Clinical Presentation: Case History # 1 Ms. C is a 35 year old white female. She came to Neurology Clinic for evaluation of her long-term neurologic complaints. The patient relates that for many years she had noticed some significant changes in neurologic functions, particularly heat intolerance precipitating a stumbling gait and a tendency to fall. Her visual acuity also seemed to change periodically during several years. Two months ago the patient was working very hard and was under a lot of stress. She got sick with a flu and her neurologic condition worsened. At that time, she could not hold objects in her hands, had significant tremors and severe exhaustion. She also had several bad falls. Since that time she had noticed arthralgia on the right and subsequently on the left side of her body. Then, the patient abruptly developed a right hemisensory deficit after several days of work. The MRI scan was performed at that time and revealed a multifocal white matter disease - areas of increased T2 signal in both cerebral hemispheres. Spinal tap was also done which revealed the presence of oligoclonal bands in CSF. Visual evoked response testing was abnormal with slowed conduction in optic nerves. (Q.1) (Q. 2) (Q.3) Today, the patient has multiple problems related to her disease: she remains weak and numb on the right side; she has impaired urinary bladder function which requires multiple voids in the mornings, and nocturia times 3. She became incontinent and now has to wear a pad during the day. (Q.4) She also has persistent balance problems with some sensation of spinning, and she is extremely fatigued. REVIEW OF SYSTEMS is also significant for a number of problems related to her suspected MS. The patient has a tendency to aspirate liquids and also solids. (Q.5) (Q.6) She complains of tinnitus which is continuous and associated with hearing loss, more prominent on the left. She has decreased finger dexterity and weakness of the hands bilaterally. She also complains of impaired short-term memory and irritability. FAMILY HISTORY is significant for high blood pressure, cancer and heart disease in the immediate family. PERSONAL HISTORY is significant for mumps and chicken pox as a child, and anemia and allergies with hives later in life. She also had a tubal ligation. NEUROLOGIC EXAMINATION: Cranial Nerve II - disks are sharp and of normal color. Funduscopic examination is normal. Cranial Nerves III, IV, VI - no extraocular motor palsy or difficulties with smooth pursuit or saccades are seen. Remainder of the cranial nerve exam is normal except for decreased hearing on the left, and numbness in the right face, which extends down into the entire right side. The Weber test reveals greater conductance to the right. Rinne's test reveals air greater than bone bilaterally. (Q.7) The palate elevates well. Swallow appears to be intact. Tongue movements are slowed, but tongue power appears to be intact. Motor examination reveals relatively normal strength in the upper extremities throughout. However, rapid alternating movements are decreased in both upper extremities and the patient has dysdiadochokinesia in the left hand. (Q.8) Mild paraparesis is noted in both legs without severe spasticity. Deep tendon reflexes are +2 and symmetrical in the arms, +3 at the ankles and at the knees. Bilateral extensor toe sign are present. Sensory exam reveals paresthesia on the right to touch and decreased pin sensation on the right diffusely. The patient has mild vibratory sense loss in the distal lower extremities. Romberg's is negative. (Q.9) Tandem gait is mildly unstable. Ambulation index is 7.0 seconds for 25 feet. (The patient takes 7.0 seconds to walk 25 feet.) Diagnosis: Multiple Sclerosis with laboratory support. © John W.Rose, M.D., Maria Houtchens, MSIII, Sharon G. Lynch, M.D.
- Submit a Manuscript
- Advanced search


American Journal of Neuroradiology
Advanced Search
MR Imaging in Multiple Sclerosis: Review and Recommendations for Current Practice
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- Figures & Data
- Info & Metrics
SUMMARY: MR imaging is widely used for the diagnosis and monitoring of patients with MS. Applications and protocols for MR imaging continue to evolve, prompting a need for continual reassessments of the optimal use of this technique in clinical practice. This article provides updated recommendations on the use of MR imaging in MS, based on a review of the trial evidence and personal experiences shared at a recent expert meeting of radiologists and neurologists.
- Abbreviations
MR imaging has played an increasing role in the diagnosis and management of MS. 1 It offers 3 main applications in MS. First, in combination with characteristic symptoms, it provides earlier and more confident diagnosis than symptoms alone. 2 Second, it contributes to our understanding of the pathophysiology of MS and how pathophysiologic changes relate to clinical manifestations of disease. 3 Third, it has a role in monitoring the effects of therapies in clinical trials and also has the potential to identify response to therapy in individual patients. 4 , 5
Contrast agents are conventionally included in acquisition protocols for MR imaging. Use of these agents also offers insights into pathogenesis and enhances the monitoring of treatment effects. 6
As applications for MR imaging in MS evolve and increasing numbers of techniques and protocols are adopted, there is a trend toward variation in the use of MR imaging among centers. In the face of this growing divergence, experts recognize the importance of standardizing protocols based on evidence of optimal practice. 6 This review seeks to contribute to this important objective by reporting an expert meeting that focused on discussions and recommendations for the optimal use of MR imaging in MS by neurologists and radiologists—key participants in the use of contrast-enhanced MR imaging in MS.
- Applications of MR Imaging in MS
MR imaging offers clinicians a range of applications for the management of MS, including support in diagnosis, insights into pathogenesis, an understanding of prognosis relevant to individual patients, and assistance in monitoring the effects of therapy.
Support in Diagnosis
A diagnosis of MS is founded on clinical evaluation. Several criteria have been developed to integrate MR imaging with clinical evaluation and other diagnostic methods to achieve earlier and more accurate diagnosis, including the revised McDonald criteria. 2
The McDonald criteria were the first to incorporate the brain and spinal cord lesions visualized on MR imaging into traditional diagnostic approaches, including history, examination, and laboratory tests. 7 The revised McDonald criteria included amendments to the original guidelines to facilitate use in typical practice settings. 2 Specifically, these revisions were designed to help demonstrate lesion dissemination in time, clarify the evaluation of spinal cord lesions, and simplify the diagnosis of primary-progressive disease.
The revised McDonald criteria have largely superseded earlier criteria 7 – 9 and represent an important element in the diagnosis of patients with suspected MS. The revised McDonald criteria have, however, been criticized for their perceived complexity and low (∼60%) sensitivity. 10
Less complex criteria have been produced, such as the Swanton criteria, which are claimed to offer similar specificity (87%) but increased sensitivity (72%) compared with the McDonald criteria. 10 Despite apparent advantages for the Swanton criteria, there has been hesitation among most clinicians to adopt them.
Another study investigating simplified criteria for diagnosing MS reported that a single MR imaging study performed <3 months after the onset of CIS is highly specific for the development of clinically definite MS in the presence of dissemination in space, providing that both gadolinium-enhancing and -nonenhancing lesions are found, indicative of dissemination in time. 11 These interesting results require confirmation.
Consensus Statement.
MR imaging has an important role in the diagnosis of MS. Expert meeting participants recommended adopting standardized protocols and reporting procedures based on the revised McDonald criteria.
Regional Differences in Characteristics of MS Lesions
The presentation of MS typically differs between Asian and Western populations. 12 In Asian patients, the optic-spinal type of MS is more frequent, an older age group (>35 years) is affected, fewer cases are positive for oligoclonal bands, and total protein concentrations in CSF are higher. When spinal involvement occurs, the lesion is characteristically longer. Caution should be exercised when applying the McDonald criteria in Asian patients because of the characteristic differences in lesion location compared with Western patients. Inclusion of a spinal cord lesion as a juxtacortical lesion increases sensitivity for diagnosis. 13 The criteria of Poser et al 9 are considered more reliable than the McDonald criteria in Asian populations, though they are not dependable for early diagnosis.
MR imaging evaluation in Asian populations should focus on the optic nerve and spinal cord. Given the frequency of spinal cord involvement in Asian patients with MS, McDonald criteria require modification for these patients.
Insights into Pathogenesis
MS is a complex immune disease in which self-reactive T-cells and monocytes mediate inflammation of CNS white matter and demyelination of axons, leading typically to cumulative neurologic disability. 14 MR imaging provides insights into the pathogenic processes of MS, alongside other noninvasive techniques and clinical evaluation. In particular, MR imaging by using gadolinium-containing contrast agents has helped identify the pivotal role of the BBB. 15 Breaching the BBB by immune cells mediates structural and functional changes in the CNS of patients with MS, including inflammation, demyelination, axonal loss, remyelination, and gliosis. Demonstrating BBB disruption at MR imaging may represent one of the earliest indications for a diagnosis of MS. Insights into pathogenesis provided by MR imaging have also offered greater understanding of the mechanisms of action of first-line drugs, IFNB, 16 and glatiramer acetate. 17
Understanding the Prognosis
An important objective in management is predicting the disease course in individual patients. For patients with CIS, the objective is to predict conversion to clinically definite MS. In patients with CIS suggestive of an MS attack and lesions on MR imaging, the likelihood of developing clinically definite MS is 88% during 14 years 18 and 82% during 20 years. 19 In established disease, objectives in management are to predict relapse in the short-term and predict disability and sustained disease progression in the long-term. A relapsing course is followed by sustained progression within 2 decades in 80% of cases. 20
Disease progression is highly variable between individuals, reflecting the complex nature of the disease and variations in capacity for repair and compensation. 21 Conventional MR imaging measures, including T2 lesion load, correlate poorly with clinical outcomes in MS, 19 , 22 and correlations tend to weaken further in later stages of disease. 23 Meta-analysis of the predictive value of gadolinium-enhanced MR imaging similarly indicates a low ability to predict relapses and development of impairment and disability ( Fig 1 ). 24
- Download figure
- Open in new tab
- Download powerpoint
Predictive value of gadolinium-enhanced MR imaging for relapses in MS: a meta-analysis. Reprinted with permission from Lancet . 24
A number of explanations have been suggested for why MR imaging assessments are dissociated from clinical status and the development of disability—the so-called “clinicoradiologic paradox”:
Deficiencies in clinical and MR imaging assessments.
The presence of strategic-versus-nonstrategic lesions.
The dual role of the immune system, both in destroying and promoting repair.
The role of neurodegenerative processes that gain importance as the disease progresses.
Abnormalities of apparently normal white and gray matter.
The role of adaptation and reorganization in compensating disease-related damage.
More reliable and standardized approaches to MR imaging assessments in MS may lead to better correlation with clinical course. Until the reasons that underlie the clinicoradiologic paradox are fully identified, prognosis in individual patients cannot be based on MR imaging findings alone.
Monitoring Therapy
MR imaging is widely used to investigate the anti-inflammatory effects of therapies in clinical trials. In this setting, the most widely adopted and best-supported MR imaging assessments are T2 (for lesion load and new and enlarging lesions) and gadolinium-enhanced T1 (for total lesion number, new and enlarging lesions, and lesion load).
The Prevention of Relapses and Disability by Interferon-Beta1a Subcutaneously in Multiple Sclerosis trial included MR imaging assessments in patients with clinically definite or laboratory-supported relapsing-remitting MS. T2 and gadolinium-enhanced T1 MR imaging identified the onset of maximal therapeutic effect for IFNB-1a at 3 months. 4 Other investigations, such as the fingolimod phase II study, similarly demonstrated the utility of MR imaging to monitor the effects of therapy in a trial setting. 25 In confirmation of individual studies, a recent meta-analysis reported strong correlations between the effects of therapies on relapses and their influence on MR imaging activity. 26
Less convincing evidence is available to support a role for other MR imaging measures in monitoring the effects of therapy, including T1 “black holes” (hypointense lesions) or atrophy of the brain or cord, and even less evidence supports a role for magnetization transfer MR imaging, DTI, spectroscopy, or functional MR imaging—though these remain areas of active investigation. 27
Some centers routinely use MR imaging to monitor response to therapy in individual patients. If neurologists choose to use MR imaging to monitor a patient's response to therapy, a rational approach is baseline assessment with follow-up at 3 or 6 months and again at 12 months. Reassessment with MR imaging may be sooner if there are concerns about the patient's disease course. Stable MR imaging assessments in an individual with clinically silent disease supports continuation of the current treatment. Identification of new lesions in a clinically silent individual may indicate a change in therapy or the need for more frequent follow-up. A major increase in lesion number in a modestly clinically active patient or a patient with indeterminate findings indicates that therapy should be re-evaluated.
MR imaging has utility for monitoring the effects of therapies in clinical trials. Further evidence is needed to support a role for MR imaging in monitoring therapeutic response in routine clinical practice.
Benefits of Early Therapy in MS
There is an encouraging trend toward initiating therapy early in the disease course, so now almost all patients with MS are treated following the first event. The rationale for early initiation of therapy is to reduce the frequency of relapses and slow progression to disability.
Outcomes from well-designed placebo-controlled trials of IFNB indicate that early treatment—at the first clinical demyelinating event—can slow progression to clinically definite MS. 5 , 28 – 31 Serial MR imaging assessments, including T2 and gadolinium-enhanced T1 scans, supported clinical observations of improvement in these trials.
Most trials investigating the benefits of early treatment have been short-term. An exception is a large observational study of early treatment with IFNB in 1504 patients with relapsing-remitting MS who were followed for 7 years. 32 Patients treated with IFNB showed significant reductions in secondary progression compared with a placebo, and the authors concluded that early treatment slowed long-term progression of MS.
Early initiation of treatment offers benefits in most patients, and these benefits appear to persist for the long-term. MR imaging contributes to the early initiation of treatment by facilitating early diagnosis.
- Techniques and Protocols for MR Imaging in MS
Conventional MR imaging
Conventional MR imaging is a reliable and accurate diagnostic technique, providing positive findings in approximately 95% of patients with clinically definite MS. MR imaging is widely recognized as superior to other imaging modalities, including CT, for the visualization of lesions, particularly smaller lesions, and has largely replaced alternative imaging techniques in practice.
MS plaques can be characterized at MR imaging by their location, morphology, signal intensity, and degree of gadolinium enhancement. Acute-phase plaques appear as rounded areas of high-signal intensity on T2 sequences. Gadolinium enhancement on T1 sequences is related to BBB damage associated with inflammation. There are 2 patterns of enhancement: uniform enhancement, reflecting the onset of a new lesion, and ringlike enhancement, indicating reactivation of an older lesion. 33 Nonenhancing lesions are the result of earlier episodes of disease. T2-weighted MR imaging is considered the most sensitive diagnostic test for demonstrating disease dissemination, but with moderate specificity. T1-weighted gadolinium-enhanced imaging offers increased specificity by differentiating enhancing from nonenhancing lesions. Use of both of these imaging techniques provides optimal specificity. 34
Typical MR imaging findings that are sensitive and specific for diagnosing MS include plaques along callososeptal interfaces and perivenular extension (Dawson finger) ( Fig 2 ). 35 Atypical MR imaging findings in MS include lesions that mimic tumors and autoimmune vasculitis. In these cases, characteristic differences in lesion distribution, supported by clinical and laboratory investigations, assist differential diagnosis. The revised McDonald criteria include recommendations for excluding alternative diagnoses through history, clinical evaluation, and appropriate laboratory studies, 2 while “red flags” have been developed to alert clinicians to reconsider the differential diagnosis more extensively in clinically suspected MS. 36
Typical MS with brain lesions.
Besides visualizing lesions in the brain, MR imaging may be used to image lesions associated with optic neuritis, neuromyelitis optica, and spinal cord MS. Optic neuritis is present in ≤50% of patients with MS and is frequently the presenting sign. Gadolinium enhancement is a sensitive method for visualizing optic neuritis and has a role, along with brain MR imaging and symptoms, in establishing a definitive diagnosis. 37
The spinal cord is also frequently involved in MS and, for most patients, both spinal cord and the brain are affected. 38 In ∼25% of patients, however, lesions are present in the spinal cord alone. 33 Most spinal lesions are localized to the cervical rather than the thoracic cord and tend to be multifocal and asymmetric. 39 At MR imaging, spinal lesions show increased T2 signal intensity and, frequently, gadolinium enhancement. In general, findings at spinal MR imaging are less definitive compared with brain MR imaging for diagnosing MS.
Indications and protocols for suspected spinal MR imaging remain an area of debate. Recommendations at the expert meeting for use of MR imaging in patients with and without a spinal presentation are summarized in Recommendation 4.
MR imaging is the optimal radiologic technique for supporting a diagnosis of MS in the brain and spinal cord. Full exchange of imaging and clinical information between radiologists and neurologists is essential for reaching a correct diagnosis.
Role of Contrast Enhancement
The meeting participants noted a lack of expert guidance on the role of contrast agents in MS and included extensive discussion on this topic. Estimates suggest that ≥35% of MR imaging examinations are performed with contrast agents, usually gadolinium-containing agents. Contrast enhancement in MS increases the reliability of MR imaging to depict active lesions 34 and has a pivotal role in demonstrating dissemination in time, as defined in the revised McDonald criteria. 2 Contrast enhancement also assists in excluding confounding diagnoses, including other inflammatory conditions and tumors. For these reasons, gadolinium enhancement is widely recommended for the diagnosis and initial evaluation of MS. 6 At some centers, contrast-enhanced MR imaging is additionally performed to monitor the effects of therapy.
Contrast enhancement by using gadolinium-containing agents increases the efficacy of MR imaging of MS lesions.
Characteristics of Contrast Agents
A number of gadolinium-containing contrast agents are available for use in MR imaging, including gadobenate dimeglumine (MultiHance; Bracco, Milan, Italy), gadobutrol (Gadovist; Bayer Schering Pharma, Berlin-Wedding, Germany), gadodiamide (Omniscan; Nycomed Amersham, Oslo, Norway), gadofosveset trisodium (Vasovist; EPIX Pharmaceuticals, Lexington, Massachusetts), gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany), gadoterate meglumine (Dotarem; Guerbet, Aulnay-sous-Bois, France), gadoteridol (ProHance, Bracco), gadoversetamide (OptiMARK; Mallinckrodt, St Louis, Missouri), and gadoxetic acid disodium (Primovist, Bayer Schering Pharma). Most currently available gadolinium-containing contrast agents are at a concentration of 0.5 mol/L, while gadobutrol is formulated at a higher concentration of 1.0 mol/L. 40
Gadolinium-containing contrast agents can be classified by the molecular structure of their gadolinium-chelate complex—whether macrocyclic or linear—and, within the linear class, by whether they are ionic or nonionic.
Contrast agents with macrocyclic structures demonstrate increased stability and a lower propensity to release gadolinium ions compared with linear contrast agents. 41 This was confirmed in a recent study comparing the stability of contrast agents in human serum under physiologic conditions. 42 Release of gadolinium ions was substantially reduced for macrocyclic agents—gadobutrol, gadoteridol, and gadoterate meglumine—relative to agents tested with linear structures ( Fig 3 ). The study also found that the addition of phosphate to the serum at a concentration of 10 mmol/L (to simulate end-stage renal disease) accelerated the release of gadolinium ions from nonionic linear agents and, to a lesser degree, from ionic linear agents, but that macrocyclic agents remained stable.
Comparison of rates of Gd 3+ release for 1 mmol/L solutions of gadolinium-containing contrast agents in native human serum from healthy volunteers at 37°C. Reprinted with permission from Frenzel T, Lengsfeld P, Shirmer H, et al. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37°C. Invest Radiol 2008;43:817–28.
Release of gadolinium ions from contrast agents may be relevant to the development of NSF. NSF is a rare outcome in patients with severe kidney failure, characterized by thickening, induration, and hardening of the skin. Some workers attribute NSF to gadolinium ions released from contrast agents. 43 In support of this still-debated association, clinical reports suggest that NSF is associated most commonly with nonionic linear contrast agents and rarely with agents with macrocyclic structures. 44
Contrast Dose and Characteristics.
Although there is consensus on the benefits of gadolinium-based agents in MR imaging, debate continues over how best to use these agents to optimize lesion visualization in MS. 45
The standard dose of contrast agent for MR imaging of the CNS is 0.1 mmol per kilogram of body weight, and this dose is sufficient for diagnosis in most patients. Studies investigating a range of pathologies, including brain tumors, gliomas, and MS, indicate, however, that lesion detection may be improved further with higher concentrations (0.2–0.3 mmol/kg). 46 – 50 These higher concentrations may have a role in cases of diagnostic doubt following the standard 0.1 mmol/kg dose. As an alternative to administering a contrast agent in sequential doses, a dose-comparison study of gadobutrol in MS recommended using a double dose (0.2 mmol/kg) at the initial assessment, an approach that was endorsed by expert panel experience. A single injection may offer optimal balance in terms of sensitivity, time, costs, and detection of active lesions. 51
A physicochemical property of contrast agents relevant to imaging performance is relaxivity, which defines the ability of an agent to alter tissue relaxation rates. Complementing theoretic studies, higher relaxivity relates to increased imaging performance in clinical trials comparing gadolinium-containing contrast agents. 52 – 55 Gadobutrol has a higher relaxivity than other macrocyclic agents currently available, leading to the highest T1 shortening per volume. 52 , 56
Acquisition Protocol by Using Contrast Agents.
Another consideration for optimizing lesion enhancement is the timing of image acquisition following contrast agent injection. 45 A recent study reported that the sensitivity of MR imaging to detect active MS lesions was progressively enhanced at up to between 5 and 10 minutes postinjection of gadobutrol, 47 which was supported by case study experience among expert meeting participants ( Fig 4 ). Although increasing enhancement over time is an area for further investigation, the meeting participants agreed that not all contrast agents share the characteristic of gadobutrol of progressive enhancement postinjection.
Case study shows brain lesion enhancement with gadobutrol. Images were obtained at 3, 6, 9, and 12 minutes postinjection.
Complex stability is an important safety consideration when selecting gadolinium-containing contrast agents, especially in patients with renal disease. Physicochemical characteristics (including concentration and relaxivity) and acquisition protocol influence imaging performance. Gadobutrol meets the criterion of high complex stability and provides the highest T1 shortening with high image quality.
Acquisition Protocols for MR Imaging
Acquisition protocols for MR imaging in MS vary widely between centers, reflecting practitioner preference and local availability of equipment. A comprehensive acquisition protocol may include a localizer scan, FLAIR sagittal, T2 and FLAIR axial, pre- and postcontrast T1 axial, and (optionally) DWI and 3D-T1-weighted spoiled gradient recalled-echo.
Simpler protocols offer time and cost savings relative to more comprehensive protocols, can be standardized across centers, and are likely to diagnose 90% of MS cases. A simple protocol was recommended by expert meeting participants:
Dual-echo and FLAIR, axial whole brain (to detect gray matter lesions)
Optional dual-echo or FLAIR, sagittal midline (to detect corpus callosum lesions)
Skip unenhanced T1 (provides little additional information)
Contrast-enhanced T1 scan
Optional DWI (to differentiate other diagnoses).
A standard dose of contrast agent (0.1 mmol/kg) should be injected before the first MR image. A scanner with at least 1T optimizes image quality and tissue contrast.
A simple acquisition protocol that can be standardized across centers offers advantages for diagnosing MS. A gadolinium-based contrast agent is recommended for all diagnostic procedures.
Novel MR Imaging Techniques
Conventional MR imaging is associated with shortcomings including low sensitivity to gray matter lesions and diffuse white matter involvement and a low capacity to predict clinical status. 3 Newer uses of existing MR imaging techniques, the availability of novel contrast agents (eg, high molar agents and smart nanoparticles), and emerging techniques (eg, MR spectroscopy, DWI, DTI, perfusion-weighted imaging, and permeability testing of the BBB) offer opportunities for improved specificity and sensitivity in diagnosing and monitoring MS. 3 , 33
Novel MR imaging techniques are continuously being developed and appraised for roles in the management of MS. Outside expert centers, T2 and gadolinium-enhanced T1 MR imaging remain mainstay approaches in practice.
- Summary of Expert Meeting Recommendations
From discussion during the expert meeting, the participants summarized 5 key recommendations for MR imaging in MS.
Recommendation 1: Diagnosis Versus Monitoring
Applications of MR imaging in MS should distinguish diagnosis from monitoring.
MR imaging, especially used with contrast agents, has an important role in diagnosing MS, excluding alternative diagnoses, and characterizing dissemination in space and time according to the revised McDonald criteria.
Recommendation 2: The Clinical–MR Imaging Paradox
MR imaging is currently not reliable for predicting the clinical evolution of MS.
Clinical decisions should not be based solely on the presence of lesions detected at MR imaging.
Recommendation 3: Importance of the McDonald Criteria
Standardized protocols and reporting procedures should be uniformly adopted on the basis of the revised McDonald criteria.
This message should be communicated widely to radiologists and neurologists at congresses and other educational opportunities.
The McDonald criteria need to be adapted for Asian populations.
Recommendation 4: Brain-Versus-Spinal Cord MR Imaging
For nonspinal cord presentation, brain MR imaging should be performed. MR imaging investigation may be stopped if there are sufficient lesions to support dissemination in space. If that is not the case, additional spinal MR imaging may be diagnostically helpful.
For spinal cord presentation, start investigations with spinal cord MR imaging, mainly to exclude alternative conditions. If MS remains suspected, perform brain MR imaging to identify additional lesions.
Recommendation 5: A Simple Standardized Protocol
A simple standardized MR imaging protocol should be implemented across centers.
Dual-echo and FLAIR axial whole brain, precontrast dual-echo or FLAIR sagittal midline (optional), and contrast-enhanced T1 scanning should be performed.
A scanner with at least 1T should be used.
Use specific landmarks to achieve consistent section positioning, especially for serial studies.
A gadolinium-containing contrast agent should be used for all diagnostic procedures.
Inject a standard dose of contrast agent (0.1 mmol/kg) before the first MR imaging.
For some contrast agents, there is evidence of a progressive increase in lesion-detection rate due to delayed enhancement.
Consider high signal intensity and safety as well as complex stability when selecting the contrast agent.
- Acknowledgment
PAREXEL MMS provided editorial support.
The expert meeting and the preparation of this review article were funded by an unrestricted educational grant from Bayer Schering Pharma AG.
Indicates open access to non-subscribers at www.ajnr.org
- Bydder GM ,
- Steiner RE ,
- Polman CH ,
- Reingold SC ,
- Thompson AJ ,
- Freedman MS ,
- Traboulsee A ,
- McDonald WI ,
- Compston A ,
- Barkhof F ,
- Filippi M ,
- Miller DH ,
- Scheinberg L ,
- Swanton JK ,
- Tintoré M ,
- Swanton J ,
- Fujihara K ,
- Grigoriadis N ,
- Grigoriadis S ,
- Polyzoidou E ,
- Altmann DR ,
- Rovaris M ,
- Ciccarelli O ,
- O'Riordan JI ,
- Fisniku LK ,
- Kremenchutzky M ,
- Baskerville J ,
- Neuhaus A ,
- Morrissey S ,
- Sormani MP ,
- Bonzano L ,
- Roccatagliata L ,
- Jacobs LD ,
- Barkhoff F ,
- Kinkel RP ,
- Kollman C ,
- O'Connor P ,
- for the CHAMPIONS Study Group
- for the BENEFIT Study Group
- Pellegrini F ,
- Barkhol F ,
- van Walderveen MA ,
- Herbert J ,
- Yousry TA ,
- Kupersmith MJ ,
- Zeiffer B ,
- Lycklama à Nijeholt G ,
- Lycklama G ,
- Thompson A ,
- Tombach B ,
- Sieber MA ,
- Lengsfeld P ,
- Frenzel T ,
- Schirmer H ,
- Barkovich AJ ,
- Maravilla KR ,
- Maldjian JA ,
- Schmalfuss IM ,
- Kirsch JE ,
- Erturk SM ,
- Yildirim H ,
- Wolansky LJ ,
- Bardini JA ,
- Migazzo E ,
- Saponaro A ,
- Mintorovitch J ,
- Rowley HA ,
- Scialfa G ,
- Pintaske J ,
- Martirosian P ,
- Tartaro A ,
- Tartaglione T ,
- Copyright © American Society of Neuroradiology
In this issue
- Table of Contents
- Index by author
Thank you for your interest in spreading the word on American Journal of Neuroradiology.
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Google Plus One
Jump to section
Related articles.
- No related articles found.
- Google Scholar
Cited By...
- Current and Emerging Therapies in Multiple Sclerosis: Implications for the Radiologist, Part 1--Mechanisms, Efficacy, and Safety
- Multiple Sclerosis: An Update
- MS Lesions Are Better Detected with 3D T1 Gradient-Echo Than with 2D T1 Spin-Echo Gadolinium-Enhanced Imaging at 3T
- Accuracy of Postcontrast 3D Turbo Spin-Echo MR Sequence for the Detection of Enhanced Inflammatory Lesions in Patients with Multiple Sclerosis
- Practice patterns of US neurologists in patients with SPMS and PPMS: A consensus study
- Crossref (80)
This article has been cited by the following articles in journals that are participating in Crossref Cited-by Linking.
- MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—clinical implementation in the diagnostic process Àlex Rovira, Mike P. Wattjes, Mar Tintoré, Carmen Tur, Tarek A. Yousry, Maria P. Sormani, Nicola De Stefano, Massimo Filippi, Cristina Auger, Maria A. Rocca, Frederik Barkhof, Franz Fazekas, Ludwig Kappos, Chris Polman, David Miller, Xavier Montalban Nature Reviews Neurology 2015 11 8
- Perfusion MRI: The Five Most Frequently Asked Technical Questions Marco Essig, Mark S. Shiroishi, Thanh Binh Nguyen, Marc Saake, James M. Provenzale, David Enterline, Nicoletta Anzalone, Arnd Dörfler, Àlex Rovira, Max Wintermark, Meng Law American Journal of Roentgenology 2013 200 1
- Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging Aviv Mezer, Jason D Yeatman, Nikola Stikov, Kendrick N Kay, Nam-Joon Cho, Robert F Dougherty, Michael L Perry, Josef Parvizi, Le H Hua, Kim Butts-Pauly, Brian A Wandell Nature Medicine 2013 19 12
- MR Imaging of Multiple Sclerosis Massimo Filippi, Maria A. Rocca Radiology 2011 259 3
- Evolution of the blood–brain barrier in newly forming multiple sclerosis lesions María I. Gaitán, Colin D. Shea, Iordanis E. Evangelou, Roger D. Stone, Kaylan M. Fenton, Bibiana Bielekova, Luca Massacesi, Daniel S. Reich Annals of Neurology 2011 70 1
- MRI in the assessment and monitoring of multiple sclerosis: an update on best practice Ulrike W. Kaunzner, Susan A. Gauthier Therapeutic Advances in Neurological Disorders 2017 10 6
- Multiscale Amplitude-Modulation Frequency-Modulation (AM–FM) Texture Analysis of Multiple Sclerosis in Brain MRI Images C.P. Loizou, V. Murray, M.S. Pattichis, I. Seimenis, M. Pantziaris, C.S. Pattichis IEEE Transactions on Information Technology in Biomedicine 2011 15 1
- A contrast-adaptive method for simultaneous whole-brain and lesion segmentation in multiple sclerosis Stefano Cerri, Oula Puonti, Dominik S. Meier, Jens Wuerfel, Mark Mühlau, Hartwig R. Siebner, Koen Van Leemput NeuroImage 2021 225
- Evaluation of MS related central fatigue using MR neuroimaging methods: Scoping review Jameen ARM, Karen Ribbons, Jeannette Lechner-Scott, Saadallah Ramadan Journal of the Neurological Sciences 2019 400
- OFSEP, a nationwide cohort of people with multiple sclerosis: Consensus minimal MRI protocol F. Cotton, S. Kremer, S. Hannoun, S. Vukusic, V. Dousset Journal of Neuroradiology 2015 42 3
More in this TOC Section
- Mechanisms of Healing in Coiled Intracranial Aneurysms: A Review of the Literature
- Ultra-High-Field MR Neuroimaging
- Armies of Pestilence: CNS Infections as Potential Weapons of Mass Destruction
Similar Articles
BRIEF RESEARCH REPORT article
Case report: a case of severe clinical deterioration in a patient with multiple sclerosis.

- 1 German Center for Vertigo and Balance Disorders, Ludwig Maximilian University of Munich, Munich, Germany
- 2 Department of Neurology, Ludwig Maximilian University of Munich, Munich, Germany
- 3 Department of Neurology, Medical Faculty, Heinrich Heine University Düsseldorf, Düsseldorf, Germany
- 4 Department of Neuroradiology, Medical Faculty, Heinrich Heine University Düsseldorf, Düsseldorf, Germany
- 5 Institute of Neuropathology, University Medical Center Göttingen, Göttingen, Germany
- 6 Institute of Neuropathology, Medical Faculty, Heinrich Heine University Düsseldorf, Düsseldorf, Germany
Tumefactive multiple sclerosis (MS) is a rare variant of MS that may lead to a rapidly progressive clinical deterioration requiring a multidisciplinary diagnostic workup. Our report describes the diagnostic and therapeutic approach of a rare and extremely severe course of MS. A 51-year-old man with an 8-year history of relapsing-remitting MS (RRMS) was admitted with a subacute progressive left lower limb weakness and deterioration of walking ability. After extensive investigations including repeated MRI, microbiological, serological, cerebrospinal fluid (CSF) studies, and finally brain biopsy, the diagnosis of a tumefactive MS lesion was confirmed. Despite repeated intravenous (IV) steroids as well as plasma exchanges and IV foscarnet and ganciclovir owing to low copy numbers of human herpesvirus 6 (HHV-6) DNA in polymerase chain reaction (PCR) analysis, the patient did not recover. The clinical presentation of tumefactive MS is rare and variable. Brain biopsy for histopathological workup should be considered in immunocompromised patients with rapidly progressive clinical deterioration with brain lesions of uncertain cause.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS) characterized by multiple lesions disseminated in time and space. Tumefactive MS is a rare variant of MS presenting with a large intracranial lesion, >2 cm in diameter with mass effect and perilesional edema and/or ring enhancement with gadolinium ( 1 ).
The rapidly progressive clinical deterioration of the MS patient presented here posed a diagnostic challenge requiring a multidisciplinary diagnostic workup. In the literature, various case reports describing the challenging diagnostic procedure of tumefactive MS due to varied clinical presentations as well as clinical courses can be found. Our report describes the diagnostic and therapeutic approach of a rare and at the same time extremely severe course of MS. It demonstrates that brain biopsy may be necessary for differential diagnosis in an immunocompromised MS patient with progressive brain lesions.
Case Presentation
A 51-year-old man of Mediterranean origin with an 8-year history of relapsing-remitting MS (RRMS) was admitted to our hospital on suspicion of a relapse.
After diagnosis in 2009, the patient had initially been treated with glatiramer acetate. The family medical history offered that the patient's mother and uncle (blood related) both suffered from MS. The patient's uncle died at the age of 52 years after being bedridden for a longer time. The patient had four relapses under glatiramer acetate necessitating treatment with intravenous (IV) steroids initially with a good treatment response. The first relapse leading to the diagnosis of a clinically isolated syndrome (CIS) was an acute central vestibular syndrome leading to dizziness and an ataxic gait dysfunction. At this time, MRI already revealed multiple white matter lesions in the supratentorium, cerebellum, and cervical as well as thoracic spinal cord.
In 2012, owing to an increasing relapse rate and incomplete clinical remissions, the medication was changed to natalizumab. At this time, the last relapses under glatiramer acetate had led to a residual paraparesis with emphasis on the left and a left side internuclear ophthalmoplegia. At the last relapse, brain MRI scan of the brain showed multiple white matter lesions with a cystic aspect and incomplete ring-like gadolinium enhancement. After the medication was switched to natalizumab, the disease course stabilized, and he suffered no more relapses. However, when the anti-JC virus (JCV) antibody level index (Stratify™) rose to 4.5, natalizumab was discontinued early in 2017. Subsequently, fingolimod was started 3 months prior to admission and 4 weeks after discontinuation of natalizumab.
The first symptoms appeared 8 days before admission: a progressive left lower limb weakness and deterioration of walking ability became evident. At that time, the patient was able to stand without help and walk a few steps with unilateral assistance [Expanded Disability Status Scale (EDSS) 6.0].
At this time (after treatment with natalizumab and rising anti-JCV antibody level index), the differential diagnoses were an MS relapse or progressive multifocal leukoencephalopathy (PML). The MRI scan of the brain on the day of admission showed bihemispheric confluent T2 white matter lesions without changes, typical for PML ( Figure 1A ). Cerebrospinal fluid (CSF) analysis revealed a normal white blood cell count (2/μl) with mildly increased lactate and glucose levels. The albumin quotient was normal, but oligoclonal bands were positive with intrathecal synthesis of immunoglobulins G and M. Polymerase chain reaction (PCR), microbiological, and serological study findings were all negative (including JCV PCR, JCV CSF/serum antibody index, HSV-1 PCR, HSV-2 PCR, VZV PCR, EBV PCR, and HIV PCR). Evoked potentials revealed an impairment of the corticospinal tract to the right leg, bilaterally impaired tibial nerve somatosensory reactions, and evidence of a bilateral affection of the visual system.
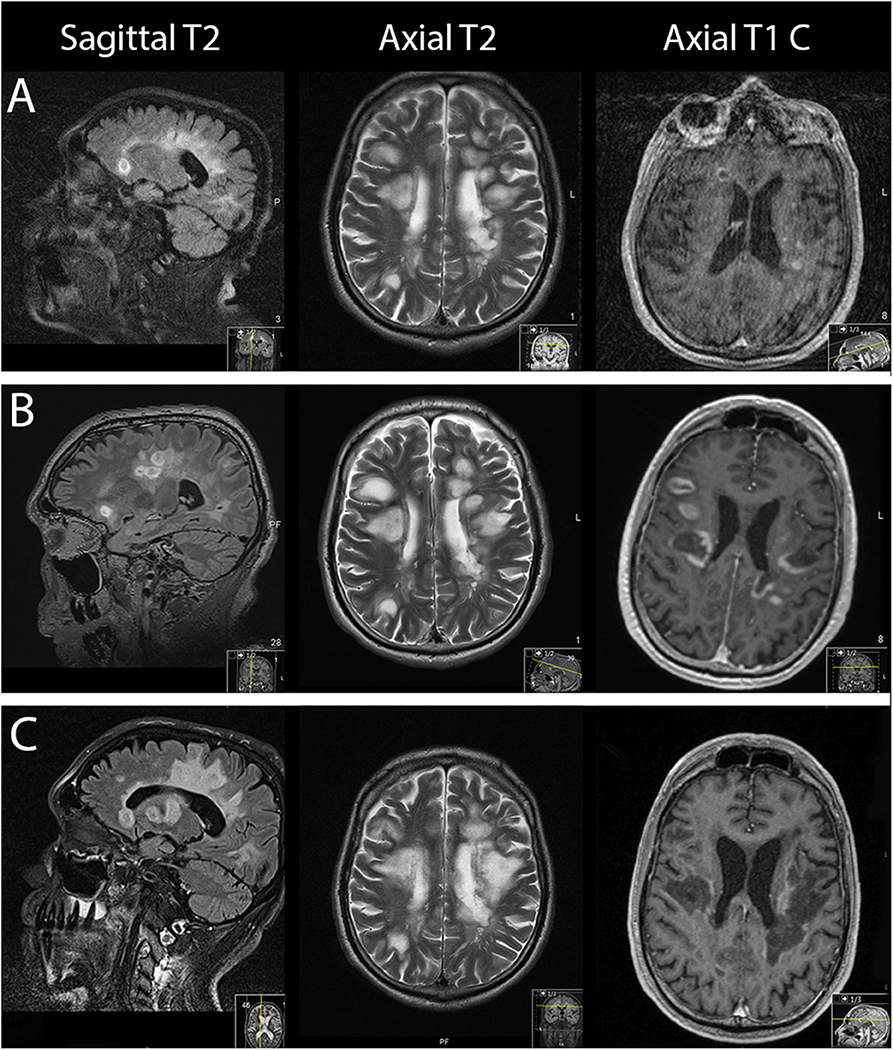
Figure 1 . From left to right: sagittal, axial MRI T2-weighted sequences and axial gadolinium contrast T1-weighted sequences. (A) On the day of admission. (B) Three days after admission. (C) Forty-one days after admission.
On suspicion of an MS relapse, the patient was treated with IV methylprednisolone 1,000 mg once daily for five consecutive days. In response to this therapy, his walking ability slightly improved. However, 2 days later, the patient's clinical status dramatically worsened, necessitating his transfer to the intensive care unit (ICU): he became somnolent and mutistic, exhibiting a bilateral horizontal gaze palsy. In addition, he became tetraplegic and had bilaterally positive Babinski signs corresponding to an EDSS score of 9.5. MRI scan of the brain at that time showed progressive bihemispheric confluent white matter lesions ( Figure 1B ). The patient developed a severe aspiration pneumonia with respiratory failure requiring intubation and subsequent tracheostomy for mechanical ventilation.
The progressive white matter lesions were judged as the radiological correlate of a clinical MS relapse.
After antibiotic treatment for pneumonia had led to a reduction of the leukocytosis and C-reactive protein, IV steroids were applied for 6 days. However, as no clinical improvement was observed on IV steroids, seven cycles of plasma exchange (PLEX) were performed. Another follow-up MRI scan of the brain revealed further progression of gadolinium enhancement and T2 lesion load.
Despite high-dose IV steroids and PLEX, the clinical condition of the patient deteriorated further. With negative laboratory results, and radiological findings atypical for PML, other differential diagnoses had to be considered. Characteristic imaging findings in PML are one or more regions of FLAIR/T2 showing hyperintense confluent white matter lesions, inconsistent in size and shape, typically involving subcortical U-fibers and sparing the cortex, leading to a sharp border between lesion and cortex. MS lesions typically present a periventricular distribution, whereas PML lesions more commonly involve the subcortical white matter.
The following differential diagnoses were considered for our patient:
• tumefactive MS relapse,
• neurocysticercosis,
• neurosarcoidosis,
• intracerebral lymphoma,
• atypic PML, and
• other viral encephalitis.
The negative results of the CSF analysis and serology argued against a neurocysticercosis, lymphoma, or PML. Cysticercosis is the most common parasitic infection of the CNS. However, the presentation on MRI imaging was judged unusual. Electroencephalography (EEG) was normal. For sarcoidosis, a CT scan of the chest and laboratory tests for ACE and sIL2R were added; both proved negative. The negative CSF findings and radiological presentation also argued against an intracerebral lymphoma but did not definitely exclude one. A tumefactive MS relapse is a rare course and commonly presents with a large intracerebral lesion (>2 cm) with mass effect and perilesional edema and/or ring enhancement with gadolinium.
To further differentiate between a tumefactive MS relapse and a less likely intracerebral lymphoma, a stereotactic biopsy of a lesion in the right frontal lobe was performed. The biopsy revealed a sharply demarcated inflammatory demyelinating lesion consistent with MS. Inflammatory infiltrates within the lesion consisted of CD3 dominated by CD8-positive T cells as well as CD138-positive plasma cells ( Figure 2 ). Deposits of complement and immunoglobulins identified the lesion as an antibody/complement mediated type of MS, described previously as pattern II MS ( 2 ). PCR analysis revealed low copy numbers of human herpesvirus 6 (HHV-6) DNA in tissue (32 copies/μg DNA). However, axons were preserved, thus ruling out a necrosis. Also, no evidence was found for lymphoma.
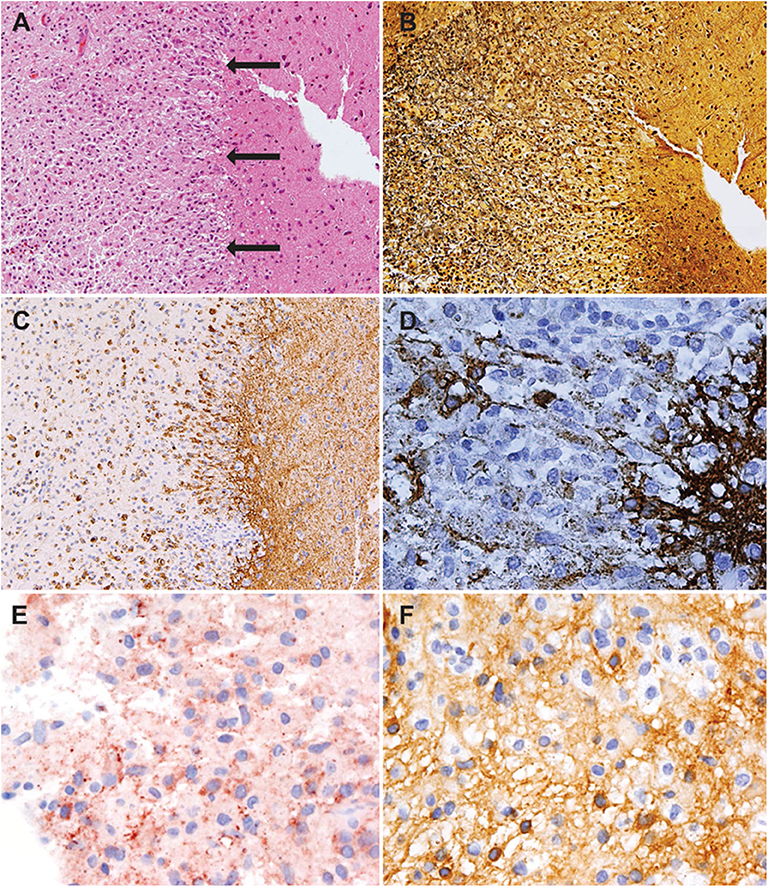
Figure 2 . Histology showed an active demyelinating multiple sclerosis (MS) lesion corresponding to immunopathological pattern II. Arrows indicate the sharply demarcated inflammatory subcortical plaque on the left; the cerebral cortex is present on the right [H&E stain, ×10 (A) ]. Axons were preserved within the lesion [Bielschowsky silver stain, ×10 (B) ], whereas myelin was lost [proteolipid protein, ×10 (C) ; cyclic nucleotide phosphodiesterase stain, ×40 (D) ]. The lesion showed early active demyelination as indicated by the presence of major (C) and minor myelin proteins (D) within the macrophages. Complement [c9neo complement stain, ×40 (E) ] and immunoglobulin G [IgG; IgG stain ×40 (F) ] were found within the macrophages, suggesting a complement and immunoglobulin-mediated demyelination (pattern II).
Given the diagnostic uncertainty between HHV-6 encephalitis and tumefactive MS lesions, we opted for a polypragmatic approach and induced a therapy with IV foscarnet and ganciclovir ( 3 ) along with another course of high-dose IV steroids for 5 days. The final neuropathologic results were suggestive of a pattern II MS lesion according to Lassmann et al. ( 2 ).
The MRI scan of the brain after therapy showed progression of the T2 lesion load; however, the regression of gadolinium enhancement suggested remission of acute inflammation ( Figure 1C ). The neurological status remained severely impaired: the tetraplegia slightly improved by developing into a severe tetraparesis of 2/5 at the upper and persisting plegia of lower limbs (EDSS 8.5).
Subsequently, the patient was transferred to a neuro-rehabilitation center. His clinical status did not improve, even after 3 months of rehabilitation. Currently, the patient is tetraparetic and lives in a special-care home where he spontaneously breathes through tracheostomy and receives enteral long-term nutrition via percutaneous endoscopic gastrostomy (PEG).
After fingolimod was discontinued during the acute phase, the possibility of administering a new highly active preventive MS treatment such as ocrelizumab or alemtuzumab in order to avoid further relapses was discussed with the patient's relatives but was discarded owing to fear of infectious complications and the patient's poor clinical status and prognosis. Unfortunately, no further MRI imaging has been performed after discharge from our clinic.
The rapid clinical deterioration posed a diagnostic challenge. Because of increasing anti-JCV index upon treatment with natalizumab, we speculated that PML might have occurred. However, the MRI findings were atypical (uncommon mass effect and degree of gadolinium enhancement). CSF analyses including PCR, microbiological, and serological studies were all negative. In view of the absence of clinical improvement and radiological progression despite high-dose IV steroids and PLEX, further differential diagnoses were considered. However, CSF findings argued against neurocysticercosis, lymphoma, or PML. Negative CSF findings for JCV (PCR) did not completely exclude PML because viral loads can be very low (<100 copies/mL); the detection threshold of commercial tests is about 200 copies/mL. With no signs of mediastinal lymphadenopathy in the CT scan of the chest and nonelevated serum ACE and sIL2-R levels, neurosarcoidosis seemed less likely. The negative CSF findings as well as the radiological presentation made the possibility of an intracerebral lymphoma or HHV-6 encephalitis less likely but could not rule them out. Therefore, a stereotactic biopsy was needed. The histopathological results yielded an inflammatory demyelinating MS lesion with low titers of HHV-6 DNA quantified by PCR. There were no morphological features of a necrotizing HHV-6-encephalitis. In correlation with low copies of HHV-6-DNA, an HHV-6 encephalitis seemed very unlikely.
HHV-6 belongs to the Herpesviridae family. In the general population, virus latency in adenoid tissues/tonsils is almost 100%, usually acquired during childhood. Cells persist in a latent viral state mainly in leukocytes and can directly integrate their DNA into host cells ( 4 ). The prevalence of integrated HHV-6 DNA in healthy blood donors is 0.5% ( 5 ). Thus, detection in a blood sample does not definitely indicate an active infection. The same applies to infection and replication in the CNS; studies have revealed the presence of HHV-6 DNA even in healthy brain tissues ( 6 ).
HHV-6 encephalitis is characterized by necrotizing brain lesions and typically high copy numbers of virus DNA in CSF and/or brain tissue. Although the disease is a rare event, it should be taken into account in an immunocompromised patient with necrotizing brain lesions. Brain biopsy should be considered because it is important that patients receive a proper diagnosis in order to possibly benefit from an antiviral therapy combining foscarnet and ganciclovir ( 3 ). In this context, advanced imaging modalities like FET-PET or MRI spectroscopy may be of help and should be further investigated for their usefulness in making the differential diagnosis.
Conclusions
In our patient, the results of the brain biopsy finally confirmed the diagnosis of a tumefactive MS lesion. All pathological features of MS were fulfilled including inflammatory demyelination, relative axonal preservation, and gliosis. The early active demyelinating nature of the lesion allowed us to classify the lesion as having the immunopathological pattern II, which has recently been shown to improve clinically in 55% in response to apheresis therapy ( 7 ). Our case exemplifies that treatment response can be dramatically negative. Possible explanations include that the onset of PLEX at 13 days after relapse onset may have been too late and/or that the damage was too severe.
Severe MS rebounds after highly effective treatment with fingolimod as well as natalizumab were previously reported ( 8 ). The differentiation between a recurrence of disease activity and a rebound, implying a more severe disease course than before natalizumab treatment, is difficult. On histological grounds, the present biopsy showed an MS lesion with an inflammatory infiltrate not exceeding the MS typical inflammation. However, a rebound can be assumed based on the clinical and MRI findings. Switching from natalizumab to fingolimod might increase the risk of tumefactive MS ( 9 ). Predictive factors to identify risk groups are warranted.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
KB gave the idea of case reporting, analyzed the case, and drafted the manuscript for intellectual content. AA revised the figures and critically reviewed the manuscript. MF, NG, HH, OA, and H-PH critically reviewed the manuscript. BK prepared the MRI scans as figures and critically reviewed the manuscript. DH critically reviewed the manuscript and revised the MRI sequences for interpretation. BT critically reviewed the manuscript and interpreted the MRI sequences. IM, WB, and GR critically reviewed the manuscript and interpreted the neuropathology results. PA critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge support by the Heinrich Heine University Düsseldorf.
Abbreviations
ACE, angiotensin-converting enzyme; CNS, central nervous system; CSF, cerebrospinal fluid; EBV, Epstein–Barr virus; EDSS, expanded disability status scale; EEG, electroencephalography; FET-PET, 18 F-fluoro-ethyl-tyrosine positron emission tomography; HHV-6, human herpesvirus 6; HIV, human immunodeficiency virus; HSV-1/HSV-2, herpes simplex virus type 1/type 2; ICU, intensive care unit; IV, intravenous; JCV, John Cunningham virus; MRI, magnet resonance imaging; MS, multiple sclerosis; PCR, polymerase chain reaction; PEG, percutaneous endoscopic gastrostomy; PLEX, plasma exchange; PML, progressive multifocal leukoencephalopathy; RRMS, relapsing-remitting multiple sclerosis; sIL-2-R, soluble interleukin-2-receptor; VZV, varicella zoster virus.
1. Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, Weigand S, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. (2008) 131:1759–75. doi: 10.1093/brain/awn098
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Lassmann H, Bruck W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med. (2001) 7:115–21. doi: 10.1016/S1471-4914(00)01909-2
3. Le Guennec L, Mokhtari K, Chauvet D, Dupuis N, Roos-Weil D, Agut H, et al. Human Herpesvirus 6 (HHV-6) necrotizing encephalitis, a rare condition in immunocompromised patients: the importance of brain biopsy associated with HHV-6 testing. J Neurol Sci. (2017) 377:112–5. doi: 10.1016/j.jns.2017.04.003
4. Arbuckle JH, Medveczky MM, Luka J, Hadley SH, Luegmayr A, Ablashi D, et al. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro . Proc Natl Acad Sci USA. (2010) 107:5563–8. doi: 10.1073/pnas.0913586107
5. Geraudie B, Charrier M, Bonnafous P, Heurte D, Desmonet M, Bartoletti MA, et al. Quantitation of human herpesvirus-6A,−6B and−7 DNAs in whole blood, mononuclear and polymorphonuclear cell fractions from healthy blood donors. J Clin Virol. (2012) 53:151–5. doi: 10.1016/j.jcv.2011.10.017
6. Luppi M, Barozzi P, Maiorana A, Marasca R, Torelli G. Human herpesvirus 6 infection in normal human brain tissue. J Infect Dis. (1994) 169:943–4. doi: 10.1093/infdis/169.4.943
7. Stork L, Ellenberger D, Beissbarth T, Friede T, Lucchinetti CF, Bruck W, et al. Differences in the reponses to apheresis therapy of patients with 3 histopathologically classified immunopathological patterns of multiple sclerosis. JAMA Neurol. (2018) 75:428–35. doi: 10.1001/jamaneurol.2017.4842
8. Faissner S, Hoepner R, Lukas C, Chan A, Gold R, Ellrichmann G. Tumefactive multiple sclerosis lesions in two patients after cessation of fingolimod treatment. Ther Adv Neurol Disord. (2015) 8:233–8. doi: 10.1177/1756285615594575
9. Jander S, Turowski B, Kieseier BC, Hartung HP. Emerging tumefactive multiple sclerosis after switching therapy from natalizumab to fingolimod. Multiple Scler. (2012) 18:1650–2. doi: 10.1177/1352458512463768
Keywords: multiple sclerosis, tumefactive multiple sclerosis, demyelinating disease, multiple sclerosis rebound, immunocompromised multiple sclerosis patient, progressive brain lesions
Citation: Breitkopf K, Aytulun A, Förster M, Kraus B, Turowski B, Huppert D, Goebels N, Hefter H, Aktas O, Metz I, Brück W, Reifenberger G, Hartung H-P and Albrecht P (2020) Case Report: A Case of Severe Clinical Deterioration in a Patient With Multiple Sclerosis. Front. Neurol. 11:782. doi: 10.3389/fneur.2020.00782
Received: 17 May 2020; Accepted: 25 June 2020; Published: 18 August 2020.
Reviewed by:
Copyright © 2020 Breitkopf, Aytulun, Förster, Kraus, Turowski, Huppert, Goebels, Hefter, Aktas, Metz, Brück, Reifenberger, Hartung and Albrecht. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philipp Albrecht, phil.albrecht@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

- Multiple sclerosis
Citation, DOI, disclosures and case data
At the time the case was submitted for publication Ahmed Abdrabou had no recorded disclosures.
Presentation
Follow up of known multiple sclerosis. Recent onset diplopia and right sided parasthesia.
Patient Data
White matter demyelination is seen involving the left peritrigonal region and extends through the forceps major fibers and splenium of corpus callosum to the contralateral side. It displays bright signal on T2 and FLAIR, low signal on T1 and enhances after contrast giving the appearance of open ring. The bright signal on DWI is believed to be T2 shine through effect as there is no evidence of restriction on ADC map. Another two plaques are seen at the left aspects of medulla oblongata and pons. Double inversion recovery sequence DIR delineates the lesions better due to suppression of signal from the white matter and CSF. Moreover, it detects involvement of the fornix as well.
Multiple intramedullary plaques are seen involving the cord starting from C4 down to D5 vertebral level displaying bright signal on T2 and STIR. Faint enhancement is noted opposite C5-C6 level. Also the left medullary and pontine lesions are evident.
Case Discussion
Multiple sclerosis is a common demyelinating disease of the central nervous septum. The diagnosis is mainly radiological and depends on detection of white matter lesions that disseminate in time and space. Plaques can be infratentorial, deep white matter, periventricular, juxta cortical or mixed white matter-grey matter lesions. MRI is the method of choice and the newly developed sequences e.g. DIR has more role in the detection and follow up. Enhancement in MS is a sign of active inflammation and usually incomplete ring.
- 1. Wattjes MP, Lutterbey GG, Gieseke J et-al. Double inversion recovery brain imaging at 3T: diagnostic value in the detection of multiple sclerosis lesions. AJNR Am J Neuroradiol. 2007;28 (1): 54-9. Pubmed citation
- 2. Zhang Q, Li Q, Zhang J et-al. Double inversion recovery magnetic resonance imaging (MRI) in the preoperative evaluation of hippocampal sclerosis: correlation with volumetric measurement and proton magnetic resonance spectroscopy (¹H MRS). J Comput Assist Tomogr. 2011;35 (3): 406-10. doi:10.1097/RCT.0b013e318219c2b6 - Pubmed citation
2 articles feature images from this case
- Double inversion recovery sequence
8 public playlists include this case
- Neuro (part 3) (part 1) by Taimur
- MR Basic by Lukasz Budynko
- kejsy #1 (part 2) by Lech Gradziński
- MRI by Tomas Jurevicius
- Rehab Registrars by Mihir Desai
- Neuroradiology by Ali
- neuroradiology #2 by Emil Michalski
Related Radiopaedia articles
Promoted articles (advertising), how to use cases.
You can use Radiopaedia cases in a variety of ways to help you learn and teach.
- Add cases to playlists
- Share cases with the diagnosis hidden
- Use images in presentations
- Use them in multiple choice question
Creating your own cases is easy.
- Case creation learning pathway
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Blood circulating microparticle species in relapsing-remitting and secondary progressive multiple sclerosis. A case-control, cross sectional study with conventional MRI and advanced iron content imaging outcomes
Affiliations.
- 1 Department of Molecular and Cellular Physiology, Louisiana State University Health-Shreveport, LA, USA.
- 2 Department Microbiology & Immunology, Center for Molecular and Tumor Virology, Louisiana State University Health-Shreveport, LA, USA.
- 3 The Jacobs Neurological Institute, Department of Neurology, University of Buffalo, Buffalo, NY, USA.
- 4 Department of Pharmaceutical Sciences, State University of New York, Buffalo, NY, USA.
- 5 Department of Neurology, Louisiana State University Health-Shreveport, LA, USA. Electronic address: [email protected].
- 6 Department of Neurology, Louisiana State University Health-Shreveport, LA, USA.
- 7 Department of Molecular and Cellular Physiology, Louisiana State University Health-Shreveport, LA, USA; Department for General and Visceral Surgery, Muenster, Germany.
- 8 Computer Sciences Department, Louisiana State University-Shreveport, LA, USA.
- 9 The Jacobs Neurological Institute, Department of Neurology, University of Buffalo, Buffalo, NY, USA; Buffalo Neuroimaging Analysis Center, Department of Neurology, University of Buffalo, Buffalo, NY, USA.
- PMID: 26073484
- PMCID: PMC4550483
- DOI: 10.1016/j.jns.2015.05.027
Background: Although multiple sclerosis (MS) is thought to represent an excessive and inappropriate immune response to several central nervous system (CNS) autoantigens, increasing evidence also suggests that MS may also be a neurovascular inflammatory disease, characterized by endothelial activation and shedding of cell membrane microdomains known as 'microparticles' into the circulation.
Objective: To investigate the relationships between these endothelial biomarkers and MS.
Methods: We examined the relative abundance of CD31(+)/PECAM-1, CD51(+)CD61(+) (αV-β3) and CD54(+) (ICAM-1) bearing microparticles in sera of healthy individuals, patients with relapsing-remitting MS, and secondary-progressive MS. We also investigated the correlation among circulating levels of different microparticle species in MS with conventional MRI (T2- and T1-lesion volumes and brain atrophy), as well as novel MR modalities [assessment of iron content on susceptibility-weighted imaging (SWI)-filtered phase].
Results: Differences in circulating microparticle levels were found among MS groups, and several microparticle species (CD31(+)/CD51(+)/CD61(+)/CD54(+)) were found to correlate with conventional MRI and SWI features of MS.
Conclusion: These results indicate that circulating microparticles' profiles in MS may support mechanistic roles for microvascular stress and injury which is an underlying contributor not only to MS initiation and progression, but also to pro-inflammatory responses.
Keywords: Atrophy; Endothelial microparticles; Iron deposition; MRI; Multiple sclerosis; Serum.
Copyright © 2015 Elsevier B.V. All rights reserved.
PubMed Disclaimer
Conflict of interest statement
Conflict of interest
There are no conflicts of interest for the authors.
A. CD31 + /CD51 +…
A. CD31 + /CD51 + CD61 + /CD54 + microparticles were elevated in…
Similar articles
- Progressive Forms of Multiple Sclerosis: Distinct Entity or Age-Dependent Phenomena. Zeydan B, Kantarci OH. Zeydan B, et al. Neurol Clin. 2018 Feb;36(1):163-171. doi: 10.1016/j.ncl.2017.08.006. Neurol Clin. 2018. PMID: 29157397 Review.
- Brain atrophy: an in-vivo measure of disease activity in multiple sclerosis. Radü EW, Bendfeldt K, Mueller-Lenke N, Magon S, Sprenger T. Radü EW, et al. Swiss Med Wkly. 2013 Nov 21;143:w13887. doi: 10.4414/smw.2013.13887. eCollection 2013. Swiss Med Wkly. 2013. PMID: 24264439 Review.
- T2 lesions and rate of progression of disability in multiple sclerosis. Mostert JP, Koch MW, Steen C, Heersema DJ, De Groot JC, De Keyser J. Mostert JP, et al. Eur J Neurol. 2010 Dec;17(12):1471-5. doi: 10.1111/j.1468-1331.2010.03093.x. Eur J Neurol. 2010. PMID: 20500805
- Chronic cerebrospinal venous insufficiency and iron deposition on susceptibility-weighted imaging in patients with multiple sclerosis: a pilot case-control study. Zivadinov R, Schirda C, Dwyer MG, Haacke ME, Weinstock-Guttman B, Menegatti E, Heininen-Brown M, Magnano C, Malagoni AM, Wack DS, Hojnacki D, Kennedy C, Carl E, Bergsland N, Hussein S, Poloni G, Bartolomei I, Salvi F, Zamboni P. Zivadinov R, et al. Int Angiol. 2010 Apr;29(2):158-75. Int Angiol. 2010. PMID: 20351672
- Serum and cerebrospinal fluid nitrite and nitrate levels in relapsing-remitting and secondary progressive multiple sclerosis patients. Yuceyar N, Taşkiran D, Sağduyu A. Yuceyar N, et al. Clin Neurol Neurosurg. 2001 Dec;103(4):206-11. doi: 10.1016/s0303-8467(01)00144-5. Clin Neurol Neurosurg. 2001. PMID: 11714562
- Possible Roles of Extracellular Vesicles in the Pathogenesis and Interventions of Immune-Mediated Central Demyelinating Diseases. Teekaput C, Thiankhaw K, Chattipakorn N, Chattipakorn SC. Teekaput C, et al. Exp Neurobiol. 2024 Apr 30;33(2):47-67. doi: 10.5607/en24002. Exp Neurobiol. 2024. PMID: 38724476 Free PMC article. Review.
- Vascular multiple sclerosis: addressing the pathogenesis, genetics, pro-angiogenic factors, and vascular abnormalities, along with the role of vascular intervention. Prajjwal P, Shree A, Das S, Inban P, Ghosh S, Senthil A, Gurav J, Kundu M, Marsool Marsool MD, Gadam S, Marsool Marsoo AD, Vora N, Amir Hussin O. Prajjwal P, et al. Ann Med Surg (Lond). 2023 Aug 14;85(10):4928-4938. doi: 10.1097/MS9.0000000000001177. eCollection 2023 Oct. Ann Med Surg (Lond). 2023. PMID: 37811110 Free PMC article. Review.
- Extracellular Vesicles in Chronic Demyelinating Diseases: Prospects in Treatment and Diagnosis of Autoimmune Neurological Disorders. Ovchinnikova LA, Zalevsky AO, Lomakin YA. Ovchinnikova LA, et al. Life (Basel). 2022 Nov 21;12(11):1943. doi: 10.3390/life12111943. Life (Basel). 2022. PMID: 36431078 Free PMC article. Review.
- Dysregulated Sulfide Metabolism in Multiple Sclerosis: Serum and Vascular Endothelial Inflammatory Responses. Veerareddy P, Dao N, Yun JW, Stokes KY, Disbrow E, Kevil CG, Cvek U, Trutschl M, Kilgore P, Ramanathan M, Zivadinov R, Alexander JS. Veerareddy P, et al. Pathophysiology. 2022 Sep 17;29(3):570-582. doi: 10.3390/pathophysiology29030044. Pathophysiology. 2022. PMID: 36136071 Free PMC article.
- Flow Cytometry Analysis of Blood Large Extracellular Vesicles in Patients with Multiple Sclerosis Experiencing Relapse of the Disease. Soukup J, Kostelanská M, Kereïche S, Hujacová A, Pavelcová M, Petrák J, Kubala Havrdová E, Holada K. Soukup J, et al. J Clin Med. 2022 May 17;11(10):2832. doi: 10.3390/jcm11102832. J Clin Med. 2022. PMID: 35628959 Free PMC article.
- Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122(4):1180–1188. http://dx.doi.org/10.1172/JCI58649 . - DOI - PMC - PubMed
- Prosperini L, Giannì C, Barletta V, et al. Predictors of freedom from disease activity in natalizumab treated-patients with multiple sclerosis. J Neurol Sci. 2012;323(1–2):104–112. http://dx.doi.org/10.1016/j.jns.2012.08.027 . - DOI - PubMed
- Wee Yong V. Inflammation in neurological disorders: a help or a hindrance? Neuro-scientist. 2010;16(4):408–420. http://dx.doi.org/10.1177/1073858410371379 . - DOI - PubMed
- Minagar A, Jy W, Jimenez JJ, et al. Elevated plasma endothelial microparticles in multiple sclerosis. Neurology. 2001;56(10):1319–1324. - PubMed
- Steinman L. Platelets provide a bounty of potential targets for therapy in multiple sclerosis. Circ Res. 2012;110(9):1157–1158. http://dx.doi.org/10.1161/CIRCRESAHA.112.269050 . - DOI - PubMed
Publication types
- Search in MeSH
Related information
Grants and funding.
- P30 GM110703/GM/NIGMS NIH HHS/United States
- R21 NS059724/NS/NINDS NIH HHS/United States
- P30GM110703/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full text sources.
- Elsevier Science
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- scite Smart Citations
- Genetic Alliance
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

- Cognitive rehab tied to MRI changes in cognition-linked brain regions
Findings may help explain why rehab leads to gains for some MS patients

by Lindsey Shapiro, PhD | June 12, 2024
Share this article:
A three month cognitive rehabilitation program, with or without aerobic exercise, increased tissue volume and activity in brain regions linked to cognition among people with progressive forms of multiple sclerosis (MS), clinical trial data suggests.
The MRI findings offer potential biological explanations about why cognitive rehabilitation leads to cognitive gains for some MS patients, the researchers said in “ Cognitive rehabilitation effects on grey matter volume and Go-NoGo activity in progressive multiple sclerosis: results from the CogEx trial ,” which was published in the Journal of Neurology, Neurosurgery, & Psychiatry .
Cognitive impairment is common in MS and is thought to affect up to 65% of patients . But people with progressive types of MS often have more severe cognitive dysfunction than those with a relapsing-remitting disease course.
Both cognitive rehabilitation, involving activities to strengthen or restore cognitive abilities, and physical exercise have each been linked to cognitive improvements in MS patients, although their exact benefits are still being explored.
The CogEx trial (NCT03679468) examined the possible cognitive benefits of a combined cognitive rehabilitation and aerobic exercise approach among more than 300 people with progressive forms of MS, ages 25-65, who had existing impairments in information processing speed, a common type of cognitive issue in MS.

Healthy Connections in Brain May Be Needed for Cognitive Rehab
Cognitive rehab’s effect on gray matter.
The participants were randomly assigned to receive cognitive rehabilitation, aerobic exercise, cognitive rehabilitation with aerobic exercise, or neither, which they performed for 12 weeks and returned for another follow-up six months after stopping the intervention.
Cognitive rehabilitation involved computer-based brain tasks. In groups where cognitive rehabilitation wasn’t performed, patients performed a sham exercise involving basic internet searches/computer use. Aerobic exercise involved a step machine. The groups not assigned to aerobic exercise performed balance and stretching activities as a sham intervention.
The trial’s primary analysis showed that combining cognitive rehabilitation with aerobic exercise failed to improve cognitive performance relative to either intervention alone. A substantial number of patients did see improvements in information processing speed, however.
A subgroup of 104 participants also underwent MRI scans to look at changes in brain tissue volume and connectivity. The recent publication reports findings from that substudy.
Here, as in the broader study population, no differences were seen in cognitive performance between the different groups.
While most structural findings on MRI scans also didn’t differ by intervention, the volume of gray matter, which is brain tissue containing mainly nerve cell bodies, showed significant differences among the groups. These differences were largely driven by an increased gray matter volume over time in those who performed only cognitive rehabilitation.
Those who performed cognitive rehabilitation with or without aerobic exercise showed significant increases in gray matter volume across multiple brain regions relevant for cognition after 12 weeks. That contrasted with the groups that didn’t involve cognitive rehabilitation, where a general decrease in gray matter volume was observed. Gray matter is relevant for cognition and studies show its loss is associated with cognitive worsening in MS. For this reason, an increase “might be beneficial for cognitive performances,” the researchers said.
Indeed, in the groups performing cognitive rehabilitation, increased gray matter volume correlated with an improved performance in a test of verbal learning and memory.
Functional MRI scans were also performed to look at differences in brain activation and connectivity with the various interventions.
Cognitive rehabilitation was associated with increased activation of a brain region called the insula relative to groups that performed the sham computer activities at week 12. The insula is involved in attention and information processing, and its dysfunction has been linked to cognitive problems in MS.
While the study shows no “synergistic effect” of cognitive rehabilitation and aerobic exercise on cognitive performance or its MRI correlates, the findings do highlight that cognitive rehabilitation itself might lead to beneficial changes in brain regions linked to cognition that may explain cognitive improvements in some patients.
“Future studies exploring insular connectivity in this cohort may provide additional insights into changes taking place in the insular network post rehabilitation,” the researchers said.
About the Author

Recent Posts
- Updated guidance eases Mavenclad MRI requirements in England
- Headaches and MS linked, and therapies may affect them: Study
Recommended reading
KYV-101 helps 2 hard-to-treat progressive MS patients: Case study
Vitamin D seen as most helpful for males in progressive MS rat model

ACTRIMS 2024: Progressive MS patients show gains in NG-01 OLE
Subscribe to our newsletter.
Get regular updates to your inbox.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Data Descriptor
- Open access
- Published: 04 June 2024
Multiparametric MRI dataset for susceptibility-based radiomic feature extraction and analysis
- Cristiana Fiscone ORCID: orcid.org/0000-0003-4306-244X 1 ,
- Giovanni Sighinolfi 2 ,
- David Neil Manners 2 , 3 ,
- Lorenzo Motta 2 ,
- Greta Venturi 2 ,
- Ivan Panzera ORCID: orcid.org/0000-0001-6114-1403 4 ,
- Fulvio Zaccagna ORCID: orcid.org/0000-0001-6838-9532 5 , 6 , 7 ,
- Leonardo Rundo 8 ,
- Alessandra Lugaresi ORCID: orcid.org/0000-0003-2902-5589 1 , 4 ,
- Raffaele Lodi 1 , 2 ,
- Caterina Tonon 1 , 2 &
- Mauro Castelli 9
Scientific Data volume 11 , Article number: 575 ( 2024 ) Cite this article
219 Accesses
Metrics details
- Multiple sclerosis
Multiple sclerosis (MS) is a progressive demyelinating disease impacting the central nervous system. Conventional Magnetic Resonance Imaging (MRI) techniques (e.g., T 2 w images) help diagnose MS, although they sometimes reveal non-specific lesions. Quantitative MRI techniques are capable of quantifying imaging biomarkers in vivo , offering the potential to identify specific signs related to pre-clinical inflammation. Among those techniques, Quantitative Susceptibility Mapping (QSM) is particularly useful for studying processes that influence the magnetic properties of brain tissue, such as alterations in myelin concentration. Because of its intrinsic quantitative nature, it is particularly well-suited to be analyzed through radiomics, including techniques that extract a high number of complex and multi-dimensional features from radiological images. The dataset presented in this work provides information about normal-appearing white matter (NAWM) in a cohort of MS patients and healthy controls. It includes QSM-based radiomic features from NAWM and its tracts, and MR sequences necessary to implement the pipeline: T 1 w, T 2 w, QSM, DWI. The workflow is outlined in this article, along with an application showing feature reliability assessment.
Similar content being viewed by others
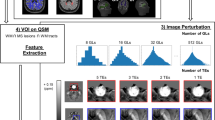
Assessing robustness of quantitative susceptibility-based MRI radiomic features in patients with multiple sclerosis
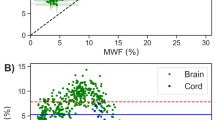
Comparison of multi echo T2 relaxation and steady state approaches for myelin imaging in the central nervous system
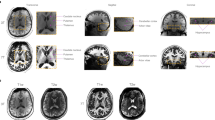
A paired dataset of T1- and T2-weighted MRI at 3 Tesla and 7 Tesla
Background & summary.
Quantitative Susceptibility Mapping (QSM) 1 , 2 is an advanced Magnetic Resonance Imaging (MRI) technique used to quantify and map the magnetic susceptibility (χ) of various structures within the body, with a primary focus on the brain. This technique is frequently employed to investigate and visualize medical conditions that alter the magnetic properties of bodily tissues, such as the presence of iron deposits, hemorrhages, or de-myelinating processes. Since the early 2010s, demand for more precise and quantitative assessments of magnetic tissue properties has driven interest in the application of QSM in neuroimaging.
The development of QSM builds on previous research in MRI and χ-based techniques. In fact, numerous neurological conditions in both adults and children have demonstrated abnormal accumulations of blood-related substances or mineral deposits. Thus, Susceptibility-Based Imaging (SBI) 3 has found application in clinical settings. While traditional SBI methods, like Susceptibility Weighted Imaging (SWI) 4 , 5 , offer valuable insights, they come with certain limitations compared to QSM 2 , which provides quantitative measurements, enables comparisons within and between different groups, and facilitates the discrimination between diamagnetic and paramagnetic substances.
One of the most investigated applications of QSM is the examination of neurodegenerative and neuroinflammatory disorders 6 , including Multiple Sclerosis (MS), which is an autoimmune demyelinating disease affecting the Central Nervous System (CNS) and presenting a wide array of symptoms such as fatigue, motor or sensory loss in limbs, cognitive decline, and visual disturbances 7 . The current diagnosis of MS relies on the McDonald criteria, which combine clinical observations, laboratory biomarkers, and imaging data 8 . Traditional MRI has played a crucial role in these criteria and is widely used to monitor disease progression. Nevertheless, conventional MRI techniques reveal established lesions without providing detailed insights into the underlying mechanisms responsible for demyelination 9 : focal or confluent white matter (WM) alterations, which are found in over 95% of MS patients, can be detected, but the presence of these lesions is not an absolute requirement for diagnosing the disease, as non-specific WM lesions can also occur in healthy individuals over 50 years old or those affected by other neurological diseases.
The development of innovative quantitative MRI (qMRI) techniques capable of quantifying imaging biomarkers in vivo holds promise for exploring the microstructure of the brain 10 and its metabolic processes 11 to unravel the pathophysiology of MS and potentially reveal signs of pre-clinical inflammatory demyelination. QSM, as a qMRI technique, may aid in the investigation of both damaged and undamaged brain tissue in patients with MS 12 , 13 , 14 , 15 .
In the last twenty years, radiomics has emerged as a quantitative analytical tool for personalized medicine using medical imaging 16 , 17 . It includes a set of techniques that extract complex and multi-dimensional features from radiological images, including characteristics such as intensity histograms and textural patterns within the Region or Volume of Interest (ROI/VOI) analyzed 18 . Since its introduction, radiomics has found applications across several medical image modalities. In the field of MR neuroimaging, a significant focus has been directed toward the study of brain tumors, making use of anatomical T 1 – and T 2 –weighted (T 1 w and T 2 w) images for purposes such as tumor characterization and grading 19 , 20 or the assessment of treatment response and clinical outcomes 21 .
The dataset 22 we provide in this work includes QSM-based radiomic features from Normal Appearing White Matter (NAWM) and its tracts, extracted in a mixed group of patients with MS and healthy controls, and all the different MR sequences necessary to implement the pipeline: for each subject, morphological T 1 w and T 2 w, QSM and Diffusion Weighted Imaging (DWI) for Diffusion Tractography Imaging (DTI). An example of an application based on those data 23 is shown in the last section.
Participants
For this work, data from 100 MS patients were obtained from the repository of the Neuroimaging Laboratory (IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy), along with data from 50 healthy control subjects. Inclusion criteria are as follows: patients had to be older than 18 years, with relapsing-remitting, primary progressive or secondary progressive MS course according to the 2017 revision of the McDonald diagnostic criteria, and to receive intravenous therapy with anti-CD20 monoclonal antibodies. Healthy controls had to be older than 18 years old; furthermore, the MRI exam had to be negative for abnormal cerebral atrophy, cortical and subcortical iron accumulation and CSF circulation disturbances. The demographic characteristics of the sample are summarized in Table 1 .
The data provided are related to scans performed between November 2020 and August 2023, except for one MR sequence for one healthy subject acquired in 2018. Acquisition and processing software and pipelines have basically remained the same throughout the relevant period, guaranteeing the homogeneity of the data used for analysis; small changes that were implemented are illustrated in the following sections.
Ethical approval and informed consent
Ethical approval for the study was obtained by the Institutional Review Board “Area Vasta Emilia Centro” (AVEC) (approval number AUSLBO 2023/CE 23043); written informed consent was obtained from all participants. Data has been anonymized and de-identified to ensure participating individuals cannot be identified from the information or images provided.
MRI data acquisition
Figure 1 shows the workflow for the acquisition and processing pipeline.

Scheme of the acquisition and processing pipeline. The brain MRI protocol provided: T 1 w, T 2 w, DWI and T 2 *w images (in this figure: patient with MS, F/38 years old): 1) T 1 w images were used to obtain white matter segmentation using 5ttgen from MRtrix3 2) LPA algorithm from FSL was applied to T 2 w images to obtain MS lesion mask. Merging WM segmentation with the MS lesion mask, the normal-appearing-white-matter segmentation mask was obtained. We run LST only on patients with MS; for controls, NAWM corresponds to WM from MRtrix3. 3) DWI images were pre-processed as explained in the specific section and diffusion tractography imaging automatic pipeline was applied, obtaining six white matter tracts (arcuate fasciculus, cortico-spinal tract, frontal aslant tract, inferior frontal-occipital fasciculus, optic radiation, uncinate fasciculus); VOIs from tractography reconstruction were merged with MS lesion mask to exclude damaged tissue. 4) T 2 *w images were processed to obtain QSM reconstructions, as explained in the specific section. All the images/masks were registered in the T 1 w space. Radiomic features were extracted from QSM images in 14 volumes (six white matter tracts and total normal appearing white matter, left and right hemisphere).
Scans were performed on a 3-T clinical scanner (Magnetom Skyra; Siemens Healthineers, Erlangen, Germany) equipped with a whole-body transmit and a 64-channel Head/Neck receiver coil. Sequences included in the MRI protocol and their details are shown in Table 2 .
We modified the DWI sequence during the study, going from single-shell (5 unweighted volumes and 64 volumes with b = 2000 s/mm 2 ) to multi-shell (8 unweighted volumes, 12 volumes with b = 300 s/mm 2 , 30 volumes with b = 1000 s/mm 2 and 64 volumes with b = 2000 s/mm 2 ) acquisition. This change should have a negligible effect on tissue contrast for equivalently weighted volumes, given the processing method employed. In the dataset, the type of sequence is indicated for each subject. Sequences were acquired with Anterior-Posterior (AP) phase encoding. An additional sequence with inverted phase encoding (PA) (~ 4’ scan time) was acquired to correct EPI distortion artifacts in the EPI volumes: for single-shell measurements, three unweighted volumes were acquired, while for multi-shell, the sequence was re-acquired by repeating each volume acquisition with weighting 0–1000 s/mm 2 . Concerning tractography reconstructions, we used volumes with a b-value of 2000 s/mm 2 for single- and multi- shell measurements without changing the pipeline. The extraction of radiomics features uses only the volumes of the reconstructed tracts, consistent between the two sequences. Raw data of unweighted and b = 2000 s/mm 2 volumes are available in the dataset 22 .
To qualify for inclusion in the dataset, complete and artifact-free MRI acquisition was essential for both patient and healthy control groups. Each examination comprises all the sequences (MPRAGE, FLAIR, DWI with both AP and PA phase encoding, QSM) necessary for the processing pipeline. The images were assessed to ensure they were of sufficient quality, devoid of significant motion artifacts or other distortions, to make them suitable for analysis using the standard pipeline.
MR sequences were derived from the same exam for all but three healthy subjects (subj-006 [F/57yo], subj-007 [F/46yo], and subj-040 [M/39yo]). In each of these instances, the FLAIR image has been acquired during a prior examination, approximately 30, 5, and 42 months before, respectively. For consistency, we include the FLAIR sequence for all the subjects, both patients and controls, even though it was used only to automatically segment patients’ MS lesions; in this context, it was not used to analyze the healthy controls. Considering this and the fact that subj-006, subj-007 and subj-040 were healthy and not elderly, the mismatch between the days of the MRI exams does not affect either the pipeline or the extracted features.
MRI data processing
Raw DICOM data were converted to NIfTI format using dcm2niix ( https://github.com/rordenlab/dcm2niix ). Over the years, the software version changed from v1.0.20171215 to v1.0.20210317. This change does not affect recorded pixel intensities.
Morphological T 1 w and T 2 w processing
Original morphological images T 1 w and T 2 w were used without further processing; T 2 w images were linearly registered onto the corresponding T 1 w using Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL) 24 (v. 6.0.4) (FSL’s Linear Image Registration Tool [FLIRT] 25 , 26 ). All inter-modality registrations were performed using 12 degrees of freedom (affine transformations).
QSM processing
To obtain χ maps, phase maps from the five echo times were processed individually by Laplacian unwrapping 27 and Variable kernel Sophisticated Harmonic Artifact Reduction for Phase data (V-SHARP) as background field removal 28 . The processing requires a brain binary mask obtained by skull-stripping the magnitude image of the first echo time using the FSL 24 Brain Extraction Tool (BET) 29 .
Multi-echo phase data were combined into a single-phase image through a weighted sum over echoes of processed phase maps, using as weights 30 :
The iterative least square ( iLSQR ) technique was used as a dipole inversion method 31 . STI Suite 32 was used for the processing. The resulting QSM image was linearly registered using FLIRT 25 , 26 to the corresponding morphological T 1 w image.
Cerebrospinal Fluid (CSF) served as the reference tissue, employing the zero-referencing method 33 . This choice is widely adopted because CSF susceptibility values do not show changes related to the subject’s age or pathological condition, and orientation dependence can be excluded. However, since the sizes of the ventricles vary and the CSF signal often appears non-uniform in QSM reconstructions, we did not consider the entire ventricles but three small volumes instead (atrium, anterior horns, and central part), as suggested by Straub and colleagues 34 . An original atlas-based method was implemented to automatically identify the three volumes on each exam. Isotropic 1-mm MPRAGE maps of 60 subjects, both healthy controls and patients, were non-linearly registered (elastic registration) using FSL 24 FNIRT ( https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FNIRT ), after brain extraction using BET 29 , onto the space of a selected reference subject. The deformation matrix returned was combined with the linear transformation mapping QSM to T 1 w in the same exam so that all 60 QSM-magnitude maps were averaged in the same space.
Three small bilateral CSF volumes were manually defined on three adjacent slices of the atlas. The volumes were back-registered onto the original QSM map for each subject and the mean susceptibility value was calculated to establish the reference.
Motion artifacts pose a significant challenge for QSM due to its reliance on phase information extracted from MRI data. Even minor movements during image acquisition can introduce errors in phase consistency across slices or time frames, undermining the accuracy of susceptibility calculations. Notably, the QSM sequence is typically lengthy - lasting approximately 9 minutes in the current study -, allowing a lot of time for artifacts to occur. These artifacts manifest as geometric distortion, ghosting effects, and signal loss, ultimately compromising the quality and reliability of QSM images. To address this issue, rigorous quality control is essential. It was implemented by evaluating each QSM reconstruction individually.
Figure 2 shows an example of a QSM reconstruction excluded from the study due to motion artifacts. In this instance, the presence of motion was apparent even in the raw magnitude maps, facilitating the decision to exclude directly after the acquisition. However, QSM images are derived from phase data, which may not exhibit such evident artifacts in their raw form because of the discontinuities present before the unwrapping stage. Such artifacts can remain undetected until post-processing is performed.

Example of one exam (patient with MS, F/29 years old) excluded because of the quality of QSM image, showing movement artifacts and not considered suitable for the analysis: in the first and second row, magnitude and phase raw data from the first echo time; in the third row, the QSM reconstruction.
DWI processing
An automated diffusion and tractography processing pipeline was implemented as previously described 35 , 36 . DWI images underwent a process of skull-stripping using BET 29 . They were denoised using the dwidenoise function from MRtrix3 37 (v. 3.0.2) with a principal component analysis approach. To address susceptibility-related distortion, we employed the FSL 24 function topup . Subsequently, we addressed susceptibility effects, eddy currents, and signal dropout using the FSL 24 eddy_openmp function 38 . Linear registration between diffusion measurements and T 1 -weighted images was conducted using the epi_reg function from FLIRT 25 , 26 . Diffusivity was modeled along the spatial eigenvector using the tensor model and a high-order fiber modeling technique. Moreover, we adopted a probabilistic streamline approach to evaluate crossing fibers.
Diffusion images were used to estimate fiber orientation distributions by the single tissue, single shell spherical deconvolution algorithm ‘csd’ implemented by the dwi2fod function 39 from MRtrix3. The Diffusion Tractography pipeline is described in the section VOI segmentation. In total, six WM tracts were defined: arcuate fasciculus (AF), cortico-spinal tract (CST), frontal aslant tract (FAT), inferior frontal-occipital fasciculus (IFOF), optic radiation (OR), and uncinate fasciculus (UF).
VOI segmentation
VOIs selected for feature extraction were segmented as follows:
White matter (WM) : WM segmentation was conducted using the MRtrix3 tool 5ttgen, which relies on FreeSurfer 40 (v. 6) segmentation, applied to the T 1 -weighted (T 1 w) image.
MS lesions : lesions were automatically segmented using the Lesion Prediction Algorithm (LPA) 41 from the Lesion Segmentation Tool (LST) (v. 3.0.0) ( www.statistical-modelling.de/lst.html ), an open-source toolbox for Statistical Parametric Mapping (SPM) (v. 12). The toolbox generates an estimation of the lesion probability map, which was utilized to create a binary map of lesions. For each examination, the NAWM was identified by multiplying the inverse of this map with the WM mask. LST was applied only for patients’ exams; for controls, the NAWM corresponds to WM identified by MRtrix3.
DTI regions : For each tract, seed and inclusion regions of interest for streamline generation and selection were defined on the Montreal Neurological Institute 152 (MNI152) standard brain. Non-linear registration between DWI and MNI spaces was performed using FNIRT from FSL 24 . Streamlines were generated using the iFOD1 method tckgen from MRtrix3 37 , inclusion regions, FOD amplitude, and deviation angle. The criteria used to define each tract are presented in Table 3 and Figs. 3 and 4 . Tracts were individually reconstructed for the two hemispheres and then combined into a single region, converted into a NIfTI image with voxel intensity representing the streamline count. To obtain a relative fiber count estimate image, a threshold was applied (referring to the maximum value). Similar to NAWM, tract VOIs were analyzed excluding the lesions.

Seed, inclusion and exclusion regions for AF, CST and FAT. Regions were defined on the MNI152 standard brain and used for streamlining generation and selection to reconstruct WM tracts. Region descriptions are in Table 2 . In the last row, the appearance of each tract reconstruction is shown in the MNI152 space, obtained by averaging tracts of 30 healthy controls (AF = Arcuate Fasciculus, CST = Cortico-Spinal Tract, FAT = Frontal Aslant Tract, OB WAY = Obligatory Waypoint(s), Excl = Exclusion mask(s), MNI152 = Montreal Neurological Institute’s 152, WM = White Matter).
Seed, inclusion and exclusion regions for IFOF, OR and UF. Regions were defined on the MNI152 standard brain and used for streamlining generation and selection to reconstruct WM tracts. Region descriptions are in Table 2 . In the last row, the appearance of each tract reconstruction is shown in the MNI152 space, obtained by averaging tracts of 30 healthy controls (IFOF = Inferior Frontal-Occipital Fasciculus, OR = Optic Radiation, UF = Uncinate fasciculus, OB WAY = Obligatory Waypoint(s), Excl = Exclusion mask(s), MNI152 = Montreal Neurological Institute’s 152, WM = White Matter).
Feature extraction
PyRadiomics 42 3.0.1 (Python 3.7.6) was used to extract features from the VOIs overlain on the QSM images registered to the corresponding T 1 w space. VOIs were likewise registered to the T 1 w space, exploiting the previously obtained linear transformation matrices of FLAIR and DWI, using nearest neighbor interpolation to maintain binary masks. Since QSM and VOIs are in the same space, no additional interpolation operation was required for feature extraction, which was performed in 3D. MR exams were performed using the same clinical scanner and following the same acquisition protocol and processing pipeline, at the same center; this ensured sufficient homogeneity within the sample, obviating the need for histogram normalization steps.
Segment-based feature extraction was performed, meaning that each feature was extracted for each region of interest. Specifically, we considered 107 features, categorized as follows:
First-order features (FO, # 18) : commonly used metrics to describe histogram intensity, including mean, median, 10 th and 90 th percentile, skewness, and kurtosis; FO measurements are independent of the number of Gray Levels (GLs);
Shape 3D features (S3D, # 14) : descriptors of the 3D size and shape of the ROI (e.g., volume, surface, minimum and maximum axes); S3D measurements are independent of the number of GLs and their intensity distributions;
Gray Level Co-occurrence Matrix features (GLCM, # 24) : describe the second-order joint probability function of an image region constrained by the mask;
Gray Level Run Length Matrix features (GLRLM, # 16) : quantify GL runs (number of consecutive pixels that have the same grey level value);
Gray Level Size Zone Matrix features (GLZM, # 16) : quantify GL zones in an image (number of the connected voxels that share the same grey level intensity);
Neighboring Gray Tone Difference Matrix features (NGTDM, # 5) : quantify the difference between a GL value and the average grey value of its neighbors;
Gray Level Dependence Matrix features (GLDM, # 14) : quantify GL dependencies in an image (number of connected voxels within a distance that are dependent on the central voxel).
Feature extraction was conducted with 64 as the number of gray levels (GLs), a parameter determined from the outcomes of a prior optimization study 23 . Categories 3 to 7 are referred to as texture and offer insights into the spatial distribution of intensity levels in the image. A complete list of the features can be found in the PyRadiomics documentation ( https://pyradiomics.readthedocs.io/en/latest/features.html ) .
Anonymization and de-identification
All images were anonymized and de-identified. The anonymization mask was created by combining: (1) the mask obtained processing T 1 w images with the automated defacing tools mri_deface 43 from FreeSurfer 40 ; (2) the mask obtained processing T 1 w images with SIENAX 44 from FSL 24 ; (3) the mask obtained with BET 29 skull-stripping of the magnitude of the first echo time of the QSM sequence. Image files contain no metadata that can be used to identify study participants.
Data Records
Files are organized according to the Brain Imaging Directory Structure (BIDS) 45 (Fig. 5 ).

Schematic diagram of the dataset organization. A list of the participants to the dataset is provided and for each subject: anatomical T 1 w and T 2 w; DWI after the correction for EPI distortions and susceptibility effects, eddy currents and signal dropout, with b-values, b-vectors and the registration matrix to T 1 w; for QSM, original magnitude and phase maps for the five echo times, final QSM reconstruction registered in T 1 w space, with registration matrix to T 1 w; VOIs (AF, CST, FAT, IFOF, OR, UF) for left and right hemisphere;.csv files containing the 107 radiomic features for each region. Images were anonymized and de-identified (T 1 w = T 1 -weighted, T 2 w = T 2 -weighted, DWI = Diffusion-Weighted Imaging, QSM = Quantitative Susceptibility Mapping, VOI = Volume of Interest).
In the dataset, files are grouped in 150 folders, each corresponding to an individual subject, and organized as follows:
In the ‘anat’ folder there are: a) T 1 -weighted (T 1 w), T 2 -FLAIR, and multi-echo GRE T 2 *w images (MEGRE) for QSM reconstruction, serving as non-structural MR images for each subject. For MEGRE, both phase and magnitude contributions are specified for each echo time; b) The QSM reconstruction, denoted with the suffix ‘chimap’, as a parametric non-structural image, along with the affine registration matrix to T 1 w. Specifically, the magnitude of the first echo time was used to register QSM to T 1 w. Given that orientation with respect to B 0 is essential information for QSM processing, raw images are stored without registration, with the registration matrix to T 1 w provided separately, while the QSM reconstruction is already registered in T 1 w space.
In the ‘dwi’ folder, there are: a) the diffusion weighted image after the correction for EPI distortions, susceptibility effects, eddy currents, and signal dropout, b) the required bvals and bvecs files, providing gradient orientation information corresponding to DWI volumes available (b = 0 and b = 2000 s/mm 2 ), and c) the affine registration matrix to T 1 w.
In the ‘derivatives’ folder there are the outputs from the processing pipeline. Two-subfolder were created: a) the segmentation folder, containing tracts and normal appearing white matter (NA-WM and tracts [AF, CST, FAT, IFOF, OR, UF] VOIs, divided for the two hemispheres) and b) the ‘radiomic_features’ folder, containing the.csv with all the features for each volume of interest (seven features for each hemisphere, resulting in a total of 14 features).
Outside the folder of individual subjects, there is the ‘code’ folder containing the scripts used for processing tasks such as image registration, radiomic feature extraction, and robustness evaluation.
Additional files are stored at the top level: a) dataset_description.json and README.txt files, describing the dataset; b) participants file (participants.xlsx and participants.json), containing the ID number, age, sex, scan date, clinical condition (denoted as ‘HC’ for healthy controls and ‘MS’ for multiple sclerosis patients), and the type of DWI sequence used (single-shell or multi-shell).
The size of the dataset 22 is ~30GB and it is available the Zenodo repository.
Usage Notes
To obtain access to the dataset 22 , the following steps must be followed:
An account must be created on the Zenodo repository, ensuring that either the username or the email address (or both) are public.
The Data Use Agreement, provided in the description of the dataset, must be completed, signed and send to [email protected].
Access will be granted within two working days, providing ‘Reader’ status to a community in which the dataset is included; the status can be monitored directly on Zenodo or via email.
Upon acceptance, access to the dataset will be granted, allowing for data download.
Technical Validation
Assessing robustness of susceptibility-based radiomic features.
Hundreds of radiomic features can be extracted from MR images, enabling automated, high-throughput quantification of image characteristics 45 . This process provides a potentially extensive source of pathology-related biomarkers. It is essential to differentiate between image measurements and biomarkers 46 . Biomarkers are objective and quantifiable descriptors of biological processes capable of consistently predicting clinical outcomes and endpoints. Interpretability and reproducibility are two critical aspects in this context. In medical applications, it is crucial to maintain a clinical perspective as the driving force behind research 47 . The validity and relevance of a biomarker, along with its utility in clinical practice and its ability to offer valuable information, must be confirmed. Furthermore, when an informative and relevant feature is identified, it is vital to ensure the generalizability and replicability of outcomes. Since these features represent the outcome of physiological processes, they should remain consistent regardless of the imaging system or processing workflow used. The stages involved, from scanning to feature extraction, are numerous and complex. Thus, many parameters can impact the reliability of the results. In radiomic applications, robustness analysis is therefore essential to maintain the consistency of outcomes across different systems and multiple centers 45 , 48 .
In 23 , we describe a novel investigation into the robustness of susceptibility-based features derived from QSM. The study involved a cohort of 121 patients with MS and 30 healthy controls. We implemented an original and robust pipeline; we analyzed NAWM both as a whole and within the six clinically relevant tracts included in our dataset. To explore feature reliability, we varied the number of gray levels and echo times used for QSM reconstructions. After optimizing the number of GLs, set at n = 64, we found at least 65% of the features demonstrated robustness for each volume of interest. Notably, WM tracts exhibited higher levels of reliability, with over 75% of robust features in all of them. Differences among these tracts were explicable due to the volume of the structure and the susceptibility variance. No significant differences were observed between the left and right hemispheres.
The research confirmed the robustness of the data processing pipeline and established the reliability of QSM-based radiomic features against gray levels and echo times. This work paves the way for future investigations, where the identified set of reliable features may be used to characterize patients with MS, distinguish clinical phenotypes, and identify different responses to treatment.
Code availability
We hosted the code used for the study in the Zenodo repository 22 ; the scripts provided can be used for image registration, radiomic feature extraction, and robustness analysis.
Deistung, A., Schweser, F. & Reichenbach, J. R. Overview of quantitative susceptibility mapping. NMR Biomed . 30 ( 4 ) (2017).
Harada, T. et al . Quantitative susceptibility mapping: basic methods and clinical applications. Radiographics. 42 (4), 1161–1176 (2022).
Article PubMed Google Scholar
Soman, S. et al . Susceptibility-based neuroimaging: standard methods, clinical applications, and future directions. Curr Radiol Rep. 5 (3), 11 (2017).
Article MathSciNet PubMed PubMed Central Google Scholar
Haacke, E. M., Mittal, S., Wu, Z., Neelavalli, J. & Cheng, Y. C. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 30 (1), 19–30 (2009).
Article CAS PubMed PubMed Central Google Scholar
Mittal, S., Wu, Z., Neelavalli, J. & Haacke, E. M. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 30 (2), 232–52 (2009).
Ravanfar, P. et al . Systematic review: quantitative susceptibility mapping (QSM) of brain iron profile in neurodegenerative diseases. Front Neurosci. 15 , 618435 (2021).
Article PubMed PubMed Central Google Scholar
Reich, D. S., Lucchinetti, C. F. & Calabresi, P. A. Multiple sclerosis. N Engl J Med. 378 (2), 169–180 (2018).
Thompson, A. J. et al . Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 17 (2), 162–173 (2018).
Filippi, M. et al . MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 15 (3), 292–303 (2016).
Sowa, P. et al . Restriction spectrum imaging of white matter and its relation to neurological disability in multiple sclerosis. Mult Scler. 25 (5), 687–698 (2019).
Grist, J. T. et al . Imaging intralesional heterogeneity of sodium concentration in multiple sclerosis: Initial evidence from 23 Na-MRI. J Neurol Sci. 387 , 111–114 (2018).
Marcille, M. et al . Disease correlates of rim lesions on quantitative susceptibility mapping in multiple sclerosis. Sci Rep. 12 (1), 4411 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar
Zivadinov, R. et al . Brain iron at quantitative MRI is associated with disability in multiple sclerosis. Radiology. 289 (2), 487–496 (2018).
Wiggermann, V. et al . Susceptibility-sensitive MRI of multiple sclerosis lesions and the impact of normal-appearing white matter changes. NMR Biomed . 30 ( 8 ) (2017).
Sibgatulin, R. et al . Magnetic susceptibility anisotropy in normal appearing white matter in multiple sclerosis from single-orientation acquisition. Neuroimage Clin. 35 , 103059 (2022).
Abbasian Ardakani, A., Bureau, N. J., Ciaccio, E. J. & Acharya, U. R. Interpretation of radiomics features - a pictorial review. Comput Methods Programs Biomed. 215 , 106609 (2022).
Guiot, J. et al . A review in radiomics: making personalized medicine a reality via routine imaging. Med Res Rev. 42 , 426–440 (2022).
Gillies, R. J., Kinahan, P. & Hricak, H. Radiomics: images are more than pictures, they are data. Radiology. 278 (2), 563–577 (2016).
Kim, J. Y. et al . Incorporating diffusion- and perfusion-weighted MRI into a radiomics model improves diagnostic performance for pseudoprogression in glioblastoma patients. Neuro Oncol. 21 (3), 404–414 (2019).
Li, G. et al . An MRI radiomics approach to predict survival and tumor-infiltrating macrophages in gliomas. Brain. 143 (3), 1151–1161 (2022).
Article Google Scholar
Kickingereder, P. & Andronesi, O. C. Radiomics, metabolic, and molecular MRI for brain tumors. Semin Neurol. 38 (1), 32–40 (2018).
Fiscone, C. et al . MRI dataset for susceptibility-based radiomic feature extraction in healthy controls and patients with multiple sclerosis [Dataset] Zenodo https://doi.org/10.5281/zenodo.10931120 (2024).
Fiscone, C. et al . Assessing robustness of quantitative susceptibility-based MRI radiomic features in patients with multiple sclerosis. Sci Rep. 13 (1), 16239 (2023).
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage. 62 (2), 782–790 (2012).
Jenkinson, M. & Smith, S. A global optimization method for robust affine registration of brain images. Medical Image Analysis. 5 (2), 143–156 (2001).
Article CAS PubMed Google Scholar
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17 (2), 825–841 (2002).
Schweser, F., Deistung, A., Lehr, B. W. & Reichenbach, J. R. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: An approach to in vivo brain iron metabolism? Neuroimage. 54 (4), 2789–2807, https://doi.org/10.1016/j.neuroimage.2010.10.070 (2011).
Li, W., Wu, B. & Liu, C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. Neuroimage. 55 (4), 1645–1656 (2011).
Smith, S. M. Fast robust automated brain extraction. Human Brain Mapping. 17 (3), 143–155 (2002).
Biondetti, E. et al . Multi-echo quantitative susceptibility mapping: how to combine echoes for accuracy and precision at 3 Tesla. MR Med. 88 (5), 2101–2116 (2022).
Google Scholar
Li, W. et al . A method for estimating and removing streaming artifacts in quantitative susceptibility mapping. Neuroimage. 108 , 111–222 (2015).
Li, W., Wu, B. & Liu, C. STI Suite. A software package for quantitative susceptibility imaging. ISMRM-ESMRMB https://archive.ismrm.org/2014/3265.html (2014).
Wang, Y. & Liu, T. Quantitative susceptibility mapping (QSM): decoding MRI data for a tissue magnetic biomarker. Magn Reson Med. 73 (1), 82–101 (2015).
Straub, S. et al . Suitable reference tissues for quantitative susceptibility mapping of the brain. Magn Reson Med. 78 (1), 204–214 (2017).
Talozzi, L. et al . Along-tract analysis of the arcuate fasciculus using the Laplacian operator to evaluate different tractography methods. Magn Reson Imaging. 54 , 183–193 (2018).
Zoli, M. et al . From neurosurgical planning to histopathological brain tumor characterization: potentialities of arcuate fasciculus along-tract diffusion tensor imaging tractography measures. Front Neurol. 12 , 633209 (2021).
Tournier, J. D. et al . MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 202 , 116–137 (2019).
Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 125 , 1063–1078 (2016).
Tournier, J. D., Calamante, F. & Connelly, A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 35 (4), 1459–72 (2007).
Fischl, B. et al . Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33 (3), 341–355 (2002).
Schmidt, P. Bayesian inference for structured additive regression models for large-scale problems with applications to medical imaging. PhD thesis, Ludwig-Maximilians-Universität München https://edoc.ub.uni-muenchen.de/20373/ (2017).
van Griethuysen, J. J. M. et al . Computational radiomics system to decode the radiographic phenotype. Cancer Res. 77 (21), e104 (2017).
Bischoff-Grethe, A. et al . A technique for the deidentification of structural brain MR images. Hum Brain Mapp. 28 (9), 892–903 (2007).
Smith, S. M. et al . Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23 (Suppl1), S208–19 (2004).
Zwanenbrug, A. et al . The image biomarker standardization initiative: standardized quantitative radiomics for high-troughput image-based phenotyping. Radiology. 295 (2), 328–338 (2020).
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 69 (3), 89–95 (2001).
Smith, E. T. S. Clinical applications of imaging biomarkers. Part 1. The neuroradiologist’s perspective. Br J Radiol. 84 (Spec Iss 2), S196–204 (2011).
Article PubMed Central Google Scholar
Zwanenbrug, A. et al . Assessing robustness of radiomic features by image perturbation. Sci Rep. 9 (1), 614 (2019).
Article ADS Google Scholar
Download references
Acknowledgements
This work was supported by national funds through the FCT (Fundação para a Ciência e a Tecnologia) by the project UIDB/04152/2020 (DOI: https://doi.org/10.54499/UIDB/04152/2020 ) - Centro de Investigação em Gestão de Informação – MagIC/NOVA IMS. This work was supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006)—A multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022). The publication of this article was supported by the ‘‘Ricerca Corrente’’ funding from the Italian Ministry of Health.
Author information
Authors and affiliations.
Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy
Cristiana Fiscone, Alessandra Lugaresi, Raffaele Lodi & Caterina Tonon
Functional and Molecular Neuroimaging Unit, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy
Giovanni Sighinolfi, David Neil Manners, Lorenzo Motta, Greta Venturi, Raffaele Lodi & Caterina Tonon
Department for Life Quality Sciences, University of Bologna, Bologna, Italy
David Neil Manners
UOSI Riabilitazione Sclerosi Multipla, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy
Ivan Panzera & Alessandra Lugaresi
Department of Imaging, Cambridge University Hospitals NHS Foundation Trust, Cambridge Biomedical Campus, Cambridge, United Kingdom
Fulvio Zaccagna
Department of Radiology, University of Cambridge, Cambridge, United Kingdom
Investigative Medicine Division, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom
Department of Information and Electrical Engineering and Applied Mathematics, University of Salerno, Fisciano, Italy
Leonardo Rundo
NOVA Information Management School (NOVA IMS), Universidade NOVA de Lisboa, Campus de Campolide, 1070-312, Lisbon, Portugal
Mauro Castelli
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the work. C.F. and M.C. played a guiding role in designing, drafting and finalizing the manuscript, which was revised by D.N.M., C.T., R.L., A.L. and L.R.. Images processing pipeline was designed by C.F., G.S., G.V. and D.N.M., radiomic features extraction pipeline was designed by C.F., L.R. and M.C. Data were collected and selected from A.L. and I.P. L.M. evaluated image quality. C.F., L.R., F.Z. and M.C. designed and conducted the study illustrated to validate the dataset. All authors read and approved the final manuscript.
Corresponding author
Correspondence to David Neil Manners .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Fiscone, C., Sighinolfi, G., Manners, D.N. et al. Multiparametric MRI dataset for susceptibility-based radiomic feature extraction and analysis. Sci Data 11 , 575 (2024). https://doi.org/10.1038/s41597-024-03418-6
Download citation
Received : 12 December 2023
Accepted : 24 May 2024
Published : 04 June 2024
DOI : https://doi.org/10.1038/s41597-024-03418-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Long-term outcomes of ADEM-like and tumefactive presentations of CNS demyelination: a case-comparison analysis
- Original Communication
- Open access
- Published: 11 June 2024
Cite this article
You have full access to this open access article

- Simon V. Arnett ORCID: orcid.org/0000-0003-4582-1171 1 , 2 , 6 ,
- Kerri Prain 3 ,
- Sudarshini Ramanathan ORCID: orcid.org/0000-0002-0294-9768 4 , 5 ,
- Sandeep Bhuta ORCID: orcid.org/0000-0002-7292-9658 5 ,
- Fabienne Brilot ORCID: orcid.org/0000-0002-7025-2276 4 &
- Simon A. Broadley ORCID: orcid.org/0000-0002-9429-4307 1 , 2
1 Altmetric
A minority of initial multiple sclerosis (MS) presentations clinically or radiologically resemble other central nervous system (CNS) pathologies, acute disseminated encephalomyelitis (ADEM) or tumefactive demyelination (atypical demyelination presentations). With the aim of better defining the long-term outcomes of this group we have performed a retrospective cohort comparison of atypical demyelination versus ‘typical’ MS presentations. Twenty-seven cases with atypical presentations (both first and subsequent demyelinating events) were identified and compared with typical MS cases. Disease features analysed included relapse rates, disability severity, whole brain and lesion volumes, lesion number and distribution. Atypical cases represented 3.9% of all MS cases. There was considerable overlap in the magnetic resonance imaging (MRI) features of ADEM-like and tumefactive demyelination cases. ADEM-like cases tended to be younger but not significantly so. Atypical cases showed a trend towards higher peak expanded disability severity score (EDSS) score at the time of their atypical presentation. Motor, cranial nerve, cerebellar, cerebral and multifocal presentations were all more common in atypical cases, and less likely to present with optic neuritis. Cerebrospinal fluid (CSF) white cell counts were higher in atypical cases ( p = 0.002). One atypical case was associated with peripheral blood myelin oligodendrocyte glycoprotein (MOG) antibodies, but subsequent clinical and radiological course was in keeping with MS. There was no difference in long-term clinical outcomes including annualised relapse rates (ARR), brain volume, lesion numbers or lesion distributions. Atypical demyelination cases were more likely to receive high potency disease modifying therapy early in the course of their illness. Despite the severity of initial illness, our cohort analysis suggests that atypical demyelination presentations do not confer a higher risk of long-term adverse outcomes.
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the CNS which often presents with recurrent episodes of focal neurological deficit in the absence of encephalopathy or fever [ 1 , 2 ]. A small number of cases present with atypical clinical or radiological features suggestive of acute disseminated encephalomyelitis (ADEM) [ 3 , 4 ] or cerebral neoplasia (tumefactive demyelination) [ 5 ]. The largest case series to date, retrospectively reviewing tumefactive demyelinating lesions over a period of 30 years at the Mayo Clinic, identified 183 cases meeting criteria for MS [ 6 ]. These atypical presentations are uncommon [ 7 ], pose a diagnostic dilemma, and data regarding treatment and prognosis are limited [ 8 , 9 ]. Despite these difficulties, a recent review proposed that combinations of imaging and paraclinical findings can be used to diagnose tumefactive demyelinating lesions [ 10 ]
ADEM typically presents in childhood and features include altered level of consciousness, seizures, fever or focal/multifocal neurological deficits. These clinical features are accompanied by widespread, poorly demarcated predominantly white matter lesions of the same age. These features have been collated into criteria for childhood ADEM [ 11 ] however similar criteria for adult presentations are yet to be defined, and previous investigations commonly define their own criteria, leading to issues of heterogeneity in case definition. MRI of the brain and spine typically shows simultaneous multifocal demyelination throughout the brain and spine and up to 50% of cases are positive for MOG antibodies. ADEM-like presentations of MS are seen in adults but typically they do not have all of the clinical features of the childhood form and MOG antibody prevalence has been less well studied [ 12 , 13 ]. Tumefactive demyelination is defined as lesions at least two centimetres in diameter and featuring gadolinium (Gd) enhancement [ 7 , 8 , 9 , 14 , 15 ]. Incomplete peripheral Gd-enhancement (‘broken ring’) is considered unique to this form of MS [ 8 , 15 ]. Whilst initially described as being mono-focal, multifocal lesions feature in many series [ 8 ]. Cases with antibodies to AQP4 and MOG have been described [ 12 , 13 , 16 , 17 , 18 , 19 ]. Expert opinion and case series analysis has led to plasma exchange and immunosuppressive therapy being advocated for atypical forms of MS [ 13 ].
With the aim of further adding to knowledge of the long-term outcomes for atypical MS presentations in adults we conducted a retrospective cohort comparison study comparing clinical and radiological outcomes with age- and sex-matched typical MS cases. Our hypotheses were: (1) atypical MS cases would have worse outcomes than typical cases in terms of disability, lesion load and brain atrophy and (2) that a proportion of atypical cases would be positive for AQP4 or MOG antibodies.
Ethics oversight and approval
Ethics approval was sought and obtained through the Griffith University and Gold Coast Hospital and Health Service, Human Research Ethics Committees. Written informed consent was provided by all participants.
Case ascertainment and data collection
Atypical MS presentations (both initial and subsequent) were identified through systematic review of medical records of patients under the care of the CNS inflammatory diseases clinic at the Gold Coast University Hospital. Cases were enrolled if they featured atypical clinical presenting symptoms (fever, seizure, encephalopathy, severe multifocal neurological deficits) and/or atypical MRI findings (see lesion definitions below). Typical cases matched for sex and age at onset were identified from a register of cases seen at the same clinic. We attempted to match up to three typical cases for every atypical case. Typical cases met the 2017 McDonald criteria for MS and atypical cases were also assessed against these criteria. Cases (atypical or typical) were excluded if there was insufficient data (clinical or MRI) to confirm a diagnosis of MS or provide a minimum dataset (demographics, disability, and relapse information).
The following clinical details were collected from available records and direct interview with cases: current age, sex, age at onset, relapse history, relapse frequency, time to first relapse (following initial presentation), time to expanded disability status scale (EDSS) score 6.0, final EDSS (last review), MS treatment, CSF cell counts, CSF protein, oligoclonal bands and MRI data (see below for details). Annualised relapse rate (ARR), EDSS and MRI parameters were recorded for the 2-year, 5-year and most recent clinical review available following disease onset. Clinical and MRI data were included if they were available within 6 months of each time point.
Serological testing
Testing for AQP4 antibodies was performed by Pathology Queensland Immunology Laboratory, Brisbane using a combination of tissue-based immunofluorescence as previously described [ 20 ] and fixed cell-based assay (Eurommun®). MOG antibodies were tested by Westmead Immunology Laboratory, Sydney using a live-cell fluorescence activated cell sorting technique as previously described [ 20 ].
Radiological lesion definitions
ADEM-like MS was defined as multiple (> 10), large (>6 mm maximum diameter in any single plane), irregularly shaped, or poorly demarcated lesions of high intensity on T2 FLAIR MRI of the brain and spine that were of the same age on DWI and Gd-enhancing sequences [ 21 ]. Tumefactive MS lesions were defined as very large (> 2 cm) lesions identified on T2 FLAIR sequences, spanning the peri-ventricular to subcortical white matter, with or without a surrounding oedema or Gd-gadolinium enhancement [ 21 ].
MRI analysis
MRI were assessed using eFilm Workstation® 4.2.3, IBM Watson Health software on Eizo® RadiForce MX270W 68 cm monitors. MRI parameters included the number of T2 FLAIR hyperintense lesions, the neuroanatomical location of these lesions, the number of large lesions (defined as >6 mm in diameter in at least one plane), the presence and number of gadolinium-enhancing lesions, and the presence and number of T1 hypointense lesions (black holes).
The following criteria were used to determine if lesions were of the same age; No established T1 black holes (minor T1 hypointensity was permitted as can be seen in acute lesions), all large lesions (> 6 mm) showed diffusion restriction or T1 Gd-enhancement and all lesions had a poorly demarcated boarder.
Volumetric analysis was performed using the open source software 3DSlicer v4.10.2 ( http://www.slicer.org ) [ 22 ]. Following importation of DICOM format imaging, cranial vault and soft tissue imaging was removed using the Swiss Skull Stripper module v4.1( https://www.slicer.org/wiki/Documentation/Nightly/Modules/SwissSkullStripper , Institute for Surgical Technology and Biomechanics, University of Bern, Switzerland). Whole brain and lesion volumes were measured using the Editor module v4. 1 ( https://www.slicer.org/w/index.php/Documentation/4.3/Modules/Editor , National Alliance for Medical Imaging Computing, Harvard University, US).
Statistical analysis
Statistical comparisons between the atypical MS cohort, and whole MS database, and the age- and sex-matched typical MS cohort were performed. The first comparison used a database of person with MS (pwMS) fulfilling the 2017 revised McDonald’s criteria [ 23 ] seen at Gold Coast University Hospital over the past 17 years. These data were used to compare demographics and disease course of the atypical MS cases against an unmatched cohort. The second comparison group was an age- and sex-matched cohort of typical MS cases identified from the same database as described. Comparison of categorical data were performed using a Χ 2 test and continuous data with the Kruskal–Wallis test. The effect of baseline characteristics on outcomes was assessed using forward stepwise linear regression analysis with p < 0.05 as the cut off for inclusion in the model. Survival analysis was undertaken using Kaplan–Meier curves and Cox proportional hazard modelling including significant predictors identified from the regression analysis of outcomes [ 24 ]. All statistical analyses were performed using the Statistical Package for Social Science (SPSS®) v25 (IBM®; Chicago, US).
Case ascertainment
A total of 28 cases were identified on clinical or radiological grounds as meeting our criteria for atypical demyelination. One case was only ever seen once in our clinic, sometime after their atypical presentation and was excluded due to lack of clinical and imaging data. This left 27 included atypical MS cases. All these cases met the McDonald criteria for MS (excluding one case in regards to requirement for an alternative diagnosis—see below). There were 712 cases in the MS Clinic database. This gives a relative frequency of 28/712 (3.9% [95%CI 2.6–5.6%]) We identified 76 age- and sex-matched typical MS cases from the database. One of these cases was also excluded due to a lack of clinical and MRI data, leaving 75 included in the analysis.

Atypical cases
Table 1 gives the demographic information, initial clinical features, MRI data, CSF results and antibody results for individual atypical MS cases. There were 13 ADEM-like cases and 14 tumefactive cases. We determined ADEM-like cases to be atypical demyelinating presentations rather than traditional ADEM on the basis of ADEM-like cases demonstrating combinations of CSF oligoclonal band positive status (6/8), remote MRI T1 black holes on initial MRI suggesting previous demyelinating events (8/13), presence of periventricular lesions (13/13) or subsequent relapses (6/13). Atypical presentations occurred at the onset of disease (first attack) in the majority of (23/27 (85%) cases), but a small number occurred as the second (1 case) or third attack (3 cases). When monophasic cases were excluded the number of atypical presentations occurring as first events 12/16 (75%) was higher than the expected number of 5/16 (34%) based on the mean number of relapses observed ( p < 0.01). One case had 6 tumefactive relapses affecting both hemispheres and posterior fossa. Atypical clinical features were seen in 12/27 (44%) of atypical MS cases. Cognitive impairment at first presentation was seen in 7/13 (54%) of ADEM-like cases compared with 2/14 (14%) of tumefactive cases ( p = 0.077). Depressed level of consciousness was seen in 4/13 (31%) of ADEM cases and none of the tumefactive cases. Two ADEM cases featured headache (7%) and one presentation involved fever in (4%). The remaining atypical MS cases (15/27 [56%]) were identified on the basis of radiological features and in some cases the symptoms were relatively mild. Lesions meeting our criteria for tumefactive demyelination were also seen in 6/13 (46%) of ADEM-like cases. The median (range) of total T2 brain lesions was greater ( p = 0.029) for ADEM-like presentations 22 (3–85) than for tumefactive cases 2.5 (1–81). Gd-enhancement was seen in 7/11 (64%) ADEM-like and 10/12 (83%) tumefactive MS cases where contrast was administered ( p = 0.549).
MRI of atypical presentations
Illustrative MRI features for ADEM-like and tumefactive presentations of MS are given in Fig. 1 . Particular features of note included multiple enhancing lesions in ADEM-like presentations (Fig. 1 B, F, J, D, H and L), peri-lesional T1 hypointensity (Fig. 1 D and P), perilesional oedema (Fig. 1 Q), central hypo-intensity on T1 (Fig. 1 D, P, R and T), complete ring-enhancement (Fig. 1 D, J and P), incomplete ring enhancement (Fig. 1 R and T), homogeneous enhancement (Fig. 1 l, O and S) and heterogeneous enhancement (Fig. 1 K and Q). We noted three patterns of ADEM-like lesion as shown in Fig. 2 which appeared to be independent of timing of the scans in relation to onset of clinical symptoms. In the first pattern there was confluent T2 hyperintensity on FLAIR imaging matched by homogeneous hyperintensity on DWI sequences and hypodensity on T1 sequences without Gd-enhancement. In the second pattern T2 hyperintense lesions on FLAIR imaging showed central relative hypo-intensity, which was matched by similar, but more pronounced changes on DWI sequences and a clear pattern of ring-enhancement with central hypo-intensity on T1 sequences. The third pattern showed patchy central T2 hyperintensities on FLAIR imaging matched by similar changes on DWI and Gd-enhanced T1 sequences. A summary of MRI features in atypical cases is given in Supplementary Table 1 .

MRI of ADEM-like and tumefactive MS cases. Images are paired (matched slices) with FLAIR images in first and third vertical panels and Gd-enhanced T1 weighted sequences in second and fourth vertical panels. Case of ADEM-like lesions with small and large lesions which all show Gadolinium enhancement ( A – D ), one larger lesion (D arrow) shows ring-enhancement with central hypointensity and peri-lesional hypointensity with surrounding oedema. Case of ADEM-like lesions with a multitude of smaller lesions all of which show either homogeneous or ring enhancement ( E – H ). Case of ADEM with multiple large lesions showing both ring enhancement and heterogeneous enhancement. Case of ADEM-like lesions showing multiple small and large lesions ( M – N ). Some lesions are non-enhancing, with some having central hypointensity on T1 sequences ( M and O open arrows) whilst other lesions show ring-enhancement (open arrows). Case of recurrent tumefactive MS showing large incomplete ring-enhancing lesion with central hypointensity and surrounding oedema (solid arrow) with mass effect ( S and T ). Case of tumefactive MS with a large incomplete ring-enhancing lesion (solid arrow) with central and perilesional hypointensity and a second non-enhancing lesion (open arrow) with central hypointensity ( S and T )

Three patterns of lesion in ADEM-like lesions. Vertical panels show FLAIR sequences (right), diffusion weighted images (centre) and T1 with contrast (left). Horizontal panels show individual cases. Upper panel ( A – C ) shows case with FLAIR and DWI hyperintensity with T1 hypointensity, but no Gd-enhancement. Middle panel ( D – F ) shows ring pattern hyperintensity on FLAIR and DWI with central hypointensity and ring-enhancement on T1 sequence. Lower panel ( G – I ) shows predominantly heterogeneous FLAIR and DWI hyperintensity with heterogeneous Gd-enhancement. There are additional lesions showing central T1 hypointensity and no enhancement.
Comparison of baseline characteristics
Comparison of baseline characteristics between the MS database cohort, the age/sex matched typical MS cohort and the atypical MS cases, as well as between ADEM-like and tumefactive cases are shown in Table 2 . There was no difference in the sex distribution of any of the groups. Whilst overall there was no difference in the age of onset between the atypical cases and MS database cases, the ADEM-like cases showed a trend towards a younger age of onset (20.5 [15–51] years) compared to tumefactive cases (29.5 [23–44] years, p = 0.068) and were younger than typical MS database cases (35 [14–71] years, p = 0.038). Peak EDSS (during the index presentation) was higher in the ADEM-like group, but this difference was not statistically significant. There was no difference in the median age of atypical and the age-matched typical MS cases. A history of recent infection was noted in 7/25 (28%) of the atypical cases and 7/54 (13%) of typical MS cases, but this difference was not statistically significant ( p = 0.19). Atypical MS cases were more likely to have motor ( p = 0.035), cranial nerve ( p = 0.003), cerebellar ( p = 0.005) and cerebral ( p < 0.001) features in their atypical episode. They were also more likely to have multifocal attacks ( p < 0.001), particularly in the ADEM-like group ( p = 0.046) and were less likely to have optic neuritis ( p = 0.037). CSF white cell count was higher in the atypical MS cases when compared to both typical MS cohorts ( p = 0.002 for the databases and p = 0.052 for the matched cohort). This difference appeared to be principally driven by the tumefactive MS cases (Supplementary Fig. 1 ). No significant differences in CSF protein and presence of oligoclonal bands were seen. Antibodies to AQP4 were tested in 24/27 (89%) atypical cases and all were negative. MOG antibodies were tested in 21/27 (78%) of atypical cases and were positive in 1/21 (5%). This case has been treated with rituximab and MOG antibodies were negative on repeat serum testing 2 years later. MRI in this case shows features typical for MS (total of 21 white matter brain lesions, periventricular lesions, Dawson finger lesions, juxta-cortical lesions and inferior temporal lobe lesions). There had been new lesions over time, but no Gd-enhancing lesions since the ADEM-like presentation and no lesions typical for MOGAD (no lesions of the optic nerve, spinal cord, brainstem or cerebellum).
Comparison of long-term clinical outcomes
A comparison of clinical outcomes is given in Table 3 . The period of follow up for typical MS cases and consequently age at last review were higher than the atypical cases ( p < 0.001). This affects several time dependent outcomes. In view of this we would be circumspect about the finding of a higher rate of monophasic/CIS disease in the atypical cohorts compared to both typical MS cohorts. With longer follow up this rate would be likely to fall (see time to event analysis below). Similarly, final EDSS was lower for the atypical MS cases. There was no difference in any of the disease duration standardised scores (e.g. 2 year and 5-year EDSS) and the final MSSS, which corrects for disease duration. Atypical MS cases were more likely to have subsequent motor ( p = 0.025) and cerebral ( p = 0.001) relapses and less likely to have optic neuritis ( p = 0.009). Atypical cases were more likely to have been commenced on highly effective disease modifying therapy as their initial treatment compared to typical MS cases ( p < 0.001). Subsequent escalation of treatment was conversely more common in the typical MS cohort ( p = 0.001). This may also reflect the greater duration of follow up for the typical MS cases and a lower availability of highly effective therapies at the time of their original diagnosis.
Regression analysis of baseline data on outcome (final MSSS) showed that both male sex (β 2.087 [95% CI 0.981–3.193] and a higher total number of FLAIR T2 hyperintense lesions on MRI brain (β 0.035 [0.012–0.058]) were associated with a worse outcome (Supplementary Table 2 and Supplementary Fig. 2 ), although the effect of FLAIR T2 lesions was small ( R 2 = 0.047). Initial treatment did not significantly affect final MSSS within the atypical MS cohort but was associated with MSSS outcomes (Supplementary Fig. 3 ) across the whole cohort ( p = 0.016), with more efficacious therapies being associated with worse outcomes for final MSSS, but not ARR.
Survival analysis
Cox proportional hazards survival analysis was used for both time to first relapse and time to EDSS 6.0 from first attack (Fig. 3 ). Only age at onset proved to be statistically significant in this analysis for time to EDSS 6.0 using a forward stepwise approach. However, because of the baseline regression analysis, age, sex and initial treatment were included in the models for both analyses (Supplementary Tables 3 and 4 ). There were no significant differences in these outcome measures for atypical MS cases compared to typical MS cases. A subgroup analysis looking at ADEM-like and tumefactive cases separately in a Kaplan–Meier analysis (Supplementary Fig. 4 ) similarly showed no differences in outcomes.

Survival curves from cox-proportional hazard models for time to first relapse ( A ) and time to reach EDSS 6.0 ( B ). Age, sex and initial treatment (low, medium or high efficacy) were included in the model. EDSS expanded disability status scale
Analyses of MRI brain with lesion counts, lesion volume and whole brain volume are shown in Fig. 4 A–C and Supplementary Table 4 . This analysis indicates no significant difference in the number of T2 lesions for typical and atypical cases. More Gd-enhancing lesions were seen in the atypical cases than typical cases ( p < 0.001) at presentation (Supplementary Table 5 . More lesions were evident for the last available MRI in typical MS cases ( p < 0.001), but this likely reflects the longer period of follow up. T2 lesion volume for atypical cases was higher at disease onset ( p < 0.001) and at Year 2 (0 = 0.004). However, subsequently there was no significant difference suggesting possible regression to the mean and similar final outcomes. There were no significant differences in whole brain volume or percentage change from baseline in whole brain volume at any timepoint (Fig. 4 D and Supplementary Table 4 ). The number of Gd-enhancing lesions was greater ( p < 0.001) in the atypical cases than typical MS cases at presentation (Fig. 4 E and Supplementary Table 4 ). As expected, there were fewer T2/FLAIR lesions at presentation in the tumefactive MS group (Fig. 4 F). There were more FLAIR lesions at onset in the ADEM-like group compared to typical MS but this difference was not statistically significant. There were no differences in the number of large T2 lesions (> 6 mm) and T1 hypointense lesions (‘old black holes’) at onset or final MRI (Supplementary Fig. 5 and Supplementary Table 5 ). The anatomical distribution of T2 brain lesions showed no significant difference at disease onset (Fig. 5 A). Subcortical lesions were more frequent in the typical MS cases at final follow up (Fig. 5 B), but this perhaps reflects the greater duration of follow and age of this group. There were no statistically significant differences in the frequency of different lesion features between ADEM-like and tumefactive presentations.

Box and whisker plots of number of T2/FLAIR lesions ( A ), T2 lesion volume ( B ) and whole brain volume ( C ) at presentation of atypical attack (Onset), 2 years, 5 years and last MRI (Final), change in whole brain volume (brain atrophy) compared to baseline at 2 year, 5 year and final follow up ( D ), Number of Gadolinium enhancing lesions at onset and final follow up ( E ), and number of T2/FLAIR lesions at onset for MS, ADEM-like and tumefactive cases ( F ). Significant differences between atypical and typical MS cases are indicated. Central bar shows median, box shows interquartile range and whiskers indicate range. Outliers indicated by circles; extreme outliers indicated by asterisks

Box and whisker plots of distribution of T2/FLAIR lesions at presentation ( A ) and last follow up ( B ). Significant differences between atypical and typical MS cases are indicated. Central bar shows median, box shows interquartile range and whiskers indicate range. Outliers indicated by circles; extreme outliers indicated by asterisks. Subcort subcortical; Perivent periventricular; Brainste brainstem; Cerebell Cerebellar; Cer Ped cerebellar peduncle
The identified cohort of 28 MS cases with atypical presentations represents approximately 3.9% (95%CI 2.6–5.6%) of cases under the care of GCUH. This prevalence of atypical MS in adults is similar to prior studies (1–5%) [ 12 , 13 , 18 , 19 ]. A disproportionately high number of atypical presentations were disproportionally first events (75%) versus what would be expected by chance (34%) amongst those with a relapsing course ( p < 0.01). This tendency has been noted for tumefactive MS [ 25 ], but we observed this pattern in both ADEM-like and tumefactive MS. Recurrent tumefactive lesions were seen in one case, a phenotype that has been previously noted [ 26 ]. Frequency of preceding infective symptoms was higher in the atypical group, but this difference was not significant. Previous studies have noted the prevalence of prior infective symptoms, but these prior investigations had no comparison group [ 13 , 27 , 28 , 29 , 30 , 31 ]. Atypical clinical features were seen in less than half of the atypical MS cases. The most common presenting symptom in both typical (38%) and atypical (48%) cohorts was sensory deficit, contrasting with previous studies in which motor deficits were the most common presentation in tumefactive MS and ADEM-like presentations [ 8 , 32 ]. In keeping with previous investigations, multifocal presentations were more common in atypical (56%) compared to typical (5%) MS cases ( p < 0.001) [ 13 , 29 , 32 , 33 ].
AQP-4 antibodies were not detected in our cohort. One ADEM-like case tested positive for MOG antibodies, out of the 21 cases available anti-MOG tested (5%). We acknowledge that this is an incomplete serological data set. Unfortunately, this deficit could not be rectified some of patients were lost to follow-up prior to MOG antibody testing being available. This finding is consistent with prior studies indicating low seroprevalence of AQP4 and anti-MOG antibodies in tumefactive and ADEM-like MS in adults [ 27 , 30 , 34 , 35 , 36 ]. One study suggested a higher prevalence (36%) of AQP4 antibodies in adult tumefactive MS [ 33 ]. The low frequency of antibodies contrasts with paediatric cases of ADEM, where MOG antibodies are found to be present in approximately one-half of cases [ 37 ]. We acknowledge the controversy of diagnosing a MOG-positive case as atypical multiple sclerosis The primary rationale for inclusion is the subsequent clinical course and radiological progression was more in line with MS than MOG. Potential explanations for this clinical course include treated MOGAD, MS with a false positive MOG antibody or co-incident MOGAD and MS.
We found that, compared to age- and sex-matched typical MS controls, and correcting for follow-up duration, atypical MS showed no difference in long-term outcomes (ARR, MSSS, time to first relapse, time to EDSS 6.0, and number of T2 lesions, T2 lesion volume and brain atrophy at 5 years). This contrasts with some differences seen with the unmatched cohort and measures that were not duration of follow-up adjusted (e.g. final EDSS). This highlights the importance of identifying suitable controls and adjusting for duration of follow up in such studies.
Atypical MS cases were more often commenced on high-efficacy therapy. This likely reflects prognostic concerns in the face of alarming radiological and clinical changes. Interestingly, initial treatment choice did not influence the survival analyses. However, the possibility that differences in initial efficacy of treatment choice may have mitigated natural history differences in long-term outcomes for the two forms of MS needs to be considered [ 38 ]. As seen in the majority of prior studies of MS, male sex was associated with greater likelihood of reaching EDSS 6.0 sooner. With a median follow up of 6 years we observed in patients with atypical MS, a conversion to MS on clinical grounds in 17/27 (63%), by MRI criteria in 18/27 (67%) and by both clinical and MRI criteria in 23/27 (85%).
Higher lesion burden within the first 5 years of diagnosis is recognised as conferring increased risk of more severe long term disability in MS [ 39 ]. We observed higher T2 lesion volume at disease onset and year 2 in the atypical MS cases (Fig. 4 ). By year 5 there were no differences and whilst the effect of the number of T2/FLAIR lesions at onset on the time to reach EDSS 6.0 was significant the effect size was small. More lesions were Gd-enhancing at first atypical presentation than in typical MS cases consistent with the florid acute presentations that are commonly seen.
The strengths of this study were that cases were ascertained through a systematically collected single centre database of demyelinating disease cases and comparisons were made with age- and sex-matched typical MS cases selected at random from the same database. In addition, data for typical and atypical cases were collected in the same manner and time-factored outcome measures have been utilised. The weaknesses of this study were that it was retrospective and that whilst matched for age at onset there was a significant difference in the duration of follow up. The inclusion of cases that pre-dated the routine use of volumetric MRI sequences necessitated the use of a less reliable tool for measuring brain volumes. The lack of histopathological correlation is another limitation. However, brain biopsy for evaluation of cerebral lesions has become increasingly rare in clinical practice, given the inherit high risk of complication and the potential of use of imaging characteristics and paraclinical information to identify likely demyelinating lesions pre-biopsy. Furthermore, given the length of follow-up for atypical cases, the presence of alternative diagnoses such as cerebral malignancy would have declared itself clinically or radiologically.
ADEM-like and tumefactive presentations of MS are uncommon. Comparison of clinical features and outcomes with a cohort of typical MS suggests that despite the initial severity of neuro-inflammatory changes, atypical MS presentations result in similar clinical and radiological outcomes (including brain atrophy) to the wider MS population.
Abbreviations
- Acute disseminated encephalomyelitis
Aquaporin-4
Central nervous system
Cerebrospinal fluid
Digital imaging and communications in medicine
Expanded disability severity score
Fluid-attenuated inversion and recovery
Gold Coast University Hospital
Myelin oligodendrocyte glycoprotein
Magnetic resonance imaging
Multiple Sclerosis
Persons with multiple sclerosis
Multiple sclerosis severity score
Neuromyelitis optica spectrum disorder
Gajofatto A, Calabrese M, Benedetti MD, Monaco S (2013) Clinical, MRI, and CSF markers of disability progression in multiple sclerosis. Dis Markers 35:687–699
Article PubMed PubMed Central Google Scholar
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50:121–127
Article CAS PubMed Google Scholar
Eckstein C, Saidha S, Levy M (2012) A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol 259:801–816
Article PubMed Google Scholar
Pohl D, Alper G, Van Haren K, Kornberg AJ, Lucchinetti CF, Tenembaum S, Belman AL (2016) Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology 87:S38-45
Karussis D (2014) The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J Autoimmun 48–49:134–142
Fereidan-Esfahani M, Decker PA, Weigand SD, Lopez Chiriboga AS, Flanagan EP, Tillema JM, Lucchinetti CF, Eckel-Passow JE, Tobin WO (2023) Defining the natural history of tumefactive demyelination: a retrospective cohort of 257 patients. Ann Clin Transl Neurol 10:1544–1555
Article CAS PubMed PubMed Central Google Scholar
Algahtani H, Shirah B, Alassiri A (2017) Tumefactive demyelinating lesions: a comprehensive review. Mult Scler Relat Disord 14:72–79
Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, Weigand S, Thomsen K, Mandrekar J, Altintas A, Erickson BJ, Konig F, Giannini C, Lassmann H, Linbo L, Pittock SJ, Bruck W (2008) Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain 131:1759–1775
Frederick MC, Cameron MH (2016) Tumefactive demyelinating lesions in multiple sclerosis and associated disorders. Curr Neurol Neurosci Rep 16:26
Sanchez P, Chan F, Hardy TA (2021) Tumefactive demyelination: updated perspectives on diagnosis and management. Expert Rev Neurother 21:1005–1017
Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, Ghezzi A, Hintzen R, Kornberg A, Pohl D, Rostasy K, Tenembaum S, Wassmer E, International Pediatric Multiple Sclerosis Study G (2013) International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 19:1261–1267
Codjia P, Ayrignac X, Carra-Dalliere C, Cohen M, Charif M, Lippi A, Collongues N, Corti L, De Seze J, Lebrun C, Vukusic S, Durand-Dubief F, Labauge P (2019) Multiple sclerosis with atypical MRI presentation: results of a nationwide multicenter study in 57 consecutive cases. Mult Scler Relat Disord 28:109–116
de Seze J, Debouverie M, Zephir H, Lebrun C, Blanc F, Bourg V, Wiertlewski S, Pittion S, Laplaud D, Le Page E, Deschamps R, Cabre P, Pelletier J, Malikova I, Clavelou P, Jaillon V, Defer G, Labauge P, Gout O, Boulay C, Edan G, Vermersch P (2007) Acute fulminant demyelinating disease: a descriptive study of 60 patients. Arch Neurol 64:1426–1432
Zaheer K, Ajmeri AN, Singh M, Suliman MS, Teka S (2018) Tumefactive multiple sclerosis, a rare variant presenting as multiple ring-enhancing lesions in an immunocompetent patient: a case report. Cureus 10:e3738
PubMed PubMed Central Google Scholar
Lin X, Yu WY, Liauw L, Chander RJ, Soon WE, Lee HY, Tan K (2017) Clinicoradiologic features distinguish tumefactive multiple sclerosis from CNS neoplasms. Neurol Clin Pract 7:53–64
Uehara T, Beck G, Baba K, Mihara M, Okuno T, Sumi H, Nakatsuji Y, Mochizuki H (2016) Tumefactive brain lesion with rapid cavity formation associated with anti-aquaporin-4 antibody. Neurol Neuroimmunol Neuroinflamm 3:e230
Katsuse K, Kurihara M, Sugiyama Y, Kodama S, Takahashi M, Momose T, Yumoto M, Kaneko K, Takahashi T, Kubota A, Hayashi T, Toda T (2019) Aphasic status epilepticus preceding tumefactive left hemisphere lesion in anti-MOG antibody associated disease. Mult Scler Relat Disord 27:91–94
Balloy G, Pelletier J, Suchet L, Lebrun C, Cohen M, Vermersch P, Zephir H, Duhin E, Gout O, Deschamps R, Le Page E, Edan G, Labauge P, Carra-Dallieres C, Rumbach L, Berger E, Lejeune P, Devos P, N’Kendjuo JB, Coustans M, Auffray-Calvier E, Daumas-Duport B, Michel L, Lefrere F, Laplaud DA, Brosset C, Derkinderen P, de Seze J, Wiertlewski S (2018) Inaugural tumor-like multiple sclerosis: clinical presentation and medium-term outcome in 87 patients. J Neurol 265:2251–2259
Liao MF, Huang CC, Lyu RK, Chen CM, Chang HS, Chu CC, Hsu WC, Wu YR, Kuo HC, Cheng MY, Hung PC, Chou ML, Lin KL, Hsieh MY, Ro LS (2011) Acute disseminated encephalomyelitis that meets modified McDonald criteria for dissemination in space is associated with a high probability of conversion to multiple sclerosis in Taiwanese patients. Eur J Neurol 18:252–259
Prain K, Woodhall M, Vincent A, Ramanathan S, Barnett MH, Bundell CS, Parratt JDE, Silvestrini RA, Bukhari W, Australian, New Zealand NMOC, Brilot F, Waters P, Broadley SA (2019) AQP4 antibody assay sensitivity comparison in the era of the 2015 diagnostic criteria for NMOSD. Front Neurol 10:1028
Marin SE, Callen DJ (2013) The magnetic resonance imaging appearance of monophasic acute disseminated encephalomyelitis: an update post application of the 2007 consensus criteria. Neuroimaging Clin N Am 23:245–266
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R (2012) 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 30:1323–1341
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintore M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173
Cox DR (1972) Regression models and life-tables. J Roy Stat Soc 34:187–220
Article Google Scholar
Tremblay MA, Villanueva-Meyer JE, Cha S, Tihan T, Gelfand JM (2017) Clinical and imaging correlation in patients with pathologically confirmed tumefactive demyelinating lesions. J Neurol Sci 381:83–87
Vakrakou AG, Tzanetakos D, Argyrakos T, Koutsis G, Evangelopoulos ME, Andreadou E, Anagnostouli M, Breza M, Tzartos JS, Gialafos E, Dimitrakopoulos AN, Velonakis G, Toulas P, Stefanis L, Kilidireas C (2020) Recurrent fulminant tumefactive demyelination with Marburg-like features and atypical presentation: therapeutic dilemmas and review of literature. Front Neurol 11:536
Vakrakou AG, Tzanetakos D, Evangelopoulos ME, Argyrakos T, Tzartos JS, Anagnostouli M, Andreadou E, Koutsis G, Velonakis G, Toulas P, Gialafos E, Dimitrakopoulos A, Psimenou E, Stefanis L, Kilidireas C (2021) Clinico-radiologic features and therapeutic strategies in tumefactive demyelination: a retrospective analysis of 50 consecutive cases. Ther Adv Neurol Disord 14:17562864211006504
Villarreal JV, Abraham MJ, Acevedo JAG, Rai PK, Thottempudi N, Fang X, Gogia B (2021) Tumefactive multiple sclerosis (TMS): a case series of this challenging variant of MS. Mult Scler Relat Disord 48:102699
Altintas A, Petek B, Isik N, Terzi M, Bolukbasi F, Tavsanli M, Saip S, Boz C, Aydin T, Arici-Duz O, Ozer F, Siva A (2012) Clinical and radiological characteristics of tumefactive demyelinating lesions: follow-up study. Mult Scler 18:1448–1453
Ketelslegers IA, Visser IE, Neuteboom RF, Boon M, Catsman-Berrevoets CE, Hintzen RQ (2011) Disease course and outcome of acute disseminated encephalomyelitis is more severe in adults than in children. Mult Scler 17:441–448
Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B (2001) Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology 56:1313–1318
Koelman DL, Chahin S, Mar SS, Venkatesan A, Hoganson GM, Yeshokumar AK, Barreras P, Majmudar B, Klein JP, Chitnis T, Benkeser DC, Carone M, Mateen FJ (2016) Acute disseminated encephalomyelitis in 228 patients: a retrospective, multicenter US study. Neurology 86:2085–2093
Jeong IH, Kim SH, Hyun JW, Joung A, Cho HJ, Kim HJ (2015) Tumefactive demyelinating lesions as a first clinical event: clinical, imaging, and follow-up observations. J Neurol Sci 358:118–124
Koelman DL, Benkeser DC, Xu Y, Neo SX, Tan K, Katsuno M, Sobue G, Natsume J, Chahin S, Mar SS, Venkatesan A, Chitnis T, Hoganson GM, Yeshokumar AK, Barreras P, Majmudar B, Carone M, Mateen FJ (2017) Acute disseminated encephalomyelitis in China, Singapore and Japan: a comparison with the USA. Eur J Neurol 24:391–396
Fereidan-Esfahani M, Decker PA, Eckel Passow JE, Lucchinetti CF, Flanagan EP, Tobin WO (2022) Population-based incidence and clinico-radiological characteristics of tumefactive demyelination in Olmsted County, Minnesota, United States. Eur J Neurol 29:782–789
Silsby M, Sanchez P, Spies JM, Frith J, Barton J, Beadnall HN, Barnett MH, Reddel SW, Hardy TA (2019) Investigation of tumefactive demyelination is associated with higher economic burden and more adverse events compared with conventional multiple sclerosis. Mult Scler Relat Disord 35:104–107
Selter RC, Brilot F, Grummel V, Kraus V, Cepok S, Dale RC, Hemmer B (2010) Antibody responses to EBV and native MOG in pediatric inflammatory demyelinating CNS diseases. Neurology 74:1711–1715
Pardo G, Jones DE (2017) The sequence of disease-modifying therapies in relapsing multiple sclerosis: safety and immunologic considerations. J Neurol 264:2351–2374
Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH (2002) A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 346:158–164
Download references
Acknowledgements
We are grateful to the study participants and would like to thank the support of the members of the Australian and New Zealand Association of Neurologists and Multiple Sclerosis Nurses Australia who assisted with data collection.
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and affiliations.
School of Medicine, Menzies Health Institute Queensland, Gold Coast Campus, Griffith University, Gold Coast, QLD, 4222, Australia
Simon V. Arnett & Simon A. Broadley
Department of Neurology, Gold Coast University Hospital, Southport, QLD, 4215, Australia
Department of Immunology, Pathology Queensland, Royal Brisbane and Women’s Hospital, Herston, QLD, 4006, Australia
Kerri Prain
Neuroimmunology Group, Kids Neurosciences Centre, Faculty of Medicine and Health, Children’s Hospital at Westmead, University of Sydney, Westmead, NSW, 2145, Australia
Sudarshini Ramanathan & Fabienne Brilot
Department of Neurology, Concord Hospital, Sydney, NSW, 2139, Australia
Sudarshini Ramanathan & Sandeep Bhuta
Griffith university, Gold Coast Campus, Gold Coast, Queensland, Australia
Simon V. Arnett
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Simon V. Arnett .
Ethics declarations
Conflicts of interest.
SAB has received honoraria for attendance at advisory boards and travel sponsorship from Bayer-Schering, Biogen-Idec, Merck-Serono, Novartis, and Sanofi-Genzyme, has received speakers honoraria from Biogen-Idec and Genzyme, is an investigator in clinical trials sponsored by Biogen Idec, Novartis and Genzyme, and was the recipient of an unencumbered research grant from Biogen-Idec. Dr Sudarshini Ramanathan has received research funding from the National Health and Medical Research Council (Australia), the Brain Foundation (Australia), the Royal Australasian College of Physicians, the Petre Foundation, and the University of Sydney. She is supported by an NHMRC Investigator Grant (GNT2008339). She serves as a consultant on an advisory board for UCB and Limbic Neurology, and has been an invited speaker for Biogen, Excemed, and Limbic Neurology. SVA has accept financial support to attend conferences supported by Biogen. KP, SR, SB, FB report no relevant financial disclosures.
Ethics approval
Supplementary information.
Below is the link to the electronic supplementary material.
Supplementary file1 (PPTX 169 KB)
Supplementary file2 (docx 14 kb), supplementary file3 (docx 30 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Arnett, S.V., Prain, K., Ramanathan, S. et al. Long-term outcomes of ADEM-like and tumefactive presentations of CNS demyelination: a case-comparison analysis. J Neurol (2024). https://doi.org/10.1007/s00415-024-12349-6
Download citation
Received : 09 December 2023
Revised : 24 March 2024
Accepted : 25 March 2024
Published : 11 June 2024
DOI : https://doi.org/10.1007/s00415-024-12349-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Multiple sclerosis
- Tumefactive
- Find a journal
- Publish with us
- Track your research

Multiple Sclerosis
What is multiple sclerosis.
Multiple sclerosis (MS) is the most common disabling neurological disease of young adults with symptom onset generally occurring between the ages of 20 to 40 years.
In MS, the immune system cells that normally protect us from viruses, bacteria, and unhealthy cells mistakenly attack myelin in the central nervous system (brain, optic nerves, and spinal cord). Myelin is a substance that makes up the protective sheath (myelin sheath) that coats nerve fibers (axons).
MS is a chronic disease that affects people differently. A small number of people with MS will have a mild course with little to no disability, whereas others will have a steadily worsening disease that leads to increased disability over time. Most people with MS, however, will have short periods of symptoms followed by long stretches of relative quiescence (inactivity or dormancy), with partial or full recovery. The disease is rarely fatal and most people with MS have a normal life expectancy.
Myelin and the immune system
MS attacks axons in the central nervous system protected by myelin, which are commonly called white matter. MS also damages the nerve cell bodies, which are found in the brain's gray matter, as well as the axons themselves in the brain, spinal cord, and optic nerves that transmit visual information from the eye to the brain. As the disease progresses, the outermost layer of the brain, called the cerebral cortex, shrinks in a process known as cortical atrophy.
The term multiple sclerosis refers to the distinctive areas of scar tissue (sclerosis—also called plaques or lesions) that result from the attack on myelin by the immune system. These plaques are visible using magnetic resonance imaging (MRI). Plaques can be as small as a pinhead or as large as a golf ball.
The symptoms of MS depend on the severity of the inflammatory reaction as well as the location and extent of the plaques, which primarily appear in the brain stem, cerebellum (involved with balance and coordination of movement, among other functions), spinal cord, optic nerves, and the white matter around the brain ventricles (fluid-filled cavaties).
Signs and symptoms of MS
The natural course of MS is different for each person, which makes it difficult to predict. The onset and duration of MS symptoms usually depend on the specific type but may begin over a few days and go away quickly or develop more slowly and gradually over many years.
There are four main types of MS, named according to the progression of symptoms over time:
- Relapsing-remitting MS—Symptoms in this type come in the form of attacks. In between attacks, people recover or return to their usual level of disability. When symptoms occur in this form of MS, it is called an attack, a relapse, or exacerbation. The periods of disease inactivity between MS attacks are referred to as remission. Weeks, months, or even years may pass before another attack occurs, followed again by a period of inactivity. Most people with MS are initially diagnosed with this form of the disease.
- Secondary-progressive MS—People with this form of MS usually have had a previous history of MS attacks but then start to develop gradual and steady symptoms and deterioration in their function over time. Most individuals with severe relapsing-remitting MS may go on to develop secondary progressive MS if they are untreated.
- Primary-progressive MS—This type of MS is less common and is characterized by progressively worsening symptoms from the beginning with no noticeable relapses or exacerbations of the disease, although there may be temporary or minor relief from symptoms.
- Progressive-relapsing MS—The rarest form of MS is characterized by a steady worsening of symptoms from the beginning with acute relapses that can occur over time during the disease course.
There are some rare and unusual variants of MS, such as:
- Marburg variant MS (also known as malignant MS) causes swift and relentless symptoms and decline in function, and may result in significant disability or even death shortly after disease onset.
- Balo's concentric sclerosis causes concentric rings of myelin destruction that can be seen on an MRI and is another variant type of MS that can progress rapidly.
Early MS symptoms often include:
- Vision problems such as blurred or double vision, or optic neuritis, which causes pain with eye movement and rapid vision loss
- Muscle weakness, often in the hands and legs, and muscle stiffness accompanied by painful muscle spasms
- Tingling, numbness, or pain in the arms, legs, trunk, or face
- Clumsiness, especially difficulty staying balanced when walking
- Bladder control problems
- Intermittent or constant dizziness
MS may also cause later symptoms, such as:
- Mental or physical fatigue which accompanies the early symptoms during an attack
- Mood changes such as depression or difficulty with emotional expression or control
- Cognitive dysfunction—problems concentrating, multitasking, thinking, learning, or difficulties with memory or judgment
Muscle weakness, stiffness, and spasms may be severe enough to affect walking or standing. In some cases, MS leads to partial or complete paralysis and the use of a wheelchair is not uncommon, particularly in individuals who are untreated or have advanced disease. Many people with MS find that weakness and fatigue are worse when they have a fever or when they are exposed to heat. MS exacerbations may occur following common infections.
Pain is rarely the first sign of MS but pain often occurs with optic neuritis and trigeminal neuralgia, a disorder that affects one of the nerves that provides sensation to different parts of the face. Painful limb spasms and sharp pain shooting down the legs or around the abdomen can also be symptoms of MS.
Conditions associated with MS
- Transverse myelitis (an inflammation of the spinal cord) may develop in those with MS. Transverse myelitis can affect spinal cord function over several hours to several weeks before partial or complete recovery. It usually begins as a sudden onset of lower back pain, muscle weakness, abnormal sensations in the toes and feet, or difficulties with bladder control or bowel movements. This can rapidly progress to more severe symptoms, including arm and/or leg paralysis. In most cases, people recover at least some function within the first 12 weeks after an attack begins.
- Neuromyelitis optica is a disorder associated with transverse myelitis as well as optic nerve inflammation (also known as optic neuritis). People with this disorder usually have abnormal antibodies (proteins that normally target viruses and bacteria) against a specific channel in optic nerves, the brain stem or spinal cord, called the aquaporin-4 channel. These individuals respond to certain treatments, which are different than those commonly used to treat MS.
- Trigeminal neuralgia is a chronic pain condition that causes sporadic, sudden burning or shock-like facial pain. The condition is more common in young adults with MS and is caused by lesions in the brain stem, the part of the brain that controls facial sensation.
Who is more likely to get multiple sclerosis?
Females are more frequently affected than males. Researchers are looking at several possible explanations for why the immune system attacks central nervous system myelin, including:
- Fighting an infectious agent (e.g., a virus) that has components that mimic components of the brain (molecular mimicry)
- Destroying brain cells because they are unhealthy
- Mistakenly identifying normal brain cells as foreign
There is also something known as the blood-brain barrier, which separates the brain and spinal cord from the immune system. If there is a break in this barrier, it exposes the brain to the immune system. When this happens, the immune system may misinterpret structures in the brain, such as myelin, as “foreign.”
Research shows that genetic vulnerabilities combined with environmental factors may cause MS.
Genetic susceptibility
MS itself is not inherited, but susceptibility to MS may be inherited. Studies show that some individuals with MS have one or more family member or relative who also have MS.
Current research suggests that dozens of genes and possibly hundreds of variations in the genetic code (gene variants) combine to create vulnerability to MS. Some of these genes have been identified, and most are associated with functions of the immune system. Many of the known genes are similar to those that have been identified in people with other autoimmune diseases as type 1 diabetes, rheumatoid arthritis, or lupus.
Infectious factors and viruses
Several viruses have been found in people with MS, but the virus most consistently linked to the development of MS is the Epstein-Barr virus (EBV) which causes infectious mononucleosis.
Only about five percent of the population has not been infected by EBV. These individuals are at a lower risk for developing MS than those who have been infected. People who were infected with EBV in adolescence or adulthood, and who therefore develop an exaggerated immune response to EBV, are at a significantly higher risk for developing MS than those who were infected in early childhood. This suggests that it may be the type of immune response to EBV that may lead to MS, rather than EBV infection itself. However, there is still no proof that EBV causes MS and the mechanisms that underlie this process are poorly understood.
Environmental factors
Several studies indicate that people who spend more time in the sun and those with relatively higher levels of vitamin D are less likely to develop MS or have a less severe course of disease and fewer relapses. Bright sunlight helps human skin produce vitamin D. Researchers believe that vitamin D may help regulate the immune system in ways that reduce the risk of MS or autoimmunity in general. People from regions near the equator, where there is a great deal of bright sunlight, generally have a much lower risk of MS than people from temperate areas such as the U.S. and Canada.
Studies have found that people who smoke are more likely to develop MS and have a more aggressive disease course. Indeed, people who smoke tend to have more brain lesions and brain shrinkage than non-smokers.
How is multiple sclerosis diagnosed and treated?
Diagnosing MS
There is no single test used to diagnose MS. The disease is confirmed when symptoms and signs develop and are related to different parts of the nervous system at more than one interval and after other alternative diagnoses have been excluded.
Doctors use different tests to rule out or confirm the diagnosis. In addition to a complete medical history, physical examination, and a detailed neurological examination, a doctor may recommend:
- MRI scans of the brain and spinal cord to look for the characteristic lesions of MS. A special dye or contrast agent may be injected into a vein to enhance brain images of the active MS lesions.
- Lumbar puncture (sometimes called a spinal tap) to obtain a sample of cerebrospinal fluid and examine it for proteins and inflammatory cells associated with the disease. Spinal tap analysis also can rule out diseases that may look like MS.
- Evoked potential tests, which use electrodes placed on the skin and painless electric signals to measure how quickly and accurately the nervous system responds to stimulation.
Treating MS
There is no cure for MS, but there are treatments that can reduce the number and severity of relapses and delay the long-term disability progression of the disease.
- Corticosteroids, such as intravenous (infused into a vein) methylprednisolone, are prescribed over the course of three to five days. Intravenous steroids quickly and potently suppress the immune system and reduce inflammation. They may be followed by a tapered dose of oral corticosteroids. Clinical trials have shown that these drugs hasten recovery from MS attacks, but do not alter the long-term outcome of the disease.
- Plasma exchange (plasmapheresis) can treat severe flare-ups in people with relapsing forms of MS who do not have a good response to methylprednisolone. Plasma exchange involves taking blood out of the body and removing components in the blood's plasma that are thought to be harmful. The rest of the blood, plus replacement plasma, is then transfused back into the body. This treatment has not been shown to be effective for secondary progressive or chronic progressive MS.
Disease-modifying treatments
Current therapies approved by the U.S. Food and Drug Administration (FDA) for MS are designed to modulate or suppress the inflammatory reactions of the disease. They are most effective for relapsing-remitting MS at early stages of the disease.
Injectable medications include:
- Beta interferon drugs are among the most common medications used to treat MS. Interferons are signaling molecules that regulate immune cells. Potential side effects of these drugs include flu-like symptoms (which usually fade with continued therapy), depression, or elevation of liver enzymes. Some individuals will notice a decrease in the effectiveness of the drugs after 18 to 24 months of treatment. If flare-ups occur or symptoms worsen, doctors may switch treatment to alternative drugs.
- Glatiramer acetate changes the balance of immune cells in the body, but how it works is not entirely clear. Side effects are usually mild and consist of local injection site reactions or swelling.
Infusion treatments include:
- Natalizumab is administered intravenously once a month. It works by preventing cells of the immune system from entering the brain and spinal cord. It is very effective but is associated with an increased risk of a serious and potentially fatal viral infection of the brain called progressive multifocal leukoencephalopathy (PML). Natalizumab is generally recommended only for individuals who have not responded well to or who are unable to tolerate other first-line therapies.
- Ocrelizumab is administered intravenously every six months and treats adults with relapsing or primary progressive forms of MS. It is the only FDA-approved disease-modifying therapy for primary-progressive MS. The drug targets the circulating immune cells that produce antibodies, which also play a role in the formation of MS lesions. Side effects include infusion-related reactions and increased risk of infections. Ocrelizumab may increase the risk of cancer as well.
- Alemtuzumab is administered for five consecutive days followed by three days of infusions one year later. It targets proteins on the surface of immune cells. Because this drug increases the risk of autoimmune disorders it is recommended for those who have had inadequate responses to two or more MS therapies.
- Mitoxantrone, which is administered intravenously four times a year, has been approved for especially severe forms of relapsing-remitting and secondary progressive MS. Side effects include the development of certain types of blood cancers in up to one percent of those with MS, as well as with heart damage. This drug should be considered as a last resort to treat people with a form of MS that leads to rapid loss of function and for whom other treatments did not work.
Oral treatments include:
- Fingolimod is a once-daily medication that reduces the MS relapse rate in adults and children. It is the first FDA-approved drug to treat MS in adolescents and children ages 10 years and older. The drug prevents white blood cells called lymphocytes from leaving the lymph nodes and entering the blood, brain, and spinal cord. Fingolimod may result in a slow heart rate and eye problems when first taken. Fingolimod can also increase the risk of infections, such as herpes virus infections, or in rare cases be associated with PML.
- Dimethyl fumarate is a twice-daily medication used to treat relapsing forms of MS. Its exact mechanism of action is not currently known. Side effects of dimethyl fumarate are flushing, diarrhea, nausea, and lowered white blood cell count.
- Teriflunomide is a once-daily medication that reduces the rate of proliferation of activated immune cells. Teriflunomide side effects can include nausea, diarrhea, liver damage, and hair loss.
- Cladribine is administered as two courses of tablets about one year apart. Cladribine targets certain types of white blood cells that drive immune attacks in MS. The drug may increase the risk of developing cancer and should be considered for individuals who have not responded well to other MS treatments.
- Diroximel fumarate is a twice-daily drug similar to dimethyl fumarate (brand name Tecfidera) but with fewer gastrointestinal side effects. Scientists suspect these drugs, which have been approved to treat secondary progressive MS, reduce damage to the brain and spinal cord by making the immune response less inflammatory, although their exact mechanism of action is poorly understood.
- Siponimod tablets (Mayzent) is taken orally and has a similar mechanism of action to fingolimod. Siponimod has been approved by the FDA to treat secondary-progressive MS.
Clinical trials have shown that cladribine, diroximel fumarate, and dimethyl fumarate decrease the number of relapses, delay the progress of physical disability, and slow the development of brain lesions.
Managing MS symptoms
MS causes a variety of symptoms that can interfere with daily activities but can usually be treated or managed. Many of these issues are best treated by neurologists who have advanced training in the treatment of MS and who can prescribe specific medications to treat these problems.
Eye and vision problems are common in people with MS but rarely result in permanent blindness. Inflammation of the optic nerve (optic neuritis) or damage to the myelin that covers the nerve fibers in the visual system can cause blurred or grayed vision, temporary blindness in one eye, loss of normal color vision, depth perception, or loss of vision in parts of the visual field. Uncontrolled horizontal or vertical eye movements (nystagmus), “jumping vision" (opsoclonus), and double vision (diplopia) are common in people with MS. Intravenous steroid medications, special eyeglasses, and periodically resting the eyes may be helpful.
Muscle weakness and spasticity is common in MS. Mild spasticity can be managed by stretching and exercising muscles using water therapy, yoga, or physical therapy. Medications such as gabapentin or baclofen can reduce spasticity. It is very important that people with MS stay physically active because physical inactivity can contribute to worsening stiffness, weakness, pain, fatigue, and other symptoms.
Tremor, or uncontrollable shaking, develops in some people with MS. Assistive devices and weights attached to utensils or even limbs are sometimes helpful for people with tremor. Deep brain stimulation and drugs, such as clonazepam, may also be useful.
Problems with walking and balance occur in many people with MS. The most common walking problem is ataxia—unsteady, uncoordinated movements—due to damage to the areas of the brain that coordinate muscle balance. People with severe ataxia generally benefit from the use of a cane, walker, or other assistive device. Physical therapy also can reduce walking problems. The FDA has approved the drug dalfampridine to improve walking speed in people with MS.
Fatigue is a common symptom of MS and may be both physical (tiredness in the arms or legs) and cognitive (slowed processing speed or mental exhaustion). Daily physical activity programs of mild to moderate intensity can significantly reduce fatigue, although people should avoid excessive physical activity and minimize exposure to hot weather conditions or ambient temperature. Other drugs that may reduce fatigue include amantadine, methylphenidate, and modafinil. Occupational therapy can help people learn how to walk using an assistive device or in a way that saves physical energy. Stress management programs, relaxation training, membership in an MS support group, or individual psychotherapy may help some people.
Pain from MS can be felt in different parts of the body. Trigeminal neuralgia (facial pain) is treated with anticonvulsant or antispasmodic drugs, or less commonly, painkillers. Central pain, a syndrome caused by damage to the brain and/or spinal cord, can be treated with gabapentin and nortriptyline. Treatments for chronic back or other musculoskeletal pain may include heat, massage, ultrasound, and physical therapy.
Problems with bladder control and constipation may include urinary frequency, urgency, or the loss of bladder control. A small number of individuals retain large amounts of urine. Medical treatments are available for bladder-related problems. Constipation is also common and can be treated with a high-fiber diet, laxatives, and stool softeners.
Sexual dysfunction can result from damage to nerves running through the spinal cord. Sexual problems may also stem from MS symptoms such as fatigue, cramped or spastic muscles, and psychological factors. Some of these problems can be corrected with medications. Psychological counseling may be helpful.
Clinical depression is frequent among people with MS. MS may cause depression as part of the disease process and chemical imbalance in the brain. Depression can intensify symptoms of fatigue, pain, and sexual dysfunction. It is most often treated with cognitive behavioral therapy, and selective serotonin reuptake inhibitor (SSRI) antidepressant medications, which are less likely than other antidepressant medications to cause fatigue.
Inappropriate and involuntary expressions of laughter, crying, or anger—symptoms of a condition called pseudobulbar affect—sometimes are associated with MS. These expressions are often incongruent with mood; for example, people with MS may cry when they are actually happy or laugh when they are not especially happy. The combination treatment of the drugs dextromethorphan and quinidine can treat pseudobulbar affect, as can other drugs such as amitriptyline or citalopram.
Cognitive impairment—a decline in the ability to think quickly and clearly and to remember easily—affects up to 75 percent of people with MS. These cognitive changes may appear at the same time as the physical symptoms or they may develop gradually over time. Drugs such as donepezil may be helpful in some cases.
Complementary and alternative therapies
Many people with MS benefit from complementary or alternative approaches such as acupuncture, aromatherapy, ayurvedic medicine, touch and energy therapies, physical movement disciplines such as yoga and tai chi, herbal supplements, and biofeedback.
Because of the risk of interactions between alternative and conventional therapies, people with MS should discuss all the therapies they are using with their doctor, especially herbal supplements. Herbal supplements have biologically active ingredients that could have harmful effects on their own or interact harmfully with other medications.
What are the latest updates on multiple sclerosis?
The National Institute of Neurological Disorders and Stroke ( NINDS ), a component of the National Institutes of Health ( NIH ), is the leading federal funder of research on the brain and nervous system, including research on MS.
In addition to NINDS , other NIH Institutes—including the National Institute of Allergy and Infectious Diseases ( NIAID )—fund research on multiple sclerosis. Find more information on NIH research efforts through NIH RePORTER , a searchable database of current and past research projects supported by NIH and other federal agencies. RePORTER also includes links to publications and patents citing support from these projects.
Although researchers have not been able to identify the cause of MS with any certainty, there has been excellent progress in other areas of MS research—especially in the development of new treatments to prevent exacerbations of the disease. New discoveries are constantly changing MS treatment options and helping to reduce MS-related disability.
Research projects conducted by NINDS scientists or through NIH grants to universities and other sites across the U.S. cover a wide range of topics such as comorbidities, mechanisms of cognitive impairment, blood-brain barrier breakdown in MS, the role of sleep and circadian rhythms, rehabilitation strategies, and telehealth. Other topics include:
- Biomarkers to accurately diagnose MS and monitor disease progression, including blood and imaging tests (e.g., MRI)
- Genetic and environmental risk factors for MS, such as low Vitamin D or the Epstein-Barr virus
- The role of the gut microbiome and diet in MS
- Mechanisms that underlie gender differences in the incidence and presentation of MS
- MS risk factors and disease course in African American and Hispanic populations, and disparities in care
- The role of the immune system in MS, including its function in the central nervous system (CNS)
- The role and crosstalk of various cell types in the CNS with relation to MS
- Basic functions of myelination, demyelilnation, and axonal degeneration, and strategies to overcome axonal and myelin loss
Scientists sponsored by NIAID are testing an experimental stem cell treatment titled, autologous hematopoietic stem cell transplantation (AHSCT), against the best available biologic therapies for severe forms of relapsing MS.
Investigators in the clinical trial BEAT-MS (BEst Available Therapy versus autologous hematopoietic stem cell transplant for Multiple Sclerosis) are removing some immune cells and then infusing some of the person's own blood-forming stem cells to reset the immune system so it no longer attacks the CNS.
Genetic research funded by NINDS is exploring the roles of "susceptibility genes"—genes that are associated with an increased risk for MS. Several candidate genes have been identified and researchers are studying their function in the nervous system to discover how they may lead to the development of MS.
Other studies aim to develop better neuroimaging tools, such as more powerful MRI methods, to diagnose MS, track disease progression, and assess treatments. NINDS scientists are collecting MRIs of the brain and spinal cord and scans of the retina, along with other clinical and biological data, from more than 100 individuals with MS and 50 individuals without the disease over a period of years to observe changes in the course of MS. Investigators are using MRI to study the natural history of MS and to help define the mechanism of action and cause of side effects of disease modifying therapies.
Intramural research programs on MS
NINDS and other NIH Institutes have a very active MS intramural research program among scientists working at NIH. Together, they have:
- Established and continue to develop MRI as a critical tool for examining the natural course of the disease in humans, monitoring disease progression, assessing effects of treatments in clinical trials, and understanding MS biology
- Played an important role in understanding why some patients develop a rare and potentially fatal brain infection (progressive multifocal leukoencephalopathy) when taking potent MS drugs, and they are developing new treatments for this infection
- Unraveled mechanisms by which viruses, especially the Epstein-Barr virus, contribute to the development of MS
- Conducted next-generation treatment trials targeting specific mechanisms of disease progression, using advanced MRI and fluid biomarkers as outcome measures
- Developed the first MRI method to visualize the lymph vessels surrounding the brain, which play a critical role in neuro-immune communication
Translational research
NIH supports translational studies to develop therapies that will stop or reverse the course of the disease, focusing on pathways that modify immune system function, repair damaged myelin, or protect neurons from damage. Researchers are also developing animal models of MS to more accurately predict drug response in human disease. However, current animal models share some of the disease mechanisms and symptoms of MS but do not fully mimic the disease, especially in its clinically progressive phase.
Focus on progressive MS therapies
Scientists continue to study the biology and mechanisms of relapsing-remitting MS while increasing efforts to stop or prevent the steady decline in function that occurs in progressive MS. In the MS-SPRINT trial, the NINDS NeuroNEXT clinical trials network tested the drug ibudilast as a potential neuroprotective drug for progressive MS and showed that the drug slowed the rate of brain shrinkage as compared to a placebo. NINDS Intramural scientists are conducting proof-of-concept clinical trials to address a key driver of clinical progression called the “slowly expanding lesion.”
Focus on biomarkers
As part of a larger effort to develop and validate effective biomarkers (signs that may indicate risk of a disease or be used to monitor its progression) for neurological disease, NINDS is supporting two definitive multicenter MS studies:
- The Central Vein Sign in MS (CAVS-MS) study, which is testing whether a rapid MRI approach designed by NINDS Intramural scientists can use the detection of a central vein passing through brain plaques to differentiate MS from other common neurological disorders that can mimic MS. The goal is to develop a reliable imaging test for MS in order to achieve rapid yet accurate diagnosis and reduce misdiagnosis, which may affect up to 20 percent of people currently diagnosed with MS.
- A study to test whether a simple new blood test that measures small amounts of neuron-derived proteins (neurofilaments) can be used to predict the severity of disease and help determine whether MS drugs are working to protect brain tissues.
How can I or my loved one help improve care for people with multiple sclerosis?
Consider participating in a clinical trial so clinicians and scientists can learn more about MS and related disorders. Clinical research uses human volunteers to help researchers learn more about a disorder and perhaps find better ways to safely detect, treat, or prevent disease.
All types of volunteers are needed— those who are healthy or may have an illness or disease— of all different ages, sexes, races, and ethnicities to ensure that study results apply to as many people as possible, and that treatments will be safe and effective for everyone who will use them.
For information about participating in clinical research visit NIH Clinical Research Trials and You . Learn about clinical trials currently looking for people with MS at Clinicaltrials.gov .
Where can I find more information about multiple sclerosis? Information may be available from the following organizations and resources: Accelerated Cure Project for Multiple Sclerosis Phone: 781-487-0008 Autoimmune Association Phone: 586-776-3900 Multiple Sclerosis Association of America (MSAA) Phone: 856-488-4500 or 800-532-7667 Multiple Sclerosis Foundation (MS Focus) Phone: 954-776-6805 or 888 673-6287 Myelin Repair Foundation (MRF) Phone: 408-871-2410 National Ataxia Foundation (NAF) Phone: 763-553-0020 National Multiple Sclerosis Society Phone: 800-344-4867 National Organization for Rare Disorders (NORD) Phone: 203-744-0100 National Rehabilitation Information Center (NARIC) Phone: 301-459-5900 or 800-346-2742; 301-459-5984 Paralyzed Veterans of America Phone: 202-872-1300 or 800-555-9140

- Allergy & Immunology
- Anesthesiology
- Critical Care
- Dermatology
- Diabetes & Endocrinology
- Emergency Medicine
- Family Medicine
- Gastroenterology
- General Surgery
- Hematology - Oncology
- Hospital Medicine
- Infectious Diseases
- Internal Medicine
- Multispecialty
- Ob/Gyn & Women's Health
- Ophthalmology
- Orthopedics
- Pathology & Lab Medicine
- Plastic Surgery
- Public Health
- Pulmonary Medicine
- Rheumatology
- Transplantation
- Today on Medscape
- Business of Medicine
- Medical Lifestyle
- Science & Technology
- Medical Students
- Pharmacists
Multiple Sclerosis Med Nearly Eliminates Disease Activity on MRI
Ted Bosworth
June 05, 2024
NASHVILLE, Tennessee — A second-generation anti-CD40L monoclonal antibody suppresses multiple sclerosis (MS) disease activity on MRI to an uncommonly high degree, new trial data suggested.
Researchers found a near absence of new brain lesions at 48 weeks in patients on the highest dose. At this level of disease suppression, there was no evidence of increased infection risk, which investigators said might relate to its mechanism of action. In addition, there were no thrombotic events, which is what defeated a first-generation drug in this same class.
Among those initially randomly assigned to receive 1200 mg every 4 weeks, 96% were free of new gadolinium-positive (Gd+ T1) lesions at 48 weeks, investigator Yang Mao-Draayer, MD, PhD, director of Clinical and Experimental Therapeutics at the Oklahoma Medical Research Foundation's Multiple Sclerosis Center of Excellence, Oklahoma City, reported. Annual relapse rates were also low.
The findings were presented on May 30 at the Consortium of Multiple Sclerosis Centers (CMSC) 2024 Annual Meeting .
No Effect on Lymphocyte Count
As previously reported by Medscape Medical News , 12-week frexalimab results were noteworthy because they provided validation for CD40L as a target in the control of MS. One of the unique features of this therapy relative to many other immunomodulatory therapies is that it has shown little, if any, effect on lymphocyte counts or immunoglobulin levels.
In the double-blind randomized phase 2 trial, 125 patients with MS of all other MS therapy were randomized in a 4:4:4:1 ratio to 1200-mg frexalimab administered intravenously every 4 weeks after a loading dose, to 300-mg frexalimab administered subcutaneously every 2 weeks after a loading dose, or to one of the two matching placebo arms.
For the primary endpoint of new Gd+ T1 lesions at the end of the blinded study, the rates at week 12 were 0.2 and 0.3 in the higher- and lower-dose treatment groups, respectively, and 1.4 in the pooled placebo groups.
At 48 weeks, the results were even better. From 12 weeks, the rate of Gd+ T1 lesions in the high-dose group continued to fall, reaching 0.1 at week 24 and 0.0 at week 48. In the lower-dose group, there was also a stepwise decline over time with a value of 0.2 at week 48. The annual relapse rate at week 48 was 0.4.
Reengineered Agent
In the placebo groups, the same type of suppression of disease activity was observed after they were switched to active therapy at the end of 12 weeks.
By 24 weeks, the number of new Gd+ T1 lesions had fallen to 0.3 in placebo patients switched to the higher dose and 1.0 in those switched to the lower dose.
By week 48, the rates were 0.2 in both of the switch arms.
The proportions of patients free of new Gd+ T1 lesions at 48 weeks were 96% in the group started and maintained on the highest dose of frexalimab, 87% in those started and maintained on the lower dose, 90% in those started on placebo and switched to the highest dose of frexalimab, and 92% of placebo patients switched to the lower dose.
"T2 lesion volume from baseline through week 48 was stable in patients who continued receiving frexalimab and decreased in placebo participants after switching to frexalimab at week 12," Mao-Draayer reported.
The CD40-CD40L co-stimulatory pathway that regulates both adaptive and innate immune responses has been pursued as a target for MS therapies for decades, Mao-Draayer said.
A first-generation monoclonal antibody directed at elevated levels of CD40L, which is implicated in the inflammation that drives MS, showed promise but was abandoned after it was associated with an increased risk for thromboembolic events in a phase 1 trial, she said.
However, the second-generation agent was engineered to avoid an interaction with platelets, which played a role in the risk for thrombosis associated with the failure of the earlier drug.
As with the first-generation agent, frexalimab had little or no impact on lymphocyte count or immunoglobulin G and immunoglobulin M levels. Both remained stable during the 12-week controlled trial and through the ongoing open-label extension, Mao-Draayer said.
This might be a factor in the low level of adverse events. Most importantly, there have been no thromboembolic events associated with frexalimab so far, but the follow-up data also show rates of infection and other events, such as nasopharyngitis, that were comparable with placebo in the 12-week controlled trial and have not increased over longer-term monitoring.
Such adverse events as headache and COVID-19 infection have also occurred at rates similar to placebo.
Two phase 3 trials are underway. FREXALT is being conducted in relapsing-remitting MS. FREVIV has enrolled patients with non-relapsing secondary progressive MS.
Impressively Low New Lesion Count
Commenting on the findings for Medscape Medical News , Jeffrey Cohen, MD, director of the Mellen Center for Multiple Sclerosis, Cleveland Clinic, Cleveland, who was not involved in the research, said that over the course of the extended follow-up, MS activity in the central nervous system as measured with new Gd+ T1 lesions was impressively low.
He noted that the phase 2 open-label follow-up continues to support the promise of frexalimab. But Cohen cautioned that this does not obviate the need for phase 3 data.
In particular, he said that an immunomodulatory agent that does not affect the lymphocyte count has a theoretical advantage but pointed out that the benefit is still presumably mediated by blocking pathways that mediate autoimmune activity.
Even if lymphocyte count is unaffected, the immunomodulatory pathway by which frexalimab does exert its benefit might pose a different set of risks, he said.
"We will not have sufficient data to judge the promise of this agent until the phase 3 trials are completed," he said.
Mao-Draayer reported financial relationships with Acorda, Bayer, Biogen, Bristol Myers Squibb, Celgene, EMD Serono, Genentech, Horizon, Janssen, Novartis, Questor, Teva, and Sanofi, which provided funding for the phase 2 frexalimab trial. Cohen reported financial relationships with Astoria, Convelo, EMD Serono, FiND, INMune, and Sandoz.
Send comments and news tips to [email protected] .
TOP PICKS FOR YOU
- Perspective
- Drugs & Diseases
- Global Coverage
- Additional Resources
- Health Spending vs 'Latitude Hypothesis' for Higher MS Prevalence
- Non-White MS Patients Rarely Included in Trials
- US FDA Declines to Approve Viatris's Injection for Multiple Sclerosis
- Diseases & Conditions Multiple Sclerosis
- Diseases & Conditions Neuro-Ophthalmologic Manifestations of Multiple Sclerosis
- Diseases & Conditions Pediatric Multiple Sclerosis
- Diagnosing Multiple Sclerosis: 5 Things to Know
- Multiple Sclerosis
- Pediatric Multiple Sclerosis
- Neuro-Ophthalmologic Manifestations of Multiple Sclerosis
- Multiple Sclerosis Spine Imaging
- Brain Imaging in Multiple Sclerosis
- The Patient's Journey Through Multiple Sclerosis
- Central Vertigo: Identifying the Hidden Cause
- Migraine a Forerunner of Multiple Sclerosis?
- Frexalimab Promising for Relapsing Multiple Sclerosis
WebMD Network
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 12, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
AI better detects prostate cancer on MRI than radiologists, study shows
by Radboud University

AI detects prostate cancer more often than radiologists. Additionally, AI triggers false alarms half as often. This is shown by an international study coordinated by Radboud university medical center and published in The Lancet Oncology . This is the first large-scale study where an international team transparently evaluates and compares AI with radiologist assessments and clinical outcomes.
Radiologists face an increasing workload as men with a higher risk of prostate cancer now routinely receive a prostate MRI. Diagnosing prostate cancer with MRI requires significant expertise, and there is a shortage of experienced radiologists. AI can assist with these challenges.
AI expert Henkjan Huisman and radiologist Maarten de Rooij, project leaders of the PI-CAI study, organized a major competition between AI teams and radiologists with an international team. Along with other centers in the Netherlands and Norway, they provided over 10,000 MRI scans. They transparently determined for each patient whether prostate cancer was present. They allowed various groups worldwide to develop AI for analyzing these images.
The top five submissions were combined into a super-algorithm for analyzing MRI scans for prostate cancer. Finally, AI assessments were compared to those of a group of radiologists on four hundred prostate MRI scans.
Accurate diagnosis
The PI-CAI community brought together over two hundred AI teams and 62 radiologists from twenty countries. They compared the findings of AI and radiologists not only with each other but also with a gold standard, as they monitored the outcomes of the men from whom the scans originated. On average, the men were followed for five years.
This first international study on AI in prostate diagnostics shows that AI detects nearly 7% more significant prostate cancers than the group of radiologists. Additionally, AI identifies suspicious areas, later found not to be cancer , 50% less often. This means the number of biopsies could be halved with the use of AI.
If these results are replicated in follow-up studies, it could greatly assist radiologists and patients in the future. It could reduce radiologists ' workload, provide more accurate diagnoses, and minimize unnecessary prostate biopsies. The developed AI still needs to be validated and is currently not yet available for patients in clinical settings.
Quality system
Huisman observes that society has little trust in AI. "This is because manufacturers sometimes build AI that isn't good enough," he explains. He is working on two things. The first is a public and transparent test to fairly evaluate AI. The second is a quality management system, similar to what exists in the aviation industry.
"If planes almost collide, a safety committee will look at how to improve the system so that it doesn't happen in the future. I want the same for AI. I want to research and develop a system that learns from every mistake so that AI is monitored and can continue to improve. That way, we can build trust in AI for health care. Optimal, governed AI can help make health care better and more efficient."
Explore further
Feedback to editors

Case study reveals important new details about rare second cancers related to CAR-T therapy
4 hours ago

Risk of secondary cancers after CAR-T cell therapy low, according to large study
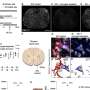
Mouse study identifies unique approach for preventing life-threatening complications after spinal cord injury
5 hours ago

Convenient at-home test identifies at-risk individuals with inadequate immunity to COVID-19
6 hours ago
Past COVID infections may help protect against certain colds. Could it lead to better vaccines?
Putting the brakes on chronic inflammation: Study discovers link between two key pathways
7 hours ago
Reproductive cells drive sex-dependent differences in lifespan, reveal role for vitamin D in improving longevity: Study
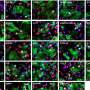
Heart regeneration: Researchers discover key role of growth factor
8 hours ago

AI-based diagnostic tool differentiates dementia diseases by analysis of eye movement patterns

Racial bias and discrimination among women of color can impact their baby's biological clock
Related stories.

AI performs as well as experienced radiologists in detecting prostate cancer
Apr 16, 2019

Researchers develop AutoProstate to automatically generate prostate cancer diagnostic reports using deep learning
Dec 17, 2021
Shorter scan to diagnose prostate cancer could increase availability and reduce cost
Apr 8, 2024
PET/MRI found to accurately classify prostate cancer patients, offer potential to avoid unnecessary biopsies
Apr 11, 2024

New test for prostate cancer could help avoid unnecessary biopsies
Oct 6, 2023

More cases of breast cancer detected with the help of AI
Sep 8, 2023
Recommended for you

Research pair finds Gen X people more susceptible to many types of cancers than prior generations
9 hours ago
CAR T cell therapy for advanced prostate cancer demonstrates positive results in Phase I clinical trial
10 hours ago
Study reveals unexpected mechanism of drug resistance in kidney cancer
11 hours ago
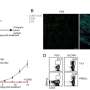
Safer virus helps eliminate cancer in mouse study
Jun 11, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Macular oct’s proficiency in identifying retrochiasmal visual pathway lesions in multiple sclerosis—a pilot study.

1. Introduction
1.1. the impact of ms on the visual pathway, 1.1.1. anatomy of the visual pathway, 1.1.2. trans-synaptic degeneration, 1.2. importance of perimetry in ms, 1.3. optical coherence tomography in ms, 1.3.1. oct as a gateway to neurological assessment, 1.3.2. global retinal changes in ms, 1.3.3. macular ganglion cell and prnfl loss beyond optic neuritis, 1.4. research objectives, 2. materials and methods, 2.1. study design, 2.2. participants, 2.3. clinical and paraclinical assessment, 2.3.1. oct measurement methodology, 2.3.2. visual field testing, 2.3.3. neuroimaging, 2.4. statistical analysis, 4. discussion, 4.1. oct in the evaluation of ms patients, 4.2. vf assessment in ms patients, 4.3. limitations, 5. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019 , 18 , 269–285. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Guerrieri, S.; Comi, G.; Leocani, L. Optical coherence tomography and visual evoked potentials as prognostic and monitoring tools in progressive multiple sclerosis. Front. Neurosci. 2021 , 15 , 692599. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ontaneda, D.; Fox, R.J. Progressive multiple sclerosis. Curr. Opin. Neurol. 2015 , 28 , 237–243. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sakata, L.M.; Deleon-Ortega, J.; Sakata, V.; Girkin, C.A. Optical coherence tomography of the retina and optic nerve—A review. Clin. Exp. Ophthalmol. 2009 , 37 , 90–99. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Xie, J.S.; Donaldson, L.; Margolin, E. The use of optical coherence tomography in neurology: A review. Brain 2022 , 145 , 4160–4177. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Maldonado, R.S.; Mettu, P.; El-Dairi, M.; Bhatti, M.T. The application of optical coherence tomography in neurologic diseases. Neurol. Clin. Pract. 2015 , 5 , 460–469. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Parisi, V.; Manni, G.; Spadaro, M.; Colacino, G.; Restuccia, R.; Marchi, S.; Bucci, M.G.; Pierelli, F. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Investig. Ophthalmol. Vis. Sci. 1999 , 40 , 2520–2527. [ Google Scholar ]
- Gallo, A.; Bisecco, A.; Bonavita, S.; Tedeschi, G. Functional plasticity of the visual system in multiple sclerosis. Front. Neurol. 2015 , 6 , 79. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Filippi, M.; Rocca, M.A. Multiple Sclerosis. In White Matter Diseases , 1st ed.; Filippi, M., Rocca, M.A., Eds.; Springer: Cham, Switzerland, 2020; Volume 1, pp. 1–35. [ Google Scholar ]
- Dasenbrock, H.H.; Smith, S.A.; Ozturk, A.; Farrell, S.K.; Calabresi, P.A.; Reich, D.S. Diffusion tensor imaging of the optic tracts in multiple sclerosis: Association with retinal thinning and visual disability. J. Neuroimaging 2011 , 21 , e41–e49. [ Google Scholar ] [ CrossRef ]
- Gabilondo, I.; Martínez-Lapiscina, E.H.; Martínez-Heras, E.; Fraga-Pumar, E.; Llufriu, S.; Ortiz, S.; Bullich, S.; Sepulveda, M.; Falcon, C.; Berenguer, J.; et al. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann. Neurol. 2014 , 75 , 98–107. [ Google Scholar ] [ CrossRef ]
- Saidha, S.; Calabresi, P.A. Optical coherence tomography should be part of the routine monitoring of patients with multiple sclerosis: Yes. Mult. Scler. 2014 , 20 , 1296–1298. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Martínez-Lapiscina, E.H.; Sanchez-Dalmau, B.; Fraga-Pumar, E.; Ortiz-Perez, S.; Tercero-Uribe, A.I.; Torres-Torres, R.; Villoslada, P. The visual pathway as a model to understand brain damage in multiple sclerosis. Mult. Scler. 2014 , 20 , 1678–1685. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Donaldson, L.; Margolin, E. Visual fields and optical coherence tomography (OCT) in neuro-ophthalmology: Structure-function correlation. J. Neurol. Sci. 2021 , 429 , 118064. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vidović, T.; Cerovski, B.; Vidović, D.H.; Cerovski, J.; Novak-Laus, K. Inapparent visual field defects in multiple sclerosis patients. Coll. Antropol. 2005 , 29 (Suppl. S1), 67–73. [ Google Scholar ]
- Banc, A.; Kedar, S. Interpretation of the visual field in neuro-ophthalmic disorders. Curr. Neurol. Neurosci. Rep. 2024 , 24 , 67–81. [ Google Scholar ] [ CrossRef ]
- Minakaran, N.; de Carvalho, E.R.; Petzold, A.; Wong, S.H. Optical coherence tomography (OCT) in neuro-ophthalmology. Eye 2021 , 35 , 17–32. [ Google Scholar ] [ CrossRef ]
- Wilson, S.W.; Houart, C. Early steps in the development of the forebrain. Dev. Cell 2004 , 6 , 167–181. [ Google Scholar ] [ CrossRef ]
- Cordano, C.; Werneburg, S.; Abdelhak, A.; Bennett, D.J.; Beaudry-Richard, A.; Duncan, G.J.; Oertel, F.C.; Boscardin, W.J.; Yiu, H.H.; Jabassini, N.; et al. Synaptic injury in the inner plexiform layer of the retina is associated with progression in multiple sclerosis. Cell Rep. Med. 2024 , 5 , 101490. [ Google Scholar ] [ CrossRef ]
- Petzold, A.; Balcer, L.J.; Calabresi, P.A.; Costello, F.; Frohman, T.C.; Frohman, E.M.; Martinez-Lapiscina, E.H.; Green, A.J.; Kardon, R.; Outteryck, O.; et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2017 , 16 , 797–812. [ Google Scholar ] [ CrossRef ]
- Saidha, S.; Sotirchos, E.S.; Ibrahim, M.A.; Crainiceanu, C.M.; Gelfand, J.M.; Sepah, Y.J.; Ratchford, J.N.; Oh, J.; Seigo, M.A.; Newsome, S.D.; et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: A retrospective study. Lancet Neurol. 2012 , 11 , 963–972. [ Google Scholar ] [ CrossRef ]
- Cellerino, M.; Cordano, C.; Boffa, G.; Bommarito, G.; Petracca, M.; Sbragia, E.; Novi, G.; Lapucci, C.; Capello, E.; Uccelli, A.; et al. Relationship between retinal inner nuclear layer, age, and disease activity in progressive MS. Neurol. Neuroimmunol. Neuroinflamm. 2019 , 6 , e596. [ Google Scholar ] [ CrossRef ]
- Gelfand, J.M.; Nolan, R.; Schwartz, D.M.; Graves, J.; Green, A.J. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain 2012 , 135 Pt 6 , 1786–1793. [ Google Scholar ] [ CrossRef ]
- Gabilondo, I.; Martínez-Lapiscina, E.H.; Fraga-Pumar, E.; Ortiz-Perez, S.; Torres-Torres, R.; Andorra, M.; Llufriu, S.; Zubizarreta, I.; Saiz, A.; Sanchez-Dalmau, B.; et al. Dynamics of retinal injury after acute optic neuritis. Ann. Neurol. 2015 , 77 , 517–528. [ Google Scholar ] [ CrossRef ]
- Balk, L.J.; Coric, D.; Knier, B.; Zimmermann, H.G.; Behbehani, R.; Alroughani, R.; Martinez-Lapiscina, E.H.; Brandt, A.U.; Sánchez-Dalmau, B.; Vidal-Jordana, A.; et al. Retinal inner nuclear layer volume reflects inflammatory disease activity in multiple sclerosis; a longitudinal OCT study. Mult. Scler. J. Exp. Transl. Clin. 2019 , 5 , 2055217319871582. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Green, A.J.; McQuaid, S.; Hauser, S.L.; Allen, I.V.; Lyness, R. Ocular pathology in multiple sclerosis: Retinal atrophy and inflammation irrespective of disease duration. Brain 2010 , 133 Pt 6 , 1591–1601. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cordano, C.; Yiu, H.H.; Oertel, F.C.; University of California, San Francisco MS-EPIC Team; Gelfand, J.M.; Hauser, S.L.; Cree, B.A.C.; Green, A.J. Retinal INL Thickness in Multiple Sclerosis: A Mere Marker of Neurodegeneration? Ann. Neurol. 2021 , 89 , 192–193. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Satue, M.; Obis, J.; Rodrigo, M.J.; Otin, S.; Fuertes, M.I.; Vilades, E.; Gracia, H.; Ara, J.R.; Alarcia, R.; Polo, V.; et al. Optical coherence tomography as a biomarker for diagnosis, progression, and prognosis of neurodegenerative diseases. J. Ophthalmol. 2016 , 2016 , 8503859. [ Google Scholar ] [ CrossRef ]
- Martinez-Lapiscina, E.H.; Arnow, S.; Wilson, J.A.; Saidha, S.; Preiningerova, J.L.; Oberwahrenbrock, T.; Brandt, A.U.; Pablo, L.E.; Guerrieri, S.; Gonzalez, I.; et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: A cohort study. Lancet Neurol. 2016 , 15 , 574–584. [ Google Scholar ] [ CrossRef ]
- Cordano, C.; Nourbakhsh, B.; Devereux, M.; Damotte, V.; Bennett, D.; Hauser, S.L.; Cree, B.A.C.; Gelfand, J.M.; Green, A.J. pRNFL as a marker of disability worsening in the medium/long term in patients with MS. Neurol. Neuroimmunol. Neuroinflamm. 2018 , 6 , e533. [ Google Scholar ] [ CrossRef ]
- Cordano, C.; Nourbakhsh, B.; Yiu, H.H.; Papinutto, N.; Caverzasi, E.; Abdelhak, A.; Oertel, F.C.; Beaudry-Richard, A.; Santaniello, A.; Sacco, S.; et al. Differences in Age-related Retinal and Cortical Atrophy Rates in Multiple Sclerosis. Neurology 2022 , 99 , e1685–e1693. [ Google Scholar ] [ CrossRef ]
- Ehrhardt, H.; Lambe, J.; Moussa, H.; Vasileiou, E.S.; Kalaitzidis, G.; Murphy, O.C.; Filippatou, A.G.; Pellegrini, N.; Douglas, M.; Davis, S.; et al. Effects of Ibudilast on Retinal Atrophy in Progressive Multiple Sclerosis Subtypes: Post Hoc Analyses of the SPRINT-MS Trial. Neurology 2023 , 101 , e1014–e1024. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Green, A.J.; Gelfand, J.M.; Cree, B.A.; Bevan, C.; Boscardin, W.J.; Mei, F.; Inman, J.; Arnow, S.; Devereux, M.; Abounasr, A.; et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): A randomised, controlled, double-blind, crossover trial. Lancet 2017 , 390 , 2481–2489. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Klistorner, A.; Garrick, R.; Barnett, M.H.; Graham, S.L.; Arvind, H.; Sriram, P.; Yiannikas, C. Axonal loss in non-optic neuritis eyes of patients with multiple sclerosis linked to delayed visual evoked potential. Neurology 2013 , 15 , 242–245. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gorczyca, W.A.; Ejma, M.; Witkowska, D.; Misiuk-Hojło, M.; Kuropatwa, M.; Mulak, M.; Szymaniec, S. Retinal antigens are recognized by antibodies present in sera of patients with multiple sclerosis. Ophthalmic Res. 2004 , 36 , 120–123. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mühlemann, F.; Grabe, H.; Fok, A.; Wagner, F.; Brugger, D.; Sheldon, C.A.; Abegg, M. Homonymous hemiatrophy of ganglion cell layer from retrochiasmal lesions in the visual pathway. Neurology 2020 , 94 , e323–e329. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018 , 17 , 162–173. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wu, C.W.; Chen, H.Y.; Chen, J.Y.; Lee, C.H. Glaucoma detection using support vector machine method based on Spectralis OCT. Diagnostics 2022 , 12 , 391. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cujba, L.; Stan, C.; Samoila, O.; Drugan, T.; Benedec Cutas, A.; Nicula, C. Identifying optical coherence tomography markers for multiple sclerosis diagnosis and management. Diagnostics 2023 , 13 , 2077. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Petzold, A.; Chua, S.Y.L.; Khawaja, A.P.; Keane, P.A.; Khaw, P.T.; Reisman, C.; Dhillon, B.; Strouthidis, N.G.; Foster, P.J.; Patel, P.J.; et al. Retinal asymmetry in multiple sclerosis. Brain 2021 , 144 , 224–235. [ Google Scholar ] [ CrossRef ]
- Riederer, I.; Mühlau, M.; Hoshi, M.M.; Zimmer, C.; Kleine, J.F. Detecting optic nerve lesions in clinically isolated syndrome and multiple sclerosis: Double-inversion recovery magnetic resonance imaging in comparison with visually evoked potentials. J. Neurol. 2019 , 266 , 148–156. [ Google Scholar ] [ CrossRef ]
- Bock, M.; Paul, F.; Dörr, J. Diagnosis and monitoring of multiple sclerosis: The value of optical coherence tomography. Nervenarzt 2013 , 84 , 483–492. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vidal-Jordana, Á.; Sastre-Garriga, J.; Montalban, X. Optical coherence tomography in multiple sclerosis. Rev. Neurol. 2012 , 54 , 556–563. [ Google Scholar ] [ PubMed ]
- Petzold, A.; de Boer, J.F.; Schippling, S.; Vermersch, P.; Kardon, R.; Green, A.; Calabresi, P.A.; Polman, C. Optical coherence tomography in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2010 , 9 , 921–932. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Costello, F.; Hodge, W.; Pan, Y.I.; Freedman, M.; DeMeulemeester, C. Differences in retinal nerve fiber layer atrophy between multiple sclerosis subtypes. J. Neurol. Sci. 2009 , 281 , 74–79. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Balk, L.J.; Tewarie, P.; Killestein, J.; Polman, C.H.; Uitdehaag, B.; Petzold, A. Disease course heterogeneity and OCT in multiple sclerosis. Mult. Scler. 2014 , 20 , 1198–1206. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Petracca, M.; Cordano, C.; Cellerino, M.; Button, J.; Krieger, S.; Vancea, R.; Ghassemi, R.; Farrell, C.; Miller, A.; Calabresi, P.A.; et al. Retinal degeneration in primary-progressive multiple sclerosis: A role for cortical lesions? Mult. Scler. 2017 , 23 , 43–50. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zehnder, S.; Wildberger, H.; Hanson, J.V.M.; Lukas, S.; Pelz, S.; Landau, K.; Wichmann, W.; Gerth-Kahlert, C. Retinal ganglion cell topography in patients with visual pathway pathology. J. Neuroophthalmol. 2018 , 38 , 172–178. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hokazono, K.; Ribeiro Monteiro, M.L. Homonymous quadrantic macular ganglion cell complex loss as a sign of trans-synaptic degeneration from occipital lobe lesion. Am. J. Ophthalmol. Case Rep. 2018 , 13 , 76–79. [ Google Scholar ] [ CrossRef ]
- Tieger, M.G.; Hedges, T.R., 3rd; Ho, J.; Erlich-Malona, N.K.; Vuong, L.N.; Athappilly, G.K.; Mendoza-Santiesteban, C.E. Ganglion cell complex loss in chiasmal compression by brain tumors. J. Neuroophthalmol. 2017 , 37 , 7–12. [ Google Scholar ] [ CrossRef ]
- Dinkin, M. Trans-synaptic retrograde degeneration in the human visual system: Slow, silent, and real. Curr. Neurol. Neurosci. Rep. 2017 , 17 , 16. [ Google Scholar ] [ CrossRef ]
- Marshall, H.N.; Andrew, N.H.; Hassall, M.; Qassim, A.; Souzeau, E.; Ridge, B.; Nguyen, T.; Fitzgerald, J.; Awadalla, M.S.; Burdon, K.P.; et al. Macular ganglion cell-inner plexiform layer loss precedes peripapillary retinal nerve fiber layer loss in glaucoma with lower intraocular pressure. Ophthalmology 2019 , 126 , 1119–1130. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhang, X.; Dastiridou, A.; Francis, B.A.; Tan, O.; Varma, R.; Greenfield, D.S.; Schuman, J.S.; Huang, D. Advanced imaging for Glaucoma Study Group. Comparison of glaucoma progression detection by optical coherence tomography and visual field. Am. J. Ophthalmol. 2017 , 184 , 63–74. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Balcer, L.J.; Balk, L.J.; Brandt, A.U.; Calabresi, P.A.; Martinez-Lapiscina, E.H.; Nolan, R.C.; Paul, F.; Petzold, A.; Saidha, S. The International Multiple Sclerosis Visual System Consortium: Advancing visual system research in multiple sclerosis. J. Neuroophthalmol. 2018 , 38 , 494–501. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Huang-Link, Y.M.; Al-Hawasi, A.; Eveman, I. Retrograde degeneration of visual pathway: Hemimacular thinning of retinal ganglion cell layer in progressive and active multiple sclerosis. J. Neurol. 2014 , 261 , 2453–2456. [ Google Scholar ] [ CrossRef ]
- Al-Louzi, O.; Button, J.; Newsome, S.D.; Calabresi, P.A.; Saidha, S. Retrograde trans-synaptic visual pathway degeneration in multiple sclerosis: A case series. Mult. Scler. 2017 , 23 , 1035–1039. [ Google Scholar ] [ CrossRef ]
- Lukewich, M.K.; Schlenker, M.B.; Micieli, J.A. Homonymous hemi-macular atrophy of the ganglion cell-inner plexiform layer with preserved visual function. J. Neurol. Sci. 2020 , 417 , 117072. [ Google Scholar ] [ CrossRef ]
- Ilardi, M.; Nolan-Kenney, R.; Fatterpekar, G.; Hasanaj, L.; Serrano, L.; Joseph, B.; Wu, S.; Rucker, J.C.; Balcer, L.J.; Galetta, S.L. Role for OCT in detecting hemi-macular ganglion cell layer thinning in patients with multiple sclerosis and related demyelinating diseases. J. Neurol. Sci. 2020 , 419 , 117159. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Remington, L.A. (Ed.) Chapter 13—Visual Pathway. In Clinical Anatomy and Physiology of the Visual System , 3rd ed.; Butterworth-Heinemann: Oxford, UK, 2012; pp. 233–252. [ Google Scholar ]
- Huang, S.C.; Pisa, M.; Guerrieri, S.; Dalla Costa, G.; Comi, G.; Leocani, L. Optical coherence tomography with voxel-based morphometry: A new tool to unveil focal retinal neurodegeneration in multiple sclerosis. Brain Commun. 2023 , 6 , fcad249. [ Google Scholar ] [ CrossRef ]
- Kedar, S.; Ghate, D.; Corbett, J.J. Visual fields in neuro-ophthalmology. Indian. J. Ophthalmol. 2011 , 59 , 103–109. [ Google Scholar ] [ CrossRef ]
- Hepworth, L.R.; Rowe, F.J. Programme choice for perimetry in neurological conditions (PoPiN): A systematic review of perimetry options and patterns of visual field loss. BMC Ophthalmol. 2018 , 18 , 241. [ Google Scholar ] [ CrossRef ]
- Ortiz-Perez, S.; Andorra, M.; Sanchez-Dalmau, B.; Torres-Torres, R.; Calbet, D.; Lampert, E.J.; Alba-Arbalat, S.; Guerrero-Zamora, A.M.; Zubizarreta, I.; Sola-Valls, N.; et al. Visual field impairment captures disease burden in multiple sclerosis. J. Neurol. 2016 , 263 , 695–702. [ Google Scholar ] [ CrossRef ]
- Chorazy, M.; Drozdowski, W.; Sherkawey, N.; Mariak, Z. Asymptomatic visual field disturbances in multiple sclerosis patients without a history of optic neuritis. Neurol. Neurochir. Pol. 2007 , 41 , 223–228. [ Google Scholar ]
- Sriram, P.; Wang, C.; Yiannikas, C.; Garrick, R.; Barnett, M.; Parratt, J.; Graham, S.L.; Arvind, H.; Klistorner, A. Relationship between optical coherence tomography and electrophysiology of the visual pathway in non-optic neuritis eyes of multiple sclerosis patients. PLoS ONE 2014 , 9 , e102546. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Villoslada, P.; Cuneo, A.; Gelfand, J.; Hauser, S.L.; Green, A. Color vision is strongly associated with retinal thinning in multiple sclerosis. Mult. Scler. 2012 , 18 , 991–999. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Trip, S.A.; Schlottmann, P.; Jones, S.J.; Altmann, D.R.; Garway-Heath, D.F.; Thompson, A.J.; Plant, G.T.; Miller, D.H. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann. Neurol. 2005 , 58 , 383–391. [ Google Scholar ] [ CrossRef ]
- Kitsos, G.; Detorakis, E.T.; Papakonstantinou, S.; Kyritsis, A.P.; Pelidou, S.H. Perimetric and peri-papillary nerve fibre layer thickness findings in multiple sclerosis. Eur. J. Neurol. 2011 , 18 , 719–725. [ Google Scholar ] [ CrossRef ]
- Walter, S.D.; Ishikawa, H.; Galetta, K.M.; Sakai, R.E.; Feller, D.J.; Henderson, S.B.; Wilson, J.A.; Maguire, M.G.; Galetta, S.L.; Frohman, E.; et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 2012 , 119 , 1250–1257. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lampert, E.J.; Andorra, M.; Torres-Torres, R.; Ortiz-Pérez, S.; Llufriu, S.; Sepúlveda, M.; Sola, N.; Saiz, A.; Sánchez-Dalmau, B.; Villoslada, P.; et al. Color vision impairment in multiple sclerosis points to retinal ganglion cell damage. J. Neurol. 2015 , 262 , 2491–2497. [ Google Scholar ] [ CrossRef ]
- Friese, M.A.; Schattling, B.; Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2014 , 10 , 225–238. [ Google Scholar ] [ CrossRef ]
- Cheng, H.; Laron, M.; Schiffman, J.S.; Tang, R.A.; Frishman, L.J. The relationship between visual field and retinal nerve fiber layer measurements in patients with multiple sclerosis. Investig. Ophthalmol. Vis. Sci. 2007 , 48 , 5798–5805. [ Google Scholar ] [ CrossRef ]
- Castro, S.M.; Damasceno, A.; Damasceno, B.P.; Vasconcellos, J.P.; Reis, F.; Iyeyasu, J.N.; Carvalho, K.M.D. Visual pathway abnormalities were found in most multiple sclerosis patients despite history of previous optic neuritis. Arq. Neuropsiquiatr. 2013 , 71 , 437–441. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schmutz, L.; Borruat, F.X. Homonymous visual field defects in patients with multiple sclerosis: Results of computerised perimetry and optical coherence tomography. Swiss Med. Wkly. 2020 , 150 , w20319. [ Google Scholar ] [ CrossRef ] [ PubMed ]
| Variable | Patients n = 52 | Controls n = 27 |
|---|---|---|
| Age (years), mean ± SD | 35.07 ± 12.73 | 31.85 ± 10.97 |
| Gender, men n (%) | 12 (23%) | 6 (22.2%) |
| Clinical form of MS, n (%) • RRMS • CIS | 45 (86.54%) 7 (13.46%) | 0 0 |
| Time since MS diagnosis (years), mean ± SD | 4.3 ± 5.39 | 0 |
| History of ON, n (%) | 16 (30.7%) | 0 |
| Comparison | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | McNemar Test p-Value |
|---|---|---|---|---|---|
| GGL vs. MRI | 0.39 | 0.68 | 0.78 | 0.27 | <0.0001 |
| Corrected GGL vs. MRI | 0.40 | 0.63 | 0.76 | 0.26 | <0.0001 |
| IPL vs. MRI | 0.45 | 0.68 | 0.80 | 0.30 | <0.0001 |
| Corrected IPL vs. MRI | 0.48 | 0.68 | 0.81 | 0.31 | 0.0001 |
| Comparison | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | McNemar Test p-Value |
|---|---|---|---|---|---|
| VF vs. MRI | 0.14 | 0.62 | 0.50 | 0.22 | <0.0001 |
| Comparison | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | McNemar Test p-Value |
|---|---|---|---|---|---|
| GGL vs. VF | 0.33 | 0.73 | 0.25 | 0.80 | 0.36 |
| Corrected GGL vs. VF | 0.38 | 0.74 | 0.29 | 0.81 | 0.34 |
| IPL vs. VF | 0.33 | 0.77 | 0.28 | 0.81 | 0.70 |
| Corrected IPL vs. VF | 0.38 | 0.77 | 0.31 | 0.82 | 0.55 |
| Comparison | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | McNemar Test p-Value |
|---|---|---|---|---|---|
| GGL vs. MRI (ipsilateral lesion) | 0.39 | 0.66 | 0.68 | 0.37 | 0.0005 |
| Corrected GGL vs. MRI (ipsilateral lesion) | 0.39 | 0.60 | 0.64 | 0.34 | 0.002 |
| IPL vs. MRI (ipsilateral lesion) | 0.48 | 0.70 | 0.75 | 0.42 | 0.002 |
| Corrected IPL vs. MRI (ipsilateral lesion) | 0.50 | 0.66 | 0.73 | 0.41 | 0.005 |
| GGL vs. MRI (contralateral lesion) | 0.41 | 0.73 | 0.78 | 0.35 | <0.0001 |
| Corrected GGL vs. MRI (contralateral lesion) | 0.43 | 0.69 | 0.76 | 0.34 | 0.0001 |
| IPL vs. MRI (contralateral lesion) | 0.46 | 0.69 | 0.77 | 0.36 | 0.0003 |
| Corrected IPL vs. MRI (contralateral lesion) | 0.50 | 0.69 | 0.78 | 0.37 | 0.0007 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Cujbă, L.; Banc, A.; Stan, C.; Drugan, T.; Nicula, C. Macular OCT’s Proficiency in Identifying Retrochiasmal Visual Pathway Lesions in Multiple Sclerosis—A Pilot Study. Diagnostics 2024 , 14 , 1221. https://doi.org/10.3390/diagnostics14121221
Cujbă L, Banc A, Stan C, Drugan T, Nicula C. Macular OCT’s Proficiency in Identifying Retrochiasmal Visual Pathway Lesions in Multiple Sclerosis—A Pilot Study. Diagnostics . 2024; 14(12):1221. https://doi.org/10.3390/diagnostics14121221
Cujbă, Larisa, Ana Banc, Cristina Stan, Tudor Drugan, and Cristina Nicula. 2024. "Macular OCT’s Proficiency in Identifying Retrochiasmal Visual Pathway Lesions in Multiple Sclerosis—A Pilot Study" Diagnostics 14, no. 12: 1221. https://doi.org/10.3390/diagnostics14121221
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Case Report
- Open access
- Published: 05 June 2024
Vitamin D add on the standard treatment for myasthenia gravis symptoms following total gastrectomy: a case report
- Tao Zhang 1 , 2 ,
- Junhong Zhong 1 ,
- Jingqing Sun 1 ,
- Yingxue Cui 1 &
- Shaosong Wang 1
BMC Neurology volume 24 , Article number: 188 ( 2024 ) Cite this article
184 Accesses
Metrics details
Myasthenia gravis (MG) is a long-term autoimmune disorder that affects the neuromuscular junction, causing muscle weakness and fatigue as its primary clinical features. Vitamin D is crucial for both the autoimmune response and skeletal muscle function.
Case presentation
Here, we presented a case report documenting the substantial improvement in symptoms experienced by a patient who underwent subtotal gastrectomy for gastric cancer following high-dose Vitamin D supplementation. The patient developed generalized MG two months after the surgery and did not respond adequately to pyridostigmine therapy, experiencing a progressive deterioration of the condition. A significant reduction in vitamin D concentration was observed following subtotal gastrectomy. In response, high-dose vitamin D supplementation was administered to the patient. Within one week of treatment, swallowing symptoms improved, enabling the consumption of a small amount of liquid food. By the second week, substantial swallowing and neck function improvements were evident. After one month, the patient regained the ability to straighten the neck while walking and consumed a regular diet despite persistent difficulties chewing hard food.
Conclusions
This case underscores the therapeutic potential of vitamin D in alleviating MG symptoms, particularly in individuals with compromised vitamin D levels following gastrectomy. The observed improvements present a new perspective on the possible involvement of vitamin D supplementation in the management of postoperative MG cases.
Peer Review reports
Myasthenia gravis (MG) is an autoimmunological condition impacting neuromuscular junction postsynaptic membrane acetylcholine signaling, mediated by antibodies including acetylcholine receptor (AchR) antibody, cellular immunity, and complement. It presents as acquired skeletal muscle weakness [ 1 ]. The participation of cytokines and activated T and B cells is crucial in producing pathogenic autoantibodies and the onset of inflammation at the neuromuscular junction in MG [ 2 ]. The activation of the immune response by dysfunctional regulatory T cells (Tregs)has been shown to contribute to the exacerbation of MG pathogenesis potentially, highlighting the significant involvement of T cells in the disease’s development and progression [ 3 ]. Research indicates that the defect in immune regulation in MG is predominantly localized in isolated Tregs [ 4 , 5 ].
The primary metabolites of vitamin D, 25-hydroxyvitamin D [25(OH)D] and 1,25-dihydroxyvitamin D [1,25(OH)2D], exhibit potent immunomodulatory properties. Vitamin D’s immunomodulatory effects include direct inhibition of effector T cells and induction of Tregs to suppress the production of inflammatory cytokines, indicating its potential significance in T-cell regulation [ 6 ]. Vitamin D deficiency is associated with various chronic autoimmune diseases such as multiple sclerosis (MS) [ 7 ], systemic lupus erythematosus (SLE) [ 8 ], and rheumatoid arthritis (RA) [ 9 ]. Recent studies and systematic reviews have demonstrated significantly lower plasma 25(OH)D levels in patients with MG relative to healthy controls, suggesting the importance of monitoring 25(OH)D levels in MG patients [ 10 ].
Limited evidence currently links vitamin D deficiency with the initiation or deterioration of MG. However, we present a case of a patient who developed gastric cancer and subsequently underwent total gastrectomy. Two months after the surgery, the patient experienced facial and systemic muscle weakness, leading to a diagnosis of MG based on symptoms, signs, immunologic tests, and electrophysiological examination. Despite initial treatment with pyridostigmine bromide, the patient’s clinical response was inadequate, and symptoms deteriorated progressively. Prior research has established a link between total gastrectomy and vitamin D deficiency [ 11 , 12 ] and an connection between MG symptoms and vitamin D levels [ 13 ]. Given the potential impact of total gastrectomy on vitamin D absorption, the patient’s serum 25-hydroxyvitamin D level was found to be notably reduced. Subsequent administration of high-dose vitamin D resulted in a marked improvement in symptoms and enhanced muscular functions. This case report offers valuable insights into how vitamin D supplementation may potentially manage postoperative MG cases.
- Case report
In April 2021, a 62-year-old male patient was diagnosed with gastric cancer and subsequently underwent multiple chemotherapy treatments at Peking Union Medical College Hospital. On January 10, 2022, he underwent a total gastrectomy at the same hospital. In early March 2022, the patient began experiencing blepharoptosis, followed by progressive dysphagia, neck weakness, and fatigue, exacerbated after physical exertion. Symptoms were milder in the morning and worsened in the evening, prompting the patient to seek medical attention at Peking Union Medical College Hospital, where he underwent relevant examinations. At admission, a paraneoplastic screen was performed and found negative for paraneoplastic syndrome. The head and neck magnetic resonance imaging (MRI) and computerized tomography angiography (CTA) revealed no abnormalities in intracranial lesions or vascular malformations. Thymus computerized tomography (CT) also showed no abnormalities. Electromyographic examination indicated stimulation of the bilateral facial nerve and accessory nerve by repetitive frequency stimulation, and the neostigmine test yielded a positive result, leading to a diagnosis of MG. The patient was prescribed oral pyridostigmine bromide at a dose of 60 mg three times a day, which initially relieved subjective symptoms. However, after two weeks, the patient experienced worsening symptoms, including difficulty straightening the neck and swallowing, faint and low voice, and overall weakness. Following daily activities, the patient’s symptoms deteriorated, necessitating the use of a wheelchair when venturing outside. Despite the addition of 240 mg of pyridostigmine bromide daily, the symptoms were not alleviated.
On April 1st, 2022, the patient was admitted to our department for inpatient care and denied a history of hypertension, other immune system disorders, and exposure to potentially harmful medications. The patient also reported regular outdoor activities to receive sunlight. Due to the potential risk of gastrointestinal bleeding post-total gastrectomy, glucocorticoid treatment was deemed unsuitable, and gamma globulin therapy was declined due to financial constraints.
To investigate a potential causal relationship between vitamin D, total gastrectomy and MG in the patient [ 11 , 12 , 13 ], we conducted tests to analyze the spectrum of acetylcholine receptor antibodies and the serum vitamin D level. The patient’s AchR antibody value (measured by enzyme-linked immunosorbent assay, ELISA) was 1.865 nmol/L, significantly exceeding the normal range of < 0.625 nmol/L. The Titin antibody value (ELISA) was 0.504 nmol/L, also significantly higher than the normal range of < 0.472 nmol/L. Additionally, compared to the typical range of > 30 ng/ml, the serum vitamin D (25-OH) level was 6.64 ng/ml, signifying a significant deficiency.
After observing a significant decline in the patient’s vitamin D levels, we commenced high-dose vitamin D supplementation. With the patient weighing 47 kg, we started with a daily dosage of 2400 IU of vitamin D in the first week, subsequently elevating the dosage to 4000 IU daily after seven days. After a month, the supplementation was reduced to 2400 IU daily for maintenance. Simultaneously, the patient continued the regimen of pyridostigmine bromide at a dosage of 60 mg thrice daily.
The severity of symptoms before and after treatment was assessed using the Absolute and Relative Score of MG (ARS-MG) scoring scale [ 14 ]. The relationship between the changes in the severity of symptoms and vitamin (25-OH) D concentration is illustrated in Fig. 1 . As the treatment went on, the vitamin (25-OH) D concentration got back to normal range, and the symptoms showed great improvements (the ARS-MG score decreased from 18 [before treatment] to 2 [after treatment] ). After one week of treatment, the patient experienced improved swallowing symptoms and was able to consume a small amount of liquid food. By the end of the second week, there was a notable enhancement in swallowing and neck function. After one month of medication, the patient could walk with a straightened neck and consume a regular diet, albeit with ongoing challenges in chewing hard food. By the third month, the patient could independently walk 300–500 m without rest and had improved chewing ability. The strength of the neck-lifting muscle had also recovered, enabling the patient to perform daily activities without difficulty. The dosage of vitamin D remained unchanged, and symptom progression was continuously monitored. A re-evaluation of vitamin D concentration (25-OH) three months later revealed a level of 39.3 ng/mL (normal range: >30 ng/ml).

Relationship between the changes in ARS-MG scores and Vit25(OH)D dosage throughout treatment
The MG-activities of daily living profile (MG-ADL) was also used to assess the patient before and after the treatment. Before treatment, the MG-ADL score was 18, and decreased to 15 after one-week treatment. The MG-ADL scores were 8, 3, and 1 respectively at the 2nd week, 4th week, and 12th week after the treatment. The MG-ADL score changes indicated the great improvement of the patient.
MG is generally an autoimmune condition that is caused by autoantibodies targeting the AChR. The role of anti-AChR antibodies in the development of MG has been firmly established, and the production of these autoantibodies relies on T-cell activity. It is crucial to regulate potentially pathogenic T cells, and the Treg is one of the primary regulators of effector T cells [ 5 ]. A mounting body of evidence has demonstrated that Tregs are functionally defective in patients with MG, emphasizing their pivotal role in immune regulation. Recent studies in healthy individuals have supported the hypothesized immune-regulating impact of vitamin D, which is believed to be connected to increased regulatory T cells [ 10 , 15 ].
Vitamin D has potential relationship with MG: first, it modulates autoimmune responses, and second, it maintains muscle function by acting on vitamin D receptors in muscle [ 16 ]. Experimental studies have demonstrated that 25(OH)D, the metabolically active form of vitamin D, exerts its immunomodulatory effects by increasing the number of Tregs [ 17 ]. Additionally, it is thought that 1,25(OH)2D3 works by reducing proinflammatory interleukin-2 and interferon-γ expression, inhibiting the proliferation and differentiation of T helper cell 1, regulating cytokine production, stimulating T helper cell two by upregulating anti-inflammatory cytokine production, inhibiting the development of Th17 cells, and inhibiting interleukin-17, which induces the proliferation of regulatory T cells [ 18 ]. Therefore, studies suggest a link between vitamin D concentration and the onset of MG. Compared with healthy individuals, MG patients are at a higher risk of having insufficient levels of vitamin D, and those with more severe diseases tend to have even lower vitamin D levels [ 19 ]. Further research is necessary to determine how vitamin D contributes to the pathogenesis of MG and the potential benefits of vitamin D supplementation.
Some studies have shown that the vitamin D receptor (VDR) acts as a transcription factor and the immunomodulatory impact of 1,25(OH)2D3 is facilitated through its binding to VDR [ 20 ]. Notably, the VDR gene Tru9I (rs757343) polymorphism has been closely linked to the susceptibility of MG in females aged over 15 years [ 21 , 22 ]. Furthermore, in the Chinese Han population, there is a potential association between rs731236 and adult non-thymoma AChRAb-negative MG patients, implying a connection between vitamin D and the onset of MG [ 23 , 24 ].
The initial clinical study on vitamin D deficiency in individuals with MG was published in 2012 [ 25 ]. The findings revealed that MG patients had significantly lower plasma 25(OH)D levels in comparison to healthy controls, attributed to reduced sun exposure and high body mass index (BMI). Administering vitamin D3 supplementation at a dosage of 800 IU/day for 2.5–10 months (mean 6 months) to MG patients without prior vitamin D3 supplementation demonstrated positive effects on autoimmune response and fatigue scores. Subsequent research has suggested a potential effect for vitamin D in MG. However, the available information on the vitamin D status and the recommended supplemental dosage for MG patients is limited and inconclusive. Some studies investigating vitamin D in individuals with MG have reported contradictory findings regarding the prevalence of deficient or insufficient vitamin D levels. Some studies have produced conflicting results and have failed to consider the association between outdoor activities and vitamin D intake [ 26 , 27 , 28 , 29 ].
The patient was administered a daily vitamin D supplementation, with the dosage ranging from 2400 to 4000 IU. However, high-dose vitamin D therapy for autoimmune diseases does not have sufficient evidence to prove its long-term safety. Although trials are investigating supraphysiological doses of vitamin D for MS, similar studies for other conditions are limited [ 13 ]. The 2022 guidelines for MG do not provide specific recommendations for the treatment of vitamin D, and the vitamin D status in MG patients remains uncertain [ 30 , 31 ]. Existing literature suggests that patients on long-term high-dose vitamin D, usually between 80,000 and 120,000 IU/day, do not experience serious adverse reactions [ 13 ]. However, no research currently supports larger doses, and further investigation is necessary.
In this reported case, the patient had a documented history of total gastrectomy, which is frequently linked to reduced vitamin D levels. High-dose vitamin D treatment was administered without interference with concurrent medications, resulting in an evident improvement of the patient’s muscle weakness symptoms. While this article constitutes a single case report and thus cannot furnish conclusive evidence for clinical practice, it suggests that physicians should consider monitoring and supplementing vitamin D levels in patients exhibiting MG symptoms following gastrointestinal surgery. This approach may yield improved clinical outcomes and increased longevity.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Myasthenia gravis
Acetylcholine receptor
25-hydroxyvitamin D
Multiple sclerosis
Systemic lupus erythematosus
Rheumatoid arthritis
Magnetic resonance imaging
Computerized tomography angiography
Narayanaswami P, Sanders DB, Wolfe G, Benatar M, Cea G, Evoli A, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021;96(3):114–22.
Article PubMed PubMed Central Google Scholar
Dalakas MC. Immunotherapy in myasthenia gravis in the era of biologics. Nat Reviews Neurol. 2019;15(2):113–24.
Article Google Scholar
Nie J, Li YY, Zheng SG, Tsun A, Li B. FOXP3 + Treg cells and gender bias in autoimmune diseases. Front Immunol. 2015;6:493.
Tarighi M, Shahbazi M, Saadat P, Daraei A, Rahimifard K, Mohammadnia-Afrouzi M. Decreased frequency of regulatory T cells and level of helios gene expression in secondary progressive multiple sclerosis patients: evidence about the development of multiple sclerosis. Int Immunopharmacol. 2023;116:109797.
Article CAS PubMed Google Scholar
Thiruppathi M, Rowin J, Ganesh B, Sheng JR, Prabhakar BS, Meriggioli MN. Impaired regulatory function in circulating CD4 + CD25highCD127low/– T cells in patients with myasthenia gravis. Clin Immunol. 2012;145(3):209–23.
Article CAS PubMed PubMed Central Google Scholar
Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, et al. 1, 25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183(9):5458–67.
Moosazadeh M, Nabinezhad-Male F, Afshari M, Nasehi MM, Shabani M, Kheradmand M, et al. Vitamin D status and disability among patients with multiple sclerosis: a systematic review and meta-analysis. AIMS Neurosci. 2021;8(2):239.
Correa-Rodríguez M, Pocovi-Gerardino G, Callejas-Rubio J-L, Ríos-Fernández R, Martín-Amada M, Cruz-Caparrós M-G, et al. Vitamin D levels are associated with disease activity and damage accrual in systemic lupus erythematosus patients. Biol Res Nurs. 2021;23(3):455–63.
Article PubMed Google Scholar
Romão VC, Fonseca JE. Etiology and risk factors for rheumatoid arthritis: a state-of-the-art review. Frontiers in Medicine. 2021; 8(689698.
Bonaccorso G. Myasthenia Gravis, Vitamin D Serum, levels: a systematic review and Meta-analysis. CNS Neurol Disorders-Drug Targets (Formerly Curr Drug Targets-CNS Neurol Disorders). 2023;22(5):752–60.
CAS Google Scholar
Hollender Å, Bjøro T, Otto Karlsen K, Kvaloy SO, Nome O, Holte H. Vitamin D deficiency in patients operated on for gastric lymphoma. Scand J Gastroenterol. 2006;41(6):673–81.
Muszyński T, Polak K, Frątczak A, Miziołek B, Bergler-Czop B, Szczepanik A. Vitamin D—The Nutritional Status of Post-gastrectomy Gastric Cancer patients—systematic review. Nutrients. 2022;14(13):2712.
Cadegiani FA. Remission of severe myasthenia gravis after massive-dose vitamin D treatment. Am J case Rep. 2016;17:51.
Sharshar T, Chevret S, Mazighi M, Chillet P, Huberfeld G, Berreotta C, et al. Validity and reliability of two muscle strength scores commonly used as endpoints in assessing treatment of myasthenia gravis. J Neurol. 2000;247:286–90.
Yamamoto E, Jørgensen TN. Immunological effects of vitamin D and their relations to autoimmunity. J Autoimmun. 2019;100:7–16.
Bischoff-Ferrari H, Borchers M, Gudat F, Dürmüller U, Stähelin H, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19(2):265–9.
Harrison SR, Li D, Jeffery LE, Raza K, Hewison M. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif Tissue Int. 2020;106:58–75.
Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020; 12(7): 2097.
Justo ME, Aldecoa M, Cela E, Leoni J, González Maglio DH, Villa AM, et al. Low vitamin D serum levels in a cohort of myasthenia gravis patients in Argentina. Photochem Photobiol. 2021;97(5):1145–9.
Rochel N. Vitamin D and its receptor from a structural perspective. Nutrients. 2022;14(14):2847.
Han J-L, Yue Y-X, Gao X, Xie Y-C, Hao H-J, Li H-Y, et al. Vitamin D receptor polymorphism and myasthenia gravis in Chinese Han population. Front Neurol. 2021;12:604052.
Han J, Li H, Xie Y, Sun L, Wang Z, Xu X, et al. Association between vitamin D receptor gene Tru9I polymorphism and myasthenia gravis. Zhonghua Yi Xue Za Zhi. 2012;92(29):2028–33.
CAS PubMed Google Scholar
Dai Y, Wu F, Ni S, Guo S, Lu L, Zhao X. Vitamin D receptor gene polymorphisms are associated with the risk and features of myasthenia gravis in the Han Chinese population. Immunol Res. 2023;71(3):404–12.
Wu Y, Xue H, Zhang W, Wu Y, Yang Y, Ji H. Application of enhanced recovery after surgery in total knee arthroplasty in patients with haemophilia A: a pilot study. Nurs Open. 2021;8(1):80–6.
Askmark H, Haggård L, Nygren I, Punga AR. Vitamin D deficiency in patients with myasthenia gravis and improvement of fatigue after supplementation of vitamin D 3: a pilot study. Eur J Neurol. 2012;19(12):1554–60.
Kang S-Y, Kang J-H, Choi JC, Song SK, Oh J-H. Low serum vitamin D levels in patients with myasthenia gravis. J Clin Neurosci. 2018;50:294–7.
Yu Z, Cheng H, Liang Y, Ding T, Yan C, Gao C, et al. Decreased serum 25-(OH)-D level associated with muscle enzyme and myositis specific autoantibodies in patients with idiopathic inflammatory myopathy. Front Immunol. 2021;12:642070.
Chroni E, Dimisianos N, Punga AR. Low vitamin D levels in healthy controls and patients with autoimmune neuromuscular disorders in Greece. Acta Neurol Belgica. 2016;116:57–63.
Guan Y, Lv F, Meng Y, Ma D, Xu X, Song Y, et al. Association between bone mineral density, muscle strength, and vitamin D status in patients with myasthenia gravis: a cross-sectional study. Osteoporos Int. 2017;28:2383–90.
Fan Y, Zeng X, Lin F, Chen Y, Chen X. Causal effect of vitamin D on myasthenia gravis: a two-sample mendelian randomization study. Front Nutr. 2023;10:1171830.
Fletcher J, Bishop EL, Harrison SR, Swift A, Cooper SC, Dimeloe SK et al. Autoimmune disease and interconnections with vitamin D. Endocr Connections. 2022; 11(3).
Download references
Acknowledgements
This study was supported by the High-level Talents Project of Talent Development Fund in Inner Mongolia Autonomous Region, Inner Mongolia Autonomous Region, China; Prairie Talent Project of Inner Mongolia Autonomous Region, Inner Mongolia Autonomous Region, China and Beijing Association for Science and Technology Youth Talent Support Programme, Beijing, China.
Author information
Authors and affiliations.
Department of Acupuncture, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, No.23 Museum Back Street, Dongcheng District, Beijing, 100010, China
Tao Zhang, Junhong Zhong, Xu Ji, Jingqing Sun, Yingxue Cui & Shaosong Wang
Department of Neurology, Inner Mongolia Hospital affiliated to Beijing Hospital of Traditional Chinese Medicine, Bayannur city, Inner Mongolia Autonomous Region, 015000, China
You can also search for this author in PubMed Google Scholar
Contributions
Tao Zhang: guarantor of integrity of the entire study; study concepts; study design; definition of intellectual content; clinical studies; manuscript preparation. Junhong Zhong: definition of intellectual content; literature research; clinical studies; manuscript preparation. Xu Ji: guarantor of integrity of the entire study; data analysis; manuscript editing; manuscript review. Jingqing Sun: study concepts; study design; definition of intellectual content. Yingxue Cui: literature research; clinical studies; manuscript preparation. Shaosong Wang: study design; definition of intellectual content; clinical studies. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Yingxue Cui or Shaosong Wang .
Ethics declarations
Ethics approval and consent to participate.
This case report followed ethical guidelines and was approved by our institutional ethics committee. Consent to participate and publish Informed consent was obtained from participant included in the study.
Consent for publication
Patients signed informed consent regarding publishing the related data and photographs.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhang, T., Zhong, J., Ji, X. et al. Vitamin D add on the standard treatment for myasthenia gravis symptoms following total gastrectomy: a case report. BMC Neurol 24 , 188 (2024). https://doi.org/10.1186/s12883-024-03687-z
Download citation
Received : 26 January 2024
Accepted : 22 May 2024
Published : 05 June 2024
DOI : https://doi.org/10.1186/s12883-024-03687-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gastrectomy
BMC Neurology
ISSN: 1471-2377
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Br J Radiol
- v.90(1074); June 2017
Multiple sclerosis update: use of MRI for early diagnosis, disease monitoring and assessment of treatment related complications
Mark s igra.
1 Department of Neuroradiology, Royal Hallamshire Hospital, Sheffield, UK
David Paling
2 Department of Clinical Neurology, Royal Hallamshire Hospital, Sheffield, UK
Mike P Wattjes
3 Department of Radiology and Nuclear Medicine, VU University Medical Center, Amsterdam, Netherlands
Daniel J A Connolly
Nigel hoggard.
4 Academic Unit of Radiology, University of Sheffield, Sheffield, UK
MRI has long been established as the most sensitive in vivo technique for detecting multiple sclerosis (MS) lesions. The 2010 revisions of the McDonald Criteria have simplified imaging criteria, such that a diagnosis of MS can be made on a single contrast-enhanced MRI scan in the appropriate clinical context. New disease-modifying therapies have proven effective in reducing relapse rate and severity. Several of these therapies, most particularly natalizumab, but also dimethyl fumarate and fingolimod, have been associated with progressive multifocal leukoencephalopathy (PML). PML-immune reconstitution inflammatory syndrome (IRIS) has been recognized in patients following cessation of natalizumab owing to PML, and discontinuation for other reasons can lead to the phenomenon of rebound MS. These complications often provide a diagnostic dilemma and have implications for imaging surveillance of patients. We demonstrate how the updated McDonald Criteria aid the diagnosis of MS and describe the imaging characteristics of conditions such as PML and PML-IRIS in the context of MS. Potential imaging surveillance protocols are considered for the diagnosis and assessment of complications. We will explain how changes in MS treatment are leading to new imaging demands in order to monitor patients for disease progression and treatment-related complications.
INTRODUCTION
Multiple sclerosis (MS) is a chronic, inflammatory neurological disease characterized by demyelination, axonal injury and gliosis within the brain and spinal cord. MRI is an important tool in the diagnosis of MS and has been part of the diagnostic criteria since 2001. 1 The latest 2010 revision has simplified the diagnostic process further and can allow a diagnosis of MS to be made following a single attack on a single contrast-enhanced MR study, by the demonstration of the simultaneous presence of asymptomatic enhancing and non-enhancing lesions. 2 , 3 MRI also has an integral role in the monitoring of disease and assessing treatment efficacy and prediction of treatment response. 4
The development of new disease-modifying drugs (DMDs) such as natalizumab, fingolimod and dimethyl fumarate has reduced MS relapse rates as well as short-term disability progression. 5 – 8 However, progressive multifocal leukoencephalopathy (PML), an opportunistic lytic infection of the white and grey matter cells in the central nervous system (CNS) by John Cunningham virus (JCV), is a recognized severe adverse event. 9 PML can cause death or permanent neurological disability. However, prompt and accurate diagnosis can reduce these risks, and PML can be detected by MRI before the patient develops symptoms. 10 , 11 PML-related immune reconstitution inflammatory syndrome (IRIS) is also a recognized complication following natalizumab cessation. 12
MS may present with atypical imaging and clinical features such as tumefactive MS and Balo concentric sclerosis and pathophysiologically different entities such as neuromyelitis optica, other neuroinflammatory conditions, vasculitis, infections and tumours, and it can mimic the neuroradiological and clinical manifestations of MS.
In this review, we focus on typical MR findings which also constitute the new McDonald Criteria. A thorough review of the imaging findings in classic MS and its variants is beyond the scope and aims of this study. For a full overview of all imaging findings related to MS and its differential diagnoses, we direct the reader to other comprehensive reviews. 13 – 15
We highlight how the most recent imaging guidelines affect diagnosis of MS and also provide information regarding the imaging characteristics of natalizumab-related complications, such as PML and PML-IRIS. We discuss recent suggested imaging protocols for MS and the potential impact that new imaging surveillance and more frequent monitoring of patients may have on radiology departments.
THE REVISED 2010 MCDONALD CRITERIA
MRI can establish a diagnosis of MS in patients with at least one clinical attack consistent with CNS inflammatory demyelinating disease in two ways. First, MRI can help by outruling alternative differential diagnoses for symptoms and signs. 16 Second, MRI can help make a positive diagnosis of MS using the McDonald Criteria which have most recently been revised in 2010. 2 , 3 These criteria allow MRI to aid diagnosis of MS, allowing demonstrating dissemination in time (DIT) and dissemination in space (DIS) of lesions where this cannot be established clinically.
For example, in a patient with a single typical clinical attack, MS can be diagnosed based on a single MRI scan, provided this scan demonstrates both DIS and DIT. DIS can be demonstrated with at least one T2 lesion in at least two of four recognized locations for MS plaques (periventricular, cortical/juxtacortical, infratentorial and spinal cord), although it should be noted that the symptomatic lesion in the case of a brainstem or spinal cord syndrome is excluded. DIT can be demonstrated as the simultaneous presence of asymptomatic contrast-enhancing and non-enhancing lesions on the same study. If this is not seen, DIT can be demonstrated by a further T2 lesion on follow-up MRI or a new clinical attack ( Figure 1 ).

A 29-year-old male presented with a 2-week history of right leg numbness, right hand weakness and brisk reflexes bilaterally with sustained clonus. T 2 weighted and fluid-attenuated inversion recovery imaging show multiple hyperintensityies. A posterior left frontal lobe lesion (arrows) shows enhancement following contrast administration. An anterior right frontal lobe lesion does not (dashed arrows). This single study demonstrates both dissemination in space and dissemination in time.
MRI is also able to detect lesions fulfilling DIS and DIT criteria in patients who may have no signs or symptoms of MS in scans performed for other indications ( i.e. headaches, head injury, research). These patients do not fulfil the McDonald Criteria for a diagnosis of MS, since they have not had at least one clinical attack. This has led to the concept of radiologically isolated syndrome, which does carry a higher risk for developing MS. Criteria for radiologically isolated syndrome including the number, location and morphology of lesions have been suggested. 17
The 2010 McDonald Criteria allow a more rapid diagnosis of MS without losing sensitivity. They should in theory result in fewer MRI examinations being performed. It should be reiterated that they only apply in those patients who have experienced a typical clinically isolated syndrome.
Suggested imaging protocol
The McDonald Criteria do not suggest specific imaging protocols. Various MRI acquisition parameters have a significant influence on the detection of MS-related pathology. Several expert groups have recently suggested protocols for baseline and follow-up MRI for the brain and spine in MS. 18 , 19
Brain imaging
The recommended minimum sequences required include three-dimensional (3D) T 1 weighted, 3D T 2 fluid-attenuated inversion-recovery (FLAIR), 3D T 2 weighted and pre- and post-single-dose gadolinium T 1 weighted imaging. 19 3.0-T MRI is preferable to 1.5 T if available owing to the improved signal-to-noise ratio and resolution. While higher field strengths increase sensitivity for lesions, they have not resulted in earlier diagnosis of MS. 20 Two-dimensional (2D) sequences should have a maximum slice thickness of no greater than 3 mm and an in-plane spatial resolution of 1 × 1 mm. 21 While spin-echo or fast spin-echo T 2 weighted and proton density (PD) sequences have been considered the reference standard to detect demyelinating lesions, they are considered optional in this new suggested protocol. 2D FLAIR sequences are more sensitive in detecting periventricular and juxtacortical lesions, although they are less sensitive for posterior fossa lesions. For this reason, the groups suggest that isotropic 3D T 2 weighted FLAIR is preferred owing to improved contrast-to-noise ratio and the ability to create multiplanar reformats. These are usually acquired in the sagittal plane to assess lesions in the corpus callosum, and this is also the fastest plane for acquisition. We recognize that 3D MR techniques are not available in all institutions. When assessing for MS lesions using a 1.5-T scanner without the option of 3D imaging, we recommend both axial 2D FLAIR and spin-echo axial T 2 weighted sequences in order to improve assessment of the posterior fossa ( Tables 1 and and2 2 ).
Standardized brain MRI protocol and standardized spinal cord protocol (adapted from Traboulsee 2016)
| Parameters | Description |
|---|---|
| Standardized brain MRI protocol | |
| Field strength | Sufficient to allow adequate signal-to-noise ratio with resolution (≤1 × 1 mm) |
| Scan orientation | Axial oblique sequences should be along the subcallosal line |
| Coverage | Whole brain |
| Section thickness and gap | ≤3 mm. No gap for 2D or 3D acquisition |
| Core sequences | Anatomic 3D inversion recovery gradient echo |
| 3D sagittal weighted imaging FLAIR | |
| 3D weighted imaging | |
| 2D axial DWI | |
| 3D FLASH post-gadolinium | |
| Optional sequences | Axial PD |
| Pre or post gadolinium T1 spin-echo | |
| SWI for central vein identification | |
| Standardized spinal cord protocol | |
| Field strength | Sufficient to allow good signal-to-noise ratio and resolution (≤1 × 1 mm) |
| Coverage | Cervical cord, although thoracic cord is recommended if lesions localize to this region |
| Core sequences | Sagittal |
| Sagittal proton attenuation, STIR or PST1-IR | |
| Axial through lesions | |
| Section thickness and gap | Sagittal ≤3 mm, no gap |
| Axial 5 mm, no gap | |
| Optional sequences | Axial through complete cervical cord |
| Post-gadolinium sagittal | |
2D, two dimensional; 3D, three dimensional; DWI, diffusion-weighted imaging; FLASH, fast low angle shot; PD, proton density; PST1-IR, indicates phase-sensitive T1 inversion recovery; STIR, short tau inversion recovery.
Clinical features and imaging characteristics of multiple sclerosis (MS) and progressive multifocal leukoencephalopathy (PML)
| Parameters | MS | PML |
|---|---|---|
| Clinical features | ||
| Onset | Acute | Subacute |
| Evolution | Hours to days | Over weeks |
| Normally stabilize. May resolve spontaneously even without therapy | Progressive | |
| Clinical presentation | Diplopia | Aphasia |
| Paraesthesia | Behavioural and neuropsychiatric alteration | |
| Paraparesis | Hemiparesis | |
| Optic neuritis | Hemianopia | |
| Myelopathy | Seizures | |
| MS | PML | |
| Imaging characteristics | ||
| Appearance | Well-defined lesions | Ill-defined lesions, white matter, often large (>3 cm), multifocal. Sharp border towards grey matter, ill-defined border towards white matter. Often surrounded by punctate lesion |
| Location | Periventricular, deep white matter, cerebellum, spinal cord | Subcortical white matter, parietal, occipital, frontal lobes. Can involve corpus callosum. Rarely brainstem and posterior fossa |
| FLAIR | Hyperintense equal to | Hyperintense, more sensitive for detection of PML in subcortical structures. |
| Isointense or hypointense | Isointense with progressive hypointensity | |
| Hyperintense, may resolve over months | Hyperintense | |
| Mass effect | Only in large lesions | Not typical. PML-IRIS may show mass effect |
| Contrast enhancement | Acute lesions enhance. Nodular or incomplete ring | 40–50% enhancement. Variable pattern—linear, nodule, punctate or peripheral |
FLAIR, fluid-attenuated inversion recovery; IRIS, immune reconstitution inflammatory syndrome.
Adapted from Yousry. Ann Neurol 2012.
A thin-section 3D inversion recovery-prepared, T 1 weighted, spoiled gradient echo sequence is useful for volumetric analysis, which may become more widespread in the future in assessing brain atrophy. 19 T 1 weighted imaging is essential in detecting “black holes”, a marker of chronic axonal loss. However, this particular sequence renders all hyperintense lesions on T 2 weighted imaging to be hypointense, thus losing the specificity of T 1 black holes. 22
Axial diffusion-weighted imaging (DWI) may detect acute inflammatory lesions as well as non-MS pathology and has a role in detecting early manifestations of PML. Given that it has histopathological specificity over conventional T 2 weighted and FLAIR imaging, inclusion of DWI sequences in an MS protocol has been advised by some authors. 23
Spinal imaging
Imaging of the spinal cord is more challenging owing to small tissue volume and artefact from vascular and cerebrospinal fluid (CSF) pulsations. At a minimum the cervical cord should be imaged, as MS lesions are more common and better visualized in this region. 19 Thoracic cord imaging should be performed if clinical symptoms localize to this region. There is no published evidence that higher field strengths improve lesion detection in the spinal cord and 1.5-T MRI is recommended. 18 Two sagittal sequences with different contrasts ( T 2 and PD and/or short tau inversion recovery) improve lesion detection rate. The diagnostic standard is considered to be sagittal 2D spin echo or fast dual-echo, although short tau inversion-recovery T 2 , PD and T 1 weighted inversion recovery sequences may also be used. As opposed to brain MRI, T 2 FLAIR sequences are no more sensitive than conventional T 2 sequences. 21 While not included in this recommended spinal imaging protocol, it is our experience that axial imaging greatly improves lesion detection and characterization, and we perform axial T 2 * gradient echo sequences of the cervical spine on all our MS protocols.
A smaller percentage of new spinal cord lesions demonstrate enhancement following gadolinium administration—61% compared with 94% of brain lesions in one study—and its value is still under consideration. 24 The updated imaging guidelines recommend that the spinal cord should be imaged directly after contrast-enhanced brain imaging, to prevent the need for additional contrast administration and to reduce repeated attendances to the department. 18
Disease-modifying drugs and progressive multifocal leukoencephalopathy
DMDs used in active relapsing MS are effective in reducing the frequency and severity of MS attacks. One of the most effective of these is natalizumab, a recombinant monoclonal (Ig)-G4 antibody directed at a4b1 and a4b7 integrins, which are cell adhesion molecules. The effect of integrin blockade is decreased T-cell migration into the CNS. 25 Phase III trials demonstrated clinical efficacy and superiority compared with interferon-B or glatiramer acetate. 5 , 26 Up to 37% of patients were free of clinical and radiological disease activity after 2 years. 5 Natalizumab is generally well tolerated with a low incidence of immediate adverse events. However, in 2005, three patients were reported to have developed PML while on natalizumab. 27 As of September 2016, 698 cases of PML associated with natalizumab had been confirmed and incidence has reached 4.18 per 1000 patients treated (Biogen MedInfo. Available from: https://medinfo.biogen.com ; Accessed September 2016). PML has additionally been occasionally reported in patients taking dimethyl fumarate and fingolimod (other disease-modifying therapies for MS). 28 – 30
Progressive multifocal leukoencephalopathy
PML is an opportunistic infection pathologically characterized by lytic infection of oligodendrocytes and astrocytes by the JCV, a double-stranded DNA polyomavirus. JCV is ubiquitous and a large percentage of the population carries it as an asymptomatic latent infection. 31 CNS infection can occur in patients who are immunosuppressed including with DMDs, following a complex reactivation and virus replication whereby latent JCV mutates to a neurotropic variant. 32 , 33
A positive anti-JCV antibody status is a risk factor for developing PML during natalizumab treatment, although patients who are serologically negative may still carry the virus, can periodically display low levels of viraemia and have very occasionally developed PML. 34 Prior use of immunosuppressants and duration of natalizumab therapy, especially beyond 2 years, are additional risk factors for the development of PML. 9
American Academy of Neurology consensus statements mandate that diagnosis can be made from brain biopsy, or more commonly from clinical findings combined with JCV DNA in CSF, typically supported by typical imaging findings. 35 , 36 Cognitive deficits are the most common clinical feature of PML, although presentation is often heterogeneous with focal and non-focal neurological deficits. 37 This may lead to early symptoms of PML being attributed to an MS relapse or exacerbation. Therefore, in the context of a JCV seropositive patient on natalizumab treated longer than 2 years presenting with new neurological symptoms, there should be a high index of suspicion for PML. CSF can occasionally be negative in early cases, particularly in patients who are asymptomatic with only MRI-based suspicion. Serial scanning and lumbar puncture supported by detailed and frequent clinical assessment is sometimes required to achieve definite diagnosis. 38
Natalizumab-associated PML has a mortality rate of 22% when picked up at the time of symptoms which is lower than the HIV-AIDS related form, although surviving patients can have significant morbidity. 39 Older patients and those with poorer baseline function have a worse prognosis. PML screening may lead to an earlier diagnosis at an asymptomatic stage with a better functional outcome. 11
Imaging characteristics of leukoencephalopathy
Attempting detection, particularly of pre-symptomatic PML on MRI can be difficult owing to the overlap of imaging findings with MS lesions. Recognized imaging characteristics of PML include one or more foci of T 2 /FLAIR hyperintensity in a subcortical location involving U-fibres with an ill-defined border towards the white matter but a well-delineated border towards the grey matter. 40 White matter involvement is typically peripheral, although periventricular white matter involvement does not exclude PML. 35 Lesions vary in shape and coalesce as they increase in size. Larger lesions may show a “granular” appearance on T 2 weighted imaging, which reflects focal areas of demyelination. 40 Lesion sizes of >3 cm are more likely to be associated with PML than MS. T 1 weighted imaging typically shows PML lesions to be hypointense. 41
The frontal lobes are most affected, followed by the parietal and occipital lobes. Callosal involvement has been reported, although isolated lesions are rare. 40 Deep grey matter structure involvement can occur, with the thalami more frequently involved than the basal ganglia. 42 Cortical involvement is increasingly recognized, 43 and in a multivariate model proposed by Wijburg et al, 44 cortical grey matter involvement along with punctate T2 lesions predicted for PML over MS lesions. Posterior fossa involvement is well recognized and “crescent”-shaped lesions involving the middle cerebellar peduncles can be seen and are not typical for MS lesions. 45 Approximately 30% of PML lesions may show enhancement or other signs of inflammation, and the enhancement pattern is varied and may be patchy, nodular, linear or peripheral. 46
Restricted diffusion can be seen on DWI sequences, although this may be less common with early or asymptomatic disease. 46 In the early stages of disease, DWI shows high signal owing to swollen and dying oligodendrocytes. 47 Treatment commencement results in the lesion rim losing its DWI hyperintensity, and over time the lesion becomes hypointense owing to tissue destruction. Apparent diffusion coefficient values rise with progressive white matter injury, in keeping with more irreversible damage. 48 This evolution of DWI signal changes is essential in monitoring disease progression and treatment response ( Figure 2 ). 48 , 49

A patient with known multiple sclerosis (MS) on treatment with natalizumab presented with progressive changes in behaviour. Coronal fluid-attenuated inversion recovery (FLAIR) imaging (a, b) shows an ill-defined focus of high signal within the medial left frontal lobe (solid arrows), without restricted diffusion on diffusion-weighted imaging/apparent diffusion coefficient (c, d). A repeat MRI scan performed 6 weeks later shows enlargement of the pre-existing left frontal lobe lesion (e and f, solid arrows). There is also faint punctate contrast enhancement at the posterior aspect of the lesion (h, dashed line). A new ill-defined high T 2 /FLAIR lesion is also now seen within the left temporal lobe (f, dotted line). Findings are consistent with progressive multifocal leukoencephalopathy. Pre-existing MS lesions within both centrum semiovale are noted.
Progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome
Treatment of natalizumab-associated PML is by rapid drug removal, usually by plasma exchange. This can hasten PML-IRIS which may worsen symptoms and is associated with significant negative effects on patient outcome. 50 IRIS was originally described in patients with AIDS who paradoxically deteriorated on starting highly active antiretroviral therapy. 51 In patients who do not receive plasma exchange on discontinuation of natalizumab, PML-IRIS occurs 90 days after the final dose, reflecting the time taken for the biological effects of the drug to clear. 52 PML-IRIS carries a mortality of up to 30%. 11
PML-IRIS is characterized by an increase in size of pre-existing PML lesions. Oedema, cerebral swelling and mass effect may be seen, imaging features which are not typical for PML. 35 The most prominent imaging finding is that of contrast enhancement, with variable, irregular, ill-defined contrast enhancement patterns described. 40 Patchy enhancement was most frequently located within the periphery of the PML lesions, which is attributed to active lytic JCV infection within preserved myelin. 47 , 53 Punctate enhancement with a perivascular distribution, outside of the main PML lesion, is another sign of PML-IRIS ( Figure 3 ). 53

Axial fluid-attenuated inversion recovery (a, b) and contrast-enhanced T 1 weighed images (c, d) of a 54-year-old patient with patients with relapsing-remitting multiple sclerosis being treated with natalizumab longer than 3 years at the time of progressive multifocal leukoencephalopathy (PML) diagnosis (a, c) and at the time of PML- immune reconstitution inflammatory syndrome (IRIS) (b, d). The PML lesion at the time of the diagnosis shows typical PML lesion characteristics without any mass effect or perilesional oedema (closed head arrows). At the PML-IRIS stage, the immune reconstitution causes substantial inflammation leading to multifocal contrast enhancement inside and outside of the main PML lesion (d, open head arrows) and swelling, mass effect and perilesional oedema (c, open head arrows).
Natalizumab rebound
Another recently recognized phenomenon is that of natalizumab rebound, where a patient develops a severe inflammatory response greater than that of their usual typical relapse severity within approximately 3 months of natalizumab cessation. 54 This rebound phenomenon has been reported in up to 40% of patients after discontinuation of natalizumab. 12 , 55 This provides another reason for clinicians to be vigilant when patients stop treatment. Typical imaging appearances are of new enhancing and non-enhancing lesions, which may be greater in number than in a normal MS relapse episode. 2 , 10 , 55
Suggested imaging surveillance protocol
Given that prompt detection and treatment of PML in the pre-symptomatic phase has been shown to improve outcomes 56 – 58 and limits permanent brain damage before immune reconstitution, 37 appropriate surveillance of patients taking natalizumab is essential. Various studies have shown that MRI is able to detect PML-related changes 3–4 months prior to development of symptoms 58 – 60
An expert group has proposed a surveillance protocol, stratifying patients into one of three risk groups based on anti-JCV antibody status: JCV negative, JCV positive with index <1.5 and JCV positive with index >1.5. Those with an index value of ≥1.5 have a relatively low risk within the first 24 months (1.17/1000), but this increases to 1 in 113 after 24 months. Therefore, these patients with higher risk should have more regular MRI monitoring for PML than those with lower risk. 57 Since JCV antibody status can change over time, it is recommended that antibody testing should be repeated every 6 months and MRI surveillance frequency adjusted if JCV becomes positive or if the index increases above 1.5. 57
The MRI surveillance strategy has been incorporated by the European Medicines Agency for the natalizumab label update. It is recommended that all patients be imaged prior to starting natalizumab and at least annually. The frequency of repeat imaging for those patients who are anti-JCV antibody positive and have been on treatment for 18 months increases to a minimum of 6 monthly for those with an index value of ≤1.5 and at least 3–4 monthly for those with a value ≥1.5. While the risk of natalizumab-associated PML increases after 24 months, the opinion of the expert group is that more frequent imaging at 18 months would aid detection of asymptomatic PML. 57 This early detection would facilitate prompt treatment cessation and further treatment.
A proposed abbreviated PML surveillance imaging protocol includes 3D or 2D FLAIR and DWI sequences 43 but not post-contrast imaging, as fewer than 50% of early PML lesions demonstrate contrast enhancement. 40 A protocol which avoids the use of contrast is important owing to the increasingly recognized phenomenon of gadolinium deposition within the brain in patients who have had multiple contrast-enhanced MRIs. Long-term effects of this deposition have yet to be determined. 61 However, it would be prudent to avoid gadolinium-based contrast agents wherever possible, particularly in those younger patients who may have large numbers of contrast-enhanced scans to assess for active disease.
There are implications of increased frequency of MRI monitoring of these patients. As of September 2016, 161,300 patients worldwide had received natalizumab (Biogen MedInfo. Available from: https://medinfo.biogen.com ). In the UK, there are an estimated 107,740 people with MS and an estimated 5110 new diagnosed cases a year. 62 Our department currently has 109 patients on natalizumab, with an estimated prevalence of MS at 2740 patients, or 3.97%. Extrapolated across the UK, this suggests a rough estimate of approximately 4286 patients treated with natalizumab. Given that 57.1% of patients in a study by Plavina et al 63 fell into the higher risk category with an index ≥1.5, 2443 patients would require frequent surveillance MRI scans. As natalizumab is well tolerated with a reduced relapse rate and reduced risk of sustained disability progression, its use in clinical practice will increase. 5 If these patients at higher risk required 3 scans per year as is suggested, nearly 7500 extra MRI scans per year could be generated from natalizumab surveillance alone. If surveillance protocols were extended to include those patients on other DMDs such as fingolimod and dimethyl fumarate (currently 144 and 272 patients, respectively, in our department), then UK radiology departments would have serious difficulty in scanning and providing reports for these patients. Certainly, PML surveillance scans would have to be performed by non-neuroradiology specialist radiologists. Therefore, suspicion for and accurate detection of MRI findings in PML will no longer be the preserve of the neuroradiologist and should be at the forefront of any radiologist who reports follow-up MRI scans on patients with MS on DMDs.
The use of MRI in the diagnosis and surveillance of MS and treatment-related complications is evolving with new MRI techniques and more DMDs available to clinicians. The most recent revision to the McDonald Criteria has simplified MS diagnosis while maintaining sensitivity and specificity and allows the diagnosis of MS on a single MRI scan. This allows earlier diagnosis and treatment.
More widespread use of newer DMDs will require improved surveillance via clinical assessment, MRI and more frequent assessment of anti-JCV antibody status, in order to accurately and promptly detect related complications such as PML, PML-IRIS and rebound episodes. The anti-JCV antibody status provides a quantitative measure of risk related to whether patients should continue with natalizumab treatment or not and stratifies them into distinct follow-up protocols. More regular repeat MRI scans as part of a surveillance protocol will provide radiology departments logistical challenges as more patients commence natalizumab therapy and effective strategies should be put in place in order to provide clinicians and patients with an effective surveillance programme.
Appointments at Mayo Clinic
Vitamin D is a nutrient your body needs for building and maintaining healthy bones. That's because your body can only absorb calcium, the primary component of bone, when vitamin D is present. Vitamin D also regulates many other cellular functions in your body. Its anti-inflammatory, antioxidant and neuroprotective properties support immune health, muscle function and brain cell activity.
Vitamin D isn't naturally found in many foods, but you can get it from fortified milk, fortified cereal, and fatty fish such as salmon, mackerel and sardines. Your body also makes vitamin D when direct sunlight converts a chemical in your skin into an active form of the vitamin (calciferol).
The amount of vitamin D your skin makes depends on many factors, including the time of day, season, latitude and your skin pigmentation. Depending on where you live and your lifestyle, vitamin D production might decrease or be completely absent during the winter months. Sunscreen, while important to prevent skin cancer, also can decrease vitamin D production.
Many older adults don't get regular exposure to sunlight and have trouble absorbing vitamin D. If your doctor suspects you're not getting enough vitamin D, a simple blood test can check the levels of this vitamin in your blood.
Taking a multivitamin with vitamin D may help improve bone health. The recommended daily amount of vitamin D is 400 international units (IU) for children up to age 12 months, 600 IU for people ages 1 to 70 years, and 800 IU for people over 70 years.
What the research says
Research on vitamin D use for specific conditions shows:
- Cancer. Findings on the benefits of vitamin D for cancer prevention are mixed. More studies are needed to determine whether vitamin D supplementation may reduce the risk of certain cancers.
- Cognitive health. Research shows that low levels of vitamin D in the blood are associated with cognitive decline. However, more studies are needed to determine the benefits of vitamin D supplementation for cognitive health.
- Inherited bone disorders. Vitamin D supplements can be used to help treat inherited disorders resulting from an inability to absorb or process vitamin D, such as familial hypophosphatemia.
- Multiple sclerosis. Research suggests that long-term vitamin D supplementation reduces the risk of multiple sclerosis.
- Osteomalacia. Vitamin D supplements are used to treat adults with severe vitamin D deficiency, resulting in loss of bone mineral content, bone pain, muscle weakness and soft bones (osteomalacia).
- Osteoporosis. Studies suggest that people who get enough vitamin D and calcium in their diets can slow bone mineral loss, help prevent osteoporosis and reduce bone fractures. Ask your doctor if you need a calcium and vitamin D supplement to prevent or treat osteoporosis.
- Psoriasis. Applying vitamin D or a topical preparation that contains a vitamin D compound called calcipotriene to the skin can treat plaque-type psoriasis in some people.
- Rickets. This rare condition develops in children with vitamin D deficiency. Supplementing with vitamin D can prevent and treat the problem.
Generally safe
Without vitamin D your bones can become soft, thin and brittle. Insufficient vitamin D is also connected to osteoporosis. If you don't get enough vitamin D through sunlight or dietary sources, you might need vitamin D supplements.
Safety and side effects
Taken in appropriate doses, vitamin D is generally considered safe.
However, taking too much vitamin D in the form of supplements can be harmful. Children age 9 years and older, adults, and pregnant and breastfeeding women who take more than 4,000 IU a day of vitamin D might experience:
- Nausea and vomiting
- Poor appetite and weight loss
- Constipation
- Confusion and disorientation
- Heart rhythm problems
- Kidney stones and kidney damage
Interactions
Possible interactions include:
- Aluminum. Taking vitamin D and aluminum-containing phosphate binders, which may be used to treat high serum phosphate levels in people with chronic kidney disease, might cause harmful levels of aluminum in people with kidney failure in the long term.
- Anticonvulsants. The anticonvulsants phenobarbital and phenytoin (Dilantin, Phenytek) increase the breakdown of vitamin D and reduce calcium absorption.
- Atorvastatin (Lipitor). Taking vitamin D might affect the way your body processes this cholesterol drug.
- Calcipotriene (Dovonex, Sorilux). Don't take vitamin D with this psoriasis drug. The combination might increase the risk of too much calcium in the blood (hypercalcemia).
- Cholestyramine (Prevalite). Taking vitamin D with this cholesterol-lowering drug can reduce your absorption of vitamin D.
- Cytochrome P-450 3A4 (CYP3A4) substrates. Use vitamin D cautiously if you're taking drugs processed by these enzymes.
- Digoxin (Lanoxin). Avoid taking high doses of vitamin D with this heart medication. High doses of vitamin D can cause hypercalcemia, which increases the risk of fatal heart problems with digoxin.
- Diltiazem (Cardizem, Tiazac, others). Avoid taking high doses of vitamin D with this blood pressure drug. High doses of vitamin D can cause hypercalcemia, which might reduce the drug's effectiveness.
- Orlistat (Xenical, Alli). Taking this weight-loss drug can reduce your absorption of vitamin D.
- Thiazide diuretics. Taking these blood pressure drugs with vitamin D increases your risk of hypercalcemia.
- Steroids. Taking steroid mediations such as prednisone can reduce calcium absorption and impair your body's processing of vitamin D.
- Stimulant laxatives. Long-term use of high doses of stimulant laxatives can reduce vitamin D and calcium absorption.
- Verapamil (Verelan, Calan SR). Taking high doses of vitamin D with this blood pressure drug can cause hypercalcemia, and might also reduce the effectiveness of verapamil.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Vitamin D: Fact sheet for health professionals. Office of Dietary Supplements. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/. Accessed Dec. 6, 2020.
- Vitamin D: Fact sheet for consumers. Office of Dietary Supplements. https://ods.od.nih.gov/factsheets/VitaminD-Consumer/. Accessed Dec. 6, 2020.
- Vitamin D. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed Dec. 6, 2020.
- AskMayoExpert. Vitamin D deficiency. Mayo Clinic; 2017.
- Cholecalciferol. IBM Microdemex. https://www.microdemexsolutions.com. Accessed Dec. 11, 2020.
- Gold J, et al. The role of vitamin D in cognitive disorders in older adults. US Neurology. 2018; doi:10.17925/USN.2018.14.1.41.
- Sultan S, et al. Low vitamin D and its association with cognitive impairment and dementia. Journal of Aging Research. 2020; doi:10.1155/2020/6097820.
- Pazirandeh S, et al. Overview of vitamin D. https://www.uptodate.com/contents/search. Accessed Dec. 13, 2020.
- Hassan-Smith ZK, et al. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 exerct distinct effects on human skeletal muscle function and gene expression. PLOS One. 2017; doi:10.1371/journal.pone.0170665.
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
We’re transforming healthcare
Make a gift now and help create new and better solutions for more than 1.3 million patients who turn to Mayo Clinic each year.

IMAGES
VIDEO
COMMENTS
MRI as a prognostic tool in MS. MRI plays an important role for the prognosis of disease development and monitoring of disease progression. Several studies have put special focus on the predictive value of T2-hyperintense lesions, T1-hypointense lesions, so-called black holes, as well as the implication of overall atrophy seen on MRI on the progression of disease.
Clinical Presentation: Case History # 1. Ms. C is a 35 year old white female. She came to Neurology Clinic for evaluation of her long-term neurologic complaints. The patient relates that for many years she had noticed some significant changes in neurologic functions, particularly heat intolerance precipitating a stumbling gait and a tendency to ...
SUMMARY: MR imaging is widely used for the diagnosis and monitoring of patients with MS. Applications and protocols for MR imaging continue to evolve, prompting a need for continual reassessments of the optimal use of this technique in clinical practice. This article provides updated recommendations on the use of MR imaging in MS, based on a review of the trial evidence and personal ...
Introduction. Magnetic resonance imaging (MRI) was formally included in the diagnostic work-up of patients presenting with a clinically isolated syndrome (CIS) suggestive of multiple sclerosis (MS) in 2001 by an International Panel of experts. 1 MS diagnosis requires demonstration of disease dissemination in space (DIS) and time (DIT) and exclusion of other conditions that can mimic MS by ...
Educational Case: Multiple sclerosis. Ariana Pape, MD, Laurie L. Wellman, PhD, and Richard M. Conran, MD, PhD, JD ... NMOSD present with relapsing-remitting neurological symptoms and lesions on magnetic resonance imaging (MRI) studies are similar to those in MS. However, lesions in NMOSD are characteristically limited to the spinal cord and ...
Multiple sclerosis (MS) is a relatively common acquired chronic demyelinating disease involving the central nervous system, and is the second most common cause of neurological impairment in young adults, after trauma 19.Characteristically, and by definition, multiple sclerosis is disseminated in space (i.e. multiple lesions in different regions of the brain) and in time (i.e. lesions occur at ...
Case Discussion. This case is very illustrative of the multiple sclerosis involvements both in the brain and in the spinal cord, with typical features inferring active lesions (open ring enhancement) in the brain and also the chronic evolution of this condition characterized by a mild diffuse brain atrophy.
Clinical and magnetic resonance imaging predictors of disease progression in multiple sclerosis: a nine-year follow-up study. Mult. Scler. 20 , 220-226 (2014).
Magnetic resonance imaging (MRI) is routinely used in clinical practice to detect and monitor inflammatory lesions in patients with multiple sclerosis (MS) [].Diagnostic criteria, as well as disease-modifying treatments (DMTs), have more recently increased the demand for MRI scans to allow early diagnosis and monitoring of treatment safety and efficacy [2, 3].
Figure 2.Histology showed an active demyelinating multiple sclerosis (MS) lesion corresponding to immunopathological pattern II. Arrows indicate the sharply demarcated inflammatory subcortical plaque on the left; the cerebral cortex is present on the right [H&E stain, ×10 (A)].Axons were preserved within the lesion [Bielschowsky silver stain, ×10 (B)], whereas myelin was lost [proteolipid ...
MRI Case Studies: Multiple Sclerosis and MS Mimics. DISCLOSURES •Speaking, consulting, and/or advisory boards for Biogen, Genzyme, Novartis, Questcor, and ... Coret, F et al. Multiple Sclerosis: 16(8) 935-941. AGGRESSIVE MS. AGGRESSIVE MS. Tumefactive MS •Lesions larger than 2 cm •May have edema, mass effect
Multiple Sclerosis (MS) is an inflammatory disorder of the central nervous system presented in roughly 2.8 million people worldwide; the disease predominantly affects women in 69% of cases, and the mean age of diagnosis is 32 years old [1]. MS is characterized by focal lymphocytic infiltration that leads to damage to myelin and axons [2].
Case Discussion. Multiple sclerosis is a common demyelinating disease of the central nervous septum. The diagnosis is mainly radiological and depends on detection of white matter lesions that disseminate in time and space. Plaques can be infratentorial, deep white matter, periventricular, juxta cortical or mixed white matter-grey matter lesions.
Case Discussion MRI of the brain and cord demonstrates numerous white matter T2 hyperintense lesions, particularly in a periventricular distribution. There are a number of juxtacortical, posterior fossa and enhancing lesions, easily fulfilling McDonald's criteria. Features are typical of multiple sclerosis.
Patient 2drelapsing remitting multiple sclerosis on disease-modifying therapy with breakthrough relapses A 39-year-old woman suffers an episode of optic neuritis and is found to have multiple white matter lesions consistent with MS on MRI. A course of intravenous (IV) steroids is given with subsequent recovery of vision after 2 weeks.
Clinical criteria were supplemented by CSF, MRI and evoked potentials in the Poser criteria in 1983. 4 With the more widespread availability of MRI, the McDonald criteria were developed by the International Panel on Diagnosis of Multiple Sclerosis in 2001 giving increasing weight to MRI in the diagnosis of MS. 5 There have been subsequent ...
Blood circulating microparticle species in relapsing-remitting and secondary progressive multiple sclerosis. A case-control, cross sectional study with conventional MRI and advanced iron content imaging outcomes J Neurol Sci. 2015 Aug 15;355(1-2):84-9. doi: 10.1016/j.jns.2015.05.027. ...
KYV-101 helps 2 hard-to-treat progressive MS patients: Case study March 20, 2024 News by Margarida Maia, PhD Vitamin D seen as most helpful for males in progressive MS rat model
Multiple Sclerosis (MS) is a chronic, inflammatory, demyelinating disease of CNS that afects cerebral cortex and grey matter including basal ganglia and Cerebellar cortex [1]. MS prevails in a total of 2.8 million people worldwide according to a study conducted in 2020 with an estimation of one person being diagnosed with MS every five minutes [2].
Multiple sclerosis (MS) is a progressive demyelinating disease impacting the central nervous system. Conventional Magnetic Resonance Imaging (MRI) techniques (e.g., T2w images) help diagnose MS ...
Multiple sclerosis (MS) is a chronic inflammatory disease of the CNS which often presents with recurrent episodes of focal neurological deficit in the absence of encephalopathy or fever [1, 2].A small number of cases present with atypical clinical or radiological features suggestive of acute disseminated encephalomyelitis (ADEM) [3, 4] or cerebral neoplasia (tumefactive demyelination) [].
A new antibody test (for kappa free light chains) may be faster and less expensive than previous spinal fluid tests for multiple sclerosis. MRI, which can reveal areas of MS ... Further studies will determine whether treatment can delay disability caused by the disease. For primary-progressive MS, ocrelizumab (Ocrevus) is the only FDA-approved ...
Multiple sclerosis (MS) is the most common disabling neurological disease of young adults with symptom onset generally occurring between the ages of 20 to 40 years. In MS, the immune system cells that normally protect us from viruses, bacteria, and unhealthy cells mistakenly attack myelin in the central nervous system (brain, optic nerves, and spinal cord).
June 05, 2024. 0. NASHVILLE, Tennessee — A second-generation anti-CD40L monoclonal antibody suppresses multiple sclerosis (MS) disease activity on MRI to an uncommonly high degree, new trial ...
Introduction. Magnetic resonance imaging (MRI) is routinely used in clinical practice to detect and monitor inflammatory lesions in patients with multiple sclerosis (MS) [].Diagnostic criteria, as well as disease-modifying treatments (DMTs), have more recently increased the demand for MRI scans to allow early diagnosis and monitoring of treatment safety and efficacy [2, 3].
More information: Artificial intelligence and radiologists in prostate cancer detection on MRI (PI-CAI): an international, paired, non-inferiority, confirmatory study, The Lancet Oncology (2024 ...
Optical coherence tomography (OCT) is a non-invasive imaging technique based on the principle of low-coherence interferometry that captures detailed images of ocular structures. Multiple sclerosis (MS) is a neurodegenerative disease that can lead to damage of the optic nerve and retina, which can be depicted by OCT. The purpose of this pilot study is to determine whether macular OCT can be ...
Background Myasthenia gravis (MG) is a long-term autoimmune disorder that affects the neuromuscular junction, causing muscle weakness and fatigue as its primary clinical features. Vitamin D is crucial for both the autoimmune response and skeletal muscle function. Case presentation Here, we presented a case report documenting the substantial improvement in symptoms experienced by a patient who ...
MRI is an important tool in the diagnosis of MS and has been part of the diagnostic criteria since 2001. 1 The latest 2010 revision has simplified the diagnostic process further and can allow a diagnosis of MS to be made following a single attack on a single contrast-enhanced MR study, by the demonstration of the simultaneous presence of ...
However, more studies are needed to determine the benefits of vitamin D supplementation for cognitive health. Inherited bone disorders. Vitamin D supplements can be used to help treat inherited disorders resulting from an inability to absorb or process vitamin D, such as familial hypophosphatemia. Multiple sclerosis.