

Upcoming Events
Recent posts.
- Harvard Business School Publishing: Best Sellers in the CRJ
- CALL FOR CASES: Special Issue on “Business at the Quarter-Century”: Disruptive Forces, Crises, and Emerging Opportunities
- Harvard Publishing Best Sellers in Information Technology
- 2024 Conference Keynote Speaker T. Grandon Gill
- What Makes a Good Case? NACRA Webinar 1/19/2024
Recent Comments
- February 2024
- January 2024
- December 2023
- December 2022
- October 2022
- Press Release
- Uncategorized
- Entries feed
- Comments feed
- WordPress.org
About NACRA
Promoting excellence in case research, writing and teaching.
The North American Case Research Association (NACRA) is a nonprofit, voluntary professional association whose mission is to promote excellence in case research, writing and teaching in business and other academic disciplines. We seek to accomplish our mission through a set of interrelated activities, including the following:
- Sponsoring an annual meeting for the presentation and improvement of new, peer-reviewed cases and papers on issues related to case pedagogy;
- Publishing a quarterly peer-reviewed journal, the Case Research Journal, the premier journal for outstanding teaching cases grounded in research;
- Promoting worldwide distribution and use of NACRA cases in multiple media throughout the world
- Providing professional development seminars and activities aimed at enhancing skills in case research, writing and teaching;
- Working to enhance the legitimacy and status of case research and pedagogy within academic institutions and professional associations; and
- Supporting the work of an international group of NACRA-affiliated strategic partners and collaboration with other professional organizations having complementary objectives.
As a collaborative organization of researchers, case writers and teachers from across the globe who support each other’s research, writing, and pedagogy of business case studies, membership is open to all persons interested in research, writing and teaching cases in business and other academic disciplines. A copy of NACRA’s constitution, revised and adopted by the membership in 2021, may be downloaded here.
On December 9th, 2021, the NACRA Executive Board unanimously adopted a statement on contract cheating, and ask all members, case teachers and instructors to abide by the following:
- Our members pledge to respect the copyright and instructor-only purpose of published cases and instructor manuals, and to keep model case answers and analyses secure.
- Case teachers and instructors educate their students about the unethical and potentially dangerous practices of contract cheating and ghostwriting, as part of the negative impact of cheating.
- Case teachers and instructors develop course assignments and assessment methods that are resistant to contract cheating, and to share effective mitigation methods.
- Stand in solidarity with the International Center for Academic Integrity’s International Day of Action Against Contract Cheating, and other initiatives to find legal and market solutions to contract cheating.
Annual Individual Membership Dues are $40. Benefits Include:
- The opportunity to connect with leading case researchers, writers and teachers. Our annual meeting offers opportunities for professional development, presentation and discussion of cases, as well as papers on case pedagogy, case research and more.
- Educator access to the Case Research Journal (CRJ). The CRJ is the premier journal for teaching cases grounded in research.
- The opportunity to serve in a leadership position for NACRA and the annual conference.
- All mailings, including the NACRA newsletter.
- Access to the Membership Directory and ability to contact members
- Canada, Mexico and USA members pay $85/year
- All other international members pay $115/year
Individual memberships are $40 a year, and run from December 1 – November 30, with the option to select auto-renew.
For more information about our association, please download our Newcomer’s Guide to NACRA .
- Member Directory
- Annual Conference
- Case Research Journal
- Privacy Policy

- Chairperson's Message
- Board of Governors
- Campus Life
- International Office
- Announcements
- All Programmes
- About Executive Education
- Short Duration Programmes
- MDP Calendar
- Long Duration Programmes (LDP)
- LDP Calendar
- Faculty & Research Home
- Faculty Directory
- Academic Groups
- Centers of Excellence
- Publications
- Conferences & Seminars
- Recruitments
- Faculty Portal
- Infrastructure
- Life @ IIMC
- Student Council
- MBA Placements
- MBAEx Placements
- PGPEX VLM Placements
- PGDBA Placements
- Alumni Magazine
- Distinguished Alumnus Award
- Alumni News
- Alumni Chapters
- Alumni Portal
- Arijit Mukherji Memorial Lecture Series
- Foundation Day
.png)
Case Research Center (IIMCCRC)
- CURRENT EVENTS
- PAST EVENTS
- FACULTY COLLABORATORS
- CDEP-IIMCCRC Case
- CASE RESEARCHERS
- MEDIA UPDATE
IIM Calcutta is a premier global management institute that uses cases in several of its courses for postgraduate students and participants in management development programs. Students at our institute are extremely responsive when it comes to case participation and the student community frequently makes it known that cases are indeed an effective tool to learn and apply theoretical concepts to practice.
The distinctive nature of Indian companies, their strategies and managerial styles, are becoming important not only to future managers of Indian companies but also to managers world-wide who are interested in seeking expansion opportunities in India. There are few institutions that specialize in Indian business cases. The Harvard Business School and the Richard Ivey School of Business are two of the largest producers of case studies in the world. In Asia, The Asia Case Research Center at the University of Hong Kong is the largest producer of Asian business cases. While cases related to Indian businesses are published by all of the above centers, they are not a major focus.
IIMC has close ties with the Indian business community and enjoys an inherent advantage in developing cases in the Indian context as well as that of other emerging markets. Over the last few years IIMC has developed a strong team of over twenty-five faculty members who have been exposed to case teaching, writing and research through recruitment of young faculty from case teaching oriented institutions, and by exposing faculty members to the Colloquium on Participant Centered Learning at the Harvard Business School.
About Case Research Centre: Prof. Ashok Banerjee
Objectives and Support offered by IIM Calcutta Case Research Centre (IIMCCRC) : Prof. Dharma Raju Bathini
We believe that with the growing importance of India in the global economy there will be an increasing demand for India focused cases among academicians and practitioners around the world. Our Case Research Centre is set to meet those demands. We have a team of accomplished faculty members who are excellent teachers, effective consultants, and prolific publishers. We have the human resources to oversee, direct, and take part in developing world-class case studies that will be sought after by business schools around the world.

International Case Research Association
A non-profit voluntary professional association whose mission is to promote emerging market cases on a global scale.
Markets Represented Include:

Latest News

Case Champion Conversations Podcast

Quality Case Research and Case Teaching Webinar No.5, May 4, 2022
Case method videos.
Watch leading academics discuss the Case Method from the 2020 NACRA Conference
ICRA shares case teaching experiences among leading academics.
Certificate
ICRA offers training and certification for Case Researchers.
Join ICRA Now!
Become a member today and share your case development and teaching experiences., the international case research association, why choose us.
The International Case Research Association is a non-profit voluntary professional association whose mission is to promote emerging market multinational cases on a global scale.
Annual Meetings
We sponsor annual meetings to help you improve your emerging market multinational cases through the peer-reviewed process.
Promote Your Emerging Market Business Cases
We will help you promote your emerging market multinational business cases through multimedia and case-competitions around the world
Professional Certification
We will provide professional development seminars and activities aimed at enhancing your skills in case research.
Increase Your Case Publications
We will work together to enhance the legitimacy and status of case research and pedagogy within emerging-market companies, institutions, and professional associations
Case Method
Comments from case researchers & scholars.

Dr. Michael Goldman
Associate Professor, University of San Francisco, President, North American Case Research Association
Dr. Liman Zhao
Senior Case Researcher, Case Centre, China Europe International Business School
Managing Editor at the Rotterdam School of Management (RSM) Case Development Centre, Erasmus University
Associate Professor, SILC Business School, Shanghai University
Dr. Chaoping Luo
Professor, Southwest University
Dr. Chongfeng Wang
Professor and Case Centre Director, Qingdao University

Case collection: Asia Case Research Centre

About the University of Hong Kong, Faculty of Business and Economics
Tracing its roots back over a century, the University of Hong Kong (HKU) today proudly stands as one of the leading institutions of higher education in Asia. And with it, the HKU Business School has grown to become a widely recognised and respected business school in the region.
The School strives to nurture first-class business leaders and to foster both academic and applied research to serve the needs of Hong Kong, China and the rest of the world in the fast-changing global economy. They engage leading scholars from all corners of the world who instil students with global knowledge. HKU Business School attracts the best and brightest students from Hong Kong and beyond.
The School’s full-time MBA programme has been ranked Asia’s no. 1 in the World MBA Rankings, released by the Economist Intelligence Unit, for nine consecutive years from 2010 to 2018. In addition to offering an elite EMBA-Global Asia programme jointly with Columbia Business School and London Business School for globally-focused senior executives and professionals, they also collaborate with top business schools throughout China and across the globe to synergise business and economics education. Their strong and extensive alumni network, with business and community leaders serving at key positions in both public and private realms, furthers their reach and strengthens their influence in different sectors and strata of society.
About the collection
The Asia Case Research Centre (“ACRC”) is part of HKU Business School. It was founded in 1997 to address the need for rich business cases with an Asian focus. The ACRC is committed to the advancement of learning and teaching in business education and strives to promote leading management thinking through research on the latest practices in the Asia Pacific business environment. The ACRC is a major producer of quality business cases. It boasts a repository of over 650 business case studies developed in collaboration with many of the region’s leading companies.
Collection contact
For any queries related to the ACRC case collection, please contact:
e [email protected] t +852 3917 7730
Browse the full collection Browse prize-winning cases
Available from The Case Centre
The ACRC collection of over 760 cases, and their accompanying instructor materials and videos, is available from The Case Centre.
ACRC materials are also available as part of our Undergraduate Case Teaching Licence and discounted pricing scheme for members in developing countries .
See what's available
There are restrictions on the distribution of some items. To see any restrictions login to our site (or register if you've not already done so) and use our online search to find the item you're interested in. Any restrictions will be shown alongside the product.

www.acrc.hku.hk
Top ten bestselling cases
Browse the top ten bestselling cases from ACRC in 2023.
Browse the full collection
View all case collections
Learning with cases can be a challenging experience.
Our interactive study guide takes students through the process, providing practical tips, tricks and tools.

Discover more

India Case Research Centre

CASE CATALOGUE

CASEPEDIA the case journal
INDUSTRY best practice case
The AIMA case research centre is established after a lot of discussion, deliberation and research. The purpose of setting up of India case research centre at AIMA is primarily to focus on developing and publishing Industry based India-focused research cases.
Submit your Case
- Case Competition 2021
- Case Competition 2022
- Case Competition 2023
- Case Competition 2024

New Arrival

Wheels of Change ...
NAVIGATING CULTURAL COMMERCE AND FI...
‘IS MY ATTITUDE GETTING ON MY WAY...
CHAT-GPT: PLAGIARIZING THE FUTURE...
A CASE STUDY ON THE ROLE OF AI/ML I...
IT Management Simulation: Cyber Attack!
15FEN: Finding The Blue Ocean in China’s Fresh Food
The Toshiba Accounting Scandal: How Corporate Governance Failed
Zandu Pharmaceutical Works: The Tak...
Ketan Logistics—Charting the Next...
SHOESTRING ARBITRAGE WITH STOCK BUY...
NEW DELHI TELEVISION ...
How To Grow On Chinese Soil
Events & Workshop
Affiliate organisations.

Due to the outbreak of the Novel Coronavirus pandemic, we at AIMA shall strive to do our bit to avoid unnecessary risk and spread of the disease.
In keeping with the Government orders, we are postponing all major events scheduled in the next few weeks. However, for the tests like MAT and UGAT, please refer to respective section of the website for their modified schedules and keep visiting those sections for periodic updates time to time. The AIMA Library shall also remain closed. We will review the situation week by week.
Adopting all modern means of technology, all staff members will "Work from Home". However, there will be no slowdown in our response to you.
All staff members will be available on phone, email, WhatsApp etc. and will respond to you as efficiently as possible.
- Biochemistry and Molecular Biology
- Biostatistics
- Environmental Health and Engineering
- Epidemiology
- Health Policy and Management
- Health, Behavior and Society
- International Health
- Mental Health
- Molecular Microbiology and Immunology
- Population, Family and Reproductive Health
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
research@BSPH
The School’s research endeavors aim to improve the public’s health in the U.S. and throughout the world.
- Funding Opportunities and Support
- Faculty Innovation Award Winners
Conducting Research That Addresses Public Health Issues Worldwide
Systematic and rigorous inquiry allows us to discover the fundamental mechanisms and causes of disease and disparities. At our Office of Research ( research@BSPH), we translate that knowledge to develop, evaluate, and disseminate treatment and prevention strategies and inform public health practice. Research along this entire spectrum represents a fundamental mission of the Johns Hopkins Bloomberg School of Public Health.
From laboratories at Baltimore’s Wolfe Street building, to Bangladesh maternity wards in densely packed neighborhoods, to field studies in rural Botswana, Bloomberg School faculty lead research that directly addresses the most critical public health issues worldwide. Research spans from molecules to societies and relies on methodologies as diverse as bench science and epidemiology. That research is translated into impact, from discovering ways to eliminate malaria, increase healthy behavior, reduce the toll of chronic disease, improve the health of mothers and infants, or change the biology of aging.
120+ countries
engaged in research activity by BSPH faculty and teams.
of all federal grants and contracts awarded to schools of public health are awarded to BSPH.
citations on publications where BSPH was listed in the authors' affiliation in 2019-2023.
publications where BSPH was listed in the authors' affiliation in 2019-2023.
Departments
Our 10 departments offer faculty and students the flexibility to focus on a variety of public health disciplines
Centers and Institutes Directory
Our 80+ Centers and Institutes provide a unique combination of breadth and depth, and rich opportunities for collaboration
Institutional Review Board (IRB)
The Institutional Review Board (IRB) oversees two IRBs registered with the U.S. Office of Human Research Protections, IRB X and IRB FC, which meet weekly to review human subjects research applications for Bloomberg School faculty and students
Generosity helps our community think outside the traditional boundaries of public health, working across disciplines and industries, to translate research into innovative health interventions and practices
Introducing the research@BSPH Ecosystem
The research@BSPH ecosystem aims to foster an interdependent sense of community among faculty researchers, their research teams, administration, and staff that leverages knowledge and develops shared responses to challenges. The ultimate goal is to work collectively to reduce administrative and bureaucratic barriers related to conducting experiments, recruiting participants, analyzing data, hiring staff, and more, so that faculty can focus on their core academic pursuits.
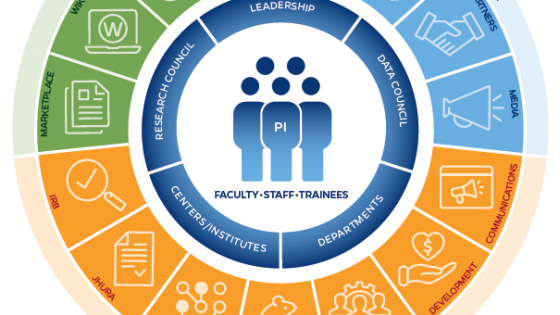
Research at the Bloomberg School is a team sport.
In order to provide extensive guidance, infrastructure, and support in pursuit of its research mission, research@BSPH employs three core areas: strategy and development, implementation and impact, and integrity and oversight. Our exceptional research teams comprised of faculty, postdoctoral fellows, students, and committed staff are united in our collaborative, collegial, and entrepreneurial approach to problem solving. T he Bloomberg School ensures that our research is accomplished according to the highest ethical standards and complies with all regulatory requirements. In addition to our institutional review board (IRB) which provides oversight for human subjects research, basic science studies employee techniques to ensure the reproducibility of research.
Research@BSPH in the News
Four bloomberg school faculty elected to national academy of medicine.
Considered one of the highest honors in the fields of health and medicine, NAM membership recognizes outstanding professional achievements and commitment to service.
The Maryland Maternal Health Innovation Program Grant Renewed with Johns Hopkins
Lerner center for public health advocacy announces inaugural sommer klag advocacy impact award winners.
Bloomberg School faculty Nadia Akseer and Cass Crifasi selected winners at Advocacy Impact Awards Pitch Competition
Welcome to Our New Website!
Smart. Open. Grounded. Inventive. Read our Ideas Made to Matter.
Which program is right for you?

Through intellectual rigor and experiential learning, this full-time, two-year MBA program develops leaders who make a difference in the world.
A rigorous, hands-on program that prepares adaptive problem solvers for premier finance careers.
A 12-month program focused on applying the tools of modern data science, optimization and machine learning to solve real-world business problems.
Earn your MBA and SM in engineering with this transformative two-year program.
Combine an international MBA with a deep dive into management science. A special opportunity for partner and affiliate schools only.
A doctoral program that produces outstanding scholars who are leading in their fields of research.
Bring a business perspective to your technical and quantitative expertise with a bachelor’s degree in management, business analytics, or finance.
A joint program for mid-career professionals that integrates engineering and systems thinking. Earn your master’s degree in engineering and management.
An interdisciplinary program that combines engineering, management, and design, leading to a master’s degree in engineering and management.
Executive Programs
A full-time MBA program for mid-career leaders eager to dedicate one year of discovery for a lifetime of impact.
This 20-month MBA program equips experienced executives to enhance their impact on their organizations and the world.
Non-degree programs for senior executives and high-potential managers.
A non-degree, customizable program for mid-career professionals.
Center for Development & Entrepreneurship at MIT

Center for Development & Entrepreneurship
Read more and be inspired by the Center's collection of researchers and ecosystem experts from throughout the world.
Research from the Legatum Center

The Women-Waste Climate Nexus in the Middle East and North Africa
Delila Khaled
This technical brief is a supplement to the white paper, The Women-Waste-Climate Nexus: Unlocking the potential of women entrepreneurs to combat the global waste crisis and Accelerate the Race to Net Zero (Khaled, 2023). Using the paper as a point of departure, it provides a brief analysis of the wo...

15 Year Impact
The Legatum Center for Development and Entrepreneurship at the Massachusetts Institute of Technology (MIT) was founded in 2007 with its vision and mission centered on the belief that entrepreneurs and their innovation-driven solutions are key to advancing global prosperity.

Pan-African Deeptech: Key to Sustainable Prosperity
Haitham Khoury, Ph.D.,
Embracing deeptech innovation – innovation grounded in advances at the frontier of science and technology – in Africa has the potential to drive sustainable economic growth, improve living standards, and bring stability and resilience. As the wellspring of new solutions, deeptech has the power to a...

Digital Health Entrepreneurship in Vietnam
Nourhan Shaaban
Despite Vietnam’s transition to an emerging economy, the healthcare sector has not witnessed the same progress seen in other sectors. Vietnam is a country of innovators and entrepreneurs, and this should be leveraged to address the country’s healthcare challenges.
On the Back of the Gray Rhino
Dina Sherif
By harnessing the power of "the gray rhino" and using the crisis as an opportunity, entrepreneurs are uniquely positioned to mitigate the myriad effects of the financial crisis.

The Women Waste-Climate Nexus
Women waste entrepreneurs were recognized as an untapped but potentially transformational force in the fight against climate change and the transition to a gender-just, net-zero future.

Print Case Studies
Our free case studies focus on innovative emerging market ventures, led by principled entrepreneurs, which have high potential for sustainable and scalable socioeconomic impact.

Making the Bad Economy Case: College Grad Edition II
• ARTICLE Beat the Press
June 16, 2024

If we wanted to know what the job market looks like for recent college graduates, we would probably start by looking at their unemployment/employment rates. On the other hand, if we wanted to try to make the labor market for recent college grads look bad, we could follow the Washington Post’s lead and look at the re-employment rates for college grads who report being unemployed.
While the latter statistic is interesting, it is only telling us about the employment prospects for the roughly 4.0 percent of college grads who report being unemployed in any given month. It tells us nothing about the job prospects for the 96 percent of college grads in the labor market who have jobs.
As it turns out, the prospects for recent college grads who end up being unemployed is not very good. This is what the Washington Post chose to highlight in a piece headlined, “Degree? Yes. Job? Maybe Not Yet.”
The article starts out by talking about the re-employment rate for new entrants to the workforce and shows us that it is far lower than for more experienced workers. This is shown with a large graph.
Next in the piece we do get the employment rate for all young people, not just recent grads, which has fallen in recent months back to levels reached in 2016, after previously reaching levels that were close to pre-pandemic peaks.
It is only towards the bottom of the piece that we get the unemployment rate for recent grads (4.3 percent). This is up slightly from the 3.9 percent low hit in 2022, but still low by most standards and very comparable to the rates we were seeing just before the pandemic.
As a practical matter, it is hard to imagine that someone could look at a 4.3 percent unemployment rate and say college grads are having a hard time finding jobs. Oddly, this is the second time in two weeks we saw this re-employment rate for recent grads highlighted as meaning that college grads are having trouble getting jobs.
The market for recent college grads continues to look pretty good, but the market for bad news on the economy is red hot.
Dean Baker | Senior Economist
Read Full Bio
Support Cepr
Apoyar a cepr, if you value cepr's work, support us by making a financial contribution., si valora el trabajo de cepr, apóyenos haciendo una contribución financiera..
Donate Apóyanos

Keep up with our latest news
Related Articles
This paper is in the following e-collection/theme issue:
Published on 10.6.2024 in Vol 26 (2024)
Creation of Standardized Common Data Elements for Diagnostic Tests in Infectious Disease Studies: Semantic and Syntactic Mapping
Authors of this article:

Original Paper
- Caroline Stellmach 1 , MSc ;
- Sina Marie Hopff 2 , Dr med ;
- Thomas Jaenisch 3 , Dr med, PhD ;
- Susana Marina Nunes de Miranda 2 , Dr rer nat ;
- Eugenia Rinaldi 1 , MSc ;
- The NAPKON, LEOSS, ORCHESTRA, and ReCoDID Working Groups 4
1 Berlin Institute of Health, Charité - Universitätsmedizin Berlin, Berlin, Germany
2 Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf, Department I of Internal Medicine, University Hospital Cologne and Faculty of Medicine, University of Cologne, Cologne, Germany
3 Heidelberg Institut für Global Health, Universitätsklinikum Heidelberg, Heidelberg, Germany
4 See Acknowledgments
Corresponding Author:
Caroline Stellmach, MSc
Berlin Institute of Health
Charité - Universitätsmedizin Berlin
Anna-Louisa-Karsch-Str 2
Berlin, 10178
Phone: 49 15752614677
Email: [email protected]
Background: It is necessary to harmonize and standardize data variables used in case report forms (CRFs) of clinical studies to facilitate the merging and sharing of the collected patient data across several clinical studies. This is particularly true for clinical studies that focus on infectious diseases. Public health may be highly dependent on the findings of such studies. Hence, there is an elevated urgency to generate meaningful, reliable insights, ideally based on a high sample number and quality data. The implementation of core data elements and the incorporation of interoperability standards can facilitate the creation of harmonized clinical data sets.
Objective: This study’s objective was to compare, harmonize, and standardize variables focused on diagnostic tests used as part of CRFs in 6 international clinical studies of infectious diseases in order to, ultimately, then make available the panstudy common data elements (CDEs) for ongoing and future studies to foster interoperability and comparability of collected data across trials.
Methods: We reviewed and compared the metadata that comprised the CRFs used for data collection in and across all 6 infectious disease studies under consideration in order to identify CDEs. We examined the availability of international semantic standard codes within the Systemized Nomenclature of Medicine - Clinical Terms, the National Cancer Institute Thesaurus, and the Logical Observation Identifiers Names and Codes system for the unambiguous representation of diagnostic testing information that makes up the CDEs. We then proposed 2 data models that incorporate semantic and syntactic standards for the identified CDEs.
Results: Of 216 variables that were considered in the scope of the analysis, we identified 11 CDEs to describe diagnostic tests (in particular, serology and sequencing) for infectious diseases: viral lineage/clade; test date, type, performer, and manufacturer; target gene; quantitative and qualitative results; and specimen identifier, type, and collection date.
Conclusions: The identification of CDEs for infectious diseases is the first step in facilitating the exchange and possible merging of a subset of data across clinical studies (and with that, large research projects) for possible shared analysis to increase the power of findings. The path to harmonization and standardization of clinical study data in the interest of interoperability can be paved in 2 ways. First, a map to standard terminologies ensures that each data element’s (variable’s) definition is unambiguous and that it has a single, unique interpretation across studies. Second, the exchange of these data is assisted by “wrapping” them in a standard exchange format, such as Fast Health care Interoperability Resources or the Clinical Data Interchange Standards Consortium’s Clinical Data Acquisition Standards Harmonization Model.
Introduction
In response to the spread of SARS-CoV-2 starting in late 2019, large-scale observational studies as well as clinical trials have been launched worldwide to gain insights into disease patterns, treatment options, prevention measures, severity, and outcomes [ 1 ]. New findings related to the diagnosis, prevention, and treatment of many infectious diseases, including COVID-19, heavily rely on data generated by diagnostic tools and laboratory analysis of the pathogen and host response [ 2 ].
Immunological testing has become a cost- and time-efficient way to monitor infections [ 3 ]. Hence, a growing number of clinical studies include biosample information as part of their data collection targets, particularly results of analytical tests performed on blood samples [ 4 ].
Data from patients enrolled in a study are commonly collected using a case report form (CRF) [ 5 ]. The International Conference on Harmonization Guidelines for Good Clinical Practice defines a CRF as a “printed, optical or electronic document designed to record all of the protocol-required information to be reported to the sponsor on each trial subject” [ 6 ]. Since the design of a CRF can affect study outcomes, time and resources need to be invested to maximize the quality of the data collected and ensure that good clinical practice guidelines are being followed [ 7 ].
The identification of common data elements (CDEs), each comprising 1 or more questions and respective answer value sets, is an approach to standardize data collection instruments (ie, CRFs) across studies [ 8 ]. A CDE may also contain standardized ontology concepts directly or include a link to the unique identifier for an appropriate ontology concept [ 9 ].
We have previously described [ 10 ] how incorporating standard codes into clinical trials metadata can increase their findability, accessibility, interoperability, and reusability (FAIR)ness [ 11 ]. The FAIR principles are recognized internationally as important guides to conducting research [ 12 ]. Interoperability, in particular, is defined as the ability of several systems to exchange information, as well as read and use the received information without requiring further preprocessing [ 13 ]. Although there are several levels of interoperability [ 14 ], the focus of this study in the context of health care data was on semantic (use of standard terminologies and classifications) and syntactic (implementation of a standard exchange format) interoperability.
The use of data standards when designing CRFs can serve multiple purposes: in addition to supporting data quality, it facilitates the merging and exchange of data from multiple sources, as well as subsequent analysis [ 5 ]. International standards development organizations (SDOs), such as Health Level Seven (HL7) or Integrating the Healthcare Enterprise (IHE), promote and coordinate the use of these standards [ 15 ]. HL7 has developed the exchange standard Fast Healthcare Interoperability Resource (FHIR), which allows for the exchange of health-related information based on packaging it into so-called resources. The FHIR can represent a wide range of data, particularly those generated in care settings [ 16 ]. In comparison, the Clinical Data Interchange Standards Consortium (CDISC) has published standards for the representation of CRF data used in clinical trials [ 17 ].
By mapping study data elements to international semantic standard codes, the included concepts receive an unambiguous definition that is tied to an identifier that makes it machine-readable [ 18 ]. Among the widely used terminologies and classifications for health care concepts are the Logical Observation Identifiers Names and Codes (LOINC) and the Systematized Nomenclature of Medicine – Clinical Terms (SNOMED CT). The National Cancer Institute Thesaurus (NCIt) is also available as a reference terminology focused, among others, on translational research and clinical care information [ 19 ]. LOINC provides standard codes (each comprising a set of an identifier, a name, and a code) for laboratory observations, documents, and questionnaires [ 20 ]. SNOMED CT covers a broad range of health care information, and each of its concepts has a unique identifier and is defined by a description and 1 or more relationships [ 21 ].
In this study, we set out to analyze CRF variables from 6 study protocols capturing information about diagnostic testing with the purpose of identifying CDEs specific to infectious diseases. The selected studies investigated 3 different infectious diseases in humans: COVID-19 [ 1 ], monkeypox (mpox) [ 22 ], and Zika [ 23 ]. The CRF variables we included originate from the International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) COVID-19 Core CRF [ 24 ], as well as from 3 of the many international research projects focused on gaining new insights into SARS-CoV-2: the ORCHESTRA project [ 25 ], the Intersectoral Platform (SUEP) of the National Pandemic Cohort Network (NAPKON SUEP) study [ 26 ], and the Lean European Open Survey on SARS-CoV‑2 (LEOSS) [ 27 ] study. Additionally, we analyzed the World Health Organization (WHO) CRF on the mpox infection [ 28 ] and the Zika CRFs of ZIKAlliance [ 29 ].
Our goal of proposing standardized paninfectious disease CRF variables for diagnostic testing information for use in CRFs was broken down into 3 subtasks: (1) identification of interstudy CDEs, (2) creation of a preliminary map of the CDEs to semantic standard codes, and (3) development of a proposed mapping of the CDEs to the FHIR syntax standards [ 30 ] and the CDISC’s standards for data collection [ 17 ].
Ethical Considerations
Since only CRF metadata (meaning definitions of questions and answers used to comprise CRFs) were used and no actual patient data were reviewed in this study, ethics approval was not required.
Study Design
Figure 1 provides a graphical overview of the steps we followed to create a standardized set of variables for use in data collection instruments in infectious disease studies focusing on diagnostic testing.
We examined 6 CRFs provided to us by 4 research consortia, and we downloaded the publicly available CRFs from the ISARIC and WHO websites [ 24 ]. We proceeded to extract diagnostic testing variables from each CRF and organized them for analysis and comparison in a Microsoft Excel sheet.
The following CRFs were included:
- ORCHESTRA work package 6 CRF [ 31 ]
- Cross-sectoral platform (SUEP) CRF of NAPKON [ 32 ]
- LEOSS study [ 27 , 33 ] electronic case report form (eCRF)
- ISARIC-WHO COVID-19 core CRF [ 24 ]
- Zika study CRF
- Mpox study CRF [ 28 ]
We translated the variables from the NAPKON SUEP study from German into English to harmonize it with the language of the other selected studies (English). The study manager verified the translation.

Common Data Elements
In the first step of analyzing the study metadata, we reviewed all CRF variables (questions and answers). Adopting the National Institutes of Health’s methodology to derive CDEs [ 34 ], we created common categories to group variables based on the key information they contained. We then reviewed the newly organized variables to determine which categories were present in at least 2 (33%) of the 6 CRFs. These common variables then formed the basis as newly identified CDEs for infectious diseases.
For each of these preliminary CDEs, the extensive value set (sum of all unique answers) across all reviewed CRFs was determined. If necessary, we created value set subsets based on informational content and pathogen type.
Mapping to Standards
Each CDE (question and value set) was then mapped to the appropriate semantic standard code(s) and FHIR element(s). We searched for available terminology codes using the NCIt browser (version 23.02d, release date February 27, 2023), the SearchLOINC tool (v2.26), and the SNOMED CT browser (version 2023-03-31). If no semantic standard code was found, we prepared a submission to request the creation of a new code, depending on the informational domain, with NCIt, SNOMED CT, or LOINC.
CRF Analysis
The analysis of the CRFs used in 6 infectious disease studies led to the identification of 216 variables focusing on diagnostic testing, which were in the scope of further analysis: 103 (47.7%) from ORCHESTRA, 51 (23.6%) from NAPKON SUEP, 27 (12.5%) from the Zika study, 16 (7.4%) from the ISARIC CRF, 13 (6%) from the LEOSS survey, and 6 (2.8%) from the mpox study (Table S1 in Multimedia Appendix 1 [ 25 , 28 , 32 , 35 - 42 ]). These diagnostic testing variables could be grouped into 22 newly defined categories, which are shown in Table S2 in Multimedia Appendix 1 .
Based on the analysis of the 6 CRFs, we identified 11 CDEs, each of which was present in at least 2 (33%) of the 6 reviewed data collection instruments and reflected diagnostic testing information applicable to infectious disease studies. We mapped these CDEs to semantic standard codes and FHIR resources (illustrated in Figure 2 ), as well as to the CDISC ( Multimedia Appendix 2 ).

Viral Lineage/Clade
The first CDE was defined as “viral lineage” or “viral clade.” Depending on the virus investigated, its value sets would vary to reflect the applicable clade and lineage details, as exemplified in Figure 3 .
Genetic diversity, as described in a phylogenetic tree, is classified by clades. A clade, also called genotype or subtype, comprises a set of lineages that are all descended from only 1 ancestor, common to them [ 43 ].
ORCHESTRA and the human mpox study contained 3 (1.4%) variables providing monkeypox virus (MPXV) and SARS-CoV-2 clade details. In addition, viral lineage information was collected from ORCHESTRA, the ISARIC CRF, and NAPKON SUEP across 4 (1.9%) variables.
There is no uniform convention for naming viral clades and lineages. In the case of SARS-CoV-2, the most widely used nomenclatures for subtypes are provided by the Global Initiative on Sharing All Influenza Data [ 44 ], Rambault et al [ 43 ], and Nextstrain [ 45 ], which differ in the position at which clades are differentiated from one another.

Specimen Identifier, Specimen Collection Date, and Specimen Type
Our analysis led to the identification of “specimen identifier,” “specimen collection date,” and “specimen type” as additional CDEs across the 6 studies. ORCHESTRA and the Zika and mpox studies included 6 (2.8%) variables that were grouped as “specimen identifiers” and had a free-text format. Any biological specimen (ie, blood, urine, cerebrospinal fluid, feces) used for laboratory analysis must be uniquely identified so that the resulting findings are associated with the right patient. Identifiers might contain a patient’s first and last names, birth date, medical facility number, or a unique, randomly generated code [ 46 ]. In addition to this internal laboratory-based specimen identifier, a particular specimen might have a second, external identifier that is assigned when results based on the analysis of said specimen are uploaded to a public/restricted databases or to a biobank [ 47 ].
Furthermore, 8 (3.7%) variables across the Zika study, NAPKON SUEP, ORCHESTRA, and ISARIC CRFs constituted the data element “specimen collection date,” requiring the input of a date format (mm/dd/yyyy). The specimen collection date marks the date on which a specimen was collected from a patient and placed in a specimen container for ensuing processing and analysis.
Details about the kind of specimen collected and used for analysis are provided by the coded “specimen type” CDE. All 6 reviewed studies included the data element “specimen type” in their variables. Our analysis led to the finding that there tended to be 2 axes involved in the value set elements of the specimen type, which covered information about the method used to collect the specimen (ie, swab) and the site of origin (ie, skin lesion). Examples are shown in Multimedia Appendix 3 .
Test Date and Test Performer
The CDEs “test date” and “test performer” included variables from the ORCHESTRA and Zika study CRFs and the ORCHESTRA and NAPKON SUEP CRFs, respectively. The test date refers to the calendar date on which a particular laboratory diagnostic test (specified by the CDE “test type”) was conducted. The “test performer” CDE captures the full name of the individual(s) executing this diagnostic test in free-text format.
Test Type, Target Gene, and Test Manufacturer
The coded CDE “test type” captures a specific laboratory test, which in this context would fall into 3 main categories: serology, sequencing, and polymerase chain reaction (PCR) analysis. All 6 reviewed CRFs included variables providing details about diagnostic tests. For serology tests, the test type in the analyzed SARS-CoV-2 studies provided details on the method, along with the analyzed target, whereas in the Zika study, only the target was given ( Multimedia Appendix 4 ).
In the context of COVID-19 research, lateral flow testing, immunofluorescence assay (IFA), enzyme-linked immunosorbent assay (ELISA), and chemiluminescence immunoassay (CLIA) are frequently used methods for the diagnosis of infections [ 48 ]. The detection of Zika and mpox infections is usually also based on serology, specifically ELISA-based antibody measurements [ 23 ].
The coded CDE “target gene” grouped 8 (3.7%) variables across the NAPKON SUEP, LEOSS, and ORCHESTRA CRFs. It refers to the target of a genome-focused diagnostic test, such as PCR or a sequencing method. Using primers that contain bases that are complementary to a conserved sequence within the target gene of a particular virus, this sequence, if present in the biological sample, is amplified and can be detected through PCR [ 49 ].
In total, 13 (6%) variables used across the Zika, NAPKON SUEP, and ORCHESTRA CRFs were grouped into the coded CDE “test manufacturer.” This data element provides information about the manufacturer of the diagnostic test (ie, kit or testing system). For example, the following PCR systems (manufacturers) were mentioned in a study variable in the NAPKON SUEP CRF: Seegene (Allplex) [ 50 ], altona Diagnostics (RealStar) [ 51 ], and Roche Deutschland Holding (cobas) [ 52 ].
Qualitative and Quantitative Results
All reported results of diagnostic testing covered by variables in the 6 CRFs we reviewed could be clustered into either qualitative or quantitative results, and thus, they formed the last 2 (18%) of 11 coded CDEs that we identified. A qualitative result details the findings about the presence or absence of a measured observable, such as virus-specific antibody or gene material. In contrast, a quantitative result constitutes numeric measurements (see Table S3 in Multimedia Appendix 1 ). In the studies that we analyzed, those numeric values were given for the titer, cycle threshold, and concentration of the same observables mentioned before.
Semantic Standards
To facilitate semantic interoperability of the proposed diagnostic testing CDEs, we suggested mapping each CDE and respective value set to the terminology standards SNOMED CT, LOINC, and NCIt. For each CDE, we created a suggested mapping that covers the variable itself and a nonexclusive list of possible value set elements (Table S4 in Multimedia Appendix 1 ).
The CDEs “viral lineage” and “viral clade” could be mapped to the following NCIt codes (code and description are shown), respectively: “C60792 Lineage” and “C179767 Clade.” Depending on the analyzed virus, the value sets (answers) could differ and be represented through mapping to either NCIt or LOINC codes. For example, in the case of detection of the SARS-CoV-2 variant B.1.1.7, the NCIt code “C179573 SARS Coronavirus 2 B.1.1.7” or the LOINC code “LA31705-9 SARS-CoV-2 B.1.1.7 lineage” is available.
The CDE “specimen identifier” could be represented in a standardized way using SNOMED CT, LOINC, and NCIt terms, as shown in Table S4 in Multimedia Appendix 1 . Likewise, codes from all 3 standards were available to represent the free-text CDEs “specimen collection date” and “specimen type.”
There are semantic standard concepts available to describe the “test date” and “test performer” CDEs. Using SNOMED CT codes from the “procedure” hierarchy or using NCIt terms, diagnostic test types, such as serology assays, sequencing, and PCR, can be described in a standardized manner. Incidentally, there are a few standard codes available to represent the value sets for “target gene” (for the envelope gene in SNOMED CT and a few in the NCIt), although not necessarily specifically meant to map viral pathogens’ genes (exception in the NCIt: “C19108 Viral Envelope Gene”). Thus, we prepared a submission to the NCIt for the creation of concepts that cover the prominently analyzed SARS-CoV-2 [ 53 ] and Zika virus (ZIKV) genes [ 54 ]. We submitted 33 concepts for code creation to the SDOs LOINC and NCIt (Table S5 in Multimedia Appendix 1 ).
No SNOMED CT codes were available to describe the value set elements for the “test manufacturer” CDE. However, both the NCIt and LOINC provide terms for this purpose; the NCIt has created concepts for specific COVID-19 diagnostic kits, detailing the manufacturer, analytical target, and method. Likewise, LOINC has created codes that bundle several kits into a single term, such as “94558-4 SARS-CoV-2 (COVID-19) Ag [Presence] in Respiratory specimen by Rapid immunoassay,” which represents 4 commercially available kits [ 55 ].
There are generic semantic terms from SNOMED CT and the NCIt to describe the “quantitative result” and “qualitative result” CDEs in a standardized manner, which can be used across viral pathogen studies, such as “Laboratory Test Result” or just “Result.” However, this would omit the distinction between “qualitative” and “quantitative.”
LOINC provides a comprehensive list of terms to describe qualitative results of laboratory diagnostic tests for SARS-CoV-2 and antibody measurements specific to ZIKV.
The use of the SNOMED CT terminology requires a country (or institutional) license. SNOMED International has, however, been releasing its Global Patient Set containing currently around 24,000 concepts, which can be used free of charge [ 56 ]. Of the 90 SNOMED CT codes, 33 (37%) that we included in the exemplary value set mappings for our proposed infectious disease diagnostic CDEs are covered by the Global Patient Set.
Syntax Standard
We proposed a preliminary mapping of the diagnostic testing CDEs to FHIR (version R4) elements as a first step toward establishing syntactical interoperability ( Figure 2 , right). Of the 11 CDEs that we identified, 8 (72.7%) were mapped to the Observation resource and the remaining 3 (27.3%) to the Specimen resource.
Additionally, we provided a preliminary suggested mapping of the FHIR elements to the CDISC according to the FHIR to CDISC Joint Mapping Implementation Guide v1.0 [ 57 ] (see Multimedia Appendix 2 ).
Principal Findings
Resulting from the review of 6 CRFs, we identified 11 panstudy CDEs that capture key diagnostic testing information commonly collected across the reviewed infectious disease studies. These CDEs were purposefully kept generic to enhance the probability that they could be adopted by researchers and integrated into data collection instruments of other infectious disease studies, even if a different pathogen was studied. The pathogen under investigation in a given study would determine the value set elements of CDEs of the coded data.
The CDEs “viral lineage” and “viral clade” provide the means to describe genetic relatedness of viruses, which is critical to pathogen surveillance and relies on the availability of well-defined nomenclature [ 58 ]. Currently, no panvirus approach to naming viral clades and lineages exists. The International Committee on Taxonomy of Viruses, founded in 1966, has the goal to develop a taxonomy for viruses and establish names for viral taxa based on international agreement. However, the International Committee on Taxonomy of Viruses does not address the naming of viral clades and lineages [ 59 ]. In the context of ensuring that diagnostic testing results are linked to the right sample (specimen) and patient, the CDEs “specimen identifier,” “specimen collection date,” and “specimen type” are important parameters. Regarding the diagnostic test itself, documentation of the CDEs “test date” and “test performer” can help identify quality problems retrospectively. Diagnostic testing results can be split into the CDEs “qualitative result” and “quantitative result,” which would confirm the presence/absence of signs of a pathogen or numeric values of measured observables, such as antibody titers. The CDEs “test type,” “target gene,” and “test manufacturer” provide all complementary details to the diagnostic tests conducted. Along with the increasing inclusion of molecular testing variables in the study of infectious diseases, we expect that this number of recurring elements (which would be candidate CDEs) that describe diagnostic tests across different studies will continue to grow.
The power of research findings can be expanded through combining data from several clinical studies for analysis in an effort to create a larger data set. Without considering privacy or legal considerations, the basis for merging data from different sources is that the correct information (ie, data variables) is linked together to ensure accuracy and avoid misinterpretation. Defining standardized CDEs that serve as a common language across clinical studies is one way to approach this challenge [ 9 ]. Lin et al [ 5 ] described a similar approach of how CRF design can be optimized for data harmonization by creating a pool of reusable CDEs. There are numerous examples for the creation of CDEs for specific medical specialties and use cases, such as stroke trials [ 60 ], pregnancy pharmacovigilance [ 61 ], and COVID-19 [ 62 ]. This includes a set of CDEs on the quality of life in neurological disorders, as well as the PhenX Toolkit to capture key information on phenotypes [ 8 ].
To facilitate interoperability of study data in particular, we proposed a mapping of the identified CDEs to semantic and syntactic standards. We also created a table with practical examples of available standard codes to identify value set concepts ambiguously for variables contained in CRFs from studies focused on SARS-CoV-2, ZIKV, and MPXV (see Table S4 in Multimedia Appendix 1 ).
In the past, we have described how semantic interoperability standard codes can be integrated directly into the study metadata to facilitate merging, sharing, and analysis of patient data that are being collected across several clinical studies and cohort types, where several methods for data storage and collection have been used [ 10 ]. Kush et al [ 9 ] and Kersloot et al [ 11 ], among others, have discussed the advantage of introducing interoperability standards prior to data collection rather than retrospectively with the aim to save time and other resources.
An important aspect of mapping study data to semantic standard concepts is choosing appropriate terminology. Although there is no universal guidance for this process, we can draw instructive conclusions from our attempt to propose a mapping for the CDEs we identified for which we searched within the LOINC, SNOMED CT and NCIt, terminologies.
The selection of semantic standards to represent CDEs and their value sets depends on the way the CDEs (and underlying CRF variables) are phrased with regard to the level of detail and the kind of information that are described. The category of information covered by a CRF variable is the first “filter” for finding the appropriate terminology. The NCIt, which is managed by the National Cancer Institute, focuses on providing a vocabulary for the cancer domain [ 63 ]; hence, it comprises many (gen)omics-related terms. Each NCIt term is represented by a code and a name and has several annotations [ 64 ].
In contrast, the LOINC coding system, which is published by the Regenstrief Institute, is used by numerous large laboratories and government agencies, such as the Centers for Disease Control and Prevention, to describe laboratory and clinical findings, as well as documents [ 65 ]. Although LOINC has a clear focus on representing laboratory terms, SNOMED CT terms have a broader coverage of information and are commonly used to represent clinical information in electronic health records [ 63 ]. SNOMED CT and th eNCIt both provide concepts that are suitable to describe variables and value sets if they are kept more generic in their wording. LOINC terms, in contrast, are specific and should only be used to represent questions, not value sets. Contrary to the NCIt, SNOMED CT comprises a limited set of concepts to describe genomic methods and results.
Unlike the use of LOINC and the NCIt, embedding SNOMED CT concepts into the metadata of research data requires a license. In recent years, many countries have purchased a SNOMED CT affiliate license or become a SNOMED CT member, including Germany, Spain, and Portugal [ 66 ].
The LOINC coding system includes suitable codes for several of the CDEs we defined. For example, we chose the concept “95609-4 SARS-CoV-2 (COVID-19) S gene [Presence] in Respiratory specimen by Sequencing” as 1 of the available standard terms for coding the “qualitative result” CDE. However, it also covers the “target gene” (S gene), “specimen type” (respiratory specimen), and “test type” (sequencing) CDEs. Another aspect that should be kept in mind, especially concerning selecting standard terms for the “quantitative result” CDE when used in a CRF, is that the units of the result should be clearly defined and match those of the standard term. Although each LOINC term has a defined unit, SNOMED CT concepts do not necessarily implicitly or explicitly define units. The concept “1240461000000109 Measurement of severe acute respiratory syndrome coronavirus 2 antibody (observable entity)” has no unit of measure attached and hence can be used if a CRF variable can be measured using several different units. A standard way to describe units is offered by the Unified Code for Units of Measure [ 67 ].
Regarding finding the appropriate standard code for viral lineage, the more general-purpose terminology of SNOMED CT does not include the required level of detail for this CDE, which is captured in the NCIt. However, the list of microorganisms defined as concepts by SNOMED CT under the hierarchy “organism” is detailed and can be used to describe a pathogen. The hierarchical organization of SNOMED CT, which also includes sublevels of concepts, provides a clear idea of the positioning of any microorganism within the complex classification of organisms overall.
As knowledge rapidly evolves in health care, missing concepts are regularly added to ontologies. The process involves concept creation requests from the public, which are submitted to the SDOs. Zheng et al [ 63 ] describe an approach of using formal concept analysis to identify missing concepts in the NCIt and SNOMED CT.
We also proposed a mapping of the 11 diagnostic testing CDEs to the corresponding FHIR (version R4) element. This provides data with a standardized exchange format, which can incorporate standard terminologies. Elements in the Specimen [ 68 ] and Observation [ 69 ] (and for the test manufacturer, also Device [ 70 ]) resources can be used to represent all 11 CDEs.
Limitations
The identified CDEs focus on diagnostic tests used in infectious disease studies. Additional CDEs that would fall into other informational categories (eg, therapeutics or comorbidities) were not considered as they were out of the scope of our study. Furthermore, since the reviewed ORCHESTRA variables include CRF variables from several COVID-19 studies, the selection of protocols might appear unbalanced.
The need to investigate COVID-19 quickly and extensively has made the pool of available variables describing diagnostic tests particularly abundant. Kush et al [ 9 ] point out that although the name “CDE” implies that these elements are common, they are not so commonly used. This is due to a lack of mandatory requirements for their use [ 9 ]. A necessary step to increase the adoption and value of CDEs would be that funding bodies (eg, the National Institutes of Health or the European Commission) in collaboration with SDOs create and impose mandatory requirements for the implementation of existent CDEs on recipients of project funding.

Acknowledgments
This material contains content from Logical Observation Identifiers Names and Codes (LOINC) [ 71 ]. LOINC is the copyright of Regenstrief Institute, Inc, and the LOINC Committee and is available at no cost under the license [ 72 ]. LOINC is a registered United States trademark of Regenstrief Institute, Inc.
This material also includes content from the National Cancer Institute Thesaurus, published by the National Cancer Institute [ 19 ].
The Systemized Nomenclature of Medicine - Clinical Terms (SNOMED CT) was used by the permission of SNOMED International. SNOMED CT was originally created by the College of American Pathologists. “SNOMED,” “SNOMED CT,” and “SNOMED Clinical Terms” are registered trademarks of SNOMED International [ 73 ].
We would like to thank all 3 SDOs for their collaboration and support with new term submissions and SNOMED International, in particular, for granting us permission to display and share the suggested terminology bindings.
The ORCHESTRA project received funding from the European Union’s Horizon 2020 Research and Innovation Program (grant agreement 101016167). The ZIKAlliance project received funding from the European Union’s Horizon 2020 Research and Innovation Program (grant agreement 734548). The ReCODID project received funding from the European Union’s Horizon 2020 Research and Innovation Program (grant agreement 825746). The Lean European Open Survey on SARS-CoV‑2 (LEOSS) registry was supported by the German Centre for Infection Research (DZIF) and the Willy Robert Pitzer Foundation. The National Pandemic Cohort Network (NAPKON) is part of the Network University Medicine and was funded by the German Federal Ministry of Education and Research (FKZ: 01KX2021). Parts of the infrastructure of the Würzburg study site were supported by the Bavarian Ministry of Research and Art to support coronavirus research projects. Parts of the NAPKON project suite and study protocols of the cross-sectoral cohort platform are based on projects funded by the DZIF.
The members of the working groups are as follows: NAPKON Working Group: Gabriele Anton, Katharina Appel, Sabine Blaschke, Isabel Bröhl, Johanna Erber, Karin Fiedler, Ramsia Geisler, Peter U. Heuschmann, Thomas Illig, Monika Kraus, Dagmar Krefting, Jens-Peter Reese, Margarete Scherer, Jörg Janne Vehreschild, Maria J.G.T. Vehreschild, and Luise Wolf. ORCHESTRA Working Group: Chiara Dellacasa, Miroslav Puskaric, Thomas Osmo, Elisa Rossi, and Anna Gorska. LEOSS Working Group: Jörg Janne Vehreschild, Carolin E. M. Koll, Margarete Scherer, and Maria J.G.T. Vehreschild. ReCoDID Working Group: Lauren Maxwell, Heather Hufstedler, and Frank Tobian.
Data Availability
The analyzed case report forms are stored and available in Excel format for review on the project’s online data repository [ 74 ].
Authors' Contributions
CS and ER created the first draft of the manuscript, together with SMH, SMNdM, and TJ. All authors reviewed the draft, commented on it, and provided revisions, and they have approved the final version of the manuscript.
Conflicts of Interest
None declared.
Supplementary tables.
Map between the proposed FHIR representation (FHIR resources) and the corresponding CDISC elements for the 11 identified diagnostic testing CDEs for infectious disease studies. CDE: common data element; CDISC: Clinical Data Interchange Standards Consortium; FHIR: Fast Healthcare Interoperability Resources.
Example specimen types listed within CRF variables in the analyzed infectious disease studies. CRF: case report form.
Example variables (question and value set) from 2 of the reviewed CRFs. One came from the ZIKV study and the other from the NAPKON SUEP study. CLIA: chemiluminescence immunoassay; CRF: case report form; ELISA: enzyme-linked immunosorbent assay; IFA: immunofluorescence assay; IgA: immunoglobulin A; IgG: immunoglobulin G; IgM: immunoglobulin M; LFT: lateral flow immunoassay; PCR: polymerase chain reaction; SGTF: S gene target failure; VNTR: variable number of tandem repeats; WES: whole exome sequencing; WGS: whole genome sequencing; ZIKV: Zika virus.
- He Z, Erdengasileng A, Luo X, Xing A, Charness N, Bian J. How the clinical research community responded to the COVID-19 pandemic: an analysis of the COVID-19 clinical studies in ClinicalTrials.gov. JAMIA Open. Apr 2021;4(2):ooab032. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Radzikowska U, Ding M, Tan G, Zhakparov D, Peng Y, Wawrzyniak P, et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. Nov 2020;75(11):2829-2845. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Fox T, Geppert J, Dinnes J, Scandrett K, Bigio J, Sulis G, et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. Nov 17, 2022;11(11):CD013652. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Courtot M, Gupta D, Liyanage I, Xu F, Burdett T. BioSamples database: FAIRer samples metadata to accelerate research data management. Nucleic Acids Res. Jan 07, 2022;50(D1):D1500-D1507. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lin C, Wu N, Liou D. A multi-technique approach to bridge electronic case report form design and data standard adoption. J Biomed Inform. Feb 2015;53:49-57. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Committee for Human Medicinal Products. Guideline for good clinical practice E6(R2). European Medicines Agency. Jan 12, 2016. URL: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-good-clinical-practice-e6r2-step-5_en.pdf [accessed 2023-02-14]
- Bellary S, Krishnankutty B, Latha M. Basics of case report form designing in clinical research. Perspect Clin Res. Oct 2014;5(4):159-166. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cohen MZ, Thompson CB, Yates B, Zimmerman L, Pullen CH. Implementing common data elements across studies to advance research. Nurs Outlook. 2015;63(2):181-188. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kush R, Warzel D, Kush M, Sherman A, Navarro E, Fitzmartin R, et al. FAIR data sharing: The roles of common data elements and harmonization. J Biomed Inform. Jul 2020;107:103421. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Rinaldi E, Stellmach C, Rajkumar NMR, Caroccia N, Dellacasa C, Giannella M, et al. Harmonization and standardization of data for a pan-European cohort on SARS- CoV-2 pandemic. NPJ Digit Med. Jun 14, 2022;5(1):75. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kersloot MG, Jacobsen A, Groenen KH, Dos Santos Vieira B, Kaliyaperumal R, Abu-Hanna A, et al. De-novo FAIRification via an Electronic Data Capture system by automated transformation of filled electronic Case Report Forms into machine-readable data. J Biomed Inform. Oct 2021;122:103897. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Boeckhout M, Zielhuis GA, Bredenoord AL. The FAIR guiding principles for data stewardship: fair enough? Eur J Hum Genet. Jul 2018;26(7):931-936. [ FREE Full text ] [ CrossRef ] [ Medline ]
- IEEE. IEEE standard computer dictionary: a compilation of IEEE standard computer glossaries. IEEE Std 610. Jan 18, 1991:1-217. [ CrossRef ]
- Lehne M, Sass J, Essenwanger A, Schepers J, Thun S. Why digital medicine depends on interoperability. NPJ Digit Med. 2019;2:79. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lee S, Do H. Comparison and Analysis of ISO/IEEE 11073, IHE PCD-01, and HL7 FHIR Messages for Personal Health Devices. Healthc Inform Res. Jan 2018;24(1):46-52. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Benson T, Grieve G. Principles of FHIR. In: Benson T, editor. Principles of Health Interoperability: SNOMED CT, HL7 and FHIR. London. Springer-Verlag London; Jul 01, 2016:329-348.
- Facile R, Muhlbradt EE, Gong M, Li Q, Popat V, Pétavy F, et al. Use of Clinical Data Interchange Standards Consortium (CDISC) Standards for Real-world Data: Expert Perspectives From a Qualitative Delphi Survey. JMIR Med Inform. Jan 27, 2022;10(1):e30363. [ FREE Full text ] [ CrossRef ] [ Medline ]
- de Mello BH, Rigo SJ, da Costa CA, da Rosa Righi R, Donida B, Bez MR, et al. Semantic interoperability in health records standards: a systematic literature review. Health Technol (Berl). 2022;12(2):255-272. [ FREE Full text ] [ CrossRef ] [ Medline ]
- NCI thesaurus. National Cancer Institute. URL: https://ncithesaurus.nci.nih.gov/ncitbrowser/ [accessed 2023-10-09]
- About LOINC. Regenstrief Institute. URL: https://loinc.org/about/ [accessed 2022-11-10]
- Millar J. The Need for a Global Language - SNOMED CT Introduction. Stud Health Technol Inform. 2016;225:683-685. [ Medline ]
- Jalilian S, Bastani MN. The Mpox, serious menace, or paper tiger? Iran J Microbiol. Dec 2022;14(6):770-777. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Panning M. Zika Virus Serology: More Diagnostic Targets, more Reliable Answers? EBioMedicine. Feb 2017;16:12-13. [ FREE Full text ] [ CrossRef ] [ Medline ]
- World Health Organization, ISARIC. COVID-19 core case report form. Acute respiratory infection clinical characterisation data tool. ISARIC. URL: https://isaricdev.wpenginepowered.com/wp-content/uploads/2021/02/ISARIC-WHO-COVID-19-CORE-CRF_EN.pdf [accessed 2023-03-20]
- Work packages. ORCHESTRA. URL: https://orchestra-cohort.eu/work-packages/ [accessed 2023-05-09]
- Vehreschild J. Intersectoral platform (SÜP) of the National Pandemic Cohort Network (NAPKON) (SUEP-NAPKON). ClinicalTrials.gov. Feb 24, 2021. URL: https://clinicaltrials.gov/ct2/show/record/NCT04768998 [accessed 2023-03-20]
- Study protocol: LEOSS; Lean European Open Survey on SARS-CoV-2. Deutsche Gesellschaft für Infektiologie (DGI). URL: https://www.dgi-net.de/wp-content/uploads/2020/03/LEOSS-Protocol-Submission-1-20200316.pdf [accessed 2023-04-04]
- Mpox case investigation form (CIF). World Health Organization. Dec 22, 2022. URL: https://cdn.who.int/media/docs/default-source/documents/health-topics/monkeypox/mpox_cif-narrative_epi_20221222.pdf?sfvrsn=d52108e5_1 [accessed 2023-04-04]
- ZIKAlliance and ReCoDID working together to promote data and sample sharing across infectious disease cohort studies. ZIKAlliance. URL: https://zikalliance.tghn.org/articles/zikalliance-and-recodid-working-together-promote-data-and-sample-sharing-across-infectious-disease-cohort-studies/ [accessed 2023-04-04]
- Benson T, Grieve G. HL7 dynamic model. In: Benson T, editor. Principles of Health Interoperability: SNOMED CT, HL7 and FHIR. Cham. Springer International Publishing; Jul 01, 2016:303-309.
- Gupta A, Konnova A, Smet M, Berkell M, Savoldi A, Morra M, mAb ORCHESTRA working group, et al. Host immunological responses facilitate development of SARS-CoV-2 mutations in patients receiving monoclonal antibody treatments. J Clin Invest. Mar 15, 2023;133(6):e166032. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Schons M, Pilgram L, Reese J, Stecher M, Anton G, Appel KS, et al. NAPKON Research Group. The German National Pandemic Cohort Network (NAPKON): rationale, study design and baseline characteristics. Eur J Epidemiol. Aug 2022;37(8):849-870. [ FREE Full text ] [ CrossRef ] [ Medline ]
- FAQ - Lean European Open Survey on SARS-CoV-2 infected patients. LEOSS. URL: https://leoss.net/faq/ [accessed 2023-03-20]
- Glossary - NINDS common data elements. National Institutes of Health. URL: https://www.commondataelements.ninds.nih.gov/glossary [accessed 2023-10-09]
- ISARIC Clinical Characterisation Group. The value of open-source clinical science in pandemic response: lessons from ISARIC. Lancet Infect Dis. Dec 2021;21(12):1623-1624. [ FREE Full text ] [ CrossRef ] [ Medline ]
- About - REDCap. Vanderbilt. URL: https://projectredcap.org/about/ [accessed 2024-06-07]
- Pilgram L, Schons M, Jakob CEM, Claßen AY, Franke B, Tscharntke L, et al. [The COVID-19 pandemic as an opportunity and challenge for registries in health services research: lessons learned from the Lean European Open Survey on SARS-CoV-2 infected patients (LEOSS)]. Gesundheitswesen. Nov 2021;83(S 01):S45-S53. [ CrossRef ] [ Medline ]
- Lean European Open Survey on SARS-CoV‑2 infected patients (LEOSS). URL: https://leoss.net/ [accessed 2023-06-07]
- Avelino-Silva VI, Mayaud P, Tami A, Miranda MC, Rosenberger KD, Alexander N, et al. ZIKAlliance Clinical Study Group. Study protocol for the multicentre cohorts of Zika virus infection in pregnant women, infants, and acute clinical cases in Latin America and the Caribbean: the ZIKAlliance consortium. BMC Infect Dis. Dec 26, 2019;19(1):1081. [ FREE Full text ] [ CrossRef ] [ Medline ]
- NAPKON. Nationales Pandemie Kohorten Netz. 2023. URL: https://napkon.de/ [accessed 2023-06-05]
- Follow-up of COVID-19 long term sequelae. ClinicalTrials.gov. Dec 2021. URL: https://clinicaltrials.gov/ct2/show/NCT05097677 [accessed 2024-05-29]
- Monitoring COVID-19 vaccination response in fragile populations (ORCHESTRA-4). ClinicalTrials.gov. Feb 2022. URL: https://clinicaltrials.gov/ct2/show/NCT05222139 [accessed 2023-03-21]
- Rambaut A, Holmes EC, O'Toole Á, Hill V, McCrone JT, Ruis C, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. Nov 2020;5(11):1403-1407. [ FREE Full text ] [ CrossRef ] [ Medline ]
- GISAID. Re3data.org. URL: https://www.re3data.org/repository/r3d100010126 [accessed 2022-10-03]
- Genomic epidemiology of SARS-CoV-2 with subsampling focused globally over the past 6 months. Nextstrain. Apr 23, 2024. URL: https://nextstrain.org/ncov/gisaid/global/6m [accessed 2023-03-28]
- Ernst DJ, Martel AM, Astin D, Dew TR, Dietz, Jr RL, Dubrowny N, et al. GP33: accuracy in patient and specimen identification. Clinical and Laboratory Standards Institute. Apr 2019. URL: https://clsi.org/media/3121/gp33-ed2_sample.pdf [accessed 2023-10-03]
- Durant TJ, Gong G, Price N, Schulz WL. Bridging the Collaboration Gap: Real-time Identification of Clinical Specimens for Biomedical Research. J Pathol Inform. 2020;11:14. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gong F, Wei HX, Li Q, Liu L, Li B. Evaluation and Comparison of Serological Methods for COVID-19 Diagnosis. Front Mol Biosci. 2021;8:682405. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kuchinski KS, Loos KD, Suchan DM, Russell JN, Sies AN, Kumakamba C, et al. Targeted genomic sequencing with probe capture for discovery and surveillance of coronaviruses in bats. Elife. Nov 08, 2022;11:e79777. [ FREE Full text ] [ CrossRef ] [ Medline ]
- AllplexTM 2019-nCoV assay. Seegene. URL: https://www.seegene.com/assays/allplex_2019_ncov_assay [accessed 2023-10-07]
- RealStar® product range. altona Diagnostics. URL: https://www.altona-diagnostics.com/en/realstar-product-range-a.html [accessed 2023-10-07]
- cobas® modular platform. Roche Deutschland Holding. URL: https://www.roche.de/diagnostik/produkte-loesungen/systeme/cobas-modular-platform [accessed 2023-10-07]
- Brant AC, Tian W, Majerciak V, Yang W, Zheng Z. SARS-CoV-2: from its discovery to genome structure, transcription, and replication. Cell Biosci. Jul 19, 2021;11(1):136. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lundberg R, Melén K, Westenius V, Jiang M, Österlund P, Khan H, et al. Zika Virus Non-Structural Protein NS5 Inhibits the RIG-I Pathway and Interferon Lambda 1 Promoter Activation by Targeting IKK Epsilon. Viruses. Nov 04, 2019;11(11):1024. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Center for Surveillance, Epidemiology and Laboratory Services (CSELS). LOINC in vitro diagnostic (LIVD) test code mapping. Centers for Disease Control and Prevention. URL: https://www.cdc.gov/csels/dls/livd-codes.html [accessed 2023-04-03]
- Global Patient Set. SNOMED International. URL: https://www.snomed.org/gps [accessed 2023-10-10]
- FHIR to CDISC joint mapping implementation guide 1.0.0 - STU 1. HL7 International. URL: http://hl7.org/fhir/uv/cdisc-mapping/STU1/ [accessed 2023-10-04]
- Alm E, Broberg EK, Connor T, Hodcroft EB, Komissarov AB, Maurer-Stroh S, et al. Geographical and temporal distribution of SARS-CoV-2 clades in the WHO European Region, January to June 2020. Euro Surveill. Aug 2020;25(32):2001410. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Siddell SG, Walker PJ, Lefkowitz EJ, Mushegian AR, Dutilh BE, Harrach B, et al. Binomial nomenclature for virus species: a consultation. Arch Virol. Feb 2020;165(2):519-525. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Jabal MS, Ibrahim MK, Thurnham J, Kallmes KM, Kobeissi H, Ghozy S, et al. Common Data Elements Analysis of Mechanical Thrombectomy Clinical Trials for Acute Ischemic Stroke with Large Core Infarct. Clin Neuroradiol. Jun 2023;33(2):307-317. [ CrossRef ] [ Medline ]
- Richardson JL, Moore A, Bromley RL, Stellfeld M, Geissbühler Y, Bluett-Duncan M, et al. Core Data Elements for Pregnancy Pharmacovigilance Studies Using Primary Source Data Collection Methods: Recommendations from the IMI ConcePTION Project. Drug Saf. May 2023;46(5):479-491. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Weissman A, Cheng A, Mainor A, Gimbel E, Nowak K, Pan H, et al. Development and implementation of the National Heart, Lung, and Blood Institute COVID-19 common data elements. J Clin Transl Sci. 2022;6(1):e142. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zheng L, Min H, Chen Y, Keloth V, Geller J, Perl Y, et al. Outlier concepts auditing methodology for a large family of biomedical ontologies. BMC Med Inform Decis Mak. Dec 15, 2020;20(Suppl 10):296. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Fragoso G, de Coronado S, Haber M, Hartel F, Wright L. Overview and utilization of the NCI thesaurus. Comp Funct Genomics. 2004;5(8):648-654. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Stram M, Gigliotti T, Hartman D, Pitkus A, Huff SM, Riben M, et al. Logical Observation Identifiers Names and Codes for Laboratorians. Arch Pathol Lab Med. Feb 2020;144(2):229-239. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Our members. SNOMED International. URL: https://www.snomed.org/members [accessed 2023-04-04]
- Rychert J. In support of interoperability: A laboratory perspective. Int J Lab Hematol. Aug 2023;45(4):436-441. [ CrossRef ] [ Medline ]
- Specimen - FHIR v4.0.1. HL7 International. URL: http://hl7.org/fhir/R4/specimen.html [accessed 2023-03-13]
- Observation - FHIR v4.0.1. HL7 International. URL: http://hl7.org/fhir/R4/observation.html [accessed 2023-04-04]
- Device - FHIR v4.0.1. HL7 International. URL: http://hl7.org/fhir/R4/device.html [accessed 2023-04-06]
- Home - LOINC. Regenstrief Institute. URL: https://loinc.org/ [accessed 2023-10-09]
- Knowledge base - LOINC. Regenstrief Institute. URL: https://loinc.org/kb/ [accessed 2023-10-09]
- Home - SNOMED International. SNOMED International. URL: https://www.snomed.org [accessed 2023-10-09]
- Stellmach C, Rinaldi E. Raw study data - CDEs for infectious disease studies. ORCHESTRA Cloud. URL: https://cloud.orchestra-cohort.eu/s/HnG8YzDC4WNFsBY [accessed 2023-10-09]
Abbreviations
| common data element |
| Clinical Data Interchange Standards Consortium |
| case report form |
| enzyme-linked immunosorbent assay |
| findability, accessibility, interoperability, and reusability |
| Fast Healthcare Interoperability Resource |
| Health Level Seven |
| Integrating the Healthcare Enterprise |
| International Severe Acute Respiratory and emerging Infection Consortium |
| Lean European Open Survey on SARS-CoV‑2 |
| Logical Observation Identifiers Names and Codes |
| monkeypox |
| monkeypox virus |
| Intersectoral Platform (SUEP) of the National Pandemic Cohort Network |
| National Cancer Institute Thesaurus |
| polymerase chain reaction |
| standards development organization |
| Systematized Nomenclature of Medicine - Clinical Terms |
| World Health Organization |
| Zika virus |
Edited by A Mavragani; submitted 19.06.23; peer-reviewed by K Ndlovu, S Hume; comments to author 19.09.23; revised version received 10.10.23; accepted 18.01.24; published 10.06.24.
©Caroline Stellmach, Sina Marie Hopff, Thomas Jaenisch, Susana Marina Nunes de Miranda, Eugenia Rinaldi, The NAPKON, LEOSS, ORCHESTRA, and ReCoDID Working Groups. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 10.06.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

An Open Comparative Study of the Effectiveness and Incomparable Study of the Immunogenicity and Safety of the Vaccine (CoviVac) for Adults Aged 60 Years and Older
| The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read our for details. |
| ClinicalTrials.gov Identifier: NCT05765773 |
| : Active, not recruiting : March 13, 2023 : March 13, 2023 |
- Study Details
- Tabular View
- No Results Posted

| Condition or disease | Intervention/treatment | Phase |
|---|---|---|
| Corona Virus Infection Vaccine COVID-19 | Biological: CoviVac vaccine (inactivated whole-virion concentrated purified) manufactured by FSBSI "Chumakov FSC R&D IBP RAS" | Phase 2 |
| Study Type : | Interventional (Clinical Trial) |
| Actual Enrollment : | 200 participants |
| Allocation: | Non-Randomized |
| Intervention Model: | Parallel Assignment |
| Masking: | Single (Participant) |
| Primary Purpose: | Prevention |
| Official Title: | An Open Comprative Study of the Prophylactic Efficacy and a Non-comparative Study of the Immunogenicity and Safety of the Inactivated Whole-virion Concentrated Purified Coronavirus Vaccine (CoviVac) for Adults Aged 60 Years and Older |
| Actual Study Start Date : | July 1, 2021 |
| Actual Primary Completion Date : | October 1, 2022 |
| Estimated Study Completion Date : | March 1, 2023 |

| Arm | Intervention/treatment |
|---|---|
| Experimental: The study group consisted of 200 volunteers | Biological: CoviVac vaccine (inactivated whole-virion concentrated purified) manufactured by FSBSI "Chumakov FSC R&D IBP RAS" |
| No Intervention: Control group |
| Ages Eligible for Study: | 60 Years to 99 Years (Adult, Older Adult) |
| Sexes Eligible for Study: | All |
| Accepts Healthy Volunteers: | Yes |
Inclusion Criteria:
Volunteers must meet the following inclusion criteria:
Type of participants
• Healthy volunteers or volunteers with a history of stable diseases that do not meet any of the criteria for non-inclusion in the study.
Other inclusion criteria
- Written informed consent of volunteers to participate in a clinical trial
- Volunteers who are able to fulfill the Protocol requirements (i.e., fill out a self-observation Diary, come to control visits).
Exclusion Criteria:
SARS-CoV-2 infection • A case of established COVID-19 disease confirmed by PCR and/or ELISA in the last 6 months.
Diseases or medical conditions
- Serious post-vaccination reaction (temperature above 40 C, hyperemia or edema more than 8 cm in diameter) or complication (collapse or shock-like condition that developed within 48 hours after vaccination; convulsions, accompanied or not accompanied by a feverish state) to any previous vaccination.
- Burdened allergic history (anaphylactic shock, Quincke's edema, polymorphic exudative eczema, serum sickness in the anamnesis, hypersensitivity or allergic reactions to the introduction of any vaccines in the anamnesis, known allergic reactions to vaccine components, etc.).
- Guillain-Barre syndrome (acute polyradiculitis) in the anamnesis.
- The axillary temperature at the time of vaccination is more than 37.0 ° C.
- Acute infectious diseases (recovery earlier than 4 weeks before vaccination) according to anamnesis.
- Donation of blood or plasma (in the amount of 450 ml or more) less than 2 months before inclusion in the study.
- Severe and/or uncontrolled diseases of the cardiovascular, bronchopulmonary, neuroendocrine systems, gastrointestinal tract, liver, kidneys, hematopoietic, immune systems.
- Is registered at the dispensary for tuberculosis, leukemia, oncological diseases, autoimmune diseases.
- Any confirmed or suspected immunosuppressive or immunodeficiency condition in the anamnesis.
- Splenectomy in the anamnesis.
- Neutropenia (decrease in the absolute number of neutrophils less than 1000/mm3), agranulocytosis, significant blood loss, severe anemia (hemoglobin less than 80 g/l) according to anamnesis.
- Anorexia according to anamnesis.
Prior or concomitant therapy
- Vaccination with any vaccine carried out within 30 days before vaccination / the first dose of the studied vaccine or planned administration within 30 days after vaccination / the last dose of the studied vaccine.
- Prior vaccination with an experimental or registered vaccine that may affect the interpretation of the study data (any coronavirus or SARS vaccines).
- Long-term use (more than 14 days) of immunosuppressants or other immunomodulatory drugs (immunoregulatory peptides, cytokines, interferons, immune system effector proteins (immunoglobulins), interferon inducers (cycloferon) during the six months preceding the study, according to anamnesis.
- Treatment with systemic glucocorticosteroids (≥ 20 mg of prednisone, or an analog, for more than 15 days during the last month).
- Volunteers who received immunoglobulin preparations or blood transfusion during the last 3 months prior to the start of the study according to anamnesis.
Other non-inclusion criteria
• Participation in any other clinical trial within the last 3 months.
Exclusion criteria:
- Withdrawal of Informed consent by a volunteer;
- The volunteer was included in violation of the inclusion/non-inclusion criteria of the Protocol;
- Any condition of a volunteer that requires, in the reasoned opinion of a medical researcher, the withdrawal of a volunteer from the study;
- Taking unauthorized medications (see section 6.2);
- The volunteer refuses to cooperate or is undisciplined (for example, failure to attend a scheduled visit without warning the researcher and/or loss of communication with the volunteer), or dropped out of observation;
- For administrative reasons (termination of the study by the Sponsor or regulatory authorities), as well as in case of gross violations of the Protocol that may affect the results of the study.
| Russian Federation | |
| State Budgetary Healthcare Institution of the Moscow region "Elektrostal Central City Hospital" | |
| Elektrostal, Moscow Oblast, Russian Federation, 144000 | |
| Federal State Budgetary Scientific Institution "I.I. Mechnikov Scientific Research Institute of Vaccines and Serums" | |
| Moscow, Russian Federation, 105064 | |
| FSBSI Chumakov FSC R&D IBP RAS | |
| Moscow, Russian Federation, 108819 | |
| Private healthcare institution "Clinical Hospital "Russian Railways-Medicine" named after N.A. Semashko" | |
| Moscow, Russian Federation, 109386 | |
| Limited Liability Company "Scientific Research Center Ecosecurity" | |
| Moscow, Russian Federation, 196143 | |
| Federal State Budgetary Healthcare Institution "Medical and Sanitary Unit No. 163 of the Federal Medical and Biological Agency" | |
| Novosibirsk, Russian Federation, 630559 | |
| Federal State Budgetary Educational Institution of Higher Education "Perm State Medical University named after Academician E.A. Wagner" of the Ministry of Health of the Russian Federation | |
| Perm, Russian Federation, 614990 | |
| Responsible Party: | Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products |
| ClinicalTrials.gov Identifier: | |
| Other Study ID Numbers: | № VKI-P-II-07/21 |
| First Posted: | March 13, 2023 |
| Last Update Posted: | March 13, 2023 |
| Last Verified: | February 2023 |
| Studies a U.S. FDA-regulated Drug Product: | No |
| Studies a U.S. FDA-regulated Device Product: | No |
| COVID-19 SARS-Cov-2 |
| COVID-19 Coronavirus Infections Pneumonia, Viral Pneumonia Respiratory Tract Infections Infections | Virus Diseases Coronaviridae Infections Nidovirales Infections RNA Virus Infections Lung Diseases Respiratory Tract Diseases |
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
- Collaboration
- Funded Research Projects
- Recent Publications
Shopping cart

College of Arts and Sciences
Elderly care research center.

Eva Kahana, PhD, Director
The Elderly Care Research Center (ECRC) is a multidisciplinary, social research organization affiliated with the Department of Sociology at Case Western Reserve University. The Center was established in 1967 by its Director, Dr. Eva Kahana, who is Robson Professor of Sociology, Humanities, Medicine, Nursing, and Applied Social Science at CWRU. Research related to aging, health, and mental health is conducted by center staff and associates. Funding for these projects has been obtained from the National Cancer Institute (NCI), National Institute on Nursing Research (NINR) and the National Institute on Aging (NIA). Senior research scientists and faculty from other universities regularly participate in research projects conducted at the center.
In addition to its research activities, the center serves as a teaching facility, training graduate and postdoctoral students from diverse social and health science disciplines in the theory and methods of social gerontological research. Students are given an opportunity to obtain “hands on” experience in conducting research and to translate formal coursework into practical applications within a research setting. Center staff also serve in an advisory capacity to various educational programs and community agencies serving the elderly.
Primary activities of the center include theory based research on diverse topics relevant to adaptation and well-being of the elderly. A programmatic thrust at the center has been the focus on health and mental health outcomes of stress, coping, and adaptation.
Research has focused on predictors of wellness as well as of vulnerability. Study samples have ranged from the frail and institutionalized, aged to adventurous, older adults undertaking long distance moves. Cross-national and cross-cultural comparisons and focus on ethnic differences also represent a unique aspect of our orientation to research. In recognition of the diverse environmental and social influences on well-being of the elderly, research has been interdisciplinary in nature, bringing to bear qualitative as well as quantitative methods of sociology, psychology, and other social science disciplines on the issues under study. In addition to publishing results of research in professional journals and presenting them to the scientific community, ECRC is committed to broad dissemination of research in a readily understood format to community organizations, professionals, and to elderly participants in diverse studies. Effective intervention programs have been developed and implemented based on findings of our research projects. The Elderly Care Research Center is affiliated with the Center on Aging and Health, Case Comprehensive Cancer Center, Case School of Medicine, and Frances Payne Bolton School of Nursing at CWRU.
ELDERLY CARE RESEARCH CENTER BROCHURE
IN THE NEWS…
Eva kahana awarded frank and dorothy humel hovorka prize, eva kahana and jeffrey kahana’s new book released.

What is the lived experience of previously healthy older adults as they face disability in late life, and how is disability assimilated in their identity? How do prevailing practices facilitate—or limit options for elders living with new disabilities? In their forthcoming book, Disability and Aging: Learning from Both to Empower the Lives of Older Adults , Jeffrey Kahana and Eva Kahana synthesize disability and gerontological perspectives to explore both the unfolding challenges of aging and the practices and policies that can enhance the lives of older adults. The book was published by Lynne Rienner Publishers, 2017.
Eva Kahana named one of Cleveland Jewish News Difference Makers
Eva kahana elected to asa office.

- Programs and Projects
- Work with us
- Diversity, Equity, and Inclusion at NTI
- Annual Reports and Financials
Machine-Building Plant (Elemash)
- Location Elektrostal, Moscow Oblast
- Type Nuclear-Weaponization
- Facility Status Operational
Want to dive deeper?
Visit the Education Center
My Resources
Send saved resources to:
This paper is in the following e-collection/theme issue:
Published on 14.6.2024 in Vol 12 (2024)
Remote Inclusion of Vulnerable Users in mHealth Intervention Design: Retrospective Case Analysis
Authors of this article:

Original Paper
- Ingjerd J Straand 1 , MSc ;
- Kimberley A Baxter 2, 3 , PhD ;
- Asbjørn Følstad 4 , PhD
1 Department of Social Work, University of Stavanger, Stavanger, Norway
2 School of Exercise and Nutrition Sciences, Queensland University of Technology, Brisbane, Australia
3 Centre for Childhood Nutrition Research, Faculty of Health, Queensland University of Technology, Brisbane, Australia
4 Department of Sustainable Communication Technologies, Sintef Digital, Oslo, Norway
Corresponding Author:
Ingjerd J Straand, MSc
Department of Social Work
University of Stavanger
Kjell Arholms hus
Stavanger, 4021
Phone: 47 93222289
Email: [email protected]
Background: Mobile health (mHealth) interventions that promote healthy behaviors or mindsets are a promising avenue to reach vulnerable or at-risk groups. In designing such mHealth interventions, authentic representation of intended participants is essential. The COVID-19 pandemic served as a catalyst for innovation in remote user-centered research methods. The capability of such research methods to effectively engage with vulnerable participants requires inquiry into practice to determine the suitability and appropriateness of these methods.
Objective: In this study, we aimed to explore opportunities and considerations that emerged from involving vulnerable user groups remotely when designing mHealth interventions. Implications and recommendations are presented for researchers and practitioners conducting remote user-centered research with vulnerable populations.
Methods: Remote user-centered research practices from 2 projects involving vulnerable populations in Norway and Australia were examined retrospectively using visual mapping and a reflection-on-action approach. The projects engaged low-income and unemployed groups during the COVID-19 pandemic in user-based evaluation and testing of interactive, web-based mHealth interventions.
Results: Opportunities and considerations were identified as (1) reduced barriers to research inclusion; (2) digital literacy transition; (3) contextualized insights: a window into people’s lives; (4) seamless enactment of roles; and (5) increased flexibility for researchers and participants.
Conclusions: Our findings support the capability and suitability of remote user methods to engage with users from vulnerable groups. Remote methods facilitate recruitment, ease the burden of research participation, level out power imbalances, and provide a rich and relevant environment for user-centered evaluation of mHealth interventions. There is a potential for a much more agile research practice. Future research should consider the privacy impacts of increased access to participants’ environment via webcams and screen share and how technology mediates participants’ action in terms of privacy. The development of support procedures and tools for remote testing of mHealth apps with user participants will be crucial to capitalize on efficiency gains and better protect participants’ privacy.
Introduction
Mobile health (mHealth) interventions, which use mobile technology such as smartphone apps to promote healthy behaviors or mindsets [ 1 ], are a promising avenue to reach vulnerable groups [ 2 ]. Meaningful user involvement is critical for such interventions [ 3 ] to ensure that end user needs and perspectives are adequately represented in the design process [ 4 ]. Conducting such feedback and evaluations with users face to face (local testing) [ 5 - 7 ] involves efficiency drawbacks, particularly travel, time, and cost [ 8 ]. Researchers and practitioners have thus experimented with remote testing and research [ 9 , 10 ] using both specialized tools (eg, UserTesting and Lookback) and videoconferencing (eg, Zoom, Hangout, and Teams). Studies comparing local and remote research practices have concluded comparable results in the quality of the research output [ 11 ]. However, before the COVID-19 pandemic, local testing was the usual practice in research and among practitioners [ 4 , 6 ]. Reasons may include network variance, poor audio or video quality, unfamiliarity with remote technology, and the lack of contextual information or nonverbal cues inherent in remote methods. Local testing, by contrast, removes users from the intended context of use; this is significant for user involvement in the design of mobile solutions such as mHealth interventions.
Traditional research methods tend to involve users from high socioeconomic backgrounds, who are easy to reach and have the means to participate, including resources of time, transport, and social support [ 12 ]. Human-computer interaction research calls for adequate reach and engagement with the people affected by the design to ensure an alignment of needs and, ultimately, an effective program [ 13 , 14 ]. This can be challenging when working with community groups who are marginalized or experience social disadvantage, such as racial or ethnic minority groups, individuals who have low income and who are unemployed, people with disabilities [ 15 ], or those with gender or sexual diversity [ 14 , 16 ]. This risks diminishing the validity of the findings to the target population and reduces the authenticity of engagement. While mHealth interventions may be particularly relevant for these groups, the suitability of remote practices for user involvement should be explored. More evidence is needed to support the appropriateness and effectiveness of remote user-centered research methods when engaging with vulnerable participants.
Accelerated by the pandemic, remote research and participation tools have become more available and ready-to-hand [ 17 ]. Researchers expedited the incorporation of remote methods that allowed for project continuity, highlighting the research community’s resilience and researchers’ and participants’ willingness to experiment with technology. A recent meta-analysis found that one of the most significant effects of the pandemic on user involvement in design was shifting to web-based platforms [ 11 ]. At the community level, the increased use of telehealth services across populations to provide continuity of health care and education [ 18 ] has increased familiarity and comfort with videoconferencing and other web-based tools.
While the COVID-19 pandemic was a catalyst for innovation and creativity in remote user design methods, now that the pandemic has resolved [ 19 ], the opportunity to learn and adopt effective remote methods remains. Conducting meta-research to capture these experiences is important for future research applications. Some examples of such research exist: Hill et al [ 20 ] reviewed practical approaches for remote user testing in older adults. Other researchers have compared findings between remote and nonremote methods [ 21 ] or discussed specific aspects of the testing, including moderator and observer roles [ 5 , 22 ]. However, few studies have detailed the implementation of user-centered design in mHealth [ 3 ] or reflected on the researcher and participant experiences [ 23 ] in intervention design targeting vulnerable or diverse population groups.
Thus, the research question addressed by this qualitative and retrospective study is as follows: what are the opportunities and considerations emerging from involving vulnerable user groups remotely in mHealth intervention design? This study will highlight what was learned by adapting to agile remote user involvement during COVID-19 to inform future applications of such involvement with vulnerable user groups. Research practices from 2 projects, which applied remote inclusion of vulnerable population groups to designing and developing mHealth interventions within child health (parental feeding) and social psychology (mindset), were used as cases.
This study is structured as follows: an overview of the research projects and the methodology of this study is provided, followed by case descriptions and lessons learned before the analytical findings and implications are presented.
Research Context
The 2 research projects in this study used human-centered design (HCD) methodology [ 24 ] to design and develop web-based mHealth interventions targeting vulnerable populations. The project aims were to create digital health interventions collaboratively with and for end users and then evaluate these as part of ongoing research. The Responsive Feeding in Tough Times (RFiTT) project in Australia aimed to develop and evaluate a parenting program to promote responsive feeding practices in parents with young children in low-income families. The Career Learning App (CL-APP) project in Norway aimed to design, develop, and test positive psychology intervention apps targeting unemployed adolescents and young adults to promote job-seeking mindset and behaviors. These projects from different contexts have shared characteristics, including transdisciplinary work across design and health and applying an HCD process where users’ ideas and feedback were central to the final intervention designs. Both project outcomes were web-based interventions designed for self-administered use on users’ mobile phones, and remote user testing was applied with research participants from vulnerable groups.
In the 2 projects, the respective authors (IJS and KAB) developed user-centered design approaches, which were predominantly formative user-based evaluation [ 4 , 6 ] in the form of qualitative, moderated early testing [ 25 ] and feedback on intervention prototypes. This included conducting the posttest analysis of the collected data. From March 2020 to December 2022, a total of 38 sessions were conducted across the 2 research projects. Participants were recruited intentionally with the characteristics of potential end users of the interventions to include their input into the designed outcome.
The projects’ remote user engagement timing aligned with different phases of the COVID-19 pandemic. The Norway project experienced acute disruption during user testing (March 2020-April 2021), coinciding with the initial COVID-19 response. In contrast, the Australian project conducted user testing (November 2022-December 2022) during a more stable “living with” COVID-19 phase.
Research Design
Given the unprecedented COVID-19 pandemic during user testing, the retrospective reflection-on-action approach [ 26 , 27 ] was selected to explore the remote research setting. This study’s research question and topic were explored [ 28 , 29 ] through “reflecting on action” [ 26 ]. Reflection was both internal and in dialogue between the authors and fellow researchers. This approach enabled researchers to reflect on the cases after the upheaval period of the pandemic had receded to uncover knowledge through analyzing and integrating experiences and practices.
We conducted a descriptive and retrospective examination of the research practices and experiences across the 2 projects. This was done through an iterative process using visual mapping (ie, affinity mapping or KJ-method) to sort findings visually [ 30 ] in Miro [ 31 ]. Affinity mapping builds upon abductive thinking and is commonly used by user experience practitioners [ 32 , 33 ]. This method was selected because of our heterogeneous data set [ 32 ] and the need to synthesize ideas from unstructured data. Our data included multiple sources: protocol documents, user test setups and documentation, researcher notes and reflections, postanalysis reports, and photos and screenshots from recordings.
We took a constructivist approach to our analysis, where synthesis and connections are formed through the researchers’ critical reflection, and learnings are identified through active engagement and “discussions with the data” [ 34 , 35 ]. Our analysis was conducted stepwise ( Figure 1 ), where we first added our data to the diagrams and started making clusters and groupings of findings relevant to the research question and labeling these on a case-by-case basis. Second, we identified learnings across cases in a collaborative process by regrouping our initial categories and findings of interest into broader categories or constructed themes [ 34 ] that represent the opportunities and considerations from different cases.

Participants
The participants included in this study are considered potentially “vulnerable” due to socioeconomic factors such as unemployment, low income, and economic hardship. Vulnerability is viewed as an inclusive term in line with the study by Culén and van der Velden [ 36 ], assuming that all users may be “vulnerable” at some point. The 2 research projects had different participant groups and ethical considerations; therefore, we describe them separately below. Table 1 summarizes participants across projects.
| Participants | ||
| Target group (inclusion criteria) | ||
| Recruitment channel | or IPS program | |
| Participants | ; 2 from ethnic minority groups (immigrant or BIPOC ) | |
| Format user test | ||
| Target group (inclusion criteria) | ||
| Recruitment channel | ||
| Participants | ; 1 ethnic minority group (immigrant or BIPOC) | |
| Format user test | ||
| Target group (inclusion criteria) | ||
| Recruitment channel | ||
| Number of participants | ||
| Format user test | ||
a NAV: Norwegian Labour and Welfare Administration.
b IPS: Individual Placement and Support program.
c On the basis of observation, not self-reported.
d BIPOC: Black, indigenous, and people of color.
Ethical Considerations
Norway: participants, ethical considerations, and approval.
Participants recruited to the Norwegian project were 18 to 29 years old and either registered as unemployed at the Norwegian Labour and Welfare Administration (NAV) or participating in a regional Individual Placement and Support program. Furthermore, they needed to speak Norwegian because of the in-app language. All participants provided explicit and written consent to participate in the study and were compensated for their time with a gift card of US $30 per session. The study was evaluated and approved by the Norwegian Centre for Research Data (approval number 131074).
Australia: Participants, Ethical Considerations, and Approval
Participants were recruited Australia-wide and self-identified as experiencing economic hardship during screening. All participants were aged >18 years and caregivers of a child between 6 months and 3 years of age. Individuals were recruited from a pool of potential participants who had previously taken part in a web-based survey and had expressed interest in being contacted about other research activities. Participants were given an electronic gift voucher worth US $18 to thank them for their time. The Children’s Health Queensland Hospital and Health Service Human Research Ethics Committee (LNR/21/QCHQ/72314) and the Queensland University of Technology Human Research Ethics Committee (2021000193) approved the study.
Case Descriptions
This section outlines the 2 research projects and details the user involvement protocols. The Norwegian project included 2 instances of user involvement; the Australian project involved 1. Hence, 3 cases are presented across the 2 research projects ( Figure 2 ). Each case is divided into case description , pandemic restrictions , test setup , participants , and case-specific reflections.

Case 1: Remote and Hybrid Testing of a Game-Based Intervention Concept, Norway
Case description.
The CL-APP project explored an interactive gaming concept to make a positive psychology intervention more engaging and relevant to unemployed young adults. The intervention design explored a 3D-based game. The development work was planned and executed in 3 sprints, with end users involved in formative user testing toward the end of each sprint. Further elaboration of the game concept and user feedback can be found in the study by Straand et al [ 37 ], with screenshots provided in Figure 3 .

Pandemic Situation
On March 12, 2020, the Norwegian government ordered a nationwide lockdown, closing nonessential workplaces, schools, and child care centers. Schools and child care reopened with reduced hours for younger children toward the end of the following month. In May 2020, social and mobility restrictions were eased or replaced with mask mandates and sanitation requirements. However, in September 2020 and October 2020, infections again peaked, and in late October, new restrictions were announced, returning nonessential workers such as university staff to home offices.
A total of 12 participants (female participants: 7/12, 58%; male participants: 5/12, 42%), aged 18 to 27 years, participated in the study.
In mid-March 2020, amidst pandemic uncertainty and lacking established remote protocols, plans for moderated, in-person usability testing were improvised. Discord, chosen for its familiarity among young gamers, served as the platform for remote testing. The test setup involved several manual operations due to the lack of functionality in Discord, including scheduling, consent, and provision of gift cards. The moderator and observer met a few minutes before and then added the participant to a group call once the participant had logged in to Discord. Despite its suitability for gamers and developers, approximately half of the users encountered startup issues due to unfamiliarity with the software. External software (Apple QuickTime) was used for recording, and this lack of a built-in recorder led to missing audio for some sessions. Platforms designed for usability testing or videoconferencing were rejected at the time from the premise of introducing complexity for the team and the participant users for a relatively short time of need.
As the team transitioned toward testing a functional prototype, concerns over network variance and load time prompted plans for face-to-face testing once restrictions eased in May 2020. A single participant signed up who had been involved in early-stage interviews. The test was conducted with strict sanitation and social distancing. However, with only 1 participant, it had limited value. A subsequent round of testing was planned for October 2020, when COVID-19 restrictions were expected to ease. This time, participants self-enrolled via a website and received SMS text messaging confirmation and reminders. We set up a testing space within the NAV offices. The team adapted its research strategy to allow participants to choose between in-person and remote testing on Zoom on the enrollment website. Remote participants signed digital consent forms and received digital gift cards, while in-person participants completed forms upon arrival and received physical gift cards (see Figure 4 for the hybrid test setup). The team completed tests with 7 participants, with the majority (5/7, 70%) opting for Zoom sessions.

Case-Specific Reflections
This case involved improvisation to enable continuity of the research, both with software and tools and with testing procedures. This iteration allowed us to observe how the videoconferencing software impacted the interaction with the participant, creating a new setting for the interaction depending on the software used. In the first rounds of testing using Discord, we all had our camera off. Discord users mostly use illustrations or avatars for their profile pictures and audio-only calls. Thus, although it is possible to share a camera view, none of the sessions using Discord had the participants with camera on; this included our webcams as researchers in the role of moderator and observers. The sessions done via videoconferencing software always had the camera-on mode for the moderator and nearly always for the participants, offering a richer data set for later analysis.
The “hybrid” strategy toward the end of the study meant the moderator and observer were usually in the same room, calling in as 1 user on Zoom. After the first session, it became the established practice for the moderator and observer to join in as individual users; the observer would mute the camera and microphone after a brief introduction at the beginning. This improved the interaction of the session, as the participant did not have to address 2 people. This remote setup allowed the observer to “disappear” into the background, overcoming the issue with the silent notetaker in a face-to-face session.
Some tasks were more challenging to deliver in the remote setup since the test tasks were designed for in-person sessions rather than remote participation. For instance, idea cards were created that participants could sort according to their preferences. When the testing was on the web, we had to send them a copy of the cards in PDF format; this made the task less engaging and cumbersome. After preliminary user-derived findings, the development of the gaming-based intervention app presented in case 1 was discontinued.
Case 2: Remote Testing of mHealth Intervention Web App Concept, Norway
Building on case 1, the CL-APP project redirected the design and development process to a mobile phone web app based on user preferences. The intervention target was foremost to promote a “growth mindset.” A growth mindset [ 38 - 40 ] encourages a different interpretation of challenges faced by the young unemployed, normalizing struggles and setbacks to offer a more positive and flexible view of one’s intelligence and ability to learn new things. The key objective of engaging with end users was to explore users’ motivation to enhance reach and adherence. HCD methods ensured that the intervention was relevant, user-friendly, and motivating (see Figure 5 for screenshots of the app). In this process, researchers collaborated with designers, developers, and stakeholders, including end users, from October 2020 until the app’s completion in December 2022. The user testing took place between November 2020 and April 2021.

On October 26, 2020, the Norwegian government announced new health restrictions to reduce social interaction at work and home, strongly recommending that people return to home offices where possible [ 41 ].
Participants were recruited for 4 rounds of testing. A total of 13 participants (female participants: 6/13, 46%; male participants: 7/13, 54%) aged 18 to 29 years participated in the study.
Given the work-from-home directive at the time, the design process, including interaction with end users, was planned remotely via Zoom. During the design process, 4 rounds of testing were performed: the initial test to understand what should be altered in the existing intervention (November: 4 participants) and 3 instances to get feedback on new designs with increasing levels of fidelity as the design progressed (January: 5 participants, February: 2 participants, and March-April: 4 participants). Prototypes were tested using the design tool Figma. The sessions were completed at times that were suitable to the participant. There was 1 session in the evening, but most participants opted for midday sessions (around 11 AM-2 PM).
Our main challenge was that the prototype was designed for mobile use, and screen sharing from devices was troublesome in Zoom. Thus, for most of the tests on the new designs, we relied on desktop use and screen sharing from the browser ( Figure 6 ). When a participant dialed in from their phone, the prototype view became unreadable, and we had to ask the participant to switch over to a device with a larger screen. All participants and moderators had their cameras switched on (unprompted). After an initial round of introductions, we continued switching the camera off for the observer or notetaker to reduce their presence in the user-researcher interaction.

Case 3: Remote Testing of an mHealth Microlearning Concept−Australia
The RFiTT research program aimed to develop and evaluate an intervention to promote optimal child-feeding practices among low-income families. The secondary aims were to determine the feasibility, satisfaction, and acceptability of the mode of delivery. Families experiencing socioeconomic disadvantages face challenges feeding their children and following optimal feeding guidelines. The early years are crucial for establishing optimal feeding practices among parents and developing healthy child eating behaviors [ 42 ]. Therefore, the target of the intervention was parents or caregivers of children aged 6 to 24 months.
An mHealth digital microlearning concept was developed in response to parent engagement during the project’s development phase [ 43 ]. Project constraints dictated a technology platform that required no software engineering or development phase and could be generated within a 4- to 6-month time frame. Web-based no-code technologies were researched and piloted to determine a suitable platform.
A learning technology platform (7taps), which used microlearning education, was selected. This platform enabled researchers to create contents that included videos, images, text, and interactions without external input from software engineers or app developers. This platform had a mobile-first design and learning management capability where modules could be delivered with preset timing in customized SMS text messages. Functional prototypes could be created and tested with users using this platform with little to no moderation. A total of 3 test modules that would form part of a microlearning responsive feeding parenting intervention were created ( Figure 7 ).

The RFiTT research program commenced in April 2020. The first case of COVID-19 was confirmed in Australia on January 25, 2020 [ 43 ]. On March 18, 2020, the federal government declared a biosecurity emergency, and all Australian States and Territories subsequently implemented lockdown measures [ 44 ]. Australia only fully opened its international borders to visitors in February 2022.
During the data collection and engagement phase of RFiTT (2021-2022), recruitment was impacted by the COVID-19 pandemic, and face-to-face data collection attempts were challenging. These recruitment challenges led to experimentation with remote research methods (telephone, web-based survey, noncontact equipment drop-offs) for research activities. By the time of the user testing sessions (November to December 2022), the RFiTT research program had adopted a complete remote research methods approach, and the scope of the population target for the intervention had shifted from a local context (Brisbane, Queensland) to Australia-wide.
A total of 12 participants tested the prototypes. Of the group, 42% (5/12) had a university degree, and 3 individuals expressed that they had neurodiversity, which impacted their ability to learn and process information (attention-deficit/hyperactivity disorder, dyslexia, and aphantasia). Further details are available in Table 1 .
Potential participants were telephoned to invite them to participate in the user testing sessions. Interested participants were sent a digital web link to the Participant Information Statement, a web-based consent form, and a short demographic survey. The web-based form and survey were hosted on REDCap (Research Electronic Data Capture; Vanderbilt University), a secure web application for building and managing web-based surveys [ 45 ]. The aim of the testing was twofold: (1) to test the acceptability, readability, and accessibility of 3 examples of microlearning content and (2) to co-design aspects of the content and structure of the intervention.
The sessions were completed at times suitable to the participant, including out-of-hour sessions from November 7, 2022, to December 1, 2022. Most participants joined the session using their mobile phones (10/12, 83%). A total of 3 modules were designed to present different styles of videos, content, and imagery to elicit feedback on the different formats and parents’ preferences. The module web links were sent via mobile phone SMS text messaging to participants on the day of the arranged session. Parents viewed the content unmoderated. A Zoom session with the lead researcher (KAB) was arranged on the same day to capture parents’ impressions and feedback. All Zoom sessions were video and audio recorded. During the sessions, the researcher shared a preview screen of the digital modules and guided the parent through a talk-out-loud walkthrough of the content ( Figure 8 ). Open-ended questions regarding the usability, accessibility, and satisfaction of the modules were asked. Perspectives from parents were sought on recruitment and retention strategies, the language of key intervention messages, structure, and program timing. The parent intervention was renamed to “ Eat, Learn, Grow ” to reflect parents’ feedback.

Remote inclusion of participants allowed for representation across Australia and of employed parents, who indicated that they would not have been able to participate if the session had been in person. The Zoom platform was effective; no participants had difficulty downloading or using the software. Most of the group (10/12, 83%) used a mobile phone. The mobile phone screen size restricted viewing the content via screen share (see Figure 8 ). However, it was acceptable, as participants had just engaged with the content. There were minimal connectivity or audio difficulties, but given the participants’ home environment, there were interruptions from young children being supervised during sessions. These disruptions did not reduce the effectiveness of the sessions and are a common occurrence in research with parents, where young children need to accompany parents. The researcher (KAB) is experienced with children and conducting research with families.
The most significant downside noted for the remote sessions in this case was that the parents engaged with the digital modules unmoderated; therefore, researchers did not observe parents interacting with the content for the first time. In the remote user setup, moderated sessions of parents viewing the content on their mobile device were not possible, given that the device needed to be used for videoconference for the feedback session. Moderating the session may have provided helpful information about parents’ responses to the digital content. However, given that the platform used (7taps) was a purpose-built learning technology designed for first-time users, this was not the aim of the user testing sessions. This was mitigated by conducting the follow-up Zoom session the same day the digital content was sent to parents.
We identified opportunities and considerations of conducting remote research with vulnerable users by reflecting on action and through visual diagramming across cases. These are represented as reduced barriers to research inclusion, digital literacy transition, contextualized insights: a window into people’s lives, seamless enactment of roles, and increased flexibility for researchers and participants.
Reduced Barriers to Research Inclusion
During the user testing, the need to quickly design procedures for remote and hybrid research was necessitated by the evolving COVID-19 pandemic. Across the 3 cases, we found that remote research methods effectively engaged the targeted population groups of unemployed young adults and parents experiencing economic hardship. Enabling participants to participate in their home environment removed some systemic barriers to engaging in traditional research. For both groups, there were barriers to meeting face to face beyond the practicalities of travel, time, and capacity. Young people and parent participants displayed increased comfort with digital technologies and remote interactions, facilitating their participation in these research programs.
Remote and agile research methods enabled a broader and more diverse participant pool. RFiTT (case 3) widened the recruitment pool to Australia-wide rather than a small geographical area focus. In the Norway project, the recruitment pool was not widened geographically. However, remote methods enabled continued research during the acute response phase of COVID-19. Toward the end of our testing of the gaming concept (case 1), in-person participation was planned since restrictions had been lifted. However, recruitment was difficult, and participants who did consent failed to attend booked appointments despite a monetary incentive. Through this recruitment period, the population group expressed a high concern about the pandemic to researchers. This experience was confirmed in discussions with stakeholders such as welfare administration staff. Shifting to remote testing via web-conferencing (Zoom) facilitated continued participation.
Remote methods mitigated accessibility barriers and eased participants’ potential fear, whether related to the pandemic or the unknown of being involved in a research project. Furthermore, many tests were conducted in the evening to adapt to the needs of parent participants (case 3). Across our populations, catering for continued remote participation was relevant even after restrictions were relaxed and was demonstrated by participants’ strong preference for remote methods.
Digital Literacy Transition
Initially, the tools used for remote research were improvised, and methodological planning took an iterative approach. As the pandemic unfolded, users and researchers gained experience with relevant digital technology, reaching greater technology awareness and control. The different time frames in which the case studies were conducted during the COVID-19 pandemic provided a context to explore this trend of what we may refer to as a transition to digital literacy.
Initially, researchers and project stakeholders were reluctant to transition to remote participation (cases 1 and 2), whereas users seemed to prefer remote modes. The preferences of researchers and project stakeholders partly grew out of a desire to conduct the research “as planned” and to use established methods. There was also uncertainty about whether users had the necessary skills to use videoconferencing. Researchers had concerns about the limited opportunity for rapport building through informal conversation before the session started. However, the research team underestimated how digitally literate the participants were. This is unsurprising given the amount of time spent on the internet and the degree of web-based communication and collaboration in both groups across many aspects of life [ 46 , 47 ]. This was coupled with COVID-19 pandemic–driven increases in the use of technology for communication and services, such as telehealth [ 48 - 51 ] and work-from-home needs [ 52 , 53 ].
Contextualized Insights: A Window Into People’s Lives
Despite our target participants’ familiarity with web-based communication, the rapid adoption of these technologies also required sensitivity in protecting participants’ privacy. Contrary to our perception of poorer conversations with the loss of face-to-face conversation, we experienced more entry into users’ lives than participation at a research site. The recording was done easily as a part of the natural flow of conversation with the participant on the web in Zoom. In contrast, introducing video recording devices into physical meetings is cumbersome and can make people uncomfortable. Furthermore, it was found that the type of software used either increased or reduced the likelihood of data sharing due to its internal logics, customs, or vibe [ 54 ]. With Discord, it is not customary to use a real profile photo; in most instances, people use an avatar, and it did not feel natural to turn the webcam on. Therefore, this channel collected much less personal information than Zoom. Zoom encourages turning webcams on and recording seamlessly and unobtrusively. The tools used for supporting the research, such as Discord and Zoom and systems for issuing electronic gift cards, required collecting more personal data (such as name, email, phone number, and usernames) than in-person research methods.
Web-based and remote methods were a more natural and relevant environment for the user, revealing more contextual information than expected and providing a temporal window into people’s lives. Sometimes, this may include unintended information, such as username, browsing history, or open tabs when participants were screen sharing. The less professional nature of the Zoom session also meant some participants were less formal. In one instance, a participant wore a bath robe, while others had babies crying in the background, pets, or others who entered the conversation. This provided a richer contextual backdrop to who the participants were and sparked informal conversation and trust building. At the same time, this contextualized information from the user tests does introduce privacy concerns.
Seamless Enactment of Roles
It is sometimes necessary to have observers during user testing. For face-to-face sessions, 2-way mirrors or screencasting to another location may be used to enable observation. Additional observers may also be needed in a physical space to take notes; this can be disruptive. The user may feel uncomfortable talking to 2 people, not knowing who to look at when talking and when someone is writing intensively. Remote user sessions may require fewer observers, and they may be less intrusive when they are present.
In the Norway cases, the observer’s role as a notetaker was improved by videoconferencing. The observer and interviewer would have the camera on for the start of the testing. Then, after introductions, the observer could mute the camera and microphone and continue taking notes without impacting the session. If the observer wanted to ask follow-up questions, it was easy and natural to either bring the observer back into the conversation or allow the observer to post questions via a chat channel for the interviewer to follow up. With this more silent observer role, there was little disturbance to the flow of the conversation. It was easy and natural to switch roles during the session, which was done in case 2, where the author (IJS) moderated most of the session, and one of the designers ran through the prototype with the participants. In the Australian case, no person other than the author (KAB) was present for the testing.
Increased Flexibility for Researchers and Participants
Remote-only testing was found to be more streamlined and flexible compared with both in-person and hybrid models. Research participation, which is planned to be hybrid (case 1), requires booking and setting up the room. This introduces limitations on the remote research imposed by the physical meetings, such as the timeline and availability of physical space.
Remote testing (cases 2 and 3) allowed for more flexibility; meetings could be conducted in the evenings or during weekends or holidays to accommodate participants, with minor disruption to researchers who could dial in from home but with great benefits to participants. Without the booking and timeline constraints of physical space, sessions could take place over time (case 2), allowing revision and adaptation of design prototypes that could be tested again. This maximized the data collection capacity of the sessions and led to a more agile approach to our research and engagement with participants.
For RFiTT (case 3), most participants (10/12, 83%) engaged with the remote user testing sessions via mobile phone. Participants did not have access to a working computer, and using a mobile device enabled participants to perform essential tasks such as supervising young children. This suggests that flexibility and convenience to do other things may contribute to the preference for remote participation. For CL-APP (cases 1 and 2), nearly all participants connected to the remote testing sessions on their computers (23/25, 92%). Participants had good access to both computers and smartphones. The preference for remote participation in this project was considered to be convenience factors, social anxiety, and COVID-19–related concerns. Further research is needed to verify the reasons for preferring digital and remote engagement with research across different populations.
Principal Findings
The global pandemic necessitated the reevaluation of traditional research methodologies, compelling researchers across disciplines to adapt to the changing environment and adopt agile approaches. This study explored opportunities and considerations from involving vulnerable user groups remotely to provide lessons learned for future research. We did this by reflecting on research practices that involved user-centric evaluation of interactive behavioral and psychological intervention designs. A total of five topics emerged from our analysis: (1) reduced barriers to research inclusion; (2) digital literacy transition; (3) contextualized insights: a window into people’s lives; (4) seamless enactment of roles; and (5) increased flexibility for researchers and participants.
Across the 3 cases, remote participation contributed to a more accessible inclusion of users in design. The emerging technology on modern mobile phones offers the potential to engage with participants effectively across digital platforms such as Zoom. Mobile smartphones are prolific, and with the declining cost of data [ 55 ], remote methods that seamlessly integrate with mobile devices are becoming more accessible and equitable for user engagement. Low-income user groups may have limited access to working laptops or home computers, as was the situation in case 3 of this study. Adequate provision or access to suitable digital devices is important in digital equity and research in vulnerable groups [ 56 ].
Remote methods mitigate accessibility barriers such as travel costs and logistical challenges, which may deter participation from vulnerable groups. In countries such as Australia and Norway, with a diverse and “spread out” geographical landscape, this was highly valuable in the intervention development phase, enabling wider recruitment reach. This also has significant implications for scalability and implementation. A broader recruitment scope may make it easier to include more participants who are less represented in research, such as those living in rural areas [ 14 ]. Web-based and remote methods of research engagement, such as social media, may facilitate engagement with vulnerable groups not connected with organizations, workplaces, or other services [ 12 ].
With an increased focus on digital health interventions and programs delivered remotely, remote user methods align with the design process of such programs. The benefits of a wider recruitment pool and efficiency gains, such as reduced travel time or inconvenience, were expected from past studies on remote research methods [ 15 , 20 , 57 ]. In past research, these gains are often contrasted against other shortcomings of being remote [ 58 ], such as lack of contextual insight, connection problems, audio or video problems, low digital literacy, and the like. Emerging from the technological leap through the COVID-19 pandemic, these shortcomings are diminishing, while the perception of benefits for researchers and participants is increasing. The research teams’ initial reservations were that remote research would be complex for potentially vulnerable user participants and could increase stress or fatigue [ 59 ]. There were also reservations that remote participation would not provide rich enough user data; however, in the cases presented here, it was found that this method did provide contextualized insights and increased ecological relevance.
Implications
This study provides insight into the broader learnings from adapting to remote research practices during the COVID-19 pandemic and beyond. From the findings, we have extracted 4 significant implications for future research and practice.
Potential for More Agile Research
Remote research practices may come closer to the ideal of an agile approach to testing (“microtesting”), involving briefer and more frequent evaluation sessions with users. This has also been recommended by other recent publications within mHealth [ 3 , 60 ]. For researchers to take advantage of this potential for mHealth apps and interventions, it will bring mHealth research closer to agile user experience practice [ 61 - 64 ] and continuous testing of minimum viable products or prototypes as a form of hypothesis testing [ 65 ]. Remote methods facilitate fast cycle iterations and testing in a research design process of sensemaking through trial and error [ 66 ].
Remote Research Increases Ecologic Relevance
The interventions developed in these research programs were designed to be used within the context of users’ lives, usually the home. Thus, a remote testing method was more ecologically relevant than a traditional face-to-face user test in an office setting. We evaluated the interventions using remote methods in the user’s home and on their devices. This enables contextual inquiry and enhances the representativeness of research findings and the applicability of the designed solution. Screen sharing from a mobile device has also improved [ 67 ] compared with during our data collection; this will reduce the problems of remote testing of mHealth interventions, enabling testing and feedback sessions with users in their own contexts and on their devices with direct interaction on the app [ 68 ].
Technology Impacts Privacy and Human Action
Our research found that there is a risk of capturing more personal data than planned through the ease of recording and screen sharing when engaging with participants through web-based modes. As the participant joins from home, their home context is recorded, including background information and activity. Screen sharing from the participant’s device may enable accidental capturing of on-screen activity, such as open tabs and browsing history, which may be unintentional on the participant’s part. Digital ethnographers have highlighted this factor in previous studies [ 69 ]. This highlights the need to safeguard participants’ privacy, as participants may not fully grasp the need to protect their privacy [ 70 ]. Throughout the research, participants became more aware of how to protect their privacy, which is represented by the increasing use of video filters such as blurred backgrounds, muting cameras, or strategically placing the webcam. However, some participants perhaps showed unintended details of their personal lives. Researchers should be aware of the ethical considerations of recording videos of participants in their home environment and take care to protect their privacy. Consenting protocols, which include preparing participants for digital interactions, are essential so that participants are adequately informed and aware. As researchers, we may also incorporate practices from web-based counseling and telehealth. Researchers in telehealth also call for revisiting ethical guidelines and procedures following the “ongoing natural experiment” of the pandemic [ 71 ].
Our research suggests that when selecting technologies for remote research, it is necessary to consider their functionality regarding privacy protection and the mediating role of technology [ 58 ] on human action [ 72 , 73 ]. For instance, when we choose Zoom, Discord, or any other technology, we should consider the norms of how these technologies are being used in other contexts, how these patterns might influence researchers and participants, and how this may influence the data collected.
Remote Research Leads to User Involvement on Participants’ Terms
Researchers were concerned by the limited opportunity that remote methods present for informal conversation and rapport building. This interaction style enables trust building and may make research participation more comfortable and less intimidating. However, we found that remote methods shifted control to participants and offered greater comfort than attending unfamiliar institutional settings for face-to-face sessions. Remote methods have the potential for enhanced anonymity as participants have more control over what they share. This may be particularly pertinent for research that involves sensitive or taboo topics, allowing individuals to feel more at ease sharing their experiences and perspectives [ 74 , 75 ].
It was our experience, during work-from-home COVID-19 mandates, that power imbalances were diminished as both researchers and participants were dialing in from a home setting. Thus, there was a more equal grounding and reduced power differential [ 76 ]. This is worth considering for future research, specifically setting up the research so that participants and researchers are in similar settings during interaction. When 2 researchers dial in from the same physical location, that introduces a new imbalance, and future research should consider applying the principle of “one remote, all remote” [ 77 , 78 ] when there is a need to do hybrid remote research to ensure equal participation.
Limitations
For the cases in this study, participants were involved in design processes to capture their experience with iterative designs and provide feedback on design revisions. This took place at different time points during the pandemic. The original research was not designed to answer the research questions of this study. Instead, this topic emerged [ 28 ] through practice and through reflecting on practice [ 26 ]. Retrospective studies have limitations since they may depend on a review of data not planned for research use [ 79 ], and information may be missing. This has been mitigated by the participation of the 2 lead authors who conducted the original research. However, our interpretation may be biased despite taking a critical stance on our reflections and interpretations.
The cases and findings presented spark conceptual development and analytical discussion [ 80 ] on remote user design methods. However, there are also limitations regarding participants and to whom the findings are relevant. Across cases, specific inclusion criteria and requirements related to recruitment likely impacted our ability to recruit participants. For instance, in cases 1 and 2, we could not advertise for participants and relied on third parties to share information about the research project with potential participants. There was also a requirement to speak Norwegian fluently due to the in-app language. These factors may have reduced the number of people with minority or immigrant backgrounds who registered for the research in the Norway project. Bearing in mind that the young unemployed are twice as likely as other young people to have come to Norway as migrants, this is a weakness. Both projects called for narrow recruitment strategies to target specific population groups. Findings from this study reflect the experiences of the population groups that were involved and may not be generalizable. Further research should explore the applicability and benefit of remote user methods across other population groups.
Conclusions
The COVID-19 pandemic has reshaped the research landscape in many ways, driving rapid innovation and the adoption of remote research methods. These methods proved crucial in overcoming recruitment challenges and enabling researchers to engage with diverse participant groups across geographical areas. Applying remote methods within hard-to-reach groups reduced participation barriers, facilitated recruitment, and cultivated a more inclusive and comfortable research environment. As researchers and designers navigate the evolving research landscape, the lessons learned underscore the enduring value of remote research methods in promoting user participation in the design of mHealth interventions. Furthermore, they may serve as a reminder to question persistent assumptions about technological competence and access in vulnerable populations.
Acknowledgments
The authors would like to thank the participants who took part in the study and gave their feedback on the different prototypes. Furthermore, the authors thanks researchers at the University of Stavanger (UiS) who contributed to the Career Learning App (CL-APP) project: Venke F Haaland, Espen Sagen, Mari Rege, Hilde Ness Sandvold, Jone Bjørnestad, and Wenche Ten Velden Hegelstad. They would also like to thank the Norwegian Labour and Welfare Administration in Sandnes and Skole- og Jobbresept at the University Hospital of Stavanger. CL-App is funded by The Norwegian Research Council (grant number 296390).
They would also like to thank the researchers at the Queensland University of Technology (QUT) who contributed to the Responsive Feeding in Tough Times (RFiTT) project: Rebecca Byrne, Smita Nambiar, Danielle Gallegos, Jeremy Kerr, Robyn Penny, and Rachel Laws. RFiTT is funded by the Queensland Children’s Hospital Foundation thanks to the generosity of Woolworths customers and team members. They further acknowledge the QUT Design Lab for organizing a research seminar that allowed authors 1 and 2 to meet and share ideas in March 2023, which resulted in this study.
Conflicts of Interest
None declared.
- Fedele DA, Cushing CC, Fritz A, Amaro CM, Ortega A. Mobile health interventions for improving health outcomes in youth: a meta-analysis. JAMA Pediatr. May 01, 2017;171(5):461-469. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Stowell E, Lyson MC, Saksono H, Wurth RC, Jimison H, Parker AG. Designing and evaluating mHealth interventions for vulnerable populations: a systematic review. In: Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems. 2018. Presented at: CHI '18; April 21-26, 2018:1-17; Montreal, QC. URL: https://dl.acm.org/doi/10.1145/3173574.3173589 [ CrossRef ]
- Cornet VP, Toscos T, Bolchini D, Rohani Ghahari R, Ahmed R, Daley C, et al. Untold stories in user-centered design of mobile health: practical challenges and strategies learned from the design and evaluation of an app for older adults with heart failure. JMIR Mhealth Uhealth. Jul 21, 2020;8(7):e17703. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lewis JR. Usability: lessons learned … and yet to be learned. Int J Hum Comput Interact. Jun 18, 2014;30(9):663-684. [ CrossRef ]
- Alshar’e M, Albadi A, Mustafa M, Tahir N, Al Amri M. A framework of the training module for untrained observers in usability evaluation motivated by COVID-19: enhancing the validity of usability evaluation for children’s educational games. Adv Hum Comput Interact. Mar 9, 2022;2022:1-11. [ CrossRef ]
- Moran K. Usability testing 101. Nielsen Norman Group. URL: https://www.nngroup.com/articles/usability-testing-101/ [accessed 2023-08-15]
- Lowgren J, Carroll JM, Hassenzahl M, Erickson T, Blackwell A, Overbeeke K, et al. The Encyclopedia of Human-Computer Interaction. 2nd edition. Aarhus, Denmark. Interaction Design Foundation; 2019.
- Vasalou A, Ng BD, Wiemer-Hastings P, Oshlyansky L. Human-moderated remote user testing: protocols and applications. In: Proceedings of the 8th ERCIM Workshop 'User Interfaces for All'. 2004. Presented at: UIA '04; June 28-29, 2004:1-10; Wien, Austria. URL: https://reed.cs.depaul.edu/peterh/papers/VasalouERCIM2004.pdf
- Alhadreti O. Assessing academics’ perceptions of blackboard usability using SUS and CSUQ: a case study during the COVID-19 pandemic. Int J Hum Comput Interact. Dec 26, 2020;37(11):1003-1015. [ CrossRef ]
- Hill JR, Harrington AB, Adeoye P, Campbell NL, Holden RJ. Going remote-demonstration and evaluation of remote technology delivery and usability assessment with older adults: survey study. JMIR Mhealth Uhealth. Mar 04, 2021;9(3):e26702. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Costagliola G, De RM, Fuccella V, Tabari P. The impact of the COVID-19 pandemic on human–computer interaction empirical research. Interact Comput. 2023;35(5):578-589. [ FREE Full text ] [ CrossRef ]
- Gallegos D, Durham J, Rutter C, McKechnie R. Working towards the Active Participation of Underrepresented Populations in Research: A Scoping Review and Thematic Synthesis. Health Soc Care Community. Apr 21, 2023;2023:1-26. [ CrossRef ]
- Bratteteig T, Wagner I. Unpacking the Notion of Participation in Participatory Design. Comput Support Coop Work. Sep 2, 2016;25(6):425-475. [ FREE Full text ] [ CrossRef ]
- Ellard-Gray A, Jeffrey NK, Choubak M, Crann SE. Finding the hidden participant: solutions for recruiting hidden, hard-to-reach, and vulnerable populations. Int J Qual Methods. Dec 17, 2015;14(5):160940691562142. [ CrossRef ]
- Petrie H, Hamilton F, King N, Pavan P. Remote usability evaluations with disabled people. In: Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. 2006. Presented at: CHI '06; April 22-27, 2006:1133-1141; Montréal, QC. URL: https://dl.acm.org/doi/10.1145/1124772.1124942 [ CrossRef ]
- Bonevski B, Randell M, Paul C, Chapman K, Twyman L, Bryant J, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. Mar 25, 2014;14(1):42. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Heidegger M. Being and Time. Hoboken, NJ. Wiley-Blackwell; 1978.
- Moorhouse BL, Wong KM. The COVID-19 pandemic as a catalyst for teacher pedagogical and technological innovation and development: teachers’ perspectives. Asia Pac J Educ. Oct 11, 2021;42(sup1):105-120. [ CrossRef ]
- Statement on the fifteenth meeting of the IHR (2005) emergency committee on the COVID-19 pandemic. World Health Organization. May 5, 2023. URL: https://tinyurl.com/3k786y5e [accessed 2023-08-22]
- Hill JR, Brown JC, Campbell NL, Holden RJ. Usability-in-place-remote usability testing methods for homebound older adults: rapid literature review. JMIR Form Res. Nov 02, 2021;5(11):e26181. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Currie H, Harvey A, Bond R, Magee J, Finlay D. Remote synchronous usability testing of public access defibrillators during social distancing in a pandemic. Sci Rep. Aug 26, 2022;12(1):14575. [ CrossRef ] [ Medline ]
- Lobchuk M, Bathi PR, Ademeyo A, Livingston A. Remote moderator and observer experiences and decision-making during usability testing of a web-based empathy training portal: content analysis. JMIR Form Res. Aug 03, 2022;6(8):e35319. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Archibald MM, Ambagtsheer RC, Casey MG, Lawless M. Using Zoom videoconferencing for qualitative data collection: perceptions and experiences of researchers and participants. Int J Qual Methods. Sep 11, 2019;18:160940691987459. [ CrossRef ]
- ISO 9241-210:2019: ergonomics of human-system interaction- part 210: human-centred design for interactive systems. International Organization for Standardization. 2019. URL: https://www.iso.org/standard/77520.html [accessed 2023-10-19]
- Cockton G. Usability evaluation. In: Ahmad A, Blackwell A, Dix A, Schmidt A, Sutcliffe AG, Crabtree A, et al, editors. The Encyclopedia of Human-Computer Interaction. 2nd edition. Aarhus, Denmark. Interaction Design Foundation; 2019.
- Schon DA. The Reflective Practitioner: How Professionals Think in Action. London, UK. Basic Books; 1984.
- Palmer AM, Burns S. Reflective Practice in Nursing: The Growth of the Professional Practitione. Hoboken, NJ. Wiley-Blackwell; 1994.
- Gaver W, Krogh PG, Boucher A, Chatting D. Emergence as a feature of practice-based design research. In: Proceedings of the 2022 ACM Designing Interactive Systems Conference. 2022. Presented at: DIS '22; June 13-17, 2022:517-526; Virtual Event. URL: https://dl.acm.org/doi/abs/10.1145/3532106.3533524 [ CrossRef ]
- Suchman L. Human-Machine Reconfigurations: Plans and Situated Actions. Cambridge, MA. Cambridge University Press; 2006.
- Pernice K, Krause R. Affinity diagramming for collaboratively sorting UX findings and design ideas. Nielsen Norman Group. URL: https://www.nngroup.com/articles/affinity-diagram/ [accessed 2023-08-22]
- Miro. URL: http://www.miro.com [accessed 2023-08-10]
- Scupin R. The KJ method: a technique for analyzing data derived from Japanese ethnology. Hum Organ. 1997;56(2):233-237. [ CrossRef ]
- Fischer C, Gregor S. Forms of reasoning in the design science research process. In: Proceedings of the 6th International Conference on Service-Oriented Perspectives in Design Science Research. 2011. Presented at: DESRIST '11; May 5-6, 2011:17-31; Milwaukee, WI. URL: https://link.springer.com/chapter/10.1007/978-3-642-20633-7_2 [ CrossRef ]
- Braun V, Clarke V. Thematic Analysis: A Practical Guide. Thousand Oaks, CA. Sage Publications; 2021.
- Charmaz K. Constructivist grounded theory. J Posit Psychol. Dec 14, 2016;12(3):299-300. [ CrossRef ]
- Culén AL, van der Velden M. The digital life of vulnerable users: designing with children, patients, and elderly. In: Proceedings of the 4th Scandinavian Conference on Information Systems on Nordic Contributions in IS Research. 2013. Presented at: SCIS '13; August 11-14, 2013:53-71; Oslo, Norway. URL: https://link.springer.com/chapter/10.1007/978-3-642-39832-2_4 [ CrossRef ]
- Straand IJ, Følstad A, Bjørnestad JR. Exploring a gaming-based intervention for unemployed young adults: thematic analysis. JMIR Hum Factors. Jan 18, 2024;11:e44423. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Dweck CS. Mindset - Updated Edition: Changing The Way You think To Fulfil Your Potential. Paris, France. Hachette Livre; 2017.
- Dweck CS, Yeager DS. A growth mindset about intelligence. In: Walton GM, Crum AJ, editors. Handbook of Wise Interventions: How Social Psychology Can Help People Change. New York, NY. The Guilford Press; 2020.
- Yeager DS, Dweck CS. What can be learned from growth mindset controversies? Am Psychol. Dec 2020;75(9):1269-1284. [ FREE Full text ] [ CrossRef ] [ Medline ]
- New national restrictions. The Office of the Prime Minister. Oct 26, 2020. URL: https://www.regjeringen.no/en/historical -archive/solbergs-government/Ministries/smk/Press-releases/2020/new-national-restrictions/id2776995/ [accessed 2023-12-12]
- Scaglioni S, De Cosmi V, Ciappolino V, Parazzini F, Brambilla P, Agostoni C. Factors influencing children's eating behaviours. Nutrients. May 31, 2018;10(6):706. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Baxter KA, Kerr J, Nambiar S, Gallegos D, Penny RA, Laws R, et al. A design thinking-led approach to develop a responsive feeding intervention for Australian families vulnerable to food insecurity: eat, Learn, Grow. Health Expect. Apr 20, 2024;27(2):e14051. [ FREE Full text ] [ CrossRef ] [ Medline ]
- COVID-19: a chronology of state and territory government announcements (up until 30 June 2020). Parliament House, Parliament of Australia. URL: https://www.aph.gov.au/About_Parliament/Parliamentary_departments/Parliamentary_Library/pubs/rp/rp2021/Chronologies/COVID-19StateTerritoryGovernmentAnnouncements#_Toc52275790 [accessed 2023-11-01]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. Jul 2019;95:103208. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lozano-Blasco R, Robres AQ, Sánchez AS. Internet addiction in young adults: a meta-analysis and systematic review. Comput Human Behav. May 2022;130:107201. [ CrossRef ]
- Bakken IJ, Wenzel HG, Götestam KG, Johansson A, Oren A. Internet addiction among Norwegian adults: a stratified probability sample study. Scand J Psychol. Apr 24, 2009;50(2):121-127. [ CrossRef ] [ Medline ]
- Shaver J. The state of telehealth before and after the COVID-19 pandemic. Prim Care. Dec 2022;49(4):517-530. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Anderson JT, Bouchacourt LM, Sussman KL, Bright LF, Wilcox GB. Telehealth adoption during the COVID-19 pandemic: a social media textual and network analysis. Digit Health. 2022;8:20552076221090041. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nicol TL, Roberts J, Jordan R. Removing regulatory barriers to telehealth before and after COVID-19. Brookings Institution. 2020. URL: https://www.brookings.edu/wp-content/uploads/2020/05/Removing-barriers-to-telehealth-before-and-after- COVID-19_PDF.pdf [accessed 2024-05-19]
- Appleton R, Williams J, Vera San Juan N, Needle JJ, Schlief M, Jordan H, et al. Implementation, adoption, and perceptions of telemental health during the COVID-19 pandemic: systematic review. J Med Internet Res. Dec 09, 2021;23(12):e31746. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bick A, Blandin A, Mertens K. Work from Home before and after the COVID-19 Outbreak. Am Econ J-Macroecon. Oct 01, 2023;15(4):1-39. [ CrossRef ]
- Bick A, Blandin A, Mertens K. Work from home after the COVID-19 outbreak. Federal Reserve Bank of Dallas. Jul 2020. URL: https://www.dallasfed.org/~/media/documents/research/papers/2020/wp2017r2.pdf [accessed 2024-04-29]
- Kender K. Tumblr is Queer and Twitter is toxic: speculating about the vibe of social media spaces. In: Proceedings of the 2022 Nordic Human-Computer Interaction Conference. 2022. Presented at: NordiCHI '22; October 8-12, 2022:1-8; Aarhus, Denmark. URL: https://dl.acm.org/doi/fullHtml/10.1145/3546155.3547279 [ CrossRef ]
- Median mobile retail cost per GB in Australia FY 2015-2019, by service type. Statista. 2023. URL: https://www.statista.com/statistics/1099607/australia-mobile-service-median-cost-per-gb-by-service-type/ [accessed 2023-09-24]
- Lyles CR, Wachter RM, Sarkar U. Focusing on digital health equity. JAMA. Nov 09, 2021;326(18):1795-1796. [ CrossRef ] [ Medline ]
- Shields MM, McGinnis MN, Selmeczy D. Remote research methods: considerations for work with children. Front Psychol. Oct 28, 2021;12:703706. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Frittgen EM, Haltaufderheide J. 'Can you hear me?': communication, relationship and ethics in video-based telepsychiatric consultations. J Med Ethics. Jan 05, 2022;48(1):22-30. [ CrossRef ] [ Medline ]
- Anh LE, Whelan E, Umair A. ‘You’re still on mute’. A study of video conferencing fatigue during the COVID-19 pandemic from a technostress perspective. Behav Inf Technol. Jul 01, 2022;42(11):1758-1772. [ CrossRef ]
- Buck Harleah G. Does health care technology work for or against older adults? J Gerontol Nurs. Aug 2017;43(8):3-4. [ CrossRef ]
- Gothelf J. Lean UX: Applying Lean Principles to Improve User Experience. Sebastopol, CA. Shroff/O'Reilly; Mar 2013.
- Knapp J, Zeratsky J, Kowitz B. Sprint: How to Solve Big Problems and Test New Ideas in Just Five Days. New York, NY. Simon & Schuster; 2016.
- Reis E. The Lean Startup: How Today's Entrepreneurs Use Continuous Innovation to Create Radically Successful Businesses. New York, NY. Crown Currency Publishing; 2011.
- Felin T, Gambardella A, Stern S, Zenger T. Lean startup and the business model: experimentation revisited. Long Range Plann. Aug 2020;53(4):101889. [ FREE Full text ] [ CrossRef ]
- Aarlien D, Colomo-Palacios R. Lean UX: a systematic literature review. In: Proceedings of the 20th International Conference on Computational Science and Its Applications. 2020. Presented at: ICCSA '20; July 1-4, 2020:500-510; Cagliari, Italy. URL: https://link.springer.com/chapter/10.1007/978-3-030-58817-5_37 [ CrossRef ]
- Swann C. Action research and the practice of design. Design Issues. Jan 2002;18(1):49-61. [ FREE Full text ] [ CrossRef ]
- Sharing your iOS screen from the Zoom desktop client. Zoom Support. Dec 29, 2023. URL: https://support.zoom.com/hc/en/article?id=zm_kb&sysparm_article=KB0066704 [accessed 2024-03-21]
- Shahil-Feroz A, Yasmin H, Saleem S, Bhutta Z, Seto E. Remote Moderated Usability Testing of a Mobile Phone App for Remote Monitoring of Pregnant Women at High Risk of Preeclampsia in Karachi, Pakistan. Informatics. Oct 17, 2023;10(4):79. [ CrossRef ]
- Addeo F, Masullo G. Studying the digital society: digital methods between tradition and innovation in social research. Ital Sociol Rev. 2021;11(4S):153. [ CrossRef ]
- Lee EW, McCloud RF, Viswanath K. Designing effective eHealth interventions for underserved groups: five lessons from a decade of eHealth intervention design and deployment. J Med Internet Res. Jan 07, 2022;24(1):e25419. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kaplan B. Revisiting health information technology ethical, legal, and social issues and evaluation: telehealth/telemedicine and COVID-19. Int J Med Inform. Nov 2020;143:104239. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Verbeek PP. Materializing morality: design ethics and technological mediation. Sci Technol Hum Values. Aug 19, 2016;31(3):361-380. [ CrossRef ]
- Goldman SL, Ihde D, Zimmerman ME, Hickman LA. Technology and the lifeworld: from garden to earth. Technol Cult. Oct 1991;32(4):1135. [ CrossRef ]
- Frost J, Vermeulen IE, Beekers N. Anonymity versus privacy: selective information sharing in online cancer communities. J Med Internet Res. May 14, 2014;16(5):e126. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Helou S, Abou-Khalil V, El Helou E, Kiyono K. Factors related to personal health data sharing: data usefulness, sensitivity and anonymity. Stud Health Technol Inform. May 27, 2021;281:1051-1055. [ CrossRef ] [ Medline ]
- Goffman E. Stigma: Notes on the Management of Spoiled Identity. New York, NY. Simon & Schuster; 2009.
- Frisch B, Greene C. What it takes to run a great hybrid meeting. Harvard Business Review. Jun 3, 2021. URL: https://hbr.org/2021/06/what-it-takes-to-run-a-great-hybrid-meeting [accessed 2023-12-07]
- Bowtell S. How to facilitate a hybrid meeting or workshop. RedAgile. Mar 13, 2023. URL: https://www.redagile.com/post/facilitate-a-hybrid-meeting [accessed 2023-12-07]
- Talari K, Goyal M. Retrospective studies - utility and caveats. J R Coll Physicians Edinb. Dec 2020;50(4):398-402. [ CrossRef ] [ Medline ]
- Straand IJ, Jevnaker BH. Leading transformation in an uncertain world: a case for strategic speculative design. In: Proceedings of the 19th European Conference on Management Leadership and Governance. 2023. Presented at: ECMLG '23; November 23-24, 2023:380-387; London, UK. URL: https://papers.academic-conferences.org/index.php/ecmlg/article/view/1947/1824 [ CrossRef ]
Abbreviations
| Career Learning App |
| human-centered design |
| mobile health |
| Norwegian Labour and Welfare Administration |
| Research Electronic Data Capture |
| Responsive Feeding in Tough Times |
Edited by L Buis; submitted 18.12.23; peer-reviewed by J Weber, G Costagliola, P Tabari, D Singh; comments to author 20.02.24; revised version received 24.03.24; accepted 24.04.24; published 14.06.24.
©Ingjerd J Straand, Kimberley A Baxter, Asbjørn Følstad. Originally published in JMIR mHealth and uHealth (https://mhealth.jmir.org), 14.06.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in JMIR mHealth and uHealth, is properly cited. The complete bibliographic information, a link to the original publication on https://mhealth.jmir.org/, as well as this copyright and license information must be included.
- Moscow Oblast
- »
- Elektrostal
State Housing Inspectorate of the Moscow Region
Phone 8 (496) 575-02-20 8 (496) 575-02-20
Phone 8 (496) 511-20-80 8 (496) 511-20-80
Public administration near State Housing Inspectorate of the Moscow Region
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Welcome to the Purdue Online Writing Lab

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
The Online Writing Lab (the Purdue OWL) at Purdue University houses writing resources and instructional material, and we provide these as a free service at Purdue. Students, members of the community, and users worldwide will find information to assist with many writing projects. Teachers and trainers may use this material for in-class and out-of-class instruction.
The On-Campus and Online versions of Purdue OWL assist clients in their development as writers—no matter what their skill level—with on-campus consultations, online participation, and community engagement. The Purdue OWL serves the Purdue West Lafayette and Indianapolis campuses and coordinates with local literacy initiatives. The Purdue OWL offers global support through online reference materials and services.
Social Media
Facebook twitter.
Get the Reddit app
A subreddit for those who enjoy learning about flags, their place in society past and present, and their design characteristics
Every Russian city/town flag that has an atom in it.
Agidel, the Republic of Bashkortostan.
Desnogorsk, Smolensk Oblast.
Dubna, Moscow Oblast.
Zheleznogorsk, Krasnoyarsk Krai.
Zelenogorsk, Krasnoyarsk Krai.
Krasnokamensk, Zabaykalsky Krai.
Lesnoy, Sverdlovsk Oblast.
Novouralsk, Sverdlovsk Oblast.
Obninsk, Kaluga Oblast.
Seversk, Tomsk Oblast.
Tryokhgorny, Chelyabinsk Oblast.
Elektrostal, Moscow Oblast.
Shchukino District of Moscow. This is not a city, but it looks funny, so I had to include it.
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
How Americans View the Coronavirus, COVID-19 Vaccines Amid Declining Levels of Concern
Continued decline in share of u.s. adults with up-to-date vaccination, table of contents.
- COVID-19 vaccination among adults ages 65 and older, by party
- Views of long COVID
- Views of the threat posed by the coronavirus
- Personal concern about getting or spreading COVID-19
- Uptake of the flu shot
- Acknowledgments
- The American Trends Panel survey methodology
- Appendix: Detailed chart and tables

Pew Research Center conducted this study to understand Americans’ views of the coronavirus and COVID-19 vaccines. For this analysis, we surveyed 10,133 U.S. adults from Feb. 7 to 11, 2024.
Everyone who took part in the survey is a member of the Center’s American Trends Panel (ATP), an online survey panel that is recruited through national, random sampling of residential addresses. This way, nearly all U.S. adults have a chance of selection. The survey is weighted to be representative of the U.S. adult population by gender, race, ethnicity, partisan affiliation, education and other categories. Read more about the ATP’s methodology .
Here are the questions used for this report , along with responses, and its methodology .
A new Pew Research Center survey finds that just 20% of Americans view the coronavirus as a major threat to the health of the U.S. population today and only 10% are very concerned they will get it and require hospitalization. This data represents a low ebb of public concern about the virus that reached its height in the summer and fall of 2020, when as many as two-thirds of Americans viewed COVID-19 as a major threat to public health.
Just 28% of U.S. adults say they have received the updated COVID-19 vaccine, which the Centers for Disease Control and Prevention (CDC) recommended last fall to protect against serious illness. This stands in stark contrast to the spring and summer of 2021, when long lines and limited availability characterized the initial rollout of the first COVID-19 vaccines. A majority of U.S. adults (69%) had been fully vaccinated by August 2021.
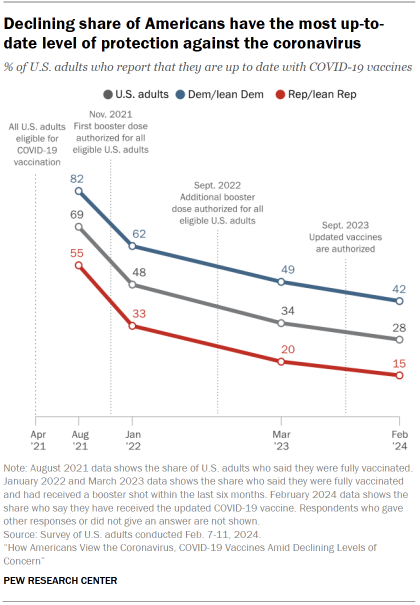
Underscoring the limited demand for the updated COVID-19 vaccines, a larger share of U.S. adults say they’ve gotten a flu shot in the last six months than the updated coronavirus vaccine (44% vs. 28%). And despite a public health push encouraging adults to get both vaccines at the same time, almost half of those who received a flu shot from a health care provider chose not to get the updated COVID-19 vaccine.
The vast majority of Americans have some level of protection from the coronavirus because of vaccination, prior infection or a combination of the two. This has led to a decline in severe illness from the disease.
Still, the virus continues to circulate widely in the United States , with wastewater data suggesting that cases in the early part of 2024 were among the highest they have been since the first omicron wave in 2022.
Long COVID ranks among the concerns of public health experts. Long COVID refers to a variety of symptoms such as fatigue and brain fog that last longer than a month after a COVID-19 infection.
The survey – conducted among 10,133 U.S. adults from Feb. 7 to 11, 2024 – finds that 50% of Americans say it is extremely or very important for medical researchers and health care providers to understand and treat long COVID; 27% see this as a less important issue and 22% of Americans say they haven’t heard of long COVID.
Continuity and change: Partisan views of COVID-19
Partisanship remains one of the most powerful factors shaping views about COVID-19 vaccines and the virus. But the size and nature of differences between Republicans and Democrats have evolved since earlier stages of the outbreak.
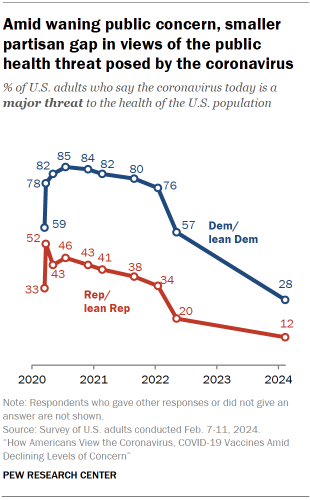
For instance, the gap between the shares of Democrats and Republicans who view the coronavirus as a major threat to public health has fallen from 37 percentage points in May 2022 to 16 points today. In the pandemic’s first year, Democrats were routinely about 40 points more likely than Republicans to view the coronavirus as a major threat to the health of the U.S. population. This gap has waned as overall levels of concern have fallen.
When it comes to vaccination, Democrats and Democratic-leaning independents remain more likely than Republicans and GOP leaners to say they’ve received an updated COVID-19 vaccine (42% vs. 15%). This 27-point gap in recent vaccination is about the same as in January 2022 when 62% of Democrats and 33% of Republicans said they were up to date (i.e., fully vaccinated and recently boosted).
In addition to partisanship, age continues to matter a great deal in attitudes and behaviors tied to the coronavirus. And the intersection of partisanship and age reveals one of the biggest recent changes in the public’s response to the outbreak: a growing divergence between the oldest Republicans and oldest Democrats in vaccine uptake, which is explored below.
Older adults continue to be one of the most at-risk groups for severe illness and death from COVID-19.
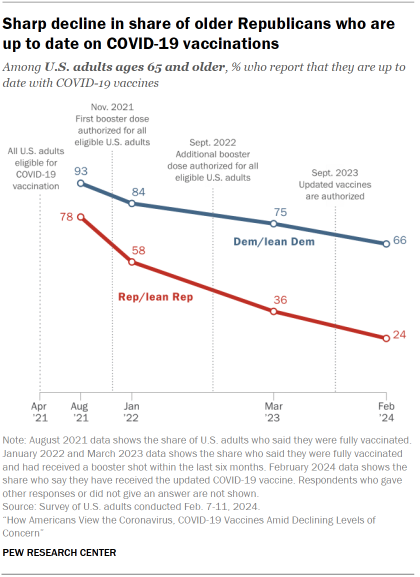
When vaccines first became available in 2021, large majorities of both Republicans and Democrats ages 65 and older said they had received the vaccine. But as additional doses have become available, uptake among older Republicans has declined at a faster rate than among older Democrats.
In the current survey, 66% of Democrats ages 65 and older say they have received the updated COVID-19 vaccine, compared with 24% of Republicans ages 65 and older.
This 42-point partisan gap is much wider now than at other points since the start of the outbreak. For instance, in August 2021, 93% of older Democrats and 78% of older Republicans said they had received all the shots needed to be fully vaccinated (a 15-point gap). Go to the Appendix for more details .
How COVID-19 vaccination varies by age within parties
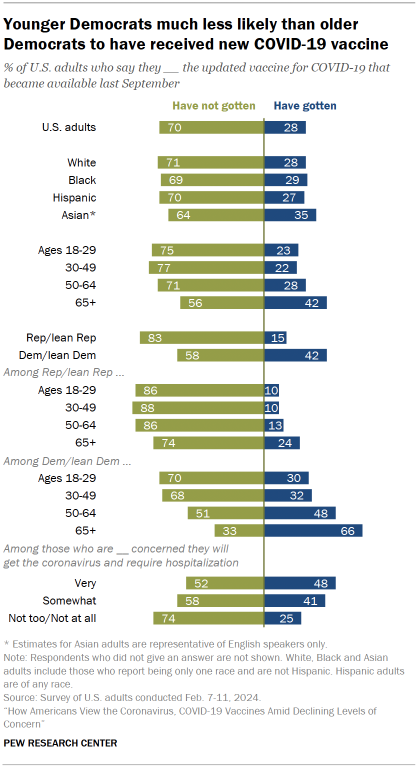
The impact of age is also striking when looking within political parties.
Among Democrats, about three-in-ten adults under 50 have received an updated COVID-19 vaccine, compared with 48% of those ages 50 to 64 and 66% of Democrats ages 65 and older.
Age differences within the GOP run in the same direction, but are much more modest, reflecting, in part, low overall levels of vaccine uptake.
How COVID-19 vaccination varies by race and ethnicity
Similar shares of White (28%), Black (29%) and Hispanic (27%) adults say they have gotten the updated vaccine. English-speaking Asian adults (35%) are slightly more likely to report receiving the updated vaccine.
As in past Center surveys, there are racial and ethnic differences in vaccine uptake among Democrats.
For instance, 50% of White Democrats and 42% of English-speaking Asian Democrats report having received the updated vaccine, compared with somewhat smaller shares of Black and Hispanic Democrats (32% each).
Half of Americans say it is extremely or very important for medical researchers and health care providers to understand and treat long COVID, considering all the different priorities they face.
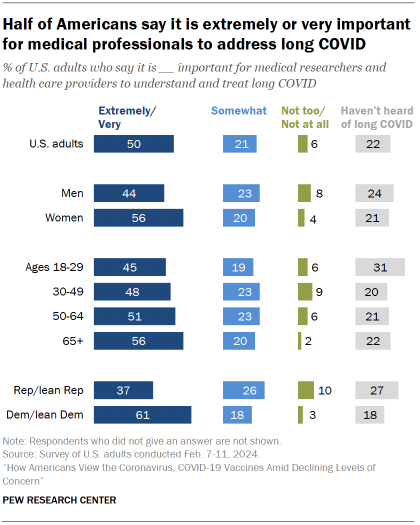
About two-in-ten (21%) say it’s somewhat important for those in medicine to address long COVID, while 6% say it is not too or not at all important. Another 22% say they haven’t heard of long COVID.
More Democrats (61%) than Republicans (37%) say it is extremely or very important for medical researchers and health care providers to understand and treat long COVID.
A majority of women (56%) consider this extremely or very important; a smaller share of men (44%) say the same. The CDC has reported that women are more likely than men to develop long COVID symptoms.
Awareness of long COVID also shapes views on its importance: Those who have heard a lot about long COVID are more likely than those who have heard a little about it to say it’s extremely or very important for medical professional to address it (76% vs. 60%).
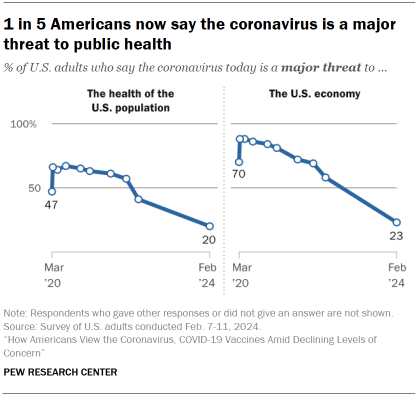
One-in-five Americans now say the coronavirus is a major threat to the health of the U.S. population, down from a high of 67% in July 2020.
Concern about the coronavirus as a major threat to the U.S. economy has also declined dramatically. Today, 23% of Americans say it’s a major threat to the economy, compared with 88% in May 2020. The pandemic spurred an economic recession in 2020 and a spike in unemployment that reached the highest levels since the Great Recession.
Federal policy on the coronavirus has changed as public concern – and the incidence of severe illness – has fallen. The Biden administration ended the public health emergency for the coronavirus pandemic in May 2023. And the CDC recently released updated guidelines with shorter isolation periods for adults testing positive for the disease.
While large partisan gaps characterized views of the coronavirus as a major threat to public health for much of the pandemic, those gaps were far smaller on views of the virus as a major threat to the economy. In the current survey, just a 6-point gap separates Republicans and Democrats with this view (20% vs. 26%, respectively) – similar to the 9-point party gap seen in May 2022.
About a quarter of Americans (27%) are very or somewhat concerned about getting a serious case of COVID-19 that would require hospitalization. A somewhat higher share (40%) say they are very or somewhat concerned they might spread the coronavirus to other people without knowing it.
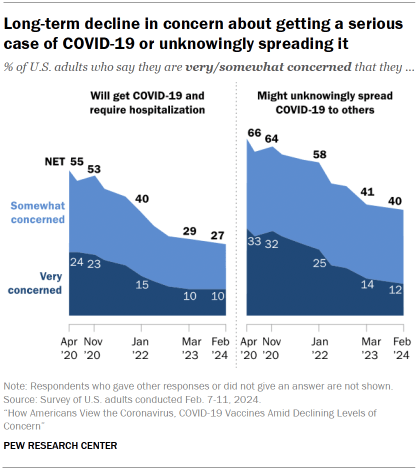
Levels of concern for getting or spreading the coronavirus are about the same as they were in March 2023 and remain down dramatically from early in the pandemic.
The share of Americans who are very or somewhat concerned about getting a serious case is 26 points lower than in November 2020, before a COVID-19 vaccine was available to the public. And the share of Americans who are at least somewhat concerned about spreading COVID-19 without knowing it is down 24 points since November 2020.
Still, the current data shows how the virus remains a concern in daily life for many Americans, more than four years after the first confirmed coronavirus cases appeared in the U.S.
Consistent with past Center surveys, there are demographic and political differences in personal concern about getting a serious case of COVID-19 and unknowingly spreading the virus:
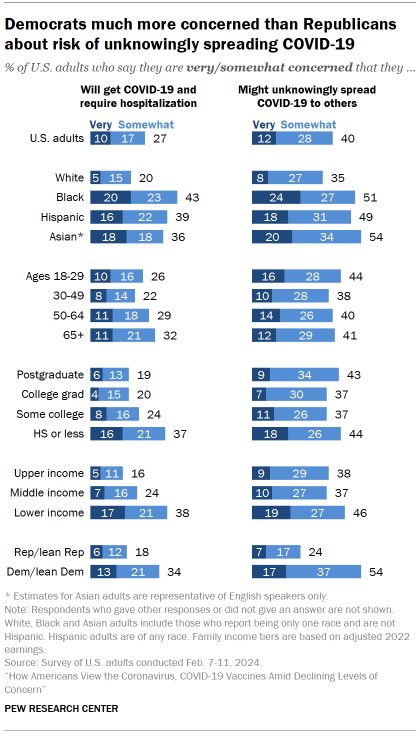
- Income: Lower-income Americans continue to be particularly concerned (38%) about getting a serious case of COVID-19. They’re also more likely than middle- and upper-income Americans to worry about unknowingly spreading COVID-19, but the differences are more modest.
- Party: Democrats (54%) are more than twice as likely as Republicans (24%) to be very or somewhat concerned about unknowingly spreading COVID-19. And they’re 16 points more likely to express concern about getting a serious case of the disease.
- Race and ethnicity: White Americans (20%) are less likely to be concerned about getting a serious case of COVID-19 than Black (43%), Hispanic (39%) and English-speaking Asian Americans (36%).
Some of the groups most personally concerned about getting a severe case of COVID-19 are also among the groups most concerned about the public health threat from the coronavirus. For example, Black adults and adults with lower incomes express more concern about the personal health and public health impact of the coronavirus than White adults and those with upper incomes.
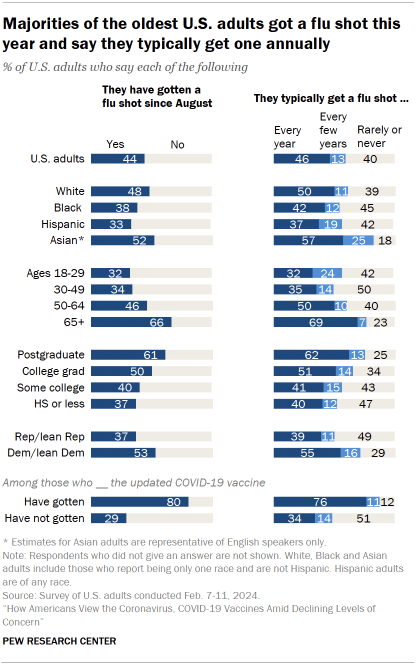
The survey finds 44% of U.S. adults say they have gotten a flu shot since August. This share is down slightly from last March, when 49% of Americans said they had recently gotten a flu shot.
Uptake varies by the following factors:
- Age: Older Americans continue to be more likely to report getting the flu shot. Two-thirds of Americans ages 65 and older say they have gotten the flu shot since August. By comparison, only about a third of those under age 50 say the same. These large age differences are seen among both Democrats and Republicans.
- Race and ethnicity: English-speaking Asian Americans (52%) and White Americans (48%) are more likely than Black Americans (38%) and Hispanic Americans (33%) to say they have gotten a flu shot since August. These racial and ethnic differences are consistent with past Center surveys.
- Partisan affiliation: Democrats are more likely than Republicans to say they got a flu shot this year (53% vs. 37%). This 16-point gap is twice as big now as it was in November 2020, during the pandemic’s first year. The current partisan difference in flu shot uptake is similar to the one recorded in March 2023.
The flu shot and updated COVID-19 vaccines are both recommended to protect against severe illness, but Americans approach these vaccines differently.
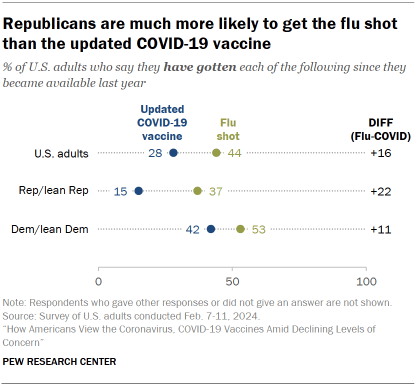
Americans are more likely to report that they received a flu shot than the updated COVID-19 vaccine this year (44% vs. 28%).
This gap in uptake between the flu shot and updated COVID-19 vaccine is more pronounced among Republicans than Democrats.
Republicans are more than twice as likely to say they’ve gotten a flu shot since August as to say they’ve received an updated COVID-19 vaccine (37% vs. 15%). Among Democrats, this difference is more modest (53% vs. 42%).
Sign up for our weekly newsletter
Fresh data delivery Saturday mornings
Sign up for The Briefing
Weekly updates on the world of news & information
- Coronavirus (COVID-19)
- COVID-19 & Politics
- COVID-19 & Science
- Medicine & Health
Americans’ Trust in Scientists, Positive Views of Science Continue to Decline
Americans’ largely positive views of childhood vaccines hold steady, lack of preparedness among top reactions americans have to public health officials’ covid-19 response, about half of recent online daters in u.s. say it’s important to see covid-19 vaccination status on profiles, partisan differences are common in the lessons americans take away from covid-19, most popular, report materials.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
© 2024 Pew Research Center
The state of AI in early 2024: Gen AI adoption spikes and starts to generate value
If 2023 was the year the world discovered generative AI (gen AI) , 2024 is the year organizations truly began using—and deriving business value from—this new technology. In the latest McKinsey Global Survey on AI, 65 percent of respondents report that their organizations are regularly using gen AI, nearly double the percentage from our previous survey just ten months ago. Respondents’ expectations for gen AI’s impact remain as high as they were last year , with three-quarters predicting that gen AI will lead to significant or disruptive change in their industries in the years ahead.
About the authors
This article is a collaborative effort by Alex Singla , Alexander Sukharevsky , Lareina Yee , and Michael Chui , with Bryce Hall , representing views from QuantumBlack, AI by McKinsey, and McKinsey Digital.
Organizations are already seeing material benefits from gen AI use, reporting both cost decreases and revenue jumps in the business units deploying the technology. The survey also provides insights into the kinds of risks presented by gen AI—most notably, inaccuracy—as well as the emerging practices of top performers to mitigate those challenges and capture value.
AI adoption surges
Interest in generative AI has also brightened the spotlight on a broader set of AI capabilities. For the past six years, AI adoption by respondents’ organizations has hovered at about 50 percent. This year, the survey finds that adoption has jumped to 72 percent (Exhibit 1). And the interest is truly global in scope. Our 2023 survey found that AI adoption did not reach 66 percent in any region; however, this year more than two-thirds of respondents in nearly every region say their organizations are using AI. 1 Organizations based in Central and South America are the exception, with 58 percent of respondents working for organizations based in Central and South America reporting AI adoption. Looking by industry, the biggest increase in adoption can be found in professional services. 2 Includes respondents working for organizations focused on human resources, legal services, management consulting, market research, R&D, tax preparation, and training.
Also, responses suggest that companies are now using AI in more parts of the business. Half of respondents say their organizations have adopted AI in two or more business functions, up from less than a third of respondents in 2023 (Exhibit 2).
Gen AI adoption is most common in the functions where it can create the most value
Most respondents now report that their organizations—and they as individuals—are using gen AI. Sixty-five percent of respondents say their organizations are regularly using gen AI in at least one business function, up from one-third last year. The average organization using gen AI is doing so in two functions, most often in marketing and sales and in product and service development—two functions in which previous research determined that gen AI adoption could generate the most value 3 “ The economic potential of generative AI: The next productivity frontier ,” McKinsey, June 14, 2023. —as well as in IT (Exhibit 3). The biggest increase from 2023 is found in marketing and sales, where reported adoption has more than doubled. Yet across functions, only two use cases, both within marketing and sales, are reported by 15 percent or more of respondents.
Gen AI also is weaving its way into respondents’ personal lives. Compared with 2023, respondents are much more likely to be using gen AI at work and even more likely to be using gen AI both at work and in their personal lives (Exhibit 4). The survey finds upticks in gen AI use across all regions, with the largest increases in Asia–Pacific and Greater China. Respondents at the highest seniority levels, meanwhile, show larger jumps in the use of gen Al tools for work and outside of work compared with their midlevel-management peers. Looking at specific industries, respondents working in energy and materials and in professional services report the largest increase in gen AI use.
Investments in gen AI and analytical AI are beginning to create value
The latest survey also shows how different industries are budgeting for gen AI. Responses suggest that, in many industries, organizations are about equally as likely to be investing more than 5 percent of their digital budgets in gen AI as they are in nongenerative, analytical-AI solutions (Exhibit 5). Yet in most industries, larger shares of respondents report that their organizations spend more than 20 percent on analytical AI than on gen AI. Looking ahead, most respondents—67 percent—expect their organizations to invest more in AI over the next three years.
Where are those investments paying off? For the first time, our latest survey explored the value created by gen AI use by business function. The function in which the largest share of respondents report seeing cost decreases is human resources. Respondents most commonly report meaningful revenue increases (of more than 5 percent) in supply chain and inventory management (Exhibit 6). For analytical AI, respondents most often report seeing cost benefits in service operations—in line with what we found last year —as well as meaningful revenue increases from AI use in marketing and sales.
Inaccuracy: The most recognized and experienced risk of gen AI use
As businesses begin to see the benefits of gen AI, they’re also recognizing the diverse risks associated with the technology. These can range from data management risks such as data privacy, bias, or intellectual property (IP) infringement to model management risks, which tend to focus on inaccurate output or lack of explainability. A third big risk category is security and incorrect use.
Respondents to the latest survey are more likely than they were last year to say their organizations consider inaccuracy and IP infringement to be relevant to their use of gen AI, and about half continue to view cybersecurity as a risk (Exhibit 7).
Conversely, respondents are less likely than they were last year to say their organizations consider workforce and labor displacement to be relevant risks and are not increasing efforts to mitigate them.
In fact, inaccuracy— which can affect use cases across the gen AI value chain , ranging from customer journeys and summarization to coding and creative content—is the only risk that respondents are significantly more likely than last year to say their organizations are actively working to mitigate.
Some organizations have already experienced negative consequences from the use of gen AI, with 44 percent of respondents saying their organizations have experienced at least one consequence (Exhibit 8). Respondents most often report inaccuracy as a risk that has affected their organizations, followed by cybersecurity and explainability.
Our previous research has found that there are several elements of governance that can help in scaling gen AI use responsibly, yet few respondents report having these risk-related practices in place. 4 “ Implementing generative AI with speed and safety ,” McKinsey Quarterly , March 13, 2024. For example, just 18 percent say their organizations have an enterprise-wide council or board with the authority to make decisions involving responsible AI governance, and only one-third say gen AI risk awareness and risk mitigation controls are required skill sets for technical talent.
Bringing gen AI capabilities to bear
The latest survey also sought to understand how, and how quickly, organizations are deploying these new gen AI tools. We have found three archetypes for implementing gen AI solutions : takers use off-the-shelf, publicly available solutions; shapers customize those tools with proprietary data and systems; and makers develop their own foundation models from scratch. 5 “ Technology’s generational moment with generative AI: A CIO and CTO guide ,” McKinsey, July 11, 2023. Across most industries, the survey results suggest that organizations are finding off-the-shelf offerings applicable to their business needs—though many are pursuing opportunities to customize models or even develop their own (Exhibit 9). About half of reported gen AI uses within respondents’ business functions are utilizing off-the-shelf, publicly available models or tools, with little or no customization. Respondents in energy and materials, technology, and media and telecommunications are more likely to report significant customization or tuning of publicly available models or developing their own proprietary models to address specific business needs.
Respondents most often report that their organizations required one to four months from the start of a project to put gen AI into production, though the time it takes varies by business function (Exhibit 10). It also depends upon the approach for acquiring those capabilities. Not surprisingly, reported uses of highly customized or proprietary models are 1.5 times more likely than off-the-shelf, publicly available models to take five months or more to implement.
Gen AI high performers are excelling despite facing challenges
Gen AI is a new technology, and organizations are still early in the journey of pursuing its opportunities and scaling it across functions. So it’s little surprise that only a small subset of respondents (46 out of 876) report that a meaningful share of their organizations’ EBIT can be attributed to their deployment of gen AI. Still, these gen AI leaders are worth examining closely. These, after all, are the early movers, who already attribute more than 10 percent of their organizations’ EBIT to their use of gen AI. Forty-two percent of these high performers say more than 20 percent of their EBIT is attributable to their use of nongenerative, analytical AI, and they span industries and regions—though most are at organizations with less than $1 billion in annual revenue. The AI-related practices at these organizations can offer guidance to those looking to create value from gen AI adoption at their own organizations.
To start, gen AI high performers are using gen AI in more business functions—an average of three functions, while others average two. They, like other organizations, are most likely to use gen AI in marketing and sales and product or service development, but they’re much more likely than others to use gen AI solutions in risk, legal, and compliance; in strategy and corporate finance; and in supply chain and inventory management. They’re more than three times as likely as others to be using gen AI in activities ranging from processing of accounting documents and risk assessment to R&D testing and pricing and promotions. While, overall, about half of reported gen AI applications within business functions are utilizing publicly available models or tools, gen AI high performers are less likely to use those off-the-shelf options than to either implement significantly customized versions of those tools or to develop their own proprietary foundation models.
What else are these high performers doing differently? For one thing, they are paying more attention to gen-AI-related risks. Perhaps because they are further along on their journeys, they are more likely than others to say their organizations have experienced every negative consequence from gen AI we asked about, from cybersecurity and personal privacy to explainability and IP infringement. Given that, they are more likely than others to report that their organizations consider those risks, as well as regulatory compliance, environmental impacts, and political stability, to be relevant to their gen AI use, and they say they take steps to mitigate more risks than others do.
Gen AI high performers are also much more likely to say their organizations follow a set of risk-related best practices (Exhibit 11). For example, they are nearly twice as likely as others to involve the legal function and embed risk reviews early on in the development of gen AI solutions—that is, to “ shift left .” They’re also much more likely than others to employ a wide range of other best practices, from strategy-related practices to those related to scaling.
In addition to experiencing the risks of gen AI adoption, high performers have encountered other challenges that can serve as warnings to others (Exhibit 12). Seventy percent say they have experienced difficulties with data, including defining processes for data governance, developing the ability to quickly integrate data into AI models, and an insufficient amount of training data, highlighting the essential role that data play in capturing value. High performers are also more likely than others to report experiencing challenges with their operating models, such as implementing agile ways of working and effective sprint performance management.
About the research
The online survey was in the field from February 22 to March 5, 2024, and garnered responses from 1,363 participants representing the full range of regions, industries, company sizes, functional specialties, and tenures. Of those respondents, 981 said their organizations had adopted AI in at least one business function, and 878 said their organizations were regularly using gen AI in at least one function. To adjust for differences in response rates, the data are weighted by the contribution of each respondent’s nation to global GDP.
Alex Singla and Alexander Sukharevsky are global coleaders of QuantumBlack, AI by McKinsey, and senior partners in McKinsey’s Chicago and London offices, respectively; Lareina Yee is a senior partner in the Bay Area office, where Michael Chui , a McKinsey Global Institute partner, is a partner; and Bryce Hall is an associate partner in the Washington, DC, office.
They wish to thank Kaitlin Noe, Larry Kanter, Mallika Jhamb, and Shinjini Srivastava for their contributions to this work.
This article was edited by Heather Hanselman, a senior editor in McKinsey’s Atlanta office.
Explore a career with us
Related articles.

Moving past gen AI’s honeymoon phase: Seven hard truths for CIOs to get from pilot to scale

A generative AI reset: Rewiring to turn potential into value in 2024
Implementing generative AI with speed and safety
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals
You are here
- Volume 83, Issue Suppl 1
- AB1399 SHORT-TERM OUTCOMES OF IMMUNOSUPPRESSIVE TREATMENT IN SEVERE PERIPHERAL ULCERATIVE KERATITIS: A RETROSPECTIVE STUDY FROM A TERTIARY HEALTHCARE CENTRE
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- A. Patankar 1 ,
- S. Yadav 1 ,
- R. Shukla 2 ,
- S. Mulkutkar 2 ,
- S. Shaha 2 ,
- G. Sachdev 1 ,
- B. Swain 1 ,
- C. Balakrishnan 1 ,
- R. Samant 1
- 1 P D Hinduja Hospital and Medical Research Centre, Department of Clinical Immunology and Rheumatology, Mumbai, India
- 2 P D Hinduja Hospital and Medical Research Centre, Department of Ophthalmology, Mumbai, India
Background: Peripheral Ulcerative Keratitis (PUK) is a rare corneal disorder characterised by progressive juxta-limbal corneal stromal degradation, thinning, perforation, and an increased risk of visual loss [1]. This inflammation can be idiopathic (Mooren’s ulcer) or secondary to systemic inflammatory diseases, such as ANCA vasculitis, Rheumatoid arthritis and SLE. Management of PUK depends on its severity and underlying cause [2]. It includes aggressive systemic immunosuppressive therapy with or without surgical intervention to prevent permanent visual loss.
Objectives: To assess the short-term outcome of immunosuppressive therapy used in treating severe PUK.
Methods: This retrospective case series encompassed all cases of severe PUK treated at our tertiary healthcare centre from 2015 to 2023. Severe PUK was defined as more than 50% corneal thinning, with impending perforation or corneal melt. The absence of infection was confirmed through a corneal scrape. Responses were evaluated as complete response (absence of clinical symptoms, total improvement in visual acuity, no stromal inflammation or progressive corneal thinning), incomplete response (lack of clinical symptoms, with incomplete improvement in visual acuity and persistent stromal inflammation but no progressive corneal thinning) and no response (persistent clinical symptoms, with no improvement in visual acuity, persistent stromal inflammation and progressive corneal thinning).
Results: 12 patients and 18 eyes were included (6 bilateral and 6 unilateral). Of these, 9 were female, with a median age of 56.5 years (IQR 49-64.5 years). RA was the primary cause in 9 patients (66%), followed by Mooren’s (Idiopathic) in 3(25%), and GPA in 1(9%). PUK was the presenting feature in 4 patients (33%). In the RA group, PUK appeared after a median period of 16 years (IQR 6-14 years). Common symptoms included eye pain, redness, diminished vision and photophobia. All the patients had visual acuity below 6/18. Corneal thinning was a prevalent ocular manifestation, with 8 (67%) patients having corneal melt.
All patients received pulse methylprednisolone (500mgx3 doses) followed by oral steroids (0.5mg/kg/day) and immunosuppressive medications. 11 patients received intravenous cyclophosphamide (500mg every 2 weekly x 6 doses) followed by oral methotrexate (15-25mg/week) in 4 patients. Surgical intervention was performed in 9 patients and comprised of amniotic membrane graft, bandage contact lens adhesive with glue and conjunctival resection (Figure 1). Outcomes assessed at 1, 3, and 6 months, indicated a favourable response to treatment, with a progressive increase in complete response over time. The median prednisolone dose showed a decreasing trend, reflecting the therapeutic efficacy of the other interventions (Figure 2). At the end of 6 months, 9 (75%) patients showed a good response to treatment. However, even after the above immunosuppressive regimen, 5 (28%) eyes were lost (visual acuity <6/60), showing an increasing need for better treatment along with early referral and collaborative management.
Conclusion: These findings provide valuable insights into management outcomes of severe PUK, shedding light on potential areas for further research and therapeutic advancement in the field.
REFERENCES: [1] Sharma N, Sinha G, Shekhar H, Titiyal JS, Agarwal T, Chawla B, et al. Demographic profile, clinical features and outcome of peripheral ulcerative keratitis: a prospective study. Br J Ophthalmol. 2015 Nov;99(11):1503–8.
[2] Ashar JN, Mathur A, Sangwan VS. Immunosuppression for Mooren’s ulcer: evaluation of the stepladder approach—topical, oral and intravenous immunosuppressive agents. Br J Ophthalmol. 2013 Nov;97(11):1391–4.
- Download figure
- Open in new tab
- Download powerpoint
Clinical characteristics and profile of patients in the study
Response to immunosuppression in severe PUK
Acknowledgements: NIL.
Disclosure of Interests: None declared.
- Interdisciplinary research
- Real-world evidence
- Glucocorticoids
https://doi.org/10.1136/annrheumdis-2024-eular.4113
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Read the full text or download the PDF:

IMAGES
VIDEO
COMMENTS
The North American Case Research Association (NACRA) is a nonprofit, voluntary professional association whose mission is to promote excellence in case research, writing and teaching in business and other academic disciplines. We seek to accomplish our mission through a set of interrelated activities, including the following: Supporting the work ...
About Case Research Centre: Prof. Ashok Banerjee. Objectives and Support offered by IIM Calcutta Case Research Centre (IIMCCRC) : Prof. Dharma Raju Bathini. We believe that with the growing importance of India in the global economy there will be an increasing demand for India focused cases among academicians and practitioners around the world.
The International Case Research Association is a non-profit voluntary professional association whose mission is to promote emerging market multinational cases on a global scale. Annual Meetings We sponsor annual meetings to help you improve your emerging market multinational cases through the peer-reviewed process.
The Asia Case Research Centre ("ACRC") is part of HKU Business School. It was founded in 1997 to address the need for rich business cases with an Asian focus. The ACRC is committed to the advancement of learning and teaching in business education and strives to promote leading management thinking through research on the latest practices in ...
The purpose of setting up of India case research centre at AIMA is primarily to focus on developing and publishing Industry based India-focused research cases. There are two major users of the management cases. While B-schools faculties are looking for relevant and high-quality cases, which meet the curriculum requirement and learning outcomes. ...
Advancing Research and Scholarly Activities. Centers and institutes are central to our work at Case Western Reserve University, from supporting various academic efforts to advancing research pursuits. More than 100 hubs enrich the campus community, covering such topics as environmental law, popular music, cancer research and more.
SHEIN vs. Zara: Digital Transformation in the Fast-fashion Industry; Uniqlo: A Supply Chain Going Global; Dieselgate - Heavy Fumes Exhausting the Volkswagen Group
Research at the Bloomberg School is a team sport. In order to provide extensive guidance, infrastructure, and support in pursuit of its research mission, research@BSPH employs three core areas: strategy and development, implementation and impact, and integrity and oversight. Our exceptional research teams comprised of faculty, postdoctoral ...
In 1938, it was granted town status. [citation needed]Administrative and municipal status. Within the framework of administrative divisions, it is incorporated as Elektrostal City Under Oblast Jurisdiction—an administrative unit with the status equal to that of the districts. As a municipal division, Elektrostal City Under Oblast Jurisdiction is incorporated as Elektrostal Urban Okrug.
The Legatum Center for Development and Entrepreneurship at the Massachusetts Institute of Technology (MIT) was founded in 2007 with its vision and mission centered on the belief that entrepreneurs and their innovation-driven solutions are key to advancing global prosperity. Apr 30, 2024. Read More.
As a practical matter, it is hard to imagine that someone could look at a 4.3 percent unemployment rate and say college grads are having a hard time finding jobs. Oddly, this is the second time in two weeks we saw this re-employment rate for recent grads highlighted as meaning that college grads are having trouble getting jobs. The market for ...
Background: It is necessary to harmonize and standardize data variables used in case report forms (CRFs) of clinical studies to facilitate the merging and sharing of the collected patient data across several clinical studies. This is particularly true for clinical studies that focus on infectious diseases. Public health may be highly dependent on the findings of such studies.
Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products: ClinicalTrials.gov Identifier: NCT05765773 Other Study ID Numbers: № VKI-P-II-07/21 : First Posted: March 13, 2023 Key Record Dates: Last Update Posted: March 13, 2023 Last Verified: February 2023
CASE - Center for Social and Economic Research. ul. Zamenhofa 5/1b, 00-165 Warsaw, Poland. tel.: +48 798 023 759. [email protected]. NIP: 5260205291. Careers: [email protected]. Business Development Team: [email protected]. contact for the media. [email protected] +48 798 317 869 . news. latest news; Medium ...
Case Comprehensive Cancer Center. We are one of only 56 comprehensive cancer centers designated by the National Cancer Institute. Learn more about this important designation. With our partners CWRU, University Hospitals, and Cleveland Clinic, we are a leader in cancer-related research in the community. Learn how we collaborate to fight cancer.
Centre for Finance and Security. The Centre for Finance and Security (CFS) (formerly the Centre for Financial Crime and Security Studies) specialises in the intersection of finance and global security. Our analysis and actionable ideas aim to challenge the status quo and build resilience in the global response to illicit finance.
MORE Center User Research. The Materials for Opto/Electronics Research and Education (MORE) Center at Case Western Reserve University offers a collaborative and student-led research environment and shared lab space for faculty, researchers and students engaged in a broad range of scientific pursuits. Our community specializes in nanoscale ...
The Elderly Care Research Center (ECRC) is a multidisciplinary, social research organization affiliated with the Department of Sociology at Case Western Reserve University. The Center was established in 1967 by its Director, Dr. Eva Kahana, who is Robson Professor of Sociology, Humanities, Medicine, Nursing, and Applied Social Science at CWRU.
In 1954, Elemash began to produce fuel assemblies, including for the first nuclear power plant in the world, located in Obninsk. In 1959, the facility produced the fuel for the Soviet Union's first icebreaker. Its fuel assembly production became serial in 1965 and automated in 1982. 1. Today, Elemash is one of the largest TVEL nuclear fuel ...
As a case study, the proposed framework is applied to the road network surrounding the Port of Los Angeles---an infrastructure of crucial importance---for assessing resilience and losses at a high resolution. It is found that the port area is disproportionately impacted in the hypothetical earthquake scenario, and delays in bridge repair can ...
Background: Mobile health (mHealth) interventions that promote healthy behaviors or mindsets are a promising avenue to reach vulnerable or at-risk groups. In designing such mHealth interventions, authentic representation of intended participants is essential. The COVID-19 pandemic served as a catalyst for innovation in remote user-centered research methods.
State Housing Inspectorate of the Moscow Region Elektrostal postal code 144009. See Google profile, Hours, Phone, Website and more for this business. 2.0 Cybo Score. Review on Cybo.
The Online Writing Lab (the Purdue OWL) at Purdue University houses writing resources and instructional material, and we provide these as a free service at Purdue. Students, members of the community, and users worldwide will find information to assist with many writing projects. Teachers and trainers may use this material for in-class and out ...
Mission, Role and Pledge. Equitably protecting health, safety & security. In April 2022, CDC launched an effort to refine and modernize its structures, systems, and processes around developing and deploying our science and programs. The goal was to learn how to pivot our long-standing practices and adapt to pandemics and other public health ...
Clinical Research Office (CRO) The Case Comprehensive Cancer Center (Case CCC) is committed to the translation of laboratory insights into routine clinical care. This requires a deep infrastructure to support the conduct of clinical trials of new interventions designed to prevent, diagnose and treat cancer, and improve the long-term outcomes ...
My point is that "everyone designs their own" v "symbols are assigned by some completely external body" is a false dichotomy, and a weird thing to focus on when the biggest difference between the two is actually that there is a national body influencing things - it's a lot more systematic than about how much individual cities or oblasts care about these things.
June 12, 2024. Donald Trump became the first former American president to be convicted of felony charges when a New York jury found him guilty of falsifying business records in the case stemming from a hush money payment to a woman who said he had an affair with her.. About half of the public approves of Trump's conviction on the felony charges, while 3 in 10 disapprove.
A new Pew Research Center survey finds that just 20% of Americans view the coronavirus as a major threat to the health of the U.S. population today and only 10% are very concerned they will get it and require hospitalization. This data represents a low ebb of public concern about the virus that reached its height in the summer and fall of 2020, when as many as two-thirds of Americans viewed ...
About the research. The online survey was in the field from February 22 to March 5, 2024, and garnered responses from 1,363 participants representing the full range of regions, industries, company sizes, functional specialties, and tenures. Of those respondents, 981 said their organizations had adopted AI in at least one business function, and ...
Objectives: To assess the short-term outcome of immunosuppressive therapy used in treating severe PUK. Methods: This retrospective case series encompassed all cases of severe PUK treated at our tertiary healthcare centre from 2015 to 2023. Severe PUK was defined as more than 50% corneal thinning, with impending perforation or corneal melt.