- Download PDF
- Share X Facebook Email LinkedIn
- Permissions

The Ketogenic Diet for Obesity and Diabetes—Enthusiasm Outpaces Evidence
- 1 Division of General Internal Medicine, Department of Medicine, New York University School of Medicine, New York
- 2 Department of Medicine, NYC Health + Hospitals/Bellevue, New York
- 3 Division of Cardiology, Montefiore Health System, Bronx, New York
- JAMA Patient Page Ketogenic Diets Zhaoping Li, MD, PhD; David Heber, MD, PhD JAMA
- Correction Error in References JAMA Internal Medicine
- Comment & Response Ketogenic Diets for Diabetes and Obesity Frederick M. Hecht, MD JAMA Internal Medicine
- Comment & Response Ketogenic Diet for Obesity and Diabetes William S. Yancy Jr, MD, MHS; Nia S. Mitchell, MD, MPH; Eric C. Westman, MD, MHS JAMA Internal Medicine
- Comment & Response Ketogenic Diets for Diabetes and Obesity—Reply Shivam Joshi, MD; Robert J. Ostfeld, MD, MSc; Michelle McMacken, MD JAMA Internal Medicine
The ketogenic diet has recently received much attention for its promise of treating obesity and type 2 diabetes. However, the enthusiasm for its potential benefits exceeds the current evidence supporting its use for these conditions. Although the temptation is great to recommend a potentially novel approach for otherwise difficult-to-treat diseases, it is important to remain grounded in our appraisal of the risks, benefits, and applicability of the diet to avoid unnecessary harm and costs to patients.
Read More About
Joshi S , Ostfeld RJ , McMacken M. The Ketogenic Diet for Obesity and Diabetes—Enthusiasm Outpaces Evidence. JAMA Intern Med. 2019;179(9):1163–1164. doi:10.1001/jamainternmed.2019.2633
Manage citations:
© 2024
Artificial Intelligence Resource Center
Best of JAMA Network 2022
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 30 November 2020
Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis
- Xiaojie Yuan 1 na1 ,
- Jiping Wang 1 na1 ,
- Shuo Yang 2 na1 ,
- Mei Gao 2 ,
- Lingxia Cao 2 ,
- Xumei Li 1 ,
- Dongxu Hong 1 ,
- Suyan Tian 3 &
- Chenglin Sun ORCID: orcid.org/0000-0003-3570-1918 1 , 2
Nutrition & Diabetes volume 10 , Article number: 38 ( 2020 ) Cite this article
37k Accesses
106 Citations
495 Altmetric
Metrics details
- Type 2 diabetes
At present, the beneficial effect of the ketogenic diet (KD) on weight loss in obese patients is generally recognized. However, a systematic research on the role of KD in the improvement of glycemic and lipid metabolism of patients with diabetes is still found scarce.
This meta-study employed the meta-analysis model of random effects or of fixed effects to analyze the average difference before and after KD and the corresponding 95% CI, thereby evaluating the effect of KD on T2DM.
After KD intervention, in terms of glycemic control, the level of fasting blood glucose decreased by 1.29 mmol/L (95% CI: −1.78 to −0.79) on average, and glycated hemoglobin A1c by 1.07 (95% CI: −1.37 to −0.78). As for lipid metabolism, triglyceride was decreased by 0.72 (95% CI: −1.01 to −0.43) on average, total cholesterol by 0.33 (95% CI: −0.66 to −0.01), and low-density lipoprotein by 0.05 (95% CI: −0.25 to −0.15); yet, high-density lipoprotein increased by 0.14 (95% CI: 0.03−0.25). In addition, patients’ weight decreased by 8.66 (95% CI: −11.40 to −5.92), waist circumference by 9.17 (95% CI: −10.67 to −7.66), and BMI by 3.13 (95% CI: −3.31 to −2.95).
KD not only has a therapeutic effect on glycemic and lipid control among patients with T2DM but also significantly contributes to their weight loss.
Similar content being viewed by others
Divergent age-associated and metabolism-associated gut microbiome signatures modulate cardiovascular disease risk

Gut microbiome remodeling and metabolomic profile improves in response to protein pacing with intermittent fasting versus continuous caloric restriction
Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial
Introduction.
Diabetes mellitus (DM) is the world’s leading cause for motility and morbidity, and the disease has become a major public health burden worldwide. It is estimated that the prevalence of diabetes in adults worldwide is over 300 million, and it will increase by 55% by 2035 1 . Obesity or overweight is one of the essential risk factors for diabetes and contributes to a twice-higher risk to develop DM 2 , 3 . Thus, dietary therapy aiming at weight loss is typically recommended in clinical practice 4 . Due to the fact that diabetes and its complications affect many aspects of physiology, the benefits of weight reduction are not limited to glycemic control but are also related to many cardiovascular risk factors such as blood pressure, high-density lipoprotein (HDL), total cholesterol (TC) and triglyceride (TG) 2 .
Medical nutrition, as part of the comprehensive treatment of DM with obesity with a primary goal of weight reduction, is the most simple, effective and economical choice of intervention. The dietary approach for body weight reduction can be obtained from many strategies, including a low-calorie diet, a very low-calorie diet, high-protein diet, and so on. Ketogenic diet (KD), which contains a very low level of carbohydrates (<55 g/d) with the main energy sources of lipid and protein, and which causes ketosis and simulates the physiological state of fasting, has been well reported to be effective for weight loss and glycemic control 4 , 5 , 6 , 7 , 8 , 9 . Previous meta-analyses have proved the efficacy of KD in body weight reduction 2 , 10 , 11 ; however, systemic reviews on the effect of KD on weight reduction and glycolipid metabolism in patients with DM are still limited. Westman et al. 12 and Partsalaki et al. 13 demonstrated that KD improved type 2 diabetes mellitus (T2DM) by reducing the glycemic response caused by carbohydrate and improving potential insulin resistance. Leonetti et al. 14 and Walton et al. 15 reported reduced TG and TC with increased HDL levels after KD consumption for a lipid profile. However, controversies are still existing; studies revealed that a low-carbohydrate, high-fat diet may exacerbate the lipid profile in patients with diabetes, although glycemic control improved with hypoglycemic medications 16 , 17 , 18 . Therefore, the purpose of the current review was to conduct a meta-analysis on the effects of a KD in patients with diabetes.
Considering the potential benefits of KD in diabetes management and weight reduction, and considering fasting blood glucose and glycated hemoglobin A1c (HbA1c) as common biomarkers for long-term glycemic control, HDL, LDL, TC, and TG levels are included in the current analysis to determine the changes of metabolic disorders in glucose and lipid metabolism. In addition, the homeostatic model assessment of insulin resistance (HOMA-IR) is considered as a reflection of insulin resistance reversal.
Materials and methods
Literature search.
In this meta-analysis, only studies published in English were considered, which were identified by searching the PubMed and MEDLINE databases. The keywords used for this literature search are T2DM or diabetes mellitus, ketogenic diet, obesity, and human. The search was finished on September 20, 2019. This meta-analysis was planned and performed according to the Preferred Reporting Items for Systemic Reviews (PRISM) guideline (Fig. 1 ).
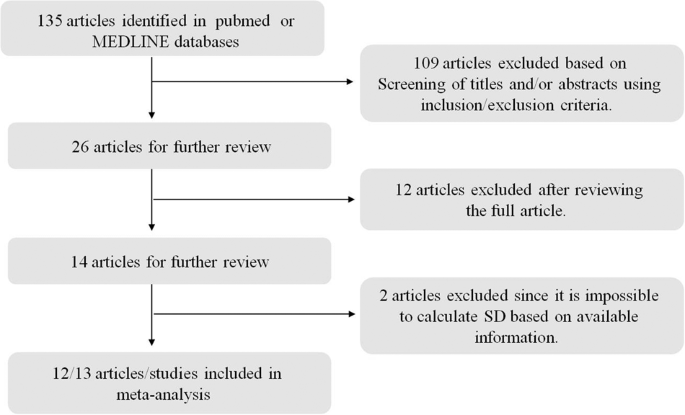
Only studies published in English were considered, which were identified by searching the PubMed and MEDLINE databases. The keywords used for this literature search are T2DM or diabetes mellitus, ketogenic diet, obesity, and human. The search was finished on September 20, 2019.
Inclusive/exclusive criteria
Studies that met the following inclusive criteria were included: (1) the disease of interest is type II diabetes; (2) the therapeutic diet under consideration is KD; (3) the study was carried out on humans; animal experiments are not included; and (4) the summary statistics of the mean difference between before and after KD (if both means for before and after measurements are available, then we took the difference of these two statistics to obtain the desired mean difference), their corresponding standard error or 95% CI (according to this, the standard error was calculated) or p values (according to this, the corresponding t statistics and subsequently the standard error were calculated) are available.
Exclusive criteria: (1) case report studies were excluded; (2) meta-analysis or review studies were excluded; (3) studies on other diseases rather than type II diabetes were excluded; and (4) if only the respective mean and standard errors were available, such studies were excluded given it is hard to get an accurate estimation for the standard error of mean difference (since both measurements were on the same patient, they should be correlated to each other, and hence it is impossible to estimate this correlation).
Statistical analysis
The effects of KD on type II diabetes were estimated by the mean difference after KD versus before KD and their corresponding 95% CIs in random-effects meta-analysis models or fixed-effect meta-analysis models. To determine which model should be used, heterogeneity among studies was evaluated by the Cochrane’s Q statistic corresponding p values and the I 2 statistics. If the p value was <0.05 and I 2 > 0.5, a random-effect meta-analysis model was used. Otherwise, a fixed-effect meta-analysis model was chosen. Additionally, potential bias was assessed by using funnel plots, in which effect sizes versus standard errors were diagrammed. All statistical analysis was carried out in the R software, version 3.5 ( www.r-project.org ) 19 , 20 , 21 .
There are 13 studies included in this meta-analysis; the details of these 13 studies are presented in Table 1 . In total, 567 subjects were included in the final meta-analysis. From the perspective of glucose metabolism, lipid metabolism, and weight control, the effects of KD on T2DM were systemically reviewed by comparing the after-intervention measures with before-intervention measures of several biomarkers for the same patient. The variables used to surrogate for carbohydrate metabolism are included fasting glucose level and HbA1c; for lipid metabolism TC, TG, HDL and LDL; and for weight loss body weight, BMI and waist circumference. For all variables except BMI and waist, random-effect models were adopted according to the Q statistic p value and I 2 statistics.
Using the meta-analysis method, we found that the fasting blood glucose level was decreased 1.29 mmol/l (95% CI: −1.78 to −0.79) after the intervention of KD, compared to before such an intervention (based on ten articles that have the summary statistics for the difference between after- and before-intervention measures). As far as HbA1c is concerned, we found that the reduced proportion of HbA1c is more significant after the KD implementation, with a difference of −1.07% (95% CI: −1.37 to −0.78), which is regarded as the ideal therapeutic effect of drugs that is possible to be achieved on HbA1c. The forest plots for these two carbohydrates metabolism indices are given in Fig. 2 .
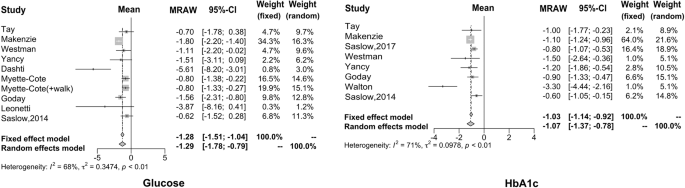
The reduced proportion of HbA1c is more significant after the KD implementation, which is regarded as the ideal therapeutic effect of drugs that is possible to be achieved on HbA1c.
In this study, eight articles investigated the effect of KD on the lipid metabolism of diabetic patients, but only five papers analyzed total cholesterol. It can be seen that after KD consumption, TG decreased by 0.72 mmol/L (95% CI: −1.01 to −0.43), TC decreased by 0.33 mmol/L (95% CI: −0.66 to −0.01), and LDL decreased by 0.05 mmol/L (95% CI: −0.25 to −0.15). On the other hand, HDL increased by 0.14 mmol/L (95% CI: 0.03−0.25). The forest plots for these four biomarkers are shown in Fig. 3 .
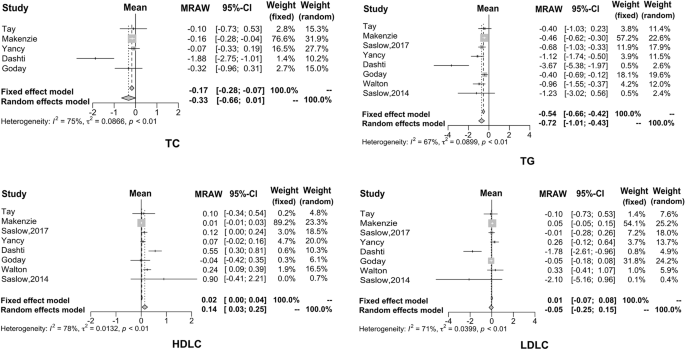
It can be seen that after KD consumption, TG, TC, and LDL decreased. On the other hand, HDL increased.
Regarding weight loss, many studies have demonstrated that KD has a positive effect by providing effective control over obesity. The results of our meta-analysis are consistent with previous results. Specifically, the average weight decreased by 8.66 kg (95% CI: −11.40 to −5.92), waist circumference reduced by 9.17 cm (95% CI: −10.67 to −7.66) and BMI reduced by 3.13 kg/m 2 (95% CI: −3.31 to −2.95), as shown in Fig. 4 .
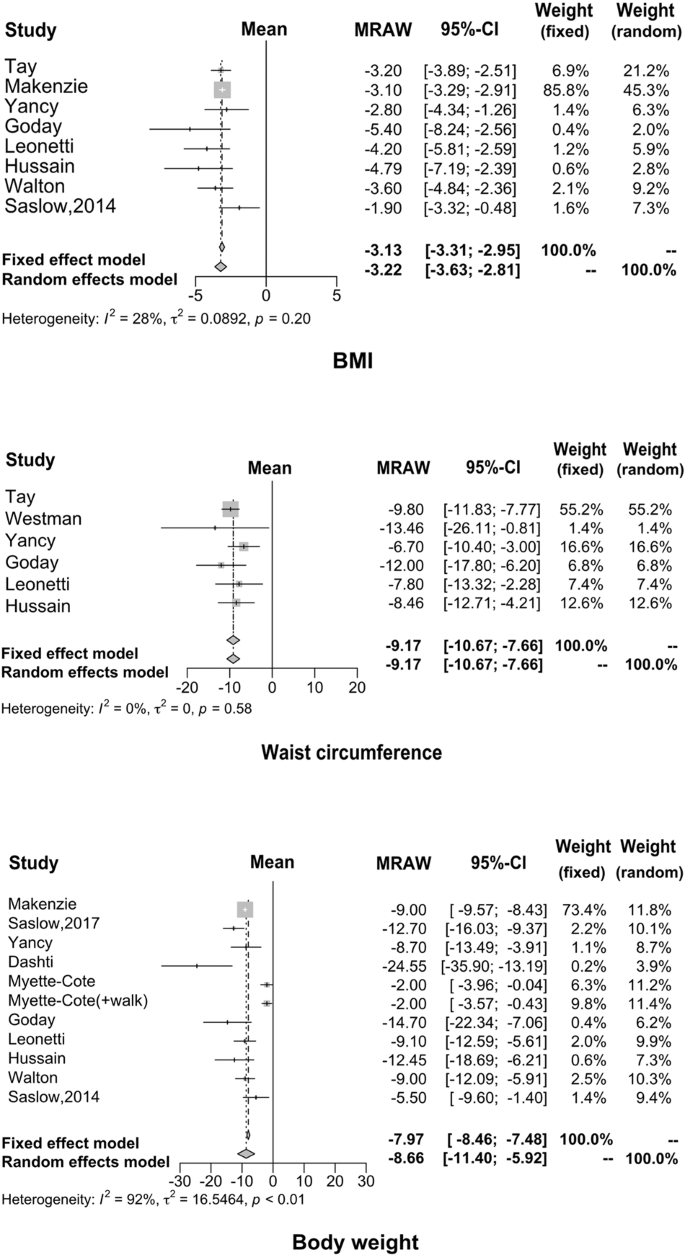
Many studies have demonstrated that KD has a positive effect by providing effective control over obesity; our findings were consistent with the previous reports.
The American Diabetes Society (ADA) recommended physical activity, dietary management, and medical intake and other approaches should be applied simultaneously to manage blood glucose levels, and other abnormal metabolic factors. KD showed numerous health benefits to patients with T2DM 22 , 23 . KD provides energy through fat oxidation. When the human body experienced extreme hunger or very limited carbohydrate, the ketone body was produced and released to circulation by hepatic transformation of fatty acids 24 , 25 . Nutritional ketosis is different from severe pathological diabetic ketosis; the blood ketone body remained at 0.5−3.0 mmol/L with reduced blood glucose and normal blood pH, with no symptoms in nutritional ketosis 26 .
The possible mechanism for the health benefit of KD on patients with T2DM is that the extreme restriction of carbohydrate reduces the intestinal absorption of mono-saccharide, which leads to lower blood glucose level and reduces the fluctuation of blood glucose, and its effectiveness on regulating glucose metabolism was confirmed by a large body of evidence 27 , 28 . The current study analyzed 13 studies from literature focusing on diabetic patients; the results showed that the reduction of blood glucose ranges from 0.62 to 5.61 mmol/L. Higher reduction amplitudes were reported by Dashti 29 and Leonetti et al. 14 of 5.61 mmol/L (weight random 3.0%) and 3.87 mmol/L (weight random 1.2%), respectively; other reductions in blood glucose were all lower than 1.8 mmol/L. The possible reason for the higher reduction found in these two studies could be the higher blood glucose level included in the studies, and also that the average blood glucose concentration was above 10.0 mmol/L, leading to the possibility of a larger reduction; however, their contribution to the overall effect estimations in the meta-analysis was low. The average changes in fasting blood glucose after the KD consumption among the selected studies were −1.29 mmol/L, indicating the effectiveness of the KD in lowering fasting blood glucose.
No studies included in this meta-analysis evaluated the effect of KD on postprandial glucose level; unlike medications, dietetic therapy showed a long-term effect on glucose regulation 4 , 16 , and HbA1c was analyzed to evaluate the long-term effect of KD. HbA1c effectively reflects the blood glucose control in the past 2−3 months in patients with diabetes. It is reported that the risk of cardiac infarction and micro-vascular complications reduced by 14% and 37%, respectively, when HbA1c reduced by 1%. Therefore, the HbA1c level showed essential clinical significance in evaluating the blood glucose control, revealing the potential problems in the treatment and thereby guiding the therapeutic schedule 30 , 31 . Eight of the selected studies showed a reduction of HbA1c after KD consumption, the changes ranging from −0.6% to −3.3%; HbA1c reduced <1.5% in the majority of the studies included in the current analysis besides the study conducted by Walton (−3.3%; weight random 5.1%) 15 . The possible explanation for such strong improvement of HbA1c could be that Walton’s study had enrolled a limited number of patients and thus the compliance of patients to KD therapy can be guaranteed. Moreover, the studied subjects were newly diagnosed diabetic patients who were under dietary management without taking glucose-lowering medications; newly diagnosed subjects persist well in the study. Considering the causal factors comprehensively, the above study showed an ideal reduction in HbA1c. The average reduction of HbA1c was 1.07 in the current analysis of the selected eight studies, indicating that dietary management may also achieve the ideal therapeutic effects of medication.
HOMA-IR is considered as an indicator to evaluate the status of insulin resistance. Insulin resistance as a clinical characteristic of T2DM is closely related to obesity. Improving insulin resistance is one of the major targets in diabetes treatment 32 , 33 , 34 . However, studies focusing on the role of KD in the improvement of insulin resistance in patients with diabetes are very limited; most of the studies focused on the effect in obese subjects 35 , 36 . For instance, a controlled clinical trial aiming at the effects of KD consumption in obese people without diabetes revealed that HOMA-IR decreased by about 2.0 after KD consumption for 6 weeks 37 . The current analysis showed consistent changes in the studies that included HOMA-IR evaluation, with reduction ranging from −0.4 to −3.4; the reason for the significant reduction of 3.4 in the study by Tay et al. 38 is that the population included was obese diabetic patients with BMI higher than 30 kg/m 2 . Obesity is closely related to insulin resistance; KD consumption is confirmed to be effective in reducing body weight, and it is expected that KD may improve insulin resistance in obese diabetic patients 39 . During the ketogenesis, the sensitivity of the insulin receptor is promoted; therefore, KD not only ensures the supply of basic nutrients but also maintains a negative balance of energy, and reduces the fluctuation and reduction of insulin secretion caused by reduced carbohydrate intake as well, which eventually leads to improved insulin sensitivity 40 , 41 , 42 , 43 .
Consumption of KD not only improved glucose metabolism, but a large body of evidence has reported that KD improved lipid metabolism as well. Hussain et al. 4 reported that KD reduced TG and TC, and increased HDL level, thus ameliorating the status of dyslipidemia. In the present study, eight studies included showed results of lipid metabolism in diabetic patients after KD consumption; however, only five analyzed the TC levels. The current results showed the mean reduction of TG was 0.72 mmol/L, TC was 0.33 mmol/L, and LDL was 0.05 mmol/L, while the increase of HDL was 0.14 mmol/L. The higher amplitude of variation occurred in the Dashti et al. study 29 . This study reported that TG reduced by 3.67 mmol/L, TC reduced by 1.88 mmol/L, and LDL reduced by 1.78 mmol/L, while HDL increased by 0.14 mmol/L. Changes in the amplitude of the lipid biomarkers were all at the higher end in the above study. Both glucose and lipid metabolism showed great improvement after KD consumption in such a study; the characteristics of subjects recruited were closely correlated. The study recruited 31 obese subjects with hyperglycemia, dyslipidemia, and BMI over 30 kg/m 2 . The baseline TG, TC, and LDL were higher than those of typical patients with T2DM, which may contribute to the significant changes after the intervention. Consumption of KD showed a significant therapeutic effect in common patients with T2DM, including the Dashti 29 study. Disorders of lipid metabolism are particularly strong among patients with insulin resistance in T2DM. Dyslipidemia is lipotoxic to cells, leading to and/or aggravating insulin resistance. Its typical manifestation is the increase of TG and free fatty acid (FFA) 44 , 45 , 46 , 47 . Increased FFA is an independent pathogenic factor for insulin resistance and can possibly increase the risk for cardiovascular diseases 48 , 49 . Therefore, the improvement of dyslipidemia is beneficial for not only regulating insulin sensitivity but also controlling the occurrence and progression of diabetic complications 50 , 51 .
Numerous studies have confirmed the role of KD consumption in weight reduction in obese patients 35 , 36 , 37 , 40 , 41 , 42 , 43 , 52 ; the current meta-analysis focused on the effect of KD on weight reduction in obese diabetic patients. The results showed the average reduction of body weight was 8.66 kg, waist circumference was 9.17 cm, and BMI was 3.22 kg/m 2 , which were consistent with previous studies in nondiabetic patients. We also found that KD reduced systolic blood pressure by 4.30 (95% CI: −7.02 to 1.58) and diastolic blood pressure by 5.14 (95% CI: −10.18 to 0.10) in patients with T2DM, which possibly benefit from the improvement of body weight 51 .
Besides the mediation of glucose and lipid metabolism, KD may also benefit other clinical symptoms in diabetic patients, including insomnia, chills, constipation, pruritus, numbness of limbs, hypopsia, fatty liver, hypertension, and reduced cardiac function.
The potential side effects of KD were only mentioned in two of the studies 14 , 41 included in the meta-analysis; thus it is impossible to perform a systematic review in terms of the risks associated with KD consumption. Specifically, Goday and Leonetti’s 14 , 41 study investigated the adverse reactions of KD. Goday et al. 41 mentioned that fatigue, headache, nausea and vomiting were more common in the KD diet group after a 2-week intervention, while constipation and orthostatic hypotension were more common after 10 weeks. It was revealed by Leonetti et al. 14 that in the early stages of applying the KD, patients reported a sense of hunger, but it could be significantly alleviated with the progress of the intervention. Even though headache, nausea, vomiting, constipation, diarrhea, and other symptoms were reported during the study, the symptoms were mild and lasted for a short time, not relating to clinical practice.
Limitations
Only 13 studies were included in the current analysis, with limited studies focusing on the effect of KD in patients with T2DM worldwide. For instance, no analysis was conducted on HOMA-IR even though there was a trend of improvement; also, very limited literature was available. All studies included in this meta-analysis were carried out among Caucasian diabetic patients (no East Asians included); however, the majority of East Asian diabetic patients showed insulin resistance with central obesity and defect in insulin secretion. Therefore, clinical trials conducted among East Asians are highly desirable to confirm whether there is an improvement in the secretion function of islet cells other than improved regulation of glucose and lipid metabolism. Moreover, the current study analyzed the data without assigning studies into time duration due to the limited number of studies and the missing data of insulin and lipid biomarkers; in addition, the duration of the follow-up was decentralized into days, months, and years. The available studies concerning the effects of ketogenic diet in patients with diabetes are very limited; it is impossible to summarize a similar follow-up interval for statistical analysis of time points. However, the current results suggested that ketogenic diet consumption contributed to therapeutic effects despite the length of the term of intervention. The analysis of the difference before and after the intervention may also give credit to the clinical efficacy of the diet therapy. In current clinical practice, a majority of the patients have to use a combination of multiple drugs to improve their glycolipid metabolism. Drug therapy is a heavy mental and economical burden to patients. Given the fact that most of the patients are confused regarding a proper dietary therapy plan, it is essential to recommend a feasible dietary therapy plan to transmit a positive message to both patients with diabetes and physicians majored in the area of diabetes.
Based on a meta-analysis that systematically reviewed 13 relevant studies, we found that ketogenic diet can not only control fasting blood glucose and reduce glycosylated hemoglobin, but also improve lipid metabolism. Additionally, ketogenic diet can reduce BMI and body weight. Therefore, ketogenic diet may be used as part of the integrated management of type 2 diabetes.
Guariguata, L. et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pr. 103 , 139–149 (2014).
Google Scholar
Yu, Z. et al. Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in T2DM: a systematic review and metaanalysis of randomized controlled trials. Clin. Nutr. 39 , 1724–1734 (2020).
Article CAS Google Scholar
Guh, D. P. et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9 , 88 (2009).
Article Google Scholar
Hussain, T. A. et al. Effect of low-calorie versus low-carbohydrate ketogenic diet in T2DM. Nutrition 28 , 1061–1021 (2012).
Hall, K. D. et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am. J. Clin. Nutr. 104 , 324–333 (2016).
Hamdy, O. et al. Fat versus carbohydrate-based energy-restricted diets for weight loss in patients with T2DM. Curr. Diab. Rep. 18 , 128 (2018).
Joshi, S., Ostfeld, R. J. & McMacken, M. The ketogenic diet for obesity and diabetes-enthusiasm outpaces evidence. JAMA Intern. Med. 179 , 1163–1164 (2019).
Saslow, L. R. et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with T2DM: a randomized controlled trial. J. Med. Internet Res. 19 , e36 (2017).
Saslow, L. R. et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with T2DM mellitus or prediabetes. Nutr. Diabetes 7 , 304 (2017).
Meng, Y. et al. Efficacy of low carbohydrate diet for T2DM mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pr. 131 , 124–131 (2017).
Castellana, M. et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 21 , 5–16 (2019).
Westman, E. C., Yancy, W. S. Jr., Mavropoulos, J. C., Marquart, M. & McDuffie, J. R. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in T2DM mellitus. Nutr. Metab. 5 , 36 (2008).
Partsalaki, I., Karvela, A. & Spiliotis, B. E. Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 25 , 697–704 (2012).
Leonetti, F. et al. Very low-carbohydrate ketogenic diet before bariatric surgery: prospective evaluation of a sequential diet. Obes. Surg. 25 , 64–71 (2015).
Walton, C. M., Perry, K., Hart, R. H., Berry, S. L. & Bikman, B. T. Improvement in glycemic and lipid profiles in type 2 diabetics with a 90-day ketogenic diet. J. Diabetes Res. 14 , 8681959 (2019).
Leow, Z. Z. X., Guelfi, K. J., Davis, E. A., Jones, T. W. & Fournier, P. A. The glycaemic benefits of a very-low-carbohydrate ketogenic diet in adults with Type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet. Med. 35 , 9 (2018).
Azevedo, D. L. P. et al. Effect of classic ketogenic diet treatment on lipoprotein subfractions in children and adolescents with refractory epilepsy. Nutrition 33 , 271–277 (2017).
Krebs, J. D. et al. Improvements in glucose metabolism and insulin sensitivity with a low-carbohydrate diet in obese patients with T2DM. J. Am. Coll. Nutr. 32 , 11–17 (2013).
Lau, J., Ioannidis, J. P. & Schmid, C. H. Quantitative synthesis in systematic reviews. Ann. Intern. Med. 127 , 820–826 (1997).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 , 1539–1558 (2002).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 7 , 177–188 (1986).
McKenzie, A. L. et al. A Novel Intervention Including Individualized Nutritional Recommendations Reduces Hemoglobin A1c Level, Medication Use, and Weight in Type 2 Diabetes. JMIR Diabetes 2 , e5 (2017).
Yancy, W. S. et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. (Lond) 2 , 34 (2005).
Athinarayanan, S. J. et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of T2DM: a 2-year non-randomized clinical trial. Front. Endocrinol. 10 , 348 (2019).
Laffel, L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 15 , 412–426 (1999).
Gershuni, V. M., Yan, S. L. & Medici, V. Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr. Nutr. Rep. 7 , 97–106 (2018).
Yancy, W. S., Vernon, M. C. & Westman, E. C. A pilot trial of a low-carbohydrate, ketogenic diet in patients with T2DM. Metab. Syndr. Relat. Disord. 1 , 239–243 (2003).
Bolla, A. M., Caretto, A., Laurenzi, A., Scavini, M. & Piemonti, L. Low-carb and ketogenic diets in type 1 and type 2 diabetes. Nutrients 26 , 962 (2019).
Dashti, H. M. et al . Beneficial effects of ketogenic diet in obese diabetic subjects. Mol. Cell Biochem . 302 , 249–256 (2007).
Yancy, W. S. Jr. et al. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch. Intern. Med . 170 , 136–145 (2010).
Stratton, I. M. et al. Association of glycaemia with macrovascular and microvascular complications of T2DM (UKPDS 35): prospective observational study. BMJ 321 , 405–412 (2000).
Nakanishi, S. et al. Comparison of HbA1c levels and body mass index for prevention of diabetic kidney disease: a retrospective longitudinal study using outpatient clinical data in Japanese patients with T2DM mellitus. Diabetes Res. Clin. Pr . 155 , 107807 (2019).
Dehghan, P. & Abbasalizad, F. M. Dietary acid load, blood pressure, fasting blood sugar and biomarkers of insulin resistance among adults: findings from an updated systematic review and meta-analysis. Int. J. Clin. Pr. 74 , 4 (2019).
Avtanski, D., Pavlov, V. A., Tracey, K. J. & Poretsky, L. Characterization of inflammation and insulin resistance in high-fat diet-induced male C57BL/6J mouse model of obesity. Anim. Model Exp. Med . 2 , 252–258 (2019).
Saslow, L. R. et al. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLoS One 9 , e91027 (2014).
Stocker, R. K., Reber, A. E., Bally, L., Nuoffer, J. M. & Stanga, Z. Ketogenic diet and its evidence-based therapeutic implementation in endocrine diseases. Praxis 108 , 541–553 (2019).
Handley, R. T., Bentley, R. E., Brown, T. L. & Annan, A. A. Successful treatment of obesity and insulin resistance via ketogenic diet status post Roux-en-Y. BMJ Case Rep. 2018 , bcr2018225643 (2018).
Tay, J. et al. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: a randomized trial. Am. J. Clin. Nutr . 102 , 780–790 (2015).
Kinzig, K. P., Honors, M. A. & Hargrave, S. L. Insulin sensitivity and glucose tolerance are altered by maintenance on a ketogenic diet. Endocrinology 151 , 3105–3114 (2010).
Cox, N., Gibas, S., Salisbury, M., Gomer, J. & Gibas, K. Ketogenic diets potentially reverse Type II diabetes and ameliorate clinical depression: a case study. Diabetes Metab. Syndr . 13 , 1475–1479 (2019).
Goday, A. et al. Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutr Diabetes 6 , e230 (2016).
Cohen, C. W. et al. A ketogenic diet reduces central obesity and serum Insulin in women with ovarian or endometrial cancer. J. Nutr . 148 , 1253–1260 (2018).
Myette-Cote, E. et al. The effect of a short-term low-carbohydrate, high-fat diet with or without postmeal walks on glycemic control and inflammation in type 2 diabetes: a randomized trial. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315 , R1210–R1219 (2018).
Zhu, B. et al. Lipid oversupply induces CD36 sarcolemmal translocation via dual modulation of PKCzeta and TBC1D1: an early event prior to insulin resistance. Theranostics 10 , 1332–1354 (2020).
Akhtar, D. H., Iqbal, U., Vazquez-Montesino, L. M., Dennis, B. B. & Ahmed, A. Pathogenesis of insulin resistance and atherogenic dyslipidemia in nonalcoholic fatty liver disease. J. Clin. Transl. Hepatol. 7 , 362–370 (2019).
Iqbal, J., Al Qarni, A., Hawwari, A., Alghanem, A. F. & Ahmed, G. Metabolic syndrome, dyslipidemia and regulation of lipoprotein metabolism. Curr. Diabetes Rev . 14 , 427–433 (2018).
Laakso, M. & Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol . 10 , 293–302 (2014).
Han, L. et al. Free fatty acid can induce cardiac dysfunction and alter insulin signaling pathways in the heart. Lipids Health Dis. 17 , 185 (2018).
Yu, S. et al. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res. Ther . 10 , 333 (2019).
Ponce, A. J. et al. Low prolactin levels are associated with visceral adipocyte hypertrophy and insulin resistance in humans. Endocrine 67 , 331–343 (2020).
Karasek, D. & Vaverkova, H. Diabetic dyslipidemia and microvascular complications of diabetes. Vnitr. Lek. 64 , 17–24 (2018).
Sajoux, I. et al. Effect of a very-low-calorie ketogenic diet on circulating myokine levels compared with the effect of bariatric surgery or a low-calorie diet in patients with obesity. Nutrients 11 , 2368 (2019).
Download references
Acknowledgements
This study was supported by the Science Technology Department of Jilin Province (20180623006TC and 20200404213YY) and the Interdisciplinary Project of First Hospital of Jilin University (JDYYJC010) and Transformation Project of First Hospital of Jilin University (JDYYZH-1902019) and Education Department of Jilin Province (JJKH20190032KJ and JJKH20201081KJ).
Author information
These authors contributed equally: Xiaojie Yuan, Jiping Wang, Shuo Yang
Authors and Affiliations
Department of Clinical Nutrition, First Hospital of Jilin University, 1 Xinmin Street, 130021, Changchun, Jilin, China
Xiaojie Yuan, Jiping Wang, Xumei Li, Dongxu Hong & Chenglin Sun
Department of Endocrinology and Metabolism, First Hospital of Jilin University, 1 Xinmin Street, 130021, Changchun, Jilin, China
Shuo Yang, Mei Gao, Lingxia Cao & Chenglin Sun
Division of Clinical Research, First Hospital of Jilin University, 1 Xinmin Street, 130021, Changchun, Jilin, China
You can also search for this author in PubMed Google Scholar
Contributions
X.Y., J.W., S.Y., S.T. and C.S. were responsible for writing, M.G., L.C., X.L. and S.Y. were responsible for the literature collection and data management, D.H. and S.T. for statistical analysis, C.S. and S.T. are in charge of the overall research design and supervision.
Corresponding authors
Correspondence to Suyan Tian or Chenglin Sun .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yuan, X., Wang, J., Yang, S. et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr. Diabetes 10 , 38 (2020). https://doi.org/10.1038/s41387-020-00142-z
Download citation
Received : 06 July 2020
Revised : 19 October 2020
Accepted : 06 November 2020
Published : 30 November 2020
DOI : https://doi.org/10.1038/s41387-020-00142-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
A randomized feasibility trial of medium chain triglyceride-supplemented ketogenic diet in people with parkinson's disease.
- Alexander H. Choi
- Melanie Delgado
- Debra J. Ehrlich
BMC Neurology (2024)
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
- Yu-Ling Xiao
- Yi-Zhou Jiang
Signal Transduction and Targeted Therapy (2024)
The Ketogenic Diet as a Treatment for Mood Disorders
- Virginie-Anne Chouinard
- Christopher M. Palmer
Current Treatment Options in Psychiatry (2024)
Effects of ketogenic diet on health outcomes: an umbrella review of meta-analyses of randomized clinical trials
- Chanthawat Patikorn
- Pantakarn Saidoung
- Nathorn Chaiyakunapruk
BMC Medicine (2023)
Adherence to ketogenic diet in lifestyle interventions in adults with overweight or obesity and type 2 diabetes: a scoping review
Nutrition & Diabetes (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Ketogenic Diet: Overview Presentation
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
Diet as the Way to Lose Weight
- Many people want to lose weight.
- Every person has their reasons.
- The issue of weight loss is relevant, but requires careful monitoring.
Often, many people think about losing extra weight. Every person has their reasons for doing so – one wants to lead a healthier life, while others want to attract people. One way or another, the questions of weight loss are acute for humanity; for this reason, quite often among the already known classic methods of weight loss new and radical options that can aggravate human health appear. In order to prevent unnecessary health issues and achieve the desired effect, nutritionists offer patients to use the rules of the popular ketogenic diet.
Everything New is Actually Well-forgotten Old
- In the early twentieth century, ketogenic diet was used to treat epilepsy.
- Carbohydrate deficiency in the diet caused insulin reduction.
- With the advent of drugs, the diet has been relegated to the background.
- It was only thirty years ago that nutritionists rediscovered the ketogenic diet.
Recently, ketogenic diets have been used by a large number of young and adult people, and this method of starvation has become incredibly popular. However, if one looks at the historical context of ketogenic diets, it is clear that the diet has a rather long and mixed history. This diet was first introduced in the early 20th century to treat diseases of the nervous system, particularly epilepsy (Rho 5). A prerequisite for the appearance of the diet was the use of fasting: when food ceased to arrive, insulin stopped being produced in the body, significantly affecting the central nervous system (Rho 5). Such fasting was indeed valid, but it was impossible to use it for a long time. The situation was particularly difficult for children, as deprivation of food posed a serious health hazard (Rho 6). At that time, a diet similar to healing fasting was developed, but it gave the body energy from fats. It showed excellent results – people with epilepsy practically stopped having seizures. Indeed, the success of the ketogenic diet was not celebrated for long: soon, there were specially developed drugs, as a result of which the diet was forgotten for some time. It was only in the 1990s, when the problem of non-universal antiepileptic drugs for patients matured, that such a diet became discussed again. Information about ketogenic diets started to spread in the media, and the “new” weight-loss remedy began to be popularized.

The Mechanism of the Diet
The rule is simple: lack of carbohydrates leads to burning of fats. The body draws energy from burning its own deposits or with food – as a result, the body weight is reduced.
The meaning of the ketogenic diet is that when carbohydrates are stopped when glucose concentration in blood decreases, the body searches for other sources of energy and burns fat. This process involves both fats from food and the body’s supplies. In the reorganization of diet, the liver produces ketone bodies (acetoacetate), which participate in the oxidation of fatty acids and are used as energy by many organs, including the brain. This condition is called ketosis (Yancy, Mitchell, & Westman 1734). As glucose disappears from the blood, the production of insulin, a hormone that prevents fat burning, is reduced.

Scientific Evidence
- There are many scientific proofs of the optimality of this model.
- Zajac et al. confirmed the short-term effect of the diet on athletes.
- Carbohydrate elimination strengthens muscles, normalizes the biochemical composition and helps to quickly lose weight.
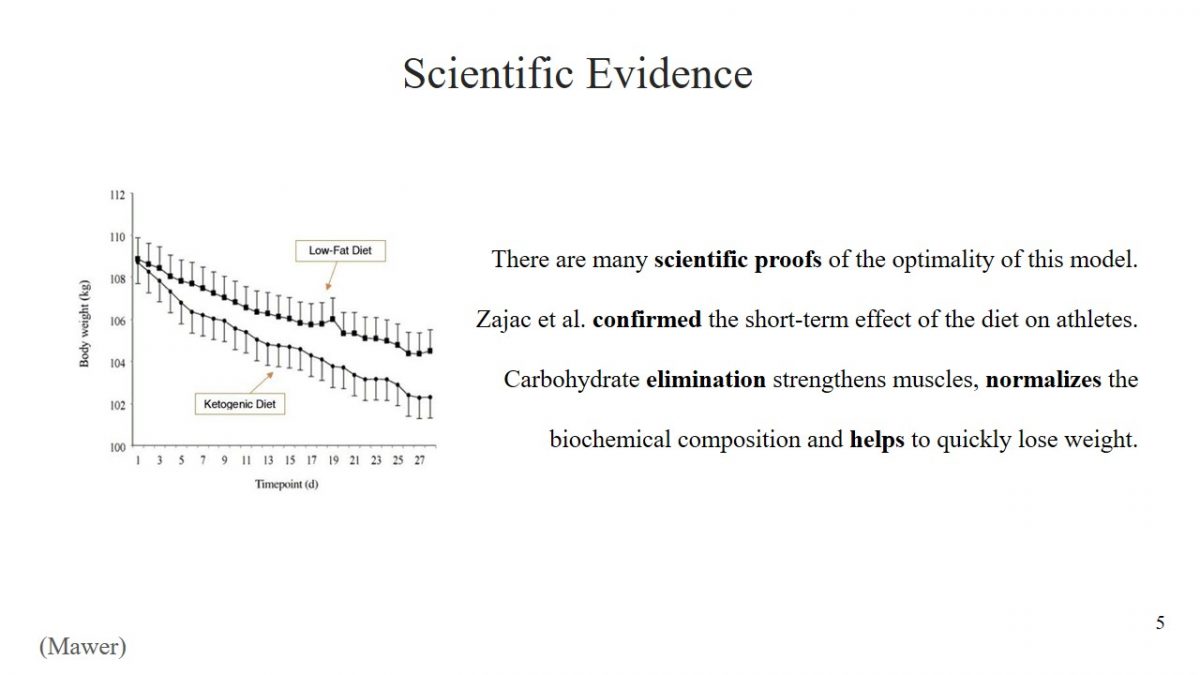
The decision to use any diet must be meaningful, and the effectiveness of starvation has been scientifically proven. For example, Zajac et al. found that a ketogenic diet has a positive effect on the performance of athletes (2496). The study determined what changes would result from changing diets to predominantly fatty young cyclists. Scientists concluded that the short-term effect of a ketogenic diet could preserve muscle structures in athletes after exercise, reduce body weight, and improve biochemical composition. An incredible achievement of Zajac et al. is the discovery that a ketogenic diet has a positive effect on a person’s breathing ability (2498).
Who is Ketogenic Diet Good for
- Meat lovers;
- Those who want to save muscle mass;
- Patients with diabetes mellitus.
Despite the complexity of the first days and strict diet restrictions, for some people’s ketogenic diets are ideal. It is worth trying out for the following categories of people who want to lose weight:
- Meat lovers. Many diets offer a drastic reduction in meat-eating or complete rejection, but a ketogenic diet does not restrict it.
- Those who want to save muscle mass. Normally diets can reduce muscle mass, but a ketogenic diet does not destroy muscle fiber structures, which is more suitable for professional athletes.
- Patients with diabetes mellitus. Ketosis can control blood sugar levels (Yancy, Mitchell, & Westman 1734). It is a type of professional diet, so a doctor’s consultation is necessary.

Mistakes that are Made
Lack of education can lead to:
- Carbohydrate consumption.
- Consumption of large quantities of proteins.
Due to their lack of education, beginners often make typical mistakes when using a ketogenic diet. Most of these errors will neutralize the positive effects of the diet, but others can have adverse effects on health. The most common mistake is when a person neglects the basic rule of diet and eats small amounts of carbohydrates. However, it is essential to understand that even a slice of eaten bread delays the start of ketosis. The second error is the opposite: people often completely give up carbohydrates, but in addition, they increase the amount of protein they consume. Protein poisoning leads to the destruction of the liver, kidneys, and digestive system problems.
The most challenging part of the keto diet is getting used to an entirely new way of eating, especially the rejection of most foods with high carbohydrate content and the addition of fat. Large amounts of natural fat can be found in vegetable and animal oils. Low-carbohydrate meat products may include beef, poultry and eggs, and fish. Non-starchy foods such as avocados, tomatoes, cabbage, and broccoli are allowed types of vegetables. On a ketogenic diet, it is possible to eat fruits and berries such as raspberries, kiwi, lemons, and blueberries.
What Does a Person Get: Arguments “For”
- The scientific validity of diet.
- Rapidity of fat burning.
- Comfortable process.
- Long-term result.
- Cholesterol Control.
- Reducing epileptic seizures.
- Improving skin condition.
Those who decide to use ketogenic diet rules for weight loss should understand the benefits that await them. First, the effectiveness of such a diet has been scientifically proven (Zajac et al. 2493). Secondly, with such a diet, a person burns excess fat very quickly. Also, unlike other diets, the patient has no sense of hunger, so fasting is comfortable. Also, it should be noted that the result obtained is quite long term as the person is not hungry, and the body is not stressed. Several scientific studies demonstrate that a ketogenic diet improves cholesterol levels (“Pros and Cons” 1). This reduces the likelihood of cardiovascular disease. In addition, it should be remembered that a diet is right for people with epilepsy – the number of seizures and convulsions decreases (Feldman 36). In the world of cosmetology, there is a belief that a ketogenic diet has a positive effect on skin health by inhibiting the growth of acne and pimples (Feldman 36).
What a Person will Face: Arguments “Against”
- This diet is unbalanced
- Like any diet, it requires willpower.
- It is quite long, can be used for years.
- Side effects are possible.
It is essential to understand that there are no ideal weight loss models. A ketogenic diet is rich in deficiencies that can significantly worsen a person’s condition (Pros and Cons” 2). For this reason, it is necessary to consult a nutritionist before using such a diet.
- First, it should be noted that the ketogenic diet is unbalanced – it excludes the intake of several nutrients, vitamins, and trace elements.
- Secondly, it requires motivation and responsibility – one has to give up favorite dishes and regularly calculate the number of calories.
- The third disadvantage of the diet is duration: a ketogenic diet can last from 3-4 weeks to a year. There is no point in staying on a diet for less than three weeks because, during this time, the body will go through ketogenic adaptation and only begin to get all the benefits of this diet.
- It also has many side effects: constipation, nausea and vomiting, growth disorders, kidney stones, and changes in blood lipids.
What Else Should be Mentioned
What must be said is :
- This diet is not suitable for pregnant and sick patients.
- Long-term effects require careful consideration.
- Each diet is selected individually and requires analysis by a specialist.
The ketogenic diet has another disadvantage, which, however, cannot be unequivocally attributed to the general disadvantages. The fact is that this type of diet is not suitable for all population groups, and is contraindicated for medical reasons to pregnant women and people with kidney and liver diseases (Feldman 34). The effects of a long-term diet on healthy people have not yet been well understood. Dieticians are advised to choose a diet that can be sustained throughout life. Short-term effects may be successful, but the method is not suitable for everyone. Dietary recommendations are tailored to each individual and following the next trend without consulting a doctor is not recommended.
Works Cited
Feldman, Ellen. “Ketogenic Diet for Refractory Pediatric Seizures.” Integrative Medicine Alert , vol. 22, no. 9, 2019, pp. 32-37.
Mawer, Rudy. “ 10 Graphs That Show the Power of a Ketogenic Diet. ” heathline . 2018. Web.
Mawer, Rudy. “ The Ketogenic Diet: A Detailed Beginner’s Guide to Keto. ” heathline . 2018. Web.
“Pros and Cons of Low-Carb/Ketogenic Diets.” Nutrition Letter , vol. 37, no. 10, 2019, pp. 1-2.
Rho, Jong M. “How Does the Ketogenic Diet Induce Anti-Seizure Effects?” Neuroscience letters , vol. 637, no. 1, 2017, pp. 4-10.
Yancy, William S., Nia S. Mitchell, and Eric C. Westman. “Ketogenic Diet for Obesity and Diabetes.” JAMA Internal Medicine , vol. 179, no. 12, 2019, pp. 1734-1735.
Zajac, Adam, et al. “The Effects of a Ketogenic Diet on Exercise Metabolism and Physical Performance in Off-Road Cyclists.” Nutrients , vol. 6, no. 7, 2014, pp. 2493-2508.
- Going Keto: Is It Worth It?
- Very-Low-Calorie Ketogenic Diet and Kidney Failure
- Analysis of Low Carb and High-Fat Diets
- Canada Food Guide Overview
- Dietary Specifications: Medicinal Meals in China
- Steps in Ensuring a Healthy Diet in College
- The Process of Cheese Production
- Unhealthy Fad Diets: Fact or Hoax
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, July 15). Ketogenic Diet: Overview. https://ivypanda.com/essays/ketogenic-diet-overview/
"Ketogenic Diet: Overview." IvyPanda , 15 July 2022, ivypanda.com/essays/ketogenic-diet-overview/.
IvyPanda . (2022) 'Ketogenic Diet: Overview'. 15 July.
IvyPanda . 2022. "Ketogenic Diet: Overview." July 15, 2022. https://ivypanda.com/essays/ketogenic-diet-overview/.
1. IvyPanda . "Ketogenic Diet: Overview." July 15, 2022. https://ivypanda.com/essays/ketogenic-diet-overview/.
Bibliography
IvyPanda . "Ketogenic Diet: Overview." July 15, 2022. https://ivypanda.com/essays/ketogenic-diet-overview/.
- Research article
- Open access
- Published: 25 May 2023
Effects of ketogenic diet on health outcomes: an umbrella review of meta-analyses of randomized clinical trials
- Chanthawat Patikorn 1 , 2 ,
- Pantakarn Saidoung 1 ,
- Tuan Pham 3 ,
- Pochamana Phisalprapa 4 ,
- Yeong Yeh Lee 5 ,
- Krista A. Varady 6 ,
- Sajesh K. Veettil 1 &
- Nathorn Chaiyakunapruk 1 , 7
BMC Medicine volume 21 , Article number: 196 ( 2023 ) Cite this article
17k Accesses
15 Citations
90 Altmetric
Metrics details
Systematic reviews and meta-analyses of randomized clinical trials (RCTs) have reported the benefits of ketogenic diets (KD) in various participants such as patients with epilepsy and adults with overweight or obesity . Nevertheless, there has been little synthesis of the strength and quality of this evidence in aggregate.
To grade the evidence from published meta-analyses of RCTs that assessed the association of KD, ketogenic low-carbohydrate high-fat diet (K-LCHF), and very low-calorie KD (VLCKD) with health outcomes, PubMed, EMBASE, Epistemonikos, and Cochrane database of systematic reviews were searched up to February 15, 2023. Meta-analyses of RCTs of KD were included. Meta-analyses were re-performed using a random-effects model. The quality of evidence per association provided in meta-analyses was rated by the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) criteria as high, moderate, low, and very low.
We included 17 meta-analyses comprising 68 RCTs (median [interquartile range, IQR] sample size of 42 [20–104] participants and follow-up period of 13 [8–36] weeks) and 115 unique associations. There were 51 statistically significant associations (44%) of which four associations were supported by high-quality evidence (reduced triglyceride ( n = 2), seizure frequency ( n = 1) and increased low-density lipoprotein cholesterol (LDL-C) ( n = 1)) and four associations supported by moderate-quality evidence (decrease in body weight, respiratory exchange ratio (RER), hemoglobin A 1c , and increased total cholesterol). The remaining associations were supported by very low (26 associations) to low (17 associations) quality evidence. In overweight or obese adults, VLCKD was significantly associated with improvement in anthropometric and cardiometabolic outcomes without worsening muscle mass, LDL-C, and total cholesterol. K-LCHF was associated with reduced body weight and body fat percentage, but also reduced muscle mass in healthy participants.
Conclusions
This umbrella review found beneficial associations of KD supported by moderate to high-quality evidence on seizure and several cardiometabolic parameters. However, KD was associated with a clinically meaningful increase in LDL-C. Clinical trials with long-term follow-up are warranted to investigate whether the short-term effects of KD will translate to beneficial effects on clinical outcomes such as cardiovascular events and mortality.
Peer Review reports
Ketogenic diets (KD) have received substantial attention from the public primarily due to their ability to produce rapid weight loss in the short run [ 1 , 2 ]. The KD eating pattern severely restricts carbohydrate intake to less than 50 g/day while increasing protein and fat intake [ 3 , 4 , 5 , 6 ]. Carbohydrate deprivation leads to an increase in circulating ketone bodies by breaking down fatty acids and ketogenic amino acids. Ketones are an alternative energy source from carbohydrates that alter physiological adaptations. These adaptions have been shown to produce weight loss with beneficial health effects by improving glycemic and lipid profiles [ 7 , 8 ]. KD has also been recommended as a nonpharmacological treatment for medication-refractory epilepsy in children and adults [ 8 , 9 ]. Evidence suggests that KD has reduced seizure frequency in patients with medication-refractory epilepsy, and even allowing some patients to reach complete and sustained remission. 11 However, the exact anticonvulsive mechanism of KD remains unclear [ 10 , 11 ].
Several systematic reviews and meta-analyses of randomized clinical trials (RCTs) have reported on the use of KD in patients with obesity or type 2 diabetes mellitus (T2DM) to control weight and improve cardiometabolic parameters [ 1 , 12 , 13 , 14 , 15 ], in patients with refractory epilepsy to reduce seizure frequency [ 16 ], and in athletes to control weight and improve performance [ 17 ]. To date, there has been little synthesis of the strength and quality of this evidence in aggregate. This umbrella review therefore aims to systematically identify relevant meta-analyses of RCTs of KD, summarize their findings, and assess the strength of evidence of the effects of KD on health outcomes.
The protocol of this study was registered with PROSPERO (CRD42022334717). We reported following the 2020 Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (Additional file 1 ) [ 18 ]. Difference from the original review protocol is described with rationale in Additional file 2 : Table S1.
Search strategy and eligibility criteria
We searched PubMed, EMBASE, Epistemonikos, and the Cochrane database of systematic reviews (CDSR) from the database inception to February 15, 2023 (Additional file 2 : Table S2). No language restriction was applied. Study selection was independently performed in EndNote by two reviewers (C.P. and PS). After removing duplicates, the identified articles' titles and abstracts were screened for relevance. Full-text articles of the potentially eligible articles were retrieved and selected against the eligibility criteria. Any discrepancies were resolved by discussion with the third reviewer (SKV).
We included studies that met the following eligibility criteria: systematic reviews and meta-analyses of RCTs investigating the effects of any type of KD on any health outcomes in participants with or without any medical conditions compared with any comparators. When more than 1 meta-analysis was available for the same research question, we selected the meta-analysis with the largest data set [ 19 , 20 , 21 ]. Articles without full-text and meta-analyses that provided insufficient or inadequate data for quantitative synthesis were excluded.

Data extraction and quality assessment
Two reviewers (CP and PS) independently performed data extraction and quality assessment (Additional file 2 : Method S1). Discrepancies were resolved with consensus by discussing with the third reviewer (SKV). We used AMSTAR- 2 -A Measurement Tool to Assess Systematic Reviews- to grade the quality of meta-analyses as high, moderate, low, or critically low by assessing the following elements, research question, a priori protocol, search, study selection, data extraction, quality assessment, data analysis, interpretation, heterogeneity, publication bias, source of funding, conflict of interest [ 22 ].
Data synthesis
For each association, we extracted effect sizes (mean difference [MD], the standardized mean difference [SMD], and risk ratio [RR]) of individual studies included in each meta-analysis and performed the meta-analyses to calculate the pooled effect sizes and 95% CIs using a random-effects model under DerSimonian and Laird [ 23 ], or the Hartung-Knapp- Sidik-Jonkman approach for meta-analyses with less than five studies [ 24 ]. p < 0.05 was considered statistically significant in 2-sided tests. Heterogeneity was evaluated using the I 2 statistic. The evidence for small-study effects was assessed by the Egger regression asymmetry test [ 25 ]. Statistical analyses were conducted using Stata version 16.0 (StataCorp). We presented effect sizes of statistically significant associations with the known or estimated minimally clinically important difference (MCID) thresholds for health outcomes [ 14 , 26 , 27 , 28 , 29 , 30 ].
We assessed the quality of evidence per association by applying the GRADE criteria (Grading of Recommendations, Assessment, Development, and Evaluations) in five domains, including (1) risk of bias in the individual studies, (2) inconsistency, (3) indirectness, (4) imprecision, and (5) publication bias [ 31 ]. We graded the strength of evidence (high, moderate, low, and very low) using GRADEpro version 3.6.1 (McMaster University).
Sensitivity analyses
Sensitivity analyses were performed by excluding small-size studies (< 25 th percentile) [ 32 ] and excluding primary studies having a high risk of bias rated by the Cochrane’s risk of bias 2 tool (RoB 2) for RCTs from the identified associations [ 19 , 20 , 21 , 33 ].
Seventeen meta-analyses were included (Fig. 1 and Additional file 2 : Table S3) [ 1 , 2 , 15 , 16 , 17 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. These meta-analyses comprised 68 unique RCTs with a median (interquartile range, IQR) sample size per RCT of 42 (20–104) participants and a median (IQR) follow-up period of 13 (8–36) weeks. The quality of meta-analyses assessed using AMSTAR-2 found that none were rated as high confidence, 2 (12%) as moderate confidence, 2 (12%) as low confidence, and 13 (76.0%) as critically low confidence (Table 1 and Additional file 2 : Table S4).
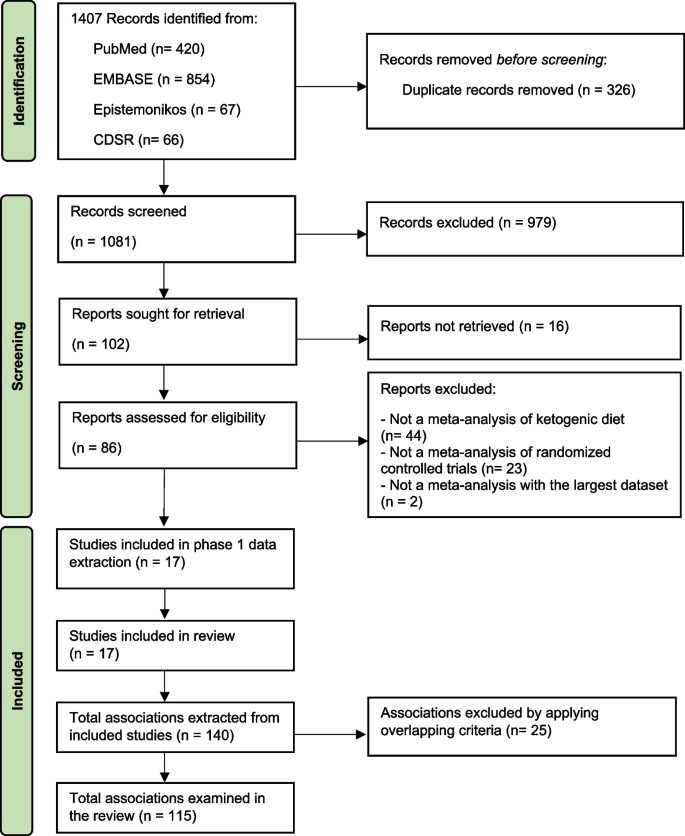
Study selection flow of meta-analyses. Abbreviation: CDSR, Cochrane database of systematic review
Types of KD identified in this umbrella review were categorized as (1) KD, which limits carbohydrate intake to < 50 g/day or < 10% of the total energy intake (TEI) [ 35 ], (2) ketogenic low-carbohydrate, high-fat diet (K-LCHF), which limits carbohydrate intake to < 50 g/day or < 10% of TEI with high amount of fat intake (60–80% of TEI) [ 38 , 46 ], (3) very low-calorie KD (VLCKD), which limits carbohydrate intake to < 30–50 g/day or 13–25% of TEI with TEI < 700–800 kcal/day, and (4) modified Atkins diet (MAD), which generally limits carbohydrate intake to < 10 g/day while encouraging high-fat foods [ 15 , 47 ]. Meta-analyses of long-chain triglyceride KD, medium-chain triglyceride KD, and low glycemic index treatment were not identified.
Description and summary of associations
We identified 115 unique associations of KD with health outcomes (Additional file 2 : Table S5). The median (IQR) number of studies per association was 3 [ 4 , 5 , 6 ], and the median (IQR) sample size was 244 (127–430) participants. Outcomes were associated with KD types, including 40 (35%) KD, 18 (16%) K-LCHF, 13 (11%) VLCKD, 25 (22%) KD or K-LCHF, 5 (4%) KD or VLCKD, 1 (1%) KD or MAD, and 13 (11%) KD, K-LCHF, or VLCKD.
The associations involved 40 (35%) anthropometric measures (i.e., body weight, body mass index [BMI] [calculated as weight in kilograms divided by height in meters squared], waist circumference, muscle mass, fat mass, body fat percentage, and visceral adipose tissue), 37 (32%) lipid profile outcomes (i.e., triglyceride, total cholesterol, high-density lipoprotein cholesterol [HDL-C], and low-density lipoprotein cholesterol [LDL-C]), 22 (19%) glycemic profile outcomes (i.e., hemoglobin A 1c [HbA 1c ], fasting plasma glucose, fasting insulin, and homeostatic model assessment of insulin resistance [HOMA-IR]), 6 (5%) exercise performance (i.e., maximal heart rate, respiratory exchange ratio [RER], maximal oxygen consumption (VO 2 max), 5 (4%) blood pressure outcomes (i.e., systolic blood pressure [SBP], diastolic blood pressure [DBP], and heart rate), 1 (1%) outcome associated with seizure frequency reduction ≥ 50% from baseline, and 3 other outcomes (i.e., serum creatinine, C-peptide, and C-reactive protein). In addition, there is 1 association (1%) of adverse events.
Participants in the identified associations included 68 (59%) associations in adults with overweight or obesity with or without T2DM or dyslipidemia, 15 (13%) athletes or resistance-trained adults, 12 (10%) adults with T2DM, 11 (10%) healthy participants ≥ 16 years old, 8 (7%) cancer patients, and 1 (1%) in children and adolescents with epilepsy.
Using GRADE, 115 associations were supported by very low strength of evidence ( n = 66, 57%), with the remaining being low ( n = 36, 31%), moderate ( n = 9, 8%), and high quality of evidence ( n = 4, 3%) (Additional file 2 : Table S5). Almost half, or 44% (51 associations), were statistically significant based on a random-effects model, of which 51% (26 associations) were supported by a very low level of evidence, followed by low (17 associations [33%]), moderate (4 associations [8%]), and high (4 associations [8%]) levels of evidence. Overall beneficial outcomes associated with KD were BMI [ 37 , 42 ], body weight [ 1 , 2 , 35 , 36 , 37 , 41 ], waist circumference [ 37 , 42 ], fat mass [ 37 , 42 ], body fat percentage [ 38 , 40 ], visceral adipose tissue [ 37 ], triglyceride [ 1 , 2 , 36 , 42 ], HDL-C [ 1 , 2 , 42 ], HbA 1c [ 2 , 34 , 35 ], HOMA-IR [ 2 , 42 ], DBP [ 1 ], seizure frequency reduction ≥ 50% from baseline [ 16 ], and respiratory exchange ratio [ 17 , 39 ]. Adverse outcomes associated with KD were reduced muscle mass [ 37 , 38 ], and increased LDL-C [ 2 , 35 ], and total cholesterol [ 2 , 17 ]. In terms of safety, one association showed no significant increase in adverse events (e.g., constipation, abdominal pain, and nausea) with KD [ 44 ].
Eight out of 13 associations supported by moderate to high-quality evidence were statistically significant (Table 2 ). There were 4 statistically significant associations supported by high-quality evidence, including the following: (1) KD or MAD for 3–16 months was associated with a higher proportion of children and adolescents with refractory epilepsy achieving seizure frequency reduction ≥ 50% from baseline compared with regular diet (RR, 5.11; 95% CI, 3.18 to 8.21) [ 16 ], (2) KD for 3 months was associated with reduced triglyceride in adults with T2DM compared with regular diet (MD, -18.36 mg/dL; 95% CI, -24.24 to -12.49, MCID threshold 7.96 mg/dL) [ 14 , 35 ], (3) KD for 12 months was associated with reduced triglyceride in adults with T2DM compared with regular diet (MD, -24.10 mg/dL; 95% CI, -33.93 to -14.27, MCID threshold 7.96 mg/dL) [ 14 , 35 ], and (4) KD for 12 months was associated with increased LDL-C in adults with T2DM compared with regular diet (MD, 6.35 mg/dL; 95% CI, 2.02 to 10.69, MCID threshold 3.87 mg/dL) [ 14 , 35 ]. In addition, there were 4 statistically significant associations supported by moderate-quality evidence: (1) KD for 3 months was associated with reduced HbA 1c in adults with T2DM compared with regular diet (MD, -0.61%; 95% CI, -0.82 to -0.40, MCID threshold 0.5%) [ 14 , 35 ], (2) VLCKD for 4–6 weeks was associated with reduced body weight in T2DM adults with overweight or obesity compared with a low-fat diet or regular diet (MD, -9.33 kg; 95% CI, -15.45 to -3.22, MCID threshold 4.40 kg) [ 14 , 15 ], (3) K-LCHF for 4–6 weeks was associated with reduced respiratory exchange ratio in athletes compared with a high-carbohydrate diet (SMD, -2.66; 95% CI, -3.77 to -1.54) [ 39 ], and (4) K-LCHF for 11–24 weeks was associated with increased total cholesterol in athletes compared with regular diet (MD, 1.32 mg/dL; 95% CI, 0.64 to 1.99) [ 14 , 17 ].
Types of KD showed different effects on health outcomes with changes more than the MCID thresholds in different populations (Fig. 2 ). KD or MAD for 3–16 months was associated with a 5-times higher proportion of children and adolescents with refractory epilepsy achieving seizure frequency reduction ≥ 50% from baseline compared with a regular diet (RR, 5.11; 95% CI, 3.18 to 8.21) [ 16 ]. In healthy participants, K-LCHF for 3–12 weeks could reduce body weight by 3.68 kg (95% CI, -4.45 to -2.90) but also significantly reduced muscle mass by 1.27 kg (95% CI, -1.83 to -0.70, MCID threshold 1.10 kg) [ 14 , 26 , 38 ]. In adults with T2DM, KD for 3–12 months was found to have significant associations with changes more than the MCID thresholds, including reduction of triglyceride and HbA 1c ; however, KD for 12 months led to a clinically meaningful increase in LDL-C by 6.35 mg/dL (95% CI, 2.02 to 10.69, MCID threshold 3.87 mg/dL) [ 14 , 35 ]. In adults with overweight or obesity and/or metabolic syndrome, VLCKD for 4–6 weeks demonstrated a clinically meaningful weight loss of 9.33 kg (95% CI, -15.45 to -3.22, MCID threshold 4.40 kg) [ 14 , 15 ]. VLCKD for 3–96 weeks led to a clinically meaningful improvement in BMI, body weight, waist circumference, triglyceride, fat mass, and insulin resistance, while preserving muscle mass [ 42 ].
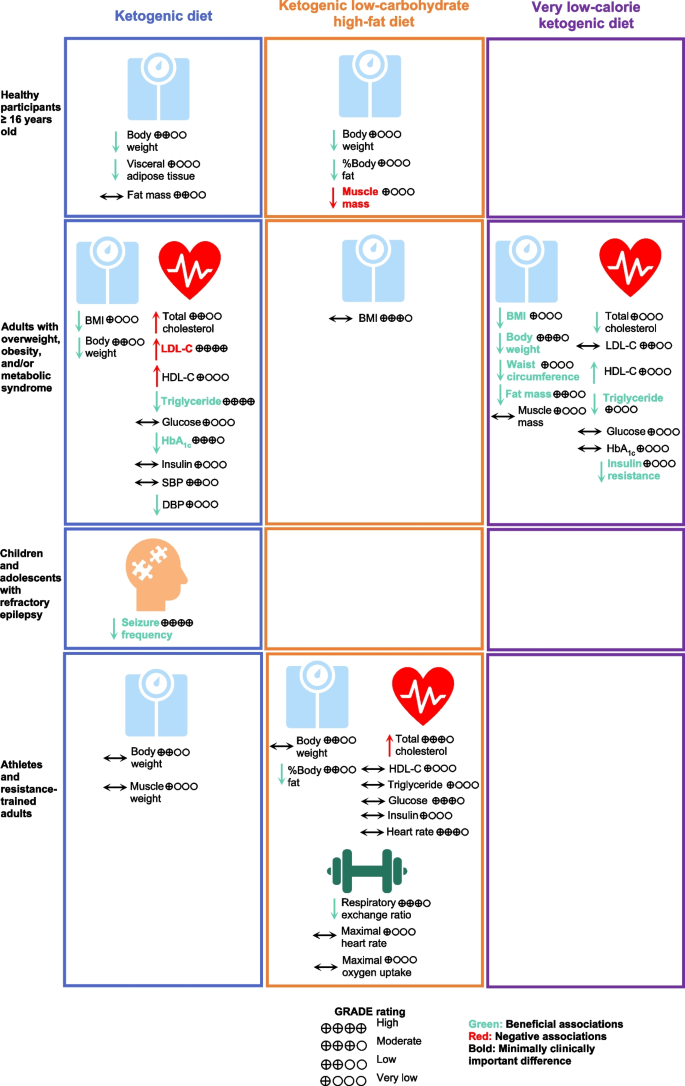
Associations of Types of Ketogenic Diet with Health Outcomes. Abbreviations: BMI, body mass index, DBP, diastolic blood pressure; GRADE, Grading of Recommendations, Assessment, Development, and Evaluations; HbA 1c , hemoglobin A 1c ; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model of insulin resistance; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TEI, total energy intake
Excluding RCTs with small sizes in 7 associations found that the strength of evidence of one association was downgraded to very low quality, i.e., KD for 12 months, and the increase of LDL-C in adults with T2DM compared with a control diet. Another association was downgraded to low quality, i.e., KD for 12 months and the reduction of triglyceride in adults with T2DM compared with the control diet (Additional file 2 : Table S6). The remaining associations retained the same rank.
This umbrella review was performed to systematically assess the potential associations of KD and health outcomes by summarizing the evidence from meta-analyses of RCTs. Sensitivity analyses were performed to provide additional evidence from high-quality RCTs, which further increased the reliability of results. We identified 115 associations of KD with a wide range of outcomes. Most associations were rated as low and very low evidence according to the GRADE criteria because of serious imprecision and large heterogeneity in findings, and indirectness due to a mix of different interventions and comparators.
Our findings showed that KD or MAD resulted in better seizure control in children and adolescents with medication-refractory epilepsy (approximately a third of cases) for up to 16 months [ 10 , 11 , 16 ]. Anti-epileptic mechanisms of KD remain unknown but are likely multifactorial. Enhanced mitochondrial metabolism and an increase in ketone bodies or reduction in glucose across the blood–brain barrier resulted in synaptic stabilization [ 48 , 49 , 50 ]. Other mechanisms include an increase in gamma-aminobutyric acid (GABA) [ 51 ], more beneficial gut microbiome [ 52 ], less pro-inflammatory markers [ 53 ], and epigenetic modifications (e.g. beta-hydroxybutyrate [beta-OHB]) [ 54 ].
In adults, KD was associated with improved anthropometric measures, cardiometabolic parameters, and exercise performance. Our findings, however, demonstrated differences in the level of associations with type of KD. On the one hand, VLCKD is very effective in producing weight loss while preserving muscle mass in adults with overweight or obesity, with specific benefits on anthropometric and cardiometabolic parameters [ 15 , 42 ]. On the other hand, a significant portion of the weight loss seen in K-LCHF was due to muscle mass loss [ 17 , 38 ]. Overall KD was negatively associated with reduced muscle mass and increased LDL-C and total cholesterol.
Our findings demonstrated that KD could induce a rapid weight loss in the initial phase of 6 months, after which time further weight loss was hardly achieved [ 35 ]. Furthermore, weight loss induced by KD is relatively modest and appears comparable to other dietary interventions that are effective for short-term weight loss, e.g., intermittent fastingand Mediterranean diet [ 55 , 56 , 57 ].
KD is one of the dietary interventions employed by individuals to achieve rapid weight loss, which usually comes with reduced muscle mass [ 58 ]. However, KD has been hypothesized to preserve muscle mass following weight loss based on several mechanisms, including the protective effect of ketones and its precursors on muscle tissue [ 59 , 60 , 61 ], and increased growth hormone secretion stimulated by low blood glucose to increase muscle protein synthesis [ 58 , 62 , 63 ].
With regards to KD effects on lipid profiles, our results demonstrate an effective reduction in serum triglyceride levels with 3 months of lowered dietary carbohydrate intake, with even further reduction by month 12 [ 35 ]. Triglyceride levels are consistently shown to decrease after KD. Acute ketosis (beta-OHB ≈ 3 mM) due to ketone supplementation also shows decreases in triglycerides, indicating a potential effect of ketones on triglycerides independent of weight loss. One possible mechanism is the decreased very low-density lipoprotein content in the plasma due to low insulin levels. Due to a lack of insulin, lipolysis increases in fat cells [ 2 , 13 , 15 ]. Of note, the converse has also been observed as a phenomenon known as carbohydrate-induced hypertriglyceridemia, whereby higher dietary carbohydrate intake leads to higher serum triglycerides levels, potentially mediated by changes in triglyceride clearance and hepatic de novo lipogenesis rates [ 64 ]. Though our aggregate results also confirm an increase in LDL-C and total cholesterol with KD and K-LCHF, respectively, it is important to note that an increase in either of these levels does not necessarily signify a potentially deleterious cardiovascular end-point. This qualification derives from the fact that LDL particles are widely heterogeneous in composition and size, with small dense LDL particles being significantly more atherogenic than larger LDL particles [ 65 ]. Our observed aggregate effect of KD on cholesterol levels does not account for the difference in LDL particle size, nor does it distinguish the sources of dietary fat, which can also be a significant effector of LDL particle size distribution and metabolism [ 66 ].
Most RCTs of KD were conducted in patients with a limited group of participants, such as those with overweight, obesity, metabolic syndrome, cancer, and refractory epilepsy. In addition, most outcomes measured were limited to only surrogate outcomes. Thus, more clinical trials with a broader scope in populations and outcomes associated with KD would expand the role of KD in a clinical setting. For example, participant selection could be expanded from previous trials to include elderly patients, nonalcoholic fatty live disease (NAFLD) patients, and polycystic ovarian syndrome patients. Outcomes of interest of could be expanded to include (1) clinical outcomes such as cardiovascular events and liver outcomes, (2) short- and long-term safety outcomes such as adverse events (e.g., gastrointestinal, neurological, hepatic, and renal), eating disorder syndrome, sleep parameters, lipid profiles, and thyroid function and (3) other outcomes such as adherence and quality of life. More importantly, long-term studies are needed to investigate the sustainability of the clinical benefits of KD.
Our findings are useful to support the generation of evidence-based recommendations for clinicians contemplating use of KD in their patients, as well as for the general population. We further emphasize the importance of consultation with healthcare professionals before utilizing KD and any other dietary interventions. We demonstrated the benefits of KD on various outcomes in the short term. However, these improvements may prove difficult to sustain in the long term because of challenges in adherence. As for any diet interventions to achieve sustainable weight loss, factors of success include adherence, negative energy balance, and high-quality foods. Thus, communication and education with KD practitioners are important to ensure their adherence to the diet. Some individuals might benefit from switching from KD to other dietary interventions to maintain long-term weight loss.
Limitations
This umbrella review has several limitations. Firstly, we focused on published meta-analyses which confined us from assessing the associations of KD on outcomes and populations that were not included in existing meta-analyses. Secondly, most of the included meta-analyses were rated with AMSTAR-2 as critically low confidence, mainly due to a lack of study exclusion reasons, unexplained study heterogeneity, and unassessed publication bias. However, these domains unlikely affected our findings. Thirdly, we could not perform a dose–response analysis to understand the effects of different levels of carbohydrate intake on health outcomes because of insufficient details of carbohydrate intake reported in the meta-analyses. Fourthly, most RCTs of KD were limited to a relatively small number of participants with a short-term follow-up period, which limited our assessment of sustained beneficial effects after stopping KD. Lastly, due to decreased adherence, carbohydrate intake most likely increased across the course of the trials. For example, subjects in the KD arm of the A TO Z Weight Loss Study [ 67 ], started with a carbohydrate intake < 10 g/day but ended at 12 months with a carbohydrate intake accounting for 34% of TEI. In the DIRECT trial, subjects in the KD group started with carbohydrate intake of 20 g/day and ended at 12 months with 40% of TEI from carbohydrate intake [ 68 ]. Thus, we cannot be certain how the precise degree of ketosis contributed to the beneficial effects noted.
Beneficial associations of practicing KD were supported by moderate- to high-quality evidence, including weight loss, lower triglyceride levels, decreased HbA 1c , RER, and decreased seizure frequency. However, KD was associated with a clinically meaningful increase in LDL-C. Clinical trials with long-term follow-up are warranted to investigate whether these short-term effects of KD will translate to beneficial effects on more long-term clinical outcomes such as cardiovascular events and mortality.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
Beta-hydroxybutyrate
Body mass index
Diastolic blood pressure
Gamma-aminobutyric acid
High-density lipoprotein cholesterol
Hemoglobin A 1c
Homeostatic model assessment of insulin resistance
Ketogenic low-carbohydrate high-fat diet
Ketogenic diets
Low-density lipoprotein cholesterol
Modified Atkins diet
Minimally clinically important difference
Nonalcoholic fatty liver disease
Randomized clinical trials
Respiratory exchange ratio
Systolic blood pressure
Type 2 diabetes mellitus
Total energy intake
Very low-calorie ketogenic diet
Maximal oxygen consumption
Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110(7):1178–87.
Article CAS PubMed Google Scholar
Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12(7):2005.
Article CAS PubMed PubMed Central Google Scholar
Kirkpatrick CF, Bolick JP, Kris-Etherton PM, Sikand G, Aspry KE, Soffer DE, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13(5):689-711.e1.
Article PubMed Google Scholar
Dahlin M, Singleton SS, David JA, Basuchoudhary A, Wickström R, Mazumder R, et al. Higher levels of Bifidobacteria and tumor necrosis factor in children with drug-resistant epilepsy are associated with anti-seizure response to the ketogenic diet. eBioMedicine. 2022;80:104061.
Crosby L, Davis B, Joshi S, Jardine M, Paul J, Neola M, et al. Ketogenic diets and chronic disease: weighing the benefits against the risks. Front Nutr. 2021;8:702802.
Article PubMed PubMed Central Google Scholar
Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789–96.
Gershuni VM, Yan SL, Medici V. Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr Nutr Rep. 2018;7(3):97–106.
Zhu H, Bi D, Zhang Y, Kong C, Du J, Wu X, et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct Target Ther. 2022;7(1):11.
Lefevre F, Aronson N. Ketogenic diet for the treatment of refractory epilepsy in children: a systematic review of efficacy. Pediatrics. 2000;105(4):E46.
Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3(2):175–92.
Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48(1):43–58.
Yudkoff M, Daikhin Y, Melø TM, Nissim I, Sonnewald U, Nissim I. The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect. Annu Rev Nutr. 2007;27:415–30.
Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10(1):38.
Goldenberg JZ, Day A, Brinkworth GD, Sato J, Yamada S, Jönsson T, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743.
Castellana M, Conte E, Cignarelli A, Perrini S, Giustina A, Giovanella L, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21(1):5–16.
Sourbron J, Klinkenberg S, van Kuijk SMJ, Lagae L, Lambrechts D, Braakman HMH, et al. Ketogenic diet for the treatment of pediatric epilepsy: review and meta-analysis. Childs Nerv Syst. 2020;36(6):1099–109.
Lee HS, Lee J. Influences of ketogenic diet on body fat percentage, respiratory exchange rate, and total cholesterol in athletes: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(6):2912.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Dragioti E, Solmi M, Favaro A, Fusar-Poli P, Dazzan P, Thompson T, et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiat. 2019;76(12):1241–55.
Article Google Scholar
Patikorn C, Roubal K, Veettil SK, Chandran V, Pham T, Lee YY, et al. Intermittent Fasting and Obesity-Related Health Outcomes: An Umbrella Review of Meta-analyses of Randomized Clinical Trials. JAMA Netw Open. 2021;4(12):e2139558-e.
Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348: g2035.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–59.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014;15(4):310–21.
Mulligan AA, Lentjes MA, Luben RN, Wareham NJ, Khaw K-T. Changes in waist circumference and risk of all-cause and CVD mortality: results from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) cohort study. BMC Cardiovasc Disord. 2019;19(1):1–15.
Jayedi A, Khan TA, Aune D, Emadi A, Shab-Bidar S. Body fat and risk of all-cause mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Int J Obes. 2022;46(9):1573–81.
Article CAS Google Scholar
Keller HH, Østbye T. Body mass index (BMI), BMI change and mortality in community-dwelling seniors without dementia. J Nutr Health Aging. 2005;9(5):316–20.
CAS PubMed Google Scholar
Kelley GA, Kelley KS, Stauffer BL. Walking and resting blood pressure: an inter-individual response difference meta-analysis of randomized controlled trials. Sci Prog. 2022;105(2):00368504221101636.
Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in cochrane reviews. Syst Rev. 2013;2(1):81.
Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA. 2014;312(6):623–30.
Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Sainsbury E, Kizirian NV, Partridge SR, Gill T, Colagiuri S, Gibson AA. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;139:239–52.
Rafiullah M, Musambil M, David SK. Effect of a very low-carbohydrate ketogenic diet vs recommended diets in patients with type 2 diabetes: a meta-analysis. Nutr Rev. 2022;80(3):488–502.
Alarim RA, Alasmre FA, Alotaibi HA, Alshehri MA, Hussain SA. Effects of the ketogenic diet on glycemic control in diabetic patients: meta-analysis of clinical trials. Cureus. 2020;12(10):e10796.
PubMed PubMed Central Google Scholar
Amini MR, Aminianfar A, Naghshi S, Larijani B, Esmaillzadeh A. The effect of ketogenic diet on body composition and anthropometric measures: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2022;62(13):3644–57.
Ashtary-Larky D, Bagheri R, Asbaghi O, Tinsley GM, Kooti W, Abbasnezhad A, et al. Effects of resistance training combined with a ketogenic diet on body composition: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2022;62(21):5717–32.
Cao J, Lei S, Wang X, Cheng S. The effect of a ketogenic low-carbohydrate, high-fat diet on aerobic capacity and exercise performance in endurance athletes: a systematic review and meta-analysis. Nutrients. 2021;13(8):2896.
Lee HS, Lee J. Effects of combined exercise and low carbohydrate ketogenic diet interventions on waist circumference and triglycerides in overweight and obese individuals: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(2):828.
López-Espinoza M, Chacón-Moscoso S, Sanduvete-Chaves S, Ortega-Maureira MJ, Barrientos-Bravo T. Effect of a ketogenic diet on the nutritional parameters of obese patients: a systematic review and meta-analysis. Nutrients. 2021;13(9):2946.
Muscogiuri G, El Ghoch M, Colao A, Hassapidou M, Yumuk V, Busetto L. European guidelines for obesity management in adults with a very low-calorie ketogenic diet: a systematic review and meta-analysis. Obes Facts. 2021;14(2):222–45.
Smith ES, Smith HA, Betts JA, Gonzalez JT, Atkinson G. A Systematic review and meta-analysis comparing heterogeneity in body mass responses between low-carbohydrate and low-fat diets. Obesity. 2020;28(10):1833–42.
Yang YF, Mattamel PB, Joseph T, Huang J, Chen Q, Akinwunmi BO, et al. Efficacy of low-carbohydrate ketogenic diet as an adjuvant cancer therapy: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2021;13(5):1388.
Vargas-Molina S, Gómez-Urquiza JL, García-Romero J, Benítez-Porres J. Effects of the ketogenic diet on muscle hypertrophy in resistance-trained men and women: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(19):12629.
El-Rashidy OF, Nassar MF, Abdel-Hamid IA, Shatla RH, Abdel-Hamid MH, Gabr SS, et al. Modified Atkins diet vs classic ketogenic formula in intractable epilepsy. Acta Neurol Scand. 2013;128(6):402–8.
Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47(2):421–4.
Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60(2):223–35.
Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, et al. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol. 2015;78(1):77–87.
Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP–dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9(11):1382–7.
Wang ZJ, Bergqvist C, Hunter JV, Jin D, Wang DJ, Wehrli S, et al. In vivo measurement of brain metabolites using two-dimensional double-quantum MR spectroscopy—exploration of GABA levels in a ketogenic diet. Magn Reson Med. 2003;49(4):615–9.
Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728-41.e13.
Dupuis N, Curatolo N, Benoist JF, Auvin S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56(7):e95–8.
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–4.
O’Neill B, Raggi P. The ketogenic diet: Pros and cons. Atherosclerosis. 2020;292:119–26.
Freire R. Scientific evidence of diets for weight loss: different macronutrient composition, intermittent fasting, and popular diets. Nutrition. 2020;69: 110549.
Becker A, Gaballa D, Roslin M, Gianos E, Kane J. Novel nutritional and dietary approaches to weight loss for the prevention of cardiovascular disease: ketogenic diet, intermittent fasting, and bariatric surgery. Curr Cardiol Rep. 2021;23(7):1–9.
Ashtary-Larky D, Bagheri R, Bavi H, Baker JS, Moro T, Mancin L, et al. Ketogenic diets, physical activity and body composition: a review. Br J Nutr. 2022;127(12):1898–920.
Thomsen HH, Rittig N, Johannsen M, Møller AB, Jørgensen JO, Jessen N, et al. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: anticatabolic impact of ketone bodies. Am J Clin Nutr. 2018;108(4):857–67.
Koutnik AP, D’Agostino DP, Egan B. Anticatabolic effects of ketone bodies in skeletal muscle. Trends Endocrinol Metab. 2019;30(4):227–9.
Parker BA, Walton CM, Carr ST, Andrus JL, Cheung EC, Duplisea MJ, et al. β-hydroxybutyrate elicits favorable mitochondrial changes in skeletal muscle. Int J Mol Sci. 2018;19(8):2247.
Huang Z, Huang L, Waters MJ, Chen C. Insulin and growth hormone balance: implications for obesity. Trends Endocrinol Metab. 2020;31(9):642–54.
Møller N, Copeland KC, Nair KS. Growth hormone effects on protein metabolism. Endocrinol Metab Clin North Am. 2007;36(1):89–100.
Parks EJ. Effect of dietary carbohydrate on triglyceride metabolism in humans. J Nutr. 2001;131(10):2772S-S2774.
Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, et al. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men: prospective results from the Qué bec Cardiovascular Study. Circulation. 1997;95(1):69–75.
Dreon DM, Fernstrom HA, Campos H, Blanche P, Williams PT, Krauss RM. Change in dietary saturated fat intake is correlated with change in mass of large low-density-lipoprotein particles in men. Am J Clin Nutr. 1998;67(5):828–36.
Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z weight loss study: a randomized trial. JAMA. 2007;297(9):969–77.
Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–41.
Download references
Acknowledgements
The authors would like to acknowledge Thunchanok Ingkaprasert and Wachiravit Youngjanin for their editorial assistance.
No funding was obtained for the conduct of this study.
Author information
Authors and affiliations.
Department of Pharmacotherapy, College of Pharmacy, University of Utah, 30 2000 E, Salt Lake City, Utah, 84112, USA
Chanthawat Patikorn, Pantakarn Saidoung, Sajesh K. Veettil & Nathorn Chaiyakunapruk
Department of Social and Administrative Pharmacy, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, Thailand
Chanthawat Patikorn
Division of Gastroenterology, Hepatology & Nutrition, Department of Internal Medicine, University of Utah, Salt Lake City, Utah, USA
Division of Ambulatory Medicine, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Pochamana Phisalprapa
School of Medical Sciences, Universiti Sains Malaysia, Kota Bharu, Malaysia
Yeong Yeh Lee
Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, Illinois, USA
Krista A. Varady
IDEAS Center, Veterans Affairs Salt Lake City Healthcare System, Salt Lake City, Utah, USA
Nathorn Chaiyakunapruk
You can also search for this author in PubMed Google Scholar
Contributions
CP, PS, SKV, and NC conceived and designed the study protocol. CP, PS, and SKV performed a literature review and data analysis. CP, PS, TP, PP, YYL, KAV, SKV, and NC interpreted the study findings. CP and PS were major contributors to writing the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Sajesh K. Veettil or Nathorn Chaiyakunapruk .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
PRISMA 2020 Main Checklist.
Additional file 2:
Method S1. Data extraction. Table S1. Difference from original review protocol. Table S2. Search strategy. Table S3. Excluded studies with reasons. Table S4. Quality assessment. Table S5. Summary of associations. Table S6. Sensitivity analyses.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Patikorn, C., Saidoung, P., Pham, T. et al. Effects of ketogenic diet on health outcomes: an umbrella review of meta-analyses of randomized clinical trials. BMC Med 21 , 196 (2023). https://doi.org/10.1186/s12916-023-02874-y
Download citation
Received : 25 November 2022
Accepted : 19 April 2023
Published : 25 May 2023
DOI : https://doi.org/10.1186/s12916-023-02874-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ketegenic diet
- Umbrella review
- Systematic review
- Meta-analysis
- Weight loss
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Get the Reddit app
r/KetoScience is dedicated to being the center for online discussion on the latest scientific discoveries in the broad and expanding role of the ketogenic diet in reversing chronic disease. We post RCTs, prospective cohorts, epidemiology, and case studies and discuss the pro's and con's of each. We discuss type 2 diabetes, gout, Alzheimer's, mild cognitive impairment, obesity, epilepsy, mental illness, autoimmune diseases, metabolic syndrome, sugar, omega 6 polyunsaturated seed oils, & more!
Thesis: The Impact of the Ketogenic Diet on Depression and Psychological Wellbeing: A Randomised Controlled Trial with Integrated Qualitative Analysis
https://repository.uel.ac.uk/item/8x2x3
Background and aims: There is evidence to suggest that a ketogenic diet (KD) may help to alleviate psychiatric symptoms, including depression, but this has not been studied extensively or compared directly to the impact of the more common low carbohydrate diet (LCD). The aim of this research was to understand the impact of a non-calorie-restricted low carbohydrate diet and ketogenic diet on depression and aspects of psychological well-being in those with either mild to moderate depressive symptoms or low or no depressive symptoms.
Materials and methods: In a randomised control trial with quasi experimental design, participants with mild to moderate depressive symptoms and low depressive symptoms were randomised into either a LCD, a KD, or a control diet (diet as usual) generating a total of 6 participant groups. The dietary interventions (LCD and KD) were delivered through an online education platform for 12 weeks, followed by 12 weeks of unsupported continued diet. The control diet was maintained for a total of 6 weeks. Examinations at baseline (T0), day 1, week 6, week 12, and week 24 included questionnaires and psychological measures stress, anxiety, mental wellbeing, positive and negative affect, depression, self-compassion, social support, and body appreciation. Demographical data was also collected and analysed. Attrition rates were explored post intervention, and a qualitative thematic analysis was carried out on participants interview data following the KD to better understand their experience of the dietary intervention.
Results: From study 1, the KD group saw no improvements in psychological wellbeing. The LCD group reported significant improvements in stress, anxiety, and negative affect after 12 weeks and in depressive symptoms after 24 weeks compared to the KD and control group. Significant improvements in positive affect, mental well-being and depressive symptoms were found in those with lower levels of body appreciation compared to those with higher levels, regardless of diet type. From study 2, dropout rates peaked during the 12-week intervention compared to post intervention and the end of the study at 24 weeks. Those with depressive symptoms were less likely to drop out of the study compared to those who were ‘healthy’. From the qualitative study 3, participants in the KD group experienced both physical and mental health improvements. They lost weight and experienced an increase in confidence, energy, and self-esteem. Some reported a renewed meaning and purpose in life.
Conclusion: The ketogenic diet did not improve quantitatively measured depressive symptoms or aspects of psychological well-being from self-reported questionnaires. However, from interview data, improvements were experienced by those on the ketogenic diet suggesting that the diet worked for some. Reasons for this contradiction are explored and may be explained in part, by reviewing the intervention design. A low carbohydrate diet was found to improve some aspects of psychological well-being in those with mild to moderate depressive symptoms over 24 weeks. Adverse events experienced were mild and temporary, but retention of participants was challenging. Further well-designed randomised control trials are warranted to identify whether a ketogenic diet would improve psychological well-being in those with more severe depression akin to antidepressant efficacy.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- News & Events
- FDA Newsroom
- Press Announcements
FDA Warns Consumers to Avoid Certain Male Enhancement and Weight Loss Products Sold Through Amazon, eBay and Other Retailers Due to Hidden, Potentially Dangerous Drug Ingredients
FDA News Release
Agency Urges Online Marketplaces, Other Websites and Retailers to Stop Selling These Male Enhancement and Weight Loss Products to American Consumers
The FDA continues to find potentially dangerous products available for purchase. On Dec. 8, 2021, the FDA warned consumers not to purchase or use nine potentially dangerous sexual enhancement products available for purchase from Walmart.com. The FDA will continue to alert the public when products and companies place consumers’ health at risk.
On July 26, 2021, the FDA issued an untitled letter to notify Amazon about its distribution of sexual enhancement and weight loss products in violation of the Federal Food, Drug, and Cosmetic Act. The FDA continues to find potentially dangerous products available for purchase and urges stores, websites, and online marketplaces, including Amazon, to stop selling these potentially dangerous products.
The U.S. Food and Drug Administration is warning consumers not to use nearly 50 male enhancement or weight loss products that have been found to contain hidden ingredients and may pose a significant health risk. The FDA purchased these products on Amazon and eBay and agency testing found that the products contain active pharmaceutical ingredients not listed on their labels, including some with ingredients found in prescription drugs. These products may cause potentially serious side effects and may interact with medications or dietary supplements a consumer is taking.
Despite FDA consumer warnings about similar products over the past decade, the agency continues to find potentially dangerous products available for purchase on the internet, including from online marketplaces like Amazon and eBay, as well as in retail stores. The agency urges consumers to beware of purchasing or taking these products.
“Protecting the health and safety of Americans is the FDA’s highest priority, and we will remain vigilant and communicate about products and companies that place U.S. consumers at risk,” said Donald D. Ashley, J.D., director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research. “While the FDA has engaged in discussions with online marketplaces like Amazon and eBay regarding these issues in the past, we believe they can do more to protect consumers from these fraudulent and potentially dangerous products. We continue to urge stores, websites and online marketplaces, like Amazon and eBay, to take appropriate steps to protect the American public by not selling or facilitating the sale of illegal FDA-regulated products.”
All 26 of the products the FDA purchased on Amazon and 20 of 25 products, or 80 percent, purchased on eBay contained undeclared active pharmaceutical ingredients. The FDA’s laboratory testing found the products contained various undeclared active ingredients, including sildenafil, tadalafil, vardenafil, sibutramine, desmethylsibutramine, phenolphthalein and/or fluoxetine. Many of these are active ingredients for use in FDA-approved prescription drugs, which are restricted to use under the supervision of a licensed health care professional.
Many of the products the agency purchased from Amazon and eBay have names that are the same as, or similar to, tainted products that have been the subject of previous FDA consumer warnings . Several of the Amazon products are designated as an “Amazon Choice” or “#1 Best Seller.” Products with undeclared drug ingredients violate federal law. In general, these products are unapproved new drugs and/or adulterated dietary supplements. In addition, they are misbranded because their labels do not accurately reflect their ingredients.
The FDA’s tainted products database can help consumers identify nearly 1,000 of these potentially dangerous products. However, the agency is unable to test and identify all products that have potentially harmful hidden ingredients. Even if a product is not included in the list, consumers should be cautious about using certain products, especially those promoted for sexual enhancement, weight loss, bodybuilding, sleep aids or pain relief. Consumers should also be on alert for products that offer immediate or quick results and that sound too good to be true. The FDA is committed to protecting consumers by identifying and removing these potentially dangerous products from the market.
Consumers using or considering using any over-the-counter product marketed for sexual enhancement, weight loss or bodybuilding, or any product marketed as a dietary supplement for pain relief, should talk to a health care professional first, as some ingredients may interact with medications or dietary supplements. Additionally, consumers should search for product information from sources other than sellers and ask a doctor for help distinguishing between reliable and questionable information.
The FDA encourages consumers and health care professionals to report any adverse events to the agency’s MedWatch Adverse Event Reporting program so the agency can take action to protect the public from any unsafe products. The FDA is also committed to protecting consumers from the risks of buying medicines online and helping them be more aware of how to buy online safely.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Related Information
- Tainted Sexual Enhancement Products
- Tainted Weight Loss Products
- Subscribe to the RSS feed
- Beware of Fraudulent Dietary Supplements
- Presentation: Tainted Products Marketed as Dietary Supplements
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
The Effects of a 6-Week Controlled, Hypocaloric Ketogenic Diet, With and Without Exogenous Ketone Salts, on Body Composition Responses
1 Department of Human Sciences, The Ohio State University, Columbus, OH, United States
Madison L. Kackley
Christopher d. crabtree, teryn n. sapper, lauren mccabe, brandon fell, rich a. lafountain, parker n. hyde, emily r. martini, jessica bowman.
2 Department of Radiology, Davis Heart & Lung Research Institute, The Ohio State University, Columbus, OH, United States
3 Division of Cardiovascular Medicine, Department of Internal Medicine, The Ohio State University, Columbus, OH, United States
4 Department of Radiology, Wexner Medical Center, The Ohio State University, Columbus, OH, United States
Debbie Scandling
Milene l. brownlow.
5 Research and Development Department, Metagenics, Inc., Aliso Viejo, CA, United States
Annalouise O'Connor
Orlando p. simonetti, william j. kraemer, jeff s. volek, associated data.
The original contributions presented in the study are included in the article/ Supplementary Material , further inquiries can be directed to the corresponding author/s.
Background: Ketogenic diets ( KDs ) that elevate beta-hydroxybutyrate ( BHB ) promote weight and fat loss. Exogenous ketones, such as ketone salts ( KS ), also elevate BHB concentrations with the potential to protect against muscle loss during caloric restriction. Whether augmenting ketosis with KS impacts body composition responses to a well-formulated KD remains unknown.
Purpose: To explore the effects of energy-matched, hypocaloric KD feeding (<50 g carbohydrates/day; 1.5 g/kg/day protein), with and without the inclusion of KS, on weight loss and body composition responses.
Methods: Overweight and obese adults were provided a precisely defined hypocaloric KD (~75% of energy expenditure) for 6 weeks. In a double-blind manner, subjects were randomly assigned to receive ~24 g/day of a racemic BHB-salt (KD + KS; n = 12) or placebo (KD + PL; n = 13). A matched comparison group ( n = 12) was separately assigned to an isoenergetic/isonitrogenous low-fat diet (LFD). Body composition parameters were assessed by dual x-ray absorptiometry and magnetic resonance imaging.
Results: The KD induced nutritional ketosis (>1.0 mM capillary BHB) throughout the study ( p < 0.001), with higher fasting concentrations observed in KD + KS than KD + PL for the first 2 weeks ( p < 0.05). There were decreases in body mass, whole body fat and lean mass, mid-thigh muscle cross-sectional area, and both visceral and subcutaneous adipose tissues ( p < 0.001), but no group differences between the two KDs or with the LFD. Urine nitrogen excretion was significantly higher in KD + PL than LFD ( p < 0.01) and trended higher in KD + PL compared to KD + KS ( p = 0.076), whereas the nitrogen excretion during KD + KS was similar to LFD ( p > 0.05).
Conclusion: Energy-matched hypocaloric ketogenic diets favorably affected body composition but were not further impacted by administration of an exogenous BHB-salt that augmented ketosis. The trend for less nitrogen loss with the BHB-salt, if manifested over a longer period of time, may contribute to preserved lean mass.
Introduction
Ketogenic diets ( KDs ) have been demonstrated to promote weight loss, often to a greater extent than low-fat diets, and without explicit instructions to limit calories ( 1 – 4 ). Some authors have suggested that low-carbohydrate diets may facilitate a metabolic advantage by increasing energy expenditure ( 5 ), but others have argued against such an effect ( 6 ). Irrespective of whether KDs promote greater weight loss than non-KDs, an important unresolved question is how KDs impact the tissue composition and distribution of weight loss, especially when matched for calories. Ideally, weight loss diets promote loss of fat mass ( FM ), especially visceral adipose tissue ( VAT ), while preserving lean tissue.
A plausible approach to support muscle anabolism during a hypocaloric KD is the use of exogenous ketone salts ( KS ), which enhance ketosis while also providing additional sodium and potentially other minerals (e.g., potassium, calcium, magnesium). Oral ingestion of ketone salts consisting of beta-hydroxybutyrate ( BHB ) and minerals is a safe and effective method of transiently increasing circulating ketones as we ( 7 ) and others ( 8 ) have shown. Notably, prior studies have demonstrated that ketosis achieved by intravenous infusion of sodium BHB preserves fat free mass ( FFM ) during very low-calorie diets and starvation ( 9 , 10 ). Sodium BHB infusion to levels within the higher range of nutritional ketosis (i.e., ~2–4 mM) has also been shown to increase skeletal muscle protein synthesis, decrease leucine oxidation, and inhibit protein breakdown ( 11 , 12 ). There is also evidence that ketone bodies may protect from muscle loss in catabolic settings such as sarcopenia, cachexia, and excessive inflammation ( 12 – 15 ), perhaps through mTORC1 signaling ( 16 , 17 ). Based on evidence from these prior studies, we hypothesized that augmenting ketosis by oral ingestion of BHB-salts might lead to preservation of muscle during a hypocaloric KD intervention in humans.
Whereas, many studies have demonstrated that short-term hypocaloric, low-carbohydrate diets result in preservation of FFM and preferential loss of fat ( 18 – 21 ), including VAT ( 4 ), other studies have reported similar ( 22 , 23 ) or greater loss of lean body mass in subjects on KDs ( 24 , 25 ). Several factors may explain the discrepant results on the composition of weight loss to KDs. First, consuming protein at a level <1 g/kg ideal body mass impairs nitrogen balance while an intake closer to 1.5 g/kg ideal body mass appears optimal during hypocaloric diets for maintaining lean mass ( 20 , 24 , 26 ). Second, many KD studies failed to provide adequate sodium to compensate for the natriuretic effect of these diets, resulting in counter-regulatory hormonal responses that adversely impacts sodium and potassium balance ( 23 ). In the KD studies that did not provide adequate sodium, nitrogen balance, and lean mass were compromised ( 24 , 25 ). Lastly, common methods of assessing body composition, such as hydro-densitometry and dual-energy x-ray absorptiometry ( DXA ), detect water loss as a decrease in lean tissue, but this does not reflect a change in whole-body intracellular protein. For example, reduced extra-vascular volume and intra-cellular glycogen (~3 g of water is stored with each gram of glycogen), which can easily account for 2–3 kg during the first few weeks of a KD ( 22 , 27 ), would be artifactually detected as a loss of lean tissue.
Collectively, these data highlight a potential role of well-formulated KDs with adequate protein and sodium to improve preservation and lean body mass during weight loss, with potential added benefit of exogenous ketones. Using a randomized, controlled-feeding, double-blind design, the primary objective of this study was to explore the composition of weight loss, including advanced imaging assessment of fat and lean mass as well as nitrogen excretion and 3-methylhistidine ( 3MH ), to a well-formulated KD with and without KS supplementation. After completing this primary objective, we decided it would be valuable to compare these KD group responses to a matched group of obese adults fed a hypocaloric low-fat diet. We used DXA to assess whole body adiposity and lean mass, magnetic resonance imaging ( MRI ) to assess VAT mass and thigh skeletal muscle cross-sectional areas, as well as nitrogen balance and 3MH to provide additional measures of protein metabolism.
Materials and Methods
Experimental design and subjects.
This was a prospective, placebo-controlled, double-blind study design. For the primary aim, we planned to randomize and balance n = 28 (14 men/14 women) in two KD groups. Eligibility was determined based on BMI (27–35 kg/m 2 ) and age (21–65 years). Twenty-eight participants were consented and 25 completers were analyzed. We performed a stratified randomization where “age” and “BMI” were divided “below” or “above” the median range of the inclusion criteria. Following stratification, we used an online number generator ( www.randomizer.org ) to randomize eight rounds of three participants to either a ketogenic diet + ketone supplement ( KD + KS , n = 12, 6 men/6 women) or ketogenic diet + placebo ( KD + PL , n = 13, 6 men/7 women). An extra male participant was added to KD + PL due to enrolment errors. For the secondary aim, we enrolled a separate, non-randomized group of age and BMI-matched participants who received an isoenergetic/isonitrogenous low-fat diet ( LFD , n = 12, 6 men/6 women) and participated in the same testing as the KD groups.
Exclusion criteria comprised: major weight loss events (<10% body mass) 6 months prior to enrollment; habitually consuming a low-carbohydrate diet (<50 g CHO/day); pre-existing gastrointestinal disorders or food allergies; excess alcohol consumption (>14 drinks/week); disease conditions (diabetes, liver, kidney, or other metabolic or endocrine dysfunction); and use of diabetic medications. Participants who met the qualifying criteria were scheduled for an in-person screening meeting where the study was described in greater detail followed by completing questionnaires about food frequency, medical history, physical activity, MRI readiness, and a menstrual history survey. There were no significant differences in baseline characteristics of completers between groups ( Table 1 ). All participants were Caucasian. For added clarity a CONSORT diagram is available in ( Supplementary Figure 1 ).
Baseline characteristics.
| | | | -value | |
|---|---|---|---|---|
| Age (years) | 35 ± 3 | 35 ± 3 | 35 ± 3 | 0.99 |
| Height (cm) | 171.5 ± 2.9 | 172.1 ± 2.7 | 172.6 ± 2.9 | 0.96 |
| Weight (kg) | 90.4 ± 3.4 | 94.1 ± 3.2 | 92.4 ± 3.4 | 0.73 |
| BMI (kg/m ) | 30.6 ± 0.7 | 31.8 ± 0.7 | 30.9 ± 0.7 | 0.50 |
| Waist Circumference (cm) | 96 ± 3 | 95 ± 2 | 92 ± 3 | 0.66 |
| Hip Circumference (cm) | 109 ± 2 | 114 ± 2 | 111 ± 2 | 0.14 |
| Lean Mass (kg) | 55.8 ± 3.1 | 55.3 ± 3.0 | 56.0 ± 3.1 | 0.99 |
| Fat Mass (kg) | 31.1 ± 2.2 | 34.5 ± 2.1 | 33.4 ± 2.2 | 0.52 |
| Body Fat Percentage (%) | 35%±2% | 38%±2% | 36%±2% | 0.68 |
| Capillary β-hydroxybutyrate (mmol/L) | 0.18 ± 0.03 | 0.18 ± 0.04 | 0.13 ± 0.02 | 0.40 |
Values reported as mean ± SEM. p-value obtained from one-way ANOVA.
BMI, body mass index; KD, ketogenic diet; KS, ketone salts; PL, placebo; LFD, low-fat diet; M/F, men/women randomized to each diet.
Feeding Intervention
All meals for the three experimental diets were prepared in a state-of-the-art metabolic kitchen. All the ingredients were precisely weighed (±0.1 g) by research staff. Individual menu composition had custom macro- and micro-nutrients calculated by a team of registered dietitians using advanced nutrient analysis software ( Table 2 ) (Nutritionist Pro, Axxya Systems, Redmond, WA). Both KDs were designed based on our previous work ( 28 , 29 ). The LFD was developed in accordance with the USDA's Dietary Guidelines for Healthy Americans 2015–2020 ( 30 ). Ideal body weight was calculated based on the Metropolitan Life Tables for medium frame populations to establish a 1.5 g protein/kg of ideal body weight for each participant ( 31 ). The total daily energy required for weight-maintenance was derived from Harris-Benedict basal metabolic rate multiplied by physical activity level (PAL) ( 32 ). PAL was determined based on previous guidelines for sedentary individuals created by the Institute of Medicine ( 33 ). Average PAL multiplier was estimated at 1.33 (range: 1.20–1.55). The individual energy needs to achieve weight loss were calculated as 75% of estimated energy requirements for weight maintenance, which provided on average 20 kcal/kg/day (range: 16–26 kcal/kg/day).
Diet composition.
| Energy (kcal/day) | 1845 ± 102 | 1752 ± 98 | 1900 ± 102 |
| Protein (g) | 99 ± 3 | 100 ± 3 | 100 ± 3 |
| Carbohydrate (g) | 40 ± 8 | 38 ± 7 | 259 ± 8 |
| Sugar (g) | 17 ± 3 | 17 ± 3 | 101 ± 3 |
| Fiber (g) | 10 ± 1 | 10 ± 1 | 34 ± 1 |
| Added sugars (g) | n/a | n/a | <25 g/day |
| Fat (g) | 143 ± 9 | 131 ± 8 | 51 ± 9 |
| SFA (g) | 63 ± 4 | 63 ± 4 | 17 ± 4 |
| MUFA (g) | 38 ± 3 | 38 ± 3 | 10 ± 3 |
| PUFA (g) | 8 ± 1 | 8 ± 1 | 7 ± 1 |
| Cholesterol (g) | 414 ± 27 | 402 ± 26 | 154 ± 27 |
| Sodium (mg) | 6100 ± 32 | 2351 ± 30 | 1974 ± 31 |
| Potassium (mg) | 2211 ± 73 | 2243 ± 75 | 2758 ± 78 |
| Calcium (mg) | 2001 ± 36 | 880 ± 34 | 1008 ± 35 |
Values reported as mean ± SEM. Distinct letters denote group differences (p < 0.05).
SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Both KDs provided ~40 g/day of carbohydrates and the remaining non-protein calories were derived from fat, with an emphasis on monounsaturated and saturated fat sources. The LFD provided 25% of energy from lipids with <10% saturated fat and <30 g added oils. Carbohydrates were primarily complex and provided at least 32 g/fiber per day with limited and added sugars (<25 g). Ad libitum intake of calorie-free/sodium-free products was allowed during the entire intervention. A variety of whole foods were used to develop both ketogenic and low-fat meals. Meal plans were developed to include a wide range of high-quality protein sources to be distributed equally between breakfast, lunch, and dinner ( Supplementary Table 1 ) with minor differences in total amino acid (AA) intake, particularly histidine (LFD vs. KD: −520 ± 20 mg/day). Because the LFD was designed in accordance with Dietary Guidelines for Americans, a minor percentage of the total protein was derived from plant sources such as quinoa, whole grain pasta/rice/bread, oatmeal, legumes (8–10%), compared to predominantly animal protein in the KD. A portion of the protein for the KD groups was provided as two daily chocolate or vanilla shakes that contained whey protein isolate (~15 g/serving) along with fat in the form of high oleic sunflower oil and medium chain fatty acids (MCT). Assessment of capillary glycemic response over 2 h following the ketogenic shake in healthy participants ( n = 10) compared with a white bread control matched for total carbohydrate content (9 g), demonstrated that there was no glycemic response to these shakes ( Supplementary Figure 2 ). In addition, subjects in the KD groups consumed one serving of 10 g MCT oil (caprylic and capric acid; Metagenics, Inc) with their breakfast and one serving in the afternoon snack.
The KD + KS group consumed a ketone supplement, twice daily, consisting of BHB salts and non-caloric flavoring (provided by Metagenics, Inc., Aliso Viejo, CA). This particular KS has been previously reported to raise BHB 4 to 5-fold in capillary blood of non-keto adapted individuals, with significant effects observed up to 1-h post-ingestion ( 34 ). One KS serving contained 11.8 g BHB, 1,874 mg sodium, 570 mg calcium, and 57 mg magnesium. BHB content was determined in our laboratory to contain a racemic BHB enantiomer mixture of R-BHB and S-BHB. Participants in the KD-KS group were instructed to mix the KS in at least 250 ml of water and stir vigorously to ensure proper mixing. One dose was taken in the morning and one 6h later after lunch. Because the ketone supplement contained calories in the form of BHB, the fat content of the KD + KS group was reduced by ~120 kcal/day to ensure that all three diets were isocaloric. The KD + PL group and the LFD received a calorie-free flavored placebo that contained no BHB or minerals. The placebo was specifically formulated by the sponsor to mimic the taste and appearance of active supplement including flavoring but contain no BHB-salts. The same placebo was used in a previous trial with no blinding problems ( 33 ). In our pilot testing with the BHB salt we compared the supplement to the placebo in a double-blind setting with members of the group who were not involved in data collection and analysis ( n = 3). Based on their feedback there were no discernable traits between the BHB salt and placebo drinks. Based on conversations with participants throughout the study, we feel confident that they were not aware which supplement they received.
Lab Procedures
A battery of tests were performed biweekly at WK0 (baseline), WK2, WK4, and WK6. All participants reported to the testing facility between 5:00 and 7:00 a.m. for their assessments (PAES Building, 305 Annie and John Glenn Ave, Columbus, OH). Arrival conditions stipulated that subjects consume no caffeine for >12 h, no food for >8 h, sleep 8–10 h the night before testing, and abstain from strenuous exercise <48 h prior to the visit. Weight and height were measured on an electronic stadiometer (SECA 703 Digital, Hamburg, Germany) calibrated to the nearest ±0.1 cm and ±0.01 kg, respectively, while participants wore light clothing and no shoes. Urine specific gravity was measured with a light refractometer (Reichert™, Buffalo, NY). If values were >1.025, the participant was instructed to drink at least 250 ml of water until euhydration was attained. Waist and hip measurements were performed by the same investigator using a manual tape (Gulick Spring Tape) to measure circumference at the narrowest part of the waist and the greater trochanter region according to previous testing guidelines ( 35 ). Resting energy expenditure (REE) and respiratory exchange ratio (RER) were measured via indirect calorimetry (ParvoMedics TrueOne® 2,400) ( 36 ). After a 20 min supine rest, gas exchanges were measured for 25 min at 15-s intervals. REE was selected from a stable, average interval recorded within the last 5 min of continuous readings to avoid artificial number inflations caused by early REE fluctuations during calibration.
Fasting BHB concentrations were assessed in capillary blood using reagent strips and a monitoring device (Abbot FreeStyle®, Columbus, OH). Outside of test days, participants reported fasted ketones every morning during the study via image texts sent to research staff. Fasting venous blood samples were collected on testing days via venipuncture in the antecubital fossa. A single plasma tube (10ml) was inverted and spun immediately after blood draw at 1200 × G for 10 min at 4°C. Plasma fractions were aliquoted in screwcap vial containers, snap frozen in liquid nitrogen, and stored at −80°C for subsequent analysis. Plasma 3MH was assayed in duplicate using a commercially available enzyme-linked immunosorbent assay kit (Wuhan Fine Biotech). Intra- and inter-assay coefficients of variation were 4.3 and 6.8%, respectively.
For urine collection, participants were provided with a 3.7-L rated container to void their 24 h urine after each biweekly visit. Of note, the first 24 h urine collection was not a true baseline because it occurred during day one of the KD and KS intervention. Thus, the baseline 24 h period included initial exposure to ketogenic meals as well as two doses of the KS or PL. The containers were pre-treated with a stabilizer (Gentamicin 20 mg, Germall II 1.25 g) to prevent metabolite degradation and disappearance at room temperature. All urine samples were returned immediately after the 24 h period, the total volume recorded, and aliquots stored at −20°C before shipping to an off-campus collaborator (Litholink Corporation, Chicago, IL, USA). Urine creatinine and urea nitrogen were measured on a Beckman AU 680 autoanalyzer (Beckman Instruments, Brea CA); creatinine by a kinetic alkaline picrate method and urea nitrogen by an enzymatic method incorporating urease and glutamate dehydrogenase. Urine urea nitrogen ( UUN ) was standardized to creatinine excretion. Nitrogen balance was calculated using a previously validated formula ( 37 ):
Body Composition
FM and FFM were quantified by dual X-ray absorptiometry (DXA) on a Lunar iDXA system (GE, Madison, WI, USA). The DXA was quality assured by a licensed technician every 2 days to ensure accuracy. Subjects were carefully aligned within detection limits on the DXA bed. The average duration of a whole-body scan lasted 7 min. A single, whole-body, DXA measurement was performed on each subject in order to estimate lean and fat mass (CoreScan TM enCORE software version 14.10) using two-dimensional projection data created by low energy, fan beam x-ray to create a model consisting of bone, adipose, and lean tissue compartments.
We used MRI to obtain a more precise measure of VAT, subcutaneous adipose tissue ( SAT ) and mid-thigh muscle cross-sectional area ( CSA ). Each participant was imaged once at baseline and once post-intervention on a 3T scanner (MAGNETOM Prisma Fit, Siemens Healthineers, Erlangen, Germany). The VARiable PROjection (VARPRO) pulse sequence was used to acquire the in-phase, out-phase, water, water percentage, fat, and fat percentage images that were used to measure abdominal fat volumes. The VARPRO pulse sequence is a single breath hold acquisition that collects the multiple echo time images required for fat/water separation. This rapid scan technique acquires 3D volumetric images covering the entire abdominal region in a single breath-hold. The total duration of the testing session averaged 45 min to 1 h.
Analysis of abdominal fat was performed using a custom built, previously described MatLab algorithm ( 38 ) using semi-automated segmentation and calculation of VAT and SAT across the full abdomen defined as 20% of the distance from the top of the iliac crests to the base of the skull. Mid-thigh CSA was measured as a single slice at the mid-point between the inguinal crease and the proximal patella. Based on previous studies that have validated this site-specific technique in sarcopenia or muscular atrophy ( 39 , 40 ) we used MRI mid-thigh CSA as a specific measure of muscle mass to complement whole-body FFM measures detected by DXA. Manual traces were drawn in the anterior, posterior, and medial compartment of the thigh then combined to obtain a whole muscle CSA value. The contours were drawn so as to avoid inter-muscular fat and artifacts using ImageJ ( 41 ).
The sample size chosen was based on what has been done in similar feeding studies (21, 23–25, 27) and what was feasible to accomplish based on the available funding and resources. Analyses were performed using SPSS Ver. 25 (SPSS, Inc., Chicago, IL, USA). Two-tail α significance was set at p < 0.05. Baseline measures were compared for significant differences with one-way analysis of variance (ANOVA). We considered this an exploratory study and therefore analyzed main effects and interactions between KDs using a 2 (group) × 4 (time) repeated measures ANOVA without adjusting for multiple comparisons. To provide additional perspective, we also explored the comparison between the two randomized KD groups and the non-randomized LFD group in a 3 × 4 ANOVA. Independent t -tests were utilized for the pre-post MRI variables. Due to baseline variability and differences in histidine intakes, we subtracted the individual 3-methylhistidine values from their respective baseline (normalized values) and analyzed the normalized means for main effects and interactions using baseline as covariate. Fisher's Least Significant Difference (LSD) correction was used for all post-hoc analyses. All data are presented as mean ± SEM.
KD + KS vs. KD + PL
In both KD groups, fasted capillary BHB increased progressively to concentrations above 1.0 mmol/L by WK2 and stayed elevated throughout the 6-week intervention ( Figure 1 ). Fasting BHB was higher in KD + KS at WK1 and WK2 but was similar to KD + PL thereafter. REE decreased at WK2 and stayed lower throughout the intervention with no differences between groups. RER decreased at WK2 and remained lower throughout the intervention ( Table 3 ).

Fasting capillary BHB responses to a ketogenic diet plus ketone salt (KD + KS) vs. placebo (KD + PL). Values at each week represent mean (± SEM) of 7-day BHB values. Primary Statistics: 2 (group) × 7 (time point) ANOVA followed by secondary 3×7 ANOVA that included a third low-fat diet (LFD) group (dashed line). Time effects: *** p < 0.001 from WK0 for primary statistics only. Interaction effects: distinct letters at each time point denote between group differences ( p < 0.05).
Diet effects and interactions.
| Weight (kg) | KD + KS | 90.4 ± 3.4 | 86.6 ± 3.3 | 84.6 ± 3.3 | 83.1 ± 3.3 | −7.3 | −8% | 0.35 | 0.47 | 0.45 | |
| KD + PL | 94.1 ± 3.2 | 89.5 ± 3.2 | 87.9 ± 3.2 | 86.1 ± 3.2 | −8.0 | −9% | |||||
| BMI (kg/m ) | KD + KS | 30.6 ± 0.7 | 29.4 ± 0.7 | 28.7 ± 0.7 | 28.2 ± 0.8 | −2.4 | −8% | 0.36 | 0.34 | 0.49 | |
| KD + PL | 31.8 ± 0.7 | 30.3 ± 0.7 | 29.7 ± 0.7 | 29.1 ± 0.7 | −2.6 | −8% | |||||
| Waist circumference (cm) | KD + KS | 95.7 ± 2.5 | 93.5 ± 2.7 | 91.0 ± 2.5 | 87.7 ± 2.6 | −8.0 | −8% | 0.20 | 0.65 | 0.32 | |
| KD + PL | 94.7 ± 2.4 | 91.0 ± 2.6 | 89.0 ± 2.4 | 87.4 ± 2.5 | −7.3 | −8% | |||||
| Hip circumference (cm) | KD + KS | 108.6 ± 1.8 | 106.7 ± 1.7 | 103.9 ± 1.9 | 103.8 ± 1.8 | −4.7 | −4% | 0.61 | 0.06 | 0.26 | |
| KD + PL | 113.7 ± 1.8 | 110.8 ± 1.7 | 109.0 ± 1.8 | 107.5 ± 1.8 | −6.3 | −6% | |||||
| Waist: hip ratio (cm/cm) | KD + KS | 0.88 ± 0.03 | 0.88 ± 0.02 | 0.88 ± 0.02 | 0.85 ± 0.02 | −0.04 | −4% | 0.38 | 0.13 | 0.23 | |
| KD + PL | 0.84 ± 0.02 | 0.82 ± 0.02 | 0.82 ± 0.02 | 0.81 ± 0.02 | −0.02 | −3% | |||||
| Lean body mass (kg) | KD + KS | 55.8 ± 3.1 | 54.2 ± 3.1 | 54.2 ± 3.0 | 53.9 ± 3.1 | −1.9 | −3% | 0.04 | 0.91 | 0.92 | |
| KD + PL | 55.3 ± 3.0 | 53.9 ± 3.0 | 53.6 ± 2.9 | 53.3 ± 2.9 | −1.9 | −4% | |||||
| Body fat mass (kg) | KD + KS | 31.1 ± 2.2 | 29.4 ± 2.1 | 27.7 ± 2.1 | 26.4 ± 2.1 | −4.8 | −15% | 0.24 | 0.20 | 0.63 | |
| KD + PL | 34.5 ± 2.1 | 32.8 ± 2.0 | 31.4 ± 2.0 | 30.2 ± 2.1 | −4.4 | −13% | |||||
| Lean: fat mass ratio (kg/kg) | KD + KS | 1.9 ± 0.2 | 1.9 ± 0.3 | 2.0 ± 0.3 | 2.1 ± 0.3 | 0.3 | 15% | 0.31 | 0.56 | 0.44 | |
| KD + PL | 1.7 ± 0.2 | 1.8 ± 0.2 | 1.8 ± 0.3 | 1.9 ± 0.3 | 0.2 | 13% | |||||
| Body fat percentage (%) | KD + KS | 35.0 ± 2.2 | 34.6 ± 2.2 | 33.2 ± 2.3 | 32.2 ± 2.3 | −2.8 | 0.08 | 0.41 | 0.68 | ||
| KD + PL | 37.7 ± 2.1 | 36.9 ± 2.1 | 35.9 ± 2.2 | 35.0 ± 2.2 | −2.7 | ||||||
| Visceral adipose tissue (g) | KD + KS | 2978 ± 589 | 2378 ± 520 | −600 | −20% | 0.24 | 0.98 | 0.56 | |||
| KD + PL | 2947 ± 542 | 2434 ± 479 | −513 | −17% | |||||||
| Subcutaneous adipose tissue (g) | KD + KS | 5220 ± 639 | 4282 ± 539 | −938 | −18% | 0.05 | 0.44 | 0.90 | |||
| KD + PL | 5869 ± 588 | 4894 ± 496 | −974 | −17% | |||||||
| Resting energy expenditure (kcal/day) | KD + KS | 1885 ± 93 | 1774 ± 86 | 1621 ± 82 | 1653 ± 87 | −231 | −12% | 0.35 | 0.41 | 0.30 | |
| KD + PL | 1739 ± 90 | 1605 ± 83 | 1609 ± 79 | 1604 ± 84 | −135 | −8% | |||||
| Respiratory exchange ratio (V /V ) | KD + KS | 0.83 ± 0.02 | 0.75 ± 0.01 | 0.76 ± 0.01 | 0.77 ± 0.01 | −0.07 | −8% | 0.28 | 0.06 | 0.69 | |
| KD + PL | 0.86 ± 0.02 | 0.78 ± 0.01 | 0.77 ± 0.01 | 0.78 ± 0.01 | −0.08 | −10% | |||||
| Urea nitrogen (g) | KD + KS | 7.4 ± 0.3 | 7.8 ± 0.4 | 7.1 ± 0.3 | 6.7 ± 0.3 | −0.7 | −9% | 0.12 | 0.08 | 0.98 | |
| KD + PL | 7.8 ± 0.3 | 8.3 ± 0.4 | 7.8 ± 0.3 | 7.3 ± 0.3 | −0.5 | −6% | |||||
| Nitrogen balance | KD + KS | 4.5 ± 0.6 | 4.0 ± 0.7 | 4.7 ± 0.6 | 5.2 ± 0.6 | 0.7 | 16% | 0.12 | 0.58 | 0.99 | |
| KD + PL | 4.1 ± 0.5 | 3.6 ± 0.7 | 4.2 ± 0.6 | 4.6 ± 0.6 | 0.5 | 12% | |||||
| 3-Methylhistidine (nmol/mL) | KD + KS | 43 ± 8 | 53 ± 21 | 31 ± 13 | 46 ± 14 | 4 | 8% | 0.51 | 0.29 | 0.19 | 0.46 |
| KD + PL | 24 ± 3 | 39 ± 11 | 34 ± 5 | 51 ± 10 | 27 | 110% | |||||
Values reported as mean ± SEM. Time effects:
Δ = absolute change from WK0. %Δ = percent change from WK0. ES = effect size (Cohen's d). Bold face higlights significant effects .
There were significant changes over time in all DXA and MRI parameters of body composition, but no group differences ( Figures 2A–C ). Body mass, FM, and FFM decreased at the end of the feeding intervention by −7.7 ± 0.4, −4.6 ± 0.4, and −1.9 ± 0.3 kg, respectively ( p < 0.001). The trajectory of body weight loss was most pronounced in the first 2 weeks (−4.6 ± 0.2% BW) and accounted for more than half (57%) of the total body weight that was lost during the intervention. Additionally, more than three-quarters of lean mass were lost by WK2 (77%) and continued toward a non-significant decrease thereafter. MRI analysis indicated a significant loss of both VAT (−19 ± 2 %; p < 0.001) and SAT (−17 ± 3%; p < 0.001). The whole-body lean-to-fat mass ratio improved in both KD groups (+0.3 ± 0.1 kg/kg; p < 0.001), showing a preference in body fat reduction over muscle. Mid-thigh whole quadriceps muscle CSA decreased over time (−5 ± 1%; p < 0.001) and corroborated the trends seen in DXA ( Table 4 ). Waist circumference (WC) decreased in KD + KS (−8.0 ± 1.0 cm; p < 0.001) and KD + PL (−7.3 ± 1.0 cm; p < 0.001) starting at WK2 and continuing through WK6. Waist-to-hip ratio (WHR) also decreased in both KD + KS (−0.04 ± 0.01; p < 0.001) and KD + PL (−0.02 ± 0.01; p < 0.05) by WK6 ( Table 3 ).

Diet effects on body weight and weight loss composition. Solid lines are primary KD outcomes; dashed lines show LFD for comparison. Results are presented as absolute change (± SEM) from baseline. Panels: (A) scale body weight, (B) DXA whole-body fat and (C) DXA whole-body lean mass. Statistics: 2 (group) × 4 (time) ANOVA. Time effects: *** p < 0.001 from WK0. Body weight and fat mass decreased steadily and significantly between each timepoint after WK0 ( p < 0.001). Lean mass decreased significantly by WK2 ( p < 0.001), followed by no other significant reductions thereafter. With LFD included in the secondary 3 (group) × 4 (time) ANOVA analysis – No major changes were detected besides a higher FFM WK2 value compared to WK6 ( p < 0.05).
Mid-thigh cross-sectional area.
| ANTERIOR (mm ) | KD + KS | 7599 ± 543 | 7340 ± 511 | −259 | −3% | 0.03 | 0.22 | 0.94 | |
| KD + PL | 6717 ± 522 | 6450 ± 491 | −267 | −4% | |||||
| POSTERIOR (mm ) | KD + KS | 3527 ± 205 | 3361 ± 183 | −166 | −5% | 0.47 | 0.26 | 0.25 | |
| KD + PL | 3257 ± 197 | 2986 ± 176 | −272 | −8% | |||||
| MEDIAL (mm ) | KD + KS | 3675 ± 322 | 3462 ± 315 | −213 | −6% | 0.06 | 0.40 | 0.88 | |
| KD + PL | 3385 ± 309 | 3154 ± 303 | −230 | −7% | |||||
| WHOLE (mm ) | KD + KS | 14801 ± 961 | 14163 ± 893 | −638 | −4% | 0.22 | 0.21 | 0.58 | |
| KD + PL | 13359 ± 923 | 12590 ± 858 | −769 | −6% | |||||
Values reported as mean ± SEM. ES = effect size (Cohen's d).
Time effects:
Anterior compartment: quadriceps (vastus lateralis/medialis, rectus, sartorius); Posterior compartment: biceps femoris, semimembranosus, semitendinosus; Medial compartment: adductor longus/magnus, gracilis. Bold face higlights significant effects .
Concentrations of UUN demonstrated a slight increase at WK2 and then declined significantly from that point at WK4 ( p < 0.05) and WK6 ( p < 0.01). There was a group trend for lower UUN excretion in the KD + KS group (−8%, p = 0.082) ( Figure 3A ). Nitrogen balance (NB) demonstrated a minor decrease at WK2 that increased significantly from that point at WK4 ( p < 0.05) and WK6 ( p < 0.01), but there were no significant group trends ( p = 0.58) ( Figure 3B ). Normalized plasma 3MH values did not change significantly ( Figure 4 ).

Diet effects on urinary nitrogen and nitrogen balance. Solid lines are primary KD outcomes; dashed lines show LFD for comparison. Panels: (A) 24 h urine urea nitrogen standardized per gram of creatinine; (B) nitrogen balance derived from validated formula. Primary analysis: 2 (group) × 4 (time) ANOVA. WK2 UUN was significantly higher than WK4 ( p < 0.05) and WK6 ( p < 0.01). Group effects show on average 8% lower UUN during KD + KS compared to KD + PL ( p = 0.082). With LFD included in the secondary 3 (group) × 4 (time) ANOVA analysis – Time effects: * p < 0.05 from WK0. Between-group effects: KD + PL had significantly higher UUN than LFD ( p = 0.004) and trended higher than KD + KS ( p = 0.076).

Change in normalized plasma 3-methylhistidine relative to baseline. Values were subtracted from WK0 plasma 3MH concentrations and analyzed for significant covariance effects. Primary statistic: 2 (group) × 4 (time) ANOVA with normalized baseline held as covariate. No significant effects were detected between KD groups. With LFD included in the secondary 3 (group) × 4 (time) ANOVA – Time effect: * p < 0.05; ** p < 0.01 from WK0.
KD + KS vs. KD + PL vs. LFD
For most parameters, the LFD responded in a similar manner as the KDs with a few notable exceptions ( Supplementary Table 2 ). The KDs significantly altered ketones and RER, but these variables were not altered by the LFD. WC reductions were evident only by WK6 on LFD (−2.9 ± 1.0 cm; p = 0.008) whereas, WHR was not significantly altered in this group. We discovered a significant interaction for WC and WHR, with both KD groups showing a greater decrease. The main group effect for UUN was significant in the 3 × 4 ANOVA ( p = 0.015) and revealed that the KD + PL group excreted significantly more urea nitrogen than LFD ( p = 0.004) ( Figure 3A ) while trending higher compared to KD + KS ( p = 0.076). There was a significant time effect for 3MH that indicated higher concentrations at WK2 ( p < 0.05) and WK6 ( p < 0.01) compared to baseline ( Figure 4 ).
This study investigated the effects of daily exogenous ketone BHB supplementation (in the form of a racemic BHB salt) in the context of a 6-week energy-controlled ketogenic feeding intervention that produced clinically meaningful weight loss. Although the KS transiently elevated BHB concentrations beyond the KD alone, it did not alter the magnitude of weight loss or changes in whole body and regional tissue composition including VAT, SAT, and thigh muscle CSA. There was a trend for lower UUN with the addition of a KS to the KD, but this was not of sufficient magnitude to manifest in a detectable change in FFM by DXA or muscle CSA by MRI over a 6-week period. The body composition responses to the KDs were similar to those observed after a calorically matched LFD.
As expected, the KD elevated fasting BHB (mean 1.1 mM), which was augmented by addition of the KS (mean 1.4 mM) during the first 2 weeks of the intervention. Ketosis was achieved in as early as 3 days, similar to what we have reported before with implementation of a well-formulated KD ( 4 ). It is important to point out that the fasting measures of BHB were all done in the morning after an overnight fast and at least 10 h following the ingestion of the last dose of KS the evening of the previous day. In keto-naïve people (fasting BHB 0.1–0.2 mM), the exact same KS and dose has previously been shown to result in a rapid increase in capillary BHB peaking at 1 mM after approximately 15 min and gradually returning to baseline by 2 h ( 34 ). Considering the transient nature of BHB elevation after consumption of the KS, the fact that we were able to detect higher fasting BHB 10–12 h later in the KD + KS group was somewhat unexpected. The augmentation of fasting BHB in response to exogenous ketones only lasted 2 weeks, suggesting that adaptations in the metabolic regulation of ketone homeostasis (e.g., BHB oxidation, renal clearance, etc.) occurred.
We hypothesized that augmentation of ketosis by twice daily ingestion of a KS might enhance preservation of lean mass in response to a hypocaloric KD that produced clinically meaningful weight loss, but results from our 6-week intervention suggest that was not the case. For every kilogram of FFM lost, FM decreased between 2.3 and 2.5 kg, a ratio that is consistent with previously published KD studies ( 42 ). These results must be viewed with an understanding that DXA determines fluid loss as a decrease in FFM, and KDs are associated with initial loss of body water ( 22 , 43 ). KDs have been shown to acutely reduce glycogen stores by 50% ( 44 ), which could account for nearly 1 kg of lean tissue. KDs are also associated with a natriuretic and diuretic effect that could lead to greater loss of fluid, especially if not counter-acted by increased sodium and fluid intake, again being reflected by increased loss of lean tissue. It is notable that the two KDs contained significantly different amounts of sodium due to the high sodium content of the KS (KD + PL = 2,351, KD + KS = 6,100 mg/day), yet this did not impact DXA determined FFM responses. Furthermore, whole body FFM analyses can be affected by the trunk area due to the presence of confounding organs in the region of interest, so appendicular regions, such as the thigh, have been used to circumvent this limitation in FFM assessment ( 45 ). Given these limitations of DXA we also used MRI assessment of thigh musculature, which demonstrated significant CSA reductions after 6 weeks in all mid-thigh muscle subdivisions starting in highest order from posterior, then medial, and least from the anterior compartment ( p < 0.001). The aggregate between these three compartments was expressed as whole thigh CSA and corroborated with the significant FFM reductions showed by DXA ( p < 0.001). The differences between DXA and MRI expressed as a total percentage FFM loss from baseline did not correlate significantly within KD + KS ( r = 0.42; p = 0.20) or KD + PL ( r = 0.26; p = 0.40). However, the correlation was significant within LFD ( r = 0.64; p = 0.025). The observed discrepancy with KD raises some caution in FFM assessments after significant weight loss due to technological limitations. Nevertheless, our results suggest that KS inclusion does not significantly alter FFM during a short-term KD that provides adequate protein.
In addition to imaging lean tissue, we measured markers of protein metabolism including UUN and 3MH, which may be more sensitive to change. The trend for lower UUN in the KD + KS group supports our initial hypothesis that the addition of a KS to a well-formulated KD may improve whole nitrogen turnover compared to PL (−8%), but the relatively low effect size casts doubt that there was a meaningful difference between the two ketogenic diets. It is noteworthy that the baseline 24 h urine collection started on the first day of the KD and also included two doses of the KS (i.e., UNN may have been influenced at baseline by exposure to the first two doses of KS). Thus, the group effect in this case is strengthened by the consistently lower UUN in KD + KS at baseline, WK2, WK4, and WK6 compared to KD + PL. However, the difference between baseline UUN values and WK6 trended toward significance ( p = 0.073), and inclusion of LFD in secondary statistical analyses showed that WK6 UUN was significantly lower than baseline ( p < 0.05). Secondary analyses also revealed that non-supplemented KDs may excrete more nitrogen than LFD ( p = 0.008). Others have demonstrated similar results by showing acute increases in nitrogen excretion (<2 weeks), followed by a prompt restoration of nitrogen balance thereafter explained by ketonemia maintenance ( 26 , 44 , 46 – 48 ). The trend for less nitrogen excretion with use of a KS is consistent with studies that demonstrate nitrogen balance improvements with BHB salts ( 9 ) and infusions ( 10 ). Some caution is warranted when comparing UUN and NBAL to FFM measured by DXA. Discrepancies in the NBAL formula can be attributed to both over- and underestimating rates of muscle protein synthesis, nitrogen clearance, unaccounted integumentary losses and fluid loss ( 49 – 51 ). Other authors used UUN and NBAL data to qualitatively support their diets rather than quantitatively correlate each gram of nitrogen lost to FFM (1 g UUN ~30 g FFM) to avoid erroneous conclusions ( 9 , 49 , 50 ). Longer interventions (>6 weeks) may be needed to accrue the benefits of enhanced nitrogen preservation that could be detected in better preservation of lean tissue as determined by conventional body composition and imaging technologies.
While UUN provides a global indication of nitrogen loss from a variety of proteins, circulating 3MH is a specific surrogate for actin and myosin degradation. Although we hypothesized higher levels of BHB might act in an anti-catabolic manner to decrease 3MH ( 9 ), our results did not confirm this response. We speculate based on previous evidence that well-formulated KDs, especially ones that include exogenous BHB, can interact with essential AA ( 11 , 52 ) and decrease essential AA efflux from muscle into circulation ( 10 , 12 , 15 , 44 ). While some authors have previously shown that BHB does not always interact with AAs ( 13 , 53 ), and others advise caution using plasma AA concentrations alone as evidence ( 11 ), these recommendations suggest that more work is needed to explore the efficacy of KS on AA catabolism during weight loss.
BHB inhibits adipose tissue lipolysis acting through PUMA-G receptors ( 54 ). Some human and rat studies have shown that intravenous D-BHB infusion or racemic salts decreased lipolysis ( 53 , 55 , 56 ), suggesting that use of KS could attenuate fat loss and affect body composition. However, the similar loss of whole-body fat from DXA and decreased VAT/SAT from MRI show no indication of KS impacting adiposity. Fat loss occurred at a similar rate, independent of the KS, which was expected given the caloric deficit was similar between all diets. We were motivated to expand on our DXA adipose tissue findings with MRI measures of VAT and SAT after previous authors demonstrated higher instrument accuracy over DXA, particularly toward SAT measurements ( 38 ). MRI revealed that the KS does not impact the normal loss of adipose tissue from visceral and subcutaneous depots in response to a KD. The decreased VAT/SAT aligned with the changes in waist and hip circumference. There was a more rapid decrease in waist circumference with the KD than LFD, perhaps due to early loss of fluid, although similar to anthropometry findings of another controlled low-carbohydrate and low-fat comparison trial ( 57 ).
We attempted to standardize the caloric intake across all participants (i.e., 75% of their estimated energy needs for weight maintenance) to provide a similar relative caloric deficit between groups which was approximately 600 kcal/day. We acknowledge our methods of determining energy needs may have introduced error in determining both basal metabolic rate and physical activity correction factors, thereby contributing to variation in weight loss among participants. Since we relied on equations as opposed to more accurate methods (e.g., whole room calorimetry or doubly labeled water), it is possible we may have prescribed a >25% energy deficit (e.g., by underestimating PAL). Even if this was the case, our approach was applied equally across all participants and should not have influenced the outcomes.
Conclusions
In summary, results of our exploratory investigation suggests that inclusion of a KS to a well-formulated hypocaloric KD does not significantly alter weight loss or body composition responses compared to a calorie-matched isonitrogenous KD + PL. Thus, short-term changes in body composition during caloric restriction are driven by caloric restriction more so than level of ketosis or macronutrient distribution.
Data Availability Statement
Ethics statement.
The studies involving human participants were reviewed and approved by the study protocol was approved by The Ohio State University Institutional Review Board (IRB #: 2017H0395). All eligible participants signed an informed consent document approved by IRB before participating in the study. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AB, MK, CC, and JV contributed to the data collection, analysis, and writing the initial draft. TS, LM, and BF created the diets, conducted nutrient analysis, and organized the feeding operations. RL and PH implemented the double-blind protocol between staff and participants and provided help with clinical research duties. CC, EM, JB, YP, DS, and OS operated all the advanced imaging scans. MB and AO'C contributed to the development of the supplement, placebo, and pilot study data. OS, WK, and JV reviewed and modified all versions of the manuscript. All authors agreed on the final version.
Conflict of Interest
JV receives royalties for low-carbohydrate nutrition books; is founder, consultant, and stockholder of Virta Health, Inc. and is a member of the advisory boards for Simply Good Foods. OS receives research funding support from The Robert F. Wolfe and Edgar T. Wolfe Foundation and from Siemens Healthineers. MB and AO'C are employees of Metagenics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Metagenics, Inc. for providing the funds and supplements that made this study possible. Additionally, we want to thank Chef David Wolf and Ava Dicke for the help and efforts during food preparation sessions, and Dr. Jackie Buell for assisting with body composition assessments.
Abbreviations
| 3MH | 3-mtehylhistidine |
| ANOVA | analysis of variance |
| ANCOVA | analysis of covariance |
| BHB | beta-hydroxybutyrate |
| BMI | body mass index |
| BW | body weight |
| CHO | carbohydrate |
| CSA | cross sectional area |
| DXA | dual x-ray absorptiometry |
| FM | fat mass |
| FFM | fat free mass |
| KD | ketogenic diet |
| KS | ketogenic salts |
| LSD | least significant difference |
| MRI | magnetic resonance imaging |
| NB | nitrogen balance |
| PL | placebo |
| UUN | urine urea nitrogen |
| SAT | subcutaneous adipose tissue |
| VAT | visceral adipose tissue. |
Funding. This work was funded by a project grant received from Metagenics, Inc.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.618520/full#supplementary-material

IMAGES
VIDEO
COMMENTS
Thus, the aim of this review is to highlight the role the ketogenic diet has in altering the microbiome, epigenetics, weight loss, diabetes, cardiovascular disease, and cancer as summarized below ( Figure 3 ). Figure 3. The potential therapeutic impacts of the ketogenic diet on the microbiome, epigenome, diabetes, weight loss and cardiovascular ...
The ketogenic diet has become increasing popular in recent years. With 25.4 million unique searches, the keto diet was the most Googled diet in the United States in 2020. 1 With increased consumer interest, the "keto" food industry has grown rapidly, and as a result, the global ketogenic diet market was valued at $9.57 billion in 2019. 2 The ketogenic diet has been discussed in popular ...
The term "Ketogenic Diet" may lead to apprehension in diabetic patients given its association with the well-known, life-threatening condition of ketoacidosis. It is important to note that during nutritional ketosis, the concentrations of beta-hydroxybutyrate and acetone are of low levels and do not cause any alterations in the pH of blood ...
Keto diet benefits may include helping people lose weight, manage acne, and improve heart health. But, there may be some risks. The keto diet is an eating plan that focuses on foods that provide a ...
1. ). Notably, diets full of unrefined carbohydrates are equally as healthful, if not more, and may confer less actual and potential risks to patients (. 2. ). 3. ). Furthermore, research suggests that some of the weight lost with low-carbohydrate diets is water loss or, worse, lean body mass loss (. 4.
English Composition I (ENGL1301) Institution. Kilgore College. An argument essay arguing in support of the ketogenic diet, a low-carb, high-fat diet. Formatted according to MLA style. 1165 words with a works cited page that has five sources. Preview 2 out of 5 pages.
39yo female, have lost ~60lbs on Keto since May 1st of this year. Energy: I have fibromyalgia, which is characterized by fatigue (often extreme), joint and muscle pain, low stamina, brain fog. Pre-Keto and since my late teens, this was my life. I could, and often did, easily sleep 18 hours a day and never feel rested.
The ketogenic diet is the best way by which ketosis is achieved and ketosis is clearly a natural human metabolic process that is advantageous for healthy development of an infant and beneficial for healthy body recovery and maintenance for adults. ... Consensus Statements; Coronavirus (COVID-19) Critical Care Medicine; Cultural Competency ...
Ketogenic diet (KD), which contains a very low level of carbohydrates (<55 g/d) with the main energy sources of lipid and protein, and which causes ketosis and simulates the physiological state of ...
Thesis Statement Generator Paraphrasing Tool Title Page Generator Lit. Guides; More. Expert Q&A Study Blog About Us Writing Help ... " The Ketogenic Diet: A Detailed Beginner's Guide to Keto. " heathline. 2018. Web. "Pros and Cons of Low-Carb/Ketogenic Diets." Nutrition Letter, vol. 37, no. 10, 2019, pp. 1-2.
Types of KD showed different effects on health outcomes with changes more than the MCID thresholds in different populations (Fig. 2).KD or MAD for 3-16 months was associated with a 5-times higher proportion of children and adolescents with refractory epilepsy achieving seizure frequency reduction ≥ 50% from baseline compared with a regular diet (RR, 5.11; 95% CI, 3.18 to 8.21) [].
The very low-calorie ketogenic diet (VLCKD) has been recognized as a promising dietary regimen for the treatment of several diseases. Short-chain fatty acids (SCFAs) produced by anaerobic ...
side effect of ketogenic -diet which then leads to osteoporosis [1]. As in this typ e of dieting, calories a re restricte d so it also gives benefit by r e-. ducing the r isk of d ifferent d ...
the effects of the ketogenic diet (kd) on inflammatory pain by livia s. wyss . a thesis submitted to the faculty of the neuroscience program in candidacy for the baccalaureate degree with honors in neuroscience neuroscience program hartford, connecticut may 16, 2016
University of Toronto
The ketogenic diet, which is a high-fat, adequate-protein, and low-carbohydrate diet, gained a resurgence of interest during the past decade for the treatment of difficult-to-control seizures in children . Also called a very low-carbohydrate diet, the ketogenic diet has been in use since the 1920s for epilepsy therapy; in some cases, it can ...
The ketogenic diet did not improve quantitatively measured depressive symptoms or aspects of psychological well-being from self-reported questionnaires. However, from interview data, improvements were experienced by those on the ketogenic diet suggesting that the diet worked for some.
Week 11 The Keto Diet Mini-Research Paper Plan Topic's Stance, Working Title, Working Thesis Statement & Works Cited ALL students in this course will write about the Ketogenic Diet. Discuss your topic's stance on the Keto Diet--pro (for) or con (against) the Keto Diet, select your three (3) online websites and/or online articles, compose your three-part working thesis statement (in at least 50 ...
Thesis statement: Although ketogenic diet (KD) has showed a lot of benefit in terms of reducing disease and loss fat, the question is whether the KD is good for the health or not. Recently, the KD seems to be a hot topic in public, the fact is that this is indeed not something new, according to Wheless "To mimic the metabolism of fasting, the ketogenic diet (KD) was introduced by modern ...
Outline Format : KETO DIET I. Thesis statement. II. What foods, vitamins, and rituals are typical of this fad diet, and why? A. Subpoint 1. Details of Subpoint 2. More details of the Subpoint B. Next Subpoint 1. Details of the Subpoint 2. More details of the Subpoint III.
The Keto diet is increasingly being considered as an adjuvant approach in cancer therapy due to its observed effects on slowing tumor growth (10-12). Mechanistically, the Keto diet counteracts the Warburg effect, where cancer cells primarily depend upon glycolysis for adenosine 5′-triphosphate production rather than oxidative phosphorylation.
A ketogenic diet primarily consists of high fat intake, moderate protein consumption, and low carbohydrate intake. The macronutrient distribution typically ranges from approximately 55% to 60% fat, 30% to 35% protein, and 5% to 10% carbohydrates. For instance, in a 2000 kcal per day diet, the carbohydrate allowance would amount to approximately ...
FDA is warning consumers not to use nearly 50 male enhancement or weight loss products that have been found to contain hidden ingredients and may pose a significant health risk.
DJT1: Annotated Bibliography Annotated Bibliography Template Thesis Statement: Research shows that a Ketogenic diet is a good idea for those with diabetes because it can help patients reduce or eliminate insulin requirements for type 2 diabetes, decrease blood cholesterol, and treat epilepsy. Annotated Bibliography: Reference Cleveland Clinic: Health Essentials (July 25, 2022) Nutrition: Here ...
Introduction. Ketogenic diets (KDs) have been demonstrated to promote weight loss, often to a greater extent than low-fat diets, and without explicit instructions to limit calories (1-4).Some authors have suggested that low-carbohydrate diets may facilitate a metabolic advantage by increasing energy expenditure (), but others have argued against such an effect ().