Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Consensus Statement
- Open access
- Published: 07 February 2019
POSITION PAPER

Challenges to curing primary brain tumours
- Kenneth Aldape 1 ,
- Kevin M. Brindle 2 ,
- Louis Chesler 3 ,
- Rajesh Chopra 3 ,
- Amar Gajjar 4 ,
- Mark R. Gilbert 5 ,
- Nicholas Gottardo 6 ,
- David H. Gutmann 7 ,
- Darren Hargrave 8 ,
- Eric C. Holland 9 ,
- David T. W. Jones 10 ,
- Johanna A. Joyce 11 ,
- Pamela Kearns 12 ,
- Mark W. Kieran 13 ,
- Ingo K. Mellinghoff 14 ,
- Melinda Merchant 15 ,
- Stefan M. Pfister 16 ,
- Steven M. Pollard 17 ,
- Vijay Ramaswamy ORCID: orcid.org/0000-0002-6557-895X 18 ,
- Jeremy N. Rich 19 ,
- Giles W. Robinson ORCID: orcid.org/0000-0001-7441-9486 4 ,
- David H. Rowitch 20 ,
- John H. Sampson 21 ,
- Michael D. Taylor 22 ,
- Paul Workman 3 &
- Richard J. Gilbertson ORCID: orcid.org/0000-0001-7539-9472 2 , 23
Nature Reviews Clinical Oncology volume 16 , pages 509–520 ( 2019 ) Cite this article
45k Accesses
503 Citations
131 Altmetric
Metrics details
- Cancer models
- Cancer therapy
- Translational research
Despite decades of research, brain tumours remain among the deadliest of all forms of cancer. The ability of these tumours to resist almost all conventional and novel treatments relates, in part, to the unique cell-intrinsic and microenvironmental properties of neural tissues. In an attempt to encourage progress in our understanding and ability to successfully treat patients with brain tumours, Cancer Research UK convened an international panel of clinicians and laboratory-based scientists to identify challenges that must be overcome if we are to cure all patients with a brain tumour. The seven key challenges summarized in this Position Paper are intended to serve as foci for future research and investment.
Similar content being viewed by others

A common goal to CARE: Cancer Advocates, Researchers, and Clinicians Explore current treatments and clinical trials for breast cancer brain metastases
Brain metastasis.

Current approaches to the management of brain metastases
Introduction.
Brain tumours are among the most feared of all forms of cancer. More than two-thirds of adults diagnosed with glioblastoma — the most aggressive type of brain cancer — will die within 2 years of diagnosis 1 , 2 . Brain cancers are also the most common and most lethal of all paediatric solid tumours 3 . Furthermore, children with these tumours who survive and enter adulthood will often be affected by the long-term consequences of exposing the developing brain to medical interventions, including surgery, radiotherapy and/or chemotherapy 4 , 5 .
Brain tumours have proved challenging to treat, largely owing to the biological characteristics of these cancers, which often conspire to limit progress. First, by infiltrating one of the body’s most crucial organs, these tumours are often located beyond the reach of even the most skilled neurosurgeon. These tumours are also located behind the blood–brain barrier (BBB) — a system of tight junctions and transport proteins that protect delicate neural tissues from exposure to factors in the general circulation, thus also impeding exposure to systemic chemotherapy 6 , 7 . Furthermore, the unique developmental, genetic, epigenetic and microenvironmental features of the brain frequently render these cancers resistant to conventional and novel treatments alike 8 , 9 , 10 . These challenges are compounded by the rarity of brain tumours relative to many other forms of cancer, which limits the level of funding and interest from the pharmaceutical industry and attracts a relatively small and fragmented research community.
To begin to address these issues and improve the outcomes of patients with brain tumours, Cancer Research UK (CRUK) convened an international panel of brain cancer researchers with interests in neurobiology, preclinical tumour modelling, genomics, pharmacology, drug discovery and/or development, neuropathology, neurosurgery, imaging, radiotherapy and medical oncology, with the task of identifying the most important challenges that must be overcome if we are to eventually be in the position to cure all patients with a brain tumour. In this Position Paper, we summarize seven key challenges identified by the panel that should serve as the foci for future research and investment (Fig. 1 ). Each of these challenges is worthy of extensive discussion and review, which are beyond the scope of this manuscript. Therefore, we highlight these challenges as a ‘call-to-arms’, summarizing the nature and importance of each challenge rather than providing an exhaustive review of the current level of understanding.
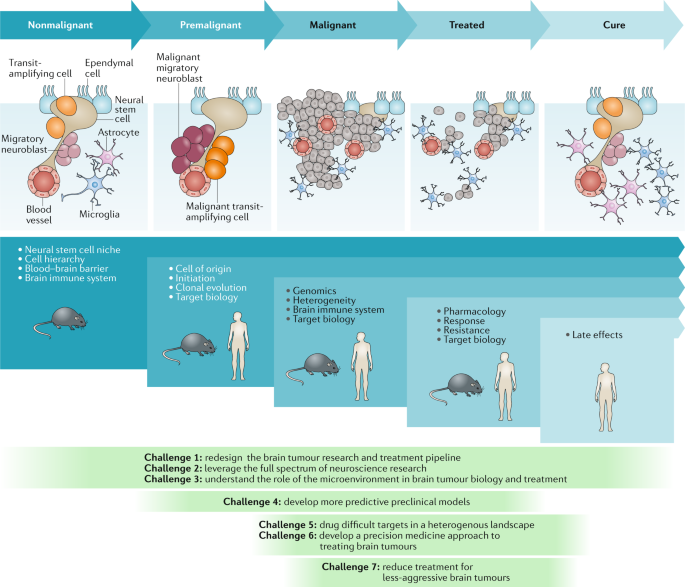
The nonmalignant cellular composition of the ventricular–subventricular zone includes neural stem cells that divide and produce transit-amplifying cells. Transit-amplifying cells give rise to migratory neuroblasts 90 . Ependymal cells are also present in the neural stem cell niche. The niche is intimately associated with blood vessels and might also communicate with other cell types, including microglia and astrocytes. Malignant transformation of neural stem cells presumably leads to the premalignant expansion of transit-amplifying cells and migratory neuroblasts as the nonmalignant hierarchy begins to transform. This unregulated hierarchy generates the malignant brain tumour. As these lesions are treated, tumour cells are killed, with the ultimate goal of eventual cure. The blue panels below these cartoons denote the focus of key research questions at each stage, from nonmalignant brain tissue to the development of cancer and remission following successful treatment. The green panels depict how the seven challenges to progress relate to these specific stages of disease development.
Challenge 1: redesign research pipeline
Clinical trials have yet to reveal an effective therapy for most brain tumours. This harsh reality stems, in part, from an incomplete understanding of brain tumour biology and the existence of a disconnect between preclinical drug development and rigorous testing in the clinic. Each element of the brain tumour research pipeline, from basic neurobiology to clinical trials, requires careful scrutiny and increased investment, although the development of an overarching strategy that facilitates and promotes interdisciplinary research is equally important (Fig. 1 ). This strategy would bring an end to the previous ‘siloed’ organization of working practices, in which basic and clinical researchers performed their studies independently and collaborated only when laboratory research was judged to be ready for the clinic or when the laboratory is engaged to understand the reasons why a promising drug failed to achieve the expected level of efficacy in clinical trials. Much deeper, longitudinal collaboration is essential in order to drive progress as rapidly as possible.
Nascent attempts to unify the brain tumour research pipeline are underway. The international paediatric brain tumour community has demonstrated remarkable levels of collaborative activity over many years and is now working to discover and define the genomic subtypes of paediatric brain tumours and form multidisciplinary teams in order to design preclinical studies that better inform the design of clinical trials 11 , 12 . However, the community must go further, by forming international collaborations that extend beyond the borders of traditional research disciplines and by engaging the entire breadth of expertise available within the biological and physical sciences (see Challenge 2). Such congregations of experts could provide a more comprehensive understanding of the workings of the brain and how these processes are subverted during malignant transformation.
The community should also explore innovative methods of designing and delivering clinical trials. Interest among the brain tumour research community in the use of adaptive trial designs is particularly welcomed 13 , 14 . Such trials could provide prospective opportunities to modify ongoing studies and integrate the investigation of new hypotheses as data are accumulated and analysed (Fig. 2 ). This approach has distinct advantages over the less flexible, traditional trial designs, which are typically designed to test one or two primary hypotheses, typically in heavily pretreated patients over a number of years. However, several hurdles must be overcome before novel trial designs can be implemented successfully, including identifying a cadre of rational therapies to feed into these new trials; developing robust biomarker-based patient selection criteria; constructing systems that enable the provision of real-time, in-trial, biomarker and end point data; and formulating optimal mechanisms of generating and sharing complex research data among centres worldwide (Fig. 2 ). Notwithstanding these challenges, improving the current approach to clinical trials is an urgent objective for the community. In the context of small, genomically defined patient subgroups, international collaborative clinical trials are crucial. A comprehensive review of the current logistical, regulatory and financial hurdles that inhibit the initiation of such trials is therefore warranted.
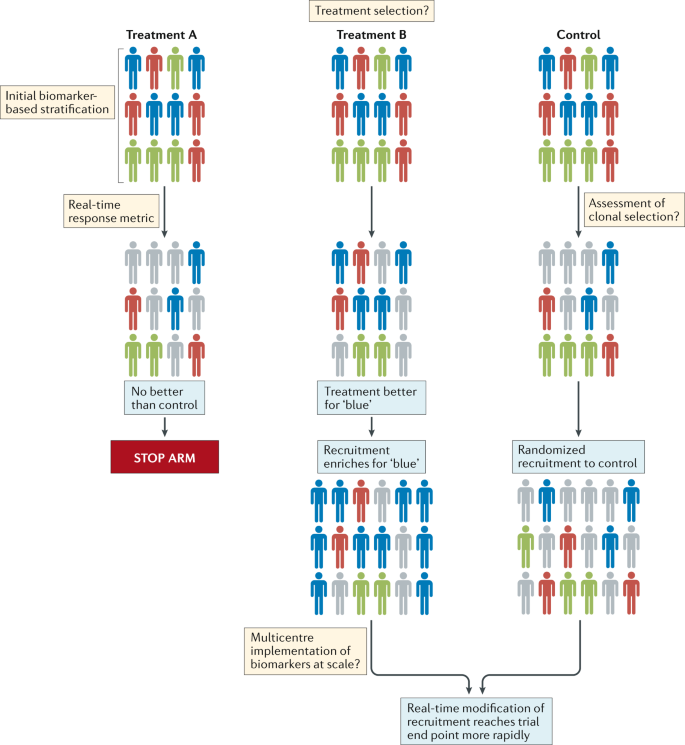
Patients with different molecular subtypes of a specific type of brain tumour (represented as blue, red and green figures) are enrolled into the trial. Patients in the control group receive the optimal standard of care. New treatments can then be tested as comparators in groups A and B. Following a defined period of treatment, the responses of patients in these groups are compared with those of patients in the control group. Responding patients are represented by bold colours; nonresponding patients are coloured grey. In this example, new treatment B produced a biased favourable response in patients with a single disease subtype (blue figures) relative to the control treatment. No advantage of new treatment A is observed relative to the control treatment. Therefore, in the next phase of the study, the new treatment A group is discontinued, while the new treatment B arm proceeds, with enrichment for patients with tumours of the ‘blue’ subtype. The control group continues with randomized recruitment of patients with all disease subtypes. The success of this approach is dependent upon addressing the key challenges summarized in the thought bubbles emerging at each stage of the trial. Many of these challenges are yet to be addressed in patients with brain tumours.
Greater integration of research disciplines will also require a change in the current research culture. The principal metrics used to reward success in most academic environments are focused on competitive activities, such as publication in high-impact journals and the acquisition of grant funding. These metrics have some value in enabling the assessment and quantification of the extent of innovative thought and discovery, although they arguably also work against the development of the deeper levels of collaborative activity that will ultimately be required to cure patients with brain tumours. This change in academic culture must go beyond a cursory recognition of the value of ‘team science’. Rather, akin to the complex and multidisciplinary groups that operate in the pharmaceutical industry, new reward and promotion structures should be developed for laboratory and clinical researchers alike that inspire and encourage their participation in collaborative academic brain tumour research.
Challenge 2: use neuroscience research
We now have a considerable level of understanding of the cellular hierarchies and signals that generate and maintain brain activity in the absence of cancer 15 . Specialized glial cells serve as neural stem cells (NSCs) in both the embryonic and adult brain, in which they reside in neurogenic niches and produce daughter cells that differentiate into neurons, astrocytes and oligodendrocytes (Fig. 1 ). These niches also include supporting cells and secreted factors that are critical for the close regulation of both NSC self-renewal and the early stages of daughter cell differentiation: an intimate relationship between NSCs and blood vessels seems to be especially important in the neurogenic niche 16 .
Several lines of evidence suggest that brain tumours arise within, or are driven by, cells that recapitulate the neurogenic niche (Fig. 3 ). Stem-like cells have been isolated from paediatric and adult brain tumours 17 , 18 , 19 , and brain tumours have been shown to contain malignant niches that seem to recapitulate the micro-architectural features and signalling properties of the nonmalignant neurogenic niche 20 , 21 . Recurrent mutations in brain tumours can also perturb the signalling pathways that regulate brain development 12 , and subgroups of brain tumours have been shown to contain the transcriptomes and epigenomes of their originating parental NSCs that generated these tumours in the mouse brain 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 .
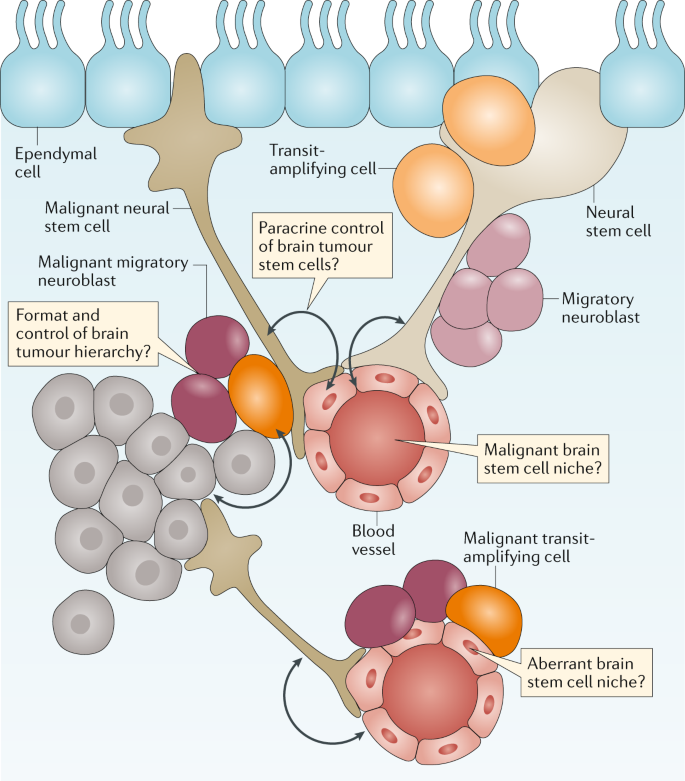
Brain tumours are thought to arise from a transformed and malignant brain tumour hierarchy generated from transformed neuronal stem cells. Nonmalignant and malignant stem cells in the brain are believed to reside in perivascular niches formed by the blood vessels of the brain. Unaddressed key research questions that could provide a better understanding of brain tumour development and treatment are shown in the thought bubbles.
Evidence that brain tumours are a consequence of aberrant brain development or repair underscores the importance of closer integration between neurobiological and cancer research; however, clear examples of such collaborations are rare. Only ~1% (US$260 million) of the $21 billion invested in neuroscience research by the US NIH in 2018 is aimed specifically at brain tumour research. Furthermore, although the PubMed database currently contains ~320,000 and 198,000 articles on neuroscience and brain tumour research, respectively, only 6,000 (~2% and ~3.5%, respectively) overlap. These observations are crude indicators of the level of interaction, but they suggest that the brain tumour research community is not capitalizing fully on the available research data. An increased focus on areas of common interest, such as immune dysregulation in patients with cancer and in those with dementia, should drive joint funding initiatives and ultimately result in progress for patients 30 , 31 .
Challenge 3: understand the TME
A thorough understanding of the properties and functions of the tumour microenvironment (TME) is required in order to obtain a complete understanding of brain tumour biology and treatment 9 , 32 (Fig. 4 ). As discussed with regard to Challenge 2, closer interactions with scientists investigating nonmalignant brain diseases will be especially important to this effort. Over many decades, research groups studying neurodegeneration and other nonmalignant brain diseases have generated a considerable level of understanding of the biology of the extracellular matrix, of specific brain-resident cellular populations (such as microglia, astrocytes and neurons) and of blood vessels (including those forming the BBB) that comprise the TME.
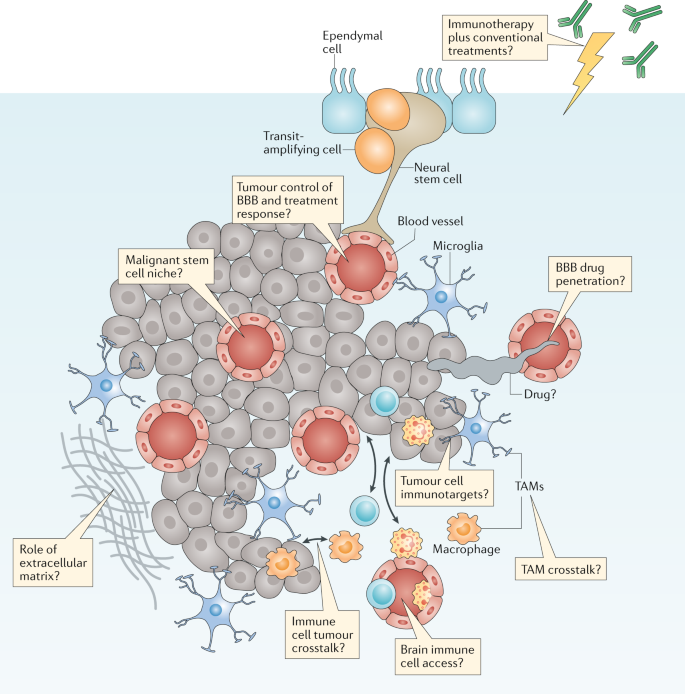
Brain tumours typically comprise a complex mixture of malignant cells and a great variety of nonmalignant cells. These include immune, vascular and nonmalignant brain cells, all of which are able to communicate with malignant cells and contribute to the development and progression of cancer. These features also form a key part of the response of a tumour to treatment. Key research questions focused on understanding how these elements affect tumour biology and responses to treatment are shown in the thought bubbles. BBB, blood–brain barrier; TAM, tumour-associated macrophage.
The immune and vascular components of the TME are likely to have particular relevance for improving the treatment of brain tumours 6 , 33 . For example, the discovery of the immune or glymphatic system of the brain and evidence that immunotherapies are effective treatments for an increasing number of cancers have prompted studies of the efficacy of such therapies in patients with brain tumours 31 , 34 , 35 , 36 . The major myeloid cell populations of the brain TME comprise macrophages and microglia — together referred to as tumour-associated macrophages (TAMs) 37 , 38 . Glioblastoma cells stimulate TAMs to produce immunosuppressive, tumour-promoting cytokines and enhanced apoptosis of T cells 39 . Glioblastoma cells can also inhibit the production of immunostimulatory cytokines and activate the recruitment of regulatory T cells, thus inhibiting antitumour immune responses 40 . These data suggest that inhibiting the activity of TAMs might provide therapeutic benefit in patients with brain tumours, although the clinical reality is likely to be more complex. Research conducted over the past decade suggests that re-educating TAMs to adopt phenotypes that are likely to prevent or inhibit the progression of brain tumours might be more effective than depleting all TAM populations 32 , 41 . A range of additional immune-based therapies are also currently in development, including vaccines, cellular therapies (such as chimeric antigen receptor (CAR) T cells) and immune-checkpoint inhibitors 9 , 31 , 34 , 40 , 41 , 42 .
Regardless of the type of immunotherapy that is being developed, several key challenges must be addressed if we are to deploy these agents effectively in the treatment of brain tumours (Fig. 4 ). Once again, a better understanding of tumour biology will be important to the selection of patients who are most likely to benefit from therapies and to improve the level of understanding of treatment-related toxicities (see Challenge 1). Addressing these challenges will not be straightforward for immunotherapies, not least because even the imprecise predictors of a response to immune-checkpoint inhibition in other cancers, such as tumour mutational burden and/or DNA methylation, remain to be proved in patients with brain tumours 43 . Furthermore, the identity of the optimal targets of other immunotherapies, such as CAR T cells, which might be patient specific, also remains unclear. Other important and unanswered questions surrounding the use of immunotherapies in patients with brain tumours include the degree to which the BBB will impede the access of cellular and molecular immunotherapies to tumours; whether immunotherapies will synergize with or counteract the effects of existing treatments, such as temozolomide, steroids and/or radiotherapy; and the type and severity of neurological adverse effects associated with immunotherapies and how best to prevent, monitor and manage these. Thus, although immunotherapy holds great promise as a new therapeutic approach for the treatment of patients with brain tumours, many aspects of the unique immunological and microenvironmental status of the brain present challenges that need to be overcome in order to develop and validate these therapies efficiently 9 .
As previously alluded to, the BBB is a major hurdle to the successful treatment of brain tumours 44 . This dynamic structure of tight junctions and molecular pumps comprises endothelial, ependymal and tanycytic cells that communicate with other brain cells and with circulating immune cells 45 , 46 , 47 , 48 . Single-cell transcriptomics has revealed a remarkable level of regional heterogeneity along the arteriovenous axis of the BBB, including a seamless continuum of endothelial cells versus a punctuated continuum of mural cells 49 . These insights could prove important in determining the functionality of the BBB in tumours located in different regions of the brain.
Discrete subtypes of brain tumours might also alter the BBB (Fig. 4 ). In this regard, the WNT subtype of medulloblastomas, which are highly sensitive to treatment, has been shown to secrete WNT antagonists that ‘re-educate’ the BBB, thus causing it to adopt a phenotype that is highly permeable to systemic chemotherapy 6 . Similarly, the therapeutic response of gliomas to temozolomide might be improved by use of this agent in combination with small-molecule inhibitors of WNT signalling 33 . Greater research efforts should be invested in cataloguing the integrity and other alterations of the BBB in each brain tumour subtype. This information might enable the more widespread use of systemic therapies that are currently regarded as BBB non-penetrating in those tumours that lack an intact BBB and support the use of WNT inhibitors and other approaches to enhance the delivery of chemotherapy to those tumours in which the BBB is intact 50 .
Challenge 4: develop preclinical models
The limited progress made in the treatment of brain tumours relates, in part, to the inaccuracy of preclinical models that have thus far failed to consistently show responses to agents with therapeutic activity in patients. Preclinical drug development pipelines that enable the accurate prediction of effective drugs are especially important for rare cancers, including brain tumours, owing to the low numbers of patients available for participation in clinical research 12 , 51 . Current pipelines are limited in their ability to identify new, more effective treatments of brain tumours for several reasons: they typically involve poorly characterized in vitro systems or subcutaneous tumour xenografts, rather than more accurate orthotopic models of brain tumours; they do not enable the assessment of benefit from new treatments in terms of survival relative to that provided by the existing combinations of neurosurgery, radiotherapy and/or chemotherapy; and finally, they usually lack rigorous characterization of other clinically important features, such as those of the BBB. Thus, curing all patients with a brain tumour will require a new approach that leverages an improved understanding of neuroscience and brain tumour biology to develop and deploy more accurate preclinical models.
A better understanding of the processes that drive the development and progression of brain tumours should promote the development of more accurate genetically engineered mouse models (GEMMs) and patient-derived xenografts (PDXs). We recommend that the brain tumour community maintains a centralized catalogue of all existing and newly developed preclinical models that have been subjected to a minimum agreed set of rigorous histological, genomic and imaging evaluations. Models from this catalogue should be made readily available and would provide the community with universally accessible, validated models that closely mimic the characteristics of human tumours. Initiatives such as the ITCC Paediatric Preclinical Proof of Concept Platform are already underway, with the intention of creating such a resource.
In addition to minimum standards of model characterization, the community should also adopt minimum standards for preclinical studies, thus enabling more effective comparisons of the results of different studies (Fig. 5 ). Of particular note, although patients with brain tumours receive complex, multimodality therapy, mice in preclinical studies typically receive monotherapies that are rarely comparable to the standard of care for the malignancy that is being modelled. Thus, efforts to establish preclinical ‘mouse hospitals’, in which potential new therapies are tested in the context of combination with neurosurgery, fractionated radiotherapy and/or conventional chemotherapy, should become the new standard 11 . Researchers with access to such preclinical hospitals should also perform robust pharmacokinetic (with particular attention to BBB penetration), pharmacodynamic and biomarker (tumour, liquid biopsy and imaging) studies for novel agents, thus maximizing the speed and likelihood of success of clinical translation (Fig. 5 ).
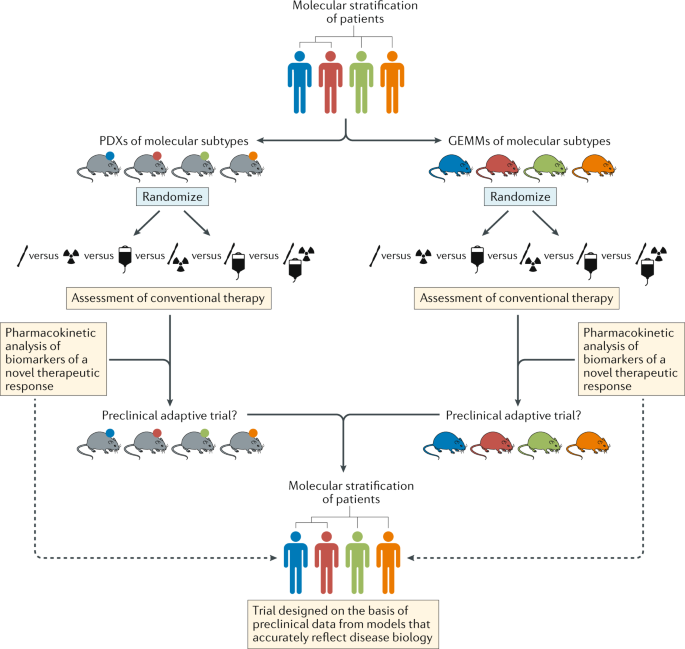
Schema depicting pathways for improvements in the preclinical development of new treatments for subsets of patients with brain tumours of distinct molecular and/or clinical subtypes (represented by different colours). This approach should include both patient-derived xenografts (PDXs) and genetically engineered mouse models (GEMMs) that accurately reflect the principle molecular and/or clinical subtypes. In order to provide the optimal level of clinical relevance, these models must also involve an assessment of responses to conventional combination therapies. These existing treatments could be included in adaptive preclinical trials designed to test novel treatments and inform the ultimate clinical trial design.
The failure of many drugs to penetrate the BBB to any clinically meaningful extent is an important reason for the poor treatment responses of patients with brain tumours to systemic therapies 52 . Functional imaging studies in particular can provide evidence of early treatment responses, although a failure to detect a response cannot distinguish between a drug that is ineffective and one that simply does not cross the BBB. Contrast enhancement of tumours on MRI, using various agents, can reveal disruption of the BBB, for example, following ultrasonography-mediated opening of the BBB 53 ; however, this approach does not provide direct evidence of drug delivery. More direct evidence can be provided by observing tumour accumulation on PET imaging of drugs labelled with a positron-emitting isotope, such as [methyl- 11 C] temozolomide in patients with gliomas 54 . However, conjugating all new drugs with such isotopes — particularly with 11 C, which will not perturb drug chemistry and thus drug–target interactions but has a sufficiently short half-life (20.3 minutes) — would be a formidable undertaking. Thus, the community should agree on acceptable preclinical and clinical standards for demonstrating successful drug delivery to brain tumours.
In addition to being grown as PDXs, patient-derived brain tumour cells can be consistently and successfully encouraged to propagate under NSC culture conditions as organoids 55 , 56 . These short-term culture systems have enormous potential for studying interactions between different cells and drug sensitivities and for interrogation using genome-wide interference and/or editing techniques 20 , 55 , 56 , 57 , 58 . Preclinical pipelines that deploy these novel screening approaches, together with the use of matched GEMMs and PDX models (ideally in humanized mice), should provide a more comprehensive and more accurate means of delivering optimized drugs and their associated biomarkers into clinical trials 11 .
Preclinical and clinical research efforts to evaluate the value of new treatments, relative to that of the conventional treatments of brain tumours, should also consider re-evaluating some long-established treatment approaches that might be of questionable value. For example, corticosteroids, such as dexamethasone, continue to be used perioperatively and might be continued throughout subsequent treatment. The original reasons for using dexamethasone in this way probably reflect antiproliferative activity that confers protection of nonmalignant cells from radiotherapy-induced and chemotherapy-induced genotoxic stress. However, both clinical data and preclinical data from mouse models suggest that corticosteroids might decrease the effectiveness of treatments and shorten the survival durations of patients with glioblastoma. Thus, greater efforts should be invested in identifying alternative agents, on the basis of robust preclinical and clinical evidence, such as anti-VEGF agents for the treatment of brain tumour-associated oedema 59 .
Challenge 5: drugging complex cancers
The so-called omics revolution is providing a comprehensive view of the genomes, epigenomes and transcriptomes of brain tumours; this additional information has enabled the segregation of histologically similar subsets of tumours into clinically and molecularly distinct subgroups. However, despite a few notable exceptions, such as inhibitors of mTOR or BRAF in selected patients 60 , 61 , genomics has yet to deliver on the promise of identifying new therapeutic targets in patients with brain tumours. Discovering new treatments of brain tumours will require a much deeper level of understanding of the biology of potential drug targets, combined with the application of cutting-edge drug discovery approaches to inhibit these targets.
Progress in drug development will not come easily. Alterations identified in brain tumours in the past decade include poorly understood targets that are frequently regarded as undruggable, including amplification of genes encoding transcription factors, such as MYC and MYCN ; gene translocations, such as FGFR–TACC and C11OF95–RELA 27 , 62 ; oncogene ‘enhancer hijacking’, for example, by GFI1 (ref. 63 ); and mutated histones, such as H3F3A and HIST1H3B variants 64 , 65 . Furthermore, these alterations exist in a shifting landscape of tumour heterogeneity, in which the populations of clones carrying each alteration fluctuate as the disease evolves in response to therapy 66 , 67 . This clonal evolution means that the proportion of tumour cells carrying a specific drug target can vary markedly during the course of disease, thus complicating efforts to target specific alterations. This heterogeneity can be measured in terms of mutational load, epigenetic alterations, rewiring of transcriptional circuits and microenvironmental influences and is widely considered to underpin the diversity of responses to treatment. The serial analysis of tumour biology using additional measures, such as advanced metabolic imaging and analyses involving serial sampling of circulating tumour DNA, is discussed with regard to Challenge 6 and might provide a more complete and accurate picture of tumour evolution.
Although daunting, the data provided by these novel approaches reflect an important principle that must be adopted by brain tumour researchers and clinicians — the notion that brain tumours are monogenetic and monoclonal must be dispelled. Comprehensive mapping of genomic evolution before, during and following treatment, including the use of single-cell sequencing approaches, should be used to better identify and prioritize treatment targets 68 , 69 . This objective will be best achieved in the context of prospective clinical trials, in which molecular imaging and other diagnostic approaches can be used to provide real-time assessments of disease evolution 70 . Prioritized targets should also be interrogated using approaches, such as deep mutational scanning, that can reveal information on the intrinsic properties of proteins and their function and how this changes with mutation 71 . Reverse translation efforts involving detailed mechanistic studies after a clinical trial of appropriately prioritized targets should include functional studies that enable the accurate definition of target function in tumours. Such serial analyses will likely be complex and resource-intensive and are probably best organized as part of a community-wide approach. This approach might be promoted in several ways: development of international tumour subtype-specific trials; the widespread availability of brain tumour stem cell banks (such as the UK glioma cellular genetics resource (GCGR) , the human glioblastoma cell culture (HGCC) resource in Sweden and the Stand Up to Cancer (SU2C) Canada Cancer Stem Cell Dream Team ); established efforts to understand complex cancer targets (such as the US National Cancer Institute RAS Initiative ); and closer collaborations with industry.
Challenge 6: precision medicine
The current classifications of brain tumours are based primarily on microscopic morphology and immunohistochemistry 72 . This approach enables the broad characterization of tumour type and a certain level of prognostication, although it fails to capitalize on the wealth of clinically relevant insights produced by the genomic subtyping of brain tumours. This dilemma is exemplified by medulloblastoma and ependymoma, both of which consist of clinically relevant subtypes with discrete cells of origin, driver mutations and global transcriptomic and epigenomic patterns that are yet to be incorporated into the WHO classification of central nervous system tumours 10 , 12 , 72 , 73 , 74 , 75 .
Concerns that genome-wide classification tools might prove impractical for the routine diagnosis of brain tumours have been dispelled by the successful large-scale methylome subtyping of formalin-fixed, paraffin-embedded tumours 75 , 76 . Therefore, the challenge to the brain tumour community is not whether but how to deploy diagnostic genomic profiling. In the UK, the centralization of genomic profiling through the National Health Service provides an enormous opportunity to begin this process at a population level. In doing so, it will be important to note that not all brain tumours are created equally: paediatric medulloblastomas and ependymomas comprise robust subgroups with distinct characteristics. However, the degree of diagnostic separation of other brain tumours, including gliomas in adults, is less clear.
The genomic classification of brain tumours will increase the level of diagnostic precision that is currently achievable, although intratumoural heterogeneity and the often limited amounts of tumour material available for analysis are likely to confound the accurate diagnosis of some tumours 68 , 77 . Therefore, the research community should aim to urgently investigate the use of more advanced imaging techniques, such as 13 C-hyperpolarized MRI, radiomics and sequencing of cell-free DNA obtained from plasma and/or cerebrospinal fluid, as ancillary approaches to the diagnosis and classification of brain tumours 70 , 78 , 79 , 80 . The challenge of validating and combining these new diagnostic modalities is enormous but also holds the potential to greatly improve the accuracy of brain tumour classification. Implementation of such approaches might also improve the assessment of novel therapies as part of the federal drug approval process. In this regard, the current standards, such as the Response Assessment in Neuro-Oncology criteria 81 , which describe complete and partial drug responses, are of limited value for assessing new treatments of slow-growing tumours, such as low-grade glioma. Early molecular and/or metabolic responses that are predictive of ultimate clinical benefit might, therefore, provide a more accurate means of evaluating the efficacy of treatments.
Once validated, new classification tools, such as those outlined above, must be adopted within the WHO guidelines in order to enable use by the wider community. A comprehensive strategy to achieve this goal should formally include genomic metrics, such as transcriptomic analysis, in WHO diagnosis and classification schemes; involve appropriate training of experienced and newly qualified pathologists in genomic methodologies; be accompanied by appropriate levels of investment in the resources (both capital and personnel) needed to deliver genomic assays routinely in diagnostic pathology labs; and incorporate standardized genomic characterization within both conventional and clinical trial management strategies. Histology will always have a place in the diagnosis of patients, although only when used in concert with advanced molecular and imaging-based diagnostics will this provide a comprehensive prediction of tumour behaviour.
In addition to developing advanced diagnostic approaches, we must also learn how best to integrate these rich data streams if they are to revolutionize the treatment of patients with cancer (Fig. 5 ). Continual, iterative and integrated analysis of these complementary and complex data streams should be made possible by machine learning, artificial intelligence and/or other mathematical and computational approaches. This will require a concerted effort to invent and deploy new analytical approaches and visualization technologies in order to generate a single flow of information that travels with a patient throughout his or her treatment journey, enabling better-informed management decisions and guiding the patient towards the maximum opportunity for cure (Fig. 6 ). Reporting the clinically relevant findings from such large-scale data sets to health-care professionals and patients will require a multidisciplinary approach, supported by appropriate information technology infrastructure, genetic counsellors, medical geneticists and clinical scientists. This longitudinal and integrated approach will also enable the development of the next generation of adaptive precision medicine clinical trials.
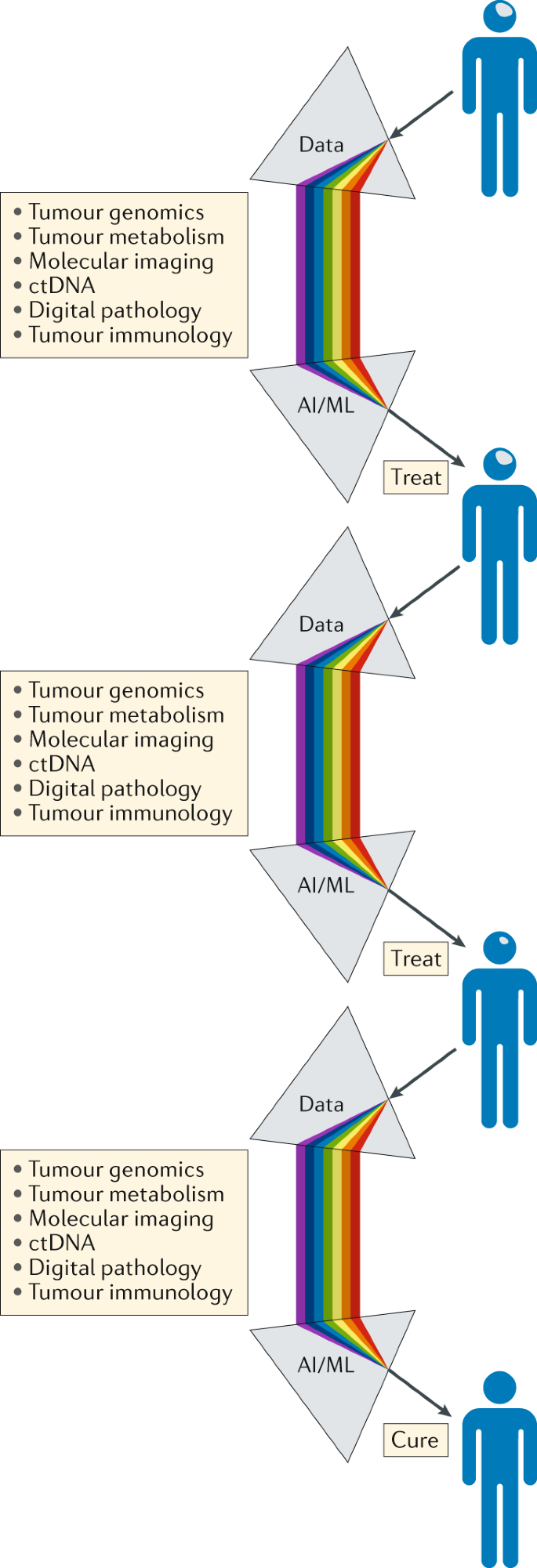
Serving as a ‘biological prism’, the use of artificial intelligence (AI) and machine learning (ML) approaches should enable the integration of separate data streams at critical points in the patient journey, providing an unprecedented level of comprehensive decision-making to guide the selection of the most appropriate treatment and support the management of patients with brain tumours. The knowledge gained through the management of each patient should be analysed iteratively over time, enabling constant refinement and improvements in clinical decision-making. ctDNA, circulating tumour DNA.
Challenge 7: reduce treatment for some
Certain brain tumours, particularly those arising in children, can be cured with aggressive surgery, radiotherapy and chemotherapy, although these cures often carry a considerable cost, particularly for young children who have lifelong neurocognitive and endocrine adverse effects 4 , 82 . Reducing the intensity of treatment in children with other cancers, such as acute lymphoblastic leukaemia, has enabled a reduction in the incidence of adverse effects while maintaining high cure rates; however, this approach has proved challenging to implement in patients with brain tumours, the majority of whom have disease progression following surgery alone. Identifying which patients might be cured with less-intense adjuvant radiotherapy and chemotherapy and to what extent the intensity of these modalities can be safely reduced remains a major challenge.
Medulloblastomas provide a clear example of how a detailed understanding of tumour biology can guide adjustments of treatment intensity. Historical attempts to limit the damaging effects of craniospinal radiotherapy on the developing brain by reducing the radiation dose have failed, largely owing to an inadequate understanding of medulloblastoma biology, which precluded the accurate selection of patients with truly low-risk disease 83 , 84 , 85 . A breakthrough was achieved with the recognition that almost all patients with the WNT subtype of medulloblastoma are cured 12 . This discovery has led to a series of highly selective ongoing studies testing reduced-intensity radiotherapy in patients with this disease subtype (NCT02724579, NCT02066220, NCT02212574 and NCT01878617). Evidence that WNT-subtype tumours (but not other forms of medulloblastoma) lack a BBB and are therefore remarkably vulnerable to systemic chemotherapy provides an explanation for the curability of these tumours, thus opening further options for alternatives to radiation-based treatments 6 . Paediatric low-grade gliomas provide another example of how molecularly guided treatments (such as inhibitors of activated BRAF and MEK) are enabling reductions in radiation dose 86 , 87 . Studies involving patients with other brain tumours are likely to identify similar approaches for the rational de-escalation of therapy.
A comprehensive approach to preventing and ameliorating the adverse effects associated with long-term treatment should include prospective functional and patient-reported outcome measures, as well as innovative predictive assessments of risk. Novel ways of supplementing these data are also emerging in the form of genetic polymorphisms and somatic genomic characteristics that might predict the risk of subsequent intellectual disability and/or hearing loss 88 , 89 .
Conclusions
The past 15 years have witnessed a revolution in our understanding of cancer. The integration of genomic and developmental biology has shown that morphologically similar cancers comprise discrete subtypes, driven by different genetic alterations, which likely arise from distinct cell lineages. These data help to explain why cancers once regarded as histologically homogeneous diseases have a discrepant range of characteristics. Improved understanding is also leading to the development of completely new treatment approaches for cancer, such as immunotherapies, and novel ways to test such therapies, such as adaptive trial designs. However, the successes achieved with these improvements have not occurred equally across all forms of cancer. Of particular note, the treatment of most childhood and adult brain tumours is at an impasse, with no new, more effective therapies being developed in the past 30 years. Thus, all available evidence suggests that the current preclinical and clinical research approaches to curing brain tumours are ineffective.
Herein, we have summarized seven major challenges to progress that must be overcome if we are to produce the sea change in brain tumour therapy that has long been awaited by patients and their families. Some of these challenges might seem to be self-evident; however, each will require a profound change in research and clinical practice and investment. In order to truly redesign the brain tumour research and treatment pipeline and to leverage the full spectrum of neuroscience research, modest improvements in the level of interdisciplinary collaborations among otherwise siloed research groups will not be sufficient. We envisage a much more substantial congregation of experts and infrastructure, focused on the task of curing brain tumours. This call reflects the need to practise brain tumour research differently and to identify, convene and support teams of scientists and clinicians who focus specifically and urgently on the need to improve the lives of patients with brain tumours. This approach must be different from the largely ad hoc and predominantly poorly structured brain tumour collaborations and programmes that currently exist in most academic centres. The harsh reality is that the current efforts at various universities and aligned clinical environments around the world have failed to adequately improve the understanding and treatment of patients with brain tumours. Nevertheless, this approach would probably be easiest to implement within the context of existing academic settings in order to engage with and closely incorporate disciplines not traditionally involved in brain tumour research, such as engineering, chemistry, physics and mathematics.
As we seek to better understand the role of the TME and aim to develop more predictive preclinical model systems, we need to take a long, hard look at the current model systems. Many of these clearly do not adequately reflect all the nonmalignant and malignant cell types that compose brain tumours and have failed to accurately predict treatments that will be effective for testing in clinical trials. Only systems that are proven to model clinically relevant aspects of tumour biology should be used as evidence to guide the initiation of clinical trials. Furthermore, through reverse translation, we should refine the use of or entirely remove models that fail to predict clinical efficacy.
Concerns relating to safety and patient acceptability limit the ability to obtain repeat biopsy samples from brain tumours in most patients, thus preventing longitudinal studies of tumour biology and treatment response and the deployment of a precision medicine approach. The use of liquid biopsies and advanced imaging approaches should be explored immediately to provide additional approaches to determine tumour response earlier in the course of treatment. Finally, the challenge most likely to yield patient benefit in the immediate future will be the use of advanced stratification approaches to reduce the risk of treatment-related toxicities, especially among children.
Gilbert, M. R. et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 370 , 699–708 (2014).
Article CAS PubMed PubMed Central Google Scholar
Chinot, O. L. et al. Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 370 , 709–722 (2014).
Article CAS PubMed Google Scholar
Smith, M. A. & Reaman, G. H. Remaining challenges in childhood cancer and newer targeted therapeutics. Pediatr. Clin. North Am. 62 , 301–312 (2015).
Article PubMed Google Scholar
Brinkman, T. M. et al. Long-term neurocognitive functioning and social attainment in adult survivors of pediatric CNS tumors: results from the St Jude Lifetime Cohort Study. J. Clin. Oncol. 34 , 1358–1367 (2016).
Chemaitilly, W., Armstrong, G. T., Gajjar, A. & Hudson, M. M. Hypothalamic-pituitary axis dysfunction in survivors of childhood CNS tumors: importance of systematic follow-up and early endocrine consultation. J. Clin. Oncol. 34 , 4315–4319 (2016).
Phoenix, T. N. et al. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell 29 , 508–522 (2016).
Gerstner, E. R. & Fine, R. L. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J. Clin. Oncol. 25 , 2306–2312 (2007).
Mackay, A. et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32 , 520–537 (2017).
Quail, D. F. & Joyce, J. A. The microenvironmental landscape of brain tumors. Cancer Cell 31 , 326–341 (2017).
Gilbertson, R. J. Mapping cancer origins. Cell 145 , 25–29 (2011).
Nimmervoll, B. et al. Establishing a preclinical multidisciplinary board for brain tumors. Clin. Cancer Res. 24 , 1654–1666 (2018).
Northcott, P. A. et al. Medulloblastomics: the end of the beginning. Nat. Rev. Cancer 12 , 818–834 (2012).
Chow, S. C. Adaptive clinical trial design. Annu. Rev. Med. 65 , 405–415 (2014).
Alexander, B. M. et al. Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin. Cancer Res. 24 , 737–743 (2018).
Kriegstein, A. & Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32 , 149–184 (2009).
Bjornsson, C. S., Apostolopoulou, M., Tian, Y. & Temple, S. It takes a village: constructing the neurogenic niche. Dev. Cell 32 , 435–446 (2015).
Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432 , 396–401 (2004).
Ignatova, T. N. et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 39 , 193–206 (2002).
Taylor, M. D. et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell 8 , 323–335 (2005).
Calabrese, C. et al. A perivascular niche for brain tumor stem cells. Cancer Cell 11 , 69–82 (2007).
Lathia, J. D., Mack, S. C., Mulkearns-Hubert, E. E., Valentim, C. L. L. & Rich, J. N. Cancer stem cells in glioblastoma. Genes Dev. 29 , 1203–1217 (2015).
Pei, Y. et al. WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Development 139 , 1724–1733 (2012).
Goodrich, L. V., Milenkovic, L., Higgins, K. M. & Scott, M. P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277 , 1109–1113 (1997).
Chen, J. et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488 , 522–526 (2012).
Gibson, P. et al. Subtypes of medulloblastoma have distinct developmental origins. Nature 468 , 1095–1099 (2010).
Johnson, R. A. et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature 466 , 632–636 (2010).
Parker, M. et al. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature 506 , 451–455 (2014).
Mohankumar, K. M. et al. An in vivo screen identifies ependymoma oncogenes and tumor-suppressor genes. Nat. Genet. 47 , 878–887 (2015).
Tirosh, I. et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature 539 , 309–313 (2016).
Article PubMed PubMed Central CAS Google Scholar
Abbott, A. Is ‘friendly fire’ in the brain provoking Alzheimer’s disease? Nature 556 , 426–428 (2018).
Sampson, J. H., Maus, M. V. & June, C. H. Immunotherapy for brain tumors. J. Clin. Oncol. 35 , 2450–2456 (2017).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19 , 1423–1437 (2013).
Griveau, A. et al. A glial signature and Wnt7 signaling regulate glioma-vascular interactions and tumor microenvironment. Cancer Cell 33 , 874–889 (2018).
Louveau, A., Harris, T. H. & Kipnis, J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 36 , 569–577 (2015).
Tivnan, A., Heilinger, T., Lavelle, E. C. & Prehn, J. H. M. Advances in immunotherapy for the treatment of glioblastoma. J. Neurooncol. 131 , 1–9 (2017).
Zacharakis, N. et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 24 , 724–730 (2018).
Graeber, M. B., Scheithauer, B. W. & Kreutzberg, G. W. Microglia in brain tumors. Glia 40 , 252–259 (2002).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330 , 841–845 (2010).
Hambardzumyan, D., Gutmann, D. H. & Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 19 , 20 (2015).
Article CAS Google Scholar
Razavi, S.-M. et al. Immune evasion strategies of glioblastoma. Front. Surg. 3 , 11 (2016).
Article PubMed PubMed Central Google Scholar
Bowman, R. L. & Joyce, J. A. Therapeutic targeting of tumor-associated macrophages and microglia in glioblastoma. Immunotherapy 6 , 663–666 (2014).
Weller, M. et al. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat. Rev. Neurol. 13 , 363–374 (2017).
Campbell, B. B. et al. Comprehensive analysis of hypermutation in human cancer. Cell 171 , 1042–1056 (2017).
Banks, W. A. From blood–brain barrier to blood–brain interface: new opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 15 , 275 (2016).
Larochelle, C. et al. EGFL7 reduces CNS inflammation in mouse. Nat. Commun. 9 , 819 (2018).
Hawkins, B. T. & Davis, T. P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 57 , 173–185 (2005).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468 , 562–566 (2010).
Abbott, N. J., Ronnback, L. & Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7 , 41–53 (2006).
Vanlandewijck, M. et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554 , 475–480 (2018).
Kung, Y. et al. Focused shockwave induced blood-brain barrier opening and transfection. Sci. Rep. 8 , 2218 (2018).
Pajtler, K. W. et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell 27 , 728–743 (2015).
Jacus, M. O. et al. Pharmacokinetic properties of anticancer agents for the treatment of central nervous system tumors: update of the literature. Clin. Pharmacokinet. 55 , 297–311 (2016).
Carpentier, A. et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl Med. 8 , 343re342 (2016).
Rosso, L. et al. A new model for prediction of drug distribution in tumor and normal tissues: pharmacokinetics of temozolomide in glioma patients. Cancer Res. 69 , 120–127 (2009).
Hubert, C. G. et al. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 76 , 2465–2477 (2016).
Toledo, C. M. et al. Genome-wide CRISPR-Cas9 screens reveal loss of redundancy between PKMYT1 and WEE1 in glioblastoma stem-like cells. Cell Rep. 13 , 2425–2439 (2015).
Atkinson, J. M. et al. An integrated in vitro and in vivo high-throughput screen identifies treatment leads for ependymoma. Cancer Cell 20 , 384–399 (2011).
Housden, B. E. et al. Improved detection of synthetic lethal interactions in Drosophila cells using variable dose analysis (VDA). Proc. Natl Acad. Sci. USA 114 , E10755–E10762 (2017).
Pitter, K. L. et al. Corticosteroids compromise survival in glioblastoma. Brain 139 , 1458–1471 (2016).
Krueger, D. A. et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N. Engl. J. Med. 363 , 1801–1811 (2010).
Kieran, M. W. et al. CNS tumours: the first study of dabrafenib in pediatric patients with BRAF V600–mutant relapsed or refractory low-grade gliomas [abstract]. Ann. Oncol. 27 (Suppl. 6), LBA19_PR (2016).
Google Scholar
Singh, D. et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 337 , 1231–1235 (2012).
Northcott, P. A. et al. Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma. Nature 511 , 428–434 (2014).
Schwartzentruber, J. et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482 , 226–231 (2012).
Wu, X. et al. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature 482 , 529–533 (2012).
Morrissy, A. S. et al. Divergent clonal selection dominates medulloblastoma at recurrence. Nature 529 , 351–357 (2016).
Wang, J. et al. Clonal evolution of glioblastoma under therapy. Nat. Genet. 48 , 768–776 (2016).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344 , 1396–1401 (2014).
Darmanis, S. et al. Single-cell RNA-seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 21 , 1399–1410 (2017).
Brindle, K. M., Izquierdo-Garcia, J. L., Lewis, D. Y., Mair, R. J. & Wright, A. J. Brain tumor imaging. J. Clin. Oncol. 35 , 2432–2438 (2017).
Fowler, D. M. & Fields, S. Deep mutational scanning: a new style of protein science. Nat. Methods 11 , 801 (2014).
Louis, D. N. et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131 , 803–820 (2016).
Pajtler, K. W. et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 133 , 5–12 (2017).
Aldape, K., Zadeh, G., Mansouri, S., Reifenberger, G. & von Deimling, A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 129 , 829–848 (2015).
Sturm, D. et al. New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell 164 , 1060–1072 (2016).
Capper, D. et al. DNA methylation-based classification of central nervous system tumours. Nature 555 , 469–474 (2018).
Reinartz, R. et al. Functional subclone profiling for prediction of treatment-induced intratumor population shifts and discovery of rational drug combinations in human glioblastoma. Clin. Cancer Res. 23 , 562–574 (2017).
Day, S. E. et al. Detecting response of rat C6 glioma tumors to radiotherapy using hyperpolarized [1–13C]pyruvate and 13C magnetic resonance spectroscopic imaging. Magn. Reson. Med. 65 , 557–563 (2011).
Wan, J. C. M. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17 , 223–238 (2017).
Pentsova, E. I. et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J. Clin. Oncol. 34 , 2404–2415 (2016).
Wen, P. Y. et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 28 , 1963–1972 (2010).
Moxon-Emre, I. et al. Intellectual outcome in molecular subgroups of medulloblastoma. J. Clin. Oncol. 34 , 4161–4170 (2016).
Duffner, P. K. et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N. Engl. J. Med. 328 , 1725–1731 (1993).
Packer, R. J. et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children’s Cancer Group Study. J. Clin. Oncol. 17 , 2127–2136 (1999).
Geyer, J. R. et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J. Clin. Oncol. 23 , 7621–7631 (2005).
Ater, J. L. et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J. Clin. Oncol. 30 , 2641–2647 (2012).
Krishnatry, R. et al. Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: a population-based study. Cancer 122 , 1261–1269 (2016).
Cole, P. D. et al. Polymorphisms in genes related to oxidative stress are associated with inferior cognitive function after therapy for childhood acute lymphoblastic leukemia. J. Clin. Oncol. 33 , 2205–2211 (2015).
Xu, H. et al. Common variants in ACYP2 influence susceptibility to cisplatin-induced hearing loss. Nat. Genet. 47 , 263–266 (2015).
Lim, D. A. & Alvarez-Buylla, A. The adult ventricular–subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb. Perspect. Biol. 8 , a018820 (2016).
Download references
Acknowledgements
R.J.G. gratefully acknowledges financial support from the US NIH (grants P01CA96832 and R0CA1129541), Cancer Research UK, the Mathile Family Foundation, Cure Search and the Sohn Foundation.
Reviewer information
Nature Reviews Clinical Oncology thanks D. Reardon and other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and affiliations.
Department of Pathology, University Health Network, Toronto, Ontario, Canada
Kenneth Aldape
CRUK Cambridge Institute, Li Ka Shing Centre, Cambridge, UK
Kevin M. Brindle & Richard J. Gilbertson
The Institute of Cancer Research, London, UK
Louis Chesler, Rajesh Chopra & Paul Workman
Department of Oncology, St Jude Children’s Research Hospital, Memphis, TN, USA
Amar Gajjar & Giles W. Robinson
Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA
Mark R. Gilbert
Telethon Kids Institute, Subiaco, WA, Australia
Nicholas Gottardo
Department of Neurology, Washington University School of Medicine, St Louis, MO, USA
David H. Gutmann
Great Ormond Street Hospital for Children, London, UK
Darren Hargrave
Human Biology Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA
Eric C. Holland
Pediatric Glioma Research Group, Hopp Children’s Cancer Center at the NCT Heidelberg, Heidelberg, Germany
David T. W. Jones
Ludwig Institute for Cancer Research, University of Lausanne, Lausanne, Switzerland
Johanna A. Joyce
Cancer Research UK Clinical Trials Unit, Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, UK
Pamela Kearns
Dana–Farber/Boston Children’s Cancer and Blood Disorders Center and Harvard Medical School, Boston, MA, USA
Mark W. Kieran
Human Oncology and Pathogenesis Program and Department of Neurology, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Ingo K. Mellinghoff
Oncology, AstraZeneca IMED Biotech Unit, Boston, MA, USA
Melinda Merchant
Division of Pediatric Oncology, Hopp Children’s Cancer Center at the NCT Heidelberg, Heidelberg, Germany
Stefan M. Pfister
Cancer Research UK Edinburgh Centre and Medical Research Council Centre for Regenerative Medicine, University of Edinburgh, Edinburgh, UK
Steven M. Pollard
Department of Paediatrics, The Hospital for Sick Children, Toronto, Ontario, Canada
Vijay Ramaswamy
Division of Regenerative Medicine, Department of Medicine, University of California, San Diego, CA, USA
Jeremy N. Rich
Department of Paediatrics, University of Cambridge and Wellcome Trust-MRC Stem Cell Institute, Cambridge, UK
David H. Rowitch
The Preston Robert Tisch Brain Tumor Center, Duke Cancer Center, Durham, NC, USA
John H. Sampson
The Arthur and Sonia Labatt Brain Tumour Research Centre and Division of Neurosurgery, The Hospital for Sick Children, Toronto, Ontario, Canada
Michael D. Taylor
CRUK Cambridge Institute and Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre, Cambridge Biomedical Campus, Cambridge, UK
Richard J. Gilbertson
You can also search for this author in PubMed Google Scholar
Contributions
All authors made substantial contributions to researching data for the article, to discussions of content and to writing the manuscript. R.J.G. reviewed and edited the manuscript before submission.
Corresponding author
Correspondence to Richard J. Gilbertson .
Ethics declarations
Competing interests.
P.W. and R.C. are employees of The Institute of Cancer Research (ICR), which has a commercial interest in a range of drug targets. The ICR operates a Rewards to Inventors scheme whereby employees of the ICR may receive financial benefit following commercial licensing of a project. P.W. is a consultant/scientific advisory board member for NextechInvest, Storm Therapeutics, Astex Pharmaceuticals and CV6 and holds stock in Chroma Therapeutics, NextInvest and Storm Therapeutics; he is also a Non-Executive Director of Storm Therapeutics and the Royal Marsden NHS Trust and a Director of the non-profit Chemical Probes Portal. R.C. is an advisor to Syncona Limited and holds equity in Celgene Corporation, e-Therapeutics and Monte Rosa Therapeutics. The other authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Human glioblastoma cell culture (HGCC) resource: http://www.hgcc.se/#about
ITCC Paediatric Preclinical Proof of concept Platform: http://www.itccp4.eu
Stand Up to Cancer (SU2C) Canada Cancer Stem Cell Dream Team: http://www.standuptocancer.ca/en/dream_teams/view/cancer_stem_cell_dream_team
UK glioma cellular genetics resource (GCGR): https://www.ed.ac.uk/cancer-centre/research/s-pollard-group/edinburghbraincancer/about-us
US National Cancer Institute RAS Initiative: https://www.cancer.gov/research/key-initiatives/ras
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Aldape, K., Brindle, K.M., Chesler, L. et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol 16 , 509–520 (2019). https://doi.org/10.1038/s41571-019-0177-5
Download citation
Published : 07 February 2019
Issue Date : August 2019
DOI : https://doi.org/10.1038/s41571-019-0177-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Microglia and macrophage metabolism: a regulator of cerebral gliomas.
- Qinyan Chen
Cell & Bioscience (2024)
Patient-derived organoids in human cancer: a platform for fundamental research and precision medicine
- Shanqiang Qu
- Rongyang Xu
- Guanglong Huang
Molecular Biomedicine (2024)
ZDHHC5-mediated S-palmitoylation of FAK promotes its membrane localization and epithelial-mesenchymal transition in glioma
- Zhangjie Wang
Cell Communication and Signaling (2024)
Artificial intelligence in neuro-oncology: advances and challenges in brain tumor diagnosis, prognosis, and precision treatment
- Sirvan Khalighi
- Kartik Reddy
- Malak Abedalthagafi
npj Precision Oncology (2024)
Redox-responsive polymer micelles co-encapsulating immune checkpoint inhibitors and chemotherapeutic agents for glioblastoma therapy
- Zhiqi Zhang
- Xiaoxuan Xu
- Shenghong Ju
Nature Communications (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Clear as a bell

Women who follow Mediterranean diet live longer

Harvard-led study IDs statin that may block pathway to some cancers
‘Dramatic’ inroads against aggressive brain cancer
Cutting-edge therapy shrinks tumors in early glioblastoma trial
Haley Bridger
Mass General Communications
A collaborative project to bring the promise of cell therapy to patients with a deadly form of brain cancer has shown dramatic results among the first patients to receive the novel treatment.
In a paper published Wednesday in The New England Journal of Medicine, researchers from Mass General Cancer Center shared the results for the first three patient cases from a Phase 1 clinical trial evaluating a new approach to CAR-T therapy for glioblastoma.
Just days after a single treatment, patients experienced dramatic reductions in their tumors, with one patient achieving near-complete tumor regression. In time, the researchers observed tumor progression in these patients, but given the strategy’s promising preliminary results, the team will pursue strategies to extend the durability of response.
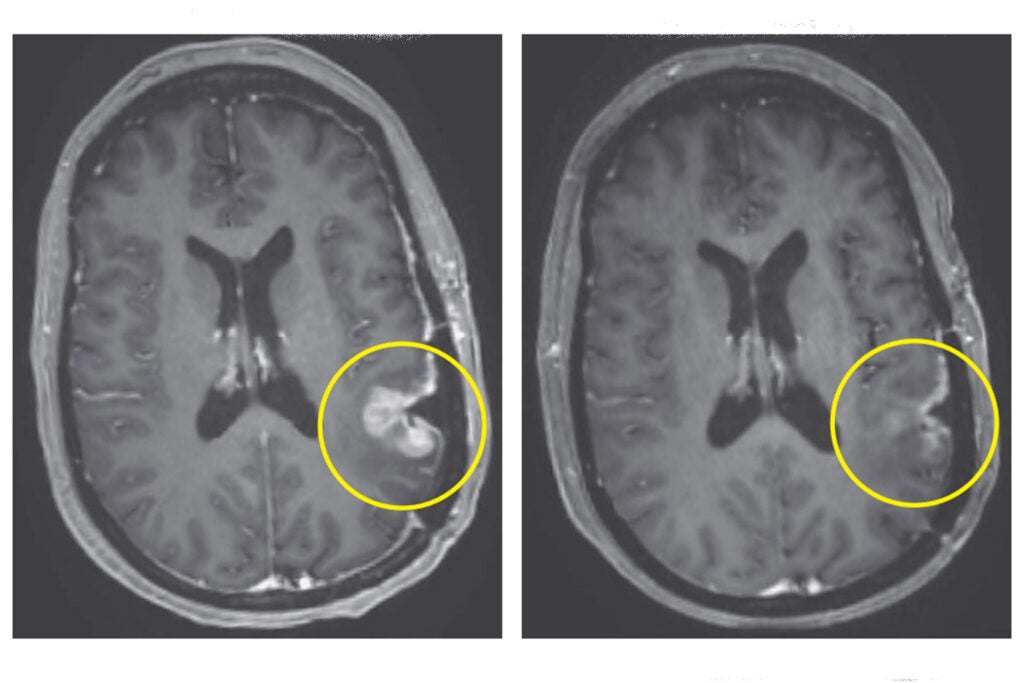
Left: MRI in Participant 3 before infusion. Right: After infusion on day five.
Image courtesy of The New England Journal of Medicine
“This is a story of bench-to-bedside therapy, with a novel cell therapy designed in the laboratories of Massachusetts General Hospital and translated for patient use within five years, to meet an urgent need,” said co-author Bryan Choi , a neurosurgeon at Harvard-affiliated Mass General and an assistant professor at Harvard Medical School. “The CAR-T platform has revolutionized how we think about treating patients with cancer, but solid tumors like glioblastoma have remained challenging to treat because not all cancer cells are exactly alike and cells within the tumor vary. Our approach combines two forms of therapy, allowing us to treat glioblastoma in a broader, potentially more effective way.”
The new approach is a result of years of collaboration and innovation springing from the lab of Marcela Maus , director of the Cellular Immunotherapy Program and an associate professor at the Medical School. Maus’ lab has set up a team of collaborating scientists and expert personnel to rapidly bring next-generation genetically modified T cells from the bench to clinical trials in patients with cancer.
“We’ve made an investment in developing the team to enable translation of our innovations in immunotherapy from our lab to the clinic, to transform care for patients with cancer,” said Maus. “These results are exciting, but they are also just the beginning — they tell us that we are on the right track in pursuing a therapy that has the potential to change the outlook for this intractable disease. We haven’t cured patients yet, but that is our audacious goal.”
CAR-T (chimeric antigen receptor T-cell) therapy works by using a patient’s own cells to fight cancer — it is known as the most personalized way to treat the disease. A patient’s cells are extracted, modified to produce proteins on their surface called chimeric antigen receptors, and then injected back into the body to target the tumor directly. Cells used in this study were manufactured by the Connell and O’Reilly Families Cell Manipulation Core Facility of the Dana-Farber/Harvard Cancer Center.
CAR-T therapies have been approved for the treatment of blood cancers, but the therapy’s use for solid tumors is limited. Solid tumors contain mixed populations of cells, allowing some malignant cells to continue to evade the immune system’s detection even after treatment with CAR-T. Maus’ team is working to overcome this challenge by combining two previously separate strategies: CAR-T and bispecific antibodies, known as T-cell engaging antibody molecules. The version of CAR-TEAM for glioblastoma is designed to be directly injected into a patient’s brain.
In the new study, the three patients’ T cells were collected and transformed into the new version of CAR-TEAM cells, which were then infused back into each patient. Patients were monitored for toxicity throughout the duration of the study. All patients had been treated with standard-of-care radiation and temozolomide chemotherapy and were enrolled in the trial after disease recurrence.
- A 74-year-old man had his tumor regress rapidly but transiently after a single infusion of the new CAR-TEAM cells.
- A 72-year-old man was treated with a single infusion of CAR-TEAM cells. Two days after receiving the cells, an MRI showed a decrease in the tumor’s size by 18 percent. By day 69, the tumor had decreased by 60 percent, and the response was sustained for more than six months.
- A 57-year-old woman was treated with CAR-TEAM cells. An MRI five days after the infusion showed near-complete tumor regression.
The authors note that despite the remarkable responses among the first three patients, they observed eventual tumor progression in all the cases, though in one case, there was no progression for over six months. Progression corresponded in part with the limited persistence of the CAR-TEAM cells over the weeks following infusion. As a next step, the team is considering serial infusions or preconditioning with chemotherapy to prolong the response.
“We report a dramatic and rapid response in these three patients. Our work to date shows signs that we are making progress, but there is more to do,” said co-author Elizabeth Gerstner, a Mass General neuro-oncologist.
In addition to Choi, Maus, and Gerstner, other authors are Matthew J. Frigault, Mark B. Leick. Christopher W. Mount, Leonora Balaj, Sarah Nikiforow, Bob S. Carter, William T. Curry, and Kathleen Gallagher.
The study was supported in part by the National Gene Vector Biorepository at Indiana University, which is funded under a National Cancer Institute contract.
Share this article
You might like.
New successful, expanded trial of groundbreaking therapy for genetic deafness suggests it may be available relatively soon

Large study shows benefits against cancer, cardiovascular mortality, also identifies likely biological drivers of better health

Cholesterol-lowering drug suppresses chronic inflammation that creates dangerous cascade
Bringing back a long extinct bird
Scientists sequence complete genome of bush moa, offering insights into its natural history, possible clues to evolution of flightless birds

Brain Cancer
Brain Cancer research articles are listed. Brain cancer news covers topics such as diagnosis, brain tumors, chemotherapy, gamma knife technology, brain cancer treatments, glioblastomas, stem cell research, neurosurgery, medicine, genetics, neurology, and other brain research.

Artificial Lymph Node Shows Promise in Cancer Treatment
New Hope for Treating Brain Cancer with Ultrasound
Glioblastoma Drug Resistance Pathway Identified
AI Tool Speeds Up Brain Tumor Classification
Breakthrough in Immunotherapy for Brain Cancer
Nanoparticles Penetrate Blood-Brain Barrier to Treat Cancer
Novel mRNA Vaccine Shows Promise Against Deadly Brain Cancer
Vitamin D Boosts Gut Bacteria for Cancer Immunity
CAR-T Trial Targets Glioblastoma Brain Cancer
Revolutionizing Glioblastoma Treatment
Prolonged Progestogen Use Linked to Brain Tumor Risk
Stress Plays Key Role in Cancer Spread
Pet Ownership Boosts Men’s Empathy Towards Animals

Viagra Shows Promise in Boosting Brain Blood Flow for Dementia Prevention

Intense Exercise May Sabotage Weight Loss Efforts

Pandemic Reveals Unexpected Resilience in Isolated Seniors
Brain tumor detection and classification using machine learning: a comprehensive survey
- Original Article
- Open access
- Published: 08 November 2021
- Volume 8 , pages 3161–3183, ( 2022 )
Cite this article
You have full access to this open access article

- Javaria Amin ORCID: orcid.org/0000-0003-1080-5446 1 , 2 ,
- Muhammad Sharif 2 ,
- Anandakumar Haldorai 3 ,
- Mussarat Yasmin 2 &
- Ramesh Sundar Nayak 4
52k Accesses
120 Citations
Explore all metrics
Brain tumor occurs owing to uncontrolled and rapid growth of cells. If not treated at an initial phase, it may lead to death. Despite many significant efforts and promising outcomes in this domain, accurate segmentation and classification remain a challenging task. A major challenge for brain tumor detection arises from the variations in tumor location, shape, and size. The objective of this survey is to deliver a comprehensive literature on brain tumor detection through magnetic resonance imaging to help the researchers. This survey covered the anatomy of brain tumors, publicly available datasets, enhancement techniques, segmentation, feature extraction, classification, and deep learning, transfer learning and quantum machine learning for brain tumors analysis. Finally, this survey provides all important literature for the detection of brain tumors with their advantages, limitations, developments, and future trends.
Similar content being viewed by others
Convolutional neural networks: an overview and application in radiology
A review on deep learning in medical image analysis
Deep CNN for Brain Tumor Classification
Avoid common mistakes on your manuscript.
Introduction
The central nervous system disseminates sensory information and its corresponding actions throughout the body [ 1 , 2 , 3 ]. The brain, along with the spinal cord, assists in this dissemination. The brain’s anatomy [ 4 ] contains three main parts; brain stem, cerebrum, and cerebellum. The weight of a normal human brain is approximately 1.2–1.4 K, with a volume of 1260 cm 3 (male brain) and 1130 cm 3 (female brain) [ 5 ]. The frontal lobe of brain assists in problem-solving, motor control, and judgments. The parietal lobe manages body position. The temporal lobe controls memory and hearing functions, and occipital lobe supervises the brain’s visual processing activities. The outer part of cerebrum is known as cerebral cortex, and is a greyish material; it is composed of cortical neurons [ 6 ]. The cerebellum is relatively smaller than the cerebrum. It is responsible for motor control, i.e., systematic regulation of voluntary movements in living organisms with a nervous system. Due to variable size and stroke territory, ALI, lesionGnb, and LINDA methods fail to detect the small lesion region. Cerebellum is well-structured and well-developed in human beings as compared to other species [ 7 ]. The cerebellum has three lobes; an anterior, a posterior, and a flocculonodular. A round-shaped structure named vermis connects the anterior and posterior lobes. The cerebellum consists of an inner area of white matter (WM) and an outer greyish cortex, which is a bit thinner than that of the cerebrum. The anterior and posterior lobes assist in the coordination of complex motor movements. The flocculonodular lobe maintains the body’s balance [ 4 , 8 ]. The brain stem, as the name states, is a 7–10 cm-long stem-like structure. It contains cranial and peripheral nerve bundles and assists in eye movements and regulations, balance and maintenance, and some essential activities such as breathing. The nerve tracks originating from the cerebrum’s thalamus pass through the brain stem to reach the spinal cord. From there, they spread throughout the body. The main parts of the brain stem are midbrain, pons, and medulla. The midbrain assists in functions such as motor, auditory, and visual processing, as well as eye movements. The pons assists in breathing, intra-brain communication, and sensations, and medulla oblongata helps in blood regulation, swallowing, sneezing, etc. [ 9 ].
Brain tumor and stroke lesions
Brain tumors are graded as slow-growing or aggressive [ 2 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 ]. A benign (slow-growing) tumor does not invade the neighboring tissues; in contrast, a malignant (aggressive) tumor propagates itself from an initial site to a secondary site [ 16 , 17 , 21 , 22 , 23 , 24 , 25 , 26 , 27 ]. According to WHO, a brain tumor is categorized into grades I–IV. Grades I and II tumors are considered as slow-growing, whereas grades III and IV tumors are more aggressive, and have a poorer prognosis [ 28 ]. In this regard, the detail of brain tumor grades is as follows.
Grade I : These tumors grow slowly and do not spread rapidly. These are associated with better odds for long-term survival and can be removed almost completely by surgery. An example of such a tumor is grade 1 pilocyticastrocytoma.
Grade II : These tumors also grow slowly but can spread to neighboring tissues and become higher grade tumors. These tumors can even come back after surgery. Oligodendroglioma is a case of such a tumor.
Grade III : These tumors develop at a faster rate than grade II, and can invade the neighboring tissues. Surgery alone is insufficient for such tumors, and post-surgical radiotherapy or chemotherapy is recommended. An example of such a tumor is anaplastic astrocytoma.
Grade IV : These tumors are the most aggressive and are highly spreadable. They may even use blood vessels for rapid growth. Glioblastoma multiforme is such a type of tumor [ 29 ].
Ischemic stroke : Ischemic stroke is an aggressive disease of brain and it is major cause of disability and death around the globe [ 30 ]. An ischemic stroke occurs when the blood supply to the brain is cut off, resulting underperfusion (in tissue hypoxia) and dead the advanced tissues in hours [ 31 ]. Based on the severity, stroke lesions are categories into different stages such as acute (0–24 h), sub-acute (24 h–2 weeks) and chronic (> 2 weeks) [ 32 ].
- Brain imaging modalities
Three major methods (PET, CT, DWI and MRI) for brain tumors are widely used to analyze the brain structure.
Positron emission tomography
Positron emission tomography (PET) uses a special type of radioactive tracers. Metabolic brain tumor features such as blood flow, glucose metabolism, lipid synthesis, oxygen consumption, and amino acid metabolism are analyzed through PET. It is still considered as one of the most powerful metabolic techniques and utilizes the best nuclear medicine named as fluorodeoxyglucose (FDG) [ 33 ]. FDG is a widely used PET tracer in brain images. Nevertheless, FDG-PET images have limitations, e.g., an inability to differentiate between necrosis radiation and a recurrent high-grade (HG) tumor [ 34 ]. Moreover, during a PET scan, radioactive tracers can cause harmful effects to the human body, causing a post-scan allergic reaction. Some patients are allergic to aspartame and iodine. In addition, PET tracers do not provide accurate localization of anatomical structure, because they have a relatively poor spatial resolution as compared to an MRI scan [ 35 ].
Computed tomography
Computed tomography (CT) images provide more in-depth information than images obtained from normal X-rays. The CT scan has received widespread recommendation and adoption since its inception. A study [ 36 ] determined that in the USA alone, the annual CT scan rate is 62 million, with 4 million for children. CT scans show soft tissues, blood vessels, and bones of different human body parts. It uses more radiation than normal X-rays. This radiation may increase the risk of cancers when multiple CT scans are performed. The associated risks of cancers have been quantified according to CT radiation doses [ 37 , 38 ]. MRI can even help in evaluating structures obscured in a CT scan, and provides high contrast among the soft tissues, providing a clearer anatomical structure [ 39 ].
Magnetic resonance imaging
An MRI scan is used to completely analyze different bodyparts, and it also helps to detect abnormalities in the brain at earlier stages than other imaging modalities [ 40 ]. Hence, complex brain structures make tumor segmentation a challenging task [ 41 , 42 , 43 , 44 , 45 , 46 , 47 ]. This review discusses preprocessing approaches, segmentation techniques [ 48 , 49 ], feature extraction and reduction methods, classification methods, and deep learning approaches. Finally, benchmark datasets and performance measures are presented.
Diffusion weighting imaging
MRI sequences are utilized to analyze the stroke lesions based on the several parameters such as age, location and extent regions [ 50 ]. In the context of treatment, a computerized method might be utilized for accurate diagnosis of the disease progression rate [ 51 ]. The neuroscientists of cognitive, who frequently conduct research in which cerebral impairments are linked to cognitive function They observed that segmentation of the stroke lesions is a vital task to analyze the total infected region of brain that provide help in the treatment process [ 52 ]. However, segmentation of the stroke lesions is a difficult task, because stroke appearance is change as the passage of time. The MRI sequence such as diffusion weighted imaging (DWI) and FLAIR are utilized for stroke lesions detection. In acute stoke stage DWI sequence highlight the infection part as a hyperintensity. The underperfusion region represents the mapping magnitude of the perfusion [ 53 ]. The dis-similarity among two regions might be considered as penumbra tissue. Stroke lesions appear in distinct locations and shapes. Different types of lesions are appeared in a variable size and shape and these lesions are not aligned with vascular patterns and more than one lesions might appeared on similar time. The size of the stroke lesions is in radii of the few millimeters and appears in a full hemisphere. The structure of the hemisphere is dissimilar, and its intensity might significantly vary within the infected region. Furthermore, automated stroke segmentation is difficult due to the similar appearance of the pathology such as white matter hyperintensities and chronic stroke lesions [ 54 ].
Evaluation and validation
In the existing literature, experimental results are evaluated on publicly available datasets to verify the robustness of algorithms.
Publicly available datasets
Several datasets are publicly available that are used by the researchers to evaluate the proposed methods. Some important and challenging datasets are discussed in this section. BRATS are the most challenging MRI datasets [ 55 , 56 , 57 ]. BRATS Challenge is published in different years with more challenges having 1 mm 3 voxels resolution. The detail of datasets is given in Fig. 1 as well as in Table 1 .
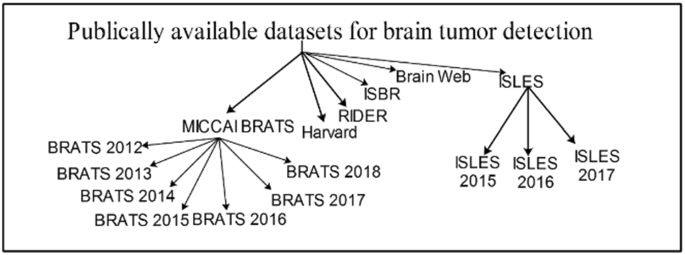
Datasets for brain tumor detection
Performance metrics
The performance measures play a significant role to compute the method’s effectiveness. A list of performance metrics is provided in Fig. 2 .
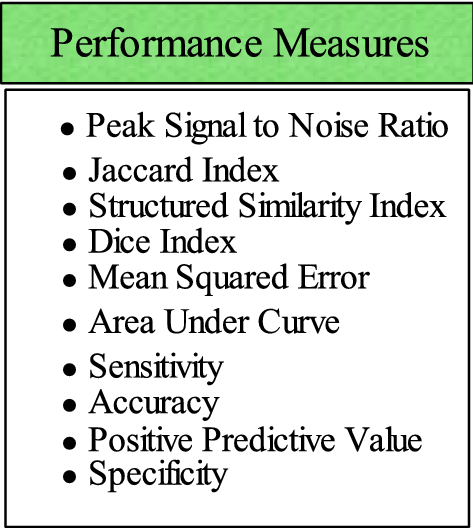
List of performance measures for evaluation of brain tumor
Preprocessing
Preprocessing is a critical task [ 61 ] to extract the requisite region. 2D brain extraction algorithm (BEA) [ 62 ], FMRIB software library [ 63 ], and BSE [ 64 ] are used for non-brain tissue removal as shown in Fig. 3 . The bias field is a key problem that arises in MRI due to imperfections of radio frequency coil called intensity inhomogeneity [ 65 , 66 ]. It is corrected as shown in Fig. 4 [ 67 ]. The preprocessing methods like linear, nonlinear [ 68 ], fixed, multi-scale, and pixel-based are used in distinct circumstances [ 69 , 70 , 71 , 72 ]. The small variations among normal and abnormal tissues due to noise [ 68 ] and artifacts often provide difficulty in direct image analysis [ 73 , 74 ]. AFINITI is used for brain tumor segmentation [ 63 ]. Consequently, automated techniques are adopted in which computer software performs segmentation and eliminates the need for manual human interaction [ 75 , 76 ]. Fully and semi-automated techniques are used widely [ 77 , 78 ]. The results of brain tumor segmentation are mentioned in Table 2 . The segmentation methods are divided into the following categories.
Conventional methods.
Machine learning methods.
Different inhomogeneities related to MRI noise have shading artifacts and partial volume effects.
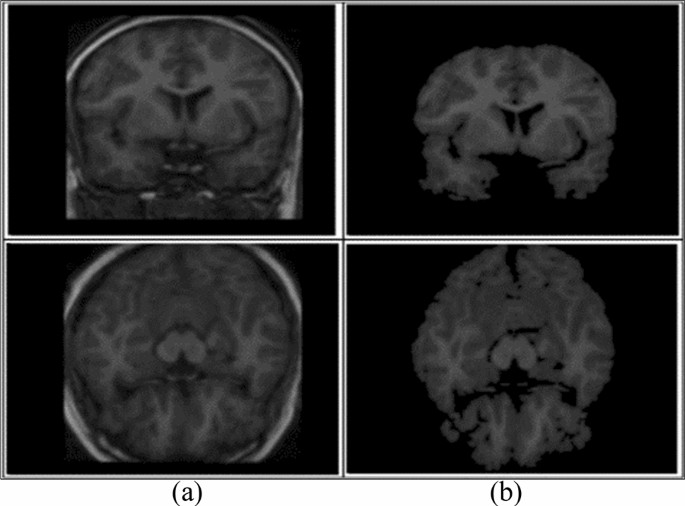
Skull removal a input, b skull removed [ 1 ]
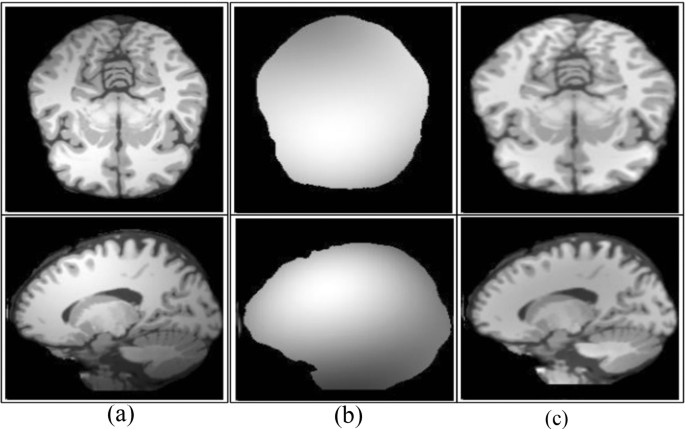
Bias field correction a input, b estimated, c corrected [ 67 ]
When different types of tissues [ 61 ] take the same pixel, then it is called partial volume effect [ 92 ]. The random noise related to MRI [ 19 , 93 , 94 ] has Rician distribution [ 95 ]. In the literature, different filters such as wavelet, anisotropic diffusion, and adaptive are presented to enhance edges [ 96 ]. An anisotropic diffusion filter is more suitable in practical applications due to low computational speed [ 97 , 98 ]. When the noise level is high in the image, it is difficult to recover the edges [ 99 ]. Normalizing the image intensity is another part of the preprocessing phase [ 2 , 100 , 101 ] and modified curvature diffusion equation (MCDE) [ 102 ] are applied for intensity normalization. Wiener filter is used to enhance the local and spatial information in medical imaging [ 103 ]. The widely utilized preprocessing methods are N4ITK [ 104 ] for the correction of bias field, median filter [ 104 ] for image smoothing, anisotropic diffusion filter [ 105 ], image registration [ 106 ], sharpening [ 107 ], and skull stripping through brain extraction tool (BET) [ 108 ].
Conventional methods
The conventional methods [ 46 ] are further categorized into the following:
Thresholding methods.
Region growing methods.
Watershed methods.
- Segmentation
Segmentation extracts the required region from input images. Thus, segmenting accurate lesion regions is a more crucial task [ 109 ]. As manual segmentation process is erroneous [ 110 ]; therefore, semi- and fully automated methods are utilized [ 46 ]. Segmentation of tumor region using semi-automated methods achieves acceptable outcomes over manual segmentation [ 111 , 112 ]. Semi-automated methods are further divided into three forms: initialization, evaluation, and feedback response [ 113 , 114 ].
Thresholding methods
The thresholding method is a basic and powerful method to segment the required objects [ 18 ] and the selection of an optimized threshold is a difficult task in low-contrast images. Histogram analysis is used to select threshold values based on image intensity [ 115 ]. Thresholding methods are classified into local and global. If high homogeneous contrast or intensity exists among the objects and background, then the global thresholding method is the best option for segmentation. The optimal threshold value can be determined by Gaussian distribution method [ 116 ]. These methods are utilized when the threshold value cannot be measured from the whole image histogram or single value of the threshold does not provide good results of segmentation [ 117 ]. In most cases, the thresholding method is applied at the first stage for segmentation and many distinct regions are segmented within the gray-level images as shown in Fig. 5 .
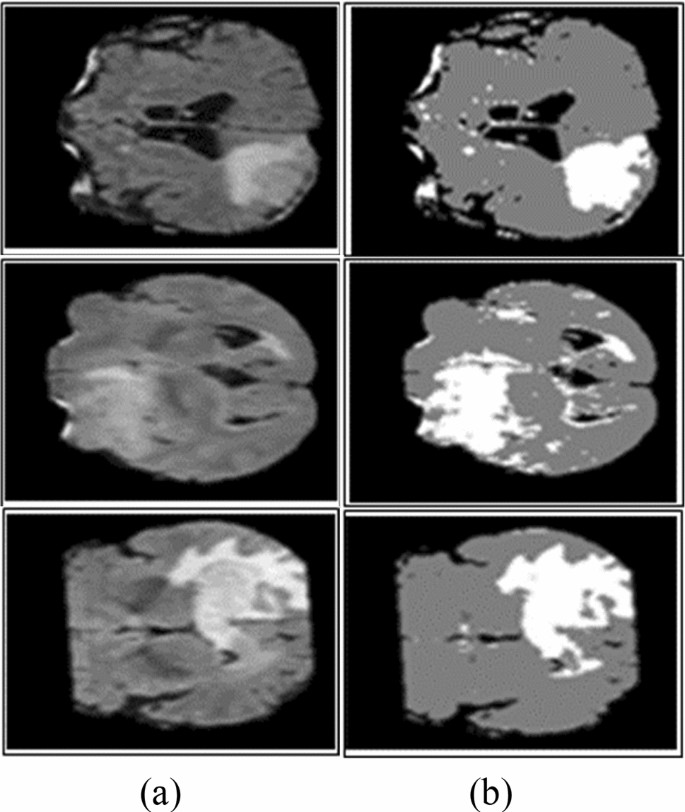
Segmentation using Otsu thresholding a original images, b Otsu thresholding [ 82 ]
Region growing (RG) methods
In RG approaches, image pixels form disjoint areas are analyzed through neighboring pixels, which are merged with homogeneousness characteristics based on pre-defined similitude criteria. The region growing might fail to provide better accuracy due to the partial volume effect [ 118 , 119 ]. To overcome this effect, MRGM is preferred [ 86 , 120 ]. The region growing with BA methods is also introduced [ 87 ].
Watershed methods
As MR images have more proteinaceous fluid intensity, therefore, watershed methods are utilized to analyze the intensity of the image [ 114 , 121 , 122 ]. Due to noise [ 123 ], watershed method leads to over-segmentation [ 124 ]. The accurate segmentation [ 125 ] results can be obtained by the combination of watershed transform with the merging of statistical methods [ 126 , 127 ]. Some watershed algorithms are topological watershed [ 128 ], image foresting transform (IFT) watershed [ 129 ], and marker-based watershed [ 130 ].
The comprehensive literature review [ 131 ] on brain tumor detection shows that there is room for improvement [ 72 ]. As a brain tumor appears in variable sizes and shapes, existing segmentation approaches require additional improvements for tumor segmentation. In overcoming the limitations of existing methods, enhancement [ 132 , 133 , 134 ] and segmentation [ 135 , 136 , 137 ] have significance in tumor detection.
Feature extraction methods
The feature extraction approaches [ 12 , 138 , 139 , 140 ] including GLCM [ 15 , 141 , 142 ], geometrical features (area, perimeter, and circularity) [ 15 ], first-order statistical (FOS), GWT [ 143 , 144 ], Hu moment invariants (HMI) [ 145 ], multifractal features [ 146 ], 3D Haralick features [ 147 ], LBP [ 148 ], GWT [ 11 ], HOG [ 14 , 137 ], texture and shape [ 82 , 143 , 149 , 150 ], co-occurrence matrix, gradient, run-length matrix [ 151 ], SFTA, curvature features [ 152 , 153 ], Gabor like multi-scale texton features [ 154 ], Gabor wavelet and statistical features [ 142 , 143 ] are utilized for classification. Table 3 lists the summary of feature extraction methods.
Feature selection methods or feature selection/reduction methods
In machine learning and computer vision applications, high-dimensional features maximize the system execution time and memory requirement for processing. Therefore, to distinguish between relevant and non-relevant features, several feature selection methods are required to minimize redundant information [ 168 ]. The optimal feature extraction is still a challenging task [ 47 ]. The single-point heuristic search method, ILS, genetic algorithm (GA) [ 169 ], GA+ fuzzy rough set [ 170 ], hybrid wrapper-filter [ 171 ], TRSFFQR, tolerance rough set (TRS), firefly algorithm (FA) [ 172 ], minimum redundancy maximum relevance (mRMR) [ 152 ], Kullback–Leibler divergence measure [ 173 ], iterative sparse representation [ 174 ], recursive feature elimination (RFE) [ 175 ], CSO-SIFT [ 176 ], entropy [ 11 , 177 , 178 ], PCA [ 179 ], and LDA [ 180 ] are utilized to remove redundant features. A summary of classification methods as shown in Table 4 .
Classification methods
The classification approaches are used to categorize input data into different classes in which training and testing are performed on known and unknown samples [ 16 , 24 , 25 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 ]. Machine learning is widely used for tumor classification into appropriate classes, e.g., tumor substructure (complete/non-enhanced/enhanced) [ 193 ], tumor and non-tumor [ 26 ], and benign and malignant tumor [ 15 , 47 , 163 , 194 , 195 ]. KNN [ 196 ], SVM, nearest subspace classifier, and representation classifier [ 143 ] are supervised, whereas FCM [ 197 , 198 ], hidden Markova random field [ 199 ] self-organization map [ 101 ], and SSAE [ 200 ] are unsupervised methods.
Recent trends in medical imaging to detect malignancy
Deep learning and quantum machine learning methodologies are widely utilized for tumor localization and classification [ 201 ]. In these techniques, automatic feature learning helps to discriminate complicated patterns [ 186 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 ].

Deep learning methods
The variety of state of the art deep learning methodologies are used to learn the data in the medical domain [ 214 ] including CNN [ 215 , 216 ], Deep CNN, cascaded CNN [ 217 ], 3D-CNN [ 218 ], convolutional encoder network, LSTM, CRF [ 218 ], U-Net CNN [ 219 ], dual-force CNN [ 220 ] and WRN-PPNet [ 221 ].
The brain tumor classification problem has been solved by employing a LSTM model. In this method, input MRI images smooth using N4ITK and 5 × 5 Gaussian filter and passed as input to the four LSTM model. The LSTM model is constructed on the four hidden Units such as 200, 225, 200, 225, respectively. The performance of this model has been tested on BRATS (2012–2015 and 2018) series and SISS-2015 benchmark datasets [ 222 ]. In this work, a new framework is presented based on the fusion of different kinds of MRI sequences. The fused sequence provides more information as compared to single sequence. Later, fused sequence has been supplied to the 23 CNN model. The suggested model is trained on brat’s series for the detection of glioma [ 16 ]. The 14 layers CNN model has been trained from the scratch on six Brats series datasets for detection of glioma and stroke lesions [ 25 ]. The classification is performed using ELM and RELM classifiers. This method has been tested on BRATS series such as 2012 to 2015 [ 189 ]. The 09-layer CNN model is trained from the scratch for classification of different types of tumors such as pituitary, glioma and meningioma. The method achieved an accuracy of the classification is 98.71% [ 223 ]. This model is trained from the scratch on publicly 696 weighted-T1 sequences. The model provides an accuracy of greater than 99% for tumor classification [ 224 ]. The existing methods are summarized in Table 5 .
Although much work is done on deep learning methods, still there exist many challenges. The present methods do not achieve maximum results in the sub-structure of the tumor region. For example, if the accuracy of the complete tumor is increased, then the accuracy of the core and the enhanced tumor is decreased (as shown in Table 5 ).
Brain tumor detection using transfer learning
The manual detection of brain tumors is difficult due to asymmetrical lesions shape, location flexibility, and unclear boundaries. Therefore, a transfer-learning model has been suggested based on the super-pixel. The VGG-19 is a pre-trained model that has been utilized for the classification of the different grades of the glioma such as high/low glioma. The method achieved 0.99 AUC on the brats 2019 series[ 232 ]. The three different types of pre-trained models i.e., VGG network, Google network and Alex network are employed on the brain datasets for the classification of glioma, pituitary and meningioma. In this method, augmentation methods are also employed on MRI slices to generalize the outcomes and reduced the overfitting problem by increasing the quantity of the input data. After the experimental analysis using different pre-trained models, we conclude that VGG-16 provides greater than 98% classification accuracy [ 233 ]. The classification of brain tumors has been done using two different types of networks, i.e., visual attention network and CNN are utilized for classification of different types of brain tumor i.e., glioma, pituitary I and meningioma [ 234 ]. A pre-trained model i.e., VGG-16, Alex and Google net are investigated for the analysis of brain tumors. The frequency domain techniques have been applied on input slices to improve the image contrast. The contrast improved images are passed in the next phase. Where pre-trained VGG-16 provides maximum classification outcomes [ 235 ]. The Laplacian filter with a multi-layered dictionary model is utilized for the recognition of brain tumors. The model performed better as compared to existing works [ 236 ]. The method consists of the three major steps such as pre-processing, augmentation of data, and segmentation and classification using transfer learning models. In which ResNet-50, DenseNet-201, MobileNet-v2 and Inceptionv3 are utilized to classify the brain lesions with 0.95 IoU [ 237 ]. The deep features are extracted from the transfer learning AlexNet model. The model has eight layers, five of which are convolutional and three of which are fully linked. The SoftMax layer has been employed for classification between the different types of brain lesions [ 238 ]. The transfer learning ResNet-50 model with average global pooling is utilized to reduce the gradient vanishing and overfitting issues. The performance of this model has been evaluated on three distinct types of brain imaging benchmark samples that contain 3064 input images. The method achieved an accuracy of the 97.08% that is maximum as compared to latest existing works [ 239 ]. A deep CNN was used in this study that based on transfer learning such as ResNet, Xception and Mobilenetv2 are utilized for the extraction of deep features has been for tumors classification using MRI images. This method achieved an accuracy of up to 98% [ 240 ]. In this method, Grab Cut method has been employed for segmentation of the brain lesions. Later hand-crafted such as LBP features dimension of 1 × 20 and HOG features dimension of 1 × 100 are extracted and serially fused to the deep features dimension of 1 × 1000 that are extracted from the pre-trained VGG-19 model and final fused features vector length of 1 × that is supplied to the different kind of classifiers. The experimental analysis proves that fused features vector provide good results as compared to existing work in this domain [ 16 , 187 ]. The global thresholding method is applied to segment the actual lesion region. After segmentation, texture features such as LBP and GWF are extracted from the segmented images. After that, the retrieved features are fused to form a single fused feature vector, which is then provided to the classifiers for differentiation between healthy and unhealthy images [ 26 ]. There are two key stages to the procedure. The brain lesions are enhanced and segmented using spatial domain approaches in the first stage, then deep information’s are extracted using pre-trained models, i.e., Alex and Google-network and score vector is achieved from softmax layer that is supplied to the classifiers such as for discrimination between the glioma/non-glioma images of brain. The Brats series dataset was used to test this technique’s efficiency [ 241 ]. For brain tumor segmentation, the superpixel approach has been suggested. From the segmented images, Gabor wavelet information are retrieved and given to SVM and CRF for discrimination between the healthy/un-healthy MRI images [ 242 ].The transfer learning models such as inceptionv3, densenet-201, and to form a single vector, extracted features are merged serially and passed to softmax for tumor classification. Furthermore, different dense blocks of the densenet201 are extracted and classify the brain tumor using softmax. The approach had a 99% accuracy rate. The evaluation outcomes clearly state that the fused vector outperformed as compared to the single vector [ 243 ]. A novel U-net model with the RESnet model has been trained on the input MRI images. The classifiers are fed the salient features derived from its pictures. This method has been tested on BRATS 2017, 2018 and 2019 datasets [ 244 ]. The tumor region is localized on Flair sequences of brats 2012 series. The skull is removed from of the input pictures, and a noise-reduction filter is applied bilaterally. During the segmentation, texton features are recovered from the input images using the superpixel approach. For brain tumor classification, the leave out validation technique is used. This strategy yielded an 88 percent dice score [ 245 ]. The deep segmentation has been designed that contains two major parts such as encoder and decoder. The spatial information is extracted using a CNN in the encoder section. For determining the whole probability map resolution, the semantic mappings information is entered into the decoder component. On the basis of U-network distinct CNN networks such as ResNetwork, dense network and Nas-network are utilized for features extraction. This model has been tested successfully on Brats-2019 series. The method achieved dice scores of 0.84 [ 246 ]. The wavelet homomorphic filter has been employed for noise removal. The tumor infected region has been localized using improved YOLOv2 model [ 230 ]. The summary of the transfer learning methods is mentioned in Table 6 .
Brain tumor detection using quantum machine learning
Superposition of quantum states/parallelism/entanglement can all be used to establish quantum computer supremacy [ 258 ]. However, exploring entanglement of quantum features for efficient computation is a difficult undertaking due to a shortage of computational resources for execution of quantum algorithms. With the progress of quantum techniques, classical computers based on quantum theory and influenced through qubits are no longer able to fully exploit the benefits of quantum state and entanglement. QANN has been found to be effective in a variety of computer tasks, including classification and pattern recognition due to the intrinsic properties supplied by quantum physics [ 259 ]. On the other hand, quantum models based on genuine quantum computers use big bits of the quantum/qubits as a simple representation of matrix and the linear functions. However, the computational complexity of the quantum-inspired neural network (QINN) designs increases several fold due to complicated and time-consuming back-propagation quantum model [ 260 ]. The automatic segmentation of brain lesions from I (MRI), which removes the onerous manual work of human specialists or radiologists, greatly aids brain tumor detection. Manually, brain tumor diagnosis, on the other hand, suffers from large variances in size, shape, orientation, illumination variations, greyish overlaying, and cross-heterogeneity. Scientists in the computer vision field have paid a lot of emphasis in recent years to building robust and efficient automated segmentation approaches. The current research focuses on a unique quantum fully supervised learning process which is defined by qutrits for timely and effective lesions segmentation. The proposed work’s main goal is to speed up the QFS-convergence Net’s and make it appropriate for computerized segmentation of the brain lesions without the need for any learning/supervision. To leverage the properties of quantum correlation, suggested a quantum fully self-supervised neural network (QFS-Net) model uses qutrits/three states of quantum for segmentation of the brain lesions [ 261 ]. The QFS-Net uses a revolutionary fully supervised qutrit-based counter propagation method to replace the sophisticated quantum back-propagation method that utilized in supervised QINN networks. This approach allows for iterative quantum state that propagates among the layers of network.
Limitations of existing’s machine/deep learning methods
In this survey, recent literature regarding the detection of brain tumors is reviewed, and it is indicated that there is still room for improvement. During image acquisition, noise is included in MRI, and noise removal is an intricate task [ 2 , 262 , 263 , 264 ]. Accurate segmentation is a difficult task [ 265 ], as brain tumors have tentacles and diffused structures [ 43 , 193 , 220 , 266 ]. Selecting and extracting optimal features and appropriate number of training/testing samples for better classification is also an important task [ 191 , 192 ]. Deep learning models are gaining attention as the learning of features is accomplished automatically; however, they require high computing power and large memory. Therefore, still there is a need to design a lightweight model that provides high ACC in less computational time. Some existing machine learning methods with their limitations are mentioned in Table 7 .
The following are the main challenges of brain tumor detection.
The glioma and stroke tumors are not well contrasted. It consists of tentacle and diffused structures that make segmentation and classification processes more challenging [ 270 ].
A small volume of tumor detection is still a challenge as it can be detected as a normal region [ 269 , 273 ].
Some of the existing methods work well for only a complete tumor region and do not provide good results for other regions (enhanced, non-enhanced) and vice versa [ 267 , 271 , 274 ].
Research findings and discussion
After a comprehensive review of the state-of-the-art exiting methods, the following challenges are found:
The size of a brain tumor grows rapidly. Therefore, tumor diagnosis at an initial stage is an exigent task.
Brain tumor segmentation is difficult owing to the following factors.
MRI image owing to magnetic field fluctuations in the coil.
Gliomas are infiltrative, owing to fuzzy borders. Thus, they become more difficult to segment [ 43 ].
Stroke lesion segmentation is a very intricate task, as stroke lesions appear in complex shapes and with ambiguous boundaries and intensity variations.
The optimized and best feature extraction and selection is another difficult process inaccurate classification of brain tumors.
The accurate brain tumor detection is still very demanding because of tumor appearance, variable size, shape, and structure. Although tumor segmentation methods have shown high potential in analyzing and detecting the tumor in MR images, still many improvements are required to accurately segment and classify the tumor region. Existing work has limitations and challenges for identifying substructures of tumor region and classification of healthy and unhealthy images.
In short, this survey covers all important aspects and latest work done so far with their limitations and challenges. It will be helpful for the researchers to develop an understanding of doing new research in a short time and correct direction.
The deep learning methods have contributed significantly but still require a generic technique. These methods provided better results when training and testing are performed on similar acquisition characteristics (intensity range and resolution); however, a slight variation in the training and testing images directly affects the robustness of the methods. In future work, research can be conducted to detect brain tumors more accurately, using real patient data from any medium (different image acquisition (scanners). Handcrafted and deep features can be fused to improve the classification results. Similarly, lightweight methods such as quantum machine learning play significant role to improve the accuracy and efficacy that save the time of radiologists and increase the survival rate of patients.
Park JG, Lee C (2009) Skull stripping based on region growing for magnetic resonance brain images. Neuroimage 47:1394–1407
Article Google Scholar
Khan MA, Lali IU, Rehman A, Ishaq M, Sharif M, Saba T et al (2019) Brain tumor detection and classification: A framework of marker-based watershed algorithm and multilevel priority features selection. Microsc Res Tech 82:909–922
Raza M, Sharif M, Yasmin M, Masood S, Mohsin S (2012) Brain image representation and rendering: a survey. Res J Appl Sci Eng Technol 4:3274–3282
Google Scholar
Watson C, Kirkcaldie M, Paxinos G (2010) The brain: an introduction to functional neuroanatomy. Academic Press, New York
(2015). https://en.wikipedia.org/wiki/Brain_size . Accessed 19 Oct 2019
Dubin MW (2013) How the brain works. Wiley, New York
Koziol LF, Budding DE, Chidekel D (2012) From movement to thought: executive function, embodied cognition, and the cerebellum. Cerebellum 11:505–525
Knierim J (1997) Neuroscience Online Chapter 5: Cerebellum. The University of Texas Health Science Center, Houston
Nuñez MA, Miranda JCF, de Oliveira E, Rubino PA, Voscoboinik S, Recalde R et al (2019) Brain stem anatomy and surgical approaches. Comprehensive overview of modern surgical approaches to intrinsic brain tumors. Elsevier, Amsterdam, pp 53–105
Chapter Google Scholar
DeAngelis LM (2001) Brain tumors. N Engl J Med 344:114–123
Amin J, Sharif M, Raza M, Saba T, Sial R, Shad SA (2020) Brain tumor detection: a long short-term memory (LSTM)-based learning model. Neural Comput Appl 32:15965–15973
Sajjad S, Hanan Abdullah A, Sharif M, Mohsin S (2014) Psychotherapy through video game to target illness related problematic behaviors of children with brain tumor. Curr Med Imaging 10:62–72
Yasmin M, Sharif M, Masood S, Raza M, Mohsin S (2012) Brain image reconstruction: a short survey. World Appl Sci J 19:52–62
Amin J, Sharif M, Raza M, Yasmin M (2018) Detection of brain tumor based on features fusion and machine learning. J Ambient Intell Human Comput:1–17
Amin J, Sharif M, Yasmin M, Fernandes SL (2020) A distinctive approach in brain tumor detection and classification using MRI. Pattern Recogn Lett 139:118–127
Saba T, Mohamed AS, El-Affendi M, Amin J, Sharif M (2020) Brain tumor detection using fusion of hand crafted and deep learning features. Cogn Syst Res 59:221–230
Sharif M, Amin J, Nisar MW, Anjum MA, Muhammad N, Shad SA (2020) A unified patch based method for brain tumor detection using features fusion. Cogn Syst Res 59:273–286
Sharif M, Tanvir U, Munir EU, Khan MA, Yasmin M (2018) Brain tumor segmentation and classification by improved binomial thresholding and multi-features selection. J Ambient Intell Human Comput:1–20
Sharif MI, Li JP, Khan MA, Saleem MA (2020) Active deep neural network features selection for segmentation and recognition of brain tumors using MRI images. Pattern Recogn Lett 129:181–189
Sharif MI, Li JP, Naz J, Rashid I (2020) A comprehensive review on multi-organs tumor detection based on machine learning. Pattern Recogn Lett 131:30–37
Ohgaki H, Kleihues P (2013) The definition of primary and secondary glioblastoma. Clin Cancer Res 19:764–772
Cachia D, Kamiya-Matsuoka C, Mandel JJ, Olar A, Cykowski MD, Armstrong TS et al (2015) Primary and secondary gliosarcomas: clinical, molecular and survival characteristics. J Neurooncol 125:401–410
Amin J, Sharif M, Gul N, Yasmin M, Shad SA (2020) Brain tumor classification based on DWT fusion of MRI sequences using convolutional neural network. Pattern Recogn Lett 129:115–122
Sharif M, Amin J, Raza M, Yasmin M, Satapathy SC (2020) An integrated design of particle swarm optimization (PSO) with fusion of features for detection of brain tumor. Pattern Recogn Lett 129:150–157
Amin J, Sharif M, Anjum MA, Raza M, Bukhari SAC (2020) Convolutional neural network with batch normalization for glioma and stroke lesion detection using MRI. Cogn Syst Res 59:304–311
Amin J, Sharif M, Raza M, Saba T, Anjum MA (2019) Brain tumor detection using statistical and machine learning method. Comput Methods Progr Biomed 177:69–79
Amin J, Sharif M, Gul N, Raza M, Anjum MA, Nisar MW et al (2020) Brain tumor detection by using stacked autoencoders in deep learning. J Med Syst 44:32
Johnson DR, Guerin JB, Giannini C, Morris JM, Eckel LJ, Kaufmann TJ (2017) 2016 updates to the WHO brain tumor classification system: what the radiologist needs to know. Radiographics 37:2164–2180
Wright E, Amankwah EK, Winesett SP, Tuite GF, Jallo G, Carey C et al (2019) Incidentally found brain tumors in the pediatric population: a case series and proposed treatment algorithm. J Neurooncol 141:355–361
Pellegrino MP, Moreira F, Conforto AB (2021) Ischemic stroke. Neurocritical care for neurosurgeons. Springer, New York, pp 517–534
Garrick R, Rotundo E, Chugh SS, Brevik TA (2021) Acute kidney injury in the elderly surgical patient. Emergency general surgery in geriatrics. Springer, New York, pp 205–227
Lehmann ALCF, Alfieri DF, de Araújo MCM, Trevisani ER, Nagao MR, Pesente FS, Gelinski JR, de Freitas LB, Flauzino T, Lehmann MF, Lozovoy MAB (2021) Carotid intima media thickness measurements coupled with stroke severity strongly predict short-term outcome in patients with acute ischemic stroke: a machine learning study. Metab Brain Dis 36:1747–1761
Scott AM (2005) PET imaging in oncology. In: Bailey DL, Townsend DW, Valk PE, Maisey MN (eds) Positron emission tomography. Springer, London, pp 311–325
Wong TZ, van der Westhuizen GJ, Coleman RE (2002) Positron emission tomography imaging of brain tumors. Neuroimaging Clin 12:615–626
Wong KP, Feng D, Meikle SR, Fulham MJ (2002) Segmentation of dynamic PET images using cluster analysis. IEEE Trans Nuclear Sci 49:200–207
Brenner DJ, Hall EJ (2007) Computed tomography—an increasing source of radiation exposure. N Engl J Med 357:2277–2284
Smith-Bindman R, Lipson J, Marcus R, Kim K-P, Mahesh M, Gould R et al (2009) Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 169:2078–2086
Fink JR, Muzi M, Peck M, Krohn KA (2015) Multimodality brain tumor imaging: MR imaging, PET, and PET/MR imaging. J Nucl Med 56:1554–1561
Hess CP, Purcell D (2012) Exploring the brain: Is CT or MRI better for brain imaging. UCSF Dep Radiol Biomed Imaging 11:1–11
Saad NM, Bakar SARSA, Muda AS, Mokji MM (2015) Review of brain lesion detection and classification using neuroimaging analysis techniques. J Teknol 74:1–13
Huang M, Yang W, Wu Y, Jiang J, Chen W, Feng Q (2014) Brain tumor segmentation based on local independent projection-based classification. IEEE Trans Biomed Eng 61:2633–2645
Khan MA, Arshad H, Nisar W, Javed MY, Sharif M (2021) An integrated design of Fuzzy C-means and NCA-based multi-properties feature reduction for brain tumor recognition. Signal and image processing techniques for the development of intelligent healthcare systems. Springer, New York, pp 1–28
Rewari R (2021) Automatic tumor segmentation from MRI scans. Stanford University, Stanford
Tandel GS, Biswas M, Kakde OG, Tiwari A, Suri HS, Turk M et al (2019) a review on a deep learning perspective in brain cancer classification. Cancers 11:1–32
El-Dahshan E-SA, Mohsen HM, Revett K, Salem A-BM (2014) Computer-aided diagnosis of human brain tumor through MRI: A survey and a new algorithm. Expert Syst Appl 41:5526–5545
Gordillo N, Montseny E, Sobrevilla P (2013) State of the art survey on MRI brain tumor segmentation. Magn Reson Imaging 31:1426–1438
Mohan G, Subashini MM (2018) MRI based medical image analysis: Survey on brain tumor grade classification. Biomed Signal Process Control 39:139–161
Amin J, Sharif M, Yasmin M (2016) Segmentation and classification of lung cancer: a review. Immunol Endocr Metab Agents Med Chem 16:82–99
Shahzad A, Sharif M, Raza M, Hussain K (2008) Enhanced watershed image processing segmentation. J Inf Commun Technol 2:9
Joo L, Jung SC, Lee H, Park SY, Kim M, Park JE et al (2021) Stability of MRI radiomic features according to various imaging parameters in fast scanned T2-FLAIR for acute ischemic stroke patients. Sci Rep 11:1–11
Chen H, Zou Q, Wang Q (2021) Clinical manifestations of ultrasonic virtual reality in the diagnosis and treatment of cardiovascular diseases. J Healthc Eng 2021:1–12
Henneghan AM, Van Dyk K, Kaufmann T, Harrison R, Gibbons C, Heijnen C, Kesler SR (2021) Measuring self-reported cancer-related cognitive impairment: recommendations from the Cancer Neuroscience Initiative Working Group. JNCI:1–9
Drake-Pérez M, Boto J, Fitsiori A, Lovblad K, Vargas MI (2018) Clinical applications of diffusion weighted imaging in neuroradiology. Insights Imaging 9:535–547
Okorie CK, Ogbole GI, Owolabi MO, Ogun O, Adeyinka A, Ogunniyi A (2015) Role of diffusion-weighted imaging in acute stroke management using low-field magnetic resonance imaging in resource-limited settings. West Afr J Radiol 22:61
Menze BH, Jakab A, Bauer S, Kalpathy-Cramer J, Farahani K, Kirby J et al (2014) The multimodal brain tumor image segmentation benchmark (BRATS). IEEE Trans Med Imaging 34:1993–2024
Bakas S, Akbari H, Sotiras A, Bilello M, Rozycki M, Kirby JS et al (2017) Advancing the cancer genome atlas glioma MRI collections with expert segmentation labels and radiomic features. Sci Data 4:170117
Kistler M, Bonaretti S, Pfahrer M, Niklaus R, Büchler P (2013) The virtual skeleton database: an open access repository for biomedical research and collaboration. J Med Internet Res 15:e245
Summers D (2003) Harvard whole brain Atlas: www. med. harvard. edu/AANLIB/home. html. J Neurol Neurosurg Psychiatry 74:288–288
Armato S, Beichel R, Bidaut L, Clarke L, Croft B, Fenimore C, Gavrielides M et al (2008) RIDER (Reference Database to Evaluate Response) Committee Combined Report, 9/25/2008 Sponsored by NIH, NCI, CIP, ITDB Causes of and Methods for Estimating/Ameliorating variance in the evaluation of tumor change in response-to therapy. https://wiki.cancerimagingarchive.net/display/Public/Collections
Kistler M, Bonaretti S, Pfahrer M, Niklaus R, Büchler P (2013) The virtual skeleton database: an open access repository for biomedical research and collaboration. J Med Internet Res 15:1–14
Yasmin M, Mohsin S, Sharif M, Raza M, Masood S (2012) Brain image analysis: a survey. World Appl Sci J 19:1484–1494
Somasundaram K, Kalaiselvi T (2010) Fully automatic brain extraction algorithm for axial T2-weighted magnetic resonance images. Comput Biol Med 40:811–822
Zhu Y, Young GS, Xue Z, Huang RY, You H, Setayesh K et al (2012) Semi-automatic segmentation software for quantitative clinical brain glioblastoma evaluation. Acad Radiol 19:977–985
Prabhu LAJ, Jayachandran A (2018) Mixture model segmentation system for parasagittal meningioma brain tumor classification based on hybrid feature vector. J Med Syst 42:1–6
Park CR, Kim K, Lee Y (2019) Development of a bias field-based uniformity correction in magnetic resonance imaging with various standard pulse sequences. Optik 178:161–166
Patel P, Bhandari A (2019) A review on image contrast enhancement techniques. Int J Online Sci 5:14–18
Zhang Z, Song J (2019) A robust brain MRI segmentation and bias field correction method integrating local contextual information into a clustering model. Appl Sci 9:1332
Irum I, Sharif M, Yasmin M, Raza M, Azam F (2014) A noise adaptive approach to impulse noise detection and reduction. Nepal J Sci Technol 15:67–76
Robb RA (2000) 3-dimensional visualization in medicine and biology. Handb Med Imaging Process Anal:685–712
Mehmood I, Ejaz N, Sajjad M, Baik SW (2013) Prioritization of brain MRI volumes using medical image perception model and tumor region segmentation. Comput Biol Med 43:1471–1483
Lu X, Huang Z, Yuan Y (2015) MR image super-resolution via manifold regularized sparse learning. Neurocomputing 162:96–104
Irum I, Sharif M, Raza M, Mohsin S (2015) A nonlinear hybrid filter for salt & pepper noise removal from color images. J Appl Res Technol 13:79–85
Stadler A, Schima W, Ba-Ssalamah A, Kettenbach J, Eisenhuber E (2007) Artifacts in body MR imaging: their appearance and how to eliminate them. Eur Radiol 17:1242–1255
Masood S, Sharif M, Masood A, Yasmin M, Raza M (2015) A survey on medical image segmentation. Curr Med Imaging 11:3–14
Irum I, Sharif M, Raza M, Yasmin M (2014) Salt and pepper noise removal filter for 8-bit images based on local and global occurrences of grey levels as selection indicator. Nepal J Sci Technol 15:123–132
Sharif M, Irum I, Yasmin M, Raza M (2017) Salt & pepper noise removal from digital color images based on mathematical morphology and fuzzy decision. Nepal J Sci Technol 18:1–7
Prastawa M, Bullitt E, Moon N, Van Leemput K, Gerig G (2003) Automatic brain tumor segmentation by subject specific modification of atlas priors1. Acad Radiol 10:1341–1348
Wu Y, Yang W, Jiang J, Li S, Feng Q, Chen W (2013) Semi-automatic segmentation of brain tumors using population and individual information. J Digit Imaging 26:786–796
Xie K, Yang J, Zhang Z, Zhu Y (2005) Semi-automated brain tumor and edema segmentation using MRI. Eur J Radiol 56:12–19
Agn M, Puonti O, af Rosenschöld PM, Law I, Van Leemput K (2015) Brain tumor segmentation using a generative model with an RBM prior on tumor shape. In: BrainLes vol 2015, pp 168–180
Haeck T, Maes F, Suetens P (2015) ISLES challenge 2015: Automated model-based segmentation of ischemic stroke in MR images. BrainLes 2015:246–253
Abbasi S, Tajeripour F (2017) Detection of brain tumor in 3D MRI images using local binary patterns and histogram orientation gradient. Neurocomputing 219:526–535
Sauwen N, Acou M, Sima DM, Veraart J, Maes F, Himmelreich U et al (2017) Semi-automated brain tumor segmentation on multi-parametric MRI using regularized non-negative matrix factorization. BMC Med Imaging 17:29
Ilunga-Mbuyamba E, Avina–Cervantes JG, Garcia-Perez A, de Jesus Romero–Troncoso R, Aguirre–Ramos H, Cruz–Aceves I et al (2017) Localized active contour model with background intensity compensation applied on automatic MR brain tumor segmentation. Neurocomputing 220:84–97
Akbar S, Akram MU, Sharif M, Tariq A, Khan SA (2018) Decision support system for detection of hypertensive retinopathy using arteriovenous ratio. Artif Intell Med 90:15–24
Banerjee S, Mitra S, Shankar BU (2018) Automated 3D segmentation of brain tumor using visual saliency. Inf Sci 424:337–353
Article MathSciNet Google Scholar
Raja NSM, Fernandes SL, Dey N, Satapathy SC, Rajinikanth V (2018) Contrast enhanced medical MRI evaluation using Tsallis entropy and region growing segmentation. J Ambient Intell Human Comput:1–12
Subudhi A, Dash M, Sabut S (2020) Automated segmentation and classification of brain stroke using expectation-maximization and random forest classifier. Biocybern Biomed Eng 40:277–289
Gupta N, Bhatele P, Khanna P (2019) Glioma detection on brain MRIs using texture and morphological features with ensemble learning. Biomed Signal Process Control 47:115–125
Myronenko A, Hatamizadeh A (2020) Robust semantic segmentation of brain tumor regions from 3D MRIs. arXiv:2001.02040
Karayegen G, Aksahin MF (2021) Brain tumor prediction on MR images with semantic segmentation by using deep learning network and 3D imaging of tumor region. Biomed Signal Process Control 66:102458
Prima S, Ayache N, Barrick T, Roberts N (2001) Maximum likelihood estimation of the bias field in MR brain images: Investigating different modelings of the imaging process. In: International conference on medical image computing and computer-assisted intervention, pp 811–819
Haider W, Sharif M, Raza M (2011) Achieving accuracy in early stage tumor identification systems based on image segmentation and 3D structure analysis. Comput Eng Intell Syst 2:96–102
Irum I, Shahid MA, Sharif M, Raza M (2015) A review of image denoising methods. J Eng Sci Technol Rev 8:1–11
Kumar SS, Dharun VS (2016) A study of MRI segmentation methods in automatic brain tumor detection. Int J Eng Technol 8:609–614
Dhas A, Madheswaran M (2018) An improved classification system for brain tumours using wavelet transform and neural network. West Indian Med J 67:243–247
Krissian K, Aja-Fernández S (2009) Noise-driven anisotropic diffusion filtering of MRI. IEEE Trans Image Process 18:2265–2274
Article MathSciNet MATH Google Scholar
Tahir B, Iqbal S, Usman Ghani Khan M, Saba T, Mehmood Z, Anjum A et al (2019) Feature enhancement framework for brain tumor segmentation and classification. Microsc Res Tech 82:803–811
Said AB, Hadjidj R, Foufou S (2019) Total variation for image denoising based on a novel smart edge detector: an application to medical images. J Math Imaging Vision 61:106–121
Bojorquez JAZ, Jodoin P-M, Bricq S, Walker PM, Brunotte F, Lalande A (2019) Automatic classification of tissues on pelvic MRI based on relaxation times and support vector machine. PLoS ONE 14:1–17
Sandhya G, Kande GB, Satya ST (2019) An efficient MRI brain tumor segmentation by the fusion of active contour model and self-organizing-map. J Biomim Biomater Biomed Eng 40:79–91
Yang Y, Huang S (2006) Novel statistical approach for segmentation of brain magnetic resonance imaging using an improved expectation maximization algorithm. Opt Appl 36:125–136
Mittal M, Goyal LM, Kaur S, Kaur I, Verma A, Hemanth DJ (2019) Deep learning based enhanced tumor segmentation approach for MR brain images. Appl Soft Comput 78:346–354
Roy S, Bandyopadhyay SK (2012) Detection and quantification of brain tumor from MRI of brain and it’s symmetric analysis. Int J Inf Commun Technol Res 2:1–7
Gao J, Xie M (2009) Skull-stripping MR brain images using anisotropic diffusion filtering and morphological processing. In: 2009 IEEE international symposium on computer network and multimedia technology, pp 1–4
Maintz JA, Viergever MA (1998) A survey of medical image registration. Med Image Anal 2:1–36
Sharma P, Diwakar M, Choudhary S (2012) Application of edge detection for brain tumor detection. Int J Comput Appl 58:1–6
Popescu V, Battaglini M, Hoogstrate W, Verfaillie SC, Sluimer I, van Schijndel RA et al (2012) Optimizing parameter choice for FSL-brain extraction tool (BET) on 3D T1 images in multiple sclerosis. Neuroimage 61:1484–1494
Despotović I, Goossens B, Philips W (2015) MRI segmentation of the human brain: challenges, methods, and applications. Comput Math Methods Med 2015:1–23
Wong KP (2005) Medical image segmentation: methods and applications in functional imaging. In: Handbook of biomedical image analysis, pp 111–182
Chae SY, Suh S, Ryoo I, Park A, Noh KJ, Shim H et al (2017) A semi-automated volumetric software for segmentation and perfusion parameter quantification of brain tumors using 320-row multidetector computed tomography: a validation study. Neuroradiology 59:461–469
Sauwen N, Acou M, Sima DM, Veraart J, Maes F, Himmelreich U et al (2017) Semi-automated brain tumor segmentation on multi-parametric MRI using regularized non-negative matrix factorization. BMC Med Imaging 17:1–14
Foo JL (2006) A survey of user interaction and automation in medical image segmentation methods. In: Tech rep ISUHCI20062, Human Computer Interaction Department, Iowa State Univ, pp 1–11
Işın A, Direkoğlu C, Şah M (2016) Review of MRI-based brain tumor image segmentation using deep learning methods. Procedia Comput Sci 102:317–324
Shanthi KJ, Kumar MS (2007) Skull stripping and automatic segmentation of brain MRI using seed growth and threshold techniques. In: 2007 International conference on intelligent and advanced systems, pp 422–426
Yao J (2006) Image processing in tumor imaging, new techniques in oncologic imaging. Zhang, F., & Hancock, ER Zhang. New Riemannian techniques for directional and tensorial image data. Pattern Recogn 43:1590–1606
Stadlbauer A, Moser E, Gruber S, Buslei R, Nimsky C, Fahlbusch R et al (2004) Improved delineation of brain tumors: an automated method for segmentation based on pathologic changes of 1H-MRSI metabolites in gliomas. Neuroimage 23:454–461
Lakare S, Kaufman A (2000) 3D segmentation techniques for medical volumes, Center for Visual Computing. Department of Computer Science, State University of New York, New York, pp 59–68
Sato M, Lakare S, Wan M, Kaufman A, Nakajima M (2000) A gradient magnitude based region growing algorithm for accurate segmentation. In: Proceedings 2000 International Conference on Image Processing, vol 3, pp 448–451
Salman YM (2009) Modified technique for volumetric brain tumor measurements. J Biomed Sci Eng 2:16
Singh NP, Dixit S, Akshaya AS, Khodanpur BI (2017) Gradient magnitude based watershed segmentation for brain tumor segmentation and classification. In: Proceedings of the 5th international conference on frontiers in intelligent computing: theory and applications, pp 611–619
Husain RA, Zayed AS, Ahmed WM, Elhaji HS (2015) Image segmentation with improved watershed algorithm using radial bases function neural networks. In: 2015 16th International conference on sciences and techniques of automatic control and computer engineering (STA), pp 121–126
Masood S, Sharif M, Yasmin M, Raza M, Mohsin S (2013) Brain image compression: a brief survey. Res J Appl Sci Eng Technol 5:49–59
Mughal B, Muhammad N, Sharif M (2018) Deviation analysis for texture segmentation of breast lesions in mammographic images. Eur Phys J Plus 133:1–15
Anjum MA, Amin J, Sharif M, Khan HU, Malik MSA, Kadry S (2020) Deep semantic segmentation and multi-class skin lesion classification based on convolutional neural network. IEEE Access 8:129668–129678
Gies V, Bernard TM (2004) Statistical solution to watershed over-segmentation. In: 2004 International Conference on Image Processing. ICIP'04, vol 3, pp 1863–1866
Kong J, Wang J, Lu Y, Zhang J, Li Y, Zhang B (2006) A novel approach for segmentation of MRI brain images. In: MELECON 2006-2006 IEEE Mediterranean Electrotechnical Conference, pp 525–528
Couprie M, Bertrand G (1997) Topological gray-scale watershed transformation. In: Vision Geometry VI International Society for Optics and Photonics, vol 3168, pp 136–146
Lotufo RA, Falcão AX, Zampirolli FA (2002) IFT-watershed from gray-scale marker. In: Proceedings. XV Brazilian Symposium on Computer Graphics and Image Processing, pp 146–152
Benson CC, Lajish VL, Rajamani K (2015) Brain tumor extraction from MRI brain images using marker based watershed algorithm. In: 2015 International Conference on advances in computing, communications and informatics (ICACCI), pp 318–323
Nasir M, Attique Khan M, Sharif M, Lali IU, Saba T, Iqbal T (2018) An improved strategy for skin lesion detection and classification using uniform segmentation and feature selection based approach. Microsc Res Tech 81:528–543
Yasmin M, Sharif M, Masood S, Raza M, Mohsin S (2012) Brain image enhancement—a survey. World Appl Sci J 17:1192–1204
Shah GA, Khan A, Shah AA, Raza M, Sharif M (2015) A review on image contrast enhancement techniques using histogram equalization. Sci Int 27:1-10
Khan MA, Akram T, Sharif M, Shahzad A, Aurangzeb K, Alhussein M et al (2018) An implementation of normal distribution based segmentation and entropy controlled features selection for skin lesion detection and classification. BMC Cancer 18:1–20
Khan MA, Akram T, Sharif M, Saba T, Javed K, Lali IU et al (2019) Construction of saliency map and hybrid set of features for efficient segmentation and classification of skin lesion. Microsc Res Tech 82:741–763
Akram T, Khan MA, Sharif M, Yasmin M (2018) Skin lesion segmentation and recognition using multichannel saliency estimation and M-SVM on selected serially fused features. J Ambient Intell Human Comput:1–20
Yasmin M, Sharif M, Mohsin S, Azam F (2014) Pathological brain image segmentation and classification: a survey. Curr Med Imaging 10:163–177
Mughal B, Muhammad N, Sharif M (2018) Deviation analysis for texture segmentation of breast lesions in mammographic images. Eur Phys J Plus 133:455
Hameed M, Sharif M, Raza M, Haider SW, Iqbal M (2012) Framework for the comparison of classifiers for medical image segmentation with transform and moment based features. Res J Recent Sci 2277:2502
Irum I, Raza M, Sharif M (2012) Morphological techniques for medical images: a review. Res J Appl Sci Eng Technol 4:2948–2962
Jafarpour S, Sedghi Z, Amirani MC (2012) A robust brain MRI classification with GLCM features. Int J Comput Appl 37:1–5
Mughal B, Muhammad N, Sharif M (2019) Adaptive hysteresis thresholding segmentation technique for localizing the breast masses in the curve stitching domain. Int J Med Inf 126:26–34
Nabizadeh N, Kubat M (2015) Brain tumors detection and segmentation in MR images: Gabor wavelet vs. statistical features. Comput Electr Eng 45:286–301
Tiwari P, Sachdeva J, Ahuja CK, Khandelwal N (2017) Computer aided diagnosis system—a decision support system for clinical diagnosis of brain tumours. Int J Comput Intell Syst 10:104–119
Zhang Y, Yang J, Wang S, Dong Z, Phillips P (2017) Pathological brain detection in MRI scanning via Hu moment invariants and machine learning. J Exp Theor Artif Intell 29:299–312
Lahmiri S (2017) Glioma detection based on multi-fractal features of segmented brain MRI by particle swarm optimization techniques. Biomed Signal Process Control 31:148–155
Xu X, Zhang X, Tian Q, Zhang G, Liu Y, Cui G et al (2017) Three-dimensional texture features from intensity and high-order derivative maps for the discrimination between bladder tumors and wall tissues via MRI. Int J Comput Assist Radiol Surg 12:645–656
Shanthakumar P, Ganeshkumar P (2015) Performance analysis of classifier for brain tumor detection and diagnosis. Comput Electr Eng 45:302–311
Zhang B, Chang K, Ramkissoon S, Tanguturi S, Bi WL, Reardon DA et al (2017) Multimodal MRI features predict isocitrate dehydrogenase genotype in high-grade gliomas. Neuro Oncol 19:109–117
Srinivas B, Rao GS (2019) Performance evaluation of fuzzy C means segmentation and support vector machine classification for MRI brain tumor. In: Soft computing for problem solving. Springer, New York, pp 355–367
Herlidou-Meme S, Constans J, Carsin B, Olivie D, Eliat P, Nadal-Desbarats L et al (2003) MRI texture analysis on texture test objects, normal brain and intracranial tumors. Magn Reson Imaging 21:989–993
Soltaninejad M, Yang G, Lambrou T, Allinson N, Jones TL, Barrick TR et al (2017) Automated brain tumour detection and segmentation using superpixel-based extremely randomized trees in FLAIR MRI. Int J Comput Assist Radiol Surg 12:183–203
Yang D, Rao G, Martinez J, Veeraraghavan A, Rao A (2015) Evaluation of tumor-derived MRI-texture features for discrimination of molecular subtypes and prediction of 12-month survival status in glioblastoma. Med Phys 42:6725–6735
Islam A, Reza SM, Iftekharuddin KM (2013) Multifractal texture estimation for detection and segmentation of brain tumors. IEEE Trans Biomed Eng 60:3204–3215
Pei L, Bakas S, Vossough A, Reza SM, Davatzikos C, Iftekharuddin KM (2020) Longitudinal brain tumor segmentation prediction in MRI using feature and label fusion. Biomed Signal Process Control 55:101648
Khan H, Shah PM, Shah MA, ul Islam S, Rodrigues JJ (2020) Cascading handcrafted features and convolutional neural network for IoT-enabled brain tumor segmentation. Comput Commun 153:196–207
Dixit A, Nanda A (2021) An improved whale optimization algorithm-based radial neural network for multi-grade brain tumor classification. Visual Comput:1–16
Zhang Y, Dong Z, Wu L, Wang S (2011) A hybrid method for MRI brain image classification. Expert Syst Appl 38:10049–10053
Zöllner FG, Emblem KE, Schad LR (2012) SVM-based glioma grading: optimization by feature reduction analysis. Z Med Phys 22:205–214
Arakeri MP, Reddy GRM (2015) Computer-aided diagnosis system for tissue characterization of brain tumor on magnetic resonance images. SIViP 9:409–425
Nachimuthu DS, Baladhandapani A (2014) Multidimensional texture characterization: on analysis for brain tumor tissues using MRS and MRI. J Digit Imaging 27:496–506
Pinto A, Pereira S, Dinis H, Silva CA, Rasteiro DM (2015) Random decision forests for automatic brain tumor segmentation on multi-modal MRI images. In: 2015 IEEE 4th Portuguese meeting on bioengineering (ENBENG), pp 1–5
Tustison NJ, Shrinidhi K, Wintermark M, Durst CR, Kandel BM, Gee JC et al (2015) Optimal symmetric multimodal templates and concatenated random forests for supervised brain tumor segmentation (simplified) with ANTsR. Neuroinformatics 13:209–225
Yang G, Zhang Y, Yang J, Ji G, Dong Z, Wang S et al (2016) Automated classification of brain images using wavelet-energy and biogeography-based optimization. Multimedia Tools Appl 75:15601–15617
Wang S, Du S, Atangana A, Liu A, Lu Z (2018) Application of stationary wavelet entropy in pathological brain detection. Multimedia Tools Appl 77:3701–3714
Padlia M, Sharma J (2019) Fractional Sobel filter based brain tumor detection and segmentation using statistical features and SVM. In: Nanoelectronics, circuits and communication systems, pp 161–175
Ayadi W, Elhamzi W, Charfi I, Atri M (2021) Deep CNN for brain tumor classification. Neural Process Lett 53:671–700
Afza F, Khan MA, Sharif M, Rehman A (2019) Microscopic skin laceration segmentation and classification: a framework of statistical normal distribution and optimal feature selection. Microsc Res Tech 82:1471–1488
Adair J, Brownlee A, Ochoa G (2017) Evolutionary algorithms with linkage information for feature selection in brain computer interfaces. In: Advances in computational intelligence systems. Springer, New York, pp 287–307
Sharma M, Mukharjee S (2012) Brain tumor segmentation using hybrid genetic algorithm and artificial neural network fuzzy inference system (anfis). Int J Fuzzy Logic Syst 2:31–42
Huda S, Yearwood J, Jelinek HF, Hassan MM, Fortino G, Buckland M (2016) A hybrid feature selection with ensemble classification for imbalanced healthcare data: a case study for brain tumor diagnosis. IEEE Access 4:9145–9154
Jothi G (2016) Hybrid tolerance rough set-firefly based supervised feature selection for MRI brain tumor image classification. Appl Soft Comput 46:639–651
Ahmed S, Iftekharuddin KM, Vossough A (2011) Efficacy of texture, shape, and intensity feature fusion for posterior-fossa tumor segmentation in MRI. IEEE Trans Inf Technol Biomed 15:206–213
Wu G, Chen Y, Wang Y, Yu J, Lv X, Ju X et al (2018) Sparse representation-based Radiomics for the diagnosis of brain tumors. IEEE Trans Med Imaging 37:893–905
Fernandez-Lozano C, Seoane JA, Gestal M, Gaunt TR, Dorado J, Campbell C (2015) Texture classification using feature selection and kernel-based techniques. Soft Comput 19:2469–2480
Dandu JR, Thiyagarajan AP, Murugan PR, Govindaraj V (2019) Brain and pancreatic tumor segmentation using SRM and BPNN classification. Health Technol:1–9
Saritha M, Joseph KP, Mathew AT (2013) Classification of MRI brain images using combined wavelet entropy based spider web plots and probabilistic neural network. Pattern Recogn Lett 34:2151–2156
Sharif M, Khan MA, Akram T, Javed MY, Saba T, Rehman A (2017) A framework of human detection and action recognition based on uniform segmentation and combination of Euclidean distance and joint entropy-based features selection. EURASIP J Image Video Process 2017:1–18
Lakshmi A, Arivoli T, Rajasekaran MP (2018) A Novel M-ACA-based tumor segmentation and DAPP feature extraction with PPCSO-PKC-based MRI classification. Arab J Sci Eng 43:7095–7111
Rathi V, Palani S (2012) Brain tumor MRI image classification with feature selection and extraction using linear discriminant analysis. arXiv:1208.2128
Naqi SM, Sharif M, Yasmin M (2018) Multistage segmentation model and SVM-ensemble for precise lung nodule detection. Int J Comput Assist Radiol Surg 13:1083–1095
Amin J, Anjum MA, Sharif M, Kadry S, Nam Y, Wang S (2021) Convolutional bi-LSTM based human gait recognition using video sequences. CMC Comput Mater Contin 68:2693–2709
Amin J, Sharif M, Anjum MA, Nam Y, Kadry S, Taniar D (2021) Diagnosis of COVID-19 infection using three-dimensional semantic segmentation and classification of computed tomography images. Comput Mater Contin:2451–2467
Amin J, Sharif M, Raza M, Saba T, Rehman A (2019) Brain tumor classification: feature fusion. In: 2019 international conference on computer and information sciences (ICCIS), pp 1–6
Amin J, Sharif M, Yasmin M, Ali H, Fernandes SL (2017) A method for the detection and classification of diabetic retinopathy using structural predictors of bright lesions. J Comput Sci 19:153–164
Amin J, Sharif M, Yasmin M, Fernandes SL (2018) Big data analysis for brain tumor detection: deep convolutional neural networks. Futur Gener Comput Syst 87:290–297
Muhammad N, Sharif M, Amin J, Mehboob R, Gilani SA, Bibi N et al (2018) Neurochemical alterations in sudden unexplained perinatal deaths—a review. Front Pediatr 6:6
Sharif M, Amin J, Raza M, Anjum MA, Afzal H, Shad SA (2020) Brain tumor detection based on extreme learning. Neural Comput Appl:1–13
Umer MJ, Amin J, Sharif M, Anjum MA, Azam F, Shah JH (2021) An integrated framework for COVID-19 classification based on classical and quantum transfer learning from a chest radiograph. Concurr Comput Pract Exp 19:153–164
Jiang J, Wu Y, Huang M, Yang W, Chen W, Feng Q (2013) Brain tumor segmentation in multimodal MR images based on learning population-and patient-specific feature sets. Comput Med Imaging Graph 37:512–521
Ortiz A, Gorriz JM, Ramírez J, Salas-Gonzalez D, Alzheimer’s Disease Neuroimaging Initiative (2013) Improving MRI segmentation with probabilistic GHSOM and multiobjective optimization 114:118–131
Havaei M, Davy A, Warde-Farley D, Biard A, Courville A, Bengio Y, Pal C, Jodoin PM, Larochelle H (2017) Brain tumor segmentation with deep neural networks. Med Image Anal 35:18–31
Zhang N, Ruan S, Lebonvallet S, Liao Q, Zhu Y (2011) Kernel feature selection to fuse multi-spectral MRI images for brain tumor segmentation. Comput Vis Image Underst 115:256–269
Ortega-Martorell S, Lisboa PJ, Vellido A, Simoes RV, Pumarola M, Julià-Sapé M et al (2012) Convex non-negative matrix factorization for brain tumor delimitation from MRSI data. PLoS ONE 7:e47824
Ali AH, Al-hadi SA, Naeemah MR, Mazher AN (2018) Classification of brain lesion using K-nearest neighbor technique and texture analysis. J Phys Conf Ser:012018
Supot S, Thanapong C, Chuchart P, Manas S (2007) Segmentation of magnetic resonance images using discrete curve evolution and fuzzy clustering. In: 2007 IEEE International Conference on Integration Technology, pp 697–700
Fletcher-Heath LM, Hall LO, Goldgof DB, Murtagh FR (2001) Automatic segmentation of non-enhancing brain tumors in magnetic resonance images. Artif Intell Med 21:43–63
Abdulbaqi HS, Mat MZ, Omar AF, Mustafa ISB, Abood LK (2014) Detecting brain tumor in magnetic resonance images using hidden Markov random fields and threshold techniques. In: 2014 IEEE student conference on research and development, pp 1–5
Parekh VS, Laterra J, Bettegowda C, Bocchieri AE, Pillai JJ, Jacobs MA (2019) Multiparametric deep learning and radiomics for tumor grading and treatment response assessment of brain cancer: preliminary results, pp 1–6. arXiv:1906.04049
Zadeh Shirazi A, Fornaciari E, McDonnell MD, Yaghoobi M, Cevallos Y, Tello-Oquendo L et al (2020) The Application of Deep Convolutional Neural Networks to Brain Cancer Images: A Survey. J Pers Med 10:224
Guan B, Yao J, Zhang G, Wang XJPRL (2019) Thigh fracture detection using deep learning method based on new dilated convolutional feature pyramid network 125:521–526
Raza M, Sharif M, Yasmin M, Khan MA, Saba T, Fernandes SL (2018) Appearance based pedestrians’ gender recognition by employing stacked auto encoders in deep learning. Future Gener Comput Syst 88:28–39
Liaqat A, Khan MA, Shah JH, Sharif M, Yasmin M, Fernandes SL (2018) Automated ulcer and bleeding classification from WCE images using multiple features fusion and selection. J Mech Med Biol 18:1850038
Naqi S, Sharif M, Yasmin M, Fernandes SL (2018) Lung nodule detection using polygon approximation and hybrid features from CT images. Curr Med Imaging 14:108–117
Ansari GJ, Shah JH, Yasmin M, Sharif M, Fernandes SL (2018) A novel machine learning approach for scene text extraction. Futur Gener Comput Syst 87:328–340
Fatima Bokhari ST, Sharif M, Yasmin M, Fernandes SL (2018) Fundus image segmentation and feature extraction for the detection of glaucoma: a new approach. Curr Med Imaging 14:77–87
Jain VK, Kumar S, Fernandes SL (2017) Extraction of emotions from multilingual text using intelligent text processing and computational linguistics. J Comput Sci 21:316–326
Fernandes SL, Gurupur VP, Lin H, Martis RJ (2017) A novel fusion approach for early lung cancer detection using computer aided diagnosis techniques. J Med Imaging Health Inf 7:1841–1850
Raja N, Rajinikanth V, Fernandes SL, Satapathy SC (2017) Segmentation of breast thermal images using Kapur’s entropy and hidden Markov random field. J Med Imaging Health Inf 7:1825–1829
Rajinikanth V, Madhavaraja N, Satapathy SC, Fernandes SL (2017) Otsu’s multi-thresholding and active contour snake model to segment dermoscopy images. J Med Imaging Health Inf 7:1837–1840
Shah JH, Chen Z, Sharif M, Yasmin M, Fernandes SL (2017) A novel biomechanics-based approach for person re-identification by generating dense color sift salience features. J Mech Med Biol 17:1740011
Fernandes SL, Bala GJ (2017) A comparative study on various state of the art face recognition techniques under varying facial expressions. Int Arab J Inf Technol 14:254–259
Yasmin M, Sharif M, Mohsin S (2013) Neural networks in medical imaging applications: a survey. World Appl Sci J 22:85–96
Chen L, Bentley P, Rueckert D (2017) Fully automatic acute ischemic lesion segmentation in DWI using convolutional neural networks. NeuroImage Clin 15:633–643
Abd-Ellah MK, Awad AI, Khalaf AA, Hamed HF (2018) Two-phase multi-model automatic brain tumour diagnosis system from magnetic resonance images using convolutional neural networks. EURASIP J Image Video Process 2018:97
Larochelle H, Jodoin P-M (2016) A convolutional neural network approach to brain tumor segmentation. In: Brainlesion: Glioma, multiple sclerosis, stroke and traumatic brain injuries: first international workshop, brainles 2015, Held in Conjunction with MICCAI 2015, Munich, Germany, 5 Oct 5, revised selected papers
Kamnitsas K, Ledig C, Newcombe VF, Simpson JP, Kane AD, Menon DK, Rueckert D, Glocker B (2017) Efficient multi-scale 3D CNN with fully connected CRF for accurate brain lesion segmentation. Med Image Anal 36:61–78
Dong H, Yang G, Liu F, Mo Y, Guo Y (2017) Automatic brain tumor detection and segmentation using U-Net based fully convolutional networks. In: Annual conference on medical image understanding and analysis, pp 506–517
Chen S, Ding C, Liu M (2019) Dual-force convolutional neural networks for accurate brain tumor segmentation. Pattern Recogn 88:90–100
Wang Y, Li C, Zhu T, Zhang J (2019) Multimodal brain tumor image segmentation using WRN-PPNet. Comput Med Imaging Graph 75:56–65
Chelghoum R, Ikhlef A, Hameurlaine A, Jacquir S (2020) Transfer learning using convolutional neural network architectures for brain tumor classification from MRI images. In: IFIP International Conference on Artificial Intelligence Applications and Innovations, pp 189–200
Mehrotra R, Ansari MA, Agrawal R, Anand RS (2020) A transfer learning approach for AI-based classification of brain tumors. Mach Learn Appl 2:1–12
Zikic D, Ioannou Y, Brown M, Criminisi A (2014) Segmentation of brain tumor tissues with convolutional neural networks. In: Proceedings MICCAI-BRATS, pp 36–39
Dvořák P, Menze B (2015) Local structure prediction with convolutional neural networks for multimodal brain tumor segmentation. In: International MICCAI workshop on medical computer vision, pp 59–71
Pereira S, Pinto A, Alves V, Silva CA (2016) Brain tumor segmentation using convolutional neural networks in MRI images. IEEE Trans Med Imaging 35:1240–1251
Zhao X, Wu Y, Song G, Li Z, Zhang Y, Fan Y (2018) A deep learning model integrating FCNNs and CRFs for brain tumor segmentation. Med Image Anal 43:98–111
Hussain S, Anwar SM, Majid M (2018) Segmentation of glioma tumors in brain using deep convolutional neural network. Neurocomputing 282:248–261
Sharif MI, Li JP, Amin J, Sharif A (2021) An improved framework for brain tumor analysis using MRI based on YOLOv2 and convolutional neural network. Complex Intell Syst:1–14
Kao PY, Shailja S, Jiang J, Zhang A, Khan A, Chen JW, Manjunath BS (2020) Improving patch-based convolutional neural networks for MRI brain tumor segmentation by leveraging location information. Front Neurosci 13:1–12
Ahuja S, Panigrahi B, Gandhi T (2020) Transfer learning based brain tumor detection and segmentation using superpixel technique. In: 2020 International Conference on Contemporary Computing and Applications (IC3A), pp 244–249
Rehman A, Naz S, Razzak MI, Akram F, Imran M (2020) A deep learning-based framework for automatic brain tumors classification using transfer learning. Circuits Syst Signal Process 39:757–775
Guy-Fernand KN, Zhao J, Sabuni FM, Wang J (2020) Classification of brain tumor leveraging goal-driven visual attention with the support of transfer learning. In: 2020 Information Communication Technologies Conference (ICTC), pp 328–332
Kaur T, Gandhi TK (2020) Deep convolutional neural networks with transfer learning for automated brain image classification. Mach Vis Appl 31:1–16
Gu Y, Li K (2021) A Transfer Model Based on Supervised Multi-Layer Dictionary Learning for Brain Tumor MRI Image Recognition. Front Neurosci 15:550
Sadad T, Rehman A, Munir A, Saba T, Tariq U, Ayesha N et al (2021) Brain tumor detection and multi-classification using advanced deep learning techniques. Microsc Res Tech 84:1296–1308
Panwar SA, Munot MV, Gawande S, Deshpande PS (2021) A reliable and an efficient approach for diagnosis of brain tumor using transfer learning. Biomed Pharmacol J 14:283–294
Kumar RL, Kakarla J, Isunuri BV, Singh M (2021) Multi-class brain tumor classification using residual network and global average pooling. Multimedia Tools Appl 80:13429–13438
Arbane M, Benlamri R, Brik Y, Djerioui M (2021) Transfer learning for automatic brain tumor classification using MRI images. In: 2020 2nd International Workshop on Human-Centric Smart Environments for Health and Well-being (IHSH), pp 210–214
Amin J, Sharif M, Yasmin M, Saba T, Anjum MA, Fernandes SL (2019) A new approach for brain tumor segmentation and classification based on score level fusion using transfer learning. J Med Syst 43:1–16
Wu W, Chen AY, Zhao L, Corso JJ (2014) Brain tumor detection and segmentation in a CRF (conditional random fields) framework with pixel-pairwise affinity and superpixel-level features. Int J Comput Assist Radiol Surg 9:241–253
Noreen N, Palaniappan S, Qayyum A, Ahmad I, Imran M, Shoaib M (2020) A deep learning model based on concatenation approach for the diagnosis of brain tumor. IEEE Access 8:55135–55144
Zhang J, Jiang Z, Dong J, Hou Y, Liu B (2020) Attention gate resU-Net for automatic MRI brain tumor segmentation. IEEE Access 8:58533–58545
Rehman ZU, Zia MS, Bojja GR, Yaqub M, Jinchao F, Arshid K (2020) Texture based localization of a brain tumor from MR-images by using a machine learning approach. Med Hypotheses 141:109705
Zeineldin RA, Karar ME, Coburger J, Wirtz CR, Burgert O (2020) DeepSeg: deep neural network framework for automatic brain tumor segmentation using magnetic resonance FLAIR images. Int J Comput Assist Radiol Surg 15:909–920
Swati ZNK, Zhao Q, Kabir M, Ali F, Ali Z, Ahmed S et al (2019) Content-based brain tumor retrieval for MR images using transfer learning. IEEE Access 7:17809–17822
Pravitasari AA, Iriawan N, Almuhayar M, Azmi T, Fithriasari K, Purnami SW et al (2020) UNet-VGG16 with transfer learning for MRI-based brain tumor segmentation. Telkomnika 18:1310–1318
Deepak S, Ameer P (2019) Brain tumor classification using deep CNN features via transfer learning. Comput Biol Med 111:3345
Lu S, Lu Z, Zhang Y-D (2019) Pathological brain detection based on AlexNet and transfer learning. J Comput Sci 30:41–47
Wacker J, Ladeira M, Nascimento JEV (2019) Transfer learning for brain tumor segmentation. arXiv:1912.12452
Soumik MFI, Hossain MA (2020) Brain tumor classification with inception network based deep learning model using transfer learning. In: 2020 IEEE Region 10 Symposium (TENSYMP), pp 1018–1021
Khamparia A, Gupta D, de Albuquerque VHC, Sangaiah AK, Jhaveri RH (2020) Internet of health things-driven deep learning system for detection and classification of cervical cells using transfer learning. J Supercomput 76:1–19
Yang Y, Yan LF, Zhang X, HanY, Nan HY, Hu YC, Hu B, Yan SL, Zhang J, Cheng DL, Ge XW (2018) Glioma grading on conventional MR images: a deep learning study with transfer learning. Front Neurosci 12:1–10
Banerjee S, Mitra S, Masulli F, Rovetta S (2019) Deep radiomics for brain tumor detection and classification from multi-sequence MRI. arXiv:1903.09240
Hao R, Namdar K, Liu L, Khalvati F (2021) A transfer learning–based active learning framework for brain tumor classification. Front Artif Intell 4
Saxena P, Maheshwari A, Maheshwari S (2021) Predictive modeling of brain tumor: a deep learning approach. In: Innovations in computational intelligence and computer vision, pp 275–285
Bergholm V, Izaac J, Schuld M, Gogolin C, Alam MS, Ahmed S et al (2018) Pennylane: automatic differentiation of hybrid quantum-classical computations. arXiv:1811.04968
Zhou R, Zhou L, Jiang N, Ding Q (2006) Dynamic analysis and application of QANN. In: First International Multi-Symposiums on Computer and Computational Sciences (IMSCCS’06), pp 347–351
Li P, Xiao H (2014) Sequence input-based quantum-inspired neural networks with applications. Neural Process Lett 40:143–168
Konar D, Bhattacharyya S, Panigrahi BK, Behrman EC (2021) Qutrit-inspired fully self-supervised shallow quantum learning network for brain tumor segmentation. IEEE Trans Neural Netw Learn Syst
Agrawal U, Brown EN, Lewis LD (2020) Model-based physiological noise removal in fast fMRI. Neuroimage 205:1–18
Dubey YK, Mushrif MM (2016) FCM clustering algorithms for segmentation of brain MR images. Adv Fuzzy Syst 2016:8
MathSciNet Google Scholar
Sharif MI, Khan MA, Alhussein M, Aurangzeb K, Raza M (2021) A decision support system for multimodal brain tumor classification using deep learning. Complex Intell Syst:1–14
Irshad M, Muhammad N, Sharif M, Yasmeen M (2018) Automatic segmentation of the left ventricle in a cardiac MR short axis image using blind morphological operation. Eur Phys J Plus 133:1–14
Han C, Rundo L, Araki R, Furukawa Y, Mauri G, Nakayama H et al (2020) Infinite brain MR images: PGGAN-based data augmentation for tumor detection. Neural approaches to dynamics of signal exchanges. Springer, New York, pp 291–303
Juan-Albarracín J, Fuster-Garcia E, Manjon JV, Robles M, Aparici F, Martí-Bonmatí L et al (2015) "Automated glioblastoma segmentation based on a multiparametric structured unsupervised classification. PLoS ONE 10:e0125143
Soltaninejad M, Yang G, Lambrou T, Allinson N, Jones TL, Barrick TR et al (2018) "Supervised learning based multimodal MRI brain tumour segmentation using texture features from supervoxels. Comput Methods Progr Biomed 157:69–84
Ito KL, Kim H, Liew SL (2019) A comparison of automated lesion segmentation approaches for chronic stroke T1-weighted MRI data. Hum Brain Mapp 40:4669–4685
Chen X, You S, Tezcan KC, Konukoglu EJMIA (2020) Unsupervised lesion detection via image restoration with a normative prior
Liu P, Dou Q, Wang Q, Heng PA (2020) An encoder-decoder neural network with 3D squeeze-and-excitation and deep supervision for brain tumor segmentation. IEEE Access 8:34029–34037
Deb D, Roy S (2021) Brain tumor detection based on hybrid deep neural network in MRI by adaptive squirrel search optimization. Multimedia Tools Appl 80:2621–2645
Feng X, Tustison NJ, Patel SH, Meyer CH (2020) "Brain tumor segmentation using an ensemble of 3d u-nets and overall survival prediction using radiomic features. Front Comput Neurosci 14:25
Zhou C, Ding C, Wang X, Lu Z, Tao D (2020) "One-pass multi-task networks with cross-task guided attention for brain tumor segmentation. IEEE Trans Image Process 29:4516–4529
Download references
Author information
Authors and affiliations.
Department of Computer Science, University of Wah, Wah Cantt, Pakistan
Javaria Amin
Department of Computer Science, COMSATS University Islamabad, Wah Campus, Islamabad, Pakistan
Javaria Amin, Muhammad Sharif & Mussarat Yasmin
Department of Computer Science and Engineering, Sri Eshwar College of Engineering, Coimbatore, Tamil Nadu, India
Anandakumar Haldorai
Department of IS&E, Canara Engineering College, Mangaluru, Karnataka, India
Ramesh Sundar Nayak
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Javaria Amin .
Ethics declarations
Conflict of interest.
There is no grant received from any resources. All authors declare that they have no conflict of interest.
Research involving human participants and/or animals
It is declared that research has not involved any human participants and animals.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Amin, J., Sharif, M., Haldorai, A. et al. Brain tumor detection and classification using machine learning: a comprehensive survey. Complex Intell. Syst. 8 , 3161–3183 (2022). https://doi.org/10.1007/s40747-021-00563-y
Download citation
Received : 28 July 2021
Accepted : 12 October 2021
Published : 08 November 2021
Issue Date : August 2022
DOI : https://doi.org/10.1007/s40747-021-00563-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Feature extraction
- Find a journal
- Publish with us
- Track your research
IEEE Account
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
- Neoplasms by Site
- Nervous System Neoplasms
- Central Nervous System Neoplasms
- Brain Tumors
An Overview of Brain Tumor
- January 2022
- In book: Brain Tumors [Working Title]

- PRIST University
- This person is not on ResearchGate, or hasn't claimed this research yet.
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- NEURAL COMPUT APPL

- Yılmaz Kaya

- Jacob Vinoo

- Abdullah A. Asiri

- Daria Petrenko

- Bhawna Sachdeva
- Jeebananda Panda
- INT J MOL SCI

- Barbara Mroczko

- Jared Becerril-Rico

- Nuersimanguli Maimaitiming
- Yusufu Mahemuti
- Zengliang Wang
- NEURAL NETWORKS

- Tianfu Wang

- Pathol Res Pract
- Georgia Karpathiou
- Maroa Dridi

- J CONTROL RELEASE

- Chih-Kuang Yeh
- MED HYPOTHESES

- Giridhar Reddy Bojja

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Comprehensive Brain Tumor Center
Brain tumor research studies.
Johns Hopkins has an unparalleled history of introducing new forms of treatment for the patients affected by malignant brain tumors. For example, the only two new effective surgical treatments for malignant brain tumors approved by FDA (Gliadel and GliaSite) were discovered and developed right here at Johns Hopkins.
There have been tremendous advances made in the last two decades in the battle against malignant brain tumors. Advances in neuroradiology have lead to more timely and accurate diagnoses; improved neurosurgical approaches have permitted more complete and safer tumor removal, and new chemotherapies and radiation therapies have extended survival.
Our national leading role in the design and the completion of multi-center clinical trials allows us to apply the most promising discoveries from the laboratory to patient care in an effective and expeditious fashion.
At present our most exciting areas of research include immunotherapy of brain tumors, adult stem cells and local delivery of a variety of new drug treatment options for brain tumors. Learn more about current areas of investigation:
Research Areas
- Drug Delivery
- Drug Targets
- Immune Response
- Meningioma Research
Transforming your care
Find diseases & conditions by first letter.

Healing starts here
The right answers the first time
Effective treatment depends on getting the right diagnosis. Our experts diagnose and treat the toughest medical challenges.
Top-ranked in the U.S.
Mayo Clinic has more No. 1 rankings than any other hospital in the nation according to U.S. News & World Report. Learn more about our top-ranked specialties.

World-class care for global patients
We make it easy for patients around the world to get care from Mayo Clinic.
Learn more about Mayo Clinic locations or choose a specific location.

Mayo Clinic in Arizona

Mayo Clinic in Florida

Mayo Clinic in Minnesota

Mayo Clinic Health System

Mayo Clinic Healthcare
Featured care areas.
We solve the world's most serious and complex medical challenges.
- Bone marrow transplant
- Brain aneurysm
- Brain tumor
- Breast cancer
- Colon cancer
- Congenital heart disease
- Heart arrhythmia
- Heart valve disease
- Living-donor transplant
- Lung transplant
- Testicular cancer
We’re transforming healthcare
Make a gift now and help create new and better solutions for more than 1.3 million patients who turn to Mayo Clinic each year.
Cancer Types
Find a type of cancer to learn about treatment, causes and prevention, screening, and the latest research.
- Bladder Cancer
- Breast Cancer
- Colorectal Cancer
- Kidney (Renal Cell) Cancer
- Lung Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer
- Uterine Cancer
- Metastatic Cancer
- Recurrent Cancer
- Advanced Cancer
- Coping with Cancer
- Managing Cancer Care
- Adolescents and Young Adults with Cancer
- Childhood Cancers
- Acute Lymphoblastic Leukemia (ALL)
- Acute Myeloid Leukemia (AML)
- Adolescents, Cancer in
- Adrenocortical Carcinoma
- Kaposi Sarcoma (Soft Tissue Sarcoma)
- AIDS-Related Lymphoma (Lymphoma)
- Primary CNS Lymphoma (Lymphoma)
- Anal Cancer
- Appendix Cancer - see Gastrointestinal Neuroendocrine Tumors
- Astrocytomas, Childhood (Brain Cancer)
- Atypical Teratoid/Rhabdoid Tumor, Childhood, Central Nervous System (Brain Cancer)
- Basal Cell Carcinoma of the Skin - see Skin Cancer
- Bile Duct Cancer
- Bone Cancer (includes Ewing Sarcoma and Osteosarcoma and Malignant Fibrous Histiocytoma)
- Brain Tumors
- Bronchial Tumors (Lung Cancer)
- Burkitt Lymphoma - see Non-Hodgkin Lymphoma
- Carcinoma of Unknown Primary
- Atypical Teratoid/Rhabdoid Tumor, Childhood (Brain Cancer)
- Medulloblastoma and Other CNS Embryonal Tumors, Childhood (Brain Cancer)
- Germ Cell Tumor, Childhood (Brain Cancer)
- Primary CNS Lymphoma
- Cervical Cancer
- Childhood Cardiac Tumors Treatment
- Cancers of Childhood, Rare
- Cholangiocarcinoma - see Bile Duct Cancer
- Chordoma, Childhood (Bone Cancer)
- Chronic Lymphocytic Leukemia (CLL)
- Chronic Myelogenous Leukemia (CML)
- Chronic Myeloproliferative Neoplasms
- Craniopharyngioma, Childhood (Brain Cancer)
- Cutaneous T-Cell Lymphoma - see Lymphoma (Mycosis Fungoids and Sézary Syndrome)
- Ductal Carcinoma In Situ (DCIS) - see Breast Cancer
- Diffuse Intrinsic Pontine Glioma (DIPG) (Brain Cancer)
- Embryonal Tumors, Medulloblastoma and Other Central Nervous System, Childhood (Brain Cancer)
- Endometrial Cancer (Uterine Cancer)
- Ependymoma, Childhood (Brain Cancer)
- Esophageal Cancer
- Esthesioneuroblastoma (Head and Neck Cancer)
- Ewing Sarcoma (Bone Cancer)
- Extracranial Germ Cell Tumor, Childhood
- Extragonadal Germ Cell Tumor
- Intraocular Melanoma
- Retinoblastoma
- Fallopian Tube Cancer
- Gallbladder Cancer
- Gastric (Stomach) Cancer
- Gastrointestinal Neuroendocrine Tumors
- Gastrointestinal Stromal Tumors (GIST) (Soft Tissue Sarcoma)
- Childhood Central Nervous System Germ Cell Tumors (Brain Cancer)
- Childhood Extracranial Germ Cell Tumors
- Extragonadal Germ Cell Tumors
- Ovarian Germ Cell Tumors
- Testicular Cancer
- Gestational Trophoblastic Disease
- Hairy Cell Leukemia
- Head and Neck Cancer
- Heart Tumors, Childhood
- Hepatocellular (Liver) Cancer
- Histiocytosis, Langerhans Cell
- Hodgkin Lymphoma
- Hypopharyngeal Cancer (Head and Neck Cancer)
- Islet Cell Tumors, Pancreatic Neuroendocrine Tumors
- Langerhans Cell Histiocytosis
- Laryngeal Cancer (Head and Neck Cancer)
- Lip and Oral Cavity Cancer (Head and Neck Cancer)
- Liver Cancer
- Lung Cancer (Non-Small Cell, Small Cell, Pleuropulmonary Blastoma, Pulmonary Inflammatory Myofibroblastic Tumor, and Tracheobronchial Tumor)
- Male Breast Cancer
- Melanoma, Intraocular (Eye)
- Merkel Cell Carcinoma (Skin Cancer)
- Mesothelioma, Malignant
- Metastatic Squamous Neck Cancer with Occult Primary (Head and Neck Cancer)
- Midline Tract Carcinoma With NUT Gene Changes
- Mouth Cancer (Head and Neck Cancer)
- Multiple Endocrine Neoplasia Syndromes
- Multiple Myeloma/Plasma Cell Neoplasms
- Mycosis Fungoides (Lymphoma)
- Myelodysplastic Syndromes , Myelodysplastic/Myeloproliferative Neoplasms
- Myelogenous Leukemia, Chronic (CML)
- Myeloid Leukemia, Acute (AML)
- Myeloproliferative Neoplasms, Chronic
- Nasal Cavity and Paranasal Sinus Cancer (Head and Neck Cancer)
- Nasopharyngeal Cancer (Head and Neck Cancer)
- Neuroblastoma
- Neuroendocrine Tumors (Gastrointestinal)
- Non-Hodgkin Lymphoma
- Non-Small Cell Lung Cancer
- Oral Cancer, Lip and Oral Cavity Cancer and Oropharyngeal Cancer (Head and Neck Cancer)
- Osteosarcoma and Undifferentiated Pleomorphic Sarcoma of Bone Treatment
- Ovarian Cancer
- Pancreatic Neuroendocrine Tumors (Islet Cell Tumors)
- Papillomatosis (Childhood Laryngeal)
- Paraganglioma
- Paranasal Sinus and Nasal Cavity Cancer (Head and Neck Cancer)
- Parathyroid Cancer
- Penile Cancer
- Pharyngeal Cancer (Head and Neck Cancer)
- Pheochromocytoma
- Pituitary Tumor
- Plasma Cell Neoplasm/Multiple Myeloma
- Pleuropulmonary Blastoma (Lung Cancer)
- Pregnancy and Breast Cancer
- Primary Central Nervous System (CNS) Lymphoma
- Primary Peritoneal Cancer
- Pulmonary Inflammatory Myofibroblastic Tumor (Lung Cancer)
- Rare Cancers of Childhood
- Rectal Cancer
- Renal Cell (Kidney) Cancer
- Rhabdomyosarcoma, Childhood (Soft Tissue Sarcoma)
- Salivary Gland Cancer (Head and Neck Cancer)
- Childhood Rhabdomyosarcoma (Soft Tissue Sarcoma)
- Childhood Vascular Tumors (Soft Tissue Sarcoma)
- Osteosarcoma (Bone Cancer)
- Soft Tissue Sarcoma
- Uterine Sarcoma
- Sézary Syndrome (Lymphoma)
- Small Cell Lung Cancer
- Small Intestine Cancer
- Squamous Cell Carcinoma of the Skin - see Skin Cancer
- Squamous Neck Cancer with Occult Primary, Metastatic (Head and Neck Cancer)
- Stomach (Gastric) Cancer
- T-Cell Lymphoma, Cutaneous - see Lymphoma (Mycosis Fungoides and Sèzary Syndrome)
- Nasopharyngeal Cancer
- Oropharyngeal Cancer
- Hypopharyngeal Cancer
- Thymoma and Thymic Carcinoma
- Thyroid Cancer
- Tracheobronchial Tumors (Lung Cancer)
- Transitional Cell Cancer of the Renal Pelvis and Ureter (Kidney (Renal Cell) Cancer)
- Unknown Primary, Carcinoma of
- Ureter and Renal Pelvis, Transitional Cell Cancer (Kidney (Renal Cell) Cancer)
- Urethral Cancer
- Uterine Cancer, Endometrial
- Vaginal Cancer
- Vascular Tumors (Soft Tissue Sarcoma)
- Vulvar Cancer
- Wilms Tumor and Other Childhood Kidney Tumors
- Young Adults, Cancer in

American Psychological Association

The impact of transgender legislation
Psychological science points to an increased risk of suicide and poor mental health amid a record number of bills aimed at restricting the rights of the LGBTQ+ population
APA policy supporting transgender, gender diverse, nonbinary individuals
Membership in APA

APA Community
A new exclusive destination tailored for APA members

Membership benefits
Unlock the tools, discounts, and services included with your membership

Renew your membership
Keep your benefits and access to leading psychological information
Psychology topics spotlight

Misinformation and disinformation

Resources to navigate trauma

Tips to foster a healthy workplace
Science and practice of psychology

Ethics Code

Continuing Education

Grants, Awards, and Funding

Standards and Guidelines
Networks and communities

Network with peers, enhance your professional development, expand your personal growth, and more

APA Divisions

High school teachers

Undergraduate educators

Graduate students

Early career psychologists

Managing your career
Resources to help you throughout your career in psychology, including finding a job, salary data, finances and money management, mentoring and supervision, and training and professional development
Explore career paths

Psychologist profiles

How did you get that job?

Events and training
Featured jobs
Apa publications and products.

Write with clarity, precision, and inclusion
Children’s books
Monitor on Psychology
Newsletters
Reports and surveys
Continuing education
Merchandise

Real Siblings

Jacob's Missing Book

Harper Becomes a Big Sister
Attachment-Based Family Therapy for Sexual and Gender Minority Young Adults and Their Non-Accepting Parents
Dismantling Everyday Discrimination
APA Services

Learn how you can help APA advocate for psychology-informed federal policy and legislation, and support psychological research

APA Services, Inc.
A companion professional organization to APA, serving all members and advocating for psychology
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Study models how ketamine’s molecular action leads to its effects on the brain
Press contact :.
Previous image Next image
Ketamine, a World Health Organization Essential Medicine, is widely used at varying doses for sedation, pain control, general anesthesia, and as a therapy for treatment-resistant depression. While scientists know its target in brain cells and have observed how it affects brain-wide activity, they haven’t known entirely how the two are connected. A new study by a research team spanning four Boston-area institutions uses computational modeling of previously unappreciated physiological details to fill that gap and offer new insights into how ketamine works.
“This modeling work has helped decipher likely mechanisms through which ketamine produces altered arousal states as well as its therapeutic benefits for treating depression,” says co-senior author Emery N. Brown , the Edward Hood Taplin Professor of Computational Neuroscience and Medical Engineering at The Picower Institute for Learning and Memory at MIT, as well as an anesthesiologist at Massachusetts General Hospital and a professor at Harvard Medical School.
The researchers from MIT, Boston University (BU), MGH, and Harvard University say the predictions of their model, published May 20 in Proceedings of the National Academy of Sciences , could help physicians make better use of the drug.
“When physicians understand what's mechanistically happening when they administer a drug, they can possibly leverage that mechanism and manipulate it,” says study lead author Elie Adam , a research scientist at MIT who will soon join the Harvard Medical School faculty and launch a lab at MGH. “They gain a sense of how to enhance the good effects of the drug and how to mitigate the bad ones.”
Blocking the door
The core advance of the study involved biophysically modeling what happens when ketamine blocks the “NMDA” receptors in the brain’s cortex — the outer layer where key functions such as sensory processing and cognition take place. Blocking the NMDA receptors modulates the release of excitatory neurotransmitter glutamate.
When the neuronal channels (or doorways) regulated by the NMDA receptors open, they typically close slowly (like a doorway with a hydraulic closer that keeps it from slamming), allowing ions to go in and out of neurons, thereby regulating their electrical properties, Adam says. But, the channels of the receptor can be blocked by a molecule. Blocking by magnesium helps to naturally regulate ion flow. Ketamine, however, is an especially effective blocker.
Blocking slows the voltage build-up across the neuron’s membrane that eventually leads a neuron to “spike,” or send an electrochemical message to other neurons. The NMDA doorway becomes unblocked when the voltage gets high. This interdependence between voltage, spiking, and blocking can equip NMDA receptors with faster activity than its slow closing speed might suggest. The team’s model goes further than ones before by representing how ketamine’s blocking and unblocking affect neural activity.
“Physiological details that are usually ignored can sometimes be central to understanding cognitive phenomena,” says co-corresponding author Nancy Kopell , a professor of mathematics at BU. “The dynamics of NMDA receptors have more impact on network dynamics than has previously been appreciated.”
With their model, the scientists simulated how different doses of ketamine affecting NMDA receptors would alter the activity of a model brain network. The simulated network included key neuron types found in the cortex: one excitatory type and two inhibitory types. It distinguishes between “tonic” interneurons that tamp down network activity and “phasic” interneurons that react more to excitatory neurons.
The team’s simulations successfully recapitulated the real brain waves that have been measured via EEG electrodes on the scalp of a human volunteer who received various ketamine doses and the neural spiking that has been measured in similarly treated animals that had implanted electrode arrays. At low doses, ketamine increased brain wave power in the fast gamma frequency range (30-40 Hz). At the higher doses that cause unconsciousness, those gamma waves became periodically interrupted by “down” states where only very slow frequency delta waves occur. This repeated disruption of the higher frequency waves is what can disrupt communication across the cortex enough to disrupt consciousness.
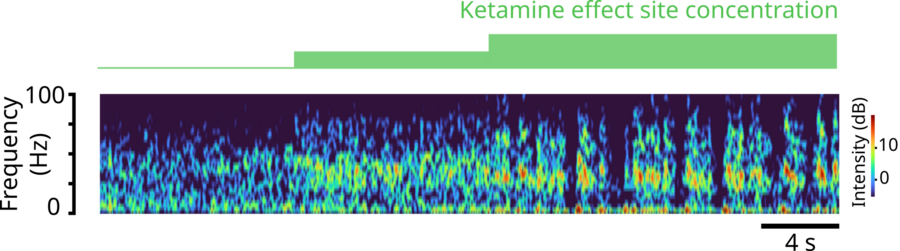
Previous item Next item
But how? Key findings
Importantly, through simulations, they explained several key mechanisms in the network that would produce exactly these dynamics.
The first prediction is that ketamine can disinhibit network activity by shutting down certain inhibitory interneurons. The modeling shows that natural blocking and unblocking kinetics of NMDA-receptors can let in a small current when neurons are not spiking. Many neurons in the network that are at the right level of excitation would rely on this current to spontaneously spike. But when ketamine impairs the kinetics of the NMDA receptors, it quenches that current, leaving these neurons suppressed. In the model, while ketamine equally impairs all neurons, it is the tonic inhibitory neurons that get shut down because they happen to be at that level of excitation. This releases other neurons, excitatory or inhibitory, from their inhibition allowing them to spike vigorously and leading to ketamine’s excited brain state. The network’s increased excitation can then enable quick unblocking (and reblocking) of the neurons’ NMDA receptors, causing bursts of spiking.
Another prediction is that these bursts become synchronized into the gamma frequency waves seen with ketamine. How? The team found that the phasic inhibitory interneurons become stimulated by lots of input of the neurotransmitter glutamate from the excitatory neurons and vigorously spike, or fire. When they do, they send an inhibitory signal of the neurotransmitter GABA to the excitatory neurons that squelches the excitatory firing, almost like a kindergarten teacher calming down a whole classroom of excited children. That stop signal, which reaches all the excitatory neurons simultaneously, only lasts so long, ends up synchronizing their activity, producing a coordinated gamma brain wave.
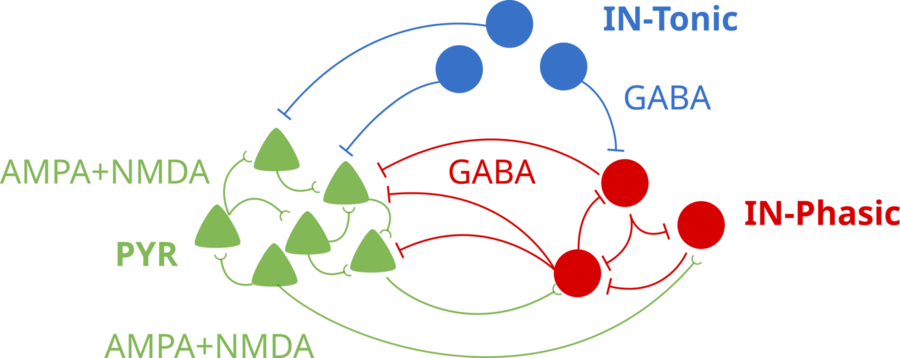
“The finding that an individual synaptic receptor (NMDA) can produce gamma oscillations and that these gamma oscillations can influence network-level gamma was unexpected,” says co-corresponding author Michelle McCarthy , a research assistant professor of math at BU. “This was found only by using a detailed physiological model of the NMDA receptor. This level of physiological detail revealed a gamma time scale not usually associated with an NMDA receptor.”
So what about the periodic down states that emerge at higher, unconsciousness-inducing ketamine doses? In the simulation, the gamma-frequency activity of the excitatory neurons can’t be sustained for too long by the impaired NMDA-receptor kinetics. The excitatory neurons essentially become exhausted under GABA inhibition from the phasic interneurons. That produces the down state. But then, after they have stopped sending glutamate to the phasic interneurons, those cells stop producing their inhibitory GABA signals. That enables the excitatory neurons to recover, starting a cycle anew.
Antidepressant connection?
The model makes another prediction that might help explain how ketamine exerts its antidepressant effects. It suggests that the increased gamma activity of ketamine could entrain gamma activity among neurons expressing a peptide called VIP. This peptide has been found to have health-promoting effects, such as reducing inflammation, that last much longer than ketamine’s effects on NMDA receptors. The research team proposes that the entrainment of these neurons under ketamine could increase the release of the beneficial peptide, as observed when these cells are stimulated in experiments. This also hints at therapeutic features of ketamine that may go beyond antidepressant effects. The research team acknowledges, however, that this connection is speculative and awaits specific experimental validation.
“The understanding that the subcellular details of the NMDA receptor can lead to increased gamma oscillations was the basis for a new theory about how ketamine may work for treating depression,” Kopell says.
Additional co-authors of the study are Marek Kowalski, Oluwaseun Akeju, and Earl K. Miller.
The work was supported by the JPB Foundation; The Picower Institute for Learning and Memory; The Simons Center for The Social Brain; the National Institutes of Health; George J. Elbaum ’59, SM ’63, PhD ’67; Mimi Jensen; Diane B. Greene SM ’78; Mendel Rosenblum; Bill Swanson; and annual donors to the Anesthesia Initiative Fund.
Share this news article on:
Related links.
- Institute for Medical Engineering and Science
- The Picower Institute for Learning and Memory
- Department of Brain and Cognitive Sciences
Related Topics
- Pharmaceuticals
- Neuroscience
- Brain and cognitive sciences
- Health sciences and technology
- Health care
- Picower Institute
- Institute for Medical Engineering and Science (IMES)
Related Articles
Anesthesia technology precisely controls unconsciousness in animal tests
Study finds tracking brain waves could reduce post-op complications

3 Questions: Emery Brown on improving anesthesia with neuroscience
Statistical model defines ketamine anesthesia’s effects on the brain
More mit news.

Through econometrics, Isaiah Andrews is making research more robust
Read full story →
Students research pathways for MIT to reach decarbonization goals

Improving working environments amid environmental distress

A data-driven approach to making better choices

Paying it forward

John Fucillo: Laying foundations for MIT’s Department of Biology
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pharmaceutics
- PMC10747851

Drug Delivery to the Brain: Recent Advances and Unmet Challenges
Sukanya bhunia.
1 Department of Immunology and Nano-Medicine, Herbert Wertheim, College of Medicine, Florida International University, Miami, FL 33199, USA
2 Institute of Neuroimmune Pharmacology, Herbert Wertheim College of Medicine, Florida International University, Miami, FL 33199, USA
Nagesh Kolishetti
Arti vashist, adriana yndart arias, deborah brooks, madhavan nair.
Brain cancers and neurodegenerative diseases are on the rise, treatments for central nervous system (CNS) diseases remain limited. Despite the significant advancement in drug development technology with emerging biopharmaceuticals like gene therapy or recombinant protein, the clinical translational rate of such biopharmaceuticals to treat CNS disease is extremely poor. The blood–brain barrier (BBB), which separates the brain from blood and protects the CNS microenvironment to maintain essential neuronal functions, poses the greatest challenge for CNS drug delivery. Many strategies have been developed over the years which include local disruption of BBB via physical and chemical methods, and drug transport across BBB via transcytosis by targeting some endogenous proteins expressed on brain-capillary. Drug delivery to brain is an ever-evolving topic, although there were multiple review articles in literature, an update is warranted due to continued growth and new innovations of research on this topic. Thus, this review is an attempt to highlight the recent strategies employed to overcome challenges of CNS drug delivery while emphasizing the necessity of investing more efforts in CNS drug delivery technologies parallel to drug development.
1. Introduction
Despite the recent advances in genomics and neurobiology, central nervous system (CNS) diseases from brain tumors to neurological diseases continue to remain a global concern due to its complex protective structure [ 1 ]. As per the estimation of WHO in 2016, one-third of the global population is impacted by neurological or psychiatric conditions at some point in their lifetime with Alzheimer’s disease (AD) alone is estimated to cost US$2 trillion by 2030 [ 2 ]. In addition, brain tumor remains to be the most diagnosed solid tumor in children and adolescents and the leading cause of cancer death among young adults [ 3 ]. In spite of the advancement of drug discovery technologies, drug development for CNS diseases remains a formidable task with a probability of only a few percentage (~8.2%) of drugs developed to be translated for clinical use [ 4 ]. The heterogeneity of CNS diseases and the lack of proper preclinical models to accurately mimic human pathology play crucial roles, these challenges of drug delivery to the brain are the major factors behind the poor clinical translation rate of CNS drugs.
Introducing drugs into the brain poses a unique set of challenges compared to other body tissues due to protective mechanisms that safeguard brain tissue externally and internally. Externally, the brain is protected by the skull and three inner layers of membranes known as meninges which regulate intracranial tissue pressure by constraining the volume [ 5 ] while it is cushioned by cerebrospinal fluid (CSF) that flows within the meninges and acts as a shock absorber to protect the brain from injury. Internally, the brain benefits from the presence of the blood–brain barrier (BBB). BBB prevents random entry of solutes, including bloodborne pathogens and neurotoxins to maintain the highly regulated CNS microenvironment essential for neuronal functions while permitting the exchange of nutrients and metabolic waste to maintain brain homeostasis. The intracranial tissue pressure in addition to the hurdle of invasive drilling of the skull largely limits the scope of local drug delivery to the brain while the systemic drug delivery to the brain is greatly hampered by the blood–brain barrier which rejects ~98% of substances. Biopharmaceuticals such as recombinant proteins or monoclonal antibodies (mAb) which have emerged as a promising part of drug development in the past two decades have failed in treating CNS disease due to their poor access to the brain across the BBB. For instance, Bevacizumab (Avastin) [ 6 ] and Natalizumab (Tysabri) [ 7 ], which are FDA approved monoclonal antibody-based therapeutics for treating brain cancer and multiple sclerosis, respectively, do not cross the BBB [ 8 ]. Thus, there is an urgent need for efficient technology that can effectively deliver pharmaceuticals to brain with minimum adverse effect. To this end, several strategies have been developed and evaluated in pre-clinical and clinical settings over the past decades, but none of these have yet turned out to be groundbreaking.
Herein, we have provided a overview on the structural aspects of the blood–brain barrier limiting the systemic drug delivery to the brain and discussed the drug delivery strategies to overcome it with a focus on the physical and cellular stimulation of BBB for enhanced permeation of pharmaceutics across BBB. In addition, we have discussed the localized drug delivery strategies for getting drugs into the brain and finally, have shed light on the importance of research efforts not only to drug development but also to delivery strategies for brain.
2. Structure and Function of Blood–Brain barrier
BBB is an endothelial membrane barrier within brain microvascular formed by tight junction of brain capillary endothelial cells (BCEC) sheathed by mural cells and astrocytes end-feet that separate CNS from systemic blood circulation ( Figure 1 A,B) [ 9 , 10 ]. The BBB protects the brain by preventing random entry of substances like neurotoxins and bloodborne pathogens while maintaining the brain homeostasis by selective passage of nutrients. BBB is composed of a continuous layer of BCEC tightly connected by junction proteins sheathed by mural cells (pericyte at the microcapillary and vascular smooth muscle cells in arteries and arterioles), basement membrane, glial cells (astrocyte, microglia and oligodendrocyte) and neurons which are together known as neurovascular unit (NVU) [ 1 , 11 ]. Cellular components of NVU functionally interact to maintain integrity of microvasculature including BBB and regulate cerebral blood flow. The CNS microvascular differs from the peripheral microvascular, the former can be extremely thin (thickness ~200 nm) and the intercellular junction of BCEC is ~50–100 times tighter compared to the peripheral. In addition, there is no fenestration in BCEC with limited number of pinocytotic vesicles unlike peripheral blood endothelial cells. This requires energy dependent active transport pathway for nutrient transport across BBB which further supports the presence of ~5–6 times more mitochondria in BCEC. Furthermore, presence of proteolytic enzymes capable of rupturing neuroactive bloodborne solutes and drugs in BCEC offers an additional enzymatic barrier [ 11 ].

( A ) Schematic representation of the neurovascular unit (NVU) comprised of neurons, vascular cells (endothelial cells-EC, smooth muscle cells-SMC at arterioles, and pericytes-PC at capillary), glial cells (astrocytes-Ast and microglia). EC are covered by PC and astrocyte end-feet which are embedded in the basement membrane (BM). Neurons communicate with adjacent mural cells (PC and SMC) to regulate blood flow in the brain microvasculature. Microglia lies around the open area of brain capillary that is not covered by astrocyte end-feet. ( B-i ) Transmission electron micrograph (TEM) of brain tissue depicting NVU ( B-ii ) component EC, PC, Ast, and tight junction; Adopted with permission from Ref. [ 1 ] ( C ) schematic of blood–brain barrier comprising interconnected brain capillary endothelial cells via junction proteins ( D ) along with the other cellular components of NVU.
Pericytes are embedded in vascular basement membrane and cover approximately 20% of abluminal (outer) surface of the BBB. These cells possess contractile proteins, allowing them to regulate blood flow in brain capillaries through contraction and relaxation. There are two basal lamina basement membranes (BMs), the inner vascular BM is formed by the extracellular matrix (ECM) secreted by BCEC and pericytes and the outer parenchymal BM is formed by secreted ECM from astrocytic process. The BMs anchor for signaling process and acts as an additional barrier. Astrocytes, a major glial cell type, play essential role in maintaining structure and function of the BBB [ 12 ]. The end-feet of astrocytes create an intricate network that surrounds BCECs, strengthening the tight junctions, almost completely ensheathe the brain endothelial capillary and maintain structural integrity of the BBB. It also connects BCEC with neurons and mediate inter-cellular communication to regulate vascular contraction/dilution and blood flow in response to neuronal response [ 12 ]. In addition, astrocytes play crucial role in maintaining brain homeostasis, clearing synapses, injury protection and considered as the primary workhorse of the CNS for such versatile roles [ 13 ]. Microglia mediates immune regulation in brain and plays crucial role in maintaining CNS homeostasis. In addition, recent studies support that activated microglia can increase the expression of tight junction proteins [ 14 ].
Three types of junctions, namely, tight junctions (TJ), adherens junctions (AJ), and gap junctions play role behind the extremely tight connection among adjacent BCEC ( Figure 1 C,D). Tight junctions contains both transmembrane proteins such as Junction adhesion molecules (JAMs), claudins, occludins, etc., and cytoplasmic proteins like zonula occludins (ZO), afadin (e.g., AF-6), cingulin, etc. [ 15 ] Claudins (~27 kDa) is the most crucial transmembrane tight junction proteins within the BBB. Claudins extracellular segments create TJ that tightly seal the space between neighboring BCECs, while their intracellular domains connect with actin filaments ( Figure 1 D). Occludins is another transmembrane protein exclusively localized at the tight junctions and perform similar function like claudins. JAMs are expressed in tight junction of BCEC regulate migration of leukocyte and platelet via integrin receptor based adhesive interaction. The cytoplasmic domain of TJs interacts with cytoskeletal and the basal adherens junction proteins which are also essential to maintain barrier property of the BBB.
The Adherens junction, which is formed by homodimeric transmembrane cadherin protein at the basal side, is important for proper assembly of tight junction proteins and structural integrity of the BBB [ 16 ]. The extracellular domain of vascular endothelial (VE)-cadherin of one BCEC span across the paracellular cleft to dimerize with another extracellular domain of VE-cadherin from neighboring BCEC to provide the structural support while the cytoplasmic domains are connected to actin filament via catenin proteins. Stabilization of catenin induces expression of claudin-3 which supports assembly of the tight junction. Platelet EC adhesion molecule 1 (PECAM-1), nectin, CD99 are other transmembrane protein components of adherens junction, and their roles are still under investigation. Gap junction, which structurally mimic an intercellular channel formed by hexamer of integral proteins connexins and pannexins (e.g., Cx37, Cx40, Cx43) connecting to adjacent endothelial cells, is located between the TJs and AJs [ 17 ]. It permits the exchange of ions, small metabolites, and signals between adjacent BCEC and plays important role in maintaining homeostasis of the BBB. Furthermore, it governs the permeability by engaging with cytoplasmic proteins like ZO-1 through afadin-6 protein. In combination, the presence of these particular endothelial junctions, especially TJ, markedly hinders the transit of random substances across the BBB. The encapsulation of brain endothelial cell capillaries by the astrocytes and pericytes further contributes to the tightness of the BBB which can be estimated by a parameter known as transendothelial electrical resistance (TEER) as 1500–2000 Ω cm 2 [ 18 ].
The BBB undergoes structural and functional alteration in CNS diseases which often compromises its structural integrity. For instance, in the context of brain tumors, BBB is referred as blood–tumor barrier (BTB) and it exhibits distinct features, including the loss of junctional proteins in endothelial cells, aberrant distribution of pericyte, loss of astrocytic endfeet and neuronal connections, and an increased infiltration of circulating immune cells into glioma tissue [ 19 , 20 ]. Furthermore, with the progression of tumor, vascularization greatly hampered the structural integrity of BBB. With an average-sized tumor, approximately 10% of the BBB may display open junctions, while around 30% may develop fenestrations that allow the passage of molecules up to 330 kDa in size [ 21 , 22 ]. It is important to note that, despite the disruption in the core of the tumor, the BBB may still maintain its barrier properties intact in other areas of brain. The BTB shares common characteristics with the BBB, including the expression of efflux transporters in endothelial cells and tumor cells. Additionally, the BTB often exhibit higher expression of certain receptors that promote tumor growth, such as GLUT1 and BCRP [ 23 ]. Similar pathological breakdown of barrier property is also observed in neurological diseases [ 10 ]. For leukocytes, altered expression of ion-channel receptors, and transporters are also observed that compromises protective function of the BBB.
3. Approaches for Drug Delivery through the Blood–Brain Barrier
Over the past few decades, diverse strategies have emerged to enhance the transportation of drugs through the BBB ( Figure 2 ). These strategies include temporary disruption of BBB via physical or chemical means as well as targeting some endogenous transporter systems over-expressed on BBB.

Summary of drug delivery strategies to brain.
3.1. Temporary Disruption of BBB
3.1.1. osmotic blood–brain barrier disruption.
In this process ( Figure 3 ), BBB permeation is achieved using a hyperosmotic agent which causes dehydration and shrinkage in BCEC resulting tight-junction dysfunction and transient disruption of BBB. This process of osmotic BBB disruption was first hypothesized by Rapoport et al. in 1972 [ 24 ] following an improved BBB permeation of a dye Evan’s blue when co-administered with hypertonic arabinose and later supported by Brightman et al. who visualize the opening of tight junction with electron microscopy after intra-carotid infusion of mannitol [ 25 ]. A variety of substances have been used as osmotic disruptors of the BBB including urea, lactamide, saline but mannitol has been most used for this purpose. Since 1980, intracarotid artery hyperosmotic mannitol (ICAHM) infusions has been used for drug delivery to brain in several pre-clinical and clinical studies [ 26 ] many of which have produced encouraging results of enhanced survivability with clinical safety. For instance, a clinical study conducted in 17 patients with primary CNS lymphoma receiving cyclophosphamide and mannitol followed by radiotherapy significantly enhances the mean survivability (from 17.8 months to 44.5 months) compared with the control group receiving radiotherapy alone [ 27 ]. Combination of carboplatin and etoposide delivered in this method exhibits an effective delivery in brain and dramatic responses in inhibiting CNS tumor in patients although unexpected high-frequency hearing loss limits the application of the combined chemotherapy [ 28 ]. Some studies in animal models have demonstrated variable and inconsistent results in BBB permeability like nonselective opening of BBB, CNS toxicity and neuroinflammatory response become the major limitation of this approach [ 29 , 30 , 31 ]. The success of this strategy depends on multiple factors, including injection speed, optimum mannitol dose, cerebral hemodynamics, and vascular anatomy. Strategies to overcome the limitation are currently under investigation, e.g., use of real time MRI guidance for optimum and targeted delivery of therapeutics [ 32 , 33 ]. Overall, mannitol mediated osmotic disruption of BBB for drug delivery to brain is safe and hold promise while further investigation is needed to improve its reproducibility and clinical effectiveness.

Schematic of focus ultrasound-mediated ( A ) and osmotic ( B ) disruption of BBB.
3.1.2. BBB Disruption with Focused Ultrasound
In this method ( Figure 3 ) local BBB permeation can be achieved by using focused ultrasound (FUS) in combination with intravenous microbubbles and can be monitored by using MR-imaging system. This method has several advantages over other methods. It is reproducible, non-invasive, and targeted opening of the BBB can be achieved. In addition, the BBB opening is transient which can be restored within 6 to 24 h allowing accumulation of therapeutics in the region of interest for a desired time window.
The FUS technology was first introduced in 1950s initially to treat psychiatric disorders and brain tumors although those early attempts were invasive involving craniectomy to introduce sonication into brain which has been evolved to non-invasive over time by decades of research [ 34 , 35 , 36 , 37 ]. Although the minimal invasiveness to reduce surgical trauma and recovery time are the driving force, the skull bone which varies in thickness and density among individuals greatly attenuates and distorts ultrasound. In addition, hair, which introduces air, significantly (up to 80%) distorts the delivery of ultrasound. The implementation of phased array transducers along with real-time MRI-thermal monitoring has been a breakthrough in this century to made non-invasive transcranial FUS feasible [ 37 ].
The cellular and molecular mechanism of FUS-mediated enhanced BBB permeation is poorly understood. The sheer stress from the stable acoustic cavitation of the microbubbles induces structural and functional modulation in the BBB like higher caveolae formation, sonoporation, as well as opening of some tight junctions which enhance intracellular and paracellular transport [ 38 , 39 , 40 ]. Although stable cavitation contributes to loosening of tight junction, inertial cavitation may contribute to hemorrhage and ischemia. Nonetheless, microbubble cavitation can be controlled by tuning ultrasound pressure amplitude and low-frequency FUS-mediated BBB opening rule out the thermal effect on the BBB. Notably, FUS activates PI3kinase/Akt pathway in neuronal cells which may play role in modulation of tight junction proteins ZO-1 and occludin [ 41 ]. Cerebral vessels are resilient to such mechanical stress caused by stable microbubbles cavitation and quickly recover their integrity after the FUS.
As indicated before, FUS can induce local and targeted opening of the BBB with a desired time window. The extent of BBB opening can be controlled by tuning ultrasound pressure amplitude, transducer frequency, microbubble size and dosage, exposure duration and burst parameters [ 42 , 43 , 44 ]. For instance, a study by Chen et al. has demonstrated that FUS can enable trans-BBB delivery of dextran molecule up to 2000 kDa (hydrodynamic diameter 2.3 to 54.4 nm) at a 0.84 MPa acoustic pressure [ 45 ]. However, small opening (70 kDa) can be achieved by stable cavitation whereas larger BBB opening (>500 kDa) is associated with inertial cavitation. Thus, FUS has been demonstrated to markedly enhance the trans-BBB delivery of therapeutic antibodies (~150 kDa, e.g., Herceptin) [ 7 , 44 , 46 , 47 , 48 ], chemotherapeutics [ 49 ], and cells [ 50 , 51 , 52 ] and shows clinical promise in treating brain tumor and other CNS diseases [ 37 , 53 ]. Furthermore, studies indicate that FUS can be utilized to target therapeutics in different regions of the brain such as the hippocampus [ 54 ], striatum [ 55 ], cortical targets [ 46 ], and brainstem [ 49 ]. The safety of FUS-mediated BBB opening is promising. A mild and short term (<2 weeks) immune response is reported after repeated administration [ 56 , 57 , 58 , 59 ]. Importantly, behavioral, morphological, and neuroimaging characteristics are retained even after long-term repeated administration of FUS in animal models (biweekly over 6 months in rats or 4 months in non-human primates) [ 60 , 61 ].
3.1.3. Radiation-Mediated BBB Disruption
Few studies have reported that radiation therapy, an important modality in treating brain tumor, may play a role in disrupting the BBB and enhance drug entry to brain [ 62 ]. However, the role of radiation in increasing drug accumulation in brain and its underlying mechanisms are still uncertain. In addition, radiation induced BBB disruption is not temporary and the recovery time is much higher (in years) which often lead to radiation induced toxicity including headache, neurologic deficits or nausea [ 63 ].
3.1.4. Interfering the Tight Junction of BBB with Chemicals
Disengaging the tight junctions of BBB is another strategy to improve permeability across BBB. Bradykinin (BK), a peptide containing 9 aminoacids upon administration causes dilation of arterioles and enhances paracellular transport by down-regulating expression of the tight junction proteins (occluding, ZO-1, and claudin-5) and improves transcellular transport by upregulating caveolin mediated pinocytotic vesicles [ 64 ]. The BBB opening potential of bradykinin, and its synthetic analogs, have been explored [ 65 , 66 , 67 ] especially in brain tumors due to the high expression of BK receptor at BTB [ 68 ]. However, it did not go through Phase-II mainly because the extremely transient opening of BBB and the adverse side effects due to the wide distribution of BK receptors at numerous additional sites beyond the BBB [ 69 ]. BBB disruption via targeting claudin-5, a major component of BBB tight junctions, via siRNA mediated knockdown or using anti-claudin5-antibody also demonstrated to enhance BBB permeation transiently and reversibly [ 70 , 71 ]. It also suffers similar limitations of transient effects and adverse side effects due to wider distribution of receptor expression. To this end, targeting Angulin-1, another functional constituent of BBB tricellular tight junctions which is majorly expressed in BBB and selectively blocks entry of macromolecules into the brain, can address the adverse effects [ 72 ]. Angubindin-1, a ligand of angulin-1, is demonstrated to enhance the entry of macromolecules across BBB by removing angulin-1 and disrupting the tricellular tight junctions [ 73 ].
3.2. Drug Transport without Disrupting BBB: Active and Passive Transport Pathways
Recent strategies of drug delivery to brain without disrupting BBB can be classified into two types based on their energy (adenosine triphosphate (ATP)) requirements during the process: passive and active transport ( Figure 4 ) [ 74 ]. Passive transport is an energy-independent process that lacks specificity. It includes the diffusion of small molecules through paracellular and transcellular pathways. Paracellular diffusion involves solute molecules moving between adjacent endothelial cells due to a negative concentration gradient. Only water-soluble molecules can pass through the paracellular space. In transcellular diffusion, non-ionic solute (molecular weight < 400 Da) with a desirable lipophilicity (e.g., hormones and steroids) can diffuse through the endothelial cells to brain [ 75 ]. However, in addition to the tight junction, some efflux pumps present at the luminal side of BCEC also limit the drug transport across BBB. Efflux pumps function in two phases, it initially inhibit cellular uptake of drug molecules in BCEC and later expel the drugs molecules (like doxorubicin, daunorubicin etc.) into blood against a negative concentration gradient in ATP dependent pathway [ 76 ]. P-glycoprotein (P-gp) is an example of efflux pump that plays a role in drug resistance in tumors. Regulating efflux pumps at the BBB represents another strategy for drug delivery to brain tumors. It is important to note that efflux pumps, although beneficial for protecting the healthy brain from harmful neurotoxins, can also pose challenges in drug delivery to brain tumors.

Schematics for drug transport pathways across BBB.
The active transport pathway often exploits endogenous receptor or transporter proteins that are expressed on the luminal side of BBB. Active transport routes include receptor mediated transcytosis (RMT), carrier mediated transcytosis, adsorption-mediated transcytosis, and cell-mediated transcytosis, all of which require ATP. In RMT, particles cross BBB via interaction with specific receptors expressed on apical surface of BCEC. It is an important pathway and is widely being explored for delivery of macromolecular biopharmaceuticals (e.g., protein or recombinant peptide-based therapeutics) or nanocarrier-mediated drug transport to the brain. The mechanism of RMT centers on endocytosis, where a ligand selectively binds to a receptor. This binding leads to the creation of an intracellular vesicle through membrane invagination. These vesicles are then transported and fused with the basolateral membrane, subsequently releasing the payloads as they detach from the membrane. It is worth mentioning that, like general endocytosis, in addition to the transcytosis from blood to brain some vesicles undergo lysosomal degradation while some others are recycled to the apical side in RMT. This process often targets specific receptors, including transferrin receptors, low-density lipoprotein (LDL) receptors, and insulin receptors for drug delivery to the brain.
Carrier- or transporter- mediated transcytosis (CMT) represents another dynamic active transport mechanism across the BBB, facilitating the transportation of essential nutrients such as amino acids and glucose into the brain. Nutrient molecules bind to the specific transporter proteins on the luminal side, causing conformational changes that enable the transfer of these nutrients into the brain. Glucose transporter isoform (GLUT-1) and large amino-acid transporter (LAT) are examples of such transporter. Small molecule drugs like L-DOPA and gabapentin utilize CMT to reach the CNS. However, the high specificity required for the interaction between transporters and ligands in this process limits its applicability in transporting macromolecular therapeutics [ 77 ]. Charged particles such as nanocarriers, predominantly traverse the BBB through adsorptive mediated transcytosis (AMT), relying on the electrostatic interactions between the negatively charged cell surface of BCEC and the particles [ 78 ]. Such interactions are non-specific, and many nanocarriers can be delivered; however, this is not devoid of the non-specific accumulation in other organs under systemic circulation. Cell-mediated transcytosis, which utilizes blood cells capable of BBB crossing for delivering drug to brain, has recently emerged as biomimetic strategy. In this method, immune cells or platelets are incorporated with drug-loaded nanocarriers which then cross BBB and navigate towards the inflammation sites within the brain by responding to chemotaxis signals and undergoing diapedesis [ 79 ]. More recently, extracellular vesicles, e.g., exosomes, have attracted significant attention as biomimetic drug carrier for CNS drug delivery. In addition, viral vectors have shown promise for gene delivery to brain. Further nanocarrier-mediated approaches have gained significant interest for efficient delivery to brain.
3.2.1. Nanocarriers Mediated Drug Transport across BBB
Nanoparticles (NPs) such as liposomes, polymeric NPs, inorganic NPs, etc., are being used as drug carriers for decades [ 80 , 81 , 82 , 83 , 84 , 85 ]. Drug loading in NPs enhances circulation life of hydrophobic drugs in blood, protect nucleic-acid-based therapeutics from serum nucleases, or reduce the adverse off-target effects of drugs [ 86 , 87 , 88 , 89 , 90 ]. Surface of NPs can be engineered with PEG to achieve longer circulation life or with cell-penetrating peptide to enhance cellular uptake [ 91 ], or with targeting ligand to selectively deliver the payloads at targeted tissue [ 92 ]. Furthermore, drug release at the targeted tissue can be externally controlled by using stimuli-responsive nanocarriers [ 82 ]. Over the past few decades, many nanocarriers with size range ~10–300 nm have been explored for delivering small molecules, nucleotides, peptides, or proteins-based therapeutics to brain for combating various CNS diseases including brain tumor, neurodegenerative disorders, neuroHIV, stroke, etc. [ 82 , 88 , 89 , 90 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 ]. Such nanocarriers can cross the BBB by passive diffusion or can be engineered with some ligand at their exo-surfaces actively targeting some endogenous receptor/transporter protein on the BBB. For instance, liposomal encapsulation of cytotoxic anti-neoplastic agent doxorubicin has significantly mitigated the adverse effect of systemic chemotherapy as indicated by the enhanced safety index in a phase I trial involving 13 children with recurrent/refractory high-grade glioma ( {"type":"clinical-trial","attrs":{"text":"NCT02861222","term_id":"NCT02861222"}} NCT02861222 ) [ 104 ]. Similarly, liposomal encapsulation of irinotecan has improved the safety profile of systemic chemotherapy in another phase I study with 34 high-grade glioma patients ( {"type":"clinical-trial","attrs":{"text":"NCT02022644","term_id":"NCT02022644"}} NCT02022644 ) permitting its progression for Phase II trial [ 105 ]. However, although such encapsulation of cytotoxic chemotherapeutics improved the safety index of systemic chemotherapy in patients, the efficacy of nanoformulations might be facilitated by their passive accumulation via compromised integrity of the BBB around high-grade tumors. Clearly, there is a need for an active transport mechanism across the BBB for delivering drugs to combat low-grade tumor or other CNS diseases with intact or less compromised integrity of BBB.
Active targeting of receptor or transporter proteins expressed in brain capillary endothelial cells (BCEC) is the most widely explored nanocarrier-based drug delivery strategy across BBB. In this method, nanocarriers are surface engineered with targeting ligands of such receptors/transporters to deliver payload in brain via RMT or CMT which has been reviewed elsewhere in detail [ 106 , 107 ]. Although Transferrin, LDL family receptors (LDLR), insulin, and integrin receptors are widely explored receptors due to their high receptor-ligand affinity, GLUT and LAT-1 are some transporter proteins that are explored for drug delivery to brain.
Transferrin Receptor: Transferrin receptors (TfRs) control iron homeostasis via their natural ligand transferrin. TfRs are highly expressed in the luminal side of BBB and in brain tumors which makes them an attractive target for drug delivery to the brain [ 108 ]. Different TfRs ligands such as transferrin (Tf) itself (~80 kDa) [ 109 ], antibodies or antibody fragments [ 110 ], and peptides [ 111 , 112 ] are explored to examine their brain targeting efficacy by grafting such ligands with the biopharmaceuticals or at the exo-surface of nanocarriers which is reviewed in detail elsewhere [ 113 ]. For instance, Lam et al. have developed a transferrin-functionalized PEGylated liposomes for simultaneous delivery of temozolomide (TMZ) and bromodomain inhibitor in brain tumor. The combined chemotherapy regimen overcome the drug resistance of TMZ, reduced the tumor size, and improved the survival of mice with glioma compared to control groups, all while showing minimal systemic drug toxicity [ 109 ]. To overcome the plausible inhibition of RMT by competitive binding of endogenous Tf, nanocarriers are also surface engineered with monoclonal antibody (mAb), or peptide fragments targeting TfR. For instance, Yue et al. has conjugated OX26 antibody, a monoclonal antibody against TfR1, with micelles to develop an immunomicelle which shows high BBB-crossing ability [ 110 ]. A TfR specific heptapeptide T7 (HAIYPRH) with high binding affinity (K d = 10 nM) has also been explored to target nucleotides and neoplastic drugs in glioma tissue in preclinical model [ 111 , 112 ]. Although such studies are at the preclinical stages, some have shown initial clinical promise [ 95 ]. For instance, a fusion of lysosomal enzyme iduronate 2- sulfatase (IDS) with anti- TfR antibody (JR-141) enabled successful delivery of the fusion protein into the CNS of patients with Hunter Syndrome under systemic settings (i.v.) in a phase I/II trial ( {"type":"clinical-trial","attrs":{"text":"NCT03128593","term_id":"NCT03128593"}} NCT03128593 ) which shows promising therapeutic efficacy with no significant safety issue [ 114 ]. Notably, the use of TfR-targeting Tf-toxin conjugates has demonstrated clinical potential in anti-glioma therapy. Human Tf is linked to a diphtheria toxin featuring a CRM107 point mutation, resulting in the creation of Tf-CRM107. This conjugate displayed tumor growth inhibition when administered directly into the tumor in a U251 mouse model [ 115 ]. Subsequently, a phase I study following intra-tumoral injection revealed no adverse effects, leading to a phase II study involving patients with recurrent high-grade brain tumors. Although 35% of the patients displayed positive tumor responses and improved survival, the phase III was discontinued due to probable CNS toxicity with an indication for more targeted delivery of the toxin [ 116 ].
The sub-optimal clinical efficiency of TfRs targeting may be related to the high recycling rate (~90%) of endocytosed TfRs by BCEC to the luminal side as indicated by studies in mouse brain [ 117 ] where only 10% of TfR-NPs are able to reach brain parenchyma. Efforts to improve rate of transcytosis via varying ligand density on nanocarrier [ 118 ] or increasing receptor-ligand affinity are being examined [ 119 ]. Bivalent TfR antibodies with high receptor-affinity diverts the trafficking into lysosomes and subsequent degradation of the therapeutics indicating requirement of optimum receptor-ligand affinity for effective transcytosis [ 120 , 121 ]. Furthermore, interspecies variation of receptors, such as 2.5 times higher expression level of TfRs in mouse brain microvessels compared with that in human also contributes to the reduced efficacy during clinical translation of such active targeting strategies. Finally, ubiquitous expression of TfRs in other organs (liver, spleen, and bone-marrow) and uptakes of drugs in non-peripheral tissues also contribute to the compromised therapeutic efficacy of such targeting strategy [ 118 ].
LDL family receptors: LDL receptor (LDL-R) and receptors for LDL-R related proteins (LRP) are the most explored among LDL family receptors for drug delivery to CNS. This is a class of receptors that help lipid transport to the brain [ 122 ] and are expressed in CNS cells, BBB endothelium, and upregulated in cell surface of glioma cells [ 123 , 124 ]. Apolipoprotein B (ApoB) and ApoE are the ligands of LDL-R. ApoE, which is prevalent in the brain, especially in CSF and plasma, has been widely explored for drug delivery to brain. LDL-R mediated transcytosis of NPs are generally two types which rely on (i) avidity-based surface attachment of ApoE to NPs or (ii) surface-functionalization of NPs via conjugation with ApoE or its derivatives. For instance, poly-butylcyanoacrylate (PBCA) NPs coated with surfactant polysorbate 80 (PS-80) [ 125 ] show enhanced cellular internalization of the NPs by 20-fold in human and bovine endothelial cells compared to the uncoated NPs [ 126 ]. Later mechanistic study reveals that this surface coating with PS-80 enables adsorption of plasma ApoE onto NPs which is then recognized LDL-R expressed in BCEC and undergoes RMT to brain parenchymas [ 127 ]. Study indicates that such receptor–ligand interaction is strong to exclude the size-effect of NPs in the BBB crossing when examined with NPs of varying sizes from 87 nm to 464 nm [ 128 ]. Such PS-80 coating approach to enhance brain delivery has also been explored for other nanoparticles (e.g., polylactic acid (PLA), solid lipid nanoparticles (SLN), in different combination of therapeutics which has been reviewed in detail elsewhere [ 129 ]. In the second strategy, synthetic peptides containing short binding sequence to LDL-R has shown some pre-clinical promise. For instance, Grafals-Ruiz et al. designed gold-liposome nanocarriers with ApoE peptides on their exo-surface for the systemic delivery of small-nucleic acids to the brain of mice with gliomas [ 130 ].
LDL-R related proteins (LRP), especially LRP1 has also been targeted for RMT-based drug delivery to brain due to its high expression level on human BBB which is comparable to TfR and insulin receptors [ 131 , 132 ]. Particularly, Angiopep-2 peptide has gained enormous attention as targeting ligand for LRP1 mediated drug delivery to brain tumor in pre-clinical study [ 133 , 134 , 135 ]. In a phase I clinical investigation involving ANG1005 (Angiopep-2 peptide conjugated to paclitaxel), the progression of disease was reduced among 8 out of 27 patients, leading to its advancement into a phase II clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT01967810","term_id":"NCT01967810"}} NCT01967810 ) for patients with high-grade glioma [ 136 ]. Furthermore, ANG1005 has recently demonstrated clinical benefits in a phase II clinical trial aimed at treating patients with recurrent brain metastasis originating from breast cancer [ 137 ].
Insulin receptors (IR) and insulin-like growth factors receptor 1 (IGFR-1), which are expressed in the brain and BBB, are also explored for drug delivery to brain. For instance, intravenous infusion of enzyme laronidase fused to an IR binding antibody (AGT-181) in a phase II trial ( {"type":"clinical-trial","attrs":{"text":"NCT03053089","term_id":"NCT03053089"}} NCT03053089 ) to treat Hurler syndrome (alternatively known as mucopolysaccharidosis type I) have shown well-tolerated safety profile with satisfactory efficacy in pediatric patient [ 138 ].
Cell adhesion molecules (CAMs) such as integrin, selectin, and gap junction proteins connexin have also been explored as drug delivery target to brain [ 139 , 140 , 141 , 142 , 143 ]. For instance, the use of paclitaxel (PTX) loaded nanoparticles targeting integrin, specifically PTX-c(RGDyK)-NP based on poly(trimethylene carbonate), extended the survival of U87MG glioma-bearing Balb/c mice by 22 days compared to free PTX [ 144 ]. Similarly, Nukolova et al. used nanogels conjugated with a monoclonal antibody of connexin 43 (Cx43), a gap junction protein, to deliver cisplatin in C6 gliomas. This approach was reported to significantly enhance the survival of animals [ 145 ].
Other Receptor and Transporter-Mediated Targeting Systems: Other than the afore-mentioned receptors, many other endogenous receptors, or transporters on the BBB such as acetylcholine receptor (nAChRs), glutathione (GSH) transporter, GLUT, LAT-1, etc., are being explored for the delivery of drugs to the brain using nanocarriers. For instance, Chaudhuri and co-workers have developed a nAChRs receptor-targeted liposomes by decorating with nicotine at their exo-surface [ 146 ]. They also designed another liposome that target LAT-1, with L-DOPA grafted onto the surface [ 147 ], to transport the STAT-3 inhibitor WP-1066 in mouse brain tumor. Overall, such active targeting strategies hold significant promise for macromolecular pharmaceuticals like recombinant proteins, although efficacy of such nanocarriers mediated RMT is still in pre-clinical stages.
3.2.2. Magnetic Field Assisted Crossing of BBB
Application of an external magnetic field is another physical method for drug delivery to the brain which not only spatially guides the magnetic nanoparticle to the targeted region but also significantly improves the speed and time for drug delivery. In this method, paramagnetic nanoparticles (PMNP), especially superparamagnetic iron oxide nanoparticles (SPIONs) with sizes ~10–100 nm, are used. Although magnetic nanoparticles (MNPs) and liposomes in diameters of 70 nm do not cross the BBB, the application of a static magnetic field facilitates its delivery across BBB. Particle size controls the magnetic susceptibility under a fixed static magnetic field. Small SPIONs exhibit higher magnetic susceptibility (highest with crystalline domain with 10–30 nm) than larger paramagnetic nanoparticles containing many crystalline domains mutually diminish the net magnetic moment. In addition, nanoparticles of 10–100 nm are considered optimum due to their longer systemic circulation times. The size of the nanoparticles determines their effect on BBB. For instance, SPION with ~117 nm under 0.39 Tesla did not disrupt BBB integrity whereas magnetic nanoparticles with a size of 800 nm cause leakage in BBB under the same magnetic field strength. In addition, the lower particle size with higher magnetic susceptibility requires less field strength, although no adverse effect in cells is reported with the static magnetic field as strong as up to 10 Tesla. Although the transcellular migration through BCEC via uptake or nanoporation is presumed to be the major pathway, some recent studies indicate interaction of SPIONs with junction proteins such as VE-cadherin may contribute to additional involvement of the paracellular pathway for BBB crossing [ 148 , 149 ].
SPIONs are used in clinics for MRI as a contrast agent and hold potential for other biomedical purposes including targeted drug delivery, image-guided drug delivery, hyperthermia, etc., for the management of CNS diseases [ 96 , 150 , 151 ]. SPIONs can be surface-functionalized with different polymers, lipids etc. to achieve desired drug loading or pharmacokinetic property. For instance, polystyrene-coated SPIONs (~100 nm) under 0.1 T external magnetic field cross the BBB, accumulate in the brain parenchyma, and exhibit 25 times greater retention with minimal neurotoxicity. Similarly, transferrin-coated PEGylated magneto liposomes (~130 nm) exhibit complete transmigration across an in vitro BBB under 0.08 T magnetic field without affecting the BBB [ 152 ]. Beyond small molecule anti-cancer drugs [ 150 ], magneto liposomes also have been used to facilitate delivery of therapeutic peptide [ 153 ], brain-derived neurotrophic factor (BDNF) [ 154 ] or antiretroviral agents across the BBB [ 155 , 156 ]. For instance, to enhance the BBB permeation of antiretroviral agent 3′Azido-3′deoxythymidine-5′-triphosphate (AZT), it is complexed with SPIONs (~25 nm) followed by coating with liposome. This magneto liposome containing encapsulated AZT (~150 nm) crosses the BBB (in vitro) under 0.3 T field and results in three times higher accumulation of AZT across BBB compared to the only AZT [ 155 ]. Importantly, to further gain control for on-demand drug release, MNP are modified to electromagnetic nanoparticles (MENP) [ 157 ] which exhibit brain accumulation under low ac magnetic field with no adverse effect in rodents [ 158 ] and non-human primates [ 159 ] and can facilitate delivery of hydrophilic therapeutics including siRNA [ 160 ], CRISPR [ 161 ] across in vitro BBB ( Figure 5 ). It is worth mentioning that such MENP can also be used for non-invasive deep brain stimulation to control neuroactivity in Parkinson’s disease [ 162 ]. Many studies have claimed lysosomal degradation of SPIONs as histopathological evaluation of major organs involved in systemic circulation revealed no iron-positive pigment or related macrophage accumulation [ 158 , 163 ]. However, some recent studies have reported toxicity of SPIONs as it causes an imbalance in iron homeostasis which may induce oxidative stress and inflammation leading to genotoxicity due to its differential interaction with mitochondria [ 164 , 165 ]. Clearly, in-depth evaluation of in vivo toxicity in long-term exposure to SPION is needed.

Magnetic field assisted drug delivery to the brain: ( A ) TEM of a magneto electric nanoparticle (MENP) containing CoFe 2 O 4 at the core with a shell of BaTiO 3 ( B ) which enables controlled drug release under alternating electric stimuli, ( C ) MENP (~30 nm) can be loaded with CRISPR via hydrophilic interaction which allows their non-invasive delivery across BBB (in vitro) under electromagnetic field, ( D ) TEM of brain tissue from ( i ) untreated mice and ( ii ) mice intravenously administered with 10 mg/kg BW of MENP indicating brain accumulation of MENP (black dot in D ( ii )). ( E ) Schematic for potential simultaneous delivery of hydrophilic and hydrophobic payload to the brain using MENP-liposome composed of a lipid coating embedded with hydrophobic drug onto the hydrophilic drug-loaded MENP. Adopted with permission from Refs. [ 148 , 157 , 158 , 161 ].
3.2.3. Cell-Based Biomimetic Strategy of BBB Crossing
Bioinspired carriers such as blood cells, cell-membrane-coated nanocarriers, exosomes, etc., are being explored recently for drug delivery across BBB due to their longer circulation life and biocompatibility [ 79 ]. Leukocytes such as macrophages, monocytes, and neutrophils are most explored for brain delivery due to their inherent chemotactic recruitment property, especially brain diseases with inflammation. In such methods, drugs are first loaded in nanocarriers which are then incorporated into cells to facilitate delivery across the BBB. For instance, Xue et al. have used neutrophils to deliver paclitaxel loaded liposomes in residual tissue post-surgery which have suppressed the recurrence of glioma growth [ 166 ]. To treat ischemic stroke, Xu et al. have developed a ‘nanoplatelet’ by coating a neuroprotective agent loaded dextran-based nanocarrier with platelet membrane surface-engineered with thrombin-responsive anti-ischemic drug and TAT peptide. This ‘nanoplatelet’ crosses the BBB, clears the thrombus clog at the ischemic site in the brain and delivers neuroprotective agent to combat ischemic stroke [ 167 ]. In another study, to combat encephalitis Yuan et al. have utilized a macrophage-derived exosome for delivering brain-derived neurotrophic factor (BDNF) to inflamed brain [ 168 ]. Such BDNF-loaded exosomes crosses BBB via intercellular adhesion molecule 1 (ICAM-1) which is upregulated under encephalitis-related inflammation. However, cell-based carriers suffer from some common limitations such as viability of cell-based carriers arising due to leaching of drug from nanocarriers. In addition, there are some cell-specific limitations like risk of immune activation while using leukocytes or activation of platelets while using it as drug carrier that may cause undesired thrombosis or bleeding. Exosomes are extracellular vesicles which show ~0.5% passive brain accumulation and have attracted attention for drug delivery to brain and treating neurological conditions. For instance, i.v. administration of dopamine-loaded exosomes enhanced the dopamine levels (15 times) in mouse brain [ 169 ]. Further understanding of the interaction between the BBB and bio-mimetic carriers are necessary for proper engineering of such carrier to maximize therapeutic benefit. Extracellular vesicles (EVs) derived from the cells have been explored for neuroprotective applications including traumatic brain injury. In one example, the EVs derived from mesenchymal stromal cells (MSCs) were examined from neuronal cell protection using in vitro models [ 170 ].
3.2.4. Viral Vector for Drug Delivery to Brain
Neurotropic viruses that can specifically infect the brain are currently being explored as drug carriers to the brain. For instance, adeno-associated viruses (AAVs) and lentivirus which have been widely used in gene therapy, are being explored for BBB trafficking. AAVs can stably transduce genes to CNS cells, e.g., neurons, astrocytes, BCECs, oligodendrocytes, and ependymal cells, and are observed to retain their expression in pre-clinical models (longer than 6 years in monkeys) [ 171 , 172 ]. Despite the shortcomings of strict packaging limit (~4.7 kb) [ 173 ] and pre-existing immunity in certain serotypes [ 174 ], the transduction efficacy and tolerability [ 175 , 176 ] of some serotypes, e.g., AAV9, AAV2 have affirmed their emerging exploration in different pre-clinical CNS disease models [ 177 ]. Importantly, the recent FDA approval of Zolgensma which is the first AAV-based (AAV9) gene therapy for spinal muscular atrophy type 1, has created a milestone for viral vectors mediated gene therapy for CNS diseases with many other ongoing clinical trials.
The packaging capacity can be improved by using lentiviral vectors which possess a bigger packaging capacity. However, the tendency to integrate with host gnome and thereby, potential adverse effect by insertional mutagenesis in CNS target cells has greatly limited its clinical scope [ 178 , 179 ]. For instance, even the non-integrating lentivirus also have residual propensity to integrate with the host genome which may have adverse effect due to insertional mutagenesis in CNS target cells. This effect can be minimized by using ex vivo transduction of cells; however, the efficacy of such lentiviral-based therapy will further depend on CNS entry efficiency of the transduced cells.
Despite the recent clinical success, application of viral vectors is greatly limited to gene therapy and often requires invasive mode of administration. Efforts to improve BBB permeability using different serotypes including AAV8, AAV9, and AAV10 have not shown satisfactory outcomes yet, as such neurotropic viral vectors poorly transduced BCECs [ 180 , 181 ]. In addition, a high dose of such vectors may be required for BBB crossing which may create challenges like autoimmunity risk and reduced rate of BBB transport due to neutralization of such serotypes by their pre-existing antibodies [ 182 ]. Finally, factors like vector purity, self-inactivating or non-integrating vectors, transgene sequence, etc., which influence their therapeutic efficacy, are under investigation, more studies are required for robust safety assessment in selecting an optimal viral vector.
4. Localized Drug Delivery Strategies
To bypass the hurdle of BBB crossing and enhance therapeutic efficacy of drugs, localized drug delivery strategies such as injections, convection enhanced delivery (CED), and administration of implants are developed. Although these strategies are invasive, site-specific delivery of the therapeutics with high bioavailability and minimal drug loss can be achieved.
4.1. Injection
In this method drugs are directly injected or infused into the disease site or remaining cavity after the resection of the tumor which is less toxic and much effective than systemic administration. However, this method is not a breakthrough for treating CNS disease due to their high risk of side effects such as edema, infections, and backflow of drugs into the catheter, narrow drug distribution in the injection site [ 183 ].
4.2. Convection Enhanced Delivery (CED)
To prevent the backflow of drugs and improve the drug distribution in the brain tissue, CED is developed [ 184 ]. In this method, an implantable pump is connected to the catheter to maintain a convective flow. Usually, the catheter is introduced stereotactically while the constant pressure from the pump maintains a convection flow (independent of drug diffusivity) of the drug solution into the delivery site. In addition, the convective flow allows the drug solution to cover longer distance in the brain compared to direct injection/infusion [ 185 ]. CED can be further connected to a real time magnetic resonance imaging method to monitor the drug distribution [ 186 ]. CED has been widely tested for the delivery of a broad spectrum of therapeutic agents, including small molecules [ 185 , 187 , 188 ], macromolecules [ 189 , 190 ], nanocarriers [ 191 , 192 ], and immunotoxins [ 193 ]. CED for delivering drug from liposomal and polymeric nanocarrier is of importance which can offer many advantages like less systemic side effects, sustained drug release, and larger distribution with targeted delivery. CED is a real progress in the field of infusion mediated drug delivery to the brain; however, despite its significant promise, invasiveness, and the common side effects of infusion methods, such as infection and edema, are common limitations. The safety and efficacy of this method is yet to clearly determined as it fails to meet clinical end points in many Phase III trials [ 194 ].
4.3. Implants
Polymeric implants including wafers, gels, microspheres, and nanospheres have been tested for localized delivery of therapeutics in brain in different shape and sizes [ 183 ]. Among these, wafers attracted attention since FDA-approval of Gliadel in 1996 [ 195 , 196 ]. Wafers are drug loaded polymeric implants that look like coins in shape and size. Wafers are implanted in the remaining cavity post-surgical resection of a tumor where they act as platforms for sustained drug release. The safety and effectiveness of Giladel, a wafer made of co-polymer of phosphino carboxylic acid and sebacic acid and loaded with chemotherapeutics BCNU (1,3-Bis(2-chloroethyl)-1-nitrosourea) alone and in combination with radiotherapy led to its FDA-approval in treating recurrent and newly diagnosed glioma [ 196 , 197 ]. Since then, various other polymers like poly(lactide-co-glycolide), poly(vinyl acetate) extrudates, etc., in combination with different chemotherapeutics are tested in various trials, though only Gliadel ® is legally approved so far for the treatment of malignant gliomas. However, the major drawbacks of wafers as standard delivery platform in treating glioma are lack of deep tissue penetration of therapeutics which is crucial for invasive tumor like glioma. The low diffusivity of the therapeutics allows drug distribution only over a few millimeters from the delivery site. In addition, leakage of the drug into the CSF, adverse neurological complication arising from mechanical mismatch of the patch, control over the spatial and temporal drug release, biodegradability, and tissue adhesiveness of the patch, etc., need attention to improve therapeutic efficacy. Efforts are being made to overcome such limitations. For instance, Lee et al. [ 198 ] have developed an adhesive, flexible, bioresorbable device covering a drug-loaded patch integrated with wireless electronics for intracranial drug release via mild-thermic actuation ( Figure 6 B,C). The thermic actuation in presence of an alternative magnetic field allows deep tissue penetration while preventing leakage towards CSF ( Figure 6 C(iii)). The softness and optimum hydrophobicity/hydrophilicity in the bioresorbable device material containing PLA layer (on top) and oxidized starch (OST) at bottom layer ( Figure 6 B) allow conformal adhesion and assimilation to the host target brain tissue over time ( Figure 6 C(iv,v)) while minimizing the neurological complication of rigid implant. Other than wafers or large implants, gels and micro/nanosphere-based or polymeric microchips have been tested as alternate drug delivery implants; however, none of these are clinically approved and share common limitation of lack of deep tissue penetration of therapeutics [ 183 ].

Localized drug delivery in the brain: ( A ) Schematic for conventional injection and convection-enhanced stereotactic drug delivery method; ( B ) a biodegradable wireless electronic patch (1 cm) made of tissue adhesive bifacial soft polymeric material PLA and OST and containing a temperature sensor, heater, and drug reservoir for intra-cranial local drug delivery via thermic actuation, Stereotactic implantation of the patch into brain ( C , i , ii ) allows local drug delivery and prevention of drug diffusion to CSF ( C , iii ), tissue assimilation ( C , iv ) and resorption with host tissue ( C , v ). Sustained drug delivery from this wireless patch under thermic actuation (OST + heating group) enhances the survivability of glioma-bearing mice compared to control groups including OST (device without actual), control wafer (custom Giladel), and heating (actuation with the empty patch). ( D ) Kaplan–Meier survival rate plots of the indicated treatment group in the mouse model, * p < 0.05, *** p < 0.001 by log-rank test with Bonferroni correction. Adopted with permission from refs. [ 183 , 198 ] under the CC-BY license, version 4.0.
4.4. Intranasal Delivery (IN)
Delivery of therapeutics to the brain through nasal route is another non-invasive approach to circumvent the BBB. Although the mechanism of intranasal drug delivery to brain is not well understood, the olfactory/trigeminal pathway is presumed to be the significant route for nose-to-brain drug delivery [ 199 ]. The drug molecules into the nasal cavity first experience the mucociliary clearance in the vestibular region and afterwards move to the posterior regions of the nasal cavity respiratory region and the olfactory region from where drug molecules are transported to mid-brain and brainstem along the trigeminal and olfactory neurons, respectively. Then, drug molecules are distributed in other regions of the brain via convection flow or perivascular routes. Some clinical trials of IN for the management of neurological disorders including Alzheimer’s diseases, Parkinson’s disease, etc., are ongoing [ 200 , 201 ]. Mucosal clearance, limited dosing volume (100–150 μL or 20–50 mg powders), and metabolic stability of drugs against nasal cavity enzymes are the limiting factors. Mucoadhesive polymeric nanoparticles and non-irritant drug formulations may hold promises to overcome such limitations; however, further research may be needed.
5. Conclusions and Future Perspective
With the growing number of neurological disorders in the aged population and brain tumors, there is an urgent need for safe and efficacious targeted drug delivery to brain. Despite years of efforts in CNS drug development, recent FDA approval of Zolgensma, viral-based gene therapy, is the first approved BBB-crossing biologics. However, the invasive mode of administration of such therapy induces vulnerability towards infection by neurotoxins or other pathogens. In addition, viral vectors are limited to gene therapy which is only effective for diseases with single gene mutation. In an era of biopharmaceuticals such as recombinant proteins being the most approved drugs for other diseases, its scope is greatly limited by poor delivery of such macromolecules across BBB which reflect the poor rate of clinical translation (~8%) of CNS drugs. With the vast spectrum of CNS drug delivery strategies, each having distinct pros and cons, it may be challenging to establish a universal platform technology for CNS drug delivery. In addition, with recent advances in tissue engineering efforts are also being made to overcome the gap in interspecies tissue homogeneity. Nanotechnologies have demonstrated enhancements in BBB permeability, region-specific targeting, drug stability, and delivery. Nevertheless, RMT and CMT have shown initial promises in delivery of macromolecular biopharmaceuticals and small molecules, respectively, across the BBB. However post-discovery, challenges include navigating regulatory hurdles, managing costs, and ensuring accessibility of these new technologies. Overall, more efforts should be invested in CNS drug delivery technologies in parallel to drug development, and fostering concentrated efforts through industry–academia collaboration may lead to safe and effective drug delivery to the brain in the near future.
Funding Statement
M.N. thanks support from the National Institutes of Health (NIH) grants DA042706, DA052271, N.K. thanks support from the H&N Wertheim Research Pilot Project from Florida International University (FIU), and A.V. thanks support from FIU Foundation via grant number AWD0011094.
Author Contributions
Conceptualization, S.B., N.K. and M.N.; resources, M.N.; writing—original draft preparation, S.B.; writing—review, revision and editing, N.K., A.V., A.Y.A., D.B., M.N.; visualization, S.B., N.K. and M.N.; supervision, N.K. and M.N.; project administration, M.N.; funding acquisition, M.N. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.

IMAGES
VIDEO
COMMENTS
The seven key challenges summarized in this Position Paper are intended to serve as foci for future research and investment. Brain cancer encompasses a diverse range of complex malignancies, many ...
Posted: August 24, 2023. An NCI-supported study called OPTIMUM, part of the Cancer Moonshot, was launched to improve the care of people with brain tumors called low-grade glioma in part by bringing them into glioma-related research. Targeted Drug Combo May Change Care for Rare Brain Tumor Craniopharyngioma. Posted: August 10, 2023.
This Frontiers Research Topic Proposal on " Brain Cancers: New Perspectives and Therapies " joined contributions from scientists and physicians who investigate on etiopathogenesis and treatment of brain cancers. In fact, studies exploiting the existing link between enhancing the knowledge of cellular and molecular pathways involved in the ...
Introduction. Brain tumours are among the most feared of all forms of cancer. More than two-thirds of adults diagnosed with glioblastoma — the most aggressive type of brain cancer — will die within 2 years of diagnosis 1,2.Brain cancers are also the most common and most lethal of all paediatric solid tumours 3.Furthermore, children with these tumours who survive and enter adulthood will ...
1. Introduction. Brain tumor accounts for about 1.6% of incidence and 2.5% of mortality of all tumors respectively according to the latest global cancer data released by the World Health Organization (WHO) in 2020 (Sung et al., 2021).While, in China, the morbidity and mortality of brain tumors rank first in the global brain tumors, up to 32% and 26% respectively, and the incidence rate is ...
resolved by consultation with a third reviewer. Results: Statistical analysis showed that 50% of the papers used a virus, 36% used polymers, and 14% used cells as carriers to transfect. the genes ...
March 14, 2024 5 min read. A collaborative project to bring the promise of cell therapy to patients with a deadly form of brain cancer has shown dramatic results among the first patients to receive the novel treatment. In a paper published Wednesday in The New England Journal of Medicine, researchers from Mass General Cancer Center shared the ...
Credit: NCI-CONNECT Staff. NCI-funded researchers are working to improve our understanding of how to treat tumors that arise in the brain or the spinal cord (together known as the central nervous system, or CNS). Such tumors can be either benign (not cancer) or malignant (cancer). But the tissues of the nervous system are so important and so ...
A to Z. Brain Cancer. Brain Cancer research articles are listed. Brain cancer news covers topics such as diagnosis, brain tumors, chemotherapy, gamma knife technology, brain cancer treatments, glioblastomas, stem cell research, neurosurgery, medicine, genetics, neurology, and other brain research. Brain Cancer Featured Neurology Neuroscience.
With the high number of brain cancer cases and deaths each year, there has been a rise in research interest in the automatic detection of brain tumors [16]. This paper reviews all articles on computer-aided diagnostic (CAD) systems developed for the automated detection of brain tumors published from 2000 to 2022. The primary intention of CAD ...
Brain tumor occurs owing to uncontrolled and rapid growth of cells. If not treated at an initial phase, it may lead to death. Despite many significant efforts and promising outcomes in this domain, accurate segmentation and classification remain a challenging task. A major challenge for brain tumor detection arises from the variations in tumor location, shape, and size. The objective of this ...
The research was supported in part by the National Cancer Institute grants 1R01NS110703-01A1, 1U19CA264338-01 and 1R01CA245969-01A1 of the National Institutes of Health, grant P50CA221747 SPORE ...
Advances in Brain Tumor Clinical Care and Research. April 29, 2020 , by Brittany Cordeiro, NCI-CONNECT Program Manager. Dr. Jing Wu. Credit: NCI-CONNECT. The NCI Center for Cancer Research's Neuro-Oncology Branch is advancing care and treatment for people with brain tumors through collaborative scientific workshops, research discoveries, and ...
The paper is organized as follows: Section 2 provides an overview of the pathophysiology of brain cancer. Section 3 , Section 4 , Section 5 and Section 6 discuss various imaging modalities, the WHO guidelines on brain cancer grading, brain cancer tests and characterization methodologies, respectively.
Brain tumours pose a significant health risk, and early detection plays a crucial role in improving patient outcomes. Deep learning techniques have emerged as a promising approach for automated brain tumor detection, leveraging the power of artificial intelligence to analyse medical images accurately and efficiently. This research study aims to explore the current state-of-the-art deep ...
A brain tumor is one of the most malignant tumors in humans. It accounts for. approximately 1.35% of all malignant neoplasm and 29.5% of cancer-related death. [1]. Brain and CNS tumors include ...
Brain Tumor Research Studies. Johns Hopkins has an unparalleled history of introducing new forms of treatment for the patients affected by malignant brain tumors. For example, the only two new effective surgical treatments for malignant brain tumors approved by FDA (Gliadel and GliaSite) were discovered and developed right here at Johns Hopkins.
MIT graduate student Jason Hou and MIT postdoc Md Osman Goni Nayeem are the lead authors of the paper, along with collaborators from MIT's McGovern Institute for Brain Research, Boston University, and Caltech. The study appears today in Nature Communications. Deep in the brain
FULL STORY. Johns Hopkins Medicine scientists say they have developed an artificial lymph node with the potential to treat cancer, according to a new study in mice and human cells. The newly ...
According to the International Agency for Research on Cancer (IARC), in 2020 there were approximately 19.3 million new cases of cancer, and 10 million deaths by this disease, 6 while 23.8 million cases and 13.0 million deaths are projected to occur by 2030. 73 In this regard, it is clear the increasing role that environmental factors ...
Make a gift now and help create new and better solutions for more than 1.3 million patients who turn to Mayo Clinic each year. Mayo Clinic is a top-ranked hospital in the U.S., with campuses in Arizona, Florida, and Minnesota.
E. Embryonal Tumors, Medulloblastoma and Other Central Nervous System, Childhood (Brain Cancer) Endometrial Cancer (Uterine Cancer) Ependymoma, Childhood (Brain Cancer) Esophageal Cancer. Esthesioneuroblastoma (Head and Neck Cancer) Ewing Sarcoma (Bone Cancer) Extracranial Germ Cell Tumor, Childhood. Extragonadal Germ Cell Tumor.
The American Psychological Association (APA) is a scientific and professional organization that represents psychologists in the United States. APA educates the public about psychology, behavioral science and mental health; promotes psychological science and practice; fosters the education and training of psychological scientists, practitioners and educators; advocates for psychological ...
The aim of the work presented in this paper is to develop and test a Deep Learning approach for brain tumor classification and segmentation using a Multiscale Convolutional Neural Network. To train and test the proposed neural model, a T1-CE MRI image dataset from 233 patients, including meningiomas, gliomas, and pituitary tumors in the common ...
"The finding that an individual synaptic receptor (NMDA) can produce gamma oscillations and that these gamma oscillations can influence network-level gamma was unexpected," says co-corresponding author Michelle McCarthy, a research assistant professor of math at BU."This was found only by using a detailed physiological model of the NMDA receptor.
2. Structure and Function of Blood-Brain barrier . BBB is an endothelial membrane barrier within brain microvascular formed by tight junction of brain capillary endothelial cells (BCEC) sheathed by mural cells and astrocytes end-feet that separate CNS from systemic blood circulation (Figure 1 A,B) [9,10].The BBB protects the brain by preventing random entry of substances like neurotoxins and ...