- Search Menu
- Sign in through your institution
- Advance articles
- Author Guidelines
- Submission Site
- Open Access
- Self-Archiving Policy
- About Food Quality and Safety
- About Zhejiang University Press
- Editorial Board
- Outstanding Reviewers
- Advertising & Corporate Services
- Journals Career Network
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Introduction, antioxidant compounds in banana fruits, carotenoids, phenolic compounds, health benefits of bioactive components in banana fruits.
- < Previous
Bioactive compounds in banana fruits and their health benefits
- Article contents
- Figures & tables
- Supplementary Data
Jiwan S Sidhu, Tasleem A Zafar, Bioactive compounds in banana fruits and their health benefits, Food Quality and Safety , Volume 2, Issue 4, December 2018, Pages 183–188, https://doi.org/10.1093/fqsafe/fyy019
- Permissions Icon Permissions
Banana is an edible fruit and is herbaceous flowering plant belonging to the genus Musa and the family Musaceae . Banana is also eaten as cooked vegetable (and is then called plantains). All the edible banana fruits are seedless (parthenocarpic) and belong to two main species, Musa acuminata Colla and Musa balbisiana Colla. The hybrid from these two species Musa x paradisiaca L. is also available nowadays. Although banana is native to Indomalaya and Australia, Papua New Guinea was the first to domesticate this fruit. Banana has now spread to almost 135 countries around the world. As per 2016 data, nearly 28 per cent of the total world’s banana production comes from India and China. Cavendish group banana, being the main export item from the banana-exporting countries, usually refers to soft, sweet, and dessert banana in the Western countries, but the plantain bananas have firm, starchy fruit which is suitable for cooking as a vegetable. Banana is known to be rich not only in carbohydrates, dietary fibres, certain vitamins and minerals, but is also rich in many health-promoting bioactive phytochemicals. General composition including various bioactives and their health contributions has been reviewed in this paper.
The consumption of fruits and fruit products is known not only to promote general good health but also lower the risk of various chronic diseases, such as heart diseases, stroke, gastrointestinal disorders, certain types of cancer, hypertension, age-related macular degeneration, cataract of the eye, skin conditions, lowering of low-density lipoprotein (LDL) cholesterol, and improved immune function. To promote healthy eating lifestyle, the USDA recommends filling up half the plate with fruits and vegetables, because these provide a good amount of dietary fibres, certain vitamins ( e.g . ascorbic acid, folic acid, and vitamin A precursors), many minerals ( e.g . potassium, magnesium, iron, and calcium), and many other important phytochemicals having strong antioxidative properties. Fruits make an important part of the balanced diet adopted by the humans. USDA recommends daily five servings of fruits to obtain most of the health benefits. Depending upon their origin and production area temperature, fruits are classified into temperate fruits, sub-tropical fruits, and tropical fruits. Banana belongs to the tropical fruits as it grows more profusely in tropical rain forest areas. Interestingly, banana fruit has flesh not only rich in starch which changes into sugars on ripening but is also a good source of resistant starch. Banana is known to be rich in carbohydrates, dietary fibres, certain vitamins, and minerals ( Table 1 ). The presence of various bioactive phytochemicals and their nutritional significance has been discussed in this review paper ( Figure 1 ).
Chemical composition of banana fruit (as is basis per 100 g)
Adopted from: Wikipedia, Internet, USDA databases.

Banana tree and banana fruits of various maturities. (Source: Internet Wikipedia.)
The reactive oxygen species (ROS) and reactive nitrogen species (RON), such as hydroxyl radicals, superoxide ions, nitric oxide radicals, and singlet oxygen and hydrogen peroxide, have now been implicated in the causation of many disorders like arthritis, diabetes, arteriosclerosis, age-related macular degeneration, certain types of cancer, inflammation, genotoxicity, and Alzheimer disease. The exact mechanism is not known but the reaction of these ROS and RNS species with biomolecules such as lipids, proteins, and DNA may be the cause of these disease conditions ( Shukla et al ., 2009 ; Septembre-Malaterre et al ., 2016 ). Kandaswamy and Aradhya (2014) have shown the banana rhizome to be a rich source of many polyphenolic compounds having antioxidant activities. Pazmino-Duran et al. (2001) have suggested the use of anthocyanins from banana bracts (florets) as natural colourants. They identified various anthocyanins such as cyanidin-3-rutinoside (main one as 80 per cent of total pigments, being 32.3 mg/100 g) and 3-rutinoside derivatives of delphinidin, pelargonidin, peonidin, and malvidin. Interestingly, the addition of heat-treated onion extract was found to inhibit the polyphenol oxidase (PPO) during ripening of banana fruit at room temperature ( Lee, 2007 ). Even the Maillard reaction products (MRP) significantly affected the banana PPO activity. The phytochemistry and pharmacology of wild banana ( Musa acuminata Colla) have been reviewed by Mathew and Negi (2017) and they suggested the use of banana pulp and peel for the development of drugs and use in functional foods.
Not only banana pulp, but pseudo stem and banana fruit peel have been found to be the good sources of antioxidants ( Table 2 ). Aziz et al . (2011) have analysed the native banana pseudo-stem flour (NBPF) and tender core of pseudo-stem flour (TCBPF) for chemical and functional properties. They found higher content of polyphenols, flavonoids, total dietary fibre, insoluble dietary fibre, lignin, hemicellulose, cellulose, antioxidant capacity, and free-radical scavenging capacity in NBPF than TCBPF. In exhaustive reviews, Pereira and Maraschin (2015) and Singh et al . (2016) have reported that banana is rich in many bioactive compounds, such as carotenoids, flavonoids, phenolics, amines, vitamin C, and vitamin E having antioxidant activities to provide many human health benefits. Recently, Vu et al . (2018) have also reviewed the phenolic compounds and their potential health benefits coming from banana peel. They have suggested the use of this valuable by-product from banana fruit processing industry in food and pharmaceutical industry. Anyasi et al . (2018) have analysed the essential macro and trace minerals as well as phenolic compounds in unripe banana flour obtained from the pulp of four cultivars treated with ascorbic, citric, and lactic acids before drying in a forced air dryer at 70°C. Results of their liquid chromatography-mass spectrometry-electrospray ionization (LC-MS-ESI) assay of phenolics revealed the presence of two flavonoids, epicatechin and 3-O-rhmnosyl-glucoside in varying concentrations. Among the essential minerals, zinc had the lowest concentration of 3.55 mg/kg, but the potassium was the highest, 14746.73 mg/kg in these cultivars.
Antioxidant activity, total polyphenol, and individual polyphenolic compounds present in organic acid treated (20 g/l) unripe banana flour
Means with different letters across rows are significantly different at P < 0.05. Values are Means ± SE of triplicate measurements. DPPH, 1,1-diphenyl-2-picrylhydrazyl. (Adopted and modified from Anyasi et al ., 2018 .)
Carotenoids is a class of compounds having some 600 members in this family. Some of these are precursors for vitamin A, and others are known to have strong antioxidant capacity to scavenge ROS. Among the carotenoids present in banana fruit, α-carotene, β-carotene, and β-cryptoxanthin have provitamin A activity, but others like lycopene and lutein have a strong antioxidant capacity ( Erdman et al ., 1993 ). Lycopene is known to provide protection against prostate cancer among men, and lutein offers human health benefits to serve as an inhibitor of age-related macular degeneration ( Davey et al ., 2006 ). Later, Davey et al . (2009) have analysed 171 different genotypes of Musa spp. for provitamin A carotenoids and 47 genotypes for two minerals (iron and zinc). They found a great variability in provitamin A among the various cultivars, but a low variability in iron and zinc, irrespective of the soil type and growing environmental conditions. They suggested the use of high provitamin A and trace mineral cultivars as development strategies to improve the nutritional health and alleviation of micronutrient deficiencies among the Musa -consuming populations.
Yellow- and orange-fleshed banana cultivars are known to be richer in trans-β-carotene content ( Englberger et al ., 2006 ). Carotenoid content of some of the banana cultivars is presented in Table 3 . Consumption of fruits rich in carotenoids is reported to boost immunity and reduce the risk of various diseases, such as cancer, type II diabetes, and cardiovascular problems ( Krinsky and Johnson, 2005 ). Certain banana cultivars rich in provitamin A carotenoids can be grown and consumed by the poor population of the world that is having serious vitamin A deficiency, and the consumption of such banana fruit would alleviate vitamin A deficiency ( Fungo and Pillay, 2013 ).
Carotenoid content of different banana cultivars (µg/100 g)
Source: Adopted and modified from: Singh et al ., 2016 .
Phenolics present in banana fruit are the major bioactive compounds having antioxidant properties and are known for providing health benefits ( Table 4 ). Various phenolics present in banana have been identified as follows: gallic acid, catechin, epicatechin, tannins, and anthocyanins. Banana rhizome is used as food and for medicinal properties as well in South India as it is very rich in phenolics ( Kandasamy and Aradhya, 2014 ). Russel et al . (2009) have detected many phenolics in banana, such as ferulic, sinapic, salicylic, gallic, p-hydroxybenzoic, vanillic, syringic, gentisic, and p-coumaric acids as major components. However, ferulic acid content was the highest (69 per cent of cinnamic acids) among these phenolics. Banana peel is also a rich source of phenolic compounds. Tsamo et al . (2015) analysed banana pulp and peel from nine plantain cultivars and two dessert banana cultivars. According to their results, hydroxycinnamic derivatives, such as ferulic acid-hexoside, were the major ones (4.4–85.1 µg/g DW) in plantain pulp. They observed large variations in the phenolic contents among the cultivars tested. In the peel from plantain cultivars, rutin was the most abundant flavonol glycoside (242.2–618.7 µg/g DW). Thus, the banana peel and pulp both are good sources of health-promoting phenolic compounds. Among the flavonoids detected in banana are as follows: quercetin, myricetin, kaempferol, and cyanidin which provide health benefits mainly because they act as free radicals, ROS, and RNS scavengers ( Kevers et al ., 2007 ). Most of these phenolics are known to also exhibit antibacterial, antiviral, anti-inflammatory, antiallergenic, antithrombotic, and vasodilatory activities ( Cook and Sammon, 1996 ). Sulaiman et al . (2011) have determined the total phenolic and mineral contents in pulp and peel from eight banana ( Musa spp.) cultivars grown in Malaysia. With a few exceptions, the peel extracts had the higher total phenolics and total antioxidant activities than the pulp. Among minerals, potassium was the major element found in both the peel and pulp followed by phosphorus, magnesium, and sodium.
Uses and health benefits of bioactive compounds in banana
Health benefits of phenolics
A flavonoid, leucocyanidin, has been identified as a predominant component of aqueous extract of unripe banana pulp that showed significant anti-ulcerogenic activity ( Lewis et al ., 1999 ). Thus, many flavonoids, especially leucocyanidin analogues, may offer immense therapeutic potential in the treatment of gastric disease conditions.
The structure–activity relationship of flavonoids indicates that their antioxidant capacity, scavenging free radicals, and chelating action are related to the presence of functional groups in their nuclear structure ( Heim et al ., 2002 ). They also attributed most of the health benefits from the consumption of flavonoids to their antioxidant and chelating properties. Because of these properties, flavonoids are also shown to exhibit antimutagenic and antitumoral activities ( Rice-Evans et al ., 1996 ). The flavonoids can also inhibit many enzymes, such as oxygenases (prostaglandin synthase), required in the synthesis of eicosanoids. Thus, the flavonoids inhibit hyaluronidase activity and help in maintaining the proteoglycans of connective tissues. This would prevent the spread of bacterial or tumour metastases ( Havsteen, 2002 ). As the flavonoids get preferentially oxidized, they are reported to prevent the oxidation of body’s natural water-soluble antioxidants like ascorbic acid ( Korkina and Afanas’ev, 1997 ). Generally, after the consumption of banana fruit, the peel ends up as a feed for the animals only. The disposal of peel (pomace) and other by-products from banana-processing industry causes a serious environmental problem ( Zhang et al ., 2005 ). Banana peel is reported to be rich in many high-value health-promoting antioxidant phytochemicals, such as anthocyanins, delphinidin, and cyanidins ( Seymour, 1993 ). In a recent study, Rebello et al . (2014) have also shown the banana peel extract to be a rich source of total phenolics (29 mg/g as GAE), which are responsible for the very high antioxidant activity. They also determined various other antioxidant compounds, namely, highly polymerized prodelphinidins (~3952 mg/kg), flavonol glycosides (mainly 3-rutinosides and predominantly quercetin-based compounds, ~129 mg/kg), B-type procyanidin dimers, and monomeric flavan-3-ols (~126 mg/kg).
Health benefits of biogenic amines
Banana peel and pulp are known to be good sources of certain biogenic amines (catecholamines) which are produced by the decarboxylation of amino acids or by the amination of aldehydes and ketones. Catecholamines include dopamine, serotonin, epinephrine, and norepinephrine and are reported to occur in many plants in considerable amounts ( Ponchet et al ., 1982 ). In animals, these biogenic amines are reported to work as neurotransmitters for the hormonal regulation of glycogen metabolism ( Kimura, 1968 ). When banana is consumed by humans, serotonin present in the pulp (ranging from 8 to 50 µg/g) creates a feeling of well-being and happiness. Banana contains a large amount of dopamine and norepinephrine ( Buckley, 1961 ). Waalkes et al . (1958) were the first to report the amount of various catecholamines in banana pulp as follows: serotonin, 28 µg/g; norepinephrine, 1.9 µg/g; and dopamine, 7.9 µg/g. The concentrations of dopamine in the pulp of yellow banana ( M. acuminata ), red banana ( Musa sapientum ), and plantain has been reported to be 42, 54, and 5.5 µg/g, respectively ( Feldman et al ., 1987 ). They highlighted the role of dopamine in human brain and body as a neurotransmitter having a strong influence on mood and emotional stability. Dopamine in the peel and pulp of commercially ripened Musa Cavendish is reported to range from 80 to 560 mg/100 g, and 2.5 to 10 mg/100 g, respectively ( Kanazawa and Sakakhibara, 2000 ). Tryptophan being one of the precursors for the synthesis of dopamine, the presence of this amino acid in banana peel increases the interest in possibilities of preventing neurodegenerative diseases like Parkinson’s using this by-product of food-processing industry by developing pharmaceutical formulations. However, the increase in dopamine content from unripe to the ripened stage in both the peel and pulp has been reported by many workers ( Romphophak et al ., 2005 ; Gonzalez-Montelongo et al ., 2010 ). They also suggested that the decline in dopamine concentration during over-ripening stage may be due to its oxidation to quinones which may further polymerize to melanin pigments.
Using peroxide value and thiobarbituric activity determination, the antioxidant compounds present in water extract of banana peel have shown to suppress the autooxidation of linoleic acid by 65 to 70 per cent after 5 days of incubation ( Kanazawa and Sakakhibara, 2000 ). When they compared dopamine with other natural antioxidants, such as ascorbic acid, reduced glutathione, and phenolic acids ( e.g. gallocatechin gallate), the dopamine showed higher antioxidant activity in vitro (DPPH assay). Gonzalez-Montelongo et al . (2010) have reported the banana peel extracts to be rich in dopamine, L-dopa, and catecholamines with a significant antioxidant capacity. They found no significant difference in the antioxidant activity in the banana peel extracts from different cultivars. The biogenic amines are also shown to play an important role in offering plants’ resistance to various invading pathogens through their interaction with phytohormones ( via auxin oxidation), thus affecting the growth and development of plants ( Newman et al ., 2001 ; Roepenack-Lahaye et al ., 2003 ).
Health benefits of phytosterols
These naturally occurring plant sterols have attracted the attention of food manufacturers to produce functional foods having higher health benefits. Because of their structural similarity with cholesterol, they compete with cholesterol for absorption in the gut, thus lowering the blood cholesterol levels ( Marangoni and Poli, 2010 ). They reported that a daily intake of 3 g of phytosterols results in marked reduction of LDL cholesterol levels. Various phytosterols reported in the banana peel are stigmasterol, -sitosterol, campesterol, 24-methylene cycloartenol, cycloeucalenol, and cycloartenol ( Knapp and Nicholas, 1969 ). Now the health professionals recommend the consumption of plant sterols–rich diet to lower the LDL cholesterol in patients who do not tolerate cholesterol-lowering statin drugs ( Ostlund et al ., 2003 ). Banana fruit has been shown to contain a good amount of phytosterols both in the peel and pulp ( Akihisa et al ., 1986 ). The phytosterols content in unripe banana in the range of 2.8 to 12.4 g·kg DW has been reported by Vilaverde et al. (2013) . According to their results, the Musa balbisiana cultivars, such as ‘Dwarf Red’ and ‘Silver’, had higher amounts of phytosterols than the M. acuminata cultivars. The lipophilic extract of ripe banana pulp from several cultivars of the M. acuminata and M. balbisiana species has been found to be a source of ω-3 and ω-6 fatty acids, phytosterols, long-chain aliphatic alcohols, and α-tocopherol, thus offering well-established nutritional and health benefits ( Vilela et al ., 2014 ).
The above discussion brings out the importance of consuming banana fruits for obtaining various health benefits. It is not only the banana fruit pulp, but also the peel of this fruit is known to contain many important phytochemicals and offers many health benefits. More research is needed to be carried out to find ways of using banana fruit peel in the development of many new functional foods.
Akihisa , T. , Shimizu , N. , Tamura , T. , Matsumoto , T . ( 1986 ). (24S)-14a, 24- Dimethyl-9b, 19-cyclo-5a-cholest-25-en-3b-ol: a new sterol and other sterols in Musa sapientum . Lipids , 21 : 494 – 497 .
Google Scholar
Anyasi , T. A. , Jideani , A. I. O. , Mchau , G. R. A . ( 2018 ). Phenolics and essential mineral profile of organic acid pretreated unripe banana flour . Food Research International (Ottawa, Ont.) , 104 : 100 – 109 .
Arora , A. , Choudhary , D. , Agarwal , G. , Singh , V. P . ( 2008 ). Compositional variation in b-carotene content, carbohydrate and antioxidant enzymes in selected banana cultivars . International Journal of Food Science and Technology , 43 : 1913 – 1921 .
Aziz , N. A. A. , Ho , L. H. , Azahari , B. , Bhat , R. , Cheng , L. H. , Ibrahim , M. N. M . ( 2011 ). Chemical and functional properties of the native banana ( Musa acuminate x balbisiana Colla cv. Awak) pseudo-stem and pseudo-stem tender core flours . Food Chemistry , 128 : 748 – 753 .
Beatrice , E. , Deborah , N. , Guy , B . ( 2015 ). Provitamin A carotenoid content of unripe and ripe banana cultivars for potential adoption in eastern . African Journal of Food Composition and Analysis , 43 : 1 – 6 .
Buckley , E. H . ( 1961 ). Further studies on the biosynthesis of 3-hydroxytyramine in the peel of the banana . Plant Physiology , 36 : 315 – 320 .
Choudhary , S. P. , Tran , L. S . ( 2011 ). Phytosterols: perspectives in human nutrition and clinical therapy . Current Medicinal Chemistry , 18 : 4557 – 4567 .
Cook , N. C. , Sammon , S . ( 1996 ). Flavanoids chemistry, metabolism, cardioprotective effects, and dietary sources . Nutritional Biochemistry , 7 : 66 – 76 .
Davey , M. W. , Bergh , I. V. , Markham , R. , Swennen , R. , Keulemans , J . ( 2009 ). Genetic variability in Musa fruit provitamin A carotenoids, lutein and mineral micronutrient contents . Food Chemistry , 115 : 806 – 813 .
Davey , M. W. , Keulemans , J. , Swennen , R . ( 2006 ). Methods for the efficient quantification of fruit provitamin A contents . Journal of Chromatography. A , 1136 : 176 – 184 .
DeLorenze , G. N. , et al. ( 2010 ). Daily intake of antioxidants in relation to survival among adult patients diagnosed with malignant glioma . BMC Cancer , 10 : 215 .
Englberger , L. , Wills , R. B. , Blades , B. , Dufficy , L. , Daniells , J. W. , Coyne , T . ( 2006 ). Carotenoid content and flesh color of selected banana cultivars growing in Australia . Food and Nutrition Bulletin , 27 : 281 – 291 .
Erdman , J. W. Jr , Bierer , T. L. , Gugger , E. T . ( 1993 ). Absorption and transport of carotenoids . Annals of the New York Academy of Sciences , 691 : 76 – 85 .
Feldman , J. M. , Lee , E. M. , Castleberry , C. A . ( 1987 ). Catecholamine and serotonin content of foods: effect on urinary excretion of homovanillic and 5-hydroxyindoleacetic acid . Journal of the American Dietetic Association , 87 : 1031 – 1035 .
Ferguson , L. R. , Zhu , S. T. , Harris , P. J . ( 2005 ). Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells . Molecular Nutrition & Food Research , 49 : 585 – 593 .
Fungo , R. , Pillay , M . ( 2013 ). β-Carotene content of selected banana genotypes from Uganda . African Journal of Biotechnology , 10 : 5423 – 5430 .
Garbe , D. ( 2000 ). Cinnamic Acid . Ullmann’s Encyclopedia of Industrial Chemistry . Wiley-VCH Verlag GmbH & Co, KGaA .
Google Preview
González-Montelongo , R. , Lobo , M. G. , González , M . ( 2010 ). Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds . Food Chemistry , 119 : 1030 – 1039 .
Havsteen , B. H . ( 2002 ). The biochemistry and medical significance of the flavonoids . Pharmacology & Therapeutics , 96 : 67 – 202 .
Heim , K. E. , Tagliaferro , A. R. , Bobilya , D. J . ( 2002 ). Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships . The Journal of Nutritional Biochemistry , 13 : 572 – 584 .
Ikeda , I. , et al. ( 2003 ). Heat-epimerized tea catechins rich in gallocatechin gallate and catechin gallate are more effective to inhibit cholesterol absorption than tea catechins rich in epigallocatechin gallate and epicatechin gallate . Journal of Agricultural and Food Chemistry , 51 : 7303 – 7307 .
Kanazawa , K. , Sakakibara , H . ( 2000 ). High content of dopamine, a strong antioxidant, in Cavendish banana . Journal of Agricultural and Food Chemistry , 48 : 844 – 848 .
Kandasamy , S. , Aradhya , S. M . ( 2014 ). Polyphenolic profile and antioxidant properties of rhizome of commercial banana cultivars grown in India . Food Bioscience , 8 : 22 – 32 .
Kevers , C. , Falkowski , M. , Tabart , J. , Defraigne , J. O. , Dommes , J. , Pincemail , J. ( 2007 ). Evolution of antioxidant capacity during storage of selected fruits and vegetables . Journal of Agricultural and Food Chemistry , 55 : 8596 – 8603 .
Kimura , M . ( 1968 ). Fluorescence histochemical study on serotonin and catecholamine in some plants . Japanese Journal of Pharmacology , 18 : 162 – 168 .
Knapp , F. F. , Nicholas , H. J . ( 1969 ). The sterols and triterpenes of banana peel . Phytochemistry , 8 : 207 – 214 .
Korkina , L. G. , Afanas’ev , I. B . ( 1997 ). Antioxidant and chelating properties of flavonoids . Advances in Pharmacology (San Diego, Calif.) , 38 : 151 – 163 .
Krinsky , N. I. , Johnson , E. J . ( 2005 ). Carotenoid actions and their relation to health and disease . Molecular Aspects of Medicine , 26 : 459 – 516 .
Kuklin , A. I. , Conger , B. V . ( 1995 ). Catecholamines in plants . Journal of the Plant Growth Regulation , 14 : 91 – 97 .
Kumar , N. , Pruthi , V . ( 2014 ). Potential applications of ferulic acid from natural sources . Biotechnology Reports (Amsterdam, Netherlands) , 4 : 86 – 93 .
Lee , M. K . ( 2007 ). Inhibitory effect of banana polyphenol oxidase during ripening of banana by onion extract and Maillard reaction products . Food Chemistry , 102 : 146 – 149 .
Lewis , D. A. , Fields , W. N. , Shaw , G. P . ( 1999 ). A natural flavonoid present in unripe plantain banana pulp ( Musa sapientum L. Var. paradisiaca ) protects the gastric mucosa from aspirin-induced erosions . Journal of Ethnopharmacology , 65 : 283 – 288 .
Li , C. , Ford , E. S. , Zhao , G. , Balluz , L. S. , Giles , W. H. , Liu , S . ( 2011 ). Serum α-carotene concentrations and risk of death among US adults: the third national health and nutrition examination survey follow-up study . Archives of Internal Medicine , 171 : 507 – 515 .
Marangoni , F. , Poli , A . ( 2010 ). Phytosterols and cardiovascular health . Pharmacological Research , 61 : 193 – 199 .
Mathew , N. S. , Negi , P. S . ( 2017 ). Traditional uses, phytochemistry and pharmacology of wild banana ( Musa acuminata Colla): a review . Journal of Ethnopharmacology , 196 : 124 – 140 .
Newman , M. A. , von Roepenack-Lahaye , E. , Parr , A. , Daniels , M. J. , Dow , J. M . ( 2001 ). Induction of hydroxycinnamoyl-tyramine conjugates in pepper by xanthomonas campestris , a plant defense response activated by hrp gene-dependent and hrp gene-independent mechanisms . Molecular Plant-Microbe Interactions , 14 : 785 – 792 .
Oliveira , T. Í. , et al. ( 2016 ). Optimization of pectin extraction from banana peels with citric acid by using response surface methodology . Food Chemistry , 198 : 113 – 118 .
Ostlund , R. E. Jr , Racette , S. B. , Stenson , W. F . ( 2003 ). Inhibition of cholesterol absorption by phytosterol-replete wheat germ compared with phytosterol-depleted wheat germ . The American Journal of Clinical Nutrition , 77 : 1385 – 1389 .
Pazmino-Duran , E. A. , Giusti , M. M. , Wrolstad , R. E. , Gloria , M. B. A . ( 2001 ). Anthocyanins from banana bracts ( Musa x paradisiaca ) as potential food colorants . Food Chemistry , 73 : 327 – 332 .
Pereira , A. , Maraschin , M . ( 2015 ). Banana ( musa spp) from peel to pulp: ethnopharmacology, source of bioactive compounds and its relevance for human health . Journal of Ethnopharmacology , 160 : 149 – 163 .
Perez-Vizcaino , F. , Duarte , J . ( 2010 ). Flavonols and cardiovascular disease . Molecular Aspects of Medicine , 31 : 478 – 494 .
Ponchet , M. , Martin-Tanguy , J. , Marais , A. , Martin , C . ( 1982 ). Hydroxycinnamoyl acid amides and aromatic amines in the inflorescences of some Araceae species . Phytochemistry , 21 : 2865 – 2869 .
Rasool , M. K. , et al. ( 2010 ). Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice . The Journal of Pharmacy and Pharmacology , 62 : 638 – 643 .
Rebello , L. P. G. , Ramos , A. M. , Pertuzatti , P. B. , Barcia , M. T. , Castillo-Munoz , N. , Hermosin- Gutierrez , I . ( 2014 ). Flour of banana ( Musa AAA) peel as a source of antioxidant phenolic compounds . Food Research International , 55 : 397 – 403 .
Rice-Evans , C. A. , Miller , N. J. , Paganga , G . ( 1996 ). Structure-antioxidant activity relationships of flavonoids and phenolic acids . Free Radical Biology & Medicine , 20 : 933 – 956 .
Romphophak , T. , Siriphanich , J. , Ueda , Y. , Abe , K. , Chachin , K. ( 2005 ). Changes in concentrations of phenolic compounds and polyphenol oxidase activity in banana peel during storage . Food Preservation Science , 31 : 111 – 115 .
Russell , W. R. , Labat , A. , Scobbie , L. , Duncan , G. J. , Duthie , G. G. ( 2009 ). Phenolic acid content of fruits commonly consumed and locally produced in Scotland . Food Chemistry , 115 : 100 – 104 .
Von Roepenack-Lahaye , E. , et al. ( 2003 ). P-coumaroylnoradrenaline, a novel plant metabolite implicated in tomato defense against pathogens . The Journal of Biological Chemistry , 278 : 43373 – 43383 .
Septembre-Malaterre , A. , Stanislas , G. , Douraguia , E. , Gonthier , M. P . ( 2016 ). Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in réunion french island . Food Chemistry , 212 : 225 – 233 .
Seymour , G. B . ( 1993 ). Banana . In: Seymour , J. E. , Tucker , G. A . (eds.) Biochemistry of Fruit Ripening . Chapman and Hall , NY , pp. 83 – 106 .
Shukla , S. , Mehta , A. , John , J. , Singh , S. , Mehta , P. , Vyas , S. P . ( 2009 ). Antioxidant activity and total phenolic content of ethanolic extract of caesalpinia bonducella seeds . Food and Chemical Toxicology , 47 : 1848 – 1851 .
Siang , S. T . ( 1983 ). Use of combined traditional Chinese and Western medicine in the management of burns . Panminerva medica , 25 : 197 – 202 .
Singh , B. , Singh , J. P. , Kaur , A. , Singh , N . ( 2016 ). Bioactive compounds in banana and their associated health benefits – A review . Food Chemistry , 206 : 1 – 11 .
Sulaiman , S. F. , Yusoff , N. A. M. , Eldeen , I. M. , Seow , E. M. , Sajak , A. A. B. , Supriatno , Ooi , K. L . ( 2011 ). Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas ( Musa sp.) . Journal of Food Composition and Analysis , 24 : 1 – 10 .
Passo Tsamo , C. V. , et al. ( 2015 ). Phenolic profiling in the pulp and peel of nine plantain cultivars ( musa sp.) . Food Chemistry , 167 : 197 – 204 .
Vilaverde , J. J. , et al. ( 2013 ). High valuable compounds from the unripe peel of several Musa species cultivated in Madeira Island (Portugal) . Industrial Crops Production , 42 : 507 – 512 .
Vilela , C. , et al. ( 2014 ). Lipophilic phytochemicals from banana fruits of several musa species . Food Chemistry , 162 : 247 – 252 .
Vu , H. T. , Scarlett , C. J. , Vuong , Q. V . ( 2018 ). Phenolic compounds within banana peel and their potential uses: a review . Journal of Functional Foods , 40 : 238 – 248 .
Waalkes , T. P. , Sjoerdsma , A. , Creveling , C. R. , Weissbach , H. , Udenfriend , S . ( 1958 ). Serotonin, norepinephrine, and related compounds in bananas . Science (New York, N.Y.) , 127 : 648 – 650 .
Williamson , G. , Manach , C . ( 2005 ). Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies . The American Journal of Clinical Nutrition , 81 : 243S – 255S .
Wilt , T. , Ishani , A. , MacDonald , R. , Stark , G. , Mulrow , C. , Lau , J . ( 1999 ). Betasitosterols for benign prostatic hyperplasia . Cochrane Database Systematic Reviews , 3 .
Yin , X. , Quan , J. , Kanazawa , T. ( 2008 ). Banana prevents plasma oxidative stress in healthy individuals . Plant Foods for Human Nutrition , 63 : 71 – 76 .
Young , S. N . ( 2007 ). How to increase serotonin in the human brain without drugs . Journal of Psychiatry & Neuroscience: JPN , 32 : 394 – 399 .
Zhang , P. , Whistler , R. L. , BeMiller , J. N. , Hamaker , B. R . ( 2005 ). Banana starch: production, physicochemical properties, and digestibility—a review . Carbohydrate Polymers , 59 : 443 – 458 .
Email alerts
Citing articles via.
- Advertising and Corporate Services
Affiliations
- Online ISSN 2399-1402
- Print ISSN 2399-1399
- Copyright © 2024 Zhejiang University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
ORIGINAL RESEARCH article
Functional characterisation of banana ( musa spp.) 2-oxoglutarate-dependent dioxygenases involved in flavonoid biosynthesis.

- 1 Genetics and Genomics of Plants, Faculty of Biology, Bielefeld University, Bielefeld, Germany
- 2 Fondazione Edmund Mach, Research and Innovation Centre, San Michele All’ Adige, Italy
Bananas ( Musa ) are non-grass, monocotyledonous, perennial plants that are well known for their edible fruits. Their cultivation provides food security and employment opportunities in many countries. Banana fruits contain high levels of minerals and phytochemicals, including flavonoids, which are beneficial for human nutrition. To broaden the knowledge on flavonoid biosynthesis in this major crop plant, we aimed to identify and functionally characterise selected structural genes encoding 2-oxoglutarate-dependent dioxygenases, involved in the formation of the flavonoid aglycon. Musa candidates genes predicted to encode flavanone 3-hydroxylase (F3H), flavonol synthase (FLS) and anthocyanidin synthase (ANS) were assayed. Enzymatic functionalities of the recombinant proteins were confirmed in vivo using bioconversion assays. Moreover, transgenic analyses in corresponding Arabidopsis thaliana mutants showed that MusaF3H , MusaFLS and MusaANS were able to complement the respective loss-of-function phenotypes, thus verifying functionality of the enzymes in planta . Knowledge gained from this work provides a new aspect for further research towards genetic engineering of flavonoid biosynthesis in banana fruits to increase their antioxidant activity and nutritional value.
Introduction
Banana ( Musa spp.) plants are well known for their edible fruit and serve as a staple food crop in Africa, Central and South America ( Arias et al., 2003 ). With more than 112 million tons produced in 2016, bananas are among the most popular fruits in the world and provide many employment opportunities ( FAO, 2019 ). Furthermore, banana fruits are rich in health promoting minerals and phytochemicals, including flavonoids, a class of plant specialised metabolites, which contribute to the beneficial effects through their antioxidant characteristics ( Forster et al., 2003 ; Wall, 2006 ; Singh et al., 2016 ). Flavonoid molecules share a C6-C3-C6 aglycon core, which can be reorganised or modified, e.g. by oxidation or glycosylation ( Tanaka et al., 2008 ; Le Roy et al., 2016 ). Modifications at the central ring structure divide flavonoids into 10 major subgroups (i.e. chalcones, aurones, flavanones, flavones, isoflavones, dihydroflavonols, flavonols, leucoanthocyanidins, anthocyanidins and flavan-3-ols). The diversity in chemical structure is closely related to diverse bioactivities of flavonoids in plant biology and human nutrition ( Falcone Ferreyra et al., 2012 ). For example, anthocyanins are in many cases well known to colour flowers and fruits to attract animals and thus promoting pollination and dispersion of seeds ( Ishikura and Yoshitama, 1984 ; Gronquist et al., 2001 ; Grotewold, 2006 ). Flavonols can interact with anthocyanins to modify the colour of fruits ( Andersen and Jordheim, 2010 ) and play a prominent role in protection against UV-B irradiation ( Li et al., 1993 ) and also in plant fertility ( Mo et al., 1992 ).
Many researchers have attributed positive effects on human health to flavonoids: e.g. antigenotoxic ( Dauer et al., 2003 ), anticarcinogenic and antioxidative ( Kandil et al., 2002 ) effects, as well as the prevention of cardiovascular diseases has been suggested (summarised in Perez-Vizcaino and Duarte, 2010 ). Additionally, Sun et al. (2019) suggested an involvement of flavonoids in the plant defence against the tropical race 4 (TR4) of the Musa Fusarium wilt (commonly known as ‘panama disease’) pathogen Fusarium oxysporum f. sp. cubense (Foc), which is a threat to the global banana production. It is certainly interesting to take a closer look at the biosynthesis of flavonoids in Musa .
Flavonoids are derived from the amino acid L-phenylalanine and malonyl-coenzyme A. Their biosynthesis ( Figure 1 ) has been analysed in many different species including the dicotyledonous model plant Arabidopsis thaliana ( Hahlbrock and Scheel, 1989 ; Lepiniec et al., 2006 ) and the monocotyledonous crop plants Zea mays (summarised in Tohge et al., 2017 ) and Oryza sativa (summarised in Goufo and Trindade, 2014 ). The first committed step, catalysed by the enzyme chalcone synthase (CHS), is the formation of naringenin chalcone from p -coumaroyl CoA and malonyl CoA ( Kreuzaler and Hahlbrock, 1972 ). A heterocyclic ring is introduced during the formation of naringenin (a flavanone) from naringenin chalcone, which can occur spontaneously or catalysed by chalcone isomerase (CHI; Bednar and Hadcock, 1988 ). The enzyme flavanone 3-hydroxylase (F3H or FHT) converts flavanones to dihydroflavonols by hydroxylation at the C-3 position ( Forkmann et al., 1980 ). Alternatively, flavanones can be converted to flavones by flavone synthase I or II (FNSI or II; Britsch, 1990 ). Flavonol synthase (FLS) catalyses the conversion of dihydroflavonols to the corresponding flavonols by introducing a double bond between C-2 and C-3 ( Forkmann et al., 1986 ; Holton et al., 1993 ). Moreover, dihydroflavonols can be converted to leucoanthocyanidins by dihydroflavonol 4-reductase (DFR; Heller et al., 1985 ), which competes with FLS for substrates ( Luo et al., 2016 ). Anthocyanidin synthase (ANS; also termed leucoanthocyanidin dioxygenase, LDOX) converts leucoanthocyanidins to anthocyanidins ( Saito et al., 1999 ).
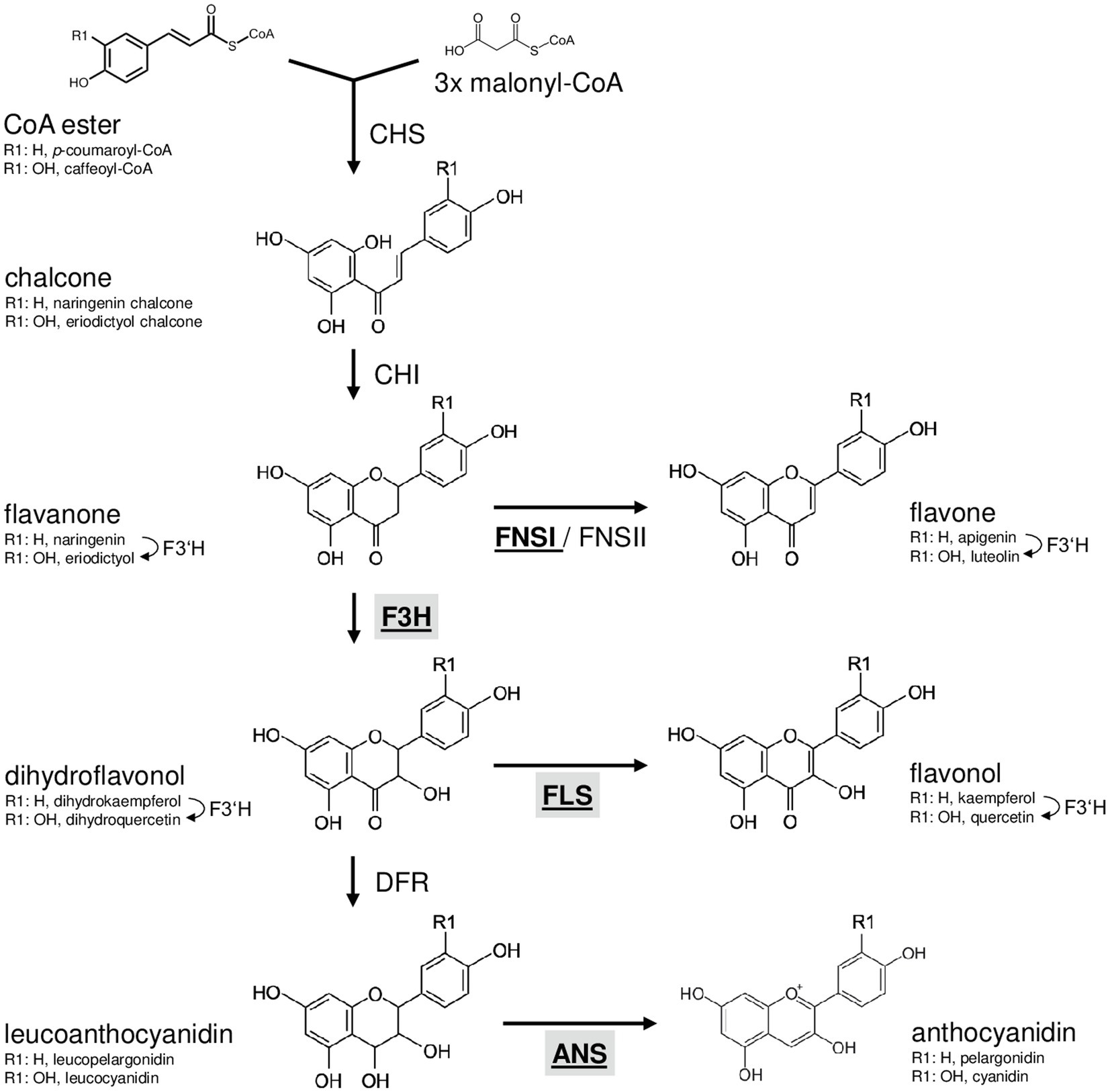
Figure 1 . Schematic, simplified illustration of the core flavonoid aglycon biosynthesis pathway in plants. Chalcone synthase, the first committed enzyme in flavonoid biosynthesis, connects a CoA ester and malonyl CoA, forming a chalcone. Chalcone isomerase introduces the heterocyclic ring. The resulting flavanone is converted to dihydroflavonol by flavanone 3-hydroxylase (F3H) or to flavones by flavone synthase (FNSI or FNSII). Dihydroflavonols are converted to flavonols by flavonol synthase (FLS). Alternatively, dihydroflavonol 4-reductase can reduce dihydroflavonols to the corresponding leucoanthocyanidin, which is converted to anthocyanidin by the activity of anthocyanidin synthase (ANS). Flavonoid 3' hydroxylase hydroxylates 3' position of the B-ring using different flavonoid substrates. 2-oxoglutarate-dependant oxygenases are given in bold underlined. Enzymes in the focus of this work are highlighted by grey boxes.
The flavonoid biosynthesis enzymes belong to different functional classes (summarised in Winkel, 2006 ): polyketide synthases (e.g. CHS), 2-oxoglutarate-dependent dioxygenases (2-ODD; e.g. F3H, ANS, FLS and FNSI), short-chain dehydrogenases/reductases (e.g. DFR), aldo-keto reductases (e.g. chalcone reductase, CHR) and cytochrome P450 monooxygenases (e.g. FNSII and F3'H). Flavonoid biosynthesis is evolutionary old in plants ( Wen et al., 2020 ) and, although differences exist, quite similar in dicots like A. thaliana and monocots-like Musa .
In the present study, we focus on the functional class of flavonoid aglycon forming enzymes, namely, the 2-ODDs. 2-ODDs occur throughout the kingdom of life and play important roles in many biological processes including oxygen sensing and DNA repair ( Jaakkola et al., 2001 ; Trewick et al., 2002 ). They are a class of non-heme iron-containing enzymes, which require 2-oxoglutarate, Fe 2+ and ascorbate for substrate conversion (summarised in Prescott and John, 1996 ).
Plant 2-ODDs share conserved amino acid residues which coordinate ferrous iron binding (HxDxnH) and binding of 2-oxoglutarate (RxS; Cheng et al., 2014 ). They can be divided into three distinct evolutionary classes, DOXA, DOXB and DOXC, based on amino acid similarity ( Kawai et al., 2014 ). The 2-ODDs involved in specialised metabolism were classified into the DOXC class. Yet, all flavonoid biosynthesis related 2-ODDs were found in the DOXC subclades 28 (F3H and FNSI) or 47 (FLS and ANS; Kawai et al., 2014 ). In some cases, the high amino acid similarity between the different enzymes leads to overlapping functions. For example, FLSs from Ginkgo biloba ( Xu et al., 2012 ) and O. sativa ( Park et al., 2019 ) can accept flavanones and dihydroflavonols as substrates. Also, ANSs from A. thaliana ( Stracke et al., 2009 ) and Malus domestica ( Yan et al., 2005 ) can catalyse the formation of flavonol glycosides.
Based on the Musa acuminata genome sequence annotation ( DHont et al., 2012 ; Pandey et al., 2016 ) identified 28 putative Musa flavonoid biosynthesis enzymes. This includes seven 2-ODD-type enzymes, namely, two F3H, four FLS and one ANS, while no suitable FNSI candidate was identified. While the expression of the respective genes and correlations of expression to flavonoid metabolite accumulation have been studied, the functionality of these enzymes has not been analysed until now.
Here, we describe the sequence-based and functional characterisation of 2-ODDs from the non-grass monocot Musa . Functionalities of recombinant Musa enzymes were analysed using in vivo bioconversion assays and by in planta complementation of corresponding A. thaliana loss-of-function mutants. This resulted in the experimental confirmation that the Musa flavonoid 2-ODDs studied have the predicted functions. The presented results contribute to the understanding of flavonoid biosynthesis in Musa . They provide a strong basis for further research to enhance the efficiency of flavonoid production in banana, in order to increase the fruits’ health promoting effects.
Materials and Methods
Plant material.
Banana plants (Grand Naine, plantain) for RNA extraction were grown in the field in Lucknow, India. Musa gene annotation identifiers refer to the study of Martin et al. (2016) . Columbia-0 (Col-0, NASC ID N1092) and Nössen-0 (Nö-0, NASC ID N3081) were used as wild-type controls. The A. thaliana mutants tt6-2 ( f3h , GK-292E08, Col-0 background; Appelhagen et al., 2014 ) and ans/fls1-2 (synonym ldox/fls1-2, ldox: SALK_028793 , Col-0 background; fls1-2: RIKEN_PST16145, Nö-0 background; Stracke et al., 2009 ) were used for complementation experiments.
Phylogenetic Analysis
Multiple protein sequence alignments were created with MAFFT v7 ( Katoh and Standley, 2013 ) using default settings. The approximately maximum-likelihood phylogenetic tree of 33 plant 2-ODDs with proven F3H, FNSI, FLS and ANS functionality ( Supplementary Table S1 ) and seven Musa 2-ODDs was constructed as described by Pucker et al. (2020b) : MAFFT alignments were cleaned with pxclsq ( Brown et al., 2017 ) and the tree was constructed with FastTree v2.1.10 using the WAG+CAT model ( Price et al., 2010 ). The tree was visualised with interactive tree of life ( Letunic and Bork, 2019 ), branch lengths were neglected.
Expression Analysis
Expression data for Musa 2-ODD genes were extracted from previous analyses ( Pucker et al., 2020a ). Short Read Archive IDs can be found in Supplementary Table S2 .
Total RNA Extraction, cDNA Synthesis and Molecular Cloning
Isolation of RNA from different Musa plant organs (leaf, pseudostem, bract, fruit peel and fruit pulp) was performed according to a protocol from Asif et al. (2000) . cDNA synthesis was performed from 1 μg total RNA using the ProtoScript ® First Strand cDNA Synthesis Kit [New England Biolabs, (NEB)] with the provided random primer mix according to the suppliers’ instructions. Amplification of predicted full-length CDSs ( Pandey et al., 2016 ) were done using Q5 ® High-Fidelity DNA polymerase (NEB) and gene-specific primers ( Supplementary Table S3 ) according to standard protocols. Creation of full-length coding sequence constructs (CDS) was performed using the GATEWAY ® Technology (Invitrogen). MusaF3H1 (Ma02_t04650), MusaF3H2 (Ma07_t17200), MusaFLS1 (Ma03_t06970), MusaFLS3 (Ma10_t25110) and MusaANS (Ma05_t03850) CDSs were successfully amplified on different cDNA pools. The resulting PCR products were recombined into pDONR ™ /Zeo (Invitrogen) with BP clonase (Invitrogen) resulting in Entry plasmids, which were sequenced by Sanger technology ( Sanger et al., 1977 ) on 3730XL sequencers using BigDye terminator v3.1 chemistry (Thermo Fisher). Entry plasmids for AtF3H , AtFLS1 and AtMYB12 were available from previous studies ( Preuss et al., 2009 ; Stracke et al., 2017 ), Petroselinum crispum FNSI ( PcFNSI ) was amplified on a plasmid from a previous study ( Martens et al., 2003 ). The full-length CDSs were introduced from the Entry plasmids into the inducible Escherichia coli expression vector pDEST17 (Invitrogen) and the binary expression vector pLEELA ( Jakoby et al., 2004 ) using GATEWAY LR reaction (Invitrogen).
Heterologous Expression in E. coli
pDEST17-based plasmids containing proT7 -RBS-6xHis-CDS- T7term expression cassettes ( proT7: T7 promoter , RBS: ribosome-binding site, 6xHis: polyhistidine tag and T7term: T7 transcription terminator ) were transformed into BL21-AI cells (Invitrogen). Cultures were grown in LB to an OD 600 of about 0.4 and expression was induced with 0.2% L-arabinose.
F3H and FLS Bioconversion Assay in E. coli
The enzyme assay was performed using 20 ml E. coli cultures expressing the respective constructs right after induction with L-arabinose. 100 μl substrate [10 mg/ml naringenin, eriodictyol or dihydroquercetin (DHQ)], 50 μl 2-oxoglutaric acid, 50 μl FeSo 4 and 50 μl 1 M sodium ascorbate were added. The cultures were incubated at 28°C overnight. To extract flavonoids, 1 ml was removed from each culture and mixed with 200 μl ethyl acetate by vortexing for 30 s. Samples were taken after 0 h, 1 h, 2 h, 3 h, 4 h and 24 h. After centrifugation for 2 min at 14,000 g, the organic phase was transferred into a fresh reaction tube. Flavonoid content was analysed by high-performance thin-layer chromatography (HPTLC). Naringenin (Sigma), dihydrokaempferol (DHK; Sigma), kaempferol (Roth), eriodictyol (TransMIT PlantMetaChem), apigenin (TransMIT PlantMetaChem), DHQ (Roth) and quercetin (Sigma) were dissolved in methanol and used as standards. 3 μl of each methanolic extract was spotted on a HPTLC Silica Gel 60 plate (Merck). The mobile phase was composed of chloroform, acetic acid and water mixed in the ratio (50:45:5). Flavonoid compounds were detected as described previously ( Stracke et al., 2007 ), using diphenylboric acid 2-aminoethyl ester (DPBA) and UV light ( Sheahan and Rechnitz, 1992 ).
Agrobacterium -Mediated Transformation of A. thaliana
T-DNA from pLEELA-based plasmids containing 2xpro35S -driven Musa2-ODDs was transformed into A. thaliana plants via Agrobacterium tumefaciens [Agrobacterium, GV101::pMP90RK, ( Koncz and Schell, 1986 )] mediated gene transfer using the floral dip method ( Clough and Bent, 1998 ). Successful T-DNA integration was verified by BASTA selection and PCR-based genotyping.
Flavonoid Staining of A. thaliana Seedlings
In situ visualisation of flavonoids in norflurazon-bleached A. thaliana seedlings was performed according to Stracke et al. (2007) using DPBA/Triton X-100 and epifluorescence microscopy.
Analysis of Flavonols in Methanolic Extracts
Flavonol glycosides were extracted and analysed as previously described ( Stracke et al., 2009 ). A. thaliana rosette leaves were homogenised in 80% methanol, incubated for 15 min at 70°C and centrifuged for 10 min at 14,000 g . Supernatants were vacuum dried. The dried pellets were dissolved in 80% methanol and analysed by HPTLC on a Silica Gel 60 plate (Merck). Ethyl acetate, formic acid, acetic acid and water (100:26:12:12) were used as a mobile phase. Flavonoid compounds were detected as described above.
Determination of Anthocyanin Content
To induce anthocyanin production, A. thaliana seedlings were grown on 0.5 MS plates with 4% sucrose and 16 h of light illumination per day at 22°C. Six-day-old seedlings were used to photometrically quantify anthocyanins as described by Mehrtens et al. (2005) . All samples were measured in three independent biological replicates. Error bars indicate the standard error of the average anthocyanin content. Statistical analysis was performed using the Mann–Whitney U test ( Mann and Whitney, 1947 ).
Creation of cDNA Constructs and Sequence-Based Characterisation of Musa Flavonoid 2-ODDs
Previously described putative Musa flavonoid biosynthesis enzymes encoded in the M. acuminata , Pahang DH reference genome sequence were used. This included two F3Hs, four FLSs and one ANS, which were classified as 2-ODDs. To functionally characterise these Musa enzymes, we amplified the corresponding CDSs from a cDNA template collection derived from different Musa organs, using primers designed on the Pahang DH reference genome sequence. The successfully amplified cDNAs of MusaF3H1 and MusaF3H2 were derived from plantain pulp, MusaFLS1 from Grand Naine young leaf, MusaFLS3 on Grand Naine bract and MusaANS on Grand Naine peel. Unfortunately, we were not able to amplify MusaFLS2 and MusaFLS4 from our template collection.
Comparison of the resulting 2-ODD cDNA sequences with the reference sequence revealed several single-nucleotide polymorphisms ( Supplementary File S1 ). The derived amino acid sequences show close similarity to other plant 2-ODD proteins known to be involved in flavonoid biosynthesis ( Supplementary File S2 ). The amino acids well known to coordinate ferrous iron binding (HxDxnH) and binding of 2-oxoglutarate (RxS) are also conserved in the Musa 2-ODDs. Additionally, the At FLS1 residues which have been shown to be involved in flavonoid substrate binding ( Supplementary File S2 ) are conserved. The residue F293 (all positions refer to At FLS1) is conserved in all Musa 2-ODD proteins, F134 and K202 are found in the Musa FLSs and Musa ANS and E295 is conserved in Musa ANS.
To analyse the evolutionary relationship between Musa 2-ODDs and 33 known flavonoid biosynthesis-related 2-ODDs, a phylogenetic tree was built ( Figure 2 ). The phylogenetic tree revealed two distinct clades, which correspond to the DOXC28 and DOXC47 classes of the 2-ODD superfamily. In the F3H- and FNSI-containing DOXC28 class, Musa F3H1 and Musa F3H2 cluster with other F3Hs from monocotyledonous plants. In the FLS- and ANS-containing DOXC47 class, the Musa ANS clusters with ANSs from monocotyledonous plants, while the FLSs from monocotyledonous plants do not form a distinct group, although the Mus aFLSs are in proximity to Zm FLS and Os FLS.
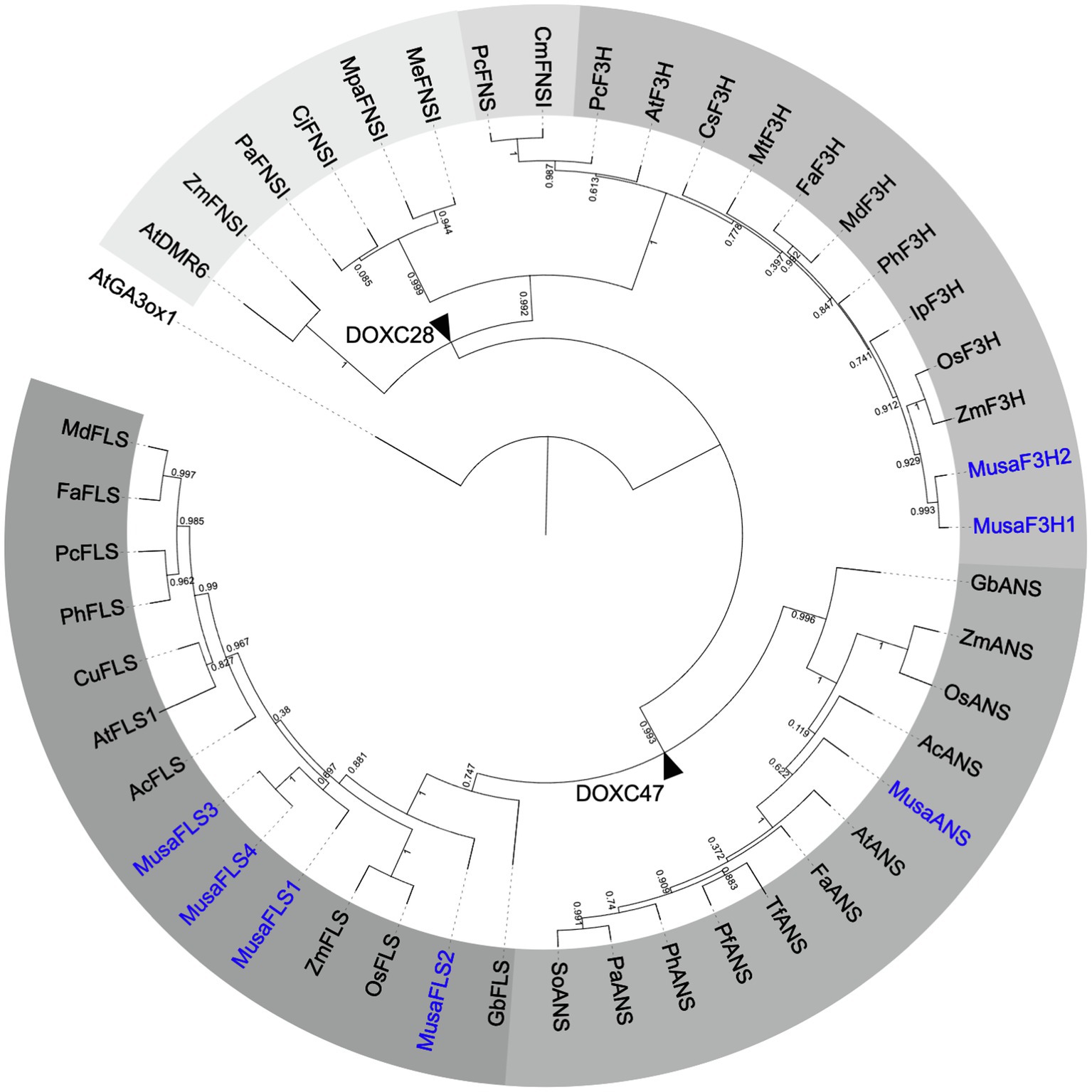
Figure 2 . Rooted approximately maximum-likelihood (ML) phylogenetic tree of 2-ODDs involved in flavonoid biosynthesis. ODDs from banana are given in blue, 39 enzymes with proven F3H, FNSI, FLS or ANS activity were included. Different grey scales indicate F3H, FLS, ANS and two evolutionary FNSI clades. At GA3ox1 (gibberellin 3 beta-hydroxylase1) was used as an outgroup. Branch points to DOXC28 and DOXC47 classes are marked with black arrowheads.
Our analyses clearly show that the CDSs identified for Musa F3H1, Musa F3H2, Musa FLS1, Musa FLS3 and Musa ANS have the potential to encode functional flavonoid 2-ODDs.
Expression Profiles of Musa 2-ODD Genes
Analysis of the expression patterns of the genes studied was performed using published RNA-Seq data. We obtained normalised RNA-Seq read values for Musa 2-ODD genes from several organs and developmental stages ( Table 1 ). MusaF3H1 and MusaF3H2 are expressed in almost all analysed organs and developmental stages. MusaF3H1 expression is highest in early developmental stages of pulp (S1 and S2), followed by intermediate developmental stages of peel (S2 and S3). MusaF3H2 shows highest expression in adult leaves. MusaFLS2 was not expressed in any of the analysed samples. All four MusaFLS genes show low or no expression in seedlings and embryogenic cell suspension. MusaFLS1 shows variance in transcript abundance with particularly high levels in peel (S2) and in young and adult leaves. MusaFLS3 and MusaFLS4 transcript levels are comparatively constant and low, with highest transcript abundance in root and peel (S1 and S3, respectively). While MusaFLS1 transcript abundance is highest in adult leaf, MusaFLS3 and MusaFLS4 lack expression in this tissue. MusaANS shows highest transcript abundance in pulp (S1, S2 and S4) and peel (S3) and very low expression in embryogenic cell suspension, seedlings and leaves.
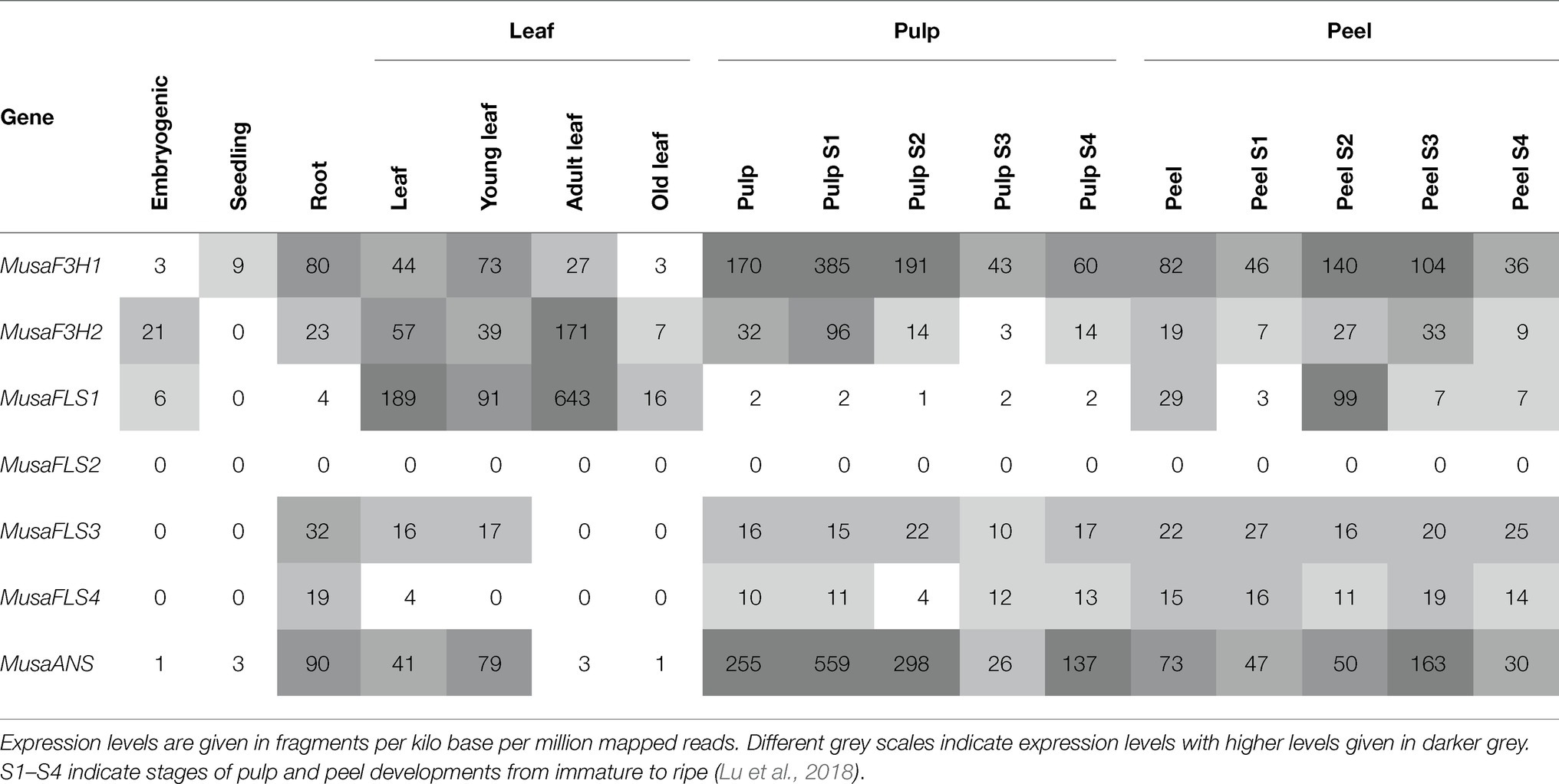
Table 1 . Expression profiles of Musa2 -ODD genes in different organs and developmental stages based on RNA-Seq data.
Musa F3H1 and Musa F3H2 Are Functional F3H
To confirm F3H activity in vivo , we used a bioconversion assay. MusaF3H1 and MusaF3H2 were heterologously expressed in E. coli and the bacterial cultures were fed with naringenin or eriodictyol as substrate of F3H. Both recombinant proteins, Musa F3H1 and Musa F3H2, were able to convert naringenin to DHK in the presence of 2-oxoglutarate and ferrous iron ( Figure 3A ). After 24 h of incubation, only small amounts of naringenin remained un-converted. Conversion of the formed DHK to kaempferol by Musa F3H1 or Musa F3H2 was not observed. Furthermore, eriodictyol was converted to DHQ by Musa F3H1 and Musa F3H2 ( Supplementary File S3 ). A further conversion to the quercetin was not observed. Since Musa F3H2 was previously considered as FNSI candidate, we analysed FNSI activity in a bioconversion assay. While Musa F3H2 was able to convert naringenin to DHK we detected apigenin as product only for PcFNSI but not for Musa F3H2 ( Supplementary File S4 ). Accordingly, Musa F3H2 does not show FNSI activity in our assay. For further in planta analysis, we chose a complementation assay with an A. thaliana f3h mutant (tt6-2) which expresses FLS and ANS but no F3H . MusaF3H1 or MusaF3H2 were expressed in tt6-2 plants under the control of the constitutive 2x35S promoter. The accumulation of flavonol glycosides was analysed in herbizide-bleached seedlings using DPBA staining ( Figure 3B ). While Col-0 wild-type seedlings appeared yellow under UV light, indicating the accumulation of flavonol glycosides, tt6-2 seedlings showed a red fluorescence. tt6-2 mutants expressing MusaF3H1 or MusaF3H2 were able to complement the mutant phenotype, showing the characteristic yellow flavonol glycoside fluorescence of the wild type. These results demonstrate that Musa F3H1 and Musa F3H2 are functional enzymes with F3H activity and are able to catalyse the conversion of naringenin to DHK.
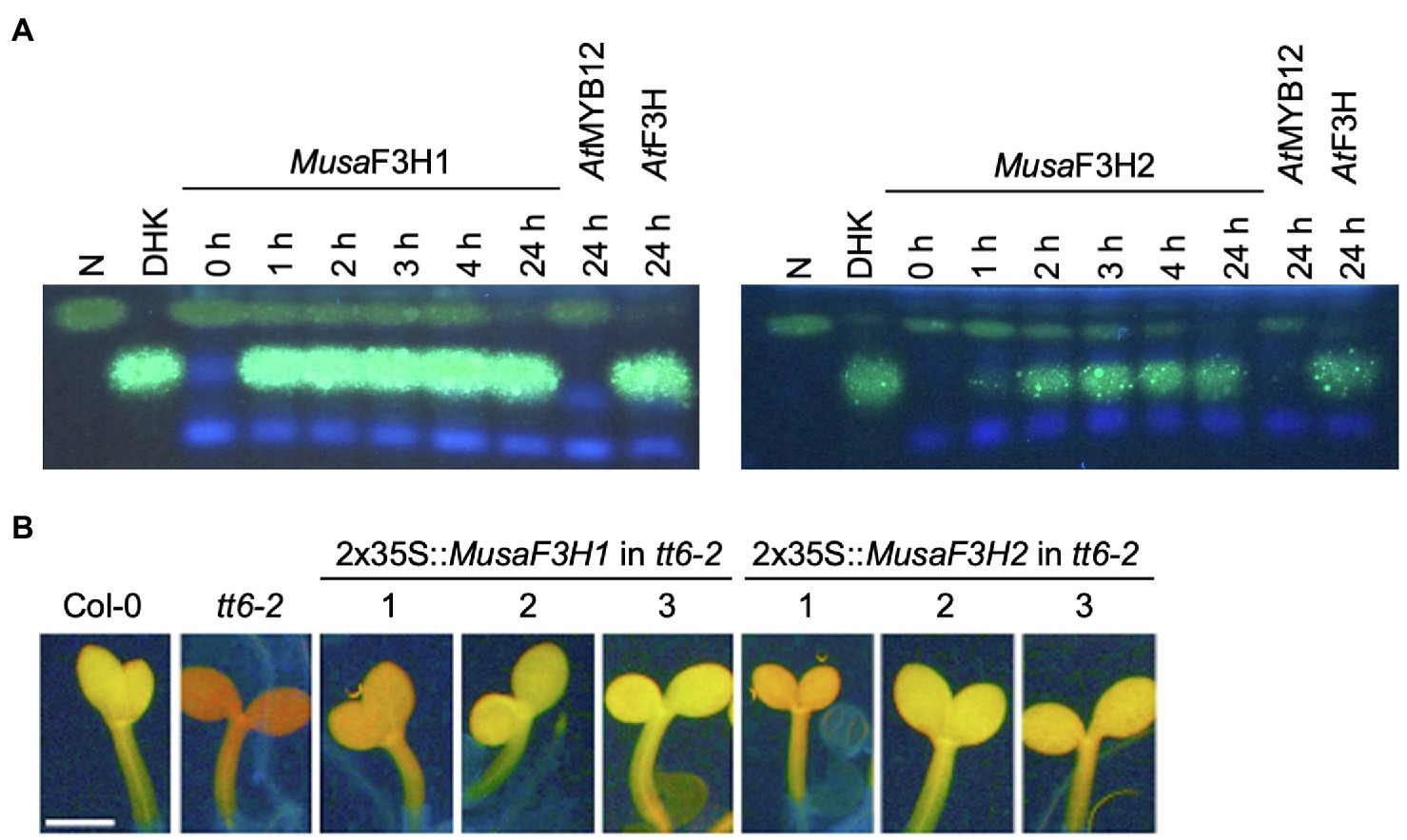
Figure 3 . Musa F3H1 and Musa F3H2 are functional F3Hs. (A) HPTLC analysis of F3H bioconversion assays using extracts from Escherichia coli expressing recombinant Musa F3H1 or Musa F3H2. The substrate naringenin (N) and the product dihydrokaempferol (DHK) were used as standards. At F3H was used as a positive control. At MYB12 was used as a negative control. (B) Analysis of tt6-2 mutant seedlings complemented with Musa F3H1 and Musa F3H2. Col-0 wild type and tt6-2 were used as controls. Representative pictures of DPBA-stained seedlings under UV light are given. Yellow fluorescence indicates flavonol glycoside accumulation. The different numbers indicate individual transgenic lines. The scale bar indicates 0.5 mm.
Musa FLS1 and Musa FLS3 Are Functional FLS
To confirm FLS activity in vivo , the enzymes were assayed in a coupled bioconversion experiment ( Figure 4A ). Two E. coli cultures expressing MusaF3H1 and a MusaFLS were mixed and fed with naringenin in the presence of 2-oxoglutarate and ferrous irons. Here, Musa F3H1 converts naringenin to DHK, thus providing the substrate for the FLS enzyme to be tested. While Musa FLS1 was found to be able to convert DHK to kaempferol under the assay conditions, Musa FLS3 was not. In a second bioconversion experiment, E. coli cultures expressing MusaFLS1 or MusaFLS3 were fed with DHQ ( Supplementary File S5 ). Again, Musa FLS1 was able to convert the dihydroflavonol to the corresponding flavonol, while Musa FLS3 was not. We checked the expression of recombinant Musa FLS1 and Musa FLS3 in the E. coli cultures by SDS-PAGE and found both Musa FLS proteins being expressed at similar levels ( Supplementary File S7 ). Moreover, we analysed a possible F3H/FLS biofunctional activity of Musa FLS1 and Musa FLS3 by feeding naringenin to E. coli cultures. While Musa FLS1 was not able to convert naringenin to DHK in the assay, Musa FLS3 showed F3H activity but not a further conversion to kaempferol ( Supplementary File S6 ). To further analyse the enzymatic properties in planta , MusaFLS1 or MusaFLS3 was expressed in the flavonol and anthocyanin-deficient A. thaliana ans/fls1-2 double mutant. HPTLC analyses of methanolic extracts from rosette leaves from greenhouse-grown plants ( Figure 4B ) showed that wild-type plants (Col-0 and Nö-0) contained flavonol glycosides, including the prominent derivatives kaempferol 3-O-rhamnoside-7-O-rhamnoside (K-3R-7R), kaempferol 3-O-glucoside-7-O-rhamnoside (K-3G-7R) and kaempferol 3[-O-rhamnosyl-glucoside]-7-O-rhamnoside (K-3[G-R]-7R). ans/fls1-2 plants accumulated several dihydroflavonols but did not show flavonol derivatives. ans/fls1-2 mutants transformed with 2x35S:: MusaFLS1 or 2x35S:: MusaFLS3 constructs were able to form several flavonol derivatives. Nevertheless, intensities and accumulation patterns of flavonol glycosides varied. We also analysed if MusaFLS1 and MusaFLS3 are able to complement the anthocyanin deficiency of the ans/fls1-2 double mutant. For this, seedlings were grown on 4% sucrose to induce anthocyanin accumulation. As shown in Figure 4C , 6-day-old Col-0 and Nö-0 seedlings were able to accumulate red anthocyanin pigments, while ans/fls1-2 transformed with MusaFLS1 or MusaFLS3 did not show visible anthocyanins. These results confirm that Musa FLS1 and Musa FLS3 are functional proteins with FLS activity and are enzymes able to catalyse the conversion of dihydroflavonol to flavonol.
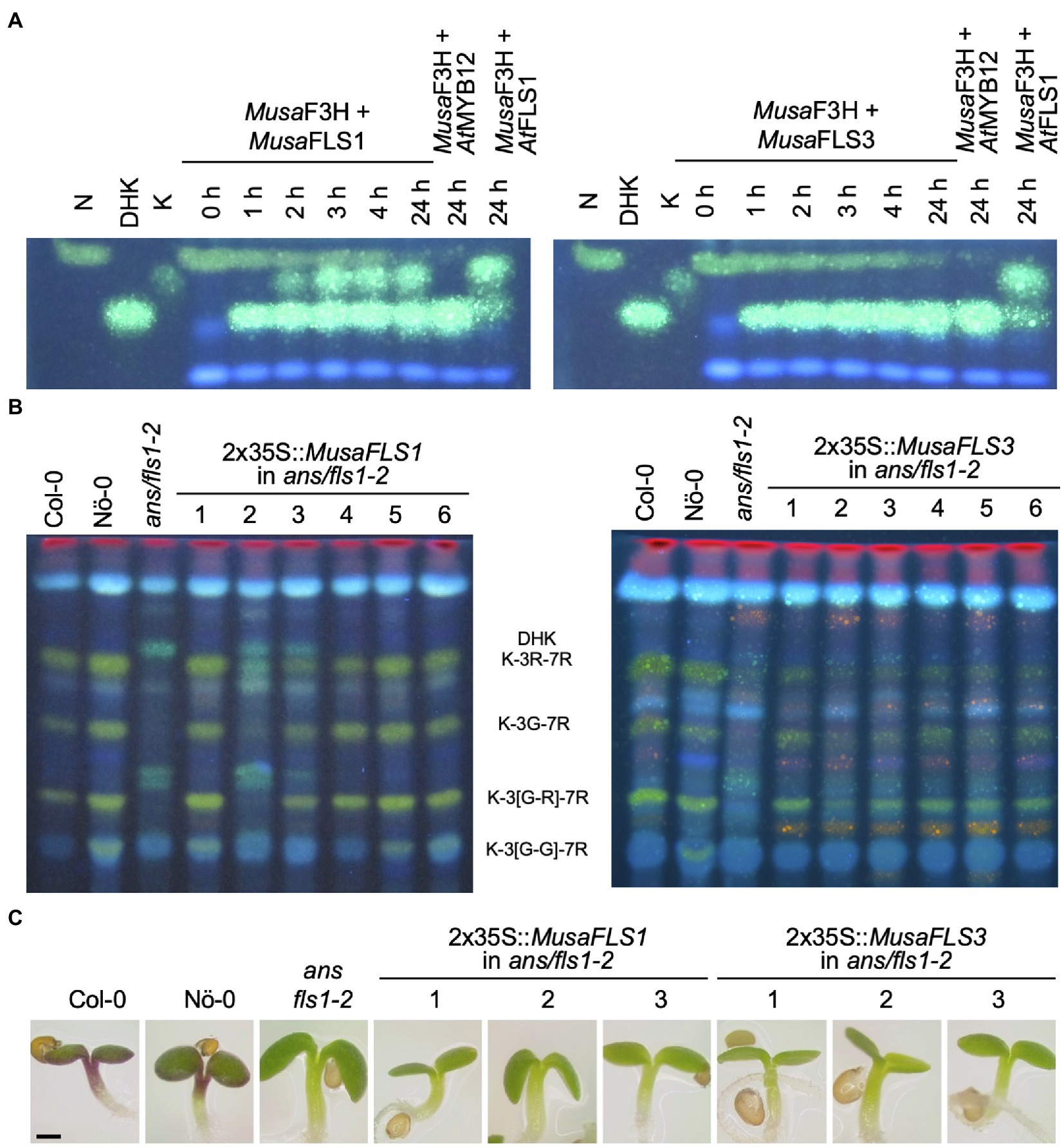
Figure 4 . Musa FLS1 and Musa FLS3 are functional FLSs. (A) HPTLC analysis of a FLS bioconversion assays using extracts from E. coli expressing recombinant Musa FLS1 or Musa FLS3. The F3H substrate naringenin (N), the FLS substrate DHK and the product kaempferol (K) were used as standards. At FLS1 served as a positive control and At MYB12 was used as a negative control. (B) Flavonol glycoside accumulation in Musa FLS-complemented ans/fls1-2 seedlings analysed by HPTLC analysis. Col-0, Nö-0 (both wild type) and ans/fls1-2 were used as controls. Bright green spots belong to derivatives of kaempferol, orange spots are derivatives of quercetin and faint blue shows sinapate derivatives. Dark green and yellow spots indicate DHK and DHQ, respectively. G, glucose; K, kaempferol; Q, quercetin; and R, rhamnose. (C) Representative pictures of anthocyanin (red) accumulation in 6-day-old Musa FLS-complemented ans/fls1-2 seedlings growing on 4% sucrose. The scale bar indicates 0.5 mm.
Musa ANS Is a Functional ANS
We analysed Musa ANS functionality in a complementation assay with the ans/fls1-2 A. thaliana double mutant. To examine the ability of MusaANS to complement the ans/fls1-2 anthocyanin deficiency phenotype, seedlings were grown on anthocyanin-inducing media. Anthocyanin accumulation was analysed visually and quantified photometrically ( Figures 5A , B ). While wild-type seedlings showed red pigmentation, the ans/fls1-2 seedlings did not. ans/fls1-2 seedlings expressing MusaANS showed accumulation of anthocyanins. The anthocyanin content in the complemented seedlings was strongly increased compared to ans/fls1-2 knockout plants, indicating ANS activity. Furthermore, we analysed the ability of MusaANS to complement the flavonol deficiency phenotype of ans/fls1-2 plants. For this, methanolic extracts of seedlings were analysed by HPTLC followed by DPBA staining ( Figure 5C ). While wild-type seedlings accumulated several kaempferol and quercetin glycosides, ans/fls1-2 mutants expressing MusaANS showed a flavonoid pattern identical to the ans/fls1-2 mutant, accumulating dihydroflavonol derivatives, but no flavonol derivatives. These results indicate that Musa ANS is a functional enzyme with ANS activity and is able to catalyse the conversion of leucoanthocyanidin to anthocyanidin.
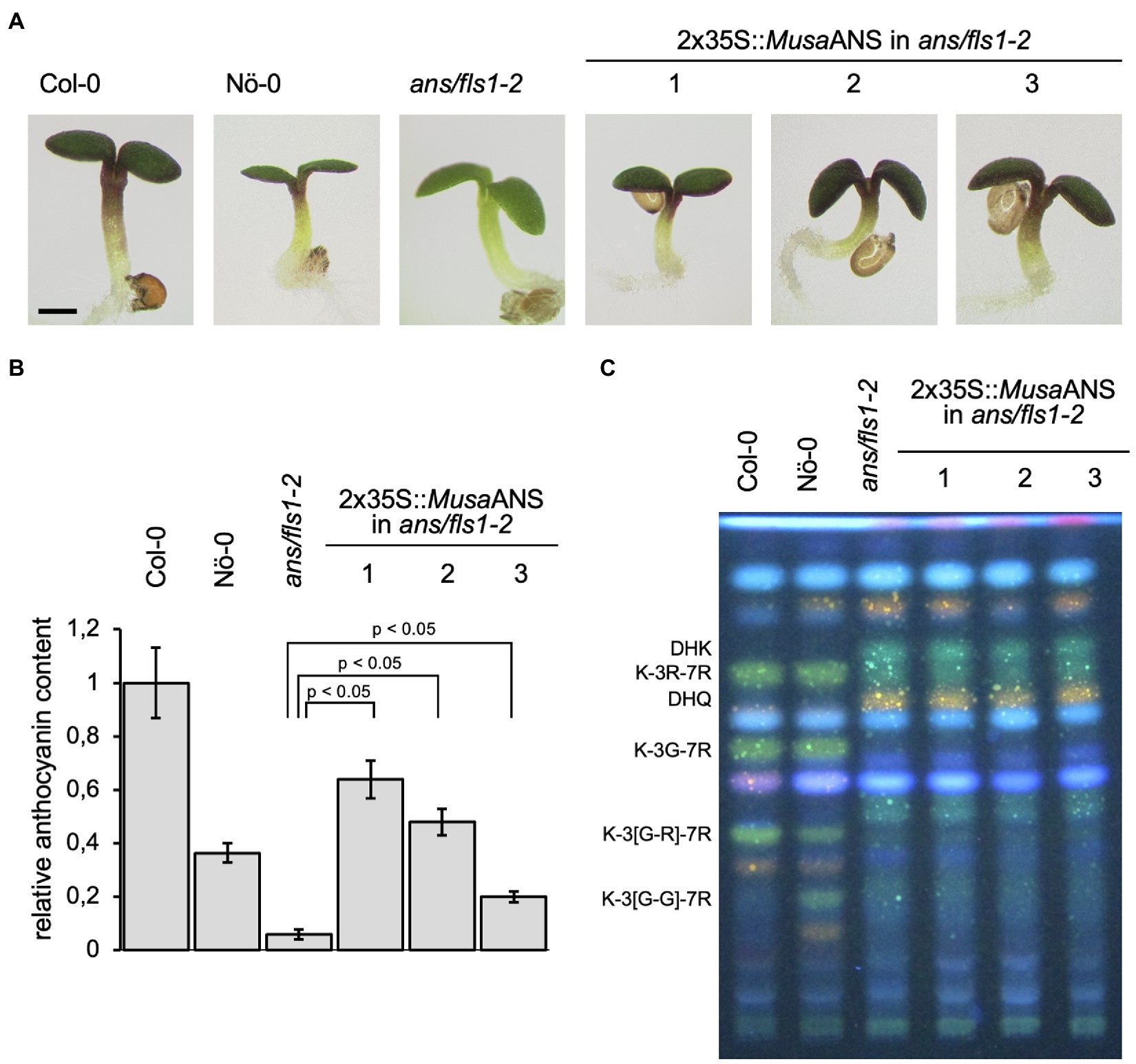
Figure 5 . Musa ANS is a functional anthocyanin synthase. Analysis of ans/fls1-2 double mutant seedlings complemented with 2x35S-driven MusaANS demonstrate in planta ANS functionality of Musa ANS by anthocyanin accumulation. The different numbers indicate individual transgenic lines. (A,B) Sucrose induced anthocyanin accumulation in 6-day-old Arabidopsis thaliana seedlings. (A) Representative pictures of seedlings (the scale bar indicates 0.5 mm) and (B) corresponding relative anthocyanin content. Error bars indicate the standard error for three independent measurements. (C) Musa ANS does not show in planta FLS activity. Flavonol glycoside accumulation in MusaANS -complemented ans/fls1-2 seedlings analysed by HPTLC analysis. Col-0, Nö-0 (both wild type) and ans/fls1-2 were used as controls. Bright green spots belong to derivatives of kaempferol, orange spots are derivatives of quercetin and faint blue shows sinapate derivatives. Dark green and yellow spots indicate DHK and DHQ, respectively. G, glucose; K, kaempferol; Q, quercetin; and R, rhamnose.
In this study, we isolated several cDNAs of in silico annotated 2-ODD-type flavonoid biosynthesis enzyme coding genes from Musa and tested the in vivo functionality of the encoded proteins in E. coli and A. thaliana .
Musa F3H1 and Musa F3H2
In vivo E. coli bioconversion assays revealed that Musa F3H1 and Musa F3H2 can covert naringenin to DHK. Moreover, MusaF3H1 and MusaF3H2 are able to complement the loss-of-function phenotype of A. thaliana tt6-2 seedlings, showing in planta F3H activity. Therefore, we conclude that Musa F3H1 and Musa F3H2 are functional F3Hs.
Previous studies annotated Ma02_g04650 ( MusaF3H1 ) and Ma07_g17200 ( MusaF3H2 ) as genes encoding F3Hs ( Martin et al., 2016 ; Pandey et al., 2016 ; Pucker et al., 2020b ). However, the automatic approach developed by Pucker et al. (2020b) also considered Ma07_g17200 as a candidate to encode a FNSI enzyme. For a Petroselinum crispum F3H protein, it has been found that a replacement of three amino acids was sufficient to cause FNSI side activity and seven amino acid exchanges almost lead to a complete change in enzyme activity towards FNSI functionality ( Gebhardt et al., 2007 ). These findings underline the particular high similarity between FNSI and F3H. A closer inspection of the deduced peptide sequence of Ma07_g17200 gene revealed a lack of conservation of amino acid residues known to be relevant for FNSI function (i.e. T106M, T115I, I116V, F131I, E195D, I200V, V215L and R216K). Furthermore, Ma07_g17200 did not show FNSI activity in our bioconversion assay. Together with the confirmed F3H activity, this indicates that the classification of Ma07_g17200 as a FNSI encoding gene was inaccurate. Even though Ma07_p17200 is no functional FNSI, flavone derivatives have been identified in Musa ( Fu et al., 2018 ). In Gerbera and Glycine max , FNSII is responsible for the formation of flavones ( Martens and Forkmann, 1999 ; Fliegmann et al., 2010 ). A candidate Musa FNSII, encoded by Ma08_g26160 , has been identified ( Pucker et al., 2020b ) and is probably responsible for the accumulation of flavones in Musa . Since FNSII enzymes are NADPH-dependent cytochrome P450 monooxygenases ( Jiang et al., 2016 ), the putative Musa FNSII (encoded by Ma08_g26160 ) was not studied in this work.
Our expression study that was based on published RNA-Seq data ( Pucker et al., 2020a ), revealed highest MusaF3H1 transcript abundance in early developmental stages of pulp and intermediate stages of peel development, while highest MusaF3H2 expression was found in adult leaves ( Table 1 ). This is, at first glance, in contrast to the quantitative real-time PCR-derived expression data presented by Pandey et al. (2016) , reporting high transcript levels of MusaF3H1 and MusaF3H2 in young leaves and bract. In this study, the authors also show increased MusaF3H1 expression in pseudostem, root and ripe pulp, compared to MusaF3H2 . These discrepancies are probably due to the different growth conditions, germplasms/cultivars and sampling time points in the generation of the expression data and cannot be reasonably analysed further at this point. It should be noted, however, that MusaF3H2 expression in leaves of plantlets was found to be increased following treatment with the phytohormone methyl jasmonate (MJ), while MusaF3H1 expression was not ( Pandey et al., 2016 ). As MJ is involved in various regulatory processes, including the response against biotic and abiotic stresses (summarised in Cheong and Choi, 2003 ), the MJ-dependent induction of MusaF3H2 expression could imply that the Musa F3H2 enzyme plays a specialised role in the formation of flavonoids in response to stresses. Such stress response induction of F3H encoding genes was previously shown for a F3H gene from the dessert plant Reaumuria soongorica , which is induced by UV light ( Liu et al., 2013 ) and two F3H genes from Camellia sinensis , which are induced by UV light and by treatment with abscisic acid (ABA) or sucrose ( Han et al., 2017 ). In addition, overexpression of F3H from C. sinensis ( Mahajan and Yadav, 2014 ) and from Lycium chinense ( Song et al., 2016 ) in tobacco improved the tolerance to salt stress and fungal pathogens in the first case and to drought stress in the latter case. In conclusion, our results clearly show that Musa F3H1 and Musa F3H2 are functional F3Hs and that Musa F3H2 might play a role in stress response.
Musa FLS1 and Musa FLS3
Musa FLS1 was found to be able to convert DHK and DHQ to the corresponding flavonol in the E. coli bioconversion assays. This ability was validated in planta by successful complementation of the flavonol deficiency of A. thaliana ans/fls1-2 double mutant seedlings, while the anthocyanin deficiency phenotype was not restored. These observations could hint to an exclusive FLS activity of Musa FLS1. FLS and DFR, the first enzymes of the flavonol and anthocyanin branches of flavonoid biosynthesis, compete for dihydroflavonol substrates ( Figure 1 ). This is demonstrated by several A. thaliana fls1 single mutants which accumulate higher levels of anthocyanin pigments ( Owens et al., 2008 ; Stracke et al., 2009 ). Falcone Ferreyra et al. (2010) found that overexpression of ZmFLS1 in an A. thaliana fls1 mutant decreases the accumulation of anthocyanins to wild-type level, again indicating that the anthocyanin and flavonol branches of flavonoid biosynthesis can compete for dihydroflavonol substrates in the same cell or tissue. Accordingly, the overlapping substrate usage of FLS and ANS is difficult to analyse in a fls1 single mutant. Here, the use of an A. thaliana ans/fls1-2 double mutant is a simple way to analyse the FLS and a possible ANS side activity of FLSs and vice versa in planta . To analyse further side activities, a f3h fls ans mutant would be beneficial. A. thaliana ans/fls1-2 plants expressing MusaFLS3 showed the accumulation of several kaempferol derivatives. In contrast, plants complemented with MusaFLS1 also accumulate quercetin derivatives. This is most likely an effect of different environmental conditions in plant growth. One set of plants was grown in the greenhouse ( MusaFLS1 ), another set was grown in a growth chamber ( MusaFLS3 ). In the plants used for Musa FLS3 experiments, these conditions promoted the accumulation of DHQ, while the other growth conditions did not in the plants used for MusaFLS1 experiments. Accordingly, DHQ was probably not available as a substrate for Musa FLS1 and could not be converted to quercetin. A possible explanation for the varying DHQ amounts could be the light-induced expression of flavonoid 3'hydroxylase (F3’H), which converts DHK to DHQ. The expression of F3’H in cultured A. thaliana cells can be induced by UV light ( Schoenbohm et al., 2000 ) and the activity of the F3’H promoter from Vitis vinifera is increased under light exposure ( Sun et al., 2015 ). Consequently, a different light quality or higher light intensity could be responsible for increased F3’H expression, causing the accumulation of DHQ in the plants grown in the growth chamber. While MusaFLS3 was able to complement the flavonol deficiency in the ans/fls1-2 mutant, it did not lead to an accumulation of anthocyanins. In contrast to the in planta results, Musa FLS3 did not convert DHK or DHQ to the corresponding flavonol in the in vivo bioconversion assays, even though the protein was successfully expressed in the E. coli culture. We used the bioconversion assays as a simple and versatile tool to analyse enzymatic activities. Nevertheless, heterologous expression of eukaryotic proteins in E. coli is an artificial system. It can cause a divergent pattern of post-translational modifications or production of high amounts of protein in inclusion bodies, leading to inactive protein ( Sahdev et al., 2008 ). Preuss et al. (2009) reported that At FLS3 did not show FLS activity in E. coli , but did convert dihydroflavonols to the corresponding flavonols upon expression in yeast, indicating that the stability of At FLS3 was improved under the latter assay conditions. The approach carried out in this work might have similar limitations. Further limitations can be caused by different substrate preferences, as reported for Os FLS ( Park et al., 2019 ). Despite the lack of FLS activity in the bioconversion assay, Musa FLS3 showed F3H activity. Nevertheless, the in planta complementation of the flavonol deficiency in the A. thaliana ans/fls1-2 mutant and the lacking complementation of the anthocyanin deficiency phenotype in the ans/fls1-2 mutant indicate that Musa FLS3 is a bifunctional enzyme with FLS and F3H activity, which does not exhibit significant ANS activity.
MusaFLS1 transcript abundance was found to be high in adult leaves and low in roots. MusaFLS3 and MusaFLS4 revealed opposed transcript levels. Such divergent expression patterns have previously been observed for FLS1 and FLS2 from Freesia hybrida and FLS1 and FLS2 from Cyclamen purpurascens ( Akita et al., 2018 ; Shan et al., 2020 ). These opposed expression patterns could point to differential activity in distinct organs. Furthermore, the tandemly arranged MusaFLS3 and MusaFLS4 genes show very similar expression patterns ( Table 1 ), possibly indicating functional redundancy. While we could not find expression of MusaFLS2 in our expression analyses, Pandey et al. (2016) observed MusaFLS2 expression in several organs (including bract, pseudostem and root), as well as in different developmental stages of peel and pulp. Again, these results show that different cultivars, growth conditions, sampling time points and analysed organs can have a strong influence on the resulting data. Accordingly, data from different studies should be evaluated carefully. MusaFLS1 and MusaFLS2 expression in leaves of plantlets does not significantly increase after MJ treatment ( Pandey et al., 2016 ). However, UV radiation induces the expression of FLS from Z. mays ( Falcone Ferreyra et al., 2010 ) and M. domestica ( Henry-Kirk et al., 2018 ) and the relative expression of FLS from Triticum aestivum increases during drought stress ( Ma et al., 2014 ). Together with the knowledge that flavonols act as antioxidants ( Wang et al., 2006 ), an involvement of Musa FLSs in stress response seems feasible and should be further analysed under a broader range of conditions. In summary, our results indicate that Musa FLS1 and Musa FLS3 are functional FLS enzymes and hint at possible differential organ-specific activities of Musa FLS1 and Musa FLS3/ Musa FLS4.
A. thaliana ans/fls1-2 seedlings expressing MusaANS show a strong, red pigmentation, revealing that MusaANS can complement the anthocyanin deficiency caused by mutation of AtANS . However, the seedlings did not display flavonol derivatives in HPTLC analyses, which have been reported to detect flavonol glycosides at levels of 50 pMol ( Stracke et al., 2010 ). These results indicate that Musa ANS is a functional ANS but does not have FLS activity. The RNA-Seq data-derived expression profiles revealed high MusaANS transcript abundance in pulp and peel. Additionally, MusaANS expression has been reported to be high in bract tissue ( Pandey et al., 2016 ), the specialised leaves surrounding the flowers and usually coloured red or purple due to anthocyanin accumulation ( Pazmiño-Durána et al., 2001 ). This spatial correlation of MusaANS transcripts and anthocyanin metabolites in bract tissue supports the proposed biological functionality of this enzyme, catalysing the conversion of leucoanthocyanidins to anthocyanidins in Musa . MusaANS expression increases 24 h after MJ treatment and decreases following dark treatment ( Pandey et al., 2016 ). As supposed for MusaF3H2 , the increased expression of MusaANS after MJ treatment could imply an involvement in the formation of anthocyanins as a consequence of stress response. ABA, salicylic acid (SA), UV-B and cold treatments have been shown to enhance ANS transcript abundance in G. biloba ( Xu et al., 2008 ) and overexpression of OsANS raises the antioxidant potential in O. sativa ( Reddy et al., 2007 ). The accumulation of anthocyanins has often been shown to be induced by (UV-) light ( Takahashi et al., 1991 ; Stapleton and Walbot, 1994 ; Meng et al., 2004 ). We therefore assume that also Musa ANS could be involved in such stress response.
Very recently, a R2R3-MYB-type transcription factor (MaMYB4) has been identified as a negative regulator of anthocyanin biosynthesis in Musa acting as repressor on the MusaANS promoter ( Deng et al., 2021 ). These results give a first insight into the transcriptional regulation of MusaANS expression and confirm the role of MusaANS in the anthocyanin biosynthesis in Musa . Taking all available evidence into account, MusaANS encodes a functional ANS with a possible involvement in the plants’ stress response.
To deepen the knowledge about 2-ODDs and other enzymes involved in Musa flavonoid biosynthesis, it would be beneficial to acquire even more spatially and timely highly resolved transcriptome and in particular metabolite data. This data could serve as a starting point for the analysis of organ-, stress- or development-specific enzyme activities and functions, as well as possible substrate preferences. It could also be used to elucidate the regulatory network of Musa flavonoid biosynthesis. Furthermore, knowledge about the influence of different stresses (e.g. pathogens, light and temperature) on specific transcriptomes and metabolomes of the Musa plant could help to widen the knowledge of flavonoid biosynthesis and particular the functionality of the Musa 2-ODD enzymes. This could also lead to the detection of possible restricted side activities or overlapping functionalities as described for 2-OODs in some other plant species ( Falcone Ferreyra et al., 2010 ; Park et al., 2019 ) and to further decode the cause of these multifunctionalities in 2-ODDs.
To conclude, in this study, the functionality of five 2-ODDs involved in flavonoid biosynthesis in Musa was demonstrated in vivo in bacterial cells and in planta . Knowledge gained about the structural genes MusaF3H1 , MusaF3H2 , MusaFLS1 , MusaFLS3 and MusaANS in a major crop plant provides a basis for further research towards engineered, increased flavonoid production in banana, which could contribute to the fruits’ antioxidant activity and nutritional value, and possibly even enhanced the plants’ defence against Foc -TR4.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material , and further inquiries can be directed to the corresponding author.
Author Contributions
MB and RS planned the experiments. MB and CA performed the experiments and analysed the data. MB and SM interpreted the TLC data. RS and BW supervised the project and revised the manuscript. MB wrote the initial draft. All authors read and approved the final manuscript version.
This work was supported by the basic funding of the chair of Genetics and Genomics of Plants provided by the Bielefeld University/Faculty of Biology and the Open Access Publication Fund of Bielefeld University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Melanie Kuhlmann for her excellent assistance in the laboratory and to Andrea Voigt for her competent help in the greenhouse. We thank Anika Beckers who contributed to creation of constructs with MusaFLS cDNAs and Prisca Viehöver for sequencing. In addition, we thank Thomas Baier for his support with the SDS-PAGE and Ashutosh Pandey for providing us with the banana cDNAs. We acknowledge support for the publication costs by the Open Access Publication Fund of Bielefeld University.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fpls.2021.701780/full#supplementary-material
Akita, Y., Kitamura, S., Mikami, R., and Ishizaka, H. (2018). Identification of functional flavonol synthase genes from fragrant wild cyclamen ( Cyclamen purpurascens ). J. Plant Biochem. Biotechnol. 27, 147–155. doi: 10.1007/s13562-017-0423-9
CrossRef Full Text | Google Scholar
Andersen, Ø. M., and Jordheim, M. (2010). “Anthocyanins,” in Encyclopedia of Life Sciences (Chichester: John Wiley & Sons, Ltd).
Google Scholar
Appelhagen, I., Thiedig, K., Nordholt, N., Schmidt, N., Huep, G., Sagasser, M., et al. (2014). Update on transparent testa mutants from Arabidopsis thaliana : characterisation of new alleles from an isogenic collection. Planta 240, 955–970. doi: 10.1007/s00425-014-2088-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Arias, P., Dankers, C., Liu, P., and Pilkauskas, P. (2003). The World Banana Economy, 1985–2002. Rome, Italy: Food and Agricultural Organization (FAO).
Asif, M. H., Dhawan, P., and Nath, P. (2000). A simple procedure for the isolation of high quality RNA from ripening banana fruit. Plant Mol. Biol. Report. 18, 109–115. doi: 10.1007/BF02824018
Bednar, R. A., and Hadcock, J. R. (1988). Purification and characterizationo of chalcone isomerase from soybeans. J. Biol. Chem. 263, 9582–9588. doi: 10.1016/S0021-9258(19)81556-9
Britsch, L. (1990). Purification and characterization of flavone synthase I, a 2-oxoglutarate-dependent desaturase. Arch. Biochem. Biophys. 282, 152–160. doi: 10.1016/0003-9861(90)90099-K
Brown, J. W., Walker, J. F., and Smith, S. A. (2017). Phyx: phylogenetic tools for unix. Bioinformatics 33, 1886–1888. doi: 10.1093/bioinformatics/btx063
Cheng, A. X., Han, X. J., Wu, Y. F., and Lou, H. X. (2014). The function and catalysis of 2-oxoglutarate-dependent oxygenases involved in plant flavonoid biosynthesis. Int. J. Mol. Sci. 15, 1080–1095. doi: 10.3390/ijms15011080
Cheong, J.- J., and Choi, Y. D. (2003). Methyl jasmonate as a vital substance in plants. Trends Genet. 19, 409–413. doi: 10.1016/S0168-9525(03)00138-0
Clough, S. J., and Bent, A. F. (1998). Floral dip: a simplified method for agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J. 16, 735–743. doi: 10.1046/j.1365-313x.1998.00343.x
Dauer, A., Hensel, A., Lhoste, E., Knasmüller, S., and Mersch-Sundermann, V. (2003). Genotoxic and antigenotoxic effects of catechin and tannins from the bark of Hamamelis virginiana L. in metabolically competent, human hepatoma cells (hep G2) using single cell gel electrophoresis. Phytochemistry 63, 199–207. doi: 10.1016/S0031-9422(03)00104-3
Deng, G. M., Zhang, S., Yang, Q. S., Gao, H. J., Sheng, O., Bi, F. C., et al. (2021). MaMYB4, an R2R3-MYB repressor transcription factor, negatively regulates the biosynthesis of anthocyanin in banana. Front. Plant Sci. 11:600704. doi: 10.3389/fpls.2020.600704
DHont, A., Denoeud, F., Aury, J. M., Baurens, F. C., Carreel, F., Garsmeur, O., et al. (2012). The banana ( Musa acuminata ) genome and the evolution of monocotyledonous plants. Nature 488, 213–217. doi: 10.1038/nature11241
Falcone Ferreyra, M. L., Rius, S. P., and Casati, P. (2012). Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 3:222. doi: 10.3389/fpls.2012.00222
Falcone Ferreyra, M. L., Rius, S., Emiliani, J., Pourcel, L., Feller, A., Morohashi, K., et al. (2010). Cloning and characterization of a UV-B-inducible maize flavonol synthase. Plant J. 62, 77–91. doi: 10.1111/j.1365-313X.2010.04133.x
FAO (2019). The State of Food and Agriculture 2019. Rome, Italy: Food and Agricultural Organization (FAO).
Fliegmann, J., Furtwangler, K., Malterer, G., Cantarello, C., Schuler, G., Ebel, J., et al. (2010). Flavone synthase II (CYP93B16) from soybean ( Glycine max L.). Phytochemistry 71, 508–514. doi: 10.1016/j.phytochem.2010.01.007
Forkmann, G., de Vlaming, P., Spribille, R., Wiering, H., and Schram, W. (1986). Genetic and biochemical studies on the conversion of dihydroflavonols to flavonols in flowers of Petunia hybrida . Zeitschrift für Naturforschung C 41, 179–186. doi: 10.1515/znc-1986-1-227
Forkmann, G., Heller, W., and Grisebach, H. (1980). Anthocyanin biosynthesis in flowers of Matthiola incana flavanone 3-and flavonoid 3'-hydroxylases. Zeitschrift für Naturforschung C 35, 691–695. doi: 10.1515/znc-1980-9-1004
Forster, M., Rodríguez, E. R., Martín, J. D., and Romero, C. D. (2003). Distribution of nutrients in edible banana pulp. Food Technol. Biotechnol. 41, 167–171.
Fu, X., Cheng, S., Liao, Y., Huang, B., Du, B., Zeng, W., et al. (2018). Comparative analysis of pigments in red and yellow banana fruit. Food Chem. 239, 1009–1018. doi: 10.1016/j.foodchem.2017.07.046
Gebhardt, Y. H., Witte, S., Steuber, H., Matern, U., and Martens, S. (2007). Evolution of flavone synthase I from parsley flavanone 3beta-hydroxylase by site-directed mutagenesis. Plant Physiol. 144, 1442–1454. doi: 10.1104/pp.107.098392
Goufo, P., and Trindade, H. (2014). Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci. Nutr. 2, 75–104. doi: 10.1002/fsn3.86
Gronquist, M., Bezzerides, A., Attygalle, A., Meinwald, J., Eisner, M., and Eisner, T. (2001). Attractive and defensive functions of the ultraviolet pigments of a flower (Hypericum calycinum) . Proc. Natl. Acad. Sci. U. S. A. 98, 13745–13750. doi: 10.1073/pnas.231471698
Grotewold, E. (2006). The Science of Flavonoids. Columbus: The Ohio State University.
Hahlbrock, K., and Scheel, D. (1989). Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 347–369. doi: 10.1146/annurev.pp.40.060189.002023
Han, Y., Huang, K., Liu, Y., Jiao, T., Ma, G., Qian, Y., et al. (2017). Functional analysis of two flavanone-3-hydroxylase genes from Camellia sinensis : a critical role in flavonoid accumulation. Genes 8:300. doi: 10.3390/genes8110300
Heller, W., Forkmann, G., Britsch, L., and Grisebach, H. (1985). Enzymatic reduction of (+)-dihydroflavonols to flavan-3,4-cis-diols with flower extracts from Matthiola incana and its role in anthocyanin biosynthesis. Planta 165, 284–287. doi: 10.1007/BF00395052
Henry-Kirk, R. A., Plunkett, B., Hall, M., McGhie, T., Allan, A. C., Wargent, J. J., et al. (2018). Solar UV light regulates flavonoid metabolism in apple (Malus x domestica). Plant Cell Environ. 41, 675–688. doi: 10.1111/pce.13125
Holton, T. A., Brugliera, F., and Tanaka, Y. (1993). Cloning and expression of flavonol synthase from Petunia hybrida . Plant J. 4, 1003–1010. doi: 10.1046/j.1365-313X.1993.04061003.x
Ishikura, N., and Yoshitama, K. (1984). Anthocyanin-Flavonol co-pigmentation in blue seed coats of Ophiopogon jaburan . J. Plant Physiol. 115, 171–175. doi: 10.1016/S0176-1617(84)80064-4
Jaakkola, P., Mole, D. R., Tian, Y. M., Wilson, M. I., Gielbert, J., Gaskell, S. J., et al. (2001). Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472. doi: 10.1126/science.1059796
Jakoby, M., Wang, H. Y., Reidt, W., Weisshaar, B., and Bauer, P. (2004). FRU (BHLH029) is required for induction of iron mobilization genes in Arabidopsis thaliana . FEBS Lett. 577, 528–534. doi: 10.1016/j.febslet.2004.10.062
Jiang, N., Doseff, A. I., and Grotewold, E. (2016). Flavones: from biosynthesis to health benefits. Plan. Theory 5:27. doi: 10.3390/plants5020027
Kandil, F. E., Smith, M. A., Rogers, R. B., Pepin, M. F., Song, L. L., Pezzuto, J. M., et al. (2002). Composition of a chemopreventive proanthocyanidin-rich fraction from cranberry fruits responsible for the inhibition of 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced ornithine decarboxylase (ODC) activity. J. Agric. Food Chem. 50, 1063–1069. doi: 10.1021/jf011136z
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kawai, Y., Ono, E., and Mizutani, M. (2014). Evolution and diversity of the 2-oxoglutarate-dependent dioxygenase superfamily in plants. Plant J. 78, 328–343. doi: 10.1111/tpj.12479
Koncz, C., and Schell, J. (1986). The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of agrobacterium binary vector. Mol. Genet. Genomics 204, 383–396. doi: 10.1007/BF00331014
Kreuzaler, F., and Hahlbrock, K. (1972). Enzymatic synthesis of aromatic compounds in higher plants: formation of naringenin (5,7,4'-trihydroxyflavanone) from p-coumaroyl coenzyme A and malonyl coenzyme A. FEBS Lett. 28, 69–72. doi: 10.1016/0014-5793(72)80679-3
Le Roy, J., Huss, B., Creach, A., Hawkins, S., and Neutelings, G. (2016). Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant Sci. 7:735. doi: 10.3389/fpls.2016.00735
Lepiniec, L., Debeaujon, I., Routaboul, J. M., Baudry, A., Pourcel, L., Nesi, N., et al. (2006). Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 57, 405–430. doi: 10.1146/annurev.arplant.57.032905.105252
Letunic, I., and Bork, P. (2019). Interactive tree Of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47, W256–W259. doi: 10.1093/nar/gkz239
Li, J., Ou-Lee, T. M., Raba, R., Amundson, R. G., and Last, R. L. (1993). Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5, 171–179. doi: 10.2307/3869583
Liu, M., Li, X., Liu, Y., and Cao, B. (2013). Regulation of flavanone 3-hydroxylase gene involved in the flavonoid biosynthesis pathway in response to UV-B radiation and drought stress in the desert plant, Reaumuria soongorica. Plant Physiol. Biochem. 73, 161–167. doi: 10.1016/j.plaphy.2013.09.016
Lu, P., Yu, S., Zhu, N., Chen, Y. R., Zhou, B., Pan, Y., et al. (2018). Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat. Plants 4, 784–791. doi: 10.1038/s41477-018-0249-z
Luo, P., Ning, G., Wang, Z., Shen, Y., Jin, H., Li, P., et al. (2016). Disequilibrium of flavonol synthase and dihydroflavonol-4-reductase expression associated tightly to white vs. red color flower formation in plants. Front. Plant Sci. 6:1257. doi: 10.3389/fpls.2015.01257
Ma, D. Y., Sun, D. X., Wang, C. Y., Li, Y. G., and Guo, T. C. (2014). Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 80, 60–66. doi: 10.1016/j.plaphy.2014.03.024
Mahajan, M., and Yadav, S. K. (2014). Overexpression of a tea flavanone 3-hydroxylase gene confers tolerance to salt stress and Alternaria solani in transgenic tobacco. Plant Mol. Biol. 85, 551–573. doi: 10.1007/s11103-014-0203-z
Mann, H. B., and Whitney, D. R. (1947). On a test of Whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 18, 50–60. doi: 10.1214/aoms/1177730491
Martens, S., and Forkmann, G. (1999). Cloning and expression of flavone synthase II from Gerbera hybrids. Plant J. 20, 611–618. doi: 10.1046/j.1365-313X.1999.00636.x
Martens, S., Forkmann, G., Britsch, L., Wellmann, F., Matern, U., and Lukacin, R. (2003). Divergent evolution of flavonoid 2-oxoglutarate-dependent dioxygenases in parsley. FEBS Lett. 544, 93–98. doi: 10.1016/S0014-5793(03)00479-4
Martin, G., Baurens, F. C., Droc, G., Rouard, M., Cenci, A., Kilian, A., et al. (2016). Improvement of the banana “ Musa acuminata ,” reference sequence using NGS data and semi-automated bioinformatics methods. BMC Genomics 17:243. doi: 10.1186/s12864-016-2579-4
Mehrtens, F., Kranz, H., Bednarek, P., and Weisshaar, B. (2005). The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol. 138, 1083–1096. doi: 10.1104/pp.104.058032
Meng, X., Xing, T., and Wang, X. (2004). The role of light in the regulation of anthocyanin accumulation in Gerbera hybrida. Plant Growth Reg. 44, 243–250. doi: 10.1007/s10725-004-4454-6
Mo, Y., Nagel, C., and Taylor, L. P. (1992). Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc. Natl. Acad. Sci. U. S. A. 89, 7213–7217. doi: 10.1073/pnas.89.15.7213
Owens, D. K., Alerding, A. B., Crosby, K. C., Bandara, A. B., Westwood, J. H., and Winkel, B. S. (2008). Functional analysis of a predicted flavonol synthase gene family in Arabidopsis . Plant Physiol. 147, 1046–1061. doi: 10.1104/pp.108.117457
Pandey, A., Alok, A., Lakhwani, D., Singh, J., Asif, M. H., and Trivedi, P. K. (2016). Genome-wide Expression analysis and metabolite profiling elucidate transcriptional regulation of flavonoid biosynthesis and modulation under abiotic stresses in banana. Sci. Rep. 6:31361. doi: 10.1038/srep31361
Park, S., Kim, D. H., Park, B. R., Lee, J. Y., and Lim, S. H. (2019). Molecular and functional characterization of Oryza sativa Flavonol synthase (OsFLS), a Bifunctional dioxygenase. J. Agric. Food Chem. 67, 7399–7409. doi: 10.1021/acs.jafc.9b02142
Pazmiño-Durána, E. A., Giustib, M. M., Wrolstad, R. E., and Glóriaa, M. B. A. (2001). Anthocyanins from banana bracts (Musa X paradisiaca) as potential food colorants. Food Chem. 73, 327–332. doi: 10.1016/S0308-8146(00)00305-8
Perez-Vizcaino, F., and Duarte, J. (2010). Flavonols and cardiovascular disease. Mol. Asp. Med. 31, 478–494. doi: 10.1016/j.mam.2010.09.002
Prescott, A. G., and John, P. (1996). DIOXYGENASES: molecular structure and role in plant metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 245–271. doi: 10.1146/annurev.arplant.47.1.245
Preuss, A., Stracke, R., Weisshaar, B., Hillebrecht, A., Matern, U., and Martens, S. (2009). Arabidopsis thaliana expresses a second functional flavonol synthase. FEBS Lett. 583, 1981–1986. doi: 10.1016/j.febslet.2009.05.006
Price, M. N., Dehal, P. S., and Arkin, A. P. (2010). FastTree 2-approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490
Pucker, B., Pandey, A., Weisshaar, B., and Stracke, R. (2020a). The R2R3-MYB gene family in banana ( Musa acuminata ): genome-wide identification, classification and expression patterns. PLoS One 15:e0239275. doi: 10.1371/journal.pone.0239275
Pucker, B., Reiher, F., and Schilbert, H. M. (2020b). Automatic identification of players in the flavonoid biosynthesis with application on the biomedicinal plant Croton tiglium . Plan. Theory 9:1103. doi: 10.3390/plants9091103
Reddy, A. M., Reddy, V. S., Scheffler, B. E., Wienand, U., and Reddy, A. R. (2007). Novel transgenic rice overexpressing anthocyanidin synthase accumulates a mixture of flavonoids leading to an increased antioxidant potential. Metab. Eng. 9, 95–111. doi: 10.1016/j.ymben.2006.09.003
Sahdev, S., Khattar, S. K., and Saini, K. S. (2008). Production of active eukaryotic proteins through bacterial expression systems: a review of the existing biotechnology strategies. Mol. Cell. Biochem. 307, 249–264. doi: 10.1007/s11010-007-9603-6
Saito, K., Kobayashi, M., Gong, Z. Z., Tanaka, Y., and Yamazaki, M. (1999). Direct evidence for anthocyanidin synthase as a 2-oxoglutarate-dependent oxygenase: molecular cloning and functional expression of cDNA from a red forma of Perilla frutescens . Plant J. 17, 181–189. doi: 10.1046/j.1365-313X.1999.00365.x
Sanger, F., Nicklen, S., and Coulson, A. R. (1977). DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A. 74, 5463–5467. doi: 10.1073/pnas.74.12.5463
Schoenbohm, C., Martens, S., Eder, C., Forkmann, G., and Weisshaar, B. (2000). Identification of the Arabidopsis thaliana flavonoid 3'-hydroxylase gene and functional expression of the encoded P450 enzyme. Biol. Chem. 381, 749–753. doi: 10.1515/BC.2000.095
Shan, X., Li, Y., Yang, S., Yang, Z., Qiu, M., Gao, R., et al. (2020). The spatio-temporal biosynthesis of floral flavonols is controlled by differential phylogenetic MYB regulators in Freesia hybrida. New Phytol. 228, 1864–1879. doi: 10.1111/nph.16818
Sheahan, J. J., and Rechnitz, G. A. (1992). Flavonoid-specific staining of Arabidopsis thaliana . BioTechniques 13, 880–883.
PubMed Abstract | Google Scholar
Singh, B., Singh, J. P., Kaur, A., and Singh, N. (2016). Bioactive compounds in banana and their associated health benefits - A review. Food Chem. 206, 1–11. doi: 10.1016/j.foodchem.2016.03.033
Song, X., Diao, J., Ji, J., Wang, G., Guan, C., Jin, C., et al. (2016). Molecular cloning and identification of a flavanone 3-hydroxylase gene from Lycium chinense , and its overexpression enhances drought stress in tobacco. Plant Physiol. Biochem. 98, 89–100. doi: 10.1016/j.plaphy.2015.11.011
Stapleton, A. E., and Walbot, V. (1994). Flavonoids can protect maize DNA from the induction of ultraviolet radiation damage. Plant Physiol. 105, 881–889. doi: 10.1104/pp.105.3.881
Stracke, R., De Vos, R. C. H., Bartelniewoehner, L., Ishihara, H., Sagasser, M., Martens, S., et al. (2009). Metabolomic and genetic analyses of flavonol synthesis in Arabidopsis thaliana support the in vivo involvement of leucoanthocyanidin dioxygenase. Planta 229, 427–445. doi: 10.1007/s00425-008-0841-y
Stracke, R., Ishihara, H., Huep, G., Barsch, A., Mehrtens, F., Niehaus, K., et al. (2007). Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 50, 660–677. doi: 10.1111/j.1365-313X.2007.03078.x
Stracke, R., Jahns, O., Keck, M., Tohge, T., Niehaus, K., Fernie, A. R., et al. (2010). Analysis of PRODUCTION OF FLAVONOL GLYCOSIDES-dependent flavonol glycoside accumulation in Arabidopsis thaliana plants reveals MYB11-, MYB12- and MYB111-independent flavonol glycoside accumulation. New Phytol. 188, 985–1000. doi: 10.1111/j.1469-8137.2010.03421.x
Stracke, R., Turgut-Kara, N., and Weisshaar, B. (2017). The AtMYB12 activation domain maps to a short C-terminal region of the transcription factor. Z. Naturforsch. C. J. Biosci. 72, 251–257. doi: 10.1515/znc-2016-0221
Sun, R. Z., Pan, Q. H., Duan, C. Q., and Wang, J. (2015). Light response and potential interacting proteins of a grape flavonoid 3'-hydroxylase gene promoter. Plant Physiol. Biochem. 97, 70–81. doi: 10.1016/j.plaphy.2015.09.016
Sun, J., Zhang, J., Fang, H., Peng, L., Wei, S., Li, C., et al. (2019). Comparative transcriptome analysis reveals resistance-related genes and pathways in Musa acuminata banana ‘Guijiao 9’ in response to Fusarium wilt. Plant Physiol. Biochem. 141, 83–94. doi: 10.1016/j.plaphy.2019.05.022
Takahashi, A., Takeda, K., and Ohnishi, T. (1991). Light-induced anthocyanin reduces the extent of damage to DNA in UV-irradiated Centaurea cyanus cells in culture. Plant Cell Physiol. 32, 541–547. doi: 10.1093/oxfordjournals.pcp.a078113
Tanaka, Y., Sasaki, N., and Ohmiya, A. (2008). Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J. 54, 733–749. doi: 10.1111/j.1365-313X.2008.03447.x
Tohge, T., de Souza, L. P., and Fernie, A. R. (2017). Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 68, 4013–4028. doi: 10.1093/jxb/erx177
Trewick, S. C., Henshaw, T. F., Hausinger, R. P., Lindahl, T., and Sedgwick, B. (2002). Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature 419, 174–178. doi: 10.1038/nature00908
Wall, M. M. (2006). Ascorbic acid, vitamin A, and mineral composition of banana (Musa sp.) and papaya ( Carica papaya ) cultivars grown in Hawaii. J. Food Compost. Anal. 19, 434–445. doi: 10.1016/j.jfca.2006.01.002
Wang, L., Tu, Y.- C., Lian, T.- W., Hung, J.- T., Yen, J.- H., and Wu, M.- J. (2006). Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food Chem. 54, 9798–9804. doi: 10.1021/jf0620719
Wen, W., Alseekh, S., and Fernie, A. R. (2020). Conservation and diversification of flavonoid metabolism in the plant kingdom. Curr. Opin. Plant Biol. 55, 100–108. doi: 10.1016/j.pbi.2020.04.004
Winkel, B. S. J. (2006). “The biosynthesis of flavonoids,” in The Science of Flavonoids. ed. E. Grotewold (New York, USA: Springer Scienceand Business Media, Inc), 71–96.
Xu, F., Cheng, H., Cai, R., Li, L. L., Chang, J., Zhu, J., et al. (2008). Molecular cloning and function analysis of an anthocyanidin synthase gene from Ginkgo biloba, and its expression in abiotic stress responses. Mol. Cells 26, 536–547.
Xu, F., Li, L., Zhang, W., Cheng, H., Sun, N., Cheng, S., et al. (2012). Isolation, characterization, and function analysis of a flavonol synthase gene from Ginkgo biloba . Mol. Biol. Rep. 39, 2285–2296. doi: 10.1007/s11033-011-0978-9
Yan, Y., Chemler, J., Huang, L., Martens, S., and Koffas, M. A. (2005). Metabolic engineering of anthocyanin biosynthesis in Escherichia coli . Appl. Environ. Microbiol. 71, 3617–3623. doi: 10.1128/AEM.71.7.3617-3623.2005
Keywords: banana, specialised metabolites, flavanone 3-hydroxylases, flavonol synthase, anthocyanidin synthase
Citation: Busche M, Acatay C, Martens S, Weisshaar B and Stracke R (2021) Functional Characterisation of Banana ( Musa spp.) 2-Oxoglutarate-Dependent Dioxygenases Involved in Flavonoid Biosynthesis. Front. Plant Sci . 12:701780. doi: 10.3389/fpls.2021.701780
Received: 28 April 2021; Accepted: 20 July 2021; Published: 17 August 2021.
Reviewed by:
Copyright © 2021 Busche, Acatay, Martens, Weisshaar and Stracke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ralf Stracke, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Advertisement
Utilization of banana waste as a resource material for biofuels and other value-added products
- Original Article
- Published: 17 January 2022
- Volume 13 , pages 12717–12736, ( 2023 )
Cite this article

- Geetika Gupta 1 ,
- Manoj Baranwal 1 ,
- Sanjai Saxena 1 &
- M. Sudhakara Reddy 1
2192 Accesses
16 Citations
1 Altmetric
Explore all metrics
Banana is one of the most important food crops which is generally planted in tropical countries and has beneficial applications in the food industry. A large amount of by-products such as leaves, inflorescence, pseudostem, and rhizomes serves as a source for different industries. Most of these by-products may serve as an undervalued commodity with a limited commercial value, application and in some cases, it is considered as an agricultural waste. This also paves the way to utilize a huge amount of untapped biomass and resolve some of the environmental issues. Most of the edible bananas are cultivated mainly for their fruits, thus, banana farms could generate several tons of underused by-products and wastes. The present review mainly discusses the utilization of banana by-products such as peels, leaves, pseudostem, pseudostem juice, stalk, and inflorescence in various industries as a thickening agent, alternative source for renewable energy, nutraceuticals, livestock feed, natural fibers, coloring agents, bioactive compounds, and bio-fertilizers. Banana waste serves as a potential source for the production of valuable products and preserves renewable resources and provides additional income to the farming industries.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Sugarcane bagasse-based biochar and its potential applications: a review

Bamboo for producing charcoal and biochar for versatile applications
Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin, data availability.
All relevant data are included in the paper.
Abbreviations
Indium tin oxide
Poly(3,4-ethylenedioxythiophene)
Polystyrene sulfonate
Polyethylene glycol
Carboxymethyl cellulose
Schopper-Riegler
Kumar KS, Bhowmik D, Duraivel S, Umadevi M (2012) Traditional and medicinal uses of banana. J Pharmacogn Phytochem 1:51–63
Google Scholar
Simmonds NW, The evolution of the bananas. The Evolution of the Bananas (1962). https://www.cabdirect.org/cabdirect/abstract/19630303919
Khanum F, Swamy MS, Krishna KS, Santhanam K, Viswanathan KR (2000) Dietary fiber content of commonly fresh and cooked vegetables consumed in India. Plant Foods Hum Nutr 55:207–218
Article Google Scholar
Mohiuddin AKM, Saha MK, Hossian MS, Ferdoushi A (2014) Usefulness of banana ( Musa paradisiaca ) wastes in manufacturing of bio-products: a review. The Agriculturists 12:148–158
Mohapatra D, Mishra S, Sutar N (2010) Banana and its by-product utilisation: an overview. Food Bioprocess Technol 69:323–329
Sharrock S and Lusty C, Nutritive value of banana, INIBAP Annual Report (2000)
Muraleedharan H, Perumal K (2010) Ecofriendly handmade paper making. Booklet published from Shri AMM Murugappa Chettiar Research Centre, Taramani, Chennai
Cordeiro N, Belgacem MN, Torres IC, Moura JCVP (2004) Chemical composition and pulping of banana pseudo-stems. Indus Crops Prod 19:147–154
Arvanitoyannis IS, Mavromatis A (2009) Banana cultivars, cultivation practices, and physicochemical properties. Crit Rev Food Sci Nutr 49:113–135
Padam BS, Tin HS, Chye FY, Abdullah MI (2014) Banana by-products: an under-utilized renewable food biomass with great potential. J Food Sci Technol 51:3527–3545
Seth K, Top Banana Producing Countries In The World (2014). Retrieved from http://www.worldatlas.com/articles/top-banana-producing-countries-in-the-world.html
Pei P, Zhang C, Li J, Chang S, Li S, Wang J, Zhao M (2014) Li, Yu M, and Chen X, Optimization of NaOH pretreatment for enhancement of biogas production of banana pseudo-stem fiber using response surface methodology. BioResources 9:5073–5087
Kokab S, Asghar M, Rehman K, Asad MJ, Adedyo O (2003) Bio-processing of banana peel for α-amylase production by Bacillus subtilis. Int J Agri Biol 5:36–39
Clarke WP, Radnidge P, Lai TE, Jensen PD, Hardin MT (2008) Digestion of waste bananas to generate energy in Australia. Waste Manage 28:527–533
Doran I, Sen B, Kaya Z (2005) The effects of compost prepared from waste material of banana on the growth, yield and quality properties of banana plants. J Environ Biol 26:7–12
Emaga TH, Ronkart SN, Robert C, Wathelet B, Paquot M (2008) Characterisation of pectins extracted from banana peels (Musa AAA) under different conditions using an experimental design. Food Chem 108:463–471
Kuo JM, Hwang A, Yeh DB, Pan MH, Tsai ML, Pan BS (2006) Lipoxygenase from banana leaf: purification and characterization of an enzyme that catalyzes linoleic acid oxygenation at the 9-position. J Agric Food Chem 54:3151–3156
Rosli WW, Law KN, Zainuddin Z, Asro R (2004) Effect of pulping variables on the characteristics of oil-palm frond-fiber. Bioresour Technol 93:233–240
Vigneswaran C, Pavithra V, Gayathri V, Mythili K (2015) Banana fiber: scope and value-added product development. J Text Appar Technol Manag 9:1–7
Jamal P, Saheed OK, Alam Z (2012) Bio-valorization potential of banana peels (Musa sapientum): an overview. Asian Journal of Biotechnology 4:1–14
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev 14:578–597
Kadirvelu K, Kavipriya M, Karthika C, Radhika M, Vennilamani N, Pattabhi S (2003) Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour Technol 87:129–132
Ogunleye OO, Ajala MA, Agarry SE (2014) Evaluation of biosorptive capacity of banana (Musa paradisiaca) stalk for lead (II) removal from aqueous solution. J Environ Prot Sci 5:1451
Ahmed T, Danish M (2018) Prospects of banana waste utilization in waste water treatment: a review. J Environ Manage 206:330–348
Prachpreecha O, Pipatpanyanugoon K, Sawangwong P (2016) A study of characterizations and efficiency of activated carbon prepared from peel and bunch of banana for methyl orange dye adsorption. IOSR J Environ Sci Toxicol Food Technol 10:17–26
Abbas MN, Abbas FS, Ibrahim SA (2014) Cyanide removal from wastewater by using banana peel. J Asian Sci Res 4:239–247
Bhaumik R, Mondal NK (2016) Optimizing adsorption of fluoride from water by modified banana peel dust using response surface modelling approach. Appl Water Sci 6:115–135
Moran M, How to grow banana plants (2020) Retrieved from https://www.wikihow.com/Grow-Banana-Plants
Macrae R, Robinson RK, Sadler MJ (1993) Bananas and plantains. Food Technology and Nutrition Academic Press, Encyclopaedia of Food Science
School Science Lessons, Banana Project (2021) Retrieved from https://www.uq.edu.au/_School_Science_Lessons/BaProj.html
Alwi H, Idris J, Musa M and Ku Hamid KH, A preliminary study of banana stem juice as a plant-based coagulant for treatment of spent coolant wastewater. J. Chem. (2013)
Asif M, Kaur A (2018) Biologically active phytochemical contents and biological activities of whole Musa Acuminata (banana) plant. International Journal of Recent Advances in Medical and Pharma Research 1:5–33
Feriotti DG and Iguti AM (2012)Proposal for use of pseudostem from banana tree (Musa cavendish). In: International Congress of Engineering and Food
Zhang P, Whistler RL, BeMiller JN, Hamaker BR (2005) Banana starch: production, physicochemical properties, and digestibility- a review. Carbohydr Polym 59:443–458
Apsara M, Pushpalatha PB (2002) Quality upgradation of jellies prepared using pectin extracted from fruit wastes. J Trop Agric 40:31–34
Elanthikkal S, Gopalakrishnapanicker U, Varghese S, Guthrie JT (2010) Cellulose microfibres produced from banana plant wastes: isolation and characterization. Carbohydr Polym 80:852–859
Aziz NAA, Ho LH, Azahari B, Bhat R, Cheng LH, Ibrahim MNM (2011) Chemical and functional properties of the native banana (Musa acuminata× balbisiana colla cv Awak) pseudo-stem and pseudo-stem tender core flours. Food Chem 128:748–753
da Mota RV, Lajolo FM, Cordenunsi BR, Ciacco C (2000) Composition and functional properties of banana flour from different varieties. Starch-Stärke 52:63–68
Emaga TH, Robert C, Ronkart SN, Wathelet B, Paquot M (2008) Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour Technol 99:4346–4354
Jadhav SA, Kataria PK, Bhise KK, Chougule SA (2013) Amylase production from potato and banana peel waste. Int J Curr Microbiol Appl Sci 2:410–414
Oshoma CE, Eguakun-Owie SO, Obuekwe IS (2019) Utilization of banana peel as a substrate for single cell protein and amylase production by Aspergillus niger. African Scientist 18:143–150
Liang S, Gliniewicz K, Gerritsen AT, McDonald AG (2016) Analysis of microbial community variation during the mixed culture fermentation of agricultural peel wastes to produce lactic acid. Bioresour Technol 208:7–12
Alsaheb RAA, Aladdin A, Othman NZ, Malek RA, Leng OM, Aziz R, Enshasy HAE (2015) Lactic acid applications in pharmaceutical and cosmeceutical industries. J Chem Pharm Res 7:729–735
Manach C, Scalbert A, Morand C, Remesy C, Jimenez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
from plant to health (2008) de Pascual-Teresa S. and Sanchez-Ballesta MT, Anthocyanins. Phytochem Rev 7:281–299
Sui X, Zhang Y, Zhou W (2016) Bread fortified with anthocyanin-rich extract from black rice as nutraceutical sources: its quality attributes and in vitro digestibility. Food Chem 196:910–916
Pazmiño-Durán EA, Giusti MM, Wrolstad RE, Glória MBA (2001) Anthocyanins from banana bracts (Musa X paradisiaca) as potential food colorants. Food Chem 73:327–332
Sutikno NMD, Rahayu S (2014) Fabrication and characterization of banana flower extract anthocyanin-based organic solar cell. J Adv Agric Technol 1:89–93
Ove TA, Kamal MM, Nasim SMNI, Momin MMI, Mondal SC (2019) Extraction and quantification of anthocyanin from banana bracts using different pH and solvent concentration. Int J 4:060–064
Kitdamrongsont K, Pothavorn P, Swangpol S, Wongniam S, Atawongsa K, Svasti J, Somana J (2008) Anthocyanin composition of wild bananas in Thailand. J Agric Food Chem 56:10853–10857
Chen L, Li D, Hao D, Ma X, Song S, Rong Y (2020) Study on chemical compositions, sensory properties, and volatile compounds of banana wine. J Food Process Pres 44:14924
Hasbullah UHAA, Mentari AB, Kholisoh SN and Hidayat TN (2021) Sensory properties of analog coffee from banana peels. AGROINTEK 15
Kennedy J (2009) Bananas and people in the homeland of genus Musa: not just pretty fruit. Ethnobot Res Appl 7:179–197
Emaga TH, Andrianaivo RH, Wathelet B, Tchango JT, Paquot M (2007) Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem 103:590–600
Someya S, Yoshiki Y, Okubo K (2002) Antioxidant compounds from bananas (Musa Cavendish). Food Chem 79:351–354
Anhwange BA (2008) Chemical composition of Musa sapientum (banana) peels. J Food Technol 6:263–266
Mokbel MS, Hashinaga F (2005) Antibacterial and antioxidant activities of banana (Musa, AAA cv. Cavendish) fruits peel. Am J Biochem Biotechnol 1:125–131
Devatkal SK, Kumboj R, Paul D (2014) Comparative antioxidant effect of BHT and water extracts of banana and sapodilla peels in raw poultry meat. J Food Sci Technol 51:387–391
Saravanan K, Aradhya SM (2011) Polyphenols of pseudostem of different banana cultivars and their antioxidant activities. J Agric Food Chem 59:3613–3623
Chye FY, Sim KY (2009) Antioxidative and antibacterial activities of Pangium edule seed extracts. Int J Pharmacol 5:285–297
Chanwitheesuk A, Teerawutgulrag A, Kilburn JD, Rakariyatham N (2007) Antimicrobial gallic acid from Caesalpinia mimosoides Lamk. Food Chem 100:1044–1048
Lu Z, Nie G, Belton PS, Tang H, Zhao B (2006) Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem Int 48:263–274
Artali R, Beretta G, Morazzoni P, Bombardelli E, Meneghetti F (2009) Green tea catechins in chemoprevention of cancer: a molecular docking investigation into their interaction with glutathione S-transferase (GST P1–1). J Enzyme Inhib Med Chem 24:287–295
Zheng J, Ding C, Wang L, Li G, Shi J, Li H, Wang H, Suo Y (2011) Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem 126:859–865
Kulma A, Szopa J (2007) Catecholamines are active compounds in plants. Plant Sci 172:433–440
Vu HT, Scarlett CJ, Vuong QV (2018) Phenolic compounds within banana peel and their potential uses: a review. J Funct Foods 1:238–248
Correa M, Bombardelli MC, Fontana PD, Bovo F, Messias-Reason IJ, Maurer JBB, Corazza ML (2017) Bioactivity of extracts of Musa paradisiaca L. obtained with compressed propane and supercritical CO2. J Supercrit Fluids 122:63–69
Rattanavichai W, Cheng W (2014) Effects of hot-water extract of banana (Musa acuminata) fruit’s peel on the antibacterial activity, and anti-hypothermal stress, immune responses and disease resistance of the giant freshwater prawn. Macrobrachium rosenbegii Fish Shellfish Immunol 39:326–335
Saleem M (2009) Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett 285:109–115
Umar Lule S, Xia W (2005) Food phenolics, pros and cons: a review. Food Rev Int 21:367–388
Tomás-Barberán FA, Clifford MN (2000) Dietary hydroxybenzoic acid derivatives–nature, occurrence and dietary burden. J Sci Food Agric 80:1024–1032
Locatelli C, Filippin-Monteiro FB, Creczynski-Pasa TB (2013) Alkyl esters of gallic acid as anticancer agents: a review. Eur J Med Chem 60:233–239
Rocha LD, Monteiro MC, Teodoro AJ (2012) Anticancer properties of hydroxycinnamic acids-a review. Cancer Clin Oncol 1:109–121
Gülçin İ (2006) Antioxidant activity of caffeic acid (3, 4-dihydroxycinnamic acid). Toxicology 217:213–220
Shukla S, Gupta S (2010) Apigenin: a promising molecule for cancer prevention. Pharm Res 27:962–978
Hosseinzadeh H, Nassiri-Asl M (2014) Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J Endocrinol Invest 37:783–788
Tapas AR, Sakarkar DM, Kakde RB (2008) Flavonoids as nutraceuticals: a review. Trop J Pharm Res 7:1089–1099
Patel K, Singh GK, Patel DK (2018) A review on pharmacological and analytical aspects of naringenin. Chin J Integr Med 24:551–560
Luo Y, Umegaki H, Wang X, Abe R, Roth GS (1998) Dopamine induces apoptosis through an oxidation-involved SAPK/JNK activation pathway. J Biol Chem 273:3756–3764
Cadet JL, Brannock C (1998) Invited review free radicals and the pathobiology of brain dopamine systems. Neurochem Int 32:117–131
Kanazawa K, Sakakibara H (2000) High content of dopamine, a strong antioxidant, in cavendish banana. J Agric Food Chem 20:844–848
Pothavorn P, Kitdamrongsont K, Swangpol S, Wongniam S, Atawongsa K, Svasti J, Somana J (2010) Sap phytochemical compositions of some bananas in Thailand. J Agric Food Chem 58:8782–8787
Singh B, Singh JP, Kaur A, Singh N (2016) Bioactive compounds in banana and their associated health benefits–a review. Food Chem 206:1–1
Akinyele BJ, Agbro O (2007) Increasing the nutritional value of plantain wastes by the activities of fungi using the solid state fermentation technique. Res J Microbiol 2:117–124
Amarnath R, Balakrishnan V (2007) Evaluation of the banana ( Musa paradisiaca ) plant by-product’s fermentation characteristics to assess their fodder potential. Int J Dairy Sci 2:217–225
Subagyo A and Chafidz A (2018) Book chapter published by Intechopen. Available on internet
Oliveira L, Evtuguin DV, Cordeiro N, Silvestre AJ, Silva AM, Torres IC (2006) Structural characterization of lignin from leaf sheaths of “Dwarf Cavendish” banana plant. J Agric Food Chem 54:2598–2605
Zuluaga R, Putaux JL, Cruz J, Vélez J, Mondragon I, Gañán P (2009) Cellulose microfibrils from banana rachis: effect of alkaline treatments on structural and morphological features. Carbohydr Polym 76:51–59
Cherian BM, Pothan LA, Nguyen-Chung T, Mennig G, Kottaisamy M, Thomas S (2008) A novel method for the synthesis of cellulose nanofibril whiskers from banana fibers and characterization. J Agric Food Chem 56:5617–5627
Pothan LA, Potschke P, Habler R, Thomas S (2005) The static and dynamic mechanical properties of banana and glass fiber woven fabric-reinforced polyester composite. J Compos Mater 39:1007–1025
Maleque M, Belal FY, Sapuan SM (2007) Mechanical properties study of pseudo-stem banana fiber reinforced epoxy composite. Arab J Sci Eng 32:359–364
Jacob N, Prema P (2008) Novel process for the simultaneous extraction and degumming of banana fibers under solid-state cultivation. Br J Microbiol 39:115–121
Mire MA, Benjelloun-Mlayah B, Delmas M, Bravo R (2005) Formic acid/acetic acid pulping of banana stem (Musa Cavendish). Appita Journal: Journal of the Technical Association of the Australian and New Zealand Pulp and Paper Industry 58:393
Fagbemigun TK, Fagbemi OD, Buhari F, Mgbachiuzo M, Igwe CC (2016) Fibre characteristics and strength properties of Nigerian pineapple leaf ( Ananas cosmosus ), banana peduncle and banana leaf ( Musa sapientum ) - potential green resources for pulp and paper production. J Sci Res Reports 12:1–13
Nassar M, Ebrahim EE, Sherif HM, Ali MA (2021) Optimization of banana stem pulp to substitute softwood pulp for high quality paper. Egypt J Chem 64:1461–1469
Marella JBR, Madireddy S, Maripi AN (2014) Production of pulp from banana pseudo stem for grease proof paper. Table of Content Topics 61(2):61–99
Sakare P, Bharimalla AK, Dhakane-Lad J, Patil PG (2020) Development of greaseproof paper from banana pseudostem fiber for packaging of butter. J Nat Fibers 18:1974–1982
Puvvada N, Kumar BP, Konar S, Kalita H, Mandal M, Pathak A (2012) Synthesis of biocompatible multicolor luminescent carbon dots for bioimaging applications. Sci Technol Adv Mater 13:045008
Vandarkuzhali SAA, Jeyalakshmi V, Sivaraman G, Singaravadivel S, Krishnamurthy KR, Viswanathan B (2017) Highly fluorescent carbon dots from pseudo-stem of banana plant: applications as nanosensor and bio-imaging agents. Sens Actuators, B Chem 252:894–900
Santoyo-Aleman D, Sanchez LT, Villa CC (2019) Citric-acid modified banana starch nanoparticles as a novel vehicle for β-carotene delivery. J Sci Food Agric 99:6392–6399
Nayak S, Sajankila SP, Rao CV (2021) Green synthesis of gold nanoparticles from banana pith extract and its evaluation of antibacterial activity and catalytic reduction of malachite green dye. J Microbiol, Biotechnol Food Sci 7:641–645
Xu YX, Hanna MA, Isom L (2008) “Green” Chemicals from renewable agricultural biomass—a mini review. Open Agr J 2:54–61
Mohanty AK, Misra M and Drzal LT (2018) Sustainable bio-composites from renewable resources: opportunities and challenges in the green materials world. Renew Energy 396–409
Saxena RC, Adhikari DK, Goyal HB (2009) Biomass-based energy fuel through biochemical routes: a review. Renewable Sustainable Energy Rev 13:167–178
Reddy N, Yang YQ (2005) Biofibers from agricultural by-products for industrial applications. Trends Biotechnol 23:22–27
Pimentel D, Patzek TW (2005) Ethanol production using corn, switchgrass and wood; biodiesel production using soybean and sunflower. Nat Resour Res 14:65–76
Raposo S, Pardão JM, Diaz I, Lima-Costa ME (2009) Kinetic modelling of bioethanol production using agro-industrial by-products. Int J of Energy Env 3:8
Manikandan K, Saravanan V, Viruthagiri T (2008) Kinetics studies on ethanol production from banana peel waste using mutant strain of Saccharomyces cerevisiae. Indian J Biotechnol 7:83–88
Harish KRY, Srijana M, Madhusudhan RD, Gopal R (2010) Coculture fermentation of banana agro-waste to ethanol by cellulolytic thermophilic Clostridium thermocellum CT2. Afr J Biotechnol 9:1926–1934
Hammond JB, Egg R, Diggins D, Coble CG (1996) Alcohol from bananas. Bioresour Technol 56:125–130
Brooks AA (2008) Ethanol production potential of local yeast strains isolated from ripe banana peels. Afr J Biotechnol 7:3749–3752
Chanakya HN, Sharma I, Ramachandra TV (2009) Micro-scale anaerobic digestion of point source components of organic fraction of municipal solid waste. Waste Manage 29:1306–1312
Bardiya N, Somayaji D, Khanna S (1996) Biomethanation of banana peel and pineapple waste. Bioresour Technol 58:73–76
Kalia VC, Sonakya V, Raizada N (2000) Anaerobic digestion of banana stem waste. Bioresour Technol 73:191–193
Ilori MO, Adebusoye SA, Lawal AK and Awotiwon OA, Production of biogas from banana and plantain peels Adv Environ. Biol 33–39 (2007)
El-Sayed M, Mansour OY, Selim IZ, Ibrahim MM (2001) Identification and utilization of banana plant juice and its pulping liquor as anti-corrosive materials. J Sci Ind Res 60:738–747
Barhanpurkar S, Kumar A, Purwar R (2015) Charcterisation of banana pseudostem sap used as a mordant for dying. SSRG Int J Polym Text Eng 2:1–7
Gupta G, Baranwal M, Saxena S, Reddy MS (2019) Utilization of banana stem juice as a feedstock material for bioethanol production. CLEAN–Soil. Air, Water 47:1–5
Tripathi SK, Kaur D, Alam I, Bhardwaj NK, Pathak P and Kumar S, Utilization of different microbes to enhance the biogas production from banana stem juice. J Environ Eng 7: (2020)
Klotoé JR, Dougnon TV, Sacramento TI, Dandjesso C, Edorh AP, Fanou VBA, Fah L, Atego JM, Loto F, Dramane K (2012) Hemostatic potential of the sap of Musa sapientum L. (Musaceae). J Appl Pharm Sci 2:65–69
Weremfo A, Adinortey MB, Pappoe ANM (2011) Haemostatic effect of the stem juice of Musa paradisiaca L.(Musaceae) in guinea pigs. Adv Biol Res 5:190–192
Sari Y, Isworo A, Upoyo AS, Sumeru A, Kurniawan DW and Sutrisna E, A comparison of the effectiveness banana stem sap and virgin coconut oil on diabetic wound healing. In IOP Conference Series: Earth and Environmental Science, 746: (2021)
Nguyen D, Novakova A, Spurna K (2017) Antidiabetic compounds in stem juice from banana. Czech J Food Sci 35:407–413
Singh SK, Kesari AN, Rai PK, Watal G (2007) Assessment of glycemic potential of Musa paradisiaca stem juice. Indian J Clin Biochem 22:48–52
Ravi U, Menon L, Gomathy G (2011) Development and quality assessment of value added plantain stem juice incorporated with grape juice. Indian J Nat Prod Resour 2:204–210
Sharma M, Patel SN, Sangwan RS, Singh SP (2017) Biotransformation of banana pseudostem extract into a functional juice containing value added biomolecules of potential health benefits. Indian J Exp Biol 55:453–462
Prasad KV, Bharathi K, Srinivasan KK (1993) Evaluation of Musa (Paradisiaca Linn. cultivar)—“Puttubale” stem juice for antilithiatic activity in albino rats. Indian J Physiol Phannacol 37:337–341
Paul V, Kanny K, Redhi GG (2013) Formulation of a novel bio-resin from banana sap. Ind Crops Prod 43:496–505
Russ W, Pittroff RM (2004) Utilizing waste products from the food production and processing industries. Crit Rev Food Sci Nutr 44:57–62
Rosentrater K, Todey D, Persyn R (2009) Quantifying total and sustainable agricultural biomass resources in South Dakota—a preliminary assessment. Agricultural Engineering International: the CIGR Journal of Scientific Research and Development Manuscript 1059(1058):1
Jayathilakan K, Sultana K, Radhakrishna K, Bawa AS (2012) Utilization of byproducts and waste materials from meat, poultry and fish processing industries: a review. J Food Sci Technol 49:278–293
Weathers PJ, Arsenault PR, Covello PS, McMickle A, Teoh KH, Reed DW (2011) Artemisinin production in Artemisia annua : studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem Rev 10:173–183
Tsamo CV, Herent MF, Tomekpe K, Emaga TH, Quetin-Leclercq J, Rogez H, Larondelle Y, Andre C (2015) Phenolic profiling in the pulp and peel of nine plantain cultivars (Musa sp.). Food Chem 167:197–204
Mahouachi Mahouachi J, López-Climent MF, Gómez-Cadenas A (2014) Hormonal and Hydroxycinnamic acids profiles in banana leaves in response to various periods of water stress. Sci World J 145:496–504
China R, Dutta S, Sen S, Chakrabarti R, Bhowmik D, Ghosh S, Dhar P (2011) In vitro antioxidant activity of different cultivars of banana flower (Musa paradicicus L.) extracts available in India. J Food Sci 76:C1292–C1299
Borges CV, de Oliveira Amorim VB, Ramlov F, da Silva Ledo CA, Donato M, Maraschin M, Amorim EP (2014) Characterisation of metabolic profile of banana genotypes, aiming at biofortified Musa spp. cultivars. Food Chem 145:496–504
Bennett RN, Shiga TM, Hassimotto NMA, Rosa EAS, Lajolo FM, Cordenunsi BR (2010) Phenolics and antioxidant properties of fruit pulp and cell wall fractions of postharvest banana (Musa acuminata Juss.) Cultivars. J Agr 58:7991–8003
Alothman M, Bhat R, Karim AA (2009) Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem 115:785–788
Saravanan K, Aradhya SM (2011) Potential nutraceutical food beverage with antioxidant properties from banana plant bio-waste (pseudostem and rhizome). Food Funct 2:603–610
Loganayaki N, Rajendrakumaran D, Manian S (2010) Antioxidant capacity and phenolic content of different solvent extracts from banana (Musa paradisiaca) and mustai (Rivea hypocrateriformis). Food Sci Biotechnol 5:1251–1258
Kumar PR, Srivastava S, Singh KK, Mathad C, Thin PS (2014) Study of antioxidant and antimicrobial properties, phytochemical screening and analysis of sap extracted from banana (Musa acuminata) pseudostem. Int J Adv Biotechnol Res 5:649–658
Karuppiah P, Mustaffa M (2013) Antibacterial and antioxidant activities of Musa sp. leaf extracts against multidrug resistant clinical pathogens causing nosocomial infection. Asian Pac J Trop Biomed 3:737–42
Jain P, Bhuiyan MH, Hossain KR, Bachar SC (2011) Antibacterial and antioxidant activities of local seeded banana fruits. Afr J Pharmacy Pharmacol 5:1398–1403
Naikwade PV, Gaurav S, Sharayu, D. and Kailas J, Evaluation of antibacterial properties of Musa paradisiaca L. leaves. In Proceeding of the national conference on conservation of natural resources & biodiversity for sustainable development (2014)
Ehiowemwenguan G, Emoghene AO, Inetianbor JE (2014) Antibacterial and phytochemical analysis of banana fruit peel. Iosr J Pharm 4:18–25
Padam BS, Tin HS, Chye FY, Abdullah MI (2012) Antibacterial and antioxidative activities of the various solvent extracts of banana (Musa paradisiaca cv. Mysore) inflorescences. Journal Biol Sci 12:62–73
Zafar IM, Saleha A, Hoque MME, Sohel RM (2011) Antimicrobial and cytotoxic properties of different extracts of Musa sapientum L. subsp. sylvestris. International Res. J. Pharm 2:62–65
Dahham SS, Mohamad TA, Tabana YM, Majid AM (2015) Antioxidant activities and anticancer screening of extracts from banana fruit (Musa sapientum). Academic J Cancer Res 8:28–34
Li F, Li S, Li HB, Deng GF, Ling WH, Wu S, Xu XR, Chen F (2013) Antiproliferative activity of peels, pulps and seeds of 61 fruits. J Funct Foods 5:1298–1309
Nadumane VK, Timsina B (2014) Anticancer potential of banana flower extract: an in vitro study. Bangladesh J Pharmacol 9:628–635
El Zawawy NA (2015) Antioxidant, antitumor, antimicrobial studies and quantitative phytochemical estimation of ethanolic extracts of selected fruit peels. Int J Curr Microbiol Appl Sci 4:298–309
Manthey, John A and Jaitrong S. An HPLC-MS analysis of phenolic antioxidants in banana peel (2016)
Rabbani GH, Teka T, Zaman B, Majid N, Khatun M, Fuchs GJ (2001) Clinical studies in persistent diarrhea: dietary management with green banana or pectin in Bangladeshi children. Gastroenterology 121:554–560
Horigome T, Sakaguchi E, Kishimoto C (2002) Hypocholesterolaemic effect of banana (Musa sapientum L. var. Cavendishii) pulp in the rat fed on a cholesterol-containing. Diet.x Br J Nutr 68:231–244
Yin X, Quan J, Kanazawa T (2008) Banana prevents plasma oxidative stress in healthy individuals. Plant Foods Hum Nutr 63:71–76
Agarwal PK, Singh A, Gaurav K, Goel S, Khanna HD, Goel RK (2009) Evaluation of wound healing activity of extracts of plantain banana (Musa sapientum var. paradisiaca) in rats. Indian J Exp Biol 47:32–40
Tewtrakul T, Subhadhirasakul S (2007) Anti-allergic activity of some selected plants in the Zingiberaceae family. J Ethnopharmacol 109:535–538
Itelima J, Onwuliri F, Onwuliri E, Onyimba I, Oforji S (2013) Bio-ethanol production from banana, plantain and pineapple peels by simultaneous saccharification and fermentation process. Int J Env Sci Dev 4:213–216
Singh AK, Rath S, Kumar Y, Masih H, Jyotsna K (2014) Peter, Benjamin JC, Singh PK and Singh DP, Bio-ethanol production from banana peel by simultaneous saccharification and fermentation process using cocultures Aspergillus niger and Saccharomyces cerevisiae . Int J Curr Microbiol App Sci 3:84–96
Gebregergs A, Gebresemati M, Sahu O (2016) Industrial ethanol from banana peels for developing countries: response surface methodology. Pac Sci Rev A: Nat Sci Eng 18:22–29
Waghmare AG, Arya SS (2016) Utilization of unripe banana peel waste as feedstock for ethanol production. Bioethanol 2:146–156
Nyandiga GO, Siagi ZO, Makokha AB (2014) Optimization of anaerobic fermentation conditions for bioethanol production from banana (Ngombe) peels using yeast in a still reactor. In Proceedings of Sustainable Research and Innovation Conference 5:120–123
Patel H, Patel A, Surati T, Shah G (2012) Potential use of banana peels for the production of fermented products. International Journal of Ecology & Development (IJED) 9:1–7
Palacios S, Ruiz HA, Ramos-Gonzalez R, Martínez J, Segura E, Aguilar M, Aguilera A, Cristóbal GM, Ilyina A (2017) Comparison of physicochemical pretreatments of banana peels for bioethanol production. Food Sci Biotechnol 26:993–1001
Chaudhary N, Chand S and Kaur N, Bioethanol production from fruit peels using simultaneous saccharification and fermentation. GSTF J Bio Sci 3: (2014)
Arumugam R, Manikandan M (2011) Fermentation of pretreated hydrolyzates of banana and mango fruit wastes for ethanol production. Asian J Exp Biol Sci 2:246–256
Thakur S, Shrivastava B, Ingale S, Kuhad RC, Gupte A (2013) Degradation and selective ligninolysis of wheat straw and banana stem for an efficient bioethanol production using fungal and chemical pretreatment. 3 Biotech 3:365–372
Thancharoen T (2014) Rotten banana waste management for bioethanol producing ethanologenic yeasts. In International Conference on Biological, Civil and Environmental Engineering (BCEE-20153–4) (3–4)
Jagessar RC, Fraser C (2016) The fermentation of banana (Musa acuminata), mango (Mangifera indica L.) and pineapple (Ananas comosus) mash in the absence and presence of additives. Am J Res Commun 4:131–140
Ingale S, Joshi SJ, Gupte A (2014) Production of bioethanol using agricultural waste: banana pseudo stem. Br J Microbiol 45:885–892
Download references
Acknowledgements
Authors thankfully acknowledge the financial assistance provided by the Department of Science and Technology, Ministry of Science and Technology, Government of India under the project “Utilization of Banana Stem Juice for renewable energy and value added products.” The authors are grateful to the Director, Thapar Institute of Engineering and Technology, Patiala, India, for providing the facilities to complete the research work.
The financial support for carrying out the study was received under project entitled “Utilization of banana stem juice for renewable energy and value added products” from Department of Science and Technology, Govt. of India (DST/SSTP/Haryana/345).
Author information
Authors and affiliations.
Department of Biotechnology, Thapar Institute of Engineering and Technology, Patiala, 147004, India
Geetika Gupta, Manoj Baranwal, Sanjai Saxena & M. Sudhakara Reddy
You can also search for this author in PubMed Google Scholar
Contributions
MSR involved in conceptualization and funding acquisition; GG participated in writing—original draft preparation; MBW and SS participated in review and editing. All authors participated in the final improvement of the manuscript.
Corresponding author
Correspondence to Geetika Gupta .
Ethics declarations
Conflict of interest.
The authors declare no conflict of interests.

Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Gupta, G., Baranwal, M., Saxena, S. et al. Utilization of banana waste as a resource material for biofuels and other value-added products. Biomass Conv. Bioref. 13 , 12717–12736 (2023). https://doi.org/10.1007/s13399-022-02306-6
Download citation
Received : 09 August 2021
Revised : 24 December 2021
Accepted : 03 January 2022
Published : 17 January 2022
Issue Date : September 2023
DOI : https://doi.org/10.1007/s13399-022-02306-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Banana waste
- Renewable resources
- Waste utilization
- Bioconversion
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 12 August 2019
AI-powered banana diseases and pest detection
- Michael Gomez Selvaraj ORCID: orcid.org/0000-0003-2394-0399 1 ,
- Alejandro Vergara 1 ,
- Henry Ruiz 2 ,
- Nancy Safari 3 ,
- Sivalingam Elayabalan 4 ,
- Walter Ocimati 5 &
- Guy Blomme 6
Plant Methods volume 15 , Article number: 92 ( 2019 ) Cite this article
47k Accesses
208 Citations
256 Altmetric
Metrics details
Banana ( Musa spp.) is the most popular marketable fruit crop grown all over the world, and a dominant staple food in many developing countries. Worldwide, banana production is affected by numerous diseases and pests. Novel and rapid methods for the timely detection of pests and diseases will allow to surveil and develop control measures with greater efficiency. As deep convolutional neural networks (DCNN) and transfer learning has been successfully applied in various fields, it has freshly moved in the domain of just-in-time crop disease detection. The aim of this research is to develop an AI-based banana disease and pest detection system using a DCNN to support banana farmers.
Large datasets of expert pre-screened banana disease and pest symptom/damage images were collected from various hotspots in Africa and Southern India. To build a detection model, we retrained three different convolutional neural network (CNN) architectures using a transfer learning approach. A total of six different models were developed from 18 different classes (disease by plant parts) using images collected from different parts of the banana plant. Our studies revealed ResNet50 and InceptionV2 based models performed better compared to MobileNetV1. These architectures represent the state-of-the-art results of banana diseases and pest detection with an accuracy of more than 90% in most of the models tested. These experimental results were comparable with other state-of-the-art models found in the literature. With a future view to run these detection capabilities on a mobile device, we evaluated the performance of SSD (single shot detector) MobileNetV1. Performance and validation metrics were also computed to measure the accuracy of different models in automated disease detection methods.
Our results showed that the DCNN was a robust and easily deployable strategy for digital banana disease and pest detection. Using a pre-trained disease recognition model, we were able to perform deep transfer learning (DTL) to produce a network that can make accurate predictions. This significant high success rate makes the model a useful early disease and pest detection tool, and this research could be further extended to develop a fully automated mobile app to help millions of banana farmers in developing countries.
Bananas ( Musa spp.) are one of the world’s most important fruit crops in terms of production volume and trade [ 1 ]. Though a major staple food in Africa, Asia, and Latin America, only 13% of bananas produced are globally traded [ 2 ], clearly indicating the fruit’s importance in domestic markets and food security. In East and Central Africa, it is a substantial dietary component, accounting for over 50% of daily total food intake in parts of Uganda and Rwanda [ 3 ]. Smallholder farmers, representing 85% of the world’s farms [ 4 ], face many abiotic and biotic constraints. Several banana pests and diseases have caused significant yield losses across production landscapes [ 5 ] and are a significant threat to global food security. Therefore, early detection of pests and diseases in the field is a first crucial step. Traditional pest and disease identification approaches rely on agricultural extension specialists, but these approaches are limited in developing countries with low human infrastructure capacity. Many smallholder farmers rely on empirical knowledge, which is less effective in overcoming farming challenges [ 6 ]. The early identification of a crop disease or pest can lead to faster interventions with resulting reduced impacts on food supply chains.
Artificial intelligence (AI) with deep learning models which help to identify plant diseases by the plant’s appearance and visual symptoms that mimic human behavior should be considered [ 7 ]. Smartphone-based AI apps could alert farmers and expedite disease diagnosis, thus preventing the possible outbreak of pests and diseases [ 8 ]. Even though many farmers of developing countries do not have access to these advanced tools, internet infiltration and smartphone penetration offer new outfits for in-field crop disease detection. The Global System for Mobile Association (GMSA) predicted that global smartphone subscriptions would reach 5 billion by 2020, of which nearly one billion in Africa [ 9 ]. We do believe that cutting-edge technologies like AI, IoT (Internet of Things), robotics, satellites, cloud computing, and machine learning are transfiguring agriculture and helping farmers foresee their near future.
Deep learning is a novel method for image processing and object detection with greater accuracy in the classification of various crop diseases [ 10 ]. Transfer learning is one such popular approach in deep learning, where pre-trained models are adapted to do a new task. Deep transfer learning (DTL) generates a fresh framework for digital image processing and predictive analytics, with greater accuracy and has huge potential in crop disease detection. DTL approach also offers a promising avenue for in-field disease recognition using large trained image datasets and bids a shortcut to the developed models to meet the restrictions that are offered by mobile application [ 11 ]. This would have a distinct practical value for real field environment.
Earlier investigations have validated AI-based recognition of crop diseases in wheat [ 12 ], cassava [ 11 ] and on datasets of healthy and diseased plants [ 8 , 13 ]. Crop disease recognition based on a computerized image system through feature extraction has revealed promising results [ 14 ] but extracting features is computationally rigorous and involves expert knowledge for robust depiction. Only few restricted large, curated image datasets of crop disease library exists [ 10 ]. The PlantVillage platform holds over 50,000 images of different crops and diseases [ 15 ]. However, most of these images were taken with detached leaves on a plain background, and CNN trained on these images did not achieve well when using real field images [ 8 ]. To build robust and more practical detection models, plenty of healthy and diseased images taken from different infected parts of the plants, and growing under different environmental conditions are needed. These images subsequently need to be labeled and pre-screened by plant pathology experts. So far, existing crop disease detection models are mostly focusing on leaf symptoms. Unfortunately, numerous symptoms also appear in other parts of the plant and the best examples are banana pest and disease linked symptoms.
The objective of this study was to apply state-of-the-art deep learning techniques for the detection of visible banana disease and pest symptoms on different parts of the banana plant. We also considered the potential for adapting pre-trained deep learning CNN models to detect banana disease and pest symptoms using a large dataset of experts’ pre-screened real field images collected from Africa and India.
Materials and methods
System description.
Our DTL system dataset consists of five major banana diseases along with their respective healthy classes; dried/old age leaves and banana corm weevil ( Cosmopolites sordidus ) damage symptom classes (Table 1 ). Since these major diseases and pest can affect different parts of the banana plant, we ended up with six different models (entire plant, leaves, pseudostem, fruit bunch, cut fruits and corm) and 18 different classes (Table 1 ) to achieve maximum accuracy. An overview of the DTL system is illustrated in Fig. 1 .

Overview of deep transfer learning (DTL) system for banana disease and pest detection
Dataset collection
Our dataset comprises of about 18,000 field images of banana, collected by banana experts, from Bioversity International (Africa) and Tamil Nadu Agricultural University (TNAU, Southern India) (Additional file 2 : Table S1). These field images were captured under different environmental conditions to build a robust model. For that purpose, various banana experts visited several banana farms located in disease/pest hotspots of Africa (Eastern Democratic Republic of Congo, Central Uganda, Burundi and Benin Republic) and Southern India (Tamil Nadu and Kerala). Our current dataset consists of various types of data, including images with various resolutions (cell phone, tablets, standard RGB camera); light conditions depending on time of image taking (e.g., illumination), season (e.g., temperature, humidity), and different environmental locations (e.g., Africa, India). We have collected the images at different growing phases of the crop (i.e., vegetative and reproductive). To prevent our model from being confused between dried/old leaves and diseased leaves, we also collected numerous images of dried and old age leaves at different plant growth stages. Images of a specific disease were collected from different varieties, at different plant growth stages and in different environments (Africa and India) in order to enrich the image library (Additional file 2 : Table S1).
Our current CIAT banana image library consists of approximately 18,000 real field images. But in this present study, our datasets cover healthy plants (HP), dried/old age leaves (DOL) and a balanced number of images (700 images) from five major diseases such as, Xanthomonas wilt of banana (BXW), Fusarium wilt of banana (FWB), black sigatoka (BS), yellow sigatoka (YS) and banana bunchy top disease (BBTV) along with the banana corm weevil (BCW) pest class. The major pest (corm weevil) and disease class symptoms and their control measures are presented in Additional file 2 : Table S2. Since symptoms of different diseases and pests are seen at different parts of the banana plants, we captured images of all the plant parts (Fig. 2 ). Our current library was structured based on the disease and the affected plant parts so each part of the plant represents a model.
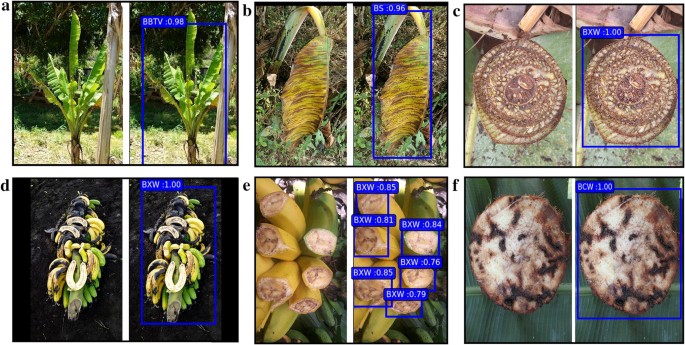
Detected classes and expected output from each model. a Entire plant affected by banana bunchy top virus (BBTV), b leaves affected by black sigatoka (BS), c cut pseudostem of Xanthomonas wilt (BXW) affected plant showing yellow bacterial ooze, d fruit bunch affected by Xanthomonas wilt (BXW), e cut fruit affected by Xanthomonas wilt (BXW), f corm affected by banana corm weevil (BCW)
Data labeling
The image tagging process was done using LabelImg software [ 16 ]. Labels and coordinates of the boxes were saved as an XML file, in the same format (PASCAL VOC) used by ImageNet [ 17 ]. The number of annotated samples corresponded to the number of bounding boxes labeled in each image. Every image could contain more than one annotation depending on the number of infected areas of the plant parts (Fig. 3 ).

Demonstration of the disease detection process during training. a Original raw images, b labeled process (desired output), c disease detection
CNN architectures
To train the models, we used three different architectures, such as ResNet50 [ 18 ], InceptionV2 [ 19 ] and MobileNetV1 [ 20 ]. For the object detector model architecture, we chose Faster RCNN with ResNet50 and InceptionV2 due to their accuracy. Single Shot Multibox (SSD) model was selected with the MobileNetV1 since this was one of the fastest object detection models available in TensorFlow [ 21 ]. To train these models, we used a python deep learning library called TensorFlow and its object detection Application Programming Interface (API) with the Graphics Process Unit (GPU) version [ 22 ]. Pre-trained models were trained with COCO (Common objects in context) data set [ 23 ], and it is openly available in the TensorFlow object detection API zoo models. These three architectures were re-trained using the transfer learning approach from the pre-trained versions. To finetune the original hyperparameters, the following configuration changes were executed, batch size and epoch number. The batch size was changed only in the MobileNetV1 from 24 to 6, and the epoch number was kept 15,000 for all the architectures trained.
One of the most challenging tasks in machine learning is splitting the data without suffering from overfitting, under fitting or generalization hitches. Nevertheless, there are several refined statistical sampling methods which provide a path to deal with these common disputes [ 24 ]. For developing banana model, our dataset was divided into the following proportions of 70%, 20%, 10%, for training (Ttr), validation (Tv) and testing (Tt), respectively. The simple random sampling (SRS) technique was selected, considering that it is efficient and simple to implement [ 24 ].
Performance metrics
Loss function.
Classification loss is used to measure the model’s confidence by classifying the pixels region delimitated by the bounding box [ 25 ] and the localization loss measures the geometric distance between the predicted bounding box and the ground truth annotation (validation bounding boxes). In this paper, we used the object detection API [ 26 ] to estimate the total loss function to measure model performance. The overall loss function or total loss was a weighted combination of the classification loss (classif) and the localization loss (loc).
The mean average precision (mAP) was used as the validation metric for banana disease and pest detection. Precision refers to the accuracy. mAP score was calculated as follows: Average across the number of classes of the true positive divided by the true positives plus false positive as in the following equation
Confusion matrix
In addition to mAP score, we also computed a confusion matrix (CM) for each selected model based on the object detection script [ 27 ]. Computation of CM protocol is described below. For each detection, the algorithm mines all the ground-truth boxes and classes, along with the detected boxes, classes, and scores of Intersection over Union (IoU). Only detections with a score ≥ 0.5 were considered and anything under this threshold were excluded. For each ground-truth box, the algorithm creates the IoU with each detected box. A match was found if both boxes had an IoU ≥ 0.5. The list of matches was trimmed to remove duplicates (ground-truth boxes that match with more than one detection box or vice versa). If there are duplicates, the best match (greater IoU) was continually selected. The CM was updated to reflect the resultant matches between ground-truth and detections. A detected box was reflected as correct where the intersection over union (IoU) of that box and the corresponding ground-truth box was ≥ 0.5. The formula for calculating IoU is shown in Fig. 4 . In the final step, the CM was normalized.
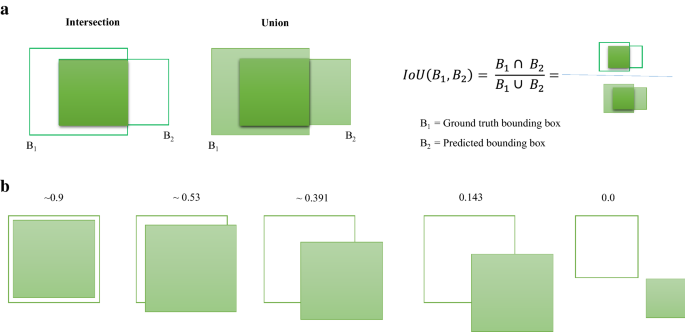
Diagram explaining intersect over union (IOU) calculation. a Intersection over union (IoU) formula where B 1 : ground truth bounding box and B 2 : predicted bounding box, b samples of calculated scores
Software and hardware system
The list of hardware and software used in this study was depicted in Table 2 . For algorithm implementation, and data wrangling scripts, python 3.6 was used. Then models were re-trained using the powerful library called TensorFlow object detection API [ 28 ] developed by Google, this library support control process unit (CPU) and GPU training and inference.
Results and discussion
Banana dataset collection and annotation.
Banana is liable to various types of pests and diseases for which symptoms occur in different parts of the plant (Table 1 ). The occurrences of these diseases depends on many factors, such as environment, temperature, humidity, rainfall, variety, season, nutrition, etc. For instance, certain diseases are localized in a particular country, region or continent, such as, Xanthomonas wilt of banana which is very specific to Africa. Therefore, reliable and accurate image collection at hotspots and strong labeling is very important. Since we are aiming for a global solution, we collected the image dataset of major banana diseases from different disease hotspots through our CGIAR network. Publicly available datasets poorly cover banana disease/pest symptom images, and the PlantVillage public dataset so far doesn’t include banana images. We collected our own datasets of leaves infected by specific pathogens at different infection stages and other infected plant parts such as entire plants, fruit bunch, cut fruits, pseudostem and corms etc. with the help of well-trained banana experts using different cameras with various resolutions (Table 1 , Fig. 2 ). Currently our CIAT-Bioversity, CGIAR dataset contains more than 18,000 expert pre-screened original field images, but in this study we utilized only 12,600 images to create banana image data sets. Since our ultimate aim is to develop a mobile-assisted banana disease detection tool targeting banana farmers across the globe/wordwide and the scientific community around the world, we enriched our image library with a diverse collection of images from different disease hot spots (Additional file 2 : Table S1). To build a robust model, images were captured in real field scenarios on banana farms. A heterogeneous background is an essential feature of any real field images, most of the publicly available datasets are images of leaves in a controlled environment and simple background. For this reason, we tried to create many variations while collecting data from the field. The more the variation in the dataset, the better is the generalization of the trained model. The images were captured with different camera devices (Additional file 2 : Table S1) with diverse background. Furthermore, the challenging part of our image dataset is the background variations caused by the surroundings of the field, dried leaves on the floor, overlapping leaves from neighboring plants etc. This made our model more robust to adapt any changes in the real-time background.
We annotated the images to train our CNN by setting the images of different classes in distinct folders. We randomly picked 75% of images of each class and put them into a training set. Likewise, another 25% of images of each class were put into a test set. The training and the test set both contained 700 real field images per class (700 × 18 classes = 12,600) which has made the data set well balanced. The categories and the number of annotated samples used in our system can be seen in Table 1 . We carried out a strong labeling approach whereby the banana experts confirmed the typical symptoms on each and every image of the data set, as a result we ended up with a total of 30,952 annotations (Table 1 ). Even though this strategy is time-consuming, we worked with three human experts to annotate the whole banana dataset which took almost 4 weeks. The tediousness of data collection and labeling had forced earlier studies [ 29 , 30 ] to use small datasets to train and test classifiers. The use of small labeled datasets is also a limiting factor in machine learning, and it can lead to over or underfitting [ 31 ]. Most of the publicly available data sets are weakly labeled and resulted in poor performance.
We summarized the total loss function (Additional file 1 : Fig. S1a–f) only for the winner models (Additional file 2 : Table S3). In general, we could observe that the accuracy increased while loss decreased gradually with epoch. For Corm damage images, the reported error was high until the 1500th iteration, then started to go down and after the 4000th step remained constant (Additional file 1 : Fig. S1f), the same behavior was noticed in Pseudostem and Cut Fruits (Additional file 1 : Fig. S1c, e), where after 2000 iterations the error remained constant until the end. For the entire plant and leaves (Additional file 1 : Fig. S1a, b), although a loss was found to be below 0.3 in the last iteration, it suffered due to lot of variations, which was evident since these two models (entire plant and leaf) were found to be low accurate compared to other models studied (Table 3 ). The probable reason was clearly explained further by other performance metrics below.
Performance metrics and validation of developed models
In recent years, deep learning techniques, and in particular convolutional neural networks (CNNs), recurrent neural networks and long-short term memories (LSTMs), have shown great success in visual data recognition, classification, and sequence learning tasks. In the field of computer vision specifically, a set of CNN architectures have been emerging and they have proved to achieve tasks like object classification, detection and segmentation. In this paper, we retrained MobileNetV1, InceptionV2 and RestNet50 architectures using transfer learning to detect the banana pest and diseases. In order to improve the accuracy, the diseases were grouped by plant parts, and a different model was trained for each plant part (Table 1 ). Transfer learning is a progress that has the huge potential of being extensively used in crop phenomics and pest and disease detection. Transfer learning is particularly interesting, as its improved performance of deep neural networks by evading intricate data mining and labeling efforts [ 32 ].
There are different metrics to measure the accuracy and effectiveness in object detection models. In this study, we used mAP which is one of the widely used metrics in the literature [ 33 , 34 ], especially for detection. Additionally, for each best model, a confusion matrix was generated. Earlier studies on detection revealed that the mAP score had become the accepted and standard way in competitions such as PASCAL VOC [ 35 ], ImageNet, and COCO datasets. More detail results are described below.
The accuracy of the models based on mAP score is presented in Table 3 . For the entire plant, leaves, pseudostem and fruit bunch models performed better in Faster R-CNN (faster regions with convolutional neural network) ResNet50 than others tested, which achieved an mAP score of 73%, 70%, 99%, and 97%, respectively. For cut fruits and corm, Faster R-CNN InceptionV2 worked better with the mAP accuracy of 95% and 98%, respectively. Fuentes et al. 2017 [ 33 ], used three CNN-based systems (Faster R-CNN, R-FCN and SSD) which performed object localization and disease diagnosis processes simultaneously and their system achieved more than 86.0% mean average precision on annotated tomato leaf images. In this present study, ResNet50 and InceptionV2 models have almost similar performance in all the cases compared to MobileNetV1 (Table 3 ). In generalized recognition, Faster R-CNN [ 36 ], models have been widely used and have achieved good results.
In this research, to achieve greater accuracy, we considered the complexity of the model as an important factor to select the best architectures for the training set. This characteristic could be measured by counting the total amount of learnable parameters or the number of operations. As a result, we selected three architectures (Inception, ResNet, MobileNet). Since complexity is associated with the capacity of the model to extract more features from the images, it is expected inceptionV2 to be the most accurate among three architectures. However, it is always a trade-off between complex and simple architecture especially when you specifically think about mobile application.
We also noticed higher accuracy (more than 95%) for pseudostem, fruit bunch and corm compared to entire plant (73%) and leaf models (70%). This was expected in the entire plant model due to background noise in field environment, multiple classes (Fig. 5 b) in the single image and wide angle. Wide-angle images are often more complex due to the substantial overlap of multiple leaves and symptoms are scattered in different leaves. In the case of banana it is much more complex because of the specific plant morphology and large leaf size. We also observed that during the labeling process, a single class per image was working as a ground-truth for the model (Fig. 5 a, b). But in real life scenario, one image could have multiple classes as seen in Fig. 3 . Developed entire plant and leaf model from this study is finding multiple classes in single image (Fig. 5 b) which is more practical and useful in the real-time field application, since our ground-truth data is solely grouped on single class (Fig. 5 a) which brought the mAP score lower than expected and it was the main cause. It was a little surprise for leaf model where we expect more than 90% accuracy since it is not very wide angle like the entire plant class. But these wide-angle images from field environment expected to have more background noises. To confirm these results in leaf model, we did an additional test to select images containing only one class per image, which reflected on higher accuracy more than 70% (Additional file 2 : Table S4). Moreover, this accuracy was further increased (more than 90%) when the new image dataset contain only one focused leaf per image (Additional file 2 : Table S4). From these results, it is clear the complexity of the banana leaf morphology, disease symptoms, multiple classes in single image, field background noises etc. Unlike other crops such as rice, wheat, and cassava, banana leaves are very big, that makes the angle wider than other crops which increases the complexity in real-time field images (Fig. 5 ). In the case of pseudostem, fruit bunch, cut fruits and corm field images used in this study have more focused images towards the object with less background variation and single class per image which reduced the complexity and improved mAP score (Table 3 ).
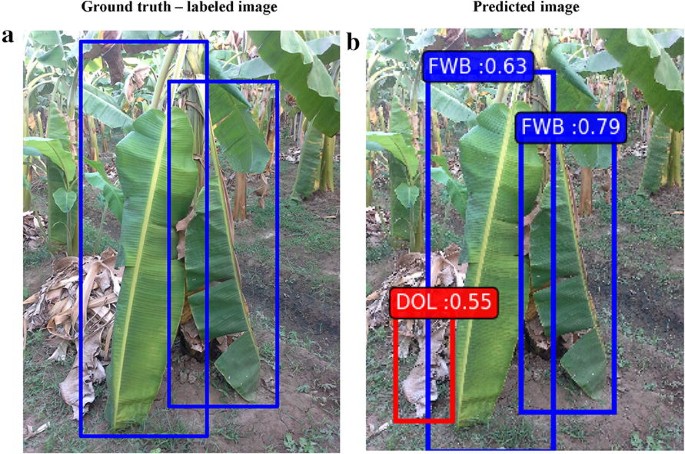
Comparison between ground-truth labeled image and the predicted classes by model. a Ground-truth labeled image of FWB, b image after predicted by a model
In the field of deep learning, specifically the problem of statistical classification, the confusion matrix, also known as an error matrix, is a specific table layout that allows visualization of the performance of an algorithm. It considers different metrics: the true positives (TP), true negatives (TN), false positives (FP) and false negatives (FN) etc. Based on the results obtained on the test dataset, we generated a confusion matrix for each of the best architectures (Fig. 6 a–f). Each confusion matrix gave us a accuracy per disease (classes) and quantitative representation of the classes in which the model is misclassified or confused (Fig. 6 a–f). Due to the complexity of the patterns shown in each class from different plant parts, the system tends to be confused on several classes that results in lower performance. Based on the results, we can visually evaluate the performance of the classifier and determine which classes and features are more prone to confusion. If the number of misclassifications between two particular classes becomes high, it indicates that we need to collect more data on those classes to properly train the convolutional architecture so that it can differentiate between those two classes. For this purpose, we also generated confusion matrix on our validation set for each best CNN architecture. Furthermore, it helps us to identify a future solution in order to avoid those inter-class confusions.
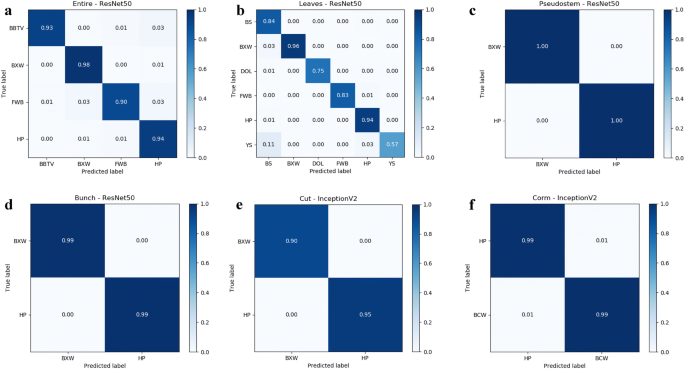
Confusion matrix for the best models identified in this study. a Entire plant—ResNet, b leaves—ResNet, c pseudostem—ResNet, d fruit bunch—ResNet, e cut fruits—inception, f corm—inception
On comparing among the models, leaves produced a lot of confusion and low accuracy (57%) especially yellow sigatoka leaf spot classes (Fig. 6 b), this was expected since YS and BS commonly produce similar symptoms in advanced stages, but early stage symptoms are unique (Additional file 1 : Fig. S2). It is worth mentioning that yellow leaf spot disease appearing more frequently in Asia and Latin America and black leaf spot in Africa, and their treatment and disease controlling measures are almost similar. To handle these issues, we are currently collecting and labeling images of early stage symptoms for improving the accuracy of the model, and the ability to generalize. Because the dataset is not big enough, it was not considered in this study. We also observed medium prediction accuracy in the dried/old age leaf classes (Fig. 6 b), it was obvious that, advanced stages of all leaf diseases will turn to be like dried/old age leaves and we expected this results. So early and mid-stage leaf symptoms are very important to detect the diseases with more accuracy. As we expected, the entire plant, corm, pseudostem, fruit bunch and cut fruits models, we had not found any accuracy or misclassification problems (Fig. 6 a, c–f), which was ranged between 90 and 100% accuracy.
Conclusions and future directions
Many computer visioned approaches for automated crop disease detection and classification have been reported, but still, a detailed exploration of real-time pest and diseases recognition is lagging. In this paper, a novel method of using deep transfer learning method was explored in order to automatically detect banana pest and disease symptoms on different parts of the banana plants using real-time field images. This system introduces a practical and applicable solution for detecting the class and location of diseases in banana plants, which represents a main comparable difference with other methods for plant diseases classification. The developed model was able to detect the difference between healthy and infected plant parts for different banana diseases. All images used in this study are available upon formal request through PestDisPlace ( https://pestdisplace.org/ ) [ 37 ]. It consists of more than 18,000 original expertly pre-screened banana images collected on real farmer’s field in Africa, Latin America and South India and was extended to more than 30,952 annotations. The experimental results achieved accuracy between 70 and 99%, of the different models tested. The robust models developed from this research will be more useful to develop the decision-support system to help early identification of pest and diseases and their management. Models developed in this study are currently utilized to develop a banana mobile app which is currently being tested by collaborative partners in Benin, DR Congo, Uganda, Colombia, and India (Additional file 1 : Fig. S3). The developed model system from this study is easily transferable to other CGIAR mandatory crops.
Future work will comprise the development of a broad structure consisting of server side machinery containing a trained model and an application for smartphone devices with features such as displaying recognized diseases in other CGIAR mandatory crop such as Brachiaria, common bean, cassava, potato and sweet potato. Additionally, future work will involve disseminating the usage of the model by training it for banana disease recognition on wider applications, merging aerial images of banana growing regions captured by drones and convolution neural networks for instant segmentation of multiple diseases. By extending this research, we are hoping to achieve a valuable impact on sustainable development and strengthen banana value chains.
Availability of data and materials
The remotely sensed and field sampling data used in this study is available from the corresponding author upon reasonable request.
Abbreviations
artificial intelligence
Application Programming Interface
banana bunchy top virus
banana corm weevil
black sigatoka
Xanthomonas wilt of banana
Consultative Group on International Agricultural Research
International Center for Tropical Agriculture
convolutional neural network
common objects in context
confusion matrix
control process unit
deep convolutional neural networks
dried/old leaves
deep transfer learning
faster regions with convolutional neural network
false negatives
false positives
Fusarium wilt of banana
graphics process unit
healthy plant
Internet of Things
intersection over union
long-short term memories (LSTMs)
mean average precision
single random sampling
single shot detector
true negatives
true positives
yellow sigatoka
FAO. Banana market review and banana statistics 2012–2013. Market and policy analyses of raw materials, horticulture and tropical (RAMHOT) Products Team. Rome; 2014.
Lescot T. World plantain and banana production systems. In: Proceedings XX international meeting ACORBAT: 9–13 September 2013; Fortaleza; 2013. p. 26–34.
Abele S, Twine E, Legg C. Food security in eastern Africa and the great lakes. Crop Crisis Control Project final report. Ibadan: Int Instit Trop Agric; 2007.
Google Scholar
Nagayets O. Small farms: current status and key trends. In: The future of small farms; 2005. p. 355.
Blomme G, Dita M, Jacobsen KS, Perez Vicente L, Molina A, Ocimati W, Poussier S, Prior P. Bacterial diseases of bananas and enset: current state of knowledge and integrated approaches toward sustainable management. Front Plant Sci. 2017;8:1290.
Article Google Scholar
Hillnhuetter C, Mahlein AK. Early detection and localisation of sugar beet diseases: new approaches. Gesunde Pflanzen. 2008;60(4):143–9.
Camargo A, Smith J. An image-processing based algorithm to automatically identify plant disease visual symptoms. Biosyst Eng. 2009;102(1):9–21.
Mohanty SP, Hughes DP, Salathe M. Using deep learning for image-based plant disease detection. Front Plant Sci. 2016;7:1419.
Intelligence G. The mobile economy Africa 2016. London: GSMA; 2016.
Kamilaris A, Prenafeta-Boldu FX. Deep learning in agriculture: a survey. Comput Elect Agric. 2018;147:70–90.
Ramcharan A, Baranowski K, McCloskey P, Ahmed B, Legg J, Hughes DP. Deep learning for image-based cassava disease detection. Front Plant Sci. 2017;8:1852.
Siricharoen P, Scotney B, Morrow P, Parr G. A lightweight mobile system for crop disease diagnosis. International conference on image analysis and recognition. Berlin: Springer; 2016. p. 783–91.
Chapter Google Scholar
Wiesner-Hanks T, Stewart EL, Kaczmar N, DeChant C, Wu H, Nelson RJ, Lipson H, Gore MA. Image set for deep learning: field images of maize annotated with disease symptoms. BMC Res Notes. 2018;11(1):440.
Mwebaze E, Owomugisha G. Machine learning for plant disease incidence and severity measurements from leaf images. 2016 15th IEEE international conference on machine learning and applications (ICMLA). New York: IEEE; 2016. p. 158–63.
Hughes D, Salathe M. An open access repository of images on plant health to enable the development of mobile disease diagnostics. arXiv preprint arXiv:1511.08060 ; 2015.
LabelImg Software. https://github.com/tzutalin/labelImg/ . Accessed 1 Feb 2019.
ImageNet Data Set. http://www.image-net.org/ . Accessed 12 Mar 2019.
He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. In: Proceedings of the IEEE conference on computer vision and pattern recognition; 2016. p. 770–8.
Ioffe S, Szegedy C. Batch normalization: Accelerating deep network training by reducing internal covariate shift. arXiv preprint arXiv:1502.03167 . 2015.
Howard AG, Zhu M, Chen B, Kalenichenko D, Wang W, Weyand T, Andreetto M, Adam H. Mobilenets: efficient convolutional neural networks for mobile vision applications. arXiv preprint arXiv:1704.04861 . 2017.
Huang J, Rathod V, Sun C, Zhu M, Korattikara A, Fathi A, Fischer I, Wojna Z, Song Y, Guadarrama S. Speed/accuracy trade-offs for modern convolutional object detectors. In: Proceedings of the IEEE conference on computer vision and pattern recognition; 2017. p. 7310–1.
TensorFlow Python API. https://www.tensorflow.org/api_docs/python . Accessed 10 Feb 2019.
COCO Data Set. http://cocodataset.org/ . Accessed 15 Feb 2019.
Reitermanova Z. Data splitting. In: WDS’10 proceedings of contributed papers, Part I, vol 10; 2010. p. 31–6.
Liu W, Anguelov D, Erhan D, Szegedy C, Reed S, Fu CY, Berg AC. Ssd: Single shot multibox detector. In: European conference on computer vision. Springer; 2016. p. 21–37.
Object Detection API Loss Functions Implementation, Tensorflow. https://github.com/tensorflow/models/blob/master/research/object_detection/core/losses.py . Accessed 5 Mar 2019.
Confusion Matrix for Object Detection. https://github.com/svpino/tf_object_detectioncm/blob/master/confusion_matrix.py . Accessed 10 Mar 2019.
Object Detection API, Tensorflow. https://github.com/tensorflow/models/tree/master/research/object_detection . Accessed 20 Feb 2019.
Dandawate Y, Kokare R. An automated approach for classification of plant diseases towards development of futuristic decision support system in Indian perspective. In: 2015 international conference on advances in computing, communications and informatics (ICACCI), IEEE; 2015. p. 794–9.
Mokhtar U, El Bendary N, Hassenian AE, Emary E, Mahmoud MA, Hefny H, Tolba MF. Svm-based detection of tomato leaves diseases. In: Intelligent Systems’ 2014. Springer; 2015. p. 641–52.
Brahimi M, Arsenovic M, Laraba S, Sladojevic S, Boukhalfa K, Moussaoui A. Deep learning for plant diseases: detection and saliency map visualisation. In: Human and machine learning. springer; 2018. p. 93–117.
Pan SJ, Yang Q. A survey on transfer learning. IEEE Trans Knowl Data Eng. 2010;22(10):1345–59.
Fuentes A, Yoon S, Kim S, Park D. A robust deep-learning-based detector for real-time tomato plant diseases and pests recognition. Sensors. 2017;17(9):2022.
Sun J, He X, Ge X, Wu X, Shen J, Song Y. Detection of key organs in tomato based on deep migration learning in a complex background. Agriculture. 2018;8(12):196.
Everingham M, Eslami SA, Van Gool L, Williams CK, Winn J, Zisserman A. The pascal visual object classes challenge: a retrospective. Int J Comput Vision. 2015;111(1):98–136.
Zhang L, Lin L, Liang X, He K. Is faster r-cnn doing well for pedestrian detection? In: European conference on computer vision. Springer; 2016. p. 443–57.
Cuellar W, Mwanzia L, Lourido D, Garcia C, Martínez A, Cruz P, Pino L, Tohme J. PestDisPlace: monitoring the distribution of pests and diseases, version 2.0. International Center for Tropical Agriculture (CIAT); 2018.
Download references
Acknowledgements
The authors would like to thank the International Center for Tropical Agriculture (CIAT) IT unit for providing facilities and logistics support. Joe Tohme, Manabu Ishitani and Wilmer Cuellar from CIAT for guidance and support to do the research. The authors would also like to acknowledge Milton Valencia, Jorge Casas, Maria Montoya, Crysthian Delgado and Frank Montenegro for their help in image annotation and data collection. Thanks to Jules Ntamwira, Jean-Pierre Mafuta and Aman Omondi of Bioversity International, Africa and Deo Kantungeko of IITA, Burundifor their immense support to collect smartphone images. The farmers of Tamil Nadu Banana grower’s federation, Trichy and planters of Tamil Nadu Hill Banana Growers Federation, Lower Palani Hills, Tamil Nadu India are also acknowledged for helping to collect data images. The authors also thank two anonymous reviewers for their detailed suggestions for improving the manuscript. As well as Angela Fernando, CIAT and Escalin Fernando, India for formatting and technical editing.
Funding for field smartphone image collection was provided by Bioversity International in the framework of the RTB-CC3.1 cluster and by the CIAT Agrobiodiversity Research Area to carry out the image processing work & preliminary app development (AGBIO1). This study was supported by the CGIAR Research Program on Roots, Tubers and Bananas (RTB). We thank the RTB Program Management Unit that supported this study and the CGIAR Fund Donors who support RTB ( www.cgiar.org/who-we-are/cgiar-fund/fund-donors-2 ).
Author information
Authors and affiliations.
International Center for Tropical Agriculture (CIAT), A.A. 6713, Cali, Colombia
Michael Gomez Selvaraj & Alejandro Vergara
Department of Soil and Crop Sciences, Texas A&M University, College Station, TX, USA
Bioversity International, Bukavu, South Kivu Province, Democratic Republic of Congo
Nancy Safari
Department of Biotechnology, Imayam Institute of Agriculture and Technology (IIAT), Affiliated to Tamil Nadu Agricultural University (TNAU), Tiruchirappalli, Tamil Nadu, India
Sivalingam Elayabalan
Bioversity International, Kampala, Uganda
Walter Ocimati
Bioversity International, c/o ILRI, Addis Ababa, Ethiopia
You can also search for this author in PubMed Google Scholar
Contributions
MGS, HR and AV designed the study, performed the experiments and are the main contributing authors of the paper. HR, AV, and MGS carried out data annotations, trained algorithms and analyzed the data. GB, SE, NS and WO collected over 17,000 images of disease and pest symptoms/damage, confirmed the symptoms and pre-screened all the images collected in Africa, Malaysia and India. MGS written the paper. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Michael Gomez Selvaraj .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
All authors agreed to publish this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1: figure s1..
Loss function curve for the winner models. a Entire plant—ResNet, b Leaves—ResNet, c Pseudostem—ResNet, d Fuit bunch—ResNet, e Cut fruits—Inception, f Corm—Inception. Fig. S2. Early and late stage symptoms of banana leafspots. a Black sigatoka (BS) late stage, b Yellow sigatoka (YS) late stage, c Black sigatoka (BS) early stage, d Yellow sigatoka (YS) early stage. Fig. S3. Developed mobile application for Banana disease and pest detection. a Initial screen, b Image taking and Scan, c Diagnostic screen, d Recommendations and management.
Additional file 2: Table S1.
Overview of banana data set collections, locations and image acquisition. Table S2. Description of major banana diseases and pest symptoms with their control measures. Table S3. Winner architecture for the models developed in this study. Table S4. mAP score metrics of leaf classes before and after segmentation.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Selvaraj, M.G., Vergara, A., Ruiz, H. et al. AI-powered banana diseases and pest detection. Plant Methods 15 , 92 (2019). https://doi.org/10.1186/s13007-019-0475-z
Download citation
Received : 03 May 2019
Accepted : 30 July 2019
Published : 12 August 2019
DOI : https://doi.org/10.1186/s13007-019-0475-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Artificial intelligence
- Deep learning
- Disease detection
- Transfer learning
- Convolutional neural networks
Plant Methods
ISSN: 1746-4811
- Submission enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Food Sci Technol
- v.56(2); 2019 Feb

Production, application and health effects of banana pulp and peel flour in the food industry
Amir amini khoozani.
Department of Food Science, University of Otago, PO Box 56, Dunedin, 9054 New Zealand
Alaa El-Din Ahmed Bekhit
The past 20 years has seen rapid development of value-added food products. Using largely wasted fruit by-products has created a potential for sustainable use of these edible materials. The high levels of antioxidant activity, phenolic compounds, dietary fibres and resistant starch in banana pulp and peel have made this tropical fruit an outstanding source of nutritive ingredient for enrichment of foodstuffs. Accordingly, processing of separate banana parts into flour has been of interest by many researchers using different methods (oven drying, spouted bed drier, ultrasound, pulsed vacuum oven, microwave, spray drying and lyophilization). Regarding the high level of bioactive compounds, especially resistant starch in banana flour, the application of its flour in starchy foods provides a great opportunity for product development, even in gluten free foods. This review aims to provide concise evaluation of the health benefits of banana bioactive components and covers a wide range of literature conducted on the application of different parts of banana and the flour produced at various ripeness stages in the food industry. Of particular interest, the impact of drying methods on banana flour properties are discussed.
Introduction
Banana is a tropical climacteric fruit and universally comprises a number of species in the genus Musa of the family Musaceae . It is one of the most favored fruits in the world and the fourth most important crop produced globally (Aurore et al. 2009 ). Nearly all of the identified cultivars derived from two diploid species, Musa acuminata and Musa balbisiana , in which the Cavendish variety is the most common. Plantain is related to the hybrid triploid cultivars of banana and is longer, more angular and diverse in shape. Even in the mature state, plantain is firmer than Cavendish and thus it is less valued as a fresh product (Zhang et al. 2005 ). According to the latest FAO statistics, Asia is the largest producer of banana with a share of 54.4% of the world’s banana production. With an average banana consumption of 12 kg per capita (FAOSTAT 2017 ), banana is amongst the world’s major food crops, after rice, wheat and maize.
Banana fruit consists of two parts: peel and pulp. Peel, which is the main by-product of banana, is about 40% of total weight of the fruit. Until recently, banana peel (Bpe) had no useful applications and was dumped as waste, contributing massive amounts of organic materials to be managed. Since researchers have begun to focus on studying the composition of BPe, several possible applications have emerged (Agama-Acevedo et al. 2016 ). Banana pulp (BP), which is the edible part of the fruit, has an abundant amount of nutrients. Studies conducted on BP have investigated different aspects ranging from its use as an ingredient for food enrichment to extraction and isolation of many health-beneficial components, such as different types of starch, cellulose and bioactive compounds (Singh et al. 2016 ). As stated by Kitts ( 1994 ), bioactive compounds are constituents with extra nutritional advantages that are naturally occurring in plants and foods in small amounts. They exert their beneficial biological effects by stimulating the probiotic growth and help in the prevention of cardiovascular disease and cancer (Kris-Etherton et al. 2002 ). Phenolics, carotenoids, flavonoids, biogenic amines, phytosterols, and other phytochemicals can be found in BP and peel (Pereira and Maraschin 2015 ). Due to the presence of these compounds, bananas have a higher antioxidant capacity than some berries, herbs and vegetables (Moongngarm et al. 2014 ). Bioactive compounds in different cultivars of banana and their health benefits were reviewed in details by Singh et al. ( 2016 ).
The increased attention to functional food products and health and wellbeing of consumers in the last decade led to an increased interest in vitamins, minerals, unsaturated fatty acids, bioactive compounds and fibre in food products. The utilization of by-products of fruits, especially banana, has become a trend as of late and many studies are in progress to evaluate their effects on food properties (Chávez-Salazar et al. 2017 ; Kaur et al. 2014 ). As approximately one-third of banana is lost due to the public tendency to consume only ripened fruit, utilization of different parts of the banana at different ripening stages has also gained interest over the past years (Sheikh et al. 2017 ).
This review discusses the health benefits of banana bioactive compounds and utilization of different parts of the banana and the flour produced at various ripeness stages in food applications. Of particular interest, various methods for producing banana flour are evaluated to highlight processing options and their influence on flour nutritional and functional properties.
Composition of banana
Banana pulp is a rich source of essential phytonutrients, including phenolic compounds and vitamins (B3, B6, B12, C and E). It also contains carotenoids, flavonoids, amine compounds and dietary fibre (DF).
Dietary fibres are indigestible carbohydrate polymers that are classified based on their water solubility into two types, soluble fibres (pectin and some hemicelluloses) and insoluble fibres (cellulose, lignin and resistant starch) (Alba et al. 2018 ). In general, it has been reported that Bpe contains more DF than BP (Garcia-Amezquita et al. 2018 ). Extracting pectin from the peels could increase their added value. In addition, BPe has high amount of lignin, cellulose and hemicelluloses fractions which can be extracted as a formed complex substrate named lignocellulosic biomass which could be used to produce bioethanol (Happi Emaga et al. 2008 ). Also Khamsucharit et al. ( 2018 ) signified that the extracted pectin from Bpe could be an alternative source for commercial pectin.
One of the most important DF which has gained a lot of attention in recent years is resistant starch (RS). It is mainly composed of the linear part of starch (amylose) which is fermented by probiotics in the colon, specifically Bifidobacterium and Lactobacillus species (Kale et al. 2002 ). This brings about the production of short chain fatty acids, mainly butyric acid, which has a key role in prevention of colorectal cancer (Amini et al. 2015 ). There are five types of RS introduced up to now: starch that is physically inaccessible in crop’s cell walls (RS1), granular native starch with high crystalline structure (RS2), retrograded starch achieved by heating and cooling of starchy foods (RS3), chemically modified starch (RS4) and amylose-lipid complex (RS5) (Amini Khoozani et al. 2019 ). Fractionation of the DF and RS is different parts of banana in different levels of maturation is shown in Table 1 .
Table 1
Composition of banana flour produced from different ripening stage (dry basis percentage)
RS resistant starch, DF dietary fiber
Unripe banana is rich in RS2, which is beneficial to colon health, while ripe banana contains more digestible starch and protein (Singh et al. 2016 ). Banana peel is a rich source of minerals, bioactive compounds and DF (Kusuma et al. 2018 ). Several studies reported the use of banana peel flour (BPeF) as a functional food source (Agama-Acevedo et al. 2016 ; Ramli et al. 2009 ; Ramli et al. 2010 ; Türker et al. 2016 ). According to some reports, both pulp and peel have high antioxidant activity (Agama-Acevedo et al. 2016 ; González-Montelongo et al. 2010 ). As lipid oxidation in food components is one of the unwanted reactions causing rancidity, food producers rely on synthetic antioxidants to minimize lipid deterioration. Potential health risks, however, is a limiting factor of using these preservatives extensively in food products, especially staple ones (Pathak et al. 2017 ). Given that BPe extract has been found to be non-toxic to human cells, more information has become available on using it as an inexpensive fruit by-product source of antioxidants (Segundo et al. 2017b ). The amount of ash, protein, crude fibre and digestible starch of BPeF was reported to be significantly higher than that of pulp, which makes the BPeF more effective as a functional additive (Nasrin et al. 2015 ). For instance, the higher quantity of ash can be valuable in treating deficiencies of minerals caused by celiac disease (Presutti et al. 2007 ). Additionally, several studies have shown the application of BPe as a low-cost precursor for producing materials such as anionic dye and heavy metal adsorbents (Mahindrakar and Rathod 2018 ; Munagapati et al. 2018 ; Oyewo et al. 2018 ; Singh et al. 2018 ; Vilardi et al. 2018 ), recovering phenolic compounds (Vu et al. 2018 ), producing cellulose nanofibers (Costa et al. 2018 ; Harini et al. 2018 ; Tibolla et al. 2018 ), as well as bioethanol (Berawi and Bimandama 2018 ; Prakash et al. 2018 ) and pectin extract (Khamsucharit et al. 2018 ). In the following sections, some of the exclusive added value components in banana are introduced.
Health effects of banana bioactive compounds
Carotenoids are natural antioxidants which contribute to the stability of foods during storage. Previous studies documented the existence of various carotenoids in banana fruit (Davey et al. 2006 ). Although some suggested that the cultivars genotype specifies the quantity of carotenoids, they mostly concurred that the amount of trans-alpha and trans-beta carotene comprised the majority of pro-vitamin A compounds (Yan et al. 2016 ). Another significant carotenoid reported was lutein, which exhibited antioxidant properties and an inhibitory effect on the age-related macular degeneration. Interestingly, it has been identified that green banana peel (GBPe) has substantially higher carotenoids than the pulp (Davey et al. 2006 ).
Phytochemicals, especially phenolic acids, are the main bioactive compounds known for exerting health benefits. Unexpectedly, the percentage of phenolic compounds have been reported to be greater in peel than in pulp (Kanazawa and Sakakibara 2000 ). Similarly, it was demonstrated that the quantity of gallocatechins in peel was five times greater than pulp. With regard to presented results, BPe extract was found to inhibit lipid oxidation better than pulp extract (Someya et al. 2002 ). Recently, it has been shown that gallocatechin extracted from GBPe was effective in the healing of surgical wounds in rats (Von Atzingen et al. 2015 ). Correspondingly, a unique flavonoid named leucocyanidin was found in aqueous extract of unripe plantain pulp, which is now known to be effective in the treatment of gastric diseases (Lewis et al. 1999 ).
Biogenic amines play a key role in the prevention of depression. Catecholamines, dopamine, norepinephrine (noradrenaline) and epinephrine (adrenaline) are the best-known examples of these bioactive compounds which regulate hormones in glycogen metabolism (González-Montelongo et al. 2010 ). Results of dopamine levels in different ripening stages of banana revealed an inverse relation between its concentration and fruit’s maturity, noting that BPe contained more dopamine than pulp (Kanazawa and Sakakibara 2000 ). The possibility of BPe application as a dopamine biomass source for the prevention of Parkinson’s disease was also discussed (Pereira and Maraschin 2015 ). Furthermore, in comparison to other plant residual biomasses, dopamine has exerted higher antioxidant capacity in vitro (Babbar et al. 2011 ).
There is a wide range of DF in banana fruit, including pectin, cellulose, lignin and hemicellulose which can be found naturally in banana flour (BF). Amongst them, RS is the most notable one which provides bioactive effects (Thebaudin et al. 1997 ). Resistant starch, which is mainly composed of the linear part of starch (amylose), is resistant to digestion in the small intestine after 2 h incubation (Homayouni et al. 2014 ). After it reaches the colon undigested, it will be fermented by membrane microbiota (mainly probiotics) and cause pH reduction. Therefore, the environment will be undesirable for the growth of pathogenic microbiota and formation of carcinogenic cells (Khalili and Amini 2015 ).
A considerable amount of literature has been published on the direct relationship between RS intake and reduction of the large bowel cancer risks either in vitro or in vivo; it seems that RS may be a major protective factor against colorectal cancer (Panebianco et al. 2017 ; Yin and Zhao 2017 ).
It was shown that the amount of RS decreased from 8 to 2% with progression in peel ripeness. However, the percentage of total dietary fibre (TDF) slightly increased in yellow banana peel (YBPe) (Ramli et al. 2010 ). In a parallel report, the RS and TDF content of green banana pulp flour (GBPF) reported 49.9% and 7.2%, respectively (Menezes et al. 2011 ). In general, it can be concluded that because approximately 70% (dry basis) of the peeled green banana comprises RS2, the unripe pulp is a remarkable source of this kind of bioactive compound (Wang et al. 2017a ).
It is well-known that phytosterols act as immune system modulators and exert cholesterol-lowering and anticancer properties in the intestine (González-Montelongo et al. 2010 ).
As reported by (Marangoni and Poli 2010 ), a daily intake of phytosterols up to 3 grams/day significantly reduced total and LDL cholesterol. A study pointed to a high amount of phytosterols, mainly beta-sitosterol, campesterol and stigmasterol, which can be found in whole green banana flour (GBF). After they investigated the effect of pre-treatments on BF and although citric acid treatment gave rise to phytosterol reduction in flour obtained from the peel, no change was detected between phytosterols of acidified and control samples from the pulp (Bertolini et al. 2010 ). Consequently, the known properties of phytosterols suggest these flours could be used as functional food components.
Methods for banana flour preparation
Due to the climacteric nature of the banana, it is highly perishable and requires drying during processing for preserving for a longer period of time. Banana flour is a product with high storability potential and long shelf life and can be readily applied to food products. The proximate composition of the flour also depends on the origin, variety, time of harvest, and drying procedure of bananas (Haslinda et al. 2009 ). Table 1 depicts a summary of the proximate composition of both green and yellow BF made from pulp and peel.
Processing steps used for flour preparation from banana pulp and peel are similar, except for the heating procedure used. Pretreatment process steps of banana flour preparation are summarized in Fig. 1 .

Pretreatment process steps of banana flour preparation
While most researchers applied oven drying for banana fruit (Gomes et al. 2016 ; Kurhade et al. 2016 ; Nasrin et al. 2015 ; Segundo et al. 2017a , b ; Türker et al. 2016 ), spouted bed drying (Bezerra et al. 2013a , b ) and lyophilization (Da Mota et al. 2000 ; Türker et al. 2016 ; Wang et al. 2012 ) were also applied in previous studies. In order to minimize enzymatic browning, soaking in sodium metabisulfite, sodium hypochlorite or citric acid solutions was a general pretreatment and considered as the first step after rinsing bananas with water.
The composition of samples obtained by lyophilization and spouted drying techniques showed a significant increase in phenolic acid content, heat sensitive vitamins and minerals compared to traditional drying methods such as solar or hot air-oven drying. Using a spouted drier for producing GBF resulted in high DF and RS content with an average of 21.91% and 68.02%, respectively. This technique did not alter the RS content; however, this effect was consistently reported since a similar study reported lower values, 13.89% and 40.14% for DF and RS, respectively (Bezerra et al. 2013a ).
Likewise, post-treatments could affect the composition of BF produced. For example, smaller particle sizes of GBPF (less than 80 µm diameter) had a higher amount of RS, while flour particles bigger than 156 µm had more TDF, ash, protein and phenolic compounds (Segundo et al. 2017a ). Briefly, depending on the enrichment purpose, selecting a proper procedure is imperative. Table 2 demonstrates the effect of various drying process on produced banana flour properties regarding the type and part of banana used.
Table 2
The effect of different drying methods on banana flours
GBPeF green banana peel flour, GBPF green banana pulp flour, YBPeF yellow banana peel flour, YBPF yellow banana pulp flour, RS resistant starch
Banana flour applications
The high dietary fibre content of GBPF and high levels of mentioned bioactive compounds have enabled the production of BF foodstuffs with remarkable functionalities. Moreover, discovering high nutrition value of BPeF represents a low-cost by-product for industrial application. As discussed earlier, depending on the ripening stage, there are four flour products produced from banana with different chemical composition. Due to the structure of BF, cereal-based products have gained more attention than other food products. The main starch-based foods targeted for enrichment with BF products are as follows.
In a study by Juarez-Garcia et al. ( 2006 ), GBF was obtained from a Mexican species ( Musa paradisiacal L. ) to develop a high gluten bread using 37% GBF in the formulation. Chemical composition analysis showed an increase in ash, protein, TDF and starch percentage of banana bread compared to wheat bread. Even though RS was decreased from 17.5% in flour to 6.7% in banana bread, it was significantly higher than the control sample. The authors also reported a significant decrease in predicted Glycemic Index (pGI) and Hydrolysis Index (HI) of the final product; a result that was in accordance with a higher value of total indigestible fraction (TIF), the main ingredients unavailable for digestion in the small intestine. However, rheological and sensory properties and shelf life of banana bread were not determined. In another study, with more attention to the sensory characteristics, GBF substitution with wheat flour resulted in lower sensory scores in 20% enriched-samples (Gomes et al. 2016 ). Moreover, darker colour, higher hardness and lower specific volume compared to control sample indicated negative effects of this enrichment. While TDF and ash content increased significantly in 20% substitution, the 10% substituted samples was regarded the best as it had no significant technological defects (Gomes et al. 2016 ).
In a study on gluten-added bread, substitution of 25% of ready freeze-dried banana powder (maturity level was not mentioned) with wheat flour resulted in increased volume and viscosity of leavened bread (Mohamed et al. 2010 ). Banana bread was darker than the control due to excess sugar in the BF that caused the Maillard Reaction to occur between reducing sugars and proteins. Regarding the evaluation of shelf life, while bread staling (firmness) increased in higher BF concentration regardless of storage temperature (25 °C, 4 °C and − 20 °C), the stiffness of the control was higher than BF bread (Mohamed et al. 2010 ). By comparing storage temperature, it was stated that bread stored at − 20 °C up to 7 days, experienced lower firmness compared to other samples (Mohamed et al. 2010 ). Similar findings reported by (Ho et al. 2013 ) who prepared a steam bread with 30% GBPF wheat flour substitution. In terms of mineral evaluation, the percentage of Mg, K, Na and Ca were higher than the control, and yielded consequently higher ash content. Increased TDF and RS were also reported to a level of 9% and 5%, respectively. However, the adverse impact of GBPF and added gluten caused an increase in hardness and adhesiveness in the produced bread. The cohesiveness, elasticity and chewiness of bread supplemented with 30% GBPF were decreased due to the lack of consistency in gluten structure. With reference to higher specific volume in banana bread samples containing 8% gluten, researchers explained that GBPF could affect the gluten network and attenuate the gas holding capability of the dough, which leads to low elasticity and expansion in leavened bread. These findings were in agreement with (Steel et al. 2013 ) and consistent with those reported by (Gomes et al. 2016 ; Kurhade et al. 2016 ) which focused on yellow banana peel flour (YBPeF) and GBF, correspondingly.
Considering nutritional properties, a reduction in TDF level and a slight increase in RS level (2.6%) were observed in a freeze-dried tortilla bread containing 40% GBPF substituted with corn flour. Moreover, higher values of pGI and HI than control showed that GBPF addition may not be a proper enrichment strategy for the purpose of producing a low-calorie tortilla bread (Aparicio-Saguilan et al. 2013 ). Additionally, the high amount of RS2 in corn flour, should lead to a major increase in RS amount. It can be concluded that RS2 is sensitive to the cooking process and most of it gelatinizes during the process (Robles-Ramírez et al. 2012 ).
A comprehensive research was carried out on composite bread with 10% peeled yellow banana pseudo-stem flour substitution with wheat flour. Due to the dilution of gluten in substituted samples, crumb and crust lightness improved in the banana bread. A significant increase in total phenolic content, ash and TDF demonstrated the fact that peeled mature banana pseudo-stem flour could be effective in producing a functional composite bread. Besides, no significant difference was observed between samples and control, except for colour and softness in a sensory analysis (Ho et al. 2013 ). In their following study, researchers also found an interaction between gum type and BF effect on volume, in which the addition of 0.8% sodium carboxymethyl cellulose (NaCMC) improved specific volume, minerals (Na, K, Mg and Ca) and RS content (14.98%) more than the same amount of xanthan. NaCMC, which is a soluble DF, acts like a sponge and absorbs water in the intestine. Therefore, it helps in mixing with the starchy food’s structure to form a dense structure that results in slowing down the rate of digestion (Ho et al. 2015 ). As a result, it can be a suitable additive together with peeled yellow banana pseudo-stem flour.
In two separate studies, different types of flat bread were enriched with the addition of BPeF in two stages of ripeness. In another report, (Kurhade et al. 2016 ) found that with the substitution of YBPeF at any ratio with wheat flour in chapatti bread, lightness will decline. Because of the increased water absorption, chapatti containing 10% YBPeF was found to be softer owing to a decrease in the tear force. In terms of antioxidants, total phenolic content, flavonoids and free radical scavenging activity were higher to than that of the control; which justified the high concentration of these elements in YBPeF. Though, overall acceptability score was not distinguished by panelists up to 10% of substitution. Nevertheless, when green banana peel flour (GBPeF) was added to a level of 10% in balady bread in another study, the control sample scored better in all sensory parameters, except taste and chewiness. Contrary to previous findings (Eshak 2016 ) also reported a higher amount of protein in banana bread (12.52%) which was ascribed to the slightly higher amount of protein in GBPeF (8.74%) compared to wheat flour (8.68%). As there was no textural analysis of balady bread in this research, further work is needed to address the technological quality of GBPeF enriched bread. Similarly, increased protein and TDF content were reported in leavened bread containing 20% and 30% fermented green banana slurry substituted with wheat flour. However, in accordance with the application of GBPeF in previous studies, sensory scores diminished for all treatments with more than 10% substitution (Adebayo-Oyetoro et al. 2016 ). Again, textural analysis and firmness during storage were not considered, therefore it is hard to validate the quality of bread produced.
It follows from the above that there are contrary results on BF usage in bread products. Also, more information of bread enrichment with GBPeF and its technological effect on the final product is required.
Pasta products are foodstuffs with an important role in diets. In addition to being easily produced with a long shelf life, pasta products also have a lower glycemic index (GI) in comparison with white bread or rice (Nilsson et al. 2008 ). Hence, enrichment of pasta products with different DF-enriched flours and micronutrients has been considered in the last decade (Filipović et al. 2010 ).
In 2009, the properties of spaghetti enriched with different substitution ratios of GBPF with semolina flour was investigated in two studies. Following a similar trend in most of the banana bread products, a reduction in lightness, protein and fat content of spaghetti containing 20, 35 and 40% GBPF was reported (Ovando-Martinez et al. 2009 ). However, textural results indicated increased adhesiveness and chewiness compared to the control, which was because of the release of amylose from starch granules during cooking. This also caused a rise in cooking loss (less than 7%) with increasing GBPF in the formulation. Yet, since a cooking loss below than 8% is considered as acceptable for semolina-based pasta products, it can be said it was not a negative effect. On account of the high amount of RS (12%) and incomplete gelatinization of starch granules, enriched samples showed lower values in vitro digestion tests.
In a study conducted by Agama-Acevedo et al. ( 2009 ), produced GBPF comprised 42.54% RS2 (dwb). With regard to a higher percentage of polyphenols and antioxidant capacity of banana pasta, it was concluded that enrichment of spaghetti with 30% GBPF could provide a product without off-flavours (Agama-Acevedo et al. 2009 ; Ovando-Martinez et al. 2009 ). These results were confirmed by Krishnan and Prabhasankar ( 2010 ) findings a year later. Besides, they found a synergic relation between sprouted Ragi Flour and GBPF; in which a combination of 15% sprouted Ragi Flour and 15% GBPF proved to be the best for nutritional and technological attributes.
Incorporation of green banana parts, individually, has also been considered in pasta products.
A separate substitution of 10% pulp and peel (obtained from two different varieties) with wheat flour was conducted to produce alkaline noodles. Since the GBPeF was higher in TDF but lower in RS content than the GBPF, the low pGI of banana peel noodles was primarily because of its high TDF. Whilst pulp enriched noodles originated from the Cavendish variety, exhibited higher elasticity, enriched samples with GBPeF from the Dream variety showed the lowest values. In discussion, it was declared that higher sugar content was responsible for high levels of total solid content which led to a rise in the density of the molecular structure (Ramli et al. 2009 ).
In a very recent study, banana flour was prepared from whole green banana and added to tagliatelle pasta up to 30% substitution with wheat flour. Contrary to previous findings, there was no evidence of darker colour in banana pasta which possibly was due to the use of wheat flour instead of semolina flour. But in support of previous researches, banana pasta presented higher ash, TDF and total phenolic content than the control, though 15% substitution showed more ash content. Likewise, the mentioned sample showed better sensory attributes by panelist scores (Zheng et al. 2016 ). Because rheological properties and some important physical properties, such as cooking loss, were not assessed, it is hard to determine the effect of GBF on quality of the final pasta product.
Confectionaries
Due to its high sugar content, utilization of BF in confectionaries has been heightened by the food industry, specifically cereal-based ones. The growth of pathogens in a cake premix made with 60% GBPF instead of wheat flour over 4 months of storage was investigated. Despite a high sugar concentration, the pre-mixture remained significantly unaltered in pH and pathogenic growth, fungus or yeasts (Borges et al. 2010 ). In another study, foaming stability and overall acceptability increased in the presence of 10% BPF. Also, with increased content of BPF to 20% of the formulation, higher hardness was reported, although chewiness and adhesiveness were not significantly different amongst samples. The reason behind this phenomenon was the lower amount of moisture content in banana cakes (Park et al. 2010 ).
With the aim of increasing DF and RS in layer and sponge cakes, GBPF was added at different particle sizes; ranging from 80 µm (fine) to 200 µm (coarse) in diameter. Researchers showed that the fine flour comprised 40% RS compared to 25% RS in the coarse flour. This fact specified higher RS content (about 3%) in 30% replacement samples with fine flours in both layer and sponge cakes. However, the percentage of TDF, protein, ash, lipid, phenolic compounds and amylose was higher in the coarse flour. In terms of technological properties, sponge cakes were noticeably worsened with the presence of banana flours (lower specific volume, inferior sensory characteristics and higher hardness), which was diminished at the 15% ratio; except for cohesiveness that showed a dramatic decrease in all samples compared to the control. The authors accounted for different gelatinization and retrogradation behaviour of banana starch compared to wheat starch for textural changes and decreased sensory scores in banana cakes. Still, samples made by fine particle sizes of GBPF showed better nutritional properties without negatively affecting textural attributes (Segundo et al. 2017a ). The same results were reported in a similar study by selection of YBPF in 40% substitution with sugar. Both sponge and layer cakes depicted enhancement in DF, polyphenols and antioxidant capacity values. In concurrence with their previous work, increased hardness and decreased volume led to the decline of the acceptability of cakes by panelists, especially in 40% banana cakes. Considering the correlation between volume and hardness was more significant in sponge than in layer cakes, the maturity of banana was not correlated to improvements of textural properties (Segundo et al. 2017b ). The same behavior was observed by (Oliveira de Souza et al. 2018 ), even though they used a higher concentration of GPB puree instead of flour for pound cakes.
Cookies containing 20% of YBPeF were rich in TDF and were categorized as a low-calorie product (Agama-Acevedo et al. 2012 ). The same pattern was reported by Elaveniya and Jayamuthunagai ( 2014 ) in which banana blossom powder was added at 5 g in 100 g of biscuit formulation. However, consumer acceptance of samples for colour, crispiness and taste decreased with the addition of BPe, while it was acceptable at 5% substitution.
In another study on biscuit, YBPeF was substituted up to 75% with wheat flour and caused a decline in hardness. According to the discussions, prior treatments of mashed peels together with DF led to an increase in softness of the product. While a high level of consistency and crispiness is required in a biscuit product, dilution of gluten in higher concentrations of YBPeF led to insignificant decrease of these attributes in all enriched samples. Organoleptic results were not significantly different in terms of colour, flavour, after taste and mouthfeel at even the 75% level of substitution (Joshi 2007 ). These findings were consistent with those reported by Carvalho and Conti-Silva ( 2018 ), where enrichment of cereal bars with 14% YBPeF exerted no negative effect on aroma or taste, while further incorporation caused a darker colour, more hardness and adhesiveness together with a bitter aftertaste.
Overall, replacement of BF, regardless of ripeness degree, was feasible up to 15% for achieving optimal quality and maximum 30% for functional purposes in confectionaries. Still, quality control of produced different types of cakes with BPeF is needed to be instigated.
Gluten-free products
Because of the rise of the gluten related disorders, such as celiac disease and dermatitis herpetiformis , it has been essential to expand the gluten-free (GF) food market. Moreover, considering that untreated celiac disease contributes to intestinal cancer, nutrient deficiencies and oxidative stress, developing GF products with the consideration of additional nutritional values is highly important (Wang et al. 2017b ). In this regard, bioactive compounds are a prominent ingredient in GF foods. As most of GF starchy products do not provide proper technological qualities, application of different types of BF has been considered as of late (Torres et al. 2017 ).
By incorporation of 47% GBPF in 100 g pasta formulation, Zandonadi et al. ( 2012 ) produced a GF pasta with an additional proportion of egg white and hydrocolloids. Regarding excess amount of water absorption during cooking, increased stickiness of the product led to a weakened structure. Another negative effect was the poor nutritional value of the product that was exhibited by a reduction in TDF and protein content. On the other hand, improvements in ash content and sensory scores were reported. These findings were in agreement to Radoi et al. ( 2015 ), who reported a growth in total phenolic acids, cinnamic acids and minerals (mainly Fe, Cu, Zn, Mn, Ni) with presence of 30–40% dried banana. Yet, textural and cooking properties of GF pasta were missing important assessments.
Gluten free bread has also been produced by the use of BF. In a study conducted by Sarawong et al. ( 2014 ), while crumb firmness increased in 15% of green plantain flour addition, bread volume improved by 25% addition at a lower baking temperature over a longer time. An elevated proportion of green plantain flour in GF bread contributed to higher water binding capacity and a reduction in starch retrogradation owing to the presence of extra water. In addition to darker crumb at 5% addition level, RS increased only up to 2.5% compared to the control sample.
In sweetened bread, both green and yellow banana GF bread showed lower volume, lesser height and darker colour, but, when black banana pulp flour was utilized at 20% of the formulation, those variables improved to a significant level. However, sensory evaluation, shelf life and textural properties of bread produced were overlooked (Seguchi et al. 2014 ).
The substitution of 15% of GBPeF was with rice flour in GF cake gave rise to a decrease in GF cake volume. Due to an increase in viscosity, the density of banana GF cakes rose (Türker et al. 2016 ). Once more, without considering textural, sensory and storability properties of the final product in the later study, it is of importance to note that more studies are needed to be done in GF starch-based food products with the aim of considering of high nutritional value and technological quality of final product.
It is desirable to find proper food applications for banana peel for achieving two goals; first, helping the environment through sustainability by utilizing secondary processing products and second, creating a new outlook for consumers and producers for generating value-added food products. Besides adding nutritional value to food products, GBF also stands out for not creating production waste, thus representing the complete use of the fruit, increasing the yield and reducing manpower costs due to peeling which is not required.
In gluten-added starchy products, such as bread, cake and pasta, textural properties were not negatively affected by banana flour addition to a certain extent. However, in gluten-free products, more research on improving rheological and cooking properties need to be undertaken; especially investigation on the application of green and yellow banana peel.
As discussed in this review, there are several methods for producing banana flour. Comparing new drying technologies with existing methods and their effect on bioactive components of produced products would be an important subject for future research. Also, further studies, especially clinical trials are needed to be considered in order to confirm the health benefits of banana flour enriched food products.
Acknowledgements
We would like to show our gratitude to the University of Otago for their support of this study.
Compliance with ethical standards
Conflict of interest.
The authors declare that they do not have any conflict of interest.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amir Amini Khoozani, Email: [email protected] .
John Birch, Phone: 64 3 479 7566, Email: [email protected] .
Alaa El-Din Ahmed Bekhit, Email: [email protected] .
- Adebayo-Oyetoro AO, Ogundipe OO, Adeeko KN. Quality assessment and consumer acceptability of bread from wheat and fermented banana flour. Food Sci Nutr. 2016; 4 :364–369. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Agama-Acevedo E, Islas-Hernandez JJ, Osorio-Díaz P, Rendón-Villalobos R, Utrilla-Coello RG, Angulo O, Bello-Pérez LA. Pasta with unripe banana flour: physical, texture, and preference study. J Food Sci. 2009; 74 :S263–S267. [ PubMed ] [ Google Scholar ]
- Agama-Acevedo E, Islas-Hernández JJ, Pacheco-Vargas G, Osorio-Díaz P, Bello-Pérez LA. Starch digestibility and glycemic index of cookies partially substituted with unripe banana flour LWT - Food. Sci Technol. 2012; 46 :177–182. [ Google Scholar ]
- Agama-Acevedo E, Sañudo-Barajas JA, Vélez De La Rocha R, González-Aguilar GA, Bello-Peréz LA. Potential of plantain peels flour ( Musa paradisiaca L.) as a source of dietary fiber and antioxidant compound CYTA. J Food. 2016; 14 :117–123. [ Google Scholar ]
- Alba K, MacNaughtan W, Laws AP, Foster TJ, Campbell GM, Kontogiorgos V. Fractionation and characterisation of dietary fibre from blackcurrant pomace Food Hydrocoll. 2018; 81 :398–408. [ Google Scholar ]
- Alkarkhi AFM, Ramli SB, Yong YS, Easa AM. Comparing physicochemical properties of banana pulp and peel flours prepared from green and ripe fruits. Food Chem. 2011; 129 :312–318. [ PubMed ] [ Google Scholar ]
- Amini Khoozani A, Birch J, El-Din Ahmed Bekhit A. Resistant starch preparation methods. In: Melton L, Shahidi F, Varelis P, editors. Encyclopedia of food chemistry. Oxford: Academic Press; 2019. pp. 390–394. [ Google Scholar ]
- Amini A, Khalili L, Keshtiban AK, Homayouni A (2015) Resistant starch as a bioactive compound in colorectal cancer prevention. In: Probiotics, prebiotics, and synbiotics: bioactive foods in health promotion. Elsevier Inc., pp 773–780. 10.1016/b978-0-12-802189-7.00058-7
- Aparicio-Saguilan A, Osorio-Díaz P, Agama-Acevedo E, Islas-Hernández JJ, Bello-Perez LA. Tortilla added with unripe banana and cassava flours: chemical composition and starch digestibility CYTA J Food. 2013; 11 :90–95. [ Google Scholar ]
- Aurore G, Parfait B, Fahrasmane L. Bananas, raw materials for making processed food products. Trends Food Sci Technol. 2009; 20 :78–91. [ Google Scholar ]
- Babbar N, Oberoi HS, Uppal DS, Patil RT. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res Int. 2011; 44 :391–396. [ Google Scholar ]
- Berawi KN, Bimandama MA. The effect of giving extract etanol of kepok banana peel ( Musa Acuminata ) toward total cholesterol level on male mice ( Mus Musculus L.) strain deutschland-denken-yoken (ddy) Obese. Biomed Pharmacol J. 2018; 11 :769–774. [ Google Scholar ]
- Bertolini AC, Bello-Pérez LA, Méndez-Montealvo G, Almeida CAS, Lajolo F. Rheological and functional properties of flours from banana pulp and peel Starke. 2010; 62 :277–284. [ Google Scholar ]
- Bezerra CV, Amante ER, de Oliveira DC, Rodrigues AMC, da Silva LHM. Green banana ( Musa cavendishii ) flour obtained in spouted bed—effect of drying on physico-chemical, functional and morphological characteristics of the starch. Ind Crop Prod. 2013; 41 :241–249. [ Google Scholar ]
- Bezerra CV, Rodrigues AMC, Amante ER, da Silva LHM. Nutritional potential of green banana flour obtained by drying in spouted bed. Rev Bras Frutic. 2013; 35 :1140–1146. [ Google Scholar ]
- Borges AM, Pereira J, Silva Júnior A, de Lucena EMP, de Sales JC. Stability of cake pre-mixture made with 60% of green banana flour. Cienc Agrotecnol. 2010; 34 :173–181. [ Google Scholar ]
- Carvalho VS, Conti-Silva AC. Cereal bars produced with banana peel flour: evaluation of acceptability and sensory profile. J Sci Food Agric. 2018; 98 :134–139. [ PubMed ] [ Google Scholar ]
- Chávez-Salazar A, Bello-Pérez LA, Agama-Acevedo E, Castellanos-Galeano FJ, Álvarez-Barreto CI, Pacheco-Vargas G. Isolation and partial characterization of starch from banana cultivars grown in Colombia. Int J Biol Macromol. 2017; 98 :240–246. [ PubMed ] [ Google Scholar ]
- Costa ALR, Gomes A, Tibolla H, Menegalli FC, Cunha RL. Cellulose nanofibers from banana peels as a pickering emulsifier: high-energy emulsification processes. Carbohydr Polym. 2018; 194 :122–131. [ PubMed ] [ Google Scholar ]
- Da Mota RV, Lajolo FM, Ciacco C, Cordenunsi BR. Composition and functional properties of banana flour from different varieties. Starke. 2000; 52 :63–68. [ Google Scholar ]
- Davey MW, Keulemans J, Swennen R. Methods for the efficient quantification of fruit provitamin A contents. J Chromatogr A. 2006; 1136 :176–184. [ PubMed ] [ Google Scholar ]
- Elaveniya E, Jayamuthunagai J. Functional, physicochemical and anti-oxidant properties of dehydrated banana blossom powder and its incorporation in biscuits. Int J Chemtech Res. 2014; 6 :4446–4454. [ Google Scholar ]
- Eshak NS. Sensory evaluation and nutritional value of balady flat bread supplemented with banana peels as a natural source of dietary fiber AOAS. 2016; 61 :229–235. [ Google Scholar ]
- FAOSTAT (2017) Top production-bananas-2016. Food and Agriculture Organisation of the United Nations. http://www.fao.org/economic/est/est-commodities/bananas/en/
- Filipović J, Filipović N, Filipović V. The effects of commercial fibres on frozen bread dough. J Serb Chem Soc. 2010; 75 :195–207. [ Google Scholar ]
- Garcia-Amezquita LE, Tejada-Ortigoza V, Heredia-Olea E, Serna-Saldívar SO, Welti-Chanes J. Differences in the dietary fiber content of fruits and their by-products quantified by conventional and integrated AOAC official methodologies. J Food Compost Anal. 2018; 67 :77–85. [ Google Scholar ]
- Gomes AAB, Ferreira ME, Pimentel TC. Bread with flour obtained from green banana with its peel as partial substitute for wheat flour: physical, chemical and microbiological characteristics and acceptance. Int Food Res J. 2016; 23 :2214–2222. [ Google Scholar ]
- González-Montelongo R, Gloria Lobo M, González M. Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds. Food Chem. 2010; 119 :1030–1039. [ Google Scholar ]
- Happi Emaga T, Robert C, Ronkart SN, Wathelet B, Paquot M. Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour Technol. 2008; 99 :4346–4354. [ PubMed ] [ Google Scholar ]
- Harini K, Ramya K, Sukumar M. Extraction of nano cellulose fibers from the banana peel and bract for production of acetyl and lauroyl cellulose. Carbohydr Polym. 2018; 201 :329–339. [ PubMed ] [ Google Scholar ]
- Haslinda WH, Cheng LH, Chong LC, Aziah AAN. Chemical composition and physicochemical properties of green banana ( Musa acuminata × balbisiana Colla cv. Awak) flour. Int J Food Sci Nutr. 2009; 60 :232–239. [ PubMed ] [ Google Scholar ]
- Ho LH, Abdul Aziz NA, Azahari B. Physico-chemical characteristics and sensory evaluation of wheat bread partially substituted with banana ( Musa acuminata X balbisiana cv. Awak) pseudo-stem flour. Food Chem. 2013; 139 :532–539. [ PubMed ] [ Google Scholar ]
- Ho LH, Tan TC, Abdul Aziz NA, Bhat R. In vitro starch digestibility of bread with banana ( Musa acuminata X balbisiana ABB cv. Awak) pseudo-stem flour and hydrocolloids. Food Biosci. 2015; 12 :10–17. [ Google Scholar ]
- Homayouni A, Amini A, Keshtiban AK, Mortazavian AM, Esazadeh K, Pourmoradian S. Resistant starch in food industry: a changing outlook for consumer and producer. Starke. 2014; 66 :102–114. [ Google Scholar ]
- Joshi RV. Low calorie biscuits from banana peel pulp. J Solid Waste Technol Manag. 2007; 33 :142–147. [ Google Scholar ]
- Juarez-Garcia E, Agama-Acevedo E, Sáyago-Ayerdi SG, Rodríguez-Ambriz SL, Bello-Pérez LA. Composition, digestibility and application in breadmaking of banana flour. Plant Foods Hum Nutr. 2006; 61 :131–137. [ PubMed ] [ Google Scholar ]
- Kale CK, Kotecha PM, Chavan JK, Kadam SS. Effect of processing conditions of bakery products on formation of resistant starch. J Food Sci Technol. 2002; 39 :520–524. [ Google Scholar ]
- Kanazawa K, Sakakibara H. High content of dopamine, a strong antioxidant, in cavendish banana. J Agric Food Chem. 2000; 48 :844–848. [ PubMed ] [ Google Scholar ]
- Kaur A, Kaur S, Singh M, Singh N, Shevkani K, Singh B. Effect of banana flour, screw speed and temperature on extrusion behaviour of corn extrudates. J Food Sci Technol. 2014; 52 :4276–4285. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Khalili L, Amini A (2015) Resistant starch in food industry. In: Polysaccharides: bioactivity and biotechnology. pp 663–673. 10.1007/978-3-319-16298-0_42
- Khamsucharit P, Laohaphatanalert K, Gavinlertvatana P, Sriroth K, Sangseethong K. Characterization of pectin extracted from banana peels of different varieties. Food Sci Biotechnol. 2018; 27 :623–629. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kitts DD. Bioactive substances in food: identification and potential uses. Can J Physiol Pharmacol. 1994; 72 :423–434. [ PubMed ] [ Google Scholar ]
- Kris-Etherton PM, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002; 113 :71S–88S. [ PubMed ] [ Google Scholar ]
- Krishnan M, Prabhasankar P. Studies on pasting, microstructure, sensory, and nutritional profile of pasta influenced by sprouted finger millet ( Eleucina coracana ) and green banana ( Musa paradisiaca ) flours. J Texture Stud. 2010; 41 :825–841. [ Google Scholar ]
- Kurhade A, Patil S, Sonawane SK, Waghmare JS, Arya SS. Effect of banana peel powder on bioactive constituents and microstructural quality of chapatti: unleavened Indian flat bread. J Food Meas Charact. 2016; 10 :32–41. [ Google Scholar ]
- Kusuma SAF, Febrianti M, Saraswati A. Comparison of unripe banana peel of kepok ( Musa paradisiaca L.) and klutuk ( Musa balbisiana colla): phytochemical and anti- dysenteriae activity. J Pharm Sci Res. 2018; 10 :911–914. [ Google Scholar ]
- La Fuente CIA, Zabalaga RF, Tadini CC. Combined effects of ultrasound and pulsed-vacuum on air-drying to obtain unripe banana flour. Innov Food Sci Emerg Technol. 2017; 44 :123–130. [ Google Scholar ]
- Lewis DA, Fields WN, Shaw GP. A natural flavonoid present in unripe plantain banana pulp ( Musa sapientum L. var. paradisiaca ) protects the gastric mucosa from aspirin-induced erosions. J Ethnopharmacol. 1999; 65 :283–288. [ PubMed ] [ Google Scholar ]
- Mahindrakar KV, Rathod VK. Utilization of banana peels for removal of strontium (II) from water. Environ Technol Innov. 2018; 11 :371–383. [ Google Scholar ]
- Marangoni F, Poli A. Phytosterols and cardiovascular health. Pharmacol Res. 2010; 61 :193–199. [ PubMed ] [ Google Scholar ]
- Menezes EW, et al. Chemical composition and nutritional value of unripe banana flour ( Musa acuminata , var. Nanicão ) Plant Foods Hum Nutr. 2011; 66 :231–237. [ PubMed ] [ Google Scholar ]
- Mohamed A, Xu J, Singh M. Yeast leavened banana-bread: formulation, processing, colour and texture analysis. Food Chem. 2010; 118 :620–626. [ Google Scholar ]
- Moongngarm A, Tiboonbun W, Sanpong M, Sriwong P, Phiewtong L, Prakitrum R, Huychan N. Resistant starch and bioactive contents of unripe banana flour as influenced by harvesting periods and its application. Am J Agric Biol Sci. 2014; 9 :457–465. [ Google Scholar ]
- Munagapati VS, Yarramuthi V, Kim Y, Lee KM, Kim DS. Removal of anionic dyes (reactive black 5 and congo red) from aqueous solutions using banana peel powder as an adsorbent. Ecotoxicol Environ Saf. 2018; 148 :601–607. [ PubMed ] [ Google Scholar ]
- Nasrin TAA, Noomhorm A, Anal AK. Physico-chemical characterization of culled plantain pulp starch, peel starch, and flour. Int J Food Prop. 2015; 18 :165–177. [ Google Scholar ]
- Nilsson A, Östman E, Preston T, Björck I. Effects of GI vs content of cereal fibre of the evening meal on glucose tolerance at a subsequent standardized breakfast. Eur J Clin Nutr. 2008; 62 :712–720. [ PubMed ] [ Google Scholar ]
- Oi RK, Santana JCC, Tambourgi EB, Júnior M (2013) Feasibility study for production of green banana flour in a spray dryer, vol 32. 10.3303/cet1332305
- Oliveira de Souza NC, de Lacerda de Oliveira L, Rodrigues de Alencar E, Moreira GP, Santos Leandro ED, Ginani VC, Zandonadi RP. Textural, physical and sensory impacts of the use of green banana puree to replace fat in reduced sugar pound cakes. LWT Food Sci Technol. 2018; 89 :617–623. [ Google Scholar ]
- Ovando-Martinez M, Sáyago-Ayerdi S, Agama-Acevedo E, Goñi I, Bello-Pérez LA. Unripe banana flour as an ingredient to increase the undigestible carbohydrates of pasta. Food Chem. 2009; 113 :121–126. [ Google Scholar ]
- Oyewo OA, Onyango MS, Wolkersdorfer C. Lanthanides removal from mine water using banana peels nanosorbent. Int J Environ Sci Technol (Tehran) 2018; 15 :1265–1274. [ Google Scholar ]
- Panebianco C, et al. Engineered resistant-starch (ERS) diet shapes colon microbiota profile in parallel with the retardation of tumor growth in in vitro and in vivo pancreatic cancer models. Nutrients. 2017 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Park JS, Lee YJ, Chun SS. Quality characteristics of sponge cake added with banana powder. J Korean Soc Food Sci Nutr. 2010; 39 :1509–1515. [ Google Scholar ]
- Pathak PD, Mandavgane SA, Kulkarni BD. Fruit peel waste: characterization and its potential uses. Curr Sci. 2017; 113 :444–454. [ Google Scholar ]
- Pereira A, Maraschin M. Banana ( Musa spp) from peel to pulp: ethnopharmacology, source of bioactive compounds and its relevance for human health. J Ethnopharmacol. 2015; 160 :149–163. [ PubMed ] [ Google Scholar ]
- Prakash H, Chauhan PS, General T, Sharma AK. Development of eco-friendly process for the production of bioethanol from banana peel using inhouse developed cocktail of thermo-alkali-stable depolymerizing enzymes. Bioproc Biosyst Eng. 2018; 41 :1003–1016. [ PubMed ] [ Google Scholar ]
- Presutti RJ, Cangemi JR, Cassidy HD, Hill DA. Celiac disease. Am Fam Phys. 2007; 76 :1795–1802+1809–1810. [ PubMed ] [ Google Scholar ]
- Radoi PB, Alexa E, Radulov I, Morvay A, Mihai CSS, Trasca TI, Trasca TI. Total phenolic, cinnamic acids and selected microelements in gluten free pasta fortified with banana. Rev Chim. 2015; 66 :1162–1165. [ Google Scholar ]
- Ramli S, Alkarkhi AFM, Shin Yong Y, Min-Tze L, Easa AM. Effect of banana pulp and peel flour on physicochemical properties and in vitro starch digestibility of yellow alkaline noodles. Int J Food Sci Nutr. 2009; 60 :326–340. [ PubMed ] [ Google Scholar ]
- Ramli S, Ismail N, Alkarkhi AFM, Easa AM. The use of principal component and cluster analysis to differentiate banana peel flours based on their starch and dietary fibre components. Trop Life Sci Res. 2010; 21 :91–100. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rayo LM, Chagurie Carvalho L, Sardá FAH, Dacanal GC, Menezes EW, Tadini CC. Production of instant green banana flour ( Musa cavendischii , var. Nanicão ) by a pulsed-fluidized bed agglomeration. LWT Food Sci Technol. 2015; 63 :461–469. [ Google Scholar ]
- Robles-Ramírez MC, Flores-Morales A, Mora-Escobedo R (2012) Corn tortillas: physicochemical, structural and functional changes. In: Maize: cultivation, uses and health benefits. Nova Science Publishers, Inc., pp 89–111
- Sarawong C, Gutiérrez ZR, Berghofer E, Schoenlechner R. Effect of green plantain flour addition to gluten-free bread on functional bread properties and resistant starch content. Int J Food Sci Technol. 2014; 49 :1825–1833. [ Google Scholar ]
- Seguchi M, Tabara A, Iseki K, Takeuchi M, Nakamura C. Development of gluten-free bread baked with banana ( musa spp.) flour. Food Sci Technol Res. 2014; 20 :613–619. [ Google Scholar ]
- Segundo C, Román L, Gómez M, Martínez MM. Mechanically fractionated flour isolated from green bananas ( M. cavendishii var. nanica ) as a tool to increase the dietary fiber and phytochemical bioactivity of layer and sponge cakes. Food Chem. 2017; 219 :240–248. [ PubMed ] [ Google Scholar ]
- Segundo C, Román L, Lobo M, Martinez MM, Gómez M. Ripe banana flour as a source of antioxidants in layer and sponge cakes. Plant Foods Hum Nutr. 2017; 72 :365–371. [ PubMed ] [ Google Scholar ]
- Sheikh BY, Sarker MMR, Kamarudin MNA, Ismail A. Prophetic medicine as potential functional food elements in the intervention of cancer: a review. Biomed Pharmacother. 2017; 95 :614–648. [ PubMed ] [ Google Scholar ]
- Singh B, Singh JP, Kaur A, Singh N. Bioactive compounds in banana and their associated health benefits—a review. Food Chem. 2016; 206 :1–11. [ PubMed ] [ Google Scholar ]
- Singh S, Parveen N, Gupta H. Adsorptive decontamination of rhodamine-B from water using banana peel powder: a biosorbent. Environ Technol Innov. 2018; 12 :189–195. [ Google Scholar ]
- Someya S, Yoshiki Y, Okubo K. Antioxidant compounds from bananas ( Musa Cavendish ) Food Chem. 2002; 79 :351–354. [ Google Scholar ]
- Steel CJ, Da Silva CB, Ormenese RCSC, Clerici MTPS, Cáceres MC, Chang YK (2013) Technological evaluation of pan bread produced with the addition of commercial resistant starch or unripe banana flour. In: Bread consumption and health. Nova Science Publishers, Inc., pp 107–119
- Thebaudin JY, Lefebvre AC, Harrington M, Bourgeois CM. Dietary fibres: nutritional and technological interest. Trends Food Sci Technol. 1997; 8 :41–48. [ Google Scholar ]
- Tibolla H, Pelissari FM, Martins JT, Vicente AA, Menegalli FC. Cellulose nanofibers produced from banana peel by chemical and mechanical treatments: characterization and cytotoxicity assessment. Food Hydrocoll. 2018; 75 :192–201. [ Google Scholar ]
- Torres MD, Arufe S, Chenlo F, Moreira R. Coeliacs cannot live by gluten-free bread alone—every once in awhile they need antioxidants. Int J Food Sci Technol. 2017; 52 :81–90. [ Google Scholar ]
- Tribess TB, Hernández-Uribe JP, Méndez-Montealvo MGC, Menezes EW, Bello-Perez LA, Tadini CC. Thermal properties and resistant starch content of green banana flour ( Musa cavendishii ) produced at different drying conditions. LWT Food Sci Technol. 2009; 42 :1022–1025. [ Google Scholar ]
- Türker B, Savlak N, Kaşikci MB. Effect of green banana peel flour substitution on physical characteristics of gluten-free cakes. Curr Res Nutr Food Sci. 2016; 4 :197–204. [ Google Scholar ]
- Vilardi G, Di Palma L, Verdone N. Heavy metals adsorption by banana peels micro-powder: equilibrium modeling by non-linear models. Chin J Chem Eng. 2018; 26 :455–464. [ Google Scholar ]
- Von Atzingen DANC, et al. Repair of surgical wounds in rats using a 10% unripe Musa sapientum peel gel. Acta Cir Bras. 2015; 30 :586–592. [ PubMed ] [ Google Scholar ]
- Vu HT, Scarlett CJ, Vuong QV. Effects of drying conditions on physicochemical and antioxidant properties of banana ( Musa cavendish ) peels drying. Technol. 2017; 35 :1141–1151. [ Google Scholar ]
- Vu HT, Scarlett CJ, Vuong QV. Phenolic compounds within banana peel and their potential uses: a review. J Funct Foods. 2018; 40 :238–248. [ Google Scholar ]
- Wang Y, Zhang M, Mujumdar AS. Influence of green banana flour substitution for cassava starch on the nutrition, color, texture and sensory quality in two types of snacks. LWT Food Sci Technol. 2012; 47 :175–182. [ Google Scholar ]
- Wang J, Huang HH, Chen PS. Structural and physicochemical properties of banana resistant starch from four cultivars. Int J Food Prop. 2017; 20 :1338–1347. [ Google Scholar ]
- Wang K, Lu F, Li Z, Zhao L, Han C. Recent developments in gluten-free bread baking approaches: a review Food. Sci Technol. 2017; 37 :1–9. [ Google Scholar ]
- Yan L, Fernando WMADB, Brennan M, Brennan CS, Jayasena V, Coorey R. Effect of extraction method and ripening stage on banana peel pigments. Int J Food Sci Technol. 2016; 51 :1449–1456. [ Google Scholar ]
- Yangilar F. Effects of green banana flour on the physical, chemical and sensory properties of ice cream. Food Technol Biotechnol. 2015; 53 :315–323. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yin DT, Zhao XH. Impact of exogenous strains on in vitro fermentation and anti-colon cancer activities of maize resistant starch and xylo-oligosaccharides. Starke. 2017; 69 :15–20. [ Google Scholar ]
- Zandonadi RP, Botelho RBA, Gandolfi L, Ginani JS, Montenegro FM, Pratesi R. Green banana pasta: an alternative for gluten-free diets. J Acad Nutr Diet. 2012; 112 :1068–1072. [ PubMed ] [ Google Scholar ]
- Zhang P, Whistler RL, Bemiller JN, Hamaker BR. Banana starch: production, physicochemical properties, and digestibility—a review. Carbohydr Polym. 2005; 59 :443–458. [ Google Scholar ]
- Zheng Z, Stanley R, Gidley MJ, Dhital S. Structural properties and digestion of green banana flour as a functional ingredient in pasta. Food Funct. 2016; 7 :771–780. [ PubMed ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Banana tree parts, such as the peels and leaves, have antioxidant activities and biological functions, including anti-diabetic, anti-diarrheal, anti-tumor, anti-mutagenic, and anti-ulcerogenic properties (Kora, 2019).Bananas have also been shown to be the source of bioactive compounds that inhibit bacterial or fungal growth (Chiang et al., 2020; Evbuomwan et al., 2018; Ismail et al., 2018 ...
Other than banana, its by‐products such as peel, pseudo‐stems, leaves, and blossoms are also rich in several nutrients, for example, carbohydrates, protein, dietary fiber, vitamins, and so on.
Banana fibre obtained from the pseudo-stem of plant has appearance similar to that of ramie and bamboo fibres. Pseudo-stem is formed of about 14 to 18 sheaths and produces fibres of different quality depending upon position, viz. (a) course fibre (outermost 2-3 sheaths) that breaks easily so these sheaths are rejected, (b) soft lustrous fibre (middle sheaths) and (c) very soft fibre (some ...
Banana plants are traditionally propagated through vegetative means using suckers (Nkengla-Asi et al., 2021).However, plants produced through suckers have their own limitations as it leads to disease transmission, low productivity, and poor preservation of original plant genetic material (Hussein, 2012).Moreover, there is a huge demand for quality planting materials to narrow the gap between ...
The presence of various bioactive phytochemicals and their nutritional significance has been discussed in this review paper . Table 1. ... Banana tree and banana fruits of various maturities. (Source: Internet Wikipedia.) ... More research is needed to be carried out to find ways of using banana fruit peel in the development of many new ...
Banana (Musaceae) is one of the world's most important fruit crops that is widely cultivated in tropical countries for its valuable applications in food industry. Its enormous by-products are an excellent source of highly valuable raw materials for other industries by recycling agricultural waste. This prevents an ultimate loss of huge amount ...
To extract UAV-MS features, we annotated all banana plants (individual and clusters, i.e., mats) located in three MicaSense-derived high-quality MS orthomosaics from the Kabare district (Supplementary Table 1). ... The output of this research paper are being integrated into other banana disease surveillance platforms of the ...
Introduction. Banana (Musa spp.) plants are well known for their edible fruit and serve as a staple food crop in Africa, Central and South America (Arias et al., 2003).With more than 112 million tons produced in 2016, bananas are among the most popular fruits in the world and provide many employment opportunities ().Furthermore, banana fruits are rich in health promoting minerals and ...
According to recent research, banana by-products such as green culled bananas, peels, and pseudo-stems may be a valuable raw material and a cheap source of high-quality pectin, starch, and cellulose for the food industry. The various chemical compounds extracted from banana by-products are listed in Table 2 (Zhang et al., 2005).
Banana is one of the most important food crops which is generally planted in tropical countries and has beneficial applications in the food industry. A large amount of by-products such as leaves, inflorescence, pseudostem, and rhizomes serves as a source for different industries. Most of these by-products may serve as an undervalued commodity with a limited commercial value, application and in ...
Abstract. Bananas (Musa spp.), belonging to the family Musaceae, are the perennial monocotyledons commonly grown in the tropics situated at latitude 20° above and below the equator, where there ...
Developing a successful protocol for banana in vitro culture is a guarantee for the mass propagation of pathogen-free, high-quality, true-to-type planting materials with low production costs. The current work aimed to investigate the influence of increasing copper levels in an MS medium on endophytic bacterial contamination; shoot multiplication; rooting and the acclimatization of in vitro ...
some of these substances. The pharmacological. effects of banana stem juice include an ti oxidant, immunomo dulator y, antibac terial, anti ulcerog enic, hypolipidemic, hypoglycemic ...
Background Banana (Musa spp.) is the most popular marketable fruit crop grown all over the world, and a dominant staple food in many developing countries. Worldwide, banana production is affected by numerous diseases and pests. Novel and rapid methods for the timely detection of pests and diseases will allow to surveil and develop control measures with greater efficiency. As deep convolutional ...
The average global banana production rose from 69 million tonnes in 2000-2002 to 116 million tonnes in 2017-2019, at an approximate value of 31 billion USD. The main driver of the expansion in production has been the increasing consumption requirements of rising populations in producing countries.
Banana plants are derived from three genera ... In the research by Arun et al. (2015), functional cookies were developed from ... Based on the previous empirical paper, banana peel has great application potential in the food-processing fields as an alternative ingredient. With regard to nutritional content, banana peel is appreciated for its ...
The P4 treatment, namely the ratio of 60% instant oats and 40% banana mas, produced gluten-free pancakes with the best characteristics with the criteria of water content of 48.28%, ash content of ...
Introduction. Banana is a tropical climacteric fruit and universally comprises a number of species in the genus Musa of the family Musaceae.It is one of the most favored fruits in the world and the fourth most important crop produced globally (Aurore et al. 2009).Nearly all of the identified cultivars derived from two diploid species, Musa acuminata and Musa balbisiana, in which the Cavendish ...
Banana(Musasp.) is the leading fruit species in the country in terms of hectarage, volume and value of production. In 1999, a total of 338 277 hectares producing 3.7 million metric tonnes valued at P15 billion (BAS 2000). It is also a consistent top dollar earner, with export revenues of more than US$200 million annually.
Optimization and modeling of density of banana stem fiber paper using response surface methodology. Effect of operating parameters on density of banana stem fiber paper was estimated using Box - Behnken Design. The optimum conditions of banana stem fiber amount, water amount and blending time to achieve a density of 675.75 g/m 3 were determined.
Banana fruits are known for a balanced source of nutrients with a major quantity of carbohydrates, rich in minerals (calcium, potassium, magnesium, iron, copper, and phosphorus), low in protein as ...
This paper examines the banana tree data structure, specifically designed to efficiently maintain persistent homology -- a multi-scale topological descriptor -- for dynamically changing time series data. We implement this data structure and conduct an experimental study to assess its properties and runtime for update operations. Our findings ...
banana plants are useable, but in our country huge amount of banana tree have thrown after collecting the banana fruits. This banana when cut down it causes the pollution of environment [[ 30].
Banana Stem Fiber Paper Properties. The fiber morphology and chemical composition study of banana pseudo-. stem used for this investigation are shown in Table 1. The first remark. concerns the ...