

Xanthophyta (Yellow Green Algae): An Overview
Animesh sahoo.
- October 22, 2023
The members of the Xanthophyta or Heterokontae (class Xanthophyceae) are characterized by their yellow-green pigments, including xanthophylls, which distinguish them from other algae.
They are commonly known as yellow-green algae .
Table of Contents
Characteristics of Xanthophyta
Xanthophyta show the following general characteristics:
- Members of the Xanthophyta are mostly freshwater algae .
- The plant body may be unicellular or multicellular (colonial, palmelloid, or coccoid).
- The cell wall is often absent, but when present, it contains a higher content of pectic substances.
- The chromatophores are discoid, with many in each cell.
- Pyrenoids are usually absent.
- Plastids are yellow-green in colour. They contain chlorophyll-a, chlorophyll-c, β-carotene, and xanthophyll (diadinoxanthin, violaxanthin, and lutein).
- Food reserves are oil, lipids, and leucosin.
- Motile bodies often bear two flagella. The flagella are unequal and inserted at the anterior end.
- Asexual reproduction takes place by zoospores, aplanospores, or akinetes.
- Sexual reproduction is rare. It’s mostly isogamous.

Distribution of Xanthophyta
There are about 100 genera and 600 species in the division Xanthophyta.
Most of the species are found in freshwater ponds and lakes (e.g., Tribonema). Some species can grow on drying mud (e.g., Botrydium) and also on damp walls and tree trunks (e.g., Ophiocytium).
A few members are marine.
Thallus Structure of Xanthophyta
There are varied forms of vegetative thalli, ranging from unicellular motile (e.g., Chlorochromonas), palmelloid siphonaceous (e.g., Botrydium), to multicellular filamentous form (e.g., Tribonema).

Vegetative cells are uninucleate, except in siphonaceous forms. The cell wall is composed mainly of pectic substances (either pectic acid or pectose) with a smaller amount of cellulose.
In Ophiocytium, the wall is made up of two halves, and as the cell grows, the tabular position elongated, with its smaller portions overlapping each other. In filamentous genera like Tribonema, H-shaped walls are present.
Each cell contains one or more discoid chromatophores in the protoplast. Due to the presence of excess xanthophyll (β-carotene, diadinoxanthin, violaxanthin, and lutein), the colour of chromatophores is yellow-green. There are also chlorophyll-a and chlorophyll-e present.
Chromatophores usually lack pyrenoids; in some species (e.g., Botrydium), pyrenoids are present.
In the form of a photosensitive organ, the eye spot can be seen, and near the eyespot, flagella arise.
Oil is the main food reserve accumulated in the cytoplasm.

Reproduction in Xanthophyta
Members of Xanthophyta reproduce by vegetative , asexual , and sexual methods.
Vegetative Reproduction
The vegetative reproduction in Xanthophyta mainly takes place by fragmentation .
The thallus can break into small fragments due to accidental breakage. Each fragment grows independently to form a new thallus.
Vegetative reproduction also occurs by cell division . Algal cells divide mitotically into two daughter cells. These cells may develop a new independent organism.
Asexual Reproduction
Xanthophyta reproduces asexually by the formation of zoospores and aplanospores .
Zoospores are large multiflagellate, ovoid structure and develop singly or in numbers within a club-shaped zoosporangium.
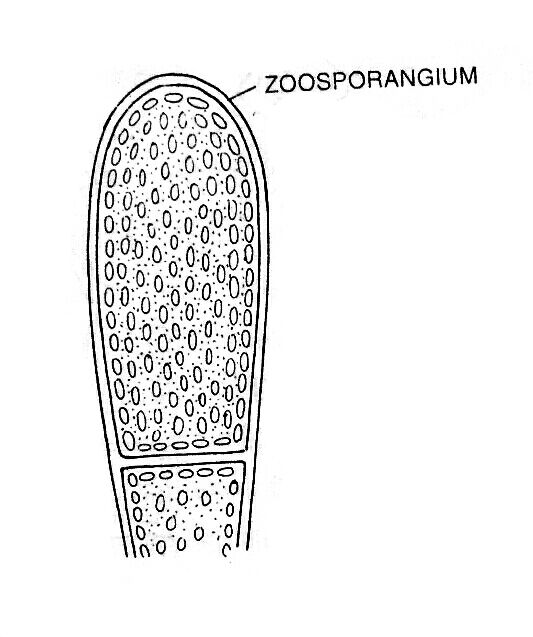
In some members of Xanthophyta (e.g., Vaucheria ), the zoospore contains many nuclei and chloroplasts in addition to numerous pairs of heterokont flagella, almost equal in length.
After maturation, zoospores are liberated. Then the resting period started, and the zoospores withdrew their flagella.
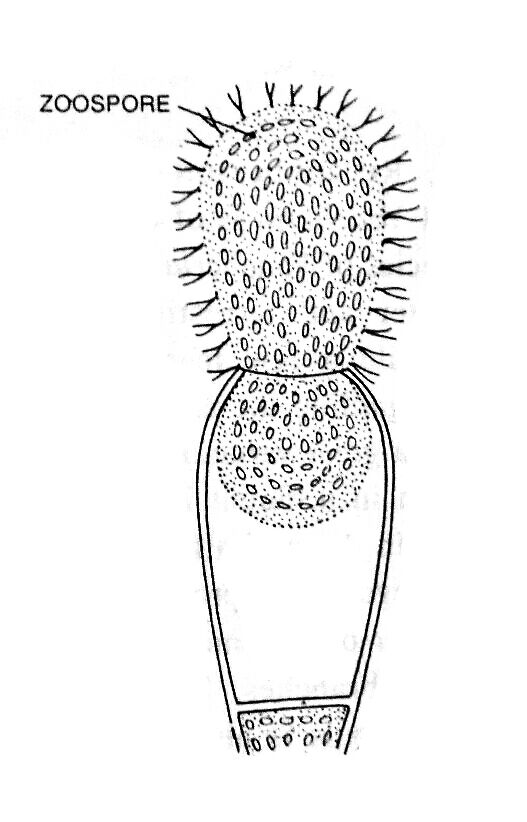
Each zoospore germinates, which forms tubular structures. One of them develops into a new thallus.
Aplanospores
Aplanospores are thin-walled, non-flagellated zoospores formed inside the aplanosporangium. After liberation, the aplanospore may germinate into a new plant.
Cysts Formation
It is formed during unfavourable conditions.
The entire protoplast, segmented by transverse division and then rounding off, secretes a thick wall. Such thick-walled segments are known as akinetes or hypnospores .

The akinetes may divide further into a number of thin-walled bodies called cysts . Under favorable conditions, the akinetes and cysts germinate into new thalli.
Sexual Reproduction
Sexual reproduction is very rare in Xanthophyta.
In Botrydium , an isogamous type of reproduction is found.
Gametes are formed during the rainy season. The protoplast of a vesicle is divided into uninucleate segments, which form biflagellate, pear-shaped gametes. These gametes have one to four chromatophores, with or without an eye spot.
Later, any two gametes come close to each other, flagella disappear, and fusion occurs. As a result, a diploid zygote is formed.
The nucleus of the zygote undergoes meiosis and forms 4–8 motile meiospores. These haploid meiospores develop new plants.
In Vaucheria , an advanced oogamous type of sexual reproduction takes place.
The species of Vaucheria may be homothallic or heterothallic. In homothallic species, the formation of antheridium and oogonium is always associated with the formation of transverse septa.
The antheridium is a slender, curved, hook-like structure, opened by a terminal pore. It is formed at the end of a short lateral branch, slightly before the development of oogonium.
The tip of the branch becomes densely filled with cytoplasm, containing many nuclei and a few chloroplasts. This portion bends like a horn and gets cut off from the rest of the filament by a cross wall. The protoplast divides into uninucleate biflagellate antherozoids, or sperm.
The oogonium development begins simultaneously with the accumulation of a colorless multinucleate mass of cytoplasm called wonderplasm in the main filament near the base of the antheridial branch. The mature oogonium contains a large nucleus at its center. The nucleus of an oogonium is filled with protoplasm to form a single egg or ovum.
After fertilisation, a diploid zygote or oospore is formed. During favorable conditions, the oogonial wall disintegrates, releasing the oospore. The oospore germinates into a new thallus.
Classification of Xanthophyta
The division Xanthophyta includes only one class Xanthophyceae , which contains only one order Vaucheriales ( Heterosiphonales ).
The order Vaucheriales is divided into two families. One is Botrydiaceae and the other is Vaucheriaceae .
Phyllogeny and Interrelationships
Three major evolutionary lines may be traced among Xanthophyta, like Chlorophyta. These are-
- Development of non-motile solitary or colonial forms from an unicellular motile ancestor.
- Development of tubular or siphonaceous forms.
- Development of multicellular filamentous types.
Due to morphological similarities with the chlorophyceae, many phycologists advocate the theory of parallel evolution and similarity in the origin of the group from the flagellate. Since the members of Xanthophyta lack elaborate thalli as found in Chlorophyceae, this sequence of parallel evolution does not go too far.
Fritsch (1935) believes that the group is still in the process of evolution, while some consider it a reduced stock.
Due to similarities in food reserves, the composition of the cell wall, and other physiological and biological features, members of Xanthophyta are compared to those of Chrysophyta.
But the presence of chlorophyll-e and other features led modern phycologists to propose an independent status for the division Xanthophyta .
Animesh Sahoo is a scientific blogger who is passionate about biology, nature, and living organisms. He enjoys sharing his knowledge through his writings. During his free time, Animesh likes to try new activities, go on adventures, experiment with different biological aspects, and learn about various organisms.
Related Notes
Bacillariophyta (diatoms): an overview.
- February 24, 2024
Phaeophyta (Brown Algae): An Overview
- December 19, 2023
Rhodophyta (Red Algae): An Overview
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Name *
Email *
Add Comment *
Save my name, email, and website in this browser for the next time I comment.
Post Comment

- Games & Quizzes
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

Xanthophyta
Our editors will review what you’ve submitted and determine whether to revise the article.
- University of California, Berkeley: Museum of Paleontology - Introduction to the Xanthophyta
Xanthophyta , division or phylum of algae commonly known as yellow-green algae ( q.v. ).
- Subscriber Services
- For Authors
- Publications
- Archaeology
- Art & Architecture
- Bilingual dictionaries
- Classical studies
- Encyclopedias
- English Dictionaries and Thesauri
- Language reference
- Linguistics
- Media studies
- Medicine and health
- Names studies
- Performing arts
- Science and technology
- Social sciences
- Society and culture
- Overview Pages
- Subject Reference
- English Dictionaries
- Bilingual Dictionaries
Recently viewed (0)
- Save Search
- Share This Facebook LinkedIn Twitter
Related Content
Related overviews.
chloroplast
See all related overviews in Oxford Reference »
More Like This
Show all results sharing these subjects:
- Life Sciences
Xanthophyta
Quick reference.
A phylum of mostly freshwater eukaryotic organisms, traditionally known as yellow-green algae, that possess carotenoid pigments (including xanthins), which are responsible for their colour, in addition to chlorophylls. Xanthophytes occur in a variety of forms – unicellular, colonial, filamentous, and siphonaceous; motile cells have two unequal-sized undulipodia (flagella). Storage products are oil and the polysaccharide chrysolaminarin. Xanthophytes are included in a eukaryotic assemblage known as the stramenopiles.
From: Xanthophyta in A Dictionary of Biology »
Subjects: Science and technology — Life Sciences
Related content in Oxford Reference
Reference entries.
View all related items in Oxford Reference »
Search for: 'Xanthophyta' in Oxford Reference »
- Oxford University Press
PRINTED FROM OXFORD REFERENCE (www.oxfordreference.com). (c) Copyright Oxford University Press, 2023. All Rights Reserved. Under the terms of the licence agreement, an individual user may print out a PDF of a single entry from a reference work in OR for personal use (for details see Privacy Policy and Legal Notice ).
date: 29 June 2024
- Cookie Policy
- Privacy Policy
- Legal Notice
- Accessibility
- [185.80.151.41]
- 185.80.151.41
Character limit 500 /500
Last updated 27/06/24: Online ordering is currently unavailable due to technical issues. We apologise for any delays responding to customers while we resolve this. For further updates please visit our website: https://www.cambridge.org/news-and-insights/technical-incident
We use cookies to distinguish you from other users and to provide you with a better experience on our websites. Close this message to accept cookies or find out how to manage your cookie settings .
Login Alert

- > The Freshwater Algal Flora of the British Isles
- > Phylum Xanthophyta (Tribophyta) (Yellow-Green Algae)

Book contents
- Frontmatter
- The online material (formerly provided in DVD format)
- List of Contributors
- Acknowledgements
- Introduction
- Distribution and Ecology
- History of Freshwater Algal Studies in the British Isles
- Field Methods
- Laboratory Methods
- Water Framework Directive
- Cultures of British Freshwater Algae
- Classification
- Key to phyla
- Cyanobacteria (Cyanophyta)
- Phylum Rhodophyta (Red Algae)
- Phylum Euglenophyta (Euglenoids)
- Phylum Cryptophyta (Cryptomonads)
- Phylum Dinophyta (Dinoflagellates)
- Phylum Raphidophyta
- Phylum Haptophyta (Prymnesiophyta)
- Phylum Chrysophyta (Golden Algae)
- Phylum Xanthophyta (Tribophyta) (Yellow-Green Algae)
- Phylum Eustigmatophyta
- Phylum Bacillariophyta (Diatoms)
- Phylum Phaeophyta (Brown Algae)
- Primitive Green Algae (‘PRASINOPHYTA’)
- Phylum Chlorophyta (Green Algae)
- Phylum Glaucophyta
- Standard Form of Authors of Algal Names
- Sources of Illustrations or Material
- Taxonomic Index
- Subject Index
- Plate Saction
- Miscellaneous Endmatter
Published online by Cambridge University Press: 12 January 2024
The Xanthophyta are much less species-diverse than the Chlorophyta, with about 600 species and many of the 100 known genera containing only a few species. However, they show a wide range of form and include biflagellate and non-motile unicells, simple or branched uniseriate filaments, and others are coenocytic or siphonous (large multinucleate cells). Colonial forms may or may not have a well-defined shape. Some species are epiphytes and sessile or attached by a stalk.
Most are non-motile, single-celled or colonial, although there a few more advanced filamentous forms and coenocytic forms such as Vaucheria . If motile, they are biflagellate, and often possess associated photoreceptors. Asexual reproduction is mainly by fragmentation into portions of one or more cells in multicellular species, aplanospores or zoospores that each have two unequal flagella and sometimes an eyespot. Sexual reproduction is apparently comparatively rare, although well-known in Vaucheria , where it is distinctive and provides important taxonomic characters. Resting structures or cysts are known and often have walls impregnated with silica. Heterogamy is uncommon and isogametes known only for a few genera.
The distinction between the Xanthophyta and the Chlorophyta rests largely on chloroplast pigments and food storage products rather than on morphological characters. Traditionally, there are three features that distinguish the xanthophytes: (i) yellow or yellowgreen colour of the chloroplasts; (ii) carbohydrate storage as oil droplets or chrysolaminarin (usually termed leucosin) granules, with starch and pyrenoids rare; (iii) walls of pectin or pectic acid (sometimes in association with cellulose or siliceous substances) and consisting of two spliced and overlapping sections (most conspicuous in Tribonema ), which on dissociation of the filaments tend to break into H-shaped sections or pieces. Although a useful taxonomic character, these sections are not present in all genera, and certainly not always readily visible even in genera where they occur.
In practice, the yellow-green colour of these plants is not always easy to distinguish, and in the case of Vaucheria , the colour is indistinguishable from that of green algae ( Vaucheria was initially described as a green alga). The xanthophytes differ in containing no chlorophyll b , but in addition to chlorophyll a have chlorophylls c 1 and c 2 ; other pigments are carotenoids (especially b-carotene) and at least three xanthophylls. These xanthophylls can give these algae a blue-green colour when treated with hot hydrochloric acid in the laboratory.
Access options
Save book to kindle.
To save this book to your Kindle, first ensure [email protected] is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about saving to your Kindle .
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service .
- By L.R. Johnson
- David M. John , Brian A. Whitton , Alan J. Brook
- Book: The Freshwater Algal Flora of the British Isles
- Online publication: 12 January 2024
- Chapter DOI: https://doi.org/10.1017/CHOL9781108784122.019
Save book to Dropbox
To save content items to your account, please confirm that you agree to abide by our usage policies. If this is the first time you use this feature, you will be asked to authorise Cambridge Core to connect with your account. Find out more about saving content to Dropbox .
Save book to Google Drive
To save content items to your account, please confirm that you agree to abide by our usage policies. If this is the first time you use this feature, you will be asked to authorise Cambridge Core to connect with your account. Find out more about saving content to Google Drive .
Update image
- Xanthophyta: The Yellow-green Algae
| Sexual reproduction in Vaucheria. (a) An egg cell in the oogonium; (b) antheridium; (c) maturing sperm cells; (d) sperm cells emerging from the antheridium; (e) and (f) the zygote and growth of a new filament. |
| Asexual reproduction in Vaucheria. (a) The multinucleatedfilament. (b) A terminal sporangiumf orms and a cross wall develops at the sporangium’s base. (c) A single, multiciliated zoospore emerges through an opening. (d) Zoospore at rest,( e), and producing a new filament, (f). |
Support our developers

More in this section
- Other Algae
- Euglenophyta
- Chrysophyta
- Acetabularia: A Green Alga
© 2012 - 2024 Biocyclopedia
Disclaimer Privacy Policy Feedback
Login to your account
Login / signup with.

Sciencing_Icons_Science SCIENCE
Sciencing_icons_biology biology, sciencing_icons_cells cells, sciencing_icons_molecular molecular, sciencing_icons_microorganisms microorganisms, sciencing_icons_genetics genetics, sciencing_icons_human body human body, sciencing_icons_ecology ecology, sciencing_icons_chemistry chemistry, sciencing_icons_atomic & molecular structure atomic & molecular structure, sciencing_icons_bonds bonds, sciencing_icons_reactions reactions, sciencing_icons_stoichiometry stoichiometry, sciencing_icons_solutions solutions, sciencing_icons_acids & bases acids & bases, sciencing_icons_thermodynamics thermodynamics, sciencing_icons_organic chemistry organic chemistry, sciencing_icons_physics physics, sciencing_icons_fundamentals-physics fundamentals, sciencing_icons_electronics electronics, sciencing_icons_waves waves, sciencing_icons_energy energy, sciencing_icons_fluid fluid, sciencing_icons_astronomy astronomy, sciencing_icons_geology geology, sciencing_icons_fundamentals-geology fundamentals, sciencing_icons_minerals & rocks minerals & rocks, sciencing_icons_earth scructure earth structure, sciencing_icons_fossils fossils, sciencing_icons_natural disasters natural disasters, sciencing_icons_nature nature, sciencing_icons_ecosystems ecosystems, sciencing_icons_environment environment, sciencing_icons_insects insects, sciencing_icons_plants & mushrooms plants & mushrooms, sciencing_icons_animals animals, sciencing_icons_math math, sciencing_icons_arithmetic arithmetic, sciencing_icons_addition & subtraction addition & subtraction, sciencing_icons_multiplication & division multiplication & division, sciencing_icons_decimals decimals, sciencing_icons_fractions fractions, sciencing_icons_conversions conversions, sciencing_icons_algebra algebra, sciencing_icons_working with units working with units, sciencing_icons_equations & expressions equations & expressions, sciencing_icons_ratios & proportions ratios & proportions, sciencing_icons_inequalities inequalities, sciencing_icons_exponents & logarithms exponents & logarithms, sciencing_icons_factorization factorization, sciencing_icons_functions functions, sciencing_icons_linear equations linear equations, sciencing_icons_graphs graphs, sciencing_icons_quadratics quadratics, sciencing_icons_polynomials polynomials, sciencing_icons_geometry geometry, sciencing_icons_fundamentals-geometry fundamentals, sciencing_icons_cartesian cartesian, sciencing_icons_circles circles, sciencing_icons_solids solids, sciencing_icons_trigonometry trigonometry, sciencing_icons_probability-statistics probability & statistics, sciencing_icons_mean-median-mode mean/median/mode, sciencing_icons_independent-dependent variables independent/dependent variables, sciencing_icons_deviation deviation, sciencing_icons_correlation correlation, sciencing_icons_sampling sampling, sciencing_icons_distributions distributions, sciencing_icons_probability probability, sciencing_icons_calculus calculus, sciencing_icons_differentiation-integration differentiation/integration, sciencing_icons_application application, sciencing_icons_projects projects, sciencing_icons_news news.
- Share Tweet Email Print
- Home ⋅
- Science ⋅
- Biology ⋅
- Cell (Biology): An Overview of Prokaryotic & Eukaryotic Cells
The Morphology of Algae

Structural Characteristics of Blue-Green Algae
In the not too distant future, advances in DNA identification could potentially change the way that ambiguous organisms like algae are classified. In the meantime, phycologists will continue to rely on a naming and classification system of morphology introduced by Carl Linnaeus in the 1700s. Like other members of the kingdom Protista, algae are eukaryotic organisms with a nuclear envelope, cell walls and organelles.
Main Characteristics of Algae
Algae are protists, an incredibly large group of organisms with markedly different features. The form and structure of algae sets them apart from plants. Although algae and plants both contain chlorophyll and photosynthesize, algae don’t have an actual root system, stem or leaves. Algae cells are typically simpler than plant cells and have fewer organelles in their cell cytoplasm.
There are few places on Earth where algae cannot be found. Algae thrive in places where few plants would dare to go. Habitats include everything from the deepest ocean to snowy mountain caps to hot springs and salt marshes.
Most species of algae are single-celled micro-organisms living in aquatic environments. Algae are primary producers on the bottom of the food chain that feed consumers. Algae are often distinguishable by their color.
Golden Brown Algae (Chrysophytes)
Golden algae (Chrysophytes) are common microscopic organisms that provide food for zooplankton in fresh water. Most are functionally photosynthetic, but under the right conditions, golden algae feed on bacteria. Structurally, golden algae are mostly unicellular and free-swimming, but some species exist as colonial algae and stringy filaments. Chrysophytes like diatoms can be seen in fossil records dating back to the Cretaceous age.
Common Green Algae
More than 7,000 species of green algae have been identified, according to the UC Museum of Paleontology. Freshwater green algae like Spirogyra in the Charophyta phylum are more closely related to plants than marine green algae (Chlorophyta). Green algae resembles a plant because it contains chlorophyll and uses sun energy to drive photosynthesis. The structure of green algae can be single- or multiple-celled.
Red Algae (Rhodophyta)
The typical red algae (Rhodophyta) is a rose-colored multicellular organism found in marine environments around the world. Accessory pigments called phycobiliproteins are responsible for the distinctive red coloring. Like green algae, red algae traces back to ancestral cyanobacteria. Certain types of red algae are edible and used to make products like agar and food additives.
Brown Algae (Phaeophyta)
Brown algae (Phaeophyta) are multicellular organisms that derive their color from the brownish pigment fucoxanthin in chloroplasts along with chlorophyll. According to the Seaweeds of Alaska website for phycologists, brown algae are bigger and more morphologically complex than any other type of marine algae. Brown algae make their food through photosynthesis and store polymers of glucose in a vacuole within the cell cytoplasm. Familiar examples of brown algae are seaweed and kelp.
Fire Algae (Pyrrophyta)
Phytoplankton are microalgae divided into two subgroups: diatoms and dinoflagellates. Phytoplankton play an important role in the food chain and ecosystem by converting nitrates, sulfur and phosphates into carbon-based nutrients. Runoff from farm fields and other pollutants can result in phytoplankton overgrowth and the formation of highly toxic harmful algal blooms (HABs).
Deadly HABs, referred to as “red tides,” form large, putrid-smelling masses over bodies of water. Bioluminescent types of dinoflagellates are called fire algae because they chemically emit light and glow like flames. At night the bioluminescent HAB appears on fire.
Yellowish Green Algae (Xanthophyta)
Xanthophyta are yellow-green algae that live in fresh water. They may be unicellular in morphology or colonial algae, bunched together. Color is derived from green, yellow and orange pigments involved in photosynthesis. Flagella make this type of algae motile in water.
Related Articles
Is algae a decomposer, a scavenger or a producer, role of algae in the ecosystem, the structure of algae, characteristics of seaweed, facts about seaweed, characteristics of protozoa & algae, types of organisms that can use photosynthesis, the major producers found in aquatic ecosystems, what do volvox eat, differences between protista & monera, characteristics of the six kingdoms of organisms, how does seaweed conduct photosynthesis, what is the function of air bladders in seaweed, cell wall composition of the six kingdoms, plants that are in the biome of the coral reef, types of heterotrophic bacteria, what are the characteristics of the protista kingdom, what are good protists, explain photosynthesis.
- UC Museum of Paleontology: Introduction to the "Green Algae"
- UC Museum of Paleontology: Introduction to the Rhodophyta
- UC Museum of Paleontology: Introduction to the Chrysophyta
- Seaweeds of Alaska: Ochrophyta
- "Freshwater Dinoflagellates of North America"; Susan Carty
- NOAA: What Is a Red Tide?
- NOAA: What Are Phytoplankton?
About the Author
Dr. Mary Dowd studied biology in college where she worked as a lab assistant and tutored grateful students who didn't share her love of science. Her work history includes working as a naturalist in Minnesota and Wisconsin and presenting interactive science programs to groups of all ages. She enjoys writing online articles sharing information about science and education. Currently, Dr. Dowd is a dean of students at a mid-sized university.
Photo Credits
Hemera Technologies/Photos.com/Getty Images
Find Your Next Great Science Fair Project! GO
Xanthophyceae
- Living reference work entry
- First Online: 01 January 2016
- Cite this living reference work entry

- Silvia Maistro 8 ,
- Paul Broady 9 ,
- Carlo Andreoli 8 &
- Enrico Negrisolo 10
1663 Accesses
3 Citations
The Xanthophyceae is a clade of stramenopilan photoautotrophs containing about 118 genera and 600 species. Morphology ranges from free-living or attached unicells to colonies and unbranched or branched filaments and siphons. A large majority are found in freshwater and soil, while some occur in brackish and marine habitats. Although abundant growth of a few species can occur in nature, none are known to be of practical importance. They are characterized by possession of chlorophylls a , c 1 , and c 2 and a range of xanthophylls, but not fucoxanthin, in generally yellowish-green, discoidal, parietal chloroplasts. Thylakoids are in groups of three, and most species investigated have a single thylakoid forming a girdle band around the periphery of the chloroplast. Chloroplasts are surrounded by chloroplast endoplasmic reticulum. Pyrenoids, when present, are typically semi-immersed and are not associated with granules of storage products. A cell wall consisting of two overlapping parts occurs in some coccoid and filamentous species. Reproduction is generally asexual but some, e.g., Vaucheria , exhibit sexual reproduction. The taxonomic status of a significant number of species is uncertain, especially those that are rarely observed, e.g., species of Chloramoebales, Heterogloeales, and Rhizochloridales. Transfer of species to the Eustigmatophyceae and other groups is likely. There is molecular phylogenetic data for fewer than 20 % of species. Four major clades are recognized. Two of these contain both coccoid and filamentous species. Many traditional orders, families, and genera are paraphyletic or polyphyletic. It is presently convenient to retain the traditional classification of seven orders based on morphology until these difficulties are resolved following the inclusion of more species in phylogenetic analyses.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Similar content being viewed by others

Zygnematophyta
Chrysophyta.
Åberg, H., & Fries, L. (1976). On cultivation of the alga Vaucheria dichotoma (Xanthophyceae) in axenic culture. Phycologia, 15 , 133–141.
Article Google Scholar
Adl, S. M., Simpson, A. G. B., Farmer, M. A., Andersen, R. A., Anderson, O. R., Barta, J. R., Bowser, S. A. S., Brugerolle, G., Fensome, R. A., Fredericq, S., James, T. Y., Karpov, S., Kugrensi, P., Krug, J., Lane, C. E., Lewis, L. A., Lodge, J., Lynn, D. H., Mann, D. G., McCourt, R. M., Mendoza, L., Moestrup, Ø., Mozley-Standrige, S. E., Nerad, T. A., Shearer, C. A., Smirnov, A. V., Spiegel, F. W., & Taylor, M. F. J. R. (2005). The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. Journal of Eukaryotic Microbiology, 52 , 399–451.
Article PubMed Google Scholar
Allorge, P. (1930). Hétérocontes ou Xanthophycées? Revue Algologique, 5 , 230.
Google Scholar
Andersen, R. A., Brett, R. W., Potter, D., & Sexton, J. (1998). Phylogeny of the Eustigmatophyceae based upon 18S rDNA, with emphasis on Nannochloropsis. Protist, 149 , 61–74.
Article CAS PubMed Google Scholar
Andersen, R. A., & Bailey, J. C. (2002). Phylogenetic analysis of 32 strains of Vaucheria (Xanthophyceae) using the rbcL gene and its two flanking region spacers. Journal of Phycology, 38 , 583–592.
Article CAS Google Scholar
Andreoli, C., Moro, I., La Rocca, N., Rigoni, F., Dalla, V. L., & Bargelloni, L. (1999). Pseudopleurochloris antarctica gen. et sp. nov., a new coccoid xanthophycean from pack-ice of Wood Bay (Ross Sea, Antarctica): Ultrastructure, pigments and 18S rRNA gene sequence. European Journal of Phycology, 34 (2), 149–159.
Bailey, J. C., & Andersen, R. C. (1998). Phylogenetic relationships among nine species of the Xanthophyceae inferred from rbcL and 18S rRNA gene sequences. Phycologia, 37 , 458–466.
Begum, A., & Broady, P. A. (2001). Chlorellidium pyrenoidosum spec. nova (Mischococcales, Xanthophyceae) from New Zealand. Algological Studies, 107 , 163–172.
Böger, P., & Kiermayer, O. (1974). Electron microscopy of plastids of Bumilleriopsis filiformis. Archiv für Mikrobiologie, 98 , 207–214.
Bohlin, K. (1897a). Studier ofver några slågten af alggruppen Confervales Borzi. Bihang till Kongliga Svenska Vetenskaps-Academiens Handlingar, 23 , Afd. III, No. 3, 1–56.
Bohlin, K. (1897b). Zur morphologie und biologie einzelliger algen. Öfversigt Kongliga Svenska Vetenskaps-Academiens Forhandlingar, 54 , 507–529.
Borzi, A. (1889). Botrydiopsis nuovo genere di alghe verdi. Bollettino della Societá Italiana di Microbiologia, 1 , 60–70.
Borzi, A. (1895). Studi Algologici. Fasc. II. . Palermo: A. Reber.
Bouck, G. B. (1971). The structure, origin and composition of the tubular mastigonemes of the Ochromonas flagellum. Journal of Cell Biology, 50 , 362–384.
Article CAS PubMed PubMed Central Google Scholar
Bouck, G. B. (1972). Architecture and assembly of mastigonemes. Advances in Cell and Molecular Biology, 2 , 237–271.
CAS Google Scholar
Braun, A. (1855). Algarum Unicellularium Genera Nova et Minus Cognita, Praemissis Observationibus de Algis Unicellularibus in Genere . Leipzig: Engelmann.
Book Google Scholar
Broady, P. A. (1976). Six new species of terrestrial algae from Signy Island, South Orkney Islands, Antarctica. British Phycological Journal, 11 , 387–405.
Broady, P. A., Ohtani, S., & Ingerfeld, M. (1997). A comparison of strains of Xanthonema (= Heterothrix , Tribonematales, Xanthophyceae) from Antarctica, Europe and New Zealand. Phycologia, 36 , 164–171.
Butterfield, N. J. (2004). A vaucheriacean alga from the middle Neoproterozoic of Spitsbergen: Implications for the evolution of Proterozoic eukaryotes and the Cambrian explosion. Paleobiology, 30 , 231–252.
Casper, S. J. (1972). Zum Feinbau der Geisseen der Chrysomonaden. I. Uroglena americana Calkins. Archiv für Protistenkunde, 114 , 65–82.
Cavalier-Smith, T., & Chao, E. E.-Y. (2006). Phylogeny and megasystematics of phagotrophic heterokonts (Kingdom Chromista). Journal of Molecular Evolution, 62 , 388–420.
Deason, T. R. (1971a). The origin of flagellar hairs in the xanthophycean alga Pseudobumilleriopsis pyrenoidosa. Transactions of the American Microscopical Society, 90 , 441–448.
Deason, T. R. (1971b). The fine structure of sporogenesis in the xanthophycean alga Pseudobumilleriopsis pyrenoidosa. Journal of Phycology, 7 , 101–107.
Ettl, H. (1978). Xanthophyceae. In H. Ettl, H.J. Gerloff, & H. Heynig (Eds.), Süsswasserflora von Mitteleuropa (Bd. 3, I. Teil). Stuttgart: Gustav Fischer.
Ettl, H., & Gärtner, G. (1995). Syllabus der Boden-, Luft- und Flechtenalgen . Stuttgart: Gustav Fischer Verlag.
Falk, H. (1967). Zum Feinbau von Botrydium granulatum Grev. (Xanthophyceae). Archiv für Mikrobiologie, 58 , 212–227.
Falk, H., & Kleinig, H. (1968). Feinbau und Carotinoide von Tribonema (Xanthophyceae). Archiv für Mikrobiologie, 61 , 347–362.
Fritsch, F. E. (1935). The structure and reproduction of the algae (Vol. 1). Cambridge: Cambridge University Press.
Greenwood, A. D. (1959). Observations on the structure of the zoospore of Vaucheria. II. Journal of Experimental Botany, 10 , 55–68.
Greenwood, A. D., Manton, I., & Clarke, B. (1957). Observations on the structure of the zoospores of Vaucheria. Journal of Experimental Botany, 8 , 71–86.
Hibberd, D. J. (1976). The ultrastructure and taxonomy of the Chrysophyceae and Prymnesiophyceae (Haptophyceae); a survey with some new observations on the ultrastructure of the Chrysophyceae. Botanical Journal of the Linnean Society, 72 , 55–80.
Hibberd, D. J. (1979). The structure and phylogenetic significance of the flagellar transition region in the chlorophyll c -containing algae. BioSystems, 11 , 243–261.
Hibberd, D. J. (1980). Xanthophytes. In E. R. Cox (Ed.), Phytoflagellates: Form and function (pp. 243–271). New York/Amsterdam/Oxford: Elsevier/North Holland.
Hibberd, D. J. (1981). Notes on the taxonomy and nomenclature of the algal classes Eustigmatophyceae and Tribophyceae (synonym Xanthophyceae). Botanical Journal of the Linnean Society, 82 , 93–119.
Hibberd, D. J. (1990). Phylum Xanthophyta. In L. Margulis, J. O. Corliss, M. Melkonian, & D. J. Chapman (Eds.), Handbook of Protoctista (pp. 686–697). Boston: Jones & Bartlett.
Hibberd, D. J., & Leedale, G. F. (1971). Cytology and ultrastructure of the Xanthophyceae. II. The zoospore and vegetative cell of coccoid forms, with special reference to Ophiocytium majus Nägeli. British Phycological Journal, 6 , 1–23.
Hibberd, D. J., & Leedale, G. F. (1972). Observations on the cytology and ultrastructure of the new algal class, Eustigmatophyceae. Annals of Botany, 36 , 49–71.
Iorya, T. (1974). Chromosome numbers in four species of Tribonema (Xanthophyceae). Journal of Japanese Botany, 49 , 29–31.
Kai, A., Yoshii, Y., Nakayama, T., & Inouye, I. (2008). Aurearenophyceae classis nova, a new class of Heterokontophyta based on a new marine unicellular alga Aurearena cruciata gen. et sp. nov. inhabiting sandy beaches. Protist, 159 , 435–457.
Koch, W. J. (1951). A study of the motile cells of Vaucheria. Journal of the Elisha Mitchell Scientific Society, 67 , 123–131.
Lokhorst, G. M. (2003). The genus Tribonema (Xanthophyceae) in the Netherlands – An integrated field and culture study. Nova Hedwigia, 77 , 19–53.
Lokhorst, G. M., & Star, W. (2003a). The flagellar apparatus in Tribonema (Xanthophyceae) reinvestigated. Phycologia, 42 , 31–43.
Lokhorst, G. M., & Star, W. (2003b). Mitosis and cytokinesis in Tribonema regulare (Tribophyceae, Chrysophyta). Protoplasma, 145 , 7–15.
Luther, A. (1899). Über Chlorosaccus eine neue Gattung der Süsswasseralgen, nebst Bemerkungen zur systematik verwandter algen. Bihang till Kongliga Svenska Vetenskaps-Academiens Handlingar , 24 , Afd, III, No. 13, 1–22.
Maistro, S., Broady, P. A., Andreoli, C., & Negrisolo, E. (2007). Molecular phylogeny and evolution of the order Tribonematales (Heterokonta, Xanthophyceae) based on analysis of plastidial genes rbcL and psaA. Molecular Phylogenetics and Evolution, 43 , 407–417.
Maistro, S., Broady, P. A., Andreoli, C., & Negrisolo, E. (2009). Phylogeny and taxonomy of Xanthophyceae (Stramenopiles, Chromalveolata). Protist, 160 , 412–426.
Manton, I., Clarke, B., Greenwood, A. D., & Flint, E. A. (1952). Further observations on the structure of plant cilia, by a combination of visual and electron microscopy. Journal of Experimental Botany, 3 , 204–215.
Marchant, H. (1972). Pyrenoids of Vaucheria woroniniana Heering. British Phycological Journal, 7 , 81–84.
Massalski, A., & Leedale, G. F. (1969). Cytology and ultrastructure of the Xanthophyceae. I. Comparative morphology of the zoospores of Bumilleria sicula Borzi and Tribonema vulgare Pascher. British Phycological Journal, 4 , 159–180.
Mizuta, S., Roberts, E. M., & Brown, R. M., Jr. (1989). A new cellulose synthesizing complex in Vaucheria hamata and its relation to microfibril assembly. In C. Schuerch (Ed.), Cellulose and wood chemistry and technology (pp. 656–676). New York: Wiley.
Moestrup, ∅. (1970). On the fine structure of the spermatozoids of Vaucheria sescuplicaria and on the later stages in spermatogenesis. Journal of the Marine Biological Association of the United Kingdom, 50 , 513–523.
Moestrup, ∅. (1982). Flagellar structure in algae: A review, with new observations particularly on the Chrysophyceae, Phaeophyceae (Fucophyceae), Euglenophyceae and Reckertia. Phycologia, 21 , 427–528.
Nagao, M., Arakawa, K., Takezawa, D., Yoshida, S., & Fujikawa, S. (1999). Akinete formation in Tribonema bombycinum Derbes et Solier (Xanthophyceae) in relation to freezing tolerance. Journal of Plant Research, 112 , 163–174.
Negrisolo, E., Maistro, S., Moro, I., Incarbone, M., Dalla Valle, L., Broady, P. A., & Andreoli, C. (2004). Morphological convergence characterizes the evolution of Xanthophyceae (Heterokontophyta): Evidence from nuclear SSU rDNA and plastidial rbcL genes. Molecular Phylogenetics and Evolution, 33 , 156–170.
Nichols, H. W. (1973). Growth media—freshwater. In J. R. Stein (Ed.), Handbook of phycological methods. Culture methods and growth measurements (pp. 7–24). Cambridge: Cambridge University Press.
Ott, D. W. (1982). Tribophyceae (Xanthophyceae): Introduction and bibliography. In J. R. Rosowski & B. C. Parker (Eds.), Selected papers in phycology II (pp. 723–727). Lawrence: Phycological Society of America.
Ott, D. W., & Brown, R. M. (1972). Light and electron microscopical observations on mitosis in Vaucheria litorea Hofman ex C. Agardh. British Phycological Journal, 7 , 361–374.
Ott, D. W., & Brown, R. M. (1974). Developmental cytology of the genus Vaucheria. I. Organisation of the vegetative filament. British Phycological Journal, 9 , 111–126.
Ott, D. W., & Brown, R. M. (1975). Developmental cytology of the genus Vaucheria III. Emergence, settlement and germination of the mature zoospore of V. fontinalis (L.) Christensen. British Phycological Journal, 10 , 49–56.
Pascher, A. (1937–1939). Heterokonten. In: L. Rabenhorst’s Kryptogamen-Flora von Deutschland, Österreich und der Schweiz (2. Aufl., Bd. XI). Leipzig: Akademische Verlagsgesellschaft m.b.h.
Pierce, S. K., Curtis, G. W., & Schwartz, J. A. (2009). Vaucheria litorea and its relationship with Elysia chlorotica. Symbiosis, 49 , 121–131.
Potter, D., Sauder, G. W., & Andersen, R. A. (1997). Phylogenetic relationships of Raphidophyceae and Xanthophyceae as inferred from nucleotide sequence of the 18S ribosomal RNA gene. American Journal of Botany, 84 , 966–972.
Prat, S. (1947). The reaction of algal cells with Schiff’s reagent. Spisy Prirodovedecke Fakulty Karlovy University, 177 , 1–16.
Ragan, M. A., & Chapman, D. J. (1978). A biochemical phylogeny of the protists . New York: Academic.
Rieth, A. (1980). Xanthophyceae. In H. Ettl, J. Gerloff, H. Heynig, (Eds.), Süsswasserflora von Mitteleuropa (Bd. 3, 2. Teil). Stuttgart und Jena: Gustav Fischer.
Riisberg, I., Orr, R. S., Kluge, R., Shalchian-Tabrizi, K., Bowers, H. A., Patil, V., Edvardsen, B., & Jakobsen, K. S. (2009). Seven gene phylogeny of Heterokonts. Protist, 160 , 191–204.
Rowan, K. S. (1989). Photosynthetic pigments of algae . Cambridge: Cambridge University Press.
Rybalka, N., Andersen, R. A., Kostikov, I., Mohr, K. I., Massalski, A., Olech, M., & Friedl, T. (2009). Testing for endemism, genotypic diversity and species concepts in Antarctic terrestrial microalgae of the Tribonemataceae (Stramenopiles, Xanthophyceae). Environmental Microbiology, 11 , 554–565.
Rybalka, N., Wolf, M., Andersen, R. A., & Friedl, T. (2013). Congruence of chloroplast- and nuclear-encoded DNA sequence variations used to assess species boundaries in the soil microalga Heterococcus (Stramenopiles, Xanthophyceae). BMC Evolutionary Biology, 13 , 39. doi:10.1186/1471-2148-13-39.
Silva, P. C. (1979). Review of the taxonomic history and nomenclature of the yellow-green algae. Archiv für Protistenkunde, 121 , 20–63.
Stransky, H., & Hager, A. (1970). Das Carotinoidmuster und die Verbreitung des lichtinduzierten Xanthophyllcyclus in verschiedenen Algenklassen. II. Xanthophyceae. Archiv für Mikrobiologie, 71 , 164–190.
Tomaselli, L. (2004). The microalgal cell. In A. Richmond (Ed.), Handbook of microalgal culture (pp. 3–19). Oxford: Blackwell.
Tschermak-Woess, E. (1988). The algal partner. In M. Galun (Ed.), CRC handbook of lichenology (Vol. I, pp. 39–94). Boca Raton: CRC Press.
Van den Hoek, C., Mann, D. G., & Jahns, H. M. (1995). Algae. An introduction to phycology . Cambridge: Cambridge University Press.
Vischer, W. (1945). Heterokonten aus Alpinen Boden, Speziell dem Schweizerischen Nationalpark. Ergebnisse der Wissenschaftlichen Untersuchung der Schweizerischen Nationalparks, 1 , 481–511.
Vlk, W. (1931). Über die Struktur der Heterokontengeisseln. Beihefte zum Botanischen Zentralblatt, 48 , 214–220.
Vlk, W. (1938). Über den Bau der Geissel. Archiv für Protistenkunde, 90 , 448–488.
Whittle, S. J. (1976). The major chloroplast pigments of Chlorobotrys regularis (West) Bohlin (Eustigmatophyceae) and Ophiocytium majus Nägeli (Xanthophyceae). British Phycological Journal, 11 , 111–114.
Whittle, S. J., & Casselton, P. J. (1975). The chloroplast pigments of the algal classes Eustigmatophyceae and Xanthophyceae. II. Xanthophyceae. British Phycological Journal, 10 , 192–204.
Yang, E. C., Boo, G. H., Kim, H. J., Cho, S. M., Boo, S. M., Andersen, R. A., & Yoon, H. S. (2012). Supermatrix data highlight the phylogenetic relationships of photosynthetic Stramenopiles. Protist, 163 , 217–231.
Zuccarello, G. C., & Lokhorst, G. M. (2005). Molecular phylogeny of the genus Tribonema (Xanthophyceae) using rbcL gene sequence data: Monophyly of morphologically simple algal species. Phycologia, 44 , 384–392.
Download references
Acknowledgments
This revised version of the chapter is indebted to the original written by David J. Hibberd (1990). We have left unchanged large parts of his sections on morphology, biochemical characteristics, and life history. However, his treatment of taxonomy and phylogeny has been largely rewritten as understanding has advanced dramatically.
Author information
Authors and affiliations.
Department of Biology, University of Padova, Via 8 Febbraio, 2-35122, Padova, Italy
Silvia Maistro & Carlo Andreoli
School of Biological Sciences, University of Canterbury, Christchurch, New Zealand
Paul Broady
Department of Comparative Biomedicine and Food Safety, University of Padova, Via 8 Febbraio, 2-35122, Padova, Italy
Enrico Negrisolo
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Enrico Negrisolo .
Editor information
Editors and affiliations.
Department of Biochemistry and Molecular Sir Charles Tupper Medical Building, Dalhousie University, Halifax, Nova Scotia, Canada
John M. Archibald
Department of Biology Life Sciences Centre, Dalhousie University, Halifax, Nova Scotia, Canada
Alastair G.B. Simpson
Department of Biochemistry and Mole, Dalhousie University Department of Biochemistry and Mole, HALIFAX, Nova Scotia, Canada
Claudio H. Slamovits
Ashland, Oregon, USA
Lynn Margulis
Biozentrum Köln, Universität zu Köln Biozentrum Köln, Köln, Nordrhein-Westfalen, Germany
Michael Melkonian
Dept. Ecology, Evolution, Marine Biology, University of California Dept. Ecology, Evolution, Marine Biology, Santa Barbara, California, USA
David J. Chapman
Libertyville, Illinois, USA
John O. Corliss
Rights and permissions
Reprints and permissions
Copyright information
© 2016 Springer International Publishing Switzerland

About this entry
Cite this entry.
Maistro, S., Broady, P., Andreoli, C., Negrisolo, E. (2016). Xanthophyceae. In: Archibald, J., et al. Handbook of the Protists. Springer, Cham. https://doi.org/10.1007/978-3-319-32669-6_30-1
Download citation
DOI : https://doi.org/10.1007/978-3-319-32669-6_30-1
Received : 04 November 2015
Accepted : 25 February 2016
Published : 12 July 2016
Publisher Name : Springer, Cham
Online ISBN : 978-3-319-32669-6
eBook Packages : Springer Reference Biomedicine and Life Sciences Reference Module Biomedical and Life Sciences
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Life Cycle of Vaucheria (With Diagram) | Xanthophyta
In this article we will discuss about the vegetative and sexual methods of reproduction that occur in the life cycle of vaucheria .
1. Vegetative Reproduction in Vaucheria:
The vegetative reproduction takes place by fragmentation. The thallus can break into small fragments due to mechanical injury or insect bites etc. A septum develops at the place of breaking to seal the injury. The broken fragment develops thick wall and later on develops into Vaucheria thallus.
2. Asexual Reproduction in Vaucheria:
The asexual reproduction takes place by formation of zoospores, aplanospores and akinetes
(a) By Zoospores:
The zoospores formation is the most common method of reproduction in aquatic species. In terrestrial species it takes place when the plants are flooded. Zoospore formation takes place in favourable seasons or can be induced if aquatic species are transferred from light to darkness or from running water to still water.
Zoospores are formed singly within elongated club shaped zoosporangium (Fig. 2A, B). The development of zoosporangium begins with a club shaped swelling at the tip of a side branch. A large number of nuclei and chloroplasts along with the cytoplasm move into it. A colourless protoplasmic region becomes visible at the base of cytoplasm and it is separated from rest of the cytoplasm of thallus.
Each separated protoplast secretes thin membrane and zoosporangium gets separated by a cross wall. Inside zoosporangium the vacuole decreases, the contents of sporangium become very dense and round off. The change takes place in relative position of chloroplasts and nuclei, the nuclei become peripheral and chloroplasts enter in inner layer of cytoplasm.
The entire protoplasm of the zoosporangium contracts to form oval zoospore. Opposite to each nucleus two flagella are produced making zoospore a multi-flagellate structure. A terminal aperture develops in zoosporangium by gelatinization of wall. The zoospore is liberated through aperture in morning hours (Fig. 2 C, D).
Each zoospore is large yellow green, oval structure. It has a central vacuole which has cell sap and may be traversed by cytoplasmic strands. The protoplasm outer to vacuole has many nuclei towards the walls and chromatophores towards vacuoles. Two flagella arise opposite to each nucleus. This part of cytoplasm can be regarded equivalent to one zoospore.
Fritsch (1948) regarded this kind of zoospore as compound zoospore or synzoospore as a number of biflagellate zoospores have failed to separate from one another.
According to Greenwood, Manton and Clarke (1957) the flagella of a pair are heterokontic and whiplash type. The shorter flagellum of each pair is directed towards the anterior end of the zoospore. The flagellar bases are united together in pairs and are firmly attached to the tip of nuclei.
According to Greenwood (1957), there is large anterior vacuole and small ones in the posterior region of the zoospores. Mitochondria are present in the peripheral layer of cytoplasm. Fat bodies and plastids are present in the cytoplasm. Chlorophyll has also been reported from the zoospores.
The zoospores swim in water for 5-15 minutes and germinate without undergoing any significant period of rest. The zoospores get attached to the substratum, withdraw flagella and secrete thin walls (Fig. 2 E, F). The chromatophores move outwards and nuclei inwards as in vegetative condition.
The two tube like outgrowths develop in opposite directions. One of the two outgrowths elongates, branches to form colourless lobed holdfast and the other outgrowth forms yellow-green tubular coenocytic filament (Fig. 2 G, H).

(b) By Aplanospores:
Aplanospores are commonly observed in species. V. geminata, V. uncinata and in marine species V. pitoboloides. The aplanospores are generally formed by terrestrial species.
Aquatic species form aplanspores under unfavorable condition of drought. The aplanospores are non-motile asexual spores formed in special structures called aplanosporangia (Fig. 3 A-C). The aplanospores are produced singly in cells at the terminal end of the short lateral or terminal branch.
The protoplasm of aplanosporangium gets metamorphosed into single multinucleate aplanospore which is thin walled. In V. germinata aplanospores are oval and are liberated from apical pore formed by gelatinization.
In V. uncinata aplanospores are spherical and are liberated by rupture of the sporangial wall. The formation and structure of aplanospores and zoospores is similar except that the zoospores lack flagella. The aplanospores soon after liberation germinate into new thalli (Fig. 3D).

(c) By Akinetes:
Akinetes are thick walled structures formed during unfavorable conditions like drought, and low temperature. The akinetes have been commonly observed in V. geminata, V. megaspora and V. uncinata.
The akinetes are formed on the terminal part of lateral branches where protoplasm migrates to the tips followed by cross-wall formation (Fig. 4). These multinucleate, thick walled segments are called akinetes or hypnospores.

The akinetes by successive divisions may form numerous thin walled bodies called cysts. When many akinetes remain attached to the parent thallus, the thallus gives the appearance of another alga Gongrosira.
Hence this stage of Vaucheria is called Gongrosira stage. During favourable conditions the akinetes and cysts develop into new thalli. Randhawa (1939) has reported that in V. uncinata the submerged parts of thallus develop sex organs whereas exposed parts of thallus form brick shaped akinetes.
(iii) Sexual Reproduction in Vaucheria :
In Vaucheria sexual reproduction is of advanced oogamous type. The male and female sex organs are antheridia and oogonia, respectively.
Majority of the freshwater species are monoecious or homothallic while some species like V dichotoma, V. litorea and V. mayyanadensis are dioecious or heterothallic. There are different types of arrangement of antheridia and oogonia in homothallic species. The position, structure and shape of antheridia are of taxonomic importance in Vaucheria.
The common patterns of arrangement of sex organs are as follows:
(a) Antheridia and oogonia develop close to each other on the filament at intervals (Fig. 5 A-C).
(b) The antheridia and oogonia are borne on special side branches with a terminal antheridium and a number of lateral oogonia (Fig. 5D).
In V. hamata the reproductive branches bear a median terminal antheridium and two oogonia, one on either side of antheridium.
In V. geminata and V. terrestris the sex organs are produced at the ends of the lateral branches with a terminal antheridium and a group of oogonia (Fig. 5D). The sex organs are unilateral when they are arranged on one side of the filament or bilateral when they are on both sides of the filament.
(c) Antheridia and oogonia are borne on adjacent branches (Fig. 5E).

Structure and Development of Antheridium:
The mature antheridia may be cylindrical, tubular, straight or strongly curved. The antheridium is separated from main filament by a septum. The antheridia can be sessile (without stalk) arising directly from main branch e.g., V. civersa. The antheridia may be placed high on the branch the antheridia are situated on androphore V. synandra.
The young antheridium is usually green in colour. It contains cytoplasm, nuclei and chloroplasts. The mature antheridia are yellow and contain many spindle shaped antherozoids. The antherozoids are liberated through a terminal pore e.g., V. aversa or through many pores e.g., V. debaryana
In monoecious species the antheridium arises as a small bulging or lateral outgrowth along with or before the oogonium development (Fig. 6A). Many nuclei along with cytoplasm enter into it and it gets cut off from the lower part forming a septum.
The antheridium grows and becomes high curved structured, its upper part is main antheridium and the lower part is stalk. The nuclei of antheridium get surrounded by cytoplasm and develop into biflagellate, yellow coloured antherozoids The antherozoids are liberated from the tip of antheridium through apical pore shortly before day break (Fig. 6D-1).

Structure and Development of Oogonium :
The oogonium development starts with accumulation of colourless multinucleate mass of cytoplasm near the base of antheridial branch. This accumulated cytoplasm has been termed as “wanderplasm”. The wanderplasm enters into the outgrowth or bulging of the main filament. This outgrowth is called as oogonial initial.
Large amount of cytoplasm and nuclei enter into oogonia, making it a large globular structure called as oogonium (Fig. 6 B-E). As the oogonium matures, it gets separated from main branch by the development of septum at its base. The mature oogonium is uninucleate structure. The nucleus of oogonium with protoplasm develops into a single egg.
There are three hypothesis regarding the fate of extra nuclei of oogonium of Vaucheria:
(a) According to Oltmanns (1895) accept a single nucleus which forms female nucleus, all other nuclei migrate back into the filament. This was supported by Heidinger (1908) and Couch (1932).
(b) According to Davis (1904), the single nucleus forms the egg and all other nuclei degenerate.
(c) According to Brehens (1890) all nuclei fuse to form a single nucleus.
The mature oogonia are globose, obovoid, hemispherical or pyriform in shape. The oogonia may be sessile or stalked structure. The protoplast of oogonium is separated from main filament by- septum formation.
The entire protoplasm with single nucleus makes a central spherical mass called as oosphere or ovum. In mature oogonium a distinct vertical or oblique beak develops in apical part. Opposite to beak develops a colourless receptive spot. A pore develops just opposite to receptive spot (Fig. 6 F).
Fertilization :
The oogonium secretes a gelatinous drop through a pore near the beak. A large number of liberated antherozoids stick to the drop. Many antherozoids push into the oogonium. The antherozoids strike violently, fall back and push forward again and fall back. Only one antherozoid enters into the oogonium.
After its entry the membrane develops at the pore to stop the further entry of antherozoids. The male nucleus increases in size and fuses with the egg nucleus to make diploid zygote. The zygote secretes a thick 3-7 layered wall and is now called as oospore (Fig. 6 G-I). The chromatophores degenerate and lie in the centre of the cell.
Germination of oospore:
The oospore undergoes a period of rest before germination. During favourable season the oogonial wall disintegrates and the oospore is liberated. The oospore germinates directly into new filaments.
Although the exact stage at which the reduction division takes place in Vaucheria is not clear, it is believed that reduction division occurs in first nuclear division in the germinating oospore (Fig. 7 A-D). The oospore germinates to make haploid thallus of Vaucheria.

According to Williams, Hanatsche and Gross the life cycle of Vaucheria is haplontic, the oospore being the only diploid structure in life cycle (Figs. 8, 9). Vaucheria thallus is haploid. It is aseptate, branched, tubular and coenocytic structure.
Vegetative re-production takes place by fragmentation. Asexual reproduction takes place by zoospore in aquatic species and by aplanospores in terrestrial species.
The zoospore is large multi flagellate structure and is supposed to be compound:
Zoospore or Synzoospore:
The sexual reproduction is advanced oogoinous type, the male and female sex organs are antheridia and oogonia. Most of the species are homothallic, some are heterothallic. After fertilization, a diploid zygote is formed which converts into oospore and undergoes a period of res The reduction division takes place in oospore during germination and an haploid thallus is formed (Fig. 8, 9).

Related Articles:
- Vaucheria: Occurrence, Reproduction and Life Cycle
- Reproductive Structures of Vaucheria (With Diagram) | Algae
- Genus Vaucheria: Useful Notes on Genus Vaucheria (1392 Words)
- Vaucheria: Occurrence, Structure and Affinities | Xanthophyta
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids
Antia g. pereira.
1 Nutrition and Bromatology Group, Analytical and Food Chemistry Department, Faculty of Food Science and Technology, Ourense Campus, University of Vigo, E-32004 Ourense, Spain; [email protected] (A.G.P.); [email protected] (P.O.); [email protected] (J.E.); se.ogivu@ccoxna (A.C.-C.); moc.liamg@4891orromahc (F.C.); moc.liamg@zenemijozallocsalocin (N.C.); [email protected] (A.J.); se.ogivu@sepol (C.L.-L.)
2 Centro de Investigação de Montanha (CIMO), Instituto Politécnico de Bragança, Campus de Santa Apolonia, 5300-253 Bragança, Portugal
Javier Echave
Anxo carreira-casais, franklin chamorro, nicolas collazo, amira jaboui, catarina lourenço-lopes, jesus simal-gandara, miguel a. prieto, associated data.
Not applicable.
Algae are considered pigment-producing organisms. The function of these compounds in algae is to carry out photosynthesis. They have a great variety of pigments, which can be classified into three large groups: chlorophylls, carotenoids, and phycobilins. Within the carotenoids are xanthophylls. Xanthophylls (fucoxanthin, astaxanthin, lutein, zeaxanthin, and β-cryptoxanthin) are a type of carotenoids with anti-tumor and anti-inflammatory activities, due to their chemical structure rich in double bonds that provides them with antioxidant properties. In this context, xanthophylls can protect other molecules from oxidative stress by turning off singlet oxygen damage through various mechanisms. Based on clinical studies, this review shows the available information concerning the bioactivity and biological effects of the main xanthophylls present in algae. In addition, the algae with the highest production rate of the different compounds of interest were studied. It was observed that fucoxanthin is obtained mainly from the brown seaweeds Laminaria japonica , Undaria pinnatifida , Hizikia fusiformis , Sargassum spp., and Fucus spp. The main sources of astaxanthin are the microalgae Haematococcus pluvialis , Chlorella zofingiensis, and Chlorococcum sp. Lutein and zeaxanthin are mainly found in algal species such as Scenedesmus spp. , Chlorella spp. , Rhodophyta spp., or Spirulina spp. However, the extraction and purification processes of xanthophylls from algae need to be standardized to facilitate their commercialization. Finally, we assessed factors that determine the bioavailability and bioaccesibility of these molecules. We also suggested techniques that increase xanthophyll’s bioavailability.
1. Introduction
In recent years, consumer demand for naturally sourced products to promote health and reduce disease has grown steadily [ 1 ]. This demand has entailed an increased interest in new natural sources of food, pharmaceutical, and cosmetic products [ 2 , 3 ]. In this context, the marine environment has been considered a potential reservoir of natural compounds [ 4 ]. Among the organisms present in this environment, it is worth highlighting algae. Algae constitute a polyphyletic group of photosynthetic primary producers organisms, which represent an interesting source of chemical components with high-value biological activities. [ 5 ]. Although the total number of algal species is unknown, it is thought to vary between one and ten million [ 6 ].
The high value of algal extracts is due to their large number of molecules such as carbohydrates, proteins, peptides, lipids (including oils and polyunsaturated fatty acids, PUFAs), minerals, iodine, phenols (polyphenols, tocopherols), alkaloids, terpenes, and pigments (as chlorophylls, carotenoids, and phycobilins) [ 7 , 8 ]. Within these compounds, one of the groups with greater interest are pigments due to the concentrations in which they are present, these being higher than that of other compounds such as phenolic compounds. In fact, algae are considered pigment-producing organisms. They have a great variety of pigments, which can be classified into three large groups: chlorophylls, carotenoids, and phycobilins. Therefore, different carotenoids (CA) profiles can be used as a medium for algal classification [ 9 ]. In this way, a first classification of the algae allows us to make a division according to the size of the algae (microalgae or macroalgae) and the following divisions according to their tones, among other characteristics. As a result, the first group comprises greenish algae (Cyanophyceae), green algae (Chlorophyceae), diatoms (Bacillariophyceae), and golden algae (Chrysophyceae), among others. Meanwhile, the macroalgae family includes red (Rhodophyta), brown (Ochrophyta), and green algae (Chlorophyta) [ 10 , 11 , 12 ]. This diversity of species and, therefore, of its chemical compositions is interesting, since once compounds are properly isolated or extracted from algae, they may show a diverse range of biological activities, such as antioxidant, antimicrobial, anticancer, anti-allergic, antiviral, and anticoagulant activities, among others [ 7 , 8 ]. This diversity of biological activities implies that there is also a significant variety of potential applications in human health, agriculture, and in food and cosmetic industries [ 4 ], in which its application depends on its chemical composition.
On an industrial scale, the most interesting species are those that produce high percentages of CA. CA are usually located in chloroplasts or stored in vesicles and a cytoplasmic matrix of plants, algae, photosynthetic bacteria, and some fungi [ 9 ]. All CA are tetraterpenes, which are compounds that have a skeleton composed of 40 carbon atoms conjugated in polyene chains [ 9 ]. They are classified into two main groups: (i) compounds that have a hydrocarbon long chain known as carotenes and (ii) compounds that have an oxygen atom in its structure, known as xanthophylls. The first group includes α-carotene, β-carotene, lycopene, and phytoene, among others. The most representative molecules of the second group are fucoxanthin, astaxanthin, lutein, zeaxanthin, and β-cryptoxanthin. This difference in its structure makes xanthophylls more polar than carotenes due to the presence of oxygen in the form of methoxy, hydroxy, keto, carboxy, and epoxy positions. However, except for lutein, they are still non-polar compounds [ 13 ]. Its structure with alternating double bonds is responsible for many of its biological functions, being the main function in photosynthetic organisms to act as accessory pigments for the capture of light in photosynthesis, and to protect photosynthetic machinery against self-oxidation [ 14 ]. However, despite the wide diversity of molecules in the carotenoid family, with more than 700 compounds currently known, only about 30 CA have a significant role in photosynthesis [ 13 ]. In recent years, numerous studies have highlighted CA multiple effects on human health due to their antioxidant properties, preventing the damage caused by oxidative stress and therefore declining the risk of chronic diseases [ 14 , 15 ]. However, the biological properties of CA are not limited to their antioxidant properties. The scientific literature has shown CA actions as anti-tumor [ 16 , 17 , 18 ], anti-inflammatory [ 19 , 20 ], neuroprotective, antimicrobial, antidiabetic, and antiobesity [ 21 , 22 ]. Therefore, algae have several CA with market interest (β-carotene, fucoxanthin, astaxanthin, lutein, zeaxanthin, and violaxanthin), representing a natural and sustainable source of these compounds [ 9 ].
Among the xanthophylls of interest is fucoxanthin, which is one of the most abundant marine CA, accounting for approximately 10% of the total production of natural CA [ 23 ]. It is found in abundant concentrations in the chloroplasts of several brown seaweeds, such as Laminaria japonica , Undaria pinnatifida , Sargassum fusiformis , in several species belonging to the genera Sargassum ( Sargassum horneri ) and Fucus ( Fucus serratus, Fucus vesiculosus ) and in diatoms ( Bacillariophyta ) [ 9 , 24 , 25 , 26 ]. Another xanthophyll of interest is astaxanthin (AS), which is a red pigment. AS is considered a potent antioxidant as it has about ten times more antioxidant activity than other CA [ 27 ]. The main natural sources of this pigment are the microalgae Haematococcus pluvialis , Chlorella zofingiensis, and Chlorococcum sp. [ 28 ]. H. pluvialis is a single-celled green freshwater alga. It is the richest source of natural AS and is already produced on an industrial scale [ 26 ]. Procedures have been technologically advanced to grow Haematococcus containing 1.5–3.0% AS dry weight [ 27 , 29 ]. The richest source of β-carotene is the halotolerant green microalgae Dunaliella salina , accumulating up to 10% of it based on the dry weight of the microalgae [ 30 , 31 ]. When H. pluvialis and D. salina are cultivated in extreme conditions (such as high salinity, high luminosity, or lack of nutrients), AS and β-carotene, respectively, can reach more than 90% of the total carotenoids [ 7 ]. Lutein and zeaxanthin are pigments found in algal species such as Scenedesmus spp. , Chlorella spp. , Rhodophyta spp., or Spirulina spp. respectively [ 32 ]. Esteban et al., 2009 [ 33 ], reported that red algae ( Rhodophyta ) show a common carotenoid pattern of β-carotene and one to three xanthophylls: lutein, zeaxanthin, or anteraxanthin. Corallina elongata and Jania rubenseran were the only algae that contained anteraxanthin as the main xanthophyll. Spirulina platensis (strain pacifica) microalgae is a source of β-cryptoxantine, β-carotene, and zeaxanthin. β-cryptoxantine is a pigment that can also be found in plants [ 34 ]. The siphonaxanthin content in green algae such as Umbraulva japonica, Caulerpa lentillifera, and Codium fragile constitutes about 0.03%–0.1% of the dry weight [ 35 ]. The cyanobacteria Synechococcus sp. strain PCC7002 produces a monocyclic myxoxanthophyll, which is identified as Myxol-2 Fucoside (Myxoxanthophyll), in addition to producing other CA such as β-carotene, zeaxanthin, and sinecoxanthin [ 36 ]. The CA composition in cyanobacteria is very different from that of other algae, including for example β-carotene, zeaxanthin, myxol pentosides, and echineone [ 32 ].
Animals should get all these CA through the diet, as they are unable to synthesize them. CA are commonly incorporated as dietary supplements, feed additives, and food colorants in several sorts of food, such as dairy products and beverages, and also in the pharmaceutical and cosmetic industries [ 37 ]. As shown in Figure 1 , CA have a high repertoire of commercial applications due to their multiple biological properties. Among the most notable applications are cosmetic, nutraceuticals, pharmaceutical purposes, and other human applications.

Positive effects on human health and industrial applications of carotenoids from natural sources.
Attributable to the various positive activities on human health and the multiple industrial applications of CA, global demand continues to increase. It is estimated that in 2026, the CA market will grow to USD 2.0 billion, registering an annual growth rate for CA of 4.2% [ 38 ]. The most relevant and important pigments on the market today are β-carotene and AS, followed by lutein, lycopene, and canthaxanthin [ 13 , 31 ]. So far, most commercial CA are artificially produced. However, the strong global interest in food of natural origin that is safe, healthy, and environmentally friendly has increased the demand for natural sources of CA [ 22 ]. Algae and algal extracts are a sustainable option for CA and have numerous benefits in comparation to alternative natural sources. For instance, its cultivation and production is cheap, easy, and ecological, its removal has higher yields and is simple, and raw materials are not scarce, nor are there seasonal limitations [ 32 , 39 , 40 ]. In order to obtain high concentrations of a certain compound, culture conditions and environmental stress can be modified to manipulate the biochemical composition of microalgae [ 39 ]. However, under optimal growth conditions, the concentration of CA pigments is often too low to produce microalgal-based pigments, making it economically unviable [ 13 , 40 ]. To improve its economic viability, it is vital to explore and understand how environmental factors and the integration of nutrients into the environment affect the production of compounds. Understanding how the metabolic pathways of species vary according to the culture conditions, the co-production and accumulation of multiple compounds in microalgae will be improved [ 41 ]. The purpose of this review is to highlight the impact of xanthophylls from algae on human health, and to study the factors affecting the feasibility of their production and use as a sustainable alternative source of CA in the coming years.
2. Main Xanthophylls Present in Algae
From examining the findings, algae are a raw material of interest due to their pigment content and the potential bioactivities they possess. However, at present, relatively few species are used for such purposes since their exploitation at an industrial level is scarce. Table 1 lists some cases on algae exploitation to obtain high value xanthophylls. It includes information about the main algae species producing xanthophylls and their applications together with the main extraction techniques used to obtain the high-value molecules. The amount obtained in each case provides necessary information to estimate whether the process is viable.
Xanthophylls in algae: mass production, concentration, and application.
| Mol. | Algae | Extraction | Concentration | Applications | Ref. |
|---|---|---|---|---|---|
| Enzyme-assisted extraction | 0.66 mg/g DW | Development of value-added nutraceutical products from seaweed | [ ] | ||
| Supercritical fluid extraction | 2.18 mg/g DW | Obtaining high-purity fucoxanthin | [ ] | ||
| Microwave-assisted extraction | 0.04 mg/g DW | Obtaining high-purity fucoxanthin | [ ] | ||
| Maceration | 0.10 mg/g DW | Drug against chronic kidney disease | [ ] | ||
| Microwave-assisted extraction | 0.90 mg/g DW | Obtention of high-purity fucoxanthin | [ ] | ||
| Maceration | 3.09 mg/g DW | Scones | [ ] | ||
| Supercritical fluid extraction | 0.99 mg/g DW | Carotenoid isolation | [ ] | ||
| Maceration | 2.67 mg/g DW | Drug development | [ ] | ||
| Ultrasonic-assisted extraction | 0.75 mg/g DW | Nutraceuticals and biomedical applications | [ ] | ||
| Maceration | 3.47 mg/g DW | Optimization of the environmental conditions | [ ] | ||
| Maceration | 18.60 mg/g DW | Commercial fucoxanthin production | [ ] | ||
| Ultrasonic-assisted extraction | 0.25 mg/g DW | Nutraceutical, cosmetic and pharmaceutical applications, such as for the treatment of metastatic melanoma | [ ] | ||
| Ultrasonic-assisted extraction | 0.03 mg/g DW | Yogurt | [ ] | ||
| Maceration | 0.1 mg/g DW | Milk | [ ] | ||
| Conventional extraction | 900 kg/2 ha/year | Antioxidant, anti-tumor, anti-inflammatory, ocular protective effect, antidiabetic, coloring agent | [ ] | ||
| Two-stage system | 3.8% dw | [ ] | |||
| Enzyme | 3.6% dw | [ ] | |||
| Conventional extraction | 2–3% dw | [ ] | |||
| Pressurized extraction | 99% of total AS | [ ] | |||
| Maceration | 83.8 mg/L | Antioxidant, light-filtering, eye protection, colorant, potential therapeutic use against several chronic diseases, lower risk of cancer, anti-inflammatory benefits | [ ] | ||
| Mechanical | 83.8 mg/L | [ ] | |||
| Mechanical | 4.92 mg/g | [ ] | |||
| Heptane–ethanol–water extraction | 30 mg/g | [ ] | |||
| - | 0.54% wt | [ ] | |||
| Conventional extraction | 15.4 mg m d | [ ] | |||
| Supercritical fluids extraction | 13.17 mg/g | Antioxidant, anti-inflammatory, eyes and UV light protection, prevention of coronary syndromes, anti-tumoral, anti-cardiovascular diseases, and structural actions in neural tissue | [ ] | ||
| Pressurized liquid extraction | 4.26 mg/g | [ ] | |||
| Pulse electric field | 1.64 mg/g | [ ] | |||
| Pulse electric field | 0.13 mg/g | [ ] | |||
| Moderate electric field | 244 µg/g | [ ] | |||
| Supercritical fluid extraction | 7.5 mg/100 g | Antioxidant, anti-inflammatory, anticancer (lung, oral, pharyngeal), improves respiratory function, stimulation of bone formation and protection, modulation response to phytosterols in post-menopausal women, decreases risk of degenerative diseases | [ , ] | ||
| Conventional extraction | 14.2% total carotenoids | [ ] | |||
| Conventional extraction | 10.2% total carotenoids | [ ] | |||
| Maceration | 2.38 µg/g DW | [ ] | |||
| Enzyme extraction | - | [ ] | |||
| Maceration | 16 mg/kg fresh algae | Anti-angiogenic, antioxidant, cancer-preventing action; inhibit adipogenesis | [ ] | ||
| Maceration | 0.1% DW | [ ] | |||
| Maceration | 0.1% DW | [ ] | |||
| MeOH extraction | 19% of total pigments | Antioxidant | [ ] | ||
| MeOH extraction | - | [ ] | |||
| EtOH extraction | 10% total carotenoids | [ ] | |||
| Whole | 14 µg/L | [ ] | |||
| MeOH extraction | 17% of total pigments | Antioxidant | [ ] |
Mol: Molecules/compounds; FU: Fucoxanthin; AS: Astaxanthin; LU: Lutein; ZEA: Zeaxanthin; CRY: β-cryptoxanthin; SIP: Siphonaxanthin; DIAD: Diadinoxanthin; DIAT: Diatoxanthin. dw: Dry weight.
2.1. Fucoxanthin
Fucoxanthin (FU) ( Figure 2 ) is produced by many algae as a secondary metabolite. It is present in the chloroplasts of eukaryotic algae and is involved in the process of photosynthesis performed by algae, which is thought to be more efficient than the photosynthesis of plants [ 77 ]. This molecule is considered one of the most abundant pigments in brown algae, and it represents up to 10% of the total CA found in nature [ 78 ]. It has been studied primarily in microalgae and brown macroalgae from several families such as Undaria , Laminaria, Sargassum , Eisenia , Himathalia , Alaria, or Cystoseira [ 79 , 80 ]. FU has a chemical structure derived from carotene but with an oxygenated backbone. In addition, this compound has several different functional groups such as hydroxyl, carboxyl, epoxy, and carbonyl moieties, and it also has an allenic bond [ 25 ]. FU is orange to brown in color, and it is responsible for the coloration of algae from the Phaeophyceae family. This lipophilic pigment absorbs light in a range from 450 to 540 nm, which translates in the blue-green to yellow-green part of the visible spectrum, and it behaves as the primary light-harvesting CA for many algae transferring energy to the chlorophyll–protein complexes with high efficiency thanks to its unique CA structure [ 81 ].

Chemical structure of the main xanthophylls present in algae [ 82 ].
Many bioactivities have been reposted regarding FU. Several articles have been published about its antioxidant, anticancer, anti-inflammatory, antimicrobial, antihypertensive, anti-obesity, antidiabetic, and anti-angiogenic activities, and also its photoprotective and neuroprotective effects ( Table 1 ) [ 79 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 ]. Considering all these properties, FU has a great potential for applications in all sectors, from supplements and enriched foods to anti-aging cosmetics and to the pharmaceutical sector in the development of new innovative drugs for all kinds of pathologies including different types of cancer. For all these reasons, the FU market is expected to keep growing and reach 120 million dollars by 2022 [ 92 ].
Even though the artificial laboratory synthesis of FU is possible, it is a very expensive process that makes the extraction of FU from algae so appealing. However, the extraction and purification processes of FU from algae need to be standardized to facilitate its future commercialization and incorporation to new profitable products on the market [ 48 ]. Nevertheless, some companies already overcame these problems, and valuable products with FU have reached the market. For example, food supplements with FU intended to contribute to the loss of weight and improve eye, brain, liver, and joint health, are being sold with the commercial name of ThinOgen ® and Fucovital ® . These products can be found in the form of oils or microencapsulated powders [ 93 ]. Furthermore, FU is being studied to help combat cancer-related diseases, showing different anticancer mechanisms of action, such as inhibition of cell proliferation, induction of apoptosis, cell cycle arrest, an increase of intracellular reactive oxygen species, and anti-angiogenic effects [ 84 , 88 , 94 , 95 , 96 , 97 ]. Many studies have been made applying FU extracts to human cell lines, such as human bronchopulmonary carcinoma cell line NSCLC-N6, erythromyeloblastoid leukemia cell line K562, and the human lymphoblastoid cell line TK6, all with positive results [ 98 ]. Similar results were observed in prostate cancer (PC-3) cells, leukemia cells (HL-60), and cervical adenocarcinoma cells (HeLa). In addition, in vivo studies were also performed. For example, in mice, the administration of FU suppressed tumor growth of primary effusion lymphoma, sarcomas, and osteosarcoma [ 51 , 55 , 96 ]. Due to its anti-inflammatory activity, FU is also being tested to prevent and treat inflammatory-related diseases, thanks to fucoxanthin’s strong antioxidant capacity and gut microbiota regulation [ 99 ], and its capacity to inhibit the production of nitric oxide, which is one of the determinants of inflammation in cells [ 100 ]. Some examples of FU incorporations in several food matrixes can already be found in the literature such as fortified yogurt [ 52 ] and milk [ 53 ], enriched canola oil [ 101 ], baked products such as scones [ 46 ], and even ground chicken breast meat [ 102 ].
2.2. Astaxanthin
Astaxanthin (AS) is a ketocarotenoid that fits in the group of terpenes and is formed from five carbon precursors, isopentenyl diphosphate, and dimethylallyl diphosphate. It is produced by a restricted number of algae (mainly microalgae), plants, bacteria, and fungi [ 103 ]. In microalgae, this compound is a secondary CA, which means that its accumulation in cytosolic lipid bodies ensues exclusively beneath environmental stress or adverse culture conditions, such as high light, high salinity, and nutrient deprivation. Despite this, algae represent the most important natural source of this compound in the aquatic food chain [ 104 ].
The commercial manufacture of this pigment has conventionally been executed by chemical synthesis. However, current studies proved that some microalgae might be the most capable source for its industrial biological production [ 105 ]. The best known and most used microalgae for its production are Haematococcus pluvialis and Chlorella zofingiensis [ 106 ]. Haematococcus pluvialis is one of the organisms with the highest concentrations of AS; thus, it is the main industrial source for the natural production of this compound [ 107 ]. It is common to reach yields of 38–40 g/kg (3.8–4%) of dried algae, and its scale at an industrial level is possible due to the high reproduction rate of this microalga [ 78 , 79 ]. The amount of AS found in cells corresponds to 85–95% of the total CA content; thus, it is relatively easy to purify it from the remaining CA [ 108 ]. Other species such as C. zofingiensis have also been studied, but the content of AS found was 50% AS of total CA, being the other main CA canthaxanthin and adonixanthin [ 109 ]. The extraction of AS, which is a lipophilic compound, can be carried out with organic solvents and oils, and it is common to combine its extraction with solvents with other types of extractions such as enzymatic or microwave extraction [ 107 ].
This compound is known as one of the most potent antioxidants; its capacity is due to the large amount of conjugated double bonds (thirteen). Different studies confirm that its antioxidant capacity is 65 times more potent than that produced by ascorbic acid; 10 times stronger than β-carotene, canthaxanthin, lutein, and zeaxanthin; and 100 times more effective than α-tocopherol, all of which are antioxidants used routinely [ 108 ]. For this reason, various products containing AS are already available on the market in various forms including oils, tablets, capsules, syrups, soft, creams, biomass, or ground [ 107 ]. An example is AstaPure ® (Algatech LTD) produced from the microalgae H. pluvialis. Moreover, the consumption as a supplement does not represent any risk of toxicity, since the human body is not capable of transforming AS into vitamin A [ 107 ]. In 2019, the European Food Safety Authority (EFSA) has established an acceptable daily intake of 0.2 mg per kg body weight [ 110 ]. However, in order to be used as a food additive, more studies are still required due to stability, conservation, handling, and storage problems in this type of matrix [ 111 ].
AS has also anti-inflammatory activity, which is mainly due to its antioxidant properties and has been concerned in meliorate lifestyle-related illnesses and dealing health. AS additionally has anti-aging activity [ 105 ]. These beneficial effects have been demonstrated for both animals and humans [ 107 ].
2.3. Lutein
Chemically, lutein (LU) is a polyisoprenoid with 40 carbon atoms and cyclic structures at each end of its conjugated chain. Therefore, it has a similar structure to zeaxanthin (explaining below), differing from it in the site of the double bond in one ring, giving three chiral centers compared to the two of zeaxanthin [ 112 ]. LU is already used regularly in sectors such as cosmetics, pharmaceuticals, and food, which is mainly due to its color and bioactivities, and its anticancer properties are worth noting [ 61 ]. In fact, different studies demonstrate the antitumor effects of LU. For example, it was found that oral LU supplementation reduced the influence of ultraviolet irradiation by diminishing acute inflammatory responses and hyperproliferative rebound induced by ultraviolet rays [ 113 ]. In addition, this compound is widely known for its preventative effects against age-related macular degeneration and cataracts [ 62 ]. These health-promoting properties of LU along with its potential as a natural food colorant have led to improved research on the potential of LU as a high-value nutraceutical ingredient [ 114 ].
In general terms and for healthy people, food is a proper source of LU, and it does not require being added in a balanced diet, as it is safe to consume 60 mg/day for an adult of 60 kg [ 115 ]. This dietary contribution of LU is mainly due to the consumption of vegetables. However, algae is being considered as a new reservoir of lutein [ 59 ]. Among them, the best source at the commercial level is microalgae, especially those belonging to the Chlorella genus. This alga is an effective source of LU production, and it is safer than that of chemical origin whose use remains questionable. For this reason, the growth optimization studies of this alga are gaining interest owing to the high growth rates of the alga, along with their high pigment content. Several studies analyze the effect of LU production under different microalgae growth conditions in bioreactors. In most of them, the optimized parameters are the concentration of nitrate, ammonium, and urea in the batch [ 60 , 61 ]. However, the cultivation conditions of other newer species such as Scenedesmus almeriensis have also been optimized to increase their LU production. In this case, the contribution of nutrients has a lesser effect due to the high tolerance of this microalga to varied ranges of temperature, pH, salinity, and nutrient concentration [ 63 ]. Other widely studied species for its high content of LU are D. salina and Galdieria sulphuraria [ 59 ]. Mostly, it is still necessary to reduce costs regarding the growth and extraction process of LU from microalgae to be profitable. For this, it is not only necessary to optimize the consumption of nutrients, but also to analyze the subsequent processes such as harvesting and drying that entail large energy costs. In this regard, the currently available studies seem to indicate that the best option may be tubular photobioreactors [ 114 , 116 ].
2.4. Zeaxanthin
Zeaxanthin (ZEA) is a structural isomer of LU. Both isomers are usually found in various foods, being mainly present in green leafy vegetables and algae [ 117 ]. It is formed by a polyene chain with 11 conjugated double bonds and ionone rings. The ionone rings have a hydroxyl group that can attach to the fatty acids during esterification [ 118 ]. This compound, as well as some derivatives (meso-zeaxanthin), has a high antioxidant effect due to its chemical structure and distribution of the bonds. Furthermore, it also has a powerful anti-inflammatory effect attributable to the down-regulated expression of several inflammatory mediator genes. Consequently, these compounds may also be used in cancer prevention, as tumors are considered inflammatory diseases. Therefore, their use in chemotherapy may be of great interest [ 119 ]. Other bioactivities include photoprotection as well as the prevention and treatment of some eye diseases such as progress of macular degeneration and cataracts [ 120 , 121 ]. Moreover, ZEA has been proved to possess anti-tyrosinase activity, an enzyme associated with the production of melanin. Therefore, the inhibition effect of ZEA on this enzyme may avoid the formation of skin spots, which point to the use of this pigment as a whitening agent [ 122 ]. Hence, ZEA is a CA with promising nutraceutical implications.
Humans are not able to synthetize ZEA, as there is no biosynthetic pathway for this compound; thus, it has to be obtained from the diet. For this reason, its extraction from natural sources including vegetables, plants, macroalgae, cyanobacteria, and microalgae is of great interest [ 123 ]. There are several species of microalgae that produce this pigment. One of them is Dunaliella salina , which has also been genetically modified to increase its yield under all growth conditions, reaching 6 mg ZEA per gram of algae [ 124 ]. Other species that synthesize ZEA include Spirulina, Corallina officinalis, Cyanophora paradoxa and Glaucocystis nostochinearum [ 117 ]. These organisms can accumulate ZEA in a concentration up to nine times higher than traditional sources of this compound such as red peppers. This is the case of Chlorella ellipsoidea . In addition, algae have the advantage over plant matrices that the ZEA present in algae is in free form, while in plants, it is present as mono and diesters of ZEA [ 66 ]. As a consequence, numerous studies show the development of protocols to obtain ZEA from microalgae on a large scale [ 125 ]. Moreover, the production of this compound can be increased by varying the conditions in which algae cultivation takes place. One option is to increase photosynthetic irradiance over that required for the saturation of photosynthesis [ 117 ].
2.5. Minor Carotenoids
In addition to FU, AS, LU, and ZEA, algae can synthetize low amounts of other CA that belong to the xanthophyll group. In this section, we assessed these minor molecules also susceptible to be exploited by the nutraceutical industry. These include β-cryptoxanthin, siphonaxanthin, saproxanthin, myxol, diatoxanthin, and diadinoxanthin. They are only present in some bacteria and marine algae.
2.5.1. β-Cryptoxanthin
β-cryptoxanthin is an oxygenated CA with a chemical structure close to that of β-carotene, being the most important difference the higher polarity of β-cryptoxanthin. The interest of this compound shows a positive correlation between the intake of β-cryptoxanthin and the prevention of several diseases. In fact, this molecule is characterized by having provitamin A activity, anti-obesity effects, antioxidant activities, and anti-inflammatory, and anti-tumor activity [ 126 ]. Furthermore, the influence of β-cryptoxanthin on some inflammatory markers is probably stronger than other CA [ 127 ]. This compound is much less common than β-carotene, and it can only be found in a small number of foods. Some of them are fruits and vegetables such as tangerines, red peppers, and pumpkin [ 128 ]. It is also possible to find this compound in algae, mainly in red algae due to its hue [ 68 ]. Its concentration on each product will depend on environmental factors such as season, processing techniques, and storage temperatures [ 126 ].
2.5.2. Siphonaxanthin
Siphonaxanthin is a specific keto-carotenoid current in comestible green algae such as Codium fragile, Caulerpa lentillifera , and Umbraulva japonica , constituting around 0.1% of their dry weight [ 35 ]. This compound is present mainly in species belonged to the Siphonales order, which is characterized by grouping green algae inhabiting deep waters from both freshwater and marine environments [ 67 ].
Some studies have been carried out with this molecule, showing the potential beneficial effects on health, including anticancer activities and its suitability in the treatment of leukemia, with even better results than those obtained with FU [ 35 ]. This greater capacity to produce an apoptosis-inducing effect may be due to the fact that siphonaxanthin, unlike FU, does not have an epoxide or an allenic bond in its structure, but it does contain an additional hydroxyl group at carbon 19 that might be responsible for this activity [ 129 ]. Other activities include anti-angiogenic, antioxidant and anti-inflammatory. The anti-inflammatory effect is due to the suppression of mast cell degranulation in vivo as it alters the functions of lipid rafts by localizing in the cell membrane and inhibiting the translocation of immunoglobulin E (IgE) / IgE receptor (FcεRI) to lipid rafts [ 130 ].
2.5.3. Saproxanthin
Saproxanthin is an uncommon and recently described natural CA found in algae, bacteria, and archaea [ 131 ], being bacteria the main source. Chemically, it is a tetraterpene with a CA β-cycle additionally hydroxylated at C3 as one end group and simple hydration of the most distant double bond at the other termination of the compound [ 132 ]. Therefore, this compound is also a xanthophyll. It was initially reported and described by Aasen and Jensen in Saprospira grandis [ 67 ]. This compound is a potent antioxidant. It is produced by algae with the aim to protect itself from the activated oxygen produced by light [ 133 ]. In vitro studies have shown its pure form pose high antioxidant activity against lipid peroxidation in the rat brain homogenate model and a neuroprotective effect of l -glutamate toxicity [ 133 , 134 ].
2.5.4. Myxol
Myxol is a derivative of γ-carotene and is present in different forms in nature (free or combined with fucosides or nitrogen groups). Nevertheless, in the free state, it is found primarily in marine environments [ 67 ]. It should be noted that this pigment is glycosylated in the 2′-OH position instead of the usual position (1′-OH) of the molecule [ 36 ]. The main group of organisms that produce this compound are cyanobacteria [ 135 ]. Cyanobacteria were previously called myxophyceae, which is named after the characteristic molecule of this family [ 36 ]. Some cyanobacteria in which this pigment has been characterized are Anabaena and Nostoc [ 136 ]. Nonetheless, algae not only contain free myxol; thus, it is also possible to quantify some combined forms of myxol. One study detected the presence of pro-glyoxylate derivative compounds such as pro-2′-O-methyl-methylpentoside and 4-keto-myxol-2′-methylpentoside in freshwater algae Oscillatoria limosa [ 137 ]. All variants of this molecule have been proved to have antioxidant properties. In fact, its antioxidant activity is greater than that of other frequently used antioxidant molecules such as ZEA and β-carotene [ 138 ]. For example, one study was able to demonstrate significant antioxidant activities against lipid peroxidation in the rat brain homogenate model and a neuroprotective effect of l -glutamate toxicity [ 134 ]. Other in vitro studies have concluded that myxol might also be effective in strengthening biological membranes, reducing permeability to oxygen. Nonetheless, these novel and rare CA require meticulous assessments before their execution [ 138 ].
2.5.5. Diatoxanthin
Diatoxanthin, a ZEA analogue, is a type of xanthophyll found in phytoplankton and diatoms. Diatoms are often called golden brown microalgae, due to their content of pigments, mainly CA, comprising FU, diadinoxanthin, and diatoxanthin [ 139 ]. These compounds have the function of serving as a protection system for algae against the harmful effects of light saturation. Thanks to its presence, the algae are able to quickly acclimatize to the difference in the amount of light received and therefore continue to carry out their vital functions without alterations [ 140 ]. Therefore, an effective way to increase the production of this compound, and hence its performance, is to increase the blue-light irradiation; 300 μmol photons m −2 ·s −1 is enough for Euglena gracilis [ 141 ].
2.5.6. Diadinoxanthin
Similar to diatoxanthin, diadinoxanthin is present only in limited algal groups, including diatoms. In fact, these pigments might be considered as diatom-specific CA [ 73 ]. Both compounds are interrelated, since diadinoxanthin is the inactive precursor of diatoxanthin, and it can be transferred to the active compound very quickly when subjected to high light stress [ 140 ]. Diadinoxanthin, together with FU, can be obtained from neoxanthin. For this, it is necessary to have a simple isomerization of one of the allenic double bonds of neoxanthin molecule [ 74 ]. Its antioxidant activity is based on deepoxidized diadinoxanthin to diatoxanthin, which leads to reduction of the singlet oxygen inside the cell, avoiding cellular damage [ 142 ].
3. Mechanism of Action of Xanthophylls
3.1. metabolism.
The mechanism of action of xanthophylls is the specific binding through which the molecule produces its pharmacological effect. This effect will depend on the absorption, distribution, and metabolism of the compound, which are critical parameters of the pharmacokinetics of the xanthophylls. This can be seen in various studies that show the low presence of this type of compound in human tissues, which directly depends on their metabolism and intestinal absorption, and therefore, its bioavailability [ 143 ]. The metabolism of xanthophylls is poorly studied, especially for those that do not have provitamin A activity. Hence, more studies are needed to understand its metabolism and, therefore, be able to develop different applications according to the mechanism by which its biological activities occur.
In turn, this would allow the development of safe and effective applications in humans as well as increase its bioavailability [ 144 ]. For example, studies on FU metabolism revealed that this compounds itself is not present in plasma but rather its metabolites due to oxidative reactions that take place on FU in mammals. This reaction transforms both compounds into ketocarotenoids [ 145 ]. In addition, when FU is administered orally, it undergoes a process of hydrolysis at the intestinal level, giving rise to fucoxanthinol, while liver metabolization results in other active metabolites such as amarouciaxanthin A [ 146 , 147 ]. In fact, it was reported that dietary FU accumulated in the heart and liver as fucoxanthinol and in adipose tissue as amarouciaxanthin A, the latter being non-detectable by HPLC in human serum [ 148 ]. Therefore, the oral administration of this compound may only provide some bioactive metabolites, as it is completely metabolized. To release products that maintain its biological activities, it is necessary to develop alternatives that prolong its biological half-life [ 146 ], such as emulsions or encapsulations ( Table 2 ).
Delivery systems used to increase marine carotenoids’ bioavailability.
| Mol. | Delivery System | Assay | Benefits | Results | Use | Ref. |
|---|---|---|---|---|---|---|
| Palm stearin solid lipid core | In vitro | Increase stability during storage | Release of FU of 22.92% during 2 h in SGF and 56.55% during 6 h SIF | Oral supplements | [ ] | |
| Nanoparticles of zein | ABTS DPPH | Increase antioxidant activity | More antioxidant than free FU | Foods and beverages | [ ] | |
| Nanoemulsion | In vitro | Increase stability during storage; antiobesity | 95% of FU remains in the emulsion after 4 weeks | Food, beverages, nutraceutics | [ ] | |
| Nanoemulsion (LCT) | In vitro digestion and bioability assays in rats | Increase stability | Increase FU level in serum blood (LCT > MCT) | Functional foods and nutraceutics | [ ] | |
| Chitosan–glycolipid nanogels | In vitro | Significant increase in bioavailability | Lpx levels (nmol MDA/mL) higher in control (30.9) than in emulsions (17.0–12.15) | Foods and nutraceutics | [ ] | |
| Fish oil | In vitro | Useful for supplementation | Better antioxidant effect | Oral supplements | [ ] | |
| Encapsulation | TBARS Peroxide enzymes | Increase stability | Better antioxidant effect | Foods | [ ] | |
| Pectin–chitosan multilayer | Stability Assays | Increase stability | Better stability than monolayer | Nutraceuticals, functional, medical foods | [ ] | |
| l-lacic acid | Release and stability test | Increase stability | Enhance stability | Functional foods and nutraceutics | [ ] | |
| Ascobyl palmitate emulsion | Stability assay | Sublingual delivery | Enhance sports performance, skin protection, cardioprotective | Dietetic supplementation in sports | [ ] | |
| β-CD | In vitro | Increase stability | More stable against oxidating agents | Foods | [ ] | |
| Glycyrrhizic acid, arabinogalactan | In vitro | Solubility enhancement | Prevention of H-aggregates formation, increase of photostability | Foods | [ ] | |
| Sea Buckthorn oil and water emulsion | Stability and digestive assays | Increase bioaccesibility | Increase 64.55% | Functional foods and nutraceutics | [ ] | |
| High-pressure treatment | Stability and digestive assays | Improve sp. ZEA disponibility | Foods | [ ] | ||
| Glycyrrhizic acid, arabinogalactan | In vitro | Solubility enhancement | Prevention of H-aggregates formation, increase of photostability | Foods | [ ] |
SGF: Simulated gastric fluid; SIF: Simulated intestinal fluid; LCT: Long-chain triglycerides; MCT: Medium-chain triglycerides.
A study carried out on rats reported that the pharmacokinetic parameters of AS only depend on the dose when it is administered intravenously due to the metabolism that takes place in the liver as a result of saturation of hepatic metabolism of AS [ 162 ]. As for AS metabolites described in humans, these are fundamentally 3-hydroxy-4-oxo-β-ionone and 3-hydroxy-4-oxo-7,8-dihydro-β-ionone [ 163 ]. The metabolization of AS after oral intake leads to 3-hydroxy-4-oxo-7,8-dihydro-β-ionol and 3-hydroxy-4-oxo-7,8-dihydro-β-ionone, being both compounds detected in plasma [ 164 ]. Several researchers hypothesize that the rate at which these reactions take place is determined by the structure of the ring, as well as by the length of the fatty acyl residue formed. Moreover, several enzymes, such as for example diacylglycerol acyltransferase 1, can catalyze the synthesis of AS esters in some strain. This is the case of the microalga Haematococcus pluvialis [ 165 ].
As for LU and its structural isomer, ZEA, studies carried out in humans have shown that both undergo an in vivo oxidation process that gives rise to several metabolites [ 166 ]. LU gives rise to a series of compounds (3′-epilutein, 3′-oxolutein) due to the presence of the enzyme that also mediated the conversion of fucoxanthinol to amarouciaxanthin A [ 167 ]. Other compounds such as 3-hydroxy-3′,4′-didehydro-β,γ-carotene and 3-hydroxy-2′,3′-didehydro-β,ε-carotene appear as result of acid hydrolysis in the stomach [ 168 ]. However, this compound is capable of remaining intact in its intact form in human ocular tissue due to the inability of the enzyme β-carotene-9′,10′-oxygenase to act on said organ. In this way, there is an extraordinary accumulation of these compounds in the ocular tissue, serving as a mechanism for the prevention of ocular diseases [ 169 ]. ZEA, being an isomer of LU, will undergo similar processes to LU. However, it is a much less studied molecule. In this way, ZEA will also be accumulated in the ocular tissue due to the inactivity of the enzymes responsible for the metabolism of ZEA in the organs of sight [ 170 ]. Therefore, to determine the bioavailability of LU it is necessary to quantify said metabolites, which also may have different bioactivities, with complementary studies.
3.2. Bioavailability and Bioaccessibility
Xanthophylls have been subjected to numerous studies due to its antioxidant activity and protective effect against several diseases [ 171 ]. In recent years, different studies have been carried out comparing the properties of synthetic CA with those of natural origin [ 172 ], noting that some of them can only be obtained from natural sources, where there is much more diversity. In addition, these CA obtained from algae can be co-extracted with other bioactive components such as polysaccharides or fatty acids. Therefore, the idea of incorporating CA in foods, nutraceuticals, or cosmetic products is of increasing interest due to their effective bioactive properties [ 173 ]. However, to develop and evaluate the viability of any food or cosmetic products that maintain these activities, it is necessary to know its bioactivity, bioavailability, and bioaccesibility [ 174 ]. These three parameters are influenced by several factors such as the food matrix; the type of cooking; the time of cooking; the CA involved; the presence of fats, fibers, proteins, and other nutrients in the diet; and the health or nutritional status in humans [ 175 , 176 , 177 , 178 , 179 ].
In humans, once CA are ingested, they are released from the food matrix through the action of gastric enzymes and must be emulsified with lipids in order to improve their absorption [ 180 ]. Moreover, its absorption mechanism will be determined by the concentration in which the compound is present. At low concentrations, absorption is mainly due to the action of type 1 class B scavenger receptor, which also captures high-density lipoproteins, platelet glycoprotein 4, and NPC1-like intracellular cholesterol transporter 1 [ 181 ]. At high concentrations, the main mechanism is passive diffusion through mucosa [ 182 ]. Enzymes released in the duodenum will also play an important role in the absorption, since in this part of the small intestine, pancreatic lipase is released. This enzyme assists the formation of mixed micelles of emulsified droplets with CA. This process depends on the concentration of bile acids among others [ 183 ]. Once the micelles are formed, they pass into the blood. Then, micelles are taken up by enterocytes, in which metabolization takes places due to the presence of the enzyme β-carotene oxygenase. The non-metabolized CA, such as LU and ZEA, are incorporated into chylomicrons or low-density lipoproteins (LDL) and are transported to the liver where they can be eliminated by the bile or metabolized and secreted in very low-density lipoprotein (VLDL) or high-density lipoproteins (HDL) to the peripheral tissues, as it can be seen in Figure 3 [ 180 , 184 ].

Uptake, transport, and secretion pathways of marine carotenoids in the human body.
All these absorption processes involve passing through membranes, which will be determined by the polarity of the membrane and the compounds. CA are frequently esterified with fatty acids, which decreases the polarity, so except for lutein, they are considered non-polar molecules. Among CA, xanthophylls have a bit higher polarity than carotenes. This is due to the small number of oxygen atoms in their structure ( Figure 2 ). In addition, the polar groups of the molecules are at opposite ends of the molecule, so their forces cancel out. Therefore, the presence of hydroxyl groups makes them a bit more polar than carotenes, which do not contain oxygen but are still considered non-polar molecules [ 185 ]. CA polarity and flexibility seem to be correlate with bioaccessibility and uptake efficiency. This may be due to the fact that this type of CA presents a better affinity for lipid transporters and/or for plasma membranes, which would increase absorption [ 186 ]. Therefore, these compounds may be the CA with highest bioavailability. Different mechanisms have also been developed to increase the bioavailability of these compounds, of which the most common are the elaboration of emulsions or encapsulations.
3.2.1. Fucoxanthin
Different in vitro, in vivo, and clinical studies show that FU digestion and absorption gives rise to metabolites such as fucoxanthinol. In a study carried out with mice, FU was transformed into fucoxanthinol in the gastrointestinal mucosa by deacetylation due to the action of lipase and cholesterol esterase enzymes. Then, the fucoxanthinol that reached the liver was transformed to amarouciaxanthin by deoxidation. As a result, fucoxanthinol could be detected in the heart, spleen, liver, and lung, and amarouciaxanthin could be found in adipose tissue [ 145 , 148 ]. During all this process, pH is a limiting factor since, as it was observed in an in vitro simulated digestion study, enzymes can be inactivated due to low pH and, consequently, FU would remain intact [ 187 ]. A study of the colonic fermentation of FU reported that 50% of FU can be metabolized by action of the human microbiota, ensuring that the compound is bioaccessible [ 187 ]. However, the absorption of FU is lower than the rest of the CA despite achieving better accumulation [ 188 ]. This may be due to digestion of the compound. In fact, FU supplementation in adults correlated with fucoxanthinol increase in serum [ 189 ]. A human trial carried out with FU extracted from Undaria pinnatifida concluded that after the supplementation of an extract with 6.1 mg of FU, FU could not be detected in blood, and the metabolite fucoxanthinol was at very low concentration, which confirms the limited intestinal absorption of FU [ 190 ]. In order to improve its absorption, different mechanisms have been developed, of which the most common encapsulation is in micelles or liposomes [ 149 ]. The best results are obtained when long or medium-chain triglycerides are used to carry out the encapsulation [ 152 ]. Encapsulation can also be done with chitosan-glycolipid nanogels, which increase FU bioavailability by 68% according to in vitro studies [ 153 ]. Other options include encapsulation with proteins such as zein and caseinate, which provide better stability to FU and enhance its antioxidant and anti-tumor activity compared to free FU [ 150 ]. Yet, human studies are scarce and contradictory, since numerous factors that influence bioavailability are reported, such as the dietary fiber of the food matrix; the interaction with other nutrients such as lipids and proteins; the solubility of the molecule; or the affinity with intestinal transporters.
3.2.2. Astaxanthin
AS is considered the compound with the highest bioavailability among CA, followed by lutein, β-carotene, and lycopene [ 185 ]. However, its bioavailability depends on the type of matrix and on the stresses of this molecule in colonic Caco-2/TC7 cells [ 191 ]. A study carried out in an in vitro digestion model with human intestinal Caco-2 cells of three geometric isomers of AS conclude that the isomerization occurs at a gastrointestinal level, with the 13-cis-astaxanthin isomer showing the greater bioaccesibility and the higher concentrations in blood [ 192 ]. In human plasma, AS increases in a dose-dependent manner, achieving stimulation of the immune system, and decreasing oxidative stress and inflammation [ 193 ]. High doses (100 mg) present maximum levels of absorption at 11.5 h, while low doses (10 mg) reach them at 6.5 h [ 194 ]. Moreover, the bioavailability of said compound can be improved by emulsion with lipids, becoming between 1.7 and 3.7 times better compared to the reference formulation [ 195 ]. Other options include encapsulation with lipoprotein aggregates, maltodextrin, pectin, or chitosan [ 155 ]. Newer encapsulation methods have also been developed such as oleic acid–bovine serum albumin complexes nanoparticles [ 196 ], which are able to find products that, for example, use nanoemulsions with ascorbyl palmitate in sublingual application to favor the absorption and bioavailability of AS [ 158 ]. Nevertheless, as AS may be easily degraded by digestive acids, intake after digestion has shown increased levels of absorption [ 197 ]. Moreover, the consumption of AS in synergy with fish oil increased the lipid-lowering effects and increased phagocytic activity compared to the consumption of free AS [ 154 ]. On the contrary, sociological factors such as smoking habits also play an important role in bioavailability, since tobacco inhibits the bioavailability of AS [ 194 ]. AS has already been studied as dietary supplements in Europe, Japan, and the United States, demonstrating their safety in human clinical trials of up to 40 mg/day. Based on these data, the US Food and Drug Administration has approved AS from H. pluvialis for human consumption at 12 mg per day and up 24 mg per day for no more than 30 days [ 194 ].
3.2.3. β-Cryptoxanthin
The bioaccesibility of various xanthophylls has been demonstrated in numerous studies. In this regard, an in vitro gastric simulation study proved that all-trans-β-cryptoxanthin has 31.87% of bioaccesibility that could be improved by modifying the nature of the matrix [ 198 ]. Additional studies suggest a mechanism for the digestion and intestinal absorption of β-cryptoxanthin in its free and esterified forms. The study was made in a digestion model with Caco-2 cells and intestinal cells clone Caco-2 TC7, reporting that β-cryptoxanthin is more bioaccessible than β-carotene, but having worse uptake with Caco-2 TC7 cells [ 199 ]. At present, this lack of knowledge makes this compound subject to controversy, since there are studies with disparate results. For example, some of the sources that were consulted state that serum β-cryptoxanthin bioavailability is greater than β-carotene measured in humans after dietary intake [ 200 ].
3.2.4. Zeaxanthin
ZEA constitutes one of the three macular pigments, and it is characterized by having a preventive effect in age-related eye diseases [ 201 ]; consequently, its consumption is important, as humans are not able to synthesize it or store it at the ocular level [ 202 ]. In this sense, the bioavailability and bioavailability of this compound is essential to meet its beneficial effects on health [ 202 ]. However, in the case of the ZEA, temperature plays a fundamental role, since thermal processing promotes ZEA release and solubilization in the gastric environment [ 67 ]. In addition, its consumption associated with diets or foods rich in fat favors the formation of micelles. These micelles will increase the absorption of the compound at the intestinal level [ 203 ]. This is the reason why foods such as sea buckthorn, with a carotenoid-rich oil, possess high bioavailability of ZEA [ 160 ]. Thanks to this property, it is relatively easy to increase the bioaccesibility of ZEA, as shown by various studies. One of them endorses the use of coconut oil to increase 6% of ZEA bioaccesibility in goji berries [ 204 ]. However, despite the increase in the solubility of ZEA in lipid emulsions, it is necessary to subject the walls of the matrix to microstructural modifications, especially with microalgae, since they can influence the digestibility and bioaccesibility of CA [ 161 ]. Nevertheless, microalgae are useful as a source of ZEA in food formulations due to its good bioaccesibility and storage in studies carried out with mice [ 205 ]. Additionally, the relationship between ZEA content and bioavailability is another aspect to consider. For example, the bioaccesibility of ZEA in egg yolk is high [ 206 ], although the ZEA content is low.
3.3. Experimental Studies
The effects of CA on health have been long studied. As mentioned, some CA such as β-cryptoxanthin or β-carotene are precursors of retinol (vitamin A), while others such as fucoxanthin, lutein, or lycopene are not. As such, their intake relates to their role in retinol production, and to their antioxidant, anti-inflammatory, and anti-tumor activities [ 207 ]. In this regard, several in vitro as well as in vivo and observational or epidemiologic studies have been carried out in the last decades. Furthermore, the antioxidant role of CA has been long-known and evidenced for its use as antioxidant additive as well as antioxidant test assay [ 208 ]. The great majority of studies have assessed the intake of CA to test their effects, as it is the major ingress pathway of these molecules. As with other antioxidants of natural origin with observed health-promoting properties, it has been suggested that the potential chemopreventive effects of these molecules are derived from the synergy of their antioxidant and anti-inflammatory properties, besides their direct inhibition of certain factors involved in cell cycle and apoptosis [ 209 ]. This is due to the intimate relationship of oxidative stress as both a cause and result of inflammation and their relationship toward developing cancer [ 210 , 211 ]. Hence, the properties and effectiveness of CA have been tested and evaluated through various ways, both with molecular methods and relating their intake or serum levels with disease or mortality incidence. A summary of relevant findings will be addressed. Experimental designs and outcomes are shown in Table 3 .
Summary of studies and meta-analysis on the health-related properties and effects of carotenoids and observed results.
| Study | Model | Dose | Experimental Design | Observations | Ref. |
|---|---|---|---|---|---|
| Anti-inflammatory | In vitro. RAW 264.7 macrophages with LPS-induced inflammation | 15–60 μM | Expression of inflammatory mediators | D-d reduction of expression of IL6-IL-1, NO, and TNF-α | [ ] |
| In vitro (Apo-9′). RAW 264.7 macrophages and zebrafish model | 25–100 μg/mL | Reduction of LPS-induced inflammation | D-d reduction of NO, ROS, TNF-α, and COX production | [ ] | |
| In vitro and in vivo. RAW 264.7 and aqueous humor of rats | 10 mg/kg | Reduction of LPS-induced inflammation | D-d reduction of PGE2, NO, TNF-α by inhibiting iNOS and COX-2 | [ ] | |
| Anti-cancer | Ex vivo. B16F10 cell culture implanted in mice | 200 μM | Growth inhibition of melanoma | D-d growth inhibition by inducing G /G cell cycle arrest and apoptosis; inhibition production of retinoblastoma protein | [ ] |
| In vitro. Human leukemic HL-60 cells | 15.2 μM | Inhibited the proliferation | DNA fragmentation | [ ] | |
| Anti-inflammatory | In vitro. RAW 264.7, splenocytes, and bone-narrow macrophages | 25 μM | Expression of inflammatory mediators in LPS-induced inflammation | D-d significant reduction of IL-6, IL-1β, and ROS production | [ ] |
| In vivo. Mice with induced acute lung injury | 60 mg/kg/day for 14 days | Analysis of inflammation markers, tissue damage | Significant reduction of mortality, histological damage, inflammatory infiltration, and iNOS and NF-κβ levels | [ ] | |
| Anti-cancer | In vitro. Human colon cancer lines HCT-116, SW480, WiDr, HT-29 and LS-174 | 5–25 µg/mL | Growth inhibition of with astaxanthin-rich extract | D-d cell cycle arrest and apoptosis induction by lowering expression of Bcl-2, AKT and induced expression of apoptotic MAPK | [ ] |
| In vivo. Chemically induced colitis and colon carcinogenesis mice | 200 ppm | Analysis of inflammatory biomarkers | D-d inhibition of NF-κβ, TNF-α, IL-1β, IL-6, and COX-2 expression; lower iNOS expression at high dosage | [ ] | |
| Anti-inflammatory | Observational study. Early atherosclerosis patients ( = 65) | 20 mg/day for 3 months | Differences in serum cytokines, and metabolic biomarkers | Significant reduction in serum IL-6 MCP-1 and LDL-cholesterol after 3 months of supplementation | [ ] |
| Observational study. Preterm infants ( = 203) | 30 mL/ kg/ day until 40 weeks post-menstrual age | Differences in inflammation biomarkers | Enhanced retinal development and reduced C-reactive protein levels | [ ] | |
| Anti-cancer | In vivo. Rats | 3–30 g/L | Inhibition of N-methylnitrosourea-induced colon crypt foci formation | Significantly lowered formation of aberrant crypt foci | [ ] |
| Anti-cancer | Prospective cohort study. Smokers and non-smokers from NHANES III ( = 10,382) | Dietary contribution | 20-year cohort | Higher serum levels of β-CRY were associated with lower death risk, but not for non-smokers | [ , ] |
| Ex vivo. Human gastric cell lines AGS and SGC-7901 implanted in mice | 0–40μM | Growth and proliferation inhibition | D-d growth and proliferation inhibitory activity by reducing cyclins, endothelial growth factor, PKA and increasing cleaved caspases expression | [ ] | |
| In vivo. Mice | 10 mg/kg diet | Induced emphysema and lung tumorigenesis | D-d tumor mass reduction, decreased levels of IL-6 and AKT and restoration of silenced tumor-suppressor genes | [ ] | |
| In vivo. Cigarette smoke-exposed ferrets | 7.5–37.5 μg/kg/day | Inflammation biomarkers and tissue damage analysis | D-d inhibition of NF-κβ, TNF-α, AP-1 expression as well as lung tissue squamous metaplasia and inflammation | [ ] | |
| Anti-cancer | In vitro. Human leukemia (HL-60) cells | 5–20 μM | Analysis on cell viability and apoptosis | D-d reduction of cell viability and induction of apoptosis by increasing levels of DR5, lower expression of Bcl-2 and increase in caspase-3 | [ ] |
D-d: Dose-dependent; LPS: Lipoplysaccharide, ROS: Reactive oxygen species, IL: Interleukin, NRF2: Nuclear factor E2-related factor 2, PKA: Protein kinase A, AKT: Protein kinase B, ERK: Extracellular signal-regulated kinase, PAI-1: Plasminogen activator inhibitor-1, MMP: Metalloproteinases, Bcl-2: B-cell lymphoma 2, PG: Prostaglandin, RR: Relative risk, CI: Confidence interval.
3.3.1. Observation In Vitro
In vitro experiments testing properties of CA are of great value to analyze the role of specific molecules and discern potential participating molecules. Their apparent results have been reinforced in multiple animals and human studies, while in some cases, results have been mixed. In fact, most experiments with CA have been made in vitro. The in vitro studies analyzed in this article can be divided into two large groups. The first corresponds to those methods that quantify the antioxidant properties of xanthophylls. The second group includes those anti-inflammatory or anti-cancer tests in cell cultures. Inflammatory models usually comprise the use of human or murine macrophage cell cultures and measure differences in the expression or translation of pro-inflammatory mediators such as cytokines (tumor necrosis factor alpha (TNF-α), interleukins (IL)-1β and IL-6), nuclear factor (NF)-κβ (which mediates the expression of these cytokines), and the production of nitric oxide (NO) or enzymes related to the inflammatory process (cyclooxygenase (COX)-2, nitric oxide synthase (iNOS)) [ 209 ]. A study on RAW 264.7 murine macrophages, splenocytes, and bone marrow-derived mice macrophages obtained from mice fed with AS reported a significant reduction of IL-1β and IL-6 and generated ROS. Moreover, the authors described that AS inhibit nuclear translocation of NF-κβ and increase the expression of nuclear factor E2-related factor (NRF)-2, which subsequently involves a lower production of reactive oxygen species (ROS) and inflammatory response [ 217 ]. Experiments involving FU or some of its metabolites such as fucoxanthinol or apo-9′-fucoxanthinone in vitro have proven anti-inflammatory activities. On murine macrophages RAW 264.7 with a lipopolysaccharide (LPS)-induced inflammation model, FU and fucoxanthin isomers such as 9′-cis or 13′-fucoxanthin all displayed a significant dose-dependent inhibition of pro-inflammatory mediators IL6-IL-1, NO, and TNF-α [ 212 ]. Likewise, apo-9′-fucoxanthinone notably reduced levels of NO, ROS, TNF-α, and COX enzyme both in RAW 264.7 macrophages and zebrafish juveniles [ 213 ]. A study with different human colon and prostate cancer cell lines elucidated that besides the anti-inflammatory and antioxidant effect of β-carotene, it exerts a direct pro-apoptotic activity on cancerous cells by reducing the expression of caveolin-1 and inducing the activity of several caspases. This protein is heavily involved in cell cycle regulation, and its expression leads to increased protein kinase B levels, being both liable of cell proliferation. Conversely, caspases are signals for apoptosis. The authors were able to elucidate this significant pathway of cell growth inhibition, as this was observed in human colon and prostate cell lines that expressed caveolin-1 (HCT-116, PC-3), but not in those that do not produce it (Caco-2, LNCaP) [ 229 ].
3.3.2. Observation In Vivo
Although most of the articles studied dealt with in vitro studies, it is also possible to find various articles about in vivo studies of the activities of xanthophylls. Most of these in vivo studies have been carried out with model animals, including mice, rats, and ferrets. Regarding the results obtained, numerous studies reported that in both animals and humans, retinol levels decrease related to inflammatory responses [ 230 ]. For instance, β-cryptoxanthin displayed lower levels of TNF-α, as well as pro-inflammatory transcription factors such as NF-κβ and activator protein (AP)-1. Similarly, another study on the anticancer effect of β-cryptoxanthin on nicotin-induced lung carcinogenesis in mice reported significantly lower levels of IL-6 and AKT alongside the re-expression of tumor-suppressor genes that were silenced by nicotine administration [ 227 ]. This interaction between nicotine and β-cryptoxanthin was also analyzed in another in vivo study carried out in this case with ferrets. These ferrets were exposed to cigarette smoke for 3 months in order to induce pulmonary tissue inflammation and carcinogenesis, showing a dose-dependent reduction of both in the groups treated with β-cryptoxanthin [ 228 ]. On non-provitamin A CA, dextran sulfate sodium-induced colitis and colon carcinogenesis mice were treated with AS food supplementation. Tissue and gut mucose analysis displayed showed significantly lower NF-κβ, TNF-α, IL-1β, IL-6, iNOS, and COX-2 expression, relating these differences to the near nullification of the induced colitis and a lowered risk of colon carcinogenesis [ 220 ]. Regarding FU, which is one of the most promising xanthophylls, a study analyzed the anti-inflammatory activity of injected FU by inducing inflammation with LPS in mice and measuring pro-inflammatory mediators in their aqueous humor. FU exerted a significant reduction of prostaglandin (PG)E-2, NO, and TNF-α levels, also showing a lower infiltration of cells and proteins by the induced inflammation. The most relevant outcome of this study is that the effectiveness shown by FU was highly similar to prednisolone, which was used to establish a feasible comparison [ 214 ]. It is noteworthy that most carotenoids display anti-inflammatory and anticancer activities in a dose-dependent fashion, as in cell culture studies.
3.3.3. Observational and Epidemiological Studies
In the last decades, case-control and observational studies have also been carried out in humans to test the effectiveness of CA to extend life expectancy and other health-promoting effects such as reducing the risk of developing cancers, chronic inflammatory diseases, or cardiovascular diseases. Results on the possible chemopreventive effect of CA, especially of β-carotene, are mixed [ 231 ]. Nevertheless, this effectiveness has been reported in other studies. Various studies are available, for example, evaluating the potential health-promoting effects of LU. One of them analyzed the effect of LU supplementation in subjects from the Shanghai region with early symptoms of atherosclerosis. Albeit the study was carried out with a small sample ( n = 65), it was observed that the levels of IL-6, MCP-1, and LDL-cholesterol were significantly lower [ 221 ]. In another study, food supplementation with β-carotene, lycopene, and lutein was provided to preterm infants. Although only C reactive was used as an inflammation marker, treated groups displayed significantly lower levels alongside improved retinal development in comparison with the control group [ 222 ]. The Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study, which was carried out in 1994 with more than 25,000 ( n = 29,133) median age male smokers, determined that intake of β-carotene and α-tocopherol supplements could increase the risk of lung cancer, after a ≤8 year follow-up [ 232 ]. Additionally, a 24-year follow-up of these subjects did not find a significant chemopreventive effect for supplementing β-carotene toward liver cancer incidence, but it did seem to exert a protective effect in diabetic subjects [ 233 ]. However, a recent prospective cohort study of a 30-year follow-up from these subjects determined a significant ( p < 0.0001) correlation between CA serum levels and reduced all-cause mortality risk in the study quintiles that displayed higher CA in serum as a result of supplement intake, despite their advanced age and smoking habits [ 234 ]. These mixed results, also reported in other prospective cohort studies, show a general trend of a protective effect of CA toward cancer development and inflammation, of which research has focused extensively in β-carotene. However, the increased risks of lung cancer development observed in some studies could arguably be due to an excess of retinol in treated groups, as many studies used high-dosage CA supplements as treatment, while subjects may also intake these CA through diet [ 233 ]. Taking the case of the ATBC study, the β-carotene dose was of 20 mg, as much as three times the recommended dietary allowance of retinol [ 232 ]. Conversely, α-carotene, lycopene, and β-cryptoxanthin have been inversely correlated with developing lung cancer or at least showing a consistent chemopreventive effect [ 235 ]. Another study assessed serum CA levels from individuals from the US Third Nutrition and Health Examination Survey (NHANES III) [ 224 ], which evaluated health habits and analyzed the serum samples of the participants. In this prospective cohort study, α-carotene and β-cryptoxanthin also displayed effectiveness in lowering the risk of lung cancer development in smokers, but this effect was not apparent in non-smokers [ 225 ]. An extensive meta-analysis of human observational studies with a total sample size of more than 150,000 individuals ( n = 174,067) assessed results from 13 studies, determining that provitamin A CA may exert a protective effect against cancer or cardiovascular mortality [ 236 ]. Yet, the authors noted that as mentioned, an excessive production of retinol because of supplementation may be responsible for the reported increased risks of lung cancer development in some case-control studies that considered these variables. It is noteworthy that the greatest meta-analysis up to date to our knowledge evaluated 34 observational studies with a total sample size of 592,479 participants and established correlations between intake or serum levels of α-carotene and lycopene but not β-carotene with lowered risk of developing prostate cancer [ 237 ]. These findings also noted that even if these carotenoids had an apparent chemopreventive activity, they were ineffective in preventing malignancy of prostate cancer once it was diagnosed. Altogether, albeit more extensive research with bigger sample sizes and the isolation of potential confusion factors is required, there is a great body of evidence suggesting that in controlled dose ranges, both provitamin A and non-provitamin carotenoids have chemopreventive effects on oxidative stress, inflammation, and cancer development through indirect and direct pathways.
4. Algae as Source of Carotenoids
Algae are recognized as a good source for numerous bioactive compounds of great interest, xanthophylls being among them, as reflected on this work. However, the application of these compounds is not linked only to food safety and human health, but factors such as economic costs, efficacy of the designed product, or current legislation are also of vital importance when deciding whether a product it is viable or not and, therefore, it is produced in a commercial way or not. Despite this complexity, algae have become a powerful industry due to its biotechnological applications, advancements in extraction methods, and increasing consumer demand for natural products. As a result, a wide range of products are and have been developed, ranging from nutraceuticals, food additives, or animal feed to drugs or cosmetics [ 67 ]. CA play a very important role in all these applications with even better results that their synthetic counterpart [ 238 ]. All of these progresses mean that the demand and market of CA are growing significantly, and this year is expected to reach $1.53 billion [ 239 ]. Despite this, more advances are still needed to reduce the cost of obtaining it from natural sources. It is estimated that CA derived from algae can reach the cost of $7500/kg [ 240 ], whereas synthetic CA could be obtained at roughly half the cost [ 241 ]. Nevertheless, despite the great diversity of natural and synthetic CA, only a few of them are commercially produced, including carotenes (β-carotene and lycopene) and xanthophylls (astaxanthin, lutein, zeaxanthin, canthaxanthin, and capsanthin) [ 242 ]. Some processes have been developed to increase the benefits. For example, high costs production can be reduced through the development of green technologies as they are considered more profitable, efficient, and ecological, transforming it into an environmentally friendly process [ 243 ]. Another important parameter when optimizing is the selection of algae used as source. In this regard, the genomic characterization of these species and identifying relevant target genes involved in CA synthesis and accumulation, paired with efficient culture and harvest techniques; has proven to be an efficient way to maximize CA production [ 116 ].
However, there are still barriers that must be solved for the commercialization of CA from algae, such as optimization of their extraction and purification, storage alternatives, and technologies that increase the bioaccessibility and bioavailability of the compounds present in algae [ 151 , 157 , 198 ]. Currently, different processes such as encapsulation or emulsification arise for CA to achieve their biological functions in humans. In addition, the research has provided data through in vitro and in vivo digestion studies that clarify the absorption mechanism of the different CA, which can be used by industries to improve the formulation of their products. However, more human studies of the nutritional efficiency of these CA extracted from algae are needed [ 203 ].
The lack of uniformity of legislation between the different countries makes its study complex. That is why in order to carry out the commercialization of the products obtained, it is necessary to carry out some modifications to adapt them to current legislation. In the case of the EU, as algae were not being used in a traditional or habitual way in food before 15 May 1997, they are considered as novel food as reflected in EU Regulation 2015/2283. This regulation is also applicable to all products obtained from algae such as food supplements of their bioactive components or food additives ( i.e. , phlorotannins from Ecklonia cava ) [ 244 ]. Therefore, its commercialization request authorization for its incorporation into the market from the European Food Safety Authority (EFSA), which requires health risk studies. These food safety analyses must also be in accordance with current legislation on food safety and food hygiene, respectively included in Regulation (EU) 178/2002 [ 244 ] and Regulation (EU) 852/2004 [ 245 ], ensuring consumer safety. Moreover, these products can be sold as nutraceuticals without scientific evidence conducted by the EFSA, which is legislated by Regulation CE No. 1924/2006 [ 246 ]. However, this same regulation dictates that the health claims alleged to these same products must be backed by proper and significant scientific evidence, which must be submitted to EFSA.
5. Conclusions
The use of algae as raw material for obtaining carotenoids, and especially xanthophylls, is an alternative that is gaining interest due to its potential and the bioactivities of the extracted compounds. Currently, CA are used commercially as food additives, feed and nutrient supplements, pigments, and, more recently, as nutraceuticals for cosmetic and pharmaceutical purposes. Despite this, there is little information on the impact of some of these xanthophylls on human health, with most of the studies focusing on FU and AS, which are compounds that also represent the main marine CA. These molecules are characterized by having a high antioxidant activity, and this may be one of the main mechanisms in their anticancer and anti-inflammatory activity. These activities will vary between the different compounds due to the nature of their terminal groups or the length of the chain, among others. However, for these proposals to be viable, it is necessary to carry out a series of advances. These advancements include increased biomass production, increased extraction, and purification performance, as well as reduced implementation costs. Some ways to solve these problems go through genetic engineering or the development of green extraction techniques.
Acknowledgments
The research leading to these results was supported by MICINN supporting the Ramón y Cajal grant for M.A. Prieto (RYC-2017-22891) and the FPU grant for Anxo Carreira Casais (FPU2016/06135); by Xunta de Galicia for supporting the pre-doctoral grant of Antía González Pereira (ED481A-2019/0228) and the program EXCELENCIA-ED431F 2020/12 that supports the work of F. Chamorro; by UP4HEALTH Project that supports the work of P. Otero and C. Lourenço-Lopes.
Author Contributions
Conceptualization, A.G.P., P.O., J.E., A.C.-C., F.C., N.C., A.J., C.L.-L., J.S.-G. and M.A.P.; methodology, A.G.P., P.O. and J.E.; investigation, A.G.P., P.O., J.E., A.C.-C., F.C., N.C., A.J. and C.L.-L.; resources, A.G.P., P.O., J.E., A.C.-C., F.C., N.C., A.J. and C.L.-L.; data curation, A.G.P., P.O., J.E., A.C.-C., F.C., N.C., A.J. and C.L.-L.; writing—original draft preparation, A.G.P., P.O., J.E., A.C.-C., F.C., N.C., A.J., C.L.-L., J.S.-G. and M.A.P.; writing—review and editing, A.G.P., P.O., J.E., A.C.-C., F.C., N.C., A.J., C.L.-L., J.S.-G. and M.A.P.; visualization, A.G.P., P.O. and J.E.; supervision, J.S.-G. and M.A.P.; project administration, A.G.P., P.O. and J.E.; funding acquisition, J.S.-G. and M.A.P. All authors have read and agreed to the published version of the manuscript.
The research leading to these results was funded by Xunta de Galicia supporting the Axudas Conecta Peme, the IN852A 2018/58 NeuroFood Project and the program EXCELENCIA-ED431F 2020/12; to Ibero-American Program on Science and Technology (CYTED—AQUA-CIBUS, P317RT0003) and to the Bio Based Industries Joint Undertaking (JU) under grant agreement No 888003 UP4HEALTH Project (H2020-BBI-JTI-2019). The JU receives support from the European Union’s Horizon 2020 research and innovation program and the Bio Based Industries Consortium. The project SYSTEMIC Knowledge hub on Nutrition and Food Security, has received funding from national research funding parties in Belgium (FWO), France (INRA), Germany (BLE), Italy (MIPAAF), Latvia (IZM), Norway (RCN), Portugal (FCT), and Spain (AEI) in a joint action of JPI HDHL, JPI-OCEANS and FACCE-JPI launched in 2019 under the ERA-NET ERA-HDHL (n° 696295).
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- BiologyDiscussion.com
- Follow Us On:
- Google Plus
- Publish Now

9 Main Characteristics of Xanthophyceae
ADVERTISEMENTS:
The following points highlight the nine main characteristics of Xanthophyceae.
1. Members of Xanthophyceae are commonly fresh water (Tribonema) and most of them are free floating. [Few members are found to grow on mud (Botrydium) and also on walls or tree trunks (Characiopsis, Ophiocytium etc.). A few members like Halosphaera are marine.
2. Plant body is unicellular (Heterochloris) or multicellular. [The multicellular bodies also exhibit various forms like palmelloid (Chlorogloea), dendroid (Mischococcus), coccoid (Chlorobotrys), rhizopodial (Stipitococcus), filamentous (Heterococcus) and siphona- ceous (Botrydium).
3. The cell wall is often absent, but when present it contains more pectic compounds than the members of Chlorophyceae. Occasionally cellulose is also present. [In some non- motile forms the cell wall is silicified and made up of two halves, those overlap each other.
4. The motile forms bear usually two flagella but rarely one. They are unequal and inserted at the anterior end. The flagella are of two types. The larger one is tinsel (or pantonematic or pleuronematic i.e., bearing halr-like appendages) and the shorter one is whiplash (or acronematic i.e., without hairs and their surface is smooth) type.
5. The chromatophores are discoid in shape and are numerous in each cell.
6. The pyrenoides are absent or rarely present.
7. The plastids are yellow-green in colour. The photosynthetic pigments are chlorophyll a, chlorophyll e (very little), P-carotene (fairly high concentration) and xanthophylls. The chief xanthophyll is diadinoxanthin. The other xanthophylis are violaxanthin, lutein, neoxanthin, flavoxanthin and flavacin. The carotenoides are normally present in excess amount than chlorophyll. Chlorophyll b is absent.
8. The reserve food is oil, lipid and lucosin. Starch is not formed.
9. Plants reproduce commonly by vegetative and asexual means. Vegetative reproduction takes place by cell division. Asexual reproduction by zoospores, aplanospores or akinetes. Sexual reproduction, though rare, may be isogamous, anisogamous or oogamous. Isogamy is common. Both iso- and anisogamy are found in Botrydium.

Related Articles:
- Rhodophyceae: Description, Characteristics and Classification
- 6 Most Important Orders of Xanthophyceae Class (436 Words)
Botany , Algae , Phylum Heterokontophyta , Classes , Xanthophyceae
- Anybody can ask a question
- Anybody can answer
- The best answers are voted up and rise to the top
Forum Categories
- Animal Kingdom
- Biodiversity
- Biological Classification
- Biology An Introduction 11
- Biology An Introduction
- Biology in Human Welfare 175
- Biomolecules
- Biotechnology 43
- Body Fluids and Circulation
- Breathing and Exchange of Gases
- Cell- Structure and Function
- Chemical Coordination
- Digestion and Absorption
- Diversity in the Living World 125
- Environmental Issues
- Excretory System
- Flowering Plants
- Food Production
- Genetics and Evolution 110
- Human Health and Diseases
- Human Physiology 242
- Human Reproduction
- Immune System
- Living World
- Locomotion and Movement
- Microbes in Human Welfare
- Mineral Nutrition
- Molecualr Basis of Inheritance
- Neural Coordination
- Organisms and Population
- Photosynthesis
- Plant Growth and Development
- Plant Kingdom
- Plant Physiology 261
- Principles and Processes
- Principles of Inheritance and Variation
- Reproduction 245
- Reproduction in Animals
- Reproduction in Flowering Plants
- Reproduction in Organisms
- Reproductive Health
- Respiration
- Structural Organisation in Animals
- Transport in Plants
- Trending 14
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
Xanthophyll
Xanthophyll is a type of accessory pigment or phytochemicals, which belongs to the class of “ Carotenoids ”. In many vascular plants and algae, xanthophylls act as the light-harvesting protein complexes. Xanthophylls are rich in antioxidants , which prevent the cells from damaging. In photosynthetic eukaryotes, the xanthophylls are usually bound to the chlorophyll molecules.
Xanthophylls are the pigment molecules present within the light-harvesting complex, which protect the photosynthetic organisms from the toxic effect of light. In this context, you will get to know the meaning, molecular structure, occurrence, types, cycle, functions and isolation of xanthophyll.
Content: Xanthophyll
Meaning of xanthophyll, molecular structure, xanthophyll cycle, isolation of xanthophyll.
Xanthophyll merely refers to the light-harvesting accessory pigments , which work coordinately with the chlorophyll-a. It can absorb light of a wavelength in a range of 425-475 nm. Xanthophylls are primarily of three types, namely lutein, zeaxanthin and cryptoxanthin. They are highly antioxygenic molecules, which protect the cell from damage and ageing.
Xanthophyll is highly beneficial for eye health, as it reduces the risk of eye cataract and macular degeneration. Xanthophylls or Phylloxanthins are the yellow colour pigment naturally present in the plants. A xanthophyll can isolate from the plant extract.
You can isolate the xanthophyll pigment from the plant extract by performing chromatography , which results in the formation of a yellow colour band over a chromatography paper. Let us look into some of the general properties of the xanthophyll.
| Properties | Xanthophyll |
|---|---|
| Molecular formula | C40H56O2 |
| Molecular weight | 568.886 g/mol |
| Charge | Neutral |
| Physical state | Liquid |
| Colour | Yellow |
| Nature | Polar compound |

Xanthophylls occur naturally in the plants, which regulate the light energy and act as “ Photochemical quenching agent ” that deals with the exciting form of chlorophyll or triplet chlorophyll. The triplet chlorophyll produces at a higher rate during the photosynthetic process. Xanthophylls are also found in the body of humans and animals, which comes ultimately by the source of green plants .
Xanthophylls mainly include accessory pigments like lutein, Zeaxanthin and cryptoxanthin.
- Lutein : It is the most common xanthophyll, which is synthesized by the green plants itself. Spinach, kale, kiwi, green apples, egg yolk, corn etc. are the sources of lutein. Lutein is a lipophilic molecule (insoluble in polar solvent like water). In plants, lutein is present as fatty acid esters, in which one or two fatty acids attach with the two –OH groups. Lutein mainly absorbs blue light , and thereby it protects the eye from the blue light that leads to an eye impairment.
- Zeaxanthin : It merely refers to the carotenoids alcohols , which can be synthesized naturally by the plants and certain microorganisms. It acts as a non-photochemical quenching agent. Zeaxanthin is an accessory pigment, which gives distinct colour to the corn, wolfberries etc. It consists of two chiral centres. Kale, spinach, turnip greens, mustard greens etc. are the sources.
- Cryptoxanthin : Its molecular structure is quite similar to the β-carotene, but a hydroxyl group is present in addition. Cryptoxanthin is found as red crystalline solid in its pure form. It also refers to provitamin-A , as during the xanthophyll cycle, cryptoxanthin converts into vitamin A (retinol).
The xanthophyll cycle occurs inside the thylakoid membrane of the chloroplast. Xanthophyll cycle facilitates the interconversion of oxygenated carotenoids. There are many types of xanthophyll cycle, but violaxanthin and Diadino xanthin cycle are the most common.
Violaxanthin Cycle
Firstly, violaxanthin (has two epoxide group) converts into antheraxanthol (has one epoxide group). Antheraxanthol further turns into Zeaxanthin (has no epoxide group). VDE , i.e. violaxanthin de-epoxidase catalyses the conversion of violaxanthin to zeaxanthin as a result of the de-epoxidation reaction.
ZE , i.e. zeaxanthin epoxidase is an enzyme, which catalyses the conversion of zeaxanthin into violaxanthin as a result of epoxidation reaction. The epoxidation reaction will occur at a low pH <5.8 under low light, whereas de-epoxidation reaction occurs at a high pH of 7.5 under a high light source.
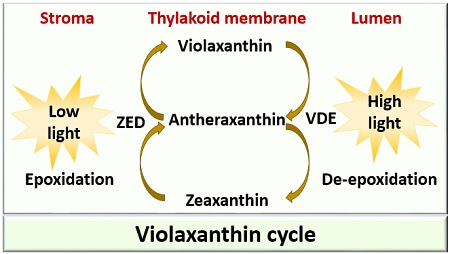
Diadinoxanthin Cycle
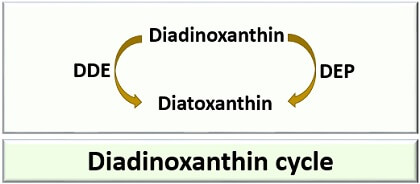
Xanthophylls perform two central roles:
- Harvesting of light : Xanthophylls are the accessory pigments, which act as a photosynthetic light-harvesting complex of algae and vascular plants.
- Dissipation of energy as heat : Xanthophyll helps in photoprotection, i.e. it protects the photosynthetic apparatus from the photo-oxidative damage in the condition of excessive light by dissipating energy.
A xanthophyll can be isolated by the method of chromatography. For this, prepare a plant extract by crushing the fresh leaves. Place a drop of leaf extract at one end (above 1 cm) on chromatography paper (considered as a stationary phase ). Then, take acetone ligroin mixture as a non-polar hydrophobic solvent (considered as a mobile phase ), which will run through the filter paper.
Add solvent and hang the filter paper inside the chromatography chamber. Cover the chromatography chamber with a lid to prevent gas exchange. As the solvent mixture comes in contact with the leaf extract, it will help in the migration of the different plant pigments at a different rate.
The separation and the travelling distance of plant pigment are based on their solubility with the solvent used. The different plant pigments like chlorophyll, xanthophyll and carotene will travel at different rates and appear as different bands. Chlorophyll being highly polar, it will adhere to the polar surface of the paper.
Thus, chlorophyll moves the shortest distance and appears as “ Green band ”. Xanthophyll being less polar will move a shorter distance and appear as “ Yellow band ”. Carotene being non-polar will attract more strongly to the non-polar solvent and move along with it. Thus, carotene will move the longest distance and appear as “ Orange band ”.
The solvent is allowed to run to the distance (by leaving 1 cm distance from the top) and called a “ Solvent front ”. The formation of different bands on the chromatography paper commonly refers to the “ Chromatogram ”. The Rf value is calculated by the ratio of distance travelled by the pigment, and the total distance travelled by a solvent.
Related Topics:
- Stomata in Plants
- Parenchyma in Plants
- Difference Between Water-Soluble and Fat-Soluble Vitamins
- Digestive Glands in Human Body
- How to Take Care of a Bonsai?
1 thought on “Xanthophyll”
I liked the article.
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Start typing and press enter to search
What is Xanthophyll?
Xanthophyll is a phytochemical or accessory pigment that belongs to the “Carotenoids” class. Xanthophylls are light-harvesting protein complexes found in many vascular plants and algae. Antioxidants are abundant in xanthophylls, which protect cells from damage. Xanthophylls are generally linked to chlorophyll molecules in photosynthetic eukaryotes.
Xanthophylls are pigment molecules found inside the photosynthesis complex that shield photosynthetic organisms from the harmful effects of light. We will learn about xanthophyll’s significance, occurrence, molecular structure, types, and functions in this article.
Table of Contents
Xanthophyll definition, occurrence of xanthophyll, molecular structure of xanthophyll, types of xanthophyll, functions of xanthophyll, xanthophyll cycle.
- Frequently Asked Questions – FAQs
The light-harvesting accessory pigments that function in combination with chlorophyll-a are referred to as xanthophyll. It can absorb light with a wavelength between 425 and 475 nm. Lutein, zeaxanthin, and α- and β-cryptoxanthin are the three main types of xanthophylls. They are antioxidizing chemicals that protect cells from damaging and ageing.
Like anthocyanins, carotenes, and phycobiliproteins, Xanthophylls are classified as accessory pigments in plants. When leaves become orange in the fall season, xanthophylls and carotenic pigments are visible.
Animals cannot make xanthophylls; hence xanthophylls are discovered in them (for example, in the eye) are derived from their diet. Ingested xanthophylls are also responsible for the yellow tint of chicken egg yolks.
Xanthophylls are oxidised carotenoid derivatives. Because they contain hydroxyl groups and are more polar, they are the pigments that will travel the most distance in paper chromatography.
|
|
|
|---|---|
| Molecular formula | C H O |
| Molecular weight | 568.886 g/mol |
| Nature | Polar compound |
| Colour | Yellow |
| Physical state | Liquid |
| Charge | Neutral |
Xanthophylls are photochemical cooling agents that deal with the active state of chlorophyll or triplet chlorophyll. They are found naturally in plants and regulate light energy. During the photosynthetic process, triplet chlorophyll develops at a faster rate. Xanthophylls can also be found in the bodies of humans and animals, and they are derived from green vegetation.
Xanthophylls and carotenes have similar structures since they are both carotenoids; however, xanthophylls have oxygen atoms, whilst carotenes are completely hydrocarbons with no oxygen.
Xanthophylls are far more polar (in molecular structure) to carotenes due to their oxygen content, and they separate from carotenes in several forms of chromatography. When serving as a bridge to generate epoxides, xanthophylls exhibit their oxygen as hydroxyl groups and/or hydrogen atoms replaced by oxygen atoms.
Xanthophylls primarily encompass accessory pigments such as Lutein, Zeaxanthin, and Cryptoxanthin.
- Lutein: This is the most prevalent xanthophyll, a lipophilic component produced by green plants themselves. Lutein can be found in kale, spinach, green apples, kiwi, corn, egg yolk, and other foods. Lutein is found in plants as fatty acid esters, which are made up of one or two fatty acids attached to two –OH groups. Lutein absorbs blue light and hence guards the eye against blue light, which can cause vision problems.
- Zeaxanthin: This refers to carotenoid alcohols, which are produced naturally by plants and microbes. It works as a non-photochemical quencher. Zeaxanthin is an additional pigment that gives corn, wolfberries, and other plants their distinctive colour. It is made up of two chiral centres. The sources include turnip greens, kale, mustard greens, spinach, and others.
- Cryptoxanthin: The molecular structure is identical to that of β-carotene, but it also contains a hydroxyl group. In its purest form, cryptoxanthin appears as a red crystalline solid. It also refers to provitamin A, as cryptoxanthin transforms into vitamin A during xanthophyll cycle (retinol).
Xanthophylls are light-harvesting pigments that can also serve as structural entities within the LHC and compounds that protect photosynthetic creatures from the potentially damaging effects of light.
- Light-harvesting: The accessory pigments xanthophylls operate as a photosynthetic light-harvesting compound in algae and vascular plants.
- Dissipation of energy as heat: Xanthophyll assists in photoprotection, or the protection of the photosynthetic system from photo-oxidative degradation in the presence of excessive light, by dissipating energy.
The xanthophyll cycle takes place within the chloroplast’s thylakoid membrane. Interconversion of oxidised carotenoids is facilitated by the xanthophyll cycle. The Diadinoxanthin and violaxanthin cycles are the most common kinds of xanthophyll cycle.
Conversions of pigments from non-energy-quenching to energy-quenching forms are part of the xanthophyll cycle. This strategy limits the light-harvesting antenna’s absorption cross-section and hence the quantity of energy that reaches the photosynthetic reaction centres. One of the key ways to guard against photoinhibition is to reduce the light-harvesting antenna, and changes in xanthophyll cycling occur in a time frame of minutes to several hours.
The xanthophyll cycle in higher plants is dominated by three carotenoid pigments: violaxanthin, antheraxanthin, and zeaxanthin. Violaxanthin is transformed into photoprotective pigments antheraxanthin and zeaxanthin when exposed to light. The enzyme violaxanthin de-epoxidase is responsible for this conversion.
The pigment diadinoxanthin is converted into diatoxanthin (diatoms) or dinoxanthin (dinoflagellates) in the xanthophyll cycle of diatoms and dinoflagellates in the high light.
Related Links:
- What are the plant pigments?
- Is Xanthophyll An Accessory Pigment?
- What Is Difference Between Dyes and Pigment?
- Where Are Carotenoid Pigments Found?
Frequently Asked Questions
What is xanthophyll used for, what is the difference between carotene and xanthophylls.
The basic distinction between carotene and xanthophyll would be that carotene is orange, while xanthophyll is yellow. Moreover, carotene is a hydrocarbon with no oxygen atoms in its structure, whereas xanthophyll is a hydrocarbon with an oxygen atom in its structure.
Are the pigments in tomato, papaya and carrot the same?
Carrot root cells contain large, crystalline accumulations of β-carotene, whereas papaya fruits contain nanoscale fragments of lipid-dissolved and liquid-crystalline-carotene. At the same time, crystalloid lycopene accumulations have been discovered in papayas and tomatoes.
| NEET Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Register with Aakash BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

COMMENTS
Xanthophyta Hibberd, 1990 [6] Yellow-green algae or the Xanthophyceae ( xanthophytes) are an important group of heterokont algae. Most live in fresh water, but some are found in marine and soil habitats. They vary from single-celled flagellates to simple colonial and filamentous forms. Xanthophyte chloroplasts contain the photosynthetic ...
Members of the Xanthophyta are mostly freshwater algae. The plant body may be unicellular or multicellular (colonial, palmelloid, or coccoid). The cell wall is often absent, but when present, it contains a higher content of pectic substances. The chromatophores are discoid, with many in each cell. Pyrenoids are usually absent.
The Xanthophyta include more than 600 species. Members of this group are photosynthetic organisms which live primarily in freshwater, though some are found in marine waters, in damp soil, or on tree trunks. They generally are not abundant when they are found at all, and many species have only been found once. Despite this, they are the dominant ...
All members are obligate parasites of algae, fungi, or plants, causing cell enlargement, especially of the plant roots. They are distinguished by the production of motile cells (zoospores) with two unequal anterior flagella. This article was most recently revised and updated by Melissa Petruzzello. Xanthophyta, division or phylum of algae ...
Search for: 'Xanthophyta' in Oxford Reference ». A phylum of mostly freshwater eukaryotic organisms, traditionally known as yellow-green algae, that possess carotenoid pigments (including xanthins), which are responsible for their colour, in addition to chlorophylls. Xanthophytes occur in a variety of forms - unicellular, colonial ...
Xanthophyta are important contaminants of source of water or drinking water supplies.These are able to change the quality of water but these are so small that mostly scientist ignore their role which they play for water. Some species of xanthophyte are presumably in response to elevate nutrients from fertilizers.
This view was supported by Chapman (1962), Taylor, Prescott (1969) and Morris (1968). A. Affinities of Vaucheria with Xanthophyceae: (i) Siphonaceous, acellular thallus. (ii) Predominance of carotenoids, over chlorophylls. Absence of chlorophyll b from Vaucheria which is a characteristic pigment of Chlorophyceae.
The Xanthophyta are much less species-diverse than the Chlorophyta, with about 600 species and many of the 100 known genera containing only a few species. However, they show a wide range of form and include biflagellate and non-motile unicells, simple or branched uniseriate filaments, and others are coenocytic or siphonous (large multinucleate ...
Commonly known as yellow-green algae, the Xanthophyta include one Class, Xanthophyceae, characterized by the following general features: (1) the photosynthetic pigments consist of chlorophyll-a and -e, β carotene and xanthophylls; of these, β carotene is usually present in fairly high concentrations; (2) the food reserves are oil, lipid, and a glucose polymer termed leucosin or ...
Abstract. Xanthophyceae, Euglenophyceae and Dinophyceae are among those important algal groups which are less diverse than other major groups of algae. Members of Xanthophyceae and Euglenophyceae do not contaminate water, whereas Dinoflagellates causes various types of poisoning. Many important algal members have been included and excluded in ...
Xanthophyta: The Yellow-green Algae The pigments in Xanthophyta are chlorophyll a, possibly chlorophyll e (although there is some uncertainty related to a suspicion that its presence may be connected to limitations in extraction methods), and an abundance of carotenoid pigments. Motile cells have two unequal flagella: a tinsel-type flagellum ...
ADVERTISEMENTS: The Xanthophyta or Heterokontae are commonly known as yellow-green algae include only one class Xanthophyceae. This division has close relationship with the Ghlorophyta comprising both marine and fresh-water forms. Certain species grow on drying mud, on trunk of trees, on damp walls, and similar other habitat. There are varied forms of vegetative body ranging […]
The typical red algae (Rhodophyta) is a rose-colored multicellular organism found in marine environments around the world. Accessory pigments called phycobiliproteins are responsible for the distinctive red coloring. Like green algae, red algae traces back to ancestral cyanobacteria. Certain types of red algae are edible and used to make ...
Presence or absence of chlorophyll b is the most reliable taxonomic criterion when the gross assignment of very small planktonic species is in question; ... D. J. (1990). Phylum Xanthophyta. In L. Margulis, J. O. Corliss, M. Melkonian, & D. J. Chapman (Eds.), Handbook of Protoctista (pp. 686-697). Boston: Jones & Bartlett. Google Scholar
Sexual reproduction in Vaucheria is of oogamous type. The male sex organ is known as antheridium, and the female sex organ is known as oogonium. The plant body of Vaucheria can either be homothallic, meaning both the male and female sex organs are borne close to each other, on the same filament, or heterothallic, such that male and female sex ...
The xanthophylls comprise a diverse group of oxygenated carotenoids with varied structures and multiple functions ().In almost all photosynthetic eukaryotes, the majority of xanthophylls are bound with chlorophyll (Chl) molecules to proteins of integral membrane, light-harvesting complexes (LHCs) (2-5).The LHCs absorb and transfer excitation energy to the photosynthetic reaction centers to ...
In this article we will discuss about the vegetative and sexual methods of reproduction that occur in the life cycle of vaucheria. 1. Vegetative Reproduction in Vaucheria: The vegetative reproduction takes place by fragmentation. The thallus can break into small fragments due to mechanical injury or insect bites etc. A septum develops at the place of breaking to seal the injury. The broken ...
The function of these compounds in algae is to carry out photosynthesis. They have a great variety of pigments, which can be classified into three large groups: chlorophylls, carotenoids, and phycobilins. Within the carotenoids are xanthophylls. Xanthophylls (fucoxanthin, astaxanthin, lutein, zeaxanthin, and β-cryptoxanthin) are a type of ...
The following points highlight the nine main characteristics of Xanthophyceae. 1. Members of Xanthophyceae are commonly fresh water (Tribonema) and most of them are free floating. [Few members are found to grow on mud (Botrydium) and also on walls or tree trunks (Characiopsis, Ophiocytium etc.). A few members like Halosphaera are marine.
Xanthophyll. The characteristic color of egg yolk results from the presence of a xanthophyll pigment typical in color of lutein or zeaxanthin of the xanthophylls, a division of the carotenoids group. Xanthophylls (originally phylloxanthins) are yellow pigments that occur widely in nature and form one of two major divisions of the carotenoid ...
Xanthophyll. Xanthophyll is a type of accessory pigment or phytochemicals, which belongs to the class of " Carotenoids ". In many vascular plants and algae, xanthophylls act as the light-harvesting protein complexes. Xanthophylls are rich in antioxidants, which prevent the cells from damaging. In photosynthetic eukaryotes, the xanthophylls ...
Xanthophylls constitute a major part of carotenoids in nature. They are an oxidized version of carotenoid. Xanthophyll has widely drawn scientists' attentions in terms of its functionality, bioavailability and diversity. An assortment of xanthophyll varieties includes lutein, zeaxanthin, β-cryptoxanthin, capsanthin, astaxanthin, and fucoxanthin.
Xanthophyll is a phytochemical or accessory pigment that belongs to the "Carotenoids" class. Xanthophylls are light-harvesting protein complexes found in many vascular plants and algae. Antioxidants are abundant in xanthophylls, which protect cells from damage. Xanthophylls are generally linked to chlorophyll molecules in photosynthetic ...