- Open access
- Published: 26 February 2019

Stem cells: past, present, and future
- Wojciech Zakrzewski 1 ,
- Maciej Dobrzyński 2 ,
- Maria Szymonowicz 1 &
- Zbigniew Rybak 1
Stem Cell Research & Therapy volume 10 , Article number: 68 ( 2019 ) Cite this article
563k Accesses
845 Citations
54 Altmetric
Metrics details
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation. Quality control and teratoma formation assays are important procedures in assessing the properties of the stem cells tested. Derivation methods and the utilization of culturing media are crucial to set proper environmental conditions for controlled differentiation. Among many types of stem tissue applications, the use of graphene scaffolds and the potential of extracellular vesicle-based therapies require attention due to their versatility. The review is summarized by challenges that stem cell therapy must overcome to be accepted worldwide. A wide variety of possibilities makes this cutting edge therapy a turning point in modern medicine, providing hope for untreatable diseases.
Stem cell classification
Stem cells are unspecialized cells of the human body. They are able to differentiate into any cell of an organism and have the ability of self-renewal. Stem cells exist both in embryos and adult cells. There are several steps of specialization. Developmental potency is reduced with each step, which means that a unipotent stem cell is not able to differentiate into as many types of cells as a pluripotent one. This chapter will focus on stem cell classification to make it easier for the reader to comprehend the following chapters.
Totipotent stem cells are able to divide and differentiate into cells of the whole organism. Totipotency has the highest differentiation potential and allows cells to form both embryo and extra-embryonic structures. One example of a totipotent cell is a zygote, which is formed after a sperm fertilizes an egg. These cells can later develop either into any of the three germ layers or form a placenta. After approximately 4 days, the blastocyst’s inner cell mass becomes pluripotent. This structure is the source of pluripotent cells.
Pluripotent stem cells (PSCs) form cells of all germ layers but not extraembryonic structures, such as the placenta. Embryonic stem cells (ESCs) are an example. ESCs are derived from the inner cell mass of preimplantation embryos. Another example is induced pluripotent stem cells (iPSCs) derived from the epiblast layer of implanted embryos. Their pluripotency is a continuum, starting from completely pluripotent cells such as ESCs and iPSCs and ending on representatives with less potency—multi-, oligo- or unipotent cells. One of the methods to assess their activity and spectrum is the teratoma formation assay. iPSCs are artificially generated from somatic cells, and they function similarly to PSCs. Their culturing and utilization are very promising for present and future regenerative medicine.
Multipotent stem cells have a narrower spectrum of differentiation than PSCs, but they can specialize in discrete cells of specific cell lineages. One example is a haematopoietic stem cell, which can develop into several types of blood cells. After differentiation, a haematopoietic stem cell becomes an oligopotent cell. Its differentiation abilities are then restricted to cells of its lineage. However, some multipotent cells are capable of conversion into unrelated cell types, which suggests naming them pluripotent cells.
Oligopotent stem cells can differentiate into several cell types. A myeloid stem cell is an example that can divide into white blood cells but not red blood cells.
Unipotent stem cells are characterized by the narrowest differentiation capabilities and a special property of dividing repeatedly. Their latter feature makes them a promising candidate for therapeutic use in regenerative medicine. These cells are only able to form one cell type, e.g. dermatocytes.
Stem cell biology
A blastocyst is formed after the fusion of sperm and ovum fertilization. Its inner wall is lined with short-lived stem cells, namely, embryonic stem cells. Blastocysts are composed of two distinct cell types: the inner cell mass (ICM), which develops into epiblasts and induces the development of a foetus, and the trophectoderm (TE). Blastocysts are responsible for the regulation of the ICM microenvironment. The TE continues to develop and forms the extraembryonic support structures needed for the successful origin of the embryo, such as the placenta. As the TE begins to form a specialized support structure, the ICM cells remain undifferentiated, fully pluripotent and proliferative [ 1 ]. The pluripotency of stem cells allows them to form any cell of the organism. Human embryonic stem cells (hESCs) are derived from the ICM. During the process of embryogenesis, cells form aggregations called germ layers: endoderm, mesoderm and ectoderm (Fig. 1 ), each eventually giving rise to differentiated cells and tissues of the foetus and, later on, the adult organism [ 2 ]. After hESCs differentiate into one of the germ layers, they become multipotent stem cells, whose potency is limited to only the cells of the germ layer. This process is short in human development. After that, pluripotent stem cells occur all over the organism as undifferentiated cells, and their key abilities are proliferation by the formation of the next generation of stem cells and differentiation into specialized cells under certain physiological conditions.
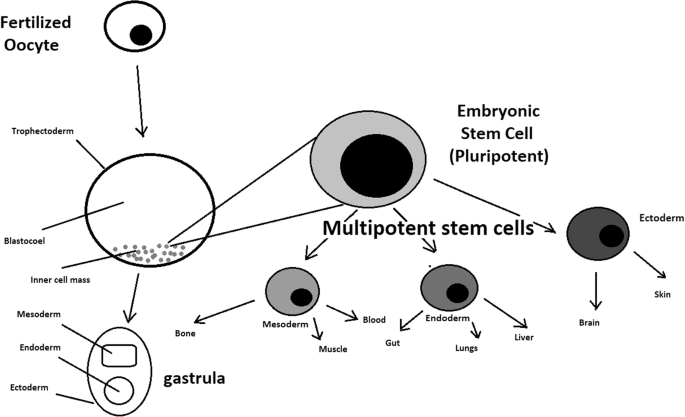
Oocyte development and formation of stem cells: the blastocoel, which is formed from oocytes, consists of embryonic stem cells that later differentiate into mesodermal, ectodermal, or endodermal cells. Blastocoel develops into the gastrula
Signals that influence the stem cell specialization process can be divided into external, such as physical contact between cells or chemical secretion by surrounding tissue, and internal, which are signals controlled by genes in DNA.
Stem cells also act as internal repair systems of the body. The replenishment and formation of new cells are unlimited as long as an organism is alive. Stem cell activity depends on the organ in which they are in; for example, in bone marrow, their division is constant, although in organs such as the pancreas, division only occurs under special physiological conditions.
Stem cell functional division
Whole-body development.
During division, the presence of different stem cells depends on organism development. Somatic stem cell ESCs can be distinguished. Although the derivation of ESCs without separation from the TE is possible, such a combination has growth limits. Because proliferating actions are limited, co-culture of these is usually avoided.
ESCs are derived from the inner cell mass of the blastocyst, which is a stage of pre-implantation embryo ca. 4 days after fertilization. After that, these cells are placed in a culture dish filled with culture medium. Passage is an inefficient but popular process of sub-culturing cells to other dishes. These cells can be described as pluripotent because they are able to eventually differentiate into every cell type in the organism. Since the beginning of their studies, there have been ethical restrictions connected to the medical use of ESCs in therapies. Most embryonic stem cells are developed from eggs that have been fertilized in an in vitro clinic, not from eggs fertilized in vivo.
Somatic or adult stem cells are undifferentiated and found among differentiated cells in the whole body after development. The function of these cells is to enable the healing, growth, and replacement of cells that are lost each day. These cells have a restricted range of differentiation options. Among many types, there are the following:
Mesenchymal stem cells are present in many tissues. In bone marrow, these cells differentiate mainly into the bone, cartilage, and fat cells. As stem cells, they are an exception because they act pluripotently and can specialize in the cells of any germ layer.
Neural cells give rise to nerve cells and their supporting cells—oligodendrocytes and astrocytes.
Haematopoietic stem cells form all kinds of blood cells: red, white, and platelets.
Skin stem cells form, for example, keratinocytes, which form a protective layer of skin.
The proliferation time of somatic stem cells is longer than that of ESCs. It is possible to reprogram adult stem cells back to their pluripotent state. This can be performed by transferring the adult nucleus into the cytoplasm of an oocyte or by fusion with the pluripotent cell. The same technique was used during cloning of the famous Dolly sheep.
hESCs are involved in whole-body development. They can differentiate into pluripotent, totipotent, multipotent, and unipotent cells (Fig. 2 ) [ 2 ].
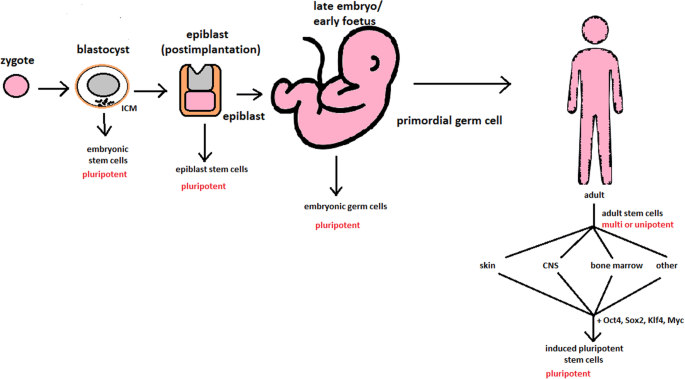
Changes in the potency of stem cells in human body development. Potency ranges from pluripotent cells of the blastocyst to unipotent cells of a specific tissue in a human body such as the skin, CNS, or bone marrow. Reversed pluripotency can be achieved by the formation of induced pluripotent stem cells using either octamer-binding transcription factor (Oct4), sex-determining region Y (Sox2), Kruppel-like factor 4 (Klf4), or the Myc gene
Pluripotent cells can be named totipotent if they can additionally form extraembryonic tissues of the embryo. Multipotent cells are restricted in differentiating to each cell type of given tissue. When tissue contains only one lineage of cells, stem cells that form them are called either called oligo- or unipotent.
iPSC quality control and recognition by morphological differences
The comparability of stem cell lines from different individuals is needed for iPSC lines to be used in therapeutics [ 3 ]. Among critical quality procedures, the following can be distinguished:
Short tandem repeat analysis—This is the comparison of specific loci on the DNA of the samples. It is used in measuring an exact number of repeating units. One unit consists of 2 to 13 nucleotides repeating many times on the DNA strand. A polymerase chain reaction is used to check the lengths of short tandem repeats. The genotyping procedure of source tissue, cells, and iPSC seed and master cell banks is recommended.
Identity analysis—The unintentional switching of lines, resulting in other stem cell line contamination, requires rigorous assay for cell line identification.
Residual vector testing—An appearance of reprogramming vectors integrated into the host genome is hazardous, and testing their presence is a mandatory procedure. It is a commonly used procedure for generating high-quality iPSC lines. An acceptable threshold in high-quality research-grade iPSC line collections is ≤ 1 plasmid copies per 100 cells. During the procedure, 2 different regions, common to all plasmids, should be used as specific targets, such as EBNA and CAG sequences [ 3 ]. To accurately represent the test reactions, a standard curve needs to be prepared in a carrier of gDNA from a well-characterized hPSC line. For calculations of plasmid copies per cell, it is crucial to incorporate internal reference gDNA sequences to allow the quantification of, for example, ribonuclease P (RNaseP) or human telomerase reverse transcriptase (hTERT).
Karyotype—A long-term culture of hESCs can accumulate culture-driven mutations [ 4 ]. Because of that, it is crucial to pay additional attention to genomic integrity. Karyotype tests can be performed by resuscitating representative aliquots and culturing them for 48–72 h before harvesting cells for karyotypic analysis. If abnormalities are found within the first 20 karyotypes, the analysis must be repeated on a fresh sample. When this situation is repeated, the line is evaluated as abnormal. Repeated abnormalities must be recorded. Although karyology is a crucial procedure in stem cell quality control, the single nucleotide polymorphism (SNP) array, discussed later, has approximately 50 times higher resolution.
Viral testing—When assessing the quality of stem cells, all tests for harmful human adventitious agents must be performed (e.g. hepatitis C or human immunodeficiency virus). This procedure must be performed in the case of non-xeno-free culture agents.
Bacteriology—Bacterial or fungal sterility tests can be divided into culture- or broth-based tests. All the procedures must be recommended by pharmacopoeia for the jurisdiction in which the work is performed.
Single nucleotide polymorphism arrays—This procedure is a type of DNA microarray that detects population polymorphisms by enabling the detection of subchromosomal changes and the copy-neutral loss of heterozygosity, as well as an indication of cellular transformation. The SNP assay consists of three components. The first is labelling fragmented nucleic acid sequences with fluorescent dyes. The second is an array that contains immobilized allele-specific oligonucleotide (ASO) probes. The last component detects, records, and eventually interprets the signal.
Flow cytometry—This is a technique that utilizes light to count and profile cells in a heterogeneous fluid mixture. It allows researchers to accurately and rapidly collect data from heterogeneous fluid mixtures with live cells. Cells are passed through a narrow channel one by one. During light illumination, sensors detect light emitted or refracted from the cells. The last step is data analysis, compilation and integration into a comprehensive picture of the sample.
Phenotypic pluripotency assays—Recognizing undifferentiated cells is crucial in successful stem cell therapy. Among other characteristics, stem cells appear to have a distinct morphology with a high nucleus to cytoplasm ratio and a prominent nucleolus. Cells appear to be flat with defined borders, in contrast to differentiating colonies, which appear as loosely located cells with rough borders [ 5 ]. It is important that images of ideal and poor quality colonies for each cell line are kept in laboratories, so whenever there is doubt about the quality of culture, it can always be checked according to the representative image. Embryoid body formation or directed differentiation of monolayer cultures to produce cell types representative of all three embryonic germ layers must be performed. It is important to note that colonies cultured under different conditions may have different morphologies [ 6 ].
Histone modification and DNA methylation—Quality control can be achieved by using epigenetic analysis tools such as histone modification or DNA methylation. When stem cells differentiate, the methylation process silences pluripotency genes, which reduces differentiation potential, although other genes may undergo demethylation to become expressed [ 7 ]. It is important to emphasize that stem cell identity, together with its morphological characteristics, is also related to its epigenetic profile [ 8 , 9 ]. According to Brindley [ 10 ], there is a relationship between epigenetic changes, pluripotency, and cell expansion conditions, which emphasizes that unmethylated regions appear to be serum-dependent.
hESC derivation and media
hESCs can be derived using a variety of methods, from classic culturing to laser-assisted methodologies or microsurgery [ 11 ]. hESC differentiation must be specified to avoid teratoma formation (see Fig. 3 ).
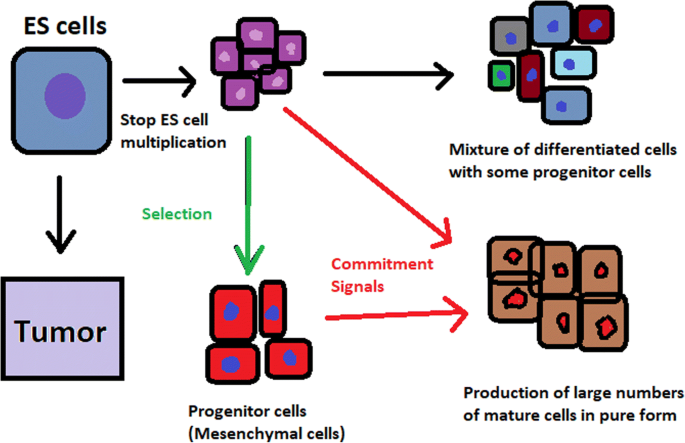
Spontaneous differentiation of hESCs causes the formation of a heterogeneous cell population. There is a different result, however, when commitment signals (in forms of soluble factors and culture conditions) are applied and enable the selection of progenitor cells
hESCs spontaneously differentiate into embryonic bodies (EBs) [ 12 ]. EBs can be studied instead of embryos or animals to predict their effects on early human development. There are many different methods for acquiring EBs, such as bioreactor culture [ 13 ], hanging drop culture [ 12 ], or microwell technology [ 14 , 15 ]. These methods allow specific precursors to form in vitro [ 16 ].
The essential part of these culturing procedures is a separation of inner cell mass to culture future hESCs (Fig. 4 ) [ 17 ]. Rosowski et al. [ 18 ] emphasizes that particular attention must be taken in controlling spontaneous differentiation. When the colony reaches the appropriate size, cells must be separated. The occurrence of pluripotent cells lasts for 1–2 days. Because the classical utilization of hESCs caused ethical concerns about gastrulas used during procedures, Chung et al. [ 19 ] found out that it is also possible to obtain hESCs from four cell embryos, leaving a higher probability of embryo survival. Additionally, Zhang et al. [ 20 ] used only in vitro fertilization growth-arrested cells.
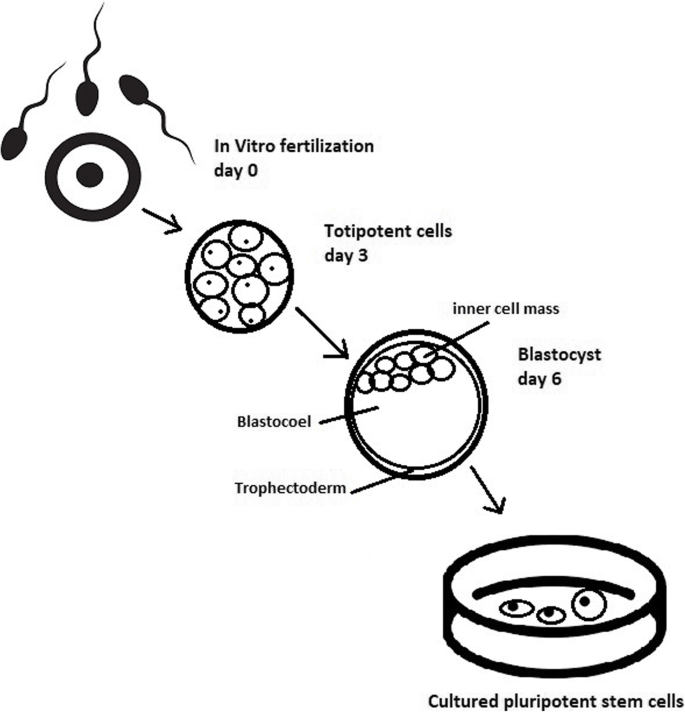
Culturing of pluripotent stem cells in vitro. Three days after fertilization, totipotent cells are formed. Blastocysts with ICM are formed on the sixth day after fertilization. Pluripotent stem cells from ICM can then be successfully transmitted on a dish
Cell passaging is used to form smaller clusters of cells on a new culture surface [ 21 ]. There are four important passaging procedures.
Enzymatic dissociation is a cutting action of enzymes on proteins and adhesion domains that bind the colony. It is a gentler method than the manual passage. It is crucial to not leave hESCs alone after passaging. Solitary cells are more sensitive and can easily undergo cell death; collagenase type IV is an example [ 22 , 23 ].
Manual passage , on the other hand, focuses on using cell scratchers. The selection of certain cells is not necessary. This should be done in the early stages of cell line derivation [ 24 ].
Trypsin utilization allows a healthy, automated hESC passage. Good Manufacturing Practice (GMP)-grade recombinant trypsin is widely available in this procedure [ 24 ]. However, there is a risk of decreasing the pluripotency and viability of stem cells [ 25 ]. Trypsin utilization can be halted with an inhibitor of the protein rho-associated protein kinase (ROCK) [ 26 ].
Ethylenediaminetetraacetic acid ( EDTA ) indirectly suppresses cell-to-cell connections by chelating divalent cations. Their suppression promotes cell dissociation [ 27 ].
Stem cells require a mixture of growth factors and nutrients to differentiate and develop. The medium should be changed each day.
Traditional culture methods used for hESCs are mouse embryonic fibroblasts (MEFs) as a feeder layer and bovine serum [ 28 ] as a medium. Martin et al. [ 29 ] demonstrated that hESCs cultured in the presence of animal products express the non-human sialic acid, N -glycolylneuraminic acid (NeuGc). Feeder layers prevent uncontrolled proliferation with factors such as leukaemia inhibitory factor (LIF) [ 30 ].
First feeder layer-free culture can be supplemented with serum replacement, combined with laminin [ 31 ]. This causes stable karyotypes of stem cells and pluripotency lasting for over a year.
Initial culturing media can be serum (e.g. foetal calf serum FCS), artificial replacement such as synthetic serum substitute (SSS), knockout serum replacement (KOSR), or StemPro [ 32 ]. The simplest culture medium contains only eight essential elements: DMEM/F12 medium, selenium, NaHCO 3, l -ascorbic acid, transferrin, insulin, TGFβ1, and FGF2 [ 33 ]. It is not yet fully known whether culture systems developed for hESCs can be allowed without adaptation in iPSC cultures.
Turning point in stem cell therapy
The turning point in stem cell therapy appeared in 2006, when scientists Shinya Yamanaka, together with Kazutoshi Takahashi, discovered that it is possible to reprogram multipotent adult stem cells to the pluripotent state. This process avoided endangering the foetus’ life in the process. Retrovirus-mediated transduction of mouse fibroblasts with four transcription factors (Oct-3/4, Sox2, KLF4, and c-Myc) [ 34 ] that are mainly expressed in embryonic stem cells could induce the fibroblasts to become pluripotent (Fig. 5 ) [ 35 ]. This new form of stem cells was named iPSCs. One year later, the experiment also succeeded with human cells [ 36 ]. After this success, the method opened a new field in stem cell research with a generation of iPSC lines that can be customized and biocompatible with the patient. Recently, studies have focused on reducing carcinogenesis and improving the conduction system.
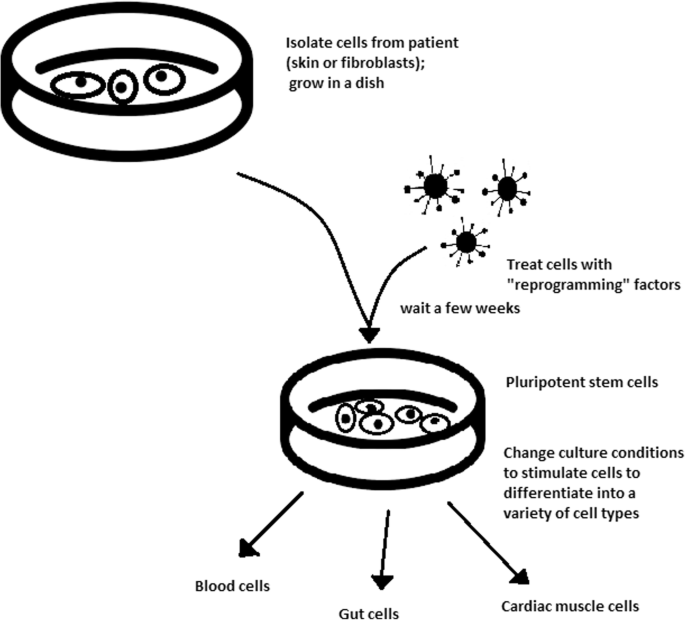
Retroviral-mediated transduction induces pluripotency in isolated patient somatic cells. Target cells lose their role as somatic cells and, once again, become pluripotent and can differentiate into any cell type of human body
The turning point was influenced by former discoveries that happened in 1962 and 1987.
The former discovery was about scientist John Gurdon successfully cloning frogs by transferring a nucleus from a frog’s somatic cells into an oocyte. This caused a complete reversion of somatic cell development [ 37 ]. The results of his experiment became an immense discovery since it was previously believed that cell differentiation is a one-way street only, but his experiment suggested the opposite and demonstrated that it is even possible for a somatic cell to again acquire pluripotency [ 38 ].
The latter was a discovery made by Davis R.L. that focused on fibroblast DNA subtraction. Three genes were found that originally appeared in myoblasts. The enforced expression of only one of the genes, named myogenic differentiation 1 (Myod1), caused the conversion of fibroblasts into myoblasts, showing that reprogramming cells is possible, and it can even be used to transform cells from one lineage to another [ 39 ].
Although pluripotency can occur naturally only in embryonic stem cells, it is possible to induce terminally differentiated cells to become pluripotent again. The process of direct reprogramming converts differentiated somatic cells into iPSC lines that can form all cell types of an organism. Reprogramming focuses on the expression of oncogenes such as Myc and Klf4 (Kruppel-like factor 4). This process is enhanced by a downregulation of genes promoting genome stability, such as p53. Additionally, cell reprogramming involves histone alteration. All these processes can cause potential mutagenic risk and later lead to an increased number of mutations. Quinlan et al. [ 40 ] checked fully pluripotent mouse iPSCs using whole genome DNA sequencing and structural variation (SV) detection algorithms. Based on those studies, it was confirmed that although there were single mutations in the non-genetic region, there were non-retrotransposon insertions. This led to the conclusion that current reprogramming methods can produce fully pluripotent iPSCs without severe genomic alterations.
During the course of development from pluripotent hESCs to differentiated somatic cells, crucial changes appear in the epigenetic structure of these cells. There is a restriction or permission of the transcription of genes relevant to each cell type. When somatic cells are being reprogrammed using transcription factors, all the epigenetic architecture has to be reconditioned to achieve iPSCs with pluripotency [ 41 ]. However, cells of each tissue undergo specific somatic genomic methylation. This influences transcription, which can further cause alterations in induced pluripotency [ 42 ].
Source of iPSCs
Because pluripotent cells can propagate indefinitely and differentiate into any kind of cell, they can be an unlimited source, either for replacing lost or diseased tissues. iPSCs bypass the need for embryos in stem cell therapy. Because they are made from the patient’s own cells, they are autologous and no longer generate any risk of immune rejection.
At first, fibroblasts were used as a source of iPSCs. Because a biopsy was needed to achieve these types of cells, the technique underwent further research. Researchers investigated whether more accessible cells could be used in the method. Further, other cells were used in the process: peripheral blood cells, keratinocytes, and renal epithelial cells found in urine. An alternative strategy to stem cell transplantation can be stimulating a patient’s endogenous stem cells to divide or differentiate, occurring naturally when skin wounds are healing. In 2008, pancreatic exocrine cells were shown to be reprogrammed to functional, insulin-producing beta cells [ 43 ].
The best stem cell source appears to be the fibroblasts, which is more tempting in the case of logistics since its stimulation can be fast and better controlled [ 44 ].
- Teratoma formation assay
The self-renewal and differentiation capabilities of iPSCs have gained significant interest and attention in regenerative medicine sciences. To study their abilities, a quality-control assay is needed, of which one of the most important is the teratoma formation assay. Teratomas are benign tumours. Teratomas are capable of rapid growth in vivo and are characteristic because of their ability to develop into tissues of all three germ layers simultaneously. Because of the high pluripotency of teratomas, this formation assay is considered an assessment of iPSC’s abilities [ 45 ].
Teratoma formation rate, for instance, was observed to be elevated in human iPSCs compared to that in hESCs [ 46 ]. This difference may be connected to different differentiation methods and cell origins. Most commonly, the teratoma assay involves an injection of examined iPSCs subcutaneously or under the testis or kidney capsule in mice, which are immune-deficient [ 47 ]. After injection, an immature but recognizable tissue can be observed, such as the kidney tubules, bone, cartilage, or neuroepithelium [ 30 ]. The injection site may have an impact on the efficiency of teratoma formation [ 48 ].
There are three groups of markers used in this assay to differentiate the cells of germ layers. For endodermal tissue, there is insulin/C-peptide and alpha-1 antitrypsin [ 49 ]. For the mesoderm, derivatives can be used, e.g. cartilage matrix protein for the bone and alcian blue for the cartilage. As ectodermal markers, class III B botulin or keratin can be used for keratinocytes.
Teratoma formation assays are considered the gold standard for demonstrating the pluripotency of human iPSCs, demonstrating their possibilities under physiological conditions. Due to their actual tissue formation, they could be used for the characterization of many cell lineages [ 50 ].
Directed differentiation
To be useful in therapy, stem cells must be converted into desired cell types as necessary or else the whole regenerative medicine process will be pointless. Differentiation of ESCs is crucial because undifferentiated ESCs can cause teratoma formation in vivo. Understanding and using signalling pathways for differentiation is an important method in successful regenerative medicine. In directed differentiation, it is likely to mimic signals that are received by cells when they undergo successive stages of development [ 51 ]. The extracellular microenvironment plays a significant role in controlling cell behaviour. By manipulating the culture conditions, it is possible to restrict specific differentiation pathways and generate cultures that are enriched in certain precursors in vitro. However, achieving a similar effect in vivo is challenging. It is crucial to develop culture conditions that will allow the promotion of homogenous and enhanced differentiation of ESCs into functional and desired tissues.
Regarding the self-renewal of embryonic stem cells, Hwang et al. [ 52 ] noted that the ideal culture method for hESC-based cell and tissue therapy would be a defined culture free of either the feeder layer or animal components. This is because cell and tissue therapy requires the maintenance of large quantities of undifferentiated hESCs, which does not make feeder cells suitable for such tasks.
Most directed differentiation protocols are formed to mimic the development of an inner cell mass during gastrulation. During this process, pluripotent stem cells differentiate into ectodermal, mesodermal, or endodermal progenitors. Mall molecules or growth factors induce the conversion of stem cells into appropriate progenitor cells, which will later give rise to the desired cell type. There is a variety of signal intensities and molecular families that may affect the establishment of germ layers in vivo, such as fibroblast growth factors (FGFs) [ 53 ]; the Wnt family [ 54 ] or superfamily of transforming growth factors—β(TGFβ); and bone morphogenic proteins (BMP) [ 55 ]. Each candidate factor must be tested on various concentrations and additionally applied to various durations because the precise concentrations and times during which developing cells in embryos are influenced during differentiation are unknown. For instance, molecular antagonists of endogenous BMP and Wnt signalling can be used for ESC formation of ectoderm [ 56 ]. However, transient Wnt and lower concentrations of the TGFβ family trigger mesodermal differentiation [ 57 ]. Regarding endoderm formation, a higher activin A concentration may be required [ 58 , 59 ].
There are numerous protocols about the methods of forming progenitors of cells of each of germ layers, such as cardiomyocytes [ 60 ], hepatocytes [ 61 ], renal cells [ 62 ], lung cells [ 63 , 64 ], motor neurons [ 65 ], intestinal cells [ 66 ], or chondrocytes [ 67 ].
Directed differentiation of either iPSCs or ESCs into, e.g. hepatocytes, could influence and develop the study of the molecular mechanisms in human liver development. In addition, it could also provide the possibility to form exogenous hepatocytes for drug toxicity testing [ 68 ].
Levels of concentration and duration of action with a specific signalling molecule can cause a variety of factors. Unfortunately, for now, a high cost of recombinant factors is likely to limit their use on a larger scale in medicine. The more promising technique focuses on the use of small molecules. These can be used for either activating or deactivating specific signalling pathways. They enhance reprogramming efficiency by creating cells that are compatible with the desired type of tissue. It is a cheaper and non-immunogenic method.
One of the successful examples of small-molecule cell therapies is antagonists and agonists of the Hedgehog pathway. They show to be very useful in motor neuron regeneration [ 69 ]. Endogenous small molecules with their function in embryonic development can also be used in in vitro methods to induce the differentiation of cells; for example, retinoic acid, which is responsible for patterning the nervous system in vivo [ 70 ], surprisingly induced retinal cell formation when the laboratory procedure involved hESCs [ 71 ].
The efficacy of differentiation factors depends on functional maturity, efficiency, and, finally, introducing produced cells to their in vivo equivalent. Topography, shear stress, and substrate rigidity are factors influencing the phenotype of future cells [ 72 ].
The control of biophysical and biochemical signals, the biophysical environment, and a proper guide of hESC differentiation are important factors in appropriately cultured stem cells.
Stem cell utilization and their manufacturing standards and culture systems
The European Medicines Agency and the Food and Drug Administration have set Good Manufacturing Practice (GMP) guidelines for safe and appropriate stem cell transplantation. In the past, protocols used for stem cell transplantation required animal-derived products [ 73 ].
The risk of introducing animal antigens or pathogens caused a restriction in their use. Due to such limitations, the technique required an obvious update [ 74 ]. Now, it is essential to use xeno-free equivalents when establishing cell lines that are derived from fresh embryos and cultured from human feeder cell lines [ 75 ]. In this method, it is crucial to replace any non-human materials with xeno-free equivalents [ 76 ].
NutriStem with LN-511, TeSR2 with human recombinant laminin (LN-511), and RegES with human foreskin fibroblasts (HFFs) are commonly used xeno-free culture systems [ 33 ]. There are many organizations and international initiatives, such as the National Stem Cell Bank, that provide stem cell lines for treatment or medical research [ 77 ].
Stem cell use in medicine
Stem cells have great potential to become one of the most important aspects of medicine. In addition to the fact that they play a large role in developing restorative medicine, their study reveals much information about the complex events that happen during human development.
The difference between a stem cell and a differentiated cell is reflected in the cells’ DNA. In the former cell, DNA is arranged loosely with working genes. When signals enter the cell and the differentiation process begins, genes that are no longer needed are shut down, but genes required for the specialized function will remain active. This process can be reversed, and it is known that such pluripotency can be achieved by interaction in gene sequences. Takahashi and Yamanaka [ 78 ] and Loh et al. [ 79 ] discovered that octamer-binding transcription factor 3 and 4 (Oct3/4), sex determining region Y (SRY)-box 2 and Nanog genes function as core transcription factors in maintaining pluripotency. Among them, Oct3/4 and Sox2 are essential for the generation of iPSCs.
Many serious medical conditions, such as birth defects or cancer, are caused by improper differentiation or cell division. Currently, several stem cell therapies are possible, among which are treatments for spinal cord injury, heart failure [ 80 ], retinal and macular degeneration [ 81 ], tendon ruptures, and diabetes type 1 [ 82 ]. Stem cell research can further help in better understanding stem cell physiology. This may result in finding new ways of treating currently incurable diseases.
Haematopoietic stem cell transplantation
Haematopoietic stem cells are important because they are by far the most thoroughly characterized tissue-specific stem cell; after all, they have been experimentally studied for more than 50 years. These stem cells appear to provide an accurate paradigm model system to study tissue-specific stem cells, and they have potential in regenerative medicine.
Multipotent haematopoietic stem cell (HSC) transplantation is currently the most popular stem cell therapy. Target cells are usually derived from the bone marrow, peripheral blood, or umbilical cord blood [ 83 ]. The procedure can be autologous (when the patient’s own cells are used), allogenic (when the stem cell comes from a donor), or syngeneic (from an identical twin). HSCs are responsible for the generation of all functional haematopoietic lineages in blood, including erythrocytes, leukocytes, and platelets. HSC transplantation solves problems that are caused by inappropriate functioning of the haematopoietic system, which includes diseases such as leukaemia and anaemia. However, when conventional sources of HSC are taken into consideration, there are some important limitations. First, there is a limited number of transplantable cells, and an efficient way of gathering them has not yet been found. There is also a problem with finding a fitting antigen-matched donor for transplantation, and viral contamination or any immunoreactions also cause a reduction in efficiency in conventional HSC transplantations. Haematopoietic transplantation should be reserved for patients with life-threatening diseases because it has a multifactorial character and can be a dangerous procedure. iPSC use is crucial in this procedure. The use of a patient’s own unspecialized somatic cells as stem cells provides the greatest immunological compatibility and significantly increases the success of the procedure.
Stem cells as a target for pharmacological testing
Stem cells can be used in new drug tests. Each experiment on living tissue can be performed safely on specific differentiated cells from pluripotent cells. If any undesirable effect appears, drug formulas can be changed until they reach a sufficient level of effectiveness. The drug can enter the pharmacological market without harming any live testers. However, to test the drugs properly, the conditions must be equal when comparing the effects of two drugs. To achieve this goal, researchers need to gain full control of the differentiation process to generate pure populations of differentiated cells.
Stem cells as an alternative for arthroplasty
One of the biggest fears of professional sportsmen is getting an injury, which most often signifies the end of their professional career. This applies especially to tendon injuries, which, due to current treatment options focusing either on conservative or surgical treatment, often do not provide acceptable outcomes. Problems with the tendons start with their regeneration capabilities. Instead of functionally regenerating after an injury, tendons merely heal by forming scar tissues that lack the functionality of healthy tissues. Factors that may cause this failed healing response include hypervascularization, deposition of calcific materials, pain, or swelling [ 84 ].
Additionally, in addition to problems with tendons, there is a high probability of acquiring a pathological condition of joints called osteoarthritis (OA) [ 85 ]. OA is common due to the avascular nature of articular cartilage and its low regenerative capabilities [ 86 ]. Although arthroplasty is currently a common procedure in treating OA, it is not ideal for younger patients because they can outlive the implant and will require several surgical procedures in the future. These are situations where stem cell therapy can help by stopping the onset of OA [ 87 ]. However, these procedures are not well developed, and the long-term maintenance of hyaline cartilage requires further research.
Osteonecrosis of the femoral hip (ONFH) is a refractory disease associated with the collapse of the femoral head and risk of hip arthroplasty in younger populations [ 88 ]. Although total hip arthroplasty (THA) is clinically successful, it is not ideal for young patients, mostly due to the limited lifetime of the prosthesis. An increasing number of clinical studies have evaluated the therapeutic effect of stem cells on ONFH. Most of the authors demonstrated positive outcomes, with reduced pain, improved function, or avoidance of THA [ 89 , 90 , 91 ].
Rejuvenation by cell programming
Ageing is a reversible epigenetic process. The first cell rejuvenation study was published in 2011 [ 92 ]. Cells from aged individuals have different transcriptional signatures, high levels of oxidative stress, dysfunctional mitochondria, and shorter telomeres than in young cells [ 93 ]. There is a hypothesis that when human or mouse adult somatic cells are reprogrammed to iPSCs, their epigenetic age is virtually reset to zero [ 94 ]. This was based on an epigenetic model, which explains that at the time of fertilization, all marks of parenteral ageing are erased from the zygote’s genome and its ageing clock is reset to zero [ 95 ].
In their study, Ocampo et al. [ 96 ] used Oct4, Sox2, Klf4, and C-myc genes (OSKM genes) and affected pancreas and skeletal muscle cells, which have poor regenerative capacity. Their procedure revealed that these genes can also be used for effective regenerative treatment [ 97 ]. The main challenge of their method was the need to employ an approach that does not use transgenic animals and does not require an indefinitely long application. The first clinical approach would be preventive, focused on stopping or slowing the ageing rate. Later, progressive rejuvenation of old individuals can be attempted. In the future, this method may raise some ethical issues, such as overpopulation, leading to lower availability of food and energy.
For now, it is important to learn how to implement cell reprogramming technology in non-transgenic elder animals and humans to erase marks of ageing without removing the epigenetic marks of cell identity.
Cell-based therapies
Stem cells can be induced to become a specific cell type that is required to repair damaged or destroyed tissues (Fig. 6 ). Currently, when the need for transplantable tissues and organs outweighs the possible supply, stem cells appear to be a perfect solution for the problem. The most common conditions that benefit from such therapy are macular degenerations [ 98 ], strokes [ 99 ], osteoarthritis [ 89 , 90 ], neurodegenerative diseases, and diabetes [ 100 ]. Due to this technique, it can become possible to generate healthy heart muscle cells and later transplant them to patients with heart disease.
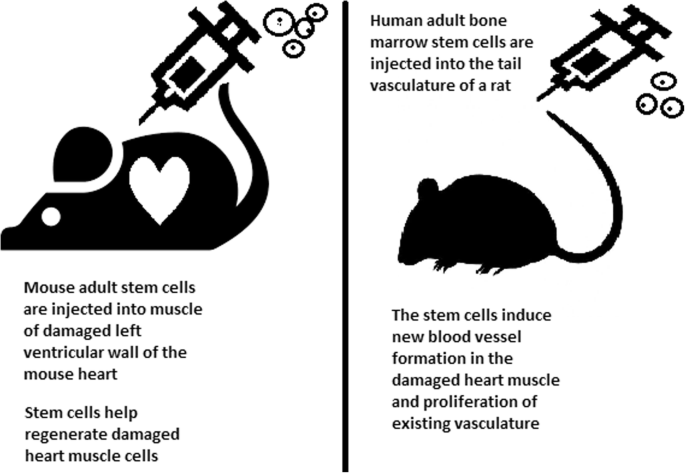
Stem cell experiments on animals. These experiments are one of the many procedures that proved stem cells to be a crucial factor in future regenerative medicine
In the case of type 1 diabetes, insulin-producing cells in the pancreas are destroyed due to an autoimmunological reaction. As an alternative to transplantation therapy, it can be possible to induce stem cells to differentiate into insulin-producing cells [ 101 ].
Stem cells and tissue banks
iPS cells with their theoretically unlimited propagation and differentiation abilities are attractive for the present and future sciences. They can be stored in a tissue bank to be an essential source of human tissue used for medical examination. The problem with conventional differentiated tissue cells held in the laboratory is that their propagation features diminish after time. This does not occur in iPSCs.
The umbilical cord is known to be rich in mesenchymal stem cells. Due to its cryopreservation immediately after birth, its stem cells can be successfully stored and used in therapies to prevent the future life-threatening diseases of a given patient.
Stem cells of human exfoliated deciduous teeth (SHED) found in exfoliated deciduous teeth has the ability to develop into more types of body tissues than other stem cells [ 102 ] (Table 1 ). Techniques of their collection, isolation, and storage are simple and non-invasive. Among the advantages of banking, SHED cells are:
Guaranteed donor-match autologous transplant that causes no immune reaction and rejection of cells [ 103 ]
Simple and painless for both child and parent
Less than one third of the cost of cord blood storage
Not subject to the same ethical concerns as embryonic stem cells [ 104 ]
In contrast to cord blood stem cells, SHED cells are able to regenerate into solid tissues such as connective, neural, dental, or bone tissue [ 105 , 106 ]
SHED can be useful for close relatives of the donor
Fertility diseases
In 2011, two researchers, Katsuhiko Hayashi et al. [ 107 ], showed in an experiment on mice that it is possible to form sperm from iPSCs. They succeeded in delivering healthy and fertile pups in infertile mice. The experiment was also successful for female mice, where iPSCs formed fully functional eggs .
Young adults at risk of losing their spermatogonial stem cells (SSC), mostly cancer patients, are the main target group that can benefit from testicular tissue cryopreservation and autotransplantation. Effective freezing methods for adult and pre-pubertal testicular tissue are available [ 108 ].
Qiuwan et al. [ 109 ] provided important evidence that human amniotic epithelial cell (hAEC) transplantation could effectively improve ovarian function by inhibiting cell apoptosis and reducing inflammation in injured ovarian tissue of mice, and it could be a promising strategy for the management of premature ovarian failure or insufficiency in female cancer survivors.
For now, reaching successful infertility treatments in humans appears to be only a matter of time, but there are several challenges to overcome. First, the process needs to have high efficiency; second, the chances of forming tumours instead of eggs or sperm must be maximally reduced. The last barrier is how to mature human sperm and eggs in the lab without transplanting them to in vivo conditions, which could cause either a tumour risk or an invasive procedure.
Therapy for incurable neurodegenerative diseases
Thanks to stem cell therapy, it is possible not only to delay the progression of incurable neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease (AD), and Huntington disease, but also, most importantly, to remove the source of the problem. In neuroscience, the discovery of neural stem cells (NSCs) has nullified the previous idea that adult CNS were not capable of neurogenesis [ 110 , 111 ]. Neural stem cells are capable of improving cognitive function in preclinical rodent models of AD [ 112 , 113 , 114 ]. Awe et al. [ 115 ] clinically derived relevant human iPSCs from skin punch biopsies to develop a neural stem cell-based approach for treating AD. Neuronal degeneration in Parkinson’s disease (PD) is focal, and dopaminergic neurons can be efficiently generated from hESCs. PD is an ideal disease for iPSC-based cell therapy [ 116 ]. However, this therapy is still in an experimental phase ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4539501 /). Brain tissue from aborted foetuses was used on patients with Parkinson’s disease [ 117 ]. Although the results were not uniform, they showed that therapies with pure stem cells are an important and achievable therapy.
Stem cell use in dentistry
Teeth represent a very challenging material for regenerative medicine. They are difficult to recreate because of their function in aspects such as articulation, mastication, or aesthetics due to their complicated structure. Currently, there is a chance for stem cells to become more widely used than synthetic materials. Teeth have a large advantage of being the most natural and non-invasive source of stem cells.
For now, without the use of stem cells, the most common periodontological treatments are either growth factors, grafts, or surgery. For example, there are stem cells in periodontal ligament [ 118 , 119 ], which are capable of differentiating into osteoblasts or cementoblasts, and their functions were also assessed in neural cells [ 120 ]. Tissue engineering is a successful method for treating periodontal diseases. Stem cells of the root apical areas are able to recreate periodontal ligament. One of the possible methods of tissue engineering in periodontology is gene therapy performed using adenoviruses-containing growth factors [ 121 ].
As a result of animal studies, dentin regeneration is an effective process that results in the formation of dentin bridges [ 122 ].
Enamel is more difficult to regenerate than dentin. After the differentiation of ameloblastoma cells into the enamel, the former is destroyed, and reparation is impossible. Medical studies have succeeded in differentiating bone marrow stem cells into ameloblastoma [ 123 ].
Healthy dental tissue has a high amount of regular stem cells, although this number is reduced when tissue is either traumatized or inflamed [ 124 ]. There are several dental stem cell groups that can be isolated (Fig. 7 ).
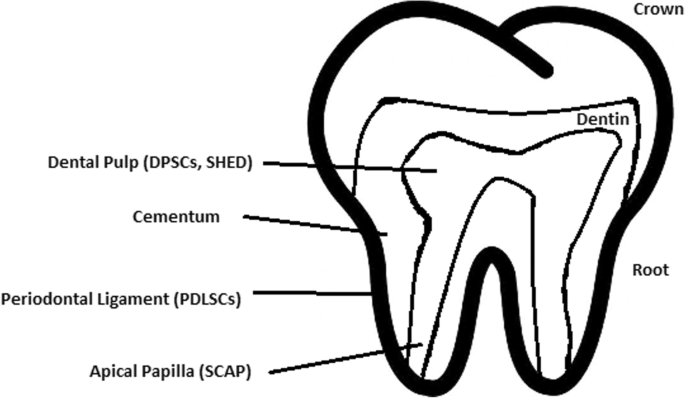
Localization of stem cells in dental tissues. Dental pulp stem cells (DPSCs) and human deciduous teeth stem cells (SHED) are located in the dental pulp. Periodontal ligaments stem cells are located in the periodontal ligament. Apical papilla consists of stem cells from the apical papilla (SCAP)
Dental pulp stem cell (DPSC)
These were the first dental stem cells isolated from the human dental pulp, which were [ 125 ] located inside dental pulp (Table 2 ). They have osteogenic and chondrogenic potential. Mesenchymal stem cells (MSCs) of the dental pulp, when isolated, appear highly clonogenic; they can be isolated from adult tissue (e.g. bone marrow, adipose tissue) and foetal (e.g. umbilical cord) [ 126 ] tissue, and they are able to differentiate densely [ 127 ]. MSCs differentiate into odontoblast-like cells and osteoblasts to form dentin and bone. Their best source locations are the third molars [ 125 ]. DPSCs are the most useful dental source of tissue engineering due to their easy surgical accessibility, cryopreservation possibility, increased production of dentin tissues compared to non-dental stem cells, and their anti-inflammatory abilities. These cells have the potential to be a source for maxillofacial and orthopaedic reconstructions or reconstructions even beyond the oral cavity. DPSCs are able to generate all structures of the developed tooth [ 128 ]. In particular, beneficial results in the use of DPSCs may be achieved when combined with other new therapies, such as periodontal tissue photobiomodulation (laser stimulation), which is an efficient technique in the stimulation of proliferation and differentiation into distinct cell types [ 129 ]. DPSCs can be induced to form neural cells to help treat neurological deficits.
Stem cells of human exfoliated deciduous teeth (SHED) have a faster rate of proliferation than DPSCs and differentiate into an even greater number of cells, e.g. other mesenchymal and non-mesenchymal stem cell derivatives, such as neural cells [ 130 ]. These cells possess one major disadvantage: they form a non-complete dentin/pulp-like complex in vivo. SHED do not undergo the same ethical concerns as embryonic stem cells. Both DPSCs and SHED are able to form bone-like tissues in vivo [ 131 ] and can be used for periodontal, dentin, or pulp regeneration. DPSCs and SHED can be used in treating, for example, neural deficits [ 132 ]. DPSCs alone were tested and successfully applied for alveolar bone and mandible reconstruction [ 133 ].
Periodontal ligament stem cells (PDLSCs)
These cells are used in periodontal ligament or cementum tissue regeneration. They can differentiate into mesenchymal cell lineages to produce collagen-forming cells, adipocytes, cementum tissue, Sharpey’s fibres, and osteoblast-like cells in vitro. PDLSCs exist both on the root and alveolar bone surfaces; however, on the latter, these cells have better differentiation abilities than on the former [ 134 ]. PDLSCs have become the first treatment for periodontal regeneration therapy because of their safety and efficiency [ 135 , 136 ].
Stem cells from apical papilla (SCAP)
These cells are mesenchymal structures located within immature roots. They are isolated from human immature permanent apical papilla. SCAP are the source of odontoblasts and cause apexogenesis. These stem cells can be induced in vitro to form odontoblast-like cells, neuron-like cells, or adipocytes. SCAP have a higher capacity of proliferation than DPSCs, which makes them a better choice for tissue regeneration [ 137 , 138 ].
Dental follicle stem cells (DFCs)
These cells are loose connective tissues surrounding the developing tooth germ. DFCs contain cells that can differentiate into cementoblasts, osteoblasts, and periodontal ligament cells [ 139 , 140 ]. Additionally, these cells proliferate after even more than 30 passages [ 141 ]. DFCs are most commonly extracted from the sac of a third molar. When DFCs are combined with a treated dentin matrix, they can form a root-like tissue with a pulp-dentin complex and eventually form tooth roots [ 141 ]. When DFC sheets are induced by Hertwig’s epithelial root sheath cells, they can produce periodontal tissue; thus, DFCs represent a very promising material for tooth regeneration [ 142 ].
Pulp regeneration in endodontics
Dental pulp stem cells can differentiate into odontoblasts. There are few methods that enable the regeneration of the pulp.
The first is an ex vivo method. Proper stem cells are grown on a scaffold before they are implanted into the root channel [ 143 ].
The second is an in vivo method. This method focuses on injecting stem cells into disinfected root channels after the opening of the in vivo apex. Additionally, the use of a scaffold is necessary to prevent the movement of cells towards other tissues. For now, only pulp-like structures have been created successfully.
Methods of placing stem cells into the root channel constitute are either soft scaffolding [ 144 ] or the application of stem cells in apexogenesis or apexification. Immature teeth are the best source [ 145 ]. Nerve and blood vessel network regeneration are extremely vital to keep pulp tissue healthy.
The potential of dental stem cells is mainly regarding the regeneration of damaged dentin and pulp or the repair of any perforations; in the future, it appears to be even possible to generate the whole tooth. Such an immense success would lead to the gradual replacement of implant treatments. Mandibulary and maxillary defects can be one of the most complicated dental problems for stem cells to address.
Acquiring non-dental tissue cells by dental stem cell differentiation
In 2013, it was reported that it is possible to grow teeth from stem cells obtained extra-orally, e.g. from urine [ 146 ]. Pluripotent stem cells derived from human urine were induced and generated tooth-like structures. The physical properties of the structures were similar to natural ones except for hardness [ 127 ]. Nonetheless, it appears to be a very promising technique because it is non-invasive and relatively low-cost, and somatic cells can be used instead of embryonic cells. More importantly, stem cells derived from urine did not form any tumours, and the use of autologous cells reduces the chances of rejection [ 147 ].
Use of graphene in stem cell therapy
Over recent years, graphene and its derivatives have been increasingly used as scaffold materials to mediate stem cell growth and differentiation [ 148 ]. Both graphene and graphene oxide (GO) represent high in-plane stiffness [ 149 ]. Because graphene has carbon and aromatic network, it works either covalently or non-covalently with biomolecules; in addition to its superior mechanical properties, graphene offers versatile chemistry. Graphene exhibits biocompatibility with cells and their proper adhesion. It also tested positively for enhancing the proliferation or differentiation of stem cells [ 148 ]. After positive experiments, graphene revealed great potential as a scaffold and guide for specific lineages of stem cell differentiation [ 150 ]. Graphene has been successfully used in the transplantation of hMSCs and their guided differentiation to specific cells. The acceleration skills of graphene differentiation and division were also investigated. It was discovered that graphene can serve as a platform with increased adhesion for both growth factors and differentiation chemicals. It was also discovered that π-π binding was responsible for increased adhesion and played a crucial role in inducing hMSC differentiation [ 150 ].
Therapeutic potential of extracellular vesicle-based therapies
Extracellular vesicles (EVs) can be released by virtually every cell of an organism, including stem cells [ 151 ], and are involved in intercellular communication through the delivery of their mRNAs, lipids, and proteins. As Oh et al. [ 152 ] prove, stem cells, together with their paracrine factors—exosomes—can become potential therapeutics in the treatment of, e.g. skin ageing. Exosomes are small membrane vesicles secreted by most cells (30–120 nm in diameter) [ 153 ]. When endosomes fuse with the plasma membrane, they become exosomes that have messenger RNAs (mRNAs) and microRNAs (miRNAs), some classes of non-coding RNAs (IncRNAs) and several proteins that originate from the host cell [ 154 ]. IncRNAs can bind to specific loci and create epigenetic regulators, which leads to the formation of epigenetic modifications in recipient cells. Because of this feature, exosomes are believed to be implicated in cell-to-cell communication and the progression of diseases such as cancer [ 155 ]. Recently, many studies have also shown the therapeutic use of exosomes derived from stem cells, e.g. skin damage and renal or lung injuries [ 156 ].
In skin ageing, the most important factor is exposure to UV light, called “photoageing” [ 157 ], which causes extrinsic skin damage, characterized by dryness, roughness, irregular pigmentation, lesions, and skin cancers. In intrinsic skin ageing, on the other hand, the loss of elasticity is a characteristic feature. The skin dermis consists of fibroblasts, which are responsible for the synthesis of crucial skin elements, such as procollagen or elastic fibres. These elements form either basic framework extracellular matrix constituents of the skin dermis or play a major role in tissue elasticity. Fibroblast efficiency and abundance decrease with ageing [ 158 ]. Stem cells can promote the proliferation of dermal fibroblasts by secreting cytokines such as platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), and basic fibroblast growth factor. Huh et al. [ 159 ] mentioned that a medium of human amniotic fluid-derived stem cells (hAFSC) positively affected skin regeneration after longwave UV-induced (UVA, 315–400 nm) photoageing by increasing the proliferation and migration of dermal fibroblasts. It was discovered that, in addition to the induction of fibroblast physiology, hAFSC transplantation also improved diseases in cases of renal pathology, various cancers, or stroke [ 160 , 161 ].
Oh [ 162 ] also presented another option for the treatment of skin wounds, either caused by physical damage or due to diabetic ulcers. Induced pluripotent stem cell-conditioned medium (iPSC-CM) without any animal-derived components induced dermal fibroblast proliferation and migration.
Natural cutaneous wound healing is divided into three steps: haemostasis/inflammation, proliferation, and remodelling. During the crucial step of proliferation, fibroblasts migrate and increase in number, indicating that it is a critical step in skin repair, and factors such as iPSC-CM that impact it can improve the whole cutaneous wound healing process. Paracrine actions performed by iPSCs are also important for this therapeutic effect [ 163 ]. These actions result in the secretion of cytokines such as TGF-β, interleukin (IL)-6, IL-8, monocyte chemotactic protein-1 (MCP-1), vascular endothelial growth factor (VEGF), platelet-derived growth factor-AA (PDGF-AA), and basic fibroblast growth factor (bFGF). Bae et al. [ 164 ] mentioned that TGF-β induced the migration of keratinocytes. It was also demonstrated that iPSC factors can enhance skin wound healing in vivo and in vitro when Zhou et al. [ 165 ] enhanced wound healing, even after carbon dioxide laser resurfacing in an in vivo study.
Peng et al. [ 166 ] investigated the effects of EVs derived from hESCs on in vitro cultured retinal glial, progenitor Müller cells, which are known to differentiate into retinal neurons. EVs appear heterogeneous in size and can be internalized by cultured Müller cells, and their proteins are involved in the induction and maintenance of stem cell pluripotency. These stem cell-derived vesicles were responsible for the neuronal trans-differentiation of cultured Müller cells exposed to them. However, the research article points out that the procedure was accomplished only on in vitro acquired retina.
Challenges concerning stem cell therapy
Although stem cells appear to be an ideal solution for medicine, there are still many obstacles that need to be overcome in the future. One of the first problems is ethical concern.
The most common pluripotent stem cells are ESCs. Therapies concerning their use at the beginning were, and still are, the source of ethical conflicts. The reason behind it started when, in 1998, scientists discovered the possibility of removing ESCs from human embryos. Stem cell therapy appeared to be very effective in treating many, even previously incurable, diseases. The problem was that when scientists isolated ESCs in the lab, the embryo, which had potential for becoming a human, was destroyed (Fig. 8 ). Because of this, scientists, seeing a large potential in this treatment method, focused their efforts on making it possible to isolate stem cells without endangering their source—the embryo.
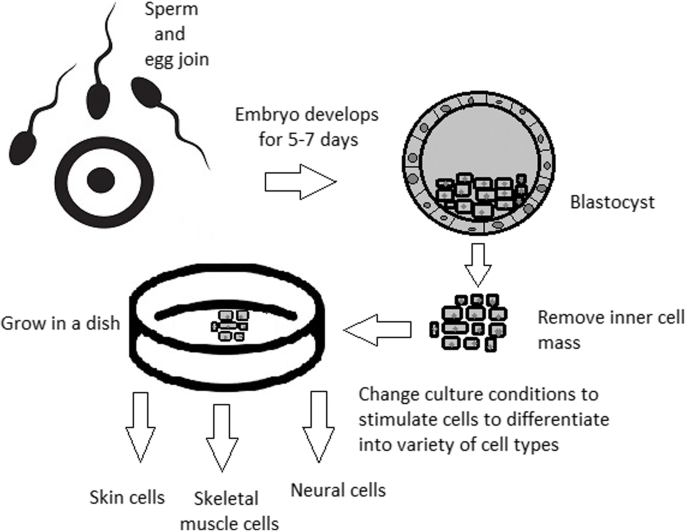
Use of inner cell mass pluripotent stem cells and their stimulation to differentiate into desired cell types
For now, while hESCs still remain an ethically debatable source of cells, they are potentially powerful tools to be used for therapeutic applications of tissue regeneration. Because of the complexity of stem cell control systems, there is still much to be learned through observations in vitro. For stem cells to become a popular and widely accessible procedure, tumour risk must be assessed. The second problem is to achieve successful immunological tolerance between stem cells and the patient’s body. For now, one of the best ideas is to use the patient’s own cells and devolve them into their pluripotent stage of development.
New cells need to have the ability to fully replace lost or malfunctioning natural cells. Additionally, there is a concern about the possibility of obtaining stem cells without the risk of morbidity or pain for either the patient or the donor. Uncontrolled proliferation and differentiation of cells after implementation must also be assessed before its use in a wide variety of regenerative procedures on living patients [ 167 ].
One of the arguments that limit the use of iPSCs is their infamous role in tumourigenicity. There is a risk that the expression of oncogenes may increase when cells are being reprogrammed. In 2008, a technique was discovered that allowed scientists to remove oncogenes after a cell achieved pluripotency, although it is not efficient yet and takes a longer amount of time. The process of reprogramming may be enhanced by deletion of the tumour suppressor gene p53, but this gene also acts as a key regulator of cancer, which makes it impossible to remove in order to avoid more mutations in the reprogrammed cell. The low efficiency of the process is another problem, which is progressively becoming reduced with each year. At first, the rate of somatic cell reprogramming in Yamanaka’s study was up to 0.1%. The use of transcription factors creates a risk of genomic insertion and further mutation of the target cell genome. For now, the only ethically acceptable operation is an injection of hESCs into mouse embryos in the case of pluripotency evaluation [ 168 ].
Stem cell obstacles in the future
Pioneering scientific and medical advances always have to be carefully policed in order to make sure they are both ethical and safe. Because stem cell therapy already has a large impact on many aspects of life, it should not be treated differently.
Currently, there are several challenges concerning stem cells. First, the most important one is about fully understanding the mechanism by which stem cells function first in animal models. This step cannot be avoided. For the widespread, global acceptance of the procedure, fear of the unknown is the greatest challenge to overcome.
The efficiency of stem cell-directed differentiation must be improved to make stem cells more reliable and trustworthy for a regular patient. The scale of the procedure is another challenge. Future stem cell therapies may be a significant obstacle. Transplanting new, fully functional organs made by stem cell therapy would require the creation of millions of working and biologically accurate cooperating cells. Bringing such complicated procedures into general, widespread regenerative medicine will require interdisciplinary and international collaboration.
The identification and proper isolation of stem cells from a patient’s tissues is another challenge. Immunological rejection is a major barrier to successful stem cell transplantation. With certain types of stem cells and procedures, the immune system may recognize transplanted cells as foreign bodies, triggering an immune reaction resulting in transplant or cell rejection.
One of the ideas that can make stem cells a “failsafe” is about implementing a self-destruct option if they become dangerous. Further development and versatility of stem cells may cause reduction of treatment costs for people suffering from currently incurable diseases. When facing certain organ failure, instead of undergoing extraordinarily expensive drug treatment, the patient would be able to utilize stem cell therapy. The effect of a successful operation would be immediate, and the patient would avoid chronic pharmacological treatment and its inevitable side effects.
Although these challenges facing stem cell science can be overwhelming, the field is making great advances each day. Stem cell therapy is already available for treating several diseases and conditions. Their impact on future medicine appears to be significant.
After several decades of experiments, stem cell therapy is becoming a magnificent game changer for medicine. With each experiment, the capabilities of stem cells are growing, although there are still many obstacles to overcome. Regardless, the influence of stem cells in regenerative medicine and transplantology is immense. Currently, untreatable neurodegenerative diseases have the possibility of becoming treatable with stem cell therapy. Induced pluripotency enables the use of a patient’s own cells. Tissue banks are becoming increasingly popular, as they gather cells that are the source of regenerative medicine in a struggle against present and future diseases. With stem cell therapy and all its regenerative benefits, we are better able to prolong human life than at any time in history.
Abbreviations
Basic fibroblast growth factor
Bone morphogenic proteins
Dental follicle stem cells
Dental pulp stem cells
Embryonic bodies
Embryonic stem cells
Fibroblast growth factors
Good Manufacturing Practice
Graphene oxide
Human amniotic fluid-derived stem cells
Human embryonic stem cells
Human foreskin fibroblasts
Inner cell mass
Non-coding RNA
Induced pluripotent stem cells
In vitro fertilization
Knockout serum replacement
Leukaemia inhibitory factor
Monocyte chemotactic protein-1
Fibroblasts
Messenger RNA
Mesenchymal stem cells of dental pulp
Myogenic differentiation
Osteoarthritis
Octamer-binding transcription factor 3 and 4
Platelet-derived growth factor
Platelet-derived growth factor-AA
Periodontal ligament stem cells
Rho-associated protein kinase
Stem cells from apical papilla
Stem cells of human exfoliated deciduous teeth
Synthetic Serum Substitute
Trophectoderm
Vascular endothelial growth factor
Transforming growth factors
Sukoyan MA, Vatolin SY, et al. Embryonic stem cells derived from morulae, inner cell mass, and blastocysts of mink: comparisons of their pluripotencies. Embryo Dev. 1993;36(2):148–58
Larijani B, Esfahani EN, Amini P, Nikbin B, Alimoghaddam K, Amiri S, Malekzadeh R, Yazdi NM, Ghodsi M, Dowlati Y, Sahraian MA, Ghavamzadeh A. Stem cell therapy in treatment of different diseases. Acta Medica Iranica. 2012:79–96 https://www.ncbi.nlm.nih.gov/pubmed/22359076 .
Sullivan S, Stacey GN, Akazawa C, et al. Quality guidelines for clinical-grade human induced pluripotent stem cell lines. Regenerative Med. 2018; https://doi.org/10.2217/rme-2018-0095 .
Amps K, Andrews PW, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat. Biotechnol. 2011; 29 (12):1121–44.
Google Scholar
Amit M, Itskovitz-Eldor J. Atlas of human pluripotent stem cells: derivation and culturing. New York: Humana Press; 2012.
Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–46.
CAS PubMed Google Scholar
Kang MI. Transitional CpG methylation between promoters and retroelements of tissue-specific genes during human mesenchymal cell differentiation. J. Cell Biochem. 2007;102:224–39.
Vaes B, Craeye D, Pinxteren J. Quality control during manufacture of a stem cell therapeutic. BioProcess Int. 2012;10:50–5.
Bloushtain-Qimron N. Epigenetic patterns of embryonic and adult stem cells. Cell Cycle. 2009;8:809–17.
Brindley DA. Peak serum: implications of serum supply for cell therapy manufacturing. Regenerative Medicine. 2012;7:809–17.
Solter D, Knowles BB. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci U S A. 1975;72:5099–102.
CAS PubMed PubMed Central Google Scholar
Hoepfl G, Gassmann M, Desbaillets I. Differentiating embryonic stem cells into embryoid bodies. Methods Mole Biol. 2004;254:79–98 https://doi.org/10.1385/1-59259-741-6:079 .
Lim WF, Inoue-Yokoo T, Tan KS, Lai MI, Sugiyama D. Hematopoietic cell differentiation from embryonic and induced pluripotent stem cells. Stem Cell Res Ther. 2013;4(3):71. https://doi.org/10.1186/scrt222 .
Article CAS PubMed PubMed Central Google Scholar
Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27(36):6032–42. https://doi.org/10.1016/j.biomaterials.2006.07.012 .
Article CAS PubMed Google Scholar
Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of the visceral yolk sac, blood islands, and myocardium. J Embryol Exp Morphol. 1985;87:27–45.
Kurosawa HY. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–98.
Heins N, Englund MC, Sjoblom C, Dahl U, Tonning A, Bergh C, Lindahl A, Hanson C, Semb H. Derivation, characterization, and differentiation of human embryonic stem cells. Stem Cells. 2004;22:367–76.
Rosowski KA, Mertz AF, Norcross S, Dufresne ER, Horsley V. Edges of human embryonic stem cell colonies display distinct mechanical properties and differentiation potential. Sci Rep. 2015;5:Article number:14218.
PubMed Google Scholar
Chung Y, Klimanskaya I, Becker S, Li T, Maserati M, Lu SJ, Zdravkovic T, Ilic D, Genbacev O, Fisher S, Krtolica A, Lanza R. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell. 2008;2:113–7.
Zhang X, Stojkovic P, Przyborski S, Cooke M, Armstrong L, Lako M, Stojkovic M. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24:2669–76.
Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc. 2012;7:2029–40.
Ellerström C, Hyllner J, Strehl R. single cell enzymatic dissociation of human embryonic stem cells: a straightforward, robust, and standardized culture method. In: Turksen K, editor. Human embryonic stem cell protocols. Methods in molecular biology: Humana Press; 2009. p. 584.
Heng BC, Liu H, Ge Z, Cao T. Mechanical dissociation of human embryonic stem cell colonies by manual scraping after collagenase treatment is much more detrimental to cellular viability than is trypsinization with gentle pipetting. Biotechnol Appl Biochem. 2010;47(1):33–7.
Ellerstrom C, Strehl R, Noaksson K, Hyllner J, Semb H. Facilitated expansion of human embryonic stem cells by single-cell enzymatic dissociation. Stem Cells. 2007;25:1690–6.
Brimble SN, Zeng X, Weiler DA, Luo Y, Liu Y, Lyons IG, Freed WJ, Robins AJ, Rao MS, Schulz TC. Karyotypic stability, genotyping, differentiation, feeder-free maintenance, and gene expression sampling in three human embryonic stem cell lines deri. Stem Cells Dev. 2004;13:585–97.
Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–6.
Nie Y, Walsh P, Clarke DL, Rowley JA, Fellner T. Scalable passaging of adherent human pluripotent stem cells. 2014. https://doi.org/10.1371/journal.pone.0088012 .
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7.
Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cellsexpress an immunogenic nonhuman sialic acid. Nat. Med. 2005;11:228–32.
Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336(6200):688–90. https://doi.org/10.1038/336688a0 .
Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. 2001;19:971–4. https://doi.org/10.1038/nbt1001-971 .
Article CAS Google Scholar
Weathersbee PS, Pool TB, Ord T. Synthetic serum substitute (SSS): a globulin-enriched protein supplement for human embryo culture. J. Assist Reprod Genet. 1995;12:354–60.
Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–9.
Sommer CA, Mostoslavsky G. Experimental approaches for the generation of induced pluripotent stem cells. Stem Cell Res Ther. 2010;1:26.
PubMed PubMed Central Google Scholar
Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140(12):2457–61 https://doi.org/10.1242/dev.092551 .
Shi D, Lu F, Wei Y, et al. Buffalos ( Bubalus bubalis ) cloned by nuclear transfer of somatic cells. Biol. Reprod. 2007;77:285–91. https://doi.org/10.1095/biolreprod.107.060210 .
Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Development. 1962;10:622–40 http://dev.biologists.org/content/10/4/622 .
CAS Google Scholar
Kain K. The birth of cloning: an interview with John Gurdon. Dis Model Mech. 2009;2(1–2):9–10. https://doi.org/10.1242/dmm.002014 .
Article PubMed Central Google Scholar
Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;24(51(6)):987–1000.
Quinlan AR, Boland MJ, Leibowitz ML, et al. Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell. 2011;9(4):366–73.
Maherali N, Sridharan R, Xie W, Utika LJ, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70.
Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, Yu J, Hebrok M, Hochedlinger K, Costello JF, Song JS, Ramalho-Santos M. Incomplete DNA methylation underlines a transcriptional memory of somatic cells in human IPS cells. Nat Cell Biol. 2011;13:541–9.
Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32 https://doi.org/10.1038/nature07314 .
Hilfiker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396:489–97.
Zhang Wendy, Y., de Almeida Patricia, E., and Wu Joseph, C. Teratoma formation: a tool for monitoring pluripotency in stem cell research. StemBook, ed. The Stem Cell Research Community . June 12, 2012. https://doi.org/10.3824/stembook.1.53.1 .
Narsinh KH, Sun N, Sanchez-Freire V, et al. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121(3):1217–21.
Gertow K, Przyborski S, Loring JF, Auerbach JM, Epifano O, Otonkoski T, Damjanov I, AhrlundRichter L. Isolation of human embryonic stem cell-derived teratomas for the assessment of pluripotency. Curr Protoc Stem Cell Biol . 2007, Chapter 1, Unit 1B 4. 3: 1B.4.1-1B.4.29.
Cooke MJ, Stojkovic M, Przyborski SA. Growth of teratomas derived from human pluripotent stem cells is influenced by the graft site. Stem Cells Dev. 2006;15(2):254–9.
Przyborski SA. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells. 2005;23:1242–50.
Tannenbaum SE, Turetsky TT, Singer O, Aizenman E, Kirshberg S, Ilouz N, Gil Y, Berman-Zaken Y, Perlman TS, Geva N, Levy O, Arbell D, Simon A, Ben-Meir A, Shufaro Y, Laufer N, Reubinoff BE. Derivation of xeno-free and GMP-grade human embryonic stem cells- platforms for future clinical applications. PLoS One. 2012;7:e35325.
Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12:243–52.
Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2007;60(2):199–214. https://doi.org/10.1016/j.addr.2007.08.036 .
Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29.
Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–806.
Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–714.
Efthymiou AG, Chen G, Rao M, Chen G, Boehm M. Self-renewal and cell lineage differentiation strategies in human embryonic stem cells and induced pluripotent stem cells. Expert Opin Biol Ther. 2014;14:1333–44.
Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDRþembryonic-stem-cell-derived population. Nature. 2008;453:524–8.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52. https://doi.org/10.1038/nbt1393 .
Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–21.
Burridge PW, Zambidis ET. Highly efficient directed differentiation of human induced pluripotent stem cells into cardiomyocytes. Methods Mol Biol. 2013;997:149–61.
Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X, Guo Y, Ding M, Deng H. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–39.
Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH. Directing human embryonic stem cell differentiation towards a renal lineage generates a selforganizing kidney. Nat Cell Biol. 2014;16:118–26.
Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, VunjakNovakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84–91.
Kadzik RS, Morrisey EE. Directing lung endoderm differentiation in pluripotent stem cells. Cell Stem Cell. 2012;10:355–61.
Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–97.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9.
Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, Brison DR, Hardingham TE, Kimber SJ. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28:1187–94.
Jun Cai, Ann DeLaForest, Joseph Fisher, Amanda Urick, Thomas Wagner, Kirk Twaroski, Max Cayo, Masato Nagaoka, Stephen A Duncan. Protocol for directed differentiation of human pluripotent stem cells toward a hepatocyte fate. 2012. DOI: https://doi.org/10.3824/stembook.1.52.1 .
Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang FY, Jones S, Shulok J, Rubin LL, Porter JA. Small-molecule modulators of hedgehog signaling: identification and characterization of smoothened agonists and antagonists. J Biol. 2002;1:10.
Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair celllike cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–16.
Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122:3169–79.
Kshitiz PJ, Kim P, Helen W, Engler AJ, Levchenko A, Kim DH. Control of stem cell fate and function by engineering physical microenvironments. Intergr Biol (Camb). 2012;4:1008–18.
Amps K, Andrews PW, Anyfantis G, Armstrong L, Avery S, Baharvand H, Baker J, Baker D, Munoz MB, Beil S, Benvenisty N, Ben-Yosef D, Biancotti JC, Bosman A, Brena RM, Brison D, Caisander G, Camarasa MV, Chen J, ChiaoE CYM, Choo AB, Collins D, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29:1132–44.
Nukaya D, Minami K, Hoshikawa R, Yokoi N, Seino S. Preferential gene expression and epigenetic memory of induced pluripotent stem cells derived from mouse pancreas. Genes Cells. 2015;20:367–81.
Murdoch A, Braude P, Courtney A, Brison D, Hunt C, Lawford-Davies J, Moore H, Stacey G, Sethe S, Procurement Working Group Of National Clinical H, E. S. C. F, National Clinical H, E. S. C. F. The procurement of cells for the derivation of human embryonic stem cell lines for therapeutic use: recommendations for good practice. Stem Cell Rev. 2012;8:91–9.
Hewitson H, Wood V, Kadeva N, Cornwell G, Codognotto S, Stephenson E, Ilic D. Generation of KCL035 research grade human embryonic stem cell line carrying a mutation in HBB gene. Stem Cell Res. 2016;16:210–2.
Daley GQ, Hyun I, Apperley JF, Barker RA, Benvenisty N, Bredenoord AL, Breuer CK, Caulfield T, Cedars MI, Frey-Vasconcells J, Heslop HE, Jin Y, Lee RT, Mccabe C, Munsie M, Murry CE, Piantadosi S, Rao M, Rooke HM, Sipp D, Studer L, Sugarman J, et al. Setting global standards for stem cell research and clinical translation: the 2016 ISSCR guidelines. Stem Cell Rep. 2016;6:787–97.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. https://doi.org/10.1016/j.cell.2006.07.024 .
Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40.
Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, SuberbielleBoissel C, Tartour E, Desnos M, Larghero J. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–7.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–16.
Ilic D, Ogilvie C. Concise review: human embryonic stem cells-what have we done? What are we doing? Where are we going? Stem Cells. 2017;35:17–25.
Rocha V, et al. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12(1):34–4.
Longo UG, Ronga M, Maffulli N. Sports Med Arthrosc 17:112–126. Achilles tendinopathy. Sports Med Arthrosc. 2009;17:112–26.
Tempfer H, Lehner C, Grütz M, Gehwolf R, Traweger A. Biological augmentation for tendon repair: lessons to be learned from development, disease, and tendon stem cell research. In: Gimble J, Marolt D, Oreffo R, Redl H, Wolbank S, editors. Cell engineering and regeneration. Reference Series in Biomedical Engineering. Cham: Springer; 2017.
Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34.
Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177–82.
Li R, Lin Q-X, Liang X-Z, Liu G-B, et al. Stem cell therapy for treating osteonecrosis of the femoral head: from clinical applications to related basic research. Stem Cell Res Therapy. 2018;9:291 https://doi.org/10.1186/s13287-018-1018-7 .
Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49(5):1005–9.
Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–30.
Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplast. 2012;27(5):679–86.
Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, Lehmann S, Lemaitre JM. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011;25:2248–53.
Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–8.
Petkovich DA, Podolskiy DI, Lobanov AV, Lee SG, Miller RA, Gladyshev VN. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 2017;25:954–60 https://doi.org/10.1016/j.cmet.2017.03.016 .
Gerontology, Rejuvenation by cell reprogramming: a new horizon in. Rodolfo G. Goya, Marianne Lehmann, Priscila Chiavellini, Martina Canatelli-Mallat, Claudia B. Hereñú and Oscar A. Brown. Stem Cell Res Therapy . 2018, 9:349. https://doi.org/10.1186/s13287-018-1075-y .
Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T, Vazquez-Ferrer E, Donoso D, Roman JLXJ, Rodriguez-Esteban C, Nuñez G, Nuñez Delicado E, Campistol JM, Guillen I, Guillen P, Izpisua. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016;167:1719–33.
de Lázaro I, Cossu G, Kostarelos K. Transient transcription factor (OSKM) expression is key towards clinical translation of in vivo cell reprogramming. EMBO Mol Med. 2017;9:733–6.
Sun S, Li ZQ, Glencer P, Cai BC, Zhang XM, Yang J, Li XR. Bringing the age-related macular degeneration high-risk allele age-related maculopathy susceptibility 2 into focus with stem cell technology. Stem Cell Res Ther. 2017;8:135 https://doi.org/10.1186/s13287-017-0584-4 .
Liu J. Induced pluripotent stem cell-derived neural stem cells: new hope for stroke? Stem Cell Res Ther. 2013;4:115 https://doi.org/10.1186/scrt326 .
Shahjalal HM, Dayem AA, Lim KM, Jeon TI, Cho SG. Generation of pancreatic β cells for treatment of diabetes: advances and challenges. Stem Cell ResTher. 2018;9:355 https://doi.org/10.1186/s13287-018-1099-3 .
Kroon E, Martinson LA, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26;443–52.
Arora V, Pooja A, Munshi AK. Banking stem cells from human exfoliated deciduous teeth. J Clin Pediatr Dent. 2009;33(4):289–94.
Mao JJ. Stem cells and the future of dental care. New York State Dental J. 2008;74(2):21–4.
Reznick, Jay B. Continuing education: stem cells: emerging medical and dental therapies for the dental Professional. Dentaltown Magazine . 2008, pp. 42–53.
Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26(7):1787–95.
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith A. Dental pulp tissue engineering with stem cells from exfoliated. J Endod. 2008;34(8):962–9.
Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146(4):519–32. https://doi.org/10.1016/j.cell.2011.06.052 .
Sadri-Ardekani H, Atala A. Testicular tissue cryopreservation and spermatogonial stem cell transplantation to restore fertility: from bench to bedside. Stem Cell ResTher. 2014;5:68 https://doi.org/10.1186/scrt457 .
Zhang Q, Xu M, Yao X, Li T, Wang Q, Lai D. Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Res Ther. 2015;6:152 https://doi.org/10.1186/s13287-015-0148-4 .
Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672–82. https://doi.org/10.1038/cr.2009.56 .
Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell ResTher. 2010;1:37 https://doi.org/10.1186/scrt37 .
Wang Q, Matsumoto Y, Shindo T, Miyake K, Shindo A, Kawanishi M, Kawai N, Tamiya T, Nagao S. Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Invest. 2006;53:61–9. https://doi.org/10.2152/jmi.53.61 .
Article PubMed Google Scholar
Moghadam FH, Alaie H, Karbalaie K, Tanhaei S, Nasr Esfahani MH, Baharvand H. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation. 2009;78:59–68. https://doi.org/10.1016/j.diff.2009.06.005 .
Byrne JA. Developing neural stem cell-based treatments for neurodegenerative diseases. Stem Cell ResTher. 2014;5:72. https://doi.org/10.1186/scrt461 .
Awe JP, Lee PC, Ramathal C, Vega-Crespo A, Durruthy-Durruthy J, Cooper A, Karumbayaram S, Lowry WE, Clark AT, Zack JA, Sebastiano V, Kohn DB, Pyle AD, Martin MG, Lipshutz GS, Phelps PE, Pera RA, Byrne JA. Generation and characterization of transgene-free human induced pluripotent stem cells and conversion to putative clinical-grade status. Stem Cell Res Ther. 2013;4:87. https://doi.org/10.1186/scrt246 .
Peng J, Zeng X. The role of induced pluripotent stem cells in regenerative medicine: neurodegenerative diseases. Stem Cell ResTher. 2011;2:32. https://doi.org/10.1186/scrt73 .
Wright BL, Barker RA. Established and emerging therapies for Huntington’s disease. 2007;7(6):579–87 https://www.ncbi.nlm.nih.gov/pubmed/17896994/579-87 .
Lin NH, Gronthos S, Bartold PM. Stem cells and periodontal regeneration. Aust Dent J. 2008;53:108–21.
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55.
Ramseier CA, Abramson ZR, Jin Q, Giannobile WV. Gene therapeutics for periodontal regenerative medicine. Dent Clin North Am. 2006;50:245–63.
Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. OrthodCraniofac Res. 2005;8:191–9.
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein. J Dent Res. 2004;83:590–5.
Hu B, Unda F, Bopp-Kuchler S, Jimenez L, Wang XJ, Haikel Y, et al. Bone marrow cells can give rise to ameloblast-like cells. J Dent Res. 2006;85:416–21.
Liu Y, Liu W, Hu C, Xue Z, Wang G, Ding B, Luo H, Tang L, Kong X, Chen X, Liu N, Ding Y, Jin Y. MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells. 2011;29(11):1804–16. https://doi.org/10.1002/stem.728 .
Raspini G, Wolff J, Helminen M, Raspini G, Raspini M, Sándor GK. Dental stem cells harvested from third molars combined with bioactive glass can induce signs of bone formation in vitro. J Oral Maxillofac Res. 2018;9(1):e2. Published 2018 Mar 31. https://doi.org/10.5037/jomr.2018.9102 .
Christodoulou I, Goulielmaki M, Devetzi M, Panagiotidis M, Koliakos G, Zoumpourlis V. Mesenchymal stem cells in preclinical cancer cytotherapy: a systematic review. Stem Cell Res Ther. 2018;9(1;336). https://doi.org/10.1186/s13287-018-1078-8 .
Bansal R, Jain A. Current overview on dental stem cells applications in regenerative dentistry. J Nat Sci Biol Med. 2015;6(1):29–34. https://doi.org/10.4103/0976-9668.149074 .
Article PubMed PubMed Central Google Scholar
Edgar Ledesma-Martínez, Víctor Manuel Mendoza-Núñez, Edelmiro Santiago-Osorio. Mesenchymal stem cells derived from dental pulp: a review. Stem Cells Int . 2016, 4,709,572, p. doi: https://doi.org/10.1155/2016/4709572 ].
Grzech-Leśniak K. Making use of lasers in periodontal treatment: a new gold standard? Photomed Laser Surg. 2017;35(10):513–4.
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–12. https://doi.org/10.1073/pnas.0937635100 .
Yasui T, Mabuchi Y, Toriumi H, Ebine T, Niibe K, Houlihan DD, Morikawa S, Onizawa K, Kawana H, Akazawa C, Suzuki N, Nakagawa T, Okano H, Matsuzaki Y. Purified human dental pulp stem cells promote osteogenic regeneration. J Dent Res. 2016;95(2):206–14. https://doi.org/10.1177/0022034515610748 .
Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res. 2014;78:16–20. https://doi.org/10.1016/j.neures.2013.10.010 .
d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;12, PMID: 19908196:75–83.
Wang L, Shen H, Zheng W, Tang L, Yang Z, Gao Y, Yang Q, Wang C, Duan Y, Jin Y. Characterization of stem cells from alveolar periodontal ligament. Tissue Eng. Part A. 2011;17(7–8):1015–26. https://doi.org/10.1089/ten.tea.2010.0140 .
Iwata T, Yamato M, Zhang Z, Mukobata S, Washio K, Ando T, Feijen J, Okano T, Ishikawa I. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol. 2010;37(12):1088–99. https://doi.org/10.1111/j.1600-051X.2010.01597.x .
Chen F-M, Gao L-N, Tian B-M, Zhang X-Y, Zhang Y-J, Dong G-Y, Lu H, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. https://doi.org/10.1186/s13287-016-0288-1 .
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol. 2011;56(7):709–21. https://doi.org/10.1016/j.archoralbio.2010.12.008 .
Han C, Yang Z, Zhou W, Jin F, Song Y, Wang Y, Huo N, Chen L, Qian H, Hou R, Duan Y, Jin Y. Periapical follicle stem cell: a promising candidate for cementum/periodontal ligament regeneration and bio-root engineering. Stem Cells Dev. 2010;19(9):1405–15. https://doi.org/10.1089/scd.2009.0277 .
Luan X, Ito Y, Dangaria S, Diekwisch TG. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15(4):595–608. https://doi.org/10.1089/scd.2006.15.595 .
Handa K, Saito M, Tsunoda A, Yamauchi M, Hattori S, Sato S, Toyoda M, Teranaka T, Narayanan AS. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect Tissue Res. 2002;43(2–3):406–8 PMID: 12489190.
Guo W, Chen L, Gong K, Ding B, Duan Y, Jin Y. Heterogeneous dental follicle cells and the regeneration of complex periodontal tissues. Tissue Engineering. Part A. 2012;18(5–6):459–70 https://doi.org/10.1089/ten.tea.2011.0261 .
Bai, Yudi et al. Cementum- and periodontal ligament-like tissue formation by dental follicle cell sheets co-cultured with Hertwig’s epithelial root sheath cells. Bone. 2011, 48, Issue 6, pp. 1417–1426, https://doi.org/10.1016/j.bone.2011.02.016 .
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. 2008, 34, pp. 962–969.
Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate, hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect Tissue Res 2. 2002;43:387–90.
Friedlander LT, Cullinan MP, Love RM. Dental stem cells and their potential role in apexogenesis and apexification. Int Endod J. 2009;42:955–62.
Cai J, Zhang Y, Liu P, Chen S, Wu X, Sun Y, Li A, Huang K, Luo R, Wang L, Liu Y, Zhou T, Wei S, Pan G, Pei D, Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen (Lond). July 30, 2013, 2(1), pp. 6, doi: https://doi.org/10.1186/2045-9769-2-6 .
Craig J. Taylor, Eleanor M. Bolton, and J. Andrew Bradley 2011 Aug 12 and https://doi.org/10.1098/rstb.2011.0030 ], 366(1575): 2312–2322. [doi:. Immunological considerations for embryonic and induced pluripotent stem cell banking,. Philos Trans R SocLond B Biol Sci. 2011, 366(1575), pp. 2312–2322, doi: https://doi.org/10.1098/rstb.2011.0030 .
T.R. Nayak, H. Andersen, V.S. Makam, C. Khaw, S. Bae, X.F. Xu, P.L.R. Ee, J.H. Ahn, B.H. Hong, G. Pastorin, B. Ozyilmaz, ACS Nano, 5 (6) (2011), pp. 4. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells,. ACS Nano. 2011, pp. 4670–4678.
Lee WC, Lim C, Shi H, Tang LAL, Wang Y, Lim CT, Loh KP. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5(9):7334–41.
Kenry LWC, Loh KP, Lim CT. When stem cells meet graphene: opportunities and challenges in regenerative medicine. Biomaterials. 2018;155:236–50.
Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, et al. Transfer of microRNAs by embryonic stem cell microvesicles. 2009. 2009, 4(3), p. https://doi.org/10.1371/journal.pone . 0004722.
Oh, Myeongsik, et al. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 2018, 19(6), p. 1715.
Ramirez MI. et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881–906.
Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–9.
Mateescu B, et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—an ISEV position paper. J. Extracell. Vesicles. 2017;6(1). https://doi.org/10.1080/20013078.2017.1286095 .
Nawaz M, et al. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016:17. Article ID 1073140.
Helfrich, Y.R., Sachs, D.L. and Voorhees, J.J. Overview of skin aging and photoaging. Dermatol. Nurs. 20, pp. 177–183, https://www.ncbi.nlm.nih.gov/pubmed/18649702 .
Julia Tigges, Jean Krutmann, Ellen Fritsche, Judith Haendeler, Heiner Schaal, Jens W. Fischer, Faiza Kalfalah, Hans Reinke, Guido Reifenberger, Kai Stühler, Natascia Ventura, Sabrina Gundermann, Petra Boukamp, Fritz Boege. The hallmarks of fibroblast ageing, mechanisms of ageing and development, 138, 2014, Pages 26–44. 2014, 138, pp. 26–44, ISSN 0047–6374, https://doi.org/10.1016/j.mad.2014.03.004 .
Huh MI, Kim MS, Kim HK, et al. Effect of conditioned media collected from human amniotic fluid-derived stem cells (hAFSCs) on skin regeneration and photo-aging. Tissue Eng Regen Med. 2014;11:171 https://doi.org/10.1007/s13770-014-0412-1 .
Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31.
Liu J, Han G, Liu H, et al. Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling. PLoS One. 2013;8:e62844.
Oh M, et al. Promotive effects of human induced pluripotent stem cell-conditioned medium on the proliferation and migration of dermal fibroblasts. Biotechnol. Bioprocess Eng. 2017;22:561–8.
Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS One. 2008;3:e1886.
Bae J-S, Lee S-H, Kim J-E, Choi J-Y, Park R-W, Park JY, Park H-S, Sohn Y-S, Lee D-S, Lee EB. βig-h3 supports keratinocyte adhesion, migration, and proliferation through α3β1 integrin. Biochem. Biophys. Res. Commun. 2002;294:940–8.
Zhou B-R, Xu Y, Guo S-L, Xu Y, Wang Y, Zhu F, Permatasari F, Wu D, Yin Z-Q, Luo D. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. BioMed Res. Int. 2013;519:126.
Peng Y, Baulier E, Ke Y, Young A, Ahmedli NB, Schwartz SD, et al. Human embryonic stem cells extracellular vesicles and their effects on immortalized human retinal Müller cells. PLoS ONE. 2018, 13(3), p. https://doi.org/10.1371/journal.pone.019400 .
Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003.
Mascetti VL, Pedersen RA. Human-mouse chimerism validates human stem cell pluripotency. Cell Stem Cell. 2016;18:67–72.
Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, Sanchez-Torrijos J, Payá R, Mirabet V, Carbonell-Uberos F, Llop M, Montero JA, Sepúlveda P. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2007;26(3):638–45.
Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14(2):149–56.
Garcia-Olmo D, Garcia-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–23.
de Mendonça CA, Bueno DF, Martins MT, Kerkis I, Kerkis A, Fanganiello RD, Cerruti H, Alonso N, Passos-Bueno MR. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg. 2008;19(1):204–10.
Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, Lee JS, Shi S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14(5):428–34.
Abbas, Diakonov I., Sharpe P. Neural crest origin of dental stem cells. Pan European Federation of the International Association for Dental Research (PEF IADR). 2008, Vols. Seq #96 - Oral Stem Cells.
Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Gaiad TP, Morini AC, Vieira NM, Marina P, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs. J Transl Med. 2008;6:35.
Xianrui Yang, Li Li, Li Xiao, Donghui Zhang. Recycle the dental fairy’s package: overview of dental pulp stem cells. Stem Cell Res Ther . 2018, 9, 1, 1. https://doi.org/10.1186/s13287-018-1094-8 .
Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, Wang S. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 2010;19:1375–83.
Wang J, et al. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly (L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010;6(10):3856–63.
Davies OG, Cooper PR, Shelton RM, Smith AJ, Scheven BA. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J Bone Miner Metab. 2015;33:371–82.
Huang GT-J, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066–73.
Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29(6):532–9.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30.
Nuti N, Corallo C, Chan BMF, Ferrari M, Gerami-Naini B. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev Rep. 2016;12:511–23.
Ferro F, et al. Dental pulp stem cells differentiation reveals new insights in Oct4A dynamics. PloS One. 2012;7(7):e41774.
Conde MCM, Chisini LA, Grazioli G, Francia A, Carvalho RVd, Alcázar JCB, Tarquinio SBC, Demarco FF. Does cryopreservation affect the biological properties of stem cells from dental tissues? A systematic review. Braz Dent J. 2016;1210(6):633-40. https://doi.org/10.1590/0103-6440201600980 .
Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, Menditti D, De Rosa A, Carinci F, Laino G. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208:319–25.
Alge DL, Zhou D, Adams LL, et. al. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4(1):73–81.
Jo Y-Y, Lee H-J, Kook S-Y, Choung H-W, Park J-Y, Chung J-H, Choung Y-H, Kim E-S, Yang H-C, Choung P-H. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–73.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5.
Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005;20:1394–402.
Zainal A, Shahrul H, et al. In vitro chondrogenesis transformation study of mouse dental pulp stem cells. Sci World J. 2012;2012:827149.
Wei X, et al. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33(6):703–8.
Dai J, et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials. 2012;33(31):7699–711.
Vasandan AB, et al. Functional differences in mesenchymal stromal cells from human dental pulp and periodontal ligament. J Cell Mol Med. 2014;18(2):344–54.
Werle SB, et al. Carious deciduous teeth are a potential source for dental pulp stem cells. Clin Oral Investig. 2015;20:75–81.
Nemeth CL, et al. Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG-GelMA-HA hydrogels. Tissue Eng A. 2014;20(21–22):2817–29.
Paino F, Ricci G, De Rosa A, D’Aquino R, Laino L, Pirozzi G, et al. Ecto-mesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. Eur. Cell Mater. 2010;20:295–305.
Ferro F, Spelat R, Baheney CS. Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. In: Kioussi C, editor. Stem cells and tissue repair. Methods in molecular biology (methods and protocols): Humana Press. 2014;1210.
Ishkitiev N, Yaegaki K, Imai T, Tanaka T, Nakahara T, Ishikawa H, Mitev V, Haapasalo M. High-purity hepatic lineage differentiated from dental pulp stem cells in serum-free medium. J Endod. 2012;38:475–80.
Download references
Acknowledgements
Not applicable.
This work is supported by Wrocław Medical University in Poland.
Availability of data and materials
Please contact author for data requests.
Author information
Authors and affiliations.
Department of Experimental Surgery and Biomaterials Research, Wroclaw Medical University, Bujwida 44, Wrocław, 50-345, Poland
Wojciech Zakrzewski, Maria Szymonowicz & Zbigniew Rybak
Department of Conservative Dentistry and Pedodontics, Krakowska 26, Wrocław, 50-425, Poland
Maciej Dobrzyński
You can also search for this author in PubMed Google Scholar
Contributions
WZ is the principal author and was responsible for the first draft of the manuscript. WZ and ZR were responsible for the concept of the review. MS, MD, and ZR were responsible for revising the article and for data acquisition. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Wojciech Zakrzewski .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Zakrzewski, W., Dobrzyński, M., Szymonowicz, M. et al. Stem cells: past, present, and future. Stem Cell Res Ther 10 , 68 (2019). https://doi.org/10.1186/s13287-019-1165-5
Download citation
Published : 26 February 2019
DOI : https://doi.org/10.1186/s13287-019-1165-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Differentiation
- Pluripotency
- Induced pluripotent stem cell (iPSC)
- Stem cell derivation
- Growth media
- Tissue banks
- Tissue transplantation
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Search Menu
- Advance Articles
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- Call for Papers
- Why Publish with Us?
- Advertising & Corporate Services
- Reprints, ePrints, Supplements
- About STEM CELLS
- Editorial Board
- Reviewer Guidelines
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
I ntroduction, acknowledgements, d isclosures, r eferences.
- < Previous

Current State of Human Embryonic Stem Cell Research: An Overview of Cell Lines and Their Use in Experimental Work
- Article contents
- Figures & tables
- Supplementary Data
Anke Guhr, Andreas Kurtz, Kelley Friedgen, Peter Löser, Current State of Human Embryonic Stem Cell Research: An Overview of Cell Lines and Their Use in Experimental Work, Stem Cells , Volume 24, Issue 10, October 2006, Pages 2187–2191, https://doi.org/10.1634/stemcells.2006-0053
- Permissions Icon Permissions
Research in human embryonic stem cells (hESCs) is a rapidly developing scientific field. In this study we collect and evaluate a thorough body of data on the current number of publicly disclosed hESC lines and the extent and impact of scientific work involving the use of these cells. These data contribute to the substantiation of the discussion on the current status of hESC research, provide a basis for the analysis of the status of such research, and uncover further needs in terms of registration, banking, standardization, and tracing.
Since human embryonic stem cell (hESC) lines were first derived in 1998 [ 1 ], these cells have been in high demand as objects of research. The ability of hESCs to reproduce almost limitlessly and to differentiate into many, if not all, cell types of the human body has generated an enormous amount of scientific interest. These unique capabilities provide a means of exploring many promising lines of research, which are likely to reveal a deeper understanding of human cellular biology and which may lead to potential cures for many diseases [ 2 , 3 ]. On the other hand, considerable controversy has arisen regarding this type of research, because derivation of hESCs requires destruction of early human embryos [ 4 ]. Consequently, national legislation regulating research involving hESCs varies widely across many countries [ 5 ]. The present paper offers information that can provide needed substance to the debate on hESC research by presenting comprehensive data on the number of currently existing hESC lines and on the actual extent of experimental work undertaken with these cells and published as of December 2005 worldwide, based on the exploration of verifiable public sources.
Figure 1A provides an overview of the number of hESC lines currently derived and in existence according to published data available from various sources. In total, information on 414 human ES cell lines was available. According to data published as of January 1, 2006, hESC lines have been established in at least 20 countries. Although the number of existing hESC lines is quite impressive, only limited data on characterization of these cell lines are publicly available. Currently it is not clear whether all lines are indeed pluripotent hESC lines. According to our database searches, derivation and at least partial characterization of only 43.2% (179, of which 171 are in English‐language journals) of these cell lines have been published in peer‐reviewed journals, and the use of an additional 6% ( n = 25) has been published without detailed characterization data in peer‐reviewed journals. Consequently, 49.2% of all cell lines have been published so far in scientific journals ( Fig. 1B ). Publication in a peer‐reviewed journal provides some information about the hESC‐like characteristics, but it does not provide absolute certainty on their quality. Especially in cases where multiple cell lines are described in a single study, characterization data are only shown for selected cell lines. Seventy‐one different hESC lines are listed in the NIH Stem Cell Registry. Of these, only 22 are currently available to researchers. Of the remaining 49 hESC lines, only three have been characterized with respect to their stem cell nature and have been published in a peer‐reviewed journal; two additional cell lines were published but have been withdrawn by the providers. Although the vast majority of hESC lines were derived using classic cultivation in the presence of feeder cells, 32 were derived under conditions free of animal cells and media containing animal‐derived serum. However, it has to be taken into account that this is not equivalent to “animal‐free” or “xerofree” conditions, as stated by some authors, since serum replacement used in some of these derivations may also contain animal‐derived compounds. Twenty‐seven hESC lines harbor defined genetic defects characteristic for distinct inheritable genetic disorders, and eight cell lines have been shown to have an abnormal karyotype. In addition to the 414 cell lines, 14 hESC lines have been clonally derived from existing hESC lines, some of which are listed in the NIH registry. Almost no information is publicly available on 106 of the 144 cell lines provided by the Reproductive Genetics Institute in Chicago and available from Stemride International Ltd. Remarkably, there were four times more new hESC lines published in peer‐reviewed journals in 2005 ( n = 88) than obtainable (available) from the NIH ( n = 22). A detailed list of currently existing and publicly known hESC lines (including their respective references) is provided as supplemental online Data.

Derived hESC lines (as of January 1, 2006). Data were extracted from the NIH registry, from work published in scientific journals listed in the PubMed database, and from information either available online, presented at conferences, or provided in press releases. To date, only a portion of these cell lines have been published in scientific journals. No detailed information on the geographic origin of the 144 cell lines derived at the Reproductive Genetics Institute and distributed by STEMRIDE International Ltd. is publicly available from sources used in this study (marked as unknown). hESC lines derived at ES Cell International were assigned to Australia. Detailed information on the hESC lines is shown in supplemental online table. (A): Total number of cell lines sorted by country of origin. (B): Cell lines described or used in work published in peer‐reviewed journals. Abbreviation: hESC, human embryonic stem cell.
We next wished to determine the number of scientific publications reporting on derivation and characterization of hESC lines or their experimental use. To cover all relevant papers on experimental use of hESCs, we performed searches of the PubMed database with no restriction to a publication category (such as “journal article”) introduced into the search criteria. Although such restrictions do not reliably exclude papers without relevance for our study, this practice often results in the exclusion of relevant publications. Therefore, the broader search criteria reported in the online supporting material were applied. The searches resulted in more than 2,500 hits over the period spanning from January 1, 1998 to December 31, 2005. These hits were manually evaluated to exclude those articles in which hESCs were not used experimentally (e.g., reviews, tutorials, news, comments, work on mouse ES cells, etc.). Practical work using human embryonic carcinoma or embryonic germ cells but not hESCs was omitted, as was work in which hESC‐derived materials (e.g., RNA or cDNA) but not hESCs were used. We found a total of 315 research papers describing derivation and/or experimental use of hESC lines that had been published (including online publication ahead of print) through December 31, 2005. Most of these research papers came from groups in the United States, followed by Israel, the U.K., and South Korea ( Fig. 2 ). Interestingly, the number of scientific reviews and papers on ethical or legal aspects of hESC research by far exceeded the number of original publications describing experimental work conducted with these cells, suggesting that discussion about hESC research has outpaced actual research activities and continues to do so. According to the list of impact factors of 2004 (Institute for Scientific Information, Thomson Scientific, Philadelphia, http://www.thomson.com/scientific/scientific.jsp ), the average impact factor for journals that have been publishing experimental work with hESCs was 6.03, indicating that an outstanding interest exists for this kind of research. Approximately 28% of published research focused on differentiation of hESCs into specialized cell or tissue types, with a noticeable emphasis on neural, heart, and blood cells. A comparable number of papers (approximately 27.5%) dealt with the molecular characterization of hESCs, including signal transduction, gene expression patterns, or early differentiation. Another 33% of papers described derivations of hESC lines or the establishment of improved culture conditions. In Table 1 , the top 20 publications in the field (cited most frequently since the establishment of the first stem cell line in 1998) are listed. Table 2 gives an overview of the leading journals in the field of experimental hESC research with respect to the number of published research papers. The complete list of publications detected by our method is available as online supporting material. It is worth noting that according to information provided in the papers, at least 29% ( n = 92) of all studies and 54% ( n = 68) of the U.S. studies were conducted with at least partial financial support from the NIH.

Overview of published work reporting on experimental use of hESCs. Searches of the PubMed database were performed as described in the supplemental online methods, and results were evaluated manually to exclude false‐positive hits. (A): Number of papers extracted from the PubMed database by the described search string and the number of research papers reporting on derivation and/or experimental use of hESC lines. Data were sorted by publication year. Advance online publications available as of December 31, 2005, have been included. (B): Number of publications describing derivation and/or experimental use of hESC lines sorted by location of corresponding authors. Abbreviation: hESC, human embryonic stem cell.
Most frequently cited papers reporting on derivation of or experimental work with hESCs and published from 1998 to 2004
Top journals with respect to publication of experimental work involving human embryonic stem cell (hESC) lines
We have also determined the frequency at which specific hESC lines have been used in published research. Information on the hESC line used was available from 91.4% of studies. In total, 681 uses of hESC lines have been reported in these studies. Notably, 210 cell lines (50.7%) have never been described or used in published experimental work so far, and 150 cell lines (36.2%) were only described or used in a single scientific report. Of the remaining 54 hESC lines (13.1%), only 15 (3.6%) have been used in more than 10 studies published in the period investigated ( Table 3 ). Among these, the cell line H9 and its clonally derived derivates were used most frequently in work (16.1% of all uses), followed by cell line H1 and its clonal derivate H1.1 (13.6%). Our data show that NIH‐approved cell lines have been used in the majority of studies published through 2005. This might be due to the good knowledge of these cell lines, to the high proportion of research funding by NIH in the field, or to the fact that use of NIH‐approved cell lines has been in accordance with regulation in other countries, such as Germany. A considerable portion of work published in 2005 (161 uses/derivations; 43.5% of all published hESC uses in 2005) reported on derivation of novel hESC lines or use of hESC lines not registered at the NIH (supplemental online Fig. 1 ). However, in most cases, the non‐NIH‐approved cell lines were used only in a single study (97 of 161 uses/derivations; 60.2% of reports on use/derivation of novel cell lines in 2005). This clearly suggests that an increasing number of researchers are establishing and using their own hESC lines. Although there is a need for new and easily accessible hESC lines derived under animal‐free conditions, increased use of a multitude of hESC lines in research might diminish the comparability of results. Availability of few well‐characterized and easily accessible hESC lines derived under animal‐free conditions and provided by specialized institutions, such as stem cell banks, might be an alternative for research.
Most frequently used human embryonic stem cell (hESC) lines
While this paper was under review, Owen‐Smith and McCormick [ 6 ] published a study of experimental work performed by the end of 2004 and using hESCs. As a basis for their study, they used those reports that cited the first derivation of hESCs by Thomson et al. [ 1 ] 1998. Although some of their data is in good agreement with our findings, there are also some notable differences. For example, we found evidence for 91 ES cell lines published by the end of 2004 in peer‐reviewed papers, whereas Owen‐Smith and McCormick [ 6 ] reported 70 hESC lines. This might be due to differences in the initial search method and to the different databases used for the studies. For example, 18.5% (58) of the papers detected by our search method did not cite the work of Thomson et al. [ 1 ]. In addition, whereas Owen‐Smith and McCormick [ 6 ] conclude that U.S. research started to lag behind international hESC research in the last years, our data revealed that 43% ( n = 40) of hESC papers published in 2004 came from U.S. groups. Similarly, in 2005, 38% ( n = 62) of hESC papers were published by researchers located in the United States. These divergent findings are probably due to the fact that international collaborations of U.S. groups have been marked as “collaborative research” by Owen‐Smith and McCormick [ 6 ]. Because there is an ongoing globalization of hESC research, we considered the localization of the corresponding author's laboratory as more appropriate for assigning a study on hESCs to a country.
In summary, we provide data on the current state of experimental research involving human embryonic stem cells. Although the completeness of this review is contingent upon the limitations of the search methodology, incomplete information in some published articles, and the lack of accepted registry and tracing mechanisms for hESC lines, we provide evidence that hESC research is a field that has been developing rapidly, especially within the last 3 years. Although the number of published hESC lines has markedly increased within the last 3 years, most published research has been performed with cell lines derived before the end of 2001. However, the growing number of a variety of well‐characterized new hESC lines partially harboring defined genetic defects or more suitable for future clinical applications all but guarantees that an increasing number of hESC research laboratories will begin using these lines in the near future.
A.G. and A.K. contributed equally to this work.
The authors indicate no potential conflicts of interest.
Thomson JA , Itskovitz‐Eldor J , Shapiro SS et al. Embryonic stem cell lines derived from human blastocysts . Science 1998 ; 282 : 1145 – 1147 .
Google Scholar
Pera MF , Trounson AO. Human embryonic stem cells: prospects for development . Development 2004 ; 131 22 : 5515 – 5525 .
Liew CG , Moore H , Ruban L et al. Human embryonic stem cells: Possibilities for human cell transplantation . Ann Med 2005 ; 37 : 521 – 532 .
de Wert G , Mummery C. Human embryonic stem cells: research, ethics and policy . Hum Reprod 2003 ; 18 : 672 – 682 .
Knowles LP. A regulatory patchwork–human ES cell research oversight . Nat Biotechnol 2004 ; 22 : 157 – 163 .
Owen‐Smith J , McCormick J. An international gap in human ES cell research . Nat Biotechnol 2006 ; 24 : 391 – 392 .
Supplementary data
Email alerts, citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations

- Online ISSN 1549-4918
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

- Table of Contents
- Random Entry
- Chronological
- Editorial Information
- About the SEP
- Editorial Board
- How to Cite the SEP
- Special Characters
- Advanced Tools
- Support the SEP
- PDFs for SEP Friends
- Make a Donation
- SEPIA for Libraries
- Entry Contents
Bibliography
Academic tools.
- Friends PDF Preview
- Author and Citation Info
- Back to Top
Ethics of Stem Cell Research
Human embryonic stem cell (HESC) research offers much hope for alleviating the human suffering brought on by the ravages of disease and injury. HESCs are characterized by their capacity for self-renewal and their ability to differentiate into all types of cells of the body. The main goal of HESC research is to identify the mechanisms that govern cell differentiation and to turn HESCs into specific cell types that can be used for treating debilitating and life-threatening diseases and injuries.
Despite the tremendous therapeutic promise of HESC research, the research has met with heated opposition because the harvesting of HESCs involves the destruction of the human embryo. HESCs are derived in vitro around the fifth day of the embryo’s development (Thomson et al . 1998). A typical day-5 human embryo consists of 200–250 cells, most of which comprise the trophoblast, which is the outermost layer of the blastocyst. HESCs are harvested from the inner cell mass of the blastocyst, which consists of 30–34 cells. The derivation of HESC cultures requires the removal of the trophoblast. This process of disaggregating the blastocyst’s cells eliminates its potential for further development. Opponents of HESC research argue that the research is morally impermissible because it involves the unjust killing of innocent human beings.
Scientists recently succeeded in converting adult human skin cells into cells that appear to have the properties of HESCs by activating four genes in the adult cells (Takahashi et al . 2007; Yu et al . 2007). The reprogrammed cells—“induced pluripotent stem cells” (iPSCs)—could ultimately eliminate the need for HESCs. However, at present, the consensus in the scientific community is that both HESC and iPSC research should be pursued, as we do not yet know whether iPSCs have the same potential as HESCs or whether it is safe to transplant them into humans. Thus, the controversies around HESC research will continue, at least in the near-term.
While the principal source of the controversy surrounding HESC research lies in competing views about the value of human embryonic life, the scope of ethical issues in HESC research is broader than the question of the ethics of destroying human embryos. It also encompasses questions about, among other things, whether researchers who use but do not derive HESCs are complicit in the destruction of embryos, whether there is a moral distinction between creating embryos for research purposes and creating them for reproductive ends, the permissibility of cloning human embryos to harvest HESCs, and the ethics of creating human/non-human chimeras. This entry provides an overview of all but the last two issues just listed; cloning and human-non-human chimeras are addressed in separate entries.
1.1 When does a human being begin to exist?
1.2 the moral status of human embryos, 1.3 the case of “doomed embryos”, 2. the ethics of using human embryonic stem cells in research, 3. the ethics of creating embryos for stem cell research and therapy, 4. stem cell-derived gametes, 5. stem cell-derived organoids, gastruloids, and synthetic embryos, cited resources, other resources, related entries, 1. the ethics of destroying human embryos for research.
The potential therapeutic benefits of HESC research provide strong grounds in favor of the research. If looked at from a strictly consequentialist perspective, it’s almost certainly the case that the potential health benefits from the research outweigh the loss of embryos involved and whatever suffering results from that loss for persons who want to protect embryos. However, most of those who oppose the research argue that the constraints against killing innocent persons to promote social utility apply to human embryos. Thus, as long as we accept non-consequentialist constraints on killing persons, those supporting HESC research must respond to the claim that those constraints apply to human embryos.
In its most basic form, the central argument supporting the claim that it is unethical to destroy human embryos goes as follows: It is morally impermissible to intentionally kill innocent human beings; the human embryo is an innocent human being; therefore it is morally impermissible to intentionally kill the human embryo. It is worth noting that this argument, if sound, would not suffice to show that all or even most HESC research is impermissible, since most investigators engaged in HESC research do not participate in the derivation of HESCs but instead use cell lines that researchers who performed the derivation have made available. To show that researchers who use but do not derive HESCs participate in an immoral activity, one would further need to establish their complicity in the destruction of embryos. We will consider this issue in section 2. But for the moment, let us address the argument that it is unethical to destroy human embryos.
A premise of the argument against killing embryos is that human embryos are human beings. The issue of when a human being begins to exist is, however, a contested one. The standard view of those who oppose HESC research is that a human being begins to exist with the emergence of the one-cell zygote at fertilization. At this stage, human embryos are said to be “whole living member[s] of the species homo sapiens … [which] possess the epigenetic primordia for self-directed growth into adulthood, with their determinateness and identity fully intact” (George & Gomez-Lobo 2002, 258). This view is sometimes challenged on the grounds that monozygotic twinning is possible until around days 14–15 of an embryo’s development (Smith & Brogaard 2003). An individual who is an identical twin cannot be numerically identical to the one-cell zygote, since both twins bear the same relationship to the zygote, and numerical identity must satisfy transitivity. That is, if the zygote, A, divides into two genetically identical cell groups that give rise to identical twins B and C, B and C cannot be the same individual as A because they are not numerically identical with each other. This shows that not all persons can correctly assert that they began their life as a zygote. However, it does not follow that the zygote is not a human being, or that it has not individuated. This would follow only if one held that a condition of an entity’s status as an individual human being is that it be impossible for it to cease to exist by dividing into two or more entities. But this seems implausible. Consider cases in which we imagine adult humans undergoing fission (for example, along the lines of Parfit’s thought experiments, where each half of the brain is implanted into a different body) (Parfit 1984). The prospect of our going out of existence through fission does not pose a threat to our current status as distinct human persons. Likewise, one might argue, the fact that a zygote may divide does not create problems for the view that the zygote is a distinct human being.
There are, however, other grounds on which some have sought to reject that the early human embryo is a human being. According to one view, the cells that comprise the early embryo are a bundle of homogeneous cells that exist in the same membrane but do not form a human organism because the cells do not function in a coordinated way to regulate and preserve a single life (Smith & Brogaard 2003, McMahan 2002). While each of the cells is alive, they only become parts of a human organism when there is substantial cell differentiation and coordination, which occurs around day-16 after fertilization. Thus, on this account, disaggregating the cells of the 5-day embryo to derive HESCs does not entail the destruction of a human being.
This account is subject to dispute on empirical grounds. That there is some intercellular coordination in the zygote is revealed by the fact that the development of the early embryo requires that some cells become part of the trophoblast while others become part of the inner cell mass. Without some coordination between the cells, there would be nothing to prevent all cells from differentiating in the same direction (Damschen, Gomez-Lobo and Schonecker 2006). The question remains, though, whether this degree of cellular interaction is sufficient to render the early human embryo a human being. Just how much intercellular coordination must exist for a group of cells to constitute a human organism cannot be resolved by scientific facts about the embryo, but is instead an open metaphysical question (McMahan 2007a).
Suppose that the 5-day human embryo is a human being. On the standard argument against HESC research, membership in the species Homo sapiens confers on the embryo a right not to be killed. This view is grounded in the assumption that human beings have the same moral status (at least with respect to possessing this right) at all stages of their lives.
Some accept that the human embryo is a human being but argue that the human embryo does not have the moral status requisite for a right to life. There is reason to think that species membership is not the property that determines a being’s moral status. We have all been presented with the relevant thought experiments, courtesy of Disney, Orwell, Kafka, and countless science fiction works. The results seem clear: we regard mice, pigs, insects, aliens, and so on, as having the moral status of persons in those possible worlds in which they exhibit the psychological and cognitive traits that we normally associate with mature human beings. This suggests that it is some higher-order mental capacity (or capacities) that grounds the right to life. While there is no consensus about the capacities that are necessary for the right to life, some of the capacities that have been proposed include reasoning, self-awareness, and agency (Kuhse & Singer 1992, Tooley 1983, Warren 1973).
The main difficulty for those who appeal to such mental capacities as the touchstone for the right to life is that early human infants lack these capacities, and do so to a greater degree than many of the nonhuman animals that most deem it acceptable to kill (Marquis 2002). This presents a challenge for those who hold that the non-consequentialist constraints on killing human children and adults apply to early human infants. Some reject that these constraints apply to infants, and allow that there may be circumstances where it is permissible to sacrifice infants for the greater good (McMahan 2007b). Others argue that, while infants do not have the intrinsic properties that ground a right to life, we should nonetheless treat them as if they have a right to life in order to promote love and concern towards them, as these attitudes have good consequences for the persons they will become (Benn 1973, Strong 1997).
Some claim that we can reconcile the ascription of a right to life to all humans with the view that higher order mental capacities ground the right to life by distinguishing between two senses of mental capacities: “immediately exercisable” capacities and “basic natural” capacities. (George and Gomez-Lobo 2002, 260). According to this view, an individual’s immediately exercisable capacity for higher mental functions is the actualization of natural capacities for higher mental functions that exist at the embryonic stage of life. Human embryos have a “rational nature,” but that nature is not fully realized until individuals are able to exercise their capacity to reason. The difference between these types of capacity is said to be a difference between degrees of development along a continuum. There is merely a quantitative difference between the mental capacities of embryos, fetuses, infants, children, and adults (as well as among infants, children, and adults). And this difference, so the argument runs, cannot justify treating some of these individuals with moral respect while denying it to others.
Given that a human embryo cannot reason at all, the claim that it has a rational nature has struck some as tantamount to asserting that it has the potential to become an individual that can engage in reasoning (Sagan & Singer 2007). But an entity’s having this potential does not logically entail that it has the same status as beings that have realized some or all of their potential (Feinberg 1986). Moreover, with the advent of cloning technologies, the range of entities that we can now identify as potential persons arguably creates problems for those who place great moral weight on the embryo’s potential. A single somatic cell or HESC can in principle (though not yet in practice) develop into a mature human being under the right conditions—that is, where the cell’s nucleus is transferred into an enucleated egg, the new egg is electrically stimulated to create an embryo, and the embryo is transferred to a woman’s uterus and brought to term. If the basis for protecting embryos is that they have the potential to become reasoning beings, then, some argue, we have reason to ascribe a high moral status to the trillions of cells that share this potential and to assist as many of these cells as we reasonably can to realize their potential (Sagan & Singer 2007, Savulescu 1999). Because this is a stance that we can expect nearly everyone to reject, it’s not clear that opponents of HESC research can effectively ground their position in the human embryo’s potential.
One response to this line of argument has been to claim that embryos possess a kind of potential that somatic cells and HESCs lack. An embryo has potential in the sense of having an “active disposition” and “intrinsic power” to develop into a mature human being (Lee & George 2006). An embryo can mature on its own in the absence of interference with its development. A somatic cell, on the other hand, does not have the inherent capacity or disposition to grow into a mature human being. However, some question whether this distinction is viable, especially in the HESC research context. While it is true that somatic cells can realize their potential only with the assistance of outside interventions, an embryo’s development also requires that numerous conditions external to it are satisfied. In the case of embryos that are naturally conceived, they must implant, receive nourishment, and avoid exposure to dangerous substances in utero . In the case of spare embryos created through in vitro fertilization—which are presently the source of HESCs for research—the embryos must be thawed and transferred to a willing woman’s uterus. Given the role that external factors—including technological interventions—play in an embryo’s realizing its potential, one can question whether there is a morally relevant distinction between an embryo’s and somatic cell’s potential and thus raise doubts about potentiality as a foundation for the right to life (Devolder & Harris 2007).
Some grant that human embryos lack the properties essential to a right to life, but hold that they possess an intrinsic value that calls for a measure of respect and places at least some moral constraints on their use: “The life of a single human organism commands respect and protection … no matter in what form or shape, because of the complex creative investment it represents and because of our wonder at the divine or evolutionary processes that produce new lives from old ones.” (Dworkin l992, 84). There are, however, divergent views about the level of respect embryos command and what limits exist on their use. Some opponents of HESC research hold that the treatment of human embryos as mere research tools always fails to manifest proper respect for them. Other opponents take a less absolutist view. Some, for example, deem embryos less valuable than more mature human beings but argue that the benefits of HESC research are too speculative to warrant the destruction of embryos, and that the benefits might, in any case, be achieved through the use of noncontroversial sources of stem cells (e.g., adult stem cells) (Holm 2003).
Many, if not most, who support the use of human embryos for HESC research would likely agree with opponents of the research that there are some circumstances where the use of human embryos would display a lack of appropriate respect for human life, for example, were they to be offered for consumption to contestants in a reality TV competition or destroyed for the production of cosmetics. But proponents of the research hold that the value of human embryos is not great enough to constrain the pursuit of research that may yield significant therapeutic benefits. Supporters of the research also frequently question whether most opponents of the research are consistent in their ascription of a high value to human embryos, as opponents generally display little concern about the fact that many embryos created for fertility treatment are discarded.
When spare embryos exist after fertility treatment, the individuals for whom the embryos were created typically have the option of storing for them for future reproductive use, donating them to other infertile couples, donating them to research, or discarding them. Some argue that as long as the decision to donate embryos for research is made after the decision to discard them, it is morally permissible to use them in HESC research even if we assume that they have the moral status of persons. The claim takes two different forms. One is that it is morally permissible to kill an individual who is about to be killed by someone else where killing that individual will help others (Curzer, H. 2004). The other is that researchers who derive HESCs from embryos that were slated for destruction do not cause their death. Instead, the decision to discard the embryos causes their death; research just causes the manner of their death (Green 2002).
Both versions of the argument presume that the decision to discard spare embryos prior to the decision to donate them to research entails that donated embryos are doomed to destruction when researchers receive them. There are two arguments one might marshal against this presumption. First, one who wants to donate embryos to research might first elect to discard them only because doing so is a precondition for donating them. There could be cases in which one who chooses the discard option would have donated the embryos to other couples were the research donation option not available. The fact that a decision to discard embryos is made prior to the decision to donate the embryos thus does not establish that the embryos were doomed to destruction before the decision to donate them to research was made. Second, a researcher who receives embryos could choose to rescue them, whether by continuing to store them or by donating them to infertile couples. While this would violate the law, the fact that it is within a researcher’s power to prevent the destruction of the embryos he or she receives poses problems for the claim that the decision to discard the embryos dooms them or causes their destruction.
Assume for the sake of argument that it is morally impermissible to destroy human embryos. It does not follow that all research with HESCs is impermissible, as it is sometimes permissible to benefit from moral wrongs. For example, there is nothing objectionable about transplant surgeons and patients benefiting from the organs of murder and drunken driving victims (Robertson 1988). If there are conditions under which a researcher may use HESCs without being complicit in the destruction of embryos, then those who oppose the destruction of embryos could support research with HESCs under certain circumstances.
Researchers using HESCs are clearly implicated in the destruction of embryos where they derive the cells themselves or enlist others to derive the cells. However, most investigators who conduct research with HESCs obtain them from an existing pool of cell lines and play no role in their derivation. One view is that we cannot assign causal or moral responsibility to investigators for the destruction of embryos from which the HESCs they use are derived where their “research plans had no effect on whether the original immoral derivation occurred.” (Robertson 1999). This view requires qualification. There may be cases in which HESCs are derived for the express purpose of making them widely available to HESC investigators. In such instances, it may be that no individual researcher’s plans motivated the derivation of the cells. Nonetheless, one might argue that investigators who use these cells are complicit in the destruction of the embryos from which the cells were derived because they are participants in a research enterprise that creates a demand for HESCs. For these investigators to avoid the charge of complicity in the destruction of embryos, it must be the case that the researchers who derived the HESCs would have performed the derivation in the absence of external demand for the cells (Siegel 2004).
The issue about complicity goes beyond the question of an HESC researcher’s role in the destruction of the particular human embryo(s) from which the cells he or she uses are derived. There is a further concern that research with existing HESCs will result in the future destruction of embryos: “[I]f this research leads to possible treatments, private investment in such efforts will increase greatly and the demand for many thousands of cell lines with different genetic profiles will be difficult to resist.” (U.S. Conference of Catholic Bishops 2001). This objection faces two difficulties. First, it appears to be too sweeping: research with adult stem cells and non-human animal stem cells, as well as general research in genetics, embryology, and cell biology could be implicated, since all of this research might advance our understanding of HESCs and result in increased demand for them. Yet, no one, including those who oppose HESC research, argues that we should not support these areas of research. Second, the claim about future demand for HESCs is speculative. Indeed, current HESC research could ultimately reduce or eliminate demand for the cells by providing insights into cell biology that enable the use of alternative sources of cells (Siegel 2004).
While it might thus be possible for a researcher to use HESCs without being morally responsible for the destruction of human embryos, that does not end the inquiry into complicity. Some argue that agents can be complicit in wrongful acts for which they are not morally responsible. One such form of complicity arises from an association with wrongdoing that symbolizes acquiescence in the wrongdoing (Burtchaell 1989). The failure to take appropriate measures to distance oneself from moral wrongs may give rise to “metaphysical guilt,” which produces a moral taint and for which shame is the appropriate response (May 1992). The following question thus arises: Assuming it is morally wrongful to destroy human embryos, are HESC researchers who are not morally responsible for the destruction of embryos complicit in the sense of symbolically aligning themselves with a wrongful act?
One response is that a researcher who benefits from the destruction of embryos need not sanction the act any more than the transplant surgeon who uses the organs of a murder or drunken driving victim sanctions the homicidal act (Curzer 2004). But this response is unlikely to be satisfactory to opponents of HESC research. There is arguably an important difference between the transplant case and HESC research insofar as the moral wrong associated with the latter (a) systematically devalues a particular class of human beings and (b) is largely socially accepted and legally permitted. Opponents of HESC research might suggest that the HESC research case is more analogous to the following kind of case: Imagine a society in which the practice of killing members of a particular racial or ethnic group is legally permitted and generally accepted. Suppose that biological materials obtained from these individuals subsequent to their deaths are made available for research uses. Could researchers use these materials while appropriately distancing themselves from the wrongful practice? Arguably, they could not. There is a heightened need to protest moral wrongs where those wrongs are socially and legally accepted. Attempts to benefit from the moral wrong in these circumstances may be incompatible with mounting a proper protest (Siegel 2003).
But even if we assume that HESC researchers cannot avoid the taint of metaphysical guilt, it is not clear that researchers who bear no moral responsibility for the destruction of embryos are morally obligated not to use HESCs. One might argue that there is a prima facie duty to avoid moral taint, but that this duty may be overridden for the sake of a noble cause.
Most HESCs are derived from embryos that were created for infertility treatment but that were in excess of what the infertile individual(s) ultimately needed to achieve a pregnancy. The HESCs derived from these leftover embryos offer investigators a powerful tool for understanding the mechanisms controlling cell differentiation. However, there are scientific and therapeutic reasons not to rely entirely on leftover embryos. From a research standpoint, creating embryos through cloning technologies with cells that are known to have particular genetic mutations would allow researchers to study the underpinnings of genetic diseases in vitro . From a therapeutic standpoint, the HESCs obtained from leftover IVF embryos are not genetically diverse enough to address the problem of immune rejection by recipients of stem cell transplants. (Induced pluripotent stem cells may ultimately prove sufficient for these research and therapeutic ends, since the cells can (a) be selected for specific genetic mutations and (b) provide an exact genetic match for stem cell recipients.) At present, the best way to address the therapeutic problem is through the creation of a public stem cell bank that represents a genetically diverse pool of stem cell lines (Faden et al . 2003, Lott & Savulescu 2007). This kind of stem cell bank would require the creation of embryos from gamete donors who share the same HLA-types (i.e., similar versions of the genes that mediate immune recognition and rejection).
Each of these enterprises has its own set of ethical issues. In the case of research cloning, some raise concerns, for example, that the perfection of cloning techniques for research purposes will enable the pursuit of reproductive cloning, and that efforts to obtain the thousands of eggs required for the production of cloned embryos will result in the exploitation of women who provide the eggs (President’s Council on Bioethics 2002, Norsigian 2005). With respect to stem cell banks, it is not practically possible to create a bank of HESCs that will provide a close immunological match for all recipients. This gives rise to the challenge of determining who will have biological access to stem cell therapies. We might construct the bank so that it provides matches for the greatest number of people in the population, gives everyone an equal chance of finding a match, or ensures that all ancestral/ethnic groups are fairly represented in the bank (Faden et al . 2003, Bok, Schill, & Faden 2004, Greene 2006).
There are, however, more general challenges to the creation of embryos for research and therapeutic purposes. Some argue that the creation of embryos for non-reproductive ends is morally problematic, regardless of whether they are created through cloning or in vitro fertilization. There are two related arguments that have been advanced to morally distinguish the creation of embryos for reproductive purposes from the creation of embryos for research and therapeutic purposes. First, each embryo created for procreative purposes is originally viewed as a potential child in the sense that each is a candidate for implantation and development into a mature human. In contrast, embryos created for research or therapies are viewed as mere tools from the outset (Annas, Caplan & Elias 1996, President’s Council on Bioethics 2002). Second, while embryos created for research and therapy are produced with the intent to destroy them, the destruction of embryos created for reproduction is a foreseeable but unintended consequence of their creation (FitzPatrick 2003).
One response to the first argument has been to suggest that we could, under certain conditions, view all research embryos as potential children in the relevant sense. If all research embryos were included in a lottery in which some of them were donated to individuals for reproductive purposes, all research embryos would have a chance at developing into mature humans (Devander 2005). Since those who oppose creating embryos for research would likely maintain their opposition in the research embryo lottery case, it is arguably irrelevant whether embryos are viewed as potential children when they are created. Of course, research embryos in the lottery case would be viewed as both potential children and potential research tools. But this is also true in the case of embryos created for reproductive purposes where patients are open to donating spare embryos to research.
As to the second argument, the distinction between intending and merely foreseeing harms is one to which many people attach moral significance, and it is central to the Doctrine of Double Effect. But even if one holds that this is a morally significant distinction, it is not clear that it is felicitous to characterize the destruction of spare embryos as an unintended but foreseeable side-effect of creating embryos for fertility treatment. Fertility clinics do not merely foresee that some embryos will be destroyed, as they choose to offer patients the option of discarding embryos and carry out the disposal of embryos when patients request it. Patients who elect that their embryos be discarded also do not merely foresee the embryos’ destruction; their election of that option manifests their intention that the embryos be destroyed. There is thus reason to doubt that there is a moral distinction between creating embryos for research and creating them for reproductive purposes, at least given current fertility clinic practices.
Recent scientific work suggests it is possible to derive gametes from human pluripotent stem cells. Researchers have generated sperm and eggs from mouse ESCs and iPSCs and have used these stem cell-derived gametes to produce offspring (Hayashi 2011; Hayashi 2012). While it may take several years before researchers succeed in deriving gametes from human stem cells, the research holds much promise for basic science and clinical application. For example, the research could provide important insights into the fundamental processes of gamete biology, assist in the understanding of genetic disorders, and provide otherwise infertile individuals a means of creating genetically related children. The ability to derive gametes from human stem cells could also reduce or eliminate the need for egg donors and thus help overcome concerns about exploitation of donors and the risks involved in egg retrieval. Nonetheless, the research gives rise to some controversial issues related to embryos, genetics, and assisted reproductive technologies (D. Mathews et al . 2009).
One issue arises from the fact that some research on stem cell-derived gametes requires the creation of embryos, regardless of whether one is using ESCs or iPSCs. To establish that a particular technique for deriving human gametes from stem cells produces functional sperm and eggs, it is necessary to demonstrate that the cells can produce an embryo. This entails the creation of embryos through in vitro fertilization. Since it would not be safe to implant embryos created during the early stages of the research, the likely disposition of the embryos is that they would be destroyed. In such instances, the research would implicate all of the moral issues surrounding the creation and destruction of embryos for research. However, the creation of embryos for research in this situation would not necessitate the destruction of the embryos, as it does when embryos are created to derive stem cell lines. One could in principle store them indefinitely rather than destroy them. This would still leave one subject to the objection that life is being created for instrumental purposes. But the force of the objection is questionable since it is not clear that this instrumental use is any more objectionable than the routine and widely accepted practice of creating excess IVF embryos in the reproductive context to increase the probability of generating a sufficient number of viable ones to produce a pregnancy.
Further issues emerge with the prospect of being able to produce large quantities of eggs from stem cells. As the capacity to identify disease and non-disease related alleles through preimplantation genetic diagnosis (PGD) expands, the ability to create large numbers of embryos would substantially increase the chances of finding an embryo that possesses most or all of the traits one wishes to select. This would be beneficial in preventing the birth of children with genetic diseases. But matters would become morally contentious if it were possible to select for non-disease characteristics, such as sexual orientation, height, superior intelligence, memory, and musical ability. One common argument against using PGD in this way is that it could devalue the lives of those who do not exhibit the chosen characteristics. Another concern is that employing PGD to select for non-disease traits would fail to acknowledge the “giftedness of life” by treating children as “objects of our design or products of our will or instruments of our ambition” rather accepting them as they are given to us (Sandel 2004, 56). There is additionally a concern about advances in genetics heightening inequalities where certain traits confer social and economic advantages and only the well-off have the resources to access the technology (Buchanan 1995). Of course, one can question whether the selection of non-disease traits would in fact lead to devaluing other characteristics, whether it would alter the nature of parental love, or whether it is distinct enough from currently permitted methods of gaining social and economic advantage to justify regulating the practice. Nonetheless, the capacity to produce human stem cell-derived gametes would make these issues more pressing.
There have been a number of recent scientific studies in which stem cells have, under certain in vitro culture conditions, self-organized into three-dimensional structures that resemble and recapitulate some of the functions of human organs (Lancaster & Knoblich 2014; Clevers 2016). These “organoids” have been established with human stem cells for a variety of organs, including, among others, the kidney, liver, gut, pancreas, retina, and brain. In addition to organoids, stem cells have been shown to self-organize into embryo-like structures in vitro . Human embryonic stem cells have formed structures – referred to as “gastruloids” – that bear some resemblance to embryos during gastrulation, which is the stage several days after implantation where the body plan and some tissues tissue types, including the central nervous system, start to develop (Warmflash et al. 2014; Deglincerti et al . 2016; Shahbazi 2016). Researchers have also combined mouse embryonic stem cells and trophoblast stem cells to create “synthetic embryos,” which have a structure akin to pre-implantation embryos (Rivron et al . 2018). Synthetic embryos have been shown to implant into the mouse uterus, though their potential to develop to term has not been demonstrated.
While these scientific advances offer promising avenues for better understanding human development and disease, they also raise some novel and challenging ethical issues. In the case of organoids, cerebral organoids raise the most vexing issues. Researchers have produced cerebral organoids with a degree of development similar to that of a few-months-old embryo, and have already used them to study how the Zika virus causes microcephaly in fetuses (Garcez et al . 2016). At present, there is some evidence that cerebral organoids may be able to receive afferent stimulations that produce simple sensations (Quadrato et al . 2017). However, they currently lack the kind of mature neural networks and sensory inputs and outputs essential to the development of cognition. If, through bioengineering, human cerebral organoids were to develop the capacity for cognition, that would provide grounds for ascribing an elevated moral status to them, and it would raise concomitant issues about our moral obligations towards them. In the nearer term, it is more likely that cerebral organoids will develop some degree of consciousness Assuming we have a shared understanding of consciousness (e.g., phenomenal consciousness), one challenge is to identify means of measuring the presence of consciousness, since a cerebral organoid cannot communicate its internal states (Lavazza & Massimini 2018). But even if we can verify that an organoid is conscious, there remains the question of the moral significance of consciousness (Shepherd 2018). There is debate over whether consciousness has intrinsic value (Lee 2018), and whether in some cases it is better for a conscious being to not possess it (Kahane & Savulescu 2009). Those who reject the intrinsic value and moral significance of consciousness might find the case of a conscious entity that has led a solely disembodied existence, emerges and persists in the absence of any social or cultural nexus, and lacks beliefs and desires, to be a paradigmatic case where the value of consciousness is doubtful.
With respect to gastruloids and synthetic embryos (if the latter are successfully produced with human stem cells), the central question is whether these entities are sufficiently like human embryos in their structure and functions to give rise to moral concerns about their use in research. Gastruloids do not possess all the characteristics of an embryo, as they do not form all of the embryonic tissues (e.g., they do not have the trophectoderm, which mediates the attachment to the uterus). At the same time, gastruloids may, with extra-embryonic tissues, achieve a developmental stage in which they manifest a whole body plan. Recall that one argument (discussed in Section 1.1 above) for rejecting that human embryos are human beings is that the cells that comprise the early embryo do not function in a coordinated way to regulate and preserve a single organism. Gastruloids can in principle operate with this higher level of coordination. While one may still reject that this characteristic of gastruloids confers human rights on them, their more advanced stage of development might ground reasonable claims for according them greater respect than embryos at an earlier stage. In the case of both gastruloids and human synthetic embryos, the possibility that they ultimately lack the potential to develop into mature human beings may be of significance in morally distinguishing them from normal human embryos. As noted previously (in section 1.2 above), one argument for ascribing a high moral status to human embryos and for distinguishing the potential of human embryos from the potential of somatic cells and embryonic stem cells is that embryos have an “active disposition” and “intrinsic power” to develop into mature humans on their own. If synthetic embryos and gastruloids do not possess this disposition and power, then those who oppose some forms of human embryo research might not object to the creation and use of human gastruloids and synthetic embryos for research.
- Annas, G., Caplan, A., and Elias, S., 1996, “The Politics of Human-Embryo Research—Avoiding Ethical Gridlock,” New England Journal of Medicine , 334: 1329–32.
- Benn, S.I., 1973, “Abortion, Infanticide, and Respect for Persons,” in The Problem of Abortion , Joel Feinberg (ed.), Belmont, CA: Wadsworth: 92–103.
- Bok H., Schill K.E., and Faden R.R., 2004, “Justice, Ethnicity, and Stem-Cell Banks,” Lancet , 364(9429): 118–21.
- Buchanan, A., 1995, “Equal Opportunity and Genetic Intervention,” Social Philosophy and Policy , 12(2): 105–35.
- Burtchaell, J.T., 1989, “The Use of Aborted Fetal Tissue in Research: A Rebuttal,” IRB: A Review of Human Subjects Research , 11(2): 9–12.
- Curzer, H., 2004, “The Ethics of Embryonic Stem Cell Research,” Journal of Medicine and Philosophy , 29(5): 533–562.
- Damschen, G., Gomez-Lobo, A., and Schonecker, D., 2006, “Sixteen Days? A reply to B. Smith and B. Brogaard on the Beginning of Human Individuals,” Journal of Medicine and Philosophy , 31: 165–175.
- Clevers, H., 2016, “Modeling Development and Disease with Organoids,” Cell , 165: 1586–1597.
- Deglincerti, A., et al ., 2016, “Self-Organization of the Attached In Vitro Human Embryo,” Nature , 533: 251–54.
- Devolder, K., 2005, “Human Embryonic Stem Cell Research: Why the Discarded-Created Distinction Cannot Be Based on the Potentiality Argument,” Bioethics , 19(2): 167–86.
- Devolder, K., and Harris, J., 2007, “The Ambiguity of the Embryo: Ethical Inconsistency in the Human Embryonic Stem Cell Debate,” Metaphilosophy , 38(2–3): 153–169.
- Dworkin, R., 1992, Life’s Dominion , New York: Vintage.
- Faden, R.R., et al ., 2003, Public Stem Cell Banks: Considerations of Justice in Stem Cell Therapy, Hastings Center Report , 33: 13–27.
- Feinberg, J., 1986, “Abortion,” in Matters of Life and Death , T. Regan (ed.), New York: Random House.
- FitzPatrick, W., 2003, “Surplus Embryos, Nonreproductive Cloning, and the Intend/Foresee Distinction,” Hastings Center Report , 33: 29–36.
- Garzes, P.P., et al ., 2016, “Zika Virus Impairs Growth in Human Neurospheres and Brain Organoids,” Science , 352: 816–18.
- George, R.P., and Gomez-Lobo, A., 2002, “Statement of Professor George (Joined by Dr. Gomez-Lobo),” in Human Cloning and Human Dignity: An Ethical Inquiry , report by the President’s Council on Bioethics: 258–266.
- Green, R., 2002, “Benefiting from ‘Evil’; An Incipient Moral Problem in Human Stem Cell Research,” Bioethics , 16(6): 544–556.
- Greene, M., 2006, “To Restore Faith and Trust: Justice and Biological Access to Cellular Therapies,” Hastings Center Report , 36(1): 57–63.
- Hayashi, K., et al ., 2011, “Reconstitution of the Mouse Germ Cell Specification Pathway in Culture by Pluripotent Stem Cells,” Cell , 146(4): 519–532.
- Hayashi, K., et al ., 2012, “Offspring from Oocytes Derived from In Vitro Primordial Germ Cell-Like Cells in Mice,” Science , 338(6109): 971–975.
- Holm, S., 2003, “The Ethical Case Against Stem Cell Research,” Cambridge Quarterly of Healthcare Ethics , 12: 372–83.
- Kahane, G. and Savulescu, J., 2009, “Brain Damage and the Moral Significance of Consciousness,” Journal of Medicine and Philosophy , 34: 6–26.
- Kuhse, H., and Singer, P., 1992, “Individuals, Human, and Persons: The Issue of Moral Status,” in Embryo Experimentation: Ethical, Legal, and Social Issues , P. Singer, et al. (eds.), Cambridge: Cambridge University Press, 65–75
- Lancaster, M.A., and Knoblich, J.A., 2014, “Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies,” Science , 345(6194): 1247125.
- Lavazza, A. and Massimini, M., 2018, “Cerebral Organoids: Ethical Issues and Consciousness Assessment,” Journal of Medical Ethics , 44: 606–10.
- Lee, A.Y., 2018, “Is Consciousness Intrinsically Valuable,” Philosophical Studies , 20: 1–17.
- Lee, P., and George R., 2006, “Human-Embryo Liberation: A Reply to Peter Singer,” National Review Online (25 January). [ Available online ]
- Lott, J.P., and Savulescu, J., 2007, “Towards a Global Human Embryonic Stem Cell Bank,” American Journal of Bioethics , 7(8): 37–44.
- Marquis, D., 2002, “Stem Cell Research: The Failure of Bioethics,” Free Inquiry , 23(1): 40–44.
- Mathews, D., et al ., 2009, “Pluripotent Stem Cell-Derived Gametes: Truth and (Potential) Consequences,” Cell: Stem Cell , 5: 11–14.
- May, L., 1992, Sharing Responsibility , Chicago: University of Chicago Press.
- McMahan, J., 2002, The Ethics of Killing: Problems at the Margins of Life , New York: Oxford University Press.
- McMahan, J., 2007a, “Killing Embryos for Stem Cell Research,” Metaphilosophy , 38(2–3): 170–189.
- –––, 2007b, “Infanticide,” Utilitas , 19: 131–159.
- Norsigian, J., 2005, “Risks to Women in Embryo Cloning,” Boston Globe , February 25.
- Parfit, D., 1984, Reasons and Persons , Oxford: Clarendon Press.
- Quadrato, G., et al ., 2017, “Cell Diversity and Network Dynamics in Photosensitive Human Brain Organoids,” Nature , 545: 48–53.
- Rivron, N.C., et al ., 2018, “Blastocyst-Like Structures Generated Solely from Stem Cells,” Nature , 557: 106–11.
- Robertson, J., 1988, “Fetal Tissue Transplant Research is Ethical,” IRB: A Review of Human Subjects Research , 6(10): 5–8.
- –––, 1999, “Ethics and Policy In Embryonic Stem Cell Research,” Kennedy Institute of Ethics Journal , 2(9): 109–36.
- Sagan, A., and Singer, P., 2007, “The Moral Status of Stem Cells,” Metaphilosophy , 38(2–3): 264–284
- Sandel, M., 2004, “The Case Against Human Perfection,” Atlantic Monthly , 293(3): 51–62.
- Savulescu, J., 1999, “Should We Clone Human Beings?” Journal of Medical Ethics , 25(2): 87–98.
- Shahbazi, M.N., et al ., 2016, “Self-Organization of the Human Embryo in the Absence of Maternal Tissues,” Nature Cell Biology , 18: 700–08.
- Shepherd, J., 2018, “Ethical (and Epistemological) Issues Regarding Consciousness in Cerebral Organoids,” Journal of Medical Ethics , 44: 611–12.
- Smith, B., and Brogaard, B., 2003, “Sixteen Days,” Journal of Medicine and Philosophy , 28: 45–78.
- Siegel, A., 2003, “Locating Convergence: Ethics, Public Policy, and Human Stem Cell Research,” in The Stem Cell Controversy , M. Ruse and C. Pynes (eds.), Amherst, NY: Prometheus Books.
- –––, 2004, “Temporal Restrictions and the Impasse on Human Embryonic Stem Cell Research,” The Lancet , 364(9429): 215–18.
- Strong, C., 1997, “The Moral Status of Preembryos, Embryos, Fetuses, and Infants,” Journal of Medicine and Philosophy , 22(5): 457–78
- Takahashi, K., et al ., 2007, “Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors,” Cell , 131: 861–872.
- Thomson, J.A., et al ., 1998, “Embryonic Stem Cell Lines Derived from Human Blastocysts,” Science , 282: 1145–47.
- Tooley, M., 1983, Abortion and Infanticide , New York: Oxford University Press.
- Warmflash, A., et al ., 2014, “A Method to Recapitulate Early Embryonic Spatial Patterning in Human Embryonic Stem Cells,” Nature Methods , 11: 847–854.
- Warren, M.A., 1973, “On the Moral and Legal Status of Abortion,” Monist , 57: 43–61.
- Yu, J., et al ., 2007, “Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells,” Science , 318: 1917–1920.
How to cite this entry . Preview the PDF version of this entry at the Friends of the SEP Society . Look up topics and thinkers related to this entry at the Internet Philosophy Ontology Project (InPhO). Enhanced bibliography for this entry at PhilPapers , with links to its database.
Other Internet Resources
- President’s Council on Bioethics, 2002, Human Cloning and Human Dignity: An Ethical Inquiry
- U.S. Conference of Catholic Bishops, 2001, Fact Sheet: President Bush’s Stem Cell Decision
- International Society for Stem Cell Research
- Stem Cell Resources from the American Association for the Advancement of Science
- Stem Cell Research and Applications , recommendations and findings from the AAAS and the Institute for Civil Society.
- Medline Plus: Stem Cells
- The Pew Forum on Religion and Public Life: Bioethics
- The Hinxton Group: An International Consortium on Stem Cell, Ethics, and Law
-->abortion, ethics of --> | cloning | double effect, doctrine of | ethics, biomedical: chimeras, human/non-human | parenthood and procreation | systems and synthetic biology, philosophy of
Copyright © 2018 by Andrew Siegel < asiegel @ jhu . edu >
- Accessibility
Support SEP
Mirror sites.
View this site from another server:
- Info about mirror sites
The Stanford Encyclopedia of Philosophy is copyright © 2023 by The Metaphysics Research Lab , Department of Philosophy, Stanford University
Library of Congress Catalog Data: ISSN 1095-5054
- Utility Menu
GA4 tracking code

Examining the ethics of embryonic stem cell research

Following the recent passage by both houses of Congress of the Stem Cell Research Enhancement Act of 2007, which would permit federal funding of research using donated surplus embryonic stem cells from fertility clinics, the president has once again threatened a veto.
Because neither the House nor the Senate had sufficient votes to override a presidential veto, it appears unlikely this new bill will be enacted into law, further stalling the pace of this research. “This bill crosses a moral line that I and others find troubling,” stated Bush, following the Senate’s vote.
SCL : What are th e main arguments for and against embryonic stem cell research? MS : Proponents argue that embryonic stem cell research holds great promise for understanding and curing diabetes, Parkinson’s disease, spinal cord injury, and other debilitating conditions. Opponents argue that the research is unethical, because deriving the stem cells destroys the blastocyst, an unimplanted human embryo at the sixth to eighth day of development. As Bush declared when he vetoed last year’s stem cell bill, the federal government should not support “the taking of innocent human life.”
It is surprising that, despite the extensive public debate—in Congress, during the 2004 and 2006 election campaigns, and on the Sunday morning talk shows—relatively little attention has been paid to the moral issue at the heart of the controversy: Are the opponents of stem cell research correct in their claim that the unimplanted human embryo is already a human being, morally equivalent to a person?

“It is important to be clear about the embryo from which stem cells are extracted. It is not implanted and growing in a woman’s uterus. It is not a fetus. It has no recognizable human features or form. It is, rather, a blastocyst, a cluster of 180 to 200 cells, growing in a petri dish, barely visible to the naked eye.”
SCL : What are the contradictions in Bush’s stance? MS : Before we address that, it is important to be clear about the embryo from which stem cells are extracted. It is not implanted and growing in a woman’s uterus. It is not a fetus. It has no recognizable human features or form.
It is, rather, a blastocyst, a cluster of 180 to 200 cells, growing in a petri dish, barely visible to the naked eye. Such blastocysts are either cloned in the lab or created in fertility clinics. The bill recently passed by Congress would fund stem cell research only on excess blastocysts left over from infertility treatments.
The blastocyst represents such an early stage of embryonic development that the cells it contains have not yet differentiated, or taken on the properties of particular organs or tissues—kidneys, muscles, spinal cord, and so on. This is why the stem cells that are extracted from the blastocyst hold the promise of developing, with proper coaxing in the lab, into any kind of cell the researcher wants to study or repair.
The moral and political controversy arises from the fact that extracting the stem cells destroys the blastocyst. It is important to grasp the full force of the claim that the embryo is morally equivalent to a person, a fully developed human being.
For those who hold this view, extracting stem cells from a blastocyst is as morally abhorrent as harvesting organs from a baby to save other people’s lives. This is the position of Senator Sam Brownback, Republican of Kansas, a leading advocate of the right-to-life position. In Brownback’s view, “a human embryo . . . is a human being just like you and me; and it deserves the same respect that our laws give to us all.
If Brownback is right, then embryonic stem cell research is immoral because it amounts to killing a person to treat other people’s diseases.
SCL : What is the basis for the belief that personhood begins at conception? MS : Some base this belief on the religious conviction that the soul enters the body at the moment of conception. Others defend it without recourse to religion, by the following line of reasoning: Human beings are not things. Their lives must not be sacrificed against their will, even for the sake of good ends, like saving other people’s lives. The reason human beings must not be treated as things is that they are inviolable. At what point do humans acquire this inviolability? The answer cannot depend on the age or developmental stage of a particular human life. Infants are inviolable, and few people would countenance harvesting organs for transplantation even from a fetus.
Every human being—each one of us—began life as an embryo. Unless we can point to a definitive moment in the passage from conception to birth that marks the emergence of the human person, we must regard embryos as possessing the same inviolability as fully developed human beings.
SCL : By this line of reasoning, human embryos are inviolable and should not be used for research, even if that research might save many lives. MS : Yes, but this argument can be challenged on a number of grounds. First, it is undeniable that a human embryo is “human life” in the biological sense that it is living rather than dead, and human rather than, say, bovine.
But this biological fact does not establish that the blastocyst is a human being, or a person. Any living human cell (a skin cell, for example) is “human life” in the sense of being human rather than bovine and living rather than dead. But no one would consider a skin cell a person, or deem it inviolable. Showing that a blastocyst is a human being, or a person, requires further argument.
Some try to base such an argument on the fact that human beings develop from embryo to fetus to child. Every person was once an embryo, the argument goes, and there is no clear, non-arbitrary line between conception and adulthood that can tell us when personhood begins. Given the lack of such a line, we should regard the blastocyst as a person, as morally equivalent to a fully developed human being.
SCL : What is the flaw in this argument? MS : Consider an analogy: although every oak tree was once an acorn, it does not follow that acorns are oak trees, or that I should treat the loss of an acorn eaten by a squirrel in my front yard as the same kind of loss as the death of an oak tree felled by a storm. Despite their developmental continuity, acorns and oak trees differ. So do human embryos and human beings, and in the same way. Just as acorns are potential oaks, human embryos are potential human beings.
The distinction between a potential person and an actual one makes a moral difference. Sentient creatures make claims on us that nonsentient ones do not; beings capable of experience and consciousness make higher claims still. Human life develops by degrees.
SCL : Yet there are people who disagree that life develops by degrees, and believe that a blastocyst is a person and, therefore, morally equivalent to a fully developed human being. MS : Certainly some people hold this belief. But a reason to be skeptical of the notion that blastocysts are persons is to notice that many who invoke it do not embrace its full implications.
President Bush is a case in point. In 2001, he announced a policy that restricted federal funding to already existing stem cell lines, so that no taxpayer funds would encourage or support the destruction of embryos. And in 2006, he vetoed a bill that would have funded new embryonic stem cell research, saying that he did not want to support “the taking of innocent human life.”
“The distinction between a potential person and an actual one makes a moral difference. Sentient creatures make claims on us that nonsentient ones do not; beings capable of experience and consciousness make higher claims still. Human life develops by degrees.”
But it is a striking feature of the president’s position that, while restricting the funding of embryonic stem cell research, he has made no effort to ban it. To adapt a slogan from the Clinton administration, the Bush policy might be summarized as “don’t fund, don’t ban.” But this policy is at odds with the notion that embryos are human beings.
SCL : If Bush’s policy were consistent with his stated beliefs, how, in your opinion, would it differ from his current “don’t fund, don’t ban” policy? MS : If harvesting stem cells from a blastocyst were truly on a par with harvesting organs from a baby, then the morally responsible policy would be to ban it, not merely deny it federal funding.
If some doctors made a practice of killing children to get organs for transplantation, no one would take the position that the infanticide should be ineligible for federal funding but allowed to continue in the private sector. In fact, if we were persuaded that embryonic stem cell research were tantamount to infanticide, we would not only ban it but treat it as a grisly form of murder and subject scientists who performed it to criminal punishment.
SCL : Couldn’t it be argued, in defense of the president’s policy, that Congress would be unlikely to enact an outright ban on embryonic stem cell research? MS : Perhaps. But this does not explain why, if the president really considers embryos to be human beings, he has not at least called for such a ban, nor even called upon scientists to stop doing stem cell research that involves the destruction of embryos. In fact, Bush has cited the fact that “there is no ban on embryonic stem cell research” in touting the virtues of his “balanced approach.”
The moral oddness of the Bush “don’t fund, don’t ban” position confused even his spokesman, Tony Snow. Last year, Snow told the White House press corps that the president vetoed the stem cell bill because he considered embryonic stem cell research to be “murder,” something the federal government should not support. When the comment drew a flurry of critical press attention, the White House retreated. No, the president did not believe that destroying an embryo was murder. The press secretary retracted his statement, and apologized for having “overstated the president’s position.”
How exactly the spokesman had overstated the president’s position is unclear. If embryonic stem cell research does constitute the deliberate taking of innocent human life, it is hard to see how it differs from murder. The chastened press secretary made no attempt to parse the distinction. His errant statement that the president considered embryo destruction to be “murder” simply followed the moral logic of the notion that embryos are human beings. It was a gaffe only because the Bush policy does not follow that logic.
SCL : You have stated that the president’s refusal to ban privately funded embryonic stem cell research is not the only way in which his policies betray the principle that embryos are persons. How so? MS : In the course of treating infertility, American fertility clinics routinely discard thousands of human embryos. The bill that recently passed in the Senate would fund stem cell research only on these excess embryos, which are already bound for destruction. (This is also the position taken by former governor Mitt Romney, who supports stem cell research on embryos left over from fertility clinics.) Although Bush would ban the use of such embryos in federally funded research, he has not called for legislation to ban the creation and destruction of embryos by fertility clinics.
SCL : If embryos are morally equivalent to fully developed human beings, doesn’t it then follow that allowing fertility clinics to discard thousands of embryos is condoning mass murder? MS : It does. If embryos are human beings, to allow fertility clinics to discard them is to countenance, in effect, the widespread creation and destruction of surplus children. Those who believe that a blastocyst is morally equivalent to a baby must believe that the 400,000 excess embryos languishing in freezers in U.S. fertility clinics are like newborns left to die by exposure on a mountainside. But those who view embryos in this way should not only be opposing embryonic stem cell research; they should also be leading a campaign to shut down what they must regard as rampant infanticide in fertility clinics.
Some principled right-to-life opponents of stem cell research meet this test of moral consistency. Bush’s “don’t fund, don’t ban” policy does not. Those who fail to take seriously the belief that embryos are persons miss this point. Rather than simply complain that the president’s stem cell policy allows religion to trump science, critics should ask why the president does not pursue the full implications of the principle he invokes.
If he does not want to ban embryonic stem cell research, or prosecute stem cell scientists for murder, or ban fertility clinics from creating and discarding excess embryos, this must mean that he does not really consider human embryos as morally equivalent to fully developed human beings after all.
But if he doesn’t believe that embryos are persons, then why ban federally funded embryonic stem cell research that holds promise for curing diseases and saving lives?
Scaling up human mesenchymal stem cell manufacturing using bioreactors for clinical uses
Affiliations.
- 1 University of Kragujevac, Serbia, Faculty of Medical Sciences, Department of Genetics, Serbia. Electronic address: [email protected].
- 2 SPEBO Medical Fertility Hospital, Leskovac, Serbia.
- 3 Newcastle University, School of Computing, Newcastle upon Tyne, UK.
- 4 University of Kragujevac, Serbia, Faculty of Medical Sciences, Department of Histology and Embryology, Serbia.
- 5 University of Kragujevac, Serbia, Faculty of Medical Sciences, Department of Genetics, Serbia.
- 6 Department of Orthopaedic Surgery, Clinical Centre of Montenegro, 81110 Podgorica, Montenegro.
- 7 University of Kragujevac, Serbia, Faculty of Medical Sciences, Department of Surgery, Serbia.
- PMID: 37163885
- DOI: 10.1016/j.retram.2023.103393
Human mesenchymal stem cells (hMSCs) are multipotent cells and an attractive therapeutic agent in regenerative medicine and intensive clinical research. Despite the great potential, the limitation that needs to be overcome is the necessity of ex vivo expansion because of insufficient number of hMSCs presented within adult organs and the high doses required for a transplantation. As a result, numerous research studies aim to provide novel expansion methods in order to achieve appropriate numbers of cells with preserved therapeutic quality. Bioreactor-based cell expansion provide high-level production of hMSCs in accordance with good manufacturing practice (GMP) and quality standards. This review summarizes current knowledge about the hMSCs manufacturing platforms with a main focus to the application of bioreactors for large-scale production of GMP-grade hMSCs.
Keywords: Bioreactor; Manufacturing; Mesenchymal stem cells.
Copyright © 2023 Elsevier Masson SAS. All rights reserved.
Publication types
- Bioreactors
- Cell Culture Techniques* / methods
- Cell Proliferation
- Cells, Cultured
- Mesenchymal Stem Cells*
- Skip to navigation
- Skip to content
- UMB Shuttle
University of Maryland, Baltimore
About UMB History, highlights, administration, news, fast facts
- Accountability and Compliance
- Administration and Finance
- Center for Information Technology Services
- Communications and Public Affairs
- Community Engagement
- Equity, Diversity, and Inclusion
- External Relations
- Government Affairs
- Philanthropy
- Office of the President
- Office of the Provost
- Research and Development
- University Counsel
- Administrative Officers
- Boards of Visitors
- Faculty Senate
- Staff Senate
- Center for Health and Homeland Security
- Council for the Arts & Culture
- Interprofessional Education
- Leaders in Education: Academy of Presidential Scholars
- Middle States Self-Study
- President's Council for Women
- President's Symposium and White Paper Project
- For the Media
- Steering Committee Roster
- Logistics Committee Roster
- UMB Police and Public Safety
- Graduation Celebration 2024
- Founders Week
- UMB Holiday Craft Fair
Academics Schools, policies, registration, educational technology
- School of Dentistry
- Graduate School
- School of Medicine
- School of Nursing
- School of Pharmacy
- School of Social Work
- Carey School of Law
- Health Sciences and Human Services Library
- Thurgood Marshall Law Library
Admissions Admissions at UMB are managed by individual schools.
- Carey School of Law Admissions
- Graduate School Admissions
- School of Dentistry Admissions
- School of Medicine Admissions
- School of Nursing Admissions
- School of Pharmacy Admissions
- School of Social Work Admissions
- Tuition and Fees
- Student Insurance
- Academic Calendar
- Financial Assistance for Prospective Students
- Financial Assistance for Current Students
- Financial Assistance for Graduating Students
Research Offices, contracts, investigators, UMB research profile
- Organized Research Centers and Institutes
- UMB Institute for Clinical & Translational Research
- Sponsored Programs Administration
- Sponsored Projects Accounting and Compliance (SPAC)
- Kuali Research
- Clinical Trials and Corporate Contracts
- CICERO Log-in
- Conflict of Interest
- Human Research Protections
- Environmental Health and Safety
- Export Compliance
- Effort Reporting
- Research Policies and Procedures
- Center for Innovative Biomedical Resources
- Baltimore Life Science Discovery Accelerator (UM-BILD)
- Find Funding
- File an Invention Disclosure
- Global Learning for Health Equity Network
- Manage Your Grant
- Research Computing
- UM Research HARBOR
- Center for Violence Prevention
- Office of Research and Development
- Center for Clinical Trials and Corporate Contracts
- Technology Transfer/UM Ventures
- Contact Research and Development
Services For students, faculty, and staff, international and on-campus
- Student Health Resources
- Educational Support and Disability Services
- Writing Center
- URecFit and Wellness
- Intercultural Leadership and Engagement
- Educational Technology
- Student Counseling Center
- UMB Scholars for Recovery
- UMB Student Affairs
- Human Resource Services
- Travel Services
- Strategic Sourcing and Acquisition Services
- Office of the Controller
- Office of the Ombuds
- Employee Assistance Program (EAP)
- Workplace Mediation Service
- Faculty Center for Teaching and Learning
- UMB Travel: Start Here
- International Students, Scholars, and Employees
- Center for Global Engagement
- International Travel SOS
- International Operations
- Parking and Transportation Services
- UMB shuttle
- SMC Campus Center Event Services
- Donaldson Brown Riverfront Event Center
- All-Gender Bathrooms
- Environmental Services
- Interprofessional Program for Academic Community Engagement
University Life Alerts, housing, dining, calendar, libraries, and recreation
- Emergency Reference Guide
- Campus Life Weekly with USGA
- Starting a New Universitywide Organization
- University Student Government Association
- Planned Closures
- Intramural Sports
- Safety Education
- About URecFit and Wellness
- How to Get Your One Card
- One Card Uses
- Lost One Card
- One Card Policies
- Photo Services
- One Card Forms
- One Card FAQs
- Office Hours and Directions
Give to UMB Sustain excellence and meet UMB's educational needs for today and tomorrow.

Thank You for Your Gift to UMB
The University of Maryland, Baltimore (UMB) is excited to share its new online giving page.
With enhanced searchability, a streamlined checkout process, and new ways to give such as Venmo, PayPal, Apple Pay, and Google Pay in addition to credit card, donors can support UMB quickly and securely.
- Ways to Give
- Where to Give
- Staying Connected: You and UMB
- The UMB Foundation
- Office of Philanthropy
- Maryland Charity Campaign

- Archived News
Search UMB News:
There are no reported emergencies on campus at this time. Sign up for UMB Alerts.
Stem Cell Therapy Boosts Natural Repair After Cardiac Arrest
May 9, 2024 | By Holly Moody-Porter
Researchers at the University of Maryland School of Medicine (UMSOM) have identified an innovation in stem cell therapy to regenerate neural cells in the brain after cardiac arrest in an animal model. The study led by Xiaofeng Jia, BM, MS, PhD, FCCM , professor of neurosurgery, found that the application of modified sugar molecules on human neural stem cells improved the likelihood of the therapy's success. The application of these sugar molecules both enhanced the stem cells' proliferation and their transition into neurons to help repair critical connections in the brain. The finding could eventually lead to improved recovery of patients with cardiac-arrest induced brain injuries.
The pivotal study was funded by the National Institute of Neurological Disorders and Stroke (R01NS125232, R01NS110387), and featured on the April Vol. 34 No. 17 front cover of Advanced Functional Materials Journal .
Brain injury is the most common consequence of cardiac arrest, due to the impaired blood flow and oxygen to the brain. About 70 percent of the nearly 7 million people who suffer from cardiac arrest each year experience a long-term brain injury that leads to permanent disability.
[Figure 1. Schematic of novel stem cell therapy with metabolic glycoengineering (MGE) for brain repair after cardiac arrest (CA). Conditions were characterized and optimized in vitro to modify human neural stem cells by using the TProp sugar analog (Ac 5 ManNTProp). The efficacy of the transplanted metabolically glycoengineered neural stem cells (MGE-NSC) for brain repair and how TProp analog affected the fate of neural stem cells were evaluated within a cardiac-arrest-induced brain injury rat model. Results indicated that MGE improved the viability and differentiation of neural stem cells, inhibited neuroinflammation, and provided neuroprotection after in vivo transplantation.]
The potential of stem cell therapy to address neurological dysfunction has long been fraught with challenges due to the harsh in vivo microenvironment of the brain; this results in poor stem cell retention and integration at the site of injuries.
Recent advances in manipulating a cell’s complex carbohydrate structure through metabolic glycoengineering, has enabled UMSOM researchers to explore the efficacy of a modified sugar molecule, known as the TProp sugar analog, to help stem cells remain more viable in the brain.
“All cells in a person’s body are enveloped in sugar molecules called 'glycans,' ” said Jia. “Through our previous research, we were able to find that these sugar molecules are vital to cell function. Glycoengineering has enabled us to further enhance stem cell viability so they may provide therapeutic effects for cardiac-arrest-induced brain injuries. This is a very important step forward in regenerative medicine for patients.”
In the study, researchers examined the efficacy in a rat model and compared the effects of “naïve” human neural stem cells to neural stem cells that were pretreated with the “TProp” sugar analog. The study found that stem cells pretreated with TProp substantially improved brain function and reduced anxiety and depression-associated behaviors through various behavioral tests.
The treatment also activated the related inflammatory Wnt/β-catenin signaling pathway, which regulates critical aspects of cell function. This upregulated pathway by TProp promotes the transition of stem cells into neurons, the nerve cells responsible for sending and receiving signals from the brain.
The TProp-pretreated group also demonstrated improved synaptic plasticity, the ability of neurons to modify the strength of their connections, and reduced neuroinflammation in the central nervous system, providing a superior ability to regenerate and recover from damaged brain functions.
The results indicate that glycoengineered stem cells have the potential to promote the growth of new connections among surviving or regenerated neurons, leading to regenerated circuits in the brain.
“This innovative research has been an important proof of concept study suggesting that stem cells could be used to regenerate neural connections in the brain of patients who suffer a devastating injury after cardiac arrest,” said UMSOM Dean Mark T. Gladwin, MD, who is the John Z. and Akiko K. Bowers Distinguished Professor, UMSOM, and vice president for medical affairs, University of Maryland, Baltimore. “Next steps for this translational application include determining the optimal delivery route and timing of metabolically glycoengineered stem cell therapy, as well as systemic evaluation on large animals before this can move into clinical studies.”
UMSOM faculty and postdoc co-authors of the paper include Jian Du, PhD , Xiao Liu, MD, MS , Subash Marasini, PhD , Zhuoran Wang, MD, PhD , and Xiaofeng Jia, MD, MS, PhD, FCCM.
Faculty from the Department of Biomedical Engineering and Translational Cell and Tissue Engineering Center at the Johns Hopkins University School of Medicine also contributed to this research.
The University of Maryland, Baltimore is the founding campus of the University System of Maryland. 620 W. Lexington St., Baltimore, MD 21201 | 410-706-3100 © 2023-2024 University of Maryland, Baltimore. All rights reserved.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 10 May 2024
Role of umbilical cord mesenchymal stromal cells in skin rejuvenation
- Le Chang 1 na1 ,
- Wei-Wen Fan 1 na1 ,
- He-Ling Yuan 1 na1 ,
- Xin Liu 1 na1 ,
- Qiang Wang 1 ,
- Guang-Ping Ruan 1 ,
- Xing-Hua Pan 1 &
- Xiang-Qing Zhu 1
npj Regenerative Medicine volume 9 , Article number: 20 ( 2024 ) Cite this article
6 Altmetric
Metrics details
- Stem-cell research
Aging is the main cause of many degenerative diseases. The skin is the largest and the most intuitive organ that reflects the aging of the body. Under the interaction of endogenous and exogenous factors, there are cumulative changes in the structure, function, and appearance of the skin, which are characterized by decreased synthesis of collagen and elastin, increased wrinkles, relaxation, pigmentation, and other aging characteristics. skin aging is inevitable, but it can be delayed. The successful isolation of mesenchymal stromal cells (MSC) in 1991 has greatly promoted the progress of cell therapy in human diseases. The International Society for Cellular Therapy (ISCT) points out that the MSC is a kind of pluripotent progenitor cells that have self-renewal ability (limited) in vitro and the potential for mesenchymal cell differentiation. This review mainly introduces the role of perinatal umbilical cord-derived MSC(UC-MSC) in the field of skin rejuvenation. An in-depth and systematic understanding of the mechanism of UC-MSCs against skin aging is of great significance for the early realization of the clinical transformation of UC-MSCs. This paper summarized the characteristics of skin aging and summarized the mechanism of UC-MSCs in skin rejuvenation reported in recent years. In order to provide a reference for further research of UC-MSCs to delay skin aging.
Introduction
The skin is the largest organ of the human body and has a surface area of 1.5–2 m 2 , covering the surface of the human body. It is in direct contact with the external environment and protects us from environmental factors 1 . The skin consists of three parts: the epidermis, dermis, and subcutaneous tissue, which jointly protect internal organs and perform different biological functions. The epidermis is located in the outermost layer of the body and plays a major defensive role 2 . The dermis is mainly responsible for the synthesis, deposition, and remodeling of the dermal extracellular matrix (ECM), which supports the structural integrity of the skin 3 , 4 . Dermal fibroblasts are the main cells in the dermis and synthesize and secrete collagen, elastin and proteoglycan to give strength and elasticity to the skin 5 . Subcutaneous tissue is located in the deepest layer of the skin and is rich in fat cells and blood vessels; it can support, warm, and provide nutrition for the dermis 6 . Skin appearance is the main factor used to evaluate age and health status. With the emergence of aging complications and the improvement in quality of life, people are highly motivated to maintain a youthful appearance. Therefore, how to prevent and delay skin aging is important for the general public, thereby stimulating the in-depth study of antiaging by researchers.
Under aging and the decline in the structure and function of skin tissue stimulated by external factors, many functional cells in skin tissue undergo senescence and apoptosis, while new cells lack the ability to self-renew. To resolve the insufficient self-renewal ability of cells in skin tissue, some researchers have proposed that skin cells can be replenished by activating stem cells in skin tissue. However, long-term activation and mobilization will lead to the depletion of stem cells in the body and the complete loss of the ability of cells in the skin to self-renew 7 . It has been reported that MSCs transplantation can improve skin conditions to some extent 8 . Therefore, exogenous supplementation of MSCs may be an effective method. The term “MSCs” originated from the isolation of the bone marrow in 1988 Marrow Stromal Stem Cells 9 , and named Mesenchymal Stem cells by A.I. Caplan in 1991 10 , ISCT changed to Mesenchymal Stromal Cells in 2006 11 , A.I. Caplan himself applied to ISCT in 2017 to change Mesenchymal Stem Cells to Medicinal Signaling Cells 12 , ISCT stated in 2019 that it was not in favor of dropping the term “mesenchymal” and recommended that the acronym “MSC” continue to be used, but with a note on the functional definition 13 . The naming of MSCs is still controversial, but with further research, increasing evidence suggests that the therapeutic role of MSCs is largely attributed to their paracrine function.
In this paper, we focus on the study of UC-MSCs in skin aging. On the one hand, umbilical cords are medical waste, and as a result, using them avoids the limitation of source and ethical issues 14 , 15 , 16 , 17 , 18 . On the other hand, the efficacy of MSCs decreases with the increase of their number of divisions, because cell division shortens telomeres and leads to cell senescence 19 , 20 . An earlier 2012 follow-up study of patients with the acute graft-versa-host disease (GVHD) treated with MSCs showed a significant increase in one-year survival (75% vs 21%) in MSCs receiving early passage (from generation 1–2) compared to MSC patients receiving late passage (from generation 3–4) 21 . In addition, the regenerative potential of MSCs also decreases with the age of donors 22 . Therefore, the UC-MSCs are isolated from neonatal tissues and seem to be “younger” than other sources of MSCs 23 . Their high activity, increased pluripotency, low immunogenicity, and suitable paracrine effects have been indicated 24 , 25 . Previous studies have shown that UC-MSCs can be induced to differentiate into various types of functional cells in vitro, such as keratinocytes and dermal fibroblasts, which provides a variety of potential strategies for the treatment of skin diseases and the development of medical beauty products 26 . It also made many researchers once believe that the efficacy of MSCs is mainly played by their differentiation into specific functional cells, so that the efficacy of MSCs is infinitely amplified, resulting in over-marketing of “Stemcells” on the market. However, subsequent studies have seen little evidence that MSCs can differentiate into specific functional cells in vivo, so it is believed that the paracrine role of MSCs is the main way to exert their therapeutic effect. This may also be the reason why A.I. Caplan applied to ISCT in 2017 to change Mesenchymal Stem Cells to Medicinal Signaling Cells.
Characteristics of skin aging
Human skin is a dynamic and complex organ that is composed of different cell types and functional regions. Like other organs, the skin ages and is characterized by structural destruction and gradual loss of function. Aging caused by genetic, metabolic, and other internal factors is called intrinsic aging, while aging caused by environmental factors such as ultraviolet rays, nutrition, air pollution, cigarettes, temperature, and pressure is called extrinsic aging 27 .
For naturally aging skin, histological changes mainly occur in the basal layer and dermis. The basal layer of the skin is located in the deepest layer of the epidermis and participates in the repair and regeneration of the skin. Studies have shown that the proliferation of basal cells of the skin, such as keratinocytes and melanocytes, decreases with age, resulting in a thinning of the skin epidermis 28 , 29 . Moreover, the fibrous ECM components such as elastin, fibrin, and collagen in the dermis degenerate, the skin is dehydrated, elasticity decreases, and wrinkles appear 30 , 31 . With age, the repair ability of skin cells decreases, resulting in intrinsic skin aging.
Extrinsic aging is far more serious than endogenous aging; ultraviolet radiation (UVR) has the greatest effect, accounting for 80% of facial skin aging 32 . In contrast to intrinsic aging, UVR thickens the epidermis and promotes the activation of epidermal melanocytes in exposed skin, resulting in pigmentation 33 . UVR on the skin leads to senescence and apoptosis of skin cells by directly damaging the deoxyribonucleic Acid (DNA), Ribonucleic Acid (RNA), and protein of skin cells 34 . Moreover, skin cells produce free radicals and reactive oxygen species (ROS) when subjected to UVR, which causes inflammation and promotes mitochondrial membrane potential (MMP) synthesis, indirectly leading to oxidative damage and ECM degradation of skin cells 35 , 36 . Photoaging also accelerates skin aging by superimposing intrinsic aging in chronological order.
The ultimate goal of researchers’ efforts investigating skin aging is to find ways to slow down the rate of aging and improve quality of life by regulating the mechanisms of skin aging. At present, it has been reported that plant extracts 37 , antioxidants 38 , growth factors, and cytokines 39 , as well as MSCs 40 , can alleviate skin aging 41 . Since cell therapy was first proposed by Swiss doctors in 1931, the field has made a breakthrough in the research of human diseases. Skin tissue is composed of a large number of mature functional cells, progenitor cells, and a small number of stem cells. Although adult tissue stem cells are rare, they play a major role in human health. The number of adult stem cells gradually decreases after birth, so supplementation with exogenous MSCs may be an effective way to promote tissue repair and regeneration. Umbilical cord mesenchymal stromal cells (UC-MSCs) have become a more promising therapeutic method because of their powerful paracrine function and the ability to secret various cytokines, growth factors, and exogenes to promote tissue regeneration and inhibit inflammatory response. However, MSCs therapy is still in the research stage, and a large amount of experimental data is needed to accelerate its clinical transformation. As of December 2023, over 2000 MSCs clinical trials have been registered at https://clinicaltrials.gov/ , including over 400 UC-MSCs clinical trials. Including UC-MSCs for Diabetic Nephropathy, Ulcerative Colitis, Oral Chronic Graft-versus-host Disease, Diabetic Foot, Skin Grafts in Donor Site Wounds, Skin Rejuvenation, Skin Ulcers, and other diseases. This large amount of data reflects the broad interest of the scientific community in the potential therapeutic applications of MSCs. However, among the many clinical trials at different stages, we have collated nine clinical trials of UC-MSCs for skin-related diseases that have been completed and have reported results (Table 1 ). By combing through these trials, we can gain a clearer understanding of the application of UC-MSCs in clinical practice, as well as the challenges and future directions. Clinical trials are designed to evaluate the efficacy and safety of MSCs in the treatment of various diseases, but clinical trials currently face many difficulties, including developing standardized treatment protocols, monitoring cell survival and function in vivo, and the safety and long-term efficacy of cell therapy. These problems not only increase the complexity of clinical trials, but also limit their wide application in practice. In order to solve the challenges faced by clinical trials, pre-clinical basic research is crucial to provide a reliable theoretical and experimental basis for clinical trials. In the basic research, the establishment of an ideal experimental model is the premise of further research, here we mainly introduce the skin aging research model. Aging research on animal models can simulate the complex environment of human skin aging in combination with in vitro and in vivo aging factors and relatively accurately reflect the characteristics of skin aging, but the accuracy of these results still needs to be verified at the cellular and molecular levels. Cells are the basic unit of the human body; they can be isolated and expanded in vitro under suitable conditions and can reflect the process and law of human aging at the cellular level, so they are widely used as an experimental model in vitro. In order to facilitate the work of subsequent researchers, we have listed in detail the modeling conditions of the currently widely used research models in Table 2 , aiming to provide clearer and convenient guidance for future basic research. However, it is worth noting that none of these studies calculated the percentage of actual engrafted cells relative to the total implanted cells, as the actual number of engrafted cells is crucial for assessing therapeutic efficacy. Therefore, future research may need to pay more attention to and carefully consider the calculation of the actual number of engrafted cells to comprehensively understand the effectiveness and mechanisms of MSC therapy.
The role and mechanism of UC-MSCs in skin aging
UC-MSCs are a kind of mesenchymal stromal cell derived from neonatal umbilical cord tissue with abundant material sources, easy amplification, strong plasticity, low immunogenicity, high migration and homing activity, exocrine secretion, and the secretion of a variety of cytokines 25 . Compared with other MSCs currently used in basic and clinical research, such as adipose mesenchymal stromal cells (ADMSCs), bone marrow mesenchymal stromal cells (BMSCs), dental pulp stromal cells (DPSCs), embryonic stromal cells (ASCs), and neural stem cells (NSCs); UC-MSCs are derived from a wider range of sources; have no ethical or safety challenges; are easier to obtain, expand and store; and can fully meet clinical needs 42 . UC-MSCs can be induced to differentiate into many types of functional cells in vitro, which is of great significance for the clinical treatment of corresponding diseases. UC-MSCs have been used in the study of cardiovascular disease 43 , inflammatory bowel disease (IBD) 44 , chronic obstructive pneumonia (COPD) 45 , premature ovarian failure (POF) 46 , skin aging 23 , and other diseases, and their effectiveness has been proven. This paper mainly summarizes the research progress of UC-MSCs in skin aging. The mechanism of UC-MSCs in the treatment of skin aging can be summarized as promoting injury repair and skin regeneration through anti-inflammatory, antioxidative, and anti-glycosylation mechanisms, as shown in Fig. 1 .
The skin shows structural and functional degradation under the action of internal and external factors, and UC-MSCs rejuvenate it by promoting injury repair and regeneration through anti-inflammatory, antioxidative, and anti-glycosylation mechanisms.
Damage repair
Skin tissue integrity, function, and regeneration decrease with age. An increasing number of studies have reported that UC-MSCs can promote the repair of damaged skin through the secretion of cytokines. The homing property of UC-MSCs is the key to their direct participation in the repair of skin injury. Many animal experiments have confirmed that when there is injury in the body, transplanted UC-MSCs can migrate to the injured site, differentiate, and replace injured cells using the chemotaxis of the injured tissue microenvironment 47 , 48 , 49 . However, with the deepening of the research, the view that MSCs differentiate and replace injured cells is no longer supported. After the importation of MSCs into the body, the amount of MSCs in the body is very small (<1%), suggesting that the repair of injuries may primarily involve the paracrine functions of MSCs (Table 3 ).
To study the role and fate of transfused MSCs, Yin’s research team explored the fate of type 2 diabetes (T2DM) mice intravenously injected with UC-MSCs compared with that in control mice. This study showed that UC-MSCs first reached the lungs and then migrated through the circulatory system to the spleen and liver. Compared with the control mice, the T2DM mice injected with UC-MSCs showed that the UC-MSCs homed to the islets. UC-MSC infusion not only effectively restored blood glucose homeostasis and reduced insulin resistance in mice but also improved hyperlipidemia and liver function in T2DM mice, suggesting that UC-MSC migration is closely related to tissue injury and can participate in tissue repair 50 . Zhang et al. 51 applied UC-MSCs and UC-MSC-CM locally to the skin wounds of diabetic mice to study their therapeutic effects on wound healing. The results showed that UC-MSCs and UC-MSC-CM significantly increased the overall wound healing rate, improved angiogenesis, and increased the percentage of M2 macrophages in the wound area. Further observation of the local microenvironment of the wound tissue showed that the secreted levels of the anti-inflammatory factors IL-10 and VEGF increased, while the secreted levels of the proinflammatory factors TNF-α and IL-6 were inhibited. It is suggested that UC-MSCs can play a role in injury repair by improving angiogenesis and regulating the local tissue microenvironment.
Promotion of skin regeneration
“Repair” and “regeneration” are often mistaken for the same concept. In fact, “repair” mainly refers to the recovery of tissue structure and function. In the context of skin, repair indicates that it may have scars and may not have hair follicles. “Regeneration” essentially refers to achieving a completely normal state through the proliferation of cells in skin tissue 52 . UC-MSCs can secrete and synthesize a variety of cytokines that promote cell growth and differentiation to regulate the local microenvironment, including FGF, EGF, VEGF, NGF, PDGF, CSF, and TNF 53 . These cytokines carry signaling information that can regenerate blood vessels, improve blood circulation, and promote tissue regeneration.
After skin injury model rats were treated with UCBMSC-exo and UCBMSCs, the skin appendages, blood vessels, and nerves were regenerated, the wound closure rate was significantly accelerated, and scarring was reduced 54 . Li et al. 23 used an aging nude mouse model and HDF model to prove that UC-MSCs can increase the thickness of aging skin and the production of matrix collagen fibers, increase the proliferation and migration of human dermal fibroblasts (HDFs), and promote skin regeneration. In addition, an interesting study showed that UC-MSCs can also be used as carriers for gene transfer and drug delivery to enhance the expression of the target gene and can interact with cytokines to change the secretion level to enhance regeneration. The Wnt protein is the key mediator of skin development. Researchers obtained conditioned medium (Wnt-CM) containing Wnt7a from the supernatant of UC-MSCs overexpressing Wnt7a and injected it into the wounds of mice. It was found that the supernatant promoted wound healing, induced hair follicle regeneration, and enhanced the expression of the ECM and the migration of fibroblasts 55 .
Anti-inflammation
Inflammation is a pathophysiological reaction after tissue injury and a protective defense response of tissues and organs to harmful stimuli. A certain degree of inflammation is beneficial, but excessive inflammation can lead to local tissue cell necrosis and dysfunction, and persistent chronic inflammation can hinder the growth or regeneration of functional cells in tissue 56 , 57 . Moreover, the human body is always exposed to various stimuli, and long-term inflammatory stimulation eventually leads to the degeneration of the structure and function of tissues and organs. Therefore, the reduction in inflammatory reactions may be beneficial to the regeneration of tissues and organs. Experiments showed that the gradual accumulation of senescent cells in the body increased the release of proinflammatory factors such as IL-6, IL-8, and TNF-α and further promoted the occurrence of senescence 58 . Photoaging is the main form of skin aging. Long-term exposure to UVR accelerates the aging of skin under the action of inflammatory cells and proinflammatory cytokines 59 .
UC-MSCs exert their anti-inflammatory effect mainly by secreting cytokines, growth factors, anti-inflammatory factors, and exocrine factors to reduce the inflammatory response and enhance tissue repair. They can also directly interact with the surface molecules of immune cells and regulate the downstream pathways of immune cells, thus affecting cell proliferation, effector production, and cell survival 60 . Several Korean researchers have used antibody arrays to evaluate the concentrations of growth factors and cytokines in UC-MSC-CM. The results showed that UC-MSC-CM contained high concentrations of anti-inflammatory-related growth factors and cytokines, including EGF, TIMP-1, IGFBP-7, thrombin reactive protein-1, fibrinogen, and fibronectin 61 . The authors further tested the anti-inflammatory activity of UC-MSCs-CM on HaCaT cells stimulated with TNF-α and INF-γ. The results showed that UC-MSC-CM had an inhibitory effect on the inflammatory cytokines TARC, TNF-β, IL-1β, and IL-6 and suggested that UC-MSC-CM had an anti-inflammatory effect. Li et al. 62 confirmed that UCMSC treatment can reduce the expression levels of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 in an LPS-induced rat model and concluded that UCMSC treatment can reduce systemic inflammation associated with LPS.
We know that continuous inflammation stimulates tissue fibroplasia, leading to tissue and organ fibrosis. Liu et al. 63 used a rat model of renal interstitial fibrosis to evaluate the effect of UCMSC-CM on tubulointerstitial inflammation and fibrosis. The results showed that UCMSC-CM reduced the deposition of ECM, the infiltration of inflammatory cells, and the release of inflammatory factors in renal fibrosis by inhibiting the activation of the TLR4/NFκB signaling pathway. Chen’s team 64 injected UC-MSCs subcutaneously into psoriatic arthritis model mice and found that UC-MSCs inhibited skin inflammation and significantly ameliorated the pathological features of mice.
Antioxidant properties
Oxidation is a process in which substances are decomposed to release energy and take place in the body regularly. When the body is in a normal physiological state, the oxidation capacity and antioxidant capacity are in dynamic balance. Once the production of free radicals (such as ROS) exceeds the body’s antioxidant capacity, the redox state is out of balance, and oxidative stress is induced 65 , 66 . Oxidative stress is accompanied by the processes of cell injury, inflammation, and metabolic disorders, which are involved in the pathology of many diseases and are considered to be the cause of aging. It has been reported that excessive ROS can directly oxidize DNA, proteins, and lipids, resulting in DNA damage, mitochondrial damage, protein damage, cell senescence, and even death 67 , 68 , 69 . According to the theory of free radical aging, ROS are mainly produced as a result of cell metabolism dysfunction and UVR; are generated by the mitochondrial electron transport chain, peroxisomes, and endoplasmic reticulum; and play a major role in skin aging 70 . ROS can activate the MAPK signaling pathway through a series of intermediates to promote the production of MMPs. MMPs can degrade collagen and elastin, resulting in increased and deepened skin wrinkles and a lack of elasticity 71 . ROS and the activated MAPK signaling pathway can also activate NFκB , mediate the expression of inflammatory cytokines, further promote the production of ROS, and accelerate skin aging 72 , 73 .
There are few reports on the antioxidant effect of UC-MSCs on skin aging. Some scholars believe that UC-MSCs can directly alleviate mitochondrial dysfunction, thus blocking the production of more free radicals from dysfunctional mitochondria that accelerate aging, but the specific mechanism is not clear 74 . However, an increasing number of researchers have observed the antioxidant stress effect of UC-MSCs in aging animal models. Recently, it was reported that after UCMSC treatment of D-galactose-induced skin aging model nude mice, the levels of superoxide dismutase (SOD) in skin tissue increased significantly, while the levels of malondialdehyde decreased significantly, essentially returning to normal levels 23 . It is suggested that UCMSC treatment can enhance the ability of cells to scavenge free radicals, improve the antioxidant stress function of skin, and play a positive role in reducing cell senescence caused by oxidative stress. However, while the antioxidant effects of UCMSC treatment have been observed, the underlying mechanism is still not completely clear. Some scholars believe that UC-MSCs play an antioxidant stress role by directly scavenging free radicals, secreting bioactive enzymes, and regulating the function of mitochondria, but there is insufficient evidence 75 .
Anti-glycosylation
Advanced glycation end products (AGEs) are the products of nonenzymatic glycosylation and oxidation of proteins and lipids, which accumulate in inflammatory environments and during aging. The accumulated AGEs easily interact with collagen fibers in the dermis to produce glycosylated collagen in the body. The structural changes of glycosylated collagen increase skin fragility and decrease skin strength so that its biological function is reduced 76 , 77 . A new study showed that UC-MSCs can protect fibroblasts from AGE cytotoxicity by secreting cytokines and activating the PI3K/AKT/PTEN pathway 78 .
Senile diabetes is a very common chronic disease related to aging. The difficulty of healing skin wounds in patients with diabetes is a problem that urgently needs to be solved 78 . An in-depth study of the pathogenesis of diabetic dermatopathy found the root cause of diabetic wound formation and healing difficulty to be the accumulation of AGEs in the dermis. However, to date, only a few effective methods can inhibit and remove AGEs in wounds, and the emergence of MSCs therapy brings great hope to these diabetic patients 79 , 80 , 81 , 82 . Many researchers have studied the promoting effect of mesenchymal stromal cells from different sources on diabetic wound healing. The results show that BMSCs and UC-MSCs can effectively promote diabetic skin wound healing 83 , 84 .
Data sources
This review conducted extensive searches across PubMed, ClinicalTrials.gov using the search terms “umbilical mesenchymal stromal cells,” “skin aging,” “regeneration,” “rejuvenation,” “mechanism,” “review,” “clinical trial,” and “retrospective study” to retrieve relevant reviews and studies published within the past 20 years. A total of 108 eligible literature pieces were screened.
Inclusion criteria
The selection criteria are as follows: 1. Literature discussing the nomenclature, biological characteristics, and mechanisms of action of MSCs in depth; 2. Articles involving experiments and clinical studies on UC-MSCs in promoting skin regeneration and repair; 3. Literature covering the construction of skin aging models and MSC treatment strategies; 4. High-quality literature provides rigorous explanations of facts and viewpoints.
Exclusion criteria
The exclusion criteria are lack of relevance, repetitive studies, and outdated articles.
Chambers, E. S. & Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 160 , 116–125 (2020).
Article CAS PubMed Google Scholar
Hsu, Y. C., Li, L. & Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 20 , 847–856 (2014).
Article CAS PubMed PubMed Central Google Scholar
Zhu, Z. et al. Exosomes derived from umbilical cord mesenchymal stem cells treat cutaneous nerve damage and promote wound healing. Front. Cell Neurosci. 16 , 913009 (2022).
Muiznieks, L. D. & Keeley, F. W. Molecular assembly and mechanical properties of the extracellular matrix: a fibrous protein perspective. Biochim. Biophys. Acta 1832 , 866–875 (2013).
Lee, H., Hong, Y. & Kim, M. Structural and functional changes and possible molecular mechanisms in aged skin. Int. J. Mol. Sci . 22 , 12489 (2021).
Driskell, R. R. et al. Defining dermal adipose tissue. Exp. Dermatol. 23 , 629–631 (2014).
Article PubMed PubMed Central Google Scholar
Mi, L. et al. The mechanism of stem cell aging. Stem Cell Rev. Rep. 18 , 1281–1293 (2022).
Mojallal, A. et al. Improvement of skin quality after fat grafting: clinical observation and an animal study. Plast. Reconstr. Surg. 124 , 765–774 (2009).
Owen, M. Marrow stromal stem cells. J. Cell Sci. Suppl. 10 , 63–76 (1988).
Caplan, A. I. Mesenchymal stem cells. J. Orthop. Res. 9 , 641–650 (1991).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8 , 315–317 (2006).
Caplan, A. I. Mesenchymal stem cells: time to change the name! Stem Cells Transl. Med. 6 , 1445–1451 (2017).
Viswanathan, S. et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 21 , 1019–1024 (2019).
Kong, P. et al. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem. Biophys. Res. Commun. 438 , 410–419 (2013).
Son, W. C., Yun, J. W. & Kim, B. H. Adipose-derived mesenchymal stem cells reduce MMP-1 expression in UV-irradiated human dermal fibroblasts: therapeutic potential in skin wrinkling. Biosci. Biotechnol. Biochem. 79 , 919–925 (2015).
Lai, P. et al. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD. J. Hematol. Oncol. 11 , 135 (2018).
De Gregorio, C. et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res. Ther. 11 , 168 (2020).
Lee, D. E., Ayoub, N. & Agrawal, D. K. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 7 , 37 (2016).
Koliada, A. K., Krasnenkov, D. S. & Vaiserman, A. M. Telomeric aging: mitotic clock or stress indicator? Front. Genet. 6 , 82 (2015).
Baxter, M. A. et al. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem cells 22 , 675–682 (2004).
Bahr, L. et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol. Blood Marrow Transplant. 18 , 557–564 (2012).
Article Google Scholar
Geissler, S. et al. Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PloS One 7 , e52700 (2012).
Li, T. et al. Human umbilical cord-derived mesenchymal stem cells ameliorate skin aging of nude mice through autophagy-mediated anti-senescent mechanism. Stem Cell Rev. Rep. 18 , 2088–2103 (2022).
Omar, R. et al. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng. Part B Rev. 20 , 523–544 (2014).
Article PubMed Google Scholar
Nagamura-Inoue, T. & He, H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J. Stem Cells 6 , 195–202 (2014).
Fatima, Q., Choudhry, N. & Choudhery, M. S. Umbilical cord tissue derived mesenchymal stem cells can differentiate into skin cells. Open Life Sci. 13 , 544–552 (2018).
Csekes, E. & Račková, L. Skin aging, cellular senescence and natural polyphenols. Int. J. Mol. Sci. 22 , 12641 (2021).
Makrantonaki, E., Zouboulis, C. C., William, J. & Cunliffe, M. Scientific awards. Characteristics and pathomechanisms of endogenously aged skin. Dermatology 214 , 352–360 (2007).
Moragas, A., Castells, C. & Sans, M. Mathematical morphologic analysis of aging-related epidermal changes. Anal. Quant. Cytol. Histol. 15 , 75–82 (1993).
CAS PubMed Google Scholar
Naylor, E. C., Watson, R. E. & Sherratt, M. J. Molecular aspects of skin ageing. Maturitas 69 , 249–256 (2011).
Krutmann, J., Schikowski, T., Morita, A. & Berneburg, M. Environmentally-induced (extrinsic) skin aging: exposomal factors and underlying mechanisms. J. Invest. Dermatol. 141 , 1096–1103 (2021).
Friedman, O. Changes associated with the aging face. Facial Plast. Surg. Clin. North Am. 13 , 371–380 (2005).
Kligman, L. H. Photoaging. Manifestations, prevention, and treatment. Clin. Geriatr. Med. 5 , 235–251 (1989).
Kammeyer, A. & Luiten, R. M. Oxidation events and skin aging. Ageing Res. Rev. 21 , 16–29 (2015).
Trenam, C. W., Blake, D. R. & Morris, C. J. Skin inflammation: reactive oxygen species and the role of iron. J. Invest. Dermatol. 99 , 675–682 (1992).
Pillai, S., Oresajo, C. & Hayward, J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation - a review. Int. J. Cosmet. Sci. 27 , 17–34 (2005).
Cavinato, M. et al. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 18 , 499–516 (2017).
Koh, E. K. et al. Protective effects of the antioxidant extract collected from Styela clava tunics on UV radiation‑induced skin aging in hairless mice. Int. J. Mol. Med. 38 , 1565–1577 (2016).
Ha, J. H. et al. Recombinant human acidic fibroblast growth factor (aFGF) expressed in nicotiana benthamiana potentially inhibits skin photoaging. Planta Med. 83 , 862–869 (2017).
Xu, Y. et al. Cell-free fat extract increases dermal thickness by enhancing angiogenesis and extracellular matrix production in nude mice. Aesthet. Surg. J. 40 , 904–913 (2020).
Chen, S., He, Z. & Xu, J. Application of adipose-derived stem cells in photoaging: basic science and literature review. Stem Cell Res. Ther. 11 , 491 (2020).
Chang, L., Fan, W., Pan, X. & Zhu, X. Stem cells to reverse aging. Chin. Med. J. 135 , 901–910 (2022).
Wu, Y. et al. Human umbilical cord-derived stem cell sheets improve left ventricular function in rat models of ischemic heart failure. Eur. J. Pharmacol. 925 , 174994 (2022).
Yang, S. et al. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res. Ther. 12 , 315 (2021).
Ridzuan, N. et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles ameliorate airway inflammation in a rat model of chronic obstructive pulmonary disease (COPD). Stem Cell Res. Ther. 12 , 54 (2021).
Lv, X. et al. Effects of single and multiple transplantations of human umbilical cord mesenchymal stem cells on the recovery of ovarian function in the treatment of premature ovarian failure in mice. J. Ovarian Res. 14 , 119 (2021).
Peyvandi, A. A. et al. Deferoxamine promotes mesenchymal stem cell homing in noise-induced injured cochlea through PI3K/AKT pathway. Cell Prolif. 51 , e12434 (2018).
Yuan, M. et al. Mesenchymal stem cell homing to improve therapeutic efficacy in liver disease. Stem Cell Res. Ther. 13 , 179 (2022).
Shin, M. J., Park, J. Y., Lee, D. H. & Khang, D. Stem cell mimicking nanoencapsulation for targeting arthritis. Int. J. Nanomed. 16 , 8485–8507 (2021).
Article CAS Google Scholar
Yin, Y. et al. The homing of human umbilical cord-derived mesenchymal stem cells and the subsequent modulation of macrophage polarization in type 2 diabetic mice. Int. Immunopharmacol. 60 , 235–245 (2018).
Zhang, S., Chen, L., Zhang, G. & Zhang, B. Umbilical cord-matrix stem cells induce the functional restoration of vascular endothelial cells and enhance skin wound healing in diabetic mice via the polarized macrophages. Stem Cell Res. Ther. 11 , 39 (2020).
Wells, J. M. & Watt, F. M. Diverse mechanisms for endogenous regeneration and repair in mammalian organs. Nature 557 , 322–328 (2018).
Meng, X., Sun, B. & Xiao, Z. Comparison in transcriptome and cytokine profiles of mesenchymal stem cells from human umbilical cord and cord blood. Gene 696 , 10–20 (2019).
Zhang, Y. et al. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Res. Ther. 12 , 434 (2021).
Dong, L. et al. A conditioned medium of umbilical cord mesenchymal stem cells overexpressing Wnt7a promotes wound repair and regeneration of hair follicles in mice. Stem Cells Int. 2017 , 3738071 (2017).
Uyar, B. et al. Single-cell analyses of aging, inflammation and senescence. Ageing Res. Rev. 64 , 101156 (2020).
Mohammed, S. et al. Necroptosis contributes to chronic inflammation and fibrosis in aging liver. Aging Cell 20 , e13512 (2021).
Mazini, L. et al. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int. J. Mol. Sci. 21 , 1306 (2020).
Bang, E., Kim, D. H. & Chung, H. Y. Protease-activated receptor 2 induces ROS-mediated inflammation through Akt-mediated NF-κB and FoxO6 modulation during skin photoaging. Redox Biol. 44 , 102022 (2021).
Markov, A. et al. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res. Ther. 12 , 192 (2021).
Kim, Y. J. et al. Effects of conditioned media from human umbilical cord blood-derived mesenchymal stem cells in the skin immune response. Biomed. Pharmacother. 131 , 110789 (2020).
Li, J. et al. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J. Inflamm. 9 , 33 (2012).
Liu, B. et al. Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-κB signaling pathway in vivo and in vitro. Stem Cell Res. Ther. 9 , 7 (2018).
Chen, Y. et al. Human umbilical cord-derived mesenchymal stem cells ameliorate psoriasis-like dermatitis by suppressing IL-17-producing γδ T cells. Cell Tissue Res. 388 , 549–563 (2022).
Lennicke, C. & Cochemé, H. M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 81 , 3691–3707 (2021).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24 , R453–R462 (2014).
Gu, Y., Han, J., Jiang, C. & Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 59 , 101036 (2020).
Nakamura, T., Naguro, I. & Ichijo, H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta Gen. Subj. 1863 , 1398–1409 (2019).
Theurey, P. & Pizzo, P. The aging mitochondria. Genes . 9 , 22 (2018).
Rinnerthaler, M. et al. Oxidative stress in aging human skin. Biomolecules 5 , 545–589 (2015).
Pittayapruek, P. et al. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci . 17 , 868 (2016).
Bell, S. et al. Involvement of NF-kappaB signalling in skin physiology and disease. Cell. Signal. 15 , 1–7 (2003).
Jomova, K. et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 97 , 2499–2574 (2023).
Perico, L. et al. Human mesenchymal stromal cells transplanted into mice stimulate renal tubular cells and enhance mitochondrial function. Nat. Commun. 8 , 983 (2017).
Ebrahimi, M. J. et al. Human umbilical cord matrix stem cells reverse oxidative stress-induced cell death and ameliorate motor function and striatal atrophy in rat model of Huntington disease. Neurotox. Res. 34 , 273–284 (2018).
Chen, Y. S., Wang, X. J., Feng, W. & Hua, K. Q. Advanced glycation end products decrease collagen I levels in fibroblasts from the vaginal wall of patients with POP via the RAGE, MAPK and NF-κB pathways. Int. J. Mol. Med. 40 , 987–998 (2017).
Nonaka, K. et al. Advanced glycation end-products increase IL-6 and ICAM-1 expression via RAGE, MAPK and NF-κB pathways in human gingival fibroblasts. J. Periodontal Res. 53 , 334–344 (2018).
Li, L. et al. The cytotoxicity of advanced glycation end products was attenuated by UCMSCs in human vaginal wall fibroblasts by inhibition of an inflammatory response and activation of PI3K/AKT/PTEN. Biosci. Trends 14 , 263–270 (2020).
Niu, Y. et al. Reduced dermis thickness and AGE accumulation in diabetic abdominal skin. Int. J. Low. Extrem. Wounds 11 , 224–230 (2012).
Tian, M. et al. The relationship between inflammation and impaired wound healing in a diabetic rat burn model. J. Burn Care Res. 37 , e115–e124 (2016).
Saheli, M. et al. Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Arch. Dermatol. Res. 312 , 325–336 (2020).
Kouroupis, D., Kaplan, L. D., Ricordi, C. & Best, T. M. Mesenchymal stem/stromal cell-derived small extracellular vesicles (MSC-sEVs): a promising treatment modality for diabetic foot ulcer. Bioengineering 10 , 1140 (2023).
Pomatto, M. et al. Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. Int. J. Mol. Sci. 22 , 3851 (2021).
Shrestha, C. et al. Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. Int. J. Endocrinol. 2013 , 592454 (2013).
Cheng, L. et al. Human umbilical cord mesenchymal stem cells for psoriasis: a phase 1/2a, single-arm study. Signal Transduct. Target. Ther. 7 , 263 (2022).
Fan, D. et al. Efficacy and safety of umbilical cord mesenchymal stem cells in treatment of cesarean section skin scars: a randomized clinical trial. Stem Cell Res. Ther. 11 , 244 (2020).
Alhaddad, M., Boen, M., Wu, D. C. & Goldman, M. P. Red deer umbilical cord lining mesenchymal stem cell extract cream for rejuvenation of the face. J. Drugs Dermatol. 18 , 363–366 (2019).
PubMed Google Scholar
Lee, S. E. et al. Intravenous allogeneic umbilical cord blood-derived mesenchymal stem cell therapy in recessive dystrophic epidermolysis bullosa patients. JCI Insight. 6 , e143606 (2021).
Kim, J. et al. The effect of human umbilical cord blood-derived mesenchymal stem cell media containing serum on recovery after laser treatment: a double-blinded, randomized, split-face controlled study. J. Cosmet. Dermatol. 19 , 651–656 (2020).
Kim, H. S. et al. Clinical trial of human umbilical cord blood-derived stem cells for the treatment of moderate-to-severe atopic dermatitis: phase I/IIa studies. Stem Cells 35 , 248–255 (2017).
Qin, H. L. et al. Clinical evaluation of human umbilical cord mesenchymal stem cell transplantation after angioplasty for diabetic foot l . Exp. Clin. Endocrinol. Diabetes. 124 , 497–503 (2016).
Hashemi, S. S. et al. The healing effect of Wharton’s jelly stem cells seeded on biological scaffold in chronic skin ulcers: a randomized clinical trial. J. Cosmet. Dermatol. 18 , 1961–1967 (2019).
Harn, H. J. et al. Rejuvenation of aged pig facial skin by transplanting allogeneic granulocyte colony-stimulating factor-induced peripheral blood stem cells from a young pig. Cell Transplant 22 , 755–765 (2013).
Eltony, S. A. & Ali, S. S. Histological study on the effect of nicotine on adult male guinea pig thin skin. Anat. Cell Biol. 50 , 187–199 (2017).
Wang, P. W. et al. Red raspberry extract protects the skin against UVB-induced damage with antioxidative and anti-inflammatory properties. Oxid. Med. Cell. Longev. 2019 , 9529676 (2019).
PubMed PubMed Central Google Scholar
Kimura, T. & Doi, K. Depigmentation and rejuvenation effects of kinetin on the aged skin of hairless descendants of Mexican hairless dogs. Rejuvenation Res. 7 , 32–39 (2004).
Liu, Y. et al. The effects of HSP27 against UVB-induced photoaging in rat skin. Biochem. Biophys. Res. Commun. 512 , 435–440 (2019).
Chung, M. G. et al. Bitter taste receptors protect against skin aging by inhibiting cellular senescence and enhancing wound healing. Nutr. Res. Pract. 16 , 1–13 (2022).
Ma, T. et al. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J. Cell. Biochem. 120 , 10847–10854 (2019).
Wedel, S. et al. tBHP treatment as a model for cellular senescence and pollution-induced skin aging. Mech. Ageing Dev. 190 , 111318 (2020).
Wu, Y. et al. Protective and anti-aging effects of 5 cosmeceutical peptide mixtures on hydrogen peroxide-induced premature senescence in human skin fibroblasts. Ski. Pharm. Physiol. 34 , 194–202 (2021).
Warnon, C. et al. HDAC2 and 7 down-regulation induces senescence in dermal fibroblasts. Aging 13 , 17978–18005 (2021).
Liu, Z. et al. Human umbilical cord mesenchymal stem cells improve irradiation-induced skin ulcers healing of rat models. Biomed. Pharmacother. 101 , 729–736 (2018).
Yang, Y. et al. Human umbilical cord mesenchymal stem cells ameliorate skin fibrosis development in a mouse model of bleomycin-induced systemic sclerosis. Exp. Ther. Med. 20 , 257 (2020).
Shi, R. et al. Role and effect of vein-transplanted human umbilical cord mesenchymal stem cells in the repair of diabetic foot ulcers in rats. Acta Biochim. Biophys. Sin. 52 , 620–630 (2020).
Wu, P. et al. HucMSC exosome-delivered 14-3-3ζ alleviates ultraviolet radiation-induced photodamage via SIRT1 pathway modulation. Aging 13 , 11542–11563 (2021).
Vu, D. M. et al. Effects of extracellular vesicles secreted by TGFβ-stimulated umbilical cord mesenchymal stem cells on skin fibroblasts by promoting fibroblast migration and ECM protein production. Biomedicines 10 , 1810 (2022).
Kim, Y. J. et al. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem. Biophys. Rep. 16 , 96–102 (2018).
Download references
Acknowledgements
This work was supported by grants from the Project of Yunnan Province Key projects (202301AY070001-034). The project entitled “Transformation of subtotipotent stem cells based on the tree shrew model of multiple organ dysfunction syndrome” (SYDW [2020]19). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. Figure 1 was created with licensed Biorender.com online website.
Author information
These authors contributed equally: Le Chang, Wei-Wen Fan, He-Ling Yuan, Xin Liu.
Authors and Affiliations
The Basic Medical Laboratory of the 920th Hospital of Joint Logistics Support Force of PLA, The Transfer Medicine Key Laboratory of Cell Therapy Technology of Yunan Province, The Integrated Engineering Research Center of Cell Biological Medicine of State and Regions, Kunming, 650032, Yunnan Province, China
Le Chang, Wei-Wen Fan, He-Ling Yuan, Xin Liu, Qiang Wang, Guang-Ping Ruan, Xing-Hua Pan & Xiang-Qing Zhu
You can also search for this author in PubMed Google Scholar
Contributions
X.-Q.Z. and L.C. conceptualize the content and wrote the paper. All authors (X.-Q.Z., L.C., W. -W.F., X.L., H.-L.Y., Q.W., G.-P.R. and X.-H.P) made substantial contributions to the conception of the project and provided critical review of the final document.
Corresponding authors
Correspondence to Xing-Hua Pan or Xiang-Qing Zhu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Chang, L., Fan, WW., Yuan, HL. et al. Role of umbilical cord mesenchymal stromal cells in skin rejuvenation. npj Regen Med 9 , 20 (2024). https://doi.org/10.1038/s41536-024-00363-1
Download citation
Received : 21 September 2023
Accepted : 26 April 2024
Published : 10 May 2024
DOI : https://doi.org/10.1038/s41536-024-00363-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
JavaScriptが無効になっています。 JavaScriptが有効になっていない場合、当ウェブサイトは正常に機能しません。 すべての機能を利用するためには、有効に設定してください。
About Top Page
Message from the President
Philosophy, Vision and Policy
- Philosophy, Vision and Policy Top Page
- Philosophy and Vision
- Basic Policy
- Education Vision
- Research Vision
- Social Liaison Vision
- International Exchange Vision
- Code of Conduct
- Our Policies
- Organization
- History and Tradition
Publications
Maps and Directions
- Maps and Directions Top Page
- Matsumoto Campus
- Nagano Education Campus
- Nagano-Engineering Campus
- Ueda Campus
- LIFE at Shinshu
Sustainability
- Sustainability Top Page
- At a Glance
- Green Campus
Admissions Top Page
Undergraduate (Japanese site only)
- Graduate Schools
Admission Policy
Research Students
Japanese Government Scholarship Students
Exchange Students
Admission FAQ
Guide to Studying at Shinshu University
- Academics Top Page
- Education Vision and Policy
- Academics Topics
- Undergraduate
- Unique Programs of Study
- Campus Life at Shinshu University
- Interviews with Graduates
Research Top Page
Research Vision and Policy
Research Topics
Interdisciplinary Research
Featured Research Projects
Researcher Interviews
Find Research
Administrative Organizations
Outreach Top Page
Social Liaison Vision and Policy
Outreach Topics
Industrial Liaison and Social Engagement Organization
- Outreach Projects
Centers and Institutes
Collaborate
Collaborate Top Page
Collaborate Topics
Shinshu University Online System of General Academic Resources
- Researchers & Corporations
- Prospective Students
- Current Students
- Site Policy
Using Stem Cell-Derived Heart Muscle Cells to Advance Heart Regenerative Therapy
- Send to email
Researchers showcase an innovative strategy for regenerative heart therapy in a primate model, paving the way to clinical trials
Regenerative heart therapies involve transplanting cardiac muscle cells into damaged areas of the heart to recover lost function. However, the risk of arrhythmias following this procedure is reportedly high. In a recent study, researchers from Japan tested a novel approach that involves injecting ‘cardiac spheroids,’ cultured from human stem cells, directly into damaged ventricles. The highly positive outcomes observed in primate models highlight the potential of this strategy.
Image title: A new protocol for regenerative heart therapy Image caption: Study shows that cardiac spheroids, derived from human induced pluripotent stem cells, can be easily transported and injected into damaged areas of the heart to promote its regeneration and recovery of function. Image credit: Hideki Kobayashi from Shinshu University License type: Original content Usage restrictions: Cannot be reused without permission.
Cardiovascular diseases are still among the top causes of death worldwide, and especially prevalent in developed countries. Myocardial infarctions, commonly known as “heart attacks,” are on the rise, resulting in a significant number of deaths each year.
Heart attacks typically kill millions of cardiac muscle cells, leaving the heart in a weakened state. Since mammals cannot regenerate cardiac muscle cells on their own, heart transplants are currently the only clinically viable option for patients suffering (or likely to suffer) heart failure. Given that full heart transplants are expensive and donors difficult to come by, it is no surprise that alternative therapies are highly sought after by the medical community.
One promising strategy that has been steadily gaining traction is using human induced pluripotent stem cells (HiPSCs) for regenerative heart therapy. Simply put, HiPSCs are cells derived from mature cells that can be effectively ‘reprogrammed’ into a completely different cell type, such as cardiac muscle cells (cardiomyocytes). By transplanting or injecting cardiomyocytes derived from HiPSCs into damaged areas of the heart, it is possible to recover some lost functionality. Unfortunately, studies have reported that this approach can increase the risk of arrythmias, posing a major hurdle to clinical trials.
In a recent study, a Japanese research team from Shinshu University and Keio University School of Medicine, tested a new strategy for regenerative heart therapy that involves injecting ‘cardiac spheroids’ derived from HiPSCs into monkeys with myocardial infarction. This study, published on April 26, 2024, in the journal Circulation , was led by Professor Yuji Shiba from the Department of Regenerative Science and Medicine, Shinshu University.
The team included Hideki Kobayashi, the first author, and Koichiro Kuwahara from the Department of Cardiovascular Medicine, Shinshu University School of Medicine, as well as Shugo Tohyama, and Keiichi Fukuda from the Department of Cardiology, Keio University School of Medicine, among others.
In their novel approach, the researchers cultivated HiPSCs in a medium that led to their differentiation into cardiomyocytes. After carefully extracting and purifying cardiac spheroids (three-dimensional clusters of cardiac cells) from the cultures, they injected approximately 6 × 10 7 cells into the damaged hearts of crab-eating macaques ( Macaca fascicularis ). They monitored the condition of the animals for twelve weeks, taking regular measurements of cardiac function. Following this, they analyzed the monkeys’ hearts at the tissue level to assess whether cardiac spheroids could regenerate the damaged heart muscles.
First, the team verified the correct reprogramming of HiPSCs into cardiomyocytes. They observed, via cellular-level electrical measurements, that the cultured cells exhibited potential patterns typical of ventricular cells. The cells also responded as expected to various known drugs. Most importantly, they found that the cells abundantly expressed adhesive proteins such as connexin 43 and N-cadherin, which would promote their vascular integration into an existing heart.
Afterwards, the cells were transported from the production facility at Keio University to Shinshu University, located 230 km away. The cardiac spheroids, which were preserved at 4 °C in standard containers, withstood the four-hour journey without problem. This means that no extreme cryogenic measures would be needed when transporting the cells to clinics, which would make the proposed approach less expensive and easier to adopt.
Finally, the monkeys received injections of either cardiac spheroids or a placebo directly into the damaged heart ventricle. During the observation period, the researchers noted that arrythmias were very uncommon, with only two individuals experiencing transient tachycardia (fast pulse) in the first two weeks among the treatment group. Through echocardiography and computed tomography exams, the team confirmed that the hearts of monkeys that received treatment had better left ventricular ejection after four weeks compared to the control group, indicating a superior blood pumping capability.
Histological analysis ultimately revealed that the cardiac grafts were mature and properly connected to pre-existing existing tissue, cementing the results of previous observations. “ HiPSC-derived cardiac spheroids could potentially serve as an optimal form of cardiomyocyte products for heart regeneration, given their straightforward generation process and effectiveness ,” remarks Assistant Professor Kobayashi. “ We believe that the results of this research will help solve the major issue of ventricular arrhythmia that occurs after cell transplantation and will greatly accelerate the realization of cardiac regenerative therapy , ” he further adds.
Although tested in monkeys, it is worth noting that the cardiac spheroid production protocol used in this study was designed for clinical application in humans. “ The favorable results obtained thus far are sufficient to provide a green light for our clinical trial, called the LAPiS trial. We are already employing the same cardiac spheroids on patients with ischemic cardiomyopathy , ” comments Asst. Prof. Kobayashi.
Let us all hope for a resounding success in the LAPiS trial, paving the way for expanded and effective treatment avenues for people suffering from heart problems.
Related SDGs

Related Topics
SWCNT ropes with polyurethane show high energy density
It's in the Blood: Donor Diets Can Trigger Allergic Reactions in Blood Recipients
Unveiling Inaoside A: An Antioxidant Derived from Mushrooms

IMAGES
VIDEO
COMMENTS
In recent years, hADSCs, including human amniotic epithelial stem cells (hAESCs) and human amniotic mesenchymal stem cells (hAMSCs) have been attractive cell sources for clinical trials and medical research, and have been shown to have advantages over other stem cells types. 35,37 These advantages include low immunogenicity and high ...
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation.
The discovery of hPSCs, including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), has revolutionized stem cell research and cell-based therapy. 98 hESCs were ...
Interview with Dr. Helen Blau on stem cells in the treatment of disease. 9m 12s Download. The derivation of induced pluripotent stem cells (iPSCs) has revolutionized stem-cell research (see the ...
Research Open Access 09 May 2024 Nature ... Hamidzada et al. show that human pluripotent stem cell-derived macrophages are educated into a tissue-resident fate within human cardiac microtissues ...
Introduction. Stem cells have the capacity to divide and give rise to identical daughter stem cells (symmetrical division) or to differentiate into specific cells of somatic tissues (asymmetrical division). 1 Commonly, stem cells are derived from two main sources: (i) early embryos (embryonic stem cells, ESCs) and (ii) adult tissue (adult stem cells, ASCs).
Stem-cell research articles from across Nature Portfolio. Stem-cell research is the area of research that studies the properties of stem cells and their potential use in medicine. As stem cells ...
Research in human embryonic stem cells (hESCs) is a rapidly developing scientific field. ... We found a total of 315 research papers describing derivation and/or experimental use of hESC lines that had been published (including online publication ahead of print) through December 31, 2005. Most of these research papers came from groups in the ...
Ethics of Stem Cell Research. First published Fri Apr 25, 2008; substantive revision Wed Dec 19, 2018. Human embryonic stem cell (HESC) research offers much hope for alleviating the human suffering brought on by the ravages of disease and injury. HESCs are characterized by their capacity for self-renewal and their ability to differentiate into ...
Stem cells are increasingly being used to model human development and disease in the form of self-organizing embryo models, brain organoids, and neurological chimeras. These new research directions are resurrecting old embryo debates around moral status and personhood. Hyun considers how these old questions are tackled in these new contexts.
MS: Proponents argue that embryonic stem cell research holds great promise for understanding and curing diabetes, Parkinson's disease, spinal cord injury, and other debilitating conditions. Opponents argue that the research is unethical, because deriving the stem cells destroys the blastocyst, an unimplanted human embryo at the sixth to ...
This article highlights a recent document developed by the International Society for Stem Cell Research (ISSCR) that provides recommendations, including reporting criteria, for scientists using human stem cells in basic research laboratories. These criteria are designed to be technically and financially feasible and, when implemented, enhance the reproducibility and rigor of stem cell research.
Stem-Cell Aging and Pathways to Precancer Evolution. C.H. Jamieson and I.L. WeissmanN Engl J Med 2023;389:1310-1319. Stem cells can renew themselves without differentiating. Aging, inflammation ...
Ethical Dilemmas and Stem Cell Research. Harold T. Shapiro Authors Info & Affiliations. Science. 24 Sep 1999. Vol 285, Issue 5436. p. 2065. DOI: 10.1126/science.285.5436.2065. eLetters (0) S cientific progress is both planned and spontaneous, a science and an art, and is always subject to social, political, and cultural forces.
Stem Cell Research is dedicated to publishing high-quality manuscripts focusing on the biology and applications of stem cell research. Submissions to Stem Cell Research, may cover all aspects of stem cells, including embryonic stem cells, tissue-specific stem cells, cancer stem cells, developmental …. View full aims & scope.
The Ethical Implications of Human Cloning. and on embryos created for research (whether natural or cloned) are morally on a par.This conclusion can be accepted by people who hold very different views about the moral status of the embryo. If cloning for stem cell research violates the respect the embryo is due,then so does stem cell research on ...
0. CHICAGO — Early results from a first-in-human trial some 20 years in the making suggest that neural stem cell transplantation is safe and improves motor function starting at 1 month after ...
Human mesenchymal stem cells (hMSCs) are multipotent cells and an attractive therapeutic agent in regenerative medicine and intensive clinical research. Despite the great potential, the limitation that needs to be overcome is the necessity of ex vivo expansion because of insufficient number of hMSCs presented within adult organs and the high ...
It is demonstrated that low inoculation density promoted endothelial-to-hematopoietic transition, followed by improved hematopoietic progenitor formation and erythrocyte generation, and the low cell culture density will improve RBC generation from human PSCs. Human pluripotent stem cell (hPSC)-derived red blood cells (RBCs) possess great potential for compensating shortages in transfusion ...
The objective of the current study is to develop a new method for tracking transplanted human induced pluripotent stem cells-derived cardiomyocytes (hiPSC-CMs) using magnetic resonance imaging (MRI). The CRISPR/dCas9 activation system is employed to overexpress ferritin heavy chain (FHC) in hiPSC-CMs.
[Figure 1. Schematic of novel stem cell therapy with metabolic glycoengineering (MGE) for brain repair after cardiac arrest (CA). Conditions were characterized and optimized in vitro to modify human neural stem cells by using the TProp sugar analog (Ac 5 ManNTProp). The efficacy of the transplanted metabolically glycoengineered neural stem cells (MGE-NSC) for brain repair and how TProp analog ...
Chen, Y. et al. Human umbilical cord-derived mesenchymal stem cells ameliorate psoriasis-like dermatitis by suppressing IL-17-producing γδ T cells. Cell Tissue Res. 388 , 549-563 (2022).
One promising strategy that has been steadily gaining traction is using human induced pluripotent stem cells (HiPSCs) for regenerative heart therapy. Simply put, HiPSCs are cells derived from mature cells that can be effectively 'reprogrammed' into a completely different cell type, such as cardiac muscle cells (cardiomyocytes).
Human exceptionalism has a strong lineage in stem cell ethics. Research with human embryos is often governed by a 'principle of subsidiarity', such that human embryo research should proceed only if there are no alternative ways of achieving one's scientific objectives that are less ethically contentious.16 Indeed, this was the view of the ...