An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Global prevalence of nosocomial infection: A systematic review and meta-analysis

Samira Raoofi
1 School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran
2 Student Research Committee, School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran
Fatemeh Pashazadeh Kan
3 Student Research Committee, School of Nursing and Midwifery, Iran University of Medical Sciences, Tehran, Iran
Sima Rafiei
4 Social Determinants of Health Research Center, Research Institute for Prevention of Non-Communicable Diseases, Qazvin University of Medical Sciences, Qazvin, Iran
Zahra Hosseinipalangi
Zahra noorani mejareh.
5 Student Research Committee, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
Saghar Khani
Bahare abdollahi, fatemeh seyghalani talab.
6 Social Determinants of Health Research Center, Qazvin University of Medical Sciences, Qazvin, Iran
Mohaddeseh Sanaei
Farnaz zarabi.
7 Department of Anesthesia, School of Allied Medical Sciences, Iran University of Medical Sciences, Tehran, Iran
Yasamin Dolati
Niloofar ahmadi, neda raoofi.
8 Cardiovascular Research Center Kermanshah, Kermanshah, Iran
Yasamin Sarhadi
Maryam masoumi.
9 Clinical Research and Development Center, Qom University of Medical Sciences, Qom, Iran
Batool sadat Hosseini
10 Shahid AkbarAbadi Clinical Research Development unit (SHACRDU), Iran University of Medical Sciences, Tehran, Iran
Negin Gholamali
11 Health Management and Economics Research Center, Health Management Research Institute, Iran University of Medical Sciences, Tehran, Iran
Saba Ahmadi
Behrooz ahmadi.
12 Clinical Research Development Center, Imam Ali Hospital, Kermanshah University of Medical Sciences, Kermanshah, Iran
Zahra Beiramy Chomalu
Elnaz asadollahi, mona rajabi, dorsa gharagozloo.
13 Department of Molecular and Cellular Sciences, Faculty of Advanced Sciences and Technology, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
Zahra Nejatifar
Rana soheylirad, shabnam jalali, farnaz aghajani, mobina navidriahy, sama deylami, mahmoud nasiri.
14 Researcher at Toward Evidence (http://towardevidence.co.uk/), Glasgow, United Kingdom
Mahsa Zareei
Zahra golmohammadi, hamideh shabani, fatemeh torabi, hosein shabaninejad.
15 Population Health Sciences Institute (PHSI), Newcastle University, Newcastle, United Kingdom
Mohammad Amerzadeh
Aidin aryankhesal.
16 Department of Health Services Management, School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran
Ahmad Ghashghaee
17 School of Medicine, Dentistry & Nursing, University of Glasgow, Glasgow, United Kingdom
Associated Data
All relevant data are within the paper and its Supporting Information files.
Hospital-acquired infections (HAIs) are significant problems as public health issues which need attention. Such infections are significant problems for society and healthcare organizations. This study aimed to carry out a systematic review and a meta-analysis to analyze the prevalence of HAIs globally.
We conducted a comprehensive search of electronic databases including EMBASE, Scopus, PubMed and Web of Science between 2000 and June 2021. We found 7031 articles. After removing the duplicates, 5430 studies were screened based on the titles/ abstracts. Then, we systematically evaluated the full texts of the 1909 remaining studies and selected 400 records with 29,159,630 participants for meta-analysis. Random-effects model was used for the analysis, and heterogeneity analysis and publication bias test were conducted.
The rate of universal HAIs was 0.14 percent. The rate of HAIs is increasing by 0.06 percent annually. The highest rate of HAIs was in the AFR, while the lowest prevalence were in AMR and WPR. Besides, AFR prevalence in central Africa is higher than in other parts of the world by 0.27 (95% CI, 0.22–0.34). Besides, E. coli infected patients more than other micro-organisms such as Coagulase-negative staphylococci, Staphylococcus spp. and Pseudomonas aeruginosa. In hospital wards, Transplant, and Neonatal wards and ICU had the highest rates. The prevalence of HAIs was higher in men than in women.
We identified several essential details about the rate of HAIs in various parts of the world. The HAIs rate and the most common micro-organism were different in various contexts. However, several essential gaps were also identified. The study findings can help hospital managers and health policy makers identify the reason for HAIs and apply effective control programs to implement different plans to reduce the HAIs rate and the financial costs of such infections and save resources.
Introduction
Hospital-acquired infections (HAIs) are significant problems which need serious attention worldwide. HAIs refer to a group of infections a patient does not have before admission to the hospital. HAIs do not even exist in the latency period; they occur upon arrival at the hospital or within 48–72 hours after admission to the hospital [ 1 – 4 ]. Nowadays, such infections are significant problems for societies and healthcare organizations. They prolong the treatment period and make both patients and health centers pay excessive costs, including increased drug intakes and tests [ 5 ]. Therefore, by preventing and reducing nosocomial infections, significant savings will be made in the costs imposed on health centers, the health system and society consequently [ 6 ].
Due to financial constraints, there are many problems in controlling HAIs in emerging countries. Besides the problems caused by the extension of hospital stay for the patient, HAIs can be transmitted to the patient’s relatives through casual contacts and jeopardize their physical conditions [ 6 ]. Such infections are not limited to specific patients. They may occur to every patient or hospital employee and increase the mortality rate of hospitals [ 7 ].
According to studies, the most prevalent causes of HAIs include urinary tract infections (UTIs), respiratory tract infections (RTIs), circulatory system infections, and surgical site infections [ 8 – 10 ]. According to a report of the World Health Organization (WHO) on 55 hospitals in 14 countries, 8.7% of the hospitalized patients had HAIs, which were more prevalent in the Eastern Mediterranean Region and less prevalent in the West of the Pacific [ 11 – 13 ]. The prevalence rate of these infections was reported to be 5% in the North of America and some parts of Europe, and was about 40% in some Asian, Latin American, and African countries [ 14 , 15 ]. According to the findings of a study conducted in Europe, the prevalence of HAIs was nearly 2.9%. Medical interventions, poor health standards of the hospital environment, and poor personal hygiene of hospital staff and patients poor practice of personal hygiene among hospital staff and patients can cause HAIs [ 16 ]. However, the major/leading cause of HAIs is lack of compliance to health and safety guidelines of hospitals [ 17 ]. Although it is impossible to eliminate such infections even in the most advanced hospitals, standards and guidelines can be complied with the intention of reducing or managing them [ 18 , 19 ]. Nowadays, with technological advances and high expectations of high quality care services, it is highly essential to analyze the frequency and causes of HAIs [ 20 ]. Therefore, it is necessary to know the prevalence rate of different HAIs to devise infection control programs in hospitals and help develop a reliable and effective plan. Lack of accurate data on the prevalence of HAIs makes the execution of control plans challenging and causes higher costs for health systems and patients [ 21 , 22 ].
Due to the presence of developing and underdeveloped countries in the EMRO (the Eastern Mediterranean Regional Office of the World Health Organization), AFRO (African Regional Office of the World Health Organization) and other countries with high prevalence of HAIs, the issue of HAIs is a significant concern, thereby spending hefty sums for controlling and reducing such infections by governments [ 23 ].
Although a number of studies have been conducted on different parts of WHO regions to determine the prevalence rate of HAIs, no systematic review has been conducted globally. This study aimed to carry out a systematic review and a meta-analysis to analyze the prevalence of HAIs globally. The research findings will contribute to the development of effective control programs by managers and policymakers of the health sector to reduce the financial costs of HAIs and save financial resources.
Databases and search terms
We conducted a comprehensive search of electronic databases including EMBASE, Scopus, PubMed and Web of Science between 2000 and June 2021. Search terms included (“infection cross”[Title] OR “cross infections”[Title] OR “healthcare associated infections”[Title] OR “healthcare associated infection”[Title] OR “health care associated infection”[Title] OR “health care associated infections”[Title] OR “hospital infection”[Title] OR “infections hospital”[Title] OR “nosocomial infection”[Title] OR “nosocomial infections”[Title] OR “hospital infections”[Title]). We found 7031 articles through searching the databases. After entering the records into EndNote software and removing the duplicates, 5430 studies were screened on the basis of their titles/ abstracts. We reviewed the reference list of all included articles to ensure the comprehensiveness of the search.
Inclusion and exclusion criteria
On the basis of the research keywords, we included studies reporting quantitative data on HAIs prevalence and their determining factors among the general population. Different observational studies, including cross-sectional, prospective, case-study, and cohort, were included. We considered articles with available full texts published in English between 2000 and June 2021 for further consideration in this review. The reason we included articles from 2000 was to estimate the trend of the current century. We excluded interventional studies, reviews, reports, letters to the editor, books, case-control, and commentaries. We also excluded the review studies using invalid methods or containing insufficient data focused on diagnostic approaches, treatment methods, and medication.
Study selection
Searching electronic databases resulted in 7031 articles. After removing the duplicates, two researchers reviewed the remaining 5630 records independently, based on the titles and abstracts. In the next step, we systematically evaluated the full texts of the 1909 remaining studies to determine whether they met the eligibility criteria defined in the study. Finally, we selected 400 records with 29159630 participants to evaluate in this meta-analysis ( Fig 1 ).

Quality assessment
We evaluated the methodological quality of the articles, using the Newcastle-Ottawa Scale (NOS) based on the procedures suggested in the Cochrane Handbook of Systematic Reviews. The NOS comprises a star system in which a study is evaluated in three areas, including four items regarding the selection of study groups, two items regarding the comparability of groups, and three items in terms of exposure or outcome ascertainment. If any of the items in the NOS were not reported in the article, a zero score was assigned; and for each of the areas addressed in the study, one was given. We categorized studies based on their methodological quality in different groups, from poor (score between 0 and 3) to high quality (score between 7 and 9). Two independent reviewers performed the quality assessment process; in case of any disagreement, a third investigator resolved the issues [ 24 ].
Data extraction
One of the reviewers used a data extraction form to enter data of the included studies. The form included items such as author/ authors’ name, the title of the study, publication year, study setting, sample size, characteristics of the study population including their age and gender, the total prevalence of hospital-acquired infection, the prevalence of hospital-acquired infection based on the infection type and related organisms ( S1 File ).
Statistical analysis
We used a random-effects model to estimate the pooled prevalence of HAIs, measuring the effect size with a 95% confidence interval (CI) and illustrating the graphical results with Forest plots. The I 2 test quantified the statistical heterogeneity, and the Egger test was applied to assess publication bias. We used subgroup analyses due to the variability of estimates based on different study settings, type of infection, and socio-demographic characteristics of study populations. We carried out all analyses, using the Comprehensive Meta-Analysis and R software. All figures with p<0.05 were considered statistically significant.
Patient and public involvement
We considered articles with available full texts published in English between 2000 and June 2021 for further consideration in this review.
According to the inclusion and exclusion criteria and PRISMA checklist (Preferred reporting items for systematic reviews and meta-analyses) [ 25 ], we selected 400 articles for the final review stage (see Fig 1 ). The total number of patients participating in these studies was 29,159,630, of which 5,441,722 had various HAIs. On the basis of the data analysis, we estimated the rate of the global HAIs to be 0.14 (95% CI, 0.12–0.15) ( Table 1 ).
As Fig 2 shows, the prevalence of nosocomial infections is increasing, as with a one-year increase, 0.06 would be added to nosocomial infections ( Fig 2 ). Moreover, to clarify the findings, we divided it into five range. The findings show that the highest prevalence of nosocomial infections was 0.20 (95% CI, 0.11–0.32) between 2011–2015, but it decreased to 0.17 (95% CI, 0.08–0.23) between 2016–2011 ( Table 6 ).

Abbreviations: Confidence Interval (CI).
Since we included 400 studies in this study and there were different sample sizes, we performed a pooled analysis based on the sample size. The results revealed no significant relationship between sample size and HAIs, and with changing the sample size, we observed no significant difference in the rate of HAIs ( Fig 2 ).
Meta-analysis based on WHO regions and countries
As illustrated in Table 2 , the highest rate of HAIs was in the AFR, and based on 94 studies analyzed, this rate was equal to 0.27 (95% CI, 0.22–0.34). The lowest infection rates were in AMR and WPR which were 0.09 (95% CI, 0.07–0.11) and 0.09 (95% CI, 0.06–0.13), respectively. Fig 3 demonstrates the distribution map of HAIs. The map shows that the rate of HAIs in central Africa is higher than anywhere else in the world ( Fig 3 ).

Map created with PhotoshopCC, using political borders.
Meta-analysis based on micro-organism and infection types
Based on the analysis of microorganisms and various HAIs, the findings showed that among all major microorganisms responsible for the HAIs, patients were infected by E. coli more than other microorganisms, 0.18 (95% CI, 0.16–0.20). However, according to WHO regions, Coagulase-negative staphylococci was the most common microorganisms in WPRO and EURO with 0.21 (95% CI, 0.11–0.36) and 0.14 (95% CI, 0.10–0.20). Also, in South-East Asian Region Office (SEARO) and EMRO, the highest rate of infection was related to E. coli with 0.19 (95% CI, 0.13–0.26) and 0.16 (95% CI, 0.13–0.20). Finally, Pseudomonas aeruginosa and Staphylococcus spp . microorganisms were the most common infectious agents in AMRO and AFRO, respectively ( Table 3 ).
The results of analyzes based on the type of infections showed that the highest type of infection among all HAIs was wound infection, with a rate of 0.34 (95% CI, 0.24–0.47). Regarding the WHO regions, the analyses showed that each region was more involved with a particular infection. For example, in the WPRO and SEARO, respiratory tract infections and surgical site infections were the most common infections. However, wound infection was more prevalent in the EMRO and AFRO than in other infections ( Table 3 ).
Meta-analysis based on hospital ward
The findings showed that the highest prevalence of HAIs in hospital wards was related to the transplant wards with the prevalence rate of 0.77 (95% CI, 0.38–0.90), followed by Neonatal and ICU wards, with a prevalence rate of 0.69 (95% CI, 0.47–0.85) and 0.68 (95% CI, 0.61–0.73), respectively ( Table 4 ).
Meta-analysis based on gender
Overall, the prevalence of HAIs is higher in men than women. However, the prevalence of this type of infection is higher in women in AMR and EMR. In AFR, EUR and SEAR, men showed higher prevalence rate, while the rates were the same for both genders in the WPR ( Table 5 ).
Meta-regression on other sub-groups
The results of the analysis showed that the prevalence of HAIs decreases with increasing age. For every one year increase in age, the prevalence decreases by 0.04 ( Fig 4 ). We categorized the participants by age to make our results clear, and we noticed that the highest prevalence of nosocomial infections was in the age range of 0–5 years 0.21 (95% CI, 0.5–0.22) ( Table 6 ).

Length of stay
According to our findings, No significant relationship was found between length of stay and the prevalence of HAIs ( Fig 4 ). days, We divided the length of stay in the hospital into more than 15 days and less than 15, based on the division of other studies [ 26 ]. The results showed that the prevalence rate was estimated to be 0.15 (95% CI, 0.11–0.34) in people who were in the hospital for more than 15 days and 0.12 (95% CI, 0.6–0.28) for those who were in the hospital for 15 days or less than 15 days ( Table 6 ).
Countries based on income
According to the findings of the analysis, countries with lower incomes had higher prevalence of infection. For example, in low-income countries, the prevalence was 0.32 (95% CI, 0.15–0.49) and the prevalence of high-income countries was estimated 0.06 (95% CI, 0.03–0.12) ( Table 6 )
Quality of study
The findings revealed that the prevalence rate in lower quality studies was 0.16 (95% CI, 0.06–0.19) whereas it was 0.14 (95% CI, 0.10–0.16) in high quality studies ( Table 6 ).
Publication bias
Based on Fig 5 , the analysis showed that this study has a Publication bias. This claim is true since the result of the Egger test was greater than 0.01. (P-value 2-tailed = 0.091).

HAIs are one of the most severe public health issues with high morbidity, mortality, and costs [ 27 ]. This study aimed to conduct a systematic review and meta-analysis to determine the prevalence rate of HAIs globally. This is the first comprehensive SLR investigating HAIs from all key aspects. In this systematic review, we screened 7031 journal articles and selected 400 articles that contained quantitative information about the global prevalence of HAIs for evaluation in the meta-analysis.
On the basis of the findings of this study, the rate of universal HAIs is estimated to be 0.14 with an annual increasing rate of 0.06 worldwide. According to our findings, the highest rate of HAIs was in the AFR, while the lowest rates were in AMRO and WPRO, at 0.09. Besides, the central Africa had higher rate than other parts of the world. This may be due to the lack of health facilities and resources in this area. The continent is also facing natural crises such as water shortages and droughts, which in turn are increasing nosocomial infections. On the other hand, economic conditions in this region are one of the most important causes of these infections. Another study revealed that the HAIs rate is 7.5% in high-income countries, while it varies between 5.7 and 19.2 in low-income countries percent [ 28 ]. According to the WHO data, the HAIs rate is 25% in developing countries and 5–15% in developed countries [ 29 , 30 ]. Another study estimated the HAIs rate at 16 percent in the Eastern Mediterranean Region [ 8 ]. In Roberts et al. study, 159 patients (12.7%) developed HAIs from among 1,253 patients in the United States [ 31 ]. The findings of our study suggest that patients are at a higher risk of nosocomial infections due to lack of facilities and poor conditions of hospitals and medical centers in low-income and underdeveloped countries than developed countries.
According to the analysis of microorganisms, the E. coli infected patients with HAIs more than other microorganisms (0.18). Based on the WHO regions, Coagulase-negative staphylococci were the most common microorganisms in WPRO and EURO with the incidence of 0.21 and 0.14, respectively. In SEARO and EMRO, the highest infectivity was E. coli, with 0.19 and 0.16. Moreover, Pseudomonas aeruginosa and Staphylococcus spp . microorganisms were the most common infectious agents in AMRO and AFRO, respectively. One of the studies on this issue revealed that Staphylococcus aureus , Pseudomonas aeroginosa , and Klebsiella species are the most common pathogens in Africa and South America [ 32 ]. Another review in Africa reported Klebsiella , Staphylococcus aureus , Pseudomonas aeroginosa , and E . coli the most common microorganisms in HAIs. These three microorganisms, in addition to being easier to transport than others, have significant resistance to antibiotics. On the other hand, they are more resistant to sterilization and disinfection methods than the others. Due to these characteristics, these microorganisms have a higher prevalence rate than others. Because these bacteria are more resistant to antibiotics than others [ 33 ].
Analysis based on the type of infections revealed that the highest type of infection in all HAIs was wound infection, with a rate of 0.34. In terms of the WHO regions, each region represents a specific type of infection; in the WPRO and SEARO. Respiratory tract infections and surgical site infections were the most widespread infections while wound infection was more prevalent in the EMRO and AFRO. A similar study showed that lower respiratory tract infections were the leading cause of HAIs [ 34 ]. Other common infections were urinary tract infections, surgical site infections, and bloodstream infections [ 9 ].
In terms of the prevalence of HAIs in hospital wards, transplant unit had the highest rate at 0.77, followed by neonatal and ICU wards 0.69 and 0.68, respectively. Nonetheless, a study in Ethiopia found that the infection rate at the surgical site was high % [ 35 ]. Another study found the surgical site as the most frequent type of HAI in Low and Middle-Income Countries [ 9 ].
Regarding HAIs in terms of gender, the prevalence of HAIs was higher in men than in women. In line with our study, the HAIs burden was shown to be greater in men in another study [ 36 ]. In the WHO regions, the rate was higher in women in AMRO and EMRO, whereas, in AFRO, EURO and SEARO, men were reported to have a higher rate. However, in the WPRO, the rates were the same for both sexes. Another study of about 633,000 people in China reported that the prevalence rate was higher in men than in women, supporting our findings [ 37 ]. Similarly, another study in the United States on about 530,000 people showed similar results to our findings [ 10 ].
With the increasing length of stay in the hospital and despite the fact that we divided the patients into two groups of more than 15 LoS and less than 15 LoS to determine the effect of length of stay on NI, the difference was not too large and we did not find any significant changes in the HAIs rate. However, the AlemkereI’sstudy found that the HAIs risk in patients with a longer stay was 24 times more than in patients with a shorter stay [ 38 ]. But in another study, the findings showed that an increase in length of stay could affect the rate of nosocomial infection, but this effect was considered significant after 9 days [ 39 ]. In another study of 65,000 people, the findings showed that although longer stays could affect the prevalence of nosocomial infections, the effect was not significant [ 40 ]. We think that this variable can affect nosocomial infections, but this effect can manifest itself after a long time stay in the hospital. A short time stay in the hospital does not have much effect and cannot increase the prevalence. According to studies reviewed, this effect becomes more severe after 15 days and increases the prevalence.
The study showed that with the increasing age, the prevalence of nosocomial infections decreases. On the other hand, by age classification, we found that the prevalence of infection is higher in the age group of 0–5 years and in the age group over 50 years. In a study conducted in Argentina on people under the age of five, the prevalence rate of nosocomial infections was reported to be 50%, which was much higher than the average for our study and the global average [ 41 ]. In another study conducted in Turkey on people of similar age range, the prevalence rate was about 25 percent [ 42 ].
This systematic and meta-analysis review was conducted to determine the rate of HAIs worldwide. The review identified a number of essential details about the rate of HAIs in various parts of the world. It revealed that the rate of universal HAIs and the number of publications in this regard has risen in recent years. The HAIs rate and the most common micro-organism were different in various regions. However, several important gaps were identified such as lack of data in different regions and territories and different domains like the cause of HAIs. The study findings can help managers and policymakers of the health sector identify the reason for HAIs and apply effective control programs to implement different plans to reduce the HAIs rate and the financial costs of such infections and save resources. We recommend that more studies be carried out to identify strategies and plans for preventing HAIs in all countries, particularly in Low and Middle-Income Countries. Nosocomial infection is one of the most important indicators of hospitals to evaluate the performance of the hospital in terms of patient safety. Our study is done on a global scale so it can be very generalizable and help health decision makers to plan to prevent these types of infections. By reducing nosocomial infections, in addition to improving the patient’s safety index, a large amount of the costs incurred by the hospital due to these types of infections will be reduced.
We suggest to decision makers that by focusing on different aspects of nosocomial infections such as age, gender, causes, etc. that we mentioned in this study, comprehensive and practical programs can be used to prevent these infections.
Limitations
There are some limitations that should be considered when interpreting our study results. First, there might be a language bias in the study as we only included the studies published in English. We focused on peer-reviewed articles; thus, grey literatures and unpublished articles were not included in this review. In addition, in some countries, reliable and published data was not available, so we could not analyze all countries in the world. Finally, studies reviewed did not address many of the variables directly related to nosocomial infections such as type of hospital, number of hospital beds, etc. We also did not include Covid-19 disease in nosocomial infections because they have different definitions, and if we included Covid-19 infections in our study, it would falsely increase the prevalence of nosocomial infections in recent years, it would be a significant bias.
We suggest that researchers work on the gaps in our study. For example, conduct studies in countries where no articles on nosocomial infections have been found. On the other hand, studies on the cause and transmission of these infections can greatly help the health system to reduce these types of diseases.
Supporting information
S1 checklist, acknowledgments.
Our research team would like to thank all those who are trying to improve the fields related to health service management, especially the (@health.care.management) team ( hcmanagers.ir ), who have made great efforts to increase the credibility of this field in the Iranian health system.
Funding Statement
The authors received no specific funding for this work.
Data Availability
- PLoS One. 2023; 18(1): e0274248.
Decision Letter 0
PONE-D-22-04059Global, regional, and the national prevalence of nosocomial infection: A systematic review and meta-analysisPLOS ONE
Dear Dr. Ghashghaee,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
Please submit your revised manuscript by Apr 21 2022 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
- A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
- A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
- An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: https://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols . Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols .
We look forward to receiving your revised manuscript.
Kind regards,
Yong-Hong Kuo
Academic Editor
Journal Requirements:
When submitting your revision, we need you to address these additional requirements.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
https://journals.plos.org/plosone/s/file?id=wjVg/PLOSOne_formatting_sample_main_body.pdf and
https://journals.plos.org/plosone/s/file?id=ba62/PLOSOne_formatting_sample_title_authors_affiliations.pdf
2. In your Data Availability statement, you have not specified where the minimal data set underlying the results described in your manuscript can be found. PLOS defines a study's minimal data set as the underlying data used to reach the conclusions drawn in the manuscript and any additional data required to replicate the reported study findings in their entirety. All PLOS journals require that the minimal data set be made fully available. For more information about our data policy, please see http://journals.plos.org/plosone/s/data-availability .
Upon re-submitting your revised manuscript, please upload your study’s minimal underlying data set as either Supporting Information files or to a stable, public repository and include the relevant URLs, DOIs, or accession numbers within your revised cover letter. For a list of acceptable repositories, please see http://journals.plos.org/plosone/s/data-availability#loc-recommended-repositories . Any potentially identifying patient information must be fully anonymized.
Important: If there are ethical or legal restrictions to sharing your data publicly, please explain these restrictions in detail. Please see our guidelines for more information on what we consider unacceptable restrictions to publicly sharing data: http://journals.plos.org/plosone/s/data-availability#loc-unacceptable-data-access-restrictions . Note that it is not acceptable for the authors to be the sole named individuals responsible for ensuring data access.
We will update your Data Availability statement to reflect the information you provide in your cover letter.
3. Thank you for stating the following financial disclosure:
“The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.”
At this time, please address the following queries:
a) Please clarify the sources of funding (financial or material support) for your study. List the grants or organizations that supported your study, including funding received from your institution.
b) State what role the funders took in the study. If the funders had no role in your study, please state: “The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.”
c) If any authors received a salary from any of your funders, please state which authors and which funders.
d) If you did not receive any funding for this study, please state: “The authors received no specific funding for this work.”
Please include your amended statements within your cover letter; we will change the online submission form on your behalf.
4. We note that Figure 3 in your submission contain [map/satellite] images which may be copyrighted. All PLOS content is published under the Creative Commons Attribution License (CC BY 4.0), which means that the manuscript, images, and Supporting Information files will be freely available online, and any third party is permitted to access, download, copy, distribute, and use these materials in any way, even commercially, with proper attribution. For these reasons, we cannot publish previously copyrighted maps or satellite images created using proprietary data, such as Google software (Google Maps, Street View, and Earth). For more information, see our copyright guidelines: http://journals.plos.org/plosone/s/licenses-and-copyright .
We require you to either (1) present written permission from the copyright holder to publish these figures specifically under the CC BY 4.0 license, or (2) remove the figures from your submission:
1. You may seek permission from the original copyright holder of Figure 3 publish the content specifically under the CC BY 4.0 license.
We recommend that you contact the original copyright holder with the Content Permission Form ( http://journals.plos.org/plosone/s/file?id=7c09/content-permission-form.pdf ) and the following text:
“I request permission for the open-access journal PLOS ONE to publish XXX under the Creative Commons Attribution License (CCAL) CC BY 4.0 ( http://creativecommons.org/licenses/by/4.0/ ). Please be aware that this license allows unrestricted use and distribution, even commercially, by third parties. Please reply and provide explicit written permission to publish XXX under a CC BY license and complete the attached form.”
Please upload the completed Content Permission Form or other proof of granted permissions as an "Other" file with your submission.
In the figure caption of the copyrighted figure, please include the following text: “Reprinted from [ref] under a CC BY license, with permission from [name of publisher], original copyright [original copyright year].”
2. If you are unable to obtain permission from the original copyright holder to publish these figures under the CC BY 4.0 license or if the copyright holder’s requirements are incompatible with the CC BY 4.0 license, please either i) remove the figure or ii) supply a replacement figure that complies with the CC BY 4.0 license. Please check copyright information on all replacement figures and update the figure caption with source information. If applicable, please specify in the figure caption text when a figure is similar but not identical to the original image and is therefore for illustrative purposes only.
The following resources for replacing copyrighted map figures may be helpful:
USGS National Map Viewer (public domain): http://viewer.nationalmap.gov/viewer/
The Gateway to Astronaut Photography of Earth (public domain): http://eol.jsc.nasa.gov/sseop/clickmap/
Maps at the CIA (public domain): https://www.cia.gov/library/publications/the-world- factbook/index.html and https://www.cia.gov/library/publications/cia-maps-publications/index.html
NASA Earth Observatory (public domain): http://earthobservatory.nasa.gov/
Landsat: http://landsat.visibleearth.nasa.gov/
USGS EROS (Earth Resources Observatory and Science (EROS) Center) (public domain): http://eros.usgs.gov/#
Additional Editor Comments (if provided):
The manuscript has been reviewed by two experts in the area. Both of them find the significance of the study and are positive about the submission. They have provided constructive and very helpful comments to improve the article. Based on their recommendations and comment, I suggest Major Revision.
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: Yes
Reviewer #2: Yes
2. Has the statistical analysis been performed appropriately and rigorously?
3. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
4. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
Reviewer #1: No
5. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: This is an interesting review article and the authors have collected a unique dataset. The paper is generally well written and organized. However, in my opinion, there are some shortcomings. Some sentences are not well structured and do not bring out the argument clearly. What is the impact of this review to the public health? What recommendations do you have for stakeholders and policymakers?
Reviewer #2: This is a large meta-analysis evaluating the global prevalence of Hospital-acquired infection (HAI). This is an important topic, and the manuscript includes interesting data on causative pathogens for HAI globally and prevalence rates of HAI. The main issue with this manuscript is the number of studies and heterogenous data. With the number of studies, it is difficult to draw broad conclusions. HAI can have different definitions between studies, countries, and institutions. Several co-variates based on different healthcare systems may be unaccounted for. For example, it is surprising that duration of hospitalization was not an associated factor with HAI as seen in previous studies. I would consider limiting the number of studies and potentially decreasing the time the studies were conducted over. With less studies, the manuscript could include a more direct summary and comparison of data from studies.
6. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy .
Reviewer #2: No
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/ . PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at gro.solp@serugif . Please note that Supporting Information files do not need this step.
Submitted filename: Reviewer comments.docx
Author response to Decision Letter 0
22 Jun 2022
Reviews 1 This is an interesting review article and the authors have collected a unique dataset. The paper is generally well written and organized. However, in my opinion, there are some shortcomings. Some sentences are not well structured and do not bring out the argument clearly. What is the impact of this review to the public health? What recommendations do you have for stakeholders and policymakers?
Answer: Nosocomial infection is one of the most important indicators of hospitals to evaluate the performance of the hospital in terms of patient safety. Our study is done on a global scale so it can be very generalizable and help health decision makers to plan to prevent these types of infections. By reducing nosocomial infections, in addition to improving the patient's safety index, a large amount of the costs incurred by the hospital due to these types of infections will be reduced.
Reviews 2 This is a large meta-analysis evaluating the global prevalence of Hospital-acquired infection (HAI). This is an important topic, and the manuscript includes interesting data on causative pathogens for HAI globally and prevalence rates of HAI. The main issue with this manuscript is the number of studies and heterogenous data. With the number of studies, it is difficult to draw broad conclusions. HAI can have different definitions between studies, countries, and institutions. Several co-variates based on different healthcare systems may be unaccounted for. For example, it is surprising that duration of hospitalization was not an associated factor with HAI as seen in previous studies. I would consider limiting the number of studies and potentially decreasing the time the studies were conducted over. With less studies, the manuscript could include a more direct summary and comparison of data from studies.
Answer: We explained in the inclusion and exclusion criteria that we only included studies that provided a clear definition of nosocomial infections. On the other hand, the main purpose of our study was to examine the trend of nosocomial infections in the new century, so if the number of articles is reduced, the main purpose of the article will change.
In relation to heterogeneity, we used subgroup analyzes to solve this problem, which were significantly more efficient.
Reviewers Comments Make the title more concise. National and regional prevalence? We changed it to : Global prevalence of nosocomial infection: A systematic review and meta-analysis
Reviewers Comments Results: AFR higher in Central Africa than the world. By how much? We wrote this information in full in the original version of the article, but in the submitted version we had to make corrections due to the word limit.
We added : 0.27 (95% CI, 0.22-0.34)
Reviewers Comments Besides E. coli infected patients…which other microorganisms are you comparing? We added: such as Coagulase-negative staphylococci, Pseudomonas aeruginosa and Staphylococcus spp.
Reviewers Comments Conclusion. Managers? What is their role? Hospital managers and health policy makers
Reviewers Comments Rephrase last sentence in first paragraph. Not clear.
Thus, by preventing the prevalence of HAIs instead of allocating hefty sums to the treatment of such infections, managers of healthcare centers can bear much lower costs to manage HAIs. Therefore, by preventing and reducing nosocomial infections, significant savings will be made in the costs imposed on health centers, the health system and society consequently
Reviewers Comments Which studies?
According to studies, the most prevalent causes of HAIs include urinary tract infections (UTIs), respiratory tract infections (RTIs), circulatory system infections, and surgical site infections. We add a reference for this statement.
Reviewers Comments Sentence is hanging
Although a number of studies have been conducted on different parts of WHO regions to determine the prevalence rate of HAIs. Although a number of studies have been conducted on different parts of WHO regions to determine the prevalence rate of HAIs, no systematic review has been conducted globally.
Reviewers Comments You need to show up to which date you acquired the 7031 articles. For future references. between 2000 and June 2021
Reviewers Comments Clarify
Increasing rate of HAIs by 0.06% in abstract or 0.6% in results. 0.06 is correct
Reviewers Comments Italicize scientific names We changed them
Reviewers Comments What could result in high HAIs in central Africa? This may be due to the lack of health facilities and resources in this area. The continent is also facing natural crises such as water shortages and droughts, which in turn are increasing nosocomial infections. On the other hand, economic conditions in this region are one of the most important causes of these infections.
Reviewers Comments Why is S. aureus, P. aeroginosa and Klebsiella the most common HAIs? These three microorganisms, in addition to being easier to transport than others, have significant resistance to antibiotics. On the other hand, they are more resistant to sterilization and disinfection methods than others. Due to these characteristics, these microorganisms have a higher prevalence rate than others.
Reviewers Comments Are there any recommendations? We suggest that researchers work on the gaps in our study. For example, conduct studies in countries where no articles on nosocomial infections have been found. On the other hand, studies on the cause and transmission of these infections can greatly help the health system to reduce these types of diseases.
Reviewers Comments Some studies consider COVID 19 as a nosocomial infection. Why did you exclude it? We did not include Covid-19 disease in nosocomial infections because they have different definitions, and if we included Covid-19 infections in our study, it would falsely increase the prevalence of nosocomial infections in recent years, it would be a significant bias.
Submitted filename: Response to reviewers.docx
Decision Letter 1
PONE-D-22-04059R1Global prevalence of nosocomial infection: A systematic review and meta-analysisPLOS ONE
Please submit your revised manuscript by Sep 22 2022 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
Additional Editor Comments:
There are still minor concerns from the reviewer. Please address them before the final publication.
1. If the authors have adequately addressed your comments raised in a previous round of review and you feel that this manuscript is now acceptable for publication, you may indicate that here to bypass the “Comments to the Author” section, enter your conflict of interest statement in the “Confidential to Editor” section, and submit your "Accept" recommendation.
Reviewer #1: All comments have been addressed
2. Is the manuscript technically sound, and do the data support the conclusions?
3. Has the statistical analysis been performed appropriately and rigorously?
4. Have the authors made all data underlying the findings in their manuscript fully available?
5. Is the manuscript presented in an intelligible fashion and written in standard English?
6. Review Comments to the Author
Reviewer #1: This is a well written review paper. The author needs to polish up Discussion section as we do not clearly understand why some microorganisms have a higher prevalence rate as nosocomial infections as compared to others.
7. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
Submitted filename: renamed_fcd50.docx
Author response to Decision Letter 1
Reviews 1 Abstract:
Objectives- Which needs immediate attention. Specific sounds redundant. “ Specific “ was omitted
Reviews 1 Introduction:
Remove the irrelevant sentence-It might happen with any kind of infection.
According to studies- Which ones?
Which studies in Europe? Which countries? Any citations?
In the last paragraph, can is subject to speculation. Replace with will All the comments were corrected
Reviews 1 Results:
Length of stay- Rewrite the first sentence
Countries based on income- Prevalence in high income countries? The first sentence was rewrote.
The prevalence of high-income countries was added
Reviews 1 Discussion-
Why is certain organisms rated as more common HAIs than others? Because these bacteria are more resistant to antibiotics than others.
Reviews 1 Conclusion-
Which gaps? However, several important gaps were identified such as lack of data in different regions and territories and different areas like the cause of HAIs
Decision Letter 2
25 Aug 2022
PONE-D-22-04059R2
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/ , click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at gro.solp@gnillibrohtua .
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact gro.solp@sserpeno .
Additional Editor Comments (optional):
Based on the Referees' recommendations, I recommend Accept.
Reviewer #1: It is an interesting article. This is a comprehensive meta-analysis that looks at the global prevalence of hospital-acquired infections (HAI). This is an essential issue, and the paper contains intriguing statistics on HAI causative microorganisms and HAI prevalence rates throughout the world. Submitted comments have been addressed.
Acceptance letter
18 Jan 2023
Dear Dr. Ghashghaee:
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now with our production department.
If your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information please contact gro.solp@sserpeno .
If we can help with anything else, please email us at gro.solp@enosolp .
Thank you for submitting your work to PLOS ONE and supporting open access.
PLOS ONE Editorial Office Staff
on behalf of
Dr. Yong-Hong Kuo
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Global prevalence of nosocomial infection: A systematic review and meta-analysis
Roles Project administration, Writing – original draft, Writing – review & editing
Affiliations School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran, Student Research Committee, School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran
Roles Data curation, Project administration
Affiliation Student Research Committee, School of Nursing and Midwifery, Iran University of Medical Sciences, Tehran, Iran
Roles Writing – original draft, Writing – review & editing
Affiliation Social Determinants of Health Research Center, Research Institute for Prevention of Non-Communicable Diseases, Qazvin University of Medical Sciences, Qazvin, Iran
Roles Data curation, Supervision
Roles Data curation, Project administration, Supervision
Affiliation Student Research Committee, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
Roles Data curation, Methodology, Supervision
Roles Data curation, Formal analysis
Roles Data curation, Investigation
Affiliation Social Determinants of Health Research Center, Qazvin University of Medical Sciences, Qazvin, Iran
Roles Data curation, Software, Supervision
Affiliation Department of Anesthesia, School of Allied Medical Sciences, Iran University of Medical Sciences, Tehran, Iran
Roles Data curation, Resources, Software
Roles Data curation, Investigation, Methodology
Affiliation Cardiovascular Research Center Kermanshah, Kermanshah, Iran
Roles Data curation
Affiliation Clinical Research and Development Center, Qom University of Medical Sciences, Qom, Iran
Roles Data curation, Investigation, Software
Roles Data curation, Methodology, Software
Affiliation Shahid AkbarAbadi Clinical Research Development unit (SHACRDU), Iran University of Medical Sciences, Tehran, Iran
Roles Data curation, Formal analysis, Validation
Roles Data curation, Resources
Affiliation Health Management and Economics Research Center, Health Management Research Institute, Iran University of Medical Sciences, Tehran, Iran
Affiliation Clinical Research Development Center, Imam Ali Hospital, Kermanshah University of Medical Sciences, Kermanshah, Iran
Roles Data curation, Formal analysis, Investigation
Roles Data curation, Validation, Visualization
Affiliation Student Research Committee, School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran
Roles Data curation, Methodology, Resources
Affiliation Department of Molecular and Cellular Sciences, Faculty of Advanced Sciences and Technology, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
Roles Data curation, Methodology
Affiliation Researcher at Toward Evidence (http://towardevidence.co.uk/), Glasgow, United Kingdom
Roles Software, Supervision, Validation, Visualization
Roles Data curation, Software, Validation
Affiliation Population Health Sciences Institute (PHSI), Newcastle University, Newcastle, United Kingdom
Roles Writing – review & editing
Roles Writing – original draft
Roles Visualization, Writing – original draft, Writing – review & editing
Affiliation Department of Health Services Management, School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran
- [ ... ],
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation School of Medicine, Dentistry & Nursing, University of Glasgow, Glasgow, United Kingdom
- [ view all ]
- [ view less ]
- Samira Raoofi,
- Fatemeh Pashazadeh Kan,
- Sima Rafiei,
- Zahra Hosseinipalangi,
- Zahra Noorani Mejareh,
- Saghar Khani,
- Bahare Abdollahi,
- Fatemeh Seyghalani Talab,
- Mohaddeseh Sanaei,

- Published: January 27, 2023
- https://doi.org/10.1371/journal.pone.0274248
- Peer Review
- Reader Comments
Hospital-acquired infections (HAIs) are significant problems as public health issues which need attention. Such infections are significant problems for society and healthcare organizations. This study aimed to carry out a systematic review and a meta-analysis to analyze the prevalence of HAIs globally.
We conducted a comprehensive search of electronic databases including EMBASE, Scopus, PubMed and Web of Science between 2000 and June 2021. We found 7031 articles. After removing the duplicates, 5430 studies were screened based on the titles/ abstracts. Then, we systematically evaluated the full texts of the 1909 remaining studies and selected 400 records with 29,159,630 participants for meta-analysis. Random-effects model was used for the analysis, and heterogeneity analysis and publication bias test were conducted.
The rate of universal HAIs was 0.14 percent. The rate of HAIs is increasing by 0.06 percent annually. The highest rate of HAIs was in the AFR, while the lowest prevalence were in AMR and WPR. Besides, AFR prevalence in central Africa is higher than in other parts of the world by 0.27 (95% CI, 0.22–0.34). Besides, E. coli infected patients more than other micro-organisms such as Coagulase-negative staphylococci, Staphylococcus spp. and Pseudomonas aeruginosa. In hospital wards, Transplant, and Neonatal wards and ICU had the highest rates. The prevalence of HAIs was higher in men than in women.
We identified several essential details about the rate of HAIs in various parts of the world. The HAIs rate and the most common micro-organism were different in various contexts. However, several essential gaps were also identified. The study findings can help hospital managers and health policy makers identify the reason for HAIs and apply effective control programs to implement different plans to reduce the HAIs rate and the financial costs of such infections and save resources.
Citation: Raoofi S, Pashazadeh Kan F, Rafiei S, Hosseinipalangi Z, Noorani Mejareh Z, Khani S, et al. (2023) Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS ONE 18(1): e0274248. https://doi.org/10.1371/journal.pone.0274248
Editor: Yong-Hong Kuo, University of Hong Kong, HONG KONG
Received: February 9, 2022; Accepted: August 24, 2022; Published: January 27, 2023
Copyright: © 2023 Raoofi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: The authors received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Hospital-acquired infections (HAIs) are significant problems which need serious attention worldwide. HAIs refer to a group of infections a patient does not have before admission to the hospital. HAIs do not even exist in the latency period; they occur upon arrival at the hospital or within 48–72 hours after admission to the hospital [ 1 – 4 ]. Nowadays, such infections are significant problems for societies and healthcare organizations. They prolong the treatment period and make both patients and health centers pay excessive costs, including increased drug intakes and tests [ 5 ]. Therefore, by preventing and reducing nosocomial infections, significant savings will be made in the costs imposed on health centers, the health system and society consequently [ 6 ].
Due to financial constraints, there are many problems in controlling HAIs in emerging countries. Besides the problems caused by the extension of hospital stay for the patient, HAIs can be transmitted to the patient’s relatives through casual contacts and jeopardize their physical conditions [ 6 ]. Such infections are not limited to specific patients. They may occur to every patient or hospital employee and increase the mortality rate of hospitals [ 7 ].
According to studies, the most prevalent causes of HAIs include urinary tract infections (UTIs), respiratory tract infections (RTIs), circulatory system infections, and surgical site infections [ 8 – 10 ]. According to a report of the World Health Organization (WHO) on 55 hospitals in 14 countries, 8.7% of the hospitalized patients had HAIs, which were more prevalent in the Eastern Mediterranean Region and less prevalent in the West of the Pacific [ 11 – 13 ]. The prevalence rate of these infections was reported to be 5% in the North of America and some parts of Europe, and was about 40% in some Asian, Latin American, and African countries [ 14 , 15 ]. According to the findings of a study conducted in Europe, the prevalence of HAIs was nearly 2.9%. Medical interventions, poor health standards of the hospital environment, and poor personal hygiene of hospital staff and patients poor practice of personal hygiene among hospital staff and patients can cause HAIs [ 16 ]. However, the major/leading cause of HAIs is lack of compliance to health and safety guidelines of hospitals [ 17 ]. Although it is impossible to eliminate such infections even in the most advanced hospitals, standards and guidelines can be complied with the intention of reducing or managing them [ 18 , 19 ]. Nowadays, with technological advances and high expectations of high quality care services, it is highly essential to analyze the frequency and causes of HAIs [ 20 ]. Therefore, it is necessary to know the prevalence rate of different HAIs to devise infection control programs in hospitals and help develop a reliable and effective plan. Lack of accurate data on the prevalence of HAIs makes the execution of control plans challenging and causes higher costs for health systems and patients [ 21 , 22 ].
Due to the presence of developing and underdeveloped countries in the EMRO (the Eastern Mediterranean Regional Office of the World Health Organization), AFRO (African Regional Office of the World Health Organization) and other countries with high prevalence of HAIs, the issue of HAIs is a significant concern, thereby spending hefty sums for controlling and reducing such infections by governments [ 23 ].
Although a number of studies have been conducted on different parts of WHO regions to determine the prevalence rate of HAIs, no systematic review has been conducted globally. This study aimed to carry out a systematic review and a meta-analysis to analyze the prevalence of HAIs globally. The research findings will contribute to the development of effective control programs by managers and policymakers of the health sector to reduce the financial costs of HAIs and save financial resources.
Databases and search terms
We conducted a comprehensive search of electronic databases including EMBASE, Scopus, PubMed and Web of Science between 2000 and June 2021. Search terms included (“infection cross”[Title] OR “cross infections”[Title] OR “healthcare associated infections”[Title] OR “healthcare associated infection”[Title] OR “health care associated infection”[Title] OR “health care associated infections”[Title] OR “hospital infection”[Title] OR “infections hospital”[Title] OR “nosocomial infection”[Title] OR “nosocomial infections”[Title] OR “hospital infections”[Title]). We found 7031 articles through searching the databases. After entering the records into EndNote software and removing the duplicates, 5430 studies were screened on the basis of their titles/ abstracts. We reviewed the reference list of all included articles to ensure the comprehensiveness of the search.
Inclusion and exclusion criteria
On the basis of the research keywords, we included studies reporting quantitative data on HAIs prevalence and their determining factors among the general population. Different observational studies, including cross-sectional, prospective, case-study, and cohort, were included. We considered articles with available full texts published in English between 2000 and June 2021 for further consideration in this review. The reason we included articles from 2000 was to estimate the trend of the current century. We excluded interventional studies, reviews, reports, letters to the editor, books, case-control, and commentaries. We also excluded the review studies using invalid methods or containing insufficient data focused on diagnostic approaches, treatment methods, and medication.
Study selection
Searching electronic databases resulted in 7031 articles. After removing the duplicates, two researchers reviewed the remaining 5630 records independently, based on the titles and abstracts. In the next step, we systematically evaluated the full texts of the 1909 remaining studies to determine whether they met the eligibility criteria defined in the study. Finally, we selected 400 records with 29159630 participants to evaluate in this meta-analysis ( Fig 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0274248.g001
Quality assessment
We evaluated the methodological quality of the articles, using the Newcastle-Ottawa Scale (NOS) based on the procedures suggested in the Cochrane Handbook of Systematic Reviews. The NOS comprises a star system in which a study is evaluated in three areas, including four items regarding the selection of study groups, two items regarding the comparability of groups, and three items in terms of exposure or outcome ascertainment. If any of the items in the NOS were not reported in the article, a zero score was assigned; and for each of the areas addressed in the study, one was given. We categorized studies based on their methodological quality in different groups, from poor (score between 0 and 3) to high quality (score between 7 and 9). Two independent reviewers performed the quality assessment process; in case of any disagreement, a third investigator resolved the issues [ 24 ].
Data extraction
One of the reviewers used a data extraction form to enter data of the included studies. The form included items such as author/ authors’ name, the title of the study, publication year, study setting, sample size, characteristics of the study population including their age and gender, the total prevalence of hospital-acquired infection, the prevalence of hospital-acquired infection based on the infection type and related organisms ( S1 File ).
Statistical analysis
We used a random-effects model to estimate the pooled prevalence of HAIs, measuring the effect size with a 95% confidence interval (CI) and illustrating the graphical results with Forest plots. The I 2 test quantified the statistical heterogeneity, and the Egger test was applied to assess publication bias. We used subgroup analyses due to the variability of estimates based on different study settings, type of infection, and socio-demographic characteristics of study populations. We carried out all analyses, using the Comprehensive Meta-Analysis and R software. All figures with p<0.05 were considered statistically significant.
Patient and public involvement
We considered articles with available full texts published in English between 2000 and June 2021 for further consideration in this review.
According to the inclusion and exclusion criteria and PRISMA checklist (Preferred reporting items for systematic reviews and meta-analyses) [ 25 ], we selected 400 articles for the final review stage (see Fig 1 ). The total number of patients participating in these studies was 29,159,630, of which 5,441,722 had various HAIs. On the basis of the data analysis, we estimated the rate of the global HAIs to be 0.14 (95% CI, 0.12–0.15) ( Table 1 ).
https://doi.org/10.1371/journal.pone.0274248.t001
As Fig 2 shows, the prevalence of nosocomial infections is increasing, as with a one-year increase, 0.06 would be added to nosocomial infections ( Fig 2 ). Moreover, to clarify the findings, we divided it into five range. The findings show that the highest prevalence of nosocomial infections was 0.20 (95% CI, 0.11–0.32) between 2011–2015, but it decreased to 0.17 (95% CI, 0.08–0.23) between 2016–2011 ( Table 6 ).
Since we included 400 studies in this study and there were different sample sizes, we performed a pooled analysis based on the sample size. The results revealed no significant relationship between sample size and HAIs, and with changing the sample size, we observed no significant difference in the rate of HAIs ( Fig 2 ).
https://doi.org/10.1371/journal.pone.0274248.g002
Meta-analysis based on WHO regions and countries
As illustrated in Table 2 , the highest rate of HAIs was in the AFR, and based on 94 studies analyzed, this rate was equal to 0.27 (95% CI, 0.22–0.34). The lowest infection rates were in AMR and WPR which were 0.09 (95% CI, 0.07–0.11) and 0.09 (95% CI, 0.06–0.13), respectively. Fig 3 demonstrates the distribution map of HAIs. The map shows that the rate of HAIs in central Africa is higher than anywhere else in the world ( Fig 3 ).
Map created with PhotoshopCC, using political borders.
https://doi.org/10.1371/journal.pone.0274248.g003
https://doi.org/10.1371/journal.pone.0274248.t002
Meta-analysis based on micro-organism and infection types
Based on the analysis of microorganisms and various HAIs, the findings showed that among all major microorganisms responsible for the HAIs, patients were infected by E. coli more than other microorganisms, 0.18 (95% CI, 0.16–0.20). However, according to WHO regions, Coagulase-negative staphylococci was the most common microorganisms in WPRO and EURO with 0.21 (95% CI, 0.11–0.36) and 0.14 (95% CI, 0.10–0.20). Also, in South-East Asian Region Office (SEARO) and EMRO, the highest rate of infection was related to E. coli with 0.19 (95% CI, 0.13–0.26) and 0.16 (95% CI, 0.13–0.20). Finally, Pseudomonas aeruginosa and Staphylococcus spp . microorganisms were the most common infectious agents in AMRO and AFRO, respectively ( Table 3 ).
https://doi.org/10.1371/journal.pone.0274248.t003
The results of analyzes based on the type of infections showed that the highest type of infection among all HAIs was wound infection, with a rate of 0.34 (95% CI, 0.24–0.47). Regarding the WHO regions, the analyses showed that each region was more involved with a particular infection. For example, in the WPRO and SEARO, respiratory tract infections and surgical site infections were the most common infections. However, wound infection was more prevalent in the EMRO and AFRO than in other infections ( Table 3 ).
Meta-analysis based on hospital ward
The findings showed that the highest prevalence of HAIs in hospital wards was related to the transplant wards with the prevalence rate of 0.77 (95% CI, 0.38–0.90), followed by Neonatal and ICU wards, with a prevalence rate of 0.69 (95% CI, 0.47–0.85) and 0.68 (95% CI, 0.61–0.73), respectively ( Table 4 ).
https://doi.org/10.1371/journal.pone.0274248.t004
Meta-analysis based on gender
Overall, the prevalence of HAIs is higher in men than women. However, the prevalence of this type of infection is higher in women in AMR and EMR. In AFR, EUR and SEAR, men showed higher prevalence rate, while the rates were the same for both genders in the WPR ( Table 5 ).
https://doi.org/10.1371/journal.pone.0274248.t005
Meta-regression on other sub-groups
The results of the analysis showed that the prevalence of HAIs decreases with increasing age. For every one year increase in age, the prevalence decreases by 0.04 ( Fig 4 ). We categorized the participants by age to make our results clear, and we noticed that the highest prevalence of nosocomial infections was in the age range of 0–5 years 0.21 (95% CI, 0.5–0.22) ( Table 6 ).
https://doi.org/10.1371/journal.pone.0274248.g004
https://doi.org/10.1371/journal.pone.0274248.t006
Length of stay
According to our findings, No significant relationship was found between length of stay and the prevalence of HAIs ( Fig 4 ). days, We divided the length of stay in the hospital into more than 15 days and less than 15, based on the division of other studies [ 26 ]. The results showed that the prevalence rate was estimated to be 0.15 (95% CI, 0.11–0.34) in people who were in the hospital for more than 15 days and 0.12 (95% CI, 0.6–0.28) for those who were in the hospital for 15 days or less than 15 days ( Table 6 ).
Countries based on income
According to the findings of the analysis, countries with lower incomes had higher prevalence of infection. For example, in low-income countries, the prevalence was 0.32 (95% CI, 0.15–0.49) and the prevalence of high-income countries was estimated 0.06 (95% CI, 0.03–0.12) ( Table 6 )
Quality of study
The findings revealed that the prevalence rate in lower quality studies was 0.16 (95% CI, 0.06–0.19) whereas it was 0.14 (95% CI, 0.10–0.16) in high quality studies ( Table 6 ).
Publication bias
Based on Fig 5 , the analysis showed that this study has a Publication bias. This claim is true since the result of the Egger test was greater than 0.01. (P-value 2-tailed = 0.091).
https://doi.org/10.1371/journal.pone.0274248.g005
HAIs are one of the most severe public health issues with high morbidity, mortality, and costs [ 27 ]. This study aimed to conduct a systematic review and meta-analysis to determine the prevalence rate of HAIs globally. This is the first comprehensive SLR investigating HAIs from all key aspects. In this systematic review, we screened 7031 journal articles and selected 400 articles that contained quantitative information about the global prevalence of HAIs for evaluation in the meta-analysis.
On the basis of the findings of this study, the rate of universal HAIs is estimated to be 0.14 with an annual increasing rate of 0.06 worldwide. According to our findings, the highest rate of HAIs was in the AFR, while the lowest rates were in AMRO and WPRO, at 0.09. Besides, the central Africa had higher rate than other parts of the world. This may be due to the lack of health facilities and resources in this area. The continent is also facing natural crises such as water shortages and droughts, which in turn are increasing nosocomial infections. On the other hand, economic conditions in this region are one of the most important causes of these infections. Another study revealed that the HAIs rate is 7.5% in high-income countries, while it varies between 5.7 and 19.2 in low-income countries percent [ 28 ]. According to the WHO data, the HAIs rate is 25% in developing countries and 5–15% in developed countries [ 29 , 30 ]. Another study estimated the HAIs rate at 16 percent in the Eastern Mediterranean Region [ 8 ]. In Roberts et al. study, 159 patients (12.7%) developed HAIs from among 1,253 patients in the United States [ 31 ]. The findings of our study suggest that patients are at a higher risk of nosocomial infections due to lack of facilities and poor conditions of hospitals and medical centers in low-income and underdeveloped countries than developed countries.
According to the analysis of microorganisms, the E. coli infected patients with HAIs more than other microorganisms (0.18). Based on the WHO regions, Coagulase-negative staphylococci were the most common microorganisms in WPRO and EURO with the incidence of 0.21 and 0.14, respectively. In SEARO and EMRO, the highest infectivity was E. coli, with 0.19 and 0.16. Moreover, Pseudomonas aeruginosa and Staphylococcus spp . microorganisms were the most common infectious agents in AMRO and AFRO, respectively. One of the studies on this issue revealed that Staphylococcus aureus , Pseudomonas aeroginosa , and Klebsiella species are the most common pathogens in Africa and South America [ 32 ]. Another review in Africa reported Klebsiella , Staphylococcus aureus , Pseudomonas aeroginosa , and E . coli the most common microorganisms in HAIs. These three microorganisms, in addition to being easier to transport than others, have significant resistance to antibiotics. On the other hand, they are more resistant to sterilization and disinfection methods than the others. Due to these characteristics, these microorganisms have a higher prevalence rate than others. Because these bacteria are more resistant to antibiotics than others [ 33 ].
Analysis based on the type of infections revealed that the highest type of infection in all HAIs was wound infection, with a rate of 0.34. In terms of the WHO regions, each region represents a specific type of infection; in the WPRO and SEARO. Respiratory tract infections and surgical site infections were the most widespread infections while wound infection was more prevalent in the EMRO and AFRO. A similar study showed that lower respiratory tract infections were the leading cause of HAIs [ 34 ]. Other common infections were urinary tract infections, surgical site infections, and bloodstream infections [ 9 ].
In terms of the prevalence of HAIs in hospital wards, transplant unit had the highest rate at 0.77, followed by neonatal and ICU wards 0.69 and 0.68, respectively. Nonetheless, a study in Ethiopia found that the infection rate at the surgical site was high % [ 35 ]. Another study found the surgical site as the most frequent type of HAI in Low and Middle-Income Countries [ 9 ].
Regarding HAIs in terms of gender, the prevalence of HAIs was higher in men than in women. In line with our study, the HAIs burden was shown to be greater in men in another study [ 36 ]. In the WHO regions, the rate was higher in women in AMRO and EMRO, whereas, in AFRO, EURO and SEARO, men were reported to have a higher rate. However, in the WPRO, the rates were the same for both sexes. Another study of about 633,000 people in China reported that the prevalence rate was higher in men than in women, supporting our findings [ 37 ]. Similarly, another study in the United States on about 530,000 people showed similar results to our findings [ 10 ].
With the increasing length of stay in the hospital and despite the fact that we divided the patients into two groups of more than 15 LoS and less than 15 LoS to determine the effect of length of stay on NI, the difference was not too large and we did not find any significant changes in the HAIs rate. However, the AlemkereI’sstudy found that the HAIs risk in patients with a longer stay was 24 times more than in patients with a shorter stay [ 38 ]. But in another study, the findings showed that an increase in length of stay could affect the rate of nosocomial infection, but this effect was considered significant after 9 days [ 39 ]. In another study of 65,000 people, the findings showed that although longer stays could affect the prevalence of nosocomial infections, the effect was not significant [ 40 ]. We think that this variable can affect nosocomial infections, but this effect can manifest itself after a long time stay in the hospital. A short time stay in the hospital does not have much effect and cannot increase the prevalence. According to studies reviewed, this effect becomes more severe after 15 days and increases the prevalence.
The study showed that with the increasing age, the prevalence of nosocomial infections decreases. On the other hand, by age classification, we found that the prevalence of infection is higher in the age group of 0–5 years and in the age group over 50 years. In a study conducted in Argentina on people under the age of five, the prevalence rate of nosocomial infections was reported to be 50%, which was much higher than the average for our study and the global average [ 41 ]. In another study conducted in Turkey on people of similar age range, the prevalence rate was about 25 percent [ 42 ].
This systematic and meta-analysis review was conducted to determine the rate of HAIs worldwide. The review identified a number of essential details about the rate of HAIs in various parts of the world. It revealed that the rate of universal HAIs and the number of publications in this regard has risen in recent years. The HAIs rate and the most common micro-organism were different in various regions. However, several important gaps were identified such as lack of data in different regions and territories and different domains like the cause of HAIs. The study findings can help managers and policymakers of the health sector identify the reason for HAIs and apply effective control programs to implement different plans to reduce the HAIs rate and the financial costs of such infections and save resources. We recommend that more studies be carried out to identify strategies and plans for preventing HAIs in all countries, particularly in Low and Middle-Income Countries. Nosocomial infection is one of the most important indicators of hospitals to evaluate the performance of the hospital in terms of patient safety. Our study is done on a global scale so it can be very generalizable and help health decision makers to plan to prevent these types of infections. By reducing nosocomial infections, in addition to improving the patient’s safety index, a large amount of the costs incurred by the hospital due to these types of infections will be reduced.
We suggest to decision makers that by focusing on different aspects of nosocomial infections such as age, gender, causes, etc. that we mentioned in this study, comprehensive and practical programs can be used to prevent these infections.
Limitations
There are some limitations that should be considered when interpreting our study results. First, there might be a language bias in the study as we only included the studies published in English. We focused on peer-reviewed articles; thus, grey literatures and unpublished articles were not included in this review. In addition, in some countries, reliable and published data was not available, so we could not analyze all countries in the world. Finally, studies reviewed did not address many of the variables directly related to nosocomial infections such as type of hospital, number of hospital beds, etc. We also did not include Covid-19 disease in nosocomial infections because they have different definitions, and if we included Covid-19 infections in our study, it would falsely increase the prevalence of nosocomial infections in recent years, it would be a significant bias.
We suggest that researchers work on the gaps in our study. For example, conduct studies in countries where no articles on nosocomial infections have been found. On the other hand, studies on the cause and transmission of these infections can greatly help the health system to reduce these types of diseases.
Supporting information
https://doi.org/10.1371/journal.pone.0274248.s001
S1 Checklist. PRISMA 2020 checklist.
https://doi.org/10.1371/journal.pone.0274248.s002
Acknowledgments
Our research team would like to thank all those who are trying to improve the fields related to health service management, especially the (@health.care.management) team ( hcmanagers.ir ), who have made great efforts to increase the credibility of this field in the Iranian health system.
- View Article
- PubMed/NCBI
- Google Scholar
- 24. Wells G.A., et al., The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2000, Oxford.
- 34. Jagt E.W. and Short S., Healthcare-Associated Infections, in Pediatric Critical Care. 2021, Springer. p. 1105–1143.
- Open access
- Published: 01 September 2023
Nurses’ knowledge on nosocomial infections preventive measures and its associated factors in Ghana: a cross-sectional study
- Samuel Salu 1 ,
- Joshua Okyere 2 , 3 ,
- Veronica Okwuchi Charles-Unadike 4 &
- Mark Kwame Ananga 4
BMC Health Services Research volume 23 , Article number: 941 ( 2023 ) Cite this article
4644 Accesses
Metrics details
Nosocomial infections (NCIs) have been associated with several adverse outcomes including extended hospitalization, persistent disability, heightened antimicrobial resistance, amplified socio-economic disruption, and elevated mortality rates. The adoption of infection prevention strategies has the greatest tendency to significantly reduce the risk and occurrence of NCIs among the population, particularly in resource constrained health systems. This study assessed nurses’ knowledge on NCI preventive measures and its associated factors in Ghana.
A cross-sectional study was conducted from July to August 2021. A sample of 237 healthcare workers in the Hohoe Municipality was selected to participate in the study. Data was collected with a questionnaire designed in Google Forms and analyzed using Stata version 16.0.
Overall, most of the participants (69.2%) were not knowledgeable about the preventive measures of NCIs. Nurses who were within the age group of 20–40 years [aOR = 0.25 (95% CI = 0.09–0.69), p = 0.007] and 41–60 years [aOR = 0.05 (95% CI = 0.01–0.29), p = 0.001] were significantly less likely to be knowledgeable about the preventive measures of NCIs compared to those who those aged less than 20 years. Nurses who attended in-service training or workshop were approximately 10 times more likely to be knowledgeable about preventive measures of nosocomial infection compared to those who had never attended in-service training or workshop [aOR = 9.55 (95% CI = 1.23–74.36), p = 0.031].
The study concludes that age and participation in-service training or workshop are significant factors that influence the knowledge of healthcare workers in preventive measures for nosocomial infections. These results highlight the importance of providing ongoing training and professional development opportunities to nurses to enhance their knowledge and improve their ability to prevent and control nosocomial infections. Additionally, the study emphasizes the need for targeted training programs that consider the age of nurses, to ensure that training is tailored to their specific needs.
Peer Review reports
Globally, infections are considered a serious public health concern [ 1 ]. While infections occur in different settings including at home, work and in outdoor settings, infections acquired at the healthcare facility pose a significant threat to the overall quality of healthcare delivery. According to the World Health Organisation [ 2 ], nosocomial infections (NCIs) or hospital-acquired infections (HAIs) refer to “an infection occurring in a patient in a hospital or other health care facility in whom the infection was not present or incubating at the time of admission. This includes infections acquired in the hospital but appearing after discharge, and also occupational infections among staff of the facility” . These infections include urinary tract infections, surgical site infections (e.g., Staphylococcus aureus), bloodstream infections, and lower respiratory tract infections [ 3 ].
There are approximately 1.7 million patients worldwide who contract NCIs each year [ 4 ]. A systematic review [ 5 ] has also estimated NCIs to be increasing worldwide with an annual increasing rate of 0.06, and with the African region having the highest rates of NCIs. Ghana, for instance, has an estimated NCI prevalence of 8.2% [ 6 ]. If left unabated, the existence of NCIs would have serious repercussions for health care delivery, time spent at the hospital, and healthcare expenditure. NCIs have been associated with several adverse outcomes including extended hospitalization, persistent disability, heightened antimicrobial resistance, amplified socio-economic disruption, and elevated mortality rates [ 6 , 7 , 8 ]. However, the adoption of infection prevention strategies has the greatest tendency to significantly reduce the risk and occurrence of NCI among the population, particularly in resource constrained health systems [ 9 ].
Available evidence suggests that adopting NCI prevention involves establishing a protective barrier between vulnerable host and microorganisms [ 10 ]. According to the WHO [ 2 ], NCI can primarily be prevented either by reducing person-to-person transmission or by preventing transmission from the environment. Reducing person-to-person transmission involves implementing various measures to minimize the spread of infections between patients, healthcare workers, and visitors. This includes promoting proper hand hygiene practices (i.e., handwashing or using hand sanitizers), and practicing safe injection practices [ 11 ]. On the other hand, preventing transmission from the environment involves maintaining a clean and hygienic healthcare setting. It encapsulates practices such as sterilization, disinfection of patient equipment, proper waste management and cleaning of the hospital environment [ 2 ].
In Ghana, the Ministry of Health and Ghana Health Service has emphasized the importance of adopting preventive measures to control the burden of NCIs [ 6 ]. This keen interest in NCI prevention in Ghana is evident in the country’s implementation of a national infection prevention policy and guidelines for healthcare settings [ 12 ]. However, it must be noted that having the support of the government agencies and a policy framework is not enough for healthcare workers to implement NCI preventive measures. Their knowledge level is quintessential to the implementation of NCI preventive measures [ 13 ]. Nurses’ knowledge on NCI preventive measures is critical for successful implementation and compliance with infection control protocols. Moreover, adequate knowledge has the potential to empower nurses to identify potential risks, implement preventive measures effectively, and respond promptly to infection control challenges [ 14 ].
Limited research has been conducted in Ghana to assess the current state of nurses’ knowledge with respect to NCI prevention. While the existing body of literature has examined the extent to which knowledge influences the practice of NCI preventive measures [ 15 , 16 ], it fails to assess what factors predict the knowledge level of nurses regarding NCI prevention in Ghana. In terms of geographical boundaries, none of the existing studies have investigated the dynamics of nurses’ knowledge on NCI preventive measures in the Volta Region of Ghana. Understanding the level of knowledge and identifying factors associated with nurses’ knowledge gaps can inform targeted interventions and educational programs aimed at improving infection control practices. By addressing these gaps, healthcare facilities in Ghana can enhance their infection prevention and control efforts, leading to a reduction in the burden of NCI and improved patient outcomes. Hence, we aimed to assess nurses’ knowledge on nosocomial infection (NCI) preventive measures and its associated factors in Ghana.
Study design
A cross-sectional study design was employed in this study. This study was carried out in the Hohoe Municipality, in the Volta Region of Ghana. The Hohoe Municipality is one of the eighteen (18) districts in the Volta Region. The city of Hohoe, of which the district was named, serves as the capital and the administrative or local government centre. It shares borders with the Republic of Togo on the east; on the southeast by the Afadzato district and southwest by Kpando Municipality; on the north with Jasikan district; and on the northwest with the Biakoye districts. Its capital, Hohoe, is about 78 km from Ho, the regional capital and 220 km from Accra, the national capital. The Municipality consists of one hundred and two (102) communities with a population of 167,016 projected from the 2010 National Population Census.
Sample size and sampling procedure
Using Cochrane’s single proportion formula, a sample size of 215 was estimated as follows;
\(n=\frac{{z}^{2} p(1-p)}{{d}^{2}}\) , considering 5% margin of error, 95% confidence interval = 1.96 and a proportion knowledge of 83.21% from a study conducted by [ 17 ]. Where;
n = Estimated sample size.
p = 0.8321.
d = margin of error (0.05).
Z = Test Statistic (1.96).
Adding 10% to cater for non-response increased the estimated sample size to 237.
The Hohoe Municipal comprises of four (4) sub-districts and all these sub-districts have health facilities. A simple random sampling was used to select two health facilities from the four (4) sub-districts. This was done by writing the names of all the health facilities from the four (4) sub-districts on pieces of paper, folded for concealment and placed into a container. These papers were thoroughly mixed, and two neutral persons were asked to pick one piece of paper each from the container without opening. In each of the two facilities sampled, nurses who met the inclusion criteria and consented to participate in the study were conveniently selected.
Inclusion and exclusion criteria
Nurses were selected based on the following inclusion criteria: (1) they must be registered nurses currently working in any of the study sites, (2) they must be present at the time of the survey, and (3) they must express a voluntary interest in participating in the study. Therefore, student nurses and nursing interns were excluded from the study. Additionally, nurses who were on leave during the data collection period were excluded from the study.
Definition of variables
Outcome variable.
Knowledge on preventive measures of nosocomial infections was the study’s outcome variable. This was assessed using eleven (11) items on the questionnaire. These items assessed whether respondents (I) have heard about infection prevention (II) could tell if gloves provide complete protection against acquiring or transmitting infections (III) knows if washing hands with soap or an alcohol-based antiseptic decreases the risk of transmission of nosocomial infections (IV) knows if the use of an alcohol-based antiseptic for hand hygiene is as effective as soap and water if hands are not visibly dirty (V) knows if gloves should be worn if blood or body fluid exposure is anticipated or not (VI) knows if there is a need to wash hands before doing procedures that do not involve bodily fluids or not (VII) knows if there is a need to wear the same pair of gloves for multiple patients as long as there is no visible contamination or not (VIII) knows the specific waste disposal buckets according to the level of their contamination (VIV) knows the written formula for preparing 0.5% chlorine solution (X) knows how long instrument or equipment should be disinfected and (XI) knows disease that are transmitted by needle stick injury.
For each of the items (I-X), respondents were asked to choose from the two responses “yes” or “no” provided. For question (XI), respondents were provided with a list of diseases (HBV, HCV, TB, HIV) to choose from. Respondents were allowed to choose from the list multiple times. A composite knowledge score was obtained by assigning a score of 1 to all the positive responses to the eleven (11) questions. All negative responses on the other hand were assigned the score of 0. A mean score was generated by adding all these responses and nurses who scored below the mean were considered “not knowledgeable” on the preventive measures of nosocomial infections, whereas nurses who scored above the mean were considered “knowledgeable”. The study incorporated the classification of knowledge on the preventive measures of nosocomial infections into “not knowledgeable” and “knowledgeable” from previous studies [ 18 , 19 , 20 ].
Explanatory variable
For the study, five (5) explanatory variables were considered in our estimations. These variables included age, sex, level of education, years of working experience and in-service training or workshop. None of these variables were chosen at random; rather, they were chosen based on the findings of previous studies on knowledge in preventive measures of nosocomial infections among healthcare workers [ 21 , 22 , 23 ]. In assessing these socio-demographic information of the respondents, age and years of working experience were collected as continuous variables and categorized into (< 20 years, 20–40 years, 41–60 years) and (1–10 years, 11–20 years and > 20 years) respectively. Respondents’ sex (male or female), level of education (certificate, diploma, degree, masters or doctoral), and in-service training or workshop (yes or no) were collected as categorical variables.
Data collection
The study covered a period from July 2021 to August 2021. Nurses working in the Hohoe Municipality and whose consents are been sought during the time of the study were recruited to participate in the study. Data was collected using a well-structured questionnaire comprising of both open and close-ended questions which were pretested. The questionnaire was designed in Google Forms. It comprised of 35 items related to the socio-demographic factors, knowledge in preventive measures and practice of preventive measures of NCIs.
Data analysis
The data was extracted from Google Forms to Excel Sheet for cleaning and then exported into STATA V.16.0 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.) analysis. To ensure the quality of the data extracted, double entry was done to address discrepancies which may have occurred during data extraction. The data was extensively cleaned again in STATA V. 16.0 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.) before analysis was carried out. Descriptive statistics were performed to interpret the socio-demographic features including age, sex, level of education, work experience, and in-service training or workshop on nosocomial infection prevention. However, inferential statistics were done to test the association between socio-demographic factors and healthcare workers’ knowledge in preventive measures of nosocomial infections. Frequencies and percentages related to the study findings were presented using tables and graphs.
Table 1 provides a distribution of the socio-demographic characteristics of the respondents. The majority of the healthcare workers were females 124 (52.3%) while males were 113 (47.7%). More than two-thirds of them, 195 (82.7%) were within the age group of (20–40) years. Most of the participants, 127 (53.6%) were Diploma holders. Regarding their work experience, almost all of them 204 (86.1%) had work experience ranging between (1–10) years. Out of the total 237 healthcare workers, 218 (92.0%) had attended in-service training or workshop on nosocomial infection prevention while 19 (8.0%) had never attended any in-service training or workshop on nosocomial infection prevention.
Knowledge of nurses on preventive measures of nosocomial infections
Table 2 shows the distribution of nurses’ knowledge on NCI preventive measures. Out of the total 237 nurses who participated in the study, more than 90% 216 (91.1%) indicated that they had heard about infection prevention. More than half 148 (68.5%) out of the 216 (91.1%) who had heard about infection prevention believed that gloves cannot provide complete protection against acquiring or transmitting infections. Almost all of them 213 (98.6%) believed that washing your hands with soap or using an alcohol-based antiseptic decreases the risk of transmission of NCI. More than 80% of them 187 (86.6%) also indicated that the use of an alcohol-based antiseptic for hand hygiene is as effective as soap and water if hands are not visibly dirty. All of them 216 (100%) agreed that there is a need to wash hands before doing procedures that do not involve bodily fluids. Furthermore, 198 (83.5%) of the nurses know the specific waste disposal buckets according to the level of their contamination. Most of the participants, 210 (88.6%), indicated that instruments or equipment should be disinfected for 10 min.
A knowledge mean score was generated using the items used to measure the level of knowledge among the nurses. Those who scored below the mean were considered not knowledgeable on the preventive measures of NCIs, whereas those who scored above the mean were considered knowledgeable. The findings from this study revealed that 164 (69.2%) of the participants were not knowledgeable on the preventive measures of NCIs (Fig. 1 ).
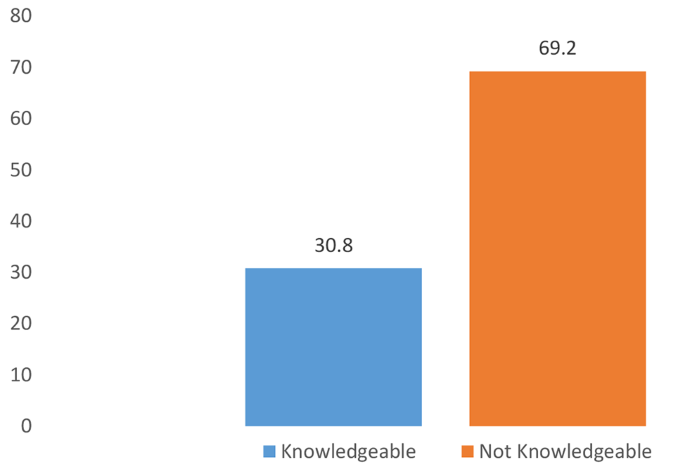
Overall level of knowledge of healthcare workers in preventive measures of nosocomial infections
Factors associated with nurses’ knowledge on preventive measures of nosocomial infections
Age and in-service training or workshop were the only factors that were significantly associated with the participants’ knowledge on NCI preventive measures. Sex, level of education and years of working experience showed no significant association with nurses’ knowledge on NCI preventive measures. Specifically, nurses who were within the age group of 20–40 years [aOR = 0.25 (95% CI = 0.09–0.69), p = 0.007] and 41–60 years [aOR = 0.05 (95% CI = 0.01–0.29), p = 0.001] were significantly less likely to be knowledgeable about the preventive measures of NCIs compared to those who those aged less than 20 years. Nurses who attended in-service training or workshop were approximately 10 times more likely to be knowledgeable about preventive measures of nosocomial infection compared to those who had never attended in-service training or workshop [aOR = 9.55 (95% CI = 1.23–74.36), p = 0.031] (Table 3 ).
Recognizing the importance of NCI prevention to the healthcare system of Ghana [ 6 , 12 ], we assessed nurses’ knowledge on NCI preventive measures and its associated factors in Ghana. Our study revealed that more than two-thirds of nurses (69.2%) were not knowledgeable about NCI preventive measures. The observed proportion of nurses who were knowledge about NCI prevention measures is less when compared a previous study conducted in Ethiopia [ 10 ] where 90% of nurses had good knowledge on NCI preventive measures. Nevertheless, our findings align with a prior study conducted in Tamale, Ghana [ 21 ] which revealed that only 50% of nurses were knowledgeable about infection prevention measures, including NCIs. The observed low knowledge on NCI preventive measures among nurses poses a significant threat to patient safety. This is in the sense that patients often rely on nurses to provide safe and effective care. Therefore, having a low knowledge about NCI preventive measures implies that nurses may be involved in practices that exacerbate the risk of NCI transmission [ 13 , 15 ]. Our findings, thus, underscore a need for the Ministry of Health, Ghana Health Service and hospital administrators to intensify education and sensitization initiatives to improve nurses’ knowledge regarding NCI preventive measures.
The study revealed that having participated in an in-service training or workshop was positively associated with nurses’ knowledge on NCI preventive measures. That is, the likelihood of being knowledgeable about NCI preventive measures was significantly higher among those who had participated in an in-service training or workshop compared to those who had not participated in such initiatives. Similar findings have been reported in North-East Ethiopia [ 23 ] and Nigeria [ 25 ]. A plausible explanation for this result could be that in-service training and workshops serve as reinforcement mechanisms for existing knowledge. Even if nurses have received prior education on infection control, attending training sessions provides an opportunity to refresh their knowledge, identify areas for improvement, and correct any misconceptions or outdated practices. The repetition of key concepts and information during the training sessions has the potential to reinforce the importance of NCI prevention and increases retention of knowledge among nurses [ 25 ].
Another finding from this study was the significant association between age and nurses’ knowledge on NCI prevention. Contrary to previous studies that have shown that nurses’ knowledge on NCI prevention increases with increasing age [ 22 , 23 , 24 ], we found that older age was associated with lower odds of being knowledgeable about NCI prevention compared to those of younger age. That is, the present study challenges the existing literature that posits that older nurses tend to be more knowledgeable about NCI prevention through years of experience and working collaboratively with senior staff [ 22 ]. It is possible that younger nurses, who may have recently completed their education or training, are likely to have been exposed to more up-to-date information and guidelines regarding NCI prevention. They may have received more comprehensive training that includes the latest research, technological advancements, and evidence-based practices. In contrast, older nurses may not have had the same exposure to these updated resources, leading to a knowledge gap between the age groups. We also postulate that older nurses may be less inclined to adopt new practices or update their knowledge base, especially if they have been practicing for a long time without encountering significant issues related to NCI. This resistance to change can result in a slower uptake of new information and guidelines, hence, explaining their lower knowledge levels regarding NCI prevention.

Implications for policy and practice
The study highlights the urgent need for the Ministry of Health, Ghana Health Service, and hospital administrators to prioritize education and sensitization initiatives on NCI preventive measures for nurses. Also, the positive association between participation in in-service training or workshops and nurses’ knowledge on NCI preventive measures emphasizes the importance of these initiatives. Healthcare institutions should provide regular opportunities for nurses to attend such training sessions, as they serve as reinforcement mechanisms for existing knowledge and contribute to improved understanding and implementation of NCI prevention measures. To bridge the knowledge gap observed among older nurses, healthcare institutions should design and implement tailored training programs that specifically address their needs. These programs should focus on updating their knowledge base, addressing resistance to change, and providing them with the necessary skills to adopt current NCI preventive measures.
Strengths and limitations
The strength of this study lies in the use of appropriate methodology to estimate the sample and analyze the data. Nevertheless, there are some limitations that must be taken into consideration. As the study relied on a cross-sectional design, it is not possible to establish a causal pathway between the age and in-service training as determinants of nurses’ knowledge on preventive measure for NCIs. The quantitative approach to this study does not provide an in-depth insight into other underlying factors that might influence the observed associations. Therefore, there is a need for qualitative research to gain a more comprehensive and nuanced understanding of nurses’ knowledge on preventive measures for NCIs. Another limitation of this study is that it focused only on nurses. Hence, the findings may not reflect the current knowledge of other healthcare workers including general medical practitioners, surgeons, and laboratory technicians.
A significant proportion of nurses in Ghana lack knowledge on NCI prevention. The study concludes that age and participation in-service training or workshop are significant factors associated with nurses’ knowledge NCI prevention. These results highlight the importance of providing ongoing training and professional development opportunities to nurses to enhance their knowledge and improve their ability to prevent and control nosocomial infections. Additionally, the study emphasizes the need for targeted training programs that consider the age of nurses, to ensure that training is tailored to their specific needs and learning styles.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
Adjusted odds ratio
Crude odds ratio
Hospital Acquired Infections
Human Immunodeficiency virus
Hepatitis B Virus
Hepatitis C Virus
Infection prevention and Control
Intensive Care Unit
- Nosocomial infection
Nosocomial infections
World Health Organization
Saleem Z, Godman B, Hassali MA, Hashmi FK, Azhar F, Rehman IU. Point prevalence surveys of health-care-associated infections: a systematic review. Pathogens and Global Health. 2019;113(4):191–205.
Article CAS PubMed PubMed Central Google Scholar
World Health Organization. Prevention of hospital-acquired infections: a practical guide. World Health Organization; 2002.
Jenkins DR. Nosocomial infections and infection control. Medicine. 2017;45(10):629–33.
Article Google Scholar
Haque M, Sartelli M, McKimm J, Bakar MA. Health care-associated infections–an overview. Infection and drug resistance. 2018 Nov 11:2321–33.
Raoofi S, Pashazadeh Kan F, Rafiei S, Hosseinipalangi Z, Noorani Mejareh Z, Khani S, Abdollahi B, Seyghalani Talab F, Sanaei M, Zarabi F, Dolati Y. Global prevalence of nosocomial infection: a systematic review and meta-analysis. PLoS ONE. 2023;18(1):e0274248.
Labi AK, Obeng-Nkrumah N, Owusu E, Bjerrum S, Bediako-Bowan A, Sunkwa-Mills G, Akufo C, Fenny AP, Opintan JA, Enweronu-Laryea C, Debrah S. Multi-centre point-prevalence survey of hospital-acquired infections in Ghana. J Hosp Infect. 2019;101(1):60–8.
Article PubMed Google Scholar
Khan HA, Baig FK, Mehboob R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed. 2017;7(5):478–82.
Nimer NA. Nosocomial infection and antibiotic-resistant threat in the Middle East. Infection and drug resistance. 2022 Jan 1:631–9.
Hazard D, von Cube M, Kaier K, Wolkewitz M. Predicting potential prevention effects on hospital burden of nosocomial infections: a multistate modeling approach. Value in Health. 2021;24(6):830–8.
Bayleyegn B, Mehari A, Damtie D, Negash M. Knowledge, attitude and practice on hospital-acquired infection prevention and associated factors among healthcare workers at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Infection and drug resistance. 2021 Jan 27:259–66.
Gilbert GL, Kerridge I. Hospital infection prevention and control (IPC) and antimicrobial stewardship (AMS): dual strategies to reduce antibiotic resistance (ABR) in hospitals. Ethics and Drug Resistance: collective responsibility for global Public Health. Oct. 2020;26:89–108.
Google Scholar
Ministry of Health, Ghana. National Policy and Guidelines for Infection Prevention and Control in Health Care Settings. 2015.
Assefa J, Diress G, Adane S. Infection prevention knowledge, practice, and its associated factors among healthcare providers in primary healthcare unit of Wogdie District, Northeast Ethiopia, 2019: a cross-sectional study. Antimicrob Resist Infect Control. 2020;9(1):1–9.
Chapman HJ, Veras-Estévez BA, Pomeranz JL, Pérez-Then EN, Marcelino B, Lauzardo M. The role of powerlessness among health care workers in tuberculosis infection control. Qual Health Res. 2017;27(14):2116–27.
Ocran I, Tagoe DN. Knowledge and attitude of healthcare workers and patients on healthcare associated infections in a regional hospital in Ghana. Asian Pac J Trop Disease. 2014;4(2):135–9.
Tahiru MM, KNOWLEDGE OF AND, COMPLIANCE TO INFECTION PREVENTION. AND CONTROL AMONG NURSES IN THE NORTHERN REGIONAL HOSPITAL (Doctoral dissertation).
Chitimwango PC. Knowledge, attitudes and practices of nurses in infection prevention and control within a tertiary hospital in Zambia [Thesis]. Stellenbosch University; 2017.
Bayleyegn B, Mehari A, Damtie D, Negash M, Knowledge. Attitude and practice on hospital-acquired infection Prevention and Associated factors among Healthcare Workers at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Infect Drug Resist. 2021;14:259–66.
Article PubMed PubMed Central Google Scholar
Fawzi SE, Sleem WF, Shahien ES, Mohamed HA. Assessment of knowledge and practice regarding nosocomial infections. Port Said Sci J Nurs. 2019;6(1):83–100.
Qin YL, Bangura HS, Li B, Zhou YS, Yuan Y, Sun Y, et al. Self-reported knowledge and Practices of Healthcare Workers on Occupational exposure and Protection from Infectious Disease at the Military Hospital in Sierra Leone. Glob J Med Res. 2018;9(5):25–32.
Algarni SS, Sofar SM, Wazqar DY. Nurses’ knowledge and Practices toward Prevention of Catheter-Associated urinary tract infection at King Abdulaziz University. J Health Med Nurs. 2019;14:50–73.
Desta M, Ayenew T, Sitotaw N, Tegegne N, Dires M, Getie M. Knowledge, practice and associated factors of infection prevention among healthcare workers in Debre Markos referral hospital, Northwest Ethiopia. BMC Health Serv Res. 2018;18(1):465.
Teshager FA, Engeda EH, Worku WZ. Knowledge, practice, and Associated factors towards Prevention of Surgical Site infection among nurses working in Amhara Regional State Referral Hospitals, Northwest Ethiopia. Surg Res Pract. 2015;736–75.
Alhassan AR, Kuugbee ED, Der EM. Surgical healthcare workers knowledge and attitude on infection prevention and control: A case of tamale teaching hospital, Ghana. Canadian Journal of Infectious Diseases and Medical Microbiology. 2021;2021.
Iliyasu G, Dayyab FM, Habib ZG, Tiamiyu AB, Abubakar S, Mijinyawa MS, Habib AG. Knowledge and practices of infection control among healthcare workers in a Tertiary Referral Center in North-Western Nigeria. Ann Afr Med. 2016;15(1):34.
Download references
Acknowledgements
We would like to acknowledge all our study participants for their cooperation and voluntary participation during the data collection. We also thank the Hohoe Municipal Health Directorate for its support in the study.
This research was self-funded by the corresponding author.
Author information
Authors and affiliations.
Department of Epidemiology and Biostatistics, University of Health and Allied Sciences, Ho, Ghana
Samuel Salu
Department of Population and Health, University of Cape Coast, Cape Coast, Ghana
Joshua Okyere
School of Nursing & Midwifery, College of Health Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Department of Population and Behavioral Sciences, University of Health and Allied Sciences, Ho, Ghana
Veronica Okwuchi Charles-Unadike & Mark Kwame Ananga
You can also search for this author in PubMed Google Scholar
Contributions
SS and VOC conceived and designed the study. VOC and MKA supervised the research work in the field. SS conducted the whole research work and wrote the drafts and revised manuscript. JO thoroughly reviewed and revised the manuscript and checked the references. SS and JO finalized the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Samuel Salu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were in accordance with the Declaration of Helsinki. The University of Health and Allied Sciences Research Ethics Committee (UHAS-REC) reviewed the study and approved it with a reference [ID: UHAS-REC A.11 {79} 20–21]. Permission was also obtained from the Hohoe Municipal Health Directorate before the commencement of the study. During data collection, permission was also sought from all the in-charges of the facilities before the administration of the questionnaire. Written informed consent was obtained from all of the participants.
Consent for publication
Not applicable.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Salu, S., Okyere, J., Charles-Unadike, V.O. et al. Nurses’ knowledge on nosocomial infections preventive measures and its associated factors in Ghana: a cross-sectional study. BMC Health Serv Res 23 , 941 (2023). https://doi.org/10.1186/s12913-023-09942-2
Download citation
Received : 26 May 2023
Accepted : 21 August 2023
Published : 01 September 2023
DOI : https://doi.org/10.1186/s12913-023-09942-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Prevention Measures
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
- Open access
- Published: 23 May 2024
Incidence and mortality of community-acquired and nosocomial infections in Japan: a nationwide medical claims database study
- Nozomi Takahashi 1 , 2 ,
- Taro Imaeda 2 ,
- Takehiko Oami 2 ,
- Toshikazu Abe 3 , 4 ,
- Nobuaki Shime 5 , 6 ,
- Kosaku Komiya 6 , 7 ,
- Hideki Kawamura 6 , 8 ,
- Yasuo Yamao 2 ,
- Kiyohide Fushimi 9 &
- Taka‑aki Nakada 2 , 6
BMC Infectious Diseases volume 24 , Article number: 518 ( 2024 ) Cite this article
166 Accesses
Metrics details
It is important to determine the prevalence and prognosis of community-acquired infection (CAI) and nosocomial infection (NI) to develop treatment strategies and appropriate medical policies in aging society.
Patients hospitalized between January 2010 and December 2019, for whom culture tests were performed and antibiotics were administered, were selected using a national claims-based database. The annual trends in incidence and in-hospital mortality were calculated and evaluated by dividing the patients into four age groups.
Of the 73,962,409 inpatients registered in the database, 9.7% and 4.7% had CAI and NI, respectively. These incidences tended to increase across the years in both the groups. Among the patients hospitalized with infectious diseases, there was a significant increase in patients aged ≥ 85 years (CAI: + 1.04%/year and NI: + 0.94%/year, P < 0.001), while there was a significant decrease in hospitalization of patients aged ≤ 64 years (CAI: -1.63%/year and NI: -0.94%/year, P < 0.001). In-hospital mortality was significantly higher in the NI than in the CAI group (CAI: 8.3%; NI: 14.5%, adjusted mean difference 4.7%). The NI group had higher organ support, medical cost per patient, and longer duration of hospital stay. A decreasing trend in mortality was observed in both the groups (CAI: -0.53%/year and NI: -0.72%/year, P < 0.001).
The present analysis of a large Japanese claims database showed that NI is a significant burden on hospitalized patients in aging societies, emphasizing the need to address particularly on NI.
Peer Review reports
Infectious diseases play an important role in hospital admissions, mortality, and medical costs worldwide [ 1 , 2 ]. Among these, bacterial and fungal infections can cause sepsis, which is characterized by organ dysfunction and leads to a worse prognosis in these patients [ 3 , 4 , 5 ]. Several methods have been formulated to classify these infections for epidemiological investigation, including the pathogen, focus of infection, and setting of onset. According to setting of onset, infections can be divided into community-acquired infection (CAI) or nosocomial infection (NI) [ 6 ]. The setting of infection onset is crucial because these two settings differ in terms of pathogenic organs, type of infection, therapeutic strategy, and clinical course [ 7 , 8 , 9 ].
There have been several epidemiological studies on infectious diseases, including specific infectious sites or backgrounds, as well as individual pathogens, which have provided insight into the disease and its burden and informed future policy decisions [ 2 , 10 , 11 ]. However, few studies have reported the current situation and trends in the incidence and clinical outcomes of patients with bacterial and fungal infections from the perspective of onset of infection, despite many studies that have reported on sepsis showing increasing trends of incidence and worse clinical outcomes in patients with NI [ 12 , 13 , 14 ]. Considering the appropriate management of medical resources and decisions regarding healthcare policy, understanding the current situation of bacterial and fungal infections along with their trends of prevalence and prognosis in these two settings of infection onset should be considered important factors, along with other factors such as age and focus of infection [ 15 ]. In particular, since Japan has had the world's largest aging population since 2005, the original Japanese analysis could make an important contribution to future healthcare policy in a country with aging population.
The aim of this study was to describe the epidemiology of the incidence and characteristics of CAI and NI, and to verify the hypothesis that NI has a higher mortality rate than CAI in Japan using data from the national claims-based database, which has more than 70 million inpatients.
Study design and data source
We conducted a retrospective observational study using the Japanese Diagnosis Procedure Combination (DPC) system, which consists of large administrative claims data on reimbursement, covering more than 80% of acute care hospitals [ 16 , 17 ]. This DPC system contains two types of codes, diagnostic and procedure codes, in addition to basic information such as age, sex, and patient outcomes. Each required code is registered for each hospitalized patient on a daily basis. The International Classification of Diseases, 10th Revision (ICD-10) codes were used as the diagnostic code, in addition to the name of the disease that was the main cause of hospitalization, comorbidities at the time of admission, and new names of diseases added during hospitalization, which were registered with up to six codes on admission and four codes after admission. The procedure code was originally defined in Japanese, and all procedure devices needed during hospitalization, including organ support or drugs used regardless of the route of administration, were coded and registered [ 18 , 19 ]. Data of patients admitted between January 2011 and December 2019 were extracted from the DPC database. This study was approved by the Institutional Review Board of Chiba University Graduate School of Medicine and was performed in accordance with the tenets of Declaration of Helsinki. The need for informed consent was waived according to the review board (approval number, 3429).
Definition and data collection
All inpatient datasets with codes for both culture tests and antibiotic administration via intravenous infusion were extracted from the DPC database. CAI group was defined as the group of patients who received antibiotics within two days of hospitalization, and NI group was defined as the group of patients who received antibiotics after the third day of hospitalization. To exclude prophylactic administration of antibiotics, we extracted those who received antibiotics for ≥ 3 days, but we also included patients who received antibiotics for < 3 days in the context of transferred and deceased patients. Accordingly, the CAI group satisfied the following conditions: (i) antibiotic administration commenced within two days of hospitalization and continued for at least four days, or (ii) antibiotics were started within two days of hospitalization and continued for less than four days, and the outcome was death or transfer. The NI group satisfied the following conditions: (i) antibiotic administration commenced at least three days after hospitalization and continued for at least four days, or (ii) antibiotics were started at least three days after hospitalization and continued for less than four days, and the outcome was death or transfer.
Basic patient characteristics, including age, sex, along with admission and discharge dates, were available in DPC. Whether or not the patients were admitted to the ICU could be referenced from the procedure code. Comorbidities and focus of infection were classified and extracted using ICD-10 codes (Supplementally file; Table S 1 , S 2 ). Comorbidities included malignant tumor, hypertension, diabetes mellitus, heart failure, cerebrovascular disease, chronic respiratory disease, ischemic heart disease, and chronic renal failure. According to the current definition of sepsis, we defined sepsis using a combination of ICD-10 and procedure codes that indicated acute organ dysfunction, as previously reported [ 20 ] (Supplementally file; Table S 3 ). Organ supportive therapy was investigated using procedure codes, including oxygen therapy, mechanical ventilation, vasopressor use, or renal replacement therapy. The duration of administration of antibiotics agents and therapeutic drugs was extracted from the procedure codes. Antibiotics agents were based on the international classification [ 21 ]. It should be noted that organ dysfunction and organ supportive therapy were extracted during the whole period of hospitalization, and the contribution of infection to length of hospitalization and cost was unknown. Therefore, the causal association to the corresponding infectious disease was unknown and should be interpreted as indicative of the nature of each patient group.
Medical cost in Japanese yen, which was calculated as the summary of medical fees including medical procedure, fee of drugs, or medical material cost, was converted into U.S. dollars in accordance with the exchange rate on January 14, 2023 (127 yen = $1 USD).
Statistical analysis
The primary outcomes were annual changes in hospitalization and mortality rates comparing CAI and NI. Secondary outcomes were in-hospital mortality in all investigated years, ICU admission, organ support, duration of initial antibiotics and all antibiotics (days), length of hospital stay, and medical cost. Primary outcomes were analyzed in the four age groups according to the criteria previously reported for older adult patients [ 22 , 23 ]. Hospitalization rates were analyzed separately for each focus of infection in a subgroup analysis. Patients with repeat hospitalizations were excluded from the analysis of in-hospital mortality for accurate analysis of the factors associated with death. Comorbidities were scored using the Elixhauser comorbidity scores for mortality analysis [ 24 , 25 ].
Cochran-Armitage test was used for 10-year trend analyses of CAI and NI prevalence relative to total hospitalizations and in-hospital mortality. Analysis of covariance was used to test the difference in the slope of linear regression analysis of the 10-year trend between the two groups obtained using the least-squares method.
Associations between CAI or NI and the outcomes were analyzed using a Poisson regression generalized linear mixed-effects model adjusted for patient age, sex, and Elixhauser comorbidity scores. Unadjusted and adjusted differences in outcomes were reported with 95% confidence intervals (CIs).
Continuous variables were presented as medians with interquartile ranges. Categorical variables were presented as numbers and percentages. Statistical significance was set at P < 0.05. Analyses were performed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-roject.org/ ) and PRISM version 8 (GraphPad Software, Inc., La Jolla, California, USA).
Of the 73,962,409 hospitalized cases recorded between 2010–2019, a total of 7,145,755 (9.7%) and 3,473,513 (4.7%) patients met the definitions of CAI and NI, respectively (Fig. 1 ).

Flow chart demonstrating the selection of study population
Regarding the baseline characteristics, patients with CAI were significantly younger (CAI: 74 years and NI: 75 years, P < 0.001) and comprised lower proportion of males (CAI: 3,965,319 patients [55.5%] and NI: 2,010,875 patients [57.9%], P < 0.001) in comparison to than that of patients with NI (Table 1 ). The proportion of comorbidities was lower in the CAI group than in the NI group for all factors except chronic pulmonary disease (CAI: 737,483 patients [10.3%] and NI: 247,372 patients [7.1%], P < 0.001). Respiratory infection was the most common focus of infection in the CAI group (2,447,113 patients [34.2%]), whereas abdominal infection was the most common infection in the NI group (923,357 patients [26.6%]). The rate of sepsis was nearly twice as high in the NI group (CAI: 784,123 patients [11.0%] and NI: 692,266 patients [19.9%], P < 0.001). Moreover, organ dysfunction was more common in the NI group in comparison to the CAI group (CAI:1,048,022 patients [14.7%] and NI: 814,034 patients [23.4%], P < 0.001). The duration of administration of initial and all antibiotics were longer in the NI group than in the CAI group (initial antibiotics, CAI: 4 days and NI: 7 days, P < 0.001; all antibiotics, CAI: 6 days and NI: 12 days, P < 0.001).
The annual proportion of patients with CAI and NI across inpatients significantly increased over the years (CAI: + 0.49%/year, P < 0.001; NI: + 0.15%/year, P = 0.001), and the difference in trends between the two groups was significant ( P = 0.0012) (Fig. 2 A). Among the patients hospitalized with infectious diseases, in both groups, there was a significant decrease in hospitalization in patients aged ≤ 64 years (CAI: -1.63%/year, P < 0.001 and NI: -0.94%/year, P < 0.001), while there was a significant increase in patients aged ≥ 85 years (CAI: + 1.04%/year, P < 0.001; NI: + 0.94%/year, P < 0.001) (Fig. 2 B, C). Patients aged ≥ 65 years and < 75 years, and aged ≥ 75 years and < 85 showed significant increase in only CAI. This trend was also observed when the ≥ 75 years group was integrated (Supplementary file; Figure S 1 ). In terms of trends by focus of infection, respiration decreased significantly in CAI but not in NI (CAI: -0.57%/year, P = 0.0031 and NI: P = 0.081), and genitourinary infection increased significantly in both groups (CAI: + 0.46%/year, P < 0.001 and NI: + 0.45%/year, P < 0.001) (Supplementary file; Figure S 2 ).

Annual changes in hospitalization and in-hospital mortality by infectious disease. A Incidence of hospitalization across all inpatients (community acquired infection: + 0.49%/year [95%CI; 0.31%–0.67%], adjusted R 2 = 0.81, P < 0.001 and nosocomial infection: + 0.15%/year [95%CI; 0.08%–0.23%], adjusted R 2 = 0.71, P = 0.001); B Proportion of hospitalization in community-acquired infections by age subgroups (≤ 64 years: -1.63%/year [95%CI; -1.77%– -1.48%], adjusted R 2 = 0.99, P < 0.001; 65–74 years: + 0.26%/year [95%CI; 0.11%–0.41%], adjusted R 2 = 0.63, P = 0.0037; 75–84 years: + 0.33%/year [95%CI; 0.29%–0.37%], adjusted R 2 = 0.97, P < 0.001; ≥ 85 years: + 1.04%/year [95%CI; 0.96%–1.12%], adjusted R 2 = 0.99, P < 0.001); C Proportion of hospitalization in nosocomial infections by age subgroups (≤ 64 years: -0.95%/year [95%CI; -1.13%– -0.76%], adjusted R 2 = 0.94, P < 0.001; 65–74 years: P = 0.95; 75–84 years: P = 0.99; ≥ 85 years: + 0.94%/year [95%CI; 0.76%–1.12%], adjusted R 2 = 0.94, P < 0.001); D In-hospital mortality of community-acquired and nosocomial infection (community acquired infection: -0.53%/year [95%CI; -0.79%– -0.27%], adjusted R 2 = 0.71, P = 0.0015 and nosocomial infection: -0.72%/year [95%CI; -1.05%– -0.38%], adjusted R 2 = 0.72, P = 0.0011); E Community-acquired infections by age subgroups (≤ 64 years: -0.15%/year [95%CI; -0.25%– -0.05%], adjusted R 2 = 0.56, P = 0.075; 65–74 years: -0.89%/year [95%CI; -1.24%– -0.54%], adjusted R 2 = 0.79, P < 0.001; 75–84 years: -1.02%/year [95%CI; -1.38%– -0.66%], adjusted R 2 = 0.82, P < 0.001; ≥ 85 years: -1.15%/year [95%CI; -1.61%– -0.69%], adjusted R 2 = 0.78, P < 0.001); F Nosocomial infections by age subgroups (≤ 64 years: -0.44%/year [95%CI; -0.64%– -0.24%], adjusted R 2 = 0.73, P = 0.001; 65–74 years: -0.86%/year [95%CI; -1.23%– -0.48%], adjusted R 2 = 0.75, P = 0.001; 75–84 years: -1.01%/year [95%CI; -1.40%– -0.62%], adjusted R 2 = 0.79, P < 0.001; ≥ 85 years: -1.04%/year [95%CI; -1.48%– -0.60%], adjusted R . 2 = 0.76, P < 0.001)
The 30-day mortality and in-hospital mortality was significantly higher in the NI group than in the CAI group after adjusting for age, sex, and Elixhauser comorbidity score (30-day mortality: unadjusted mean difference, 1.3% [95%CI, 1.2–1.3]; adjusted mean difference, 1.0% [95%CI; 1.0–1.1], in-hospital mortality: unadjusted mean difference, 6.2% [95%CI, 6.2–6.3]; adjusted mean difference, 4.7% [95%CI; 4.7–4.7]) (Table 2 ). Even after excluding transfers, both 30-day mortality (CA; 259,993/4,229,528patisnts [6.1%], NI; 151,470/1,717,715patients [8.8%]) and in-hospital mortality (CA; 413,513/4,229,528patisnts [9.8%], NI; 336,805/1,717,715patients [19.6%]) were both significantly higher in the NI group ( P < 0.001). The ICU admission rate was significantly higher in the NI group (unadjusted mean difference, 3.0%; adjusted mean difference, 2.3%), but it should be noted that the causal relationship between ICU admission and infection and the timing of onset of infection were unknown in this analysis. The annual in-hospital mortality significantly decreased in both CAI and NI (CAI: -0.53%/year, P = 0.0015 and NI: -0.72%/year, P = 0.0011) (Fig. 2 D). In terms of age group, the in-hospital mortality for both CAI and NI significantly decreased in the four age groups: ≥ 65 and < 75 years, ≥ 75 and < 85 years, and ≥ 85 years (Fig. 2 E, F, Supplementary file; Figure S 1 ). NI had a higher in-hospital mortality rate than CAI for all infection foci ( P < 0.001) (Table 3 ). In each infection focus, respiratory infections had the highest mortality in both groups (CAI: 154,566 patients [8.8%]; NI: 115,117 patients [18.4%]), followed by endocarditis/circulatory infections in CAI (4,417 patients [7.9%]) and abdominal infections in NI (86,669 patients [13.4%]). Liver dysfunction was associated with the highest mortality for both CAI and NI (CAI: 3,072 patients [32.3%]; NI: 2,679 patients [41.7%]), and all organ dysfunctions, except hematological dysfunction, were associated with significantly higher mortality for NI ( P < 0.001) (Table 3 ). The central nervous system mortality rate did not decrease in either group (CAI, P = 0.38; NI, P = 0.062) (Supplementary file; Figure S 3 ).
Furthermore, NI had higher organ support (unadjusted mean difference 3.0% [95%CI; 2.9–3.0]; adjusted mean difference 2.3% [95%CI; 2.2–2.3]), medical cost per patient (unadjusted mean difference $9,210 [95%CI; 9,171–9,250]; adjusted mean difference $9,209 [95%CI; 9,147–9,242]), and longer duration of hospital stay (unadjusted mean difference 23 days [95%CI; 23–24]; adjusted mean difference 23 days [95%CI; 22–23]).
The present analysis of a large Japanese claims database consisting of more than 10 million patients showed that the incidences of both CAI and NI tended to increase across the years especially in patients aged ≥ 85 years. Moreover, in-hospital mortality was found to be significantly higher in the NI group compared to the CAI group, while there was a decreasing trend of mortality in both groups.
CAI and NI differ in background disease, cause, treatment including antimicrobial therapy, and prevention, which indicates the importance of understanding the characteristics, prevalence, and mortality of each [ 26 , 27 ]. The present study is the most extensive epidemiological study on the incidence and mortality of CAI and NI worldwide till date, and at the same time, the most comprehensive nationwide study conducted in Japan. The median age of the study population was approximately 75 years, which indicates that Japan is the most aged country in the world. Therefore, these results suggest the importance of future healthcare policies for older adults and nosocomial infections in an aging society.
Our results indicate that the incidence of both infections increased. Few studies have reported changes in the prevalence of CAI and NI, although some have only reported the prevalence of NI. A 2010 Centers for Disease Control and Prevention (CDC) prevalence study of 183 hospitals in the US reported that approximately 4% of hospitalized patients had NI, which is in concordance with our findings [ 28 ]. Furthermore, a previous study that conducted surveys in 2002 and 2009 to measure the prevalence and characteristics of NI in Canadian hospitals showed an 11.7% increase in the prevalence of NI, with the increment being maximum in terms of urinary tract infections during this period [ 29 ]. Additionally, a meta-analysis of the global prevalence of NI from 2000–2021 reported an annual increase of 0.06% [ 30 ]. This trend is similar to our findings, suggesting that the incidence of NI has been increasing despite appropriate infection prevalence. However, our results showed a decrease in the last year of data collection, suggesting that the prevalence may decrease further in the future. However, estimating changes in hospitalizations due to infectious diseases after 2020 is an arduous task as COVID-19 changed the landscape of hospitalizations due to infectious diseases [ 31 ]. It should be noted that the incidence of patients aged ≥ 85 years, called the oldest-old, among those hospitalized for infectious diseases clearly increased in both groups (1.04%/year for CAI, 0.94%/year for NI). Statistics in Japan shows that the population aged ≥ 85 years increased from 3.8 million in 2010 to 6.2 million in 2020 (6.3%/year increase) [ 32 ], which may reflect the aging of Japanese population.
In-hospital mortality was nearly twice as high in the NI group as in the CAI group regardless of the focus of infection, whereas the incidence was twice as high in the CAI group in comparison to the NI group. Previous studies on sepsis have also shown that the mortality rate for NI was higher than that for CAI, and Japanese studies have reported the same two-fold increase [ 12 , 13 , 14 , 33 , 34 , 35 ]. A Canadian study in 2017 showed that the overall mortality rate among patients with at least one nosocomial infection was 16.6%, which is similar to our finding of 14.5% [ 36 ]. Some studies have suggested that the underlying conditions of patients, including immunosuppression, type and severity of the infection, in-hospital interventions, and increased bacteremia-induced septic shock, are associated with worse outcomes in NI [ 37 ]. In our study, comorbidities excluding chronic lung disease were significantly higher, and sepsis was approximately twice as common in the NI group, which may have contributed to the high mortality rate. In addition, ICU admission and organ support were significantly higher in the NI group after adjustment for other factors, reflecting the higher severity of NI compared to CAI, indicating that NI consumes more medical resources and creates a burden. Conversely, the incidence increased in both groups, but the mortality rate decreased over time. This may be due to the fact that the incidence of respiratory infections, which have a high mortality rate, has decreased, whereas the incidence of genitourinary infections, which have a low mortality rate, has increased. Mortality rates decreased for each infection, especially for abdominal infections. This could be due to appropriate antimicrobial stewardship and decrease in the number of antimicrobial-resistant organisms as a result of the development of guidelines for specific focus or sepsis, which are associated with high mortality and spread of antimicrobial stewardship in clinical practice [ 38 , 39 ]. In Japan, routine pneumococcal vaccination of elderly people aged 65 years or older and patients at high risk of the infectious disease began in 2014. In the same year, the Japanese government issued a ministerial ordinance directing nosocomial infection control measures, which became an additional target for the medical reimbursement. These medical policies may have contributed to the change in the mortality rate. On the other hand, the incidence continues to increase, especially among the elderly, suggesting the need to implement medical policies to prevent both community-acquired and nosocomial infections, with particular emphasis on the elderly aged 75 years or older. This study may serve as a basis for publicizing such policies. However, this study did not clearly identify the causes of mortality, and future research on related factors is warranted. Furthermore, it should be noted that linear regression may not necessarily be optimal for 10-year trends in incidence and mortality of the present study. Although the Cochran-Armitage test, which is a pre-defined method for trend analysis, uses a regression line, the actual annual proportion of patients with CAI and NI across inpatients may have peaked from 2017 to 2018 and then started to decline. Also, the in-hospital mortality for both CAI and NI may have changed since around 2014. Further long-term data accumulation would be helpful to establish appropriate analytical methods and models, and the trend results should be interpreted with caution.
The present study had a few limitations. First, this study includes only some bacterial and fungal infections in the broad sense of the term, and does not include viral or special infections, limiting information on treatment methods. Furthermore, the database does not include information on the microorganisms causing the infection or the culture results, so it is uncertain whether they were truly causing the infection. Therefore, the potential selection bias occurred in the selection of infections, and is an issue in research design and methodology, while these findings may contribute useful information for the interpretation of prognosis, as NI is expected to be associated with a higher proportion of bacteria that are resistant to antimicrobial agents. Second, the last year of data used in this analysis was just before the COVID-19 pandemic and did not reflect COVID-19, which continues to have a significant impact worldwide. The impact of COVID-19 on community-acquired pneumonia should be considered in future data collection and analysis as it may have caused particularly large changes in CAI. Third, patients who were administered antimicrobials within two days of transfer were included in the CAI group. We were unable to distinguish this because it was not possible to refer to the pretransfer records in conjunction with each other. Fourth, some nosocomial infections, such as catheter-related and surgical site infections, were not categorized as the focus of infection in this study. This is because ICD-10 defines T81.4 as 'Infection following a procedure, not elsewhere classified', which may include these diseases and other abscesses regardless of site. Similarly, T82.7 is defined as 'Infection and inflammatory reaction due to other cardiac and vascular devices, implants, and grafts', was not included in the focus of infection since it may include infections such as catheter and surgical site infections. It is difficult to completely isolate the focus of infection using only the ICD-10 codes, which is a limitation of the method used in this study. Fifth, the results found on the incidence and mortality rate of CAI and NI might be specific to the Japanese medical environment including medical insurance system and the access to healthcare, which leads that these are not necessarily applicable to other regions or countries. Therefore, it is important to adapt the results to each local health care policy and situation. Sixth, although this study included more than 80% hospitals in Japan that were members of the DPC system, the number of eligible hospitals changed over the 10-year period, showing an increasing trend (Supplementary file; Table S 4 ). Hospitals with a large number of beds did not show an increasing trend, while hospitals with a small number of beds showed an increasing trend. Therefore, it should be noted that the decrease in mortality in particular may have reflected an increase in the proportion of patients with non-severe illnesses included, which has limitations in its interpretation.
Conclusions
Japanese claims database of more than 10 million patients revealed an increase in trends of hospitalization in both CAI and NI groups especially in patients aged ≥ 85 years and a significant burden in hospitalized patients with NI in the aging societies. In-hospital mortality was significantly higher in the NI group than that in the CAI group, whereas there was a decreasing trend in mortality in both the groups.
Availability of data and materials
The datasets used during the study are available from the corresponding author on reasonable request.
Abbreviations
Community-acquired infection
Nosocomial infection
Intensive care unit
Diagnosis procedure combination
International statistical classification of diseases and related health problems 10th revision
Confidence interval
Sequential organ failure assessment
Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. https://doi.org/10.1016/S0140-6736(20)30925-9 .
Article Google Scholar
Collaborators GBDAR. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400(10369):2221–2248. https://doi.org/10.1016/S0140-6736(22)02185-7 .
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10. https://doi.org/10.1001/jama.2016.0287 .
Article CAS PubMed PubMed Central Google Scholar
Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–11. https://doi.org/10.1016/s0140-6736(19)32989-7 .
Article PubMed PubMed Central Google Scholar
Jawad I, Lukšić I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health. 2012;2(1): 010404. https://doi.org/10.7189/jogh.02.010404 .
Doron S, Gorbach SL. Bacterial Infections: Overview. International Encyclopedia of Public Health. 2008:273–82. https://doi.org/10.1016/b978-012373960-5.00596-7 .
Magill SS, O’Leary E, Janelle SJ, et al. Changes in prevalence of health care-associated infections in U.S. Hospitals N Engl J Med. 2018;379(18):1732–44. https://doi.org/10.1056/NEJMoa1801550 .
Article PubMed Google Scholar
Rüden H, Gastmeier P, Daschner FD, Schumacher M. Nosocomial and community-acquired infections in Germany. Summary of the results of the First National Prevalence Study (NIDEP). Infection. 1997;25(4):199–202. https://doi.org/10.1007/bf01713142 .
Ott E, Saathoff S, Graf K, Schwab F, Chaberny IF. The prevalence of nosocomial and community acquired infections in a university hospital: an observational study. Dtsch Arztebl Int. 2013;110(31–32):533–40. https://doi.org/10.3238/arztebl.2013.0533 .
Dwyer LL, Harris-Kojetin LD, Valverde RH, et al. Infections in long-term care populations in the United States. J Am Geriatr Soc. 2013;61(3):342–9. https://doi.org/10.1111/jgs.12153 .
Baker RE, Mahmud AS, Miller IF, et al. Infectious disease in an era of global change. Nat Rev Microbiol. 2022;20(4):193–205. https://doi.org/10.1038/s41579-021-00639-z .
Article CAS PubMed Google Scholar
Tonai M, Shiraishi A, Karumai T, et al. Hospital-onset sepsis and community-onset sepsis in critical care units in Japan: a retrospective cohort study based on a Japanese administrative claims database. Crit Care. 2022;26(1):136. https://doi.org/10.1186/s13054-022-04013-0 .
Page DB, Donnelly JP, Wang HE. Community-, healthcare-, and hospital-acquired severe sepsis hospitalizations in the university healthsystem consortium. Crit Care Med. 2015;43(9):1945–51. https://doi.org/10.1097/ccm.0000000000001164 .
Rhee C, Wang R, Zhang Z, Fram D, Kadri SS, Klompas M. Epidemiology of hospital-onset versus community-onset sepsis in U.S. Hospitals and association with mortality: a retrospective analysis using electronic clinical data. Crit Care Med. 2019;47(9):1169–76. https://doi.org/10.1097/ccm.0000000000003817 .
Šuljagić V, Bajčetić M, Mioljević V, et al. A nationwide assessment of the burden of healthcare-associated infections and antimicrobial use among surgical patients: results from Serbian point prevalence survey, 2017. Antimicrob Resist Infect Control. 2021;10(1):47. https://doi.org/10.1186/s13756-021-00889-9 .
Hayashida K, Murakami G, Matsuda S, Fushimi K. History and Profile of Diagnosis Procedure Combination (DPC): Development of a real data collection system for acute inpatient care in Japan. J Epidemiol. 2021;31(1):1–11. https://doi.org/10.2188/jea.JE20200288 .
Miyamoto Y, Aso S, Iwagami M, et al. Association Between IV thiamine and mortality in patients with septic shock: a nationwide observational study. Crit Care Med. 2020;48(8):1135–9. https://doi.org/10.1097/ccm.0000000000004394 .
Iwagami M, Yasunaga H, Doi K, et al. Postoperative polymyxin B hemoperfusion and mortality in patients with abdominal septic shock: a propensity-matched analysis. Crit Care Med. 2014;42(5):1187–93. https://doi.org/10.1097/ccm.0000000000000150 .
Sasabuchi Y, Yasunaga H, Matsui H, et al. The volume-outcome relationship in critically Ill patients in relation to the ICU-to-hospital bed ratio. Crit Care Med. 2015;43(6):1239–45. https://doi.org/10.1097/ccm.0000000000000943 .
Sasabuchi Y, Matsui H, Lefor AK, Fushimi K, Yasunaga H. Risks and benefits of stress ulcer prophylaxis for patients with severe sepsis. Crit Care Med. 2016;44(7):e464–9. https://doi.org/10.1097/ccm.0000000000001667 .
Zanichelli V, Sharland M, Cappello B, et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull World Health Organ. 2023;101(4):290–6. https://doi.org/10.2471/blt.22.288614 .
Article PubMed Central Google Scholar
Campion EW. The oldest old. N Engl J Med. 1994;330(25):1819–20. https://doi.org/10.1056/nejm199406233302509 .
Guidet B, Leblanc G, Simon T, et al. Effect of systematic intensive care unit triage on long-term mortality among critically Ill Elderly patients in france: a randomized clinical trial. JAMA. 2017;318(15):1450–9. https://doi.org/10.1001/jama.2017.13889 .
Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: The AHRQ elixhauser comorbidity index. Med Care. 2017;55(7):698–705. https://doi.org/10.1097/mlr.0000000000000735 .
Sharma N, Schwendimann R, Endrich O, Ausserhofer D, Simon M. Comparing Charlson and Elixhauser comorbidity indices with different weightings to predict in-hospital mortality: an analysis of national inpatient data. BMC Health Serv Res. 2021;21(1):13. https://doi.org/10.1186/s12913-020-05999-5 .
Boev C, Kiss E. Hospital-acquired infections: current trends and prevention. Crit Care Nurs Clin North Am. 2017;29(1):51–65. https://doi.org/10.1016/j.cnc.2016.09.012 .
Hazard D, von Cube M, Kaier K, Wolkewitz M. Predicting potential prevention effects on hospital burden of nosocomial infections: a multistate modeling approach. Value Health. 2021;24(6):830–8. https://doi.org/10.1016/j.jval.2021.02.002 .
Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–208. https://doi.org/10.1056/NEJMoa1306801 .
Taylor G, Gravel D, Matlow A, et al. Assessing the magnitude and trends in hospital acquired infections in Canadian hospitals through sequential point prevalence surveys. Antimicrob Resist Infect Control. 2016;5:19. https://doi.org/10.1186/s13756-016-0118-3 .
Raoofi S, Pashazadeh Kan F, Rafiei S, et al. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS ONE. 2023;18(1): e0274248. https://doi.org/10.1371/journal.pone.0274248 .
Rennert-May E, Leal J, Thanh NX, et al. The impact of COVID-19 on hospital admissions and emergency department visits: A population-based study. PLoS ONE. 2021;16(6): e0252441. https://doi.org/10.1371/journal.pone.0252441 .
Nakatani H. Population aging in Japan: policy transformation, sustainable development goals, universal health coverage, and social determinates of health. Glob Health Med. 2019;1(1):3–10. https://doi.org/10.35772/ghm.2019.01011 .
Dabar G, Harmouche C, Salameh P, et al. Community- and healthcare-associated infections in critically ill patients: a multicenter cohort study. Int J Infect Dis. 2015;37:80–5. https://doi.org/10.1016/j.ijid.2015.05.024 .
Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the United States-an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018;46(12):1889–97. https://doi.org/10.1097/ccm.0000000000003342 .
Jones SL, Ashton CM, Kiehne LB, et al. Outcomes and resource use of sepsis-associated stays by presence on admission, severity, and hospital type. Med Care. 2016;54(3):303–10. https://doi.org/10.1097/mlr.0000000000000481 .
Mitchell R, Taylor G, Rudnick W, et al. Trends in health care-associated infections in acute care hospitals in Canada: an analysis of repeated point-prevalence surveys. CMAJ. 2019;191(36):E981-e988. https://doi.org/10.1503/cmaj.190361 .
Matta R, Hallit S, Hallit R, Bawab W, Rogues AM, Salameh P. Epidemiology and microbiological profile comparison between community and hospital acquired infections: A multicenter retrospective study in Lebanon. J Infect Public Health May-Jun. 2018;11(3):405–11. https://doi.org/10.1016/j.jiph.2017.09.005 .
Gu Y, Fujitomo Y, Ohmagari N. Outcomes and Future Prospect of Japan's National Action Plan on Antimicrobial Resistance (2016–2020). Antibiotics (Basel). 2021;10(11). https://doi.org/10.3390/antibiotics10111293 .
Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–64. https://doi.org/10.1086/649554 .
Download references
Acknowledgements
Not applicable.
No funding and support.
Author information
Authors and affiliations.
Centre for Heart Lung Innovation, St. Paul’s Hospital, The University of British Columbia, 1081 Burrard Street, Vancouver, BC, V6Z 1Y6, Canada
Nozomi Takahashi
Department of Emergency and Critical Care Medicine, Chiba University Graduate School of Medicine, Chiba, Japan
Nozomi Takahashi, Taro Imaeda, Takehiko Oami, Yasuo Yamao & Taka‑aki Nakada
Health Services Research and Development Center, University of Tsukuba, Tsukuba, Japan
Toshikazu Abe
Department of Emergency and Critical Care Medicine, Tsukuba Memorial Hospital, Tsukuba, Japan
Department of Emergency and Critical Care Medicine, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan
Nobuaki Shime
Ad Hoc Committee On Clinical Research Using DPC, The Japanese Association for Infectious Diseases, Tokyo, Japan
Nobuaki Shime, Kosaku Komiya, Hideki Kawamura & Taka‑aki Nakada
Respiratory Medicine and Infectious Diseases, Faculty of Medicine, Oita University, Oita, Japan
Kosaku Komiya
Department of Infection Control and Prevention, Kagoshima University Hospital, Kagoshima, Japan
Hideki Kawamura
Department of Health Policy and Informatics, Tokyo Medical and Dental University Graduate School of Medical and Dental Sciences, Tokyo, Japan
Kiyohide Fushimi
You can also search for this author in PubMed Google Scholar
Contributions
NT, TI, NS and TN contributed to the conception and study design. NT performed statistical analysis and drafting the manuscript, and NT, TO and TA contributed to the interpretation of data. All authors contributed to subsequent drafts and approved manuscript. NT and KF had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Correspondence to Nozomi Takahashi .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Institutional Review Board of Chiba University Graduate School of Medicine (approval number, 3429), and performed in accordance with the tenets of Declaration of Helsinki. The need for informed consent was waived according to the Institutional Review Board of Chiba University Graduate School of Medicine.
Consent for publication
Competing interests.
The authors declared no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Takahashi, N., Imaeda, T., Oami, T. et al. Incidence and mortality of community-acquired and nosocomial infections in Japan: a nationwide medical claims database study. BMC Infect Dis 24 , 518 (2024). https://doi.org/10.1186/s12879-024-09353-6
Download citation
Received : 20 December 2023
Accepted : 26 April 2024
Published : 23 May 2024
DOI : https://doi.org/10.1186/s12879-024-09353-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Infectious disease
- Hospital-acquired infections
- Organ dysfunction
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 31 May 2024
The real-time infection hospitalisation and fatality risk across the COVID-19 pandemic in England
- Thomas Ward ORCID: orcid.org/0000-0001-8801-747X 1 ,
- Martyn Fyles 1 ,
- Alex Glaser 1 ,
- Robert S. Paton 1 ,
- William Ferguson ORCID: orcid.org/0000-0002-1327-4997 1 &
- Christopher E. Overton 1 , 2
Nature Communications volume 15 , Article number: 4633 ( 2024 ) Cite this article
Metrics details
- Epidemiology
- Statistical methods
- Viral infection
The COVID-19 pandemic led to 231,841 deaths and 940,243 hospitalisations in England, by the end of March 2023. This paper calculates the real-time infection hospitalisation risk (IHR) and infection fatality risk (IFR) using the Office for National Statistics Coronavirus Infection Survey (ONS CIS) and the Real-time Assessment of Community Transmission Survey between November 2020 to March 2023. The IHR and the IFR in England peaked in January 2021 at 3.39% (95% Credible Intervals (CrI): 2.79, 3.97) and 0.97% (95% CrI: 0.62, 1.36), respectively. After this time, there was a rapid decline in the severity from infection, with the lowest estimated IHR of 0.32% (95% CrI: 0.27, 0.39) in December 2022 and IFR of 0.06% (95% CrI: 0.04, 0.08) in April 2022. We found infection severity to vary more markedly between regions early in the pandemic however, the absolute heterogeneity has since reduced. The risk from infection of SARS-CoV-2 has changed substantially throughout the COVID-19 pandemic with a decline of 86.03% (80.86, 89.35) and 89.67% (80.18, 93.93) in the IHR and IFR, respectively, since early 2021. From April 2022 until March 2023, the end of the ONS CIS study, we found fluctuating patterns in the severity of infection with the resumption of more normative mixing, resurgent epidemic waves, patterns of waning immunity, and emerging variants that have shown signs of convergent evolution.
Similar content being viewed by others

Cryptic transmission of SARS-CoV-2 and the first COVID-19 wave
Disease burden and clinical severity of the first pandemic wave of COVID-19 in Wuhan, China
Reconstruction of the full transmission dynamics of COVID-19 in Wuhan
Introduction.
The COVID-19 pandemic has been attributed to 6.91 million mortalities globally, up to the 31st March 2023 1 . This has had far reaching implications for public health policy worldwide and led to unprecedented interventions. The clinical severity observed in response to an infection with SARS-CoV-2 has evolved over time as a consequence of emerging variants of concern, vaccination campaigns, high infection attack rates, and changes to the clinical management of patients.
Emerging variants of concern have been the impetus behind resurgent waves of SARS-CoV-2 incidence and changes to the severity profile of infections. The Alpha variant was first sequenced in September 2020 and became the dominant variant in the UK. Relative to wild type, Alpha 2 was estimated to have a 62% (Hazard Ratio (HR) – 1.62 (95% CI: 1.48, 1.78)) and 73% (HR – 1.73 (95% CI: 1.41, 2.13)) increased risk of hospitalisation and mortality, respectively. The discernible replacement of Alpha by Delta was detected in early 2021 3 and it became the dominant variant by June 4 . Delta was found to have a substantially increased risk of hospitalisation relative to Alpha with a HR of 1.85 (95% CI: 1.39, 2.47) 5 . Delta was replaced by the Omicron BA.1 in December 2021 6 with increased vaccine escape noted as a significant factor. Omicron BA.1 was estimated to have an almost threefold reduction in the risk of hospital admission relative to Delta 7 . In March 2022, Omicron BA.2 replaced Omicron BA.1 8 , and there was found to be limited evidence of a difference in the severity of infection 7 . Omicron BA.5 replaced Omicron BA.2, in June 2022, as the dominant variant in the UK 9 . There was evidence that Omicron BA.5 10 infections may be associated with an increased risk of hospitalisation relative to Omicron BA.2. Following a summer epidemic wave of SARS-CoV-2 infections in 2022, Omicron further diversified. Several of these lineages convergently acquired mutations on the receptor binding domain that are associated with immune evasion 11 . These lineages include BF.7 (a BA.5.2 derivative), BA.5.3 sub lineages BQ.1 and BQ.1.1, as well as lineages derived from BA.2.75. Notably, a BA.2 recombinant carrying many of these mutations (XBB) drove a wave of incidence in Singapore and later became dominant in the UK 12 .
The vaccination campaign in the United Kingdom began on the 8th December 2020 13 . The campaign was implemented in phases with the groups prioritised by clinical risk. Phase 1 included 9 high priority groups and began with care home residents, individuals over the age of 70, the clinically extremely vulnerable, frontline healthcare staff and social care workers 14 . The remaining phase 1 groups included those aged 50 to 69 years old. Subsequently phase 2 15 was implemented in April 2021 and began a further age stratified approach beginning with those aged 40–49 and concluding with the 18-29 age group. Phase 3, 4, and 5 focused on booster campaigns, the clinically vulnerable, and children over the age of 12.
The primary vaccinations administered in the UK were the AstraZeneca vaccine, Vaxzevria, and the Pfizer vaccine, Comirnaty. The impact of vaccination campaigns on the disease severity in the population has been influenced by the timing of the campaign and the variant specific response. An early study found that Vaxzevria had a vaccine efficacy of 66.7% (95% Confidence Interval (CI): 57.4, 74.0), 14 days after the second dose 16 for wild type. The efficacy of the vaccine was estimated to be 81.3% (95% CI: 60.3, 91.2) for individuals that had a longer prime-boost interval. The vaccine efficacy of 2 doses of Vaxzevria against symptomatic infection was estimated to be 70.4% (95% CI: 43.6, 84.5) 17 for Alpha. It was later estimated that the vaccine effectiveness for Delta was 67.0% (95% CI: 61.3, 71.8) 18 and limited protection against symptomatic disease was found for Omicron BA.1 19 . There was no significant difference found in the vaccine effectiveness for Omicron BA.1 and Omicron BA.2 20 and there was limited evidence for the effectiveness of two doses of Vaxzevria for Omicron BA.4/BA.5. Vaxzevria was the primary vaccine administered to those aged over 40 in the UK after concerns of haemostatic complications in younger ages. Comirnaty was administered at the start of the vaccination campaign and subsequently it was primarily administered to those aged under 40. The vaccine efficacy for two doses of Comirnaty was estimated to be 96.2% (95% CI: 93.3, 98.1) 21 early in the pandemic. Later studies estimated the vaccine effectiveness to be 89.5% (95% CI: 85.9, 92.3) for Alpha 22 and 88.0% (95% CI: 85.3, 90.1) for Delta 18 . Evidence of the two dose effectiveness of Comirnaty for Omicron subvariants 23 was limited with wide uncertainty 24 . For the 3rd and 4th booster vaccinations, the Joint Committee on Vaccination and Immunisation stated 25 a preference for Comirnaty or a half dose of Spikevax and where mRNA vaccines could not be used then individuals were offered Vaxzevria.
On the 15th August 2022, the Medicines and Healthcare products Regulatory Agency (MHRA) approved the use of a bivalent COVID-19 vaccine made by Moderna, which targeted both the 2020 SARS-CoV-2 viral strain and Omicron BA.1 26 . The Pfizer/BioNTech bivalent vaccine was approved by the MHRA, less than a month later, on the 3rd September 2022 27 , which also targeted the 2020 SARS-CoV-2 viral strain and Omicron BA.1. A second bivalent vaccine from Pfizer/BioNTech was approved by the MHRA in November 2022, which targeted Omicron BA.4/BA.5 and the 2020 viral strain. A study published near the end of 2023 28 found that the vaccine effectiveness of bivalent BA.1 boosters against hospitalisation peaked at 53.0% (95% CI: 47.9, 57.5) 2 to 4 weeks after a dose was administered and at 10 weeks this had reduced to 35.9% (95% CI: 31.4, 40.1). In September 2023 the MHRA approved Pfizer/BioNTech 29 and Moderna’s 30 bivalent vaccine to target Omicron XBB.1.5; with ongoing work to understand this vaccine’s effectiveness against emerging variants. Analysis of vaccine effectiveness through population-based studies in the UK has been impacted by the cessation of free testing in the UK. This limits the understanding of variant prevalence and impacts the means to adjust for past infection in statistical analyses, with limited information on the infection ascertainment rates.
Improvements in the medical management of patients infected with COVID-19 has reduced the hospitalisation and fatality risk for those infected with the virus. Research found the use of non-invasive continuous positive airways pressure and awake prone positioning to be associated with improved patient outcomes 31 . To try and reduce the risk of severe disease in the clinically extremely vulnerable, anti-viral medicine, and neutralising monoclonal antibodies have been made available in the community and within Secondary Care 32 . This has included nirmatrelvir and ritonavir (Paxlovid), sotrovimab (Xevudy), remdesivir (Veklury), and molnupiravir (Lagevrio) 33 .
This paper describes changes over time to the real-time infection hospitalisation risk (IHR) and infection fatality risk (IFR) using the Office for National Statistics Coronavirus Infection Survey (ONS CIS) and Real-time Assessment of Community Transmission (REACT) prevalence survey. We assess the impact by region and age over the length of the pandemic.
Parameter estimation
The estimated PCR positivity length for every dominant variant across the epidemic in the UK can be seen in Supplementary Fig. 1 and Supplementary Table 1 . The temporal changes in the time from symptom onset to hospitalisation and death by age groups can be seen in Supplementary Figs. 2 and 3 , respectively. The PCR test sensitivity modelling for every dominant variant and each age group can be seen in Supplementary Fig. 4 . The modelling estimates for the time from symptom onset to a first positive test by age and region can be seen in Supplementary Figs. 5 – 9 .
Real-time infection hospitalisation risk – national
The IHR in England peaked at 3.39% (95% Credible Intervals (CrI): 2.79, 3.97) in January 2021, during the period when the Alpha variant was dominant and most of the population were unvaccinated (Fig. 1 ). After the rollout of the vaccination programme, the IHR started declining rapidly. Near the end of the Delta period, in November 2021, the IHR had reduced to 0.58% (95% CrI: 0.50, 0.67) and the lowest IHR was estimated to be 0.32% (95% CrI: 0.27, 0.39) in December 2022. Since this time, the IHR has fluctuated and it was estimated to be 0.47% (95% CrI: 0.39, 0.59) by February 2023. Overall, the IHR has declined by 86.03% (80.86, 89.35) since January 2021. The REACT and ONS CIS prevalence estimates and hospital admissions attributed to COVID-19 can be seen in Supplementary Figs. 10 and 11 , respectively.

A The posterior estimates of the median infection hospitalisation risk for England, based on the combined REACT and ONS sampling, with 95% credible intervals. B Posterior estimates of the median infection hospitalisation risk for England, based on REACT sampling, with 95% credible intervals. C Posterior estimates of the median infection hospitalisation risk for England, based on ONS sampling, with 95% credible intervals. Not all estimates derived from the ONS CIS study have been plotted. The data for the figure are provided as a Source Data file.
Real-time infection hospitalisation risk – age groups
The IHR peaked in January and February 2021 for the age groups over 44 (Table 1 and Fig. 2 ). The IHR peaked later for those aged between 6 to 24 (March 2021) and 25 to 44 (April 2021). The IHR declined in every age group from May 2021 and reached the lowest estimated value in April 2022 for the age groups over 54 and in December 2022 for the age groups under 55. Since this time, we have seen fluctuations in the estimated IHR for every age group. Since the peak in early 2021 until February 2023 the IHR has decreased by 92.51% (88.84, 94.52) for the ≥ 75 age group; 92.25% (88.04, 94.63) for the 65 to 74 age group; 91.90% (87.86, 94.37) for the 55 to 64 age group; 91.51% (87.90, 94.00) for the 45 to 54 age group; 92.72% (89.50, 94.89) for the 25 to 44 age group; and 88.30% (80.48, 92.25) for the 6 to 24 age group. The REACT and ONS CIS prevalence estimates and hospital admissions attributed to COVID-19 for each age group can be seen in Supplementary Figs. 12 and 13 , respectively. The full results for each prevalence study and age group can be seen in Supplementary Figs. 14 – 19 .
The posterior estimates of the median infection hospitalisation risk by age group, based on the combined REACT and ONS sampling, with 95% credible intervals. The data for the figure are provided as a Source Data file.
Real-time infection hospitalisation risk – regions
Following the national estimates, we estimated the highest IHR in all NHS regions was in January and February 2021 (Fig. 3 and Table 2 ). After early 2021, the IHR rapidly declined in all NHS regions. The IHR reached the lowest estimated value in March and April 2022 in the East of England, London, South East, and the North East and Yorkshire when Omicron BA.2 was dominant. The IHR continued declining until October 2022 in the North West and until December 2022 in the Midlands and South West. All regions have since seen a fluctuating pattern in the IHR. The REACT and ONS CIS prevalence estimates and hospital admissions attributed to COVID-19 for each NHS region can be seen in Supplementary Figs. 20 and 21 , respectively. The full results for each study and region can be seen in Supplementary Figs. 22 to 28 .
The posterior estimates of the median infection hospitalisation risk for the regions of England, based on the combined REACT and ONS sampling, with 95% credible intervals. The data for the figure are provided as a Source Data file.
Real-time infection fatality risk – national
The infection fatality risk in England peaked at 0.97% (95% CrI: 0.62, 1.36) in January 2021, after which time the IFR began to rapidly decline (Fig. 4 ). In November 2021, at the end of the Delta period, the IFR had reduced to 0.11% (95% CrI: 0.08, 0.15). The IFR continued to decrease through the Omicron BA.1 and Omicron BA.2 period reaching 0.06% (95% CrI: 0.04, 0.08) in April 2022. Since this time, we have observed fluctuations in the IFR and at the end of Februrary 2023 it was estimated to be 0.10% (95% CrI: 0.07, 0.16). Since the peak in January 2021 the IFR had declined, overall, by 89.67% (80.18, 93.93) up to February 2023. The national REACT and ONS CIS prevalence estimates and deaths attributed to COVID-19 can be seen in Supplementary Figs. 10 and 29 .

A The posterior estimates of the median infection fatality risk for England, based on the combined REACT and ONS sampling, with 95% credible intervals. B Posterior estimates of the median infection fatality risk for England, based on REACT sampling, with 95% credible intervals. C Posterior estimates of the median infection fatality risk for England, based on ONS sampling, with 95% credible intervals. Not all estimates derived from the ONS CIS study have been plotted. The data for the figure are provided as a Source Data file.
Real-time infection fatality risk – age groups
In every age group we estimated the highest IFRs to be in January and February 2021 (Table 3 and Fig. 5 ). For most of the age groups the IFR reached the lowest estimated value in March and April 2022 with the exception of the 6 to 24 age group which reached its lowest estimated value in February 2023. From early 2021 until February 2023 we have seen a decline in the IFR of 95.95% (91.95, 97.70) for the ≥ 75 age group; 95.19% (91.00, 97.18) for the 65 to 74 age group; 94.19% (88.93, 96.71) for the 55 to 64 age group; 92.92% (87.74, 95.72) for the 45 to 54 age group; 90.71% (82.53, 95.05) for the 25 to 44 age group; and 92.74% (78.17, 98.35) for the 6 to 24 age group. The REACT and ONS CIS prevalence estimates and deaths attributed to COVID-19 for each age group can be seen in Supplementary Figs. 12 and 30 , respectively. The full results for each study and age group can be seen in Supplementary Figs. 31 – 36 .

The posterior estimates of the median infection fatality risk by age group, based on the combined REACT and ONS sampling, with 95% credible intervals. The data for the figure are provided as a Source Data file.
Real-time infection fatality risk – regions
Similar to the trends seen in the IHR, we found the highest estimated IFR for most regions to be in January 2021 with the exception of London and the North East that peaked in November 2020 (Table 4 and Fig. 6 ). The IFR reached the lowest estimated value in March and April 2022 for every English region except the South West, South East, and East of England (estimated to be in July 2022). We subsequently have observed fluctuations in the estimated IFR for every region. The REACT and ONS CIS prevalence estimates and deaths attributed to COVID-19 for each region can be seen in Supplementary Figs. 20 and 37 , respectively. The full results for each study and region can be seen in Supplementary Figs. 38 to 46 . The regional age composition and index of multiple deprivation scores can be seen in Supplementary Fig. 47 .

The posterior estimates of the median infection fatality risk for the regions of England, based on the combined REACT and ONS sampling, with 95% credible intervals. The data for the figure are provided as a Source Data file.
Over the course of the pandemic in England, the severity from infection of SARS-CoV-2 has substantially decreased. Changes to the IHR and IFR have been driven by a combination of vaccination, immunity from infection, patient management, and the demographic distribution of infections. We observe that since the January 2021 peak until February 2023, there has been a decline of 86.03% (80.86, 89.35) and 89.67% (80.18, 93.93) in the IHR and IFR, respectively. The early decline, since January 2021, was likely a consequence of the rollout of the vaccination programme, which reached the oldest and most vulnerable individuals in December 2020 and January 2021. Consequently, we observe a later peak in March and April 2021 in the IHR for the age groups under 45. We observed considerable regional heterogeneity at the start of the pandemic, which has substantially reduced post vaccination and following high infection attack rates in the population. Nationally, the IHR and IFR continued to decline until December and April 2022, respectively, which followed Autumn and Winter booster campaigns in England. However, the period following early 2022 has been characterised by an undulatory pattern in the IFR and IHR in response to the timing of vaccination campaigns, resurgent epidemic waves, and emerging variants. We estimated by the end of the study that 4.73 (3.85, 5.93) individuals in 1,000 that are infected with SARS-CoV-2 will be hospitalised and that 1.00 (0.67, 1.56) individual in 1,000 that are infected will die.
Early point estimates of the IFR in 2020, calculated from antibody surveys, ranged from 1.15% (95% Prediction Interval Range (PI): 0.78%, 1.79%) in high income countries to 0.23% (95% PI: 0.14%, 0.42%) in low income countries 34 . Further IFR estimates retrospectively calculated, of the largely pre-vaccination period in the UK, have ranged from 1.57% (95% Uncertainty Interval (UI): 1.22, 2.47) in April 2020 to 1.20% (95% UI: 0.88%, 1.73%) in January 2021 35 . The study period for this paper began on the 8 th November 2020 and therefore does not cover the early pandemic. However, we did not find a reduction in the IFR until after January 2021. Nonetheless, the considerable uncertainty in these estimates overlap with the credible intervals of this study’s estimates in January 2021. Some of these early serological studies were not adequately powered, with regards to sample size, and draw from existing surveys 34 , 35 , 36 , 37 that may not be representative of the general population.
To calculate incidence and the time to a clinical event we used temporally variable parameters. The time from symptom onset date (used here as a proxy for infection date) to hospitalisation and death evolves in response to epidemic phases 38 , changes to clinical management, prior immunity, and the pathogenesis of novel variants 39 . The length of the infectious period of a randomised sampled cohort changes in response to epidemic phases. PCR positivity was found to vary across the variants that became dominant in the UK. We found the Alpha variant to have the longest PCR positivity and a reduction in length was estimated for the Omicron variants.
The criteria for a hospital admission or mortality attributed to COVID-19 can be multifaceted, obfuscated by the comorbidities, clinical policy, and extrinsic factors including hospital pressure. The absolute value of the IHR and IFR estimates are sensitive to the criteria used to define a hospitalisation or mortality from COVID-19. The definition commonly used in the pandemic within the UK has been 28-day deaths 40 , which was thought to be likely an underestimate of true deaths from COVID-19 41 . However, the 60-day deaths definition could overestimate COVID-19 deaths in some subgroups, particularly in older individuals who have higher baseline mortality rates. Death certificate confirmed COVID-19 deaths suffered from changes to death reporting practices across the pandemic 40 . Determining the cause of a hospital admission from surveillance data requires assumptions without further clinical information. Hospital surveillance data can include some nosocomial patients as well as patients admitted to hospital for other illnesses who tested positive for COVID-19 on admission. Although the absolute values of the IHR and IFR are sensitive to these criteria, the temporal trends are robust, provided the definitions remain consistent over time.
The method used in this paper calculates the proportion of infections that led to deaths and hospital admissions attributed to COVID-19. Since we are interested in the IFR/IHR across grouped time-periods, or rounds, we made an approximation to the method that relies on the assumption that the risk is constant within each round. This assumption simplifies the method, without substantially affecting the within round estimates. This method is slightly affected by the epidemic phase severity bias 42 , whereby severity is overestimated during phases of growth and underestimated during phases of decline. To adjust for this bias, we would need to adjust for different incubation period distributions conditional on patient outcomes. That is, we would need to construct two prevalence time-series, prevalence among individuals that will get admitted to hospital or die and prevalence among individuals that will not. These two time-series would need to be deconvolved using different incubation periods, corresponding to the outcome, to estimate the bias-corrected infection incidence time-series. To obtain these two mutually exclusive prevalence time-series, data linking prevalence to patient outcome and exposure date is needed. The REACT survey data, available to this study, did not include the personally identifiable information needed to link to patient outcomes and this was not possible with ONS CIS due to the conditions of the participant consent agreement.
In this paper we have assessed temporal changes to the real-time infection hospitalisation and fatality risk from COVID-19. There has been considerable regional heterogeneity, which is likely a consequence of differing infection attack rates, distinct age compositions, and the relative differences in deprivation across England. At the end of February 2023, the IHR and IFR in England are estimated to be 0.47% (95% CrI: 0.39%, 0.59%) and 0.10% (95% CrI: 0.067%, 0.16%), respectively.
This section describes the methods used to calculate the real-time IFR and IHR from the REACT and ONS CIS studies. We discuss the calculations for the viral parameter history, which includes calculating the length of PCR positivity by variant, PCR test sensitivity by variant, and the time delay modelling from symptom onset to a first positive test, hospital admission and death. For each study (Supplementary Fig. 48 ) we describe the modelling to calculate prevalence, incidence, and infection severity risk.
Epidemiological data
There were two large surveys in the United Kingdom that provided real-time estimates of SARS-CoV-2 positivity: the ONS Coronavirus Infection Survey 43 and the REACT 1 antigen survey coordinated by Imperial College London in conjunction with Ipsos MORI 44 . ONS data was sourced through the Secure Research Service (SRS) 45 where we extracted demographic and regional breakdowns of the Reverse Transcription Polymerase Chain Reaction (RT-PCR) test results. Non-identifiable aggregated REACT study data was provided through a data agreement between UKHSA and Imperial College London.
REACT 1 was a repeat cross-sectional study that estimated SARS-CoV-2 prevalence in England 46 from May 2020 until March 2022. The study aimed to sample between 95,000 to 175,000 individuals randomly for each survey round over the age of 5 47 , which was updated from 100,000 to 150,000 individuals in the original study protocol 46 . The study sent out recruitment letters on a 4–6-week basis to a randomised sample from the National Health Service patient register that aimed to be nationally representative. For children under the age of 18 the recruitment letters were sent to a parent or guardian. Individuals that chose to participate were sent throat and nasal swabs, which were sent for RT-PCR testing. The participants were then invited to complete an online questionnaire, which included demographics, infection history, and behavioural topics. Overall, the REACT 1 and REACT 2 studies found a response rate of 23.4% across the study period 48 . The REACT reports and study protocol have been published through Imperial College London and Welcome Open Research 46 , 49 . Supplementary Figs. 49 – 51 describes the sample sizes for each age group and region over time.
The ONS CIS study was produced by the ONS in collaboration with the Wellcome Trust, University of Oxford, IQVIA, Lighthouse Laboratories, Joint Biosecurity Centre, UKHSA and the University of Manchester 50 . The study began in April 2020 as a pilot and invited 20,000 households from the ongoing Labour Force Survey 51 and those that had agreed previously to participate in the Opinions and Lifestyle Survey 52 . Then in August 2020, the survey expanded to invite a randomised household sample from AddressBase 53 . The extended household study aimed to achieve around 150,000 swabs a fortnight in England between October 2020 and March 2023. The study requested that the entire household (over the age of 2) take a nose and throat swab, which was sent to the Lighthouse Laboratory for RT-PCR testing. Tests were conducted by home visits from a study worker and in April 2022 a proportion of participants of the study were asked to post their samples. At this time the number of swabs required reduced by 25% with an aim to swab 227,300 individuals every 28 days in England. From the 1st August 2022, the study collections had moved to being entirely remote. The ONS reported 50 an attrition rate of 0.62% in December 2020 that fluctuated between a high of 1.37% in July 2021 to the lowest rate of 0.32% in December 2021. The ONS study paused at the end of March 2023 with the intention to restart later in the year. Supplementary Figs. 49 – 51 describes the sample sizes for each age group and region over time.
Mortality data, subset by age and geography, were sourced from the UKHSA COVID-19 death linelist. To limit capturing deaths that were less likely to be linked to a COVID-19 infection we only included deaths that had occurred 60 days following a positive RT-PCR test. Hospitalisation attributed to an infection with COVID-19 were collected from the NHSE&I situational report data 54 . Mortality data was available for the 9 English regions 55 and hospital data was reported for the 7 NHS English Health regions 56 .
Population size data, stratified either by age group or region, were sourced from the ONS at a yearly resolution. This data excludes communal establishments, as these were not sampled during the REACT or ONS surveys. Linear interpolation was used to provide estimates of population sizes for the midpoint of each round for each of the prevalence studies.
In order to provide context for the estimated temporal changes in IHR and IFR, we extracted data on sequenced cases from the Second Generation Surveillance System at the UKHSA, appending metadata on the age and residential region for the case. To complement the national, regional and age stratified analyses we calculated the proportion of sequenced cases associated with wild type, Alpha, Delta, and Omicron lineages.
Due to data protection regulations the model methodologies were developed to work across different data platforms including the ONS SRS, the UKHSA Halo system and Public Health England data infrastructure. The study period of this paper is from the 8 th November 2020 until March 2023.
Methodology outline
Our methodology for calculating the infection severity risk features two key steps. Firstly, we must take the number of positive and negative tests for each survey round and estimate the number of new infections for that round, referred to as the incidence for that round. This requires us to adjust for several quantities: positivity duration, delay from infection to testing positive, test sensitivity and test specificity. Given an estimate of the round incidence, we then produce an estimate of how many clinical outcomes, such as deaths or hospitalisations, are attributed to a given round. This is achieved by estimating the delay from symptom onset to clinical outcome, and then temporally adjusting clinical outcomes. Furthermore, many of these key parameters are known to vary over time, variant type, and age group. We first detail the models used to estimate these key disease history parameters. Then, we will outline our final methodology for calculating the rate of severe outcomes, first using each prevalence study in isolation, then combining the results of the two studies.
A Bayesian methodology is used throughout, with all statistical models implemented using CmdStanR (version 0.6.1) 57 , and posterior sampling performed using Hamiltonian Markov Chain Monte Carlo (MCMC). For each model we run 4 chains, each with 1000 warmup iterations followed by 1000 sampling iterations. Convergence was assessed using the \(\hat{R}\) statistic 58 , with convergence declared if \(\hat{R} \, < \, 1.01\) .
Propagation of uncertainty
The data used in this paper is stored in several different research environments, therefore it is not possible for this method to be implemented in a single Stan Bayesian program. Consequently, to propagate the uncertainty it is necessary to pass the posterior estimates from the models that estimate key disease parameters to later models that estimate the incidence or calculate the rate of severe outcomes. We have not made this explicit in the following methods and instead summarise our approach here.
If a model \({{{{{{\mathcal{M}}}}}}}_{1}\) depends on a parameter \({{{{{\rm{\theta }}}}}}\) that is obtained from model \({{{{{{\mathcal{M}}}}}}}_{0}\) , then this is provided to model \({{{{{{\mathcal{M}}}}}}}_{1}\) via the prior
where \({{{{{\mathcal{D}}}}}}\) is an appropriate parametric distribution. For example, a beta distribution is an appropriate choice to describe the posterior distribution of parameters bounded between 0 and 1. The values of \(\hat{{{{{{{\rm{\mu }}}}}}}_{{{{{{\rm{\theta }}}}}}}},\hat{{{{{{{\rm{\sigma }}}}}}}_{{{{{{\rm{\theta }}}}}}}}\) were obtained by using maximum likelihood to fit \({{{{{\mathcal{D}}}}}}\) to the posterior draws of \({{{{{\rm{\theta }}}}}}\) from model \({{{{{{\mathcal{M}}}}}}}_{0}\) . For the parameters that do need to be provided between models, we found that either normal or beta distributions were able to provide satisfactory fits for the parameters.
Disease history parameter estimation
Positivity duration.
From the ONS COVID Infection Survey, we have longitudinal testing data for individuals. After initially entering the survey, individuals test weekly for the first four weeks, before testing monthly for the remainder of their time on the survey. We estimate the duration of positivity \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{pos}}}}}}\in {{\mathbb{R}}}_{+}\) , defined as the delay from when a case first becomes positive to when a case ceases to be positive, by decomposing into two delays such that \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{pos}}}}}}={{{{{{\rm{\tau }}}}}}}_{{{{{\rm{fp}}}}}}+{{{{{{\rm{\tau }}}}}}}_{{{{{\rm{EoP}}}}}}\) , where \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{fp}}}}}}\in {{\mathbb{R}}}_{+}\) is the delay from symptom onset to first testing positive, and \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{EoP}}}}}}\in {{\mathbb{R}}}_{+}\) is the delay from when the case first tests positive to when the case ceases to test positive. As part of this, we make the assumption that time of symptom onset approximates the time at which the case becomes positive 59 . While it would be possible to not make this assumption and use an interval censoring model to estimate the delay from the infection becoming positive to the infection testing positive, due to the size of the intervals relative to the delay, the uncertainty on any estimates produced using this approach would be too large and would consequently degrade results (please see a schematic in Supplementary Fig. 52 ).
Delay from first positive test, to the end of positivity
For each case, data can be obtained on two delays: the delay from the first positive test to the last positive test followed by a negative test \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{lp}}}}}}\in {{\mathbb{R}}}_{+}\) , and the delay from the first positive test to the first negative test \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{fn}}}}}}\in {{\mathbb{R}}}_{+}\) . Therefore, we have that \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{EoP}}}}}}\in \left[{{{{{{\rm{\tau }}}}}}}_{{{{{\rm{lp}}}}}},{{{{{{\rm{\tau }}}}}}}_{{{{{\rm{fn}}}}}}\right]\) .
It is likely that the test positivity duration has changed with the emergence of different variants, therefore we condition \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{EoP}}}}}}\) upon the dominant variant at the time of the case’s infection. We also make the assumption that the positive duration of each case is distributed according to a Weibull distribution, and for each variant we perform interval-censored regression to estimate the positive duration distribution. This is achieved by defining a date range, over which individuals who first test positive within this range are assumed to be infected with that variant. The date ranges used are:
Letting \({\tau }_{{{{{\rm{EoP}}}}}}^{\left(i\right)}\) denote the delay for the \({i}^{{th}}\) case, we assume that \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{EoP}}}}}}^{\left(i\right)} \sim {{{{\rm{Weibull}}}}}\left(\alpha,{{{{{{\rm{\lambda }}}}}}}_{{{{{{\rm{v}}}}}}}\right)\) . Here, the shape parameter \({{{{{\rm{\alpha }}}}}}\in {{\mathbb{R}}}_{+}\) is shared across all variants with the rate \({{{{{{\rm{\lambda }}}}}}}_{{{{{{\rm{v}}}}}}}\in {{\mathbb{R}}}_{+}\) parameter conditional upon the variant assigned to the \({i}^{{th}}\) case, where \({{{{{\rm{v}}}}}}\in \{1,\ldots,7\}\) denotes which variant the \(i\) th case is assigned to.
For the \(i\) th case, we must compute the likelihood for the event
which has a likelihood given by
For our priors, we let \({{{{{\rm{\alpha }}}}}}\sim {{{{\rm{Exponential}}}}}\left(0.1\right)\) , and \({\lambda }_{{{{{{\rm{v}}}}}}}{{{{{\mathcal{\sim }}}}}}{{{{{\mathcal{N}}}}}}\left({{{{\mathrm{15,10}}}}}\right)\) .
Our assumption that the shape parameter is shared across all variants is due to the presence of large censoring intervals that make inferring the shape of the distribution difficult. Consequently, we find it necessary to use data from multiple variants to infer the shape of the Weibull distribution.
Onset to first positive test delay
For symptomatic individuals with a positive COVID-19 test, the ONS COVID Infection Survey reports symptom onset date. This allows data to be extracted on symptom onset and first positive test time for each patient. The mean time from symptom onset to a positive test is used as a proxy measure to estimate the temporal variation in PCR positivity by approximating the average time from becoming positive to testing positive.
This distribution is likely to vary significantly compared to the community COVID-19 testing, because community testing is based on healthcare seeking behaviour among the general population as opposed to randomised testing within the population. This delay is further affected by epidemic phases, whereby during times of growth the observed delays are shorter, and during times of decay the observed delays are longer, consequently it is necessary for the parameters of this delay to be modelled as time-varying. After visualising the observed delays, we find that the skew-normal distribution is the only positive unbounded continuous distribution that would be appropriate to model this distribution that is available in Stan. Other distributions available in Stan were either symmetric, featured heavy tails or had other undesirable properties that meant that they were not appropriate for modelling the data.
Let \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{fp}}}}}}^{\left(i\right)}\in {\mathbb{R}}\) be the delay from the \(i\) th case developing symptoms to first testing positive. Under our assumptions we have that
where \(\underline{\xi }\in {\mathbb{R}}^{K},\,\underline{\omega }\in {\mathbb{R}}_{+}^{K}\,{{{{{\rm{and}}}}}}\,\underline{\upsilon }\in {\mathbb{R}}^{K}\) are the location, scale, and shape parameters of the skew normal distribution respectively, and \({{{{{\rm{k}}}}}}\in \{{{{{\mathrm{1,2}}}}},\ldots,{{{{{\rm{K}}}}}}\}\) denotes which survey round the \(i\) th observation belongs to. The parameters of the skew normal are modelled using first order random walk smoothing priors, which enforce smoothness by assuming that increments of the random walk are normally distributed, i.e. let \(\underline{x }=(x_{1},\ldots,x_{K})\in {{\mathbb{R}}}^{ K }\) be a first order random walk ( \({RW}1\) ), then we have that \({x}_{i+1}-{x}_{i}{{{{{\mathcal{ \sim }}}}}}{{{{{\mathcal{N}}}}}}\left(0,{{{{{{\rm{\sigma }}}}}}}^{2}\right)\) where \({{{{{\rm{\sigma }}}}}}\in {{\mathbb{R}}}_{+}\) is a hyperparameter to be estimated that controls the smoothness of the random walk. Hence, for modelling the parameters of the skew normal, we let
where \({{{{{{\rm{\sigma }}}}}}}_{{{{{{\rm{\xi }}}}}}},{{{{{{\rm{\sigma }}}}}}}_{{{{{{\rm{\omega }}}}}}},{{{{{{\rm{\sigma }}}}}}}_{{{{{{\rm{\upsilon }}}}}}}\sim {{{{{{\mathcal{N}}}}}}}_{+}\left(0,1\right)\) .
A survey round is defined as the sampling period that was determined by the REACT (typically 2 weeks) and the ONS CIS study.
Onset to clinical event delay
To measure the distribution of delays from onset to clinical event, we use the Secondary Uses Service (SUS) 60 data from the NHS and the UKHSA death line list data sets. These data are daily censored, so we consider this as doubly-interval censored data. In these data, we only observe patients conditional on the clinical event occurring, which introduces right-truncation, since data are only observed before the final day of data collection T , which in this case is 23 rd April 2023.
To estimate the mean delay from onset (as reported by patients) to hospitalisation or death, we fit to the data using a Weibull and lognormal distribution respectively, with both models accounting for interval censoring and right truncation. These distributions were selected as the best performing distributions out of the gamma, Weibull, and lognormal distributions, according to the Pareto-smoothed importance sampling leave-one-out cross-validation scores 61 . We fit the models to data aggregated into three-month periods by symptom onset date, in order to obtain time-varying delay parameters. The method here adapts the methods from Ward & Johnsen 38 , Ward et al. 62 , and Vekaria, et al. 63 .
In this method, we assume that symptom onset time \(S\in {\mathbb{Z}}\) for each individual sits within an interval \(\left[{s}_{1},{s}_{2}\right]\) , where \({s}_{1}\) is the reported symptom onset date and \({s}_{2}\) is the day after, i.e., \({s}_{2}={s}_{1}+1\) . Similarly, the clinical event time \(E\in {\mathbb{Z}}\) sits within an interval \(\left[{e}_{1},{e}_{2}\right]\) where \({e}_{2}={e}_{1}+1\) . The likelihood of observing a given clinical event time, conditional on the observed onset interval, is given by:
This likelihood could be modelled by numerically integrating across the observation intervals. However, this would be very computationally expensive. Instead, we can include estimated event times for each patient as latent variables within our model 62 , which we assume to be uniformly distributed across the observation interval. Introducing these latent variables \({e}^{*}\) and \({s}^{*}\) , our likelihood function simplifies to
where \({f}_{\theta }(.)\) is the probability density function of the parametric distributions with parameters \({\theta }_{1}\) and \({\theta }_{2}\) . We combine this likelihood with prior distributions for our latent variables given by
We assume \({\theta }_{1}\) represents the mean for admissions and the log mean for mortalities, and follows a weakly informative normal prior distribution. For the delay to hospitalisation, we assume
For the delay to death, we assume
We assume \({\theta }_{2}\) represents the shape parameter for admissions, and has the prior
For mortalities, we assume \({\theta }_{2}\) represents the log of the standard deviation, and has the prior
This model is fit using MCMC implemented in Stan, with full model formula
We consider time varying onset to clinical outcome delays, fitting the delays independently to each three-month time period, starting from September 2020.
Sensitivity and specificity
The REACT and ONS studies both use RT-PCR tests that can have variable sensitivity and specificity, which were not adjusted for in the reported results of either study. These values are influenced by swabbing protocol, laboratory, specimen storage, days since symptom onset, site of swab, age, and variant mutations. Primers are adjusted if a drop in sensitivity is observed for a variant 64 , 65 . In addition, given that test sensitivity is conditional upon days since symptom onset, it is known that the average test sensitivity will be affected by epidemic phase bias 66 .
The average RT-PCR test sensitivity for individuals in each round is calculated from two key components: an estimate of the test sensitivity as a function of the time to symptom onset, and an estimated delay distribution of the time between symptom onset and first positive test. Given the existence of potential differences in viral dynamics between different variants, we estimate a different test sensitivity trajectory for each variant, and we estimate the time from symptom onset to testing positive for each round and each study.
To calculate RT-PCR test sensitivity, we have assessed repeat tests by age group from ONS CIS where an individual must have a symptom onset date, with at least one positive test up to 12 days prior and 30 days after symptom onset date.
To fit the RT-PCR test sensitivity, we adapt the method of Binny et al. 67 who fit a piecewise linear logistic regression model, with the binary outcome of a positive or negative test, using days relative to symptom onset date, \({{{{{\rm{d}}}}}}\in {\mathbb{R}}\) , as the primary explanatory variable.
In practice, we found a piecewise linear logistic regression to be a poor fit for our data, and as such we instead modify this function by: removing the changepoint and replacing with a sigmoid, which results in a smoothed out changepoint that is more biologically plausible; and replacing the piecewise linear terms with piecewise polynomial terms, which allows for greater flexibility in fitting to the data. The derived function form is given by:
where \({{{{{{\rm{\beta }}}}}}}_{1},{{{{{{\rm{\lambda }}}}}}}_{1},{{{{{{\rm{\lambda }}}}}}}_{2}\in {{\mathbb{R}}}_{+}\) and \({{{{{{\rm{\beta }}}}}}}_{0},{{{{{\rm{D}}}}}}\in {\mathbb{R}}\) , \({{{{{{\rm{\beta }}}}}}}_{2}\in {{\mathbb{R}}}_{-}\) . We use \(\Phi\) to denote the cumulative distribution function of the standard normal distribution, however for this purpose we are using it as a sigmoidal function rather than for its probabilistic interpretation. The following priors are used in fitting this function:
Infection hospitalisation and fatality risk modelling
The ONS and REACT studies reported positive testing rates over time to help inform the public health response to the pandemic via calculations of the effective reproduction number and acting as inputs into government modelling. With results at both a national level as well as geographic and demographic subdivisions, it is possible to detect higher risk areas in need of greater intervention. By combining the estimated incidence with clinical outcome data in the form of hospital admissions and mortalities, the IHR and IFR can be calculated by the method set out below.
Estimating prevalence from positivity
For each round \(k\in \left[1,\ldots,K\right]\) and subgroup (i.e., region or age group) indexed according to \(s\in \left[1,\ldots,S\right]\) , we calculate the expected test sensitivity p avg_sens for that round using
where \({p}_{{{{{\rm{sens}}}}}}\left(t,k,s\right)\) is our estimate of the probability of testing positive \(t\) days after symptom onset in the \(k\) th round for the \(s\) th subgroup given in equation ( 8 ), \({f}_{{SN}}\) is the probability density function of the skew normal distribution that we use to model the delay from symptom onset to testing for positive tests, and \({{{{{{\rm{\xi }}}}}}}_{k,s},{{{{{{\rm{\alpha }}}}}}}_{k,s},{{{{{{\rm{\omega }}}}}}}_{k,s}\) are the estimated parameters of the skew normal distribution for the \(k\) th estimate of positivity and the \(s\) th subgroup.
Test specificity \({p}_{{{{{\rm{spec}}}}}}\in \left[{{{{\mathrm{0,1}}}}}\right]\) , does not have sufficient data available to produce an estimate. We apply a strong prior that encodes prior beliefs that RT-PCR tests are highly sensitivity, with a false positive rate of approximately 1 in 10,000, i.e., \({p}_{{{{{\rm{spec}}}}}}\sim {{{{{\mathcal{B}}}}}}{eta}\left({{{{\mathrm{10000,1}}}}}\right)\) .
We assume that the prevalence is constant across each round \({p}_{{{{{\rm{prev}}}}}}\in \left[{{{{\mathrm{0,1}}}}}\right]\) . Given \({p}_{{{{{\rm{prev}}}}}}\) , \({p}_{{{{{\rm{spec}}}}}}\) , and \({p}_{{{{{\rm{avg}}}}}\_{{{{\rm{sens}}}}}}\) , the probability that a randomly tested individual will test positive \({p}_{{{{{\rm{pos}}}}}}\in \left[{{{{\mathrm{0,1}}}}}\right]\) , is given by
Let \({N}_{k,s}\in {{\mathbb{Z}}}_{+}\) be the number of tests performed for a given round and stratum, and \({P}_{k,s}\in \left[0,{N}_{k,s}\right]\) be the number of tests that were positive for that round and stratum. Then the likelihood is given by
A hierarchal model structure with second order random walk smooths and random effects is used when estimating \({p}_{{{{{\rm{prev}}}}}}\left({{{k}}},{{{s}}}\right)\) . To maintain identifiability of the model in the presence of both smoothing and random effects, which are effectively two different smooths at the round-subgroup level, we use a special formulation adapted from a BYM2 framework 68 given by
where \({f}_{{{{{\rm{avg}}}}}\_{{{{\rm{prev}}}}}}\) and \({f}_{{{{{\rm{prev}}}}}}\) are logit-scaled smooth functions, and \({{{{{\rm{\xi }}}}}}\sim {{{{{\mathcal{N}}}}}}\left({{{{\mathrm{0,1}}}}}\right)\) are the random effects, \({{{{{{\rm{\beta }}}}}}}_{{{{{{\rm{k}}}}}}}\in {\mathbb{R}}\) the intercept term, \({{{{{\rm{\gamma }}}}}}\in {{\mathbb{R}}}_{+}\) an overall scale term, and \({{{{{{\rm{\alpha }}}}}}}_{1},{{{{{{\rm{\alpha }}}}}}}_{2},{{{{{{\rm{\alpha }}}}}}}_{3}\in \left[{{{{\mathrm{0,1}}}}}\right]\) the elements of a 2-simplex, i.e. \({{{{{{\rm{\alpha }}}}}}}_{1}+{{{{{{\rm{\alpha }}}}}}}_{2}+{{{{{{\rm{\alpha }}}}}}}_{3}=1.\)
Both \({f}_{{{{{\rm{avg}}}}}\_{{{{\rm{prev}}}}}}\) and \({f}_{{{{{\rm{prev}}}}}}\left(\cdot,s\right)\) are constrained to have a mean of zero to maintain identifiability. In addition, we ensure that both \({f}_{{{{{\rm{avg}}}}}\_{{{{\rm{prev}}}}}}\) and \({f}_{{{{{\rm{prev}}}}}}\left(\cdot,s\right)\) are on approximately the same scale as the random effects by placing a standard normal distribution prior on them \({f}_{{{{{\rm{avg}}}}}\_{{{{\rm{prev}}}}}},{f}_{{{{{\rm{prev}}}}}}{{{{{\mathcal{\sim }}}}}}{{{{{\mathcal{N}}}}}}\left({{{{\mathrm{0,1}}}}}\right)\) , in addition to their improper smoothing prior. Therefore, the overall scale is controlled by \({{{{{\rm{\gamma }}}}}}\) given that
which results in a well-identified model structure. For the random walk smoothing priors we let
where \({RW}2\left({{{{{\rm{\sigma }}}}}}\right)\) implies a penalty on the second order derivative in the form of
The \({{{{{\rm{\alpha }}}}}}\) terms control the relative contribution to the variance from each of the components, and we let \({{{{{\rm{\alpha }}}}}} \sim {{{{\rm{Dirichlet}}}}}\left({{{{\mathrm{2,2,2}}}}}\right)\) .
In addition to the prevalence for each age group, we also estimated the national prevalence by Multilevel Regression and Poststratification, which allows us to perform statistical adjustment for demographics that are over/under represented in the sample. The above method for calculating the prevalence in each age group uses a multilevel regression approach, and it remains to perform a poststratification step to estimate the national prevalence by reweighting the prevalence for each age group. Letting \({p}_{{{{{\rm{prev}}}}}}^{{{{{\rm{nat}}}}}}\left(s\right)\) be the poststratified estimate of national prevalence, calculated as
where \({N}_{k}\) is the population of the \({k}^{{th}}\) strata. We poststratified our results according to the age breakdown of our sample, on the basis that age is the most important variable to account for when producing nationally representative estimates of the IHR.
Calculating incidence attributed to round
For each survey (REACT and ONS), we converted the estimated prevalence rates \({p}_{{{{{\rm{prev}}}}}}\left(k,s\right)\) for population stratum \(s\) and round \(k\) into an incidence time series \(I\left(k,s\right)\) using this expression:
Here, \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{pos}}}}}}\left(s\right)\) is the expected duration for which an individual tests positive, \(\Omega \left(k,s\right)\) is the population size of stratum \(s\) during round \(k\) , and \(l\left(k\right)\in {\mathbb{N}}\) is the length of the round in days. We note that \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{pos}}}}}}\) , and other parameters used in calculating it, are derived from the ONS study, since REACT surveying did not provide adequate data to estimate these values.
One way to think of this equation is that initial positive test frequencies are shifted back in time to more accurately reflect when individuals were infected. In this paper, we shift the testing dates to symptom onset date rather than infection date, since we have more reliable data on the delay distributions post symptom onset date. Finally, we multiply by the population \(\Omega\) to scale up our sample to population wide numbers, but also divide by the time for which someone tests positive \({{{{{{\rm{\tau }}}}}}}_{{{{{\rm{pos}}}}}}\) .
Calculating outcome counts attributed to round
Given an estimate of the number of new infections that occurred during a round, it remains to estimate the number of clinical outcomes attributed to individuals infected during that round, which then finally allows us the calculate the rate out severe outcomes.
There is a time delay between symptom onset and clinical outcome 38 , which must be accounted for in the relationship between incidence and hospitalisation or death 69 , 70 , 71 . For a given stratum \(s\) , we must establish a time series \({d}_{s}\left({{{{{\rm{t}}}}}}\right)\) that models clinical outcomes \({c}_{s}\left({{{{{\rm{t}}}}}}\right)\) in that stratum by date of symptom onset rather than by date of outcome. We model \({d}_{s}\left({{{{{\rm{t}}}}}}\right)\) as
where \({p}_{s}\left({t}^{{\prime} },|,t\right)\) is the probability that the time from symptom onset to outcome is \({t}^{{\prime} }\) for someone in stratum \(s\) , given that they were infected on day \(t\) . This approximates the method for mapping outcomes to date of symptom onset 69 , under the assumption that each round has a constant risk.
From the daily-level time series \({d}_{s}\left(t\right)\) , it remains to estimate the number of clinical events attributed to the \({k}^{{{{{\rm{th}}}}}}\) round in stratum \(s\) , denoted by \({D}_{s}\left(k\right)\) , using
where \({{{{{\mathscr{I}}}}}}\left(k\right)\) is the set of timepoints that correspond to the \(k\) th round.
Outcome risk modelling
Given posterior draws of \({I}_{s}\left(k\right),{D}_{s}\left(k\right)\) , we compute the clinical outcome rate, however the resulting estimates are noisy, implying that some smoothing is required.
We first fit a normal distribution to the posterior draws of \({{{{{{\rm{D}}}}}}}_{{{{{{\rm{s}}}}}}}\left({{{{{\rm{k}}}}}}\right),{{{{{{\rm{I}}}}}}}_{{{{{{\rm{s}}}}}}}\left({{{{{\rm{k}}}}}}\right)\) for each round and subgroup, as we are able to easily provide this parametric summary of the posterior as an input to the model. Letting \({{{{{{\rm{\mu }}}}}}}_{s}\left(k\right),{{{{{{\rm{\sigma }}}}}}}_{s}\left(k\right)\) be the parameters of the normal distributions for each round and stratum, we have that
Employing the Jeffrey’s prior for the clinical outcome rate, the posterior distribution for the infection risk \({R}_{s}\left(k\right)\in \left[{{{{\mathrm{0,1}}}}}\right]\) is given by
In addition, we place a second order random walk smoothing prior, with random effects present on \({R}_{k}\left(t\right)\) . A similar structure is used to the hierarchal model employed when estimating prevalence to maintain identifiability in the presence of both a random walk smooth and a random effect smooth;
Infection study model combination
To combine the prevalence studies, each study was matched temporally over the same sampling periods, using test results from the ONS study matched to REACT round sampling dates. We weight the model so that the two samples are assigned weights by adjusting for the relative sample sizes. This weighting method follows the approach of Balcome, et al. 72 adapting the work of Haddad et al. 73 .
Here we let \({D}_{O}\) and \({I}_{O}\) denote \({D}_{s}\left(k\right)\) and \({I}_{s}\left({{{{{\rm{k}}}}}}\right)\) , respectively, for the ONS infection survey study. In this vein, let \({D}_{R}\) and \({I}_{R}\) denote \({D}_{s}\left(k\right)\) and \({I}_{s}\left({{{{{\rm{k}}}}}}\right)\) for the REACT study. Letting \(R\) denote \({R}_{s}\left(k\right)\) then the posterior distribution for this event probability can be modelled using a Beta distribution, i.e.
where \(I\) is the number of infections, and \(D\) is the corresponding number of events. The aim of this combination method is to obtain a weighting factor \(\hat{{{{{{\rm{\alpha }}}}}}}\) such that \({D=D}_{O}+{\hat{\alpha }D}_{R}\) and \({I=I}_{O}+{\hat{\alpha }I}_{R}\) .
We weight the two studies based on their relative sample sizes, so that when the sample sizes are equal, both studies are assigned equal weight, and otherwise the largest study is assigned greater weight. That is, we set \(\hat{\alpha }=\frac{{N}_{R}}{{N}_{O}}\) , where \({N}_{O}\) is the sample size of the ONS study and \({N}_{R}\) is the sample size of the REACT study. Including \(\hat{{{{{{\rm{\alpha }}}}}}}\) into the posterior distribution for the event probability, we obtain
As in the previous section, when calculating the clinical outcome rate for a single study, we provided parametric summaries of the posteriors of \(I,D\) terms to the model as inputs. We also place the same smoothing prior on \(R\) as in the previous section.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The Office of National Statistics COVID Infection Survey (ONS CIS) data can be accessed through the Secure Research Service of the ONS . For all other datasets used in this study please contact the UKHSA. UKHSA operates a robust governance process for applying to access protected data that considers: the benefits and risks of how the data will be used. compliance with policy, regulatory and ethical obligations. data minimisation. how the confidentiality, integrity, and availability will be maintained. retention, archival, and disposal requirements. best practice for protecting data, including the application of ‘privacy by design and by default’, emerging privacy conserving technologies and contractual controls. Access to protected data is always strictly controlled using legally binding data sharing contracts. UKHSA welcomes data applications from organisations looking to use protected data for public health purposes. To request an application pack or discuss a request for UKHSA data you would like to submit, contact [email protected]. Source data are provided with this paper.
Code availability
The model code can be made available on request to [email protected].
Our world in data. “Coronavirus (COVID-19) Deaths .” https://ourworldindata.org/covid-deaths (2022).
Grint, D. J. et al. Severity of severe acute respiratory system coronavirus 2 (SARS-CoV-2) Alpha Variant (B.1.1.7) in England. Clin. Infect. Dis. 75 , e1120–e1127 (2022).
Article PubMed Google Scholar
Ward, T. et al. Growth, reproduction numbers and factors affecting the spread of SARS-CoV-2 novel variants of concern in the UK from October 2020 to July 2021: a modelling analysis. BMJ Open 11 , e056636 (2021).
Ward, T. et al. Bayesian spatial modelling of localised SARS-CoV-2 transmission through mobility networks across England. PLoS Comput. Biol. 19 , e1011580 (2023).
Article CAS PubMed PubMed Central Google Scholar
Sheikh, A., McMenamin, J., Taylor, B. & Robertson, C., Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet 397 , 2461–2462 (2021).
Paton, R. S., Overton, C. E. & Ward, T. The rapid replacement of the SARS-CoV-2 Delta variant by Omicron (B.1.1.529) in England. Sci. Transl. Med. 14 , eabo5395 (2022).
Article CAS PubMed Google Scholar
Sievers, C. et al. SARS-CoV-2 Omicron Variants BA.1 and BA.2 both show similarly reduced disease severity of COVID-19 compared to Delta, Germany. Eurosurveillance 27 , 2021–2022 (2022).
Article Google Scholar
Elliott, P. et al. Twin peaks: the Omicron SARS-CoV-2 BA.1 and BA.2 epidemics in England. Science 376 , eabq4411 (2022).
UK Health Security Agency. SARS-CoV-2 Variants of Concern and Variants under Investigation in England . https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1093275/covid-technical-briefing-44-22-july-2022.pdf (2022).
Hansen, C. H. et al. Risk of reinfection, vaccine protection, and severity of infection with the BA.5 Omicron Subvariant: A Danish Nation-Wide Population-Based Study. Lancet Infect. Dis . 23 , 167–176 (2022).
Cao, Y. et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature 614 , 521–529 (2023).
ADS CAS PubMed Google Scholar
UKHSA. SARS-CoV-2 Variants of Concern and SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 52 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1152143/variant-technical-briefing-52-21-april-2023.pdf (2023).
NHS England. Landmark Moment as First NHS Patient Receives COVID-19 Vaccination . https://www.england.nhs.uk/2020/12/landmark-moment-as-first-nhs-patient-receives-covid-19-vaccination/ (2020).
Department of Health and Social Care. UK COVID-19 Vaccines Delivery . https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/951928/uk-covid-19-vaccines-delivery-plan-final.pdf (2021).
Department of Health and Social Care. JCVI Final Statement on Phase 2 of the COVID-19 Vaccination Programme :. https://www.gov.uk/government/publications/priority-groups-for-phase-2-of-the-coronavirus-covid-19-vaccination-programme-advice-from-the-jcvi/jcvi-final-statement-on-phase-2-of-the-covid-19-vaccination-programme-13-april-2021 (202!).
Voysey, M. et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 397 , 881–891 (2021).
Emary, K. R. W. et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet 397 , 1351–1362 (2021).
Lopez Bernal, J. L. et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 385 , 585–594 (2021).
Andrews, N. et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 386 , 1532–1546 (2022).
Kirsebom, F. C. M. et al. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect. Dis. 22 , 931–933 (2022).
Thomas, S. J. et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 385 , 1761–1773 (2021).
Abu-Raddad, L. J., Chemaitelly, H. & Butt, A. A., National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 385 , 187–189 (2021).
Khan, F. L. et al. Estimated BNT162b2 vaccine effectiveness against infection with Delta and omicron variants among US children 5 to 11 years of age. JAMA Netw. Open https://doi.org/10.1001/jamanetworkopen.2022.46915 (2022).
Tartof, S. Y. et al. BNT162b2 vaccine effectiveness against SARS-CoV-2 omicron BA.4 and BA.5. Lancet Infect. Dis. 22 , 1663–1665 (2022).
NHS. Immediate Action Required for Phase 3 Booster Vaccinations . https://www.england.nhs.uk/coronavirus/documents/immediate-action-required-for-phase-3-booster-vaccinations/ (2021).
Medicines and Healthcare Products Regulatory Agency. First Bivalent COVID-19 Booster Vaccine Approved by UK Medicines Regulator . https://www.gov.uk/government/news/first-bivalent-covid-19-booster-vaccine-approved-by-uk-medicines-regulator (2022).
Medicines & Healthcare Products Regulatory Agency. Coronavirus Vaccine – Summary of Yellow Card Reporting . https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting#:~:text=1)%20and%20the%20Pfizer%2FBioNTech,1%20variant (2023).
Kirsebom, F. C. M., Andrews, N., Stowe, J., Ramsay, M. & Lopez Bernal, J. L. Duration of protection of ancestral-strain monovalent vaccines and effectiveness of bivalent BA.1 boosters against COVID-19 hospitalisation in England: a test-negative case-control study. Lancet Infect. Dis. 23 , 1235–1243 (2023).
Medicines and Healthcare Products Regulatory Agency. MHRA Approves Pfizer/BioNTech’s Adapted COVID-19 Vaccine (Comirnaty) that Targets Omicron XBB.1.5 . https://www.gov.uk/government/news/mhra-approves-pfizerbiontechs-adapted-covid-19-vaccine-comirnaty-that-targets-omicron-xbb15 .
Medicines and Healthcare Products Regulatory Agency. MHRA Approves Moderna’s adapted COVID-19 vaccine (Spikevax) that targets Omicron XBB.1.5 . https://www.gov.uk/government/news/mhra-approves-modernas-adapted-covid-19-vaccine-spikevax-that-targets-omicron-xbb15 (2023).
Hallifax, R. J. et al. Successful awake proning is associated with improved clinical outcomes in patients with COVID-19: single-centre high-dependency unit experience. BMJ Open Respir. Res . https://doi.org/10.1136/bmjresp-2020-000678 (2020).
UKHSA. COVID-19 Antibody and Antiviral Treatments . https://www.gov.uk/government/collections/covid-19-antibody-and-antiviral-treatments (2022).
NHS. Treatments for COVID-19 . https://www.nhs.uk/conditions/covid-19/treatments-for-covid-19/ (2023).
Brazeau, N. F. et al. Report 34. COVID-19 Infection Fatality Ratio: Estimates from Seroprevalence . Imperial College COVID 19 response team. https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2020-10-29-COVID19-Report-34.pdf (2020).
Variation in the COVID-19 infection–fatality ratio by age, time, and geography during the pre-vaccine era: a systematic analysis. Lancet 399 , 1469–1488 (2022). https://doi.org/10.1016/S0140-6736(21)02867-1 .
Stringhini, S. et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet 396 , 313–319 (2020).
Perez-Saez, J. et al. Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva, Switzerland. Lancet Infect. Dis. 21 , e69–e70 (2021).
Ward, T. & Johnsen, A. Understanding an evolving pandemic: an analysis of the clinical time delay distributions of COVID-19 in the United Kingdom. PLOS ONE 16 , e0257978 (2021).
Ward, T. et al. Replacement dynamics and the pathogenesis of the Alpha, Delta and Omicron variants of SARS-CoV-2. Epidemiol. Infect. 151 , e32 (2022).
Article PubMed PubMed Central Google Scholar
UK Health Security Agency. Defining a COVID-19 Death . https://ukhsa.blog.gov.uk/2020/08/12/behind-the-headlines-counting-covid-19-deaths/ (2020).
Department of Health and Social Care. New UK-wide Methodology Agreed to Record COVID-19 Deaths . https://www.gov.uk/government/news/new-uk-wide-methodology-agreed-to-record-covid-19-deaths (2020).
Seaman, S. R. et al. Adjusting for time of infection or positive test when estimating the risk of a post-infection outcome in an epidemic. Stat. Methods Med. Res. 31 , 1942–1958 (2022).
Article MathSciNet PubMed PubMed Central Google Scholar
Office of National Statistics. COVID-19 Infection Survey . https://www.ons.gov.uk/surveys/informationforhouseholdsandindividuals/householdandindividualsurveys/covid19infectionsurvey (2022).
Imperial College London. The REACT 1 Programme . https://www.imperial.ac.uk/medicine/research-and-impact/groups/react-study/the-react-1-programme/ (2022).
Office of National Statistics. Coronavirus (COVID-19) Infection Survey, UK Statistical bulletins. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/previousReleases (2022).
Riley, S. et al. REal-time Assessment of Community Transmission (REACT) of SARS-CoV-2 virus: study protocol. Wellcome Open Res. 5 , 200 (2020).
Article ADS PubMed Google Scholar
Whitaker, M. et al. ‘Variant-specific symptoms of COVID-19 among 1,542,510 people in England,’ medRxiv. https://www.medrxiv.org/content/10.1101/2022.05.21.22275368v1 (2022).
Atchison, C. J. et al. Long-term health impacts of COVID-19 among 242,712 adults in England. Nat. Commun. 14 , 6588 (2023).
Article ADS CAS PubMed PubMed Central Google Scholar
Imperial College London. Real-time Assessment of Community Transmission (REACT) Study . https://www.imperial.ac.uk/medicine/research-and-impact/groups/react-study/ (2023).
ONS. Coronavirus (COVID-19) Infection Survey: Methods and Further Information . https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/methodologies/covid19infectionsurveypilotmethodsandfurtherinformation (2023).
ONS. Labour Force Survey . https://www.ons.gov.uk/surveys/informationforhouseholdsandindividuals/householdandindividualsurveys/labourforcesurvey (2023).
ONS. Opinions and Lifestyle Survey QMI . https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/methodologies/opinionsandlifestylesurveyqmi (2022).
Ordnance Survey. AddressBase . https://beta.ordnancesurvey.co.uk/products/addressbase (2023).
NHS. COVID-19 Situation Reports . https://digital.nhs.uk/about-nhs-digital/corporate-information-and-documents/directions-and-data-provision-notices/data-provision-notices-dpns/covid-19-situation-reports (2022).
ONS. Detailed Information on the Administrative Structure within England . https://www.ons.gov.uk/methodology/geography/ukgeographies/administrativegeography/england#:~:text=There%20are%20nine%20English%20regions,sub%2Dnational%20division%20in%20England (2022).
ONS. Health Geographies . https://www.ons.gov.uk/methodology/geography/ukgeographies/healthgeography#:~:text=1.-,English%20health%20geography,Care%20Board%20Locations%20(SICBL) (2023).
Gabry, J., Češnovar, R. & Johnso, A. CmdStanR. https://mc-stan.org/cmdstanr/#cmdstanr- (2023).
Vehtari, A., Gelman, A., Simpson, D., Carpenter, B. & Bürkner, P.-C. Rank-Normalization, Folding, and Localization: an Improved Rˆ for Assessing Convergence of MCMC (with Discussion). Bayesian Anal. 16 , 667–718 (2021).
Article ADS MathSciNet Google Scholar
Walsh, K. A. et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Infect. 81 , 357–371 (2020).
NHS. Digital, Secondary Uses Service (SUS). https://digital.nhs.uk/services/secondary-uses-service-sus (2022).
Vehtari, A., Gelman, A. & Gabry, J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat. Comput. 27 , 1413–1432 (2017).
Article MathSciNet Google Scholar
Ward, T., Christie, R., Paton, R. S., Cumming, F. & Overton, C. E. Transmission dynamics of monkeypox in the United Kingdom: contact tracing study. BMJ 379 , e073153 (2022).
Vekaria, B. et al. Hospital length of stay for COVID-19 patients: Data-driven methods for forward planning. BMC Infect. Dis. 21 , 700 (2021).
Brown, K. L. et al. A comparative analysis exposes an amplification delay distinctive to SARS-CoV-2 Omicron variants of clinical and public health relevance. Emerg. Microbes Infect. 12 , 2154617 (2023).
Bei, Y. et al. Overcoming variant mutation-related impacts on viral sequencing and detection methodologies. Front. Med. (Lausanne) 9 , 989913 (2022).
Makhoul, M. et al. Analyzing inherent biases in SARS-CoV-2 PCR and serological epidemiologic metrics. BMC Infect. Dis. 22 , 458 (2022).
Binny, R. N. et al. Sensitivity of reverse transcription polymerase chain reaction tests for severe acute respiratory syndrome coronavirus 2 through time. J. Infect. Dis. 227 , 9–17 (2022).
Riebler, A., Sørbye, S. H., Simpson, D. & Rue, H. An intuitive Bayesian spatial model for disease mapping that accounts for scaling. Stat. Methods Med. Res. 25 , 1145–1165 (2016).
Article MathSciNet PubMed Google Scholar
Overton, C. E. et al. Novel methods for estimating the instantaneous and overall COVID-19 case fatality risk among care home residents in England. PLoS Comput. Biol. 18 , e1010554 (2022).
Reich, N. G., Lessler, J., Cummings, D. A. & Brookmeyer, R. Estimating absolute and relative case fatality ratios from infectious disease surveillance data. Biometrics 68 , 598–606 (2012).
Russell, T. W., et al. “Estimating the infection and case fatality ratio for coronavirus disease (covid19) using age-adjusted data from the outbreak on the diamond princess cruise ship, february 2020,” Eurosurveillance , 25, 2000256 (2020).
Balcome, S., Musgrove, D., Haddad, T., Hickey, G. & Jackson, C. Implementation of the Bayesian Discount Prior Approach for Clinical Trials . (CRAN, 2022).
Haddad, T. et al. Incorporation of stochastic engineering models as prior information in Bayesian medical device trials. J. Biopharm. Stat. 27 , 1089–1103 (2017).
Download references
Author information
Authors and affiliations.
UK Health Security Agency, Data, Analytics and Surveillance, Nobel House, London, SW1P 3JR, UK
Thomas Ward, Martyn Fyles, Alex Glaser, Robert S. Paton, William Ferguson & Christopher E. Overton
University of Liverpool, Department of Mathematical Sciences, Peach Street, Liverpool, UK
Christopher E. Overton
You can also search for this author in PubMed Google Scholar
Contributions
TW conceived and led the study. T.W., A.G., W.F., M.F., and C.E.O. developed the methods and code for the time delay models. T.W., M.F., C.E.O., R.S.P., and A.G. developed the data criteria. T.W., A.G., M.F., and R.S.P. wrote the visualisations code. T.W., A.G., and M.F. developed the parameter models. T.W., A.G., M.F., and, C.E.O., and M.F. developed the methods and code for the infection risk models. T.W., A.G., C.E.O., and M.F. wrote the original manuscript. T.W., M.F., A.G., and C.E.O. reviewed the manuscript. T.W., M.F., A.G., and C.E.O. wrote the revisions.
Corresponding author
Correspondence to Thomas Ward .
Ethics declarations
Competing interests.
The authors declares no competing interests.
Ethical statement
UKHSA have an exemption under regulation 3 of section 251 of the National Health Service Act (2006) to allow identifiable patient information to be processed to diagnose, control, prevent, or recognise trends in, communicable diseases and other risks to public health.
Peer review
Peer review information.
Nature Communications thanks Vincent Auvigne, Julio Croda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ward, T., Fyles, M., Glaser, A. et al. The real-time infection hospitalisation and fatality risk across the COVID-19 pandemic in England. Nat Commun 15 , 4633 (2024). https://doi.org/10.1038/s41467-024-47199-3
Download citation
Received : 14 February 2023
Accepted : 22 March 2024
Published : 31 May 2024
DOI : https://doi.org/10.1038/s41467-024-47199-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Molecular characterization of Staphylococcus aureus isolated from hospital-acquired infections in Ilam, Iran
Affiliations.
- 1 Department of Microbiology, School of Medicine, Ilam University of Medical Sciences, Ilam, Iran.
- 2 Clinical Microbiology Research Center, Ilam University of Medical Sciences, Ilam, Iran.
- 3 Department of Infectious Diseases, School of Medicine, Ilam University of Medical Sciences, Ilam, Iran.
- 4 Department of Microbiology, School of Medicine, Ilam University of Medical Sciences, Ilam, Iran. [email protected].
- 5 Clinical Microbiology Research Center, Ilam University of Medical Sciences, Ilam, Iran. [email protected].
- 6 Clinical Microbiology Research Center, Ilam University of Medical Sciences, Ilam, Iran. [email protected].
- PMID: 38796602
- DOI: 10.1007/s11033-024-09580-9
Objective: This research study was undertaken to investigate antimicrobial resistance patterns and the prevalence of hospital-acquired infections (HAIs). The study focuses on common microorganisms responsible for HAIs and explores emerging challenges posed by antimicrobial drug-resistant isolates.
Methods: A comprehensive analysis of 123 patients with HAIs, hospitalized in surgical department and intensive care unit (ICU) at Imam Khomeini Hospital, Ilam, Iran, was conducted over a six-month period. Pathogenic bacterial isolates, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Staphylococcus aureus (VRSA), were isolated and subjected to antibiotic susceptibility testing.
Results: The study findings revealed a significant prevalence of multidrug-resistant (MDR) isolates, of which 73.3% were MRSA. Notably, 6.7% of S. aureus isolates exhibited resistance to vancomycin, indicating the emergence of VRSA. Respiratory infections were identified as the most prevalent HAI, constituting 34.67% of cases, often arising from extended ICU stays and invasive surgical procedures. Furthermore, patients aged 60 and above, particularly those associated with MDR, exhibited higher vulnerability to HAI.
Conclusions: This research sheds light on the intricate interplay between drug resistance and HAI, highlighting the imperative role of rational antibiotic use and infection control in addressing this critical healthcare challenge.
Keywords: Antimicrobial resistance; Hospital-acquired infection; Methicillin-resistant Staphylococcus aureus; Multidrug-resistant organisms; Vancomycin-Resistant Staphylococcus aureus.
© 2024. The Author(s), under exclusive licence to Springer Nature B.V.
- Anti-Bacterial Agents* / pharmacology
- Cross Infection* / epidemiology
- Cross Infection* / microbiology
- Drug Resistance, Multiple, Bacterial / genetics
- Intensive Care Units
- Iran / epidemiology
- Methicillin-Resistant Staphylococcus aureus* / drug effects
- Methicillin-Resistant Staphylococcus aureus* / genetics
- Methicillin-Resistant Staphylococcus aureus* / isolation & purification
- Methicillin-Resistant Staphylococcus aureus* / pathogenicity
- Microbial Sensitivity Tests*
- Middle Aged
- Staphylococcal Infections* / epidemiology
- Staphylococcal Infections* / microbiology
- Staphylococcus aureus / drug effects
- Staphylococcus aureus / genetics
- Staphylococcus aureus / isolation & purification
- Staphylococcus aureus / pathogenicity
- Vancomycin-Resistant Staphylococcus aureus / genetics
- Anti-Bacterial Agents

IMAGES
VIDEO
COMMENTS
Hospital-acquired infections (HAIs) are significant problems as public health issues which need attention. ... The research findings will contribute to the development of effective control programs by managers and policymakers of the health sector to reduce the financial costs of HAIs and save financial resources. ... The paper is generally ...
Objectives Hospital-acquired infections (HAIs) are significant problems as public health issues which need attention. Such infections are significant problems for society and healthcare organizations. This study aimed to carry out a systematic review and a meta-analysis to analyze the prevalence of HAIs globally. Methods We conducted a comprehensive search of electronic databases including ...
Hospital waste serves as potential source of pathogens and about 20%-25% of hospital waste is termed as hazardous. Nosocomial infections can be controlled by practicing infection control programs, keep check on antimicrobial use and its resistance, adopting antibiotic control policy. Efficient surveillance system can play its part at national ...
A point-prevalence survey that was conducted in the United States in 2011 showed that 4% of hospitalized patients had a health care-associated infection. We repeated the survey in 2015 to assess ...
Nosocomial infections or healthcare associated infections occur in patients under medical. care. These infections occur worldwide both in developed and developing countries. Nosocomial infections ...
Background Nosocomial infections (NCIs) have been associated with several adverse outcomes including extended hospitalization, persistent disability, heightened antimicrobial resistance, amplified socio-economic disruption, and elevated mortality rates. The adoption of infection prevention strategies has the greatest tendency to significantly reduce the risk and occurrence of NCIs among the ...
Background It is important to determine the prevalence and prognosis of community-acquired infection (CAI) and nosocomial infection (NI) to develop treatment strategies and appropriate medical policies in aging society. Methods Patients hospitalized between January 2010 and December 2019, for whom culture tests were performed and antibiotics were administered, were selected using a national ...
REVIEW IN HOSPITAL-ACQUIRED INFECTION. Dr. Nagham Mahmood Aljamali*1, Dr. Muhsin Mohammed Al Najim2. 1* Professor, Ph.D., Organic Chemistry, Synthetic Chemistry Field, Iraq. 2 College of Medicine ...
Introduction. Hospital-acquired infections (HAIs) are those infections, which are not present at the time of admission and acquired during the course of hospital settings manifested 48-72 h after admission, more prevalent in critical care settings.[1 2] World Health Organisation (WHO) reported overall 5.1-11.6% prevalence rate of HAIs in high-income countries (HICs).[]
Overall, 15.7% showed good knowledge while most patients showed fair knowledge (71.6%) and a negative perception (51%) of nosocomial infections. There was a significant diference in patients ...
This review mainly focused on general features and introduction to A. baumannii and its epidemiological status, potential sources of infection, risk factors, and strategies to control infection to minimize spread. Expand. 50. PDF. 1 Excerpt. Nosocomial infections in medical intensive care units in the United States.
Wound/surgical infections 24 17 ≈15 18.7 3 6.9 7.5 Skin and soft tissue infections 9 5.7 ≈15 2.1 15 4.8 3.9 Miscellaneous (meninges, bones, genital tract, gastrointestinal tract) 18 ≈14 ≈12 ≈10 10.8 8.5 a Primary bacteraemia. EPIIC = European Prevalence of Infection in Intensive Care; NNIS = National Nosocomial Infections Surveillance.
The COVID-19 pandemic led to 231,841 deaths and 940,243 hospitalisations in England, by the end of March 2023. This paper calculates the real-time infection hospitalisation risk (IHR) and ...
Study of Nosocomial Infections among the Patients Admitted in the Intensive Care Units of a Tertiary Care Centre in North East India April 2017 International Journal of Scientific Research 6(4):55-60
%PDF-1.4 %âãÏÓ 271 0 obj /Linearized 1 /O 273 /H [ 807 1993 ] /L 415401 /E 7991 /N 72 /T 409862 >> endobj xref 271 19 0000000016 00000 n 0000000731 00000 n 0000002800 00000 n 0000003037 00000 n 0000004151 00000 n 0000004440 00000 n 0000004479 00000 n 0000004501 00000 n 0000005199 00000 n 0000005220 00000 n 0000005311 00000 n 0000005590 00000 n 0000006693 00000 n 0000006743 00000 n ...
1 Introduction. Infectious diseases are a global public health challenge that causes the loss of 13 million lives annually. [] Over the past 50 years, many new and re-emerging infections have threatened humanity, for example, Legionnaire's disease, Ebola virus, human immunodeficiency viruses, and more recently, SARS-CoV-2, to name a few. [] A particularly concerning category of infectious ...
The need for a new review on masks was highlighted by a widely publicized polarization in scientific opinion. The masks section of a 2023 Cochrane review of non-pharmaceutical interventions was—controversially—limited to randomized controlled trials (RCTs).It was interpreted by the press and by some but not all of its own authors to mean that "masks don't work" and "mask mandates ...
This research sheds light on the intricate interplay between drug resistance and HAI, highlighting the imperative role of rational antibiotic use and infection control in addressing this critical healthcare challenge. ... Molecular characterization of Staphylococcus aureus isolated from hospital-acquired infections in Ilam, Iran Mol Biol Rep ...