Alzheimer's Disease Research Center


Guidelines for reducing dementia risk
Mayo Clinic contributed to the recently released World Health Organization publication that provides evidence-based guidance for a public health response to dementia. Read the report (PDF).
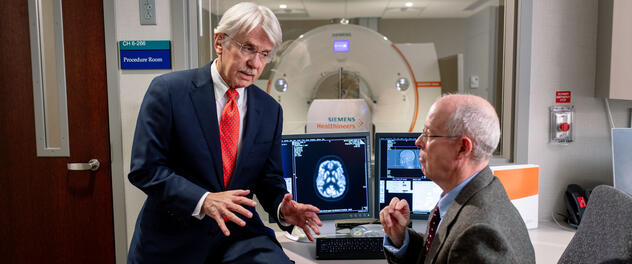
A letter from the director
Center director Ronald C. Petersen, M.D., Ph.D., provides an update on brain health and research. Read the update in the center's newsletter (PDF).

Mayo Clinic Conference on Brain Health and Dementia
The Nov. 4, 2023, Mayo Clinic Conference on Brain Health and Dementia explored paths to emotional wellness for participants and those living with dementia. Read more.

Peer-to-peer support for dementia caregivers
Mayo Clinic researchers are studying the effectiveness of peer-to-peer caregiver matching for mutual emotional support. Find enrollment information for interested participants or study information for researchers .

Coordinating Center: Lewy body dementia
The Alzheimer's Disease Research Center at Mayo Clinic in Rochester, Minnesota, is the Coordinating Center for the Lewy Body Dementia Association Research Centers of Excellence network.
The Alzheimer's Disease Research Center at Mayo Clinic promotes research and education about healthy brain aging, mild cognitive impairment, Alzheimer's disease, Lewy body dementia, frontotemporal dementia and other related dementia disorders.
The Alzheimer's Disease Research Center, which is jointly based at the Mayo Clinic campuses in Jacksonville, Florida, and Rochester, Minnesota, also provides care and services for patients with dementia disorders and their families.
Ultimately, researchers in the Alzheimer's Disease Research Center hope to prevent, delay and possibly cure Alzheimer's disease and other dementia disorders.
In creative, interdisciplinary collaboration, researchers in the Alzheimer's Disease Research Center conduct a wide range of investigations, such as the molecular workings of memory and clinical trials that test new drugs. The center is a leader in classifying and diagnosing different forms of early-stage cognitive changes and identifying predictive models of risk. The center also trains new scientists.
The Alzheimer's Disease Research Center also studies the entire spectrum of aging, including typical aging, mild cognitive impairment and dementia, in cooperation with the Mayo Clinic Study of Aging .
Research in the Alzheimer's Disease Research Center has led to the detection of biomarkers and advanced neuroimaging tests, in turn paving the way for potential new prevention therapies and treatments for early Alzheimer's disease.
Research Center of Excellence
The Alzheimer's Disease Research Center at Mayo Clinic's campus in Rochester, Minnesota, is a Lewy Body Dementia Association Research Center of Excellence and serves as the Coordinating Center for the entire Lewy Body Dementia Association Research Centers of Excellence program. This program currently consists of 33 of the nation's leading Lewy body dementia research institutions, which are committed to providing advanced Lewy body dementia care, community outreach and support.
Learn more about the Lewy Body Dementia Association Research Centers of Excellence .
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get more news and information from the Mayo Clinic Alzheimer’s Disease Research Center. Subscribe to Insights today.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Thank you for subscribing to Insights from the Mayo Clinic Alzheimer’s Disease Research Center. This newsletter provides timely information on research developments, upcoming events, support opportunities, and products and services that may be of interest to you. You may unsubscribe at any time by clicking on the unsubscribe link in any Insights e-mail.
By subscribing to Insights, you agree to receive the newsletter by email three times a year from the Mayo Clinic Alzheimer’s Disease Research Center. The newsletter may contain information on Mayo and non-Mayo products and services that may be of interest to you. If you have elected to receive other Mayo Clinic newsletters, they may be delivered more frequently. Please review our privacy policy to learn how we use and protect your information. You may opt out at any time by clicking on the unsubscribe link in any Insights email.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Patient support
For patients and families affected by Alzheimer's disease or a related dementia, the Alzheimer's Disease Research Center at Mayo Clinic offers:
- Opportunities to participate in drug trials, clinical research projects, special programs, support groups and education events
- Information, education and programs for research participants, patients and families
The director of the Alzheimer's Disease Research Center is Ronald C. Petersen, M.D., Ph.D.
The Mayo Clinic Alzheimer's Disease Research Center is one of 29 Alzheimer's disease centers in the U.S. funded by the National Institute on Aging.
Although each of the Alzheimer's disease centers has a unique focus, a common goal is to enhance research by sharing ideas, innovative strategies and research results.
- View Complete Calendar
Innovative strategies drive neuroscience research across the spectrum of aging, from healthy aging to dementia.
- Focus Areas - Research Focus Areas
- Developmental Projects - Research Developmental Projects
- Mayo Clinic Study of Aging - Research Mayo Clinic Study of Aging
- Data Requests - Research Data Requests

For Patients and Families
The center offers a variety of conferences, programs and events as well as a website, Dementia Hub, for research participants, patients, families and the community.
- Dementia Wellness and Education Programs - For Patients and Families Dementia Wellness and Education Programs
- Dementia Hub - For Patients and Families Dementia Hub
- Clinical Services - For Patients and Families Clinical Services
- Clinical Trials - For Patients and Families Clinical Trials

Contact the Alzheimer's Disease Research Center at Mayo Clinic with inquiries about research, education and support opportunities, or patient appointments.
- Contact the Center
- Patient Appointments
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Clinical Trials
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Alzheimer's Research & Therapy
Announcing the launch of mini reviews.

Concise overview articles of key topics in neurodegeneration, which can be read wherever you are, whenever suits you.
Find out more here .
New Thematic Series - AI in Dementia

Alzheimer's Research & Therapy presents a thematic series focusing on the use of artificial intelligence, machine learning and related techniques in dementia research.
Find out more about the series here .
- Most accessed
Alzheimer’s and neurodegenerative disease biomarkers in blood predict brain atrophy and cognitive decline
Authors: Heather E. Dark, Yang An, Michael R. Duggan, Cassandra Joynes, Christos Davatzikos, Guray Erus, Alexandria Lewis, Abhay R. Moghekar, Susan M. Resnick and Keenan A. Walker
Utilization of fluid-based biomarkers as endpoints in disease-modifying clinical trials for Alzheimer’s disease: a systematic review
Authors: Marlies Oosthoek, Lisa Vermunt, Arno de Wilde, Bram Bongers, Daniel Antwi-Berko, Philip Scheltens, Pieter van Bokhoven, Everard G. B. Vijverberg and Charlotte E. Teunissen
Effects of risk factors on the development and mortality of early- and late-onset dementia: an 11-year longitudinal nationwide population-based cohort study in South Korea
Authors: Min Young Chun, Wonjeong Chae, Sang Won Seo, Hyemin Jang, Jihwan Yun, Duk L. Na, Dongwoo Kang, Jungkuk Lee, Dustin B. Hammers, Liana G. Apostolova, Sung-In Jang and Hee Jin Kim
Association of midlife body-weight variability and cycles with earlier dementia onset: a nationwide cohort study
Authors: Yujin Park, Su Hwan Kim, Jiwon Ryu and Hyung-Jin Yoon
Associations of plasma neurofilament light chain with cognition and neuroimaging measures in community-dwelling early old age men
Authors: Rongxiang Tang, Erik Buchholz, Anders M. Dale, Robert A. Rissman, Christine Fennema-Notestine, Nathan A. Gillespie, Donald J Hagler Jr, Michael J. Lyons, Michael C. Neale, Matthew S. Panizzon, Olivia K. Puckett, Chandra A. Reynolds, Carol E. Franz, William S. Kremen and Jeremy A. Elman
Most recent articles RSS
View all articles
Stem cell factor and granulocyte colony-stimulating factor reduce β-amyloid deposits in the brains of APP/PS1 transgenic mice
Authors: Bin Li, Maria E Gonzalez-Toledo, Chun-Shu Piao, Allen Gu, Roger E Kelley and Li-Ru Zhao
Cerebral microbleeds: overview and implications in cognitive impairment
Authors: Sergi Martinez-Ramirez, Steven M Greenberg and Anand Viswanathan
BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease
Authors: Robert Vassar
A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody
Authors: Chad J. Swanson, Yong Zhang, Shobha Dhadda, Jinping Wang, June Kaplow, Robert Y. K. Lai, Lars Lannfelt, Heather Bradley, Martin Rabe, Akihiko Koyama, Larisa Reyderman, Donald A. Berry, Scott Berry, Robert Gordon, Lynn D. Kramer and Jeffrey L. Cummings
The Correction to this article has been published in Alzheimer's Research & Therapy 2022 14 :70
Alzheimer’s disease drug-development pipeline: few candidates, frequent failures
Authors: Jeffrey L Cummings, Travis Morstorf and Kate Zhong
Most accessed articles RSS
Early Career Researcher Reviewer Panel

Interested in an introduction to peer reviewing articles for Alzheimer's Research & Therapy?
Find out more about our ECR Peer Reviewer Panel!
Aims and scope
Alzheimer's Research & Therapy is the major forum for translational research into Alzheimer's disease. An international peer-reviewed journal, it publishes open access basic research with a translational focus, as well as clinical trials, research into drug discovery and development, and epidemiologic studies. The journal also provides reviews, viewpoints, commentaries, debates and reports.
Although the primary focus is Alzheimer's disease, the scope encompasses translational research into other neurodegenerative diseases.
Published Thematic Series

10 Years of Alzheimer's Research & Therapy
Subject Cognitive Decline
Latest Tweets
Your browser needs to have JavaScript enabled to view this timeline
Alzheimer's blogs from BMC
Failed to load RSS feed.
- Editorial Board
- Instructions for Editors
- Sign up for article alerts and news from this journal
Affiliated with

Annual Journal Metrics
2022 Citation Impact 9.0 - 2-year Impact Factor 9.2 - 5-year Impact Factor 1.849 - SNIP (Source Normalized Impact per Paper) 2.650 - SJR (SCImago Journal Rank)
2023 Speed 15 days submission to first editorial decision for all manuscripts (Median) 152 days submission to accept (Median)
2023 Usage 1,914,774 downloads 2,982 Altmetric mentions
- More about our metrics
ISSN: 1758-9193
- General enquiries: [email protected]
Study Suggests Treatments that Unleash Immune Cells in the Brain Could Help Combat Alzheimer’s
Posted on April 25th, 2024 by Dr. Monica M. Bertagnolli
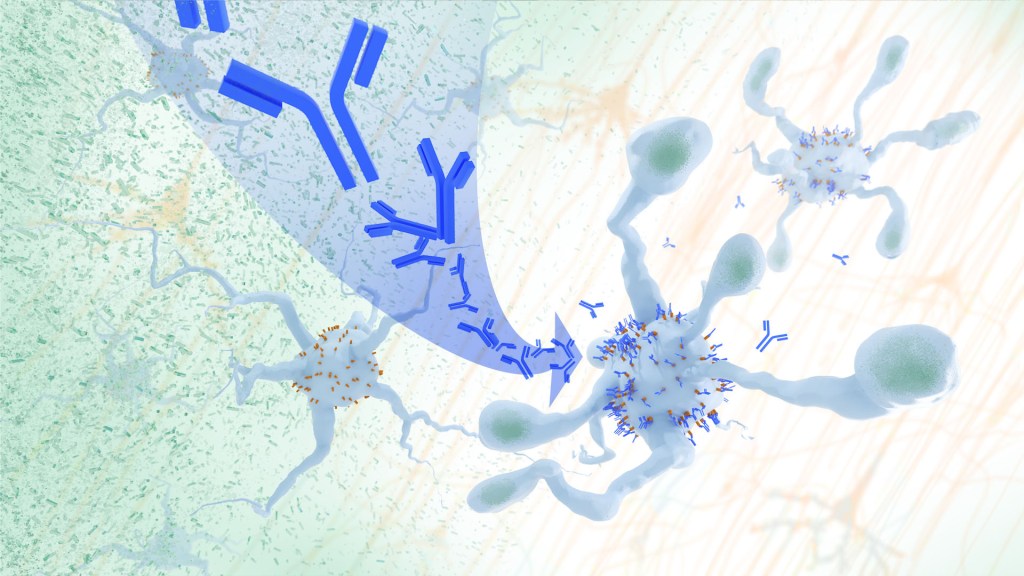
In Alzheimer’s disease, a buildup of sticky amyloid proteins in the brain clump together to form plaques, causing damage that gradually leads to worsening dementia symptoms. A promising way to change the course of this disease is with treatments that clear away damaging amyloid plaques or stop them from forming in the first place. In fact, the Food and Drug Administration recently approved the first drug for early Alzheimer’s that moderately slows cognitive decline by reducing amyloid plaques. 1 Still, more progress is needed to combat this devastating disease that as many as 6.7 million Americans were living with in 2023.
Recent findings from a study in mice, supported in part by NIH and reported in Science Translational Medicine , offer another potential way to clear amyloid plaques in the brain. The key component of this strategy is using the brain’s built-in cleanup crew for amyloid plaques and other waste products: immune cells known as microglia that naturally help to limit the progression of Alzheimer’s. The findings suggest it may be possible to develop immunotherapies—treatments that use the body’s immune system to fight disease—to activate microglia in the brains of people with Alzheimer’s and clear amyloid plaques more effectively. 2
In their report, the research team—including Marco Colonna , Washington University School of Medicine in St. Louis, and Jinchao Hou, now at Children’s Hospital of Zhejiang University School of Medicine in Zhejiang Province, China—wrote that microglia in the brain surround plaques to create a barrier that controls their spread. Microglia can also destroy amyloid plaques directly. But how microglia work in the brain depends on a fine-tuned balance of signals that activate or inhibit them. In people with Alzheimer’s, microglia don’t do their job well enough.
The researchers suspected this might have something to do with a protein called apolipoprotein E (APOE). This protein normally helps carry cholesterol and other fats in the bloodstream. But the gene encoding the protein is known for its role in influencing a person’s risk for developing Alzheimer’s, and in the Alzheimer’s brain, the protein is a key component of amyloid plaques. The protein can also inactivate microglia by binding to a receptor called LILRB4 found on the immune cells’ surfaces.
Earlier studies in mouse models of Alzheimer’s showed that the LILRB4 receptor is expressed at high levels in microglia when amyloid plaques build up. This suggested that treatments targeting this receptor on microglia might hold promise for treating Alzheimer’s. In the new study, the research team looked for evidence that an increase in LILRB4 receptors on microglia plays an important role in the brains of people with Alzheimer’s.
To do this, the researchers first studied brain tissue samples from people who died with this disease and discovered unusually high amounts of the LILRB4 receptor on the surfaces of microglia, similar to what had been seen in the mouse models. This could help explain why microglia struggle to control amyloid plaques in the Alzheimer’s brain.
Next, the researchers conducted studies of mouse brains with accumulating amyloid plaques that express the LILRB4 receptor to see if an antibody targeting the receptor could lower amyloid levels by boosting activity of immune microglia. Their findings suggest that the antibody treatment blocked the interaction between APOE proteins and LILRB4 receptors and enabled microglia to clear amyloid plaques. Intriguingly, the team’s additional studies found that this clearing process also changed the animals’ behavior, making them less likely to take risks. That’s important because people with Alzheimer’s may engage in risky behaviors as they lack memories of earlier experiences that they could use to make decisions.
There’s plenty more to learn. For instance, the researchers don’t know yet whether this approach will affect the tau protein , which forms damaging tangles inside neurons in the Alzheimer’s brain. They also want to investigate whether this strategy of clearing amyloid plaques might come with other health risks.
But overall, these findings add to evidence that immunotherapies of this kind could be a promising way to treat Alzheimer’s. This strategy may also have implications for treating other neurodegenerative conditions characterized by toxic debris in the brain, such as Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and Huntington’s disease. The hope is that this kind of research will ultimately lead to more effective treatments for Alzheimer’s and other conditions affecting the brain.
References:
[1] FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval . U.S. Food and Drug Administration (2023).
[2] Hou J, et al . Antibody-mediated targeting of human microglial leukocyte Ig-like receptor B4 attenuates amyloid pathology in a mouse model . Science Translational Medicine . DOI: 10.1126/scitranslmed.adj9052 (2024).
NIH Support: National Institute of General Medical Sciences, National Institute on Aging
Share this:
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: Health , News , Science
Tags: Alzheimer's treatment , Alzheimer’s disease , amyloid , basic research , brain , microglia , neurons , neuroscience
so, whats next? is it available? how to obtain this??
Keep up the good work
Leave a Comment Cancel reply
@nihdirector on x, nih on social media.
Kendall Morgan, Ph.D.
Comments and Questions
If you have comments or questions not related to the current discussions, please direct them to Ask NIH .
You are encouraged to share your thoughts and ideas. Please review the NIH Comments Policy

- Visitor Information
- Privacy Notice
- Accessibility
- No Fear Act
- HHS Vulnerability Disclosure
- U.S. Department of Health and Human Services
- USA.gov – Government Made Easy
Discover more from NIH Director's Blog
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading

Enter a Search Term
Alzheimer's disease research.
Driving innovative research around the world to end Alzheimer’s , we identify high-risk, high-reward projects that have the most promise to change the trajectory of the disease.
Our 360° Approach to Research
Tangling with tau, battling amyloid beta, blood and the brain in dementia, immunity and inflammation, biology of apoe and lipids, new approaches.
BrightFocus takes a 360 ° approach to fund innovative scientific research worldwide to defeat Alzheimer’s, exploring the full range of scientific paths toward better treatments and ultimately a cure. Watch this video and learn more.
Genes are the “master blueprint” that instructs our cells to make unique proteins which in turn build, operate, and repair human tissue. Humans have an estimated 24,000 genes along our 23 matched pairs of chromosomes (46 in all), and “genomics” refers to the field that studies all of them at once.

A biological marker (biomarker) is a measurable substance in an organism whose presence is indicative of some phenomenon such as disease or infection. Biomarkers can help doctors and scientists diagnose diseases and health conditions, find health risks in a person, monitor responses to treatment, and see how a person's disease or health condition changes over time.

Tau is a small protein with a short name but a large reputation because of its association with multiple brain diseases, including Alzheimer’s disease (AD). The tau protein is predominantly found in brain cells (neurons).

There are many versions of amyloid protein in the human body, and most serve a useful role. One of the hallmarks of Alzheimer’s disease (AD) is the accumulation of amyloid plaques (abnormally configured proteins) between nerve cells (neurons) in the brain.

Scientists are interested in developing a screening tool for Alzheimer’s disease (AD) in blood. A simple blood draw is much less invasive than a spinal tap and may prove more cost effective. Developing blood biomarkers that accurately depict brain changes has proven challenging, as levels of AD hallmark proteins in the blood are low, but there are some very recent promising results observing tau and the ratio of Aβ42 and Aβ40.

One theory about Alzheimer’s disease (AD) is that it may be triggered, in part, by a breakdown in the brain’s immune system.

Alzheimer's disease (AD). Its primary function is to regulate a class of proteins involved in the metabolism of fats (lipids) in the body. However, APOE has several common variants (or "alleles") whose effect vary.

The human brain has an estimated 100 billion neurons. Extending from each of them is a long fiber, known as an “axon,” which can run several feet. Each axon forms a connection, known as a “synapse” with another neuron, creating a circuit over which brain signals travel. In Alzheimer’s disease (AD), individual neurons die and do not regenerate; while others have brains that are more are resilient and respond to meeting changing demands.

Years of innovative and dedicated research have paid off with the discovery of numerous factors contributing to Alzheimer’s disease (AD) pathology. With a disease as complex as this one, it’s very helpful to find multiple points where it may be possible to slow or halt its progress.

Research We Fund
BrightFocus drives innovative research worldwide on Alzheimer’s, macular degeneration, and glaucoma. Search our grant awards to learn more.
Insights and Breakthroughs
Well-designed research pays off. With further research, each of these discoveries may contribute to the development of new treatments and preventions.

Driving Innovation in Diagnosis and Treatment: Alzheimer’s Disease Research Roundup

Searching the Eye for Signs of a Rare Form of Alzheimer’s

New Alzheimer’s Drug Leqembi Granted Full FDA Approval

What Can We Learn From 100-Year-Olds Without Alzheimer’s?

FDA Approves First Treatment for Alzheimer’s-Associated Agitation

Researchers Develop First-of-its-Kind Artificial Intelligence Model That Could Detect Alzheimer’s Through Retinal Photographs

Researchers Identify a Genetic Factor in People of African Ancestry That May Lower Alzheimer’s Risk

Moderate Alcohol Use May Accelerate Alzheimer’s Disease
Living with alzheimer's.
An Alzheimer’s diagnosis can be overwhelming. BrightFocus Foundation is a trusted resource to help you understand, manage and live with Alzheimer’s disease.
Learn from experts.
We're here to help.
Alzheimer's disease is the seventh leading cause of death in the United States. An irreversible degeneration of the brain that causes disruptions in memory, cognition, personality, and other functions, it eventually leads to death from complete brain failure.
Nearly 7 million Americans aged 65 and older are thought to have Alzheimer's disease. By 2050, that figure may increase to nearly 13 million.
Learn about Alzheimer's Disease
Alzheimer’s disease is the most common form of dementia, affecting more than six and a half million Americans aged 65 and older. In this section, you can find out more about Alzheimer’s and how you can manage care for yourself or a loved one.
- Alzheimer's Overview
- Brain Health
Donate to Alzheimer’s Disease Research
Your gift can help lead to treatments and a cure to end Alzheimer’s. Fund the latest, promising research and help provide valuable information to families living with this disease.
I would like to donate
The Eye, A Window on the Brain
It is often said that “the eyes are the window to the soul,” and while that may or may not be true, the eye is certainly a window into many health conditions.
In fact, sometimes an eye doctor will be the first physician to diagnose a medical condition because the first signs may appear in the eye. Thus, having your eyes thoroughly examined is a lot more than just getting a prescription for new glasses or contact lenses.
Useful Resources
BrightFocus Foundation offers vetted resources to help you and your loved ones better understand, manage and live with an Alzheimer’s diagnosis.
- Healthy Living
- Understanding Alzheimer's
- Managing Alzheimer's
- Living with Alzheimer's
Expert Advice
Useful articles to help you understand and manage symptoms, treatment, and the latest discoveries in Alzheimer's Disease Research.

When Alzheimer’s Disease Begins with Vision Problems

What’s Next for Alzheimer’s Disease Treatments: A 2024 Forecast

Alzheimer’s Treatment Coverage: 6 Facts to Know About Patient Registries

Facts About Leqembi, a New Alzheimer’s Drug

Exploring a Connection Between ADHD and Alzheimer’s

"Is It Something I'm Taking?" Medications That Can Mimic Dementia

Biohacking Brain Health: Research Exploring Fasting and Diet Changes Shows Promise in Delaying Alzheimer's Disease, Improving Cognition

Navigating Neurodegenerative Diseases: What Causes Neurodegeneration and Can It Be Stopped?

Can a Multivitamin Prevent Alzheimer’s Disease?

Alzheimer’s Blood Tests – What You Need to Know in 2023

Alzheimer’s Disease and COVID-19—What’s the Connection?
Take action, be part of the cure.
The most valuable assets we have are the individuals dedicated to our cause. People like you are the reason we are able to boost our scientific agenda so that patients’ lives may be enhanced.
Find an Alzheimer's Clinical Trial
These studies are crucial to advancing medical approaches most effective for specific conditions or groups of people. Today’s clinical trials will lead to new standards of care in the future.
Connect with @BrightFocus
Let’s stay in touch. BrightFocus is a top source of accurate, helpful information on the latest scientific research and care for Alzheimer’s, glaucoma, and macular degeneration.
Stay in touch.
Receive Alzheimer’s breakthrough news, research updates and inspiring stories

Basic science research featured at 2024 Vanderbilt Alzheimer’s Disease Research Day
Alexandra Scammell
Apr 24, 2024, 12:50 PM
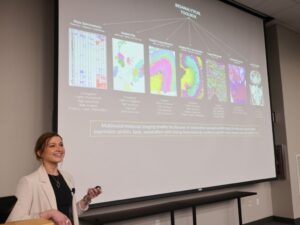
On April 10, 2024, the Vanderbilt Memory and Alzheimer’s Center hosted its fifth Annual Vanderbilt Alzheimer’s Disease Research Day, which included faculty presentations, data blitzes, a keynote presentation, and a poster reception with more than 45 posters. “Big data” was the theme of this year’s VMAC event, but any research touching on Alzheimer’s disease and dementia was welcome.
Several School of Medicine Basic Sciences faculty presented at Alzheimer’s Disease Research Day this year, including Angela Kruse , research instructor in the Department of Cell and Developmental Biology, and Timothy J. Hohman , professor of pharmacology.
In her presentation titled “Multimodal Imaging Mass Spectrometry: Connecting Omics and Imaging to Discover Molecular Drivers of Health and Disease,” Kruse highlighted the work of Claire Scott , graduate student in the Department of Cell and Developmental Biology. “Claire [said] she would heckle me if I made a mistake presenting her data,” Kruse admitted good-naturedly.

Scott’s research focuses on white matter hyperintensities, which indicate lesions in the white matter of the brain and are visible on MRI scans. “They are associated with lower cognition in Alzheimer’s disease and, importantly, they start to show up in individuals far before the development of cognitive symptoms,” Kruse said. But beyond associations with cognitive decline in Alzheimer’s disease patients, the molecular environments of white matter hyperintensities and the background of their formation is poorly understood.
“My research aims to address this knowledge gap by applying a multimodal approach to human Alzheimer’s disease brain tissue,” Scott explained after Kruse’s presentation. She combines MRI, imaging mass spectrometry, immunofluorescent microscopy, among other methods to perform her research. As more data are collected, Scott can begin to computationally distinguish the lipid alterations most indicative of white matter hyperintensities across human donors and investigate the metabolic pathways involved in these changes.
“I thoroughly enjoyed discussing my work with such thoughtful people,” Scott said about the event’s attendees. “The conversations were invigorating and I’m excited to pursue avenues that [we] discussed.”
Presentations continued throughout the day. Hohman, in his talk, discussed the ways experts analyze and integrate data from demographics, imaging, genetics, fluid biomarkers, and the clinic to create various disease models. The “hope and goal is to use these different layers of information” to provide personalized treatment for Alzheimer’s disease.
“We are pretty multidisciplinary team,” Hohman said. “We have folks on the team who focus entirely on genomics. We have people on the team who focus primarily on neuroimaging and cognitive phenotypes. Then we have folks who focus on multi-omics—transcriptomics and proteomics—and integration of the two of these.”
The all-day event also included poster and “data blitz” presentations. Various Basic Sciences community members participated, including Neil Dani , assistant professor of cell and developmental biology, Jared Phillips , graduate student in pharmacology, and Yuting Tan , postdoc in the molecular physiology and biophysics.
For Scott, Alzheimer’s Disease Research Day allowed her to make connections that will help her incorporate more human brain tissue samples into her research. Plus, she learned more about how and what patient data is collected in certain cohorts. For Scott, learning about current data management principles was “very interesting” and, as a researcher, was glad for the opportunity to learn about it. “Overall, I was struck by how nicely the research presented in talks and posters seemed to dovetail in powerful ways,” she said.
Explore Story Topics
- Discoveries
- Research, News & Discoveries
- Alzheimer’s disease
- Angela Kruse
- mass spectrometry
- Timothy J. Hohman
- Vanderbilt Memory and Alzheimer's Center
Call our 24 hours, seven days a week helpline at 800.272.3900

- Professionals

- Younger/Early-Onset Alzheimer's
- Is Alzheimer's Genetic?
- Women and Alzheimer's
- Creutzfeldt-Jakob Disease
- Dementia with Lewy Bodies
- Down Syndrome & Alzheimer's
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
- Traumatic Brain Injury (TBI)
- Know the 10 Signs
- Difference Between Alzheimer's & Dementia
- 10 Steps to Approach Memory Concerns in Others
- Medical Tests for Diagnosing Alzheimer's
- Why Get Checked?
- Visiting Your Doctor
- Life After Diagnosis
- Stages of Alzheimer's
- Earlier Diagnosis
- Part the Cloud
- Research Momentum
- Our Commitment to Research
- TrialMatch: Find a Clinical Trial
- What Are Clinical Trials?
- How Clinical Trials Work
- When Clinical Trials End
- Why Participate?
- Talk to Your Doctor
- Clinical Trials: Myths vs. Facts
- Can Alzheimer's Disease Be Prevented?
- Brain Donation
- Navigating Treatment Options
- Lecanemab Approved for Treatment of Early Alzheimer's Disease
- Aducanumab Discontinued as Alzheimer's Treatment
- Medicare Treatment Coverage
- Donanemab for Treatment of Early Alzheimer's Disease — News Pending FDA Review
- Questions for Your Doctor
- Medications for Memory, Cognition and Dementia-Related Behaviors
- Treatments for Behavior
- Treatments for Sleep Changes
- Alternative Treatments
- Facts and Figures
- Assessing Symptoms and Seeking Help
- Now is the Best Time to Talk about Alzheimer's Together
- Get Educated
- Just Diagnosed
- Sharing Your Diagnosis
- Changes in Relationships
- If You Live Alone
- Treatments and Research
- Legal Planning
- Financial Planning
- Building a Care Team
- End-of-Life Planning
- Programs and Support
- Overcoming Stigma
- Younger-Onset Alzheimer's
- Taking Care of Yourself
- Reducing Stress
- Tips for Daily Life
- Helping Family and Friends
- Leaving Your Legacy
- Live Well Online Resources
- Make a Difference
- Daily Care Plan
- Communication and Alzheimer's
- Food and Eating
- Art and Music
- Incontinence
- Dressing and Grooming
- Dental Care
- Working With the Doctor
- Medication Safety
- Accepting the Diagnosis
- Early-Stage Caregiving
- Middle-Stage Caregiving
- Late-Stage Caregiving
- Aggression and Anger
- Anxiety and Agitation
- Hallucinations
- Memory Loss and Confusion
- Sleep Issues and Sundowning
- Suspicions and Delusions
- In-Home Care
- Adult Day Centers
- Long-Term Care
- Respite Care
- Hospice Care
- Choosing Care Providers
- Finding a Memory Care-Certified Nursing Home or Assisted Living Community
- Changing Care Providers
- Working with Care Providers
- Creating Your Care Team
- Long-Distance Caregiving
- Community Resource Finder
- Be a Healthy Caregiver
- Caregiver Stress
- Caregiver Stress Check
- Caregiver Depression
- Changes to Your Relationship
- Grief and Loss as Alzheimer's Progresses
- Home Safety
- Dementia and Driving
- Technology 101
- Preparing for Emergencies
- Managing Money Online Program
- Planning for Care Costs
- Paying for Care
- Health Care Appeals for People with Alzheimer's and Other Dementias
- Social Security Disability
- Medicare Part D Benefits
- Tax Deductions and Credits
- Planning Ahead for Legal Matters
- Legal Documents
- ALZ Talks Virtual Events
- ALZNavigator™
- Veterans and Dementia
- The Knight Family Dementia Care Coordination Initiative
- Online Tools
- Asian Americans and Pacific Islanders and Alzheimer's
- Native Americans and Alzheimer's
- Black Americans and Alzheimer's
- Hispanic Americans and Alzheimer's
- LGBTQ+ Community Resources for Dementia
- Educational Programs and Dementia Care Resources
- Brain Facts
- 50 Activities
- For Parents and Teachers
- Resolving Family Conflicts
- Holiday Gift Guide for Caregivers and People Living with Dementia
- Trajectory Report
- Resource Lists
- Search Databases
- Publications
- Favorite Links
- 10 Healthy Habits for Your Brain
- Stay Physically Active
- Adopt a Healthy Diet
- Stay Mentally and Socially Active
- Online Community
- Support Groups
- Find Your Local Chapter
- Any Given Moment
- New IDEAS Study
- RFI Amyloid PET Depletion Following Treatment
- Bruce T. Lamb, Ph.D., Chair
- Christopher van Dyck, M.D.
- Cynthia Lemere, Ph.D.
- David Knopman, M.D.
- Lee A. Jennings, M.D. MSHS
- Karen Bell, M.D.
- Lea Grinberg, M.D., Ph.D.
- Malú Tansey, Ph.D.
- Mary Sano, Ph.D.
- Oscar Lopez, M.D.
- Suzanne Craft, Ph.D.
- About Our Grants
- Andrew Kiselica, Ph.D., ABPP-CN
- Arjun Masurkar, M.D., Ph.D.
- Benjamin Combs, Ph.D.
- Charles DeCarli, M.D.
- Damian Holsinger, Ph.D.
- David Soleimani-Meigooni, Ph.D.
- Donna M. Wilcock, Ph.D.
- Elizabeth Head, M.A, Ph.D.
- Fan Fan, M.D.
- Fayron Epps, Ph.D., R.N.
- Ganesh Babulal, Ph.D., OTD
- Hui Zheng, Ph.D.
- Jason D. Flatt, Ph.D., MPH
- Jennifer Manly, Ph.D.
- Joanna Jankowsky, Ph.D.
- Luis Medina, Ph.D.
- Marcello D’Amelio, Ph.D.
- Marcia N. Gordon, Ph.D.
- Margaret Pericak-Vance, Ph.D.
- María Llorens-Martín, Ph.D.
- Nancy Hodgson, Ph.D.
- Shana D. Stites, Psy.D., M.A., M.S.
- Walter Swardfager, Ph.D.
- ALZ WW-FNFP Grant
- Capacity Building in International Dementia Research Program
- ISTAART IGPCC
- Alzheimer’s Disease Strategic Fund: Endolysosomal Activity in Alzheimer’s (E2A) Grant Program
- Imaging Research in Alzheimer’s and Other Neurodegenerative Diseases
- Zenith Fellow Awards
- National Academy of Neuropsychology & Alzheimer’s Association Funding Opportunity
- Part the Cloud-Gates Partnership Grant Program: Bioenergetics and Inflammation
- Pilot Awards for Global Brain Health Leaders (Invitation Only)
- Robert W. Katzman, M.D., Clinical Research Training Scholarship
- Funded Studies
- How to Apply
- Portfolio Summaries
- Supporting Research in Health Disparities, Policy and Ethics in Alzheimer’s Disease and Dementia Research (HPE-ADRD)
- Diagnostic Criteria & Guidelines
- Annual Conference: AAIC
- Professional Society: ISTAART
- Alzheimer's & Dementia
- Alzheimer's & Dementia: DADM
- Alzheimer's & Dementia: TRCI
- International Network to Study SARS-CoV-2 Impact on Behavior and Cognition
- Alzheimer’s Association Business Consortium (AABC)
- Global Biomarker Standardization Consortium (GBSC)
- Global Alzheimer’s Association Interactive Network
- International Alzheimer's Disease Research Portfolio
- Alzheimer’s Disease Neuroimaging Initiative Private Partner Scientific Board (ADNI-PPSB)
- Research Roundtable
- About WW-ADNI
- North American ADNI
- European ADNI
- Australia ADNI
- Taiwan ADNI
- Argentina ADNI
- WW-ADNI Meetings
- Submit Study
- RFI Inclusive Language Guide
- Scientific Conferences
- AUC for Amyloid and Tau PET Imaging
- Make a Donation
- Walk to End Alzheimer's
- The Longest Day
- RivALZ to End ALZ
- Ride to End ALZ
- Tribute Pages
- Gift Options to Meet Your Goals
- Founders Society
- Fred Bernhardt
- Anjanette Kichline
- Lori A. Jacobson
- Pam and Bill Russell
- Gina Adelman
- Franz and Christa Boetsch
- Adrienne Edelstein
- For Professional Advisors
- Free Planning Guides
- Contact the Planned Giving Staff
- Workplace Giving
- Do Good to End ALZ
- Donate a Vehicle
- Donate Stock
- Donate Cryptocurrency
- Donate Gold & Sterling Silver
- Donor-Advised Funds
- Use of Funds
- Giving Societies
- Why We Advocate
- Ambassador Program
- About the Alzheimer’s Impact Movement
- Research Funding
- Improving Care
- Support for People Living With Dementia
- Public Policy Victories
- Planned Giving
- Community Educator
- Community Representative
- Support Group Facilitator or Mentor
- Faith Outreach Representative
- Early Stage Social Engagement Leaders
- Data Entry Volunteer
- Tech Support Volunteer
- Other Local Opportunities
- Visit the Program Volunteer Community to Learn More
- Become a Corporate Partner
- A Family Affair
- A Message from Elizabeth
- The Belin Family
- The Eliashar Family
- The Fremont Family
- The Freund Family
- Jeff and Randi Gillman
- Harold Matzner
- The Mendelson Family
- Patty and Arthur Newman
- The Ozer Family
- Salon Series
- No Shave November
- Other Philanthropic Activities
- Still Alice
- The Judy Fund E-blast Archive
- The Judy Fund in the News
- The Judy Fund Newsletter Archives
- Sigma Kappa Foundation
- Alpha Delta Kappa
- Parrot Heads in Paradise
- Tau Kappa Epsilon (TKE)
- Sigma Alpha Mu
- Alois Society Member Levels and Benefits
- Alois Society Member Resources
- Zenith Society
- Founder's Society
- Joel Berman
- JR and Emily Paterakis
- Legal Industry Leadership Council
- Accounting Industry Leadership Council
Find Local Resources
Let us connect you to professionals and support options near you. Please select an option below:
Use Current Location Use Map Selector
Search Alzheimer’s Association

Double Your Impact in the Fight to End Alzheimer's
Alzheimer's disease was first described in 1906. Since then, scientists have made remarkable strides in understanding how Alzheimer's affects the brain and learning how to make life better for affected individuals and families. Below are some important milestones in our progress, including the founding of the Alzheimer's Association in 1980, which has played a key role in advancing research and raising awareness of the disease.
1906-1960: First discovery
1970-1979: modern research, 1980-1989: awareness and momentum, 1990-1999: treatments emerge, 2000-2009: progress and hope, 2010-2019: setting a national agenda, 2020-present: a new era of treatment, dr. alois alzheimer first describes "a peculiar disease".
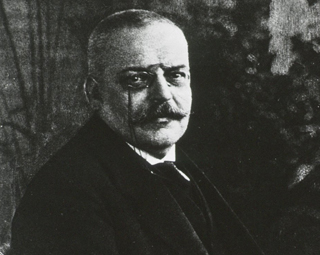
German physician Alois Alzheimer, a pioneer in linking symptoms to microscopic brain changes, describes the haunting case of Auguste D., a patient who had profound memory loss, unfounded suspicions about her family, and other worsening psychological changes. In her brain at autopsy, he saw dramatic shrinkage and abnormal deposits in and around nerve cells. Dr. Alzheimer died in 1915, never suspecting that his encounter with Auguste D. would one day touch the lives of millions and ignite a massive international research effort. Scientists recognize Dr. Alzheimer not only for his groundbreaking characterization of a major disease but also as a role model. He set a new standard for understanding neurodegenerative disorders by establishing a close clinical relationship with his patients and using new scientific tools to determine how symptoms related to physical brain changes.
Alzheimer's disease named
Emil Kraepelin, a German psychiatrist who worked with Dr. Alzheimer, first names "Alzheimer's Disease" in the eighth edition of his book "Psychiatrie."
Invention of electron microscope allows further study of brain
In 1931, Germans Max Knoll and Ernst Ruska co-invent the electron microscope, which can magnify up to 1 million times. It is not until after WWII that the electron microscope becomes common in major research settings, enabling scientists to study brain cells in more detail.
Development of cognitive measurement scales
Researchers develop the first validated measurement scale for assessing cognitive and functional decline in older adults, paving the way to correlate the level of measured impairment with estimates of the number of brain lesions and the volume of damaged tissue.
Founding of National Institute on Aging
An act of Congress establishes the National Institute on Aging (NIA) as one of our National Institutes of Health (NIH). The NIA is our primary federal agency supporting Alzheimer's research.
Alzheimer's recognized as most common cause of dementia
Neurologist Robert Katzman identifies Alzheimer's disease as the most common cause of dementia and a major public health challenge in his editorial published in Archives of Neurology.
Alzheimer's Association founded

In 1979, Jerome H. Stone and representatives from several family support groups met with the National Institute on Aging to explore the value of a national, independent, nonprofit organization to complement and stimulate federal efforts on Alzheimer's disease. That meeting resulted in the 1980 formation of the Alzheimer's Association with Mr. Stone as founding president. Today, the Alzheimer’s Association is the leading voluntary health organization in Alzheimer’s care, support and research.
Declaration of National Alzheimer's Disease Month
Awareness of Alzheimer's disease increases, leading Congress to designate November 1983 as the first National Alzheimer's Disease Month.
Beta-amyloid identified
Researchers George Glenner and Cai'ne Wong report identification of "a novel cerebrovascular amyloid protein," known as beta-amyloid — the chief component of Alzheimer's brain plaques and a prime suspect in triggering nerve cell damage.
Nationwide infrastructure for Alzheimer's research established
The NIA begins funding its network of Alzheimer's Disease Centers at flagship medical institutions, establishing a nationwide infrastructure for research, diagnosis and treatment.
Tau protein identified
Researchers discover that tau protein is a key component of tangles — the second pathological hallmark of Alzheimer's disease and another prime suspect in nerve cell degeneration.
First Alzheimer's drug trial
The Alzheimer's Association assists the NIA and Warner-Lambert Pharmaceutical Company (now Pfizer) in launching and recruiting participants for clinical trials of tacrine, the first drug specifically targeting symptoms of Alzheimer's disease.
First deterministic Alzheimer's gene identified
Researchers identify the first gene associated with rare, inherited forms of Alzheimer's disease. This gene on chromosome 21 codes amyloid precursor protein (APP), the parent molecule from which beta-amyloid is formed. Chromosome 21 is also the chromosome of which those with Down syndrome have three copies rather than two. Many individuals with Down syndrome develop Alzheimer's disease, often as young as their 30s and 40s.
Federal clinical study consortium launched
The NIA established the Alzheimer's Disease Cooperative Study (ADCS), a nationwide medical network to facilitate clinical research and conduct federally funded clinical trials.
First Alzheimer's risk factor gene identified
Researchers identify APOE-e4, a form of the apolipoprotein-E (APOE) gene on chromosome 19, as the first gene that raises risk for Alzheimer's but does not determine that a person who has it will develop the disease.
First Alzheimer's drug approved by FDA
The Food and Drug Administration (FDA) approves tacrine (Cognex) as the first drug specifically targeting Alzheimer's memory and thinking symptoms. Four additional drugs are approved over the next 10 years.
President Reagan's diagnosis announced

Former U.S. President Ronald Reagan shares with the American people that he has been diagnosed with Alzheimer's disease. In an open letter to the American people about his decision to share his diagnosis, President Reagan wrote, "In opening our hearts, we hope this might promote greater awareness of this condition. Perhaps it will encourage a clearer understanding of the individuals and families who are affected by it."

First World Alzheimer's Day
The first World Alzheimer's Day (WAD) launches on September 21 by Alzheimer's Disease International, the umbrella organization of Alzheimer's associations.
First transgenic mouse model announced
Researchers announce the first transgenic mouse model that developed Alzheimer-like brain pathology. The mouse was developed by inserting one of the human APP genes linked to a rare, inherited form of Alzheimer's disease. The Alzheimer's Association first awarded a grant to develop a mouse model of a rare neurodegenerative disorder called Gerstmann-Sträussler-Scheinker syndrome in 1989, laying the technical foundation for Alzheimer's mouse models.
"Alzheimer's vaccine" successful in mice
The first in a series of reports is published showing that injecting transgenic "Alzheimer" mice with beta-amyloid prevents the animals from developing plaques and other Alzheimer-like brain changes.
National Alzheimer's Disease Genetics Study begins
The Alzheimer's Association partners with the National Institute on Aging to recruit participants for the National Alzheimer's Disease Genetics Study, a federal initiative to collect and bank blood samples from families with several members who developed Alzheimer's disease late in life in order to identify additional Alzheimer's risk genes.
First report on Pittsburgh Compound B (PIB)
Researchers at the Alzheimer's Association International Conference on Alzheimer's Disease (AAICAD) share their first report on an imaging agent called Pittsburgh Compound B (PIB), a major potential breakthrough in disease monitoring and early detection. PIB enters the brain through the bloodstream and attaches itself to beta-amyloid deposits, where it can be detected by positron emission tomography (PET). The Alzheimer's Association provided significant support to initiatives to develop PIB and conduct preclinical testing it in animal studies.
Neuroimaging Initiative (ADNI)

The Alzheimer's Association joins public and private donors as a major sponsor of the Alzheimer's Disease Neuroimaging Initiative (ADNI), a nationwide study to establish standards for obtaining and interpreting brain images. The ultimate goal of ADNI is to determine whether standardized images, possibly combined with laboratory and psychological tests, can identify high-risk individuals; provide early detection; and track and monitor treatment effects, especially in clinical trials of disease-modifying drugs. In 2006, the Association launched the European Alzheimer's Disease Neuroimaging Initiative (E-ADNI), to expand ADNI's scope by combining data from several European brain imaging initiatives with ADNI data. This effort has now grown into World Wide ADNI (WW-ADNI), a global network of flagship research sites united in a common effort to improve diagnosis and speed treatment development with standardized protocols and data shared internationally.
Alzheimer's & Dementia® journal launched
The Alzheimer's Association launches Alzheimer's & Dementia®: The Journal of the Alzheimer's Association to further support a global, interdisciplinary exchange within the Alzheimer's research community.
Healthy Brain Initiative launched
The Alzheimer’s Association and the U.S. Centers for Disease Control and Prevention launch the Healthy Brain Initiative with the publication of A National Public Health Road Map to Maintaining Cognitive Health. The Road Map advances 44 science-based actions emphasizing primary prevention of cognitive impairment. The goal of this initiative is to maintain or improve the cognitive performance of all adults.
International Society to Advance Alzheimer's Research and Treatment formed
To further the work of the global Alzheimer's research community, the Alzheimer's Association creates the International Society to Advance Alzheimer's Research and Treatment (ISTAART), the first and only professional society dedicated to Alzheimer's and dementia.
International Conference on Alzheimer's Disease becomes an annual event
With accelerating progress intensifying the need for global information exchange, the Alzheimer's Association International Conference on Alzheimer's Disease® (AAICAD®) becomes an annual event.
Effort to standardize biomarkers begins
The Alzheimer's Association announces funding of the Alzheimer's Association QC Program for CSF Biomarkers to help overcome variation among institutions in measuring potential biomarkers in cerebrospinal fluid (CSF).
Alzheimer's researchers unite to raise awareness and concern
Dozens of Alzheimer's researchers unite with the Alzheimer's Association for an "Alzheimer's Breakthrough Ride®," a 66-day bike relay across America to raise public and congressional awareness of the urgent need for more federal funding to support the search for effective Alzheimer's treatments.
Alzheimer's clinical trial database established
The Alzheimer's Association and its partners in the Coalition Against Major Diseases (CAMD) released a first-of-its kind database of 4,000 patients who participated in 11 pharmaceutical industry-sponsored clinical trials of Alzheimer's treatments. The combined data, accessible to any qualified researcher, will offer unprecedented power to understand the course of Alzheimer's.
Alzheimer's Association TrialMatch® launched

The Association launches TrialMatch®, a free, easy-to-use clinical studies matching service that connects individuals living with Alzheimer's disease, caregivers, and healthy volunteers with current research studies. Stakeholders unanimously identified building awareness of research studies and increasing enrollment as key strategies to accelerate treatment development.
An influential model of biomarker changes during Alzheimer’s disease progression is first published
A group of researchers publish a working model relating changes in Alzheimer’s biomarkers to disease stage and symptom severity. The model has become a focal point of research into Alzheimer’s biomarkers, and is revised periodically to account for new research.
President Obama signs National Alzheimer's Project Act (NAPA) into law
Groundbreaking legislation establishes our first-ever framework for a national strategic plan to address the Alzheimer's crisis and to coordinate our response on multiple fronts, including research, care and support.
New criteria and guidelines for Alzheimer's disease diagnosis
Three workgroups convened by the Alzheimer's Association and the National Institute on Aging issue updated criteria and guidelines for diagnosing Alzheimer's disease and propose a research agenda to define a new preclinical stage.
Annual assessment for cognitive impairment for all Medicare Beneficiaries implemented as part of Annual Wellness visits
The Centers for Medicare and Medicaid Services implement Annual Wellness Visits for all Medicare Beneficiaries under the Patient Protection and Affordable Care Act. A mandatory part of the Annual Wellness Visit is an assessment for detection of cognitive impairment.
First major clinical trial for prevention of Alzheimer’s disease is initiated
A multinational research consortium, the Dominantly Inherited Alzheimer Network, launches the first major clinical trial testing drug therapy to prevent the onset of Alzheimer’s disease symptoms in people who inherited an autosomal dominant mutation putting them at high risk for the disease.
International Genomics of Alzheimer’s Project (IGAP) researchers identify new genetic risk factors for Alzheimer’s disease
Hundreds of researchers from around the world collaborate to perform a meta-analysis of genome-wide association studies intended to identify genetic variations linked with an increased risk for Alzheimer’s disease. The collaboration revealed 20 genetic variations associated with increased risk, 11 of which had not been linked with Alzheimer’s before. Some of the newly identified genetic variations are thought to be specific to the immune system, adding to mounting evidence of a role for the immune system in Alzheimer’s disease.
Rates of death caused by Alzheimer’s disease found to be much higher than reported on death certificates
Researchers at Rush University find that the annual number of deaths attributable to Alzheimer’s disease in the U.S. among people at least 75 years old is about 500,000, much higher than the number reported on death certificates (>84,000).
Alzheimer's Accountability Act signed into law
The Alzheimer's Association led the fight for this revolutionary law that allows scientists at the NIH to submit an annual research budget directly to Congress.
Historic funding increase
Historic $400 million increase for federal Alzheimer’s disease research funding signed into law, bringing annual funding to $1.4 billion.
Dementia Care Practice Recommendations published
Alzheimer’s Association released Dementia Care Practice Recommendations aimed at helping professional care providers deliver optimal quality, person-centered care for those living with Alzheimer’s and other dementias.
International consortium established to improve care and psychosocial outcomes
The National Institutes of Health (NIH) awarded the Alzheimer’s Association $1.34 million over five years for an international research network, Leveraging an Interdisciplinary Consortium to Improve Care and Outcomes for Persons Living with Alzheimer’s and Dementia (LINC-AD), to improve care and psychosocial outcomes for individuals living with dementia.
Bill Gates helps fund Part the Cloud Research Grant Program
Bill Gates joined the Alzheimer’s Association’s Part the Cloud global research grant program with a $10 million award that will stimulate an additional $20 million in funding to the Alzheimer’s Association.
Alzheimer’s funding reaches all-time high

A $350 million increase for Alzheimer’s and dementia research funding at the National Institutes of Health (NIH) was signed into law, bringing the annual funding to $2.8 billion. An additional $10 million was also approved for the BOLD Infrastructure for Alzheimer’s Act.
Aducanumab approved for treatment of Alzheimer’s disease
Aducanumab (Aduhelm®) received accelerated approval as a treatment for Alzheimer's disease by the U.S. Food and Drug Administration (FDA). This is the first FDA-approved therapy to address the underlying biology of Alzheimer's disease. (Aducanumab will be discontinued on Nov. 1, 2024. Please connect with your provider on treatment options.)
Lecanemab receives traditional approval for treatment of Alzheimer's disease
Lecanemab (Leqembi®) received traditional approval as a treatment for early Alzheimer's from the FDA. It is the first traditionally approved treatment that addresses the underlying biology of Alzheimer's and changes the course of the disease in a meaningful way for people in the early stages.
Donanemab results show drug slows progression of early Alzheimer's
Phase 3 clinical trial results showed that the drug donanemab (which is not yet FDA-approved) significantly slowed cognitive and functional decline in people living with early symptomatic Alzheimer's disease (either mild cognitive impairment or mild dementia).
CMS decides to cover PET imaging for Alzheimer's disease diagnosis
A policy change by the Centers for Medicare & Medicaid Services (CMS) expanded coverage of brain amyloid positron emission tomography (PET) imaging for the diagnosis of Alzheimer's disease, making a valuable tool more accessible across the country.
Alzheimer's Association announces milestone $100 million research investment
The Alzheimer's Association, the world's largest nonprofit funder of Alzheimer's and dementia research, invested a milestone $100 million in research initiatives in 2023, the largest single-year total since the organization's founding in 1980.
Keep Up With Alzheimer’s News and Events

Alzheimer’s Disease Research
What Has Guided Research So Far and Why It Is High Time for a Paradigm Shift
- © 2023
- Christian Behl 0
Institute of Pathobiochemistry, University Medical Center of the Johannes Gutenberg University, Mainz, Germany
You can also search for this author in PubMed Google Scholar
- Aims to answer why—after more than 100 years of Alzheimer's research—there is still no convincing therapy available
- Informs on leading perspectives and key developments of Alzheimer's research from its beginnings up until today
- Promotes a paradigm shift in Alzheimer's Disease research and a greater openness towards new disease hypotheses
10k Accesses
5 Citations
36 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
Table of contents (21 chapters)
Front matter, introduction.
Christian Behl
The Psychiatrist and Pathologist Aloysius Alzheimer and His Seminal Findings
Alzheimer’s disease research after 1945: the recommencement, alzheimer’s research goes deeper: ultrastructural electron microscopy studies, focus on neurochemistry led to the cholinergic hypothesis of alzheimer’s disease, the glutamatergic hypothesis of alzheimer’s disease, biochemistry and genetics point out a prime suspect for causing alzheimer’s disease, getting to the bottom of it: amyloid beta peptide is derived from a larger precursor, step by step toward an amyloid beta peptide-based hypothesis of alzheimer’s disease, concerns about the amyloid cascade hypothesis and reappraisals, ignorance or conspiracy or just an amyloid firewall that blocks alternative ideas, in the slip stream of amyloid: the tau and tangle hypothesis, focus on tauopathies and beyond, alzheimer’s research gains momentum and spreads out, the amyloid cascade hypothesis has to deliver, finally, beyond app , psen1 , psen2 , and apoe : what else does the genome tell us, alternative hypotheses and observations that were somehow lost on the way, is the persistence of the amyloid cascade hypothesis a result of constant confirmation bias, driving forces of alzheimer’s research directions.
- Alzheimer Clinics
- Alzheimer Therapy
- Alzheimer's Disease
- Amyloid Plaques
- Amyloid-Cascade-Hypothesis
- Agenda Setting
- Aternative Hypotheses
- Risk Factors
About this book
This book highlights the key phases and central findings of Alzheimer’s Disease research since the introduction of the label ‘Alzheimer’s Disease’ in 1910. The author, Christian Behl, puts dementia research in the context of the respective zeitgeist and summarizes the paths that have led to the currently available Alzheimer’s drugs. As the reader is taken through the major developments in Alzheimer's Disease research, particularly over the past thirty years, Behl poses critical questions: Why are the exact causes of Alzheimer's Disease still in the dark, despite all the immense, worldwide research efforts in academia as well as in the pharmaceutical industry? Why has the majority of an entire research field kept focusing on a single hypothesis that establishes the deposition of the amyloid beta peptide in the brain as the key trigger of Alzheimer's pathology, even though this concept has still not been convincingly proven in the clinics? Are there other hypotheses that might explainthe pathogenesis of this complex brain disease, and if so, why were these perspectives not adequately followed?
In this book, Behl tries to answer these questions. Starting with the historical background, the author illustrates the long and arduous research journey, its numerous setbacks, and the many alternative explanations for the disease, which have started gaining increasing attention and acceptance in the Alzheimer’s research community only more recently.
With his deep dive into the history and progression of this research, including the most recent developments, Behl explains why he believes that it is high time to promote a paradigm shift in Alzheimer’s Disease research.
Authors and Affiliations
About the author.
Christian Behl is Professor of Pathobiochemistry and Director of the Institute of Pathobiochemistry at the University Medical Center of the Johannes Gutenberg University Mainz, Germany. He has been closely following Alzheimer’s Disease research since the early 1990’s, when he first got involved into the field himself during his time at the Salk Institute for Biological Studies, La Jolla, USA. He stayed active in the field all through his research station at the Max Planck Institute of Psychiatry, Munich, Germany, and later in Mainz. There his current research (in Mainz) focuses on the cellular degradation mechanism autophagy in the context of neurodegeneration and aging. For quite some time Behl has been an active advocate for widening the focus of Alzheimer’s Disease research to improve the understanding of this complex, age-related brain disorder. Behl is member of several scientific boards, including the German Alzheimer Foundation.
Bibliographic Information
Book Title : Alzheimer’s Disease Research
Book Subtitle : What Has Guided Research So Far and Why It Is High Time for a Paradigm Shift
Authors : Christian Behl
DOI : https://doi.org/10.1007/978-3-031-31570-1
Publisher : Springer Cham
eBook Packages : Biomedical and Life Sciences , Biomedical and Life Sciences (R0)
Copyright Information : The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG 2023
Hardcover ISBN : 978-3-031-31569-5 Published: 14 July 2023
Softcover ISBN : 978-3-031-31572-5 Due: 14 August 2023
eBook ISBN : 978-3-031-31570-1 Published: 13 July 2023
Edition Number : 1
Number of Pages : XXV, 652
Number of Illustrations : 9 b/w illustrations, 107 illustrations in colour
Topics : Neurosciences , Neurology , Physiology , Cognitive Psychology , Neurosciences , Neurosciences
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Alzheimer's disease drug development pipeline: 2024
Affiliations.
- 1 Chambers-Grundy Center for Transformative Neuroscience Department of Brain Health School of Integrated Health Sciences University of Nevada, Las Vegas (UNLV) Las Vegas Nevada USA.
- 2 Genomic Medicine Institute Lerner Research Institute, Cleveland Clinic Cleveland Ohio USA.
- 3 Howard R Hughes College of Engineering Department of Computer Science University of Nevada, Las Vegas (UNLV) Las Vegas Nevada USA.
- 4 Department of Molecular Medicine Cleveland Clinic Lerner College of Medicine Case Western Reserve University Cleveland Ohio USA.
- 5 Case Comprehensive Cancer Center Case Western Reserve University School of Medicine Cleveland Ohio USA.
- 6 Cleveland Clinic Genome Center Lerner Research Institute, Cleveland Clinic Cleveland Ohio USA.
- PMID: 38659717
- PMCID: PMC11040692
- DOI: 10.1002/trc2.12465
Introduction: New therapies to prevent or delay the onset of symptoms, slow progression, or improve cognitive and behavioral symptoms of Alzheimer's disease (AD) are needed.
Methods: We interrogated clinicaltrials.gov including all clinical trials assessing pharmaceutical therapies for AD active in on January 1, 2024. We used the Common Alzheimer's Disease Research Ontology (CADRO) to classify the targets of therapies in the pipeline.
Results: There are 164 trials assessing 127 drugs across the 2024 AD pipeline. There were 48 trials in Phase 3 testing 32 drugs, 90 trials in Phase 2 assessing 81 drugs, and 26 trials in Phase 1 testing 25 agents. Of the 164 trials, 34% ( N = 56) assess disease-modifying biological agents, 41% ( N = 68) test disease-modifying small molecule drugs, 10% ( N = 17) evaluate cognitive enhancing agents, and 14% ( N = 23) test drugs for the treatment of neuropsychiatric symptoms.
Discussion: Compared to the 2023 pipeline, there are fewer trials (164 vs. 187), fewer drugs (127 vs. 141), fewer new chemical entities (88 vs. 101), and a similar number of repurposed agents (39 vs. 40).
Highlights: In the 2024 Alzheimer's disease drug development pipeline, there are 164 clinical trials assessing 127 drugs.The 2024 Alzheimer's disease drug development pipeline has contracted compared to the 2023 Alzheimer pipeline with fewer trials, fewer drugs, and fewer new chemical entities.Drugs in the Alzheimer's disease drug development pipeline target a wide array of targets; the most common processes targeted include neurotransmitter receptors, inflammation, amyloid, and synaptic plasticity.The total development time for a potential Alzheimer's disease therapy to progress from nonclinical studies to FDA review is approximately 13 years.
© 2024 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Publication types
- English Abstract
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 13 May 2021
Alzheimer disease
- David S. Knopman ORCID: orcid.org/0000-0002-6544-066X 1 ,
- Helene Amieva 2 ,
- Ronald C. Petersen 1 ,
- Gäel Chételat 3 ,
- David M. Holtzman 4 ,
- Bradley T. Hyman ORCID: orcid.org/0000-0002-7959-9401 5 ,
- Ralph A. Nixon 6 , 7 &
- David T. Jones ORCID: orcid.org/0000-0002-4807-9833 1
Nature Reviews Disease Primers volume 7 , Article number: 33 ( 2021 ) Cite this article
42k Accesses
758 Citations
217 Altmetric
Metrics details
- Alzheimer's disease
- Diagnostic markers
- Translational research
Alzheimer disease (AD) is biologically defined by the presence of β-amyloid-containing plaques and tau-containing neurofibrillary tangles. AD is a genetic and sporadic neurodegenerative disease that causes an amnestic cognitive impairment in its prototypical presentation and non-amnestic cognitive impairment in its less common variants. AD is a common cause of cognitive impairment acquired in midlife and late-life but its clinical impact is modified by other neurodegenerative and cerebrovascular conditions. This Primer conceives of AD biology as the brain disorder that results from a complex interplay of loss of synaptic homeostasis and dysfunction in the highly interrelated endosomal/lysosomal clearance pathways in which the precursors, aggregated species and post-translationally modified products of Aβ and tau play important roles. Therapeutic endeavours are still struggling to find targets within this framework that substantially change the clinical course in persons with AD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Is Alzheimer disease a disease?

The Amyloid-β Pathway in Alzheimer’s Disease

Emerging diagnostics and therapeutics for Alzheimer disease
Jessen, F. et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 10 , 844–852 (2014).
PubMed PubMed Central Google Scholar
Petersen, R. C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256 , 183–194 (2004).
CAS PubMed Google Scholar
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 7 , 263–269 (2011).
Petersen, R. C. How early can we diagnose Alzheimer disease (and is it sufficient)? The 2017 Wartenberg lecture. Neurology 91 , 395–402 (2018).
Nelson, P. T. et al. Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 121 , 571–587 (2011).
Boyle, P. A. et al. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann. Neurol. 83 , 74–83 (2018). A clinical-neuropathological analysis of >1,000 persons demonstrating how multiple aetiologies relate to late-life cognition.
CAS PubMed PubMed Central Google Scholar
Schneider, J. A., Arvanitakis, Z., Leurgans, S. E. & Bennett, D. A. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 66 , 200–208 (2009).
Kapasi, A., DeCarli, C. & Schneider, J. A. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 134 , 171–186 (2017).
Karanth, S. et al. Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol. 77 , 1299–1307 (2020).
Brodaty, H. et al. The world of dementia beyond 2020. J. Am. Geriatr. Soc. 59 , 923–927 (2011).
PubMed Google Scholar
Wu, Y. T. et al. The changing prevalence and incidence of dementia over time — current evidence. Nat. Rev. Neurol. 13 , 327–339 (2017).
Satizabal, C. L. et al. Incidence of dementia over three decades in the Framingham Heart Study. N. Engl. J. Med. 374 , 523–532 (2016).
Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 11 , 1006–1012 (2012).
Tom, S. E. et al. Trends in incident dementia and early life socieoeconomic status by birth cohort in the adult changes in thought study. JAMA Open 3 , e2011094 (2020).
Google Scholar
Kovari, E., Herrmann, F. R., Bouras, C. & Gold, G. Amyloid deposition is decreasing in aging brains: an autopsy study of 1,599 older people. Neurology 82 , 326–331 (2014).
Niu, H., Álvarez-Álvarez, I., Guillén-Grima, F. & Aguinaga-Ontoso, I. Prevalence and incidence of Alzheimer’s disease in Europe: a meta-analysis. Neurologia 32 , 523–532 (2017).
Hy, L. X. & Keller, D. M. Prevalence of AD among whites: a summary by levels of severity. Neurology 55 , 198–204 (2000).
Gillis, C., Mirzaei, F., Potashman, M., Ikram, M. A. & Maserejian, N. The incidence of mild cognitive impairment: a systematic review and data synthesis. Alzheimers Dement. 11 , 248–256 (2019).
Petersen, R. C. et al. Mild cognitive impairment due to Alzheimer’s disease: criteria in the community. Ann. Neurol. 74 , 199–208 (2013).
Degenhardt, E. K. et al. Florbetapir F18 PET amyloid neuroimaging and characteristics in patients with mild and moderate Alzheimer dementia. Psychosomatics 57 , 208–216 (2016).
Robinson, J. L. et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141 , 2181–2193 (2018).
Prince, M. et al. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res. Ther. 8 , 23 (2016).
Röhr, S. et al. Estimating prevalence of subjective cognitive decline in and across international cohort studies of aging: a COSMIC study. Alzheimers Res. Ther. 12 , 167 (2020).
Petersen, R. C. et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology. Neurology 90 , 126–135 (2018).
Petersen, R. C. et al. Prevalence of mild cognitive impairment is higher in men than in women. The Mayo Clinic Study of Aging. Neurology 75 , 889–897 (2010).
Mielke, M. M., Vemuri, P. & Rocca, W. A. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin. Epidemiol. 6 , 37–48 (2014).
Thambisetty, M., An, Y. & Tanaka, T. Alzheimer’s disease risk genes and the age-at-onset phenotype. Neurobiol. Aging 34 , 2696.e1–5 (2013).
CAS Google Scholar
Haass, C., Kaether, C., Thinakaran, G. & Sisodia, S. Trafficking and proteolytic processing of APP. Cold Spring Harb. Perspect. Med. 2 , a006270 (2012).
Jonsson, T. et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488 , 96–99 (2012).
van der Lee, S. J. et al. The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: a community-based cohort study. Lancet Neurol. 17 , 434–444 (2018).
Bellenguez, C. et al. Contribution to Alzheimer’s disease risk of rare variants in TREM2, SORL1, and ABCA7 in 1779 cases and 1273 controls. Neurobiol. Aging 59 , 220.e1–220.e9 (2017).
Leonenko, G. et al. Polygenic risk and hazard scores for Alzheimer’s disease prediction. Ann. Clin. Transl. Neurol. 6 , 456–465 (2019).
Karch, C. M., Cruchaga, C. & Goate, A. M. Alzheimer’s disease genetics: from the bench to the clinic. Neuron 83 , 11–26 (2014).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51 , 414–430 (2019).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet 396 , 413–446 (2020).
Singh-Manoux, A. et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry 74 , 712–718 (2017).
Gottesman, R. F. et al. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 74 , 1246–1254 (2017).
Samieri, C. et al. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA 320 , 657–664 (2018).
Gottesman, R. F. et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 317 , 1443–1450 (2017).
Vemuri, P. et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 138 , 761–771 (2015).
Golde, T. E., DeKosky, S. T. & Galasko, D. Alzheimer’s disease: the right drug, the right time. Science 362 , 1250–1251 (2018).
Herrup, K. The case for rejecting the amyloid cascade hypothesis. Nat. Neurosci. 18 , 794–799 (2015).
Zlokovic, B. V. et al. Vascular contributions to cognitive impairment and dementia (VCID): a report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement. 16 , 1714–1733 (2020).
Arnold, S. E., Hyman, B. T., Flory, J., Damasio, A. R. & Van Hoesen, G. W. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb. Cortex 1 , 103–116 (1991). Graphic demonstration of the different regional distributions of Aβ and tau at autopsy.
Montine, T. J. et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 123 , 1–11 (2012).
Haass, C. & Selkoe, D. J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 8 , 101–112 (2007).
Holtzman, D. M., Morris, J. C. & Goate, A. M. Alzheimer’s disease: the challenge of the second century. Sci. Transl. Med. 3 , 77sr71 (2011).
Thinakaran, G. & Koo, E. H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 283 , 29615–29619 (2008).
Haass, C. & Willem, M. Secreted APP modulates synaptic activity: a novel target for therapeutic intervention? Neuron 101 , 557–559 (2019).
Cirrito, J. R. et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron 48 , 913–922 (2005).
Kang, J. E. et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science 326 , 1005–1007 (2009).
Boespflug, E. L. & Iliff, J. J. The emerging relationship between interstitial fluid-cerebrospinal fluid exchange, amyloid-β, and sleep. Biol. Psychiatry 83 , 328–336 (2018).
Spires-Jones, T. L. & Hyman, B. T. The intersection of amyloid β and tau at synapses in Alzheimer’s disease. Neuron 82 , 756–771 (2014).
Benarroch, E. E. Glutamatergic synaptic plasticity and dysfunction in Alzheimer disease: emerging mechanisms. Neurology 91 , 125–132 (2018).
Rice, H. C. et al. Secreted amyloid-β precursor protein functions as a GABA(B)R1a ligand to modulate synaptic transmission. Science 363 , eaao4827 (2019).
Müller, U. C., Deller, T. & Korte, M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 18 , 281–298 (2017).
Small, S. A. & Petsko, G. A. Endosomal recycling reconciles the Alzheimer’s disease paradox. Sci. Transl. Med. 12 , eabb1717 (2020).
Gallardo, G. & Holtzman, D. M. Amyloid-β and tau at the crossroads of Alzheimer’s disease. Adv. Exp. Med. Biol. 1184 , 187–203 (2019).
Pooler, A. M., Noble, W. & Hanger, D. P. A role for tau at the synapse in Alzheimer’s disease pathogenesis. Neuropharmacology 76 (Pt A), 1–8 (2014).
Eftekharzadeh, B. et al. Tau protein disrupts nucleocytoplasmic transport in Alzheimer’s disease. Neuron 99 , 925–940.e7 (2018).
Kent, S. A., Spires-Jones, T. L. & Durrant, C. S. The physiological roles of tau and Aβ: implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol. 140 , 417–447 (2020).
Yamada, K. et al. Neuronal activity regulates extracellular tau in vivo. J. Exp. Med. 211 , 387–393 (2014).
Wu, J. W. et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci. 19 , 1085–1092 (2016).
de Calignon, A. et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73 , 685–697 (2012).
Dujardin, S. et al. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 26 , 1256–1263 (2020). A clinical-neuropathological-molecular demonstration of how post-translational modifications of tau might play a role in the rate of clinical progression.
Shi, Y. et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549 , 523–527 (2017).
Gratuze, M. et al. Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration. J. Clin. Invest. 130 , 4954–4968 (2020).
Shi, Y. et al. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J. Exp. Med. 216 , 2546–2561 (2019).
Braak, H., Thal, D. R., Ghebremedhin, E. & Del Tredici, K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 70 , 960–969 (2011).
Braak, H. & Braak, E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 82 , 239–259 (1991).
Delacourte, A. et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 52 , 1158–1165 (1999).
Price, J. L. & Morris, J. C. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol. 45 , 358–368 (1999).
Delacourte, A. et al. Tau aggregation in the hippocampal formation: an ageing or a pathological process? Exp. Gerontol. 37 , 1291–1296 (2002).
Crary, J. F. et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 128 , 755–766 (2014).
Jack, C. R. et al. The bivariate distribution of amyloid-β and tau: relationship with established neurocognitive clinical syndromes. Brain 142 , 3230–3242 (2019).
Hanseeuw, B. J. et al. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 76 , 915–924 (2019).
Ossenkoppele, R. et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139 , 1551–1567 (2016).
Graff-Radford, J. et al. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 20 , 222–234 (2021).
Kim, J., Basak, J. M. & Holtzman, D. M. The role of apolipoprotein E in Alzheimer’s disease. Neuron 63 , 287–303 (2009).
Huynh, T. V., Davis, A. A., Ulrich, J. D., Holtzman, D. M. & Apolipoprotein, E. and Alzheimer’s disease: the influence of apolipoprotein E on amyloid-β and other amyloidogenic proteins. J. Lipid Res. 58 , 824–836 (2017).
Reiman, E. M. et al. Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc. Natl Acad. Sci. USA 106 , 6820–6825 (2009).
Jack, C. R. Jr. et al. Age, sex, and APOE e4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol. 72 , 511–519 (2015).
van der Kant, R., Goldstein, L. S. B. & Ossenkoppele, R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 21 , 21–35 (2020).
Small, S. A. & Duff, K. Linking Aβ and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron 60 , 534–542 (2008).
Busche, M. A. & Hyman, B. T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 23 , 1183–1193 (2020).
Kurt, M. A., Davies, D. C., Kidd, M., Duff, K. & Howlett, D. R. Hyperphosphorylated tau and paired helical filament-like structures in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes. Neurobiol. Dis. 14 , 89–97 (2003).
Le, R. et al. Plaque-induced abnormalities in neurite geometry in transgenic models of Alzheimer disease: implications for neural system disruption. J. Neuropathol. Exp. Neurol. 60 , 753–758 (2001).
Leyns, C. E. G. et al. TREM2 function impedes tau seeding in neuritic plaques. Nat. Neurosci. 22 , 1217–1222 (2019).
Small, S. A., Simoes-Spassov, S., Mayeux, R. & Petsko, G. A. Endosomal traffic jams represent a pathogenic hub and therapeutic target in Alzheimer’s disease. Trends Neurosci. 40 , 592–602 (2017).
Nixon, R. A. & Yang, D. S. Autophagy failure in Alzheimer’s disease–locating the primary defect. Neurobiol. Dis. 43 , 38–45 (2011).
Palop, J. J. & Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 17 , 777–792 (2016).
Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30 , 572–580 (1991).
DeKosky, S. T. & Scheff, S. W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 27 , 457–464 (1990). A real-time demonstration of a close correspondence between synapse loss and degree of cognitive impairment in brain biopsies from patients with AD.
Spires, T. L. et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 25 , 7278–7287 (2005).
Arbel-Ornath, M. et al. Soluble oligomeric amyloid-β induces calcium dyshomeostasis that precedes synapse loss in the living mouse brain. Mol. Neurodegener. 12 , 27 (2017).
Zhou, L. et al. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun. 8 , 15295 (2017).
Masliah, E., Hansen, L., Albright, T., Mallory, M. & Terry, R. D. Immunoelectron microscopic study of synaptic pathology in Alzheimer’s disease. Acta Neuropathol. 81 , 428–433 (1991).
Henstridge, C. M. et al. Post-mortem brain analyses of the Lothian Birth Cohort 1936: extending lifetime cognitive and brain phenotyping to the level of the synapse. Acta Neuropathol. Commun. 3 , 53 (2015).
Lleo, A. et al. Changes in synaptic proteins precede neurodegeneration markers in preclinical Alzheimer’s Disease cerebrospinal fluid. Mol. Cell Proteom. 18 , 546–560 (2019).
DeVos, S. L. et al. Synaptic tau seeding precedes tau pathology in human Alzheimer’s disease brain. Front. Neurosci. 12 , 267 (2018).
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53 , 337–351 (2007).
Keskin, A. D. et al. BACE inhibition-dependent repair of Alzheimer’s pathophysiology. Proc. Natl Acad. Sci. USA 114 , 8631–8636 (2017).
Busche, M. A. et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321 , 1686–1689 (2008).
Kuchibhotla, K. V. et al. Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59 , 214–225 (2008).
Menkes-Caspi, N. et al. Pathological tau disrupts ongoing network activity. Neuron 85 , 959–966 (2015).
Busche, M. A. et al. Tau impairs neural circuits, dominating amyloid-β effects, in Alzheimer models in vivo. Nat. Neurosci. 22 , 57–64 (2019).
Anderson, K. J., Scheff, S. W. & DeKosky, S. T. Reactive synaptogenesis in hippocampal area CA1 of aged and young adult rats. J. Comp. Neurol. 252 , 374–384 (1986).
Geddes, J. W. & Cotman, C. W. Plasticity in hippocampal excitatory amino acid receptors in Alzheimer’s disease. Neurosci. Res. 3 , 672–678 (1986).
Hyman, B. T., Kromer, L. J. & Van Hoesen, G. W. Reinnervation of the hippocampal perforant pathway zone in Alzheimer’s disease. Ann. Neurol. 21 , 259–267 (1987).
Liu, L. et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE 7 , e31302 (2012).
Rauch, J. N. et al. LRP1 is a master regulator of tau uptake and spread. Nature 580 , 381–385 (2020).
Kounnas, M. Z. et al. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell 82 , 331–340 (1995).
Rebeck, G. W., Reiter, J. S., Strickland, D. K. & Hyman, B. T. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron 11 , 575–580 (1993).
Wilton, D. K., Dissing-Olesen, L. & Stevens, B. Neuron-glia signaling in synapse elimination. Annu. Rev. Neurosci. 42 , 107–127 (2019).
Nixon, R. A. The role of autophagy in neurodegenerative disease. Nat. Med. 19 , 983–997 (2013).
Menzies, F. M. et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93 , 1015–1034 (2017).
Nixon, R. A. Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer’s disease: inseparable partners in a multifactorial disease. FASEB J. 31 , 2729–2743 (2017).
Shehata, M. et al. Autophagy enhances memory erasure through synaptic destabilization. J. Neurosci. 38 , 3809–3822 (2018).
Glatigny, M. et al. Autophagy is required for memory formation and reverses age-related memory decline. Curr. Biol. 29 , 435–448.e8 (2019).
Cataldo, A. M. et al. Endocytic pathway abnormalities precede amyloid β deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am. J. Pathol. 157 , 277–286 (2000).
Van Acker, Z. P., Bretou, M. & Annaert, W. Endo-lysosomal dysregulations and late-onset Alzheimer’s disease: impact of genetic risk factors. Mol. Neurodegener. 14 , 20 (2019).
Suire, C. N. et al. Cathepsin D regulates cerebral Aβ42/40 ratios via differential degradation of Aβ42 and Aβ40. Alzheimers Res. Ther. 12 , 80 (2020).
Kwart, D. et al. A large panel of isogenic APP and PSEN1 mutant human iPSC neurons reveals shared endosomal abnormalities mediated by APP β-CTFs, not Aβ. Neuron 104 , 256–270.e5 (2019). Evidence from induced pluripotent stem cells on the critical role of endosomal dysregulation in the pathogenesis of Aβ and tau-induced disease.
Pensalfini, A. et al. Endosomal dysfunction induced by directly over-activating Rab5 recapitulates prodromal and neurodegenerative features of Alzheimer’s disease. Cell Rep. 33 , 108420 (2020).
Lauritzen, I. et al. Intraneuronal aggregation of the β-CTF fragment of APP (C99) induces Aβ-independent lysosomal-autophagic pathology. Acta Neuropathol. 132 , 257–276 (2016).
Lee, J. H. et al. Presenilin 1 maintains lysosomal Ca 2+ homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep. 12 , 1430–1444 (2015).
Lee, J. H. et al. β2-adrenergic agonists rescue lysosome acidification and function in PSEN1 deficiency by reversing defective ER-to-lysosome delivery of ClC-7. J. Mol. Biol. 432 , 2633–2650 (2020).
Morel, N. & Poea-Guyon, S. The membrane domain of vacuolar H + ATPase: a crucial player in neurotransmitter exocytotic release. Cell. Mol. Life Sci. 72 , 2561–2573 (2015).
Higashida, H., Yokoyama, S., Tsuji, C. & Muramatsu, S. I. Neurotransmitter release: vacuolar ATPase V0 sector c-subunits in possible gene or cell therapies for Parkinson’s, Alzheimer’s, and psychiatric diseases. J. Physiol. Sci. 67 , 11–17 (2017).
El Far, O. & Seagar, M. A role for V-ATPase subunits in synaptic vesicle fusion? J. Neurochem. 117 , 603–612 (2011).
Peng, K. Y. et al. Apolipoprotein E4 genotype compromises brain exosome production. Brain 142 , 163–175 (2019).
Liu, R. Q. et al. Membrane localization of β-amyloid 1-42 in lysosomes: a possible mechanism for lysosome labilization. J. Biol. Chem. 285 , 19986–19996 (2010).
Morishita, H. & Mizushima, N. Diverse cellular roles of autophagy. Annu. Rev. Cell Dev. Biol. 35 , 453–475 (2019).
Levine, B. & Kroemer, G. Biological functions of autophagy genes: a disease perspective. Cell 176 , 11–42 (2019).
Bordi, M. et al. Autophagy flux in CA1 neurons of Alzheimer hippocampus: Increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy 12 , 2467–2483 (2016).
Moreira, P. I., Carvalho, C., Zhu, X., Smith, M. A. & Perry, G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta 1802 , 2–10 (2010).
Nixon, R. A. The aging lysosome: an essential catalyst for late-onset neurodegenerative diseases. Biochim. Biophys. Acta Proteins Proteom. 1868 , 140443 (2020).
Whyte, L. S., Lau, A. A., Hemsley, K. M., Hopwood, J. J. & Sargeant, T. J. Endo-lysosomal and autophagic dysfunction: a driving factor in Alzheimer’s disease? J. Neurochem. 140 , 703–717 (2017).
Pensalfini, A. et al. Intracellular amyloid and the neuronal origin of Alzheimer neuritic plaques. Neurobiol. Dis. 71 , 53–61 (2014).
Adalbert, R. et al. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain 132 , 402–416 (2009).
Nixon, R. A. & Yang, D. S. Autophagy and neuronal cell death in neurological disorders. Cold Spring Harb. Perspect. Biol. 4 , a008839 (2012).
Nakanishi, H. Microglial cathepsin B as a key driver of inflammatory brain diseases and brain aging. Neural Regenerat. Res. 15 , 25–29 (2020).
Lowry, J. R. & Klegeris, A. Emerging roles of microglial cathepsins in neurodegenerative disease. Brain Res. Bull. 139 , 144–156 (2018).
Sarlus, H. & Heneka, M. T. Microglia in Alzheimer’s disease. J. Clin. Invest. 127 , 3240–3249 (2017).
Deretic, V., Saitoh, T. & Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 13 , 722–737 (2013).
Cho, M. H. et al. Autophagy in microglia degrades extracellular β-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy 10 , 1761–1775 (2014).
Jay, T. R. et al. Disease progression-dependent effects of TREM2 deficiency in a mouse model of Alzheimer’s disease. J. Neurosci. 37 , 637–647 (2017).
Lewcock, J. W. et al. Emerging microglia biology defines novel therapeutic approaches for Alzheimer’s disease. Neuron 108 , 801–821 (2020).
Kim, H. J. et al. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry 22 , 1576–1584 (2017).
Saido, T. & Leissring, M. A. Proteolytic degradation of amyloid β-protein. Cold Spring Harb. Perspect. Med. 2 , a006379 (2012).
Rasmussen, M. K., Mestre, H. & Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 17 , 1016–1024 (2018).
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124 , 1–38 (2008). Demonstration of the relationship between the default mode network and amyloid accumulation.
Greicius, M. D., Srivastava, G., Reiss, A. L. & Menon, V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl Acad. Sci. USA 101 , 4637–4642 (2004).
Buckner, R. L. et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. 29 , 1860–1873 (2009).
Agosta, F. et al. Resting state fMRI in Alzheimer’s disease: beyond the default mode network. Neurobiol. Aging 33 , 1564–1578 (2012).
Whitwell, J. L. et al. Working memory and language network dysfunctions in logopenic aphasia: a task-free fMRI comparison with Alzheimer’s dementia. Neurobiol. Aging 36 , 1245–1252 (2015).
Townley, R. A. et al. Progressive dysexecutive syndrome due to Alzheimer’s disease: a description of 55 cases and comparison to other phenotypes. Brain Commun. 2 , fcaa068 (2020).
Franzmeier, N. et al. Functional brain architecture is associated with the rate of tau accumulation in Alzheimer’s disease. Nat. Commun. 11 , 347 (2020).
Sintini, I. et al. Tau and amyloid relationships with resting-state functional connectivity in atypical Alzheimer’s disease. Cereb. Cortex https://doi.org/10.1093/cercor/bhaa319 (2020).
Article PubMed Central Google Scholar
Jones, D. T. et al. Tau, amyloid, and cascading network failure across the Alzheimer’s disease spectrum. Cortex 97 , 143–159 (2017).
Sepulcre, J. et al. Hierarchical organization of tau and amyloid deposits in the cerebral cortex. JAMA Neurol. 74 , 813–820 (2017).
Jack, C. R. Jr. et al. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology 81 , 1732–1740 (2013).
Protas, H. D. et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 70 , 320–325 (2013).
Franzmeier, N. et al. Patient-centered connectivity-based prediction of tau pathology spread in Alzheimer’s disease. Sci. Adv. 6 , eabd1327 (2020). Analysis showing how regional tau expansion follows connectivity patterns.
D’Onofrio, G. et al. Neuropsychiatric symptoms and functional status in Alzheimer’s disease and vascular dementia patients. Curr. Alzheimer Res. 9 , 759–771 (2012).
Lehmann, M. et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain 136 , 844–858 (2013).
Crutch, S. J. et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 13 , 870–884 (2017).
Gorno-Tempini, M. L. et al. The logopenic/phonological variant of primary progressive aphasia. Neurology 71 , 1227–1234 (2008).
Bergeron, D. et al. Prevalence of amyloid-β pathology in distinct variants of primary progressive aphasia. Ann. Neurol. 84 , 729–740 (2018).
Ossenkoppele, R. et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain 138 , 2732–2749 (2015).
Murray, M. E. et al. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 10 , 785–796 (2011).
Clarfield, A. M. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch. Intern. Med. 163 , 2219–2229 (2003).
Beach, T. G., Monsell, S. E., Phillips, L. E. & Kukull, W. Accuracy of the clinical diagnosis of Alzheimer disease at national institute on aging Alzheimer disease centers, 2005-2010. J. Neuropathol. Exp. Neurol. 71 , 266–273 (2012).
McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89 , 88–100 (2017).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134 , 2456–2477 (2011).
Gorno-Tempini, M. L. et al. Classification of primary progressive aphasia and its variants. Neurology 76 , 1006–1014 (2011).
Botha, H. et al. FDG-PET in tau-negative amnestic dementia resembles that of autopsy-proven hippocampal sclerosis. Brain 141 , 1201–1217 (2018).
Petersen, R. C. & Yaffe, K. Issues and questions surrounding screening for cognitive impairment in older patients. JAMA 323 , 722–724 (2020).
Patnode, C. D. et al. Screening for cognitive impairment in older adults: updated evidence report and systematic review for the US Preventive Services TaskFforce. JAMA 323 , 764–785 (2020).
Jack, C. R. Jr. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14 , 535–562 (2018).
Chetelat, G. et al. Amyloid-PET and 18 F-FDG-PET in the diagnostic investigation of Alzheimer”s disease and other dementias. Lancet Neurol. 19 , 951–962 (2020).
Klunk, W. E. et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 55 , 306–319 (2004).
Jack, C. R. J. et al. Introduction to revised criteria for the diagnosis of Alzheimer’s disease: National Institute on Aging and the Alzheimer Association Workgroups. Alzheimer’s Dement. 7 , 257–262 (2011).
Knopman, D. S. et al. Evolution of neurodegeneration-imaging biomarkers from clinically normal to dementia in the Alzheimer disease spectrum. Neurobiol. Aging 46 , 32–42 (2016).
Schröder, J. & Pantel, J. Neuroimaging of hippocampal atrophy in early recognition of Alzheimer’s disease–a critical appraisal after two decades of research. Psychiatry Res. Neuroimaging 247 , 71–78 (2016).
Petersen, C. et al. Alzheimer’s disease clinical variants show distinct regional patterns of neurofibrillary tangle accumulation. Acta Neuropathol. 138 , 597–612 (2019).
Ridha, B. H. et al. Tracking atrophy progression in familial Alzheimer’s disease: a serial MRI study. Lancet Neurol. 5 , 828–834 (2006).
Greenberg, S. M. et al. Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat. Rev. Neurol. 16 , 30–42 (2020).
Graff-Radford, J. et al. Cerebral microbleeds: prevalence and relationship to amyloid burden. Neurology 92 , e253–e262 (2019).
Li, X. et al. The significant effects of cerebral microbleeds on cognitive dysfunction: an updated meta-analysis. PLoS ONE 12 , e0185145 (2017).
Laforce, R. Jr. et al. Parallel ICA of FDG-PET and PiB-PET in three conditions with underlying Alzheimer’s pathology. Neuroimage Clin. 4 , 508–516 (2014).
Caroli, A. et al. Mild cognitive impairment with suspected nonamyloid pathology (SNAP): prediction of progression. Neurology 84 , 508–515 (2015).
Iaccarino, L., Sala, A. & Perani, D. Predicting long-term clinical stability in amyloid-positive subjects by FDG-PET. Ann. Clin. Transl. Neurol. 6 , 1113–1120 (2019).
Villemagne, V. L., Doré, V., Burnham, S. C., Masters, C. L. & Rowe, C. C. Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat. Rev. Neurol. 14 , 225–236 (2018).
Clark, C. M. et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 11 , 669–678 (2012).
Murray, M. E. et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain 138 , 1370–1381 (2015).
Klunk, W. E. et al. The centiloid project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 11 , 1–15.e1–4 (2015).
Landau, S. M. et al. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann. Neurol. 74 , 826–836 (2013).
Leuzy, A. et al. Pittsburgh compound B imaging and cerebrospinal fluid amyloid-β in a multicentre European memory clinic study. Brain 139 , 2540–2553 (2016).
Grothe, M. J. et al. In vivo staging of regional amyloid deposition. Neurology 89 , 2031–2038 (2017).
Jagust, W. J. & Landau, S. M. Temporal dynamics of β-amyloid accumulation in aging and Alzheimer’s disease. Neurology 96 , e1347–e1357 (2021).
Jack, C. R. Jr. et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using THE National Institute on Aging-Alzheimer’s association research framework. JAMA Neurol. 76 , 1174–1183 (2019).
PubMed Central Google Scholar
Johnson, K. A. et al. Appropriate use criteria for amyloid PET: a report of the amyloid imaging task force, the society of nuclear medicine and molecular imaging, and the Alzheimer’s association. J. Nucl. Med. 54 , 476–490 (2013).
Rabinovici, G. D. et al. Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA 321 , 1286–1294 (2019). A very large pragmatic trial of the value of Aβ imaging in clinical dementia practice.
de Wilde, A. et al. Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE project. JAMA Neurol. 75 , 1062–1070 (2018).
Jeong, H. J. et al. [ 18 F]THK5351 PET imaging in patients with mild cognitive impairment. J. Clin. Neurol. 16 , 202–214 (2020).
Pascoal, T. A. et al. 18 F-MK-6240 PET for early and late detection of neurofibrillary tangles. Brain 143 , 2818–2830 (2020).
Leuzy, A. et al. Diagnostic performance of RO948 F 18 tau positron emission tomography in the differentiation of Alzheimer disease from other neurodegenerative disorders. JAMA Neurol. 77 , 955–965 (2020).
Ossenkoppele, R. et al. Discriminative accuracy of [ 18 F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA 320 , 1151–1162 (2018). Demonstration of the remarkable specificity of an elevated tau PET signal outside of the medial temporal lobe for persons with elevated Aβ.
Aschenbrenner, A. J., Gordon, B. A., Benzinger, T. L. S., Morris, J. C. & Hassenstab, J. J. Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology 91 , e859–e866 (2018).
Brier, M. R. et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci. Transl. Med. 8 , 338ra366 (2016).
Harrison, T. M. et al. Longitudinal tau accumulation and atrophy in aging and Alzheimer disease. Ann. Neurol. 85 , 229–240 (2019).
Lu, M. et al. Aggregated tau measured by visual interpretation of flortaucipir positron emission tomography and the associated risk of clinical progression of mild cognitive impairment and Alzheimer disease: results from 2 phase III clinical trials. JAMA Neurol. 78 , 445–453 (2021).
Lowe, V. J. et al. Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141 , 271–287 (2018).
Pontecorvo, M. J. et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain 140 , 748–763 (2017).
Shaw, L. M. et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease. Alzheimers Dement. 14 , 1505–1521 (2018).
Molinuevo, J. L. et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol. 136 , 821–853 (2018).
Hansson, O. et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 1 , 30029–30023 (2018).
Hansson, O. et al. Prediction of Alzheimer’s disease using the CSF Aβ42/Aβ40 ratio in patients with mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 23 , 316–320 (2007).
Wiltfang, J. et al. Amyloid β peptide ratio 42/40 but not Aβ42 correlates with phospho-Tau in patients with low- and high-CSF Aβ40 load. J. Neurochem. 101 , 1053–1059 (2007).
Mattsson, N. et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302 , 385–393 (2009).
Skillbäck, T. et al. Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 138 , 2716–2731 (2015).
Buerger, K. et al. No correlation between CSF tau protein phosphorylated at threonine 181 with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 130 , e82 (2007).
Seppälä, T. T. et al. CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology 78 , 1568–1575 (2012).
Barthélemy, N. R. et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat. Med. 26 , 398–407 (2020).
Janelidze, S. et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 11 , 1683 (2020).
Kern, S. et al. Association of cerebrospinal fluid neurofilament light protein with risk of mild cognitive impairment among individuals without cognitive impairment. JAMA Neurol. 76 , 187–193 (2019).
Zetterberg, H. & Bendlin, B. B. Biomarkers for Alzheimer’s disease-preparing for a new era of disease-modifying therapies. Mol. Psychiatry 26 , 296–308 (2020).
Tarawneh, R. et al. Diagnostic and prognostic utility of the synaptic marker neurogranin in Alzheimer disease. JAMA Neurol. 73 , 561–571 (2016).
Schindler, S. E. et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93 , e1647–e1659 (2019).
Karikari, T. K. et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 19 , 422–433 (2020).
Palmqvist, S. et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 324 , 772–781 (2020).
de Wolf, F. et al. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain 143 , 1220–1232 (2020).
Ashton, N. J. et al. An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat. Rev. Neurol. 16 , 265–284 (2020).
Wattmo, C. & Wallin A, K. Early- versus late-onset Alzheimer’s disease in clinical practice: cognitive and global outcomes over 3 years. Alzheimers Res. Ther. 9 , 70 (2017).
Wilson, R. S. et al. Cognitive decline in incident Alzheimer’s disease in a community population. Neurology 74 , 951–955 (2010).
van Harten, A. C. et al. Subjective cognitive decline and risk of MCI: the Mayo Clinic Study of Aging. Neurology 91 , e300–e312 (2018).
Kryscio, R. J. et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology 83 , 1359–1365 (2014).
Stewart, R. et al. Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. Br. J. Psychiatry 198 , 199–205 (2011).
Amariglio, R. E. et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50 , 2880–2886 (2012).
Petersen, R. C. et al. Randomized controlled trials in mild cognitive impairment: sources of variability. Neurology 88 , 1751–1758 (2017).
Roberts, R. O. et al. Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol. 75 , 970–979 (2018).
Vos, S. J. et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain 138 , 1327–1338 (2015).
Jack, C. R. Jr. et al. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. JAMA 321 , 2316–2325 (2019).
Knopman, D. S. et al. Entorhinal cortex tau, amyloid-β, cortical thickness and memory performance in non-demented subjects. Brain 142 , 1148–1160 (2019).
Vos, S. J. et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 12 , 957–965 (2013).
Serrano-Pozo, A., Qian, J., Monsell, S. E., Betensky, R. A. & Hyman, B. T. APOEε2 is associated with milder clinical and pathological Alzheimer disease. Ann. Neurol. 77 , 917–929 (2015).
Craft, S. et al. Accelerated decline in apolipoprotein E-epsilon4 homozygotes with Alzheimer’s disease. Neurology 51 , 149–153 (1998).
Butler, M. et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann. Intern. Med. 168 , 52–62 (2018).
Fink, H. A. et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann. Intern. Med. 168 , 39–51 (2018).
Brasure, M. et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann. Intern. Med. 168 , 30–38 (2018).
Kane, R. L. et al. in Interventions to Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer’s-Type Dementia (Agency for Healthcare Research and Quality, 2017).
Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385 , 2255–2263 (2015). A first demonstration of non-pharmacological means of delaying cognitive decline in elderly persons at risk for dementia.
Debette, S. et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77 , 461–468 (2011).
Williamson, J. D. et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 321 , 553–561 (2019).
Reuben, D. B. et al. D-CARE: the dementia care study: design of a pragmatic trial of the effectiveness and cost effectiveness of health system-based versus community-based dementia care versus usual dementia care. J. Am. Geriatr. Soc. 68 , 2492–2499 (2020).
Fisk, J. D., Beattie, B. L., Donnelly, M., Byszewski, A. & Molnar, F. J. Disclosure of the diagnosis of dementia. Alzheimers Dement. 3 , 404–410 (2007).
Alpinar-Sencan, Z. & Schicktanz, S. Addressing ethical challenges of disclosure in dementia prediction: limitations of current guidelines and suggestions to proceed. BMC Med. Ethics 21 , 33 (2020).
Amieva, H. et al. Group and individual cognitive therapies in Alzheimer’s disease: the ETNA3 randomized trial. Int. Psychogeriatr. 28 , 707–717 (2016).
Tricco, A. C. et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA 318 , 1687–1699 (2017).
Mohs, R. C. et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology 57 , 481–488 (2001).
Panza, F., Lozupone, M., Logroscino, G. & Imbimbo, B. P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 15 , 73–88 (2019).
Rogers, M. B. https://www.alzforum.org/news/research-news/aducanumab-still-needs-prove-itself-researchers-say (2020).
Mintun, M. A. et al. Donanemab in early Alzheimer’s disease. N. Engl. J. Med . https://doi.org/10.1056/NEJMoa2100708 (2021).
Shugart, J. https://www.alzforum.org/news/conference-coverage/banish-av-ban2401-antibody-makes-its-move-phase-3-program (2020).
VandeVrede, L., Boxer, A. L. & Polydoro, M. Targeting tau: clinical trials and novel therapeutic approaches. Neurosci. Lett. 731 , 134919 (2020).
Rios-Romenets, S. et al. Baseline demographic, clinical, and cognitive characteristics of the Alzheimer’s prevention initiative (API) autosomal-dominant Alzheimer’s disease Colombia trial. Alzheimers Dement 16 , 1023–1030 (2020).
Insel, P. S., Donohue, M. C., Sperling, R., Hansson, O. & Mattsson-Carlgren, N. The A4 study: β-amyloid and cognition in 4432 cognitively unimpaired adults. Ann. Clin. Transl. Neurol. 7 , 776–785 (2020).
Ballard, C. et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 17 , 213–222 (2018).
Jennings, L. A. et al. Patient and caregiver goals for dementia care. Qual. Life Res. 26 , 685–693 (2017).
Bannon, S. et al. In it together: a qualitative meta-synthesis of common and unique psychosocial stressors and adaptive coping strategies of persons with young-onset dementia and their caregivers. Gerontologist https://doi.org/10.1093/geront/gnaa169 (2020).
Moon, H. & Adams, K. B. The effectiveness of dyadic interventions for people with dementia and their caregivers. Dementia 12 , 821–839 (2013).
van Charante, E. P. et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 388 , 797–805 (2016).
Andrieu, S. et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 16 , 377–389 (2017).
Sink, K. M. et al. Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the life randomized trial. JAMA 314 , 781–790 (2015).
De Jager, P. L., Yang, H. S. & Bennett, D. A. Deconstructing and targeting the genomic architecture of human neurodegeneration. Nat. Neurosci. 21 , 1310–1317 (2018).
Rehiman, S. H. et al. Proteomics as a reliable approach for discovery of blood-based Alzheimer’s disease biomarkers: a systematic review and meta-analysis. Ageing Res. Rev. 60 , 101066 (2020).
Mahajan, U. V. et al. Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: a targeted metabolomic and transcriptomic study. PLoS Med. 17 , e1003012 (2020).
Dawson, T. M., Golde, T. E. & Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 21 , 1370–1379 (2018).
van der Kant, R. et al. Cholesterol metabolism is a druggable axis that independently regulates tau and amyloid-β in iPSC-derived Alzheimer’s disease neurons. Cell Stem Cell 24 , 363–375.e9 (2019).
American Psychiatric Association. DSM-5: Diagnostic and Statistical Manual of Mental Disorders 5th ed. (American Psychiatric Association, 2013).
Albert, M. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging– Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7 , 270–279 (2011).
Dubois, B. et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13 , 614–629 (2014).
Thal, D. R., Rub, U., Orantes, M. & Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58 , 1791–1800 (2002).
Download references
Acknowledgements
The authors acknowledge research support from NIH (D.S.K. and R.C.P, P30 AG062677 and U01 AG006786; B.T.H., P30AG062421; R.A.N. P01 AG017617 and R01 AG062376).
Author information
Authors and affiliations.
Department of Neurology, Mayo Clinic, Rochester, MN, USA
David S. Knopman, Ronald C. Petersen & David T. Jones
Inserm U1219 Bordeaux Population Health Center, University of Bordeaux, Bordeaux, France
Helene Amieva
Normandie Univ, UNICAEN, INSERM, U1237, PhIND “Physiopathology and Imaging of Neurological Disorders”, Institut Blood and Brain @ Caen-Normandie, Cyceron, Caen, France
Gäel Chételat
Department of Neurology, Washington University School of Medicine, St. Louis, MO, USA
David M. Holtzman
Department of Neurology, Massachusetts General Hospital, Boston, MA, USA
Bradley T. Hyman
Departments of Psychiatry and Cell Biology, New York University Langone Medical Center, New York University, New York, NY, USA
Ralph A. Nixon
NYU Neuroscience Institute, New York University Langone Medical Center, New York University, New York, NY, USA
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (D.S.K.); Epidemiology (H.A.); Mechanisms/pathophysiology (D.T.J., R.A.N., B.T.H. and D.M.H.); Diagnosis, screening and prevention (G.C., R.C.P. and D.S.K.); Management (R.C.P. and D.S.K.); Quality of life (D.S.K.); Outlook (D.S.K.); Overview of Primer (D.S.K.).
Corresponding author
Correspondence to David S. Knopman .
Ethics declarations
Competing interests.
D.S.K. served on a Data Safety Monitoring Board for the DIAN study. He serves on a Data Safety Monitoring Board for a tau therapeutic for Biogen but receives no personal compensation. He is a site investigator in a Biogen aducanumab trial. He is an investigator in a clinical trial sponsored by Lilly Pharmaceuticals and the University of Southern California. He serves as a consultant for Samus Therapeutics, Third Rock, Roche and Alzeca Biosciences but receives no personal compensation. He receives research support from the NIH. G.C. serves on the Scientific Advisory Board of the Fondation Vaincre Alzheimer but receives no personal compensation. She receives personal fees from Fondation d’entreprise MMA des Entrepreneurs du Futur and from Fondation Alzheimer as she serves in the Operational Committee. She receives research support from European Union Horizon 2020 research and innovation programme (grant agreement number 667696), Inserm, Fondation d’entreprise MMA des Entrepreneurs du Futur, Fondation Alzheimer, Programme Hospitalier de Recherche Clinique, Région Normandie, Association France Alzheimer et maladies apparentées and Fondation Vaincre Alzheimer. R.C.P. is a consultant for Biogen, Inc., Roche, Inc., Merck, Inc., Genentech Inc. and Eisai, Inc., has given educational lectures for GE Healthcare, receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), UpToDate, and receives research support from the NIH. B.T.H. has a family member who works at Novartis and owns stock in Novartis; he serves on the SAB of Dewpoint and owns stock. He serves on a scientific advisory board or is a consultant for Biogen, Novartis, Cell Signalling, the US Dept of Justice, Takeda, Vigil, W20 group and Seer. His laboratory is supported by sponsored research agreements with Abbvie, F Prim, and research grants from the National Institutes of Health, Cure Alzheimer’s Fund, Tau Consortium, Brightfocus and the JPB Foundation. H.A. serves on the Scientific Advisory Board of the Observatoire des Mémoires but receives no personal compensation. She receives research support from Spoelberch Foundation, Association France Alzheimer et maladies apparentées, the Regional Health Agency of Aquitaine and National Research Agency. D.M.H. reports being a Co-founder for C2N Diagnostics LLC and participating in scientific advisory boards/consulting for Genentech, C2N Diagnostics, Denali, Merck and Idorsia. He is an inventor on patents licensed by Washington University to C2N Diagnostics on the therapeutic use of anti-tau antibodies (this anti-tau antibody programme is licensed to Abbvie) and to Eli Lilly on the therapeutic use of an anti-amyloid-β antibody. His laboratory receives research grants from the National Institutes of Health, Cure Alzheimer’s Fund, Tau Consortium, the JPB Foundation, Good Ventures, Centene, BrightFocus and C2N Diagnostics. All other authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks K. Iqbal, D. Selkoe, H. Soininen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Knopman, D.S., Amieva, H., Petersen, R.C. et al. Alzheimer disease. Nat Rev Dis Primers 7 , 33 (2021). https://doi.org/10.1038/s41572-021-00269-y
Download citation
Accepted : 09 April 2021
Published : 13 May 2021
DOI : https://doi.org/10.1038/s41572-021-00269-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Variation of the essential oil components of citrus aurantium leaves upon using different distillation techniques and evaluation of their antioxidant, antidiabetic, and neuroprotective effect against alzheimer’s disease.
- Esraa A. Elhawary
- Nilofar Nilofar
- Omayma A. Eldahshan
BMC Complementary Medicine and Therapies (2024)
Gut microbiota-host lipid crosstalk in Alzheimer’s disease: implications for disease progression and therapeutics
- Ling-Ling Yang
- Xiu-Qing Yao
Molecular Neurodegeneration (2024)
Loss of GDE2 leads to complex behavioral changes including memory impairment
- Daniel Daudelin
- Anna Westerhaus
- Shanthini Sockanathan
Behavioral and Brain Functions (2024)
Profiles of subgingival microbiomes and gingival crevicular metabolic signatures in patients with amnestic mild cognitive impairment and Alzheimer’s disease
- Zhongchen Song
Alzheimer's Research & Therapy (2024)
Early onset diagnosis in Alzheimer’s disease patients via amyloid-β oligomers-sensing probe in cerebrospinal fluid
- Kyeonghwan Kim
- Jong Seung Kim
Nature Communications (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 29, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Researchers discover the mechanism that links a diet rich in fats with Alzheimer's disease
by University of Rovira i Virgili

A study led by the Universitat Rovira i Virgili (URV) has revealed the mechanism behind the link between a diet high in saturated fats and Alzheimer's disease. The research focused on how this kind of diet affects certain molecules found in the blood and in other tissues such as the brain that act as markers and regulators of the disease.
The study was headed by Mònica Bulló, professor at the Department of Biochemistry and Biotechnology and member of the Metabolic Health and Nutrition unit and the Environmental, Food and Toxicological Technology Centre (TecnATox) of the URV, in collaboration with the Pere Virgili Health Research Institute (IISPV), CIBERobn and the University of Barcelona. The results have been published in the journal Nutrients .
The research was conducted on mice models who developed Alzheimer's disease in adulthood. Previous studies in these animals had already shown that after a diet high in saturated fats the mice developed Alzheimer's much earlier than mice on a conventional diet. However, the mechanisms that led to the onset of Alzheimer's remained unknown. That is, until now.
The researchers analyzed the expression of 15 miRNAs, small molecules of RNA that play a crucial role in genetic regulation in both plasma and brain tissues. The team examined changes in insulin-related miRNAs in mouse models predisposed to Alzheimer's not on a diet low in saturated fats.
The results demonstrated that their metabolism worsened after being on this diet for six months: their body weight increased significantly and their response to glucose and insulin decreased. These same characteristics can also be found in people with obesity or type 2 diabetes.
Furthermore, researchers found changes to various miRNAs in both the blood and the brain. These changes were related to processes that can cause brain damage , such as the accumulation of β-amyloid plaques (protein deposits that form in the brain and which are markers of Alzheimer's), excessive production of the tau protein (which can damage brain cells when it gets out of control) and inflammation in the brain.
"The results of this study are a step forward in our understanding of this disease and may explain the relationship between obesity, type 2 diabetes and the onset of Alzheimer's. The findings also offer new targets for the possible prevention and treatment of the disease," said researcher Bulló.
The study not only provides new data on how a high-fat diet can affect the health of the brain, but also opens the door to future research into dietary strategies as a means of treating Alzheimer's. The results underline the importance of a balanced diet in preventing neurodegenerative diseases and highlight miRNAs as targets for therapeutic interventions.
Explore further
Feedback to editors

Popular teenagers sleep less than their peers, study finds
2 hours ago

Patients with rheumatoid arthritis have unique and complex autoantibody patterns, study reveals

Study gauges effectiveness of COVID-19 burden mitigation policies
9 hours ago
Neuroscientists discover two specific brain differences linked to how brains respond during tasks

An electrifying discovery may help doctors deliver more effective gene therapies

Exercise programs benefit a wide range of long-term health conditions, finds health data analysis
10 hours ago

Treatment-related pain may be 'socially contagious'
11 hours ago

Gene expression analyses identify potential drivers of chronic allergic inflammation

Researchers reveal a new approach for treating degenerative diseases
12 hours ago

Early gestational diabetes treatment shown to reduce birth complications, health costs for those at higher risk
Related stories.

How diabetes might lead to Alzheimer's: Study suggests the liver is key
Mar 25, 2024

Long term high-fat diet expands waistline and shrinks brain
Jul 8, 2022
Understanding diet's role in modifying risk of Alzheimer's disease
Dec 4, 2023
Obesity + aging linked to Alzheimer's markers in the brain
Jun 28, 2018
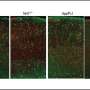
Eating more ketones may fight against Alzheimer's disease
Dec 9, 2019

High fat diet impairs new neuron creation in female mice
Dec 23, 2019
Recommended for you
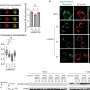
Regulating cholesterol levels might be the key to improving cancer treatment
13 hours ago

Cranberry extracts could boost microbiota and counter cardiometabolic diseases
14 hours ago

Omega-6 fatty acids could cut risk of bipolar disorder, study suggests

Does obesity really increase your risk of dementia?
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Alzheimer's drug adoption in US slowed by doctors' skepticism
- Medium Text

'SIGNIFICANT RISKS, MARGINAL BENEFIT'
'your enemy is nihilism', 'peace and quiet'.
Sign up here.
Reporting by Julie Steenhuysen in Chicago; editing by Caroline Humer and Suzanne Goldenberg
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab
Business Chevron

GSK raises full-year profit forecast, says first-half will see stronger sales
GSK raised its full-year profit forecast on Wednesday and said its sales would be higher in the first six months of the year than the second half on the back of strong demand for its common respiratory virus and shingles vaccines.


IMAGES
COMMENTS
Learn how the Association is committed to accelerating the global progress of new treatments, preventions and a cure for Alzheimer's disease. Find the latest information on research funding, grants, clinical trials and global research news.
Learn about Alzheimer's disease, the Center, and its research and education programs. The Center aims to understand and improve the care of patients with early and symptomatic stages of the disease.
Alzheimer's disease is a progressive neurodegenerative disease that impairs memory and cognitive judgment and is often accompanied by mood swings, disorientation and eventually delirium. It is the ...
NIH highlights the scientific advances and efforts in Alzheimer's and related dementia diseases between March 2021 and early 2022. The report covers drug development, lifestyle interventions, biomarker research, and more, and provides a budget estimate for future research.
NIH has released a 2022 scientific progress report on the progress and opportunities of Alzheimer's disease and related dementias research. The report covers drug development, lifestyle interventions, biomarker research, and more, and provides an estimate of the funds needed to fully pursue the research.
Researchers propose a new approach to measure how long a drug can delay or halt the progression of Alzheimer's disease. They use time-based models to calculate the benefits of disease-modifying drugs, such as lecanemab and donanemab, in clinical trials.
Learn how the Alzheimer's Association is funding and advancing research on Alzheimer's disease and other dementias, from earlier diagnosis to prevention to treatment. Find out how you can participate in clinical trials, donate your brain, or support the global effort to end Alzheimer's.
1. Introduction. Alzheimer's disease (AD) is a polygenic and multifactorial disease characterized by the deposition of amyloid-β (Aβ) fibrils in the brain, leading to the formation of plaques and neurofibrillary tangles (NFTs), and ultimately resulting in dendritic dysfunction, neuronal cell death, memory loss, behavioral changes, and organ shutdown [1,2,3,4,5].
Office of the Director (OD). NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care (PDF, 17.5M), a 2022 scientific progress report. This report provides a comprehensive overview of the meaningful progress researchers made from April 2021 through March 2022 to address ...
Abstract. Alzheimer disease (AD) is the most common contributor to dementia in the world, but strategies that slow or prevent its clinical progression have largely remained elusive, until recently ...
Learn about the research and education activities of the Alzheimer's Disease Research Center at Mayo Clinic, which is funded by the National Institute on Aging. The center studies healthy brain aging, mild cognitive impairment, Alzheimer's disease, Lewy body dementia and other dementia disorders.
1. Introduction. Alzheimer's disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles (Figure 1) as a result of amyloid-beta peptide's (Aβ) accumulation in the most affected area of the brain, the medial temporal ...
The National Institute on Aging (NIA) highlights seven recent papers from the Accelerating Medicines Partnership Alzheimer's Disease (AMP AD) program that reveal new insights into the molecular mechanisms and biomarkers of Alzheimer's disease. The papers cover topics such as gene expression, microglia, RNA sequencing, protein panels, and metabolites. The data and methods are available through the AD Knowledge Portal.
Alzheimer's Research & Therapy is the major forum for translational research into Alzheimer's disease. An international peer-reviewed journal, it publishes open access basic research with a translational focus, as well as clinical trials, research into drug discovery and development, and epidemiologic studies. The journal also provides reviews ...
We used the Common Alzheimer's Disease Research Ontology (CADRO) to classify the targets of therapies in the pipeline. RESULTS. There are 164 trials assessing 127 drugs across the 2024 AD pipeline. There were 48 trials in Phase 3 testing 32 drugs, 90 trials in Phase 2 assessing 81 drugs, and 26 trials in Phase 1 testing 25 agents.
In Alzheimer's disease, ... This suggested that treatments targeting this receptor on microglia might hold promise for treating Alzheimer's. In the new study, the research team looked for evidence that an increase in LILRB4 receptors on microglia plays an important role in the brains of people with Alzheimer's.
Learn about the federal government's efforts to find solutions for Alzheimer's and related dementias, including drug discovery, early detection, genetic research, and lifestyle interventions. Explore the advances, challenges, and opportunities in this complex and urgent field.
An irreversible degeneration of the brain that causes disruptions in memory, cognition, personality, and other functions, it eventually leads to death from complete brain failure. Nearly 7 million Americans aged 65 and older are thought to have Alzheimer's disease. By 2050, that figure may increase to nearly 13 million.
Late-onset Alzheimer's disease is an irreversible neurodegenerative disorder which accounts for the majority of dementia cases 1.Despite major private and public investments in research, there ...
The Vanderbilt Memory and Alzheimer's Center hosted its fifth Annual Vanderbilt Alzheimer's Disease Research Day on April 10 with the theme of "big data." Aside from presentations from faculty from across campus and the School of Medicine Basic Sciences, the day featured data blitzes, a keynote presentation, and a poster reception with more than 45 posters.
Alzheimer's, brain and dementia research milestones - track key advances in treatments, clinical trials, raising awareness and setting a national agenda. ... Historic $400 million increase for federal Alzheimer's disease research funding signed into law, bringing annual funding to $1.4 billion. 2018 Dementia Care Practice Recommendations ...
This book highlights the key phases and central findings of Alzheimer's Disease research since the introduction of the label 'Alzheimer's Disease' in 1910. The author, Christian Behl, puts dementia research in the context of the respective zeitgeist and summarizes the paths that have led to the currently available Alzheimer's drugs.
The Alzheimer's Disease Research Centers (ADRCs) offer local resources, support, and opportunities to participate in research on Alzheimer's disease and related dementias. These centers are dedicated to developing and testing new ways to detect, diagnose, treat, and prevent dementia and to improving care for people with these diseases and ...
We used the Common Alzheimer's Disease Research Ontology (CADRO) to classify the targets of therapies in the pipeline. Results: There are 164 trials assessing 127 drugs across the 2024 AD pipeline. There were 48 trials in Phase 3 testing 32 drugs, 90 trials in Phase 2 assessing 81 drugs, and 26 trials in Phase 1 testing 25 agents.
Find a center near you that offers diagnosis, care, and research for people with Alzheimer's disease and related dementias. The NIA funds 33 ADRCs at major medical institutions across the US, as well as four Exploratory ADRCs. Learn about the services, programs, and resources they provide.
Alzheimer's disease (AD) is a neurodegenerative disease that usually starts slowly and progressively worsens, and is the cause of 60-70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems with language, disorientation (including easily getting lost), mood swings, loss of motivation, self ...
Indeed, in one study of 184 individuals who met research neuropathological criteria for AD 9, ... C. et al. Contribution to Alzheimer's disease risk of rare variants in TREM2, SORL1, and ABCA7 ...
A study led by the Universitat Rovira i Virgili (URV) has revealed the mechanism behind the link between a diet high in saturated fats and Alzheimer's disease. The research focused on how this ...
Dr. Nathaniel Chin, a geriatrician with the University of Wisconsin's Alzheimer's Disease Research Center, said he was the target of negative comments on social media after he urged geriatricians ...