
Health & Medicine News
Top headlines, latest headlines.
- Advanced Cell Atlas
- Food in Sight? The Liver Is Ready!
- Antipsychotics for the Elderly?
- Acid Reflux Drugs and Risk of Migraine
- Chemotherapy With Closed-Loop Drug-Delivery
- When Neurons Wreak Havoc On Metabolism: Injury
- Key Tumor Defense Against Immune Attack
- Climate Change and Transmission of Malaria?
- Do Cells Have a Hidden Communication System?
- Neurosurgery: Microdisplay of Brain Activity
Earlier Headlines
Thursday, april 25, 2024.
- Early Trauma Cuts Life Short for Squirrels, and Climate Change Could Make Matters Worse
Wednesday, April 24, 2024
- Solving the Riddle of the Sphingolipids in Coronary Artery Disease
- Tumor Cells Evade the Immune System Early On: Newly Discovered Mechanism Could Significantly Improve Cancer Immunotherapies
- AI Designs New Drugs Based on Protein Structures
- It Takes Two to TANGO: New Strategy to Tackle Fibrosis and Scarring
- CAR T Cell Therapy Targeting HER2 Antigen Shows Promise Against Advanced Sarcoma in Phase I Trial
- Social Media Can Be Used to Increase Fruit and Vegetable Intake in Young People
- Low Intensity Exercise Linked to Reduced Depression
- Positive Effect of Midazolam After Cardiac Arrest
- Researchers Unveil PI3K Enzyme's Dual Accelerator and Brake Mechanisms
- Biophysics: Testing How Well Biomarkers Work
- Discovering Cancers of Epigenetic Origin Without DNA Mutation
- Understaffed Nursing Homes in Disadvantaged Neighborhoods More Likely to Overuse Antipsychotics
- Apply Single-Cell Analysis to Reveal Mechanisms of a Common Complication of Crohn's Disease
Tuesday, April 23, 2024
- Researching Cancer by Studying Lipids Cell by Cell
- Genetics Predict Type 2 Diabetes Risk and Disparities in Childhood Cancer Survivors
- New Study Uncovers Lasting Financial Hardship Associated With Cancer Diagnosis for Working-Age Adults in the U.S.
- Innovative Microscopy Demystifies Metabolism of Alzheimer's
- Study Compares Salmonella Rates in Backyard, Commercial Poultry Farm Samples
- In the Brain, Bursts of Beta Rhythms Implement Cognitive Control
- Magnetic Microcoils Unlock Targeted Single-Neuron Therapies for Neurodegenerative Disorders
- Odor-Causing Bacteria in Armpits Targeted Using Bacteriophage-Derived Lysin
- Liver Cancer: Molecular Signaling Pathway of Tumor Development Decoded
- Gentle Defibrillation for the Heart
- Dengue Fever Infections Have Negative Impacts on Infant Health for Three Years
Monday, April 22, 2024
- Bella Moths Use Poison to Attract Mates: Scientists Are Closer to Finding out How
- Scientists Discover the Cellular Functions of a Family of Proteins Integral to Inflammatory Diseases
- 3 in 5 Parents Play Short Order Cook for Young Children Who Don't Like Family Meal
- Study Explores Possible Future for Early Alzheimer's Diagnostics
- New Approach to Tackle Muscle Loss in Aging
- Predicting Cardiac Arrhythmia 30 Minutes Before It Happens
- Protein Network Dynamics During Cell Division
- Pressure in the Womb May Influence Facial Development
- The Enemy Within: How Pathogens Spread Unrecognized in the Body
- Breakthrough Rice Bran Nanoparticles Show Promise as Affordable and Targeted Anticancer Agent
- Genetically Engineering a Treatment for Incurable Brain Tumors
- People Think 'old Age' Starts Later Than It Used To, Study Finds
- Despite AI Advancements, Human Oversight Remains Essential
- Mosaics of Predisposition Cause Skin Disease
- Social Programs Save Millions of Lives, Especially in Times of Crisis
Friday, April 19, 2024
- Shoe Technology Reduces Risk of Diabetic Foot Ulcers
- Researchers Develop a New Way to Safely Boost Immune Cells to Fight Cancer
- Glial Hyper-Drive for Triggering Epileptic Seizures
- Toxic Chemicals from Microplastics Can Be Absorbed Through Skin
- New Research Defines Specific Genomic Changes Associated With the Transmissibility of the Monkeypox Virus
- Signs of Multiple Sclerosis Show Up in Blood Years Before Symptoms
- Study Opens New Avenue for Immunotherapy Drug Development
- Analyzing the Progression in Retinal Thickness Could Predict Cognitive Progression in Parkinson's Patients
- Dietary Treatment More Effective Than Medicines in IBS
- Key Protein Regulates Immune Response to Viruses in Mammal Cells
Thursday, April 18, 2024
- Why Can Zebrafish Regenerate Damaged Heart Tissue, While Other Fish Species Cannot?
- Mutations in Noncoding DNA Become Functional in Some Cancer-Driving Genes
- Coal Train Pollution Increases Health Risks and Disparities
- A Common Pathway in the Brain That Enables Addictive Drugs to Hijack Natural Reward Processing
- Metabolic Health Before Vaccination Determines Effectiveness of Anti-Flu Response
- Perfect Balance: How the Brain Fine-Tunes Its Sensitivity
- Scientists Uncover 95 Regions of the Genome Linked to PTSD
- AI Tool Predicts Responses to Cancer Therapy Using Information from Each Cell of the Tumor
- How Data Provided by Fitness Trackers and Smartphones Can Help People With MS
- Siblings With Unique Genetic Change Help Scientists Progress Drug Search for Type 1 Diabetes
- New Urine-Based Test Detects High-Grade Prostate Cancer, Helping Men Avoid Unnecessary Biopsies
Wednesday, April 17, 2024
- Study Identifies New Metric for Diagnosing Autism
- Researchers Create New AI Pipeline for Identifying Molecular Interactions
- Paper: To Understand Cognition--and Its Dysfunction--Neuroscientists Must Learn Its Rhythms
- Protecting Brain Cells With Cannabinol
- Does Using Your Brain More at Work Help Ward Off Thinking, Memory Problems?
- Guidance on Energy and Macronutrients Across the Lifespan
- Calorie Restriction Study Reveals Complexities in How Diet Impacts Aging
- Global Study Reveals Health Impacts of Airborne Trace Elements
- New Data Identifies Trends in Accidental Opioid Overdoses in Children
- Artificial Intelligence Beats Doctors in Accurately Assessing Eye Problems
- Researchers Find That Accelerated Aging Biology in the Placenta Contributes to a Rare Form of Pregnancy-Related Heart Failure
- Genetic Variant Identified That Shaped the Human Skull Base
- Adults With Congenital Heart Disease Faced Higher Risk of Abnormal Heart Rhythms
- AI Speeds Up Drug Design for Parkinson's by Ten-Fold
- Tracking a Protein's Fleeting Shape Changes
- Research Explores How a Father's Diet Could Shape the Health of His Offspring
- Novel Robotic Training Program Reduces Physician Errors Placing Central Lines
- Researchers Uncover Human DNA Repair by Nuclear Metamorphosis
Tuesday, April 16, 2024
- Researchers Discover Urine-Based Test to Detect Head and Neck Cancer
- Nanoparticle Delivery of FZD4 to Lung Endothelial Cells Inhibits Lung Cancer Progression and Metastases
- Real-Time Detection of Infectious Disease Viruses by Searching for Molecular Fingerprinting
- New Treatment Method Using Plasma Irradiation Promotes Faster Bone Healing
- Common HIV Treatments May Aid Alzheimer's Disease Patients
- Bacteria Behind Meningitis in Babies Explained
- New Inflammatory Bowel Disease Testing Protocol Could Speed Up Diagnosis
- Health Behaviors Accumulate and Remain Relatively Stable Throughout Middle Adulthood
- New Insights Could Unlock Immunotherapy for Rare, Deadly Eye Cancer
- New Study Focuses on the Placenta for Clues to the Development of Gestational Diabetes
- 'One Ring to Rule Them All': How Actin Filaments Are Assembled by Formins
- Deadly Bacteria Show Thirst for Human Blood
- Teen Stress May Raise Risk of Postpartum Depression in Adults
- Scientists Identify Cell Vulnerability 'fingerprint' Related to Parkinson's, Lewy Body Dementia
Monday, April 15, 2024
- Take It from the Rats: A Junk Food Diet Can Cause Long-Term Damage to Adolescent Brains
- Family and Media Pressure to Lose Weight in Adolescence Linked to How People Value Themselves Almost Two Decades Later
- Illuminating the Path to Hearing Recovery
- Next-Generation Treatments Hitch a Ride Into Cancer Cells
- AI Enhances Physician-Patient Communication
- Vaccine Breakthrough Means No More Chasing Strains
- Microplastics Make Their Way from the Gut to Other Organs
- LATEST NEWS
- Health & Medicine
- Diseases & Conditions
- Alzheimer's Research
- Amyotrophic Lateral Sclerosis
- Attention Deficit Disorder
- Back and Neck Pain
- Birth Defects
- Bladder Disorders
- Blood Clots
- COVID and SARS
- Cervical Cancer
- Bladder Cancer
- Multiple Myeloma
- Pancreatic Cancer
- Brain Tumor
- Colon Cancer
- Breast Cancer
- Ovarian Cancer
- Lung Cancer
- Mesothelioma
- Skin Cancer
- Prostate Cancer
- Cerebral Palsy
- Chikungunya
- Chronic Fatigue Syndrome
- Cold and Flu
- Crohn's Disease
- Cystic Fibrosis
- Dengue Fever
- Down Syndrome
- Eating Disorder Research
- Encephalitis
- Epilepsy Research
- Erectile Dysfunction
- Fibromyalgia
- Gastrointestinal Problems
- HIV and AIDS
- Headache Research
- Hearing Loss
- Heart Health
- Cholesterol
- Stroke Prevention
- Heart Disease
- Hormone Disorders
- Hypertension
- Infectious Diseases
- Insomnia Research
- Irritable Bowel Syndrome
- Kidney Disease
- Liver Disease
- Lung Disease
- Lyme Disease
- Mental Health Research
- Multiple Sclerosis Research
- Mumps, Measles, Rubella
- Muscular Dystrophy
- Osteoporosis
- Parkinson's Research
- Prostate Health
- Restless Leg Syndrome
- Sickle Cell Anemia
- Sleep Disorder Research
- Thyroid Disease
- Triglycerides
- Tuberculosis
- Medical Topics
- Accident and Trauma
- Alternative Medicine
- Birth Control
- Bone and Spine
- Chronic Illness
- Controlled Substances
- Dietary Supplements and Minerals
- Epigenetics
- Food Additives
- Foodborne Illness
- Foot Health
- Gene Therapy
- Health Policy
- Human Biology
- Immune System
- Joint Health
- Medical Imaging
- Nervous System
- Pain Control
- Personalized Medicine
- Pharmacology
- Psychology Research
- Wounds and Healing
- PHYSICAL/TECH
- ENVIRONMENT
- SOCIETY & EDUCATION
- Mice Given Mouse-Rat Brains Can Smell Again
- How Do Birds Flock? New Aerodynamics
- Cancer: Epigenetic Origin Without DNA Mutation
- Climate Change Driving Biodiversity Loss
- Why Can't Robots Outrun Animals?
- Evolution of Gliding in Marsupials
- Novel One-Dimensional Superconductor
Trending Topics
Strange & offbeat.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih research matters.
December 22, 2021
2021 Research Highlights — Promising Medical Findings
Results with potential for enhancing human health.
With NIH support, scientists across the United States and around the world conduct wide-ranging research to discover ways to enhance health, lengthen life, and reduce illness and disability. Groundbreaking NIH-funded research often receives top scientific honors. In 2021, these honors included Nobel Prizes to five NIH-supported scientists . Here’s just a small sample of the NIH-supported research accomplishments in 2021.
Printer-friendly version of full 2021 NIH Research Highlights
20210615-covid.jpg
Advancing COVID-19 treatment and prevention
Amid the sustained pandemic, researchers continued to develop new drugs and vaccines for COVID-19. They found oral drugs that could inhibit virus replication in hamsters and shut down a key enzyme that the virus needs to replicate. Both drugs are currently in clinical trials. Another drug effectively treated both SARS-CoV-2 and RSV, another serious respiratory virus, in animals. Other researchers used an airway-on-a-chip to screen approved drugs for use against COVID-19. These studies identified oral drugs that could be administered outside of clinical settings. Such drugs could become powerful tools for fighting the ongoing pandemic. Also in development are an intranasal vaccine , which could help prevent virus transmission, and vaccines that can protect against a range of coronaviruses .
202211214-alz.jpg

Developments in Alzheimer’s disease research
One of the hallmarks of Alzheimer’s is an abnormal buildup of amyloid-beta protein. A study in mice suggests that antibody therapies targeting amyloid-beta protein could be more effective after enhancing the brain’s waste drainage system . In another study, irisin, an exercise-induced hormone, was found to improve cognitive performance in mice . New approaches also found two approved drugs (described below) with promise for treating AD. These findings point to potential strategies for treating Alzheimer’s. Meanwhile, researchers found that people who slept six hours or less per night in their 50s and 60s were more likely to develop dementia later in life, suggesting that inadequate sleep duration could increase dementia risk.
20211109-retinal.jpg
New uses for old drugs
Developing new drugs can be costly, and the odds of success can be slim. So, some researchers have turned to repurposing drugs that are already approved for other conditions. Scientists found that two FDA-approved drugs were associated with lower rates of Alzheimer’s disease. One is used for high blood pressure and swelling. The other is FDA-approved to treat erectile dysfunction and pulmonary hypertension. Meanwhile, the antidepressant fluoxetine was associated with reduced risk of age-related macular degeneration. Clinical trials will be needed to confirm these drugs’ effects.
20210713-heart.jpg
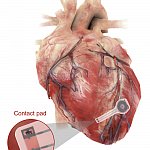
Making a wireless, biodegradable pacemaker
Pacemakers are a vital part of medical care for many people with heart rhythm disorders. Temporary pacemakers currently use wires connected to a power source outside the body. Researchers developed a temporary pacemaker that is powered wirelessly. It also breaks down harmlessly in the body after use. Studies showed that the device can generate enough power to pace a human heart without causing damage or inflammation.
20210330-crohns.jpg

Fungi may impair wound healing in Crohn’s disease
Inflammatory bowel disease develops when immune cells in the gut overreact to a perceived threat to the body. It’s thought that the microbiome plays a role in this process. Researchers found that a fungus called Debaryomyces hansenii impaired gut wound healing in mice and was also found in damaged gut tissue in people with Crohn’s disease, a type of inflammatory bowel disease. Blocking this microbe might encourage tissue repair in Crohn’s disease.
20210406-flu.jpg
Nanoparticle-based flu vaccine
Influenza, or flu, kills an estimated 290,000-650,000 people each year worldwide. The flu virus changes, or mutates, quickly. A single vaccine that conferred protection against a wide variety of strains would provide a major boost to global health. Researchers developed a nanoparticle-based vaccine that protected against a broad range of flu virus strains in animals. The vaccine may prevent flu more effectively than current seasonal vaccines. Researchers are planning a Phase 1 clinical trial to test the vaccine in people.
20211002-lyme.jpg

A targeted antibiotic for treating Lyme disease
Lyme disease cases are becoming more frequent and widespread. Current treatment entails the use of broad-spectrum antibiotics. But these drugs can damage the patient’s gut microbiome and select for resistance in non-target bacteria. Researchers found that a neglected antibiotic called hygromycin A selectively kills the bacteria that cause Lyme disease. The antibiotic was able to treat Lyme disease in mice without disrupting the microbiome and could make an attractive therapeutic candidate.
20211102-back.jpg

Retraining the brain to treat chronic pain
More than 25 million people in the U.S. live with chronic pain. After a treatment called pain reprocessing therapy, two-thirds of people with mild or moderate chronic back pain for which no physical cause could be found were mostly or completely pain-free. The findings suggest that people can learn to reduce the brain activity causing some types of chronic pain that occur in the absence of injury or persist after healing.
2021 Research Highlights — Basic Research Insights >>
Connect with Us
- More Social Media from NIH
- History, Facts & Figures
- YSM Dean & Deputy Deans
- YSM Administration
- Department Chairs
- YSM Executive Group
- YSM Board of Permanent Officers
- FAC Documents
- Current FAC Members
- Appointments & Promotions Committees
- Ad Hoc Committees and Working Groups
- Chair Searches
- Leadership Searches
- Organization Charts
- Faculty Demographic Data
- Professionalism Reporting Data
- 2022 Diversity Engagement Survey
- State of the School Archive
- Faculty Climate Survey: YSM Results
- Strategic Planning
- Mission Statement & Process
- Beyond Sterling Hall
- COVID-19 Series Workshops
- Previous Workshops
- Departments & Centers
- Find People
- Biomedical Data Science
- Health Equity
- Inflammation
- Neuroscience
- Global Health
- Diabetes and Metabolism
- Policies & Procedures
- Media Relations
- A to Z YSM Lab Websites
- A-Z Faculty List
- A-Z Staff List
- A to Z Abbreviations
- Dept. Diversity Vice Chairs & Champions
- Dean’s Advisory Council on Lesbian, Gay, Bisexual, Transgender, Queer and Intersex Affairs Website
- Minority Organization for Retention and Expansion Website
- Office for Women in Medicine and Science
- Committee on the Status of Women in Medicine Website
- Director of Scientist Diversity and Inclusion
- Diversity Supplements
- Frequently Asked Questions
- Recruitment
- By Department & Program
- News & Events
- Executive Committee
- Aperture: Women in Medicine
- Self-Reflection
- Portraits of Strength
- Mindful: Mental Health Through Art
- Event Photo Galleries
- Additional Support
- MD-PhD Program
- PA Online Program
- Joint MD Programs
- How to Apply
- Advanced Health Sciences Research
- Clinical Informatics & Data Science
- Clinical Investigation
- Medical Education
- Visiting Student Programs
- Special Programs & Student Opportunities
- Residency & Fellowship Programs
- Center for Med Ed
- Organizational Chart
- Leadership & Staff
- Committee Procedural Info (Login Required)
- Faculty Affairs Department Teams
- Recent Appointments & Promotions
- Academic Clinician Track
- Clinician Educator-Scholar Track
- Clinican-Scientist Track
- Investigator Track
- Traditional Track
- Research Ranks
- Instructor/Lecturer
- Social Work Ranks
- Voluntary Ranks
- Adjunct Ranks
- Other Appt Types
- Appointments
- Reappointments
- Transfer of Track
- Term Extensions
- Timeline for A&P Processes
- Interfolio Faculty Search
- Interfolio A&P Processes
- Yale CV Part 1 (CV1)
- Yale CV Part 2 (CV2)
- Samples of Scholarship
- Teaching Evaluations
- Letters of Evaluation
- Dept A&P Narrative
- A&P Voting
- Faculty Affairs Staff Pages
- OAPD Faculty Workshops
- Leadership & Development Seminars
- List of Faculty Mentors
- Incoming Faculty Orientation
- Faculty Onboarding
- Past YSM Award Recipients
- Past PA Award Recipients
- Past YM Award Recipients
- International Award Recipients
- Nominations Calendar
- OAPD Newsletter
- Fostering a Shared Vision of Professionalism
- Academic Integrity
- Addressing Professionalism Concerns
- Consultation Support for Chairs & Section Chiefs
- Policies & Codes of Conduct
- Health & Well-being
- First Fridays
- Fund for Physician-Scientist Mentorship
- Grant Library
- Grant Writing Course
- Mock Study Section
- Research Paper Writing
- Funding Opportunities
- Join Our Voluntary Faculty
- Child Mental Health: Fostering Wellness in Children
- Faculty Resources
- Research by Keyword
- Research by Department
- Research by Global Location
- Translational Research
- Research Cores & Services
- Program for the Promotion of Interdisciplinary Team Science (POINTS)
- CEnR Steering Committee
- Experiential Learning Subcommittee
- Goals & Objectives
- Issues List
- Print Magazine PDFs
- Print Newsletter PDFs
- YSM Events Newsletter
- Social Media
- Patient Care
INFORMATION FOR
- Residents & Fellows
- Researchers
The machinery of medicine: how technology influences medical research and clinical care
- Features The machinery of medicine From raw idea to finished product The artificial pancreas If you have a lemon, make lemonade The tools of medicine Human body as machine Keeping the “Goldilocks” organ cool New artery? We can print that A hunch leads to an anti-HIV compound Not by the numbers Greek drama’s lessons for veterans
- News Our collective will, says the Surgeon General, can give “every man, woman, and child a fair shot at good health” Vulnerable populations at home and abroad Yale and University of Puerto Rico collaborate on M.D./Ph.D. studies Alumnus makes gift of instructional websites to School of Medicine A drug for women tested mostly in men After a heart attack, a disparity in outcomes Fresh vegetables and primary care Still against all odds Why Nicole Sitkin adds a plus sign to LGBTQI+ Green lawns and frogs’ sex An encyclopedia of medicine from the Amazon
- People Match Day 2016 Service drives M.D./Ph.D. student Helping doctors help themselves From the United States to the Balkans, and back Alum sees place for e-cigarettes in smoking cessation
- Dialogue Winning the war on cancer From the Editor The machinery of medicine: how technology influences medical research and clinical care Second Opinion
Since Neolithic humans fashioned the first scalpel out of stone, new machines and methods have changed the way we practice medicine and learn about the human body. Physicians moved on from those early scalpels to stethoscopes, X-rays, and MRIs, the better to understand the workings of the human body. With these new understandings has come translational research that transfers findings from the lab into new, more effective treatments and medicines. Dean Robert J. Alpern, M.D., Ensign Professor of Medicine, discussed basic science and advances in clinical care; technology and patient care; and the role of serendipity in research with Yale Medicine .
What have been some of the key inventions or discoveries that have advanced clinical care and medical research? In the past 50 to 100 years, there have been so many advances that it’s hard to rank any one above the other. Obviously, some come to mind—the discovery of the structure of DNA, recombinant DNA, electron microscopy, knockout technology. The new gene editing technology, CRISPR, is really going to transform research. It’s important to point out that the major advances in health care have been based on basic scientific findings. DNA technology and the structure of DNA were basic science findings that now drive clinical genetics. The understanding of how cells grow has transformed cancer care. Basic understandings of the immune system have led to immunotherapy for cancer.
How do physicians integrate new technologies into medicine while maintaining the doctor-patient relationship? Technology is always good for improving what physicians can do, but you run the risk that doctors won’t hone their clinical skills as well as they could because they know that the technology will end up defining the diagnosis. There needs to be a combination of the two. I don’t see technology replacing the need for outstanding clinicians. Technology should enhance clinical skills, not replace them.
How important is serendipity in scientific discovery? There are stories of serendipity, but the best investigators always appear to have good luck. The best investigators are asking the right questions, the important questions. It’s a matter of staying knowledgeable about all of the technologies, including those from other fields, and thinking about how to apply them to your field. When you ask the right question and use the right technology, serendipity falls upon you.
Featured in this article
- Robert Alpern, MD Ensign Professor of Medicine (Nephrology) and Professor of Cellular and Molecular Physiology
On Medicine

Latest posts
Beyond sensors and alerts: diabetic foot prevention requires more than the odd sock.

20/03/2024 Jenny Corser
In a follow-up to her recent blog post, Jenny Corser discusses the SOCKSESS study of smart sensing socks for monitoring diabetic feet, registered at the ISRCTN… Read more »
A smartphone app for the self-management of Perthes’ Disease – a blog for Rare Disease Day 2024

28/02/2024 Adam Galloway
For Rare Disease Day 2024, Adam Galloway discusses the NON-STOP trial, registered at the ISRCTN registry, which evaluates the acceptability and usability of a… Read more »
Prescriptions aren’t one-way tickets: how to stop what we started

08/02/2024 Dave Taylor and Jackie Martin-Kerry
In this blog, Dave Taylor and Jackie Martin-Kerry discuss the CHARMER (CompreHensive geriAtRician led MEdication Review) trial, registered at the ISRCTN registry.… Read more »
More On Medicine posts
Pear-tree: understanding the usefulness of the pear-bio platform in patients with kidney cancer.

08/01/2024 Maxine Tran & Matt Williams
For World Cancer Day, Maxine Tran and Matt Williams discuss the PEAR-TREE study, which is registered at the ISRCTN registry.
Intensive interaction for children and young people with profound and multiple learning disabilities: the INTERACT trial

27/11/2023 Jill Bradshaw
On the International Day of Persons with Disabilities, Jill Bradshaw and the Interact Trial Team describe the INTERACT trial,… Read more »
Improving Romanian teachers’ LGBT+ related attitudes through an online intervention – the LGBT inclusion project

17/10/2023 Dr Ioana Latu and Dr Nastasia Sălăgean
Nastasia Sălăgean discusses the Teacher LGBT Inclusion study that is testing the effectiveness of an intervention to improve… Read more »
Advances in nursing assessment: a fundamental step in patient care

27/09/2023 Irene Llagostera Reverter and Víctor M. González Chordá
Irene Llagostera Reverter discusses her study of the VALENF Instrument, a nursing assessment meta-tool, in adult inpatients units,… Read more »
Sharing health education and exercise videos on TikTok to prevent sarcopenia

27/09/2023 Ya Shi
Ya Shi discusses a social-media-based intervention, SHEEP, to prevent sarcopenia in young-older adults in the community,… Read more »
Photobiomodulation therapy to prevent chemotherapy-induced oral mucositis

21/09/2023 Marwa Khalil
Marwa Khalil discusses her study of photobiomodulation therapy as a preconditioning treatment against chemotherapy-induced oral… Read more »
Teaching recovery techniques plus parenting: a cluster randomized controlled trial in Ukrainian schools in Ternopil (TRUST)

12/09/2023 Saba Masood and Dennis Ougrin
For World Mental Health Day 2023, Saba Masood and Dennis Ougrin discuss teaching the recovery techniques plus parenting TRUST… Read more »
Popular On Medicine tags
- BMC Medicine
- clinical trials
- medical evidence
- Genome Medicine
- ISRCTN Registry
- Alzheimer's
- Alzheimer's Research & Therapy
- breast cancer
Popular posts
- Most shared
Sorry. No data so far.
Most Shared Posts
- World Suicide Prevention Day 2023: the Lifeguard Pharmacy study
- Brain Tumor Awareness Month: Can proton beam therapy improve the long-term quality of life of patients with oligodendroglioma brain tumors?
- Winners of the 2022 Cardio-Oncology Journal Prize
- A unique approach. A unique population. Investing in nurses’ mental health
- Does the midwife-led continuity of carer model improve birth outcomes and maternal mental health in vulnerable women?
- March 2024 (1)
- February 2024 (2)
- January 2024 (1)
- November 2023 (1)
- October 2023 (1)
- September 2023 (7)
- July 2023 (1)
- June 2023 (1)
- May 2023 (4)
- April 2023 (1)
- March 2023 (2)
- February 2023 (7)
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on Health Research and the Privacy of Health Information: The HIPAA Privacy Rule; Nass SJ, Levit LA, Gostin LO, editors. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research. Washington (DC): National Academies Press (US); 2009.

Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research.
- Hardcopy Version at National Academies Press
3 The Value, Importance, and Oversight of Health Research
The previous chapter reviewed the value of privacy, while this chapter examines the value and importance of health research. As noted in the introduction to Chapter 2 , the committee views privacy and health research as complementary values. Ideally, society should strive to facilitate both for the benefit of individuals as well as the public.
In addition to defining health research and delineating its value to individuals and society, this chapter provides an overview and historical perspective of federal research regulations that were in place long before the Privacy Rule was implemented. Because a great deal of medical research falls under the purview of multiple federal regulations, it is important to understand how the various rules overlap or diverge. The chapter also explains how the definition of research has become quite complex under the various federal regulations, which make a distinction between research and some closely related health practice activities that also use health data, such as quality improvement initiatives.
The chapter also reviews the available survey data regarding public perceptions of health research and describes the importance of effective communication about health research with patients and the public.
- CONCEPTS AND VALUE OF HEALTH RESEARCH
Definitions
Under both the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule and the Common Rule , “research” is defined as “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.” This is a broad definition that may include biomedical research, epidemiological studies, 1 and health services research, 2 as well as studies of behavioral, social, and economic factors that affect health.
Perhaps the most familiar form of health research is the clinical trial, in which patients volunteer to participate in studies to test the efficacy and safety of new medical interventions. But an increasingly large portion of health research is now information based. A great deal of research entails the analysis of data and biological samples that were initially collected for diagnostic, treatment, or billing purposes, or that were collected as part of other research projects, and are now being used for new research purposes. This secondary 3 use of data is a common research approach in fields such as epidemiology, health services research, and public health research, and includes analysis of patterns of occurrences, determinants, and natural history of disease; evaluation of health care interventions and services; drug safety surveillance; and some genetic and social studies ( Lowrance, 2002 ; Lowrance and Collins, 2007 ).
The Importance of Health Research
Like privacy, health research has high value to society. It can provide important information about disease trends and risk factors, outcomes of treatment or public health interventions, functional abilities, patterns of care, and health care costs and use. The different approaches to research provide complementary insights. Clinical trials can provide important information about the efficacy and adverse effects of medical interventions by controlling the variables that could impact the results of the study, but feedback from real-world clinical experience is also crucial for comparing and improving the use of drugs, vaccines, medical devices, and diagnostics. For example, Food and Drug Administration (FDA) approval of a drug for a particular indication is based on a series of controlled clinical trials, often with a few hundred to a few thousand patients, but after approval it may be used by millions of people in many different contexts. Therefore, tracking clinical experience with the drug is important for identifying relatively rare adverse effects and for determining the effectiveness in different populations or in various circumstances. It is also vital to record and assess experience in clinical practice in order to develop guidelines for best practices and to ensure high-quality patient care.
Collectively, these forms of health research have led to significant discoveries, the development of new therapies, and a remarkable improvement in health care and public health. 4 Economists have found that medical research can have an enormous impact on human health and longevity, and that the resulting increased productivity of the population contributes greatly to the national economy ( Hatfield et al., 2001 ; Murphy and Topel, 1999 ) in addition to the individual benefits of improved health. If the research enterprise is impeded, or if it is less robust, important societal interests are affected.
The development of Herceptin as a treatment for breast cancer is a prime example of the benefits of research using biological samples and patient records ( Box 3-1 ) ( Slamon et al., 1987 ). Many other examples of findings from medical records research have changed the practice of medicine as well. Such research underlies the estimate that tens of thousands of Americans die each year from medical errors in the hospital, and research has provided valuable information for reducing these medical errors by implementing health information technology, such as e-prescribing ( Bates et al., 1998 ; IOM, 2000b ). This type of research also has documented that disparities in health care and lack of access to care in inner cities and rural areas result in poorer health outcomes ( Mick et al., 1994 ). Furthermore, medical records research has demonstrated that preventive services (e.g., mammography) substantially reduce mortality and morbidity at reasonable costs ( Mandelblatt et al., 2003 ), and has established a causal link between the nursing shortage and patient health outcomes by documenting that patients in hospitals with fewer registered nurses are hospitalized longer and are more likely to suffer complications, such as urinary tract infections and upper gastrointestinal bleeding ( Needleman et al., 2002 ). These findings have all informed and influenced policy decisions at the national level. As the use of electronic medical records increases, the pace of this form of research is accelerating, and the opportunities to generate new knowledge about what works in health care are expanding ( CHSR, 2008 ).
Examples of Important Findings from Medical Database Research. Herceptin and breast cancer: Data were collected from a cohort of more than 9,000 breast cancer patients whose tumor specimens were consecutively received at the University (more...)
Advances in health information technology are enabling a transformation in health research that could facilitate studies that were not feasible in the past, and thus lead to new insights regarding health and disease. As noted by the National Committee on Vital and Health Statistics, “Clinically rich information is now more readily available, in a more structured format, and able to be electronically exchanged throughout the health and health care continuum. As a result, the information can be better used for quality improvement, public health, and research, and can significantly contribute to improvements in health and health care for individuals and populations” ( NCVHS, 2007a ). The informatics grid recently developed with support from the National Cancer Institute (Cancer Biomedical Informatics Grid, or caBIG) is an example of a how information technologies can facilitate health research by enabling broader sharing of health data while still ensuring regulatory compliance and protecting patient privacy ( Box 3-2 ).
caBIG (Cancer Biomedical Informatics Grid). The National Cancer Institute’s caBIG Data Sharing and Intellectual Capital Workspace’s mission is to enable all constituencies in the cancer community—including researchers, physicians, (more...)
Science today is also changing rapidly and becoming more complex, so no single researcher or single site can bring all the expertise to develop and validate medical innovations or to ensure their safety. Thus, efficient sharing of information between institutions has become even more important than in previous eras, when there were fewer new therapies introduced. The expansion of treatment options, as well as the escalating expense of new therapies, mandates greater scrutiny of true effectiveness, 5 once efficacy has been demonstrated. This requires registries of patient characteristics, outcomes, and adverse events. Large populations are required to facilitate comparison of patient populations and to calculate risk/benefit estimates. For example, INTERMACS 6 (Interagency Registry for Mechanically Assisted Circulatory Support) is a national registry for patients who are receiving mechanical circulatory support device therapy to treat advanced heart failure. This registry was devised as a joint effort of the National Heart, Lung and Blood Institute, Centers for Medicare & Medicaid Services, FDA, clinicians, scientists and industry representatives. Analysis of the data collected is expected to facilitate improved patient evaluation and management while aiding in better device development. Registry results are also expected to influence future research and facilitate appropriate regulation and reimbursement of such devices. Similarly, the Extracorporeal Life Support Organization (ELSO), 7 an international consortium of health care professionals and scientists who focus on the development and evaluation of novel therapies for support of failing organ systems, maintains a registry of extracorporeal membrane oxygenation and other novel forms of organ system support. Registry data are used to support clinical practice and research, as well as regulatory agencies. Another example is the database developed by the United Network for Organ Sharing (UNOS) for the collection, storage, analysis and publication of data pertaining to the patient waiting list, organ matching, and transplants. 8 Launched in 1999, this secure Internet-based system contains data regarding every organ donation and transplant event occurring in the United States since 1986.
Information-based research, such as research using health information databases has many advantages (reviewed by Lowrance, 2002 ). It is often faster and less expensive than experimental studies; it can analyze very large sets of data and may detect unexpected phenomena or differences among subpopulations that might not be included in a controlled experimental study; it can often be undertaken when controlled trials are simply not possible for ethical, technical, or other reasons, and it can be used to study effectiveness of a specific test or intervention in clinical practice, rather than just the efficacy as determined by a controlled experimental study. It can also reexamine data accrued in other research studies, such as clinical trials, to answer new questions quickly and inexpensively. However, information-based research does have limitations. Often it has less statistical rigor than controlled clinical studies because it lacks scientific control over the original data collection, quality, and format that prospective experimental research can dictate from the start. In addition to these scientific limitations, because of its relational and often distant physical separation from the data subjects, and the sheer volume of the records involved, obtaining individual consent for the research can be difficult or impossible.
Advances in information-based medical research could also facilitate the movement toward personalized medicine, which will make health research more meaningful to individuals. The goal of personalized medicine is to tailor prevention strategies and treatments to each individual based on his/her genetic composition and health history. In spite of the strides made in improving health through new treatments, it is widely known that most drugs are effective in only a fraction of patients who have the condition for which the drug is indicated. Moreover, a small percentage of patients are likely to have adverse reactions to drugs that are found to be safe for the majority of the population at the recommended dose. Both of these phenomena are due to variability in the patient population. Revolutionary advances in the study of genetics and other markers of health and disease are now making it possible to identify and study these variations, and are leading to more personalized approaches to health care—that is, the ability to give “the appropriate drug, at the appropriate dose, to the appropriate patient, at the appropriate time.” Achieving the goals of personalized medicine will lead to improvements in both the effectiveness and the safety of medical therapies.
Public Perceptions of Health Research
A number of studies have been undertaken to gauge the public’s attitude toward research and the factors that influence individuals’ willingness to participate in research. The surveys reviewed in this chapter focus on interventional clinical trials. A review of survey questions to gauge the public willingness to allow their medical records to be used in research can be found in Chapter 2 .
The Public Values Health Research
A number of studies suggest that most Americans have a positive view of medical research and believe that research is beneficial to society. A recent Harris poll found that nearly 80 percent of respondents were interested in health research findings, consistent with previous survey results ( Westin, 2007 ). A study in 2005 compiled data from 70 state surveys and 18 national surveys and found that the majority of Americans believe maintaining world leadership in health-related research is important. Seventy-eight percent of respondents said that it is very important, and 17 percent said that it is somewhat important. Only 4 percent of Americans reported that maintaining world leadership in health-related research is not impor tant ( Woolley and Propst, 2005 ). Similar results were found in a 2007 survey—76 percent of respondents reported that science plays a very important role in our health, and 78 percent reported that science plays a very important role in our competitiveness ( Research!America, 2007 ).
The Virginia Commonwealth University 2004 Life Sciences Survey also found that most Americans have a positive view of research. In this study, 90 percent of respondents agreed that developments in science have made society better; 92 percent reported that “scientific research is essential for improving the quality of human lives”; and 84 percent agreed that “the benefits of scientific research outweigh the harmful results” ( NSF, 2006 ).
Overall Experience When Participating in Research
Little is known about the attitudes of individuals who have actually participated in medical research. However, the available evidence suggests that most research participants have positive experiences. A recent Harris Poll found that 13 percent of respondents had participated in some form of health research, and 87 percent of those felt comfortable about their experience ( Westin, 2007 ). In a study focused on cancer, 93 percent of respondents who participated in research reported it as a very positive experience; 76 percent said they would recommend participation in a clinical trial to someone with cancer. Most physicians surveyed in this study stated that they believe clinical trial participants receive the best possible care, and have outcomes at least as good as patients receiving standard cancer treatment ( Comis et al., 2000 ). Another study found that 55 percent of individuals who participated in a research study would be willing to participate again in a future research study ( Trauth et al., 2000 ).
Willingness to Participate in Research
Public opinion surveys indicate that a majority of Americans are willing to participate in clinical research studies. In 2001, a compilation of studies commissioned by Research !America found that 63 percent of Americans would be willing to participate in a clinical research study ( Woolley and Propst, 2005 ). This percentage has remained stable over time. A 2007 Research!America survey also found that 63 percent of Americans would be very likely to participate in a clinical research study if asked ( Research!America, 2007 ); 68 percent of respondents reported that their desire to improve their own health or the health of others was a major factor in deciding whether to participate in a clinical research project ( Research!America, 2007 ).
Other surveys also suggest that willingness to participate in research focused on specific diseases is quite high. In one survey, the percentage of respondents indicating a willingness to participate in a medical research study was 88 percent for cancer, 86 percent for heart disease, 83 percent for a noncurable fatal disease, 79 percent for addiction, 78 percent for depression, and 76 percent for schizophrenia ( Trauth et al., 2000 ). Respondents with greater knowledge of how research is conducted were more willing to participate ( Trauth et al., 2000 ). Another study found that 8 of 10 Americans would consider participating in a clinical trial if faced with cancer. More than two-thirds of respondents said they would be willing to participate in a clinical trial designed to prevent cancer ( Comis et al., 2000 ).
Americans also seem to be very supportive of medical research that relies on genetic data. A 2007 survey found that 93 percent of Americans supported the use of genetic testing if the information collected is used by researchers to find new ways to diagnose, prevent, or treat disease ( Genetics & Public Policy Center, 2007 ). Two separate surveys found that 66 percent of Americans would be willing to donate their genetic material for medical research ( Genetics & Public Policy Center, 2007 ; Research!America, 2007 ). However, despite this apparent positive view of genetic research, 92 percent of Americans reported they were concerned about their genetic information being used in a “harmful way” ( Genetics & Public Policy Center, 2007 ).
Many factors, in addition to concerns about privacy and confidentiality ( Genetics & Public Policy Center, 2007 ; Research!America, 2007 ), may influence an individual’s willingness to participate in a medical research study. The Trauth survey found that individuals with higher income levels, with a college or graduate degree, or with children were more likely to participate in research. Age affected willingness to participate: 57 percent of respondents ages 18–34 were willing to participate in research, but only 31 percent of respondents ages 65 or older were willing ( Trauth et al., 2000 ).
Other factors that potentially influence an individual’s willingness to participate in research are race and ethnicity. It is well documented that minorities participate in health research at a much lower percentage than white Americans. Many cultural, linguistic, and socioeconomic barriers could be responsible for this difference ( Giuliano et al., 2000 ), and study results have been variable on this issue. Several studies suggest that the low participation rates by racial and ethnic minority groups are due to their strong distrust of the medical research community compared to the general population ( Braunstein et al., 2008 ; Corbie-Smith et al., 1999 ; Farmer et al., 2007 ; Grady et al., 2006 ; Shavers et al., 2002 ).
However, other evidence suggests that the low percentage of minorities participating in research is related to minority groups’ lack of access to the research community ( Brown et al., 2000 ; Wendler et al., 2006 ; Williams and Corbie-Smith, 2006 ). Thus, it is likely that the low number of minority individuals participating in medical research is at least partly due to recruitment techniques that are ineffective for minority populations.
The survey that focused on cancer research suggests that one of the main reasons why individuals do not participate in research is lack of knowledge about the availability of clinical trials. In a survey of nearly 6,000 cancer patients, 85 percent said they were unaware of the opportunity to participate in a clinical trial. Respondents who did participate said they did so because of one of the following beliefs: (1) trials provide access to the best quality of care (76 percent), (2) their participation would benefit future cancer patients (72 percent), (3) they would receive newer and better treatment (63 percent), and (4) participation would get them more care and attention (40 percent) ( Comis et al., 2000 ).
A recommendation from a physician can also impact participation. In the United States, 48 percent of respondents to one survey reported that a physicians’ recommendation would be a major factor in deciding whether to take part in a research study. Nearly three-fourths of respondents also cited an institution’s reputation as a key factor to consider when deciding whether to participate in a study ( Research!America, 2007 ). Twenty percent of respondents in an Italian public survey indicated that the presence of a physician as a reference during a research study influenced their willingness to participate ( Mosconi et al., 2005 ).
In sum, surveys indicate that the vast majority of Americans have a positive view of medical research, believe that research is beneficial to society, and are interested in health research findings. Although little is known about the attitudes of individuals who have actually participated in medical research, the available evidence suggests that most research participants have positive experiences. Surveys also suggest that a majority of Americans are willing to participate in clinical research studies. Similar to the findings in Chapter 2 , surveys indicate that many factors, in addition to concerns about privacy and confidentiality, can potentially influence an individual’s willingness to participate in medical research, including the type of research and personal characteristics such as health status, age, education, and race. Notably, respondents with greater knowledge of how research is conducted were more willing to participate in research.
- OVERSIGHT OF HEALTH RESEARCH
Historical Development of Federal Protections of Health Information in Research
The development of international codes, federal legislation, and federal regulation of human subjects often occurred in response to past abuses in biomedical experiments (reviewed by Pritts, 2008 ) ( Box 3-3 ). The most well-known examples included (1) reported abuses of concentration camp prisoners in Nazi experiments during World War II, and (2) the Tuskegee syphilis study begun in 1932, in which researchers withheld effective treatment from affected African American men long after a cure for syphilis was found. Most of the current principles and standards for conducting human subjects research were developed primarily to protect against the physical and mental harms that can result from these types of biomedical experiments. Therefore, they focus on the principles of autonomy and consent. Although the standards apply to research that uses identifiable health information, research based solely on information is not their primary focus.
The Basis for Human Subjects Protections in Biomedical Research. Nuremberg Code The Nuremberg Code, created by the international community after the Nazi War Crimes Trials, is generally seen as the first codification (more...)
In the United States, perhaps the most influential inquiry into the protection of human subjects in research was the Belmont Report. The Belmont principles have been elaborated on in many settings, and served as the basis for formal regulation of human subjects research in the United States. In general, states do not directly regulate the activity of most researchers ( Burris et al., 2003 ). However, the Belmont Commission’s recommendations were reflected in the Department of Health and Human Services’ (HHS’s) Policy for Protection of Human Subjects Research , Subpart A of 45 C.F.R. 46 (“Subpart A”) in 1979. 9 These protections were considered a benchmark policy for federal agencies, and in December 1981, the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research recommended 10 that all federal departments and agencies adopt the HHS regulations. 11
In 1982, the President’s Office of Science and Technology Policy appointed a Committee for the Protection of Human Research Subjects to respond to the recommendations of the President’s commission. The committee agreed that uniformity of federal regulations on human subjects protection is desirable to eliminate unnecessary regulations and to promote increased understanding by institutions that conduct federally supported or regulated research. As a result, in 1991, other federal departments and agencies joined HHS in adopting a uniform set of rules for the protection of human subjects of research, identical to Subpart A of 45 C.F.R. 46, which is now informally known as the “ Common Rule .” Eighteen federal agencies have now adopted the Common Rule as their own respective regulations.
Overview of the Common Rule
The Common Rule governs most federally funded research conducted on human beings and aims to ensure that the rights of human subjects are protected during the course of a research project. The Common Rule stresses the importance of individual autonomy and consent; requires independent review of research by an Institutional Review Board (IRB); and seeks to minimize physical and mental harm. Privacy and confidentiality protections, although not defined in a detailed and prescriptive manner, are included as important components of risk in research.
The framework for achieving the goal of protecting human subjects is based on two foundational requirements: the informed consent of the research participant and the review of proposed research by an IRB. This section describes some of the basic parameters of the Common Rule (reviewed by Pritts, 2008 ). Particular provisions that interact with the HIPAA Privacy Rule are described in more detail in Chapter 4 .
Scope of the Common Rule
In general, the Common Rule applies only to research on human subjects that is supported by the federal government. 12 As noted previously, research is defined as “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.” 13
Under the Common Rule , a “human subject” is defined as “a living individual about whom an investigator … conducting research obtains (1) Data through intervention or interaction with the individual, or (2) Identifiable private information.” Private information is considered to be personally identifiable if the identity of the subject is or may readily be ascertained by the investigator or associated with the information.
The Common Rule applies to most human subjects research conducted using federal funds, but its influence is broader because most institutions that accept federal funds sign an agreement (a Federalwide Assurance or FWA) with HHS to abide by the Common Rule requirements in all research, regardless of funding source. Nonetheless, some privately funded human subjects research is conducted outside the purview of federal regulation ( Goldman and Choy, 2001 ; Williams, 2005 ). Companies and other organizations may voluntarily choose to apply the Common Rule to their research projects, and many do. However, research projects in which compliance is voluntary are not subject to oversight or disciplinary action by HHS ( Goldman and Choy, 2001 ; Williams, 2005 ).
Informed Consent 14
The Common Rule requires that a researcher obtain informed consent (usually in writing) from a person before he/she can be admitted to a study ( Williams, 2005 ). Informed consent is sought through a process in which a person learns key facts about a research study, including the potential risks and benefits, so that he/she can then agree voluntarily to take part or decide against it.
The Common Rule informed consent regulations focus primarily on the elements and documentation of informed consent rather than on the process used to obtain it. As to the process, the regulations require that informed consent be sought only under circumstances that provide the prospective subject with adequate opportunity to consider whether to participate. The Common Rule requires that information pertaining to informed consent be given in language understandable to the subject, and that the consent does not imply that the subject is giving up his/her legal rights or that the investigator is released from liability for negligence during the conduct of the study. 15
The Common Rule also specifies a number of elements that must be provided when informed consent is sought. These elements include:
- an explanation of the purposes of the research,
- the expected duration of the subject’s participation,
- the potential risks and benefits of the research,
- how confidentiality will be maintained,
- the fact that participation is strictly voluntary, and
- who the subject can contact to answer questions about the study or about his/her rights as a research participant.
In certain limited circumstances, the Common Rule allows an informed consent to be for unspecified future research. For example, under the Common Rule an informed consent can be used to obtain a person’s permission to study personally identifiable information maintained in a repository for future, unspecified research purposes ( HHS, 2003 ).
For the most part, the required elements of an informed consent address all types of research, although some are more relevant to biomedical research (e.g., the consent must include a disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject). One required element of informed consent is particularly relevant to research involving personally identifiable health information. The Common Rule requires an informed consent to include a statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained. 16
Institutional Review Boards
Adopting the principles of the Belmont Report, the Common Rule requires that protocols for human subjects research be reviewed by an IRB ( Box 3-4 ) before research may begin. 17 The IRB must meet certain membership requirements, including having members with different expertise and at least one member who is not affiliated with the investigator’s institution. The Common Rule specifies which level of IRB review is needed for various types of research and provides criteria for the IRB to consider during the review. Although the Common Rule does not specify the procedures an IRB must follow in its review of protocols, it does require the IRB to have written procedures for how it will review protocols and document IRB decisions.
Institutional Review Boards. According to the Department of Health and Human Services (HHS) Institutional Review Board (IRB) guidebook, “the IRB is an administrative body established to protect the rights and welfare of human research subjects (more...)
The Common Rule requires that an IRB determine the following factors are satisfied to approve proposed research:
- Risks to subjects are minimized;
- Risks to subjects are reasonable in relation to anticipated benefits, if any, to subjects, and the importance of the knowledge that may reasonably be expected to result;
- The selection of subjects is equitable;
- Informed consent will be sought in accordance with the rules and will be documented;
- When appropriate, the research plan makes adequate provision for monitoring the data collected to ensure the safety of subjects; and
- When appropriate, adequate provisions are in place to protect the privacy of subjects and to maintain the confidentiality of data. 18
An IRB may waive the requirement to obtain informed consent or approve an alteration of the consent form for some minimal risk research. The IRB may also waive the requirement for signed consent in certain circumstances. 19
Anonymized Data
As noted above, the Common Rule considers use of “private identifiable information” to be human subjects research. Data are considered personally identifiable if the identity of the subject is or may be readily ascertained by the investigator or associated with the information accessed by the researcher. 20 However, the Common Rule exempts from its requirements research that involves:
[T]he collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. 21
Otherwise identifiable data may be deidentified or “anonymized” for purposes of the Common Rule if it is coded and certain other conditions are met ( HHS, 2004 ). Under Guidance issued by the Office for Human Research Protection, information is “coded” if identifying information (such as name or Social Security number) that would enable the investigator to readily ascertain the identity of the individual to whom the private information or specimens pertain has been replaced with a number, letter, symbol, or combination thereof (the code), and a key to decipher the code exists, enabling linkage of the identifying information to the private information or specimen.
Research involving only coded private information or specimens is not considered to involve human subjects under the Common Rule if the following conditions are met:
- The private information or specimens were not collected specifically for the currently proposed research project through an interaction or intervention with living individuals; and
- —The key to decipher the code is destroyed before the research begins;
- —The investigators and the holder of the key enter into an agreement prohibiting the release of the key to the investigators under any circumstances, until the individuals are deceased;
- —IRB-approved written policies and operating procedures for a repository or data management center prohibit the release of the key to investigators under any circumstances, until the individuals are deceased; or
- —Other legal requirements prohibit the release of the key to the investigators, until the individuals are deceased.
Under this standard, when a researcher accesses or receives data that have been coded and does not have access to the identifying key, the research is not considered human subjects research and is not subject to the Common Rule ’s requirements of informed consent or IRB review and approval of protocol.
Enforcement of the Common Rule
The Common Rule requirements for informed consent do not preempt any applicable federal, state, or local laws that require additional information to be disclosed to a subject in order for informed consent to be legally effective. 22
Federal funding can be suspended or withdrawn from an institution when it is found to be in material violation of the Common Rule . 23 There is no authority to impose penalties directly on individual researchers for violations. Neither does the Common Rule expressly provide a research participant with a private right of action. It should be noted, however, that recent cases indicate that courts may be willing to hold an institution liable under common law negligence theories where the approved informed consent form is determined to be less than adequate ( Shaul et al., 2005 ). 24
FDA Protection of Human Research Subjects
Some health research is also subject to FDA regulations. The FDA is charged by statute with ensuring the protection of the rights, safety, and welfare of human subjects who participate in clinical investigations 25 involving articles subject to the Federal Food, Drug, and Cosmetic Act 26 (the Act), as well as clinical investigations that support applications for research or marketing permits for products regulated by the FDA, including drugs, medical devices, and biological products for human use ( Box 3-5 ).
FDA Protection of Human Subjects Regulations. The Food and Drug Administration (FDA) Protection of Human Subjects Regulations aim to protect the rights of human subjects enrolled in research involving products that the FDA regulates (i.e., drugs, medical (more...)
In January 1981, the FDA adopted regulations governing informed consent of human subjects 27 and regulations establishing standards for the composition, operation, and responsibilities of IRBs that review clinical investigations involving human subjects. 28 At the same time, HHS adopted the Common Rule regulations on the protection of human research subjects. 29 The FDA’s regulations were harmonized with the Common Rule in 1991 to the extent permitted by statute. Key differences between FDA and HHS regulations include that the FDA does not allow for waiver or alteration of informed consent and requires that subjects be informed that the FDA may inspect their medical records. In addition, studies of efficacy based solely on medical records research are not permitted to support registration. Remaining differences in the rules are due to differences in the statutory scope or requirements ( Lee, 2000 ).
- DISTINGUISHING HEALTH RESEARCH FROM PRACTICE
The Common Rule and Privacy Rule make a somewhat artificial distinction between health research and some closely related health care practices, such as public health practice, quality improvement activities, program evaluations, 30 and utilization reviews, 31 all of which may involve collection and analysis of personally identifiable health information. However, determining which activities meet the definition of “research” is a major challenge for IRBs, Privacy Boards , 32 investigators, and health care practitioners because neither the regulations nor their interpretations by HHS provide clear guidance on how to distinguish research from activities that use similar techniques to analyze health information ( IOM, 2000a ).
It is important for IRBs and Privacy Boards to correctly distinguish among activities that are or are not subject to the various provisions of the Privacy Rule and the Common Rule . Only research requires formal IRB or Privacy Board review and informed consent. 33 Inappropriate classification of an activity as research can make it difficult or impossible for important health care activities, such as public health practice and quality improvement, to be undertaken. On the other hand, failure to correctly identify an activity as research could potentially allow improper disclosure of personally identifiable health information without sufficient oversight.
Thus, standard criteria are urgently needed for IRBs and Privacy Boards to use when making distinctions between health research and related activities, and the committee recommends that HHS consult with relevant stake holders to develop such standard criteria. HHS is aware of this need, and created a working document titled “What Is Research ?” However, the work on this project apparently has been delayed for unknown reasons ( NCURA, 2007 ). 34 As described below, a number of other models have already been proposed to help determine whether activities should be classified as research in the fields of public health and quality improvement, and these could be instructive for developing HHS guidance. Any criteria adopted by HHS should be regularly evaluated to ensure that they are helpful and producing the desired outcomes.
The following sections describe some ongoing efforts to develop such criteria in the fields of public health and quality improvement. The intent of the committee is not to endorse these particular models, but rather to illustrate the challenges associated with making these distinctions and establishing standard criteria.
Public Health Practice Versus Public Health Research
The Belmont Report defined health practice as “interventions designed solely to enhance the well-being of the person, patient or client, and which have reasonable expectation of success” ( CDC, 1999 ). To apply this definition to “public” health practice, the targeted beneficiary of the intervention must be expanded to include benefit to the community, rather than just a particular person. Neither the Common Rule nor the Privacy Rule provides a specific definition for public health research; rather public health research is included in the general definition of research. However, the Privacy Rule regulates public health practice differently from public health research (see Chapter 4 ).
An early model for distinguishing public health research from public health practice focused on the intent for which the activity was designed, noting that the intent of public health research is to “contribute to or generate generalizable knowledge,” while the intent of public health practice is to “conduct programs to prevent disease and injury and improve the health of communities” ( Snider and Stroup, 1997 ). The Centers for Disease Control and Prevention developed a similar method with an expanded assessment of intent. For example, the model posits that in public health research, the intended benefits of the project extend beyond the study participants, and the data collected exceed the requirements for the care of the study participants. But for public health practice, the intended benefits of the project are primarily for the participants in the activity, or for the participants’ community, and the only data collected are those needed to assess or improve a public health program or service, or the health of the participants and their community. The model also assumes that public health practice is based on well-established medical interventions and is nonexperimental ( CDC, 1999 ). However, these models both have been criticized as too subjective and too dependent on the opinion of the person conducting the activity ( Gostin, 2008 ; Hodge, 2005 ).
A new, more comprehensive model incorporating much of the previous two was recently proposed as a more objective checklist to be used by IRBs, Privacy Boards , and interested parties ( Hodge, 2005 ; Hodge and Gostin, 2004 ). The foundations for this model are specific definitions of public health research: “the collection and analysis of identifiable health data by a public health authority for the purpose of generating knowledge that will benefit those beyond the participating community who bear the risks of participation,” and public health practice: “the collection and analysis of identifiable health data by a public health authority for the purpose of protecting the health of a particular community, where the benefits and risks are primarily designed to accrue to the participating community.”
The model is based on two primary assumptions. First, the actor performing the activity in question is a governmental public health official, agent, agency, or entity at the federal, tribal, state, or local level. Second, the activity in question involves the acquisition, use, or disclosure of personally identifiable health data. The model is then divided into two stages. Stage 1 is applied to all activities, and can be used to distinguish practice from research in the easiest cases. Stage 2 is only applied to those cases that are hard to distinguish, and where Stage 1 failed to lead to a definitive IRB/ Privacy Board decision ( Box 3-6 ).
A Model for Distinguishing Public Health Practice from Research. Stage 1 Public health practice:
Quality Improvement Versus Health Research
Quality improvement has been defined as “systematic, data-guided activities designed to bring about immediate, positive change in the delivery of health care in a particular setting” ( Baily, 2008 ). Quality improvement activities do not require IRB or Privacy Board approval under the Common Rule or the Privacy Rule, which classify quality improvement as a component of health care operations. 35
However, in many cases, it is difficult for health care providers, IRBs, and Privacy Boards to determine whether a particular activity is purely for quality improvement, or whether it also entails research. One survey 36 exploring opinions in the health care community about the need for IRBs to review various quality-related activities found that physicians conducting quality improvement were less likely than IRB chairs to believe that IRB review was required for a given hypothetical activity, or that informed consent was necessary ( Lindenauer et al., 2002 ). Recently, a highly publicized case has again brought the issue to the forefront for all the stakeholders ( Box 3-7 ).
A Case Study of Quality Improvement and Research. Peter Pronovost of Johns Hopkins University (JHU) led a quality improvement effort at 103 intensive care units (ICUs) in Michigan hospitals to reduce the number of catheter-related bloodstream infections. (more...)
Some members of the health care community have proposed requiring that all prospective quality improvement activities go through external review ( Bellin and Dubler, 2001 ), while others have outlined specific criteria to differentiate quality improvement activities from research.
For example, Casarett and colleagues developed a two-part test to identify quality improvement activities. The first test is whether the majority of patients are expected to benefit directly from “the knowledge to be gained” from the initiative. This means that the patients must actually benefit from the knowledge learned during the evaluation, not just from being a recipient of the protocol itself. If the patients are generally expected to directly benefit from the knowledge gained during the activity, then the activity is quality improvement. If not, the activity is research. The second test is whether the participants would be subjected to additional risks or burdens, including the risk of privacy breach, beyond the usual clinical practice in order to make the results of the initiative generalizable. If yes, then the initiative should be reviewed as research ( Casarett et al., 2000 ).
More recently, the Hastings Center published a report exploring the similarities and differences between research and quality improvement. The report emphasized three fundamental characteristics of quality improvement and three fundamental characteristics of research. The authors argue that individuals have a responsibility to participate in the quality improvement activities because all patients have an interest in receiving high-quality medical care, and the success of a quality improvement activity depends on the cooperation of all patients. In addition, the report notes that quality improvement activities are a low risk to the patient, so there is little justification for not participating. The report also assumes that quality improvement activities are based on existing knowledge about human health and should lead to immediate local improvements in the provision of medical care.
In contrast, the report notes that participation in research should be voluntary, and decisions to participate should be based on researchers’ full disclosure of all the potential risks and benefits. In addition, the authors assert that research is designed to create new knowledge about human health, rather than relying solely on existing knowledge, and that most research does not result in any direct benefit to the institution where the research is being conducted.
The authors concluded that IRBs are not the appropriate body for the ethical oversight of quality improvement activities. They argue that IRBs unnecessarily impose high transaction costs on these activities because of the difference in the way they are conducted compared to research. For example, in research, any changes in methodology require further IRB approval. In contrast, quality improvement activities involve frequent adjustments in the intervention, measurement, and goals of the activity based on the experience of the investigators. Requiring the investigator to revisit an IRB every time a small adjustment is needed in such an activity significantly increases the amount of time and effort required to conduct the initiative and to produce meaningful data. Also, the investigators involved in quality improvement activities ordinarily are already involved in the clinical care of participants and bear responsibility for the quality and safety of an intervention. Thus, the authors argue that there is no need for the additional oversight by an IRB to protect participant safety.
Rather, the report recommended integrating the ethical oversight of quality improvement activities into the ongoing management of an institution’s health care delivery system, suggesting that oversight of quality improvement could be left with the managers of clinical care organizations, and that consent to receive treatment should include consent to participate in any quality improvement project that is minimal risk. However, the report stated that if a project has the characteristics of both quality improvement and research, the project should be reviewed as both human subjects research and quality improvement ( Baily et al., 2006 ; Lynn et al., 2007 ).
In response to the ongoing confusion over when quality improvement rises to the level of research and requires IRB review, the IOM jointly hosted a meeting with the American Board of Internal Medicine in May 2008 to discuss this issue. Key members of the quality improvement community attended, and short- and long-term solutions to this problem were proposed. However, no written report from this meeting was produced and no general consensus was reached.
- THE IMPORTANCE OF EFFECTIVE COMMUNICATION WITH THE PUBLIC
As noted previously in this chapter, surveys indicate that the vast majority of Americans believe that health research is important and are interested in the findings of research studies. The majority of patients also appear to be willing to participate in health research, either by volunteering for a study to test a medical intervention or by allowing access to their medical records or stored biospecimens, under certain conditions. Their willingness to participate depends on trust in researchers to safeguard the rights and well-being of patients, including assurance of privacy and confidentiality, and the belief that it is a worthwhile endeavor that warrants their involvement. Yet patients often lack information about how research is conducted, and are rarely informed about research results that may have a direct impact on their health. The committee’s recommendations in this section are intended to address both the public’s desire for more information about health research and to help fulfill two of the committees overarching goals of the report: (1) improving the privacy and security of health information, and (2) improving the effectiveness of health research.
Disseminating Health Research Results
Ethicists have long suggested greater community involvement in health research studies, including more communication about research results (reviewed by Shalowitz and Miller, 2008a , b ). In addition, the IOM committee identified transparency—the responsibility to disclose clearly how and why personally identifiable information is being collected—as an important component of comprehensive privacy protections. A previous IOM report also recommended improved communication with the public and research participants to ensure that the protection process is open and accessible to all interested parties ( IOM, 2002 ). Effective communication would build the public’s trust of the research community and is consistent with the principles of fair information practices.
When patients consent to the use of their medical records in a particular study, health researchers should make greater efforts at the conclusion of the study to inform study participants about the results, and the relevance and importance of those results. Learning about clinically relevant findings from a study in which a patient has participated could make patients feel more integrated into the process and could encourage more to participate in future studies. A recent United Kingdom report on the use of personal data in health research concluded that public involvement in research is necessary for the success of information-based research, and that a public informed about the value of research is likely to have greater enthusiasm and confidence in research and the research community ( AMS, 2006 ). Moreover, direct feedback with study participants could lead to improved health care for the individuals if the results indicate that an altered course of care is warranted.
Nonetheless, there are multiple impediments, beyond cost, to providing meaningful feedback to participants. A summary of the results alone, while necessary and reasonable, can be seen as a token, and also raises questions about issues such as how best to write summaries, the stage at which results should be disseminated, and how to present research with uninformative outcomes. For example, one recent study found that sharing results directly with study participants was met with overwhelmingly favorable reactions from patients, but the study also revealed some obstacles ( Partridge et al., 2008 ). In a survey of women who had participated in a randomized trial of breast cancer therapy and had received a summary of the study results by mail, 95 percent reported that they were glad they received the results. Most respondents interpreted the results correctly, although incorrect interpretation of the results was associated with increased anxiety, as was dissatisfaction with treatment.
Although some guidelines for providing and explaining study results to research participants have been proposed, they differ in details because limited data are available on this subject, and thus standards are lacking ( Partridge and Winer, 2002 ; Partridge et al., 2008 ; Shalowitz and Miller, 2008b ; Zarin and Tse, 2008 ). Because transparency is best achieved by providing graded levels of information and guidance to interested parties ( IOM, 2002 ), it will be important to develop effective and efficient ways to communicate with various sectors of the population. A commitment to the principles of “plain language” 37 will be important. Broader adoption of electronic medical records may also be helpful in accomplishing this goal.
Research Registries
One way to make information about research studies more broadly available to the public is through registration of trials and other studies in public databases. HHS should encourage such registration of trials and other studies, particularly when research is conducted with an IRB/ Privacy Board approved waiver of consent or authorization (see Chapter 4 ). Numerous clinical trial registries already exist, and registration has increased in recent years (reviewed by Zarin and Tse, 2008 ). In 2000, the National Library of Medicine established a clinical trials registry ( ClinicalTrials.gov ), which has expanded to include information from several other trial registries and to serve as the FDA-required site for submissions about clinical trials subject to the FDA databank requirement. The FDA Amendments Act of 2007 38 expanded the scope of required registrations at ClinicalTrials.gov and provided the first federally funded trials results database. It mandates registrations of controlled clinical investigations, except for Phase I trials, of drugs, biologics, and devices subject to FDA regulation.
A policy of the International Committee of Medical Journal Editors (ICMJE), adopted in fall 2005, also requires prospective trial registration as a precondition for publication ( DeAngelis et al., 2004 ). This policy led to a 73 percent increase in trial registrations of all intervention types from around the world ( Zarin et al., 2005 ). Nearly 45,000 trials had been registered by fall 2007.
However, although the development of such registries is an important first step toward providing high-quality clinical trial information to the public, no centralized system currently exists to disseminate information about clinical trials of drugs or other interventions, making it difficult for consumers and their health care providers to identify ongoing studies. The current statutory requirements for registration and data reporting in the United States are not as broad as the transnational policies of the ICMJE or the World Health Organization, which call for the registration of all interventional studies in human beings regardless of intervention type ( Laine et al., 2007 ; Sim et al., 2006 ). Moreover, noninterventional studies, such as observational studies that play an increasingly critical role in biomedical research, are not generally included in these databases. Because many noninterventional studies are conducted with an IRB/ Privacy Board approved waiver of consent or authorization, including those studies in a registry could be an important method for increasing public knowledge of such studies.
Informing the Public About the Methods and Value of Research
As noted previously, clinical trials are the most visible of the various types of health research, but a great deal of information-based health research entails analysis of thousands of patient records to better understand human diseases, to determine treatment effectiveness, and to identify adverse side effects of therapies. This form of research is likely to increase in frequency as the availability of electronic records continues to expand. As we move toward the goal of personalized medicine, research results will be even more likely to be directly relevant to patients, but more study subjects will be necessary to derive meaningful results.
However, many patients probably are not aware that their medical records are being used in information-based research. For example, the recent study that used focus groups to examine the views of veterans toward the use of medical records in research found that the majority of participants (75 percent) were not aware that “under some circumstances, [their] medical records could be used in some research studies without [their] permission,” despite the fact that a notice of privacy practices, which included a statement that such research could occur, had been mailed to all participants less than a year prior to the study ( Damschroder et al., 2007 ).
Moreover, surveys show that many patients desire not only notice, but also the opportunity to decide whether to consent to such research with medical records. Those surveys further indicate that patients who wish to be asked for consent for each study are most concerned about the potentially detrimental affects of inappropriate disclosure of their personally identifiable health information, including discrimination in obtaining health or life insurance or employment.
As noted in Chapter 2 , strengthening security protections of health data should reduce the risk of security breaches and their potential negative consequences, and thus should help to alleviate patient concerns in this regard. But educating patients about how health research is conducted, monitored, and reported on could also help to ease patient concerns about privacy and increase patients’ trust in the research community, which as noted above is important for the public’s continued participation in health research. For example, datasets are most often provided to researchers without direct identifiers such as name and Social Security number. Furthermore, identifiers are not included in publications about research results. Also, under both the Privacy Rule and the Common Rule , a waiver of consent and authorization is possible only under the supervision of an IRB or Privacy Board , and a waiver is granted only when the research entails minimal risk and when obtaining individual consent and authorization is impracticable (see the previous section and also Chapter 4 ). Finally, professional ethics dictate that researchers safeguard data and respect privacy.
Conveying the value of medical records research to patients will be important. Surveys show that people are more supportive of research that is relevant to them and their loved ones. At the same time, educational efforts should stress the negative impact of incomplete datasets on research findings. Representative samples are essential to ensure the validity and generalizability of health research ( Box 3-8 ), but datasets will not represent the entire population if some people withhold access to their health information.
Selection Bias in Health Research. When researchers are required to obtain consent or authorization to access each individual’s medical record for a research study, it is likely that individuals’ willingness to grant access will not be (more...)
In addition, an educated public could also decrease the potential for biased research samples. A universal requirement for consent or authorization in medical records research leads to incomplete datasets, and thus to biased results and inaccurate conclusions. Some large medical institutions with a strong research history and reputation (e.g., Mayo Clinic) can obtain authorization and consent rates as high as 80 percent, but the 20 percent who refuse have distinct demographic and health characteristics. In fact, even a refusal rate of less than 5 percent can create selection bias in the data ( Jacobsen et al., 1999 ; see Chapter 5 for more detail). Conveying to the public the importance of health care improvements derived from medical records research and stressing the negative impact of incomplete datasets on research findings may increase the public’s participation in research and their willingness to support information-based research that is conducted with IRB or Privacy Board oversight, under a waiver of patient consent or authorization.
Numerous examples of important research findings from medical records research would not have been possible if direct patient consent and authorization were always required ( Box 3-1 ). For example, analysis of medical records showed that infants exposed to diethylstilbesterol (DES) during the first trimester of pregnancy had an increased risk of breast, vaginal, and cervical cancer as well as reproductive anomalies as adults. Similarly, studies of medical records led to the discovery that folic acid supplementation during pregnancy can prevent neural tube defects.
Thus, HHS and the health research community should work to edu cate the public about how research is done and the value it provides. All stakeholders, including professional organizations, nonprofit funders, and patient organizations, have different interests and responsibilities to make sure that their constituencies are well informed. For example, the American Society of Clinical Oncology and the American Heart Association already have some online resources to help patients gather information about research that may be relevant to their conditions. But coordination and identification of best practices by HHS would be helpful, and research is needed to identify which segments of the population would be receptive to and benefit from various types of information about how research is done and its value in order to create and implement an effective plan.
Greater use of community-based participatory research, in which community-based organizations or groups bring community members into the research process as partners to help design studies and disseminate the knowledge gained, 39 could help achieve this goal. These groups help researchers to recruit research participants by using the knowledge of the community to understand health problems and to design activities that the community is likely to value. They also inform community members about how the research is done and what comes out of it, with the goal of providing immediate community benefits from the results when possible.
- CONCLUSIONS AND RECOMMENDATIONS
Based on its review of the information described in this chapter, the committee agreed on a second overarching principle to guide the formation of recommendations. The committee affirms the importance of maintaining and improving health research effectiveness. Research discoveries are central to achieving the goal of extending the quality of healthy lives. Research into causes of disease, methods for prevention, techniques for diagnosis, and new approaches to treatment has increased life expectancy, reduced infant mortality, limited the toll of infectious diseases, and improved outcomes for patients with heart disease, cancer, diabetes, and other chronic diseases. Patient-oriented clinical research that tests new ideas makes rapid medical progress possible. Today, the rate of discovery is accelerating, and we are at the precipice of a remarkable period of investigative promise made possible by new knowledge about the genetic underpinnings of disease. Genomic research is opening new possibilities for preventing illness and for developing safer, more effective medical care that may eventually be tailored for specific individuals. Further advances in relating genetic information to predispositions to disease and responses to treatments will require the use of large amounts of existing health-related information and stored tissue specimens. The increasing use of electronic medical records will further facilitate the generation of new knowledge through research and accelerate the pace of discovery. These efforts will require broad participation of patients in research and broad data sharing to ensure that the results are valid and applicable to different segments of the population. Collaborative partnerships among communities of patients, their physicians, and teams of researchers to gain new scientific knowledge will bring tangible benefits for people in this country and around the world.
Surveys indicate that the majority of Americans believe that health research is important, are interested in the findings of research studies, and are willing to participate in health research. But patients often lack information about how research is conducted and are rarely informed about research results that may have a direct impact on their health. Effective communication could build the public’s trust of the research community, which is important because trust is necessary for the public’s continued participation in research. Moreover, direct feedback could lead to improved health care for study participants if the results indicate that an altered course of care is warranted.
Thus, the committee recommends that when patients consent to the use of their medical records in a particular study, health researchers should make greater efforts when the study ends to inform study participants about the results, and the relevance and importance of those results. Broader adoption of electronic health records may be helpful in accomplishing this goal, but standards and guidelines for providing and explaining study results to research participants or various sectors of the public are needed.
HHS should also encourage registration of trials and other studies in public databases, particularly when research is conducted with an IRB/ Privacy Board approved waiver of consent or authorization, as a way to make information about research studies more broadly available to the public. Numerous clinical trial registries already exist, and registration has increased in recent years, but no centralized system currently exists for disseminating information about clinical trials of drugs or other interventions, making it difficult for consumers and their health care providers to identify ongoing studies. Moreover, noninterventional studies, such as observational studies that play an increasingly critical role in biomedical research, are not generally included in these databases. Because many noninterventional studies are conducted with an IRB/Privacy Board approved waiver of consent or authorization, including such studies in a registry could be an important method for increasing public knowledge of those studies.
Interventional clinical trials are the most visible of the various types of health research, but a great deal of information-based health research entails analysis of thousands of patient records to better understand human diseases, to determine treatment effectiveness, and to identify adverse side effects of therapies. This form of research is likely to increase in frequency as the availability of electronic health records continues to expand. As we move toward the goal of personalized medicine, research results will be even more likely to be directly relevant to patients, but more study participants will be necessary to derive meaningful results.
However, many patients are likely not aware that their medical records are being used in information-based research, and surveys show that many patients desire not only notice, but also the opportunity to decide about whether to consent to such research with medical records. As noted in Chapter 2 , strengthening security protections of health data should reduce the risk of security breaches and their potential negative consequences, and thus should help to alleviate patient concerns in this regard. But educating patients about how health research is conducted, monitored, and reported could also increase patients’ trust in the research community. Thus, HHS and the health research community should work to educate the public about how research is done.
It will also be important for HHS and researchers to convey the value of health care improvements derived from medical records research, and to stress the negative impact of incomplete datasets on research findings. Representative samples are essential to ensure the validity and generalizability of health research, but datasets will not be representative of the entire population if some people withhold access to their health information. A universal requirement for consent or authorization in information-based research may lead to incomplete datasets, and thus to biased results and inaccurate conclusions. Numerous examples of important research findings from medical records research would not have been possible if direct patient consent and authorization were always required.
To ensure that beneficial health research and related activities continue to be undertaken with appropriate oversight under federal regulations, it will be important for HHS to also provide more guidance on how to distinguish the various activities. The Privacy Rule makes a distinction between health research and some closely related endeavors, such as public health and quality improvement activities, which also may involve collection and analysis of personally identifiable health information. Under the Privacy Rule (as well as the Common Rule ), these activities, which aim to protect the public’s health and improve the quality of patient care, are considered health care “practice” rather than health research. Therefore, they can be undertaken without consent or authorization, or an IRB/ Privacy Board waiver of consent or authorization. However, it can be a challenge for IRBs and Privacy Boards to distinguish among activities that are or are not subject to the various provisions of the Privacy Rule and the Common Rule, and inappropriate decisions may prevent important activities from being undertaken or could potentially allow improper disclosure of personally identifiable health information.
To address these difficulties, a number of models have been proposed that outline the criteria IRBs and Privacy Boards should use to distinguish practice and research. For example, one recent model provides a detailed checklist for IRBs and Privacy Boards to use in determining whether an activity is public health research and required to comply with the research provisions of the Privacy Rule, or public health practice that does not need IRB/Privacy Board review. The committee believes that standardizing the criteria is essential to support the conduct of these important health care activities.
Thus, HHS should convene the relevant stakeholders to develop standard criteria for IRBs and Privacy Boards to use when making decisions about whether protocols entail research or practice. There should be flexibility in the regulation to allow important activities to go forward with appropriate levels of oversight. Also, it will be important to evaluate whether these criteria are effective in aiding IRB/Privacy Board reviews of proposed protocols, and whether they lead to appropriate IRB/Privacy Board decisions.
These changes suggested above could be accomplished without any changes to HIPAA by making them a condition of funding from HHS and other research sponsors and by providing some additional funds to cover the cost.
- AMS (Academy of Medical Sciences). Personal data for public good: Using health information in medical research. 2006. [accessed August 28, 2008]. http://www .acmedsci.ac .uk/images/project/Personal.pdf .
- Baily MA. Harming through protection? New England Journal of Medicine. 2008; 258 (8):768–769. [ PubMed : 18287599 ]
- Baily MA, Bottrell M, Lynn J, Jennings B. The ethics of using QI methods to improve health care quality and safety. A Hastings Center Special Report. 2006; 36 (4):S1–S40. [ PubMed : 16898359 ]
- Bates DW, Leape LL, Cullen DJ, Laird N, Petersen LA, Teich JM, Burdick E, Hickey M, Kleefield S, Shea B, Vander VM, Seger DL. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998; 280 (15):1311–1316. [ PubMed : 9794308 ]
- Bellin E, Dubler NN. The quality improvement-research divide and the need for external oversight. American Journal of Public Health. 2001; 91 (9):1512–1517. [ PMC free article : PMC1446813 ] [ PubMed : 11527790 ]
- Big Health. Big health consortium. 2008. [accessed October 29, 2008]. http://www .bighealthconsortium .org/about/
- Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine. 2008; 87 (1):1–9. [ PubMed : 18204365 ]
- Brown DR, Fouad MN, Basen-Engquist K, Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention and treatment trials. Annals of Epidemiology. 2000; 10 :S13–S21. [ PubMed : 11189088 ]
- Burris S, Gable L, Stone L, Lazzarini Z. The role of state law in protecting human subjects of public health research and practice. Journal of Law, Medicine & Ethics. 2003; 31 :654. [ PubMed : 14968667 ]
- Casarett D, Karlawish J, Sugarman J. Determining when quality improvement initiatives should be considered research: Proposed criteria and potential implications. JAMA. 2000; 284 (7):2275–2280. [ PubMed : 10807388 ]
- Casarett D, Karlawish J, Andrews E, Caplan A. Bioethical issues in pharmacoepidemiological research. In: Strom BL, editor. Pharmacoepidemiology. West Sussex, England: John Wiley & Sons, Ltd.; 2005. pp. 417–432.
- CDC (Centers for Disease Control and Prevention). Guidelines for defining public health research and public health non-research. 1999. [accessed March 4, 2008]. http://www .cdc.gov/od /science/regs/hrpp/researchdefinition .htm .
- CHSR (Coalition for Health Services Research). Framework for health services research policy for 2008. 2008. [accessed August 21, 2008]. http://www .chsr.org/Policy_Priorities .pdf .
- Comis RL, Aldige CR, Stovall EL, Krebs LU, Risher PJ, Taylor HJ. A quantitative survey of public attitudes towards cancer clinical trials. Philadelphia, PA: Coalition of National Cancer Cooperative Groups, Cancer Research Foundation of America, Cancer Leadership Council, and Oncology Nursing Society; 2000.
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans towards participation in medical research. Journal of General Internal Medicine. 1999; 14 :537–546. [ PMC free article : PMC1496744 ] [ PubMed : 10491242 ]
- Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: Patients’ willingness to allow researchers to access their medical records. Social Science & Medicine. 2007; 64 (1):223–235. [ PubMed : 17045717 ]
- DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJPM, Schroeder TV, Sox HC, Van Der Weyden MB. Clinical trial registration: A statement from the International Committee of Medical Journal Editors. JAMA. 2004; 292 (11):1363–1364. [ PubMed : 15355936 ]
- Farmer D, Jackson SA, Camacho F, Hall MA. Attitudes of African American and low socioeconomic status white women toward medical research. Journal of Health Care for the Poor and Underserved. 2007; 18 :85–99. [ PubMed : 17337800 ]
- FDA (Food and Drug Administration). Certain estrogens for oral or parenteral use. Drugs for human use; drug efficacy study implementation. Federal Register. 1971; 36 (217):21537–21538.
- FDA. FDA public health advisory, deaths with antipsychotics in elderly patients with behavioral disturbances. 2005. [accessed August 18, 2008]. http://www .fda.gov/cder /drug/advisory/antipsychotics.htm .
- FDA. FDA alert [6/16/2008]: Information for healthcare professionals. Antipsychotics. 2008. [accessed August 18, 2008]. http://www .fda.gov/cder /drug/infosheets/hcp /antipsychotics_conventional.htm .
- Finkelstein EA, Fiebelkorn IC, Wang G. National medical spending attributable to overweight and obesity: How much, and who’s paying? Health Affairs Web Exclusive. 2003. [accessed August 21, 2008]. http://content .healthaffairs .org/cgi/content /abstract/hlthaff.w3.219v1 . [ PubMed : 14527256 ]
- Furrow BR, Greaney TL, Johnson SH, Jost TS, Schwartz RL. Bioethics: Health care law and ethics. St. Paul, MN: Thomson/West; 2004.
- GAO (Government Accountability Office). Scientific research: Continued vigilance critical to protecting human subjects. Washington, DC: GAO; 1996.
- Genetics & Public Policy Center. U.S. public opinion on uses of genetic information and genetic discrimination. 2007. [accessed August 21, 2008]. http://www .dnapolicy .org/resources/GINAPublic _Opinion_Genetic _Information_Discrimination.pdf .
- Gill S, Bronskill S, Normand S, Anderson G, Sykora K, Lam K, Bell C, Lee P, Fischer H, Herrmann N, Gurwitz J, Rochon P. Antipsychotic drug use and mortality in older adults with dementia. Annals of Internal Medicine. 2007; 146 :775–786. [ PubMed : 17548409 ]
- Giuliano AR, Mokuau N, Hughes C, Tortelero-Luna G, Risendal B, Ho RCS, Prewitt TE, McCaskill-Stevens WJ. Participation of minorities in cancer research: The influence of structural, cultural, and linguistic factors. Annals of Epidemiology. 2000; 10 :S22–S34. [ PubMed : 11189089 ]
- Goldman J, Choy A. Ethical and policy issues in research involving human participants. Bethesda, MD: National Bioethics Advisory Commission; 2001. Privacy and confidentiality in health research; pp. C1–C34.
- Gostin LO. Public health law: Power, duty, restraint. Berkeley, CA: University of California Press; 2008. Surveillance and public health research: Personal privacy and the “right to know.”
- Grady C, Hampson LA, Wallen GR, Rivera-Goba MV, Carrington KL, Mittleman BB. Exploring the ethics of clinical research in an urban community. American Journal of Public Health. 2006; 96 (11):1996–2001. [ PMC free article : PMC1751807 ] [ PubMed : 17018826 ]
- Hatfield M, Sonnenschein HF, Rosenberg LE. Exceptional returns: The economic value of America’s investment in medical research. 2001. [accessed August 21, 2008]. http://www .laskerfoundation .org/advocacy/pdf/exceptional.pdf .
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. New England Journal of Medicine. 1971; 284 (15):878–881. [ PubMed : 5549830 ]
- HEW (Department of Health, Education and Welfare). The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research. 1979. [accessed August 21, 2008]. http://ohsr .od.nih.gov /guidelines/belmont.html . [ PubMed : 25951677 ]
- HHS (Department of Health and Human Services). Institutional review boards and the HIPAA Privacy Rule. 2003. [accessed August 21, 2008]. http: //privacyruleandresearch .nih.gov/pdf/IRB_Factsheet.pdf .
- HHS. Guidance on research involving coded private information or biological specimens. 2004. [accessed August 21, 2008]. http://www .hhs.gov/ohrp /humansubjects/guidance/cdebiol.pdf .
- Hodge JG Jr. An enhanced approach to distinguishing public health practice and human subjects research. Journal of Law, Medicine & Ethics. 2005; 33 (1):125–141. [ PubMed : 15934670 ]
- Hodge JG, Gostin LO. Public health practice vs. research: A report for public health practitioners including cases and guidance for making distinctions. Atlanta, GA: Council of State and Territorial Epidemiologists; 2004.
- IOM (Institute of Medicine). Health services research: Work force and educational issues. Washington, DC: National Academy Press; 1995.
- IOM. Protecting data privacy in health services research. Washington, DC: National Academy Press; 2000a. [ PubMed : 25057723 ]
- IOM. To err is human: Building a safer health system. Washington, DC: National Academy Press; 2000b. [ PubMed : 25077248 ]
- IOM. Responsible research: A systems approach to protecting research participants. Washington, DC: The National Academies Press; 2002. [ PubMed : 20669487 ]
- Jacobsen S, Xia Z, Campion M, Darby C, Plevak M, Seltman K, Melton L. Potential effect of authorization bias on medical record research. Mayo Clinic Proceedings. 1999; 74 :330–338. [ PubMed : 10221460 ]
- Laine C, Horton R, DeAngelis CD, Drazen JM, Frizelle FA, Godlee F, Haug C, Hébert PC, Kotzin S, Marusic A, Sahni P, Schroeder TV, Sox HC, Van Der Weyden MB, Verheugt FWA. Clinical trial registration—looking back and moving ahead. New England Journal of Medicine. 2007; 356 (26):2734–2736. [ PubMed : 17548427 ]
- Lee B. Comparison of FDA and HHS human subject protection regulations. 2000. [accessed August 21, 2008]. http://www .fda.gov/oc/gcp/comparison .html .
- Lindenauer PK, Benjamin EM, Naglieri-Prescod D, Fitzgerald J, Pekow P. The role of the institutional review board in quality improvement: A survey of quality officers, institutional review board chairs, and journal editors. The American Journal of Medicine. 2002; 113 :575–579. [ PubMed : 12459404 ]
- Lowrance WW. Learning from experience, privacy and the secondary use of data in health research. London: The Nuffield Trust; 2002.
- Lowrance WW, Collins FS. Identifiability in genomic research. Science. 2007; 317 :600–602. [ PubMed : 17673640 ]
- Lynn J, Baily MA, Bottrell M, Jennings B, Levine RJ, Davidoff F, Casarett D, Corrigan J, Fox E, Wynia MK, Agich GJ, Speroff T, Schyve P, Batalden P, Tunis S, Berlinger N, Cronenwett L, Fitzmaurice M, Dubler NN, James B. The ethics of using quality improvement methods in health care. Annals of Internal Medicine. 2007; 146 (6):666–674. [ PubMed : 17438310 ]
- Mandelblatt J, Saha S, Teutsch S, Hoerger T, Siu AL, Atkins D, Klein J, Helfand M. The cost-effectiveness of screening mammography beyond age 65: A systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2003; 139 :835–842. [ PubMed : 14623621 ]
- Mick S, Morlock LL, Salkever D, de Lissovoy G, Malitz F, Wise CG, Jones A. Strategic activity and financial performance of U.S. rural hospitals: A national study, 1983 to 1988. Journal of Rural Health. 1994; 10 (3):150–167. [ PubMed : 10138031 ]
- Mosconi P, Poli P, Giolo A, Apolone G. How Italian health consumers feel about clinical research: A questionnaire survey. European Journal of Public Health. 2005; 15 (4):372–379. [ PubMed : 16014662 ]
- Murphy K, Topel R. The economic value of medical research. Chicago, IL: University of Chicago Press; 1999.
- NCI (National Cancer Institute). Getting connected with caBIG: Data sharing and security framework. 2008. [accessed October 27, 2008]. https://cabig .nci.nih .gov/working_groups /DSIC_SLWG/data_sharing_policy/
- NCURA (National Council of University Research Administrators). Report on research compliance. 2007. [accessed March 4, 2008]. http://www .reportonresearchcompliance .com .
- NCVHS (National Committee on Vital and Health Statistics). Enhanced protections for uses of health data: A stewardship framework for “secondary uses” of electronically collected and transmitted health data. 2007a. [accessed December 19, 2007]. http://ncvhs .hhs.gov/071221lt.pdf .
- NCVHS, Ad Hoc Work Group on Secondary Uses of Health Data. Testimony of the cancer Biomedical Informatics Grid (caBIG) Data Sharing and Intellectual Capital (DSIC) workspace. 2007b August 1, 2007
- Needleman J, Buerhaus P, Mattke S, Stewart M, Zelevinsky K. Nurse-staffing levels and the quality of care in hospitals. New England Journal of Medicine. 2002; 346 (22):1715–1722. [ PubMed : 12037152 ]
- NSF (National Science Foundation). Science and Engineering Indicators 2006. Arlington, VA: National Science Foundation; 2006. Science and technology: Public attitudes and understanding. Chapter 7.
- OHRP (Office for Human Research Protections). IRB guidebook, part I.A. 2008a. [accessed August 21, 2008]. http://www .hhs.gov/ohrp /irb/irb_guidebook.htm .
- OHRP. OHRP concludes case regarding Johns Hopkins University research on hospital infections. 2008b. [accessed August 21, 2008]. http://www .hhs.gov/ohrp/news/recentnews .html#20080215 .
- Partridge A, Winer E. Informing clinical trial participants about study results. JAMA. 2002; 288 :363–365. [ PubMed : 12117402 ]
- Partridge AH, Wolff AC, Marcom PK, Kaufman PA, Zhang L, Gelman R, Moore C, Lake D, Fleming GF, Rugo HS, Atkins J, Sampson E, Collyar D, Winer EP. The impact of sharing results of a randomized breast cancer clinical trial with study participants. Breast Cancer Research and Treatment. 2008 June 10 [ PubMed : 18543100 ]
- Pitkin R. Folate and neural tube defects. American Journal of Clinical Nutrition. 2007; 85 (1):285S–288S. [ PubMed : 17209211 ]
- Pritts J. The importance and value of protecting the privacy of health information: Roles of HIPAA Privacy Rule and the Common Rule in health research. 2008. [accessed March 15, 2008]. http://www .iom.edu/CMS/3740/43729/53160 .aspx .
- Research!America. America speaks: Poll summary. Vol. 7. Alexandria, VA: United Health Foundation; 2007.
- Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang P. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. Canadian Medical Association Journal. 2007; 176 :672–632. [ PMC free article : PMC1800321 ] [ PubMed : 17325327 ]
- Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: Attitudes, practices, and future directions. PLoS Medicine. 2008a May 13; 5 (5):e91. [ PMC free article : PMC2375946 ] [ PubMed : 18479180 ]
- Shalowitz DI, Miller FG. The search for clarity in communicating research results to study participants. Journal of Medical Ethics. 2008b September; 34 (9):e17. [ PubMed : 18757617 ]
- Shaul RZ, Birenbaum S, Evans M. Legal liability in research: Early lessons from North America. BMC Medical Ethics. 2005; 6 (4):1–4. [ PMC free article : PMC1182131 ] [ PubMed : 15953387 ]
- Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Annals of Epidemiology. 2002; 12 :248–256. [ PubMed : 11988413 ]
- Sim I, Chan A-W, Gülmezoglu AM, Evans T, Pang T. Clinical trial registration: Transparency is the watchword. The Lancet. 2006; 367 (9523):1631–1633. [ PubMed : 16714166 ]
- Slamon D, Clark G, Wong S, Levin W, Ullrich A, McGuire W. Human breast cancer: Correlation of relapse and survival with amplification of the her-2/neu oncogene. Science. 1987; 235 (4785):177–182. [ PubMed : 3798106 ]
- Snider DE, Stroup DF. Defining research when it comes to public health. Public Health Reports. 1997; 112 :29–32. [ PMC free article : PMC1381834 ] [ PubMed : 9018284 ]
- Thorpe KE, Florence CS, Howard DH, Joski P. The impact of obesity in rising medical spending. Health Affairs Web Exclusive. 2004. [accessed August 21, 2008]. http://content .healthaffairs .org/cgi/content /abstract/hlthaff.w4.480v1 . [ PubMed : 15496437 ]
- Trauth JM, Musa D, Siminoff L, Jewell IK, Ricci E. Public attitudes regarding willingness to participate in medical research studies. Journal of Health & Social Policy. 2000; 12 (2):23–43. [ PubMed : 11184441 ]
- Veurink M, Koster M, Berg L. The history of DES, lessons to be learned. Pharmacy World & Science. 2005; 27 (3):139–143. [ PubMed : 16096877 ]
- Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, Brawley OW, Gross CP, Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Medicine. 2006; 3 (2):201–210. [ PMC free article : PMC1298944 ] [ PubMed : 16318411 ]
- Westin A. How the public views privacy and health research. 2007. [accessed November 11, 2008]. http://www .iom.edu/Object .File/Master/48 /528/%20Westin%20IOM %20Srvy%20Rept%2011-1107.pdf .
- Williams ED. Federal protection for human research subjects: An analysis of the Common Rule and its interactions with FDA regulations and the HIPAA Privacy Rule, CRS report for Congress. Washington, DC: Congressional Research Service; 2005.
- Williams IC, Corbie-Smith G. Investigator beliefs and reported success in recruiting minority participants. Contemporary Clinical Trials. 2006; 27 :580–586. [ PubMed : 16839822 ]
- Winston FK, Durbin DR, Kallan MJ, Moll EK. The danger of premature graduation to seat belts for young children. Pediatrics. 2000; 105 (6):1179–1183. [ PubMed : 10835054 ]
- WMA (World Medical Association). Declaration of Helsinki: Ethical principles for medical research involving human subjects. 1964. [accessed August 21, 2008]. http://ohsr .od.nih.gov /guidelines/helsinki.html . [ PubMed : 16010903 ]
- Woolley M, Propst SM. Public attitudes and perceptions about health-related research. JAMA. 2005; 294 (11):1380–1384. [ PubMed : 16174697 ]
- Zarin DA, Tse T. Moving toward transparency of clinical trials. Science. 2008; 319 :1340–1342. [ PMC free article : PMC2396952 ] [ PubMed : 18323436 ]
- Zarin DA, Tse T, Ide NC. Trial registration at ClinicalTrials.gov between May and October 2005. New England Journal of Medicine. 2005; 353 (26):2779–2787. [ PMC free article : PMC1568386 ] [ PubMed : 16382064 ]
Epidemiology is the study of the occurrence, distribution, and control of diseases in populations.
Health services research has been defined as a multidisciplinary field of inquiry, both basic and applied, that examines the use, costs, quality, accessibility, delivery, organization, financing, and outcomes of health care services to increase knowledge and understanding of the structure, processes, and effects of health services for individuals and populations ( IOM, 1995 ).
The National Committee on Vital and Health Statistics has noted that “secondary uses” of health data is an ill-defined term, and urges abandoning it in favor of precise description of each use ( NCVHS, 2007a ). Thus, the committee chose to minimize use of the term in this report.
See Standards for Privacy of Individually Identifiable Health Information , 64 Fed. Reg. 59918, 59967 (preamble to rule proposed November 3, 1999) for a discussion on the benefits of health records research.
Effectiveness can be defined as the extent to which a specific test or intervention, when used under ordinary circumstances, does what it is intended to do. Efficacy refers to the extent to which a specific test or intervention produces a beneficial result under ideal conditions (e.g., in a clinical trial).
See http://www .intermacs.org .
See http://www .elso.med.umich.edu .
See http://www .unos.org/Data .
The Department of Health, Education and Welfare (now HHS) had previously issued policy and guidance on the protection of human subjects. See Williams (2005) .
In its report “First Biennial Report on the Adequacy and Uniformity of Federal Rules and Policies, and their Implementation, for the Protection of Human Subjects in Biomedical and Behavioral Research , Protecting Human Subjects.”
45 C.F.R. part 46 (2005).
See 45 C.F.R. § 46.101 (2005).
See 45 C.F.R. § 46.102(d) (2005).
This section on informed consent is based largely on a Congressional Research Service report ( Williams, 2005 ), as adapted by Pritts (2008) .
See 45 C.F.R. § 46.116 (2005).
See 45 C.F.R. § 46.116(b) (2005).
See 45 C.F.R. § 46.103 (2005).
See 45 C.F.R. § 46.111 (2005). There are additional factors if the study includes subjects who are likely to be vulnerable to coercion or undue influence.
See 45 C.F.R. § 46.116(d); 46.117(c) (2005).
See 45 C.F.R. § 46.102(f) (2005).
See 45 C.F.R. § 46.101(b)(4) (2005).
See 45 C.F.R. § 46.116(e) (2005).
See 45 C.F.R. § 46.123 (2005).
See also Grimes v. Kennedy Krieger Institute , 782 A. 2d 807 (Md. Ct. App. 2001); Gelsinger v. University of Pennsylvania (Philadelphia County Court of Common Pleas filed September 18, 2000), available at http://www .sskrplaw.com /links/healthcare2.html .
The FDA has defined “clinical investigation” to be synonymous with “research.”
The Food, Drug, and Cosmetic Act Section 505(i), 507(d), or 520(g) of 21 U.S.C. 355(i), 357(d), or 360j(g) (1972).
See 21 C.F.R. part 50 (2008); 46 Fed. Reg. 8942 (1981).
See 21 C.F.R. part 56 (2008); 46 Fed. Reg. 8958 (1981).
See 45 C.F.R. part 46 (2005); 46 Fed. Reg. 8366 (1981).
The Centers for Disease Control and Prevention defines program evaluation as the “systematic investigation of the merit, worth, or significance of organized public health action,” noting that such evaluations are “systematic ways to improve and account for public health actions by involving procedures that are useful, feasible, ethical, and accurate.” They can be based on goals, processes, outcomes, or value ( http://www .cdc.gov/mmwr /preview/mmwrhtml/rr4811a1.htm ).
The Utilization Review Accreditation Commission defines utilization review as “the evaluation of the medical necessity, appropriateness, and efficiency of the use of health care services, procedures, and facilities under the provisions of the applicable health benefits plans” ( http://www .urac.org/about/ ).
Another type of oversight board defined by the Privacy Rule. See Chapter 4 .
Under the Privacy Rule, consent is referred to as authorization. See Chapter 4 .
Personal communication, C. Heide, Office for Civil Rights, HHS, May 29, 2008.
The Privacy Rule defines the term “health care operations” by listing a number of specific activities that qualify as health care operations. These include “conducting quality assessment and improvement activities, population-based activities relating to improving or reducing health care costs, and case management and care coordination.” See 45 C.F.R. § 164.501 (2006).
A total of 444 surveys were mailed to the medical directors of quality improvement and IRB chairs at hospitals with 400 or more beds that belong to the Council of Teaching Hospitals of the Association of American Medical Colleges, and to the editors of all U.S.-based medical journals that publish original research and appear in the Abridged Index Medicus. 236 surveys were returned, for a 53 percent response rate. The survey consisted of six brief scenarios that asked respondents to determine whether the described project needed IRB review and informed consent.
See http: //plainlanguage.gov/index.cfm .
FDA, Public Law 110–85 § 801 (2007).
See http://www .ahrq.gov/research/cbprrole .htm .
- Cite this Page Institute of Medicine (US) Committee on Health Research and the Privacy of Health Information: The HIPAA Privacy Rule; Nass SJ, Levit LA, Gostin LO, editors. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research. Washington (DC): National Academies Press (US); 2009. 3, The Value, Importance, and Oversight of Health Research.
- PDF version of this title (1.6M)
- Disable Glossary Links
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- The Value, Importance, and Oversight of Health Research - Beyond the HIPAA Priva... The Value, Importance, and Oversight of Health Research - Beyond the HIPAA Privacy Rule
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- News Feature
- Published: 13 January 2021
Eight ways machine learning is assisting medicine
- Mike May 1
Nature Medicine volume 27 , pages 2–3 ( 2021 ) Cite this article
26k Accesses
49 Citations
336 Altmetric
Metrics details
There has been a lot of hype around the applications of machine learning in medicine. But how is machine learning actually helping bench-to-bedside scientists and clinicians do their jobs?
The idea of improving medicine with computation is almost as old as digital computers. In the early 1960s, scientists used a computer in diagnosing blood diseases, and that was just one pioneering example in this field. In the branch of artificial intelligence (AI) called ‘machine learning’, computer software learns from experience. The results teach medical researchers and clinicians new ways of studying diseases, making medicines and treating patients.
Computation in general enhances several key areas of clinical research, and AI-based methods promise even more applications for researchers. Despite not being in wide use so far, machine-learning systems already influence several areas of clinical research, such as appreciating the value of big data.
1. Reconstructing diseases
Combining machine learning with multi-modal datasets and almost unlimited computing power allows clinical researchers to “reconstruct the underlying mechanisms of disease,” says Colin Hill, CEO and cofounder of GNS Healthcare. For example, GNS Healthcare’s AI-driven simulation platform Gemini provides a computer model of the progression of multiple myeloma and drug responses. This model “harnesses the power of causal machine learning and simulation and in-depth clinical and molecular patient data to allow pharma companies to simulate drug response at the individual patient level,” Hill explains.
2. Hypothesis testing
In any medical research, predicting the outcome for a particular scenario proves very difficult. “With partial or imperfect biological knowledge, statistical models are the best way to reveal structure and predict outcomes,” says David Watson, doctoral candidate at the Oxford Internet Institute of the University of Oxford and founding member of the Digital Ethics Lab. “It is not always obvious how to do this with clinical data alone, and although clinicians are often confident that genomic information can solve the problem, this data is so noisy that it may make the task harder rather than simpler.” By combining information from clinicians and data-science tools, including machine learning, scientists can develop a hypothesis, model it, adjust it, and replicate the process in an iterative manner. “This requires close collaboration between clinicians and data scientists, who tend to conceptualize problems rather differently, but getting people with different realms of expertise to work together on challenging problems is what good research is all about,” Watson says.
3. Recruiting patients
Clinical trials provide a key element of medical research, and one complicated challenge is recruiting patients. As pharmacologist Mira Desai of the Nootan Medical College & Research Centre in India wrote , “Surprisingly, participant enrollment issues are the major reasons for trial terminations.” Machine learning could help medical researchers solve that problem. At the Commonwealth Scientific and Industrial Research Organisation in Australia, a group of scientists developed a machine-learning technique that explores patient medical records to find people who would fit specific trials. This example is one of many in which machine learning is just getting started in improving clinical trials.
4. Big data
“In the past, a large dataset for clinical researchers would often mean hundreds of patients only,” says Pearse Keane, consultant ophthalmologist at the UK-based Moorfields Eye Hospital NHS Foundation Trust. “As a byproduct of the infrastructure required for machine learning, we are starting to be able to aggregate much, much larger datasets.” Keane studies age-related macular degeneration, which is the most common cause of blindness in Europe, the UK and the USA, as well as in many other countries. “In the next decade,” Keane says, “I anticipate we will be doing clinical studies using images from every patient diagnosed with [age-related macular degeneration], perhaps running into the hundreds of thousands of patients per year.”
In the clinic
In the clinic, machine learning offers great promise, but much work lies ahead. As Keane says, “There is a huge gap between showing a proof of concept in a research paper and actually deploying a machine learning system in the real world — something that Eric Topol of Scripps Research and I have described as ‘the AI chasm’.” He adds, “There is undoubtedly huge potential for machine learning to transform healthcare, but going ‘from code to clinic’ is the hard part.”
5. Developing diagnostics
From military applications to medicine, computation can be used to analyze images. Imaging and clinical experts Dineo Mpanya and Nqoba Tsabedze of the Charlotte Maxeke Johannesburg Academic Hospital in Johannesburg, South Africa, teamed up to describe the impact of machine learning on the interpretation of medical images, such as chest X-rays. Subtypes of machine learning, such as convolutional neural networks, “can identify subtle changes in chest X-ray films, and in some instances, the accuracy levels for diagnosing conditions, such as pneumonia, are equivalent or superior to that of clinicians,” the scientists note. “Unlike traditional statistical methods, where inferences are made based on the population studied, machine-learning algorithms mimic human cognitive processes when making decisions.”
In April 2018, the US Food and Drug Administration approved the first AI-based diagnostic, IDx-DR, which detects diabetic retinopathy in people with diabetes by analyzing retinal images. Machine learning will soon be applied to many other medical conditions, from cardiology to neurodegenerative diseases and beyond.
6. Improving prognostics
In addition to using it to diagnose conditions, clinicians can use machine learning to predict a patient’s prognosis. The first application that comes to mind here is usually cancer. For example, one international team of scientists developed a machine learning–based tool that analyzes the prognosis of patients with stage III colon cancer, and the group reported that the results “could provide crucial information to aid treatment planning” for people with this disease. Plus, John Halamka, president of the Mayo Clinic Platform, and his colleagues suggested that machine learning might improve a clinician’s ability to determine the likely outcome of a patient with COVID-19. As with the use of machine language in clinical diagnosis, work in prognosis promises many improvements ahead.
7. Patient monitoring
Traditionally, physicians come in contact with patients after symptoms appear — sometimes not even until an illness creates a health crisis. “This is slowly changing with the development and increasing use of predictive analytics linked to machine learning and artificial intelligence models,” says Ali Rezai, the John D. Rockefeller IV Chair in neuroscience at West Virginia University. One day, machine learning and wearable technology could continuously monitor a person’s health. “Two of the most commercially available AI systems are incorporated in devices like the Apple Watch or the Kardia Alivecor devices, which can detect arrhythmias and send alerts to patients through their smartphone apps,” Rezai says. “While this is not fully integrated into the current clinical flow, AI will likely have a big impact in cardiology, cancer, and neurosciences by helping stratify and profile patients, enabling more proactive management and care.”

8. Requiring collaborations
Perhaps more than anything else in medicine, machine learning promises to drive collaboration — in fact, getting the most from machine learning–based applications depends on it. That is exactly what Maria Littmann, a doctoral candidate in bioinformatics at Technical University Munich, and her colleagues found when they analyzed 250 articles that described applications on machine learning in biology or medicine. These scientists discovered that 73% of the machine-learning applications in these articles arose from interdisciplinary collaborations of computational scientists, biologists and medical experts.
Experts from different fields bring varying perspectives and different modes of data, such as genomic and patient information, as well as different ways to analyze the data. Such collaborations will build bigger datasets. “As the volume of multi-modal data grows, the potential for machine learning’s impact on clinical research grows with it,” Hill says. “So far, we have just scratched the surface, and the impact is clear: Machine learning fueled by the right data has the power to transform the development of breakthrough, new medicines and optimize their use in patient care.”
Author information
Authors and affiliations.
Mike May is a freelance writer and editor based in Bradenton, Florida, USA
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
About this article
Cite this article.
May, M. Eight ways machine learning is assisting medicine. Nat Med 27 , 2–3 (2021). https://doi.org/10.1038/s41591-020-01197-2
Download citation
Published : 13 January 2021
Issue Date : January 2021
DOI : https://doi.org/10.1038/s41591-020-01197-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Use of extreme gradient boosting, light gradient boosting machine, and deep neural networks to evaluate the activity stage of extraocular muscles in thyroid-associated ophthalmopathy.
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
A comparison of machine learning algorithms in predicting COVID-19 prognostics
- Serpil Ustebay
- Abdurrahman Sarmis
Internal and Emergency Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
77 interesting medical research topics for 2024
Last updated
25 November 2023
Reviewed by
Brittany Ferri, PhD, OTR/L
Medical research is the gateway to improved patient care and expanding our available treatment options. However, finding a relevant and compelling research topic can be challenging.
Use this article as a jumping-off point to select an interesting medical research topic for your next paper or clinical study.
- How to choose a medical research topic
When choosing a research topic , it’s essential to consider a couple of things. What topics interest you? What unanswered questions do you want to address?
During the decision-making and brainstorming process, here are a few helpful tips to help you pick the right medical research topic:
Focus on a particular field of study
The best medical research is specific to a particular area. Generalized studies are often too broad to produce meaningful results, so we advise picking a specific niche early in the process.
Maybe a certain topic interests you, or your industry knowledge reveals areas of need.
Look into commonly researched topics
Once you’ve chosen your research field, do some preliminary research. What have other academics done in their papers and projects?
From this list, you can focus on specific topics that interest you without accidentally creating a copycat project. This groundwork will also help you uncover any literature gaps—those may be beneficial areas for research.
Get curious and ask questions
Now you can get curious. Ask questions that start with why, how, or what. These questions are the starting point of your project design and will act as your guiding light throughout the process.
For example:
What impact does pollution have on children’s lung function in inner-city neighborhoods?
Why is pollution-based asthma on the rise?
How can we address pollution-induced asthma in young children?
- 77 medical research topics worth exploring in 2023
Need some research inspiration for your upcoming paper or clinical study? We’ve compiled a list of 77 topical and in-demand medical research ideas. Let’s take a look.
- Exciting new medical research topics
If you want to study cutting-edge topics, here are some exciting options:
COVID-19 and long COVID symptoms
Since 2020, COVID-19 has been a hot-button topic in medicine, along with the long-term symptoms in those with a history of COVID-19.
Examples of COVID-19-related research topics worth exploring include:
The long-term impact of COVID-19 on cardiac and respiratory health
COVID-19 vaccination rates
The evolution of COVID-19 symptoms over time
New variants and strains of the COVID-19 virus
Changes in social behavior and public health regulations amid COVID-19
Vaccinations
Finding ways to cure or reduce the disease burden of chronic infectious diseases is a crucial research area. Vaccination is a powerful option and a great topic to research.
Examples of vaccination-related research topics include:
mRNA vaccines for viral infections
Biomaterial vaccination capabilities
Vaccination rates based on location, ethnicity, or age
Public opinion about vaccination safety
Artificial tissues fabrication
With the need for donor organs increasing, finding ways to fabricate artificial bioactive tissues (and possibly organs) is a popular research area.
Examples of artificial tissue-related research topics you can study include:
The viability of artificially printed tissues
Tissue substrate and building block material studies
The ethics and efficacy of artificial tissue creation
- Medical research topics for medical students
For many medical students, research is a big driver for entering healthcare. If you’re a medical student looking for a research topic, here are some great ideas to work from:
Sleep disorders
Poor sleep quality is a growing problem, and it can significantly impact a person’s overall health.
Examples of sleep disorder-related research topics include:
How stress affects sleep quality
The prevalence and impact of insomnia on patients with mental health conditions
Possible triggers for sleep disorder development
The impact of poor sleep quality on psychological and physical health
How melatonin supplements impact sleep quality
Alzheimer’s and dementia
Cognitive conditions like dementia and Alzheimer’s disease are on the rise worldwide. They currently have no cure. As a result, research about these topics is in high demand.
Examples of dementia-related research topics you could explore include:
The prevalence of Alzheimer’s disease in a chosen population
Early onset symptoms of dementia
Possible triggers or causes of cognitive decline with age
Treatment options for dementia-like conditions
The mental and physical burden of caregiving for patients with dementia
- Lifestyle habits and public health
Modern lifestyles have profoundly impacted the average person’s daily habits, and plenty of interesting topics explore its effects.
Examples of lifestyle and public health-related research topics include:
The nutritional intake of college students
The impact of chronic work stress on overall health
The rise of upper back and neck pain from laptop use
Prevalence and cause of repetitive strain injuries (RSI)
- Controversial medical research paper topics
Medical research is a hotbed of controversial topics, content, and areas of study.
If you want to explore a more niche (and attention-grabbing) concept, here are some controversial medical research topics worth looking into:
The benefits and risks of medical cannabis
Depending on where you live, the legalization and use of cannabis for medical conditions is controversial for the general public and healthcare providers.
Examples of medical cannabis-related research topics that might grab your attention include:
The legalization process of medical cannabis
The impact of cannabis use on developmental milestones in youth users
Cannabis and mental health diagnoses
CBD’s impact on chronic pain
Prevalence of cannabis use in young people
The impact of maternal cannabis use on fetal development
Understanding how THC impacts cognitive function
Human genetics
The Human Genome Project identified, mapped, and sequenced all human DNA genes. Its completion in 2003 opened up a world of exciting and controversial studies in human genetics.
Examples of human genetics-related research topics worth delving into include:
Medical genetics and the incidence of genetic-based health disorders
Behavioral genetics differences between identical twins
Genetic risk factors for neurodegenerative disorders
Machine learning technologies for genetic research
Sexual health studies
Human sexuality and sexual health are important (yet often stigmatized) medical topics that need new research and analysis.
As a diverse field ranging from sexual orientation studies to sexual pathophysiology, examples of sexual health-related research topics include:
The incidence of sexually transmitted infections within a chosen population
Mental health conditions within the LGBTQIA+ community
The impact of untreated sexually transmitted infections
Access to safe sex resources (condoms, dental dams, etc.) in rural areas
- Health and wellness research topics
Human wellness and health are trendy topics in modern medicine as more people are interested in finding natural ways to live healthier lifestyles.
If this field of study interests you, here are some big topics in the wellness space:
Gluten sensitivity
Gluten allergies and intolerances have risen over the past few decades. If you’re interested in exploring this topic, your options range in severity from mild gastrointestinal symptoms to full-blown anaphylaxis.
Some examples of gluten sensitivity-related research topics include:
The pathophysiology and incidence of Celiac disease
Early onset symptoms of gluten intolerance
The prevalence of gluten allergies within a set population
Gluten allergies and the incidence of other gastrointestinal health conditions
Pollution and lung health
Living in large urban cities means regular exposure to high levels of pollutants.
As more people become interested in protecting their lung health, examples of impactful lung health and pollution-related research topics include:
The extent of pollution in densely packed urban areas
The prevalence of pollution-based asthma in a set population
Lung capacity and function in young people
The benefits and risks of steroid therapy for asthma
Pollution risks based on geographical location
Plant-based diets
Plant-based diets like vegan and paleo diets are emerging trends in healthcare due to their limited supporting research.
If you’re interested in learning more about the potential benefits or risks of holistic, diet-based medicine, examples of plant-based diet research topics to explore include:
Vegan and plant-based diets as part of disease management
Potential risks and benefits of specific plant-based diets
Plant-based diets and their impact on body mass index
The effect of diet and lifestyle on chronic disease management
Health supplements
Supplements are a multi-billion dollar industry. Many health-conscious people take supplements, including vitamins, minerals, herbal medicine, and more.
Examples of health supplement-related research topics worth investigating include:
Omega-3 fish oil safety and efficacy for cardiac patients
The benefits and risks of regular vitamin D supplementation
Health supplementation regulation and product quality
The impact of social influencer marketing on consumer supplement practices
Analyzing added ingredients in protein powders
- Healthcare research topics
Working within the healthcare industry means you have insider knowledge and opportunity. Maybe you’d like to research the overall system, administration, and inherent biases that disrupt access to quality care.
While these topics are essential to explore, it is important to note that these studies usually require approval and oversight from an Institutional Review Board (IRB). This ensures the study is ethical and does not harm any subjects.
For this reason, the IRB sets protocols that require additional planning, so consider this when mapping out your study’s timeline.
Here are some examples of trending healthcare research areas worth pursuing:
The pros and cons of electronic health records
The rise of electronic healthcare charting and records has forever changed how medical professionals and patients interact with their health data.
Examples of electronic health record-related research topics include:
The number of medication errors reported during a software switch
Nurse sentiment analysis of electronic charting practices
Ethical and legal studies into encrypting and storing personal health data
Inequities within healthcare access
Many barriers inhibit people from accessing the quality medical care they need. These issues result in health disparities and injustices.
Examples of research topics about health inequities include:
The impact of social determinants of health in a set population
Early and late-stage cancer stage diagnosis in urban vs. rural populations
Affordability of life-saving medications
Health insurance limitations and their impact on overall health
Diagnostic and treatment rates across ethnicities
People who belong to an ethnic minority are more likely to experience barriers and restrictions when trying to receive quality medical care. This is due to systemic healthcare racism and bias.
As a result, diagnostic and treatment rates in minority populations are a hot-button field of research. Examples of ethnicity-based research topics include:
Cancer biopsy rates in BIPOC women
The prevalence of diabetes in Indigenous communities
Access inequalities in women’s health preventative screenings
The prevalence of undiagnosed hypertension in Black populations
- Pharmaceutical research topics
Large pharmaceutical companies are incredibly interested in investing in research to learn more about potential cures and treatments for diseases.
If you’re interested in building a career in pharmaceutical research, here are a few examples of in-demand research topics:
Cancer treatment options
Clinical research is in high demand as pharmaceutical companies explore novel cancer treatment options outside of chemotherapy and radiation.
Examples of cancer treatment-related research topics include:
Stem cell therapy for cancer
Oncogenic gene dysregulation and its impact on disease
Cancer-causing viral agents and their risks
Treatment efficacy based on early vs. late-stage cancer diagnosis
Cancer vaccines and targeted therapies
Immunotherapy for cancer
Pain medication alternatives
Historically, opioid medications were the primary treatment for short- and long-term pain. But, with the opioid epidemic getting worse, the need for alternative pain medications has never been more urgent.
Examples of pain medication-related research topics include:
Opioid withdrawal symptoms and risks
Early signs of pain medication misuse
Anti-inflammatory medications for pain control
- Identify trends in your medical research with Dovetail
Are you interested in contributing life-changing research? Today’s medical research is part of the future of clinical patient care.
As your go-to resource for speedy and accurate data analysis , we are proud to partner with healthcare researchers to innovate and improve the future of healthcare.
Get started today
Go from raw data to valuable insights with a flexible research platform
Editor’s picks
Last updated: 21 December 2023
Last updated: 16 December 2023
Last updated: 6 October 2023
Last updated: 25 November 2023
Last updated: 12 May 2023
Last updated: 15 February 2024
Last updated: 11 March 2024
Last updated: 12 December 2023
Last updated: 18 May 2023
Last updated: 6 March 2024
Last updated: 10 April 2023
Last updated: 20 December 2023
Latest articles
Related topics, log in or sign up.
Get started for free
2023-2024 Best Medical Schools: Research
Ranked in 2023
A medical career starts with finding the program that best fits your needs. With the
A medical career starts with finding the program that best fits your needs. With the U.S. News rankings of the top medical schools for research, narrow your search by location, tuition, school size and test scores. Footnotes below specify schools that declined to fill out the U.S. News statistical survey. Please review our methodology to see how those schools' data were used in the ranking. Read the methodology »
For full rankings, MCAT scores and student debt data, sign up for the U.S. News Medical School Compass .
Here are the 2023-2024 Best Medical Schools: Research
Harvard university, johns hopkins university, university of pennsylvania (perelman), columbia university, duke university, stanford university, university of california--san francisco, vanderbilt university, washington university in st. louis.
SEE THE FULL RANKINGS
- Clear Filters

- # 1 in Best Medical Schools: Research
$66,284 (full-time) TUITION AND FEES
699 ENROLLMENT (FULL-TIME)
Its tuition is full-time: $66,284. The faculty-student ratio at Harvard University is 14.6:1. The Medical School has... Read More »
TUITION AND FEES
$66,284 (full-time)
ENROLLMENT (FULL-TIME)
Mcat total score.

Baltimore , MD
- # 2 in Best Medical Schools: Research
$59,700 (full-time) TUITION AND FEES
470 ENROLLMENT (FULL-TIME)
The School of Medicine at Johns Hopkins University has an application deadline of Oct. 15. The application fee at Johns... Read More »
$59,700 (full-time)

Philadelphia , PA
- # 3 in Best Medical Schools: Research
$61,586 (full-time) TUITION AND FEES
626 ENROLLMENT (FULL-TIME)
The Perelman School of Medicine at University of Pennsylvania (Perelman) has an application deadline of Oct. 15. The... Read More »
$61,586 (full-time)

New York , NY
- # 4 in Best Medical Schools: Research
$66,816 (full-time) TUITION AND FEES
577 ENROLLMENT (FULL-TIME)
The College of Physicians and Surgeons at Columbia University has an application deadline of Oct. 15. The application... Read More »
$66,816 (full-time)

Durham , NC
- # 5 in Best Medical Schools: Research (tie)
$63,310 (full-time) TUITION AND FEES
507 ENROLLMENT (FULL-TIME)
The School of Medicine at Duke University has an application deadline of Oct. 15. The application fee at Duke... Read More »
$63,310 (full-time)

Stanford , CA
$63,747 (full-time) TUITION AND FEES
491 ENROLLMENT (FULL-TIME)
The School of Medicine at Stanford University has an application deadline of Oct. 3. The application fee at Stanford... Read More »
$63,747 (full-time)
San Francisco , CA
$38,073 (in-state, full-time) TUITION AND FEES
$50,318 (out-of-state, full-time) TUITION AND FEES
680 ENROLLMENT (FULL-TIME)
The School of Medicine at University of California--San Francisco has an application deadline of Oct. 15. The... Read More »
$38,073 (in-state, full-time)
$50,318 (out-of-state, full-time)

Nashville , TN
$64,882 (full-time) TUITION AND FEES
407 ENROLLMENT (FULL-TIME)
The School of Medicine at Vanderbilt University has an application deadline of Nov. 1. The application fee at... Read More »
$64,882 (full-time)

St. Louis , MO
$65,001 (full-time) TUITION AND FEES
448 ENROLLMENT (FULL-TIME)
The School of Medicine at Washington University in St. Louis has an application deadline of Nov. 30. The application... Read More »
$65,001 (full-time)

Cornell University (Weill)
- # 10 in Best Medical Schools: Research (tie)
$62,650 (full-time) TUITION AND FEES
451 ENROLLMENT (FULL-TIME)
The Weill Cornell Medical College at Cornell University (Weill) has an application deadline of Oct. 16. The application... Read More »
$62,650 (full-time)
U.S. News Grad Compass
See expanded profiles for more than 2,000 programs. Unlock entering class stats including MCAT, GMAT and GRE scores for business, medicine, engineering, education and nursing programs.
Driving Innovations in Biostatistics with Denise Scholtens, PhD
“I'm continually surprised by new data types. I think that we will see the emergence of a whole new kind of technology that we probably can't even envision five years from now…When I think about where the field has come over the past 20 years, it's just phenomenal.” — Denise Scholtens, PhD
- Director, Northwestern University Data Analysis and Coordinating Center (NUDACC)
- Chief of Biostatistics in the Department of Preventive Medicine
- Professor of Preventive Medicine in the Division of Biostatistics and of Neurological Surgery
- Member of Northwestern University Clinical and Translational Sciences Institute (NUCATS)
- Member of the Robert H. Lurie Comprehensive Cancer Center
Episode Notes
Since arriving at Feinberg in 2004, Scholtens has played a central role in the dramatic expansion of biostatistics at the medical school. Now the Director of NUDACC, Scholtens brings her expertise and leadership to large-scale, multicenter studies that can lead to clinical and public health practice decision-making.
- After discovering her love of statistics as a high school math teacher, Scholtens studied bioinformatics in a PhD program before arriving at Feinberg in 2004.
- Feinberg’s commitment to biostatistics has grown substantially in recent decades. Scholtens was only one of five biostatisticians when she arrived. Now she is part of a division with almost 50 people.
- She says being a good biostatistician requires curiosity about other people’s work, knowing what questions to ask and tenacity to understand subtitles of so much data.
- At NUDACC, Scholtens and her colleagues specialize in large-scale, multicenter prospective studies and clinical trials that lead to clinical or public health practice decision-making. They operate at the executive level and oversee all aspects of the study design.
- Currently, Scholtens is involved with the launch of a large study, along with The Ohio State University, that received a $14 million grant to look at the effectiveness of aspirin in the prevention of hypertensive disorders in pregnancy.
- Scholtens first started her work in data coordinating through the Hyperglycemia Adverse Pregnancy Outcome (HAPO) study, which looked at 25,000 pregnant individuals. This led to a continued interest in fetal and maternal health.
- When it comes to supportive working environments, Scholtens celebrates the culture at Feinberg, and especially her division in biostatistics, for being collaborative as well as genuinely supportive of each other’s projects. She attributes this to strong leadership which established a culture with these guiding principles.
Additional Reading
- Read more about the ASPIRIN trial and other projects taking place at NUDACC
- Discover a study linking mothers’ obesity-related genes to babies’ birth weight, which Scholtens worked in through the HAPO study
- Browse all of Scholtens recent publications
Recorded on February 21, 2024.
Continuing Medical Education Credit
Physicians who listen to this podcast may claim continuing medical education credit after listening to an episode of this program..
Target Audience
Academic/Research, Multiple specialties
Learning Objectives
At the conclusion of this activity, participants will be able to:
- Identify the research interests and initiatives of Feinberg faculty.
- Discuss new updates in clinical and translational research.
Accreditation Statement
The Northwestern University Feinberg School of Medicine is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Credit Designation Statement
The Northwestern University Feinberg School of Medicine designates this Enduring Material for a maximum of 0.50 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Surgery Continuous Certification Program
Successful completion of this CME activity enables the learner to earn credit toward the CME requirement(s) of the American Board of Surgery’s Continuous Certification program. It is the CME activity provider's responsibility to submit learner completion information to ACCME for the purpose of granting ABS credit.
All the relevant financial relationships for these individuals have been mitigated.
Disclosure Statement
Denise Scholtens, PhD, has nothing to disclose. Course director, Robert Rosa, MD, has nothing to disclose. Planning committee member, Erin Spain, has nothing to disclose. FSM’s CME Leadership, Review Committee, and Staff have no relevant financial relationships with ineligible companies to disclose.
Read the Full Transcript
[00:00:00] Erin Spain, MS: This is Breakthroughs, a podcast from Northwestern University Feinberg School of Medicine. I'm Erin Spain, host of the show. Northwestern University Feinberg School of Medicine is home to a team of premier faculty and staff biostatisticians, who are the driving force of data analytic innovation and excellence here. Today, we are talking with Dr. Denise Scholtens, a leader in biostatistics at Northwestern, about the growing importance of the field, and how she leverages her skills to collaborate on several projects in Maternal and Fetal Health. She is the Director of the Northwestern University Data Analysis and Coordinating Center, NUDACC, and Chief of Biostatistics in the Department of Preventive Medicine, as well as Professor of Preventive Medicine and Neurological Surgery. Welcome to the show.
[00:01:02] Denise Scholtens, PhD: Thank you so much.
[00:01:02] Erin Spain, MS: So you have said in the past that you were drawn to this field of biostatistics because you're interested in both math and medicine, but not interested in becoming a clinician. Tell me about your path into the field and to Northwestern.
[00:01:17] Denise Scholtens, PhD: You're right. I have always been interested in both math and medicine. I knew I did not want to be involved in clinical care. Originally, fresh out of college, I was a math major and I taught high school math for a couple of years. I really enjoyed that, loved the kids, loved the teaching parts of things. Interestingly enough, my department chair at the time assigned me to teach probability and statistics to high school seniors. I had never taken a statistics course before, so I was about a week ahead of them in our classes and found that I just really enjoyed the discipline. So as much as I loved teaching, I did decide to go ahead and invest in this particular new area that I had found and I really enjoyed. So I wanted to figure out how I could engage in the field of statistics. Decided to see, you know, exactly how studying statistics could be applied to medicine. At the time, Google was brand new. So I literally typed in the two words math and medicine to see what would come up. And the discipline of biostatistics is what Google generated. And so here I am, I applied to grad school and it's been a great fit for me.
[00:02:23] Erin Spain, MS: Oh, that's fantastic. So you went on to get a PhD, and then you came to Northwestern in 2004. And so tell me a little bit about the field then and how it's changed so dramatically since.
[00:02:36] Denise Scholtens, PhD: So yes, I started here at Northwestern in 2004, just a few months after I had defended my thesis. At the time there was really an emerging field of study called bioinformatics. So I wrote my thesis in the space of genomics data analysis with what at the time was a brand new technology, microarrays. This was the first way we could measure gene transcription at a high throughput level. So I did my thesis work in that space. I studied at an institution with a lot of strengths and very classical statistics. So things that we think of in biostatistics like clinical trial design, observational study analysis, things like that. So I had really classic biostatistics training and then complimented that with sort of these emerging methods with these high dimensional data types. So I came to Northwestern here and I sort of felt like I lived in two worlds. I had sort of classic biostat clinical trials, which were certainly, you know, happening here. And, that work was thriving here at Northwestern, but I had this kind of new skillset, and I just didn't quite know how to bring the two together. That was obviously a long time ago, 20 years ago. Now we think of personalized medicine and genomic indicators for treatment and, you know, there's a whole variety of omics data variations on the theme that are closely integrated with clinical and population level health research. So there's no longer any confusion for me about how those two things come together. You know, they're two disciplines that very nicely complement each other. But yeah, I think that does speak to how the field has changed, you know, these sort of classic biostatistics methods are really nicely blended with a lot of high dimensional data types. And it's been fun to be a part of that.
[00:04:17] Erin Spain, MS: There were only a handful of folks like you at Northwestern at the time. Tell me about now and the demand for folks with your skill set.
[00:04:26] Denise Scholtens, PhD: When I came to Northwestern, I was one of a very small handful of biostatistics faculty. There were five of us. We were not even called a division of biostatistics. We were just here as the Department of Preventive Medicine. And a lot of the work we did was really very tightly integrated with the epidemiologists here in our department and we still do a lot of that for sure. There was also some work going on with the Cancer Center here at Northwestern. But yeah, a pretty small group of us, who has sort of a selected set of collaborations. You know, I contrast that now to our current division of biostatistics where we are over 20s, pushing 25, depending on exactly how you want to count. Hoping to bring a couple of new faculty on board this calendar year. We have a staff of about 25 statistical analysts. And database managers and programmers. So you know, when I came there were five faculty members and I think two master's level staff. We are now pushing, you know, pushing 50 people in our division here so it's a really thriving group.
[00:05:26] Erin Spain, MS: in your opinion, what makes a good biostatistician? Do you have to have a little bit of a tough skin to be in this field?
Denise Scholtens, PhD: I do think it's a unique person who wants to be a biostatistician. There are a variety of traits that can lead to success in this space. First of all, I think it's helpful to be wildly curious about somebody else's work. To be an excellent collaborative biostatistician, you have to be able to learn the language of another discipline. So some other clinical specialty or public health application. Another trait that makes a biostatistician successful is to be able to ask the right questions about data that will be collected or already have been collected. So understanding the subtleties there, the study design components that lead to why we have the data that we have. You know, a lot of our data, you could think of it in a simple flat file, right? Like a Microsoft Excel file with rows and columns. That certainly happens a lot, but there are a lot of incredibly innovative data types out there: wearables technology, imaging data, all kinds of high dimensional data. So I think a tenacity to understand all of the subtleties of those data and to be able to ask the right questions. And then I think for a biostatistician at a medical school like ours, being able to blend those two things, so understanding what the data are and what you have to work with and what you're heading toward, but then also facilitating the translation of those analytic findings for the audience that really wants to understand them. So for the clinicians, for the patients, for participants and the population that the findings would apply to.
Erin Spain, MS: It must feel good, though, in those situations where you are able to help uncover something to improve a study or a trial.
[00:07:07] Denise Scholtens, PhD: It really does. This is a job that's easy to get out of bed for in the morning. There's a lot of really good things that happen here. It's exciting to know that the work we do could impact clinical practice, could impact public health practice. I think in any job, you know, you can sometimes get bogged down by the amount of work or the difficulty of the work or the back and forth with team members. There's just sort of all of the day to day grind, but to be able to take a step back and remember the actual people who are affected by our own little niche in this world. It's an incredibly helpful and motivating practice that I often keep to remember exactly why I'm doing what I'm doing and who I'm doing it for.
[00:07:50] Erin Spain, MS: Well, and another important part of your work is that you are a leader. You are leading the center, NUDACC, that you mentioned, Northwestern University Data Analysis and Coordinating Center. Now, this has been open for about five years. Tell me about the center and why it's so crucial to the future of the field.
[00:08:08] Denise Scholtens, PhD: We specialize at NUDACC in large scale, multicenter prospective studies. So these are the clinical trials or the observational studies that often, most conclusively, lead to clinical or public health practice decision making. We focus specifically on multicenter work. Because it requires a lot of central coordination and we've specifically built up our NUDACC capacity to handle these multi center investigations where we have a centralized database, we have centralized and streamlined data quality assurance pipelines. We can help with central team leadership and organization for large scale networks. So we have specifically focused on those areas. There's a whole lot of project management and regulatory expertise that we have to complement our data analytics strengths as well. I think my favorite part of participating in these studies is we get involved at the very beginning. We are involved in executive level planning of these studies. We oversee all components of study design. We are intimately involved in the development of the data capture systems. And in the QA of it. We do all of this work on the front end so that we get all of the fun at the end with the statistics and can analyze data that we know are scientifically sound, are well collected, and can lead to, you know, really helpful scientific conclusions.
[00:09:33] Erin Spain, MS: Tell me about that synergy between the clinicians and the other investigators that you're working with on these projects.
[00:09:41] Denise Scholtens, PhD: It is always exciting, often entertaining. Huge range of scientific opinion and expertise and points of view, all of which are very valid and very well informed. All of the discussion that could go into designing and launching a study, it's just phenomenally interesting and trying to navigate all of that and help bring teams to consensus in terms of what is scientifically most relevant, what's going to be most impactful, what is possible given the logistical strengths. Taking all of these well informed, valid, scientific points of view and being a part of the team that helps integrate them all toward a cohesive study design and a well executed study. That's a unique part of the challenge that we face here at NUDACC, but an incredibly rewarding one. It's also such an honor and a gift to be able to work with such a uniformly gifted set of individuals. Just the clinical researchers who devote themselves to these kinds of studies are incredibly generous, incredibly thoughtful and have such care for their patients and the individuals that they serve, that to be able to sit with them and think about the next steps for a great study is a really unique privilege.
[00:10:51] Erin Spain, MS: How unique is a center like this at a medical school?
[00:10:55] Denise Scholtens, PhD: It's fairly unique to have a center like this at a medical school. Most of the premier medical research institutions do have some level of data coordinating center capacity. We're certainly working toward trying to be one of the nation's best, absolutely, and build up our capacity for doing so. I'm actually currently a part of a group of data coordinating centers where it's sort of a grassroots effort right now to organize ourselves and come up with, you know, some unified statements around the gaps that we see in our work, the challenges that we face strategizing together to improve our own work and to potentially contribute to each other's work. I think maybe the early beginnings of a new professional organization for data coordinating centers. We have a meeting coming up of about, I think it's 12 to 15 different institutions, academic research institutions, specifically medical schools that have centers like ours to try to talk through our common pain points and also celebrate our common victories.
[00:11:51] Erin Spain, MS: I want to shift gears a little bit to talk about some of your research collaborations, many of which focus on maternal and fetal health and pregnancy. You're now involved with a study with folks at the Ohio State University that received a 14 million grant looking at the effectiveness of aspirin in the prevention of hypertensive disorders in pregnancy. Tell me about this work.
[00:12:14] Denise Scholtens, PhD: Yes, this is called the aspirin study. I suppose not a very creative name, but a very appropriate one. What we'll be doing in this study is looking at two different doses of aspirin for trying to prevent maternal hypertensive disorders of pregnancy in women who are considered at high risk for these disorders. This is a huge study. Our goal is to enroll 10,742 participants. This will take place at 11 different centers across the nation. And yes, we at NUDACC will serve as the data coordinating center here, and we are partnering with the Ohio State University who will house the clinical coordinating center. So this study is designed to look at two different doses to see which is more effective at preventing hypertensive disorders of pregnancy. So that would include gestational hypertension and preeclampsia. What's really unique about this study and the reason that it is so large is that it is specifically funded to look at what's called a heterogeneity of treatment effect. What that is is a difference in the effectiveness of aspirin in preventing maternal hypertensive disorders, according to different subgroups of women. We'll specifically have sufficient statistical power to test for differences in treatment effectiveness. And we have some high priority subgroups that we'll be looking at. One is a self-identified race. There's been a noted disparity in maternal hypertensive disorders, for individuals who self identify according to different races. And so we will be powered to see if aspirin has comparable effectiveness and hopefully even better effectiveness for the groups who really need it, to bring those rates closer to equity which is, you know, certainly something we would very strongly desire to see. We'll also be able to look at subgroups of women according to obesity, according to maternal age at pregnancy, according to the start time of aspirin when aspirin use is initiated during pregnancy. So that's why the trial is so huge. For a statistician, the statisticians out there who might be listening, this is powered on a statistical interaction term, which doesn't happen very often. So it's exciting that the trial is funded in that way.
[00:14:27] Erin Spain, MS: Tell me a little bit more about this and how your specific skills are going to be utilized in this study.
[00:14:32] Denise Scholtens, PhD: Well, there are three biostatistics faculty here at Northwestern involved in this. So we're definitely dividing and conquering. Right now, we're planning this study and starting to stand it up. So we're developing our statistical analysis plans. We're developing the database. We are developing our randomization modules. So this is the piece of the study where participants are randomized to which dose of aspirin they're going to receive. Because of all of the subgroups that we're planning to study, we need to make especially sure that the assignments of which dose of aspirin are balanced within and across all of those subgroups. So we're going to be using some adaptive randomization techniques to ensure that that balance is there. So there's some fun statistical and computer programming innovation that will be applied to accomplish those things. So right now, there are usually two phases of a study that are really busy for us. That's starting to study up and that's where we are. And so yes, it is very busy for us right now. And then at the end, you know, in five years or so, once recruitment is over, then we analyze all the data,
[00:15:36] Erin Spain, MS: Are there any guidelines out there right now about the use of aspirin in pregnancy. What do you hope that this could accomplish?
Prescribing aspirin use for the prevention of hypertension during pregnancy is not uncommon at all. That is actually fairly routinely done, but that it's not outcomes based in terms of which dosage is most effective. So 81 milligrams versus 162 milligrams. That's what we will be evaluating. And my understanding is that clinicians prescribe whatever they think is better, and I'm sure those opinions are very well informed but there is very little outcome based evidence for this in this particular population that we'll be studying. So that would be the goal here, would be to hopefully very conclusively say, depending on the rates of the hypertensive disorders that we see in our study, which of the two doses of aspirin is more effective. Importantly, we will also be tracking any side effects of taking aspirin. And so that's also very much often a part of the evaluation of You know, taking a, taking a drug, right, is how safe is it? So we'll be tracking that very closely as well. Another unique part of this study is that we will be looking at factors that help explain aspirin adherence. So we are going to recommend that participants take their dose of aspirin daily. We don't necessarily expect that's always going to happen, so we are going to measure how much of their prescribed dose they are actually taking and then look at, you know, factors that contribute to that. So be they, you know, social determinants of health or a variety of other things that we'll investigate to try to understand aspirin adherence, and then also model the way in which that adherence could have affected outcomes.
Erin Spain, MS: This is not the first study that you've worked on involving maternal and fetal health. Tell me about your interest in this particular area, this particular field, and some of the other work that you've done.
[00:17:31] Denise Scholtens, PhD: So I actually first got my start in data coordinating work through the HAPO study. HAPO stands for Hyperglycemia Adverse Pregnancy Outcome. That study was started here at Northwestern before I arrived. Actually recruitment to the study occurred between 2000 and 2006. Northwestern served as the central coordinating center for that study. It was an international study of 25,000 pregnant individuals who were recruited and then outcomes were evaluated both in moms and newborns. When I was about mid career here, all the babies that were born as a part of HAPO were early teenagers. And so we conducted a follow up study on the HAPO cohort. So that's really when I got involved. It was my first introduction to being a part of a coordinating center. As I got into it, though, I saw the beauty of digging into all of these details for a huge study like this and then saw these incredible resources that were accumulated through the conduct of such a large study. So the data from the study itself is, was of course, a huge resource. But then also we have all of these different samples that sit in a biorepository, right? So like usually blood sample collection is a big part of a study like this. So all these really fun ancillary studies could spin off of the HAPO study. So we did some genomics work. We did some metabolomics work. We've integrated the two and what's called integrated omics. So, you know, my work in this space really started in the HAPO study. And I have tremendously enjoyed integrating these high dimensional data types that have come from these really rich data resources that have all, you know, resulted because of this huge multicenter longitudinal study. So I kind of accidentally fell into the space of maternal and fetal health, to be honest. But I just became phenomenally interested in it and it's been a great place.
[00:19:24] Erin Spain, MS: Would you say that this is also a population that hasn't always been studied very much in biomedical science?
[00:19:32] Denise Scholtens, PhD: I think that that is true, for sure. There are some unique vulnerabilities, right, for a pregnant individual and for the fetus, right, and in that situation. You know, the vast majority of what we do is really only pertaining to the pregnant participant but, you know, there are certainly fetal outcomes, newborn outcomes. And so, I think conducting research in this particular population is a unique opportunity and there are components of it that need to be treated with special care given sort of this unique phase of human development and this unique phase of life.
[00:20:03] Erin Spain, MS: So, as data generation just really continues to explode, and technology is advancing so fast, faster than ever, where do you see this field evolving, the field of biostatistics, where do you see it going in the next five to ten years?
[00:20:19] Denise Scholtens, PhD: That's a great question. I think all I can really tell you is that I'm continually surprised by new data types. I think that we will see an emergence of a whole new kind of technology that we probably can't even envision five years from now. And I think that the fun part about being a biostatistician is seeing what's happening and then trying to wrap your mind around the possibilities and the actual nature of the data that are collected. You know, I think back to 2004 and this whole high throughput space just felt so big. You know, we could look at gene transcription across the genome using one technology. And we could only look at one dimension of it. Right now it just seems so basic. When I think about where the field has come over the past 20 years, it's just phenomenal. I think we're seeing a similar emergence of the scale and the type of data in the imaging space and in the wearable space, with EHR data, just. You know, all these different technologies for capturing, capturing things that we just never even conceived of before. I do hope that we continue to emphasize making meaningful and translatable conclusions from these data. So actionable conclusions that can impact the way that we care for others around us. I do hope that remains a guiding principle in all that we do.
[00:21:39] Erin Spain, MS: Why is Northwestern Medicine and Northwestern Feinberg School of Medicine such a supportive environment to pursue this type of work?
[00:21:47] Denise Scholtens, PhD: That's a wonderful question and one, honestly, that faculty candidates often ask me. When we bring faculty candidates in to visit here at Northwestern, they immediately pick up on the fact that we are a collaborative group of individuals who are for each other. Who want to see each other succeed, who are happy to share the things that we know and support each other's work, and support each other's research, and help strategize around the things that we want to accomplish. There is a strong culture here, at least in my department and in my division that I've really loved that continues to persist around really genuinely collaborating and genuinely sharing lessons learned and genuinely supporting each other as we move toward common goals. We've had some really strong, generous leadership who has helped us to get there and has helped create a culture where those are the guiding principles. In my leadership role is certainly something that I strive to maintain. Really hope that's true. I'm sure I don't do it perfectly but that's absolutely something I want to see accomplished here in the division and in NUDACC for sure.
[00:22:50] Erin Spain, MS: Well, thank you so much for coming on the show and telling us about your path here to Northwestern and all of the exciting work that we can look forward to in the coming years.
[00:22:59] Denise Scholtens, PhD: Thank you so much for having me. I've really enjoyed this.
[00:23:01] Erin Spain, MS: You can listen to shows from the Northwestern Medicine Podcast Network to hear more about the latest developments in medical research, health care, and medical education. Leaders from across specialties speak to topics ranging from basic science to global health to simulation education. Learn more at feinberg. northwestern.edu/podcasts.
Related News

9th Annual Research Conference Highlights Innovation and Discoveries from Across the Valley

Nanoparticle Delivery of FZD4 to Lung Endothelial Cells Inhibits Lung Cancer Progression and Metastases

Five Guidelines for Safe Solar Eclipse Viewing
Pharmacogenomics Symposium Showcased Progress and Potential in the Field
Chase Congleton
Researchers from across the United States and Canada gathered at the University of Arizona College of Medicine – Phoenix’s campus to discuss recent trends, new therapies and what the future will hold for the field of pharmacogenomics during the annual Precision Medicine and Pharmacogenomics Symposium April 8.
Pharmacogenomics is the study of how genes affect a person’s response to drugs and combines pharmacology and genomics to prescribe effective medications based on a patient’s genetic makeup. Central to the college’s focus on precision medicine, its promise for improved patient outcomes by preventing adverse interactions is why medical students at the college are instructed on the foundational principles of the field.
“One of the key things we have done at the college is implement a precision medicine longitudinal theme with our medical students,” Will Heise, MD , assistant professor at the Division of Clinical Data Analytics and Decision Support at the UArizona College of Medicine – Phoenix, said. “Students have been subject to a number of sessions in pharmacogenomics and precision medicine with the goal of providing tools for them to integrate precision medicine into their future practice.”
The symposium is an extension of those efforts; and throughout five oral presentations, members of the audience learned about the latest research on gene-drug interactions. Special topics also included gene-drug interactions in psychiatric conditions, as well as responses in anticoagulant and antiplatelet drugs.

Nita Limdi, PharmD, PhD, MSPH, is a professor of Neurology at the University of Alabama at Birmingham, School of Medicine – Personalized Medicine Institute. Her presentation discussed pharmacogenomics for anticoagulant and antiplatelet response.
Anticoagulants are medicines that help prevent blood clots and are commonly known as blood thinners. People who are at high risk of developing blood clots are prescribed anticoagulants to reduce their chances of developing serious conditions such as stroke, heart attack and pulmonary embolism.
Currently, anticoagulants are the leading cause of medication-related hospitalizations in the United States. Adverse drug reactions are the fourth-leading cause of death, according to the United States Food and Drug Administration.
The aim of Dr. Limdi’s research is to identify common and rare genetic variants on anticoagulation response to examine what other factors affect comorbid conditions and co-medications. These include ancestry, diet, exercise and other medications.
Her team’s goal was to develop and validate polygenic risk scores for 10 diseases across various ancestral groups. The study is almost finished and has recruited 25,000 patients in primary care. Dr. Limdi said that the data collected from patients contains genomics, family history and rich social determinants of health data.
“When reports on genetic testing come in, if the patient has no genetic risk, the providers should still be made aware,” Dr. Limdi said. “If there is a high polygenic risk, providers will call to notify the patient or there is a video visit to explain it.”
Chad Bousman, PhD, works as an associate professor at the University of Calgary’s Department of Medical Genetics in Alberta, Canada. His presentation discussed pharmacogenomics with an emphasis on prescription for psychiatric diseases.

Among the hurdles for conducting pharmacogenomics testing is building provider confidence and determining who should be tested. In regard to working around clinical workflow integration, Dr. Bousman found that there is no correct answer.
“There are so many workflows out there, and I would say that all of them are good for particular settings,” Dr. Bousman said. “The key that I find between a successful workflow and a less successful workflow are the ones that disrupt existing prescribing practice as little as possible.”
The future of pharmacogenomics in psychiatry will continue to grow. According to Dr. Bousman, 64% of the top 25 prescribed psychiatric medications do not have pharmacogenomics guidelines.
“Fluoxetine is the only SSRI that does not have a guideline and is also the first line therapy for depression in adolescents,” Dr. Bousman said. “It’s a huge gap in our ability to implement pharmacogenetic testing in child adolescent psychiatry.”
Vignesh Subbian, PhD, MS, is an associate professor in the University of Arizona Department of Biomedical Engineering and Department of Systems and Industrial Engineering and is a member of the BIO5 Institute.
Dr. Subbian’s presentation focused on past and current work related to patient-centered clinical decision support for pharmacogenomics. He discussed integrating pharmacogenomic testing into electronic health records with clinical decision support and selecting the correct workflow for patients to receive data.
“If you want a holistic pharmacogenomics clinical support, it depends on the knowledge bases,” Dr. Subbian said. “Successful adoption of pharmacogenomics into routine clinical care requires a machine-readable vault of knowledge suitable for use within an electronic health record.”
Tyler Gallo, PharmD, an assistant professor in the Division of Clinical Data Analytics and Decision Support at the college, focused his presentation on the impact and outcomes of pharmacogenomic clinical decision support.

His presentation built upon Dr. Subbian’s talk by providing examples of how clinical decision support is used in the field. Dr. Gallo mentioned that there are two main applications of clinical decision support in pharmacogenomics.
“One is to identify patients who should undergo pharmacogenomic testing, and the other is post-test alerts for when pharmacogenomic testing results in a change or modification of that therapy,” Dr. Gallo said. “Recommendations can change over time, so we need to continually evaluate these things and make sure they’re functioning as intended.”
Dr. Heise’s talk focused on the future of pharmacogenomic clinical decision support and automation.
“We have to consider the opportunity that patients have to be in a place where they can receive precision medicine,” Dr. Heise said. “We recognize we have disparities in delivery of pharmacogenomics, precision medicine and research. Our pharmacogenomic panels are inadequate if we do not address variants in populations.”
Dr. Heise discussed the limitations of AI to provide accurate medical diagnoses and recommended therapeutics. He demonstrated that ChatGPT is useful for background information but not for actual patient care because it lacks updated information and data personalized to the patient.
“What we recognize is that AI, while it can be incredibly helpful and meaningful, is limited, and it will never have human understanding and capacity,” Dr. Heise said. “Many of the strides that have been made in AI are not because we’ve been able to make it more human-like, but rather because we have higher computational power.”
The event was sponsored by the Arizona Biomedical Research Centre and the Flinn Foundation.
About the College
Founded in 2007, the University of Arizona College of Medicine – Phoenix inspires and trains exemplary physicians, scientists and leaders to optimize health and health care in Arizona and beyond. By cultivating collaborative research locally and globally, the college accelerates discovery in a number of critical areas — including cancer, stroke, traumatic brain injury and cardiovascular disease. Championed as a student-centric campus, the college has graduated more than 800 physicians, all of whom received exceptional training from nine clinical partners and more than 2,700 diverse faculty members. As the anchor to the Phoenix Bioscience Core , which is projected to have an economic impact of $3.1 billion by 2025, the college prides itself on engaging with the community, fostering education, inclusion, access and advocacy.
- Work & Careers
- Life & Arts
Become an FT subscriber
Try unlimited access Only $1 for 4 weeks
Then $75 per month. Complete digital access to quality FT journalism on any device. Cancel anytime during your trial.
- Global news & analysis
- Expert opinion
- Special features
- FirstFT newsletter
- Videos & Podcasts
- Android & iOS app
- FT Edit app
- 10 gift articles per month
Explore more offers.
Standard digital.
- FT Digital Edition
Premium Digital
Print + premium digital, weekend print + standard digital, weekend print + premium digital.
Today's FT newspaper for easy reading on any device. This does not include ft.com or FT App access.
- 10 additional gift articles per month
- Global news & analysis
- Exclusive FT analysis
- Videos & Podcasts
- FT App on Android & iOS
- Everything in Standard Digital
- Premium newsletters
- Weekday Print Edition
- FT Weekend Print delivery
- Everything in Premium Digital
Essential digital access to quality FT journalism on any device. Pay a year upfront and save 20%.
- Everything in Print
Complete digital access to quality FT journalism with expert analysis from industry leaders. Pay a year upfront and save 20%.
Terms & Conditions apply
Explore our full range of subscriptions.
Why the ft.
See why over a million readers pay to read the Financial Times.
International Edition
This paper is in the following e-collection/theme issue:
Published on 25.4.2024 in Vol 26 (2024)
Effect of Prosocial Behaviors on e-Consultations in a Web-Based Health Care Community: Panel Data Analysis
Authors of this article:

Original Paper
- Xiaoxiao Liu 1, 2 , PhD ;
- Huijing Guo 3 , PhD ;
- Le Wang 4 , PhD ;
- Mingye Hu 5 , PhD ;
- Yichan Wei 1 , BBM ;
- Fei Liu 6 , PhD ;
- Xifu Wang 7 , MCM
1 School of Management, Xi’an Jiaotong University, Xi'an, China
2 China Institute of Hospital Development and Reform, Xi'an Jiaotong University, Xi'an, China
3 School of Economics and Management, China University of Mining and Technology, Xuzhou, China
4 College of Business, City University of Hong Kong, Hong Kong, China (Hong Kong)
5 School of Economics and Management, Xi’an University of Technology, Xi'an, China
6 School of Management, Harbin Engineering University, Harbin, China
7 Healthcare Simulation Center, Guangzhou First People’s Hospital, Guangzhou, China
Corresponding Author:
Xifu Wang, MCM
Healthcare Simulation Center
Guangzhou First People’s Hospital
1 Pan Fu Road
Yuexiu District
Guangzhou, 510180
Phone: 86 13560055951
Email: [email protected]
Background: Patients using web-based health care communities for e-consultation services have the option to choose their service providers from an extensive digital market. To stand out in this crowded field, doctors in web-based health care communities often engage in prosocial behaviors, such as proactive and reactive actions, to attract more users. However, the effect of these behaviors on the volume of e-consultations remains unclear and warrants further exploration.
Objective: This study investigates the impact of various prosocial behaviors on doctors’ e-consultation volume in web-based health care communities and the moderating effects of doctors’ digital and offline reputations.
Methods: A panel data set containing information on 2880 doctors over a 22-month period was obtained from one of the largest web-based health care communities in China. Data analysis was conducted using a 2-way fixed effects model with robust clustered SEs. A series of robustness checks were also performed, including alternative measurements of independent variables and estimation methods.
Results: Results indicated that both types of doctors’ prosocial behaviors, namely, proactive and reactive actions, positively impacted their e-consultation volume. In terms of the moderating effects of external reputation, doctors’ offline professional titles were found to negatively moderate the relationship between their proactive behaviors and their e-consultation volume. However, these titles did not significantly affect the relationship between doctors’ reactive behaviors and their e-consultation volume ( P =.45). Additionally, doctors’ digital recommendations from patients negatively moderated both the relationship between doctors’ proactive behaviors and e-consultation volume and the relationship between doctors’ reactive behaviors and e-consultation volume.
Conclusions: Drawing upon functional motives theory and social exchange theory, this study categorizes doctors’ prosocial behaviors into proactive and reactive actions. It provides empirical evidence that prosocial behaviors can lead to an increase in e-consultation volume. This study also illuminates the moderating roles doctors’ digital and offline reputations play in the relationships between prosocial behaviors and e-consultation volume.
Introduction
e-Consultations, offered through web-based health care communities [ 1 ], are increasingly becoming vital complements to traditional hospital services [ 2 - 4 ]. In hospital consultations, patients can only passively accept treatment [ 5 ] from a limited pool of medical resources within a geographical radius. However, when engaging with web-based health care communities, patients can search for primary care solutions [ 6 ] from an extensive digital market in a relatively short time [ 7 ]. Given that the diagnostic accuracy of e-consultations matches that of hospital consultations [ 8 - 10 ], e-consultations are becoming increasingly attractive to patients [ 3 , 11 ].
Doctors are also showing a growing interest in e-consultations, motivated by economic and social benefits. First, doctors can achieve economic gains by participating in e-consultations [ 7 , 12 ]. Web-based consultation platforms facilitate an efficient reputation system, enabling patients to easily provide feedback about doctors. Consequently, doctors can use e-consultation to strengthen their relationship with patients [ 13 , 14 ] and foster positive word-of-mouth [ 15 ]. More e-consultations can benefit doctors by retaining current patients, attracting new ones, and boosting in-person hospital visits [ 16 , 17 ]. Second, doctors could also receive social returns from engaging in e-consultation [ 7 ]. Active participation in e-consultations allows doctors to demonstrate their skills, attitude, and experience, aiding in accumulating professional capital [ 7 ], building their reputation [ 18 ], and increasing their social influence [ 19 ]. Given these tangible and intangible benefits, it is essential for doctors to diligently provide the desired e-consultations and make additional efforts to highlight their service attributes to stand out [ 6 , 20 , 21 ]. This involves engaging in prosocial behaviors in web-based health care communities, which is the primary research focus of this study.
Prior studies have examined the effects of prosocial behaviors on financial outcomes, such as actions reflecting social responsibility in the workplace [ 22 ]. In the health care sector, previous research has explored doctors’ prosocial behaviors within traditional, offline medical services. Doctors, working in established medical institutes and serving patients with limited choices of clinical service providers, often aim for self-satisfaction and patient satisfaction with their offline prosocial behaviors. For example, research indicates that doctors may act prosocially to regulate their self-oriented feelings [ 23 ] and foster a caring and understanding attitude toward patients [ 24 , 25 ]. Additionally, doctors who demonstrate more empathy and care can elicit positive emotions in patients and improve the doctor-patient relationship [ 26 , 27 ].
Compared to the offline context, doctors’ prosocial behaviors in a digital context may differ in 2 aspects. First, the internet allows patients to choose from a broader, more diverse range of doctors without the constraints of time and space [ 7 ]. However, the uncertainty inherent in the digital environment creates a more pronounced information asymmetry between patients and doctors [ 28 ], consequently making it more challenging for patients to establish trust. Therefore, doctors’ prosocial behaviors are crucial in building their self-image, establishing patients’ trust, and assisting patients in identifying suitable doctors [ 29 , 30 ]. Second, unlike offline environments, web-based medical platforms offer a range of functions, including asynchronous activities such as publishing articles, as well as real-time interactional actions such as answering questions during live streams. This array of functions facilitates the adoption of more diverse prosocial behaviors by doctors.
Although these differences underscore the importance of studying doctors’ prosocial behavior, there has been limited research focusing on the impact of such behaviors in the digital context. One previous study has scrutinized the impact of prosocial behaviors, such as answering patients’ questions freely, on patient engagement within web-based health care communities [ 31 ]. An aspect that requires further exploration is how doctors’ motivations and patients’ involvement vary in doctors’ helping behaviors. Consequently, studies on web-based health care communities should differentiate between diverse prosocial actions to understand their effects on doctors’ web-based service outcomes. This study aims to contribute new knowledge regarding the full breadth of doctors’ prosocial behaviors.
Unlike the previous study that exclusively investigated doctors’ asynchronous behaviors in web-based health care communities [ 31 ], this study also explores the role of synchronous reactive actions in achieving optimal doctors’ e-consultation volume. Recently, web-based health care communities have developed and released live-streaming functions to assist doctors in providing voluntary interactions with patients. The effect of doctors’ engagement in medical live streaming on e-consultation services remains unexplored. While these behaviors could demonstrate doctors’ ethical traits and ability to fulfill an e-consultation workflow, a potential trade-off with e-consultations may exist when doctors engage in prosocial behaviors.
In summary, this study examines the effects of doctors’ proactive and reactive prosocial behaviors, considering their digital and offline reputations as potential moderating factors. First, drawing from functional motives theory (FMT), we explore the impact of doctors’ web-based proactive actions on their e-consultation volume. Proactive behaviors are actions in which individuals exceed their assigned work, focusing on long-term goals to prevent future problems [ 32 , 33 ]. According to FMT, these behaviors reflect helping actions that satisfy personal needs [ 34 ], driven by self-focused motivations [ 35 ], such as impression management and the realization of self-worth goals. For example, knowledge-based proactive behaviors, such as disseminating expertise to preempt future issues, are self-initiated and not reactions to immediate requests [ 36 ]. This study categorizes doctors’ sharing of professional articles as a form of proactive behavior that creates a professional image for their patient audience. This is because these actions aim to assist patients with future health concerns rather than directly responding to patients’ immediate needs.
Second, this study explores the role of doctors’ reactive prosocial behaviors in increasing e-consultations, guided by social exchange theory (SET). Unlike proactive behaviors, reactive behaviors are characterized by instances of individuals engaging in helping activities [ 35 ], typically in response to others’ needs [ 34 ]. SET posits that individuals incurring additional social costs in relationships may anticipate reciprocal value [ 37 , 38 ]. Reactive prosocial behaviors, per SET, are initiated by the motivation to satisfy others’ desires, leading to the development of cooperative social values. In our context, medical live streams facilitate real-time, synchronized interactions, enabling patients to ask questions and doctors to provide immediate responses. Patients’ health questions during these streams indicate their immediate needs. Thus, a higher frequency of live streams within a certain period suggests doctors are increasingly responding to patients’ needs during that time. Therefore, this study uses the number of medical live-streaming sessions conducted by doctors as a measure for their synchronous reactive behaviors.
Finally, considering that doctors’ reputations play a crucial role in their workflow on web-based health care communities [ 39 , 40 ], we test the moderating roles of digital and offline reputation—measured by doctors’ offline professional titles and patients’ recommendations in the digital context, respectively—on the main effects.
Based on previous studies and practices within web-based health care communities, we aim to extend the literature by testing the impact of 2 types of web-based prosocial behaviors by doctors: proactive and synchronous reactive actions on e-consultation volume. We then explore the moderating roles of doctors’ offline and digital reputations on these main effects.
Research Framework and Hypothesis Development
We have developed a research framework, shown in Figure 1 , to identify effective prosocial strategies used by doctors within web-based health care communities to achieve a preferred e-consultation volume from the supply side.

Primarily, we explore the relationships between doctors’ prosocial behaviors and e-consultation volume, drawing on FMT and SET. These theories are widely adopted for measuring and classifying the outcomes of prosocial behaviors from 2 fundamental perspectives based on human nature [ 34 ]. While doctors’ offline prosocial behaviors may help satisfy patients [ 24 , 25 ], who are already service acceptors, the outcomes of doctors’ web-based prosocial behaviors still need careful distinction. It is essential to clearly differentiate between various types of doctors’ prosocial behaviors to identify their nature. In this study, following the leads of FMT and SET, we test 2 kinds of prosocial behavior: proactive (posting professional articles to achieve self-worth) and reactive (conducting medical live streaming to create cooperative social values).
Subsequently, we examine how doctors’ external reputation moderates the impacts of doctors’ proactive and reactive prosocial behaviors. This examination is conducted from the perspectives of reducing uncertainty and building trust, respectively.
Doctors’ Proactive Behaviors and e-Consultation Volume
FMT places emphasis on the primary motivations behind individuals’ behaviors, adopting an atheoretical stance [ 41 ]. Through the exploratory process, previous studies have provided examples to identify the functional motivations behind prosocial behaviors [ 42 ], such as expressing important personal values. In web-based health care communities, doctors have the opportunity to demonstrate personal traits through proactive behaviors. According to FMT, these proactive behaviors stem from the actors’ active efforts to satisfy their own needs and achieve self-worth [ 34 , 35 ].
Doctors might post professional articles, such as clinical notes and scientific papers, on web-based health care communities to help patient readers handle future health problems. These proactive prosocial behaviors are primarily driven by a desire to showcase personal medical competence, a crucial characteristic of a professional image [ 43 ], in medical consultations. By posting professional articles, doctors can display their medical knowledge, care delivery capability, and service quality, thereby enhancing their professional image. We hypothesize that this effort will lead to an increase in the e-consultation volume. Therefore, we propose the following hypothesis:
- Hypothesis 1: The posting of professional articles by doctors positively impacts their e-consultation volume on web-based health care communities.
Doctors’ Reactive Behaviors and e-Consultation Volume
Considering the social environment in the working context, SET suggests that reactive prosocial behaviors stem from responding to others’ needs [ 34 ]. Engaging in such behaviors can foster positive perceptions among the audience and build cooperative social values [ 44 ] through reactive social exchange. People with a high orientation toward cooperative social values act to maximize mutual interests [ 45 ], a trait highly valued in the medical field.
We use medical live streaming as a measure of doctors’ reactive behaviors on web-based health care communities. Volunteering to provide interactional live streaming, a typical reactive behavior that may generate cooperative social value, gives the patient audience the impression that the doctors will prioritize demand-side interests during e-consultation services. Additionally, engaging in medical live streaming allows doctors to present themselves as authentic and recognized experts. This enhances their social presence [ 46 ], potentially leading to increased service use [ 47 ] and greater popularity [ 48 ]. Consequently, patients are more likely to perceive doctors who participate in medical live streaming as trustworthy for consultations. Given that e-consultations are closely related to the health conditions of the demand side, a credible doctor is likely to attract more e-consultations. Therefore, we propose the following hypothesis:
- Hypothesis 2: The conduct of medical live streaming by doctors positively impacts their e-consultation volume on web-based health care communities.
Moderating Roles of Offline and Digital Reputation
As doctors’ proactive and reactive behaviors potentially affect their consultation performance, based on 2 distinct theoretical foundations of human nature, there exists a discrepancy in how doctors’ reputations influence the relationship between various prosocial behaviors and e-consultation.
We formulate hypotheses regarding the moderating effects within the context of digital health care, by taking into account the inherent information asymmetry and the significance of establishing patient trust. Specifically, our hypotheses explore the influence of reputation on the relationship between doctors’ proactive behaviors and e-consultation volume, with a focus on reducing uncertainty. Additionally, we examine how reputation moderates the impact of doctors’ reactive behaviors, emphasizing the perspective of trust building.
First, in the marketing literature, service providers’ reputations, which can reduce information asymmetry and purchase uncertainty [ 49 ], are key factors influencing purchasing behavior and sales performance in the digital context [ 50 - 52 ]. Similarly, for doctors, reputations are related to the experiences and beliefs of other stakeholders [ 53 ]. As health care services are credence goods [ 54 ]—whose quality patients cannot discern even after experiencing the services—and given the nature of web-based platforms (eg, the absence of face-to-face meetings), there is a significant information asymmetry [ 51 ]. This increases patients’ uncertainty regarding the quality of doctors. Consequently, doctors’ reputations play crucial roles in patients’ decision-making processes [ 18 , 39 ]. We use doctors’ professional titles and patients’ recommendations on web-based health care communities to measure doctors’ offline and digital reputations.
Proactive behaviors by low-reputation doctors can create deeper professional impressions [ 34 , 35 ] to reduce uncertainty in e-consultations than high-reputation doctors, who are less uncertain in medical services. Then, doctors’ reputations—measured by offline professional titles and digital patients’ recommendations on web-based health care communities—will negatively moderate the relationship between proactive behavior and e-consultation volume. Thus, we propose the following hypotheses:
- Hypothesis 3a: Doctors’ offline professional titles negatively moderate the relationship between the posting of professional articles and e-consultation volume on web-based health care communities.
- Hypothesis 3b: Doctors’ digital recommendations from patients negatively moderate the relationship between the posting of professional articles and e-consultation volume on web-based health care communities.
Second, one of the central elements of SET is the concept of trust between actors in the exchange process [ 55 - 58 ]. In the context of digital health, patient’s trust in doctors is important to establish in order to refine the doctor-patient relationship. Doctors’ reputations can reflect their personality traits [ 39 ] and promote trust from patients [ 53 ]. Conducting medical live streaming, a form of reactive prosocial behavior, includes doctors’ cooperative social value orientations that are preferred in e-consultations. For low-reputation doctors, such as those with relatively junior professional titles and few digital patient recommendations, conducting medical live streaming will build patients’ confidence in e-consultations to a greater extent than doctors with high reputations, who are usually already highly trusted. Then, offline and digital reputation may negatively moderate the relationship between engaging in medical live streaming and e-consultation volume. Thus, we propose the final hypotheses:
- Hypothesis 4a: Doctors’ offline professional titles will negatively moderate the relationship between conducting medical live streaming and e-consultation volume on web-based health care communities.
- Hypothesis 4b: Doctors’ digital recommendations from patients will negatively moderate the relationship between conducting medical live streaming and e-consultation volume on web-based health care communities.
Research Context and Data Collection
Our research context is one of the largest web-based health care communities in China. This platform, established in 2006, offers e-consultation services to patients. As of July 2023, it boasts over 260,000 active doctors from 10,000 hospitals nationwide and has provided web-based medical services to 79 million patients.
The platform allows doctors to create home pages where they can display relevant information such as offline professional titles, experiences shared by other patients, and personal introductions. Patients can select doctors for e-consultation by browsing this information. Besides e-consultation, doctors can engage in prosocial behavior primarily focused on knowledge sharing. This includes posting professional articles in various formats (text, voice, and short videos) and conducting medical live streams for real-time interaction with patients.
We collected data over a 22-month period, from January 2021 to October 2022, focusing on common diseases such as diabetes, depression, infertility, skin diseases, and gynecological diseases. To ensure that our findings are generalizable to a typical and active doctor on the platform, we included doctors who had posted at least 1 article and conducted at least 1 live stream before the end of the study period in our analysis [ 59 - 61 ]. Our sample consists of 2880 doctors and includes the following information for each doctor: professional title, patient recommendations, records of experiences shared by the doctor’s patients, records of professional articles posted, records of live streams conducted, and records of the doctor’s e-consultations.
Variable Operationalization
Our unit of analysis is each doctor. We investigate how doctors’ prosocial behaviors, including proactive behaviors (posting professional articles) and reactive behaviors (conducting medical live streams), influence their e-consultation volume.
Dependent Variable
Our dependent variable is the doctors’ e-consultation volume, denoted as Consultation it , which is measured by the number of e-consultations of doctor i in month t .
Independent Variables
Our independent variables are doctors’ proactive behaviors and reactive behaviors. Doctors’ proactive behavior is operationalized as the posting of professional articles. Specifically, we denote proactive behavior as Articles it , which is measured by the number of professional articles posted by doctor i in month t . Doctors’ reactive behavior is operationalized as medical live streaming. This variable is denoted as LiveStreaming it , which is calculated as the number of medical live streams conducted by doctor i in month t .
Moderating Variables
We are also interested in how doctors’ external reputation, including their offline professional titles and digital recommendations from patients, influences the relationship between prosocial behaviors and e-consultation volume. A doctor’s offline professional title is denoted as Title i , which is a dummy variable indicating whether doctor i is a chief doctor ( Title i =1 indicates the doctor is a chief doctor, and Title i =0 indicates the doctor has a lower-ranked title). Digital recommendations are captured by Recommendations i , which is the digital recommendation level of doctor i as calculated by the platform based on the recommendations provided by their past patients.
Control Variables
We incorporated several control variables to account for factors that may influence patient’s choices of doctors in the digital context. The shared experiences of patients regarding a doctor’s treatment [ 39 ], as well as the number of patients who have previously consulted with the doctors [ 17 , 62 ], can indicate the doctor’s overall popularity. This, in turn, may affect patient choice. Therefore, we controlled for (1) the total number of patients who consulted with doctor i in the digital context before month t ( TotalPatients it ) and (2) the total number of patient-shared experiences about offline treatment by doctor i before month t ( TotalExperiences it ). Furthermore, doctors’ past behaviors, including article publishing and live streaming, can influence their current practices in posting articles and conducting live streams. Simultaneously, these factors may also act as signals affecting patients’ judgments and selection of doctors [ 12 ]. To account for these influences, we also controlled for (1) the total number of articles posted by doctor i before month t ( TotalArticles it ) and (2) the total number of medical live streams conducted by doctor i before month t ( TotalLiveStreaming it ).
To control for both observed and unobserved doctor-specific factors that do not change over time, individual-fixed effects were added. Additionally, time-fixed effects were introduced into our analysis to account for both observed and unobserved factors that vary over time but remain constant across doctors. Table 1 shows the variables and their definitions.
Estimation Model
To estimate the direct impact of doctors’ proactive behaviors and reactive behaviors on their e-consultation volume, the following 2-way fixed effects regression model was used:
Consultation it = β 0 + β 1 Articles it + β 2 LiveStreaming it + β 3 TotalPatients it + β 4 TotalExperiences it + β 5 TotalArticles it + β 6 TotalLiveStreaming it + α i + δ t + μ it (1)
where i denotes doctor, t denotes month, α i is doctor-fixed effects, δ t is month-fixed effects, Consultation it is the number of e-consultations of doctor i in month t , Articles it is the number of professional articles posted by doctor i in month t , LiveStreaming it is the number of medical live streams conducted by doctor i in month t , TotalPatients it is the total number of patients who consulted doctor i in the digital context before month t , TotalExperiences it is the total number of patient-shared experiences about offline treatment by doctor i before month t , TotalArticles it is the total number of articles posted by doctor i before month t , TotalLiveStreaming it is the total number of medical live streams doctor i conducted before month t , β is the coefficient, and μ it is the error term. We took the log transformation for our continuous variables in the model to reduce the skewness of the variables [ 63 ].
Next, the moderating effects of doctors’ offline professional titles and digital recommendations by patients were investigated based on the following specification:
Consultation it = β 0 + β 1 Articles it + β 2 LiveStreaming it + β 3 Articles it × Title i + β 4 LiveStreaming it × Title i + β 5 Articles it × Recommendation i + β 6 LiveStreaming it × Recommendation i + β 7 TotalPatients it + β 8 TotalExperiences it + β 9 TotalArticles it + β 10 TotalLiveStreaming it + α i + δ t + μ it (2)
where Title i indicates whether doctor i is a chief doctor ( Title i =1 indicates the doctor is a chief doctor, and Title i =0 indicates the doctor has a lower-ranked title). Recommendations i is the digital recommendation level of doctor i by other patients.
Ethical Considerations
This study used secondary publicly available data obtained from a website and did not involve the collection of original data pertaining to human participants. As such, there is no evidence of unethical behavior in the study. Consequently, ethics approval by an ethics committee or institutional review board was not deemed necessary.
In this section, we present our empirical results. The descriptive statistics are shown in Table 2 , and the correlation matrix is shown in Table 3 .
Empirical Results
Results for direct effects.
The analysis was conducted progressively. We first estimated the equation without control variables (model 1) and then added control variables in model 2. The estimated results are shown in Table 4 . From the results, we can see that the coefficient of Articles is significant and positive in model 2 (β=.093; P <.001), indicating that doctors’ proactive behaviors (ie, posting professional articles) can help them obtain more e-consultations. Thus, hypothesis 1 is supported. Regarding doctors’ engagement in medical live streaming, the results show that the coefficient of LiveStreaming is significantly positive (β=.214; P <.001), which suggests that doctors’ reactive behaviors (ie, conducting medical live streaming) can increase their e-consultation volume. This supports hypothesis 2.
a All models include doctor-fixed effects and month-fixed effects; robust SEs clustered by doctors are reported; the number of doctors is 2880, and the number of observations is 63,360.
b R 2 =0.843; F 2,2879 =175.98; P <.001.
c R 2 =0.851; F 6,2879 =119.72; P <.001.
d N/A: not applicable.
Results for Moderating Effects
The results for moderating effects are shown in Table 5 . In model 1, interaction terms were initially introduced between Title and Articles , as well as between Title and LiveStreaming , to estimate the moderating effect of doctors’ offline professional titles. The interaction terms were then added between Recommendations and Articles , as well as between Recommendations and LiveStreaming , to estimate the moderating effect of doctors’ digital recommendations in model 2. Finally, a full model was estimated by incorporating all interaction terms. We find that the results are consistent across all models. Wald tests and likelihood ratio were used to compare the fit among nested models [ 64 , 65 ], and the results show that the inclusion of moderating variables significantly enhances the model’s fit.
Regarding the moderating effect of doctors’ offline professional titles, we find that the coefficient of Articles × Title in model 1 of Table 5 is significantly negative (β=–.058; P <.001), which supports hypothesis 3a that doctors’ offline professional titles have a negative moderating effect on the relationship between doctors’ proactive behaviors and e-consultation volume. However, the coefficient of LiveStreaming × Title is insignificant (β=–.024; P =.45), which suggests that doctors’ offline professional titles have no moderating effect on the relationship between doctors’ reactive behaviors and e-consultation volume. Thus, hypothesis 4a is not supported.
b R 2 =0.851; F 8,2879 =89.98; P <.001; Wald test: P <.001; likelihood ratio: P <.001.
c R 2 =0.851; F 8,2879 =89.13; P <.001; Wald test: P <.001; likelihood ratio: P <.001.
d R 2 =0.852; F 10,2879 =71.44; P <.001; Wald test: P <.001; likelihood ratio: P <.001.
e N/A: not applicable.
For the moderating effect of digital patient recommendations, we find that both of the coefficients of Articles × Recommendations and LiveStreaming × Recommendations are negative and significant (β=–.055; P <.001 and β=–.100; P <.001, respectively, in model 2 of Table 5 ). This indicates that digital recommendations from patients have negative moderating effects on the relationship between doctors’ proactive behaviors and e-consultation volume as well as on the relationship between doctors’ reactive behaviors and e-consultation volume; this finding supports hypotheses 3b and 4b.
Robustness Check
First, additional analysis was performed to check whether our findings are robust to different measures of doctors’ reactive behaviors. In the main analysis, we used the number of medical live streams to construct doctors’ reactive behaviors. In the robustness check, doctors’ reactive behaviors were measured using the following measures: (1) the length of time spent in medical live streaming ( LSDuration it ), which is calculated as the total duration of all medical live streams conducted by doctor i in month t ; and (2) the number of doctor-patient interactions in the medical live streams ( LSInteractions it ), which is calculated as the total number of interactions between doctor i and patients in medical live streams in month t . This measure is likely to more effectively capture the reactive element of the behavior. The estimated results are shown in Table 6 , and we can see that the results are consistent with the main results.
Second, in the above analysis, the total number of articles posted by the doctors was used to measure doctors’ proactive behaviors. As doctors can post articles that are either their own original work or reposts from others, we further used the number of original articles ( OriArticles it ) to measure doctors’ proactive prosocial behaviors. Specifically, the number of articles was replaced with the number of original articles posted by doctor i in month t ( OriArticles it ). Models 1 and 2 in Table 7 show the results. We can see that using this alternative measure of proactive behavior does not materially change the results.
Third, as our dependent variable takes nonnegative values, negative binomial regression was further used to re-estimate our models. We find that the results (models 3 and 4 in Table 7 ) are similar to the main results.
Fourth, to further enhance the robustness and validity of our findings, article quality was used as a measure of doctors’ proactive behaviors. This approach is based on the premise that article quality more accurately reflects the effort and time invested by doctors in content creation. Specifically, we assessed article quality based on either the length of each article or the number of likes it received and then re-estimated our model. As indicated in Table 8 , the results remain consistent with our main findings, thereby further reinforcing the validity of our conclusions.
b R 2 =0.851; F 6,2879 =131.71; P <.001.
c R 2 =0.851; F 10,2879 =79.29; P <.001; Wald test: P <.001; likelihood ratio: P <.001.
d R 2 =0.850; F 6,2879 =112.52; P <.001.
e R 2 =0.851; F 10,2879 =68.43; P <.001; Wald test: P <.001; likelihood ratio: P <.001.
f N/A: not applicable.
a All models include doctor-fixed effects and month-fixed effects; robust SEs clustered by doctors are reported in models 1 and 2; bootstrap SEs in models 3 and 4.
b R 2 =0.851; F 6,2879 =118.99; P <.001.
c R 2 =0.852; F 10,2879 =71.04; P <.001; Wald test: P <.001; likelihood ratio: P <.001.
d Log likelihood=–150,015.36.
e Log likelihood=–149,888.24.
b R 2 =0.851; F 6,2879 =127.75; P <.001.
c R 2 =0.851; F 10,2879 =89.97; P <.001; Wald test: P <.001; likelihood ratio: P <.001.
d R 2 =0.851; F 6 , 2879 =133.39; P <.001.
e R 2 =0.852; F 10 , 2879 =84.94; P <.001; Wald test: P <.001; likelihood ratio: P <.001.
Analysis of Results
Web-based medical platforms offer a variety of functions to support doctors’ engagement in different types of prosocial behaviors. However, few studies have investigated the effects of these behaviors. Drawing on FMT and SET, this study categorized doctors’ prosocial practices in web-based health care communities into proactive and reactive actions and examined their effects on e-consultation volume. Briefly, prosocial behaviors positively impact on e-consultation, and a doctor’s digital and offline reputation moderates the relationship between prosocial behavior and e-consultation, albeit with some nuances.
First, we expanded upon existing literature on proactive prosocial behaviors, concluding that these actions can help doctors create professional images [ 43 ] in the medical consultation context. Our panel data analysis reveals that doctors’ posting of professional articles, which contribute to their professional image in the digital context, attracts more e-consultations. This finding aligns with the prior study [ 31 ], which observed that a health professional’s previous asynchronous prosocial behavior positively influences their future economic performance.
Second, drawing from SET, we analyzed the impact of synchronous reactive prosocial behaviors, a less explored area in prior literature. Our findings confirm that engaging in medical live streaming, a form of reactive prosocial behavior, leads to higher e-consultation volumes. Interestingly, we found that the positive impact of conducting a live stream exceeds that of posting an article.
Third, we expanded our research by testing the moderating roles of digital and offline reputations, measured by doctors’ offline professional titles and patients’ recommendations on web-based health care communities. We found that digital reputations significantly moderate the relationships between both types of prosocial behaviors and e-consultation volume. Specifically, doctors who post professional articles or conduct medical live streams attract more e-consultations when they have fewer patient recommendations compared to those with higher recommendations. Regarding offline professional titles, our results indicate a significant moderating effect on the relationship between proactive prosocial behaviors and e-consultation volume. Notably, junior doctors should focus more on posting articles in web-based health care communities to compensate for limitations associated with their titles [ 66 ]. However, the moderating effect of offline titles on the impact of reactive prosocial behaviors was found to be insignificant. We attribute this to the unique dynamics of trust conversion in Chinese health care settings. As doctors’ offline titles are granted by medical institutions, these titles could enhance patients’ trust in doctors only if there is a conversion of trust from the organization to the individual doctor, which represents different types of trust [ 67 ]. Consequently, doctors with the same offline titles from different hospitals may be perceived differently. For example, a senior doctor from a 3-A hospital is usually seen as highly professional in their clinical field, while a doctor with the same title in a 1-A hospital might typically handle primary diseases. Due to this trust conversion phenomenon, patients may not uniformly trust doctors from different hospitals with the same offline titles, leading to the insignificant moderating effect of offline titles on the impact of reactive prosocial behaviors.
In summary, this study underscores the importance of prosocial behaviors and reputation in shaping doctors’ e-consultation volumes on web-based health care communities, offering valuable insights for health care professionals aiming to increase their consultation outreach.
Implications
This study makes several theoretical implications. First, this study contributes to web-based health care community literature by offering a nuanced understanding of how doctors’ prosocial behaviors enhance e-consultation volume. While a limited number of studies have examined the effects of doctors’ freely provided behaviors in the digital context [ 31 ], the specific impact of different types of prosocial behaviors on e-consultation volume remains largely unexplored. This study addresses this knowledge gap by theoretically categorizing doctors’ prosocial behaviors in web-based health care communities into proactive and reactive types and exploring their impacts on e-consultations.
Second, this study enriches web-based health care communities and live streaming literature by validating the role of medical live streaming in web-based health services. Prior research on live streaming has mainly concentrated on e-commerce [ 68 ], web-based gaming [ 69 ], and web-based learning [ 70 ]. Our study extends this research to the health care context, highlighting the importance of live streaming on web-based health care platforms. Specifically, this study delves into how doctors’ synchronous, reactive volunteer interactions via live streaming influence patient decision-making.
Finally, this study advances FMT and SET by highlighting the importance of context in theory development and providing guidance for context-specific theorizing on web-based health platforms. It also sheds light on how the impact of different prosocial behaviors on e-consultation volume varies depending on a doctor’s offline and digital reputations. Notably, this study validates that proactive behaviors work more effectively in promoting e-consultations for doctors with lower titles or fewer digital recommendations, while reactive behaviors are more effective for doctors with fewer digital recommendations.
This study offers several practical implications for doctors and platform managers. First, the beneficial effects of prosocial behaviors suggest that doctors should adapt their engagement activities when participating in web-based health care platforms. Nowadays, an increasing number of doctors are joining web-based health care communities and focusing on e-consultations, attracted by the economic and social benefits. Based on our results, posting professional articles can help doctors establish a professional image, potentially leading to more e-consultations. Additionally, conducting medical live streams can bolster e-consultations by fostering cooperative social value for doctors and enhancing their credibility among patient audiences. Therefore, doctors may prefer engaging in both proactive and reactive prosocial activities in web-based health care communities to attract more patients to their e-consultation services.
Second, the boundary conditions of the effects of prosocial behaviors imply that doctors should strategically leverage the beneficial effect of proactive and reactive behaviors according to their offline and digital reputations. Doctors with fewer digital recommendations should focus more on prosocial behavior to attract patients to e-consultations. Meanwhile, doctors with lower titles should devote their efforts to proactive behaviors to demonstrate their capability in fulfilling the e-consultations, thereby reducing information asymmetry between patients and themselves.
Third, our findings offer implications for web-based health care platform managers in designing effective functions. An increasing number of platforms are launching various features to better serve doctors and patients, meeting the needs of both groups more effectively. Our empirical findings suggest that doctors’ proactive and reactive prosocial behaviors, such as posting professional articles and conducting medical live streams, can help them establish professional image and enhance patient trust, leading to improved performance. Importantly, these behaviors also benefit patients by enhancing their health knowledge and literacy. Thus, platform managers could introduce functions (eg, article posting, live streaming, and doctor-driven communities) to encourage more prosocial behaviors by doctors. Additionally, platform managers might consider incorporating guidelines or incentive mechanisms for prosocial behaviors into their platforms. For example, it is recommended that platforms collect and analyze doctors’ proactive and reactive prosocial behaviors and guide them on how to effectively use these functions and engage in different types of activities.
Limitations
Despite its contributions, this study also presents several limitations that future research should consider. First, various classifications of prosocial behavior are available; for instance, Richaud et al [ 71 ] classified such behavior as altruistic, compliant, emotional, public, anonymous, or dire actions. Given the intricacy of web-based medical services, future studies would benefit from further exploring the roles of these other types of prosocial behavior exhibited by doctors on web-based health care communities. Second, our research model was constructed primarily from the doctor’s perspective and thus did not investigate the influence of doctors’ prosocial behaviors on patients’ satisfaction and well-being. Future research should delve into these relationships to obtain a more comprehensive understanding of the impacts of doctors’ prosocial behaviors. Finally, this study focused only on the quantity of medical live-streaming sessions, overlooking the quality aspect, which could be a crucial factor influencing e-consultation volume. Future research will concentrate on exploring this aspect.
Conclusions
Building upon prior studies on doctors’ prosocial behaviors on web-based health care communities, this study further delineates doctors’ beneficial actions into proactive and synchronous reactive behaviors. This distinction is based on the divergence in doctors’ motives for engaging and patients’ levels of involvement. Drawing from FMT and SET, this study offers insights that could aid doctors in increasing their e-consultation volume by adopting these beneficial behaviors. Concurrently, this research augments our understanding of the roles a doctor’s reputation plays in the relationships between various prosocial behaviors—specifically, proactive and reactive actions—and their e-consultation volume. This study may inspire doctors with comparatively lower offline professional titles and digital popularity to achieve their desired e-consultation volume.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (72001170 and 72102179), the Fundamental Research Funds for the Central Universities (SK2024028), the Ministry of Education in China Project of Humanities and Social Sciences (21XJC630003), the China Postdoctoral Science Foundation (2022T150515, 2023M742818, and 2020M673432), the National Natural Science Foundation of China (72004042), and the Heilongjiang Natural Science Foundation (YQ2023G003), and the grants from City University of Hong Kong (projects 7005959, 7006152, and 7200725).
Conflicts of Interest
None declared.
- Qi M, Cui J, Li X, Han Y. Influence of e-consultation on the intention of first-visit patients to select medical services: results of a scenario survey. J Med Internet Res. 2023;25:e40993. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cao B, Huang W, Chao N, Yang G, Luo N. Patient activeness during online medical consultation in China: multilevel analysis. J Med Internet Res. 2022;24(5):e35557. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Jiang S. Talk to your doctors online: an internet-based intervention in China. Health Commun. 2021;36(4):405-411. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Jiang J, Cameron AF, Yang M. Analysis of massive online medical consultation service data to understand physicians' economic return: observational data mining study. JMIR Med Inform. 2020;8(2):e16765. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Street RL, Millay B. Analyzing patient participation in medical encounters. Health Commun. 2001;13(1):61-73. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ren D, Ma B. Effectiveness of interactive tools in online health care communities: social exchange theory perspective. J Med Internet Res. 2021;23(3):e21892. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Guo S, Guo X, Fang Y, Vogel D. How doctors gain social and economic returns in online health-care communities: a professional capital perspective. J Manag Inf Syst. 2017;34(2):487-519. [ FREE Full text ] [ CrossRef ]
- Tande AJ, Berbari EF, Ramar P, Ponamgi SP, Sharma U, Philpot L, et al. Association of a remotely offered infectious diseases eConsult service with improved clinical outcomes. Open Forum Infect Dis. 2020;7(1):ofaa003. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Richardson BR, Truter P, Blumke R, Russell TG. Physiotherapy assessment and diagnosis of musculoskeletal disorders of the knee via telerehabilitation. J Telemed Telecare. 2017;23(1):88-95. [ CrossRef ] [ Medline ]
- Castaneda PR, Duffy B, Andraska EA, Stevens J, Reschke K, Osborne N, et al. Outcomes and safety of electronic consult use in vascular surgery. J Vasc Surg. 2020;71(5):1726-1732. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Jiang S. The relationship between face-to-face and online patient-provider communication: examining the moderating roles of patient trust and patient satisfaction. Health Commun. 2020;35(3):341-349. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Li J, Tang J, Jiang L, Yen DC, Liu X. Economic success of physicians in the online consultation market: a signaling theory perspective. Int J Electron Commer. 2019;23(2):244-271. [ FREE Full text ] [ CrossRef ]
- Guo S, Guo X, Zhang X, Vogel D. Doctor–patient relationship strength’s impact in an online healthcare community. Inf Technol Dev. 2017;24(2):279-300. [ FREE Full text ] [ CrossRef ]
- Audrain-Pontevia AF, Menvielle L. Do online health communities enhance patient-physician relationship? an assessment of the impact of social support and patient empowerment. Health Serv Manage Res. 2018;31(3):154-162. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Yang Y, Zhang X, Lee PK. Improving the effectiveness of online healthcare platforms: an empirical study with multi-period patient-doctor consultation data. Int J Prod Econ. 2019;207:70-80. [ FREE Full text ] [ CrossRef ]
- Fan W, Zhou Q, Qiu L, Kumar S. Should doctors open online consultation services? An empirical investigation of their impact on offline appointments. Inf Syst Res. 2023;34(2):629-651. [ FREE Full text ] [ CrossRef ]
- Liu QB, Liu X, Guo X. The effects of participating in a physician-driven online health community in managing chronic disease: evidence from two natural experiments. MIS Q. 2020;44(1):391-419. [ FREE Full text ] [ CrossRef ]
- Yan Z, Wang T, Chen Y, Zhang H. Knowledge sharing in online health communities: a social exchange theory perspective. Inf Manag. 2016;53(5):643-653. [ FREE Full text ] [ CrossRef ]
- Luo P, Chen K, Wu C, Li Y. Exploring the social influence of multichannel access in an online health community. J Assoc Inf Sci Technol. 2018;69(1):98-109. [ FREE Full text ] [ CrossRef ]
- Chai S, Bagchi-Sen S, Morrell C, Rao HR, Upadhyaya SJ. Internet and online information privacy: an exploratory study of preteens and early teens. IEEE Trans Profess Commun. 2009;52(2):167-182. [ FREE Full text ] [ CrossRef ]
- Ahearne M, Rapp A, Hughes DE, Jindal R. Managing sales force product perceptions and control systems in the success of new product introductions. J Mark Res. 2010;47(4):764-776. [ FREE Full text ] [ CrossRef ]
- Awaysheh A, Heron RA, Perry T, Wilson JI. On the relation between corporate social responsibility and financial performance. Strateg Manag J. 2020;41(6):965-987. [ FREE Full text ] [ CrossRef ]
- Coll MP, Grégoire M, Eugène F, Jackson PL. Neural correlates of prosocial behavior towards persons in pain in healthcare providers. Biol Psychol. 2017;128:1-10. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kapıkıran NA. Sources of ethnocultural empathy: personality, intergroup relations, affects. Curr Psychol. 2021;42(14):11510-11528. [ FREE Full text ] [ CrossRef ]
- Yin Y, Wang Y. Is empathy associated with more prosocial behaviour? a meta‐analysis. Asian J Soc Psychol. 2023;26(1):3-22. [ FREE Full text ] [ CrossRef ]
- Finset A. "I am worried, Doctor!" emotions in the doctor-patient relationship. Patient Educ Couns. 2012;88(3):359-363. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Larson EB, Yao X. Clinical empathy as emotional labor in the patient-physician relationship. JAMA. 2005;293(9):1100-1106. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bloom G, Standing H, Lloyd R. Markets, information asymmetry and health care: towards new social contracts. Soc Sci Med. 2008;66(10):2076-2087. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Guo F, Zou B, Guo J, Shi Y, Bo Q, Shi L. What determines academic entrepreneurship success? A social identity perspective. Int Entrep Manag J. 2019;15(3):929-952. [ FREE Full text ] [ CrossRef ]
- Yang H, Zhang X. Investigating the effect of paid and free feedback about physicians' telemedicine services on patients' and physicians' behaviors: panel data analysis. J Med Internet Res. 2019;21(3):e12156. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Yan Z, Kuang L, Qiu L. Prosocial behaviors and economic performance: evidence from an online mental healthcare platform. Prod Oper Manag. 2022;31(10):3859-3876. [ FREE Full text ] [ CrossRef ]
- Frese M, Fay D. 4. Personal initiative: an active performance concept for work in the 21st century. Res Organ Behav. 2001;23:133-187. [ FREE Full text ] [ CrossRef ]
- Frese M, Kring W, Soose A, Zempel J. Personal initiative at work: differences between East and West Germany. Acad Manage J. 1996;39(1):37-63. [ FREE Full text ] [ CrossRef ]
- Spitzmuller M, Van Dyne L. Proactive and reactive helping: contrasting the positive consequences of different forms of helping. J Organ Behavior. 2013;34(4):560-580. [ FREE Full text ] [ CrossRef ]
- Bandura A. Social cognitive theory: an agentic perspective. Annu Rev Psychol. 2001;52(1):1-26. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mittal S, Sengupta A, Agrawal NM, Gupta S. How prosocial is proactive: developing and validating a scale and process model of knowledge-based proactive helping. J Manag Organ. 2018;26(4):625-650. [ FREE Full text ] [ CrossRef ]
- Walter A, Ritter T, Gemünden HG. Value creation in buyer–seller relationships: theoretical considerations and empirical results from a supplier's perspective. Ind Mark Manag. 2001;30(4):365-377. [ FREE Full text ] [ CrossRef ]
- Eggert A, Ulaga W, Schultz F. Value creation in the relationship life cycle: a quasi-longitudinal analysis. Ind Mark Manag. 2006;35(1):20-27. [ FREE Full text ] [ CrossRef ]
- Deng Z, Hong Z, Zhang W, Evans R, Chen Y. The effect of online effort and reputation of physicians on patients' choice: 3-wave data analysis of China's good doctor website. J Med Internet Res. 2019;21(3):e10170. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kurihara H, Maeno T, Maeno T. Importance of physicians' attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cooper ML, Shapiro CM, Powers AM. Motivations for sex and risky sexual behavior among adolescents and young adults: a functional perspective. J Pers Soc Psychol. 1998;75(6):1528-1558. [ CrossRef ] [ Medline ]
- Clary EG, Snyder M, Ridge RD, Copeland J, Stukas AA, Haugen J, et al. Understanding and assessing the motivations of volunteers: a functional approach. J Pers Soc Psychol. 1998;74(6):1516-1530. [ CrossRef ] [ Medline ]
- Epstein RM, Hundert EM. Defining and assessing professional competence. JAMA. 2002;287(2):226-235. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhang L, Qiu Y, Zhang N, Li S. How difficult doctor‒patient relationships impair physicians' work engagement: the roles of prosocial motivation and problem-solving pondering. Psychol Rep. 2020;123(3):885-902. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Dabholkar PA, Johnston WJ, Cathey AS. The dynamics of long-term business-to-business exchange relationships. J Acad Mark Sci. 1994;22(2):130-145. [ FREE Full text ] [ CrossRef ]
- Ochs M, Mestre D, de Montcheuil G, Pergandi JM, Saubesty J, Lombardo E, et al. Training doctors’ social skills to break bad news: evaluation of the impact of virtual environment displays on the sense of presence. J Multimodal User Interfaces. 2019;13(1):41-51. [ FREE Full text ] [ CrossRef ]
- Schroeder T, Seaman K, Nguyen A, Gewald H, Georgiou A. Enablers and inhibitors to the adoption of mHealth apps by patients—a qualitative analysis of German doctors' perspectives. Patient Educ Couns. 2023;114:107865. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Liu X, Hu M, Xiao BS, Shao J. Is my doctor around me? investigating the impact of doctors’ presence on patients’ review behaviors on an online health platform. J Assoc Inf Sci Technol. 2022;73(9):1279-1296. [ FREE Full text ] [ CrossRef ]
- Chen Y, Xie J. Online consumer review: word-of-mouth as a new element of marketing communication mix. Manage Sci. 2008;54(3):477-491. [ FREE Full text ] [ CrossRef ]
- Ye Q, Li Y, Kiang M, Wu W. The impact of seller reputation on the performance of online sales: evidence from TaoBao Buy-It-Now (BIN) data. SIGMIS Database. 2009;40(1):12-19. [ FREE Full text ] [ CrossRef ]
- Liu X, Guo X, Wu H, Wu T. The impact of individual and organizational reputation on physicians’ appointments online. Int J Electron Commer. 2016;20(4):551-577. [ FREE Full text ] [ CrossRef ]
- Dewan S, Hsu V. Adverse selection in electronic markets: evidence from online stamp auctions. J Ind Econ. 2004;52(4):497-516. [ CrossRef ]
- Torres E, Vasquez-Parraga AZ, Barra C. The path of patient loyalty and the role of doctor reputation. Health Mark Q. 2009;26(3):183-197. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gottschalk F, Mimra W, Waibel C. Health services as credence goods: a field experiment. Econ J. 2020;130(629):1346-1383. [ FREE Full text ] [ CrossRef ]
- Nunkoo R, Ramkissoon H. Power, trust, social exchange and community support. Ann Tour Res. 2012;39(2):997-1023. [ FREE Full text ] [ CrossRef ]
- Blau PM. Exchange and Power in Social Life. New Brunswick. Transaction Publishers; 1964.
- Molm LD, Takahashi N, Peterson G. Risk and trust in social exchange: an experimental test of a classical proposition. Am J Sociol. 2000;105(5):1396-1427. [ FREE Full text ] [ CrossRef ]
- Lioukas CS, Reuer JJ. Isolating trust outcomes from exchange relationships: social exchange and learning benefits of prior ties in alliances. Acad Manage J. 2015;58(6):1826-1847. [ FREE Full text ] [ CrossRef ]
- Malgonde OS, Saldanha TJ, Mithas S. Resilience in the open source software community: how pandemic and unemployment shocks influence contributions to others’ and one’s own projects. MIS Q. 2023;47(1):361-390. [ FREE Full text ] [ CrossRef ]
- Mayya R, Ye S, Viswanathan S, Agarwal R. Who forgoes screening in online markets and why? Evidence from Airbnb. MIS Q. 2021;45(4):1745-1776. [ FREE Full text ] [ CrossRef ]
- Moqri M, Mei X, Qiu L, Bandyopadhyay S. Effect of “following” on contributions to open source communities. J Manag Inf Syst. 2018;35(4):1188-1217. [ FREE Full text ] [ CrossRef ]
- Chen Q, Jin J, Zhang T, Yan X. The effects of log-in behaviors and web reviews on patient consultation in online health communities: longitudinal study. J Med Internet Res. 2021;23(6):e25367. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mo J, Sarkar S, Menon S. Competing tasks and task quality: an empirical study of crowdsourcing contests. MIS Q. 2021;45(4):1921-1948. [ FREE Full text ] [ CrossRef ]
- Gourieroux C, Holly A, Monfort A. Likelihood ratio test, Wald test, and Kuhn-Tucker test in linear models with inequality constraints on the regression parameters. Econometrica. 1982;50(1):63. [ CrossRef ]
- Peng CH, Yin D, Zhang H. More than words in medical question-and-answer sites: a content-context congruence perspective. Inf Syst Res. 2020;31(3):913-928. [ FREE Full text ] [ CrossRef ]
- Guo L, Jin B, Yao C, Yang H, Huang D, Wang F. Which doctor to trust: a recommender system for identifying the right doctors. J Med Internet Res. 2016;18(7):e186. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zheng S, Hui SF, Yang Z. Hospital trust or doctor trust? A fuzzy analysis of trust in the health care setting. J Bus Res. 2017;78:217-225. [ FREE Full text ] [ CrossRef ]
- Mao Z, Du Z, Yuan R, Miao Q. Short-term or long-term cooperation between retailer and MCN? new launched products sales strategies in live streaming e-commerce. J Retail Consum Serv. 2022;67:102996. [ FREE Full text ] [ CrossRef ]
- Hilvert-Bruce Z, Neill JT, Sjöblom M, Hamari J. Social motivations of live-streaming viewer engagement on Twitch. Comput Hum Behav. 2018;84:58-67. [ FREE Full text ] [ CrossRef ]
- Tang YM, Chen PC, Law KMY, Wu CH, Lau YY, Guan J, et al. Comparative analysis of student's live online learning readiness during the coronavirus (COVID-19) pandemic in the higher education sector. Comput Educ. 2021;168:104211. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Richaud MC, Mesurado B, Cortada AK. Analysis of dimensions of prosocial behavior in an Argentinean sample of children. Psychol Rep. 2012;111(3):687-696. [ FREE Full text ] [ CrossRef ] [ Medline ]
Abbreviations
Edited by G Eysenbach; submitted 11.09.23; peer-reviewed by P Luo, Y Zhu, C Fu; comments to author 05.10.23; revised version received 30.12.23; accepted 09.03.24; published 25.04.24.
©Xiaoxiao Liu, Huijing Guo, Le Wang, Mingye Hu, Yichan Wei, Fei Liu, Xifu Wang. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 25.04.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.
Our approach
- Responsibility
- Infrastructure
- Try Meta AI

In medicine, access to the right information at the right time in the right place can determine everything—from deciding to take preventative measures to seeking care when needed, receiving a timely diagnosis, and taking medications as prescribed.
Meditron , a suite of open-source large multimodal foundation models tailored to the medical field and designed to assist with clinical decision-making and diagnosis, was built on Meta Llama 2 and trained on carefully curated, high-quality medical data sources with continual input from clinicians and experts in humanitarian response.
Researchers at EPFL ’s School of Computer and Communication Sciences and Yale School of Medicine teamed up on the project, working closely with humanitarian organizations like the International Committee of the Red Cross (ICRC). Meditron has been downloaded over 30,000 times within its first months of release, filling an important gap in innovation in low-resource medical settings. And following last week’s release of Meta Llama 3 , the team fine-tuned the new 8B model within 24 hours to deliver Llama-3[8B]-MeditronV1.0 , which outperforms all state-of-the-art open models within its parameter class on standard benchmarks such as MedQA and MedMCQA.
“Foundation models have become modern-day intellectual and cultural assets,” says Yale professor Mary-Anne Hartley, who is co-leading the project. “When applied to the medical domain, they have the potential to provide life-saving advice and guidance. Yet the lowest-resource settings have the most to gain and remain the least represented.”
LLMs like Llama can compress complex information into an accessible conversational interface. Meditron adapted Llama 2 to ensure that the information provided better aligns with evidence-based care, contextually aware recommendations, and professional standards. The Meditron suite has the potential to serve crucial needs in a variety of settings, including emergency scenarios requiring fast and accurate medical response and assisting healthcare workers in diagnosing and treating patients in underserved areas.
The hope, says Hartley, is that releasing it fully open-access and open-source—from data to weights, with clear getting-started documentation—can empower innovation in resource-constrained settings to better ensure representation and create equitable access to medical knowledge.
“Low-resource settings should not be forced to ‘reinvent the wheel’ in order to have their populations and needs represented in this critical technology,” Hartley says.
When funding is an issue, start small and focus on quality
Funding can be a major challenge for anyone, but particularly for groups working in humanitarian and low-resource settings. Hartley says the team chose not to commercialize in order to maintain the neutrality required for impartial validation.
To conserve costs, experiments started on the smaller Llama 2 7B to narrow down optimal pre-training data mixtures and parameters for the scale-up to 70B. That conservative approach is also why the team released 7B and 70B Meditron models. While Meditron 7B is less performant, it is still very useful for modeling experimental scale-up, Hartley notes.
The multimodal implementation has followed a similar path. Meditron 7B integrates image interpretation, and while extremely promising (outperforming the 562B Medpalm M on medical image interpretation), it would be even better on 70B and deserves investment, Hartley says.

This focus on quality over quantity also meant the team spent most of its time carefully curating medically validated textual documents representing evidence-based guidelines in high- and low-resource settings. Continued pretraining, which updates all the parameters of the model rather than just focusing on a subset for fine-tuning, minimized the risk of contamination and bias from the general text corpus on which Llama was trained, Hartley says. It also maximized its retention of medical knowledge.
Because continued pretraining on a multi-GPU, multi-node cluster is very technically challenging, the team integrated the Llama architecture into a high-performing distributed trainer, Megatron-LM. Recognizing that this is an issue many others could also face, they made sure to open-source the adapted version of Megatron.
Putting Meditron to the test with open validation and evaluation
Hartley says that by far the most exciting real-world result from the Meditron work is the massive scale interest from medical professionals and humanitarian organizations across the world to participate in the Meditron MOOVE (Massive Online Open Validation and Evaluation).
Doctors from around the world, especially in low-resource settings, are asking Meditron challenging questions and critically evaluating its answers so the team can adapt it accordingly.
Meditron is currently the best-performing open-source LLM for medicine according to the leading benchmarks in the field, such as question-answering of biomedical exams, Hartley says. The team opted for a MOOVE to make the community aware that these benchmarks do not fully represent the real-world clinical practice of medicine or the challenges in low-resource settings and humanitarian response.
“That these time-constrained professionals are volunteering their time in our open-source community to independently validate Meditron is a recognition of its value,” Hartley says. “We are in a unique position to take all this feedback and incorporate it in a new model. We hope funders will recognize the social and commercial value of investing in our academic open-source initiative.”
Open-source technology has a time-tested history of empowering innovation and, critically, making it equitably accessible, Hartley says. “We are constantly hearing from researchers in low-resource settings about how Meditron has enabled their research,” she explains. “While open source is not new, the scale and cost of the contribution are. We need to be more audacious in seeking neutral philanthropic support for efforts like these.”
Our latest updates delivered to your inbox
Subscribe to our newsletter to keep up with Meta AI news, events, research breakthroughs, and more.
Join us in the pursuit of what’s possible with AI.

Product experiences
Foundational models
Latest news
Meta © 2024

IMAGES
VIDEO
COMMENTS
Medical research involves research in a wide range of fields, such as biology, chemistry, pharmacology and toxicology with the goal of developing new medicines or medical procedures or improving ...
Medicine, the practice concerned with the maintenance of health and the prevention, alleviation, or cure of disease. Learn about the organization of health services, medical practices around the world, fields of medicine, alternative medicine, and clinical research.
Nature Medicine - To celebrate the end of our 25th anniversary year, we asked thought leaders and experts in the field to answer one question: What will shape the next 25 years of medical research ...
Medical Research News. Health news on everything from cancer to nutrition. Updated daily. ... to create cells that look and act like cells from the body. This accomplishment, a first in the field ...
Basic medical research (otherwise known as experimental research) includes animal experiments, cell studies, biochemical, genetic and physiological investigations, and studies on the properties of drugs and materials. ... Interventional studies are experimental in character and are further subdivided into field studies (sample from an area ...
Healthcare research is a systematic inquiry intended to generate trustworthy evidence about issues in the field of medicine and healthcare. The three principal approaches to health research are the quantitative, the qualitative, and the mixed methods approach.
Although it is too soon to know when countries around the world will control the COVID-19 pandemic, there is already much to be learned. To explore trends for 2021, we talked to experts from ...
The findings suggest that people can learn to reduce the brain activity causing some types of chronic pain that occur in the absence of injury or persist after healing. 2021 Research Highlights — Basic Research Insights >>. NIH findings with potential for enhancing human health include new drugs and vaccines in development for COVID-19 ...
The University of Florida Cancer and Genetics Research Complex is an integrated medical research facility. Medical research (or biomedical research ), also known as health research, refers to the process of using scientific methods with the aim to produce knowledge about human diseases, the prevention and treatment of illness, and the promotion ...
With these new understandings has come translational research that transfers findings from the lab into new, more effective treatments and medicines. Dean Robert J. Alpern, M.D., Ensign Professor of Medicine, discussed basic science and advances in clinical care; technology and patient care; and the role of serendipity in research with Yale ...
Investigators in the Division of Precision Medicine are conducting research in several areas, including inflammation and anti-inflammatory drugs, wound healing, hepatic cirrhosis, angiogenesis and atherosclerosis, new cancer therapies, and pathways linking perceived stress and altered immunity. Learn more about our precision medicine research.
Therefore, to advance medicine in any field ongoing research is crucial. Once new knowledge is generated, it needs to be critically interpreted, verified and distributed to the scientific community by means of the medical literature so that it can be finally implemented into good clinical practice.
Insights and opinion on the latest medical research, clinical practices, and developments in the field.
Majority of parents experience isolation, loneliness and burnout, survey reveals. A new national survey conducted by The Ohio State University Wexner Medical Center finds a broad majority of ...
Artificial intelligence (AI) is a powerful and disruptive area of computer science, with the potential to fundamentally transform the practice of medicine and the delivery of healthcare. In this review article, we outline recent breakthroughs in the application of AI in healthcare, describe a roadmap to building effective, reliable and safe AI ...
A research field is being developed to produce nanoparticles and nanotechnology-based treatments. 6.4. ... In the medical field, nanomedicine uses nanotechnological expertise to prevent and treat severe conditions, including cardiovascular disease and cardiac illness. Nanoscale materials, including biocompatible nanoparticles and medical ...
When asked about the purpose of medical research most people would hopefully reply: to advance knowledge for the good of society; to improve the health of people worldwide; or to find better ways to treat and prevent disease. The reality is different. The research environment, with its different players, is now much less conducive to thinking ...
Advances in information-based medical research could also facilitate the movement toward personalized medicine, which will make health research more meaningful to individuals. ... Health services research has been defined as a multidisciplinary field of inquiry, both basic and applied, that examines the use, costs, quality, accessibility ...
Arrhythmia, cardiomyopathy, heart failure, preventative cardiology and vascular topics research. Heart Institute. Biomechanics research, gait and mobility disorders, swallowing dysfunction research. Department of Physical Medicine and Rehabilitation. Brain tumor, MS, pediatric neurosurgery and transverse myelitis research.
Here are 10 careers you can pursue in the field of medical research: 1. Clinical laboratory scientist. National average salary: $89,291 per year Primary duties: A clinical laboratory scientist is a scientist who specializes in using lab equipment to perform tests on biological specimens. This can involve extracting and testing bodily fluids to ...
Abstract. Artificial intelligence (AI) is poised to broadly reshape medicine, potentially improving the experiences of both clinicians and patients. We discuss key findings from a 2-year weekly ...
Machine learning will soon be applied to many other medical conditions, from cardiology to neurodegenerative diseases and beyond. 6. Improving prognostics. In addition to using it to diagnose ...
Since 2020, COVID-19 has been a hot-button topic in medicine, along with the long-term symptoms in those with a history of COVID-19. Examples of COVID-19-related research topics worth exploring include: The long-term impact of COVID-19 on cardiac and respiratory health. COVID-19 vaccination rates.
Here are the 2023-2024 Best Medical Schools: Research. Harvard University. Johns Hopkins University. University of Pennsylvania (Perelman) Columbia University. Duke University. Stanford University ...
Northwestern University Feinberg School of Medicine is home to a team of premier faculty and staff biostatisticians who are a driving force of data analytic innovation and excellence. In this episode, Denise Scholtens, PhD, a leader in biostatistics at Feinberg, discusses the growing importance of the field of biostatistics and how she leverages her skills to collaborate on several projects in ...
Researchers from across the United States and Canada gathered at the University of Arizona College of Medicine - Phoenix's campus to discuss recent trends, new therapies and what the future will hold for the field of pharmacogenomics during the annual Precision Medicine and Pharmacogenomics Symposium April 8.
Industry Updates From the Field of Stem Cell Research and Regenerative Medicine in April 2023 Dusko Ilic 1 Stem Cell Laboratories, Guy's Assisted Conception Unit, Department of Women & Children's Health, Faculty of Life Sciences & Medicine, King's College London, London, SE1 9RT, UK Correspondence [email protected]
OpenAI's latest artificial intelligence model has almost matched expert doctors in analysing eye conditions, according to research that highlights the technology's potential in medicine.
Background: Patients using web-based health care communities for e-consultation services have the option to choose their service providers from an extensive digital market. To stand out in this crowded field, doctors in web-based health care communities often engage in prosocial behaviors, such as proactive and reactive actions, to attract more users.
Meditron is currently the best-performing open-source LLM for medicine according to the leading benchmarks in the field, such as question-answering of biomedical exams, Hartley says. The team opted for a MOOVE to make the community aware that these benchmarks do not fully represent the real-world clinical practice of medicine or the challenges ...