- ASH Foundation
- Log in or create an account
- Publications
- Diversity Equity and Inclusion
- Global Initiatives
- Resources for Hematology Fellows
- American Society of Hematology
- Hematopoiesis Case Studies

Case Study: Sickle Cell Disease A 25-Year-Old in Transition
- Agenda for Nematology Research
- Precision Medicine
- Genome Editing and Gene Therapy
- Immunologic Treatment
- Research Support and Funding
A 25-year-old woman with a history of sickle cell disease (SCD) presents to the clinic for follow-up after a hospitalization for a vaso-occlusive pain crisis complicated by influenza A. She has a history of an acute ischemic stroke at age 5 years and has received monthly, simple red cell transfusions since the stroke. Her last transfusion was approximately four months prior. She is taking deferasirox 20 mg/kg daily but occasionally misses doses.
Laboratory results show the following:
Which of the following is the next best step in diagnosis
- Restart scheduled red blood cell transfusions
- Start prophylactic penicillin
- Discontinue transfusions and start hydroxyurea
- Order transcranial doppler ultrasonography (TCD) to assess risk of stroke
- Increase dose of deferasirox to 25 mg/kg/day
Explanation
The incidence of primary stroke in children with SCD is 0.6 to 0.8 events per 100 patient-years, with a cumulative incidence of 7.8 percent by age 14 years in the Jamaican cohort and 11 percent by age 20 years in the U.S. Cooperative Study of Sickle Cell Disease. Once stroke has occurred, the incidence of recurrent (secondary) stroke ranges from 47 to 93 percent in patients not started on regular transfusions. The Stroke Prevention Trial in SCD (STOP) randomized 130 high-risk children with SCD to either transfusion therapy (to maintain HbS 30%) or observation. These high-risk children had an increased blood flow in the internal carotid or middle cerebral artery by TCD. This study showed a 92 percent reduction in incidence of first stroke in transfused high-risk patients. A follow-up study, STOP2, randomly assigned 72 children whose TCD had normalized after 30 months of transfusion therapy to either ongoing or discontinued transfusions. The study was closed early due to a significant increase in abnormal TCD velocity and stroke risk for those who halted transfusion therapy.
The multicenter phase III TWiTCH trial evaluated children with SCA and abnormal TCD velocities without a history of stroke on chronic transfusions. Data showed that hydroxyurea at maximal tolerated dose was noninferior to chronic transfusions for maintaining TCD velocities as primary stroke prophylaxis (choice C). This patient has a history of ischemic stroke, so the results of TWiTCH do not apply to her.
The Stroke with Transfusions Changing to Hydroxyurea (SWiTCH) study was designed as a phase III multicenter trial to determine the efficacy of hydroxyurea/phlebotomy, compared with transfusions/chelation for children with SCA, stroke, and iron overload in secondary stroke prophylaxis. The primary endpoint was a composite of noninferiority for stroke prevention and superiority for reduction of liver iron content. The trial was terminated at the first scheduled interim analysis for futility for the composite endpoint, which required superiority of phlebotomy over iron chelation for reducing excess iron stores. The incidence of stroke on the hydroxyurea plus phlebotomy arm was higher (7 of 67 patients; 10.4%) than in the transfusion plus chelation arm (1 of 66 patients; 1.5%). These results, though not powered for inferiority, showed a trend towars increased stroke risk with transition to hydroxyurea. In patients with prior stroke, cessation of transfusion therapy is currently not recommended.
Whether chronic transfusion therapy can be stopped after a longer period of transfusions in a patient with a prior stroke remains unclear even though risk of recurrent stroke remains high in adolescence and young adulthood. In patients older than 16 years, TCD velocity criteria to determine stroke risk is not reliable (choice D).
In the Prophylaxis with Oral Penicillin in Children with Sickle Cell Anemia trial, children with SCA were randomly assigned to receive oral prophylactic penicillin or placebo PROPS 1986 ). The trial ended eight months early after the occurrence of 15 cases of pneumococcal sepsis, 13 in the placebo group and two in the penicillin group, showing an 84 percent reduction in pneumococcal sepsis with penicillin prophylaxis. The follow-up study, PROPS II, did not show an increased risk in pneumococcal infections with discontinuation of prophylactic penicillin after age 5 years. Therefore, prophylactic penicillin is not recommended in adults with SCA (choice B).
The trajectory of ferritin in this patient has not been established and an increase in oral iron chelation is not indicated at this time.
Case Study submitted by Marquita Nelson, MD, of University of Chicago, Chicago, IL.
- Hirst C, Owusu-Ofori S Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease . Cochrane Database Syst Rev. 2014 6:CD003427.
- Valadi N, Silva GS, Bowman LS, et al Transcranial Doppler ultrasonography in adults with sickle cell disease . Neurology. 2006 22:572-574.
- Ware RE, Davis BR, Schultz WH, et al Stroke with transfusions changing to hydroxyurea (SWiTCH) . Blood. 2012 119:3925-3932.
- Kumar N, Gross JB Jr, Ahlskog JE TCD with transfusions changing to hydroxyurea (TWiTCH): hydroxyurea therapy as an alternative to transfusions for primary stroke prevention in children with sickle cell anemia . Blood. 2015 126:3.
American Society of Hematology. (1). Case Study: Sickle Cell Disease A 25-Year-Old in Transition. Retrieved from https://www.hematology.org/education/trainees/fellows/case-studies/sickle-cell-disease-a-25-year-old-in-transition .
American Society of Hematology. "Case Study: Sickle Cell Disease A 25-Year-Old in Transition." Hematology.org. https://www.hematology.org/education/trainees/fellows/case-studies/sickle-cell-disease-a-25-year-old-in-transition (label-accessed June 08, 2024).
"American Society of Hematology." Case Study: Sickle Cell Disease A 25-Year-Old in Transition, 08 Jun. 2024 , https://www.hematology.org/education/trainees/fellows/case-studies/sickle-cell-disease-a-25-year-old-in-transition .
Citation Manager Formats
- Main Navigation
- Account Navigation
- Main Content
JavaScript is disabled on your browser
Please enable JavaScript or upgrade to a JavaScript-capable browser to use the ASH Image Bank.
- ASH Academy

- ABOUT IMAGE BANK
- PERMISSIONS
- COLLECTION Images of peripheral blood and/or bone marrow of blood disorders and normal hematopoiesis.
- ATLAS Normal and abnormal blood cells
- REFERENCE CASES Complete cases of common blood disorders (peripheral blood, bone marrow, and diagnostic studies).
- UPLOAD IMAGES
- Home / Reference Cases
Case history of a child with sickle cell anemia in India
A three years old male child, native of Jharkhand, Central India presented with mild pallor, icterus, and history of on and off abdominal and joint pains. On examination the child had mild splenomegaly. He had history of two prior hospital admissions. First at the age of 1 year, when he was diagnosed to have pneumonia and second, at the age of 3 years (3 months prior to coming to our institution) for fever, anemia and jaundice. He has had three transfusions till now, last transfusion was 3 months back. There is history of sibling death at 5 years of age due to fever and jaundice.
The hemogram showed anemia with leukocytosis. Red cell morphology (Figure 1) revealed severe anisopoikilocytosis with macrocytes, microcytic hypochromic red cells, target cells, many boat cells, sickled RBCs, polychromatic cells and occasional nucleated RBCs. Results of the automated blood cell counts showed Hb 7.7 g/dl, RBC 2.44 x 109/l, MCV 97.1 fl, MCH 31.4 pg, MCHC 32.3 g/dl, RDW 26.6%. There were occasional nucleated red cells and relative neutrophilia. Further to confirm HbS, a sickling test using freshly prepared 2% sodium meta-bisulphite was performed which was positive (Figure 2).
Hemoglobin HPLC on Bio-Rad Variant 2 showed raised fetal hemoglobin (HbF) and a variant peak in S window (71.9%) at retention time of 4.36 mins. Adult Hb (HbA) of 8.5% was noted (Figure 3). Figure 4 shows Cellulose acetate hemoglobin electrophoresis at alkaline pH (8.6), which showed a prominent band in S/D/G region and a faint band in F region. Investigations of the father showed also showed a variant peak in S window (32.9%) at retention time of 4.36 mins along with HbA (57.1%) on HPLC with Bio-Rad Variant II which is diagnostic of Sickle cell trait (Figure 5).
Sickle cell disease (SCD) is the most common symptomatic hemoglobinopathy caused as a result of inheritance of two copies of the sickle β-globin gene variant (βS). A single nucleotide substitution leading to replacement of glutamic acid by valine at position 6 of the β-globin polypeptide chain leads to formation of HbS which is responsible for disease manifestation. SCD has a wide geographical distribution throughout major parts of Africa, the Middle East, India and in some regions of Mediterranean countries. In India, it is mainly concentrated in the central region including parts of Madhya Pradesh, Chattisgarh, Orissa, Maharashtra, Gujrat and Jharkhand. HbS has carrier frequencies varying from 5 to 35% and are especially seen amongst the scheduled tribes, scheduled castes and other backward castes.
Sickle cell mutation is believed to be originated five times in history spontaneously. This can be elucidated by five βS-globin haplotypes. These haplotypes include Senegal (SEN), Benin (BEN), Bantu or the Central African Republic (CAR), Cameroon (CAM) and Arab-Indian (ARAB). They enable us to understand the origin, evolution, migration and natural selection of genetic defects. They can be identified by specific restriction sites within the β-globin gene cluster. Different haplotypes are known to have different HbF levels. Senegal and Arab-Indian haplotypes have higher HbF levels when compared to other haplotypes. However, recently a study has investigated the origins of the sickle mutation by using whole-genome-sequence data to conclude that there might be single origin of sickle allele.
LEARNING POINTS
1. Sickle cell disease (SCD) is the most common symptomatic hemoglobinopathy in the world, largely seen in parts of Africa, the Middle East, India and in some regions of Mediterranean countries.
2. SCA is a monogenic disorder with an autosomal recessive inheritance. The parents are clinically asymptomatic and have normal blood counts. They are usually diagnosed incidentally or as a result of family studies in SCA patients.
3. Neonates are asymptomatic due to high HbF, but symptoms begin to appear by six months of age. Many infants present with lethal complications at first presentation. This emphasizes the importance of newborn screening in these susceptible pre-symptomatic cases in endemic regions.
4. SCA has a variable clinical course amongst different individuals depending upon various genetic determinants like βs haplotype, factors affecting HbF levels and co-inheritance of other disease modifying factors.
5. Diagnosis mainly relies upon identification of HbS (by any of the following HPLC, Hb Electrophoresis, Iso-electric focusing or sickling test). Once HbS is identified, it has to be validated by alternative method.
6. Treatment of sickle cell disease generally aims at relieving symptoms and preventing infections, sickle cell crises and long-term complications. Stem cell transplant is the only potential cure available presently.
HPLC pattern of the index case with sickle cell anemia showing HbS and HbF peaks.
Hemoglobin electrophoresis at alkaline pH. Black arrow shows the index case with HbS and HbF bands.
Loading. Please wait.
Uploading files. please wait..
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Case Rep Med
- v.2021; 2021

Varied Age of First Presentation of Sickle Cell Disease: Case Presentations and Review
Alexis claeys.
1 Pediatric Department, ZNA Paola Children's Hospital, Antwerp, Belgium
2 Pediatric Department, UZ Gent, Gent, Belgium
Susanne Van Steijn
Lydia van kesteren, elizabet damen.
3 Pediatric Hematology, Oncology Unit, UZ Brussel, Jette, Belgium
Machiel Van Den Akker
4 Pediatric Hematology, Oncology Unit, Queen Mathilde Mother and Child Center, Antwerp University Hospital, Edegem, Belgium
Associated Data
The data used to support the findings of this study are available in the archives of the ZNA Paola Children's Hospital, Antwerp, Belgium.
Sickle cell disease is a multisystem condition characterized by hemolytic anemia and vasoocclusion. Not only are the symptoms of the first presentation but also the ages of presentation are very variable. Following three case reports, different causes of possible late presentation are discussed. Many factors are responsible for the age at which sickle cell disease is diagnosed: doctor's delay (unfamiliarity with the disease), patient's delay (education and financial position of the parents, cultural factors), high- versus low-resource country (availability of newborn screening), fetal hemoglobin, reticulocyte count, and genetic modulators, such as SCD genotype, alpha-thalassemia, fetal hemoglobin concentration, and G6PD deficiency. The individual course of sickle cell disease depends on (epi) genetic and environmental properties and the underlying interactions. In further studies, the role of each factor should be evaluated more deeply, and its use as a marker of disease severity or activity should be assessed.
1. Introduction
Sickle cell disease (SCD) is a common group of life-threatening, genetic disorders caused by the synthesis of abnormal hemoglobin (sickle hemoglobin), which when deoxygenated, polymerizes and causes sickling of red blood cells. SCD is characterized by chronic hemolytic anemia, vasoocclusion, and progressive vascular injury causing multiorgan damage [ 1 ]. The clinic of SCD is quite variable and reflects the interactions with other genetic and environmental factors and can include painful vasoocclusive crises (VOCs), acute chest syndrome, cerebral stroke, and acute and chronic hemolysis. Due to functional hyposplenism, there is susceptibility to invasive bacterial infection with encapsulated bacteria [ 2 , 3 ].
In sub-Saharan Africa, the Middle East, India, and the Mediterranean area, SCD is the most prevalent, where more than 90% of the annual SCD births take place, but due to migration, SCD can be diagnosed also in other parts of the world [ 4 ]. While newborn screening and follow-up with prophylactic interventions are common in high-resource countries, most children die before the age of 5 years, often before a diagnosis was made, in low-resource countries [ 5 ].
Sickle cell anemia (SCA) refers solely to patients homozygous for the sickle mutation (HbSS) and is together with sickle β °-thalassemia responsible for more severe diseases in the group of SCD. Hemoglobin SC disease and sickle β ⁺-thalassemia are common and in general, less severe presentations of SCD. Hemoglobin SD and hemoglobin SE are much less common.
The individual course of SCD is heterogeneous and depends not only on several genetic factors, such as the concomitant presence of alpha-thalassemia, sickle β -haplotypes, fetal hemoglobin levels, and G6PD (glucose-6-phosphate dehydrogenase) deficiency, but also on environmental properties and their underlying interactions [ 2 ]. There can be a widespread diversity in clinic and first presentation between different communities, but sometimes also in the same family [ 6 – 8 ]. A late first presentation is not always so straightforward, and patient and doctor's delay is not uncommon as we will show in two cases.
2. Case Presentations
2.1. case 1.
A twenty-month-old boy with no relevant medical history presented at the outpatient clinic with limping for 7 days ( D 7 = day 7 of disease) without a prior trauma. Fever up to 38.5 degrees Celsius was noted over the last week. The boy was born at term by an uncomplicated vaginal delivery following a normal pregnancy; his weight at birth was 3.6 kilograms. He was the first child of healthy, nonconsanguineous parents from Angola. The family history did not reveal any relevant information except that the mother is a carrier of sickle cell anemia (HbAS), which was denied to the father. Physical examination showed a mild sick, male with normal cardiovascular and respiratory parameters, with 38.5 degrees Celsius. The skin was pale, without signs of jaundice. There were no signs of dehydration. ENT examination showed mild rhinorrhea with some pharyngeal redness. Heart and lung auscultation was normal. There was no hepatosplenomegaly. Neurological examination was normal. We saw mild limping of the right leg with mildly painful abduction right hip. The initial complete blood count showed a mild, microcytic anemia with hemoglobin of 9.4 g/dL (normal 10.5–13.6 g/dL), MCV 68 (normal 70–85 fL), reticulocytes 60/1000 RBC (normal 11–29/1000 RBC), leukocytes of 12.3 × 10E9/L (normal 6.0–17.0 × 10E9/L) with normal differentiation, and thrombocytes of 559 × 10E9/L (normal 304–574 × 10E9/L). Blood smear revealed poikilocytosis, tear cells, schistocytes, and some target cells. C-reactive protein (CRP) was elevated up to 144 mg/L (normal ≤ 5 mg/L). An ultrasound of the ankles, knees, and hips did not show any abnormalities. Under suspicion of reactive arthritis, the patient was sent home with bedrest, pain medication, and follow-up appointment.
At D13, he was again seen in the outpatient clinic; his temperature normalized over the last few days, while his physical examination did not reveal changes. The ultrasound of the hip was normal. A radiograph of the pelvis showed irregularities of the right iliac wing. Bone scintigraphy with 99mTc-MDP revealed an elevated distribution at the right pelvic wing anterolaterally, possible of an inflammatory nature. Because CRP spontaneously improved (23.9 mg/L (normal ≤ 5 mg/L)) and of the absence of fever, an MRI of the pelvis was requested as an outpatient.
At D19, MRI (magnetic resonance imaging) revealed an increased signal of the right iliac wing on the STIR (short-TI inversion recovery) images. The adjacent soft tissue captures the contrast. The image suggests osteomyelitis. The patient was admitted. CRP normalized to 3 mg/l. His temperature was normal. After blood cultures were drawn, IV (intravenously) antibiotics, cefotaxime and flucloxacillin, were started. Blood culture did not reveal any growth. Because of an atypical course, more extensive research into possible explanation has been carried out, including cultures for Salmonella typhi and Kingella kingae , the tuberculin skin test, and serology for HIV. They were all negative. Before a biopsy of the bone lesion was performed, hemoglobin electrophoresis confirmed sickle cell anemia (HbA2 2.3%, HbF 24.5%, and HbS 73.2%). Due to the absence of clear sickle cells in the blood smear, denial of carrier status (HbAS) of the father, late recognition that the limping is pain due to VOC, and the presence of a high HbF, the diagnose of sickle cell anemia was delayed.
2.2. Case 2
A three-year-old boy was followed by a pediatric rheumatologist on suspicion of systemic onset juvenile idiopathic arthritis due to varying pains in his hands and feet for 18 months. Sporadically, the child refused to walk. Often, these episodes were preceded by fever and eased with ibuprofen. No rash was reported. He presented in the clinic of the pediatric rheumatologist with fever (39 degrees Celsius) for 1-2 weeks with limping and painful swelling of the right tibia above the ankle. He was initially seen by the pediatric rheumatologist who made the diagnosis of systemic onset juvenile idiopathic arthritis (JIA), and treatment with ibuprofen and corticosteroids was started. The body temperature normalized, but pain and swelling of the tibia remained, after which the child was admitted.
The boy was born at term by an uncomplicated vaginal delivery following a normal pregnancy; his weight at birth was 3 kilograms. He was the first child of healthy, nonconsanguineous parents from Guinea. The family history did not reveal any relevant information.
Physical examination at the first presentation showed a moderate sick male with normal cardiovascular and respiratory parameters, with a temperature of 37.6 degrees Celsius. The skin was pale. No signs of dehydration. No signs of upper respiratory tract infection. Heart and lung auscultation were normal.
The abdomen was showing mild hepatomegaly (3-4 cm under the costal arch), and the spleen was not palpable. Extremities: at the right tibia, warm, painful swelling several centimeters proximal of the distal metaphysis. The left tibia was painful on palpation, but no other clinical signs.
The initial complete blood count showed a severe, normocytic anemia with hemoglobin of 6.4 g/dL (normal 11.5–13.5 g/dL), reticulocytes 77/1000 RBC (normal 8–20/1000 RBC), leukocytes of 15.3 × 10E9/L (normal 5.5–15.5 × 10E9/L) with normal differentiation, and thrombocytes of 448 × 10E9/L (normal 228–433 × 10E9/L). Blood smear revealed hypochromasia, target cells, poikilocytosis, and sickle cells. There were no signs of hemolysis with normal lactate dehydrogenase (707 U/L (normal 425–975 U/L)) and total bilirubin (0.7 mg/dL (normal 0.2–1.3)). C-reactive protein was 56 mg/L (normal ≤ 5 mg/L).
After admission to the pediatric department, within hours, his clinical condition worsened with prolonged capillary refill with tachycardia (192/minute) but normal blood pressure. After blood cultures were drawn, IV ceftriaxone (100 mg/kg) was started together with IV fluid resuscitation (NaCl 0.9%, 20 mL/kg) and red blood cell (RBC) transfusion. X-ray of the right tibia showed significant soft tissue swelling from the distal lower leg and at the distal metaphysis, a slight clearance with possible cortex interruption. Bone scintigraphy with 99mTc-MDP reveals a bone injury at the right distal tibia with a hyperemic component, compatible with an abscess. Surgical drainage showed a lot of pus with a culture showing growth of Salmonella typhimurium . Antibiotic treatment was switched to high dose amoxicillin according to the sensitivity. Blood and stool cultures remained negative. Hemoglobin electrophoresis confirmed the diagnosis of sickle cell disease (HbA2 2.7%, HbF 24.1%, and HbS 73%). The initial wrong diagnosis (doctor's delays) by not recognizing sickle cell disease despite high risk based on ethnicity and VOC and the high HbF were responsible for the late diagnosis.
2.3. Case 3
A three-year-old girl without a relevant medical history was admitted to the hospital under the suspicion of pneumonia. She was born at term by an uncomplicated vaginal delivery following a normal pregnancy; her weight at birth was 3.2 kilogram. She was the second child of healthy, nonconsanguineous parents from Mali. The family history did not reveal any relevant information. Physical examination showed a sick female with mild tachycardia (pulse 134/minute) and moderate dyspnea (34/minute), with 39.1 degrees Celsius. The skin was pale, no signs of jaundice, and no signs of dehydration. ENT examination showed the presence of mild rhinorrhea with some pharyngeal redness. Systolic murmur 1/6 was over the heart and diminished air-entry over the right lower lobe. The abdomen was not showing hepatosplenomegaly. The initial complete blood count showed a mild, microcytic anemia with hemoglobin of 8.5 g/dL (normal 10.5–13.6 g/dL), reticulocytes of 30/1000 RBC (normal 11–29/1000 RBC), leukocytes of 10.3 × 10E9/L (normal 6.0–17.0 × 10E9/L) with normal differentiation, and thrombocytes of 316 × 10E9/L (normal 229–435 × 10E9/L). Blood smear revealed poikilocytosis, polychromasia, and target cells. C-reactive protein (CRP) was only mildly elevated up to 30 mg/L (normal ≤ 5 mg/L). X-thorax showed a consolidation in the region of the right lower lobe. Under suspicion of pneumonia, intravenous amoxicillin-clavulanic acid was initiated. Despite the ethnicity and the clinic of an acute chest syndrome, only in the context of the anemia, a hemoglobin electrophoresis was performed, revealing the presence of hemoglobin C (42%) and hemoglobin S (46%). Family research showed that the parents are carriers (mother HbC 32% and father HbS 42%), and their first-born male has the same condition as his sister, namely, hemoglobin SC disease. After discharge, antibiotic prophylaxis and folic acid were initiated.
3. Discussion
The patients presented in this case series are born in Belgium. They are of sub-Saharan African immigrant families and demonstrate a high frequency of SCD mutations. In their native countries, newborn screening and comprehensive healthcare only rarely takes place at an early age [ 9 ], therefore, resulting in a wide variation of the age at diagnosis of SCD. The medium age reported varies from 2 until 6 years [ 10 – 12 ]. Sarat et al. report that in Brazil, the medium age at diagnosis of the adults receiving care for HbSS was five years and for HSC, 21 years [ 13 ]. Even in high-resource countries such as Belgium, universal newborn screening and prenatal counseling are not always performed. According to the German SCD registry, most children were discovered after the age of one year, usually by presenting with symptoms [ 14 , 15 ]. The median age at diagnosis of a cohort of children with SCD in the Netherlands was 25 months [ 16 ]. Often due to the lack of knowledge of the physicians regarding SCD, even symptoms, typically associated with clinical manifestations of SCD, are misinterpreted, resulting in inadequate treatment. It is important to address these important public health issues.
There can be several other factors also responsible for the manner and the time at which the disease presents itself, some of which will be discussed ( Table 1 ).
Factors influencing the age of presentation of SCD in children.
3.1. Western World versus Developing Countries
Although patients with SCA have an identical monogenic condition, its clinical phenotypes are highly variable [ 17 , 18 ]. Different pathways play a role in the pathophysiology, and different genes have been under evolutionary pressure. It is fair to assume that ethnic differences influence the course of SCA [ 17 ]. The vast majority and burden of disease is in sub-Saharan Africa. Because of economic limitations, newborn screening of hemoglobin disorders is not achieved. Diagnosis is mostly delayed until children have clinical signs. Screening because of a positive family history is possible yet less applied because of financial considerations [ 19 ]. Also, not infrequently, the belief in evil spirits may be one obstacle for seeking timely medical care [ 20 ].
In sub-Saharan countries, without intervention, 50–90% of children with SCD will die in childhood, mostly due to infection/sepsis [ 2 , 21 ], often before the diagnosis of SCD is made. There is no increased mortality in children with severe malaria when they also have sickle cell anemia [ 22 ].
Worldwide, we also have a genetic explanation for different ways of presentation, as we know there are different haplotypes in different regions of the world. For example, in the Kingdom of Saudi Arabia, there are two major clinical phenotypes. Patients from the Western Province have a severe course consistent with the Benin haplotype. They are prone to recurrent episodes of acute chest syndrome (ACS), stroke, and dactylitis and in general have a lower level of hemoglobin and hemoglobin F (HbF). They typically present early with painful crises. Patients from the Eastern Province have a milder course of disease; they act more like the Arab and Indian phenotypes. This is associated with higher levels of HbF and frequently a later date of the first presentation [ 23 , 24 ].
3.2. Fetal Hemoglobin
HbF is a physiologic protein tetramer that is of major importance during fetal life. Its affinity for oxygen is higher than the mother's hemoglobin and thereby facilitates the transfer of oxygen from maternal to fetal blood [ 25 ]. A higher HbF level in the postnatal period is known to have a positive effect on the course of SCD [ 2 , 25 – 27 ]. Patients with SCD and hereditary persistence of fetal hemoglobin (HPFH) have a significantly milder phenotype [ 27 ] with a longer lifespan of the red blood cells [ 18 ]. HbF prevents polymerization of HbS by reducing the concentration of HbS. The sufficient amount of HbF to prevent the clinical manifestations of SCD in vivo is not completely clarified [ 27 ], while in vitro studies suggest that 20% HbF is necessary to prevent polymerization of HbS [ 28 ]. In a cross-sectional single center study in Nigeria, it was found that stroke was related to lower levels of HbF; while on the other hand, complications such as priapism, osteomyelitis, and septic arthritis or ulcers were not linked to lower levels of HbF. In this study, lower levels were mostly found in boys, which were not seen in adults. Possibly, a hormonal role during puberty is responsible. In addition, there is a relationship between the percentage of HbF and age, in which the percentage of HbF decreases with age [ 26 ].
A different explanation might be the haplotype of the disease. Senegal, Saudi, and Indian haplotypes are generally associated with higher levels of HbF and therefore known for a milder course [ 24 – 26 ]. HbF is also increased in different inherited conditions, such as HPFH, hereditary spherocytosis, and thalassemia. Family studies suggest an inheritance that does not follow the Mendelian pattern. HbF levels are multifactorial, influenced with 89% of variability because of genetic factors, and the other is dependent on sex, age, and environmental factors. The level of HbF rises in several acquired states, such as pregnancy, aplastic anemia, thyrotoxicosis, hepatoma, myeloproliferative disorders, or hypoplastic myelodysplastic syndrome [ 25 ].
3.3. Reticulocytes
The role of the reticulocyte in the pathophysiology of SCD is not fully understood and still understudied [ 18 ]. The reticulocyte count is commonly used as a marker of the bone marrow's response to hemolysis and anemia [ 18 , 27 ]. Under normal conditions, each second, the bone marrow produces about 2 million reticulocytes. In SCD, this can increase up to 20-fold due to hemolytic anemia. During this increased production, the bone marrow releases reticulocytes prematurely in the blood in order to meet the demands. As SCD is accompanied by chronic hemolysis, there is a continuous stress erythropoiesis and therefore a persistent reticulocytosis. These prematurely released reticulocytes are less rigid than the sickle cell, but they contribute to vasoocclusion due to their big size and membrane properties promoting adhesion [ 18 ]. The splenic dysfunction in SCD is responsible for the lack of removal of the reticulocytes, leading to an overrepresentation (up to 25%) of the reticulocytes of all circulating red blood cells. The benefit of these cells might not transcend the pathological consequences. Data and studies are lacking to identify a thorough link between reticulocytosis and disease severity [ 18 ].
In infancy, in children with SCD, the physiologic anemia will show an exaggerated increase in the reticulocyte count and could be a marker for SCD. In the cooperative study of sickle cell disease, the absolute reticulocyte count above 200 000/ µ l in the age group 2–6 months was associated with a three-time risk for SCD-related events which needed hospitalization within the first three years of life [ 18 , 29 , 30 ]. Ambrose et al. showed in a prospective cohort study conducted in Tanzania that children with SCA and sickle β °-thalassemia had a lower Hb with a high red cell distribution width (RDW) at 3- and 6-month follow-ups, compared to carriers of sickle cell disease and sickle β + -thalassemia. The results of a simple blood test could suspect the presence of SCD already in this age group [ 19 ].
3.4. Genetic Modulators
Some genetic modulators of SCD severity are well known as concurrent α -thalassemia, fetal hemoglobin concentration, and ß -globin haplotype. Possible G6PD deficiency can have an impact on SCD. More recently, BCL11 A and HBS1L-MYP were added to the list [ 27 ].
3.5. SCD Genotype
While sickle cell anemia (HbSS) and sickle β 0 -thalassemia (HbS- β 0 ) are responsible for more severe diseases; hemoglobin SC disease (HbSC) and sickle β ⁺-thalassemia (HbS- β ⁺) are common and in general, less severe presentations of SCD. HbSC is the most common type in the African American population in the United States [ 27 ]. HbC does not polymerize, but it potentiates the polymerization of HbS by increasing the mean corpuscular hemoglobin concentration. As the course is rather mild, the patients still can suffer from the classic vasoocclusive complications as priapism, splenic sequestration, and acute chest syndrome. HbSC patients rarely present with a stroke as they have higher hemoglobin levels [ 27 ], but presumable, due to the higher blood viscosity, retinopathy is seen more often [ 31 ]. HbS- β ⁺ is milder with less severe pain crises and less severe splenic dysfunction. Therefore, the disease can present itself later in life [ 32 ]. On the other hand, this genotype has a broad spectrum of phenotypes, and therefore, caution should be taken [ 27 ].
3.6. Alpha-Thalassemia
The decrease in α -globin chains in SCD reduces the mean corpuscular hemoglobin concentration and, thereby, the polymerization within the RBC. Homozygous alpha-thalassemia decreases the oxidative stress in SCA and could be an explanation for the protected role of alpha-thalassemia from various hemolysis-related complications of SCA. In contrast, the rate of vasoocclusive events is not related to their oxidative stress level [ 33 ]. In 2014, Cox et al. reported in a study of 601 stroke-free Tanzanian SCA patients aged <24 years, the importance of alpha-thalassemia of reducing risk of abnormal cerebral blood flow velocity [ 34 ]. Olatunya et al. performed a cross-sectional retrospective study on 100 young Nigerian patients with SCA and 63 controls. Their study confirms that coexistence of alpha-thalassemia with SCA significantly influences both the clinical and laboratory manifestations with increased bone pain crisis and protection against leg ulcers [ 35 ].
3.7. Fetal Hemoglobin Concentration
The Xmn1 polymorphism on chromosome 11p is the first genetic variant associated with increased levels of HbF [ 36 ], but it is assumed that additional factors are also necessary to have a clinical impact [ 27 ]. Another gene linked to higher HbF levels is BCL11 A on chromosome 2p16. This is a transcriptional repressor of the γ -globin in helping control the developmental switch from embryonic to adult ß -globin [ 37 ]. Variations in the BCL11 A binding site are found concurrent with HPFH [ 27 ]. The MYB transcription factor of the c - Myb gene on chromosome 6q23 is an important regulator of hematopoiesis, erythropoiesis, and HbF levels [ 27 , 38 ]. Low MYB levels stimulate the erythroid differentiation, causing the release of larger cells expressing predominantly γ -globin [ 39 ]. Using whole genome sequencing of many SCD genomes, many other HbF regulators will be identified. With all the acquired new data, it will be difficult to define and harmonize the variable phenotypes [ 27 ].
There are conflicting data on the impact of concurrent G6PD deficiency on laboratory and clinical parameters of SCD. Belisario et al. investigated a cohort of 395 Brazilian children with SCA and demonstrated that G6PD molecular deficiency or enzyme activity was not associated either with clinical ischemic stroke or high-risk transcranial Doppler [ 40 ], while Joly et al. studied 121 children with SCA and concluded that G6PD deficiency and absence of a -thalassemia increases the risk for cerebral vasculopathy in children with sickle cell anemia [ 41 ]. Antwi-Baffour et al. examined the blood of 120 SCD patients of genotypes HbSS and HbSC and found statistically significant differences between the Hb concentration of the participants having a G6PD deficiency (males) and those with normal G6PD activity (females), concluding that G6PD deficiency may increase the severity of anemia in SCD patients and therefore a possible earlier presentation [ 42 ].
4. Conclusion
SCD is a well-studied but not completely understood entity. When no newborn screening has taken place, a child's sickle cell disease can present for the first time in different ways and at different ages. Many factors are responsible for this. Familiarity and knowledge of SCD are of course essential with a low threshold for additional research (peripheral blood smear and Hb electrophoresis) to prevent delay of the diagnosis and performance of unnecessary tests.
Data Availability
Written consent was obtained from the patient's legal guardian(s) of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Conflicts of Interest
The authors declare that they have no conflicts of interest.

Authors' Contributions
Alexis Claeys drafted the initial manuscript and approved the final manuscript submitted. Susanne van Steijn, Lydia van Kesteren, and Elizabet Damen critically reviewed the manuscript and approved the final manuscript submitted. Machiel van den Akker carried out the initial analyses, coordinated and supervised the writing of the manuscript, critically reviewed the manuscript, and approved the final manuscript submitted.
From Gene to Disease: Sickle Cell Anemia
Lesson summary.

- Describe how a mutation at one point in the DNA can change an organism’s phenotype.
- Draw and interpret pedigree and Punnett square models.
- Analyze data tables and maps to make and support claims.
- Explain the principle of an evolutionary trade-off, and how environmental conditions influence fitness.
Science and Engineering Practices:
Ma science and technology/engineering (2016):, ngss (2013):, common core math/language arts standards:, educator soundbites.
"I used this as half of my final exam in High School Biology, mostly 9th and 10th grade students. It worked well a performance task since it integrated a lot of concepts we studies second semester. It was right on point. The instructions were clear, and students needed very little help. They seemed to be very interested in it and were all immediately engaged. I thought it was a great and worthwhile exercise, integrating genetics and evolution, and having students complete tables, interpret data, and choose a claim and support it with evidence at the end." – Julie Bookman, Lancaster High School in Lancaster, CA
Lesson Documents
Download PDF View Google Doc
- Name First Last
- Which lesson do you have feedback for? *
- Yes, with my name and school included
- Yes, but with just my school, not my name included
- Your name and/or school as you'd like them to appear
- May we contact you with further questions and add you to our mailing list?
- Hidden Lesson Title
Email address:
Who are you? Educator K–5 Educator 6–8 Educator 9–12 Educator 13+ Researcher Other
State Alabama Alaska Arizona Arkansas California Colorado Connecticut Delaware Florida Georgia Hawaii Idaho Illinois Indiana Iowa Kansas Kentucky Louisiana Maine Maryland Massachusetts Michigan Minnesota Mississippi Missouri Montana Nebraska Nevada New Hampshire New Jersey New Mexico New York North Carolina North Dakota Ohio Oklahoma Oregon Pennsylvania Puerto Rico Rhode Island South Carolina South Dakota Tennessee Texas Utah Vermont Virgin Islands Virginia Washington Washington D.C. West Virginia Wisconsin Wyoming Outside US
You may download and use for educational purposes the free resources made available on the BiteScis webpages. These resources should not be used for any commercial purpose without the express permission of BiteScis. Email [email protected] for more information.
If you have any question, send us an email and we'll get back to you, soon.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases and Disorders Education Program
- Publications and Resources
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- Clinical Trials
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- < Back To Health Topics
- What Is Sickle Cell Disease?
- Causes and Risk Factors
- Sickle Cell Trait
- How Sickle Cell Disease May Affect Your Health
- Living With Sickle Cell Disease
- Pregnancy, Reproduction, and Sickle Cell Disease
MORE INFORMATION
Sickle Cell Disease What Is Sickle Cell Disease?
Language switcher.

Sickle cell disease is a group of inherited red blood cell disorders that affect hemoglobin, the protein that carries oxygen through the body. Normally, red blood cells are disc-shaped and flexible enough to move easily through the blood vessels. In sickle cell disease, red blood cells become crescent- or “sickle”-shaped due to a genetic mutation. These sickled red blood cells do not bend or move easily and can block blood flow to the rest of the body.
The blocked blood flow through the body can lead to serious problems, including stroke , eye problems, infections, and episodes of pain called pain crises.
Sickle cell disease is a lifelong illness. Until recently, a bone marrow transplant was the only cure for sickle cell disease. However, in December 2023, the U.S. Food and Drug Administration approved two new therapies to treat the disease. One approach adds a gene to the body and the other makes changes to a gene that is already in the body. NHLBI researchers are continuing to explore treatments that impact genes, but there are other types of treatments that can reduce symptoms and prolong life. If you have sickle cell disease, your healthcare team will work with you on a treatment plan to reduce your symptoms and manage the condition .
The condition affects more than 100,000 people in the United States and 20 million people worldwide. In the United States, most people who have sickle cell disease are of African ancestry or identify themselves as Black:
- About 1 in 13 Black or African American babies are born with sickle cell trait .
- About 1 in every 365 Black or African American babies are born with sickle cell disease.
Many people who come from Hispanic, Southern European, Middle Eastern, or Asian Indian backgrounds also have sickle cell disease.
The NHLBI leads and supports research and clinical trials to find a cure for sickle cell disease.

Sickle Cell Disease: Milestones in Research and Clinical Progress Booklet
Learn about the history of sickle cell disease in the United States, from its discovery in 1910 to the NHLBI legacy of research that has advanced the understanding of sickle cell disease, improved clinical progress, and paved the way for widely available cures.
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- Skip to footer
- Image & Use Policy
- Translations
UC MUSEUM OF PALEONTOLOGY
Understanding Evolution
Your one-stop source for information on evolution
DNA and Mutations
A case study of the effects of mutation: sickle cell anemia.
Sickle cell anemia is a genetic disease with severe symptoms, including pain and anemia. The disease is caused by a mutated version of the gene that helps make hemoglobin — a protein that carries oxygen in red blood cells. People with two copies of the sickle cell gene have the disease. People who carry only one copy of the sickle cell gene do not have the disease, but may pass the gene on to their children.
The mutations that cause sickle cell anemia have been extensively studied and demonstrate how the effects of mutations can be traced from the DNA level up to the level of the whole organism. Consider someone carrying only one copy of the gene. She does not have the disease, but the gene that she carries still affects her, her cells, and her proteins:
Normal hemoglobin (left) and hemoglobin in sickled red blood cells (right) look different; the mutation in the DNA slightly changes the shape of the hemoglobin molecule, allowing it to clump together.
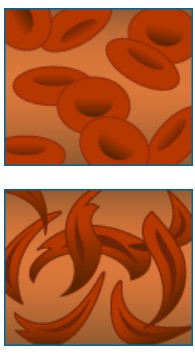
- There are negative effects at the whole organism level Under conditions such as high elevation and intense exercise, a carrier of the sickle cell allele may occasionally show symptoms such as pain and fatigue.
- There are positive effects at the whole organism level Carriers of the sickle cell allele are resistant to malaria, because the parasites that cause this disease are killed inside sickle-shaped blood cells.
This is a chain of causation. What happens at the DNA level propagates up to the level of the complete organism. This example illustrates how a single mutation can have a large effect, in this case, both a positive and a negative one. But in many cases, evolutionary change is based on the accumulation of many mutations, each having a small effect. Whether the mutations are large or small, however, the same chain of causation applies: changes at the DNA level propagate up to the phenotype.
The effects of mutations
Mutations are random
Subscribe to our newsletter
- Teaching resource database
- Correcting misconceptions
- Conceptual framework and NGSS alignment
- Image and use policy
- Evo in the News
- The Tree Room
- Browse learning resources
Clinical and biochemical characteristics, and outcome in 33 patients with ceftriaxone-induced liver injury
- Published: 29 May 2024
Cite this article

- Cai-Xia Feng 1 ,
- Wen-Yu Ye 1 &
- Qing-Wen Shan 1
79 Accesses
Explore all metrics
To summarize the clinical and biochemical characteristics of patients with ceftriaxone-induced liver injury and guide the selection of safe medication.
Retrieved domestic and foreign databases from inception to October 2023, collected case data conforming to ceftriaxone-induced liver injury, and statistically analyzed the data.
A total of 617 articles were retrieved, and 16 articles with 33 cases (10 children, 23 adults) were included. Males represented 60% (18/30), with a male-to-female ratio of 1.5:1. The age of onset ranged from 2 days to 96 years, with 15 of 23 adults (65%) over 55 years old. The time from ceftriaxone use to liver injury fluctuated between 0.5 and 47 days. Only 9 patients (27.3%, 9/33) had clinical symptoms, and the clinical classification was dominated by cholestatic injury (46.2%, 12/26). There was a significant difference in the clinical classification of ceftriaxone-induced liver injury between children and adults ( P = 0.0126), with hepatocellular injury predominating in children and cholestatic injury predominating in adults. The severity of liver injury was mainly mild (66.7%, 12/18). Peak values of alanine aminotransferase ranging from 228.5 to 8098 U/L, aspartate aminotransferase ranging from 86.7 to 21575 U/L, alkaline phosphatase ranging from 143 to 2434 U/L, and total bilirubin ranging from 3.35 to 66.1 mg/dL. There was a significant difference in peak values of alkaline phosphatase between children and adults ( P = 0.027), with a higher peak value of alkaline phosphatase in adults (1039 ± 716.4 U/L vs. 257 ± 134.9 U/L ) . Patients with normal imaging examinations accounted for the majority (61.5%, 7/13). The prognosis of 32 patients (97%, 32/33) was good, and one child with sickle cell anemia who developed immune hemolysis, progressive renal failure, and acute liver injury after using ceftriaxone died in the end.
Ceftriaxone-induced liver injury can occur at any age, with a higher risk in the elderly, and age may be related to the clinical classification. Although the clinical manifestations are not specific, close monitoring of liver biochemical indicators during the use can detect liver injury early. Most cases have a good prognosis, but for people with concomitant sickle cell anemia, it is necessary to be vigilant about the occurrence of severe hemolytic anemia.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Drug-induced liver injury associated with high-dose ceftriaxone: a retrospective cohort study adjusted for the propensity score.

Liver Injury and Failure in Critically Ill Children

Antibiotic-Induced Liver Injury in Paediatric Outpatients: A Case-Control Study in Primary Care Databases
Data availability.
No datasets were generated or analysed during the current study.
European Association for the Study of the Liver (2019) EASL Clinical Practice guidelines: drug-induced liver injury. J Hepatol 70(6):1222–1261. https://doi.org/10.1016/j.jhep.2019.02.014
Article Google Scholar
Andrade RJ, Chalasani N, Björnsson ES et al (2019) Drug-induced liver injury. Nat Rev Dis Primers 5(1):58. https://doi.org/10.1038/s41572-019-0105-0
Article PubMed Google Scholar
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury (2012) Bethesda (MD). National Institute of Diabetes and Digestive and Kidney Diseases
Technology Committee on DILI Prevention and Management, Chinese Medical Biotechnology Association, Study Group of Drug-Induced Liver Disease (2023) Chinese Medical Association for the Study of Liver Diseases (2023). Zhonghua Gan Zang Bing Za Zhi 31(4):355–384. https://doi.org/10.3760/cma.j.cn501113-20230419-00176-1
Stephens C, Lucena MI, Andrade RJ (2018) Host risk modifiers in Idiosyncratic Drug-Induced Liver Injury (DILI) and its interplay with Drug Properties. Drug-Induced Liver toxicity. Springer New York, New York, pp 477–496. https://doi.org/10.1007/978-1-4939-7677-5_23
Chapter Google Scholar
Garcia-Cortes M, Robles-Diaz M, Stephens C et al (2020) Drug induced liver injury: an update. Arch Toxicol 94(10):3381–3407. https://doi.org/10.1007/s00204-020-02885-1
Article CAS PubMed Google Scholar
Clare KE, Miller MH, Dillon JF (2017) Genetic factors influencing Drug-Induced Liver Injury: do they have a role in Prevention and diagnosis? Curr Hepatol Rep 16(3):258–264. https://doi.org/10.1007/s11901-017-0363-9
Article PubMed PubMed Central Google Scholar
Stephens C, Robles-Diaz M, Medina-Caliz I et al (2021) Comprehensive analysis and insights gained from long-term experience of the Spanish DILI Registry. J Hepatol 75(1):86–97. https://doi.org/10.1016/j.jhep.2021.01.029
Chalasani N, Bonkovsky HL, Fontana R et al (2015) Features and outcomes of 899 patients with Drug-Induced Liver Injury: the DILIN prospective study. Gastroenterology 148(7):1340–1352. https://doi.org/10.1053/j.gastro.2015.03.006
Bjornsson ES, Bergmann OM, Bjornsson HK et al (2013) Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 144(7):1419–e20. https://doi.org/10.1053/j.gastro.2013.02.006
Bessone F, Hernandez N, Mendizabal M et al (2019) When the creation of a Consortium provides useful answers: experience of the latin American DILI Network (LATINDILIN). Clin Liver Dis (Hoboken) 13(2):51–57. https://doi.org/10.1002/cld.778
DiPaola F, Molleston JP, Gu J et al (2019) Antimicrobials and antiepileptics are the leading causes of idiosyncratic drug-induced Liver Injury in American Children. J Pediatr Gastroenterol Nutr 69(2):152–159. https://doi.org/10.1097/MPG.0000000000002383
Article CAS PubMed PubMed Central Google Scholar
Nakaharai K, Sakamoto Y, Yaita K et al (2016) Drug-induced liver injury associated with high-dose ceftriaxone: a retrospective cohort study adjusted for the propensity score. Eur J Clin Pharmacol 72(8):1003–1011. https://doi.org/10.1007/s00228-016-2064-7
Floreani A, Bizzaro D, Shalaby S et al (2023) Sex disparity and drug-induced liver injury. Dig Liver Dis 55(1):21–28. https://doi.org/10.1016/j.dld.2022.06.025
Moore TJ, Cohen MR, Furberg CD (2007) Serious adverse drug events reported to the Food and Drug Administration, 1998–2005. Arch Intern Med 167(16):1752–1759. https://doi.org/10.1001/archinte.167.16.1752
Danan G, Benichou C (1993) Causality assessment of adverse reactions to drugs–I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 46(11):1323–1330. https://doi.org/10.1016/0895-4356(93)90101-6
Guarino M, Perna B, Pastorelli A et al (2022) A case of CILI and literature review. Infez Med 30(2):293–297. https://doi.org/10.53854/liim-3002-16
Castellazzi ML, Agostoni CV, Palella J et al (2022) Ceftriaxone-induced cholestatic hepatitis in a child: a case report and a review of the literature. Front Pediatr 10:1051887. https://doi.org/10.3389/fped.2022.1051887
Peker E, Cagan E, Dogan M (2009) Ceftriaxone-induced toxic hepatitis. World J Gastroenterol 15(21):2669–2671. https://doi.org/10.3748/wjg.15.2669
Dear JW, Clarke JI, Francis B et al (2018) Risk stratification after paracetamol overdose using mechanistic biomarkers: results from two prospective cohort studies. Lancet Gastroenterol Hepatol 3(2):104–113. https://doi.org/10.1016/S2468-1253(17)30266-2
McGill MR, Staggs VS, Sharpe MR et al (2014) Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology 60(4):1336–1345. https://doi.org/10.1002/hep.27265
Clarke JI, Dear JW, Antoine DJ (2016) Recent advances in biomarkers and therapeutic interventions for hepatic drug safety - false dawn or new horizon? Expert Opin Drug Saf 15(5):625–634. https://doi.org/10.1517/14740338.2016.1160057
Cuzzolin L, Oggiano AM, Clemente MG et al (2021) Ceftriaxone-associated biliary pseudolithiasis in children: do we know enough? Fundam Clin Pharmacol 35(1):40–52. https://doi.org/10.1111/fcp.12577
Zinberg J, Chernaik R, Coman E et al (1991) Reversible symptomatic biliary obstruction associated with ceftriaxone pseudolithiasis. Am J Gastroenterol 86(9):1251–1254
CAS PubMed Google Scholar
Neuman G, Boodhan S, Wurman I et al (2014) Ceftriaxone-induced immune hemolytic anemia. Ann Pharmacother 48(12):1594–1604. https://doi.org/10.1177/1060028014548310
Bell MJ, Stockwell DC, Luban NL et al (2005) Ceftriaxone-induced hemolytic anemia and hepatitis in an adolescent with hemoglobin SC disease. Pediatr Crit Care Med 6(3):363–366. https://doi.org/10.1097/01.PCC.0000161285.12396.FF
Shen T, Liu Y, Shang J et al (2019) Incidence and etiology of drug-induced liver injury in Mainland China. Gastroenterology 156(8):2230–2241. https://doi.org/10.1053/j.gastro.2019.02.002
Hunt CM, Yuen NA, Stirnadel-Farrant HA et al (2014) Age-related differences in reporting of drug-associated liver injury: data-mining of WHO Safety Report Database. Regul Toxicol Pharmacol 70(2):519–526. https://doi.org/10.1016/j.yrtph.2014.09.007
Zhu Y, Li YG, Wang JB et al (2015) Causes, features, and outcomes of drug-induced liver injury in 69 children from China. Gut Liver 9(4):525–533. https://doi.org/10.5009/gnl14184
Morgan RE, van Staden CJ, Chen Y et al (2013) A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol Sci 136(1):216–241. https://doi.org/10.1093/toxsci/kft176
Schmucker DL, Sanchez H (2011) Liver regeneration and aging: a current perspective. Curr Gerontol Geriatr Res 2011:526379. https://doi.org/10.1155/2011/526379
Sunil Kumar N, Remalayam B, Thomas V et al (2021) Outcomes and predictors of mortality in patients with drug-induced liver injury at a tertiary hospital in South India: a single-centre experience. J Clin Exp Hepatol 11(2):163–170. https://doi.org/10.1016/j.jceh.2020.08.008
Bjornsson ES (2021) Clinical management of patients with drug-induced liver injury (DILI). United Eur Gastroenterol J 9(7):781–786. https://doi.org/10.1002/ueg2.12113
Download references
This study was supported by the Key Laboratory of Children’s Disease Research in Guangxi’s Colleges and Universities, the Education Department of Guangxi Zhuang Autonomous Region and the Guangxi Clinical Research Center for Pediatric Disease (NO: AD22035219).
Author information
Authors and affiliations.
Department of Pediatrics, The First Affiliated Hospital of Guangxi Medical University, Difficult and Critical illness Center, Pediatric Clinical Medical Research Center of Guangxi, Nanning, Guangxi Zhuang Autonomous Region, China
Cai-Xia Feng, Wen-Yu Ye & Qing-Wen Shan
You can also search for this author in PubMed Google Scholar
Contributions
CX F and QW S designed the study; CX F and WY Y were involved in the development of retrieval strategies, data acquisition, and data analysis and wrote the paper; QW S participated in the critical revision of the manuscript. All authors approved the final manuscript.
Corresponding author
Correspondence to Qing-Wen Shan .
Ethics declarations
Ethics approval.
Not applicable.
Consent to participate
Consent for publication, competing interest.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Feng, CX., Ye, WY. & Shan, QW. Clinical and biochemical characteristics, and outcome in 33 patients with ceftriaxone-induced liver injury. Eur J Clin Pharmacol (2024). https://doi.org/10.1007/s00228-024-03701-w
Download citation
Received : 08 January 2024
Accepted : 10 May 2024
Published : 29 May 2024
DOI : https://doi.org/10.1007/s00228-024-03701-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ceftriaxone
- Drug-induced liver injury
- Literature analysis
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Rationale. Sickle cell anemia is a disorder of the red blood cells characterized by abnormally shaped red cells that block and damage blood vessels leading to oxygen deprivation, pain, anemia, serious infections, and damage to vital organs. I AM JUST PUTTING THE QUESTION AND ANSWER.
Case Outcome. The child goes back to school the next day, and her caregiver returns to work. Study with Quizlet and memorize flashcards containing terms like Meet the Client, The nurse meets with the child and her caregiver to discuss her health condition. The caregiver asks the nurse, "I have heard of sickle cell disease (SCD) and I know it ...
Anthony is 15 years old and has sickle cell. He was playing sports on a 90F day when he started to get pain in his knees. Eval by camp nurse and brought to ED. Sickle Cell Crisis. Sickle Cell diagnosed at birth. Has frequent pneumonia and many incidents of SC crisis. ##### Continue plan of care with education to help reduce chances of
Answer. Restart scheduled red blood cell transfusions; Explanation. The incidence of primary stroke in children with SCD is 0.6 to 0.8 events per 100 patient-years, with a cumulative incidence of 7.8 percent by age 14 years in the Jamaican cohort and 11 percent by age 20 years in the U.S. Cooperative Study of Sickle Cell Disease.
Sickle Cell, Case Study 27. Scenario. V. is a 29-year-old African American married man who has sickle cell disease (SCD) marked by frequent episodes of severe pain. His anemia has been managed with multiple transfusions. Six months ago, he started showing signs of chronic renal failure.
SIM Sickle Cell Unfolding Case Study. Course. Advanced Adult Health Care. 214 Documents. Students shared 214 documents in this course. University Keiser University. Academic year: 2020/2021. ... Anthony was diagnosed with sickle cell anemia at birth during a routine newborn screening. He was a term newborn with normal childhood illnesses.
On examination the child had mild splenomegaly. He had history of two prior hospital admissions. First at the age of 1 year, when he was diagnosed to have pneumonia and second, at the age of 3 years (3 months prior to coming to our institution) for fever, anemia and jaundice. He has had three transfusions till now, last transfusion was 3 months ...
Dactylitis, often referred to as hand-foot syndrome, is frequently the 1st manifestation of pain in children with sickle cell anemia, occurring in 50% of children by 2 years of age. [ 5] A 3.5-year-old girl from race of Arab reffered to Shafa Hospital with severe anemia, thrombocytopenia, leucocytosis and elevated ESR and LDH.
Sickle cell anemia is a genetic disease with severe symptoms, including pain and anemia. The disease is caused by a mutated version of the gene that helps make hemoglobin — a protein that carries oxygen in red blood cells. People with two copies of the sickle cell gene have the disease. People who carry only one copy of the sickle cell gene ...
Sickle cell disease (SCD) is a common group of life-threatening, genetic disorders caused by the synthesis of abnormal hemoglobin (sickle hemoglobin), which when deoxygenated, polymerizes and causes sickling of red blood cells. SCD is characterized by chronic hemolytic anemia, vasoocclusion, and progressive vascular injury causing multiorgan ...
Sickle Cell at the Molecular Level. In sickle cell anemia, there is a mutation in the gene that encodes the β chain of hemoglobin. Within this gene (located on Chromosome 11), ONE BASE in the DNA is replaced with another base, and this mutation causes the normal amino acid #6 to be replaced by another amino acid. 1.
Nurse Maggie works in a pediatric hematology unit and is caring for Marcus, a 9-year-old with a history of sickle cell disease who was admitted for a vaso-occlusive crisis, or VOC. After settling Marcus in his room, Nurse Maggie goes through the steps of the Clinical Judgment Measurement Model to make clinical decisions about Marcus' care by ...
Case Study-Sickle Cell Anemia. Mark is a 14-year-old patient diagnosed with Sickle Cell Anemia. He is the youngest of 3 children and lives with his mother and two older siblings. Additionally, his grandmother, aunt and 2 cousins live in the household. Mark recently returned from a football training camp held in the mountains.
Sickle cell disease (SCD) and its variants are genetic disorders resulting from the presence of a mutated form of hemoglobin, hemoglobin S (HbS) (see the image below). The most common form of SCD found in North America is homozygous HbS disease (HbSS), an autosomal recessive disorder first described by Herrick in 1910.
The case explores the initial presentation of sickle cell symptoms in a heterozygote, the assembly of a pedigree and calculation of genetic risk for transmission of the mutation, and the biochemical and genetic testing options that are available for diagnostic and preconception genetic testing in sickle cell disease. This case also covers the ...
Study with Quizlet and memorize flashcards containing terms like A younger school aged client is admitted to the pediatric unit with an exacerbation of Sickle cell disease (SCD). The client is accompanied by the caregiver., Which is the best initial response by the nurse to explain SCD to the clients caregiver?, How should the nurse respond? and more.
Through a case study approach, students learn about sickle cell anemia, a deadly recessive disease that remains prevalent in the human population because being a carrier of the disease confers resistance against malaria. Students explore the evolutionary trade-offs involved in this classic example of heterozygote advantage.
Nurse Maggie works in a pediatric hematology unit and is caring for Marcus, a 9-year-old with a history of sickle cell disease who was admitted for a vaso-occlusive crisis, or VOC. After settling Marcus in his room, Nurse Maggie goes through the steps of the Clinical Judgment Measurement Model to make clinical decisions about Marcus' care by ...
STEM CELL TRANSPLANT REPORT Visit: VISIT NBR EDT STAT seq. e: SEQ_NBR Visit Date: VISIT DT SUBJECT ID Letter Code: LETTER_CD 1 Checked for completeness and accuracy A Certification number B Signature C General Comments Keyer: KEY CERT MOD Status: Mcdified: Edited: EDT DT
Sickle cell anemia results from a point mutation at the HBB gene that codes for the beta- globin on chromosome 11. It is a point mutation in which glutamic acid is replaced by valine at the sixth position of the beta-globin chain, and the product formed is called hemoglobin S (HbS) instead of the normal hemoglobin A (HbA). In low oxygen levels, HbS precipitates, and red blood cells take on a ...
Sickle cell disease is a group of inherited red blood cell disorders that affect hemoglobin, the protein that carries oxygen through the body. Normally, red blood cells are disc-shaped and flexible enough to move easily through the blood vessels. In sickle cell disease, red blood cells become crescent- or "sickle"-shaped due to a genetic ...
DNA and Mutations. A case study of the effects of mutation: Sickle cell anemia. Sickle cell anemia is a genetic disease with severe symptoms, including pain and anemia. The disease is caused by a mutated version of the gene that helps make hemoglobin — a protein that carries oxygen in red blood cells. People with two copies of the sickle cell ...
In our study, one child with sickle cell anemia developed immune hemolytic anemia, progressive renal failure, and acute liver injury after using ceftriaxone, eventually leading to multiple organ failure and death . The mechanism of hemolysis in this case may involve the formation of immune complexes further damaging red blood cells.