An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Heart Views
- v.18(3); Jul-Sep 2017

Guidelines To Writing A Clinical Case Report
What is a clinical case report.
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant literature on the topic. The case report is a rapid short communication between busy clinicians who may not have time or resources to conduct large scale research.
WHAT ARE THE REASONS FOR PUBLISHING A CASE REPORT?
The most common reasons for publishing a case are the following: 1) an unexpected association between diseases or symptoms; 2) an unexpected event in the course observing or treating a patient; 3) findings that shed new light on the possible pathogenesis of a disease or an adverse effect; 4) unique or rare features of a disease; 5) unique therapeutic approaches; variation of anatomical structures.
Most journals publish case reports that deal with one or more of the following:
- Unusual observations
- Adverse response to therapies
- Unusual combination of conditions leading to confusion
- Illustration of a new theory
- Question regarding a current theory
- Personal impact.
STRUCTURE OF A CASE REPORT[ 1 , 2 ]
Different journals have slightly different formats for case reports. It is always a good idea to read some of the target jiurnals case reports to get a general idea of the sequence and format.
In general, all case reports include the following components: an abstract, an introduction, a case, and a discussion. Some journals might require literature review.
The abstract should summarize the case, the problem it addresses, and the message it conveys. Abstracts of case studies are usually very short, preferably not more than 150 words.
Introduction
The introduction gives a brief overview of the problem that the case addresses, citing relevant literature where necessary. The introduction generally ends with a single sentence describing the patient and the basic condition that he or she is suffering from.
This section provides the details of the case in the following order:
- Patient description
- Case history
- Physical examination results
- Results of pathological tests and other investigations
- Treatment plan
- Expected outcome of the treatment plan
- Actual outcome.
The author should ensure that all the relevant details are included and unnecessary ones excluded.
This is the most important part of the case report; the part that will convince the journal that the case is publication worthy. This section should start by expanding on what has been said in the introduction, focusing on why the case is noteworthy and the problem that it addresses.
This is followed by a summary of the existing literature on the topic. (If the journal specifies a separate section on literature review, it should be added before the Discussion). This part describes the existing theories and research findings on the key issue in the patient's condition. The review should narrow down to the source of confusion or the main challenge in the case.
Finally, the case report should be connected to the existing literature, mentioning the message that the case conveys. The author should explain whether this corroborates with or detracts from current beliefs about the problem and how this evidence can add value to future clinical practice.
A case report ends with a conclusion or with summary points, depending on the journal's specified format. This section should briefly give readers the key points covered in the case report. Here, the author can give suggestions and recommendations to clinicians, teachers, or researchers. Some journals do not want a separate section for the conclusion: it can then be the concluding paragraph of the Discussion section.
Notes on patient consent
Informed consent in an ethical requirement for most studies involving humans, so before you start writing your case report, take a written consent from the patient as all journals require that you provide it at the time of manuscript submission. In case the patient is a minor, parental consent is required. For adults who are unable to consent to investigation or treatment, consent of closest family members is required.
Patient anonymity is also an important requirement. Remember not to disclose any information that might reveal the identity of the patient. You need to be particularly careful with pictures, and ensure that pictures of the affected area do not reveal the identity of the patient.
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- Medical Studies
How to Write a Medical Case Study Report
Last Updated: April 18, 2024 Fact Checked
This article was medically reviewed by Mark Ziats, MD, PhD and by wikiHow staff writer, Jennifer Mueller, JD . Dr. Mark Ziats is an Internal Medicine Physician, Scientist, Entrepreneur, and the Medical Director of xBiotech. With over five years of experience, he specializes in biotechnology, genomics, and medical devices. He earned a Doctor of Medicine degree from Baylor College of Medicine, a Ph.D. in Genetics from the University of Cambridge, and a BS in Biochemistry and Chemistry from Clemson University. He also completed the INNoVATE Program in Biotechnology Entrepreneurship at The Johns Hopkins University - Carey Business School. Dr. Ziats is board certified by the American Board of Internal Medicine. There are 15 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 187,119 times.
You've encountered an interesting and unusual case on your rounds, and a colleague or supervising physician says, "Why don't you write up a case study report?" If you've never written one before, that might sound intimidating, but it's a great way to get started in medical writing. Case studies always follow a standard structure and format, so the writing is very formulaic once you get the hang of it. Read on for a step-by-step guide to writing your first case study report.
What is a case study report?

- Medical students or residents typically do the bulk of the writing of the report. If you're just starting your medical career, a case study report is a great way to get a publication under your belt. [2] X Research source

- If the patient is a minor or is incapable of giving informed consent, get consent from their parents or closest relative. [4] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source
- Your hospital likely has specific consent forms to use. Ask your supervising physician if you're not sure where to get one.
- Some journals also have their own consent form. Check your target journal's author or submission information to make sure. [5] X Research source
How is a case study report structured?

- Even though the introduction is the first part of a case study report, doctors typically write it last. You'll have a better idea of how to introduce your case study to readers after you've written it.
- Your abstract comes at the top, before the introduction, and provides a brief summary of the entire report. Unless your case study is published in an open-access journal, the abstract is the only part of the article many readers will see.

- Many journals offer templates and checklists you can use to make sure your case study includes everything necessary and is formatted properly—take advantage of these! Some journals, such as BMJ Case Reports , require all case studies submitted to use their templates.
Drafting Your Medical Case Study Report

- Patient description
- Chronological case history
- Physical exam results
- Results of any pathological tests, imaging, or other investigations
- Treatment plan
- Expected outcome of treatment
- Actual outcome of treatment

- Why the patient sought medical help (you can even use their own words)
- Important information that helped you settle on your diagnosis
- The results of your clinical examination, including diagnostic tests and their results, along with any helpful images
- A description of the treatment plan
- The outcome, including how and why treatment ended and how long the patient was under your care [11] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source

- You will need references to back up symptoms of the condition, common treatment, and the expected outcome of that common treatment.
- Use your research to paint a picture of the usual case of a patient with a similar condition—it'll help you show how unusual and different your patient's case is.
- Generally, aim for around 20 references—no fewer than 15, but no more than 25. [13] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source

- Close your discussion section with a summary of the lessons learned from the case and why it's significant to consider when treating similar cases in the future.
- Outline any open questions that remain. You might also provide suggestions for future research.

- In your conclusion, you might also give suggestions or recommendations to readers based on what you learned as a result of the case.
- Some journals don't want a separate conclusion section. If that's the case for one of your target journals, just move this paragraph to the end of your discussion section.
Polishing Your Report for Submission to Publishers

- Most titles are fewer than 10 words long and include the name of the disease or condition treated.
- You might also include the treatment used and whether the outcome was successful. When deciding what to include, think about the reason you wrote the case study in the first place and why you think it's important for other clinicians to read.

- Made a significant intellectual contribution to the case study report
- Was involved in the medical care of the patient reported
- Can explain and defend the data presented in the report
- Has approved the final manuscript before submission for publication

- Keep in mind that the abstract is not just going to be the first thing people read—it will often be the only thing people read. Make sure that if someone is going to walk away having only read the abstract, they'll still get the same message they would have if they read the whole thing.
- There are 2 basic types of abstract: narrative and structured. A narrative abstract is a single paragraph written in narrative prose. A structured abstract includes headings that correspond with the sections of the paper, then a brief summary of each section. Use the format preferred by your target journal.

- Look for keywords that are relevant to your field or sub-field and directly related to the content of your article, such as the name of the condition or specific treatments you used.
- Most journals allow 4-8 keywords but check the submission guidelines of your target journal to make sure.

- Blur out the patient's face as well as any tattoos, birthmarks, or unrelated scars that are visible in diagnostic images.

- It's common to thank the patient, but that's up to you. Even if you don't, include a statement indicating that you have the patient's written, informed consent to publish the information.
- Read the journal's submission guidelines for a definition of what that journal considers a conflict of interest. They're generally the same, but some might be stricter than others. [22] X Research source

- If you're not familiar with the citation style used by your target journal, check online for a guide. There might also be one available at your hospital or medical school library.
- Medical librarians can also help with citation style and references if you run into something tricky—don't just wing it! Correct citation style insures that readers can access the materials you cite.

- It's also a good idea to get a beta reader who isn't a medical professional. Their comments can help you figure out where you need to clarify your points.
- Read a lot of case studies published in your target journals—it will help you internalize the tone and style that journal is looking for.
Submitting Your Report to Publishers

- Look into the background and reputation of journals before you decide to submit to them. Only seek publication from reputable journals in which articles go through a peer-review process.
- Find out what publishing fees the journals charge. Keep in mind that open-access journals tend to charge higher publishing fees. [26] X Research source
- Read each journal's submission and editorial guidelines carefully. They'll tell you exactly how to format your case study, how long each section should be, and what citation style to use. [27] X Research source
- For electronic journals that only publish case reports, try BMJ Case Reports , Journal of Medical Case Reports , or Radiology Case Reports .

- If your manuscript isn't suitable for the journal you submitted to, the journal might offer to forward it to an associated journal where it would be a better fit.
- When your manuscript is provisionally accepted, the journal will send it to other doctors for evaluation under the peer-review process.
- Most medical journals don't accept simultaneous submissions, meaning you'll have to submit to your first choice, wait for their decision, then move to the next journal on the list if they don't bite.

- Along with your revised manuscript, include a letter with your response to each of the reviewer's comments. Where you made revisions, add page numbers to indicate where the revisions are that address that reviewer's comments.
- Sometimes, doctors involved in the peer review process will indicate that the journal should reject the manuscript. If that's the case, you'll get a letter explaining why your case study report won't be published and you're free to submit it elsewhere.

- Some journals require you to have your article professionally copy-edited at your own cost while others do this in-house. The editors will let you know what you're responsible for.

- With your acceptance letter, you'll get instructions on how to make payment and how much you owe. Take note of the deadline and make sure you pay it as soon as possible to avoid publication delays.
- Some journals will publish for free, with an "open-access option" that allows you to pay a fee only if you want open access to your article. [32] X Research source

- Through the publishing agreement, you assign your copyright in the article to the journal. This allows the journal to legally publish your work. That assignment can be exclusive or non-exclusive and may only last for a specific term. Read these details carefully!
- If you published an open-access article, you don't assign the copyright to the publisher. The publishing agreement merely gives the journal the right to publish the "Version of Record." [33] X Research source
How do I find a suitable case for a report?

- A rare disease, or unusual presentation of any disease
- An unusual combination of diseases or conditions
- A difficult or inconclusive diagnosis
- Unexpected developments or responses to treatment
- Personal impact
- Observations that shed new light on the patient's disease or condition

- There might be other members of your medical team that want to help with writing. If so, use one of these brainstorming sessions to divvy up writing responsibilities in a way that makes the most sense given your relative skills and experience.
- Senior doctors might also be able to name some journals that would potentially publish your case study. [36] X Research source
Expert Q&A
You Might Also Like

- ↑ https://www.elsevier.com/connect/authors-update/the-dos-and-donts-of-writing-and-publishing-case-reports
- ↑ https://www.bmj.com/content/350/bmj.h2693
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5686928/
- ↑ https://health.usf.edu/medicine/internalmedicine/im-impact/~/media/B3A3421F4C144FA090AE965C21791A3C.ashx
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2597880/
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6476221/
- ↑ https://www.springer.com/gp/authors-editors/authorandreviewertutorials/writing-a-journal-manuscript/title-abstract-and-keywords/10285522
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2597880/
- ↑ https://thelancet.com/pb/assets/raw/Lancet/authors/tl-info-for-authors.pdf
- ↑ https://jmedicalcasereports.biomedcentral.com/articles/10.1186/s13256-017-1351-y
- ↑ https://guides.himmelfarb.gwu.edu/casereports
- ↑ https://casereports.bmj.com/pages/authors/
- ↑ https://jmedicalcasereports.biomedcentral.com/articles/10.1186/1752-1947-7-239
- ↑ https://research.chm.msu.edu/students-residents/writing-a-case-report
- ↑ https://authorservices.taylorandfrancis.com/publishing-your-research/moving-through-production/copyright-for-journal-authors/#
About This Article

Medical Disclaimer
The content of this article is not intended to be a substitute for professional medical advice, examination, diagnosis, or treatment. You should always contact your doctor or other qualified healthcare professional before starting, changing, or stopping any kind of health treatment.
Read More...
To start a medical case study report, first choose a title that clearly reflects the contents of the report. You’ll also need to list any participating authors and develop a list of keywords, as well as an abstract summarizing the report. Your report will need to include an introduction summarizing the context of the report, as well as a detailed presentation of the case. Don’t forget to include a thorough citation list and acknowledgements of anyone else who participated in the study. For more tips from our Medical co-author, including how to get your case study report published, keep reading! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Sep 5, 2020
Did this article help you?

Asfia Banu Pasha
Apr 10, 2017
Jun 20, 2021
Mar 1, 2017

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
wikiHow Tech Help Pro:
Level up your tech skills and stay ahead of the curve
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 30 January 2023
A student guide to writing a case report
- Maeve McAllister 1
BDJ Student volume 30 , pages 12–13 ( 2023 ) Cite this article
24 Accesses
Metrics details
As a student, it can be hard to know where to start when reading or writing a clinical case report either for university or out of special interest in a Journal. I have collated five top tips for writing an insightful and relevant case report.
A case report is a structured report of the clinical process of a patient's diagnostic pathway, including symptoms, signs, diagnosis, treatment planning (short and long term), clinical outcomes and follow-up. 1 Some of these case reports can sometimes have simple titles, to the more unusual, for example, 'Oral Tuberculosis', 'The escapee wisdom tooth', 'A difficult diagnosis'. They normally begin with the word 'Sir' and follow an introduction from this.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Guidelines To Writing a Clinical Case Report. Heart Views 2017; 18 , 104-105.
British Dental Journal. Case reports. Available online at: www.nature.com/bdj/articles?searchType=journalSearch&sort=PubDate&type=case-report&page=2 (accessed August 17, 2022).
Chate R, Chate C. Achenbach's syndrome. Br Dent J 2021; 231: 147.
Abdulgani A, Muhamad, A-H and Watted N. Dental case report for publication; step by step. J Dent Med Sci 2014; 3 : 94-100.
Download references
Author information
Authors and affiliations.
Queen´s University Belfast, Belfast, United Kingdom
Maeve McAllister
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Maeve McAllister .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
McAllister, M. A student guide to writing a case report. BDJ Student 30 , 12–13 (2023). https://doi.org/10.1038/s41406-023-0925-y
Download citation
Published : 30 January 2023
Issue Date : 30 January 2023
DOI : https://doi.org/10.1038/s41406-023-0925-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Writing a Case Report
This page is intended for medical students, residents or others who do not have much experience with case reports, but are planning on writing one.
What is a case report? A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient. The most important aspect of a case report, i.e. the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
Case reports are commonly of the following categories :
- Rare diseases
- Unusual presentation of disease
- Unexpected events
- Unusual combination of diseases or conditions
- Difficult or inconclusive diagnosis
- Treatment or management challenges
- Personal impact
- Observations that shed new light on a disease or condition
- Anatomical variations
It is important that you recognize what is unique or interesting about your case, and this must be described clearly in the case report.
Case reports generally take the format of :
1. Background
2. Case presentation
3. Observations and investigation
4. Diagnosis
5. Treatment
7. Discussion
Does a case report require IRB approval?
Case reports typically discuss a single patient. If this is true for your case report, then it most likely does not require IRB approval because it not considered research. If you have more than one patient, your study could qualify as a Case Series, which would require IRB review. If you have questions, you chould check your local IRB's guidelines on reviewing case reports.
Are there other rules for writing a case report?
First, you will be collecting protected health information, thus HIPAA applies to case reports. Spectrum Health has created a very helpful guidance document for case reports, which you can see here: Case Report Guidance - Spectrum Health
While this guidance document was created by Spectrum Health, the rules and regulations outlined could apply to any case report. This includes answering questions like: Do I need written HIPAA authorization to publish a case report? When do I need IRB review of a case report? What qualifies as a patient identifier?
How do I get started?
1. We STRONGLY encourage you to consult the CARE Guidelines, which provide guidance on writing case reports - https://www.care-statement.org/
Specifically, the checklist - https://www.care-statement.org/checklist - which explains exactly the information you should collect and include in your case report.
2. Identify a case. If you are a medical student, you may not yet have the clinical expertise to determine if a specific case is worth writing up. If so, you must seek the help of a clinician. It is common for students to ask attendings or residents if they have any interesting cases that can be used for a case report.
3. Select a journal or two to which you think you will submit the case report. Journals often have specific requirements for publishing case reports, which could include a requirement for informed consent, a letter or statement from the IRB and other things. Journals may also charge publication fees (see Is it free to publish? below)
4. Obtain informed consent from the patient (see " Do I have to obtain informed consent from the patient? " below). Journals may have their own informed consent form that they would like you to use, so please look for this when selecting a journal.
Once you've identified the case, selected an appropriate journal(s), and considered informed consent, you can collect the required information to write the case report.
How do I write a case report?
Once you identify a case and have learned what information to include in the case report, try to find a previously published case report. Finding published case reports in a similar field will provide examples to guide you through the process of writing a case report.
One journal you can consult is BMJ Case Reports . MSU has an institutional fellowship with BMJ Case Reports which allows MSU faculty, staff and students to publish in this journal for free. See this page for a link to the journal and more information on publishing- https://lib.msu.edu/medicalwriting_publishing/
There are numerous other journals where you can find published case reports to help guide you in your writing.
Do I have to obtain informed consent from the patient?
The CARE guidelines recommend obtaining informed consent from patients for all case reports. Our recommendation is to obtain informed consent from the patient. Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports before publishing. The CARE guidelines recommend obtaining informed consent AND the patient's perspective on the treatment/outcome (if possible). Please consider this as well.
If required, it is recommended you obtain informed consent before the case report is written.
An example of a case report consent form can be found on the BMJ Case Reports website, which you can access via the MSU library page - https://casereports.bmj.com/ . Go to "Instructions for Authors" and then "Patient Consent" to find the consent form they use. You can create a similar form to obtain consent from your patient. If you have identified a journal already, please consult their requirements and determine if they have a specific consent form they would like you to use.
Seek feedback
Once you have written a draft of the case report, you should seek feedback on your writing, from experts in the field if possible, or from those who have written case reports before.
Selecting a journal
Aside from BMJ Case Reports mentioned above, there are many, many journals out there who publish medical case reports. Ask your mentor if they have a journal they would like to use. If you need to select on your own, here are some strategies:
1. Do a PubMed search. https://pubmed.ncbi.nlm.nih.gov/
a. Do a search for a topic, disease or other feature of your case report
b. When the results appear, on the left side of the page is a limiter for "article type". Case reports are an article type to which you can limit your search results. If you don't see that option on the left, click "additional filters".
c. Review the case reports that come up and see what journals they are published in.
2. Use JANE - https://jane.biosemantics.org/
3. Check with specialty societies. Many specialty societies are affiliated with one or more journal, which can be reviewed for ones that match your needs
4. Search through individual publisher journal lists. Elsevier publishes many different medical research journals, and they have a journal finder, much like JANE ( https://journalfinder.elsevier.com/ ). This is exclusive to Elsevier journals. There are many other publishers of medical journals for review, including Springer, Dove Press, BMJ, BMC, Wiley, Sage, Nature and many others.
Is it free to publish ?
Be aware that it may not be free to publish your case report. Many journals charge publication fees. Of note, many open access journals charge author fees of thousands of dollars. Other journals have smaller page charges (i.e. $60 per page), and still others will publish for free, with an "open access option". It is best practice to check the journal's Info for Authors section or Author Center to determine what the cost is to publish. MSU-CHM does NOT have funds to support publication costs, so this is an important step if you do not want to pay out of pocket for publishing
*A more thorough discussion on finding a journal, publication costs, predatory journals and other publication-related issues can be found here: https://research.chm.msu.edu/students-residents/finding-a-journal
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. 2013. The CARE guidelines: Consensus-based clinical case reporting guideline development. Glob Adv Health Med . 2:38-43. doi: 10.7453/gahmj.2013.008
Riley DS, Barber MS, Kienle GS, AronsonJK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG, Sox H, Werthmann PG, Moher D, Rison RA, Shamseer L, Koch CA, Sun GH, Hanaway P, Sudak NL, Kaszkin-Bettag M, Carpenter JE, Gagnier JJ. 2017. CARE guidelines for case reports: explanation and elaboration document . J Clin Epidemiol . 89:218-234. doi: 10.1016/j.jclinepi.2017.04.026
Guidelines to writing a clinical case report. 2017. Heart Views . 18:104-105. doi: 10.4103/1995-705X.217857
Ortega-Loubon C, Culquichicon C, Correa R. The importance of writing and publishing case reports during medical education. 2017. Cureus. 9:e1964. doi: 10.7759/cureus.1964
Writing and publishing a useful and interesting case report. 2019. BMJ Case Reports. https://casereports.bmj.com/pages/wp-content/uploads/sites/69/2019/04/How-to-write-a-Case-Report-DIGITAL.pdf
Camm CF. Writing an excellent case report: EHJ Case Reports , Case of the Year 2019. 2020. European Heart Jounrnal. 41:1230-1231. https://doi.org/10.1093/eurheartj/ehaa176
*content developed by Mark Trottier, PhD
A guide to writing and using case reports
A thematic series published in Journal of Medical Case Reports .
A valuable resource for clinicians in the form of a special series of editorials, which comprise a guide to writing and using case reports.
Another important publication in the journal for reference when writing and using case reports is, “ A guide to writing case reports for the Journal of Medical Case Reports and BioMed Central Research Notes ”, which was published in 2013 and written by Richard Rison.
When to write a neurology case report
- View Full Text
How to write a neurology case report
Neurology case reports have a long history of transmitting important medical information across many generations for the improvement of patient care. Case reports contribute much to the physician’s knowledge b...
How to review a case report
How to write a case report in nephrology, case reports in medical education: a platform for training medical students, residents, and fellows in scientific writing and critical thinking.
A case report is a detailed narrative that usually illustrates a diagnostic or therapeutic problem experienced by one or several patients. Case reports commonly serve as the first line of evidence for new inte...
How to apply clinical cases and medical literature in the framework of a modified “failure mode and effects analysis” as a clinical reasoning tool – an illustration using the human biliary system
Clinicians use various clinical reasoning tools such as Ishikawa diagram to enhance their clinical experience and reasoning skills. Failure mode and effects analysis, which is an engineering methodology in ori...
How to apply case reports in clinical practice using surrogate models via example of the trigeminocardiac reflex
Case reports are an increasing source of evidence in clinical medicine. Until a few years ago, such case reports were emerged into systematic reviews and nowadays they are often fitted to the development of cl...
Case Report: A Beginner’s Guide with Examples
A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient’s history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
A similar design involving a group of patients (with the similar problem) is referred to as case series.
Advantages of case reports
Case reports offer, in general a fast, easy and cheap way to report an unusual observation or a rare event in a clinical setting, as these have very small probability of being detected in an experimental study because of limitations on the number of patients that can be included.
These events deserve to be reported since they might provide insights on some exceptions to general rules and theories in the field.
Case reports are great to get first impressions that can generate new hypotheses (e.g. detecting a potential side effect of a drug) or challenge existing ones (e.g. shedding the light on the possibility of a different biological mechanism of a disease).
In many of these cases, additional investigation is needed such as designing large observational studies or randomized experiments or even going back and mining data from previous research looking for evidence for theses hypotheses.
Limitations of case reports
Observing a relationship between an exposure and a disease in a case report does not mean that it is causal in nature.
This is because of:
- The absence of a control group that provides a benchmark or a point of reference against which we compare our results. A control group is important to eliminate the role of external factors which can interfere with the relationship between exposure and disease
- Unmeasured Confounding caused by variables that influence both the exposure and the disease
A case report can have a powerful emotional effect (see examples of case reports below). This can lead to overrate the importance of the evidence provided by such case. In his book Against Empathy: The Case for Rational Compassion , Paul Bloom explains how a powerful story affects our emotions, can distort our judgement and even lead us to make bad moral choices.
When a case report describes a rare event it is important to remember that what we’re reading about is exceptional and most importantly resist generalizations especially because a case report is, by definition, a study where the sample is only 1 patient.
Selection bias is another issue as the cases in case reports are not chosen at random, therefore some members of the population may have a higher probability of being included in the study than others.
So, results from a case report cannot be representative of the entire population.
Because of these limitations, case reports have the lowest level of evidence compared to other study designs as represented in the evidence pyramid below:
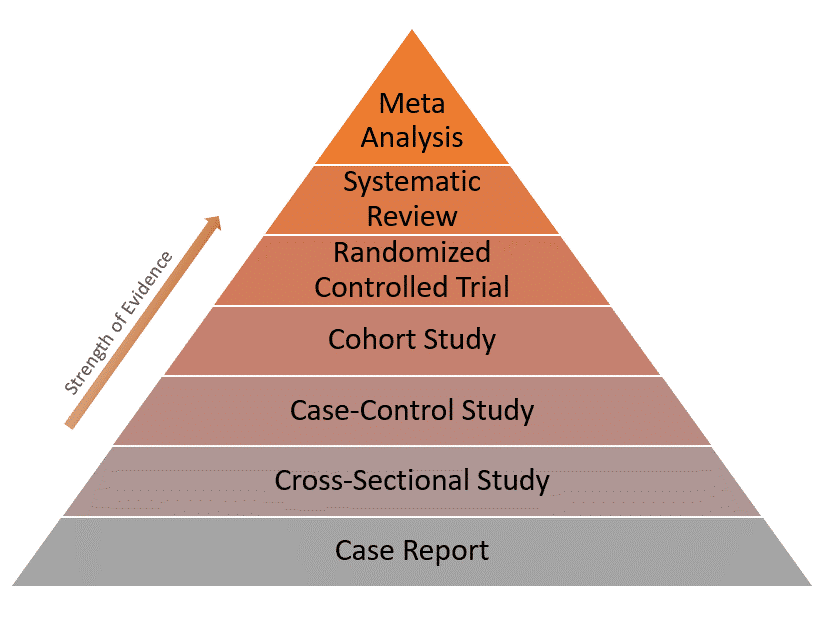
Real-world examples of case reports
Example 1: normal plasma cholesterol in an 88-year-old man who eats 25 eggs a day.
This is the case of an old man with Alzheimer’s disease who has been eating 20-30 eggs every day for almost 15 years. [ Source ]
The man had an LDL-cholesterol level of only 142 mg/dL (3.68 mmol/L) and no significant clinical atherosclerosis (deposition of cholesterol in arterial walls)!
His body adapted by reducing the intestinal absorption of cholesterol, lowering the rate of its synthesis and increasing the rate of its conversion into bile acid.
This is indeed an unusual case of biological adaptation to a major change in dietary intake.
Example 2: Recovery from the passage of an iron bar through the head
This is an interesting case of a construction foreman named Phineas Gage. [ Source ]
In 1848, due to an explosion at work, an iron bar passed through his head destroying a large portion of his brain’s frontal lobe. He survived the event and the injury only affected 1 thing: His personality!
After the accident, Gage became profane, rough and disrespectful to the extent that he was no longer tolerable to people around him. So he lost his job and his family.
His case inspired further research that focused on the relationship between specific parts of the brain and personality.
- Sayre JW, Toklu HZ, Ye F, Mazza J, Yale S. Case Reports, Case Series – From Clinical Practice to Evidence-Based Medicine in Graduate Medical Education . Cureus . 2017;9(8):e1546. Published 2017 Aug 7. doi:10.7759/cureus.1546.
- Nissen T, Wynn R. The clinical case report: a review of its merits and limitations . BMC Res Notes . 2014;7:264. Published 2014 Apr 23. doi:10.1186/1756-0500-7-264.
Further reading
- Case Report vs Cross-Sectional Study
- Cohort vs Cross-Sectional Study
- How to Identify Different Types of Cohort Studies?
- Matched Pairs Design
- Randomized Block Design
Case report
Journal of Medical Case Reports welcomes well-described reports of cases that include the following:
- Unreported or unusual side effects or adverse interactions involving medications.
- Unexpected or unusual presentations of a disease.
- New associations or variations in disease processes.
- Presentations, diagnoses and/or management of new and emerging diseases.
- An unexpected association between diseases or symptoms.
- An unexpected event in the course of observing or treating a patient.
- Findings that shed new light on the possible pathogenesis of a disease or an adverse effect.
Case reports submitted to Journal of Medical Case Reports should make a contribution to medical knowledge and must have educational value or highlight the need for a change in clinical practice or diagnostic/prognostic approaches. The journal will not consider case reports describing preventive or therapeutic interventions, as these generally require stronger evidence.
Authors are encouraged to describe how the case report is rare or unusual as well as its educational and/or scientific merits in the covering letter that accompanies the submission of the manuscript.
Any images should protect the patient’s anonymity as far as possible. Any photos or medical imaging should not show the patient's name, medical record number, or date of birth. Images should be cropped only to show the key feature. As per journal policy, JMCR does not consider images with patient faces or patient facial features. If an image of a face must be published, this should be cropped so that only the affected area is shown.
Consent for publication is a mandatory journal requirement for all case reports . Written informed consent for publication must be obtained from the patient (or their parent or legal guardian in the case of children under 18, or from the next of kin if the patient has died). For more information, please see our editorial policies .
Patient ethnicity must be included in the Abstract and in the Main Body under the Case Presentation section.
Reporting standards
For case reports, Journal of Medical Case Reports requires authors to follow the CARE guidelines . The CARE checklist should be provided as an additional files. Submissions received without these elements will be returned to the authors as incomplete.
The checklist will not be used as a tool for judging the suitability of manuscripts for publication in Journal of Medical Case Reports , but is intended as an aid to authors to clearly, completely, and transparently let reviewers and readers know what authors did and found. Using the CARE guideline to write the case report and completing the CARE checklist are likely to optimize the quality of reporting and make the peer review process more efficient.
Preparing your manuscript
The information below details the section headings that you should include in your manuscript and what information should be within each section.
Please note that your manuscript must include a 'Declarations' section including all of the subheadings (please see below for more information).
Title page
The title page should:
- "A versus B in the treatment of C: a randomized controlled trial", "X is a risk factor for Y: a case control study", "What is the impact of factor X on subject Y: A systematic review, A case report etc."
- or, for non-clinical or non-research studies: a description of what the article reports
- if a collaboration group should be listed as an author, please list the Group name as an author. If you would like the names of the individual members of the Group to be searchable through their individual PubMed records, please include this information in the “Acknowledgements” section in accordance with the instructions below
- Large Language Models (LLMs), such as ChatGPT , do not currently satisfy our authorship criteria . Notably an attribution of authorship carries with it accountability for the work, which cannot be effectively applied to LLMs. Use of an LLM should be properly documented in the Methods section (and if a Methods section is not available, in a suitable alternative part) of the manuscript
- indicate the corresponding author
The Abstract should not exceed 350 words. Please minimize the use of abbreviations and do not cite references in the abstract. The abstract must include the following separate sections:
- Background: why the case should be reported and its novelty
- Case presentation: a brief description of the patient’s clinical and demographic details, the diagnosis, any interventions and the outcomes
- Conclusions: a brief summary of the clinical impact or potential implications of the case report
Keywords
Three to ten keywords representing the main content of the article.
The Background section should explain the background to the case report or study, its aims, a summary of the existing literature.
Case presentation
This section should include a description of the patient’s relevant demographic details, medical history, symptoms and signs, treatment or intervention, outcomes and any other significant details.
Discussion and Conclusions
This should discuss the relevant existing literature and should state clearly the main conclusions, including an explanation of their relevance or importance to the field.
List of abbreviations
If abbreviations are used in the text they should be defined in the text at first use, and a list of abbreviations should be provided.
Declarations
All manuscripts must contain the following sections under the heading 'Declarations':
Ethics approval and consent to participate
Consent for publication, availability of data and materials, competing interests, authors' contributions, acknowledgements.
- Authors' information (optional)
Please see below for details on the information to be included in these sections.
If any of the sections are not relevant to your manuscript, please include the heading and write 'Not applicable' for that section.
Manuscripts reporting studies involving human participants, human data or human tissue must:
- include a statement on ethics approval and consent (even where the need for approval was waived)
- include the name of the ethics committee that approved the study and the committee’s reference number if appropriate
Studies involving animals must include a statement on ethics approval and for experimental studies involving client-owned animals, authors must also include a statement on informed consent from the client or owner.
See our editorial policies for more information.
If your manuscript does not report on or involve the use of any animal or human data or tissue, please state “Not applicable” in this section.
If your manuscript contains any individual person’s data in any form (including any individual details, images or videos), consent for publication must be obtained from that person, or in the case of children, their parent or legal guardian. All presentations of case reports must have consent for publication.
You can use your institutional consent form or our consent form if you prefer. You should not send the form to us on submission, but we may request to see a copy at any stage (including after publication).
See our editorial policies for more information on consent for publication.
If your manuscript does not contain data from any individual person, please state “Not applicable” in this section.
All manuscripts must include an ‘Availability of data and materials’ statement. Data availability statements should include information on where data supporting the results reported in the article can be found including, where applicable, hyperlinks to publicly archived datasets analysed or generated during the study. By data we mean the minimal dataset that would be necessary to interpret, replicate and build upon the findings reported in the article. We recognise it is not always possible to share research data publicly, for instance when individual privacy could be compromised, and in such instances data availability should still be stated in the manuscript along with any conditions for access.
Authors are also encouraged to preserve search strings on searchRxiv https://searchrxiv.org/ , an archive to support researchers to report, store and share their searches consistently and to enable them to review and re-use existing searches. searchRxiv enables researchers to obtain a digital object identifier (DOI) for their search, allowing it to be cited.
Data availability statements can take one of the following forms (or a combination of more than one if required for multiple datasets):
- The datasets generated and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]
- The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
- All data generated or analysed during this study are included in this published article [and its supplementary information files].
- The datasets generated and/or analysed during the current study are not publicly available due [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
- Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
- The data that support the findings of this study are available from [third party name] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [third party name].
- Not applicable. If your manuscript does not contain any data, please state 'Not applicable' in this section.
More examples of template data availability statements, which include examples of openly available and restricted access datasets, are available here .
BioMed Central strongly encourages the citation of any publicly available data on which the conclusions of the paper rely in the manuscript. Data citations should include a persistent identifier (such as a DOI) and should ideally be included in the reference list. Citations of datasets, when they appear in the reference list, should include the minimum information recommended by DataCite and follow journal style. Dataset identifiers including DOIs should be expressed as full URLs. For example:
Hao Z, AghaKouchak A, Nakhjiri N, Farahmand A. Global integrated drought monitoring and prediction system (GIDMaPS) data sets. figshare. 2014. http://dx.doi.org/10.6084/m9.figshare.853801
With the corresponding text in the Availability of data and materials statement:
The datasets generated during and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]. [Reference number]
If you wish to co-submit a data note describing your data to be published in BMC Research Notes , you can do so by visiting our submission portal . Data notes support open data and help authors to comply with funder policies on data sharing. Co-published data notes will be linked to the research article the data support ( example ).
All financial and non-financial competing interests must be declared in this section.
See our editorial policies for a full explanation of competing interests. If you are unsure whether you or any of your co-authors have a competing interest please contact the editorial office.
Please use the authors initials to refer to each authors' competing interests in this section.
If you do not have any competing interests, please state "The authors declare that they have no competing interests" in this section.
All sources of funding for the research reported should be declared. If the funder has a specific role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript, this should be declared.
The individual contributions of authors to the manuscript should be specified in this section. Guidance and criteria for authorship can be found in our editorial policies .
Please use initials to refer to each author's contribution in this section, for example: "FC analyzed and interpreted the patient data regarding the hematological disease and the transplant. RH performed the histological examination of the kidney, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript."
Please acknowledge anyone who contributed towards the article who does not meet the criteria for authorship including anyone who provided professional writing services or materials.
Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements section.
See our editorial policies for a full explanation of acknowledgements and authorship criteria.
If you do not have anyone to acknowledge, please write "Not applicable" in this section.
Group authorship (for manuscripts involving a collaboration group): if you would like the names of the individual members of a collaboration Group to be searchable through their individual PubMed records, please ensure that the title of the collaboration Group is included on the title page and in the submission system and also include collaborating author names as the last paragraph of the “Acknowledgements” section. Please add authors in the format First Name, Middle initial(s) (optional), Last Name. You can add institution or country information for each author if you wish, but this should be consistent across all authors.
Please note that individual names may not be present in the PubMed record at the time a published article is initially included in PubMed as it takes PubMed additional time to code this information.
Authors' information
This section is optional.
You may choose to use this section to include any relevant information about the author(s) that may aid the reader's interpretation of the article, and understand the standpoint of the author(s). This may include details about the authors' qualifications, current positions they hold at institutions or societies, or any other relevant background information. Please refer to authors using their initials. Note this section should not be used to describe any competing interests.
Footnotes can be used to give additional information, which may include the citation of a reference included in the reference list. They should not consist solely of a reference citation, and they should never include the bibliographic details of a reference. They should also not contain any figures or tables.
Footnotes to the text are numbered consecutively; those to tables should be indicated by superscript lower-case letters (or asterisks for significance values and other statistical data). Footnotes to the title or the authors of the article are not given reference symbols.
Always use footnotes instead of endnotes.
Examples of the Vancouver reference style are shown below.
See our editorial policies for author guidance on good citation practice
Web links and URLs: All web links and URLs, including links to the authors' own websites, should be given a reference number and included in the reference list rather than within the text of the manuscript. They should be provided in full, including both the title of the site and the URL, as well as the date the site was accessed, in the following format: The Mouse Tumor Biology Database. http://tumor.informatics.jax.org/mtbwi/index.do . Accessed 20 May 2013. If an author or group of authors can clearly be associated with a web link, such as for weblogs, then they should be included in the reference.
Example reference style:
Article within a journal
Smith JJ. The world of science. Am J Sci. 1999;36:234-5.
Article within a journal (no page numbers)
Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjønneland A, et al. Meat consumption and mortality - results from the European Prospective Investigation into Cancer and Nutrition. BMC Medicine. 2013;11:63.
Article within a journal by DOI
Slifka MK, Whitton JL. Clinical implications of dysregulated cytokine production. Dig J Mol Med. 2000; doi:10.1007/s801090000086.
Article within a journal supplement
Frumin AM, Nussbaum J, Esposito M. Functional asplenia: demonstration of splenic activity by bone marrow scan. Blood 1979;59 Suppl 1:26-32.
Book chapter, or an article within a book
Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. In: Bourne GH, Danielli JF, Jeon KW, editors. International review of cytology. London: Academic; 1980. p. 251-306.
OnlineFirst chapter in a series (without a volume designation but with a DOI)
Saito Y, Hyuga H. Rate equation approaches to amplification of enantiomeric excess and chiral symmetry breaking. Top Curr Chem. 2007. doi:10.1007/128_2006_108.
Complete book, authored
Blenkinsopp A, Paxton P. Symptoms in the pharmacy: a guide to the management of common illness. 3rd ed. Oxford: Blackwell Science; 1998.
Online document
Doe J. Title of subordinate document. In: The dictionary of substances and their effects. Royal Society of Chemistry. 1999. http://www.rsc.org/dose/title of subordinate document. Accessed 15 Jan 1999.
Online database
Healthwise Knowledgebase. US Pharmacopeia, Rockville. 1998. http://www.healthwise.org. Accessed 21 Sept 1998.
Supplementary material/private homepage
Doe J. Title of supplementary material. 2000. http://www.privatehomepage.com. Accessed 22 Feb 2000.
University site
Doe, J: Title of preprint. http://www.uni-heidelberg.de/mydata.html (1999). Accessed 25 Dec 1999.
Doe, J: Trivial HTTP, RFC2169. ftp://ftp.isi.edu/in-notes/rfc2169.txt (1999). Accessed 12 Nov 1999.
Organization site
ISSN International Centre: The ISSN register. http://www.issn.org (2006). Accessed 20 Feb 2007.
Dataset with persistent identifier
Zheng L-Y, Guo X-S, He B, Sun L-J, Peng Y, Dong S-S, et al. Genome data from sweet and grain sorghum (Sorghum bicolor). GigaScience Database. 2011. http://dx.doi.org/10.5524/100012 .
Figures, tables and additional files
See General formatting guidelines for information on how to format figures, tables and additional files.
Submit manuscript
- Editorial Board
- Manuscript editing services
- Meet the Editors
- Instructions for Editors
- Sign up for article alerts and news from this journal
Annual Journal Metrics
2022 Citation Impact 1.0 - 2-year Impact Factor 0.628 - SNIP (Source Normalized Impact per Paper) 0.284 - SJR (SCImago Journal Rank)
2023 Speed 33 days submission to first editorial decision for all manuscripts (Median) 148 days submission to accept (Median)
2023 Usage 4,048,208 downloads 2,745 Altmetric mentions
- More about our metrics

- Follow us on Twitter
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 20 May 2024
The quality of reporting in case reports of permanent neonatal diabetes mellitus: a cross-sectional study
- Pengli Jia 1 ,
- Ling Wang 2 ,
- Xi Yang 3 ,
- WenTing Pei 2 ,
- Chang Xu 4 ,
- Jinglin Feng 1 &
- Ying Han 1
BMC Medical Research Methodology volume 24 , Article number: 117 ( 2024 ) Cite this article
Metrics details
Although randomized trials and systematic reviews provide the best evidence to guide medical practice, many permanent neonatal diabetes mellitus (PNDM) studies have been published as case reports. However, the quality of these studies has not been assessed. The purpose of this study was to assess the extent to which the current case reports for PNDM comply with the Case Report (CARE) guidelines and to explore variables associated with the reporting.
Six English and four Chinese databases were searched from their inception to December 2022 for PNDM case reports. The 23 items CARE checklist was used to measure reporting quality. Primary outcome was the adherence rate of each CARE item and second outcome was total reporting score for each included PNDM case report. Linear and logistic regression analyses were used to examine the connection between five pre-specified predictor variables and the reporting quality. The predictor variables were impact factor of the published journal (<3.4 vs. ≥3.4, categorized according to the median), funding (yes vs. no), language (English vs. other language), published journal type (general vs. special) and year of publication (>2013 vs. ≤ 2013).
In total, 105 PNDM case reports were included in this study. None of the 105 PNDM case reports fulfilled all 23 items of the CARE checklist. The response rate of 11 items were under 50%, including prognostic characteristics presentation (0%), patient perspective interpretation (0%), diagnostic challenges statement (2.9%), clinical course summary (21.0%), diagnostic reasoning statement (22.9%), title identification (24.8%), case presentation (33.3%), disease history description (34.3%), strengths and limitations explanation (41.0%), informed consent statement (45.7%), and lesson elucidation (47.6%). This study identified that the PNDM case reports published in higher impact factor journals were statistically associated with a higher reporting quality.
The reporting of case reports for PNDM is generally poor. As a result, this information may be misleading to providers, and the clinical applications may be detrimental to patient care. To improve reporting quality, journals should encourage strict adherence to the CARE guidelines.
Peer Review reports
Neonatal diabetes mellitus (NDM) is a rare metabolic disease with an incidence of 90,000-160,000 neonates [ 1 ]. The permanent form of neonatal diabetes mellitus (PNDM) accounts for approximately half of all cases, with an incidence of one in 260,000 live births [ 2 ]. PNDM is a lifelong disease without remission that requires treatment throughout life [ 3 ]. The main clinical manifestations are hyperglycemia, intrauterine growth retardation, ketoacidosis, weight loss and reduced quality of life [ 4 ]. Given the severe condition and substantial medical need of PNDM, there is an urgent need for high-quality clinical research to guide PNDM clinical practice [ 5 ].
However, traditional clinical research methods for PNDM are often impeded by the scarcity and geographical dispersion of patients and the involvement of children, which can result in deficiencies in the development of clinical research evidence [ 6 ]. For example, Tudur found that compared to non-rare disease clinical trials, rare disease clinical trials are single-arm, non-randomized, non-blind, open-label, and too fragile to be terminated early [ 7 ]. Given the problems with recruitment in PNDM research, innovative strategies for rare disease clinical research are urgently required for high-quality diagnosis and treatment evidence [ 5 ].
Case reports have been used to recognize the genetic cause, main symptoms, medical, family, or psychosocial history, and clinical diagnostic, therapeutic, and prognostic information of PNDM [ 8 , 9 , 10 , 11 ]. However, there is a continuing debate about the validity of PNDM case reports and their value to practicing clinicians [ 12 ]. These case reports are generally regarded as having poor evidential quality because of their prose and spontaneous reporting [ 13 ]. Written without the benefit of reporting guidelines, case reports are often insufficiently rigorous to be aggregated for data analysis, to inform research design, or to guide clinical practice [ 13 ].
Surprisingly, general international reporting guidelines for case reports did not exist until the CARE (CAse REport) Guidelines were published [ 13 ]. Although PNDM case reports are overrepresented in the literature, little is known about reporting quality. A lack of adequate reporting of details would make the effective use of such case reports evidence less likely. Under certain circumstances, this can lead misinformed healthcare decisions. Therefore, this study conducted a cross-sectional study to specifically assess the extent to which the current case reports for PNDM complied with the CARE guidelines and explore factors associated with reporting.
Inclusion criteria
All case reports enrolled patient diagnosed with PNDM will be included. PNDM was defined as a diagnosis of diabetes within 4 or 6 weeks of birth [ 3 ]. An included case report should report useful clinical information on PNDM, such as clinical findings, patient characteristics, diagnosis or therapeutic information. There was no limitation on the publication language.
Literature search and screening
This study searched PubMed, EMBASE, Scopus, Web of Science, CINAHL, Medrxiv, and four Chinese Databases, SinoMed, National Knowledge Infrastructure (CNKI), Wanfang, and VIP, from inception to 1st of December 2022. A combination of keywords and Medical Subject Headings related to PNDM and case report was used ("pediatric”, “PNDM”, “NDM”, "permanent neonatal diabetes mellitus”, "case report”, "WRS” and "Wolcott-Rallison syndrome"). The reference lists of eligible papers were also manually screened for articles that were not identified by the computerized search. Further details are provided in Appendix 1 .
Pairs of well-trained authors, independently and in duplicate, scanned titles and abstracts to exclude obviously irrelevant studies, and potentially eligible articles were investigated in full text. Disagreements were resolved by discussion between the two reviewers; if no consensus was achieved, a third reviewer was involved.

Data collection
Data extraction was performed by two authors using a predefined data sheet that included general publication information: name of the first author, year of publication, published language, region of the first author, funding information, journal where the care report was published, and the journal’s impact factor.
The CARE guidelines checklist was used to assess the reporting quality of case reports [ 15 ]. We slightly modified the checklist by merging some sub-items into one item: 1) the four sub-item “the main symptoms of the patient, main clinical findings, the main diagnoses and interventions and the main outcomes” were merged as item 3b “Case Presentation”; 2) types of intervention (eg, pharmacologic, surgical, preventive, self-care), administration of intervention (eg, dosage, strength, duration) and changes in intervention (with rationale) were merged as item 9 “therapeutic intervention”; 3) clinician and patient-assessed outcomes, important follow-up test results (positive or negative), intervention adherence and tolerability (and how this was assessed) and adverse and unanticipated events were merged as item 10 “clinical course of all follow-up visits”; The merging resulting in 23 items of the finally CARE guideline checklist, see details in the Appendix 2 .
For each included PNDM case report, quality of reporting against the 23 items was determined as “Yes”, “Partially yes”, or “No”. The primary outcome was Adherence Rate. The Adherence rate (AR=n/N) and 95% confidence interval (CI) were used to reflect the degree of compliance of each case report to each item of CARE checklist, where n is the number of PNMD case reports adhering to the requirement of a certain item, and N is the total number of PNMD case reports. The present study summarized the AR of each item at three levels: met by 80% or above was well complied, 50 to 79% was moderately complied, and less than 50% was poorly complied.
The second outcome was the total score of reporting. The item rated as “Yes” “Partially yes” or “No” was given a point of 2, 1 or 0 respectively. Possible scores ranged from 0 to 46. Higher scores indicated better quality. The purpose of the score was to explore the connections between some pre-specified factor and reporting quality.
Data analysis
Baseline characteristics which included multinomial (language, region of first author, impact factor of the published journal) and dichotomous variables (year of publication, published journal type, sources of funding) were described as number and percentages.
This study pre-specified five variables to explore their connection to reporting quality. These were impact factor of the published journal (<3.4 vs. ≥3.4, categorized according to the median), funding (yes vs. no), language (English vs. other language), journal type (general vs. special) and year of publication (≤ 2013 vs. >2013). The year was categorized based on the year CARE was published. Reporting scores of the five pre-specified group were calculated as median and interquartile ranges (IQR). Standardized β coefficient with 95% confidence intervals (CI) were calculated using univariate and multivariate linear regression analyses to examine the association between reporting score and the pre-specified variables.
In order to avoid the bias of the score system on the results, we conducted a logistic regression in which the adherence to each 23 items CARE checklist was categorized as two group (Yes or No), the predictor factor was “published journal (<3.4 vs. ≥3.4, categorized according to the median), funding (yes vs. no), language (English vs. other language), published journal type (general vs. special) and year of publication (≤ 2013 vs. >2013). Standardized Odds Ratio (OR) with 95% CI was estimated by the logistic regression to examine the association between response quality and the five variables.
All the analyses were conducted using Stata14.0/SE software (STATA, College Station, TX, Serial number: 10699393), and alpha = 0.05 was the criterion for statistical significance.
The initial search yielded 1664 reports, of which 1316 were eliminated due to duplication or title and abstract screening. After full-text reading, 105 case reports on PNDM were included. No additional case reports were identified through the reference list screening (Fig. 1 ).
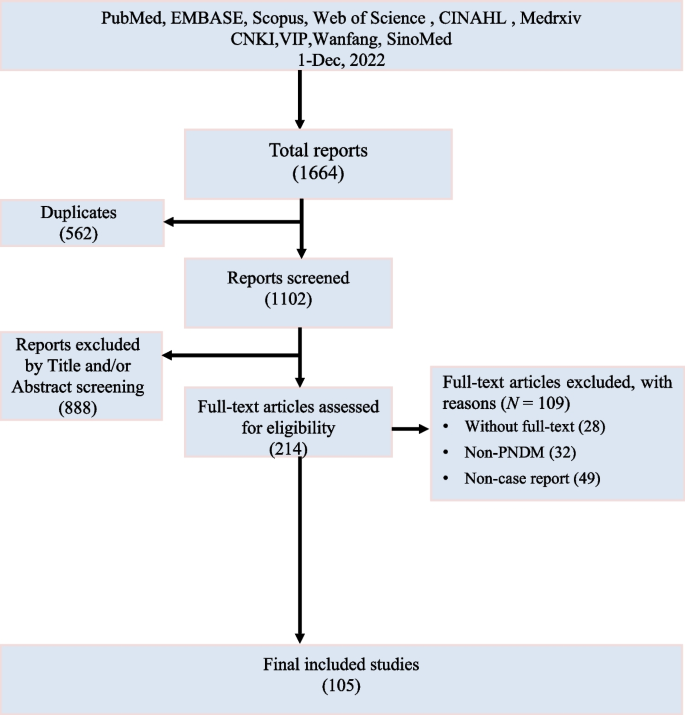
Flow plot of literature search and screening
Characteristics of included studies
A total of 105 PNDM case reports were published between 1971 and December 2022. The majority were published in English (93.33%). Research groups from Asian contributed most (40.00%), followed by European (38.09%), and North American (17.14%) groups. Majority of case reports were published in specialized journals (86.67%), such as pediatrics and endocrinology. The median impact factor for the published journals was 3.40 (IQR: 1.48, 4.50). Almost half of the included cases reported funding resources (57/105), all of which were provided by nonprofit funding agencies (Table 1 ).
Adherence rate of each reporting item
The overall CARE scores resulted in a median score of 28 (IQR: 23, 30). None of the 105 PNDM case reports fulfilled all 23 items of the CARE checklist: five out of 23 items were well complied, seven were moderately complied, and 11 were poorly complied. The adherence rates for the items reported in the CARE checklist are listed in Table 2 .
The title section item, which was identified as “elucidated the study as ‘case report’ along with phenomenon of greatest interest”, was poorly complied (AR=24.8%, 95% CI: 16.4, 33.2%). The keyword element describing the key information of the case as 2-5 words was moderately complied with 61.9% (95% CI: 52.5, 71.3%) of the PNDM case reports adhering this item.
Of the three items in the abstract section, the item of introduction narration was moderately complied (AR=60.0%, 95% CI:50.5, 69.5%), while the other two items were poorly complied: case presentation (AR=33.3%, 95% CI:24.2, 42.5%) and lesson elucidation (AR=47.6%, 95% CI:37.9, 57.3%). The background summary was complied by 79.0% (95% CI: 71.1, 87.0%) of the PNDM case repots.
In terms of the patient information (three items), 59 (AR=56.2%, 95 CI:46.5, 65.8%) provided details of demographic information, and a large proportion (AR=96.2%, 95% CI: 92.5, 99.9%) specified the main symptoms of the patient, while only a small proportion (AR=34.3%, 95% CI:25.1, 43.5%) specified details regarding the medical, family, and psychosocial history.
Within the diagnostic assessment element, there were 4 items identified, including clarifying the diagnostic methods (AR=94.3%, 95% CI: 89.8, 98.8%), diagnostic reasoning (AR=22.9%, 95% CI: 14.7, 31.0%.), diagnostic challenges (AR=2.9%, 95% CI: -0.4, 6.1%) and prognostic characteristics (AR=0%).
Of the four items in the discussion section, relevant medical literature, rationale for conclusion and main take-away’ lessons were evaluated completely in 90 (AR=85.7%, 95% CI: 78.9, 92.5%), 89 (AR=84.8%, 95% CI: 77.8, 91.8%) and 69 (AR=65.7%, 95% CI: 56.5, 74.9%) PNDM case reports, respectively. Total compliance was less than 50% in the strengths and limitations item (41.0%, 95%CI: 31.4, 50.5%).
With regard to the four separately specified items, description of physical examination (AR=89.5%, 95%CI:83.6, 95.5%) was highly adhered, types of intervention (AR=75.2%, 95%CI:66.8, 83.6%) and important dates and times (AR=56.2%, 95%CI:46.5, 65.8%) were moderately adhered. The remaining item summarized the clinical course of all follow-up visits (AR=21.0%, 95%CI:13.0, 28.9%) was poorly addressed.
For the two alternative items, informed consent was poorly complied (AR=45.7%, 95CI: 36.0, 55.4), while the reporting of patient perspective was seriously limited (AR=0%).
Factors associated with the reporting quality
The median and IQR of reporting score in the case reports published with funding, in English language and after year 2013 were 27.0 (23.5 to 30.5), 27.5 (23.7, 30.0) and 28.0 (24.0, 31.0). For those case reports that in general and impact factor ≥3.4 journals, the median and IQR of reporting score were 25.0 (21.2, 29.0) and 27.0 (22.0, 29.0).
Multivariable linear regression analyses showed that PNDM case reports published in higher impact factor journals were statistically associated with a higher total score (standardized β coefficient 0.27, 95% CI: -4.98 to 0.59), while those published in recent years (standardized β coefficient 0.12, 95% CI: -0.89 to 3.46), in English (standardized β coefficient -0.14, 95% CI: -7.08 to 1.48), in a general journal (standardized β coefficient -0.17, 95% CI: -5.79 to 0.50), and with funding supporting (standardized β coefficient -0.90, 95% CI: -3.09 to 1.29) were not associated with the reporting (Table 3 ).
The multiple logistic regression showed that PNDM case reports published in English (OR 15.94, 95% CI 1.59, 160.16) and higher impact factor journals (impact factor ≥3.4) (OR 2.77, 95% CI 1.03, 7.40) were associated with a higher likelihood of case presentation. Similarly, PNDM case reports published in the higher impact factor journals were more likely to achieve reporting the conclusion (OR 3.21, 95% CI 1.29, 8.00) and brief background summary (OR 6.23, 95% CI 1.50, 25.71). PNDM case reports published in general journals (OR 7.53, 95% CI 1.43, 39.76) and with funding support (OR 3.78, 95% CI 1.45, 9.85) were associated with a higher likelihood of achieving informed consent (Table 4 ).
The present study collected case reports on PNDM over the past half century. To the best of our knowledge, this is the first epidemiological study to systematically assess the extent to which case reports comply with reporting guidelines in this specific field. A total of 105 case reports for PNDM were identified. Across these case reports, this study found that the critical details regarding prognostic characteristics, patient perspectives, diagnostic challenges, follow-up visits, diagnostic reasoning, title and case presentation were often omitted. The apparent low adherence rate was primarily due to poor reporting; however, the non-mandatory requirement (patient perspective or prognostic characteristics) of the items may also affect the assessment [ 14 ]. The failure to report diagnostic information was probably due to the lack and disarray of diagnostic criteria in the area of rare diseases [ 5 ]. The under-reporting of follow-up visits could be partly because this information was not available, as the patient did not revisit the physician or died because of progressive disease [ 16 ].
Conversely, this study found that the items related to therapeutic intervention were better reported (more than 70% of case studies complied completely), such as the type, administration and changes in intervention. This finding was consistent with studies addressing the reporting quality using CARE guidelines in high-impact journals (AR=79.9%) [ 17 ], coronavirus disease (AR=84.0%) [ 18 ] and dental trauma field (AR=98.0%) [ 19 ]. A study conducted in emergency medicine used self-made 11 items scale by referring to clinical epidemiology textbooks, guidelines for critical appraisal studies, and the Users’ Guides to Evidence-Based Medicine also found similar result (AR=79.9%) [ 12 ]. Although the evaluation tools are different, these studies reflected the attentions of clinical intervention by authors, editors, and peer reviewers.
The inconsistent and suboptimal reporting across items implies that certain items may have been treated differently, as to their importance [ 20 ]. Retaining more clinically significant content and removing details about the methodology was often suggested by the editor, as journals usually pay more attention to the clinical value of research [ 21 ]. Given that some PNDM case reports were published as letters that may have strict word limitations, the deletion of “non-sense” information is even more common [ 12 ]. We would argue that while journal space is valuable, editors must balance the need to be concise with the importance of adequate case descriptions.
Both our linear and logistic regression analyses identified that the PNDM case reports published in higher impact factor journals were statistically associated with a higher reporting quality. This was consistent with the research published in 2018 and 2020 [ 17 , 22 ]. Even though the use of journal impact factor as surrogate metric to measure journal quality is controversial [ 23 ], but it’s worth to mention that the overall completeness in reporting was high for CARE endorsing journals, such as the BMJ Case Reports and JAMA [ 17 ].
Strengths and limitations
This study has several strengths. We innovatively assessed the quality of the PNDM case reports using the widely accepted CARE checklist. Second, a comprehensive search, explicit eligibility criteria, rigorous methods for screening studies and data collection ensured transparency and reproducibility of judgments. Third, the use of two independent reviewers for the preselection of case reports, assessment quality and data extraction was of great help in avoiding errors and subjective judgments.
This study has some limitations. First, the results were confined to PNDM case reports, which constituted a small fraction of case reports. Second, we scored reporting quality and added a category “Partially yes” to each item that may skew the results. Third, we did not include any grey literature and the reporting quality of these case reports was unknown. We expect such a report to be rare. Fourth, the non-mandatory requirement of some items may underestimate the results of the reporting quality.
Reporting of PNDM case reports is generally suboptimal. Substantial effort is needed to improve reporting, especially the reporting of case presentation, diagnostic assessment, follow-up, and outcomes. A larger word count may be beneficial for better reporting. To improve reporting quality, journals should encourage strict adherence to the CARE guidelines.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
Adherence rate
Confidence interval
CAse REport
Interquartile range
Neonatal diabetes mellitus
- Permanent neonatal diabetes mellitus
Lemelman MB, Letourneau L, Greeley SAW. Neonatal diabetes mellitus: an update on diagnosis and management. Clin Perinatol. 2018;45(1):41–59.
Article PubMed Google Scholar
Habeb AM, Al-Magamsi MS, Eid IM, Ali MI, Hattersley AT, Hussain K, et al. Incidence, genetics, and clinical phenotype of permanent neonatal diabetes mellitus in northwest Saudi Arabia. Pediatr Diabetes. 2012;13(6):499–505.
Article CAS PubMed Google Scholar
Slingerland AS, Shields BM, Flanagan SE, Bruining GJ, Noordam K, Gach A, et al. Referral rates for diagnostic testing support an incidence of permanent neonatal diabetes in three European countries of at least 1 in 260,000 live births. Diabetologia. 2009;52(8):1683–5.
Article CAS PubMed PubMed Central Google Scholar
Beltrand J, Elie C, Busiah K, Fournier E, Boddaert N, Bahi-Buisson N, et al. Sulfonylurea therapy benefits neurological and psychomotor functions in patients with neonatal diabetes owing to potassium channel mutations. Diabetes Care. 2015;38(11):2033–41.
Dunoyer M. Accelerating access to treatments for rare diseases. Nat Rev Drug Discovery. 2011;10(7):475–6.
van der Lee JH, Wesseling J, Tanck MW, Offringa M. Efficient ways exist to obtain the optimal sample size in clinical trials in rare diseases. J Clin Epidemiol. 2008;61(4):324–30.
Bell SA, Tudur Smith C. A comparison of interventional clinical trials in rare versus non-rare diseases: an analysis of ClinicalTrials.gov. Orphanet J Rare Dis. 2014;9:170.
Article PubMed PubMed Central Google Scholar
Ille J, Putarek NR, Radica A, Hattersley A, Ellard S, Dumić M. Low doses of sulphonyluria as a successful replacement for insulin therapy in a patient with neonatal diabetes due to a mutation of KCNJ11 gene encoding Kir6.2. Lijecnicki Vjesnik. 2010;132(3–4):90–3.
PubMed Google Scholar
Kim MS, Kim SY, Kim GH, Yoo HW, Lee DW, Lee DY. Sulfonylurea therapy in two Korean patients with insulin-treated neonatal diabetes due to heterozygous mutations of the KCNJ11 gene encoding Kir6.2. J Korean Med Sci. 2007;22(4):616–20.
Mirza A, Dhillon RA, Irfan O, Amin A, Salat M. Neonatal diabetes mellitus - is trisomy 21 associated with refractory hyperglycaemia? J Ayub Med Coll Abbottabad. 2022;34(Suppl 1)(3):S717–s19.
Razzaghy-Azar M, Nourbakhsh M, Talea A, Mohammad Amoli M, Nourbakhsh M, Larijani B. Meglitinide (repaglinide) therapy in permanent neonatal diabetes mellitus: two case reports. J Med Case Rep. 2021;15(1):535.
Richason TP, Paulson SM, Lowenstein SR, Heard KJ. Case reports describing treatments in the emergency medicine literature: missing and misleading information. BMC Emerg Med. 2009;9:10.
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med. 2013;2(5):38–43.
Rison RA, Kidd MR, Koch CA. The CARE (CAse REport) guidelines and the standardization of case reports. J Med Case Reports. 2013;7:261.
Article Google Scholar
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013:bcr2013201554.
Kaszkin-Bettag M, Hildebrandt W. Case reports on cancer therapies: the urgent need to improve the reporting quality. Glob Adv Health Med. 2012;1(2):8–10.
Calvache JA, Vera-Montoya M, Ordoñez D, Hernandez AV, Altman D, Moher D. Completeness of reporting of case reports in high-impact medical journals. Eur J Clin Invest. 2020;50(4):e13215.
Scaffidi MA, Gimpaya N, Li J, Bansal R, Verma Y, Elsolh K, et al. Completeness of reporting for COVID-19 case reports, January to April 2020: a meta-epidemiologic study. CMAJ Open. 2021;9(1):E295–e301.
Seguel-Moraga P, Onetto JE, Uribe SE. Reporting quality of case reports about dental trauma published in international journals 2008–2018 assessed by CARE guidelines. Dent Traumatol. 2021;37(2):345–53.
Jia P, Tang L, Yu J, Liu J, Kang D, Sun X. The quality of reporting in randomized controlled trials of acupuncture for knee osteoarthritis: a cross-sectional survey. PLoS One. 2018;13(4):e0195652.
Kim KH, Kang JW, Lee MS, Lee JD. Assessment of the quality of reporting for treatment components in cochrane reviews of acupuncture. BMJ Open. 2014;4(1):e004136.
Ravi R, Mulkalwar A, Thatte UM, Gogtay NJ. Medical case reports published in PubMed-indexed Indian journals in 2015: adherence to 2013 CARE guidelines. Indian J Med Ethics. 2018;3(3):192–5.
Bornmann L, Marx W. The journal impact factor and alternative metrics: a variety of bibliometric measures has been developed to supplant the impact factor to better assess the impact of individual research papers. EMBO Rep. 2016;17(8):1094–7.
Download references
Acknowledgements
Not applicable.
This project was supported by the Fundamental Research Program of Shanxi Province No. 202303021222159.
Author information
Authors and affiliations.
School of Management, Shanxi Medical University, Taiyuan, China
Pengli Jia, Jinglin Feng & Ying Han
The Second Clinical Medical College of Anhui Medical University, Hefei, China
Ling Wang & WenTing Pei
Department of Maternal, Child and Adolescent Health, School of Public Health, Anhui Medical University, Hefei, China
Proof of Concept Center, Eastern Hepatobiliary Surgery Hospital, Third Affiliated Hospital, Second Military Medical University, Naval Medical University, Shanghai, China
You can also search for this author in PubMed Google Scholar
Contributions
PLJ conceived and designed the study, analyzed the data, and drafted the manuscript; LW, XY and WTF collected the data, assessed the quality; CX and JLF screened the literature; YH and PLJ provided methodological comments and revised the manuscript. All authors revised the manuscript.
Corresponding author
Correspondence to Ying Han .
Ethics declarations
Ethics approval and consent to participate.
Not applicable. This study did not report on or involve any animal or human participants, human material, or human data.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publishers’ note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Jia, P., Wang, L., Yang, X. et al. The quality of reporting in case reports of permanent neonatal diabetes mellitus: a cross-sectional study. BMC Med Res Methodol 24 , 117 (2024). https://doi.org/10.1186/s12874-024-02226-1
Download citation
Received : 14 November 2023
Accepted : 22 April 2024
Published : 20 May 2024
DOI : https://doi.org/10.1186/s12874-024-02226-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Case report
- Reporting quality
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]

IRIS MABRY-HERNANDEZ, MD, MPH, Medical Officer, U.S. Preventive Services Task Force, Agency for Healthcare Research and Quality
SUSAN J. CHING, DO, Preventive Medicine Resident, Uniformed Services University of the Health Sciences
Am Fam Physician. 2024;109(5):457-458
Related editorial: Anxiety Screening Is Unlikely to Improve Mental Health Outcomes
Related USPSTF Clinical Summary: Screening for Anxiety Disorders in Adults
Author disclosure: No relevant financial relationships.
A 34-year-old patient (gravida 2, para 2) presents for a well-woman examination and Papanicolaou smear. She feels healthy and has no significant medical history, aside from her uncomplicated pregnancies, which did not include postpartum depression or anxiety. She reports increased stress at home due to an upcoming move and some difficulty sleeping.
Case Study Questions
1 . According to the U.S. Preventive Services Task Force (USPSTF) recommendation, which one of the following is advised for this patient?
A. Screen for anxiety disorder.
B. Assess her anxiety in 6 months.
C. Refer her to an obstetrician-gynecologist for postpartum anxiety screening.
D. Recommend melatonin.
E. Refer her to a behavioral health professional for sleep management.
2 . According to the Diagnostic and Statistical Manual of Mental Disorders , 5th ed. (DSM-5), which of the following can be categorized as anxiety disorders?
A. Generalized anxiety disorder.
B. Obsessive-compulsive disorder.
C. Separation anxiety disorder.
D. Social anxiety disorder.
3 . Which one of the following populations should be screened for anxiety disorders, according to the USPSTF recommendation?
A. People already diagnosed with anxiety or another mental health disorder.
B. People younger than 18 years.
C. People older than 65 years.
D. People with no recognized signs or symptoms of anxiety disorders.
The correct answer is A . The USPSTF recommends screening all adults 19 to 64 years of age for anxiety disorder, including those who are pregnant and postpartum. The USPSTF notes there is little evidence for the ideal timing and frequency of anxiety screening for perinatal and general adult populations. 1 However, clinical judgment, particularly considering risk factors, comorbid conditions, and life events, can determine whether additional screening of high-risk patients is warranted. There is a lack of evidence on screening rates for anxiety disorders. Underdetection appears to be common. Patients with anxiety disorders may present with other concerns, such as sleep disturbances or somatic issues.
The correct answers are A, C, and D . The DSM-5 recognizes the following types of anxiety disorders: generalized anxiety disorder, social anxiety disorder, panic disorder, agoraphobia, specific phobias, separation anxiety disorder, selective mutism, substance or medication-induced anxiety disorder, anxiety disorder due to another medical condition, and anxiety not otherwise specified. 2 Obsessive-compulsive disorder is not considered an anxiety disorder.
The correct answer is D . The USPSTF recommendation statement applies to adults (defined as those 19 to 64 years of age), including people who are pregnant or postpartum, who do not have a diagnosed mental health disorder and are not showing recognized signs or symptoms of anxiety disorders. 2 For people 65 years or older, the USPSTF concludes that the evidence is insufficient to recommend for or against screening for anxiety disorders.
The views expressed in this work are those of the authors and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences, the U.S. Department of Defense, or the U.S. government.
This PPIP quiz is based on the recommendations of the USPSTF. More information is available in the USPSTF Recommendation Statement and supporting documents on the USPSTF website ( https://www.uspreventiveservicestaskforce.org ). The practice recommendations in this activity are available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/anxiety-adults-screening .
O’Connor EA, Henninger ML, Perdue LA, et al. Anxiety screening: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2023;329(24):2171-2184.
Barry MJ, Nicholson WK, Silverstein M, et al. Screening for anxiety disorders in adults: US Preventive Services Task Force recommendation statement. JAMA. 2023;329(24):2163-2170.
This series is coordinated by Joanna Drowos, DO, contributing editor.
A collection of Putting Prevention Into Practice published in AFP is available at https://www.aafp.org/afp/ppip.
Continue Reading
More in AFP
Copyright © 2024 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 13, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Study shows ChatGPT can accurately analyze medical charts for clinical research, other applications
by UT Southwestern Medical Center
ChatGPT, the artificial intelligence (AI) chatbot designed to assist with language-based tasks, can effectively extract data for research purposes from physicians' clinical notes, UT Southwestern Medical Center researchers report in a new study.
Their findings , published in npj Digital Medicine , could significantly accelerate clinical research and lead to new innovations in computerized clinical decision-making aids.
"By transforming oceans of free-text health care data into structured knowledge, this work paves the way for leveraging artificial intelligence to derive insights, improve clinical decision-making, and ultimately enhance patient outcomes ," said study leader Yang Xie, Ph.D., Professor in the Peter O'Donnell Jr. School of Public Health and the Lyda Hill Department of Bioinformatics at UT Southwestern.
Dr. Xie is also Associate Dean of Data Sciences at UT Southwestern Medical School, Director of the Quantitative Biomedical Research Center, and a member of the Harold C. Simmons Comprehensive Cancer Center.
Much of the research in the Xie Lab focuses on developing and using data science and AI tools to improve biomedical research and health care. She and her colleagues wondered whether ChatGPT might speed the process of analyzing clinical notes—the memos physicians write to document patients' visits, diagnoses, and statuses as part of their medical record—to find relevant data for clinical research and other uses.
Clinical notes are a treasure trove of information, Dr. Xie explained; however, because they are written in free text, extracting structured data typically involves having a trained medical professional read and annotate them. This process requires a huge investment of time and often resources—and can also introduce human bias.
Existing programs that use natural language processing require extensive human annotation and model training. As a result, clinical notes are largely underused for research purposes.
To determine whether ChatGPT could convert clinical notes to structured data, Dr. Xie and her colleagues had it analyze more than 700 sets of pathology notes for lung cancer patients to find the major features of primary tumors, whether lymph nodes were involved, and the cancer stage and subtype.
Overall, Dr. Xie said, the average accuracy of ChatGPT to make these determinations was 89%, based on reviews by human readers.
Their analysis took several weeks of full-time work compared with the few days it took to fine-tune data extraction from the ChatGPT model. This accuracy was significantly better than other traditional natural language processing methods tested for this use.
To test whether this approach is applicable to other diseases, Dr. Xie and her colleagues used ChatGPT to extract information about cancer grade and margin status from 191 clinical notes on patients from Children's Health with osteosarcoma, the most common type of bone cancer in children and adolescents. Here, ChatGPT returned information with nearly 99% accuracy on grade and 100% accuracy on margin status.
Dr. Xie noted that the results were strongly influenced by what prompts ChatGPT was given to perform each task—a phenomenon called prompt engineering. Providing multiple options to choose from, giving examples of appropriate responses, and directing ChatGPT to rely on evidence to draw conclusions improved its performance.
She added that using ChatGPT or other large language models to extract structured data from clinical notes could not only speed clinical research but also help clinical trial enrollment by matching patients' information to clinical trial protocols. However, she said, ChatGPT won't replace the need for human physicians.
"Even though this technology is an extremely promising way to save time and effort, we should always use it with caution. Rigorous and continuous evaluation is very important," Dr. Xie said.
Explore further
Feedback to editors

Study sheds light on bacteria associated with pre-term birth
44 minutes ago

Researchers find intriguing connections between Alzheimer's disease and other common conditions
Research shows linked biological pathways driving skin inflammation

Bioluminescence and 3D-printed implants shed light on brain–spinal interactions
2 hours ago

Researchers uncover biological trigger of early puberty

Social media-related nightmares: Study explores links between social media use, mental health and sleep quality

New device helps paraplegics regain partial use of hands
3 hours ago

New CRISPR screening method could reveal what drives brain diseases

Happy or angry: Researchers discover brain network that recognizes emotions
Study shows exercise spurs neuron growth and rewires the brain, helping mice forget traumatic and addictive memories
Related stories.
ChatGPT found to display lower concern for child development 'warning signs' than physicians
May 3, 2024

Study: ChatGPT extracts data for ischemic stroke almost perfectly, is useful for thrombectomy data transfer
Apr 19, 2024
Good evidence confuses ChatGPT when used for health information, study finds
Apr 3, 2024

Study shows ChatGPT can produce medical record notes 10 times faster than doctors without compromising quality
Mar 26, 2024

ChatGPT shows poor performance in answering drug-related questions
Dec 12, 2023

Study shows ChatGPT can be helpful for Black women's self-education about HIV, PrEP
Recommended for you.

New AI algorithm may improve autoimmune disease prediction and therapies
4 hours ago
New analysis estimates the effects of race-neutral lung function testing on patients, hospitals, and beyond
May 19, 2024

Artificial intelligence and the future of surgery
May 17, 2024

Research team develops new AI tool to help classify brain tumors

Global life expectancy projected to increase by nearly 5 years by 2050 despite various threats
May 16, 2024

New tool can help surgeons quickly search videos and create interactive feedback
May 15, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

Writing a BMJ Case Report
- BMJ Library Resource Centre
- BMJ Case Reports resources
Resource Centre
- BMJ Case Reports
- Print Resources
- Digital resources
- Support and training
Do you want to make sure your BMJ Case Report is accepted, but not sure where to start? Then simply download this guide and follow these easy steps to publishing your first Case Report.
BMJ publishes the world's largest repository of case reports, BMJ Case Reports, now with nearly 19,000 cases and growing.

Study: More people dying on Hampton Roads highways
H AMPTON ROADS, Va. (WAVY) — Too many people are dying on roads in our region, according to a study presented Thursday to the Hampton Roads Regional Transportation Planning Organization.
Consider, distracted driving, alcohol use, safety belt use, speeding — 70% of traffic fatalities include at least one of those. Taking away those four would save 100 lives per year, according to a regional traffic study.
The study laid out those reasons, and what can be done about them.
The Safety Study on Crash Fatalities found reckless speeding was the number one cause of death. The study conducted by Keith Nichols who is Principal Transportation Engineer for the Hampton Roads Transportation Organization (HRTPO)
“There is a lot of speeding in our region and it is becoming a bigger problem, especially reckless driving, and speeding 20 miles an hour above the speed limit,” said Keith Nichols, principal transportation engineer for the HRTPO.
Nichols, along with chief transportation engineer Rob Case, presented their findings on traffic fatalities to the HRTPO.
They say they went through thousands of VDOT records to get speeding data.
Nothing was more stunning during the meeting than Case’s deep dive into the connection between police not giving speeding tickets and increasing speeds on our roads.
“This is a much more perfect relationship between citations coming down in orange and speeding going up in black,” Case said.
The graph made the connection between the low number of police citations and a hike in speeding.
The finding — drivers speed more when police are not writing tickets.
They argue in the safety study that too many people are dying on our roads.
“So, the major takeaways we are seeing are especially when it comes to the number of fatalities in our region,” Case said. “Unfortunately, the number is increasing, especially since the pandemic, we have seen a large increase in the number of people killed on our roadways in our region each year.”
In 2011, there were 120 to 140 crash fatalities. By 2021, there were 179 fatalities, 166 fatalities in 2022 and 166 fatalities in 2023. The totals are similar over the three year period, but they’re well above the 2011 total.
“The number of fatalities is up, so crashes have become more fatal more dangerous more problematic,” Case said.
People are also driving differently, and more dangerously, since the pandemic.
“People’s moods, I guess,” Nichols said. “First and foremost, people are just driving faster. They are taking more reckless actions in their vehicles, things like that.”
Congestion levels on the roads dropped during the pandemic.
“So, less congestion on the road makes for more ability to drive faster,” Nichols said. “Once again, we are still seeing that continue even as we return to normal traffic volumes and normal congestion level. So, there is something beyond just that. It is just people driving differently post-pandemic.”
He added: “We have a different frame of mind, and the pandemic changed our driving habits. There is anger on the road. … People are hitting the gas. They are yelling at people around them more. We are just seeing these trends post-pandemic. It is just that people have a different frame of mind.”
This regional safety study comes ahead of Andy Fox’s special report on Danger Ahead.
10 On Your Side takes you to the most dangerous intersections based on the number of crashes, deaths, and serious injuries in Hampton Roads.
See below to see the full Hampton Roads Transportation Planning Organization safety report.
For the latest news, weather, sports, and streaming video, head to WAVY.com.

A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- About Violence Prevention
- Resources for Action
- Cardiff Model
Violence Prevention

About The Public Health Approach to Violence Prevention
Violence Topics

About Adverse Childhood Experiences
About Child Abuse and Neglect
About Community Violence
About Firearm Injury and Death
About Intimate Partner Violence
About Sexual Violence
About Youth Violence
About Abuse of Older Persons
About Violence Against Children and Youth Surveys
About The National Intimate Partner and Sexual Violence Survey (NISVS)
About The National Violent Death Reporting System (NVDRS)
Featured Resources

Essentials for Parenting Teens

Essentials for Parenting Toddlers and Preschoolers

Cardiff Violence Prevention Model Toolkit
Violence is an urgent public health problem. CDC is committed to preventing violence so that everyone can be safe and healthy.
For Everyone
Public health.
Staff Members
222 results returned.

Samantha Agbeblewu
Lending Assistant

Jack (John) Ahern
Director of Annual Giving

Kimberly Allen
Projects and Gov Docs Metadata Assistant
Chris (Christopher) Allen
Library Facilities Manager

Nate (Nathaniel) Allen
Carolina Blu Delivery & Weekend Supervisor
Meaghan Alston
Assistant Curator for African American Collections

Katelyn Ander
Media Center Manager

Juan Arango
Records Management & Searching Coordinator

Baz Armstrong
Circulation Supervisor/Technology Lending Coordinator

Jess (Jessica) Aylor
Executive Director of Library Development

IMAGES
VIDEO
COMMENTS
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant ...
Writing up. Write up the case emphasising the interesting points of the presentation, investigations leading to diagnosis, and management of the disease/pathology. Get input on the case from all members of the team, highlighting their involvement. Also include the prognosis of the patient, if known, as the reader will want to know the outcome.
3. Write an abstract summarizing the entire article. Your abstract should be one of the last things you write—and it's often the most challenging. In about 150 words, provide readers with the nuts and bolts of your case study: the case itself, the problem it presents, and the overall message conveyed.
Begin with the case presentation (box 2): describe your encounter with the patient, their symptoms, and their signs. You should already have an idea what your take home messages will be. If the journal presentation of the case report allows, you can write these take home messages as bullet points (box 3).
Writing the core of your case report: clinical findings and outcome Step 1: state the obvious ... 1 The following article was published in Oxford Medical Case Reports. Bottineau et al. A misleading appearance of a common diseases: tuberculosis with generalized lymphodenopathy - a Case Report." ... and in many studies. It is recommended for use
It is best to write the actual report in one stretch if possible, including as much detail as you think is relevant. You can always edit the discussion and trim down the article at a later stage. Below is the general format adopted for most case reports. Introduction. Summarise your case report in a sentence. Mention how rare this condition is ...
Sometimes case reports include a short literature review, if you think it is worthwhile, include it. 2. Describe your patient and follow the diagnostic pathway. For example - Patient X is a 10 ...
Title of case. You do not need to include "a case report" in the title - you may be cryptic if you wish. Summary. This will be freely available online. Up to 150 words summarising the case presentation and outcome. We need a good flavour of the case - emphasise the learning points. Background.
A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient. The most important aspect of a case report, i.e. the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
Case reports are a time-honored tradition in the medical profession. From Hippocrates (460 B.C. to 370 B.C.), and even arguably further back since the papyrus records of ancient Egyptian medicine (c. 1600 B.C.) to modern day, physicians of all specialties have described interesting cases involving all specialties [1, 2].Published case reports provide essential information for optimal patient ...
Case reports in medical education: a platform for training medical students, residents, and fellows in scientific writing and critical thinking. A case report is a detailed narrative that usually illustrates a diagnostic or therapeutic problem experienced by one or several patients. Case reports commonly serve as the first line of evidence for ...
A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient's history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
A clinical case report or case study is a means of disseminating new knowledge gained from clinical practice. Clinical case reports are the first-line evidence in medical literature as they present original observations. This article provides detailed guidance on how to identify, write, and publish a case report.
can introduce very effective treatment paradigms. Preparing the manuscript for a case report may be the first exposure to scientific writing for a budding clinician/researcher. This manuscript describes the steps of writing a case report and essential considerations when publishing these articles. Individual components of a case report and the "dos and don'ts" while preparing these ...
Case Report abstract s that do not provide meaningful teaching points will not be accepted. Title . The abstract title should emphasize the clinical condition and main teaching point. Format . Case Report abstracts must be submitted in the following structured format: • Introduction or Background • Clinical Case (including diagnostic ...
For case reports, Journal of Medical Case Reports requires authors to follow the CARE guidelines.The CARE checklist should be provided as an additional files. Submissions received without these elements will be returned to the authors as incomplete. The checklist will not be used as a tool for judging the suitability of manuscripts for publication in Journal of Medical Case Reports, but is ...
Although randomized trials and systematic reviews provide the best evidence to guide medical practice, many permanent neonatal diabetes mellitus (PNDM) studies have been published as case reports. However, the quality of these studies has not been assessed. The purpose of this study was to assess the extent to which the current case reports for PNDM comply with the Case Report (CARE ...
She reports increased stress at home due to an upcoming move and some difficulty sleeping. A 34-year-old patient (gravida 2, para 2) presents for a well-woman examination and Papanicolaou smear.
Traumatic Brain Injury & Concussion A traumatic brain injury, or TBI, is an injury that affects how the brain works. TBI is a major cause of death and disability in the United States.
Clinical notes are a treasure trove of information, Dr. Xie explained; however, because they are written in free text, extracting structured data typically involves having a trained medical ...
BMJ publishes the world's largest repository of case reports, BMJ Case Reports, now with nearly 19,000 cases and growing. Do you want to make sure your BMJ Case Report is accepted, but not sure where to start? Then simply download this guide and follow these easy steps to publishing your first Case Report. Download 'Writing and publishing a ...
This regional safety study comes ahead of Andy Fox's special report on Danger Ahead. 10 On Your Side takes you to the most dangerous intersections based on the number of crashes, deaths, and ...
Each case study will have 11 questions to be answered. Key Requirements: - Medical Background: The ideal candidate should have a medical background or experience in writing medical content. - Attention to Detail: It's crucial to provide accurate and concise answers to each question. - Professionalism: The case studies should be written in a ...
Outcomes. ACEs can have lasting effects on health and well-being in childhood and life opportunities well into adulthood. 9 Life opportunities include things like education and job potential. These experiences can increase the risks of injury, sexually transmitted infections, and involvement in sex trafficking.
208 Raleigh Street CB #3916 Chapel Hill, NC 27515-8890 919-962-1053