a few common types:
- Inductive generalization : You use observations about a sample to come to a conclusion about the population it came from.
- Statistical generalization: You use specific numbers about samples to make statements about populations.
- Causal reasoning: You make cause-and-effect links between different things.
- Sign reasoning: You make a conclusion about a correlational relationship between different things.
- Analogical reasoning: You make a conclusion about something based on its similarities to something else.
Inductive reasoning is a bottom-up approach, while deductive reasoning is top-down.
Inductive reasoning takes you from the specific to the general, while in deductive reasoning, you make inferences by going from general premises to specific conclusions.
In inductive research , you start by making observations or gathering data. Then, you take a broad scan of your data and search for patterns. Finally, you make general conclusions that you might incorporate into theories.
Inductive reasoning is a method of drawing conclusions by going from the specific to the general. It’s usually contrasted with deductive reasoning, where you proceed from general information to specific conclusions.
Inductive reasoning is also called inductive logic or bottom-up reasoning.
A hypothesis states your predictions about what your research will find. It is a tentative answer to your research question that has not yet been tested. For some research projects, you might have to write several hypotheses that address different aspects of your research question.
A hypothesis is not just a guess — it should be based on existing theories and knowledge. It also has to be testable, which means you can support or refute it through scientific research methods (such as experiments, observations and statistical analysis of data).
Triangulation can help:
- Reduce research bias that comes from using a single method, theory, or investigator
- Enhance validity by approaching the same topic with different tools
- Establish credibility by giving you a complete picture of the research problem
But triangulation can also pose problems:
- It’s time-consuming and labor-intensive, often involving an interdisciplinary team.
- Your results may be inconsistent or even contradictory.
There are four main types of triangulation :
- Data triangulation : Using data from different times, spaces, and people
- Investigator triangulation : Involving multiple researchers in collecting or analyzing data
- Theory triangulation : Using varying theoretical perspectives in your research
- Methodological triangulation : Using different methodologies to approach the same topic
Many academic fields use peer review , largely to determine whether a manuscript is suitable for publication. Peer review enhances the credibility of the published manuscript.
However, peer review is also common in non-academic settings. The United Nations, the European Union, and many individual nations use peer review to evaluate grant applications. It is also widely used in medical and health-related fields as a teaching or quality-of-care measure.
Peer assessment is often used in the classroom as a pedagogical tool. Both receiving feedback and providing it are thought to enhance the learning process, helping students think critically and collaboratively.
Peer review can stop obviously problematic, falsified, or otherwise untrustworthy research from being published. It also represents an excellent opportunity to get feedback from renowned experts in your field. It acts as a first defense, helping you ensure your argument is clear and that there are no gaps, vague terms, or unanswered questions for readers who weren’t involved in the research process.
Peer-reviewed articles are considered a highly credible source due to this stringent process they go through before publication.
In general, the peer review process follows the following steps:
- First, the author submits the manuscript to the editor.
- Reject the manuscript and send it back to author, or
- Send it onward to the selected peer reviewer(s)
- Next, the peer review process occurs. The reviewer provides feedback, addressing any major or minor issues with the manuscript, and gives their advice regarding what edits should be made.
- Lastly, the edited manuscript is sent back to the author. They input the edits, and resubmit it to the editor for publication.
Exploratory research is often used when the issue you’re studying is new or when the data collection process is challenging for some reason.
You can use exploratory research if you have a general idea or a specific question that you want to study but there is no preexisting knowledge or paradigm with which to study it.
Exploratory research is a methodology approach that explores research questions that have not previously been studied in depth. It is often used when the issue you’re studying is new, or the data collection process is challenging in some way.
Explanatory research is used to investigate how or why a phenomenon occurs. Therefore, this type of research is often one of the first stages in the research process , serving as a jumping-off point for future research.
Exploratory research aims to explore the main aspects of an under-researched problem, while explanatory research aims to explain the causes and consequences of a well-defined problem.
Explanatory research is a research method used to investigate how or why something occurs when only a small amount of information is available pertaining to that topic. It can help you increase your understanding of a given topic.
Clean data are valid, accurate, complete, consistent, unique, and uniform. Dirty data include inconsistencies and errors.
Dirty data can come from any part of the research process, including poor research design , inappropriate measurement materials, or flawed data entry.
Data cleaning takes place between data collection and data analyses. But you can use some methods even before collecting data.
For clean data, you should start by designing measures that collect valid data. Data validation at the time of data entry or collection helps you minimize the amount of data cleaning you’ll need to do.
After data collection, you can use data standardization and data transformation to clean your data. You’ll also deal with any missing values, outliers, and duplicate values.
Every dataset requires different techniques to clean dirty data , but you need to address these issues in a systematic way. You focus on finding and resolving data points that don’t agree or fit with the rest of your dataset.
These data might be missing values, outliers, duplicate values, incorrectly formatted, or irrelevant. You’ll start with screening and diagnosing your data. Then, you’ll often standardize and accept or remove data to make your dataset consistent and valid.
Data cleaning is necessary for valid and appropriate analyses. Dirty data contain inconsistencies or errors , but cleaning your data helps you minimize or resolve these.
Without data cleaning, you could end up with a Type I or II error in your conclusion. These types of erroneous conclusions can be practically significant with important consequences, because they lead to misplaced investments or missed opportunities.
Data cleaning involves spotting and resolving potential data inconsistencies or errors to improve your data quality. An error is any value (e.g., recorded weight) that doesn’t reflect the true value (e.g., actual weight) of something that’s being measured.
In this process, you review, analyze, detect, modify, or remove “dirty” data to make your dataset “clean.” Data cleaning is also called data cleansing or data scrubbing.
Research misconduct means making up or falsifying data, manipulating data analyses, or misrepresenting results in research reports. It’s a form of academic fraud.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement but a serious ethical failure.
Anonymity means you don’t know who the participants are, while confidentiality means you know who they are but remove identifying information from your research report. Both are important ethical considerations .
You can only guarantee anonymity by not collecting any personally identifying information—for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, or videos.
You can keep data confidential by using aggregate information in your research report, so that you only refer to groups of participants rather than individuals.
Ethical considerations in research are a set of principles that guide your research designs and practices. These principles include voluntary participation, informed consent, anonymity, confidentiality, potential for harm, and results communication.
Scientists and researchers must always adhere to a certain code of conduct when collecting data from others .
These considerations protect the rights of research participants, enhance research validity , and maintain scientific integrity.
In multistage sampling , you can use probability or non-probability sampling methods .
For a probability sample, you have to conduct probability sampling at every stage.
You can mix it up by using simple random sampling , systematic sampling , or stratified sampling to select units at different stages, depending on what is applicable and relevant to your study.
Multistage sampling can simplify data collection when you have large, geographically spread samples, and you can obtain a probability sample without a complete sampling frame.
But multistage sampling may not lead to a representative sample, and larger samples are needed for multistage samples to achieve the statistical properties of simple random samples .
These are four of the most common mixed methods designs :
- Convergent parallel: Quantitative and qualitative data are collected at the same time and analyzed separately. After both analyses are complete, compare your results to draw overall conclusions.
- Embedded: Quantitative and qualitative data are collected at the same time, but within a larger quantitative or qualitative design. One type of data is secondary to the other.
- Explanatory sequential: Quantitative data is collected and analyzed first, followed by qualitative data. You can use this design if you think your qualitative data will explain and contextualize your quantitative findings.
- Exploratory sequential: Qualitative data is collected and analyzed first, followed by quantitative data. You can use this design if you think the quantitative data will confirm or validate your qualitative findings.
Triangulation in research means using multiple datasets, methods, theories and/or investigators to address a research question. It’s a research strategy that can help you enhance the validity and credibility of your findings.
Triangulation is mainly used in qualitative research , but it’s also commonly applied in quantitative research . Mixed methods research always uses triangulation.
In multistage sampling , or multistage cluster sampling, you draw a sample from a population using smaller and smaller groups at each stage.
This method is often used to collect data from a large, geographically spread group of people in national surveys, for example. You take advantage of hierarchical groupings (e.g., from state to city to neighborhood) to create a sample that’s less expensive and time-consuming to collect data from.
No, the steepness or slope of the line isn’t related to the correlation coefficient value. The correlation coefficient only tells you how closely your data fit on a line, so two datasets with the same correlation coefficient can have very different slopes.
To find the slope of the line, you’ll need to perform a regression analysis .
Correlation coefficients always range between -1 and 1.
The sign of the coefficient tells you the direction of the relationship: a positive value means the variables change together in the same direction, while a negative value means they change together in opposite directions.
The absolute value of a number is equal to the number without its sign. The absolute value of a correlation coefficient tells you the magnitude of the correlation: the greater the absolute value, the stronger the correlation.
These are the assumptions your data must meet if you want to use Pearson’s r :
- Both variables are on an interval or ratio level of measurement
- Data from both variables follow normal distributions
- Your data have no outliers
- Your data is from a random or representative sample
- You expect a linear relationship between the two variables
Quantitative research designs can be divided into two main categories:
- Correlational and descriptive designs are used to investigate characteristics, averages, trends, and associations between variables.
- Experimental and quasi-experimental designs are used to test causal relationships .
Qualitative research designs tend to be more flexible. Common types of qualitative design include case study , ethnography , and grounded theory designs.
A well-planned research design helps ensure that your methods match your research aims, that you collect high-quality data, and that you use the right kind of analysis to answer your questions, utilizing credible sources . This allows you to draw valid , trustworthy conclusions.
The priorities of a research design can vary depending on the field, but you usually have to specify:
- Your research questions and/or hypotheses
- Your overall approach (e.g., qualitative or quantitative )
- The type of design you’re using (e.g., a survey , experiment , or case study )
- Your sampling methods or criteria for selecting subjects
- Your data collection methods (e.g., questionnaires , observations)
- Your data collection procedures (e.g., operationalization , timing and data management)
- Your data analysis methods (e.g., statistical tests or thematic analysis )
A research design is a strategy for answering your research question . It defines your overall approach and determines how you will collect and analyze data.
Questionnaires can be self-administered or researcher-administered.
Self-administered questionnaires can be delivered online or in paper-and-pen formats, in person or through mail. All questions are standardized so that all respondents receive the same questions with identical wording.
Researcher-administered questionnaires are interviews that take place by phone, in-person, or online between researchers and respondents. You can gain deeper insights by clarifying questions for respondents or asking follow-up questions.
You can organize the questions logically, with a clear progression from simple to complex, or randomly between respondents. A logical flow helps respondents process the questionnaire easier and quicker, but it may lead to bias. Randomization can minimize the bias from order effects.
Closed-ended, or restricted-choice, questions offer respondents a fixed set of choices to select from. These questions are easier to answer quickly.
Open-ended or long-form questions allow respondents to answer in their own words. Because there are no restrictions on their choices, respondents can answer in ways that researchers may not have otherwise considered.
A questionnaire is a data collection tool or instrument, while a survey is an overarching research method that involves collecting and analyzing data from people using questionnaires.
The third variable and directionality problems are two main reasons why correlation isn’t causation .
The third variable problem means that a confounding variable affects both variables to make them seem causally related when they are not.
The directionality problem is when two variables correlate and might actually have a causal relationship, but it’s impossible to conclude which variable causes changes in the other.
Correlation describes an association between variables : when one variable changes, so does the other. A correlation is a statistical indicator of the relationship between variables.
Causation means that changes in one variable brings about changes in the other (i.e., there is a cause-and-effect relationship between variables). The two variables are correlated with each other, and there’s also a causal link between them.
While causation and correlation can exist simultaneously, correlation does not imply causation. In other words, correlation is simply a relationship where A relates to B—but A doesn’t necessarily cause B to happen (or vice versa). Mistaking correlation for causation is a common error and can lead to false cause fallacy .
Controlled experiments establish causality, whereas correlational studies only show associations between variables.
- In an experimental design , you manipulate an independent variable and measure its effect on a dependent variable. Other variables are controlled so they can’t impact the results.
- In a correlational design , you measure variables without manipulating any of them. You can test whether your variables change together, but you can’t be sure that one variable caused a change in another.
In general, correlational research is high in external validity while experimental research is high in internal validity .
A correlation is usually tested for two variables at a time, but you can test correlations between three or more variables.
A correlation coefficient is a single number that describes the strength and direction of the relationship between your variables.
Different types of correlation coefficients might be appropriate for your data based on their levels of measurement and distributions . The Pearson product-moment correlation coefficient (Pearson’s r ) is commonly used to assess a linear relationship between two quantitative variables.
A correlational research design investigates relationships between two variables (or more) without the researcher controlling or manipulating any of them. It’s a non-experimental type of quantitative research .
A correlation reflects the strength and/or direction of the association between two or more variables.
- A positive correlation means that both variables change in the same direction.
- A negative correlation means that the variables change in opposite directions.
- A zero correlation means there’s no relationship between the variables.
Random error is almost always present in scientific studies, even in highly controlled settings. While you can’t eradicate it completely, you can reduce random error by taking repeated measurements, using a large sample, and controlling extraneous variables .
You can avoid systematic error through careful design of your sampling , data collection , and analysis procedures. For example, use triangulation to measure your variables using multiple methods; regularly calibrate instruments or procedures; use random sampling and random assignment ; and apply masking (blinding) where possible.
Systematic error is generally a bigger problem in research.
With random error, multiple measurements will tend to cluster around the true value. When you’re collecting data from a large sample , the errors in different directions will cancel each other out.
Systematic errors are much more problematic because they can skew your data away from the true value. This can lead you to false conclusions ( Type I and II errors ) about the relationship between the variables you’re studying.
Random and systematic error are two types of measurement error.
Random error is a chance difference between the observed and true values of something (e.g., a researcher misreading a weighing scale records an incorrect measurement).
Systematic error is a consistent or proportional difference between the observed and true values of something (e.g., a miscalibrated scale consistently records weights as higher than they actually are).
On graphs, the explanatory variable is conventionally placed on the x-axis, while the response variable is placed on the y-axis.
- If you have quantitative variables , use a scatterplot or a line graph.
- If your response variable is categorical, use a scatterplot or a line graph.
- If your explanatory variable is categorical, use a bar graph.
The term “ explanatory variable ” is sometimes preferred over “ independent variable ” because, in real world contexts, independent variables are often influenced by other variables. This means they aren’t totally independent.
Multiple independent variables may also be correlated with each other, so “explanatory variables” is a more appropriate term.
The difference between explanatory and response variables is simple:
- An explanatory variable is the expected cause, and it explains the results.
- A response variable is the expected effect, and it responds to other variables.
In a controlled experiment , all extraneous variables are held constant so that they can’t influence the results. Controlled experiments require:
- A control group that receives a standard treatment, a fake treatment, or no treatment.
- Random assignment of participants to ensure the groups are equivalent.
Depending on your study topic, there are various other methods of controlling variables .
There are 4 main types of extraneous variables :
- Demand characteristics : environmental cues that encourage participants to conform to researchers’ expectations.
- Experimenter effects : unintentional actions by researchers that influence study outcomes.
- Situational variables : environmental variables that alter participants’ behaviors.
- Participant variables : any characteristic or aspect of a participant’s background that could affect study results.
An extraneous variable is any variable that you’re not investigating that can potentially affect the dependent variable of your research study.
A confounding variable is a type of extraneous variable that not only affects the dependent variable, but is also related to the independent variable.
In a factorial design, multiple independent variables are tested.
If you test two variables, each level of one independent variable is combined with each level of the other independent variable to create different conditions.
Within-subjects designs have many potential threats to internal validity , but they are also very statistically powerful .
Advantages:
- Only requires small samples
- Statistically powerful
- Removes the effects of individual differences on the outcomes
Disadvantages:
- Internal validity threats reduce the likelihood of establishing a direct relationship between variables
- Time-related effects, such as growth, can influence the outcomes
- Carryover effects mean that the specific order of different treatments affect the outcomes
While a between-subjects design has fewer threats to internal validity , it also requires more participants for high statistical power than a within-subjects design .
- Prevents carryover effects of learning and fatigue.
- Shorter study duration.
- Needs larger samples for high power.
- Uses more resources to recruit participants, administer sessions, cover costs, etc.
- Individual differences may be an alternative explanation for results.
Yes. Between-subjects and within-subjects designs can be combined in a single study when you have two or more independent variables (a factorial design). In a mixed factorial design, one variable is altered between subjects and another is altered within subjects.
In a between-subjects design , every participant experiences only one condition, and researchers assess group differences between participants in various conditions.
In a within-subjects design , each participant experiences all conditions, and researchers test the same participants repeatedly for differences between conditions.
The word “between” means that you’re comparing different conditions between groups, while the word “within” means you’re comparing different conditions within the same group.
Random assignment is used in experiments with a between-groups or independent measures design. In this research design, there’s usually a control group and one or more experimental groups. Random assignment helps ensure that the groups are comparable.
In general, you should always use random assignment in this type of experimental design when it is ethically possible and makes sense for your study topic.
To implement random assignment , assign a unique number to every member of your study’s sample .
Then, you can use a random number generator or a lottery method to randomly assign each number to a control or experimental group. You can also do so manually, by flipping a coin or rolling a dice to randomly assign participants to groups.
Random selection, or random sampling , is a way of selecting members of a population for your study’s sample.
In contrast, random assignment is a way of sorting the sample into control and experimental groups.
Random sampling enhances the external validity or generalizability of your results, while random assignment improves the internal validity of your study.
In experimental research, random assignment is a way of placing participants from your sample into different groups using randomization. With this method, every member of the sample has a known or equal chance of being placed in a control group or an experimental group.
“Controlling for a variable” means measuring extraneous variables and accounting for them statistically to remove their effects on other variables.
Researchers often model control variable data along with independent and dependent variable data in regression analyses and ANCOVAs . That way, you can isolate the control variable’s effects from the relationship between the variables of interest.
Control variables help you establish a correlational or causal relationship between variables by enhancing internal validity .
If you don’t control relevant extraneous variables , they may influence the outcomes of your study, and you may not be able to demonstrate that your results are really an effect of your independent variable .
A control variable is any variable that’s held constant in a research study. It’s not a variable of interest in the study, but it’s controlled because it could influence the outcomes.
Including mediators and moderators in your research helps you go beyond studying a simple relationship between two variables for a fuller picture of the real world. They are important to consider when studying complex correlational or causal relationships.
Mediators are part of the causal pathway of an effect, and they tell you how or why an effect takes place. Moderators usually help you judge the external validity of your study by identifying the limitations of when the relationship between variables holds.
If something is a mediating variable :
- It’s caused by the independent variable .
- It influences the dependent variable
- When it’s taken into account, the statistical correlation between the independent and dependent variables is higher than when it isn’t considered.
A confounder is a third variable that affects variables of interest and makes them seem related when they are not. In contrast, a mediator is the mechanism of a relationship between two variables: it explains the process by which they are related.
A mediator variable explains the process through which two variables are related, while a moderator variable affects the strength and direction of that relationship.
There are three key steps in systematic sampling :
- Define and list your population , ensuring that it is not ordered in a cyclical or periodic order.
- Decide on your sample size and calculate your interval, k , by dividing your population by your target sample size.
- Choose every k th member of the population as your sample.
Systematic sampling is a probability sampling method where researchers select members of the population at a regular interval – for example, by selecting every 15th person on a list of the population. If the population is in a random order, this can imitate the benefits of simple random sampling .
Yes, you can create a stratified sample using multiple characteristics, but you must ensure that every participant in your study belongs to one and only one subgroup. In this case, you multiply the numbers of subgroups for each characteristic to get the total number of groups.
For example, if you were stratifying by location with three subgroups (urban, rural, or suburban) and marital status with five subgroups (single, divorced, widowed, married, or partnered), you would have 3 x 5 = 15 subgroups.
You should use stratified sampling when your sample can be divided into mutually exclusive and exhaustive subgroups that you believe will take on different mean values for the variable that you’re studying.
Using stratified sampling will allow you to obtain more precise (with lower variance ) statistical estimates of whatever you are trying to measure.
For example, say you want to investigate how income differs based on educational attainment, but you know that this relationship can vary based on race. Using stratified sampling, you can ensure you obtain a large enough sample from each racial group, allowing you to draw more precise conclusions.
In stratified sampling , researchers divide subjects into subgroups called strata based on characteristics that they share (e.g., race, gender, educational attainment).
Once divided, each subgroup is randomly sampled using another probability sampling method.
Cluster sampling is more time- and cost-efficient than other probability sampling methods , particularly when it comes to large samples spread across a wide geographical area.
However, it provides less statistical certainty than other methods, such as simple random sampling , because it is difficult to ensure that your clusters properly represent the population as a whole.
There are three types of cluster sampling : single-stage, double-stage and multi-stage clustering. In all three types, you first divide the population into clusters, then randomly select clusters for use in your sample.
- In single-stage sampling , you collect data from every unit within the selected clusters.
- In double-stage sampling , you select a random sample of units from within the clusters.
- In multi-stage sampling , you repeat the procedure of randomly sampling elements from within the clusters until you have reached a manageable sample.
Cluster sampling is a probability sampling method in which you divide a population into clusters, such as districts or schools, and then randomly select some of these clusters as your sample.
The clusters should ideally each be mini-representations of the population as a whole.
If properly implemented, simple random sampling is usually the best sampling method for ensuring both internal and external validity . However, it can sometimes be impractical and expensive to implement, depending on the size of the population to be studied,
If you have a list of every member of the population and the ability to reach whichever members are selected, you can use simple random sampling.
The American Community Survey is an example of simple random sampling . In order to collect detailed data on the population of the US, the Census Bureau officials randomly select 3.5 million households per year and use a variety of methods to convince them to fill out the survey.
Simple random sampling is a type of probability sampling in which the researcher randomly selects a subset of participants from a population . Each member of the population has an equal chance of being selected. Data is then collected from as large a percentage as possible of this random subset.
Quasi-experimental design is most useful in situations where it would be unethical or impractical to run a true experiment .
Quasi-experiments have lower internal validity than true experiments, but they often have higher external validity as they can use real-world interventions instead of artificial laboratory settings.
A quasi-experiment is a type of research design that attempts to establish a cause-and-effect relationship. The main difference with a true experiment is that the groups are not randomly assigned.
Blinding is important to reduce research bias (e.g., observer bias , demand characteristics ) and ensure a study’s internal validity .
If participants know whether they are in a control or treatment group , they may adjust their behavior in ways that affect the outcome that researchers are trying to measure. If the people administering the treatment are aware of group assignment, they may treat participants differently and thus directly or indirectly influence the final results.
- In a single-blind study , only the participants are blinded.
- In a double-blind study , both participants and experimenters are blinded.
- In a triple-blind study , the assignment is hidden not only from participants and experimenters, but also from the researchers analyzing the data.
Blinding means hiding who is assigned to the treatment group and who is assigned to the control group in an experiment .
A true experiment (a.k.a. a controlled experiment) always includes at least one control group that doesn’t receive the experimental treatment.
However, some experiments use a within-subjects design to test treatments without a control group. In these designs, you usually compare one group’s outcomes before and after a treatment (instead of comparing outcomes between different groups).
For strong internal validity , it’s usually best to include a control group if possible. Without a control group, it’s harder to be certain that the outcome was caused by the experimental treatment and not by other variables.
An experimental group, also known as a treatment group, receives the treatment whose effect researchers wish to study, whereas a control group does not. They should be identical in all other ways.
Individual Likert-type questions are generally considered ordinal data , because the items have clear rank order, but don’t have an even distribution.
Overall Likert scale scores are sometimes treated as interval data. These scores are considered to have directionality and even spacing between them.
The type of data determines what statistical tests you should use to analyze your data.
A Likert scale is a rating scale that quantitatively assesses opinions, attitudes, or behaviors. It is made up of 4 or more questions that measure a single attitude or trait when response scores are combined.
To use a Likert scale in a survey , you present participants with Likert-type questions or statements, and a continuum of items, usually with 5 or 7 possible responses, to capture their degree of agreement.
In scientific research, concepts are the abstract ideas or phenomena that are being studied (e.g., educational achievement). Variables are properties or characteristics of the concept (e.g., performance at school), while indicators are ways of measuring or quantifying variables (e.g., yearly grade reports).
The process of turning abstract concepts into measurable variables and indicators is called operationalization .
There are various approaches to qualitative data analysis , but they all share five steps in common:
- Prepare and organize your data.
- Review and explore your data.
- Develop a data coding system.
- Assign codes to the data.
- Identify recurring themes.
The specifics of each step depend on the focus of the analysis. Some common approaches include textual analysis , thematic analysis , and discourse analysis .
There are five common approaches to qualitative research :
- Grounded theory involves collecting data in order to develop new theories.
- Ethnography involves immersing yourself in a group or organization to understand its culture.
- Narrative research involves interpreting stories to understand how people make sense of their experiences and perceptions.
- Phenomenological research involves investigating phenomena through people’s lived experiences.
- Action research links theory and practice in several cycles to drive innovative changes.
Hypothesis testing is a formal procedure for investigating our ideas about the world using statistics. It is used by scientists to test specific predictions, called hypotheses , by calculating how likely it is that a pattern or relationship between variables could have arisen by chance.
Operationalization means turning abstract conceptual ideas into measurable observations.
For example, the concept of social anxiety isn’t directly observable, but it can be operationally defined in terms of self-rating scores, behavioral avoidance of crowded places, or physical anxiety symptoms in social situations.
Before collecting data , it’s important to consider how you will operationalize the variables that you want to measure.
When conducting research, collecting original data has significant advantages:
- You can tailor data collection to your specific research aims (e.g. understanding the needs of your consumers or user testing your website)
- You can control and standardize the process for high reliability and validity (e.g. choosing appropriate measurements and sampling methods )
However, there are also some drawbacks: data collection can be time-consuming, labor-intensive and expensive. In some cases, it’s more efficient to use secondary data that has already been collected by someone else, but the data might be less reliable.
Data collection is the systematic process by which observations or measurements are gathered in research. It is used in many different contexts by academics, governments, businesses, and other organizations.
There are several methods you can use to decrease the impact of confounding variables on your research: restriction, matching, statistical control and randomization.
In restriction , you restrict your sample by only including certain subjects that have the same values of potential confounding variables.
In matching , you match each of the subjects in your treatment group with a counterpart in the comparison group. The matched subjects have the same values on any potential confounding variables, and only differ in the independent variable .
In statistical control , you include potential confounders as variables in your regression .
In randomization , you randomly assign the treatment (or independent variable) in your study to a sufficiently large number of subjects, which allows you to control for all potential confounding variables.
A confounding variable is closely related to both the independent and dependent variables in a study. An independent variable represents the supposed cause , while the dependent variable is the supposed effect . A confounding variable is a third variable that influences both the independent and dependent variables.
Failing to account for confounding variables can cause you to wrongly estimate the relationship between your independent and dependent variables.
To ensure the internal validity of your research, you must consider the impact of confounding variables. If you fail to account for them, you might over- or underestimate the causal relationship between your independent and dependent variables , or even find a causal relationship where none exists.
Yes, but including more than one of either type requires multiple research questions .
For example, if you are interested in the effect of a diet on health, you can use multiple measures of health: blood sugar, blood pressure, weight, pulse, and many more. Each of these is its own dependent variable with its own research question.
You could also choose to look at the effect of exercise levels as well as diet, or even the additional effect of the two combined. Each of these is a separate independent variable .
To ensure the internal validity of an experiment , you should only change one independent variable at a time.
No. The value of a dependent variable depends on an independent variable, so a variable cannot be both independent and dependent at the same time. It must be either the cause or the effect, not both!
You want to find out how blood sugar levels are affected by drinking diet soda and regular soda, so you conduct an experiment .
- The type of soda – diet or regular – is the independent variable .
- The level of blood sugar that you measure is the dependent variable – it changes depending on the type of soda.
Determining cause and effect is one of the most important parts of scientific research. It’s essential to know which is the cause – the independent variable – and which is the effect – the dependent variable.
In non-probability sampling , the sample is selected based on non-random criteria, and not every member of the population has a chance of being included.
Common non-probability sampling methods include convenience sampling , voluntary response sampling, purposive sampling , snowball sampling, and quota sampling .
Probability sampling means that every member of the target population has a known chance of being included in the sample.
Probability sampling methods include simple random sampling , systematic sampling , stratified sampling , and cluster sampling .
Using careful research design and sampling procedures can help you avoid sampling bias . Oversampling can be used to correct undercoverage bias .
Some common types of sampling bias include self-selection bias , nonresponse bias , undercoverage bias , survivorship bias , pre-screening or advertising bias, and healthy user bias.
Sampling bias is a threat to external validity – it limits the generalizability of your findings to a broader group of people.
A sampling error is the difference between a population parameter and a sample statistic .
A statistic refers to measures about the sample , while a parameter refers to measures about the population .
Populations are used when a research question requires data from every member of the population. This is usually only feasible when the population is small and easily accessible.
Samples are used to make inferences about populations . Samples are easier to collect data from because they are practical, cost-effective, convenient, and manageable.
There are seven threats to external validity : selection bias , history, experimenter effect, Hawthorne effect , testing effect, aptitude-treatment and situation effect.
The two types of external validity are population validity (whether you can generalize to other groups of people) and ecological validity (whether you can generalize to other situations and settings).
The external validity of a study is the extent to which you can generalize your findings to different groups of people, situations, and measures.
Cross-sectional studies cannot establish a cause-and-effect relationship or analyze behavior over a period of time. To investigate cause and effect, you need to do a longitudinal study or an experimental study .
Cross-sectional studies are less expensive and time-consuming than many other types of study. They can provide useful insights into a population’s characteristics and identify correlations for further research.
Sometimes only cross-sectional data is available for analysis; other times your research question may only require a cross-sectional study to answer it.
Longitudinal studies can last anywhere from weeks to decades, although they tend to be at least a year long.
The 1970 British Cohort Study , which has collected data on the lives of 17,000 Brits since their births in 1970, is one well-known example of a longitudinal study .
Longitudinal studies are better to establish the correct sequence of events, identify changes over time, and provide insight into cause-and-effect relationships, but they also tend to be more expensive and time-consuming than other types of studies.
Longitudinal studies and cross-sectional studies are two different types of research design . In a cross-sectional study you collect data from a population at a specific point in time; in a longitudinal study you repeatedly collect data from the same sample over an extended period of time.
| Longitudinal study | Cross-sectional study |
|---|---|
| observations | Observations at a in time |
| Observes the multiple times | Observes (a “cross-section”) in the population |
| Follows in participants over time | Provides of society at a given point |
There are eight threats to internal validity : history, maturation, instrumentation, testing, selection bias , regression to the mean, social interaction and attrition .
Internal validity is the extent to which you can be confident that a cause-and-effect relationship established in a study cannot be explained by other factors.
In mixed methods research , you use both qualitative and quantitative data collection and analysis methods to answer your research question .
The research methods you use depend on the type of data you need to answer your research question .
- If you want to measure something or test a hypothesis , use quantitative methods . If you want to explore ideas, thoughts and meanings, use qualitative methods .
- If you want to analyze a large amount of readily-available data, use secondary data. If you want data specific to your purposes with control over how it is generated, collect primary data.
- If you want to establish cause-and-effect relationships between variables , use experimental methods. If you want to understand the characteristics of a research subject, use descriptive methods.
A confounding variable , also called a confounder or confounding factor, is a third variable in a study examining a potential cause-and-effect relationship.
A confounding variable is related to both the supposed cause and the supposed effect of the study. It can be difficult to separate the true effect of the independent variable from the effect of the confounding variable.
In your research design , it’s important to identify potential confounding variables and plan how you will reduce their impact.
Discrete and continuous variables are two types of quantitative variables :
- Discrete variables represent counts (e.g. the number of objects in a collection).
- Continuous variables represent measurable amounts (e.g. water volume or weight).
Quantitative variables are any variables where the data represent amounts (e.g. height, weight, or age).
Categorical variables are any variables where the data represent groups. This includes rankings (e.g. finishing places in a race), classifications (e.g. brands of cereal), and binary outcomes (e.g. coin flips).
You need to know what type of variables you are working with to choose the right statistical test for your data and interpret your results .
You can think of independent and dependent variables in terms of cause and effect: an independent variable is the variable you think is the cause , while a dependent variable is the effect .
In an experiment, you manipulate the independent variable and measure the outcome in the dependent variable. For example, in an experiment about the effect of nutrients on crop growth:
- The independent variable is the amount of nutrients added to the crop field.
- The dependent variable is the biomass of the crops at harvest time.
Defining your variables, and deciding how you will manipulate and measure them, is an important part of experimental design .
Experimental design means planning a set of procedures to investigate a relationship between variables . To design a controlled experiment, you need:
- A testable hypothesis
- At least one independent variable that can be precisely manipulated
- At least one dependent variable that can be precisely measured
When designing the experiment, you decide:
- How you will manipulate the variable(s)
- How you will control for any potential confounding variables
- How many subjects or samples will be included in the study
- How subjects will be assigned to treatment levels
Experimental design is essential to the internal and external validity of your experiment.
I nternal validity is the degree of confidence that the causal relationship you are testing is not influenced by other factors or variables .
External validity is the extent to which your results can be generalized to other contexts.
The validity of your experiment depends on your experimental design .
Reliability and validity are both about how well a method measures something:
- Reliability refers to the consistency of a measure (whether the results can be reproduced under the same conditions).
- Validity refers to the accuracy of a measure (whether the results really do represent what they are supposed to measure).
If you are doing experimental research, you also have to consider the internal and external validity of your experiment.
A sample is a subset of individuals from a larger population . Sampling means selecting the group that you will actually collect data from in your research. For example, if you are researching the opinions of students in your university, you could survey a sample of 100 students.
In statistics, sampling allows you to test a hypothesis about the characteristics of a population.
Quantitative research deals with numbers and statistics, while qualitative research deals with words and meanings.
Quantitative methods allow you to systematically measure variables and test hypotheses . Qualitative methods allow you to explore concepts and experiences in more detail.
Methodology refers to the overarching strategy and rationale of your research project . It involves studying the methods used in your field and the theories or principles behind them, in order to develop an approach that matches your objectives.
Methods are the specific tools and procedures you use to collect and analyze data (for example, experiments, surveys , and statistical tests ).
In shorter scientific papers, where the aim is to report the findings of a specific study, you might simply describe what you did in a methods section .
In a longer or more complex research project, such as a thesis or dissertation , you will probably include a methodology section , where you explain your approach to answering the research questions and cite relevant sources to support your choice of methods.
Ask our team
Want to contact us directly? No problem. We are always here for you.
- Email [email protected]
- Start live chat
- Call +1 (510) 822-8066
- WhatsApp +31 20 261 6040

Our team helps students graduate by offering:
- A world-class citation generator
- Plagiarism Checker software powered by Turnitin
- Innovative Citation Checker software
- Professional proofreading services
- Over 300 helpful articles about academic writing, citing sources, plagiarism, and more
Scribbr specializes in editing study-related documents . We proofread:
- PhD dissertations
- Research proposals
- Personal statements
- Admission essays
- Motivation letters
- Reflection papers
- Journal articles
- Capstone projects
Scribbr’s Plagiarism Checker is powered by elements of Turnitin’s Similarity Checker , namely the plagiarism detection software and the Internet Archive and Premium Scholarly Publications content databases .
The add-on AI detector is powered by Scribbr’s proprietary software.
The Scribbr Citation Generator is developed using the open-source Citation Style Language (CSL) project and Frank Bennett’s citeproc-js . It’s the same technology used by dozens of other popular citation tools, including Mendeley and Zotero.
You can find all the citation styles and locales used in the Scribbr Citation Generator in our publicly accessible repository on Github .
- Applied Ethics
- Moral Philosophy
Research Ethics
- Publisher: e-PG Pathshala

- Aligarh Muslim University

Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up

- Games & Quizzes
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction & Top Questions
Introduction of moral codes
- Problems of divine origin
- Nonhuman behaviour
- Kinship and reciprocity
- Anthropology and ethics
- The Middle East
- Ancient Greece
- The Epicureans
- Ethics in the New Testament
- St. Augustine
- St. Thomas Aquinas and the Scholastics
- Machiavelli
- The first Protestants
- Early intuitionists: Cudworth, More, and Clarke
- Shaftesbury and the moral sense school
- Butler on self-interest and conscience
- The climax of moral sense theory: Hutcheson and Hume
- The intuitionist response: Price and Reid
- Moore and the naturalistic fallacy
- Modern intuitionism
- Existentialism
- Universal prescriptivism
- Moral realism
- Kantian constructivism: a middle ground?
- Irrealist views: projectivism and expressivism
- Ethics and reasons for action
- The debate over consequentialism
- Varieties of consequentialism
- Objections to consequentialism
- An ethics of prima facie duties
- Rawls’s theory of justice
- Rights theories
- Natural law ethics
- Virtue ethics
- Feminist ethics
- Ethical egoism
- Environmental ethics
- War and peace
- Abortion, euthanasia, and the value of human life

What is ethics?
How is ethics different from morality, why does ethics matter, is ethics a social science.
- What did Aristotle do?

Our editors will review what you’ve submitted and determine whether to revise the article.
- Business LibreTexts - What is Ethics?
- Internet Encyclopedia of Philosophy - Ethics and Contrastivism
- Internet Encyclopedia of Philosophy - Empathy and Sympathy in Ethics
- VIVA Open Publishing - Ethics and Society - Ethical Behavior and Moral Values in Everyday Life
- Philosophy Basics - Ethics
- American Medical Association - Journal of Ethics - Triage and Ethics
- Psychology Today - Ethics and Morality
- Government of Canada - Treasury Board of Canada Secretariat - What is ethics?
- Cornell Law School - Legal Information Institute - Ethics
- ethics and morality - Student Encyclopedia (Ages 11 and up)
- Table Of Contents

The term ethics may refer to the philosophical study of the concepts of moral right and wrong and moral good and bad, to any philosophical theory of what is morally right and wrong or morally good and bad, and to any system or code of moral rules, principles, or values. The last may be associated with particular religions , cultures, professions, or virtually any other group that is at least partly characterized by its moral outlook.
Traditionally, ethics referred to the philosophical study of morality, the latter being a more or less systematic set of beliefs, usually held in common by a group, about how people should live. Ethics also referred to particular philosophical theories of morality. Later the term was applied to particular (and narrower) moral codes or value systems. Ethics and morality are now used almost interchangeably in many contexts, but the name of the philosophical study remains ethics .
Ethics matters because (1) it is part of how many groups define themselves and thus part of the identity of their individual members, (2) other-regarding values in most ethical systems both reflect and foster close human relationships and mutual respect and trust, and (3) it could be “rational” for a self-interested person to be moral, because his or her self-interest is arguably best served in the long run by reciprocating the moral behaviour of others.
No. Understood as equivalent to morality, ethics could be studied as a social-psychological or historical phenomenon, but in that case it would be an object of social-scientific study, not a social science in itself. Understood as the philosophical study of moral concepts, ethics is a branch of philosophy , not of social science.
Trusted Britannica articles, summarized using artificial intelligence, to provide a quicker and simpler reading experience. This is a beta feature. Please verify important information in our full article.
This summary was created from our Britannica article using AI. Please verify important information in our full article.
ethics , the discipline concerned with what is morally good and bad and morally right and wrong. The term is also applied to any system or theory of moral values or principles.
(Read Britannica’s biography of this author, Peter Singer.)
How should we live? Shall we aim at happiness or at knowledge, virtue , or the creation of beautiful objects? If we choose happiness, will it be our own or the happiness of all? And what of the more particular questions that face us: is it right to be dishonest in a good cause? Can we justify living in opulence while elsewhere in the world people are starving? Is going to war justified in cases where it is likely that innocent people will be killed? Is it wrong to clone a human being or to destroy human embryos in medical research? What are our obligations, if any, to the generations of humans who will come after us and to the nonhuman animals with whom we share the planet?
Ethics deals with such questions at all levels. Its subject consists of the fundamental issues of practical decision making , and its major concerns include the nature of ultimate value and the standards by which human actions can be judged right or wrong .
The terms ethics and morality are closely related. It is now common to refer to ethical judgments or to ethical principles where it once would have been more accurate to speak of moral judgments or moral principles. These applications are an extension of the meaning of ethics. In earlier usage, the term referred not to morality itself but to the field of study, or branch of inquiry, that has morality as its subject matter. In this sense, ethics is equivalent to moral philosophy .
Although ethics has always been viewed as a branch of philosophy, its all-embracing practical nature links it with many other areas of study, including anthropology , biology , economics , history , politics , sociology , and theology . Yet, ethics remains distinct from such disciplines because it is not a matter of factual knowledge in the way that the sciences and other branches of inquiry are. Rather, it has to do with determining the nature of normative theories and applying these sets of principles to practical moral problems.
This article, then, will deal with ethics as a field of philosophy, especially as it has developed in the West. For coverage of religious conceptions of ethics and the ethical systems associated with world religions, see Buddhism ; Christianity ; Confucianism ; Hinduism ; Jainism ; Judaism ; Sikhism .
The origins of ethics
Mythical accounts.
When did ethics begin and how did it originate? If one has in mind ethics proper—i.e., the systematic study of what is morally right and wrong—it is clear that ethics could have come into existence only when human beings started to reflect on the best way to live. This reflective stage emerged long after human societies had developed some kind of morality, usually in the form of customary standards of right and wrong conduct . The process of reflection tended to arise from such customs, even if in the end it may have found them wanting. Accordingly, ethics began with the introduction of the first moral codes .
Virtually every human society has some form of myth to explain the origin of morality. In the Louvre in Paris there is a black Babylonian column with a relief showing the sun god Shamash presenting the code of laws to Hammurabi (died c. 1750 bce ), known as the Code of Hammurabi . The Hebrew Bible ( Old Testament ) account of God’s giving the Ten Commandments to Moses (flourished 14th–13th century bce ) on Mount Sinai might be considered another example. In the dialogue Protagoras by Plato (428/427–348/347 bce ), there is an avowedly mythical account of how Zeus took pity on the hapless humans, who were physically no match for the other beasts. To make up for these deficiencies, Zeus gave humans a moral sense and the capacity for law and justice , so that they could live in larger communities and cooperate with one another.
That morality should be invested with all the mystery and power of divine origin is not surprising. Nothing else could provide such strong reasons for accepting the moral law. By attributing a divine origin to morality, the priesthood became its interpreter and guardian and thereby secured for itself a power that it would not readily relinquish. This link between morality and religion has been so firmly forged that it is still sometimes asserted that there can be no morality without religion. According to this view, ethics is not an independent field of study but rather a branch of theology ( see moral theology ).
There is some difficulty, already known to Plato, with the view that morality was created by a divine power. In his dialogue Euthyphro , Plato considered the suggestion that it is divine approval that makes an action good . Plato pointed out that, if this were the case, one could not say that the gods approve of such actions because they are good. Why then do they approve of them? Is their approval entirely arbitrary? Plato considered this impossible and so held that there must be some standards of right or wrong that are independent of the likes and dislikes of the gods. Modern philosophers have generally accepted Plato’s argument, because the alternative implies that if, for example, the gods had happened to approve of torturing children and to disapprove of helping one’s neighbours, then torture would have been good and neighbourliness bad.

ESSAY SAUCE
FOR STUDENTS : ALL THE INGREDIENTS OF A GOOD ESSAY
Essay: Research ethics
Essay details and download:.
- Subject area(s): Education essays
- Reading time: 5 minutes
- Price: Free download
- Published: 16 February 2021*
- File format: Text
- Words: 1,298 (approx)
- Number of pages: 6 (approx)
Text preview of this essay:
This page of the essay has 1,298 words. Download the full version above.
Ethics is a system of moral principles or the moral values that influence the proper conduct of an individual or group. The term originated from the Greek word ‘ethos’ meaning habit or character, and it speaks to how we ought to live, that is, how we ought to treat others. Any research which involves human subjects or participants is bound to raise challenging ethical, social, legal and political considerations. Ethics in the context of research is particularly interested in the analysis of ethical issues that arise in research with people as participants. The primary concern of any research project is to protect the participants involved in the study and to ensure that the research is carried out in order to serve the interest of individuals, groups or the society as a whole. Another objective in research ethics is to analyse specific research projects and activities to decipher its ethical soundness. Ethical issues involving the protection of confidentiality, the management of risks, the process of obtaining informed consent, physical or legal harm, deception, the protection of privacy and anonymity should be properly addressed in any research project. A study involving human subjects’ especially vulnerable people as participants raises unique issues in any research context. In light of this, the sensitive nature of my research project which is on “police brutality in Nigeria: a human rights perspective”, raises questions on how ethical issues are to be addressed as it involves victims of police brutality and prisoners as participants in the study. The research seeks to identify the nature and causes of police brutality in Nigeria as well as proffer possible solutions to the problem. Also, through research this study is looking to discover the potential victims of this form of brutality, the human rights issues involved and the extent to which the international community or international human rights groups are aware of police brutality in Nigeria and what steps have or have not been taken to curb it. Furthermore, the methodology of this research project is via a qualitative approach as it acknowledges ethical issues, finds meaning through the eyes of the participants, and it’s ideal to explore and understand people’s experiences, attitudes, behaviour and interactions. The use of focus groups, personal experiences of victims and telephone interviews with international human rights groups like ‘Human Rights watch’ and ‘Amnesty International’ would be pertinent to carry out this research. Reports from credible human rights organisations would also be considered. Ethical issues are bound to arise within this research project considering its sensitivity. The ethical responsibility of the researcher is necessary throughout all the stages of the research process, from recruiting participants to the treatment of participants and to the consequences of their participation. The issue of informed consent is an ethical issue that could arise within this project. According to Miller and Brewer (2003), it is important that clear and accurate information regarding the research is communicated to the participants, including its possible benefits and risk. Information pertaining to the aims and objectives of the research, its methodology and intended outcomes should be given, presented in lay terms to enhance easy understanding. In addressing the issue of consent, the researcher should ensure that the participant is adequately informed and the consent is explicitly and voluntarily given. The researcher should never coerce anyone to participate in a research study and should make participants aware of their rights which include the fact that they could withdraw at anytime without penalty (Endacott, 2004). Confidentiality and anonymity are other ethical issues that could come up in this research project as the process can divulge sensitive and confidential information such as the disclosure of names, addresses, location and occupational details. The researcher should ensure that information shared by the participants is protected from unauthorized observation. It is not enough for the researcher to say he/she will ensure confidentiality, rather the researcher should demonstrate to the participants how. To do this, the researcher should present data publicly only in form of an aggregate e.g. as statistics or percentages (Neuman, 2009). Referring to the research study in question, the researcher should ensure that information concerning the mistreatment and abuse of inmates by police officers should be held in strict confidentiality which cannot be traced to a particular person. Another ethical issue is the protection of participants from harm, although obviously evident in medical research, social research can also cause great psychological or emotional distress. Qualitative interviews on sensitive topics could trigger powerful emotional responses from a participant and as such, the researcher should anticipate risks e.g. screening out high risk participants like people with heart conditions, mental problems or seizures. (Neuman, 2009). Research should not cause the participants harm whether physical or mental. The problem of access, which is how to get hold of participants in any research study, is of immense importance to the success of the study; participants could be in terms of an organisation or individual. To gain access to an institution or organisation, it is important to negotiate with gatekeepers who could deny access to the organisation, ration it or impose certain Conditions (Burnham et al, 2004). Access when denied, restricts the research process. There are certain ethical issues related to access, a research study could be viewed as an invasion of privacy or an intrusion to the institution to be studied as it interrupts routines and schedules causing inconveniences and disturbances; it is highly unethical to invade other people’s privacy and as such gaining access could be a really slow process. Furthermore, the fact that research could disclose the limitations of the activities of an institution or individual could further impede access (Flick, 2009). To gain access within the context of this research project, the researcher should be competent in establishing relationships and gaining the trust of its participants and institutions as much as possible to forge a working alliance in which research becomes possible. Qualitative research in cross- cultural studies is difficult, problematic, challenging and time consuming. Ethical issues in cross-cultural research include issues relating to values and world views which involve the misunderstanding of participants by researchers from a different culture. It is important for the researcher to respect the cultural views and belief systems of the participants and not to impose one’s values in the research process. The researcher should be sensitive to cultural and social differences. Ethical considerations should be in place when representing or in the dissemination of results in a cross-cultural research. According to Marshall and Batten (2003), the researcher should portray findings so that it does not damage the reputation of a community or group of people. Sue & Sue (1990) states that research procedures can be ethically sound by acknowledging and incorporating the cultural practices of participants and their larger communities. In the course of the research, there are ethical guidelines the researcher should adhere to while implementing the project. The researcher must responsibly conduct research morally and legally while conforming to ethical standards, the researcher should be informed and not knowingly contravene the legislation of a country in cross-cultural research. The researcher should not use deception to gain information from participants and should pursue objectivity while upholding their integrity without fear or favour. The researcher should be informative and descriptive rather than rigidly prescriptive or authoritative. The researcher should avoid undue intrusion, obtain informed consent and be confidential. The safety of both the researcher and participants in a study should be ensured and the risk of harm minimised. In conclusion, ethics in research should be concerned with finding a balance between benefits and risk for harm (Boeije, 2010). The results of findings based on data gathered unethically could lead to harm, possible conflicts and enormous dilemmas. As such, it is considered good practice for a research project to fully comply with ethical standards. Bakare Ibironke Helen 09281206 Page 1
...(download the rest of the essay above)
About this essay:
If you use part of this page in your own work, you need to provide a citation, as follows:
Essay Sauce, Research ethics . Available from:<https://www.essaysauce.com/education-essays/research-ethics/> [Accessed 26-06-24].
These Education essays have been submitted to us by students in order to help you with your studies.
* This essay may have been previously published on Essay.uk.com at an earlier date.
Essay Categories:
- Accounting essays
- Architecture essays
- Business essays
- Computer science essays
- Criminology essays
- Economics essays
- Education essays
- Engineering essays
- English language essays
- Environmental studies essays
- Essay examples
- Finance essays
- Geography essays
- Health essays
- History essays
- Hospitality and tourism essays
- Human rights essays
- Information technology essays
- International relations
- Leadership essays
- Linguistics essays
- Literature essays
- Management essays
- Marketing essays
- Mathematics essays
- Media essays
- Medicine essays
- Military essays
- Miscellaneous essays
- Music Essays
- Nursing essays
- Philosophy essays
- Photography and arts essays
- Politics essays
- Project management essays
- Psychology essays
- Religious studies and theology essays
- Sample essays
- Science essays
- Social work essays
- Sociology essays
- Sports essays
- Types of essay
- Zoology essays
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Proposed Increases in Government Authority Over Research Misconduct Proceedings
- 1 Ropes & Gray LLP, Boston, Massachusetts
- 2 Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard, Boston, Massachusetts
- 3 Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts
- Viewpoint Protecting Participants Is Not the Top Priority in Clinical Research Jerry Menikoff, MD, JD JAMA
Public scrutiny of academic research continues to intensify, as evidenced by recent resignations of prominent university leaders, retractions of articles by leading scientists, and a proliferation of media coverage concerning research misconduct, which is most often defined as falsification or fabrication of research data, or plagiarism. Science bloggers have emerged as powerful contributors of allegations, with critical blog posts frequently triggering formal research misconduct proceedings. Physician-scientists are in the crosshairs of intensified enforcement with regard to their research publications, making more important the intricacies of research misconduct proceedings for all reported research, from bench science to data research to clinical trials. While a small number of misconduct cases dominate the media, physician-scientists and the public generally have little visibility into the thousands of research misconduct proceedings conducted each year at institutions across the US. This is by design, as federal regulations underpinning and dictating how institutions process allegations require confidentiality. However, the US Department of Health and Human Services’ Office of Research Integrity (ORI) has proposed substantial amendments to the regulations that, if adopted, would steer the entire process for reviewing allegations of misconduct into a more legalistic framework less familiar to physician-scientists than the peer review approach that the current regulations approximate.
Read More About
Caron MM , Barnes M , Bierer BE. Proposed Increases in Government Authority Over Research Misconduct Proceedings. JAMA. Published online June 20, 2024. doi:10.1001/jama.2024.9244
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu
- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Genealogical ethics in the united states and the popularization of genealogical research in the digital age.

1. Introduction
2. genealogy and ethics, 3. sensitive issues and ethical considerations.
Content Warning : Materials in the Library of Virginia’s collections contain historical terms, phrases, and images that are offensive to modern readers. These include demeaning and dehumanizing references to race, ethnicity, and nationality; enslaved or free status; physical or mental ability; gender and sexual orientation ( Library of Virginia 2023 , https://lva-virginia.libguides.com/land-grants , accessed on 21 January 2024).
Some content (or its descriptions) found on this site may be harmful and difficult to view. These materials may be graphic or reflect personal biases. In some cases, they may conflict with strongly held cultural values, beliefs or restrictions. We provide access to these materials to preserve the historical record, but we do not endorse the attitudes, prejudices, or behaviors found within them ( Georgia Historic Newspapers 2023 , https://gahistoricnewspapers.galileo.usg.edu/search/advanced/ , accessed on 21 January 2024).
3.1. Case A: A Troubled Past
3.2. case b: a personal regret, 3.3. case c: a hidden secret, 3.4. common themes, 4. unexpected genetic discoveries and ethical considerations.
- First, is there an ethical responsibility to Mary in telling her story? Did she know that the father who reared her was not biologically related to her?
- Second, is there an ethical responsibility to Mary’s mother in telling this story? Unless further records are located, it is impossible to know the circumstances through which Mary was conceived. Might her mother have been raped? Might Mary’s conception have been the result of a clandestine relationship hidden from her family? Did the man refuse to marry her after learning she was pregnant? Or, might she have made an informed decision to marry someone else who would help her achieve a more stable and productive life? Or, perhaps, was she already involved in a relationship with the father who raised Mary, thus opening the possibility that she may not have known herself that he was Mary’s biological father? Without further evidence, we cannot know the answer to any of these questions.
- Third, is there an ethical responsibility to the father who reared Mary? Did he know he was not Mary’s biological father? If he did, he would seem to have made the conscious choice to marry a woman already with child and to have given his name to her. He recorded her name first in the family Bible, and he left to Mary—with whom he lived the final years of his life—a share of his estate that equaled all his other children. He likely married Mary’s mother before her pregnancy began to be noticeable. They had been married for five and a half months by the time Mary was born, and he played a prominent role in her life. Soon after Mary’s birth, the family moved about seventy miles westwards, into what was then Georgia’s most western frontier. Had any suspicions lingered in the neighborhood about Mary’s origins, they likely would have been removed by the family’s relocation, and they seem never to have crossed paths with Progenitor X, her offspring, and her extended family again.
- Fourth, is there an ethical responsibility to Mary’s descendants? In this particular case, Mary’s social and legal father’s family has maintained a strong sense of identity, including regular family gatherings emphasizing connections between his family and those of his two brothers as well as among descendants of his other children. This discovery would effectively remove Mary’s descendants from this still active family circle while at the same time perhaps creating tensions among the descendants of Mary’s mother herself, who might question the identification or feel some embarrassment at having these obscure details of their ancestors’ lives resurrected after more than two centuries.
5. Identity Confusion and Ethical Considerations
5.1. example a: william burk and william l. burk, 5.2. example b: william thomas ellerbee and thomas william ellerbee, 5.3. example c: two john hammacks or one, 5.4. common themes, 6. conclusions, informed consent statement, data availability statement, conflicts of interest.
| 1 |
- Adams, Athur, and Frederick L. Weiss. 1955. The Magna Charta Sureties, 1215; and Some of Their Descendants Who Settled in America, 1607–1650 , 1st ed. Boston: The Authors. [ Google Scholar ]
- American Antiquarian Society. 1945. “George Dudley Seymour,” The American Antiquarian Society Quarterly , Obituaries. Available online: https://www.americanantiquarian.org/proceedings/44807115.pdf (accessed on 4 February 2024).
- American Antiquarian Society. 2024. “About,” American Antiquarian Society. Available online: https://www.americanantiquarian.org/about (accessed on 4 February 2024).
- American Society of Genealogists. 1999–2023. “About,” The American Society of Genealogists. Available online: https://fasg.org/about/ (accessed on 4 February 2024).
- Ancestry. 2024a. Ancestry Academy. Available online: https://www.ancestryacademy.com/browse (accessed on 3 May 2024).
- Ancestry. 2024b. AncestryDNA ® ThruLines ® . Available online: https://support.ancestry.com/s/article/AncestryDNA-ThruLines?language=en_US (accessed on 30 April 2024).
- Ancestry. 2024c. Genealogy.com FAQs. Available online: https://www.ancestry.com/cs/faq/genealogy-faq (accessed on 3 May 2024).
- Anderson, Robert Charles. 2015. The Great Migration Directory: Immigrants to New England, 1620–1640 . Boston: New England Historic Genealogical Society. [ Google Scholar ]
- Askin, Jayne. 1998. A Handbook for Adoptees and Birthparents , 3rd ed. Phoenix: Oryx Press. [ Google Scholar ]
- Aulicino, Emily. 2013. Genetic Genealogy: The Basics and Beyond . Bloomington: AuthorHouse, Inc. [ Google Scholar ]
- Barratt, Nick, Sarah Newberry, Jenny Thomas, Matthew Helm, and April Leigh Helm. 2009. Researching Your Family History Online for Dummies , 2nd ed. Chichester: John Wiley and Sons. [ Google Scholar ]
- Bettinger, Blaine T. 2019. The Family Tree Guide to DNA Testing and Genetic Genealogy . Cincinnati: Family Tree Books. [ Google Scholar ]
- Bettinger, Blaine T., and Debbie Wane Parker. 2016. Genetic Genealogy in Practice . Arlington: National Genealogical Society. [ Google Scholar ]
- Board for Certification of Genealogists. 2000. The BCG Genealogical Standards Manual: Millenium Edition . Nashville: Ancestry.com, an Imprint of Turner Publishing Company. [ Google Scholar ]
- Board for Certification of Genealogists. 2014. Genealogy Standards: 50th Anniversary Edition . Nashville: Ancestry.com, an Imprint of Turner Publishing Company. [ Google Scholar ]
- Board for Certification of Genealogists. 2021. Genealogy Standards , 2nd rev. ed. Nashville: Ancestry.com, an Imprint of Turner Publishing Company. First published 2019. [ Google Scholar ]
- Boddie, John Bennett. 1938. Seventeenth Century Isle of Wight County, Virginia . Chicago: Chicago Law Printing. [ Google Scholar ]
- Botkin, Jeffrey R. 2001. Protecting the privacy of family members in survey and pedigree research. JAMA: Journal of the American Medical Association 285: 207–11. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Broglin, Jana Sloan. 2007. Hookers, Crooks, and Kooks . 2 vols. Westminster: Heritage Books, Inc. [ Google Scholar ]
- Brown, Teresa A. 2008. Adoption Records Handbook . Las Vegas: Crary Publications. [ Google Scholar ]
- Byrnes, Thomas. 1886. Rogues’ Gallery: 247 Professional Criminals of 19th-Century America . New York: Cassell and Company. [ Google Scholar ]
- CCEd: Clergy of the Church of England Database. 2024. Available online: https://theclergydatabase.org.uk/cced-explained/ (accessed on 3 May 2024).
- Chroninger, Kelly. 2020. Lyon Gardiner Tyler (1853–1935) in Encyclopedia Virginia . Available online: https://encyclopediavirginia.org/entries/tyler-lyon-gardiner-1853-1935 (accessed on 15 January 2024).
- Coddington, John Insley. 1954. Nathaniel 1 Ely of Springfield, Massachusetts, is not (Alas!) the Man We Thought He Was. The American Genealogist 30: 78–81. [ Google Scholar ]
- Cornell Law School. 2024. LII: Legal Information Institute: Wex: Legal Dictionary and Legal Encyclopedia. Available online: https://www.law.cornell.edu/wex (accessed on 28 April 2024).
- Crowe, Elizabeth Powell. 2014. Genealogy Online , 10th ed. New York: McGraw Hill. [ Google Scholar ]
- Davis, Richard Beale. 1963. William Fitzhugh and his Chesapeake World, 1676–1701: The Fitzhugh Letters and Other Documents . Chapel Hill: University of North Carolina Press. [ Google Scholar ]
- Davis, Robert Scott, Jr. 1982–1987. The Georgia Black Book: Morbid, Macabre, and Sometimes Disgusting Records of Genealogical Value . 2 vols. Greenville: Southern Historical Press. [ Google Scholar ]
- Demos, John Putnam. 1995. The Unredeemed Captive: A Family Story from Early America , 2nd ed. New York: Vintage Books. [ Google Scholar ]
- Demos, John Putnam. 2004. Entertaining Satan: Witchcraft and Culture in Early New England , 2nd ed. New York and Oxford: Oxford University Press. [ Google Scholar ]
- deRoche, John Estano, and Constance deRoche. 2009. Ethics. In Encyclopedia of Case Study Research . Edited by Albert J. Mills, Gabrielle Durepos and Elden Wiebe. Los Angeles: Sage. [ Google Scholar ]
- Dowell, David. 2011. Crash Course in Genealogy . Santa Barbara: Libraries Unlimited. [ Google Scholar ]
- Early Colonial Settlers. 2024. Early Colonial Settlers of Southern Maryalnd and Virginia’s Northern Neck Counties. Available online: https://www.colonial-settlers-md-va.us/ (accessed on 3 May 2024).
- Ellerbee, Bobby Graham. 1995. The Family History and Lineage of Mark Ellerbee . Augusta: Insty Prints. [ Google Scholar ]
- Estes, Roberta. 2021. DNA for Native American Genealogy . Baltimore: Genealogical Publishing Company. [ Google Scholar ]
- FamilySearch. 2024. Research Process. Available online: https://www.familysearch.org/en/wiki/Research_Process (accessed on 3 May 2024).
- Farmer, John. 1829. Genealogical Register of the First Settlers of New England . Lancaster: Carter, Matthews, and Co. [ Google Scholar ]
- Feagin, Joe R., Anthony M. Orum, and Gideon Sjoeberg. 1991. A Case for the Case Study . Chapel Hill: University of North Carolina Press. [ Google Scholar ]
- Findagrave. 2011. Findagrave Memorial ID 77500543, Created 2 October 2011. Available online: https://www.findagrave.com/memorial/77500543/william_l-burke (accessed on 10 February 2024).
- Flannery, Kent, and Joyce Marcus. 2021. Origins of Hieroglyphic Writing. University of Michigan Museum of Anthropological Archaeology. Available online: https://sites.lsa.umich.edu/oaxaca-archaeology/origins-of-hieroglyphic-writing-2/) (accessed on 7 January 2024).
- Fleet, Beverly. 1988. Virginia Colonial Abstracts: The Original 34 Volumes Reprinted in 3 . 3 vols. Baltimore: Genealogical Publishing Company. [ Google Scholar ]
- Flekke, Mary M. 2011. Telling Our Stories: Oral and Family History: A Bibliography , 5th ed. Westminster: Heritage Books, Inc. [ Google Scholar ]
- Foster, Janet, and Julia Sheppard. 2002. British Archives: A Guide to Archive Resources in the UK , 4th ed. New York: Palgrave. [ Google Scholar ]
- Galford, Ellen. 2005. The Genealogy Handbook: The Complete Guide to Tracing Your Family Tree . Pleasantville and Montreal: Reader’s Digest. [ Google Scholar ]
- Georgia Historic Newspapers. 2023. Statement on Potentially Harmful Content. Available online: https://gahistoricnewspapers.galileo.usg.edu/about/harmful_content (accessed on 1 December 2023).
- Glynn, Claire L. 2022. Bridging Disciplines to Form a New One: The Emergence of Forensic Genetic Genealogy. Genes 13: 1381. Available online: https://www.mdpi.com/2073-4425/13/8/1381 (accessed on 2 May 2024).
- Greenblatt, Stephen Jay. 1980. Renaissance Self-Fashioning: From More to Shakespeare . Chicago: University of Chicago Press. [ Google Scholar ]
- Hansen, Charles M. 1995. The Claimed Gentry Descent of Oliver1 Cope, Immigrant to Pennsylvania: A Nineteenth Century Genealogical Fraud. The American Genealogist 70: 156–61. [ Google Scholar ]
- Hatcher, Patricia Law. 1996. Producing a Quality Family History . Salt Lake City: Ancestry. [ Google Scholar ]
- Horowitz, Lois. 1999. Dozens of Cousins: Blue Genes, Horse Thieves, and Other Relative Surprises in Your Family Tree . Berkeley: Ten Speed Press. [ Google Scholar ]
- Hyde, Myrtle S. 1994. Edmund Tilson’s English Origin Not Found. The American Genealogist 69: 37–44. [ Google Scholar ]
- Jacobus, Donald Lines. 1930. Genealogy as Pastime and Profession . New Haven: Tuttle, Morehouse, & Taylor Company. [ Google Scholar ]
- Jacobus, Donald Lines. 1930–1932. History and Genealogy of the Families of Old Fairfield . 2 vols. Fairfield: Eunice Dennie Burr Chapter, Daughters of the American Revolution. [ Google Scholar ]
- Jacobus, Donald Lines. 1968. Genealogy as Pastime and Profession , 2nd ed. With Forward by Milton Rubincam. Baltimore: Genealogical Publishing Company. [ Google Scholar ]
- Jester, Annie Lash, and Martha Woodroof Hiden. 1956. Adventurers of Purse and Person in Virginia, 1607–1626 , 1st ed. Princeton: Order of First Families of Virginia and Princeton University Press. [ Google Scholar ]
- Jordan, Winthrop. 1996. Tumult and Silence at Second Creek: An Inquiry into a Civil War Slave Conspiracy , 2nd ed. Baton Rouge: Louisiana State University Press. [ Google Scholar ]
- JSTOR. 2024. JSTOR: Explore the World’s Knowledge, Cultures, and Ideas. Available online: https://www.jstor.org/ (accessed on 5 May 2024).
- King James Bible. 2020. King James Bible Online. Available online: https://www.kingjamesbibleonline.org (accessed on 5 January 2024).
- Knight, Thomas Daniel. 1995. ‘To Ride a Native of Our Own’: Social and Political Tensions between Anglicization and Creolization in Virginia, 1676–1727. Master’s thesis, University of Oxford, Oxford, UK. [ Google Scholar ]
- Knight, Thomas Daniel. 2004. The Social Construction of Gentility in Virginia, 1607–1776. Ph.D.’s thesis, University of Oxford, Oxford, UK. [ Google Scholar ]
- Knight, Thomas Daniel. 2011. Hammack and Hammock Families in England and America, 1569–2010 . Rockport: Penobscot Press. [ Google Scholar ]
- Knight, Thomas Daniel. 2015. The Yorkshire Family of Edmund 1 Jenings and Peter 1 Jenings of Virginia. The American Genealogist 87: 161–71, 308–17. [ Google Scholar ]
- Knight, Thomas Daniel. 2019. The Family of William Thomas Ellerbee and Jane Johnston. Unpublished manuscript. [ Google Scholar ]
- Knight, Thomas Daniel. 2022. Documenting Difficulty Cases: A Mixed Method Analysis. Genealogy 6: 69. Available online: https://www.mdpi.com/2313-5778/6/3/69 (accessed on 10 February 2024).
- Knight, Thomas Daniel. 2023. John Hammack in Colonial Virginia: A Study of Men Named John Hammack (or Hammock) Born Prior to 1750, Including a Discussion of the Origins of John Hammack, Sr. of Lincoln Co., GA . Unpublished manuscript. [ Google Scholar ]
- Krasner, Barbara. 2020. DNA Testing and Privacy . New York: Greenhaven Publishing. [ Google Scholar ]
- Leclerk, Michael J., and Henry B. Hoff, eds. 2006. Genealogical Writing in the 21st Century: A Guide to Register Style and More . Boston: New England Historic Genealogical Society. [ Google Scholar ]
- Library of Virginia. 2023. Content Warning. Available online: https://lva-virginia.libguides.com/catalog (accessed on 5 January 2024).
- Lifton, Betty J. 2009. Lost & Found: The Adoption Experience , 2nd ed. Ann Arbor: The University of Michigan Press. [ Google Scholar ]
- Mahler, Leslie. 2006. An Addition to the Ancestry of Oliver 1 Cope of Pennsylvania: With Further Evidence of Fraud in the Claimed Gentry Pedigree. The American Genealogist 81: 314–15. [ Google Scholar ]
- Massachusetts Historical Society. 2024. History of the MHS: The Founding. Available online: https://www.masshist.org/about (accessed on 4 February 2024).
- McCarney, Martha. 2017. Virginia Immigrants and Adventurers, 1607–1635: A Biographical Dictionary , annotated ed. Baltimore: Genealogical Publishing Company. [ Google Scholar ]
- McCracken, George E. 1976. Towards an Index Expurgatorius. The American Genealogist 52: 182–93. [ Google Scholar ]
- McGoldrick, Monica, Randy Gerson, and Sueli Petry. 2008. Genograms: Assessment and Intervention , 3rd ed. New York and London: W. W. Norton & Company. [ Google Scholar ]
- McKibbin, Kyle, Mahsa Shabani, and Maarten H. D. Larmueau. 2023. From collected stamps to hair locks: Ethical and legal implications of testing DNA found on privately owned family artifacts. Human Genetics 142: 331–41. Available online: https://link.springer.com/article/10.1007/s00439-022-02508-y (accessed on 2 May 2024).
- McMillan, Joseph. 2014. The Honorable Philip Steptoe, Esquire: Supposedly of Virginia: A Cautionary Heraldic Tale. The American Genealogist 87: 117–24. [ Google Scholar ]
- MilesFiles. 2024. MilesFiles 23.0: Hundreds of Eastern Shore Families from Charlemagne to the Present. Available online: https://espl-genealogy.org/ (accessed on 3 May 2024).
- Mills, Albert J., Gabrielle Durepos, and Elden Wiebe. 2009. Encyclopedia of Case Study Research . Los Angeles: Sage. [ Google Scholar ]
- Mills, Elizabeth Shown. 1997. Evidence Explained: Citation & Analysis for the Family Historian . Baltimore: Genealogical Publishing Company. [ Google Scholar ]
- Mills, Elizabeth Shown. 2015. Evidence Explained: Citing History Sources from Artifacts to Cyberspace , 3rd ed. Baltimore: Genealogical Publishing Company. [ Google Scholar ]
- Mills, Elizabeth Shown, ed. 2001. Professional Genealogy: A Manual For Researchers, Writers, Editors, Lecturers, and Librarians . Baltimore: Genealogical Publishing Company. [ Google Scholar ]
- Mills, Elizabeth Shown, ed. 2018. Professional Genealogy: Preparation, Practice, and Standards . Baltimore: Genealogical Publishing Company. [ Google Scholar ]
- Moore, Susan M. 2023a. Ethical Dilemmas in Family History: A Psychological Approach. Genealogy 7: 67. [ Google Scholar ] [ CrossRef ]
- Moore, Susan M. 2023b. Family History Research and Distressing Emotions. Genealogy 7: 26. Available online: https://www.mdpi.com/2313-5778/7/2/26 (accessed on 28 April 2024).
- Moyers, Bill, and Joseph Campbell. 1988. The Power of Myth . Edited by Betty Sue Flowers. New York: Doubleday. [ Google Scholar ]
- National Park Service. 2024. Facilitating Respectful Return. Available online: www.nps.gov/subjects/nagpra/index.htm (accessed on 9 February 2024).
- Neagles, James C. 1990. The Library of Congress: A Guide to Genealogical and Historical Research . Salt Lake City: Ancestry, Incorporated. [ Google Scholar ]
- New England Historic Genealogical Society. 2024a. History of New England Historic Genealogical Society. Available online: www.americanancestors.org/about/history (accessed on 4 February 2024).
- New England Historic Genealogical Society. 2024b. The Great Migration Study Project . Boston: New England Historic Genealogical Society. Available online: https://www.americanancestors.org/publications/great-migration-study-project (accessed on 10 February 2024).
- Nichols, Barbara J. 1993. Francis 1 Nichols of Stratford, Connecticut, Was Not a Brother of Deputy Governor Richard Nicholls of New York. The American Genealogist , 68. [ Google Scholar ]
- Noble, Mark. 1805. A History of the College of Arms and The Lives of All the Kings, Heralds, and Pursuivants, from the Reign of Richard III . London: T. Egerton. [ Google Scholar ]
- O’Brien, Michael, ed. 1962. Corpus Genealogiarum Hiberniae . Dublin: Dublin Institute for Advanced Studies, vol. 1. [ Google Scholar ]
- Oxford University Press. 2024. Oxford Languages. Available online: https://languages.oup.com/google-dictionary-en/ (accessed on 2 May 2024).
- Quality Matters. 2024. QM: Quality Matters: Helping You Deliver on Your Online Promise. Available online: https://www.qualitymatters.org/index.php/ (accessed on 7 May 2024).
- Randolph County, AL, Probate Court. 1917. Estate Case File of Wiley Burke. Alabama, U.S., Wills and Probate Records, 1753–1999. Available online: https://obu.edu/wp-content/blogsdir/archives/files/2019/04/ProbateCourtIndex.pdf (accessed on 10 February 2024).
- Ray, Paul. 2016. The Role and Functions of Biblical Genealogies. Faculty Publications 192. Available online: https://digitalcommons.andrews.edu/cgi/viewcontent.cgi?article=1196&context=pubs (accessed on 10 January 2024).
- Remensnyder, Amy Goodrich. 1995. Remembering Kings Past: Monastic Foundation Legends in Medieval Southern France . Ithaca: Cornell University Press. [ Google Scholar ]
- Rockett, Kay. 1998. Signature Penmanship—Identifying Reverend Mr. Jacob Ware. National Genealogical Society Quarterly 86: 212–18. [ Google Scholar ]
- Rootsweb. 2024. Retiring and Migrating Portions of Rootsweb. Available online: https://support.rootsweb.com/s/article/Retiring-and-Migrating-Portions-of-RootsWeb (accessed on 3 May 2024).
- Rubincam, Milton. 1980. Genealogical Research: Methods and Sources . Washington, DC: The American Society of Genealogists. [ Google Scholar ]
- Rubincam, Milton. 1987. Pitfalls in Genealogical Research . Salt Lake City: Ancestry, Inc. [ Google Scholar ]
- Santa Clara University. 2019. Ethics in Life and Business. Available online: https://www.scu.edu/mobi/resources--tools/blog-posts/ethics-in-life-and-business/ethics-in-life-and-business.html (accessed on 2 May 2024).
- Scholar, Helen. 2023. Special Issue Information. Genealogy . Available online: https://www.mdpi.com/journal/genealogy/special_issues/1C57XX05C3 (accessed on 2 May 2024).
- Seymour, George Dudley. 1941. Documentary Life of Nathan Hale . New Haven: Donald L. Jacobus. [ Google Scholar ]
- Seymour, George Dudley, and Donald Lines Jacobus. 1939. The Seymour Family: Descendants of Richard Seymour of Hartford, Connecticut, for Six Generations . New Haven: Tuttle, Morehouse, & Taylor Company. [ Google Scholar ]
- Shieber, Stuart. 2009. Is open-access journal publishing a vanity publishing industry. The Occasional Pamphlet on Scholarly Communication . Available online: https://archive.blogs.harvard.edu/pamphlet/2009/10/16/is-open-access-publishing-a-vanity-publishing-industry/ (accessed on 30 April 2024).
- Smith, Amy M. 2008. Family Webs: The Impact of Women’s Genealogy Research on Family Communication. Ph.D. dissertation, Bowling Green State University, Bowling Green, OH, USA. Available online: https://scholarworks.bgsu.edu/media_comm_diss/99 (accessed on 15 June 2023).
- Smith, Dean Crawford, and Melinda Lutz Sanborn. 1991. The Angells and Roger Williams: Thomas 1 Angell of Providence, R.I., was Not a Son of James Angell of London. The American Genealogist 66: 119–32. [ Google Scholar ]
- Social Security Administration. 2023. Program Operations Manual System: GN 03315.010. Disclosing a Deceased Individual’s Information. Available online: https://secure.ssa.gov/poms.nsf/lnx/0203315010#:~:text=Under%20both%20the%20Freedom%20of,deceased%20individual%20to%20any%20party (accessed on 9 February 2024).
- Steele, Carolyn M. 2008. Preserving Family Legends for Future Generations . Denton: Roots and Branches. [ Google Scholar ]
- Stolley, Kathy Shepherd, and Vern L. Bullough. 2006. The Praeger Handbook of Adoption . 2 vols. Westport: Praeger. [ Google Scholar ]
- Story, Joanna. 2003. Carolingian Connections: Anglo-Saxon England and Carolingian France, c. 750–870 . Oxford: Routledge. [ Google Scholar ]
- Stratton, Penelope L., and Henry B. Hoff. 2014. Guide to Genealogical Writing , 3rd ed. Boston: New England Historic Genealogical Society. [ Google Scholar ]
- Strother, Edward L. 1998. Three John Strothers But Which One Died in Georgia? National Genealogical Society Quarterly 86: 189–203. [ Google Scholar ]
- TallBear, Kim. 2013. Native American DNA: Tribal Belonging and the False Promise of Genealogical Science . Minneapolis and London: University of Minnesota Press. [ Google Scholar ]
- Taylor, Nathaniel Lane. 2022. Donald Lines Jacobus and the Scholarly Genealogical Revolution in the United States, 1922–1964. Genealogica & Heraldica 35: 130–38. [ Google Scholar ]
- Taylor, Robert M., and Ralph J. Crandall, eds. 1986. Generations and Change: Genealogical Perspectives in Social History . Macon: Mercer University Press. [ Google Scholar ]
- The Reeves Project. 2024. The Reeves Project . Available online: https://thereevesproject.org/data/tiki-index.php?page=HomePage_Wiki (accessed on 3 May 2024).
- Thorndale, William. 2005. Christopher Martin of the Mayflower was Not the Virginia Company Shareholder. The American Genealogist 80: 264. [ Google Scholar ]
- Torrence, Clayton. 1935. Old Somerset on the Eastern Shore of Maryland . Richmond: Whitney & Shepperson. [ Google Scholar ]
- Triola, Mario. 2021. Elementary Statistics . Upper Saddle River: Pearson. [ Google Scholar ]
- Turabian, Kate L., Wayne C. Booth, Gregory C. Colomb, Joseph M. Williams, Joseph Bizup, and William T. FitzGerald. 2021. A Manual for Writers of Research Papers, Theses, and Dissertations, Ninth Edition: Chicago Style for Students and Researchers (Chicago Guides to Writing, Editing, and Publishing) , 9th ed. Chicago: University of Chicago Press. [ Google Scholar ]
- Tyler, Lyon G., and Earl Gregg Swem. 1919–1952. Tyler’s Quarterly Historical and Genealogical Magazine . 33 vols. Richmond: Richmond Press. [ Google Scholar ]
- USGenWeb. 2024. Tthe USGENWEB Project: Keeping Free Genealogy on the Internet. Available online: https://usgenweb.org/ (accessed on 3 May 2024).
- USGenWeb Archives. 2024. Available online: http://www.usgwarchives.net/ (accessed on 3 May 2024).
- Vance, J. David. 2024. The Genealogist’s Guide to Y-DNA Testing for Genetic Genealogy , 2nd ed. Privately Printed. First published 2020. [ Google Scholar ]
- Vandagriff, G. G. 1993. Voices in Your Blood: Discovering Identity Through Family History . Kansas City: Andrews McNeel Publishing. [ Google Scholar ]
- Virginia Museum of History and Culture. 2024. Our History. Virginia Museum of History and Culture. Available online: https://virginiahistory.org/our-history (accessed on 4 February 2024).
- Wakefield, Robert S. 1983. Not All the Children of Experience Mitchell are Mayflower Descendants. The American Genealogist 59: 28–31. [ Google Scholar ]
- Wallace, Peggy. 2009. Anonymity and Confidentiality. In Encyclopedia of Case Study Research . Edited by Albert J. Mills, Gabrielle Durepos and Elden Wiebe. Los Angeles: Sage. [ Google Scholar ]
- Weil, François. 2007. John Farmer and the Making of American Genealogy. New England Quarterly 80: 408–34. [ Google Scholar ] [ CrossRef ]
- Weinberg, Tamar. 2017. The Adoptee’s Guide to DNA Testing: How to Use Genetic Genealogy to Discover Your Long-Lost Family . Cincinnati: Family Tree Books. [ Google Scholar ]
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Knight, T.D. Genealogical Ethics in the United States and the Popularization of Genealogical Research in the Digital Age. Genealogy 2024 , 8 , 78. https://doi.org/10.3390/genealogy8030078
Knight TD. Genealogical Ethics in the United States and the Popularization of Genealogical Research in the Digital Age. Genealogy . 2024; 8(3):78. https://doi.org/10.3390/genealogy8030078
Knight, Thomas Daniel. 2024. "Genealogical Ethics in the United States and the Popularization of Genealogical Research in the Digital Age" Genealogy 8, no. 3: 78. https://doi.org/10.3390/genealogy8030078
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

Traffic engineers build roads that invite crashes because they rely on outdated research and faulty data
Professor of Civil Engineering, University of Colorado Denver
Disclosure statement
Wes Marshall receives funding from the US Department of Transportation's University Transportation Centers program and various state transportation departments.
View all partners
“Can you name the truck with four-wheel drive, smells like a steak, and seats 35?”
Back in 1998, “The Simpsons” joked about the Canyonero, an SUV so big that they were obviously kidding. At that time, it was preposterous to think anyone would drive something that was “12 yards long, two lanes wide, 65 tons of American Pride.”
In 2024, that joke isn’t far from reality.
And our reality is one where more pedestrians and bicyclists are getting killed on U.S. streets than at any time in the past 45 years – over 1,000 bicyclists and 7,500 pedestrians in 2022 alone.
Vehicle size is a big part of this problem. A recent paper by urban economist Justin Tyndall found that increasing the front-end height of a vehicle by roughly 4 inches (10 centimeters) increases the chance of a pedestrian fatality by 22% . The risk increases by 31% for female pedestrians or those over 65 years, and by 81% for children.
It’s hard to argue with physics, so there is a certain logic in blaming cars for rising traffic deaths. In fact, if a bicyclist is hit by a pickup truck instead of a car, Tyndall suggests that they are 291% more likely to die.
Yet automakers have long asserted that if everyone simply followed the rules of the road, nobody would die. Vehicle size is irrelevant to that assertion.
My discipline, traffic engineering , acts similarly. We underestimate our role in perpetuating bad outcomes, as well as the role that better engineering can play in designing safer communities and streets.

Millions of road deaths
How bad are the bad outcomes? The U.S. has been tracking car-related road deaths since 1899. As a country, we hit the threshold of 1 million cumulative deaths in 1953, 2 million in 1975 and 3 million in 1998. While the past several years of data have not yet been released, I estimate that the U.S. topped 4 million total road deaths sometime in the spring of 2024.
How many of those are pedestrians and bicyclists? Analysts didn’t do a great job of separating out the pedestrian and cyclist deaths in the early years , but based on later trends, my estimate is that some 930,000 pedestrians and bicyclists have been killed by automobiles in the U.S.
How many of those deaths do we blame on big cars or bad streets? The answer is, very few.
As I show in my new book, “ Killed by a Traffic Engineer: Shattering the Delusion that Science Underlies our Transportation System ,” the National Highway Traffic Safety Administration calls road user error the “ critical reason” behind 94% of crashes, injuries and deaths .
Crash data backs that up.
Police investigate crashes and inevitably look to see which road users, including drivers, pedestrians and cyclists, are most at fault. It’s easy to do because in almost any crash, road user error appears to be the obvious problem.
This approach helps insurance companies figure out who needs to pay. It also helps automakers and traffic engineers rationalize away all these deaths. Everyone – except the families and friends of these 4 million victims – goes to sleep at night feeling good that bad-behaving road users just need more education or better enforcement.
But road user error only scratches the surface of the problem.
Who creates dangerous streets?
When traffic engineers build an overly wide street that looks more like a freeway , and a speeding driver in a Canyonero crashes, subsequent crash data blames the driver for speeding.
When traffic engineers provide lousy crosswalks separated by long distances , and someone jaywalks and gets hit by that speeding Canyonero driver, one or both of these road users will be blamed in the official crash report.
And when automakers build gargantuan vehicles that can easily go double the speed limit and fill them with distracting touchscreens , crash data will still blame the road users for almost anything bad that happens.
These are the sorts of systemic conditions that lead to many so-called road user errors. Look just below the surface, though, and it becomes clear that many human errors represent the typical, rational behaviors of typical, rational road users given the transportation system and vehicle options we put in front of them.
Look more deeply, and you can start to see how our underlying crash data gives everyone a pass but the road users themselves. Everyone wants a data-driven approach to road safety, but today’s standard view of crash data lets automakers, insurance companies and policymakers who shape vehicle safety standards off the hook for embiggening these ever-larger cars and light-duty trucks.
It also absolves traffic engineers, planners and policymakers of blame for creating a transportation system where for most Americans, the only rational choice for getting around is a car .
Understanding road behavior
Automakers want to sell cars and make money. And if bigger SUVs seem safer to potential customers, while also being much more profitable , it’s easy to see how interactions between road users and car companies – making seemingly rational decisions – have devolved into an SUV arms race.
Even though these same vehicles are less safe for pedestrians, bicyclists and those in opposing vehicles , the current data-driven approach to road safety misses that part of the story.
This can’t all be fixed at once. But by pursuing business as usual, automakers and traffic engineers will continue wasting money on victim-blaming campaigns or billboards placed high over a road telling drivers to pay attention to the road .
A better starting point would be remaking the U.S.’s allegedly data-driven approach to road safety by reinventing our understanding of the crash data that informs it all.
The key is starting to ask why. Why did these road users act as they did? Why didn’t they follow the rules that were laid out for them? Bad road user behavior shouldn’t be excused, but a bit of digging below the surface of crash data unearths a completely different story.
Figuring out which road user is most at fault may be useful for law enforcement and insurance companies, but it doesn’t give transportation engineers, planners, policymakers or automakers much insight into what they can do better. Even worse, it has kept them from realizing that they might be doing anything wrong.
- Urban policy
- Civil engineering
- Pedestrians
- Car accidents
- Sustainable cities
- Transportation policy
- Safe streets
- Bicycle safety
- Motorcycles

Research Fellow – Magnetic Refrigeration

Centre Director, Transformative Media Technologies

Postdoctoral Research Fellowship

Social Media Producer

Dean (Head of School), Indigenous Knowledges
- Share full article
Advertisement
Supported by
Guest Essay
What Happened to Stanford Spells Trouble for the Election

By Renée DiResta
Ms. DiResta is the former research director of the Stanford Internet Observatory, a unit of Stanford University that studies abuse of online platforms.
In 2020 the Stanford Internet Observatory, where I was until recently the research director, helped lead a project that studied election rumors and disinformation. As part of that work, we frequently encountered conspiratorial thinking from Americans who had been told the 2020 presidential election was going to be stolen.
The way theories of “the steal” went viral was eerily routine . First, an image or video, such as a photo of a suitcase near a polling place, was posted as evidence of wrongdoing. The poster would tweet the purported evidence, tagging partisan influencers or media accounts with large followings. Those accounts would promote the rumor, often claiming, “Big if true!” Others would join, and the algorithms would push it out to potentially millions more. Partisan media would follow.
If the rumor was found to be false — and it usually was — corrections were rarely made and even then, little noticed. The belief that “the steal” was real led directly to the events of Jan. 6, 2021.
Within a couple of years, the same online rumor mill turned its attention to us — the very researchers who documented it. This spells trouble for the 2024 election.
For us, it started with claims that our work was a plot to censor the right. The first came from a blog related to the Foundation for Freedom Online, the project of a man who said he “ran cyber” at the State Department. This person, an alt-right YouTube personality who’d gone by the handle Frame Game, had been employed by the State Department for just a couple of months .
Using his brief affiliation as a marker of authority, he wrote blog posts styled as research reports contending that our project, the Election Integrity Partnership, had pushed social media networks to censor 22 million tweets. He had no firsthand evidence of any censorship, however: his number was based on a simple tally of viral election rumors that we’d counted and published in a report after the election was over. Right-wing media outlets and influencers nonetheless called it evidence of a plot to steal the election, and their followers followed suit.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .

IMAGES
VIDEO
COMMENTS
The ideas and opinions expressed in this essay are the author's own and do not necessarily represent those of the NIH, NIEHS, or US government. ... Education in research ethics is can help people get a better understanding of ethical standards, policies, and issues and improve ethical judgment and decision making. ...
Research ethics are a set of principles that guide your research designs and practices in both quantitative and qualitative research. In this article, you will learn about the types and examples of ethical considerations in research, such as informed consent, confidentiality, and avoiding plagiarism. You will also find out how to apply ethical principles to your own research projects with ...
Research Ethics is defined here to be the ethics of the planning, conduct, and reporting of research. It is clear that research ethics should include: Protections of human and animal subjects. However, not all researchers use human or animal subjects, nor are the ethical dimensions of research confined solely to protections for research ...
business. Ethical norms also serve the aims or goals of research and apply to people who conduct scientific research or other scholarly or creative activities. There is even a specialized discipline, research ethics, which studies these norms. There are several reasons why it is important to adhere to ethical norms in research. First, norms ...
Introduction. Ethics are a guiding principle that shapes the conduct of researchers. It influences both the process of discovery and the implications and applications of scientific findings 1.Ethical considerations in research include, but are not limited to, the management of data, the responsible use of resources, respect for human rights, the treatment of human and animal subjects, social ...
Introduction. In science and medical research, ethics is essential in enhancing the safety and well-being of the subjects or participants. Different studies globally expose vulnerable populations or subjects to abuse, affecting their overall health. In the same case, researchers are employing diverse strategies to enhance ethics and reduce ...
Once you find a suitable topic and are ready, start to write your ethics essay, conduct preliminary research, and ascertain that there are enough sources to support it. 2. Conduct In-Depth Research. Once you choose a topic for your essay, the next step is gathering sufficient information about it.
Ethics in research, writing, and publication are critical in medicine and nursing—decisions that affect human lives often are influenced by knowledge that is disseminated in healthcare journals. While it may seem less critical that healthcare design adhere to strong ethical principles in research, writing, and publication of findings, huge ...
Abstract. Ethics, as an integral component of human decision-making, undeniably shape the landscape of scientific research. This article delves deeply into the nuanced realm of ethical ...
Reputable journals screen for ethics at submission—and inability to pass ethics checks is one of the most common reasons for rejection. Unfortunately, once a study has begun, it's often too late to secure the requisite ethical reviews and clearances. Learn how to prepare for publication success by ensuring your study meets all ethical requirements before work begins.
question. Such alternative answers can appear in your essay as counterarguments. Each time you address a counterargument, your thesis becomes more plausible, since you have eliminated one of the possible alternative answers to your question. 2. Counterarguments lend tension and structure to your argument.-Counter
ethics in research as the disclosure of methods applied during. the study, where the researcher must always maintain an. impartial position, being honest in his intentions and not. giving away the ...
Abstract. Published articles in scientific journals are a key method for knowledge-sharing. Researchers can face the pressures to publish and this can sometimes lead to a breach of ethical values, whether consciously or unconsciously. The prevention of such practices is achieved by the application of strict ethical guidelines applicable to ...
Research ethics is unambiguously concerned in the examination of ethical issues that are upraised when individuals are involved as participants in the study. Research ethics committee/Institutional Review Board (IRB) reviews whether the research is ethical enough or not to protect the rights, dignity and welfare of the respondents. ...
Research ethics are moral principles that guide researchers to conduct and report research without deception or intention to harm the participants of the study or members of the society as a whole, whether knowingly or unknowingly. Practising ethical guidelines while conducting and reporting research is essential to establish the validity of ...
Ethics and ethical behaviour are the fundamental pillars of a civilised society. The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law. In fact, ethics gets precedence with anything that would include, affect, transform, or influence upon individuals, communities or any living creatures. Many institutions within Europe have set up their own ...
6.3 Principles of Research Ethics There are general ethical principles that guide and underpin the proper conduct of research. The term "ethical principles" refers to those general rules that operate as a foundational rationale for the numerous specific ethical guidelines and assessments of human behaviour. 7 The National Statement on 'ethical conduct in human research' states that ...
Ethical oversight in the form of review boards and research ethics committees provide protection for research subjects as well as guidance for safe conduct of studies. As the number of collaborative emergency care research studies carried out in low- and middle-income countries increases, it is crucial to have a shared understanding of how ...
Why do research ethics matter? Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe. ... In shorter scientific papers, where the aim is to report the findings of a specific study, you ...
Ethics of Science is a comprehensive and student-friendly introduction to the study of ethics in science and scientific research. The book covers: * Science and Ethics * Ethical Theory and ...
The term ethics may refer to the philosophical study of the concepts of moral right and wrong and moral good and bad, to any philosophical theory of what is morally right and wrong or morally good and bad, and to any system or code of moral rules, principles, or values. The last may be associated with particular religions, cultures, professions, or virtually any other group that is at least ...
Ethics is a system of moral principles or the moral values that influence the proper conduct of an individual or group. The term originated from the Greek word 'ethos' meaning habit or character, and it speaks to how we ought to live, that is, how we ought to treat others. Any research which involves human subjects or participants is bound ...
personal or research-related work during class time. Final Assignment Short Essay: Each student is required to complete a final writing assignment. For this short essay, please choose a session topic and connect it to a reallife - situation or case through . your own research. (For example, essays on scientific misconduct
The model's third step, "Applied Ethics," is similar to bioethicist's role, and applies ethical theories to assess the real-world impact of technology developments like, for example, the use of animals in research, the provenance of human biological specimens, questions of privacy and consent, the benefits and risks of new technologies ...
Public scrutiny of academic research continues to intensify, as evidenced by recent resignations of prominent university leaders, retractions of articles by leading scientists, and a proliferation of media coverage concerning research misconduct, which is most often defined as falsification or fabrication of research data, or plagiarism.
This article examines genealogical ethics in the digital age. At a time when more resources for research are available digitally than ever previously, digital media also pose challenges for the large-scale dissemination of false or misleading information as well as the incautious presentation of more careful research that then might be misconstrued by some. This article first reviews the ...
Transportation engineer Wesley Marshall explains why he believes traffic engineers systematically fail to design safer streets. Understanding road behavior
Research Ethics is defined here to be the ethics of the planning, conduct, and reporting of research. This introduction covers what research ethics is, its ethical distinctions, approaches to teaching research ethics, and other resources on this topic. Descriptions of educational settings, including in the classroom, and in research contexts.
Ms. DiResta is the former research director of the Stanford Internet Observatory, a unit of Stanford University that studies abuse of online platforms. In 2020, the Stanford Internet Observatory ...