
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases and Disorders Education Program
- Publications and Resources
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- Clinical Trials
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- < Back To Research Topics

Cystic Fibrosis Research
Language switcher.
Cystic fibrosis is a life-shortening genetic disease that affects many organs of the body, especially the lungs. No cure for cystic fibrosis exists yet, but decades of NHLBI leadership in and support for research have led to more and better treatment options.
Effective treatments now allow many people with cystic fibrosis to live well into adulthood. Close to 40,000 children and adults are living with cystic fibrosis in the United States.
The condition is caused by mutations, or changes, in the gene that codes for the cystic fibrosis transmembrane conductance regulator (CFTR) protein. CFTR helps control how chloride – a component of salt – and other ions are secreted by cells. The chloride attracts water and thins out the mucus the cells produce.
Current research supported by the NHLBI focuses on understanding how changes in the CFTR protein lead to the development of cystic fibrosis. The Institute carries out and supports studies that could lead to gene therapies and other treatments that may help the lives of people who have the condition.
NHLBI research that really made a difference
- Medicines that address underlying causes: NHLBI’s lung program supported decades of research to understand the structure and function of a lung protein that is genetically changed by cystic fibrosis. This foundational work led to the industry discovery of ivacaftor , the first drug to treat the underlying cause of cystic fibrosis, which the U.S. Food and Drug Administration (FDA) approved in 2012.
- Effective combination therapies: NHLBI-supported clinical trials led to the approval of a triple combination of CFTR modulator medicines that improves lung function in about 90% of people who have cystic fibrosis. However, further studies have shown that people of color are less likely than white people to have mutations that are eligible for treatment with current CFTR modulators. Researchers supported in part by the NHLBI are investigating these disparities. The Institute is also funding projects to develop treatments that will work for people who have less common CFTR mutations.
- Remote monitoring of lung health: As COVID-19 made in-person healthcare visits more challenging, an NHLBI-funded study developed an at-home diagnostic tool for measuring how well the lungs work. The FDA-cleared system uses a handheld breathing device and a mobile app called Breathe Easy. Patients blow into the device and communicate directly with their healthcare provider, who can monitor how well their lungs are working and adjust medicines as needed. Between April 2020 and May 2021, the Cystic Fibrosis Foundation distributed nearly 20,000 of the devices to people with cystic fibrosis in the United States. A study showed results were reliable 81% of the time for a sample of 48 patients who used the portable device with the app.
Find funding opportunities and program contacts for cystic fibrosis research.
Current research funded by the NHLBI
Our Division of Lung Diseases and its Airway Biology and Disease Branch oversee much of the research on cystic fibrosis that we fund.
- Biomarkers of declining lung function: The NHLBI Catalyze Program is supporting the development of a biomarker-based platform that predicts lung function decline in patients with cystic fibrosis and a web-based application to inform physicians when a patient may require therapeutic interventions.
- More options for disease modulators: Investigators are studying CFTR protein folding and the use of modulators for patients with rare variant mutations not typically eligible for cystic fibrosis modulator therapy.
- Monitoring methods with fewer side effects: Researchers are combining noninvasive, radiation-free imaging and proteomic biomarkers to diagnose and monitor lung disease progression in kids with cystic fibrosis.
Current research on cystic fibrosis treatments
- New treatment strategies: Although treatments improve lung function for many living with cystic fibrosis, some people have CFTR mutations that do not respond to available CFTR modulators. Even those whose disease responds to CFTR modulators still have trouble clearing bacteria out of their lungs. We are supporting a project, Novel Strategies to Clear Bacteria from the CF Lung , that aims to develop an inhaled medicine to shift the lung’s immune balance to help clear bacteria from lungs of people with cystic fibrosis.
- New treatment mechanisms: NHLBI-funded studies are testing whether a medicine to correct acid problems in the blood can also help reduce acid levels in the airways , which can then prevent or slow the development of cystic fibrosis.
- Better medicines to fight mucus: Researchers are developing new medicines to help clear and target the thick mucus found in cystic fibrosis lungs and improve how well the lungs work. This can also help prevent inflammation and infection.
- Antibiotic alternatives: Investigators are studying the thiocyanate (SCN−) analog selenocyanate (SeCN−) as an alternative therapeutic for the treatment of cystic fibrosis lung pathogens that are difficult to treat with current antibiotics.

Watch videos of a 2022 workshop that brought together experts from the NHLBI and other parts of NIH, as well as the Cystic Fibrosis Foundation, to identify future research needs for treating cystic fibrosis.
Current research on gene editing and cystic fibrosis
The NHLBI is supporting research on new genetic therapies to treat cystic fibrosis. For example, researchers are studying state-of-the-art gene delivery tools and technologies that may be better at delivering a corrected gene to lung cells. Researchers are also working on better methods to improve genetic therapies in the laboratory before moving to clinical trials.
Through the NIH Common Fund Somatic Cell Genome Editing (SCGE) Program , the NHLBI supports studies that explore new genetic therapy approaches to repair the cystic fibrosis gene, among others.
- Gene editing for lung disease: Studies using CRISPR gene editing tools will correct genes in the cells that line the airways. Using these tools could lead to new treatments for genetic and acquired lung disease.
- Gene editing for cystic fibrosis: A research program is developing combinatorial nonviral and viral CRISPR delivery for lung diseases. These studies focus on efficiently targeting gene editing tools to diseased lung cells in people who have cystic fibrosis.
To identify research barriers and challenges to using gene editing as a means to cure cystic fibrosis, the NHLBI participated in a joint workshop with the Cystic Fibrosis Foundation in 2018. The Institute also participated in a 2020 virtual workshop to discuss challenges and opportunities that could be addressed in a potential second phase of the SCGE program.
The NHLBI funds other studies of gene editing for cystic fibrosis as well.
- Molecular targets to treat cystic fibrosis: The NHLBI supports research for new molecular therapies , including gene editing. Molecular therapies have helped restore the CFTR protein function for some but not all people with cystic fibrosis. New research uses airway cells and animal models to look for more ways to prevent and treat this condition.
- How nanoparticles may improve treatment effectiveness: To improve the delivery of gene editing tools and other therapeutics, researchers are developing more effective virus-inspired nanoparticles to penetrate mucus barriers in diseases with thick mucus like cystic fibrosis.
Find more NHLBI-funded studies on gene editing and cystic fibrosis at NIH RePORTER.
Read more about NHLBI-supported research on gene editing, which involves making changes to a specific DNA sequence to correct the mutation in the cystic fibrosis gene: Genome editing for cystic fibrosis: A Q&A with Peter Glazer, Ph.D., M.D.
Current research on understanding the causes of cystic fibrosis
- New treatment and prevention: NHLBI-supported scientists are carrying out Multi-Scale Investigations of Respiratory Mucus/Mucin Structure and Function in Health and Disease designed to build a solid foundation of knowledge about mucus, how it forms, and how it works to protect the body from infections. The resulting knowledge could lead to new ways to treat and prevent lung problems that result from the thick, sticky mucus caused by CFTR mutations.
- Origins of disease: Scientists are using a cystic fibrosis animal model to study the origins of cystic fibrosis airway disease . Researchers hope this will help speed up the development of new treatments for early lung disease.
- High-resolution imaging to better understand lung disease mechanisms: Another NHLBI-funded study uses an imaging method called optical coherence tomography (OCT) to take high-resolution images of the lungs and the nose. OCT can help researchers better understand how mucus is cleared and how cystic fibrosis affects this process.
Find more NHLBI-funded studies on the causes of cystic fibrosis at NIH RePORTER.

Learn more about how cystic fibrosis changes the airways’ cellular makeup: Molecular analysis identifies differences between healthy lung and lungs of people who have cystic fibrosis .
Cystic fibrosis research labs at the NHLBI
The Division of Intramural Research , which includes investigators from the Pulmonary Branch , is actively engaged in the study of cystic fibrosis.
Related cystic fibrosis programs
- The Trans-Omics for Precision Medicine (TOPMed) program includes participants who have cystic fibrosis, which may help researchers understand how genes affect people and how individuals respond to treatment.
- The NHLBI-funded LungMAP research centers are creating molecular maps of the lungs to better understand rare lung diseases in children, including cystic fibrosis.
Explore more NHLBI research on cystic fibrosis
The sections above provide you with the highlights of NHLBI-supported research on cystic fibrosis. You can explore the full list of NHLBI-funded studies on the NIH RePORTER .
To find more studies:
- Type your search words into the Quick Search box and press enter.
- Check Active Projects if you want current research.
- Select the Agencies arrow, then the NIH arrow, then check NHLBI .
If you want to sort the projects by budget size — from the biggest to the smallest — click on the FY Total Cost by IC column heading.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Transl Med

Cystic fibrosis: current therapeutic targets and future approaches
Misbahuddin m. rafeeq.
1 Department of Pharmacology, Faculty of Medicine, King Abdulaziz University, Rabigh Campus, Jeddah, 21589 Saudi Arabia
Hussam Aly Sayed Murad
2 Department of Pharmacology, Faculty of Medicine, Ain Shams University, Cairo, 11562 Egypt
Study of currently approved drugs and exploration of future clinical development pipeline therapeutics for cystic fibrosis, and possible limitations in their use.
Extensive literature search using individual and a combination of key words related to cystic fibrosis therapeutics.
Key findings
Cystic fibrosis is an autosomal recessive disorder due to mutations in CFTR gene leading to abnormality of chloride channels in mucus and sweat producing cells. Respiratory system and GIT are primarily involved but eventually multiple organs are affected leading to life threatening complications. Management requires drug therapy, extensive physiotherapy and nutritional support. Previously, the focus was on symptomatic improvement and complication prevention but recently the protein rectifiers are being studied which are claimed to correct underlying structural and functional abnormalities. Some improvement is observed by the corrector drugs. Other promising approaches are gene therapy, targeting of cellular interactomes, and newer drugs for symptomatic improvement.
Conclusions
The treatment has a long way to go as most of the existing therapeutics is for older children. Other limiting factors include mutation class, genetic profile, drug interactions, adverse effects, and cost. Novel approaches like gene transfer/gene editing, disease modeling and search for alternative targets are warranted.
Introduction and pathophysiology
Cystic fibrosis (henceforth CF) is autosomal recessive disease involving mucus and sweat producing cells affecting multiple organs with lungs most severely affected leading to death in 90% of patients [ 1 ]. A mutation in Cystic fibrosis trans-membrane conductance regulator (henceforth CFTR) gene changes a protein (a regulated chloride channel), which regulate the activity of other chloride and sodium channels at the cell surface epithelium [ 2 – 4 ]. There are about 70,000 worldwide cases and approximately 1000 new cases are added each year. CF is most common in white people of north European ancestry having 1 in 2000–3000 births [ 5 ] and least in Asian-Americans having 1:30,000 newborns [ 6 ].
The CFTR protein lets chloride to pass through the mucus producing cells after which the water follows and mucus becomes thin. However, defective CFTR results in thick and sticky mucus obstructing the pathways [ 7 ], leading to serious lung infections especially pseudomonas. There is massive neutrophil infiltration releasing elastase, which overpowers the lung antiproteases contributing to tissue destruction [ 8 ]. Additionally, degranulating neutrophils release large quantities of nucleic acids and cytosol matrix proteins contributing to the mucus hyper-viscosity [ 9 ].
In the GIT, the mucous plugs obstruct the canaliculi of pancreas and gall bladder duct preventing enzyme and bile flow into duodenum triggering malabsorption and digestion abnormalities. Additionally, Distal Intestinal Obstruction Syndrome (DIOS), which is distinctive to CF, may occur especially in those with pancreatic insufficiency. This is characterized by ileo-cecal obstruction of inspissated intestinal contents [ 10 , 11 ]. There is also imbalance of minerals in blood due to loss of extra salt in sweat leading to dehydration, arrhythmias, fatigue, weakness, heat stroke and rarely death.
The CFTR gene is located at 7q31.2. More than 1900 mutation have been identified of which ‘F508del’ (deletion of three bases coding for phenylalanine at the 508th position) is the most common [ 12 ]. Six classes of mutations are described as depicted in Table 1 .
Table 1
Depicting various classes of mutations, the primary defect and the outcome with examples
| Mutation class | Defect | Outcome | Common mutations |
|---|---|---|---|
| I | Protein production | Complete absence of CFTR protein due to premature mRNA termination (nonsense or frame shift mutation) [ , , ] | G542X, W1282X, R553X, 621+G>T |
| II | Protein processing | Inability of protein to localize to correct cellular location due to abnormal post-translational modifications [ ] | F508del, N1303K, A455E |
| III | Protein regulation | Decreased activity of protein (chloride channel) in response to ATP due to abnormalities of the nuclear binding fold regions [ ] | G551D |
| IV | Protein conduction | Frequency of flow of ions and channel opening duration are reduced though there is generation of chloride currents on stimulation with cAMP [ ] | R117H |
| V | Reduced amount of functional CFTR | Stability of mRNA and/or mature protein is compromised [ , ] | A455E |
| VI | Normal amount of functional CFTR | Enhanced turnover due to C-terminus abnormalities | Q1412X |
Class I mutations contribute to protein production defect leading to complete absence of CFTR protein, found in 2–5% cases worldwide with the exception of Ashkenazi Jews, in whom 60% of patients carry at least one copy. Class II mutations contribute to protein processing abnormality leading to aberrant localization. It includes F508del which is the most common mutation accounting for 70% of the disease-causing alleles in US. Approximately 50% of the CF patients are homozygous and 90% are heterozygous for this allele. Class III mutations contribute to protein regulation abnormalities leading to a decreased activity. It also includes other mutations especially in regulatory domain. G551D is most common class III mutation. Class IV mutations contribute to protein conduction abnormalities leading to altered frequency of ion flow. Most common mutation is R117H. Class V mutations lead to a reduced amount of functional CFTR protein [ 13 – 17 ] and class VI mutation cause an enhanced protein turnover. Patients carrying Class I–III mutations manifest a more severe form of disease.
However, possible influences of gene modifiers for example TGF-beta1 and mannose-binding lectin have downplayed the clinical significance of a specific combination of mutations [ 18 ].
Complications
Respiratory system complications include Bronchiectasis, Chronic infections leading to pneumonia, growths (nasal polyps), hemoptysis, pneumothorax and eventually respiratory failure.
Digestive system complications include nutritional deficiencies including fat and fat soluble vitamins and diabetes (Nearly 20% of people with cystic fibrosis develop diabetes by age 30). Additionally, progressive hepatic dysfunction, gallstones, intestinal obstruction, intussusception, small intestine bacterial overgrowth (SIBO) and distal intestinal obstruction syndrome (DIOS) may also manifest.
Other complications may include Infertility, Osteoporosis, Electrolyte imbalances and dehydration manifesting as increased heart rate, fatigue, weakness and low blood pressure.
Because of better quality medical interventions and comprehensive care, there is a remarkable increase in percentage of patients (29.2% in 1986 to 49.7% in 2013) surviving above the age of 18 years.
The goals of treatment primarily include:
Respiratory system
Preventing and controlling lung infections—antibiotics are prescribed. These mainly consist of inhaled forms of azithromycin, tobramycin, aztreonam and levofloxacin. Other antibiotics recommended are ciprofloxacin, cephalexin, amoxicillin and doxycycline depending on the sensitivity patterns [ 19 , 20 ].
Control of airway inflammation—NSAIDs, inhaled and systemic steroids and cromolyn [ 21 ].
Reducing viscoelasticity and removing thick, sticky mucus from the lungs and dilating the airways—inhaled β agonists with humidified oxygen; a 3–6% hypertonic saline solution and dornase alfa are recommended [ 22 – 24 ].
Additionally exercise and physiotherapy including positive expiratory pressure (PEP) device or a high frequency chest wall oscillation device (a percussion vest) is recommended [ 25 ].
Preventing or treating intestinal blockages—oral rehydration and osmotic laxatives (incomplete blockage) and hyperosmolar contrast enemas (complete DIOS). A balanced electrolyte intestinal lavage solution or enema containing (diatrizoate meglumine and diatrizoate sodium) depending on vomiting status [ 26 ]. To prevent recurrence, regular administration of oral polyethylene glycol 3350 may be given for 6 months to 1 year.
Pancreatic insufficiency—pancreatic enzyme replacement therapy (PERT) containing multiple combinations of proteases, lipases and amylases [ 27 ].
Nutrition and electrolyte
Providing appropriate nutrition and preventing dehydration—a high-calorie-fat diet, supplemental vitamins ADEK, and minerals including fluoride and zinc are recommended. Additionally sodium chloride supplementation is given tailored to patient’s age and environmental conditions [ 28 ].
Figure 1 summarizes the main abnormalities and treatment approaches in CF patients.

The main pathophysiological dysfunctions and treatment modalities for CF patients. Inner trapezoid boxes depict the pathophysiological abnormalities and outer rectangular boxes depict the main treatments. The texts connecting the outer boxes show non-pharmacological management
In the recent past, Denufosol, an agonist of P2Y2 receptors was tried in CF patients but it eventually failed after early promising results. The detailed analysis is beyond the scope of this review.
Current and future medicinal products
The current and future therapeutic targets are mainly focused on correcting structural and functional abnormalities of CFTR protein. Additionally, some agents for symptomatic improvement are also in pipeline.
CFTR modulators
A new group of drugs called CFTR modulators are available which are able to correct the basic defect in CF, i.e. CFTR protein itself though the exact mechanism is not fully elucidated.
Developed by vertex pharmaceuticals and approved by FDA in 2012 for children ≥6 years having rare mutation, G551D (class III), ivacaftor (Kalydeco) [ 29 ] was the first successful medicine to rectify the defective protein and has proven to be very effective in two large multi-centric trials, STRIVE and ENVISION [ 30 , 31 ]. Marked improvement in FEV 1 , body weight and quality of life were observed. Now FDA has expanded its use in other mutations and also children aged 2–5 years based on the results of KIWI trial [ 32 ]. Additionally, a phase IV study (GOAL) also reported improvements in FEV 1 and FVC, BMI, quality of life and decreased sweat chloride concentration in patients carrying at least one G551D allele. More than 72% patients in this trial also carried F508del as second allele [ 33 ]. The G551D mutation causes the channel to act like a locked gate, preventing the trans-conductance of chloride and fluid. The location of channel is proper but the function is impaired. Ivacaftor increases the time of channel in open state. But the main limitation of this therapy is that G551D mutation is present in only 2.3% patients [ 34 ]. It is not found to be effective in the most common F508del (class II) mutation because of decreased availability of protein. Additionally, the high cost of therapy may also be a limiting factor (ICER: £335,000–£1,274,000/QALYs gained [ 35 ].
Another CFTR modulator, lumacaftor has shown favorable results in F508del mutation. This is the most common mutation affecting approximately 1/3rd CF population in US and nearly 70% in EU. This mutation affects the heat stability due to misfolding of NBD1 domain and restricts the CFTR in ER for subsequent degradation. It fails to localize to correct epithelial location and achieve normal structure. Increased transport of protein to cell surface was observed in vitro using cultured human bronchial epithelium [ 36 ]. Nevertheless, despite increased transport of protein to proper location, no correction of the underlying functional impairment was observed. Moreover, another in vitro study revealed contrasting negative results [ 37 ] which were further reinforced by a clinical trial. No significant improvement was observed in FEV 1 , CFQR scores and respiratory exacerbation rates [ 38 ].
Based on the individual mechanisms, a combination of lumacaftor and ivacaftor (Orkambi) was proposed to correct both, including protein trafficking as well as channel gating abnormalities.
Initially, phase II trials were conducted for both homozygous and heterozygous F508del patients >12 years old but only homozygous patients showed clinically significant results. Two large phase III trials, TRAFFIC and TRANSPORT were conducted with the combination therapy (600 + 250 and 400 + 250 mg versus placebo) in patients ≥12 years with primary endpoint as FEV 1 improvement at 24 weeks. Patients completing the study were progressed to 48 weeks PROGRESS trial. The isolated as well as pooled results showed a significant improvement in parameters including FEV 1 , reduction of exacerbations, decrease in hospitalizations, increase in BMI and CFQR scores; consistent across different dosage regimens and patient groups. The adverse effects were comparable to placebo group except one case of death during extension phase [ 39 , 40 ].
Additionally, a phase I study in homozygous children ≤12 years also showed promising results but further advance phase studies are needed [ 41 ]. However, when compared to ivacaftor monotherapy in patients having G551D mutation in a separate study, there was significantly less improvement in pulmonary function with combination therapy [ 42 ].
Orkambi (lumacaftor + ivacaftor) is approved recently for homozygous F508del patients ≥12 years. Orkambi acts by a two-step method. Lumacaftor assists in moving the defective protein to its correct location and ivacaftor rectifies and enhances its activity eventually increasing the conductance of ions and fluid. Figure 2 shows the possible mechanism of action of Orkambi and other drugs.

Depicting the action of Orkambi (lumacaftor + ivacaftor) and other agents in cystic fibrosis. Orkambi is a combination of two drugs which acts by a two-step method. Lumacaftor assists in moving the defective protein to its correct location and ivacaftor rectifies the gate opening time and enhances its activity eventually increasing the conductance of chloride ions followed by water. Read through agents for example Ataluren increase read through of Premature Termination codon enhancing production of immature protein. Possible “Corrector” drugs act on post translational modifications to increasing stability and reducing degradation by binding to various domains of CFTR. p-TM post translational modifications, GB golgi bodies, ER endoplasmic reticulum
Possible limitations
Though the arrival of CFTR modulators have improved the CF management but there are still some limitation which include (a) non-significant response in F508del mutation heterozygotes by ivacaftor; (b) need to continue other daily symptomatic treatment; (c) interaction with CYP3A inducers and inhibitors; (d) side effects including elevated transaminases, cataract, oropharyngeal pain and URTI; (e) negligible benefit in <12 years old; (f) need of higher dose up to 600 mg (in case of lumacaftor); and (g) mutual interaction of lumacaftor and ivacaftor leading to increased metabolism of ivacaftor and need of a higher dose combination.
Additionally, because of the multi domain structure and sequential folding of CFTR, no single “corrector drug” can fix all the misfolding in different domains, so a combination of drugs is a must. Moreover, from a clinical trial perspective, there are sample size issues as specific criteria (primary and secondary endpoints) make selection more difficult in already narrowed mutation specific population warranting unique adaptive trial designs.
Corrector/modifier therapeutic agents in clinical development pipeline
Many other compounds depicting corrector/potentiator activity besides read-through and gene transfer agents and are undergoing various phases of studies as mentioned below.
- 4PBA (sodium 4-phenylbutarate): though the mechanism of action is not certain but this compound has shown to enhance chloride transport in vitro and in patients having F508del [ 43 ].
- VRT - 532: preclinical studies confirmed that this compound corrects the CFTR structural abnormality and increases the protein expression and stability in patients carrying F508del and G551D mutations but showing more affinity for F08del [ 44 , 45 ].
- N6022: increases quantity of CFTR at the epithelium and decrease inflammation. This compound inhibits S-nitrosoglutathione reductase which cause the breakdown of S-nitrosoglutathione, as a result, levels of S-nitrosoglutathione increase which functions as a signaling molecule for protein production [ 46 ]. They also limit the binding of HOP (Hsp70/Hsp90 organizing protein) to mature form of CFTR, eventually preventing its degradation. Currently this is in phase 1/2 trials in F508del homozygotes [ 47 ].
- Ataluren (PTC124): it promotes the read-through of non-sense codons in the mRNA of CFTR (Fig. 1 ). After promising results in phase II, a phase 3 trial in 2011 also showed a lower rate of decline in lung function. However, another phase 3 trial showed non-significant results (FEV 1 similar in placebo and drug group) and increased creatinine which was later attributed to inhaled tobramycin [ 48 ]. Other trials are currently going on. Additionally a synthetic aminoglycoside NB124 has also depicted a restoration of CFTR activity up to 7% based on the same mechanism of increased PTC suppression [ 49 ].
- Tezacaftor (VX - 661) in combination with ivacaftor: a phase 2 trial showed promising results and now this combination is being tried in phase 3. VX-661 is expected to move the defective protein to correct location [ 50 ].
- Riociguat: though originally approved for treatment of pulmonary hypertension due to its synergistic effect with nitric oxide and stimulation of guanylate cyclase [ 51 ], it is currently in phase 2 and preclinical studies have shown positive results regarding expression of CFTR.
- QBW251: anticipated as a facilitator/potentiator for channel dynamics, its mechanism is similar to ivacaftor i.e. increasing the channel opening and is expected not to affect the membrane stability of CFTR as by lumacaftor. It is currently undergoing phase 2 [ 52 ].
- {"type":"entrez-nucleotide","attrs":{"text":"N91115","term_id":"1444442","term_text":"N91115"}} N91115 (Cavosonstat): it increases levels of S-nitrosoglutathione which is decreased in CF patients. It has shown to increase the CFTR protein quantity. Just completed phase 1b trial with demonstrated safety and undergoing phase 2a [ 53 ].
- QR - 010: it is an antisense oligonucleotide intended to rectify the defective CFTR mRNA. It is given by inhalational route and under phase 1b at present [ 54 ].
- Triple therapy of GLPG2665 (corrector) with GLPG2222 (another corrector) and GLPG1837 (potentiator) has shown promising results in early preclinical studies depicting a sixfold more efficacy than Orkambi while early phase human trials are still going on [ 55 – 57 ].
- CTP656 : it is deuterated ivacaftor which in a phase I trial showed a reduced rate of clearance, increased half-life, significantly improved exposure and better plasma levels [ 58 ]. Other corrector drug candidates are FDL160, FDL169 (phase I), FDL392 and FDL304 are also in early developmental pipeline [ 59 ].
- PGM169/GL67A (plasmid + cationic liposome): is a non-viral CFTR gene transfer agent. It is in phase IIb trial. Repeated nebulization in patients ≥12 years of age showed a modest but significant improvement in lung function but the response is highly varied even FEV 1 deteriorating in some patients [ 60 ]. Additionally, the placebo group received saline based instead of saline based nebulization. Moreover, secondary outcomes like CFTR expression levels, inflammatory markers and NPD showed non-significant results. Another gene transfer agents, Ad5-CB-CFTR, H5.001CBCFTR (with adenovirus) are in phase 1.
- PTI428 : is projected as a ‘CFTR amplifier’ drug candidate currently in phase I has shown promising results in preclinical studies on HBE cells. Additionally, the PTI-NC-733 is a combination of PTI428 with a corrector and potentiator and has revealed better outcomes in vitro than a combination of ivacaftor and lumacaftor [ 61 , 62 ].
- Duramycin (Moli1901/Lancovutide ): activates CaCC in airway epithelium. Has shown improved FEV 1 in a phase II trial.
- PDE5 inhibitors : a study on F508del murine model of CF showed an increased chloride transport in respiratory mucosa on inhalation of PDE5 inhibitors [ 63 ]. Also a decrease in sputum neutrophil elastase was found in a clinical study [ 64 ]. So, a phase II trial [ {"type":"clinical-trial","attrs":{"text":"NCT01132482","term_id":"NCT01132482"}} NCT01132482 ] is underway to test for the NPD, sweat sodium and chloride concentration, spirometric parameters and lung clearance index. Additionally, the PDE5 inhibitors are also being tested for increased exercise tolerance [ {"type":"clinical-trial","attrs":{"text":"NCT02057458","term_id":"NCT02057458"}} NCT02057458 ].
Cysteamine: by inhibiting transglutaminase 2 (an important multifunctional intermediary in cell autophagy), increases the expression of CFTR and restores its function and decrease inflammatory mediators in murine CF models and epithelial cell lines [ 65 ]. A phase II study is done in homo and heterozygous for F508del CF patients in combination with epigallocatechin gallate (which inhibits overactive pleiotropic protein kinase eventually increasing stability of F508del CFTR). The results reported a decrease in sweat chloride concentrations, increased CFTR function, repair of autophagy, decrease in inflammatory cytokines, and improvement in FEV 1 [ 66 ].
Other possible strategy is proteostasis modulation. Growing evidence suggests that CFTR do not works as an isolated ion channel but is a component of wider cellular signaling environment. The functional state of CFTR is modulated by the cellular environment and heterogeneity in cellular signaling. Differences in cellular protein interactomes were found between wild and mutant CFTR, so proteostatis modulation may shift the interactome to a more healthy state thus normalizing the CFTR [ 67 ]. As a matter of fact, a deficiency in autophagy pathways as evidenced by reduced autophagosome formation, and the buildup of sequestrosome 1 was discovered in CF airway cells. Myriad proteostasis network interactions have been identified but no human study is done yet. Detailed discussion on these proteostasis networks is beyond the scope of this review.
Another approach is exploration of role of chemical and pharmacological chaperones. They do not act directly on CFTR but on smaller cellular proteins increasing their stability thus modifying the CFTR interactome. Some examples include curcumin and thapsigargin (reducing ER calcium and increasing F508del-CFTR release), Bortezomib (preventing ER associated degradation of F508del-CFTR by increasing HsP70) and Miglustat (inhibitor of α-1,2-glucosidase eventually reducing cytoplasmic degradation of CFTR). Some other compounds include VRT-325, VRT-532 and Corr-4a reducing ubiquitination susceptibility of CFTR. But for most compounds, no corrector activity in humans is yet established. Moreover, there are technical difficulties in bringing these agents to clinical trials and a significant improvement is not anticipated.
Another promising approach is gene editing with the help of nucleases like zinc finger nucleases, transcription activator-like effector nuclease and especially CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats associated with Cas9 nuclease). Engineered nucleases cut the mutant DNA precisely and then wild type DNA is recombined to produce a normal transcript. There is one proof of concept study in intestinal stem cell organoids of CF patients carrying two F508del mutations with CRISPR/Cas9 methodology [ 68 ].
In addition, there are many other compounds identified through HTS that may bind and regulate different domains of CFTR and regulate channel activity but these are still in animal experimentation phase.
Newer therapeutic agents for symptomatic improvement
CF management not only requires CFTR correction and modification but intensive symptomatic treatment targeting inflammation, infection, bronchial hydration and nutrition. Newer drugs targeting these issues are summarized below briefly.
Inflammation
Andecaliximab, which is an antibody to Matrix metalloproteinase 9 (MMP9) is undergoing phase IIb and expected to reduce inflammation and improve lung function. However, the baseline FEV1 required for this drug is between 40 and 80% limiting its use in very severe CF [ 69 ].
Another compound in phase 1 is POL6014 which is synthesized to block neutrophil elastase function, eventually reducing the tissue destruction and lung inflammation.
LAU - 7b is a fenretinide, a member of retinoid compounds related to vitamin A. Phase 2 study is yet to begin and it is expected to reduce the inflammatory response in CF lungs.
CTX - 4430 decreases the production of leukotriene B4, an inflammatory mediator elevated in CF. It is presently undergoing a phase 2 trial [ 70 ].
Other anti-inflammatory compounds in clinical development pipeline are α - 1 anti - trypsin, CTX - 4430, Elastase Inhibitor AZD9668, JBT - 101 (phase 2) for reducing inflammation.
Hydration and mucus clearance
AZD5634 is undergoing phase 1b study. It is anticipated to block the sodium channel in CF airway, thus rehydrating and thinning the mucus in the lungs, making it easier to clear.
SPX - 101 is another compound designed to block sodium channel function in the lungs, currently undergoing phase II study.
OrPro (ORP - 100) is a modified form of thioredoxin, expected to decrease mucus viscosity in the lungs and improve clearance from the CF airway.
OligoG (Alginate Oligosaccharide) has shown to decrease mucus thickness in CF airway. It is currently being tested in phase IIb in Europe and UK. It can be used either as a dry powder or liquid for nebulization [ 71 ].
Other agents for rehydration of airway secretions include bronchitol currently in phase 3 in US and already approved in UK, Australia and Russia (for patients >18 years); VX - 371 (P1037 ) presently in phase II for blocking sodium channel and prolonging the duration of hypertonic saline stay [ 72 ]; GSK2225745 acting by silencing ENaC through RNA interference are underway to reach the patients.
Liprotamase (Anthera AN-EPI3332) is a pancreatic enzyme replacement for CF-related pancreatic insufficiency undergoing phase 3 study [ 73 ].
AquADEKs-2 undergoing phase II is a balanced combination of fat-soluble vitamins and several antioxidants including beta-carotene, mixed tocopherols, coenzyme Q10, mixed carotenoids, and minerals like zinc and selenium.
Oral Glutathione is being tried in phase II as this antioxidant is important for normal lung and GIT function. CF patients have depicted lower glutathione levels and oral glutathione is anticipated to improve growth and decrease gut inflammation [ 74 ].
Other agents like enzyme burlulipase for pancreatic insufficiency, lubiprostone for constipation and roscovitine for pulmonary infection are currently being tested at various centers.
Though there is an improvement in management of CF patients after the approval of CFTR modulators but it is still falling short of mark because in CF, management needs not only the protein rectifiers but also symptomatic treatment and intensive physiotherapy which require concomitant therapies. Myriad genotypes also pose a challenge. Most of the ‘corrector’ drugs in the pipeline are for older children either >12 or 6–12 years of age. Additionally most of these drugs are having serious hepatic and other side effects in addition to high costs. Moreover, many drugs discussed above are still in early clinical phase with limited data and a confirmed beneficial outcome cannot be guaranteed. There is also a need to address the psychological and social burden of disease.
Future research
Gene engineering techniques and new molecular targets may be explored besides CFTR. Help of modern biology approaches like DNA nanotechnology, systems biology, metabolomics, disease modeling and intracellular protein kinetics may help to unravel new pathways and networks associated with cystic fibrosis and eventually new therapeutic targets. Additionally, the focus should also not be minimized on novel physiotherapy techniques, new drugs for symptomatic improvement and complications prevention.

Authors’ contributions
MMR conceived the idea, MMR and HASM searched the literature, MMR drafted the manuscript with help of HASM. HASM draw the figure and corrected the references. All authors read and approved the final manuscript.
Acknowledgements
Competing interests.
The authors declare that they have no competing interests.
Consent for publication
All the authors have read and approved the final version of manuscript and give consent.
No source of funding was obtained for this work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Misbahuddin M. Rafeeq, Phone: +96655030963, Email: as.ude.uak@qeefaram .
Hussam Aly Sayed Murad, Email: as.ude.uak@darumah .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The future of cystic fibrosis care: a global perspective
Affiliations.
- 1 Department of Thoracic Medicine, The Prince Charles Hospital, Brisbane, QLD, Australia; QIMR Berghofer Medical Research Institute, Brisbane, QLD, Australia. Electronic address: [email protected].
- 2 Charité - Universitätsmedizin Berlin, Berlin Institute of Health, Berlin, Germany; German Center for Lung Research, Berlin, Germany.
- 3 University of Alabama at Birmingham, Birmingham, AL, USA.
- 4 Department of Biology and Medical Genetics, Second Faculty of Medicine, Motol University Hospital, Charles University, Prague, Czech Republic.
- 5 Royal Brompton and Harefield NHS Foundation Trust, London, UK.
- 6 Royal Brompton and Harefield NHS Foundation Trust, London, UK; National Heart and Lung Institute, Imperial College, London, UK.
- 7 Hôpital Cochin, Assistance Publique-Hôpitaux de Paris, Paris, France; Université Paris Descartes, Institut Cochin, Paris, France.
- 8 St Michael's Hospital, Toronto, ON, Canada; University of Toronto, Toronto, ON, Canada.
- 9 Hospital de Pediatria "Juan P Garrahan", Buenos Aires, Argentina.
- 10 Cystic Fibrosis Centre, IRCCS Istituto Giannina Gaslini, Genoa, Italy.
- 11 Starship Children's Hospital, Auckland, New Zealand; University of Auckland, Auckland, New Zealand.
- 12 Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore.
- 13 Cystic Fibrosis Trust, London, UK.
- 14 European Medicines Agency, Amsterdam, Netherlands.
- 15 University of Washington, Seattle, WA, USA.
- 16 Department of Medical Microbiology, Second Faculty of Medicine, Motol University Hospital, Charles University, Prague, Czech Republic.
- 17 University of Wisconsin, Madison, WI, USA.
- 18 Cystic Fibrosis Clinic, British Columbia Children's Hospital, Vancouver, BC, Canada.
- 19 Cystic Fibrosis Centre, University Hospital Leuven, Leuven, Belgium.
- 20 University of Washington, Seattle, WA, USA; Seattle Children's Research Institute, Seattle, WA, USA.
- 21 Research Center for Medical Genetics, Moscow, Russia.
- 22 Hadassah Medical Centre, Jerusalem, Israel.
- 23 Post Graduate Institute of Medical Education and Research, Chandigarh, India.
- 24 School of Medicine, St Vincent's University Hospital, Dublin, Ireland; University College Dublin School of Medicine, Dublin, Ireland.
- 25 Universities of Giessen and Marburg Lung Center, German Center of Lung Research, Justus-Liebig-University Giessen, Giessen, Germany.
- 26 CS Mott Children's Hospital, Ann Arbor, MI, USA; University of Michigan, Ann Arbor, MI, USA.
- 27 Queen's University of Belfast, Belfast, UK.
- 28 Jessa Ziekenhuis, Holsbeek, Belgium.
- 29 Johns Hopkins University, Baltimore, MD, USA.
- 30 Alder Hey Children's Hospital, Liverpool, UK; University of Liverpool, Liverpool, UK.
- 31 Royal Prince Alfred Hospital, Sydney, NSW, Australia; Woolcock Institute of Medical Research, Sydney, NSW, Australia.
- 32 Division of Paediatric Pulmonology and MRC Unit for Child and Adolescent Health, University of Cape Town, Cape Town, South Africa; Red Cross War Memorial Children's Hospital, Cape Town, South Africa.
- 33 University of Toronto, Toronto, ON, Canada; Division of Respiratory Medicine, Department of Paediatrics, Translational Medicine Research Program, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada. Electronic address: [email protected].
- PMID: 31570318
- PMCID: PMC8862661
- DOI: 10.1016/S2213-2600(19)30337-6
- Correction to Lancet Respir Med 2019; published online Sept 27. https://doi.org/10.1016/S2213-2600(19)30337-6. [No authors listed] [No authors listed] Lancet Respir Med. 2019 Dec;7(12):e40. doi: 10.1016/S2213-2600(19)30408-4. Epub 2019 Oct 25. Lancet Respir Med. 2019. PMID: 31669225 No abstract available.
The past six decades have seen remarkable improvements in health outcomes for people with cystic fibrosis, which was once a fatal disease of infants and young children. However, although life expectancy for people with cystic fibrosis has increased substantially, the disease continues to limit survival and quality of life, and results in a large burden of care for people with cystic fibrosis and their families. Furthermore, epidemiological studies in the past two decades have shown that cystic fibrosis occurs and is more frequent than was previously thought in populations of non-European descent, and the disease is now recognised in many regions of the world. The Lancet Respiratory Medicine Commission on the future of cystic fibrosis care was established at a time of great change in the clinical care of people with the disease, with a growing population of adult patients, widespread genetic testing supporting the diagnosis of cystic fibrosis, and the development of therapies targeting defects in the cystic fibrosis transmembrane conductance regulator (CFTR), which are likely to affect the natural trajectory of the disease. The aim of the Commission was to bring to the attention of patients, health-care professionals, researchers, funders, service providers, and policy makers the various challenges associated with the changing landscape of cystic fibrosis care and the opportunities available for progress, providing a blueprint for the future of cystic fibrosis care. The discovery of the CFTR gene in the late 1980s triggered a surge of basic research that enhanced understanding of the pathophysiology and the genotype-phenotype relationships of this clinically variable disease. Until recently, available treatments could only control symptoms and restrict the complications of cystic fibrosis, but advances in CFTR modulator therapies to address the basic defect of cystic fibrosis have been remarkable and the field is evolving rapidly. However, CFTR modulators approved for use to date are highly expensive, which has prompted questions about the affordability of new treatments and served to emphasise the considerable gap in health outcomes for patients with cystic fibrosis between high-income countries, and low-income and middle-income countries (LMICs). Advances in clinical care have been multifaceted and include earlier diagnosis through the implementation of newborn screening programmes, formalised airway clearance therapy, and reduced malnutrition through the use of effective pancreatic enzyme replacement and a high-energy, high-protein diet. Centre-based care has become the norm in high-income countries, allowing patients to benefit from the skills of expert members of multidisciplinary teams. Pharmacological interventions to address respiratory manifestations now include drugs that target airway mucus and airway surface liquid hydration, and antimicrobial therapies such as antibiotic eradication treatment in early-stage infections and protocols for maintenance therapy of chronic infections. Despite the recent breakthrough with CFTR modulators for cystic fibrosis, the development of novel mucolytic, anti-inflammatory, and anti-infective therapies is likely to remain important, especially for patients with more advanced stages of lung disease. As the median age of patients with cystic fibrosis increases, with a rapid increase in the population of adults living with the disease, complications of cystic fibrosis are becoming increasingly common. Steps need to be taken to ensure that enough highly qualified professionals are present in cystic fibrosis centres to meet the needs of ageing patients, and new technologies need to be adopted to support communication between patients and health-care providers. In considering the future of cystic fibrosis care, the Commission focused on five key areas, which are discussed in this report: the changing epidemiology of cystic fibrosis (section 1); future challenges of clinical care and its delivery (section 2); the building of cystic fibrosis care globally (section 3); novel therapeutics (section 4); and patient engagement (section 5). In panel 1, we summarise key messages of the Commission. The challenges faced by all stakeholders in building and developing cystic fibrosis care globally are substantial, but many opportunities exist for improved care and health outcomes for patients in countries with established cystic fibrosis care programmes, and in LMICs where integrated multidisciplinary care is not available and resources are lacking at present. A concerted effort is needed to ensure that all patients with cystic fibrosis have access to high-quality health care in the future.
Copyright © 2020 Elsevier Ltd. All rights reserved.
PubMed Disclaimer
Figure 1. Pathophysiology of Cystic Fibrosis
A-D, Role of CFTR in healthy airways and molecular…
Figure 2. Classes of CFTR Mutations.
Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR)…
Figure 3. Median FEV 1 % predicted…
Figure 3. Median FEV 1 % predicted for people with Cystic Fibrosis in the United…
Figure 4. The Number of Children and…
Figure 4. The Number of Children and Adults with Cystic Fibrosis (USA),1987–2017
Cystic fibrosis patients…
Figure 5. Maps of Countries with Cystic…
Figure 5. Maps of Countries with Cystic Fibrosis Registries and their Population Size
Countries with…
Figure 6. CFTR Function and Clinical Phenotype
CBAVD – congenital bilateral absence of toe vas…
Figure 7. Cystic fibrosis Standards of Care
Green – comprehensive, Blue – partial, Orange –…
Figure 8. Schematic of approach to CFTR…
Figure 8. Schematic of approach to CFTR restoration with small molecules, by mutation class.
Figure 9. Cystic fibrosis drug development pipeline…
Figure 9. Cystic fibrosis drug development pipeline of the US Cystic Fibrosis Foundation
Reproduced with…
Figure 10. CFTR Modulators – countries where…
Figure 10. CFTR Modulators – countries where approval and funded
Panel A Countries with significant…
- Cystic fibrosis lung disease and bronchiectasis. Chalmers JD. Chalmers JD. Lancet Respir Med. 2020 Jan;8(1):12-14. doi: 10.1016/S2213-2600(19)30335-2. Epub 2019 Sep 27. Lancet Respir Med. 2020. PMID: 31570316 No abstract available.
- A patient's experience of cystic fibrosis care. Brennan S. Brennan S. Lancet Respir Med. 2020 Jan;8(1):14-16. doi: 10.1016/S2213-2600(19)30336-4. Epub 2019 Sep 27. Lancet Respir Med. 2020. PMID: 31570317 No abstract available.
- Progress in understanding the molecular pathology and microbiology of cystic fibrosis. Tümmler B. Tümmler B. Lancet Respir Med. 2020 Jan;8(1):8-10. doi: 10.1016/S2213-2600(19)30333-9. Epub 2019 Sep 27. Lancet Respir Med. 2020. PMID: 31570319 No abstract available.
- Clinical care for cystic fibrosis: preparing for the future now. Konstan MW, Flume PA. Konstan MW, et al. Lancet Respir Med. 2020 Jan;8(1):10-12. doi: 10.1016/S2213-2600(19)30334-0. Epub 2019 Sep 27. Lancet Respir Med. 2020. PMID: 31570320 No abstract available.
- Cystic fibrosis in Turkey. Dogru D, Çakır E, Eyüboğlu TŞ, Pekcan S, Özçelik U; board members and the working group of the Cystic Fibrosis Registry of Turkey. Dogru D, et al. Lancet Respir Med. 2020 Apr;8(4):e17. doi: 10.1016/S2213-2600(20)30055-2. Lancet Respir Med. 2020. PMID: 32246929 No abstract available.
Similar articles
- The future of Cochrane Neonatal. Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
- Re-imagining cystic fibrosis care: next generation thinking. Rang C, Keating D, Wilson J, Kotsimbos T. Rang C, et al. Eur Respir J. 2020 May 27;55(5):1902443. doi: 10.1183/13993003.02443-2019. Print 2020 May. Eur Respir J. 2020. PMID: 32139465 Review.
- Tuberculosis. Bloom BR, Atun R, Cohen T, Dye C, Fraser H, Gomez GB, Knight G, Murray M, Nardell E, Rubin E, Salomon J, Vassall A, Volchenkov G, White R, Wilson D, Yadav P. Bloom BR, et al. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. PMID: 30212088 Free Books & Documents. Review.
- Correctors (specific therapies for class II CFTR mutations) for cystic fibrosis. Southern KW, Patel S, Sinha IP, Nevitt SJ. Southern KW, et al. Cochrane Database Syst Rev. 2018 Aug 2;8(8):CD010966. doi: 10.1002/14651858.CD010966.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Dec 17;12:CD010966. doi: 10.1002/14651858.CD010966.pub3. PMID: 30070364 Free PMC article. Updated. Review.
- Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis. Patel S, Sinha IP, Dwan K, Echevarria C, Schechter M, Southern KW. Patel S, et al. Cochrane Database Syst Rev. 2015 Mar 26;(3):CD009841. doi: 10.1002/14651858.CD009841.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 Jan 07;1:CD009841. doi: 10.1002/14651858.CD009841.pub3. PMID: 25811419 Updated. Review.
- [Cystic fibrosis primarily presenting with pseudo-Bartter syndrome: a report of three cases and literature review]. Zhang JY, Sun LJ, Duan XJ, Zhang ZM, Xiao ZH, Chen YP, You JY. Zhang JY, et al. Zhongguo Dang Dai Er Ke Za Zhi. 2024 May 15;26(5):506-511. doi: 10.7499/j.issn.1008-8830.2310080. Zhongguo Dang Dai Er Ke Za Zhi. 2024. PMID: 38802912 Free PMC article. Review. Chinese.
- Cystic fibrosis management in pediatric population-from clinical features to personalized therapy. Azoicai AN, Lupu A, Trandafir LM, Alexoae MM, Alecsa M, Starcea IM, Cuciureanu M, Knieling A, Salaru DL, Hanganu E, Mocanu A, Lupu VV, Ioniuc I. Azoicai AN, et al. Front Pediatr. 2024 May 10;12:1393193. doi: 10.3389/fped.2024.1393193. eCollection 2024. Front Pediatr. 2024. PMID: 38798310 Free PMC article. Review.
- Physical activity and its correlates in people with cystic fibrosis: a systematic review. Kinaupenne M, De Craemer M, Schaballie H, Vandekerckhove K, Van Biervliet S, Demeyer H. Kinaupenne M, et al. Eur Respir Rev. 2022 Sep 7;31(165):220010. doi: 10.1183/16000617.0010-2022. Print 2022 Sep 30. Eur Respir Rev. 2022. PMID: 38743505 Free PMC article. Review.
- Musculoskeletal aspects of respiratory function in cystic fibrosis: a cross-sectional comparative study. Sinderholm Sposato N, Bjerså K, Gilljam M, Lannefors L, Fagevik Olsén M. Sinderholm Sposato N, et al. Eur Clin Respir J. 2024 May 8;11(1):2350206. doi: 10.1080/20018525.2024.2350206. eCollection 2024. Eur Clin Respir J. 2024. PMID: 38726022 Free PMC article.
- Harnessing inter-kingdom metabolic disparities at the human-fungal interface for novel therapeutic approaches. Costantini C, Pariano M, Puccetti M, Giovagnoli S, Pampalone G, Dindo M, Cellini B, Romani L. Costantini C, et al. Front Mol Biosci. 2024 Apr 24;11:1386598. doi: 10.3389/fmolb.2024.1386598. eCollection 2024. Front Mol Biosci. 2024. PMID: 38721278 Free PMC article.
- Andersen DH, Hodges RG. Celiac syndrome; genetics of cystic fibrosis of the pancreas, with a consideration of etiology. Am J Dis Child 1946; 72: 62–80. - PubMed
- Di Sant’Agnese PA, Darling RC, Perera GA, Shea E. Abnormal electrolyte composition of sweat in cystic fibrosis of the pancreas; clinical significance and relationship to the disease. Pediatrics 1953; 12(5): 549–63. - PubMed
- Gibson LE, Cooke RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 1959; 23(3): 545–9. - PubMed
- Quinton PM. Chloride impermeability in cystic fibrosis. Nature 1983; 301(5899): 421–2. - PubMed
- Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science 1989; 245(4922): 1073–80. - PubMed
Publication types
- Search in MeSH
Related information
Grants and funding.
- P30 DK072482/DK/NIDDK NIH HHS/United States
- P30 DK089507/DK/NIDDK NIH HHS/United States
- UM1 HL119073/HL/NHLBI NIH HHS/United States
- R35 HL135816/HL/NHLBI NIH HHS/United States
- R01 DK109692/DK/NIDDK NIH HHS/United States
- R01 FD003704/FD/FDA HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
- R01 AI101307/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full text sources.
- Elsevier Science
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- The Lens - Patent Citations
- Genetic Alliance
- MedlinePlus Health Information
Research Materials
- NCI CPTC Antibody Characterization Program
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Cystic fibrosis articles from across Nature Portfolio
Cystic fibrosis is a genetic disorder driven by CFTR mutations that affects the exocrine glands. Manifesting with excessively viscous mucus production, patients typically experience obstruction of passageways (e.g., pancreatic and bile ducts, intestines, and bronchi). Lung disease is responsible for the majority of morbidity in patients.
Latest Research and Reviews
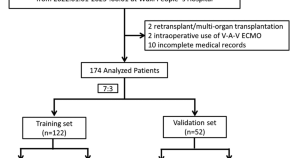
A novel nomogram for predicting prolonged mechanical ventilation in lung transplantation patients using extracorporeal membrane oxygenation
- Chenhao Xuan
- Jingxiao Gu
- Hongyang Xu
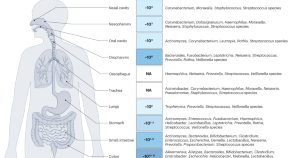
The gut–airway microbiome axis in health and respiratory diseases
In this Review, Özçam and Lynch examine recent findings reporting the interaction between the gut and the airway microbiomes and explore the role of gut–airway crosstalk in human health and respiratory diseases.
- Mustafa Özçam
- Susan V. Lynch
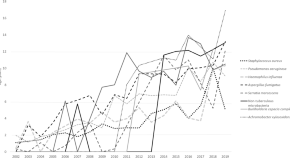
The changing epidemiology of pulmonary infection in children and adolescents with cystic fibrosis: an 18-year experience
- Jagdev Singh
- Sharon Hunt
- Hiran Selvadurai
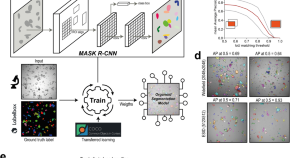
OrgaSegment: deep-learning based organoid segmentation to quantify CFTR dependent fluid secretion
A deep learning model—OrgaSegment—is presented for segmentation of individual intestinal patient-derived organoid structures from bright-field images. This enables quantification of organoid swelling and discrimination between organoids with different levels CFTR function and response to therapy.
- Juliet W. Lefferts
- Suzanne Kroes
- Sam F. B. van Beuningen
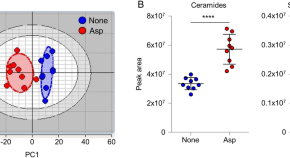
Dual species sphingosine-1-phosphate lyase inhibitors to combine antifungal and anti-inflammatory activities in cystic fibrosis: a feasibility study
- Barbara Cellini
- Gioena Pampalone
- Claudio Costantini
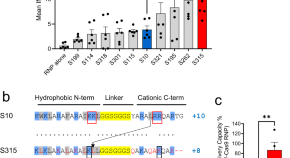
Shuttle peptide delivers base editor RNPs to rhesus monkey airway epithelial cells in vivo
Gene editing strategies for cystic fibrosis are challenging. Here the authors improve on their previously reported shuttle peptide noncovalently combined with Cas ribonucleoprotein (RNP), and derive the S315 peptide for delivery: they show base editing in the respiratory tract of the rhesus macaques.
- Katarina Kulhankova
- Soumba Traore
- Paul B. McCray Jr.
News and Comment
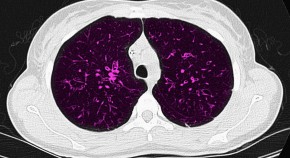
Sweat sticker for cystic fibrosis
A wearable device improves the collection and analysis of sweat for the diagnosis of cystic fibrosis.
- Michael Basson
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
Cystic fibrosis is an autosomal recessive disease caused by mutations of the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). We summarize clinical and genetic characteristics of cystic fibrosis gene mutations, as well as animal models used to study human cystic fibrosis disease. 1.
Cystic fibrosis (CF) is a recessive genetic disease caused by a mutation in the epithelial chloride channel—cystic fibrosis transmembrane conductance regulator (CFTR). CF is a predominant genetic disorder with a disease severity ranging from mild to life-threatening.
This success is based on fundamental research, which led to the identification of the disease-causing CFTR gene and our subsequent understanding of the disease mechanisms underlying the pathogenesis of cystic fibrosis, working together with a continuously evolving clinical research and drug development pipeline.
This article reviews the pathophysiology, evaluation, and treatment of cystic fibrosis, including recent advances with the use of highly effective modulator therapy.
Current research supported by the NHLBI focuses on understanding how changes in the CFTR protein lead to the development of cystic fibrosis. The Institute carries out and supports studies that could lead to gene therapies and other treatments that may help the lives of people who have the condition.
Cystic fibrosis is an autosomal recessive disorder due to mutations in CFTR gene leading to abnormality of chloride channels in mucus and sweat producing cells. Respiratory system and GIT are primarily involved but eventually multiple organs are affected leading to life threatening complications.
We are currently witnessing transformative change for people with cystic fibrosis with the introduction of small molecule, mutation-specific drugs capable of restoring function of the defective...
In considering the future of cystic fibrosis care, the Commission focused on five key areas, which are discussed in this report: the changing epidemiology of cystic fibrosis (section 1); future challenges of clinical care and its delivery (section 2); the building of cystic fibrosis care globally (section 3); novel therapeutics (section 4); and ...
Cystic fibrosis is a genetic disorder driven by CFTR mutations that affects the exocrine glands. Manifesting with excessively viscous mucus production, patients typically experience...
Cystic fibrosis is a monogenic disease considered to affect at least 100 000 people worldwide. Mutations in CFTR, the gene encoding the epithelial ion channel that normally transports chloride and bicarbonate, lead to impaired mucus hydration and clearance.