Have an account?


History of Periodic Table
8th - 10th grade.
10 questions

Introducing new Paper mode
No student devices needed. Know more
- 1. Multiple Choice Edit 30 seconds 1 pt What was Dmitri Mendeleev's greatest contribution to the history of the periodic table? He arranged all of the known elements by their atomic number He realised that there was a pattern of reactivity which repeated every 8 elements He predicted the existence (and properties) of new elements He identified the "law of triads" which became the groups
- 2. Multiple Choice Edit 30 seconds 1 pt Which of these was not proposed by John Dalton in 1805? All matter is made of atoms Atoms are made of protons, neutrons and electrons During chemical reactions, atoms rearrange themselves The atoms of a particular element are all identical
- 3. Multiple Choice Edit 30 seconds 1 pt Who arranged the first periodic table. Mosely Einstein Mendeleev Bohr
- 4. Multiple Choice Edit 30 seconds 1 pt Mendeleev classified elements based on not only atomic mass but what else? reactivity boiling points physical properties only chemical & physical properties
- 5. Multiple Choice Edit 30 seconds 1 pt Why were there blank spaces left in Mendeleev's periodic table? He didn't know what to put there. Undiscovered elements not yet known. Multiple elements could have fit He forgot to add the elements in.
- 6. Multiple Choice Edit 30 seconds 1 pt How many elements were discovered during Mendeleev's time? 63 56 101 34
- 7. Multiple Choice Edit 30 seconds 1 pt Which statement is not a difference between Mendeleev's table and the modern periodic table? there are no transition elements Mendeleev's table. Elements are arranged according to atomic no. not atomic mass. no gaps in the modern periodic table. much more than 60 elements in the modern periodic table.
- 8. Multiple Choice Edit 2 minutes 1 pt A ___________ is an arrangement of elements in columns columns rows periodic table electron shell
- 9. Multiple Choice Edit 1 minute 1 pt Each column in the periodic table is called a period group cluster unit
- 10. Multiple Choice Edit 30 seconds 1 pt Particles in an atom's nucleus that are neutral and have no charge are megatrons. electrons. neutrons. protons.
Explore all questions with a free account

Continue with email
Continue with phone
A brief history of the periodic table

The periodic table of elements is a common sight in classrooms, campus hallways and libraries, but it is more than a tabular organization of pure substances. Scientists can use the table to analyze reactivity among elements, predict chemical reactions, understand trends in periodic properties among different elements and speculate on the properties of those yet to be discovered.
The modern periodic table arranges the elements by their atomic numbers and periodic properties. Several scientists worked over almost a century to assemble the elements into this format.
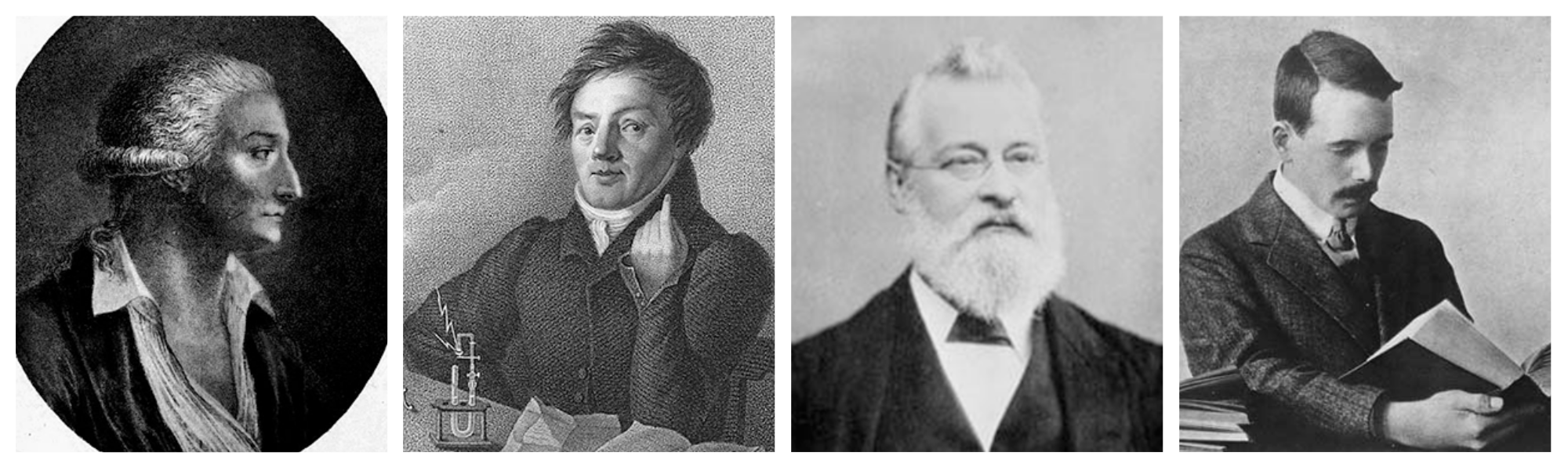
In 1789, French chemist Antoine Lavoisier tried grouping the elements as metals and nonmetals. Forty years later, German physicist Johann Wolfang Döbereiner observed similarities in physical and chemical properties of certain elements. He arranged them in groups of three in increasing order of atomic weight and called them triads, observing that some properties of the middle element, such as atomic weight and density, approximated the average value of these properties in the other two in each triad.
A breakthrough came with the publication of a revised list of elements and their atomic masses at the first international conference of chemistry in Karlsruhe, Germany, in 1860. They concluded that hydrogen would be assigned the atomic weight of 1 and the atomic weight of other elements would be decided by comparison with hydrogen. For example, carbon, being12 times heavier than hydrogen, would have an atomic weight of 12.

British chemist John Newlands was the first to arrange the elements into a periodic table with increasing order of atomic masses. He found that every eight elements had similar properties and called this the law of octaves. He arranged the elements in eight groups but left no gaps for undiscovered elements.
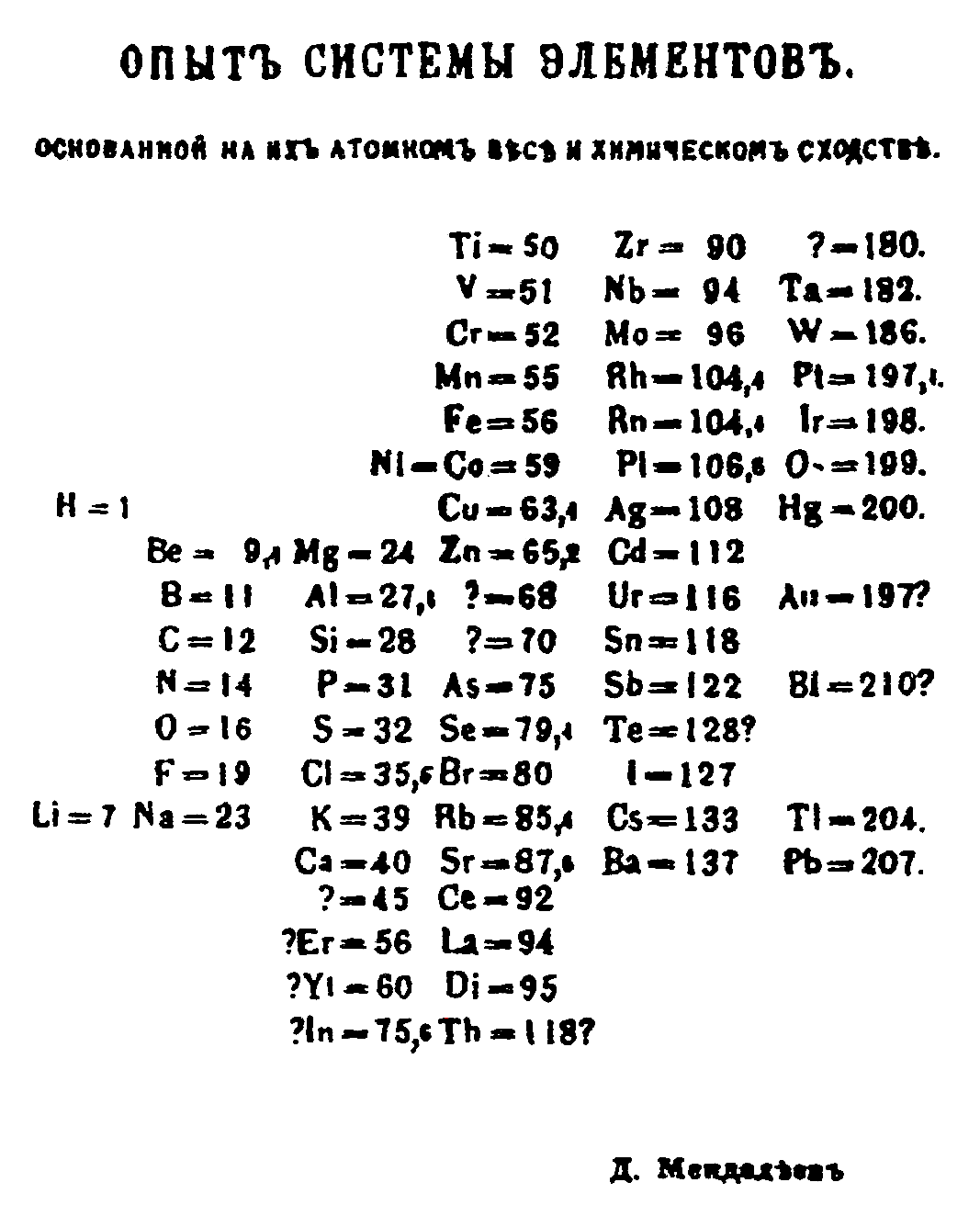
In 1869, Russian chemist Dmitri Mendeleev created the framework that became the modern periodic table, leaving gaps for elements that were yet to be discovered. While arranging the elements according to their atomic weight, if he found that they did not fit into the group he would rearrange them. Mendeleev predicted the properties of some undiscovered elements and gave them names such as "eka-aluminium" for an element with properties similar to aluminium. Later eka-aluminium was discovered as gallium. Some discrepancies remained; the position of certain elements, such as iodine and tellurium, could not be explained.
German chemist Lothar Meyer produced a version of the periodic table similar to Mendeleev’s in 1870. He left gaps for undiscovered elements but never predicted their properties. The Royal Society of London awarded the Davy Medal in 1882 to both Mendeleev and Meyer. The later discovery of elements predicted by Mendeleev, including gallium (1875), scandium (1879) and germanium (1886), verified his predictions and his periodic table won universal recognition. In 1955 the 101st element was named mendelevium in his honor.
The concept of sub-atomic particles did not exist in the 19 th century. In 1913, English physicist Henry Moseley used X-rays to measure the wavelengths of elements and correlated these measurements to their atomic numbers. He then rearranged the elements in the periodic table on the basis of atomic numbers. This helped explain disparities in earlier versions that had used atomic masses.
In the periodic table, the horizontal rows are called periods, with metals in the extreme left and nonmetals on the right. The vertical columns, called groups, consist of elements with similar chemical properties. The periodic table provides information about the atomic structure of the elements and the chemical similarities or dissimilarities between them. Scientists use the table to study chemicals and design experiments. It is used to develop chemicals used in the pharmaceutical and cosmetics industries and batteries used in technological devices.
UNESCO named 2019 the International Year of the Periodic Table to mark the 150 th anniversary of Mendeleev’s publication. Researchers and teachers worldwide took this opportunity to reflect on the importance of the periodic table and spread awareness about it in classrooms and beyond. Workshops and conferences encouraged people to use the knowledge of the periodic table to solve problems in health, technology, agriculture, environment and education. Publication houses organized monthly activities such as quiz contests, podcasts, personal story sections and industry site tours. These initiatives demonstrated how the elements are integral to our daily lives in medicines, pesticides and lithium batteries.
On its website marking the celebration, UNESCO wrote, “The Periodic Table of Chemical Elements is more than just a guide or catalogue of the entire known atoms in the universe; it is essentially a window on the universe, helping to expand our understanding of the world around us.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition monthly and the digital edition weekly.
Deboleena M. Guharay earned her Ph.D. in chemistry from Virginia Commonwealth University. She is very enthusiastic and passionate about science communication.
Related articles
Get the latest from asbmb today.
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles.

Harnessing a natural plant insecticide for commercial use
Researchers in Australia have identified circular peptides, called cyclotides, that affect the formation of cell membranes, causing death or restricted growth.

New study discovers tiny target on RNA to short-circuit inflammation
Paper details high-throughput process for rapid screening, identification of mysterious ‘long non-coding RNA.’


How to control chronic wasting disease
A prion sickness similar to mad cow is spreading rapidly through North America’s deer and elk populations. A veterinary microbiologist discusses the options for keeping it in check.
From the journals: MCP
Unlocking a metabolic disorder mystery. The many roles of lactate. Tools can unveil dementia biomarkers. Read about these recent papers.

Cardiolipin helps fruit flies take flight
Researchers at New York University have found that this long-lived phospholipid may underlie the insect’s extraordinary wing strength.

Simple trick could improve accuracy of plant genetics research
Researchers at North Carolina State University have found that a technique used to study gene activity in other organisms can also be used to make studies in plants more accurate.
Development of the periodic table
Table formation, alexandre-emile béguyer de chancourtois.

Can France claim the first periodic table? Probably not, but a French Geology Professor made a significant advance towards it, even though at the time few people were aware of it.
Alexandre Béguyer de Chancourtois was a geologist, but this was at a time when scientists specialised much less than they do today. His principal contribution to chemistry was the 'vis tellurique' (telluric screw), a three-dimensional arrangement of the elements constituting an early form of the periodic classification, published in 1862.
The telluric screw plotted the atomic weights of the elements on the outside of a cylinder, so that one complete turn corresponded to an atomic weight increase of 16. As the diagram shows, this arrangement means that certain elements with similar properties appear in a vertical line. Although the telluric screw did not correctly display all the trends that were known at the time, de Chancourtois was the first to use a periodic arrangement of all of the known elements, showing that similar elements appear at periodic atom weights.
|
|
|
John Newlands

John Newlands was British; his father was a Scottish Presbyterian minister. He was educated by his father at home, and then studied for a year (1856) at the Royal College of Chemistry, which is now part of Imperial College London. Later he worked at an agricultural college trying to find patterns of behaviour in organic chemistry. However, he is remembered for his search for a pattern in inorganic chemistry.
Just four years before Mendeleev announced his periodic table, Newlands noticed that there were similarities between elements with atomic weights that differed by seven. He called this The Law of Octaves, drawing a comparison with the octaves of music. The noble gases (Helium, Neon, Argon etc.) were not discovered until much later, which explains why there was a periodicity of 7 and not 8 in Newlands table. Newlands did not leave any gaps for undiscovered elements in his table, and sometimes had to cram two elements into one box in order to keep the pattern. Because of this, the Chemical Society refused to publish his paper, with one Professor Foster saying he might have equally well listed the elements alphabetically.
Even when Mendeleev had published his table, and Newlands claimed to have discovered it first, the Chemical Society would not back him up. In 1884 he was asked to give a lecture of the Periodic Law by the Society, which went some way towards making amends. Finally, in 1998 the Royal Society of Chemistry oversaw the placing a blue commemorative plaque on the wall of his birthplace, recognising his discovery at last.
|
|
Julius Lothar Meyer

Meyer trained at Heidelberg University under Bunsen and Kirchhoff, as did Mendeleev. So the two scientists would certainly have known each other although neither was aware of all the work done by the other. Meyer's roots, however, were firmly in Germany. Meyer was just four years older than Mendeleev, and produced several Periodic Tables between 1864-1870.
His first table contained just 28 elements, organised by their valency (how many other atoms they can combine with). These elements were almost entirely main group elements, but in 1868 he incorporated the transition metals in a much more developed table. This 1868 table listed the elements in order of atomic weight, with elements with the same valency arranged in vertical lines, strikingly similar to Mendeleev’s table. Unfortunately for Meyer, his work wasn’t published until 1870, a year after Mendeleev’s periodic table had been published. Even after 1870, Meyer and Mendeleev were still unaware of each other’s work, although Meyer later admitted that Mendeleev had published his version first.
Meyer did contribute to the development of the periodic table in another way though. He was the first person to recognise the periodic trends in the properties of elements, and the graph shows the pattern he saw in the atomic volume of an element plotted against its atomic weight.
Dmitri Mendeleev

As we have seen, Mendeleev was not the first to attempt to find order within the elements, but it is his attempt that was so successful that it now forms the basis of the modern periodic table.
Mendeleev did not have the easiest of starts in life. He was born at Tobolsk in 1834, the youngest child of a large Siberian family. His father died while he was young, and so his mother moved the family 1500 km to St. Petersburg, where she managed to get Dmitri into a “good school“, recognising his potential. In his adult life he was a brilliant scientist, rising quickly in academic circles. He wrote a textbook, Chemical Principles, because he couldn’t find an adequate Russian book.
Mendeleev discovered the periodic table (or Periodic System, as he called it) while attempting to organise the elements in February of 1869. He did so by writing the properties of the elements on pieces of card and arranging and rearranging them until he realised that, by putting them in order of increasing atomic weight, certain types of element regularly occurred. For example, a reactive non-metal was directly followed by a very reactive light metal and then a less reactive light metal. Initially, the table had similar elements in horizontal rows, but he soon changed them to fit in vertical columns, as we see today.
Not only did Mendeleev arrange the elements in the correct way, but if an element appeared to be in the wrong place due to its atomic weight, he moved it to where it fitted with the pattern he had discovered. For example, iodine and tellurium should be the other way around, based on atomic weights, but Mendeleev saw that iodine was very similar to the rest of the halogens (fluorine, chlorine, bromine), and tellurium similar to the group 6 elements (oxygen, sulphur, selenium), so he swapped them over.
The real genius of Mendeleev’s achievement was to leave gaps for undiscovered elements. He even predicted the properties of five of these elements and their compounds. And over the next 15 years, three of these elements were discovered and Mendeleev’s predictions shown to be incredibly accurate. The table below shows the example of Gallium, which Mendeleev called eka-aluminium, because it was the element after aluminium. Scandium and Germanium were the other two elements discovered by 1886, and helped to cement the reputation of Mendeleev’s periodic table.
The final triumph of Mendeleev’s work was slightly unexpected. The discovery of the noble gases during the 1890s by William Ramsay initially seemed to contradict Mendeleev’s work, until he realised that actually they were further proof of his system, fitting in as the final group on his table. This gave the table the periodicity of 8 which we know, rather than 7 as it had previously been. Mendeleev never received a Nobel Prize for his work, but element 101 was named Mendelevium after him, an even rarer distinction.
|
| |
| About 68 | 69.72 |
| 6.0 g/cm³ | 5.9 g/cm³ |
| Low | 29.78°C |
| 3 | 3 |
| Probably from its spectrum | Spectroscopically |
| Formula Ea O , density 5.5 g/cm . Soluble in both acids and alkalis | Formula Ga O , density 5.88 g/cm . Soluble in both acids and alkalis |
A comparison of Mendeleev’s predicted “Eka-aluminium” and Gallium, discovered by Paul Emile Lecoq in 1875
|
|
Henry Moseley

It wasn’t until 1913, six years after Mendeleev’s death that the final piece of the puzzle fell into place. The periodic table was arranged by atomic mass, and this nearly always gives the same order as the atomic number. However, there were some exceptions (like iodine and tellurium, see above), which didn’t work. Mendeleev had seen that they needed to be swapped around, but it was Moseley that finally determined why.
He fired the newly-developed X-ray gun at samples of the elements, and measured the wavelength of X-rays given. He used this to calculate the frequency and found that when the square root of this frequency was plotted against atomic number, the graph showed a perfect straight line. He’d found a way to actually measure atomic number. When the First World War broke out, Moseley turned down a position as a professor at Oxford and became an officer in the Royal Engineers. He was killed by a sniper in Turkey in August 15, and many people think that Britain lost a future Nobel prize winner.
Within 10 years of his work, the structure of the atom had been determined through the work of many prominent scientists of the day, and this explained further why Moseley’s X-rays corresponded so well with atomic number. The idea behind the explanation is that when an electron falls from a higher energy level to a lower one, the energy is released as electromagnetic waves, in this case X-rays. The amount of energy that is given out depends on how strongly the electrons are attracted to the nucleus. The more protons an atom has in its nucleus, the more strongly the electrons will be attracted and the more energy will be given out. As we know, atomic number is also known as proton number, and it is the amount of protons that determine the energy of the X-rays.
After years of searching, at last we had a periodic table that really worked, and the fact that we still use it today is testament to the huge achievement of these and many other great minds of the last two centuries of scientific discovery.
- Periodic Table

- Membership & professional community
- Campaigning & outreach
- Journals, books & databases
- Teaching & learning
- News & events
- Locations & contacts
- Awards & funding
- Help & legal
- Become a member
- Connect with others
- Supporting individuals
- Supporting organisations
- Manage my membership

COMMENTS
Study with Quizlet and memorize flashcards containing terms like What is the correct chronological order of scientists contributing to the modern periodic table?
Quiz yourself with questions and answers for The History and Arrangement of the Periodic Table Assignment and Quiz, so you can be ready for test day. Explore quizzes and practice tests created by teachers and students or create one from your course material.
How did the work of Dmitri Mendeleev differ from that of John Newlands in the development of the periodic table? A. Mendeleev arranged the elements according to increasing atomic mass. B. Mendeleev predicted elements that would later be discovered.
The modern periodic table has evolved through a long history of attempts by chemists to arrange the elements according to their properties as an aid in predicting chemical behavior.
What was Dmitri Mendeleev's greatest contribution to the history of the periodic table? He arranged all of the known elements by their atomic number. He realised that there was a pattern of reactivity which repeated every 8 elements. He predicted the existence (and properties) of new elements.
The periodic law was developed independently by Dmitri Mendeleev and Lothar Meyer in 1869. Mendeleev created the first periodic table and was shortly followed by Meyer. They both arranged the elements by their mass and proposed that certain properties periodically reoccur.
The periodic table is an arrangement of the chemical elements, structured by their atomic number, electron configuration and recurring chemical properties. In the basic form, elements are presented in order of increasing atomic number, in the reading sequence.
Study with Quizlet and memorize flashcards containing terms like The Periodic Table, Early Attempts at Creating a Periodic Table, Early Attempts at Creating a Periodic Table and more.
In 1869, Russian chemist Dmitri Mendeleev created the framework that became the modern periodic table, leaving gaps for elements that were yet to be discovered. While arranging the elements according to their atomic weight, if he found that they did not fit into the group he would rearrange them.
Mendeleev discovered the periodic table (or Periodic System, as he called it) while attempting to organise the elements in February of 1869. He did so by writing the properties of the elements on pieces of card and arranging and rearranging them until he realised that, by putting them in order of increasing atomic weight, certain types of ...