- Login / Register

‘Sport has the power to unite people in a way that is almost unique’
STEVE FORD, EDITOR
- You are here: COPD

Diagnosis and management of COPD: a case study
04 May, 2020
This case study explains the symptoms, causes, pathophysiology, diagnosis and management of chronic obstructive pulmonary disease
This article uses a case study to discuss the symptoms, causes and management of chronic obstructive pulmonary disease, describing the patient’s associated pathophysiology. Diagnosis involves spirometry testing to measure the volume of air that can be exhaled; it is often performed after administering a short-acting beta-agonist. Management of chronic obstructive pulmonary disease involves lifestyle interventions – vaccinations, smoking cessation and pulmonary rehabilitation – pharmacological interventions and self-management.
Citation: Price D, Williams N (2020) Diagnosis and management of COPD: a case study. Nursing Times [online]; 116: 6, 36-38.
Authors: Debbie Price is lead practice nurse, Llandrindod Wells Medical Practice; Nikki Williams is associate professor of respiratory and sleep physiology, Swansea University.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here (if the PDF fails to fully download please try again using a different browser)
Introduction
The term chronic obstructive pulmonary disease (COPD) is used to describe a number of conditions, including chronic bronchitis and emphysema. Although common, preventable and treatable, COPD was projected to become the third leading cause of death globally by 2020 (Lozano et al, 2012). In the UK in 2012, approximately 30,000 people died of COPD – 5.3% of the total number of deaths. By 2016, information published by the World Health Organization indicated that Lozano et al (2012)’s projection had already come true.
People with COPD experience persistent respiratory symptoms and airflow limitation that can be due to airway or alveolar abnormalities, caused by significant exposure to noxious particles or gases, commonly from tobacco smoking. The projected level of disease burden poses a major public-health challenge and primary care nurses can be pivotal in the early identification, assessment and management of COPD (Hooper et al, 2012).
Grace Parker (the patient’s name has been changed) attends a nurse-led COPD clinic for routine reviews. A widowed, 60-year-old, retired post office clerk, her main complaint is breathlessness after moderate exertion. She scored 3 on the modified Medical Research Council (mMRC) scale (Fletcher et al, 1959), indicating she is unable to walk more than 100 yards without stopping due to breathlessness. Ms Parker also has a cough that produces yellow sputum (particularly in the mornings) and an intermittent wheeze. Her symptoms have worsened over the last six months. She feels anxious leaving the house alone because of her breathlessness and reduced exercise tolerance, and scored 26 on the COPD Assessment Test (CAT, catestonline.org), indicating a high level of impact.
Ms Parker smokes 10 cigarettes a day and has a pack-year score of 29. She has not experienced any haemoptysis (coughing up blood) or chest pain, and her weight is stable; a body mass index of 40kg/m 2 means she is classified as obese. She has had three exacerbations of COPD in the previous 12 months, each managed in the community with antibiotics, steroids and salbutamol.
Ms Parker was diagnosed with COPD five years ago. Using Epstein et al’s (2008) guidelines, a nurse took a history from her, which provided 80% of the information needed for a COPD diagnosis; it was then confirmed following spirometry testing as per National Institute for Health and Care Excellence (2018) guidance.
The nurse used the Calgary-Cambridge consultation model, as it combines the pathological description of COPD with the patient’s subjective experience of the illness (Silverman et al, 2013). Effective communication skills are essential in building a trusting therapeutic relationship, as the quality of the relationship between Ms Parker and the nurse will have a direct impact on the effectiveness of clinical outcomes (Fawcett and Rhynas, 2012).
In a national clinical audit report, Baxter et al (2016) identified inaccurate history taking and inadequately performed spirometry as important factors in the inaccurate diagnosis of COPD on general practice COPD registers; only 52.1% of patients included in the report had received quality-assured spirometry.
Pathophysiology of COPD
Knowing the pathophysiology of COPD allowed the nurse to recognise and understand the physical symptoms and provide effective care (Mitchell, 2015). Continued exposure to tobacco smoke is the likely cause of the damage to Ms Parker’s small airways, causing her cough and increased sputum production. She could also have chronic inflammation, resulting in airway smooth-muscle contraction, sluggish ciliary movement, hypertrophy and hyperplasia of mucus-secreting goblet cells, as well as release of inflammatory mediators (Mitchell, 2015).
Ms Parker may also have emphysema, which leads to damaged parenchyma (alveoli and structures involved in gas exchange) and loss of alveolar attachments (elastic connective fibres). This causes gas trapping, dynamic hyperinflation, decreased expiratory flow rates and airway collapse, particularly during expiration (Kaufman, 2013). Ms Parker also displayed pursed-lip breathing; this is a technique used to lengthen the expiratory time and improve gaseous exchange, and is a sign of dynamic hyperinflation (Douglas et al, 2013).
In a healthy lung, the destruction and repair of alveolar tissue depends on proteases and antiproteases, mainly released by neutrophils and macrophages. Inhaling cigarette smoke disrupts the usually delicately balanced activity of these enzymes, resulting in the parenchymal damage and small airways (with a lumen of <2mm in diameter) airways disease that is characteristic of emphysema. The severity of parenchymal damage or small airways disease varies, with no pattern related to disease progression (Global Initiative for Chronic Obstructive Lung Disease, 2018).
Ms Parker also had a wheeze, heard through a stethoscope as a continuous whistling sound, which arises from turbulent airflow through constricted airway smooth muscle, a process noted by Mitchell (2015). The wheeze, her 29 pack-year score, exertional breathlessness, cough, sputum production and tiredness, and the findings from her physical examination, were consistent with a diagnosis of COPD (GOLD, 2018; NICE, 2018).
Spirometry is a tool used to identify airflow obstruction but does not identify the cause. Commonly measured parameters are:
- Forced expiratory volume – the volume of air that can be exhaled – in one second (FEV1), starting from a maximal inspiration (in litres);
- Forced vital capacity (FVC) – the total volume of air that can be forcibly exhaled – at timed intervals, starting from a maximal inspiration (in litres).
Calculating the FEV1 as a percentage of the FVC gives the forced expiratory ratio (FEV1/FVC). This provides an index of airflow obstruction; the lower the ratio, the greater the degree of obstruction. In the absence of respiratory disease, FEV1 should be ≥70% of FVC. An FEV1/FVC of <70% is commonly used to denote airflow obstruction (Moore, 2012).
As they are time dependent, FEV1 and FEV1/FVC are reduced in diseases that cause airways to narrow and expiration to slow. FVC, however, is not time dependent: with enough expiratory time, a person can usually exhale to their full FVC. Lung function parameters vary depending on age, height, gender and ethnicity, so the degree of FEV1 and FVC impairment is calculated by comparing a person’s recorded values with predicted values. A recorded value of >80% of the predicted value has been considered ‘normal’ for spirometry parameters but the lower limit of normal – equal to the fifth percentile of a healthy, non-smoking population – based on more robust statistical models is increasingly being used (Cooper et al, 2017).
A reversibility test involves performing spirometry before and after administering a short-acting beta-agonist (SABA) such as salbutamol; the test is used to distinguish between reversible and fixed airflow obstruction. For symptomatic asthma, airflow obstruction due to airway smooth-muscle contraction is reversible: administering a SABA results in smooth-muscle relaxation and improved airflow (Lumb, 2016). However, COPD is associated with fixed airflow obstruction, resulting from neutrophil-driven inflammatory changes, excess mucus secretion and disrupted alveolar attachments, as opposed to airway smooth-muscle contraction.
Administering a SABA for COPD does not usually produce bronchodilation to the extent seen in someone with asthma: a person with asthma may demonstrate significant improvement in FEV1 (of >400ml) after having a SABA, but this may not change in someone with COPD (NICE, 2018). However, a negative response does not rule out therapeutic benefit from long-term SABA use (Marín et al, 2014).
NICE (2018) and GOLD (2018) guidelines advocate performing spirometry after administering a bronchodilator to diagnose COPD. Both suggest a FEV1/FVC of <70% in a person with respiratory symptoms supports a diagnosis of COPD, and both grade the severity of the condition using the predicted FEV1. Ms Parker’s spirometry results showed an FEV1/FVC of 56% and a predicted FEV1 of 57%, with no significant improvement in these values with a reversibility test.
GOLD (2018) guidance is widely accepted and used internationally. However, it was developed by medical practitioners with a medicalised approach, so there is potential for a bias towards pharmacological management of COPD. NICE (2018) guidance may be more useful for practice nurses, as it was developed by a multidisciplinary team using evidence from systematic reviews or meta-analyses of randomised controlled trials, providing a holistic approach. NICE guidance may be outdated on publication, but regular reviews are performed and published online.
NHS England (2016) holds a national register of all health professionals certified in spirometry. It was set up to raise spirometry standards across the country.
Assessment and management
The goals of assessing and managing Ms Parker’s COPD are to:
- Review and determine the level of airflow obstruction;
- Assess the disease’s impact on her life;
- Risk assess future disease progression and exacerbations;
- Recommend pharmacological and therapeutic management.
GOLD’s (2018) ABCD assessment tool (Fig 1) grades COPD severity using spirometry results, number of exacerbations, CAT score and mMRC score, and can be used to support evidence-based pharmacological management of COPD.

When Ms Parker was diagnosed, her predicted FEV1 of 57% categorised her as GOLD grade 2, and her mMRC score, CAT score and exacerbation history placed her in group D. The mMRC scale only measures breathlessness, but the CAT also assesses the impact COPD has on her life, meaning consecutive CAT scores can be compared, providing valuable information for follow-up and management (Zhao, et al, 2014).
After assessing the level of disease burden, Ms Parker was then provided with education for self-management and lifestyle interventions.
Lifestyle interventions
Smoking cessation.
Cessation of smoking alongside support and pharmacotherapy is the second-most cost-effective intervention for COPD, when compared with most other pharmacological interventions (BTS and PCRS UK, 2012). Smoking cessation:
- Slows the progression of COPD;
- Improves lung function;
- Improves survival rates;
- Reduces the risk of lung cancer;
- Reduces the risk of coronary heart disease risk (Qureshi et al, 2014).
Ms Parker accepted a referral to an All Wales Smoking Cessation Service adviser based at her GP surgery. The adviser used the internationally accepted ‘five As’ approach:
- Ask – record the number of cigarettes the individual smokes per day or week, and the year they started smoking;
- Advise – urge them to quit. Advice should be clear and personalised;
- Assess – determine their willingness and confidence to attempt to quit. Note the state of change;
- Assist – help them to quit. Provide behavioural support and recommend or prescribe pharmacological aids. If they are not ready to quit, promote motivation for a future attempt;
- Arrange – book a follow-up appointment within one week or, if appropriate, refer them to a specialist cessation service for intensive support. Document the intervention.
NICE (2013) guidance recommends that this be used at every opportunity. Stead et al (2016) suggested that a combination of counselling and pharmacotherapy have proven to be the most effective strategy.
Pulmonary rehabilitation
Ms Parker’s positive response to smoking cessation provided an ideal opportunity to offer her pulmonary rehabilitation (PR) – as indicated by Johnson et al (2014), changing one behaviour significantly increases a person’s chance of changing another.
PR – a supervised programme including exercise training, health education and breathing techniques – is an evidence-based, comprehensive, multidisciplinary intervention that:
- Improves exercise tolerance;
- Reduces dyspnoea;
- Promotes weight loss (Bolton et al, 2013).
These improvements often lead to an improved quality of life (Sciriha et al, 2015).
Most relevant for Ms Parker, PR has been shown to reduce anxiety and depression, which are linked to an increased risk of exacerbations and poorer health status (Miller and Davenport, 2015). People most at risk of future exacerbations are those who already experience them (Agusti et al, 2010), as in Ms Parker’s case. Patients who have frequent exacerbations have a lower quality of life, quicker progression of disease, reduced mobility and more-rapid decline in lung function than those who do not (Donaldson et al, 2002).
“COPD is a major public-health challenge; nurses can be pivotal in early identification, assessment and management”
Pharmacological interventions
Ms Parker has been prescribed inhaled salbutamol as required; this is a SABA that mediates the increase of cyclic adenosine monophosphate in airway smooth-muscle cells, leading to muscle relaxation and bronchodilation. SABAs facilitate lung emptying by dilatating the small airways, reversing dynamic hyperinflation of the lungs (Thomas et al, 2013). Ms Parker also uses a long-acting muscarinic antagonist (LAMA) inhaler, which works by blocking the bronchoconstrictor effects of acetylcholine on M3 muscarinic receptors in airway smooth muscle; release of acetylcholine by the parasympathetic nerves in the airways results in increased airway tone with reduced diameter.
At a routine review, Ms Parker admitted to only using the SABA and LAMA inhalers, despite also being prescribed a combined inhaled corticosteroid and long-acting beta 2 -agonist (ICS/LABA) inhaler. She was unaware that ICS/LABA inhalers are preferred over SABA inhalers, as they:
- Last for 12 hours;
- Improve the symptoms of breathlessness;
- Increase exercise tolerance;
- Can reduce the frequency of exacerbations (Agusti et al, 2010).
However, moderate-quality evidence shows that ICS/LABA combinations, particularly fluticasone, cause an increased risk of pneumonia (Suissa et al, 2013; Nannini et al, 2007). Inhaler choice should, therefore, be individualised, based on symptoms, delivery technique, patient education and compliance.
It is essential to teach and assess inhaler technique at every review (NICE, 2011). Ms Parker uses both a metered-dose inhaler and a dry-powder inhaler; an in-check device is used to assess her inspiratory effort, as different inhaler types require different inhalation speeds. Braido et al (2016) estimated that 50% of patients have poor inhaler technique, which may be due to health professionals lacking the confidence and capability to teach and assess their use.
Patients may also not have the dexterity, capacity to learn or vision required to use the inhaler. Online resources are available from, for example, RightBreathe (rightbreathe.com), British Lung Foundation (blf.org.uk). Ms Parker’s adherence could be improved through once-daily inhalers, as indicated by results from a study by Lipson et al (2017). Any change in her inhaler would be monitored as per local policy.
Vaccinations
Ms Parker keeps up to date with her seasonal influenza and pneumococcus vaccinations. This is in line with the low-cost, highest-benefit strategy identified by the British Thoracic Society and Primary Care Respiratory Society UK’s (2012) study, which was conducted to inform interventions for patients with COPD and their relative quality-adjusted life years. Influenza vaccinations have been shown to decrease the risk of lower respiratory tract infections and concurrent COPD exacerbations (Walters et al, 2017; Department of Health, 2011; Poole et al, 2006).
Self-management
Ms Parker was given a self-management plan that included:
- Information on how to monitor her symptoms;
- A rescue pack of antibiotics, steroids and salbutamol;
- A traffic-light system demonstrating when, and how, to commence treatment or seek medical help.
Self-management plans and rescue packs have been shown to reduce symptoms of an exacerbation (Baxter et al, 2016), allowing patients to be cared for in the community rather than in a hospital setting and increasing patient satisfaction (Fletcher and Dahl, 2013).
Improving Ms Parker’s adherence to once-daily inhalers and supporting her to self-manage and make the necessary lifestyle changes, should improve her symptoms and result in fewer exacerbations.
The earlier a diagnosis of COPD is made, the greater the chances of reducing lung damage through interventions such as smoking cessation, lifestyle modifications and treatment, if required (Price et al, 2011).
- Chronic obstructive pulmonary disease is a progressive respiratory condition, projected to become the third leading cause of death globally
- Diagnosis involves taking a patient history and performing spirometry testing
- Spirometry identifies airflow obstruction by measuring the volume of air that can be exhaled
- Chronic obstructive pulmonary disease is managed with lifestyle and pharmacological interventions, as well as self-management
Related files
200506 diagnosis and management of copd – a case study.
- Add to Bookmarks
Related articles
Have your say.
Sign in or Register a new account to join the discussion.

COPD Case Study: Patient Diagnosis and Treatment (2024)
by John Landry, BS, RRT | Updated: May 16, 2024
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease that affects millions of people around the world. It is primarily caused by smoking and is characterized by a persistent obstruction of airflow that worsens over time.
COPD can lead to a range of symptoms, including coughing, wheezing, shortness of breath, and chest tightness, which can significantly impact a person’s quality of life.
This case study will review the diagnosis and treatment of an adult patient who presented with signs and symptoms of this condition.
25+ RRT Cheat Sheets and Quizzes
Get access to 25+ premium quizzes, mini-courses, and downloadable cheat sheets for FREE.
COPD Clinical Scenario
A 56-year-old male patient is in the ER with increased work of breathing. He felt mildly short of breath after waking this morning but became extremely dyspneic after climbing a few flights of stairs. He is even too short of breath to finish full sentences. His wife is present in the room and revealed that the patient has a history of liver failure, is allergic to penicillin, and has a 15-pack-year smoking history. She also stated that he builds cabinets for a living and is constantly required to work around a lot of fine dust and debris.

Physical Findings
On physical examination, the patient showed the following signs and symptoms:
- His pupils are equal and reactive to light.
- He is alert and oriented.
- He is breathing through pursed lips.
- His trachea is positioned in the midline, and no jugular venous distention is present.
Vital Signs
- Heart rate: 92 beats/min
- Respiratory rate: 22 breaths/min
Chest Assessment
- He has a larger-than-normal anterior-posterior chest diameter.
- He demonstrates bilateral chest expansion.
- He demonstrates a prolonged expiratory phase and diminished breath sounds during auscultation.
- He is showing signs of subcostal retractions.
- Chest palpation reveals no tactile fremitus.
- Chest percussion reveals increased resonance.
- His abdomen is soft and tender.
- No distention is present.
Extremities
- His capillary refill time is two seconds.
- Digital clubbing is present in his fingertips.
- There are no signs of pedal edema.
- His skin appears to have a yellow tint.
Lab and Radiology Results
- ABG results: pH 7.35 mmHg, PaCO2 59 mmHg, HCO3 30 mEq/L, and PaO2 64 mmHg.
- Chest x-ray: Flat diaphragm, increased retrosternal space, dark lung fields, slight hypertrophy of the right ventricle, and a narrow heart.
- Blood work: RBC 6.5 mill/m3, Hb 19 g/100 mL, and Hct 57%.
Based on the information given, the patient likely has chronic obstructive pulmonary disease (COPD) .
The key findings that point to this diagnosis include:
- Barrel chest
- A long expiratory time
- Diminished breath sounds
- Use of accessory muscles while breathing
- Digital clubbing
- Pursed lip breathing
- History of smoking
- Exposure to dust from work
What Findings are Relevant to the Patient’s COPD Diagnosis?
The patient’s chest x-ray showed classic signs of chronic COPD, which include hyperexpansion, dark lung fields, and a narrow heart.
This patient does not have a history of cor pulmonale ; however, the findings revealed hypertrophy of the right ventricle. This is something that should be further investigated as right-sided heart failure is common in patients with COPD.
The lab values that suggest the patient has COPD include increased RBC, Hct, and Hb levels, which are signs of chronic hypoxemia.
Furthermore, the patient’s ABG results indicate COPD is present because the interpretation reveals compensated respiratory acidosis with mild hypoxemia. Compensated blood gases indicate an issue that has been present for an extended period of time.
What Tests Could Further Support This Diagnosis?
A series of pulmonary function tests (PFT) would be useful for assessing the patient’s lung volumes and capacities. This would help confirm the diagnosis of COPD and inform you of the severity.
Note: COPD patients typically have an FEV1/FVC ratio of < 70%, with an FEV1 that is < 80%.
The initial treatment for this patient should involve the administration of low-flow oxygen to treat or prevent hypoxemia .
It’s acceptable to start with a nasal cannula at 1-2 L/min. However, it’s often recommended to use an air-entrainment mask on COPD patients in order to provide an exact FiO2.
Either way, you should start with the lowest possible FiO2 that can maintain adequate oxygenation and titrate based on the patient’s response.
Example: Let’s say you start the patient with an FiO2 of 28% via air-entrainment mask but increase it to 32% due to no improvement. The SpO2 originally was 84% but now has decreased to 80%, and his retractions are worsening. This patient is sitting in the tripod position and continues to demonstrate pursed-lip breathing. Another blood gas was collected, and the results show a PaCO2 of 65 mmHg and a PaO2 of 59 mmHg.
What Do You Recommend?
The patient has an increased work of breathing, and their condition is clearly getting worse. The latest ABG results confirmed this with an increased PaCO2 and a PaO2 that is decreasing.
This indicates that the patient needs further assistance with both ventilation and oxygenation .
Note: In general, mechanical ventilation should be avoided in patients with COPD (if possible) because they are often difficult to wean from the machine.
Therefore, at this time, the most appropriate treatment method is noninvasive ventilation (e.g., BiPAP).
Initial BiPAP Settings
In general, the most commonly recommended initial BiPAP settings for an adult patient include this following:
- IPAP: 8–12 cmH2O
- EPAP: 5–8 cmH2O
- Rate: 10–12 breaths/min
- FiO2: Whatever they were previously on
For example, let’s say you initiate BiPAP with an IPAP of 10 cmH20, an EPAP of 5 cmH2O, a rate of 12, and an FiO2 of 32% (since that is what he was previously getting).
After 30 minutes on the machine, the physician requested another ABG to be drawn, which revealed acute respiratory acidosis with mild hypoxemia.
What Adjustments to BiPAP Settings Would You Recommend?
The latest ABG results indicate that two parameters must be corrected:
- Increased PaCO2
- Decreased PaO2
You can address the PaO2 by increasing either the FiO2 or EPAP setting. EPAP functions as PEEP, which is effective in increasing oxygenation.
The PaCO2 can be lowered by increasing the IPAP setting. By doing so, it helps to increase the patient’s tidal volume, which increased their expired CO2.
Note: In general, when making adjustments to a patient’s BiPAP settings, it’s acceptable to increase the pressure in increments of 2 cmH2O and the FiO2 setting in 5% increments.
Oxygenation
To improve the patient’s oxygenation , you can increase the EPAP setting to 7 cmH2O. This would decrease the pressure support by 2 cmH2O because it’s essentially the difference between the IPAP and EPAP.
Therefore, if you increase the EPAP, you must also increase the IPAP by the same amount to maintain the same pressure support level.
Ventilation
However, this patient also has an increased PaCO2 , which means that you must increase the IPAP setting to blow off more CO2. Therefore, you can adjust the pressure settings on the machine as follows:
- IPAP: 14 cmH2O
- EPAP: 7 cmH2O
After making these changes and performing an assessment , you can see that the patient’s condition is improving.
Two days later, the patient has been successfully weaned off the BiPAP machine and no longer needs oxygen support. He is now ready to be discharged.
The doctor wants you to recommend home therapy and treatment modalities that could benefit this patient.
What Home Therapy Would You Recommend?
You can recommend home oxygen therapy if the patient’s PaO2 drops below 55 mmHg or their SpO2 drops below 88% more than twice in a three-week period.
Remember: You must use a conservative approach when administering oxygen to a patient with COPD.
Pharmacology
You may also consider the following pharmacological agents:
- Short-acting bronchodilators (e.g., Albuterol)
- Long-acting bronchodilators (e.g., Formoterol)
- Anticholinergic agents (e.g., Ipratropium bromide)
- Inhaled corticosteroids (e.g., Budesonide)
- Methylxanthine agents (e.g., Theophylline)
In addition, education on smoking cessation is also important for patients who smoke. Nicotine replacement therapy may also be indicated.
In some cases, bronchial hygiene therapy should be recommended to help with secretion clearance (e.g., positive expiratory pressure (PEP) therapy).
It’s also important to instruct the patient to stay active, maintain a healthy diet, avoid infections, and get an annual flu vaccine. Lastly, some COPD patients may benefit from cardiopulmonary rehabilitation .
By taking all of these factors into consideration, you can better manage this patient’s COPD and improve their quality of life.
Final Thoughts
There are two key points to remember when treating a patient with COPD. First, you must always be mindful of the amount of oxygen being delivered to keep the FiO2 as low as possible.
Second, you should use noninvasive ventilation, if possible, before performing intubation and conventional mechanical ventilation . Too much oxygen can knock out the patient’s drive to breathe, and once intubated, these patients can be difficult to wean from the ventilator .
Furthermore, once the patient is ready to be discharged, you must ensure that you are sending them home with the proper medications and home treatments to avoid readmission.

Written by:
John Landry is a registered respiratory therapist from Memphis, TN, and has a bachelor's degree in kinesiology. He enjoys using evidence-based research to help others breathe easier and live a healthier life.
- Faarc, Kacmarek Robert PhD Rrt, et al. Egan’s Fundamentals of Respiratory Care. 12th ed., Mosby, 2020.
- Chang, David. Clinical Application of Mechanical Ventilation . 4th ed., Cengage Learning, 2013.
- Rrt, Cairo J. PhD. Pilbeam’s Mechanical Ventilation: Physiological and Clinical Applications. 7th ed., Mosby, 2019.
- Faarc, Gardenhire Douglas EdD Rrt-Nps. Rau’s Respiratory Care Pharmacology. 10th ed., Mosby, 2019.
- Faarc, Heuer Al PhD Mba Rrt Rpft. Wilkins’ Clinical Assessment in Respiratory Care. 8th ed., Mosby, 2017.
- Rrt, Des Terry Jardins MEd, and Burton George Md Facp Fccp Faarc. Clinical Manifestations and Assessment of Respiratory Disease. 8th ed., Mosby, 2019.
Recommended Reading
How to prepare for the clinical simulations exam (cse), faqs about the clinical simulation exam (cse), 7+ mistakes to avoid on the clinical simulation exam (cse), copd exacerbation: chronic obstructive pulmonary disease, epiglottitis scenario: clinical simulation exam (practice problem), guillain barré syndrome case study: clinical simulation scenario, drugs and medications to avoid if you have copd, the pros and cons of the zephyr valve procedure, the 50+ diseases to learn for the clinical sims exam (cse).
- Open access
- Published: 13 December 2023
The patient journey in Chronic Obstructive Pulmonary Disease (COPD): a human factors qualitative international study to understand the needs of people living with COPD
- Nicola Scichilone 1 ,
- Andrew Whittamore 2 ,
- Chris White 3 ,
- Elena Nudo 4 ,
- Massimo Savella 4 &
- Marta Lombardini 4
BMC Pulmonary Medicine volume 23 , Article number: 506 ( 2023 ) Cite this article
2719 Accesses
1 Altmetric
Metrics details
Chronic obstructive pulmonary disease (COPD) is a common condition that causes irreversible airway obstruction. Fatigue and exertional dyspnoea, for example, have a detrimental impact on the patient’s daily life. Current research has revealed the need to empower the patient, which can result in not only educated and effective decision-making, but also a considerable improvement in patient satisfaction and treatment compliance.
The current study aimed to investigate the perspectives and requirements of people living with COPD to possibly explore new ways to manage their disease.
Adults with COPD from 8 European countries were interviewed by human factor experts to evaluate their disease journey through the gathering of information on the age, performance, length, and impact of diagnosis, symptoms progression, and family and friends' reactions. The assessment of present symptoms, services, and challenges was performed through a 90-min semi-structured interview. To identify possible unmet needs of participants, a generic thematic method was used to explore patterns, themes, linkages, and sequences within the data collected. Flow charts and diagrams were created to communicate the primary findings. Following analysis, the data was consolidated into cohesive insights and conversation themes relevant to determining the patient's unmet needs.
The 62, who voluntarily accepted to be interviewed, were patients (61% females, aged 32–70 years) with a COPD diagnosis for at least 6 months with stable symptoms of different severity. The main challenges expressed by the patients were the impact on their lifestyle, reduced physical activity, and issues with their mobility. About one-fourth had challenges with their symptoms or medication including difficulty in breathing. Beyond finding a cure for COPD was the primary goal for patients, their main needs were to receive adequate information on the disease and treatments, and to have adequate support to improve physical activity and mobility, helpful both for patients and their families.
Conclusions
These results could aid in the creation of new ideas and concepts to improve our patient’s quality of life, encouraging a holistic approach to people living with COPD and reinforcing the commitment to understanding their needs.
Peer Review reports
Introduction
Chronic obstructive pulmonary disease (COPD) is defined by irreversible airway obstruction linked with comorbidities or systemic effects [ 1 ]. COPD is a worldwide epidemic that contributes significantly to healthcare expenses due to high morbidity and mortality rates [ 2 , 3 ]. The clinical assessment of fixed airflow limitation and symptoms such as coughing and wheezing determine a COPD diagnosis; nevertheless, COPD symptoms negatively impact the patient's daily activities and lifestyle [ 4 ]. Patients may encounter a variety of debilitating physical symptoms, resulting in functional loss and high degrees of psychosocial anguish [ 5 , 6 , 7 ].
Integrated approaches to disease assessment and management are required to better understand and address the burden of COPD symptoms from a patient's perspective [ 8 ].
According to a recent observational study, regardless of disease severity, more than half of COPD patients experienced symptoms during the whole 24-h day, and almost 80% of patients reported experiencing symptoms at least twice a day. Symptoms are linked to poor health, depression, anxiety, and poor sleep quality [ 9 , 10 ].
Patients with COPD and comorbidities remain particularly challenging to manage because in Europe there is, generally, no guidance at the national level except in the UK, Slovenia, and Germany [ 11 , 12 , 13 ]. In Nordic countries and France, the management of patients with COPD is mainly performed by general practitioners with an inadequate level of assistance [ 14 , 15 , 16 ]. In other countries, patient management is performed at the discretion of the local structures, and the need for a comprehensive, holistic approach is looked forward [ 17 , 18 , 19 , 20 ]. Other chronic conditions increase symptom load, impair functional performance, and negatively impact health status; thus, management strategies must be adjusted accordingly [ 10 ].
Care plans, within the healthcare system, emphasize the importance of addressing these patients' particular physical, psychological, social, and spiritual needs through holistic supportive input offered as person-centered care [ 21 ]. Understanding the patient's perspective on their support requirements (those areas of living with COPD for which they require assistance, such as help controlling symptoms or accessing financial benefits) is critical to facilitating this approach. A recent systematic literature review has identified a whole range of support needs for COPD patients, based on the perspectives of the patients themselves [ 7 ].
Our human factor study aims to explore how COPD has affected the patients’ daily lives and the lives of those around them, through the assessment of symptoms, treatment, and service availability, identifying what challenges the patient faces in living with COPD, and which are the unmet needs in the different stages of the journey of care.
This human factors COPD patient needs study was conducted in November 2022 by an ISO 13485 certified specialist human factors consultant (Rebus Medical Ltd), both in-person or remotely, via video call using the Zoom platform. Remote interviews were needed to enable more severe patients to attend the sessions and to ensure that the intended study sample was achieved. As for other qualitative analyses, a minimum of 48 participants were planned to be interviewed.
Interviews were conducted on a 1–1 basis, with patients who voluntarily accepted to be interviewed from 8 countries: Denmark, France, Germany, Italy, Slovenia, Spain, Sweden, and the UK. Each interview was 90 min long and followed a semi-structured approach allowing for unscripted discussion when the participants’ responses raised new questions. For interviews that took place outside of the UK, a native-speaking moderator conducted the interview, whilst an interpreter translated the conversation live to a data analyst (Fig. 1 ).
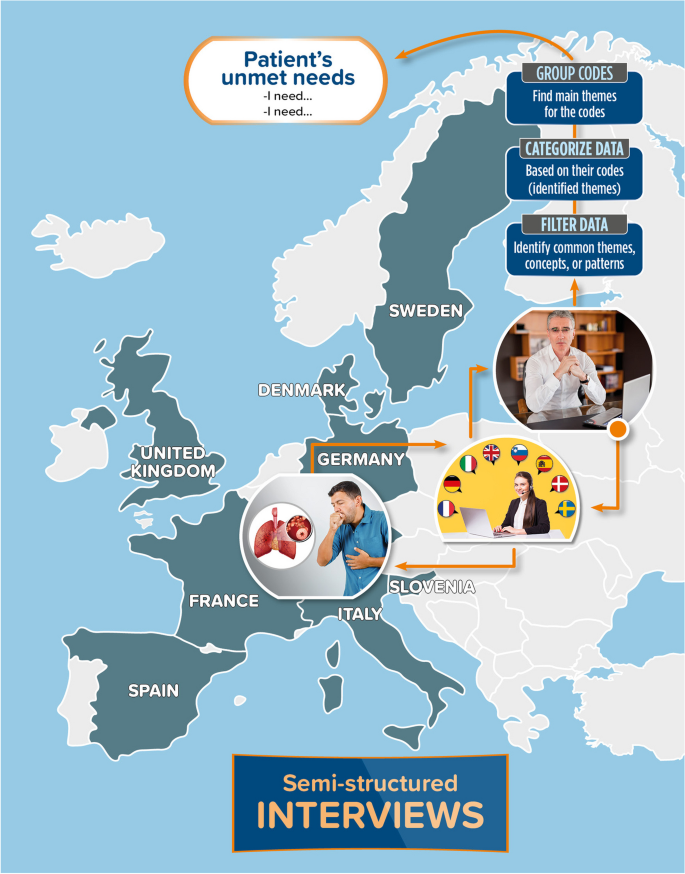
Summary of the study methods. Countries involved in the study are indicated in grey
Participants included in the study, aged 18 years or older, with a current COPD diagnosis, were screened for COPD severity according to GOLD criteria-2020-document [ 22 ] and voluntarily provided their informed consent.
Because the objectives were connected to identifying unmet requirements through video conference, the formative interviews were deemed low to minimal risk to participants and, thus, no formal approval to an Ethical Committee was required.
For interviews conducted in a language other than English, a simultaneous translator was recruited to enable a member of Rebus Medical staff to watch the interview listen to the translation, and record notes. Digital video recordings were collected to accurately account for each test session. Notes were verified at the end of each interview, while participant faces recorded on the videos were blurred to anonymize the footage. When all interviews were complete, the raw notes from each interview were collated and verified using the recorded videos in a master data capture spreadsheet.
The interviews were conducted to evaluate the journey of care through the collection of information on the gender, age, performance, length, and impact of diagnosis, symptoms progression, and family and friends’ reactions through questions that were designed on purpose to identify the unmet need and main challenges of each step of the patient’s journey. The evaluation of the current symptoms (fluctuations, flare-ups, alleviations, effect on sleep and daily activities including the use of electronic devices), services (health care providers support, insurance, available information on COPD), and challenges (in lifestyle, daily activities, treatments, symptoms management, emotional and environmental) was included in the semi-structured interview (Table 1 ).
As this was an exploratory insight interview, protocol deviations like alterations to the interviewer’s script to reformulate questions, ad hoc addition of questions and probes to the interviewer’s script to focus on points of interest specific to each participant, and changes to the interviewer’s script as the study progresses to allow for study learnings were permitted and expected.
A generic thematic approach was employed to uncover patterns, themes, links, and sequences within the data collected to identify probable unmet needs of participants through the patient journey of people living with COPD.
To communicate the major findings, flow charts, and diagrams were constructed. Following analysis, the data were synthesized and refined into cohesive insights and discussion themes pertinent to identifying the patient's unmet needs along the different stages of the patient journey.
A total of 62 patients (38—61% females) with COPD aged between 32 and 70 years ( N = 1 aged 25–40 years, N = 42 aged 41–65 years, N = 19 aged > 65 years) were interviewed. Most of the patients (35—56%) had severe COPD (Table 2 ).
Current- or past smokers were 49 (80%) of the 61 respondents. A larger proportion of patients with severe COPD (9/35, 26%) had never smoked compared to the moderate COPD patient group (3/27, 11%); in fact, 26 (74%) severe patients and 24 (89%) moderate were smokers or had smoked in the past (Fig. 2 ).
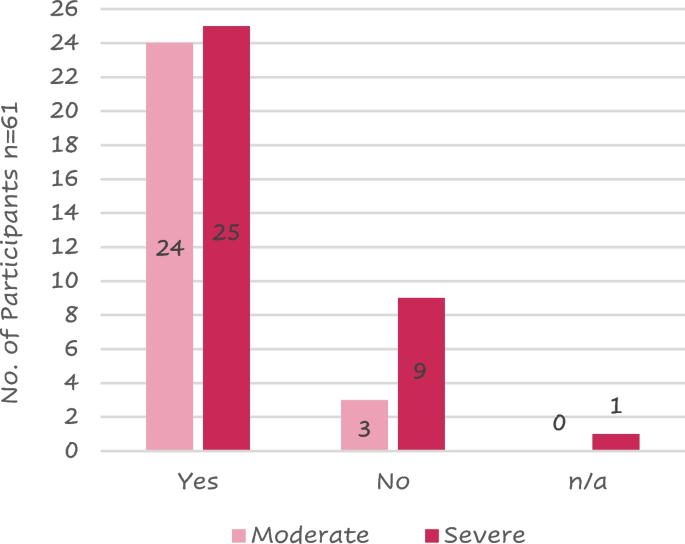
Distribution of patients that have ever been a smoker against COPD severity
Legend: n/a = not available
Patient journey
A total of 113 symptoms of COPD were recorded because most patients reported more than 1 symptom at the onset of the disease; 78 (69%) of these symptoms were related to dyspnoea. The highest reported symptoms were difficulty breathing and coughing (Fig. 3 ).
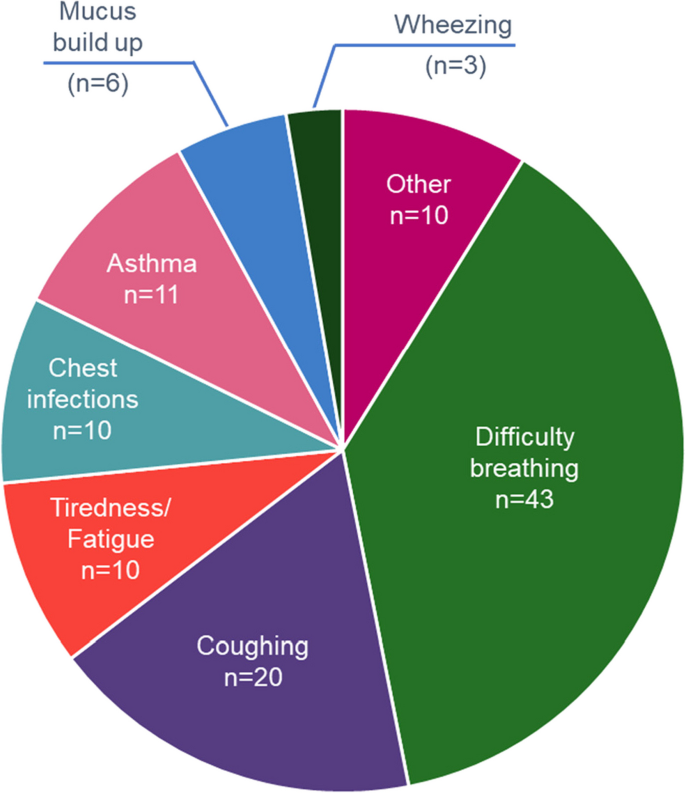
Patient’s reported signs and symptoms leading to COPD diagnosis
Note—Other includes chest tightness, hereditary respiratory issues, persistent flare ups, unable to walk upstairs, difficulty talking, difficulty walking, difficulty swallowing, bronchitis as a child and headaches
Fourteen (30%) of the 46 respondents referred to being diagnosed with COPD more than 1 year after initial symptoms, while 6 (13%) were diagnosed from 7 to 12 months from the onset of symptoms. Ten (64%) of the 14 requiring > 1 year for their diagnosis had severe COPD.
Most of the 56 patients who answered (41 – 73%) were diagnosed by a lung specialist mainly using spirometry (Fig. 4 ).
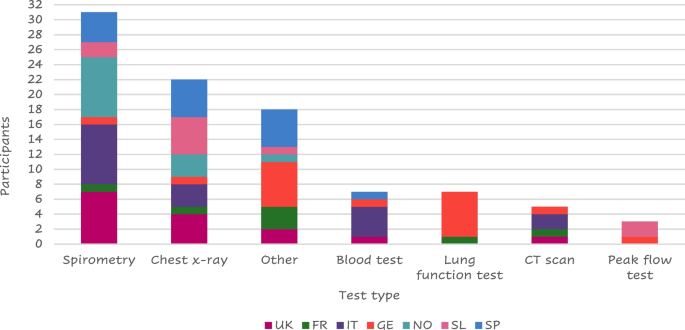
Tests performed at the visit of diagnosis
Legend: FR = France, GE = Germany, IT = Italy, SL = Slovenia, SP = Spain, NO = Northern (Sweden Denmark), UK = United Kingdom. “Other” includes: MRI, pressure cabin test, swabs collected, endoscope to check lungs, chamber, PET scan, Blood taken from the ear, blood gas test, oxygen saturation, walking/ running tests, echocardiogram, pulse oximeter/O 2 saturation, sleep test
About half of the responders (23 of 45 – 51%) felt their symptoms stable from the diagnosis (Fig. 5 ).
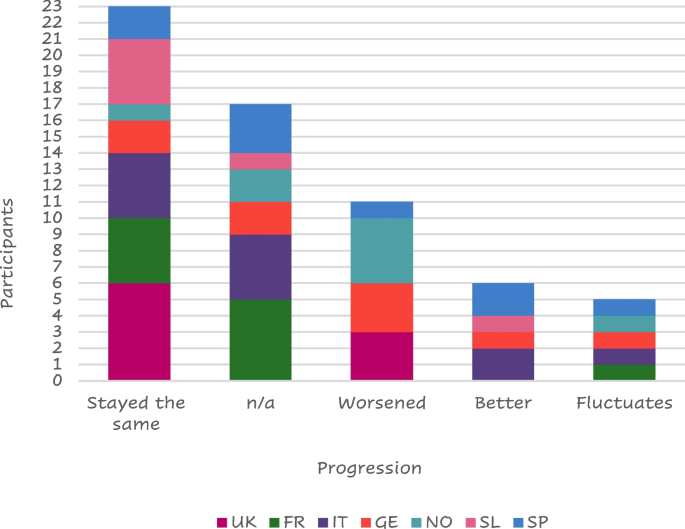
Symptom progression
Legend: FR = France, GE = Germany, IT = Italy, SL = Slovenia, SP = Spain, NO = Northern (Sweden Denmark), UK = United Kingdom, n/a = not available
Thirteen (29%) of those interviewed stated that their family and friends were supportive at the time of COPD diagnosis while 8 (18%) were worried about the diagnosis. Seven of them received no reaction from their family or friends and a further 7 did not tell anyone about their diagnosis. ‘Other’ reactions that were received from family and friends included: acceptance, anger, fear, shock, anguish, and expected, while some patients “prefer not to speak about it”.
The COPD diagnosis hurt 26 (58%) of the responders who described a negative impact of their COPD diagnosis, mainly because of their inability to be active, while 13 of them (29%) felt a positive impact mainly because they stopped or reduced smoking (Table 3 ).
Six (19%) of the 31 patients who provided details on the reason for quitting smoking reported they received more information about how to give up smoking and the risks associated with smoking, 3 patients mentioned some form of medication to support smoking cessation may have helped them give up, and 2 patients reported that they would give up for a family member but would struggle to have the motivation to do it themselves. Three patients reported that nothing would have helped them stop smoking while 8 patients reported that, despite knowing the impact smoking has, they still chose to smoke. Other suggestions to stop smoking reported by participants included: the threat of death, vaping if the smoking affected their fitness, cigarettes stopped being sold, stopping because of asthma and its diagnosis, quitting when they were in the hospital for a week giving it up after then, or because the smell was horrible.
A total of 59 patients answered about their changes in symptoms throughout the day; seventeen (29%) felt no changes while 13 (22%) worsened in the morning, 11 (19%) worsened at night, and 6 (10%) worsened both in the morning and at night.
Twenty-five (41%) of the 61 responders were hospitalized due to a COPD flare-up at least once after their COPD diagnosis; most of them had severe disease (Fig. 6 ).
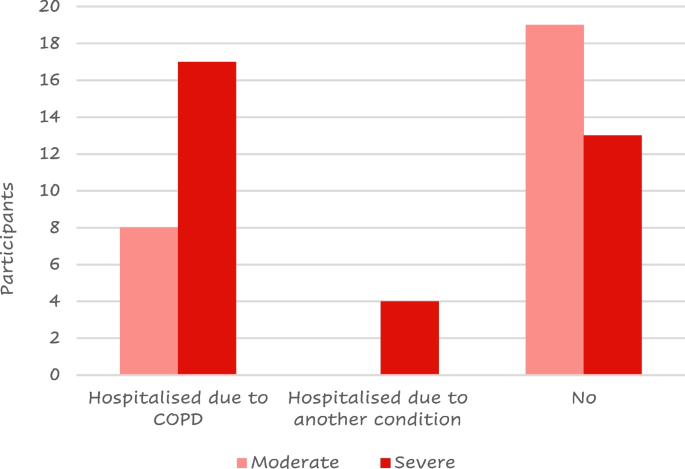
Number of patients that have experienced a COPD flare-up by COPD severity
Seven (30%) of the 23 patients who took any action to alleviate their symptoms, before seeing a doctor and getting a diagnosis, reduced their physical exercise to not trigger symptoms. While others were more vigilant with their health, received help from family and friends, or used inhalers, a rescue pack, or menthol sweets.
Thirty seven out of 58 participants reported sleep disruption. Of these, 12 (32%), reported disruption due to COPD while 10 (17%) had sleep negatively affected by another condition. Other causes for patients’ sleep disruption included coughing, the need to change sleeping positions, and cold weather.
Patients reported needing more support including more information about their condition, financial support for transportation, improved treatment options, accessibility badges, and help in carrying out chores in the house such as cooking, cleaning, and general housekeeping. Some patients also indicated a wish for personal training. Some patients were unaware of what type of support they may require or what type of support could be available to them while others were looking for a different inhaler or treatment to alleviate their cough or a device that assists deep breathing, transplant, a dog or a sport requiring a limited physical effort that would help them be more active, and/or meeting a COPD support group.
About half of the respondents (26/56 – 46%) used electronic devices to monitor their health status including a finger pulse oximeter ( n = 9), smartwatch ( n = 8), or a blood pressure cuff ( n = 5).
A total of 64 responses were collected from the 58 patients who shared their opinion on the treatment they were utilizing; 33 (52%) of the feedback was positive (Table 4 ).
While 20 (31%) of the respondents felt neutral about their current prescribed treatment, 11 (17%) reported either that their medicine had "no therapeutic impact", that they faced "psychological restraint" with their prescribed regime, or that they had issues with treatment compliance.
Six (12%) of the 52 respondents confirmed using digital or analogic reminders to take their dose. Three patients were currently using a dose counter on their device to remind them if their doses had not been taken, and two patients were using a timer on their mobile phones to remind them when their next dose was due. One participant used digital/analogic support but did not indicate which.
The main strategies used to remind them to take their medication include:
leaving the medication in a specific location to prompt them to take their dose at the correct time,
relying on habit or routine to prompt them to take their medication,
taking the COPD medication at the same time as other medications,
feeling unwell to prompt themselves to take their medication.
A total of 32 (56%) of the 57 respondents reported missing a medicine dose; eight of them cited a change in their schedule or routine as its cause. Other reasons for missing a dose reported by patients included: not taking the medication seriously, forgetting to take their dose in the evening, forgetting to bring their medication with them when leaving the house, a change in their environment, a missed medication delivery, and “not taking regular doses”.
The primary reasons why patients appreciate their present treatments were the drug's functionality ( n = 18), the device design ( n = 10), the convenience of use ( n = 8), and the medication's quick and uncomplicated administration ( n = 5). Other patients expressed liking for current medication including feeling comfortable with their present treatment, feeling in charge, and independence.
On the other hand, the device design ( n = 14), the necessity to take their medication ( n = 8), and the side effects of the drug ( n = 5) were the most reported characteristics that patients disliked therapy. Other reported reasons included uncertainty about what the treatment is supposed to do, a sense of guilt when their medication is forgotten, the fact that they are still limited in their activity, and the sensation or taste inside their mouth. Three patients stated that they did not enjoy their current prescribed treatment. "You have to accept what is available," one patient said. Other patients referred detest having to take their medications daily.
About two-thirds ( n = 34 – 67%) of those polled ( n = 51) claimed no involvement with the selection of their present treatment option.
Most of the patients ( n = 42 – 69% of the 61 respondents) reported receiving training for the use of their current treatment. The remaining 31% of the patients did not receive any training, reporting that they “would have liked more formal training, the current device is more complex”, or believed it “could have been useful to receive training and would have loved the explanation, demo training”. Three patients also stated that they did not need training, whether they received it or not.
Twenty-two (52%) of the 42 patients that received training, thought that it was effective and only 5 (12%) did not believe their training to be effective. Fifteen (36%) of patients who received training did not provide feedback on the efficacy of the training they received.
Eight Italian patients reported receiving instruction mostly from a lung specialist, while the majority of British ( n = 5) and Nordic ( n = 4) patients reported receiving training primarily from a nurse (Fig. 7 ); this is probably due to the different structures of the national health systems.
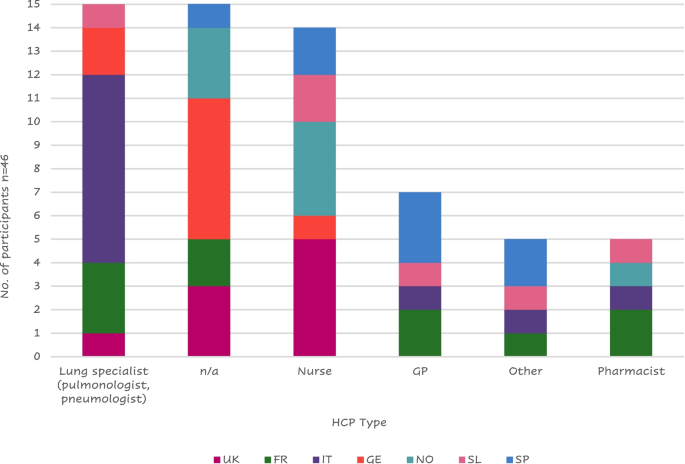
Health care provider (HCP) that administered training to patients by country
Legend: FR = France, GE = Germany, GP = General Practitioner; IT = Italy, SL = Slovenia, SP = Spain, n/a = not applicable; NO = Northern (Sweden Denmark), UK = United Kingdom
One Italian patient stated he received no specific training but was told by his pneumologist to look inside the package and read the instructions; a Frenchman mentioned that his wife was a doctor, so she just showed him how to use the device. Other participants’ training was received at meetings of a lung association from the pharmacists or at a live course organized by the doctor or during rehabilitation.
Six (18% of the 34 respondents) received help from their family or friends to find training materials or treatment information. Most patients received help to find further information and one participant mentioned that he was able to speak to a relative with COPD.
Six (15%) of the 41 respondents had gone online for help with their equipment (looking for tutorials online on forums and finding animated videos on how to use their inhalers). The main reasons for not using the internet for support were a lack of trust in online information ("would rather trust a doctor than go online"), an unwillingness to read more about their condition due to a fear of "reading too much" and becoming "depressed" if they investigated their disease. Other patients did not feel the need for additional support from the internet because their devices were "easy to use" or they wouldn't need further support due to their disease. One patient stated that he looked online and "found it strange that the messages were exclusively for persons with moderate to severe COPD, with only a few messages from people with mild COPD".
Lung specialists were the health care providers (HCPs) who most frequently provided support to patients with COPD ( n = 24/60—40%) followed by general practitioners (23 – 38%) (Fig. 8 ); only 3 patients reported not having received any support.
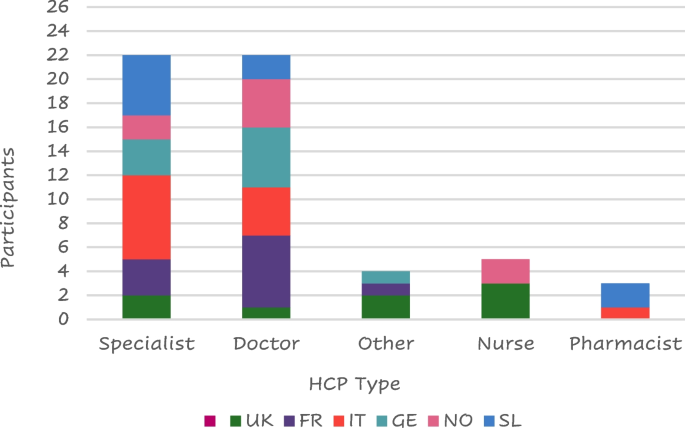
Type of HCP support by country
Legend: FR = France, GE = Germany, IT = Italy, SL = Slovenia, SP = Spain, NO = Northern (Sweden Denmark), UK = United Kingdom
The most frequent answers to the question “If you had a magic wand what would you wish for to improve your life with COPD?” were to find a cure ( n = 18), followed by more regular visits from their doctor/specialist ( n = 11), stop smoking ( n = 5), more information ( n = 4), HCP contact number and COPD support group ( n = 3), and digital monitoring ( n = 2) (Fig. 9 ).
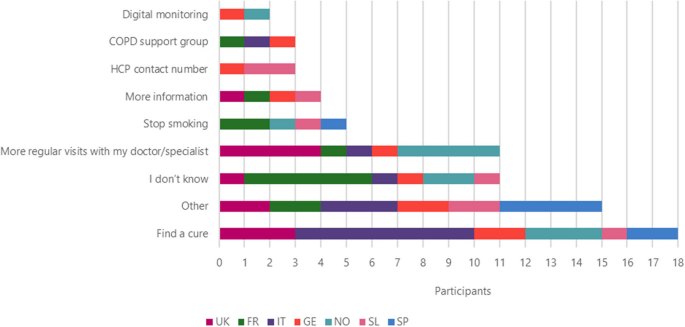
Improvements that patients wish to be made to improve their life by country
Other improvements that patients wish for include: access to new drugs, information about COPD, current and new drugs, reduced side effects, holding COPD workshops, investment in more research, provide cheaper treatment options, new lungs, something to help be more active, to be told that they would not need to take medication anymore, a new type of drug delivery that wouldn’t need to be taken with patient everywhere (like a nicotine patch), instant relief and doctors and nurses to be more humane.
Other services they felt were useful for them included physiotherapy ( n = 12), the use of support groups ( n = 8), exercise classes and psychological assistance ( n = 6), nutrition ( n = 4) while 1 patient from the UK suggested lifestyle (Fig. 10 ).
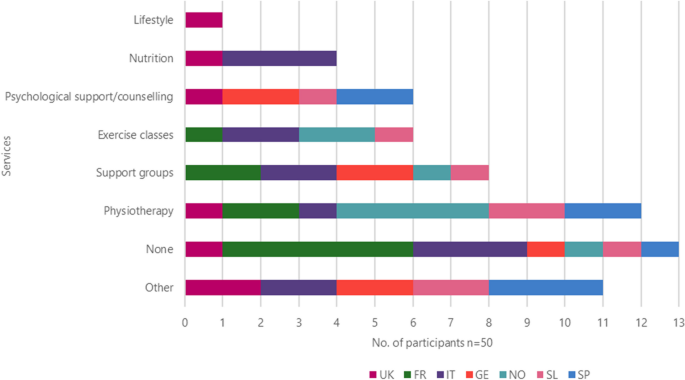
Other services the patient would like to use by country
Other services that patients would like to use included easier access to their HCP, paid, private physiotherapy sessions, smoking cessation support, disability card, training (videos and tutorials) including emergencies, lung transplants, more information about new drugs and the benefits of medication, hear more from doctors and pharmacists, and workshops for families and friends to help them understand what patients are going through.
Even if 3 patients reported having insurance covering additional services, they were generally unaware of the support they could receive through medical insurance. Many had concerns that such services would cost more money.
All the patients included in the study provided a total of 122 daily challenges they must face. 53 (43%) of the responses were related to their lifestyle. Reduced physical activity was referred by more than half ( n = 32) of them and difficulty in mobility was reported by 16; 28 (23%) reported challenges with their symptoms or medication (mainly difficult breathing, n = 15) (Fig. 11 ) while 13 (11%) reported emotional challenges including anxiety, depression, embarrassment due to symptoms or treatment, fear of the conditioning worsening, people recognizing they have a condition, acceptance of the condition and dependence on the medication.
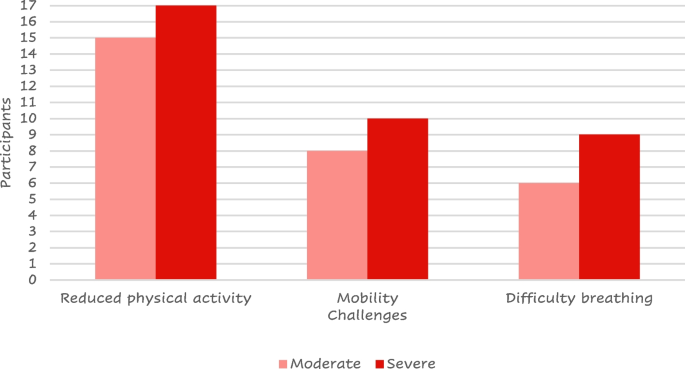
Most reported challenges by COPD severity
The objective of this human factors research was to identify the unmet needs along the different stages of people living with COPD through a one-to-one, semi-structured interview exploring the patient’s feelings and attitudes toward their journeys with the disease.
Differently from other studies exploring similar aspects of the impact of the disease on patient’s daily life where the data belong to medical databases, [ 4 , 6 , 7 , 9 , 10 , 23 ] the current approach is unique, in that it systematically investigates the patient’s feelings in a structured fashion, thus allowing us to better understand the patient’s emotions, which is becoming a relevant aspect of COPD management [ 7 , 24 ]. Furthermore, because of the consistent and wide heterogeneity between the different countries, patients included in this study could have been considered representative of the entire population of European patients with COPD.
The patient reported feelings highlighted that reduced physical activity, mobility challenges, and difficulty breathing resulted as the main challenges in daily life. According to the current international guidelines on COPD management, [ 22 , 25 , 26 ] physical activity is encouraged and monitored to evaluate the prognosis or looked forward to as a target for the evaluation of the treatment efficacy. [ 25 ] Our results confirm that patients perceive COPD as the cause of their reduced physical activity, [ 27 ] having a strong impact on their self-perception. Differently from other studies where increased physical activity was observed independently from patients’ counseling, [ 28 ] general psychological support and accepting their mobility challenges were described as important aims by the patients. Our patients felt reduced mobility as one of their main challenges; aids to improve mobility were described in the available literature as crucial to maintaining the patient’s independence [ 7 ] and have been included in the 2023 GOLD guidelines [ 29 ].
The HCP approach is mainly focused on improving the patient’s breathlessness and exercise intolerance [ 22 , 25 ]; the feeling depicted by the interviewed patients confirms the lack of information about how to manage breathlessness. [ 30 ] The only positive aspect of the COPD diagnosis, reported by 6 of the interviewees, was smoke quitting. Patients frequently feel angry and depressed when they think about the difficulties they have described. Participants discussed a variety of coping mechanisms to deal with these difficulties, including cutting back on physical activity, making sure they stayed active (as much as possible), and utilizing their rescue inhaler as a preventative step.
About one-fourth of the patients did not report having performed spirometry at diagnosis; as spirometry is the landmark of diagnosis; any other method is not gold standard and subjected to criticism [ 22 ]. Because of the qualitative nature of this study, we cannot exclude that this issue was linked to the patient’s reduced memory at the time of diagnosis.
As observed in other studies, [ 31 ] negative behavior has a strong influence on the patient’s quality of life. Patients in the current study generally felt negative emotions before receiving their diagnosis; however, a supportive role of relatives and caregivers was referred by interviewed subjects at the time of diagnosis. About forty percent of patients complained of having waited long before the diagnosis. When asked about the impact of their current treatment, participants gave primarily positive feedback and commonly described their current therapy as “good” and doing its job. Even if most of the patients included in our study felt stable symptoms, some were still looking for a “miraculous” cure. The need for support beyond just pharmacological treatments, such as psychological support and physiotherapy, became clear through the in-depth discussions with patients, confirming the requirement for an integrated and patient-tailored interview to identify the profile of each patient [ 27 , 32 ] to share the most appropriate interventions in the periodic visits, without the need of the patient’s hospitalizations to allow the introduction of new therapies suggested by other research [ 33 ].
As expected, our results show that the information about COPD and the training on both the disease and treatment were provided by different HCPs in various European countries. However, patients often felt that they were not provided with enough information at the point of diagnosis regarding the condition itself or the range of treatment options available. Some felt they did not receive adequate training on how to take their medication correctly, whereas others highlighted that the public should be made more aware of the condition, in general, to help them feel accepted and understood by their family and friends. When asked about the current support they were receiving for their disease, patients reported wanting more information about their clinical condition or treatment options, more regular visits with their HCP, smoking cessation assistance, and support in their day-to-day lives such as housework and improved accessibility, confirming the need of self-management education and skills training highlighted by other authors [ 22 , 25 , 26 ]. However, many patients were unsure or unaware of what support/services were available to them or did not feel they needed any additional support.
This study had a qualitative approach and was, thus, not designed to provide any definitive answer to a study hypothesis. Differently from other studies on general populations of patients with COPD where males and elderly are the most frequent patients [ 34 , 35 ], those who agreed to participate in this study were mostly women and aged between 42 and 65 years. Due to the inclusion of patients that could not be fully representative of the global patients with COPD and the study approach, the outcomes have to be properly generalized. Furthermore, the nature of the study required interviews to be carried out in the participant’s local language with the use of translators to support analysis leading to a potential loss of nuance in meaning.
In conclusion, the current findings show that an apparent discrepancy exists between the traditional lung functional and pharmacological approaches in diagnosing and managing COPD and patient’s needs and challenges in daily activities. In this respect, human factor studies play a relevant role in intercepting gaps in the care of people suffering from COPD, encouraging a novel holistic approach when designing clinical research or shepherding patients along their COPD daily journey.
Availability of data and materials
The data that support the findings of this study are available from Chris White (Rebus Medical), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are not available without permission of Chiesi Farmaceutici.
Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012 [cited 2023 May 29];379(9823):1341–51. Available from: https://pubmed.ncbi.nlm.nih.gov/22314182/ .
Roggeri A, Micheletto C, Roggeri DP. Outcomes and costs of treating chronic obstructive pulmonary disease with inhaled fixed combinations: the Italian perspective of the PATHOS study. Int J Chron Obstruct Pulmon Dis. 2014 Jun 5 [cited 2023 May 29];9:569–76. Available from: https://pubmed.ncbi.nlm.nih.gov/24940053/ .
Blasi F, Cesana G, Conti S, Chiodini V, Aliberti S, Fornari C, et al. The clinical and economic impact of exacerbations of chronic obstructive pulmonary disease: a cohort of hospitalized patients. PLoS One. 2014 Jun 27 [cited 2023 May 29];9(6). Available from: https://pubmed.ncbi.nlm.nih.gov/24971791/ .
Rennard S, Decramer M, Calverley PMA, Pride NB, Soriano JB, Vermeire PA, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International Survey. Eur Respir J. 2002 Oct 1 [cited 2023 May 29];20(4):799–805. Available from: https://pubmed.ncbi.nlm.nih.gov/12412667/ .
Sundh J, Ekström M. Persistent disabling breathlessness in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016 Nov 9 [cited 2023 May 29];11(1):2805–12. Available from: https://pubmed.ncbi.nlm.nih.gov/27877034/ .
Ouellette DR, Lavoie K. Recognition, diagnosis, and treatment of cognitive and psychiatric disorders in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017 Feb [cited 2023 May 29];12:639–50. Available from: https://pubmed.ncbi.nlm.nih.gov/28243081/ .
Gardener AC, Ewing G, Kuhn I, Farquhar M. Support needs of patients with COPD: a systematic literature search and narrative review. Int J Chron Obstruct Pulmon Dis. 2018 Mar 26 [cited 2023 May 29];13:1021–35. Available from: https://pubmed.ncbi.nlm.nih.gov/29628760/ .
Spruit MA, Singh SJ, Garvey C, Zu Wallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013 Oct 15 [cited 2023 May 29];188(8). Available from: https://pubmed.ncbi.nlm.nih.gov/24127811/ .
Miravitlles M, Worth H, Soler Cataluña JJ, Price D, De Benedetto F, Roche N, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res. 2014 Oct 21 [cited 2023 May 29];15(1). Available from: https://pubmed.ncbi.nlm.nih.gov/25331383/ .
Jones PW, Watz H, Wouters FM, Cazzola M, COPD: the patient perspective. cited 2023 May 29. Available from: 2016. https://doi.org/10.2147/COPD.S85977 .
Article PubMed Central Google Scholar
Škrgat S, Triller N, Košnik M, Susič TP, Petek D, Jamšek VV, et al. Priporočila za obravnavo bolnika s kronično obstruktivno pljučno boleznijo na primarni in specialistični pulmološki ravni v Sloveniji. Zdravniski Vestnik. 2017;86(1–2):1–12.
Google Scholar
Mehring M, Donnachie E, Fexer J, Hofmann F, Schneider A. Disease management programs for patients with COPD in Germany: a longitudinal evaluation of routinely collected patient records. Respir Care. 2014 [cited 2023 Nov 15];59(7):1123–32. Available from: https://pubmed.ncbi.nlm.nih.gov/24222706/ .
Overview | Chronic obstructive pulmonary disease in over 16s: diagnosis and management | Guidance | NICE. [cited 2023 Nov 15]. Available from: https://www.nice.org.uk/guidance/ng115 .
Molin KR, Søndergaard J, Lange P, Egerod I, Langberg H, Lykkegaard J. Danish general practitioners’ management of patients with COPD: a nationwide survey. Scand J Prim Health Care. 2020 [cited 2023 Nov 15];38(4):391–8. Available from: https://pubmed.ncbi.nlm.nih.gov/33164618/ .
Sandelowsky H, Natalishvili N, Krakau I, Modin S, Ställberg B, Nager A. COPD management by Swedish general practitioners – baseline results of the PRIMAIR study. Scand J Prim Health Care. 2018 Jan 2 [cited 2023 Nov 15];36(1):5. Available from: /pmc/articles/PMC5901441/.
Meeraus W, Wood R, Jakubanis R, Holbrook T, Bizouard G, Despres J, et al. COPD treatment pathways in France: a retrospective analysis of electronic medical record data from general practitioners. Int J Chron Obstruct Pulmon Dis. 2018 Dec 18 [cited 2023 Nov 15];14:51–63. Available from: https://www.dovepress.com/copd-treatment-pathways-in-france-a-retrospective-analysis-of-electron-peer-reviewed-fulltext-article-COPD .
Come migliorare la qualità di vita dei pazienti colpiti da bronco-pneumopatia cronica e dei loro caregiver? | Azienda Ospedaliera Nazionale SS. Antonio e Biagio e Cesare Arrigo Alessandria. [cited 2023 Nov 15]. Available from: https://www.ospedale.al.it/it/comunicazione/notizie/come-migliorare-qualita-vita-pazienti-colpiti-bronco-pneumopatia-cronica-loro-caregiver .
Aiello F, Alunni A, Berardi M, Bordoni F, Calzolari M, Coviello AP, et al. Medicina Pratica L’associazione LABA-LAMA nella gestione del paziente con BPCO-Il punto di vista della Medicina Generale. Rivista Società Italiana di Medicina Generale n 3 • [Internet]. [cited 2023 Nov 15];29:2022. Available from: https://goldcopd.org/ .
ALLEGATOA alla Dgr n. 206 del 24 febbraio 2015.
Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Spanish Guidelines for Management of Chronic Obstructive Pulmonary Disease (GesEPOC) 2017. Pharmacological Treatment of Stable Phase. Arch Bronconeumol. 2017 Jun 1 [cited 2023 Nov 15];53(6):324–35. Available from: https://pubmed.ncbi.nlm.nih.gov/28477954/ .
NHS England » Ambitions for Palliative and End of Life Care: A national framework for local action 2021–2026. [cited 2023 May 29]. Available from: https://www.england.nhs.uk/publication/ambitions-for-palliative-and-end-of-life-care-a-national-framework-for-local-action-2021-2026/ .
POCKET GUIDE TO COPD DIAGNOSIS, MANAGEMENT, AND PREVENTION A Guide for Health Care Professionals. 2020 [cited 2023 May 29]; Available from: www.goldcopd.org .
Rayner J, Khan T, Chan C, Wu C. Illustrating the patient journey through the care continuum: leveraging structured primary care electronic medical record (EMR) data in Ontario, Canada using chronic obstructive pulmonary disease as a case study. Int J Med Inform. 2020 Aug 1 [cited 2023 Jun 3];140. Available from: https://pubmed.ncbi.nlm.nih.gov/32473567/ .
Walker S, Andrew S, Hodson M, Roberts CM. Stage 1 development of a patient-reported experience measure (PREM) for chronic obstructive pulmonary disease (COPD). NPJ Prim Care Respir Med. 2017 Dec 1 [cited 2023 Jun 3];27(1). Available from: https://pubmed.ncbi.nlm.nih.gov/28740181/ .
Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023 Apr 1 [cited 2023 May 29];61(4):2300239. Available from: https://erj.ersjournals.com/content/61/4/2300239 .
Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global Initiative for Asthma Strategy 2021 Executive Summary and Rationale for Key Changes. Am J Respir Crit Care Med [Internet]. 2022 Jan 1 [cited 2023 Jun 3];205(1):17–35. Available from: https://pubmed.ncbi.nlm.nih.gov/34658302/ .
Jones PW, Watz H, Wouters EFM, Cazzola M. COPD: the patient perspective. Int J Chron Obstruct Pulmon Dis. 2016 [cited 2023 Jun 3];11 Spec Iss(Spec Iss):13–20. Available from: https://pubmed.ncbi.nlm.nih.gov/26937186/ .
Aggarwal AN, Gupta D, Jindal SK. The relationship between FEV1 and peak expiratory flow in patients with airways obstruction is poor. Chest. 2006 [cited 2023 Jun 3];130(5):1454–61. Available from: https://pubmed.ncbi.nlm.nih.gov/17099024/ .
2023 GOLD guidelines for chronic obstructive pulmonary disease. [cited 2023 Jun 26]. Available from: https://www.jwatch.org/na56004/2023/04/20/2023-gold-guidelines-chronic-obstructive-pulmonary-disease .
Oliver SM. Living with failing lungs: the doctor–patient relationship. Fam Pract. 2001 Aug 1 [cited 2023 Jun 3];18(4):430–9. Available from: https://academic.oup.com/fampra/article/18/4/430/620192 .
JIACI · J Invest Allergol Clin Immunol. [cited 2023 Jun 3]. Available from: https://www.jiaci.org/summary/vol16-issue4-num90 .
Martínez-Guiu J, Arroyo-Fernández I, Rubio R. Impact of patients’ attitudes and dynamics in needs and life experiences during their journey in COPD: an ethnographic study. Expert Rev Respir Med. 2022 [cited 2023 Jun 3];16(1):121–32. Available from: https://pubmed.ncbi.nlm.nih.gov/34238094/ .
Lainscak M, Gosker HR, Schols AMWJ. Chronic obstructive pulmonary disease patient journey: hospitalizations as window of opportunity for extra-pulmonary intervention. Curr Opin Clin Nutr Metab Care. 2013 May [cited 2023 Jun 3];16(3):278–83. Available from: https://pubmed.ncbi.nlm.nih.gov/23507875/ .
Kim-Dorner SJ, Schmidt T, Kuhlmann A, Graf von der Schulenburg JM, Welte T, Lingner H. Age- and gender-based comorbidity categories in general practitioner and pulmonology patients with COPD. NPJ Prim Care Respir Med. 2022 Dec 1 [cited 2023 Nov 15];32(1). Available from: /pmc/articles/PMC9061861/.
Maestri R, Vitacca M, Paneroni M, Zampogna E, Ambrosino N. Gender and age as determinants of success of pulmonary rehabilitation in individuals with chronic obstructive pulmonary disease. Arch Bronconeumol. 2023 Mar 1 [cited 2023 Nov 15];59(3):174–7. Available from: https://www.archbronconeumol.org/en-gender-age-as-determinants-success-articulo-S0300289622005683 .
Download references
Acknowledgements
Rebus Medical (St Nicholas House, 31-34 High St, Bristol BS1 2AW, United Kingdom) was responsible for contacting the patients, data collection, and statistical analyses. The authors thank Andrea Rossi for the medical writing support and the Chiesi and Rebus study team for the management of the Human factors study (Marta Lombardini, Ilaria Milesi, Lorenzo Ventura, Veronica Giminiani, Massimo Savella, Elena Zeni, Elena Nudo, Lisa Forde, Shivani Bhalsod, Elsie Barker, and Chris White).
The authors thank the patients, the interviewers, and the translators who made this study possible.
All the activities were funded by Chiesi Farmaceutici S.p.A. (Parma, Italy).
Author information
Authors and affiliations.
Division of Respiratory Medicine, Department PROMISE, “Giaccone” University Hospital, University of Palermo, Palermo, Italy
Nicola Scichilone
GP, Portsdown Group Practice, Portsmouth, UK
Andrew Whittamore
Rebus Medical LTD, Bristol, UK
Chris White
Chiesi Farmaceutici S.P.A, Via Paradigna 131/A – 43122, Parma, Italy
Elena Nudo, Massimo Savella & Marta Lombardini
You can also search for this author in PubMed Google Scholar
Contributions
NS contributed to the design of the study and critically revised the outcomes according to the clinical needs from a specialistic point of view. AW contributed to the design of the study and critically revised the outcomes according to the clinical needs from a general practitioner’s point of view. CW designed the study, managed and coordinated the study activities. EN, MS, and ML contributed to the design of the study and critically revised the outcomes from a treatment producer’s point of view. All authors critically revised the drafted article and read and approved the final manuscript.
Corresponding author
Correspondence to Nicola Scichilone .
Ethics declarations
Ethics approval and consent to participate.
The DL 20 marzo 2008 specifies that interviews to the patients without any clinical intervention (as the present study) are not considered observational studies and, thus, don't need to be submitted to the revision and approval of an Ethical committee.
All patients provided their informed consent to participate in this study. The informed consent included statements that required participants to agree to maintain confidentiality regarding the information shared during the study session, as well as described the conditions for the collection, use, processing, retention, and transfer of their personal data (including personally identifiable information and personal health information).
Consent for publication
Not applicable.
Competing interests
NS declares no competing interests.
AW declares no competing interests.
CW is a full-time employee of Rebus Medical.
EN, MS, and ML are full-time employees of Chiesi Farmaceutici.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Scichilone, N., Whittamore, A., White, C. et al. The patient journey in Chronic Obstructive Pulmonary Disease (COPD): a human factors qualitative international study to understand the needs of people living with COPD. BMC Pulm Med 23 , 506 (2023). https://doi.org/10.1186/s12890-023-02796-8
Download citation
Received : 18 September 2023
Accepted : 29 November 2023
Published : 13 December 2023
DOI : https://doi.org/10.1186/s12890-023-02796-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chronic obstructive pulmonary disease
- Assessment of healthcare needs
- Qualitative research
- Patient-centered care
- Human-centered design
- Human factors science
- Pharmacologic therapy
- Qualitative evaluation
- Determination of healthcare needs
- Multinational perspective
- Quality of life
- Quality of healthcare
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
It seems you are using an outdated browser. Please upgrade to a modern browser to improve your experience on this website.

A COPD Case Study: Jim B.

This post was written by Jane Martin, BA, LRT, CRT, Assistant Director of Education at the COPD Foundation .
We're interested in your thoughts on our latest COPD case study: Jim B., a 68-year-old man here for his Phase II Pulmonary Rehabilitation intake interview.
A bit more about Jim:
Medical history: COPD, FEV1 six weeks ago was 38% of normal predicted, recent CXR shows flattened diaphragm with increased AP diameter, appendectomy age 34, broken nose and broken right arm as a child.
Labs: Lytes plus and CBC all within normal limits.
Physical exam: Breath sounds markedly diminished bilaterally with crackles right lower lobe and wheeze left upper lobe. Visible use of accessory muscles. O2 Saturation 93% room air, 95% O2 on 2lpm. Respiratory rate 24 and shallow, HR 94, BP 150/88, 1+ pitting pedal edema.
Current Medications: Prednisone 10mg q day / DuoNeb q 4 hrs. / Ibuprofen 400mg BID / Tums prn (estimates he takes two per day).
Respiratory history: 80-pack-year cigarette history, quit last year. He has developed a dry, hacking, non-productive cough over the last six months. Had asthma as a child and was exposed to second-hand smoke and cooking fumes while working at family-owned restaurant as a child. Lately, he has noticed slight chest tightness and increased cough when visiting his wife’s art studio.
Family history: Father had emphysema, died at age 69, mother died of breast cancer at 62. Grandfather died at age 57, grandmother died in her 40s of suicide. Six adult children, alive and well.
Previous respiratory admissions: Inpatient admission for six days last winter for acute exacerbation of COPD with bacterial pneumonia requiring 24-hour intubation and mechanical ventilation.
Psych: Jim presents to his Phase II Pulmonary Rehab intake interview appearing disheveled, wearing a sweatshirt, pajama pants and bedroom slippers. He is accompanied by his wife and adult daughter who appear neat, clean and well dressed. Patient states, “I don’t think you people can do anything to help me. I’m only here because they (referring to wife and daughter) made me go.” Jim states that he has been doing less and less at home since discharged from the hospital last winter. Wife states, “He walked outside a little with our grandchildren last Sunday and got so short of breath, he almost collapsed.” Became emotional when saying, “It scared the kids. It tore me up for them to see me that way. Besides that, with this darn shoulder I can’t even pick up the little ones anymore.”

Social: Lives at home with his wife of 43 years who works as an artist. Two out of his six children live within 30 miles of Jim’s home.
Occupation: Building contractor, retired three years ago. Jim states, “I made a good living. All the kids were able to go to college. I was strong. I could work circles around anybody in my crew. And now look at me. I’m tied to that darn breathing machine (referring to nebulizer) and I might as well hang it up.” Wife states, “He used to have all kinds of energy. Now all he does is sit in his chair watching TV, eating potato chips and peanuts.”
Tell us your impressions!
- What co-morbidities should be explored?
- How would you change Jim’s medication regime?
- What psych/social recommendations would you make?
- What other medical disciplines should do a consult on this patient?
- This is a real case. What are your thoughts on what took place following Jim’s pulmonary rehab intake interview?
This page was reviewed on March 3, 2020 by the COPD Foundation Content Review and Evaluation Committee
15 Comments
Join Us on COPD360social
Join the Conversation
Already a Member?
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Respir Med Case Rep
Early COPD diagnosis and treatment: A case report
Associated data.
Not applicable.
Chronic obstructive pulmonary disease (COPD) refers to a group of widely diffuse diseases that cause airflow blockage characterized by persistent respiratory symptoms such as dyspnea, chronic cough, recurrent wheezing, chronic sputum production, and progressive restricted airflow associated with exacerbations. COPD is the third leading cause of death worldwide and can only be treated not cured.
Pulmonary function tests do not permit the identification of initial obstructive airways disease. Forced expiratory flow (FEF 25-75 ), which calculates obstruction severity at small and medium bronchial airways levels, allows an early COPD diagnosis.
We report a 72-year-old ex-smoker male not exposed to occupational risk with symptoms suggesting early COPD. Baseline pulmonary function tests were normal, except FEF 25-75 . The patient did not respond to the first 6 months of treatment with long-acting muscarinic antagonist (LAMA), whereas he showed a clear clinical and FEF 25-75 response to 1-year treatment with LAMA associated with long-acting β2 agonist (LABA).
This clinical case report highlights the usefulness of FEF 25-75 evaluation in early COPD diagnosis and monitoring and confirms the efficacy of LAMA–LABA association for small airways obstruction treatment.
1. Introduction
Chronic obstructive pulmonary disease (COPD) refers to a group of widely diffuse diseases, including emphysema and chronic bronchitis, that cause airflow blockage. It is characterized by persistent respiratory symptoms such as dyspnea, chronic cough, chronic production of sputum, recurrent wheezing, and restricted airflow [ 1 ]. COPD is characterized by airflow limitation that is not fully reversible. Airflow limitation is progressive and associated with abnormal inflammatory response of the lungs, particularly caused by cigarette smoking [ 2 ].
COPD is the third leading cause of death worldwide because of associated comorbidities such as smoking, environmental pollution, and occupational risk [ 3 ]. Despite accurate studies and clinical trials analyzing COPD etiology and phenotypes, currently it can only be treated not cured.
Although COPD affects the lungs, it causes systemic damages with heart involvement developing pulmonary hypertension and cor pulmonale [ 4 ].
The importance of exacerbations is closely related to COPD clinical course, and their reduction increases the outcome and improves the quality of life (QOL). Exacerbations are events requiring therapy with antibiotics, systemic steroids, or both. The severity of exacerbations is defined by the location of care such as home and emergency room or hospital. Mild exacerbations involve upper airways and are treated with adjustments in bronchodilator or inhaled corticosteroid therapy, and moderate exacerbations involve lower respiratory tracts and are treated with antibiotics and systemic corticosteroids. Based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [ 1 ], the COPD classification of airflow limitation severity is defined as follows: (i) mild forced expiratory volume in 1 second (FEV 1 ) ≥ 80% predicted; (ii) moderate 50% ≤ FEV 1 < 80% predicted; (iii) severe 30% ≤ FEV 1 <50% predicted; and (iv) very severe FEV 1 <30% predicted. An additional sensitive parameter to evaluate early airflow obstruction in peripheral airways is forced expiratory flow (FEF) rate between 25% and 75% of forced vital capacity (FVC). The normal value of FEF 25–75% depends on age and height, and the results of FEF 25–75% are little effort-dependent and representative of air movement through small airways [ 5 , 6 ].
The aim of this clinical case is to highlight the usefulness of pulmonary function tests and particularly FEF 25–75% in early COPD diagnosis to improve therapeutic approach, reduce acute exacerbations, QOL, and disease outcome.
2. Case report
A 72-year-old ex-smoker male, 5 pack-years, was admitted with exertional dyspnea lasting 6 months and productive cough lasting 3 months suggesting early COPD. The patient was not exposed to occupational risk and was neither affected by allergy nor other relevant comorbidities. Physical chest examination showed fine rhonchi without wheezing and crackling sounds. Cyanosis was absent, and pulse oximetry was 96% in room air. No signs of cardiovascular failure were detected.
Pulmonary function tests at baseline, including FEV 1 and FEV 1 /FVC ratio, were in the normal range and did not suggest an initial obstructive airways disease. Note that the FEF 25-75 values were suggestive of moderate small airways obstruction ( Table 1 ). The patient was first treated with long-acting muscarinic antagonist (LAMA – umeclidinium bromide 65 mcg/daily). However, dyspnea and cough did not ameliorate, and the patient suffered from exacerbation responsive to antibiotics. Spirometry after 6 months of LAMA therapy showed a decrease of FEF 25-75 , especially of FEF 25 from 59% to 50%. Then, the treatment was modified using the association of LAMA with long-acting β2 agonist (LABA – vilanterol 22 mcg/daily) according to GOLD guidelines. Patient evaluation after 12 months of LAMA–LABA therapy revealed excellent response with decreased dyspnea and cough and absence of exacerbations. Spirometry showed clear regression of small airways obstructive parameters. Notably FEF 25 increased from 50% to 62% ( Table 1 and Fig. 1 ).
FEF 25-75 values before and after treatment.
| Baseline | After 6 months of LAMA therapy | After 1 year of LAMA–LABA therapy | |
|---|---|---|---|
| 59 | 50 | 62 | |
| 42 | 41 | 42 | |
| 43 | 43 | 47 |
FEF: forced expiratory flow; LAMA: long-acting muscarinic antagonist; LABA: long-acting β2 agonist.

FEF 25-75 behavior during LAMA and LAMA–LABA treatment.
3. Discussion
In the reported case, the patient's symptoms such as productive cough associated with dyspnea under exertion worsened and were poorly controlled, particularly in the course of an exacerbation occurring during 6-month LAMA therapy. The persistent symptoms required more effective treatment and led, according to GOLD guidelines, to a therapeutic change replacing LAMA alone with LAMA–LABA association [ 7 ] ( Fig. 2 ).

Efficacy of LAMA–LABA therapy in COPD.
The efficacy of umeclidinium plus vilanterol led to an evident reduction in symptoms avoiding COPD exacerbations at 6, 12, and 18 months of follow-up. The therapeutic benefit was confirmed by spirometry, particularly by FEF 25-75 increase. These findings confirm the usefulness of the association of antimuscarinic agent with LABA in COPD treatment [ 1 ].
Finding normal parameters in pulmonary function tests does not permit, as in this case, to identify an initial obstructive airways disease. Only FEF 25-75, which calculates obstruction severity at several small and medium bronchial airways levels, allows an early COPD diagnosis.
Recently, Kwon Sun et al. [ 5 ] confirmed that FEF 25-75 is reduced in early COPD and associated with small airway disease . Interestingly, the previously performed Boston early-onset COPD study identified FEF 25-75 as a potential indicator of genetic susceptibility to develop COPD. However, the same authors suggested that this measure does not provide information beyond FEV 1 /FVC for demonstrating small airways disease [ 8 ] .
Note that many clinical trials assessing COPD diagnosis and treatment used only FVC and FEV 1 to evaluate the severity of airways obstruction and therapeutic response [ 3 , [9] , [10] , [11] , [12] , [13] , [14] ].
The hallmarks of the present case are that it (i) underlines that FEF 25-75 decrease is the earliest and sensitive pulmonary function test parameter suggesting COPD diagnosis; (ii) FEF 25-75 decrease reflects the loss of elastic recoil and air trapping from emphysema and COPD small airways; and (iii) highlights that lower FEF 25-75 is closely associated with an increase in COPD severity, providing new knowledge of pathophysiological and anatomical mechanisms that lead to clinical outcomes in COPD [ 6 ].
Authors’ contributions
RGC and GB: conceptualization and investigation. FP: reviewing and editing.
Ethics approval
Consent to publication, availability of data and material.
University of Genoa Grant n.:100007-2020-SD-FRA_001.
Declaration of competing interest
The Authors do not have any conflict of interest.
Handling Editor: DR AC Amit Chopra
Abbreviations
- Open access
- Published: 21 July 2021
Evaluating the implementation of a chronic obstructive pulmonary disease management program using the Consolidated Framework for Implementation Research: a case study
- Stefan Paciocco 1 ,
- Anita Kothari 2 ,
- Christopher J. Licskai 3 ,
- Madonna Ferrone 4 &
- Shannon L. Sibbald 5
BMC Health Services Research volume 21 , Article number: 717 ( 2021 ) Cite this article
2753 Accesses
2 Citations
Metrics details
Chronic obstructive pulmonary disease (COPD) is a prevalent chronic disease that requires comprehensive approaches to manage; it accounts for a significant portion of Canada’s annual healthcare spending. Interprofessional teams are effective at providing chronic disease management that meets the needs of patients. As part of an ongoing initiative, a COPD management program, the Best Care COPD program was implemented in a primary care setting. The objectives of this research were to determine site-specific factors facilitating or impeding the implementation of a COPD program in a new setting, while evaluating the implementation strategy used.
A qualitative case study was conducted using interviews, focus groups, document analysis, and site visits. Data were deductively analyzed using the Consolidated Framework for Implementation Research (CFIR) to assess the impact of each of its constructs on Best Care COPD program implementation at this site.
Eleven CFIR constructs were determined to meaningfully affect implementation. Five were identified as the most influential in the implementation process. Cosmopolitanism (partnerships with other organizations), networks and communication (amongst program providers), engaging (key individuals to participate in program implementation), design quality and packaging (of the program), and reflecting and evaluating (throughout the implementation process). A peer-to-peer implementation strategy included training of registered respiratory therapists (RRT) as certified respiratory educators and the establishment of a communication network among RRTs to discuss experiences, collectively solve problems, and connect with the program lead.
Conclusions
This study provides a practical example of the various factors that facilitated the implementation of the Best Care COPD program. It also demonstrates the potential of using a peer-to-peer implementation strategy. Focusing on these factors will be useful for informing the continued spread and success of the Best Care COPD program and future implementation of other chronic care programs.
Peer Review reports
The prevalence of chronic diseases in Canada has increased dramatically within the last few decades [ 1 , 2 ]. The number of individuals with chronic obstructive pulmonary disease (COPD) has almost doubled since 2000–2001 [ 3 ]. COPD is a debilitating chronic respiratory disease that accounts for the greatest number of chronic illness-related hospital admissions in Canada [ 4 ].
The use of team-based primary care has been explored to manage and combat the rise of chronic illnesses [ 5 ]. Chronic disease management programs using team-based primary care have been successful at mitigating the negative impacts of chronic diseases such as diabetes [ 6 ], chronic kidney disease [ 7 ], and congestive heart failure [ 8 ]. Using primary care to manage chronic diseases has become a successful part of comprehensive care, resulting in these models becoming a standard for chronic disease management and care around the world [ 9 ]. Unfortunately, there is limited literature describing how best to engage primary care in the management of COPD utilizing an integrated disease management approach. Indeed, in Canada and other jurisdictions the management of COPD falls below guideline standards, is reactive, not proactive, and in this way distinct from other conditions such as diabetes, where the obverse is true [ 10 – 14 ].
Canada has a universal health care system delivered under provincial jurisdiction. Ontario, Canada’s most populous province (14.7 million), implemented family health teams (FHTs) as a collaborative primary care model consisting of providers from multiple disciplines including primary care clinicians and allied health professionals [ 15 ]. Since their implementation in 2005, FHTs have resulted in improved health outcomes and increased access to interprofessional care for patients in Ontario [ 16 ]. For patients with COPD who may struggle to navigate the health system, interprofessional team-based primary care is often a better alternative to emergency department or solo practitioner care [ 17 ].
Accessibility limits the impact of FHTs in general, and on patients with COPD specifically, as only approximately 20% of the population in Ontario has access to team care within an FHT [ 15 , 18 ]. The Best Care COPD program (BCC), the subject of this case study, is an efficacious interprofessional team care program that was developed within the FHT context [ 10 ]. The impact of BCC and other chronic disease management programs in primary care is dependent on effective implementation.
In order to effectively implement chronic disease management programs context-specific guidance is needed [ 19 ]. The implementation of any program into a new setting requires a rich understanding of local context, analysis of stakeholders, and evaluation of provider, organization, and system factors [ 1 ].
Using an evidence-based implementation framework for evaluation ensures research is theoretically grounded [ 20 ]. There are a number of available frameworks such as promoting action on research implementation in health services [ 21 ], the theoretical domains framework [ 22 ], and the consolidated framework for implementation research (CFIR) [ 23 ]. CFIR, an amalgamation of 19 different theories [ 23 ] considers constructs known to affect implementation (see Table 1 and Additional file 1 ) [ 24 ]. Information from CFIR can be used both prescriptively to facilitate the implementation of a program into specific local contexts or retroactively to evaluate implementation efforts [ 25 ]. We chose CFIR to evaluate the implementation of the BCC program.
In Ontario, a team-based COPD management program (BCC) based in primary care focusing on patient self-management through education, skills training and case management, was spread from the region where it was originally developed and implemented, to a neighbouring region using a peer-to-peer implementation approach. While current literature lacks a clear definition, or common name for peer-to-peer approaches in implementation, in general terms, peer-to-peer approaches involve using peer-led education and peer assessment as a method to support learning about the intervention [ 26 – 28 ]. Using a peer-to-peer approach implementation processes can facilitate buy-in and successful uptake/program implementation [ 27 ]. Although more research is needed, preliminary evidence shows peer-to-peer learning as facilitating improved clinical education [ 29 , 30 ]. The purpose of this research was to explore the implementation of the BCC management program at a new clinical site in a different region, using the CFIR framework. Two research objectives guided this study.
Determine the enabling and impeding factors to implementation and spread of an interprofessional team-based primary care model, and
Explore the peer-to-peer approach to implementing a team-based primary care model.
Current literature lacks context empirical examples of the implementation of team-based primary chronic care models specifically for patients with COPD [ 31 ] as well as examples of using a peer-to-peer approach for implementation. Our research set out to fill this gap.
Case description
The BCC management program is a model of care consisting of primary care practitioners (physicians and nurse practitioners), nurses, a respirologist, RRT’s with certified respiratory educator training, and health administrators all working together to provide COPD-specific care to patients. This model was created for the purposes of “delivering standardized, high-impact best-practices, within an interdisciplinary care model” ([ 32 ] p.6). BCC has demonstrated improved patient outcomes (such as reduced severe exacerbations) and reduced urgent health services use (including emergency department visits) [ 10 ]. Best practices in the program include creating action plans, skills training (including inhaler and breathing techniques), how to handle exacerbations, spirometry pre- and post-intervention to measure progress, and medication and exercise prescriptions [ 33 ] . Program standardization and evaluation was supported by a program specific technology solution that guided every encounter and captured performance and outcome metrics [ 32 ]. In Canada, health care providers can obtain a certification as certified respiratory educator (a CRE program recognized by the Canadian Network of Respiratory Care)[ 34 ]; in this case, all RRTs providing care within BCC had (or obtained) a certified respiratory educator designation.
An important component of the BCC program is an advisory committee, called the Primacy Care Innovation Collaborative (PCIC). The PCIC focuses on healthcare system innovation within primary care including participating in the development of provincial standards [ 35 ], and work to better integrate services within primary care through a ‘medical home’ approach [ 36 ]. Specific to BCC, PCIC supported and facilitated the robust evaluation and spread of the program outside the original region [ 32 ]. As a proof of concept project to demonstrate the programs ability to spread as well as to support the feasibility of the peer-led implementation approach, BCC was spread into a five-site FHT within Ontario (B-FHT). The unit of analysis in this case was considered the FHT. All individuals and organizations external to this were considered the outer setting.
Peer-to-peer implementation of this program was multi-pronged and began with BCC program leads presenting to healthcare teams and practitioners promoting the program. In this case, after the presentation to B-FHT, the BCC RRT program lead worked directly with the RRT at B-FHT to commence program implementation. This began with training providers (RRTs) through both an internal three-day intensive didactic training BCC training process along with the external CRE training requirement. Peer-training continues as patients are recruited and enrolled into the BCC program and new RRTs shadow existing RRTs (and vice versa as new RRTs take on new patients). Peer implementation is also happening concurrently for executive directors – who can reach out to current executive directors already running the BCC program as well as for physicians, who can call on BCC physicians and/or the specialist physician (respirologist) for support. Research is currently on-going exploring the peer-to-peer implementation process in greater detail.
Qualitative case study methodology was selected because it allows an in-depth exploration of a single selected entity or case [ 37 ]. Stake’s constructivist case study was chosen specifically because he advocates for the researcher’s active involvement in the case [ 38 ]. CFIR was used as a theoretical background to collect and analyze the data. CFIR has been used extensively in implementation and evaluation to become aware of influential factors, facilitate analysis, and organize the findings of an implementation [ 39 ].
Setting and participants
The BCC management program evaluated in this study was implemented within one FHT with five clinical sites in Southwestern Ontario. The FHT included different types of providers (physician, nurses, RRTs) and FHT staff working collaboratively to deliver healthcare and management education to patients. Implementation was evaluated at all sites as a single case, since providers interviewed worked across all five sites.
A convenience sample was used for recruitment with all having specific roles on the FHT. Participants included providers implementing BCC within the FHT (i.e., RRTs), providers referring patients to the BCC management program (primary care providers), and patients enrolled in the program (Table 2 ). Access to participants was granted through the FHT’s executive director. We relied on providers to assist in patient recruitment; recruitment remained ongoing throughout the course of data collection and analysis.
Data collection
Data were collected from a variety of qualitative sources which collectively contributed to the analysis to ensure that the individual, collective, and documented experiences of participants were obtained. Focus groups were conducted to gather the collective experience of the participants [ 40 ]. Provider and patient focus groups were conducted independently during sites visits. Observational field notes were taken during the site visits. The purpose of the site visits and field notes were to allow for substantiation of the data through triangulation, as well as to provide an element of reflexivity [ 38 ]. Data collection tools were guided by CFIR [ 24 ]; interview and focus group questions were built from CFIR as well as from expert opinion (i.e., those involved in the program delivery and implementation). Questions were considered and subsequently selected by the research team with the main goal of eliciting important information about implementation. All questions were piloted and used in previous research [ 41 ]. Final focus group and interview guides are available upon request.
One-on-one phone interviews with additional primary care providers who referred patients to the BCC program were conducted. The goal was to gather additional views about the implementation of the program from individuals working indirectly with the program.
Review of FHT documents such as memorandums of understanding, reports, and data sharing agreements produced contextual data that was primarily used to support analysis. Documents were accessed through the executive director of the site and the PCIC.
Throughout the entire research process, written reflexive notes were created by the researchers to ensure that the interviewer’s thoughts and assumptions could be incorporated during data analysis and interpretation [ 38 ].
Data analysis
To ensure a thorough understanding of the context of the site and data collected, an ongoing deductive coding strategy based on CFIR was used, supported by NVivo. Data were coded by 3 researchers (SP, SLS, SM) into related CFIR constructs and sub-constructs. To acknowledge and account for data that did not directly fit into CFIR more effectively (such as patient experience), inductive coding was also performed. Discrepancies were discussed and if there were multiple agreeable codes, segments of data were double-coded. Data categorization methods were performed as per Stake [ 38 ]‘s recommendations: direct interpretation and categorical aggregation. All analysis was discussed by the entire research team including a primary care physician. The key enabling constructs were identified as most important during the data analysis process due to being discussed most frequently by the participants and were determined to have a greater impact on implementation through member checking and research team discussion.
While implementation at the FHT took place over 5 sites, the implementation was viewed and evaluated as a single case. Implementation success was qualitatively assessed, using data collected from the provider and patient participants. Throughout data collection, participants reported a high level of satisfaction with the program implementation and delivery.
Field notes and collected documents were integrated into our analysis iteratively. This was done by deductively coding information related to implementation in a similar manner as described above. This additional data was then used in conjunction with focus group and interview data to identify facilitators and barriers to implementation. Participants received an interim report which was discussed during a focus group. Feedback was incorporated into our results. A member check (method of qualitative data triangulation) was conducted to explore the validity of our findings. Member checking involved returning to the implementation site after initial data collection and analysis for a follow-up visit. This allowed the researchers to confirm their interpretation of the data with the participants as well as ask additional questions.
In total, three focus groups (2 provider, 1 patient), n = 1 phone interview, and n = 1 key informant interview were conducted involving a total of 28 participants. n = 24 providers and n = 4 patients (Table 3 ). All FHT providers invited to participate took part in the study. Informed consent was obtained from all participants prior to any data collection. Response rate for the patients was unknown due to the recruitment of patients being performed by RRTs.
Factors affecting Implementation
Our results are presented according to the 5 main categories of CFIR, while incorporating results from the patient perspective and peer-to-peer implementation. Quotes are provided to illustrate our findings.
Intervention characteristics
Design quality and packaging.
The design quality and packaging of the program was discussed as a critical factor in the decision to adapt the model and its successful implementation. This included the presence of highly trained team members with experience implementing and delivering the BCC program as part of the peer-to-peer approach. They acted in a hands-on and advisory capacity during and following implementation. They trained individuals on how to execute the program as well as offered continued advice post-implementation.
When [the BCC Program Leads] came in, they knew what the expectations were, they knew what the outcomes would look like. They had that experience, where we were just fishing and hoping we would get the outcomes we were hoping for, but we didn’t really have the experience with that to confidently approach all those physician groups (Provider #3, Provider Focus Group #1) .
Participants believed previous program success translated to a smooth process for B-FHT staff in terms of program implementation. “Right away we were sold … it’s an easy sell because they… drop a program and a person attached to it in your lap. It is zero work” (Provider #2, Key Informant Interview #1). The participants further discussed the low complexity of the implementation. “Once… the patients were being seen, there’s not a lot of other admin, oversight really required. It’s the simplest honestly. So simple… everything just fell into place.” (Provider #2, Provider Focus Group #1). Providers elaborated on how they felt the implementation was done effectively and efficiently. “It seems really simple … it didn’t really disrupt … your everyday (Provider #1). If we could roll out every single program that way, it’d be great” (Provider #2, Provider Focus Group #1).
Providers felt the recruiting of patients into the program was smooth and effective. Patients concurred with this statement, saying: “[I] flowed right through [into the new program]” (Patient Focus Group #1). Even though patient awareness of the transition into the program was low; providers believed this facilitated implementation because it did not disrupt usual patient appointments. Providers appreciated being able to spend more time focusing on patient transition and less on other aspects of implementation.
Relative advantage
Providers discussed that prior to implementation of the BCC program, B-FHT had been unsuccessful in their attempts to create their own COPD management program. The relative advantage of the BCC program offered a successful and adaptable solution for their patients.
There [were] challenges. One, that there wasn’t an established program, for [the RRT] to mimic. And two … we are a multi-site organization, and with a 0.5 [full-time equivalent RRT] position it is really hard to establish any programming without a consistence presence. Which … just wasn’t possible (Provider #2, Key Informant Interview #1) .
Physician #1 echoed this explaining the advantage of having a comprehensive COPD specific care program in “free (ing) me up to focus on other things during appointments” (Phone Interview #1). Providers agreed this was a clear benefit of the program. The physician was confident in the abilities of the newly educated RRTs to provide COPD-specific care to the patients. As a result, they were able to focus their time on a patient’s other concerns and needs, allowing for more efficient use of time during appointments.
Patients also appreciated the coordinate afforded by the BCC program explaining how they prefer this program to alternatives they have previously experienced. “I’ve got a specialist that I’m not agreeing with and that’s not helping me, I might as well not even go to him. [The RRT in this program] is doing [more for me] than he is” (Patient #4, Patient Focus Group #1). From the patient’s view, the RRT was delivering better care for their COPD than was the specialist working external to the program.
Providers discussed the challenges of adding the program’s new reporting technology on top of existing technology. “They have their own system … I hate adding systems. That was the one thing probably that I really was not happy about … we have an [electronic medical record] (EMR). We’re seeing our patients but will be documenting in [the BCC’s system]” (Provider #2, Key Informant Interview #1).
Outer setting
Patient needs and resources.
Providers discussed the patient needs in the community as one of the reasons B-FHT proceeded with program implementation. “COPD was a problem. And COPD patients are complex, time-consuming, and costly. There’s plenty of patients and ongoing work to keep you busy full-time” (Provider #3, Provider Focus Group #1).
Cosmopolitanism
Participants described B-FHT’s cosmopolitanism efforts (i.e., efforts to collaboration with external organizations) to be instrumental in implementing the program. There was a shared agreement amongst providers about how “[the BCC’s] guidance was key for us being successful so quickly” (Provider #3, Provider Focus Group #1). In addition to working with the BCC program team, the B-FHT had the chance to learn from the PCIC and strengthen their coordinated efforts with the local hospital.
We actually built it to [be] part of one [program] to refer hospital discharges... with the COPD diagnosis... to automatically send a message to the RRT saying that, that person was discharged (Provider #5, Provider Focus Group #2) .
B-FHT had a pre-existing relationship with the local hospital, which had been responsible for performing diagnostic lung function testing (spirometry). The collaboration was challenged because the BCC program standard was for the RRT to complete the spirometry in the local B-FHT office. Transferring spirometry from the hospital to the B-FHT office was a concern because it meant shifting care away from the hospital. A compromise was reached allowing B-FHT to maintain the relationship with the hospital and respecting the requisite program fidelity.
Inner setting
Networks and communication.
In implementing BCC, a network of RRTs was created to enhance communication and facilitate a peer-to-peer approach. This was discussed as a key factor for implementation success because it facilitated information sharing across a broad context which informed practice. This peer-to-peer approach facilitated the training of providers within B-FHT and supported implementation of the program. “The RRTs have their own network where they communicate with each other” (Provider #6, Provider Focus Group #2). “It’s an opportunity for them to … say what’s working, what’s not working, what are they finding out there in the field. They [also] have a [messaging] group” (Provider #4, Provider Focus Group #2). The peer-to-peer approach strengthened communication between providers within the program. BCC Program leads were seen as a highly valued resource for providers. “[She is] always available if we run into any problems or have questions … [we] just reach out to her directly” (Provider #4, Provider Focus Group #2).
Occasionally, poor communication amongst leadership and providers acted as a barrier to implementation. One provider noted “if [meetings are announced] last minute or we forget… it’s just not going to be priority to move all our other appointments around … (Provider #7, Provider Focus Group #2). This was especially significant when the meetings included training or were meant to connect new providers.
Readiness for Implementation - available resources
Resource support was also discussed as a factor for successful implementation. Typically, RRTs were newly hired to support program delivery, however “[B-FHT] (used) their existing [RRT] to deliver [the BCC] model” (Provider #4, Provider Focus Group #2). This was both seen as a facilitator (i.e., using available resources) and as a barrier (i.e., requiring unlearning of existing, possibly hindering practices and habits). With the BCC program, the current RRT role was expanded into a full-time position, making it easier to implement the program in B-FHT’s multi-site clinical setting.
Administrative support including affirmation from senior management, secretarial/scheduling support, and chart audit support from the BCC leads was particularly important during the initial stages of implementation. Without this support, RRTs believed they would have had to spend excessive time doing administrative work rather than focusing on patient care. “If you’re rolling a program like this into a [FHT] office without a lot of allied health, those cold calls … for [a patient’s] first visit might be time-consuming if they didn’t have that support” (Provider #3, Provider Focus Group #1).
However, the providers discussed how data in the primary care EMR, distinct from the program electronic health record, was of poor quality. This hampered the ability for the program RRT to identify high risk individuals. Providers felt “better data in [the EMR] would’ve helped. But that’s not… likely or possible” (Provider #2, Provider Focus Group #1).
Characteristics of individuals
Self-efficacy.
Peer-to-peer implementation allowed providers to learn about the program and its intended implementation first-hand from experienced RRTs. Providers felt this increased their confidence in program delivery. “I really appreciate having people who are experts in COPD care that can give me recommendations. The more knowledge I start to feel comfortable with … in COPD in particular is because of [the RRT]” (Provider #3, Provider Focus Group #1).
Throughout focus group discussion, patients remarked how “you follow what [the RRT] says and [what] the doctor says and … my quality of life is better” (Patient #2, Patient Focus Group #1). This trust built between the providers and patients was important for implementation success. Patients reported they felt empowered to manage their care and talked about sharing that with peers and family members.
Knowledge and beliefs about the intervention
Providers and patients valued the program from the start. This buy-in of the program enhanced implementation. Providers and patients highly valued the RRT role and expressed many positive views about the RRTs: “If we could clone [the B-FHT RRT], that’s part of what has … made it so successful for us is that she was able to just come in” (Provider #2, Provider Focus Group #1).
Support from senior leadership was essential during the implementation process. The initial impetus to implement the program stemmed from collaboration between RRTs, however, the executive director of the site fully supported and actively facilitated implementation. Participants reported buy-in from senior leadership as a major facilitator to implementation. In addition, other primary care providers engaged with the program throughout the implementation process by learning about program offerings and supporting patient recruitment into the program.
Initially… we were reminded to refer any of our COPD patients for the [RRTs] to make sure that there was a demand. I would really emphasize the importance of frequent reminders to … everyone who would refer patients to the program, reminding them of what kinds of [patients] they can and should refer (Physician #1, Phone Interview #1) .
Providers praised the BCC’s intensive approach to early implementation. They explained how it resulted in buy-in from the start.
That initial, really strong blitz on talking to, providing the education to the physicians, speaking with the physician groups individually, getting the searches ready to go... It seemed like that period was probably short, but intense, and necessary (Provider #3, Provider Focus Group #1) .
Providers also reported the peer-to-peer approach enabled rapid implementation the program. Peer-to-peer training was conducted by an RRT and supported by a respirologist. Provider #1 said that she “sat with somebody who’d been doing it for twenty, five, and three years” respectively (Provider Focus Group #1). Provider #2 elaborated: “the training piece was also big … that training then does ensure that there’s a consistency in [program delivery]” (Provider Focus Group #1).
Reflecting and evaluating
In an effort to collect data on the implementation and performance of the BCC management program, feedback data was collected by B-FHT and the PCIC. Data was collected using patient satisfaction surveys as well as regular debriefing with stakeholders. This data was then used to facilitate adjustments to the implementation and execution of the program as needed.
When discussing the results of this data during reflection of the implementation process, many participants boasted at its success. “I don’t have a single criticism about the program. I really can’t think of how it could have been done better” (Provider #2, Key Informant Interview #1). “We always look at outcome measures, which are always really positive.” (Provider #3, Provider Focus Group #1). Providers explained that not only is patient satisfaction increasing but “hospital admissions had been decreased” (Provider #1, Provider Focus Group #1). When asked to provide advice to other teams considering implementing the program, a key informant said:
Take advantage of this program it is zero work on your end. They will come in and do everything and they will also return. If you are struggling at any point... having trouble identifying patients or... with physician buy-in, if you’re having process issues, they are happy to return... my only advice actually, is “say yes” (Provider #2, Key Informant Interview #1) .
Patients echoed provider’s positive views of the program and focused their conversation on the care they received from the program. Patients reflected on their own care and found value in their improved overall quality of life: “[The RRT] was very thorough … with their explanations of your puffers [and] your medication … [the RRT] gave you [advice]… I find it very good, helpful (Patient Focus Group #1).
This implementation case study was the pilot site to evaluate the opportunity for program spread to multiple sites across multiple regions. All of the CFIR constructs analyzed affected implementation, however five were determined as key enabling constructs (based on the frequency of their occurrence in participant comments and in documents) to consider when implementing a team-based chronic care program such as the BCC program: cosmopolitanism, networks and communication, engaging, design quality and packaging, and reflecting and evaluating.
CFIR constructs acting as barriers to be managed during implementation were also identified. These were: complexity (of the new patient reporting system); communication (between providers and management as well as between providers and specialists); and lack available resources (in this case, lack of quality data in the clinic-based primary care EMR).
In a systematic review, Kadu and Stolee [ 25 ] evaluated the implementation of chronic care management models in primary care settings. To do this, they used CFIR constructs to determine facilitators and barriers to implementation. Although within each of the 22 studies included there were many combinations of all 39 CFIR constructs affecting implementation, they identified seven constructs which had a meaningful effect on implementation across the studies included in their review. These were: networks and communication, culture, implementation climate, structural characteristics, engaging, executing, readiness for implementation, and knowledge and beliefs about the intervention [ 25 ]. Our analysis approach overlapped the Kadu and Stolee [ 25 ] review in three constructs: networks and communication, engaging, and knowledge and beliefs about the intervention. This is not to say the others were not important during the implementation of the BCC program, they simply did not appear frequently in our analysis. Our study shows, as others do, that it is a combination of multiple CFIR constructs which meaningfully affect successful implementation [ 24 ]. Although Kadu and Stolee [ 25 ] evaluated the implementation of chronic care management models in primary care settings, there were no studies among the 22 included that focused specifically on COPD. This may be one reason why only 3 of Kadu and Stolee [ 25 ]‘s primary constructs aligned with our finding. Our study adds to this work by providing insight into the implementation of a COPD-specific management program.

Key enabling CFIR constructs
The strong cosmopolitan relationship developed between B-FHT and the BCC leadership was supported by the evidence-based characteristic of the program. Participants could easily align cognitively and philosophically on evidence-based treatment standards. It also facilitated networking with external organizations. When implementing a chronic care management program, it is important to first consider collaboration with external organizations [ 1 ]. The established networks present with PCIC and other RRT networks gave B-FHT providers opportunities to collaborate and gain access to knowledge from a broad network of providers.
Literature on the implementation of chronic care models states that when a collaborative effort is made with external organizations, implementation and sustainability efforts are more effective [ 1 ] and factors such as communication, cohesion, and role primacy increase [ 42 ]. This finding is mimicked in recent works by Brown et al. [ 43 ] and Huang et al. [ 44 ]. In our case study, B-FHT’s partnership with the BCC program team acted as a key facilitator to implementation.
Networks and communication or information and communication as termed in Davy et al. [ 1 ] are facilitators within literature that were important in this case study. The BCC program in its initial stages created strong networks and communication which supported program delivery [ 45 ]. Enhancing communication among providers and establishing provider-specific networks are key components to facilitating the peer-to-peer implementation [ 46 ]. Kadu and Stolee [ 25 ] uphold that strong implementation efforts require established internal communication networks. This helps improve long-term sustainability, keep track of patients, and proactively notice gaps in service provision [ 1 ]. When information and communication systems are not in place or are insufficient, they can become a significant barrier to implementation [ 47 ].
Engaging champions in implementation efforts is a key factor to success [ 48 ]. Champions can help increase provider support through enthusiasm and support [ 48 ]. Participants identified champions within the PCIC, B-FHT management, and the RRT as peer leaders during implementation. When leadership is engaged, there is more likely to be support from other providers [ 1 , 25 ]. Alternatively, if leadership is not engaged, stakeholders may begin to lose interest [ 48 ].
The excellent design and packaging of the intervention positively influenced B-FHT’s decision to implement and supported the ease of implementation. The BCC structured program coupled with the support from BCC program team, the PCIC and other RRT networks infused quality within the whole implementation. Literature has shown that poor design quality or lack of attention to packaging can be a barrier to implementation [ 47 – 49 ]. Well-designed educational materials, such as those used by BCC, can also facilitating implementation by fostering engagement and increasing clarity [ 50 ].
In our study, evidence-based data, specifically regular reflecting on performance metrics and peer-to-peer feedback methods were used effectively to support implementation. Integrating regular monitoring and evaluation throughout program delivery can support implementation efforts [ 10 ]. Regular debriefing with stakeholders to allow for critical reflection and evaluation is also important and should be embedded early on in implementation [ 1 , 38 ]. Feedback systems that are used to support implementation can also work to support program sustainability [ 1 , 49 ].
Other CFIR factors affecting implementation
There were other CFIR constructs found to be supportive of implementation, but not necessarily as impactful as those already discussed. Complexity: When stakeholders believe an implementation is simple, the program is more easily implemented into practice [ 51 ]; overly complex programs or processes can impede the implementation of chronic care models [ 1 ]. In our case, the simplicity of implementation, attributed to the high level of support from the BCC program team, was a facilitator – often supporting other key constructs such as engagement and reflection. Patient Needs and Resources: There is consensus in the literature around the importance of considering context in implementation efforts - this should consider factors at multiple levels including patient, provider, team, organization, and community [ 1 , 24 , 51 – 53 ]. A systematic review by Davy et al. [ 1 ] described how implementation is enabled when providers believe their intervention helps their patients, rather than a change for change’s sake. The BCC program in our study addressed a clear and growing need for COPD-specific care in the B-FHT community. Relative Advantage: Providers believed the program had a relative advantage to what was currently being offered to patients; this, combined with the perceived need within their community, facilitated implementation. Our results echo the findings of Greenhalgh et al. and others [ 47 , 51 ] who have explained the importance of programs having a “clear, unambiguous advantage in either effectiveness or cost-effectiveness [as] more easily adopted and implemented” [ 51 p.594]. Readiness for Implementation – Available Resources: There is consensus in the literature that a lack of resources, or a misuse of available resources (ex. time, funding, and space) can hinder implementation [ 1 , 54 , 55 ]. In our study, the addition of a full-time RRT position made a meaningful difference in the overall provision of services. While the addition of resources can support implementation, it is important to ensure any resource is added or appropriate for the context [ 1 ]. Self-Efficacy/Knowledge and Beliefs about the Intervention: Ensuring providers possess the necessary skills to achieve implementation goals is essential [ 1 ]. BCC’s training helped build confidence and empowerment in providers, and the peer-to-peer approach facilitated buy-in to the possibilities of the program (i.e., positive outcomes for patients and providers) [ 43 ]. Low self-efficacy and high staff turnover can undermine the implementation process [ 56 , 57 ]. When providers feel more confident in their scope of practice, they can build trust and support implementation.
Peer-to-peer implementation
Literature describes peer-led education as a powerful approach to achieving program goals and objectives [ 26 – 28 ]. To our knowledge, using a peer-led approach to implementation has not been directly studied, however our case shows the potential for such an approach. In this case RRTs, as regulated health professionals with specialized COPD training as certified respiratory educators, worked directly with other RRTs in implementation and program delivery. Their professional-efficacy and commitment to program goals was amplified by regular peer-to-peer education and training. The providers mentioned how regular training sessions, along with the program lead’s availability throughout implementation, as essential in the success of program implementation and ultimately the program overall. The creation of the RRT network, which allowed RRTs to share emerging ideas and concerns about the program and their patients throughout and following implementation, was seen as a key component successful implementation. Positive views surrounding the program and the critical role of the RRTs during implementation were shared by all participants echoing the findings of Pfadenhauer et al. [ 58 ] who explained how key individuals who can be champions during an implementation can enhance overall program success.
Limitations
Our small sample size and in depth look at one case meant there was a potential for both social desirability bias and the Dunning-Kreuger effect (the belief that implementation was better than it actually was) [ 59 ]. The latter point is mitigated by positive outcomes reported in the seminal randomized control trial from which the spread initiative was launched [ 10 ]. Participants all worked together which may have affected their willingness to share experiences; however, we ensured there were multiple opportunities for feedback, including our formal member checking. We acknowledge our results are specific to this case study, however our standardized methodology and evaluation framework support our results can being interpreted in other contexts. Triangulation of data and member checking supported the rigor and trustworthiness of our data. Due to the fact this is a single case study, determination of important factors affecting implementation success was based on in-depth discussion with our research team and research participants; we also relied on inference from factors including the frequency with which particular barriers/facilitators were mentioned. Future research is ongoing examining the variability in implementation success across multiple sites and examining differences in facilitators at successful sites versus barrier’s unsuccessful sites. Even though the findings came from a single case study, our results will be useful in planning spread and implementation efforts of the BCC program at other sites and for others wanting to implement a chronic disease management program.
This study was conducted to understand the facilitators and barriers that affect the implementation of a chronic care management program for patients with COPD in a primary care context. Our aim was to determine enabling factors of implementation and spread of an interprofessional team-based primary care model to support future spread efforts. The five most influential constructs to implementation according to CFIR were cosmopolitanism, networks and communication, engaging, design quality and packaging, and reflecting and evaluating. Our results align with those from the literature including Kadu and Stolee [ 25 ]‘s systematic review using CFIR of factors affecting the implementation of chronic disease management programs. The successful implementation of the BCC program within B-FHT can be attributed to multiple factors. The program’s overall success was well regarded by both providers for its positive outcomes and by patients for the improvement in their COPD-specific care. Overall, CFIR was a suitable determinant framework for conducting our study. It provided a broad and useful set of constructs from which was able to determine factors affecting the implementation of the BCC management program. We also aimed to understand the peer-to-peer approach to implementation. This implementation was understood as vital to assist in communication, engagement, and self-efficacy of providers.
This study provides a practical example of the various factors that facilitate the implementation of the BCC management program. It also demonstrates the potential of using a peer-to-peer implementation strategy. Focusing on these factors will be useful for informing the continued spread and success of the BCC program and future implementation of other chronic care programs.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available in order to maintain the confidentiality of the participants but are available from the corresponding author on reasonable request.
Abbreviations
Chronic Obstructive Pulmonary Disease
Family Health Team
Consolidated Framework for Implementation Research
Electronic Medical Record
Registered Respiratory Therapist
Best Care COPD
Primary Care Innovation Collaborative
Davy C, Bleasel J, Liu H, Tchan M, Ponniah S, Brown A. Factors influencing the implementation of chronic care models: A systematic literature review. BMC Family Pract. 2015;16(1). [cited 2019 Feb 05] Available from: https://doi.org/10.1186/s12875-015-0319-5 DOI: https://doi.org/10.1186/s12875-015-0319-5 .
Yeoh E, Wong MC, Wong EL, Yam C, Poon C, Chung RY, et al. Benefits and limitations of implementing chronic care model (CCM) in primary care programs: A systematic review. Int J Cardiol. 2018;258:279–88. [cited 2019 Feb 01] Available from: https://doi.org/10.1016/j.ijcard.2017.11.057 .
Prevalence of chronic diseases among Canadian adults. Canada: Public Health Agency of Canada; 2019 Dec 9. 1 p.
Benady S. The human and economic burden of COPD: A leading cause of hospital admission in Canada. Ottawa: Canadian Thoracic Society; 2010.
Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young, B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–20. [cited 2019 Jan 19] Available from: https://doi.org/10.1056/nejmoa1003955 .
Stellefson M, Dipnarine K, Stopka C. The chronic care model and diabetes management in US primary care settings: A systematic review. Prev Chronic Dis. 2013;10. [cited 2019 Mar 09] Available from: https://doi.org/10.5888/pcd10.120180 DOI: https://doi.org/10.5888/pcd10.120180
Armstrong N, Herbert G, Brewster L. Contextual barriers to implementation in primary care: an ethnographic study of a programme to improve chronic kidney disease care. Fam. Pract. 2016;33(4):426–31. [cited 2019 Mar 09] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4957013/ DOI: https://doi.org/10.1093/fampra / cmw049.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(15):1909. [cited 2019 Apr 06] Available from: https://doi.org/10.1001/jama.288.15.1909 DOI: 10.1001/jama.288.14. 1775.
Garland-Baird L, Fraser K. Conceptualization of the chronic care model. Home Healthc Now. 2018 [cited 2019 Mar 09];36(6):379–85. Available from: https://doi.org/10.1097/nhh.0000000000000699 .
Ferrone M, Masciantonio MG, Malus N, Stitt L, O’Callahan T, Roberts Z, et al. The impact of integrated disease management in high-risk COPD patients in primary care. NPJ Prim. Care Respir. Med.. 2019;29(1). [cited 2019 Jan 01] Available from: https://doi.org/10.1038/s41533-019-0119-9 .
Kruis AL, Smidt N, Assendelft WJJ, Gussekloo J, Boland MRS, Mölken MR, et al. Integrated disease management interventions for patients with Chronic obstructive pulmonary disease. Cochrane Database Syst. Rev 2013;(10). [cited 2021 Feb 10] Available from: https://pubmed.ncbi.nlm.nih.gov/24108523/
GOLD Committee. Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, measurement, and prevention of chronic obstructive pulmonary disease (2021 Report). Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf
Khan A, Dickens AP, Adab P, Jordan RE. Self-management behaviour and support among primary care COPD patients: cross-sectional analysis of data from the Birmingham Chronic Obstructive Pulmonary Disease Cohort. npj Prim. Care Respir. Med. 2017;27:46. [cited 2021 Mar 4] Available from: https://pubmed.ncbi.nlm.nih.gov/28729620/
Lundell S, Tistad M, Rehn B, Wiklund M, Holmner Å, and Wadell K. Building COPD care on shaky ground: a mixed methods study from Swedish primary care professional perspective. BMC Health Serv Res. 2017;17, 467. [cited 2021 Mar 12] Available from: https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-017-2393-y#citeas
Ministry of Health and Long-Term Care. Family Health Teams. Canada: Ministry of Health and Long-Term Care / Ministère de la Santé et des Soins de longue durée; 2016. Available from: http://www.health.gov.on.ca/en/pro/programs/fht/
Nisbet G, Dunn S, Lincoln M, Shaw J. Development and initial validation of the interprofessional team learning profiling questionnaire. J Interprof Care. 2016;30(3):278–87. [cited 2020 Feb 14] Available from: https://doi.org/10.3109/13561820.2016.1141188 .
Rosser W, Colwill JM, Kasperski J, Wilson L. Progress of Ontario's family health team model: A patient-centered medical home. Ann. Fam. Med. 2011;9(2):165–71. [cited 2019 Jan 07] Available from: https://doi.org/10.1370/afm.1228 DOI: https://doi.org/10.1370/afm.1228 .
Statistics Canada. Population estimates on July 1 st , by age and sex. Canada: Statistics Canada; 2021 03 15. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501&pickMembers%5B0%5D=1.7&pickMembers%5B1%5D=2.1&cubeTimeFrame.startYear=2016&cubeTimeFrame.endYear=2020&referencePeriods=20160101%2C20200101
Wensing M. Implementation science in healthcare: Introduction and perspective. Z Evid Fortbild Qual Gesundhwes. 2015 ;109(2):97–102. [cited 2019 Jan 05]Available from: https://doi.org/10.1016/j.zefq.2015.02.014 DOI: https://doi.org/10.1016/j.zefq.2015 .02.014.
Nilsen, P. Making sense of implementation theories, models and frameworks. Imp Sci. 2015;10(1). [cited 2020 May 19] Available from: https://doi.org/10.1186/s13012-015-0242-0 DOI: https://doi.org/10.1186/s13012-015-0242-0 .
Kitson A, Harvey G, McCormack B. Enabling the implementation of evidence based practice: A conceptual framework. Qual Safety in HCare 1998 ;7(3):149–158. [cited 2020 Jan 07] Available from: https://doi.org/10.1136/qshc.7.3.149 .
Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Imp Sci. 2012;7(1). [cited 2020 Jan 07] Available from: https://doi.org/10.1186/1748-5908-7-37 .
Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder, L. A systematic review of the use of the consolidated framework for implementation research. Imp Sci. 2016;11(1). [cited 2019 mar 09]Available from: https://doi.org/10.1186/s13012-016-0437-z DOI: https://doi.org/10.1186/s13012-016-0437-z .
Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Imp Sci. 2009;4(1). [cited 2019 mar 09] Available from: https://doi.org/10.1186/1748-5908-4-50 .
Kadu MK, Stolee P. Facilitators and barriers of implementing the chronic care model in primary care: A systematic review. BMC Fam Pract. 2015;16(1). [cited 2019 Feb 05] Available from: https://doi.org/10.1186/s12875-014-0219-0 DOI: https://doi.org/10.1186/s12875-014-0219-0 .
Aimola L, Jasim S, Tripathi N, Tucker S, Worrall A, Quirk A, et al. Impact of peer-led quality improvement networks on quality of inpatient mental health care: Study protocol for a cluster randomized controlled trial. BMC Psychiatry. 2016;16(1). [cited 2020 Feb 19] Available from: https://doi.org/10.1186/s12888-016-1040-1 DOI: https://doi.org/10.1186/s128880161040 .
Pronovost PJ, Hudson DW. Improving healthcare quality through organisational peer-to-peer assessment: Lessons from the nuclear power industry. BMJ Qual Saf. 2012;21(10):872–75. [cited 2020 Feb 19] Available from: https://doi.org/10.1136/bmjqs-2011-000470 DOI: https://doi.org/10.1136/bmjqs-2011-000470 .
Walpola RL, McLachlan AJ, Chen TF. A scoping review of peer-led education in patient safety training. Am J Pharm Educ. 2018 ;82(2):6110. [cited 2020 Feb 19] Available from: https://doi.org/10.5688/ajpe6110 DOI: https://doi.org/10.5688/ajpe6110 , A Scoping Review of Peer-led Education in Patient Safety Training.
Bennett D, O’Flynn S, Kelly M. Peer assisted learning in the clinical setting: An activity systems analysis. Adv Health Sci Educ Theory Pract. 2015;20(3):595–610. [cited 2020 Feb 20] Available from: https://doi.org/10.1007/s1045 9-014-9557-x DOI: https://doi.org/10.1007/s10459-014-9557-x .
Roberts D. Friendship fosters learning: The importance of friendships in clinical practice. Nurse Educ Pract. 2009;9(6):367–71. [cited 2020 Feb 21] Available from: https://doi.org/10.1016/j.nepr.2008.10.016 DOI: https://doi.org/10.1016/j.nepr.2008.10.016 .
Clini E, Castaniere I, Tonelli R. Looking for a chronic care model in COPD patients. Eur Respir J. 2017;51(1):1702087. [cited 2019 Mar 01] Available from: https://doi.org/10.1183/13993003.02087-2017 DOI: https://doi.org/10.1183/13993003.02087-2017 .
Primary Care Innovation Collaborative Report. 2019. Best Care Chronic Obstructive Pulmonary Disease Program for Primary Care.
Best Care Chronic Obstructive Pulmonary Disease Report. 2020. Primary Care Innovation Collaborative.
Canadian Network for Respiratory Care. Certification for Asthma, Respiratory, COPD and Tobacco Educators. Canada: Canadian Network for Respiratory Care; n.d.. [cited 2021 Apr 30] Available from: http://cnrchome.net/certification.html
Health Quality Ontario. Chronic Obstructive Pulmonary Disease – Care in the Community for Adults. 2018. Availale from: https://www.hqontario.ca/evidence-to-improve-care/quality-standards/view-all-quality-standards/chronic-obstructive-pulmonary-disease
Kondro W. Family physicians urged creation of ‘medical homes’ for every Canadian. CMAJ. 2009 ;181(12). [cited 2021 Apr 30] Available from: https://www.cmaj.ca/content/181/12/885
Abma TA, Stake RE. Science of the particular: An Advocacy of Naturalistic Case Study in Health Research. Qual Health Res. 2014 ;24(8):1150–61. [cited 2019 Feb 15] Available from: https://doi.org/10.1177/1049732314543196 DOI: https://doi.org/10.1177/1049732314543196 .
Stake RE. The art of case study research. USA: SAGE; 1995.
Breimaier HE, Heckemann B, Halfens RJ, Lohrmann C. The consolidated framework for implementation research (CFIR): A useful theoretical framework for guiding and evaluating a guideline implementation process in a hospital-based nursing practice. BMC Nurs. 2015;14(1). [cited 2019 Jan 08] Available from: https://doi.org/10.1186/s12912-015-0088-4 DOI: https://doi.org/10.1186/s12912-015-0088-4 .
Litosseliti L. Using focus groups in research. London: A&C Black; 2003.
Google Scholar
Sibbald SL, Ziegler BR, Maskell R, Schouten K. Implementation of interprofessional team-based care: A cross-case analysis. J of Interprof Care. 2020 :1-8. [cited 2021 Apr 24] Available from: https://doi.org/10.1080/13561820.2020.1803228 , 1, 8.
Bauer MS, Miller CJ, Kim B, Lew R, Stolzmann K, Sullivan J, et al. Effectiveness of implementing a collaborative chronic care model for clinician teams on patient outcomes and health status in mental health. JAMA Netw Open. 2019;2(3);e190230. [cited 2019 Feb 17] Available from: https://doi.org/10.1001/jamanetworkopen.2019.0230 DOI: https://doi.org/10.1001/jamanetworkopen.2019.0230 .
Brown CH, Kellam SG, Kaupert S, Muthén BO, Wang W, Muthén LK, et al. Partnerships for the design, conduct, and analysis of effectiveness, and implementation research: Experiences of the prevention science and methodology group. Adm Policy Ment Health. 2012;39(4):301–16. [cited 2019 Feb 17] Available from: https://doi.org/10.1007/s10488-011-0387-3 DOI: https://doi.org/10.1007/s10488-011-0387-3 .
Huang K, Kwon SC, Cheng S, Kamboukos D, Shelley D, Brotman LM, et al. Unpacking partnership, engagement, and collaboration research to inform implementation strategies development: Theoretical frameworks and emerging methodologies. Front Public Health. 2018;6. [cited 2019 Feb 17] Available from: https://doi.org/10.3389/fpubh.2018.00190 DOI: https://doi.org/10.3389/fpubh.2018.00190 , 6
Sibbald S, Schouten K, Sedig K, Maskell R, Licskai, C. Key Characteristics and Critical Juctures for Successful Interprofessional Networks in Healthcare – A case study. 2020 [cited 2020 Jul 17].
Kim CR, Free C. Recent evaluations of the peer-led approach in adolescent sexual health education: A systematic review. Perspect Sex Reprod Health. 2008 40(3):144–51. [cited 2020 Feb 19] Available from: https://doi.org/10.1363 /4014408 DOI: https://doi.org/10.1363/ifpp.34.0089.08 .
Weir NM, Newham R, Dunlop E, Bennie M. Factors influencing national implementation of innovations within community pharmacy: A systematic review applying the consolidated framework for implementation research. Imp Sci. 2019;14(1). [cited 2020 Apr 16] Available from: https://doi.org/10.1186/s13012-019-0867-5 DOI: https://doi.org/10.1186/s13012-019-0867-5 .
Hagedorn HJ, Wisdom JP, Gerould H, Pinsker E, Brown R, Dawes M, et al. Implementing alcohol use disorder pharmacotherapy in primary care settings: A qualitative analysis of provider-identified barriers and impact on implementation outcomes. Addict Sci Clin Pract. 2019;14(1). [cited 2020 Apr 12] Available from: https://doi.org/10.1186/s13722-019-0151-7 DOI: https://doi.org/10.1186/s13722-019-0151-7 .
Stevenson L, Ball S, Haverhals LM, Aron DC, Lowery J. Evaluation of a national telemedicine initiative in the Veterans Health Administration: Factors associated with successful implementation. J Telemed Telecare. 2018;24(3):168–78. [cited 2020 Apr 12] Available from: https://doi.org/10.1177/1357633x16677676 DOI: https://doi.org/10.1177/1357633X16677676 .
King ES, Moore CJ, Wilson HK, Harden SM, Davis M, Berg AC. Mixed methods evaluation of implementation and outcomes in a community-based cancer prevention intervention. BMC Public Health. 2019;19(1). [cited 2020 Apr 11] Available from: https://doi.org/10.1186/s12889-019-7315-y DOI: https://doi.org/10.1186/s12889-019-7315-y .
Greenhalgh T, Robert G, MacFarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: Systematic review and recommendations. Milbank Q. 2004;82(4):581–629. [cited 2020 May 28] Available from: https://doi.org/10.1111/j.0887-378x.2004.00325.x DOI: https://doi.org/10.1111/j.0887-378X.2004.00325.x .
Aarons GA, Green AE, Palinkas LA, Self-Brown S, Whitaker DJ, Lutzker JR, et al. Dynamic adaptation process to implement an evidence-based child maltreatment intervention. Imp Sci. 2012 [cited 2020 Jan 15];7(1). Available from: https://doi.org/10.1186/1748-5908-7-32 DOI: 10.1186/17485908732.
Ault-Brutus A, Lee C, Singer S, Allen M, Alegría M. Examining implementation of a patient activation and self-management intervention within the context of an effectiveness trial. Adm Policy Ment Health. 2014 [cited 2019 Feb 14];41(6):777–87. Available from: https://doi.org/10.1007/s10488-013-0527-z DOI: https://doi.org/10.1007/s104880130527z .
Uvhagen H, Hasson H, Hansson J, Von Knorring M. Leading top-down implementation processes: A qualitative study on the role of managers. BMC Health Serv Res. 2018;18(1). [cited 2020 May 01] Available from: https://doi.org/10.1186/s12913-018-3360-y DOI: https://doi.org/10.1186/s12913-018-3360-y .
Yapa HM, Bärnighausen T. Implementation science in resource-poor countries and communities. Imp Sci. 2018;13(1). [cited 2020 May 01] Available from: https://doi.org/10.1186/s13012-018-0847-1 .
Feifer C, Ornstein SM, Nietert PJ, Jenkins RG. System Supports for Chronic Illness Care and Their Relationship to Clinical Outcomes. Top Health Inf Manage. 2001;22(2):65–72. [cited 2020 Apr 18] Available from: https://pubmed.ncbi.nlm.nih.gov/11761794/
Feifer C, Mora A, White B, Barnett BP. Challenges to improving chronic disease care and training in residencies. Acad Med. 2006;81(8):696–701. [cited 2020 Apr 18] Available from: https://doi.org/10.1097/00001888-200608000-00004 DOI: https://doi.org/10.1097/00001888-200608000-00004 .
Pfadenhauer LM, Gerhardus A, Mozygemba K, Lysdahl KB, Booth A, Hofmann B, et al. Making sense of complexity in context and implementation: The context and implementation of complex interventions (CICI) framework. Imp Sci. 2017;12(1). [cited 2020 Apr 16] Available from: https://doi.org/10.1186/s13012-017-0552-5 .
Kruger J, Dunning D. Unskilled and unaware of it: How difficulties in recognizing one’s own incompetence lead to inflated self-assessments. J Personality and Psych. 1999;77(6). [cited 2020 Nov 18] Available from: https://doi.apa.org/doiLanding?doi=10.1037%2F0022-3514.77.6.1121
Download references
Acknowledgements
We would like to acknowledge the participants in this study. Thank you to Dr. Judy Belle Brown, Dr. Sayra Cristancho and the entire research team for your guidance throughout this process. Thank you especially to Dr. Shiraz Malik for your assistance during the data analysis process. Thank you also to Alvina Asif Jiwani who assisted with referencing for this manuscript.
This study was funded through a 2-year grant from Lawson Research. Lawson Research had no role in the design of the study, data collection, data analysis, or preparation of the manuscript.
Author information
Authors and affiliations.
Health and Rehabilitation Sciences, Western University, London, Canada
Stefan Paciocco
School of Health Studies, Faculty of Health Sciences, Western University, London, Canada
Anita Kothari
Department of Family Medicine, Schulich School of Medicine and Dentistry, Western University, London, Canada
Christopher J. Licskai
RRT-CRE, Asthma Research Group Inc., London, Canada
Madonna Ferrone
School of Health Studies, Faculty of Health Sciences, Department of Family Medicine, Schulich School of Medicine and Dentistry, The Schulich Interfaculty Program in Public Health, Schulich School of Medicine and Dentistry, Western University, London, Canada
Shannon L. Sibbald
You can also search for this author in PubMed Google Scholar
Contributions
SP was the graduate student during the research process. SP was lead interviewer during focus groups and interviews. SP performed all of the initial deductive and inductive coding and drafted the first version of the manuscript. AK was a member of the advisory committee and provided advice on the scope and direction of the research. AK reviewed the manuscript during writing, provided feedback and made edits throughout the entire process. SLS supported data collection and analysis. SLS created field notes during focus groups. SLS was involved in the deductive and inductive coding of the data. SLS contributed to the writing of the manuscript. CJL an MF made substantial contributions to the development of the BCC program as members of the PCIC, supported data collection at B-FHT sites, and in the interpretation of data for the work. CJL and MF reviewed the manuscript critically for important intellectual content. By design, CJL and MF did not participate in the direct acquisition, coding, or primary interpretation of the data. All authors read and approved the final manuscript.
Authors’ information
Stefan Paciocco 1 , MSc; Anita Kothari 2 , PhD; Christopher J. Licskai, MD 3 ; Madonna Ferrone 4 ; Shannon L. Sibbald 2,3,5 , PhD.
Health and Rehabilitation Sciences -Western University 1 , School of Health Studies, Faculty of Health Sciences - Western University 2 , Department of Family Medicine, Schulich School of Medicine and Dentistry 3 , Asthma Research Group Incorporated 4 , The Schulich Interfaculty Program in Public Health, Schulich School of Medicine and Dentistry 5 .
When doing constructivist research, we must have an active personal role in data collection and analysis. This will be done by stating preconceived notions and assumptions and discussing how they affected the interpretation of the data. SLS had worked alongside the PCIC in the past and knew their program and implementation strategy well. The initial iteration of the program was evaluated and deemed a notable success. As a result, SP possessed an understanding that the BCC management program was a successful program. Although SP found the majority of results to be positive, on occasion, he may have been more likely to code in a positive manner rather than neutral or negative. Therefore, there may exist other interpretations of which the researchers were not immediately aware of which may have been understood by another researcher unaware of the program’s successful origins.
Corresponding author
Correspondence to Shannon L. Sibbald .
Ethics declarations
Ethics approval and consent to participate.
The Western University Ethics and Review Board granted ethics approval for this study (Project Number: 108415). Additionally, Lawson Research funded this project, and we were required to submit a Research Database Application (ReDA ID: 6416), which was also approved. As this research involved human participants and human data, all research was performed in accordance with the Declaration of Helsinki. Informed consent to participate was obtained from each participant including the executive director through the reading, explanation, and signing of a letter of information and informed consent for participation and publication.
Consent for publication
Informed consent for publication was included in the letter of information and informed consent forms signed by all participants including the executive director of B-FHT. The letter of information and informed consent form signed by participants were obtained for informed consent for both participation and publication. No information was published identifying an individual person, therefore not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Consolidated Framework for Implementation Research Constructs. This document provides an organized list including the categories and constructs of the consolidated framework for implementation research, as well as a short definition for each construct.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Paciocco, S., Kothari, A., Licskai, C.J. et al. Evaluating the implementation of a chronic obstructive pulmonary disease management program using the Consolidated Framework for Implementation Research: a case study. BMC Health Serv Res 21 , 717 (2021). https://doi.org/10.1186/s12913-021-06636-5
Download citation
Received : 17 March 2021
Accepted : 14 June 2021
Published : 21 July 2021
DOI : https://doi.org/10.1186/s12913-021-06636-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chronic obstructive pulmonary disease
- Implementation science
- Implementation evaluation
- Consolidated framework for Implementation research
- Chronic disease management
- Primary healthcare
- Interprofessional teams
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Data Descriptor
- Open access
- Published: 06 June 2024
Multi-center Korean cohort study based on RNA-sequencing data targeting COPD patients
- Minseok Seo ORCID: orcid.org/0000-0002-5364-7524 1 ,
- Sinwoo Park 1 ,
- WooJin Kim 2 ,
- Ji Ye Jung 3 ,
- So Hyeon Bak 4 ,
- Edwin K. Silverman 5 &
- Jinkyeong Park ORCID: orcid.org/0000-0002-8833-9062 6
Scientific Data volume 11 , Article number: 593 ( 2024 ) Cite this article
40 Accesses
Metrics details
- Respiratory tract diseases
In 2023, WHO ranked chronic obstructive pulmonary disease (COPD) as the third leading cause of death, with 3.23 million fatalities in 2019. The intricate nature of the disease, which is influenced by genetics, environment, and lifestyle, is evident. The effect of air pollution and changes in atmospheric substances because of global warming highlight the need for this research. These environmental shifts are associated with the emergence of various respiratory infections such as COVID-19. RNA sequencing is pivotal in airway diseases, including COPD, as it enables comprehensive transcriptome analysis, biomarker discovery, and uncovers novel pathways. It facilitates personalized medicine by tracking dynamic changes in gene expression in response to various triggers. However, the limited research on East Asian populations may overlook the unique nuances of COPD development and progression. Bridging this gap and using peripheral blood samples for systemic analysis are crucial for comprehensive and globally applicable COPD diagnosis and treatment.
Similar content being viewed by others

Single-cell and spatial transcriptomics analysis of non-small cell lung cancer

Identifying proteomic risk factors for cancer using prospective and exome analyses of 1463 circulating proteins and risk of 19 cancers in the UK Biobank

Multiomic analyses uncover immunological signatures in acute and chronic coronary syndromes
Background & summary.
In 2023, the World Health Organization (WHO) released a comprehensive report affirming that chronic obstructive pulmonary disease (COPD) has ascended to rank as the third foremost contributor to global mortality 1 , characterized by a heterogeneous lung condition manifested as chronic respiratory symptoms (dyspnea, cough, expectoration, and exacerbations) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction 2 . The report revealed that COPD accounted for a staggering tally of approximately 3.23 million fatalities in 2019 3 . This multifaceted disease arises from intricate interactions among genetic predisposition, environmental exposure, and lifestyle factors, leading to diverse clinical manifestations and variable treatment responses 4 . The current era, fraught with global infectious threats, such as coronavirus disease 2019 (COVID-19), indicates the urgency of comprehending the underlying pathophysiology of COPD, especially considering its heightened vulnerability to infections 5 .
In the landscape of COPD research, RNA sequencing (RNA-seq) from peripheral blood samples provides a critical lens through which we can view the systemic nature of the disease 6 , 7 . This approach aligns with our aim to understand COPD beyond its primary lung involvement, as it manifests systemic inflammation and immune responses that are detectable and quantifiable in blood 8 . By analyzing peripheral blood, our study captures these systemic changes, offering insights into COPD’s impact on overall health, which is especially relevant for East Asian populations exposed to unique environmental factors like biomass 9 , 10 and post-tuberculosis sequelae 11 .
Our comprehensive RNA-seq analysis encompasses an extensive range of genetic expressions from protein-coding genes to noncoding RNAs and alternative splicing events, uncovering potential biomarkers and pathways critical for disease progression and response to therapies. Blood samples, which reflect systemic physiological responses to COPD, are less invasive and more accessible for patients, making them a valuable tool for broad epidemiological studies and personalized medicine. Through this method, we aim to address the lack of research in East Asian populations whose COPD characteristics may diverge from the patterns seen in Western cohorts due to distinct genetic and environmental influences.
Specifically, our study focuses on uncovering novel molecular signatures within blood that could indicate COPD in East Asians, facilitating early detection and monitoring of the disease. Identifying such biomarkers could lead to the development of targeted interventions and contribute to the nuanced understanding needed for personalized treatment strategies. Our findings reveal the roles of particular non-coding RNAs and gene expression changes related to COPD’s systemic effects, emphasizing the potential of RNA-seq in blood samples to inform targeted therapeutic interventions.
We are pioneering this inclusive approach to COPD research, which not only advances our understanding of the disease’s management and treatment but also fosters a framework for precision medicine. Considering the unique clinical and environmental context of East Asian populations, our work aims to ensure that COPD research reflects the diversity of patient experiences and meets the global health challenge with tailored solutions.
A list of abbreviations can be found in Table 1 .
Ethics statement
This study was approved by the Institutional Review Board of Dongguk University Ilsan Hospital (DUIH 2020-04-012), Kyung Hee University Hospital at Gangdong (KHNMC 2022-03-063-008), Kangwon University Hospital (KNUH 2012-06-007), and Severance Hospital, Yonsei University College of Medicine (YUHS 4-2021-064). Prior to sample preparation, all samples were anonymized by assigning patient ID numbers. This study was performed in adherence to the Declaration of Helsinki.
Patients and acquisition of blood samples
This study was designed to compare characteristics and clinical outcomes using radiological findings and blood transcriptome in prospective COPD cohorts from three different regions: Seoul (YUHS and KHNMC), Gyeonggi province (DUIH), and Kangwon province (KNUH) in the Republic of Korea (Fig. 1a ). Seoul is an urban region, Gyeonggi Province is a suburban region, and Kangwon Province is a rural area in Korea. Patients with a smoking history of more than 10 pack-years or who visited a respiratory outpatient clinic with chronic bronchitis were prospectively recruited since 2020. Patients with the following conditions were excluded from this study: (1) < 18 years of age, (2) had undergone a tracheostomy or experienced difficulty performing a pulmonary function test, (3) pregnant women, (4) previous history pulmonary resection, (5) suspected lung cancer or undergoing chemotherapy, (6) recently undergone ophthalmic surgery or been recently hospitalized for acute coronary artery disease, and (7) received radiation therapy to the thorax. Total blood RNA was collected from all participants after obtaining written informed consent to participate in the study and for their RNA data to be shared in a repository. Data on demographics, spirometry findings, imaging findings, smoking burden, respiratory symptoms and comorbidities were collected. Total RNA was collected using PAXgene™ Blood RNA tubes and extracted from all 294 samples with the Qiagen PreAnalytiX PAXgene Blood Kit (Qiagen, Valencia, CA) to ensure consistency in RNA extraction.

Schematic diagram to collect RNA-seq data from multi-centers and multi-platforms. (a) Schematic diagram of the overall study progress. (b) Diagram of the sample size and the experimental batches of the collected patients and the RNA-seq data.
Library preparation and sequencing
A total of 294 blood samples were sequentially RNA-sequenced once in the order in which blood was collected at the three hospitals (Fig. 1b ). This was a prospective study in which patients visiting three hospitals were enrolled; thus, we conducted sequencing at seven different time points (Phase 1 to Phase 7). Two sequencing platforms were employed: the Illumina NovaSeq-6000 for Phases 1 to 5 and the MGISeq-2000 for Phases 6 to 7. This strategic choice aimed to broaden our data range and mitigate any bias specific to a single sequencing platform. A rigorously standardized protocol across RNA extraction and library preparation stages ensured the fidelity and comparability of our sequencing data.
The initial step of RNA extraction was meticulously conducted across all samples using the TRI-reagent-based method (QIAZOL Lysis Reagent, Qiagen, Germany), ensuring efficient and reproducible isolation of total RNA. RNA concentration and integrity were measured with the Quant-iT RiboGreen assay (Invitrogen, #R11490) and Agilent TapeStation RNA screentape (Agilent, #5067-5576), advancing only samples with an RNA integrity number (RIN) of 6.0 or higher to library construction to maintain high-quality standards for sequencing.
The library preparation for Illumina-based sequencing followed the TruSeq Stranded Total RNA protocol, leveraging the Ribo-Zero Human kit (Illumina, Inc., San Diego, CA, USA) for thorough rRNA depletion, enriching the libraries for a comprehensive array of coding and non-coding RNA species. The protocol included an initial RNA fragmentation, followed by first-strand cDNA synthesis using SuperScript II (Invitrogen, #18064014). Strand specificity was maintained in the subsequent synthesis of the second cDNA strand by incorporating dUTP instead of dTTP. Akin to this procedure, the MGISeq-2000 library preparation maintained identical steps, employing the MGIEasy RNA Directional Library Prep Set for rRNA depletion, followed by the same fragment size distribution, reverse transcriptase enzymes, and primers to preserve the strand orientation of the RNA.
Subsequent steps for both platforms—including “A” base addition, adapter ligation, and PCR enrichment—were conducted per the established Illumina protocol. Quality control for the final library involved using the same KAPA Library Quantification kits and Agilent TapeStation D1000 ScreenTape to quantify and validate the libraries, confirming uniform quality across both platforms.
Sequencing on the MGISeq-2000 employed 150-bp paired-end reads, mirroring those of the NovaSeq, to directly compare data between the platforms. Such meticulous parallelism in read length and sequencing depth between NovaSeq and MGISeq enabled us to seamlessly integrate and compare datasets, enhancing our findings’ reliability and the transcriptomic landscape’s interpretability.
RNA-seq data processing
FastQC v.0.11.9 12 was used to confirm the base quality of the raw reads generated by RNA-seq. Trimmomatic (v0.39) was used to remove poor bases and adapter sequences from the raw reads. The clean reads were aligned to the Grch38 human reference genome derived from the Ensembl Genome Database using Hisat2 (v2.2.1) 13 . Alignment results were recorded in sorted BAM format using Samtools view (v1.14), and mapping-related statistics were collected using Samtools stats and Hisat2 outcomes. Mapped reads were quantified using featureCounts (v2.0.1) 14 according to the Ensembl gene annotation. All quality indicators for technical validation were organized and visualized using R software (v4.1.3). Statistical analysis was performed to identify differentially expressed genes (DEGs) using limma Voom (v3.56.2) 15 . In a statistical hypothesis test to find differentially expressed genes according to recent smoking status (Current vs Former/Never smokers), in addition to the main effect (current smoking status), seven different sequencing batches, gender, age, height, and weight, were considered as covariates. Similarly, in the analysis of differentially expressed genes between two different sequencing platforms (Nova-seq and MGI-seq), modeling was performed with the same covariate correction in addition to the two sequencing platform information as the main fixed effect. In this study, the false discovery rate (FDR) adjustment method was used to correct for multiple testing problems 16 , and a 5% significance level was considered as the cutoff.
Data Records
Raw and preprocessed RNA-seq data were deposited in the GEO database under the accession number GSE24065615 17 . Quality control data, preprocessed data, clinical phenotypes, and R codes can be found on figshare 18 .
Technical Validation
In our investigation of the COPD patient cohort, we performed a rigorous quality control analysis on RNA-seq data sourced from blood samples of 294 individuals. These patients were selected based on their proximity to cement factories and coal mines in South Korea, smoking history, or exposure to other environmental pollutants. A total of 294 patients were enrolled in the study, with 83.3% reporting a smoking history averaging 26.7 pack-years. These details, along with other demographic information such as age and sex distribution, which mirrors established COPD cohorts, are presented in Table 2 . The mean age was 68.9 years with a majority being male (79.6%). This demographic breakdown supports the comparability of our study with existing literature 19 , 20 . Pulmonary function tests indicated that 54.4% of patients experienced airflow limitations, with 16.6% displaying moderate-to-severe airflow limitation, reflected by an average FEV1 of 2.14 liters. A summary of these clinical findings is provided in the Table 2 , which includes the COPD status and spirometry results.
Each participant’s sample, subjected to sequencing, met strict quality standards: RIN greater than 6 and a minimum concentration of 25 μg/ul, ensuring the integrity of our RNA data. The sequencing, detailed in Fig. 1 , was conducted on two platforms, NovaSeq and MGI-seq, with each sample being sequenced once to eliminate the possibility of batch effects and ensure consistent data. The sequencing was methodically phased into seven parts, corresponding with the sample collection order, to maintain RNA integrity and reduce degradation risks.
The average number of raw reads generated across the entire RNA-seq dataset was 68,610,798 reads per sample (range: 43,812,379–141,160,930 reads) (Fig. 2a ). Considering that the recommended number of reads in RNA-seq research is over 20 million 21 , 22 , we confirmed that a sufficient number of raw reads were generated across all samples. For technical verification of the generated raw reads, we checked their basic characteristics using fastQC software. A review of the generated reads’ GC ratio confirmed no special sequencing GC bias at 47.724% across batches, which aligns with the expected genomic range and shows no particular sequencing bias (Fig. 2b ). It is widely known that the percentage of GC in generated reads should ideally be close to 50% to minimize sequencing bias. Using the Phred score, an index that can intuitively indicate sequencing quality, we confirmed that all samples had a base call accuracy of ≥99.9%, which was 35.883, reflecting the high quality of our sequencing data (Fig. 2c ). We found that “N” contained an average of 0.01%, and a slightly higher ratio was observed in the MGI-seq platform (Fig. 2d ).

Quality control of raw reads generated through RNA-sequencing. In all plots, colors represent different sequencing batches. (a) Number of raw generated reads across all samples. (b) G/C ratio per each sequencing batch. (d) Phred scores of raw reads generated from each sequencing batch. (d) Proportion of unsequenced bases among total raw reads generated from each sequencing batch. (e) Illumina’s universal adapter discovery rate per each sequencing batch.
When examined for inclusion of universal adapters from Illumina based on the FastQC tool, adapter sequences were detected only in samples included in phases 1 to 4 generated using the Illumina sequencing platform. As expected, no Illumina universal adapter seuqence was detected in samples from stages 5 to 7 sequenced via MGI-seq, indicating that all samples were sequenced as planned without mislabeling issues (Fig. 2e ). Particularly noteworthy was the high quality of reads in phase 2, as highlighted in Fig. 3a and b . While the reasons for the elevated quality in this phase are multifaceted—ranging from variations in sample collection to operational shifts in the sequencing facilities—these did not impact the overall integrity of our research findings. Our rigorous QC standards across all phases ensured data reliability and robustness, and the minor variations observed did not influence the conclusions of our research.

Statistics of cleaned reads after quality control process through Trimmomatic. In all plots, colors represent different sequencing batches. (a) Proportion of reads that passed the filtering process among all raw reads. (b) Ratio of only forward reads among paired-end reads passing the filtering process. (c) Ratio of only reverse reads among paired-end reads passing the filtering process. (d) Number of clean reads that passed the filtering process across the entire samples.
After completing the technical validation of the raw reads generated through RNA-seq, we proceeded to remove adapter sequences that might be attached to the ends of the reads and eliminate reads of poor quality using the Trimmomatic software. On average, 98.259% of the raw reads successfully passed through this filtering process (Fig. 3a ). Among the paired-end reads, only forward reads survived at a rate of 0.013% (Fig. 3b ), and only reverse reads survived at a rate of 0.003% (Fig. 3c ) across all the samples. Due to their very low numbers, these surviving reads were excluded from the subsequent alignment step. We primarily utilized the paired-end information, which is relatively reliable. After confirming the number of cleaned reads that remained following the removal of low-quality bases and adapter sequences, it was technically confirmed that the average number of reads was 67,452,913, exceeding the expected number of 20 million reads (ranging from 42,843,793 to 149,526,153 reads) (Fig. 3d ).
As a result of aligning these quality-verified cleaned reads to the human reference genome, an average mapping rate of 98.867% was observed, indicating excellent sequencing quality (Fig. 4a ). While most sequencing quality indicators did not significantly differ between the two sequencing platforms, a notable discrepancy was observed in the multiple alignment ratio during the process of mapping reads to the reference genome (Fig. 4b ). On average, the ratio of multiple mapped reads for reads generated through the Nova-seq platform was 0.085, whereas for reads generated via MGI-seq, it was 0.171. This is a relative characteristic that may occur during library preparation in the entire process of securing RNA-seq samples. After the technical validation of mapping quality, we proceeded to the final step, gene annotation-based quantification. As we targeted the entire transcriptome and adopted a conservative approach that did not consider multiple mapped reads during the quantification process, we expected an assignment rate of 30% at the gene level during quantification based on the current quality control outcome of benchmark study 23 . Upon quantification based on Ensembl human gene annotation, it was confirmed that an average of 31.981% of mapped reads were successfully quantified across all samples (Fig. 4c ). In total, an average of 42,611,086 mapped reads were quantified as expression of specific human genes emphasizing the high-quality quantification process that was carried out (Fig. 4d ).

Mapping and quantification quality Based on the GRCH38 reference genome and gene annotation. In all plots, colors represent different sequencing batches. (a) Proportion of clean reads successfully mapped to the Grch38 human reference genome. (b) Percentage of mapped reads to multiple regions (two or more) on the reference genome. (c) Proportion of reads quantified as specific transcripts based on Ensembl gene annotation. (d) Number of reads successfully quantified across entire samples.
Following the execution of dimensionality reduction analysis to assess the spread of 294 samples, for which expression were measured across a total of 57,791 genes, significant disparities in expression were discerned between the two sequencing platforms (Fig. 5a ). It was substantiated that 32,600 genes, which comprises more than half of the total genes (56.41%), exhibited notable variations in expression between the two sequencing platforms at a significance level of 5%, after FDR adjustment (Supplementary Data S1 ). In our exploration of whether genes demonstrating differences in expression depending on the sequencing platform could be characterized according to detailed gene types outlined in the Ensembl gene annotation, we identified an enrichment of protein-coding genes (Fig. 5b and Supplementary Data S2 ). When designing this study, we hypothesized that distinctions might arise across different sequencing platforms due to various experimental factors, including differences in the library preparation process, biotechnological characteristics of sequencing, and other related variables. Although previous studies have yet to be able to consider experimental bias factors stemming from disparities in sequencing technologies, mainly owing to the high costs associated with next-generation sequencing technology, this study attempted to consider that. As a result, we suggest the possibility that the relative expression of transcripts could be measured differently depending on the sequencing technology and/or library preparation method used. We expect that this observation will be revealed in the near future through research in the field of biotechnology that confirms expression levels by only varying the conditions of specific experimental stages.

Identifying differentially expressed genes between two different sequencing platforms or smoking status. (a) The result of dimensionally reducing the expression levels of all quantified genes using the multi-dimensional scaling method. (b) Investigation of types of differentially expressed genes in two sequencing platforms. The RNA type used information described in Ensembl biomart. DEGs identified between the two sequencing platforms were determined at a 5% significance level after FDR adjustment. (c) Top 8 genes whose expression levels significantly change depending on current smoking status.
It is well known that the key risk factor for COPD, which is the primary subject of this study, is smoking history. Although large-scale RNA-seq studies examining the association between COPD and smoking status in Asian populations are limited, there are many outcomes from large-scale studies reporting Western populations, such as COPDGene 24 . Within our cohort, we included a diverse range of smoking statuses to examine the effects of smoking on gene expression comprehensively. Our comparative analysis encompassed 294 samples from both smokers and non-smokers, providing a broad representation of the population. Additionally, we paid particular attention to current smokers, with 100 samples from this subgroup undergoing sequencing. This allowed us to draw more nuanced comparisons and identify genes differentially expressed with current smoking status. Current smokers were compared to both former smokers and never smokers. This approach enabled us to examine the differential gene expression associated with current smoking status in comparison to both groups, offering a comprehensive understanding of the effects of smoking on gene expression.
In this study, we sequenced 294 samples, utilizing two different sequencing platforms to enhance the robustness of our data. 177 samples were sequenced using the Illumina NovaSeq-6000 platform, and 117 samples were sequenced using the MGISeq-2000 platform. This dual-platform strategy was instrumental in broadening our analytical scope and ensuring that platform-specific biases did not limit our findings. Our dataset identified 20 genes that exhibited differential expression according to the current smoking status, with statistical significance at the 5% level after adjusting for the FDR (Supplementary Data S3 ). As expected, the top five genes discovered in this study were markers validated in previous large-scale transcriptome studies based on smoking status. For example, the three top genes identified in this study (Fig. 5c ), GPR15 (P adj :5.29e-22), LRRN3 (P adj :6.47e-11), and PID1 (P adj :3.04e-06), were also identified as representative biomarkers that were differentially expressed depending on smoking status in the ECLIPSE study, an international representative cohort for COPD 25 . Moreover, among the identified markers, CLDND1 (P adj :0.003), LRRN3 , and SASH1 (P adj :0.0001) were identified as biomarkers related to smoking status using microarray and qRT-PCR techniques in a previous study 26 . This provides direct evidence that the generated data are technically valid.
We further verified whether findings such as ENSG00000286285 lncRNA, which was not reported to be associated with current smoking status, were East Asian-specific based on large-scale RNA-seq data from an independent Western cohort. To clarify this observation, we performed the same analysis using RNA-seq data from COPDGene, the largest representative cohort studying COPD in Western populations. As a result, five representative genes, GPR15 (P adj :5.93e-38), LRRN3 (P adj :4.19e-36), PID1 (P adj :3.98e-19), CLDND1 (P adj :3.88e-21), and SASH1 (P adj :1.68e-17), which are differentially expressed according to current smoking status revealed in this study, were most significantly found in the Western population as well 27 . Furthermore, it was technically verified that ENSG00000286285 lncRNA showed no significant difference according to the current smoking status in the study for the Western population.
In the quest to unravel the complexity of COPD and its diverse manifestations, our study has identified several novel lncRNAs that may serve as potential biomarkers in the East Asian population. To validate these lncRNAs, we are extending our research through planned collaborations with established Korean cohorts, including Korean Obstructive Lung Disease (KOLD) 28 and Korean COPD Subgroup Study (KOCOSS) 29 , and with the international COPDGene project. Such collaborations will allow us to validate our findings across varied population subsets. These efforts aim to confirm the potential of these novel lncRNAs as universal or population-specific biomarkers, offering an unprecedented depth of understanding of COPD in various racial and environmental settings. We anticipate that this collaborative work will solidify the foundation of our study, contributing to the global initiative of tailoring COPD management and therapeutic interventions to individual patient profiles.
Code availability
The R code used to perform differential expression analysis is available in figshare 18 .
WHO. Chronic obstructive pulmonary disease (COPD) , https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (2023).
Disease, G. I. f. C. O. L. GLOBAL STRATEGY FOR PREVENTION, DIAGNOSIS AND MANAGEMENT OF COPD: 2023 Report. Report No. 2023, (2022, 2023 Global Initiative for Chronic Obstructive Lung Disease, Inc., 2023).
Collaborators, G. B. D. C. R. D. Global burden of chronic respiratory diseases and risk factors, 1990-2019: an update from the Global Burden of Disease Study 2019. EClinicalMedicine 59 , 101936, https://doi.org/10.1016/j.eclinm.2023.101936 (2023).
Article Google Scholar
Alfahad, A. J. et al . Current views in chronic obstructive pulmonary disease pathogenesis and management. Saudi Pharm J 29 , 1361–1373, https://doi.org/10.1016/j.jsps.2021.10.008 (2021).
Article CAS PubMed PubMed Central Google Scholar
Beltramo, G. et al . Chronic respiratory diseases are predictors of severe outcome in COVID-19 hospitalised patients: a nationwide study. Eur Respir J 58 , https://doi.org/10.1183/13993003.04474-2020 (2021).
Morrow, J. D. et al . RNA-sequencing across three matched tissues reveals shared and tissue- specific gene expression and pathway signatures of COPD. Respir Res 20 , https://doi.org/10.1186/s12931-019-1032-z
Edmiston, J. S. et al . Gene expression profiling of peripheral blood leukocytes identifies po tential novel biomarkers of chronic obstructive pulmonary disease in current and former smokers. Biomarkers 15 , 715-730, https://doi.org/10.3109/1354750x.2010.512091 (2010).
Wouters, E., Groenewegen, K., Dentener, M. & Vernooy, J. Systemic Inflammation in Chronic Obstructive Pulmonary Disease.
Ghafari, M. & Taghizadieh, A. A Description of the Lung Disease Found in Iranian Women Exposed to Du ng Smoke.
Agarwal, D. M. et al . Disruptions in oral and nasal microbiota in biomass and tobacco smoke associated chronic obstructive pulmonary disease. Arch Microbiol 203 , 2087–2099, https://doi.org/10.1007/s00203-020-02155-9 (2021).
Kumar, H. & Samaria, J. Post tubercular sequels as a important non-smoking risk factor for dev eloping COPD.
Andrews, S. (Babraham Bioinformatics, Babraham Institute, Cambridge, United Kingdom, 2010).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nature biotechnology 37 , 907–915 (2019).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30 , 923–930 (2014).
Article CAS PubMed Google Scholar
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome biology 15 , 1–17 (2014).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 57 , 289–300 (1995).
Article MathSciNet Google Scholar
Park, J. & Seo, M. GEO . https://identifiers.org/geo/GSE240656 (2024).
Seo, M. et al . (2024). Figshare https://doi.org/10.6084/m9.figshare.c.7182627.v1 (2024).
Genetic Epidemiology of COPD (COPDGene) Funded by the National Heart, L., and Blood Institute. (the National Heart, Lung, and Blood Institute, 2019).
Vestbo, J. et al . Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). European Respiratory Journal 31 , 869–873 (2008).
Ching, T., Huang, S. & Garmire, L. X. Power analysis and sample size estimation for RNA-Seq differential expression. Rna 20 , 1684–1696 (2014).
Hart, T., Komori, H. K., LaMere, S., Podshivalova, K. & Salomon, D. R. Finding the active genes in deep RNA-seq gene expression studies. BMC genomics 14 , 1–7 (2013).
Park, S. et al . Benchmark study for evaluating the quality of reference genomes and gene annotations in 114 species. Frontiers in Veterinary Science 10 , 1128570 (2023).
Article PubMed PubMed Central Google Scholar
Ghosh, A. J. et al . Blood RNA sequencing shows overlapping gene expression across COPD phenotype domains. Thorax 77 , 115–122 (2022).
Article PubMed Google Scholar
Obeidat, M. et al . The effect of different case definitions of current smoking on the discovery of smoking-related blood gene expression signatures in chronic obstructive pulmonary disease. Nicotine & Tobacco Research 18 , 1903–1909 (2016).
Article CAS Google Scholar
Beineke, P. et al . A whole blood gene expression-based signature for smoking status. BMC medical genomics 5 , 1–9 (2012).
Parker, M. M. et al . RNA sequencing identifies novel non-coding RNA and exon-specific effects associated with cigarette smoking. BMC medical genomics 10 , 1–10 (2017).
Park, T. S. et al . Study Design and Outcomes of Korean Obstructive Lung Disease (KOLD) Co hort Study. Tuberc Respir Dis 76 , 169, https://doi.org/10.4046/trd.2014.76.4.169 (2014).
Lee, J. Y. et al . Characteristics of Patients with Chronic Obstructive Pulmonary Disease at the First Visit to a Pulmonary Medical Center in Korea: The KOrea COpd Subgroup Study Team Cohort. J Korean Med Sci 31 , 553, https://doi.org/10.3346/jkms.2016.31.4.553 (2016).
Download references
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) No.2020R1C1C1009091, RS-2024-00341669 (to J.P.) and Nos. 2021R1C1C1008199 and 2021R1A5A8032895 (to M.S.S). The current study was conducted after obtaining approval from the Institutional Review Board (IRB) under protocol numbers DUIH2020-04-012, KNUH 2012-06-007, YUHS 4-2021-064, and KHNMC 2022-03-063-008, and all participants provided written informed consent.
Author information
Authors and affiliations.
Department of Computer and Information Science, Korea University, Sejong, Republic of Korea
Minseok Seo & Sinwoo Park
Department of Internal Medicine, School of Medicine, Kangwon National University, Kangwon National University Hospital, Chuncheon, Republic of Korea
Division of Pulmonology, Department of Internal Medicine, Institute of Chest Diseases, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea
Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Republic of Korea
So Hyeon Bak
Division of Pulmonary and Critical Care Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA
Edwin K. Silverman
Department of Pulmonary, Allergy and Critical Care Medicine, Kyung Hee University College of Medicine, Kyung Hee University Hospital at Gangdong, Seoul, Republic of Korea
Jinkyeong Park
You can also search for this author in PubMed Google Scholar
Contributions
M.S. and J.P. designed the study and drafted the manuscript. J.P., W.J.K., S.H.B., and J.Y.J. screened the study participants, obtained informed consent for their inclusion, conducted research-related examinations, and followed up with patients. M.S., S.P., and J.P. performed the experiments and analyzed the data. J.P. supervised the experiments. M.S. and J.P. supervised data analysis. E.S. and J.P. reviewed the overall quality of the research and the data analysis methods. All authors, including E.S., have participated in the manuscript review and given their final approval of the version to be published.
Corresponding author
Correspondence to Jinkyeong Park .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
3rd_revision_scientific_datar, supplementary data s1, supplementary data s2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Seo, M., Park, S., Kim, W. et al. Multi-center Korean cohort study based on RNA-sequencing data targeting COPD patients. Sci Data 11 , 593 (2024). https://doi.org/10.1038/s41597-024-03389-8
Download citation
Received : 09 October 2023
Accepted : 20 May 2024
Published : 06 June 2024
DOI : https://doi.org/10.1038/s41597-024-03389-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Differences in anthropometric, sleep and respiratory characteristics between hypercapnic and normocapnic patients with copd-osa overlap syndrome.

1. Introduction
2. materials and methods, 2.1. participants, 2.2. study variables, 2.3. polysomnography, 2.4. statistical analysis, 4. discussion, 5. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Newton, K.; Malik, V.; Lee-Chiong, T. Sleep and Breathing. Clin. Chest Med. 2014 , 35 , 451–456. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- McNicholas, W.T. COPD-OSA Overlap Syndrome: Evolving Evidence Regarding Epidemiology, Clinical Consequences, and Management. Chest 2017 , 152 , 1318–1326. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Voulgaris, A.; Archontogeorgis, K.; Apessos, I.; Paxinou, N.; Nena, E.; Steiropoulos, P. Is COPD the Determinant Factor for Myocardial Injury and Cardiac Wall Stress in OSA Patients? Medicina 2023 , 59 , 1759. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. NIHR RESPIRE Global Respiratory Health Unit Global, Regional, and National Prevalence of, and Risk Factors for, Chronic Obstructive Pulmonary Disease (COPD) in 2019: A Systematic Review and Modelling Analysis. Lancet Respir. Med. 2022 , 10 , 447–458. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the Global Prevalence and Burden of Obstructive Sleep Apnoea: A Literature-Based Analysis. Lancet Respir. Med. 2019 , 7 , 687–698. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shawon, M.S.R.; Perret, J.L.; Senaratna, C.V.; Lodge, C.; Hamilton, G.S.; Dharmage, S.C. Current Evidence on Prevalence and Clinical Outcomes of Co-Morbid Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease: A Systematic Review. Sleep. Med. Rev. 2017 , 32 , 58–68. [ Google Scholar ] [ CrossRef ]
- Czerwaty, K.; Dżaman, K.; Sobczyk, K.M.; Sikorska, K.I. The Overlap Syndrome of Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease: A Systematic Review. Biomedicines 2022 , 11 , 16. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Voulgaris, A.; Archontogeorgis, K.; Papanas, N.; Pilitsi, E.; Nena, E.; Xanthoudaki, M.; Mikhailidis, D.P.; Froudarakis, M.E.; Steiropoulos, P. Increased Risk for Cardiovascular Disease in Patients with Obstructive Sleep Apnoea Syndrome-Chronic Obstructive Pulmonary Disease (Overlap Syndrome). Clin. Respir. J. 2019 , 13 , 708–715. [ Google Scholar ] [ CrossRef ]
- Lv, R.; Liu, X.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological Mechanisms and Therapeutic Approaches in Obstructive Sleep Apnea Syndrome. Signal Transduct. Target. Ther. 2023 , 8 , 218. [ Google Scholar ] [ CrossRef ]
- McNicholas, W.T.; Hansson, D.; Schiza, S.; Grote, L. Sleep in Chronic Respiratory Disease: COPD and Hypoventilation Disorders. Eur. Respir. Rev. 2019 , 28 , 190064. [ Google Scholar ] [ CrossRef ]
- Calverley, P.M.A. Respiratory Failure in Chronic Obstructive Pulmonary Disease. Eur. Respir. J. 2003 , 22 , 26s–30s. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Resta, O.; Foschino Barbaro, M.P.; Brindicci, C.; Nocerino, M.C.; Caratozzolo, G.; Carbonara, M. Hypercapnia in Overlap Syndrome: Possible Determinant Factors. Sleep Breath. 2002 , 6 , 11–18. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Marin, J.M.; Soriano, J.B.; Carrizo, S.J.; Boldova, A.; Celli, B.R. Outcomes in Patients with Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea: The Overlap Syndrome. Am. J. Respir. Crit. Care Med. 2010 , 182 , 325–331. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jaoude, P.; Kufel, T.; El-Solh, A.A. Survival Benefit of CPAP Favors Hypercapnic Patients with the Overlap Syndrome. Lung 2014 , 192 , 251–258. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- van Zeller, M.; Basoglu, O.K.; Verbraecken, J.; Lombardi, C.; McNicholas, W.T.; Pepin, J.-L.; Steiropoulos, P.; Sliwinski, P.; Correia, D.; Bonsignore, M.R.; et al. Sleep and Cardiometabolic Comorbidities in the Obstructive Sleep Apnoea–COPD Overlap Syndrome: Data from the European Sleep Apnoea Database. ERJ Open Res. 2023 , 9 , 00676–02022. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lacedonia, D.; Carpagnano, G.E.; Aliani, M.; Sabato, R.; Foschino Barbaro, M.P.; Spanevello, A.; Carone, M.; Fanfulla, F. Daytime PaO2 in OSAS, COPD and the Combination of the Two (Overlap Syndrome). Respir. Med. 2013 , 107 , 310–316. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013 , 310 , 2191–2194. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tsara, V.; Serasli, E.; Amfilochiou, A.; Constantinidis, T.; Christaki, P. Greek Version of the Epworth Sleepiness Scale. Sleep Breath. 2004 , 8 , 91–95. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Laaban, J.-P.; Chailleux, E. Daytime Hypercapnia in Adult Patients with Obstructive Sleep Apnea Syndrome in France, before Initiating Nocturnal Nasal Continuous Positive Airway Pressure Therapy. Chest 2005 , 127 , 710–715. [ Google Scholar ] [ CrossRef ]
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2023 , 207 , 819–837. [ Google Scholar ] [ CrossRef ]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012 , 8 , 597–619. [ Google Scholar ] [ CrossRef ]
- Vanfleteren, L.E.; Beghe, B.; Andersson, A.; Hansson, D.; Fabbri, L.M.; Grote, L. Multimorbidity in COPD, Does Sleep Matter? Eur. J. Intern. Med. 2020 , 73 , 7–15. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Soler, X.; Gaio, E.; Powell, F.L.; Ramsdell, J.W.; Loredo, J.S.; Malhotra, A.; Ries, A.L. High Prevalence of Obstructive Sleep Apnea in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2015 , 12 , 1219–1225. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sanders, M.H.; Newman, A.B.; Haggerty, C.L.; Redline, S.; Lebowitz, M.; Samet, J.; O’Connor, G.T.; Punjabi, N.M.; Shahar, E. Sleep Heart Health Study Sleep and Sleep-Disordered Breathing in Adults with Predominantly Mild Obstructive Airway Disease. Am. J. Respir. Crit. Care Med. 2003 , 167 , 7–14. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Laghi, F.; Owens, R.L. COPD+OSA: Can Two Bad Things Be Good for You? Thorax 2017 , 72 , 204–205. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schreiber, A.; Cemmi, F.; Ambrosino, N.; Ceriana, P.; Lastoria, C.; Carlucci, A. Prevalence and Predictors of Obstructive Sleep Apnea in Patients with Chronic Obstructive Pulmonary Disease Undergoing Inpatient Pulmonary Rehabilitation. COPD J. Chronic Obstr. Pulm. Dis. 2018 , 15 , 265–270. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wang, D.; Piper, A.J.; Yee, B.J.; Wong, K.K.; Kim, J.-W.; D’Rozario, A.; Rowsell, L.; Dijk, D.-J.; Grunstein, R.R. Hypercapnia Is a Key Correlate of EEG Activation and Daytime Sleepiness in Hypercapnic Sleep Disordered Breathing Patients. J. Clin. Sleep. Med. 2014 , 10 , 517–522. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chaouat, A.; Weitzenblum, E.; Krieger, J.; Ifoundza, T.; Oswald, M.; Kessler, R. Association of Chronic Obstructive Pulmonary Disease and Sleep Apnea Syndrome. Am. J. Respir. Crit. Care Med. 1995 , 151 , 82–86. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Resta, O.; Foschino Barbaro, M.P.; Bonfitto, P.; Talamo, S.; Mastrosimone, V.; Stefano, A.; Giliberti, T. Hypercapnia in Obstructive Sleep Apnoea Syndrome. Neth. J. Med. 2000 , 56 , 215–222. [ Google Scholar ] [ CrossRef ]
- Böing, S.; Randerath, W.J. Chronic Hypoventilation Syndromes and Sleep-Related Hypoventilation. J. Thorac. Dis. 2015 , 7 , 1273–1285. [ Google Scholar ] [ CrossRef ]
- Lin, C.-K.; Lin, C.-C. Work of Breathing and Respiratory Drive in Obesity. Respirology 2012 , 17 , 402–411. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wang, S.; Sun, X.; Hsia, T.-C.; Lin, X.; Li, M. The Effects of Body Mass Index on Spirometry Tests among Adults in Xi’an, China. Medicine 2017 , 96 , e6596. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- He, B.-T.; Lu, G.; Xiao, S.-C.; Chen, R.; Steier, J.; Moxham, J.; Polkey, M.I.; Luo, Y.-M. Coexistence of OSA May Compensate for Sleep Related Reduction in Neural Respiratory Drive in Patients with COPD. Thorax 2017 , 72 , 256–262. [ Google Scholar ] [ CrossRef ]
- Wang, X.-N.; Luo, J.-M.; Xiao, Y.; Zhang, D.-M.; Huang, R. Daytime Hypercapnia in Adult Patients with Obstructive Sleep Apnea in China. Chin. Med. J. 2021 , 134 , 2237–2239. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kawata, N.; Tatsumi, K.; Terada, J.; Tada, Y.; Tanabe, N.; Takiguchi, Y.; Kuriyama, T. Daytime Hypercapnia in Obstructive Sleep Apnea Syndrome. Chest 2007 , 132 , 1832–1838. [ Google Scholar ] [ CrossRef ]
- Mathews, A.M.; Wysham, N.G.; Xie, J.; Qin, X.; Giovacchini, C.X.; Ekström, M.; MacIntyre, N.R. Hypercapnia in Advanced Chronic Obstructive Pulmonary Disease: A Secondary Analysis of the National Emphysema Treatment Trial. Chronic Obstr. Pulm. Dis. 2020 , 7 , 336–345. [ Google Scholar ] [ CrossRef ]
| Patients without Hypercapnia n = 108 | Patients with Hypercapnia n = 55 | p | |
|---|---|---|---|
| Gender (males/females) | 92/16 | 50/5 | 0.302 |
| Age (years) | 62 (51–68) | 61 (55–68) | 0.689 |
| BMI (kg/m ) | 35.2 (31.1–39.5) | 39.7 (34.4–43.2) | 0.001 |
| Neck circumference (cm) | 44 (41–47) | 47 (44–50) | 0.017 |
| Waist circumference (cm) | 124 (110–132) | 132 (117.5–138) | 0.013 |
| Hip circumference (cm) | 117 (110–123) | 122 (110.5–129) | 0.171 |
| WHR | 0.89 (0.79–0.95) | 0.90 (0.81–1.03) | 0.128 |
| Former smokers (%) | 81.5% | 81.8% | 0.357 |
| Patients without Hypercapnia n = 108 | Patients with Hypercapnia n = 55 | p | |
|---|---|---|---|
| Recording time (min) | 382 (360.3–402) | 378.5 (359.5–396) | 0.654 |
| TST (min) | 303 (248.3–338.5) | 310 (264–354) | 0.088 |
| N1 (%) | 20 (10.8–37.3) | 12 (4–21) | 0.001 |
| N2 (%) | 55.5 (42–70) | 67 (50–83) | 0.005 |
| N3 (%) | 6 (0–11.3) | 7 (1–17) | 0.204 |
| REM (%) | 8 (3–16) | 6 (2–10) | 0.237 |
| AHI (events/hour) | 39.5 (18–61) | 41 (16–66.5) | 0.403 |
| ODI (events/hour) | 35.5 (20.3–60.8) | 63 (28–80) | 0.004 |
| Aver SpO (%) | 91 (89–94) | 89 (85.5–92) | <0.001 |
| Min SpO (%) | 77 (70–83) | 68 (57–78) | <0.001 |
| T < 90% (%) | 20.7 (4–44.9) | 38 (14.8–80.5) | 0.006 |
| Arousal index | 30 (17–49.3) | 21 (5.5–50) | 0.118 |
| Sleep efficiency (%) | 79.3 (68.5–87.7) | 85.5 (74.4–91.9) | 0.033 |
| ESS score | 9 (6–12) | 13 (7.5–17.5) | 0.008 |
| Patients without Hypercapnia n = 108 | Patients with Hypercapnia n = 55 | p | |
|---|---|---|---|
| FEV (% predicted) | 69.5 (58–79) | 59 (45–75) | 0.003 |
| FVC (% predicted) | 74.5 (62–91) | 63 (54–82) | 0.004 |
| FEV /FVC (%) | 67 (62–69.1) | 68 (63–69.6) | 0.396 |
| pH | 7.43 (7.41–7.45) | 7.41 (7.39–7.43) | 0.001 |
| pO (mmHg) | 74 (66.3–82) | 65 (58–71) | <0.001 |
| pCO (mmHg) | 41 (37.8–43) | 49 (47–54) | <0.001 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Voulgaris, A.; Archontogeorgis, K.; Chadia, K.; Siopi, D.; Nena, E.; Steiropoulos, P. Differences in Anthropometric, Sleep and Respiratory Characteristics between Hypercapnic and Normocapnic Patients with COPD-OSA Overlap Syndrome. J. Pers. Med. 2024 , 14 , 600. https://doi.org/10.3390/jpm14060600
Voulgaris A, Archontogeorgis K, Chadia K, Siopi D, Nena E, Steiropoulos P. Differences in Anthropometric, Sleep and Respiratory Characteristics between Hypercapnic and Normocapnic Patients with COPD-OSA Overlap Syndrome. Journal of Personalized Medicine . 2024; 14(6):600. https://doi.org/10.3390/jpm14060600
Voulgaris, Athanasios, Kostas Archontogeorgis, Konstantina Chadia, Dimitra Siopi, Evangelia Nena, and Paschalis Steiropoulos. 2024. "Differences in Anthropometric, Sleep and Respiratory Characteristics between Hypercapnic and Normocapnic Patients with COPD-OSA Overlap Syndrome" Journal of Personalized Medicine 14, no. 6: 600. https://doi.org/10.3390/jpm14060600
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Why the Pandemic Probably Started in a Lab, in 5 Key Points

By Alina Chan
Dr. Chan is a molecular biologist at the Broad Institute of M.I.T. and Harvard, and a co-author of “Viral: The Search for the Origin of Covid-19.”
This article has been updated to reflect news developments.
On Monday, Dr. Anthony Fauci returned to the halls of Congress and testified before the House subcommittee investigating the Covid-19 pandemic. He was questioned about several topics related to the government’s handling of Covid-19, including how the National Institute of Allergy and Infectious Diseases, which he directed until retiring in 2022, supported risky virus work at a Chinese institute whose research may have caused the pandemic.
For more than four years, reflexive partisan politics have derailed the search for the truth about a catastrophe that has touched us all. It has been estimated that at least 25 million people around the world have died because of Covid-19, with over a million of those deaths in the United States.
Although how the pandemic started has been hotly debated, a growing volume of evidence — gleaned from public records released under the Freedom of Information Act, digital sleuthing through online databases, scientific papers analyzing the virus and its spread, and leaks from within the U.S. government — suggests that the pandemic most likely occurred because a virus escaped from a research lab in Wuhan, China. If so, it would be the most costly accident in the history of science.
Here’s what we now know:
1 The SARS-like virus that caused the pandemic emerged in Wuhan, the city where the world’s foremost research lab for SARS-like viruses is located.
- At the Wuhan Institute of Virology, a team of scientists had been hunting for SARS-like viruses for over a decade, led by Shi Zhengli.
- Their research showed that the viruses most similar to SARS‑CoV‑2, the virus that caused the pandemic, circulate in bats that live r oughly 1,000 miles away from Wuhan. Scientists from Dr. Shi’s team traveled repeatedly to Yunnan province to collect these viruses and had expanded their search to Southeast Asia. Bats in other parts of China have not been found to carry viruses that are as closely related to SARS-CoV-2.

The closest known relatives to SARS-CoV-2 were found in southwestern China and in Laos.
Large cities
Mine in Yunnan province
Cave in Laos
South China Sea

The closest known relatives to SARS-CoV-2
were found in southwestern China and in Laos.
philippines

The closest known relatives to SARS-CoV-2 were found
in southwestern China and Laos.
Sources: Sarah Temmam et al., Nature; SimpleMaps
Note: Cities shown have a population of at least 200,000.

There are hundreds of large cities in China and Southeast Asia.

There are hundreds of large cities in China
and Southeast Asia.

The pandemic started roughly 1,000 miles away, in Wuhan, home to the world’s foremost SARS-like virus research lab.

The pandemic started roughly 1,000 miles away,
in Wuhan, home to the world’s foremost SARS-like virus research lab.

The pandemic started roughly 1,000 miles away, in Wuhan,
home to the world’s foremost SARS-like virus research lab.
- Even at hot spots where these viruses exist naturally near the cave bats of southwestern China and Southeast Asia, the scientists argued, as recently as 2019 , that bat coronavirus spillover into humans is rare .
- When the Covid-19 outbreak was detected, Dr. Shi initially wondered if the novel coronavirus had come from her laboratory , saying she had never expected such an outbreak to occur in Wuhan.
- The SARS‑CoV‑2 virus is exceptionally contagious and can jump from species to species like wildfire . Yet it left no known trace of infection at its source or anywhere along what would have been a thousand-mile journey before emerging in Wuhan.
2 The year before the outbreak, the Wuhan institute, working with U.S. partners, had proposed creating viruses with SARS‑CoV‑2’s defining feature.
- Dr. Shi’s group was fascinated by how coronaviruses jump from species to species. To find viruses, they took samples from bats and other animals , as well as from sick people living near animals carrying these viruses or associated with the wildlife trade. Much of this work was conducted in partnership with the EcoHealth Alliance, a U.S.-based scientific organization that, since 2002, has been awarded over $80 million in federal funding to research the risks of emerging infectious diseases.
- The laboratory pursued risky research that resulted in viruses becoming more infectious : Coronaviruses were grown from samples from infected animals and genetically reconstructed and recombined to create new viruses unknown in nature. These new viruses were passed through cells from bats, pigs, primates and humans and were used to infect civets and humanized mice (mice modified with human genes). In essence, this process forced these viruses to adapt to new host species, and the viruses with mutations that allowed them to thrive emerged as victors.
- By 2019, Dr. Shi’s group had published a database describing more than 22,000 collected wildlife samples. But external access was shut off in the fall of 2019, and the database was not shared with American collaborators even after the pandemic started , when such a rich virus collection would have been most useful in tracking the origin of SARS‑CoV‑2. It remains unclear whether the Wuhan institute possessed a precursor of the pandemic virus.
- In 2021, The Intercept published a leaked 2018 grant proposal for a research project named Defuse , which had been written as a collaboration between EcoHealth, the Wuhan institute and Ralph Baric at the University of North Carolina, who had been on the cutting edge of coronavirus research for years. The proposal described plans to create viruses strikingly similar to SARS‑CoV‑2.
- Coronaviruses bear their name because their surface is studded with protein spikes, like a spiky crown, which they use to enter animal cells. T he Defuse project proposed to search for and create SARS-like viruses carrying spikes with a unique feature: a furin cleavage site — the same feature that enhances SARS‑CoV‑2’s infectiousness in humans, making it capable of causing a pandemic. Defuse was never funded by the United States . However, in his testimony on Monday, Dr. Fauci explained that the Wuhan institute would not need to rely on U.S. funding to pursue research independently.

The Wuhan lab ran risky experiments to learn about how SARS-like viruses might infect humans.
1. Collect SARS-like viruses from bats and other wild animals, as well as from people exposed to them.

2. Identify high-risk viruses by screening for spike proteins that facilitate infection of human cells.

2. Identify high-risk viruses by screening for spike proteins that facilitate infection of
human cells.

In Defuse, the scientists proposed to add a furin cleavage site to the spike protein.
3. Create new coronaviruses by inserting spike proteins or other features that could make the viruses more infectious in humans.

4. Infect human cells, civets and humanized mice with the new coronaviruses, to determine how dangerous they might be.

- While it’s possible that the furin cleavage site could have evolved naturally (as seen in some distantly related coronaviruses), out of the hundreds of SARS-like viruses cataloged by scientists, SARS‑CoV‑2 is the only one known to possess a furin cleavage site in its spike. And the genetic data suggest that the virus had only recently gained the furin cleavage site before it started the pandemic.
- Ultimately, a never-before-seen SARS-like virus with a newly introduced furin cleavage site, matching the description in the Wuhan institute’s Defuse proposal, caused an outbreak in Wuhan less than two years after the proposal was drafted.
- When the Wuhan scientists published their seminal paper about Covid-19 as the pandemic roared to life in 2020, they did not mention the virus’s furin cleavage site — a feature they should have been on the lookout for, according to their own grant proposal, and a feature quickly recognized by other scientists.
- Worse still, as the pandemic raged, their American collaborators failed to publicly reveal the existence of the Defuse proposal. The president of EcoHealth, Peter Daszak, recently admitted to Congress that he doesn’t know about virus samples collected by the Wuhan institute after 2015 and never asked the lab’s scientists if they had started the work described in Defuse. In May, citing failures in EcoHealth’s monitoring of risky experiments conducted at the Wuhan lab, the Biden administration suspended all federal funding for the organization and Dr. Daszak, and initiated proceedings to bar them from receiving future grants. In his testimony on Monday, Dr. Fauci said that he supported the decision to suspend and bar EcoHealth.
- Separately, Dr. Baric described the competitive dynamic between his research group and the institute when he told Congress that the Wuhan scientists would probably not have shared their most interesting newly discovered viruses with him . Documents and email correspondence between the institute and Dr. Baric are still being withheld from the public while their release is fiercely contested in litigation.
- In the end, American partners very likely knew of only a fraction of the research done in Wuhan. According to U.S. intelligence sources, some of the institute’s virus research was classified or conducted with or on behalf of the Chinese military . In the congressional hearing on Monday, Dr. Fauci repeatedly acknowledged the lack of visibility into experiments conducted at the Wuhan institute, saying, “None of us can know everything that’s going on in China, or in Wuhan, or what have you. And that’s the reason why — I say today, and I’ve said at the T.I.,” referring to his transcribed interview with the subcommittee, “I keep an open mind as to what the origin is.”
3 The Wuhan lab pursued this type of work under low biosafety conditions that could not have contained an airborne virus as infectious as SARS‑CoV‑2.
- Labs working with live viruses generally operate at one of four biosafety levels (known in ascending order of stringency as BSL-1, 2, 3 and 4) that describe the work practices that are considered sufficiently safe depending on the characteristics of each pathogen. The Wuhan institute’s scientists worked with SARS-like viruses under inappropriately low biosafety conditions .

In the United States, virologists generally use stricter Biosafety Level 3 protocols when working with SARS-like viruses.
Biosafety cabinets prevent
viral particles from escaping.
Viral particles
Personal respirators provide
a second layer of defense against breathing in the virus.
DIRECT CONTACT
Gloves prevent skin contact.
Disposable wraparound
gowns cover much of the rest of the body.

Personal respirators provide a second layer of defense against breathing in the virus.
Disposable wraparound gowns
cover much of the rest of the body.
Note: Biosafety levels are not internationally standardized, and some countries use more permissive protocols than others.

The Wuhan lab had been regularly working with SARS-like viruses under Biosafety Level 2 conditions, which could not prevent a highly infectious virus like SARS-CoV-2 from escaping.
Some work is done in the open air, and masks are not required.
Less protective equipment provides more opportunities
for contamination.

Some work is done in the open air,
and masks are not required.
Less protective equipment provides more opportunities for contamination.
- In one experiment, Dr. Shi’s group genetically engineered an unexpectedly deadly SARS-like virus (not closely related to SARS‑CoV‑2) that exhibited a 10,000-fold increase in the quantity of virus in the lungs and brains of humanized mice . Wuhan institute scientists handled these live viruses at low biosafet y levels , including BSL-2.
- Even the much more stringent containment at BSL-3 cannot fully prevent SARS‑CoV‑2 from escaping . Two years into the pandemic, the virus infected a scientist in a BSL-3 laboratory in Taiwan, which was, at the time, a zero-Covid country. The scientist had been vaccinated and was tested only after losing the sense of smell. By then, more than 100 close contacts had been exposed. Human error is a source of exposure even at the highest biosafety levels , and the risks are much greater for scientists working with infectious pathogens at low biosafety.
- An early draft of the Defuse proposal stated that the Wuhan lab would do their virus work at BSL-2 to make it “highly cost-effective.” Dr. Baric added a note to the draft highlighting the importance of using BSL-3 to contain SARS-like viruses that could infect human cells, writing that “U.S. researchers will likely freak out.” Years later, after SARS‑CoV‑2 had killed millions, Dr. Baric wrote to Dr. Daszak : “I have no doubt that they followed state determined rules and did the work under BSL-2. Yes China has the right to set their own policy. You believe this was appropriate containment if you want but don’t expect me to believe it. Moreover, don’t insult my intelligence by trying to feed me this load of BS.”
- SARS‑CoV‑2 is a stealthy virus that transmits effectively through the air, causes a range of symptoms similar to those of other common respiratory diseases and can be spread by infected people before symptoms even appear. If the virus had escaped from a BSL-2 laboratory in 2019, the leak most likely would have gone undetected until too late.
- One alarming detail — leaked to The Wall Street Journal and confirmed by current and former U.S. government officials — is that scientists on Dr. Shi’s team fell ill with Covid-like symptoms in the fall of 2019 . One of the scientists had been named in the Defuse proposal as the person in charge of virus discovery work. The scientists denied having been sick .
4 The hypothesis that Covid-19 came from an animal at the Huanan Seafood Market in Wuhan is not supported by strong evidence.
- In December 2019, Chinese investigators assumed the outbreak had started at a centrally located market frequented by thousands of visitors daily. This bias in their search for early cases meant that cases unlinked to or located far away from the market would very likely have been missed. To make things worse, the Chinese authorities blocked the reporting of early cases not linked to the market and, claiming biosafety precautions, ordered the destruction of patient samples on January 3, 2020, making it nearly impossible to see the complete picture of the earliest Covid-19 cases. Information about dozens of early cases from November and December 2019 remains inaccessible.
- A pair of papers published in Science in 2022 made the best case for SARS‑CoV‑2 having emerged naturally from human-animal contact at the Wuhan market by focusing on a map of the early cases and asserting that the virus had jumped from animals into humans twice at the market in 2019. More recently, the two papers have been countered by other virologists and scientists who convincingly demonstrate that the available market evidence does not distinguish between a human superspreader event and a natural spillover at the market.
- Furthermore, the existing genetic and early case data show that all known Covid-19 cases probably stem from a single introduction of SARS‑CoV‑2 into people, and the outbreak at the Wuhan market probably happened after the virus had already been circulating in humans.

An analysis of SARS-CoV-2’s evolutionary tree shows how the virus evolved as it started to spread through humans.
SARS-COV-2 Viruses closest
to bat coronaviruses
more mutations

Source: Lv et al., Virus Evolution (2024) , as reproduced by Jesse Bloom

The viruses that infected people linked to the market were most likely not the earliest form of the virus that started the pandemic.

- Not a single infected animal has ever been confirmed at the market or in its supply chain. Without good evidence that the pandemic started at the Huanan Seafood Market, the fact that the virus emerged in Wuhan points squarely at its unique SARS-like virus laboratory.
5 Key evidence that would be expected if the virus had emerged from the wildlife trade is still missing.

In previous outbreaks of coronaviruses, scientists were able to demonstrate natural origin by collecting multiple pieces of evidence linking infected humans to infected animals.
Infected animals
Earliest known
cases exposed to
live animals
Antibody evidence
of animals and
animal traders having
been infected
Ancestral variants
of the virus found in
Documented trade
of host animals
between the area
where bats carry
closely related viruses
and the outbreak site

Infected animals found
Earliest known cases exposed to live animals
Antibody evidence of animals and animal
traders having been infected
Ancestral variants of the virus found in animals
Documented trade of host animals
between the area where bats carry closely
related viruses and the outbreak site

For SARS-CoV-2, these same key pieces of evidence are still missing , more than four years after the virus emerged.

For SARS-CoV-2, these same key pieces of evidence are still missing ,
more than four years after the virus emerged.
- Despite the intense search trained on the animal trade and people linked to the market, investigators have not reported finding any animals infected with SARS‑CoV‑2 that had not been infected by humans. Yet, infected animal sources and other connective pieces of evidence were found for the earlier SARS and MERS outbreaks as quickly as within a few days, despite the less advanced viral forensic technologies of two decades ago.
- Even though Wuhan is the home base of virus hunters with world-leading expertise in tracking novel SARS-like viruses, investigators have either failed to collect or report key evidence that would be expected if Covid-19 emerged from the wildlife trade . For example, investigators have not determined that the earliest known cases had exposure to intermediate host animals before falling ill. No antibody evidence shows that animal traders in Wuhan are regularly exposed to SARS-like viruses, as would be expected in such situations.
- With today’s technology, scientists can detect how respiratory viruses — including SARS, MERS and the flu — circulate in animals while making repeated attempts to jump across species . Thankfully, these variants usually fail to transmit well after crossing over to a new species and tend to die off after a small number of infections. In contrast, virologists and other scientists agree that SARS‑CoV‑2 required little to no adaptation to spread rapidly in humans and other animals . The virus appears to have succeeded in causing a pandemic upon its only detected jump into humans.
The pandemic could have been caused by any of hundreds of virus species, at any of tens of thousands of wildlife markets, in any of thousands of cities, and in any year. But it was a SARS-like coronavirus with a unique furin cleavage site that emerged in Wuhan, less than two years after scientists, sometimes working under inadequate biosafety conditions, proposed collecting and creating viruses of that same design.
While several natural spillover scenarios remain plausible, and we still don’t know enough about the full extent of virus research conducted at the Wuhan institute by Dr. Shi’s team and other researchers, a laboratory accident is the most parsimonious explanation of how the pandemic began.
Given what we now know, investigators should follow their strongest leads and subpoena all exchanges between the Wuhan scientists and their international partners, including unpublished research proposals, manuscripts, data and commercial orders. In particular, exchanges from 2018 and 2019 — the critical two years before the emergence of Covid-19 — are very likely to be illuminating (and require no cooperation from the Chinese government to acquire), yet they remain beyond the public’s view more than four years after the pandemic began.
Whether the pandemic started on a lab bench or in a market stall, it is undeniable that U.S. federal funding helped to build an unprecedented collection of SARS-like viruses at the Wuhan institute, as well as contributing to research that enhanced them . Advocates and funders of the institute’s research, including Dr. Fauci, should cooperate with the investigation to help identify and close the loopholes that allowed such dangerous work to occur. The world must not continue to bear the intolerable risks of research with the potential to cause pandemics .
A successful investigation of the pandemic’s root cause would have the power to break a decades-long scientific impasse on pathogen research safety, determining how governments will spend billions of dollars to prevent future pandemics. A credible investigation would also deter future acts of negligence and deceit by demonstrating that it is indeed possible to be held accountable for causing a viral pandemic. Last but not least, people of all nations need to see their leaders — and especially, their scientists — heading the charge to find out what caused this world-shaking event. Restoring public trust in science and government leadership requires it.
A thorough investigation by the U.S. government could unearth more evidence while spurring whistleblowers to find their courage and seek their moment of opportunity. It would also show the world that U.S. leaders and scientists are not afraid of what the truth behind the pandemic may be.
More on how the pandemic may have started

Where Did the Coronavirus Come From? What We Already Know Is Troubling.
Even if the coronavirus did not emerge from a lab, the groundwork for a potential disaster had been laid for years, and learning its lessons is essential to preventing others.
By Zeynep Tufekci

Why Does Bad Science on Covid’s Origin Get Hyped?
If the raccoon dog was a smoking gun, it fired blanks.
By David Wallace-Wells

A Plea for Making Virus Research Safer
A way forward for lab safety.
By Jesse Bloom
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow the New York Times Opinion section on Facebook , Instagram , TikTok , WhatsApp , X and Threads .
Alina Chan ( @ayjchan ) is a molecular biologist at the Broad Institute of M.I.T. and Harvard, and a co-author of “ Viral : The Search for the Origin of Covid-19.” She was a member of the Pathogens Project , which the Bulletin of the Atomic Scientists organized to generate new thinking on responsible, high-risk pathogen research.
- Share full article
Advertisement
Academic Support for Nursing Students
No notifications.
Disclaimer: This essay has been written by a student and not our expert nursing writers. View professional sample essays here.
View full disclaimer
Any opinions, findings, conclusions, or recommendations expressed in this essay are those of the author and do not necessarily reflect the views of NursingAnswers.net. This essay should not be treated as an authoritative source of information when forming medical opinions as information may be inaccurate or out-of-date.
COPD Case Study Assignment
Info: 5325 words (21 pages) Nursing Essay Published: 14th Dec 2020
Reference this
Tagged: COPD
If you need assistance with writing your nursing essay, our professional nursing essay writing service is here to help!
Our nursing and healthcare experts are ready and waiting to assist with any writing project you may have, from simple essay plans, through to full nursing dissertations.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services

- Nursing Essay Writing Service

- Nursing Dissertation Service

- Reflective Writing Service
Related Content
Content relating to: "COPD"
Chronic Obstructive Pulmonary Disease is a group of chronic and progressive respiratory disorders that are characterized by an airway obstruction with little or no reversibility. Damage to the lungs continues to make breathing gradually more difficult over time.
Related Articles

Nursing Case Study: Patient with Drug and Alcohol Induced Paranoid Schizophrenia
This essay will focus on a patient encountered while on placement. The patient was 64-year-old male named Jim who was diagnosed with drug and alcohol induced paranoid schizophrenia thirty years previo...
Chronic Obstructive Pulmonary Disease (COPD) Causes
ADEBAYO AINA Contents Explain the physiological origin of Chronic Obstructive Pulmonary Disease Describe the physical effects of the Chronic Obstructive Pulmonary Disease Explain the contributory fa...
Nursing Care for Acute Exacerbation of COPD
Introduction This essay is focused on the signification of health assessment throughout the nursing process of a scenario of patient (Mr Lee) who diagnosed with acute exacerbation of COPD and express...
DMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on the NursingAnswers.net website then please:
Our academic writing and marking services can help you!
- Marking Service
- Samples of our Work
- Full Service Portfolio
Related Lectures
Study for free with our range of nursing lectures!
- Drug Classification
- Emergency Care
- Health Observation
- Palliative Care
- Professional Values

Write for Us
Do you have a 2:1 degree or higher in nursing or healthcare?
Study Resources
Free resources to assist you with your nursing studies!
- APA Citation Tool
- Example Nursing Essays
- Example Nursing Assignments
- Example Nursing Case Studies
- Reflective Nursing Essays
- Nursing Literature Reviews
- Free Resources
- Reflective Model Guides
- Nursing and Healthcare Pay 2021
The state of AI in early 2024: Gen AI adoption spikes and starts to generate value
If 2023 was the year the world discovered generative AI (gen AI) , 2024 is the year organizations truly began using—and deriving business value from—this new technology. In the latest McKinsey Global Survey on AI, 65 percent of respondents report that their organizations are regularly using gen AI, nearly double the percentage from our previous survey just ten months ago. Respondents’ expectations for gen AI’s impact remain as high as they were last year , with three-quarters predicting that gen AI will lead to significant or disruptive change in their industries in the years ahead.
About the authors
This article is a collaborative effort by Alex Singla , Alexander Sukharevsky , Lareina Yee , and Michael Chui , with Bryce Hall , representing views from QuantumBlack, AI by McKinsey, and McKinsey Digital.
Organizations are already seeing material benefits from gen AI use, reporting both cost decreases and revenue jumps in the business units deploying the technology. The survey also provides insights into the kinds of risks presented by gen AI—most notably, inaccuracy—as well as the emerging practices of top performers to mitigate those challenges and capture value.
AI adoption surges
Interest in generative AI has also brightened the spotlight on a broader set of AI capabilities. For the past six years, AI adoption by respondents’ organizations has hovered at about 50 percent. This year, the survey finds that adoption has jumped to 72 percent (Exhibit 1). And the interest is truly global in scope. Our 2023 survey found that AI adoption did not reach 66 percent in any region; however, this year more than two-thirds of respondents in nearly every region say their organizations are using AI. 1 Organizations based in Central and South America are the exception, with 58 percent of respondents working for organizations based in Central and South America reporting AI adoption. Looking by industry, the biggest increase in adoption can be found in professional services. 2 Includes respondents working for organizations focused on human resources, legal services, management consulting, market research, R&D, tax preparation, and training.
Also, responses suggest that companies are now using AI in more parts of the business. Half of respondents say their organizations have adopted AI in two or more business functions, up from less than a third of respondents in 2023 (Exhibit 2).
Gen AI adoption is most common in the functions where it can create the most value
Most respondents now report that their organizations—and they as individuals—are using gen AI. Sixty-five percent of respondents say their organizations are regularly using gen AI in at least one business function, up from one-third last year. The average organization using gen AI is doing so in two functions, most often in marketing and sales and in product and service development—two functions in which previous research determined that gen AI adoption could generate the most value 3 “ The economic potential of generative AI: The next productivity frontier ,” McKinsey, June 14, 2023. —as well as in IT (Exhibit 3). The biggest increase from 2023 is found in marketing and sales, where reported adoption has more than doubled. Yet across functions, only two use cases, both within marketing and sales, are reported by 15 percent or more of respondents.
Gen AI also is weaving its way into respondents’ personal lives. Compared with 2023, respondents are much more likely to be using gen AI at work and even more likely to be using gen AI both at work and in their personal lives (Exhibit 4). The survey finds upticks in gen AI use across all regions, with the largest increases in Asia–Pacific and Greater China. Respondents at the highest seniority levels, meanwhile, show larger jumps in the use of gen Al tools for work and outside of work compared with their midlevel-management peers. Looking at specific industries, respondents working in energy and materials and in professional services report the largest increase in gen AI use.
Investments in gen AI and analytical AI are beginning to create value
The latest survey also shows how different industries are budgeting for gen AI. Responses suggest that, in many industries, organizations are about equally as likely to be investing more than 5 percent of their digital budgets in gen AI as they are in nongenerative, analytical-AI solutions (Exhibit 5). Yet in most industries, larger shares of respondents report that their organizations spend more than 20 percent on analytical AI than on gen AI. Looking ahead, most respondents—67 percent—expect their organizations to invest more in AI over the next three years.
Where are those investments paying off? For the first time, our latest survey explored the value created by gen AI use by business function. The function in which the largest share of respondents report seeing cost decreases is human resources. Respondents most commonly report meaningful revenue increases (of more than 5 percent) in supply chain and inventory management (Exhibit 6). For analytical AI, respondents most often report seeing cost benefits in service operations—in line with what we found last year —as well as meaningful revenue increases from AI use in marketing and sales.
Inaccuracy: The most recognized and experienced risk of gen AI use
As businesses begin to see the benefits of gen AI, they’re also recognizing the diverse risks associated with the technology. These can range from data management risks such as data privacy, bias, or intellectual property (IP) infringement to model management risks, which tend to focus on inaccurate output or lack of explainability. A third big risk category is security and incorrect use.
Respondents to the latest survey are more likely than they were last year to say their organizations consider inaccuracy and IP infringement to be relevant to their use of gen AI, and about half continue to view cybersecurity as a risk (Exhibit 7).
Conversely, respondents are less likely than they were last year to say their organizations consider workforce and labor displacement to be relevant risks and are not increasing efforts to mitigate them.
In fact, inaccuracy— which can affect use cases across the gen AI value chain , ranging from customer journeys and summarization to coding and creative content—is the only risk that respondents are significantly more likely than last year to say their organizations are actively working to mitigate.
Some organizations have already experienced negative consequences from the use of gen AI, with 44 percent of respondents saying their organizations have experienced at least one consequence (Exhibit 8). Respondents most often report inaccuracy as a risk that has affected their organizations, followed by cybersecurity and explainability.
Our previous research has found that there are several elements of governance that can help in scaling gen AI use responsibly, yet few respondents report having these risk-related practices in place. 4 “ Implementing generative AI with speed and safety ,” McKinsey Quarterly , March 13, 2024. For example, just 18 percent say their organizations have an enterprise-wide council or board with the authority to make decisions involving responsible AI governance, and only one-third say gen AI risk awareness and risk mitigation controls are required skill sets for technical talent.
Bringing gen AI capabilities to bear
The latest survey also sought to understand how, and how quickly, organizations are deploying these new gen AI tools. We have found three archetypes for implementing gen AI solutions : takers use off-the-shelf, publicly available solutions; shapers customize those tools with proprietary data and systems; and makers develop their own foundation models from scratch. 5 “ Technology’s generational moment with generative AI: A CIO and CTO guide ,” McKinsey, July 11, 2023. Across most industries, the survey results suggest that organizations are finding off-the-shelf offerings applicable to their business needs—though many are pursuing opportunities to customize models or even develop their own (Exhibit 9). About half of reported gen AI uses within respondents’ business functions are utilizing off-the-shelf, publicly available models or tools, with little or no customization. Respondents in energy and materials, technology, and media and telecommunications are more likely to report significant customization or tuning of publicly available models or developing their own proprietary models to address specific business needs.
Respondents most often report that their organizations required one to four months from the start of a project to put gen AI into production, though the time it takes varies by business function (Exhibit 10). It also depends upon the approach for acquiring those capabilities. Not surprisingly, reported uses of highly customized or proprietary models are 1.5 times more likely than off-the-shelf, publicly available models to take five months or more to implement.
Gen AI high performers are excelling despite facing challenges
Gen AI is a new technology, and organizations are still early in the journey of pursuing its opportunities and scaling it across functions. So it’s little surprise that only a small subset of respondents (46 out of 876) report that a meaningful share of their organizations’ EBIT can be attributed to their deployment of gen AI. Still, these gen AI leaders are worth examining closely. These, after all, are the early movers, who already attribute more than 10 percent of their organizations’ EBIT to their use of gen AI. Forty-two percent of these high performers say more than 20 percent of their EBIT is attributable to their use of nongenerative, analytical AI, and they span industries and regions—though most are at organizations with less than $1 billion in annual revenue. The AI-related practices at these organizations can offer guidance to those looking to create value from gen AI adoption at their own organizations.
To start, gen AI high performers are using gen AI in more business functions—an average of three functions, while others average two. They, like other organizations, are most likely to use gen AI in marketing and sales and product or service development, but they’re much more likely than others to use gen AI solutions in risk, legal, and compliance; in strategy and corporate finance; and in supply chain and inventory management. They’re more than three times as likely as others to be using gen AI in activities ranging from processing of accounting documents and risk assessment to R&D testing and pricing and promotions. While, overall, about half of reported gen AI applications within business functions are utilizing publicly available models or tools, gen AI high performers are less likely to use those off-the-shelf options than to either implement significantly customized versions of those tools or to develop their own proprietary foundation models.
What else are these high performers doing differently? For one thing, they are paying more attention to gen-AI-related risks. Perhaps because they are further along on their journeys, they are more likely than others to say their organizations have experienced every negative consequence from gen AI we asked about, from cybersecurity and personal privacy to explainability and IP infringement. Given that, they are more likely than others to report that their organizations consider those risks, as well as regulatory compliance, environmental impacts, and political stability, to be relevant to their gen AI use, and they say they take steps to mitigate more risks than others do.
Gen AI high performers are also much more likely to say their organizations follow a set of risk-related best practices (Exhibit 11). For example, they are nearly twice as likely as others to involve the legal function and embed risk reviews early on in the development of gen AI solutions—that is, to “ shift left .” They’re also much more likely than others to employ a wide range of other best practices, from strategy-related practices to those related to scaling.
In addition to experiencing the risks of gen AI adoption, high performers have encountered other challenges that can serve as warnings to others (Exhibit 12). Seventy percent say they have experienced difficulties with data, including defining processes for data governance, developing the ability to quickly integrate data into AI models, and an insufficient amount of training data, highlighting the essential role that data play in capturing value. High performers are also more likely than others to report experiencing challenges with their operating models, such as implementing agile ways of working and effective sprint performance management.
About the research
The online survey was in the field from February 22 to March 5, 2024, and garnered responses from 1,363 participants representing the full range of regions, industries, company sizes, functional specialties, and tenures. Of those respondents, 981 said their organizations had adopted AI in at least one business function, and 878 said their organizations were regularly using gen AI in at least one function. To adjust for differences in response rates, the data are weighted by the contribution of each respondent’s nation to global GDP.
Alex Singla and Alexander Sukharevsky are global coleaders of QuantumBlack, AI by McKinsey, and senior partners in McKinsey’s Chicago and London offices, respectively; Lareina Yee is a senior partner in the Bay Area office, where Michael Chui , a McKinsey Global Institute partner, is a partner; and Bryce Hall is an associate partner in the Washington, DC, office.
They wish to thank Kaitlin Noe, Larry Kanter, Mallika Jhamb, and Shinjini Srivastava for their contributions to this work.
This article was edited by Heather Hanselman, a senior editor in McKinsey’s Atlanta office.
Explore a career with us
Related articles.

Moving past gen AI’s honeymoon phase: Seven hard truths for CIOs to get from pilot to scale

A generative AI reset: Rewiring to turn potential into value in 2024
Implementing generative AI with speed and safety

IMAGES
VIDEO
COMMENTS
This article uses a case study to discuss the symptoms, causes and management of chronic obstructive pulmonary disease, describing the patient's associated pathophysiology. Diagnosis involves spirometry testing to measure the volume of air that can be exhaled; it is often performed after administering a short-acting beta-agonist.
COPD: a case study Authors Debbie Price is lead practice nurse, Llandrindod Wells Medical Practice; Nikki Williams is associate professor of respiratory and sleep physiology, Swansea University. Abstract This article uses a case study to discuss the symptoms, causes and management of chronic obstructive pulmonary disease, describing the patient's
Clinical Case Study on COPD. Danielle McGarry. Introduction. The patient is a 76-year-old female with a history of chronic obstructive pulmonary disease (COPD). The patient is a married housewife with a 1 pack a day smoking history from age 15. The patient states that she, "only smokes a little to calm her nerves" though her husband still ...
On examination, the temperature was 36.4°C, the heart rate 103 beats per minute, the blood pressure 79/51 mm Hg, the respiratory rate 30 breaths per minute, and the oxygen saturation 99% while ...
Many patients have mild COPD; women have a 72% chance and men a 78% chance of survival at five years (British Lung Foundation, 2010). However, in patients with severe COPD requiring oxygen therapy and treatment with nebulisers, five-year survival rates are lower at 24% for women and 30% for men (National End of Life Intelligence Network, 2011 ...
This Independent Study is brought to you for free and open access by the Department of Nursing at UND Scholarly Commons. It has been accepted for inclusion in Nursing Capstones by an authorized administrator of UND Scholarly Commons. For more information, please contact. [email protected]. COPD Case Study.
Based on the information given, the patient likely has chronic obstructive pulmonary disease (COPD). The key findings that point to this diagnosis include: Barrel chest. Tachypnea. A long expiratory time. Diminished breath sounds. Use of accessory muscles while breathing. Digital clubbing. Pursed lip breathing.
Chronic obstructive pulmonary disease (COPD) is a common condition that causes irreversible airway obstruction. Fatigue and exertional dyspnoea, for example, have a detrimental impact on the patient's daily life. Current research has revealed the need to empower the patient, which can result in not only educated and effective decision-making, but also a considerable improvement in patient ...
Nurse Seema works on a medical-surgical unit and is caring for Richard, a 75-year-old male with a history of smoking, who was admitted for an acute exacerbation of chronic obstructive pulmonary disease, or COPD. After settling Richard in his room, Nurse Seema goes through the steps of the Clinical Judgment Measurement Model to make clinical ...
This essay will focus on the example of chronic obstructive pulmonary disease (COPD). ... believe that an additional contributing factor in case of COPD relates to the strong association ... mood disorders are some of the most undertreated COPD symptoms. A study of nearly four hundred patients reported that while 77% experienced low mood, it ...
Meet Susan M! Share your impressions in our latest COPD case study. Summary of in-patient admission: Susan M. is being discharged today following a 6-day ICU and step-down admission for acute exacerbation of COPD with bacterial pneumonia requiring intubation and mechanical ventilation for a period of 32 hours. Subsequent to her extubation and transfer to the step down unit she was treated with ...
COPD Case Study Essay. Chronic obstructive pulmonary disease is a chronic progressive lung disease caused predominantly by smoking National Institute for Health and Clinical Excellence NICE, 2010. It is characterised by airflow obstruction which is not fully reversible. The airflow obstruction does not change markedly over several months and is ...
COPD Case Study Examples. Decent Essays. 556 Words. 3 Pages. Open Document. COPD (chronic obstructive pulmonary disease) Subjective data: difficulty breathing, tightness of the chest, light-headed, sleep too much and feels tired all the time, and she has been smoking since her teen years. Objective data: 61 years old woman.
We're interested in your thoughts on another COPD case study: Jim B., a 68-year-old man here for his Phase II Pulmonary Rehabilitation intake interview. A bit more about Jim: Medical history: COPD, FEV1 six weeks ago was 38% of normal predicted, recent CXR shows flattened diaphragm with increased AP diameter, appendectomy age 34, broken nose and broken right arm as a child.
This clinical case report highlights the usefulness of FEF 25-75 evaluation in early COPD diagnosis and monitoring and confirms the efficacy of LAMA-LABA association for small airways obstruction treatment. Keywords: COPD, LAMA, LABA, FEF25-75, Treatment. Abbreviations: COPD, chronic obstructive pulmonary disease; LAMA, long acting muscarinic ...
COPD: a Clinical Case Study. Info: 3436 words (14 pages) Nursing Case Study Published: 11th Feb 2020. Reference this Tagged: COPD. ... COPD Case Study Essay. Chronic obstructive pulmonary disease is a chronic progressive lung disease caused predominantly by smoking National Institute for Health and Clinical Excellence NICE, 2010. It is ...
Background Chronic obstructive pulmonary disease (COPD) is a prevalent chronic disease that requires comprehensive approaches to manage; it accounts for a significant portion of Canada's annual healthcare spending. Interprofessional teams are effective at providing chronic disease management that meets the needs of patients. As part of an ongoing initiative, a COPD management program, the ...
is currently designing a multicentre study with the following aims: (1) to systematically investigate and quantify the distribution of treatable traits in a large, prospective cohort of patients with COPD who seek medical attention due to an acute increase in respiratory symptoms (≤14 days) that was diagnosed as a COPD exacerbation by the attending physician in primary care or a hospital ...
1) CASE SUMMARY. Mr TLT is a 58 year old taxi driver who was admitted to Hospital Batu Pahat due to newly diagnosed chronic obstructive pulmonary disease. He has had hypertension for the past one year and is taking T Amlodipine 5mg od. He is also a chronic smoker for the past 40 years who smokes about 20 sticks of cigarettes a day.
I believe this is the best opportunity to publish your excellent work. Both original articles and reviews are welcome. In light of scientific originality this Article Collection is not currently accepting case reports, case series, meeting reports, photo essays, poster extracts, or study protocols.
COPD Exacerbation Case Study. Introduction: A COPD exacerbation can be defined as "an event in the natural course of the disease characterized by a change in the patient's baseline dyspnea, cough, and/or sputum, that is beyond normal day-to-day variations, is acute in onset and may warrant a change in medication in a patient with underlying ...
In 2023, WHO ranked chronic obstructive pulmonary disease (COPD) as the third leading cause of death, with 3.23 million fatalities in 2019. The intricate nature of the disease, which is influenced ...
Background: Overlap syndrome (OS), the coexistence of chronic obstructive pulmonary disease and obstructive sleep apnea, is frequently characterized by the presence of daytime hypercapnia (pCO2 ≥ 45 mmHg). The aim of this study was to investigate potential differences in anthropometric, sleep and respiratory characteristics between hypercapnic and normocapnic patients with OS. Methods ...
Dr. Chan is a molecular biologist at the Broad Institute of M.I.T. and Harvard, and a co-author of "Viral: The Search for the Origin of Covid-19." This article has been updated to reflect news ...
Trump's San Francisco visit is a case study in the meaninglessness of 'moderate' politics Words like moderate and centrist are political weasel words with no set definitions By Gil Duran ...
Cloud computing is the use of comprehensive digital capabilities delivered via the internet for organizations to operate, innovate, and serve customers. It eliminates the need for organizations to host digital applications on their own servers. Group of white spheres on light blue background.
1) CASE SUMMARY. Mr TLT is a 58 year old taxi driver who was admitted to Hospital Batu Pahat due to newly diagnosed chronic obstructive pulmonary disease. He has had hypertension for the past one year and is taking T Amlodipine 5mg od. He is also a chronic smoker for the past 40 years who smokes about 20 sticks of cigarettes a day.
If 2023 was the year the world discovered generative AI (gen AI), 2024 is the year organizations truly began using—and deriving business value from—this new technology. In the latest McKinsey Global Survey on AI, 65 percent of respondents report that their organizations are regularly using gen AI, nearly double the percentage from our ...