An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMJ Open Access


Variation in use and outcomes related to midline catheters: results from a multicentre pilot study
Vineet chopra.
1 The Division of Hospital Medicine, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, Michigan, USA
2 Patient Safety Enhancement Program and Center for Clinical Management Research, VA Ann Arbor Health Care System, Ann Arbor, Michigan, USA
3 The Michigan Hospital Medicine Safety Consortium, Ann Arbor, Michigan, USA
Scott Kaatz
4 Department of Internal Medicine, Henry Ford Health System, Detroit, Michigan, USA
Lakshmi Swaminathan
5 Department of Internal Medicine, Beaumont Hospital, Dearborn, Michigan, USA
Tanya Boldenow
6 St. Josephs Health Center, Ypsilanti, Michigan, USA
Ashley Snyder
Rachel burris, steve j bernstein.
7 The Division of General Medicine, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, Michigan, USA
Scott Flanders
Associated data.
bmjqs-2018-008554supp001.pdf
While midline vascular catheters are gaining popularity in clinical practice, patterns of use and outcomes related to these devices are not well known.
Trained abstractors collected data from medical records of hospitalised patients who received midline catheters in 12 hospitals. Device characteristics, patterns of use and outcomes were assessed at device removal or at 30 days. Rates of major (upper-extremity deep vein thrombosis [DVT], bloodstream infection [BSI] and catheter occlusion) and minor complications were assessed. χ 2 tests were used to examine differences in rates of complication by number of lumens, reasons for catheter removal l, and hospital-level differences in rates of midline use.
Complete data on 1161 midlines representing 5%–72% of all midlines placed in participating hospitals between 1 January 2017 and 1 March 2018 were available. Most (70.8%) midlines were placed in general ward settings for difficult intravenous access (61.4%). The median dwell time of midlines across hospitals was 6 days; almost half (49%) were removed within 5 days of insertion. A major or minor complication occurred in 10.3% of midlines, with minor complications such as dislodgement, leaking and infiltration accounting for 71% of all adverse events. While rates of major complications including occlusion, upper-extremity DVT and BSI were low (2.2%, 1.4% and 0.3%, respectively), they were just as likely to lead to midline removal as minor complications (53.8% vs 52.5%, p=0.90). Across hospitals, absolute volume of midlines placed varied from 100 to 1837 devices, with corresponding utilisation rates of 0.97%–12.92% (p<0.001).
Midline use and outcomes vary widely across hospitals. Although rates of major complications are low, device removal as a result of adverse events is common.
Introduction
First introduced in the 1950s, midline catheters are unique vascular access devices often referred to as ‘middle ground’ intravenous catheters. 1 Like peripherally inserted central catheters (PICCs), midlines are inserted in the peripheral veins of the upper extremity; however, unlike PICCs, midlines terminate in the peripheral, not the central veins. By definition, the tip of the midline catheter should be located at or near the level of the axilla, distal to the shoulder. 2 Because they are not central venous catheters, midlines cannot lead to central line-associated bloodstream infection (CLABSI), resulting in many hospitals preferentially using to these devices to avoid CLABSI and associated financial penalties. 3 4 Additionally, as midlines are longer than traditional peripheral intravenous catheters and reach the deeper veins of the arm, they are potentially able to dwell longer than standard peripheral intravenous catheters. For these reasons, they offer a convenient alternative to PICCs for certain indications. 5
With growing reports regarding overuse of PICCs and the risk of potentially avoidable complications, 6 7 renewed interest in the use of midlines has emerged. However, midline devices are not homogeneous. Rather, they vary in composition (eg, polyurethane vs silicone), configuration (single vs dual lumen), gauge (4 and 5 French) and insertion technique. Consequently, substantial variation regarding safety and outcomes of midlines exists. For example, in a single-centre retrospective study spanning 1538 midlines, dwell times ranged from 12 to 27 days (median 26) and occlusion was a common complication (1.44 events per 1000 midline days). 8 In another study comparing outcomes between PICCs and midlines, patients who received midlines were observed to experience more complications than those with PICCs (19.5% vs 5.8%, p<0.001), with no difference in rates of severe complications. 9 In contrast, a third quasi-experimental study found that introduction of appropriateness criteria to guide the selection of midlines versus PICCs led to a reduction in hospital PICC use, rates of upper-extremity thrombosis and catheter occlusion with no major adverse events from midlines. 10 Importantly, these studies all represent single-centre experiences. Little is known about real-world use and variation of outcomes related to midlines within and across hospitals. Given this gap, we conducted a pilot study to examine use, variation and outcomes related to midline catheters in hospitals across the state of Michigan.
Study setting and design
This multicentre, prospective cohort study was conducted using data from a 48-hospital collaborative quality initiative supported by Blue Cross Blue Shield of Michigan and Blue Care Network. The design and setting of this consortium have been previously described. 11–13 In brief, adult patients admitted to a general medicine ward or intensive care unit of a participating hospital who received a midline device as part of their clinical care were eligible for inclusion. Patients (1) under the age of 18, (2) pregnant, (3) admitted to a non-medicine service (eg, general surgery) or (4) admitted under observation status were excluded.
A pilot study to evaluate midline catheter use and outcomes at participating hospitals was launched in July 2017. Twelve hospitals that represented diverse geographical regions, varying bed size and varying volume of midline utilisation were selected to participate in the pilot. At each hospital, dedicated, trained medical record abstractors used a defined protocol to collect clinical data directly from the medical records of patients who received midlines. Based on finite resources for abstractors and the time required to collect data from each case, hospitals were asked to collect data on six midline cases that met the eligibility criteria every 2 weeks (abstraction cycle). Hospitals used a combination of electronic medical records (insertion notes and billing data) and records from inserters (interventional radiology and vascular access teams) to identify eligible patients within each abstraction cycle. Inclusion and exclusion criteria were then applied to each patient on this list and data on the first six eligible cases were collected. To prevent sampling bias based on the day of placement, abstractors sorted cases by date of insertion or medical record number, and the first midline placed each day was abstracted. This process of identifying and abstracting data from eligible patients appropriate was repeated every 2 weeks (see online supplementary Appendix for protocol). All patients were prospectively followed until midline removal, death or at 30 days (whichever occurred first). If a patient was discharged with a midline in place or if the status of the midline was not clear at the time of discharge, patients were contacted by phone to determine the presence of the device and complications.
Supplementary data
Covariates and definitions.
Midlines were defined as vascular access devices inserted in the veins of the upper extremity that terminated in the brachial, basilic or cephalic veins of the arm at or near the axillary line. Thus, short peripheral intravenous catheters and PICCs were excluded from this pilot. However, a PICC placed at or trimmed to a ‘midline’ position was included. Medical diagnoses, history, physical findings, medications and baseline laboratory values at the time of midline placement were abstracted directly from patient medical records. Data regarding midline insertion (eg, indication for use, vein/arm of insertion, gauge, lumens) were obtained directly from the vascular nursing or interventional radiology note or from the physician order for midline placement. In cases where multiple indications for midline use were listed, the primary indication for midline insertion was selected according to a predefined hierarchical structure (eg, difficult intravenous access 1, antibiotics 2, intravenous fluids 3, unknown 4).
Clinical outcomes
The main outcome of interest included the proportion of midlines that experienced a major or minor complication. Major complications were defined as bloodstream infection related to the midline catheter (captured using a standardised definition from the National Healthcare Safety Network), 14 symptomatic upper-extremity deep vein thrombosis (DVT) (defined as performance of a compression or duplex ultrasound for arm symptoms with visible thrombus or non-compressibility of the vein) and catheter occlusion (defined as inability to aspirate or flush, or ‘sluggish’, ‘slow’ and ‘poor’ flow documented in the medical record). Minor complications were defined using standardised definitions and included mechanical events (accidental dislodgement, leaking from the exit site and thrombophlebitis), infiltration of infusate outside of a vein and superficial thrombophlebitis. 2 For this analysis, data from patients enrolled during the pilot period between January 2017 and March 2018 were included.
Statistical analyses
Indication for midline insertion, dwell time, device characteristics and complications were tabulated using descriptive statistics. Comparisons between minor and major complications and by number of device lumens were made using χ 2 tests. In addition to device complications, the rates of midline use (midline utilisation rate) for each hospital were estimated by expressing the proportion of midlines placed in adult non-surgical patients to the total number of non-surgical adult discharges during the same period. This statistic was useful to assess how often midlines were placed relative to patient volume. Associations between patient factors (severity of illness as defined by the Charlson-Deyo Score), hospital factors (bed size, hospital volume) and rates of midline utilisation by site were assessed using Pearson’s correlation. All statistical tests were two-sided, with p<0.05 considered statistically significant. All analyses were conducted using Stata MP/SE V.15.
Complete data on 1161 midlines placed in 12 hospitals were available and included in this analysis. The available sample of midlines represented 5%–72% of all devices placed in these hospitals during the study period ( table 1 ). Most midlines were placed in general ward settings (n=822, 70.8%); however, 24.2% (n=281) were placed in critical care units and 5.0% (n=58) in ‘other’ settings (eg, emergency room). The majority of midlines were placed by vascular access nurses at the patients bedside (n=1036, 89.2%). The documented indication for 61.4% of midlines (n=713) was difficult intravenous access; however, intravenous antibiotics represented approximately one-third of all placement indications (n=318, 27.4%). Consultation with infectious diseases for placement of midlines for intravenous antibiotics occurred for less than half of all midlines placed for this indication (46.2% [12.7% of all midlines]), suggesting a potential opportunity for device and antibiotic stewardship. Almost a quarter (23.9%, n=278) of patients had more than one documented indication for midline placement, while the indication for midline insertion was unknown (ie, none documented in medical records or procedure notes) in 16.1% of cases.
Pilot hospital characteristics and midline utilisation data, January 2017–March 2018
LOS refers to the average length of stay for patients admitted to that hospital across all services.
Midline utilisation rate=total number of midlines placed per year in adult non-surgical patients/number of adult non-surgical patients discharged per year.
The right arm was more often used for midline placement (n=597, 51.4%). The basilic (n=487, 42.0%) and brachial (n=469, 40.4%) veins were most commonly used for insertion. Single-lumen devices represented almost half of all midlines (46.0%). Ultrasound guidance was documented for placement in 80.6% of midline devices.
The median dwell time of midlines across hospitals was 6 days (IQR: 3–12 days), with almost half (n=569, 49.0%) removed within 5 days of insertion. The most common reasons for removal within 5 days (67.8% of 5-day midline dwells) were completion of therapy and patient discharge. A total of 13.5% of midlines remained in place beyond 30 days ( figure 1a ). Most patients (n=994, 85.6%) who received a midline did not have a PICC or central venous catheter placed within the past 3 months, suggesting that midlines were not used as ‘step-down’ device from more invasive lines. Informed consent for midline catheter placement was obtained in 36.2% (n=420) of patients.

(A) Median midline dwell time and (B) indication (by site). The vertical bars in Panel (A) show range of catheter dwell (in days).
Device complications
A major or minor complication occurred in 10.3% of midline insertions ( table 2 ). Minor complications accounted for the majority of adverse events (66.7% of all complications). In order of frequency, minor complications included accidental dislodgement (3.8%, n=44), leaking from the exit site (2.2%, n=26), catheter infiltration (n=7, 0.6%) and superficial thrombophlebitis (n=4, 0.3%). Although major complications accounted for a third of all adverse events, the most frequent major complication was catheter occlusion (2.2%, n=26). Rates of symptomatic upper-extremity DVT and bloodstream infection were low across patients (1.4% [n=16] and 0.3% [n=4], respectively). Rates of midline removal were similar among patients who developed major or minor complications (53.8% vs 52.5%, p=0.90). With respect to the number of lumens, single-lumen midline catheters appeared to have lower rates of complications than multilumen devices (single-lumen=9.0%, multilumen=12.8%; p=0.23). Among patients with midlines placed for <5 days, 12.7% (n=72) experienced a complication and 7.2% (n=41) had their device removed due to this complication.
Rates of midline complications across pilot hospitals, January 2017–March 2018
Midline utilisation rate=total number of midlines placed per year/number of patient discharges per year.
DVT, deep vein thrombosis.
Variation in use and outcomes of midlines across hospitals
The absolute volume of midline use varied across hospitals, ranging from 100 to 1837 devices, with corresponding midline utilisation rates of 0.97%–12.92% (p<0.001). Overall, the sample of devices in this study represented 5%–72% of all midlines placed at individual hospitals; thus, some hospitals used very few midlines, while others used more. Variation in midline utilisation rate across hospitals was not associated with the severity of patient illness by Charlson-Deyo Score (r=−0.20, p=0.540), or by hospital-level factors such as patient volume (r=−0.30, p=0.337) or bed size (−0.37, p=0.231). Additionally, dwell time and indications for midline use varied across hospitals ( figure 1A,B ). For example, placement of midlines for the indication of difficult venous access varied from 5.2% to 87.8% across sites (p<0.001). Similarly, the frequency of midline complications varied from 3.4% to 16.7% across hospitals (p=0.07) ( table 2 ). Notably, patterns of complications also differed across hospitals. For example, three hospitals reported no upper-extremity DVT events from midlines. Among the nine hospitals that did report DVT, incidence varied from 0.7% to 3.3% of patients who received midlines. Similarly, although accidental dislodgement was the most prevalent minor complication, rates for this event ranged from 1.1% to 6.1% across sites. No differences in rates of complications by device manufacturer were observed (p=0.68).
As hospitals seek to improve appropriate use of PICCs to avoid adverse events such as DVT and financial penalties associated with CLABSI, interest in use of midline devices has emerged. 15 Despite growing use of these devices, a paucity of epidemiological data regarding patterns of use and infectious and non-infectious outcomes exists. In this multihospital pilot study spanning twelve sites and 1161 midline insertions, we found that midlines were most often placed for the indications of difficult intravenous access and antibiotic therapy. Almost half of midlines placed were removed within 5 days of insertion after having met the indication they were placed for, with few of these shorter dwells (7%) removed for complications. Among patients who did experience a complication, minor complications were more common than major events. However, both types of complications often required midline removal, suggesting that minor complications are not inconsequential from a vascular access or patient perspective. Like prior work with PICCs, 12 we found substantial variation in patterns of use and outcomes from midlines across hospitals. Collectively, these data suggest that guidance to inform and improve use of midlines is needed in order to ensure patient safety.
Although rates of complications with midlines were low, significant variation in both the nature and types of adverse events across hospitals was observed. Importantly, midlines appear to be more often associated with minor than major complications, with low rates of DVT and bloodstream infection noted across hospitals. These observations are congruent with recent studies and suggest that midlines might be a ‘safer’ alternative to PICCs with respect to these outcomes. 8 9 Given their lower rate of potentially lethal events such as DVT and CLABSI, midlines appear to have an important place for hospitalised patients, especially for those who need short-term venous access. Compared with prior studies that found substantially greater rates of complications, 16 our data suggest that the complication profile of midlines has improved perhaps due to newer catheter materials or ultrasound to guide insertion.
Despite these assurances, when and how midlines should be used to minimise risk and ensure benefit remain an important question. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) provides criteria for when use of a midline versus a PICC is appropriate for hospitalised and critically ill patients and patients with cancer. 17 18 Additionally, MAGIC provides guidance on what types of infusions are appropriate for a midline device—a key component of mitigating harm from these devices. As has been the case with PICCs, 10 using MAGIC as a framework to understand and improve midline appropriateness and patient outcomes may be valuable in improving patient safety.
We observed that most midlines were placed for the indication of difficult venous access, suggesting that hospitals are turning to this device when attempts to cannulate peripheral veins fail. As midlines are longer in length, placed under sterile conditions and usually placed by ultrasound, they reach the deeper veins of the arm to secure more durable access. However, variation in indication for placement and rates of midline dwell were observed across hospitals. Thus, how best to use, insert and maintain midlines to ensure catheter dwell matches clinical need remains an important outstanding question. Studies that focus on insertion parameters related to midlines, including use of ultrasound, length of catheter within the vein and catheter-to-vein ratio, are thus needed as they may help prolong dwell. Similarly, identifying optimal care and maintenance techniques including what should be adminstered via a peripheral device such as a midline, volume, type and frequency of flush and optimal securement strategies to prevent dislodgement also appear necessary. 19 These considerations represent important questions for future research and are needed to fully realise the potential of these devices.
Our study has limitations. First, we collected data from the electronic medical records of hospitalised patients via trained data abstractors; data or events that were not documented or occurred after hospitalisation and were not reported by patients during phone conversations may be missed using this method. Second, we sampled patients at hospitals in order to understand patterns and outcome of midline use. Differences between our study sample and those from the larger population of patients who received midlines may affect our findings. Third, while we did not find an association between hospital-level and patient-level factors related to midline use or outcome, it is possible that we were underpowered for this effect given the pilot nature of this study. Fourth, complications with midlines (including major complications such as infection and DVT) may be under-reported or under-recognised as these are peripheral intravenous devices. Furthermore, variability in DVT rates between hospitals may also reflect variability in diagnostic testing thresholds, which we were unable to assess. However, our use of medical record documentation (including laboratory and imaging data) rather than administratively coded data helps to mitigate this risk. Finally, outcomes from midlines may vary based on materials, catheter design, insertion techniques, and infusates which we did not collect in this pilot study. Future iterations of this work will seek to incorporate these aspects.
Our study also has important strengths. To our knowledge, this is the first and largest multihospital study to date to examine indications, patterns of use and outcomes for midline catheters. As use of these devices expands, understanding best practices through epidemiological studies such as ours will become more necessary. Second, we report several new findings including the fact that the number of midline lumens appears associated with the rates of complications. These findings have important implications for clinical decision-making and patient safety. Third, substantial variation in hospital event rates related to midline devices appears to exist. Using a conceptual framework of patient, provider and device factors to understand complications thus appears to be just as important for midlines as it was for PICCs. 20 Future studies should now begin to examine these factors and consider strategies through which to prolong midline dwell so as to ensure best outcomes.
In conclusion, substantial variation in the rates of use and outcomes from midline catheters were observed in this multihospital study. Given lower risk of major complications, we suspect the use of midline catheters over PICCs will continue to expand. Studies to better define appropriate indications for use, optimal insertion technique, and best care and maintenance practices to ensure longevity of these devices are needed to ensure patient safety.
Contributors: All authors participated equally in the design, conception, writing, analysis, drafting and final approval of the paper. VC and AS had access to the data and vouch for the integrity of the findings.
Funding: Support for the Michigan Hospital Medicine Safety (HMS) Consortium is provided by Blue Cross and Blue Shield of Michigan (BCBSM) and Blue Care Network as part of the BCBSM Value Partnerships program. Although Blue Cross Blue Shield of Michigan and HMS work collaboratively, the opinions, beliefs and viewpoints expressed by the author do not necessarily reflect the opinions, beliefs and viewpoints of BCBSM or any of its employees.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Because the purpose of the consortium is to measure and improve the quality of existing care practices, the project received a 'Not Regulated' status from the Institutional Review Board at our hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.

Revisiting CLABSI prevention strategies: Pt 1
Follow the basics to keep your patients safe..
· Central line–associated bloodstream infection prevention requires meticulous attention to insertion, care, and maintenance of central lines.
· Collaboration between front line staff, the infection prevention team, and vascular access specialists can help identify opportunities for improving care of patients with vascular access devices.
Editor’s note: This is the first in a two-part series on central line–associated bloodstream infections (CLABSIs). Part 1 focuses on indications and insertions. Part 2 will discuss maintenance and what to do if a CLABSI occurs.
THE COST of CLABSIs is high both for patients and for organizations. Although progress in reducing these infections has been made, they remain a common problem in hospitals. CLABSIs increase mortality risk and can cost an organization about $45,000 per case. (See CLABSI defined .)
CLABSI prevention strategies fall into three categories: clinical indications, insertion, and care and maintenance. In this first of a two-part series, we’ll focus on indications and insertion.
Clinical indications
An often overlooked first step in CLABSI prevention is asking whether a central line is clinically indicated. Central line access poses risks beyond CLABSI, including deep vein thrombosis, bleeding, pneumothorax, and arrhythmias. An interprofessional team can work together to establish organizational criteria for central line use and help all units adopt them. The team should represent infectious diseases, hospitalists, nephrology, critical care, emergency, administration, pharmacy, vascular access, infection prevention, information technology, and frontline nursing staff. Use evidence-based practice to develop central line criteria, and include protocols for establishing individual patient need, choosing a device, and preventing device complications.

The Centers for Disease Control and Prevention (CDC) guidelines and Infusion Nurses Society (INS) standards discuss basic concepts for appropriate device selection. Other documents, such as The Joint Commission CLABSI Toolkit and the “Compendium of strategies to prevent healthcare-associated infections in acute care hospitals” from the Society for Healthcare Epidemiology of America (SHEA), emphasize daily review of central lines to determine if their continued use is necessary.
Recent publications (for example the Michigan Appropriateness Guide for Intravenous Catheters [MAGIC]) offer direction for clarifying the indications most broadly accepted for central line use. Based on expert consensus, recommendations for preferred vascular access devices should be based on patient characteristics (for example, difficult venous access), appropriateness of infusate for peripheral administration, and anticipated therapy duration. These broad considerations are easily adopted into order sets and are available as a free, downloadable smartphone application ( www.improvepicc.com ).
Individual patient need
Early in a patient’s admission, the healthcare team should discuss appropriate vascular access, indications, and vessel health and preservation. If a line is placed, review its continued use daily. When it’s no longer needed, develop a plan for appropriate, reliable access for the patient’s ongoing needs.
Device options and complications
Vascular access isn’t synonymous with central access. Hospitals that have added midline catheters to their vascular access options have consistently reported substantial decreases in central line days. This suggests an over-reliance on central lines without a true need for them. Midlines (longer/extended dwell peripheral catheters) may offer an option for patients with difficult venous access who may otherwise have been escalated to a central line, when no valid central line indication existed. Because midlines are considered peripheral catheters, care must be taken to ensure that only peripherally compatible infusates are given. A vascular access consult can provide the necessary recommendations for evidence-based device selection.
Excess lumens substantially increase CLABSI and deep vein thrombosis risk. Including extra lumens “just in case” isn’t acceptable. Studies show that defaulting to single-lumen central lines, establishing specific criteria for multi-lumen devices, combined with provider, nursing, and pharmacist education supported by real-time monitoring and feedback contribute to positive outcomes. The University of Michigan published these criteria for the use of multi-lumen devices:
- simultaneous administration of multiple incompatible medications
- total parenteral nutrition infusion with concurrent need for additional I.V. medications
- simultaneous use of continuous vesicant or irritant chemotherapy with other medications
- need for vasopressors.
Calculators and simulation studies ( improvepicc.com ) can help you evaluate the potential decreases in CLABSI and deep vein thrombosis risk if you change the relative percentages of triple-, double-, and single-lumen central lines in your organization.
Current reimbursement penalties focus only on CLABSI and don’t include reporting of short peripheral catheter and midline infections. Frontline staff can be patient advocates and ensure that line selection is a clinical decision guided solely by the patient’s access needs. Choosing devices without fully understanding their infection rates and other complications could expose patients to significant risk.
Shared governance, patient safety committees, and infection prevention committees can encourage organizations to expand their surveillance scope to include all device-associated infections, not just those from central lines, and request complication rate information for all devices used by the vascular access team. Fully understanding benefits and complications of vascular access devices helps frontline staff advocate for safe access.
After the care team determines that central access is required and the type of access (acute central venous catheter, tunneled catheter, peripherally inserted central catheter, totally implanted device) is chosen, plan the insertion, including use of antimicrobial lines (if indi- cated) and insertion checklists and bundles.
Antimicrobial lines
Based on organizational goals and current infection incidence, consider an antimicrobial line. Studies, including those from the author’s institution, have shown substantial reductions in CLABSI when this technology is used.
Checklists and bundles
Central line insertion checklists or bundles—hand hy- giene, maximum sterile barrier precautions (head-to-toe sterile drape, mask, cap, sterile gown, sterile gloves), chlorhexidine skin prep (unless contraindicated), and, when possible, avoidance of the femoral vein—are the cornerstone to CLABSI prevention. Incorporating the checklist into the electronic health record (EHR) will reinforce and verify its use across all central venous access devices in all settings within the organization.
Using the checklist in conjunction with a trained observer helps ensure that all elements of the insertion process are followed. The observer should record each step of the checklist in the EHR and provide real-time feedback if any element is missed. Some checklists have the option of “yes” or “yes, with coaching” to emphasize the importance of an active review process. Some organizations have adopted a “stop the line” approach, which further empowers the observer to stop a procedure that’s not complying with safety expectations. Another strategy organizations use is to have a formal process for following up (peer review or individualized coaching from division leaders, hospital epidemiologists, and administration) with clinicians who don’t adhere to the checklist even when coached to determine barriers to compliance and to reinforce that using the checklist is compulsory.
Central line insertion that doesn’t comply with the checklist (for example, a line inserted in an emergency situation) should be documented as emergent, and the line should be flagged for removal within 24 to 48 hours based on the organization’s policies. To avoid this situation, organizations may want to consider using intraosseous devices as bridges for emergent vascular access. They allow time for the patient to stabilize and provide an opportunity for safe insertion of the most appropriate device for continued therapy.
Limitations aid prevention
CLABSI prevention begins with limiting central access line use to established indications. When central lines are indicated, checklists and bundles can help ensure proper insertion. The next article in this series will focus on access line maintenance and what to do if a CLABSI occurs.
Michelle DeVries is senior infection control officer for Methodist Hospitals in Gary, Indiana, and is an adjunct research fellow at Griffith University in Australia. She serves on the speakers bureau for Access Scientific, Becton Dickinson, Ethicon, and Eloquest.
Selected references
Blanco-Mavillard I, Rodríguez-Calero MA, Castro-Sánchez E, Bennasar-Veny M, De Pedro-Gómez J. Appraising the quality standard underpinning international clinical practice guidelines for the selection and care of vascular access devices: A systematic review of reviews. BMJ Open . 2018;8(10):e021040.
Bozaan D, Skicki D, Brancaccio A, et al. Less lumens-less risk: A pilot intervention to increase the use of single-lumen peripherally inserted central catheters. J Hosp Med . 2019;14(1):42-6.
Centers for Disease Control and Prevention. Healthcare-associated infections: Current HAI progress report. March 19, 2019. cdc.gov/hai/data/portal/progress-report.html
Cheng HY, Lu CY, Huang LM, Lee PI, Chen JM, Chang LY. Increased frequency of peripheral venipunctures raises the risk of central-line associated bloodstream infection in neonates with peripherally inserted central venous catheters. J Microbiol Immunol Infect . 2016;49(2):230-6.
Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(suppl 6):S1-40.
Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infus Nurs . 2016;39(suppl 1):S1-159.
Kornbau C, Lee KC, Hughes GD, Firstenberg MS. Central line complications. Int J Crit Illn Inj Sci. 2015;5(3):170-8.
Laan BJ, Spijkerman IJ, Godfried MH, et al. De-implementation strategy to reduce the inappropriate use of urinary and intravenous CATheters: Study protocol for the RICAT-study. BMC Infect Dis. 2017;17(1):53.
Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753-71.
Moureau N, Sigl G, Hill M. How to establish an effective midline program: A case study of 2 hospitals. J Assoc Vasc Access . 2015;20(3):179-88.
O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis . 2011;52(9):e162-93.
Pathak R, Gangina S, Jairam F, Hinton K. A vascular access and midlines program can decrease hospital-acquired central line-associated bloodstream infections and cost to a community-based hospital. Ther Clin Risk Manag . 2018;14:1453-6.
Pathak R, Patel A, Enuh H, Adekunle O, Shrisgantharajah V, Diaz K. The incidence of central line-associated bacteremia after the introduction of midline catheters in a ventilator unit population. Infect Dis Clin Pract (Baltim Md) . 2015;23(3):131-4.
Pittiruti M, Scoppettuolo G, Dolcetti L, et al. Clinical experience of a subcutaneously anchored sutureless system for securing central venous catheters. Br J Nurs . 2019;28(2):S4-14.
Quan KA, Cousins SM, Porter DD, et al. Electronic health record solutions to reduce central line-associated bloodstream infections by enhancing documentation of central line insertion practices, line days, and daily line necessity. Am J Infect Control. 2016;44(4):438-43.
Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol . 2016;37(7):811-7.
Rupp ME, Karnatak R. Intravascular catheter-related bloodstream infections. Infect Dis Clin North Am. 2018;32(4):765-87.
Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-46.
2 Comments .
Hi Cindy, Thanks for the nice comment and for your question. CDC, INS and SHEA all have suggestions on when an antimicrobial line is considered. In my own organization, it is our standard for PICCs and CICCs. The specific wording from the various guidances is:
CDC Use a (chlorhexidine/silver sulfadiazine or minocycline/rifampin -) impregnated CVC in patients whose catheter is expected to remain in place >5 days if, after successful implementation of a comprehensive strategy to reduce rates of CLABSI, the CLABSI rate is not decreasing. The comprehensive strategy should include at least the following three components: educating persons who insert and maintain catheters, use of maximal sterile barrier precautions, and a >0.5% chlorhexidine preparation with alcohol for skin antisepsis during CVC insertion. (CATEGORY IA)
INS Collaborate with the interprofessional team to consider anti-infective CVADs in the following circumstances, as anti-infective CVADs have shown a decrease in colonization and/or CLABSI in some settings. (I) Expected dwell of more than 5 days. CLABSI rate remains high even after employing other preventive strategies Patients with enhanced risk of infection (ie, neutropenic, transplant, burn, or critically ill patients). Emergency insertions.
SHEA Use antiseptic- or antimicrobial-impregnated CVCs in adult patients (quality of evidence: I). a. The risk of CLABSI is reduced with some currently marketed antiseptic-impregnated (eg, chlorhexidine- silver sulfadiazine) catheters and antimicrobial- impregnated (eg, minocycline-rifampin) catheters. Use such catheters in the following instances. i. Hospital units or patient populations have a CLABSI rate above institutional goals despite compliance with basic CLABSI prevention practices. Some evidence suggests that use of anti- microbial CVCs may have no additional benefit in patient care units that have already established a low incidence of catheter infections. ii. Patients have limited venous access and a history of recurrent CLABSI. iii. Patients are at heightened risk of severe sequelae from a CLABSI (eg, patients with recently implanted intravascular devices, such as a prosthetic heart valve or aortic graft).
Hello to the editor! I would like to say how much I appreciated this article. I have been doing CLABSI surveillance for my hospital for 1 ½ years, and this information is so timely and relevant. I plan on getting with our vascular access team to learn more about our processes at my institution. I have a question. There is the statement that “antimicrobial lines should be used (if indicated)”. How is that indication determined? Are their specific criteria, or is it individually determined based on each patient and the care team making that decision? It seems to me that if a patient meets the criteria for a central line, an antimicrobial line should always be indicated. I’m still learning, so I will see what I can find out. I look forward to part 2! Thank you!!
Comments are closed.

NurseLine Newsletter
- First Name *
- Last Name *
- Hidden Referrer
*By submitting your e-mail, you are opting in to receiving information from Healthcom Media and Affiliates. The details, including your email address/mobile number, may be used to keep you informed about future products and services.
Test Your Knowledge
Recent posts.

Competent care during pregnancy loss

Knowledge of intravascular determination

Virtual reality: Treating pain and anxiety

Broken trust

Diabetes innovations and access to care

Breaking barriers: Nursing education in a wheelchair

Chronic traumatic encephalopathy and traumatic brain injury

Connecting theory and practice

Stevens-Johnson syndrome

Turning the tide

Gut microbiome and health

Addressing social determinants of health

Takotsubo cardiomyopathy: The tale of a broken heart

Nurse referrals to pharmacy

The nurse’s role in advance care planning
The Infona portal uses cookies, i.e. strings of text saved by a browser on the user's device. The portal can access those files and use them to remember the user's data, such as their chosen settings (screen view, interface language, etc.), or their login data. By using the Infona portal the user accepts automatic saving and using this information for portal operation purposes. More information on the subject can be found in the Privacy Policy and Terms of Service. By closing this window the user confirms that they have read the information on cookie usage, and they accept the privacy policy and the way cookies are used by the portal. You can change the cookie settings in your browser.
- Login or register account

INFONA - science communication portal

- advanced search
- conferences
- Collections
Case Report How to Establish an Effective Midline Program: A Case Study of 2 Hospitals $("#expandableTitles").expandable();
- Contributors
Fields of science
- Bibliography
Journal of the Association for Vascular Access > 2015 > 20 > 3 > 179-188
Identifiers
User assignment, assignment remove confirmation, you're going to remove this assignment. are you sure.

Nancy Moureau
Gordon sigl, margaret hill.
infusion intravenous catheter indwelling catheterization peripheral/method
Additional information
- Read online
- Add to read later
- Add to collection
- Add to followed

Export to bibliography

- Terms of service
Accessibility options
- Report an error / abuse
Reporting an error / abuse
Sending the report failed.
Submitting the report failed. Please, try again. If the error persists, contact the administrator by writing to [email protected].
You can adjust the font size by pressing a combination of keys:
- CONTROL + + increase font size
- CONTROL + – decrease font
You can change the active elements on the page (buttons and links) by pressing a combination of keys:
- TAB go to the next element
- SHIFT + TAB go to the previous element
This website is intended for healthcare professionals

- { $refs.search.focus(); })" aria-controls="searchpanel" :aria-expanded="open" class="hidden lg:inline-flex justify-end text-gray-800 hover:text-primary py-2 px-4 lg:px-0 items-center text-base font-medium"> Search
Search menu
Moureau N, Chopra V. Indications for peripheral, midline and central catheters: summary of the MAGIC recommendations. Br J Nurs. 2016; 25:(8)S15-S24 https://doi.org/10.12968/bjon.2016.25.8.S15
Alexandrou E, Ramjan LM, Spencer T The use of midline catheters in the adult acute care setting–clinical implications and recommendations for practice. J Assoc Vasc Access. 2011; 16:(1)35-41 https://doi.org/10.2309/java.16-1-5
Anderson NR. Midline catheters: the middle ground of intravenous therapy administration. J Infus Nurs. 2004; 27:(5)313-321 https://doi.org/10.1097/00129804-200409000-00005
Chopra V, Kaatz S, Swaminathan L Variation in use and outcomes related to midline catheters: results from a multicentre pilot study. BMJ Qual Saf. 2019; 28:(9)714-720 https://doi.org/10.1136/bmjqs-2018-008554
Dumont C, Getz O, Miller S. Evaluation of midline vascular access: a descriptive study. Nursing. 2014; 44:(10)60-66 https://doi.org/10.1097/01.NURSE.0000453713.81317.52
Moreau N, Sigl G, Hill M. How to establish an effective midline program: a case of 2 hospitals. J Assoc Vascular Access. 2015; 20:(3)179-188 https://doi.org/10.1016/j.java.2015.05.001
Gorski L, Hadaway L, Hagle M, McGoldrick M, Doellman D. Blood sampling via a vascular access device. Infusion Therapy Standards of Practice. J Infus Nurs. 2016; 39:(1S)
Frey AM. Drawing blood samples from vascular access devices: evidence-based practice. J Infus Nurs. 2003; 26:(5)285-293 https://doi.org/10.1097/00129804-200309000-00004
Lippi G, Cervellin G, Mattiuzzi C. Critical review and meta-analysis of spurious hemolysis in blood samples collected from intravenous catheters. Biochem Med (Zagreb). 2013; 23:(2)193-200 https://doi.org/10.11613/bm.2013.022
Barnard EB, Potter DL, Ayling RM, Higginson I, Bailey AG, Smith JE. Factors affecting blood sample haemolysis: a cross-sectional study. Eur J Emerg Med. 2016; 23:(2)143-146 https://doi.org/10.1097/MEJ.0000000000000195
Halm MA, Gleaves M. Obtaining blood samples from peripheral intravenous catheters: best practice?. Am J Crit Care. 2009; 18:(5)474-478 https://doi.org/10.4037/ajcc2009686
Bradford JY, Reeve NE, Killian M Clinical practice guideline: prevention of blood specimen hemolysis in peripherally-collected venous specimens. J Emerg Nurs. 2018; 44:(4)402e1-402.e22 https://doi.org/10.1016/j.jen.2018.05.017
Phelan MP, Reineks EZ, Schold JD, Hustey FM, Chamberlin J, Procop GW. Preanalytic factors associated with hemolysis in emergency department blood samples. Arch Pathol Lab Med. 2018; 142:(2)229-235 https://doi.org/10.5858/arpa.2016-0400-OA
Natali R, Wand C, Doyle K, Noguez JH. Evaluation of a new venous catheter blood draw device and its impact on specimen hemolysis rates. Pract Lab Med. 2018; 10:38-43 https://doi.org/10.1016/j.plabm.2018.01.002
Green SF. The cost of poor blood specimen quality and errors in preanalytical processes. Clin Biochem. 2013; 46:(13-14)1175-1179 https://doi.org/10.1016/j.clinbiochem.2013.06.001
Cadamuro J, Wiedemann H, Mrazek C The economic burden of hemolysis. Clin Chem Lab Med. 2015; 53:(11)e285-e288 https://doi.org/10.1515/cclm-2015-0363
Lowe G, Stike R, Pollack M Nursing blood specimen collection techniques and hemolysis rates in an emergency department: analysis of venipuncture versus intravenous catheter collection techniques. J Emerg Nurs. 2008; 34:(1)26-32 https://doi.org/10.1016/j.jen.2007.02.006
https://www.accessdata.fda.gov/cdrh_docs/pdf16/K162377.pdf
Evaluation of processes, outcomes, and use of midline peripheral catheters for the purpose of blood collection
Daleen Penoyer
Center for Nursing Research, Orlando Health, Orlando, FL
View articles
Melody Bennett
Patricia I. Geddie
Orlando Regional Medical Center, Orlando Health, Orlando, FL
Alyssa Nugent
Vascular Access Team, Orlando Regional Medical Center of Orlando Health, Orlando, FL
Tara Volkerson
Results added knowledge on use of midline catheters (MCs) for blood sampling.
Using MCs for blood withdrawal resulted in low rates of hemolysis (0.69%).
Dwell time was longer in those who had blood drawn from their MC.
Nurse practices for blood sampling from MCs varied and learned from other nurses.
Background:
Blood withdrawal from midline catheters (MCs) is done clinically, but no studies were found evaluating outcomes from this procedure, nor were clinical guidelines found. Drawing blood samples from short peripheral catheters is associated with higher hemolysis rates.
A prospective, observational, mixed methods study was used to evaluate outcomes from using MCs for blood withdrawal. Focus group sessions were held to evaluate nurses' practices for this procedure.
Data were collected over 3 months on 397 MCs in 378 patients. Hemolysis rates when the MC was used for blood withdrawal was 0.69% in 1021 tests. More than half had blood specimens drawn through the MC, and the time known for the successful withdrawal was on average 64 ± 85 hours. Mean dwell time for all MCs was 108.5 ± 98 hours, and when MCs were used for blood withdrawal, mean dwell time was 127.19 ± 109.13 hours and for MCs not used for blood withdrawal, 88.34 ± 79.86 hours (P < 0.001). In 338 patients who received therapy through their MC (n = 338), 87% completed intended therapy: 88% with blood withdrawal and 81% without blood withdrawal. Qualitative analysis from focus groups demonstrated wide variation in practice for blood sampling from MCs, and most learned techniques from their preceptors, other nurses, or patients.
Conclusions:
Findings indicated that blood withdrawal from one specific type of MC had low rates of hemolysis, increased dwell time, and completion of therapy. More studies are needed to determine best practices for blood sampling through various types of MCs and outcomes.
Midline intravenous catheters (MCs) have been in clinical use since the 1950s and are commonly used as an alternative for intravenous (IV) access to short peripheral IV catheters and central venous access devices. 1 – 5 Use of MCs is associated with lower phlebitis rates and infections than for central venous catheters (CVCs) and can be inserted without the need for radiologic verification. 2 , 6 Indications for using MCs are to administer intravenous medications and infusions for up to 30 days and are recommended when treatment is anticipated for 5 – 6 days or for long-term therapy in patients with limited IV access. 2 , 4 – 6 In addition to MC use for infusions and medication administration, using the MC for blood sampling has been included as an indication for those who have difficult IV access. 6 This option may reduce need for direct venipuncture in patients who have MCs with ongoing or frequent blood sampling needs for testing. 6
While studies have been reported on the outcomes of use with MCs, such as dwell time, complications, completion of therapy, and recommended processes for maintenance and administration of fluids and medications, 2 , 4 – 6 little is known or reported about procedures for blood sampling or outcomes from performance for this function. The Infusion Nursing Society standards 7 includes guidance for blood sampling from central venous and short peripheral catheters (SPCs). However, when referencing MCs for blood sampling, the statement in the standards is “no evidence regarding obtaining blood samples from MLCs [midline catheters].” Frey 8 also reported methods for blood sampling from various vascular access devices, but MCs were not included in this review and recommendations for practice.
Generally, intravenous catheters are designed to administer fluids and medication in a forward, antegrade motion into the venous system rather than to withdraw venous blood in a retrograde fashion, except for aspiration of blood return to verify patency. Little is known about the dynamics and physical effects on vasculature, catheter function, and clinical outcomes when retrograde maneuvers are used to withdraw samples of blood from MCs. Furthermore, no standardized procedures exist for withdrawing blood from MCs for sampling, though anecdotally, this practice is done in various clinical settings.
Hemolysis in vitro is the injury or breakdown of red blood cells that can occur during blood sampling. 9 Reports in the literature show that using SPCs for blood sampling results in higher rates of hemolysis, 9 – 12 mostly owing to mechanical shearing (Reynolds stress) and turbulence that occurs at the rough surface edges or kinks in tubing when withdrawing blood from the catheter. 13 Hemolysis of samples that occurs during the preanalytic phase of blood collection can result in rejection of those samples for a myriad of blood tests. It is estimated about 3%–32% hemolysis occurs in acute care settings, 14 accounts for 40%–70% of specimens unacceptable for processing, 15 has a significant financial impact, 16 places an additional burden on the patient for repeated venipunctures and vein injury, and results in delays in diagnosis or treatment. 15 The American Society for Clinical Pathology benchmark for hemolysis rate is at 2%, thus reported hemolysis for blood sampling generally exceeds that mark. 17
More recently, anecdotal feedback from practitioners to a company conducting clinical assessment of one of their MCs was the perception of increased hemolysis of samples when withdrawing blood from the catheter. To evaluate this further, the company conducted labora tory studies of fluid dynamics through the catheter structure and concluded that when performing fluid withdrawal maneuvers with a syringe, the distal tip of the catheter would collapse, potentially contributing to hemolysis during blood sampling. To address these challenges, the company made design changes to reinforce the tip of the MC to overcome distal catheter tip collapse with retrograde flow during blood sampling. The goal of this design change of the MC was to enhance success in collecting blood samples with reductions in hemolysis. In August 2016, the blood draw improvement with the reinforced tip was submitted to the U.S. Food and Drug Administration (FDA) as a special 510(k) application and received clearance from the FDA in September 2016.18 This MC (PowerGlide ProTM; BD, Salt Lake City, UT) demonstrated improved aspiration flow rates and reports of less hemolysis, but clinical testing of the MC for blood-sampling performance had not been previously done and was needed.
While some study findings in the literature address outcomes from MC use in general, no evidence was found about processes or clinical outcomes from using MCs for blood sampling, such as impact on dwell time, hemolysis of samples, and functional aspects of the MC to obtain samples. No clear procedures exist to guide techniques to perform blood sampling or ways to avoid hemolysis with MCs. Thus, variation in practice likely exists across many settings and caregivers with regard to blood specimen collection from MCs. Given the paucity of published data or studies related to blood collection from MCs, the investigators aimed to use exploratory and observational methods to summarize practices and outcomes of using the MC for blood collection.
The primary purpose of this study was to evaluate the processes, uses, and outcomes of using MCs for the purpose of blood collection for laboratory analysis. The aims of this study were to (1) evaluate the rate of hemolysis when the MC was used for blood collection, (2) evaluate outcomes from using the MCs for blood collection in the acute care setting (such as catheter performance during blood sampling, dwell time), and (3) evaluate nurse perceptions and practices used for withdrawing blood specimens from the MC.
This investigator-led study used a prospective, observational, and mixed methods design at a single-site hospital to evaluate the function and outcomes of MCs for blood specimen collection in two medical and two surgical units at the study facility. The study was approved by the organizational institutional review board through expedited review and waived informed consent from patients for data collection on MC use and outcomes since there were no interventions and only observation of usual care was done (IRB #19.216.07). No private health information was retained. However, informed consent was required by nurses to participate in the qualitative component of the study.
Subjects and settings
The study was conducted in a large, tertiary care center in southeastern United States. The units selected for the study were based on volumes of MC use from medical and surgical populations. As there were no interventions in the study and no comparison, we used historical MC use to drive sample estimates for the study and used a convenience sample of patients over a 3-month period to generate a reasonable sample size for this observational study. The aim was to achieve 300 total MCs inserted and to capture at least 150 cases where blood was drawn from the MC at any time during its dwell time. The MC was considered the study “subject,” and it was possible for patients to have more than one MC during their hospital stay. Criteria for inclusion were adult patients (≥18 years) whose initial MC was inserted within 24 hours during their stay on 1 of 4 study units. The study units were 2 medical progressive care units and 2 surgical units with similar patient characteristics. Excluded were those MCs on the study units that were inserted >24 hours prior to enrollment.
As a standard of practice at the study organization, all MCs are placed by specialized and trained nurses on the vascular access team (VAT) using sterile technique. Nurses used ultrasound-guided placement using an all-in-one, no-touch method with an integrated guidewire technique used with the type of MC, which is also described in the literature. 4 Figure 1 illustrates the type of MC with a reinforced tip used for all insertions in this study and exclusively at the study organization. In discussions with the VAT, they indicated that their practice was to assess each patient for the most appropriate vascular access prior to insertion of an MC.
Study measures
The investigator collected data on all MCs on the study units meeting inclusion of cases whose MCs were used for blood collection as well as those that were used only for clinical indications, such as for IV medications, fluids, difficult intravenous access, and blood administration. Difficult intravenous access was defined as those with a significant history of venous access issues requiring MC or other venous access devices or unsuccessful achievement of venous access with an SPC after two reasonable attempts.
To meet study aims 1 and 2, outcomes from catheter performance in all MCs, including both those used or not used for blood sampling, included complications, such as phlebitis, extravasation, infiltration, and catheter performance (dwell time, completion of therapy). Rates of hemolysis of blood samples sent to the laboratory were determined by direct observation of data obtained from the electronic healthcare record (EHR). Hemolysis rates were determined by the number of tests that were hemolyzed divided by the total number of blood tests done in those patients who had their MC used for blood collection. At the study facility, the laboratory result reports in the EHR customarily and reliably note when samples for blood tests are hemolyzed with instructions for resampling for testing. No other methods of reporting hemolysis were used by the laboratory system, thus manual observation and EHR reviews were needed.
Timing metrics were defined as follows:
- Dwell time–the time from insertion to removal–retrieved from the EHR.
- MC functional for blood collection time–time in hours from insertion until last known time functional to perform blood sampling or when no longer able to obtain a blood sample from the MC.
Other measures included reasons for insertion (medications, fluid administration, blood transfusions, blood specimen collection), removal (complications, completion of therapy/discharge, no longer indicated, inadvertent removal), and for stopping use of the MC for blood collection. Patient demographics of age, gender, and reason for admission were obtained from the EHR. Types of medications administered either continuously or intermittently via the MC were collected and categorized for the study using the hospital medication list for irritating or nonirritating drugs.
To meet aim 3, evaluation of current practice with regard to MC blood specimen, collection was done through qualitative methods to capture experiences to explore this unstudied practice. Nurses on the VAT and study units were given the opportunity to participate in 1 of 3 hour-long focus group discussions about the methods they used to withdraw blood from the MC, facilitators and barriers to performance, or any other information about blood sampling from the MC. All willing participants received an informed consent form describing the study and voluntary nature of participating in the focus groups. Sessions were audio-recorded and transcribed for qualitative analysis by the research team. Three of the investigators evaluated information from the transcripts using constant comparative analysis techniques to develop codes and generate themes about using MCs for blood sampling. The initial and exploratory questions used to prompt group discussion were as follows:
- Describe your experience with using MCs for blood collection for laboratory tests.
- Describe the processes you use to withdraw blood from the MC.
- Describe any facilitators or barriers you have found when using the MC for blood collection.
Data collection
Every weekday, the investigator obtained a patient list from the EHR on the study units and identified those patients whose MCs were inserted by the VAT less than 24 hours prior to enrollment. The investigator reviewed physician orders for laboratory tests requiring blood collection. The investigator was present on the study units every day during the week, usually starting at 4 am, when most blood sampling occurred, and then made rounds throughout the day for other laboratory orders. A second investigator would make rounds in the late afternoons to capture other orders or blood collections. Any new MC insertions beyond that time were usually found by the next morning on rounds. The investigator queried each nurse about the method used for blood collection: either from the MC, direct venipuncture (RN/phlebotomist), or other intravascular device, and which tools were used, that is, syringe/size, tourniquet, or vacutainer. In those cases in which the MC was used to withdraw blood, the investigator reviewed laboratory results to capture any cases of reported hemolysis from tests done.
In patients identified with MCs, the investigator directly observed the MC site daily for its appearance and any complications, such as phlebitis, using the Infusion Nurses Society 2016 Phlebitis Scale, 7 signs of extravasation or infiltration (acute pain, burning, failure to flush), and thrombosis (failure to flush). The investigator also queried the nurse for any issues encountered when using the MC for blood sampling. Timing and other metrics as described above was collected and documented daily by the investigator as described earlier.
All observational data were entered into an Excel spreadsheet (Microsoft Corp., Redmond, WA) on a password-protected computer and server. To analyze the rate of hemolysis, the total number of blood tests that were withdrawn from the MC divided by the number of reported hemolyzed samples was used. Simple, descriptive statistics were used to summarize data, such as mean, standard deviation, and median values. To detect differences between groups, nonparametric testing with independent samples (using the Mann-Whitney U test) was used since the data were skewed and not normally distributed.
After 3 months of data collection, 397 MCs in 378 patients were included for study. Of those patients, 19 had more than 1 MC, 16 had 2, and 3 had 3 MCs during their hospitalization. Reasons for removal and replacement in those patients were phlebitis, infiltration, occlusion, accidental removal, one no longer indicated, and another at a patient's request. While the sample was heterogeneous, the most typical patient was female, 62 years old, and being treated for infection. The mean age of the sample was 61.53 ± 18 years (median 63 years; range, 19–98 years). A significant difference was seen in gender, with females composing 61.5% of the total sample, but age of subjects was not significantly different ( P =0.11). A wide diversity of reasons for admission was seen in the sample. Demographics for patients in the sample are summarized in Table 1 .
VAT nurses cannulated all patients with the MC with the reinforced tip as described earlier. Most MCs (89%) were placed in the cephalic vein using a 20-gauge MC. The reasons listed in the VAT consultation form for insertion was predominately for difficult venous access, either by patient history or actual attempts at venipuncture (61%) and venous access for medication administration (36%). Table 2 demonstrates the characteristics of MCs inserted and site location.
MC = midline catheter; med = medication; PICC = peripherally inserted central venous catheter.
Outcomes from MC use for blood sampling
In this sample, 176 patients never had blood withdrawn from their MC, either because there were no orders for blood sampling, blood was collected from other existing lines or venipuncture, or it was never attempted. In 221 cases, MCs were used for blood sampling (55.7%). Since laboratory tests were not ordered regularly over the duration of the dwell time in most patients, the last known recorded time that the MC was functional for drawing blood was used for analysis. The average time recorded that the MC was known to be successfully used for blood sampling was 64 ± 85 hours (2.67 days), with a median of 34.7 hours. The longest time over which blood could be sampled from the MC was 685 hours (28.5 days). In 226 cases total, nurses reported that they were not able to sample blood from the MC after some point in time during its dwell due to no blood return when attempting to withdraw using with a syringe. This often happened later in the course of the dwell time of the MC, and its frequency significantly varied.
Focus group results
The investigators held three focus groups of nurses to determine their practices when drawing blood from the MC. Two groups were nurses working on the study units (n=16) and another group with 8 VAT nurses. Three nurse investigators attended each focus group, took field notes, and recorded sessions that were transcribed and reviewed using constant comparative analysis. After discussion and review, consensus was reached by the team. Nurses described general principles they used for safe blood withdrawal as described in the hospital policy and INS standards, such as proper use of hand hygiene, gloves, disinfection of injection surfaces, and infusion interruption. They also followed principles of flushing to remove debris, aspiration for blood, clearance of the line, and avoidance of diluted blood or incompatible fluids or drugs. Key themes emerged from qualitative analysis of these focus groups. Themes from nurses who use MCs were sources of information, methods used, and supplies used. For sources of information, nurses most often learned techniques to withdraw blood from their preceptors or other nurses on the unit. Most nurses indicated they attempt to withdraw blood from the MC first to avoid venipunctures on patients and that many patients request and expect the MC to be used for blood collection when possible. Patients also prompted nurses with “tips” to be successful with blood collection. Methods used by nurses to withdraw blood varied considerably; however most used 10 mL syringes with various flushing efforts. Nurses tended to use saline syringe flushes and varied regarding the number of syringes used. They described using “power” flushes and “pulsed” flushes to ensure line patency. They rarely used a vacutainer device, but rather used a transfer device to inject blood into the collection tube. As a last resort, nurses would, on occasion, use a tourniquet at various locations on the upper arm. They described manipulation of arm and catheter position to “get the kinks out” of the catheter where it interfaced with skin. They described this “angle” to be where the catheter kinks due to its position in the arm and the patients' excess skin and fat. They described techniques used to “straighten out the angle,” such as pulling on the catheter hub or straightening out the arm. Other manipulations used were to take the dressing off the catheter when they deemed it to be “too tight” such that no blood would flow. They also seemed to borrow techniques commonly used with CVCs to achieve blood collection, such as turning the head, coughing, raising the arm, and other such maneuvers. In contrast, different themes arose from VAT nurses who insert MCs: vein preservation and selection, insertion request burden, and nurse education. VAT nurses valued the use of the MC access for therapy over blood collection and felt vein preservation was important. They preferred to use the cephalic vein as it was easier to access, but they stated it was not the vein of choice due to its anatomical position. They indicated they believed that MCs are requested more often since in-patients are admitted with higher acuity, are older, and have comorbidities that lead to poor venous access or depletion. They also stated that the pH of medications impacted dwell time and that nurses should have more education on MC maintenance care.
In this exploratory study on MC processes and outcomes for blood sampling, few references exist to compare for previous studies, as none were found in the literature at the time of the study. Interesting findings were that hemolysis incidence was very low. Previous studies on the use of SPCs for blood sampling have shown high incidence of hemolyzed samples. 13 In our study, the <1% hemolysis rate was lower than generally acceptable levels of 2% by the American Society for Clinical Pathology17 and, as reported in the literature, ranging from 3% to 32%. 14 In the study by Green, 15 estimated costs in North American hospitals per preanalytic errors such as hemolysis from blood specimen collection is approximately $208 per specimen. Also reported in this paper was that such costs can add up to 0.23%–1.2% of hospital operating costs, which could represent about $1.2 million per year in a hospital size of 650 beds, and there is a potential of 26% unnecessary diagnostics or treatment. Recommendations from their study and others 16 are to develop improvements in blood collection techniques to reduce unnecessary costs associated with preanalytic processes. The ability to use the MC for blood specimen collection with low hemolysis occurrence is one way to potentially reduce costs associated with hemolysis and multiple phlebotomy venipunctures with its associated pain and suffering, particularly for those with difficult IV access. There may be differences in outcomes from other MC brands, as this specific catheter was designed to reduce hemolysis in blood sampling. More studies are needed to test this in other types of MCs and compare results.
Few complications occurred in all patients with the MCs, including those used for blood sampling, which is consistent with findings from other outcomes studies. 6 The average and mean dwell time in our sample was less than found in other recent studies. 4 – 6 The fact that most of the MCs in our sample were removed at discharge may have impacted our overall dwell time. Dwell time of catheters was longer in those who had MCs used for blood draws when compared to those who did not. This may indicate that the use of MCs for the indication of blood sampling may not affect dwell time or ability to use the catheter for therapeutic reasons. Additionally, more patients who had their MC used for blood sampling completed therapy than those who did not, indicating that when the MC is used for this purpose it may not impact therapy completion negatively. Overall, when the MC was used for almost any reason, dwell time was higher. It is possible that when the MC was used for infusions of medications and fluids and withdrawal of blood specimens, the MC may have been flushed more frequently (before and after), thus maintaining its patency and usability. Positive benefits were that those patients whose blood tests were sampled from the MC ( n =1021) were spared the experience of venipuncture and its associated discomfort and costs.
The main reason for catheter removal was at discharge, and the average dwell time was similar to the average length of stay (LOS) on the study units. These findings are consistent with those found in the Chopra et al.4 study, where completion of therapy and discharge were common reasons for removal. In acute care, there is an emphasis to reduce unnecessary patient LOS with its associated costs and potential for complications during hospitalization. As older patients and those with chronic and debilitating comorbid diseases are admitted to the hospital, many also have vasculopathy and need hospitalization and administration of medications and blood samples for laboratory analysis. This is particularly concerning in patients with repeated admissions requiring vascular access and loss of vein integrity. While MCs can be used up to 30 days, hospital LOS is usually much shorter, thus there is likely less duration of therapy with MCs in the acute care setting. When multiple venous actions are required, the MC can serve as a vehicle to withdraw blood for sampling safely and reduce unnecessary costs and suffering associated with multiple venipunctures. One caveat and trade-off for this privilege is a potential loss of catheter function over time and catheter dwell time. More studies are needed in this area to determine efficacy under various clinical conditions and circumstances.
The average time over which the MC was used for blood sampling was 2.67 days, with a median of 1.45 days. Anecdotal reports in the literature about withdrawing blood from MCs indicate the time that the catheter could be used for blood sampling was often short and limited to either the first day or two, and this is supported by this data. In this study, the duration of time that the MC was known to be functional for blood sampling was measured, so if blood tests were ordered later during the course of dwell time, the actual endurance of the MC for blood sampling was likely underestimated and may have been functional beyond measures used in this study. More studies are needed to verify this metric. Additionally, patients received a variety of irritating and nonirritating IV medications that may have contributed to chemically induced phlebitis or hyperplasia that may have influenced the failure to aspirate blood for sampling. Finally, only one type of MC was used in this study, and its design was purposed to reduce hemolysis and turbulent blood flow at the catheter tip. This may have affected the ability for blood withdrawal with retrograde flow and the results seen in this study. Thus, studies to evaluate time to effectiveness of blood withdrawal in other types of MCs and with patients who have more frequent and long-term blood sampling may help us to better understand the length of time MCs function for this purpose.
Since little was known about nursing practices and perceptions about the use of MCs for blood specimen collection, findings from the focus groups supported the presumption that variation in practices exist for this procedure. As no guidelines exist to direct nursing actions with regard to blood collection, there are opportunities to test and develop best practices for blood sampling from MCs for safe and effective use.
Conclusions
In this study, findings demonstrate that blood can be withdrawn successfully from this type of MC with very low rates of hemolysis. These data may further support the use of MCs for the purpose of phlebotomy. However, since only one type of MC with structural alterations intended to reduce hemolysis was used in this study, and the study was conducted at one clinical site, it is not clear that similar results would be achieved in other types of MCs or settings. Additionally, withdrawal of blood samples for testing had little impact on dwell time and completion of therapy. Since removal of MCs at the time of discharge was the main reason for removal, complete understanding about catheter longevity and usefulness over longer periods of time is limited, particularly when subjected to repeated blood withdrawal. When withdrawing blood from MCs, nurses tended to use procedures recommended for phlebotomy in other vascular access devices to guide their actions. More information was gained to better understand how nurses perform and use the MC for blood withdrawal and provides opportunities to develop and standardize practices for blood sampling from MCs.
Limitations
To our knowledge, this was the first known exploratory study to evaluate use and outcomes of MCs for blood sampling in a single, acute care hospital. For this reason, findings may not be generalizable to all patient populations and settings. Since only one type of MC was available for use in this study, findings may not reflect outcomes for other types of MCs. Another limitation is associated with manual collection of data for hemolysis; other settings with automated and robust hemolysis reports would lessen complexity of data collection. However, all historical results were available in the EHR for later capture. The investigator was present only 5 days a week and may have missed patients with a short-term LOS or was not able to capture the data in 24 hours. However, the investigator was present in early hours during usual blood specimen collection times to capture subjects, and another investigator did rounds on patients in the afternoons for possible inclusion or for other blood specimens being collected. One limitation in the study was that the investigators did not perform or observe all blood sampling events by the nurses, and some data were collected from the EHR for some of the metrics in the study. Thus, the actual techniques used by nurses likely varied. Another limitation was that the patients' LOS was not collected to compare with dwell time, so other factors may have influenced dwell time beyond LOS. More studies are needed to replicate or substantiate outcomes and uses.
Recommendations for practice
Given that this study is the first to evaluate outcomes from using MCs for blood sampling and few references exist to guide clinicians as to the best procedures to use, evaluation of these practices and outcomes at other settings is recommended. Further research is needed to explore outcomes from MCs other than the one tested in this study to compare results, particularly with regard to hemolysis and catheter performance when multiple actions are applied to MCs. Other recommendations are to develop and test procedures for blood sampling from MCs to contribute to and establish standards of practice in this area.

Midline catheter use significantly reduce CLABSI rates
Introduction: Establishing an effective midline program involves more than simply learning an insertion technique for a new product. Midline catheters provide a reliable vascular access option for those patients with difficult venous access who would otherwise require multiple venipunctures or the use of higher-risk central lines to maintain access. An effective midline program establishes a protocol for device selection and includes standing orders to facilitate speed to placement.
[ctt tweet=”ReTweet if useful… Midline catheter use significantly reduce CLABSI rates http://ctt.ec/a3pP1+ @ivteam #ivteam” coverup=”a3pP1″]
Methods: Our retrospective descriptive review evaluated the successful integration of midline programs into existing vascular access bedside insertion programs in 2 acute care hospitals. The investigator reviewed a convenience sample of hospital patients. Participants in the study included vascular access team managers and team members from the sample sites.
Results: The results of this 2-hospital study demonstrate successful integration of a midline program into a bedside insertion program with 0 midline-related infections since initiation. Documentation of overall central line-associated bloodstream infection rates for hospital 1 changed from 1.7/1000 catheter-days to 0.2/1000 catheter-days, reflecting a 78% reduction in infections and a projected cost avoidance of $531,570 annually. Both hospitals demonstrated reduced rates of infection following implementation of a midline program.
Conclusions: Midlines have a history of lower risk for both infection and thrombosis compared with central venous devices. Although more research is needed on the more recently developed midline catheters, available evidence suggests that midlines provide a safe and reliable form of vascular access, reducing costs and the risk of infection associated with central venous catheters, especially those placed solely for patients with difficult venous access.
Moureau , N., Sigl, G. and Hill, M. (2015) How to Establish an Effective Midline Program: A Case Study of 2 Hospitals. The Journal of the Association for Vascular Access. 20(3), p.179-188.
Thank you to our partners for supporting IVTEAM [slideshow_deploy id=’23788’]
About IVTEAM citation library
IVTEAM has established itself as a core global resource for infusion and vascular access practitioners. Each day 6K users have access thousands of pages of easy to access relevant intravenous news and updates. IVTEAM offers its visitors a premier service that has no financial or subscriber based restrictions. The editorial stance of IVTEAM is to ensure no editorial restrictions exist; content remains unbiased and independent.
It is crucial that IVTEAM stays up-to-date and offers visitors regular, current updates. Each day news feeds are analysed, websites searched and various additional sources of information visited. This results in large amounts of data being sifted through for relevant information every day. Only a small amount of data is relevant to IV practice and makes it onto IVTEAM.
- Home
- Article citations
- Biomedical & Life Sci.
- Business & Economics
- Chemistry & Materials Sci.
- Computer Sci. & Commun.
- Earth & Environmental Sci.
- Engineering
- Medicine & Healthcare
- Physics & Mathematics
- Social Sci. & Humanities
Journals by Subject
- Biomedical & Life Sciences
- Chemistry & Materials Science
- Computer Science & Communications
- Earth & Environmental Sciences
- Social Sciences & Humanities
- Paper Submission
- Information for Authors
- Peer-Review Resources
- Open Special Issues
- Open Access Statement
- Frequently Asked Questions
Publish with us
Article citations more>>.
Moureau, N., Sigl, G. and Hill, M. (2015) How to Establish an Effective Midline Program: A Case Study of 2 Hospitals. Journal of the Association for Vascular Access, 20, 179-188. https://doi.org/10.1016/j.java.2015.05.001
has been cited by the following article:
TITLE: Application of Midline Catheter in Patients with Oral Cavity Malignancies during Perioperative Period
KEYWORDS: Oral Cavity Malignancy , Midline Catheter , Indwelling Needle , Adverse Reactions
JOURNAL NAME: Journal of Cancer Therapy , Vol.13 No.10 , October 31, 2022
ABSTRACT: Objective: The study aims to compare the application value of midline catheter and indwelling needle in patients with oral cavity malignancies during perioperative period. Methods: 146 patients with oral cavity malignancies admitted to our hospital from January 2019 to July 2021 were selected as the research subjects. 73 patients treated with midline catheters during the treatment were the experimental group, and another 73 patients were treated with indwelling needles as the control group. The indwelling time, total number of puncturing times, and incidence of adverse reactions of two catheterization methods were compared between the two groups. Meanwhile, each patient was investigated for treatment satisfaction. Result: The indwelling time was significantly longer in the experimental group than in the control group (P 2 = 4.960, P = 0.0259) was significantly lower than that in the control group in terms of catheter occlusion (χ2 = 12.56, P = 0.0004), catheter detachment (χ2 = 8.46, P = 0.0036), drug extravasation (χ2 = 3.27, P = 0.0011), phlebitis (χ2 = 3.62, P = 0.0003), and bleeding from the puncture point (χ2 = 14.98, P = 0.0001). The satisfaction rate (χ2 = 33.45, P 2 = 16.57, P those in the control group, while the dissatisfaction rate was significantly lower than that in the control group (χ2 = 11.38, P = 0.0007). The difference is statistically significant. Conclusion: Compared with indwelling needle, the application of midline catheters in patients with oral cavity malignancies during perioperative period can effectively reduce the number of puncturing times and the incidence of catheter-related adverse reactions, with a high satisfaction rate, which is worthy of clinical promotion and application.
Related Articles:
- Open Access Articles BAX G(-248)A Gene Polymorphism and Its Association with Risk of Non-Small Cell Lung Cancer—A Case Control Study Jamsheed Javid, Rashid Mir, Pramod Kumar Julka, Prakash Chandra Ray, Alpana Saxena Open Journal of Apoptosis Vol.4 No.2 , April 29, 2015 DOI: 10.4236/ojapo.2015.42005
- Open Access Articles Challenges of E-Journal Club: A Case Study Nazila Zarghi, Seyed Reza Mazlom, Majid Rahban Creative Education Vol.3 No.6 , October 9, 2012 DOI: 10.4236/ce.2012.36105
- Open Access Articles Association between Homa Index and Vascular Endothelial Dysfunction in Type 2 Diabetic Patients N. Rama Kumari, I. Bhaskara Raju, M. Aruna Devi, M. Pallam Praveen Open Journal of Internal Medicine Vol.4 No.4 , December 15, 2014 DOI: 10.4236/ojim.2014.44019
- Open Access Articles Calculation of the Effective G-Factor for the (ns 2 S 1/2 )→(np 2 P 3/2 )→(n's 2 S 1/2 ) Transitions in Hydrogen-Like Atoms and Its Application to the Atomic Cesium Ziya Saglam, S. Burcin Bayram, Mesude Saglam Journal of Modern Physics Vol.1 No.6 , December 29, 2010 DOI: 10.4236/jmp.2010.16057
- Open Access Articles Progression of Minimally Invasive Urological Surgery at Hôpital Général Idrissa Pouye in Dakar in 20 Years of Practice Mohamed Jalloh, David M. C. Loko, Mouhamadou Moustapha Mbodji, Medina Ndoye, Abdourahmane Diallo, Thierno Amadou Diallo, Serigne Abdou Diagne, Moussa Sène, Babou Sakho, Harmonie Adanmayi, Becaye Gassama, Lamine Niang, Issa Labou, Serigne Gueye Open Journal of Urology Vol.13 No.7 , July 21, 2023 DOI: 10.4236/oju.2023.137026
- Journals A-Z
About SCIRP
- Publication Fees
- For Authors
- Peer-Review Issues
- Special Issues
- Manuscript Tracking System
- Subscription
- Translation & Proofreading
- Volume & Issue
- Open Access
- Publication Ethics
- Preservation
- Privacy Policy

Vascular Access
5 key advantages of midlines you should know of
Campus Vygon
Short Peripheral Intravenous Catheters (SPIVCs) are commonly used as default vascular access devices by practicians around the world for short and midterm therapies. However, they can be a problem in case of medium therapies as that would entail the insertion of multiple cannulas. Many professionals choose to place central lines instead. However, they can be contraindicated, and they can require expertise and additional costs. To bridge the gap between SPIVCs and central lines, midlines were introduced in the 1950s and are today referred to as “the middle ground” of intravenous therapy administration 1 .
In this article, we will define midlines and discuss their indications and their advantages compared to SPIVCs and/or central lines.
What are midlines?
The Infusion Therapy Standards of Practice (INS 2021) differentiate between mini-Midlines and Midline catheters. It defines them as follow:
Long peripheral intravenous catheter (long PIVC) or mini-midline: inserted in either superficial or deep peripheral veins and offers an option when a short PIVC is not long enough to adequately cannulate the available vein. A long PIVC can be inserted via traditional over-the-needle technique or with more advanced procedures, such as Seldinger and accelerated Seldinger techniques.
Midline catheter: inserted into a peripheral vein of the upper arm via the basilic, cephalic, or brachial vein with the terminal tip located at the level of the axilla in children and adults; for neonates, in addition to arm veins, midline catheters may be inserted via a scalp vein with the distal tip located in the jugular vein above the clavicle or in the lower extremity with the distal tip located below the inguinal crease.
In this article, we will refer to midlines based on the second definition.
Midline indications
Midlines can be used for the administration of blood, fluids and peripherally compatible medications’ and can be used in the following circumstances:
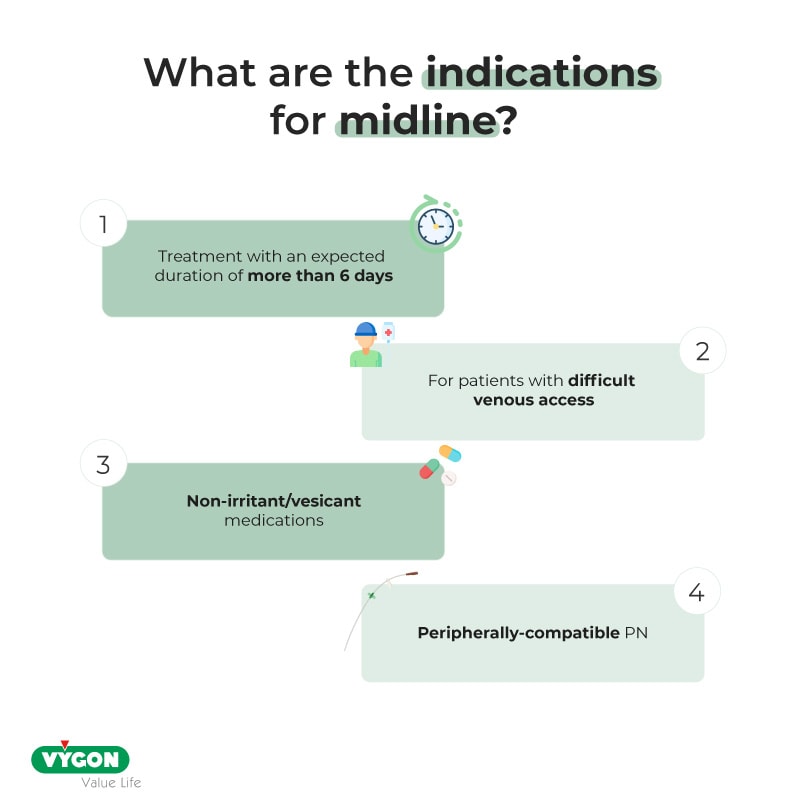
Advantages of midlines
In many situations, it can be preferable to use a midline rather than an SPIVC or a central line considering their various advantages:
- Greater dwell time than SPIVCs
Midlines have a greater dwell time than SPIVCs thanks to their length.
Devries et al., (2016) suggests that SPIVCs dwell for 2.3 to 4.3 days. For midterm therapies, practicians often have to remove or replace the cannula which leads to the multiplication of punctures. This makes SPIVCs inappropriate for therapies that are longer than this period or for patients with difficult venous access.
According to Fabiani et al., Moureau et al., 2015, Midlines have a dwell time of 7.7 to 16.4 days. It is longer than SPIVCs’ and therefore more suitable for safe and secure mid-term therapies.
- Decrease in major complications
The use of midlines can lower the risk of early and delayed complications compared to SPIVCs and central lines.
A study conducted by Marsh et al., (2023) has shown that SPIVCs had a higher risk of phlebitis and PIVC infiltration. For PICCs, literature has found that the use of PICCs was associated to a higher risk of developing serious complications such as CLABSI (Central line-associated Bloodstream Infections) and catheter occlusion. 2
Therefore, Midlines appear to be safer for patients that require mid-term therapies.
- Can be placed by nurses
The placement of midlines can be delegated to nurses. Not only is it timesaving for the teams, but it can also increase their use inside the units and therefore, reduce the risk of serious complications that come with the insertion of central lines.
To ensure the proper functioning of the VAD and the safety of the patient, training of healthcare workers in midline insertion, care and maintenance is a requirement.
- Can benefit the well-being of both patients and healthcare professionals
Midlines can have a positive impact on the well-being of patients and healthcare workers.
Literature demonstrates that midlines have a high rate of first-attempt success. 3 This makes them a more comfortable option for patients as they do not have to undergo multiple needlesticks. This can ensure venous capital preservation.
The reduction of punctures coupled with the possible insertion of midlines in DIVA and overweight patients can also benefit doctors and nurses. It would help reduce their workload and stress linked to the difficulty of finding veins.
- An economic alternative
Midlines are a cost-effective alternative compared to SPIVCs and central lines.
Literature has found that the increase of midline placement by a trained team on a two-year period significantly reduced the cost of VAD placement. 4 The cost savings through midlines can be explained by a limited use of more costly central lines with patients that do not require them, as well as the decrease of costs linked to central line-associated complications such as CLABSI.
Moreover, midlines are a more economic option compared to SPIVCs as the latter require multiple cannula replacements in prolonged therapies, that can reach four or more repeated insertions. 5 This can also be beneficial for patients with difficult-to-access peripheral veins.
With patients’ safety and well-being as a priority, midlines could be considered the most suitable VAD for midterm therapies. It offers various advantages that improve the patients’ experience and general comfort. Moreover, the device is a key asset for healthcare institutions as it has been proven to be more economic and easier to use by the personnel. Nevertheless, specific training of nurses and healthcare professionals should be conducted in order to ensure a safe patient-centred procedure.
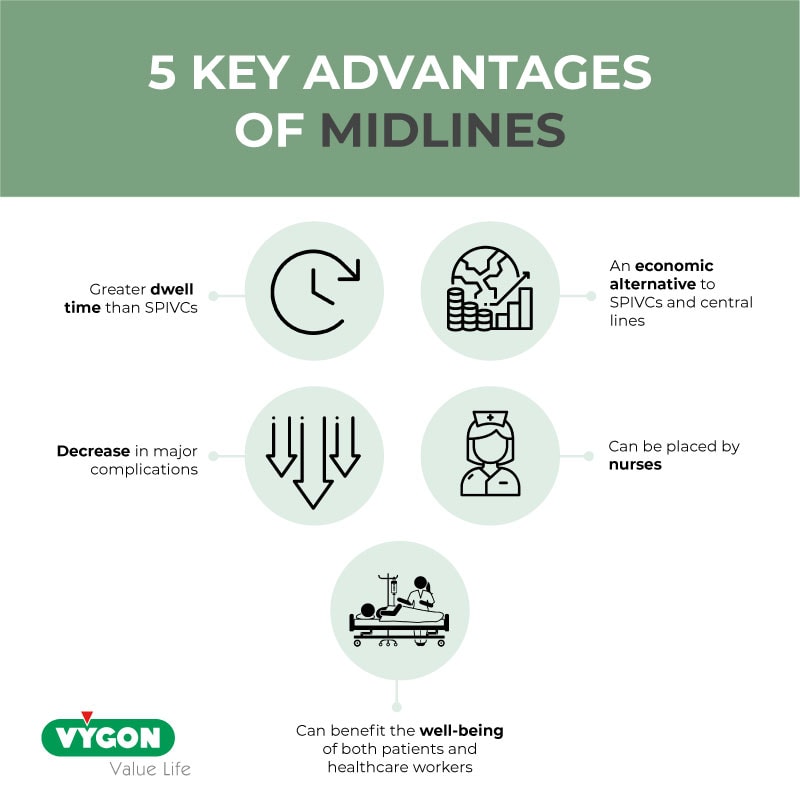
Bibliography:
- N Richard Anderson – “Midline catheters: the middle ground of intravenous therapy administration” – Journal of Infusion Nursing 2004 Sep-Oct;27(5):313-21 – Available online: https://pubmed.ncbi.nlm.nih.gov/15385895/
- Lakshmi Swaminathan, Scott Flanders, Jennifer Horowitz, Qisu Zhang, Megan O’Malley, Vineet Chopra – “Safety and Outcomes of Midline Catheters vs Peripherally Inserted Central Catheters for Patients With Short-term Indications: A Multicenter Study” – JAMA Intern Med. 2022 Jan 1;182(1):50-58 – Available online: https://pubmed.ncbi.nlm.nih.gov/34842905/
- Daniel Z.Adams MD, Andrew Little DO, Charles Vinsant MD, Sorabh Khandelwal MD – “The Midline Catheter: A Clinical Review” – The Journal of Emergency Medicine (Volume 51, Issue 3, September 2016, Pages 252 – 258 – Available online: https://www.sciencedirect.com/science/article/abs/pii/S0736467916301597
- Shanja-Grabarz, L. Santoriello, G. Ritter, V. Patel, J. Nicastro, R. Barrera. – “Midline Catheters: An Underutilized, Cost-Effective Means of Decreasing Central Venous Catheter Use” – Academic surgical congress abstracts archive (January, 2019) – Available online: https://www.asc-abstracts.org/abs2019/59-05-midline-catheters-an-underutilized-cost-effective-means-of-decreasing-central-venous-catheter-use/
- Emma Bundgaard Nielsen, Louise Antonsen, Camilla Mensel, Nikolaj Milandt, Lars Skov Dalgaard, Britta Skov Illum, Hanne Arildsen, Peter Juhl-Olsen – “The efficacy of midline catheters—a prospective, randomized, active-controlled study” – International Journal of Infectious Diseases (Volume 102, January 2021, Pages 220-225) – Available online: https://www.sciencedirect.com/science/article/pii/S1201971220322578
- DeVries, M., Valentine, M., & Mancos, P. (2016) – “Protected clinical indication of peripheral intravenous lines: Successful implementation” – Journal of the Association for Vascular Access, 21(2), 89–92. https://doi. org/10.1016/j.java.2016.03.001
- Moureau, N., Sigl, G., & Hill, M. (2015). “How to establish an effective midline program: A case study of 2 hospitals.” – Journal of the Association for Vascular Access, 20(3), 179–188. https://doi.org/10.1016/j.java. 2015.05.001
- Fabiani, A., Dreas, L., & Sanson, G. (2017). “Ultrasound-guided deep-arm veins insertion of long peripheral catheters in patients with difficult venous access after cardiac surgery. Heart & Lung”, 46(1), 46–53. https://doi.org/10.1016/j.hrtlng.2016.09.003
- Nicole Marsh, Emily N. Larsen, Catherine O’Brien, Robert S. Ware, Tricia M. Kleidon, Peter Groom, Barbara Hewer, Evan Alexandrou, Julie Flynn, Kaylene Woollett, Claire M. Rickard – “Safety and efficacy of midline catheters versus peripheral intravenous catheters: A pilot randomized controlled trial” – International Journal of Nursing Practice (Volume 29, Issue 2, April 2023) – Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/ijn.13110
Related Articles
Wireless Ultrasound in the field of Vascular Access
by Daniele Guerino Biasucci | 17 Jan 2024
The PERSEUS guidelines, recently released by the European Society of Anaesthesiology and Intensive Care (ESAIC), recommend a global use of ultrasound to assist all steps of central venous catheterization in order to minimize all immediate, early, and late...

Ultrasound in the placement of PICCs and midlines
by Campus Vygon | 20 Dec 2023
As the GAVeCeLT group (1) stated in their manual on PICCs and midlines regarding the evolution of PICC use, the introduction of ultrasound in the 2000s has been a breakthrough in the field. In this article, we delve into the utility of ultrasound in the field of...
CSSAS: Closed Safety Systems for Administration to Reduce Risks of Occupational Exposure to HMPs
by Campus Vygon | 7 Dec 2023
Healthcare professionals handling chemotherapy for preparation and administration of HMPs are likely to be exposed to potential contamination risks associated with cytotoxic drugs. These risks can lead to health implications, ranging from minor issues like headaches,...

IMAGES
VIDEO
COMMENTS
1. A midline catheter program was evaluated at two hospitals. 2. Each hospital outlined the development of their programs with outcomes. 3. Midlines were compared with central venous catheters for levels of risk. 4. Indications for midlines were included along with discussion on recommendations and issues for usage. 5.
Inclusion criteria were for a 2-cohort sample of acute care hospitals with operating midline programs consisting of bedside insertions, policies, and out-comes of >2 years. Excluded were hospitals without func-tional midline protocols and hospitals in excess of 2. This project was designed as a case study; aggregated facility public
An effective midline program establishes a protocol for device selection and includes standing orders to facilitate speed to placement. Methods: Our retrospective descriptive review evaluated the ...
Abstract. Introduction: Establishing an effective midline program involves more than simply learning an insertion technique for a new product. Midline catheters provide a reliable vascular access option for those patients with difficult venous access who would otherwise require multiple venipunctures or the use of higher-risk central lines to maintain access. An effective midline program ...
A prospective, controlled, randomized clinical trial was conducted to determine if intravenous vancomycin could be safely administered through a novel midline catheter and found that it can be safely and cost-efficiently administered in the deep vessels of the upper arm using the midline study device. Expand. 63. PDF.
9,10 Enabling specialty teams to choose devices and insert catheters based on patient need increases efficiency in treatment delivery, hospital through-put, and patient satisfaction. 11-20 Creation of a policy and referral process that includes midlines should be a part of an overall hospital strategy to reduce infections while effectively ...
Create a Free Account. ... How to establish an effective midline program: a case study of 2 hospitals. J Vasc Access. 2015; 20: 179-188. ... How to establish an effective midline program: a case study of 2 hospitals. J Vasc Access. 2015; 20: 179-188. View in Article Scopus (36) ...
How to Establish an Effective Midline Program: A Case Study of 2 Hospitals. Nancy Moureau, BSN, ... Abstract . View Article titled, How to Establish an Effective Midline Program: A Case Study of 2 Hospitals. Open the PDF for in another window. Cover Image. Cover Image. All Issues. Feedback; Advertising; Terms & Conditions; Facebook;
safety.12,16 Although recent studies reported 0 midline cath - eter BSIs during the first 2 years11 and 4 years13 of imple-menting a midline catheter program, Hogle et12 found al the incidence of midline catheter BSI to be 0.88 per 1000 midline catheter days across 5 hospitals over a 12-month period. Additionally, other non-BSI reported ...
This page is a summary of: How to Establish an Effective Midline Program: A Case Study of 2 Hospitals, Journal of the Association for Vascular Access, September 2015, Allen Press, DOI: 10.1016/j.java.2015.05.001. You can read the full text: Read
The absolute volume of midline use varied across hospitals, ranging from 100 to 1837 devices, with corresponding midline utilisation rates of .97%-12.92% (p<0.001). Overall, the sample of devices in this study represented 5%-72% of all midlines placed at individual hospitals; thus, some hospitals used very few midlines, while others used more.
How to establish an effective midline program: a case study of 2 hospitals. J Assoc Vasc Access. 2015; 20: 179-188. Crossref; ... it is difficult to establish the differential rate of complications between MCs and CVCs. ... How to establish an effective midline program: a case study of 2 hospitals. J Assoc Vasc Access. 2015; 20: ...
Abstract Introduction: Establishing an effective midline program involves more than simply learning an insertion technique for a new product. Midline catheters provide a reliable vascular access option. Abstract Introduction: Establishing an effective midline program involves more than simply learning an insertion technique for a new product. ...
How to establish an effective midline program: A case study of 2 hospitals. J Assoc Vasc Access . 2015;20(3):179-88. O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections.
Establishing an effective midline program involves more than simply learning an insertion technique for a new product. Midline catheters provide a reliable vascular access option for those patients with difficult venous access who would otherwise require multiple venipunctures or the use of higher-risk central lines to maintain access. An effective midline program establishes a protocol for ...
This investigator-led study used a prospective, observational, and mixed methods design at a single-site hospital to evaluate the function and outcomes of MCs for blood specimen collection in two medical and two surgical units at the study facility. ... Hill M. How to establish an effective midline program: a case of 2 hospitals. J Assoc ...
Create a Free Account. ACEP Member Login. ACEP Members, full access to the journal is a member benefit. ... How to establish an effective midline program: a case study of 2 hospitals. J Assoc Vasc Access. 2015; 20: 179-188. ... a cost-effective proposal for timely central line removal. J Surg Res. 2014; 191: 1-5.
In 2015, the hospital implemented a midline catheter program to increase vascular access options while reducing the use of central venous catheters. The policy permitted indefinite infusion of vasopressors and inotropic agents through a properly placed midline catheter. The study was approved by the hospital's institutional review board.
The provision of a safe clinical framework for midline insertion, and the improvement of patient care and experiences by avoiding treatment interruptions and unnecessary cannulation attempts from failed traditional peripheral vascular access devices are assessed. Introduction: Midline catheters have been reported to be an effective and safe means of providing patients with intravenous access ...
Documentation of overall central line-associated bloodstream infection rates for hospital 1 changed from 1.7/1000 catheter-days to 0.2/1000 catheter-days, reflecting a 78% reduction in infections and a projected cost avoidance of $531,570 annually. Both hospitals demonstrated reduced rates of infection following implementation of a midline program.
Common vascular access challenges (as illustrated in Table 1) must be navigated wisely to achieve the critical and at times competing goals of vessel preservation and effective delivery of needed infusion therapy. 2-5 Resources and algorithms are available to guide vascular access device (VAD) selection and placement, but the evolving nature of these situations, varied available resources, and ...
Article citations More>>. Moureau, N., Sigl, G. and Hill, M. (2015) How to Establish an Effective Midline Program: A Case Study of 2 Hospitals. Journal of the Association for Vascular Access, 20, 179-188.
Midlines can have a positive impact on the well-being of patients and healthcare workers. Literature demonstrates that midlines have a high rate of first-attempt success. 3 This makes them a more comfortable option for patients as they do not have to undergo multiple needlesticks. This can ensure venous capital preservation.