An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(10); 2023 Oct
- PMC10663705


A Comprehensive Review of Gestational Diabetes Mellitus: Impacts on Maternal Health, Fetal Development, Childhood Outcomes, and Long-Term Treatment Strategies
Vaishnavi s nakshine.
1 Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research, Wardha, IND
Sangita D Jogdand
2 Pharmacology and Therapeutics, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research, Wardha, IND
This review article conducts a comprehensive analysis of gestational diabetes mellitus (GDM) and its ramifications for both maternal health and the well-being of their offspring. GDM is a significant pregnancy complication in which women who have never had diabetes acquire chronic hyperglycemia during their gestational period. In most cases, hyperglycemia is caused by impaired glucose tolerance caused by pancreatic beta cell dysfunction in the background of chronic insulin resistance. Being overweight or obese, having an older mother age, and having a family history of any type of diabetes are all risk factors for developing GDM. GDM consequences include a higher risk of maternal cardiovascular disease (CVD) and type 2 diabetes, as well as macrosomia and delivery difficulties in the newborn. There is also a longer-term risk of obesity, type 2 diabetes, and cardiovascular disease in the infant. Premature birth, hypoglycemia at birth, and shoulder dystocia are also a few of the fetal problems that can result from GDM. Unfortunately, there is no widely acknowledged treatment or preventative strategy for GDM at the moment, except lifestyle modification (diet and exercise) and, on occasion, insulin therapy, which is only of limited value due to the insulin resistance that is commonly present. Although new oral medications for diabetes management, such as glyburide and metformin, show potential, there are ongoing worries regarding their safety over an extended period for both the mother and the child. By identifying gaps in the research, it calls for further investigations and a multidisciplinary approach, ultimately aiming to enhance the management and care for women with GDM, which would impact these affected individuals indubitably.
Introduction and background
Gestational diabetes mellitus (GDM) is a metabolic condition of pregnancy that presents as newly developing hyperglycemia in pregnant women who did not have diabetes before getting pregnant, and it normally resolves after giving birth [ 1 ]. Around 9% of pregnancies around the globe are affected by this prevalent antepartum condition [ 2 ]. Although one can develop GDM at any instance during the entire course of pregnancy, it is typically seen between weeks 24 and 28 of pregnancy. Additionally, the prevalence of GDM is growing globally due to an increase in maternal weight gain, maternal age, and inactivity [ 3 ]. The etiology of GDM is explained by the maternal pancreas' inability to adjust to the increased insulin demand throughout gestation. During pregnancy, the body becomes less responsive to insulin, which leads to an increased production of insulin by pancreatic beta cells [ 4 ]. Insulin, which is secreted by these beta cells, plays a vital role in promoting the uptake of glucose by peripheral tissues, reducing the synthesis of glucose in the liver, and controlling the release of lipids from adipose tissue. However, if regular levels of insulin fail to achieve the desired response from insulin receptors, insulin resistance can develop. Consequently, beta cells must produce more insulin than usual to maintain normal maternal blood glucose levels [ 1 ]. This insulin resistance is a natural part of a healthy pregnancy and is induced by placental hormones to ensure the fetus receives the necessary nourishment for proper growth and development. Maternal beta cells respond by increasing their number, insulin production, and release to sustain glucose balance despite insulin resistance [ 5 ]. However, when maternal beta cells cannot adapt to the metabolic changes associated with pregnancy, gestational diabetes mellitus (GDM) results in hyperglycemia.
GDM is essential to detect and treat during pregnancy due to the harmful impact it has on both the mother and the fetus, in both the short and long runs. Gestational diabetes can cause short-term pregnancy complications such as high blood pressure, the necessity for a cesarean section (C-section), pre-eclampsia, and difficulty during childbirth [ 6 ]. In the long run, it may reappear in subsequent pregnancies, increasing the mother's risk of developing type 2 diabetes later in life [ 7 , 8 ]. Many recent researches have focused on treating GDM with GM-targeting techniques. Several prior research have looked at the influence of probiotics on the progression of GDM, but results have been ambiguous. GDM treatment tries to reduce the hazards for both the mother and the infant by controlling excessive blood sugar levels. Mothers must learn about the illness in order to achieve the best possible blood sugar control in GDM patients. The primary therapies for GDM involve adopting lifestyle changes such as modifying your diet, exercising regularly, and maintaining a healthy weight. Medication may be an option if high blood sugar persists despite all of these changes. GDM medication comprises glucose-lowering drugs, metformin, glibenclamide, and insulin [ 9 ]. Women having GDM are recommended to discontinue any medication they were using for the condition postpartum due to the quick return of insulin sensitivity [ 10 ].
Search methodology
We undertook a comprehensive search through PubMed and CENTRAL in June 2023 using keywords such as "gestational diabetes mellitus" and "type 2 diabetes mellitus" ((gestational diabetes mellitus [title/abstract]) OR (GDM [title/abstract])) OR (macrosomia [title/abstract]) OR ("gestational diabetes mellitus" [MeSH terms]) AND (("type 2 diabetes mellitus" [title/abstract]) OR (T2DM [title/abstract])) OR ("type 2 diabetes mellitus" [MeSH terms]). Additionally, we looked through the bibliographies of pertinent research to find important references. In July 2023, the search was updated. Two reviewers independently checked the retrieved papers against the inclusion criteria based on the title and abstract first and then the full texts (Figure 1 ).

PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Effects on the mother
GDM subsequently causes several short- and long-term complications with regard to maternal health. Along with the challenges of a typical pregnancy, GDM may contribute to depression in prenatal [ 11 ]. In many instances, the baby must be delivered surgically due to the increased risk of issues in subsequent pregnancies, such as premature birth and hypertension [ 12 ]. Women who have been diagnosed with GDM are significantly more likely to develop diabetes mellitus later in life. Nearly 10% of women with gestational diabetes mellitus are diagnosed with diabetes mellitus shortly post giving birth [ 13 ]. Without particular interventions to lower their chance of developing diabetes mellitus, the remainder seem to develop the disease at rates of 20%-60% within 5-10 years following the index pregnancy. However, not all women having gestational diabetes will develop diabetes mellitus, according to limited long-term data from O'Sullivan, but most of them will [ 14 ]. Similar to prenatal issues, the risk of postpartum diabetes mellitus is increased by GDM. Regardless, the risk of prenatal problems brought on by GDM is substantially lower than the likelihood of the mother developing diabetes mellitus post-GDM diagnosis. Therefore, it is logical to assume that GDM is a type of prediabetes similar to glucose intolerance in non-pregnant people [ 15 ].
Plenty of patients who have diabetes mellitus after GDM meet the pre-type 2 diabetes mellitus (T2DM) profile, as was previously discussed. Studies on the regulation of glucose following GDM over time show declining beta cell remuneration for insulin resistance (chronic) that may also deteriorate as time passes [ 16 ]. Markers of rather severe decompensation, such as elevated glucose levels, noticeable insulin resistance, and impaired beta cell activity, are risk factors for the relatively quick onset of diabetes mellitus following childbirth. Women exhibiting these traits might surpass the threshold of glucose levels defining diabetes mellitus following a slight decline in their physical condition [ 17 ]. Weight increase, insulin resistance, increasing C-reactive protein levels, and declining adiponectin levels are risk factors for beta cell deterioration at comparatively high rates, which leads to diabetes mellitus [ 18 ]. These results imply that the metabolic consequences of obesity serve a key role in the degeneration of beta cells that result in diabetes mellitus. As will be covered below, the most effective defense against the emergence of T2DM after GDM is the amelioration of the detrimental consequences of obesity induced by diet and exercise or by taking medications that improve the biological makeup and operation of adipose tissue [ 19 ]. Figure Figure2 2 aids in comprehending the multifaceted nature of post-GDM diabetes development and highlights the significance of managing beta cell function and addressing obesity to mitigate this risk effectively.

GDM: gestational diabetes mellitus
Image credits: Vaishnavi Nakshine
Metabolic syndrome, which includes obesity and other associated diseases, serves as the foundation upon which T2DM develops. The probability of women with GDM displaying symptoms of metabolic syndrome is higher than it is for women without GDM [ 17 ]. A greater frequency of cardiovascular risk factors and cardiovascular events is also linked to previous episodes of GDM [ 20 ]. Most mothers who have GDM are obese, and a sizable fraction of obese people also have GDM [ 21 ]. According to a meta-analysis, pregnant women who are overweight are 2.14 times more susceptible to be diagnosed with GDM than pregnant women average in weight, obese pregnant women are 3.56 times more likely to do so, and extremely obese pregnant women are 8.56 times more likely [ 22 ].
Complications During Pregnancy
Vaginal birth will be more challenging in case the baby is very large. There is a chance of a long labor process during which the fetus could get clung in the birth/vaginal canal, an instrumental delivery may be required (using forceps or a vacuum), or even an unanticipated or emergency cesarean section might be required. A perineal tear (muscle tearing between the vagina and the anus) as well as lacerations and tears of the vaginal tissue are more likely to occur during childbirth than when the infant is of normal size [ 23 ]. Moreover, there is a high risk of uterine atony. Heavy bleeding and postpartum hemorrhage may occur as a result of the uterus' muscle failing to contract appropriately. Macrosomic births have an about three- to fivefold increased risk of postpartum hemorrhage and genital tract injury [ 24 ]. In addition, if the woman has already undergone a cesarean section, there is an increased probability of tearing of the uterus along the surgical scar tissue from the prior procedure.
Fetal complications and effects
Premature Birth
Preterm delivery is possible as a result of inducing labor early (earlier than 39 weeks and/or early rupturing of the membrane). Although every effort has been made to induce early labor, babies are still at risk for prematurity-related problems, such as breathing and feeding issues, infections, jaundice, admission to a neonatal ICU, and perinatal mortality. Preterm delivery has a prevalence of roughly 10.6% worldwide when combined with several other problematic factors such as obesity and hypertension during pregnancy [ 25 ].
Hypoglycemia at Birth
In addition to having a negative impact on mothers, GDM also harms the fetus. The growing fetus can only produce a small amount of glucose; hence, it gets the majority of its glucose from the mother's blood. While maternal insulin does not pass the placenta, maternal glucose does. The modified Pedersen's theory, therefore, states that regardless of glucose stimulation, greater fetal insulin production results from extra glucose transported across the placenta in high and uncontrolled maternal glucose levels [ 22 ]. This is corroborated by the placental expression of glucose transport proteins (GLUTs) being found to be higher in pregnancies with insulin-dependent diabetes mellitus [ 26 ]. Additionally, insulin is known to have the ability to activate mTOR, a powerful controller of cell proliferation. The placenta's system A and system L amino acid transporters boost cell division and the supply of essential nutrients to the fetus as a result of elevated maternal insulin, which also causes a surge in placental mTOR activity [ 27 ]. Maternal hyperglycemia and hyperinsulinemia can result in alterations in the fetus that are comparable to those found in GDM due to the aforementioned causes, which can result in neonatal obesity [ 28 ]. An increase in neonatal size at birth, also known as macrosomia, is the result of excessive nutrition storage. The majority of fat is centered in the fetal abdomen and shoulders. Macrosomic babies are born in 15%-45% of GDM pregnancies [ 22 ]. Additionally, GDM has been linked to a higher incidence of respiratory distress in newborns [ 1 ].
Shoulder Dystocia and Erb's Palsy
Shoulder dystocia, particularly linked to birth trauma, is one of the most serious consequences of administering delivery through the vagina, specifically in macrosomic infants. Newborns weighing 4,500 g or greater are six times more likely than others to experience birth trauma [ 23 ], and furthermore, if the birth weight is above 4,500 g, there is an almost 20-fold increased chance of brachial plexus damage [ 29 ].
Congenital Anomalies
The most prevalent birth problems include heart defects and disorders of the neural tube, including spina bifida. Congenital abnormalities can result from the growing fetus' organ damage caused by the elevated blood sugar levels of women with GDM [ 30 ]. Furthermore, it is not certain if GDM and fetal anomalies are related. Congenital abnormalities are twice as common in women with pre-existing diabetes as they are in non-diabetic individuals, demonstrating a strong association between the two diseases. The data for GDM, however, is inconsistent [ 1 ].
Fetal Nutrition
With the onset of GDM, changes in breast milk composition are seen too. Breast milk is a continuously changing fluid with bioactivity that greatly varies from female to female and from phase to phase. Numerous maternal variables, including term and preterm labor, maternal diet, metabolic problems, and diseases [ 31 ], have an impact on it. Diabetes mellitus is a long-term metabolic condition that may affect expectant mothers whether it develops before pregnancy or if it develops during pregnancy (a newly formed syndrome) [ 32 ]. Citrate, lactose, and total nitrogen levels take 15-24 hours longer for mothers with gestational diabetes to attain levels that are comparable to those of healthy women [ 33 ]. Due to the beneficial correlation between mammary gland growth during pregnancy and circulating levels of human placental lactogen, women having gestational diabetes during their pregnancy may have a delay at the beginning of breast milk [ 34 ]. Pregnant women affected by gestational DM exhibited elevated levels of cytokines and chemokines in their colostrum. Interleukin (IL)-6, IL-15, and interferon-γ levels were up, whereas IL-1ra and granulocyte-macrophage colony-stimulating factor (GM-CSF) levels were decreased. This led to a modified immune composition of the colostrum [ 35 ].
Neonatal complications
Neonatal complications can include delivery trauma, such as shoulder dystocia and a brachial plexus wound, as well as potential hypoxia, hypoglycemia, kernicterus, and jaundice. They may also include bacterial infections and newborn respiratory distress syndrome (NRDS) [ 2 ].
Neonatal Jaundice
Prematurity, inadequate nutrition, and increased enterohepatic circulation of bilirubin due to decreased hepatic conjugation of bilirubin are some factors that may contribute to jaundice. Neonates with macrosomia have an elevated oxygen demand, which leads to elevated erythropoiesis and, ultimately, polycythemia [ 36 ]. As a consequence of this, as these cells degrade, bilirubin (a by-product of red blood cells) rises, which causes newborn jaundice.
Childhood and adulthood complications
It is generally known that GDM and hyperglycemia in children are related. The research of the Pima Indians in the USA was the first concrete proof that a mother's hyperglycemia may cause her offspring to develop an adult illness. Indeed, children who have diabetic mothers experience an increased risk of obesity, hypertension, and dyslipidemia in later adulthood [ 37 ]. In 10 different countries, researchers from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study discovered a direct link between maternal hyperglycemia during pregnancy and a rise in hyperglycemia and insulin resistance in children as they grew older [ 38 ]. Additionally, compared to offspring of mothers with normal blood sugar levels, GDM progeny had a higher homeostatic model assessment of insulin resistance (HOMA-IR), waist measurement, body mass index (BMI), and triglyceride levels [ 39 ]. With about 20% of offspring resulting from GDM having type 2 diabetes and prediabetes by 22, it is plausible that the development of being resistant to insulin raises the chance of the child getting the disease [ 40 ]. Along with an increased risk of illnesses including cardiovascular conditions and resistance to insulin, the greater incidence of obesity in children of women with GDM is also linked to an increased risk of other diseases [ 40 ]. In addition to hyperglycemia and BMI, children delivered to GDM mothers were shown to have considerably greater cardiovascular risk and adiposity. GDM kids are more likely to experience cardiac arrhythmias and require hospitalization for cardiovascular diseases (CVDs) as a result of increased cardiovascular risk [ 39 ]. In addition, GDM offspring are 29% more likely to suffer early-onset cardiovascular conditions such as cardiac failure, high blood pressure, deep vein thrombosis, and pulmonary embolism [ 41 ]. All of these researches indicate that the environment in utero affects how metabolic illness is programmed in the child. Population studies have shown that all of these changes experienced throughout childhood are probably to last into adulthood. Numerous studies also indicate that the long-duration impacts of in utero GDM subjection often do not manifest themselves until adolescence, another period that is particularly vulnerable to the development of obesity [ 23 ].
A comprehensive strategy is needed to handle an individual with GDM as best as possible. This entails educating patients on managing pregnancy weight gain, dietary adjustments, nutritional monitoring, and regulating one's blood sugar levels. With enough exercise, dietary changes, and lifestyle adjustments, up to 70%-85% of those with gestational diabetes are curable [ 42 ]. For 15%-30% of people, taking medication is necessary. Insulin and oral hypoglycemics are some of them. Figure 3 provides a concise overview of the management strategies to be employed for controlling GDM.

Blood Glucose Monitoring
The majority of organizations advise daily at-home self-glucose monitoring. Presently, daily self-monitoring of postprandial and fasting blood glucose levels is encouraged. The American Diabetes Association (ADA) advises that the target blood sugar levels be 95 mg/dL for fasting and 140 mg/dL or 120 mg/dL for one to two hours, respectively, following a meal. Pre-existing diabetics are the main beneficiaries of pre-prandial glucose monitoring. Screening the levels of hemoglobin A1C is not as helpful for evaluating glucose control in GDM [ 43 ].
Dietary Modifications
Some of the dietary strategies mentioned in the literature include the DASH diet (dietary techniques to treat hypertension), calorie-restricted diets, low-glycemic index diets, low-carbohydrate diets, low-unsaturated fat diets, high-fiber diets, and soy-based diets. The emphasis of nutritional advice should be on a balanced diet with reasonable portion sizes, healthy fats, complex carbs, and 20% protein [ 44 ].
Physical Exercise
Even in pregnant women with GDM, physical activity and regular exercise have been promoted and are encouraged. The benefits of moderate exercise during pregnancy include a lower risk of gestational diabetes, a lower potential of larger-than-normal newborns, and a lower risk of high blood pressure problems, preterm birth, and fetal growth restriction [ 45 ]. Additionally, pregnancy-related lifestyle modifications affect the period of postpartum, reducing the chance of postpartum depression [ 46 ].
Pharmacotherapy for GDM Management
In about 15%-30% of GDM patients, blood glucose management is insufficient despite suggested dietary and lifestyle changes, necessitating the use of medication [ 43 ]. Usually, if hyperglycemia still exists throughout the course of the day after 10-14 days of nutritional and daily living changes, medication courses should be taken into account. Insulin and oral-route medications are administered for patients with gestational diabetes mellitus in order to control hyperglycemia [ 47 ]. Insulin provides the most secure outline during pregnancy. The oral medications that have been researched include metformin and sulfonylureas such as glyburide. Large molecules such as insulin cannot pass through the placenta. Metformin and glyburide have been demonstrated to have the capacity to pass the placental barrier and reach the fetus [ 48 ]. Figure 4 depicts functions as an illustrative guide outlining the diagnostic and therapeutic procedures for gestational diabetes mellitus.

Table Table1 1 presents an analysis of the traits and features of the articles included in the review.
GDM: gestational diabetes mellitus, rPL: rat placental lactogen, GCT: glucose challenge test, T2DM: type 2 diabetes mellitus, CVD: cardiovascular disease, LGA: large for gestational age, IDM: infants of diabetic mothers, NIDM: infants of mothers without diabetes
Conclusions
Global health continues to be seriously impacted by GDM, the most common metabolic condition during pregnancy. Characterized by elevated blood sugar levels during pregnancy, it demands our attention and a deeper understanding due to its significant impact on the health of expectant mothers and their children. This review shed light on the immediate and long-term consequences of GDM on pregnant women. Short-term consequences encompass a higher likelihood of gestational hypertension, cesarean sections, and other perinatal complications. Long-term implications involve an increased risk of developing type 2 diabetes postpartum, highlighting the importance of continued monitoring and care for women who have experienced GDM. Furthermore, GDM's influence on offspring is a matter of critical concern. This review underscores that children of GDM-affected mothers face a higher risk of developing conditions such as obesity, hypertension, and insulin resistance, which can persist into adulthood. Understanding these intergenerational health implications is vital for proactive prevention and management.
To address GDM effectively, a multifaceted approach is required. This approach includes vigilant blood glucose monitoring, dietary modifications, regular physical activity, and, when necessary, pharmaceutical interventions. The effective management of GDM relies on a collaborative effort between healthcare professionals and expectant mothers, emphasizing education and tailored care. A clinical dietitian should provide dietary advice to all women with GDM, as dietary counseling is the cornerstone of GDM treatment. Particular attention should be paid to carbohydrate intake, as carbohydrate type, amount, and distribution all play a significant role in postprandial blood glucose levels. While considerable progress has been made in GDM research and treatment, there are still gaps in knowledge and variances in clinical recommendations. Moreover, a variety of therapy alternatives for GDM are discussed, although existing data do not support the effectiveness of these approaches over the long term. Future research must focus on a more comprehensive understanding of the long-term cardiometabolic risks that the offspring of GDM-affected mothers may face. In addition, for the prevention and control of GDM, an integrated strategy combining population-wide preventive management, intensive health education, early detection, and multidisciplinary care programs should be strengthened, which could help reduce the risk of GDM and associated complications in the general population and high-risk individuals, improve maternal and neonatal pregnancy outcomes, and promote long-term health.
The authors have declared that no competing interests exist.
- Open access
- Published: 08 August 2022
A scoping review of gestational diabetes mellitus healthcare: experiences of care reported by pregnant women internationally
- Sheila Pham 1 ,
- Kate Churruca 1 ,
- Louise A. Ellis 1 &
- Jeffrey Braithwaite 1
BMC Pregnancy and Childbirth volume 22 , Article number: 627 ( 2022 ) Cite this article
3334 Accesses
6 Citations
5 Altmetric
Metrics details
Gestational diabetes mellitus (GDM) is a condition associated with pregnancy that engenders additional healthcare demand. A growing body of research includes empirical studies focused on pregnant women’s GDM healthcare experiences. The aim of this scoping review is to map findings, highlight gaps and investigate the way research has been conducted into the healthcare experiences of women with GDM.
A systematic search of primary research using a number of databases was conducted in September 2021. Studies were included if they had an explicit aim of focusing on GDM and included direct reporting of participants’ experiences of healthcare. Key data from each study was extracted into a purposely-designed form and synthesised using descriptive statistics and thematic analysis.
Fifty-seven articles were included in the analysis. The majority of studies used qualitative methodology, and did not have an explicit theoretical orientation. Most studies were conducted in urban areas of high-income countries and recruitment and research was almost fully conducted in clinical and other healthcare settings. Women found inadequate information a key challenge, and support from healthcare providers a critical factor. Experiences of prescribed diet, medication and monitoring greatly varied across settings. Additional costs associated with managing GDM was cited as a problem in some studies. Overall, women reported significant mental distress in relation to their experience of GDM.
Conclusions
This scoping review draws together reported healthcare experiences of pregnant women with GDM from around the world. Commonalities and differences in the global patient experience of GDM healthcare are identified.
Peer Review reports
Gestational diabetes mellitus (GDM) is defined as any degree of hyperglycaemia recognised for the first time during pregnancy, including type 2 diabetes mellitus diagnosed during pregnancy as well as true GDM which develops in pregnancy [ 1 ]. GDM is associated with a number of adverse maternal and neonatal outcomes, including increased birth weight and increased cord-blood serum C-peptide levels [ 2 ], as well as greater risk of future diabetes [ 3 ].
The global incidence and health burden of GDM is increasing [ 4 ] and the cost of healthcare relating to GDM significant. In 2019, the International Diabetes Federation estimated the annual global diabetes-related health expenditure, which includes GDM, reached USD$760 billion [ 4 ]. In China, for example, the annual societal economic burden of GDM is estimated to be ¥19.36 billion ($5.59 billion USD) [ 5 ].
GDM is estimated to affect 7–10% of all pregnancies worldwide, though the absence of a universal gold standard for screening means it is difficult to achieve an accurate estimation of prevalence [ 6 ], and the prevalence of GDM varies considerably depending on the data source used [ 7 ]. In Australia, for example, between 2000 and 01 and 2017-18, the rate of diagnosis for GDM tripled from 5.2 to 16.1% (3); furthermore, in 2017-18, there were around 53,700 hospitalisations for a birth event where gestational diabetes was recorded as the principal and/or additional diagnosis [ 8 ]. Important risk factors for GDM include being overweight/obese, advanced maternal age and having a family history of diabetes mellitus (DM), with all these risk factors dependent on foreign-born racial/ethnic minority status [ 9 ]. However, primarily directing research to understanding risk factors does not necessarily lead to better pregnancy care, particularly where diabetes is concerned, and developing better interventions requires consideration of women’s beliefs, behaviours and social environments [ 10 ].
To date there have been numerous systematic and scoping reviews focused on women’s experiences of GDM, which provide a comprehensive overview of numerous issues. However, gaps remain. In 2014, Nielsen et al. [ 11 ] reviewed qualitative and quantitative studies to investigate determinants and barriers to women’s use of GDM healthcare services, finding that although most women expressed commitment to following health professional advice to manage GDM, compliance with treatment was challenging. Their review also noted that only four out of the 58 included studies were conducted in low-income countries. In their follow-up review, Nielsen et al. specifically focused on research from low and middle income countries (LMIC) to examine barriers and facilitators for implementing programs and services for hyperglycaemia in pregnancy in those settings [ 12 ] and identified a range of factors such as women reporting treatment is “expensive, troublesome and difficult to follow”.
In 2014, Costi et al. [ 13 ] reviewed 22 qualitative studies on women’s experiences of diabetes and diabetes management in pregnancy, including both pre-existing diabetes and GDM. From their synthesis of study findings, they concluded that health professionals need to take a more whole-person approach when treating women with GDM, and that prescribed regimes need to be more accommodating [ 13 ]. Another 2014 review by Parsons et al. [ 14 ] conducted a narrative meta-synthesis of qualitative studies. Their 16 included studies focused on the experiences of women with GDM, including healthcare support and information, but the focus of their meta-synthesis was focused on perceptions of diabetes risk and views on future diabetes prevention.
In a systematic review of qualitative and survey studies from 2015, Van Ryswyck et al. [ 15 ] included 42 studies and had similar findings to Parsons et al. [ 14 ], also emphasising their findings regarding the emotional responses of women who have experienced GDM. Specifically, Van Ryswyck et al. [ 15 ] identified that women’s experiences ran the gamut of emotions from “very positive to difficult and confusing”, with a clear preference for non-judgmental and positively focused care. Most recently, the 2020 systematic review of qualitative studies by He et al. [ 16 ] synthesised findings from 10 studies to argue that understanding the experiences of women with GDM can aid health care professionals to better understand those under their care and to develop more feasible interventions to reduce the risk of DM. A further systematic review of qualitative studies by Craig et al. [ 17 ] focused on women’s psychosocial experiences of GDM diagnosis, one important aspect of healthcare experience, highlighting future directions for research into the psychosocial benefits and harms of a GDM diagnosis.
There has been insufficient consideration of epistemological assumptions and other aspects of research design which may affect how such studies are framed, which participants are included, how data is collected and subsequently what findings are spotlighted. While women’s experiences of GDM healthcare are often broadly included in reviews, they are not often the exclusive focus with healthcare experiences folded into accounts of living with GDM [ 11 ], healthcare service implementation [ 12 ], diabetes and pregnancy [ 13 ], understanding of future risk [ 14 ] and seeking postpartum care after GDM [ 15 ].
To address this gap, the aim of this review was to map the literature, identify gaps in knowledge and investigate the ways research has been conducted into GDM healthcare experiences. The research questions were:
When, where and how has knowledge been produced about women’s experiences of GDM healthcare?
What findings have been reported about women’s experience of GDM healthcare?
A scoping review was selected as the most appropriate method given our multiple aims relate to mapping the field of GDM healthcare experiences [ 18 ]. The reporting of this scoping review was guided by an adaptation of the PRISMA-ScR (Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews) reporting guidelines [ 19 ].
Search strategy
The search strategy was designed in consultation with a research librarian. The following databases were used: Scopus, PubMed, CINAHL, Web of Science, MEDLINE, Embase and Joanna Briggs Institute EBP. These databases were searched on 27 September 2021 by the first author using the keywords and MESH terms outlined in Table 1 . No limits were set on publication date, study design or country of origin. The reference lists of included articles were also examined to identify other potential articles (i.e. snowballing).
Study selection
References were downloaded into Endnote before being exported into the online systematic review platform Rayyan [ 20 ]. Titles and abstracts were first screened against inclusion criteria by the first author and uncertainties about article inclusion were referred to the second and third authors for a decision. A second reviewer independently screened a subset (5%) of titles and abstracts of studies for eligibility to ensure inclusion criteria were consistently applied. Studies were included if they reported primary (empirical) research in the English-language in a published peer-reviewed journal. Studies had to have an explicit aim of focusing on GDM and include direct reporting of participants’ experiences of healthcare. The experience of healthcare is here understood as being the patient experience of care occurring in formal clinical settings, including interactions with providers and other aspects of care prescribed by healthcare professionals. Exclusion criteria were reviews of any kind, research that was not empirical (e.g. personal accounts) and conference abstracts.
Data extraction and synthesis
Data from studies including authors, year published, study design, setting, sample size, recruitment site, stated theoretical approach, data collection method, languages and findings, were extracted into a custom template developed in Microsoft Excel. Findings were further summarised through an iterative coding process and used to develop a series of categories that broadly captured women’s experiences of GDM healthcare.
Search results
A total of 2856 articles were identified as potentially relevant to the research question from database searches. After removing duplicates ( n = 811) and excluding non-relevant studies by screening titles and abstracts ( n = 2045) and identifying an additional study through snowballing ( n = 1), 112 articles were examined for inclusion through a full text assessment. Of these, 57 articles were included in this review, with 55 studies being excluded with reasons for exclusion documented. Figure 1 outlines the process of data gathering and Additional file: Appendix 1 for summarised study characteristics.
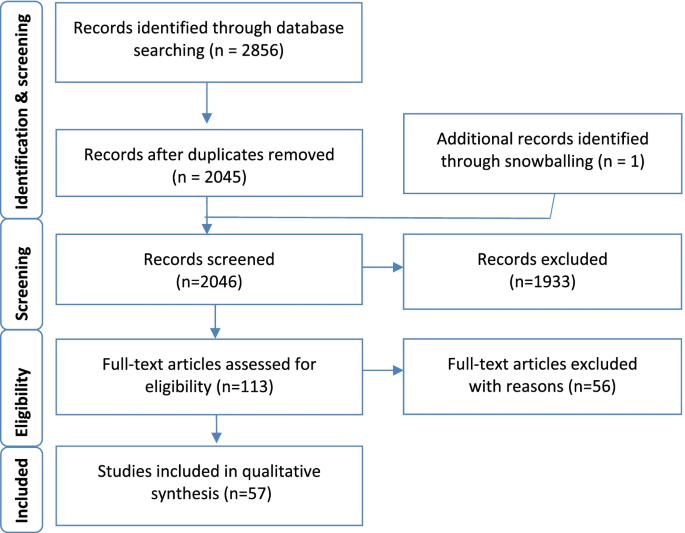
The process of data gathering
Publication dates
All of the included studies were published from 2005 onwards, except for one early study published in 1994 [ 21 ]. There has been an overall increase in the number of studies published each year to 2020 (see Fig. 2 ).
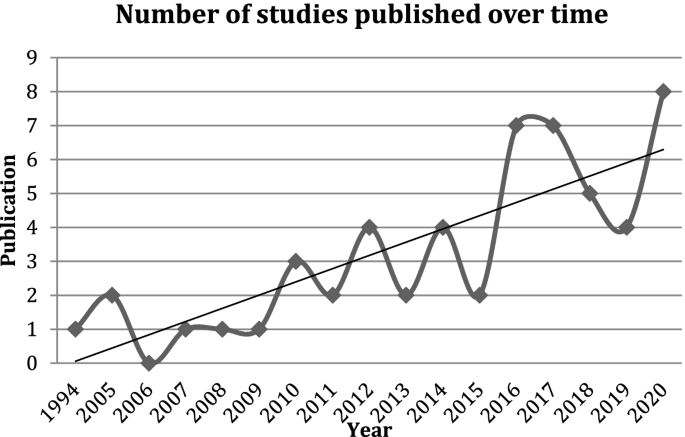
Included studies published over time
Research settings
For the vast majority of studies ( n = 55, 91%), recruitment of women with GDM was conducted via hospitals, clinics and healthcare providers, with one of these studies also conducting additional recruitment via workplaces [ 22 ]. Electronic databases were used in two studies for recruitment, with one study using a national diabetes database in Australia [ 23 ] and another using electronic health data in the United States [ 24 ]. Two studies which targeted Indigenous populations relied on pre-existing relationships; a Canadian study gained entry to an Indigenous population by building on pre-existing relationships with the Mi’kmaq communities [ 25 ] and an Australian study which focused on Aboriginal populations relied on existing research networks [ 26 ]. Only one study recruited completely outside clinical, healthcare and research settings using advertisements and community notices in targeted areas of Atlanta, Georgia in the United States [ 27 ].
A handful of studies ( n = 5, 9%) were based in countries classified as low- and lower middle-income; there were no countries considered ‘least developed’ [ 28 ]. For the most part, included studies were concentrated in a relatively small number of high-income countries, with the top six countries for research on women’s experiences of GDM healthcare being Australia ( n = 11), Canada ( n = 8), Sweden ( n = 7), the United States ( n = 6), the United Kingdom ( n = 4) and China ( n = 4). The remaining studies were spread across a number of countries, largely one study per setting: Austria [ 29 ], Brazil [ 30 ], Denmark [ 31 ], Ghana [ 32 ], India [ 33 ], Indonesia [ 34 ], Iran [ 35 , 36 ], Malaysia [ 37 ], New Zealand [ 38 , 39 ], Norway [ 40 ], Singapore [ 41 ], South Africa [ 42 , 43 ], Vietnam [ 44 ], Zimbabwe [ 45 ] (see Fig. 3 ).
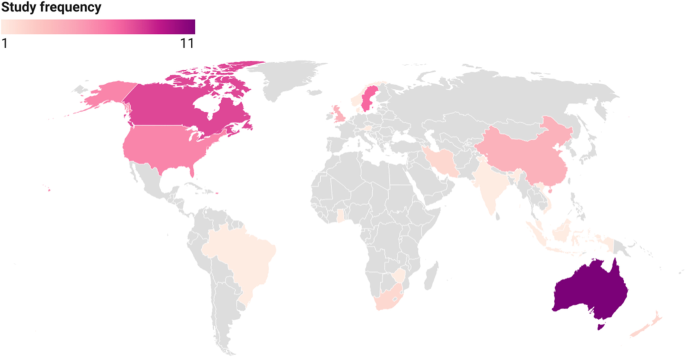
Settings of included studies
Forty-eight of the studies (84%) were conducted with participants in urban areas and the remaining studies ( n = 9) were conducted in regional and rural areas of Australia [ 26 , 46 ], Canada [ 25 , 47 , 48 , 49 ], China [ 50 ], Tamil Nadu in India [ 33 ], and the state of New York in the United States [ 51 ]. A number of studies were conducted by the same research team and published in multiple installments; these studies were conducted in Lund, Sweden (6 studies), southeastern China (4 studies) and Melbourne, Australia (4 studies).
Participants
The majority of studies specifically focused on women diagnosed with GDM as the sole target group, though two studies also interviewed comparative groups of women with different conditions such as DM [ 27 , 52 ]. Several studies targeted women as well as healthcare professionals, including nurses, clinicians, general practitioners, with data being compared between groups [ 26 , 27 , 32 , 36 , 41 , 46 , 47 , 53 , 54 ]. In one study it was noted how some participants had pre-existing medical conditions, such as hypertension and HIV, and that their co-morbidities directly contributed to their perspective on GDM [ 36 ].
Depending on the nature of the study design—whether qualitative, mixed methods or quantitative—the range of participants varied greatly, from a small number of interview and focus group participants ( n = 8) [ 55 ] through to large datasets such as the open-ended responses on a cross-sectional survey ( n = 393) [ 23 ]. While there was some stratification of participants based on individual factors, such as body mass index [ 56 ] as well as glycaemic targets set [ 38 ], the main categorisation made was often in relation to ethnicity in studies from countries such as Australia, Sweden and the United States, where the focus on ethnic differences was built into the design of studies. For example, this included directly comparing ethnic groups, such as Swedish-born versus African-born [ 57 ], or comparing groups of women by their ethnicity, namely Caucasian, Arabic and Chinese [ 58 ].
Study designs
The studies varied in how they understood, described and measured women’s experiences of GDM healthcare. Of the 57 included studies, 50 (88%) used qualitative study designs. Only four studies (7%) had quantitative designs and three (5%) employed mixed-methods [ 29 ]. The vast majority of studies ( n = 49, 86%) were cross-sectional, with seven studies [ 21 , 51 , 56 , 59 , 60 , 61 , 62 ] interviewing the same women at multiple time points. In terms of methodologies used, all the qualitative studies featured various types of interviews and/or focus groups. These were largely conducted face-to-face or via telephone. Seven studies employed more than one qualitative method to collect data [ 36 , 43 , 47 , 55 , 63 , 64 , 65 ] and, in addition, three studies used mixed methods to collect data [ 29 , 41 , 46 ]. One study focused on First Nations women in Canada used a focused ethnographic approach [ 49 ], and another 2021 study focused on South Asian women in Australia using ethnography [ 54 ]. The quantitative studies comprised four survey studies using questionnaires [ 37 , 38 , 52 , 66 ].
Theoretical approaches
The majority of studies did not specify a theoretical approach ( n = 31, 54%), and relied on general data analysis approaches such as thematic analysis. Where a theory was referred to, it was largely used as a guiding framework for study design and data collection, and data analysis where applicable (see Additional file: Appendix 1 ). The three most popular theoretical approaches were the Health Belief Model ( n = 6), Grounded Theory ( n = 3) and phenomenology ( n = 8), with the last of these specifically including hermeneutic [ 67 ] and interpretative approaches [ 63 , 68 ]. Two of the studies that focused on Indigenous populations used culturally-sensitive qualitative methodologies designed to respect and recognise Indigenous worldviews, namely the Two-Eyed Seeing Approach [ 25 ] and the Kaupapa Māori methodology [ 39 ]. Another study [ 47 ] focused on an Indigenous population discussed qualitative research in general being the most “flexible and interpretive methodology” and how using open-ended interviewing creates a dialogue which recognises Indigenous oral traditions and knowledge.
Data collection
Studies varied in when they captured data during the pregnancy and postpartum periods. Where the focus of a study was specifically on healthcare, women’s experiences were often elicited by researchers directly; otherwise, healthcare experience was generally revealed in relation to broader questions within the research framing, such as looking at factors that influence migrant women’s management of GDM [ 69 , 70 ] or examining barriers and possible solutions to nonadherence to antidiabetic therapy [ 71 ].
Almost all studies were conducted in a primary language of the research team, with fluency in the primary language largely requisite for participation. However, there were 14 studies involving multicultural populations that allowed women to use their preferred language as research teams consisted of multilingual researchers, research assistants or interpreters (see Table 2 ).
Study findings on women with GDM experiences of healthcare
The findings from the 57 included studies were categorised into a number of salient aspects of formal healthcare experience, then further categorised as being positive and/or negative experiences depending on how participants’ self-reports were described and quoted by study authors. Where there was not an explicit reference to sentiment in the study, it has not been recorded in this review.
Mental distress
Mental distress included acute emotional reactions such as shock and stress, as well as ongoing psychological challenges in coping with GDM. The vast majority of included studies noted mental distress of some kind ( n = 48, 84%), inferring that mental distress was inextricably part of women’s experiences of GDM and intertwined with healthcare experience.
Patient-provider interactions
From the moment diagnosis of GDM occurs, a cornerstone of women’s healthcare experience is interactions with providers, which differs depending on the model of care offered. ‘Interactions’ can be broadly defined as interpersonal encounters where communication occurs directly through conversations at consultations as well as group sessions, or interactions via other means such as text messages, emails and phone calls. Forty-four studies ( n = 44, 77%) discussed patient-provider interactions in their findings; these were positive experiences ( n = 9, 20%), negative experiences ( n = 16, 36%), or ambivalent, being both positive and negative ( n = 19, 43%). As an example of positive experience, one study reported “women were happy with the care provided in managing their GDM, acknowledging that the care was better than in their home country.” [ 62 ] In terms of negative experiences, women felt, for example, healthcare providers could be “preachy” [ 55 ] and discount their own expertise in their bodies [ 21 ]. One study [ 40 ] specifically examined the difference in women’s experiences with primary and secondary healthcare providers, and found that overall they received better care from the latter. More generally, the participants from one study emphasised the importance of a humanistic approach to care [ 76 ].
Treatment satisfaction
Treatment satisfaction was a measure reported in two quantitative studies [ 37 , 52 ], and the mixed-methods study [ 29 ]. The Diabetes Treatment Satisfaction Questionnaire (DTSQ) was used in two studies to measure satisfaction [ 29 , 37 ]. The study by Anderberg et al. [ 52 ] used its own purposely developed instrument and found 89% of women with GDM marked “satisfied”, 2% marked “neutral” and no one indicated dissatisfaction. In the study by Hussain et al. [ 37 ], which used the DTSQ, 122 (73.5%) patients reported they were satisfied with treatment and 44 (26.5%) were unsatisfied; overall, the majority of patients were satisfied with treatment but retained a ‘negative’ attitude towards GDM. The study by Trutnovsky et al. [ 29 ] went further in its analysis as women responded to the DTSQ at three different phases – before treatment, during early treatment and during late treatment – and found that overall treatment satisfaction was high, and significantly increased between early and late treatment.
Diet prescribed
Diet is a fundamental component of treatment for GDM. Once diagnosed, many women are prescribed modified diets to maintain blood sugar levels, which they record on paper or by using an electronic monitor at specified times. Thirty-nine studies ( n = 39, 68%) included findings and discussion about women’s experiences of prescribed diet, and of those studies ( n = 33, 84%) this is captured as generally a negative experience. In some studies, women’s experience of the prescribed diet was reported as being both positive and negative ( n = 4, 10%); only one study ( n = 1, 3%) recorded it as a positive experience [ 38 ]. The difficulty of following a new diet during pregnancy was a key reason as to why the experience was negative, as well as practical considerations such as being able to easily access fresh food in remote areas [ 26 ]. In studies with multicultural populations, negative experience related to managing the advice in conjunction with culturally-based diets. As noted in the two studies led by Bandyopadhyay, women had difficulty maintaining their traditional diet due to the new restrictions placed upon them [ 54 , 62 ].
Medication prescribed
Medication for GDM primarily involves some form of insulin, which is prescribed to manage blood sugar levels. Twenty-one studies ( n = 21, 37%) included findings and discussion about women’s experiences of GDM medication and of those, it was mostly reported as being a negative experience ( n = 13, 62%), with various reasons captured including insufficient time to “figure things out” [ 77 ] and causing feelings of anxiety and failure [ 78 ]. However, in a few studies prescribed medication was noted as being a positive experience ( n = 3, 14%), or both a positive and negative experience ( n = 5, 24%). In one study, a participant stated, “the fact that I’m on insulin makes it easy” [ 68 ].
Monitoring captures both the direct monitoring conducted by healthcare providers, primarily blood and blood sugar level tests as well as ultrasounds, as well as self-monitoring women were required to carry out and which was often then verified by healthcare professionals. Twenty studies ( n = 20, 35%) included findings and discussion about women’s experiences of monitoring and of those it was seen as being negative ( n = 14, n = 70%), both positive and negative ( n = 5, 25%) and positive ( n = 1, n = 5%). In the one study that reported positive experiences only, a participant reported that she thought it was good “they are monitoring us all the time” [ 30 ]. Studies reporting negative experiences with monitoring had participants citing reasons such as feeling over-scrutinised [ 65 ].
Access to timely healthcare
Access to healthcare can be a challenge in certain settings, and, even when access is possible, timeliness can be an issue. Of the 31 studies ( n = 31, 54%) that referred to access in their findings, the vast majority of these studies ( n = 28) reported access to timely healthcare being a negative experience, with reasons cited including geographic distance [ 39 , 46 ], difficulties in being able to make a booking to be seen at a hospital [ 79 ] and then, when being seen, not having enough time with a healthcare provider [ 27 , 44 ]. In one of the two studies reporting positive experiences [ 52 ], all questions relating to accessibility indicated satisfaction (97%); in the other of the two studies [ 38 ], the majority of women (68%) appreciated that health professionals took time to listen and explain.
Provision of information
Information to support women is critical in managing their GDM diagnosis. Ongoing management came from meetings with healthcare providers—described in one study as being “frontline support” [ 79 ]— alongside sources focused on diet, medication, exercise and other pertinent information. Across all the studies which discussed how provision of information by healthcare providers was received ( n = 38, 67%), it was noted as largely negative ( n = 24, 63%) and both positive and negative ( n = 10, 18%), though there were discussions of positive experiences ( n = 4, 7%). Considered together, all the studies suggested how crucial clear information is to a positive experience of healthcare. For women, having inadequate knowledge about how to cope was a source of disempowerment and, across the majority of studies ( n = 44, 77%), participants reported they found information from providers was insufficient. Interestingly, one of these studies found the insufficiency was actually due to the information being “too much” [ 26 ], while another study [ 59 ] found there was a desire for “more frequent controls and dietary advice”. The inappropriate timing of information was also reported in a number of studies [ 31 , 58 , 79 , 80 , 81 ]. One study noted how participants found one group of healthcare providers, midwives and nurses provided better information than general practitioners [ 40 ], while another noted the contradictory nature of advice from different providers [ 82 ]. Language barriers were also identified as a problem with information provision with a lack of information available in a woman’s preferred language [ 69 ].
Financial issues
Direct healthcare costs including out-of-pocket medical consultation fees, medication and medical equipment were primarily raised by participants in the United States [ 27 ], Ghana [ 32 ] and Zimbabwe [ 45 ], with the last of these reporting that some participants discussed “the related costs of treatment … resulted in participants foregoing some of the tests and treatments ordered” [ 45 ]. A study from Canada noted a number of participants with refugee status discussed the “economic challenge” of managing GDM and that the cost of diabetes care “was quite high and difficult to manage” [ 83 ]. Several indirect costs were also discussed across the studies. In a number of studies ( n = 7), the additional cost of purchasing healthy food to manage GDM was brought up as being a burden [ 25 , 27 , 38 , 42 , 48 , 51 , 84 ]. However, in one study, women said the costs related to food went down as being able to buy take-away (fast foods) became restricted [ 38 ]. Loss of income [ 46 ] as well as daycare costs were cited [ 25 ], as was additional transportation and hospital parking costs [ 39 , 46 , 56 ]. Finally, women in one study reported having to change occupations and even quit work to manage GDM [ 21 ].
The growing number of research studies relaying women’s GDM healthcare experience is encouraging, given increasing incidence and health burden. As this review demonstrates, there are important commonalities across all studies, suggesting that some aspects of GDM healthcare experience seem to be universal; mental distress, for example, was reported in most studies. In contrast, other aspects of GDM healthcare experience seem to relate to factors specific to local settings; financial issues were mainly raised in settings where healthcare is not universal or is not readily affordable. Related financial issues were raised by participants in a number of rural-based studies, revealing something of a difference between urban and rural healthcare settings regardless of country context.
All of the included studies relied on women’s self-reporting without necessarily involving other measures, which broadly fell into two categories: women currently undergoing care for GDM at the time of study data collection and those looking back on past experience. Included studies were overwhelmingly qualitative in design, with relatively small numbers of participants for each category; put together, though, they paint a broad picture of women’s GDM healthcare experience across a range of settings. As the phenomenon being examined here is women’s experiences, qualitative methodologies are vital given the experience of health, illness and medical intervention cannot be quantified [ 85 ]. On the other hand, quantitative studies are able to include far more participants, though it is important to note not necessarily greater applicability and generalisability; when both types of studies are considered together as in mixed-methods study designs, there is a possibility of corroboration, elaboration, complementarity and even contradiction [ 85 ].
Recruiting women through clinical and other healthcare settings, as almost all of the included studies did, necessarily leads to biased samples of participants likely to be ‘compliant’ with healthcare requirements and treatment regimens. As one study noted, compliance was high despite limited understanding of GDM and dietary requirements, as well as why change was required [ 71 ]. This scenario occurs against the backdrop of the inherent power imbalance which exists in patient-provider relationships in reproductive healthcare [ 86 ]. A few of the included studies demonstrated reflexivity for this issue, with the studies most sensitive to these concerns focused on Indigenous populations. This power imbalance also exists in patient-researcher relationships [ 87 ]; a critical way to mitigate this effect is to actively include participants in research design, which only one included study reported doing 75]. This suggests an important direction for future studies, building on recent work involving patients to establish research priorities for GDM [ 88 ]. Indeed, many of the included studies did incorporate ideas about improving healthcare as proposed by the women themselves. For example, in one study, participants reported that small group sessions with medical practitioners and more detailed leaflets would be useful [ 44 ], suggesting how current sessions could be run better.
Culturally sensitive qualitative methodologies were employed with Indigenous populations and those learnings could be further extended to other groups of research participants. GDM is known to be more common in foreign-born racial minorities [ 9 ], so it is encouraging that some studies focused on these particular groups and had study designs that included interpreters. However, this line of research is arguably under-developed given most studies excluded minoritised women who did not have a high degree of fluency in the dominant language. Language barriers were identified as a problem with information provision with GDM healthcare [ 69 , 70 ], and it is possible to extend this idea to research contexts themselves. Not being able to use the language one feels most fluent in clearly affects the way GDM healthcare experiences are reported.
Treatment satisfaction was used in both quantitative and mixed-method studies, but as a solo measure the insights it can provide is limited; we do not exactly know why or how, for example, women’s satisfaction improves later in GDM care [ 29 ]. However, a number of the studies provide possible answers. Persson et al. [ 61 ] describe the process women underwent “from stun to gradual balance” due to a process of adaptation that became easier “with increasing knowledge” about how to self-manage GDM. Ge et al. [ 89 ] found that women developed a philosophical attitude over time to reach a state of acceptance, and such a shift in attitude would clearly have an impact on how healthcare is received and understood. These findings suggest the benefit of both time and experience, and the role of these factors could be better examined with more longitudinal studies.
In this scoping review, under half of the included studies explicitly drew on theory. But as argued by Mitchell and Cody [ 90 ], regardless of whether it is acknowledged, theoretical interpretation occurs in qualitative research. Explicitly incorporating theoretical approaches are valuable in strengthening research design when such conceptual thinking clearly informs the research process; here, examining women’s lived experiences without articulating the theoretical bases which underpins research design and analysis leads to a lack of acknowledgement of relevant context as to how both treatment and research occurs. For example, gender exerts a significant influence upon help-seeking and healthcare delivery [ 91 ], and particularly for GDM. In future, it might be useful to further consider the value of theory in elucidating women’s experiences to address biases in research design to further the fields of study which relate to women’s GDM experiences [ 90 ].
Finally, much of this research has been generated in a small number of wealthy countries. GDM is a growing problem in low income settings and yet, as Nielsen et al. [ 92 ] describe, detection and treatment of GDM is hindered due to “barriers within the health system and society”. Going further, Goldenberg et al. suggest that due to competing concerns, “diagnosing and providing care to women with diabetes in pregnancy is not high on the priority lists in many LMIC”. [ 93 ] Similar barriers exist with GDM research endeavours; ensuring that evaluation of healthcare includes women’s experiences of GDM healthcare would be valuable to researchers in these settings and beyond. Thus there are clear gaps in practice as well as the research literature in considering women’s experiences of GDM healthcare internationally.
Implications
Research into women’s experience of GDM healthcare continues to accumulate and continued research efforts will contribute to far greater understanding of how we might best support women and improve healthcare outcomes. However, there is room for improvement, such as by following participants longitudinally, using mixed methods and taking more reflexive and theoretically informed approaches to researching women’s experiences of GDM healthcare. There is a need highlighted for more culturally sensitive research techniques as well as including women in the study design process, and not just as research subjects to be instrumentalised for developing recommendations for clinical delivery.
Strengths and limitations
Secondary analyses of primary research are challenging to conduct when the pool of included studies is highly heterogeneous. In this scoping review, in order to synthesise a large group of diverse studies, summarising results in terms of positive and negative experiences of GDM healthcare was reductive but necessary. This key strength of our review, inspired by sentiment analysis [ 94 ], shows the utility in capturing overall polarity of feelings as it highlights the ambivalence of healthcare experience. An additional strength was involving a research librarian to help design the searches and advise on relevant databases.
There are several limitations. For our search strategy, we used a broad set of terms relating to patient experience, but there is no standard set of terminology about this type of research, so it is possible some studies were missed. Only studies in English were included, so any studies published in other languages were missed. We did not conduct a critical appraisal on the included studies, which was a limitation; however, this was a purposeful choice in order to include a wide range of studies, including from research settings that are not as well-resourced.
This scoping review identifies commonalities in how GDM healthcare is delivered and received in settings around the world, with women’s experiences varying depending on what model of care is applied alongside other factors. Documenting experiences of GDM healthcare is a vital way to inform future policy and research directions, such as more theoretically informed longitudinal and mixed method approaches, and co-designed studies.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Dirar AM, Doupis J. Gestational diabetes from A to Z. World J Diabetes. 2017;8(12):489.
Article Google Scholar
Metzger BE, Contreras M, Sacks D, et al. Hyperglycemia and adverse pregnancy outcomes. New Engl J Med. 2008;358(19):1991–2002.
Article PubMed Google Scholar
Kim C. Gestational diabetes: risks, management, and treatment options. Int J Women’s Health. 2010;2:339.
Article CAS Google Scholar
International Diabetes Federation. IDF Diabetes Atlas. Brussels: IDF; 2019. Available from: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/159-idf-diabetes-atlas-ninth-edition-2019.html .
Xu T, Dainelli L, Yu K, et al. The short-term health and economic burden of gestational diabetes mellitus in China: a modelling study. BMJ Open. 2017;7(12):e018893.
Article PubMed PubMed Central Google Scholar
Behboudi-Gandevani S, Amiri M, Bidhendi Yarandi R, Ramezani Tehrani F. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11(1):11.
Lawrence RL, Wall CR, Bloomfield FH. Prevalence of gestational diabetes according to commonly used data sources: an observational study. BMC Pregnancy and Childbirth. 2019;19(1):349.
Australian Institute of Health and Welfare. Diabetes. Canberra: The Institute; 2020. Available from: https://www.aihw.gov.au/reports/diabetes/diabetes/contents/how-many-australians-have-diabetes/type-2-diabetes .
Pu J, Zhao B, Wang EJ, et al. Racial/ethnic differences in gestational diabetes prevalence and contribution of common risk factors. Paediatr Perinat Epidemiol. 2015;29(5):436–43.
Lavender T, Platt MJ, Tsekiri E, et al. Women’s perceptions of being pregnant and having pregestational diabetes. Midwifery. 2010;26(6):589–95.
Nielsen KK, Kapur A, Damm P, de Courten M, Bygbjerg IC. From screening to postpartum follow-up - the determinants and barriers for gestational diabetes mellitus (GDM) services, a systematic review. BMC Pregnancy and Childbirth. 2014;14:41.
Nielsen KK, Damm P, Bygbjerg IC, Kapur A. Barriers and facilitators for implementing programmes and services to address hyperglycaemia in pregnancy in low and middle income countries: a systematic review. Diabetes Res Clin Pract. 2018;145:102–18.
Costi L, Lockwood C, Munn Z, Jordan Z. Women’s experience of diabetes and diabetes management in pregnancy: a systematic review of qualitative literature. JBI Database Syst Rev Implement Rep. 2014;12(1):176–280.
Parsons J, Ismail K, Amiel S, Forbes A. Perceptions among women with gestational diabetes. Qual Health Res. 2014;24(4):575–85.
Van Ryswyk E, Middleton P, Shute E, Hague W, Crowther C. Women’s views and knowledge regarding healthcare seeking for gestational diabetes in the postpartum period: a systematic review of qualitative/survey studies. Diabetes Res Clin Pract. 2015;110(2):109–22.
He J, Chen X, Wang Y, Liu Y, Bai J. The experiences of pregnant women with gestational diabetes mellitus: a systematic review of qualitative evidence. Rev Endocr Metab Disord. 2021;22(4):777–87. Epub 2020 Nov 12.
Craig L, Sims R, Glasziou P, Thomas R. Women’s experiences of a diagnosis of gestational diabetes mellitus: a systematic review. BMC Pregnancy and Childbirth. 2020;20(1):76.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143.
Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Lawson EJ, Rajaram S. A transformed pregnancy: the psychosocial consequences of gestational diabetes. Sociol Health Illn. 1994;16(4):536–62.
Ge L, Wikby K, Rask M. Lived experience of women with gestational diabetes mellitus living in China: a qualitative interview study. BMJ Open. 2017;7(11):e017648.
Morrison MK, Lowe JM, Collins CE. Australian women’s experiences of living with gestational diabetes. Women Birth. 2014;27(1):52–7.
Gray MF, Hsu C, Kiel L, Dublin S. “It’s a very big burden on me”: women’s experiences using insulin for gestational diabetes. Matern Child Health J. 2017;21(8):1678–85.
Whitty-Rogers J, Caine V, Cameron B. Aboriginal women’s experiences with gestational diabetes mellitus: a participatory study with Mi’kmaq women in Canada. Adv Nurs Sci. 2016;39(2):181–98.
Kirkham R, King S, Graham S, Boyle JA, Whitbread C, Skinner T, et al. “No sugar”, “no junk food”, “do more exercise” - moving beyond simple messages to improve the health of Aboriginal women with Hyperglycaemia in Pregnancy in the Northern Territory - A phenomenological study. Women Birth. 2021;34(6):578–84. https://doi.org/10.1016/j.wombi.2020.10.003 . Epub 2020 Nov 2.
Article CAS PubMed Google Scholar
Collier SA, Mulholland C, Williams J, Mersereau P, Turay K, Prue C. A qualitative study of perceived barriers to management of diabetes among women with a history of diabetes during pregnancy. J Women’s Health. 2011;20(9):1333–9.
Organisation for Economic Co-operation and Development. DAC List of ODA Recipients. Paris: OECD; 2021. Available from: https://www.oecd.org/dac/financing-sustainable-development/development-finance-standards/DAC-List-ODA-Recipients-for-reporting-2021-flows.pdf .
Trutnovsky G, Panzitt T, Magnet E, Stern C, Lang U, Dorfer M. Gestational diabetes: women’s concerns, mood state, quality of life and treatment satisfaction. J Matern-Fetal Neonatal Med. 2012;25(11):2464–6.
Nicolosi BF, Lima SAM, Rodrigues MRK, et al. Prenatal care satisfaction: perception of caregivers with diabetes mellitus. Rev Bras Enferm. 2019;72(suppl 3):305–11.
Dayyani I, Maindal HT, Rowlands G, Lou S. A qualitative study about the experiences of ethnic minority pregnant women with gestational diabetes. Scand J Caring Sci. 2019;33(3):621–31.
Mensah GP, van Rooyen DRM, ten Ham-Baloyi W. Nursing management of gestational diabetes mellitus in Ghana: perspectives of nurse-midwives and women. Midwifery. 2019;71:19–26.
Kragelund Nielsen K, Vildekilde T, Kapur A, Damm P, Seshiah V, Bygbjerg IC. “If I Don’t Eat Enough, I Won't Be Healthy”. Women’s Experiences with Gestational Diabetes Mellitus Treatment in Rural and Urban South India. Int J Environ Res Public Health. 2020;17(9):3062.
Mufdlilah M, Efriani R, Rokhanawati D, Dzakiyullah NR. Mother’s obstacles in managing gestational diabetes mellitus: A Qualitative study. Ann Trop Med Public Health. 2020;23(S9):SP23942.
Khooshehchin TE, Keshavarz Z, Afrakhteh M, Shakibazadeh E, Faghihzadeh S. Perceived needs in women with gestational diabetes: a qualitative study. Electron physician. 2016;8(12):3412–20.
Kolivand M, Keramat A, Rahimi M, Motaghi Z, Shariati M, Emamian M. Self-care education needs in gestational diabetes tailored to the Iranian culture: a qualitative content analysiss. Iran J Nurs Midwifery Res. 2018;23(3):222–9.
Hussain Z, Yusoff ZM, Sulaiman SA. A study exploring the association of attitude and treatment satisfaction with glycaemic level among gestational diabetes mellitus patients. Prim Care Diabetes. 2015;9(4):275–82.
Martis R, Brown J, Crowther CA. Views and Experiences of New Zealand Women with Gestational Diabetes in Achieving Glycaemic Control Targets: The Views Study. J Diabetes Res. 2017;2017:2190812. https://doi.org/10.1155/2017/2190812 . Epub 2017 Oct 31.
Reid J, Anderson A, Cormack D, et al. The experience of gestational diabetes for indigenous Māori women living in rural New Zealand: qualitative research informing the development of decolonising interventions. BMC Pregnancy Childbirth. 2018;18:478.
Helmersen M, Sorensen M, Lukasse M, Laine HK, Garnweidner-Holme L. Women’s experience with receiving advice on diet and self-monitoring of blood glucose for gestational diabetes mellitus: a qualitative study. Scand J Prim Health Care. 2021;39(1):44–50.
Hewage S, Audimulam J, Sullivan E, Chi C, Yew TW, Yoong J. Barriers to Gestational Diabetes Management and Preferred Interventions for Women With Gestational Diabetes in Singapore: Mixed Methods Study. JMIR Form Res. 2020;4(6):e14486.
Dickson LM, Buchmann EJ, Norris SA. Women’s accounts of the gestational diabetes experience – a South African perspective. S Afr J Obstet Gynaecol. 2020;26(1):1–7.
Google Scholar
Muhwava LS, Murphy K, Zarowsky C, Levitt N. Perspectives on the psychological and emotional burden of having gestational diabetes amongst low-income women in Cape Town, South Africa. BMC Women’s Health. 2020;20(1):231.
Hirst JE, Tran TS, My ATD, Rowena F, Morris JM, Jeffery HE. Women with gestational diabetes in Vietnam: a qualitative study to determine attitudes and health behaviours. BMC Pregnancy and Childbirth. 2012;12:10.
Mukona D, Munjanja SP, Zvinavashe M, Stray-Pederson B. Barriers of adherence and possible solutions to nonadherence to antidiabetic therapy in women with diabetes in pregnancy: Patients’ perspective. J Diabetes Res. 2017;2017:3578075.
Rasekaba T, Nightingale H, Furler J, Lim WK, Triay J, Blackberry I. Women, clinician and IT staff perspectives on telehealth for enhanced gestational diabetes mellitus management in an Australian rural/regional setting. Rural Remote Health. 2021;21(1):5983.
PubMed Google Scholar
Tait Neufeld H. Patient and caregiver perspectives of health provision practices for First Nations and Métis women with gestational diabetes mellitus accessing care in Winnipeg, Manitoba. BMC Health Serv Res. 2014;14:440.
Pace R, Loon O, Chan D, Porada H, Godin C, Linton J, et al. Preventing diabetes after pregnancy with gestational diabetes in a Cree community: an inductive thematic analysis. BMJ Open Diabetes Res Care. 2020;8(1):e001286.
Oster RT, Mayan MJ, Toth EL. Diabetes in pregnancy among First Nations women. Qual Health Res. 2014;24(11):1469–80.
Ge L, Wikby K, Rask M. ’Is gestational diabetes a severe illness?‘ exploring beliefs and self-care behaviour among women with gestational diabetes living in a rural area of the south east of China. Aust J Rural Health. 2016;24(6):378–84.
Abraham K, Wilk N. Living with gestational diabetes in a rural community. MCN Am J Mater Child Nurs. 2014;39(4):239–45.
Anderberg E, Berntorp K, Crang-Svalenius E. Diabetes and pregnancy: women’s opinions about the care provided during the childbearing year. Scand J Caring Sci. 2009;23(1):161–70.
McCloskey L, Sherman ML, St John M, et al. Navigating a ‘perfect storm’ on the path to prevention of type 2 diabetes mellitus after gestational diabetes: lessons from patient and provider narratives. Matern Child Health J. 2019;23(5):603–12.
Bandyopadhyay M. Gestational diabetes mellitus: a qualitative study of lived experiences of South Asian immigrant women and perspectives of their health care providers in Melbourne, Australia. BMC Pregnancy and Childbirth. 2021;21(1):500.
Nolan JA, McCrone S, Chertok IRA. The maternal experience of having diabetes in pregnancy. J Am Acad Nurs Pract. 2011;23(11):611–8.
Jarvie R. Lived experiences of women with co-existing BMI ≥ 30 and gestational diabetes mellitus. Midwifery. 2017;49:79–86.
Hjelm K, Berntorp K, Apelqvist J. Beliefs about health and illness in Swedish and African-born women with gestational diabetes living in Sweden. J Clin Nurs. 2012;21(9–10):1374–86.
Razee H, van der Ploeg HP, Blignault I, et al. Beliefs, barriers, social support, and environmental influences related to diabetes risk behaviours among women with a history of gestational diabetes. Health Promot J Aust. 2010;21(2):130–7.
Hjelm K, Bard K, Apelqvist J. Gestational diabetes: prospective interview-study of the developing beliefs about health, illness and health care in migrant women. J Clin Nurs. 2012;21(21–22):3244–56.
Hjelm K, Bard K, Apelqvist J. A qualitative study of developing beliefs about health, illness and healthcare in migrant African women with gestational diabetes living in Sweden. BMC Women’s Health. 2018;18(1):34.
Persson M, Winkvist A, Mogren I. ’From stun to gradual balance’- women’s experiences of living with gestational diabetes mellitus. Scand J Caring Sci. 2010;24(3):454–62.
Bandyopadhyay M, Small R, Davey MA, Oats JJN, Forster DA, Aylward A. Lived experience of gestational diabetes mellitus among immigrant South Asian women in Australia. Aust New Z J Obstet Gynaecol. 2011;51(4):360–4.
Carolan M, Gill GK, Steele C. Women’s experiences of factors that facilitate or inhibit gestational diabetes self-management. BMC Pregnancy and Childbirth. 2012;12:99.
Carolan M. Women’s experiences of gestational diabetes self-management: a qualitative study. Midwifery. 2013;29(6):637–45.
Parsons J, Sparrow K, Ismail K, Hunt K, Rogers H, Forbes A. Experiences of gestational diabetes and gestational diabetes care: a focus group and interview study. BMC Pregnancy Childbirth. 2018;18(1):25.
Sayakhot P, Carolan-Olah M. Sources of information on Gestational Diabetes Mellitus, satisfaction with diagnostic process and information provision. BMC Pregnancy Childbirth. 2016;16(1):287.
Article PubMed PubMed Central CAS Google Scholar
Evans MK, O’Brien B. Gestational diabetes: the meaning of an at-risk pregnancy. Qual Health Res. 2005;15(1):66–81.
Carolan-Olah M, Gill G, Steel C. Women’s experiences of gestational diabetes self-management: A qualitative study. Women and Birth. 2013;26(1):S2-S.
Wah YYE, McGill M, Wong J, Ross GP, Harding AJ, Krass I. Self-management of gestational diabetes among Chinese migrants: A qualitative study. Women and Birth. 2019;32(1):E17–23.
Jirojwong S, Brownhill S, Dahlen HG, Johnson M, Schmied V. Going up, going down: the experience, control and management of gestational diabetes mellitus among Southeast Asian migrant women living in urban Australia. Health Promot J Aust. 2017;28(2):123–31.
Carolan-Olah M, Duarte-Gardea M, Lechuga J, Salinas-Lopez S. The experience of gestational diabetes mellitus (GDM) among Hispanic women in a U.S. border region. Sex Reprod HealthC. 2017;12:16–23.
Hjelm K, Bard K, Nyberg P, Apelqvist J. Swedish and Middle-Eastern-born women’s beliefs about gestational diabetes. Midwifery. 2005;21(1):44–60.
Hjelm K, Bard K, Nyberg P, Apelqvist J. Management of gestational diabetes from the patient’s perspective–a comparison of Swedish and Middle-Eastern born women. J Clin Nurs. 2007;16(1):168–78.
Dayyani I, Terkildsen Maindal H, Rowlands G, Lou S. A qualitative study about the experiences of ethnic minority pregnant women with gestational diabetes. Scand J Caring Sci. 2019;33(3):621–31.
Ge L, Wikby K, Rask M. Quality of care from the perspective of women with gestational diabetes in China. Int J Gynecol Obstet. 2016;134(2):151–5.
Hui AL, Sevenhuysen G, Harvey D, Salamon E. Food choice decision-making by women with gestational diabetes. Can J Diabetes. 2014;38(1):26–31.
Draffin CR, Alderdice FA, McCance DR, et al. Exploring the needs, concerns and knowledge of women diagnosed with gestational diabetes: A qualitative study. Midwifery. 2016;40:141–7.
Boyd J, McMillan B, Easton K, Delaney B, Mitchell C. Utility of the COM-B model in identifying facilitators and barriers to maintaining a healthy postnatal lifestyle following a diagnosis of gestational diabetes: a qualitative study. BMJ Open. 2020;10(8):e037318.
Hjelm K, Berntorp K, Frid A, Aberg A, Apelqvist J. Beliefs about health and illness in women managed for gestational diabetes in two organisations. Midwifery. 2008;24(2):168–82.
Hjelm K, Bard K, Nyberg P, Apelqvist J. Management of gestational diabetes from the patient’s perspective - a comparison of Swedish and Middle-Eastern born women. J Clin Nurs. 2007;16(1):168–78.
Nur Suraiya AHS, Zahara AM, Nazlena MA, Suzana S, Norazlin MI, Sameeha MJ. Perspectives of healthcare professionals and patients on management of gestational diabetes mellitus: a qualitative study in Negeri Sembilan, Malaysia. Malays J Nutr. 2016;21(3):393–9.
Siad FM, Fang XY, Santana MJ, Butalia S, Hebert MA, Rabi DM. Understanding the experiences of East African immigrant women with gestational diabetes mellitus. Can J Diabetes. 2018;42(6):632–8.
Kaptein S, Evans M, McTavish S, et al. The subjective impact of a diagnosis of gestational diabetes among ethnically diverse pregnant women: a qualitative study. Can J Diabetes. 2015;39(2):117–22.
Hammarberg K, Kirkman M, de Lacey S. Qualitative research methods: when to use them and how to judge them. Hum Reprod. 2016;31(3):498–501.
Alspaugh A, Barroso J, Reibel M, Phillips S. Women’s contraceptive perceptions, beliefs, and attitudes: an integrative review of qualitative research. J Midwifery & Women’s Health. 2020;65(1):64–84.
Råheim M, Magnussen LH, Sekse RJ, Lunde Å, Jacobsen T, Blystad A. Researcher-researched relationship in qualitative research: Shifts in positions and researcher vulnerability. Int J Qual Stud Health Well-being. 2016;11:30996.
Rees SE, Chadha R, Donovan LE, et al. Engaging patients and clinicians in establishing research priorities for gestational diabetes mellitus. Can J Diabetes. 2017;41(2):156–63.
Ge L, Albin B, Hadziabdic E, Hjelm K, Rask M. Beliefs about health and illness and health-related behavior among urban women with gestational diabetes mellitus in the south east of China. J Transcult Nurs. 2016;27(6):593–602.
Mitchell GJ, Cody WK. The role of theory in qualitative research. Nurs Sci Q. 1993;6(4):170–8.
Kuhlmann E, Annandale E. Gender and Healthcare Policy. In: Kuhlmann E, Blank RH, Bourgeault IL, Wendt C, editors. The Palgrave International Handbook of Healthcare Policy and Governance. London: Palgrave Macmillan UK; 2015. p. 578–96.
Chapter Google Scholar
Nielsen KK, de Courten M, Kapur A. Health system and societal barriers for gestational diabetes mellitus (GDM) services - lessons from World Diabetes Foundation supported GDM projects. BMC Int Health Hum Rights. 2012;12:33.
Goldenberg RL, McClure EM, Harrison MS, Miodovnik M. Diabetes during Pregnancy in Low- and Middle-Income Countries. Am J Perinatol. 2016;33(13):1227–35.
Liu B. Sentiment analysis and opinion mining. Synthesis Lectures on Human Language Technologies. 2012;5(1):1–167.
Download references
Acknowledgements
Jeremy Cullis, Clinical Librarian at Macquarie University, provided invaluable assistance with the database search strategy.
SP is being supported by a Macquarie Research Excellence Scholarship, funded by both Macquarie University and the Australian Government’s Research Training Program.
Author information
Authors and affiliations.
Centre for Healthcare Resilience and Implementation Science, Australian Institute of Health Innovation, Macquarie University, 75 Talavera Rd, North Ryde, NSW, 2113, Sydney, Australia
Sheila Pham, Kate Churruca, Louise A. Ellis & Jeffrey Braithwaite
You can also search for this author in PubMed Google Scholar
Contributions
SP led and executed the study with design support, input and advice from KC, LAE and JB. SP supported by KC assessed the literature. LAE provided statistical and methodological expertise alongside the other authors, and JB provided strategic advice. All authors reviewed and provided editorial suggestions on SP’s draft and agreed with the final submitted version. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Sheila Pham .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Characteristics of the studies included in the scoping review
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Pham, S., Churruca, K., Ellis, L.A. et al. A scoping review of gestational diabetes mellitus healthcare: experiences of care reported by pregnant women internationally. BMC Pregnancy Childbirth 22 , 627 (2022). https://doi.org/10.1186/s12884-022-04931-5
Download citation
Received : 30 March 2022
Accepted : 14 July 2022
Published : 08 August 2022
DOI : https://doi.org/10.1186/s12884-022-04931-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gestational diabetes mellitus
- Patient experience
- Delivery of healthcare
- Scoping review
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]

- Previous Article
- Next Article
Introduction
Research design and methods, conclusions, article information, gestational diabetes mellitus and diet: a systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Jennifer M. Yamamoto , Joanne E. Kellett , Montserrat Balsells , Apolonia García-Patterson , Eran Hadar , Ivan Solà , Ignasi Gich , Eline M. van der Beek , Eurídice Castañeda-Gutiérrez , Seppo Heinonen , Moshe Hod , Kirsi Laitinen , Sjurdur F. Olsen , Lucilla Poston , Ricardo Rueda , Petra Rust , Lilou van Lieshout , Bettina Schelkle , Helen R. Murphy , Rosa Corcoy; Gestational Diabetes Mellitus and Diet: A Systematic Review and Meta-analysis of Randomized Controlled Trials Examining the Impact of Modified Dietary Interventions on Maternal Glucose Control and Neonatal Birth Weight. Diabetes Care 1 July 2018; 41 (7): 1346–1361. https://doi.org/10.2337/dc18-0102
Download citation file:
- Ris (Zotero)
- Reference Manager
Medical nutrition therapy is a mainstay of gestational diabetes mellitus (GDM) treatment. However, data are limited regarding the optimal diet for achieving euglycemia and improved perinatal outcomes. This study aims to investigate whether modified dietary interventions are associated with improved glycemia and/or improved birth weight outcomes in women with GDM when compared with control dietary interventions.
Data from published randomized controlled trials that reported on dietary components, maternal glycemia, and birth weight were gathered from 12 databases. Data were extracted in duplicate using prespecified forms.
From 2,269 records screened, 18 randomized controlled trials involving 1,151 women were included. Pooled analysis demonstrated that for modified dietary interventions when compared with control subjects, there was a larger decrease in fasting and postprandial glucose (−4.07 mg/dL [95% CI −7.58, −0.57]; P = 0.02 and −7.78 mg/dL [95% CI −12.27, −3.29]; P = 0.0007, respectively) and a lower need for medication treatment (relative risk 0.65 [95% CI 0.47, 0.88]; P = 0.006). For neonatal outcomes, analysis of 16 randomized controlled trials including 841 participants showed that modified dietary interventions were associated with lower infant birth weight (−170.62 g [95% CI −333.64, −7.60]; P = 0.04) and less macrosomia (relative risk 0.49 [95% CI 0.27, 0.88]; P = 0.02). The quality of evidence for these outcomes was low to very low. Baseline differences between groups in postprandial glucose may have influenced glucose-related outcomes. As well, relatively small numbers of study participants limit between-diet comparison.
Modified dietary interventions favorably influenced outcomes related to maternal glycemia and birth weight. This indicates that there is room for improvement in usual dietary advice for women with GDM.
Gestational diabetes mellitus (GDM) is one of the most common medical complications in pregnancy and affects an estimated 14% of pregnancies, or one in every seven births globally ( 1 ). Women with GDM and their offspring are at increased risk of both short- and longer-term complications, including, for mothers, later development of type 2 diabetes, and for offspring, increased lifelong risks of developing obesity, type 2 diabetes, and metabolic syndrome ( 2 – 6 ). The adverse intrauterine environment causes epigenetic changes in the fetus that may contribute to metabolic disorders, the so-called vicious cycle of diabetes ( 7 ).
The mainstay of GDM treatment is dietary and lifestyle advice, which includes medical nutrition therapy, weight management, and physical activity ( 8 ). Women monitor their fasting and postmeal glucose levels and adjust their individual diet and lifestyle to meet their glycemic targets. This pragmatic approach achieves the glycemic targets in approximately two-thirds of women with GDM ( 8 ). However, despite the importance of medical nutrition therapy and its widespread recommendation in clinical practice, there are limited data regarding the optimal diet for achieving maternal euglycemia ( 8 – 11 ). It is also unknown whether the dietary interventions for achieving maternal glycemia are also effective for reducing excessive fetal growth and adiposity ( 12 ).
Different dietary strategies have been reported including low glycemic index (GI), energy restriction, increase or decrease in carbohydrates, and modifications of fat or protein quality or quantity ( 12 – 14 ). Three recent systematic reviews have been performed examining specific diets and pregnancy outcomes ( 15 – 17 ). Viana et al. ( 16 ) and Wei et al. ( 15 ) concluded that low-GI diets were associated with a decreased risk of infant macrosomia. However, the most recent systematic review from Cochrane, including 19 trials randomizing 1,398 women, found no clear difference in large for gestational age or other primary neonatal outcomes with the low-GI diet ( 17 ). The primary maternal outcomes were hypertension (gestational and/or preeclampsia), delivery by cesarean section, and type 2 diabetes, outcomes for which most trials lacked statistical power, even when dietary subgroups were combined. Remarkably, no systematic reviews examined the impact of modified dietary interventions on the detailed maternal glycemic parameters, including change in glucose-related variables, the outcomes that are most directly influenced by diet.
To address this knowledge gap, we performed a systematic review and meta-analysis of randomized controlled trials to investigate whether modified dietary interventions (defined as a dietary intervention different from the usual one used in the control group) in women with GDM offer improved glycemic control and/or improved neonatal outcomes when compared with standard diets.
In accordance with a published protocol (PROSPERO CRD42016042391), we performed a systematic review and meta-analysis. Reporting is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines ( 18 ). An international panel of experts was formed by the International Life Sciences Institute Europe. This panel determined the review protocol and carried out all aspects of the review.
Data Sources and Search Strategy
The following databases were searched for all available dates using the search terms detailed in Supplementary Table 1 : PubMed, MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science Core Collection, Applied Social Sciences Index and Abstracts, ProQuest, ProQuest Dissertations & Theses—A&I and UK & Ireland, National Institute for Health and Care Excellence evidence search, Scopus, UK Clinical Trials Gateway, ISRCTN, and ClinicalTrials.gov . The initial search was performed in July 2016. An updated search of MEDLINE, Embase, CENTRAL, and CINAHL was performed on 3 October 2017 using the same search terms.
A hand-search of relevant reviews and all included articles was conducted to identify studies for potential inclusion. As well, experts on the panel were consulted for the inclusion of additional articles. Reference management was carried out using EndNote.
Study Selection
All titles and abstracts were assessed independently and in duplicate to identify articles requiring full-text review. Published studies fulfilling the following criteria were included: randomized controlled trials, evaluated modified dietary interventions on women with GDM, glucose intolerance or hyperglycemia during pregnancy, reported-on primary maternal and neonatal outcomes, included women aged 18–45 years, had a duration of 2 weeks or more, and were published in English, French, Spanish, Portuguese, Italian, Dutch, German, or Chinese. We excluded studies that included participants with type 1 or type 2 diabetes if data for participants with GDM were not presented independently, if dietary characteristics were not available, if the study was in animals, or if the study did not report outcomes of interest. We did not include studies of nutritional supplements such as vitamin D or probiotics as recent reviews have addressed these topics ( 19 , 20 ).
All citations identified after title and abstract assessment were full-text reviewed in duplicate. Reasons for exclusion at the full-text review stage were recorded. Any disagreements between reviewers were resolved by consensus and with consultation with the expert group when required.
Data Extraction
Data from included studies were extracted in duplicate using prespecified data extraction forms. Extracted data elements included study and participant demographics, study design, diagnostic criteria for GDM, glucose intolerance or hyperglycemia, funding source, description of modified dietary intervention and comparator, and maternal and neonatal outcomes. For studies with missing data, inconsistencies, or other queries, authors were contacted. Record management was carried out using Microsoft Excel and RevMan.
For articles providing information on maternal weight, fasting glucose, postprandial glucose, HbA 1c , or HOMA insulin resistance index (HOMA-IR) at baseline and postintervention but not their change, change was calculated as the difference between postintervention and baseline. Standard deviations were imputed using the correlation coefficient observed in articles reporting full information on the variable at baseline and postintervention and its change or a correlation coefficient of 0.5 when this information was not available ( 21 ). As studies differed in postprandial glucose at baseline, glycemic control at study entry was not considered to be equivalent in both arms, and thus continuous glucose-related variables at follow-up are reported as change from baseline.
Data Synthesis
The primary outcomes were maternal glycemic outcomes (mean glucose, fasting glucose, postprandial glucose [after breakfast, lunch, and dinner and combined], hemoglobin A 1c [HbA 1c ], assessment of insulin sensitivity by HOMA-IR, and change in these parameters from baseline to assessment; medication treatment [defined as oral diabetes medications or insulin]) and neonatal birth weight outcomes (birth weight, macrosomia, and large for gestational age).
Data were pooled into relative risks (RRs) or mean differences with 95% CI for dichotomous outcomes and continuous outcomes, respectively. Meta-analysis was performed using random-effects models. A prespecified analysis stratified by type of diet and quality assessment was performed to explore potential reasons for interstudy variation. Heterogeneity was assessed using I 2 statistics. Small study effects were examined for using funnel plots. Analyses were conducted using RevMan version 5.3. Pooled estimation of birth weight in the study and control arms, both overall and according to the specific diet intervention, was performed using Stata 14.0.
Quality Assessment
Methodological quality and bias assessment was completed by two reviewers. Risk of bias was assessed using the Cochrane Collaboration tool, which rates seven items as being high, low, or unclear for risk of bias ( 21 ). These items included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias ( 21 ). A sensitivity analysis was performed excluding articles with relevant weaknesses in trial design or execution.
The overall quality of the evidence was also assessed using Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group guidelines ( 21 ). GRADE was assessed for all primary and secondary outcomes, both maternal and neonatal, but without subgroup analysis per different dietary intervention for each outcome measure.
We screened 2,269 records for potential inclusion, and 126 articles were reviewed in full ( Supplementary Fig. 1 ). Eighteen studies ( 12 – 14 , 22 – 36 ) were included in the meta-analysis with a total of 1,151 pregnant women with GDM.
Study Characteristics
The types of modified dietary intervention included low-GI ( n = 4), Dietary Approaches to Stop Hypertension (DASH) ( n = 3), low-carbohydrate ( n = 3), fat-modification ( n = 2), soy protein–enrichment ( n = 2), energy-restriction ( n = 1), high-fiber ( n = 1), and ethnic diets (i.e., foods commonly consumed according to participant’s ethnicity) ( n = 1) and behavioral intervention ( n = 1). Details of the study characteristics are included in Table 1 . Most trials were single centered and had small sample sizes (range 12–150). Only two trials (one each from Spain and Australia) included over 100 participants, nine had 50–100 participants, and seven studies had fewer than 50 participants. They were performed in North America, Europe, or Australasia and all had a duration of at least 2 weeks. The ethnicity of participants was reported in seven studies ( 12 , 13 , 26 , 29 , 31 , 32 , 34 ).
Characteristics of studies included
Unless otherwise stated, the units are kcal/day for energy, % for carbohydrate, protein, and fat. OGTT, oral glucose tolerance test.
*Reported actual dietary intake. When not reported, prescribed dietary intake is reported.
†Intervention is defined as dietary intervention different from the usual dietary intervention used in the control group.
‡Indicates prescribed diet.
§The control and intervention groups were reversed for the purpose of meta-analysis so it could be included in the low-carbohydrate group.
Most studies assessed individual dietary adherence using food diaries ( 13 , 23 – 36 ). Although most studies did report an overall difference in dietary composition between the intervention diet and control diet, few studies reported a detailed assessment of dietary adherence. Only five studies used a formal measure of adherence ( 24 , 25 , 29 , 33 , 34 ), and four of them reported data ( 25 , 29 , 33 , 34 ). Adherence ranged from 20% to 76% in the control groups and 60% to 80% in the intervention groups.
Participant Characteristics
When baseline characteristic data were pooled, women in the intervention group were older than women in the control group (pooled mean difference 0.60 years [95% CI 0.06, 1.14]) and had higher postprandial glucose (5.47 [0.86, 10.08]), most influenced by the DASH and ethnic diet studies. There was no overall significant difference between the intervention and control groups for BMI, gestational age at enrollment, fasting glucose, HbA 1c , or HOMA-IR.
Maternal Glycemic Outcomes for All Modified Dietary Interventions
Pooled risk ratios in 15 studies involving 1,023 women demonstrated a lower need for medication (RR 0.65 [95% CI 0.47, 0.88]; I 2 = 55) ( Table 2 ). Thirteen studies ( n = 662 women) reported fasting glucose levels, nine ( n = 475) reported combined postprandial glucose measures, and three ( n = 175) reported post-breakfast glucose measures. Pooled analysis demonstrated a larger decrease in fasting, combined postprandial, and post-breakfast glucose levels in modified dietary interventions (mean −4.07 mg/dL [95% CI −7.58, −0.57], I 2 = 86, P = 0.02; −7.78 mg/dL [−12.27, −3.29], I 2 = 63, P = 0.0007; and −4.76 mg/dL [−9.13, −0.38], I 2 = 34, P = 0.03, respectively) compared with control group. There were no significant differences in change in HbA 1c (seven studies), HOMA-IR (four studies), or in post-lunch or -dinner glucose levels (two studies).
Pooled analyses of primary maternal glycemic and infant birth weight outcomes
Neonatal Birth Weight Outcomes for All Diets
Pooled mean birth weight was 3,266.65 g (95% CI 3,172.15, 3,361.16) in the modified dietary intervention versus 3,449.88 g (3,304.34, 3,595.42) in the control group. Pooled analysis of all 16 modified dietary interventions including 841 participants demonstrated lower birth weight (mean −170.62 g [95% CI −333.64, −7.60], I 2 = 88; P = 0.04) and less macrosomia (RR 0.49 [95% CI 0.27, 0.88], I 2 = 11; P = 0.02) compared with conventional dietary advice ( Table 2 and Fig. 1 ). There was no significant difference in the risk of large-for-gestational-age newborns in modified dietary interventions as compared with control diets (RR 0.96 [95% CI 0.63, 1.46], I 2 = 0; P = 0.85).
Forest plot of birth weight for modified dietary interventions compared with control diets in women with GDM. Reference citations for studies can be found in Table 1 . CHO, carbohydrate; IV, inverse variance.
Subgroup Meta-analysis by Types of Dietary Interventions
Pooled analysis of low-GI diets showed a larger decrease in fasting ( 26 , 29 , 30 ), postprandial, and post-breakfast glucose compared with control diets ( 26 , 30 ) ( Table 2 ). However, the pooled analysis of the DASH diet showed significant favorable modifications in several outcomes, including change in fasting ( 22 , 36 ) and postprandial glucose ( 22 ), HOMA-IR ( 35 ), HbA 1c ( 22 ), medication need ( 22 , 23 , 36 ), infant birth weight ( 23 , 36 ), and macrosomia ( 23 , 36 ) ( Tables 2 and 3 ). Last, pooled analysis of the soy protein–enriched diet demonstrated a significant decrease in medication use and birth weight ( 14 , 27 ) ( Tables 2 and 3 ). One soy–protein intervention ( n = 68 participants) described significantly lower HOMA-IR ( 27 ) ( Table 2 ).
Sensitivity analysis of primary maternal glycemic and infant birth weight outcomes
Behavioral (one study) and ethnic-specific modified dietary interventions (one study) were included. The behavioral change dietary intervention reported significant differences in change in postprandial glucose and in HbA 1c ( Table 2 ) ( 24 ). The ethnic diet study demonstrated a significantly larger decrease in fasting and postprandial glucose ( Table 2 ) ( 34 ). Fat-modification, low-carbohydrate, and energy-restriction diets were not associated with a significant difference in our primary outcomes in the stratified analysis.
Secondary Outcomes
Weight gain from inclusion was lower for low-carbohydrate diets and cesarean birth for DASH diets ( Supplementary Table 2 ). Specific diet interventions did not show significant between-group differences in maternal gestational weight gain throughout pregnancy, preeclampsia/eclampsia, neonatal hypoglycemia as defined by the authors, preterm birth, neonatal intensive care unit admission, or small-for-gestational-age newborns ( Supplementary Tables 2 and 3 ).
Sensitivity Analysis of Primary Outcomes
Sensitivity analysis was performed to explore reasons for heterogeneity and to assess outcomes when studies with methodological concerns were removed. We were unable to include four studies ( 22 , 23 , 34 , 36 ), including all the DASH diet studies, where clarification of certain aspects of the results could not be obtained, even after a direct approach to the authors. The authors of the ethnic diet study responded to queries but did not provide the required information regarding gestational age at randomization ( 34 ). After these studies are removed, the changes in postprandial glucose (mean −5.90 mg/dL [95% CI −7.93, −3.88], I 2 = 0; P = 0.0001), post-breakfast glucose levels (−4.76 mg/dL [−9.13, −0.38], I 2 = 34; P = 0.03), and birth weight (−74.88 g [−144.86, −4.90], I 2 = 1; P = 0.04) remained significant when all diets were combined ( Table 3 ). Furthermore, the heterogeneity in most primary outcomes decreased after removal of these four studies.
When dietary subgroups were assessed, low-GI diets had significant differences in changes in fasting (mean −5.33 mg/dL [95% CI −6.91, −3.76]) ( 26 , 29 , 30 ), postprandial (−7.08 mg/dL [−12.07, −2.08]) ( 26 , 30 ), and post-breakfast (−8.6 mg/dL [−14.11, −3.09]) glucose ( 26 , 30 ). The soy protein–enriched diet had differences in change of HOMA-IR (mean −2.00 [95% CI −3.17, −0.83]) ( 27 ), required less medication use (RR 0.44 [95% CI 0.21, 0.91]), and had a lower birth weight (mean −184.67 g [95% CI −319.35, −49.98]) ( 14 , 27 ). The behavior modification diet had significant differences in change in postprandial glucose (mean −6.90 mg/dL [95% CI −9.85, −3.95]) and in HbA 1c (−0.19% [−0.26, −0.12]) ( 24 ) ( Table 3 ).
Assessment of Bias and Quality of the Evidence
None of the included studies were assessed as having a low risk of bias in all seven items of the Cochrane Collaboration tool ( Supplementary Fig. 2 ). Most studies were high risk for blinding of participants and personnel and for other sources of bias ( Supplementary Fig. 3 ). Studies scored high risk for other sources of bias for concerns such as baseline differences and industry funding. Most studies had an unclear risk of bias for selective outcome reporting and very few had registered protocols ( Supplementary Fig. 3 ).
GRADE assessment for the outcomes of interest reveals overall low to very low quality of evidence ( Supplementary Table 4 ). Considerations to downgrade quality of evidence involved the entire spectrum, including limitations in the study design, inconsistency in study results, and indirectness and imprecision in effect estimates.
Evaluation for Small Study Effect
Funnel plots of means and RRs of the primary outcomes for the main analysis are shown in Supplementary Figs. 4 and 5 and for the sensitivity analysis in Supplementary Figs. 6 and 7 . Overall, funnel plot asymmetry improves with the sensitivity analysis compared with the main analysis for neonatal birth weight outcomes.
In this meta-analysis, we pooled results from 18 studies including 1,151 women with a variety of modified dietary interventions. Remarkably, this is the first meta-analysis with a comprehensive analysis on maternal glucose parameters. Despite the heterogeneity between studies, we found a moderate effect of dietary interventions on maternal glycemic outcomes, including changes in fasting, post-breakfast, and postprandial glucose levels and need for medication treatment, and on neonatal birth weight. After removal of four studies with methodological concerns, we saw an attenuation of the treatment effect. Nonetheless, the change in post-breakfast and postprandial glucose levels and lowering of infant birth weight remained significant. Given the inconsistencies between the main and sensitivity analyses, we consider that conclusions should be drawn from the latter. These data suggest that dietary interventions modified above and beyond usual dietary advice for GDM have the potential to offer better maternal glycemic control and infant birth weight outcomes. However, the quality of evidence was judged as low to very low due to the limitations in the design of included studies, the inconsistency between their results, and the imprecision in their effect estimates.
Previous systematic reviews have focused on the easier-to-quantify outcomes, such as the decision to start additional pharmacotherapy and glucose-related variables at follow-up, but did not address change from baseline ( 15 – 17 ). The most recently published Cochrane systematic review by Han et al. ( 17 ) did not find any clear evidence of benefit other than a possible reduction in cesarean section associated with DASH diet. The very high-carbohydrate intake (∼400 g/day) and 12 servings of fruit and vegetables in the DASH diet ( 22 , 23 , 36 ) limit its clinical applicability and generalizability to women from lower socioeconomic, inner city backgrounds in Western countries. The Cochrane review shared one of our primary outcomes, large for gestational age ( 17 ). Neither meta-analysis detected a significant difference in risk of large for gestational age because the trials with a larger effect on birth weight (the three DASH studies) did not report on large for gestational age.
Our findings regarding pooled analysis of low-GI dietary interventions are broadly consistent with those of Viana et al. ( 16 ) and Wei et al. ( 15 ). Viana et al. ( 16 ) noted decreased birth weight and insulin use based on four studies of low-GI diet among 257 women (mean difference −161.9 g [95% CI −246.4, −77.4] and RR 0.767 [95% CI 0.597, 0.986], respectively). Wei et al. ( 15 ) also reported decreased risk of macrosomia with a low-GI diet in five studies of 302 women (RR 0.27 [95% CI 0.10, 0.71]). In our analyses of four studies in a comparable number of participants ( n = 276), we found the same direction of these effect estimates, without significant between-group differences. This is most likely due to the different studies included. For example, we were unable to obtain effect estimates stratified by type of diabetes in the study by Perichart-Perera et al. (which included women with type 2 diabetes) and therefore did not include this study ( 37 ). An important difference between our analyses and that of Wei et al. ( 15 ) is that they included DASH diet as a low-GI dietary subtype. We also included a recent study by Ma et al. ( 30 ) not included by the previous reviews.
Our sensitivity analyses highlighted concerns regarding some studies included in previous reviews. Notably, after removal of the studies with the most substantial methodological concerns in the sensitivity analysis, differences in the change in fasting plasma glucose were no longer significant. Although differences in the change in postprandial glucose and birth weight persisted, they were attenuated.
This review highlights limitations of the current literature examining dietary interventions in GDM. Most studies are too small to demonstrate significant differences in our primary outcomes. Seven studies had fewer than 50 participants and only two had more than 100 participants ( n = 125 and 150). The short duration of many dietary interventions and the late gestational age at which they were started ( 38 ) may also have limited their impact on glycemic and birth weight outcomes. Furthermore, we cannot conclude if the improvements in maternal glycemia and infant birth weight are due to reduced energy intake, improved nutrient quality, or specific changes in types of carbohydrate and/or protein.
We have not addressed the indirect modifications of nutrients. For example, reducing intake of dietary carbohydrates to decrease postprandial glucose may be compensated by a higher consumption of fat potentially leading to adverse effects on maternal insulin resistance and fetal body composition. Beneficial or adverse effects of other nutrients such as n-3 long-chain polyunsaturated fatty acid, vitamin D, iron, and selenium cannot be ruled out.
Our study has important strengths and weakness. To our knowledge, ours is the first systematic review of dietary interventions in GDM comprehensively examining the impact of diet on maternal glycemic outcomes assessing the change in fasting and postprandial glucose, HbA 1c , and HOMA-IR from baseline. This is especially important given that groups were not well balanced at baseline. Our review also benefits from the rigorous methodology used as well as the scientific, nutritional, and clinical expertise from an international interdisciplinary panel. However, it also has limitations. Baseline differences between groups in postprandial glucose may have influenced glucose-related outcomes. Furthermore, three of the included trials were pilot studies and therefore not designed to find between-group differences ( 12 , 26 , 34 ). The low number of studies reporting on adherence clearly illustrates that the quality of the evidence is far from ideal. The heterogeneity of the dietary interventions even within a specific type (varied macronutrient ratios, unknown micronutrient intake, and short length of some dietary interventions) and baseline characteristics of women included (such as prepregnancy BMI or ethnicity) may have also affected our pooled results. It should also be noted that the relatively small numbers of study participants limit between-diet comparisons. Last, we were unable to resolve queries regarding potential concerns for sources of bias because of lack of author response to our queries. We have addressed this by excluding these studies in the sensitivity analysis.
Modified dietary interventions favorably influenced outcomes related to maternal glycemia and birth weight. This indicates that there is room for improvement in usual dietary advice for women with GDM. Although the quality of the evidence in the scientific literature is low, our review highlights the key role of nutrition in the management of GDM and the potential for improvement if better recommendations based on adequately powered high-quality studies were developed. Given the prevalence of GDM, new studies designed to evaluate potential dietary interventions for these women should be based in larger study groups with appropriate statistical power. As most women with GDM are entering pregnancy with a high BMI, evidence-based recommendations regarding both dietary components and total energy intake are particularly important for overweight and obese women. The evaluation of nutrient quality, in addition to their quantity, as well as dietary patterns such as Mediterranean diet ( 39 ) would also be relevant. In particular, there is an urgent need for well-designed dietary intervention studies in the low- and middle-income countries where the global health consequences of GDM are greatest.
H.R.M. and R.C. contributed equally to this work.
See accompanying commentary, p. 1343 .
See accompanying articles, pp. 1337 , 1339 , 1362 , 1370 , 1378 , 1385 , 1391 , and e111 .
Funding. H.R.M. was funded by the U.K. National Institute for Health Research (CDF 2013-06-035). This work was conducted by an expert group of the European branch of the International Life Sciences Institute (ISLI Europe). This publication was coordinated by the ISLI Europe Early Nutrition and Long-Term Health and the Obesity and Diabetes task forces. Industry members of these task forces are listed on the ILSI Europe website at www.ilsi.eu . Experts are not paid for the time spent on this work; however, the nonindustry members within the expert group were offered support for travel and accommodation costs from the Early Nutrition and Long-Term Health and the Obesity and Diabetes task forces to attend meetings to discuss the manuscript and a small compensatory sum (honoraria) with the option to decline. The expert group carried out the work, i.e. collecting and analyzing data and information and writing the scientific paper, separate to other activities of the task forces. The research reported is the result of a scientific evaluation in line with ILSI Europe’s framework to provide a precompetitive setting for public-private partnership. ILSI Europe facilitated scientific meetings and coordinated the overall project management and administrative tasks relating to the completion of this work.
The opinions expressed herein and the conclusions of this publication are those of the authors and do not necessarily represent the views of ILSI Europe nor those of its member companies. For further information about ILSI Europe, please email [email protected] or call +32 2 771 00 14.
Duality of Interest. E.M.v.d.B. works part-time for Nutricia Research. E.C.-G. works full-time for Nestec. R.R. works full-time for Abbott Nutrition. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M.Y. contributed to data extraction, statistical analyses, and writing the first draft manuscript. J.E.K. contributed to data extraction and writing the first draft summary tables. M.B. and A.G.-P. contributed to literature extraction, statistics, and manuscript revision. E.H. contributed to data extraction and GRADE assessments. I.S. and I.G. contributed to statistics and manuscript revision. E.M.v.d.B., E.C.-G., S.H., and S.F.O. contributed to concept and design, data extraction, and manuscript review. M.H. contributed to concept and design and draft manuscript evaluation. K.L. contributed to concept and design, data extraction, and critical review for intellectual content. L.P. contributed to concept and design and manuscript review. R.R., P.R., and H.R.M. contributed to concept and design, data extraction, and revising the draft manuscript. L.v.L. contributed to data extraction and draft summary tables. B.S. contributed to data extraction and critical review for intellectual content. R.C. contributed to literature extraction, statistical analyses, and revising the draft manuscript. R.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this work were presented at the Diabetes UK National Diabetes in Pregnancy Conference, Leeds, U.K., 14 November 2017, and the XXIX National Congress of the Spanish Society of Diabetes, Oviedo, Spain, 18–20 April 2018.
Supplementary data
Email alerts.
- Gestational Diabetes Mellitus: Does One Size Fit All? A Challenge to Uniform Worldwide Diagnostic Thresholds
- A Tailored Letter Based on Electronic Health Record Data Improves Gestational Weight Gain Among Women With Gestational Diabetes Mellitus: The Gestational Diabetes’ Effects on Moms (GEM) Cluster-Randomized Controlled Trial
- Day-and-Night Closed-Loop Insulin Delivery in a Broad Population of Pregnant Women With Type 1 Diabetes: A Randomized Controlled Crossover Trial
- Gestational Diabetes Mellitus and Renal Function: A Prospective Study With 9- to 16-Year Follow-up After Pregnancy
- Neonatal Hypoglycemia Following Diet-Controlled and Insulin-Treated Gestational Diabetes Mellitus
- The Human Placenta in Diabetes and Obesity: Friend or Foe? The 2017 Norbert Freinkel Award Lecture
- Nutrition Therapy in Gestational Diabetes Mellitus: Time to Move Forward
- Gestational Diabetes Mellitus: Is It Time to Reconsider the Diagnostic Criteria?
- The Sensitivity and Specificity of the Glucose Challenge Test in a Universal Two-Step Screening Strategy for Gestational Diabetes Mellitus Using the 2013 World Health Organization Criteria
- In This Issue of Diabetes Care
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Search Menu
- Advance Articles
- Thematic Issues
- ENDO Meeting Abstracts
- Clinical Practice Guidelines
- Endocrine Reviews
- Endocrinology
- Journal of the Endocrine Society
- The Journal of Clinical Endocrinology & Metabolism
- JCEM Case Reports
- Molecular Endocrinology
- Endocrine Society Journals
- Author Guidelines
- Submission Site
- Open Access
- Why Publish with the Endocrine Society?
- Advertising & Corporate Services
- Reprints, ePrints, Supplements
- About Endocrine Reviews
- Editorial Board
- Author Resources
- Reviewer Resources
- Rights & Permissions
- Other Society Publications
- Member Access
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Current gdm diagnostic criteria, contemporary clinical evidence following the revised iadpsg gdm diagnostic criteria, current classification of hyperglycemia in pregnancy and gdm, the impact of preanalytical glucose processing standards on the diagnosis of gdm, incidence and prevalence of gdm, risk factors for gdm, pathophysiology of gdm, genetics of gdm, maturity-onset diabetes of the young, consequences of gdm, neonatal complications, short-term risk, long-term risk in the offspring, maternal complications, management of gdm, lifestyle intervention, gestational weight gain, maternal glucose targets, insulin therapy, oral pharmacotherapy, obstetric management, longer term management of women following gdm, treatment of gdm and long-term offspring outcomes, precision medicine in gdm: physiological heterogeneity, subtype classification, risk prediction, and biomarker utility, the covid-19 pandemic and gdm, financial support, disclosure summary, a clinical update on gestational diabetes mellitus.
- Article contents
- Figures & tables
- Supplementary Data
Arianne Sweeting, Jencia Wong, Helen R Murphy, Glynis P Ross, A Clinical Update on Gestational Diabetes Mellitus, Endocrine Reviews , Volume 43, Issue 5, October 2022, Pages 763–793, https://doi.org/10.1210/endrev/bnac003
- Permissions Icon Permissions
Gestational diabetes mellitus (GDM) traditionally refers to abnormal glucose tolerance with onset or first recognition during pregnancy. GDM has long been associated with obstetric and neonatal complications primarily relating to higher infant birthweight and is increasingly recognized as a risk factor for future maternal and offspring cardiometabolic disease. The prevalence of GDM continues to rise internationally due to epidemiological factors including the increase in background rates of obesity in women of reproductive age and rising maternal age and the implementation of the revised International Association of the Diabetes and Pregnancy Study Groups’ criteria and diagnostic procedures for GDM. The current lack of international consensus for the diagnosis of GDM reflects its complex historical evolution and pragmatic antenatal resource considerations given GDM is now 1 of the most common complications of pregnancy. Regardless, the contemporary clinical approach to GDM should be informed not only by its short-term complications but also by its longer term prognosis. Recent data demonstrate the effect of early in utero exposure to maternal hyperglycemia, with evidence for fetal overgrowth present prior to the traditional diagnosis of GDM from 24 weeks’ gestation, as well as the durable adverse impact of maternal hyperglycemia on child and adolescent metabolism. The major contribution of GDM to the global epidemic of intergenerational cardiometabolic disease highlights the importance of identifying GDM as an early risk factor for type 2 diabetes and cardiovascular disease, broadening the prevailing clinical approach to address longer term maternal and offspring complications following a diagnosis of GDM.

Gestational diabetes mellitus (GDM) is 1 of the most common medical complications of pregnancy and is increasing in prevalence globally.
GDM is associated with obstetric and neonatal complications primarily due to increased birthweight and is a major risk factor for future type 2 diabetes, obesity, and cardiovascular disease in mother and child.
Detecting GDM is important because perinatal complications and stillbirth risk are greatly reduced by treatment.
A precision medicine approach to GDM which recognizes severity and onset of maternal hyperglycemia as well as genetic and physiologic subtypes of GDM may address the current diagnostic controversy via accurate risk stratification and individualized treatment strategies, leading to improved clinical care models and outcomes.
The traditional focus on normalization of obstetric and neonatal outcomes achieved via short-term antenatal maternal glucose management should now shift to early postnatal prevention strategies to decrease the progression from GDM to type 2 diabetes and address longer term maternal and offspring metabolic risk given the global epidemic of diabetes, obesity, and cardiovascular disease.
Diabetes in pregnancy was first described in 1824 by Bennewitz in Germany ( 1 ), with subsequent case series in the United Kingdom and United States reporting high perinatal mortality rates in women with diabetes in pregnancy ( 2-4 ). In 1909, Williams reported arguably the first diagnostic criteria for diabetes in pregnancy in the United States, proposing physiological and pathophysiological thresholds for “transient glycosuria in pregnancy” ( 5 ).
In 1964, O’Sullivan and Mahan defined specific diagnostic criteria for gestational diabetes mellitus (GDM) in the United States derived from the 100-g 3-hour oral glucose tolerance test (OGTT) undertaken in the second and third trimester of pregnancy in 752 women ( 6 ). GDM was defined as ≥2 venous whole blood glucose values greater than 2 SD above the mean glucose values for pregnancy in their initial cohort. These glucose thresholds were primarily chosen because the resulting GDM prevalence of 2% corresponded to the background population prevalence of diabetes, while the requirement of ≥2 elevated glucose values sought to minimize the risk of preanalytical error ( 7 ). These thresholds were validated by their identification of subsequent diabetes up to 8 years postpartum in an additional cohort of 1013 women. Increased perinatal mortality was also observed in women with ≥2 glucose values exceeding the proposed diagnostic criteria ( 6 ). In 1965, the World Health Organization (WHO) concurrently recommended that GDM be diagnosed by either a 50- or 100-g OGTT using the 2-hour postload glucose value, but the threshold used was the same as for diagnosing diabetes in the nonpregnant population ( 8 ). The WHO continued to diagnose GDM based on glucose thresholds for diabetes in the nonpregnant population ( 9 , 10 ) until its endorsement of the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria in 2013 ( 11 ).
Since 1973, the screening approach to GDM frequently adopted a 2-step procedure with the 50-g 1-hour glucose challenge test (GCT) followed by the 100-g 3-hour OGTT if the GCT was positive. This was based on data from O’Sullivan et al, which showed that a 2-step diagnostic approach to GDM using the GCT as the initial screening test and a glucose threshold of 7.9 mmol/L (143 mg/dL) was 79% sensitive and 87% specific for diagnosing GDM on the 100-g 3-h OGTT in a cohort of 752 women ( 12 ). The rationale for this approach was the efficient identification of women most at risk of GDM.
In 1979, the US National Diabetes Data Group (NDDG) published conversions of the original O’Sullivan and Mahan 100-g 3-hour OGTT diagnostic criteria for GDM, reflecting the transition from venous whole blood glucose to plasma blood glucose analysis ( 13 ). These revised criteria were subsequently adopted by the American Diabetes Association (ADA) and internationally ( 9 , 14 , 15 ). In 1982, Carpenter and Coustan recommended lowering of the NDDG diagnostic criteria, reflecting newer preanalytical enzymatic methods that were more specific for plasma glucose ( 7 , 16 ). They also advised lowering the GCT glucose threshold to 7.5 mmol/L (135 mg/dL) based on their study of 381 women who underwent the 100-g 3-h OGTT after screening positive on the GCT, whereby a GCT glucose threshold ≤ 7.5 mmol/L (135 mg/dL) strongly correlated with a normal OGTT ( 17 ). However, in the absence of clear evidence supporting a specific glucose threshold for the GCT, the ADA and the American College of Obstetricians and Gynecologists (ACOG) continued to recommend a screen positive GCT glucose threshold from 7.2 to 7.8 mmol/L (130-140 mg/dL) for GDM ( 18 , 19 ).
The ADA did however recommend the modified Carpenter and Coustan diagnostic glucose thresholds for GDM from 2000 ( 20 ), supported by the findings of the Toronto Tri-Hospital Gestational Diabetes Project ( 21 , 22 ). These data demonstrated a positive correlation between increasing maternal hyperglycemia even below the NDDG diagnostic criteria for GDM and risk of obstetric and neonatal complications including preeclampsia, cesarean section, and macrosomia (neonatal birthweight > 4000 g) ( 21 , 22 ). In addition, several large cohort studies showed that women diagnosed (but not treated) with GDM based on the Carpenter and Coustan criteria were at increased risk of perinatal complications including hypertensive disorders of pregnancy, increased birthweight, macrosomia, neonatal hypoglycemia, hyperbilirubinemia, and shoulder dystocia, compared to women diagnosed and treated as GDM by NDDG diagnostic criteria ( 16 , 23-25 ). From 2003 the ADA additionally endorsed the 1-step 75-g 2-hour OGTT for the diagnosis of GDM derived from the modified Carpenter and Coustan fasting, 1- and 2-hour glucose thresholds for the 100-g 3-hour OGTT, particularly for women at high-risk ( 26 ). This approach was deemed more cost-effective, albeit less validated, than the 100-g 3-hour OGTT. The use of the modified Carpenter and Coustan thresholds was associated with an almost 50% increase in prevalence of GDM ( 16 , 23 ).
The evolution of diagnostic criteria for GDM illustrates the historic lack of consensus for the diagnosis of GDM, with the presence or absence of disease varying dependent on expert consensus. The underlying rationale for the diagnosis of GDM also shifted over time toward identifying perinatal risk rather than future maternal diabetes risk.
The seminal Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study sought to provide an evidence base to guide risk in GDM, and its results were published in 2008 ( 27 ). This large, international, prospective, observational study evaluated the relationship between glucose levels on the 75-g 2-hour OGTT performed at 24 to 32 weeks’ gestation (mean 27.8 weeks’ gestation) in over 25 000 pregnant women with the following primary perinatal outcomes: birthweight > 90th percentile for gestational age, primary cesarean section delivery, neonatal hypoglycemia, and cord blood serum C-peptide > 90th centile. Secondary outcomes were preeclampsia, preterm delivery (defined as delivery before 37 weeks’ gestation), shoulder dystocia or birth injury, hyperbilirubinemia, and neonatal intensive care admission. The results showed a continuous positive linear relationship between maternal fasting; 1- and 2-hour plasma glucose levels obtained on the OGTT, below those that were diagnostic of diabetes outside pregnancy; and risk of primary outcomes ( 27 ). Notably, there were no specific glucose thresholds at which obstetric and neonatal complications significantly increased.
Based on these findings and supported by trials [the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) and the Maternal-Fetal Medicine Units Network (MFMU) trial] showing benefit of treatment of more severe and “mild” degrees of maternal hyperglycemia, respectively ( 28 , 29 ), the IADPSG revised its diagnostic criteria for GDM. Despite the lack of a clear diagnostic glucose threshold in HAPO, the consensus of the IADPSG was to define diagnostic thresholds for the fasting, 1- and 2-hour glucose values for the 75-g 2-hour OGTT based on the average glucose values at which the odds of the primary outcomes were 1.75 times the odds of these outcomes occurring at the mean glucose levels for the HAPO cohort ( 30 ). The IADPSG consensus was also that only 1 elevated glucose level for the OGTT was required for GDM diagnosis, as each glucose threshold represented broadly comparable level of risk. Thus, the main purpose of the diagnostic criteria for GDM post-HAPO was to define the level of risk associated with increased perinatal complications.
Post-HAPO, there exist several different screening and testing approaches for the diagnosis of GDM internationally. The IADPSG and WHO recommend universal testing of all pregnant women between 24 to 28 weeks’ gestation with the 75-g 2-hour OGTT ( 11 , 30 ). These revised recommendations were largely endorsed by several organizations including the ADA ( 18 ), Endocrine Society ( 31 ), International Federation of Gynecology and Obstetrics ( 32 ), Australasian Diabetes in Pregnancy Association ( 33 ), Japan Diabetes Society ( 34 ), Ministry of Health of China ( 35 ), and the European Board of Gynecology and Obstetrics ( 36 ).
The National Institutes of Health did not endorse the IADPSG recommendations, citing the expected increase in prevalence of GDM, cost, and intervention in the context of a lack of evidence for an associated improvement in perinatal outcomes ( 37 ). The National Institutes of Health and ACOG continue to recommend a 2-step testing approach, with the initial screening GCT for all women and those who screen positive proceeding to the diagnostic 100-g 3-hour OGTT ( 19 , 37 ). This approach is also endorsed by ADA ( 18 ). However, the ACOG’s 2018 guidelines now acknowledge that individual practices and institutions may instead choose to use the IADPSG’s 1-step testing approach and diagnostic criteria if appropriate for their population ( 19 ). The UK National Institute for Health and Care Excellence (NICE) guidelines advise a selective screening approach, whereby women with risk factors for GDM are recommended to undergo a diagnostic 75-g 2-hour OGTT at 26 to 28 weeks’ gestation, with diagnostic (fasting or 2-hour) glucose thresholds higher than the IADPSG diagnostic criteria for GDM ( 38 ). Several other European bodies also currently recommend selective risk factor-based screening, with only women fulfilling specific high-risk criteria proceeding to a diagnostic OGTT, even if the IADPSG diagnostic criteria for GDM are applied ( 39 , 40 ). The revised IADPSG diagnostic criteria and testing approach to GDM in comparison to other international organizations are summarized in Table 1 .
Current international testing approach to gestational diabetes mellitus
Abbreviations: ACOG, American College of Obstetricians and Gynecologists; ADA, American Diabetes Association; ADIPS, Australasian Diabetes in Pregnancy Association; CDA, Canadian Diabetes Association; CNGOF, Organisme professionnel des médecins exerçant la gynécologie et l'obstétrique en France; DDG, German Diabetes Association; DGGG, European Board of Gynecology and Obstetrics; DIPSI, Diabetes in Pregnancy Study Group of India; FIGO, International Federation of Gynecology and Obstetrics; GCT, glucose challenge test; IADPSG, International Association of the Diabetes and Pregnancy Study Groups; JDS, Japan Diabetes Society; NDDG, US National Diabetes Data Group; NICE, National Institute for Health and Care Excellence; OGTT, oral glucose tolerance test; WHO, World Health Organization.
a The ADA states that the choice of a specific positive GCT screening threshold is based upon the trade-off between sensitivity and specificity ( 41 ). ACOG advises that in the absence of clear evidence that supports a specific GCT threshold value between 7.2 and 7.8 mmol/L, obstetricians and obstetric care providers may select a single consistent GCT threshold for their practice based on factors such as community prevalence rates of GDM ( 19 ).
b Plasma or serum glucose.
c ACOG 2018 Clinical Practice Bulletin on GDM continues to recommend 2-step testing for GDM but states that individual practices and institutions may choose to use the IADPSG’s 1-step testing approach and diagnostic criteria if appropriate for their population ( 19 ).
d ACOG 2018 Clinical Practice Bulletin on GDM acknowledges that women who have even 1 abnormal value on the 100-g 3-hour OGTT have a significantly increased risk of adverse perinatal outcomes compared to women without GDM but state that further research is needed to clarify the risk of adverse outcomes and benefits of treatment in these women ( 19 ).
e A glucose level ≥ 11.1 mmol/L following the initial screening GCT is classified as GDM, and there is no need for a subsequent 2-hour 75-g OGTT.
f BMI > 30 kg/m 2 , previous macrosomia (≥4500 g), previous GDM, family history of diabetes, and family origin with a high prevalence of diabetes (South Asian, Black Caribbean, Middle Eastern) ( 38 ).
g Maternal age ≥ 35 years, body mass index ≥ 25 kg/m 2 , family history of diabetes, previous GDM, previous macrosomia ( 39 ).
h If first trimester fasting glucose normal (ie, < 5.1 mmol/L).
i Adapted from the WHO 1999 diagnostic criteria for GDM ( 45 ), using a nonfasting 75-g 2-hour OGTT ( 44 ).
It is important to consider the increase in GDM prevalence associated with the IADPSG diagnostic criteria in the context of the rising background rates of impaired glucose tolerance, type 2 diabetes, and obesity among young adults and women of reproductive age ( 46 , 47 ). For example, almost 18% of HAPO study participants would have met the IADPSG diagnostic thresholds for GDM. By comparison, the rate of prediabetes in US adults aged between 20 and 44 years is >29% ( 48 , 49 ).
Studies in Indian, Israeli, and US cohorts have suggested that the IADPSG testing approach and intervention for GDM is cost-effective based on a combination of delaying future type 2 diabetes and preventing perinatal complications ( 50-53 ). For example, a US study found that the IADPSG diagnostic criteria would be cost-effective if associated intervention decreased the absolute incidence of preeclampsia by >0.55% and cesarean delivery by >2.7% ( 53 ). In contrast, UK health economic data show that routinely identifying GDM is not cost-effective based on perinatal outcomes ( 54 ) and that the universal WHO (IADPSG) testing approach is less cost-effective than the NICE selective screening approach ( 55 ).
The lack of randomized controlled trials (RCTs) evaluating outcomes in women diagnosed with GDM based on the IADPSG criteria and the clinical relevance of treating the resulting milder degrees of hyperglycemia remain controversial ( 56 ). Several retrospective studies have shown that women diagnosed with GDM by the IADPSG criteria but who were previously classified as having normal glucose tolerance were still at increased risk for obstetric and neonatal complications, including gestational hypertension, preeclampsia, cesarean delivery, macrosomia, large-for-gestational-age (LGA), shoulder dystocia, and neonatal intensive care admission, compared to women with normal glucose tolerance ( 57-59 ). For example, a 2015 retrospective study in Taiwan comparing pregnancy outcomes in women diagnosed and treated for GDM using the 2-step (GCT followed by the 100-g 3-hour OGTT) approach compared to the IADPSG 1-step approach found that the latter was associated with a reduction in gestational weight gain (GWG), birthweight, macrosomia, and LGA ( 60 ). Another retrospective study in the United Kingdom reported that women who were diagnosed with GDM based on modified IADPSG diagnostic glucose thresholds but who screened negative for GDM on 2015 NICE diagnostic criteria had a higher risk of LGA, cesarean delivery, and polyhydramnios ( 61 ). Other retrospective studies have also demonstrated higher birthweight, birthweight z-score, ponderal index, and increased rates of LGA and cesarean delivery in untreated women diagnosed with GDM based on the IADPSG criteria, compared to women with normal glucose tolerance ( 62 , 63 ).
The recent randomized ScreenR2GDM trial compared 1-step screening (75-g 2-hour OGTT) with 2-step screening (2 GCT thresholds ≥7.2 mmol/L and ≥7.8 mmol/L used, followed by the 100-g 3-hour OGTT) in 23 792 pregnant women in the United States ( 64 ). Despite doubling the diagnosis of GDM with the 1-step approach (16.5% vs 8.5%), there were no differences in pregnancy complications including LGA [relative risk (RR) 0.95; 97.5% CI 0.87-1.05], perinatal composite outcome (RR 1.04; 97.5% CI 0.88-1.23), gestational hypertension or preeclampsia (RR 1.00; 97.5% CI 0.93-1.08), and primary cesarean section (RR 0.98; 97.5% CI 0.93-1.02) between the different screening approaches. These findings have not resolved the diagnostic debate for GDM, with some arguing that the 1-step approach therefore demonstrates insufficient perinatal benefit for the associated increased healthcare costs ( 65 ), while others have identified potential limitations in study methodology ( 7 , 47 , 65 , 66 ). Despite randomization to either testing strategy, the pragmatic trial design allowed clinicians to select a preferred strategy. Consequently, one third of women randomized to the 1-step approach did not adhere to the assigned screening and were tested via the 2-step approach, compared to only 8% of women randomized to the 2-step approach. Although the study attempted to adjust for this difference using inverse probability weighting, residual provider bias cannot be excluded ( 47 ). Given this was a population level analysis of GDM screening, GDM (treatment) status differed only for the 8% of women not diagnosed with GDM based on the 2-step approach who may have otherwise been diagnosed with GDM based on the 1-step approach. Whether these women had potentially worse outcomes that may have been mitigated by treatment cannot be determined by this study. However, given the rates of pharmacotherapy were similar between the 1- and 2-step cohorts at 43% and 46%, respectively ( 64 ), this strategy detected women with essentially an equivalent risk of hyperglycemia warranting pharmacotherapy ( 47 ). This observation is consistent with other studies in UK cohorts comparing the IADPSG testing approach to the less sensitive NICE and Canadian criteria, whereby women demonstrated insulin resistance and required pharmacotherapy for control of hyperglycemia even at the most sensitive thresholds of the IADPSG diagnostic criteria ( 67 ).
More generally, the GCT fails to detect approximately 20% to 25% of women with GDM, particularly those diagnosed with GDM based on an elevated fasting glucose ( 68 ). The frequency of GDM diagnosed by the OGTT fasting glucose threshold in the HAPO study ranged from 24% to 26% in Thailand and Hong Kong to >70% in the United States ( 69 ). This highlights the variability and thus limitations of post-glucose load screening based on ethnicity. Moreover, a recent systematic review and meta-analysis of 25 studies (n = 4466 women) showed that even 1 abnormal value on the diagnostic 3-hour 100-g OGTT is associated with an increased risk of perinatal complications compared to women with a normal GCT, and this risk was similar to that of women actually diagnosed with GDM ( 70 ).
The degree of benefit of treating women with GDM defined by the IADPSG diagnostic criteria is yet to be determined. The potential benefit is inferred from the treatment of maternal hyperglycemia described in the ACHOIS and MFMU intervention trials ( 28 , 29 ), whereby maternal glucose levels overlapped with the thresholds recommended by the IADPSG. It is worth noting that there are differences in these 2 trials with regards to the diagnostic criteria used to define GDM and cohort characteristics (eg, women were excluded from the MFMU trial if they had an abnormal glucose screening test prior to 24 weeks’ gestation or previous GDM), and thus the generalizability of these findings in women diagnosed with GDM based on the IADPSG criteria remains contentious.
The WHO first defined GDM in 1965 as “hyperglycemia of diabetic levels occurring during pregnancy” ( 8 ). Thus, historically, the term “GDM” encompassed the entire spectrum of maternal hyperglycemia in pregnancy, from pregestational diabetes to hyperglycemia first detected in pregnancy. In 1979, the NDDG defined GDM as “glucose intolerance that has its onset or recognition during pregnancy” ( 13 ). This was subsequently modified in 1985 at the Second International Workshop-Conference on Gestational Diabetes as “carbohydrate intolerance resulting in hyperglycemia of variable severity with onset or first recognition during pregnancy” and remained the most widely used definition of GDM until recently ( 71 ).
Contemporary nomenclature and diagnostic criteria now more clearly differentiate between women with pregestational diabetes and those with hyperglycemia first detected in pregnancy ( 30 ) ( Fig. 1 ). Pregestational diabetes includes type 1 diabetes, type 2 diabetes, and other types of diabetes such as cystic fibrosis-related diabetes, steroid/medication-induced diabetes, and monogenic diabetes.

Flowchart summarizing the contemporary nomenclature for hyperglycemia in pregnancy.
Hyperglycemia in pregnancy is now subclassified by the IADPSG into 2 separate categories, namely “overt diabetes mellitus during pregnancy” (overt diabetes) and GDM ( 30 ). Similarly, the WHO has a binary definition of hyperglycemia in pregnancy but has replaced the term “overt diabetes” with “diabetes mellitus in pregnancy” (DIP) ( 11 ). The rationale for the IADPSG recommendation for early testing in high-risk women is to diagnose DIP early in pregnancy. This is because DIP, diagnosed based on nonpregnant diabetes glucose thresholds, recognizes the increasing prevalence of undiagnosed preexisting diabetes in women of childbearing age as well as the greater risk associated with this degree of hyperglycemia ( 72-74 ). For example, a recent study in almost 5000 women in France found that DIP was associated with a 3.5-fold greater risk of hypertensive disorders in pregnancy compared to women with normal glucose tolerance, while early‐diagnosed DIP was associated with an increased risk of congenital malformation (7.7% vs 1.0% for women with normal glucose tolerance), suggesting that early hyperglycemia in pregnancy may sometimes be present at conception ( 75 ). However, DIP is not synonymous with preexisting diabetes. In Australian, women with DIP who performed an OGTT at 6 to 8 weeks postpartum, 21% had diabetes, 38% had impaired fasting glucose or impaired glucose tolerance, and 41% returned to normal glucose tolerance ( 76 ).
Regardless of the specific nomenclature used, DIP is distinct from GDM, which is defined by lower glucose thresholds on the OGTT and was historically considered to be a condition of mid to late pregnancy. The ADA has not accepted this nomenclature and defines GDM based on timing of diagnosis: women diagnosed with diabetes in the first trimester are classified as having (preexisting) type 2 diabetes, while GDM is defined as diabetes diagnosed in later pregnancy and not meeting the diagnostic criteria for type 2 diabetes ( 18 ). A summary of the current international nomenclature and diagnostic criteria for hyperglycemia in pregnancy is presented in Table 2 .
Classification and diagnostic criteria for hyperglycemia in pregnancy
75-g 2-hour OGTT: only 1 plasma glucose level needs to be elevated for the diagnosis of GDM. 100 g 3-hour OGTT: at least 2 plasma glucose levels need to be elevated for the diagnosis of GDM.
Abbreviations: ADA, American Diabetes Association; ADIPS, Australasian Diabetes in Pregnancy Association; EBCOG, European Board & College of Obstetrics and Gynaecology; FIGO, International Federation of Gynecology and Obstetrics; GCT, glucose challenge test; HbA1c, hemoglobulin A1c; IADPSG/; International Association of the Diabetes and Pregnancy Study Groups; GDM, gestational diabetes mellitus; OGTT, oral glucose tolerance test; WHO, World Health Organization.
a The IADPSG recommends confirmation by fasting plasma glucose or HbA1c for the diagnosis of overt diabetes during pregnancy ( 30 ).
Most international guidelines now recommend early antenatal testing for women at high risk to identify women with DIP ( 11 , 18 , 30 , 38 , 39 , 42-44 ). This has resulted in increased detection of milder degrees of hyperglycemia below the threshold of DIP, referred to as GDM diagnosed prior to 24 weeks’ gestation or early GDM. Studies in women with GDM have reported that between 27% and 66% of GDM can be detected in early pregnancy depending on the population as well as the screening and diagnostic criteria used ( 77-81 ).
Recent studies evaluating the relationship between maternal glycemia and fetal growth trajectories confirm the early impact of maternal glycemia on excess fetal growth and adiposity prior to the diagnosis of standard GDM from 24 weeks’ gestation. A US multiethnic prospective cohort study of 2458 women enrolled between 8 and 13 weeks’ gestation included 107 (4.4%) women with GDM ( 82 ). GDM was associated with an increase in estimated fetal weight from 20 weeks’ gestation, which became significant at 28 weeks’ gestation. Similarly, Sovio et al showed that excessive fetal growth occurred between 20 to 28 weeks’ gestation, prior to the diagnosis of GDM, especially among women with higher body mass index [BMI (kg/m 2 )] ( 83 ). An Indian study also showed that excess subcutaneous abdominal adiposity was first detected at 20 weeks’ gestation, at least 4 weeks prior to the diagnosis of GDM ( 84 ). Early excess adiposity persisted despite adjustments for maternal age, BMI, GWG, fetal sex, and gestational age and remained higher at 32 weeks’ gestation ( 84 ).
Currently, there is no consensus for the preferred testing approach or diagnostic glycemic thresholds for early GDM. The IADPSG recommends diagnosing early GDM based on a fasting glucose of 5.1 mmol/L to 6.9 mmol/L (92-124 mg/dL) ( 30 ), consistent with the diagnostic fasting glucose threshold for standard GDM. The utility of a single fasting glucose measurement for early GDM diagnosis warrants consideration. First, preanalytical glucose handling variation, particularly in the setting of a single glucose measurement, is a major issue for GDM diagnostic accuracy (discussed in the following text). Second, an Israeli cohort study of 6129 women who underwent a fasting glucose test at a median of 9.5 weeks’ gestation demonstrated a positive association between first trimester fasting glucose up to 5.8 mmol/L (104.5 mg/dL) and increased risk for subsequent diagnosis of GDM, LGA, macrosomia, and cesarean section ( 85 ). Similar to the HAPO study, a clear glucose threshold was lacking, with pregnancy complications evident at fasting glucose levels <5.1 mmol/L (92 mg/dL). Third, maternal fasting glucose decreases in the first trimester, most pronounced between 6 to 10 weeks’ gestation [median decrease in glucose 0.11 mmol/L (1.98 mg/dL)] ( 86 ), while studies have consistently shown that early fasting glucose is poorly predictive of GDM at 24 to 28 weeks’ gestation ( 86-88 ), leading to potential overdiagnosis of GDM. In China, an early fasting glucose between 6.1 mmol/L to 6.9 mmol/L (110-124 mg/dL) best corresponded to later GDM diagnosis ( 88 ), but this requires further validation.
The WHO recommends the same diagnostic OGTT glucose thresholds for GDM in early pregnancy as those derived from HAPO by the IADPSG ( 11 ). However, the prognostic value of these glucose levels in early pregnancy is yet to be established. Others have proposed an hemoglobin A1c (HbA1c) risk threshold ( 89 ), based primarily on evidence that an early HbA1c ≥ 5.9% (41 mmol/mol) detected all cases of DIP and predicted adverse pregnancy outcomes in a New Zealand cohort ( 90 ). However, studies in other cohorts have found that while an elevated HbA1c in early pregnancy is highly specific, it lacks sensitivity for identifying hyperglycemia and certain perinatal complications ( 91 , 92 ), with no clear benefit of treating women with HbA1c 5.7% to 6.4% (39-46 mmol/mol) in early pregnancy ( 93 , 94 ). A summary of the various international criteria for testing of GDM in early pregnancy is presented in Table 3 .
International criteria for testing of gestational diabetes mellitus in early pregnancy
75-g 2-h OGTT: Only 1 abnormal glucose level needs to be elevated for the diagnosis of GDM. 100-g 3-h OGTT: 2 abnormal glucose levels need to be elevated for the diagnosis of GDM.
Abbreviations: ADA, American Diabetes Association; ACOG, American College of Obstetricians and Gynecologists; ADIPS, Australasian Diabetes in Pregnancy Association; CNGOF, Organisme professionnel des médecins exerçant la gynécologie et l'obstétrique en France; DDG, German Diabetes Association; DGGG, European Board of Gynecology and Obstetrics; DIPSI, Diabetes in Pregnancy Study Group of India; EBCOG, European Board & College of Obstetrics and Gynaecology; GCT, glucose challenge test; GDM, gestational diabetes mellitus; IADPSG, International Association of the Diabetes and Pregnancy Study Groups; NICE, National Institute for Health and Care Excellence; OGTT, oral glucose tolerance test; WHO, World Health Organization.
a High-risk criteria not explicitly defined.
b IADPSG does not recommend routinely performing the 75-g 2-h OGTT prior to 24 weeks’ gestation but advises that a fasting glucose ≥ 5.1 mmol/L in early pregnancy be classified as GDM ( 30 ).
c GDM diagnosed at any time in pregnancy based on an abnormal 75-g 2-h OGTT ( 11 ).
d High-risk criteria defined as previous hyperglycemia in pregnancy; previously elevated blood glucose level; maternal age ≥ 40 years; ethnicity: Asian, Indian subcontinent, Aboriginal, Torres Strait Islander, Pacific Islander, Maori, Middle Eastern, non-White African; family history of diabetes (first-degree relative with diabetes or sister with hyperglycemia in pregnancy); prepregnancy body mass index > 30 kg/m 2 ; previous macrosomia (birth weight > 4500 g or > 90th percentile); polycystic ovary syndrome; and medications: corticosteroids, antipsychotics ( 33 ).
e High-risk criteria defined as body mass index ≥ 25 kg/m 2 (≥ 23 kg/m 2 in Asian Americans) plus 1 of the following: physical inactivity; previous GDM; previous macrosomia (≥ 4000 g); previous stillbirth; hypertension; high density lipoprotein cholesterol ≤ 0.90 mmol/L; fasting triglycerides ≥ 2.82 mmol/L; polycystic ovary syndrome; acanthosis nigricans; nonalcoholic steatohepatitis; morbid obesity and other conditions associated with insulin resistance; hemoglobulin A1c ≥ 5.7%; impaired glucose tolerance or impaired fasting glucose; cardiovascular disease; family history of diabetes (first-degree relative); and ethnicity: African American, American Indian, Asian American, Hispanic, Latina, or Pacific Islander ethnicity. Note that the ADA recommends testing for GDM at 24 to 28 weeks’ gestation and have no specific definition for early GDM ( 41 ).
f ACOG states that the best test for early GDM screening is not clear but suggest the testing approach and diagnostic criteria used to diagnose type 2 diabetes in the nonpregnant population and thus have no specific definition for early GDM ( 19 ).
g High-risk criteria defined as previous GDM; overweight/obesity; family history of diabetes (first-degree relative with diabetes); previous macrosomia (>4000g or >90th percentile); polycystic ovary syndrome; ethnicity: Mediterranean, South Asian, black African, North African, Caribbean, Middle Eastern, or Hispanic ( 36 ).
h High-risk criteria defined as age ≥ 45 years; prepregnancy body mass index ≥ 30 kg/m 2 ; physical inactivity; family history of diabetes; high-risk ethnicity (eg. Asians, Latin Americans); previous macrosomia ≥ 4500 g; previous GDM; hypertension; prepregnancy dyslipidemia (high-density lipoprotein cholesterol ≤ 0.90 mmol/L, fasting triglycerides ≥ 2.82 mmol/L); polycystic ovary syndrome; prediabetes in an earlier test; other clinical conditions associated with insulin resistance (eg, acanthosis nigricans); history of coronary artery disease/peripheral artery disease/cerebral vascular disease; medications associated with hyperglycemia (eg. glucocorticoids). Note that the DDG/DGGG recommends that a 75-g 2-h OGTT be the initial early test in high-risk women (defined as women with ≥2 risk factors for GDM) ( 43 ).
i High-risk criteria are defined as previous GDM, previous impaired glucose tolerance, and/or obesity ( 39 ).
j High-risk criteria defined as body mass index> 30 kg/m 2 ; previous macrosomia (≥4500 g); previous GDM; family history of diabetes (first-degree relative with diabetes); minority ethnic family origin with a high prevalence of diabetes. The updated 2015 NICE guidelines state that women with previous GDM should undergo early self-monitoring of blood glucose or a 75-g 2-hour OGTT as soon as possible after booking (first or second trimester), and a repeat 75-g 2-hour OGTT at 24 to 28 weeks’ gestation if the initial OGTT was negative ( 38 ).
k 2-hour postload glucose measured on nonfasting 75-g OGTT ( 44 ).
Despite the lack of diagnostic clarity for early GDM, increasing evidence suggests that women with early GDM represent a high-risk cohort ( 81 ). Early studies also reported worse pregnancy outcomes and increased insulin resistance in early GDM ( 78 , 95-97 ) but were confounded by the inclusion of women with pregestational diabetes. The first large retrospective cohort study excluding women with DIP showed that women diagnosed and treated for early GDM, especially those diagnosed in the first trimester, were more insulin resistant and at significantly greater risk for obstetric and neonatal complications compared to women diagnosed and treated for GDM from 24 weeks’ gestation ( 81 ). Other studies have since confirmed these findings ( 98 , 99 ). Concerningly, an increased risk of perinatal mortality and congenital abnormalities has also been reported in the offspring of women with early GDM ( 75 , 78 , 95 , 96 ), with some data demonstrating that 5% of women with early GDM have abnormal fetal echocardiograms ( 97 ). A recent meta-analysis of 13 cohort studies showed greater perinatal mortality among women with early GDM (RR 3.58; 95% CI 1.91-6.71) compared to women with a later diagnosis of GDM despite treatment ( 100 ).
A recent study assessing the pathophysiological characteristics of women diagnosed with GDM at a median of 16 weeks’ gestation compared to those diagnosed from 24 weeks’ gestation using IADPSG diagnostic criteria reported that women with early GDM had lower insulin sensitivity (defined by insulin-mediated glucose clearance during an OGTT), even after accounting for maternal BMI ( 101 ). Consistent with the pathophysiology of GDM, women with both early and standard GDM demonstrated impairment in pancreatic β-cell function ( 102 ). These data underscore GDM phenotypic differences, specifically based on timing of diagnosis and degree of hyperglycemia ( 103 ).
A key issue is the current lack of high-quality evidence that diagnosing and treating early GDM improves pregnancy outcomes. A recent major RCT in the United States evaluating early testing for GDM in 962 women with obesity included a subgroup analysis of women diagnosed and treated for GDM [early n = 69 (15.0%) vs standard n = 56 (12.1%)] based on the 2-step testing approach ( 104 ). The average gestational age at GDM diagnosis was similar at 24.3 ± 5.2 weeks for the early screen group compared to 27.1 ± 1.7 weeks in the routine screen group. There was no difference in pregnancy outcomes, although the primary composite perinatal outcome (macrosomia, primary cesarean delivery, gestational hypertension, preeclampsia, hyperbilirubinemia, shoulder dystocia, and neonatal hypoglycemia) was nonsignificantly higher in the early-screen group (56.9% vs 50.8%; P = 0.06). Requirement for insulin therapy was almost 4-fold higher, while gestational age at delivery was lower (36.7 vs 38.7 weeks’ gestation; P = 0.001) in women with early GDM. In a post hoc analysis of the Lifestyle in Pregnancy study ( 105 ), no difference in pregnancy outcomes was shown between women randomized to either lifestyle intervention (n = 36) or standard treatment (n = 54) in early pregnancy. Whether different glycemic targets are required reflecting physiological differences in early maternal glucose or whether additional risk factors contributing to a more insulin resistant phenotype such as maternal adiposity might also have a role remain unanswered ( 81 ). The ongoing Treatment of Booking Gestational Diabetes Mellitus study, evaluating the impact of immediate vs delayed care for gestational diabetes diagnosed at booking, will seek to determine whether or not there is benefit from treating early GDM ( 106 ).
Although the contemporary testing approach to GDM remains contentious, it is important to recognize that the diagnosis of GDM is based on the laboratory measurement of maternal glucose rather than a clinical diagnosis. Arguably then, a major issue in the contemporary diagnosis of GDM is optimizing preanalytical processing and measurement of maternal plasma glucose to ensure diagnostic accuracy ( 107 , 108 ). This includes optimization of sample handling and minimization of any analytic error. Unfortunately, stringent preanalytical processing standards are not currently routinely applied. The American Association for Clinical Chemistry (AACC) and ADA recommendations on laboratory testing in diabetes advise collection of plasma glucose in sodium fluoride tubes, with immediate placement in an ice slurry and centrifugation within 30 minutes ( 109 ). Citrate tubes are recommended as an alternative where early centrifugation is not possible. These standards are important because a major source of preanalytical glucose measurement error in sodium fluoride tubes is glycolysis by erythrocytes and leukocytes, which at room temperature lowers glucose levels prior to centrifugation at a rate of 5% to 7% per hour [~0.6 mmol/L (10 mg/dL)] ( 109 , 110 ). By 1 hour, this degree of glucose lowering is higher than the total analytical error threshold for glucose based on biological variation ( 107 ).
Recent studies have shown that OGTT preanalytical glucose processing variability greatly impacts the prevalence of GDM ( 67 , 111 ). Implementation of the AACC/ADA recommendations in a UK cohort resulted in higher mean glucose concentrations and 2.7-fold increased detection of GDM based on IADPSG criteria compared with the standard practice of storing sodium fluoride tubes at room temperature and delaying centrifugation until collection of all 3 OGTT samples ( 112 ). This increase in GDM diagnosis was entirely attributable to control of glycolysis ( 107 ). Similarly, in a large Australian multiethnic cohort (n = 12317), the rate of GDM diagnosis based on IADPSG criteria increased from 11.6% to 20.6% with early (within 10 minutes) vs delayed centrifugation ( 111 ). Mean glucose concentrations for the fasting, 1-hour, and 2-hour OGTT samples were 0.24 mmol/L (5.4%), 0.34 mmol/L (4.9%), and 0.16 mmol/L (2.3%) higher with early centrifugation, with the increase in GDM diagnosis primarily due to the resulting increase in fasting glucose levels ( 111 ). Importantly, the HAPO study, upon which the IADPSG diagnostic criteria for GDM was based, followed these AACC/ADA preanalytical glucose processing standards ( 111 ).
GDM is 1 of the most common medical complications of pregnancy ( 73 ). In 2019, the International Diabetes Federation (IDF) estimated that 1 in 6 live births worldwide were complicated by GDM ( 113 ). More than 90% of cases of hyperglycemia in pregnancy occur in low- and middle-income countries ( 114 ), where the prevalence and severity of maternal and neonatal complications associated with GDM ( 47 , 113 ) contrast with the near-normal pregnancy outcomes of modern management of GDM in developed countries ( 115 ).
The prevalence of GDM varies widely, depending on the population, the specific screening and the diagnostic criteria utilized. A 2012 systematic review of the diagnostic criteria used to define GDM reported a worldwide prevalence of GDM of 2% to 24.5% for the WHO criteria, 3.6% to 38% for the Carpenter and Coustan criteria, 1.4 to 50% for the NDDG criteria, and 2% to 19% for the IADPSG criteria ( 116 ).
Regardless of the specific diagnostic criteria or population, the prevalence of GDM continues to rise internationally, corresponding to epidemiological factors including the background rates of type 2 diabetes and increased incidence of obesity in women of childbearing age and rising maternal age ( 117-124 ). Implementation of the revised IADPSG diagnostic criteria have further increased the proportion of women being diagnosed with GDM ( 69 , 125 , 126 ). The incidence of GDM in the original HAPO study cohort applying the IADPSG diagnostic criteria ranged from 9.3% to 25.5% depending on study site ( 69 ). Recent international prevalence data also demonstrate marked variability in the rate of GDM, ranging from 6.6% in Japan and Nepal to 45.3% of pregnancies in the United Arab Emirates ( 127 ).
Several modifiable and nonmodifiable risk factors for GDM have been identified ( Table 4 ). A history of GDM in a previous pregnancy is the strongest risk factor for GDM, with reported recurrence rates of up to 84% ( 128 ). The risk of recurrence varies greatly depending on ethnicity ( 128 ). Ethnicities at increased risk for development of type 2 diabetes, such as South and East Asians, Hispanic, Black and Native Americans, Aboriginal and Torres Strait Islanders, and Middle Easterners are also associated with an increased risk of GDM ( 129 , 130 ). A US study of over 123 000 women reported the prevalence of GDM using the 2000 ADA diagnostic criteria to be the highest among Filipinas (10.9%) and Asians (10.2%), followed by Hispanics (6.8%), non-Hispanic Whites (4.5%) and Black Americans (4.4%) ( 131 ). Women who have had GDM are at increased risk for subsequent type 2 diabetes, while family history of type 2 diabetes in a first-degree relative or sibling with GDM is a major risk factor for GDM ( 129 , 132-134 ).
Key risk factors for gestational diabetes mellitus
Abbreviations: BMI, body mass index; GDM, gestational diabetes mellitus.
Increasing maternal age is also a risk factor for GDM ( 129 , 133-135 ). The prospective First and Second Trimester Evaluation of Risk trial (n = 36 056) demonstrated a continuous positive relationship between increasing maternal age and risk for adverse pregnancy outcomes, including GDM ( 135 ). Maternal age 35 to 39 years and ≥40 years was associated with an adjusted odds ratio (OR) for GDM of 1.8 (95% CI 1.5-2.1) and 2.4 (95% CI 1.9-3.1), respectively ( 135 ). Other studies in high-risk cohorts have reported a lesser risk between increasing maternal age and GDM after adjustment for other risk factors ( 136 ).
Maternal prepregnancy overweight (BMI 25-29.99 kg/m 2 ) or obesity (BMI ≥ 30 kg/m 2 ) are common risk factors for GDM ( 129 , 130 , 133 , 134 , 136 , 137 ). The risk of GDM is increased almost 3-fold (95% CI 2.1-3.4) in women with class I obesity (BMI 30-34.99 kg/m 2 ) and 4-fold (95% CI 3.1-5.2) in women with class II obesity (BMI 35-39.99 kg/m 2 ), compared to women with a BMI < 30 kg/m 2 ( 138 ). High GWG, particularly in the first trimester, is also associated with an increased risk for GDM ( 131 , 139 , 140 ). Further, women with obesity and high GWG are 3- to 4-fold more likely to develop abnormal glucose tolerance compared to women who remained within the 1990 Institute of Medicine (IOM) recommendations for GWG ( 131 , 141 ). Interpregnancy weight gain is also a risk factor for GDM and perinatal complications in a subsequent pregnancy ( 142 ) and may be a potential confounder when considering the risk of GDM recurrence.
Studies have demonstrated an association between polycystic ovary syndrome and GDM, although this is significantly attenuated after adjustment for maternal BMI ( 143 , 144 ). Other risk factors for GDM include multiparity ( 133 , 134 ), twin pregnancy ( 145 , 146 ), previous macrosomia ( 123 ), a history of perinatal complications ( 134 ), maternal small-for-gestational-age (SGA) or LGA ( 134 ), physical inactivity ( 129 , 147 , 148 ), low-fiber high-glycemic load diets ( 149 ), greater dietary fat and lower carbohydrate intake ( 137 ), and medications such as glucocorticoids and anti-psychotic agents ( 150 , 151 ). Maternal pre- and early pregnancy hypertension is also associated with an increased risk of developing GDM ( 152 , 153 ).
Overall, noting the variation in performance and utility of clinical risk factors based on local population factors, previous GDM and family history of diabetes appear to be the strongest clinical risk factors for GDM ( 154-157 ). Ethnicity, higher maternal age, and BMI are also strong predictors for GDM ( 154-158 ).
Normal pregnancy is associated with marked changes in glycemic physiology ( 159 , 160 ). There is a progressive increase in insulin resistance, predominantly due to increased circulating placental hormones including growth hormone, corticotrophin-releasing hormone, human placental lactogen, prolactin, estrogen, and progesterone ( 161-166 ). Increased maternal adiposity particularly in early pregnancy also promotes insulin resistance, contributing to facilitated lipolysis by late pregnancy ( 167 , 168 ). The resultant increase in maternal free fatty acid (FFA) levels exacerbates maternal insulin resistance by inhibiting maternal glucose uptake and stimulating hepatic gluconeogenesis ( 168 , 169 ). By late pregnancy, studies have reported decreases in maternal glucose sensitivity between 40% and 80% in women with normal or increased BMI ( 170-172 ). Increased maternal insulin resistance results in higher maternal postprandial glucose levels and FFAs for maternal growth ( 164 , 167 , 173 ) and increased facilitated diffusion across the placenta, leading to greater availability of glucose for fetal growth ( 161 , 174 ). This progressive rise in maternal insulin resistance underpins the delayed testing approach to GDM, aiming to maximize detection of GDM when insulin resistance is at its greatest in mid- to late gestation.
In addition to increased insulin resistance and elevated postprandial glucose, adaptations in normal pregnancy include enhanced insulin secretion ( 160 , 165 ). Maternal glucose levels are maintained at lower levels than in healthy nonpregnant women ( 175 , 176 ), and euglycemia is maintained by a corresponding 200% to 250% increase in insulin secretion, most notable in early pregnancy ( 161 , 167 , 177 ). Human placental lactogen, in addition to prolactin and growth hormone, primarily regulate increased maternal β-cell insulin secretion and proliferation during pregnancy ( 178-180 ). Rodent studies have demonstrated a 3- to 4-fold increase in β-cell mass during pregnancy, mediated via hypertrophy, hyperplasia, neogenesis, and/or reduced apoptosis ( 181 , 182 ).
GDM is characterized by a relative insulin secretory deficit ( 177 ), in which maternal β-cell insulin secretion is unable to compensate for the progressive rise in insulin resistance during pregnancy ( 183 ). This leads to decreased glucose uptake, increased hepatic gluconeogenesis, and maternal hyperglycemia ( 167 ). It is hypothesized that this results from the failure of β-cell mass expansion ( 182 , 184 ). Hyperlipidemia, characterized predominantly by higher serum triglycerides, may also cause lipotoxic β-cell injury, further impairing insulin secretion ( 185 , 186 ). The pathogenesis of GDM therefore parallels that of type 2 diabetes, characterized by both increased insulin resistance and relative insulin deficiency arising from a reduction in β-cell function and mass ( 187 , 188 ).
Serial studies of the insulin secretory response in women who develop GDM suggest that the abnormal insulin secretory response is present from prepregnancy and increases in early pregnancy, prior to and independent of changes in insulin sensitivity ( 170 , 189-191 ). These data suggest that many women with GDM may have chronic or preexisting β-cell dysfunction, potentially mediated by circulating hormones including leptin ( 191 ).
The genetics of GDM and glucose metabolism in pregnancy remain poorly defined. Data on epigenetic mechanisms in GDM are especially lacking and primarily limited to the potential role of DNA methylation in mediating the intrauterine effects of GDM on offspring outcomes ( 192 , 193 ).
Most genetic studies have focused on variants associated with type 2 diabetes and have demonstrated a similar association with GDM ( 194 , 195 ). A meta-analysis of 28 case-control studies (n = 23425) ( 196 ) identified 6 genetic polymorphisms at loci involved in insulin secretion [insulin-like growth factor 2 messenger RNA-binding protein 2 ( IGF2BP2 ), melatonin receptor 1B ( MTNR1B ) and transcription factor 7-like 2 ( TCF7L2 )] ( 197-199 ), insulin resistance [insulin receptor substrate 1 ( IRS1 ) and peroxisome proliferator-activated receptor gamma ( PPARG )] ( 200 , 201 ), and inflammation [tumor necrosis factor alpha ( TNF-α )] ( 202 ) in type 2 diabetes. Overall, only MTNR1B , TCF7L2 , and IRS1 were also significantly associated with GDM, supporting the role of both impaired insulin secretion and insulin resistance in the pathogenesis of GDM as well as type 2 diabetes ( 196 ). Subgroup analysis showed the risk alleles of TCF7L2 and PPARG were significant only in Asian populations, while the association between IRS1 and TCF7L2 and GDM risk varied depending on diagnostic criteria and genotype methodology ( 196 ), highlighting the need for further large confirmatory studies.
Two genome-wide association studies (GWAS) have evaluated the genetic associations for GDM and glucose metabolism ( 194 , 203 ). The first, a 2-stage GWAS in Korean women, compared 468 women with GDM and 1242 normoglycemic women using 2.19 million genotyped markers before further genotyping 11 loci in 1714 women, identifying 2 loci significantly associated with GDM ( 203 ). A variant in cyclin-dependent kinase 5 regulatory subunit-associated protein 1-like 1 ( CDKAL1 ) had the strongest association with GDM, followed by a variant near MTNR1B expressed in pancreatic β-cells ( 204 ). The IGF2BP2 variant did not reach genome-wide significance with GDM in this study. CDKAL1 was significantly associated with decreased fasting insulin concentration and homeostasis model assessment of β-cell function in women with GDM, consistent with impaired β-cell compensation. MTNR1B was associated with decreased fasting insulin concentrations in women with GDM and increased fasting glucose concentrations in both women with and without GDM ( 203 ). Variants in CDKAL1 and MTNR1B have previously been associated with type 2 diabetes risk ( 205 , 206 ).
A subsequent GWAS performed in a subset of the HAPO cohort (n = 4528) comprising European, Thai, Afro-Caribbean, and Hispanic women evaluated maternal metabolic traits in pregnancy ( 194 ). This study reported 5 variants associated with quantitative glycemic traits in the general population ( 207 , 208 ) that were also associated with glucose or C-peptide levels in pregnancy, although strength of association varied across cohorts ( 194 ). Specifically, loci in glucokinase regulator ( GCKR ), glucose-6-phosphatase 2 ( G6PC2 ), proprotein convertase subtilisin/kexin type 1 ( PCSK1 ), protein phosphatase 1, regulatory subunit 3B ( PPP1R3B ), and MTNR1B were associated with fasting glucose. In addition, GCKR and PPP1R3B were associated with fasting C-peptide levels, while MTNR1B was associated with 1-hour postload glucose. These loci have also previously been associated with lipid metabolism ( GCKR and PPP1R3B ), glycogen metabolism ( PPP1R3B ), and obesity-related traits ( PCSK1 ) ( 209-214 ).
Two additional novel loci identified near hexokinase domain containing 1 ( HKDC1 ) associated with 2-hour postload glucose, and β-site amyloid polypeptide cleaving enzyme 2 ( BACE2 ) associated with fasting C-peptide, demonstrated limited association with glycemic traits outside of compared to in pregnancy ( 215 ). In general, however, studies evaluating associations between genetic risk scores, glycemic traits in pregnancy, and GDM have also confirmed that genetic determinants of fasting glucose and insulin, insulin secretion, and insulin sensitivity reported outside of pregnancy influence GDM risk ( 216 ). A summary of the genes associated with GDM is provided in Table 5 .
Genes linked to gestational diabetes mellitus
Genes were identified and selected from the genome-wide association studies ( 194 , 203 ). The name and function of each gene was determined from GeneCards ( https://www.genecards.org ).
a Collectrin, amino acid transport regulator is a stimulator of β-cell replication.
Maturity-onset diabetes of the young (MODY) is the most common form of monogenic diabetes; inherited forms of diabetes characterized by defects in single genes regulating β-cell development and function ( 217 , 218 ). MODY consists of several autosomal dominant forms of diabetes accounting for up to 2% of all diabetes diagnoses ( 219 ). A diagnosis of MODY requires confirmatory molecular genetic testing, and thus MODY is frequently misdiagnosed as preexisting diabetes or GDM, accounting for up to 5% of GDM “cases” ( 220-223 ). A UK study reported that HNF-1α (MODY3) (52%) and glucokinase (GCK)-MODY subtype (MODY2) (32%) were most frequent in probands confirmed with MODY, followed by HNF-4α (MODY1) and HNF-1β (MODY5) ( 224 ).
Women with GCK-MODY often first present following antenatal screening for GDM, with an estimated prevalence of 1% of all GDM “cases” actually GCK-MODY ( 220 , 222 ). GCK-MODY is caused by mutations in the glucokinase gene, leading to a greater set point for glucose stimulated insulin release ( 219 ). Clinically, GCK-MODY is defined by mild, stable fasting hyperglycemia [fasting glucose 98-150 mg/dL (5.4-8.3 mmol/L)] and low rates of microvascular and macrovascular complications ( 220 ). It should be suspected following a positive OGTT in pregnancy if the fasting glucose is ≥5.5 mmol/L, the glucose increment from the fasting to 2-hour (75-g) OGTT is small (<4.6 mmol/L), and there is a positive family history of mild hyperglycemia or diabetes. In addition, a combination of fasting glucose ≥ 100 mg/dL (5.6 mmol/L) and BMI < 25 kg/m 2 has been shown to have a sensitivity of 68% and a specificity of 99% for differentiating GCK-MODY from GDM ( 220 ). Importantly, management differs from that of GDM because the need for intensive maternal glycemic control largely depends on whether the GCK-MODY mutation is also present in the fetus ( 220 , 225 , 226 ). Maternal insulin therapy is therefore only recommended in the presence of increased fetal abdominal growth (>75th centile) measured on serial ultrasounds from 26 weeks’ gestation, as this indicates that the fetus does not have the GCK mutation ( 220 ).
GDM is associated with excess neonatal and maternal short- and long-term morbidity, summarized in Table 6 .
Maternal and neonatal complications of gestational diabetes mellitus
Sources: Scholtens et al ( 227 ) and Saravanan ( 228 ).
Abbreviation: GDM, gestational diabetes mellitus.
The Pedersen hypothesis describes the pathophysiology contributing to perinatal complications in GDM ( 229 ). Maternal hyperglycemia results in fetal hyperglycemia via facilitated diffusion of glucose by the glucose transporter 1 (GLUT1) ( 230 ). Fetal hyperglycemia results in fetal hyperinsulinemia, promoting fetal anabolism, excessive fetal adiposity, and accelerated growth, leading to LGA and macrosomia ( 231-239 ). Maternal hyperlipidemia also contributes to excess fetal growth ( 233 , 240 ). Macrosomia and LGA increase the risk of cesarean section, birth trauma, and perinatal complications including shoulder dystocia, brachial plexus injury and fracture, and perinatal asphyxia ( 27 , 132 , 237 , 238 , 241-243 ). Increased risk of perinatal asphyxia is associated with fetal death in utero, polycythemia, and hyperbilirubinemia ( 27 , 244-246 ). Fetal hyperinsulinemia can also increase the risk of metabolic abnormalities including neonatal hypoglycemia, hyperbilirubinemia, and respiratory distress syndrome postpartum ( 27 , 244 ). The risk appears to be greater among offspring of women with more severe hyperglycemia ( 247 ). Figure 2 summarizes the perinatal consequences of GDM.

Perinatal consequences of gestational diabetes mellitus.
In the HAPO study, higher maternal glucose levels were associated with an increased risk of LGA, shoulder dystocia or birth injury, and neonatal hypoglycemia ( 27 ). A recent systematic review (n = 207 172) confirmed similar positive linear associations for maternal glycemia based on maternal glucose thresholds for the GCT, 75-g 2-hour OGTT, or 100-g 3-hour OGTT and risk of cesarean section, induction of labor (IOL), LGA, macrosomia, and shoulder dystocia ( 248 ). GDM has also been associated with an increased risk of preterm birth, birth trauma, neonatal respiratory distress syndrome, and hypertrophic cardiomyopathy ( 27 , 244 , 249 ). An increased risk of congenital malformations in the offspring has been reported, although whether this persists after adjustment for maternal age, BMI, ethnicity, and other contributing factors is unknown ( 250 ). A French cohort study (n = 796 346) reported a 30% higher risk of cardiac malformations in the offspring of women with GDM compared to women with normal glucose tolerance, after excluding women with likely undiagnosed pregestational diabetes ( 249 ). However, this increased risk only reached statistical significance in women treated with insulin therapy. Maternal BMI, which was not evaluated in these studies, may account for these findings ( 251 , 252 ). Similarly, a reported increase in perinatal mortality after 35 weeks’ gestation in the offspring of women with GDM may also be confounded by obesity ( 253-256 ). An increased risk of perinatal mortality after 37 weeks’ gestation was demonstrated in French women with GDM on dietary intervention, possibly because these women delivered later than women treated with insulin therapy ( 249 ). In contrast, the HAPO study did not demonstrate excess perinatal mortality in their untreated cohort ( 27 ).
Modern management of GDM and associated maternal risk factors is associated with near-normal birthweight in developed countries ( 115 , 257 ). This is important because birthweight is the major risk factor for shoulder dystocia, brachial plexus injury, neonatal hypoglycemia, and neonatal respiratory distress syndrome in the offspring of women with and without GDM ( 242 ). A retrospective cohort study of 36 241 pregnancies in the United States reported that the risk of shoulder dystocia among infants of women without GDM compared to women with GDM was 0.9% vs 1.6% if birthweight was <4000 g and 6.0% vs 10.5% if birthweight was ≥4000 g (macrosomia) ( 242 ). The risk of neonatal hypoglycemia in infants with birthweight < 4000 g was 1.2% vs 2.6% and 2.4% vs 5.3% for birthweight ≥ 4000 g, in women without GDM compared to women with GDM, respectively. Similar findings were seen for brachial plexus injury and neonatal respiratory distress syndrome. Thus, GDM confers increased risk of perinatal complications independent of birthweight.
The risk of stillbirth is also greater in women with GDM. A large US retrospective analysis examined stillbirth rates at various stages of gestation in over 4 million women, including 193 028 women with GDM. The overall risk of stillbirth from 36 to 42 weeks’ gestation was higher in women with GDM compared to women without GDM (17.1 vs 12.7 per 10 000 deliveries; RR 1.34; 95% CI 1.2-1.5) ( 253 ). This increased risk of stillbirth was also observed at each gestational week: 3.3 to 8.6 per 10 000 ongoing pregnancies in women with GDM compared to 2.1 to 6.4 per 10 000 ongoing pregnancies in women without GDM from 36 to 41 weeks’ gestation ( 253 ). For women with GDM, the relative risk of stillbirth was highest in week 37 (RR 1.84, 95% CI 1.5-2.3). Notably, the risk of stillbirth is highest in women with undiagnosed GDM. In a UK prospective case-control study (n = 1024), women with undiagnosed GDM based on a fasting glucose level ≥ 5.6mmol/L (≥100 mg/dL) had a 4-fold greater risk of late stillbirth (defined as occurring ≥28 weeks’ gestation) compared to women with fasting glucose < 5.6mmol/L (<100 mg/dL) ( 74 ). In contrast, women at risk of GDM based on NICE risk factors who were diagnosed with GDM on the OGTT had a similar risk of stillbirth to women who were not at risk of GDM. This suggests that diagnosing and managing GDM reduces the risk of stillbirth to near-normal levels ( 74 ).
Recent epidemiological studies suggest an increased risk of later adverse cardiometabolic sequelae in the offspring of women with GDM ( 227 , 258 ). A large Danish population-based cohort study (n = 2 432 000) demonstrated an association between maternal diabetes and an increased rate of early onset cardiovascular disease (CVD; ≤40 years of age) among offspring ( 259 ). GDM specifically was associated with a 19% increased risk of early onset CVD (95% CI 1.07-1.32). A longitudinal UK study provides potential mechanistic insight, finding that GDM was associated with alterations in fetal cardiac function and structure, with reduced systolic and diastolic ventricular function persisting in infancy ( 260 ). This is consistent with the association between in utero exposure to maternal hyperglycemia and fetal programming first reported in the Native American Pima population, characterized by a high prevalence of obesity, type 2 diabetes, and GDM ( 261 ).
The recent HAPO Follow Up Study (HAPO-FUS), which was not confounded by treatment of maternal glycemia, included 4832 children 10 to 14 years of age whose mothers were participants of HAPO ( 227 ). The HAPO-FUS demonstrated a durable impact of maternal glycemia with long-term offspring glucose metabolism, including at glucose levels lower than those diagnostic for GDM ( 227 ). A generally linear relationship between maternal antenatal glucose and offspring glucose levels and related outcomes was observed. Increasing maternal glucose categories were associated with a higher risk of impaired fasting glucose and impaired glucose tolerance and higher timed glucose measures and HbA1c levels and were inversely associated with insulin sensitivity and disposition index by 14 years of age, independent of maternal and childhood BMI and family history of diabetes ( 227 ). A positive association was observed between GDM defined by any criteria and glucose levels and impaired glucose tolerance in the offspring at ages 10 to 14 years and an inverse association with offspring insulin sensitivity ( 262 ). Higher frequencies of childhood obesity and measures of adiposity across increasing categories of maternal OGTT glucose levels were also noted ( 262 ). Recent evidence for increased glucose-linked hypothalamic activation in offspring aged 7 to 11 years previously exposed to maternal obesity and GDM in utero, which predicted higher subsequent BMI, represents 1 possible mechanism for this increased childhood obesity risk ( 263 ).
Women with GDM are at an increased risk of obstetric intervention including IOL, cesarean section ( 27-29 , 264 , 265 ), and complications associated with delivery including perineal lacerations and uterine rupture, predominantly relating to fetal macrosomia and polyhydramnios ( 266 ).
As demonstrated in HAPO and other studies, women with GDM also have an increased risk of gestational hypertension and preeclampsia ( 267-269 ). Consistent with the association between diabetes and microvascular disease, abnormalities in glucose metabolism affect trophoblast invasion, leading to impaired placentation and greater risk for preeclampsia ( 270 ). The mechanism likely relates to insulin resistance and inflammatory pathway activation ( 271 , 272 ), with in vitro studies showing that elevated glucose concentrations inhibit trophoblast invasiveness by preventing uterine plasminogen activator activity ( 272 ).
Long-term Maternal Risk Following GDM
Women diagnosed with GDM based on pre-IADPSG diagnostic criteria are at increased risk of GDM in future pregnancies, with reported recurrence rates of 30% to 84% ( 128 ). A diagnosis of GDM is also associated with up to a 20-fold greater lifetime risk of type 2 diabetes ( 273 , 274 ). A recent large meta-analysis and systematic review (20 studies, n = 1 332 373 including 67 956 women with GDM) showed that women with a history of GDM have a 10-fold increased risk of developing type 2 diabetes, mostly within the first 5 years post-GDM ( 273 ). HAPO-FUS demonstrated that over 50% of women whose OGTT thresholds met (untreated) IADPSG diagnostic criteria for GDM had developed impaired glucose tolerance after 14 years of follow-up ( 275 ). These data highlight the importance of a management approach to GDM that focuses on early prevention of type 2 diabetes. For example, the updated NICE guidelines now recommend diabetes prevention for all women with previous GDM ( 276 , 277 ).
Previous GDM is also associated with cardiovascular risk factors such as obesity, hypertension, and dyslipidemia ( 274 , 278-280 ). The lifetime risk of cardiovascular disease following GDM is almost 3-fold higher in women who develop type 2 diabetes and 1.5 fold higher even in women without type 2 diabetes ( 280 ). Studies also report a 26% greater risk of hypertension and a 43% greater risk of myocardial infarction or stroke in women with previous GDM compared to women without GDM ( 281 , 282 ). The significance of GDM as a risk factor for type 2 diabetes and cardiovascular disease has been recently recognized by international organizations including the American Heart Association ( 283 ).
Benefits of Intervention on Perinatal Outcomes
Contemporary changes to the detection and management of GDM have been associated with almost comparable neonatal birthweight and adiposity outcomes to the background maternity population in developed countries ( 115 ).
The ACHOIS trial (n = 1000) was the first large RCT to evaluate whether treatment of women with GDM reduced the risk of perinatal complications ( 28 ). GDM was diagnosed based on a combination of fasting glucose < 7.8 mmol/L (140 mg/dL) and 2-hour postload glucose 7.8 to 11.0 mmol/L (140-199 mg/dL), respectively, using the 75-g 2-hour OGTT between 24 and 34 weeks’ gestation, following screening with either positive clinical risk factors or the GCT ( 28 ). ACHOIS demonstrated that a combination of dietary advice, self-monitoring of maternal glucose levels (SMBG), and insulin therapy, if required, to achieve SMBG targets [fasting glucose 3.5-5.5 mmol/L (63-99 mg/dL), preprandial glucose ≤ 5.5 mmol/L (99 mg/dL), and 2-hour postprandial glucose ≤ 7.0 mmol/L (126 mg/dL)], reduced the rate of serious perinatal complications (a composite of death, shoulder dystocia, nerve palsy, and fracture) compared to routine care (1% vs 4%; P = 0.01). In addition, such interventions were associated with a reduced incidence of macrosomia (10% vs 21%; P < 0.001), preeclampsia (12% vs 18%; P = 0.02), and improved maternal health-related quality of life ( 28 ).
In 2009, the MFMU trial (n = 958) reported that treatment of “mild” GDM was also associated with improved outcomes ( 29 ). Following a positive GCT between 24 and 30 + 6 weeks’ gestation, “mild” GDM was defined on a positive 100-g 3-hour OGTT by a fasting glucose < 5.3 mmol/L (95 mg/dL), and at least 2 postload glucose thresholds that exceeded the 2000 ADA diagnostic thresholds [1-, 2-, or 3-hour thresholds 10.0 mmol/L (180 mg/dL), 8.6 mmol/L (155 mg/dL), and 7.8 mmol/L (140 mg/dL), respectively]. Women with previous GDM were excluded from the study. Dietary intervention, SMBG, and insulin therapy, if required, to achieve a fasting glucose target < 5.3 mmol/L (95 mg/dL) and 2-hour postprandial glucose target < 6.7 mmol/L (121 mg/dL) was associated with reduced rates of macrosomia (5.9% vs 14.3%; P < 0.001), LGA (7.1% vs 14.5%; P < 0.001), shoulder dystocia (1.5% vs 4.0%; P = 0.02), cesarean section (26.9% vs 33.8%; P = 0.02), and preeclampsia and gestational hypertension (8.6% vs 13.6%; P = 0.01) compared to routine care. However, the intervention did not lead to a significant difference in the primary composite outcome of stillbirth, perinatal death, and neonatal complications (hyperbilirubinemia, hypoglycemia, hyperinsulinemia, and birth trauma) ( 29 ). Treatment targets in the MFMU trial were lower than that of the ACHOIS trial, and whether this may account for the reduction in cesarean section not shown in the ACHOIS trial is unclear. These key findings, supported by other studies ( 22 , 284 ), were highlighted by the IADPSG to support the lowering of the GDM diagnostic criteria and treating mild hyperglycemia ( 30 ).
A recent Cochrane review (8 RCTs; n = 1418) reported that GDM treatment, including dietary intervention and insulin therapy, reduced a composite outcome of perinatal morbidity (death, shoulder dystocia, bone fracture, and nerve palsy) by 68% compared to routine antenatal care ( 285 ). Treatment was also associated with reductions in macrosomia, LGA, and preeclampsia but an increase in IOL and neonatal intensive care admission.
The main objective of GDM management is to attain maternal normoglycemia because evidence suggests that excessive fetal growth can be attenuated by maintaining near normal glucose levels ( 286 , 287 ). The foundation of this approach is medical nutrition therapy. Given carbohydrates are the primary determinant of maternal postprandial glucose levels, current dietary practice aims to modify carbohydrate quality (glycemic index) and distribution ( 32 , 288 , 289 ). The original nutritional approach for GDM decreased total carbohydrate intake to 33% to 40% of total energy intake (EI) and was associated with reduced postprandial glycemia and fetal overgrowth ( 290 ). More recent evidence suggests that higher carbohydrate intake and quality (lower glycemic index) between 60% and 70% EI can also limit maternal hyperglycemia ( 291-293 ). Nevertheless, there remain limited data to support a specific dietary intervention for GDM ( 294 ). A recent meta-analysis (18 RCTs; n = 1151) showed that enhancing nutritional quality (modified dietary intervention, defined as a dietary intervention different from the usual one used in the control group) after GDM diagnosis, irrespective of the specific dietary approach, improved maternal fasting and postprandial glycemia, and reduced pharmacotherapy requirements, birthweight, and macrosomia ( 295 ).
Guidelines therefore currently recommend a range of carbohydrate intake between 33% and 55% EI ( 32 , 288 , 289 ). Studies have reported improved pregnancy outcomes in GDM with both lower carbohydrate (42%E) and high‐carbohydrate (55%E) diets ( 296 ), reflected in the most recent Academy of Nutrition and Dietetics guidelines, which state that beneficial effects on pregnancy outcomes in GDM are seen with a range of carbohydrate intakes ( 288 ). The IOM guidelines recommend a carbohydrate intake of at least 175 g/day and a total daily caloric intake of 2000 to 2500 kilocalories during pregnancy ( 289 ). The ACOG recommends a lower carbohydrate diet (33-40%E) ( 297 ). However, the ADA has raised concerns over the corresponding higher maternal fat intake, fetal lipid exposure, and overgrowth resulting from lowering carbohydrate intake ( 298 ) and withdrew specific dietary guidelines for GDM in 2005 ( 299 ).
Given maternal glucose primarily supports fetal growth and brain development ( 300 ), theoretically if the maternal diet is too low in carbohydrate, the maternal-fetal glucose gradient may be compromised. Restriction of total maternal EI is associated with reduced fetal growth ( 301 ). A recent systematic review similarly showed that lower carbohydrate intake correlated with lower birthweight and greater incidence of SGA ( 302 ), with a lower carbohydrate threshold of 47% EI associated with appropriate fetal growth ( 302 , 303 ). Importantly, the lower carbohydrate threshold independent of energy restriction in GDM is yet to be established. Related safety concerns with lower carbohydrate diets include the potential risk of higher fetal exposure to maternal ketones ( 304 ) and micronutrient deficiency ( 305 , 306 ). In vitro studies have shown that ketones suppress trophoblast uptake of glucose, jeopardizing glucose transfer across the placenta ( 307 ). Clinically, a prospective US cohort study of women with preexisting diabetes, GDM, or normal glucose tolerance demonstrated an inverse correlation between higher maternal third trimester beta-hydroxybutyrate and FFAs and lower offspring intellectual development scores at 2 to 5 years of age, although total carbohydrate, EI, and maternal BMI were not reported ( 304 ).
The IOM has published recommendations for weight gain during pregnancy based on prepregnancy BMI ( 289 ), but no specific recommendations for weight gain in GDM exist ( 286 ). In women with overweight or obesity, studies have suggested that weight reduction or gain ≤ 5 kg increased the risk of SGA ( 308 ). A recent systematic review based on data from almost 740 000 women demonstrated that GWG of 5 kg to 9 kg in women with class I obesity (BMI 30-34.99 kg/m 2 ), 1 to <5 kg for class II obesity (35-39.99 kg/m 2 ), and no GWG for women with class III obesity (BMI ≥ 40kg/m 2 ), minimized the combined risk of LGA, SGA, and cesarean section ( 309 ).
A meta-analysis (n = 88 599) evaluating the relationship between GWG and pregnancy outcomes in GDM specifically showed that GWG greater than the IOM recommendations was associated with an increased risk of pharmacotherapy, as well as of hypertensive disorders of pregnancy, cesarean section, LGA, and macrosomia ( 310 ). GWG below the IOM recommendations was protective for LGA (RR 0.71; 95% CI 0.56-0.90) and macrosomia (RR 0.57; 95% CI 0.40-0.83) and did not increase the risk of SGA (RR 1.40; 95% CI 0.86-2.27) ( 289 ). This suggests that GWG targets in GDM may need to be lower than the current recommendations for normal pregnancy. However, from a practical perspective, only 30% of women gained less than the recommended IOM GWG targets ( 310 ).
Fasting and postprandial glucose testing with either the 1- or 2-hour postprandial glucose value is recommended in women with GDM. The 1-hour postprandial glucose approximates to the peak glucose excursion in pregnancy in women without diabetes and those with type 1 diabetes ( 175 ). Studies have shown that the 1-hour postprandial peak glucose level correlates with amniotic fluid insulin levels, reflecting fetal hyperinsulism ( 311 ) and with fetal abdominal circumference in women with type 1 diabetes ( 286 ). An RCT that compared pre- to postprandial maternal SMBG values showed that titrating insulin therapy based on the 1-hour postprandial values was associated with improved maternal glycemic control and may better attenuate the risk of neonatal complications attributed to fetal hyperinsulinemia ( 312 ).
Treatment targets based on maternal SMBG levels vary internationally ( Table 7 ). There is some suggestion that lower glucose targets may improve pregnancy outcomes in GDM ( 176 , 313 , 314 ), but this is yet to be evaluated in adequately powered RCTs. Conversely, lower glycemic targets may be associated with an increased risk of SGA ( 315-317 ) and maternal and fetal hypoglycemia ( 318 , 319 ). A small study evaluating stringent glycemic targets in 180 women with GDM failed to demonstrate additional benefits, with no differences in the rates of cesarean section, birthweight, macrosomia, or SGA in the offspring of women randomized to intensive [preprandial glucose ≤ 5.0 mmol/L (90 mg/dL) and 1-hour postprandial glucose ≤ 6.7 mmol/L (121 mg/dL)] compared to standard treatment targets [preprandial glucose ≤ 5.8 mmol/L (104.5 mg/dL) and 1-hour postprandial glucose ≤ 7.8 mmol/L (140 mg/dL)] ( 320 ).
Recommended glycemic treatment targets in GDM
Abbreviations: ACHOIS, Australian Carbohydrate Intolerance Study in Pregnant Women Study; ADA, American Diabetes Association; ADIPS, Australasian Diabetes in Pregnancy Society; CDA, Canadian Diabetes Association; NICE, UK National Institute for Health and Care Excellence; MFMU, National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network.
Insulin has traditionally been the preferred treatment for GDM if maternal glucose levels remain elevated on medical nutrition therapy ( 267 ). Depending on targets, approximately 50% of women with GDM are prescribed insulin therapy to maintain normoglycemia ( 321 , 322 ), with a combination of evening intermediate-acting insulin if fasting glucose levels are elevated and mealtime rapid-acting insulin when indicated. Additional daytime intermediate-acting insulin may also be needed to control prelunch or predinner hyperglycemia.
Decreasing insulin doses in the third trimester may simply reflect the physiological increase in maternal insulin sensitivity observed at this stage of pregnancy ( 176 , 323 ). However, substantial insulin dose reduction, recurrent maternal hypoglycemia, and/or slowing of fetal growth or preeclampsia may indicate underlying pathophysiological placental insufficiency ( 324 ), impacting the timing of delivery and intensity of obstetric monitoring.
Risk factors for insulin therapy include earlier diagnosis of GDM ( 81 ), the pattern and degree of elevation of the 75-g 2-hour OGTT diagnostic glucose thresholds ( 325 ), and ethnicity ( 325 ). Other risk factors including gestational age and HbA1c level at the time of GDM diagnosis, BMI, and family history of diabetes account for only 9% of the attributable risk for insulin therapy ( 321 ). A recent Australian study found that maternal age > 30 years, family history of diabetes, prepregnancy obesity, previous GDM, early diagnosis of GDM, fasting glucose ≥ 5.3 mmol/L (96 mg/dL) and HbA1c ≥ 5.5% (37 mmol/mol) at diagnosis were all independent predictors for insulin therapy ( 326 ). Insulin usage could also be estimated according to the number of predictors present, with up to 93% of women with 6 to 7 predictors using insulin therapy compared with less than 15% of women with 0 to 1 predictors ( 326 ).
Oral pharmacotherapy options include glyburide and metformin. Oral pharmacotherapy is associated with improved cost effectiveness, compliance, and acceptability compared to insulin therapy ( 327 ). However, there are issues regarding efficacy and safety, particularly longer term, and thus insulin is generally preferred as first-line pharmacotherapy following lifestyle intervention.
Glyburide is commonly prescribed as first-line therapy for GDM in the United States ( 328 ). An early study evaluating the efficacy of glyburide vs insulin therapy in 404 women with GDM reported no differences in maternal glucose levels or neonatal outcomes between the treatment groups ( 329 ). However, subsequent studies show that approximately 20% of women treated with glyburide required additional insulin therapy to achieve adequate maternal glycemia ( 330 ). Moreover, a large retrospective US study of almost 111 000 women with GDM, in which 4982 women were treated with glyburide and 4191 women were treated with insulin, reported that glyburide was associated with an increased risk of neonatal complications including neonatal intensive care admission, respiratory distress syndrome, hypoglycemia, birth injury, and LGA compared to insulin therapy ( 331 ). Although transplacental transfer of glyburide to the fetus is highly variable, it can reach 50% to 70% of maternal plasma concentration ( 332 ), potentially causing direct stimulation of fetal insulin production ( 333 ).
The use of metformin in pregnancy continues to rise ( 334 ). However, its use remains controversial, due to the potential concerns regarding long-term metabolic programming effects of placental transfer of metformin to the fetus, with some studies suggesting similar plasma concentrations of metformin in the maternal and fetal circulation ( 335 ). A recent systematic review and meta-analysis of 28 studies (n = 3976) evaluating growth in offspring of women with GDM exposed to metformin compared to insulin therapy found that neonates exposed to metformin had lower birthweights (mean difference −107.7 g; 95% CI −182.3 to −32.7), decreased risk of LGA (OR 0.78; 95% CI 0.62-0.99), and macrosomia (OR 0.59; 95% CI 0.46-0.77) and lower ponderal indices than neonates whose mothers were treated with insulin ( 336 ). No difference in the risk of SGA was found, in contrast to outcomes in women with type 2 diabetes, with the Metformin in Women with Type 2 Diabetes RCT observing more than double the rate of SGA (95% CI 1.16-3.71) in the metformin treated cohort, in association with lower insulin doses, HbA1c, and GWG ( 337 ). Offspring of women with GDM exposed to metformin also demonstrate accelerated postnatal growth at 18 to 24 months of age (2 studies; n = 411; mean difference in weight 440 g; 95% CI 50-830), resulting in higher BMI at 5 to 9 years of age (3 studies; n = 520; BMI mean difference 0.78 kg/m 2 , 95% CI 0.23-1.33) ( 336 ).
The Metformin in Gestational Diabetes trial randomized 751 women to receive either metformin or insulin therapy, finding no significant difference in the composite neonatal outcome of neonatal hypoglycemia, respiratory distress syndrome, hyperbilirubinemia, low Apgar scores, birth trauma, and preterm birth ( 322 ). There was a trend toward increased preterm birth and decreased maternal GWG in women treated with metformin, while severe neonatal hypoglycemia was highest in those treated with insulin. Almost 50% of women treated with metformin required the addition of insulin therapy ( 322 ). Other studies have reported that between 14.0% and 55.8% of women treated with metformin also require insulin therapy to achieve optimal glycemic control ( 338 , 339 ). The Metformin in Gestational Diabetes: The Offspring Follow-Up 2-year follow-up study found that children exposed to metformin had increased subcutaneous fat localized to the arm compared with children whose mothers were treated with insulin alone ( 340 ). By 7 and 9 years of age the children exposed to metformin had similar offspring total and abdominal body fat percentage and metabolic biochemistry including fasting glucose, insulin, and lipids but were larger overall based on measures including weight, arm and waist circumference, waist-to-height ratio, and dual-energy X-ray absorptiometry fat mass and lean mass ( 341 ). These findings are consistent with a recent follow-up study of metformin therapy in pregnant women with polycystic ovary syndrome, which showed that children exposed to metformin in utero had higher BMI and rates of overweight and obesity at 4 years of age ( 342 ).
A recent Cochrane review (8 RCTs; n = 1487) evaluating the use of metformin, glyburide, and acarbose in women with GDM found that the benefits and potential harms of these therapies in comparison to each other are unclear ( 343 ). Other meta-analyses comparing glyburide, metformin, and insulin have shown that metformin was associated with lower GWG, gestational hypertension, and postprandial maternal glucose levels compared to either glyburide or insulin ( 344 , 345 ), but metformin was associated with an increased risk of preterm birth compared to insulin ( 345 ). Compared to metformin, glyburide was associated with a higher risk of increased birthweight, LGA, macrosomia, neonatal hypoglycemia, and increased GWG ( 344 ). More recently, a small RCT (n = 104) suggested that glyburide and metformin were comparable in terms of maternal glycemia and perinatal outcomes ( 346 ). Treatment success after second-line (oral) therapy was higher in the (first-line) metformin vs glyburide cohort (87% vs 50%; P = 0.03), suggesting that metformin may be the preferred first-line therapy. Overall, most women required either a combination of metformin and glyburide to achieve glycemic control and/or replacement of first-line oral therapy due to hypoglycemia and gastrointestinal side effects, suggesting neither agent alone is likely to be successful in most women with GDM. Combined oral pharmacotherapy had an efficacy rate of 89%, with only 11% of women required third-line therapy with insulin ( 346 ). However, the effects of dual oral therapy crossing the placenta on long-term potential fetal programming via their effects on cellular metabolism, hepatic gluconeogenesis, and insulin sensitivity (metformin) ( 347 ) and fetal hyperinsulinemia (glyburide) is unknown ( 348 ).
A recent Cochrane review consisting of only 3 small RCTs (n = 524) reported insufficient (very low certainty) evidence to evaluate the use of fetal biometry in guiding the medical management of GDM ( 349 ). Nevertheless, serial fetal growth ultrasounds, particularly assessing fetal abdominal circumference, are potentially useful in guiding the intensity of maternal glucose targets and insulin therapy ( 350-352 ). Studies have demonstrated that neonates with an estimated fetal weight ≥ 75th percentile on early third trimester ultrasound were 10-fold more likely to be LGA compared to neonates with an estimated fetal weight < 75th percentile ( 353 ). Measured fetal abdominal circumference < 90th percentile on 2 ultrasounds at 3- to 4-week intervals has also been shown to provide high reliability in excluding the risk of LGA ( 351 ). Moreover, a recent retrospective study (n = 275) found that estimated fetal weight or abdominal circumference up to the 30th percentile on third trimester ultrasound was associated with a greater risk of adverse neonatal outcomes, comparable to that observed with abdominal circumference or estimated fetal weight > 95th percentile in women with hyperglycemia in pregnancy (including GDM) ( 354 ). These findings suggest the potential utility of fetal biometry at thresholds other than defining SGA or LGA in identifying higher risk pregnancies in GDM.
The optimal timing of delivery in GDM is complex, guided by maternal glycemic control in addition to maternal and fetal factors, and has not been definitively established. Current guidelines recommend delivery by 40 + 6 weeks’ gestation in low-risk women with GDM managed with diet alone and from 39 + 0 to 39 + 6 weeks’ gestation for women with GDM well controlled with therapy ( 38 , 277 , 355 ). A recent Canadian population-based cohort study examining the week-specific risks of severe pregnancy complications in women with diabetes included 138 917 women with GDM and 2 553 243 women without diabetes over a 10-year period ( 356 ). There was no significant difference in gestational age-specific maternal mortality or morbidity (defined as ≥1 of the following in the immediate perinatal period: obstetric embolism, obstetric shock, postpartum hemorrhage with hysterectomy or other procedures to control bleeding, sepsis, thromboembolism, or uterine rupture) between iatrogenic delivery and expectant management in women with GDM. However, iatrogenic delivery was associated with an increased risk of neonatal mortality and morbidity (birth or fetal asphyxia, grade 3 or 4 intraventricular hemorrhage, neonatal convulsions, other disturbances of cerebral status of newborn, respiratory distress syndrome, birth injury, shoulder dystocia, stillbirth or neonatal death) at 36 to 37 weeks’ gestation (76.7 and 27.8 excess cases per 1000 deliveries, respectively) but a lower risk of neonatal morbidity and mortality at 38 to 40 weeks’ gestation (7.9, 27.3, and 15.9 fewer cases per 1000 deliveries, respectively) compared with expectant management, suggesting that delivery at 38, 39, or 40 weeks’ gestation may provide the best neonatal outcomes in women with GDM ( 356 ).
Up to one third of women with GDM diagnosed by pre-IADPSG criteria will have glucose levels consistent with diabetes or prediabetes on postpartum testing at 6 to 12 weeks ( 357 ). Thus, a repeat OGTT or fasting glucose as early as 6 to 12 weeks’ postpartum is recommended to confirm maternal glucose status ( 41 , 277 ). Only around 25% of women are tested at this time point with compliance with postpartum testing ranging between 23% and 58% ( 357 , 358 ). In women with GDM with overweight or obesity, a reduction in interpregnancy BMI of ≥2.0 kg/m 2 reduces the risk of subsequent GDM by 74% ( 359 ). Longer term, women should perform regular cardiometabolic health assessment and optimization of lifestyle measures to reduce their greater risk of type 2 diabetes and cardiovascular disease ( 282 , 360 , 361 ). Up to 74% of women with obesity and previous GDM develop type 2 diabetes compared with <25% of women who achieve a normal BMI postpartum following GDM ( 362 ). It is unclear how relevant these studies in older women are for current clinical care given recent data that 50% of women develop type 2 diabetes within 5 to 10 years post-GDM diagnosis ( 273 ). The Diabetes Prevention Program demonstrated that lifestyle intervention and metformin therapy improved insulin sensitivity and preserved β-cell function in women with a history of previous GDM ( 363 ). Early type 2 diabetes prevention following GDM is therefore an essential component of the contemporary GDM detection and management paradigm ( 276 ).
Importantly, despite a reduction in the risk of macrosomia at birth, the ACHOIS and MFMU follow-up studies did not demonstrate a beneficial impact on childhood obesity and glucose tolerance at 5 to 10 years of age in the offspring of women who received treatment for maternal hyperglycemia ( 364 , 365 ). Other prospective cohort studies similarly suggest that the offspring of women with treated GDM still have a greater risk of obesity, type 2 diabetes, the metabolic syndrome, and cardiovascular disease from early childhood and adolescence ( 258 , 366-380 ). For example, a 2017 Danish National Birth Cohort study (n = 561) reported increased adiposity, an adverse cardiometabolic profile, and earlier onset puberty among adolescent females of women with GDM ( 381 ). A prospective offspring cohort study of women with GDM who achieved good antenatal glycemic control demonstrated that offspring adiposity (adipose tissue quantity measured using magnetic resonance imaging) was similar in the GDM and normal glucose tolerance groups within 2 weeks postpartum but was 16.0% greater (95% CI 6.0-27.1; P = 0.002) by 2 months of age ( 382 ). The mechanism for this greater adiposity and rapid weight gain in early infancy is uncertain given both groups were predominantly breastfed. Consistent with the ACHOIS and MFMU follow-up studies ( 364 , 365 ), these data suggest that the current approach to glycemic control in GDM may not mitigate its impact on longer term infant health. Further, this pathway may be potentially mediated by excess infant adiposity, which correlates with childhood adiposity ( 383 ). Table 8 presents practical tips for managing women with GDM.
Practical tips for managing women with GDM
Abbreviations: GDM, gestational diabetes mellitus; OGTT, oral glucose tolerate test.
Precision medicine seeks to improve diagnostics, prognostics, prediction, and therapeutics in diabetes, including GDM, by evaluating and translating various biological axes including metabolomics, genomics, lipidomics, proteomics, technology, clinical risk factors and biomarkers, and mathematical and computer modeling into clinical practice ( 384 ). The Precision Medicine in Diabetes Initiative was launched in 2018 by the ADA, in partnership with the European Association for the Study of Diabetes, with their first consensus report published in 2020 ( 384 ).
In GDM, precision medicine represents the increasing understanding of heterogeneity within its genotype and phenotype ( 170 , 385-388 ) to identify and translate subclassification of GDM into more personalized clinical care ( 388 ). For example, physiologic subtypes of GDM based on the underlying mechanisms leading to maternal hyperglycemia have been recently characterized ( 386 ). Among 809 women from the Genetics of Glucose Regulation in Gestation and Growth pregnancy cohort, heterogeneity in the contribution of insulin resistance and deficiency to GDM were characterized based on validated indices of insulin sensitivity and secretory response measured during the 75-g OGTT performed between 24 and 30 weeks’ gestation ( 388 ). Compared to women with normal glucose tolerance, women with insulin resistant GDM (51% of GDM) had higher BMI and fasting glucose, hypertriglyceridemia, and hyperinsulinemia, larger infants, and almost double the risk of GDM-associated pregnancy complications. In contrast, women with predominantly insulin secretion defects had comparable BMI, fasting glucose, infant birthweight, and risk of adverse outcomes to those with normal glucose tolerance ( 388 ).
Other studies have also suggested that greater insulin resistance in GDM carries a higher risk of perinatal complications ( 389 ). A recent multicenter prospective study of 1813 women evaluating subtypes of GDM based on insulin resistance ( 389 ) found that women with GDM and high insulin resistance [n = 189 (82.9%)] had a higher BMI, systolic blood pressure, fasting glucose, and lipid levels in early pregnancy compared to women with normal glucose tolerance or those diagnoses with insulin-sensitive GDM. Insulin-sensitive women with GDM [n = 39 (17.1%)] had a significantly lower BMI than women with normal glucose tolerance but similar blood pressure, early pregnancy fasting glucose and lipid levels, and pregnancy outcomes. Despite no differences in insulin treatment and early postpartum glucose intolerance among the GDM subtypes, women with GDM and high insulin resistance had a greater than 2-fold risk of preterm birth and an almost 5-fold increased risk of neonatal hypoglycemia compared with women with normal glucose tolerance. This suggests the high insulin resistance GDM subtype has a greater risk of pregnancy complications potentially arising from the resultant fetal hyperinsulinemia ( 389 ).
The contemporary precision medicine approach to GDM also includes the increasing exploration of early pregnancy risk prediction and risk management models ( 390 ). The traditional binary clinical risk factor approach to identifying women at high risk in early pregnancy is limited by poor sensitivity and specificity, with studies showing that clinical risk factor-based screening fails to identify 10% to over 30% of women with GDM ( 391-396 ). The Pregnancy Outcome for Women with Pre-gestational Diabetes Along the Irish Atlantic Seaboard study found that the prevalence of women with GDM who had no risk factors was low, ranging from 2.7% to 5.4% ( 397 ). However, despite the absence of risk factors, these women with GDM had more pregnancy complications than those with normal glucose tolerance ( 397 ). Other studies have also reported that women without risk factors diagnosed with GDM have comparable pregnancy outcomes to women with GDM identified as high risk ( 393 ). Thus, clinical risk factors alone are not predictive of GDM risk for all women. Although some improvement in the predictive accuracy for GDM is seen in clinical risk scoring approaches ( 158 , 398 ), greater improvement via multivariate risk prediction and mathematical or computer models combining clinical risk factors and biomarkers have been reported in the GDM research setting ( 154-156 , 399-403 ).
Biomarkers are defined as a biological observation that substitutes and ideally predicts the clinically relevant endpoint (ie, GDM) ( 404 ). Biomarker discovery and application in the early detection of GDM has become a major research area. However, few biomarkers are specific enough for clinical application ( 405 ). Most novel biomarkers with potential utility for the prediction of GDM are involved in pathophysiological pathways related to insulin resistance, dyslipidemia, and type 2 diabetes ( 402 , 406 ) but are frequently mediated by maternal obesity ( 240 , 407 ). Early pregnancy risk prediction models for GDM combining clinical risk factors and biomarkers have included various measures of maternal glucose, lipids, adipokines, inflammatory markers, and pragmatic aneuploidy and preeclampsia screening markers, with model performance (area under the curve) up to 0.91 ( 153 , 154 , 399 , 402 , 403 , 408-416 ). Limitations to the clinical application of novel biomarkers and model performance include heterogeneity in the testing approach to GDM and cohort characteristics, potential overestimation of model performance due to overfitting of the data to the index study population, the lack of external clinical validation studies, and limited regulatory guidance for validating biomarker assays ( 405 ).
The COVID-19 pandemic has led to dynamic changes in the testing approach and model of care for women with GDM to minimize the risk of virus transmission and because of decreased clinical capacity. Several temporary pragmatic diagnostic strategies have been suggested as an alternative to the OGTT, including measurement of fasting plasma glucose, random plasma glucose, and HbA1c ( 417-419 ). A secondary analysis of 5974 women from the HAPO study ( 420 ), reported that the UK, Canadian, and Australian COVID-19–modified diagnostic approaches reduced the frequency of GDM by 81%, 82%, and 25%, respectively. Short-term pregnancy complications in the subgroup of women now with undiagnosed GDM (“missed GDM”) were comparable to women diagnosed with GDM based on the Canadian-modified diagnostic criteria, slightly lower for the UK-modified criteria, but significantly lower for the Australasian Diabetes in Pregnancy Association–modified criteria. While all approaches recommend universal testing, the Australian approach adopts a lower fasting glucose threshold of 4.7 mmol/L to identify women who require an OGTT and does not include HbA1c measurement ( 420 ). A retrospective UK study of over 18 000 women sought to define evidence-based recommendations for pragmatic GDM testing during the COVID-19 pandemic ( 421 ), reporting that ~5% of women would be identified as GDM based on a random glucose threshold ≥ 8.5 mmol/L (153 mg/dL) at 12 weeks’ gestation and fasting glucose ≥ 5.2 to 5.4 mmol/L (94-97 mg/dL) or HbA1c ≥ 5.7% (39 mmol/mol) measured at 28 weeks’ gestation. Each test predicted some, but not all, obstetric and perinatal complications, lacking the sensitivity of the OGTT for the diagnosis of GDM but overall may provide adequate risk stratification where the OGTT is not feasible ( 421 ).
GDM is one of the most common complications of pregnancy and is increasing in global prevalence. Diagnosing GDM is important because perinatal complications and stillbirth risk are reduced by treatment. Despite the benefit of identifying and treating GDM, much of the current (short-term) diagnostic and management approach to GDM remains contentious. These differences confound interpretation and application of trial data, preventing a single standard international approach to GDM.
Recent data indicates near normal birthweight and maternity population outcomes in women with GDM based on modern IADPSG criteria in developed countries, demonstrating that even treatment of “milder” maternal hyperglycemia improves pregnancy outcomes. However, most cases of GDM occur in low- and middle-income countries where perinatal risks are far greater and universal 1-step testing may be more practical. There are limited RCT data to guide diagnosis and management in this setting, and further evidence is urgently needed. In developed countries including the United Kingdom, the main issue arguably does not pertain to women diagnosed with GDM but rather high-risk women who remain unscreened (associated with factors such as lower socioeconomic status and higher BMI) who are at highest risk of stillbirth ( 74 ).
The background to the various GDM diagnostic criteria is informative in demonstrating that no approach clearly separates risk groups. It is also now evident that a continuum of risk for GDM exists based on both the timing and degree of maternal hyperglycemia. This underscores the difficulty of defining absolute glucose thresholds at a single timepoint in pregnancy for the diagnosis of GDM and is confounded further by variation in glucose measurement due to preanalytical glucose processing and reproducibility issues. Thus, current diagnostic glucose thresholds for GDM must inevitably reflect compromise and consensus.
A precision medicine approach that recognizes GDM subtype and heterogeneity, enhanced by further research into the genetics of GDM and validation of novel biomarkers and new technologies such as continuous glucose monitoring may improve risk stratification, optimize clinical models of care, and facilitate more individualized and consumer-friendly detection and treatment strategies.
The recent HAPO-FUS data confirming the long-term impact of maternal hyperglycemia on maternal and offspring metabolic health ( 227 , 262 ) highlight an important paradigm shift. The approach to GDM should reflect an evidence base that evaluates diagnostic glucose thresholds and measurement within a framework that includes timing of detection and treatment trials with long-term clinical and health economic outcomes. For example, if the ongoing Treatment of Booking Gestational Diabetes Mellitus trial demonstrates a benefit for early GDM detection and treatment, there are implications for the prevailing diagnostic GDM glucose thresholds in later pregnancy. This is because these thresholds were derived from the risk of perinatal complications in a heterogeneous GDM cohort, which included women who would fulfill early GDM criteria.
Other important areas for research include the evaluation of dietary interventions establishing the optimal carbohydrate threshold in GDM, further clarity on the potential long-term impact of intrauterine metformin on the offspring, as well as the efficacy of preconception and early pregnancy preventive strategies targeting risk factors other than glycemia, such as maternal obesity and GWG. Improved obstetric assessment of placental function, especially in late pregnancy, to inform timing of delivery and identify women at highest risk of stillbirth in GDM is also needed.
The complications of GDM may indeed be greater based on the severity of maternal glycemia and associated vascular risk factors. Nevertheless, the traditional focus on diagnostic criteria and short-term antenatal maternal glucose management fails to address the importance of identifying “milder” (IADPSG-defined) GDM as a risk factor for future maternal and offspring diabetes and CVD risk. It should also be apparent that the increasing prevalence of GDM largely reflects the worsening metabolic health burden including prediabetes and obesity in women of childbearing age. The clinical focus for GDM must therefore urgently shift to early postnatal prevention strategies to decrease the progression from GDM to type 2 diabetes and address longer term maternal and offspring cardiometabolic risk post-GDM via a life course management approach.
A.S. was supported by an NHMRC Fellowship Grant (GNT1148952).
A.S., J.W., H.M., and G.P.R. have nothing to declare.
Bennewitz H. De Diabete Mellito, Gravidatatis Symptomate . MD thesis, University of Berlin ; 1824 .
Google Scholar
Google Preview
Barker DJP. Mothers Babies and Diseases in Later Life. BMJ ; 1994 .
Duncan J . On puerperal diabetes . Trans Obstet Soc Lond. 1882 ; 24 : 256-285 .
Miller HC . The effect of diabetic and prediabetic pregnancies on the fetus and newborn infant . J Pediatr. 1946 ; 29 ( 4 ): 455 - 461 .
Williams J . The clinical significance of glycosuria in pregnant women . Am J Med Sci. 1909 ; 137 : 1 - 26 .
O’Sullivan JB , Mahan CM . Criteria for the oral glucose tolerance test in pregnancy . Diabetes. 1964 ; 13 : 278 - 285 .
Coustan DR . Gestational diabetes. In: Harris MI , Cowie CC , Stern MP , Boyko EJ , Reiber GE , Bennett PH , eds. Diabetes in America . National Institute of Health ; 1995 : 703 - 717 .
World Health Organization . Technical Report Series. No 310. Diabetes Mellitus . Report of a WHO Expert Committee. 1965 .
World Health Organization . Technical Report Series. No 646. Second Report on Diabetes Mellitus . Report of a WHO Expert Committee. 1980 .
World Health Organization . Technical Report Series. No 727. Second Report on Diabetes Mellitus . Report of a WHO Expert Committee. 1985 .
World Health Organisation . Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization guideline . Diabetes Res Clin Pract. 2014 ; 103 ( 3 ): 341 - 363 .
O’Sullivan JB , Mahan CM , Charles D , Dandrow RV . Screening criteria for high-risk gestational diabetic patients . Am J Obstet Gynecol. 1973 ; 116 ( 7 ): 895 - 900 .
National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance . Diabetes. 1979 ; 28 ( 12 ): 1039 - 1057 .
American College of Obstetricians and Gynecologists . Management of Diabetes Mellitus During Pregnancy . Technical Bulletin No. 92. 1986 .
American Diabetes Association . Gestational diabetes mellitus . Diabetes Care. 1986 ; 9 ( 4 ): 430 - 431 .
Ferrara A , Hedderson MM , Quesenberry CP , Selby JV . Prevalence of gestational diabetes mellitus detected by the National Diabetes Data Group or the Carpenter and Coustan plasma glucose thresholds . Diabetes Care. 2002 ; 25 ( 9 ): 1625 - 1630 .
Carpenter MW , Coustan DR . Criteria for screening tests for gestational diabetes . Am J Obstet Gynecol. 1982 ; 144 ( 7 ): 768 - 773 .
American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018 . Diabetes Care. 2018 ; 41 ( suppl 1 ): S13 - S27 .
American College of Obstetricians and Gynecologists . Practice Bulletin No. 190: gestational diabetes mellitus . Obstet Gynecol. 2018 ; 131 ( 2 ): e49 - e64 .
American Diabetes Association . Gestational diabetes mellitus . Diabetes Care. 2000 ; 23 ( suppl 1 ): S77 - S79 .
Sermer M , Naylor CD , Farine D , et al. The Toronto tri-hospital gestational diabetes project: a preliminary review . Diabetes Care. 1998 ; 21 ( Suppl 2 ): B33 - B42 .
Sermer M , Naylor CD , Gare DJ , et al. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes: the Toronto Tri-Hospital Gestational Diabetes Project . Am J Obstet Gynecol. 1995 ; 173 ( 1 ): 146 - 156 .
Berggren EK , Boggess KA , Stuebe AM , Jonsson Funk M . National Diabetes Data Group vs Carpenter-Coustan criteria to diagnose gestational diabetes . Am J Obstet Gynecol. 2011 ; 205 ( 3 ): 253 e1 - 253.e7 .
Cheng YW , Block-Kurbisch I , Caughey AB . Carpenter-Coustan criteria compared with the National Diabetes Data Group thresholds for gestational diabetes mellitus . Obstet Gynecol. 2009 ; 114 ( 2 Pt 1 ): 326 - 332 .
Chou CY , Lin CL , Yang CK , et al. Pregnancy outcomes of Taiwanese women with gestational diabetes mellitus: a comparison of Carpenter-Coustan and National Diabetes Data Group criteria . J Womens Health (Larchmt). 2010 ; 19 ( 5 ): 935 - 939 .
American Diabetes Association . Gestational diabetes mellitus . Diabetes Care. 2003 ; 26 ( suppl 1 ): S103 - S105 .
Metzger BE , Lowe LP , Dyer AR , et al. ; HAPO Study Cooperative Group. Hyperglycemia and adverse pregnancy outcomes . N Engl J Med. 2008 ; 358 ( 19 ): 1991 - 2002 .
Crowther CA , Hiller JE , Moss JR , et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes . N Engl J Med. 2005 ; 352 ( 24 ): 2477 - 2486 .
Landon MB , Spong CY , Thom E , et al. A multicenter, randomized trial of treatment for mild gestational diabetes . N Engl J Med. 2009 ; 361 ( 14 ): 1339 - 1348 .
Metzger BE , Gabbe SG , Persson B , et al. ; International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy . Diabetes Care. 2010 ; 33 ( 3 ): 676 - 682 .
Blumer I , Hadar E , Hadden DR , et al. Diabetes and pregnancy: an Endocrine Society clinical practice guideline . J Clin Endocrinol Metab. 2013 ; 98 ( 11 ): 4227 - 4249 .
Hod M , Kapur A , Sacks DA , et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care . Int J Gynaecol Obstet. 2015 ; 131 ( suppl 3 ): S173 - S211 .
Nankervis A , McIntyre HD , Moses R , et al. ADIPS Consensus Guidelines for the Testing and Diagnosis of Hyperglycaemia in Pregnancy in Australia and New Zealand. Modified November 2014 . http://adips.org/downloads/2014ADIPSGDMGuidelinesV18.11.2014_000.pdf . Accessed June 1, 2021.
Japan Diabetes Society . Evidence-based practice guideline for the treatment for diabetes in Japan 2013 . Last updated November 26, 2020. http://www.jds.or.jp/modules/en/index.php?content_id=44 . Accessed June 1, 2021.
Yang HX . Diagnostic criteria for gestational diabetes mellitus . Chin Med J. 2012 ; 125 ( 7 ): 1212 - 1213 .
Benhalima K , Mathieu C , Damm P , et al. A proposal for the use of uniform diagnostic criteria for gestational diabetes in Europe: an opinion paper by the European Board & College of Obstetrics and Gynaecology (EBCOG) . Diabetologia. 2015 ; 58 ( 7 ): 1422 - 1429 .
Vandorsten JP , Dodson WC , Espeland MA , et al. NIH consensus development conference: diagnosing gestational diabetes mellitus . NIH Consens State Sci Statements. 2013 ; 29 ( 1 ): 1 - 31 .
National Institute for Health and Care Excellence . Diabetes in Pregnancy: Management of Diabetes and its Complications from Pre-conception to the Postnatal Period . NICE Clinical Guideline NG3. 2015 .
Vambergue A . Expert consensus on gestational diabetes mellitus . Diabetes Metab. 2010 ; 36 ( 6 Pt 2 ): 511 .
Associazione Medici Diabetologi and Società Italiana di Diabetologia. Italian National Health System Guidelines for the screening of gestational diabetes mellitus . May 28, 2014. http://www.standarditaliani.it/skin/www.standarditaliani.it/pdf/STANDARD_2014_May28.pdf . Accessed June 1, 2021.
American Diabetes Association . 14. Management of diabetes in pregnancy: standards of medical care in diabetes-2020 . Diabetes Care. 2020 ; 43 ( suppl 1 ): S183 - SS92 .
Canadian Diabetes Association Clinical Practice Guidelines Expert Committee . Clinical practice guidelines for the prevention and management of diabetes in Canada . Canadian J Diabetes. 2013 ; 37(suppl 1):S1-S3 .
Kleinwechter H , Schafer-Graf U , Buhrer C , et al. Gestational diabetes mellitus (GDM) diagnosis, therapy and follow-up care: practice Guideline of the German Diabetes Association (DDG) and the German Association for Gynaecology and Obstetrics (DGGG) . Exp Clin Endocrinol Diabetes. 2014 ; 122 ( 7 ): 395 - 405 .
Seshiah V , Das AK , Balaji V , et al. Gestational diabetes mellitus—guidelines . J Assoc Physicians India. 2006 ; 54 : 622 - 628 .
World Health Organization . Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications . Report of WHO Consultation. 1999 .
Coustan DR , Lowe LP , Metzger BE , et al. ; on behalf of the International Association of Diabetes in Pregnancy Study Groups. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus . Am J Obstet Gynecol. 2010 ; 202 ( 6 ): 654 e1 - 654 e6 .
McIntyre HD , Oats JJ , Kihara AB , et al. Update on diagnosis of hyperglycemia in pregnancy and gestational diabetes mellitus from FIGO’s Pregnancy & Non-Communicable Diseases Committee . Int J Gynaecol Obstet. 2021; 154 (2):189-194.
Menke A , Casagrande S , Geiss L , Cowie CC . Prevalence of and trends in diabetes among adults in the United States, 1988-2012 . JAMA. 2015 ; 314 ( 10 ): 1021 - 1029 .
Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020 . US Department of Health and Human Services ; 2020 .
Lieberman N , Kalter-Leibovici O , Hod M . Global adaptation of IADPSG recommendations: a national approach . Int J Gynaecol Obstet. 2011 ; 115 ( suppl 1 ): S45 - S47 .
Marseille E , Lohse N , Jiwani A , et al. The cost-effectiveness of gestational diabetes screening including prevention of type 2 diabetes: application of a new model in India and Israel . J Matern Fetal Neonatal Med. 2013 ; 26 ( 8 ): 802 - 810 .
Werner EF , Pettker CM , Zuckerwise L , et al. Screening for gestational diabetes mellitus: are the criteria proposed by the International Association of the Diabetes and Pregnancy Study Groups cost-effective? Diabetes Care . 2012 ; 35 ( 3 ): 529 - 535 .
Mission JF , Ohno MS , Cheng YW , Caughey AB . Gestational diabetes screening with the new IADPSG guidelines: a cost-effectiveness analysis . Am J Obstet Gynecol. 2012 ; 207 ( 4 ): 326 e1 - 326 e9 .
Farrar D , Simmonds M , Griffin S , et al. The identification and treatment of women with hyperglycaemia in pregnancy: an analysis of individual participant data, systematic reviews, meta-analyses and an economic evaluation . Health Technol Assess. 2016 ; 20 ( 86 ): 1 - 348 .
Jacklin PB , Maresh MJ , Patterson CC , et al. A cost-effectiveness comparison of the NICE 2015 and WHO 2013 diagnostic criteria for women with gestational diabetes with and without risk factors . BMJ Open. 2017 ; 7 ( 8 ): e016621 .
Cundy T , Ackermann E , Ryan EA . Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear . BMJ. 2014 ; 348 : g1567 .
O’Sullivan EP , Avalos G , O’Reilly M , et al. Atlantic Diabetes in Pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria . Diabetologia . 2011 ; 54 ( 7 ): 1670 -167 5 .
Lapolla A , Dalfra MG , Ragazzi E , et al. New International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommendations for diagnosing gestational diabetes compared with former criteria: a retrospective study on pregnancy outcome . Diabet Med. 2011 ; 28 ( 9 ): 1074 - 1077 .
Benhalima K , Hanssens M , Devlieger R , et al. Analysis of pregnancy outcomes using the new IADPSG recommendation compared with the Carpenter and Coustan criteria in an area with a low prevalence of gestational diabetes . Int J Endocrinol. 2013 ; 2013 : 248121 .
Hung TH , Hsieh TT . The effects of implementing the International Association of Diabetes and Pregnancy Study Groups criteria for diagnosing gestational diabetes on maternal and neonatal outcomes . PLoS One. 2015 ; 10 ( 3 ): e0122261 .
Meek CL , Lewis HB , Patient C , et al. Diagnosis of gestational diabetes mellitus: falling through the net . Diabetologia. 2015 ; 58 ( 9 ): 2003 - 2012 .
Djelmis J , Pavic M , Mulliqi Kotori V , et al. Prevalence of gestational diabetes mellitus according to IADPSG and NICE criteria . Int J Gynaecol Obstet. 2016 ; 135 ( 3 ): 250 - 254 .
Ethridge JK Jr , Catalano PM , Waters TP . Perinatal outcomes associated with the diagnosis of gestational diabetes made by the international association of the diabetes and pregnancy study groups criteria . Obstet Gynecol. 2014 ; 124 ( 3 ): 571 - 578 .
Hillier TA , Pedula KL , Ogasawara KK , et al. A pragmatic, randomized clinical trial of gestational diabetes screening . N Engl J Med. 2021 ; 384 ( 10 ): 895 - 904 .
Casey B . Gestational diabetes—on broadening the diagnosis . N Engl J Med. 2021 ; 384 ( 10 ): 965 - 966 .
Dunne F , Lindsay R , Loeken M . This is the decade to find the solution for gestational diabetes mellitus screening and treatments . Diabet Med. 2021 ; 38 ( 8 ): e14602 .
O’Malley EG , Reynolds CME , O’Kelly R , et al. The diagnosis of gestational diabetes mellitus (GDM) using a 75 g oral glucose tolerance test: a prospective observational study . Diabetes Res Clin Pract . 2020 ; 163 : 108144 .
van Leeuwen M , Louwerse MD , Opmeer BC , et al. Glucose challenge test for detecting gestational diabetes mellitus: a systematic review . BJOG. 2012 ; 119 ( 4 ): 393 - 401 .
Sacks DA , Hadden DR , Maresh M , et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study . Diabetes Care. 2012 ; 35 ( 3 ): 526 - 528 .
Roeckner JT , Sanchez-Ramos L , Jijon-Knupp R , et al. Single abnormal value on 3-hour oral glucose tolerance test during pregnancy is associated with adverse maternal and neonatal outcomes: a systematic review and metaanalysis . Am J Obstet Gynecol. 2016 ; 215 ( 3 ): 287 - 297 .
Freinkel N , Metzger BE , Phelps RL , et al. Gestational diabetes mellitus: heterogeneity of maternal age, weight, insulin secretion, HLA antigens, and islet cell antibodies and the impact of maternal metabolism on pancreatic B-cell and somatic development in the offspring . Diabetes. 1985 ; 34 ( suppl 2 ): 1 - 7 .
Schaefer UM , Songster G , Xiang A , et al. Congenital malformations in offspring of women with hyperglycemia first detected during pregnancy . Am J Obstet Gynecol. 1997 ; 177 ( 5 ): 1165 - 1171 .
Omori Y , Jovanovic L . Proposal for the reconsideration of the definition of gestational diabetes . Diabetes Care. 2005 ; 28 ( 10 ): 2592 - 2593 .
Stacey T , Tennant P , McCowan L , et al. Gestational diabetes and the risk of late stillbirth: a case-control study from England, UK . BJOG. 2019 ; 126 ( 8 ): 973 - 982 .
Cosson E , Bentounes SA , Nachtergaele C , et al. Prognosis associated with sub-types of hyperglycaemia in pregnancy . J Clin Med. 2021 ; 10 ( 17 ): 3904 .
Wong T , Ross GP , Jalaludin BB , Flack JR . The clinical significance of overt diabetes in pregnancy . Diabet Med. 2013 ; 30 ( 4 ): 468 - 474 .
Bartha JL , Martinez-Del-Fresno P , Comino-Delgado R . Early diagnosis of gestational diabetes mellitus and prevention of diabetes-related complications . Eur J Obstet Gynecol Reprod Biol. 2003 ; 109 ( 1 ): 41 - 44 .
Bartha JL , Martinez-Del-Fresno P , Comino-Delgado R . Gestational diabetes mellitus diagnosed during early pregnancy . Am J Obstet Gynecol. 2000 ; 182 ( 2 ): 346 - 350 .
Berkowitz GS , Roman SH , Lapinski RH , et al. Maternal characteristics, neonatal outcome, and the time of diagnosis of gestational diabetes . Am J Obstet Gynecol. 1992 ; 167 ( 4 Pt 1 ): 976 - 8282 .
Meyer WJ , Carbone J , Gauthier DW , et al. Early gestational glucose screening and gestational diabetes . J Reprod Med. 1996 ; 41 ( 9 ): 675 - 679 .
Sweeting AN , Ross GP , Hyett J , et al. Gestational diabetes mellitus in early pregnancy: evidence for poor pregnancy outcomes despite treatment . Diabetes Care. 2016 ; 39 ( 1 ): 75 - 81 .
Li M , Hinkle SN , Grantz KL , et al. Glycaemic status during pregnancy and longitudinal measures of fetal growth in a multi-racial US population: a prospective cohort study . Lancet Diabetes Endocrinol. 2020 ; 8 ( 4 ): 292 - 300 .
Sovio U , Murphy HR , Smith GC . Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women . Diabetes Care. 2016 ; 39 ( 6 ): 982 - 987 .
Venkataraman H , Ram U , Craik S , et al. Increased fetal adiposity prior to diagnosis of gestational diabetes in South Asians: more evidence for the “thin-fat” baby . Diabetologia. 2017 ; 60 ( 3 ): 399 - 405 .
Riskin-Mashiah S , Damti A , Younes G , et al. Normal fasting plasma glucose levels during pregnancy: a hospital-based study . J Perinat Med. 2011 ; 39 ( 2 ): 209 - 211 .
Mills JL , Jovanovic L , Knopp R , et al. Physiological reduction in fasting plasma glucose concentration in the first trimester of normal pregnancy: the diabetes in early pregnancy study . Metabolism. 1998 ; 47 ( 9 ): 1140 - 1144 .
Corrado F , D’Anna R , Cannata ML , et al. Correspondence between first-trimester fasting glycaemia, and oral glucose tolerance test in gestational diabetes diagnosis . Diabetes Metab . 2012 ; 38 ( 5 ): 458 - 61 .
Zhu WW , Yang HX , Wei YM , et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china . Diabetes Care. 2013 ; 36 ( 3 ): 586 - 590 .
McIntyre HD , Sacks DA , Barbour LA , et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy . Diabetes Care. 2016 ; 39 ( 1 ): 53 - 54 .
Hughes RC , Moore MP , Gullam JE , et al. An early pregnancy HbA1c ≤5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes . Diabetes Care. 2014 ; 37 ( 11 ): 2953 - 2959 .
Sweeting AN , Ross GP , Hyett J , et al. Baseline HbA1c to identify high-risk gestational diabetes: utility in early vs standard gestational diabetes . J Clin Endocrinol Metab. 2017 ; 102 ( 1 ): 150 - 156 .
Immanuel J , Simmons D , Desoye G , et al. Performance of early pregnancy HbA1c for predicting gestational diabetes mellitus and adverse pregnancy outcomes in obese European women . Diabetes Res Clin Pract. 2020 ; 168 : 108378 .
Osmundson SS , Norton ME , El-Sayed YY , et al. Early screening and treatment of women with prediabetes: a randomized controlled trial . Am J Perinatol. 2016 ; 33 ( 2 ): 172 - 179 .
Roeder HA , Moore TR , Wolfson T , Ramos GA . Treating hyperglycemia in the first trimester: a randomized controlled trial . Am J Obstet Gynecol MFM. 2017 ; 1 ( 1 ): 33 - 41 .
Hawkins JS , Lo JY , Casey BM , et al. Diet-treated gestational diabetes mellitus: comparison of early vs routine diagnosis . Am J Obstet Gynecol. 2008 ; 198 ( 3 ): 287 e1 - 287 e6 .
Most OL , Kim JH , Arslan AA , et al. Maternal and neonatal outcomes in early glucose tolerance testing in an obstetric population in New York city . J Perinat Med. 2009 ; 37 ( 2 ): 114 - 117 .
Gupta S , Dolin C , Jadhav A , et al. Obstetrical outcomes in patients with early onset gestational diabetes . J Matern Fetal Neonatal Med. 2016 ; 29 ( 1 ): 27 - 31 .
Harreiter J , Simmons D , Desoye G , et al. IADPSG and WHO 2013 gestational diabetes mellitus criteria identify obese women with marked insulin resistance in early pregnancy . Diabetes Care. 2016 ; 39 ( 7 ): e90 - e92 .
Egan AM , Vellinga A , Harreiter J , et al. Epidemiology of gestational diabetes mellitus according to IADPSG/WHO 2013 criteria among obese pregnant women in Europe . Diabetologia. 2017 ; 60 ( 10 ): 1913 - 1921 .
Immanuel J , Simmons D . Screening and treatment for early-onset gestational diabetes mellitus: a systematic review and meta-analysis . Curr Diab Rep. 2017 ; 17 ( 11 ): 115 .
Bozkurt L , Gobl CS , Pfligl L , et al. Pathophysiological characteristics and effects of obesity in women with early and late manifestation of gestational diabetes diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria . J Clin Endocrinol Metab. 2015 ; 100 ( 3 ): 1113 - 1120 .
Bozkurt L , Gobl CS , Hormayer AT , et al. The impact of preconceptional obesity on trajectories of maternal lipids during gestation . Sci Rep. 2016 ; 6 : 29971 .
Sweeting AN , Ross GP , Hyett J , et al. Gestational diabetes in the first trimester: is early testing justified? Lancet Diabetes Endocrinol. 2017 ; 5 ( 8 ): 571 - 573 .
Harper LM , Jauk V , Longo S , et al. Early gestational diabetes screening in obese women: a randomized controlled trial . Am J Obstet Gynecol. 2020 ; 222 ( 5 ): 495 e1 - 495 e8 .
Vinter CA , Tanvig MH , Christensen MH , et al. Lifestyle intervention in Danish obese pregnant women with early gestational diabetes mellitus according to WHO 2013 criteria does not change pregnancy outcomes: results from the LiP (Lifestyle in Pregnancy) study . Diabetes Care. 2018 ; 41 ( 10 ): 2079 - 2085 .
Simmons D , Hague WM , Teede HJ , et al. Hyperglycaemia in early pregnancy: the Treatment of Booking Gestational Diabetes Mellitus (TOBOGM) study: a randomised controlled trial . Med J Aust. 2018 ; 209 ( 9 ): 405 - 406 .
Bruns DE , Metzger BE , Sacks DB . Diagnosis of gestational diabetes mellitus will be flawed until we can measure glucose . Clin Chem. 2020 ; 66 ( 2 ): 265 - 267 .
Bogdanet D , O’Shea P , Lyons C , et al. The oral glucose tolerance test-is it time for a change?-a literature review with an emphasis on pregnancy . J Clin Med. 2020 ; 9 ( 11):3451 .
Sacks DB , Arnold M , Bakris GL , et al. Position statement executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus . Diabetes Care. 2011 ; 34 ( 6 ): 1419 - 1423 .
Chan AY , Swaminathan R , Cockram CS . Effectiveness of sodium fluoride as a preservative of glucose in blood . Clin Chem. 1989 ; 35 ( 2 ): 315 - 317 .
Potter JM , Hickman PE , Oakman C , et al. Strict preanalytical oral glucose tolerance test blood sample handling is essential for diagnosing gestational diabetes mellitus . Diabetes Care. 2020 ; 43 ( 7 ): 1438 - 1441 .
Daly N , Flynn I , Carroll C , et al. Impact of implementing preanalytical laboratory standards on the diagnosis of gestational diabetes mellitus: a prospective observational study . Clin Chem. 2016 ; 62 ( 2 ): 387 - 391 .
International Diabetes Federation . IDF Diabetes Atlas . 9th ed. 2019 .
Guariguata L , Linnenkamp U , Beagley J , et al. Global estimates of the prevalence of hyperglycaemia in pregnancy . Diabetes Res Clin Pract. 2014 ; 103 ( 2 ): 176 - 185 .
Prentice PM , Olga L , Petry CJ , et al. Reduced size at birth and persisting reductions in adiposity in recent, compared with earlier, cohorts of infants born to mothers with gestational diabetes mellitus . Diabetologia. 2019 ; 62 ( 11 ): 1977 - 1987 .
Hartling L , Dryden DM . Screening and diagnosing gestational diabetes mellitus . Evid Rep Technol Assess (Full Rep). 2012 ; 210 : 1 - 327 .
Bottalico JN . Recurrent gestational diabetes: risk factors, diagnosis, management, and implications . Semin Perinatol. 2007 ; 31 ( 3 ): 176 - 184 .
Anna V , van der Ploeg H , P , Cheung NW , et al. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005 . Diabetes Care. 2008 ; 31 ( 12 ): 2288 - 2293 .
Chu SY , Callaghan WM , Kim SY , et al. Maternal obesity and risk of gestational diabetes mellitus . Diabetes Care. 2007 ; 30 ( 8 ): 2070 - 2076 .
Torloni MR , Betran AP , Horta BL , et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis . Obes Rev. 2009 ; 10 ( 2 ): 194 - 203 .
Mokdad AH , Ford ES , Bowman BA , et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003 ; 289 ( 1 ): 76 - 79 .
Mulla WR , Henry TQ , Homko CJ . Gestational diabetes screening after HAPO: has anything changed? Curr Diab Rep. 2010 ; 10 ( 3 ): 224 - 228 .
Petry CJ . Gestational diabetes: risk factors and recent advances in its genetics and treatment . Br J Nutr. 2010 ; 104 ( 6 ): 775 - 787 .
Hunt KJ , Schuller KL . The increasing prevalence of diabetes in pregnancy . Obstet Gynecol Clin North Am. 2007 ; 34 ( 2 ): 173 - 199 , vii.
Agarwal MM , Dhatt GS , Shah SM . Gestational diabetes mellitus: simplifying the international association of diabetes and pregnancy diagnostic algorithm using fasting plasma glucose . Diabetes Care. 2010 ; 33 ( 9 ): 2018 - 2020 .
Moses RG , Morris GJ , Petocz P , et al. The impact of potential new diagnostic criteria on the prevalence of gestational diabetes mellitus in Australia . Med J Aust. 2011 ; 194 ( 7 ): 338 - 340 .
Brown FM , Wyckoff J . Application of one-step IADPSG versus two-step diagnostic criteria for gestational diabetes in the real world: impact on health services, clinical care, and outcomes . Curr Diab Rep. 2017 ; 17 ( 10 ): 85 .
Kim C , Berger DK , Chamany S . Recurrence of gestational diabetes mellitus: a systematic review . Diabetes Care. 2007 ; 30 ( 5 ): 1314 - 1319 .
Solomon CG , Willett WC , Carey VJ , et al. A prospective study of pregravid determinants of gestational diabetes mellitus . JAMA. 1997 ; 278 ( 13 ): 1078 - 1083 .
Chamberlain C , McNamara B , Williams ED , et al. Diabetes in pregnancy among indigenous women in Australia, Canada, New Zealand and the United States . Diabetes Metab Res Rev. 2013 ; 29 ( 4 ): 241 - 256 .
Hedderson MM , Gunderson EP , Ferrara A . Gestational weight gain and risk of gestational diabetes mellitus . Obstet Gynecol. 2010 ; 115 ( 3 ): 597 - 604 .
Kjos SL , Buchanan TA . Gestational diabetes mellitus . N Engl J Med. 1999 ; 341 ( 23 ): 1749 - 1756 .
Di Cianni G , Volpe L , Lencioni C , et al. Prevalence and risk factors for gestational diabetes assessed by universal screening . Diabetes Res Clin Pract. 2003 ; 62 ( 2 ): 131 - 137 .
Cypryk K , Szymczak W , Czupryniak L , et al. Gestational diabetes mellitus—an analysis of risk factors . Endokrynol Pol. 2008 ; 59 ( 5 ): 393 - 397 .
Cleary-Goldman J , Malone FD , Vidaver J , et al. Impact of maternal age on obstetric outcome . Obstet Gynecol. 2005 ; 105 ( 5 Pt 1 ): 983 - 990 .
Yang H , Wei Y , Gao X , et al. Risk factors for gestational diabetes mellitus in Chinese women: a prospective study of 16,286 pregnant women in China . Diabet Med. 2009 ; 26 ( 11 ): 1099 - 1104 .
Morisset AS , St-Yves A , Veillette J , et al. Prevention of gestational diabetes mellitus: a review of studies on weight management . Diabetes Metab Res Rev. 2010 ; 26 ( 1 ): 17 - 25 .
Weiss JL , Malone FD , Emig D , et al. Obesity, obstetric complications and cesarean delivery rate—a population-based screening study . Am J Obstet Gynecol. 2004 ; 190 ( 4 ): 1091 - 1097 .
Gibson KS , Waters TP , Catalano PM . Maternal weight gain in women who develop gestational diabetes mellitus . Obstet Gynecol. 2012 ; 119 ( 3 ): 560 - 565 .
Morisset AS , Tchernof A , Dube MC , et al. Weight gain measures in women with gestational diabetes mellitus . J Womens Health (Larchmt). 2011 ; 20 ( 3 ): 375 - 380 .
Tovar A , Must A , Bermudez OI , et al. The impact of gestational weight gain and diet on abnormal glucose tolerance during pregnancy in Hispanic women . Matern Child Health J. 2009 ; 13 ( 4 ): 520 - 530 .
Teulings N , Masconi KL , Ozanne SE , et al. Effect of interpregnancy weight change on perinatal outcomes: systematic review and meta-analysis . BMC Pregnancy Childbirth. 2019 ; 19 ( 1 ): 386 .
Lo JC , Feigenbaum SL , Escobar GJ , et al. Increased prevalence of gestational diabetes mellitus among women with diagnosed polycystic ovary syndrome: a population-based study . Diabetes Care. 2006 ; 29 ( 8 ): 1915 - 1917 .
Mikola M , Hiilesmaa V , Halttunen M , et al. Obstetric outcome in women with polycystic ovarian syndrome . Hum Reprod. 2001 ; 16 ( 2 ): 226 - 229 .
Dinham GK , Henry A , Lowe SA , et al. Twin pregnancies complicated by gestational diabetes mellitus: a single centre cohort study . Diabet Med. 2016 ; 33 ( 12 ): 1659 - 1667 .
Rauh-Hain JA , Rana S , Tamez H , et al. Risk for developing gestational diabetes in women with twin pregnancies . J Matern Fetal Neonatal Med. 2009 ; 22 ( 4 ): 293 - 299 .
Chasan-Taber L , Schmidt MD , Pekow P , et al. Physical activity and gestational diabetes mellitus among Hispanic women . J Womens Health (Larchmt). 2008 ; 17 ( 6 ): 999 - 1008 .
Mottola MF . The role of exercise in the prevention and treatment of gestational diabetes mellitus . Curr Diab Rep. 2008 ; 8 ( 4 ): 299 - 304 .
Zhang C , Liu S , Solomon CG , et al. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus . Diabetes Care. 2006 ; 29 ( 10 ): 2223 - 2230 .
Kucukgoncu S , Guloksuz S , Celik K , et al. Antipsychotic exposure in pregnancy and the risk of gestational diabetes: a systematic review and meta-analysis . Schizophr Bull. 2020 ; 46 ( 2 ): 311 - 318 .
Galbally M , Frayne J , Watson SJ , et al. The association between gestational diabetes mellitus, antipsychotics and severe mental illness in pregnancy: a multicentre study . Aust N Z J Obstet Gynaecol. 2020 ; 60 ( 1 ): 63 - 69 .
Hedderson MM , Ferrara A . High blood pressure before and during early pregnancy is associated with an increased risk of gestational diabetes mellitus . Diabetes Care. 2008 ; 31 ( 12 ): 2362 - 2367 .
Lao TT , Ho LF . First-trimester blood pressure and gestational diabetes in high-risk Chinese women . J Soc Gynecol Investig. 2003 ; 10 ( 2 ): 94 - 98 .
Sweeting AN , Appelblom H , Ross GP , et al. First trimester prediction of gestational diabetes mellitus: a clinical model based on maternal demographic parameters . Diabetes Res Clin Pract. 2017 ; 127 : 44 - 50 .
Syngelaki A , Visser GH , Krithinakis K , et al. First trimester screening for gestational diabetes mellitus by maternal factors and markers of inflammation . Metabolism. 2016 ; 65 ( 3 ): 131 - 137 .
Nanda S , Savvidou M , Syngelaki A , et al. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks . Prenat Diagn. 2011 ; 31 ( 2 ): 135 - 141 .
van Leeuwen M , Opmeer BC , Zweers EJ , et al. Estimating the risk of gestational diabetes mellitus: a clinical prediction model based on patient characteristics and medical history . BJOG. 2010 ; 117 ( 1 ): 69 - 75 .
Naylor CD , Sermer M , Chen E , et al. ; Toronto Trihospital Gestational Diabetes Project Investigators. Selective screening for gestational diabetes mellitus . N Engl J Med. 1997 ; 337 ( 22 ): 1591 - 1596 .
Catalano PM . Trying to understand gestational diabetes . Diabet Med. 2014 ; 31 ( 3 ): 273 - 281 .
Catalano PM . Carbohydrate metabolism and gestational diabetes . Clin Obstet Gynecol. 1994 ; 37 ( 1 ): 25 - 38 .
Lain KY , Catalano PM . Metabolic changes in pregnancy . Clin Obstet Gynecol. 2007 ; 50 ( 4 ): 938 - 948 .
Costrini NV , Kalkhoff RK . Relative effects of pregnancy, estradiol, and progesterone on plasma insulin and pancreatic islet insulin secretion . J Clin Invest. 1971 ; 50 ( 5 ): 992 - 999 .
Kalkhoff RK , Kissebah AH , Kim HJ . Carbohydrate and lipid metabolism during normal pregnancy: relationship to gestational hormone action . Semin Perinatol. 1978 ; 2 ( 4 ): 291 - 307 .
Leturque A , Hauguel S , Sutter Dub MT , et al. Effects of placental lactogen and progesterone on insulin stimulated glucose metabolism in rat muscles in vitro . Diabete Metab. 1989 ; 15 ( 4 ): 176 - 181 .
Ryan EA , Enns L . Role of gestational hormones in the induction of insulin resistance . J Clin Endocrinol Metab. 1988 ; 67 ( 2 ): 341 - 347 .
Alvarez JJ , Montelongo A , Iglesias A , Lasuncion MA , Herrera E . Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women . J Lipid Res. 1996 ; 37 ( 2 ): 299 - 308 .
Barbour LA , McCurdy CE , Hernandez TL , Kirwan JP , Catalano PM , Friedman JE . Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes . Diabetes Care. 2007 ; 30 ( suppl 2 ): S112 - S119 .
Bomba-Opon D , Wielgos M , Szymanska M , et al. Effects of free fatty acids on the course of gestational diabetes mellitus . Neuro Endocrinol Lett. 2006 ; 27 ( 1-2 ): 277 - 280 .
Boden G , Chen X , Ruiz J , et al. Mechanisms of fatty acid-induced inhibition of glucose uptake . J Clin Invest. 1994 ; 93 ( 6 ): 2438 - 2446 .
Catalano PM , Huston L , Amini SB , et al. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus . Am J Obstet Gynecol. 1999 ; 180 ( 4 ): 903 - 916 .
Buchanan TA , Metzger BE , Freinkel N , et al. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes . Am J Obstet Gynecol. 1990 ; 162 ( 4 ): 1008 - 1014 .
Sivan E , Chen X , Homko CJ , et al. Longitudinal study of carbohydrate metabolism in healthy obese pregnant women . Diabetes Care. 1997 ; 20 ( 9 ): 1470 - 1475 .
Caro JF , Dohm LG , Pories WJ , et al. Cellular alterations in liver, skeletal muscle, and adipose tissue responsible for insulin resistance in obesity and type II diabetes . Diabetes Metab Rev. 1989 ; 5 ( 8 ): 665 - 689 .
Freinkel N , Metzger BE , Nitzan M , et al. Faciltated anabolism in late pregnancy: some novel maternal compensations for accelerated starvation. In: Malaise WJ , Pirart J , Vallence-Own J , eds. International Congress Series 312 . Excerpta Medica ; 1974 : 474 - 488 .
Yogev Y , Ben-Haroush A , Chen R , et al. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women . Am J Obstet Gynecol. 2004 ; 191 ( 3 ): 949 - 953 .
Hernandez TL , Friedman JE , Van Pelt RE , et al. Patterns of glycemia in normal pregnancy: should the current therapeutic targets be challenged? Diabetes Care. 2011 ; 34 ( 7 ): 1660 - 1668 .
Kuhl C . Etiology and pathogenesis of gestational diabetes . Diabetes Care. 1998 ; 21 ( suppl 2 ): B19 - B26 .
Parsons JA , Brelje TC , Sorenson RL . Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion . Endocrinology. 1992 ; 130 ( 3 ): 1459 - 1466 .
Sorenson RL , Brelje TC . Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones . Horm Metab Res. 1997 ; 29 ( 6 ): 301 - 307 .
Nielsen JH , Galsgaard ED , Moldrup A , et al. Regulation of beta-cell mass by hormones and growth factors . Diabetes. 2001 ; 50 ( suppl 1 ): S25 - S29 .
Parsons JA , Bartke A , Sorenson RL . Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones . Endocrinology. 1995 ; 136 ( 5 ): 2013 - 2021 .
Rieck S , White P , Schug J , et al. The transcriptional response of the islet to pregnancy in mice . Mol Endocrinol. 2009 ; 23 ( 10 ): 1702 - 1712 .
Buchanan TA , Kitzmiller JL . Metabolic interactions of diabetes and pregnancy . Annu Rev Med. 1994 ; 45 : 245 - 260 .
Rieck S , Kaestner KH . Expansion of beta-cell mass in response to pregnancy . Trends Endocrinol Metab. 2010 ; 21 ( 3 ): 151 - 158 .
Koukkou E , Watts GF , Lowy C . Serum lipid, lipoprotein and apolipoprotein changes in gestational diabetes mellitus: a cross-sectional and prospective study . J Clin Pathol. 1996 ; 49 ( 8 ): 634 - 637 .
Nolan CJ . Controversies in gestational diabetes . Best Pract Res Clin Obstet Gynaecol. 2011 ; 25 ( 1 ): 37 - 49 .
Talchai C , Xuan S , Lin HV , Sussel L , Accili D . Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure . Cell. 2012 ; 150 ( 6 ): 1223 - 1234 .
Halban PA , Polonsky KS , Bowden DW , et al. beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment . Diabetes Care. 2014 ; 37 ( 6 ): 1751 - 1758 .
Catalano PM , Tyzbir ED , Wolfe RR , et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes . Am J Physiol. 1993 ; 264 ( 1 Pt 1 ): E60 - E67 .
Homko C , Sivan E , Chen X , et al. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus . J Clin Endocrinol Metab. 2001 ; 86 ( 2 ): 568 - 573 .
Powe CE , Huston Presley LP , Locascio JJ , et al. Augmented insulin secretory response in early pregnancy . Diabetologia. 2019 ; 62 ( 8 ): 1445 - 1452 .
Dluski DF , Wolińska E , Skrzypczak M . Epigenetic changes in gestational diabetes mellitus . Int J Mol Sci. 2021 ; 22 ( 14 ):7649.
Elliott HR , Sharp GC , Relton CL , et al. Epigenetics and gestational diabetes: a review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction . Diabetologia. 2019 ; 62 ( 12 ): 2171 - 2178 .
Hayes MG , Urbanek M , Hivert MF , et al. Identification of HKDC1 and BACE2 as genes influencing glycemic traits during pregnancy through genome-wide association studies . Diabetes. 2013 ; 62 ( 9 ): 3282 - 3291 .
Liu S , Liu Y , Liao S . Heterogeneous impact of type 2 diabetes mellitus-related genetic variants on gestational glycemic traits: review and future research needs . Mol Genet Genomics. 2019 ; 294 ( 4 ): 811 - 847 .
Wu L , Cui L , Tam WH , et al. Genetic variants associated with gestational diabetes mellitus: a meta-analysis and subgroup analysis . Sci Rep. 2016 ; 6 : 30539 .
Chon SJ , Kim SY , Cho NR , et al. Association of variants in PPARgamma(2), IGF2BP2, and KCNQ1 with a susceptibility to gestational diabetes mellitus in a Korean population . Yonsei Med J. 2013 ; 54 ( 2 ): 352 - 357 .
Kim JY , Cheong HS , Park BL , et al. Melatonin receptor 1 B polymorphisms associated with the risk of gestational diabetes mellitus . BMC Med Genet. 2011 ; 12 : 82 .
Klein K , Haslinger P , Bancher-Todesca D , et al. Transcription factor 7-like 2 gene polymorphisms and gestational diabetes mellitus . J Matern Fetal Neonatal Med. 2012 ; 25 ( 9 ): 1783 - 1786 .
Alharbi KK , Khan IA , Abotalib Z , et al. Insulin receptor substrate-1 (IRS-1) Gly927Arg: correlation with gestational diabetes mellitus in Saudi women . Biomed Res Int. 2014 ; 2014 : 146495 .
Tok EC , Ertunc D , Bilgin O , et al. PPAR-gamma2 Pro12Ala polymorphism is associated with weight gain in women with gestational diabetes mellitus . Eur J Obstet Gynecol Reprod Biol. 2006 ; 129 ( 1 ): 25 - 30 .
Montazeri S , Nalliah S , Radhakrishnan AK . Is there a genetic variation association in the IL-10 and TNF alpha promoter gene with gestational diabetes mellitus? Hereditas. 2010 ; 147 ( 2 ): 94 - 102 .
Kwak SH , Kim SH , Cho YM , et al. A genome-wide association study of gestational diabetes mellitus in Korean women . Diabetes. 2012 ; 61 ( 2 ): 531 - 541 .
Lyssenko V , Nagorny CL , Erdos MR , et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion . Nat Genet. 2009 ; 41 ( 1 ): 82 - 88 .
Wellcome Trust Case Control Consortium . Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls . Nature. 2007 ; 447 ( 7145 ): 661 - 678 .
Bouatia-Naji N , Bonnefond A , Cavalcanti-Proenca C , et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk . Nat Genet. 2009 ; 41 ( 1 ): 89 - 94 .
Saxena R , Elbers CC , Guo Y , et al. Large-scale gene-centric meta-analysis across 39 studies identifies type 2 diabetes loci . Am J Hum Genet. 2012 ; 90 ( 3 ): 410 - 425 .
Manning AK , Hivert MF , Scott RA , et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance . Nat Genet. 2012 ; 44 ( 6 ): 659 - 669 .
Sparso T , Andersen G , Nielsen T , et al. The GCKR rs780094 polymorphism is associated with elevated fasting serum triacylglycerol, reduced fasting and OGTT-related insulinaemia, and reduced risk of type 2 diabetes . Diabetologia. 2008 ; 51 ( 1 ): 70 - 75 .
Vaxillaire M , Cavalcanti-Proenca C , Dechaume A , et al. The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population . Diabetes. 2008 ; 57 ( 8 ): 2253 - 2257 .
Agius L . Glucokinase and molecular aspects of liver glycogen metabolism . Biochem J. 2008 ; 414 ( 1 ): 1 - 18 .
Chasman DI , Pare G , Mora S , et al. Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis . PLoS Genet. 2009 ; 5 ( 11 ): e1000730 .
Seidah NG . The proprotein convertases, 20 years later . Methods Mol Biol. 2011 ; 768 : 23 - 57 .
Benzinou M , Creemers JW , Choquet H , et al. Common nonsynonymous variants in PCSK1 confer risk of obesity . Nat Genet. 2008 ; 40 ( 8 ): 943 - 945 .
Hayes A , Chevalier A , D’Souza M , et al. Early childhood obesity: association with healthcare expenditure in Australia . Obesity (Silver Spring) . 2016 ; 24 ( 8 ): 1752 - 8 .
Powe CE , Nodzenski M , Talbot O , et al. Genetic determinants of glycemic traits and the risk of gestational diabetes mellitus . Diabetes. 2018 ; 67 ( 12 ): 2703 - 2709 .
Fajans SS , Bell GI , Polonsky KS . Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young . N Engl J Med. 2001 ; 345 ( 13 ): 971 - 980 .
Hattersley AT , Patel KA . Precision diabetes: learning from monogenic diabetes . Diabetologia. 2017 ; 60 ( 5 ): 769 - 777 .
Dickens LT , Naylor RN . Clinical management of women with monogenic diabetes during pregnancy . Curr Diab Rep. 2018 ; 18 ( 3 ): 12 .
Chakera AJ , Steele AM , Gloyn AL , et al. Recognition and management of individuals with hyperglycemia because of a heterozygous glucokinase mutation . Diabetes Care. 2015 ; 38 ( 7 ): 1383 - 1392 .
Urbanova J , Brunerova L , Nunes M , et al. Identification of MODY among patients screened for gestational diabetes: a clinician’s guide . Arch Gynecol Obstet. 2020 ; 302 ( 2 ): 305 - 314 .
Wang Z , Ping F , Zhang Q , et al. Preliminary screening of mutations in the glucokinase gene of Chinese patients with gestational diabetes . J Diabetes Investig. 2018 ; 9 ( 1 ): 199 - 203 .
Gjesing AP , Rui G , Lauenborg J , et al. High prevalence of diabetes-predisposing variants in MODY genes among Danish women with gestational diabetes mellitus . J Endocr Soc. 2017 ; 1 ( 6 ): 681 - 690 .
Shields BM , Hicks S , Shepherd MH , et al. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010 ; 53 ( 12 ): 2504 - 2508 .
Spyer G , Hattersley AT , Sykes JE , et al. Influence of maternal and fetal glucokinase mutations in gestational diabetes . Am J Obstet Gynecol. 2001 ; 185 ( 1 ): 240 - 241 .
Hattersley AT , Beards F , Ballantyne E , et al. Mutations in the glucokinase gene of the fetus result in reduced birth weight . Nat Genet. 1998 ; 19 ( 3 ): 268 - 270 .
Scholtens DM , Kuang A , Lowe LP , et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal glycemia and childhood glucose metabolism . Diabetes Care. 2019 ; 42 ( 3 ): 381 - 392 .
Saravanan P ; Diabetes in Pregnancy Working Group . Gestational diabetes: opportunities for improving maternal and child health . Lancet Diabetes Endocrinol. 2020 ; 8 ( 9 ): 793 - 800 .
Pedersen J . Hyperglycaemia-hyperinsulinism theory and birthweight. In: The Pregnant Diabetic and Her Newborn: Problems and Management . Williams and Wilkins ; 1977 : 211 - 220 .
Illsley NP . Glucose transporters in the human placenta . Placenta. 2000 ; 21 ( 1 ): 14 - 22 .
Pedersen J . Weight and length at birth of infants of diabetic mothers . Acta Endocrinol (Copenh). 1954 ; 16 ( 4 ): 330 - 342 .
Whitelaw A . Subcutaneous fat in newborn infants of diabetic mothers: an indication of quality of diabetic control . Lancet. 1977 ; 1 ( 8001 ): 15 - 18 .
Vrijkotte TG , Krukziener N , Hutten BA , et al. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD study . J Clin Endocrinol Metab. 2012 ; 97 ( 11 ): 3917 - 3925 .
Yang X , Hsu-Hage B , Zhang H , et al. Women with impaired glucose tolerance during pregnancy have significantly poor pregnancy outcomes . Diabetes Care. 2002 ; 25 ( 9 ): 1619 - 1624 .
Spellacy WN , Miller S , Winegar A , et al. Macrosomia—maternal characteristics and infant complications . Obstet Gynecol. 1985 ; 66 ( 2 ): 158 - 161 .
Jang HC , Cho NH , Min YK , et al. Increased macrosomia and perinatal morbidity independent of maternal obesity and advanced age in Korean women with GDM . Diabetes Care. 1997 ; 20 ( 10 ): 1582 - 1588 .
Langer O , Yogev Y , Most O , et al. Gestational diabetes: the consequences of not treating . Am J Obstet Gynecol. 2005 ; 192 ( 4 ): 989 - 997 .
Weiss PA , Haeusler M , Tamussino K , et al. Can glucose tolerance test predict fetal hyperinsulinism? BJOG. 2000 ; 107 ( 12 ): 1480 - 1485 .
Boulet SL , Alexander GR , Salihu HM , et al. Macrosomic births in the united states: determinants, outcomes, and proposed grades of risk . Am J Obstet Gynecol. 2003 ; 188 ( 5 ): 1372 - 1378 .
Ryckman KK , Spracklen CN , Smith CJ , et al. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis . BJOG. 2015 ; 122 ( 5 ): 643 - 651 .
Jovanovic L , Pettitt DJ . Gestational diabetes mellitus . JAMA. 2001 ; 286 ( 20 ): 2516 - 2518 .
Esakoff TF , Cheng YW , Sparks TN , et al. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus . Am J Obstet Gynecol. 2009 ; 200 ( 6 ): 672 e1 - 672 e4 .
Henriksen T . The macrosomic fetus: a challenge in current obstetrics . Acta Obstet Gynecol Scand. 2008 ; 87 ( 2 ): 134 - 145 .
Reece EA , Leguizamon G , Wiznitzer A . Gestational diabetes: the need for a common ground . Lancet. 2009 ; 373 ( 9677 ): 1789 - 1797 .
Cetin H , Yalaz M , Akisu M , et al. Polycythaemia in infants of diabetic mothers: beta-hydroxybutyrate stimulates erythropoietic activity . J Int Med Res. 2011 ; 39 ( 3 ): 815 - 821 .
Farrar D , Fairley L , Santorelli G , et al. Association between hyperglycaemia and adverse perinatal outcomes in south Asian and white British women: analysis of data from the Born in Bradford cohort . Lancet Diabetes Endocrinol. 2015 ; 3 ( 10 ): 795 - 804 .
Balsells M , Corcoy R , Adelantado JM , et al. Gestational diabetes mellitus: metabolic control during labour . Diabetes Nutr Metab. 2000 ; 13 ( 5 ): 257 - 262 .
Farrar D , Simmonds M , Bryant M , et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis . BMJ. 2016 ; 354 : i4694 .
Billionnet C , Mitanchez D , Weill A , et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012 . Diabetologia. 2017 ; 60 ( 4 ): 636 - 644 .
Allen VM , Armson BA . SOGC Clinical Practice Guideline: teratogenicity associated with pre-existing and gestational diabetes . J Obstet Gynaecol Can. 2007 ; 29 ( 11 ): 927 - 934 .
Owens LA , O’Sullivan EP , Kirwan B , et al. ATLANTIC DIP: the impact of obesity on pregnancy outcome in glucose-tolerant women . Diabetes Care. 2010 ; 33 ( 3 ): 577 - 579 .
Dennedy MC , Avalos G , O’Reilly MW , et al. ATLANTIC-DIP: raised maternal body mass index (BMI) adversely affects maternal and fetal outcomes in glucose-tolerant women according to International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria . J Clin Endocrinol Metab. 2012 ; 97 ( 4 ): E608 - E612 .
Rosenstein MG , Cheng YW , Snowden JM , et al. The risk of stillbirth and infant death stratified by gestational age in women with gestational diabetes . Am J Obstet Gynecol. 2012 ; 206 ( 4 ): 309 e1 - 309 e7 .
Mitanchez D . Foetal and neonatal complications in gestational diabetes: perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications . Diabetes Metab. 2010 ; 36 ( 6 Pt 2 ): 617 - 627 .
Cundy T , Gamble G , Townend K , et al. Perinatal mortality in type 2 diabetes mellitus . Diabet Med. 2000 ; 17 ( 1 ): 33 - 39 .
Hutcheon JA , Kuret V , Joseph KS , et al. Immortal time bias in the study of stillbirth risk factors: the example of gestational diabetes . Epidemiology. 2013 ; 24 ( 6 ): 787 - 790 .
Poston L , Bell R , Croker H , et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial . Lancet Diabetes Endocrinol. 2015 ; 3 ( 10 ): 767 - 777 .
Tam WH , Ma RCW , Ozaki R , et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in Offspring . Diabetes Care. 2017 ; 40 ( 5 ): 679 - 686 .
Yu Y , Arah OA , Liew Z , et al. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up . BMJ. 2019 ; 367 : l6398 .
Hod M . The fetal heart in gestational diabetes: long-term effects . BJOG. 2021 ; 128 ( 2 ): 280 .
Dabelea D , Hanson RL , Lindsay RS , et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships . Diabetes. 2000 ; 49 ( 12 ): 2208 - 2211 .
Lowe WL Jr. , Lowe LP , Kuang A , et al. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study . Diabetologia. 2019 ; 62 ( 4 ): 598 - 610 .
Page KA , Luo S , Wang X , et al. Children exposed to maternal obesity or gestational diabetes mellitus during early fetal development have hypothalamic alterations that predict future weight gain . Diabetes Care. 2019 ; 42 ( 8 ): 1473 - 1480 .
Anderberg E , Kallen K , Berntorp K . The impact of gestational diabetes mellitus on pregnancy outcome comparing different cut-off criteria for abnormal glucose tolerance . Acta Obstet Gynecol Scand. 2010 ; 89 ( 12 ): 1532 - 1537 .
Ju H , Rumbold AR , Willson KJ , et al. Borderline gestational diabetes mellitus and pregnancy outcomes . BMC Pregnancy Childbirth. 2008 ; 8 : 31 .
Jastrow N , Roberge S , Gauthier RJ , et al. Effect of birth weight on adverse obstetric outcomes in vaginal birth after cesarean delivery . Obstet Gynecol. 2010 ; 115 ( 2 Pt 1 ): 338 - 343 .
Metzger BE , Coustan DR . Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee . Diabetes Care. 1998 ; 21 ( suppl 2 ): B161 - B167 .
Hiden U , Lassance L , Tabrizi NG , et al. Fetal insulin and IGF-II contribute to gestational diabetes mellitus (GDM)-associated up-regulation of membrane-type matrix metalloproteinase 1 (MT1-MMP) in the human feto-placental endothelium . J Clin Endocrinol Metab. 2012 ; 97 ( 10 ): 3613 - 3621 .
Dunne FP , Avalos G , Durkan M , et al. ATLANTIC DIP: pregnancy outcomes for women with type 1 and type 2 diabetes . Ir Med J. 2012 ; 105 ( 5 suppl ): 6 - 9 .
Roberts JM , Redman CW . Pre-eclampsia: more than pregnancy-induced hypertension . Lancet. 1993 ; 341 ( 8858 ): 1447 - 1451 .
Desoye G , Hauguel-de Mouzon S . The human placenta in gestational diabetes mellitus. The Insulin and Cytokine Network . Diabetes Care. 2007 ; 30 ( suppl 2 ): S120 - S126 .
Belkacemi L , Lash GE , Macdonald-Goodfellow SK , et al. Inhibition of human trophoblast invasiveness by high glucose concentrations . J Clin Endocrinol Metab. 2005 ; 90 ( 8 ): 4846 - 4851 .
Vounzoulaki E , Khunti K , Abner SC , et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis . BMJ. 2020 ; 369 : m1361 .
Daly B , Toulis KA , Thomas N , et al. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: a population-based cohort study. . PLoS Med. 2018 ; 15 ( 1 ): e1002488 .
Lowe WL Jr , Scholtens DM , Kuang A , et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism . Diabetes Care. 2019 ; 42 ( 3 ): 372 - 380 .
Murphy HR . 2020 NICE guideline update: good news for pregnant women with type 1 diabetes and past or current gestational diabetes . Diabet Med. 2021 ; 38 ( 6 ): e14576 .
National Institute for Health and Care Excellence . Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period . NICE Guidelines NG3. 2015 . Last updated December 16, 2020. https://www.nice.org.uk/guidance/ng3
Carr DB , Utzschneider KM , Hull RL , et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes . Diabetes Care. 2006 ; 29 ( 9 ): 2078 - 2083 .
Gunderson EP , Chiang V , Pletcher MJ , et al. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: the Coronary Artery Risk Development in Young Adults study . J Am Heart Assoc. 2014 ; 3 ( 2 ): e000490 .
Retnakaran R . Glucose tolerance status in pregnancy: a window to the future risk of diabetes and cardiovascular disease in young women . Curr Diabetes Rev. 2009 ; 5 ( 4 ): 239 - 244 .
Tobias DK , Hu FB , Forman JP , et al. Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study . Diabetes Care. 2011 ; 34 ( 7 ): 1582 - 1584 .
Tobias DK , Stuart JJ , Li S , et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US Women . JAMA Intern Med. 2017 ; 177 ( 12 ): 1735 - 1742 .
Brown HL , Warner JJ , Gianos E , et al. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American College of Obstetricians and Gynecologists . Circulation. 2018 ; 137 ( 24 ): e843 - ee52 .
Knudsen LS , Christensen IJ , Lottenburger T , et al. Pre-analytical and biological variability in circulating interleukin 6 in healthy subjects and patients with rheumatoid arthritis . Biomarkers. 2008 ; 13 ( 1 ): 59 - 78 .
Alwan N , Tuffnell DJ , West J . Treatments for gestational diabetes . Cochrane Database Syst Rev. 2009 ; 3 : CD003395 .
Jovanovic-Peterson L , Peterson CM , Reed GF , et al. Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. The National Institute of Child Health and Human Development—Diabetes in Early Pregnancy Study . Am J Obstet Gynecol. 1991 ; 164 ( 1 Pt 1 ): 103 - 111 .
Bain E , Crane M , Tieu J , et al. Diet and exercise interventions for preventing gestational diabetes mellitus . Cochrane Database Syst Rev. 0443 ; 2015 ; 4 : CD01 .
Duarte-Gardea MO , Gonzales-Pacheco DM , Reader DM , et al. Academy of nutrition and dietetics gestational diabetes evidence-based nutrition practice guideline . J Acad Nutr Diet. 2018 ; 118 ( 9 ): 1719 - 1742 .
Rasmussen KM , Yaktine AL , eds.; Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines . Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press ; 2009 .
Jovanovic-Peterson L , Peterson CM . Dietary manipulation as a primary treatment strategy for pregnancies complicated by diabetes . J Am Coll Nutr. 1990 ; 9 ( 4 ): 320 - 325 .
Hernandez TL , Van Pelt RE , Anderson MA , et al. A higher-complex carbohydrate diet in gestational diabetes mellitus achieves glucose targets and lowers postprandial lipids: a randomized crossover study . Diabetes Care. 2014 ; 37 ( 5 ): 1254 - 1262 .
Hernandez TL , Van Pelt RE , Anderson MA , et al. Women with gestational diabetes mellitus randomized to a higher-complex carbohydrate/low-fat diet manifest lower adipose tissue insulin resistance, inflammation, glucose, and free fatty acids: a pilot study . Diabetes Care. 2016 ; 39 ( 1 ): 39 - 42 .
Asemi Z , Tabassi Z , Samimi M , et al. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: a randomised clinical trial . Br J Nutr. 2013 ; 109 ( 11 ): 2024 - 2030 .
Han S , Middleton P , Shepherd E , et al. Different types of dietary advice for women with gestational diabetes mellitus . Cochrane Database Syst Rev. 2017 ; 2 : CD009275 .
Yamamoto JM , Kellett JE , Balsells M , et al. Gestational diabetes mellitus and diet: a systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight . Diabetes Care. 2018 ; 41 ( 7 ): 1346 - 1361 .
Major CA , Henry MJ , De Veciana M , et al. The effects of carbohydrate restriction in patients with diet-controlled gestational diabetes . Obstet Gynecol. 1998 ; 91 ( 4 ): 600 - 604 .
American College of Obstetrics and Gynecology . Practice Bulletin No. 137: gestational diabetes mellitus . Obstet Gynecol. 2013 ; 122 ( 2 Pt 1 ): 406 - 416 .
Metzger BE , Buchanan TA , Coustan DR , et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus . Diabetes Care. 2007 ; 30 ( suppl 2 ): S251 - S260 .
American Diabetes Association . Standards of medical care in diabetes-2011 . Diabetes Care. 2011 ; 34 ( suppl 1 ): S11 - S61 .
Battaglia FC , Meschia G. An Introduction to Fetal Physiology . Academic Press ; 1986 .
Harding JE , Johnston BM . Nutrition and fetal growth . Reprod Fertil Dev. 1995 ; 7 ( 3 ): 539 - 547 .
Sweeting A , Markovic TP , Mijatovic J , et al. The carbohydrate threshold in pregnancy and gestational diabetes: how low can we go? Nutrients. 2021 ; 13 ( 8 ): 2599 .
Morisaki N , Nagata C , Yasuo S , et al. Optimal protein intake during pregnancy for reducing the risk of fetal growth restriction: the Japan Environment and Children’s Study . Br J Nutr. 2018 ; 120 ( 12 ): 1432 - 1440 .
Rizzo T , Metzger BE , Burns WJ , et al. Correlations between antepartum maternal metabolism and intelligence of offspring . N Engl J Med. 1991 ; 325 ( 13 ): 911 - 916 .
Shaw GM , Yang W . Women’s periconceptional lowered carbohydrate intake and NTD-affected pregnancy risk in the era of prefortification with folic acid . Birth Defects Res. 2019 ; 111 ( 5 ): 248 - 253 .
Mijatovic J , Louie JCY , Buso MEC , et al. Effects of a modestly lower carbohydrate diet in gestational diabetes: a randomized controlled trial . Am J Clin Nutr. 2020 ; 112 ( 2 ): 284 - 292 .
Shubert PJ , Gordon MC , Landon MB , et al. Ketoacids attenuate glucose uptake in human trophoblasts isolated from first-trimester chorionic villi . Am J Obstet Gynecol. 1996 ; 175 ( 1 ): 56 - 62 .
Catalano PM , Mele L , Landon MB , et al. Inadequate weight gain in overweight and obese pregnant women: what is the effect on fetal growth? Am J Obstet Gynecol. 2014 ; 211 ( 2 ): 137 e1 - 137 e7 .
Faucher MA , Barger MK . Gestational weight gain in obese women by class of obesity and select maternal/newborn outcomes: a systematic review . Women Birth. 2015 ; 28 ( 3 ): e70 - e79 .
Viecceli C , Remonti LR , Hirakata VN , et al. Weight gain adequacy and pregnancy outcomes in gestational diabetes: a meta-analysis . Obes Rev. 2017 ; 18 ( 5 ): 567 - 580 .
Kainer F , Weiss PA , Huttner U , et al. Levels of amniotic fluid insulin and profiles of maternal blood glucose in pregnant women with diabetes type-I . Early Hum Dev. 1997 ; 49 ( 2 ): 97 - 105 .
de Veciana M , Major CA , Morgan MA , et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy . N Engl J Med. 1995 ; 333 ( 19 ): 1237 - 1241 .
Hernandez TL . Glycemic targets in pregnancies affected by diabetes: historical perspective and future directions . Curr Diab Rep. 2015 ; 15 ( 1 ): 565 .
Abell SK , Boyle JA , Earnest A , et al. Impact of different glycaemic treatment targets on pregnancy outcomes in gestational diabetes . Diabet Med. 2019 ; 36 ( 2 ): 177 - 183 .
Garner P , Okun N , Keely E , et al. A randomized controlled trial of strict glycemic control and tertiary level obstetric care versus routine obstetric care in the management of gestational diabetes: a pilot study . Am J Obstet Gynecol. 1997 ; 177 ( 1 ): 190 - 195 .
Langer O , Levy J , Brustman L , et al. Glycemic control in gestational diabetes mellitus--how tight is tight enough: small for gestational age versus large for gestational age? Am J Obstet Gynecol . 1989 ; 161 ( 3 ): 646 - 653 .
Langer O , Rodriguez DA , Xenakis EM , et al. Intensified versus conventional management of gestational diabetes . Am J Obstet Gynecol. 1994 ; 170 ( 4 ): 1036 - 1046 .
Pregnancy outcomes in the diabetes control and complications trial . Am J Obstet Gynecol. 1996 ; 174 ( 4 ): 1343 - 1353 .
Martis R , Brown J , Alsweiler J , et al. Different intensities of glycaemic control for women with gestational diabetes mellitus . Cochrane Database Syst Rev. 2016 ; 4 : CD011624 .
Snyder JMI , Melzter S , Nadeau J . Gestational diabetes and glycemic control: a randomized clinical trial . Am J Obstet Gynecol. 1998 ; 178 ( 1 Pt 2 ): S55 .
Pertot T , Molyneaux L , Tan K , et al. Can common clinical parameters be used to identify patients who will need insulin treatment in gestational diabetes mellitus? Diabetes Care. 2011 ; 34 ( 10 ): 2214 - 2216 .
Rowan JA , Hague WM , Gao W , et al. Metformin versus insulin for the treatment of gestational diabetes . N Engl J Med. 2008 ; 358 ( 19 ): 2003 - 2015 .
Murphy HR , Rayman G , Duffield K , et al. Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy . Diabetes Care. 2007 ; 30 ( 11 ): 2785 - 2791 .
Padmanabhan S , McLean M , Cheung NW . Falling insulin requirements are associated with adverse obstetric outcomes in women with preexisting diabetes . Diabetes Care. 2014 ; 37 ( 10 ): 2685 - 2692 .
Wong VW . Gestational diabetes mellitus in five ethnic groups: a comparison of their clinical characteristics . Diabet Med. 2012 ; 29 ( 3 ): 366 - 371 .
Barnes RA , Wong T , Ross GP , et al. A novel validated model for the prediction of insulin therapy initiation and adverse perinatal outcomes in women with gestational diabetes mellitus . Diabetologia. 2016 ; 59 ( 11 ): 2331 - 2338 .
Ryu RJ , Hays KE , Hebert MF . Gestational diabetes mellitus management with oral hypoglycemic agents . Semin Perinatol. 2014 ; 38 ( 8 ): 508 - 515 .
Camelo Castillo W , Boggess K , Sturmer T , et al. Trends in glyburide compared with insulin use for gestational diabetes treatment in the United States, 2000-2011 . Obstet Gynecol. 2014 ; 123 ( 6 ): 1177 - 1184 .
Langer O , Conway DL , Berkus MD , et al. A comparison of glyburide and insulin in women with gestational diabetes mellitus . N Engl J Med. 2000 ; 343 ( 16 ): 1134 - 1138 .
Kremer CJ , Duff P . Glyburide for the treatment of gestational diabetes . Am J Obstet Gynecol. 2004 ; 190 ( 5 ): 1438 - 1439 .
Camelo Castillo W , Boggess K , Sturmer T , et al. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes . JAMA Pediatr. 2015 ; 169 ( 5 ): 452 - 458 .
Schwartz RA , Rosenn B , Aleksa K , et al. Glyburide transport across the human placenta . Obstet Gynecol. 2015 ; 125 ( 3 ): 583 - 588 .
Cheng YW , Chung JH , Block-Kurbisch I , et al. Treatment of gestational diabetes mellitus: glyburide compared to subcutaneous insulin therapy and associated perinatal outcomes . J Matern Fetal Neonatal Med. 2012 ; 25 ( 4 ): 379 - 384 .
Lindsay RS , Loeken MR . Metformin use in pregnancy: promises and uncertainties . Diabetologia. 2017 ; 60 ( 9 ): 1612 - 1619 .
Charles B , Norris R , Xiao X , et al. Population pharmacokinetics of metformin in late pregnancy . Ther Drug Monit. 2006 ; 28 ( 1 ): 67 - 72 .
Tarry-Adkins JL , Aiken CE , Ozanne SE . Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: a systematic review and meta-analysis . PLoS Med. 2019 ; 16 ( 8 ): e1002848 .
Feig DS , Donovan LE , Zinman B , et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): a multicentre, international, randomised, placebo-controlled trial . Lancet Diabetes Endocrinol. 2020 ; 8 ( 10 ): 834 - 844 .
Niromanesh S , Alavi A , Sharbaf FR , et al. Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial . Diabetes Res Clin Pract. 2012 ; 98 ( 3 ): 422 - 429 .
Khin MO , Gates S , Saravanan P . Predictors of metformin failure in gestational diabetes mellitus (GDM) . Diabetes Metab Syndr. 2018 ; 12 ( 3 ): 405 - 410 .
Rowan JA , Rush EC , Obolonkin V , et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition at 2 years of age . Diabetes Care. 2011 ; 34 ( 10 ): 2279 - 2284 .
Rowan JA , Rush EC , Plank LD , et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age . BMJ Open Diabetes Res Care. 2018 ; 6 ( 1 ): e000456 .
Hanem LGE , Stridsklev S , Juliusson PB , et al. Metformin use in PCOS pregnancies increases the risk of offspring overweight at 4 years of age: follow-up of two RCTs . J Clin Endocrinol Metab. 2018 ; 103 ( 4 ): 1612 - 1621 .
Brown J , Martis R , Hughes B , et al. Oral anti-diabetic pharmacological therapies for the treatment of women with gestational diabetes . Cochrane Database Syst Rev. 2017 ; 1 : CD011967 .
Balsells M , Garcia-Patterson A , Sola I , et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis . BMJ. 2015 ; 350 : h102 .
Poolsup N , Suksomboon N , Amin M . Efficacy and safety of oral antidiabetic drugs in comparison to insulin in treating gestational diabetes mellitus: a meta-analysis . PLoS One. 2014 ; 9 ( 10 ): e109985 .
Nachum Z , Zafran N , Salim R , et al. Glyburide versus metformin and their combination for the treatment of gestational diabetes mellitus: a randomized controlled study . Diabetes Care. 2017 ; 40 ( 3 ): 332 - 337 .
Sacco F , Calderone A , Castagnoli L , et al. The cell-autonomous mechanisms underlying the activity of metformin as an anticancer drug . Br J Cancer. 2016 ; 115 ( 12 ): 1451 - 1456 .
Barbour LA , Davies JK . Comment on Nachum et al . Glyburide versus metformin and their combination for the treatment of gestational diabetes mellitus: a randomized controlled study. Diabetes Care. 2017;40:332-337. Diabetes Care. 2017; 40 (8):e115.
Rao U , de Vries B , Ross GP , et al. Fetal biometry for guiding the medical management of women with gestational diabetes mellitus for improving maternal and perinatal health . Cochrane Database Syst Rev. 2019 ; 9 : CD012544 .
Schaefer-Graf UM , Kjos SL , Fauzan OH , et al. A randomized trial evaluating a predominantly fetal growth-based strategy to guide management of gestational diabetes in Caucasian women . Diabetes Care. 2004 ; 27 ( 2 ): 297 - 302 .
Schaefer-Graf UM , Wendt L , Sacks DA , et al. How many sonograms are needed to reliably predict the absence of fetal overgrowth in gestational diabetes mellitus pregnancies? Diabetes Care. 2011 ; 34 ( 1 ): 39 - 43 .
Kjos SL , Schaefer-Graf UM . Modified therapy for gestational diabetes using high-risk and low-risk fetal abdominal circumference growth to select strict versus relaxed maternal glycemic targets . Diabetes Care. 2007 ; 30 ( suppl 2 ): S200 - S205 .
Nelson L , Wharton B , Grobman WA . Prediction of large for gestational age birth weights in diabetic mothers based on early third-trimester sonography . J Ultrasound Med. 2011 ; 30 ( 12 ): 1625 - 1628 .
McLean A , Katz M , Oats J , et al. Rethinking third trimester ultrasound measurements and risk of adverse neonatal outcomes in pregnancies complicated by hyperglycaemia: a retrospective study . Aust N Z J Obstet Gynaecol. 2021 ; 61 ( 3 ): 366 - 372 .
American College of Obstetricians and Gynecologists . ACOG Committee Opinion No. 560: medically indicated late-preterm and early-term deliveries . Obstet Gynecol. 2013 ; 121 ( 4 ): 908 - 910 .
Metcalfe A , Hutcheon JA , Sabr Y , et al. Timing of delivery in women with diabetes: a population-based study . Acta Obstet Gynecol Scand. 2020 ; 99 ( 3 ): 341 - 349 .
Hunt KJ , Logan SL , Conway DL , et al. Postpartum screening following GDM: how well are we doing? Curr Diab Rep. 2010 ; 10 ( 3 ): 235 - 241 .
Gabbe SG , Landon MB , Warren-Boulton E , et al. Promoting health after gestational diabetes: a National Diabetes Education Program call to action . Obstet Gynecol. 2012 ; 119 ( 1 ): 171 - 176 .
Ehrlich SF , Hedderson MM , Feng J , et al. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy . Obstet Gynecol. 2011 ; 117 ( 6 ): 1323 - 1330 .
Kim C , Newton KM , Knopp RH . Gestational diabetes and the incidence of type 2 diabetes: a systematic review . Diabetes Care. 2002 ; 25 ( 10 ): 1862 - 1868 .
Bellamy L , Casas JP , Hingorani AD , et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis . Lancet. 2009 ; 373 ( 9677 ): 1773 - 1779 .
Dornhorst A , Bailey PC , Anyaoku V , et al. Abnormalities of glucose tolerance following gestational diabetes . Q J Med. 1990 ; 77 ( 284 ): 1219 - 1228 .
Aroda VR , Christophi CA , Edelstein SL , et al. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up . J Clin Endocrinol Metab. 2015 ; 100 ( 4 ): 1646 - 1653 .
Pirc LK , Owens JA , Crowther CA , et al. Mild gestational diabetes in pregnancy and the adipoinsular axis in babies born to mothers in the ACHOIS randomised controlled trial . BMC Pediatr. 2007 ; 7 : 18 .
Landon MB , Rice MM , Varner MW , et al. Mild gestational diabetes mellitus and long-term child health . Diabetes Care. 2015 ; 38 ( 3 ): 445 - 452 .
Dabelea D , Pettitt DJ . Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility . J Pediatr Endocrinol Metab. 2001 ; 14 ( 8 ): 1085 - 1091 .
Pettitt DJ , Baird HR , Aleck KA , et al. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy . N Engl J Med. 1983 ; 308 ( 5 ): 242 - 245 .
Gillman MW , Rifas-Shiman S , Berkey CS , Field AE , Colditz GA . Maternal gestational diabetes, birth weight, and adolescent obesity . Pediatrics. 2003 ; 111 ( 3 ): e221 - e226 .
Plagemann A , Harder T , Kohlhoff R , et al. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes . Int J Obes Relat Metab Disord. 1997 ; 21 ( 6 ): 451 - 456 .
Pribylova H , Dvorakova L . Long-term prognosis of infants of diabetic mothers. Relationship between metabolic disorders in newborns and adult offspring . Acta Diabetol. 1996 ; 33 ( 1 ): 30 - 34 .
Silverman BL , Metzger BE , Cho NH , et al. Impaired glucose tolerance in adolescent offspring of diabetic mothers: relationship to fetal hyperinsulinism . Diabetes Care. 1995 ; 18 ( 5 ): 611 - 617 .
Crume TL , Ogden L , West NA , et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study . Diabetologia. 2011 ; 54 ( 1 ): 87 - 92 .
West NA , Crume TL , Maligie MA , et al. Cardiovascular risk factors in children exposed to maternal diabetes in utero . Diabetologia. 2011 ; 54 ( 3 ): 504 - 507 .
Hillier TA , Pedula KL , Schmidt MM , et al. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia . Diabetes Care. 2007 ; 30 ( 9 ): 2287 - 2292 .
Whincup PH , Kaye SJ , Owen CG , et al. Birth weight and risk of type 2 diabetes: a systematic review . JAMA. 2008 ; 300 ( 24 ): 2886 - 2897 .
Guerrero-Romero F , Aradillas-Garcia C , Simental-Mendia LE , et al. Birth weight, family history of diabetes, and metabolic syndrome in children and adolescents . J Pediatr. 2010 ; 156 ( 5 ): 719 - 723 , 23 e1.
Harder T , Roepke K , Diller N , et al. Birth weight, early weight gain, and subsequent risk of type 1 diabetes: systematic review and meta-analysis . Am J Epidemiol. 2009 ; 169 ( 12 ): 1428 - 1436 .
Reece EA . The fetal and maternal consequences of gestational diabetes mellitus . J Matern Fetal Neonatal Med. 2010 ; 23 ( 3 ): 199 - 203 .
Ornoy A . Prenatal origin of obesity and their complications: gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia . Reprod Toxicol. 2011 ; 32 ( 2 ): 205 - 212 .
Page KA , Romero A , Buchanan TA , et al. Gestational diabetes mellitus, maternal obesity, and adiposity in offspring . J Pediatr. 2014 ; 164 ( 4 ): 807 - 810 .
Grunnet LG , Hansen S , Hjort L , et al. Adiposity, dysmetabolic traits, and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: a clinical study within the Danish National Birth Cohort . Diabetes Care. 2017 ; 40 ( 12 ): 1746 - 1755 .
Logan KM , Emsley RJ , Jeffries S , et al. Development of early adiposity in infants of mothers with gestational diabetes mellitus . Diabetes Care. 2016 ; 39 ( 6 ): 1045 - 1051 .
Catalano PM , Farrell K , Thomas A , et al. Perinatal risk factors for childhood obesity and metabolic dysregulation . Am J Clin Nutr. 2009 ; 90 ( 5 ): 1303 - 1313 .
Chung WK , Erion K , Florez JC , et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) . Diabetes Care. 2020 ; 43 ( 7 ): 1617 - 1635 .
Cheney C , Shragg P , Hollingsworth D . Demonstration of heterogeneity in gestational diabetes by a 400-kcal breakfast meal tolerance test . Obstet Gynecol. 1985 ; 65 ( 1 ): 17 - 23 .
Powe CE , Allard C , Battista MC , et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus . Diabetes Care. 2016 ; 39 ( 6 ): 1052 - 1055 .
Catalano PM , Tyzbir ED , Roman NM , et al. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women . Am J Obstet Gynecol. 1991 ; 165 ( 6 Pt 1 ): 1667 - 1672 .
Powe CE , Hivert MF , Udler MS . Defining heterogeneity among women with gestational diabetes mellitus . Diabetes. 2020 ; 69 ( 10 ): 2064 - 2074 .
Benhalima K , Van Crombrugge P , Moyson C , et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance . Diabetologia. 2019 ; 62 ( 11 ): 2118 - 2128 .
Sweeting A , Park F , Hyett J . The first trimester: prediction and prevention of the great obstetrical syndromes . Best Pract Res Clin Obstet Gynaecol. 2015 ; 29 ( 2 ): 183 - 193 .
Coustan DR , Nelson C , Carpenter MW , et al. Maternal age and screening for gestational diabetes: a population-based study . Obstet Gynecol. 1989 ; 73 ( 4 ): 557 - 561 .
Lavin JP Jr . Screening of high-risk and general populations for gestational diabetes. Clinical application and cost analysis . Diabetes. 1985 ; 34 ( suppl 2 ): 24 - 27 .
Weeks JW , Major CA , de Veciana M , et al. Gestational diabetes: does the presence of risk factors influence perinatal outcome? Am J Obstet Gynecol. 1994 ; 171 ( 4 ): 1003 - 1007 .
Cosson E , Benbara A , Pharisien I , et al. Diagnostic and prognostic performances over 9 years of a selective screening strategy for gestational diabetes mellitus in a cohort of 18,775 subjects . Diabetes Care. 2013 ; 36 ( 3 ): 598 - 603 .
Chevalier N , Fenichel P , Giaume V , et al. Universal two-step screening strategy for gestational diabetes has weak relevance in French Mediterranean women: should we simplify the screening strategy for gestational diabetes in France? Diabetes Metab. 2011 ; 37 ( 5 ): 419 - 425 .
Moses RG , Moses J , Davis WS . Gestational diabetes: do lean young Caucasian women need to be tested? Diabetes Care. 1998 ; 21 ( 11 ): 1803 - 1806 .
Avalos GE , Owens LA , Dunne F , et al. Applying current screening tools for gestational diabetes mellitus to a European population: is it time for change? Diabetes Care. 2013 ; 36 ( 10 ): 3040 - 3044 .
Teede HJ , Harrison CL , Teh WT , et al. Gestational diabetes: development of an early risk prediction tool to facilitate opportunities for prevention . Aust N Z J Obstet Gynaecol. 2011 ; 51 ( 6 ): 499 - 504 .
Syngelaki A , Pastides A , Kotecha R , et al. First-trimester screening for gestational diabetes mellitus based on maternal characteristics and history . Fetal Diagn Ther. 2015 ; 38 ( 1 ): 14 - 21 .
Savvidou M , Nelson SM , Makgoba M , et al. First-trimester prediction of gestational diabetes mellitus: examining the potential of combining maternal characteristics and laboratory measures . Diabetes. 2010 ; 59 ( 12 ): 3017 - 3022 .
Lamain-de Ruiter M , Kwee A , Naaktgeboren CA , et al. Prediction models for the risk of gestational diabetes: a systematic review . Diagn Progn Res. 2017 ; 1 : 3 .
Sweeting AN , Wong J , Appelblom H , et al. A novel early pregnancy risk prediction model for gestational diabetes mellitus . Fetal Diagn Ther. 2019 ; 45 ( 2 ): 76 - 84 .
Sweeting AN , Wong J , Appelblom H , et al. A first trimester prediction model for gestational diabetes utilizing aneuploidy and pre-eclampsia screening markers . J Matern Fetal Neonatal Med. 2018 ; 31 ( 16 ): 2122 - 2130 .
Aronson JK , Ferner RE . Biomarkers-a general review . Curr Protoc Pharmacol. 2017 ; 76 : 9 23 19 17 .
Allinson JL . Clinical biomarker validation . Bioanalysis. 2018 ; 10 ( 12 ): 957 - 968 .
Sattar N , Wannamethee SG , Forouhi NG . Novel biochemical risk factors for type 2 diabetes: pathogenic insights or prediction possibilities? Diabetologia. 2008 ; 51 ( 6 ): 926 - 940 .
O’Malley EG , Reynolds CME , Killalea A , et al. The use of biomarkers at the end of the second trimester to predict Gestational Diabetes Mellitus . Eur J Obstet Gynecol Reprod Biol. 2020 ; 250 : 101 - 106 .
Richardson AC , Carpenter MW . Inflammatory mediators in gestational diabetes mellitus . Obstet Gynecol Clin North Am. 2007 ; 34 ( 2 ): 213 - 224 , viii.
Eleftheriades M , Papastefanou I , Lambrinoudaki I , et al. Elevated placental growth factor concentrations at 11-14 weeks of gestation to predict gestational diabetes mellitus . Metabolism. 2014 ; 63 ( 11 ): 1419 - 1425 .
Lovati E , Beneventi F , Simonetta M , et al. Gestational diabetes mellitus: including serum pregnancy-associated plasma protein-A testing in the clinical management of primiparous women? A case-control study . Diabetes Res Clin Pract. 2013 ; 100 ( 3 ): 340 - 347 .
White SL , Lawlor DA , Briley AL , et al. Early antenatal prediction of gestational diabetes in obese women: development of prediction tools for targeted intervention . PLoS One. 2016 ; 11 ( 12 ): e0167846 .
Rasanen JP , Snyder CK , Rao PV , et al. Glycosylated fibronectin as a first-trimester biomarker for prediction of gestational diabetes . Obstet Gynecol. 2013 ; 122 ( 3 ): 586 - 594 .
Watanabe N , Morimoto S , Fujiwara T , et al. Prediction of gestational diabetes mellitus by soluble (pro)renin receptor during the first trimester . J Clin Endocrinol Metab. 2013 ; 98 ( 6 ): 2528 - 2535 .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 05 October 2023
Precision gestational diabetes treatment: a systematic review and meta-analyses
- Jamie L. Benham 1 na1 ,
- Véronique Gingras 2 , 3 na1 ,
- Niamh-Maire McLennan 4 , 5 na1 ,
- Jasper Most ORCID: orcid.org/0000-0001-8591-5629 6 na1 ,
- Jennifer M. Yamamoto 7 na1 ,
- Catherine E. Aiken 8 , 9 na1 ,
- Susan E. Ozanne ORCID: orcid.org/0000-0001-8753-5144 10 na2 ,
- Rebecca M. Reynolds ORCID: orcid.org/0000-0001-6226-8270 4 , 5 na2 &
ADA/EASD PMDI
Communications Medicine volume 3 , Article number: 135 ( 2023 ) Cite this article
3403 Accesses
3 Citations
20 Altmetric
Metrics details
- Combination drug therapy
- Gestational diabetes
- Predictive markers
Gestational Diabetes Mellitus (GDM) affects approximately 1 in 7 pregnancies globally. It is associated with short- and long-term risks for both mother and baby. Therefore, optimizing treatment to effectively treat the condition has wide-ranging beneficial effects. However, despite the known heterogeneity in GDM, treatment guidelines and approaches are generally standardized. We hypothesized that a precision medicine approach could be a tool for risk-stratification of women to streamline successful GDM management. With the relatively short timeframe available to treat GDM, commencing effective therapy earlier, with more rapid normalization of hyperglycaemia, could have benefits for both mother and fetus.
We conducted two systematic reviews, to identify precision markers that may predict effective lifestyle and pharmacological interventions.
There was a paucity of studies examining precision lifestyle-based interventions for GDM highlighting the pressing need for further research in this area. We found a number of precision markers identified from routine clinical measures that may enable earlier identification of those requiring escalation of pharmacological therapy (to metformin, sulphonylureas or insulin). This included previous history of GDM, Body Mass Index and blood glucose concentrations at diagnosis.
Conclusions
Clinical measurements at diagnosis could potentially be used as precision markers in the treatment of GDM. Whether there are other sensitive markers that could be identified using more complex individual-level data, such as omics, and if these can feasibly be implemented in clinical practice remains unknown. These will be important to consider in future studies.
Plain language summary
Gestational diabetes (GDM) is high blood sugar first detected during pregnancy. Normalizing blood sugar levels quickly is important to avoid pregnancy complications. Many women achieve this with lifestyle changes, such as to diet, but some need to inject insulin or take tablets. We did two thorough reviews of existing research to see if we could predict which women need medication. Firstly we looked for ways to identify the characteristics of women who benefit most from changing their lifestyles to treat GDM, but found very limited research on this topic. We secondly searched for characteristics that help identify women who need medication to treat GDM. We found some useful characteristics that are obtained during routine pregnancy care. Further studies are needed to test if additional information could provide even better information about how we could make GDM treatment more tailored for individuals during pregnancy.
Similar content being viewed by others

Refining the diagnosis of gestational diabetes mellitus: a systematic review and meta-analysis
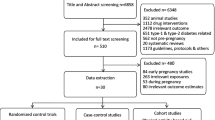
Effectiveness of pre-pregnancy lifestyle in preventing gestational diabetes mellitus—a systematic review and meta-analysis of 257,876 pregnancies
Gestational diabetes in Germany—prevalence, trend during the past decade and utilization of follow-up care: an observational study
Introduction.
Gestational diabetes (GDM) is the most common pregnancy complication, occurring in 3–25% of pregnancies globally 1 . GDM is associated with short- and long-term risks to both mothers and babies, including adverse perinatal outcomes, future obesity, type 2 diabetes and cardiovascular disease 1 , 2 , 3 . The landmark Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) demonstrated that effective treatment of GDM reduces serious perinatal morbidity 4 .
Current treatment guidelines for management of GDM assume homogeneous treatment requirements and responses, despite the known heterogeneity of GDM aetiology 5 , 6 , 7 , 8 . Standard care includes diet and lifestyle advice at a multi-disciplinary clinic, home blood glucose monitoring at least four times per day, clinic reviews every 2 to 4 weeks, and then progression to pharmacological treatment with metformin, glyburide and/or insulin if glucose targets are not met. Around a third of women cannot maintain euglycaemia with lifestyle measures alone and require treatment escalation to a pharmacological agent 3 . Yet current treatment pathways often take 4–8 weeks to achieve glucose targets. This delay resulting in continued exposure to hyperglycaemia poses a risk of accelerated foetal growth 9 , 10 . Previous research has suggested that maternal characteristics including body mass index (BMI) ≥ 30 kg/m 2 , family history of type 2 diabetes, prior history of GDM and higher glycated haemoglobin (HbA1c) increase the likelihood of need for insulin treatment in GDM 11 , indicating the potential for risk-stratification of women to streamline successful GDM management. There is emerging evidence that precision biomarkers predict treatment response in type 2 diabetes, which has similar heterogeneity to GDM 12 , 13 and thus gives rationale to investigate whether a similar precision approach could be successful in optimising outcomes in GDM.
To address this knowledge gap, we conducted two systematic reviews of the available evidence for precision markers of GDM treatment. We aimed to determine which patient-level characteristics are precision markers for predicting (i) responses to personalised diet and lifestyle interventions delivered in addition to standard of care (ii) requirement for escalation of treatment in women treated with diet and lifestyle alone, and in women receiving pharmacological agents for the treatment of GDM. For both reviews we considered whether the precision markers predicted achieving glucose targets, as well as maternal and neonatal outcomes. The Precision Medicine in Diabetes Initiative (PMDI) was established in 2018 by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes (EASD). The ADA/EASD PMDI includes global thought leaders in precision diabetes medicine who are working to address the burgeoning need for better diabetes prevention and care through precision medicine 14 . This systematic review is written on behalf of the ADA/EASD PMDI as part of a comprehensive evidence evaluation in support of the 2nd International Consensus Report on Precision Diabetes Medicine 15 .
We find a paucity of studies examining precision lifestyle-based interventions for GDM highlighting the pressing need for further research in this area. We find a number of precision markers identified from routine clinical measures that may enable earlier identification of those requiring escalation of pharmacological therapy (to metformin, sulphonylureas or insulin). These findings suggest that clinical measurements at diagnosis could potentially be used as precision markers in the treatment of GDM. Whether there are other sensitive markers that could be identified using more complex individual-level data, such as omics, and if these can feasibly be implemented in clinical practice remains unknown and will be important to consider in future studies.
The systematic reviews and meta-analyses were performed as outlined a priori in the registered protocols (PROSPERO registration IDs CRD42022299288 and CRD42022299402). The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines 16 were followed. Ethical approval was not required as these were secondary studies using published data.
Literature searches, search strategies and eligibility criteria
Search strategies for both reviews were developed based on relevant keywords in partnership with scientific librarians (see Supplementary Note 1 for full search strategies). We searched two databases (MEDLINE and EMBASE) for studies published from inception until January 1st, 2022. We also scanned the references of included manuscripts for inclusion as well as relevant reviews and meta-analyses published within the past two years for additional citations.
For both systematic reviews we included studies (randomised or non-randomised trials and observational studies) published in English and including women ≥16 years old with diagnosed GDM, as defined by the study authors. For the first systematic review (precision diet and lifestyle interventions), we included studies with any behavioural intervention using any approach (e.g., specific exercise, dietary interventions, motivational interviewing) that examined precision markers that could tailor a lifestyle intervention in a more precise way compared to a control group receiving standard care only. For the second systematic review (precision markers for escalation of pharmacological interventions to achieve glucose targets), we included studies investigating women with GDM that required escalation of pharmacological therapy (e.g., insulin, metformin, sulphonylurea) compared to women with GDM that achieved glucose targets with diet and lifestyle measures only, or women with GDM treated with oral agents that required progression to insulin to achieve glucose targets. For both reviews, we included any relevant reported outcomes; maternal (e.g., treatment adherence, hypertensive disorders of pregnancy, gestational weight gain, mode of birth), neonatal (e.g., birthweight, macrosomia, shoulder dystocia, preterm birth, neonatal hypoglycaemia, neonatal death), cost efficiency or acceptability. We excluded studies with a total sample size <50 participants to ensure sufficient data to interpret the effect of precision markers. We also excluded studies published before or during 2004, in order to consider studies with standard care similar to ACHOIS 4 .
Study selection and data extraction
The results of our two searches were imported separately into Covidence software (Veritas Health Innovation, Australia, available at www.covidence.org ) and duplicates were removed. Two reviewers independently reviewed identified studies. First, they screened titles and abstracts of all references identified from the initial search. In a second step, the full-text articles of potentially relevant publications were scrutinised in detail and inclusion criteria were applied to select eligible articles. Reason for exclusion at the full-text review stage was documented. Disagreement between reviewers was resolved through consensus by discussion with the group of authors.
Two reviewers independently extracted relevant information from each eligible study, using a pre-specified standardised extraction form. Any disagreement between reviewers was resolved as outlined above.
Data extracted included first author name, year of publication, country, study design, type and details of the intervention when applicable, number of cases/controls or cohort groups, total number of participants and diagnostic criteria used for GDM. Extracted data elements also included outcomes measures, size of the association (Odds Ratio (OR), Relative Risk (RR) or Hazard Ratio (HR)) with corresponding 95% Confidence Interval (CI) and factors adjusted for, confounding factors taken into consideration and methods used to control covariates. We prioritised adjusted values where both raw and adjusted data were available. Details of precision markers (mean (standard deviation) for continuous variables or N (%) for categorical variables) including BMI (pre-pregnancy or during pregnancy), ethnicity, age, smoking status, comorbidities, parity, glycaemic variables (e.g., oral glucose tolerance test (OGTT) diagnostic values, HbA1c), timing of GDM diagnosis, history of diabetes or of GDM, and season were also extracted.
Quality assessment (risk of bias and GRADE assessments)
We first assessed the quality and risk of bias of each individual study using the Joanna Briggs Institute (JBI) critical appraisal tools 17 . A Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach was then used to review the total evidence for each precision marker, and the quality of the included studies to assign a GRADE certainty to this body of evidence (high, moderate, low and/or very low) 18 . Quality assessment was performed in duplicate and conflicts were resolved through consensus.

Statistical analysis
Where possible, meta-analyses were conducted using random effects models for each precision marker available. The pooled effect size (mean difference for continuous outcomes and ORs for categorical outcomes) with the corresponding 95% CI was computed. The heterogeneity of the studies was quantified using I 2 statistics, where I 2 > 50% represents moderate and I 2 > 75% represents substantial heterogeneity across studies. Publication bias was assessed with visual assessment of funnel plots. Statistical analyses were performed using Review Manager software [RevMan, Version 5.4.1, The Cochrane Collaboration, Copenhagen, Denmark].
As part of the diabetes scientific community, we are sensitive in using inclusive language, especially in relation to gender. However, the vast majority of original studies that the GDM precision medicine working groups reviewed used women as their terminology to describe their population, as GDM per definition occurs in pregnancy which can only occur in individuals that are female at birth. To be consistent with the original studies defined populations, we use the word ‘women’ in our summary of the evidence, current gaps and future perspectives, but fully acknowledge that not all individuals who experienced a pregnancy may self-identify as women at all times over their life course.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Study selection and study characteristics
PRISMA flow charts (Figs. 1 and 2 ) summarise both searches and study selection processes.
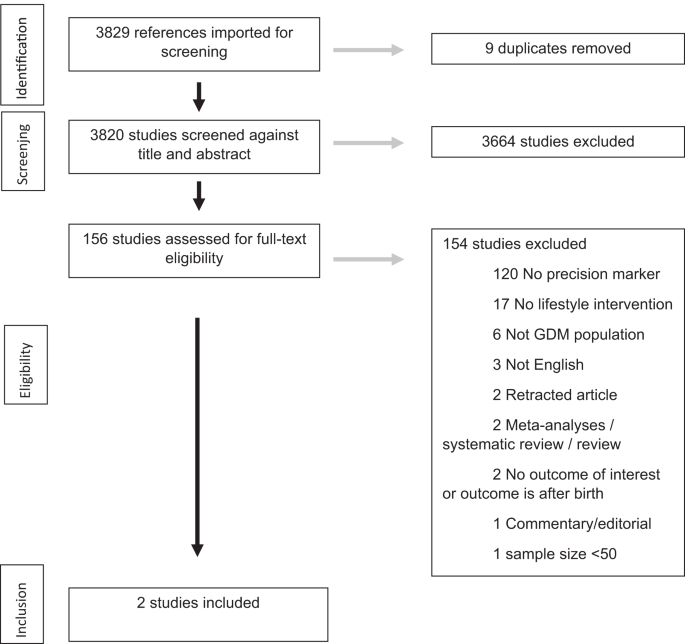
The PRISMA flow diagram details the search and selection process applied in the review.
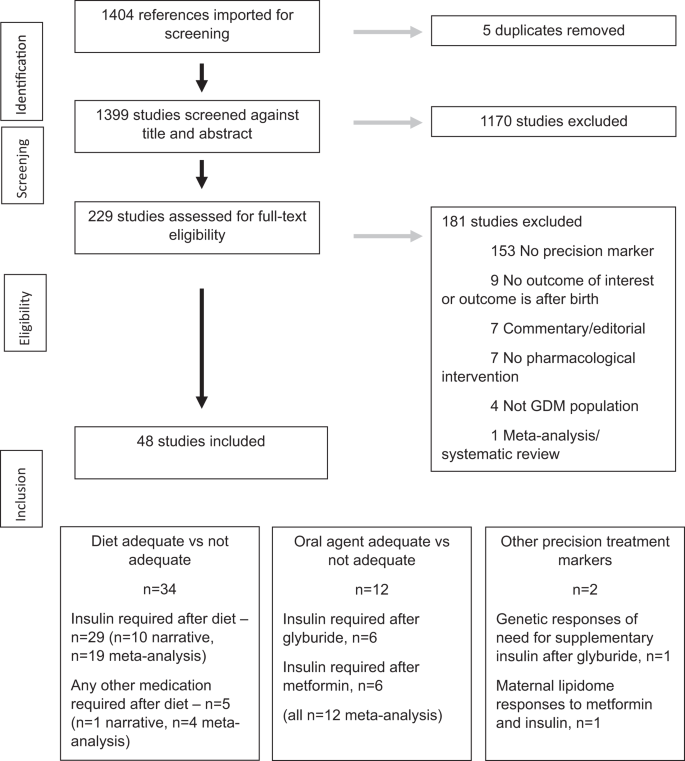
For the first systematic review (precision approaches to diet and lifestyle interventions), we identified 2 eligible studies ( n = 2354 participants), which were randomised trials from USA and Singapore (Supplementary Data 1 ) 19 , 20 .
For the second systematic review (precision markers for escalation of pharmacological interventions to achieve target glucose levels), we identified 48 eligible studies ( n = 25,724 participants) (Supplementary Data 2 ) 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 . There were 34 studies ( n = 23,831 participants) investigating precision markers for escalation to pharmacological agent(s) in addition to standard care with diet and lifestyle advice. Of these, 29 studies ( n = 20,486) reported escalation to insulin as the only option 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 and 5 ( n = 3345) reported escalation to any medication (metformin, glyburide and/or insulin) 50 , 51 , 52 , 53 , 54 . There were 12 studies ( n = 1836 participants) investigating precision markers for escalation to insulin when treatment with oral agents was not adequate to achieve target glucose levels. Initial treatment was with glyburide in 6 of these studies ( n = 527) 55 , 56 , 57 , 58 , 59 , 60 and metformin in the other 6 studies ( n = 1142) 61 , 62 , 63 , 64 , 65 , 66 . A further 2 eligible studies reported maternal genetic predictors of need for supplementary insulin after glyburide ( n = 117 participants) 67 and maternal lipidome responses to metformin and insulin ( n = 217 participants) 68 .
The majority of included studies were observational in design. Most studies reported outcomes of singleton pregnancies. The studies were from a range of geographical locations: Europe (Belgium, Finland, France, Italy, Netherlands, Poland, Portugal, Spain, Sweden), Switzerland, Middle East (Israel, Qatar, United Arab Emirates), Australasia (Australia, New Zealand), North America/Latin America (Canada, USA and Brazil) and Asia (China, Malaysia, Japan). There were a range of approaches to GDM screening, choice of diagnostic test and diagnostic glucose thresholds.
Quality assessment
Study quality assessment is presented as an overall risk of bias for the studies included in the meta-analyses in Fig. 3 and as a heat map for quality assessment for each included study in Fig. 4 . Most of the studies were rated as low risk of bias, as they adequately described how a diagnosis of GDM was assigned, defining inclusion and exclusion criteria, and reported the protocol for initiation of pharmacological therapy. Not all studies reported whether women received diet and lifestyle advice as standard care. Few studies reported whether the precision marker was measured in a valid and reliable way. Using the GRADE approach, the majority of precision markers were classified as having a low certainty of evidence with some classified as very low certainty (Tables 1 and 2 ). No publication bias (as ascertained by funnel plot analyses) was detected.
Green circle with + sign, Yes, Red circle with – sign, No, Blank – not described.

Green – low risk of bias, Grey – unclear risk of bias, Red – high risk of bias.
Precision diet and lifestyle interventions in GDM
Two studies examining different precision approaches to behavioural interventions were included in the first systematic review, so we present a narrative synthesis of the findings. Neither study examined whether a precision approach to specific lifestyle interventions facilitated achievement of glucose targets during pregnancy or improved outcomes that reflect glycaemic control during pregnancy such as macrosomia, large for gestational age, or neonatal hypoglycaemia.
In one study of women with GDM 19 , the intervention was distribution of a tailored letter based on electronic health record data detailing gestational weight gain (GWG) recommendations (as defined by the Institute of Medicine). Receipt of this tailored letter increased the likelihood of meeting the end-of-pregnancy weight goal among women with normal pre-pregnancy BMI, but not among women with overweight or obese pre-pregnancy BMI. This study identified normal pre-pregnancy BMI as a precision marker for intervention success.
The second study 20 used a Web/Smart phone lifestyle coaching programme in women with GDM. Pre-intervention excessive GWG was evaluated as a potential precision marker for the response to the Web/Smart phone lifestyle coaching programme in preventing excess GWG. There was no difference between study arms with respect to either excess GWG or absolute GWG by the end of pregnancy indicating that early GWG is not a useful precision marker with respect to this intervention.
Precision markers for escalation of pharmacological interventions to achieve glucose targets in GDM
Of the 34 studies of precision markers for escalation to pharmacological therapy to achieve glucose targets in addition to standard care with diet and lifestyle advice, 23 studies ( n = 19,112 participants) were included in the meta-analysis 21 , 22 , 23 , 25 , 26 , 31 , 32 , 33 , 34 , 35 , 36 , 38 , 40 , 41 , 43 , 44 , 45 , 46 , 48 , 50 , 51 , 52 , 53 and 11 studies ( n = 7158 participants) in the narrative synthesis 24 , 27 , 28 , 29 , 30 , 37 , 39 , 42 , 47 , 49 , 54 .
Table 1 and Supplementary Figs. 1 – 13 show that precision markers for GDM to be adequately managed with lifestyle measures were lower maternal age, nulliparity, lower BMI, no previous history of GDM, lower HbA1c, lower glucose values at the diagnostic OGTT (fasting, 1 h, 2 and/or 3 h glucose), no family history of diabetes, later gestation of diagnosis of GDM and no macrosomia in previous pregnancies. There was a similar pattern for not smoking but this did not reach statistical significance.
Twelve studies ( n = 1836 participants) of precision markers for escalation to insulin to achieve glucose targets in addition to oral agents were included in the meta-analysis 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 .
Table 2 and Supplementary Figs. 14 – 25 show that precision markers for achieving glucose targets with oral agents only were nulliparity, lower BMI, no previous history of GDM, lower HbA1c, lower glucose values at the diagnostic OGTT (fasting, 1 h, and/or 2 h glucose), later gestation of diagnosis of GDM and later gestation at initiation of the oral agent. In sensitivity analyses, there were no differences in the precision markers predicting response to metformin versus glyburide (Supplementary Data 3 ).
Similar precision markers for escalation to pharmacotherapy to achieve glucose targets were observed in the 11 studies ( n = 7158 participants) that were not included in the meta-analysis 24 , 27 , 28 , 29 , 30 , 37 , 39 , 42 , 47 , 49 , 54 (Supplementary Data 4 ). Additional precision markers including foetal sex 28 , ethnicity 30 , 47 and season of birth 37 were evaluated in some studies but there was insufficient data to draw conclusions.
There was a paucity of data in examining other precision markers with only weak evidence that the maternal lipidome 68 or genetics 67 hold potential as precision markers for escalation of pharmacological treatment (Supplementary Data 4 ).
As the factors contributing to the development of GDM and its aetiology are heterogeneous 5 , 6 , 7 , 8 , it is plausible that the most effective treatment strategies may also be variable among women with GDM. A precision medicine approach resulting in more rapid normalisation of hyperglycaemia could have substantial benefits for both mother and foetus. By synthesising the evidence from two systematic reviews, we sought to identify key precision markers that may predict effective lifestyle and pharmacological interventions. There were a paucity of studies examining precision approaches to better target lifestyle-based interventions for GDM treatment highlighting the pressing need for further research in this area. However, we found a number of precision markers to enable earlier identification of those requiring escalation of pharmacological therapy. These included characteristics such as BMI, that are easily and routinely measured in clinical practice, and thus have potential to be integrated into prediction models with the aim of achieving rapid glycaemic control. With the relatively short timeframe available to treat GDM, commencing effective therapy earlier, and thus reducing excess foetal growth, is an important target to improve outcomes. Basing treatment decisions closely on precision markers could also avoid over-medicalisation of women who are likely to achieve glucose targets with dietary counselling alone.
In our first systematic review, we identified only two studies addressing precision markers in lifestyle-based interventions for GDM, over and above the usual lifestyle intervention as standard care 19 , 20 . In both studies, precision markers were examined as secondary analyses of the trials and only two precision markers (communication of GWG goals according to pre-pregnancy BMI; and early GWG as a precision marker for the efficacy of technological enhancement to a behavioural intervention) were assessed; it is thus not possible to conclusively identify any precision marker in lifestyle-based interventions for GDM. This gap in the literature highlights the need for more research, as also echoed by patients and healthcare professionals participating in the 2020 James Lind Alliance (JLA) Priority Setting Partnership (PSP) 69 .
Our second systematic review extends the observations of a previous systematic review reporting maternal characteristics associated with the need for insulin treatment in GDM 11 . We identified a number of additional precision markers of successful GDM treatment with lifestyle measures alone, without need for additional pharmacological therapy. The same set of predictors identified women requiring additional insulin after treatment with glyburide as with metformin, despite their different mechanisms of action. However, the numbers of women included in most studies were relatively low and most studies with data in relation to need to escalation to insulin in addition to glyburide were over 10 years old 55 , 56 , 58 , 59 , 60 . We acknowledge that there are also differences in diagnostic criteria, clinical practices, and preferences for choice of which drug to start as first pharmacological agent in various global regions which may limit the generalisability of our findings.
Notably, many of the identified precision markers are routinely measured in clinical practice and so could be incorporated into prediction models of need for pharmacological treatment 70 , 71 . By identifying those who require escalation of pharmacological therapy earlier, better allocation of resources can be achieved. Additionally, some of the precision markers identified, such as BMI, are potentially modifiable. This raises the question of how women can be helped to better prepare for pregnancy 72 . Implementing interventions prior to pregnancy could help understand if these precision markers are on the causal pathway, thus providing an opportunity for prevention and improving health outcomes.
Importantly, there was a lack of data on other potential precision treatment biomarkers, with only two eligible low-quality studies reporting maternal genetic and metabolomic findings 67 , 68 . In the non-pregnancy literature, efficacy of dietary interventions has been reported to differ for patients with distinct metabolic profiles, for example high fasting glucose versus high fasting insulin, or insulin resistance versus low insulin secretion 73 , 74 , 75 . More recent evidence from appropriately designed, prospective dietary intervention studies has confirmed that dietary interventions tailored towards specific metabolic profiles have more beneficial effects than interventions not specifically designed towards a patient’s metabolic profile 76 , 77 , 78 , 79 . Ongoing studies such as the Westlake Precision Birth Cohort (WeBirth) in China (NCT04060056) and the USA Hoosier Moms Cohort (NCT03696368) are collecting additional biomarkers which will enhance knowledge in this field. However, implementing such measures in clinical practice, if they prove informative, could be complex and expensive and thus not suitable for use in all global contexts.
Our study has several limitations: Our reviews primarily relied on secondary analyses from observational studies that were not specifically designed to address the question of precision medicine in GDM treatment and were not powered for many of the comparisons made. Prior to introduction in clinical practice, any marker would have to be rigorously and prospectively tested with respect to sensitivity and specificity to predict treatment needs. The majority of data were extracted from clinical records leading to a lack of detail, such as the precise timing of BMI measurements, and limited information about whether BMI was self-reported or clinician measured. There was marked variation in approaches to GDM screening methods, choice of glucose challenge test and diagnostic thresholds as well as heterogeneity in glucose targets or criteria met to warrant escalation in treatment. Whilst we included studies from a range of geographical settings, the majority of studies were from high income settings, and therefore our findings may not be applicable to low- and middle-income countries. Pregnancy outcomes of precision medicine strategies for GDM also remain unknown, underscoring the need for tailored interventions that account for patient perspective and diverse patient populations.
Despite these limitations, our study has several strengths. We used robust methods to identify a broad range of precision markers, many of which are routinely measured and can be easily translated into prediction models. We excluded studies where the choice of drug was decided by the clinician based on participant characteristics to avoid bias. Our study also highlights the need for further research in this area, particularly in exploring whether there are more sensitive markers that could be identified through omics approaches.
In conclusion, our findings suggest that precision medicine for GDM treatment holds promise as a tool to stream-line individuals towards the most effective and potentially cost-effective care. Whether this will impact on short-term pregnancy outcomes and longer term health outcomes for both mother and baby is not known. More research is urgently needed to identify precision lifestyle interventions and to explore whether more sensitive markers could be identified. Prospective studies, appropriately powered and designed to allow assessment of discriminative abilities (sensitivity, specificity), and (external) validation studies are urgently needed to understand the utility and generalisability of our findings to under-represented populations. This is an area of active research with findings from ongoing studies (NCT04187521, NCT03029702, NCT05932251) eagerly awaited. Consideration of how identified markers can be implemented feasibly and cost effectively in clinical practice is also required. Such efforts will be critical for realising the full potential of precision medicine and empowering patients and their health care providers to optimise short and long-term health outcomes for both mother and child.
Data availability
The included studies are detailed in Supplementary Data 1 and 2 . The data underlying Tables 1 and 2 are in Supplementary Figs. 1 – 13 and 14 – 25 , respectively. Additional information is available via contact with the corresponding author.
Saravanan, P. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 8 , 793–800 (2020).
PubMed Google Scholar
Vounzoulaki, E. et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 369 , m1361 (2020).
PubMed PubMed Central Google Scholar
Metzger, B. E. et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 358 , 1991–2002 (2008).
Crowther, C. A. et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 352 , 2477–2486 (2005).
CAS PubMed Google Scholar
Powe, C. E., Hivert, M. F. & Udler, M. S. Defining heterogeneity among women with gestational diabetes mellitus. Diabetes 69 , 2064–2074 (2020).
CAS PubMed PubMed Central Google Scholar
Powe, C. E. et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care 39 , 1052–1055 (2016).
Benhalima, K. et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia 62 , 2118–2128 (2019).
Madsen, L. R. et al. Do variations in insulin sensitivity and insulin secretion in pregnancy predict differences in obstetric and neonatal outcomes? Diabetologia 64 , 304–312 (2021).
Harrison, R. K., Cruz, M., Wong, A., Davitt, C. & Palatnik, A. The timing of initiation of pharmacotherapy for women with gestational diabetes mellitus. BMC Preg, Childbirth 20 , 773 (2020).
CAS Google Scholar
Tisi, D. K., Burns, D. H., Luskey, G. W. & Koski, K. G. Fetal exposure to altered amniotic fluid glucose, insulin, and insulin-like growth factor-binding protein 1 occurs before screening for gestational diabetes mellitus. Diabetes Care 34 , 139–144 (2011).
Alvarez-Silvares, E., Bermúdez-González, M., Vilouta-Romero, M., García-Lavandeira, S. & Seoane-Pillado, T. Prediction of insulin therapy in women with gestational diabetes: a systematic review and meta-analysis of observational studies. J. Perinat. Med. 50 , 608–619 (2022).
Dennis, J. M., Shields, B. M., Henley, W. E., Jones, A. G. & Hattersley, A. T. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol. 7 , 442–451 (2019).
Dawed, A. Y. et al. Pharmacogenomics of GLP-1 receptor agonists: a genome-wide analysis of observational data and large randomised controlled trials. Lancet Diabetes Endocrinol. 11 , 33–41 (2023).
Nolan, J. J. et al. ADA/EASD Precision Medicine in Diabetes Initiative: an international perspective and future vision for precision medicine in diabetes. Diabetes Care 45 , 261–266 (2022).
Tobias, D. K., Merino, J., Ahmad, A. & PMDI, A. E. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat. Med. (in press), https://doi.org/10.1038/s41591-023-02502-5 . (2023)
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151 , 264–269 (2009).
Joanna Briggs Institute (JBI) critical appraisal tools https://jbi.global/critical-appraisal-tools . Accessed 15 April 2023.
Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) https://guidelines.diabetes.ca/cpg/chapter2 . Accessed 15 April 2023.
Hedderson, M. M. et al. A tailored letter based on electronic health record data improves gestational weight gain among women with gestational diabetes mellitus: the Gestational Diabetes’ Effects on Moms (GEM) cluster-randomized controlled trial. Diabetes Care 41 , 1370–1377 (2018).
Yew, T. W. et al. A randomized controlled trial to evaluate the effects of a smartphone application-based lifestyle coaching program on gestational weight gain, glycemic control, and maternal and neonatal outcomes in women with gestational diabetes mellitus: the SMART-GDM study. Diabetes Care 44 , 456–463 (2021).
Ares, J. et al. Gestational Diabetes Mellitus (GDM): relationship between higher cutoff values for 100g Oral Glucose Tolerance Test (OGTT) and insulin requirement during pregnancy. Matern. Child Health J. 21 , 1488–1492 (2017).
Barnes, R. A. et al. Predictors of large and small for gestational age birthweight in offspring of women with gestational diabetes mellitus. Diabet. Med. 30 , 1040–1046 (2013).
Benhalima, K. et al. Differences in pregnancy outcomes and characteristics between insulin- and diet-treated women with gestational diabetes. BMC Preg. Childbirth 15 , 271 (2015).
Google Scholar
Berg, M., Adlerberth, A., Sultan, B., Wennergren, M. & Wallin, G. Early random capillary glucose level screening and multidisciplinary antenatal teamwork to improve outcome in gestational diabetes mellitus. Acta Obstetr. Gynecol, Scand. 86 , 283–290 (2007).
Ducarme, G. et al. Predictive factors of subsequent insulin requirement for glycemic control during pregnancy at diagnosis of gestational diabetes mellitus. Int. J. Gynaecol. Obstetr. 144 , 265–270 (2019).
Durnwald, C. P. et al. Glycemic characteristics and neonatal outcomes of women treated for mild gestational diabetes. Obstetr. Gynecol. 117 , 819–827 (2011).
Elnour, A. A. Antenatal oral glucose-tolerance test values and pregnancy outcomes. Int. J. Pharm Pract. 16 , 189–197 (2008).
Giannubilo, S. R., Pasculli, A., Ballatori, C., Biagini, A. & Ciavattini, A. Fetal sex, need for insulin, and perinatal outcomes in gestational diabetes mellitus: an observational cohort study. Clin. Ther. 40 , 587–592 (2018).
Gibson, K. S., Waters, T. P. & Catalano, P. M. Maternal weight gain in women who develop gestational diabetes mellitus. Obstetr. Gynecol. 119 , 560–565 (2012).
Hillier, T. A., Ogasawara, K. K., Pedula, K. L. & Vesco, K. K. Markedly different rates of incident insulin treatment based on universal gestational diabetes mellitus screening in a diverse HMO population. Am. J. Obstetr. Gynecol. 209 , 440.e441–449 (2013).
Ikenoue, S. et al. Clinical impact of women with gestational diabetes mellitus by the new consensus criteria: two year experience in a single institution in Japan. Endocr. J. 61 , 353–358 (2014).
Ito, Y. et al. Indicators of the need for insulin treatment and the effect of treatment for gestational diabetes on pregnancy outcomes in Japan. Endocr. J. 63 , 231–237 (2016).
Kalok, A. et al. Correlation between oral glucose tolerance test abnormalities and adverse pregnancy outcomes in gestational diabetes: a cross-sectional study. Int. J. Environ. Res. Public Health 17 , 6990 (2020).
Koning, S. H. et al. Risk stratification for healthcare planning in women with gestational diabetes mellitus. Netherlands J. Med. 74 , 262–269 (2016).
Mecacci, F. et al. Different gestational diabetes phenotypes: which insulin regimen fits better? Front. Endocrinol. 12 , 630903 (2021).
Meghelli, L., Vambergue, A., Drumez, E. & Deruelle, P. Complications of pregnancy in morbidly obese patients: What is the impact of gestational diabetes mellitus? J. Gynecol. Obstetr. Hum. Reprod. 49 , 101628 (2020).
Molina-Vega, M. et al. Relationship between environmental temperature and the diagnosis and treatment of gestational diabetes mellitus: an observational retrospective study. Sci. Total Environ. 744 , 140994 (2020).
Ng, A., Liu, A. & Nanan, R. Association between insulin and post-caesarean resuscitation rates in infants of women with GDM: a retrospective study. J. Diabetes 12 , 151–157 (2020).
Nguyen, T. H., Yang, J. W., Mahone, M. & Godbout, A. Are there benefits for gestational diabetes mellitus in treating lower levels of hyperglycemia than standard recommendations? Can. J. Diabetes 40 , 548–554 (2016).
Nishikawa, T. et al. One-hour oral glucose tolerance test plasma glucose at gestational diabetes diagnosis is a common predictor of the need for insulin therapy in pregnancy and postpartum impaired glucose tolerance. J. Diabetes Investig. 9 , 1370–1377 (2018).
Ouzounian, J. G. et al. One-hour post-glucola results and pre-pregnancy body mass index are associated with the need for insulin therapy in women with gestational diabetes. J. Matern.-Fetal Neonatal Med. 24 , 718–722 (2011).
Parrettini, S. et al. Gestational diabetes: a link between OGTT, maternal-fetal outcomes and maternal glucose tolerance after childbirth. Nutr. Metab. Cardiovasc. Dis. 30 , 2389–2397 (2020).
Silva, J. K., Kaholokula, J. K., Ratner, R. & Mau, M. Ethnic differences in perinatal outcome of gestational diabetes mellitus. Diabetes Care 29 , 2058–2063 (2006).
Souza, A. et al. Can we stratify the risk for insulin need in women diagnosed early with gestational diabetes by fasting blood glucose? J. Matern-Fetal Neonatal Med. 32 , 2036–2041 (2019).
Suhonen, L., Hiilesmaa, V., Kaaja, R. & Teramo, K. Detection of pregnancies with high risk of fetal macrosomia among women with gestational diabetes mellitus. Acta Obstetr. Gynecol. Scand. 87 , 940–945 (2008).
Sun, T. et al. The effects of insulin therapy on maternal blood pressure and weight in women with gestational diabetes mellitus. BMC Preg. Childbirth 21 , 657 (2021).
Wong, V. W. Gestational diabetes mellitus in five ethnic groups: a comparison of their clinical characteristics. Diabet. Med. 29 , 366–371 (2012).
Wong, V. W. & Jalaludin, B. Gestational diabetes mellitus: who requires insulin therapy? Aust. N.Z. J. Obstetr. Gynaecol. 51 , 432–436 (2011).
Zawiejska, A., Wender-Ozegowska, E., Radzicka, S. & Brazert, J. Maternal hyperglycemia according to IADPSG criteria as a predictor of perinatal complications in women with gestational diabetes: a retrospective observational study. J. Matern.-Fetal Neonatal Med. 27 , 1526–1530 (2014).
Bashir, M. et al. Metformin-treated-GDM has lower risk of macrosomia compared to diet-treated GDM- a retrospective cohort study. J. Matern.-Fetal Neonatal Med. 33 , 2366–2371 (2020).
Gilbert, L. et al. Mental health and its associations with glucose-lowering medication in women with gestational diabetes mellitus. A prospective clinical cohort study. Psychoneuroendocrinology 124 , 105095 (2021).
Krispin, E., Ashkenazi Katz, A., Shmuel, E., Toledano, Y. & Hadar, E. Characterization of women with gestational diabetes who failed to achieve glycemic control by lifestyle modifications. Arch. Gynecol. Obstetr. 303 , 677–683 (2021).
Meshel, S. et al. Can we predict the need for pharmacological treatment according to demographic and clinical characteristics in gestational diabetes? J. Matern.-Fetal Neonatal Med. 29 , 2062–2066 (2016).
Zhu, S., Meehan, T., Veerasingham, M. & Sivanesan, K. COVID-19 pandemic gestational diabetes screening guidelines: a retrospective study in Australian women. Diabetes & Metabolic Syndrome 15 , 391–395 (2021).
Chmait, R., Dinise, T. & Moore, T. Prospective observational study to establish predictors of glyburide success in women with gestational diabetes mellitus. J. Perinatol. 24 , 617–622 (2004).
Conway, D. L., Gonzales, O. & Skiver, D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. J. Matern.-Fetal Neonatal Med. 15 , 51–55 (2004).
Harper, L. M., Glover, A. V., Biggio, J. R. & Tita, A. Predicting failure of glyburide therapy in gestational diabetes. J. Perinatol. 36 , 347–351 (2016).
Kahn, B. F., Davies, J. K., Lynch, A. M., Reynolds, R. M. & Barbour, L. A. Predictors of glyburide failure in the treatment of gestational diabetes. Obstetr. Gynecol. 107 , 1303–1309 (2006).
Rochon, M., Rand, L., Roth, L. & Gaddipati, S. Glyburide for the management of gestational diabetes: risk factors predictive of failure and associated pregnancy outcomes. Am. J. Obstetr. Gynecol. 195 , 1090–1094 (2006).
Yogev, Y. et al. Glyburide in gestational diabetes–prediction of treatment failure. J. Matern.-Fetal Neonatal Med. 24 , 842–846 (2011).
Gante, I., Melo, L., Dores, J., Ruas, L. & Almeida, M. D. C. Metformin in gestational diabetes mellitus: predictors of poor response. Eur. J. Endocrinol. 178 , 129–135 (2018).
Khin, M. O., Gates, S. & Saravanan, P. Predictors of metformin failure in gestational diabetes mellitus (GDM). Diabetes Metab. Syndr. 12 , 405–410 (2018).
McGrath, R. T., Glastras, S. J., Hocking, S. & Fulcher, G. R. Use of metformin earlier in pregnancy predicts supplemental insulin therapy in women with gestational diabetes. Diabetes Res. Clin. Pract. 116 , 96–99 (2016).
Picón-César, M. J. et al. Metformin for gestational diabetes study: metformin vs insulin in gestational diabetes: glycemic control and obstetrical and perinatal outcomes: randomized prospective trial. Am. J. Obstetr. Gynecol. 225 , 517.e511–517.e517 (2021).
Rowan, J. A., Hague, W. M., Gao, W., Battin, M. R. & Moore, M. P. Metformin versus insulin for the treatment of gestational diabetes. N. Engl J. Med. 358 , 2003–2015 (2008).
Tertti, K., Ekblad, U., Koskinen, P., Vahlberg, T. & Rönnemaa, T. Metformin vs. insulin in gestational diabetes. A randomized study characterizing metformin patients needing additional insulin. Diabetes Obes. Metab. 15 , 246–251 (2013).
Bouchghoul, H. et al. Hypoglycemia and glycemic control with glyburide in women with gestational diabetes and genetic variants of cytochrome P450 2C9 and/or OATP1B3. Clin. Pharmacol. Ther. 110 , 141–148 (2021).
Huhtala, M. S., Tertti, K. & Rönnemaa, T. Serum lipids and their association with birth weight in metformin and insulin-treated patients with gestational diabetes. Diabetes Res. Clin. Pract. 170 , 108456 (2020).
Ayman, G. et al. The top 10 research priorities in diabetes and pregnancy according to women, support networks and healthcare professionals. Diabet. Med. 38 , e14588 (2021).
Cooray, S. D. et al. Development, validation and clinical utility of a risk prediction model for adverse pregnancy outcomes in women with gestational diabetes: the PeRSonal GDM model. EClinicalMedicine 52 , 101637 (2022).
Liao, L. D. et al. Development and validation of prediction models for gestational diabetes treatment modality using supervised machine learning: a population-based cohort study. BMC Med. 20 , 307 (2022).
Cassinelli, E. H. et al. Preconception health and care policies and guidelines in the UK and Ireland: a scoping review. Lancet 400 , S61 (2022).
Hjorth, M. F. et al. Pretreatment Fasting glucose and insulin as determinants of weight loss on diets varying in macronutrients and dietary fibers-the POUNDS LOST study. Nutrients 11 , 586 (2019).
Hjorth, M. F. et al. Pretreatment fasting plasma glucose and insulin modify dietary weight loss success: results from 3 randomized clinical trials. Am. J. Clin. Nutr. 106 , 499–505 (2017).
Hjorth, M. F., Due, A., Larsen, T. M. & Astrup, A. Pretreatment fasting plasma glucose modifies dietary weight loss maintenance success: results from a stratified RCT. Obesity 25 , 2045–2048 (2017).
Bergia, R. E. et al. Differential glycemic effects of low- versus high-glycemic index Mediterranean-style eating patterns in adults at risk for type 2 diabetes: the MEDGI-Carb randomized controlled trial. Nutrients 14 , 706 (2022).
Aldubayan, M. A. et al. A double-blinded, randomized, parallel intervention to evaluate biomarker-based nutrition plans for weight loss: the PREVENTOMICS study. Clin. Nutr. 41 , 1834–1844 (2022).
Trouwborst, I. et al. Cardiometabolic health improvements upon dietary intervention are driven by tissue-specific insulin resistance phenotype: a precision nutrition trial. Cell Metab. 35 , 71–83.e75 (2023).
Cifuentes, L. et al. Phenotype tailored lifestyle intervention on weight loss and cardiometabolic risk factors in adults with obesity: a single-centre, non-randomised, proof-of-concept study. EClinicalMedicine 58 , 101923 (2023).
Download references
Acknowledgements
The ADA/EASD Precision Diabetes Medicine Initiative, within which this work was conducted, has received the following support: The Covidence license was funded by Lund University (Sweden) for which technical support was provided by Maria Björklund and Krister Aronsson (Faculty of Medicine Library, Lund University, Sweden). Administrative support was provided by Lund University (Malmö, Sweden), University of Chicago (IL, USA), and the American Diabetes Association (Washington D.C., USA). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person writing group meetings (PI: L Phillipson, University of Chicago, IL). J.M.M. acknowledges the support of the Henry Friesen Professorship in Endocrinology, University of Manitoba, Canada. N.-M.M. and R.M.R. acknowledge the support of the British Heart Foundation (RE/18/5/34216). S.E.O. is supported by the Medical Research Council (MC_UU_00014/4) and British Heart Foundation (RG/17/12/33167).
Author information
These authors contributed equally: Jamie L. Benham, Véronique Gingras, Niamh-Maire McLennan, Jasper Most, Jennifer M. Yamamoto, Catherine E. Aiken.
These authors jointly supervised this work: Susan E. Ozanne, Rebecca M. Reynolds.
Authors and Affiliations
Department of Medicine and Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
Jamie L. Benham
Department of Nutrition, Université de Montréal, Montreal, QC, Canada
Véronique Gingras
Research Center, Sainte-Justine University Hospital Center, Montreal, QC, Canada
MRC Centre for Reproductive Health, Queens’s Medical Research Institute, University of Edinburgh, Edinburgh, UK
Niamh-Maire McLennan & Rebecca M. Reynolds
Centre for Cardiovascular Science, Queens’s Medical Research Institute, University of Edinburgh, Edinburgh, UK
Department of Orthopedics, Zuyderland Medical Center, Sittard-Geleen, The Netherlands
Jasper Most
Internal Medicine, University of Manitoba, Winnipeg, MB, Canada
Jennifer M. Yamamoto
Department of Obstetrics and Gynaecology, the Rosie Hospital, Cambridge, UK
Catherine E. Aiken & Catherine Aiken
NIHR Cambridge Biomedical Research Centre, University of Cambridge, Cambridge, UK
University of Cambridge Metabolic Research Laboratories and MRC Metabolic Diseases Unit, Wellcome-MRC Institute of Metabolic Science, Cambridge, UK
Susan E. Ozanne
Division of Preventative Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Deirdre K. Tobias & Vanessa Santhakumar
Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Deirdre K. Tobias, Zhila Semnani-Azad, Marta Guasch-Ferré & Paul W. Franks
Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
Jordi Merino, Anne Cathrine B. Thuesen, Mette K. Andersen, Christoffer Clemmensen, Torben Hansen, Mariam Nakabuye & Ruth J. F. Loos
Diabetes Unit, Endocrine Division, Massachusetts General Hospital, Boston, MA, USA
Jordi Merino, Sara J. Cromer, Raymond J. Kreienkamp, Aaron Leong, Camille E. Powe, Jose C. Florez, Marie-France Hivert & Miriam S. Udler
Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA
Jordi Merino, Raymond J. Kreienkamp, Aaron J. Deutsch, Jose C. Florez & Miriam S. Udler
Department of Clinical Sciences, Lund University Diabetes Centre, Lund University, Malmö, Sweden
Abrar Ahmad, Monika Dudenhöffer-Pfeifer, Hugo Fitipaldi, Hugo Pomares-Millan, Maria F. Gomez & Paul W. Franks
Department of Molecular Genetics, Madras Diabetes Research Foundation, Chennai, India
Dhanasekaran Bodhini
Division of Pediatric Endocrinology, Department of Pediatrics, Saint Louis University School of Medicine, SSM Health Cardinal Glennon Children’s Hospital, St. Louis, MO, USA
Amy L. Clark
Department of Clinical and Biomedical Sciences, University of Exeter Medical School, Exeter, Devon, UK
Kevin Colclough, Alice Hughes, Kashyap Amratlal Patel, Katherine Young, Angus G. Jones, Elisa de Franco, Sarah E. Flanagan, Andrew McGovern, John M. Dennis, Andrew T. Hattersley & Richard Oram
CIBER-BBN, ISCIII, Madrid, Spain
Rosa Corcoy
Institut d’Investigació Biomèdica Sant Pau (IIB SANT PAU), Barcelona, Spain
Departament de Medicina, Universitat Autònoma de Barcelona, Bellaterra, Spain
Program in Metabolism and Medical & Population Genetics, Broad Institute, Cambridge, MA, USA
Sara J. Cromer, Raymond J. Kreienkamp, Magdalena Sevilla-Gonzalez, Aaron J. Deutsch, Camille E. Powe, Jose C. Florez & Miriam S. Udler
Department of Medicine, Harvard Medical School, Boston, MA, USA
Sara J. Cromer, Magdalena Sevilla-Gonzalez, Tinashe Chikowore, Aaron J. Deutsch, Aaron Leong, Camille E. Powe, Jose C. Florez, James B. Meigs & Miriam S. Udler
Division of Endocrinology, Diabetes and Metabolism, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA
Jamie L. Felton, Linda A. DiMeglio, Carmella Evans-Molina, Arianna Harris-Kawano, Heba M. Ismail, Dianna Perez, Gabriela S. F. Monaco & Emily K. Sims
Herman B Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN, USA
Center for Diabetes and Metabolic Diseases, Indiana University School of Medicine, Indianapolis, IN, USA
Department of Biostatistics and Epidemiology, Rutgers School of Public Health, Piscataway, NJ, USA
- Ellen C. Francis
University Hospital Leuven, Leuven, Belgium
Pieter Gillard & Chantal Mathieu
Department of Pediatrics, Erasmus Medical Center, Rotterdam, The Netherlands
Romy Gaillard
Division of Population Health & Genomics, School of Medicine, University of Dundee, Dundee, UK
Eram Haider, Robert Massey, Adem Y. Dawed & Ewan R. Pearson
Department of Pediatrics, Stanford School of Medicine, Stanford University, Stanford, CA, USA
Jennifer M. Ikle & Anna L. Gloyn
Stanford Diabetes Research Center, Stanford School of Medicine, Stanford University, Stanford, CA, USA
University of Florida, Gainesville, FL, USA
Laura M. Jacobsen
Department of Nutrition, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Anna R. Kahkoska
Helsinki University Hospital, Abdominal Centre/Endocrinology, Helsinki, Finland
Jarno L. T. Kettunen & Tiinamaija Tuomi
Folkhalsan Research Center, Helsinki, Finland
Jarno L. T. Kettunen
Institute for Molecular Medicine Finland FIMM, University of Helsinki, Helsinki, Finland
Department of Pediatrics, Division of Endocrinology, Boston Children’s Hospital, Boston, MA, USA
Raymond J. Kreienkamp
Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
Lee-Ling Lim
Asia Diabetes Foundation, Hong Kong SAR, China
Department of Medicine & Therapeutics, Chinese University of Hong Kong, Hong Kong SAR, China
Lee-Ling Lim, Claudia Ha-ting Tam, Chuiguo Huang, Gechang Yu, Yingchai Zhang & Ronald C. W. Ma
Department of Pediatrics and Clinical Genetics, Kuopio University Hospital, Kuopio, Finland
Jonna M. E. Männistö
Department of Medicine, University of Eastern Finland, Kuopio, Finland
Centre for Cardiovascular Science, Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, UK
Niamh-Maire Mclennan, Rebecca M. Reynolds & Robert K. Semple
Department of Epidemiology, University of Pittsburgh, Pittsburgh, PA, USA
Rachel G. Miller & Tina Costacou
Metabolic Disease Unit, University Hospital of Padova, Padova, Italy
Mario Luca Morieri
Department of Medicine, University of Padova, Padova, Italy
Department of Pediatrics and Medicine, University of Chicago, Chicago, IL, USA
Rochelle N. Naylor
Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Bige Ozkan, Mary R. Rooney, Amelia S. Wallace & Elizabeth Selvin
Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins School of Medicine, Baltimore, MD, USA
Department of Medicine, Johns Hopkins University, Baltimore, MD, USA
Scott J. Pilla, Sarah Kanbour, Sudipa Sarkar & Nestoras Mathioudakis
Department of Health Policy and Management, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA
Scott J. Pilla
Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University Düsseldorf, Auf’m Hennekamp 65, 40225, Düsseldorf, Germany
Katsiaryna Prystupa, Martin Schön & Robert Wagner
German Center for Diabetes Research (DZD), Ingolstädter Landstraße 1, 85764, Neuherberg, Germany
Katsiaryna Prystupa, Martin Schön, Norbert Stefan & Robert Wagner
Section of Academic Primary Care, US Department of Veterans Affairs Eastern Colorado Health Care System, Aurora, CO, USA
Sridharan Raghavan
Department of Medicine, University of Colorado School of Medicine, Aurora, CO, USA
Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Mary R. Rooney, Amelia S. Wallace, Caroline C. Wang, Debashree Ray & Elizabeth Selvin
Institute of Experimental Endocrinology, Biomedical Research Center, Slovak Academy of Sciences, Bratislava, Slovakia
Martin Schön
Clinical and Translational Epidemiology Unit, Massachusetts General Hospital, Boston, MA, USA
Magdalena Sevilla-Gonzalez
Mohn Center for Diabetes Precision Medicine, Department of Clinical Science, University of Bergen, Bergen, Norway
Pernille Svalastoga, Ingvild Aukrust, Janne Molnes & Pål Rasmus Njølstad
Children and Youth Clinic, Haukeland University Hospital, Bergen, Norway
Pernille Svalastoga & Pål Rasmus Njølstad
Eastern Health Clinical School, Monash University, Melbourne, VIC, Australia
Wubet Worku Takele, Gebresilasea Gendisha Ukke & Siew S. Lim
Laboratory for Molecular Epidemiology in Diabetes, Li Ka Shing Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong, China
Claudia Ha-ting Tam, Chuiguo Huang, Gechang Yu, Yingchai Zhang & Ronald C. W. Ma
Hong Kong Institute of Diabetes and Obesity, The Chinese University of Hong Kong, Hong Kong, China
Claudia Ha-ting Tam & Ronald C. W. Ma
Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA
Mustafa Tosur & Maria J. Redondo
Division of Pediatric Diabetes and Endocrinology, Texas Children’s Hospital, Houston, TX, USA
Mustafa Tosur, Marzhan Urazbayeva & Maria J. Redondo
Children’s Nutrition Research Center, USDA/ARS, Houston, TX, USA
Mustafa Tosur
Stanford University School of Medicine, Stanford, CA, USA
Jessie J. Wong & Korey K. Hood
Department of Diabetology, APHP, Paris, France
Chloé Amouyal
Sorbonne Université, INSERM, NutriOmic team, Paris, France
Department of Nutrition, Dietetics and Food, Monash University, Melbourne, VIC, Australia
Maxine P. Bonham & Gloria K. W. Leung
Monash Centre for Health Research and Implementation, Monash University, Clayton, VIC, Australia
Mingling Chen
Health Management Center, The Second Affiliated Hospital of Chongqing Medical University, Chongqing Medical University, Chongqing, China
Feifei Cheng
MRC/Wits Developmental Pathways for Health Research Unit, Department of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Tinashe Chikowore
Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, MA, USA
Sydney Brenner Institute for Molecular Bioscience, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Department of Women and Children’s health, King’s College London, London, UK
Sian C. Chivers & Sara L. White
Lifecourse Epidemiology of Adiposity and Diabetes (LEAD) Center, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Dana Dabelea, Kristen Boyle & Wei Perng
Section of Adult and Pediatric Endocrinology, Diabetes and Metabolism, Kovler Diabetes Center, University of Chicago, Chicago, USA
Laura T. Dickens
Department of Pediatrics, Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, IN, USA
Linda A. DiMeglio
Richard L. Roudebush VAMC, Indianapolis, IN, USA
Carmella Evans-Molina
Biomedical Research Institute Girona, IdIBGi, Girona, Spain
María Mercè Fernández-Balsells
Diabetes, Endocrinology and Nutrition Unit, Girona, University Hospital Dr Josep Trueta, Girona, Spain
Institute of Health System Science, Feinstein Institutes for Medical Research, Northwell Health, Manhasset, NY, USA
Stephanie L. Fitzpatrick
Department of Pediatrics, Diabetes Center, University of California at San Francisco, San Francisco, CA, USA
Stephen E. Gitelman
Division of Endocrinology, Diabetes and Metabolism, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Mark O. Goodarzi
Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Adelaide Medical School, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, SA, Australia
Jessica A. Grieger, Nahal Habibi, Kai Liu, Maleesa Pathirana & Alejandra Quinteros
Robinson Research Institute, The University of Adelaide, Adelaide, SA, Australia
Jessica A. Grieger, Nahal Habibi, Maleesa Pathirana & Shao J. Zhou
Department of Public Health and Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, 1014, Copenhagen, Denmark
Marta Guasch-Ferré
Division of Endocrinology and Diabetes, Department of Pediatrics, Sanford Children’s Hospital, Sioux Falls, SD, USA
Benjamin Hoag
University of South Dakota School of Medicine, E Clark St, Vermillion, SD, USA
Department of Biomedical Informatics, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Randi K. Johnson & Maggie A. Stanislawski
Department of Epidemiology, Colorado School of Public Health, Aurora, CO, USA
Randi K. Johnson
Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK
Angus G. Jones, Andrew T. Hattersley & Richard Oram
Oxford Centre for Diabetes, Endocrinology and Metabolism, University of Oxford, Oxford, UK
Robert W. Koivula, Katharine R. Owen & Paul W. Franks
Division of General Internal Medicine, Massachusetts General Hospital, Boston, MA, USA
Aaron Leong & James B. Meigs
UPMC Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA
Ingrid M. Libman
Center for Translational Immunology, Benaroya Research Institute, Seattle, WA, USA
S. Alice Long
Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
William L. Lowe Jr.
Department of Pathology & Molecular Medicine, McMaster University, Hamilton, ON, Canada
Robert W. Morton
Population Health Research Institute, Hamilton, ON, Canada
Robert W. Morton, Russell de Souza & Diana Sherifali
Department of Translational Medicine, Medical Science, Novo Nordisk Foundation, Tuborg Havnevej 19, 2900, Hellerup, Denmark
Robert W. Morton & Paul W. Franks
Department of Diabetes and Endocrinology, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
Ayesha A. Motala
Center for Public Health Genomics, Department of Public Health Sciences, University of Virginia, Charlottesville, VA, USA
Suna Onengut-Gumuscu & Stephen S. Rich
Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN, USA
James S. Pankow
Department of Chronic Diseases and Metabolism, Clinical and Experimental Endocrinology, KU Leuven, Leuven, Belgium
Sofia Pazmino, Nele Steenackers & Bart Van der Schueren
School of Health and Wellbeing, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, UK
John R. Petrie
Department of Obstetrics, Gynecology, and Reproductive Biology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
- Camille E. Powe
Sanford Children’s Specialty Clinic, Sioux Falls, SD, USA
Rashmi Jain
Department of Pediatrics, Sanford School of Medicine, University of South Dakota, Sioux Falls, SD, USA
Rashmi Jain & Kurt Griffin
Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Debashree Ray
Centre for Physical Activity Research, Rigshospitalet, Copenhagen, Denmark
Mathias Ried-Larsen
Institute for Sports and Clinical Biomechanics, University of Southern Denmark, Odense, Denmark
Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, Indiana University School of Medicine, Indianapolis, IN, USA
AMAN Hospital, Doha, Qatar
Sarah Kanbour
Department of Preventive Medicine, Division of Biostatistics, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Denise M. Scholtens
Institute of Molecular and Genomic Medicine, National Health Research Institutes, Taipei City, Taiwan, ROC
Wayne Huey-Herng Sheu
Divsion of Endocrinology and Metabolism, Taichung Veterans General Hospital, Taichung, Taiwan, ROC
Division of Endocrinology and Metabolism, Taipei Veterans General Hospital, Taipei, Taiwan, ROC
Center for Interventional Immunology, Benaroya Research Institute, Seattle, WA, USA
Cate Speake
Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Andrea K. Steck & Peter A. Gottlieb
University Hospital of Tübingen, Tübingen, Germany
Norbert Stefan
Institute of Diabetes Research and Metabolic Diseases (IDM), Helmholtz Center Munich, Neuherberg, Germany
Steno Diabetes Center Aarhus, Aarhus University Hospital, Aarhus, Denmark
University of Newcastle, Newcastle upon Tyne, UK
Rachael Taylor
Section on Genetics and Epidemiology, Joslin Diabetes Center, Harvard Medical School, Boston, MA, USA
Sok Cin Tye
Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, The Netherlands
Gastroenterology, Baylor College of Medicine, Houston, TX, USA
Marzhan Urazbayeva
Department of Endocrinology, University Hospitals Leuven, Leuven, Belgium
Bart Van der Schueren
Sorbonne University, Inserm U938, Saint-Antoine Research Centre, Institute of Cardiometabolism and Nutrition, Paris, 75012, France
Camille Vatier
Department of Endocrinology, Diabetology and Reproductive Endocrinology, Assistance Publique-Hôpitaux de Paris, Saint-Antoine University Hospital, National Reference Center for Rare Diseases of Insulin Secretion and Insulin Sensitivity (PRISIS), Paris, France
Royal Melbourne Hospital Department of Diabetes and Endocrinology, Parkville, VIC, Australia
John M. Wentworth
Walter and Eliza Hall Institute, Parkville, VIC, Australia
John M. Wentworth & Tiinamaija Tuomi
University of Melbourne Department of Medicine, Parkville, VIC, Australia
Deakin University, Melbourne, VIC, Australia
Wesley Hannah
Department of Epidemiology, Madras Diabetes Research Foundation, Chennai, India
Department of Diabetes and Endocrinology, Guy’s and St Thomas’ Hospitals NHS Foundation Trust, London, UK
Sara L. White
School of Agriculture, Food and Wine, University of Adelaide, Adelaide, SA, Australia
Shao J. Zhou
Institut Cochin, Inserm U 10116, Paris, France
Jacques Beltrand & Michel Polak
Pediatric endocrinology and diabetes, Hopital Necker Enfants Malades, APHP Centre, université de Paris, Paris, France
Department of Medical Genetics, Haukeland University Hospital, Bergen, Norway
Ingvild Aukrust & Janne Molnes
Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, USA
Kristin A. Maloney & Toni I. Pollin
Department of Epidemiology, Geisel School of Medicine at Dartmouth, Hanover, NH, USA
Hugo Pomares-Millan
Nephrology, Dialysis and Renal Transplant Unit, IRCCS—Azienda Ospedaliero-Universitaria di Bologna, Alma Mater Studiorum University of Bologna, Bologna, Italy
Michele Provenzano
Department of Medical Genetics, AP-HP Pitié-Salpêtrière Hospital, Sorbonne University, Paris, France
Cécile Saint-Martin
Global Center for Asian Women’s Health, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Cuilin Zhang
Department of Obstetrics and Gynecology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Kaiser Permanente Northern California Division of Research, Oakland, CA, USA
Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, CA, USA
National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA
Sungyoung Auh & Rebecca J. Brown
Department of Health Research Methods, Evidence, and Impact, Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada
Russell de Souza
Ann & Robert H. Lurie Children’s Hospital of Chicago, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Andrea J. Fawcett & Jami L. Josefson
Department of Clinical and Organizational Development, Chicago, IL, USA
Andrea J. Fawcett
American Diabetes Association, Arlington, VA, USA
Chandra Gruber
College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Eskedar Getie Mekonnen
Global Health Institute, Faculty of Medicine and Health Sciences, University of Antwerp, 2160, Antwerp, Belgium
Department of Medicine and Kovler Diabetes Center, University of Chicago, Chicago, IL, USA
Emily Mixter & Louis H. Philipson
School of Nursing, Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada
Diana Sherifali
Division of Endocrinology, Metabolism, Diabetes, University of Colorado, Boulder, CO, USA
Robert H. Eckel
Department of Clinical Medicine, School of Medicine, Trinity College Dublin, Dublin, Ireland, UK
John J. Nolan
Department of Endocrinology, Wexford General Hospital, Wexford, Ireland, UK
Division of Endocrinology, NorthShore University HealthSystem, Skokie, IL, USA
Liana K. Billings
Department of Medicine, Prtizker School of Medicine, University of Chicago, Chicago, IL, USA
Department of Genetics, Stanford School of Medicine, Stanford University, Stanford, CA, USA
Anna L. Gloyn
Faculty of Health, Aarhus University, Aarhus, Denmark
Maria F. Gomez
Department of Pediatrics and Medicine and Kovler Diabetes Center, University of Chicago, Chicago, USA
Siri Atma W. Greeley
Sanford Research, Sioux Falls, SD, USA
Kurt Griffin
University of Washington School of Medicine, Seattle, WA, USA
Irl B. Hirsch
Department of Population Medicine, Harvard Medical School, Harvard Pilgrim Health Care Institute, Boston, MA, USA
Marie-France Hivert
Department of Medicine, Universite de Sherbrooke, Sherbrooke, QC, Canada
Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Republic of Korea
Soo Heon Kwak
Joslin Diabetes Center, Harvard Medical School, Boston, MA, USA
Lori M. Laffel
Charles Bronfman Institute for Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Ruth J. F. Loos
Broad Institute, Cambridge, MA, USA
James B. Meigs
Division of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Shivani Misra
Department of Diabetes & Endocrinology, Imperial College Healthcare NHS Trust, London, UK
Department of Diabetology, Madras Diabetes Research Foundation & Dr. Mohan’s Diabetes Specialities Centre, Chennai, India
Viswanathan Mohan
Department of Medicine, Faculty of Medicine and Health Sciences, University of Auckland, Auckland, New Zealand
Rinki Murphy
Auckland Diabetes Centre, Te Whatu Ora Health New Zealand, Auckland, New Zealand
Medical Bariatric Service, Te Whatu Ora Counties, Health New Zealand, Auckland, New Zealand
Oxford NIHR Biomedical Research Centre, University of Oxford, Oxford, UK
Katharine R. Owen
University of Cambridge, Metabolic Research Laboratories and MRC Metabolic Diseases Unit, Wellcome-MRC Institute of Metabolic Science, Cambridge, UK
Department of Epidemiology & Public Health, University of Maryland School of Medicine, Baltimore, MD, USA
Toni I. Pollin
Department of Internal Medicine, Division of Metabolism, Endocrinology and Diabetes, University of Michigan, Ann Arbor, MI, USA
Rodica Pop-Busui
AdventHealth Translational Research Institute, Orlando, FL, USA
Richard E. Pratley
Pennington Biomedical Research Center, Baton Rouge, LA, USA
Leanne M. Redman
MRC Human Genetics Unit, Institute of Genetics and Cancer, University of Edinburgh, Edinburgh, UK
Robert K. Semple
Yale School of Medicine, New Haven, CT, USA
Jennifer L. Sherr
Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
Arianne Sweeting
Department of Endocrinology, Royal Prince Alfred Hospital, Sydney, NSW, Australia
Kaiser Permanente Northwest, Kaiser Permanente Center for Health Research, Portland, OR, USA
Kimberly K. Vesco
Clinial Research, Steno Diabetes Center Copenhagen, Herlev, Denmark
Tina Vilsbøll
Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
Department of Endocrinology and Diabetology, University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Moorenstr. 5, 40225, Düsseldorf, Germany
Robert Wagner
You can also search for this author in PubMed Google Scholar
- Deirdre K. Tobias
- , Jordi Merino
- , Abrar Ahmad
- , Catherine Aiken
- , Jamie L. Benham
- , Dhanasekaran Bodhini
- , Amy L. Clark
- , Kevin Colclough
- , Rosa Corcoy
- , Sara J. Cromer
- , Daisy Duan
- , Jamie L. Felton
- , Ellen C. Francis
- , Pieter Gillard
- , Véronique Gingras
- , Romy Gaillard
- , Eram Haider
- , Alice Hughes
- , Jennifer M. Ikle
- , Laura M. Jacobsen
- , Anna R. Kahkoska
- , Jarno L. T. Kettunen
- , Raymond J. Kreienkamp
- , Lee-Ling Lim
- , Jonna M. E. Männistö
- , Robert Massey
- , Niamh-Maire Mclennan
- , Rachel G. Miller
- , Mario Luca Morieri
- , Jasper Most
- , Rochelle N. Naylor
- , Bige Ozkan
- , Kashyap Amratlal Patel
- , Scott J. Pilla
- , Katsiaryna Prystupa
- , Sridharan Raghavan
- , Mary R. Rooney
- , Martin Schön
- , Zhila Semnani-Azad
- , Magdalena Sevilla-Gonzalez
- , Pernille Svalastoga
- , Wubet Worku Takele
- , Claudia Ha-ting Tam
- , Anne Cathrine B. Thuesen
- , Mustafa Tosur
- , Amelia S. Wallace
- , Caroline C. Wang
- , Jessie J. Wong
- , Jennifer M. Yamamoto
- , Katherine Young
- , Chloé Amouyal
- , Mette K. Andersen
- , Maxine P. Bonham
- , Mingling Chen
- , Feifei Cheng
- , Tinashe Chikowore
- , Sian C. Chivers
- , Christoffer Clemmensen
- , Dana Dabelea
- , Adem Y. Dawed
- , Aaron J. Deutsch
- , Laura T. Dickens
- , Linda A. DiMeglio
- , Monika Dudenhöffer-Pfeifer
- , Carmella Evans-Molina
- , María Mercè Fernández-Balsells
- , Hugo Fitipaldi
- , Stephanie L. Fitzpatrick
- , Stephen E. Gitelman
- , Mark O. Goodarzi
- , Jessica A. Grieger
- , Marta Guasch-Ferré
- , Nahal Habibi
- , Torben Hansen
- , Chuiguo Huang
- , Arianna Harris-Kawano
- , Heba M. Ismail
- , Benjamin Hoag
- , Randi K. Johnson
- , Angus G. Jones
- , Robert W. Koivula
- , Aaron Leong
- , Gloria K. W. Leung
- , Ingrid M. Libman
- , S. Alice Long
- , William L. Lowe Jr.
- , Robert W. Morton
- , Ayesha A. Motala
- , Suna Onengut-Gumuscu
- , James S. Pankow
- , Maleesa Pathirana
- , Sofia Pazmino
- , Dianna Perez
- , John R. Petrie
- , Camille E. Powe
- , Alejandra Quinteros
- , Rashmi Jain
- , Debashree Ray
- , Mathias Ried-Larsen
- , Zeb Saeed
- , Vanessa Santhakumar
- , Sarah Kanbour
- , Sudipa Sarkar
- , Gabriela S. F. Monaco
- , Denise M. Scholtens
- , Elizabeth Selvin
- , Wayne Huey-Herng Sheu
- , Cate Speake
- , Maggie A. Stanislawski
- , Nele Steenackers
- , Andrea K. Steck
- , Norbert Stefan
- , Julie Støy
- , Rachael Taylor
- , Sok Cin Tye
- , Gebresilasea Gendisha Ukke
- , Marzhan Urazbayeva
- , Bart Van der Schueren
- , Camille Vatier
- , John M. Wentworth
- , Wesley Hannah
- , Sara L. White
- , Gechang Yu
- , Yingchai Zhang
- , Shao J. Zhou
- , Jacques Beltrand
- , Michel Polak
- , Ingvild Aukrust
- , Elisa de Franco
- , Sarah E. Flanagan
- , Kristin A. Maloney
- , Andrew McGovern
- , Janne Molnes
- , Mariam Nakabuye
- , Pål Rasmus Njølstad
- , Hugo Pomares-Millan
- , Michele Provenzano
- , Cécile Saint-Martin
- , Cuilin Zhang
- , Sungyoung Auh
- , Russell de Souza
- , Andrea J. Fawcett
- , Chandra Gruber
- , Eskedar Getie Mekonnen
- , Emily Mixter
- , Diana Sherifali
- , Robert H. Eckel
- , John J. Nolan
- , Louis H. Philipson
- , Rebecca J. Brown
- , Liana K. Billings
- , Kristen Boyle
- , Tina Costacou
- , John M. Dennis
- , Jose C. Florez
- , Anna L. Gloyn
- , Maria F. Gomez
- , Peter A. Gottlieb
- , Siri Atma W. Greeley
- , Kurt Griffin
- , Andrew T. Hattersley
- , Irl B. Hirsch
- , Marie-France Hivert
- , Korey K. Hood
- , Jami L. Josefson
- , Soo Heon Kwak
- , Lori M. Laffel
- , Siew S. Lim
- , Ruth J. F. Loos
- , Ronald C. W. Ma
- , Chantal Mathieu
- , Nestoras Mathioudakis
- , James B. Meigs
- , Shivani Misra
- , Viswanathan Mohan
- , Rinki Murphy
- , Richard Oram
- , Katharine R. Owen
- , Susan E. Ozanne
- , Ewan R. Pearson
- , Wei Perng
- , Toni I. Pollin
- , Rodica Pop-Busui
- , Richard E. Pratley
- , Leanne M. Redman
- , Maria J. Redondo
- , Rebecca M. Reynolds
- , Robert K. Semple
- , Jennifer L. Sherr
- , Emily K. Sims
- , Arianne Sweeting
- , Tiinamaija Tuomi
- , Miriam S. Udler
- , Kimberly K. Vesco
- , Tina Vilsbøll
- , Robert Wagner
- , Stephen S. Rich
- & Paul W. Franks
Contributions
All authors J.L.B., V.G., N.-M.M., J.M., J.M.Y., C.E.A., S.E.O. and R.M.R. contributed to the design of the research questions, study selection, extraction of data, data analyses, quality assessment and data interpretation. The ADA/EASD PMDI consortium members provided feedback on methodology and reporting guidelines. RMR wrote the first draft of the manuscript. All authors edited the manuscript and all approved the final version.
Corresponding author
Correspondence to Rebecca M. Reynolds .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Communications Medicine thanks Rochan Agha-Jaffar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, supplementary data 1, supplementary data 2, supplementary data 3, supplementary data 4, peer review file, description of additional supplementary files, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Benham, J.L., Gingras, V., McLennan, NM. et al. Precision gestational diabetes treatment: a systematic review and meta-analyses. Commun Med 3 , 135 (2023). https://doi.org/10.1038/s43856-023-00371-0
Download citation
Received : 08 May 2023
Accepted : 25 September 2023
Published : 05 October 2023
DOI : https://doi.org/10.1038/s43856-023-00371-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine.
- Jordi Merino
- Paul W. Franks
Nature Medicine (2023)
Communications Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Veillonella and Bacteroides are associated with gestational diabetes mellitus exposure and gut microbiota immaturity
Roles Formal analysis, Investigation, Methodology, Writing – original draft
Affiliation Laboratorio de Envejecimiento Saludable, Instituto Nacional de Medicina Genómica, Centro de Investigación Sobre Envejecimiento (CIE-CINVESTAV Sur), Ciudad de México, México
Roles Resources
Affiliation Hospital de la Niñez Oaxaqueña, Oaxaca, México
Affiliation Unidad de Bioquímica e Inmunología, Tecnológico Nacional de México-Instituto Tecnológico de Oaxaca, Oaxaca, México
Affiliation Centro de Investigación Facultad de Medicina UNAM-UABJO, Facultad de Medicina y Cirugía, Universidad Autónoma “Benito Juárez” de Oaxaca, Oaxaca, México
Roles Conceptualization, Funding acquisition, Project administration, Resources, Writing – review & editing
* E-mail: [email protected] (NM-C); [email protected] (BP-G)
Affiliation Unidad de Vinculación Científica de la Facultad de Medicina UNAM en Instituto Nacional de Medicina Genómica, Ciudad de México, México
Roles Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing
- Fernanda Valdez-Palomares,
- Jaqueline Reyes Aguilar,
- Eduardo Pérez-Campos,
- Laura Pérez-Campos Mayoral,
- Noemi Meraz-Cruz,
- Berenice Palacios-González

- Published: May 14, 2024
- https://doi.org/10.1371/journal.pone.0302726
- Reader Comments
Dysbiosis during childhood impacts the configuration and maturation of the microbiota. The immaturity of the infant microbiota is linked with the development of inflammatory, allergic, and dysmetabolic diseases.
To identify taxonomic changes associated with age and GDM and classify the maturity of the intestinal microbiota of children of mothers with GDM and children without GDM (n-GDM).
Next-generation sequencing was used to analyze the V3–V4 region of 16S rRNA gene. QIIME2 and Picrust2 were used to determine the difference in the relative abundance of bacterial genera between the study groups and to predict the functional profile of the intestinal microbiota.
According to age, the older GDM groups showed a lower alpha diversity and different abundance of Enterobacteriaceae, Veillonella , Clostridiales , and Bacteroides . Regarding the functional profile, PWY-7377 and K05895 associated with Vitamin B12 metabolism were reduced in GDM groups. Compared to n-GDM group, GDM offspring had microbiota immaturity as age-discriminatory taxa in random forest failed to classify GDM offspring according to developmental age (OOB error 81%). Conclusion. Offspring from mothers with GDM have a distinctive taxonomic profile related to taxa associated with gut microbiota immaturity.
Citation: Valdez-Palomares F, Aguilar JR, Pérez-Campos E, Mayoral LP-C, Meraz-Cruz N, Palacios-González B (2024) Veillonella and Bacteroides are associated with gestational diabetes mellitus exposure and gut microbiota immaturity. PLoS ONE 19(5): e0302726. https://doi.org/10.1371/journal.pone.0302726
Editor: Michael Bader, Max Delbruck Centrum fur Molekulare Medizin Berlin Buch, GERMANY
Received: January 5, 2024; Accepted: April 10, 2024; Published: May 14, 2024
Copyright: © 2024 Valdez-Palomares et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Raw data are available in https://www.ncbi.nlm.nih.gov/bioproject/ with the accession number: PRJNA1085791.
Funding: Universidad Nacional Autónoma de México, PAPIIT-DGAPA grant IN221014, Noemi Meraz-Cruz Instituto Nacional de Medicina Genómica, INMEGEN grant 11/2016/I, Berenice Palacios-Gonzalez.
Competing interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Introduction
Gestational diabetes mellitus (GDM) according to the American Diabetes Association is defined as “diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes before gestation” [ 1 ]. In Mexico, the prevalence of GDM has been increasing; currently, its incidence is 17.7% [ 2 ]. GDM during pregnancy increases the susceptibility of offspring to develop insulin resistance, obesity, and hypertension [ 3 , 4 ].
Early alteration of the childhood microbiota is associated with allergies, inflammation, and childhood obesity [ 5 – 7 ]. According to the Developmental Origin of Health and Disease (DOHaD) theory the intrauterine exposure to excessive energy may result in permanent physiological and metabolic alterations, increasing disease risk in adulthood to developing obesity and type 2 diabetes [ 8 – 13 ].
The intestinal microbiota of the newborn is particularly interesting because, due to rapid temporal variation, the bacterial communities in the intestine are remarkably unstable. Therefore, early childhood is a crucial time window where the child’s gut microbiota can be modified [ 14 , 15 ] in contrast to an adult individual’s "mature" microbiota, which seems relatively stable over time.
The aim of this study was to identify taxonomic changes associated with age and GDM and to classify the gut-microbiota maturity of offspring from mothers with GDM and the offspring from mothers without GDM (n-GDM)
Materials and methods
Study population.
The present is a cross-sectional study conducted in “C.S.T.III Dr. Gabriel Garzón Cossa" and “Hospital de la Niñez Oaxaqueña” located in Mexico. Offspring exposed and not exposed to GDM aged 0 to 30 months, either born vaginally or via C-section were included during routine hospital visit, only mothers that underwent a 75-g oral glucose tolerance test (OGTT) between 24- and 28-weeks’ gestation as part of screening protocol to determine GDM diagnosis were included. GDM was diagnosed according to the International Association of the Diabetes and Pregnancy Study Group criteria: Plasma glucose level ≥8.5 mmol/L following a 75 g OGTT [ 16 ]. A total of 40 infants were included: 26 infants not exposed to GDM (0–6 Months (n = 6), 7–12 Months (n = 10) and 13–30 Months (n = 10)) and 14 infants exposed to GDM (0–6 Months (n = 4), 7–12 Months (n = 5) and 13–30 Months (n = 5)). The 2009 WHO child growth standards (World Health Organization 2009) were used as a reference for children’s weight and length/height [ 17 ]. Maternal and infant characteristics and feeding practices (breastfeeding, formula, or mixed) were collected from medical records during interview and medical records (data were accessed for research purposes between the 9th of January 2017 and the 30th of November 2017). The following exclusion criteria were used: Infants with history of hospitalization and or use of antibiotics six months before the study, presence of chronic illness, gastrointestinal pathology, or diarrheal illness presented one month before the study. The study was performed according to the latest version of the Declaration of Helsinki and was approved by the Human Research Ethical Committee of Universidad Nacional Autónoma de México 079/2017/CEI-HGT. All parents or legal guardians and children provided written informed consent.
Anthropometric measurements
The children were weighed, for which their clothing, diapers, and ornaments were removed. Length was measured using an infantometer, placing the child in a supine position with the head resting against the headboard; the measurer slid the stirrup along the base until it was flat against the soles of the feet, and the measurement was recorded from the digital counter (accuracy ±1.0 mm). Head circumference was measured using Seca 121 tape (Seca 212). The anthropometric measurements were obtained using standardized procedures applied by trained personnel.
Fecal bacterial DNA isolation, libraries preparation and bioinformatic analysis
Fecal samples from infants aged 0–6 Months: (n = 6 not exposed to GDM; n = 4 exposed to GDM), aged 7–12 Months: (n = 10 not exposed to GDM; n = 5 exposed to GDM) and aged 13–30 Months: (n = 10 not exposed to GDM; n = 5 exposed to GDM) were collected from diapers or digital rectal examination, and placed in a sterile plastic container. The QIAamp PowerFecal Pro DNA kit (QIAGEN) was used to isolate bacterial DNA. DNA concentrations were determined using a NanoDrop V3.8.1 spectrophotometer. The 16s rRNA gene was amplified using primers 338F and 806R, targeting the V3-V4 hypervariable regions. The libraries were sequenced at the Sequencing Unit of the Instituto Nacional de Medicina Genómica using the Illumina Miseq platform (Illumina, San Diego, CA) [ 18 ]. Fastq reads were processed using the Quantitative Insights Into Microbial Ecology 2 (QIIME 2) [ 19 ]. The dada2 denoise-paired instruction was used for denoising quality, chimera checking, and clustering. For the 97% taxonomic assignment, the SILVA 16S reference database (version_138) and the classifiers naïve Bayes algorithm. A rooted phylogenetic tree was generated for further statistical analysis for α-diversity tests and Bray-Curtis for β-diversity tests. Microbial community metagenome prediction was performed with PICRUSt2 [ 20 ]. Microbiome Analyst was used to create the heat trees with mean abundance and the non-parametric Wilcoxon rank sum test corrected by Benjamin Hochberg (FD) ( http://www.microbiomeanalyst.ca ) [ 21 ]. Raw data are available in https://www.ncbi.nlm.nih.gov/bioproject/ with the accession number: PRJNA1085791.
Classification of gut-microbiota maturity by developmental stages using Random Forests
The random forest classification model used the ’randomForest’ R package. A rarefied ASV table served as input data; the model was trained on 26 non-GDM infants and validated on 10 non-GDM infants, built using the following parameters: ntree = 10,000 and mtry of p/3 ASVs randomly sampled at each split, in which p represents the number of ASVs. The model was further refined by applying tenfold cross-validation to estimate the minimal number of top-ranking age-discriminatory taxa required for prediction, including 15 ASVs to train the final model based on a mean decrease in Gini. The Random Forests algorithm was applied to classify healthy infants by chronologic age ranging from 0–6 months, >6–12 months, and >12–30 months, thereby identifying taxa that discriminate by chronologic age in healthy children and subsequently applied on 16 GDM offspring infants.
Statistical analysis
Continuous quantitative variables (anthropometric data) will be presented as the mean ± standard deviation (SD) for variables with parametric distribution and median and 25th and 75th percentiles for variables with non-parametric distribution. To evaluate the differences between the study groups, the one-way ANOVA or Kruskall Wallis test will be used according to the homoscedasticity criterion and the normality of the data. Statistical analyses will be performed in the GraphPad 9 statistical program and R ( https://www.r-project.org/ ) [ 22 , 23 ].
Population demographics and clinical characteristics
A total of 40 children were included: 26 children not exposed to GDM (n-GDM) and 14 children exposed to GDM (GDM) ( Table 1 ) . Regarding clinical parameters, there were no significant differences between the groups.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0302726.t001
Composition of the bacterial community of children exposed to GDM
From the Illumina 330 bp paired-end sequencing of the amplicon targeting the V3–V4 region of 16S rRNA gene, two thousand five hundred seventy-nine high-quality sequences among the 30 fecal samples from the participants with an average of 30,027 sequences per sample were generated. Chao1, Shannon, and Simpson index were used to describe alpha diversity ( Fig 1A–1C ). There were no significant differences in alpha diversity between n-GDM and GDM groups. To compare microbial communities’ composition, we calculated the beta diversity by Bray-Curtis index ( Fig 1D ). No significant difference was found between the groups.
(a) Chao index in n-GDM and GDM group, no significant differences were observed between the groups; (b) Shannon index in n-GDM and GDM group, no statistically significant differences were found between the groups; (c) Simpson index in n-GDM and GDM group, no differences were found among the group; (d) Bray-Curtis index in n-GDM and GDM groups, no significant differences were shown amongst the groups, n-GDM group (light blue circles) and GDM group (gray circles); (e) Phylum-level composition (% relative abundances) among the study groups. Firmicutes, coloured yellow, followed by Bacteroidetes, coloured orange; (f) Genus-level composition (% relative abundances) among the study groups, the most abundant taxa were Bacteroides , Akkermansia , Bifidobacterium , Blautia , Clostridium , Escherichia_Shigella , Faecalibacterium , Klebsiella , Parabacteroides , Streptococcus and Veillonella ; (g) Heat tree for pair-wise comparison. Those taxa that showed statistically significant differences were Veillonella of yellow colour, enrichment in the n-GDM group; and green-coloured Bacteroides enrichment in the GDM group.
https://doi.org/10.1371/journal.pone.0302726.g001
The predominant phyla were Firmicutes, with a mean abundance of 44%, followed by 24% of Bacteroidetes, Proteobacteria, which represented 26%, while Verrucomicrobia and Actinobacteria represented 1% and 5%, respectively ( Fig 1E ). Comparisons of the relative abundance of the taxa were made to determine differences at the taxonomic level between each age group ( Fig 1F ). The fecal microbiota of the GDM group had significantly lower (p = 0.007) proportions of Firmicutes, specifically in Veillonella genus, and significantly higher (p = 0.014) proportions of Bacteroidetes, specifically in Bacteroides , compared to n-GDM group ( Fig 1G ). Fecal microbiota according to type of delivery was also compared. In vaginally born infants, the predominant phyla were Bacteroidetes , while, Proteobacteria and Actinobacteria were main constituents in C-section infants ( S1A Fig ). No significant differences in alpha or beta diversity were detected among groups ( S1B and S1C Fig ), Enrichment in members of the Proteobacteria phyla: Gammaproteobacteria , Enterobacteriales , Enterobacteriaceae and Escherichia_Shigella genus was detected in C-section infants, while enrichment in the Bacteroidetes phylum members, Bacteroides , Bacteroidales , Bacteroidaceae and Bacteroides was detected in vaginally born infants ( S1D Fig ).
Influences of age-related GDM on the microbiota composition
The effect of age on the bacterial community composition was assessed. Regarding alpha diversity, the GDM group presented lower alpha diversity as age advanced ( Fig 2A–2C ). Interestingly, the GDM group remains at all alpha diversity metrics below the n-GDM groups. The beta diversity analysis ( Fig 2D ), calculated on the Bray-Curtis dissimilarity, revealed no statistically significant difference (F-value: 1.2489; R2: 0.14782; p-value: 0.088). Subsequently, the groups were divided according to age ranges: newborns to 6 months (Group 1), 7 to 12 months (Group 2), and 13 months to 24 months (Group 3). At the phyla level, no significant differences were found ( Fig 2E ). On the other hand, the GDM-1 group presented a lower abundance of Enterobacteriaceae (family) ( Fig 2F ). The GDM-1 and GDM2 groups showed a lower abundance of Veillonella (a genus belonging to the Clostridiales order) ( Fig 2F and 2G ), while the GDM3 group only showed a lower abundance in Clostridiales without reaching a difference in any specific genus ( Fig 2H ). The GDM-1 and GDM-3 groups also presented a higher abundance of Bacteroides (genus) ( Fig 2F and 2G ) .
(a) Chao index, (b) Shannon index, (c) Simpson index across the age, n-GDM group (light blue circles) and GDM group (gray circles), the GDM group presented lower alpha diversity as age advance; (d) Bray-Curtis index across the age in n-GDM and GDM groups, no significant differences were found between the groups (F-value: 1.2489; R2: 0.14782; p-value: 0.088), newborns to 6 months (n-GDM group, red circles; GDM group, dark purple), 7 to 12 months (n-GDM group, orange circles; GDM group, light purple) and 13 months to 24 months (n-GDM group, yellow circles; GDM group, gray circles); (e) Phylum-level composition (% relative abundances) among the study groups; Heat tree for pair-wise comparison, divided by age, (f) newborns to 6 months, (g) 7 to 12 months and (h) 13 months to 24 months. Those taxa that showed statistically significant differences are shown in colour. Taxa coloured yellow are enriched in the n-GDM group and those coloured green are enriched in the GDM group. The colour of each taxon indicates the log-2 ratio of the proportions observed in each condition.
https://doi.org/10.1371/journal.pone.0302726.g002
Age-related effects on microbial functional pathways
PICRUSt2 was employed to predict functional differences in fecal microbiota. The n-GDM microbiome was enriched for the KEGG pathway oxidoreductases (K05895 (cobK-cbiJ); pre-corrin-6A/cobalt-pre corrin-6A reductase; Vitamin B12 metabolism) and PWY-7377 (adenosylcobalamin biosynthesis I (anaerobic); cob(II) urinate a c-diamide biosynthesis I (early cobalt insertion)) ( Fig 3A and 3B ). These pathways were inversely related to age in n-GDM group. Vitamin 12 biosynthesis is mediated exclusively by the bacterial fermentation process, so a correlation matrix between the predicted abundance pathway and differentially expressed taxa was performed. PWY-7377 positively correlated with Veillonella , Clostridium sensu stricto ; Escherichia-Shigella and Streptococcus , meanwhile negatively correlated with Bacteroides ( Fig 3C ).
(a) KEGG Orthology (KO; K05895). Red indicates enrichment and green shows depletion; (b) Microbial metabolic pathway PWY-7377 Cob(II)yrinate a,c-diamide bio-synthesis I (early cobalt insertion) in n-GDM and GDM groups. p <0.05 are considered statistically significant differences. Statistically significant differences among the groups are shown with letters, where a>b>c>d; (c) Heatmap correlogram containing Pearson’s rho negative and positive correlation coefficients. Blue and red color denotes positive and negative correlation coefficients correlation, respectively. *p < 0.05. Fecal samples from infants aged 0–6 Months: (n-GDM1: n = 6; GDM1: n = 4), aged 7–12 Months: (n-GDM2: n = 10; GDM2: n = 5) and aged 13–30 Months: (n-GDM3: n = 10; GDM3: n = 5).
https://doi.org/10.1371/journal.pone.0302726.g003
GDM infants have immature gut microbiota in the early stages of development
To define the maturity of the microbiota, the Random Forests (RF) model resulting from healthy children was used, thus determining those taxa that help to distinguish the different stages of postnatal life. Rated lists of all bacterial taxa, in order of ’age-discriminative importance,’ were selected by considering those whose relative abundance values, when permuted, have the most considerable mean decrease in Gini. An increase in validation error was observed when including taxa beyond the top 15 ( Fig 4A ). Log-transformed counts of the top 15 relatives of age-discriminating taxa are shown in Fig 4B . RF model of 15 top discriminatory taxa accurately classified healthy offspring according to developmental age (OOB error 0%) ( Fig 4C ). The RF model was later applied with no further parameter optimization to classify GDM offspring according to periods of postnatal life. The results revealed that compared to healthy, GDM offspring had microbiota immaturity as age-discriminatory taxa in RF failed to classify developmental age (OOB error 81%) of GDM offspring ( Fig 4D ).
A top 15 age-discriminatory taxa random forest classification model was constructed with healthy offspring samples. (a) The number of features was selected by ten-fold cross-validation (b). The 15 features in the model and their abundance are shown the model was applied to healthy offspring (c) and to GDM offspring (d).
https://doi.org/10.1371/journal.pone.0302726.g004
GDM is a public health problem that increases the risk of maternal and perinatal morbidity and mortality and contributes to the development of long-term health complications in both mothers and offspring. GDM has been associated with changes in the intestinal microbiota both in the neonate and during pregnancy [ 24 – 27 ]. Interestingly, intestinal dysbiosis during the first stages of life is associated with long-term health status, which denotes the importance of the intestinal microbiota in the first years of life on the development of metabolic and immunological diseases [ 28 , 29 ]. In the present study, no changes in microbiota composition were observed when comparing children exposed to GDM with children not exposed to GDM. However, when the groups were separated by age, the offspring of mothers with GDM maintained lower alpha diversity. As mentioned above, the gut microbiota in early childhood (≤2 years) changes over time [ 30 ], both at the taxonomic level and in alpha and beta diversity metrics [ 31 ]. Offspring that were not exposed to DMG showed continuous development and maturation of the intestinal microbiota during the first years of life, unlike offspring from mothers exposed to DMG that presented lower alpha diversity, which has been associated with overall delay in microbial maturation and subsequent adverse health outcomes, including atopy and allergic diseases [ 32 , 33 ]. This result is consistent with other studies indicating that GDM is associated with lower diversity [ 34 , 35 ]. Unlike what Crusell et al. observed, alpha diversity remains decreased in the offspring of children exposed to GDM and does not show the recovery observed at nine months [ 34 ]. Furthermore, obese children have less diversity and richness [ 36 ], suggesting that in children with GDM, the immaturity of the gut microbiota could be a determining factor for the development of obesity in the future [ 37 ].
Several studies suggest early colonization is essential for establishing and maturing the gut microbiota. During the early colonization of the newborn intestine, facultative anaerobic Proteobacteria gradually consume oxygen in the gastrointestinal tract, and, consequently, obligate anaerobes colonize the new environment [ 38 ]. Premature infants exhibit immaturity in the gut microbiota, characterized by the predominance of pathogenic bacteria within the Enterobacteriaceae family, persistently low diversity, and a scarcity of strictly anaerobic taxa, including Veillonella relative to appropriately growing [ 39 ]. Interestingly, in this study, the offspring of mothers with GDM showed a lower abundance, specifically of the taxon Veillonella , which, as mentioned above, is considered involved in developing the gut microbiota as seed species along with Streptococcus [ 40 ]. The above suggests that the presence of GDM affects the maturity of the intestinal microbiota.
In addition, the groups with GDM had a greater abundance of Bacteroides; Vatanen et al. demonstrated in vitro studies that Bacteroides species from newborns with increased susceptibility to type 1 diabetes and allergies produce a subtype of lipopolysaccharide (LPS) that inhibits the immunostimulatory activity of Escherichia coli LPS [ 41 ]. The authors also point out that in the first year of life, the metabolism of human milk oligosaccharides (HMO) is involved in the maintenance and/or establishment of an intestinal microbiota dominated by Bifidobacterium versus Bacteroides, presumably because both genera contend for the HMO as an energy source, the above is of clinical relevance to promote breastfeeding in children from pregnancies with GDM. Differential enrichment in Bacteroidetes - and Proteobacteria -related taxa according to type of delivery was detected in infants as previously reported by other authors [ 42 ]. However, the present RF classification model trained using data from infants with different type of delivery, was conducted to define chronological age and microbiota maturity in the offspring form healthy mothers regardless of the type of delivery. According to this, ASVs assigned to the discriminatory genera Escherichia_Shigella and Bacteroides in C-section and vaginally born infants were among top 15 age discriminatory taxa in n-GDM offspring group, therefore, discriminatory taxa for type of delivery can also define microbiota maturity.
On the other hand, in this study, the PWY-7377 pathway implicated in the biosynthesis of vitamin B12 (cobalamin) was inversely related to age in the n-GDM group and significantly correlated with the abundance of Veillonella . The synthesis of vitamin B12 is mainly carried out by three orders: Propionibacterales, Corynebacterales, Coriobacterales belonging to Actinobacteria, and the orders Clostridiales, Selenomonadales and Veillonellales ( Veillonella atypica and Veillonella parvula ) of Firmicutes [ 43 ]. Although cobalamin in mammals is synthesized exclusively by the gut microbiota, microbial vitamin B12 production plays a limited function due to its limited availability in the environment. Two issues prevent humans from obtaining significant levels of cobalamin from this source. First, of the total corrinoids in feces, less than 2% of cobalamin is produced by intestinal bacteria; Secondly, due to the absence of transporters of this vitamin in the colon [ 44 , 45 ]. Taking this into account, a possible explanation for our findings could come from vitamin B12-dependent reactions in bacteria. Vitamin B12 contributes to the microbiota as a modulator of intestinal microbial ecology. Since 80% of bacteria encode cobalamin-dependent enzymes [ 45 ]. Considering this information and our results, one hypothesis is that those prototrophic taxa, such as Veillonella , would help supply cobalamin to auxotrophic taxa during the first months of life. Kundra et al. demonstrated that even in the absence of B12, the presence of prototrophic bacteria could supply sufficient cobalamin for the remaining 80% of auxotrophic taxa [ 46 ]. Thus, the presence of Veillonella could help modulate the configuration of the microbiota in the early stages of life.
We found that GDM-exposed offspring had disrupted microbiota maturation characterized by persistently low diversity and reduced Veillonella compared to non-GDM-exposed infants. Finally, a random forest classification model was used to model the maturation of the microbiota of healthy infants, based on the approach of Subramanian et al. [ 47 ]. The model taxa included Bifidobacterium , Escherichia-Shigella , Bacteroides , Veillonella , Clostridium sensu stricto (group I), and Faecalibacterium . Interestingly, Bacteroides , Bifidobacterium , and Clostridium sensu stricto I are first colonizers of the infant intestine [ 48 ]. While Faecalibacterium prausnitzii is present at low abundance and, in some cases, absent during early childhood and with advancing age, its abundance increases until reaching the levels observed in adulthood [ 49 ], suggesting that GDM negatively affects the early establishment in the gut of early colonizers concerning health and delayed establishment of microbiota members that are expected to increase in abundance after the first year of life. However, limitations of the study, are the relatively small number of infants exposed and not exposed to GDM and the predictive limitations of our cross-sectional study, additional prospective studies are needed to replicate our findings and determine whether these signatures of microbiota maturation could apply to GDM-exposed offspring. However, limitations of the study, are the relatively small number of infants exposed and not exposed to GDM and the predictive limitations of a cross-sectional study, further prospective studies are needed to replicate our findings and determine whether these signatures of microbiota maturation could be applicable to GDM-exposed offspring.
In conclusion, children of mothers with GDM have a distinctive taxonomic profile related to the immaturity of the intestinal microbiota. Our results show the importance of the first months of life for the maturation and future configuration of the intestinal microbiota.
Supporting information
S1 fig. microbiota analysis in children not exposed and exposed to gdm stratified by pregnancy outcome..
(a) Phylum-level composition (% relative abundances) among the study groups. (b) Observed features, Chao and Shannon index in C-section and vaginally born infants, no significant differences were observed between the groups; (c) Bray-Curtis index in C-section and vaginally born infants, no significant differences were shown amongst the groups. (d) Heat tree for pair-wise comparison. Those taxa that showed statistically significant differences were members of Proteobacteria in C-section group (red) and members of Bacteroidetes in vaginally born infants group (blue). The colour of each taxon indicates the log-2 ratio of the proportions observed in each condition.
https://doi.org/10.1371/journal.pone.0302726.s001
- View Article
- PubMed/NCBI
- Google Scholar
- 17. Organization W.H., WHO child growth standards and the identification of severe acute malnutrition in infants and children: A Joint Statement by the World Health Organization and the United Nations Children’s Fund. 2009, World Health Organization: Geneva, Switzerland.
- Open access
- Published: 19 May 2024
Adipose stem cells-derived small extracellular vesicles transport Thrombospondin 1 cargo to promote insulin resistance in gestational diabetes mellitus
- Huaping Li 1 na1 ,
- Hao Yang 2 na1 ,
- Jingyan Liu 2 ,
- Hedi Yang 2 ,
- Xinyu Gao 2 ,
- Xiaoying Yang 2 ,
- Zhou Liu 1 &
- Qiaohui Qian 3
Diabetology & Metabolic Syndrome volume 16 , Article number: 105 ( 2024 ) Cite this article
Metrics details
Gestational diabetes mellitus (GDM) is a highly prevalent disease and poses a significant risk to the health of pregnant women. Abdominal adipose tissue (AT) contributes to insulin resistance (IR) associated with GDM. However, the underlying mechanisms remain unclear.
In this study, we developed a mouse model of GDM by subjecting mice to a high-fat diet. We collected adipose-derived stem cells (ADSCs) from the abdominal and inguinal regions and examined their role in inducing IR in normal tissues through the secretion of small extracellular vesicles (sEVs). The sEVs derived from ADSCs isolated from GDM mice (ADSC/GDM) were found to inhibit cell viability and insulin sensitivity in AML12, a normal mouse liver cell line.
Through proteomic analysis, we identified high levels of the thrombospondin 1 (Thbs1) protein in the sEVs derived from ADSC/GDM. Subsequent overexpression of Thbs1 protein in AML12 cells demonstrated similar IR as observed with ADSC/GDM-derived sEVs. Mechanistically, the Thbs1 protein within the sEVs interacted with CD36 and transforming growth factor (Tgf) β receptors in AML12 cells, leading to the activation of Tgfβ/Smad2 signaling. Furthermore, the administration of LSKL, an antagonistic peptide targeting Thbs1, suppressed Thbs1 expression in ADSC/GDM-derived sEVs, thereby restoring insulin sensitivity in AML12 cells and GDM mice in vivo.
Conclusions
These findings shed light on the intercellular transmission mechanism through which ADSCs influence hepatic insulin sensitivity and underscore the therapeutic potential of targeting the Thbs1 protein within sEVs.
Introduction
Gestational diabetes mellitus (GDM) refers to glucose intolerance that occurs or is first diagnosed during the second or third trimester of pregnancy [ 1 ]. It is considered an early stage of type 2 diabetes mellitus (T2DM). Recent changes in the population structure, including increased elderly fertility rates and obesity rates among women, have contributed to the rising prevalence of GDM has an upward trend in recent years [ 2 ]. The global prevalence of GDM varies substantially, ranging from 1% to > 30%, with Asians showing a particularly high prevalence (i.e., South-East Asia: 9.6–18.3%; Western Pacific (China): 4.5–20.3%) [ 3 ]. In China, due to the changing fertility strategy, the prevalence of high-risk pregnant women, such as those of advanced age and with pre-pregnancy overweight or obesity, has dramatically increased. A recent systematic review reported a pooled prevalence of GDM in China of 14.8% (95% confidence interval (CI) 12.8–16.7%) [ 4 ]. GDM poses significant risks of both the mother and the developing fetus. Up to 50% of GDM patients later develop T2DM [ 5 ], and affected mothers are at a greater risk of cardiovascular disease [ 6 ]. For infants, GDM can lead to neonatal hypoglycemia and insulin resistance (IR)-related obesity in young adulthood [ 7 ]. Additionally, approximately 35% to 50% of GDM progress to T2DM within 10 years after delivery [ 8 ].
The pathogenesis of GDM involves two main factors: high IR and decreased production of insulin by pancreatic β-cells [ 9 ]. Obesity and GDM are associated with elevated inflammatory markers, leading to IR. In obesity, rapid expansion of fat cells can result in lipid imbalance, chronic inflammation, and tissue fibrosis [ 10 ].Therefore, the role of inflammation in the development of GDM is considered crucial [ 11 ]. The liver, a target organ of IR-related diseases such as GDM and T2DM, exhibits dysfunction in the early pregnancy, which can increase the risk of GDM in the pregnancy period [ 12 , 13 ].
Adipose-derived stem cells (ADSCs) are a type of mesenchymal stem cells obtained by separating adipocytes. They possess the ability to differentiate into other adipocytes and secrete various paracrine cytokines, growth factors, microRNAs, and important components such as small extracellular vesicles (sEVs) that support normal cellular functions. The potential therapeutic applications of sEVs derived from ADSCs have been confirmed for "stem cell-free therapy" in neurodegenerative and metabolic diseases [ 14 ]. However, factors such as a long-term hyperglycemic environment and reactive oxygen species within the recipient can alter the biological function of ADSCs [ 15 ]. Currently, there are no reports of abnormal expression of sEVs derived from ADSCs under pathological conditions. Nevertheless, evidence suggests that sEVs isolated from plasma of GDM females can induce abnormal glucose tolerance and impair skeletal muscle sensitivity to insulin in mice [ 16 ].
The endoplasmic reticulum (ER), an organelle responsible for storing calcium ions, plays a crucial role in synthesizing and processing membrane proteins and lipid biosynthesis. Maintaining ER's internal environment stability is vital for cellular survival, proliferation, and growth [ 17 ]. Thrombospondin 1 (Thbs1), a protein secreted by small sEVs from ADSCs, possesses multiple functional domains and is predominantly expressed in the visceral adipose tissue (AT) of IR or obese individuals [ 18 ]. GDM can activate the ER stress signal, disrupt ER homeostasis, promote the accumulation of unfolded proteins within the ER lumen, and induce the unfolded protein response or ER stress (ERS). ERS plays a central role in triggering IR and T2DM [ 19 ].
Thbs1 has been identified as one of the markers in the early stages of diabetes and a key mediator in its development. The peptide antagonist, LSKL, can inhibit Thbs1 activity, thereby reducing diabetes-related complications resulting from Thbs1 upregulation [ 20 , 21 ]. The expression of Thbs1 is positively correlated with obesity and IR. Knocking out Thbs1 in AT can inhibit tissue inflammation caused by obesity and improve tissue insulin sensitivity [ 22 ]. However, little is known about the abnormal expression of ADSC-derived exosomes under GDM conditions and how they affect the insulin sensitivity of GDM target organs, particularly the liver histiocyte, promoting the development of IR in GDM. Therefore, our study aimed to investigate the intercellular transmission mechanism of ADSCs that influence insulin sensitivity.
Materials and methods
Establishment and identification of mice with gdm.
Five-week-old female C57BL/6 J mice were purchased from Shanghai Bikaikeyi Biotechnology Co., LTD. (License No. SCXK2018-0006) and housed in an SPF animal laboratory. Prior to the experiment, tail venous blood samples were collected from the mice to determine fasting blood glucose levels, with a normal range of 4–7 mmol/L. Sixteen mice were randomly divided into two groups: one group received a high-fat diet containing 60% fat (XTHF60, Xietong pharmaceutical bio, Nanjing, China; 8 mice), while the other group received a normal diet (8 mice). After 8 weeks, female mice were placed in the same cage with male C57BL/6 J mice at a ratio of 2:1. Once pregnancy was confirmed, the mice continued to be fed their respective diets for 12 days. Blood glucose levels were recorded every 3 days during this period, and GDM was considered successfully induced if random blood glucose levels exceeded 11.1 mmol/L. On day 12, plasma was collected, and the concentrations of insulin, leptin, adiponectin, and hypersensitive C-reactive protein (hs-CRP) were determined using an enzyme-linked immunosorbent assay (ELISA) kit (Mlbio, Shanghai, China) following the manufacturer's instructions. All animal experiments were approved by the Animal Ethics Committee of Shanghai University of Medicine and Health Sciences.
ADSCs isolation and cell culture
Primary adipose stem cells (ADSCs) were isolated from GDM mice and normal gestational mice, respectively. Under aseptic conditions, the abdominal cavity of the mice was opened, and AT from the abdominal and inguinal regions was obtained. The AT was then rinsed, minced, and collected in a pre-cooled Hank’s Balanced Salt Solution (Sangon BIOTECH, Shanghai, China). Next, 2 mg/ml collagenase I (Yeason, Shanghai, China) and 3 mM CaCl 2 were added in double volume to the tissue, which was then digested at 37 ℃ for 4 h. Digestion was stopped by adding an equal volume of DMEM/F12 medium (Gibco, GIBCO, NY, USA) containing 10% FBS (Merck KGaA, Darmstadt, Germany), followed by centrifugation at 1200 g for 10 min. The cell precipitates were resuspended, washed with PBS, and cultured in DMEM/F12 medium containing 10% FBS. Mouse normal liver cells, AML12, were purchased from Shanghai Fuheng Biotechnology and cultured in DMEM/F12 medium containing 10% FBS, 1% ITS media supplement (R&D Systems, MN, USA), and 40 ng/ml dexamethasone (Merck KGaA).
Plasmid and reagent
The encoding region of mouse Thbs1 mRNA (NCBI number: NM011580) was cloned onto the pcDNA3.1–3 × Flag-eGFP-C2 vector. The control vector and Flag-eGFP-Thbs1 fusion plasmid were transfected into AML12 cells using liposomal transfection reagent (Yeason). Thbs1 pharmacological inhibitory peptide LSKL and transforming growth factor (Tgf) β inhibitor ITD-1 were purchased from Selleck Chemicals (Shanghai, China).
Extraction, purification and identification of sEV
When the confluency of ADSCs reached 80–90%, the medium was changed to DMEM/F12 medium containing 10% FBS without sEVs and continued to culture for 72 h. The supernatant was then centrifuged at a low speed, and large vesicles were removed by filtration using a 0.22 μm pore size filter. The culture medium was subjected to ultracentrifugation at 120,000 × g for 90 min (OptimaTM XPN-100, Beckman Coulter, USA). The resulting sEV precipitates were resuspended in pre-cooled PBS, followed by another round of centrifugation. Subsequently, the sEV precipitates were resuspended in an appropriate volume of phosphate buffered saline (PBS) and characterized using nanoparticle tracking analysis (NTA) with ZetaView PMX 110 (Particle Metrix, Meerbusch, Germany). The sEV solution was placed on copper grids (Zhongjingkeyi Technology, Beijing, China), stained with 50 μL of uranium acetate, and visualized using transmission electron microscopy (FEI Tecnai G2 Spirit TEM, USA) for sEV visualization.
Western blotting
AML12 cells were lysed using radioimmunoprecipitation assay (RIPA) buffer (KeygenBio, Nanjing, China) containing a protease inhibitor cocktail (P8340, Merck KGaA). Total proteins in AML12 cell lysates or sEV solutions were quantified using a bicinchoninic acid (BCA) protein assay kit (Yeason). Ten micrograms of protein were resolved on SDS polyacrylamide gel before being transferred to a PVDF membrane (Millipore, USA). The membrane was then blocked with 5% bovine serum albumin (BSA, Sangon BIOTECH) at room temperature for 1 h, incubated with primary antibodies overnight at 4℃, and subsequently incubated with secondary antibody for 1 h. Images were captured using a Bioanalytical imaging system (Tanon 5200 Multi system, Shanghai, China). The primary antibodies used were as follows: anti-Cd63 (25682-1-AP, Proteintech, Wuhan, China), anti-Tsg101 (72312, Cell Signaling Technology, MA, USA), anti-Thbs1 (37879, Cell Signaling Technology), anti-Cd44 (15675-1-AP, Proteintech), anti-Phospho-Jnk (AP0631, ABclonal, Wuhan, China), anti-Jnk (A4867, ABclonal), anti-Atf4 (A0201, ABclonal), anti-Atf6 (A0202, ABclonal), anti-Ire1 (A17940, ABclonal), anti-Grp78 (A4908, ABclonal), anti-Chop (A0221, ABclonal), anti-Smad2 (A7699, ABclonal), anti-Phospho-Smad2 (AP0269, ABclonal), anti-Tgfβ2 (19999-1-AP, Proteintech), and anti-CD36 (A17339, ABclonal).
Immunofluorescence
A total of 2 × 10 4 ADSC or ADSC/GDM cells were cultured in 24-well plates with preset glass plates. After cell adhesion, they were fixed for 30 min in 4% paraformaldehyde. Subsequently, the cells were sealed in a PBS solution containing 0.5% Triton X-100 and 10% fetal bovine serum (FBS) for 2 h. The glass slides were then incubated with primary Cd44 antibody and Alexa Fluor 594-labeled secondary antibody (SA00006-4, Proteintech). Following the cleaning process with PBS, the slides were stained with 4′,6-diamidino-2-phenylindole (DAPI)solution for 5 min. Finally, the slides were embedded in fluoromount-G (SouthernBiotech, USA) and photographed using a fluorescence microscope (DS-Ri2, Nikon, Japan).
Cell viability assay
A total of 10 4 normal mouse liver cells (AML12) were seeded in 96-well plates. Once adhered, sEVs with final concentrations of 0, 10 8 , 5 × 10 8 , and 10 9 particle numbers/mL were added to serum-free DMEM/F12 medium and treated for 48 or 72 h. Each well was supplemented with 100 μL of culture medium and 10 μL of CCK8 reagent (Laisi biotech, Shanghai, China). The cell viability was measured by microplate reader, determining the OD450nm value.
Cell apoptosis assay
AML12 cells at a density of 8 × 10 5 were placed in a 6-well plate and co-cultured with sEVs at a concentration of 10 8 particle numbers/mL for 48 or 72 h. Cell apoptosis was detected using propidium iodide (PI) staining and the Annexin V apoptosis detection kit (BD Biosciences, NZ, USA) via flow cytometry (Novocyte, Agilent Technologies, CA, USA). The proportion of apoptotic cells was analyzed using NovoExpress software (version 1.5.0, Agilent Technologies).
Insulin sensitivity assay
A total of 6 × 10 5 AML12 cells were seeded in a 12-well plate. After cell adhesion, the AML12 cells were incubated in serum-free medium with sEVs at a concentration of 10 8 particles/mL for 48 or 72 h. In the control wells, the same volume of serum-free medium was added. Following co-culture, the AML12 cells were treated with or without 1 μg/mL insulin for 1 h. Glucose content was determined by collecting the culture medium and using the glucose detection kit (GAGO20, Merck KGaA). Glucose uptake was measured by subtracting the glucose level in the cultured well from that in the cell-free well, which reflected the insulin sensitivity.
Real-time polymerase chain reaction (PCR)
Total RNA was isolated from AML12 cells co-cultured with sEVs using the RNeasy Mini kit (Qiagen, Hilden, Germany). Synthesis of complementary DNA (cDNA) was performed using HiScript II Q RT SuperMix (Vazyme, Nanjing, China). Real-time PCR was conducted on QuantStudio 7 (Thermo Fisher Scientific) using SYBR Premix Ex Taq (Takara, Otsu, Japan). Gene expression was quantified using delta Ct. The primer sequences for all mRNA are provided in “Additional file 3 : Table S1”.
Proteomics of sEV
Differences in expression of the sEV proteome secreted by normal ADSCs and GDM mice-derived ADSCs were identified using label-free protein quantification. The proteins in the sEVs were extracted and analyzed by nano-high-performance liquid chromatography-tandem mass spectrometry (HPLC–MS/MS, Thermo Q Exactive). The data acquisition mode was data-dependent acquisition (DDA). The series of mass spectra were analyzed using PEAK Studio version X (Bioinformatics Solutions Inc., Waterloo, Canada), and protein databases were searched using PEAK DB. These detection procedures were performed by Guangzhou Gene denovo Biotechnology Co., Ltd. (Guangzhou, China).
Co-immunoprecipitation
AML12 cells treated with sEVs or inhibitors were lysed with RIPA buffer. AML12 cell extracts were immunoprecipitated with anti-Tgfβ2 or anti-CD36 antibodies for 24 h, then co-incubated with protein A/G magnetic beads (Bimake, Shanghai, China) for 3 h. The magnetic beads carrying interacting proteins were heated at 100 ℃ for 5 min, and the expression levels of interacting proteins were analyzed by western blotting.
Histological analysis
Liver tissue, abdominal AT, and inguinal AT from normal mice, GDM mice, and GDM mice treated with LSKL were fixed with 4% paraformaldehyde and embedded in paraffin. AT and liver tissue morphology were observed through hematoxylin–eosin (HE) staining. For immunohistochemistry (IHC), tissue sections were sequentially dewaxed, rehydrated, and treated with 3% H 2 O 2 . Sections were blocked in 5% BSA for 30 min, and then incubated overnight with anti-Tsg101, Thbs1, p-Jnk, Atf6, Ire1, and p-Smad2 antibodies at 4 ℃. After incubation with an enzyme-labeled secondary antibody at room temperature, the positive signal was observed using diaminobenzene (DAB) chromogenic agent and imaged under an optical microscope.
Statistical analysis
Data analysis was performed using GraphPad Prism 7.0 (GraphPad Software, USA) and SPSS 20.0 software (SPSS Inc., USA). Results are expressed as mean ± standard deviation (SD). Student’s t-test was used to analyze statistical differences between two groups of samples. One-way analysis of variance (ANOVA) and Bonferroni post hoc tests were used to analyze statistically significant differences among three or more groups. A P -value of < 0.05 was considered statistically significant.
Isolation of adipose stem cells and sEV from mice with GDM
High fat fed C57BL/6 J gestational mice were used as GDM models, monitoring their blood glucose levels during gestation. We observed a significant increase in blood glucose levels in GDM mice compared to normal gestational mice at day 1, 4, and 12 after gestation “Fig. 1 A”. The plasma of mice at day 12 of gestation was then determined by enzyme linked immunosorbent assay (ELISA). Compared with normal gestational mice, a significant decrease in insulin, leptin, adiponectin, and hypersensitive C-reactive protein (hs-CRP) levels in the plasma of GDM mice was observed “Fig. 1 B”. This finding suggests that GDM mice exhibit reduced insulin sensitivity in comparison to normal gestational mice. In order to investigate the role of AT in the functionality of other tissues and insulin sensitivity in GDM mice, we isolated primary adipose stem cells from the abdominal and inguinal AT of GDM mice (ADSC/GDMs), as well as from normal gestational mice (ADSCs) “Fig. 1 C, D”. Immunofluorescence analysis confirmed positive expression of the stem cell marker Cd44 in both isolated primary ADSCs and ADSC/GDMs, alongside Cd29, a mesenchymal stem cell markers (Fig. 1 E and Additional file 1 : Figure S1). Conversely, the monocyte population marker Cd14 showed negative expression in both ADSCs and ADSC/GDMs (Additional file 2 : Figure S2). Subsequently, we cultured and subcultured these primary ADSCs and ADSC/GDMs on a large scale and isolated and purified sEVs from the cell culture medium via a standard ultracentrifugal procedure. Transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA) were employed to determine the morphology, size, and concentration of sEVs derived from purified ADSCs (sEV A ) and ADSC/GDMs (sEV AG ) “Fig. 1 F, G”. Western blotting analysis confirmed the expression of stem cell marker protein Cd44, as well as extracellular vesicle marker proteins Cd63 and Tsg101, in sEV A and sEV AG “Fig. 1 H”. Consequently, we successfully isolated ADSCs from both GDM mice and normal gestational mice and purified their respective sEVs.
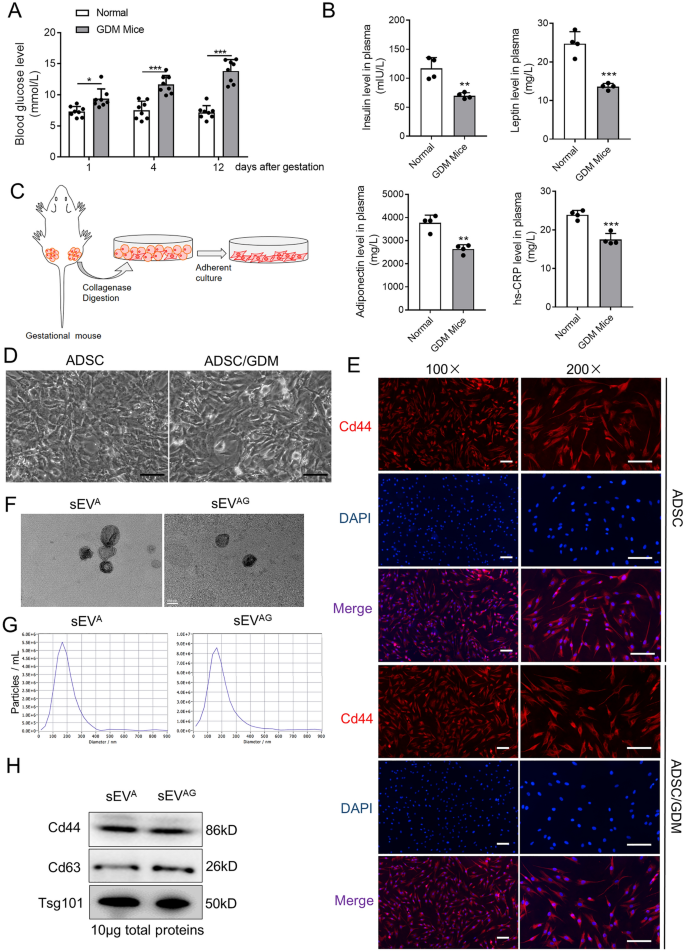
Isolation, culture and purification of GDM mice-derived ADSCs and their sEVs. A Plasma glucose values were quantified in eight normal gestation and GDM mice. B The expression of insulin, leptin, adiponectin and hs-CRP in the plasma of mice was detected by ELISA assays. C ADSCs was obtained by collagenase digestion of abdominal and inguinal adipose tissue from gestational mice and further in vitro culture. D Microscopic images of ADSCs isolated and cultured from normal gestational and GDM mice. Scale bar, 25 μm. E The expression of Cd44 marker (red) was identified by immunofluorescence. DAPI staining (blue) indicated the nucleus. Scale bar, 25 μm. F The sEV A and sEV AG were identified by transmission electron microscope. Scale bar, 100 nm. G The sEV A and sEV AG solution was diluted 500 times and particle size distribution and concentration were determined by nanoparticle tracking analysis. H The sEV markers and stem cell markers were detected by western blotting in sEV A and sEV. AG samples. Data were presented with mean ± standard deviation (SD), * P < 0.05, ** P < 0.01, *** P < 0.001
ADSC/GDM-derived sEV inhibited IR in normal liver cells
We hypothesized that ADSC/GDMs in AT affect insulin sensitivity in other tissues through the secretion of sEVs. The sEV A and sEV AG were co-cultured with normal mouse liver cells (AML12) to assess changes in cell viability, apoptosis, and insulin sensitivity. At different concentrations and time points, sEV A derived from normal gestational mice had little effects on the viability of AML12 cells “Fig. 2 A and B”. Conversely, sEV AG obtained from GDM mice significantly inhibited AML12 cell viability “Fig. 2 A, B”. We selected a concentration of 10 8 particles/mL for sEV A and sEV AG co-cultured with AML12 cells for 48 and 72 h to evaluate cell apoptosis. The results demonstrated that sEV AG significantly promoted apoptosis of AML12 cells, while sEV A did not induce such effects “Fig. 2 C, D”. Subsequently, we stimulated AML12 cells with insulin after co-culturing them with sEV A and sEV AG , and evaluated glucose metabolism in these cells. The findings revealed a significant increase in glucose uptake in control AML12 cells and sEV A co-cultured AML12 cells after insulin stimulation at 48 and 72 h of co-culture. Conversely, insulin failed to regulate glucose uptake in sEV AG co-cultured AML12 cells “Fig. 2 E, F”. These results indicate that sEV AG inhibits insulin sensitivity in AML12 cells. Previous comprehensive studies have suggested a close association between gestational diabetes-induced IR and ERS [ 17 , 23 , 24 ]. To further investigate the effects of sEV A and sEV AG on ER stress-related regulatory pathways, we conducted RT-PCR “Fig. 2 G” and western blotting “Fig. 2 H” analyses. The data revealed that sEV AG significantly increased the expression of ER stress-related genes and proteins, including phosphorylated Jnk, Atf4, Atf6, Ire1, Grp78, and Chop, in AML12 cells compared to normal AML12 cells or those co-cultured with sEV A . Consequently, sEV AG secreted by ADSCs derived from GDM mice impairs viability and insulin sensitivity in normal hepatocytes while promoting ERS.
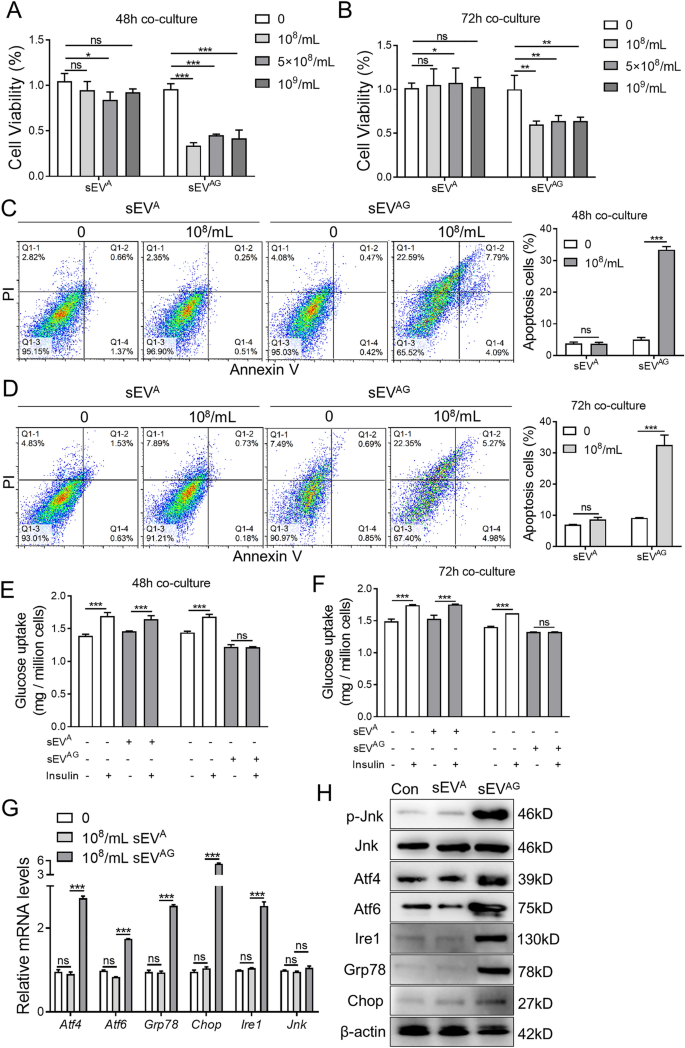
ADSC/GDM-derived inhibits hepatocyte proliferation and insulin sensitivity in normal mice. A , B The sEV A and sEV AG with different concentrations were co-cultured with AML12 cells for 48 or 72 h. Cell viability was obtained by CCK8 assays. C , D The sEV A and sEV AG with 10 8 particles / mL were co-cultured with AML12 cells for 48 or 72 h. Cell apoptosis was detected by flow cytometry. E , F The sEV A and sEV AG with 10 8 particle number / mL were co-cultured with AML12 cells for 48 or 72 h, then cells were treated with 1 μg/ml insulin for 1 h. Glucose uptake was measured to reflect insulin sensitivity of AML12 cells in each treatment. G The sEV A and sEV AG with 10. 8 particle number /mL were co-cultured with AML12 cells for 48 h, and mRNA expression of endoplasmic reticulum (ER)-related genes was detected by RT-PCR. H The expression of ER-related proteins was detected by western blotting. Data were presented with mean ± standard deviation (SD); ns, no significance; *P < 0.05, **P < 0.01, ***P < 0.001
Proteomics revealed high Thbs1 expression in ADSC/GDM-derived sEV
To unravel the mechanism underlying sEV AG -mediated IR, we conducted a comparative analysis of the proteomes of sEV A and sEV AG using protein mass spectrometry. In total, we identified 462 peptides and 178 proteins in six sEV samples (three replicates per group) from the sEV A and sEV AG groups. Out of these, 147 proteins were expressed in both groups of sEV samples “Fig. 3 A”. We further analyzed the 147 proteins expressed in both groups using R language and Student's t-test for quantification, resulting in the identification of differentially expressed proteins for each comparison group. The screening conditions included a P -value less than 0.05 obtained through the t-test, a fold-change greater than 1.2, and the presence of at least one unique peptide segment. Consequently, we identified 63 differential sEV proteins. Comparing the sEV A and sEVAG groups, 56 proteins were significantly overexpressed in the sEV AG group, while 7 proteins were significantly under-expressed “Fig. 3 B, C, Additional file 4 : Table S2″. Gene Ontology (GO) analysis revealed that these differentially expressed proteins were involved in both intracellular and extracellular life activities “Fig. 3 D”. KEGG bubble map analysis demonstrated that proteins associated with extracellular matrix (ECM)-receptor interactions exhibited the most significant differences in the sEV AG group ( P = 0.00008), “Fig. 3 E”. Gene set enrichment analysis (GSEA) showed an enrichment of ECM-mediated intercellular communication proteins in sEV AG “Fig. 3 F”. Additionally, the Search Tool for the Retrieval of Interacting Genes (STRING)protein interaction network analysis displayed interactions between hub proteins responsible for ECM and cell communication”Fig. 3 G”. Among the proteins involved in regulating ECM-receptor interactions, 10 proteins exhibited the most significant differences in sEV AG “Fig. 3 H”. Notably, Thbs1 displayed the most significant difference between the two groups “Fig. 3 I”, ( P = 2.67E-16). We further confirmed the high expression of Thbs1 in sEVAG using western blotting “Fig. 3 J”. Therefore, proteomic analysis unveiled the contents of sEVAG and indicated that the high expression of Thbs1 may contribute to IR.
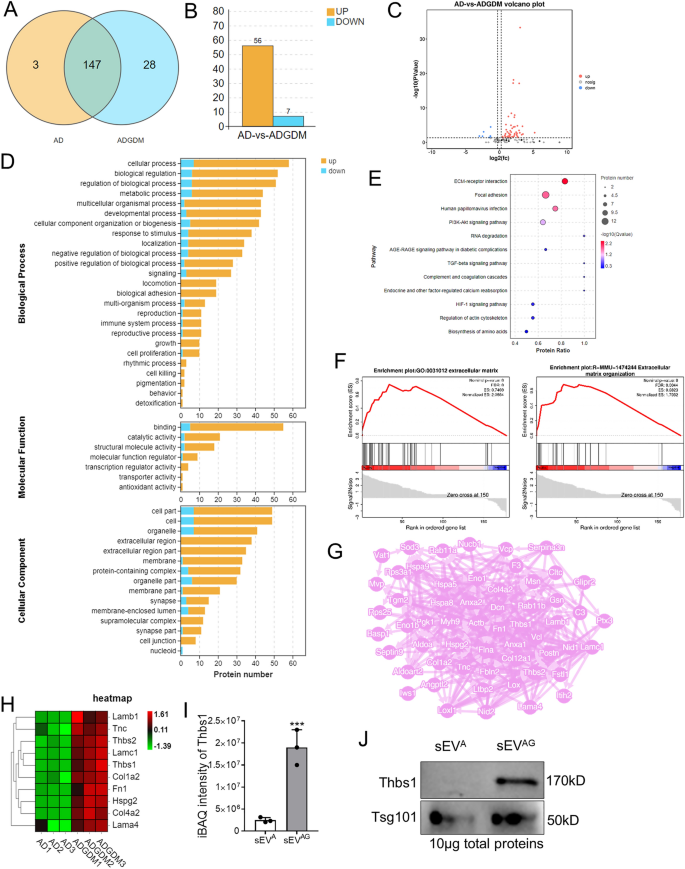
Proteomic identification of high Thbs1 expression in ADSC/GDM-derived sEV. A Venn diagram of the number of proteins detected by unlabeled protein profiles for and sEV A (AD in graph) and sEV AG (ADGDM in graph). B The number of proteins with high and low expression in sEV AG . C Volcanic map of differentially expressed protein in sEV AG . D GO signaling pathway analysis of sEV A and sEV AG proteins. E KEGG signal analysis of sEVA and sEV AG proteins. F GSEA analysis revealed enrichment of the ECm-receptor interaction pathway. G Network analysis of interactions between differential ECM regulatory proteins and other differential proteins. H. Heat maps of 10 proteins significantly overexpressed in sEV AG . I , J Mass spectrometry quantification and western blotting of Thbs1 in sEV A and sEV. AG . *** P < 0.001
Thbs1 damaged insulin sensitivity of normal liver cells
To investigate the impact of Thbs1 on the viability and insulin sensitivity of mouse liver cells, we transfected AML12 cells with Flag-eGFP-Thbs1 plasmid and a control vector. The expression of Thbs1 was confirmed in the transfected AML12 cells by detecting the GFP fusion protein “Fig. 4 A”. Overexpression of Thbs1 significantly compromised the viability of AML12 cells “Fig. 4 B” and increased cell apoptosis “Fig. 4 C”. In AML12 cells with elevated Thbs1 expression, insulin exhibited limited effectiveness in facilitating glucose uptake, indicating insulin insensitivity “Fig. 4 D”. To counteract the IR induced by sEV AG and Thbs1, AML12 cells were treated with LSKL, an inhibitory polypeptide specific to Thbs1. The results demonstrated that LSKL significantly improved cell viability “Fig. 4 E” and reduced the percentage of apoptotic cells”Fig. 4 F” in sEV AG -pretreated AML12 cells. Moreover, AML12 cells treated with sEV AG and LSKL exhibited restored insulin-induced glucose uptake “Fig. 4 G”. In terms of ER stress signaling related to the regulation of insulin sensitivity, Thbs1 overexpression in AML12 cells resulted in high mRNA levels of Atf4, Atf6, Ire1, Grp78, and Chop “Fig. 4 H and I”, as well as increased protein expressions of phosphorylated Jnk, Atf4, Atf6, Ire1, Grp78, and Chop “Fig. 4 J”. However, after LSKL treatment, the expression of these markers was suppressed. Consequently, the elevated expression of Thbs1 in sEV AG inhibited the viability and insulin sensitivity of mouse liver cells.

Overexpression of Thbs1 inhibits insulin sensitivity in AML12 cells. A Flag-eGFP-Thbs1 plasmid and control vector were transfected into AML12 cells. The expression of eGFP-thbs1 fusion protein in AML12 was identified by western blotting and fluorescence microscopy. B Plasmid transfected AML12 cells were placed in 96-well plates, and cell viability was detected by CCK8 assay. C . Apoptosis assays of AML12 cells after plasmid transfection were performed. D Plasmid transfected AML12 cells were treated with 1 μg/ml insulin for 1 h, and the culture medium was collected for glucose uptake measurement. E – G AML12 cells were treated with 10 8 /mL sEV AG , 10 μM LSKL and 10 8 /mL sEV AG + 10 μM LSKL for 48 h. Cell viability, apoptosis, and insulin-induced glucose uptake were then measured. H The expression of endoplasmic reticulum stress (ERS)-related mRNA in AML12 cells after plasmid transfection was detected by RT-PCR. I The expression of ERS-related mRNA in 10 8 /mL sEV AG and/or 10 μM LSKL treated AML12 cells was detected by RT-PCR. J The expression of ERS-related proteins in AML12 cells was detected by western blotting. Statistical data were presented as mean ± standard deviation (SD); ns, no significance; * P < 0.05, ** P < 0.01, *** P < 0.001
Thbs1 from ADSC/GDM sEV binded to CD36 receptor and activated Tgfβ1 signaling
Thbs1, a membrane protein abundant in AT, triggers the Tgfβ/Smads signaling pathway [ 25 ]. LSKL functions as a competitive antagonist that impedes the activation of Thbs1/Tgfβ by inhibiting the interaction between the KRFK sequence of Thbs1 and Tgfβ [ 26 ]. We hypothesized that Thbs1 present in sEV AG regulates insulin sensitivity by activating the Tgfβ/Smads signaling pathway in AML12 cells. Analysis of AML12 cell lysates at the protein level revealed a significant increase in phosphorylated Smad2 expression when treated with sEV AG containing high levels of Thbs1, indicating activation of the Thbs1/Tgfβ pathway “Fig. 5 A”. Moreover, the protein interactions between Thbs1 and Tgfβ were enhanced in AML12 cells co-cultured with sEVAG compared to control and sEV A co-cultured cells “Fig. 5 B”. Furthermore, ITD-1, a pharmacological inhibitor of Tgfβ “Fig. 5 C”, and LSKL as a Thbs1 antagonist “Fig. 5 D”, reduced the levels of phosphorylated Smad2 in AML12 cells co-cultured with sEVAG. Additionally, we observed increased interactions between extracellular Thbs1 and the CD36 ligand, which plays a crucial role in lipid and glucose metabolism and promotes IR [ 27 , 28 ]. Specifically, the interactions between Thbs1 and CD36 were elevated in AML12 cells co-cultured with sEV AG compared to control and sEV A “Fig. 5 E”. However, the interaction between Thbs1 and CD36 was reduced by LSKL in AML12 cells co-cultured with sEV AG “Fig. 5 F”. Our findings propose an sEV AG -mediated mechanism of IR, potentially involving the activation of Tgfβ and CD36 pathways in recipient cells through secreted Thbs1.

ADSC/GDM-derived sEV carrying Thbs1 induces Tgfβ and CD36 activation. A sEV AG was co-cultured with AML12 for 48 h, and the expression of Smad2, phosphorylated Smad2 and Tgfβ was detected by western blotting. B sEV AG was co-cultured with AML12 for 48 h, and the cell lysate was subsequently subjected to co-immunoprecipitation (co-IP) with anti-Tgfβ antibodies. Thbs1 levels in co-IP products and input samples were detected. C, D AML12 cells were treated with 108 /mL sEV AG , 2 μM ITD-1, 10 μM LSKL or a combination for 48 h. The expression of Smad2 and phosphorylated Smad2 was detected by western blotting. E Cell lysate was co-immunoprecipitated with anti-CD36 antibody. Thbs1 levels in co-IP products and input samples were detected. F AML12 cells were treated with 10 8 /mL sEV AG , 10 μM LSKL or a combination for 48 h, followed by co-IP with anti-CD36 antibody
Inhibition of Thbs1 in ADSC/GDM-derived sEV improved insulin sensitivity
To validate the role of Thbs1 in sEV AG , ADSC/GDM cells were treated with LSKL for 72 h, and sEV was isolated from the culture medium. The morphology and particle size distribution of sEV secreted by cells treated with 5 μM and 10 μM LSKL (sEV AG−L5 and sEV AG−L10 ) were examined using TEM and NTA “Fig. 6 A”. Western blotting results demonstrated that the levels of Thbs1 protein in sEV AG−L5 and sEV AG−L10 were significantly lower than those in sEV AG “Fig. 6 B”. Co-culturing AML12 cells with sEV AG−L5 and sEV AG−L10 resulted in significantly increased cell viability “Fig. 6 C”, decreased apoptosis “Fig. 6 D”, and improved insulin sensitivity “Fig. 6 E” compared to sEV AG . Mechanistically, co-culturing AML12 cells with sEV AG−L5 and sEV AG−L10 reduced the activation of the Tgfβ pathway, as evidenced by decreased expression of phosphorylated Smad2 “Fig. 6 F”. Additionally, the mRNA and protein expression levels of ER stress-related genes were reduced in AML12 cells co-cultured with sEV AG−L5 and sEV AG−L10 “Fig. 6 G and H”. Consequently, pharmacological inhibition of Thbs1 in sEV AG aided in alleviating stress signals in mouse liver cells.

ADSC/GDM sEV restore insulin sensitivity to liver cells after Thbs1 pharmacological interference. A ADSC-GDM cells were treated with different concentrations of LSKL (0, 5 μM, 10 μM) for 72 h. sEV AG , sEV AG−L5 and sEV AG−L10 were collected and identified by TEM and NTA. B The expression of Thbs1 in sEV AG , sEV AG−L5 and sEV AG−L10 was identified by western blotting. C sEV AG , sEV AG−L5 and sEV AG−L10 were co-cultured with AML12 for 24–96 h, and cell viability was detected by CCK8 assays. D sEV AG , sEV AG−L5 and sEV AG−L10 were co-cultured with AML12 for 48 h, and cell apoptosis was detected by flow cytometry. E Glucose uptake of AML12 cells after insulin treatment was measured. F. sEV AG , sEV AG−L5 and sEV AG−L10 were co-cultured with AML12 for 48 h, and the expression of phosphorylated Smad2 and total Smad2 protein was detected by western blotting. G , H RT-PCR and western blotting were used to detect the expression of ER stress related genes in AML12 cells. Statistical data were presented as mean ± standard deviation (SD); ns, no significance; * P < 0.05, ** P < 0.01, *** P < 0.001
Thbs1 pharmacological inhibition in vivo improves insulin sensitivity in GDM mice
GDM mice were intraperitoneally injected with 1 mg/kg LSKL daily for 15 days before and after pregnancy. We evaluated the effects of LSKL treatment on insulin sensitivity and marker expression in tissues. The results indicated that LSKL treatment significantly reduced blood glucose levels and increased plasma insulin, leptin, and adiponectin levels in GDM mice “(Fig. 7 A”. Immunological examination of abdominal and inguinal ATs, as well as liver tissues of GDM mice treated with either vehicle control or LSKL, revealed high expression of vesicular proteins Tsg101 and Thbs1 in the AT of GDM mice compared to normal mice. However, LSKL treatment attenuated the expression of Tsg101 and Thbs1 in the AT of GDM mice “Fig. 7 B”. Immunohistochemical analysis demonstrated that the expression of Thbs1, ER stress regulatory proteins (phosphorylated Jnk, Atf6, Ire1), and phosphorylated Smad2 in the livers of GDM mice was higher than in normal mice. Nevertheless, LSKL treatment decreased their expression in the livers of GDM mice “Fig. 7 C”. Hence, the peptide LSKL, targeting Thbs1, proved to be an effective pharmacological tool for improving IR in GDM mice.

Targeted Thbs1 pharmacological therapy improves insulin resistance phenotype in GDM mice. A Plasma was extracted from normal, GDM, and LSKL-treated GDM mice, and blood glucose and insulin, leptin, adiponectin and hs-CRP levels were measured by glucose meter and ELISA kit. B HE staining and immunohistochemical detection of adipose tissues from normal, GDM, and LSKL treated mice. C HE staining and immunohistochemical detection of liver tissues from these mice. Statistical data were presented as mean ± standard deviation (SD); ns, no significance; * P < 0.05, ** P < 0.01, *** P < 0.001
In the present study, we aimed to investigate the underlying mechanisms of insulin sensitivity suppression induced by ADSCs in GDM mice, specifically focusing on sEVs. Our findings demonstrate that sEVs derived from ADSCs were responsible for the reduction of insulin sensitivity in normal tissues. Furthermore, we have provided evidence suggesting that sEVs derived from ADSCs of GDM mice carry Thbs1 protein, which inhibits cell viability and insulin sensitivity in liver cells. Mechanistically, the Thbs1 protein in sEVs interacts with CD36 and Tgfβ receptors in liver cells, activating the Tgfβ/Smad2 signaling pathway.
Obesity measurements before and during early pregnancy have been identified as strong predictors of fasting insulin concentration throughout pregnancy [ 29 ]. Pregnancy is associated with a disproportional accumulation of visceral fat and an increased risk of metabolic disease. Transient IR during pregnancy may contribute to intra-abdominal fat accumulation [ 30 ]. Increased fat expression and IR are significant features of pregnancy. Maternal obesity causes oxidative stress and metabolic dysfunction in both maternal and fetal health [ 30 ].
With defective regulation of adipocytokines in early-stage type 2 diabetes, GDM women represent an ideal research population, thereby enhancing the understanding of interrelationships [ 31 ]. Adiponectin, leptin, and high-sensitivity CRP have shown correlations with the onset of T2DM and microvascular complications [ 32 ]. Our findings revealed a significant decrease in insulin, leptin, adiponectin, and hs-CRP levels in the plasma of GDM mice at day 12 of gestation compared to normal gestational mice. These results aligned with various cross-sectional reports [ 33 , 34 ]. Furthermore, placenta-produced leptin has been associated with weight regulation and metabolism, with reported levels being both elevated [ 35 ] and within normal ranges [ 36 ]. Notably, it seems that HsCRP does not significantly contribute to pregnancy-induced insulin resistance in GDM or in women with a healthy pregnancy [ 31 ]. A study involving 180 women found reduced adiponectin levels in GDM [ 37 ]. Additionally, previous reports indicate that when BMI and adiposity were considered, hsCRP was not significantly associated with GDM [ 38 ]. Therefore, the observed elevation of hsCRP is not deemed an important cause or consequence of reduced IR during pregnancy [ 31 ]. Our research outcomes validated the findings of the above studies.
GDM is characterized by increased adipose expression and secretion of proinflammatory cytokines. However, the mechanism behind these changes remains unclear. Previous studies have shown that GDM women release significantly higher levels of AT-derived exosomes compared to healthy pregnant women. These exosomes increase the expression of glucose metabolism-related factors in placental cells, thereby mediating IR formation [ 39 ] . ADSCs, somatic stem cells obtained from white AT [ 40 ], produce adipocytokines that play endocrine and paracrine roles and participate in the occurrence of IR [ 41 ]. The release of sEVs, observed in many pathological conditions [ 42 , 43 ], including T2DM and GDM [ 44 , 45 ], is higher in patients with GDM compared to patients with normal glucose tolerance. AT from women with GDM releases a greater number of sEVs compared to women with normal glucose tolerance, supporting our research findings that hyperglycemia can induce the secretion of sEVs from AT. However, the relationship between sEVs secreted by ADSCs and GDM-induced IR remains unclear.
ADSCs have gained significant attention in regenerative medicine and stem cell therapy due to their ability to repair tissues and regulate immunity. However, studies have shown that under continuous high glucose environments, the glucose metabolism, cell replication, apoptosis, and differentiation ability of ADSCs are impaired, with a more pronounced negative impact on ADSCs derived from patients with diabetes [ 46 ]. Liver tissue can be damaged in the hyperglycemic environment of diabetes, leading to liver fibrosis. Addition of hepatocyte growth factors to ADSCs has been proposed as a method to treat liver fibrosis in diabetic patients [ 47 ]. Our study showed differences in the biological functions of AML-12 cells between sEV A and sEV AG . Compared to sEV A , sEV AG significantly inhibited cell proliferation, promoted cell apoptosis, and enhanced IR under insulin induction, consistent with the findings from existing literature.
ER related- kinase, inositol demand enzyme 1α (IRE1-α) existing in mammalian endoplasmic, and reticulum membrane activating transcription factor 6 (ATF-6), are three ER transmembrane protein, playing important roles in the signal transduction pathway of ERS. In addition, X-box binding protein-1(XBP1) and activating transcription factor 4 (Atf4) are also crucial in inducing ERS response and can regulate genes involved in ERS [ 48 ]. ERS plays a core role in triggering IR and T2DM [ 19 ]. ERS in liver histiocytes can cause changes in insulin signaling pathways, leading to IR through overactivation of the c-Jun amino terminal kinase pathway and serine phosphorylation of insulin receptor substrate 1 [ 49 ]. There is also a connection between extracellular vesicles, ERS, and IR. For example, extracellular vesicles derived from bone marrow stem cells can significantly inhibit insulin receptor substrate induced by IR in a renal ischemia–reperfusion model [ 50 ]. Additionally, under pathological conditions, abnormally expressed extracellular vesicles can induce cellular ERS response, leading to cell apoptosis and tissue dysfunction [ 51 ]. Therefore, it is speculated that extracellular vesicles derived from ADSCs induce the expression of ERS signaling-related proteins in liver tissue cells, thereby impairing insulin sensitivity and promoting the formation of IR in GDM. Our study demonstrated that sEV AG increased the expression of ERS signaling-related proteins Atf4 and Atf6 in AML-12 cells, thereby damaging cellular insulin sensitivity. Several studies have suggested that ERS is a core mechanism underlying various diseases, including IR and T2DM [ 19 ]. The overactivation of multiple ERS signal-related genes, such as ATF4 and ATF6, in AT [ 52 ]. Therefore, the activation of ERS signals plays a crucial role in the occurrence of IR and hyperglycemia. This study shows that sEV AG increased the expression of ERS signaling related proteins ATFf4 and ATF6 in AML-12 cells, damaging the insulin sensitivity of cells. However, further research is needed to understand how ERS signaling-related proteins Atf4 and Atf6 promote IR in AML-12 cells.
Thbs1 is abundantly found in alpha granules of platelets, but normal plasma levels are typically low (100–200 ng/ml). The expression of Thbs1 increases in high glucose and high-fat environments. It is also elevated in T2DM and cardiovascular disease [ 53 ]. In the disease model of visceral fat and IR, Thbs1 is highly expressed in the visceral AT of obese rats [ 54 ] . Our results revealed specific expression of Thbs1 in the exosomes derived from ADSCs of GDM mice. Knockout of the Thbs1 gene can protect mouse AT from inflammation and invasion of IR [ 18 ]. This study demonstrated that high expression of Thbs1 in sEV AG damages cellular insulin sensitivity, while the addition of Thbs1 peptide antagonist LSKL can restore insulin sensitivity, consistent with existing research findings.
Overall, our results suggest that sEVs are responsible for inducing IR in normal tissues mediated by ADSCs in GDM mice. Treatment with exo-GDM inhibited cell viability and insulin sensitivity in normal mouse liver cells compared to exo-NGT. Additionally, exo-GDM amplified the expression of Thbs1 proteins through the activation of Tgfβ/Smad2 signaling in ADSC/GDM-derived sEVs. These findings align with previous studies demonstrating the ability of sEVs to interact with and regulate gene and miRNA expression [ 55 , 56 ], stimulate cytokines, and impair insulin response in target cells [ 57 ]. To the best of our knowledge, this is the first study to propose ADSC/GDM-derived sEVs as "switches" that upregulate Thbs1 and induce the increase of ERS signaling-related proteins Atf4 and Atf6 in AML-12 cells, thereby damaging cellular insulin sensitivity and promoting the formation of IR in GDM mice. This study provides a foundation for further understanding the molecular mechanisms underlying the formation of insulin resistance in GDM and may offer new strategies for targeted treatment of GDM and even T2DM.
Availability of data and materials
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Pinto Y, Frishman S, Turjeman S, Eshel A, Nuriel-Ohayon M, Shrossel O, Ziv O, Walters W, Parsonnet J, Ley C, et al. Gestational diabetes is driven by microbiota-induced inflammation months before diagnosis. Gut. 2023;72(5):918–28.
Article CAS PubMed Google Scholar
Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(Suppl 2):S141-146.
Article PubMed Google Scholar
McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47.
Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. J Diabetes Investig. 2019;10(1):154–62.
Geach T. Diabetes: a metabolomic signature to predict the transition from GDM to T2DM. Nat Rev Endocrinol. 2016;12(9):498.
PubMed Google Scholar
Pathirana MM, Lassi Z, Ali A, Arstall M, Roberts CT, Andraweera PH. Cardiovascular risk factors in women with previous gestational diabetes mellitus: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2021;22(4):729–61.
Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30(9):2287–92.
Rughani A, Friedman JE, Tryggestad JB. Type 2 diabetes in youth: the role of early life exposures. Curr Diab Rep. 2020;20(9):45.
Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140(3):365–71.
Article CAS PubMed PubMed Central Google Scholar
Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res. 2016;118(11):1786–807.
Abell SK, De Courten B, Boyle JA, Teede HJ. Inflammatory and other biomarkers: role in pathophysiology and prediction of gestational diabetes mellitus. Int J Mol Sci. 2015;16(6):13442–73.
Chen X, Chen H, Zhang Y, Jiang Y, Wang Y, Huang X, Wang D, Li M, Dou Y, Sun X, et al. Maternal liver dysfunction in early pregnancy predisposes to gestational diabetes mellitus independent of preconception overweight: a prospective cohort study. BJOG. 2022;129(10):1695–703.
Titchenell PM, Lazar MA, Birnbaum MJ. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol Metab. 2017;28(7):497–505.
Trzyna A, Banas-Zabczyk A. Adipose-derived stem cells secretome and its potential application in “Stem Cell-Free Therapy.” Biomolecules. 2021. https://doi.org/10.3390/biom11060878 .
Article PubMed PubMed Central Google Scholar
Fiorina P, Pietramaggiori G, Scherer SS, Jurewicz M, Mathews JC, Vergani A, Thomas G, Orsenigo E, Staudacher C, La Rosa S, et al. The mobilization and effect of endogenous bone marrow progenitor cells in diabetic wound healing. Cell Transplant. 2010;19(11):1369–81.
James-Allan LB, Rosario FJ, Barner K, Lai A, Guanzon D, McIntyre HD, Lappas M, Powell TL, Salomon C, Jansson T. Regulation of glucose homeostasis by small extracellular vesicles in normal pregnancy and in gestational diabetes. FASEB J. 2020;34(4):5724–39.
Villalobos-Labra R, Subiabre M, Toledo F, Pardo F, Sobrevia L. Endoplasmic reticulum stress and development of insulin resistance in adipose, skeletal, liver, and foetoplacental tissue in diabesity. Mol Aspects Med. 2019;66:49–61.
Inoue M, Jiang Y, Barnes RH 2nd, Tokunaga M, Martinez-Santibanez G, Geletka L, Lumeng CN, Buchner DA, Chun TH. Thrombospondin 1 mediates high-fat diet-induced muscle fibrosis and insulin resistance in male mice. Endocrinology. 2013;154(12):4548–59.
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–61.
von Toerne C, Huth C, de Heras Las GT, Kronenberg F, Herder C, Koenig W, Meisinger C, Rathmann W, Waldenberger M, Roden M, et al. MASP1, THBS1, GPLD1 and ApoA-IV are novel biomarkers associated with prediabetes: the KORA F4 study. Diabetologia. 2016;59(9):1882–92.
Article Google Scholar
Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-beta activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am J Pathol. 2011;178(6):2573–86.
Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS ONE. 2011;6(10):e26656.
Melo AM, Benatti RO, Ignacio-Souza LM, Okino C, Torsoni AS, Milanski M, Velloso LA, Torsoni MA. Hypothalamic endoplasmic reticulum stress and insulin resistance in offspring of mice dams fed high-fat diet during pregnancy and lactation. Metabolism. 2014;63(5):682–92.
Yao X, Liu R, Li X, Li Y, Zhang Z, Huang S, Ge Y, Chen X, Yang X. Zinc, selenium and chromium co-supplementation improves insulin resistance by preventing hepatic endoplasmic reticulum stress in diet-induced gestational diabetes rats. J Nutr Biochem. 2021;96:108810.
Jiang N, Zhang Z, Shao X, Jing R, Wang C, Fang W, Mou S, Ni Z. Blockade of thrombospondin-1 ameliorates high glucose-induced peritoneal fibrosis through downregulation of TGF-beta1/Smad3 signaling pathway. J Cell Physiol. 2020;235(1):364–79.
Murphy-Ullrich JE, Suto MJ. Thrombospondin-1 regulation of latent TGF-beta activation: a therapeutic target for fibrotic disease. Matrix Biol. 2018;68–69:28–43.
Gutierrez LS, Gutierrez J. Thrombospondin 1 in metabolic diseases. Front Endocrinol (Lausanne). 2021;12:638536.
Bai J, Xia M, Xue Y, Ma F, Cui A, Sun Y, Han Y, Xu X, Zhang F, Hu Z, et al. Thrombospondin 1 improves hepatic steatosis in diet-induced insulin-resistant mice and is associated with hepatic fat content in humans. EBioMedicine. 2020;57:102849.
Bernier E, Plante AS, Robitaille J, Lemieux S, Girard M, Bujold E, Gagnon C, Weisnagel SJ, Tchernof A, Morisset AS. First-trimester diet quality in association with maternal subcutaneous and visceral adipose tissue thicknesses and glucose homeostasis during pregnancy. Int J Food Sci Nutr. 2023;74(2):268–78.
Ingram KH, Hunter GR, James JF, Gower BA. Central fat accretion and insulin sensitivity: differential relationships in parous and nulliparous women. Int J Obes (Lond). 2017;41(8):1214–7.
McLachlan KA, O’Neal D, Jenkins A, Alford FP. Do adiponectin, TNFalpha, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes Metab Res Rev. 2006;22(2):131–8.
Wang LK, Wang H, Wu XL, Shi L, Yang RM, Wang YC. Relationships among resistin, adiponectin, and leptin and microvascular complications in patients with type 2 diabetes mellitus. J Int Med Res. 2020;48(4):300060519870407.
Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83(4):341–7.
Worda C, Leipold H, Gruber C, Kautzky-Willer A, Knofler M, Bancher-Todesca D. Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;191(6):2120–4.
Kautzky-Willer A, Pacini G, Tura A, Bieglmayer C, Schneider B, Ludvik B, Prager R, Waldhausl W. Increased plasma leptin in gestational diabetes. Diabetologia. 2001;44(2):164–72.
Simmons D, Breier BH. Fetal overnutrition in polynesian pregnancies and in gestational diabetes may lead to dysregulation of the adipoinsular axis in offspring. Diabetes Care. 2002;25(9):1539–44.
Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care. 2004;27(3):799–800.
Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. C-reactive protein and gestational diabetes: the central role of maternal obesity. J Clin Endocrinol Metab. 2003;88(8):3507–12.
Jayabalan N, Lai A, Ormazabal V, Adam S, Guanzon D, Palma C, Scholz-Romero K, Lim R, Jansson T, McIntyre HD, et al. Adipose tissue exosomal proteomic profile reveals a role on placenta glucose metabolism in gestational diabetes mellitus. J Clin Endocrinol Metab. 2019;104(5):1735–52.
Skubis-Sikora A, Sikora B, Witkowska A, Mazurek U, Gola J. Osteogenesis of adipose-derived stem cells from patients with glucose metabolism disorders. Mol Med. 2020;26(1):67.
Valsamakis G, Kumar S, Creatsas G, Mastorakos G. The effects of adipose tissue and adipocytokines in human pregnancy. Ann N Y Acad Sci. 2010;1205:76–81.
Salomon C, Scholz-Romero K, Sarker S, Sweeney E, Kobayashi M, Correa P, Longo S, Duncombe G, Mitchell MD, Rice GE, et al. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes. 2016;65(3):598–609.
Pillay P, Maharaj N, Moodley J, Mackraj I. Placental exosomes and pre-eclampsia: Maternal circulating levels in normal pregnancies and early and late onset pre-eclamptic pregnancies. Placenta. 2016;46:18–25.
Freeman DW, Noren Hooten N, Eitan E, Green J, Mode NA, Bodogai M, Zhang Y, Lehrmann E, Zonderman AB, Biragyn A, et al. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes. 2018;67(11):2377–88.
Bernea EG, Suica VI, Uyy E, Cerveanu-Hogas A, Boteanu RM, Ivan L, Ceausu I, Mihai DA, Ionescu-Tirgoviste C, Antohe F. Exosome proteomics reveals the deregulation of coagulation, complement and lipid metabolism proteins in gestational diabetes mellitus. Molecules. 2022. https://doi.org/10.3390/molecules27175502 .
Cramer C, Freisinger E, Jones RK, Slakey DP, Dupin CL, Newsome ER, Alt EU, Izadpanah R. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev. 2010;19(12):1875–84.
Gharbia S, Nazarie SR, Dinescu S, Balta C, Herman H, Peteu VE, Gherghiceanu M, Hermenean A, Costache M. Adipose-derived stem cells (ADSCs) supplemented with hepatocyte growth factor (HGF) attenuate hepatic stellate cell activation and liver fibrosis by inhibiting the TGF-beta/smad signaling pathway in chemical-induced liver fibrosis associated with diabetes. Cells. 2022. https://doi.org/10.3390/cells11213338 .
Sobrevia L, Salsoso R, Fuenzalida B, Barros E, Toledo L, Silva L, Pizarro C, Subiabre M, Villalobos R, Araos J, et al. Insulin is a key modulator of fetoplacental endothelium metabolic disturbances in gestational diabetes mellitus. Front Physiol. 2016;7:119.
Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529(7586):326–35.
Wang C, Zhu G, He W, Yin H, Lin F, Gou X, Li X. BMSCs protect against renal ischemia-reperfusion injury by secreting exosomes loaded with miR-199a-5p that target BIP to inhibit endoplasmic reticulum stress at the very early reperfusion stages. FASEB J. 2019;33(4):5440–56.
Wu R, Chen Z, Ma J, Huang W, Wu K, Chen Y, Zheng J. Renal cancer stem cell-derived sEVs impair renal function by inducing renal cell ERS and apoptosis in mice. Transl Androl Urol. 2022;11(5):578–94.
Foss-Freitas MC, Ferraz RC, Monteiro LZ, Gomes PM, Iwakura R, de Freitas LCC, Foss MC. Endoplasmic reticulum stress activation in adipose tissue induces metabolic syndrome in individuals with familial partial lipodystrophy of the Dunnigan type. Diabetol Metab Syndr. 2018;10:6.
Isenberg JS, Roberts DD. THBS1 (thrombospondin-1). Atlas Genet Cytogenet Oncol Haematol. 2020;24(8):291–9.
PubMed PubMed Central Google Scholar
Hida K, Wada J, Zhang H, Hiragushi K, Tsuchiyama Y, Shikata K, Makino H. Identification of genes specifically expressed in the accumulated visceral adipose tissue of OLETF rats. J Lipid Res. 2000;41(10):1615–22.
Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450–5.
Kranendonk ME, Visseren FL, van Balkom BW, Nolte-’t Hoen EN, van Herwaarden JA, de Jager W, Schipper HS, Brenkman AB, Verhaar MC, Wauben MH, et al. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity. 2014;22(5):1296–308.
Deng ZB, Poliakov A, Hardy RW, Clements R, Liu C, Liu Y, Wang J, Xiang X, Zhang S, Zhuang X, et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58(11):2498–505.
Download references
The material purchases and the data collections in this project were supported by the Discipline Group Construction Program of the Health Bureau of Shanghai Pudong in China (No. PWZxq2022-15), Fund of Shanghai Pudong New Area Science and Technology Commission in China (No. PKJ2021-Y30).
Author information
Huaping Li and Hao Yang contributed equally to this work.
Authors and Affiliations
Department of Obstetrics and Gynecology, Shanghai University of Medicine & Health Sciences Affiliated Zhoupu Hospital, Shanghai, China
Huaping Li & Zhou Liu
Shanghai Key Laboratory of Molecular Imaging, Jiading District Central Hospital Affiliated Shanghai University of Medicine and Health Sciences, Shanghai, China
Hao Yang, Jingyan Liu, Hedi Yang, Xinyu Gao & Xiaoying Yang
Endocrinology Department, Shanghai University of Medicine & Health Sciences Affiliated Zhoupu Hospital, Shanghai, China
Qiaohui Qian
You can also search for this author in PubMed Google Scholar
Contributions
HL designed and performed the research, analyzed the data, and drafted the manuscript. QQ, ZL guided and provided the final approval of the manuscript. HY, JL, HY, XG and XY completed animal and cell experiments. All authors contributed to the article and approved the submitted version.
Corresponding authors
Correspondence to Zhou Liu or Qiaohui Qian .
Ethics declarations
Competing interests.
The authors have no competing interests to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: figure s1..
Immunofluorescence analysis revealed the positive expression of Cd44 and Cd29, the mesenchymal stem cell markers, in isolated primary ADSCs and ADSC/GDMs.
Additional file 2: Figure S2.
The monocyte population marker Cd14 showed negative expression in both ADSCs and ADSC/GDMs.
Additional file 3. Supplementary Table 1.
Primer sequences for RT-PCR.
Additional file 4. Supplementary Table 2.
Differentially expressed proteins in sEVAG and sEVA group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Li, H., Yang, H., Liu, J. et al. Adipose stem cells-derived small extracellular vesicles transport Thrombospondin 1 cargo to promote insulin resistance in gestational diabetes mellitus. Diabetol Metab Syndr 16 , 105 (2024). https://doi.org/10.1186/s13098-024-01276-1
Download citation
Received : 02 October 2023
Accepted : 24 January 2024
Published : 19 May 2024
DOI : https://doi.org/10.1186/s13098-024-01276-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adipose-derived stem cells
- Gestational diabetes mellitus
- Insulin resistance
- Small extracellular vesicles
- Thrombospondin 1
Diabetology & Metabolic Syndrome
ISSN: 1758-5996
- Submission enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Introduction and background. Gestational diabetes mellitus (GDM) is a metabolic condition of pregnancy that presents as newly developing hyperglycemia in pregnant women who did not have diabetes before getting pregnant, and it normally resolves after giving birth [].]. Around 9% of pregnancies around the globe are affected by this prevalent antepartum condition [].
Gestational diabetes mellitus, one of the most common complications of pregnancy, 1,2 affects 6 to 25% of pregnant women (depending on diagnostic criteria) 3,4 and is associated with increased ...
Gestational diabetes is a complex metabolic condition thought to have a strong genetic predisposition. A large genome-wide association study of participants from Finland sheds light on the genetic ...
Objective To investigate the association between gestational diabetes mellitus and adverse outcomes of pregnancy after adjustment for at least minimal confounding factors. Design Systematic review and meta-analysis. Data sources Web of Science, PubMed, Medline, and Cochrane Database of Systematic Reviews, from 1 January 1990 to 1 November 2021. Review methods Cohort studies and control arms of ...
Gestational diabetes mellitus (GDM) is the most common complication in pregnancy and has short-term and long-term effects in both mother and offspring. This Primer discusses the definitions of GDM ...
Perinatal outcomes vary for women with gestational diabetes mellitus (GDM). ... Prior research has largely focused on the impact of pre-pregnancy overweight or obesity on adverse perinatal ...
Gestational diabetes mellitus (GDM) is a condition associated with pregnancy that engenders additional healthcare demand. A growing body of research includes empirical studies focused on pregnant women's GDM healthcare experiences. The aim of this scoping review is to map findings, highlight gaps and investigate the way research has been conducted into the healthcare experiences of women ...
The Treatment of Booking Gestational Diabetes Mellitus (TOBOGM) trial was a multicenter, randomized, controlled trial performed at 17 hospitals in Australia, Austria, Sweden, and India (Table S1 ...
Gestational diabetes mellitus (GDM) is one of the most common medical complications in pregnancy and affects an estimated 14% of pregnancies, or one in every seven births globally ().Women with GDM and their offspring are at increased risk of both short- and longer-term complications, including, for mothers, later development of type 2 diabetes, and for offspring, increased lifelong risks of ...
Diabetes Research and Clinical Practice. Volume 162, April 2020, 108044. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Author links open overlay panel Yueyi Li a 1, Xinghua Ren b 1, Lilan He a, Jing Li a, Shiyi Zhang a, Weiju Chen a.
Gestational diabetes mellitus (GDM) is 1 of the most common medical complications of pregnancy and is increasing in prevalence globally. GDM is associated with obstetric and neonatal complications primarily due to increased birthweight and is a major risk factor for future type 2 diabetes, obesity, and cardiovascular disease in mother and child.
Gestational Diabetes Mellitus (GDM) affects approximately 1 in 7 pregnancies globally. It is associated with short- and long-term risks for both mother and baby. Therefore, optimizing treatment to ...
Gestational diabetes mellitus (GDM) is the most prevalent disease among pregnant women, affecting up to 15-25% of pregnancies worldwide. ... Research gaps in gestational diabetes mellitus. There is much disagreement specifically on the criteria used to diagnose GDM. American Diabetes Association and American College of Obstetricians and ...
Summary When adjusted for confounders, gestational diabetes mellitus was signi cantly associated with a range of adverse pregnancy outcomes. Study design Systematic review and meta-analysis. Data sources 156 Overall risk of bias: 7 506 061 studies 19% low, % medium, % high pregnancies were evaluated.
Published by Elsevier Inc. 1 TITLE: Impact of Hypertensive Disorders of Pregnancy and Gestational Diabetes Mellitus 1 on Offspring Cardiovascular Health in Early Adolescence 2 3 AUTHORS: Kartik K ... (NUCATS) 35 Institute, Northwestern Memorial Foundation Dixon Translational Research Grant, American 36 Heart Association 17SFRN33660752 ...
Introduction. Gestational diabetes mellitus (GDM) is one of the commonest medical complications of pregnancy with rapidly rising Australian prevalence estimates between 12-25%. 1 For women diagnosed with GDM, the diagnosis itself can be overwhelming. 2 This diagnosis can include emotional, financial and cultural challenges for women. 2, 3 A GDM diagnosis results in an escalation of a woman's ...
Introduction. Gestational diabetes mellitus (GDM) according to the American Diabetes Association is defined as "diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes before gestation" [].In Mexico, the prevalence of GDM has been increasing; currently, its incidence is 17.7% [].GDM during pregnancy increases the susceptibility of offspring to ...
Purpose . Nutrient intake for pregnant women with gestational diabetes mellitus (GDM) is important to ensure satisfactory birth outcomes. This study aims to explore the dietary profiles of patients with GDM, compare the results with the Chinese dietary guidelines or Dietary Reference Intakes (DRIs) from China and investigate the relationship between maternal dietary intake and pregnancy outcomes.
Background Gestational diabetes mellitus (GDM) is a highly prevalent disease and poses a significant risk to the health of pregnant women. Abdominal adipose tissue (AT) contributes to insulin resistance (IR) associated with GDM. However, the underlying mechanisms remain unclear. Methods In this study, we developed a mouse model of GDM by subjecting mice to a high-fat diet. We collected adipose ...