Clinical Trials Transformation Initiative
- Registry Trials
Home - Our Work - Novel Clinical Trial Designs - Registry Trials
The primary purpose of registries has traditionally been to collect data to better understand long-term trends in specific populations. Data from registries hold great potential: they can help make clinical trials more efficient and less expensive and potentially bring new treatments to patients faster.
To achieve this potential, stakeholders can use CTTI’s recommendations for registry trials to assess, develop, and evaluate registries for the support of clinical research and help meet expectations for FDA review of new products.

Recommendation Summary
Recommendations.
Recommendations for Registry Trials
Related Items
- Determining the Suitability of Registries for Embedding Clinical Trials in the United States: A Project of the Clinical Trials Transformation Initiative
- New CTTI Recommendations Provide Path for More Efficient Clinical Trials Using Clinical Registries
- CTTI Presents Recommendations from the Registry Trials Project
- Embedding Clinical Trials within Registries: How Feasible?
- A Brave New World: Registry-Based Clinical Trials
- Join Transforming Trials 2030
- Mark B McClellan, MD, PhD
- John Alexander, MD, MHS, FACC
- M. Khair ElZarrad, PhD, MPH
- Donna R. Cryer, JD
- Pat Furlong
- Steven K. Galson, MD, MPH
- Patricia Hurley, MSC
- Michael Kolodziej, MD
- Theodore Lystig, PhD
- Rod MacKenzie, PhD
- Pierre Meulien, PhD
- Robert Temple, MD
- Spiros Vamvakas, MD
- Bram Zuckerman, MD
- Steering Committee
- Benefits & Responsibilities
- Annual Membership Dues
- Become A Member
- Become a Patient/Caregiver Rep
- Meet Our Current Patient/Caregiver Reps
- Annual Accomplishments
- Transparency
- Our Work All Recommendations & Resources
- Quality By Design
- Measuring Trials Transformation
- Informing ICH E6 Renovation
- ClinicalTrials.gov Reporting Challenges
- Recruitment
- Pregnancy Testing
- State of Clinical Trials
- Clinical Trials Issues Related to COVID-19
- Engaging All Stakeholders in Clinical Trial Design
- Developing Novel Endpoints
- Planning Decentralized Trials
- Selecting and Testing a Digital Health Technology
- Managing Data
- Supporting Sites
- Interacting with Regulators
- Real-World Data
- Disease Progression Modeling
- Master Protocol Studies
- Trials in Clinical Practice
- ABDD HABP/VABP Studies
- ABDD Peds Trials
- ABDD Streamlining HABP/VABP Trials
- ABDD Unmet Need
- Electronic Healthcare Data
- Large Simple Trials
- Patient Group Engagement
- Patient Engagement Collaborative
- Investigator Community
- Investigator Qualification
- Study Start-Up
- Informed Consent
- Safety Reporting
- Data Monitoring Committees
- CTTI History
- Publications
- Media Coverage
- CTTI Meetings
- Press Releases
- Case Studies
- CTTI Presentations
- CTTI Paper Discusses Suitability of Patient Registries for Embedded Clinical Trials Embedding clinical trials into patient registries can lead to high-quality, efficient prospective research. However, methods for assessing which registries are appropriate to serve as the platform for the conduct of ...
Demographic, disease, and outcome data collected in clinical observational registries can be a valuable resource when these data overlap with those needed to support clinical trials. Integrating clinical trials within registries may offer considerable benefits and improve overall trial conduct; however, methods are needed to inform key decisions or activities, including the following:
- Identify the appropriate registries
- Ensure data quality/comparability
- Meet regulatory/legal requirements
- Protect participant privacy/security
- Clarify processes that are necessary to implement a registry-based clinical trial
The Registry Trials Project focuses on the feasibility of using registries to conduct prospective, embedded clinical trials that can support FDA review of new products, including efficacy as well as safety evaluations.*
*Note: Study designers and sponsors should meet with FDA for official determination if registry trial data is acceptable for regulatory purposes.
Registry Trials: Conducting Clinical Trials Using Clinical Observational Registries (2013-2017)
- Identify essential elements of registries needed to successfully embed and conduct registry-based clinical trials
- Determine requirements to utilize a data registry for a clinical trial (e.g., electronic transfer of data from registry to a case report form, communication between registry personnel and site coordinators)
- IND-based submissions for drug/biologic trials and device/IDE applications
- Describe the potential barriers to the conduct of clinical trials within a registry, and leverage learning from successful trials in order to overcome those barriers
- Outline the lessons learned from post-market registry device trials that may be applied to earlier phase device trials and drug trials in the context of different regulatory requirements
- Recommend best practices for conducting randomized registry trials for regulatory purposes
Increase in the practice of leveraging clinical observational registries to facilitate high-quality clinical trials at lower costs
- Literature review
- Expert interviews
- Landscape assessment
- Expert meeting
The literature review revealed that the type and purpose of a registry are important to determine if embedding a clinical trial is possible and appropriate. Considerations include data completeness, data quality, interoperability, representativeness, informed consent, and privacy. There are also cost and operational questions, such as who will fund the registry, which party will pay for adjustments required for clinical trials, and who will be responsible for maintenance costs.
In multi-stakeholder interviews , experts described weaknesses and strengths of registries. Data quality was consistently identified as a potential weakness. Multiple strengths were noted, including efficiency/cost efficiency, recruitment, study design, large datasets, and a more real-life population. Experts suggested several issues to address to encourage the use of registries, including:
- Data harmonization and standardization
- Reliability of data
- Regulatory flexibility
- Need to persuade leaders
After input at a multi-stakeholder expert meeting, CTTI developed recommendations to facilitate determination of a registry’s suitability for conducting an embedded clinical trial intended for regulatory submission.
- EMA Guideline on good pharmacovigilance practices (GVP)
- FDA Draft Guidance on Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices
- Real-World Evidence — What Is It and What Can It Tell Us? (Sherman RE, et al. N Engl J Med 2016)
- Accelerating Development of Scientific Evidence for Medical Products Within the Existing US Regulatory Framework (Sherman RE, et al. Nat Rev Drug Disc 2017)
- RoPR Database : Registry of Patient Registries by the U.S. Department of Health and Human Services Agency for Healthcare Research and Quality (AHRQ)
Organization affiliations are listed as active affiliations during the project.
*Indicates former project manager, team leader, or team member.
Patient Registries for Clinical Research
- First Online: 08 February 2019
Cite this chapter

- Rachel L. Richesson PhD, MPH, FACMI 3 ,
- Leon Rozenblit JD, PhD 4 ,
- Kendra Vehik PhD, MPH 5 &
- James E. Tcheng MD 6
Part of the book series: Health Informatics ((HI))
1593 Accesses
1 Citations
Patient registries are fundamental to biomedical research. Registries provide consistent data for defined populations and can be used to support the study of the determinants and manifestations of disease and provide a picture of the natural history, outcomes of treatment, and experiences of individuals with a given condition or exposure. It is anticipated that electronic health record (EHR) systems will evolve to ubiquitously capture detailed clinical data that supports observational, and ultimately interventional, research. Emerging data representation and exchange standards can enable the interoperability required for automated transmission of clinical data into patient registries. This chapter describes informatics principles and approaches relevant to the design and implementation of patient registries, with emphasis on the ingestion of clinical data and the role of patient registries in research and learning health activities.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
AHRQ. In: Gliklich RE, Dreyer NA, editors. Registries for evaluating patient outcomes: a user’s guide. Rockville: Agency for Healthcare Research and Quality; 2010.
Google Scholar
Travers K, et al. Characteristics and temporal trends in patient registries: focus on the life sciences industry, 1981–2012. Pharmacoepidemiol Drug Saf. 2015;24(4):389–98.
Article Google Scholar
Muilu J, Peltonen L, Litton JE. The federated database – a basis for biobank-based post-genome studies, integrating phenome and genome data from 600,000 twin pairs in Europe. Eur J Hum Genet. 2007;15(7):718–23.
Article CAS Google Scholar
Nakamura Y. The BioBank Japan project. Clin Adv Hematol Oncol. 2007;5(9):696–7.
PubMed Google Scholar
Ollier W, Sprosen T, Peakman T. UK Biobank: from concept to reality. Pharmacogenomics. 2005;6(6):639–46.
Sandusky G, Dumaual C, Cheng L. Review paper: human tissues for discovery biomarker pharmaceutical research: the experience of the Indiana University Simon Cancer Center-Lilly Research Labs Tissue/Fluid BioBank. Vet Pathol. 2009;46(1):2–9.
Horsley K. Florence Nightingale. J Mil Veterans’ Health. 2018;18(4):2–5.
Military Records. Civil war records: basic research sources. 2018 [cited 2018 July 1, 2018]. Available from: https://www.archives.gov/research/military/civil-war/resources .
Patient registries. In: DN, Gliklich RE, Leavy MB, editors. Registries for evaluating patient outcomes: a user’s guide [Internet]. 3rd ed. Rockville: Agency for Healthcare Research and Quality (US); 2014.
CMS. Centralized repository/RoPR. 2018a. [cited 2018 June 23]. Available from: https://www.cms.gov/Regulations-and-Guidance/Legislation/EH RIncentivePrograms/CentralizedRepository-.html .
FDA. Guidance for industry and FDA staff. Procedures for handling post-approval studies imposed by PMA order. Rockville: U.S. Food and Drug Administration; 2007.
Hollak CE, et al. Limitations of drug registries to evaluate orphan medicinal products for the treatment of lysosomal storage disorders. Orphanet J Rare Dis. 2011;6:16.
Clinical Trials Transformation Initiative (CTTI). CTTI recommendations: registry trials. 2017. [cited 2018 June 23]. Available from: https://www.ctti-clinicaltrials.org/files/recommendations/registrytrials-recs.pdf .
Stey AM, et al. Clinical registries and quality measurement in surgery: a systematic review. Surgery. 2015;157(2):381–95.
CMS. Quality measures requirements. 2018b [cited 2018 June 23]. Available from: https://qpp.cms.gov/mips/quality-measures.
Platt R, et al. Clinician engagement for continuous learning discussion paper. Washington, DC: National Academy of Medicine; 2017.
AHRQ. Bringing the patient voice to evidence generation: patient engagement in disease registries. (AHRQ Views. Blog posts from AHRQ leaders). 2018. [cited 2018 June 23]. Available from: http://www.ahrq.gov/news/blog/ahrqviews/disease-registries.html .
IOM. The learning healthcare system: workshop summary. Washington, DC: The National Academies Press; 2007.
ONC. Introduction to the interoperability standards advisory. 2018a. [cited 2018 June 23]. Available from: https://www.healthit.gov/isa/ .
Chute CG. Medical concept representation. In: Chen H, et al., editors. Medical informatics. Knowledge management and data mining in biomedicine. New York: Springer; 2005. p. 163–82.
ONC. 2015 edition certification companion guide. 2015 edition common clinical data set – 45 CFR 170.102. 2018b. [cited 2018 June 23]. Available from: https://www.healthit.gov/sites/default/files/2015Ed_CCG_CCDS.pdf .
NLM. The NIH common data element (CDE) resource portal. 2013. [cited 2013 March 6]. Available from: http://www.nlm.nih.gov/cde/ .
CMS. Data element library. 2018. [cited 2018 June 23]. Available from: https://del.cms.gov/DELWeb/pubHome .
Sood HS, et al. Has the time come for a unique patient identifier for the U.S.? NEJM Catalyst. 2018.
Dusetzina SB, Tyree S, Meyer AM, et al. Linking data for health services research: a framework and instructional guide [Internet]. In:An overview of record linkage methods. Rockville: Agency for Healthcare Research and Quality (US); 2014.
21st Century Cures Act. 2018. [cited 2018 July 1]. Available from: https://www.fda.gov/RegulatoryInformation/LawsEnforcedbyFDA/SignificantAmendmentstotheFDCAct/21stCenturyCuresAct/default.htm .
Drozda JP Jr, et al. Constructing the informatics and information technology foundations of a medical device evaluation system: a report from the FDA unique device identifier demonstration. J Am Med Inform Assoc: JAMIA. 2018;25(2):111–20.
Campbell WS, et al. An alternative database approach for management of SNOMED CT and improved patient data queries. J Biomed Inform. 2015;57:350–7.
PheKB. 2012. [cited 2013 May 24]. Vanderbilt University. Available from: http://www.phekb.org/ .
NLM. NLM Value Set Authority Center (VSAC). 2015. Feb 11, 2015 [cited 2015 March 11]. Available from: https://vsac.nlm.nih.gov/ .
PheMA. PheMA wiki: phenotype execution modeling architecture project. 2015. [cited 2015 September 28]. Available from: http://informatics.mayo.edu/phema/index.php/Main_Page .
Richesson RL, et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH health care systems collaboratory. J Am Med Inform Assoc. 2013;20(e2):e226–31.
Richesson RL, Smerek MM, Blake Cameron C. A framework to support the sharing and reuse of computable phenotype definitions across health care delivery and clinical research applications. EGEMS (Washington, DC). 2016;4(3):1232.
Gliklich RE, et al. Registry of patient registries outcome measures framework: information model report. Methods research report, Prepared by L&M Policy Research, LLC, under Contract No. 290-2014-00004-C. Rockville: Agency for Healthcare Research and Quality (US); 2018.
Cochi SL, et al. Congenital rubella syndrome in the United States, 1970–1985. On the verge of elimination. Am J Epidemiol. 1989;129(2):349–61.
Tilling K. Capture-recapture methods – useful or misleading? Int J Epidemiol. 2001;30(1):12–4.
Rothman K, Greenland S. Modern epidemiology. 2nd ed. Hagerstown: Lippincott Williams and Wilkins; 1998.
AHRQ. In: Gliklich RE, Dreyer NA, editors. Registries for evaluating patient outcomes: a user’s guide. Rockville: Agency for Healthcare Research and Quality; 2007.
Sanborn TA, et al. ACC/AHA/SCAI 2014 health policy statement on structured reporting for the cardiac catheterization laboratory: a report of the American College of Cardiology Clinical Quality Committee. J Am Coll Cardiol. 2014;63(23):2591–623.
Wickham H. Tidy data. 2014., 2014;59(10):23.
Blumenthal S. The use of clinical registries in the United States: a landscape survey. eGEMs (Generating evidence & methods to improve patient outcomes). 2017;5(1):26.
Chute CG, Huff SM. The pluripotent rendering of clinical data for precision medicine. Stud Health Technol Inform. 2017;245:337–40. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29295111 .
ONC. Common clinical data set. 2015. [cited 2018 June 25]. Available from: https://www.healthit.gov/sites/default/files/commonclinicaldataset_ml_11-4-15.pdf .
S4S. Sync for science (S4S). Helping patients share EHR data with researchers. 2018. [cited 2018 June 25]. Available from: http://syncfor.science/ .
Sankar PL, Parker LS. The precision medicine initiative’s all of us research program: an agenda for research on its ethical, legal, and social issues. Genet Med: Off J Am Coll Med Genet. 2017;19(7):743–50.
Download references
Author information
Authors and affiliations.
Duke University School of Nursing, Durham, NC, USA
Rachel L. Richesson PhD, MPH, FACMI
Prometheus Research, LLC, New Haven, CT, USA
Leon Rozenblit JD, PhD
University of South Florida, Health Informatics Institute, Tampa, FL, USA
Kendra Vehik PhD, MPH
Duke University School of Medicine, Durham, NC, USA
James E. Tcheng MD
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Rachel L. Richesson PhD, MPH, FACMI .
Editor information
Editors and affiliations.
Rachel L. Richesson
School of Information, University of South Florida, Tampa, FL, USA
James E. Andrews
Rights and permissions
Reprints and permissions
Copyright information
© 2019 Springer International Publishing
About this chapter
Richesson, R.L., Rozenblit, L., Vehik, K., Tcheng, J.E. (2019). Patient Registries for Clinical Research. In: Richesson, R., Andrews, J. (eds) Clinical Research Informatics. Health Informatics. Springer, Cham. https://doi.org/10.1007/978-3-319-98779-8_13
Download citation
DOI : https://doi.org/10.1007/978-3-319-98779-8_13
Published : 08 February 2019
Publisher Name : Springer, Cham
Print ISBN : 978-3-319-98778-1
Online ISBN : 978-3-319-98779-8
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you, list of registries, frequently asked questions.
What is a registry?
A registry is a collection of information about individuals, usually focused around a specific diagnosis or condition. Many registries collect information about people who have a specific disease or condition, while others seek participants of varying health status who may be willing to participate in research about a particular disease. Individuals provide information about themselves to these registries on a voluntary basis. Registries can be sponsored by a government agency, nonprofit organization, health care facility, or private company. It’s always good to check first to know who sponsors the registry – or – look for information on a registry’s site to know about their sponsor(s).
Why are registries needed?
Registries can provide health care professionals and researchers with first-hand information about people with certain conditions, both individually and as a group, and over time, to increase our understanding of that condition. Some registries collect information that can be used to track trends about the number of people with diseases, treatments, and more. Other registries invite people to sign up to be contacted about participating in clinical research. These ask very basic questions about health history that would help determine whether someone is possibly eligible to join a research study.
It sounds like these registries collect personal health information. Is there a risk that such information could be disclosed?
Government agencies have strict privacy requirements set by law such as the Federal Information Security Management Act (FISMA), and the Health Insurance Portability and Accountability Act (HIPAA). If registries have followed all of these rules, the likelihood of identifiable personal information being shared is very small.
What benefits will someone receive from participating in a registry?
Participation in a registry is likely to increase what we know about a specific condition, help health care professionals improve treatment, and allow researchers to design better studies on a particular condition, including development and testing of new treatments. Being part of a clinical trials registry can help people interested in participating in research connect with clinical investigators. However, individuals (and their families) who choose to participate in a registry should understand that participation will not guarantee a treatment or cure for their condition or that they will be eligible to join a study.
Who has access to the information in a registry?
Usually, a federally-funded registry has a very limited list of individuals (registry coordinator) who may have access to participants’ personal, identifying information. ;Those individuals must be specially trained and certified regarding information security requirements.
Who owns the data from a registry? Who makes decisions about how these data will be used?
The data collected in a disease registry is stripped of personal information. It belongs to the sponsor of the registry, and depending on how the registry is set up, may be shared with the participants and their families, and approved health care professionals and researchers. However, personal, identifying information is kept private. Usually, a registry has a governing committee that makes decisions about how the data can be used or shared.
Can a participant withdraw from the registry?
Yes. Registries are free and voluntary; there is no penalty for choosing to withdraw at any point.
Who should the participant contact with additional questions or concerns?
For any questions about participation or any issues that may arise, registries provide a contact, usually the registry coordinator.
How is a registry different from a clinical trial?
Registries focused on specific diseases or conditions collect information voluntarily from people with those conditions. Clinical trials registries collect basic health information from people who agree to be contacted about participating in future clinical trials or studies.
A clinical trial is the study of new ways to prevent, detect or treat diseases or conditions. Volunteering for a registry does not mean a person has signed up for a clinical trial. Participation in a disease registry can sometimes become a first step toward participation in a clinical trial, but registries and specific trials are not directly linked.
Disclaimer: The following listing is not intended to be comprehensive, and the inclusion of any particular organization on this list does not imply endorsement by the National Institutes of Health or the Department of Health and Human Services. Our intent is to provide information about registry efforts at the national level and therefore have not included many local groups that can offer valuable assistance to individuals and their families within a limited geographic area.
Alzheimer’s Prevention Registry
Autoimmune registry, autoimmune research network (arnet), breast cancer surveillance consortium, cancer genetics network, cascade fh registry, cchs now registry, cerebral palsy research network mycp, chromosome 8p registry, clinical trials public data share website, collaborative islet transplant registry, colon cancer family registry, congenital heart disease genetic network study (chd genes), congenital muscle disease international registry (cmdir), creatineinfo registry, cure rtd foundation, curedrpla global patient registry, cystic fibrosis foundation patient registry, development of a national incompatible kidney transplant registry, dominantly inherited alzheimer network (dian) — expanded registry, drug inducted liver injury network (dilin), ds-connect™: the down syndrome registry, dtrf desmoid tumor patient registry, the environmental polymorphisms registry (epr) — using dna to study disease, epithelioid hemangioendothelioma (ehe) global patient registry, eyegene ® : the national ophthalmic disease genotyping and phenotyping network, fanconi anemia patient registry, fd/mas patient registry, fecal microbiota transplant national registry, fibromuscular dysplasia (fmd) registry, foundation fighting blindness, foundation for sarcoidosis patient registry, frontotemporal degeneration (ftd) registry, genomeconnect, global genes rare-x patient communities, the global paroxysmal nocturnal hemoglobinuria (pnh) patient registry (iamrare.org), global prader-willi syndrome registry, global registry for inherited neuropathies (grin) registry, impact registry, diagnostic and interventional cardiac catheterization in congenital heart disease, inherited bone marrow failure syndrome, interagency registry for mechanically assisted circulatory support (intermacs), international registry of coronavirus exposure in pregnancy (ircep), international registry of werner syndrome, itp natural history study registry, kcnt1 epilepsy, krabbe community united research and engagement study (krabbecures), leigh syndrome global patient registry, lipedema foundation, lupus family registry and repository, monogenic diabetes at the university of chicago, mother to baby, multiple myeloma research foundation’s (mmrf) curecloud, myasthenia gravis patient registry, national addiction & hiv data archive program, national alopecia areata registry, national als registry, national and state cancer registries, national pediatric cardiology quality improvement collaborative, national registry of genetically triggered thoracic aortic aneurysms and cardiovascular conditions (gentac), nida center for genetics research, nidcd national temporal bone, hearing & balance pathology resource registry, nih human embryonic stem cell registry, nih national registry of u.s. myotonic dystrophy and u.s. facioscapulohumeral muscular dystrophy (fshd), oaa natural history patient registry, pediatric cardiac critical care consortium (pc4), pediatric imaging, neurocognition, and genetics (ping), pediatric pulmonary hypertension (pphnet) informatics registry, pku patient registry, pprom registry (preterm premature rupture of membranes), pregsource ® : crowdsourcing to understand pregnancy, the preeclampsia registry, priority (pregnancy coronavirus outcomes registry), pulmonary fibrosis, rare diseases registry program (radar), research registry for neonatal lupus, ray: research accelerated by you, sample collection registry, section on neonatal-perinatal medicine (sonpm), seer registries, severe chronic neutropenia international registry, shareforcures (breast cancer research registry by susan g. komen), simons searchlight (rare genetic neurodevelopmental disorders registry), society for thoracic surgeons society, congenital heart surgery database, syngap1 (mrd5) patient registry, tatton brown rahman syndrome (tbrs), usher syndrome registry, usidnet registry for patients with primary immunodeficiency diseases, virtual pediatric systems (vps).
This page last reviewed on April 17, 2024
Connect with Us
- More Social Media from NIH
Skip to content
Initiatives and Committees
Plan your research, join a study, research registries and studies, join a research study.
A research study is an investigation of a human health issue to learn more about it or to develop new methods of health care. Many types of research studies exist. For example, clinical trials test specific interventions, such as medical strategies, treatments, or devices, to determine whether they are safe and effective for humans. Other studies use interviews or surveys to understand health or behavior.
If you are interested in finding a specific research study at Columbia University, you may do the following:
- Contact or visit the Columbia Community Partnership for Health (CCPH) to learn about research studies that are currently looking for volunteers. You can contact CCPH by calling 646-697-2274 or visiting at 390 Fort Washington Avenue, Ground Floor, New York, NY.
- Search RecruitMe for research studies that are looking for volunteers.
- Search ClinicalTrials.gov for studies happening at Columbia University Medical Center in New York, NY.
Join a Research Registry
A research registry is a collection of information about individuals. There are different types of research registries: registries of people with a specific diagnosis or condition and registries that connect people interested in being research participants with health studies. By joining a research registry, you agree to be contacted about participating in future research studies.
RecruitMe is a free online study recruitment tool launched by Columbia University’s Clinical Trials Office to connect those who want to participate in research studies to the researchers who conduct them. By using RecruitMe, you can search for a study or join the registry to be contacted about participating in future research studies. Visit the RecruitMe website to learn more .
ResearchMatch
ResearchMatch is a free national registry that connects people who are trying to find research studies and clinical trials with researchers who are looking for volunteers. ResearchMatch works by emailing you about studies that may be a good match for you. Visit the ResearchMatch website to learn more.
The All of Us Research Program is asking one million people to share health information to create the largest health database ever. Researchers will be able to use this data to better understand disease. This may create a healthier future for generations to come. Columbia University Irving Medical Center is part of the All of Us New York City Consortium. Visit the All of Us New York City website to learn more .
Columbia Community Partnership for Health Research Registry
The Columbia Community Partnership for Health (CCPH) research registry can help match your interests to health research studies in upper Manhattan. As part of our research registry you will receive information about studies that are recruiting in Harlem, Washington Heights, and Inwood. If you are interested in participating in a study, you may contact the researcher recruiting for the study and ask questions, check your eligibility, and/or volunteer to participate. To join the CCPH Research Registry, call or visit CCPH:
Columbia Community Partnership for Health 390 Fort Washington Avenue, Ground Floor New York, NY 10033 646-697-2274
You can also visit CCPH and find out about health research studies that researchers are currently recruiting for or to use our bilingual health library to learn more about health issues that affect the upper Manhattan community.
- Conference Coverage
- CRO/Sponsor
- 2023 Salary Survey

- Publications
- Conferences
Clinical Trials vs Registries
Applied Clinical Trials Supplements
Similarities exist, but successful design requires that CROs understand the differences.
As health care stakeholders, from regulators to providers to payers, demand more evidence on the safety and effectiveness of drugs and medical devices in real-world use, they are also increasingly asking life science companies to develop patient registries to fulfill this need.

Tips to Rescue a Clinical Trial Before It’s Too Late
Why constant communication and transparency are paramount to successful partnerships between pharmaceutical companies and CROs

Focus on Fundamentals for Better Collaboration Across Research Sites and Sponsors
Improving the site-sponsor relationship can get trials off on the right foot and on a path for success.

When FMVs Collide: Coming to Terms with Fair Market Value
With variation in costs between different stakeholders in clinical trials, there is often disagreement on fair market value.

Top 3 Most Read CRO/Sponsor Feature Articles of 2023
Authors highlighted outsourcing and the impact of the COVID-19 pandemic in these CRO/sponsor articles from 2023.

Anticipating Near-Term Structural Change in the Outsourcing Landscape
Can full-service outsourcing to CROs by large biopharma companies sustainably prosecute a clinical development portfolio?

Harmonizing Outsourcing to Keep Clinical Trials on Track
A shared partnership culture is critical in navigating today’s complex terrain.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

ISRCTN registry
The ISRCTN registry is a primary clinical trial registry recognised by WHO and ICMJE that accepts all clinical research studies (whether proposed, ongoing or completed), providing content validation and curation and the unique identification number necessary for publication. All study records in the database are freely accessible and searchable.
ISRCTN supports transparency in clinical research, helps reduce selective reporting of results and ensures an unbiased and complete evidence base.
ISRCTN accepts all studies involving human subjects or populations with outcome measures assessing effects on human health and well-being, including studies in healthcare, social care, education, workplace safety and economic development. The registry welcomes submissions in English from any location. Studies should ideally be registered prospectively (before recruitment starts). ISRCTN also accepts studies registered retrospectively once they are underway or after completion.
Register your study Update your record Report your results
Latest studies
Isrctn96925141: understanding the impact of resistant bugs on deaths in england.
Recording Antimicrobial Resistance during Death Certification in England
ISRCTN17909701: Implementing creative psychotherapy in primary mental health services
Feasibility study of implementation of an evidence-based creative group psychotherapy for depression (Arts for the Blues) into primary care mental health services
ISRCTN62558470: Developing a tailored digital intervention to improve adherence to statin medications
Promoting STatin Adherence with a Tailored Intervention
Supporter organisations
.png)
- Department of Health and Human Services
- National Institutes of Health

COVID-19 Research Studies
More information, about clinical center, clinical trials and you, participate in a study, referring a patient, about clinical research.
Research participants are partners in discovery at the NIH Clinical Center, the largest research hospital in America. Clinical research is medical research involving people The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953. We do not charge patients for participation and treatment in clinical studies at NIH. In certain emergency circumstances, you may qualify for help with travel and other expenses Read more , to see if clinical studies are for you.
Medical Information Disclaimer
Emailed inquires/requests.
Email sent to the National Institutes of Health Clinical Center may be forwarded to appropriate NIH or outside experts for response. We do not collect your name and e-mail address for any purpose other than to respond to your query. Nevertheless, email is not necessarily secure against interception. This statement applies to NIH Clinical Center Studies website. For additional inquiries regarding studies at the National Institutes of Health, please call the Office of Patient Recruitment at 1-800-411-1222

Find NIH Clinical Center Trials
The National Institutes of Health (NIH) Clinical Center Search the Studies site is a registry of publicly supported clinical studies conducted mostly in Bethesda, MD.

- Societies and Advocacy

The Basics of Clinical Data Registries

Healthcare’s digital transformation is long underway, yet still lagging. Calls for better and more efficient care, therapies, and outcomes have never been louder and more insistent. The role of clinical data registries has never been more significant.
It’s necessary to understand the importance of registries and how they make sense out of large volumes of disparate healthcare data to measurably improve care and advance research.
Let’s cover the basics:
What Is a Clinical Data Registry?
Types of clinical registries, purpose and uses of clinical data registries, the value of clinical data registries, examples of quality improvement with clinical data registries.
- Example of a Medical Device Registry
Getting Started with Clinical Data Registry Software Solutions
A clinical data registry is an interactive database that collects, organizes, and displays healthcare information.
Clinical data registries are also sometimes called patient registries and disease registries. Professional medical associations and specialty societies tend to use the term clinical data registry, while research and patient foundations and government organizations lean toward patient registry. Because disease registries sound condition-specific, that term is often more popular with industry.
Regardless of the name, the purpose of a data registry is the same: to evaluate and improve outcomes for a population defined by a particular condition, disease, or exposure.
Specifically, registries use observational study methods to collect and harmonize data about the treatment, outcomes, and well-being of patients who receive care over time. They aggregate large data sets and analyze trends or patterns in treatments and outcomes.
Registries can serve many purposes and provide value for a variety of healthcare stakeholders. For example:
- Physicians and other healthcare professionals use registries to evaluate available treatments, procedures, and therapies, and to understand how patients with different characteristics respond to various treatments.
- Medical device manufacturers and pharmaceutical developers use registries to track and understand the effectiveness, safety, and value of medical devices or therapies and drugs entering or on the market.
The number of registries has grown over the past several decades as healthcare information has become digitized. Yet despite their increase in use and significance, registries face real challenges in establishing the participation, engagement, and utility needed to drive their sustainability.
Modern clinical data registries address these limitations by going beyond data collection and data warehousing. They rely on advanced analytics and data science to transform data into meaningful insights that are useful , usable , and used by a variety of stakeholders to achieve a desired outcome.
Clinical registries come in many different forms. The type of registry depends on the organization managing or sponsoring it, and the patient population, disease, condition, or treatment it examines.
Although registry goals and purposes vary, when designed with the right approach and built with the right analytics technology, they can measurably improve care.
What Is a Patient Registry?
A patient registry, also called a disease registry, tracks information about the health status of patients and the care they receive for a specific disease or condition. Patient registries bring together data to evaluate longitudinal outcomes, best practices, treatment guidelines, and to support research and therapeutic development.
A growing number of patient foundations and pharmaceutical organizations are establishing patient registries to study the treatment of rare diseases and conditions, such as hemophilia and other genetic diseases.
What Is a Specialty Registry?
Specialty registries are clinical registries focused on advancing care and outcomes across a medical specialty or subspecialty, such as pathology, sleep medicine, surgery, and trauma medicine. These registries often aim to develop guidelines and decision support tools, accelerate research, and advance care through collaborative quality improvement.
What Is a Population Registry?
A population registry is more broadly focused across entire patient populations and spans both specialty care and specific diseases and conditions. These registries aim to capture the health, well-being, diagnostic, treatment, and outcome data for every patient within a population defined by demographics (age, gender, or other social determinants), geography (state, region, country and including like Health Information Exchanges and within Health Departments), or disease or condition (diabetes, cancer).
What Is a Medical Device Registry?
A medical device registry is focused on tracking the effectiveness, safety, and value of medical devices. Device registries come in several forms. Medical specialty organizations may collect data on various devices used for procedures or conditions, as part of their clinical data registries. Medical device companies establish registries and use registry data to support post-market surveillance.
What Is a Payer Registry?
A payer registry is established by a healthcare payer focused on measuring and improving value by advancing outcomes and reducing costs. Payer-sponsored registries are often organized across a specific geography or region, and by specialty – surgery, urology, emergency medicine, etc.
Healthcare organizations such as medical specialty societies, patient foundations, pharmaceutical companies, and medical device manufacturers establish registries for many purposes and uses.
Clinical Data Registry Purposes
- Quality improvement
- Benchmarking
- Clinical research
- Clinical effectiveness
- Cost effectiveness
- Device surveillance
- Treatment surveillance
- Population surveillance
Clinical Data Registry Uses
- Decision support
- Guideline development
- Measure development
- Regulatory and public reporting
- Value-based reimbursement and payment
- Patient engagement
- Post-market surveillance
- Registry-based clinical trials
- Education development
- Certification and accreditation
Clinical data registries are valuable when they measurably improve care and achieve results. Examples of this in action are advancing research, establishing and evaluating guidelines, or managing and reducing costs.
Achieving value with a registry happens when:
- Physicians and providers use high-quality, data-driven insights to better understand expected outcomes, make evidence-based decisions, and share best practices.
- Patients share timely and personal data about their condition and outcomes and gain a greater understanding of their care that leads to informed shared decision-making.
- Researchers and developers use registry data as the foundation for registry-enhanced or registry-based research, clinical trials, or post-market surveillance studies.
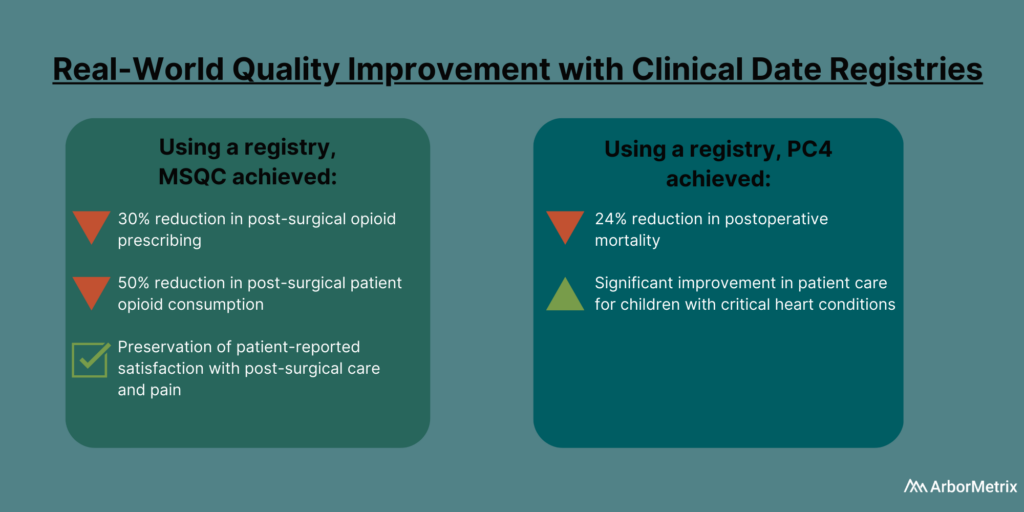
In 2019, the Michigan Surgical Quality Collaborative (MSQC) used registry data to generate knowledge in the form of procedure‐specific opioid prescribing guidelines.
The result?
Post-surgical opioid prescribing dropped by 30%, and post-surgical patient opioid consumption dropped by 50%, according to a paper published in the New England Journal of Medicine . There also was no change in patient-reported satisfaction with care and pain in the week after surgery.
The Pediatric Cardiac Critical Care Consortium (PC4) aims to improve the quality of care for pediatric heart patients through a clinical data registry that allows hospitals to evaluate their own outcomes and learn best practices.
Their efforts are paying off.
Eighteen hospitals significantly reduced mortality and improved care for children with critical heart conditions, according to a paper published in the December 2019 edition of the Journal of the American College of Cardiology . Specifically, they achieved a 24% decrease in postoperative mortality among participating sites between 2014 and 2018.
If you want to know more, read this post about how MSQC and PC4 are top examples of quality improvement in healthcare .
Today’s most successful clinical data registries use healthcare analytics technology that goes beyond data collection and data warehousing and plays a crucial role in advancing care and research.
Specifically, a clinical data registry platform should:
- Acquire various data using industry-leading technology and standards.
- Assemble the data into real-world evidence using advanced analytics and data science.
- Enable various users to act on the evidence using dashboards, reports, surveys, and other unique decision-support tools.
Leverage the Power of Clinical Data Registries
The National Quality Registry Network outlines some key considerations when approaching a clinical data registry and deciding what to outsource to a vendor. This includes your in-house availability and expertise, budget impact, convenience, and the many responsibilities that can be outsourced or kept in-house.
At ArborMetrix, we help healthcare organizations and companies demonstrate real and measurable results through robust analytics and intuitive reporting. Through our comprehensive partnerships and clinical expertise, we enable our clients to leverage their real-world evidence for real-world results.
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
Submit Studies
Related Pages
- Login to ClinicalTrials.gov PRS
Do you or someone you know want to participate in a clinical study? See information for patients and families .
Steps for Registering a Clinical Study
Considerations for observational studies and expanded access records, clinicaltrials.gov protocol information review process, required registration updates.
The steps on this page describe the overall process of registering studies. If you would like step-by-step instructions for entering registration information into the PRS, see the PRS Guided Tutorials . The tutorials include a quick overview guide called Entering a New Registration that briefly summarizes how to use the tutorials to support registering a study. [Requires a browser that supports HTML5.]
- More information on identifying the Responsible Party for National Institutes of Health (NIH) grantees is available from the NIH Office of Extramural Research.
- See How to Apply for a PRS Account to learn how to determine whether your organization already has a PRS account, contact your organization's PRS account administrator, or apply for a PRS account.
- Any applicable human subject or ethics review regulations (or equivalent)
- Any applicable regulations of the national or regional health authority (or equivalent)
- See Why Should I Register and Submit Results? for background information on the reasons for registering a clinical study.
- See the Interventional Study Protocol Registration Template for a formatted summary of the relevant interventional study data elements for each registration module. The template is intended to help investigators understand and gather the data needed to complete each registration module.
- To retrieve forgotten passwords for existing PRS accounts, click on the Forgot password link on the linkToPRSLogin().
- For basic help with using PRS, review the Quick Start Guide found in the Help section of the PRS main menu. More detailed instructions are available in the PRS User's Guide, also found on the PRS main menu.
- See the ClinicalTrials.gov Protocol Review Criteria (PDF) for a description of items that should be addressed before releasing the record to ClinicalTrials.gov.
- Verify in PRS that the Record Status is released. The record will not be processed by ClinicalTrials.gov unless it is released. Only the Responsible Party or a PRS account administrator can release the record.
Registering Observational Studies
The Observational Study Type (see Study Type data element on ClinicalTrials.gov) can be used to register studies of human beings in which biomedical and/or health outcomes are assessed in predefined groups of individuals, but the investigator does not assign specific interventions to the study participants. This will provide access to the Observational Study Design data elements on ClinicalTrials.gov, including Observational Study Model, Time Perspective, and Biospecimen information.
The Patient Registry Observational Study Subtype (see Study Type data element on ClinicalTrials.gov) can be used to indicate that an observational study is also considered to be a Patient Registry. The Agency for Healthcare Research and Quality (AHRQ) defines a Patient Registry as including an organized system that uses observational methods to collect uniform data (clinical and other) prospectively for a population defined by a particular disorder/disease, condition (including susceptibility to a disorder), or exposure (including products, health care services, or procedures) and that serves a predetermined scientific, clinical, or policy purpose. Patient registries may be single-purpose or ongoing data collection programs that address one or more questions.
Observational study records should be updated and maintained in the same manner as interventional study records.
Registering Expanded Access Records
Expanded access (sometimes also referred to as "compassionate use") is a potential pathway for a patient with a serious or immediately life-threatening disease or condition to gain access to an investigational medical product for treatment outside of clinical trials when no comparable or satisfactory alternative therapy options are available. A responsible party for an applicable clinical trial (ACT) of an investigational drug product or biological product who is both the manufacturer of the drug product and the sponsor of the trial is required to submit expanded access information to ClinicalTrials.gov. Note that a physician who submits an individual patient expanded access Investigational New Drug Application (IND), including for emergency use , to the U.S. Food and Drug Administration generally would not be required to submit expanded access information to ClinicalTrials.gov.
For an ACT studying an investigational drug or biological product for which expanded access is available under FDA regulations, the responsible party would select "Yes" for the Availability of Expanded Access data element and submit information for an expanded access record if certain other conditions are met (see Who is required to submit expanded access information to ClinicalTrials.gov and what information is required? FAQ).
If expanded access is available at the time the clinical trial registration information for an ACT of an investigational drug product or biological product is submitted, then registration information for the expanded access record must also be submitted. If expanded access becomes available after the registration information for an ACT is submitted, then the Availability of Expanded Access data element for the ACT must be updated and the expanded access record must be submitted not later than 30 calendar days after expanded access to the investigational drug or biological product becomes available.
- FDA: Expanded Access (Compassionate Use)
- FDA: For Patients - Learn About Expanded Access and Other Treatment Options
- FDA: Expanded Access for Medical Devices
- ClinicalTrials.gov FAQ: What is expanded access?
- ClinicalTrials.gov FAQ: Who is required to submit expanded access information to ClinicalTrials.gov and what information is required?
- ClinicalTrials.gov FAQ: When is expanded access information required to be submitted to ClinicalTrials.gov?
- ClinicalTrials.gov: Registration Data Element Definitions for Expanded Access
Expanded access records describe the procedure for obtaining an experimental drug or device outside of a clinical trial. To register information about expanded access, select Expanded Access for the Study Type (see Study Type data element on ClinicalTrials.gov). Any manufacturer or Sponsor accepting requests for single-patient investigational new drug applications (INDs) or protocol exceptions (including for emergency use) should provide only one expanded access record. Do not register each single-patient INDs or protocol exception separately.
Expanded access records should generally be updated and maintained in the same manner as interventional study records. For descriptions of data elements, see the Expanded Access Data Element Definitions .
When registering a clinical trial that includes a drug that is also available via expanded access, the Availability of Expanded Access data element should be answered Yes (see Expanded Access and Availability of Expanded Access on ClinicalTrials.gov). Also provide the ClinicalTrials.gov Identifier (NCT Number) for the expanded access record.
Additional information about expanded access is available on the NLM and Food and Drug Administration (FDA) Web sites:
A ClinicalTrials.gov staff member will review the study record after it is released (submitted) and before it is published on ClinicalTrials.gov. This review will focus on apparent validity (when possible), meaningful entries, logic and internal consistency, and formatting. You may be asked to clarify items or make corrections to the record before publication. Please note that the review process may take up to a few days. Ensuring that the record is consistent with the ClinicalTrials.gov Protocol Review Criteria (PDF) before releasing it will expedite publication on the site.
After you release a record and it is accepted by review staff for publication, the record, including its NCT Number, will be available on ClinicalTrials.gov within 2–5 business days.
Responsible Parties should update their records within 30 days of a change to any of the following:
- Individual Site Status and Overall Recruitment Status data elements on ClinicalTrials.gov
- Primary Completion Date data element on ClinicalTrials.gov on ClinicalTrials.gov.
As described in 42 CFR Part 11 , additional information must also be updated within 15 or 30 days of a change. Other changes or updates to the record must be made at least every 12 months. It is recommended that the Record Verification Date be updated at least every 6 months for studies that are not yet completed, even if there were no changes to the record.
See How to Edit Your Study Record for details on updating study information.
Submitting Results
For certain clinical trials subject to FDAAA 801 and 42 CFR Part 11, the Responsible Party should submit summary results no later than 12 months after the Primary Completion Date, defined as the date the final participant was examined or received an intervention for purposes of final collection of data for the primary outcome (see Primary Completion Date data element on ClinicalTrials.gov). See How to Submit Your Results and FDAAA 801 and the Final Rule for more information.
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
Primary Image
Clinical Trial
Clinical Research Registry (CRR)
- LinkedIn Logo linkedin
The purpose of this research study is to include individuals into the Shirley Ryan Ability Lab’s participant registry. The registry will provide a controlled list from which researchers at Shirley Ryan Ability Lab and Northwestern University (NU), Department of Physical Therapy and Human Movement Sciences or Department of Physical Medicine and Rehabilitation may identify potential participants for current and/or future studies.
If you are interested in participating in current and future research, have had a stroke, spinal cord injury, traumatic brain injury, cerebral palsy, limb loss, musculoskeletal conditions, multiple sclerosis or parkinson's disease/parkinson's movement disorders and want to be put on a list for us to reach out to you, then please sign up below.
Join the Clinical Research Registry.
Who can participate.
Any individual with a history of stroke, spinal cord injury, traumatic brain injury, cerebral palsy, limb loss, musculoskeletal conditions, multiple sclerosis or parkinson's disease/parkinson's movement disorders.
Compensation
Provided if selected to participate in additional testing.
Apply for this Trial
Your information will not be published.
All fields are required.
Featured Quote

Our mission, always, is to connect patients with the latest research and innovation. Monica A. Perez, PT, PhD Scientific Chair, Arms + Hands Lab, Shirley Ryan AbilityLab | Professor of Physical Medicine and Rehabilitation, Northwestern University read more
More Studies
Remote therapeutic monitoring for exercise and self-efficacy tracking.
This study aims to understand if remote therapeutic monitoring (RTM) is useful in tracking long term physical activity participation and improving self-efficacy and readiness for exercise. Participants will use their own wearable activity tracker.
Experiences and Outcomes of People Living with SCI: A Mixed Methods, International Collaboration
Intermittent hypoxia initiated motor plasticity in individuals with multiple sclerosis, latest updates.

Ask the Expert: Richard Harvey, MD, on Stroke Recovery and Research

Shirley Ryan AbilityLab Announces New Combined Residency for Pediatrics and Physical Medicine and Rehabilitation

Newlywed Jordan Embraces New Beginnings Following Stroke At 29
Patient Story
Leading Change in Cancer Clinical Research, Because Our Patients Can’t Wait
May 31, 2024 , by W. Kimryn Rathmell, M.D., Ph.D., and Shaalan Beg, M.D.

Greater use of technologies that can increase participation in cancer clinical trials is just one of the innovations that can help overcome some of the bottlenecks holding up progress in clinical research.
Thanks to advances in technology, data science, and infrastructure, the pace of discovery and innovation in cancer research has accelerated, producing an impressive range of potential new treatments and other interventions that are being tested in clinical studies . The extent of the innovative ideas that might help people live longer, improve our ability to detect cancer early, or otherwise transform care is staggering.
Our understanding of tumor biology is also evolving, and those gains in knowledge are being translated into the continued discovery of targets for potential interventions and the development of novel types of treatments. Some of these therapies are producing unprecedented clinical responses in studies, including in traditionally difficult-to-treat cancers.
These advances have contributed to a record number of Food and Drug Administration (FDA) approvals in recent years with, arguably, the most notable approvals being those for drugs that can be used for any cancer, regardless of where it is in the body .
In some instances, the activity of new agents has been so profound that clinical investigators are having to rethink their criteria for implementation in patient care and their definitions of treatment response.
For example, although HER2 has been a known therapeutic target in breast cancer for many decades, the new antibody-drug conjugates (ADCs) that target HER2 have proven to be vastly more effective than the original HER2-targeted therapies. This has forced researchers to rethink fundamental questions about how these ADCs are used in patient care: Can they be effective in people whose tumors have lower expression of HER2 than we previously thought was needed ? And, if so, do we need to redefine how we classify HER2-positive cancer?
As more innovative therapies like ADCs hit the clinic at a far more rapid cadence than ever before, the research community is being inundated with such fundamentally important questions.
However, the remarkable progress we're experiencing with novel new therapies is tempered by a critical bottleneck: the clinical research infrastructure can’t be expected to keep pace in this new landscape.
Currently, many studies struggle to enroll enough participants. At the same time, there are patients who don’t have ready access to studies from which they might benefit. Furthermore, ideas researchers have today for studies of innovative new interventions might not come to fruition for 2 or 3 years, or even longer—years that people with cancer don’t have.
The key to overcoming this bottleneck is to invite innovation to help reshape our clinical trials infrastructure. And here’s how we plan to accomplish that.
Testing Innovation in Cancer Clinical Trials
A transformation in cancer clinical research is already underway. That transformation has been led in part by the success of novel precision oncology approaches, such as those tested in the NCI-MATCH trial .
This innovative study ushered in novel ways of recruiting participants and involving oncologists at centers big and small. And NCI-MATCH has spawned several successor studies that are incorporating and building on its innovations and achievements.
An innovation that emerged from the COVID pandemic was the increase of remote work, even in the clinical trials domain. Indeed, staffing shortages have caused participation in NCI-funded trials to decline. In response, NCI is piloting a Virtual Clinical Trials Office to offer remote support staff to participating study sites. This support staff includes research nurses, clinical research associates, and data specialists, all of whom will help NCI-Designated Cancer Centers and community practices engaged in clinical research activities.
Such technology-enabled services can allow us to reimagine how clinical trials are designed and run. This includes developing technologies and processes for remotely identifying clinical trial participants, shipping medications to participants at home, having imaging performed in the health care settings where our patients live, and empowering local physicians to participate in clinical trials.
We also need mechanisms to test and implement innovations in designing and conducting clinical studies.
For example, NCI recently established the Clinical Trials Innovation Unit (CTIU) to pressure test a variety of innovations. One of the first trials to emerge from the CTIU’s initial efforts was the Pragmatica-Lung Cancer Treatment Trial , a phase 3 study designed to be easy to launch, enroll, and interpret its results.
The CTIU, which includes leadership from FDA and NCI’s National Clinical Trials Network , is already working on future innovations, including those that will streamline data collection and apply innovative approaches for other cancers, all with the goal of making cancer clinical studies less burdensome to run and easier for patients to participate.
Data-Driven Solutions
The era of data-driven health care is here, providing still more opportunities to transform cancer clinical research.
The emergence of artificial intelligence (AI) solutions, large language models, and informatics brings real potential for wholesale changes in how we match patients to clinical studies, assess side effects, and monitor events like disease progression.
Recognizing this potential, NCI is offering funding opportunities and other resources that will fuel the development of AI tools for clinical research, allow us to carefully test their usefulness, and ultimately deploy them across the oncology community.

Creating Partnerships and Expanding Health Equity
To be sure, none of this will be, or can be, done by NCI alone. All these innovations require partnerships. We will increase our engagement with partners in the public- and private-sectors, including other government agencies and nonprofits.
That includes high-level engagement with the Office of the National Coordinator for Health Information Technology (ONC), with input from FDA, Centers for Medicare & Medicaid Services, and Centers for Disease Control and Prevention.

Dr. W. Kimryn Rathmell, M.D., Ph.D.
NCI Director
One example of such a partnership is the USCDI+ Cancer program . Conducted under the auspices of the ONC, this program will further the aims of the White House's reignited Cancer Moonshot SM by encouraging the adoption and utilization of interoperable cancer health IT standards, providing resources to support cancer-specific use cases, and promoting alignment between federal partners.
And just as importantly, the new partnerships we create must include those with patients, advocates, and communities in ways we have never considered before.
A central feature of this community engagement must involve intentional efforts to expand health equity, to create study designs that are inclusive and culturally appropriate. Far too many marginalized communities and populations today are further harmed by studies that fail to provide findings that apply to their unique situations and needs.
Very importantly, the future will require educating our next generation of clinical investigators and empowering them with the tools that enable new ways of managing clinical studies. By supporting initiatives spearheaded by FDA and professional groups like the American Society of Clinical Oncology, NCI is making it easier for community oncologists to participate in clinical trials and helping clarify previously misunderstood regulatory requirements.
These efforts must also ensure that we have a clinical research workforce that is representative of the people it is intended to serve. Far too many structural barriers have prevented this from taking place in the past, and it’s time for that to change.
Expanding our capacity doesn’t mean doing more of the same, it means challenging ourselves to work differently. This will let us move forward to a new state, one in which clinical research is integrated in everyday practice. It is only with more strategic partnerships and increased inclusivity that we can open the doors to seeing clinical investigation in new ways, with new standards for success.
A Collaborative Effort

Shaalan Beg, M.D.
Senior Advisor for Clinical Research
To make the kind of progress we all desire, we have to recognize that our clinical studies system needs to evolve.
There was a time when taking years to design, launch, and complete a clinical trial was acceptable. It isn’t acceptable anymore. We are in an era where we have the tools and the research talent to make far more rapid progress than we have in the past.
And we can do that by engaging with many different communities and stakeholders in unique and dynamic ways—making them partners in our effort to end cancer as we know it.
Together, our task is to capitalize on this work so we can move faster and enable cutting-edge research that benefits as many people as possible.
We also know that there are more good ideas in this space, and part of this transformation includes grass roots efforts to drive systemic change. So, we encourage you to share your ideas on how we can transform clinical research. Because achieving this goal can’t be done by any one group alone. We are all in this together.
Featured Posts
March 27, 2024, by Edward Winstead
March 21, 2024, by Elia Ben-Ari
March 5, 2024, by Carmen Phillips
- Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)
- Open access
- Published: 30 May 2024
Utility of a virtual small group cognitive behaviour program for autistic children during the pandemic: evidence from a community-based implementation study
- Vivian Lee 1 ,
- Nisha Vashi 2 ,
- Flora Roudbarani 2 ,
- Paula Tablon Modica 2 ,
- Ava Pouyandeh 2 ,
- Teresa Sellitto 2 ,
- Alaa Ibrahim 2 ,
- Stephanie H. Ameis 3 , 4 ,
- Alex Elkader 5 ,
- Kylie M. Gray 6 ,
- Connor M. Kerns 7 ,
- Meng-Chuan Lai 3 , 4 ,
- Johanna Lake 3 , 4 ,
- Kendra Thomson 8 , 9 &
- Jonathan A. Weiss 2
BMC Health Services Research volume 24 , Article number: 685 ( 2024 ) Cite this article
66 Accesses
Metrics details
Autistic children often experience socioemotional difficulties relating to emotion regulation and mental health problems. Supports for autistic children involve the use of adapted interventions that target emotion regulation and social skills, alongside mental health symptoms. The Secret Agent Society Small Group (SAS: SG), an adapted cognitive behavioural program, has demonstrated efficacy through lab-delivered randomized control trials. However, research is still needed on its effectiveness when delivered by publicly funded, community-based autism providers under real-world ecologically valid conditions, especially within the context of a pandemic. The COVID-19 pandemic has disrupted access to community-based supports and services for autistic children, and programs have adapted their services to online platforms. However, questions remain about the feasibility and clinical utility of evidence-based interventions and services delivered virtually in community-based settings.
The 9-week SAS: SG program was delivered virtually by seven community-based autism service providers during 2020–2021. The program included the use of computer-based games, role-playing tasks, and home missions. Caregivers completed surveys at three timepoints: pre-, post-intervention, and after a 3-month follow-up session. Surveys assessed caregivers’ perception of the program’s acceptability and level of satisfaction, as well as their child’s social and emotional regulation skills and related mental health challenges.
A total of 77 caregivers (94% gender identity females; Mean = 42.1 years, SD = 6.5 years) and their children (79% gender identity males; Mean = 9.9 years, SD = 1.3 years) completed the SAS: SG program. Caregivers agreed that the program was acceptable (95%) and were highly satisfied (90%). Caregivers reported significant reduction in their child’s emotion reactivity from pre- to post-intervention (-1.78 (95% CI, -3.20 to -0.29), p = 0.01, d = 0.36), that continued to decrease after the 3-month booster session (-1.75 (95% CI, -3.34 to -0.16), p = 0.02, d = 0.33). Similarly, improvements in anxiety symptoms were observed (3.05 (95% CI, 0.72 to 5.36), p = 0.006, d = 0.39).
Conclusions
As online delivery of interventions for autistic children remains popular past the pandemic, our findings shed light on future considerations for community-based services, including therapists and agency leaders, on how best to tailor and optimally deliver virtually based programming.
Trial registration
This study has been registered with ISRCTN Registry (ISRCTN98068608) on 15/09/2023. The study was retroactively registered.
Peer Review reports
Introduction
Autistic children often experience difficulties with emotion regulation and social communication skills, which can interfere with their functioning and have a negative impact on their quality of life and well-being. Difficulties in emotion regulation (i.e., challenges in monitoring, evaluating, and expressing one’s own emotions [ 16 ]) are considered transdiagnostic symptoms [ 1 , 37 ] in that they are implicated in the development of many different mental health problems, including anxiety, depression, eating disorders, and substance use [ 18 ]. Emotion regulation is also often relational in nature [ 16 ], and in autistic children, challenges with emotion regulation have been correlated with greater social communication difficulties [ 25 ]. Pandemic-related policies (e.g., closure of schools and community-based services, lockdowns, etc.) meant to limit the spread of COVID-19 likely exacerbated the emotion regulation problems, as well as social and mental health difficulties experienced by many autistic children [ 23 , 24 , 27 , 28 , 36 , 39 ].
For verbally able autistic children, variations of adapted cognitive behaviour therapy (CBT) programs have been used to improve emotion regulation skills and social skills, alongside mental health problems. For instance, work from Wood and colleagues [ 44 ] demonstrated the effectiveness of the Behavioral Interventions for Anxiety in Children with Autism (BIACA), an intervention delivered in modular format that allows social skills to be targeted alongside coping skills for anxiety. The study found that BIACA was more effective in increasing social communication skills when compared to traditional CBT programs that focused on anxiety reduction alone. Similarly, White et al. [ 40 , 42 ] demonstrated the feasibility and clinical utility of the Multimodal Anxiety and Social Skills Intervention (MASSI), an adapted CBT program that considers the interconnectedness of anxiety and social communicative challenges in autistic children. Beyond solely treating anxiety, group-based CBT programs have also been successfully adapted to target emotion regulation and social skills [ 7 , 22 , 32 , 35 ]. A randomized control trial of a one-on-one CBT program, the Secret Agent Society: Operation Regulation (SAS: OR) [ 3 ], showed improvements in emotion regulation and adaptive skills, and reductions in externalizing symptoms and overall psychiatric symptom severity [ 38 ].
Pandemic disruptions have accelerated the need for programs that leverage existing online platforms to deliver therapeutic interventions, including using synchronous (real-time) and asynchronous (recorded) sessions, homework assignments, and peer support [ 2 , 17 ]. Even before the pandemic, emerging evidence supported the effectiveness of online-based programs. For example, Beaumont and colleagues [ 6 ] conducted a pilot randomized control trial of an online version of the Secret Agent Society Small Group (SAS: SG) program [ 4 , 33 ] for autistic children within a university-setting and found improvements in parent-reported social skills and problem behaviours compared to a control group. Lee and colleagues [ 21 ] conducted a mixed-methods evaluation of an online SAS: OR program during the first wave of the pandemic and demonstrated improvements in emotion regulation, social skills, and reductions in children’s externalizing behaviours after participation in the intervention. Other programs that target social skills and anxiety were also quickly adapted for online delivery, and preliminary results demonstrated general improvements in target behaviours (PEERS - Lee et al., 2023 [ 20 ]; Facing Your Fears - McMorris et al., in prep). Although the results of these pilot programs are promising, there is still a need to explore considerations for delivering virtual programming, particularly in community-based settings where autistic children receive most of their supports.
In Canada, community-based agencies are often publicly funded and provide the bulk of services for autistic children (e.g., behavioural interventions and supports, family workshops, parent respite, core clinical services, etc.). During the pandemic, many of these agencies continued to provide adapted virtual supports (e.g., phone consultations, online programming, etc.) for families. Group programs that are delivered by community agencies have been particularly impacted by the pandemic, as lockdowns and social distancing measures limited the availability of services [ 21 , 29 ]. There is some research suggesting that in-person community agencies were among the first to close and one of the last to re-open following pandemic restrictions in Canada [ 45 ], relative to hospital or school-based programs (Data from the Canadian Institute for Health Information, see https://www.cihi.ca/en for more information).
Online delivery of programs by community services can be beneficial and help address logistical barriers that many families face [ 11 , 27 ]. Online platforms may enhance intervention adherence and accessibility, as participants can access services from their homes, reducing barriers related to transportation, resources, and time [ 6 , 11 , 23 ]. Such interventions can also be tailored to the unique needs and preferences of families, including the ability to access services outside geographical location or service boundaries (e.g., catchment area) and to participate in sessions without leaving their home [ 2 , 6 , 23 ]. Yet changes to evidence-based interventions for online delivery, especially within the context of a pandemic, require careful considerations of feasibility and intervention clinical utility.
The current study reports on the feasibility and clinical utility of an adapted virtual socioemotional intervention (SAS: SG) delivered during the pandemic by seven community agencies in Ontario, Canada. Using an effectiveness-implementation hybrid design [ 10 ], which takes a dual focus by testing the effects of a clinical intervention on relevant participant outcomes while gathering information on implementation. For this study we tested the effectiveness of participation on child socioemotional and clinical outcomes (i.e., parent-reported changes in emotion regulation and social skills, and symptoms of anxiety and depression) post-intervention and after a 3-month follow-up session. At the same time, we gathered information on the feasibility of the program’s delivery by assessing the level of intervention acceptability reported by families, session attendance, therapist fidelity, and parent ratings of intervention acceptability and satisfaction.
Participants
Families were eligible to participate in the intervention if: (a) their child was between 8 and 12 years of age; (b) the child had a confirmed autism diagnosis from a regulated healthcare professional; (c) caregivers informally reported child difficulties with emotion regulation and social functioning, and/or were waiting for supports to address emotion regulation and social skills; and (d) a caregiver was able to participate in the program. Families were excluded if the child had (a) an intellectual disability; (b) a diagnosis of acute psychosis or conduct disorder; or (c) any behaviours that made online group participation a safety concern (e.g., self-harm behaviours, etc.).
A total of 87 families, across 7 agencies, participated in the study. Ten did not complete the intervention (see results section for more information about non-completing families). Of the 77 primary caregivers (94% mothers; Mean age = 42.5 years, SD age = 5.7 years) who completed the program, 67 completed the optional 3-month follow-up booster session. Caregivers identified as primarily White (72%), South/West/East Asian (12%), multiethnic (5%), Latin American/Hispanic (5%), and Black (2%). Children (79% identified their gender as males; Mean age = 9.9 years, SD age = 1.3 years) identified as White (66%), multiethnic (17%), South/West/East Asian (9%), Black (2%) and Latin American/Hispanic (2%). Additional participant characteristics are presented in Table 1 .
The study was approved by the research ethics board at the researchers’ institution, an academic hospital, and by the research review committee at two community-based agencies. The project was supported by a community-partner participatory framework [ 19 ], and was co-designed by a team of researchers, as well as with community agency leadership and frontline staff (e.g., therapists, child, and youth workers, etc.). Prior to implementation, researchers met with agencies to discuss agency-specific recruitment strategies and protocols, and how best to incorporate the intervention into existing programming without interfering with overall service deliverables.
Seven community-based autism service providers across Southern Ontario participated in the implementation of the SAS: SG project between October 2020 and December 2021. Prior to the delivery of each group, 21 therapists participated in a standardized four-day online training in August 2020 facilitated by the SAS: SG development team. Please see the Appendix to review therapist demographics including their level of education and discipline of practice.
Families were screened and recruited by each agency. Agencies followed their usual screening and enrollment protocol, as outlined by their own agency guidelines and policies, for offering services to children and their families on their client list. In publicly funded service providing agencies, children only require an autism diagnosis to get access to services and supports, and do not have to meet clinical cut-offs to enroll in interventions targeting emotion regulation and social skills. Therapists will use clinical judgement to determine which programs would best match the child’s and/or family needs. In some agencies, caregivers can self-refer their child if they feel that the focus of a program might be a good fit for their child. Some participants were recruited internally from agency waitlists, and some agencies recruited participants using social media posts or emailing past clients. All families were screened according to the inclusion and exclusion criteria. Once a family was deemed a good fit for the program by the SAS: SG therapist team (e.g., ready to receive intervention, available for group sessions, family goals align with program targets, etc.), researchers contacted the participants to review the research consent and provide details for participation in the study. Caregivers were then sent the pre-intervention child and family measures to be completed online. Caregivers completed post-intervention child and family measures (see below for program description and delivery schedule), and again after the 3-month follow-up booster session.
Intervention
The Secret Agent Society: Small Group Program (SAS: SG; Social Science Translated) [ 3 – 5 ] is a spy-themed manualized cognitive behavioural program focused on helping school-age children with identified emotion regulation and social skill difficulties. All caregiver and child sessions were delivered virtually through Zoom or Microsoft Teams. The program included separate caregiver and child sessions facilitated by therapists from each agency, as well as between-session practice activities and inclusive classroom tip sheets for each child’s schoolteachers. Agencies had the option to deliver the parent and child group sessions at the same time, or on different days, but the modules were synchronized to ensure that the parent session reviewed concepts covered in the child group sessions. Child sessions targeted social communication skills, working on teams, problem solving, developing and maintaining friendships, recognizing emotions in oneself and others, coping with feelings of anger and anxiety, and expressing emotions in helpful ways (for more specific information about the intervention, see https://www.secretagentsociety.com/ ). The child sessions were either provided as a weekly 9-session (90 min per session) or an 18-session (45 min per session) format. In the current study, out of 77 children, 65 (84.4%) received the 9-session format and 12 (15.6%) received the 18-session format. In the 9-session format, 92.3% attended at least 8 sessions or more, and in the 18-session format, 83% attended at least 16 sessions or more. The sessions were facilitated by either one or two trained facilitators, with a group of 3–4 or 4–6 children.
Caregiver sessions reviewed key components from the child sessions and teach caregivers how to support generalization of skills at home and beyond. Caregiver sessions were delivered in three different formats, and agencies could choose the schedule that worked best for them. The formats included (1) 9 weekly sessions of 45 min per week; (2) 18 weekly sessions of 30 min per week; or (3) three 2-hour sessions every 3 weeks. All agencies offered a 2-hour parent information session prior to beginning the program. In the current study, 56 (72.7%) caregivers received the 9 sessions module, 12 (15.6%) caregivers received the 18 sessions module, and 9 (11.7%) received the three 2-hour sessions. In the 9-session format, 96.4% attended at least 8 sessions or more; in the 18-session format, 83% attended at least 16 sessions or more; and in the 3-session format, 55.5% attended at least 2 sessions or more.
Implementation measures
Attendance. Therapists tracked attendance for the weekly sessions and the 3-month booster session.
Fidelity . Therapists tracked their adherence to the SAS: SG protocol using a weekly session checklist. The checklists were collected after completion of the program, and fidelity was calculated as the percentage of completed tasks across all sessions.
Implementation Acceptability Scale (IAS) [ 23 ]. The IAS is a 7-item lab-developed measure to assess intervention acceptability at the end of the 9-week sessions, based on Sekhon and colleagues’ theoretical framework of acceptability [ 31 ]. Caregivers were asked to describe their experience receiving the intervention using a five-point Likert scale (1 = “ strongly disagree ” to 5 = “ strongly agree ”), with higher scores reflecting greater treatment acceptability. Caregivers rated various dimensions, including affective attitude, burden, ethicality, intervention coherence, opportunity costs, perceived effectiveness, and self-efficacy. We evaluated acceptability based on the percentage of respondents that at least indicated “ agreed ” or higher (e.g., 3 or higher on the scale) for each question.
Program Satisfaction Questionnaire (PSQ) [ 3 ]. Caregivers completed the PSQ, which assessed their views on the appropriateness and effectiveness of the program. Open-ended questions asked caregivers to comment on changes in their child’s skills or behaviour, confidence in supporting their child, enjoyment of the program, and satisfaction with the therapists. Caregivers were also asked to describe their satisfaction with different components of the program on a five-point Likert scale (0 = “ not at all satisfied ” to 5 = “ very satisfied ”). The program components included: format, session dates and times, number of sessions and session length, and overall program satisfaction.
Child outcome measures
The Social Responsiveness Scale 2nd Edition (SRS-2) [ 9 ]. The SRS-2 is a 65-item caregiver-report measure used to capture school-aged (4–18-year-olds) children’s social functioning and autism-related characteristics. Caregivers are asked to respond on a 4-point Likert Scale (0 = “ Not True ” to 3 = “ Almost Always True ”) to statements related to their child’s social functioning including in areas of social awareness, social cognition, social communication, social motivation, and the presence of restricted interests and repetitive behaviours. The SRS-2 has good external reliability ( 0.90), with strong internal consistency [ 9 ]. Additionally, this measure has high predictive validity (0.92) and construct validity [ 9 ]. It is one of the most widely used measures of children’s actual social performance and it can be expected to show moderate to large changes in the context of a successful clinical intervention [ 43 ]. In the current study, the Total T-score, Social Communication and Interaction (SCI) scale T-score and the Restricted Interests and Repetitive Behavior (RRB) scale T-score were used.
The Emotion Dysregulation Inventory (EDI) [ 26 ]. The EDI is a caregiver-report measure developed to assess the severity of autistic children’s struggles with negative mood and reactivity via two subscales, Dysphoria (6 items; anhedonia, sadness, nervousness) and Reactivity (7 items; explosive outbursts, difficulty calming, rapid escalation, intense/extreme/inappropriate emotionality). Items are rated on a 5-point Likert scale (0 = “not at all” to 4 = “very severe” ). The items were summed for each subscale and converted to T - Scores. For both subscales, higher scores indicated greater dysregulation. The EDI shows strong validity and reliability for assessing Reactivity and Dysphoria in autistic children [ 26 ]. Internal consistency for Dysphoria and Reactivity within the current sample pre-intervention were very good: α = 0.88 and α = 0.89, respectively.
Child and Adolescent Symptoms Inventory-5 ( CASI-5) [ 13 ]. The CASI-5 is a caregiver-report measure that gathers information about the symptoms of Diagnostic and Statistical Manual and Mental Disorders- 5th Edition (DSM-5) defined disorders in children and adolescents between the ages of 5 to 18 years. The 173-item inventory is organized into modules where each consists of a list of symptom statements for 14 of the most commonly defined DSM-5 disorders. Caregivers are asked to rate whether their child displays any of the symptoms on a 4-point scale ranging from “ never ” to “ very often ”. The tool shows strong validity and reliability in caregivers of autistic children, including overlap with interview measures of mental health disorders [ 12 ]. This measure has been found to have very good internal consistency for assessments of anxiety (α = 0.85-0.88) [ 14 ] and depression (α = 0.83) [ 15 ] in parent-reports. In the current study, we used the total symptom severity T-Score for separation anxiety, social anxiety, generalized anxiety disorder, and major depression disorder, with higher scores indicating greater level of presenting symptoms.
Data analyses
All analyses were conducted using SPSS version 28. Implementation acceptability and feasibility were explored using descriptive statistics, while changes in child outcome measures were analyzed using paired t-tests and repeated-measures ANOVAs, with a Greenhouse-Geisser correction to account for violations of sphericity. Post hoc analyses of ANOVA outcomes used Bonferroni corrections.
Non-completer participant profiles
A preliminary evaluation of the demographic profiles and key baseline characteristics of families included those who did not complete the program are outlined in Table 2 ( n = 10). Families listed various reasons for being unable to continue with the program including scheduling issues with the group sessions ( n = 2), the program required too much time commitment ( n = 2), lack of interest in the theme ( n = 1), virtual format not a good fit for their child ( n = 3), and urgent family obligations ( n = 2). These caregivers attended on average 2.43 sessions ( Range = 1–3) and children attended 1.67 sessions ( Range = 0–4). There were no significant differences in age or gender distributions between non-completer and completer caregivers. Independent sample t-tests showed a significantly higher SRS-2 RRB T-score for non-completer children ( M = 78.3, SD = 9.60) compared to completer children ( M = 78.30, SD = 9.03, F (1,86) = 4.14, p = 0.04). There were no other significant differences in pre-intervention child outcome measures or child demographics.
Implementation results
As shown in Table 3 , almost all treatment completers attended their weekly parent and child group sessions (attended 93.8% of sessions).
On the self-reported checklists, therapists indicated above 80% fidelity for both caregiver ( M = 93.5%, Range = 88–99%) and child ( M = 86.6%, Range = 78.9–6.9%) weekly sessions. A review of the fidelity checklists suggested that therapists were unable to complete some parts because of technology issues that prevented the completion of certain activities (e.g., online board game, virtual missions with the group, poor internet connections preventing participation, etc.), ran out of time to do an activity during the session (which resulted in assigning the task as homework), and/or unexpected disruptions (e.g., child abruptly disengages from the group, home-based interference, etc.). In terms of post-program acceptability, 75% of caregivers agreed or strongly agreed that they felt positively about the program, 95% agreed or strongly agreed that it aligned with their values, 87% agreed that they understood how it worked, 77% agreed that they did not have to give up resources or opportunities to participate in the program, and 77% agreed that they felt confident in the skills they had learned. Lower ratings of acceptability related to acceptable amount of effort to participate (only 61% agreed) and feeling that it was effective in achieving its goals (only 62% agreed). A qualitative analysis of caregiver feedback ( n = 20) indicated that the virtual format required parents to spend more time monitoring their child’s group sessions in order to manage their behaviours, and to help them stay engaged. Some caregivers ( n = 10) hoped that participation would lead to new emotion regulation or social skills but instead were somewhat disappointed when the program only reinforced their child’s current skill set. On the post-intervention PSQ, 70% of the caregivers reported feeling “ moderately ” to “ very ” confident in their ability to support their child’s future social and emotional development following completion of the program, and 83% reported that the program was “ moderately ” to “ very ” enjoyable for their child. Caregivers reported being “ moderately ” to “ very ” satisfied (90%) with their SAS: SG group facilitator, and overall, 71% of caregivers reported being “ moderately ” to “ very ” satisfied with the entire program.
Child outcomes
The pre-intervention SRS-2 Total T-score ranged from 54 to 90 ( M = 71.33, SD = 8.91). For the SCI scale, the pre-intervention t-score ranged from 54 to 90 ( M = 70.52, SD = 9.07) and the RRB scale T-score ranged from 52 to 90 ( M = 72.03, SD = 9.28). In our sample, 91% of children met clinical level of concern on the SRS-2 Total T-Score. As shown in Table 4 , there was a significant difference between SRS-2 Total T-Scores, SCI, and RRB T-scores across pre-, post-, and the 3-month time points. Scores consistently decreased over time, which demonstrated improvements from pre- to post-intervention (Mean Difference = -3.14 (95% CI, -7.86 to -4.60, p = 0.001, d = 0.55)), and from post-intervention to the 3-month booster session (Mean Difference = -2.76 (95% CI, -5.03 to -0.49, p = 0.001, d = 0.52)) on the SRS-2 Total T-score.
On the emotion dysregulation measure (EDI), there were significant differences between time points on the EDI Reactivity and Dysphoria T-scores. The pre-intervention EDI Reactivity T-scores ranged from 30.1 to 66.7 ( M = 50.13, SD = 7.07) and the EDI Dysphoria T-score ranged from 36.4 to 70.3 ( M = 48.01, SD = 8.84), with 52% and 25% of the children in our sample meeting clinical cut-offs for emotion regulation difficulties across the two scales, respectively [ 8 ]. EDI Reactivity scores decreased from pre-intervention to post-intervention (-1.78 (95% CI, -3.2 to -0.29), p = 0.01, d = 0.36), and continued to decrease from post-intervention to after the 3-month booster session (-1.75 (95% CI, -3.34 to -0.16), p = 0.02, d = 0.33). While EDI Dysphoria scores decreased from pre-intervention to post-intervention, this change was not significant. EDI Dysphoria scores continued to decrease after the 3-month booster, with scores being significantly lower than the pre-intervention scores (-3.13 (95% CI, -5.02 to -1.23), p = 0.001, d = 0.51) but not the post-intervention scores (-1.67 (95% CI, -3.40 to 0.56, p = 0.061).
Scores on the CASI-5 indicated that pre-intervention, 38% of the children met clinical range of concerns for separation anxiety ( T -score range = 50.0–78.0), 49% for social anxiety ( T -score range = 50.0–76.0), 83% for general anxiety disorder ( T -score range = 50.0–78.0), and 43% for depression ( T -score range = 50.0–78.0). There were significant changes across time points with respect to symptoms related to both generalized anxiety disorder (GAD) and major depression, but not for separation anxiety and social anxiety. GAD symptom severity scores improved from pre-intervention to post-intervention (3.05 (95% CI, 0.72 to 5.36), p = 0.006, d = 0.39), and then remained stable from post-intervention to the 3-month booster session (0.80 (95% CI (-3.38 to 1.77), p = 1.00). For depression, there appeared to be no statistically significant change from pre- to post-intervention (2.29 (95% CI, -0.56 to 5.16), p = 0.16), but there was a significant improvement from pre-intervention to the 3-month booster session (3.61 (95% CI (0.84 to 6.38), p = 0.006, d = 0.39) (See Table 4 ).
The present study used an effectiveness-implementation hybrid design to evaluate the effects of an adapted virtual cognitive behaviour program, SAS: SG, on autistic children’s socioemotional outcomes while collecting information on community-based implementation. The SAS: SG program is publicly available, but existing research has largely focused on outcomes from lab- or university-based evaluations, and prior to the COVID-19 pandemic, it was delivered primarily in an in-person format. Findings from the current study suggest that the program was implemented successfully with high therapist-reported fidelity across seven community autism-focused service agencies and provides support for more rigorous research into the efficacy of group-based online programs for autistic children in the community. Results from our study suggest that families completed most of their weekly parent and child group sessions, with similar attendance rates compared to in-person adapted group programs [ 44 ] and other online versions of the program [ 23 ]. The program also saw a rather low attrition rate ( ∼ 10%) which may have reflected the strengths of an online formatting that decreased the usual barriers to participation including the cost of travel (e.g., time and financial costs).
In terms of feasibility, caregivers rated most aspects of intervention acceptability as high and described feeling positively about the program, that it aligned with their values, and that they understood how the program worked. However, it should be noted that a substantial group of caregivers did not agree that the program demanded a reasonable amount of effort from them (39%) or that it was effective in achieving its goals (38%). A review of text-based comments from caregivers indicated that the amount of time that was asked of them was sometimes overwhelming, including having to monitor their child’s participation, learn new concepts, support their child’s learning of skills, and facilitate assigned home activities on a weekly basis. For some caregivers, this led to hours of work above and beyond their own participation in the parent groups. These themes are consistent with previous findings related to delivering caregiver-involved online programs during the pandemic [ 17 , 23 , 41 ]. In terms of goal achievement, some caregivers were underwhelmed by the usefulness of the skills taught in the program. A qualitative review of text responses from caregivers indicated that that some hoped that their child could have learned new emotion regulation and social skills rather than practice skills they had already mastered. Some caregivers felt that the online format did not provide enough opportunities for their children to practice and apply the social skills being taught in the program, thus not achieving their original goals.
Considering caregiver feedback about the program, it is important for virtually delivered programs to consider the demands placed on caregivers that build upon existing stressors in their life [ 21 ]. During the pandemic, this reflected the additional demands of managing online support for their children, the evolving virtual school requirements, on top of their own work and household responsibilities, COVID-related illness, or other stressors. There is literature highlighting how this added burden is often placed upon primary caregivers, usually mothers. There is an urgent need to acknowledge these considerations around equity of supports for caregivers during the pandemic and beyond [ 30 ]. A review of non-completing families suggests that children who had higher levels of RRBs had a more difficult time engaging in online sessions. This is consistent with previous work [ 23 ] suggesting that program delivery with an online format may not be well suited to all caregivers and autistic children, especially those with behaviours that interfere with sitting and attending (e.g., compulsive behaviours, self-injurious behaviours, etc.).
Although there are benefits with delivering a program online (e.g., limiting the cost of travel, enabling further research for community service providers, etc.), some families may require different supports to make participating online more accessible to them. This might include adaptations like shorter sessions, more frequent assessment of motivation and engagement, greater use of specialized interests, and adaptations that focus on individualized care. For example, Mootz et al. [ 27 ] described modifications made to optimize participation for a single group pilot SAS: SG program delivered via telehealth in Australia during the pandemic. They described similar needs to develop procedures to support families including troubleshooting technology throughout delivery, shortening sessions, and tasking caregivers with supervision of child sessions (e.g., giving out end-of-session rewards, specifying consequences for non-engagement, etc.). Yet, despite online adaptations some children and their families may still find in person programming more beneficial and better suited to their needs. Future research by the current team includes a direct comparison of in person versus online version of the SAS: SG program in community-based services.
It should be noted that the current study took place at the beginning of the pandemic when rolling lockdowns were prevalent and there were few competing activities for families that required travel (e.g., other appointments, recreational activities, etc.). During this time, some caregivers were actively seeking access to any programs for their children, which may have contributed to the rather high engagement with the virtual program (e.g., lower than usual attrition rate). In addition, reduced demands associated with online delivery of the program (i.e., less travel time) may have contributed to increased feasibility and satisfaction with the program.
Successful implementation of this program may be the result of a community-partnered participatory framework that allowed each agency flexibility in recruitment and delivery [ 19 ]. Agencies managed their own scheduling (e.g., number of weeks, days, and times, etc.), and were supported by the research team throughout the project (e.g., troubleshooting technology issues, etc.). Therapist-reported fidelity suggested that adherence was high (87% or higher), although we could not independently verify their session fidelity as sessions were not recorded. Therapists did face some challenges completing parts of the modules, especially during child sessions, due to technology issues, running out of time, and unexpected disruptions. These are important factors to consider from an implementation perspective for future hybrid service delivery. Anecdotally, feedback from therapist teams suggests that those who spent more time troubleshooting and preparing for technological issues were able to react and respond better when issues arose during program delivery. Some therapists noted that caregiver involvement was necessary to deescalate emotionally tense situations with their children, which, as noted, often increased the demands placed upon caregivers. Therapists mentioned that specialized interests were incorporated into group sessions as necessary, and overall, most worked hard (e.g., provided visual aids, used animations, and used an abundance of reinforcers and/or tokens, etc.) to engage the children in the group sessions. Therapists should consider individual child and family needs, and screening participants for suitability for online-based group programs should consider access to technology and a family’s ability to support their child’s participation in the program [ 13 , 23 ].
In terms of clinical utility of the program, caregivers reported improvements in child emotion regulation and social communication skills from pre- to post-intervention, and these gains were sustained after the 3-month booster sessions. Caregivers also reported that their children showed statistically significant improvements in social interactions and communication behaviours, and emotion reactivity at each time point. Emotion dysphoria, however, revealed a different pattern, as scores did not show a statistically significant improvement until after the 3-month booster. These findings are consistent with the main tenets of the SAS: SG program which aims to teach children deescalating techniques to prevent emotionally reactive behaviours in the face of intense or socially frustrating situations, and these skills may take additional time to solidify.
Consistent with previous work [ 17 ], no changes were found in separation and social anxiety at post-intervention and following the 3-month booster session. Given that many children were at home during the early waves of the pandemic, they may have had fewer opportunities to socialize, or be separated from caregivers. Caregivers did report improvements in children’s generalized anxiety symptoms, even after the 3-month booster session. Similar to the emotional dysphoria findings, caregivers reported steady improvements in symptoms of depression over time, but the scores reached statistically significant levels of improvement only after the 3-month booster session. These findings may suggest that the program may indirectly benefit dysphoric or behaviours resulting in negative moods and, with practice, symptoms improve over time, even though it does not specifically target them.
Interpretation of the results should be mindful of a few study limitations. The study was a single-arm implementation trial which makes our results particularly suspectable to placebo effects, and results were interpreted without a control group or blinded independent clinical assessments. Data from the study were based mainly on caregiver reports which may differ from therapist and child perspectives. Fidelity ratings were self-reported by therapists and could not be independently verified by recordings, and future implementation trials would benefit from independent coding of recorded sessions for reliability. Interpretation of the findings should consider the variability in symptom severity of our sample, especially since not all children met clinical levels of concern pre-intervention, on emotion dysregulation (52% for reactivity and 25% for dysphoria), and mental health symptoms (e.g., only symptoms of generalized anxiety were above clinical threshold pre-intervention, with social anxiety and depression symptoms being moderately elevated). Although our results showed post-intervention improvements across these domains with small to moderate effect sizes, they may not reflect a clinically meaningful change as expected for most interventions. Clinically meaningful improvements in symptoms related to generalized anxiety, however, were observed, suggesting that the socioemotional support program may have some indirect impact on improving some aspects of mental health. Finally, there seems to be particular risks in overinterpreting improvements post-intervention that may simply be due to family acclimations to pandemics stressors. Although, the SAS: SG program was delivered between September 2020 to September 2021, and started at least 4 months after the initial shut-downs due to the pandemic, which suggests that families had some time to adjust.
Despite these limitations, our findings suggest that an evidence-based intervention targeting emotion regulation and social skills in autistic children is feasible and can be delivered by community-based service providers with success. To our knowledge, this is the first study to demonstrate the effectiveness of an adapted, virtual group-based program focused on socioemotional skills, delivered in the community for autistic children during the early waves of the pandemic. Results highlight the need for ongoing support for autistic children, especially given the unpredictable circumstances imposed by the past and future pandemic resulting in a global loss of supports (e.g., therapy, social skills groups, academic and recreational programming). Our findings encourage community-partnerships with publicly funded agencies and contribute to the emerging efforts to narrow the gap from research to practice in implementing evidence-based programs in community settings. Ultimately, training in evidence-based programs by community providers can increase access to helpful ways of supporting emotion regulation and social challenges for autistic children.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due university-imposed restrictions around data sharing agreements but are available from the corresponding author on reasonable request.
Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin Psychol Rev. 2010;30(2):217–37. https://doi.org/10.1016/j.cpr.2009.11.004 .
Article PubMed Google Scholar
Ameis SH, Lai MC, Mulsant BH, Szatmari P. Coping, fostering resilience, and driving care innovation for autistic people and their families during the COVID-19 pandemic and beyond. Mol Autism. 2020;11(1):61. https://doi.org/10.1186/s13229-020-00365-y .
Article PubMed PubMed Central Google Scholar
Beaumont R. Secret agent society: solving the mystery of social encounters—facilitator kit. Brisbane, Australia: The Social Skills Training Institute, A Subdivision of Triple P International; 2010.
Google Scholar
Beaumont R, Hinton S, Sofronoff K. (2018). The Secret Agent Society: Upskilling teachers in the delivery of a game-based social skills children program. In D. Mentor, editor, Computer-mediated learning for workforce development. IGI Global. https://doi.org/10.4018/978-1-5225-4111-0.ch002 .
Beaumont R, Sofronoff K. A multi-component social skills intervention for children with Asperger syndrome: the Junior Detective Training Program. J Child Psychol Psychiatry. 2008;49(7):743–53. https://doi.org/10.1111/j.1469-7610.2008.01920.x .
Beaumont R, Walker H, Weiss J, Sofronoff K. Randomized controlled trial of a video gaming-based social skills program for children on the autism spectrum. J Autism Dev Disord. 2021;51(10):3637–50. https://doi.org/10.1007/s10803-020-04801-z .
Conner CM, White SW, Beck KB, Golt J, Smith IC, Mazefsky CA. Improving emotion regulation ability in autism: the emotional awareness and skills enhancement (EASE) program. Autism: Int J Res Pract. 2019;23(5):1273–87. https://doi.org/10.1177/1362361318810709 .
Article Google Scholar
Connor CM, Golt J, Schaffer R, Righi G, Siegel M, Mazefsky CA. Emotion dysregulation is substantially elevated in Autism compared to the General Population: impact on Psychiatric services. Autism Res. 2021;14(1):169–81.
Constantino JN, Gruber CP. Social Responsiveness Scale- Second Edition (SRS-2). Torrance, CA. Western Psychological Services; 2012.
Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26. https://doi.org/10.1097/MLR.0b013e3182408812 .
Ellison KS, Guidry J, Picou P, Adenuga P, Davis TE 3rd. Telehealth and autism prior to and in the age of COVID-19: a systematic and critical review of the last decade. Clin Child Fam Psychol Rev. 2021;24(3):599–630. https://doi.org/10.1007/s10567-021-00358-0 .
Gadow KD, Sprafkin J. Child symptom inventory 4: CSI. Stony Brook, NY: Checkmate Plus; 1997.
Gadow KD, Sprafkin J. Fact sheet for the child and adolescent Symptom Inventory-5. Stony Brook, NY: © Checkmate Plus; 2013.
White SW, Schry AR, Maddox BB. Brief report: the assessment of anxiety in high-functioning adolescents with autism spectrum disorder. J Autism Dev Disord. 2012;42:1138–45.
Witwer AN, Lecavalier L, Norris M. Reliability and validity of the children’s interview for psychiatric syndromes-parent version in autism spectrum disorders. J Autism Dev Disord. 2012;42:1949–58.
Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. pp. 3–24.
Kalvin CB, Jordan RP, Rowley SN, Weis A, Wood KS, Wood JJ, Ibrahim K, Sukhodolsky DG. Conducting CBT for anxiety in children with Autism Spectrum Disorder during COVID-19 pandemic. J Autism Dev Disord. 2021;51(11):4239–47. https://doi.org/10.1007/s10803-020-04845-1 .
Kranzler A, Young JF, Hankin BL, Abela JRZ, Elias MJ, Selby EA. Emotional awareness: a transdiagnostic risk factor for internalizing symptoms in children and adolescents? J Clin Child Adolesc Psychology=. 2016;45(3):262–9. https://doi.org/10.1080/15374416.2014.987379 .
Kwon SC, Tandon SD, Islam N, Riley L, Trinh-Shevrin C. Applying a community-based participatory research framework to patient and family engagement in the development of patient-centred outcomes research and practice. Translational Behav Med. 2018;8(5):683–91. https://doi.org/10.1093/tbm/ibx026 .
Lee CM, Petricek C, Haga M, Smalley S, Pelletier K, Essa N, Hudock RL. Examining the feasibility and effectiveness of PEERS for adolescents via telehealth during the COVID-19 pandemic. Res Autism Spectr Disorders. 2023;109:102279.
Lee V, Albaum C, Tablon Modica P, Ahmad F, Gorter JW, Khanlou N, McMorris C, Lai J, Harrison C, Hedley T, Johnston P, Putterman C, Spoelstra M, Weiss JA. The impact of COVID-19 on the mental health and wellbeing of caregivers of autistic children and children: a scoping review. Autism Res. 2021;14(12):2477–94. https://doi.org/10.1002/aur.2616 .
Lee J, Lee TS, Lee S, Jang J, Yoo S, Choi Y, Park YR. Development and application of a metaverse-based social skills training program for children with autism spectrum disorder to improve social interaction: protocol for a randomized controlled trial. JMIR Res Protocols. 2022;11(6):e35960.
Lee V, Roudbarani F, Modica T, Pouyandeh P, A., Weiss JA. Adaptation of cognitive behavior therapy for autistic children during the pandemic: a mixed-methods program evaluation. Evidence-Based Pract Child Adolesc Mental Health. 2021;1–18. https://doi.org/10.1080/23794925.2021.1941432 .
Liu JJ, Bao Y, Huang X, Shi J, Lu L. Mental health considerations for children quarantined because of COVID-19. Lancet Child Adolesc Health. 2020;4(5):347–9. https://doi.org/10.1016/S2352-4642(20)30096-1 .
Article CAS PubMed PubMed Central Google Scholar
Mazefsky CA, Herrington J, Siegel M, Scarpa A, Maddox BB, Scahill L, White SW. The role of emotion regulation in autism spectrum disorder: emotion regulation in ASD. J Am Acad Child Adolesc Psychiatry. 2013;52(7):679–88. https://doi.org/10.1016/j.jaac.2013.05.006 .
Mazefsky CA, Yu L, White SW, Siegel M, Pilkonis PA. The emotion dysregulation inventory: psychometric properties and item response theory calibration in an autism spectrum disorder sample. Autism Res. 2018;11(6):928–41.
Mootz CA, Lemelman A, Giordano J, Winter J, Beaumont R. Brief report: feasibility of delivering the Secret Agent Society group social skills program via telehealth during COVID-19: a pilot exploration. J Autism Dev Disord. 2022;52(12):5274–9. https://doi.org/10.1007/s10803-022-05591-2 .
Mutluer T, Doenyas C, Aslan Genc H. Behavioral implications of the Covid-19 process for autism spectrum disorder, and individuals’ comprehension of and reactions to the pandemic conditions. Front Psychiatry. 2020;11:561882. https://doi.org/10.3389/fpsyt.2020.561882 .
Pavlopoulou G, Wood R, Papadopoulos C. Impact of Covid-19 on the experiences of caregivers and family carers of autistic children and young people in the UK. London, UK: UCL Institute of Education; 2020.
Pellicano E, Stears M. The hidden inequities of COVID-19. Autism. 2020;24(6):1309–10.
Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17:88. https://doi.org/10.1186/s12913-017-2031-8 .
Sofronoff K, Beaumont R, Weiss JA. (2014). Treating transdiagnostic processes in ASD: Going beyond anxiety. In T. E. Davis III, S. W. White, & T. H. Ollendick, editors, Handbook of Autism and Anxiety (pp. 171–183). Springer International Publishing. https://doi.org/10.1007/978-3-319-06796-4_12 .
Sofronoff K, Silva J, Beaumont R. The Secret Agent Society social-emotional skills program for children with a high-functioning autism spectrum disorder: a parent-directed trial. Focus Autism Other Dev Disabil. 2017;32(1):55–70. https://doi.org/10.1177/1088357615583467 .
Stahmer AC, Brookman-Frazee L, Rieth SR, Stoner JT, Feder JD, Searcy K, Wang T. Parent perceptions of an adapted evidence-based practice for toddlers with autism in a community setting. Autism. 2017;21(2):217–30. https://doi.org/10.1177/1362361316637580 .
Temkin AB, Beaumont R, Wkya K, Hariton JR, Flye BL, Sheridan E, Miranda A, Vela J, Zendegui E, Schild J, Gasparro S, Loubriel D, Damianides A, Weisman J, Silvestre A, Yadegar M, Catarozoli C, Bennett SM. (2022). Secret Agent Society: A randomized controlled trial of a transdiagnostic children social skills group treatment. Research on Child and Adolescent Psychopathology , 50 (9), 1107–1119. https://doi.org/10.1007/s10802-022-00919-z Thompson, R. A. (1994). Emotion regulation: A theme in search of definition. Monographs of the Society for Research in Child Development , 59 (2–3), 25–52.
Vasa RA, Singh V, Holingue C, Kalb LG, Jang Y, Keefer A. Psychiatric problems during the COVID-19 pandemic in children with autism spectrum disorder. Autism Res. 2021;14(10):2113–9.
Weiss JA. Transdiagnostic case conceptualization of emotional problems in children with ASD: an emotion regulation approach. Clin Psychol. 2014;21(4):331–50. https://doi.org/10.1111/cpsp.12084 .
Weiss JA, Thomson K, Burnham Riosa P, Albaum C, Chan V, Maughan A, Tablon P, Black K. A randomized waitlist-controlled trial of cognitive behavior therapy to improve emotion regulation in children with autism. J Child Psychol Psychiatry. 2018;59(11):1180–91. https://doi.org/10.1111/jcpp.12915 .
Weyland M, Maes P, Kissine M, Defresne P. (2022). Impact of Belgian COVID-19 lockdown restrictions on autistic individuals’ socio-communicative behaviors and their caregivers’ quality of life. PLoS ONE, 17(8), e0273932.
White SW, Albano AM, Johnson CR, Kasari C, Ollendick T, Klin A, Oswald D, Scahill L. Development of a cognitive-behavioral intervention program to treat anxiety and social deficits in teens with high-functioning autism. Clin Child Fam Psychol Rev. 2010;13(1):77–90. https://doi.org/10.1007/s10567-009-0062-3 .
White LC, Law JK, Daniels AM, Toroney J, Vernoia B, Xiao S, Consortium SPARK, Feliciano P, Chung WK. Brief report: impact of COVID-19 on individuals with ASD and their caregivers: a perspective from the SPARK cohort. J Autism Dev Disord. 2021;51(10):3766–73. https://doi.org/10.1007/s10803-02004816-6 .
White SW, Ollendick T, Albano AM, Oswald D, Johnson C, Southam-Gerow MA, Kim I, Scahill L. Randomized controlled trial: multimodal anxiety and social skill intervention for adolescents with autism spectrum disorder. J Autism Dev Disord. 2013;43(2):382–94. https://doi.org/10.1007/s10803012-1577-x .
Wolstencroft J, Robinson L, Srinivasan R, Kerry E, Mandy W, Skuse D. A systematic review of group social skills interventions, and meta-analysis of outcomes, for children with high functioning ASD. J Autism Dev Disord. 2018;48:2293–307.
Wood JJ, Kendall PC, Wood KS, Kerns CM, Seltzer M, Small BJ, Lewin AB, Storch EA. Cognitive behavioral treatments for anxiety in children with autism spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2020;77(5):474–83. https://doi.org/10.1001/jamapsychiatry.2019.4160 .
Yuan P, Aruffo E, Gatov E, Tan Y, Li Q, Ogden N, Collier S, Nasri BR, Moyles I, Zhu H. School and community reopening during the COVID-19 pandemic: a mathematical modelling study. Royal Soc Open Sci. 2022;9:211883. https://doi.org/10.1098/rsos.211883 .
Article CAS Google Scholar
Download references
Acknowledgements
The authors would like to thank the families who participated in the groups and our partnered community agencies who co-designed and implemented this research study.
The authors disclose receipt of the following support for the research, authorship, and/or publications of this article. This study was supported by the Kids Brain Health Network, and by the York Research Chair in Autism and Neurodevelopmental Disability Mental Health.
Author information
Authors and affiliations.
Department of Psychology, Carleton University, 214E Social Science Research Building, Ottawa, ON, K1S 5B6, Canada
Department of Psychology, York University, 230 BSB, 4700 Keele St, Toronto, ON, M3J 1P3, Canada
Nisha Vashi, Flora Roudbarani, Paula Tablon Modica, Ava Pouyandeh, Teresa Sellitto, Alaa Ibrahim & Jonathan A. Weiss
Centre for Addiction and Mental Health, 1001 Queen Street West, Toronto, ON, M6J 1H4, Canada
Stephanie H. Ameis, Meng-Chuan Lai & Johanna Lake
Department of Psychiatry, Temerty Faculty of Medicine, University of Toronto, 250 College Street, 8th Floor, Toronto, ON, M5T 1R8, Canada
Kinark Child and Family Services, 7271 Warden Ave, Markham, ON, L3R 5X5, Canada
Alex Elkader
Centre for Education Development, Appraisal, and Research, University of Warwick, Coventry, CV4 7AL, UK
Kylie M. Gray
Department of Psychology, University of British Columbia, 2136 West Mall, Vancouver, BC, V6T 1Z4, Canada
Connor M. Kerns
Department of Applied Disabilities Studies, Brock University, 1812 Sir Issac Brock Way, St. Catherines, ON, L2S 3A1, Canada
Kendra Thomson
Azrieli Adult Neurodevelopmental Centre at the Centre for Addiction and Mental Health, 1451 Queen Street West, Toronto, ON, M63 1A1, Canada
You can also search for this author in PubMed Google Scholar
Contributions
VL and JW conceptualized the study. VL, NV, FR, PTM, AP, and TS worked together under the supervision of JW to collect the data. VL and JW analyzed and interpreted the data. VL wrote the manuscript. NV, FR, PTM, AP, TS, AI, SHA, AE, KW, CMK, MCL, JL, KT, and JW provided edits and revisions. All authors read and approved the final manuscript before submission.
Corresponding author
Correspondence to Vivian Lee .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the research ethics board at the researchers’ institution (York University and Carleton University), an academic hospital (Ron Joyce Children’s Health Centre), and by the research review committee at two community-based agencies (Kerry’s Place Autism Services and Kinark Autism Services). All methods in the study were performed in accordance with the Declaration of Helsinki. Participant provided electronic informed consent to participate in the study.
Consent for publication
No individual’s personal data (details, images, or videos) are included in this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lee, V., Vashi, N., Roudbarani, F. et al. Utility of a virtual small group cognitive behaviour program for autistic children during the pandemic: evidence from a community-based implementation study. BMC Health Serv Res 24 , 685 (2024). https://doi.org/10.1186/s12913-024-11033-9
Download citation
Received : 27 July 2023
Accepted : 23 April 2024
Published : 30 May 2024
DOI : https://doi.org/10.1186/s12913-024-11033-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Virutal CBT
- Emotional regulation
- Social skills
- Community services
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
- Find a Doctor
- Appointments and Second Opinions
- Find a Location
- Patient Portals
Clinical trials show promise in treating central nervous system lymphoma, breast cancer, and glioblastoma
The findings to be presented at the 2024 Annual Meeting of the American Society of Clinical Oncology (ASCO).
Dana-Farber Cancer Institute researchers are leading 3 separate studies with encouraging results in treating patients with central nervous system (CNS) lymphoma, breast cancer, and glioblastoma. The studies support future research in these potential breakthroughs where treatment options may be limited. The research teams will present their findings at the 2024 Annual Meeting of the American Society of Clinical Oncology (ASCO) in Chicago, May 31-June 4, 2024. ASCO is the world's largest clinical cancer research meeting, attracting more than 30,000 global oncology professionals.
These findings are among more than 80 studies presented at ASCO that are led by Dana-Farber-affiliated researchers.
A full list of Dana-Farber Oral Presentations at the 2024 ASCO Annual Meeting is available here.
A full list of Dana-Farber Poster Discussions at the 2024 ASCO Annual Meeting is available here.
CAR T-cell therapy shows promise in patients with central nervous system lymphoma
A CAR T-cell therapy approved for patients with large B cell lymphoma has produced positive results in a pilot study involving patients with relapsed, treatment-resistant central nervous system (CNS) lymphoma, Dana-Farber investigators report. The therapy, axicabtagene ciloleucel, was found to be safe and well tolerated in the 18 study participants and had an overall response rate of 94%. The median progression-free survival – the time in which patients lived without the cancer worsening – was 14.3 months, and the median overall survival was 26.4 months. The most common side effects, cytokine release syndrome and immune effector cell-associated neurologic syndrome (ICANS) that are inflammatory conditions often associated with CAR T-cell therapy, were manageable. CNS lymphoma is a rare non-Hodgkin lymphoma in which malignant cells form in the lymph tissue of the brain or spinal cord. Although initial treatment is often effective, better treatments are needed when the disease recurs. CAR T-cell therapies use genetically modified versions of a patient's own immune system T cells to attack cancer cells in the body.
- Study Title: A Pilot Study of Axicabtagene Ciloleucel (axi-cel) for Relapsed/Refractory Primary and Secondary Central Nervous System Lymphoma Oral Abstract Number: 2006 Session: Oral Abstract Session - Central Nervous Tumors; June 3, 2024, 10:36am ET Presenting & Lead Author: Lakshmi Nayak, MD Co-lead Author: Caron Jacobson, MD, MMSc
Antibody-drug conjugate plus checkpoint inhibitor shows a trend toward improved progression-free survival in PD-L1-positive hormone receptor-positive, HER2-negative breast cancer
In patients with metastatic hormone receptor (HR)-positive/HER2-negative breast cancer unselected by PD-L1 status, adding the immune checkpoint inhibitor pembrolizumab to the antibody-drug conjugate sacituzumab govitecan resulted in a 1.9-month improvement in median progression-free survival that was not statistically significant. In the subgroup of patients with PD-L1-positive tumors (defined as a combined positive score ≥1), a 4.4-month increase in median progression-free survival was observed with sacituzumab govitecan plus pembrolizumab compared to sacituzumab govitecan alone.
The phase II SACI-IO HR+ trial was designed to evaluate whether these two therapies act synergistically. Sacituzumab govitecan consists of an antibody linked to a chemotherapy drug called SN-38. In cancer cells, SN-38 causes DNA damage that, via activation of pathways in the cancer cell, may draw T-cells to the cancer. The combination with pembrolizumab (an immune checkpoint inhibitor that lifts the brakes off the immune system) could enhance the ability of the immune system to recognize and attack cancer cells. The SACI-IO HR+ trial included 110 patients with previously treated advanced or metastatic HR-positive/HER2-negative breast cancer; 104 patients started therapy on the study – half of whom received sacituzumab govitecan plus pembrolizumab and half of whom received sacituzumab govitecan alone.
At a median follow-up of 12.5 months, the median progression-free survival (how long patients lived before their cancer worsened) was 8.1 months for patients receiving the combined therapy, compared to 6.2 months for those receiving sacituzumab govitecan alone. In the approximately 40% of patients who participated in the study whose tumor was PD-L1-positive, the median progression-free survival was 11.1 months with the combination vs 6.7 months with sacituzumab govitecan alone. These results support further investigation of sacituzumab govitecan plus pembrolizumab in patients with PD-L1-positive metastatic HR-positive/HER2-negative breast cancer.
- Study Title: SACI-IO HR+: A randomized phase II trial of sacituzumab govitecan with or without pembrolizumab in patients with metastatic hormone receptor-positive/HER2-negative breast cancer Abstract Number: LBA1004 Session: Oral Abstract Session - Breast Cancer Metastatic; June 1, 2024, 5:00pm ET Presenting Author: Ana Garrido-Castro, MD Senior Author: Sara Tolaney, MD, MPH
Drug targeting protein involved in control of immune response produces encouraging results in glioblastoma
A drug that targets a protein involved in regulating the immune response might be promising in patients with glioblastoma, Dana-Farber investigators report. The drug ibudilast inhibits a protein called MIF, which is produced at elevated levels in patients with glioblastoma and can hamper the immune response to cancer. In a phase 1b/2a study, 36 patients with newly diagnosed glioblastoma and 26 patients with recurrent glioblastoma were treated with daily ibudilast and monthly cycles of temozolomide, a chemotherapy agent. The six-month progression-free survival was 44% for patients with newly diagnosed glioblastoma and 31% for those with recurrent glioblastoma. Although the survival rates were comparable to historically reported rates, laboratory research suggests the drug may be more effective in patients with glioblastoma when combined with immunotherapy agents known as checkpoint inhibitors, making this combination a potentially promising therapy.
- Study Title: Phase 1b/2a study evaluating the combination of MN-166 (Ibudilast) and temozolomide in patients with newly diagnosed and recurrent glioblastoma Oral Abstract Number: 2016 Session: Rapid Oral Abstract Session - Central Nervous System Tumors; June 2, 2024, 1:00pm ET Presenting Author: Gilbert Youssef, MD Senior Author: Patrick Wen, MD
ASCO Awards and Fellows
ASCO recognized Janet L. Abrahm, MD, FACP, FAAHPM, of Dana-Farber and Brigham and Women's Hospital, as a recipient of an ASCO Special Award, the Society's highest honor. Abrahm was awarded the Walther Cancer Foundation Supportive Oncology Award, noting her as a distinguished leader in palliative and supportive oncology through the prevention, assessment, and management of cancer- and treatment-related suffering.
Each year ASCO also recognizes members with the Fellow of the American Society of Clinical Oncology (FASCO) distinction. This year Narjust Florez, MD, FASCO, and Shail Maingi, MD, FASCO, are among the recipients recognized for their extraordinary volunteer service, dedication, and commitment to ASCO.
For all ASCO-related media inquiries, call, or email Victoria Warren, 617-939-5531 , [email protected] . Follow the meeting live on X using the hashtag #ASCO24 and follow Dana-Farber News on X at @DanaFarberNews.
Media Contacts
If you are a journalist and have a question about this story, please call 617-632-4090 and ask to speak to a member of the media team, or email [email protected] .
The Media Team cannot respond to patient inquiries. For more information, please see Contact Us .

Dana-Farber Cancer Institute research presented at ASCO 2024.
- Introduction
- Conclusions
- Article Information
PNE indicates pain neuroscience education.
The KOOS 4 primary outcome includes the subscales pain, symptoms, function of daily living, and knee-related quality of life; scores range from 0 to 100, with higher scores indicating better outcomes. Data points are means; error bars represent 95% CI. PNE indicates pain neuroscience education.
The KOOS 4 primary outcome includes the subscales pain, symptoms, function of daily living, and knee-related quality of life; scores range from 0 to 100, with higher scores indicating better outcomes. Positive scores indicate improvements in KOOS 4 , and negative scores indicate a decline in KOOS 4 . PNE indicates pain neuroscience education.
Trial Protocol and Statistical Analysis Plan
eAppendix 1. CONSORT Checklist for Randomized Trials
eAppendix 2. TIDieR Checklist for Information to Include When Describing an Intervention
eAppendix 3. CERT Checklist for What to Include When Reporting Exercise Programs
eMethods 1. Pain Neuroscience Education Session 1
eMethods 2. Pain Neuroscience Education Session 2
eTable 1. Patient Baseline Characteristics for Those Attending the 12-Month Follow-up Assessment and Those who Did Not Attend
eTable 2. Intention-to-Treat Analysis for Risk Ratios for Usage of Pain Medication From Baseline to 12 Months
eTable 3. Per-Protocol Analysis for the Primary and Secondary Outcomes for Change From Baseline to 12 Months
eTable 4. Per-Protocol Analysis for the Changes in Usage of Pain Medication From Baseline to 12 Months
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Larsen JB , Skou ST , Laursen M , Bruun NH , Arendt-Nielsen L , Madeleine P. Exercise and Pain Neuroscience Education for Patients With Chronic Pain After Total Knee Arthroplasty : A Randomized Clinical Trial . JAMA Netw Open. 2024;7(5):e2412179. doi:10.1001/jamanetworkopen.2024.12179
Manage citations:
© 2024
- Permissions
Exercise and Pain Neuroscience Education for Patients With Chronic Pain After Total Knee Arthroplasty : A Randomized Clinical Trial
- 1 Musculoskeletal Health and Implementation, Department of Health Science and Technology, Faculty of Medicine, Aalborg University, Aalborg, Denmark
- 2 Research Unit for Musculoskeletal Function and Physiotherapy, Department of Sports Science and Clinical Biomechanics, University of Southern Denmark, Odense, Denmark
- 3 The Research and Implementation Unit PROgrez, Department of Physiotherapy and Occupational Therapy, Næstved-Slagelse-Ringsted Hospitals, Region Zealand, Denmark
- 4 Orthopedic Surgery Research Unit, Aalborg University Hospital, Aalborg, Denmark
- 5 Research Data and Biostatistics, Aalborg University Hospital, Aalborg, Denmark
- 6 Translational Pain Biomarkers, Department of Health Science and Technology, Faculty of Medicine, Aalborg University, Aalborg, Denmark
- 7 ExerciseTech, Department of Health Science and Technology, Faculty of Medicine, Aalborg University, Aalborg, Denmark
Question What is the effect of neuromuscular exercise and pain neuroscience education compared with pain neuroscience education alone on pain and function in patients with chronic pain for more than 1 year after total knee arthroplasty?
Findings In this randomized clinical trial of 69 patients, neuromuscular exercise and pain neuroscience education did not provide superior pain and function outcomes compared with pain neuroscience education alone, although approximately one-third of all patients experienced clinically important improvements.
Meaning Findings from this study suggest that neuromuscular exercise and pain neuroscience education do not provide superior pain and function outcomes compared with pain neuroscience education alone, but clinically important improvements in pain and function can be elicited in patients with chronic pain after total knee arthroplasty.
Importance Up to 20% of patients develop chronic pain after total knee arthroplasty (TKA), yet there is a scarcity of effective interventions for this population.
Objective To evaluate whether neuromuscular exercise and pain neuroscience education were superior to pain neuroscience education alone for patients with chronic pain after TKA.
Design, Setting, and Participants A superiority randomized clinical trial was conducted at 3 outpatient clinics at Aalborg University Hospital in Denmark. Participants with moderate-to-severe average daily pain intensity and no signs of prosthesis failure at least 1 year after primary TKA were included. Participant recruitment was initiated on April 12, 2019, and completed on October 31, 2022. The 12-month follow-up was completed on March 21, 2023.
Interventions The study included 24 sessions of supervised neuromuscular exercise (2 sessions per week for 12 weeks) and 2 total sessions of pain neuroscience education (6 weeks between each session) or the same pain neuroscience education sessions alone. The interventions were delivered in groups of 2 to 4 participants.
Main Outcomes and Measures The primary outcome was change from baseline to 12 months using the mean score of the Knee Injury and Osteoarthritis Outcome Score, covering the 4 subscales pain, symptoms, activity of daily living, and knee-related quality of life (KOOS 4 ; scores range from 0 to 100, with higher scores indicating better outcomes). The outcome assessors and statistician were blinded. All randomized participants were included in the intention-to-treat analysis.
Results Among the 69 participants (median age, 67.2 years [IQR, 61.2-71.9 years]; 40 female [58%]) included in the study, 36 were randomly assigned to the neuromuscular exercise and pain neuroscience education group, and 33 to the pain neuroscience education–alone group. The intention-to-treat analysis showed no between-group difference in change from baseline to 12 months for the KOOS 4 (7.46 [95% CI, 3.04-11.89] vs 8.65 [95% CI, 4.67-12.63] points; mean difference, −1.33 [95% CI, −7.59 to 4.92]; P = .68). Among the 46 participants who participated in the 12-month assessment in the 2 groups, 16 (34.8%) experienced a clinically important improvement (a difference of ≥10 points on the KOOS 4 ) with no between-group difference. No serious adverse events were observed.
Conclusions and Relevance In this randomized clinical trial, the results demonstrated that neuromuscular exercises and pain neuroscience education were not superior to pain neuroscience education alone in participants with chronic pain after TKA. Approximately one-third of the participants, regardless of intervention, experienced clinically important improvements. Future studies should investigate which patient characteristics indicate a favorable response to exercises and/or pain neuroscience education.
Trial Registration ClinicalTrials.gov Identifier: NCT03886259
End-stage knee osteoarthritis is commonly treated with total knee arthroplasty (TKA). 1 In 2018, more than 715 000 TKAs were performed in the US, 2 and the number is expected to rise to 1.9 million annually by 2030. 3 Most patients undergoing TKA surgery will experience a positive outcome in terms of pain relief and improved functional performance, but 15% to 20% of patients will develop chronic pain after TKA. 4 , 5 Chronic pain after TKA is defined as pain present for at least 3 to 6 months following surgery. 6
Patients have described the chronic pain after TKA as extreme, constant, and requiring maximal effort to endure. 7 Furthermore, activities of daily living (eg, walking and stair climbing) are impaired in patients with chronic pain after TKA when compared with patients with knee osteoarthritis prior to surgery. 8
Chronic pain after TKA is considered multifactorial and can be influenced by physiological factors, such as central pain mechanisms, and psychosocial factors. 6 , 8 There is a scarcity of high-quality evidence and guidelines on effective treatments of chronic pain after TKA. 6 , 9 The lack of evidence-based treatment guidelines leads to inadequate access to optimal treatment and the risk of patients feeling abandoned by the health care system. 10
Studies have evaluated the inclusion of early postoperative exercises to avoid patients developing chronic pain after TKA but have not found this approach effective. 11 , 12 However, a combination of exercise and education treatment modalities could induce beneficial treatment effects in patients with chronic pain after TKA, 13 but to our knowledge, this has never been investigated.
Therefore, we conducted a superiority randomized clinical trial with the purpose of investigating whether a 12-week treatment consisting of neuromuscular exercise and pain neuroscience education (PNE) would prove superior in terms of improving pain and function compared with receiving PNE alone. It was hypothesized that the participants randomized to neuromuscular exercise and PNE would improve significantly more from baseline to 12 months compared with participants randomized to PNE alone.
The study was designed as a parallel-group superiority randomized clinical trial, entitled the NEPNEP (Neuromuscular Exercises and Pain Neuroscience Education for Chronic Pain) trial. An open access study protocol was published to ensure research quality and transparency. 14 The trial followed the Consolidated Standards of Reporting Trials ( CONSORT ) reporting guideline for randomized clinical trials. 15 The patient flow diagram is provided as Figure 1 , and the trial protocol is provided in Supplement 1 . The CONSORT, Template for Intervention Description and Replication (TIDieR), and Consensus on Exercise Reporting Template (CERT) checklists are provided in eAppendices 1-3, respectively, in Supplement 2 . The trial was approved by the North Denmark Region Committee on Health Research Ethics. All participants signed informed consent before inclusion in the trial.
Participants were recruited from Aalborg University Hospital (Aalborg, Denmark), which included 3 hospital sites in Farsoe, Thisted, and Aalborg. The hospital research database was used to identify participants who underwent TKA at least 1 year before recruitment. Eligible participants were contacted by mail and telephone and invited to participate in the study. Participants willing to enroll and meeting the eligibility criteria of primary TKA due to knee osteoarthritis 12 months or longer after their surgery and, in the index knee, chronic pain for longer than 6 months and an average daily pain score of 4 or more (moderate to severe pain) on a numeric rating scale (ranging from 0 to 10, in which 0 is no pain, and 10 is maximum pain) over the last week were included. The major exclusion criteria were chronic pain due to loosening of an implant or a prosthesis failure requiring revision surgery or primary pain area other than the index knee (eg, low back pain or upper extremity pain). A full list of eligibility criteria can be found in the study protocol ( Supplement 1 ). 14 Participants received the interventions at 1 of the 3 outpatient clinics at Aalborg University Hospital (Farsoe, Thisted, and Aalborg) dependent on their geographical preferences and on which day and time for exercise and PNE suited them best. Recruitment was initiated on April 12, 2019, and completed on October 31, 2022. The 12-month follow-up was completed on March 21, 2023.
Two patients with chronic pain after TKA assisted in designing the trial from a patient perspective. The patients gave feedback concerning study procedures, interventions, and outcome measures and how to describe and explain the study in layperson’s terms to possible participants.
The participants were randomized in a 1:1 ratio and allocated to 1 of 2 intervention arms, neuromuscular exercises and PNE or PNE alone. Randomization with treatment group concealment was done by the project manager (J.B.L.) by using computer-generated random numbers in permuted blocks of 4 to 8 participants. Outcome assessment was performed by trained outcome assessors (not involved in the study), who were masked toward treatment allocation. The statistician (N.H.B.) conducting the analysis was masked toward group allocation.
The neuromuscular exercises and PNE group received a 12-week neuromuscular exercise program 16 and PNE. The neuromuscular exercise program has previously been found feasible for patients following TKA surgery. 17 One-hour group-based sessions consisting of 2 to 4 participants were held twice a week (24 sessions in total). Sessions were supervised by trained physiotherapists and included individualization of the exercise difficulty considering each participant’s physical ability and pain intensity. Full details of the neuromuscular exercise program can be found in the study protocol ( Supplement 1 ). 14
The PNE consisted of two 1-hour group-based educational sessions. The first session was held before the first exercise session for the neuromuscular exercise and PNE group, and the second session took place 6 weeks later. A physiotherapist trained in PNE (J.B.L.) delivered the sessions to both groups. Both intervention groups received the same content in the PNE sessions. The overall aim of PNE was to change maladaptive pain cognitions, enabling the participants to reconceptualize their pain 18 and thereby engage in self-management of their symptoms. Following both PNE sessions, a short information leaflet, summarizing the PNE topics, was given to the participants. Content for the PNE sessions can be found in eMethods 1 and 2 in Supplement 2 . Assessments of outcomes were conducted at baseline and at 3, 6, and 12 months.
The primary outcome was prespecified and reported in the study protocol 14 and the statistical analysis plan. 19 The primary outcome was the between-group change from baseline to 12 months for the Knee Injury and Osteoarthritis Outcome Score (KOOS), using the mean score of the 4 subscales: pain, symptoms, activities of daily living, and knee-related quality of life (KOOS 4 ). The subscales, which include a fifth dimension—sport and recreation—are scored on a 5-point Likert scale; the total is converted into a range of 0 (worst) to 100 (best). 20 , 21 A prespecified minimum clinically important difference of 10 points was used to indicate whether a clinically relevant between-group improvement from baseline to the 12-month follow-up had occurred. 22 The KOOS questionnaire has shown validity, reliability, and responsiveness as a patient-reported outcome measure following TKA. 23
Six prespecified secondary outcomes were evaluated as between-group changes using the mean difference from baseline to a 12-month follow-up. 19 All 5 KOOS subscales, including the sport and recreation subscale, were reported individually to support the clinical interpretation of the primary outcome. 22 The overall change in a participant’s knee condition was measured using the global perceived effect scale by their answer to the question: “How are your knee problems now compared with before you entered this study?” The global perceived effect scale was administered on a 7-point Likert scale ranging from 1 (improved, an important improvement) to 7 (worse, an important worsening). The global perceived effect scale has shown excellent reliability. 24 Three physical performance tests were included. 25 The time to complete the 40-m fast-paced walk test and the stair-climb test, a test of ascending and descending 9 steps on a staircase, was recorded. For the 30-second chair-stand test, the maximum number of chair-rise repetitions within 30 seconds was registered. 25 The physical performance tests have been found reliable. 26 , 27 Use of pain medication was evaluated by asking participants whether they had used pain medication over last week (yes or no). Adverse events occurring during the trial period were registered as either serious or nonserious events by participant self-report and/or by the physiotherapists supervising the neuromuscular exercises. Serious adverse events were defined according to the definitions from the US Food and Drug Administration, and nonserious adverse events comprised all other events. 28 Other treatments initiated because of the index knee received during the trial period were registered by self-reporting from the participants.
A statistical analysis plan was published and available before the 12-month follow-up, and any analyses were initiated. 19 The analyses were conducted as predefined in the statistical analysis plan. To avoid the risk of misleading interpretation, the results from the intention-to-treat analysis were presented to the author group in a blinded version (coded as group A and group B). In writing, the authors agreed on 2 separate interpretations of the results, 29 and documentation for the interpretations was registered online. 30 After finalizing the interpretations, the randomization code was broken, and the appropriate interpretation was chosen.
For this superiority randomized clinical trial, a sample-size calculation was conducted to estimate the sample size required to detect a between-group minimum clinically important difference in change of 10 points from baseline to the 12-month follow-up for the KOOS 4 (with an SD of 15). 17 , 22 The calculation revealed that 49 participants were required in both groups to achieve a study power of 90% from baseline to the 12-month follow-up for the between-group comparison, using a 2-sided significance level of .05. To account for a possible loss to follow-up of 20%, a total of 60 participants in each group were planned to be enrolled. However, the trial was impacted by the COVID-19 pandemic, making recruitment particularly difficult and causing a higher dropout rate than anticipated. Therefore, we were not able to recruit the preplanned number of participants and decided to stop recruitment after recruiting for 42 months.
The main analysis consisted of the between-group differences in mean change from baseline to the 12-month follow-up. Analysis of all outcomes was performed according to the intention-to-treat principle. Furthermore, a prespecified per-protocol analysis was conducted, including participants who participated in at least 18 of 24 (75%) neuromuscular exercise sessions and participated in both PNE sessions (valid for both groups).
Data were checked for normal distribution by reviewing data frequency in histograms and tests for normality (Shapiro-Wilk). Based on the observations, median and IQR were recorded. For the primary and secondary outcomes (except use of pain medication), repeated measures mixed-effects models were applied, with participants as the random effect and time for visit (baseline and 3, 6, and 12 months) and treatment arm (neuromuscular exercises and PNE or PNE alone) as fixed effects, with adjustment for baseline imbalance. Interaction between follow-up and treatment arm was also included in the models. Two models are reported: model 1, adjusted for participant, follow-up, treatment arm, and interaction between follow-up and treatment arm; model 2 further included adjustment for age, sex, and body mass index. The between-group comparison for use of pain medication within the last week was dichotomized as yes or no, and relative risks were analyzed using a Poisson regression model with robust error variance. No analysis for difference in adverse events was required because no adverse events were registered in the PNE-alone group.
A prespecified responder analysis was conducted to illustrate the proportion of participants in the 2 intervention groups who experienced a minimum clinically important difference of at least 10 points in KOOS 4 . The proportions were compared using a χ 2 test.
For all outcomes, 95% CIs are presented. A 95% CI, including 10 points or more for the primary outcome, KOOS 4 , was interpreted as a clinically meaningful difference. 22 A 2-sided P < .05 was considered significant. All analyses were performed in Stata, version 18 (StataCorp LLC).
A total of 69 patients (median age, 67.2 years [IQR, 61.2-71.9 years]; 40 female [58%]) and 29 male [42%]) were recruited. Overall, 435 patients were assessed for eligibility ( Figure 1 ). Of these, 364 were excluded, leaving 71 eligible for inclusion; 2 patients withdrew before randomization. Thirty-six participants were randomized to receive neuromuscular exercises and PNE and 33 participants to receive PNE alone. The participants’ baseline characteristics were comparable ( Table 1 ). 31 The mean body mass index in our population was greater than 33 (calculated as weight in kilograms divided by height in meters squared), most participants had at least 1 comorbidity, and there was a group-average score in the Hospital Anxiety and Depression Scale 31 that indicated clinical depression.
All participants were included in the intention-to-treat analysis. Twenty-three participants (64%) in the neuromuscular exercises and PNE group and 26 (79%) in the PNE-alone group adhered to the intervention and were included in the per-protocol analysis. The completion rates for the 12-month follow-up assessment were 24 of 36 participants (67%) for the neuromuscular exercises and PNE group and 22 of 33 (67%) for the PNE-alone group. Dropout reasons are reported in Figure 1 . The baseline characteristics for the participants adhering to the 12-month assessment and the participants lost to follow-up were comparable (eTable 1 in Supplement 2 ).
The intention-to-treat analysis showed no between-group difference in improvement from baseline to the 12-month follow-up for the primary outcome KOOS 4 , illustrated by an adjusted mean difference of −1.33 (95% CI, −7.59 to 4.92; P = .68) ( Figure 2 ). Both groups experienced significant improvements in KOOS 4 from baseline to the 12-month follow-up, with the neuromuscular exercise and PNE group improving 7.46 points (95% CI, 3.04-11.89; P = .001) and the PNE-alone group improving 8.65 points (95% CI, 4.67-12.63; P < .001) ( Table 2 ).
The responder analysis showed that 8 of 24 participants (33.3%) in the neuromuscular exercise and PNE group and 8 of 22 participants (36.4%) in the PNE-alone group (16 of 46 total participants [34.8%]) experienced clinically important improvements (10 points) from baseline to the 12-month follow-up for the primary outcome KOOS 4 . Individual changes in KOOS 4 from baseline to 12 months are shown in Figure 3 . There was no difference in the proportion of responders between the groups (relative risk, 1.09; 95% CI, 0.49-2.41; P = .83).
There were no significant between-group differences in change in the 5 KOOS subscales of pain, symptoms, activity of daily living, sport and recreation, and knee-related quality of life; the global perceived effect; time to complete the 40-m fast-paced walk test and the stair-climb test; or numbers of repetitions in the 30-second chair-stand test ( Table 2 ). Nor was there a significant between-group difference for use of pain medication (relative risk, 1.02; 95% CI, 0.73-1.43; P = .92) (eTable 2 in Supplement 2 ). Both groups experienced significant within-group improvements in all outcomes except use of pain medication, in which neither group showed an improvement; the KOOS subscale sport and recreation, in which the neuromuscular exercise and PNE group showed no improvement; and the 40-m fast-paced walk test, in which the PNE-alone group showed no improvement.
No serious adverse events were registered in either of the intervention groups during the trial. For the neuromuscular exercise and PNE group, 5 nonserious adverse events were registered during the trial: 4 participants experienced increased pain intensity, and 1 participant experienced swelling in the index knee following a neuromuscular exercise session, which subsided after a few days and did influence the next neuromuscular exercise session. No nonserious adverse events were registered in the PNE-alone group. No participants in either group reported that they had received other treatments during the trial period. The per-protocol analysis revealed no differences in changes from baseline to 12 months for neither the primary nor the secondary outcomes (eTables 3 and 4 in Supplement 2 ).
To our knowledge, the NEPNEP trial is the first randomized clinical trial evaluating exercise and education for patients with chronic pain after TKA. Our results revealed that neuromuscular exercise and PNE were not superior to PNE alone for the primary outcome KOOS 4 in patients with chronic pain after TKA or for any of the secondary outcomes. Consequently, the results did not support the hypothesis that neuromuscular exercises and PNE would lead to greater improvements in pain and function than would PNE alone. We observed clinically important improvements in approximately one-third (34.7%) of the participants with chronic pain after TKA, regardless of treatment allocation.
Studies evaluating the effect of treatments introduced in the early postoperative period 32 - 37 have not considered that patients who undergo TKA often experience spontaneous improvements in pain between 3 and 9 months after surgery. 38 Hence, the observed treatment effects could have been influenced by the natural course of improvement after TKA and are therefore not generalizable to patients with chronic pain more than 1 year after TKA. Our findings contribute insight into the treatment of the patients who do not experience spontaneous improvements postoperatively and still experience chronic pain for at least 1 year after their TKA surgery.
Qualitative research has shown that patients with chronic pain after TKA feel abandoned by the health care system and the lack of treatment options. Therefore, patients experience their pain as something they are stuck with and that nothing more can be done. 10 Our results challenge that perception. Given that both intervention groups experienced similar outcomes, the introduction of PNE as treatment could be of particular importance. By providing PNE, patients might realize the factors they can influence themselves, which could lead to improved self-management.
As illustrated in Figure 2 , the neuromuscular exercise and PNE group exhibited an improvement in KOOS 4 immediately after the 3-month supervised exercise therapy program. While the neuromuscular exercise and PNE-alone group largely maintained their improvements until the 12-month follow-up, the PNE group gradually improved from baseline to 12 months. This could indicate that exercising is effective when performed with effects diminishing over time, similarly to findings within hip and knee osteoarthritis. 39 , 40 Therefore, it would be valuable to investigate whether a longer period of exercise therapy or booster sessions could provide sustained improvements.
The KOOS was chosen as the primary outcome, as it is imperative to consider the patient perspective when evaluating treatment effect. 41 , 42 The psychometric properties of KOOS have been scrutinized, with some findings indicating the need for further validation 42 and other findings consolidating its validity and reliability. 23 , 41 However, the KOOS remains a frequently used patient-reported outcome measure for patients undergoing TKA. 17 , 43 , 44
As illustrated in Figure 3 , participants from both groups experienced large improvements in KOOS 4 , highlighting that some participants benefited substantially from neuromuscular exercise and PNE or PNE alone. On the contrary, other participants in both groups experienced little improvement or even a worsening in KOOS 4 . This supports the need for individualized approaches when seeking the best possible treatment. Future research should investigate which patient characteristics indicate a favorable response to exercises and PNE and who might not benefit from either. 45
The mean body mass index in our population was greater than 33, most participants had at least 1 comorbidity, and there was a group-average score in the Hospital Anxiety and Depression Scale 31 that indicated clinical depression. These factors have previously been associated with chronic pain after TKA 45 and emphasize the complexity of the studied population. Given the multiple factors influencing chronic pain and the characteristics of the population, a biopsychosocial and multimodal treatment approach should be considered for patients with chronic pain after TKA. 6 , 10
This trial has some limitations. The study was affected by the COVID-19 pandemic and failed in recruiting the target sample size. However, when taking the small between-group differences into consideration, it seems unlikely that a fully powered study would change the conclusion of no between-group differences. Moreover, considering that the study did not include a no-treatment control group, the true effects of neuromuscular exercises and/or PNE could not be determined. Therefore, the findings could represent fluctuations in pain intensity over time. Long-term follow-up studies have observed that some patients experience pain fluctuations after TKA, whereas other patients’ chronic pain remains stable over time. 38
The results of this randomized clinical trial suggest that neuromuscular exercises and PNE were not superior to PNE alone for the primary outcome on pain, symptoms, function, and knee-related quality of life or any of the secondary outcomes in participants with chronic pain after TKA. The study demonstrated clinically relevant improvements in approximately one-third of the participants, regardless of intervention group. This finding challenges the perception that nothing can be done to relieve pain in patients with chronic pain after TKA. Therefore, the results could have important implications for the future management of patients with chronic pain after TKA. Despite the contributions of this study, an evidence gap for the treatment and management of patients with chronic pain after TKA remains and should be further addressed in future research.
Accepted for Publication: March 15, 2024.
Published: May 24, 2024. doi:10.1001/jamanetworkopen.2024.12179
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Larsen JB et al. JAMA Network Open .
Corresponding Author: Jesper B. Larsen, PhD, Musculoskeletal Health and Implementation, Department of Health Science and Technology, Aalborg University, Selma Lagerløfs Vej 249, 9260 Gistrup, Denmark ( [email protected] ).
Author Contributions: Dr Larsen and Mr Bruun had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Larsen, Skou, Laursen, Arendt-Nielsen, Madeleine.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Larsen, Skou, Arendt-Nielsen, Madeleine.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Larsen, Bruun.
Obtained funding: Larsen, Skou, Arendt-Nielsen, Madeleine.
Administrative, technical, or material support: Larsen, Laursen, Madeleine.
Supervision: Skou, Laursen, Arendt-Nielsen, Madeleine.
Conflict of Interest Disclosures: Prof Skou reported receiving grants from the European Research Council as payment to the University of Southern Denmark and from Region Zealand (Exercise First) for payment to the Næstved-Slagelse-Ringsted Hospital, receiving personal fees from Munksgaard as royalties for book chapters and from TrustMe-Ed as royalties for online lectures, and receiving honoraria from Nestlé Health Science for 1 presentation at a webinar on osteoarthritis outside the submitted work and reported being cofounder of GLA:D, a not-for-profit initiative hosted at the University of Southern Denmark aimed at implementing clinical guidelines for osteoarthritis in clinical practice. No other disclosures were reported.
Funding/Support: This work was supported by grant R168-A5619 from the Danish Rheumatism Association and by the Svend Andersen Foundation and the Lions Club Danmark (Dr Larsen) and by grants 801790 for payment to the University of Southern Denmark and 945377 for payment to the Næstved-Slagelse-Ringsted Hospital from the European Union’s Horizon 2020 research and innovati on program (Prof Skou).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 3 .
Additional Contributions: We thank the patients for their participation in the trial. We acknowledge the Department of Occupational Therapy and Physiotherapy, Aalborg University Hospital, Denmark, for administrative and logistic support and the Department of Orthopedic Surgery, Aalborg University Hospital, Denmark, for its involvement in recruiting patients.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gliklich RE, Dreyer NA, Leavy MB, editors. Registries for Evaluating Patient Outcomes: A User's Guide [Internet]. 3rd edition. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Apr.

Registries for Evaluating Patient Outcomes: A User's Guide [Internet]. 3rd edition.
- Hardcopy Version at Agency for Healthcare Research and Quality
13 Analysis, Interpretation, and Reporting of Registry Data To Evaluate Outcomes
1. introduction.
Registries have the potential to produce databases that are an important source of information regarding health care patterns, decisionmaking, and delivery, as well as the subsequent association of these factors with patient outcomes. Registries, for example, can provide valuable insight into the safety and/or effectiveness of an intervention or the efficiency, timeliness, quality, and patient centeredness of a health care system. The utility and applicability of registry data rely heavily on the quality of the data analysis plan and its users' ability to interpret the results. Analysis and interpretation of registry data begin with a series of core questions:
- Study purpose : Were the objectives/hypotheses predefined or post hoc?
- Patient population : Who was studied?
- Data quality : How were the data collected, reviewed, and verified?
- Data completeness : How were missing data handled?
- Data analysis : How were the analyses chosen and performed?
While registry data present many opportunities for meaningful analysis, there are inherent challenges to making appropriate inferences. A principal concern with registries is that of making inferences without regard to the quality of data, since quality standards have not been previously well established or consistently reported. In some registries, comparison groups may not be robustly defined, and information provided about the external validity of a registry sample is often limited. These factors must be considered when making inferences based on analyses of registry data. 1
This chapter explains how analysis plans are constructed for registries, how they differ depending on the registry's purpose, and how registry design and conduct can affect analysis and interpretation. The analytic techniques generally used for registry data are presented, addressing how conclusions may be drawn from the data and what caveats are appropriate. The chapter also describes how timelines for data analysis can be built in at registry inception and how to determine when the registry data are complete enough to begin analysis.
2. Hypotheses and Purposes of the Registry
While it may be relatively straightforward to develop hypotheses for registries intended to evaluate safety and effectiveness, not all registries have specific, testable, or simple hypotheses. Disease registries commonly have aims that are primarily descriptive, such as describing the typical clinical features of individuals with a disease, variations in phenotype, and the clinical progression of the disease over time (i.e., natural history). These registries play a particularly important role in the study of rare diseases.
In the case of registries where the aim is to study the associations between specific exposures and outcomes, prespecification of the study methodology and presence or absence of a priori hypotheses or research questions may affect the acceptance of results of studies derived from registry data. The many possible scenarios are well illustrated by examples at the theoretical extremes.
On one extreme, a study may evolve out of a clear and explicit prespecified research question and hypothesis. In such a study, there may have been preliminary scientific work that laid the conceptual foundation and plausibility for the proposed study. The investigators fully articulate the objectives and analytic plan before embarking on any analysis. The outcome is clearly defined and the statistical approach documented. Secondary analyses are identified and may be highlighted as hypothesis generating. The investigators have no prior knowledge of analyses in this database that would bias them in the formulation of their study objective. The study is conducted and published regardless of the result. The paper states clearly that the objective and hypothesis were prespecified. For registries intended to support national coverage determinations with data collection as a condition of coverage, the specific coverage decision question may be specified a priori as the research question in lieu of a formal hypothesis.
At the other extreme, a study may evolve out of an unexpected observation in a database in the course of doing analyses for another purpose. A study could also evolve from a concerted effort to discover associations—for example, as part of a large effort to understand disease causation. In such a study, the foundation for the study is developed post hoc, or after making the observation. Because of the way in which the observation was found, the rationale for the study is developed retrospectively. The paper publishing this study's results does not clearly state that the objective and hypothesis were not prespecified.
Of course, many examples fall between these extremes. An investigator may suspect an association for many variables but find the relationship for only one of them. The investigator decides to pursue only the positive finding and develop a rationale for a study or grant. The association was sought, but it was sought along with associations for many other variables and outcomes.
Thus, while there is substantial debate about the importance of prespecified hypotheses, 2 , 3 there is general agreement that it is informative to reveal how the study was developed. Transparency in methods is needed so that readers may know whether these studies are the result of hypotheses developed independently of the study database, or whether the question and analyses evolved from experience with the database and multiple iterations of exploratory analyses. Both types of studies have value.
3. Patient Population
The purpose of a registry is to provide information about a specific patient population to which all study results are meant to apply. To determine how well the study results apply to the target population, five populations, each of which is a subset of the preceding population, need to be considered, along with how well each population represents the preceding population. These five subpopulations are shown in Figure 13–1 .
Figure 13–1
Patient populations.
The target population is defined by the study's purpose. To assess the appropriateness of the target population, one must ask the question, “Is this really the population that we need to know about?” For example, the target population for a registry of oral contraceptive users would include women of childbearing age who could become pregnant and are seeking to prevent pregnancy. Studies often miss important segments of the population in an effort to make the study population more homogeneous. For example, a study to assess a medical device used to treat patients for cardiac arrhythmias that defines only men as its target population would be less informative than it could be, because the device is designed for use in both men and women.
The accessible population is defined using inclusion criteria and exclusion criteria. The inclusion criteria define the population that will be used for the study and generally include geographic (e.g., hospitals or clinics in the New England region), demographic, disease-specific, and temporal (e.g., specification of the included dates of hospital or clinic admission), as well as other criteria. Conversely, the exclusion criteria seek to eliminate specific patients from study and may be driven by an effort to assure an adequate-sized population of interest for analysis. The same goals may be said of inclusion criteria, since it is difficult to separate inclusion from exclusion criteria (e.g., inclusion of adults aged 18 and older vs. exclusion of children younger than 18).
The accessible population may lose representativeness to the extent that convenience plays a part in its determination, because people who are easy to enroll in the registry may differ in some critical respects from the population at large. Similarly, to the extent that homogeneity plays a part in determining the accessible population, it is less likely to be representative of the entire population because certain population subgroups will be excluded.
Factors to be considered in assessing the accessible population's representativeness of the target population include all the inclusion and exclusion criteria mentioned above. One method of evaluating representativeness is to describe the demographics and other key descriptors of the registry study population and to contrast its composition with patients with similar characteristics who are identified from an external database, such as might be obtained from health insurers, health maintenance organizations, or the U.S. Surveillance Epidemiology and End Results (SEER) cancer registries. 4
However, simple numerical/statistical representativeness is not the main issue. Representativeness should be evaluated in the context of the purpose of the study—that is, whether the study results can reasonably be generalized or extrapolated to other populations of interest outside of those included in the accessible population. (See Case Example 26 .) For example, suppose that the purpose of the study is to assess the effectiveness of a drug in U.S. residents with diabetes. If the accessible population includes no children, then the study results may not apply to children, since children often metabolize drugs very differently from adults.
On the other hand, consider the possibility that the accessible population is generally drawn from a geographically isolated region, whereas the target population may be the entire United States or the world. In that case, the accessible population is not geographically representative of the target population, but that circumstance would have little or no impact on the representativeness of the study findings to the target population if the action of the drug (or its delivery) does not vary geographically (which we would generally expect to be the case, unless pertinent racial/genetic or dietary factors were involved). Therefore, in this example, the lack of geographical representativeness would not affect interpretation of results.
The reason for using an intended population rather than the whole accessible population for the study is simply a matter of convenience and practicality. The issues to consider in assessing how well the intended population represents the accessible population are similar to those for assessing how well the accessible population represents the target population. The main difference is that the intended population may be specified by a sampling scheme, which often tries to strike a balance among representativeness, convenience, and budget. If the intended population is a random sample of the accessible population, it may be reasonably assumed that it will represent the accessible population; however, for many, if not most, registries, a complete roster of the accessible population does not exist. More commonly, the intended population is compared with the accessible population in terms of pertinent variables.
To the extent that convenience or other design (e.g., stratified random sample) is used to choose the intended population, one must consider the extent to which the sampling of the accessible population—by means other than random sampling—has decreased the representativeness of the intended population. For example, suppose that, for the sake of convenience, only patients who attend clinic on Mondays are included in the study. If patients who attend clinic on Mondays are similar in every relevant respect to other patients, that may not constitute a limitation. But if Monday patients are substantially different from patients who attend clinic on other days of the week (e.g., well-baby clinics are held on Mondays) and if those differences affect the outcome that is being studied (e.g., proportion of baby visits for “well babies”), then that sampling strategy would substantially alter the interpretations from the registry and would be considered a meaningful limitation.
The extent to which the actual population is not fully representative of the intended population is generally a matter of real-world issues that prevent the initial inclusion of study subjects or adequate followup. In assessing representativeness, one must consider the likely underlying factors that caused those subjects not to be included in the analysis of study results and how that might affect the interpretations from the registry. For example, consider a study of a newly introduced medication, such as an anti-inflammatory drug that is thought to be as effective as other products and to have fewer side effects but that is more costly. Inclusion in the actual population may be influenced by prescribing practices governed by a health insurer. For example, if a new drug is approved for reimbursement only for patients who have “failed” treatment with other anti-inflammatory products, the resulting actual population will be systematically different from the target population of potential anti-inflammatory drug users. The actual population may be refractory to treatment or may have more comorbidities (e.g., gastrointestinal problems), and may be specifically selected for treatment beyond the intention of the study-specified inclusion criteria. In fact, registries of newly introduced drugs and devices may often include patients who are different from the ultimate target population.
Finally, the analytic population includes all those patients who meet the criteria for analysis. In some cases, it becomes apparent that there are too few cases of a particular type, or too few patients with certain attributes, such that these subgroups do not contribute enough information for meaningful analysis. Patients may also be excluded from the analysis population because their conditions are so rare that to include them could be considered a breach of patient confidentiality. Analytic populations are also created to meet specific needs. For example, an investigator may request a data set that will be used to analyze a subset of the registry population, such as those who had a specific treatment or condition.
A related issue is that of early adopters , 5 in which practitioners who are quick to use a novel health care intervention or therapy differ from those who use it only once it is well established. For example, a registry of the use of a new surgical technique may initially enroll largely academic physicians and only much later enroll community-based surgeons. If the outcomes of the technique differ between the academic surgeons (early adopters) and community-based surgeons (later adopters), then the initial results of the registry may not reflect the true effectiveness of the technique in widespread use. Patients selected for treatment with a novel therapy may also differ with regard to factors such as severity or duration of disease and prior treatment history, including treatment failures. For example, patients with more severe or late-stage disease who have failed other treatments might be more likely to use a newly approved product that has shown efficacy in treating their condition. Later on, patients with less severe disease may start using the product.
Finally, patients who are included in the analytic population for a given analysis of registry data may also be subject to selection or inclusion criteria (admissibility criteria), and these may affect interpretation of the resulting analyses. (See Chapter 18 .) For example, if only patients who remain enrolled and attend followup visits through 2 years after study initiation are included in analysis of adherence to therapy, it is possible or likely that adherence among those who remain enrolled in the study and have multiple followup visits will be different from adherence among those who do not. Differential loss to followup, whereby patients who are lost may be more likely to experience adverse outcomes, such as mortality, than those who remain under observation, is a related issue that may lead to biased results. (See Chapter 3 .)
4. Data Quality Issues
In addition to a full understanding of study design and methodology, analysis of registry events and outcomes requires an assessment of data quality. One must consider whether most or all important covariates were collected, whether the data were complete, and whether the problem of missing data was handled appropriately, as well as whether the data are accurate.
4.1. Collection of All Important Covariates
While registries are generally constructed for a particular purpose or purposes, registry information may be collected for one purpose (e.g., provider performance feedback) and then used for another (e.g., addressing a specific clinical research question). When using an available database for additional purposes, one needs to be sure that all the information necessary to address a specific research question was collected in a manner that is sufficient to answer the question.
For example, suppose the research question addresses the comparative effectiveness of two treatments for a given disease using an existing registry. To be meaningful, the registry should have accurate, well-defined, and complete information, including potential confounding and effect-modifying factors; population characteristics of those with the specified disease; exposures (whether patients received treatment A or B); and patient outcomes of interest. Confounding factors are variables that influence both the exposure (treatment selection) and the outcome in the analyses. These factors can include patient factors (age, gender, race, socioeconomic factors, disease severity, or comorbid illness); provider factors (experience, skills); and system factors (type of care setting, quality of care, or regional effects). While it is not possible to identify all confounding factors in planning a registry, it is desirable to give serious thought to what will be important and how the necessary data can be collected. While effect modification is not a threat to validity, it is important to consider potential effect modifiers for data collection and analysis in order to evaluate whether an association varies within specific subgroups. 6 Analysis of registries requires information about such variables so that the confounding covariates can be accounted for, using one of several analytic techniques covered in upcoming sections of this chapter. In addition, as described in Chapter 3 , eligibility for entry into the registry may be restricted to individuals within a certain range of values for potential confounding factors in order to reduce the effects of these factors. Such restrictions may also affect the generalizability of the registry.
4.2. Data Completeness
Assuming that a registry has the necessary data elements, the next step is to ensure that the data are complete. Missing data can be a challenge for any registry-based analysis. Missing data include situations in which a variable is directly reported as missing or unavailable, a variable is “nonreported” (i.e., the observation is blank), the reported data may not be interpretable, or the value must be imputed to be missing because of data inconsistency or out-of-range results. Before analyzing a registry database, the database should be “cleaned” (discussed in Chapter 11, Section 2.5 .), and attempts should be made to obtain as much missing data as realistically possible from source documents. Inconsistent data (e.g., a “yes” answer to a question at one point and “no” to the same question at another) and out-of-range data (e.g., a 500-year-old patient) should be corrected when possible. Finally, the degree of data completeness should be summarized for the researcher and eventual consumer of analyses from the registry. Detailed examples of sources of incomplete data are described in Chapter 18 .
4.3. Missing Data
The intent of any analysis is to make valid inferences from the data. Missing data can threaten this goal both by reducing the information yield of the study and, in many cases, by introducing bias. A thorough review of types of missing data with examples can be found in Chapter 18 . Briefly, the first step is to understand which data are missing. The second step is to understand why the data are missing (e.g., missing item-response or right censoring). Finally, missing data fall into three classic categories of randomness: 7
- Missing completely at random (MCAR) : Instances where there are no differences between subjects with missing data and those with complete data. In such random instances, missing data only reduce study power without introducing bias.
- Missing at random (MAR) : Instances where missing data depend on known or observed values but not unmeasured data. In such cases, accounting for these known factors in the analysis will produce unbiased results.
- Missing not at random (MNAR) : Here, missing data depend on events or factors not measured by the researcher and thus potentially introduce bias.
To gain insight into which of the three categories of missing data are in play, one can compare the distribution of observed variables for patients with specific missing data to the distribution of those variables for patients for whom those same data are present.
While pragmatically it is difficult to determine whether data are MCAR or MAR, there are, nonetheless, several means of managing missing data within an analysis. For example, a complete case strategy limits the analysis to patients with complete information for all variables. This is the default strategy used in many standard analytic packages (e.g., SAS, Cary, NC). A simple deletion of all incomplete observations, however, is not appropriate or efficient in all circumstances, and it may introduce significant bias if the deleted cases are substantively different from the retained, complete cases (i.e., not MCAR). In observational studies with prospective, structured data collection, missing data are not uncommon, and the complete case strategy is inefficient and not generally used. For example, patients with diabetes who were hospitalized because of inadequate glucose control might not return for a scheduled followup visit at which HbA1c was to be measured. Those missing values for HbA1c would probably differ from the measured values because of the reason for which they were missing, and they would be categorized as MNAR. In an example of MAR, the availability of the results of certain tests or measurements may depend on what is covered by patients' health insurance (a known value), since registries do not typically pay for testing. Patients without this particular measurement may still contribute meaningfully to the analysis. In order to include patients with missing data, one of several imputation techniques may be used to estimate the missing data.
Imputation is a common strategy in which average values are substituted for missing data using strategies such as unconditional and conditional mean, multiple hot-deck , and expectation maximum , among others. 7 , 8 For data that are captured at multiple time points or repeated measures, investigators often “carry forward” a last observation. However, such a technique can be problematic if early dropouts occur and a response variable is expected to change over time or when the effect of the exposure (or treatment) is intermittent. Worst-case imputation is another means of substitution in which investigators test the sensitivity of a finding by substituting a worst-case value for all missing results. While this is conservative, it offers a lower bound to an association rather than an accurate assessment. One particular imputation method that has received significant attention in recent analyses has been termed multiple imputation . Rubin first proposed the idea to impute more than one value for a missing variable as a means of reflecting the uncertainty around this value. 9 The general strategy is to replace a missing value with multiple values from an approximate distribution for missing values. This produces multiple complete data sets for analysis from which a single summary finding is estimated.
There are several issues concerning how prognostic models for decisionmaking can be influenced by data completeness and missing data. 10 Burton and Altman reviewed 100 multivariable cancer prognostic models published in seven leading cancer journals in 2002. They found that the proportion of complete cases was reported in only 39 studies, while the percentage missing for important prognostic variables was reported in 52 studies. Comparison of complete cases with incomplete cases was provided in 10 studies, and the methods used to handle missing data were summarized in 32 studies. The most common techniques used for handling missing data in this review article were (a) complete case analysis (12), (b) dropping variables with high numbers of missing cases from model consideration (6), and (c) using some simple author imputation rule (6). Only one study reported using multiple imputation. The reviewers concluded that there was room for improvement in the reporting and handling of missing data within registry studies
Readers interested in learning more about methods for handling missing data and the potential for bias are directed to other useful resources by Greenland and Finkle, 11 Hernán and colleagues, 12 and Lash, Fox, and Fink. 13
It is important to keep in mind that the impact of data completeness will differ, depending on the extent of missing data and the intended use of the registry. It may be less problematic with regard to descriptive research than research intended to support decisionmaking. For all registries, it is important to have a strategy for how to identify and handle missing data as well as how to explicitly report on data completeness to facilitate interpretation of study results. For more information on other specific types of missing data issues (e.g., left truncation), please see Chapter 18 .
4.4. Data Accuracy and Validation
While observational registry studies are usually not required to meet U.S. Food and Drug Administration and International Conference on Harmonisation standards of Good Clinical Practice developed for clinical trials, sponsors and contract research organizations that conduct registry studies are responsible for ensuring the accuracy of study data to the extent possible. Detailed plans for site monitoring, quality assurance, and data verification should be developed at the beginning of a study and adhered to throughout its lifespan. Chapter 11 discusses in detail approaches to data collection and quality assurance, including data management, site monitoring, and source data verification.
Ensuring the accuracy and validity of data and programming at the analysis stage requires additional consideration. The Office of Surveillance and Epidemiology (OSE) of the Food and Drug Administration's Center for Drug Evaluation and Research uses the manual Standards of Data Management and Analytic Process in the Office of Surveillance and Epidemiology for analyses of databases conducted within OSE; the manual addresses many of these issues and may be consulted for further elaboration on these topics. 14 Topics addressed that pertain to ensuring the accuracy of data just before and during analysis include developing a clear understanding of the data at the structural level of the database and variable attributes; creating analytic programs with careful documentation and an approach to variable creation and naming conventions that is straightforward and, when possible, consistent with the Clinical Data Interchange Standards Consortium initiative; and complete or partial verification of programming and analytic data set creation by a second analyst.
For more detail about validation substudies, please see Chapter 18 .
5. Data Analysis
This section provides an overview of practical considerations for analysis of data from a registry. As the name suggests, a descriptive study focuses on describing frequency and patterns of various elements of a patient population, whereas an analytical study focuses on examining associations between patients or treatment characteristics and health outcomes of interest (e.g., comparative effectiveness).
Statistical methods commonly used for descriptive purposes include those that summarize information from continuous variables (e.g., mean, median) or from categorical variables (e.g., proportions, rates). Registries may describe a population using incidence (the proportion of the population that develops the condition over a specified time interval) and prevalence (the proportion of the population that has the condition at a specific point in time). Another summary estimate that is often used is an incidence rate. The incidence rate (also known as absolute risk) takes into account both the number of people in a population who develop the outcome of interest and the person-time at risk, or the length of time contributed by all people during the period when they were in the population and the events were counted.
For studies that include patient followup, an important part of the description of study conduct is to characterize how many patients are “lost,” or drop out, during the course of the registry, at what point they are lost, and if they return. Lasagna plots are one convenient method to visually assess missing data over time when conducting a longitudinal analysis. 15 Figure 13–2 illustrates key points of information that provide a useful description of losses to followup and study dropouts.
Figure 13–2
The flow of participants into an analysis. Tooth L, Ware R, Bain C. Quality of reporting of observational longitudinal research. Am J Epidemiol 2005; 161(3):280–8. Reprinted with permission. Copyright restrictions apply. By permission of Oxford (more...)
For analytical studies, the association between a risk factor and outcome may be expressed as attributable risk, relative risk, odds ratio, or hazard ratio, depending on the nature of the data collected, the duration of the study, and the frequency of the outcome. Attributable risk, a concept developed in the field of public health and preventive medicine, is defined as the proportion of disease incidence that can be attributed to a specific exposure, and it may be used to indicate the impact of a particular exposure at a population level. The standard textbooks cited here have detailed discussions regarding epidemiologic and statistical methods commonly used for the various analyses supported by registries. 6 , 16 , 17 , 18 , 19
For analytical studies of data derived from observational studies such as registries, it is important to consider the role of confounding. Although those planning a study try to collect as much data as possible to address known confounders, there is always the chance that unknown confounders will affect the interpretation of analyses derived from observational studies. It is important to consider the extent to which bias (systematic error stemming from factors that are related to both the decision to treat and the outcomes of interest [confounders]) could have distorted the results. For example, selective prescribing (confounding by indication) results when people with more severe disease or those who have failed other treatments are more likely to receive newer treatments; these patients are systematically different from other patients who may be treated with the product under study. Misclassification in treatment can result from the patient's incorrect recall of dose, or poor adherence or treatment compliance. Other types of bias include detection bias 20 (e.g., when comparison groups are assessed at different points in time or by different methods), selective loss to followup in which patients with the outcomes of most interest (e.g., sickest) may be more likely to drop out of one treatment group than another, and performance bias (e.g., systematic differences in care other than the intervention under study, such as a public health initiative promoting healthy lifestyles directed at patients who receive a particular class of treatment).
Confounding may be evaluated using stratified analysis, multivariable analysis, sensitivity analyses, and simple or quantitative bias analysis. 12 Appropriate methods should be used to adjust for confounding. For example, if an exposure or treatment varies over time and the confounding variable also varies over time, traditional adjustment using conventional multivariable modeling will introduce selection bias. Marginal structural models use inverse probability weighting to account for time-dependent confounding without introducing selection bias. 21 The extensive information and large sample sizes available in some registries also support use of more advanced modeling techniques for addressing confounding by indication, such as the use of propensity scores to create matched comparison groups, or for stratification or inclusion in multivariable risk modeling. 22 - 25 New methods also include the high-dimensional propensity score (hd-PS) for adjustment using administrative data. 26 The uptake of these approaches in the medical literature in recent years has been extremely rapid, and their application to analyses of registry data has also been broad. Examples are too numerous for a few selections to be fully representative, but registries in nearly every therapeutic area, including cancer, 27 cardiac devices, 28 organ transplantation, 29 and rare diseases, 30 have published the results of analyses incorporating approaches based on propensity scores. As noted in Chapter 3 , instrumental variable methods present opportunities for assessing and reducing the impact of confounding by indication, 31 - 33 but verification of the assumptions are important to ensure that an instrument is valid. 34 Violations in the instrumental variable assumptions or the use of a weak instrument will lead to results more biased than those from conventional methods. 35 While a variety of methods have been developed to address confounding, particularly confounding by indication, residual confounding may still be present even after adjustment; therefore, these methods may not fully control for unmeasured confounding. 35 For specific examples of the application of these methods, please see Chapter 18 . Information bias, such as misclassification, and selection bias are also threats to the validity of our findings and examples can be found in Chapter 18 . For further information on how to quantify bias, please see Lash, Fox, and Fink. 13
Groupings within a study population, such as patients seen by a single clinician or practice, residents of a neighborhood, or other “clusters,” may themselves impact or predict health outcomes of interest. Such groupings may be accounted for in analysis through use of analytic methods including analysis of variance (ANOVA), and hierarchical or multilevel modeling. 36 - 39
Heterogeneity of treatment effect is also an important consideration for comparative effectiveness research as the effect of a treatment may vary within subgroups of heterogeneous patients. 40 Stratification on the propensity score has been used to identify heterogeneity of treatment effect and may identify clinically meaningful differences between subgroups.
For economic analyses, the analytic approaches often encountered are cost-effectiveness analyses and cost-utility studies. To examine cost-effectiveness, costs are compared with clinical outcomes measured in units such as life expectancy or years of disease avoided. 41 Cost-utility analysis, a closely related technique, compares costs with outcomes adjusted for quality of life (utility) using measures known as quality-adjusted life years. Since most new interventions are more effective but also more expensive, another analytic approach examines the incremental cost-effectiveness ratio and contrasts that to the willingness to pay. (Willingness-to-pay analyses are generally conducted on a country-by-country basis, since various factors relating to national health insurance practices and cultural issues affect willingness to pay.) The use of registries for cost-effectiveness evaluations is a fairly recent development, and consequently, the methods are evolving rapidly. More information about economic analyses can be found in standard textbooks. 42 - 47
It is important to emphasize that cost-effectiveness analyses, much like safety and clinical effectiveness analyses, require collection of specific data elements suited to the purpose. Although cost-effectiveness-type analyses are becoming more important and registries can play a key role in such analyses, registries traditionally have not collected much information on quality of life or resource use that can be linked to cost data. 48 To be used for cost-effectiveness analysis, registries must be developed with that purpose in mind.
5.1. Developing a Statistical Analysis Plan
5.1.1. need for a statistical analysis plan.
It is important to develop a statistical analysis plan (SAP) that describes the analytical principles and statistical techniques to be employed in order to address the primary and secondary objectives, as specified in the study protocol or plan. Generally, the SAP for a registry study intended to support decisionmaking, such as a safety registry, is likely to be more detailed than the SAP for a descriptive study or health economics study. A registry may require a primary “master SAP” as well as subsequent, supplemental SAPs. Supplemental SAPs might be triggered by new research questions emerging after the initial master SAP was developed or might be needed because the registry has evolved over time (e.g., additional data collected, data elements revised). Although the evolving nature of data collection practices in some registries poses challenges for data analysis and interpretation, it is important to keep in mind that the ability to answer questions emerging during the course of the study is one of the advantages (and challenges) of a registry. In the specific case of long-term rare-disease registries, many of the relevant research questions of interest cannot be defined a priori but arise over time as disease knowledge and treatment experience accrue. Supplemental SAPs can be developed only when enough data become available to analyze a particular research question. At times, the method of statistical analysis may have to be modified to accommodate the amount and quality of data available. To the extent that the research question and SAP are formulated before the data analyses are conducted and results are used to answer specific questions or hypotheses, such supplemental analysis retains much of the intent of prespecification rather than being wide-ranging exploratory analyses (sometimes referred to as “fishing expeditions”). The key to success is to provide sufficient details in the SAP that, together with the study protocol and the case report forms, describe the overall process of the data analysis and reporting.
5.1.2. Preliminary Descriptive Analysis To Assist SAP Development
During SAP development, one particular aspect of a registry that is somewhat different from a randomized controlled study is the necessity to understand the “shape” of the data collected in the study by conducting a simple stratified analysis. 15 This may be crucial for a number of reasons.
Given the broad inclusion criteria that most registries tend to propose, there might be a wide distribution of patients, treatment, and/or outcome characteristics. The distribution of age, for example, may help to determine if more detailed analyses should be conducted in the “oldest old” age group (80 years and older) to help understand health outcomes in this subgroup that might be different from those of their younger counterparts.
Unless a registry is designed to limit data collection to a fixed number of regimens, the study population may experience many “regimens,” considering the combination of various dose levels, drug names, frequency and timing of medication use (e.g., acute, chronic, intermittent), and sequencing of therapies. The scope and complexity of these variations constitute one of the most challenging aspects of analyzing a registry, since treatment is given at each individual physician's discretion. Grouping of treatment into regimens for analysis should be done carefully, guided by clinical experts in that therapeutic area. The full picture of treatment patterns may become clear only after a sizable number of patients have been enrolled. Consequently, the treatment definition in an SAP may be refined during the course of a study. Furthermore, there may be occasions where a particular therapeutic regimen is used in a much smaller number of patients than anticipated, so that specific study objectives focusing on this group of patients might become unfeasible. Also, the registry might have enrolled many patients who would normally be excluded from a clinical trial because of significant contraindications related to comorbidity or concomitant medication use. In this case, the SAP may need to define how these patients will be analyzed (either as a separate group or as part of the overall study population) and how these different approaches might affect the interpretation of the study results.
There is a need to evaluate the presence of potential sources of bias and, to the extent feasible, use appropriate statistical measures to address such biases. For example, the bias known as confounding by indication 49 results from the fact that physicians do not prescribe medicine at random: the reason a patient is put on a particular regimen is often associated with their underlying disease severity and may, in turn, affect treatment outcome. (See Chapter 18 for more detailed discussion and examples.) To detect such a bias, the distribution of various prognostic factors at baseline is compared for patients who receive a treatment of interest and those who do not. A related concept is channeling bias , in which drugs with similar therapeutic indications are prescribed to groups of patients who may differ with regard to factors influencing prognosis. 50 To detect such a bias, registry developers and users must document the characteristics of the treated and untreated participants and either demonstrate their comparability or use statistical techniques to adjust for differences where possible. (Additional information about biases often found in registries is detailed in Chapter 3, Section 10 .) In addition to such biases, analyses need to account for factors that are interrelated, also known as effect modifiers . 15 The presence of effect modification may also be identified after the data are collected. All of these issues should be taken into account in an SAP, based on understanding of the patient population in the registry.
5.2. Timing of Analyses during the Study
Unlike a typical clinical trial, registries, especially those that take several years to complete, may conduct intermediate analyses before all patients have been enrolled and/or all data collection has been completed. Such midcourse analyses may be undertaken for several reasons. First, many of these registries focus on serious safety outcomes. For such safety studies, it is important for all parties involved to actively monitor the frequency of such events at regular predefined intervals so that further risk assessment or risk management can be considered. The timing of such analyses may be influenced by regulatory requirements. Second, it may be of interest to examine treatment practices or health outcomes during the study to capture any emerging trends. Finally, it may also be important to provide intermediate or periodic analysis to document progress, often as a requirement for continued funding.
While it is useful to conduct such periodic analysis, careful planning should be given to the process and timing. The first questions are whether a sufficient number of patients have been enrolled and whether a sufficient number of events have occurred. Answers to both questions can be estimated based on the speed of enrollment and rate of patient retention, as well as the expected incidence rate of the event of interest. The second issue is whether sufficient time has elapsed after the initial treatment with a product so that it is biologically plausible for events to have occurred. (For example, some events, such as site reactions to injections, can be observed after a relatively short duration, compared with events like cancers, which may have a long induction or latency.) If there are too few patients or insufficient time has elapsed, premature analyses may lead to the inappropriate conclusion that there is no occurrence of a particular event. Similarly, uncommon events, occurring by random chance in a limited sample, may be incorrectly construed as a safety signal. However, it is inappropriate to delay analysis so long that an opportunity might be missed to observe emerging safety outcomes. Investigators should use sound clinical and epidemiological judgment when planning an intermediate analysis and, whenever possible, use data from previous studies to help to determine the feasibility and utility of such an analysis.
When planning the timing of the analysis, it may be helpful to consider substudies if emerging questions require data not initially collected. Substudies often involve data collection based on biological specimens or specific laboratory procedures. They may, for example, take the form of nested case-control studies. In other situations, a research question may be applicable only to a subset of patients, such as those who become pregnant while in the study. It may also be desirable to conduct substudies among patients in a selected site or patient group to confirm the validity of study measurement. In such instances, a supplemental SAP may be a useful tool to describe the statistical principles and methods.
5.3. Factors To Be Considered in the Analysis
Registry results are most interpretable when they are specific to well-defined endpoints or outcomes in a specific patient population with a specific treatment status. Registry analyses may be more meaningful if variations of study results across patient groups, treatment methods, or subgroups of endpoints are reported. In other words, analysis of a registry should explicitly provide the following information:
- Patient : What are the characteristics of the patient population in terms of demographics, such as age, gender, race/ethnicity, insurance status, and clinical and treatment characteristics (e.g., past history of significant medical conditions, disease status at baseline, and prior treatment history)?
- Exposure (or treatment) : Exposure could be therapeutic treatment such as medication or surgery; a diagnostic or screening tool; behavioral factors such as alcohol, smoking habits, and diet; or other factors such as genetic predisposition or environmental factors. What are the distributions of the exposure in the population? Is the study objective specific to any one form of treatment? Is a new user design being used? 51 Does the exposure definition (index and reference group) and analysis avoid immortal-time bias? 52 Are there repeated measures or is the exposure intermittent?
- Endpoints (or outcomes) : Outcomes of interest may encompass effectiveness or comparative effectiveness, the benefits of a health care intervention under real-world circumstances, 53 and safety—the risks or harms that may be associated with an intervention. Examples of effectiveness outcomes include survival, disease recurrence, symptom severity, quality of life, and cost-effectiveness. Safety outcomes may include infection, sensitivity reactions, cancer, organ rejection, and mortality. Endpoints must be precisely defined at the data collection and analysis stages. Are the study data on all-cause mortality or cause-specific mortality? Is information available on pathogen-specific infection (e.g., bacterial vs. viral)? (See Case Example 27 .) Are there competing risks? 54
- Covariates : As with all observational studies, comparative effectiveness research requires careful consideration, collection, and analysis of important confounding and effect modifying variables. For medication exposures, are dose, duration, and calendar time under consideration? Directed acyclic graphs (DAGs) can be useful tools to illustrate how the exposure (or treatment), outcome and covariates are related. 55 , 56
- Time : For valid analysis of risk or benefit that occurs over a period of time following therapy, detailed accounting for time factors is required. For exposures, dates of starting and stopping a treatment or switching therapies should be recorded. For outcomes, the dates when followup visits occur, and whether or not they lead to a diagnosis of an outcome of interest, are required in order to take into account how long and how frequently patients were followed. Dates of diagnosis of outcomes of interest, or dates when patients complete a screening tool or survey, should be recorded. At the analysis stage, results must also be described in a time-appropriate fashion. For example, is an observed risk consistent over time (in relation to initiation of treatment) in a long-term study? If not, what time-related risk measures should be reported in addition to or instead of cumulative risk? When exposure status changes frequently, what is the method of capturing the population at risk? Many observational studies of intermittent exposures (e.g., use of nonsteroidal antiinflammatory drugs or pain medications) use time windows of analysis, looking at events following first use of a drug after a prescribed interval (e.g., 2 weeks) without drug use. Different analytic approaches may be required to address issues of patients enrolling in a registry at different times and/or having different lengths of observation during the study period.
- Potential for bias : Successful analysis of observational studies also depends to a large extent on the ability to measure and analytically address the potential for bias. Refer to Chapter 3, Section 10 for a description of potential sources of bias. Directed acyclic graphs can also be useful for understanding and identifying the source of bias. 55 , 56 Details and examples of quantification of bias can be found in Chapter 18 . For details on how to quantify potential bias, see the textbook by Lash, Fox, and Fink. 13
5.3.1. Choice of Comparator
An example of a troublesome source of bias is the choice of comparator. When participants in a cohort are classified into two or more groups according to certain study characteristics (such as treatment status, with the “standard of care” group as the comparator), the registry is said to have an internal or concurrent comparator. The advantage of an internal comparator design is that patients are likely to be more similar to each other, except for their treatment status, than patients in comparisons between registry subjects and external groups of subjects. When defining the comparator group, it is important not to introduce immortal time bias. 52 In addition, consistency in measurement of specific variables and in data collection methods make the comparison more valid. Internal comparators are particularly useful for treatment practices that change over time. Comparative effectiveness studies may often necessitate use of an internal comparator in order to maximize the comparability of patients receiving different treatments within a given study, and to ensure that variables required for multivariable analysis are available and measured in an equivalent manner for all patients to be analyzed.
Unfortunately, it is not always possible to have or sustain a valid internal comparator. For example, there may be significant medical differences between patients who receive a particularly effective therapy and those who do not (e.g., underlying disease severity or contraindications), or it may not be feasible to maintain a long-term cohort of patients who are not treated with such a medication. It is known that external information about treatment practices (such as scientific publications or presentations) can result in physicians changing their practice, such that they no longer prescribe the previously accepted standard of care. There may be a systematic difference between physicians who are early adopters and those who start using the drug or device after its effectiveness has been more widely accepted. Early adopters may also share other practices that differentiate them from their later-adopting colleagues. 5
In the absence of a good internal comparator, one may have to leverage external comparators to provide critical context to help interpret data revealed by a registry. An external or historical comparison may involve another study or another database that has disease or treatment characteristics similar to those of registry subjects. Such data may be viewed as a context for anticipating the rate of an event. One widely used comparator is the U.S. SEER cancer registry data, because SEER provides detailed annual incidence rates of cancer stratified by cancer site, age group, gender, and tumor staging at diagnosis. SEER represents 28 percent of the U.S. population. 57 A procedure for formalizing comparisons with external data is known as standardized incidence rate or ratio ; 15 when used appropriately, it can be interpreted as a proxy measure of risk or relative risk.
Use of an external comparator, however, may present significant challenges. For example, SEER and a given registry population may differ from each other for a number of reasons. The SEER data cover the general population and have no exclusion criteria pertaining to history of smoking or cancer screening, for example. On the other hand, a given registry may consist of patients who have an inherently different risk of cancer than the general population, resulting from the registry's having excluded smokers and others known to be at high risk of developing a particular cancer. Such a registry would be expected to have a lower overall incidence rate of cancer, which, if SEER incidence rates are used as a comparator, may complicate or confound assessments of the impact of treatment on cancer incidence in the registry.
Regardless of the choice of comparator, similarity between the groups under comparison should not be assumed without careful examination of the study patients. Different comparator groups may result in very different inferences for safety and effectiveness evaluations; therefore, analysis of registry findings using different comparator groups may be used in sensitivity analyses or bias analyses to determine the robustness of a registry's findings. Sensitivity analysis refers to a procedure used to determine how robust the study result is to alterations of various parameters. If a small parameter alteration leads to a relatively large change in the results, the results are said to be sensitive to that parameter. Sensitivity and bias analyses may be used to determine how the final study results might change when taking into account those lost to followup. A simple hypothetical example is presented in Table 13–1 .
Table 13–1
Hypothetical simple sensitivity analysis.
Table 13–1 illustrates the extent of change in the incidence rate of a hypothetical outcome assuming varying degrees of loss to followup, and differences in incidence between those for whom there is information and those for whom there is no information due to loss to followup. In the first example, where 10 percent of the patients are lost to followup, the estimated incidence rate of 111/1,000 people is reasonably stable; it does not change too much when the (unknown) incidence in those lost to followup changes from 0.5 times the observed to 5 times the observed, with the corresponding incidence rate that would have been observed ranging from 106 to 156 per 1,000. On the other hand, when the loss to followup increases to 30 percent, the corresponding incidence rates that would have been observed range from 94 to 242. This procedure could be extended to a study that has more than one cohort of patients, with one being exposed and the other being nonexposed. In that case, the impact of loss to followup on the relative risk could be estimated by using sensitivity analysis. More examples are included in Chapter 18 .
5.3.2. Patient Censoring
At the time of a registry analysis, events may not have occurred for all patients. For these patients, the data are said to be censored , indicating that the observation period of the registry was stopped before all events occurred (e.g., mortality). In these situations, it is unclear when the event will occur, if at all. In addition, a registry may enroll patients until a set stop date, and patients entered into the registry earlier will have a greater probability of having an event than those entered more recently because of the longer followup. An important assumption, and one that needs to be assessed in a registry, is how patient prognosis varies with the time of entrance into the registry. This issue may be particularly problematic in registries that assess innovative (and changing) therapies. Patients and outcomes initially observed in the registry may differ from patients and outcomes observed later in the registry timeframe, either because of true differences in treatment options available at different points in time, or because of the shorter followup for people who entered later. Patients with censored data, however, contribute important information to the registry analysis. When possible, analyses should be planned so as to include all subjects, including those censored before the end of the followup period or the occurrence of an event. One method of analyzing censored data to estimate the conditional probability of the event occurring is to use the Kaplan-Meier method. 58 In this method, for each time period, the probability is calculated that those who have not experienced an event before the beginning of the period will still not have experienced it by the end of the period. The probability of an event occurring at any given time is then calculated from the product of the conditional probabilities of each time interval.
For information about right censoring and left truncation, please see Chapter 18 .
6. Summary of Analytic Considerations
In summary, a meaningful analysis requires careful consideration of study design features and the nature of the data collected. Most typical epidemiological study analytical methods can be applied, and there is no one-size-fits-all approach. Efforts should be made to carefully evaluate the presence of biases and to control for identified potential biases during data analysis. This requires close collaboration among clinicians, epidemiologists, statisticians, study coordinators, and others involved in the design, conduct, and interpretation of the registry.
A number of biostatistics and epidemiology textbooks cover in depth the issues raised in this section and the appropriate analytic approaches for addressing them—for example, “time-to-event” or survival analyses 59 and issues of recurrent outcomes and repeated measures, with or without missing data, 60 in longitudinal cohort studies. Other texts address a range of regression and nonregression approaches to analysis of case-control and cohort study designs 61 that may be applied to registries.
7. Interpretation of Registry Data
Interpretation of registry data is needed so that the lessons from the registry can be applied to the target population and used to change future health care and improve patient outcomes. Proper interpretation of registry data allows users to understand the precision of the observed risk or incidence estimates, to evaluate the hypotheses tested in the current registry, and often also to generate new hypotheses to be examined in future registries or in randomized controlled trials. If the purpose of the registry is explicit, the actual population studied is reasonably representative of the target population, the data quality monitored, and the analyses performed so as to reduce potential biases, then the interpretation of the registry data should allow a realistic picture of the quality of medical care, the natural history of the disease studied, or the safety, effectiveness, or value of a clinical evaluation. Each of these topics needs to be discussed in the interpretation of the registry data, and potential shortcomings should be explored. Assumptions or biases that could have influenced the outcomes of the analyses should be highlighted and separated from those that do not affect the interpretation of the registry results. The use of a comparator of the highest reasonably possible quality is integral to the proper interpretation of the analysis.
Interpretation of registry results may also be aided by comparisons with external information. Examples include rates, or prevalence, of the outcomes of interest in other studies and different data sources (taking into account reasons why they may be similar or different). Such comparisons can put the findings of registry analyses within the context of previous study results and other pertinent clinical and biological considerations as to the validity and generalizability of the results.
Once analyzed, registries provide important feedback to several groups. First analysis and interpretation of the registry will demonstrate strengths and limitations of the original registry design and will allow the registry developers to make needed design changes for future versions of the registry. Another group consists of the study's sponsors and related oversight/governance groups, such as the scientific committee and data monitoring committee. (Refer to Chapter 2, Section 2.6 for more information on registry governance and oversight.) Interpretation of the analyses allows the oversight committees to offer recommendations concerning continued use and/or adaptation of the registry and to evaluate patient safety. The final group consists of the end users of the registry output, such as patients or other health care consumers, health services researchers, health care providers, and policymakers. These are the people for whom the data were collected and who may use the results to choose a treatment or intervention, to determine the need for additional research programs to change clinical practice, to develop clinical practice guidelines, or to determine policy. Ideally, all three user groups work toward the ultimate goal of each registry—improving patient outcomes.
- Case Examples for Chapter 13
Case Example 26 Using registry data to evaluate outcomes by practice
View in own window
Although guidelines for managing cystic fibrosis patients have been widely available for many years, little is known about variations in practice patterns among care sites and their associated outcomes. To determine whether differences in lung health existed between groups of patients attending different CF care sites, and to determine whether these differences were associated with differences in monitoring and intervention, data on a large number of CF patients from a wide variety of CF sites were necessary.
As a large, observational, prospective registry, ESCF collected data on a large number of patients from a range of participating sites. At the time of the outcomes study, the registry was estimated to have data on over 80 percent of CF patients in the United States, and it collected data from more than 90 percent of the sites accredited by the U.S. Cystic Fibrosis Foundation. Because the registry contained a representative population of CF patients, the registry database offered strong potential for analyzing the association between practice patterns and outcomes.
Proposed Solution
In designing the study, the team decided to compare CF sites using lung function (i.e., FEV1 [forced expiratory volume in 1 second] values), a common surrogate outcome for respiratory studies. Data from 18,411 patients followed in 194 care sites were reviewed, and 8,125 patients from 132 sites (minimum of 50 patients per site) were included. Only sites with at least 10 patients in a specified age group (ages 6–12, 13–17, and 18 or older) were included for evaluation of that age group. For each age group, sites were ranked in quartiles based on the median FEV1 value at each site. The frequency of patient monitoring and use of therapeutic interventions were compared between upper and lower quartile sites after stratification for disease severity.
Substantial differences in lung health across different CF care sites were observed. Within-site rankings tended to be consistent across the three age groups. Patients who were cared for at higher-ranking sites had more frequent monitoring of their clinical status, measurements of lung function, and cultures for respiratory pathogens. These patients also received more interventions, particularly intravenous antibiotics for pulmonary exacerbations. The study concluded that frequent monitoring and increased use of appropriate medications in the management of CF are associated with improved outcomes.
Stratifying patients by quartile of lung function, age, and disease severity allowed comparison of practices among sites and revealed practice patterns that were associated with better clinical status. The large numbers of patients and sites allowed for sufficient information to create meaningful and informative stratification, and resulted in sufficient information within those strata to reveal meaningful differences in site practices.
For More Information
Johnson C, Butler SM, Konstan MW, et al. Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest. 2003; 123 :20–7. [ PubMed : 12527598 ].
Padman R, McColley SA, Miller DP, et al. Infant care patterns at Epidemiologic Study of Cystic Fibrosis sites that achieve superior childhood lung function. Pediatrics. 2007; 119 :E531–7. [ PubMed : 17332172 ].
Case Example 27 Using registry data to study patterns of use and outcomes
RSV is the leading cause of serious lower respiratory tract disease in infants and children and the leading cause of hospitalizations nationwide for infants under 1 year of age. Palivizumab was approved by the U.S. Food and Drug Administration (FDA) in 1998 and is indicated for the prevention of serious lower respiratory tract disease caused by RSV in pediatric patients at high risk of RSV disease. Two additional large retrospective surveys conducted after FDA approval studied the effectiveness of palivizumab in infants, again showing that it reduces the rate of RSV hospitalizations. To capture postlicensure patient demographic outcome information, the manufacturer wanted to create a prospective study that identified infants receiving palivizumab. The objectives of the study were to better understand the population receiving the prophylaxis for RSV disease and to study the patterns of use and hospitalization outcomes.
A multicenter registry study was created to collect data on infants receiving palivizumab injections. No control group was included. The registry was initiated during the 2000–2001 RSV season. Over 4 consecutive years, 256 sites across the United States enrolled infants who had received palivizumab for RSV under their care, provided that the infant's parent or legally authorized representative gave informed consent for participation in the registry. Data were collected by the primary health care provider in the office or clinic setting. The registry was limited to data collection related to subjects' usual medical care. Infants were enrolled at the time of their first injection, and data were obtained on palivizumab injections, demographics, and risk factors, as well as on medical and family history.
Followup forms were used to collect data on subsequent palivizumab injections, including dates and doses, during the RSV season. Compliance with the prescribed injection schedule was determined by comparing the number of injections actually received with the number of expected doses, based on the month that the first injection was administered. Infants who received their first injection in November were expected to receive five injections, whereas infants receiving their first injection in February would be expected to receive only two doses through March. Data were also collected for all enrolled infants hospitalized for RSV and were directly reported to an onsite registry coordinator. Testing for RSV was performed locally, at the discretion of the health care provider. Adverse events were not collected and analyzed separately for purposes of this registry. Palivizumab is contraindicated in children who have had a previous significant hypersensitivity reaction to palivizumab. Cases of anaphylaxis and anaphylactic shock, including fatal cases, were reported following initial exposure or re-exposure to palivizumab. Other acute hypersensitivity reactions, which might have been severe, were also reported on initial exposure or re-exposure to palivizumab. Adverse reactions occurring greater than or equal to 10 percent and at least 1 percent more frequently than placebo are fever and rash. In postmarketing reports, cases of severe thrombocytopenia (platelet count <50,000/microliter) and injection site reactions were reported.
From September 2000 through May 2004, the registry collected data on 19,548 infants. The analysis presented injection rates and hospitalization rates for all infants by month of injection and by site of first dose (pediatrician's office or hospital). The observed number of injections per infant was compared with the expected number of doses based on the month the first injection was given. Over 4 years of data collection, less than 2 percent (1.3%) of enrolled infants were hospitalized for RSV. This analysis confirmed a low hospitalization rate for infants receiving palivizumab prophylaxis for RSV in a large nationwide cohort of infants from a geographically diverse group of practices and clinics. The registry data also showed that the use of palivizumab was mostly consistent with the 2003 guidelines of the American Academy of Pediatrics for use of palivizumab for prevention of RSV infections. As the registry was conducted prospectively, nearly complete demographic information and approximately 99 percent of followup information was captured on all enrolled infants, an improvement compared with previously completed retrospective studies.
A simple stratified analysis was used to describe the characteristics of infants receiving injections to help prevent severe RSV disease. Infants in the registry had a low hospitalization rate, and these data support the effectiveness of this treatment outside of a controlled clinical study. Risk factors for RSV hospitalizations were described and quantified by presenting the number of infants with RSV hospitalization as a percentage of all enrolled infants who were hospitalized. These data supported an analysis of postlicensure effectiveness of RSV prophylaxis, in addition to describing the patient population and usage patterns.
Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997-1999. Ped Infect Dis J. 2002; 21 (7):629–32 [ PubMed : 12237593 ].
Frogel M, Nerwen C, Cohen A, et al. Prevention of hospitalization due to respiratory syncytial virus: Results from the Palivizumab Outcomes Registry. J Perinatol. 2008; 28 :511–7. [ PubMed : 18368063 ].
American Academy of Pediatrics—Committee on Infectious Disease. Red Book 2003: Policy Statement: Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003; 112 :1442–6. [ PubMed : 14654627 ].
- References for Chapter 13
- Cite this Page Gliklich RE, Dreyer NA, Leavy MB, editors. Registries for Evaluating Patient Outcomes: A User's Guide [Internet]. 3rd edition. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Apr. 13, Analysis, Interpretation, and Reporting of Registry Data To Evaluate Outcomes.
In this Page
- Introduction
- Hypotheses and Purposes of the Registry
- Patient Population
- Data Quality Issues
- Data Analysis
- Summary of Analytic Considerations
- Interpretation of Registry Data
Other titles in these collections
- AHRQ Methods for Effective Health Care
- Health Services/Technology Assessment Texts (HSTAT)
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Analysis, Interpretation, and Reporting of Registry Data To Evaluate Outcomes - ... Analysis, Interpretation, and Reporting of Registry Data To Evaluate Outcomes - Registries for Evaluating Patient Outcomes
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Search form
Asco family of sites.
- ASCO Connection
- ASCO Daily News
- ASCO Journals
- ASCO Practice Central
- ASCO Education
- Cancer.Net - For Patients
- Conquer Cancer
- TAPUR Study
- The ASCO Post

ASCOconnection.org features blogs from members, the online version of the membership magazine, a discussion area, working groups, and links to the Membership Directory, Career Center, and Volunteer Portal.

ASCO Daily News is the official conference reporter for ASCO meetings and symposia, providing high-quality, unbiased research summaries and oncology news to members and oncology health care providers.

ASCO’s growing roster of cutting-edge journals serves readers as the most credible, authoritative, peer-reviewed resources for significant clinical oncology research and research that informs the delivery of efficient, high-quality cancer care across the globe.

ASCO Practice Central helps oncology professionals navigate a complicated and ever-changing practice environment—while providing high-quality patient care.

Stay updated on the latest oncology has to offer with ASCO Education—your online, on-demand resource for timely information, real-world application, and practice-changing care.

A cutting-edge health information technology platform, CancerLinQ® enables practitioners to learn from individual patients. By assembling vast amounts of usable, searchable, real-world data, CancerLinQ seeks to improve the quality and value of cancer care.

Cancer.Net brings the expertise and resources of ASCO to people living with cancer and those who care for and about them to help patients and families make informed health care decisions.

Conquer Cancer, the ASCO Foundation, raises funds to support the world's leading researchers who are improving treatments and discovering cures for every cancer, every patient, everywhere.

ASCO’s Targeted Agent and Profiling Utilization Registry (TAPUR) Study is a non-randomized clinical trial aiming to describe the performance of commercially available, targeted anticancer drugs prescribed for treatment of patients with advanced cancer with a potentially actionable genomic variant.

The ASCO Post, in partnership with the American Society of Clinical Oncology, communicates news of the highest quality multidisciplinary cancer care to a broad audience of oncology professionals and members.
News Releases
Adding belantamab mafodotin to multiple myeloma treatment combinations slows disease progression or death.
ASCO Perspective Quote
“The DREAMM-8 trial demonstrates that the first-in-class BCMA-targeted antibody-drug conjugate—belantamab mafodotin—plus pomalidomide and dexamethasone (BPd) is significantly more effective than the PVd regimen (pomalidomide, bortezomib, and dexamethasone), cutting the risk of disease progression or death by nearly half in patients with relapsed or refractory multiple myeloma. The findings of this trial suggest that BPd is poised to be a potential new treatment strategy for relapsed or refractory multiple myeloma.” - Oreofe O. Odejide, MD, MPH, Assistant Professor of Medicine, Dana-Farber Cancer Institute
Study at-a-Glance
ALEXANDRIA, Va. — According to a new study, adding belantamab mafodotin to pomalidomide and dexamethasone for the treatment of relapsed or refractory multiple myeloma was more effective at slowing disease progression or death compared to current standard-of-care bortezomib plus pomalidomide and dexamethasone. The research will be presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting taking place May 31-June 4 in Chicago, Illinois.
About the Study
Belantamab mafodotin is an antibody-drug conjugate that binds to a protein called BCMA on myeloma cells and then delivers a chemotherapy drug to destroy the cell. The recent DREAMM-7 clinical trial showed that a combination of belantamab mafodotin plus bortezomib and dexamethasone slowed the progression of multiple myeloma if the first treatment did not work when compared to daratumumab plus bortezomib and dexamethasone. The DREAMM-8 study combines belantamab mafodotin with another common myeloma drug, pomalidomide (a medication that stimulates the immune system), and dexamethasone (a steroid).
In the DREAMM-8 study, people with relapsed and refractory multiple myeloma whose disease had progressed after at least one previous treatment (including lenalidomide) were randomized to receive belantamab mafodotin plus pomalidomide and dexamethasone, or BPd (n=155) or pomalidomide plus bortezomib and dexamethasone, or PVd (n=147). Of all the participants, 60% were men, 86% were White, and the average age was around 67 years. The participants were followed for a median of nearly 22 months.
Key Findings
- After a median follow-up of 22 months, the median progression-free survival (PFS) was not reached for the participants who received BPd. For those who received PVd, the median PFS was 12.7 months.
- At the end of the first year, PFS was 71% for those receiving BPd compared to 51% of those receiving PVd.
- The overall response rate was 77% for those receiving BPd compared to 72% for those receiving PVd. Additionally, 40% of patients treated with BPd achieved a complete response or better compared to 16% of patients who were treated with PVd.
- Among those with disease that responded to treatment, the median duration of response was not yet reached in those who received BPd, and it was 17.5 months in those who received PVd.
Nearly all participants (>99%) receiving BPd experienced side effects, as did 96% of those receiving PVd. Eye-related side effects were common, such as changes in the cornea and blurred vision, affecting 89% of those in the BPd group and 30% of those in the PVd group. Eye-related side effects often went away and could be managed by adjusting or holding the dose of belantamab mafodotin, which allowed most patients to keep receiving the study treatment and benefit from it.
“This regimen could become an important treatment option for patients with multiple myeloma at first relapse and for subsequent relapses. It is suitable for a broad range of patients and can be given in a community oncology setting without the need for specialized cancer center support,’ said lead study author Suzanne Trudel, MSc, MD, Associate Professor at the Princess Margaret Cancer Centre in Toronto, Ontario.
The researchers will continue to follow the study participants to evaluate if patients live longer and stay free of disease. They will also continue to collect data to find out the median PFS and duration of response for those who received BPd.
This study was funded by GSK.
View the full embargoed abstract
View author disclosures
View the News Planning Team disclosures: https://society.asco.org/sites/new-www.asco.org/files/content-files/about-asco/pdf/2024-am-ccc-disclosures.pdf
ATTRIBUTION TO THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY ANNUAL MEETING IS REQUESTED IN ALL COVERAGE
About ASCO :
Founded in 1964, the American Society of Clinical Oncology, Inc. (ASCO®) is committed to the principle that knowledge conquers cancer. Together with the Association for Clinical Oncology, ASCO represents nearly 50,000 oncology professionals who care for people living with cancer. Through research, education, and promotion of high quality, equitable patient care, ASCO works to conquer cancer and create a world where cancer is prevented or cured, and every survivor is healthy. Conquer Cancer, the ASCO Foundation, supports ASCO by funding groundbreaking research and education across cancer’s full continuum. Learn more at www.ASCO.org , explore patient education resources at www.Cancer.Net , and follow us on Facebook , Twitter , LinkedIn , Instagram , and YouTube .
Related News Releases
Related tags.
- Meetings News
- Events Calendar /calendar Events Calendar
- Research & Data ASCO Journals TAPUR Study CDK4/6 Inhibitor Dosing Knowledge (CDK) Study Clinical Trials ASCO Data Library Reports & Studies Research Survey Pool Interactive Map of Oncology /research-data Research & Data
- Guidelines /practice-patients/guidelines Guidelines
- Grants & Awards /career-development/grants-awards Grants & Awards
- Cancer Progress Timeline /news-initiatives/cancer-progress-timeline Cancer Progress Timeline
- Volunteering & Committees Apply to Volunteer FASCO Distinction International Cancer Corps Policies for Volunteers & Committee Members Regional Councils /get-involved/volunteering-committees/volunteer-asco Volunteering & Committees
- Discussion Groups https://myconnection.asco.org Discussion Groups

COMMENTS
To achieve this potential, stakeholders can use CTTI's recommendations for registry trials to assess, develop, and evaluate registries for the support of clinical research and help meet expectations for FDA review of new products.
In summary, planning a patient registry involves several key steps, including articulating its purpose, determining whether it is an appropriate means of addressing the research question, identifying stakeholders, defining the scope and target population, assessing feasibility, and securing funding.
Registry-based study: Investigation of a research question using the data collection infrastructure or patient population of one or several patient registries. registry-based study is either a clinical trial or a non-interventional study as defined in Article 2 of Regulation (EU) No 536/2014.
In summary, planning a patient registry involves several key steps, including articulating its purpose, determining whether it is an appropriate means of addressing the research question, identifying stakeholders, defining the scope and target population, assessing feasibility, and securing funding.
Join a National Registry of Research Volunteers ResearchMatch This is an NIH-funded initiative to connect 1) people who are trying to find research studies, and 2) researchers seeking people to participate in their studies. It is a free, secure registry to make it easier for the public to volunteer and to become involved in clinical research studies that contribute to improved health in the ...
Patient registries are fundamental to biomedical research. Registries provide consistent data for defined populations and can be used to support the study of the determinants and manifestations of disease and provide a picture of the natural history, outcomes of treatment, and experiences of individuals with a given condition or exposure.
ClinicalTrials.gov is a place to learn about clinical studies from around the world.
outcomes. Registries are also used more frequently to support clinical trials and track long-term outcomes in patient populations. This article reviews registry methodology, including the collection of data from crowdsourcing and real-world sources, that can be applied to nurse researcher and clinical research nurse skill sets. The authors illustrate a recently reported crowdsourced COVID-19 ...
A registry is a place to store detailed information about people with a specific disease or condition, who provide it on a voluntary basis.
A research registry is a collection of information about individuals. There are different types of research registries: registries of people with a specific diagnosis or condition and registries that connect people interested in being research participants with health studies. By joining a research registry, you agree to be contacted about participating in future research studies.
ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world.
Registry standards A patient registry is "an organized system that uses observational study methods to collect uniform data (clinical and other) to evaluate specified outcomes for a population defined by a particular disease, condition or exposure, and that serves a predetermined scientific, clinical or policy purpose."
For the purposes of registration, a clinical trial is any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes. Clinical trials may also be referred to as interventional trials.
The ISRCTN registry is a primary clinical trial registry recognised by WHO and ICMJE that accepts all clinical research studies (whether proposed, ongoing or completed), providing content validation and curation and the unique identification number necessary for publication. All study records in the database are freely accessible and searchable.
The National Institutes of Health (NIH) Clinical Center Search the Studies site is a registry of publicly supported clinical studies conducted mostly in Bethesda, MD.
A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. Clinical data registries are also sometimes called patient registries and disease registries. Professional medical associations and specialty societies tend to use the term clinical data registry, while research and patient ...
The Patient Registry Observational Study Subtype (see Study Type data element on ClinicalTrials.gov) can be used to indicate that an observational study is also considered to be a Patient Registry. The Agency for Healthcare Research and Quality (AHRQ) defines a Patient Registry as including an organized system that uses observational methods to collect uniform data (clinical and other ...
The present study was designed to assess the effectiveness and safety of IRE for Stage 3 PDAC in a real-world setting after induction chemotherapy. Methods: Patients with Stage 3 PDAC treated with IRE were prospectively enrolled in a multicenter registry study.
Registries focused on determining clinical effectiveness or cost-effectiveness or assessing safety or harm are generally hypothesis driven and concentrate on evaluating the effects of specific treatments on patient outcomes. Research questions should address the registry's purposes, as broadly described in Table 3-2.
The purpose of this research study is to include individuals into the Shirley Ryan Ability Lab's participant registry. The registry will provide a controlled list from which researchers at Shirley Ryan Ability Lab and Northwestern University (NU), Department of Physical Therapy and Human Movement Sciences or Department of Physical Medicine and Rehabilitation may identify potential ...
Leveraging genomic instability scores (GIS) to investigate BRCA variants of unknown significance (VUS) in ovarian cancer: An analysis of 14,513 patients in the Myriad Collaborative Research Registry (MCRR).
5585 Background: To compare the characteristics and genetic mutational profiles of patients with ovarian, fallopian tube and primary peritoneal cancers by age and self-identified ancestry. Methods: Data on patients with epithelial ovarian, fallopian, and peritoneal cancers who had germline, MyChoice, and/or Precise testing were obtained from the Myriad Collaborative Research Registry. Gene ...
Testing Innovation in Cancer Clinical Trials. A transformation in cancer clinical research is already underway. That transformation has been led in part by the success of novel precision oncology approaches, such as those tested in the NCI-MATCH trial. This innovative study ushered in novel ways of recruiting participants and involving ...
The study was approved by the research ethics board at the researchers' institution (York University and Carleton University), an academic hospital (Ron Joyce Children's Health Centre), and by the research review committee at two community-based agencies (Kerry's Place Autism Services and Kinark Autism Services).
Dana-Farber Cancer Institute researchers are leading 3 separate studies with encouraging results in treating patients with central nervous system (CNS) lymphoma, breast cancer, and glioblastoma. The studies support future research in these potential breakthroughs where treatment options may be limited. The research teams will present their findings at the 2024 Annual Meeting of the American ...
This randomized clinical trial investigates the effect of neuromuscular exercise and pain neuroscience education vs pain neuroscience education alone on pain and function among patients in Denmark with chronic pain for more than 1 year after total knee arthroplasty.
In the case of registries where the aim is to study the associations between specific exposures and outcomes, prespecification of the study methodology and presence or absence of a priori hypotheses or research questions may affect the acceptance of results of studies derived from registry data.
According to a new study, adding belantamab mafodotin to pomalidomide and dexamethasone for the treatment of relapsed or refractory multiple myeloma was more effective at slowing disease progression or death compared to current standard-of-care bortezomib plus pomalidomide and dexamethasone. The research will be presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting ...
5617 Background: To compare uterine cancer patient characteristics and genetic mutational profiles by age and self-identified ancestry. Methods: Data on uterine cancer patients who had germline, MyChoice, and/or Precise testing were collected from the Myriad Collaborative Research Registry. Mutations in genes of interest were obtained from TAPUR studies currently under enrollment. Graph Pad ...