- You are here:
- American Chemical Society
- Discover Chemistry
- Tiny Matters

Mad cow and the history, cause and spread of prion diseases
Mad cow disease, also known as bovine spongiform encephalopathy (BSE) was first discovered in cattle in the UK in 1986. In 1996, BSE made its way into humans for the first time, setting off panic and fascination with the fatal disease that causes rapid onset dementia. In this episode, Sam and Deboki cover the cause, spread and concern surrounding mad cow and other prion diseases.
Transcript of this Episode
Sam: As a young kid I became pretty fascinated by the things that could kill me, particularly infectious diseases. Don’t ask me why, I just was. And the first one I remember becoming obsessed with was mad cow. The formal name for mad cow disease is bovine spongiform encephalopathy or BSE. The name makes sense — the brain of a cow with BSE looks spongy under a microscope, because of holes left by the disease. Although it can take years from the time a cow is infected to the time it first shows symptoms, like issues with coordination, once it does show symptoms things escalate quickly and the cow is usually dead within a couple weeks to six months.
Deboki: Mad cow disease was first discovered in 1986 in the UK, where it wreaked havoc for over a decade, killing nearly 200,000 cows and devastating many farming communities. In 1996, BSE made its way into humans for the first time, causing a decline in coordination, issues with vision, and the rapid onset of dementia. Over 200 cases of BSE have been reported — mostly in the UK — and everyone who was infected has died.
And in December 2003, mad cow made its first appearance in the US when an infected cow was discovered on a farm in Washington State. Today cases of BSE and of BSE making its way into people are pretty much nonexistent, thanks in large part to practices designed to keep us safe.
For example, mad cow kicked off because the feed being given to cows was infected. But since August 1997, the US FDA has banned the use of most cow parts and other animals to be used to make cow feed, limiting the risk of infected meat making it into their food.
Sam: I think we’re all used to hearing about infectious diseases caused by bacteria, viruses and a bunch of different parasites. But BSE is quite unusual: it’s not caused by any of those things. It’s caused by a protein, a fundamental building block of all living things.
Welcome to Tiny Matters. I’m Sam Jones and I’m joined by my co-host Deboki Chakravarti.
Deboki: Today on the show, we’re going to be focusing on prion diseases — rare, fatal brain diseases like mad cow that are caused by a protein malfunctioning and folding in a way it shouldn’t. I know the concept might sound a little weird and confusing, but Sam and I, and the scientists we chatted with, are going to break it down for you and talk about what’s being done to detect these diseases before something like mad cow happens again.
So what is a prion? It’s a protein that can take on two forms. The first one is what we consider the normal form, which doesn’t cause disease. Normal prions are found in the brain, although researchers don’t know much about what they do. But when people say “prion,” they’re usually not talking about the normal form. They’re usually talking about the other form…the bad, misfolded form that causes disease.
Mark Zabel: You can think of the normal form as sort of a really nice three-dimensional structure. Sort of balloon looking. When it misfolds into the prion, that balloon, three-dimensional structure becomes basically almost a two-dimensional structure. Think of it as like a bathroom tile. Very small, thin, flat.
Sam: That’s Mark Zabel, who’s the associate director of the Prion Research Center in the College of Veterinary Medicine and Biomedical Sciences at Colorado State University. He told us that when the misfolded prion — the bathroom tile as he described it — comes into contact with a normal prion, it causes it to also misfold. I think of it like a domino effect.
Deboki: And once you get a bunch of misfolded prions, those tiles stack up together and form fibers that tangle around each other, which then kills your neurons. As your neurons die off, it leaves holes in your brain—like the spongy brains seen in mad cow. And when you have holes in your brain it causes dementia, difficulty walking and speaking, sometimes even hallucinations, and ultimately death.
So how many misfolded prions is enough to cause disease?
Brian Appleby: I would say we don’t know that for sure except that prions aren’t desired to have. But is one misfolded prion protein enough to cause disease? Probably not. But the problem with prion disease is they aggregate, you know, they're kind of like the bad kids in the schoolyard. The bad kids recruit the good kids, and you have more bad kids, and that keeps amplifying and amplifying until you get disease. So that's kind of what happens — you get enough bad prions in the brain that it causes a variety of diseases in animals and humans.
Deboki: That’s Brian Appleby, a professor of neurology, psychiatry, and pathology at Case Western Reserve University.
Sam with Brian Appleby: What are some of the ways that someone could develop this disease? What would allow for these proteins to misfold?
Brian Appleby: So in humans, there's three main causes of prion disease. The most common cause by far is what we call sporadic. And there's a lot of similarity to that with Alzheimer's disease and Parkinson's disease, which are also sporadic illnesses for the most part. And what that means is that for reasons that we don't really understand, that normal protein becomes misfolded and misshapen spontaneously within the body after it's already been made. I equate it a lot to cancer. We all make cancer cells as we get older, but our body's generally able to detect them and get rid of them. The same is true with our proteins — we make bad proteins every day, but the likelihood of making bad proteins increases as we age, as well as our ability to detect them and clear them. And then you get these protein misfolding diseases like prion disease and Alzheimer's. Deboki: Brian told us around 85% of prion diseases are sporadic. But there are also prion diseases caused by a mutation in the gene that codes for the prion protein PRNP. This genetic mutation makes it more likely for the prion protein to misfold over a person’s lifetime.
And in addition to sporadic and genetic causes of prion disease, there are also acquired prion diseases. This is by far the most rare version and typically happens because of a medical procedure — say brain surgery, if there’s prion contamination on surgical equipment. It can also be caused by eating meat that contains infected nervous tissue. I think this is the version most people know about, because that’s what happened with mad cow.
People came down with the human version of BSE by eating beef that had been contaminated with nervous tissue of infected cows. But the cows developed BSE in the first place because they were fed sheep products infected with a prion disease called scrapie that’s been documented in sheep for over 300 years.
Sam: Another somewhat well-known acquired prion disease is kuru, which is caused by eating contaminated human brain tissue. In the 1950s and 60s, the Fore people in the highlands of Papua New Guinea experienced high levels of the disease, which turned out to be the result of ritualistic cannibalism where relatives prepared and consumed the bodies of deceased family members, including their brains.
So overall, there are 3 main categories of prion diseases — sporadic, genetic, and acquired — and within those categories you have more specific diseases like kuru which is of course acquired, fatal familial insomnia which is passed on genetically, and Creutzfeldt-Jakob disease, or CJD, the most common prion disease that affects humans. CJD falls under all 3 categories — it can develop sporadically, genetically or be acquired. This is a form of prion disease that people exposed to BSE — mad cow — developed.
Deboki: And because the symptoms are pretty much identical throughout all of these diseases, the only way to really tell them apart is by looking at brain tissue under a microscope to see the size and distribution of the holes or prion protein deposits.
Brian Appleby’s work focuses on all three categories of human prion disease.
Sam with Brian Appleby: So what is it about prion diseases that you find so interesting?
Brian Appleby: A lot actually. So I am a trained neuropsychiatrist, geriatric psychiatrist by training. And I really got interested in the field primarily from the caregiver side because this is a very rapidly progressive neurodegenerative illness. It's horrible for families to go through and there's not a whole lot of clinical expertise to help them out. So that's how I originally got interested in it. And then of course at that time I was also kind of a dementia doctor, so there's a good overlap between the two. And then I got really interested in the science, which of course is extremely interesting. I think from the clinical side, seeing the patients, they're very difficult to diagnose sometimes. And then of course the biology and trying to understand that and how it affects public health.
Deboki: Brian is the director of the National Prion Disease Pathology Surveillance Center.
Brian Appleby: the National Prion Disease Pathology Surveillance Center was founded in 1997, mainly in response to the mad cow epidemic. Most countries wanted to develop surveillance programs to know whether or not people were being affected by mad cow disease. It's funded by the CDC and we're funded to do neuropathologic surveillance. So we collect brain tissue on patients who had CJD or another form of prion disease and examine it underneath the microscope to see whether or not it is in fact prion disease, because that's the only way to definitively diagnose it.
Deboki: They’re also working on developing tests to be able to more specifically diagnose people who appear to have a prion disease.
Brian Appleby: We also do a lot of outreach and education to clinicians, but also to funeral home providers because there's a lot of fear of potentially contracting this disease and people that deal with that.
Sam with Brian Appleby: I actually have a follow up based on what you just said, which was this sort of fear for people who are handling bodies of people who have passed away from prion diseases. There is some anxiety that you could actually get prion disease. How likely is that?
Brian Appleby: My predecessor used to say that the fear of prion disease was way more infectious than prion disease itself. And that's certainly true, right? It’s difficult to transmit prion disease and you really can only do it in certain scenarios. So you need to have infectious tissue which is almost always gonna be brain tissue. And then that either needs to be injected into a person, consumed orally by a person, or placed in another person's brain for transmission to occur. Now most of those scenarios don't happen in everyday life, right? So there are specific scenarios where it could happen though — neurosurgery, brain surgery, autopsies where we were removing the brain, and then in the past we used to reuse brain tissue and pieces that surrounded the brain in healthy individuals.
And in fact, that's how some prion disease got transmitted. One example is we used to get human growth hormone from cadavers through their pituitary gland, which is part of the brain. They would grind it up and inject it into children of short stature to treat their short stature and it would transmit prion disease. But we don't do that anymore. Now we make what we call recombinant human growth hormone or made in a laboratory human growth hormone. So we don't have to do those things. So it is hard to transmit. There are certain scenarios where you have to take precautions, but they are few.
Deboki: One place where precautions are of course necessary is if someone is doing laboratory research involving prions. In 2019, a researcher in France named Émilie Jaumain died of acquired CJD — at age 33, 10 years after pricking her thumb during an experiment with prion-infected mice. In 2021, a second lab worker in France was diagnosed with CJD, leading to a months-long moratorium on prion research at a number of public research institutions in the country.
Sam: Again, prion diseases in humans are incredibly rare and the scenarios where you’d be at risk for acquiring one are quite specific. But in other species, a prion disease called chronic wasting disease spreads easily and is on the rise.
Mark Zabel: Chronic wasting disease is a prion disease that affects cervids. Cervids include elk, deer, moose, caribou, reindeer, red deer. It’s a highly infectious disease. It's one of the most infectious prion diseases we've ever studied. It’s very similar to the sheep prion disease known as scrapie.
Sam: That’s Mark Zabel again, from Colorado State University. You heard him briefly at the top of the episode. Mark’s research focus is chronic wasting disease or CWD.
Mark Zabel: Until recently, within the past five to 10 years, it was thought that it jumped species and was caused from sheep scrapie and thought that maybe some deer came in contact with some contaminated environments, or came into contact with infected sheep. And that has been turned on its head just a little bit, based on some studies that my lab has done and others, but also the fact that CWD has most recently been found in Northern Europe, in Nordic countries, first in Norway, but since then, Sweden and Finland, and it's interesting because there's no known connection of CWD in those Nordic countries to North America.
There is sheep scrapie in Scandinavian countries, so there's a chance that it could have been a trans species event from sheep scrapie. We can't rule that out. But there's a really interesting story emerging in the Nordic countries, and that is they're finding a lot of moose with CWD. And the reason that's interesting is moose, unlike other cervid species, they're solitary animals. And we think that CWD is passed from deer to deer, elk to elk, by direct and indirect contact. But moose don’t behave that way, so how do they get it? That indicates that it's potentially a spontaneous disease.
Deboki: Remember a spontaneous disease is just that — it’s spontaneous. It’s like a form of cancer where, for no rhyme or reason, you just have cells that go rogue and start dividing like crazy. In the case of prion disease, it’s the prion proteins going rogue and misfolding like crazy.
Unlike human prion diseases, prions that cause CWD can be excreted in saliva. Deer are super social, they have nose to nose contact. Which is very cute, unless one of them has CWD. They can also excrete prions in urine and feces. And those prions can stick around in the environment for a long time, even decades.
Mark Zabel: We think they can accumulate to a point where now a deer sniffing around in the ground eating plants that have been contaminated with urine or feces can now be ingested in that way as well. So that's another indirect transmission. Also decaying carcasses in the environment from deer that passed away from CWD and other deer, elk or moose will come and kind of sniff around that carcass as well.
Deboki: The good and very important news to share is that at this point, there is no documented transmission of CWD to humans. But that doesn’t mean we should assume it will stay that way. Remember, BSE did cross the species barrier, from sheep to cows and then cows to humans.
Mark told us that one of his biggest concerns is that hunters are being exposed to CWD in large quantities. When people were exposed to mad cow, they were usually eating a burger that had been made from different cows combined into one patty, and maybe just one of those cows had the disease, so it was watered down. But for hunters, things are different.
Mark Zabel: Consider a hunter who’s killed a CWD infected animal. They're gonna feed that animal to a very small number of people, family and friends, maybe a handful, maybe a half dozen. The prion titer, the load that they get from eating that one sick animal, it's not diluted into a bunch of other animals. The infectious dose they're receiving is orders of magnitude higher than the people who ate an infected hamburger. So that could really stress the species barrier to breaking. That's one of my big fears.
Deboki: By the species barrier breaking, Mark means that with enough of that infectious protein present there’s a greater chance of infection and CWD could go from a deer problem to a human problem.
Sam: And I feel like we should say this again, because Mark reiterated it many times throughout our conversation: no cases of CWD jumping to humans have been reported. And there are ongoing studies looking at hunters to see if they’re dying of prion disease at a higher rate than the general public. Mark says that so far there's no evidence suggesting that.
I also asked Mark if there was concern about dogs contracting CWD. I’m a dog owner, and if you’ve ever owned a dog, chances are you know they're prone to sniff around and seek out gross and dead stuff. So I wondered if they were at risk.
Mark Zabel: I do have some good news for you about your dog though, and my dog. It seems that there's some species, some mammals, that are particularly resistant to prion disease — dogs are one of them. If you're a cat owner, unfortunately there is feline spongiform encephalopathy, and that was produced during the BSE outbreak. So not only did humans get it, but they also made cat and dog food out of some of those infected cattle and some cats in Europe ended up getting this new FSE, this new prion disease of cats, but no dogs. There is no canine spongiform encephalopathy.
Deboki: Mark and his colleagues are working on a bunch of things. One is developing tests that can easily detect CWD in feces found in the environment to monitor its spread. Just like human prion diseases, there are no current treatments for CWD, so they’re also working on therapeutics that could interfere with production of the diseased prion protein.
And Mark told us something else that’s really important about prion research. It’s applicable to a huge range of diseases where proteins don’t fold correctly.
Mark Zabel: Prion diseases belong to a larger family of diseases that we refer to as protein misfolding diseases. These are diseases that also are caused by normal proteins that we all express that misfold and start causing these amyloid or these plaques in the brain. Many of these diseases are much more common than prion diseases. So Alzheimer's disease, for example, Parkinson's disease, Lou Gehrig's disease, ALS — amyotrophic lateral sclerosis — traumatic brain injuries, chronic encephalopathies, are associated with proteins that misfold. So prion diseases are just a member of these much larger family of protein misfolding diseases.
Sam with Mark Zabel: That's interesting. And it also is interesting because I would imagine that, to some degree, the work that's done to try and understand those other contexts in which you have protein misfolding like a traumatic brain injury or Alzheimer's, that what you gather from those studies could often be more broadly applied.
Mark Zabel: Absolutely. And, since obviously I'm a prion researcher, I would turn that converse, because one thing that's really interesting about prion diseases that helps researchers, is that these lab animals I'm talking about rodents, especially, that we can genetically manipulate, they actually get a prion disease, and it is a bonafide prion disease, unlike Alzheimer's, right? Where we do study that in the lab and we use these genetically altered animals from mice, but it's just a model because they don't really get Alzheimer’s. We can manipulate them so that they get a form of something that looks like Alzheimer's, but it's not exactly Alzheimer's. But prion diseases can be completely recapitulated in a mouse, and that disease is exactly the same disease that humans will get from a prion disorder as well.
It’s really changing the way we think about proteins and how they function and what they really do.
Sam: Prion diseases are no doubt scary but hopefully this episode made you feel a little better about them. Unless you didn’t know they existed before this episode and in that case oops sorry. I can say with certainty that this episode would have made kid me — the one obsessed with mad cow — feel better, knowing that prion diseases are incredibly rare and being monitored, and that there are researchers making big strides to catch these diseases early, develop treatments, and prevent them altogether.
I think we can hop into this Tiny Show and Tell.
Deboki: Yeah, I can go first.
Sam: Perfect.
Deboki: My Tiny Show and Tell, it's not relevant to this episode, but it's also very related, because it's about a condition that kind of comes on very quickly and is very, very hard to test for, but that people have been making really exciting progress on recently. And this is preeclampsia, which is a condition that comes up around the middle of pregnancy that basically causes a lot of issues with blood pressure and can be really, really dangerous for people. It usually happens in around one in 25 pregnancies and in the US it affects black women more than white women.
I remember from previous experiences of being pregnant that it's like a thing that they ask you about very early on and that you're kind of like, "Ah, I don't know. I don't know how to tell you what my risk factors are for this." Doctors and nurses, they're always just trying to make sure to mitigate the risk of preeclampsia.
And one of the things that's really exciting is that the FDA has approved a blood test for helping pregnant people figure out if they're at risk for preeclampsia. So it's not necessarily something that I think you can take from my understanding super early on. But the way that it works right now, at least in Europe where this test is used, is that if you're around those middle weeks of pregnancy and you're starting to show symptoms of preeclampsia or things that could maybe be preeclampsia-like, you could take this test to figure out just how likely you are to actually have preeclampsia develop. Like I said, this is something that comes on very quickly. So you might have the symptoms of it, but you might not actually know for sure that's going to happen. But then once it does happen, it just happens so quickly that you need to be able to address it really quickly.
So having a test to help people figure out are these symptoms potentially preeclampsia earlier on, is super helpful. And it looks specifically at two proteins in the placenta and their ratios of one versus the other because if these two proteins are really unbalanced, you're more likely to develop severe preeclampsia. There's about a 96% accuracy for predicting who won't develop preeclampsia. And meanwhile, two-thirds of the people who do get a positive test result will end up developing severe preeclampsia. There's still a lot that needs to be done in terms of monitoring how well this test works, but I think it's just super important because I didn't mention this earlier, but some of the things that can happen with preeclampsia is that you can have kidney and liver failure, you can have seizures. So having some kind of test that can help people who are pregnant figure out what's going on so they can get the right treatment is super important.
Sam: Yeah. That is really important. And I think preeclampsia is something that a lot of people don't really know about maybe until they're trying to get pregnant or are pregnant. And in graduate school, actually, the research group right next to the lab I was in worked on preeclampsia.
Deboki: Oh, interesting.
Sam: And that's how I learned about it. I had no idea what it was and I like the idea of a test that could help tune a lot of people in to the fact that they could have preeclampsia, that it's likely that and not something else, so that if things do escalate, they can say to the doctor right away, "Look, I'm high risk for preeclampsia. That could be what this is," and just save that time that would be spent trying to figure out what might be going on. That's I mean lifesaving, right? So-
Deboki: Totally. Yeah.
Sam: Yeah. Thanks for sharing Deboki.
Deboki: Mm-hmm.
Sam: That's good news. I like that.
Deboki: Yeah.
Sam: In my Tiny Show and Tell this week, I'm going to take us back 5,000 years. So this is not current day testing developments. This is very different. So in 2008, archeologists discovered a 5,000 year old grave in the town of Valentina in southwest Spain. And so in this super old grave, they found ivory tusks, amber, ostrich eggshells, and a crystal dagger. And so they thought, "Okay, this probably belonged to an elite leader." And so then they dubbed the individual, The Ivory Man. But now there's a team of researchers, and they use this new technique I had not heard about before. It actually looks at this enamel forming protein, amelogenin, which I guess sticks around much better than DNA does. And the other thing is, apparently male and female chromosomes have different versions of the gene that produces this amelogenin protein.
And so you can actually use it to determine sex. And so by analyzing these proteins on two of the teeth of this person found in the 5,000 year old grave, they confirmed that's not The Ivory Man. It's The Ivory Lady. So yeah, it was a woman.
They also found a bunch of chemical traces of cannabis, wine, even some mercury, because people loved mercury back in the day. They were using it as a pigment. They were ingesting it, thinking it was curing a bunch of things. Oops. But yes, they found a lot of other stuff near her body, which would suggest that maybe she was involved in some sort of religious rituals. And this was during the Copper Age. And it seems like in the Copper Age in the Mediterranean, that this was actually pretty much in line with a lot of what was happening.
A lot of prehistoric women actually had some prestige. They held authority. And so our modern assumption, which is very paternalistic and male dominated, we're kind of viewing the past through that lens. And actually, in some ways, a lot of these societies were more progressive than the ones we have today. And it also reminded me that last October, we did an episode about some of our travels last year, the travel that I shared was going to Greece. And so one of the islands that I went to when I was in Greece was Crete, where you had the Minoan Society. So the Minoans were around during the Bronze Age. There's some overlap with the Copper Age, but it's generally slightly after. So the Copper Age ends around 2000 BCE, whereas the Bronze Age ends around 1000 BCE. Again, with the Minoans, initially people thought, "Oh, it was all men that were in charge," the usual, and then more and more evidence kept coming forward really making a compelling argument that like, "No, the people in charge, the rulers, they were women."
Deboki: That's so cool. And it's so interesting how we've developed these techniques to be able to understand these questions in different ways and to look at these remains. And I got very excited just when I heard crystal dagger too. I was just like, "That sounds so amazing."
Sam: I know. I know. Right?
Deboki: Thanks for tuning in to this week’s episode of Tiny Matters, a production of the American Chemical Society. This week’s script was written by Sam, who is also our executive producer, and was edited by me and by Michael David. It was fact-checked by Michelle Boucher. The Tiny Matters theme and episode sound design are by Michael Simonelli and the Charts & Leisure team. Our artwork was created by Derek Bressler.
Sam: Thanks so much to Brian Appleby and Mark Zabel for joining us. If you’d like to support us, pick up a Tiny Matters coffee mug! Or through August 11th send us your questions and we’ll enter you into a raffle to win a Tiny Matters mug. These can be science questions, questions about a previous podcast episode, questions about how Deboki and I made our way to science communication. Truly the sky's the limit. Send your questions to tinymatters@acs.org . You can find me on social at samjscience.
Deboki: And you can find me at okidokiboki. See you next time.
LISTEN AND SUBSCRIBE
iHeartRadio
Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society
Governing Uncertain Threats: Lessons from the Mad Cow Saga in France
- First Online: 01 January 2009
Cite this chapter

- Pierre-Benoit Joly 2 &
- Hajime Sato 3
Part of the book series: Alliance for Global Sustainability Bookseries ((AGSB,volume 16))
1014 Accesses
The mad cow crisis has often been described as emblematic of the “risk society,” for at least three reasons. First, the dangers to human health from beef consumption were not the result of fate; rather, they were “manufactured” (Giddens 1994, 1997) and a byproduct of modernization. Second, science was at the core of the crisis. Although the source of the problem was technology, scientific knowledge was instrumental in solving it by providing surveillance tools, a better understanding of the causes of the disease, early tests, and so forth. Thus, the mad cow crisis perfectly illustrates Beck's (1992) thesis of reflexive scientization. Finally, this risk appeared to be the product of the institutions and economic participants that had concealed the actual nature of the disease for a long time. Consequently, the mad cow problem was ripe for high-profile publicity and politicization. Indeed, as stated by Kasperson et al. (2001), the mad cow disease case typifies a special class of hazards – ones that trigger intense media coverage, strong public concern, and a great deal of institutional attention, and produce large-scale secondary or higher consequences.
In addition, the mad cow crisis is used to exemplify the precautionary principle “better safe than sorry,” – in other words, it is better to act at the beginning, when the risk is still hypothetical –, as evidenced by the report to the French Prime Minister on precautionary measures (Kourilsky & Viney 2000) and other reports from Europe (Harremoës et al. 2001).
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
This lack of compliance does not appear to stem from uncertainty as such – as stated by Setbon, Raude, Fischler, and Flahaukt (2005) – but from the problem definition.
Indeed, this is reflected in the transformation of the organization of scientific expertise, which began in March 1996 with the establishment of the statutorily independent Dormont Committee and later with the creation of the French Agency for the Sanitary Security of Food (AFSSA).
See the UK Philips Report pertaining to food risks (Phillips et al. 2000 ).
Interview by P. B. Joly, the Centre National d'Etudes Vétérinaires et Alimentaires (CNEVA), Maisons-Alfort, January 1999.
Françoise Cathala, of the Institut national de la santé et de la recherche médicale (INSERM), played an important role in the emergence of research on the infectious nature of encephalopathy in France in the 1960s.
Note de service , DGAL/SDSPA/N88-8114 (June 20, 1988).
Offals: brain and central nervous system products.
An avis is not compulsory but intended to inform the participants of the government's policy.
Testimony by J. P. Lugan before the Parliamentary Mission on September 18, 1996, and published later (Guilhem & Mattei 1997 ). Of course, this kind of report has to be interpreted with caution, as such a mission does not have the same power of inquiry as a tribunal. Therefore, it is fairly obvious that persons testifying before the mission might present a rather biased viewpoint, because the procedure did not force them to give a statement based on facts and evidence.
In a January 1999 interview, Savey recalled that, at that time, he was not aware of the importance of the problem of the continuing importation of UK MBM. Although the President of the SPCGA had reliable information on the matter, he might be suspected of trying to protect the SPCGA membership rather than the public interest.
Arrêté du 24/7/90 (Decree of July 24, 1990) published in the Journal Officiel de la République Française (JORF) , on August 11, 1990. Although it does not explicitly refer to the precautionary principle, the decree is clearly in the spirit of the principle, in that it refers to the potential risk of transmission that would be created by the ban.
Nallet estimated that the French decision was a breakthrough in the relationship between the Agricultural Council and the SVC: “Up to 1990, BSE was the responsibility of the Veterinary Committee which is quite autonomous and whose conclusions are usually adopted in ‘point A' of the Ministry Council, without any discussion.”
This Council Directive obliges the UK to identify the cattle and restricts the exporting of UK bovine meat (adding restrictions to Commission Decision 90/200, which was adopted on April 9, 1990, before the crisis). It also requires the examination of the MBM production equipment in all member states and further research on BSE. However, the measure had little effect.
Published in the JORF on June 13, 1990.
The December 4 (second) decree details the financial part of the ESN.
Interview, January 1999.
This information was subsequently revealed by Le Canard Enchaîné in 1996 but not reported in other journals. Information on the methods for detecting mad cow disease before 1994 remains a little fuzzy, as the accounts are not always the same. The chronology established by the DGAL in 1996 recalls that Decree 90/478 implied that, if a case of BSE is confirmed, the whole herd must be slaughtered and all SBO incinerated. However, this was not obligatory before the Arrêté of July 27, 1994; at this earlier stage, it is probable that all BSE animals were destroyed, but other animals were marked and kept isolated on the farm. When the latter were slaughtered, their SBO were incinerated. (For a different account, see Sauvadet & Vergnier 2001 , p. 88.)
To be compared with more than 1,000 infected cows in France until December 2007.
The preparation of this report was first informally requested of Marc Savey, reflecting the concern over BSE. Note that in 1991, two important conferences were organized: a consultation on BSE organized in Paris from October 30 to 31, 1991, by the Office International d'Epizooties (OIE, International Office of Animal Health), and a WHO consultation on public health issues related to animal and human spongiform encephalopathy, held in Geneva from November 12 to 14, 1991 (World Health Organization, 1991).
Note that only one veterinarian and no researchers from the Institut National de la Recherche Agronomique (INRA, National Institute for Agricultural Research) participated in this first working group. This composition shows the weak mobilization of agronomic and veterinary researchers on TSE problems at that time.
The growth hormone produced by the extraction of hypophysis was a cause of the transmission of CJD in the late 1980s (The first case in France occurred in 1989 and in the US in 1985). By late 1998 it had caused 54 cases of CJD in France, the most exposed country. The working group, largely composed of specialists in medical research and practice, were very keen to focus on such important problems.
Even at that time, research on experimental animals that had orally ingested brain and then had been tested for scrapie showed that the quantity of brain tissue ingested was very important (Broxmeyer 2005 ). The authors of the report estimated that inter-species transmission through oral ingestion would require 106 times more substance than through intra-cerebral injection. The possibility of a change in the infectious properties of the tissue was not considered. Further experiments showed that BSE had been transmitted to sheep through oral ingestion of 0.5 g of infected brain (Foster et al. 1996 ). This result changed completely the evaluation of the BSE transmission risk to humans. As Dormont commented, “… these data demonstrate that we are now completely changing the frame [for the evaluation of risk – note from the editor] that had been elaborated in the last few years” ( Le Monde , May 6, 1996).
Interview, Grenoble, February 1999.
Note that of the 23 selected projects, only 8 were directly related to TSEs (of which only one was conducted at the INRA). It is interesting to compare this lack of mobilization to what took place after March 1996. In 1997, the French TSE Program sponsored 50 projects, all directly related to TSEs.
Hearing of the Parliamentary Mission, July 9, 1996 (as cited in Guilhem & Mattei 1997 ).
Tambourin refers to the 1984 Rapport de Conjoncture et de Prospective de l'INSERM (Report of the Situation and Prospective of INSERM), recalling that it did not say a word about AIDS, despite the presence of Montagnier on the editorial board. Interestingly, a 1994 report from the INSERM on therapeutic risks cites the TSE problem and notes: “Even though rare, this pathology is all the more troubling as the infectious agent is not identified but is potentially present in our food”. However, this report had no impact.
Interview Grenoble, February 1999.
The July 31, 1992, joint decree of the Direction Générale de l'Alimentation, Ministère de l'Agriculture (DGAL, Division of Food Affairs) and the Direction Générale de la Santé (DGS, Directorate of Health) implemented a recommendation of the Commission Interministérielle d'Etude des Aliments destinés à une Alimentation Particulière (CEDAP, Inter-ministry Commission on the Study of Foods for a Particular Alimentation).
Note for guidance for minimizing the risk of transmitting agents causing BSE via medicinal products (III/3281/91) , prepared for the European Community's Committee for Proprietary Medicinal Products (Brussels, June 1991).
He notes an important difference with the way these problems were discussed at a 1993 session of the Comité Supérieur d'Hygiène Publique, Ministère de la Santé (CSHP, Higher Committee on Public Health, Ministry of Health). In this session, the committee did conclude that the situation presented no specific dangers and, thus, did not require further action.
The French demand coincided with one from Germany, thereby reinforcing the pressure on the EC and the UK.
Directive 90/667 of November 27, 1990, on the treatment of animal waste was supposed to be enforced in all member states within 30 days of its publication. Note that, recently, several of these states (France, Benelux, Germany, Spain, Sweden, Finland, Italy, Portugal, Denmark, and Greece) have been subjected to infringement proceedings to Directive 90/667/EEC, as well as Decisions 94/381/EC and 96/449/EC. The proceedings followed the European inspections in these countries. In the French Arrêté of December 30, 1991, this treatment was made obligatory for both high- and low-risk animal waste. If the shortcomings persist, the commission claims that it will pursue the proceedings further (Commission of the European Communities 1998 ).
Arrêté du 21/3/96 (Decree of March 21, 1996) published in the JORF , on March 22, 1996. It was later confirmed by the EC Decision and by the judgment of the European Court of Justice (May 1998).
The extreme reaction of the minister may be explained, inter alia, by certain biographical facts. His Cabinet director was well aware of all the problems associated with meat production and consumption, as he was previously Director of the Office Interprofessionnel de la Viande et du Lait (OFIVAL, National Inter-Professional Office of Meat, Breeding and Poultry Farming).
The Directeur Général de la Santé (DGS, Director General of Health), Ministry of Health; the Directeur Général de l'Alimentation (DGAL, Director General of Food Affairs), Ministry of Agriculture; and the Directeur Général de la Consommation, de la Concurrence et de la Répression des Fraudes (DGCCRF, Director General of Consumption, Competition and Repression of Fraud), Ministry of Economy and Finances.
Hearings of the Parliamentary Mission, July 9, 1996.
The DGAL produced a chronology, available on its website, that presents a detailed explanation of how each decision resulted from a committee recommendation.
The committee of WHO experts met in Geneva on April 2 to 3, 1996. It recommended banning SBO from human food. The DGAL published a circular completed on April 9, stipulating that all SBO from animals that may have been fed with MBM must be banned. This partial measure is another illustration of the conflicts of interest at the DGAL.
Actually, the surveillance for scrapie had been implemented in the Atlantic Pyrénées district in 1995, through the initiative of the Farmers' Veterinary Association, because, with some 100 herds infected, this district had been severely affected.
Their implementation provoked a crisis in the rendering industry because of the sharp increase in costs. The volume of “fallen stock” to be incinerated was about 450,000 tons a year, which represents a total cost of about 700 million Francs. It was necessary to change the status of this industry through the passage of a law in December 1996. The law defined rendering as a public service and imposed a tax on meat to finance the activity.
The French government considered that upstream selection was more efficient, taking into account the doubts about the efficiency of the heat treatment of MBM. The latest evidence seems to confirm this position. However, the government was obliged by an Arrêté dated February 6, 1998, to implement EC Decision 96/449, which had been rendered on July 18, 1996. Nowadays, these standards are obligatory for all factories producing MBM.
CIV is under the control of the “ filière (processes)” of meat production and receives subsidies from the Ministry of Agriculture.
Compared with the UK's 2.496 million dairy cows, Germany's 5.193 million, Portugal's 0.364 million (in 1995), and Italy's 2.113 million (in 1995). Eurostat 97 (DG Agriculture website).
Note that “estimated average use” means that the level of exposure to the infectious agent varied greatly. This surely explains the concentration of cases in large dairy herds in the western part of France.
In France, 29.3% of dairy cows are in herds numbering more than 50 cows, compared to 84% in the UK, 36.2% in Germany, 41.1% in Ireland, 50% in Italy, and 16.4% in Portugal (DG Agriculture: Eurostat 1995 website).
Law 98-535, enacted July 1, 1998, is related to the intensification of the health watch and the control of the sanitation of products intended for humans.
The decision to transfer CNEVA from the Ministry of Agriculture to the AFSSA was added to the law by an amendment of the Sénat .
Commission de Technologie Aimentaire (CTA, Food Technology Commission); Commission Interministérielle d'Etude des Produits Destinés à l'Alimentation Particulière (CEDAP, Inter-ministry Commission on the Study of Products Destined for Particular Alimentation); Centre National d'Etudes et de Recommandations sur la Nutrition et l'Alimentation (CNERNA, National Centre of Studies and Recommendations on Nutrition and Alimentation); and Observatoire des Consommations Alimentaires (OCA, Observatory of Food Consumption).
Martin Hirsch, who was Director of the Cabinet of the Minister of Health, Bernard Kouchner. Hirsch published a book on the mad cow crisis: L'affolante Histoire de la Vache Folle (The terrifying history of mad cow), cited, in which he writes that this crisis shows the need for a major change in the food safety system (Hirsch & Duneton 1996 ).
This standard distinction has been elaborated in the joint expert consultation of the FAO and the WHO (Geneva, March 13–17, 1995). It has now been integrated into the text of the Codex Alimentarius.
The first version of the DBES was submitted to the commission by the British government on October 2, 1997. The SSC at first gave a favorable notice under the conditions in place on December 9, 1997. The new version of the DBES received a favorable notice from the SSC on February 20, 1998. On November 4, 1998, the Permanent Veterinarian Committee (consisting of leading veterinarians of the member states) adopted a decision that placed an embargo on British bovine meat (eight votes to five – France, Italy, Spain, Germany, and Austria voting no). The commission made this decision on November 25, 1998 (Decision 98/692/EC). Decision 99/514/EC, rendered July 1999, fixed the starting date of the embargo as August 1, 1999.
A total of 650 cases out of a million cattle more than 2 months old, compared with the French incidence rate of 1.5 to 2, and the rate in Northern Ireland of 10 to 15.
In this view, we join Hermitte and Dormont ( 2000 ) when they suggest endowing expert committees with a power of inquiry, thereby guaranteeing them access to the relevant data for their analyses.
Phantom risks are risks the very existence of which is unproven and perhaps incapable of being proven. Nonetheless, they cause real problems at the interface between science and the law (Foster et al. 1999 ).
See: http://vetolavie.chez-alice.fr/bse/details/graphfr/graphfr.htm
The government decided to systematically publish epidemiological data on BSE in mid-2000, just a few months before the second crisis.
Declared also in the governmental acts, because at the end of 1999 the French government was opposed to lifting the ban on British bovine meat.
Nevertheless, as numerous studies have established, the perceptions of risk by consumers are not the same as those of experts. They are more qualitative and take into consideration criteria that are not explicitly considered in quantitative models of risk. Examples of these criteria include the voluntary nature of the exposure, level of awareness of the dangers, the number of people potentially concerned, and so forth. This difference between experts and the uninitiated has less to do with differences in cognitive capacity than with a difference of position: the expert considers the “risk in itself” to be truly important, whereas for the consumer it's the “risk to him or herself.” Such a difference in perception can lead to the distinction of two types of evaluation – that of a “theoretical risk,” which results from a fundamental analysis and is not concerned with the contingencies involved with the implementation of preventative measures (errors, sabotage, etc.), and that of a “real risk,” which integrates in its evaluation elements tied to effective practices (fraud, malfunctions, etc.) and actors, and to the inevitable deficiencies in institutional systems.
This sense of outrage is related to reactions to the following statements: (1) “It is shocking that herbivores have been made carnivores,” (2) “Lobbies work to prevent certain measures from being taken,” (3) “Mad cow disease is linked to blind profit-seeking,” and (4) “Profit is considered more important than public health.”
French livestock and veterinary circles have been thrown into disarray by the first case of BSE in a cow born after the implementation of the more stringent feed safeguard measures in 1996. The cow in question, in which BSE was discovered in a systematic screening of cattle over 30 months old, was a cow of the Normande breed born in August 1997 in Seine-Maritime. The cow is being described by French officials as ‘super-naif' (Ne Apres I'Interdiction des Farines animales ) i.e. born after the full ban on animal meal in mid-1996 ( Agra Europe , April 20, 2001).
In France, under the Fifth Constitution, it may happen that the President and the government come from different and opposed political parties. Such a situation existed from 1997 to 2002, with Jacques Chirac – right-wing – at the Elysée (President) and Lionel Jospin – Socialist Party – at the Matignon (Prime Minister).
Abrial, D., Calavas, D., Jarrige, N., & Ducrot, C. (2005). Spatial heterogeneity of the risk of BSE in France following the ban of meat and bone meal in cattle feed. Preventive Veterinary Medicine, 67 (1), 69–82.
Article PubMed Google Scholar
Agence Française de Sécurité Sanitaire des Aliments. (2001, April). Avis de l'Agence française de sécurité sanitaire des aliments en date du 7 avril 2001: Réponse à la saisine du 31 octobre 2000 - Les risques sanitaires liés aux différents usages des farines et graisses d'origine animale et aux conditions de leur traitement et de leur élimination. Paris: Author.
Google Scholar
Alperovitch, A. (1998). Apports et limites de l'épidémiologie [Contributions and limits of the epidemiology]. Annales des Mines: Responsabilité & Environnement , 9 , 74–79.
Barbier, M. (2003). La constitution de l'ESB comme problème public européen: Une interprétation à partir de l'étude de quelques configurations. Revue Internationale de Politique Comparée 10 (2), 233–246.
Article Google Scholar
Beck, U. (1992). Risk society: towards a new modernity. London, SAGE.
Benamouzig, D., & Besançon, J. (2005). Administrer un monde incertain: les nouvelles bureaucraties techniques. Le cas des agences sanitaires en France [Administering an uncertain world: New technical bureaucracies in French health agencies]. Sociologie du Travail, 47 (3), 301–322.
Besançon, J. (2007). French agencies. In D. Benamouzig & O. Borraz (Eds.), Food and pharmaceutical agencies in Europe: between bureaucracy and democracy (Cahiers Risques Collectifs et Situations de Crise, no 7) (Section 2). Grenoble, France: MSH Alpes.
Borraz, O. (2007). Risk and public problems. Journal of Risk Research, 10 (7), 941–957.
Borraz, O., Besançon, J., & Clergeau, C. (2006). Is it just about trust? The partial reform of French food safety regulation. In C. Ansell & D. Vogel (Eds.), What's the beef? The contested governance of European food safety (pp. 125–152). Cambridge, MA: MIT.
Broxmeyer, L. (2005). Is mad cow disease caused by a bacteria? Medical Hypotheses, 63 , 731–739.
Chevassus, B. (2000). L'analyse du risque alimentaire: quels principes, quels mode` les, quelles organisations pour demain? Paper presented at OECD Edinburgh Conference on the Scientific and Health Aspects of Genetically Modified Foods, Edinburgh (February, 2000). UK, Edinburgh.
Commission of the European Communities. (1998). First bi-annual BSE follow-up report: communication from the Commission to the Council, the European Parliament, the Economic and Social Committee and the Committee of the Regions (COM (98) 282 final). Brussels, Belgium: Author.
Coudert, M., Belli, P., Savey, M., & Martel, J.-L. (1995). Le réseau national d'épidémiosurveillance de l'encéphalopathie spongiforme bovine [The national network of epidemic-surveillance of the bovine spongiform encephalopathy]. Epidémiologie et Santé Animale, 27 , 59–67.
Council of the European Communities. (1990). Press releases, January–June, 1990: Special council meeting – Agriculture . Retrieved, November 12, 2008, from http://aei.pitt.edu/4429/01/000486_1.pdf
Deriot, G., & Bizet, M.J. (2001). Rapport de la commission d'enquête (1) sur les conditions d'utilisation des farines animales dans l'alimentation des animaux d'élevage et les conséquences qui en résultent pour la santé des consommateurs, créée en vertu d'une résolution adoptée par le Sénat le 21 novembre 2000 (Sénat Rapport, no 321). Paris: Sénat.
Dodier, N. (2003). Leçons politiques de l'épidémie de sida. Paris: EHESS.
Dormont, D. (1992). Les encéphalopathies subaigües spongiformes humaines et animales: description clinique et biologique, facteurs étiologiques, conséquences sur la santé publique, et axes de recherches développés en France (Rapport au Ministre de la recherche et de l'espace) [Human and animal spongiform encephalopathy: Clinical and biological description, ethological factors, consequences for public health, and areas of research developed in France (Report to the Ministry of Research and Space)]. Fontenay aux Roses, France.
Dormont, D. (1998). Oral presentation. In G. Paillotin (Ed.), European agricultural research in the 21st century: which innovations will contribute most to the quality of life, food and agriculture? Berlin: Springer.
Dubot, J. (1996). Les encéphalopathies subaigües spongiformes transmissibles: Etat actuel des connaissances. [Transmissible Spongiform Encephalopathy: Current status of knowledge]. Paris: Institut national de la santé et de la recherche méd.
Enjalbert, F. (1996). Les farines de viande: intérêts dans l'alimentation des ruminants et réglementation. Le Point Vétérinaire, 28 (179), 689–695.
Foster, J. D., Bruce, M., McConnell, I., Chree, A., & Fraser, H. (1996). Detection of BSE infectivity in brain and spleen of experimentally infected sheep. The Veterinary Record , 138(22), 546–548.
Article CAS PubMed Google Scholar
Foster, K. R., Bernstein, D. E., & Huber, P. W. (1999). Phantom risk: scientific inference and the law . Cambridge, MA: MIT.
Giddens, A. (1994). Living in a post-traditional society. In Beck, U., Giddens, A., Lash, S. (Eds.), Reflexive modernisation: Politics, tradition and aesthetics in the modern social order . Cambridge, UK: Polity.
Giddens, A. (1997). Risk society: the context of British politics. In J. Franklin (Ed.), The politics of risk society (pp. 23–34). Cambridge, UK: Polity.
Godard, O. (2001, February). Embargo or not embargo? La Recherche, 339 , 50–55.
Guilhem, E., & Mattei, J.-F. (1997). De la “vache folle” à la “vache émissaire” [From “mad cow” to “emissary cow”] (Par la mission d'information commune sur l'ensemble des problèmes posés par le développement de l'épidémie d'encéphalopathie spongiforme bovine, Rapport d'information, no 3291). Paris: Assemblée Nationale.
Harremoës, P., Gee, D., MacGarvin, M., Stirling, A., Keys, J., Wynne, B., et al. (Eds.) (2001). Late lessons from early warnings: the precautionary principle 1896–2000 (Environmental Issue Report No. 22). Copenhagen, Denmark: European Environmental Agency.
Hermitte, M.-A. (1996). Le sang et le droit: Essai sur la transfusion sanguine. Paris: Seuil.
Hermitte, M.-A., & Dormont, D. (2000). Propositions pour le principe de précaution à la lumière de l'affaire de la vache folle. In P. Kourilsky & G. Viney (Eds.), Le principe de precaution (pp. 341–386). Paris: Odile Jacob.
Hirsch, M., & Duneton, P. (1996). L'affolante histoire de la vache folle [The terrifying history of mad cow]. Paris: Balland.
Institut de Protection et de Sûreté Nucléaire. (2000). Perception des risques et de la sécurité. Résultats du sondage d'octobre 2000 . Fontenay-Aux-Roses: Institut de Protection et de Sûreté Nucléaire.
Ipsos. (2000, October). The French and the quality and safety of food. Paris: Ipsos.
Irwin, A., & Wynne, B. (Eds.). (1996). Misunderstanding science? The public reconstruction of science and technology. Cambridge, UK: Cambridge University Press.
Joly, P.-B. (2007). Scientific expertise in public arenas: lessons from the French experience. Journal of Risk Research, 10 (7), 905–924.
Joly, P.-B., & Barbier, M. (2001). Que faire des désaccords entre comités d'experts? Les leçons de la guerre du bœuf. Risques, 47 , 87–94.
Joly, P.-B., Le Pape, Y., & Remy, E. (1998). Quand les scientifiques traquent les prions: Le fonctionnement d'un comité d'experts dans la crise de la vache folle [When the scientists pursue prions: the functioning of an expert committee in the crisis of the mad cow]. Annales des Mines: Responsabilité & Environnement , 9, 86–95.
Joly, P.-B., Le Pape, Y., Barbier, M., Estadès, J., Lemarié, J., & Marcant, O. (1999). BSE and the France national action system (Report for the BASES EU project). Grenoble, France: Institut National de la Recherche Agronomique.
Kasperson, R. E., Jhaveri, N., & Kasperson, J. X. (2001). Stigma and the social amplification of risk: toward a framework of analysis. In J. Flynn, P. Slovic, & H. Kunreuther (Eds.), Risk, media and stigma: understanding public challenges to modern science and technology (pp. 9–27). London, Earthscan.
Kourilsky, P., & Viney, G. (2000). Le principe de précaution. Paris: Odile Jacob/La Documentation Française.
Latouche, K., Rainelli, P., & Vermersch, D. (1998). Food safety issues and the BSE scare: some lessons from the French case. Food Policy, 23 (5), 347–356.
Laufer, R. (1993). L'entreprise face aux risques majeurs: a propos de l'incertitude des normes sociales. Paris: L'Harmattan.
Mer, R. (2001). Vache folle: du r°le des médias en temps de crise … Le Courrier de l'Environnement de l'INRA, 43 , 79–92.
Millstone, E., & van Zwanenberg, P. (2001). Politics of expert advice: lessons from the early history of the BSE saga. Science and Public Policy, 28 (2), 99–112.
Ortega, M. (1997 ). Sur les allégations d'infraction ou de mauvaise administration dans l'application du droit communautaire en matière d'ESB, sans préjudice des compétences des juridictions communautaires et nationales. Brussels, Belgium: European Parliament.
Parker, R. (2006). The politics of BSE. New York, Palgrave McMillan.
Phillips, N., Bridgeman, J., & Ferguson-Smith, M. (2000). The BSE inquiry: return to an order of the Honourable the House of Commons dated October 2000 for the Report, evidence and supporting papers of the Inquiry into the emergence and identification of Bovine Spongiform Encephalopathy (BSE) and variant Creutzfeldt-Jakob Disease (vCJD) and the action taken in response to it up to 20 March 1996. Norfolk, UK: The Stationary Office.
Power, M. (2004). The risk management of everything: rethinking the politics of uncertainty. London: Demos.
Sauvadet, F., & Vergnier, M (2001). Rapport fait au nom de la Commission d'enquête sur le recours aux farines animales dans l'alimentation des animaux d'élevage, la lutte contre l'encéphalopathie spongiforme bovine et les enseignements de la crise en termes de pratiques agricoles et de santé publique (Rapport, no 3138). Paris: Assemblée Nationale.
Savey, M. (1993). BSE risk assessment and surveillance in France (Proceedings of a consultation of the Directorate-General for Agriculture at the European Community (DG VI)). Brussels, Belgium: Commission of the European Communities.
Savey, M., & Baron, T. (1994). La BSE: un risque pour la santé publique? [BSE: a risk for public health?]. Revue de Médecine Vétérinaire, 145 (11), 819–827.
Savey, M., & Espinasse, J. (1979). Scrapie in the sheep and laboratory animals: suitability of the sheep model for the study of slow virus diseases. Veterinary Research Communications, 3 (1), 87–107.
Savey, M., Parodi, A.-L., & Maillot, E. (1989). L'encéphalopathie spongiforme bovine en Europe. Bulletin de l'Académie Vétérinaire de France, 62 , 483–490.
Setbon, M., Raude, J., Fischler, C., & Flahaukt, A. (2005). Risk perception of the “mad cow disease” in France: determinants and consequences. Risk Analysis, 25 (4), 813–826.
Southwood, R. (1989). Report of the working party on bovine spongiform encephalopathy . London, UK: Department of Health.
Taylor, D. (1996). Inactivation of the causal agents of Creutzfeldt–Jakob disease and other prion diseases. Brain Pathology 6(2), 197–198.
Taylor, D.M., Fraser, H., McConnel, I., Brown, D.A., Brown, K.L., Lawza, K.A., & Smith, G.R.A. (1994). Decontamination studies with the agents of bovine spongiform encephalopathy and scrapie. Archives of Virology 139(3–4), 313–326.
Torny, D. (1997). Surveiller et contenir. Trace des alertes aux prions. Alertes et propheties. In F. Chateauraynaud (Ed.), Les risques collectifs entre vigilance, controverse et critique (volume II, pp. 138–309). Paris: EHESS, Groupe de Sociologie Politique et Morale.
Van Zwanenberg, P., & Millstone, E. (1999). BSE and the UK national action system (Report for the BASES EU project). Brighton, UK: Science and Technology Policy Research, University of Sussex.
Wells, G. A., Scott, A. C., Johnson, C. T., Gunning, R. F., Hancock, R.D., Jeffrey, M., et al. (1987). A novel progressive spongiform encephalopathy in cattle. The Veterinary Record, 121 (18), 419–420.
Will, R. G., Ironside, J. W., Zeidler, M., Cousens, S. N., Estibeiro, K., Alperovitch, A., et al. (1996). A new variant of Creutzfeldt-Jakob disease in the UK. The Lancet, 347 (9006), 921–925.
Article CAS Google Scholar
Wolfer, B. (2004). La crise des farines. Ivry sur Seine, France: INRA/MONA.
Download references
Author information
Authors and affiliations.
UR 1216 TSV (Transformations Sociales et Politiques liées au Vivant), French National Institute for Agricultural Research, Paris, France
Pierre-Benoit Joly
Department of Public Health, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
Hajime Sato
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Hajime Sato .
Editor information
Editors and affiliations.
Graduate School of Medicine &, University of Tokyo, Hongo 7-3-1, Tokyo, 113-0033, Japan
Rights and permissions
Reprints and permissions
Copyright information
© 2009 Springer Science+Business Media B.V.
About this chapter
Joly, PB., Sato, H. (2009). Governing Uncertain Threats: Lessons from the Mad Cow Saga in France. In: Sato, H. (eds) Management of Health Risks from Environment and Food. Alliance for Global Sustainability Bookseries, vol 16. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-3028-3_9
Download citation
DOI : https://doi.org/10.1007/978-90-481-3028-3_9
Published : 06 September 2009
Publisher Name : Springer, Dordrecht
Print ISBN : 978-90-481-3027-6
Online ISBN : 978-90-481-3028-3
eBook Packages : Engineering Engineering (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Mad Cow Disease (Bovine Spongiform Encephalopathy)
Mad cow disease, or bovine spongiform encephalopathy (BSE), is a disease that was first found in cattle. It's related to a disease in humans called variant Creutzfeldt-Jakob disease (vCJD). Both disorders are universally fatal brain diseases caused by a prion. A prion is a protein particle that lacks DNA (nucleic acid). It's believed to be the cause of various infectious diseases of the nervous system. Eating infected cattle products, including beef, can cause a human to develop mad cow disease.
What is mad cow disease?
Mad cow disease is a progressive, fatal neurological disorder of cattle resulting from infection by a prion. It appears to be caused by contaminated cattle feed that contains the prion agent. Most mad cow disease has happened in cattle in the United Kingdom (U.K.), a few cases were found in cattle in the U.S. between 2003 and 2006. Feed regulations were then tightened.
In addition to the cases of mad cow reported in the U.K. (78% of all cases were reported there) and the U.S., cases have also been reported in other countries, including France, Spain, Netherlands, Portugal, Ireland, Italy, Japan, Saudi Arabia, and Canada. Public health control measures have been implemented in many of the countries to prevent potentially infected tissues from entering the human food chain. These preventative measures appear to have been effective. For instance, Canada believes its prevention measures will wipe out the disease from its cattle population by 2017.
What is variant Creutzfeldt-Jakob Disease (vCJD)?
Creutzfeldt-Jakob Disease (CJD) is a rare, fatal brain disorder. It causes a rapid, progressive dementia (deterioration of mental functions), as well as associated neuromuscular disturbances. The disease, which in some ways resembles mad cow disease, traditionally has affected men and women between the ages of 50 and 75. The variant form, however, affects younger people (the average age of onset is 28) and has observed features that are not typical as compared with CJD. About 230 people with vCJD have been identified since 1996. Most are from the U.K. and other countries in Europe. It is rare in the U.S., with only 4 reported cases since 1996.
What is the current risk of acquiring vCJD from eating beef and beef products produced from cattle in Europe?
Currently this risk appears to be very small, perhaps fewer than 1 case per 10 billion servings--if the risk exists at all. Travelers to Europe who are concerned about reducing any risk of exposure can avoid beef and beef products altogether, or can select beef or beef products, such as solid pieces of muscle meat, as opposed to ground beef and sausages. Solid pieces of beef are less likely to be contaminated with tissues that may hide the mad cow agent. Milk and milk products are not believed to transmit the mad cow agent. You can't get vCJD or CJD by direct contact with a person who has the disease. Three cases acquired during transfusion of blood from an infected donor have been reported in the U.K. Most human Creutzfeldt-Jakob disease is not vCJD and is not related to beef consumption but is also likely due to prion proteins
Find a Treatment Center
- Neurology and Neurosurgery
Find Additional Treatment Centers at:
- Howard County Medical Center
- Sibley Memorial Hospital
- Suburban Hospital
Request an Appointment

Facioscapulohumeral Muscular Dystrophy in Children

Moyamoya Disease
Motor Stereotypies
Related Topics
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Animal & Veterinary
- Resources for You
- Animal Health Literacy
All About BSE (Mad Cow Disease)

The word BSE is short but it stands for a disease with a long name, bovine spongiform encephalopathy. "Bovine" means that the disease affects cows, "spongiform" refers to the way the brain from a sick cow looks spongy under a microscope, and "encephalopathy" indicates that it is a disease of the brain. BSE is commonly called “mad cow disease.”
What is BSE?
BSE is a progressive neurologic disease of cows. Progressive means that it gets worse over time. Neurologic means that it damages a cow’s central nervous system (brain and spinal cord).
What Causes BSE?
Most scientists think that BSE is caused by a protein called a prion. For reasons that are not completely understood, the normal prion protein changes into an abnormal prion protein that is harmful. The body of a sick cow does not even know the abnormal prion is there. Without knowing it is there, the cow’s body cannot fight off the disease.
What are the Signs of BSE in Cows?
A common sign of BSE in cows is incoordination. A sick cow has trouble walking and getting up. A sick cow may also act very nervous or violent, which is why BSE is often called “mad cow disease.”
It usually takes four to six years from the time a cow is infected with the abnormal prion to when it first shows symptoms of BSE. This is called the incubation period. During the incubation period, there is no way to tell that a cow has BSE by looking at it. Once a cow starts to show symptoms, it gets sicker and sicker until it dies, usually within two weeks to six months. There is no treatment for BSE and no vaccine to prevent it.
Currently, there is no reliable way to test for BSE in a live cow. After a cow dies, scientists can tell if it had BSE by looking at its brain tissue under a microscope and seeing the spongy appearance. Scientists can also tell if a cow had BSE by using test kits that can detect the abnormal prion in the brain.
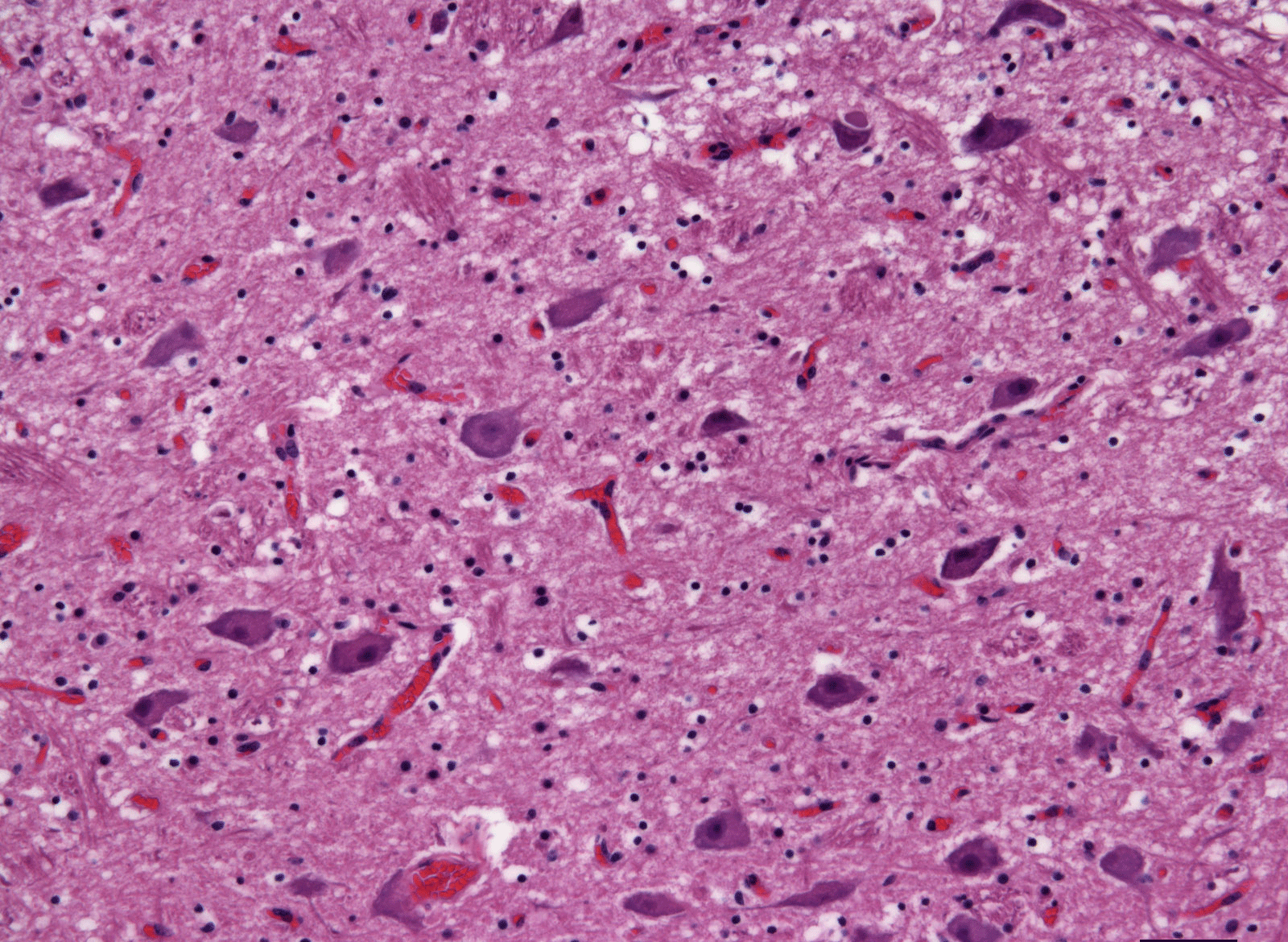
Brain from a healthy cow, as seen under a microscope using special stains.
Photo courtesy of Dr. Katie Kelly, Johns Hopkins University
Brain from a cow sick with BSE, as seen under a microscope using special stains. The large white spaces are like the "holes" of a sponge.
Photo courtesy of the late Dr. Al Jenny, USDA

How Does a Cow Get BSE?

The parts of a cow that are not eaten by people are cooked, dried, and ground into a powder. The powder is then used for a variety of purposes, including as an ingredient in animal feed. A cow gets BSE by eating feed contaminated with parts that came from another cow that was sick with BSE. The contaminated feed contains the abnormal prion, and a cow becomes infected with the abnormal prion when it eats the feed. If a cow gets BSE, it most likely ate the contaminated feed during its first year of life. Remember, if a cow becomes infected with the abnormal prion when it is one-year-old, it usually will not show signs of BSE until it is five-years-old or older.
Can People Get BSE?
People can get a version of BSE called variant Creutzfeldt-Jakob disease (vCJD). As of 2019, 232 people worldwide are known to have become sick with vCJD, and unfortunately, they all have died. It is thought that they got the disease from eating food made from cows sick with BSE. Most of the people who have become sick with vCJD lived in the United Kingdom at some point in their lives. Only four lived in the U.S., and most likely, these four people became infected when they were living or traveling overseas.
Neither vCJD nor BSE is contagious. This means that it is not like catching a cold. A person (or a cow) cannot catch it from being near a sick person or cow. Also, research studies have shown that people cannot get BSE from drinking milk or eating dairy products, even if the milk came from a sick cow.
What is the FDA Doing to Keep Your Food Safe?
The U.S. Food and Drug Administration (FDA) is doing many things to keep the food in the U.S. safe for both people and cows. Since August 1997, the FDA has not allowed most parts from cows and certain other animals to be used to make food that is fed to cows. This protects healthy cows from getting BSE by making sure that the food they eat is not contaminated with the abnormal prion.

In April 2009, the FDA took additional steps to make sure the food in the U.S. stays safe. Certain high-risk cow parts are not allowed to be used to make any animal feed, including pet food. This prevents all animal feed from being accidentally contaminated with the abnormal prion. High-risk cow parts are those parts of the cow that have the highest chance of being infected with the abnormal prion, such as the brains and spinal cords from cows that are 30 months of age or older.
By keeping the food that is fed to cows safe, the FDA is protecting people by making sure that the food they eat comes from healthy cows.
The FDA also works with the U.S. Department of Agriculture (USDA) to keep cows in the U.S. healthy and free of BSE. The USDA prevents high-risk cows and cow products from entering the U.S. from other countries. The USDA also makes sure that high-risk cow parts, such as the brains and spinal cords, and cows that are unable to walk or that show other signs of disease are not used to make food for people.
The steps the FDA and USDA have taken to prevent cows in the U.S. from getting BSE are working very well. Only six cows with BSE have been found in the U.S. The first case was reported in 2003 and the most recent case was found in August 2018.
It is worth noting that there are two types of BSE, classical and atypical. Classical is caused by contaminated feed fed to cows. Atypical is rarer and happens spontaneously, usually in cows 8-years-old or older. Of the six U.S. cows found with BSE, five were atypical. The only case of classical BSE in the U.S. was the first one, in 2003, in a cow imported from Canada.
Can Other Animals Get BSE?

Sheep, goats, mink, deer, and elk can get sick with their own versions of BSE. Cats are the only common household pet known to have a version of BSE. It is called feline spongiform encephalopathy, and the same things that are being done to protect people and cows are also protecting cats. No cat in the U.S. has ever been found to have this disease.
How Can I Get More Information?
- Contact the FDA’s Center for Veterinary Medicine at 240-402-7002 or [email protected] .
- U.S. Department of Agriculture, BSE Frequently Asked Questions
- Centers for Disease Control and Prevention, Variant Creutzfeldt-Jakob Disease (vCJD)
- Centers for Disease Control and Prevention, BSE Cases Identified in the United States
- University of Edinburgh, The National CJD Research & Surveillance Unit (NCJDRSU)
Coronavirus (COVID-19): Latest Updates | Visitation Policies Visitation Policies Visitation Policies Visitation Policies Visitation Policies | COVID-19 Testing | Vaccine Information Vaccine Information Vaccine Information
Health Encyclopedia
Mad cow disease (spongiform encephalopathy or bse).
Mad cow disease, or bovine spongiform encephalopathy (BSE), is a disease that was first found in cattle. It's related to a disease in humans called variant Creutzfeldt-Jakob disease (vCJD). Both disorders are universally fatal brain diseases caused by a prion. A prion is a protein particle that lacks DNA (nucleic acid). It's believed to be the cause of various infectious diseases of the nervous system. Eating infected cattle products, including beef, can cause a human to develop vCJD.
What is mad cow disease?
Mad cow disease is a progressive, fatal neurological disorder of cattle resulting from infection by a prion. It appears to be caused by contaminated cattle feed that contains the prion agent. Most mad cow disease has happened in cattle in the United Kingdom (U.K.), and a few cases were found in cattle in the U.S. between 2003 and 2006. Feed regulations were then tightened.
In addition to the cases of mad cow reported in the U.K. (78% of all cases were reported there) and the U.S., cases have also been reported in other countries, including France, Spain, the Netherlands, Portugal, Ireland, Italy, Japan, Saudi Arabia, and Canada. Public health control measures have been implemented in many countries to prevent potentially infected tissues from entering the human food chain. These preventative measures appear to have been effective.
What is variant Creutzfeldt-Jakob Disease (vCJD)?
Creutzfeldt-Jakob Disease (CJD) is a rare, fatal brain disorder. It causes a rapid, progressive dementia (deterioration of mental functions), as well as associated neuromuscular disturbances. The disease, which in some ways resembles mad cow disease, traditionally has affected men and women between the ages of 50 and 75. The variant form, vCJD, however, affects younger people (the average age of onset is 28) and has observed features that are not typical as compared with CJD. About 230 people with vCJD have been identified since 1996. Most are from the U.K. and other countries in Europe. It's rare in the U.S.
What is the current risk of getting vCJD from eating beef and beef products from cattle in Europe?
Currently, this risk appears to be very small, perhaps fewer than 1 case per 10 billion servings, if the risk exists at all. Travelers to Europe who are concerned about reducing any risk of exposure can avoid beef and beef products altogether, or can select beef or beef products such as solid pieces of muscle meat as opposed to ground beef and sausages. Solid pieces of beef are less likely to be contaminated with tissues that may hide the mad cow agent. Milk and milk products are not believed to transmit the mad cow agent. You can't get vCJD or CJD by direct contact with a person who has the disease. A few cases were acquired during the transfusion of blood from an infected donor. Most human Creutzfeldt-Jakob disease is not vCJD and is not related to beef consumption but is also likely due to prion proteins.
Medical Reviewers:
- Joseph Campellone MD
- Melinda Murray Ratini DO
- Raymond Kent Turley BSN MSN RN
- Ask a Medical Librarian Make an Appointment Related News International Authority Coming to Address 'Mad Cow' Fears
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Researchers link mad-cow culprit to stem cell health
What does mad cow disease have in common with stem cell research? MIT and Whitehead Institute scientists have found that the same protein that causes neurodegenerative conditions such as bovine spongiform encephalopathy (mad cow disease) is also important for helping certain adult stem cells maintain themselves.
"For years we've wondered why evolution has preserved this protein, what positive role it could possibly be playing," says MIT professor of biology and Whitehead member Susan Lindquist. "With these findings, we have our first answer."
Lindquist, Harvey Lodish (also an MIT biology professor and Whitehead member), and colleagues are co-authors on a paper to be published online in the Proceedings of the National Academy of Sciences during the week of Jan. 30.
For more than 10 years, researchers have known that a protein called PrP causes mad cow disease and its human equivalent, Creutzfeld-Jakob disease. PrP is a prion, a class of proteins that has the unusual ability to recruit other proteins to change their shape (PrP is shorthand for "prion protein."). This is significant, because a protein's form determines its function. When a prion changes shape, or "misfolds," it creates a cascade in which neighboring proteins all assume that particular conformation. In some organisms, such as yeast cells, this process can be harmless, even beneficial. But in mammals, it can lead to the fatal brain lesions that characterize diseases such as Creutzfeld-Jakob.
Curiously, however, PrP can be found throughout healthy human bodies, particularly in the brain where it's highly abundant. In fact, it's found in many mammalian species, and only on the rarest occasions does it result in disease. Clearly, scientists have reasoned, such a widely conserved protein also must play a positive role.
Chengcheng Zhang, a postdoctoral researcher in Lodish's lab, was studying hematopoietic (blood forming) stem cells in mouse fetal tissue when he discovered that PrP was expressed abundantly on the surfaces of these stem cells. "I found that while not all blood cells with PrP on their surface were stem cells, any cell that lacked PrP was definitely not a stem cell," says Zhang.
Zhang teamed up with the Lindquist lab's graduate student Andrew Steele, an expert in prions, to discover what role PrP might play in stem cell biology. Zhang and Steele took bone marrow from mice in which PrP had been knocked out, and transferred that marrow into normal mice whose blood and immune systems had been irradiated. The new bone marrow took hold, and these mice flourished, although all their blood cells lacked PrP. Zhang and Steele continued the experiment, this time taking bone marrow from the newly reconstituted mice, and transplanting it into another group of mice. They repeated this process again and again -- transplanting bone marrow from one group of mice to another like passing a baton.
Soon they noticed that with each subsequent transplant, the stem cells began to lose their ability to reconstitute. Eventually, the scientists ended up with mice whose hematopoietic stem cells completely lacked the ability to generate new cells. However, in the control group, where they mimicked the experiment with bone marrow abundant with PrP, each transplant was as good as the next, and at no point did stem cells lose their efficacy.
"Clearly, PrP is important for maintaining stem cells," says Lodish. "We're not sure yet how it does this, but the correlation is obvious."
"PrP is a real black box," Lindquist says. "This is the first clear indication we have of a beneficial role for it in a living animal. Now we need to discover its molecular mechanism."
This research was funded by the National Science Foundation, the National Institutes of Health, the Ellison Medical Research Foundation and the Leukemia and Lymphoma Society.
A version of this article appeared in MIT Tech Talk on February 1, 2006 (download PDF) .
Share this news article on:
Related links.
- Lab sheds light on prions
- Susan Lindquist
- Harvey Lodish
- Whitehead Institute for Biomedical Research
Related Topics
- Bioengineering and biotechnology
- Neuroscience
More MIT News

How AI might shape LGBTQIA+ advocacy
Read full story →

Two MIT PhD students awarded J-WAFS fellowships for their research on water

Exploring the mysterious alphabet of sperm whales

“Pathways to Invention” documentary debuts on PBS, streaming

This sound-suppressing silk can create quiet spaces

William Green named director of MIT Energy Initiative
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
![ARUK-FOR-A-CURE logo Core-colours-RGB[1] alzheimer's research uk for a cure logo](https://www.alzheimersresearchuk.org/wp-content/uploads/2023/05/ARUK-FOR-A-CURE-logo-Core-colours-RGB1-1024x192.png)
What can mad cow disease tell us about dementia?
« Back to all news

By Ed Pinches | Tuesday 02 April 2019
Earlier this month, at the Alzheimer’s Research UK Conference in Harrogate, Dr Jason Sang presented his latest research on how harmful proteins can spread through the brain.
He revealed fascinating insights on how we can learn from the science behind diseases like Creutzfeldt Jakob Disease (CJD) in humans, or BSE in cattle (also known as mad cow disease), to make progress in tackling the diseases that cause dementia.
Problem proteins
Many diseases including those like Alzheimer’s occur when important proteins in our bodies go wrong.
Proteins are the building blocks of our body’s cells. They need to be in the right place, forming the right shapes and in the right numbers for any cell to do its job properly. If just one of these factors is not as it should be, things can start to go wrong.
One protein, known as the prion protein is found on the surface of cells in number of different organs and tissues in the body. Its exact function in healthy cells is complex, but it is how it acts during disease that is unlocking clues to dementia.
The prion protein
The prion protein is infamous for its role in the development of the rare diseases CJD and BSE. These rare diseases develop when an abnormally-folded prion protein triggers a healthy one to also fold incorrectly. This kicks off a chain reaction that causes a large build-up of prion protein inside nerve cells causing disease.
In CJD, this abnormal protein can be passed from human to human or even animal to human through contaminated tissue, although not through the air or by direct contact. But how does this prion protein have anything to do with the diseases like Alzheimer’s that cause dementia?
Research winner

Dr Sang, has now received a top award at Alzheimer’s Research UK’s leading dementia conference for his work to understand how these proteins spread through the brain.
“Winning the Jean Corsan Prize is the highlight of my career so far. It’s an honour to receive this award and be given an opportunity to present my work to world experts at the Alzheimer’s Research UK conference.”
In his award-winning paper , Dr Sang used a special experimental system to study how the prion protein spreads through the brain.
His research technique involved carefully processing samples of synthetic prion protein from mice. Using powerful microscopes, Dr Sang measured how the proteins spread through the brain.
His work also sheds more light on the mechanism of another protein called alpha-synuclein. Alpha-synuclein is the protein that builds up in the brain in dementia with Lewy bodies , the third most common cause of dementia, as well as in Parkinson’s disease.
Dr Sang found that although alpha-synuclein spreads through the brain in a remarkably similar way to the prion protein, the spread is much slower.
Studying this process for Dr Sang is painstaking work. It involves studying the proteins in very controlled studies at constant temperature, shaking his samples at a precise speed. Everything must run like clock-work, day after day in the laboratory.

His research is almost an art form. But Jason say’s it’s worth it:
“Making discoveries like this requires dedication and if it advances our understanding of the way diseases develop it will get us one step closer to a real breakthrough.”
With your support, Alzheimer’s Research UK is now funding a £50,000 research study at University College London to look at blood-based markers of disease. It is hoped this will help scientists measure disease progression in prion-like diseases. It’s work like this, that will give us more insight into the disease itself and give us vital clues about how we can also overcome the diseases that cause dementia.

Donate to support pioneering research like this at https://alzheimersresearchuk.org/support-us/donate/make-a-donation/
If you yourself want to get involved in research, contact the Dementia Research Infoline on 0300 111 5 111
- share
By submitting a comment you agree to our comments policy . Please do not post any personal information about yourself or anyone else, especially any health data or other sensitive data. If you do submit sensitive data, you consent to us handling it in line with our comments policy.
Leave a Comment Cancel Reply
About the author.
Team: Science news

Privacy overview

IMAGES
COMMENTS
Bovine spongiform encephalopathy (BSE), also known as "mad cow disease," and variant Creutzfeldt-Jakob disease (vCJD) are the cattle and human forms of a group of relentlessly progressive neurodegenerative diseases known as the transmissible spongiform encephalopathies. There are acquired and hereditary forms of the diseases, and the ...
Tiny Matters July 26, 2023. Mad cow disease, also known as bovine spongiform encephalopathy (BSE) was first discovered in cattle in the UK in 1986. In 1996, BSE made its way into humans for the first time, setting off panic and fascination with the fatal disease that causes rapid onset dementia. In this episode, Sam and Deboki cover the cause ...
Emergence of a New Creutzfeldt-Jakob Disease: 26 Cases of the Human Version of Mad-Cow Disease, Days After a COVID-19 Injection January 2023 DOI: 10.5281/zenodo.7540331
A possible link between bovine spongiform encephalopathy, commonly called "mad cow" disease, and Creutzfeldt-Jakob disease, the fatal human equivalent, has been announced. Both of these diseases are forms of transmissible spongiform encephalopathies that attack the brain and destroy its nerve cells. That a disease identified with cows since ...
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on MAD COW DISEASE. Find methods information, sources, references or conduct a literature review on MAD ...
BSE is a transmissible spongiform encephalitis (TSE) caused by prion protein. Transmissible spongiform encephalopathies (TSEs) are characterised by fatal neurodegenerative changes in the brain, abnormal behaviour and death affecting man and several species of animals (Table 26.1). TSEs or prion protein diseases are inherited and infectious.
also known as 'mad cow disease', is not merely a UK phenome- ... research, both at the organismal and the molecular level. Prions ... These papers cover diverse topics: the species selectivity ...
Abstract. The mad cow crisis has often been described as emblematic of the "risk society," for at least three reasons. First, the dangers to human health from beef consumption were not the result of fate; rather, they were "manufactured" (Giddens 1994, 1997) and a byproduct of modernization. Second, science was at the core of the crisis.
Also Private Eye, 11 January 1997 for the British E coli outbreak, the deathrate from which is greater than that from the new CJD variant in humans. 10 J.R Fisher, 'British Physicians, Medical Science, and the Cattle Plague, 1865-66', Bulletin of the Htstory of Medicine, 67, 1993, 673-5. 11 L. Garrett, The Coming Plague (London 1993), 281-390 ...
A common genetic factor may give protection against the human form of mad cow disease. Researchers have pinned down a genetic variation that may help protect against variant Creutzfeldt-Jakob disease (vCJD)--the fatal neurodegenerative disease linked to eating cattle infected with "mad cow disease." The new finding--the second genetic factor ...
Exploring the Transmissible Zoonotic Potential of Animal Prion Diseases. Medicine, Environmental Science. 2016. TLDR. Mad Cow disease is a fatal and serious disease often found in Europe and is often characterized by vacuoles found within the cytoplasm of neurons, which often leads to the appearance of a spongy like brain. Expand.
Brain, Nerves and Spine. Mad cow disease, or bovine spongiform encephalopathy (BSE), is a disease that was first found in cattle. It's related to a disease in humans called variant Creutzfeldt-Jakob disease (vCJD). Both disorders are universally fatal brain diseases caused by a prion. A prion is a protein particle that lacks DNA (nucleic acid).
With the ongoing UK government-funded public inquiry into bovine spongiform encephalopathy (BSE) under Sir Nicholas Philipps, there is renewed interest and media coverage in what has been termed "the biggest governmental, economical and public health crisis in the UK". We are now looking back more than 8 years to the start of the mad-cow story with few scientific certainties but much more ...
First release papers; Archive; ... And research during the previous decade had strongly implicated prions in some human neurodegenerative diseases such as kuru, a CJD-like disease discovered in the Fore people of New Guinea and thought to be transmitted directly or indirectly through cannibalism. ... Tracking the Human Fallout From 'Mad Cow ...
First release papers; Archive; ... GET OUR E-ALERTS. Home Science Vol. 249, No. 4976 Mad Cow Disease: Uncertainty Rules. Back To Vol. 249, No. 4976. Full access. News & Comment. ... Subscribe to ScienceAdviser to get the latest news, commentary, and research, free to your inbox daily. Subscribe. LATEST NEWS. News 26 Apr 2024.
A sick cow has trouble walking and getting up. A sick cow may also act very nervous or violent, which is why BSE is often called "mad cow disease.". It usually takes four to six years from the ...
Mad cow disease is a progressive, fatal neurological disorder of cattle resulting from infection by a prion. It appears to be caused by contaminated cattle feed that contains the prion agent. Most mad cow disease has happened in cattle in the United Kingdom (U.K.), and a few cases were found in cattle in the U.S. between 2003 and 2006.
What does mad cow disease have in common with stem cell research? MIT and Whitehead Institute scientists have found that the same protein that causes neurodegenerative conditions such as bovine spongiform encephalopathy (mad cow disease) is also important for helping certain adult stem cells maintain themselves.
The government now plans to test more than 1000 of the 800,000 samples of tonsil and 45,000 samples of appendix removed and stored each year. Comparison of tissue removed before and at the height of the mad cow disease epidemic in the late 1980s may provide a rough assessment of the extent to which the population harbors nvCJD prion proteins.
If you yourself want to get involved in research, contact the Dementia Research Infoline on 0300 111 5 111. Dr Jason Sang revealed fascinating insights on how we can learn from the science behind diseases like Creutzfeldt Jakob Disease (CJD) in humans, or BSE in cattle (also known as mad cow disease), to make progress in tackling the diseases ...