Research Methods for Successful PhD
- Download Book Flyer
- Table of Content
- Browse Articles
- New Book Idea
River Publishers Series in Innovation and Change in Education - Cross-cultural Perspective
Author: Dinesh Kumar, RMIT University, Australia
ISBN: 9788793609181 e-ISBN: 9788793609174
Available: November 2017
- Buy Print € 35.00
- Buy Print $ 51.99
- Articles € 95.00 PDF Size 110.55 MB 454 Downloads 280 Reads
- Description
- Table of Contents
LET US HELP
Welcome to Capella
Select your program and we'll help guide you through important information as you prepare for the application process.
FIND YOUR PROGRAM
Connect with us
A team of dedicated enrollment counselors is standing by, ready to answer your questions and help you get started.

- Capella University Blog
- PhD/Doctorate

What are acceptable dissertation research methods?
August 16, 2023
Reading time: 3â4 minutes
Doctoral research is the cornerstone of a PhD program .
In order to write a dissertation, you must complete extensive, detailed research. Depending on your area of study, different types of research methods will be appropriate to complete your work.
âThe choice of research method depends on the questions you hope to answer with your research,â says Curtis Brant, PhD, Capella University dean of research and scholarship.
Once youâve identified your research problem, youâll employ the methodology best suited for solving the problem.
There are two primary dissertation research methods: qualitative and quantitative.
Qualitative
Qualitative research focuses on examining the topic via cultural phenomena, human behavior or belief systems. This type of research uses interviews, open-ended questions or focus groups to gain insight into peopleâs thoughts and beliefs around certain behaviors and systems.
Dr. Brant says there are several approaches to qualitative inquiry. The three most routinely used include:
Generic qualitative inquiry. The researcher focuses on peopleâs experiences or perceptions in the real world. This often includes, but is not limited to, subjective opinions, attitudes and beliefs .
Case study. The researcher performs an in-depth exploration of a program, event, activity or process with an emphasis on the experience of one or more individuals. The focus of this kind of inquiry must be defined and often includes more than one set of data, such as interviews and field notes, observations or other qualitative data.
Phenomenological. The researcher identifies lived experiences associated with how an individual encounters and engages with the real world .
Qualitative research questions seek to discover:
- A participantâs verbal descriptions of a phenomenon being investigated
- A researcherâs observations of the phenomenon being investigated
- An integrated interpretation of participantâs descriptions and researchers observations
Quantitative
Quantitative research involves the empirical investigation of observable and measurable variables. It is used for theory testing, predicting outcomes or determining relationships between and among variables using statistical analysis.
According to Dr. Brant, there are two primary data sources for quantitative research.
Surveys: Surveys involve asking people a set of questions, usually testing for linear relationships, statistical differences or statistical independence. This approach is common in correlation research designs.
Archival research (secondary data analysis). Archival research involves using preexisting data to answer research questions instead of collecting data from active human participants.
Quantitative research questions seek to address:
- Descriptions of variables being investigated
- Measurements of relationships between (at least two) variables
- Differences between two or more groupsâ scores on a variable or variables
Which method should you choose?
Choosing a qualitative or quantitative methodology for your research will be based on the nature of the questions you ask, the preferred method in your field, the feasibility of the approach and other factors. Many programs offer doctoral mentors and support teams that can help guide you throughout the process.
Capella University offers PhD and professional doctorate degree programs ranging from business to education and health to technology. Learn more about Capella doctoral programs and doctoral support.
You may also like

Can I transfer credits into a doctoral program?
January 8, 2020

What are the steps in writing a dissertation?
December 11, 2019

The difference between a dissertation and doctoral capstone
November 25, 2019
Start learning today
Get started on your journey now by connecting with an enrollment counselor. See how Capella may be a good fit for you, and start the application process.
Please Exit Private Browsing Mode
Your internet browser is in private browsing mode. Please turn off private browsing mode if you wish to use this site.
Are you sure you want to cancel?
- Institutions
- Subscription

- The Netherlands
Research Methods for Successful PhD
Information.
- River Publishers
- 9788793609174
Published at
About this book.
A PhD is the start of a research career, and these students are the backbone of universities and research institutions. It is the opportunity for youthful energy and creativity to make a global impact and train future researchers to make a difference. However, the candidature can also be a period of confusion and regret due to lack of structure and understanding. Research Methods for Successful PhD is written to help PhD students and other young researchers navigate through this phase to give them a direction and purpose. It is a candid conversation developed from the experience of supervising 30 research students and publishing 400 papers over 20 years. The book recognizes that every student is different and has unique circumstances. It teases out the fundamental questions that we forget to ask, the method of relating to the supervisor, discusses methods to improve communication skills and explains how to get the work published.
By using our website you agree to our cookie policy and the storage of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
PhD Assistance
15 kinds of research methodologies for phd. pupils, basic research.
Pure research or fundamental research or basic research zooms on enhancing scientific knowledge for the exhaustive understanding of a topic or certain natural phenomena, essentially in natural sciences; knowledge that is obtained for the purpose of knowledge it is called fundamental research.
1.Applied research
Research that covers real life applications of the natural sciences; aimed at offering an answer to particular practical issues and develops novel technologies
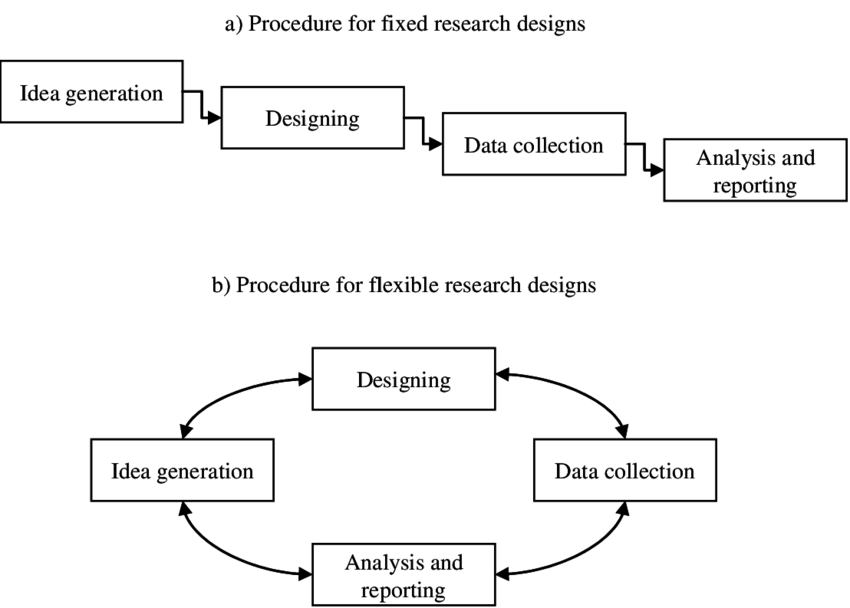
2.Fixed research versus flexible research
In fixed research, the design of the study is fixed prior to the main phase of data gathering; moreover, fixed designs are essentially theoretical. Variables that need to be controlled and measured need to be known in advance and they are measured quantitatively.
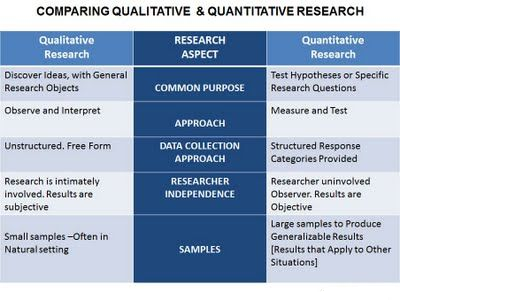
3.Quantitative research and qualitative research
Quantitative research denotes gauging phenomena in various grades; on the other hand, qualitative research sometimes deems Boolean measurements alone; solution can be studied qualitatively for its appropriateness. However, comparison between candidate solutions requires quantitative observation.
4.Experimental research and non-experimental research
In an experimental design , operationalize the variables to be measured; moreover, operationalize in the best manner. Consider the study expectations, outcome measurement, variable measurement, and the methods to answer research questions.
Think of the practical limitations such as the availability of data-sets and experimental set-ups that represent actual scenarios.
5.Exploratory research and confirmatory research
Confirmatory research tests a priori hypotheses—outcome predictions done prior to the measurement stage. Such a priori hypotheses are usually derived from a theory or the results of previous studies.
Exploratory research generates a posteriori hypotheses by investigating a data-set and ascertaining potential connection between variables.
6.Explanatory research or casual research
Causal research is also called explanatory research ; conducted to ascertain the extent and type of cause-effect relationships. Causal research are conducted to evaluate effects of specific changes on existing norms, various processes etc.
7.Descriptive research
Descriptive research is the available statement of affairs; researcher has no control over variable. Descriptive studies are characterised as simply an effort to ascertain, define or recognize. Not “why it is that way” nor “how it came to be,” which is the objective of analytical research.
8.Historical research
Historical research explores and explains the meanings, phases and traits of a phenomena or process at a certain phase of time in the past; historical research is a research strategy from the research of history.
9.Casual comparative research
Also called as “ex-post facto” research (In Latin, implies “after the fact”); researchers determine the causes or consequences of differences that already exist between or among groups of individuals.
An effort to ascertain a causative relationship between an independent variable and a dependent variable; relationship between the independent variable and dependent variable are usually a suggested relationship (not proved yet) because you do not have complete control over the independent variable
10.Correlational research
Correlational research is a form of non-experimental research technique wherein a researcher measures 2 variables and assesses the statistical connection between them with no influence from any external variable.
The correlation between two variables is given through correlation coefficient, which is a statistical measure that calculates the strength of the relationship between two variables that is a value measured between -1 and +1.
11.Evaluation research method
Evaluation research technique is known as program evaluation and refers to a research purpose instead of a particular technique; objective is to assess the effect of social involvements such as new treatment techniques, innovations in services, etc.
A form of applied research to have some real-world effect. Methods such as surveys and experiments are used in evaluation research.
12.Formative and summative evaluation
While learning is in progress, formative assessment offers feedback and information; measures participant’s progress and also assess researcher’s own progress as well.
For example, when implementing a new program, you can determine whether or not the activity should be used again (or modified) with the help of observation and/or surveying.
Summative assessment happens after the learning has ended and offers info and feedback to sum up the process; essentially, no formal learning is happening at this phase other than incidental learning which might take place through the completion of program.
13.Diagnostic research
Descriptive research studies define the characteristics of a particular individual, or of a group.
Studies showing whether certain variables are linked are examples of diagnostic research.
Researcher defines what he or she wants to measure and finds adequate methods for measuring it along with a clear description of ‘population’.
Aim is to obtain complete and accurate information. And the researcher plans the procedure carefully.
14.Prognostic research
Prognostic research (specifically in clinical research) examines chosen predictive variables and risk factors; prognostic research assesses influence on the outcome of a disease. Clinicians have a better understanding of the history of the ailment.
This understanding facilitates clinical decision-making via providing apt treatment alternatives and helps to predict accurate disease outcomes.
Assessing prognostic studies involves ascertaining the internal validity of the study design and assessing the effects of bias or systemic errors.
15.Action research
A systematic inquiry for improving and/or honing researchers’ actions. Researchers find it an empowering experience.
Action research has positive result for various reasons; most important is that action research is pertinent to the research participants.
Relevance is assured because the aim of each research project is ascertained by the researchers, who are also the main beneficiaries of the research observations.
Related Topics
Essay Writting Services
Research methods
Primary research methods
methodology example
Secondary research methods
Primary and secondary research
Academic Research
scholarly research
- 15 purposes of research
- 15 types of research
- example of phd research methodology
- methodology in phd
- methodology in phd research
- methodology phd
- phd in research methodology
- phd methodology
- PhD Research Methodology
- Phd Research Methods
- PhD Research Process
- phd types of research
- research methodology for phd
- Research Methodology for PhD Thesis
- research methodology in phd
- research methodology phd
- research methods for phd
- research methods phd
- types of phd research
- types of research
- types of research in research methodology
- types of research methodology
- types of research methods

Quick Contact

- Adversial Attacks
- Artificial Intelligence
- Artificial Intelligence (AI) and ML ( Machine Learning )
- Big Data Analysis
- Business and Management
- Categories of Research methodology – PhDAssistance
- Category of Research Proposal Services
- coding & algorithm
- Computer Data Science
- Category of Machine Learning – PhDassistance
- Computer Science/Research writing/Manuscript
- Course Work Service
- Data Analytics
- Data Processing
- Deep Networks
- Dissertation Statistics
- economics dissertation
- Editing Services
- Electrical Engineering Category
- Engineering & Technology
- finance dissertation writing
- Gap Identification
- Healthcare Dissertation Writing
- Intrusion-detection-system
- journals publishing
- Life Science Dissertation writing services
- literature review service
- Machine Learning
- medical thesis writing
- Peer review
- PhD Computer Programming
- PhD Dissertation
- Phd Journal Manuscript
- Annotated Bibliography
- PhD Publication Support
- Phd thesis writing services
- Phd Topic Selection
- Categories of PhdAssistance Dissertation
- Power Safety
- problem identification
- Quantitative Analysis
- quantitative research
- Recent Trends
- Referencing and Formatting
- Research Gap
- research journals
- Research Methodology
- research paper
- Research Proposal Service
- secondary Data collection
- Statistical Consulting Services
- Uncategorized
- Find a Library
- Browse Collections
- Research Methods for Successful PhD
ebook ∣ River Publishers Series in Innovation and Change in Education - Cross-cultural Perspective
By dinesh kumar.

Add Book To Favorites
Is this your library?
Sign up to save your library.
With an OverDrive account, you can save your favorite libraries for at-a-glance information about availability. Find out more about OverDrive accounts.
9788793609181
River Publishers Series in Innovation and Change in Education - Cross-cultural Perspective
Dinesh Kumar
River Publishers
01 September 2022
Find this title in Libby, the library reading app by OverDrive.

Search for a digital library with this title
Title found at these libraries:.

How to develop a researcher mindset as a PhD student
Entering the postgraduate sphere is a whole new ball game. Shaif Uddin Ahammed shows how to hone a PhD mindset
Shaif Uddin Ahammed

Created in partnership with

You may also like

Popular resources
.css-1txxx8u{overflow:hidden;max-height:81px;text-indent:0px;} Students using generative AI to write essays isn't a crisis
How students’ genai skills affect assignment instructions, turn individual wins into team achievements in group work, access and equity: two crucial aspects of applied learning, emotions and learning: what role do emotions play in how and why students learn.
Life as a PhD student is challenging – and one of the most testing aspects of it is the change in mindset it requires.
You switch from being a consumer of knowledge to a producer of knowledge. In other words, you transition from passively absorbing information to actively generating new insights through original research. To do that, you have to develop the mindset of a researcher. Here, I’ll reflect on my own academic journey and experiences of supervising others, to share my thoughts on how to do just that.
Have a career plan
A PhD can be long and the prospect of writing a thesis is daunting. It can even be distracting, because you’re leaving the very idea of long-term goals on the back burner.
- Viving la viva: how to answer viva questions
- What I have learned on the journey towards commercialising my PhD
- Five tips for surviving your doctorate after moving over from industry
That’s exactly why it’s worth having a career plan. It will remind you why you’re doing all of this and carry you through the more draining aspects of your studies and research. Trust me, this will help.
But there’s a difference between simply having goals and having a plan. A plan involves steps to help you achieve the goals you’re aiming towards and gives you boxes to tick. For example, your plan could involve attending conferences, publishing articles and teaching and supporting students. It should also identify skills gaps and outline plans to address them.
Make sure your targets are realistic and achievable, and discuss them with your supervisor, who will guide you accordingly. Having a well-considered plan will help to motivate you and provide a map to help you chart your progress. Aside from anything else, this is important in helping you maintain a healthy work-life balance.
Take every opportunity that you can to learn
If you’re studying towards a PhD, you have already demonstrated a desire to learn. Make sure you now take every opportunity to do so and that you learn from sources beyond your supervisor or supervisory team.
Postgraduate research students can attend regular events and workshops organised by the academic skills teams and career advisors within their universities. By leveraging these resources, you can develop the knowledge and skills required to complete your doctoral degree and also learn about the skills required to secure a job with potential employers.
It is particularly important to attend workshops organised by the university’s doctoral school. I would strongly urge you not to ignore these sessions. Some students choose to select only those workshops they believe will be beneficial, but attending all workshops – particularly in the early stages of your degree – will help you to develop skills and knowledge that could prove vital in the future.
For instance, if you are a qualitative researcher, you might choose only to attend workshops related to qualitative research. However, in a future job you might need to teach quantitative methodology or be involved in research using quantitative methods. So it’s good practice not to be selective and to attend all workshops, allowing you to gain wider knowledge and develop networks with individuals from diverse backgrounds.
Involve yourself in academic activities
In research-related careers, applicants are generally expected to have experience of teaching, so it’s hugely important to actively seek teaching and supervisory opportunities both within your university and outside of it. You should also engage in grant applications with others, including your supervisory team – this will provide hands-on experience of the daily challenges faced by academics.
Many PhD students – and even some supervisors – think these activities could delay the completion of a doctoral degree, but they really do help you to acquire the skills you will need going forward. Supervising undergraduate and postgraduate students will offer insight into mentoring and managing expectations, including those of your supervisor. Involvement with teaching and assessments will give you an intuition when it comes to academic life, and the opportunity to directly apply new skills with the students you work with. This will foster the mindset that you are not only a PhD student but also an active academic.
Attend conferences and engage with journals
Seek out opportunities to publish in academic journals and attend relevant conferences. If you don’t, your work might not have the desired impact, regardless of its merit.
Conferences offer a platform for feedback, peer review opportunities, research visibility and invaluable networking. Similarly, involvement in publications and conferences can inspire new ideas and perspectives for research.
The PhD journey is never an easy one, given the number of commitments involved. Remind yourself that you are a researcher and an academic, and that your work has the potential to shape knowledge and understanding for years to come. Research is challenging – but if you’re in a position to study for a PhD, that means you already have the tools to overcome them.
Shaif Uddin Ahammed is programme leader of MSc International Management and lecturer in strategy and leadership at the University of the West of Scotland.
If you would like advice and insight from academics and university staff delivered direct to your inbox each week, sign up for the Campus newsletter .
Students using generative AI to write essays isn't a crisis
Eleven ways to support international students, indigenising teaching through traditional knowledge, seven exercises to use in your gender studies classes, rather than restrict the use of ai, embrace the challenge, how hard can it be testing ai detection tools.
Register for free
and unlock a host of features on the THE site

- Education & Teaching
- Schools & Teaching

Download the free Kindle app and start reading Kindle books instantly on your smartphone, tablet, or computer - no Kindle device required .
Read instantly on your browser with Kindle for Web.
Using your mobile phone camera - scan the code below and download the Kindle app.

Image Unavailable

- To view this video download Flash Player
Research Methods for Successful PhD (River Publishers Series in Innovation and Change in Education - Cross-cultural Perspective) 1st Edition
Purchase options and add-ons.
- ISBN-10 8793609183
- ISBN-13 978-8793609181
- Edition 1st
- Publisher River Publishers
- Publication date November 30, 2017
- Language English
- Dimensions 6.5 x 0.5 x 9.5 inches
- Print length 192 pages
- See all details

Editorial Reviews
About the author.
Dinesh Kumar
Product details
- Publisher : River Publishers; 1st edition (November 30, 2017)
- Language : English
- Hardcover : 192 pages
- ISBN-10 : 8793609183
- ISBN-13 : 978-8793609181
- Item Weight : 16 ounces
- Dimensions : 6.5 x 0.5 x 9.5 inches
- #7,048 in Education Research (Books)
- #10,550 in Energy Production & Extraction
- #56,518 in Education (Books)
Customer reviews
Customer Reviews, including Product Star Ratings help customers to learn more about the product and decide whether it is the right product for them.
To calculate the overall star rating and percentage breakdown by star, we don’t use a simple average. Instead, our system considers things like how recent a review is and if the reviewer bought the item on Amazon. It also analyzed reviews to verify trustworthiness.
No customer reviews
- Amazon Newsletter
- About Amazon
- Accessibility
- Sustainability
- Press Center
- Investor Relations
- Amazon Devices
- Amazon Science
- Sell on Amazon
- Sell apps on Amazon
- Supply to Amazon
- Protect & Build Your Brand
- Become an Affiliate
- Become a Delivery Driver
- Start a Package Delivery Business
- Advertise Your Products
- Self-Publish with Us
- Become an Amazon Hub Partner
- › See More Ways to Make Money
- Amazon Visa
- Amazon Store Card
- Amazon Secured Card
- Amazon Business Card
- Shop with Points
- Credit Card Marketplace
- Reload Your Balance
- Amazon Currency Converter
- Your Account
- Your Orders
- Shipping Rates & Policies
- Amazon Prime
- Returns & Replacements
- Manage Your Content and Devices
- Recalls and Product Safety Alerts
- Conditions of Use
- Privacy Notice
- Consumer Health Data Privacy Disclosure
- Your Ads Privacy Choices
At the New York Fed, our mission is to make the U.S. economy stronger and the financial system more stable for all segments of society. We do this by executing monetary policy, providing financial services, supervising banks and conducting research and providing expertise on issues that impact the nation and communities we serve.

Introducing the New York Innovation Center: Delivering a central bank innovation execution

Do you have a Freedom of Information request? Learn how to submit it.

Learn about the history of the New York Fed and central banking in the United States through articles, speeches, photos and video.
Markets & Policy Implementation
- Effective Federal Funds Rate
- Overnight Bank Funding Rate
- Secured Overnight Financing Rate
- SOFR Averages & Index
- Broad General Collateral Rate
- Tri-Party General Collateral Rate
- Treasury Securities
- Agency Mortgage-Backed Securities
- Repos & Reverse Repos
- Securities Lending
- Central Bank Liquidity Swaps
- System Open Market Account Holdings
- Primary Dealer Statistics
- Historical Transaction Data
- Agency Commercial Mortgage-Backed Securities
- Agency Debt Securities
- Discount Window
- Treasury Debt Auctions & Buybacks as Fiscal Agent
- Foreign Exchange
- Foreign Reserves Management
- Central Bank Swap Arrangements
- ACROSS MARKETS
- Actions Related to COVID-19
- Statements & Operating Policies
- Survey of Primary Dealers
- Survey of Market Participants
- Annual Reports
- Primary Dealers
- Reverse Repo Counterparties
- Foreign Exchange Counterparties
- Foreign Reserves Management Counterparties
- Operational Readiness
- Central Bank & International Account Services
- Programs Archive
As part of our core mission, we supervise and regulate financial institutions in the Second District. Our primary objective is to maintain a safe and competitive U.S. and global banking system.

The Governance & Culture Reform hub is designed to foster discussion about corporate governance and the reform of culture and behavior in the financial services industry.

Need to file a report with the New York Fed? Here are all of the forms, instructions and other information related to regulatory and statistical reporting in one spot.

The New York Fed works to protect consumers as well as provides information and resources on how to avoid and report specific scams.
The Federal Reserve Bank of New York works to promote sound and well-functioning financial systems and markets through its provision of industry and payment services, advancement of infrastructure reform in key markets and training and educational support to international institutions.

The New York Fed provides a wide range of payment services for financial institutions and the U.S. government.

The New York Fed offers several specialized courses designed for central bankers and financial supervisors.

The New York Fed has been working with tri-party repo market participants to make changes to improve the resiliency of the market to financial stress.
- High School Fed Challenge
- College Fed Challenge
- Teacher Professional Development
- Classroom Visits
- Museum & Learning Center Visits
- Educational Comic Books
- Lesson Plans and Resources
- Economic Education Calendar

We are connecting emerging solutions with funding in three areas—health, household financial stability, and climate—to improve life for underserved communities. Learn more by reading our strategy.

The Economic Inequality & Equitable Growth hub is a collection of research, analysis and convenings to help better understand economic inequality.

This Economist Spotlight Series is created for middle school and high school students to spark curiosity and interest in economics as an area of study and a future career.

« Taking Stock: Dollar Assets, Gold, and Official Foreign Exchange Reserves | Main | Can Discount Window Stigma Be Cured? »
Thinking of Pursuing a PhD in Economics? Info on Graduate School and Beyond
Kasey Chatterji-Len and Anna Kovner

Becoming a PhD economist can provide a fulfilling and financially secure career path. However, getting started in the field can be daunting if you don’t know much about the preparation you’ll need and the available job opportunities. If you’re wondering what it means to be an economics researcher or how to become one, please read on. We’ll review how to prepare for a career in economics research, what an economics PhD program entails, and what types of opportunities it might bring. Economic education is a core component of the Federal Reserve Bank of New York’s mission to serve the community. To empower would-be economists, this post provides information for students who seek a career in economics research. We hope this information will be helpful to students interested in economics, regardless of their background and economic situation. This information is most applicable to students applying to programs in the United States.
The Breadth of Economics Research
Academic disciplines conduct research in different ways, so it’s important to have a basic understanding of the types of questions economists ask and how they approach answering them. There are many definitions of economics, but a broadly useful one is the study of how people, organizations, and governments make decisions under different constraints, and how those decisions may affect their outcomes.
When answering these questions, economists seek to ground their analyses in models and to be quantitatively precise about the effects they assign to any given cause. The range of topics economists can study is wide, but the accepted approaches to answering questions are stricter. Some examples of what economists might ask:
- How do different public housing programs affect the children who live there?
- Does a certain type of law encourage businesses to innovate?
- How will a change in the interest rate affect inflation and unemployment rates?
- How much does affordable health insurance improve people’s health?
- How can poor countries eradicate poverty?
There are many different subfields within economics, including, but not limited to behavioral, econometrics, energy/environmental, development, financial, international, monetary, public, and urban economics. You can familiarize yourself with the latest work in economics by subscribing to working paper series, such as NBER’s New This Week or the New York Fed’s Staff Reports . To get an idea of the breadth of questions economists can answer, you could listen to Stephen Dubner’s “ Freakonomics Radio ” podcast. You may also want to explore the Journal of Economic Perspectives , the New York Fed’s Liberty Street Economics blog, VoxDev , or VoxEU .
What Is a PhD Program Like?
Economics PhD programs typically last five to seven years. Unlike masters programs, they are often fully funded with a stipend, though most require students to complete teaching assistant and/or research assistant (RA) work as part of their funding package. In the first two years, students take classes, many of which are mathematically demanding. The rest of the program can include additional classes but is primarily devoted to original research with the aim of producing publishable papers that will constitute the dissertation.
Faculty advisors are a central part of PhD programs, as students look to them for guidance during the research process. Economics PhD programs are offered within university economics departments, but there are similar programs in public policy and business schools. You can look at their websites to understand any differences in coursework and subsequent job placements.
What Can You Do with an Economics PhD?
Upon graduation, students can obtain jobs in a variety of industries. Many PhD students hope to become university professors. Governments and public policy-related institutions such as the Federal Reserve System, the U.S. federal government, the World Bank, and the International Monetary Fund (IMF) also hire economists to work on policy, lead programs, and conduct research. Finally, economics PhD graduates can also find employment at a variety of private sector companies, including banks, economic consulting firms, and big tech companies. The pay for these different positions can vary. According to the American Economics Association (AEA), the average starting salary for economics assistant professors in 2022-23 was approximately $140,000 at PhD granting institutions and $98,000 at BA granting institutions.
Programs often publish the placements of their PhD graduates, so you can look online to see specific employment outcomes. See, for example, the University of Maryland’s placements . Ultimately, economists are highly regarded as authorities on a variety of topics. Governments, nonprofits, philanthropic foundations, financial institutions, and non-financial businesses all look to economists to answer important questions about how to best achieve their goals. Thus, earning an economics Ph.D. can potentially help you to influence issues that are important to you.
Preparing for an Economics PhD Program
There are several components to an economics PhD program application: college transcripts, GRE scores, letters of recommendation, and personal statements. Please download the Appendix linked below to learn more about transcripts and letters of recommendation. The Appendix details ways in which you can select coursework, obtain research experience, and develop relationships to position yourself for success as a PhD applicant.
If you feel that you are too far along in your academic career to take enough of the classes described in the Appendix, this does not necessarily preclude you from pursuing an economics PhD. For example, it’s possible to take some of these classes through a master’s program, or through a pre-doctoral RA job. Some pre-doctoral RA jobs, such as the one here at the New York Fed , may enable you to take classes in preparation for graduate school. If you are concerned about your transcript, reach out to an economist at your university for advice; program standards for coursework and grades vary, and it’s a good idea to get more personalized advice.
Research Experience
If you’re interested in becoming an economics researcher and applying to PhD programs, it’s best to get research experience as soon as possible. Working as an RA is a great way to learn how to conduct research and get a better idea of whether it’s the right career path for you. Additionally, it can help you obtain a letter of recommendation for graduate school applications and improve your qualifications.
All types of academic research can be enriching, but it’s beneficial to gain experience working directly with an economist. To find a position, you can reach out to professors whose work you find interesting or find an RA program at your school. Typical RA tasks may involve data collection and cleaning, as well as running analyses and creating charts to represent results. This is where coding skills become crucial; having taken math, statistics, and econometrics courses will also enable you to take on more responsibilities.
You may also have the opportunity to conduct your own research, possibly under the supervision of a professor at your university. This research could be self-initiated or part of a course such as a thesis workshop. Self-directed research is a great opportunity to learn about all stages of the research process. It’s also an excellent opportunity to create a writing sample for graduate school applications. Ultimately, though, your motivation for conducting your own research project should be that you want to answer a question. One thing economists have in common is a love of answering questions using data and theory.
Research experience is also often obtained after completing an undergraduate or master’s degree. Taking on a full-time RA position before applying to PhD programs is very common and can make you a more competitive applicant. You may either get an RA job working for a professor or participate in a pre-doctoral RA program.
Research assistant programs are more structured than positions with individual professors or projects, which could be helpful. Universities, parts of the government, think tanks, research organizations, and the Federal Reserve System are all good places to look for research assistant programs. To help you decide which opportunities are most desirable, you may want to ask potential employers : Where do people in this program tend to go afterward? Will I be working directly with an economist? How much of my time will be spent on academic research work? Will I be able to take classes as part of this program? Considering whether an economist will be able to evaluate your performance is an important factor for recommendation letters. The ability to take classes, either through tuition reimbursement or waivers, can also be an important benefit.
The Research Analyst program here at the Federal Reserve Bank of New York is one example of these programs and you should check it out here . The Federal Reserve Board of Governors also has a large program, and many other regional Federal Reserve Banks have similar programs. In addition, the PREDOC website and the NBER post listings of RA opportunities. J-PAL and IPA also tend to recruit RAs for economic development projects. Another source of RA opportunities is the @econ_ra account on X.
Who Should Get a PhD in Economics?
A PhD may not be for everyone, but it is for anyone—people of all genders, religions, ethnicities, races, and national origins have PhDs in economics. Many economists majored in economics, but others majored in math, physics, or chemistry. Because economics is such an integral part of policymaking, it is important that economists come from a wide range of backgrounds so policy can be stronger and more effective. The inclusion of differing perspectives helps ensure that the contribution of economists to work in public policy, academia, and beyond effectively serves the broadest range of society.
- Coursework Appendix

Kasey Chatterji-Len is a research analyst in the Federal Reserve Bank of New York’s Research and Statistics Group.

Anna Kovner is the director of Financial Stability Policy Research in the Bank’s Research and Statistics Group.
How to cite this post: Kasey Chatterji-Len and Anna Kovner, “Thinking of Pursuing a PhD in Economics? Info on Graduate School and Beyond,” Federal Reserve Bank of New York Liberty Street Economics , May 31, 2024, https://libertystreeteconomics.newyorkfed.org/2024/05/thinking-of-pursuing-a-phd-in-economics-info-on-graduate-school-and-beyond/.
You may also be interested in: AEA: Resources for Students
PREDOC: Guidance for Undergraduates
RA Positions-Not at the NBER
Disclaimer The views expressed in this post are those of the author(s) and do not necessarily reflect the position of the Federal Reserve Bank of New York or the Federal Reserve System. Any errors or omissions are the responsibility of the author(s).
Share this:
Post a comment
Your email address will not be published. Required fields are marked *
(Name is required. Email address will not be displayed with the comment.)
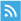
Liberty Street Economics features insight and analysis from New York Fed economists working at the intersection of research and policy. Launched in 2011, the blog takes its name from the Bank’s headquarters at 33 Liberty Street in Manhattan’s Financial District.
The editors are Michael Fleming, Andrew Haughwout, Thomas Klitgaard, and Asani Sarkar, all economists in the Bank’s Research Group.
Liberty Street Economics does not publish new posts during the blackout periods surrounding Federal Open Market Committee meetings.
The views expressed are those of the authors, and do not necessarily reflect the position of the New York Fed or the Federal Reserve System.
Economic Inequality
Most Read this Year
- Credit Card Delinquencies Continue to Rise—Who Is Missing Payments?
- The Post-Pandemic r*
- Spending Down Pandemic Savings Is an “Only-in-the-U.S.” Phenomenon
- The Evolution of Short-Run r* after the Pandemic
- Auto Loan Delinquency Revs Up as Car Prices Stress Budgets
- Economic Indicators Calendar
- FRED (Federal Reserve Economic Data)
- Economic Roundtable
- OECD Insights
- World Bank/All about Finance
We encourage your comments and queries on our posts and will publish them (below the post) subject to the following guidelines:
Please be brief : Comments are limited to 1,500 characters.
Please be aware: Comments submitted shortly before or during the FOMC blackout may not be published until after the blackout.
Please be relevant: Comments are moderated and will not appear until they have been reviewed to ensure that they are substantive and clearly related to the topic of the post.
Please be respectful: We reserve the right not to post any comment, and will not post comments that are abusive, harassing, obscene, or commercial in nature. No notice will be given regarding whether a submission will or will not be posted.
Comments with links: Please do not include any links in your comment, even if you feel the links will contribute to the discussion. Comments with links will not be posted.
Send Us Feedback
The LSE editors ask authors submitting a post to the blog to confirm that they have no conflicts of interest as defined by the American Economic Association in its Disclosure Policy. If an author has sources of financial support or other interests that could be perceived as influencing the research presented in the post, we disclose that fact in a statement prepared by the author and appended to the author information at the end of the post. If the author has no such interests to disclose, no statement is provided. Note, however, that we do indicate in all cases if a data vendor or other party has a right to review a post.
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- Request a Speaker
- International, Seminars & Training
- Governance & Culture Reform
- Data Visualization
- Economic Research Tracker
- Markets Data APIs
- Terms of Use

2024 Theses Doctoral
Artificial Intelligence vs. Human Coaches: A Mixed Methods Randomized Controlled Experiment on Client Experiences and Outcomes
Barger, Amber
The rise of artificial intelligence (AI) challenges us to explore whether human-to-human relationships can extend to AI, potentially reshaping the future of coaching. The purpose of this study was to examine client perceptions of being coached by a simulated AI coach, who was embodied as a vocally conversational live-motion avatar, compared to client perceptions of a human coach. It explored if and how client ratings of coaching process measures and outcome measures aligned between the two coach treatments. In this mixed methods randomized controlled trial (RCT), 81 graduate students enrolled in the study and identified a personally relevant goal to pursue. The study deployed an alternative-treatments between-subjects design, with one-third of participants receiving coaching from simulated AI coaches, another third engaging with seasoned human coaches, and the rest forming the control group. Both treatment groups had one 60-minute session guided by the CLEAR (contract, listen, explore, action, review) coaching model to support each person to gain clarity about their goal and identify specific behaviors that could help each make progress towards their goal. Quantitative data were captured through three surveys and qualitative input was captured through open-ended survey questions and 27 debrief interviews. The study utilized a Wizard of Oz technique from human-computer interaction research, ingeniously designed to sidestep the rapid obsolescence of technology by simulating an advanced AI coaching experience where participants unknowingly interacted with professional human coaches, enabling the assessment of responses to AI coaching in the absence of fully developed autonomous AI systems. The aim was to glean insights into client reactions to a future, fully autonomous AI with the expert capabilities of a human coach. Contrary to expectations from previous literature, participants did not rate professional human coaches higher than simulated AI coaches in terms of working alliance, session value, or outcomes, which included self-rated competence and goal achievement. In fact, both coached groups made significant progress compared to the control group, with participants convincingly engaging with their respective coaches, as confirmed by a novel believability index. The findings challenge prevailing assumptions about human uniqueness in relation to technology. The rapid advancement of AI suggests a revolutionary shift in coaching, where AI could take on a central and surprisingly effective role, redefining what we thought only human coaches could do and reshaping their role in the age of AI.
- Adult education
- Artificial intelligence--Educational applications
- Graduate students
- Educational technology--Evaluation
- Education, Higher--Technological innovations
- Education, Higher--Effect of technological innovations on
This item is currently under embargo. It will be available starting 2029-05-14.
More About This Work
- DOI Copy DOI to clipboard
- Open access
- Published: 30 May 2024
CRISPR-Cas and CRISPR-based screening system for precise gene editing and targeted cancer therapy
- Mingming Qin 1 , 2 na1 ,
- Chunhao Deng 3 na1 ,
- Liewei Wen 4 ,
- Guoqun Luo 1 &
- Ya Meng 4
Journal of Translational Medicine volume 22 , Article number: 516 ( 2024 ) Cite this article
105 Accesses
Metrics details
Target cancer therapy has been developed for clinical cancer treatment based on the discovery of CRISPR (clustered regularly interspaced short palindromic repeat) -Cas system. This forefront and cutting-edge scientific technique improves the cancer research into molecular level and is currently widely utilized in genetic investigation and clinical precision cancer therapy. In this review, we summarized the genetic modification by CRISPR/Cas and CRISPR screening system, discussed key components for successful CRISPR screening, including Cas enzymes, guide RNA (gRNA) libraries, target cells or organs. Furthermore, we focused on the application for CAR-T cell therapy, drug target, drug screening, or drug selection in both ex vivo and in vivo with CRISPR screening system. In addition, we elucidated the advantages and potential obstacles of CRISPR system in precision clinical medicine and described the prospects for future genetic therapy.
In summary, we provide a comprehensive and practical perspective on the development of CRISPR/Cas and CRISPR screening system for the treatment of cancer defects, aiming to further improve the precision and accuracy for clinical treatment and individualized gene therapy.
Introduction
Cancer therapy has been developed from the very initial surgical removal in the ancient to currently precision minimally invasive surgery; from the chemotherapy, radiotherapy to the targeted therapy and precision individualized immunotherapy, under the progress of precise and granular molecular characterization at present [ 1 ]. The newly discovered genome editing tool CRISPR (clustered regularly interspaced short palindromic repeat) /Cas system provides a powerful method for the investigation of cancer therapy [ 2 , 3 , 4 ]. It was described initially in bacteria as a primitive immune system to fight against viral infections and was universally recognized as a genomic modification system in the past decade [ 5 , 6 ]. In Prokaryotes, the short DNA repeats CRISPR exist between regular spacing units, and are recognized as intervening sequences derived from preexisting fragment of bacteriophages and conjugative plasmids, contributing to bacteria immune system [ 7 ]. The genetic sequences of the viral invaders or plasmid challengers are captured and aligned as spacer segments in the CRISPR region in bacteria or archaea [ 8 , 9 ], comprising the CRISPR-mediated adaptive immunity system [ 10 ]. Two classes of CRISPR-Cas systems have been described in prokaryotes based on their effector modules [ 11 , 12 , 13 , 14 ], characterized into 6 types, and 33 subtypes described in 2020 [ 15 ]. The Class 2 CRISPR-Cas system composed only 10% percentage but has expanded biotechnology toolbox for genome editing with 190,000 shares worldwide from 640 labs [ 16 , 17 ]. It consists of three types of effectors: type II, type V and type VI, with several widely recognized genetic editing enzymes, being Cas9 in type II, Cas12a (Cpf1), Cas12b (C2c1), Cas12c (C2c3), Cas14 subgroup in type V [ 18 ], Cas13a (C2c2), Cas13b (C2c6) and Cas13c (C2c7) in type VI [ 14 , 19 ]. Schematic representation of two classes of CRISPR/Cas systems were depicted in Fig. 1 .
Schematic representative of CRISPR/Cas loci in Class 1 and Class 2 system. Class 1 system show multi-component effectors, while the Class 2 system have one effector. Three subgroups of Class 2 CRISPR systems are presented. Representative Type II-A CRISPR protein contains: Streptococcus pyogenes Cas9 (SpCas9), Staphylococcus aureus Cas9 (SaCas9) and Streptococcus thermophilus Cas9 (StrCas9), all of which have the tracrRNA sequences. Type V CRISPR, which comprises Cas12a, Cas12b and Cas12c, exhibits distinct genome structures. Cas12b has the tracrRNA structure, while Cas12c only has one assistant protein cas1 for genome editing. Cas14 subgroup is not depicted in this figure. Type VI CRISPR systems show few assistant proteins to identify RNA virus, however, type VI-B has csx27 and csx28 proteins to regulate nuclease activity. Illustrated according to Ref [ 14 , 16 , 20 , 21 ].
CRISPR/Cas system has been utilized for cellular genetic modification [ 22 , 23 ] and the generation of animal models for cancer research [ 24 , 25 ]. Furthermore, the CRISPR/Cas-based genetic screening system was developed for cellular investigation [ 26 , 27 , 28 ], as well as in tumor studies [ 25 , 29 ]. In addition, high throughput gRNA libraries have been established to enable efficient genetic screening, specially facilitating personalized treatment strategies for cancer patients individually [ 30 ]. In this review, we provide a comprehensive overview of the CRISPR/Cas system and essential elements for successful CRISPR screening system, including gRNA libraries, gRNA validation, and clinical application for cancer research. Furthermore, we explored the application of the CRISPR screening system in cancer therapy from both ex vivo and in vivo investigation, aiming to elucidate the inherent advantages and potential obstacles for clinical precision medicine.

The application of Class 2 CRISPR-Cas effectors and genome modification in cancer therapy
Type ii effector cas9 in cancer research.
Both Streptococcus pyogenes Cas9 (SpCas9) and Staphylococcus aureus Cas9 (SaCas9), classified as the type II-A effectors, showed comparable genome editing efficiency for in vitro and in vivo study [ 21 , 31 , 32 , 33 ]. These effectors enable rapid modification of cellular or animal models for transcriptional modulation via CRISPR knockout/knockin or high throughput genomic screening [ 23 , 34 ]. The compact size of SaCas9 renders it an optimal enzyme for in vivo AAV application. However, SpCas9, one of the pioneering Cas9 proteins, has been extensively investigated and utilized in CRISPR gene editing. Three variants of SpCas9 have been developed, the wild-type Cas9, nickase Cas9 (nCas9), and dead Cas9 (dCas9).
Cas9 mediated DNA cleavage with the two distinct active sites RuvC and HNH, under the assistance of CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA) ribonucleoprotein complex [ 8 ]. The dual-tracrRNA: crRNA chimera single guide RNA (sgRNA) was created and directed Cas9 nuclease to the potential target loci for site-specific DNA cleavage, initiating the genome editing system in vitro [ 35 ]. The binding of Cas9 to the adjacent sequence of three nucleotides, known as protospacer adjacent motif (PAM), triggers DNA cleavage by inducing double-strand breaks with its scissor-like activity [ 36 ]. The recently used Cas9-gRNA ribonucleoprotein (RNP) complexes remarkably increase fidelity and efficacy for double-strand DNA breaks with minimized cell mortality [ 37 ]. It also combined with repair donor to achieve site-specific correction of cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in epithelial organoids [ 38 ]. Cre-dependent Cas9 knockin mouse was generated, and KRAS, p53 , and LKB1 depletion resulted in carcinoma formation in these transgenic mice, providing a robust cancer model for research [ 24 ].
One mutation in D10A of Cas9 protein makes a nCas9, which improves genome editing specificity [ 39 ]. The combination of sgRNA pairs with nCas9 significantly enhances cutting specificity by 50-1000 folds in cell lines and mouse zygotes [ 40 ]. CRISPR-Cas base editing using nCas9 enables precise incorporation of point mutations in genomic DNA without inducing double-strand breaks, demonstrating its potential in treating genetic diseases caused by base-pair alterations through adenine base editors (ABEs) or cytosine base editors (CBEs) [ 41 ]. In addition, DNA base editors combining with the leading platform adeno-associated virus (AAV) vector for viral delivery expanded the CRISPR-base-edit toolkit for Prime-editing (PE) [ 42 ]. Meanwhile, the recently developed genome editing technique known as NICER utilizes Cas9 D10A nickase to correct heterozygous mutations. It generates multiple DNA nicks and triggers gene correction via interhomolog homologous recombination (IH-HR) which rarely induces genomic alterations, making it a precise strategy to restore genetic diseases or single nucleotide mutations [ 43 ]. Except the precise single nucleotide restoration, cancer translocations were generated by double strand breaks and paired nicks with either Cas9 or nCas9, creating endogenous chromosomal translocations cell model for investigating tumor driving genes [ 44 ].
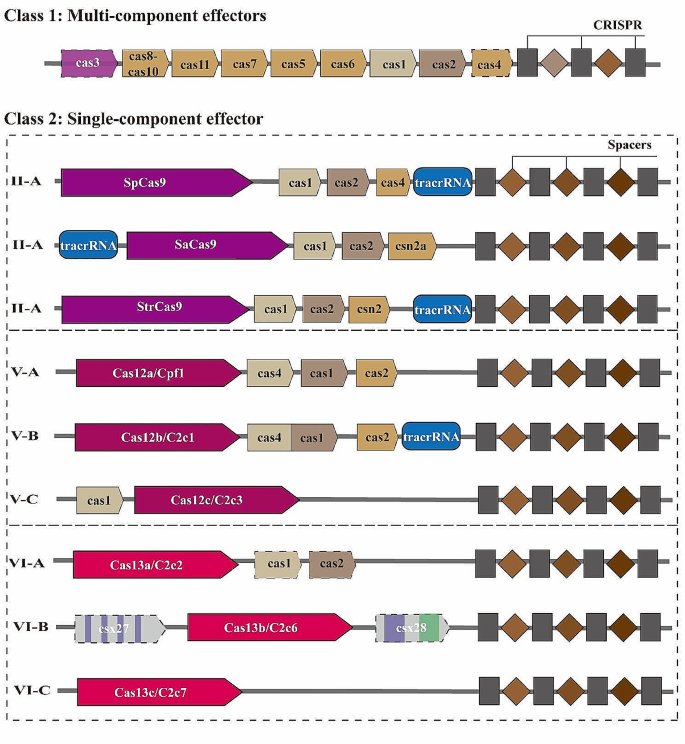
Catalytically inactive Cas9, a ‘dead’ protein (dCas9) with both mutations in D10A and H840A of RuvC and HNH domains, showed its popularity in gene regulation with inhibition, activation, and cell imaging and labeling [ 45 ]. Genome-scale screenings utilizing CRISPR inhibition (CRISPRi) and CRISPR activation (CRISPRa) have been employed to identify both known and novel genes involved in controlling cell growth and sensitivity to toxins [ 46 ]. Precise inducible gene knockdown or overexpression can be supported using dCas9-KRAB (Krüppel-associated box) or Cas9 combined with Tetracycline Inducible Expression promoter (TetO) [ 47 ]. Firstly, the fusion of dCas9 with transcriptional repressor produces the CRISPRi genetic tool [ 48 ]. The dCas9-BFP-KRAB repressor domain enables the suppression of gene expression [ 49 ]. Second, fusing dCas9 with RNA polymerase (RNAP) omega subunit upregulates gene expression [ 50 ], and dCas9-VP64 was used for transcriptional activation [ 51 ]. In addition, dCas9 protein serves as a valuable tool for labeling of endogenous genomic loci in living cells. By employing an optimized sgRNA fused with EGFP-tagged dCas9, repetitive elements in telomeres and various other regions can be robustly labeled [ 52 ]. A double-color CRISPR labeling method was established by incorporating MS2 or PP7 RNA aptamers into the sgRNA, fused with the catalytically inactive Cas9 (dCas9) for direct visualization [ 53 ]. Finally, dCas9 can be employed for in vivo imaging of chromosomal dynamics and genome organization dimensions [ 47 ], allowing systematic fluorescent labeling of up to 10 proteins [ 48 ]. Summary of the type II Cas9 enzymes was depicted in Fig. 2 .
Summary of Cas9 proteins and modified nCas9 and dCas9 genome editing tools. (A) PAM for SpCas9 is NGG, while PAM for SaCas9 is NNGRRT with the ability to cut DNA double helix. (B) Mutation of D10A leads to the formation of nCas9 while both mutations generate dCas9 protein. (C) nCas9 can be applied for base editing such as CBE and ABE, also for Base editor and developed as NICER to repair heterogenous mutation. (D) dCas9 was modified to generate CRISPRi, CRISPRa and CRISPR labeling tools. dCas9: dead Cas9. nCas9: nickase Cas9. CBE: Cytosine Base Editor, ABE: Adenine Base Editor. RT: reverse transcriptase. pegRNA: prime editing guide RNA.
Type V and type VI effectors in cancer research
Mainly three subtypes of type V effectors were investigated for gene editing, named as type V-A, V-B and V-C. The type V-A effector Cpf1 (CRISPR from Prevotella and Francisella 1 ), exhibits enhanced genome editing specificity attributed to a T-rich PAM (-5′TTTV) [ 54 ], resulting in a staggered DNA double stranded break [ 55 ]. Two candidate Cpf1 (Cas12a) enzymes, AsCpf1 from Acidominococcus sp. BV3L6 and LbCpf1 from Lachnospiraceae bacterium ND2006 , show a robust genome editing ability in human cells compared to that of Cas9 [ 56 ]. Furthermore, successful generation of gene knockout transgenic mice was achieved using both AsCpf1 (40.7%) and LbCpf1 (28.6%), providing a wonderful animal model for research [ 57 , 58 ]. Multiplex genome editing was conducted using Cpf1 from Aspergillus aculeatus strain TBRC277 [ 59 ] and AsCpf1 was engineered with adeno-associated viral vectors (AAVs) for multiplex genome editing of mouse brain in vivo [ 60 ]. One-step generation of homology-directed repair (HDR) and checkpoint knockout CAR-T (KIKO CAR-T) was achieved with the adeno-associated virus and CRISPR/Cpf1 system, establishing an efficient AAV-Cpf1 double knockin system and opening new possibilities for cancer research [ 61 ]. The type V-B CRISPR effector Cas12b (C2c1) discovered in Bacillus hisashii (BhCas12b) showed a nickase effect at 37 °C for human gene editing, while BhCas12b v4, containing K846R/S893R/E837G mutants, demonstrated strong genome editing ability in human cells comparable to SpCas9 [ 62 ]. While the type V-C CRISPR effector Cas12c (C2c3) is a site-specific ribonuclease generating mature crRNAs for DNA targeting, crRNAs direct DNA binding by Cas12c without DNA cutting, providing a DNase-free pathway for transient antiviral immunity [ 63 ].
While both type II and type V are effective for DNA targeting in the genome level, the type VI effector Cas13 exhibits efficacy in treating genetic diseases and rescuing diseased sequences at the RNA level. They provide valuable genetic tools for diagnosis and degradation of viruses such as HIV and HPV [ 64 , 65 ]. Several Cas13 proteins were characterized, such as Cas13a, Cas13b, Cas13bt and Cas13d, showed the efficiency to cleave single stranded RNAs [ 66 , 67 , 68 ]. Of which Cas13a based SHERLOCK (Specific High-Sensitivity Enzymatic Reporter UnLOCKing) system can detect Zika or Dengue Virus as well as somatic mutations in cell free DNA (cfDNA) samples such as serine/threonine kinase (BRAF) V600E cancer mutation [ 69 ]. Shortened detection time and high sensitivity were applied for virus detection via SHERLPCKv2 system [ 70 , 71 ].
SHERLOCK enables to identify EGFR-T790M mutation in patient DNA with high efficiency by detecting 0.6% mutant ratio samples [ 72 ], this system was also used for DNA and RNA detection with single-base specificity and attomolar sensitivity in cancer patients samples [ 73 ]. Cas13b was used to fight RNA viruses such as porcine reproductive and respiratory syndrome virus (PRRSV) [ 74 ], chikungunya (CHIKV) and dengue in mosquito cells [ 75 ] as well as SARS-CoV-2 resistance [ 76 , 77 ]. Since Cas13b targets RNA without interfering genome sequence of the targeted gene, it provides a potential safer alternative to Cas9 enzymes. Catalytically inactive Cas13b (dCas13b) was engineered to direct adenosine-to-inosine deaminase for precise base editing, enabling the Programmable A to I Replacement (REPAIR) RNA editing platform. This platform can be utilized in transcriptome engineering of advanced leukemias, as well as head, liver, and breast cancers, thereby demonstrating a feasible strategy for investigating gene function in cancer at the RNA level [ 78 , 79 ]. The RNA-targeting CRISPR-Cas13 system showed promising roles in cancer diagnosis, therapy, and research; with the ability for early detection of cancer markers in liquid biopsy samples, degradation and manipulation of cancer-related mutant transcripts, as well as identification of novel therapeutic drug targets described in the recent review [ 80 ].
Altogether, the class 2 effectors expanded the current CRISPR/Cas toolkit. Cas9 possesses recognition ability of specific target sequences, and has the genomic editing ability for precision cancer treatment and mutation detection [ 2 ]. Meanwhile, the recently discovered Cas12 and Cas13 expand RNA editing tool, providing novel genetic methods for cancer diagnosis and molecular examination of cancer research [ 3 ].
The application of CRISPR screening system in cancer
The development of CRISPR/Cas system and high-throughput sequencing makes genetic screening easily accessible in basic biology, drug discovery, and personalized medicine for cancer therapy [ 3 ]. Cas9 nuclease is a preferred choice for genetic screening, and has been used for genomic modification in multiple researches [ 26 , 28 , 81 , 82 ]. One-step generation of multiplex genome mutations via CRISPR/Cas9 system was successfully achieved in mice, facilitating in vivo functional analysis of redundant genes [ 83 ]. CRISPR screening system was developed based on CRISPR/Cas combined with thousands of gRNAs integrated into viral vectors [ 81 , 84 ]. These libraries harbor gRNAs targeting various genes, and have received up to 1000 annual requests globally, enabling unbiased, phenotypic forward genetic screening [ 17 ]. The first whole genomic gRNA libraries for both mouse and human were generated with mouse lentiviral gRNA library containing 87,897 gRNAs for 19,150 coding genes, naming as (GeCKOv1), and was established to screen out unknown genes for Clostridium septicum alpha-toxin or 6-thioguanine (6TG) drug resistance [ 81 ]. However, low viral titer of the lentiviral delivery systems in GeCKOv1 limited the usage for biological screening, and genome-scale CRISPR knockout v2 (GeCKOv2), contained 123,411 unique sgRNAs targeting 19,050 annotated protein-coding genes and 1000 control sgRNAs (sg-NTCs), resulting in a 10-fold increase for viral generation [ 84 ]. Optimized mouse gRNA libraries targeting 20,611 genes with 130,209 gRNAs were also established with 100-fold increase of functional viral titer [ 84 ]. Innovative strategies of CRISPR-Cas9 system have been developed for large-scale genome knockout and transcriptional activation [ 85 ], as well as combinatorial genetic screening [ 27 ]. Processes for gRNA library generation and amplification were illustrated as depicted in the following Fig. 3 .
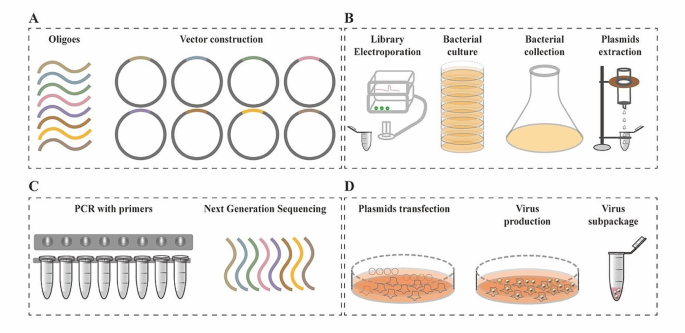
Schematic representation of gRNA library construction and virus production. (A) Oligoes synthesis and vector construction for gRNA library. (B) Amplification of gRNA library by bacterial culture, collection, and plasmid extraction. (C) PCR examination and sequence confirmation for library coverage. (D) Plasmids transfection and virus production with a certain gRNA library.
gRNA libraries for cancer research
Various of genome-scale gRNA libraries were established for CRISPR screening, and some gRNA libraries for specific selected genes were also established with small capacity. Established gRNA libraries of genome wide and specific selected targets for cancer research were summarized in the Table 1 .
Human lentiviral GeCKOv1 library (lentiCRISPRv1) was established for high throughput gene targeting of 18,080 genes, with 64,751 unique gRNAs total, and was used for cell viability-related gene screening in cancer. It was also examined for resistance to a therapeutic RAF inhibitor, vemurafenib, in a A375 melanoma model, leading to the discovery of novel genes sensitive to drug treatment [ 28 ]. GeCKOv2 library was also used to identify responsible genes related to EGF-induced apoptosis [ 86 ]. Genome-wide sgRNA library (mGeCKOa) transfection in non-metastatic mouse non-small cell lung cancer with 67,405 sgRNAs targeting 20,611 protein-coding genes. Cells were treated and transplanted into immunocompromised Nu/Nu mice, and tumor growth and migration were evaluated in vivo [ 25 ]. The pooled lentiviral sgRNA library with 73,151 gRNAs targeting 7114 gene and 100 non-targeting controls were used to screen the resistant genes for nucleotide analog 6TG treatment in human leukemic cell lines, screening resistance genes toward chemotherapeutic etoposide [ 26 ]. Patient-derived glioblastoma cell line (GBM), retinal epithelial cells (RPE1), colorectal carcinoma (HCT116 and DLD1), cervical carcinoma (Hela) and melanoma (A375) cells were subjected into genetic screening with the “90k library” containing 17,232 targeting genes and 91,320 gRNA sequences. Subsequentially, the supplemental library naming 176,500 TKO (Toronto KnockOut) library targeting 17,661 protein-coding genes were used to identify fitness genes in cancer cell lines [ 87 ]. Lentiviral vectors with genome-scale sgRNA library consisting of 70,290 guides (3 sgRNAs for each transcription start site (TSS)) were used for synthetic activation mediator (SAM)-based screening to target 200 bp upstream of the TSS and confer resistance to a BRAF inhibitor in melanoma cell line A375 and patient derived samples [ 51 ].
Although genome scale gRNA libraries are widely used in cancer research, its complexity and transcript isoform variance as well as difficulty in viral vectors cloning limited its usage. Other specific gRNA libraries for certain signal pathways or gene functions were established according to screening purpose for modulating endogenous genes. Total 5920 candidate enhancers were perturbed by the dCas9-KRAB enzyme, establishing the multiplex, expression quantitative trait locus (eQTL) framework, and total 664 cis enhancer-gene pairs were identified and enriched based on 254,974 single-cell transcriptomes in K562 derived from a chronic myologenous leukemia patient [ 49 ]. Undescribed immunotherapy targets for transplantable melanoma tumors in mice were explored with the 9992 sgRNAs targeting 2368 genes selected from transduced cells, establishing the in vivo genetic screen tumor models [ 88 ]. Recurrently mutated genes derived from pan-cancer The Cancer Genome Atlas datasets were recognized as well-known tumor suppressors genes (TSGs) or oncogenes. Total 49 orthologs of human TSGs were found in mouse genome, and the mouse TSG library containing 280 sgRNAs targeting 56 different genes (7 housekeeping genes) were used for tumor metastasis analysis [ 89 ].
The improvements of specificity and validation methods for gRNA Library
The procedure to perform pooled genome-editing experiments was clearly described, and successful CRISPR/Cas9 screening needs the specific and efficient gRNA sequence with proper quality and low off-target effect [ 91 ]. Off-target predictions calculated by algorithms indicating false positives and quantified error rates were developed by Bowtie and BWA sequencing methods, or considered by MIT-Broad score and the CFD score as summarized in previous reviews [ 92 ]. Computational tools for sgRNA designing with low off-target and high on-target efficacy and specificity have been developed and summarized in 2018 [ 93 ]. Several methods have been built for eliminating off-target results such as the utilization of high-efficiency delivery RNP tool, modification of the gRNA sequence, and improvement the specificity of Cas9 Enzymes [ 94 ]. The computational tool CRISPOR established high-quality gRNA libraries by selection according to off-target and on-target predictions, it also helps with vector cloning, gRNA validation and expression with primer designing and restriction enzymes depiction [ 95 ]. Optimized on-target efficiency prediction model was generated to illustrate the cleavage ability of gRNA sequence ( http://crispor.org ) [ 96 ]. Meanwhile, CRISPResso provides a robust and user-friendly computational pipeline to evaluate effects of coding and noncoding sequences and select off-target sites [ 97 ]. For precise gene selection analysis, the Model-based Analysis of Genome-wide CRISPR/Cas9 Knockout (MAGeCK) is the optimized method for both positive and negative selection, which offers high sensitivity and low FDR regardless of sequencing depth or sgRNA numbers for a single gene [ 98 ]. Besides that, intergration deficient lentiviral (IDLV) capture [ 99 ], and high-throughput genome-wide translocation sequencing (HTGTS) [ 100 ] are other methods for off-target detection.
Analysis of gRNAs abundance in pooled libraries plays an important role in targeting efficiency and screening accuracy and specificity. PCR products of gRNA library vectors can be sequenced on Hiseq 2500 and aligned to sgRNAs by Bowtie, an ultrafast, efficient program for aligning short DNA sequence to large genomes [ 101 ]. Rigorous analytical methods mitigate the false discovery rates generated by CRISPR screens via a Bayesian classifier of gene essentiality [ 102 ]. Sequence quality control can also be carried out under the guide of GPP Pooled Screen Analysis ( https://portals.broadinstitute.org/gpp/broad/ ), and statistical enrichment and gene depletion were calculated by hit calling algorithm STARS ( http://www.broadinstitute.org/rnai/public/software/index ) based on normalized fold changes [ 103 ]. High-content downstream gRNA library sequence validation in tumor immunology were summarized in the recent review [ 29 ]. Generally speaking, breaks labeling, enrichment on streptavidin and next-generation sequencing (BLESS) [ 104 ], genome-wide unbiased identification of DSBs enabled by sequencing (GUIDE-seq) [ 105 , 106 ] and discovery of in situ Cas off-targets and verification by sequencing (DISCOVER-seq) [ 107 , 108 , 109 ] were used as cell based methods with direct sequencing. More sensitive biochemical methods such as digested genome sequencing (Digenome-seq) [ 110 , 111 , 112 ], selective enrichment and identification of adapter-tagged DNA ends by sequencing (SITE-Seq) [ 113 ], circularization for in vitro reporting of cleavage effects by sequencing (CIRCLE-seq) [ 114 , 115 ] and circularization for high-throughput analysis of nuclease genome-wide effects by sequencing (CHANGE-seq) [ 116 ] were developed for accurate sequence confirmation.
CRISPR screening application in cancer therapy
The application of the CRISPR/Cas system for cancer therapy has been investigated using viral vectors including lentivirus, adenovirus, and AAV vectors, as well as non-viral vectors such as polymer nanoparticles, golden nanoparticles, or lipid nanoparticles in both ex vivo and in vivo circumstances as described in recent reviews [ 117 , 118 ]. Various cancer cell lines [ 2 , 4 , 87 , 119 , 120 ], T-cells via chimeric antigen receptor (CAR) integration or CAR-T system [ 90 , 121 , 122 ], and organoids derived from patient samples [ 123 ] have been explored for cancer therapy research. However, because of manipulation limitations in highly differentiated cells, in vivo clinical precision therapy involving modified cells with AAV vector delivery for the CRISPR modification system is widely used for a broad range of human diseases [ 118 ]. In this part, we mainly focus on the application of CRISPR screening system for cancer therapy, including ex vivo and in vivo approaches. Schematic representation of CRISPR screening applications for cancer research is summarized in Fig. 4 .
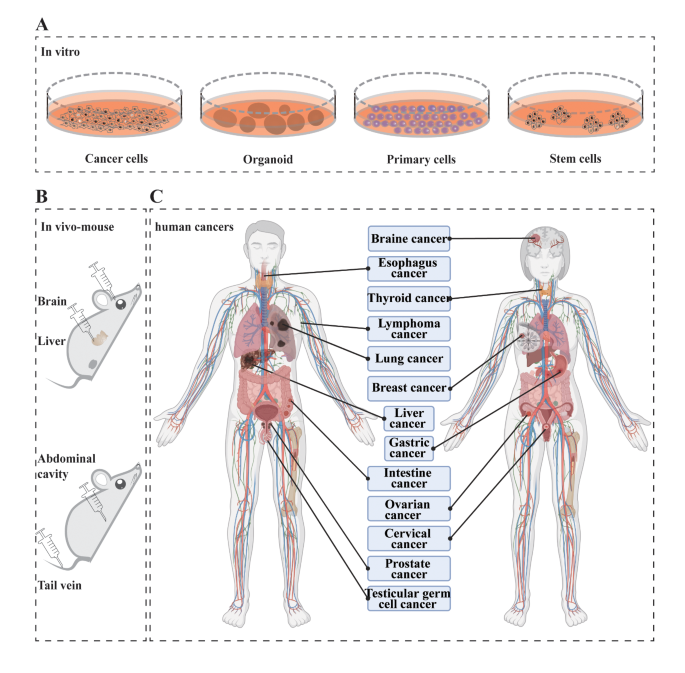
CRISPR screening and its applications in ex vivo and in vivo for cancer therapy. (A) CRISPR screening application in cultured cells. (B) CRISPR screening in vivo application in mouse with direct injection to organs and indirect injection in abdominal and tail vein. (C) Schematic representation of CRISPR screening applications for human cancers; Created with BioRender.com
CRISPR screening in vitro for cancer therapy
CRISPR screening has several potential applications in cancer therapy, including modified T cells and Chimeric antigen receptor CAR-T cancer treatment, novel target identification, drug resistance, drug selection exploration and so on [ 4 , 29 ]. The CRISPR screening system has been employed to investigate various cancer cell types originating from diverse organs including lymphatic system, esophagus, stomach, intestines, lungs, nervous system, skin, liver, blood cells as well as reproductive organs. CRISPR screening applications in Cancer therapy were summarized in Table 2 .
Modified T cell and CAR-T therapy for cancer therapy
Immune system is the most important defender to fight off cancer. Immunotherapy strategy is to make better immune cells such as tumor-infiltrating lymphocytes (TIL) or CAR-T cells to attack cancer via T-cell transfer. TIL therapy uses patient’s own lymphocytes to kill tumor, whereas CAR-T means modified T cells with specific proteins from surface of cancer cells, thus having the ability to attack tumors. In addition to Cas9 utilization, conjugated Cas12 (cCas12a) can be used for CAR-T cell generation. Using an AAV vector, Cas12a-crRNA complex showed robust efficiency to generate site-specific and precisely targeted CAR-T cells [ 149 ].
Recent review showed the importance of gamma retroviral or lentiviral vectors for CAR-T cell generation to target B-cell lymphomas and leukemias, although with complex manufacturing procedure, providing a promising “off-the-shelf” products for cancer treatment [ 150 ]. Whole-genome CRISPR/Cas9 screening was performed in CAR-T cells and co-cultured with Glioblastoma (GBM) stem cells (GSCs) to explore the PD-1 dependence genes such as TLE4 and IKZF2 for cancer treatment. Meanwhile, transduced GSCs were subjected to CAR-T challenge in order to identify enriched and depleted genes for cancer cell apoptosis [ 124 ]. Until 2021, total 3 FDA approved CAR-T therapies have been described as tisagenlecleucel, axicabtagene ciloleurel , and brexucabtagene autoleucel based one CD19-mediated CAR-T cells [ 151 ]. Although CAR-T is efficient in blood cancers, its efficiency loss impedes the treatment efficiency. To overcome refractory of B-cell malignancies, genome-scale CRISPR-Cas9 loss-of-function screens were performed, and revealed the crucial role of FADD and TNFRSF10B (TRAIL-R2) in mediating CAR-T cell cytotoxicity [ 125 ]. Except for precision CAR-T treatment, multiplexed CRISPR-Cas9 editing applications have been used to generate universal CAR-T products, with the aim of enhancing antitumor efficacy and improving safety of cell-based therapies [ 152 ].
Novel targets identification using CRISPR/Cas9 screening in cancer research
The invasion and metastasis of cancers make it more difficult to treat, and new targets should be identified for complete cure. Using genome-wide CRISPR/Cas9 screening, key drivers for invasion and metastasis of esophageal squamous cell carcinoma (ESCC) were identified by gain- and loss-of-function experiments, demonstrating that high expression of Mesoderm Specific Transcript (MEST), interacting with purine rich element binding Protein A, is associated with poor patient survival via activating SRCIN1/RASAL1-ERK-snail signaling [ 126 ]. Synergistical effect of genetic deletion and pharmacologic inhibition to increase cytotoxicity of MEK signaling inhibitors in pancreatic ductal adenocarcinoma cells was also investigated by CRISPR knockout screening [ 127 ]. Genome wide CRISPR/Cas9 knockout screening identified Zinc finger protein (ZNF) family member ZNF319 as a potent suppressor responsible for metastasis of breast cancer in an orthotopic murine model [ 153 ]. In hepatocellular carcinoma (HCC), CRISPR/Cas9 knockout library screening revealed the crucial role of pyruvate metabolism in HCC treatment, particularly when combined with a glutamine-deficient diet, showing the targetable metabolic vulnerabilities of pyruvate dehydrogenase α(PDHA), pyruvate dehydrogenase β(PDHB), and pyruvate carboxylase (PC) [ 128 ]. CRISPR-Cas9 knockout mutagenesis to exons encoding functional protein domains was performed to screen drug targets and dependencies, providing a comprehensive identification of protein domains for cancer cell sustainment [ 120 ]. In epithelial ovarian cancer (EOC), CRISPR-Cas9 screening combined with olaparib treatment successfully identified five genes, ATM, NBN, MUS81, RAD51B, and BRCA2, as predictive markers for olaparib response. Additionally, CDK12 emerged as a promising therapeutic target for EOC without compromising the efficacy of Olaparib response [ 129 ]. The whole-genome CRISPR screening in Guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) mutant uveal melanoma (UM) cells showed that a member of Gα protein family Gαq promoted PI3K/AKT signaling pathway through focal adhesion kinase (FAK) for cell growth and survival [ 130 ].
Combinatorial CRISPR screening with scRNA-seq showed that driver gene alterations influenced TSGs, and triggered tumorigenesis in human mammary epithelial cells, indicating the impact of transcriptional epistasis on oncogenic mediators and potential therapeutic targets, including CDK4, SRPK1, and DNMT1 [ 131 ]. By analyzing the CRISPR-Cas9 screening data from Depmap (Cancer Dependency Map) and TCGA data of differentially expressed genes, the cell cycle pathway was identified as a key pathway of cell viability regulation in breast cancer patients [ 154 ]. The CRISPR/Cas9 screening in chemo-resistant small-cell lung cancer (SCLC) identified serine/threonine kinase cell division cycle 7 (CDC7) as a potential synergistic target. Combination of CDC7 inhibitor XL413 and chemotherapy led to apoptosis of chemo-sensitive SCLC in xenograft tumor [ 132 ]. Acute Myeloid Leukemia (AML) cell lines such as MOLM-13, MV4-11, HL-60, OCI-AML2, OCI-AML3 were examined for therapeutic targets via genome-wide CRISPR screening, indicating KAT2A inhibition as a therapeutic strategy in AML [ 133 ].
Hepatocellular carcinoma (HCC) was examined via CRISPRa for growth and metastasis driver genes. High MYADML2 protein level reduced sensitivity to chemotherapeutic drugs and led to worse survival [ 134 ]. Essential single nucleotide polymorphisms (SNPs) for PrCa proliferation were explored via dCas9-KRAB negative screening with 2166 candidate SNP sites in 9133 gRNAs. RIGOR program analysis identified 117 SNPs which tended to reside near 5 kb flanking the transcription start sites. SNP (rs60464856) site targeting in stable dCas9 expressing cell line showed significant down regulation of RUVBL1 gene, and further validation showed that RUVBL1 was associated with tumorigenesis [ 135 ]. dCas9-KRAB perturbation genome screening identified 470 high-confidence cis enhancer-gene pairs in 5920 enhancers in chronic myelogenous leukemia cell K562, facilitating the large-scale mapping of enhancer-gene regulatory interaction network [ 49 ].
The utilization of CRISPR-Cas9 in investigating drug resistance against tumors
Resistance to nucleotide analog 6- thioguanine was examined by genome-scale knockout screen in two human cell lines, identified DNA mismatch repair pathway, DNA topoisomerase II (TOP2A) and cyclin-dependent kinase 6, (CDK) for DNA topoisomerase II (TOP2A) poison etoposide, demonstrating Cas9/ sgRNA screens as a powerful tool for systematic genetic analysis in mammalian cells [ 26 ]. CRISPR knockout screening in human A549 lung adenocarcinoma cells identified 5 EGF-resistance genes, and further RNAi validation showed DUSP1 increased survival of EGF treated cells, providing a novel target for EGFR-overexpressing cancers [ 86 ]. Genome-wide knockout screening using CRISPR-Cas9 was also carried out in respiratory cancers, including Nasopharyngeal carcinoma (NPC) and lung cancer (LC). Nine genes were found to be associated with radiosensitivity of NPC cells (C666-1R, 6-8FR). Fanconi anemia pathway and the TGF-β signaling pathway were reported to be important contributors for radiosensitivity [ 136 ]. In the nervous system, neuroblastoma tumorigenesis was investigated via CRISPR genome-wide knockout screening, showed that ubiquitin-specific proteases (USPs) stabilize and increase half-life of repressor element-1 silencing transcription factor (REST), indicating its critical role in neuroblastoma generation [ 137 ]. As for reproductive cancers, drug resistance genes as well as lethal genes for cancer cell were identified. Genome-scale screening in ovarian cancer cell lines with the GeCKO library identified one previously validated gene SULF1 and a novel gene ZNF587B responsible for cisplatin resistance [ 138 ]. Cervical cancer cell lines such as Hela and Siha were incubated with cisplatin or paclitaxel, respectively, and screened by genome-scale CRISPR/Cas knockout library and ninety-seven genes were identified to be associated with drug resistance [ 139 ]. Prostate cancer (PrCa) is one of the most lethal causes of cancer-related death in males. Resistance to Enzalutamide, docetaxel, and Cabazitaxel in metastatic castration-resistant prostate cancer (mCRPC) is a big obstacle for cancer treatment of male patients. Whole-genome CRISPR/Cas9 knockout screening in mCRPC cell line C4 dissected the potential genes responsible for drug resistance. Two genes (IP6K2, XPO4) were validated after the screening process via bioinformatic prediction, highlighting the necessity to perform individualized validation [ 140 ].
Phase III clinical trial for Aurora-A (AURKA) inhibitor alisertib (MLN8237) in breast cancer failed to prolong patients’ survival. Rational drug combinations for better therapeutic outcome were carried out based on CRISPR/Cas9 knockout screening of 507 kinases, identifying synthetic lethality interactions with MLN8237 and Haspin (GSG2). The combination of MLN8237 and Haspin inhibitor CHR-6494 reduced tumor growth both in vitro and in vivo [ 141 ]. CRISPR screening for 656 E3 ubiquitin ligases in PrCa cells identified 51 genes as tumor repressors. The novel oncodriver Ring Finger Protein 19 A (RNF19A) was frequently amplified and highly expressed in PrCa. It correlated with castration resistance and ubiquitylated Thyroid Hormone Receptor Interactor 13 (TRIP13) and was activated by androgen receptor (AR), and Hypoxia Inducible Factor 1 Subunit Alpha (HIF1A), indicating AR/HIF1A-RNF19A-TRIP13 signaling axis for PrCa therapy [ 142 ].
Colorectal cancer (CRC) was examined for drug resistance to oxaliplatin and screened by CRISPR/Cas9 genome-wide library knockdown system. It found that low expression of mitochondrial elongation factor 2 (MIEF2) contributed to oxaliplatin drug resistance by reducing mitochondrial stability and inhibiting apoptosis via decreased cytochrome C release [ 143 ]. The CRISPRa system was employed to investigate genes associated with resistance to lymphoma radiotherapy, and a total of 8 genes were screened and subsequently validated, demonstrating a significant correlation with radiotherapy resistance [ 144 ]. Patients with Cisplatin-resistant Testicular Germ Cell Tumors (TGCTs) have poor prognosis, and developments of novel therapeutic strategies are critical. CRISPRa system revealed that NEDD8-activating enzyme E1 (NAE1) was highly expressed in drug-resistant colonies of TGCT cells, and indicated that neddylation inhibitor (MLN4924) combined with cisplatin as a novel treatment option for TGCTs [ 145 ].
Utilizing CRISPR/Cas9 screening for personalized drug selection through patient-derived organoids
Organoids derived from both healthy and diseased tissues offer a valuable resource for biological or pathological investigations. Although CRISPR screening showed powerful manipulation in cancer cells lines, it is also employed for tumor organoids derived from diverse cancer patients for personalized drug selection. Suspension culture increases efficiency of culturing cancer organoids for genome-wide CRISPR-Cas9 screening and large-scale perturbation screens [ 146 ]. Human fetal hepatocyte organoids were generated to model nonalcoholic fatty liver disease (NAFLD), and CRISPR screening was utilized to identify steatosis modulators in APOB −/− and MTTP −/− organoids [ 147 ]. CRISPR-Cas9 genetic intervention and high-throughput drug screening have been applied in digestive organoids for personalized disease modeling and therapy [ 155 ]. Human Pancreatic cancer organoid biobank established from 31 distinct tumor lines was used for CRISPR/Cas9 genome editing and drug screening, indicated increased sensitivity of kinase inhibitors dasatinib and VE-821 with driver gene ARID1A mutation [ 148 ]. Drug response evaluation by in vivo CRISPR screening (DREBIC) method was used in pancreatic ductal adenocarcinoma organoid [ 127 ].
CRISPR screening in vivo for cancer therapy
In 2022, FDA approved a total of five CAR-T cell products for the treatment of B cell acute lymphoblastic leukemia or high-grade lymphomas, as well as multiple myeloma using lentiviral or γ-retroviral approaches [ 156 ]. Notably, two clinical trials (NCT05143307/NCT03872479) employed AAV as the delivery method in their studies on cancer therapy in vivo based on CAR-T cells and CRISPR/Cas system [ 117 ]. CRISPR screening system provides a robust genetic tool for in vivo elucidation of CAR-T resistance mechanisms. Loss-of-function genetic screens in an immunocompetent murine model with B-cell acute lymphoblastic leukemia (B-ALL) identified the IFNR/JAK/STAT signaling and antigen processing and presentation pathway as key factors for CAR-T resistance in vivo. In addition, natural killer (NK) cells also engage in the resistance progress [ 157 ]. Gain-of-function CRISPR activation screen in primary CD8 + T cells identified a key factor PRODH2 for improving the in vivo efficacy of CAR-T based cell killing. Augmentation of PRODH2 enhanced metabolic function of CAR-T cells as an immune booster [ 158 ].
CRISPR screening was also utilized for in vivo investigation to elucidate gene function within a whole organism or the context of complex biological systems, using lentiviral or AAV mediated sgRNA transfection in living organisms. AAV was the widely used vector for in vivo genetic therapy due to its low immunogenicity and non-pathogenic character [ 118 ]. The limitation of AAV’s vector capacity has been addressed through the recent development of a two-split intern vectors system [ 159 ], while smaller SpCas9 orthologues such as SaCas9 have demonstrated comparable editing efficiency to that of SpCas9, rendering them suitable for AAV-SaCas9 mediated in vivo genome editing [ 21 ]. Additionally, Cre-dependent and constitutive Cas9 expressing transgenic mice were established with EGFP labeling, which provides an animal model for genome-wide targeting and contributes to in vivo investigation [ 24 ].
In vivo screens were performed in mouse brain, liver, pancreases, lung and so on. The application of SpCas9 and gRNAs using AAV vectors enabled multiple gene modifications in the adult mouse brain, demonstrating its potential for genetic regulation [ 33 ]. Gliomagenesis suppressors were investigated by in vivo stereotaxic injection of AAV carrier sgRNA library in conditional-Cas9 mouse brain [ 160 ]. Autochthonous invasion of AAV-mTSGs library in Cre-inducible Cas9 mice liver led to cancer development in situ, and the mice died within 4 months [ 89 ]. NIT1 cells (a non-obese-diabetic-derived mouse beta cell line) mutated with GeCKO-v2 were subcutaneously transplanted into type 1 diabetes mouse model to identify genes contributing to autoimmune killing resistance [ 161 ]. With the AAV9-LPL gene delivery into the lung, multiple mutations of KRAS G12D , p53 and LKB1 were obtained to induce macroscopic tumors. In vivo screening for lung cancer TSGs through CRISPR/Cas9 genome-wide knockout showed that ZNF24 contributed to P65 suppression via NF-κB pathway. Combinational inhibition of KRAS, NF-κB, and PD-1 effectively shrank autochthonous Kras G12D /ZNF24 −/− lung cancers in mouse [ 162 ].
Examination of immunotherapy-treated normal and Tcra -/- mice in vivo by CRISPR screening showed the loss of CD47 caused resistance to immunotherapy. Deletion of protein tyrosine phosphatase (PTPN2) increased immunotherapy efficacy [ 88 ]. CRISPR screening identified PD-1, Tim-3, and RNA helicase Dhx37 as regulators of tumor infiltration and degranulation. Depletion of Dhx37 improved CD8 T cells efficacy towards triple-negative breast cancer in vivo, and the NF-kB signal pathway was involved in the process [ 163 ]. In vivo applications of CRISPR screening system were summarized in the following Table 3 .
Limitations and prospection
The advances of CRISPR/Cas technology and screening strategies have revolutionized genetic identification, enabling the dissection of functional genes in specific biological processes and diseases, facilitating drug selection and individualized therapy. CRISPR screening has demonstrated great potential in cancer therapy by offering methods to combat drug resistance and aggressive behaviors, as well as identifying possible gene targets for novel approaches to treat cancers. However, there are still several obstacles for CRISPR/Cas application in clinical cancer treatment, including delivery of CRISPR/Cas9 system, Off-target effect, PAM limitation, as well as multiple gene-editing [ 117 ]. In this part, we paid more attention on limitations of CRISPR screening system and CAR-T cell therapy for cancers.
Limitations of CRISPR screening system
CRISPR screening delivery primarily relies on lentiviral and AAV vectors, which are crucial tools for either ex vivo or in vivo investigation. Of which, AAV vector has the advantages with mildly immunogenic and long-term transgene expression in post-mitotic cells, making it a leading platform for in vivo cancer therapy [ 164 ]. However, AAV vector showed some drawbacks in manufacturing, packaging size limitation, vector quality control and editing specificity, as described in the recent review [ 118 ].
Except the delivery limitations, the occurrence of off-target effects and unintended mutations induced by CRISPR technology are barriers to its application in clinical therapy. SpCas9 protein showed the ability to identify PAM sequence and cut specific DNA region in the CRISPR system. Due to the tolerance of gRNA recognition and nucleotide indels in the target region, even a single guide can generate thousands of off-targets as detected by sensitive high-throughput sequencing methods such as GUIDE-Seq and CIRCLE-seq [ 138 , 143 ]. This raises concerns regarding the application of CRISPR technology in gene therapy [ 165 ]. The reason of the off-target effect is the conformational states of HNH domain. The activated conformation of HNH increases DNA cleavage efficiency for DNA double-strand break formation, leading to both on- and off-target effects [ 166 ]. To minimize the probability of off-target mutagenesis, other high-fidelity nucleases such as SpCas9-HF1, eSpCas9 and HypaCas9 were developed [ 167 , 168 ]. In addition, PAM sequence limitation for Cas9 has been broadened by the identification of KKH SaCas9 variant, which exhibits robust genome editing activities with the PAM (NNNRRT) while maintaining comparable levels of off-target effects [ 169 ].
Anti-CRISPR is another obstacle to overcome because of the restriction of targeting specificity and activities. The VI-CRISPR inhibitors acrVIA1-7 from phage exhibit the ability to block Cas13a RNA targeting and dCas13a-mediated single nucleic acid editing. Specifically, AcrVIA1, 4, 5 and 6 bind to LwaCas13a, while AcrVIA2 and 3 interact with LwaCas13-crRNA complex [ 170 ].
Limitations of CAR-T cancer therapy
Although CAR-T showed success of B-cell malignance treatment, its usage in solid tumors still have some limitations such as T-cell exhaustion, lack of CAR-T cell persistence, and cytokine-related toxicities. To address these challenges, CRISPR technology has been used to generate safe and potent allogeneic universal CAR-T cell products for cancer immunotherapy [ 152 ]. However, hurdles remain for solid tumor CAR-T therapy due to target antigen heterogeneity, unable to pass through vascular endothelium to target tumor cells, and the immunosuppressive tumor microenvironments [ 171 ]. As viral vectors are commonly used for delivering CAR-T cells, safety concerns have arisen. To address this issue, virus-free CRISPR-CAR (VFC-CAR) T cells were generated [ 172 ]. Virus-free CAR-T cells (PD1-19bbz) were generated and a clinical trial was performed and registered at www.clinicaltrials.gov (NCT04213469) [ 173 ].
Future perspectives
Given the capacity of CRISPR to precisely modify the human genome in cells, ethical considerations have emerged as a pivotal factor for its application in genetic manipulation [ 174 , 175 , 176 ]. The challenges posed by off-target effects and unintended mutations serve as barriers to the clinical implementation of CRISPR technology. However, extensive efforts have been made to mitigate these concerns through the development of novel strategies, rendering CRISPR technologies indispensable tools for elucidating gene functions and noncoding elements involved in tumorigenesis, as well as facilitating the creation of next-generation cancer immunotherapies. In summary, CRISPR/Cas system continues to play an essential role in advancing human cancer research and clinical therapy.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
Clustered regularly interspaced short palindromic repeats
CRISPR-associated protein 9
Streptococcus pyogenes Cas9
Staphylococcus aureus Cas9
Streptococcus thermophilus Cas9
CRISPR from Prevotella and Francisella 1
Adeno-associated vector
Trans-activating crRNA
Protospacer adjacent motif
Ribonucleoprotein
Cystic fibrosis transmembrane conductance regulator
Cas9 nickase
Adenine base-editors
Cytosine base-editors
Interhomolog homologous recombination
CDD conserved protein domain family
Conserved Protein Domain Family HNH, His-Asn-His (HNH)
CRISPR inhibition
CRISPR activation
Krüppel-associated box
Tetracycline Inducible Expression promoter
RNA polymerase
Transcriptional activator consists of four copies of VP16
Cpf1 enzyme from Acidominococcus sp. BV3L6
Cpf1 enzyme from Lachnospiraceae bacterium ND2006
Homology-directed repair
Cas12b enzyme from Bacillus hisashii
Specific High-Sensitivity Enzymatic Reporter UnLOCKing
SARS-coronavirus-2
Open reading frame
Cell free DNA
porcine reproductive and respiratory syndrome virus
RNA viruses’ chikungunya
Catalytically inactive Cas13b
Programmable A to I Replacement
Toronto KnockOut
Genome-scale CRISPR-Cas9 knockout version 1
Model-based Analysis of Genome-wide CRISPR/Cas9 Knockout
Synthetic activation mediator
Transcription start site
Expression quantitative trait locus
The Cancer Genome Atlas
Genome-wide CRISPR/Cas9 Knockout
Integration deficient lentiviral
High-throughput genome-wide translocation sequencing
Breaks labeling, enrichment on streptavidin and next-generation sequencing
Genome-wide unbiased identification of DSBs enabled by sequencing
Discovery of in situ Cas off-targets and verification by sequencing
Digested genome sequencing
Selective enrichment and identification of adapter-tagged DNA ends by sequencing
Circularization for in vitro reporting of cleavage effects by sequencing
Circularization for high-throughput analysis of nuclease genome-wide ffects by sequencing
Chimeric antigen receptor
Tumor-infiltrating lymphocytes
Glioblastoma (GBM) stem cells
Programmed Cell Death Ligand 1
Esophageal squamous cell carcinoma
Mesoderm Specific Transcript
Zinc finger protein
Hepatocellular carcinoma
Pyruvate carboxylase
Epithelial ovarian cancer
Guanine nucleotide-binding protein G(q) subunit alpha
Uveal melanoma
Focal adhesion kinase
Tumor suppressor genes
Cancer Dependency Map
Chemo-resistant small-cell lung cancer
Serine/threonine kinase cell division cycle 7
Acute Myeloid Leukemia
Single nucleotide polymorphisms
DNA topoisomerase II
Cyclin-dependent kinase 6
Nasopharyngeal carcinoma
Lung cancer
Ubiquitin-specific proteases
Repressor element-1 silencing transcription factor
Prostate cancer
Colorectal cancer
Mitochondrial elongation factor 2
NEDD8-activating enzyme E1
Nonalcoholic fatty liver disease
B-cell acute lymphoblastic leukemia
Protein tyrosine phosphatase
Advancing Cancer Therapy. Nat Cancer. 2021;2:245–6. https://doi.org/10.1038/s43018-021-00192-x .
Article Google Scholar
Huang CH, Lee KC, Doudna JA. Applications of CRISPR-Cas enzymes in Cancer therapeutics and detection. Trends Cancer. 2018;4:499–512. https://doi.org/10.1016/j.trecan.2018.05.006 .
Article CAS PubMed PubMed Central Google Scholar
Wang M, Chen M, Wu X, Huang X, Yu B. CRISPR applications in cancer diagnosis and treatment. Cell Mol Biol Lett. 2023;28:73. https://doi.org/10.1186/s11658-023-00483-4 .
Article PubMed PubMed Central Google Scholar
Yang X, Zhang B. A review on CRISPR/Cas: a versatile tool for cancer screening, diagnosis, and clinic treatment. Funct Integr Genomics. 2023;23:182. https://doi.org/10.1007/s10142-023-01117-w .
Article CAS PubMed Google Scholar
Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA. Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science. 2010;329:1355–8. https://doi.org/10.1126/science.1192272 .
Wiedenheft B, Zhou K, Jinek M, Coyle SM, Ma W, Doudna JA. Structural basis for DNase activity of a conserved protein implicated in CRISPR-mediated genome defense. Cell. 2009;17:904–12. https://doi.org/10.1016/j.str.2009.03.019 .
Article CAS Google Scholar
Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60:174–82. https://doi.org/10.1007/s00239-004-0046-3 .
Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012;109:E2579–2586. https://doi.org/10.1073/pnas.1208507109 .
Jore MM, Lundgren M, van Duijn E, Bultema JB, Westra ER, Waghmare SP, Wiedenheft B, Pul U, Wurm R, Wagner R, et al. Structural basis for CRISPR RNA-guided DNA recognition by Cascade. Nat Struct Mol Biol. 2011;18:529–36. https://doi.org/10.1038/nsmb.2019 .
Sternberg SH, Haurwitz RE, Doudna JA. Mechanism of substrate selection by a highly specific CRISPR endoribonuclease. RNA. 2012;18:661–72. https://doi.org/10.1261/rna.030882.111 .
Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E, Haft DH, et al. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol. 2015;13:722–36. https://doi.org/10.1038/nrmicro3569 .
Koonin EV, Makarova KS, Zhang F. Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol. 2017;37:67–78. https://doi.org/10.1016/j.mib.2017.05.008 .
Makarova KS, Zhang F, Koonin EV. SnapShot: class 1 CRISPR-Cas systems. Cell. 2017;168:946–946. https://doi.org/10.1016/j.cell.2017.02.018 .
Makarova KS, Zhang F, Koonin EV. SnapShot: class 2 CRISPR-Cas systems. Cell. 2017;168:328–328. https://doi.org/10.1016/j.cell.2016.12.038 .
Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, Brouns SJJ, Charpentier E, Cheng D, Haft DH, Horvath P, et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol. 2020;18:67–83. https://doi.org/10.1038/s41579-019-0299-x .
Tang Y, Fu Y. Class 2 CRISPR/Cas: an expanding biotechnology toolbox for and beyond genome editing. Cell Biosci. 2018;8:59. https://doi.org/10.1186/s13578-018-0255-x .
LaManna CM, Pyhtila B, Barrangou R. Sharing the CRISPR Toolbox with an Expanding Community. CRISPR J. 2020;3:248–52. https://doi.org/10.1089/crispr.2020.0075 .
Article PubMed Google Scholar
Harrington LB, Burstein D, Chen JS, Paez-Espino D, Ma E, Witte IP, Cofsky JC, Kyrpides NC, Banfield JF, Doudna JA. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science. 2018;362:839–42. https://doi.org/10.1126/science.aav4294 .
Shmakov S, Smargon A, Scott D, Cox D, Pyzocha N, Yan W, Abudayyeh OO, Gootenberg JS, Makarova KS, Wolf YI, et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat Rev Microbiol. 2017;15:169–82. https://doi.org/10.1038/nrmicro.2016.184 .
Liu TY, Doudna JA. Chemistry of Class 1 CRISPR-Cas effectors: binding, editing, and regulation. J Biol Chem. 2020;295:14473–87. https://doi.org/10.1074/jbc.REV120.007034 .
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–91. https://doi.org/10.1038/nature14299 .
Cong L, Zhang F. Genome engineering using CRISPR-Cas9 system. Methods Mol Biol. 2015;1239:197–217. https://doi.org/10.1007/978-1-4939-1862-1_10 .
Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet. 2014;23:R40–46. https://doi.org/10.1093/hmg/ddu125 .
Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159:440–55. https://doi.org/10.1016/j.cell.2014.09.014 .
Chen S, Sanjana NE, Zheng K, Shalem O, Lee K, Shi X, Scott DA, Song J, Pan JQ, Weissleder R, et al. Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell. 2015;160:1246–60. https://doi.org/10.1016/j.cell.2015.02.038 .
Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343:80–4. https://doi.org/10.1126/science.1246981 .
Joung J, Konermann S, Gootenberg JS, Abudayyeh OO, Platt RJ, Brigham MD, Sanjana NE, Zhang F. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat Protoc. 2017;12:828–63. https://doi.org/10.1038/nprot.2017.016 .
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–7. https://doi.org/10.1126/science.1247005 .
Holcomb EA, Pearson AN, Jungles KM, Tate A, James J, Jiang L, Huber AK, Green MD. High-content CRISPR screening in tumor immunology. Front Immunol. 2022;13:1041451. https://doi.org/10.3389/fimmu.2022.1041451 .
Henkel L, Rauscher B, Schmitt B, Winter J, Boutros M. Genome-scale CRISPR screening at high sensitivity with an empirically designed sgRNA library. BMC Biol. 2020;18:174. https://doi.org/10.1186/s12915-020-00905-1 .
Kumar N, Stanford W, de Solis C, Aradhana, Abraham ND, Dao TJ, Thaseen S, Sairavi A, Gonzalez CU, Ploski JE. The development of an AAV-Based CRISPR SaCas9 genome editing system that can be delivered to neurons in vivo and regulated via doxycycline and cre-recombinase. Front Mol Neurosci. 2018;11:413. https://doi.org/10.3389/fnmol.2018.00413 .
Liao HK, Hatanaka F, Araoka T, Reddy P, Wu MZ, Sui Y, Yamauchi T, Sakurai M, O’Keefe DD, Nunez-Delicado E et al. In vivo target gene activation via CRISPR/Cas9-Mediated trans-epigenetic modulation. Cell 2017, 171:1495–507 https://doi.org/10.1016/j.cell.2017.10.025 .
Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, Zhang F. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol. 2015;33:102–6. https://doi.org/10.1038/nbt.3055 .
Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–78. https://doi.org/10.1016/j.cell.2014.05.010 .
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21. https://doi.org/10.1126/science.1225829 .
Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507:62–7. https://doi.org/10.1038/nature13011 .
Lin S, Staahl BT, Alla RK, Doudna JA. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. Elife. 2014;3:e04766. https://doi.org/10.7554/eLife.04766 .
Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–8. https://doi.org/10.1016/j.stem.2013.11.002 .
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23. https://doi.org/10.1126/science.1231143 .
Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–9. https://doi.org/10.1016/j.cell.2013.08.021 .
Dickson KA, Field N, Blackman T, Ma Y, Xie T, Kurangil E, Idrees S, Rathnayake SNH, Mahbub RM, Faiz A, Marsh DJ. CRISPR single base-editing: in silico predictions to variant clonal cell lines. Hum Mol Genet. 2023;32:2704–16. https://doi.org/10.1093/hmg/ddad105 .
Kantor A, McClements ME, MacLaren RE. CRISPR-Cas9 DNA base-editing and prime-editing. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21176240 . 21.
Tomita A, Sasanuma H, Owa T, Nakazawa Y, Shimada M, Fukuoka T, Ogi T, Nakada S. Inducing multiple nicks promotes interhomolog homologous recombination to correct heterozygous mutations in somatic cells. Nat Commun. 2023;14:5607. https://doi.org/10.1038/s41467-023-41048-5 .
Renouf B, Piganeau M, Ghezraoui H, Jasin M, Brunet E. Creating cancer translocations in human cells using Cas9 DSBs and nCas9 paired nicks. Methods Enzymol. 2014;546:251–71. https://doi.org/10.1016/B978-0-12-801185-0.00012-X .
Martin Jinek KC, Ines Fonfara M, Hauer JA. Doudna,Emmanuelle Charpentier: a programmable dual RNA guided DNA endonuclease in adaptive bacterial immunity. Science. 2012. https://doi.org/10.1126/science.1225829 .
Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, et al. Genome-scale CRISPR-Mediated control of gene repression and activation. Cell. 2014;159:647–61. https://doi.org/10.1016/j.cell.2014.09.029 .
Mandegar MA, Huebsch N, Frolov EB, Shin E, Truong A, Olvera MP, Chan AH, Miyaoka Y, Holmes K, Spencer CI, et al. CRISPR Interference efficiently induces specific and reversible gene silencing in human iPSCs. Cell Stem Cell. 2016;18:541–53. https://doi.org/10.1016/j.stem.2016.01.022 .
Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–51. https://doi.org/10.1016/j.cell.2013.06.044 .
Gasperini M, Hill AJ, McFaline-Figueroa JL, Martin B, Kim S, Zhang MD, Jackson D, Leith A, Schreiber J, Noble WS, et al. A genome-wide Framework for Mapping Gene Regulation via Cellular Genetic screens. Cell. 2019;176:377–90. https://doi.org/10.1016/j.cell.2018.11.029 .
Bikard D, Jiang W, Samai P, Hochschild A, Zhang F, Marraffini LA. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013;41:7429–37. https://doi.org/10.1093/nar/gkt520 .
Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–8. https://doi.org/10.1038/nature14136 .
Chen B, Gilbert LA, Cimini BA, Schnitzbauer J, Zhang W, Li GW, Park J, Blackburn EH, Weissman JS, Qi LS, Huang B. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155:1479–91. https://doi.org/10.1016/j.cell.2013.12.001 .
Wang S, Su JH, Zhang F, Zhuang X. An RNA-aptamer-based two-color CRISPR labeling system. Sci Rep. 2016;6:26857. https://doi.org/10.1038/srep26857 .
Kim HK, Song M, Lee J, Menon AV, Jung S, Kang YM, Choi JW, Woo E, Koh HC, Nam JW, Kim H. In vivo high-throughput profiling of CRISPR-Cpf1 activity. Nat Methods. 2017;14:153–9. https://doi.org/10.1038/nmeth.4104 .
Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–71. https://doi.org/10.1016/j.cell.2015.09.038 .
Kleinstiver BP, Tsai SQ, Prew MS, Nguyen NT, Welch MM, Lopez JM, McCaw ZR, Aryee MJ, Joung JK. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat Biotechnol. 2016;34:869–74. https://doi.org/10.1038/nbt.3620 .
Kim Y, Cheong SA, Lee JG, Lee SW, Lee MS, Baek IJ, Sung YH. Generation of knockout mice by Cpf1-mediated gene targeting. Nat Biotechnol. 2016;34:808–10. https://doi.org/10.1038/nbt.3614 .
Hur JK, Kim K, Been KW, Baek G, Ye S, Hur JW, Ryu SM, Lee YS, Kim JS. Targeted mutagenesis in mice by electroporation of Cpf1 ribonucleoproteins. Nat Biotechnol. 2016;34:807–8. https://doi.org/10.1038/nbt.3596 .
Abdulrachman D, Champreda V, Eurwilaichitr L, Chantasingh D, Pootanakit K. Efficient multiplex CRISPR/Cpf1 (Cas12a) genome editing system in Aspergillus Aculeatus TBRC 277. J Biotechnol. 2022;355:53–64. https://doi.org/10.1016/j.jbiotec.2022.06.011 .
Zetsche B, Heidenreich M, Mohanraju P, Fedorova I, Kneppers J, DeGennaro EM, Winblad N, Choudhury SR, Abudayyeh OO, Gootenberg JS, et al. Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat Biotechnol. 2017;35:31–4. https://doi.org/10.1038/nbt.3737 .
Dai X, Park JJ, Du Y, Kim HR, Wang G, Errami Y, Chen S. One-step generation of modular CAR-T cells with AAV-Cpf1. Nat Methods. 2019;16:247–54. https://doi.org/10.1038/s41592-019-0329-7 .
Strecker J, Jones S, Koopal B, Schmid-Burgk J, Zetsche B, Gao L, Makarova KS, Koonin EV, Zhang F. Engineering of CRISPR-Cas12b for human genome editing. Nat Commun. 2019;10:212. https://doi.org/10.1038/s41467-018-08224-4 .
Huang CJ, Adler BA, Doudna JA. A naturally DNase-free CRISPR-Cas12c enzyme silences gene expression. Mol Cell. 2022;82:2148–60. https://doi.org/10.1016/j.molcel.2022.04.020 .
Yin L, Zhao F, Sun H, Wang Z, Huang Y, Zhu W, Xu F, Mei S, Liu X, Zhang D, et al. CRISPR-Cas13a inhibits HIV-1 infection. Mol Ther Nucleic Acids. 2020;21:147–55. https://doi.org/10.1016/j.omtn.2020.05.030 .
Zheng X, Li Y, Yuan M, Shen Y, Chen S, Duan G. Rapid detection of HPV16/18 based on a CRISPR-Cas13a/Cas12a dual-channel system. Anal Methods. 2022;14:5065–75. https://doi.org/10.1039/d2ay01536f .
Xu C, Zhou Y, Xiao Q, He B, Geng G, Wang Z, Cao B, Dong X, Bai W, Wang Y, et al. Programmable RNA editing with compact CRISPR-Cas13 systems from uncultivated microbes. Nat Methods. 2021;18:499–506. https://doi.org/10.1038/s41592-021-01124-4 .
Zhang C, Konermann S, Brideau NJ, Lotfy P, Wu X, Novick SJ, Strutzenberg T, Griffin PR, Hsu PD, Lyumkis D. Structural basis for the RNA-Guided ribonuclease activity of CRISPR-Cas13d. Cell. 2018;175:212–23. https://doi.org/10.1016/j.cell.2018.09.001 . e217.
Tong H, Huang J, Xiao Q, He B, Dong X, Liu Y, Yang X, Han D, Wang Z, Wang X, et al. High-fidelity Cas13 variants for targeted RNA degradation with minimal collateral effects. Nat Biotechnol. 2023;41:108–19. https://doi.org/10.1038/s41587-022-01419-7 .
Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, Verdine V, Donghia N, Daringer NM, Freije CA, et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356:438–42. https://doi.org/10.1126/science.aam9321 .
Fozouni P, Son S, Diaz de Leon Derby M, Knott GJ, Gray CN, D’Ambrosio MV, Zhao C, Switz NA, Kumar GR, Stephens SI, et al. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy. Cell. 2020. https://doi.org/10.1016/j.cell.2020.12.001 .
Yang L, Zhang Y, Yi W, Dong X, Niu M, Song Y, Han Y, Li H, Sun Y. A rapid and efficient platform for antiviral crRNA screening using CRISPR-Cas13a-based nucleic acid detection. Front Immunol. 2023;141116230. https://doi.org/10.3389/fimmu.2023.1116230 .
Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360:439–44. https://doi.org/10.1126/science.aaq0179 .
Kellner MJ, Koob JG, Gootenberg JS, Abudayyeh OO, Zhang F. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc. 2019;14:2986–3012. https://doi.org/10.1038/s41596-019-0210-2 .
Cui J, Techakriengkrai N, Nedumpun T, Suradhat S. Abrogation of PRRSV infectivity by CRISPR-Cas13b-mediated viral RNA cleavage in mammalian cells. Sci Rep. 2020;10:9617. https://doi.org/10.1038/s41598-020-66775-3 .
Tng PYL, Carabajal Paladino L, Verkuijl SAN, Purcell J, Merits A, Leftwich PT, Fragkoudis R, Noad R, Alphey L. Cas13b-dependent and Cas13b-independent RNA knockdown of viral sequences in mosquito cells following guide RNA expression. Commun Biol. 2020;3413. https://doi.org/10.1038/s42003-020-01142-6 .
Fareh M, Zhao W, Hu W, Casan JML, Kumar A, Symons J, Zerbato JM, Fong D, Voskoboinik I, Ekert PG, et al. Reprogrammed CRISPR-Cas13b suppresses SARS-CoV-2 replication and circumvents its mutational escape through mismatch tolerance. Nat Commun. 2021;12:4270. https://doi.org/10.1038/s41467-021-24577-9 .
Yu D, Han HJ, Yu J, Kim J, Lee GH, Yang JH, Song BM, Tark D, Choi BS, Kang SM, Heo WD. Pseudoknot-targeting Cas13b combats SARS-CoV-2 infection by suppressing viral replication. Mol Ther. 2023;31:1675–87. https://doi.org/10.1016/j.ymthe.2023.03.018 .
Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J, Zhang F. RNA editing with CRISPR-Cas13. Science. 2017;358:1019–27. https://doi.org/10.1126/science.aaq0180 .
Granados-Riveron JT, Aquino-Jarquin G. CRISPR-Cas13 Precision Transcriptome Engineering in Cancer. Cancer Res. 2018;78:4107–13. https://doi.org/10.1158/0008-5472.CAN-18-0785 .
Palaz F, Kalkan AK, Can O, Demir AN, Tozluyurt A, Ozcan A, Ozsoz M. CRISPR-Cas13 System as a Promising and Versatile Tool for Cancer diagnosis, therapy, and Research. ACS Synth Biol. 2021;10:1245–67. https://doi.org/10.1021/acssynbio.1c00107 .
Koike-Yusa H, Li Y, Tan EP, Velasco-Herrera Mdel C, Yusa K. Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat Biotechnol. 2014;32:267–73. https://doi.org/10.1038/nbt.2800 .
Shalem O, Sanjana NE, Zhang F. High-throughput functional genomics using CRISPR-Cas9. Nat Rev Genet. 2015;16:299–311. https://doi.org/10.1038/nrg3899 .
Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–8. https://doi.org/10.1016/j.cell.2013.04.025 .
Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–4. https://doi.org/10.1038/nmeth.3047 .
Najm FJ, Strand C, Donovan KF, Hegde M, Sanson KR, Vaimberg EW, Sullender ME, Hartenian E, Kalani Z, Fusi N, et al. Orthologous CRISPR-Cas9 enzymes for combinatorial genetic screens. Nat Biotechnol. 2018;36:179–89. https://doi.org/10.1038/nbt.4048 .
Kim JS, Lee JH, Jeon SR, Kim Y, Jeon SH, Wu HG. Identification of genes involved in EGF-induced apoptosis using CRISPR/Cas9 knockout screening: implications for Novel therapeutic targets in EGFR-Overexpressing cancers. Cancer Res Treat. 2023. https://doi.org/10.4143/crt.2022.1414 .
Hart T, Chandrashekhar M, Aregger M, Steinhart Z, Brown KR, MacLeod G, Mis M, Zimmermann M, Fradet-Turcotte A, Sun S, et al. High-resolution CRISPR screens Reveal Fitness genes and genotype-specific Cancer liabilities. Cell. 2015;163:1515–26. https://doi.org/10.1016/j.cell.2015.11.015 .
Manguso RT, Pope HW, Zimmer MD, Brown FD, Yates KB, Miller BC, Collins NB, Bi K, LaFleur MW, Juneja VR, et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547:413–8. https://doi.org/10.1038/nature23270 .
Wang G, Chow RD, Ye L, Guzman CD, Dai X, Dong MB, Zhang F, Sharp PA, Platt RJ, Chen S. Mapping a functional cancer genome atlas of tumor suppressors in mouse liver using AAV-CRISPR-mediated direct in vivo screening. Sci Adv. 2018;4:eaao5508. https://doi.org/10.1126/sciadv.aao5508 .
Parnas O, Jovanovic M, Eisenhaure TM, Herbst RH, Dixit A, Ye CJ, Przybylski D, Platt RJ, Tirosh I, Sanjana NE, et al. A genome-wide CRISPR screen in primary Immune cells to Dissect Regulatory Networks. Cell. 2015;162:675–86. https://doi.org/10.1016/j.cell.2015.06.059 .
Canver MC, Haeussler M, Bauer DE, Orkin SH, Sanjana NE, Shalem O, Yuan GC, Zhang F, Concordet JP, Pinello L. Integrated design, execution, and analysis of arrayed and pooled CRISPR genome-editing experiments. Nat Protoc. 2018;13:946–86. https://doi.org/10.1038/nprot.2018.005 .
Wilson LOW, O’Brien AR, Bauer DC. The current state and future of CRISPR-Cas9 gRNA Design Tools. Front Pharmacol. 2018;9:749. https://doi.org/10.3389/fphar.2018.00749 .
Cui Y, Xu J, Cheng M, Liao X, Peng S. Review of CRISPR/Cas9 sgRNA design tools. Interdiscip Sci. 2018;10:455–65. https://doi.org/10.1007/s12539-018-0298-z .
Vakulskas CA, Behlke MA. Evaluation and reduction of CRISPR off-target cleavage events. Nucleic Acid Ther. 2019;29:167–74. https://doi.org/10.1089/nat.2019.0790 .
Concordet JP, Haeussler M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018;46:W242–5. https://doi.org/10.1093/nar/gky354 .
Haeussler M, Schonig K, Eckert H, Eschstruth A, Mianne J, Renaud JB, Schneider-Maunoury S, Shkumatava A, Teboul L, Kent J, et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016;17148. https://doi.org/10.1186/s13059-016-1012-2 .
Pinello L, Canver MC, Hoban MD, Orkin SH, Kohn DB, Bauer DE, Yuan GC. Analyzing CRISPR genome-editing experiments with CRISPResso. Nat Biotechnol. 2016;34:695–7. https://doi.org/10.1038/nbt.3583 .
Li W, Xu H, Xiao T, Cong L, Love MI, Zhang F, Irizarry RA, Liu JS, Brown M, Liu XS. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 2014;15:554. https://doi.org/10.1186/s13059-014-0554-4 .
Wang X, Wang Y, Wu X, Wang J, Wang Y, Qiu Z, Chang T, Huang H, Lin RJ, Yee JK. Unbiased detection of off-target cleavage by CRISPR-Cas9 and TALENs using integrase-defective lentiviral vectors. Nat Biotechnol. 2015;33:175–8. https://doi.org/10.1038/nbt.3127 .
Frock RL, Hu J, Meyers RM, Ho YJ, Kii E, Alt FW. Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. Nat Biotechnol. 2015;33:179–86. https://doi.org/10.1038/nbt.3101 .
Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. https://doi.org/10.1186/gb-2009-10-3-r25 .
Hart T, Brown KR, Sircoulomb F, Rottapel R, Moffat J. Measuring error rates in genomic perturbation screens: gold standards for human functional genomics. Mol Syst Biol. 2014;10:733. https://doi.org/10.15252/msb.20145216 .
Lane-Reticker SK, Kessler EA, Muscato AJ, Kim SY, Doench JG, Yates KB, Manguso RT, Dubrot J. Protocol for in vivo CRISPR screening using selective CRISPR antigen removal lentiviral vectors. STAR Protoc. 2023;4:102082. https://doi.org/10.1016/j.xpro.2023.102082 .
Crosetto N, Mitra A, Silva MJ, Bienko M, Dojer N, Wang Q, Karaca E, Chiarle R, Skrzypczak M, Ginalski K, et al. Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat Methods. 2013;10:361–5. https://doi.org/10.1038/nmeth.2408 .
Tsai SQ, Zheng Z, Nguyen NT, Liebers M, Topkar VV, Thapar V, Wyvekens N, Khayter C, Iafrate AJ, Le LP, et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol. 2015;33:187–97. https://doi.org/10.1038/nbt.3117 .
Malinin NL, Lee G, Lazzarotto CR, Li Y, Zheng Z, Nguyen NT, Liebers M, Topkar VV, Iafrate AJ, Le LP, et al. Defining genome-wide CRISPR-Cas genome-editing nuclease activity with GUIDE-seq. Nat Protoc. 2021;16:5592–615. https://doi.org/10.1038/s41596-021-00626-x .
Wienert B, Wyman SK, Richardson CD, Yeh CD, Akcakaya P, Porritt MJ, Morlock M, Vu JT, Kazane KR, Watry HL, et al. Unbiased detection of CRISPR off-targets in vivo using DISCOVER-Seq. Science. 2019;364:286–9. https://doi.org/10.1126/science.aav9023 .
Wienert B, Wyman SK, Yeh CD, Conklin BR, Corn JE. CRISPR off-target detection with DISCOVER-seq. Nat Protoc. 2020;15:1775–99. https://doi.org/10.1038/s41596-020-0309-5 .
Zou RS, Liu Y, Gaido OER, Konig MF, Mog BJ, Shen LL, Aviles-Vazquez F, Marin-Gonzalez A, Ha T. Improving the sensitivity of in vivo CRISPR off-target detection with DISCOVER-Seq. Nat Methods. 2023;20:706–13. https://doi.org/10.1038/s41592-023-01840-z .
Kim D, Bae S, Park J, Kim E, Kim S, Yu HR, Hwang J, Kim JI, Kim JS. Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat Methods. 2015;12:237–43. https://doi.org/10.1038/nmeth.3284 .
Kim D, Kang BC, Kim JS. Identifying genome-wide off-target sites of CRISPR RNA-guided nucleases and deaminases with Digenome-Seq. Nat Protoc. 2021;16:1170–92. https://doi.org/10.1038/s41596-020-00453-6 .
Park J, Childs L, Kim D, Hwang GH, Kim S, Kim ST, Kim JS, Bae S. Digenome-Seq web tool for profiling CRISPR specificity. Nat Methods. 2017;14:548–9. https://doi.org/10.1038/nmeth.4262 .
Cameron P, Fuller CK, Donohoue PD, Jones BN, Thompson MS, Carter MM, Gradia S, Vidal B, Garner E, Slorach EM, et al. Mapping the genomic landscape of CRISPR-Cas9 cleavage. Nat Methods. 2017;14:600–6. https://doi.org/10.1038/nmeth.4284 .
Lazzarotto CR, Nguyen NT, Tang X, Malagon-Lopez J, Guo JA, Aryee MJ, Joung JK, Tsai SQ. Defining CRISPR-Cas9 genome-wide nuclease activities with CIRCLE-seq. Nat Protoc. 2018;13:2615–42. https://doi.org/10.1038/s41596-018-0055-0 .
Tsai SQ, Nguyen NT, Malagon-Lopez J, Topkar VV, Aryee MJ, Joung JK. CIRCLE-seq: a highly sensitive in vitro screen for genome-wide CRISPR-Cas9 nuclease off-targets. Nat Methods. 2017;14:607–14. https://doi.org/10.1038/nmeth.4278 .
Lazzarotto CR, Malinin NL, Li Y, Zhang R, Yang Y, Lee G, Cowley E, He Y, Lan X, Jividen K, et al. CHANGE-seq reveals genetic and epigenetic effects on CRISPR-Cas9 genome-wide activity. Nat Biotechnol. 2020;38:1317–27. https://doi.org/10.1038/s41587-020-0555-7 .
Khoshandam M, Soltaninejad H, Mousazadeh M, Hamidieh AA, Hosseinkhani S. Clinical applications of the CRISPR/Cas9 genome-editing system: delivery options and challenges in precision medicine. Genes Dis. 2024;11:268–82. https://doi.org/10.1016/j.gendis.2023.02.027 .
Wang D, Zhang F, Gao G. CRISPR-Based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell. 2020;181:136–50. https://doi.org/10.1016/j.cell.2020.03.023 .
Proietti L, Manhart G, Heyes E, Troester S, Grebien F. Arrayed CRISPR/Cas9 screening for the functional validation of Cancer Genetic dependencies. Bio Protoc. 2022. https://doi.org/10.21769/BioProtoc.4577 .
Shi J, Wang E, Milazzo JP, Wang Z, Kinney JB, Vakoc CR. Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat Biotechnol. 2015;33:661–7. https://doi.org/10.1038/nbt.3235 .
Schmidt R, Steinhart Z, Layeghi M, Freimer JW, Bueno R, Nguyen VQ, Blaeschke F, Ye CJ, Marson A. CRISPR activation and interference screens decode stimulation responses in primary human T cells. Science. 2022;375:eabj4008. https://doi.org/10.1126/science.abj4008 .
Shifrut E, Carnevale J, Tobin V, Roth TL, Woo JM, Bui CT, Li PJ, Diolaiti ME, Ashworth A, Marson A. Genome-wide CRISPR Screens in Primary Human T Cells Reveal Key Regulators of Immune Function. Cell 2018, 175:1958–1971 e1915. https://doi.org/10.1016/j.cell.2018.10.024 .
Geurts MH, Clevers H. CRISPR engineering in organoids for gene repair and disease modelling. Nat Reviews Bioeng. 2023;1:32–45. https://doi.org/10.1038/s44222-022-00013-5 .
Wang D, Prager BC, Gimple RC, Aguilar B, Alizadeh D, Tang H, Lv D, Starr R, Brito A, Wu Q, et al. CRISPR Screening of CAR T cells and Cancer stem cells reveals critical dependencies for cell-based therapies. Cancer Discov. 2021;11:1192–211. https://doi.org/10.1158/2159-8290.CD-20-1243 .
Dufva O, Koski J, Maliniemi P, Ianevski A, Klievink J, Leitner J, Polonen P, Hohtari H, Saeed K, Hannunen T, et al. Integrated drug profiling and CRISPR screening identify essential pathways for CAR T-cell cytotoxicity. Blood. 2020;135:597–609. https://doi.org/10.1182/blood.2019002121 .
Xu WW, Liao L, Dai W, Zheng CC, Tan XP, He Y, Zhang QH, Huang ZH, Chen WY, Qin YR, et al. Genome-wide CRISPR/Cas9 screening identifies a targetable MEST-PURA interaction in cancer metastasis. EBioMedicine. 2023;92104587. https://doi.org/10.1016/j.ebiom.2023.104587 .
Szlachta K, Kuscu C, Tufan T, Adair SJ, Shang S, Michaels AD, Mullen MG, Fischer NL, Yang J, Liu L, et al. CRISPR knockout screening identifies combinatorial drug targets in pancreatic cancer and models cellular drug response. Nat Commun. 2018;94275. https://doi.org/10.1038/s41467-018-06676-2 .
Yang C, Lee D, Zhang MS, Tse AP, Wei L, Bao MH, Wong BP, Chan CY, Yuen VW, Chen Y, Wong CC. Genome-wide CRISPR/Cas9 Library Screening revealed Dietary Restriction of glutamine in combination with inhibition of pyruvate metabolism as effective Liver Cancer Treatment. Adv Sci (Weinh). 2022;9:e2202104. https://doi.org/10.1002/advs.202202104 .
Coelho R, Tozzi A, Disler M, Lombardo F, Fedier A, Lopez MN, Freuler F, Jacob F, Heinzelmann-Schwarz V. Overlapping gene dependencies for PARP inhibitors and carboplatin response identified by functional CRISPR-Cas9 screening in ovarian cancer. Cell Death Dis. 2022;13909. https://doi.org/10.1038/s41419-022-05347-x .
Arang N, Lubrano S, Rigiracciolo DC, Nachmanson D, Lippman SM, Mali P, Harismendy O, Gutkind JS. Whole-genome CRISPR screening identifies PI3K/AKT as a downstream component of the oncogenic GNAQ-focal adhesion kinase signaling circuitry. J Biol Chem. 2023;299102866. https://doi.org/10.1016/j.jbc.2022.102866 .
Zhao X, Li J, Liu Z, Powers S. Combinatorial CRISPR/Cas9 screening reveals epistatic networks of interacting tumor suppressor genes and therapeutic targets in human breast Cancer. Cancer Res. 2021;81:6090–105. https://doi.org/10.1158/0008-5472.CAN-21-2555 .
Deng L, Yang L, Zhu S, Li M, Wang Y, Cao X, Wang Q, Guo L. Identifying CDC7 as a synergistic target of chemotherapy in resistant small-cell lung cancer via CRISPR/Cas9 screening. Cell Death Discov. 2023;9:40. https://doi.org/10.1038/s41420-023-01315-2 .
Tzelepis K, Koike-Yusa H, De Braekeleer E, Li Y, Metzakopian E, Dovey OM, Mupo A, Grinkevich V, Li M, Mazan M, et al. A CRISPR dropout screen identifies genetic vulnerabilities and therapeutic targets in Acute myeloid leukemia. Cell Rep. 2016;17:1193–205. https://doi.org/10.1016/j.celrep.2016.09.079 .
Zhang B, Ren Z, Zheng H, Lin M, Chen G, Luo OJ, Zhu G. CRISPR activation screening in a mouse model for drivers of hepatocellular carcinoma growth and metastasis. iScience. 2023;26:106099. https://doi.org/10.1016/j.isci.2023.106099 .
Tian Y, Dong D, Wu L, Park JY, Wei GH, Wang L, consortium PE. Combined CRISPR and proteomics screening reveal a cohesin-CTCF-bound allele contributing to increased expression of RUVBL1 and prostate cancer progression. bioRxiv 2023. https://doi.org/10.1101/2023.01.18.524405 .
Zhou Z, Chen G, Shen M, Li J, Liu K, Liu M, Shi S, Yang D, Chen W, Chen S, et al. Genome-scale CRISPR-Cas9 knockout screening in nasopharyngeal carcinoma for radiosensitive and radioresistant genes. Transl Oncol. 2023;30101625. https://doi.org/10.1016/j.tranon.2023.101625 .
Karapurkar JK, Kim MS, Colaco JC, Suresh B, Sarodaya N, Kim DH, Park CH, Hong SH, Kim KS, Ramakrishna S. CRISPR/Cas9-based genome-wide screening of the deubiquitinase subfamily identifies USP3 as a protein stabilizer of REST blocking neuronal differentiation and promotes neuroblastoma tumorigenesis. J Exp Clin Cancer Res. 2023;42121. https://doi.org/10.1186/s13046-023-02694-1 .
Ouyang Q, Liu Y, Tan J, Li J, Yang D, Zeng F, Huang W, Kong Y, Liu Z, Zhou H, Liu Y. Loss of ZNF587B and SULF1 contributed to cisplatin resistance in ovarian cancer cell lines based on genome-scale CRISPR/Cas9 screening. Am J Cancer Res. 2019;9:988–98.
CAS PubMed PubMed Central Google Scholar
Cao C, Liu T, Zhang Q, Li R, Zeng Z, Cui Z, Wang X, Gong D, Tian X, Hu Z. Somatic mutations and CRISPR/Cas9 library screening integrated analysis identifies cervical cancer drug-resistant pathways. Clin Transl Med. 2021;11e632. https://doi.org/10.1002/ctm2.632 .
Haldrup J, Weiss S, Schmidt L, Sorensen KD. Investigation of enzalutamide, docetaxel, and cabazitaxel resistance in the castration resistant prostate cancer cell line C4 using genome-wide CRISPR/Cas9 screening. Sci Rep. 2023;13:9043. https://doi.org/10.1038/s41598-023-35950-7 .
Chen A, Wen S, Liu F, Zhang Z, Liu M, Wu Y, He B, Yan M, Kang T, Lam EW, et al. CRISPR/Cas9 screening identifies a kinetochore-microtubule dependent mechanism for Aurora-A inhibitor resistance in breast cancer. Cancer Commun (Lond). 2021;41:121–39. https://doi.org/10.1002/cac2.12125 .
Zhang N, Huang D, Ruan X, Ng AT, Tsu JH, Jiang G, Huang J, Zhan Y, Na R. CRISPR screening reveals gleason score and castration resistance related oncodriver ring finger protein 19 A (RNF19A) in prostate cancer. Drug Resist Updat. 2023;67100912. https://doi.org/10.1016/j.drup.2022.100912 .
Xie C, Li K, Li Y, Peng X, Teng B, He K, Jin A, Wang W, Wei Z. CRISPR-based knockout screening identifies the loss of MIEF2 to enhance oxaliplatin resistance in colorectal cancer through inhibiting the mitochondrial apoptosis pathway. Front Oncol. 2022;12881487. https://doi.org/10.3389/fonc.2022.881487 .
Luo BH, Huang JQ, Huang CY, Tian P, Chen AZ, Wu WH, Ma XM, Yuan YX, Yu L. Screening of Lymphoma Radiotherapy-resistant genes with CRISPR Activation Library. Pharmgenomics Pers Med. 2023;16:67–80. https://doi.org/10.2147/PGPM.S386085 .
Funke K, Einsfelder U, Hansen A, Arevalo L, Schneider S, Nettersheim D, Stein V, Schorle H. Genome-scale CRISPR screen reveals neddylation to contribute to cisplatin resistance of testicular germ cell tumours. Br J Cancer. 2023;128:2270–82. https://doi.org/10.1038/s41416-023-02247-5 .
Price S, Bhosle S, Goncalves E, Li X, McClurg DP, Barthorpe S, Beck A, Hall C, Lightfoot H, Farrow L, et al. A suspension technique for efficient large-scale cancer organoid culturing and perturbation screens. Sci Rep. 2022;12:5571. https://doi.org/10.1038/s41598-022-09508-y .
Hendriks D, Brouwers JF, Hamer K, Geurts MH, Luciana L, Massalini S, Lopez-Iglesias C, Peters PJ, Rodriguez-Colman MJ, Chuva de Sousa Lopes S, et al. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis. Nat Biotechnol. 2023. https://doi.org/10.1038/s41587-023-01680-4 .
Hirt CK, Booij TH, Grob L, Simmler P, Toussaint NC, Keller D, Taube D, Ludwig V, Goryachkin A, Pauli C, et al. Drug screening and genome editing in human pancreatic cancer organoids identifies drug-gene interactions and candidates for off-label treatment. Cell Genom. 2022;2100095. https://doi.org/10.1016/j.xgen.2022.100095 .
Ling X, Chang L, Chen H, Liu T. Efficient generation of locus-specific human CAR-T cells with CRISPR/cCas12a. STAR Protoc. 2022;3:101321. https://doi.org/10.1016/j.xpro.2022.101321 .
Wagner DL, Koehl U, Chmielewski M, Scheid C, Stripecke R. Review: sustainable clinical development of CAR-T cells - switching from viral transduction towards CRISPR-Cas Gene Editing. Front Immunol. 2022;13:865424. https://doi.org/10.3389/fimmu.2022.865424 .
Sochacka-Cwikla A, Maczynski M, Regiec A. FDA-Approved drugs for hematological malignancies-the last Decade Review. Cancers (Basel). 2021. https://doi.org/10.3390/cancers14010087 . 14.
Dimitri A, Herbst F, Fraietta JA. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol Cancer. 2022;21:78. https://doi.org/10.1186/s12943-022-01559-z .
Wang L, Zhou L, Li M, Zhao J, Liu Y, Chen Y, Qin X, Wang S, Chen H, Piao Y, et al. Genome-wide CRISPR/Cas9 knockout screening uncovers ZNF319 as a novel tumor suppressor critical for breast cancer metastasis. Biochem Biophys Res Commun. 2022;589:107–15. https://doi.org/10.1016/j.bbrc.2021.12.023 .
Sun X, Wang Z, Chen X, Shen K. CRISPR-cas9 screening identified Lethal genes enriched in cell cycle pathway and of prognosis significance in breast Cancer. Front Cell Dev Biol. 2021;9:646774. https://doi.org/10.3389/fcell.2021.646774 .
Wang Q, Guo F, Jin Y, Ma Y. Applications of human organoids in the personalized treatment for digestive diseases. Signal Transduct Target Ther. 2022;7:336. https://doi.org/10.1038/s41392-022-01194-6 .
Cortese MJ, Sauter C. A CRISPR non-viral manufacturing approach for CAR T cell therapies. Mol Ther. 2022;30:3338–40. https://doi.org/10.1016/j.ymthe.2022.09.022 .
Ramos A, Koch CE, Liu-Lupo Y, Hellinger RD, Kyung T, Abbott KL, Frose J, Goulet D, Gordon KS, Eidell KP, et al. Leukemia-intrinsic determinants of CAR-T response revealed by iterative in vivo genome-wide CRISPR screening. Nat Commun. 2023;148048. https://doi.org/10.1038/s41467-023-43790-2 .
Ye L, Park JJ, Peng L, Yang Q, Chow RD, Dong MB, Lam SZ, Guo J, Tang E, Zhang Y, et al. A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy. Cell Metab. 2022;34:595–614. https://doi.org/10.1016/j.cmet.2022.02.009 .
Madigan V, Zhang F, Dahlman JE. Drug delivery systems for CRISPR-based genome editors. Nat Rev Drug Discov. 2023;22:875–94. https://doi.org/10.1038/s41573-023-00762-x .
Chow RD, Guzman CD, Wang G, Schmidt F, Youngblood MW, Ye L, Errami Y, Dong MB, Martinez MA, Zhang S, et al. AAV-mediated direct in vivo CRISPR screen identifies functional suppressors in glioblastoma. Nat Neurosci. 2017;20:1329–41. https://doi.org/10.1038/nn.4620 .
Li J, Lee YC, Iessi IL, Wu C, Yi P, Cai EP. Protocol for genome-scale in vivo CRISPR screening to study protection of beta cells under autoimmunity in a type 1 diabetes mouse model. STAR Protoc. 2023;4:102155. https://doi.org/10.1016/j.xpro.2023.102155 .
Liu L, Lei Y, Chen W, Zhou Q, Zheng Z, Zeng G, Liu W, Feng P, Zhang Z, Yu L, Chen L. In vivo genome-wide CRISPR screening identifies ZNF24 as a negative NF-kappaB modulator in lung cancer. Cell Biosci. 2022;12193. https://doi.org/10.1186/s13578-022-00933-0 .
Dong MB, Wang G, Chow RD, Ye L, Zhu L, Dai X, Park JJ, Kim HR, Errami Y, Guzman CD, et al. Systematic Immunotherapy Target Discovery using genome-scale in vivo CRISPR screens in CD8 T cells. Cell. 2019;178:1189–204. https://doi.org/10.1016/j.cell.2019.07.044 .
Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18:358–78. https://doi.org/10.1038/s41573-019-0012-9 .
Haeussler M. CRISPR off-targets: a question of context. Cell Biol Toxicol. 2020;36:5–9. https://doi.org/10.1007/s10565-019-09497-1 .
Sternberg SH, LaFrance B, Kaplan M, Doudna JA. Conformational control of DNA target cleavage by CRISPR-Cas9. Nature. 2015;527:110–3. https://doi.org/10.1038/nature15544 .
Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351:84–8. https://doi.org/10.1126/science.aad5227 .
Chen JS, Dagdas YS, Kleinstiver BP, Welch MM, Sousa AA, Harrington LB, Sternberg SH, Joung JK, Yildiz A, Doudna JA. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature. 2017;550:407–10. https://doi.org/10.1038/nature24268 .
Kleinstiver BP, Prew MS, Tsai SQ, Nguyen NT, Topkar VV, Zheng Z, Joung JK. Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat Biotechnol. 2015;33:1293–8. https://doi.org/10.1038/nbt.3404 .
Lin P, Qin S, Pu Q, Wang Z, Wu Q, Gao P, Schettler J, Guo K, Li R, Li G, et al. CRISPR-Cas13 inhibitors Block RNA editing in Bacteria and mammalian cells. Mol Cell. 2020;78:850–61. https://doi.org/10.1016/j.molcel.2020.03.033 .
Safarzadeh Kozani P, Safarzadeh Kozani P, Ahmadi Najafabadi M, Yousefi F, Mirarefin SMJ, Rahbarizadeh F. Recent advances in solid tumor CAR-T cell therapy: driving Tumor cells from Hero to zero? Front Immunol. 2022;13:795164. https://doi.org/10.3389/fimmu.2022.795164 .
Mueller KP, Piscopo NJ, Forsberg MH, Saraspe LA, Das A, Russell B, Smerchansky M, Cappabianca D, Shi L, Shankar K, et al. Production and characterization of virus-free, CRISPR-CAR T cells capable of inducing solid tumor regression. J Immunother Cancer. 2022. https://doi.org/10.1136/jitc-2021-004446 .
Hu Y, Zu C, Zhang M, Wei G, Li W, Fu S, Hong R, Zhou L, Wu W, Cui J, et al. Safety and efficacy of CRISPR-based non-viral PD1 locus specifically integrated anti-CD19 CAR-T cells in patients with relapsed or refractory Non-hodgkin’s lymphoma: a first-in-human phase I study. EClinicalMedicine. 2023;60:102010. https://doi.org/10.1016/j.eclinm.2023.102010 .
Johnson WG, Bowman DM. Inherited regulation for advanced ARTs: comparing jurisdictions’ applications of existing governance regimes to emerging reproductive technologies. J Law Biosci. 2022;9:lsab034. https://doi.org/10.1093/jlb/lsab034 .
Cyranoski D. China set to introduce gene-editing regulation following CRISPR-baby furore. Nature. 2019. https://doi.org/10.1038/d41586-019-01580-1 .
Scott CT, Selin C. What to expect when expecting CRISPR Baby Number Four. Am J Bioeth. 2019;19:7–9. https://doi.org/10.1080/15265161.2018.1562793 .
Download references
Acknowledgements
The authors would like to thank Prof. Guokai Chen and Dr. Weiwei Liu from University of Macau, Dr. Carlos Godoy-Parejo from Icahn School of Medicine at Mount Sinai, Ms Qinru Li from University of Toronto for critical comments and wonderful advises on the manuscript. We also thank other laboratory members for helpful discussions of our review.
This work was funded by Guangdong Basic and Applied Basic Research Foundation (File No.2020A1515110045), by Zhuhai High-level Health Personnel Team Project (File No. Zhuhai HLHPTP201702), and by Zhuhai People’s Hospital (File No. 2020XSYC-12).
Author information
Mingming Qin and Chunhao Deng contributed equally to this paper.
Authors and Affiliations
Reproductive Medical Center, Affiliated Foshan Maternity & Child Healthcare Hospital, Southern Medical University (Foshan Women and Children Hospital), Foshan, Guangdong, 528000, China
Mingming Qin & Guoqun Luo
Department of Developmental Biology, School of Basic Medical Sciences, Southern Medical University, Guangzhou, Guangdong, 510515, China
Mingming Qin
Chinese Medicine and Translational Medicine R&D center, Zhuhai UM Science & Technology Research Institute, Zhuhai, Guangdong, 519031, China
Chunhao Deng
Guangdong Provincial Key Laboratory of Tumor Interventional Diagnosis and Treatment, Zhuhai People’s Hospital, Zhuhai Clinical Medical College of Jinan University, Zhuhai, Guangdong, 519000, China
Liewei Wen & Ya Meng
You can also search for this author in PubMed Google Scholar
Contributions
MQ and CD are responsible for the manuscript drafting and revision. LW is contributed to manuscript revision. GL is responsible for manuscript revision and financial support. YM is contributed to manuscript revision. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Guoqun Luo or Ya Meng .
Ethics declarations
Ethical approval.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Qin, M., Deng, C., Wen, L. et al. CRISPR-Cas and CRISPR-based screening system for precise gene editing and targeted cancer therapy. J Transl Med 22 , 516 (2024). https://doi.org/10.1186/s12967-024-05235-2
Download citation
Received : 03 January 2024
Accepted : 24 April 2024
Published : 30 May 2024
DOI : https://doi.org/10.1186/s12967-024-05235-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- CRISPR-Cas system
- CRISPR screening
- Cancer therapy
- Precision medicine
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

- News Releases
USC researchers pioneer new brain imaging technique through clear “window” in patient’s skull
In a proof-of-concept study, a research team based at the Keck School of Medicine of USC showed that functional ultrasound imaging can record brain activity through a transparent skull implant.
Keck School of Medicine of USC
Clear experimental skull implant may enable functional ultrasound imaging of the brain for patients with serious head injuries.
Credit: Todd Patterson
In the first study of its kind, researchers from the Keck School of Medicine of USC and the California Institute of Technology (Caltech) designed and implanted a transparent window in the skull of a patient, then used functional ultrasound imaging (fUSI) to collect high-resolution brain imaging data through the window. Their preliminary findings suggest that this sensitive, non-invasive approach could open new avenues for patient monitoring and clinical research, as well as broader studies of how the brain functions.
“This is the first time anyone had applied functional ultrasound imaging through a skull replacement in an awake, behaving human performing a task,” said Charles Liu, MD, PhD , a professor of clinical neurological surgery, urology and surgery at the Keck School of Medicine and director of the USC Neurorestoration Center . “The ability to extract this type of information noninvasively through a window is pretty significant, particularly since many of the patients who require skull repair have or will develop neurological disabilities. In addition, ‘windows’ can be surgically implanted in patients with intact skulls if functional information can help with diagnosis and treatment.”
The research participant, 39-year-old Jared Hager, sustained a traumatic brain injury (TBI) from a skateboarding accident in 2019. During emergency surgery, half of Hager’s skull was removed to relieve pressure on his brain, leaving part of his brain covered only with skin and connective tissue. Because of the pandemic, he had to wait more than two years to have his skull restored with a prosthesis.
During that time, Hager volunteered for earlier research conducted by Liu, Jonathan Russin, MD , associate surgical director of the USC Neurorestoration Center, and another Caltech team on a new type of brain imaging called fPACT. The experimental technique had been done on soft tissue, but could only be tested on the brain in patients like Hager who were missing a part of their skull. When the time came for implanting the prosthesis, Hager again volunteered to team up with Liu and his colleagues, who designed a custom skull implant to study the utility of fUSI—which cannot be done through the skull or a traditional implant—while repairing Hager’s injury.
Before the reconstructive surgery, the research team tested and optimized fUSI parameters for brain imaging, using both a phantom (a scientific device designed to test medical imaging equipment) and animal models. They then collected fUSI data from Hager while he completed several tasks, both before his surgery and after the clear implant was installed, finding that the window offered an effective way to measure brain activity. The research, funded in part by the National Institutes of Health, was just published in the journal Science Translational Medicine .
Functional brain imaging, which collects data on brain activity by measuring changes in blood flow or electrical impulses, can offer key insights about how the brain works, both in healthy people and those with neurological conditions. But current methods, such as functional magnetic resonance imaging (fMRI) and intracranial electroencephalography (EEG) leave many questions unanswered. Challenges include low resolution, a lack of portability or the need for invasive brain surgery. fUSI may eventually offer a sensitive and precise alternative.
“If we can extract functional information through a patient’s skull implant, that could allow us to provide treatment more safely and proactively,” including to TBI patients who suffer from epilepsy, dementia, or psychiatric problems, Liu said.
A new frontier for brain imaging
As a foundation for the present study, Liu has collaborated for years with Mikhail Shapiro, PhD and Richard Andersen, PhD, of Caltech, to develop specialized ultrasound sequences that can measure brain function, as well as to optimize brain-computer interface technology, which transcribes signals from the brain to operate an external device.
With these pieces in place, Liu and his colleagues tested several transparent skull implants on rats, finding that a thin window made from polymethyl methacrylate (PMMA)—which resembles plexiglass—yielded the clearest imaging results. They then collaborated with a neurotechnology company, Longeviti Neuro Solutions, to build a custom implant for Hager.
Before surgery, the researchers collected fUSI data while Hager did two activities: solving a “connect-the-dots” puzzle on a computer monitor and playing melodies on his guitar. After the implant was installed, they collected data on the same tasks, then compared the results to determine whether fUSI could provide accurate and useful imaging data.
“The fidelity of course decreased, but importantly, our research showed that it’s still high enough to be useful,” Liu said. “And unlike other brain-computer interface platforms, which require electrodes to be implanted in the brain, this has far less barriers to adoption.”
fUSI may offer finer resolution than fMRI and unlike intracranial EEG, it does not require electrodes to be implanted inside the brain. It is also less expensive than those methods and could provide some clinical advantages for patients over non-transparent skull implants, said Russin, who is also an associate professor of neurological surgery at the Keck School of Medicine and director of cerebrovascular surgery at Keck Hospital of USC.
“One of the big problems when we do these surgeries is that a blood clot can form underneath the implant, but having a clear window gives us an easy way to monitor that,” he said.
Refining functional ultrasound technology
In addition to better monitoring of patients, the new technique could offer population-level insights about TBI and other neurological conditions. It could also allow scientists to collect data on the healthy brain and learn more about how it controls cognitive, sensory, motor and autonomic functions.
“What our findings shows is that we can extract useful functional information with this method,” Liu said. “The next step is: What specific functional information do we want, and what can we use it for?”
Until the new technologies undergo clinical trials, fUSI and the clear implant are experimental. In the meantime, the research team is working to improve their fUSI protocols to further enhance image resolution. Future research should also build on this early proof-of-concept study by testing more participants to better establish the link between fUSI data and specific brain functions, the researchers said.
“Jared is an amazing guy,” said Liu, who is continuing to collaborate with the study participant on refining new technologies, including laser spectroscopy, which measures blood flow in the brain. “His contributions have really helped us explore new frontiers that we hope can ultimately help many other patients.”
About this research
In addition to Liu, Russin, Shapiro and Andersen, the study’s other authors are Kay Jann, PhD, from the Mark and Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine of USC; Claire Rabut, Sumner Norman and Whitney Griggs from the California Institute of Technology; and Vasileios Christopoulos from the University of California Riverside.
This work was supported by the National Institutes of Health [R01NS123663]; the T&C Chen Brain-Machine Interface Center; the Boswell Foundation; the National Eye Institute [F30 EY032799]; the Josephine de Karman Fellowship; the UCLA-Caltech Medical Scientist Training Program [NIGMS T32 GM008042]; the Della Martin Postdoctoral Fellowship; the Human Frontier Science Program Cross-Disciplinary Fellowship [LT000217/2020-C]; the USC Neurorestoration Center; and the Howard Hughes Medical Institute.
Science Translational Medicine
Method of Research
Experimental study
Subject of Research
Article title.
Functional ultrasound imaging of human brain activity through an acoustic transparent cranial window
Article Publication Date
29-May-2024
COI Statement
CR, WSG, SLN, RAA, CL, and MGS have filed a provisional patent application based on this research, filing # CIT-9020-P entitled "A method for observing brain states using functional ultrasound imaging and a sonolucent material". The authors declare that they have no other competing interests.
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.
June 2024: Dr. Kathryn D. Coduto (COM)
- By: Shannon Landis
- June 1, 2024

What made you decide to be a social scientist/ why does social science matter to you?
I love being able to answer questions about the world; I originally wanted to be a journalist for that exact reason. As I went through my undergraduate program, I discovered social scientific research and realized just how much I could do within this field. I also feel empowered by social science; there was a time when the idea of statistics was so intimidating to me. But now, I am confident in my ability to navigate complex questions and their solutions through a variety of tools and techniques, all stemming from social scientific training and exploration.
Can you tell us about a recent research project that you’re excited about?
Dr. Allison McDonald, from the Faculty of Computing and Data Sciences, and I have been working on a project investigating sexting behaviors in romantic relationships. We specifically have asked people about how they negotiate, if they do, the sharing of this highly personal information with partners. I’m especially excited about this project, though, because we are also investigating what people do with sexual material once they’ve broken up. There isn’t much research in this area yet, and I think we’re getting really rich insights into how people are thinking about handling sensitive content throughout the lifetime of a relationship. I recently presented this work at the CHI 2024 conference and got some great feedback on potential design implications for improving technologies in these instances. We have another part of the study we’re hoping to launch soon, too.
What is the best piece of professional advice you ever received?
I had great advisors for both my master’s degree (Dr. Danielle Coombs at Kent State University) and my doctoral degree (Dr. Jesse Fox at Ohio State University). They both always encouraged me to follow my interests, and I always felt supported in exploring the ideas I was most passionate about. I think following your interests is critical to staying interested in and excited by the work you do; I love thinking about research and so much of that is because I study the things I am most interested in. I now pass that advice along to my own advisees, too—follow your interests!
What is your favorite course you’ve taught at BU?
I love teaching Communication Research (CM 722). Comm Research is many students’ first experience with social scientific research, especially in a communication context, and I love helping them realize how much they can do with research. I am always so excited when I see different concepts click into place for students, whether that be perfecting a research design, conducting a statistical analysis, or crafting implications from their research. It is always so rewarding.
Tell us a surprising fact about yourself.
Before coming to BU, I was an assistant professor at South Dakota State University in Brookings, SD. My favorite place in the world is still Badlands National Park in western South Dakota—I haven’t been anywhere that can top it!
View all posts
- Open access
- Published: 27 May 2024
Current status of community resources and priorities for weed genomics research
- Jacob Montgomery 1 ,
- Sarah Morran 1 ,
- Dana R. MacGregor ORCID: orcid.org/0000-0003-0543-0408 2 ,
- J. Scott McElroy ORCID: orcid.org/0000-0003-0331-3697 3 ,
- Paul Neve ORCID: orcid.org/0000-0002-3136-5286 4 ,
- Célia Neto ORCID: orcid.org/0000-0003-3256-5228 4 ,
- Martin M. Vila-Aiub ORCID: orcid.org/0000-0003-2118-290X 5 ,
- Maria Victoria Sandoval 5 ,
- Analia I. Menéndez ORCID: orcid.org/0000-0002-9681-0280 6 ,
- Julia M. Kreiner ORCID: orcid.org/0000-0002-8593-1394 7 ,
- Longjiang Fan ORCID: orcid.org/0000-0003-4846-0500 8 ,
- Ana L. Caicedo ORCID: orcid.org/0000-0002-0378-6374 9 ,
- Peter J. Maughan 10 ,
- Bianca Assis Barbosa Martins 11 ,
- Jagoda Mika 11 ,
- Alberto Collavo 11 ,
- Aldo Merotto Jr. ORCID: orcid.org/0000-0002-1581-0669 12 ,
- Nithya K. Subramanian ORCID: orcid.org/0000-0002-1659-7396 13 ,
- Muthukumar V. Bagavathiannan ORCID: orcid.org/0000-0002-1107-7148 13 ,
- Luan Cutti ORCID: orcid.org/0000-0002-2867-7158 14 ,
- Md. Mazharul Islam 15 ,
- Bikram S. Gill ORCID: orcid.org/0000-0003-4510-9459 16 ,
- Robert Cicchillo 17 ,
- Roger Gast 17 ,
- Neeta Soni ORCID: orcid.org/0000-0002-4647-8355 17 ,
- Terry R. Wright ORCID: orcid.org/0000-0002-3969-2812 18 ,
- Gina Zastrow-Hayes 18 ,
- Gregory May 18 ,
- Jenna M. Malone ORCID: orcid.org/0000-0002-9637-2073 19 ,
- Deepmala Sehgal ORCID: orcid.org/0000-0002-4141-1784 20 ,
- Shiv Shankhar Kaundun ORCID: orcid.org/0000-0002-7249-2046 20 ,
- Richard P. Dale 20 ,
- Barend Juan Vorster ORCID: orcid.org/0000-0003-3518-3508 21 ,
- Bodo Peters 11 ,
- Jens Lerchl ORCID: orcid.org/0000-0002-9633-2653 22 ,
- Patrick J. Tranel ORCID: orcid.org/0000-0003-0666-4564 23 ,
- Roland Beffa ORCID: orcid.org/0000-0003-3109-388X 24 ,
- Alexandre Fournier-Level ORCID: orcid.org/0000-0002-6047-7164 25 ,
- Mithila Jugulam ORCID: orcid.org/0000-0003-2065-9067 15 ,
- Kevin Fengler 18 ,
- Victor Llaca ORCID: orcid.org/0000-0003-4822-2924 18 ,
- Eric L. Patterson ORCID: orcid.org/0000-0001-7111-6287 14 &
- Todd A. Gaines ORCID: orcid.org/0000-0003-1485-7665 1
Genome Biology volume 25 , Article number: 139 ( 2024 ) Cite this article
590 Accesses
7 Altmetric
Metrics details
Weeds are attractive models for basic and applied research due to their impacts on agricultural systems and capacity to swiftly adapt in response to anthropogenic selection pressures. Currently, a lack of genomic information precludes research to elucidate the genetic basis of rapid adaptation for important traits like herbicide resistance and stress tolerance and the effect of evolutionary mechanisms on wild populations. The International Weed Genomics Consortium is a collaborative group of scientists focused on developing genomic resources to impact research into sustainable, effective weed control methods and to provide insights about stress tolerance and adaptation to assist crop breeding.
Each year globally, agricultural producers and landscape managers spend billions of US dollars [ 1 , 2 ] and countless hours attempting to control weedy plants and reduce their adverse effects. These management methods range from low-tech (e.g., pulling plants from the soil by hand) to extremely high-tech (e.g., computer vision-controlled spraying of herbicides). Regardless of technology level, effective control methods serve as strong selection pressures on weedy plants and often result in rapid evolution of weed populations resistant to such methods [ 3 , 4 , 5 , 6 , 7 ]. Thus, humans and weeds have been locked in an arms race, where humans develop new or improved control methods and weeds adapt and evolve to circumvent such methods.
Applying genomics to weed science offers a unique opportunity to study rapid adaptation, epigenetic responses, and examples of evolutionary rescue of diverse weedy species in the face of widespread and powerful selective pressures. Furthermore, lessons learned from these studies may also help to develop more sustainable control methods and to improve crop breeding efforts in the face of our ever-changing climate. While other research fields have used genetics and genomics to uncover the basis of many biological traits [ 8 , 9 , 10 , 11 ] and to understand how ecological factors affect evolution [ 12 , 13 ], the field of weed science has lagged behind in the development of genomic tools essential for such studies [ 14 ]. As research in human and crop genetics pushes into the era of pangenomics (i.e., multiple chromosome scale genome assemblies for a single species [ 15 , 16 ]), publicly available genomic information is still lacking or severely limited for the majority of weed species. Recent reviews of current weed genomes identified 26 [ 17 ] and 32 weed species with sequenced genomes [ 18 ]—many assembled to a sub-chromosome level.
Here, we summarize the current state of weed genomics, highlighting cases where genomics approaches have successfully provided insights on topics such as population genetic dynamics, genome evolution, and the genetic basis of herbicide resistance, rapid adaptation, and crop dedomestication. These highlighted investigations all relied upon genomic resources that are relatively rare for weedy species. Throughout, we identify additional resources that would advance the field of weed science and enable further progress in weed genomics. We then introduce the International Weed Genomics Consortium (IWGC), an open collaboration among researchers, and describe current efforts to generate these additional resources.
Evolution of weediness: potential research utilizing weed genomics tools
Weeds can evolve from non-weed progenitors through wild colonization, crop de-domestication, or crop-wild hybridization [ 19 ]. Because the time span in which weeds have evolved is necessarily limited by the origins of agriculture, these non-weed relatives often still exist and can be leveraged through population genomic and comparative genomic approaches to identify the adaptive changes that have driven the evolution of weediness. The ability to rapidly adapt, persist, and spread in agroecosystems are defining features of weedy plants, leading many to advocate agricultural weeds as ideal candidates for studying rapid plant adaptation [ 20 , 21 , 22 , 23 ]. The insights gained from applying plant ecological approaches to the study of rapid weed adaptation will move us towards the ultimate goals of mitigating such adaptation and increasing the efficacy of crop breeding and biotechnology [ 14 ].
Biology and ecological genomics of weeds
The impressive community effort to create and maintain resources for Arabidopsis thaliana ecological genomics provides a motivating example for the emerging study of weed genomics [ 24 , 25 , 26 , 27 ]. Arabidopsis thaliana was the first flowering plant species to have its genome fully sequenced [ 28 ] and rapidly became a model organism for plant molecular biology. As weedy genomes become available, collection, maintenance, and resequencing of globally distributed accessions of these species will help to replicate the success found in ecological studies of A. thaliana [ 29 , 30 , 31 , 32 , 33 , 34 , 35 ]. Evaluation of these accessions for traits of interest to produce large phenomics data sets (as in [ 36 , 37 , 38 , 39 , 40 ]) enables genome-wide association studies and population genomics analyses aimed at dissecting the genetic basis of variation in such traits [ 41 ]. Increasingly, these resources (e.g. the 1001 genomes project [ 29 ]) have enabled A. thaliana to be utilized as a model species to explore the eco-evolutionary basis of plant adaptation in a more realistic ecological context. Weedy species should supplement lessons in eco-evolutionary genomics learned from these experiments in A. thaliana .
Untargeted genomic approaches for understanding the evolutionary trajectories of populations and the genetic basis of traits as described above rely on the collection of genotypic information from across the genome of many individuals. While whole-genome resequencing accomplishes this requirement and requires no custom methodology, this approach provides more information than is necessary and is prohibitively expensive in species with large genomes. Development and optimization of genotype-by-sequencing methods for capturing reduced representations of newly sequence genomes like those described by [ 42 , 43 , 44 ] will reduce the cost and computational requirements of genetic mapping and population genetic experiments. Most major weed species do not currently have protocols for stable transformation, a key development in the popularity of A. thaliana as a model organism and a requirement for many functional genomic approaches. Functional validation of genes/variants believed to be responsible for traits of interest in weeds has thus far relied on transiently manipulating endogenous gene expression [ 45 , 46 ] or ectopic expression of a transgene in a model system [ 47 , 48 , 49 ]. While these methods have been successful, few weed species have well-studied viral vectors to adapt for use in virus induced gene silencing. Spray induced gene silencing is another potential option for functional investigation of candidate genes in weeds, but more research is needed to establish reliable delivery and gene knockdown [ 50 ]. Furthermore, traits with complex genetic architecture divergent between the researched and model species may not be amenable to functional genomic approaches using transgenesis techniques in model systems. Developing protocols for reduced representation sequencing, stable transformation, and gene editing/silencing in weeds will allow for more thorough characterization of candidate genetic variants underlying traits of interest.
Beyond rapid adaptation, some weedy species offer an opportunity to better understand co-evolution, like that between plants and pollinators and how their interaction leads to the spread of weedy alleles (Additional File 1 : Table S1). A suite of plant–insect traits has co-evolved to maximize the attraction of the insect pollinator community and the efficiency of pollen deposition between flowers ensuring fruit and seed production in many weeds [ 51 , 52 ]. Genetic mapping experiments have identified genes and genetic variants responsible for many floral traits affecting pollinator interaction including petal color [ 53 , 54 , 55 , 56 ], flower symmetry and size [ 57 , 58 , 59 ], and production of volatile organic compounds [ 60 , 61 , 62 ] and nectar [ 63 , 64 , 65 ]. While these studies reveal candidate genes for selection under co-evolution, herbicide resistance alleles may also have pleiotropic effects on the ecology of weeds [ 66 ], altering plant-pollinator interactions [ 67 ]. Discovery of genes and genetic variants involved in weed-pollinator interaction and their molecular and environmental control may create opportunities for better management of weeds with insect-mediated pollination. For example, if management can disrupt pollinator attraction/interaction with these weeds, the efficiency of reproduction may be reduced.
A more complete understanding of weed ecological genomics will undoubtedly elucidate many unresolved questions regarding the genetic basis of various aspects of weediness. For instance, when comparing populations of a species from agricultural and non-agricultural environments, is there evidence for contemporary evolution of weedy traits selected by agricultural management or were “natural” populations pre-adapted to agroecosystems? Where there is differentiation between weedy and natural populations, which traits are under selection and what is the genetic basis of variation in those traits? When comparing between weedy populations, is there evidence for parallel versus non-parallel evolution of weediness at the phenotypic and genotypic levels? Such studies may uncover fundamental truths about weediness. For example, is there a common phenotypic and/or genotypic basis for aspects of weediness among diverse weed species? The availability of characterized accessions and reference genomes for species of interest are required for such studies but only a few weedy species have these resources developed.
Population genomics
Weed species are certainly fierce competitors, able to outcompete crops and endemic species in their native environment, but they are also remarkable colonizers of perturbed habitats. Weeds achieve this through high fecundity, often producing tens of thousands of seeds per individual plant [ 68 , 69 , 70 ]. These large numbers in terms of demographic population size often combine with outcrossing reproduction to generate high levels of diversity with local effective population sizes in the hundreds of thousands [ 71 , 72 ]. This has two important consequences: weed populations retain standing genetic variation and generate many new mutations, supporting weed success in the face of harsh control. The generation of genomic tools to monitor weed populations at the molecular level is a game-changer to understanding weed dynamics and precisely testing the effect of artificial selection (i.e., management) and other evolutionary mechanisms on the genetic make-up of populations.
Population genomic data, without any environmental or phenotypic information, can be used to scan the genomes of weed and non-weed relatives to identify selective sweeps, pointing at loci supporting weed adaptation on micro- or macro-evolutionary scales. Two recent within-species examples include weedy rice, where population differentiation between weedy and domesticated populations was used to identify the genetic basis of weedy de-domestication [ 73 ], and common waterhemp, where consistent allelic differences among natural and agricultural collections resolved a complex set of agriculturally adaptive alleles [ 74 , 75 ]. A recent comparative population genomic study of weedy barnyardgrass and crop millet species has demonstrated how inter-specific investigations can resolve the signatures of crop and weed evolution [ 76 ] (also see [ 77 ] for a non-weed climate adaptation example). Multiple sequence alignments across numerous species provide complementary insight into adaptive convergence over deeper timescales, even with just one genomic sample per species (e.g., [ 78 , 79 ]). Thus, newly sequenced weed genomes combined with genomes available for closely related crops (outlined by [ 14 , 80 ]) and an effort to identify other non-weed wild relatives will be invaluable in characterizing the genetic architecture of weed adaptation and evolution across diverse species.
Weeds experience high levels of genetic selection, both artificial in response to agricultural practices and particularly herbicides, and natural in response to the environmental conditions they encounter [ 81 , 82 ]. Using genomic analysis to identify loci that are the targets of selection, whether natural or artificial, would point at vulnerabilities that could be leveraged against weeds to develop new and more sustainable management strategies [ 83 ]. This is a key motivation to develop genotype-by-environment association (GEA) and selective sweep scan approaches, which allow researchers to resolve the molecular basis of multi-dimensional adaptation [ 84 , 85 ]. GEA approaches, in particular, have been widely used on landscape-wide resequencing collections to determine the genetic basis of climate adaptation (e.g., [ 27 , 86 , 87 ]), but have yet to be fully exploited to diagnose the genetic basis of the various aspects of weediness [ 88 ]. Armed with data on environmental dimensions of agricultural settings, such as focal crop, soil quality, herbicide use, and climate, GEA approaches can help disentangle how discrete farming practices have influenced the evolution of weediness and resolve broader patterns of local adaptation across a weed’s range. Although non-weedy relatives are not technically required for GEA analyses, inclusion of environmental and genomic data from weed progenitors can further distinguish genetic variants underpinning weed origins from those involved in local adaptation.
New weeds emerge frequently [ 89 ], either through hybridization between species as documented for sea beet ( Beta vulgaris ssp. maritima) hybridizing with crop beet to produce progeny that are well adapted to agricultural conditions [ 90 , 91 , 92 ], or through the invasion of alien species that find a new range to colonize. Biosecurity measures are often in place to stop the introduction of new weeds; however, the vast scale of global agricultural commodity trade precludes the possibility of total control. Population genomic analysis is now able to measure gene flow between populations [ 74 , 93 , 94 , 95 ] and identify populations of origin for invasive species including weeds [ 96 , 97 , 98 ]. For example, the invasion route of the pest fruitfly Drosophila suzukii from Eastern Asia to North America and Europe through Hawaii was deciphered using Approximate Bayesian Computation on high-throughput sequencing data from a global sample of multiple populations [ 99 ]. Genomics can also be leveraged to predict invasion rather than explain it. The resequencing of a global sample of common ragweed ( Ambrosia artemisiifolia L.) elucidated a complex invasion route whereby Europe was invaded by multiple introductions of American ragweed that hybridized in Europe prior to a subsequent introduction to Australia [ 100 , 101 ]. In this context, the use of genomically informed species distribution models helps assess the risk associated with different source populations, which in the case of common ragweed, suggests that a source population from Florida would allow ragweed to invade most of northern Australia [ 102 ]. Globally coordinated research efforts to understand potential distribution models could support the transformation of biosecurity from perspective analysis towards predictive risk assessment.
Herbicide resistance and weed management
Herbicide resistance is among the numerous weedy traits that can evolve in plant populations exposed to agricultural selection pressures. Over-reliance on herbicides to control weeds, along with low diversity and lack of redundancy in weed management strategies, has resulted in globally widespread herbicide resistance [ 103 ]. To date, 272 herbicide-resistant weed species have been reported worldwide, and at least one resistance case exists for 21 of the 31 existing herbicide sites of action [ 104 ]—significantly limiting chemical weed control options available to agriculturalists. This limitation of control options is exacerbated by the recent lack of discovery of herbicides with new sites of action [ 105 ].
Herbicide resistance may result from several different physiological mechanisms. Such mechanisms have been classified into two main groups, target-site resistance (TSR) [ 4 , 106 ] and non-target-site resistance (NTSR) [ 4 , 107 ]. The first group encompasses changes that reduce binding affinity between a herbicide and its target [ 108 ]. These changes may provide resistance to multiple herbicides that have a common biochemical target [ 109 ] and can be effectively managed through mixture and/or rotation of herbicides targeting different sites of action [ 110 ]. The second group (NTSR), includes alterations in herbicide absorption, translocation, sequestration, and/or metabolism that may lead to unpredictable pleotropic cross-resistance profiles where structurally and functionally diverse herbicides are rendered ineffective by one or more genetic variant(s) [ 47 ]. This mechanism of resistance threatens not only the efficacy of existing herbicidal chemistries, but also ones yet to be discovered. While TSR is well understood because of the ease of identification and molecular characterization of target site variants, NTSR mechanisms are significantly more challenging to research because they are often polygenic, and the resistance causing element(s) are not well understood [ 111 ].
Improving the current understanding of metabolic NTSR mechanisms is not an easy task, since genes of diverse biochemical functions are involved, many of which exist as extensive gene families [ 109 , 112 ]. Expression changes of NTSR genes have been implicated in several resistance cases where the protein products of the genes are functionally equivalent across sensitive and resistant plants, but their relative abundance leads to resistance. Thus, regulatory elements of NTSR genes have been scrutinized to understand their role in NTSR mechanisms [ 113 ]. Similarly, epigenetic modifications have been hypothesized to play a role in NTSR, with much remaining to be explored [ 114 , 115 , 116 ]. Untargeted approaches such as genome-wide association, selective sweep scans, linkage mapping, RNA-sequencing, and metabolomic profiling have proven helpful to complement more specific biochemical- and chemo-characterization studies towards the elucidation of NTSR mechanisms as well as their regulation and evolution [ 47 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 ]. Even in cases where resistance has been attributed to TSR, genetic mapping approaches can detect other NTSR loci contributing to resistance (as shown by [ 123 ]) and provide further evidence for the role of TSR mutations across populations. Knowledge of the genetic basis of NTSR will aid the rational design of herbicides by screening new compounds for interaction with newly discovered NTSR proteins during early research phases and by identifying conserved chemical structures that interact with these proteins that should be avoided in small molecule design.
Genomic resources can also be used to predict the protein structure for novel herbicide target site and metabolism genes. This will allow for prediction of efficacy and selectivity for new candidate herbicides in silico to increase herbicide discovery throughput as well as aid in the design and development of next-generation technologies for sustainable weed management. Proteolysis targeting chimeras (PROTACs) have the potential to bind desired targets with great selectivity and degrade proteins by utilizing natural protein ubiquitination and degradation pathways within plants [ 125 ]. Spray-induced gene silencing in weeds using oligonucleotides has potential as a new, innovative, and sustainable method for weed management, but improved methods for design and delivery of oligonucleotides are needed to make this technique a viable management option [ 50 ]. Additionally, success in the field of pharmaceutical drug discovery in the development of molecules modulating protein–protein interactions offers another potential avenue towards the development of herbicides with novel targets [ 126 , 127 ]. High-quality reference genomes allow for the design of new weed management technologies like the ones listed here that are specific to—and effective across—weed species but have a null effect on non-target organisms.
Comparative genomics and genome biology
The genomes of weed species are as diverse as weed species themselves. Weeds are found across highly diverged plant families and often have no phylogenetically close model or crop species relatives for comparison. On all measurable metrics, weed genomes run the gamut. Some have smaller genomes like Cyperus spp. (~ 0.26 Gb) while others are larger, such as Avena fatua (~ 11.1 Gb) (Table 1 ). Some have high heterozygosity in terms of single-nucleotide polymorphisms, such as the Amaranthus spp., while others are primarily self-pollinated and quite homozygous, such as Poa annua [ 128 , 129 ]. Some are diploid such as Conyza canadensis and Echinochloa haploclada while others are polyploid such as C. sumetrensis , E. crus-galli , and E. colona [ 76 ]. The availability of genomic resources in these diverse, unexplored branches of the tree of life allows us to identify consistencies and anomalies in the field of genome biology.
The weed genomes published so far have focused mainly on weeds of agronomic crops, and studies have revolved around their ability to resist key herbicides. For example, genomic resources were vital in the elucidation of herbicide resistance cases involving target site gene copy number variants (CNVs). Gene CNVs of 5-enolpyruvylshikimate-3-phosphate synthase ( EPSPS ) have been found to confer resistance to the herbicide glyphosate in diverse weed species. To date, nine species have independently evolved EPSPS CNVs, and species achieve increased EPSPS copy number via different mechanisms [ 153 ]. For instance, the EPSPS CNV in Bassia scoparia is caused by tandem duplication, which is accredited to transposable element insertions flanking EPSPS and subsequent unequal crossing over events [ 154 , 155 ]. In Eleusine indica , a EPSPS CNV was caused by translocation of the EPSPS locus into the subtelomere followed by telomeric sequence exchange [ 156 ]. One of the most fascinating genome biology discoveries in weed science has been that of extra-chromosomal circular DNAs (eccDNAs) that harbor the EPSPS gene in the weed species Amaranthus palmeri [ 157 , 158 ]. In this case, the eccDNAs autonomously replicate separately from the nuclear genome and do not reintegrate into chromosomes, which has implications for inheritance, fitness, and genome structure [ 159 ]. These discoveries would not have been possible without reference assemblies of weed genomes, next-generation sequencing, and collaboration with experts in plant genomics and bioinformatics.
Another question that is often explored with weedy genomes is the nature and composition of gene families that are associated with NTSR. Gene families under consideration often include cytochrome P450s (CYPs), glutathione- S -transferases (GSTs), ABC transporters, etc. Some questions commonly considered with new weed genomes include how many genes are in each of these gene families, where are they located, and which weed accessions and species have an over-abundance of them that might explain their ability to evolve resistance so rapidly [ 76 , 146 , 160 , 161 ]? Weed genome resources are necessary to answer questions about gene family expansion or contraction during the evolution of weediness, including the role of polyploidy in NTSR gene family expansion as explored by [ 162 ].
Translational research and communication with weed management stakeholders
Whereas genomics of model plants is typically aimed at addressing fundamental questions in plant biology, and genomics of crop species has the obvious goal of crop improvement, goals of genomics of weedy plants also include the development of more effective and sustainable strategies for their management. Weed genomic resources assist with these objectives by providing novel molecular ecological and evolutionary insights from the context of intensive anthropogenic management (which is lacking in model plants), and offer knowledge and resources for trait discovery for crop improvement, especially given that many wild crop relatives are also important agronomic weeds (e.g., [ 163 ]). For instance, crop-wild relatives are valuable for improving crop breeding for marginal environments [ 164 ]. Thus, weed genomics presents unique opportunities and challenges relative to plant genomics more broadly. It should also be noted that although weed science at its core is an applied discipline, it draws broadly from many scientific disciplines such as, plant physiology, chemistry, ecology, and evolutionary biology, to name a few. The successful integration of weed-management strategies, therefore, requires extensive collaboration among individuals collectively possessing the necessary expertise [ 165 ].
With the growing complexity of herbicide resistance management, practitioners are beginning to recognize the importance of understanding resistance mechanisms to inform appropriate management tactics [ 14 ]. Although weed science practitioners do not need to understand the technical details of weed genomics, their appreciation of the power of weed genomics—together with their unique insights from field observations—will yield novel opportunities for applications of weed genomics to weed management. In particular, combining field management history with information on weed resistance mechanisms is expected to provide novel insights into evolutionary trajectories (e.g. [ 6 , 166 ]), which can be utilized for disrupting evolutionary adaptation. It can be difficult to obtain field history information from practitioners, but developing an understanding among them of the importance of such information can be invaluable.
Development of weed genomics resources by the IWGC
Weed genomics is a fast-growing field of research with many recent breakthroughs and many unexplored areas of study. The International Weed Genomics Consortium (IWGC) started in 2021 to address the roadblocks listed above and to promote the study of weedy plants. The IWGC is an open collaboration among academic, government, and industry researchers focused on producing genomic tools for weedy species from around the world. Through this collaboration, our initial aim is to provide chromosome-level reference genome assemblies for at least 50 important weedy species from across the globe that are chosen based on member input, economic impact, and global prevalence (Fig. 1 ). Each genome will include annotation of gene models and repetitive elements and will be freely available through public databases with no intellectual property restrictions. Additionally, future funding of the IWGC will focus on improving gene annotations and supplementing these reference genomes with tools that increase their utility.
The International Weed Genomics Consortium (IWGC) collected input from the weed genomics community to develop plans for weed genome sequencing, annotation, user-friendly genome analysis tools, and community engagement
Reference genomes and data analysis tools
The first objective of the IWGC is to provide high-quality genomic resources for agriculturally important weeds. The IWGC therefore created two main resources for information about, access to, or analysis of weed genomic data (Fig. 1 ). The IWGC website (available at [ 167 ]) communicates the status and results of genome sequencing projects, information on training and funding opportunities, upcoming events, and news in weed genomics. It also contains details of all sequenced species including genome size, ploidy, chromosome number, herbicide resistance status, and reference genome assembly statistics. The IWGC either compiles existing data on genome size, ploidy, and chromosome number, or obtains the data using flow cytometry and cytogenetics (Fig. 1 ; Additional File 2 : Fig S1-S4). Through this website, users can request an account to access our second main resource, an online genome database called WeedPedia (accessible at [ 168 ]), with an account that is created within 3–5 working days of an account request submission. WeedPedia hosts IWGC-generated and other relevant publicly accessible genomic data as well as a suite of bioinformatic tools. Unlike what is available for other fields, weed science did not have a centralized hub for genomics information, data, and analysis prior to the IWGC. Our intention in creating WeedPedia is to encourage collaboration and equity of access to information across the research community. Importantly, all genome assemblies and annotations from the IWGC (Table 1 ), along with the raw data used to produce them, will be made available through NCBI GenBank. Upon completion of a 1-year sponsoring member data confidentiality period for each species (dates listed in Table 1 ), scientific teams within the IWGC produce the first genome-wide investigation to submit for publication including whole genome level analyses on genes, gene families, and repetitive sequences as well as comparative analysis with other species. Genome assemblies and data will be publicly available through NCBI as part of these initial publications for each species.
WeedPedia is a cloud-based omics database management platform built from the software “CropPedia” and licensed from KeyGene (Wageningen, The Netherlands). The interface allows users to access, visualize, and download genome assemblies along with structural and functional annotation. The platform includes a genome browser, comparative map viewer, pangenome tools, RNA-sequencing data visualization tools, genetic mapping and marker analysis tools, and alignment capabilities that allow searches by keyword or sequence. Additionally, genes encoding known target sites of herbicides have been specially annotated, allowing users to quickly identify and compare these genes of interest. The platform is flexible, making it compatible with future integration of other data types such as epigenetic or proteomic information. As an online platform with a graphical user interface, WeedPedia provides user-friendly, intuitive tools that encourage users to integrate genomics into their research while also allowing more advanced users to download genomic data to be used in custom analysis pipelines. We aspire for WeedPedia to mimic the success of other public genomic databases such as NCBI, CoGe, Phytozome, InsectBase, and Mycocosm to name a few. WeedPedia currently hosts reference genomes for 40 species (some of which are currently in their 1-year confidentiality period) with additional genomes in the pipeline to reach a currently planned total of 55 species (Table 1 ). These genomes include both de novo reference genomes generated or in progress by the IWGC (31 species; Table 1 ), and publicly available genome assemblies of 24 weedy or related species that were generated by independent research groups (Table 2 ). As of May 2024, WeedPedia has over 370 registered users from more than 27 countries spread across 6 continents.
The IWGC reference genomes are generated in partnership with the Corteva Agriscience Genome Center of Excellence (Johnston, Iowa) using a combination of single-molecule long-read sequencing, optical genome maps, and chromosome conformation mapping. This strategy has already yielded highly contiguous, phased, chromosome-level assemblies for 26 weed species, with additional assemblies currently in progress (Table 1 ). The IWGC assemblies have been completed as single or haplotype-resolved double-haplotype pseudomolecules in inbreeding and outbreeding species, respectively, with multiple genomes being near gapless. For example, the de novo assemblies of the allohexaploids Conyza sumatrensis and Chenopodium album have all chromosomes captured in single scaffolds and most chromosomes being gapless from telomere to telomere. Complementary full-length isoform (IsoSeq) sequencing of RNA collected from diverse tissue types and developmental stages assists in the development of gene models during annotation.
As with accessibility of data, a core objective of the IWGC is to facilitate open access to sequenced germplasm when possible for featured species. Historically, the weed science community has rarely shared or adopted standard germplasm (e.g., specific weed accessions). The IWGC has selected a specific accession of each species for reference genome assembly (typically susceptible to herbicides). In collaboration with a parallel effort by the Herbicide Resistant Plants committee of the Weed Science Society of America, seeds of the sequenced weed accessions will be deposited in the United States Department of Agriculture Germplasm Resources Information Network [ 186 ] for broad access by the scientific community and their accession numbers will be listed on the IWGC website. In some cases, it is not possible to generate enough seed to deposit into a public repository (e.g., plants that typically reproduce vegetatively, that are self-incompatible, or that produce very few seeds from a single individual). In these cases, the location of collection for sequenced accessions will at least inform the community where the sequenced individual came from and where they may expect to collect individuals with similar genotypes. The IWGC ensures that sequenced accessions are collected and documented to comply with the Nagoya Protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization under the Convention on Biological Diversity and related Access and Benefit Sharing Legislation [ 187 ]. As additional accessions of weed species are sequenced (e.g., pangenomes are obtained), the IWGC will facilitate germplasm sharing protocols to support collaboration. Further, to simplify the investigation of herbicide resistance, the IWGC will link WeedPedia with the International Herbicide-Resistant Weed Database [ 104 ], an already widely known and utilized database for weed scientists.
Training and collaboration in weed genomics
Beyond producing genomic tools and resources, a priority of the IWGC is to enable the utilization of these resources across a wide range of stakeholders. A holistic approach to training is required for weed science generally [ 188 ], and we would argue even more so for weed genomics. To accomplish our training goals, the IWGC is developing and delivering programs aimed at the full range of IWGC stakeholders and covering a breadth of relevant topics. We have taken care to ensure our approaches are diverse as to provide training to researchers with all levels of existing experience and differing reasons for engaging with these tools. Throughout, the focus is on ensuring that our training and outreach result in impacts that benefit a wide range of stakeholders.
Although recently developed tools are incredibly enabling and have great potential to replace antiquated methodology [ 189 ] and to solve pressing weed science problems [ 14 ], specialized computational skills are required to fully explore and unlock meaning from these highly complex datasets. Collaboration with, or training of, computational biologists equipped with these skills and resources developed by the IWGC will enable weed scientists to expand research programs and better understand the genetic underpinnings of weed evolution and herbicide resistance. To fill existing skill gaps, the IWGC is developing summer bootcamps and online modules directed specifically at weed scientists that will provide training on computational skills (Fig. 1 ). Because successful utilization of the IWGC resources requires more than general computational skills, we have created three targeted workshops that teach practical skills related to genomics databases, molecular biology, and population genomics (available at [ 190 ]). The IWGC has also hosted two official conference meetings, one in September of 2021 and one in January of 2023, with more conferences planned. These conferences have included invited speakers to present successful implementations of weed genomics, educational workshops to build computational skills, and networking opportunities for research to connect and collaborate.
Engagement opportunities during undergraduate degrees have been shown to improve academic outcomes [ 191 , 192 ]. As one activity to help achieve this goal, the IWGC has sponsored opportunities for US undergraduates to undertake a 10-week research experience, which includes an introduction to bioinformatics, a plant genomics research project that results in a presentation, and access to career building opportunities in diverse workplace environments. To increase equitable access to conferences and professional communities, we supported early career researchers to attend the first two IWGC conferences in the USA as well as workshops and bootcamps in Europe, South America, and Australia. These hybrid or in-person travel grants are intentionally designed to remove barriers and increase participation of individuals from backgrounds and experiences currently underrepresented within weed/plant science or genomics [ 193 ]. Recipients of these travel awards gave presentations and gained the measurable benefits that come from either virtual or in-person participation in conferences [ 194 ]. Moving forward, weed scientists must amass skills associated with genomic analyses and collaborate with other area experts to fully leverage resources developed by the IWGC.
The tools generated through the IWGC will enable many new research projects with diverse objectives like those listed above. In summary, contiguous genome assemblies and complete annotation information will allow weed scientists to join plant breeders in the use of genetic mapping for many traits including stress tolerance, plant architecture, and herbicide resistance (especially important for cases of NTSR). These assemblies will also allow for investigations of population structure, gene flow, and responses to evolutionary mechanisms like genetic bottlenecking and artificial selection. Understanding gene sequences across diverse weed species will be vital in modeling new herbicide target site proteins and designing novel effective herbicides with minimal off-target effects. The IWGC website will improve accessibility to weed genomics data by providing a single hub for reference genomes as well as phenotypic and genotypic information for accessions shared with the IWGC. Deposition of sequenced germplasm into public repositories will ensure that researchers are able to access and utilize these accessions in their own research to make the field more standardized and equitable. WeedPedia allows users of all backgrounds to quickly access information of interest such as herbicide target site gene sequence or subcellular localization of protein products for different genes. Users can also utilize server-based tools such as BLAST and genome browsing similar to other public genomic databases. Finally, the IWGC is committed to training and connecting weed genomicists through hosting trainings, workshops, and conferences.
Conclusions
Weeds are unique and fascinating plants, having significant impacts on agriculture and ecosystems; and yet, aspects of their biology, ecology, and genetics remain poorly understood. Weeds represent a unique area within plant biology, given their repeated rapid adaptation to sudden and severe shifts in the selective landscape of anthropogenic management practices. The production of a public genomics database with reference genomes and annotations for over 50 weed species represents a substantial step forward towards research goals that improve our understanding of the biology and evolution of weeds. Future work is needed to improve annotations, particularly for complex gene families involved in herbicide detoxification, structural variants, and mobile genetic elements. As reference genome assemblies become available; standard, affordable methods for gathering genotype information will allow for the identification of genetic variants underlying traits of interest. Further, methods for functional validation and hypothesis testing are needed in weeds to validate the effect of genetic variants detected through such experiments, including systems for transformation, gene editing, and transient gene silencing and expression. Future research should focus on utilizing weed genomes to investigate questions about evolutionary biology, ecology, genetics of weedy traits, and weed population dynamics. The IWGC plans to continue the public–private partnership model to host the WeedPedia database over time, integrate new datasets such as genome resequencing and transcriptomes, conduct trainings, and serve as a research coordination network to ensure that advances in weed science from around the world are shared across the research community (Fig. 1 ). Bridging basic plant genomics with translational applications in weeds is needed to deliver on the potential of weed genomics to improve weed management and crop breeding.
Availability of data and materials
All genome assemblies and related sequencing data produced by the IWGC will be available through NCBI as part of publications reporting the first genome-wide analysis for each species.
Gianessi LP, Nathan PR. The value of herbicides in U.S. crop production. Weed Technol. 2007;21(2):559–66.
Article Google Scholar
Pimentel D, Lach L, Zuniga R, Morrison D. Environmental and economic costs of nonindigenous species in the United States. Bioscience. 2000;50(1):53–65.
Barrett SH. Crop mimicry in weeds. Econ Bot. 1983;37(3):255–82.
Powles SB, Yu Q. Evolution in action: plants resistant to herbicides. Annu Rev Plant Biol. 2010;61:317–47.
Article CAS PubMed Google Scholar
Thurber CS, Reagon M, Gross BL, Olsen KM, Jia Y, Caicedo AL. Molecular evolution of shattering loci in U.S. weedy rice. Mol Ecol. 2010;19(16):3271–84.
Article PubMed PubMed Central Google Scholar
Comont D, Lowe C, Hull R, Crook L, Hicks HL, Onkokesung N, et al. Evolution of generalist resistance to herbicide mixtures reveals a trade-off in resistance management. Nat Commun. 2020;11(1):3086.
Article CAS PubMed PubMed Central Google Scholar
Ashworth MB, Walsh MJ, Flower KC, Vila-Aiub MM, Powles SB. Directional selection for flowering time leads to adaptive evolution in Raphanus raphanistrum (wild radish). Evol Appl. 2016;9(4):619–29.
Chan EK, Rowe HC, Kliebenstein DJ. Understanding the evolution of defense metabolites in Arabidopsis thaliana using genome-wide association mapping. Genetics. 2010;185(3):991–1007.
Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94.
Harkess A, Zhou J, Xu C, Bowers JE, Van der Hulst R, Ayyampalayam S, et al. The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat Commun. 2017;8(1):1279.
Periyannan S, Moore J, Ayliffe M, Bansal U, Wang X, Huang L, et al. The gene Sr33 , an ortholog of barley Mla genes, encodes resistance to wheat stem rust race Ug99. Science. 2013;341(6147):786–8.
Ågren J, Oakley CG, McKay JK, Lovell JT, Schemske DW. Genetic mapping of adaptation reveals fitness tradeoffs in Arabidopsis thaliana . Proc Natl Acad Sci U S A. 2013;110(52):21077–82.
Article PubMed Central Google Scholar
Schartl M, Walter RB, Shen Y, Garcia T, Catchen J, Amores A, et al. The genome of the platyfish, Xiphophorus maculatus , provides insights into evolutionary adaptation and several complex traits. Nat Genet. 2013;45(5):567–72.
Ravet K, Patterson EL, Krähmer H, Hamouzová K, Fan L, Jasieniuk M, et al. The power and potential of genomics in weed biology and management. Pest Manag Sci. 2018;74(10):2216–25.
Hufford MB, Seetharam AS, Woodhouse MR, Chougule KM, Ou S, Liu J, et al. De novo assembly, annotation, and comparative analysis of 26 diverse maize genomes. Science. 2021;373(6555):655–62.
Liao W-W, Asri M, Ebler J, Doerr D, Haukness M, Hickey G, et al. A draft human pangenome reference. Nature. 2023;617(7960):312–24.
Huang Y, Wu D, Huang Z, Li X, Merotto A, Bai L, et al. Weed genomics: yielding insights into the genetics of weedy traits for crop improvement. aBIOTECH. 2023;4:20–30.
Chen K, Yang H, Wu D, Peng Y, Lian L, Bai L, et al. Weed biology and management in the multi-omics era: progress and perspectives. Plant Commun. 2024;5(4):100816.
De Wet JMJ, Harlan JR. Weeds and domesticates: evolution in the man-made habitat. Econ Bot. 1975;29(2):99–108.
Mahaut L, Cheptou PO, Fried G, Munoz F, Storkey J, Vasseur F, et al. Weeds: against the rules? Trends Plant Sci. 2020;25(11):1107–16.
Neve P, Vila-Aiub M, Roux F. Evolutionary-thinking in agricultural weed management. New Phytol. 2009;184(4):783–93.
Article PubMed Google Scholar
Sharma G, Barney JN, Westwood JH, Haak DC. Into the weeds: new insights in plant stress. Trends Plant Sci. 2021;26(10):1050–60.
Vigueira CC, Olsen KM, Caicedo AL. The red queen in the corn: agricultural weeds as models of rapid adaptive evolution. Heredity (Edinb). 2013;110(4):303–11.
Donohue K, Dorn L, Griffith C, Kim E, Aguilera A, Polisetty CR, et al. Niche construction through germination cueing: life-history responses to timing of germination in Arabidopsis thaliana . Evolution. 2005;59(4):771–85.
PubMed Google Scholar
Exposito-Alonso M. Seasonal timing adaptation across the geographic range of Arabidopsis thaliana . Proc Natl Acad Sci U S A. 2020;117(18):9665–7.
Fournier-Level A, Korte A, Cooper MD, Nordborg M, Schmitt J, Wilczek AM. A map of local adaptation in Arabidopsis thaliana . Science. 2011;334(6052):86–9.
Hancock AM, Brachi B, Faure N, Horton MW, Jarymowycz LB, Sperone FG, et al. Adaptation to climate across the Arabidopsis thaliana genome. Science. 2011;334(6052):83–6.
Initiative TAG. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana . Nature. 2000;408(6814):796–815.
Alonso-Blanco C, Andrade J, Becker C, Bemm F, Bergelson J, Borgwardt KM, et al. 1,135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana . Cell. 2016;166(2):481–91.
Durvasula A, Fulgione A, Gutaker RM, Alacakaptan SI, Flood PJ, Neto C, et al. African genomes illuminate the early history and transition to selfing in Arabidopsis thaliana . Proc Natl Acad Sci U S A. 2017;114(20):5213–8.
Frachon L, Mayjonade B, Bartoli C, Hautekèete N-C, Roux F. Adaptation to plant communities across the genome of Arabidopsis thaliana . Mol Biol Evol. 2019;36(7):1442–56.
Fulgione A, Koornneef M, Roux F, Hermisson J, Hancock AM. Madeiran Arabidopsis thaliana reveals ancient long-range colonization and clarifies demography in Eurasia. Mol Biol Evol. 2018;35(3):564–74.
Fulgione A, Neto C, Elfarargi AF, Tergemina E, Ansari S, Göktay M, et al. Parallel reduction in flowering time from de novo mutations enable evolutionary rescue in colonizing lineages. Nat Commun. 2022;13(1):1461.
Kasulin L, Rowan BA, León RJC, Schuenemann VJ, Weigel D, Botto JF. A single haplotype hyposensitive to light and requiring strong vernalization dominates Arabidopsis thaliana populations in Patagonia. Argentina Mol Ecol. 2017;26(13):3389–404.
Picó FX, Méndez-Vigo B, Martínez-Zapater JM, Alonso-Blanco C. Natural genetic variation of Arabidopsis thaliana is geographically structured in the Iberian peninsula. Genetics. 2008;180(2):1009–21.
Atwell S, Huang YS, Vilhjálmsson BJ, Willems G, Horton M, Li Y, et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature. 2010;465(7298):627–31.
Flood PJ, Kruijer W, Schnabel SK, van der Schoor R, Jalink H, Snel JFH, et al. Phenomics for photosynthesis, growth and reflectance in Arabidopsis thaliana reveals circadian and long-term fluctuations in heritability. Plant Methods. 2016;12(1):14.
Marchadier E, Hanemian M, Tisné S, Bach L, Bazakos C, Gilbault E, et al. The complex genetic architecture of shoot growth natural variation in Arabidopsis thaliana . PLoS Genet. 2019;15(4):e1007954.
Tisné S, Serrand Y, Bach L, Gilbault E, Ben Ameur R, Balasse H, et al. Phenoscope: an automated large-scale phenotyping platform offering high spatial homogeneity. Plant J. 2013;74(3):534–44.
Tschiersch H, Junker A, Meyer RC, Altmann T. Establishment of integrated protocols for automated high throughput kinetic chlorophyll fluorescence analyses. Plant Methods. 2017;13:54.
Chen X, MacGregor DR, Stefanato FL, Zhang N, Barros-Galvão T, Penfield S. A VEL3 histone deacetylase complex establishes a maternal epigenetic state controlling progeny seed dormancy. Nat Commun. 2023;14(1):2220.
Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci U S A. 2009;106(45):19096–101.
Davey JW, Blaxter ML. RADSeq: next-generation population genetics. Brief Funct Genomics. 2010;9(5–6):416–23.
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, et al. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE. 2011;6(5):e19379.
MacGregor DR. What makes a weed a weed? How virus-mediated reverse genetics can help to explore the genetics of weediness. Outlooks Pest Manag. 2020;31(5):224–9.
Mellado-Sánchez M, McDiarmid F, Cardoso V, Kanyuka K, MacGregor DR. Virus-mediated transient expression techniques enable gene function studies in blackgrass. Plant Physiol. 2020;183(2):455–9.
Dimaano NG, Yamaguchi T, Fukunishi K, Tominaga T, Iwakami S. Functional characterization of Cytochrome P450 CYP81A subfamily to disclose the pattern of cross-resistance in Echinochloa phyllopogon . Plant Mol Biol. 2020;102(4–5):403–16.
de Figueiredo MRA, Küpper A, Malone JM, Petrovic T, de Figueiredo ABTB, Campagnola G, et al. An in-frame deletion mutation in the degron tail of auxin coreceptor IAA2 confers resistance to the herbicide 2,4-D in Sisymbrium orientale . Proc Natl Acad Sci U S A. 2022;119(9):e2105819119.
Patzoldt WL, Hager AG, McCormick JS, Tranel PJ. A codon deletion confers resistance to herbicides inhibiting protoporphyrinogen oxidase. Proc Natl Acad Sci U S A. 2006;103(33):12329–34.
Zabala-Pardo D, Gaines T, Lamego FP, Avila LA. RNAi as a tool for weed management: challenges and opportunities. Adv Weed Sci. 2022;40(spe1):e020220096.
Fattorini R, Glover BJ. Molecular mechanisms of pollination biology. Annu Rev Plant Biol. 2020;71:487–515.
Rollin O, Benelli G, Benvenuti S, Decourtye A, Wratten SD, Canale A, et al. Weed-insect pollinator networks as bio-indicators of ecological sustainability in agriculture. A review Agron Sustain Dev. 2016;36(1):8.
Irwin RE, Strauss SY. Flower color microevolution in wild radish: evolutionary response to pollinator-mediated selection. Am Nat. 2005;165(2):225–37.
Ma B, Wu J, Shi T-L, Yang Y-Y, Wang W-B, Zheng Y, et al. Lilac ( Syringa oblata ) genome provides insights into its evolution and molecular mechanism of petal color change. Commun Biol. 2022;5(1):686.
Xing A, Wang X, Nazir MF, Zhang X, Wang X, Yang R, et al. Transcriptomic and metabolomic profiling of flavonoid biosynthesis provides novel insights into petals coloration in Asian cotton ( Gossypium arboreum L.). BMC Plant Biol. 2022;22(1):416.
Zheng Y, Chen Y, Liu Z, Wu H, Jiao F, Xin H, et al. Important roles of key genes and transcription factors in flower color differences of Nicotiana alata . Genes (Basel). 2021;12(12):1976.
Krizek BA, Anderson JT. Control of flower size. J Exp Bot. 2013;64(6):1427–37.
Powell AE, Lenhard M. Control of organ size in plants. Curr Biol. 2012;22(9):R360–7.
Spencer V, Kim M. Re"CYC"ling molecular regulators in the evolution and development of flower symmetry. Semin Cell Dev Biol. 2018;79:16–26.
Amrad A, Moser M, Mandel T, de Vries M, Schuurink RC, Freitas L, et al. Gain and loss of floral scent production through changes in structural genes during pollinator-mediated speciation. Curr Biol. 2016;26(24):3303–12.
Delle-Vedove R, Schatz B, Dufay M. Understanding intraspecific variation of floral scent in light of evolutionary ecology. Ann Bot. 2017;120(1):1–20.
Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol. 2002;5(3):237–43.
Ballerini ES, Kramer EM, Hodges SA. Comparative transcriptomics of early petal development across four diverse species of Aquilegia reveal few genes consistently associated with nectar spur development. BMC Genom. 2019;20(1):668.
Corbet SA, Willmer PG, Beament JWL, Unwin DM, Prys-Jones OE. Post-secretory determinants of sugar concentration in nectar. Plant Cell Environ. 1979;2(4):293–308.
Galliot C, Hoballah ME, Kuhlemeier C, Stuurman J. Genetics of flower size and nectar volume in Petunia pollination syndromes. Planta. 2006;225(1):203–12.
Vila-Aiub MM, Neve P, Powles SB. Fitness costs associated with evolved herbicide resistance alleles in plants. New Phytol. 2009;184(4):751–67.
Baucom RS. Evolutionary and ecological insights from herbicide-resistant weeds: what have we learned about plant adaptation, and what is left to uncover? New Phytol. 2019;223(1):68–82.
Bajwa AA, Latif S, Borger C, Iqbal N, Asaduzzaman M, Wu H, et al. The remarkable journey of a weed: biology and management of annual ryegrass ( Lolium rigidum ) in conservation cropping systems of Australia. Plants (Basel). 2021;10(8):1505.
Bitarafan Z, Andreasen C. Fecundity allocation in some european weed species competing with crops. Agronomy. 2022;12(5):1196.
Costea M, Weaver SE, Tardif FJ. The biology of Canadian weeds. 130. Amaranthus retroflexus L., A. powellii , A. powellii S. Watson, and A. hybridus L. Can J Plant Sci. 2004;84(2):631–68.
Dixon A, Comont D, Slavov GT, Neve P. Population genomics of selectively neutral genetic structure and herbicide resistance in UK populations of Alopecurus myosuroides . Pest Manag Sci. 2021;77(3):1520–9.
Kersten S, Chang J, Huber CD, Voichek Y, Lanz C, Hagmaier T, et al. Standing genetic variation fuels rapid evolution of herbicide resistance in blackgrass. Proc Natl Acad Sci U S A. 2023;120(16):e2206808120.
Qiu J, Zhou Y, Mao L, Ye C, Wang W, Zhang J, et al. Genomic variation associated with local adaptation of weedy rice during de-domestication. Nat Commun. 2017;8(1):15323.
Kreiner JM, Caballero A, Wright SI, Stinchcombe JR. Selective ancestral sorting and de novo evolution in the agricultural invasion of Amaranthus tuberculatus . Evolution. 2022;76(1):70–85.
Kreiner JM, Latorre SM, Burbano HA, Stinchcombe JR, Otto SP, Weigel D, et al. Rapid weed adaptation and range expansion in response to agriculture over the past two centuries. Science. 2022;378(6624):1079–85.
Wu D, Shen E, Jiang B, Feng Y, Tang W, Lao S, et al. Genomic insights into the evolution of Echinochloa species as weed and orphan crop. Nat Commun. 2022;13(1):689.
Yeaman S, Hodgins KA, Lotterhos KE, Suren H, Nadeau S, Degner JC, et al. Convergent local adaptation to climate in distantly related conifers. Science. 2016;353(6306):1431–3.
Haudry A, Platts AE, Vello E, Hoen DR, Leclercq M, Williamson RJ, et al. An atlas of over 90,000 conserved noncoding sequences provides insight into crucifer regulatory regions. Nat Genet. 2013;45(8):891–8.
Sackton TB, Grayson P, Cloutier A, Hu Z, Liu JS, Wheeler NE, et al. Convergent regulatory evolution and loss of flight in paleognathous birds. Science. 2019;364(6435):74–8.
Ye CY, Fan L. Orphan crops and their wild relatives in the genomic era. Mol Plant. 2021;14(1):27–39.
Clements DR, Jones VL. Ten ways that weed evolution defies human management efforts amidst a changing climate. Agronomy. 2021;11(2):284.
Article CAS Google Scholar
Weinig C. Rapid evolutionary responses to selection in heterogeneous environments among agricultural and nonagricultural weeds. Int J Plant Sci. 2005;166(4):641–7.
Cousens RD, Fournier-Level A. Herbicide resistance costs: what are we actually measuring and why? Pest Manag Sci. 2018;74(7):1539–46.
Lasky JR, Josephs EB, Morris GP. Genotype–environment associations to reveal the molecular basis of environmental adaptation. Plant Cell. 2023;35(1):125–38.
Lotterhos KE. The effect of neutral recombination variation on genome scans for selection. G3-Genes Genom Genet. 2019;9(6):1851–67.
Lovell JT, MacQueen AH, Mamidi S, Bonnette J, Jenkins J, Napier JD, et al. Genomic mechanisms of climate adaptation in polyploid bioenergy switchgrass. Nature. 2021;590(7846):438–44.
Todesco M, Owens GL, Bercovich N, Légaré J-S, Soudi S, Burge DO, et al. Massive haplotypes underlie ecotypic differentiation in sunflowers. Nature. 2020;584(7822):602–7.
Revolinski SR, Maughan PJ, Coleman CE, Burke IC. Preadapted to adapt: Underpinnings of adaptive plasticity revealed by the downy brome genome. Commun Biol. 2023;6(1):326.
Kuester A, Conner JK, Culley T, Baucom RS. How weeds emerge: a taxonomic and trait-based examination using United States data. New Phytol. 2014;202(3):1055–68.
Arnaud JF, Fénart S, Cordellier M, Cuguen J. Populations of weedy crop-wild hybrid beets show contrasting variation in mating system and population genetic structure. Evol Appl. 2010;3(3):305–18.
Ellstrand NC, Schierenbeck KA. Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci U S A. 2000;97(13):7043–50.
Nakabayashi K, Leubner-Metzger G. Seed dormancy and weed emergence: from simulating environmental change to understanding trait plasticity, adaptive evolution, and population fitness. J Exp Bot. 2021;72(12):4181–5.
Busi R, Yu Q, Barrett-Lennard R, Powles S. Long distance pollen-mediated flow of herbicide resistance genes in Lolium rigidum . Theor Appl Genet. 2008;117(8):1281–90.
Délye C, Clément JAJ, Pernin F, Chauvel B, Le Corre V. High gene flow promotes the genetic homogeneity of arable weed populations at the landscape level. Basic Appl Ecol. 2010;11(6):504–12.
Roumet M, Noilhan C, Latreille M, David J, Muller MH. How to escape from crop-to-weed gene flow: phenological variation and isolation-by-time within weedy sunflower populations. New Phytol. 2013;197(2):642–54.
Moghadam SH, Alebrahim MT, Mohebodini M, MacGregor DR. Genetic variation of Amaranthus retroflexus L. and Chenopodium album L. (Amaranthaceae) suggests multiple independent introductions into Iran. Front Plant Sci. 2023;13:1024555.
Muller M-H, Latreille M, Tollon C. The origin and evolution of a recent agricultural weed: population genetic diversity of weedy populations of sunflower ( Helianthus annuus L.) in Spain and France. Evol Appl. 2011;4(3):499–514.
Wesse C, Welk E, Hurka H, Neuffer B. Geographical pattern of genetic diversity in Capsella bursa-pastoris (Brassicaceae) -A global perspective. Ecol Evol. 2021;11(1):199–213.
Fraimout A, Debat V, Fellous S, Hufbauer RA, Foucaud J, Pudlo P, et al. Deciphering the routes of invasion of Drosophila suzukii by means of ABC random forest. Mol Biol Evol. 2017;34(4):980–96.
CAS PubMed PubMed Central Google Scholar
Battlay P, Wilson J, Bieker VC, Lee C, Prapas D, Petersen B, et al. Large haploblocks underlie rapid adaptation in the invasive weed Ambrosia artemisiifolia . Nat Commun. 2023;14(1):1717.
van Boheemen LA, Hodgins KA. Rapid repeatable phenotypic and genomic adaptation following multiple introductions. Mol Ecol. 2020;29(21):4102–17.
Putra A, Hodgins K, Fournier-Level A. Assessing the invasive potential of different source populations of ragweed ( Ambrosia artemisiifolia L.) through genomically-informed species distribution modelling. Authorea. 2023;17(1):e13632.
Google Scholar
Bourguet D, Delmotte F, Franck P, Guillemaud T, Reboud X, Vacher C, et al. Heterogeneity of selection and the evolution of resistance. Trends Ecol Evol. 2013;28(2):110–8.
The International Herbicide-Resistant Weed Database. www.weedscience.org . Accessed 20 June 2023.
Powles S. Herbicide discovery through innovation and diversity. Adv Weed Sci. 2022;40(spe1):e020220074.
Murphy BP, Tranel PJ. Target-site mutations conferring herbicide resistance. Plants (Basel). 2019;8(10):382.
Gaines TA, Duke SO, Morran S, Rigon CAG, Tranel PJ, Küpper A, et al. Mechanisms of evolved herbicide resistance. J Biol Chem. 2020;295(30):10307–30.
Lonhienne T, Cheng Y, Garcia MD, Hu SH, Low YS, Schenk G, et al. Structural basis of resistance to herbicides that target acetohydroxyacid synthase. Nat Commun. 2022;13(1):3368.
Comont D, MacGregor DR, Crook L, Hull R, Nguyen L, Freckleton RP, et al. Dissecting weed adaptation: fitness and trait correlations in herbicide-resistant Alopecurus myosuroides . Pest Manag Sci. 2022;78(7):3039–50.
Neve P. Simulation modelling to understand the evolution and management of glyphosate resistance in weeds. Pest Manag Sci. 2008;64(4):392–401.
Torra J, Alcántara-de la Cruz R. Molecular mechanisms of herbicide resistance in weeds. Genes (Basel). 2022;13(11):2025.
Délye C, Gardin JAC, Boucansaud K, Chauvel B, Petit C. Non-target-site-based resistance should be the centre of attention for herbicide resistance research: Alopecurus myosuroides as an illustration. Weed Res. 2011;51(5):433–7.
Chandra S, Leon RG. Genome-wide evolutionary analysis of putative non-specific herbicide resistance genes and compilation of core promoters between monocots and dicots. Genes (Basel). 2022;13(7):1171.
Margaritopoulou T, Tani E, Chachalis D, Travlos I. Involvement of epigenetic mechanisms in herbicide resistance: the case of Conyza canadensis . Agriculture. 2018;8(1):17.
Pan L, Guo Q, Wang J, Shi L, Yang X, Zhou Y, et al. CYP81A68 confers metabolic resistance to ALS and ACCase-inhibiting herbicides and its epigenetic regulation in Echinochloa crus-galli . J Hazard Mater. 2022;428:128225.
Sen MK, Hamouzová K, Košnarová P, Roy A, Soukup J. Herbicide resistance in grass weeds: Epigenetic regulation matters too. Front Plant Sci. 2022;13:1040958.
Han H, Yu Q, Beffa R, González S, Maiwald F, Wang J, et al. Cytochrome P450 CYP81A10v7 in Lolium rigidum confers metabolic resistance to herbicides across at least five modes of action. Plant J. 2021;105(1):79–92.
Kubis GC, Marques RZ, Kitamura RS, Barroso AA, Juneau P, Gomes MP. Antioxidant enzyme and Cytochrome P450 activities are involved in horseweed ( Conyza sumatrensis ) resistance to glyphosate. Stress. 2023;3(1):47–57.
Qiao Y, Zhang N, Liu J, Yang H. Interpretation of ametryn biodegradation in rice based on joint analyses of transcriptome, metabolome and chemo-characterization. J Hazard Mater. 2023;445:130526.
Rouse CE, Roma-Burgos N, Barbosa Martins BA. Physiological assessment of non–target site restistance in multiple-resistant junglerice ( Echinochloa colona ). Weed Sci. 2019;67(6):622–32.
Abou-Khater L, Maalouf F, Jighly A, Alsamman AM, Rubiales D, Rispail N, et al. Genomic regions associated with herbicide tolerance in a worldwide faba bean ( Vicia faba L.) collection. Sci Rep. 2022;12(1):158.
Gupta S, Harkess A, Soble A, Van Etten M, Leebens-Mack J, Baucom RS. Interchromosomal linkage disequilibrium and linked fitness cost loci associated with selection for herbicide resistance. New Phytol. 2023;238(3):1263–77.
Kreiner JM, Tranel PJ, Weigel D, Stinchcombe JR, Wright SI. The genetic architecture and population genomic signatures of glyphosate resistance in Amaranthus tuberculatus . Mol Ecol. 2021;30(21):5373–89.
Parcharidou E, Dücker R, Zöllner P, Ries S, Orru R, Beffa R. Recombinant glutathione transferases from flufenacet-resistant black-grass ( Alopecurus myosuroides Huds.) form different flufenacet metabolites and differ in their interaction with pre- and post-emergence herbicides. Pest Manag Sci. 2023;79(9):3376–86.
Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21(3):181–200.
Acuner Ozbabacan SE, Engin HB, Gursoy A, Keskin O. Transient protein-protein interactions. Protein Eng Des Sel. 2011;24(9):635–48.
Lu H, Zhou Q, He J, Jiang Z, Peng C, Tong R, et al. Recent advances in the development of protein–protein interactions modulators: mechanisms and clinical trials. Signal Transduct Target Ther. 2020;5(1):213.
Benson CW, Sheltra MR, Maughan PJ, Jellen EN, Robbins MD, Bushman BS, et al. Homoeologous evolution of the allotetraploid genome of Poa annua L. BMC Genom. 2023;24(1):350.
Robbins MD, Bushman BS, Huff DR, Benson CW, Warnke SE, Maughan CA, et al. Chromosome-scale genome assembly and annotation of allotetraploid annual bluegrass ( Poa annua L.). Genome Biol Evol. 2022;15(1):evac180.
Montgomery JS, Giacomini D, Waithaka B, Lanz C, Murphy BP, Campe R, et al. Draft genomes of Amaranthus tuberculatus , Amaranthus hybridus and Amaranthus palmeri . Genome Biol Evol. 2020;12(11):1988–93.
Jeschke MR, Tranel PJ, Rayburn AL. DNA content analysis of smooth pigweed ( Amaranthus hybridus ) and tall waterhemp ( A. tuberculatus ): implications for hybrid detection. Weed Sci. 2003;51(1):1–3.
Rayburn AL, McCloskey R, Tatum TC, Bollero GA, Jeschke MR, Tranel PJ. Genome size analysis of weedy Amaranthus species. Crop Sci. 2005;45(6):2557–62.
Laforest M, Martin SL, Bisaillon K, Soufiane B, Meloche S, Tardif FJ, et al. The ancestral karyotype of the Heliantheae Alliance, herbicide resistance, and human allergens: Insights from the genomes of common and giant ragweed. Plant Genome . 2024;e20442. https://doi.org/10.1002/tpg2.20442 .
Mulligan GA. Chromosome numbers of Canadian weeds. I Canad J Bot. 1957;35(5):779–89.
Meyer L, Causse R, Pernin F, Scalone R, Bailly G, Chauvel B, et al. New gSSR and EST-SSR markers reveal high genetic diversity in the invasive plant Ambrosia artemisiifolia L. and can be transferred to other invasive Ambrosia species. PLoS One. 2017;12(5):e0176197.
Pustahija F, Brown SC, Bogunić F, Bašić N, Muratović E, Ollier S, et al. Small genomes dominate in plants growing on serpentine soils in West Balkans, an exhaustive study of 8 habitats covering 308 taxa. Plant Soil. 2013;373(1):427–53.
Kubešová M, Moravcova L, Suda J, Jarošík V, Pyšek P. Naturalized plants have smaller genomes than their non-invading relatives: a flow cytometric analysis of the Czech alien flora. Preslia. 2010;82(1):81–96.
Thébaud C, Abbott RJ. Characterization of invasive Conyza species (Asteraceae) in Europe: quantitative trait and isozyme analysis. Am J Bot. 1995;82(3):360–8.
Garcia S, Hidalgo O, Jakovljević I, Siljak-Yakovlev S, Vigo J, Garnatje T, et al. New data on genome size in 128 Asteraceae species and subspecies, with first assessments for 40 genera, 3 tribes and 2 subfamilies. Plant Biosyst. 2013;147(4):1219–27.
Zhao X, Yi L, Ren Y, Li J, Ren W, Hou Z, et al. Chromosome-scale genome assembly of the yellow nutsedge ( Cyperus esculentus ). Genome Biol Evol. 2023;15(3):evad027.
Bennett MD, Leitch IJ, Hanson L. DNA amounts in two samples of angiosperm weeds. Ann Bot. 1998;82:121–34.
Schulz-Schaeffer J, Gerhardt S. Cytotaxonomic analysis of the Euphorbia spp. (leafy spurge) complex. II: Comparative study of the chromosome morphology. Biol Zentralbl. 1989;108(1):69–76.
Schaeffer JR, Gerhardt S. The impact of introgressive hybridization on the weediness of leafy spurge. Leafy Spurge Symposium. 1989;1989:97–105.
Bai C, Alverson WS, Follansbee A, Waller DM. New reports of nuclear DNA content for 407 vascular plant taxa from the United States. Ann Bot. 2012;110(8):1623–9.
Aarestrup JR, Karam D, Fernandes GW. Chromosome number and cytogenetics of Euphorbia heterophylla L. Genet Mol Res. 2008;7(1):217–22.
Wang L, Sun X, Peng Y, Chen K, Wu S, Guo Y, et al. Genomic insights into the origin, adaptive evolution, and herbicide resistance of Leptochloa chinensis , a devastating tetraploid weedy grass in rice fields. Mol Plant. 2022;15(6):1045–58.
Paril J, Pandey G, Barnett EM, Rane RV, Court L, Walsh T, et al. Rounding up the annual ryegrass genome: high-quality reference genome of Lolium rigidum . Front Genet. 2022;13:1012694.
Weiss-Schneeweiss H, Greilhuber J, Schneeweiss GM. Genome size evolution in holoparasitic Orobanche (Orobanchaceae) and related genera. Am J Bot. 2006;93(1):148–56.
Towers G, Mitchell J, Rodriguez E, Bennett F, Subba Rao P. Biology & chemistry of Parthenium hysterophorus L., a problem weed in India. Biol Rev. 1977;48:65–74.
CAS Google Scholar
Moghe GD, Hufnagel DE, Tang H, Xiao Y, Dworkin I, Town CD, et al. Consequences of whole-genome triplication as revealed by comparative genomic analyses of the wild radish ( Raphanus raphanistrum ) and three other Brassicaceae species. Plant Cell. 2014;26(5):1925–37.
Zhang X, Liu T, Wang J, Wang P, Qiu Y, Zhao W, et al. Pan-genome of Raphanus highlights genetic variation and introgression among domesticated, wild, and weedy radishes. Mol Plant. 2021;14(12):2032–55.
Chytrý M, Danihelka J, Kaplan Z, Wild J, Holubová D, Novotný P, et al. Pladias database of the Czech flora and vegetation. Preslia. 2021;93(1):1–87.
Patterson EL, Pettinga DJ, Ravet K, Neve P, Gaines TA. Glyphosate resistance and EPSPS gene duplication: Convergent evolution in multiple plant species. J Hered. 2018;109(2):117–25.
Jugulam M, Niehues K, Godar AS, Koo DH, Danilova T, Friebe B, et al. Tandem amplification of a chromosomal segment harboring 5-enolpyruvylshikimate-3-phosphate synthase locus confers glyphosate resistance in Kochia scoparia . Plant Physiol. 2014;166(3):1200–7.
Patterson EL, Saski CA, Sloan DB, Tranel PJ, Westra P, Gaines TA. The draft genome of Kochia scoparia and the mechanism of glyphosate resistance via transposon-mediated EPSPS tandem gene duplication. Genome Biol Evol. 2019;11(10):2927–40.
Zhang C, Johnson N, Hall N, Tian X, Yu Q, Patterson E. Subtelomeric 5-enolpyruvylshikimate-3-phosphate synthase ( EPSPS ) copy number variation confers glyphosate resistance in Eleusine indica . Nat Commun. 2023;14:4865.
Koo D-H, Molin WT, Saski CA, Jiang J, Putta K, Jugulam M, et al. Extrachromosomal circular DNA-based amplification and transmission of herbicide resistance in crop weed Amaranthus palmeri . Proc Natl Acad Sci U S A. 2018;115(13):3332–7.
Molin WT, Yaguchi A, Blenner M, Saski CA. The eccDNA Replicon: A heritable, extranuclear vehicle that enables gene amplification and glyphosate resistance in Amaranthus palmeri . Plant Cell. 2020;32(7):2132–40.
Jugulam M. Can non-Mendelian inheritance of extrachromosomal circular DNA-mediated EPSPS gene amplification provide an opportunity to reverse resistance to glyphosate? Weed Res. 2021;61(2):100–5.
Kreiner JM, Giacomini DA, Bemm F, Waithaka B, Regalado J, Lanz C, et al. Multiple modes of convergent adaptation in the spread of glyphosate-resistant Amaranthus tuberculatus . Proc Natl Acad Sci U S A. 2019;116(42):21076–84.
Cai L, Comont D, MacGregor D, Lowe C, Beffa R, Neve P, et al. The blackgrass genome reveals patterns of non-parallel evolution of polygenic herbicide resistance. New Phytol. 2023;237(5):1891–907.
Chen K, Yang H, Peng Y, Liu D, Zhang J, Zhao Z, et al. Genomic analyses provide insights into the polyploidization-driven herbicide adaptation in Leptochloa weeds. Plant Biotechnol J. 2023;21(8):1642–58.
Ohadi S, Hodnett G, Rooney W, Bagavathiannan M. Gene flow and its consequences in Sorghum spp. Crit Rev Plant Sci. 2017;36(5–6):367–85.
Renzi JP, Coyne CJ, Berger J, von Wettberg E, Nelson M, Ureta S, et al. How could the use of crop wild relatives in breeding increase the adaptation of crops to marginal environments? Front Plant Sci. 2022;13:886162.
Ward SM, Cousens RD, Bagavathiannan MV, Barney JN, Beckie HJ, Busi R, et al. Agricultural weed research: a critique and two proposals. Weed Sci. 2014;62(4):672–8.
Evans JA, Tranel PJ, Hager AG, Schutte B, Wu C, Chatham LA, et al. Managing the evolution of herbicide resistance. Pest Manag Sci. 2016;72(1):74–80.
International Weed Genomics Consortium Website. https://www.weedgenomics.org . Accessed 20 June 2023.
WeedPedia Database. https://weedpedia.weedgenomics.org/ . Accessed 20 June 2023.
Hall N, Chen J, Matzrafi M, Saski CA, Westra P, Gaines TA, et al. FHY3/FAR1 transposable elements generate adaptive genetic variation in the Bassia scoparia genome. bioRxiv . 2023; DOI: https://doi.org/10.1101/2023.05.26.542497 .
Jarvis DE, Sproul JS, Navarro-Domínguez B, Krak K, Jaggi K, Huang Y-F, et al. Chromosome-scale genome assembly of the hexaploid Taiwanese goosefoot “Djulis” ( Chenopodium formosanum ). Genome Biol Evol. 2022;14(8):evac120.
Ferreira LAI, de Oliveira RS, Jr., Constantin J, Brunharo C. Evolution of ACCase-inhibitor resistance in Chloris virgata is conferred by a Trp2027Cys mutation in the herbicide target site. Pest Manag Sci. 2023;79(12):5220–9.
Laforest M, Martin SL, Bisaillon K, Soufiane B, Meloche S, Page E. A chromosome-scale draft sequence of the Canada fleabane genome. Pest Manag Sci. 2020;76(6):2158–69.
Guo L, Qiu J, Ye C, Jin G, Mao L, Zhang H, et al. Echinochloa crus-galli genome analysis provides insight into its adaptation and invasiveness as a weed. Nat Commun. 2017;8(1):1031.
Sato MP, Iwakami S, Fukunishi K, Sugiura K, Yasuda K, Isobe S, et al. Telomere-to-telomere genome assembly of an allotetraploid pernicious weed, Echinochloa phyllopogon . DNA Res. 2023;30(5):dsad023.
Stein JC, Yu Y, Copetti D, Zwickl DJ, Zhang L, Zhang C, et al. Genomes of 13 domesticated and wild rice relatives highlight genetic conservation, turnover and innovation across the genus Oryza . Nat Genet. 2018;50(2):285–96.
Wu D, Xie L, Sun Y, Huang Y, Jia L, Dong C, et al. A syntelog-based pan-genome provides insights into rice domestication and de-domestication. Genome Biol. 2023;24(1):179.
Wang Z, Huang S, Yang Z, Lai J, Gao X, Shi J. A high-quality, phased genome assembly of broomcorn millet reveals the features of its subgenome evolution and 3D chromatin organization. Plant Commun. 2023;4(3):100557.
Mao Q, Huff DR. The evolutionary origin of Poa annua L. Crop Sci. 2012;52(4):1910–22.
Benson CW, Sheltra MR, Maughan JP, Jellen EN, Robbins MD, Bushman BS, et al. Homoeologous evolution of the allotetraploid genome of Poa annua L. Res Sq. 2023. https://doi.org/10.21203/rs.3.rs-2729084/v1 .
Brunharo C, Benson CW, Huff DR, Lasky JR. Chromosome-scale genome assembly of Poa trivialis and population genomics reveal widespread gene flow in a cool-season grass seed production system. Plant Direct. 2024;8(3):e575.
Mo C, Wu Z, Shang X, Shi P, Wei M, Wang H, et al. Chromosome-level and graphic genomes provide insights into metabolism of bioactive metabolites and cold-adaption of Pueraria lobata var. montana . DNA Research. 2022;29(5):dsac030.
Thielen PM, Pendleton AL, Player RA, Bowden KV, Lawton TJ, Wisecaver JH. Reference genome for the highly transformable Setaria viridis ME034V. G3 (Bethesda, Md). 2020;10(10):3467–78.
Yoshida S, Kim S, Wafula EK, Tanskanen J, Kim Y-M, Honaas L, et al. Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. Curr Biol. 2019;29(18):3041–52.
Qiu S, Bradley JM, Zhang P, Chaudhuri R, Blaxter M, Butlin RK, et al. Genome-enabled discovery of candidate virulence loci in Striga hermonthica , a devastating parasite of African cereal crops. New Phytol. 2022;236(2):622–38.
Nunn A, Rodríguez-Arévalo I, Tandukar Z, Frels K, Contreras-Garrido A, Carbonell-Bejerano P, et al. Chromosome-level Thlaspi arvense genome provides new tools for translational research and for a newly domesticated cash cover crop of the cooler climates. Plant Biotechnol J. 2022;20(5):944–63.
USDA-ARS Germplasm Resources Information Network (GRIN). https://www.ars-grin.gov/ . Accessed 20 June 2023.
Buck M, Hamilton C. The Nagoya Protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization to the convention on biological diversity. RECIEL. 2011;20(1):47–61.
Chauhan BS, Matloob A, Mahajan G, Aslam F, Florentine SK, Jha P. Emerging challenges and opportunities for education and research in weed science. Front Plant Sci. 2017;8:1537.
Shah S, Lonhienne T, Murray CE, Chen Y, Dougan KE, Low YS, et al. Genome-guided analysis of seven weed species reveals conserved sequence and structural features of key gene targets for herbicide development. Front Plant Sci. 2022;13:909073.
International Weed Genomics Consortium Training Resources. https://www.weedgenomics.org/training-resources/ . Accessed 20 June 2023.
Blackford S. Harnessing the power of communities: career networking strategies for bioscience PhD students and postdoctoral researchers. FEMS Microbiol Lett. 2018;365(8):fny033.
Pender M, Marcotte DE, Sto Domingo MR, Maton KI. The STEM pipeline: The role of summer research experience in minority students’ Ph.D. aspirations. Educ Policy Anal Arch. 2010;18(30):1–36.
PubMed PubMed Central Google Scholar
Burke A, Okrent A, Hale K. The state of U.S. science and engineering 2022. Foundation NS. https://ncses.nsf.gov/pubs/nsb20221 . 2022.
Wu J-Y, Liao C-H, Cheng T, Nian M-W. Using data analytics to investigate attendees’ behaviors and psychological states in a virtual academic conference. Educ Technol Soc. 2021;24(1):75–91.
Download references
Peer review information
Wenjing She was the primary editor of this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
The International Weed Genomics Consortium is supported by BASF SE, Bayer AG, Syngenta Ltd, Corteva Agriscience, CropLife International (Global Herbicide Resistance Action Committee), the Foundation for Food and Agriculture Research (Award DSnew-0000000024), and two conference grants from USDA-NIFA (Award numbers 2021–67013-33570 and 2023-67013-38785).
Author information
Authors and affiliations.
Department of Agricultural Biology, Colorado State University, 1177 Campus Delivery, Fort Collins, CO, 80523, USA
Jacob Montgomery, Sarah Morran & Todd A. Gaines
Protecting Crops and the Environment, Rothamsted Research, Harpenden, Hertfordshire, UK
Dana R. MacGregor
Department of Crop, Soil, and Environmental Sciences, Auburn University, Auburn, AL, USA
J. Scott McElroy
Department of Plant and Environmental Sciences, University of Copenhagen, Taastrup, Denmark
Paul Neve & Célia Neto
IFEVA-Conicet-Department of Ecology, University of Buenos Aires, Buenos Aires, Argentina
Martin M. Vila-Aiub & Maria Victoria Sandoval
Department of Ecology, Faculty of Agronomy, University of Buenos Aires, Buenos Aires, Argentina
Analia I. Menéndez
Department of Botany, The University of British Columbia, Vancouver, BC, Canada
Julia M. Kreiner
Institute of Crop Sciences, Zhejiang University, Hangzhou, China
Longjiang Fan
Department of Biology, University of Massachusetts Amherst, Amherst, MA, USA
Ana L. Caicedo
Department of Plant and Wildlife Sciences, Brigham Young University, Provo, UT, USA
Peter J. Maughan
Bayer AG, Weed Control Research, Frankfurt, Germany
Bianca Assis Barbosa Martins, Jagoda Mika, Alberto Collavo & Bodo Peters
Department of Crop Sciences, Federal University of Rio Grande Do Sul, Porto Alegre, Rio Grande Do Sul, Brazil
Aldo Merotto Jr.
Department of Soil and Crop Sciences, Texas A&M University, College Station, TX, USA
Nithya K. Subramanian & Muthukumar V. Bagavathiannan
Department of Plant, Soil and Microbial Sciences, Michigan State University, East Lansing, MI, USA
Luan Cutti & Eric L. Patterson
Department of Agronomy, Kansas State University, Manhattan, KS, USA
Md. Mazharul Islam & Mithila Jugulam
Department of Plant Pathology, Kansas State University, Manhattan, KS, USA
Bikram S. Gill
Crop Protection Discovery and Development, Corteva Agriscience, Indianapolis, IN, USA
Robert Cicchillo, Roger Gast & Neeta Soni
Genome Center of Excellence, Corteva Agriscience, Johnston, IA, USA
Terry R. Wright, Gina Zastrow-Hayes, Gregory May, Kevin Fengler & Victor Llaca
School of Agriculture, Food and Wine, University of Adelaide, Glen Osmond, South Australia, Australia
Jenna M. Malone
Jealott’s Hill International Research Centre, Syngenta Ltd, Bracknell, Berkshire, UK
Deepmala Sehgal, Shiv Shankhar Kaundun & Richard P. Dale
Department of Plant and Soil Sciences, University of Pretoria, Pretoria, South Africa
Barend Juan Vorster
BASF SE, Ludwigshafen Am Rhein, Germany
Jens Lerchl
Department of Crop Sciences, University of Illinois, Urbana, IL, USA
Patrick J. Tranel
Senior Scientist Consultant, Herbicide Resistance Action Committee / CropLife International, Liederbach, Germany
Roland Beffa
School of BioSciences, University of Melbourne, Parkville, VIC, Australia
Alexandre Fournier-Level
You can also search for this author in PubMed Google Scholar
Contributions
JMo and TG conceived and outlined the article. TG, DM, EP, RB, JSM, PJT, MJ wrote grants to obtain funding. MMI, BSG, and MJ performed mitotic chromosome visualization. VL performed sequencing. VL and KF assembled the genomes. LC and ELP annotated the genomes. JMo, SM, DRM, JSM, PN, CN, MV, MVS, AIM, JMK, LF, ALC, PJM, BABM, JMi, AC, MVB, LC, AFL, and ELP wrote the first draft of the article. All authors edited the article and improved the final version.
Corresponding author
Correspondence to Todd A. Gaines .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval is not applicable for this article.
Competing interests
Some authors work for commercial agricultural companies (BASF, Bayer, Corteva Agriscience, or Syngenta) that develop and sell weed control products.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13059_2024_3274_moesm1_esm.docx.
Additional file 1. List of completed and in-progress genome assemblies of weed species pollinated by insects (Table S1).
13059_2024_3274_MOESM2_ESM.docx
Additional file 2. Methods and results for visualizing and counting the metaphase chromosomes of hexaploid Avena fatua (Fig S1); diploid Lolium rigidum (Fig S2); tetraploid Phalaris minor (Fig S3); and tetraploid Salsola tragus (Fig S4).
Additional file 3. Review history.
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Montgomery, J., Morran, S., MacGregor, D.R. et al. Current status of community resources and priorities for weed genomics research. Genome Biol 25 , 139 (2024). https://doi.org/10.1186/s13059-024-03274-y
Download citation
Received : 11 July 2023
Accepted : 13 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s13059-024-03274-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Weed science
- Reference genomes
- Rapid adaptation
- Herbicide resistance
- Public resources
Genome Biology
ISSN: 1474-760X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
7 Research Proposal
Ieee account.
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.

IMAGES
VIDEO
COMMENTS
However, the candidature can also be the period of confusion and regret because of lack of structure and understanding.Research Methods for Successful PhD is written to help the PhD students and other young researchers navigate their path through this phase that will give them a direction and purpose. It is a candid conversation and developed ...
Research Methods for Successful PhD is written to help PhD students and other young researchers navigate through this phase to give them a direction and purpose. It is a candid conversation developed from the experience of supervising 30 research students and publishing 400 papers over 20 years. The book recognizes that every student is ...
A PhD is the start of the research careers, and these students are the backbone of Universities and research institutions. It is the opportunity for youthful energy and creativity to make global impact and train the future researchers to make a difference. However, the candidature can also be the period of confusion and regret because of lack of structure and understanding.Research Methods for ...
Research Methods for Successful PhD is written to help the PhD students and other young researchers navigate their path through this phase that will give them a direction and purpose. It is a candid conversation and developed over the experience of supervising 30 research students and publishing 400 papers over 20 years. The book recognizes ...
A PhD is the start of the research careers, and these students are the backbone of Universities and research institutions. It is the opportunity for youthful energy and creativity to make global impact and train the future researchers to make a difference. However, the candidature can also be the period of confusion and regret because of lack of structure and understanding.Research Methods for ...
Abstract. Doctorates are awarded for making an original contribution to knowledge and understanding and/or practice. This chapter aims to help one to think about how one can going to go about managing your doctorate. It considers how to approach and start your doctorate; how to organise it; how to manage your research project; how to complete ...
Research Methods for Successful PhD is written to help the PhD students and other young researchers navigate their path through this phase that will give them a direction and purpose. It is a candid conversation and developed over the experience of supervising 30 research students and publishing 400 papers over 20 years. The book recognizes ...
Research Methods for Successful PhD is written to help the PhD students and other young researchers navigate their path through this phase that will give them a direction and purpose. It is a ...
Research Methods for Successful PhD is written to help the PhD students and other young researchers navigate their path through this phase that will give them a direction and purpose. It is a candid conversation and developed over the experience of supervising 30 research students and publishing 400 papers over 20 years. The book recognizes ...
Research Methods for Successful PhD is written to help PhD students and other young researchers navigate through this phase to give them a direction and purpose. It is a candid conversation developed from the experience of supervising 30 research students and publishing 400 papers over 20 years. The book recognizes that every student is ...
Research Methods for Successful PhD is written to help PhD students and other young researchers navigate through this phase to give them a direction and purpose. It is a candid conversation developed from the experience of supervising 30 research students and publishing 400 papers over 20 years. The book recognizes that every student is ...
Qualitative research focuses on examining the topic via cultural phenomena, human behavior or belief systems. This type of research uses interviews, open-ended questions or focus groups to gain insight into people's thoughts and beliefs around certain behaviors and systems. Dr. Brant says there are several approaches to qualitative inquiry.
An indispensable reference for postgraduates, providing up to date guidance in all subject areas Methods for Postgraduates brings together guidance for postgraduate students on how to organise, plan and do research from an interdisciplinary perspective. In this new edition, the already wide-ranging coverage is enhanced by the addition of new chapters on social media, evaluating the research ...
Research Methods for Successful PhD Dinesh Kant Kumar Professor, RMIT University Melbourne Australia. Published 2017 by River Publishers River Publishers Alsbjergvej 10, 9260 Gistrup, Denmark www.riverpublishers.com Distributed exclusively by Routledge 4 Park Square, Milton Park, Abingdon, Oxon OX14 4RN
Research Methods for Successful PhD 1st Edition is written by Dinesh Kumar and published by River Publishers. The Digital and eTextbook ISBNs for Research Methods for Successful PhD are 9781000796162, 1000796167 and the print ISBNs are 9788793609181, 8793609183. Save up to 80% versus print by going digital with VitalSource. Additional ISBNs for this eTextbook include 9781003339281, 9781000793390.
Research Methods for Successful PhD 1st Edition is written by Dinesh Kumar and published by River Publishers. The Digital and eTextbook ISBNs for Research Methods for Successful PhD are 9781000796162, 1000796167 and the print ISBNs are 9788793609181, 8793609183. Save up to 80% versus print by going digital with VitalSource. Additional ISBNs for this eTextbook include 8793609183, 100333928X ...
Research Methods for Successful PhD is written to help PhD students and other young researchers navigate through this phase to give them a direction and purpose. It is a candid conversation developed from the experience of supervising 30 research students and publishing 400 papers over 20 years. The book recognizes that every student is ...
15.Action research. A systematic inquiry for improving and/or honing researchers' actions. Researchers find it an empowering experience. Action research has positive result for various reasons; most important is that action research is pertinent to the research participants. Relevance is assured because the aim of each research project is ...
A PhD is the start of the research careers, and these students are the backbone of Universities and research institutions. ... the candidature can also be the period of confusion and regret because of lack of structure and understanding.Research Methods for Successful PhD is written to help the PhD students and other young researchers navigate ...
Created in partnership with. Life as a PhD student is challenging - and one of the most testing aspects of it is the change in mindset it requires. You switch from being a consumer of knowledge to a producer of knowledge. In other words, you transition from passively absorbing information to actively generating new insights through original ...
However, the candidature can also be the period of confusion and regret because of lack of structure and understanding.Research Methods for Successful PhD is written to help the PhD students and other young researchers navigate their path through this phase that will give them a direction and purpose. It is a candid conversation and developed ...
Research Experience . If you're interested in becoming an economics researcher and applying to PhD programs, it's best to get research experience as soon as possible. Working as an RA is a great way to learn how to conduct research and get a better idea of whether it's the right career path for you.
Be willing to review papers for journals and proposals for funding agencies. They are the best sources of information on advances in your research area. Be willing to read over and comment on colleagues' manuscripts and proposals. That is helpful to them, and you will learn more about current ideas in the field.
In this mixed methods randomized controlled trial (RCT), 81 graduate students enrolled in the study and identified a personally relevant goal to pursue. The study deployed an alternative-treatments between-subjects design, with one-third of participants receiving coaching from simulated AI coaches, another third engaging with seasoned human ...
To help overcome these limitations, the research team applied a mix of quantum and classical computing methods. This framework could allow quantum algorithms to address the areas that are challenging for state-of-the-art classical computing, including protein size, intrinsic disorder, mutations and the physics involved in proteins folding.
Target cancer therapy has been developed for clinical cancer treatment based on the discovery of CRISPR (clustered regularly interspaced short palindromic repeat) -Cas system. This forefront and cutting-edge scientific technique improves the cancer research into molecular level and is currently widely utilized in genetic investigation and clinical precision cancer therapy. In this review, we ...
News Release 29-May-2024. USC researchers pioneer new brain imaging technique through clear "window" in patient's skull. In a proof-of-concept study, a research team based at the Keck School ...
Kathryn (Katy) Coduto is an assistant professor of media science in the Department of Mass Communication, Advertising and Public Relations, in the College of Communications. She .teaches courses in communication research methods, social media strategy, and communication theory. She earned her Ph.D. in Communication from Ohio State University.
While these methods have been successful, few weed species have well-studied viral vectors to adapt for use in virus induced gene silencing. Spray induced gene silencing is another potential option for functional investigation of candidate genes in weeds, but more research is needed to establish reliable delivery and gene knockdown [ 50 ].
Research Methods for Successful PhD is written to help the PhD students and other young researchers navigate their path through this phase that will give them a direction and purpose. It is a candid conversation and developed over the experience of supervising 30 research students and publishing 400 papers over 20 years. The book recognizes ...